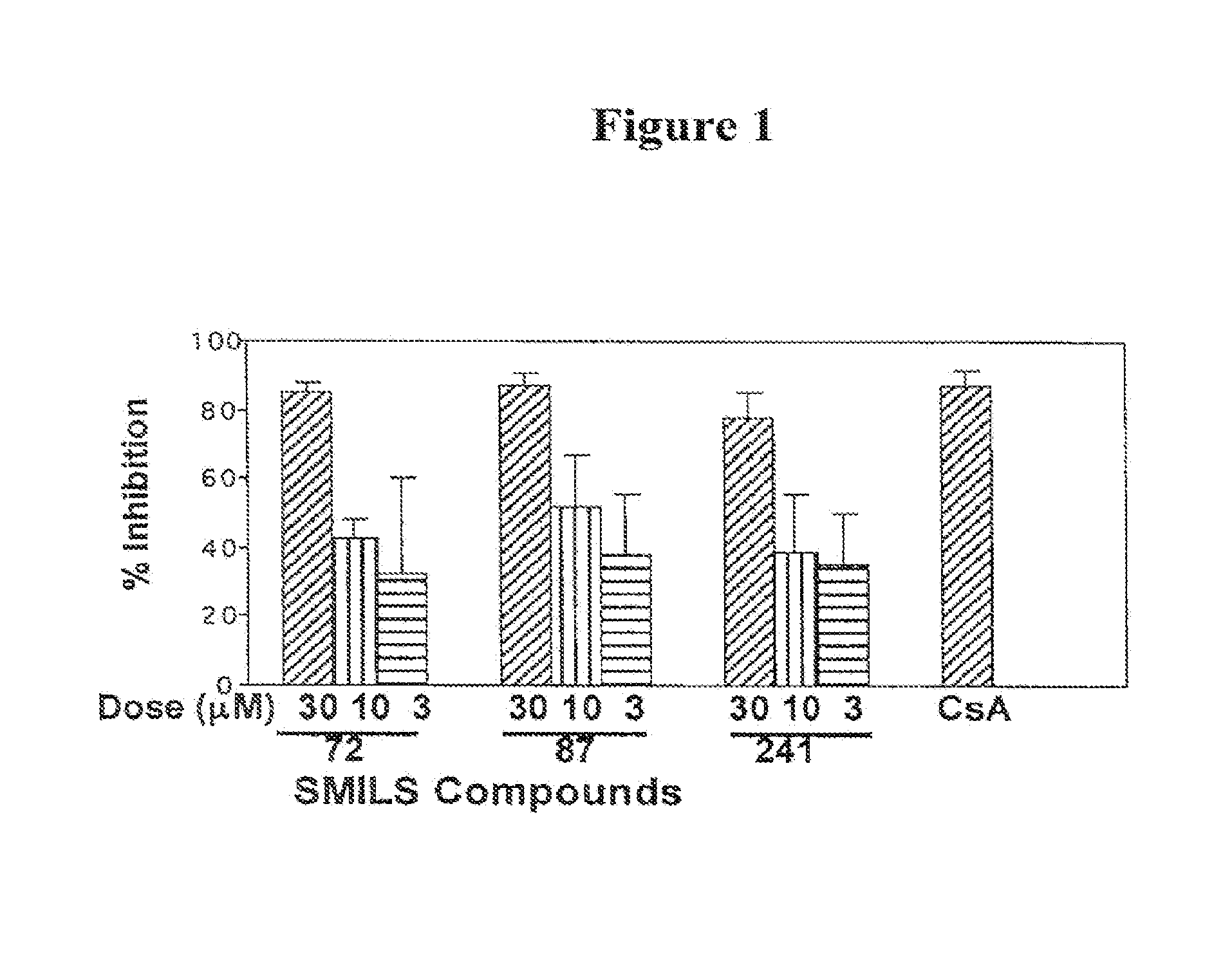
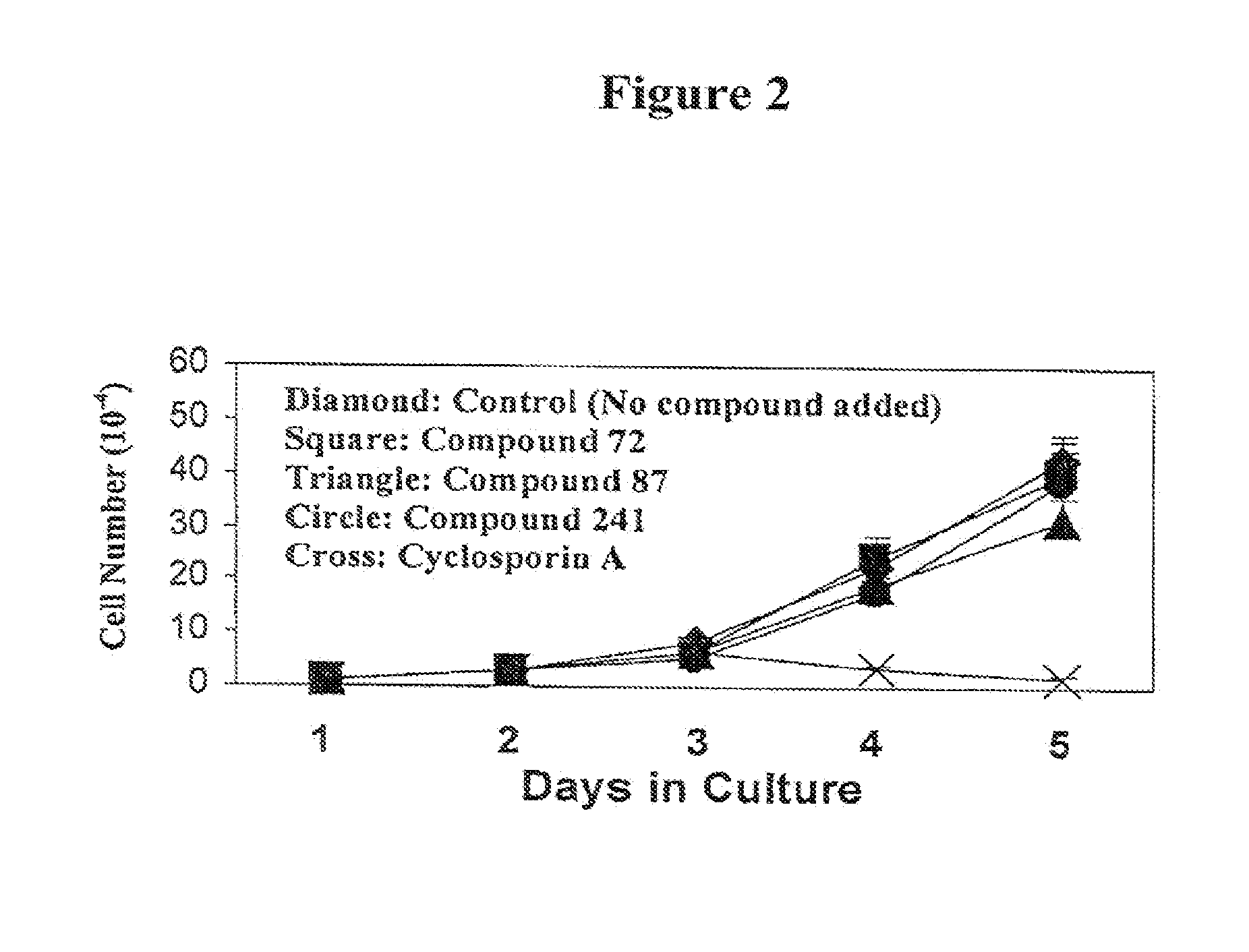
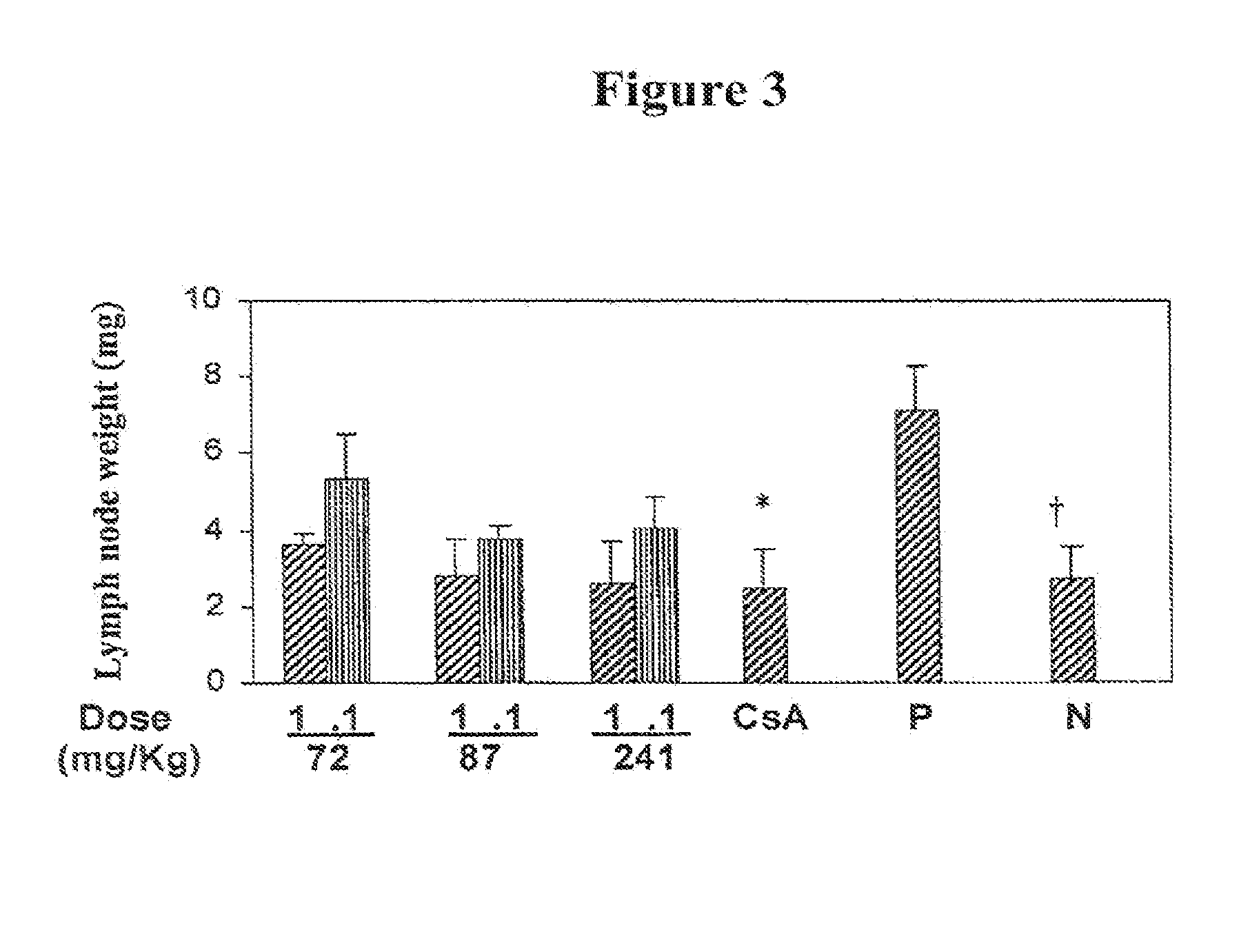
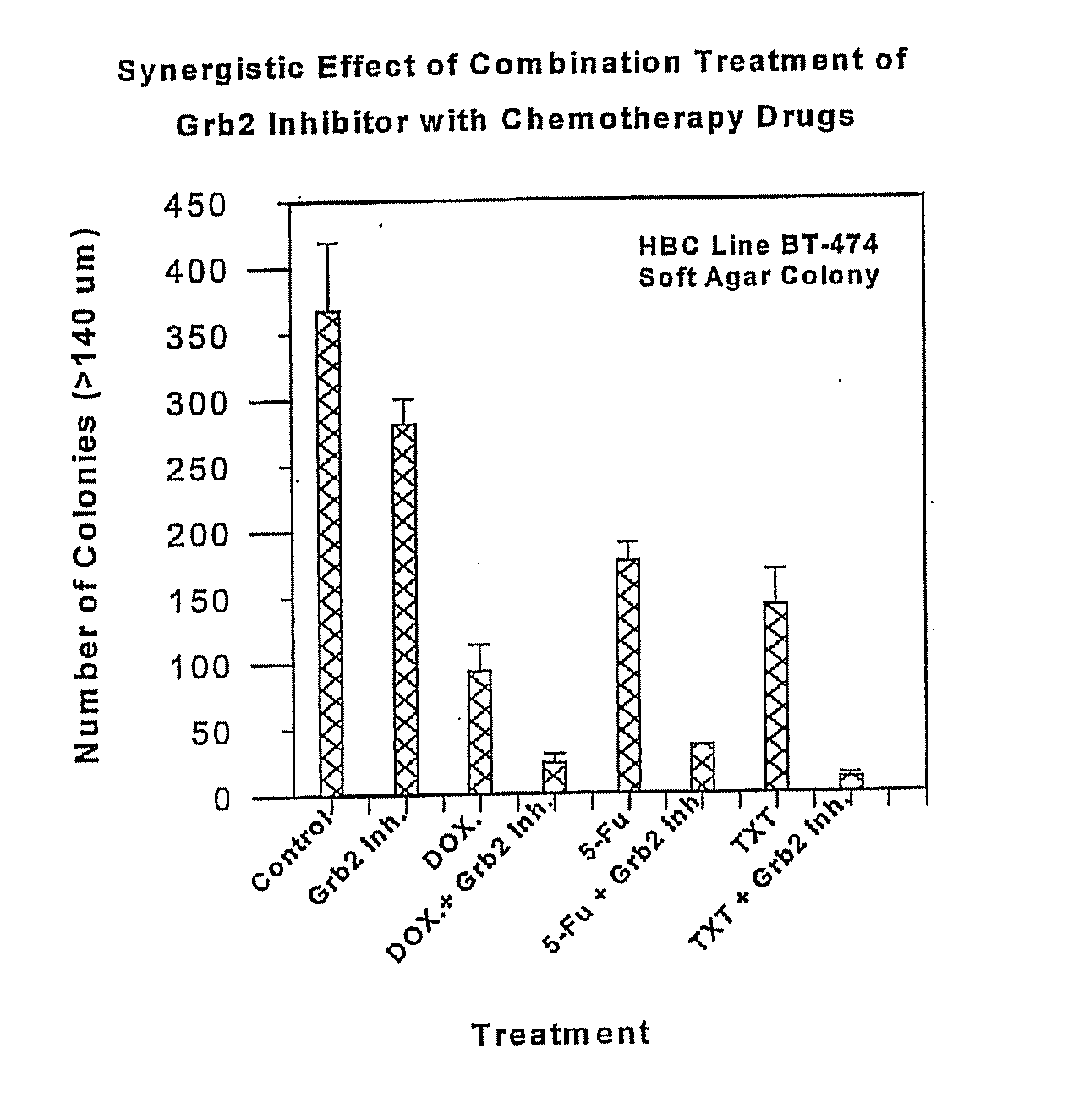
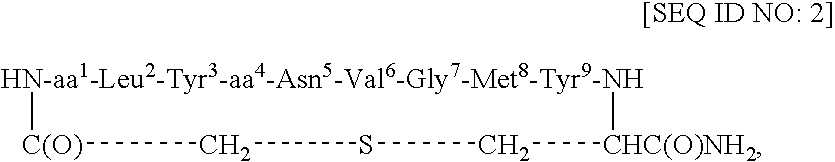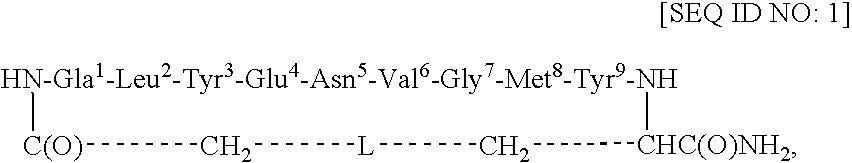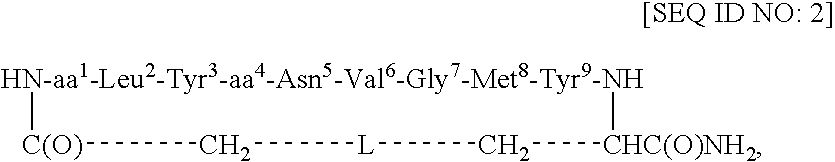Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "SH2 domain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
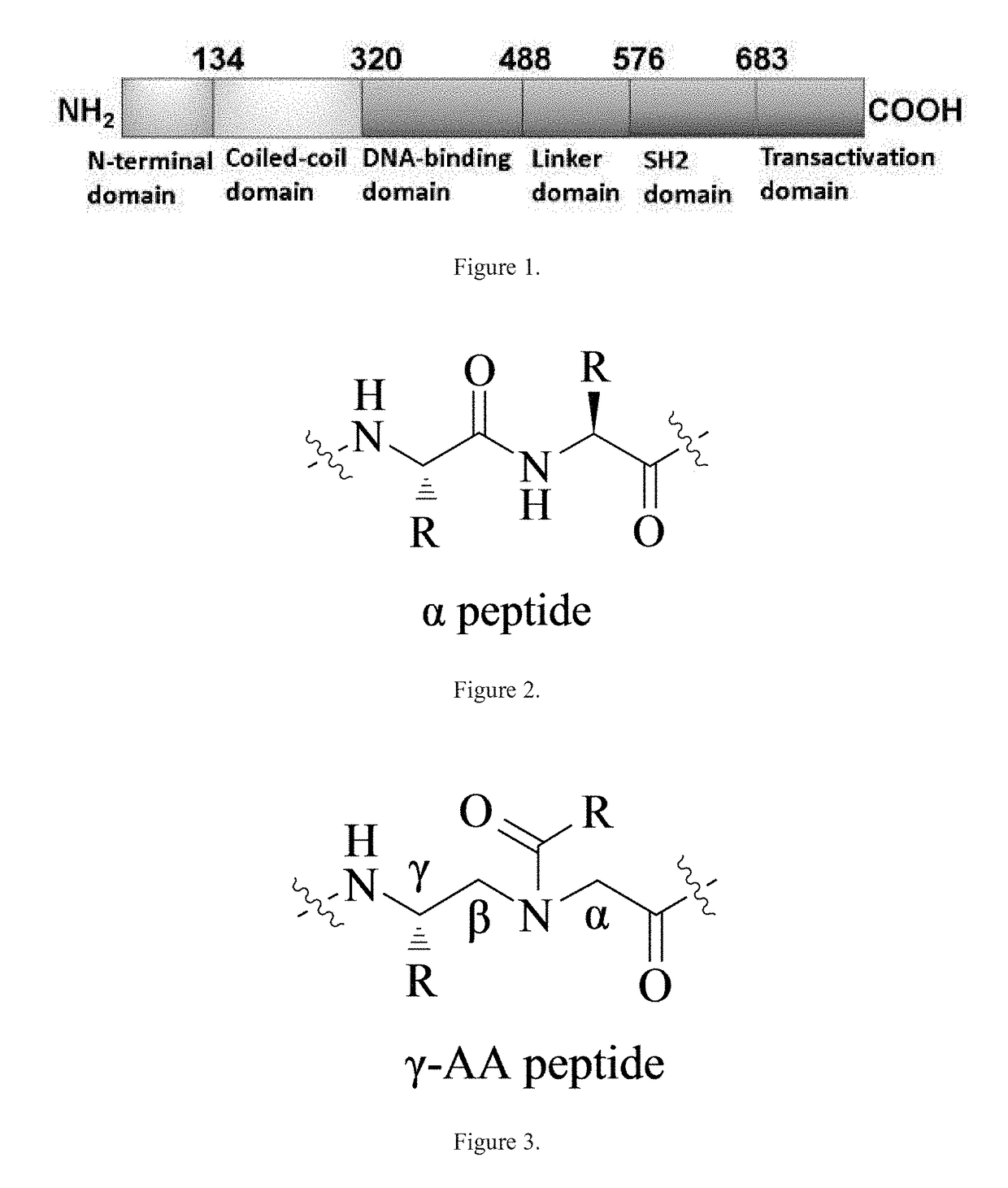
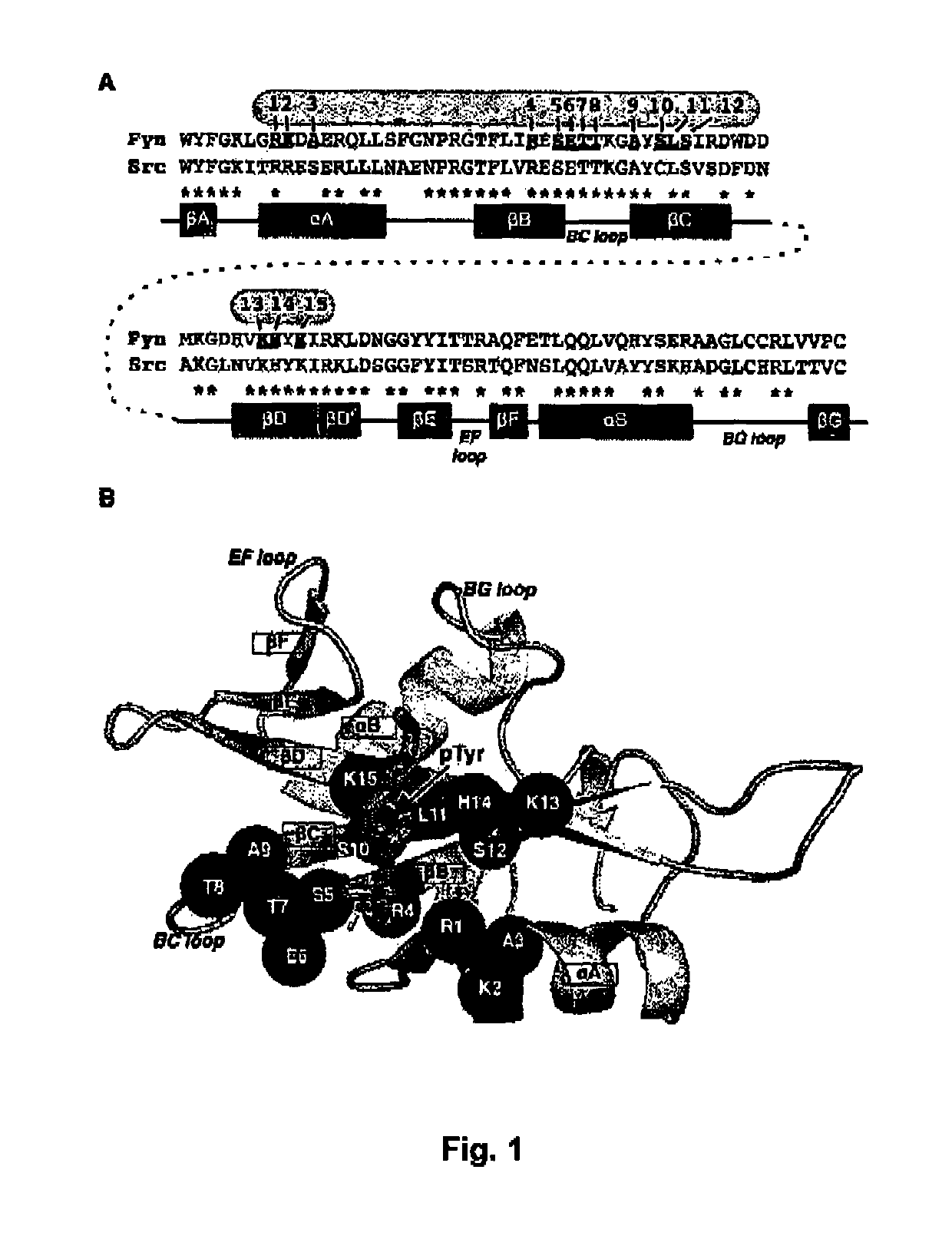
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains allow proteins containing those domains to dock to phosphorylated tyrosine residues on other proteins. SH2 domains are commonly found in adaptor proteins that aid in the signal transduction of receptor tyrosine kinase pathways.
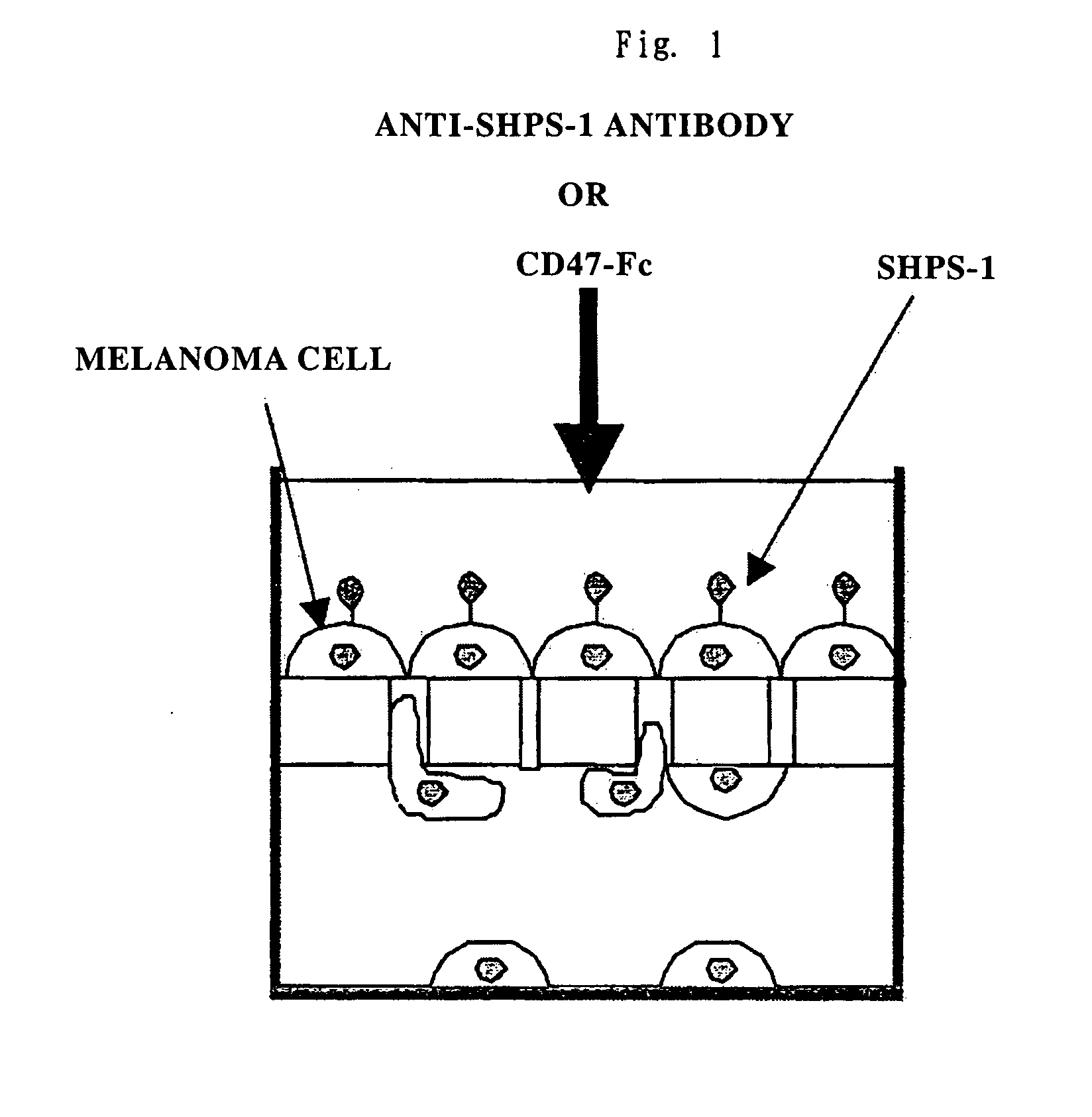
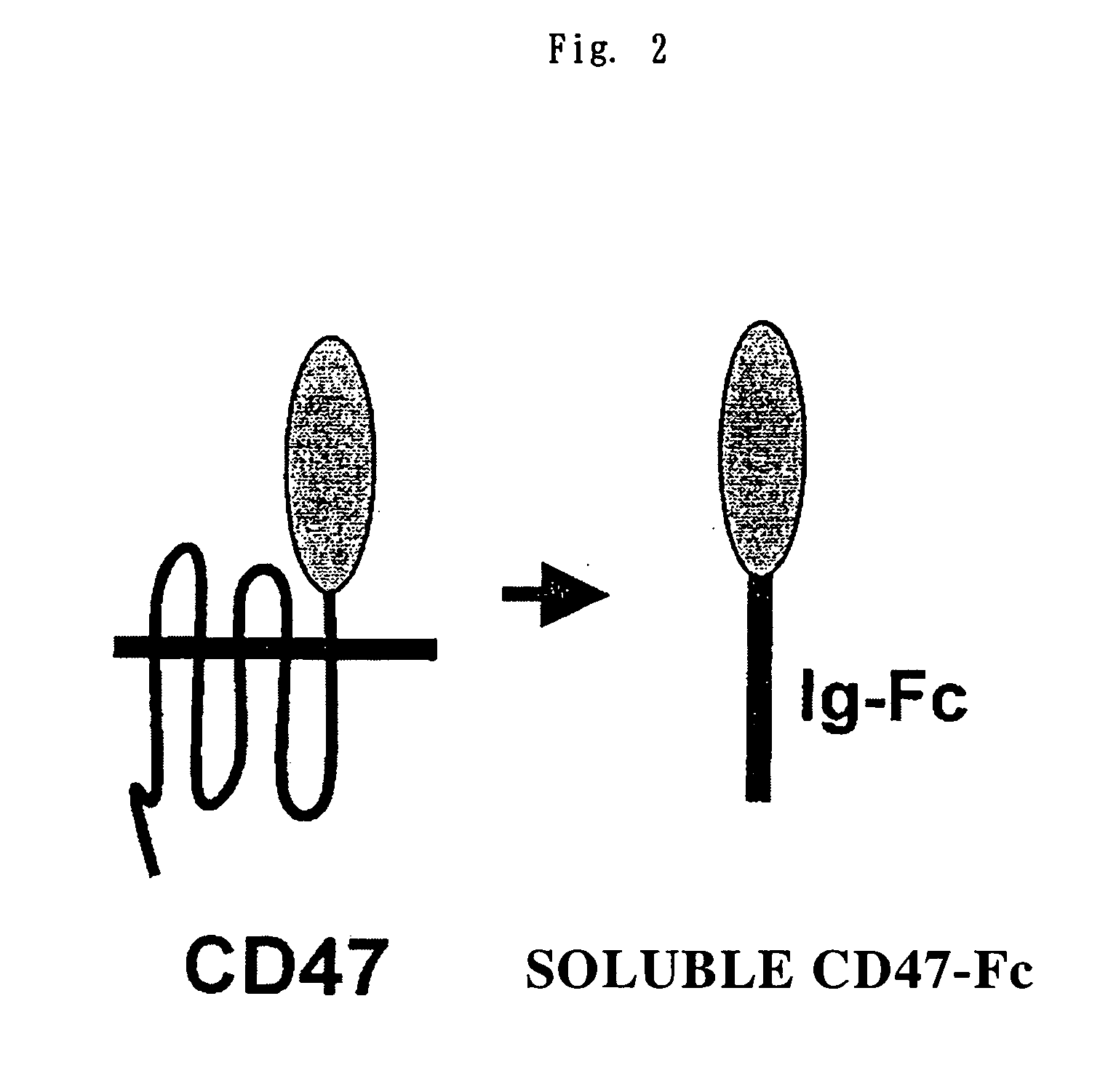
Cd47 partal peptdie and anti-shps-1 monoclonal antibody
A CD47 partial peptide which has an amino acid sequence constituting an extracellular region having an immunoglobulin-like structure of a CD47 protein, which is specifically bound to an N-terminal immunoglobulin-like structure of a dephosphorylation substrate protein SHPS-1 of an SH2 domain-containing protein, and which can act on a function of cell response mediated by SHPS-1, and an anti-SHPS-1 monoclonal antibody.
Owner:GUNMA UNIVERSITY
Compositions and methods for producing bioactive fusion proteins
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
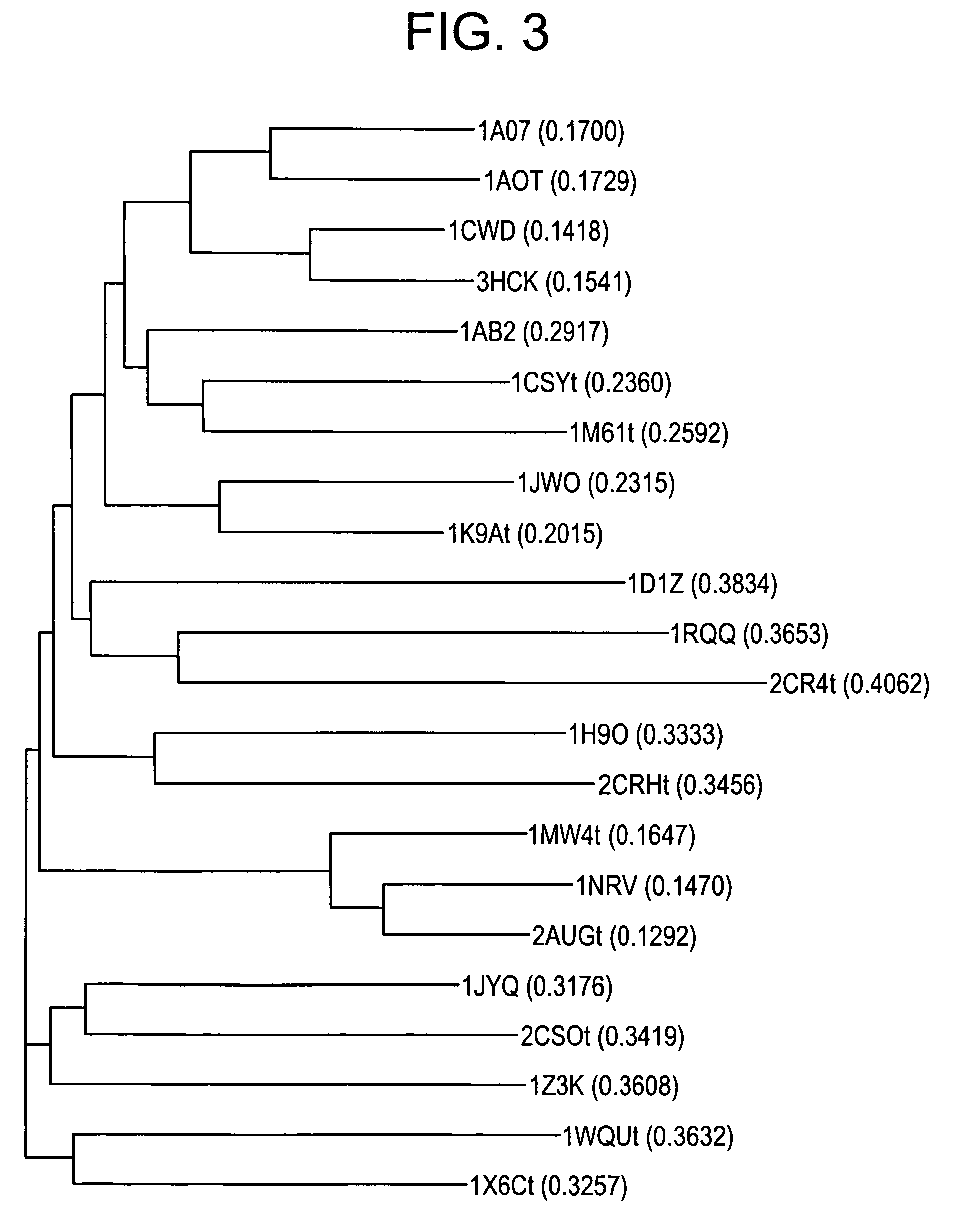
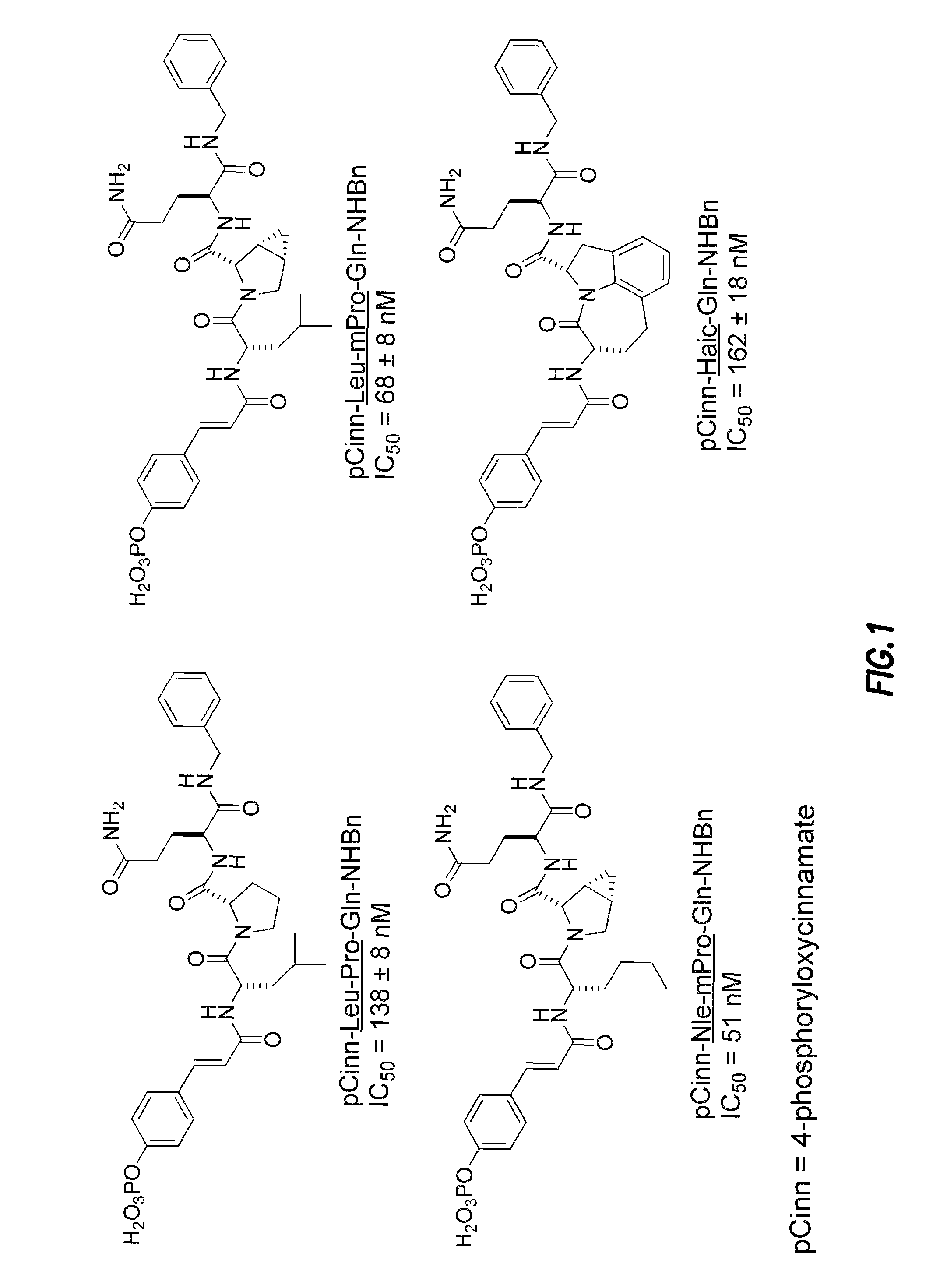
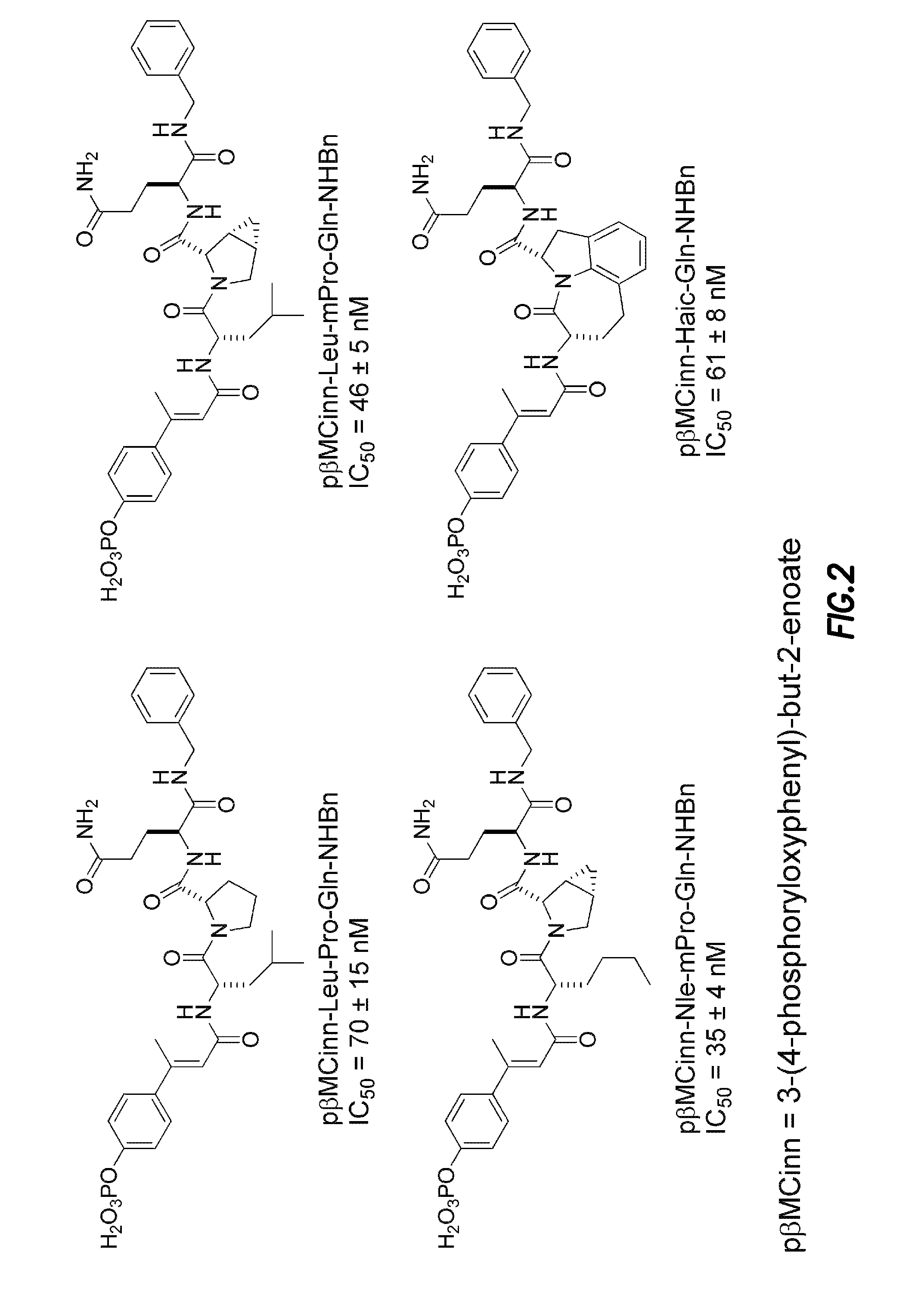
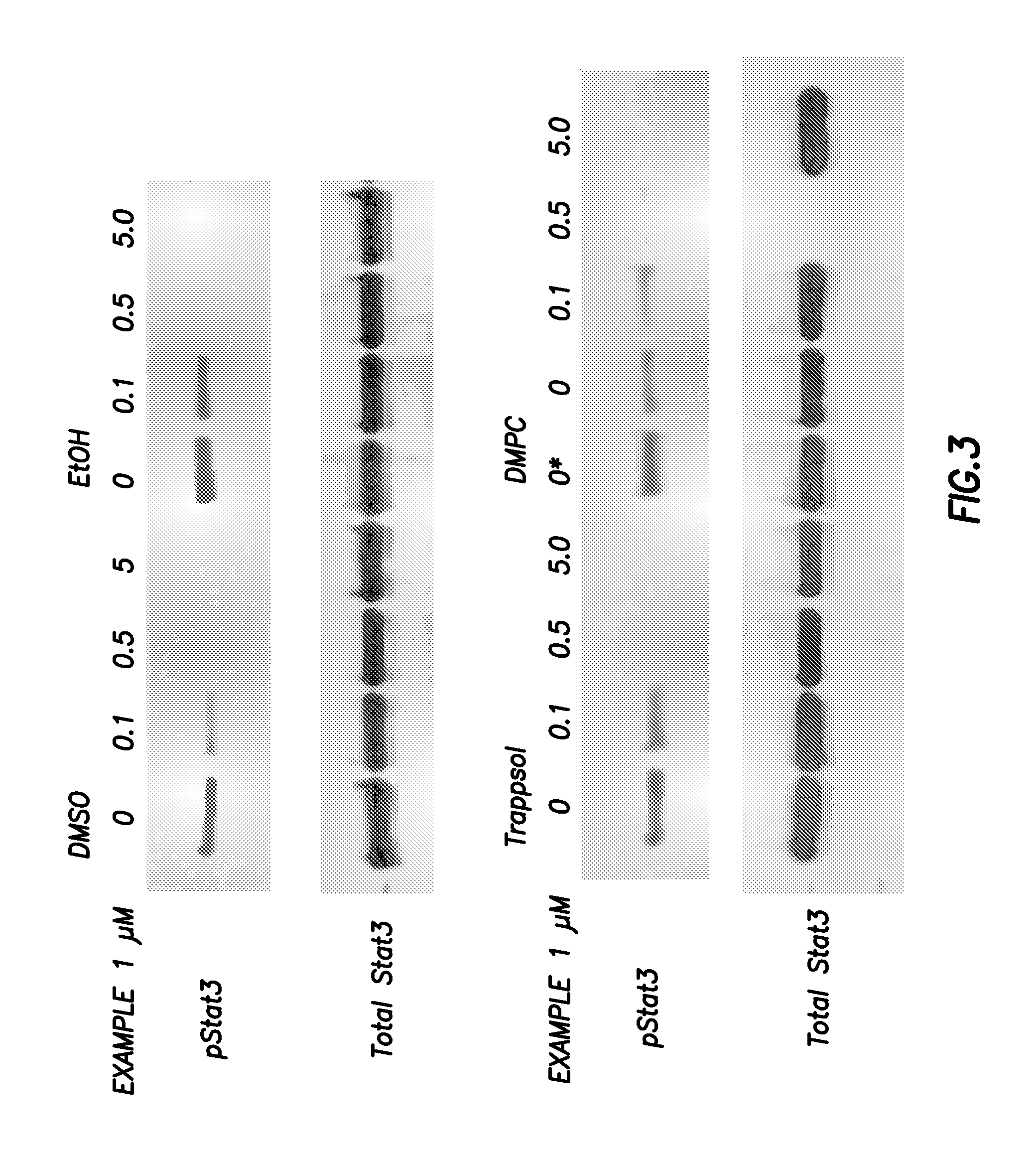
Inhibitors of signal transduction and activator of transcription 3
InactiveUS20070010428A1Dipeptide ingredientsTetrapeptide ingredientsStat3 inhibitorStructural analog
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Inhibition of the sh2-domain containing protein tyr-phosphatase, shp-1, to enhance vaccines
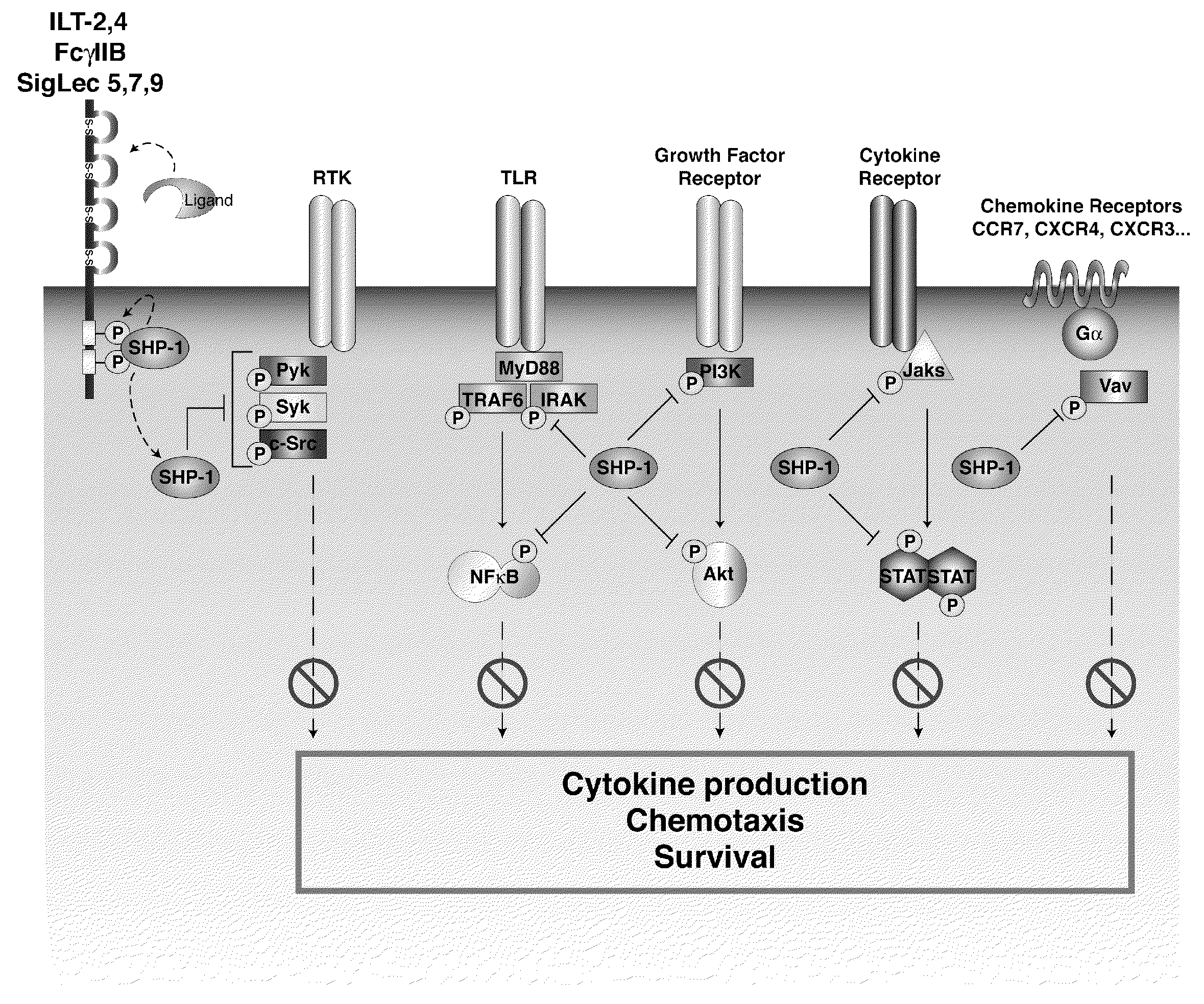
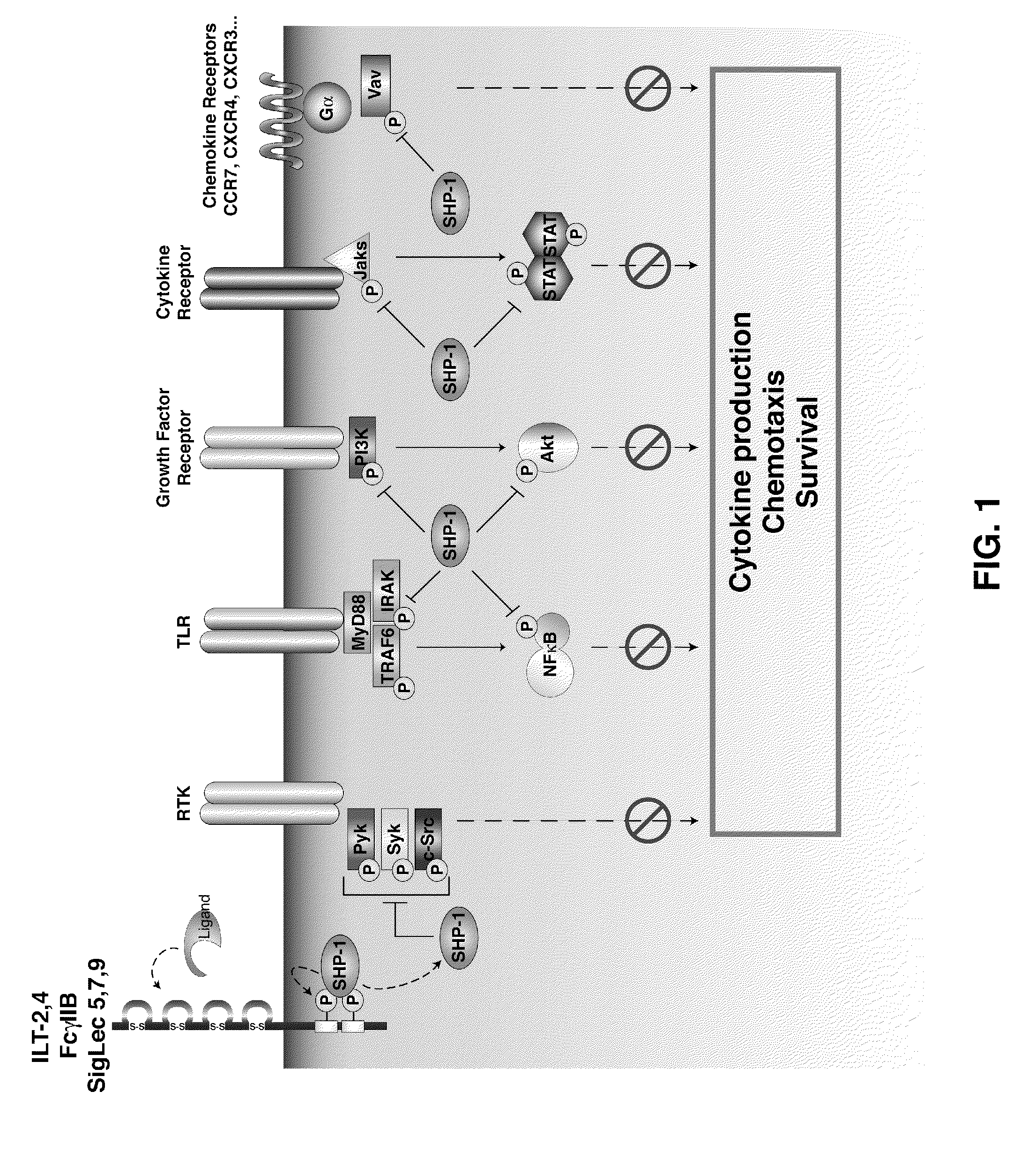
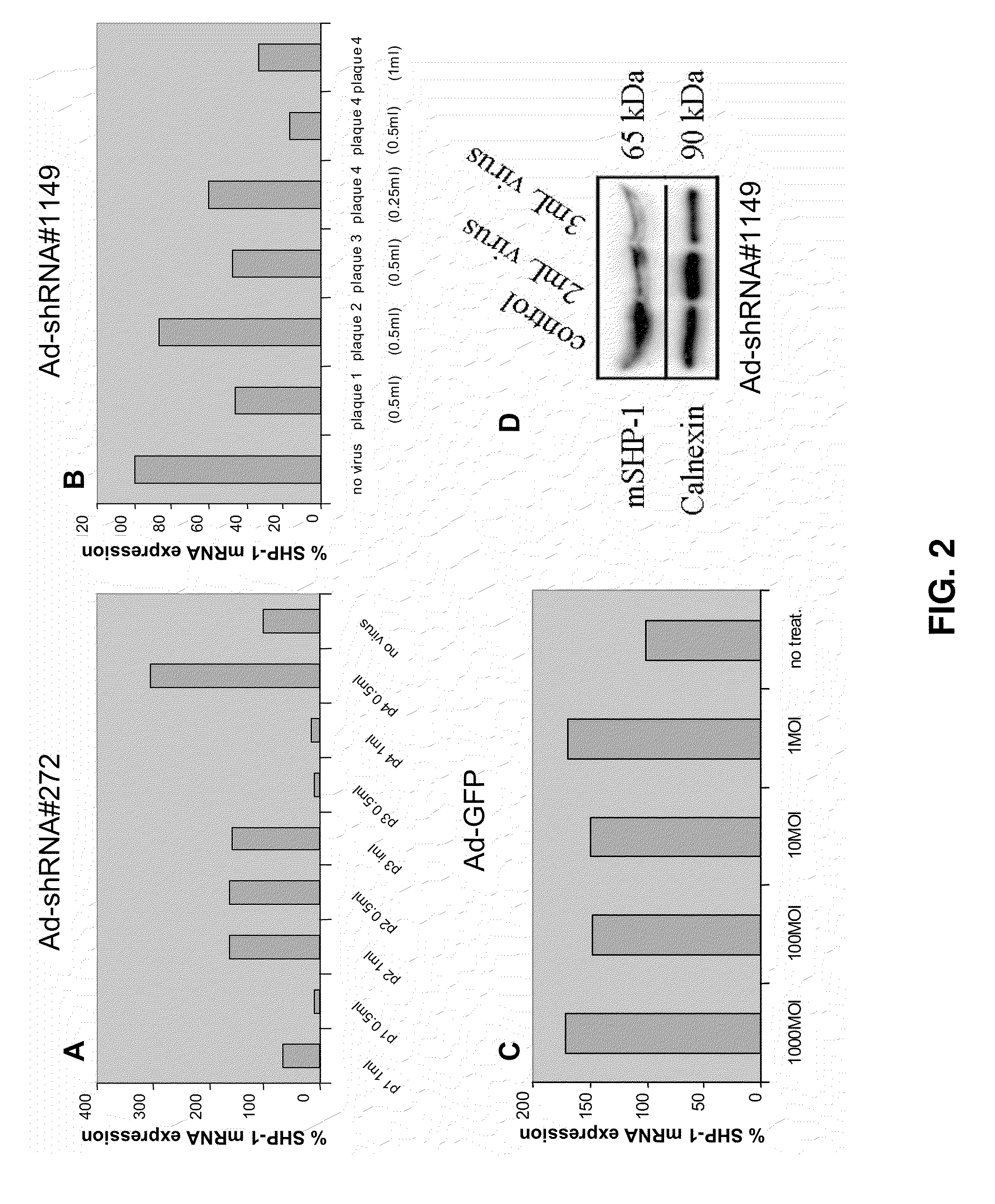
The invention describes the use of dendritic cell vaccines, wherein SHP-1 expression or activity is modulated in the dendritic cell. In particular, the invention provides dendritic cells (DC) transduced with an SHP1-shRNA adenovirus, or dominant negative (dn-SHP-1) or constitutively active (ca-SHP-1), and pulsed with an antigen. The methods and compositions of the invention are used for the prevention and / or treatment of cancers, other cell proliferation diseases and conditions, diseases caused by a pathogen, or autoimmune disorders.
Owner:BAYLOR COLLEGE OF MEDICINE
Cell
ActiveUS20190023761A1Prevent and treat diseasePolypeptide with localisation/targeting motifImmunoglobulin superfamilyHeterologousPhosphorylation
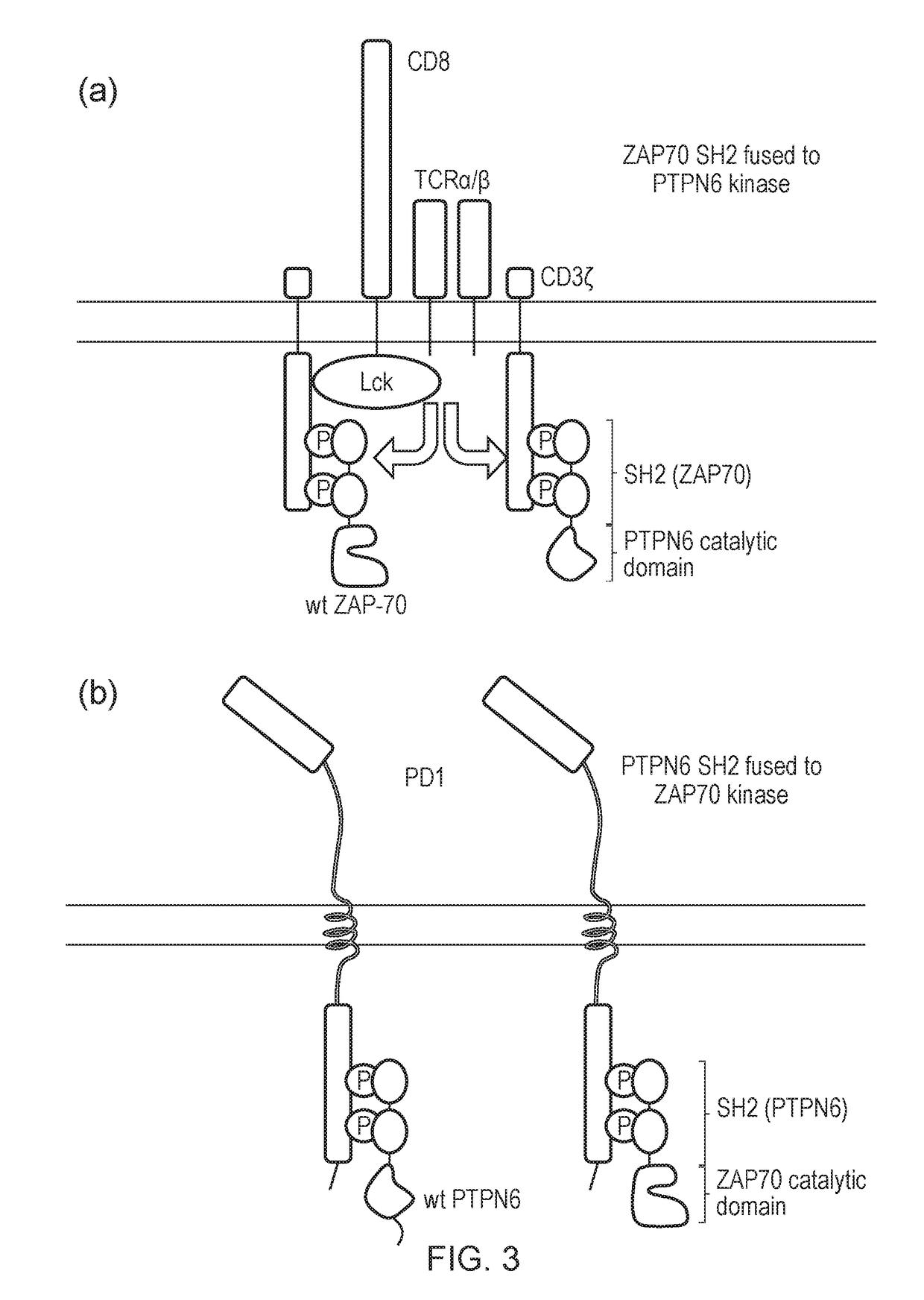
The present invention relates to a cell which comprises a chimeric antigen receptor (CAR) and a signal transduction modifying protein, selected from one of the following: (i) a truncated protein which comprises an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM), but lacks a kinase domain; (ii) a truncated protein which comprises an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based inhibition motif (ITIM) but lacks a phosphatase domain; (iii) a fusion protein which comprises (a) an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) or from a protein which binds a phosphorylated immunoreceptor tyrosine-based inhibition motif (ITIM); and (ii) a heterologous domain.
Owner:AUTOLUS LIMIED
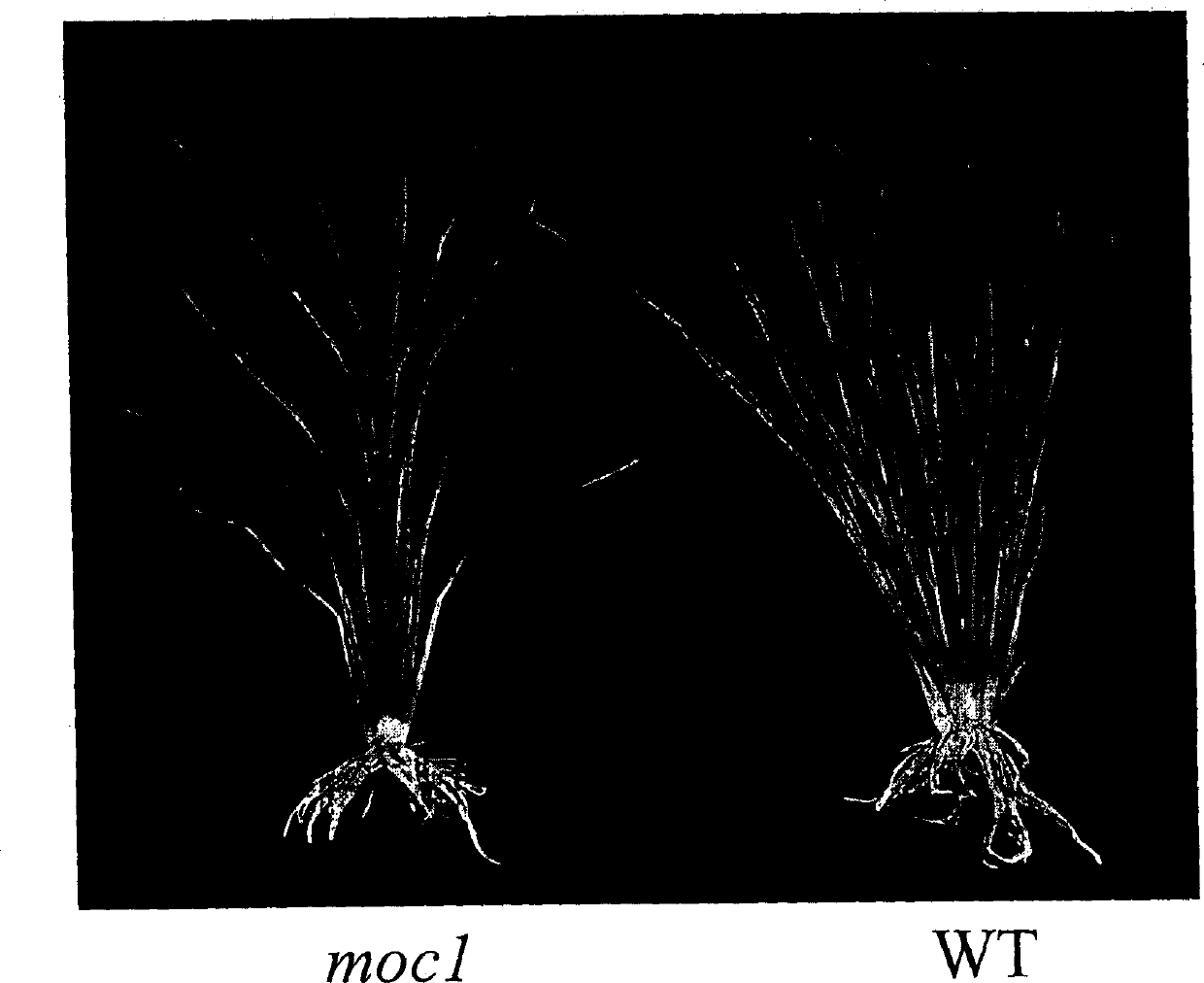
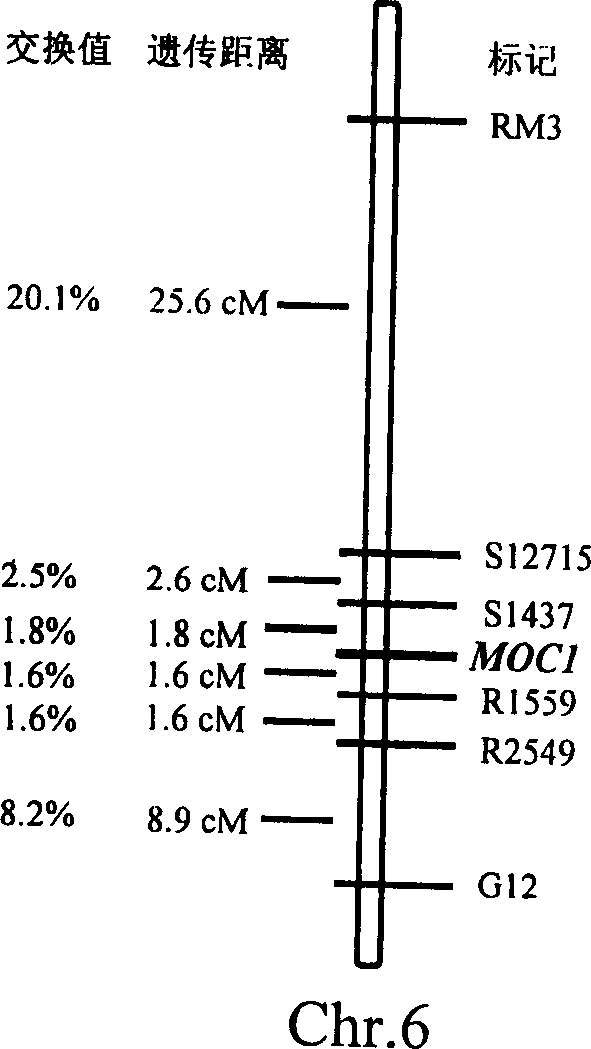
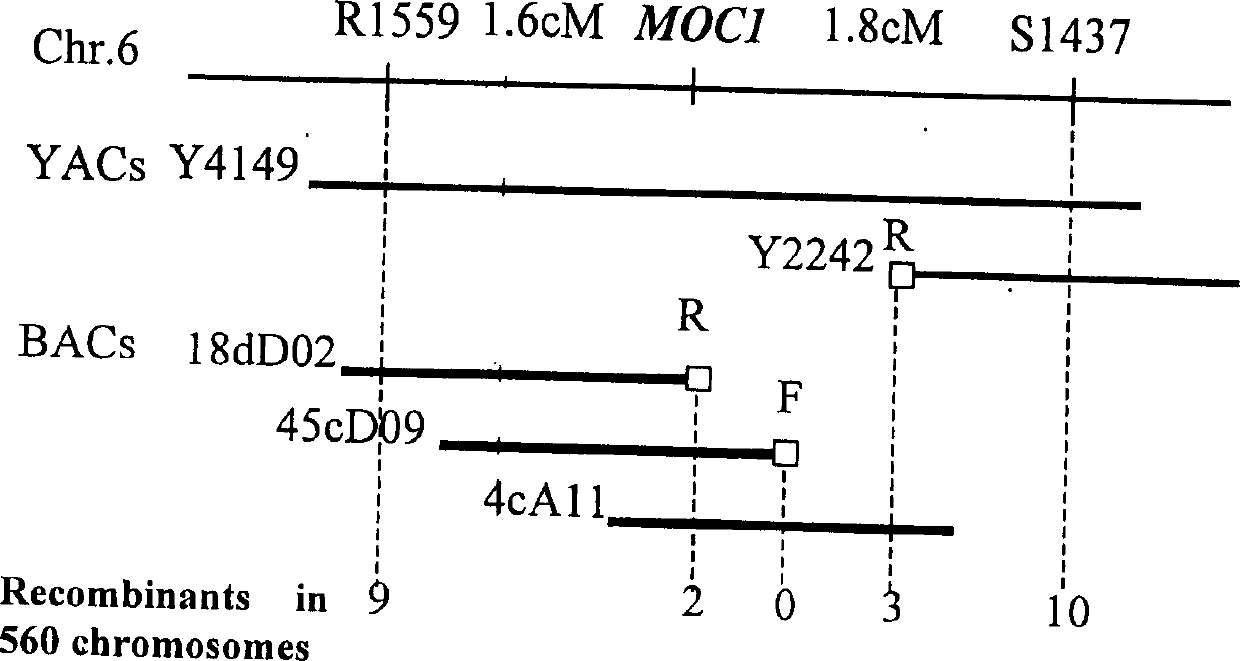
Rice tiller control gene MOC1 and its application
InactiveCN1477112AIncrease productionQuality improvementClimate change adaptationPlant peptidesGenetically modified riceAgricultural science
The present invention utilizes rice single tiller mutant Mono culm 1(moc1) to clone and identify the gene capable of controlling rice tiller, and its name is MOC1. The transgenic function complementary assay shows that MOCI is the gene for controlling rice tiller, and the amino acid sequence comparison analysis shows that said gene belongs to the GRAS transcription factor family. The gene can be used for regulating tiller character of rice.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Compositions and methods for producing bioactive fusion proteins
InactiveUS20090118181A1Low immunogenic riskReduce riskBacteriaPeptide/protein ingredientsDNA constructActive protein
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
STAT function-regulatory protein
Disclosed is a protein having the ability to inhibit the function of a STAT in a mammalian JAK / STAT signal transduction pathway, which is induced by STAT3 or STAT6, which has the ability to inhibit tyrosine phosphorylation of gp130 or STAT3 and which comprises an SH2 domain; and also disclosed is a DNA coding for the same. Further disclosed is a method for screening a substance having the capability to regulate cytokine activity, in which the protein of the present invention is used. Still further disclosed are an antisense DNA and an antisense RNA capable of inhibiting the biosynthesis of the above-mentioned protein; a monoclonal antibody capable of binding to the above-mentioned protein; and a DNA probe and an RNA probe capable of hybridizing to the above-mentioned DNA. Still further disclosed are a replicable recombinant DNA molecule comprising a replicable expression vector and, operably inserted therein, the DNA of the present invention; a cell of a microorganism or cell culture, transformed with the replicable recombinant DNA molecule; and a method for screening a substance having the capability to regulate cytokine activity in which the transformant is used.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION
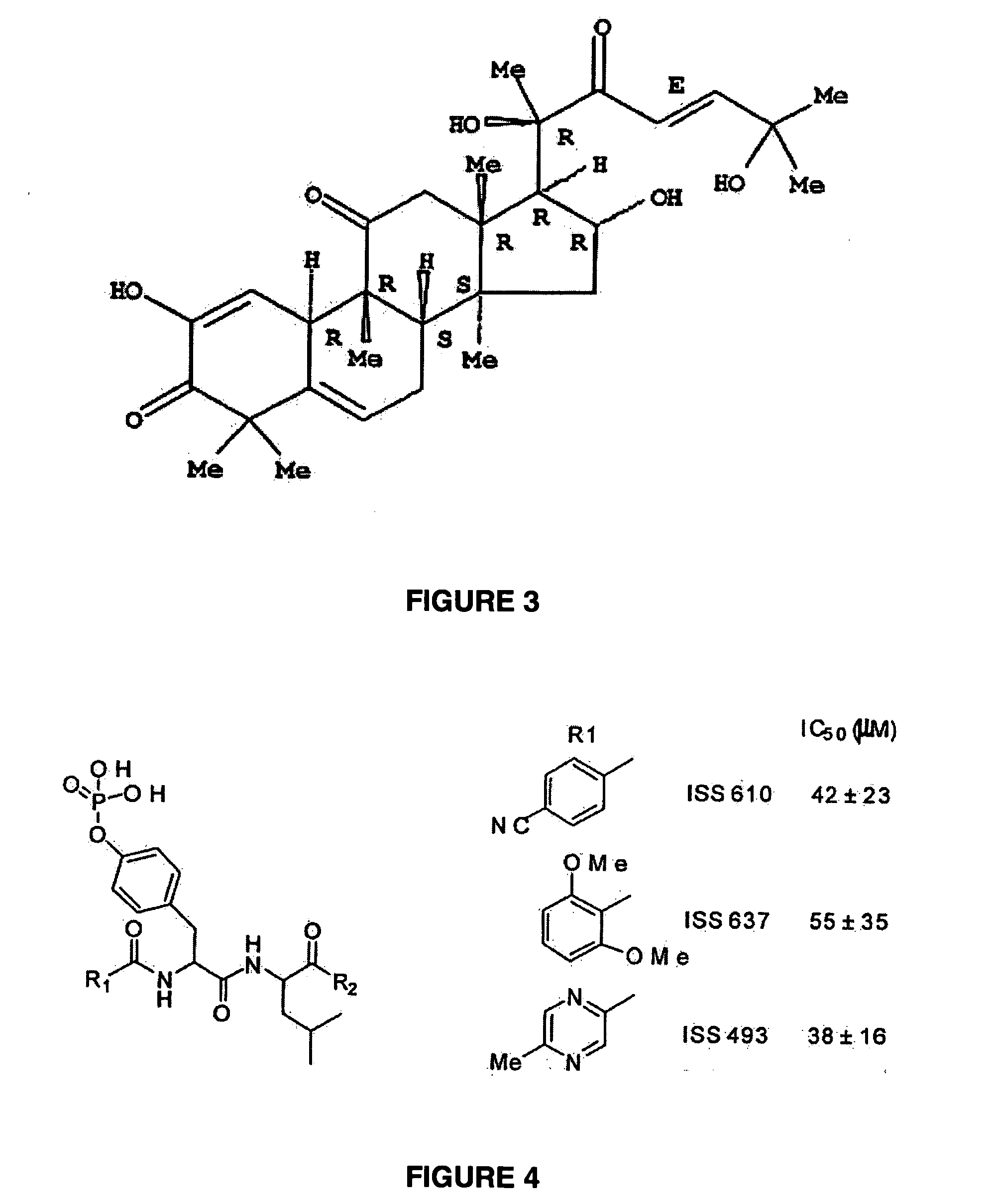
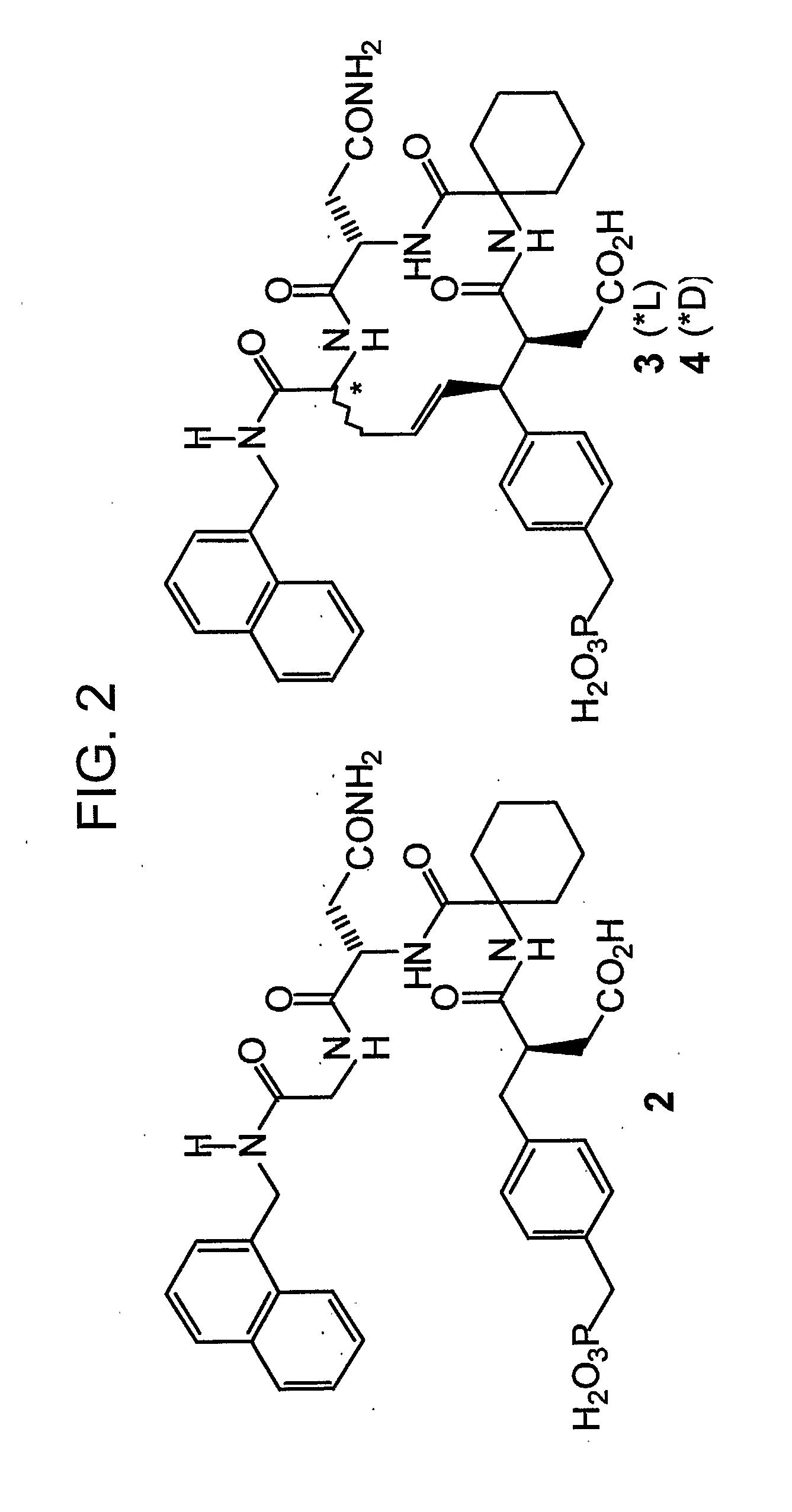
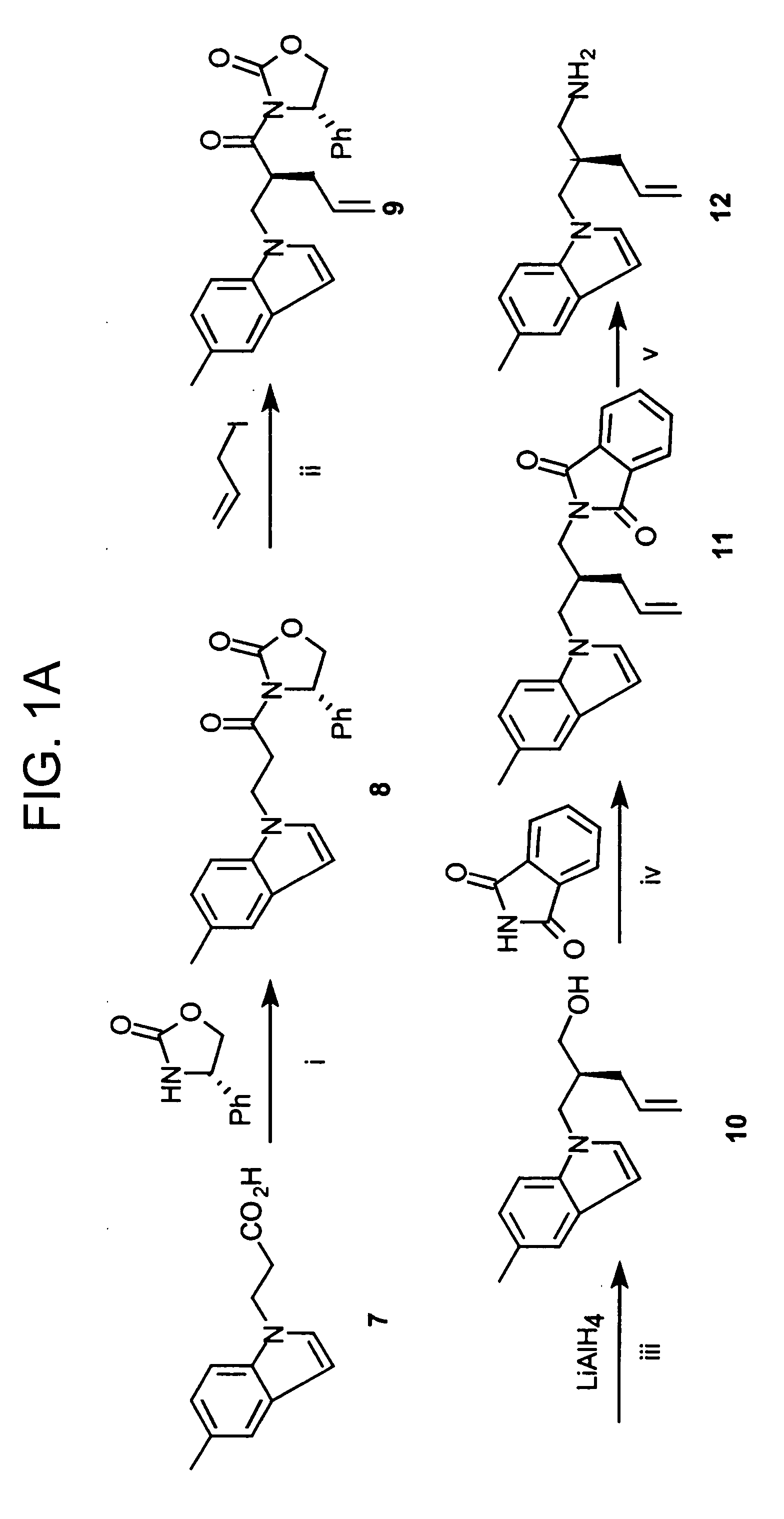
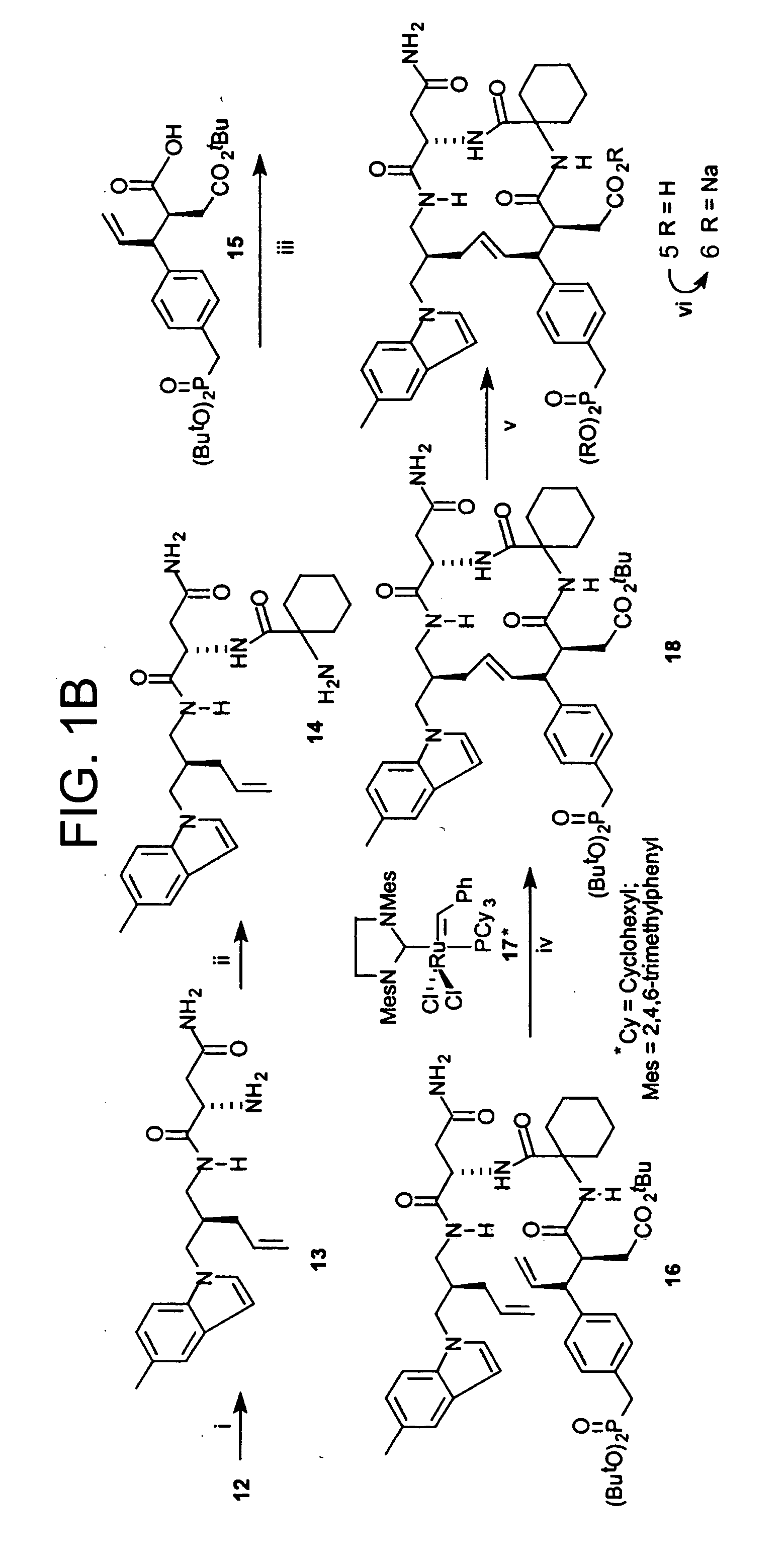
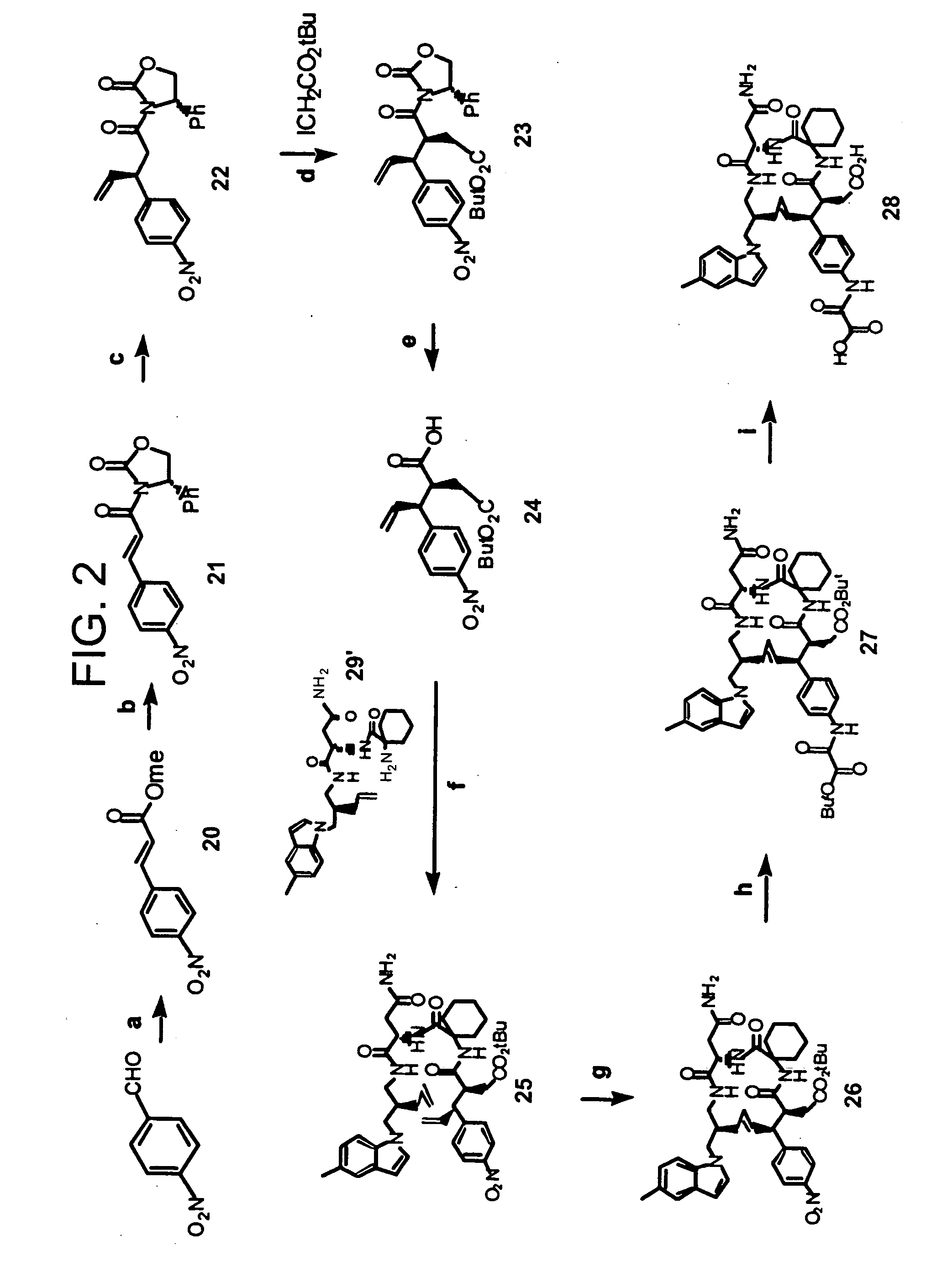
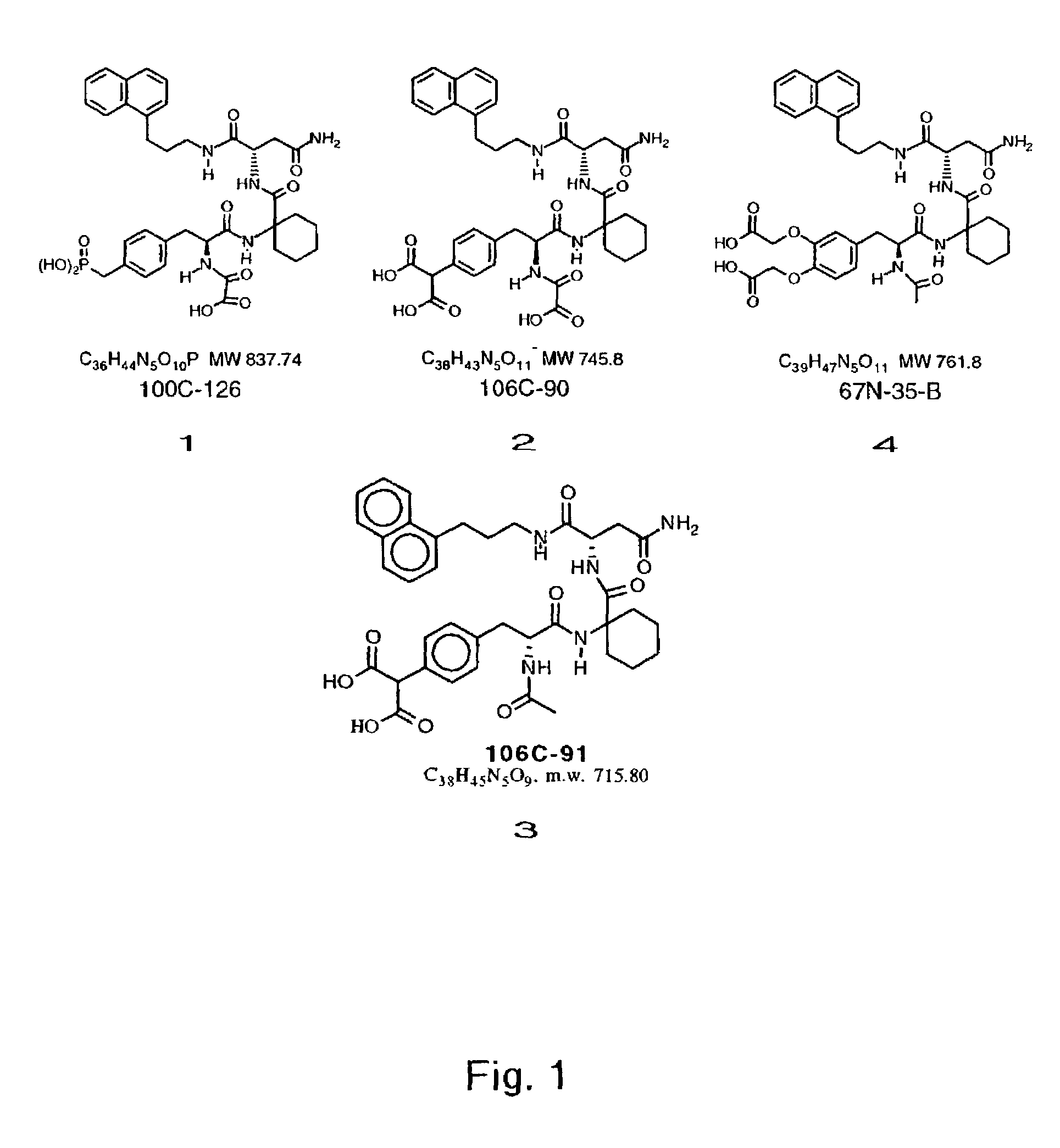
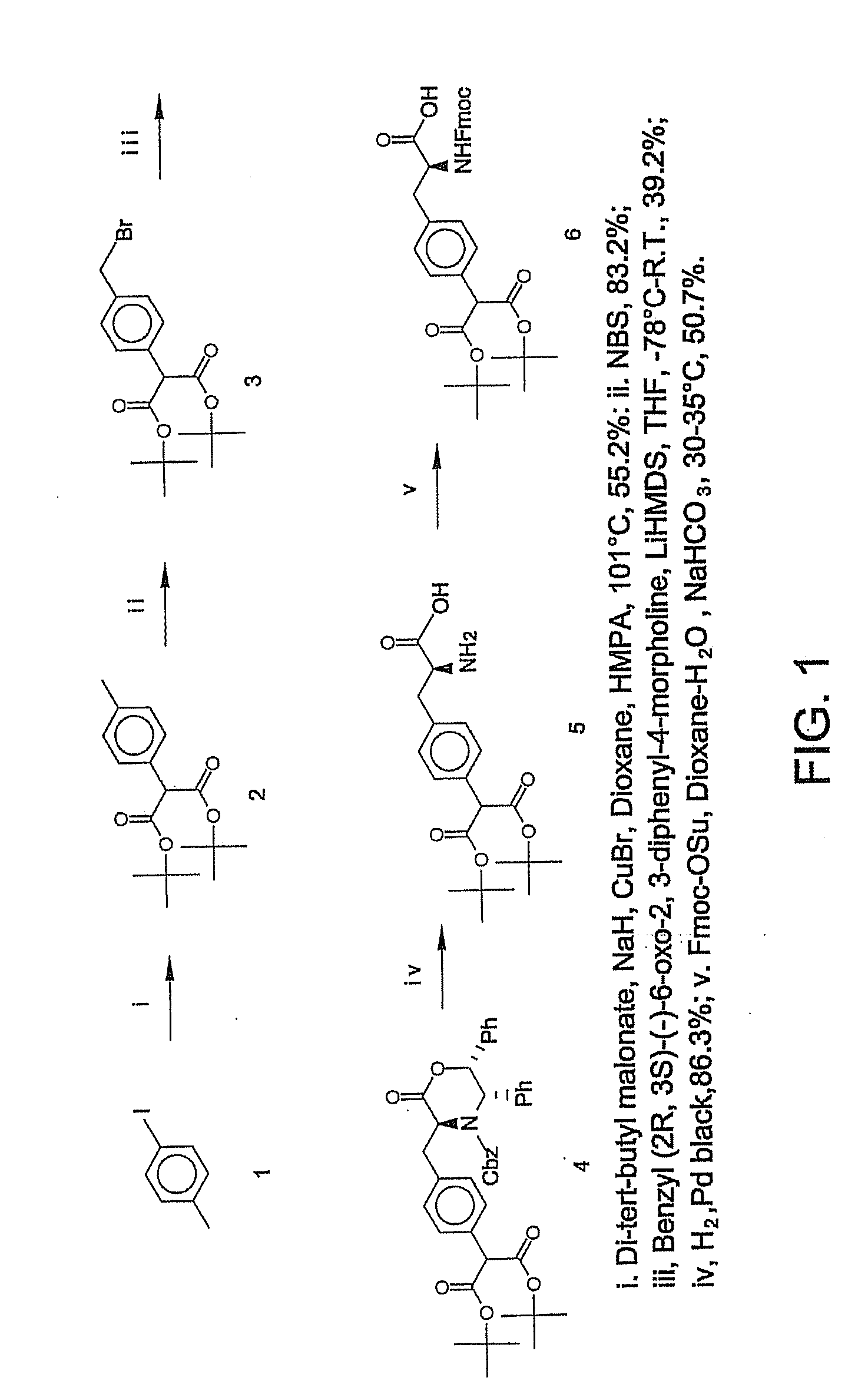
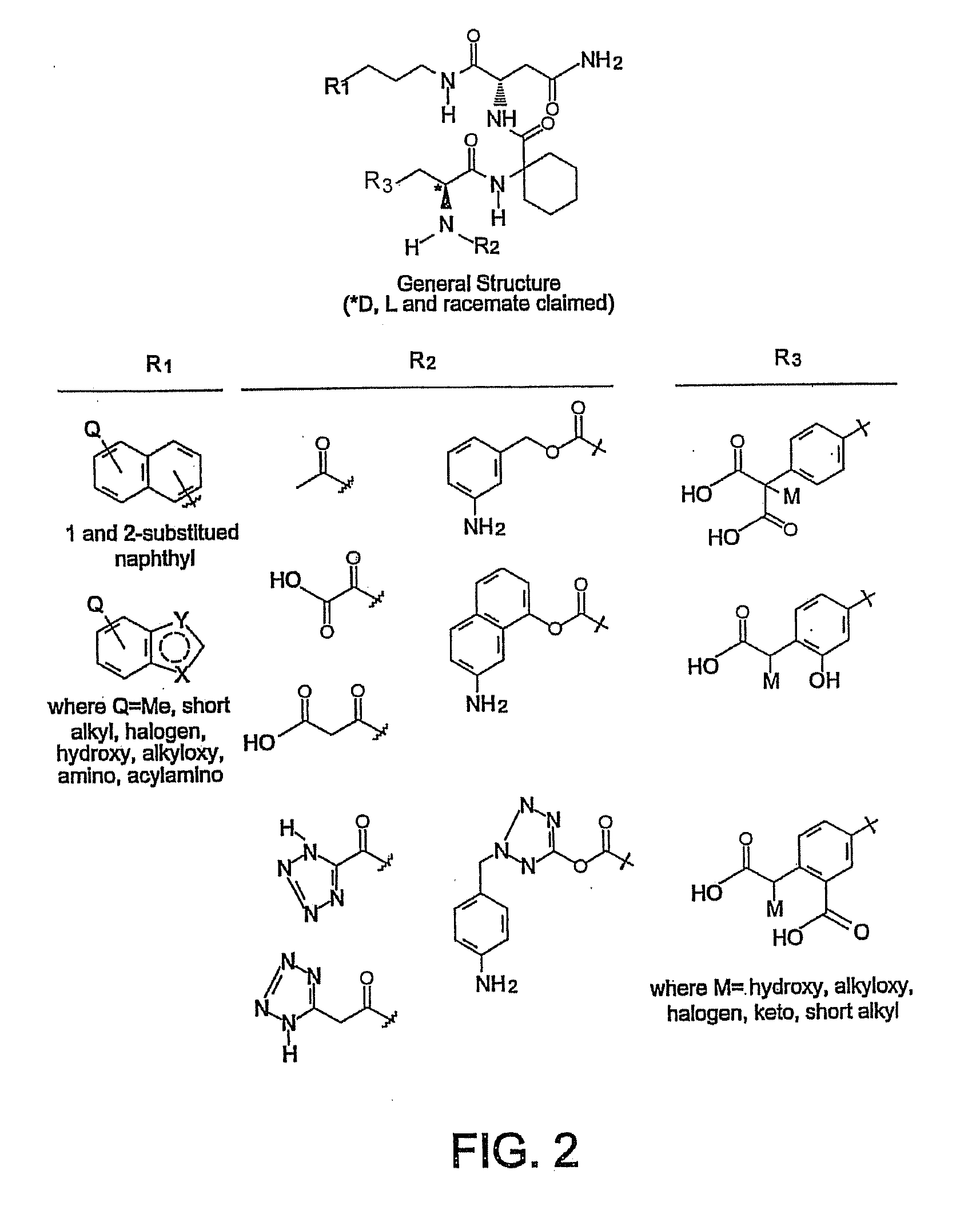
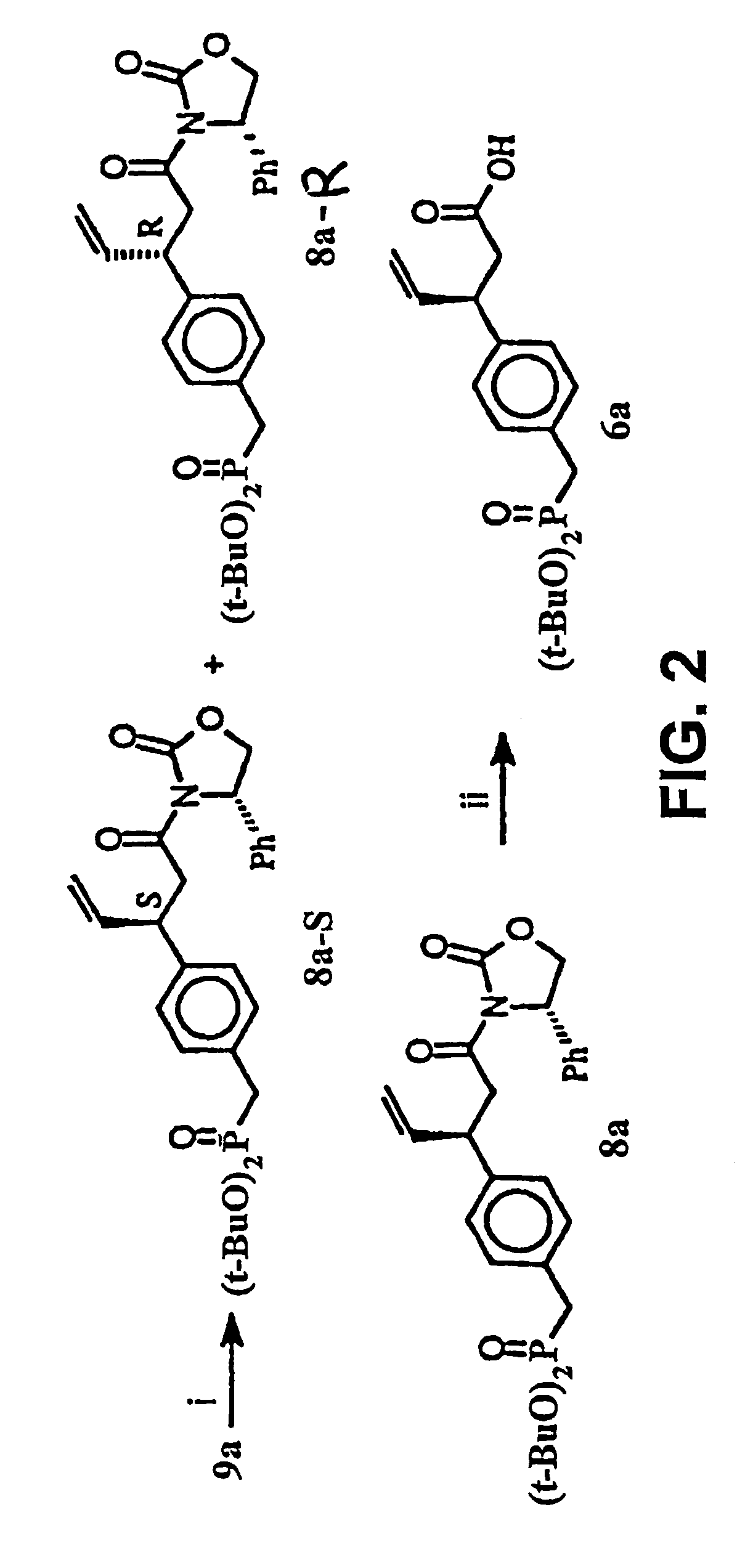
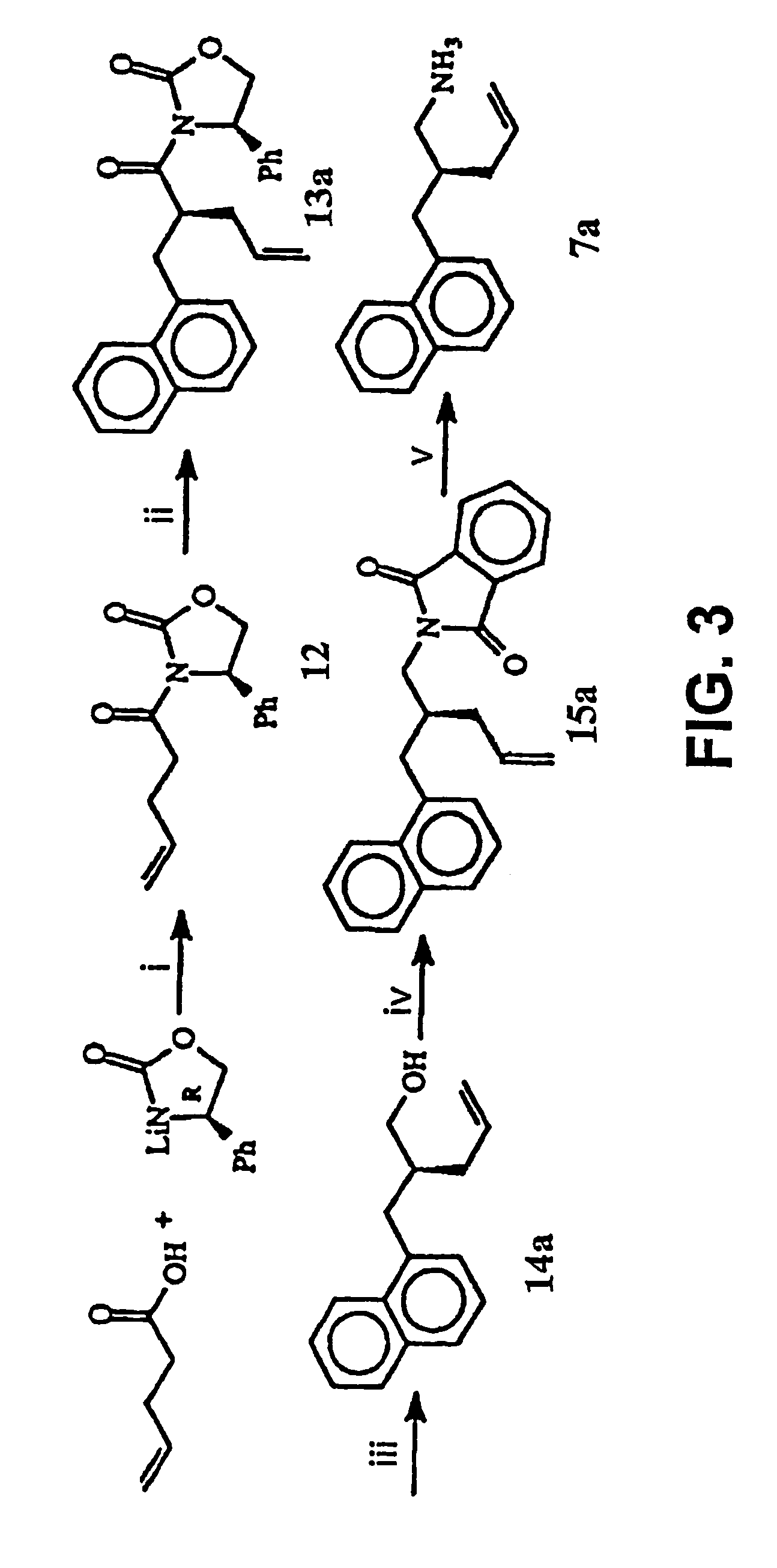
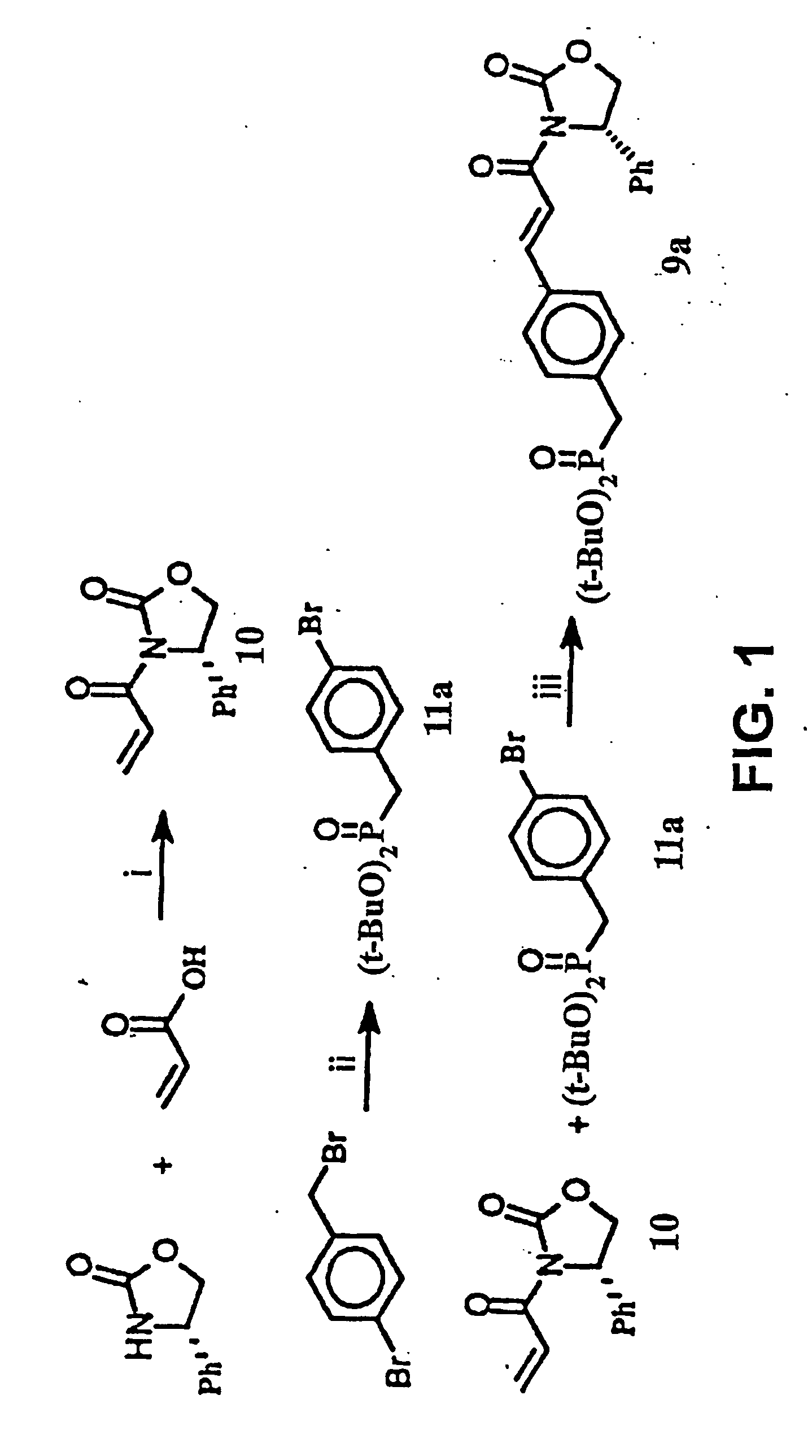
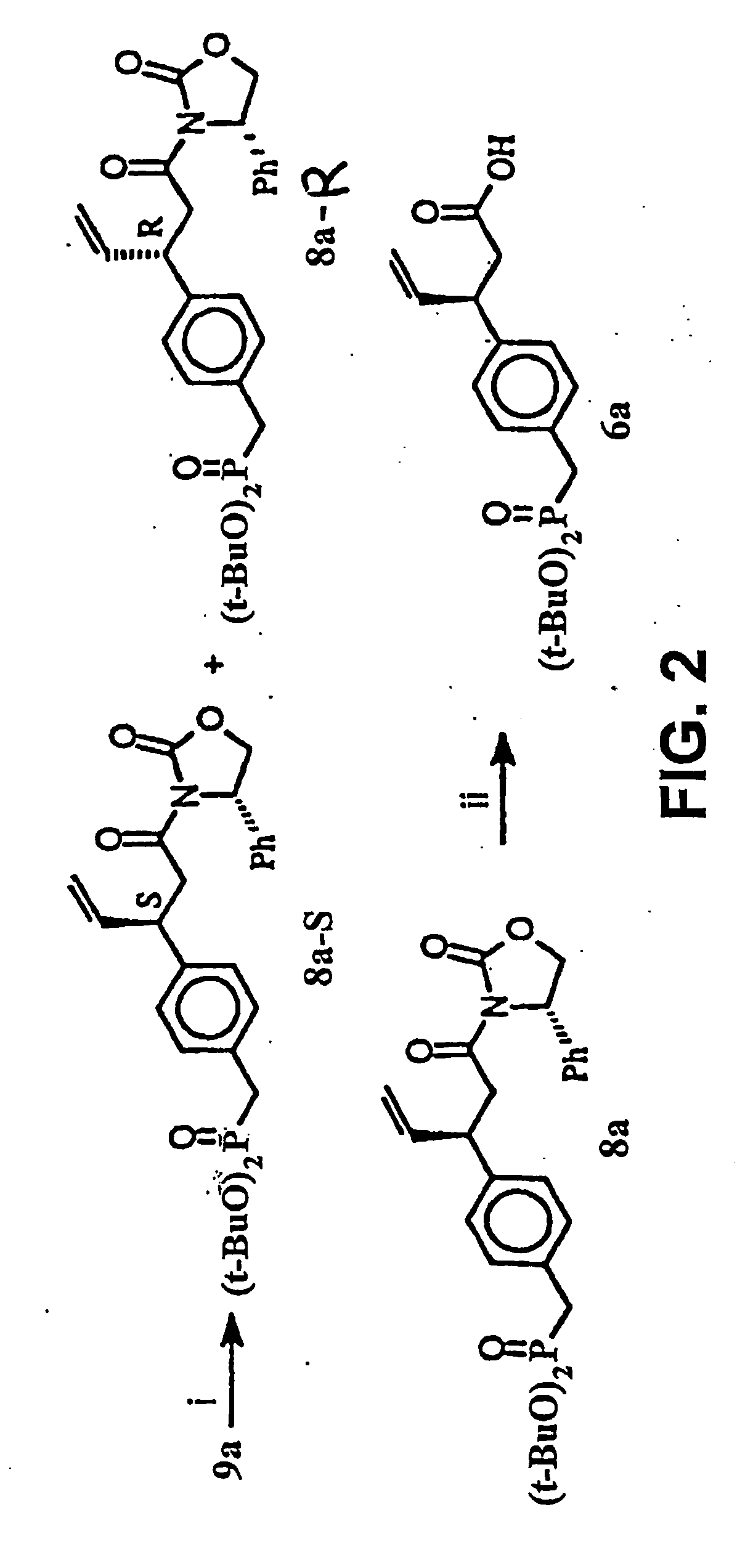
Macrocyclic Sh2 Domain Binding Inhibitors
Disclosed are compounds for inhibiting the binding of an SH2 domain-containing protein, for example, a compound of formula (I): FORMULA (I) wherein R1 is a lipophile; R2, in combination with the phenyl ring, is a phenylphosphate mimic group or a protected phenylphosphate mimic group; R3 is, for example, hydrogen, azido, amino, oxalylamino, carboxy alkyl, alkoxycarbonyl alkyl, aminocarbonyl alkyl, or alkyl carbonylamino; R6 is a linker; AA is an amino acid; and n is 1 to 6; or a pharmaceutically acceptable salt, stereoisomer, solvate, or hydrate thereof. Also disclosed are pharmaceutical compositions and methods of use of such compounds.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
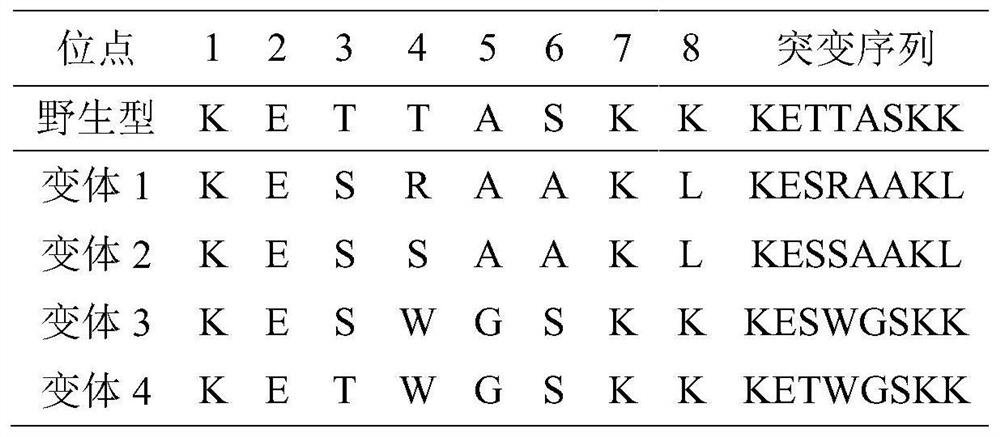
Sh2 domain variants
ActiveUS20150177258A1Inhibiting and preventing effectPeptide/protein ingredientsDepsipeptidesAmino acid substitutionO-phospho-L-tyrosine
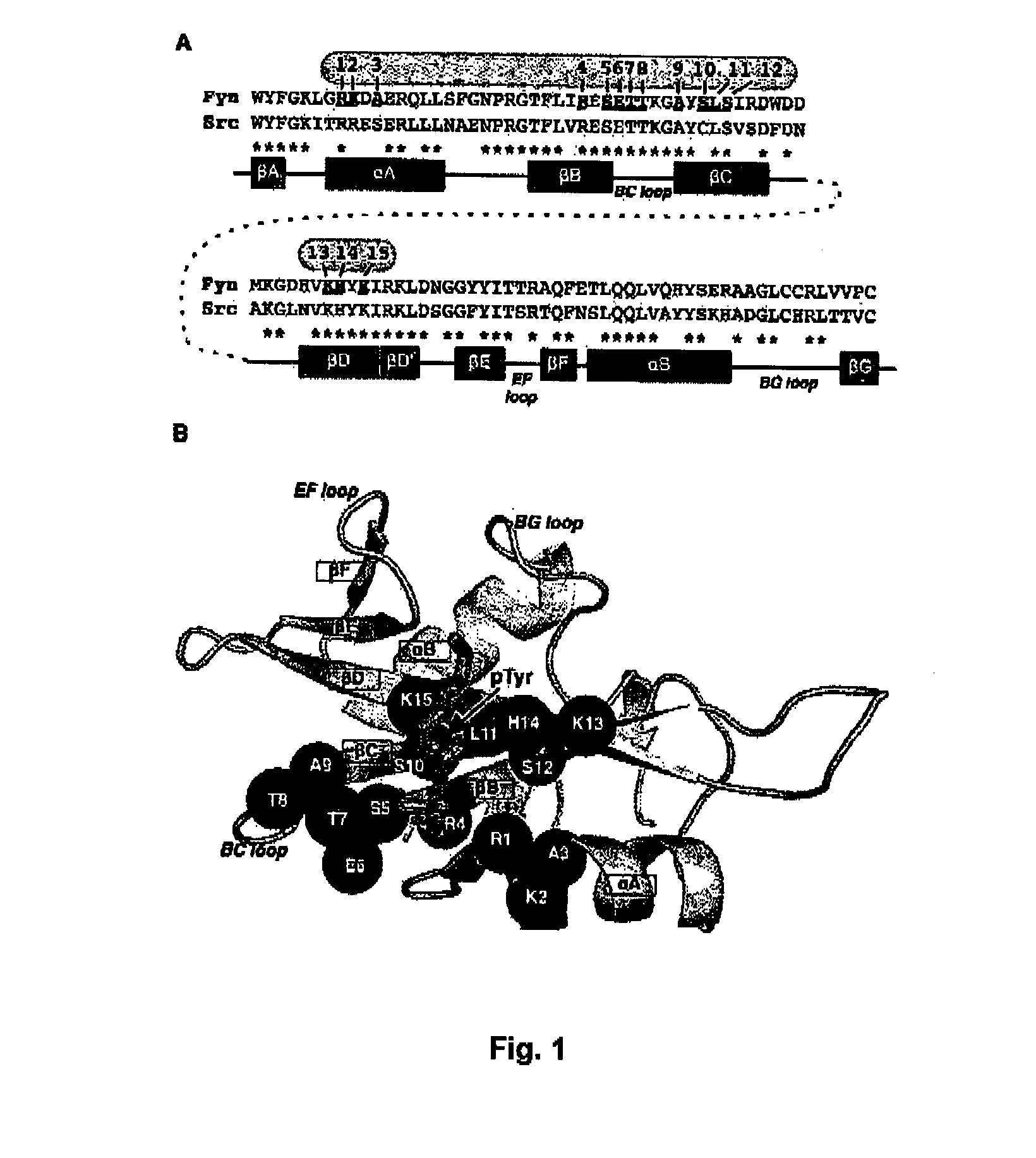
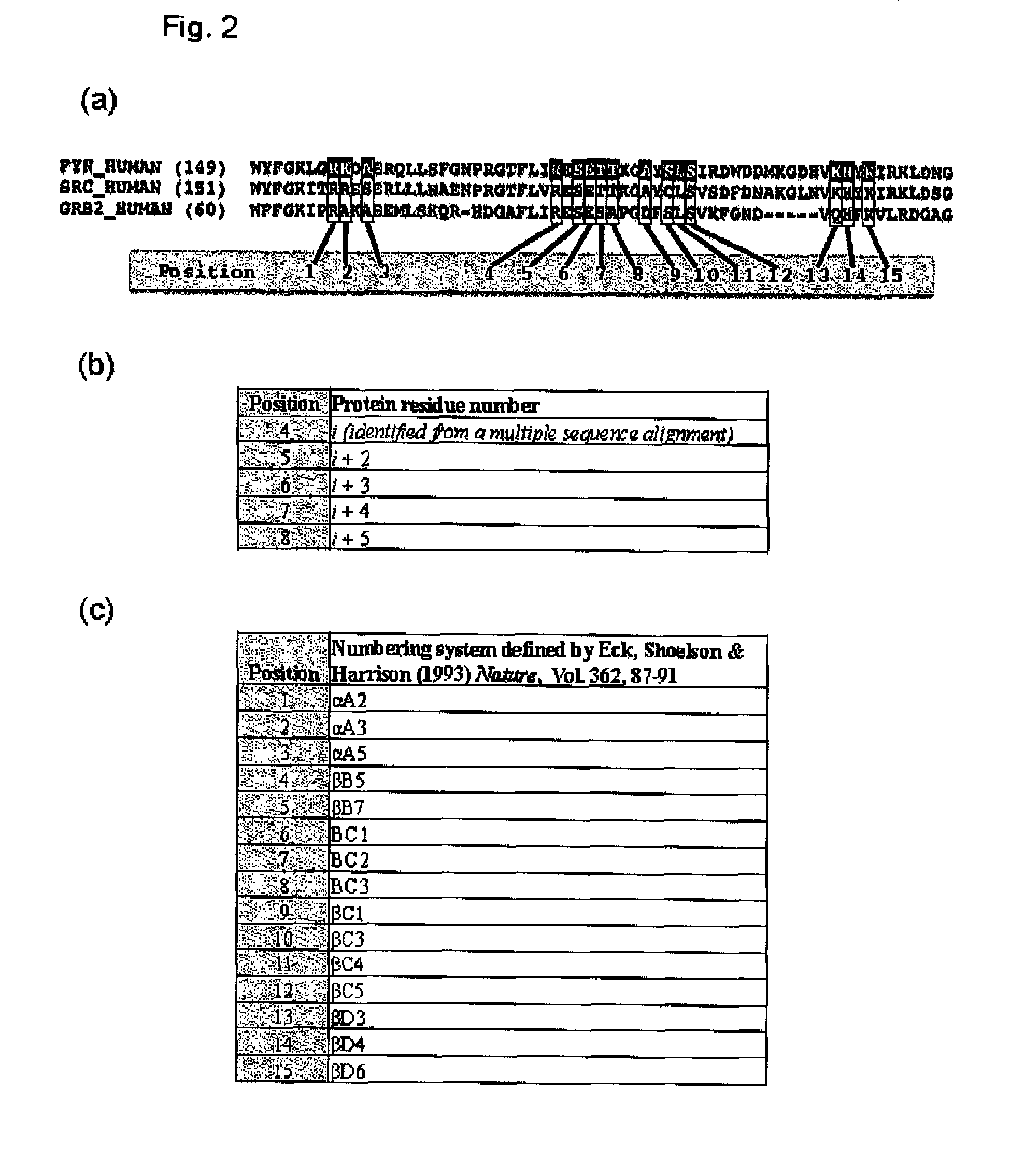
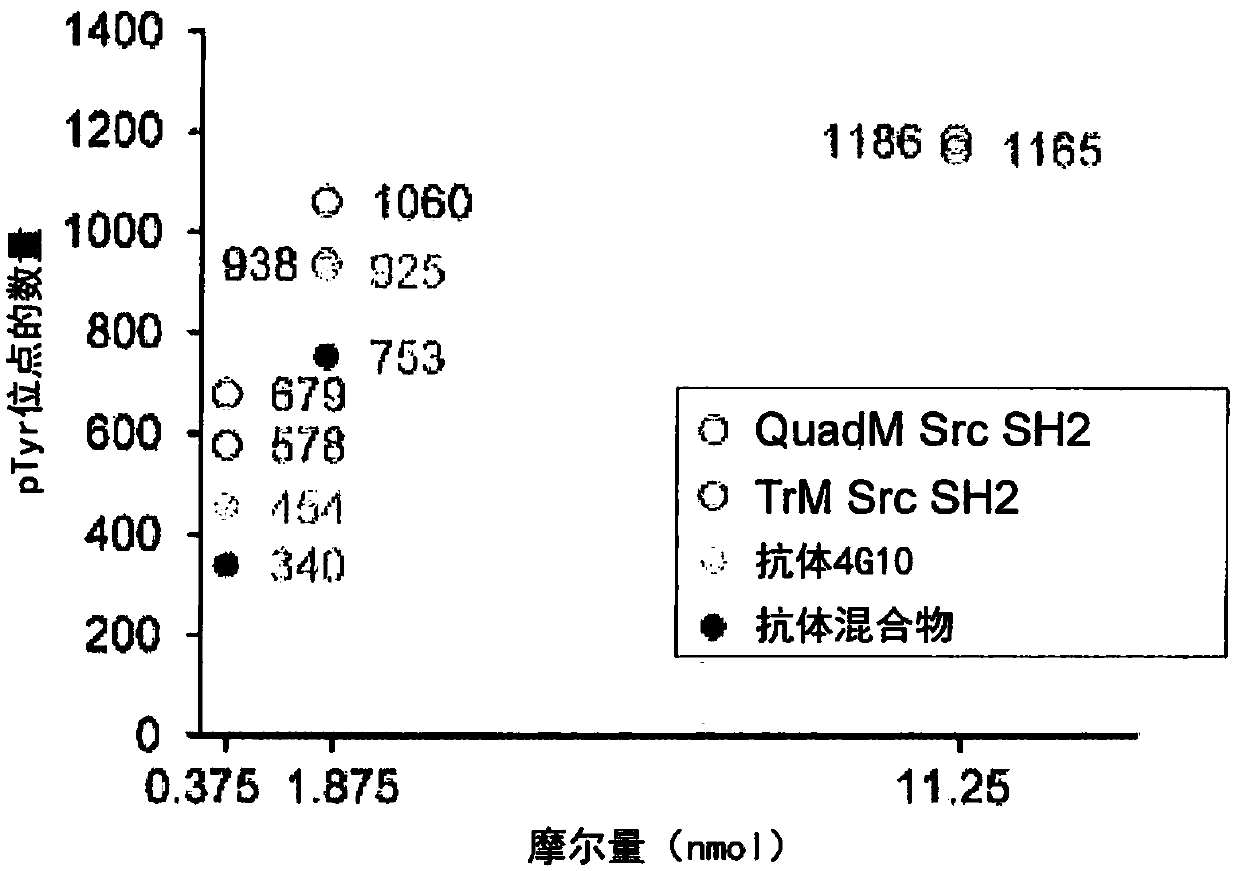
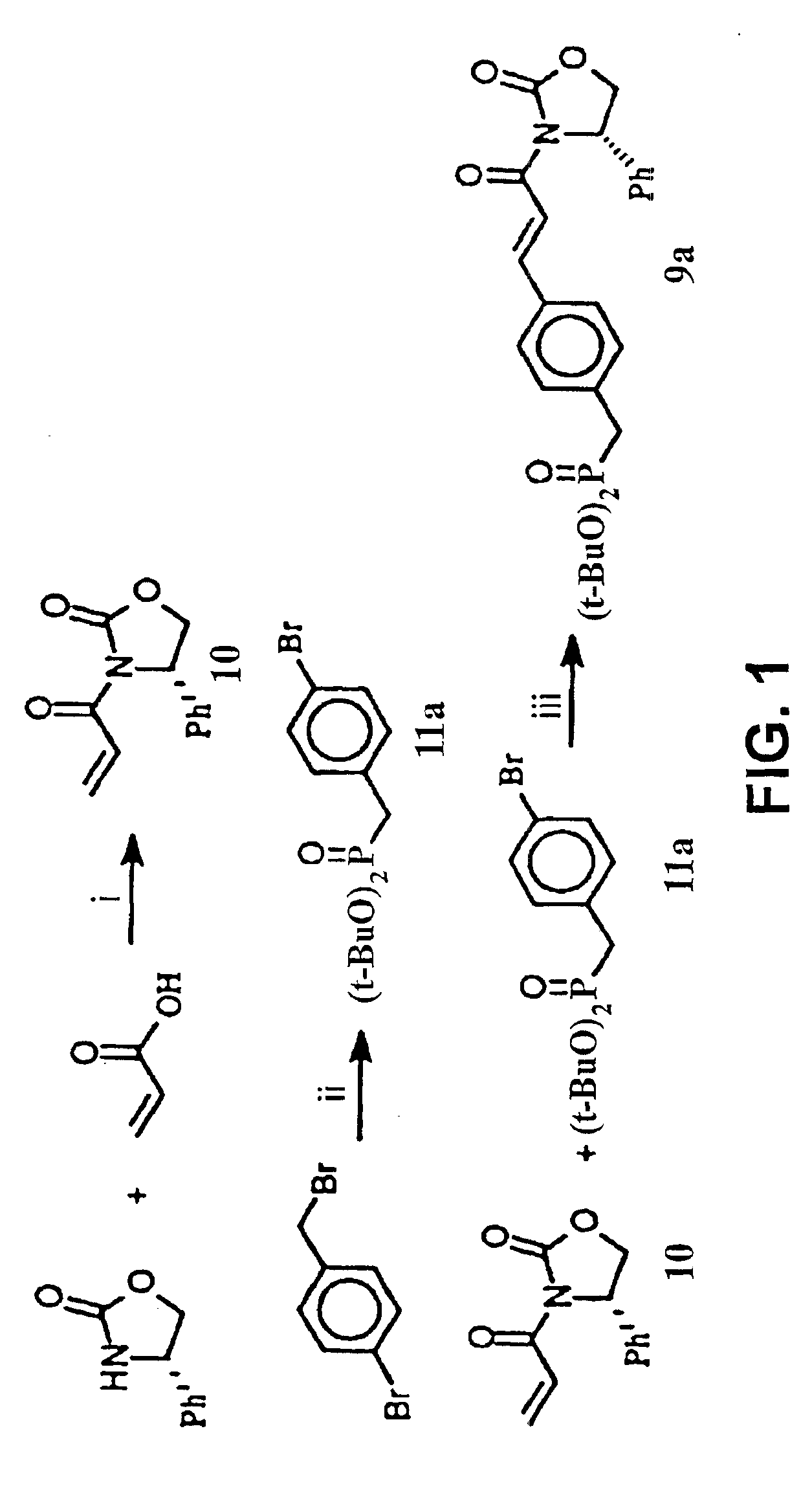
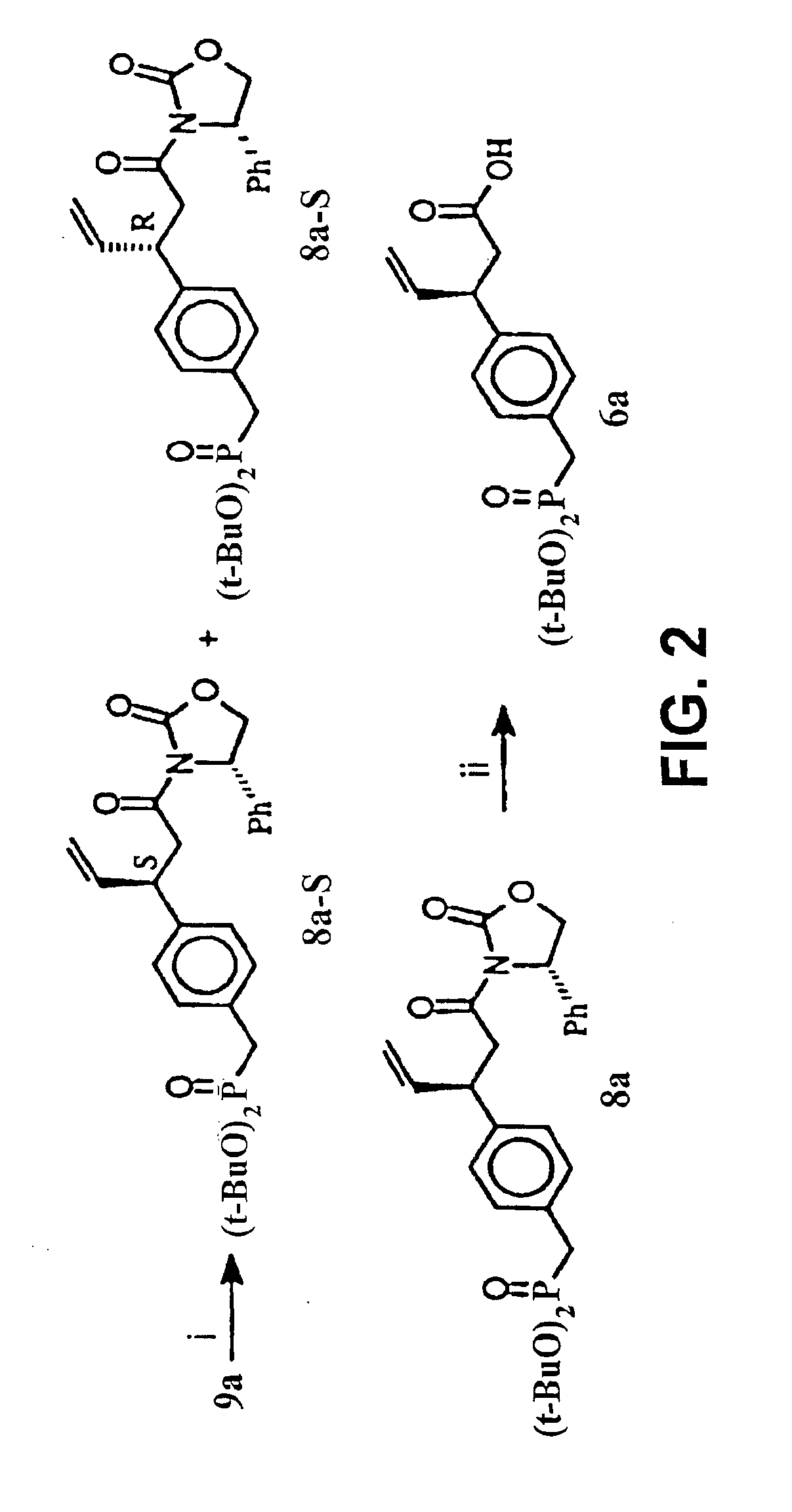
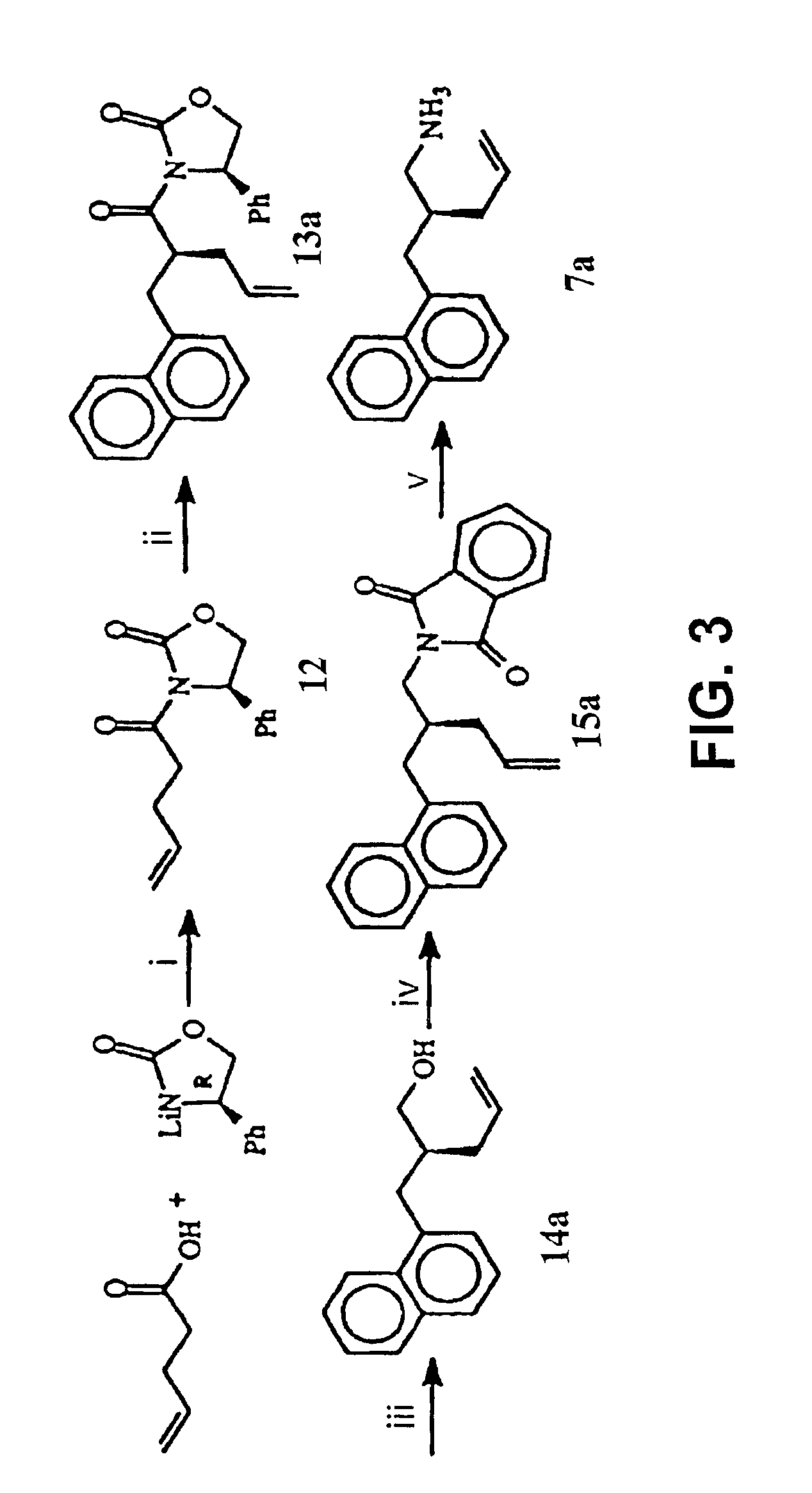
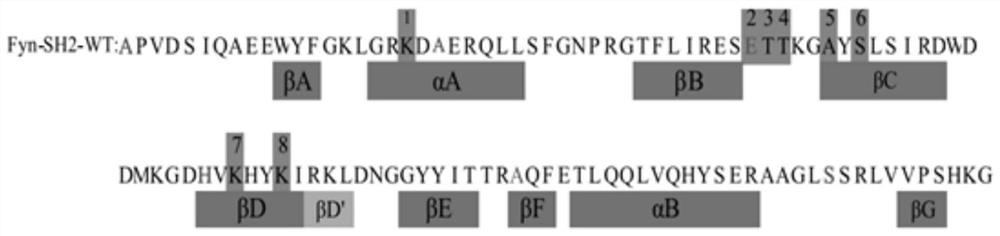
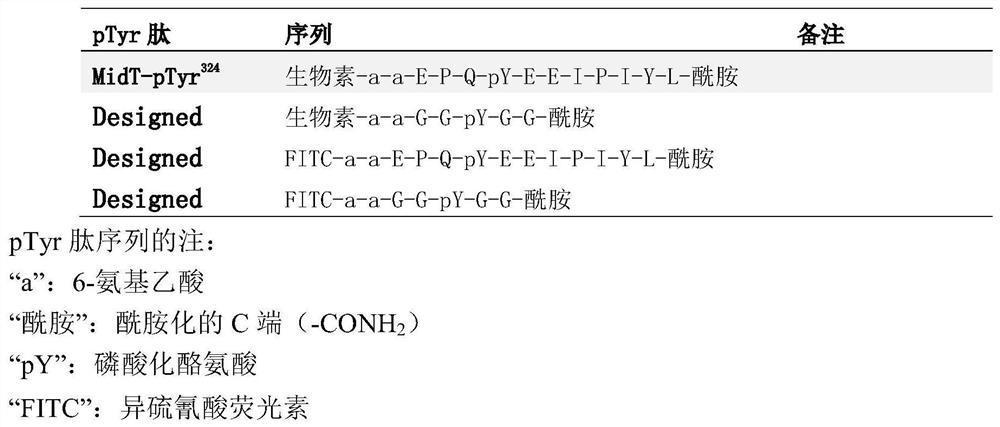
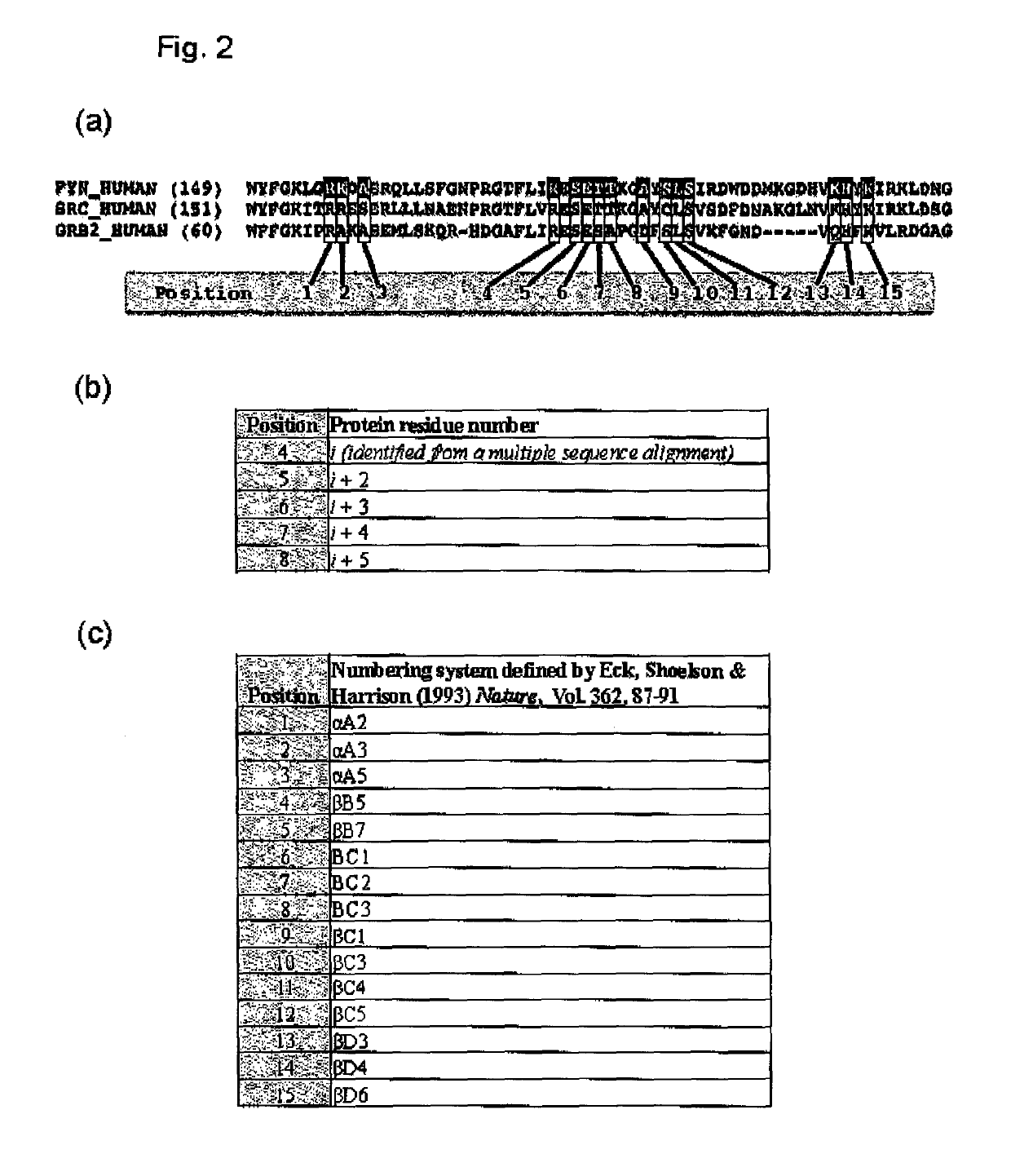
The present invention relates to variant SH2 domains for binding a phosphotyrosine (pTyr)-containing peptide. The variant SH2 domains of the present invention include a parent SH2 domain having at least one amino acid substitution in a pre-defined region of 15 amino acid positions of the parent SR2 domain, wherein said at least one amino acid substitution increases the affinity of the variant SH2 domain for the pTyr-containing peptide relative to the parent SH2 domain. The present application relates also to methods of using the variant SH2 domains in the treatment of protein kinase-associated disorders, or the diagnosis or prognosis of protein kinase-associated disorders, for isolating and measuring the concentration of pTyr-containing molecules, and as reagents in research.
Owner:UNIV OF WESTERN ONTARIO +1
SH2 domain binding inhibitors
Disclosed are compounds represented by the formula: or a pharmaceutically acceptable salt or isomer thereof, wherein R1-R6 are as defined in the specification. These compounds are targeted for use as inhibitors of SH2 domain binding with a phosphoprotein, and are contemplated for use in a number of diseases including cancer. Also disclosed are pharmaceutical compositions comprising a compound of the invention and a pharmaceutically acceptable carrier.
Owner:UNITED STATES OF AMERICA
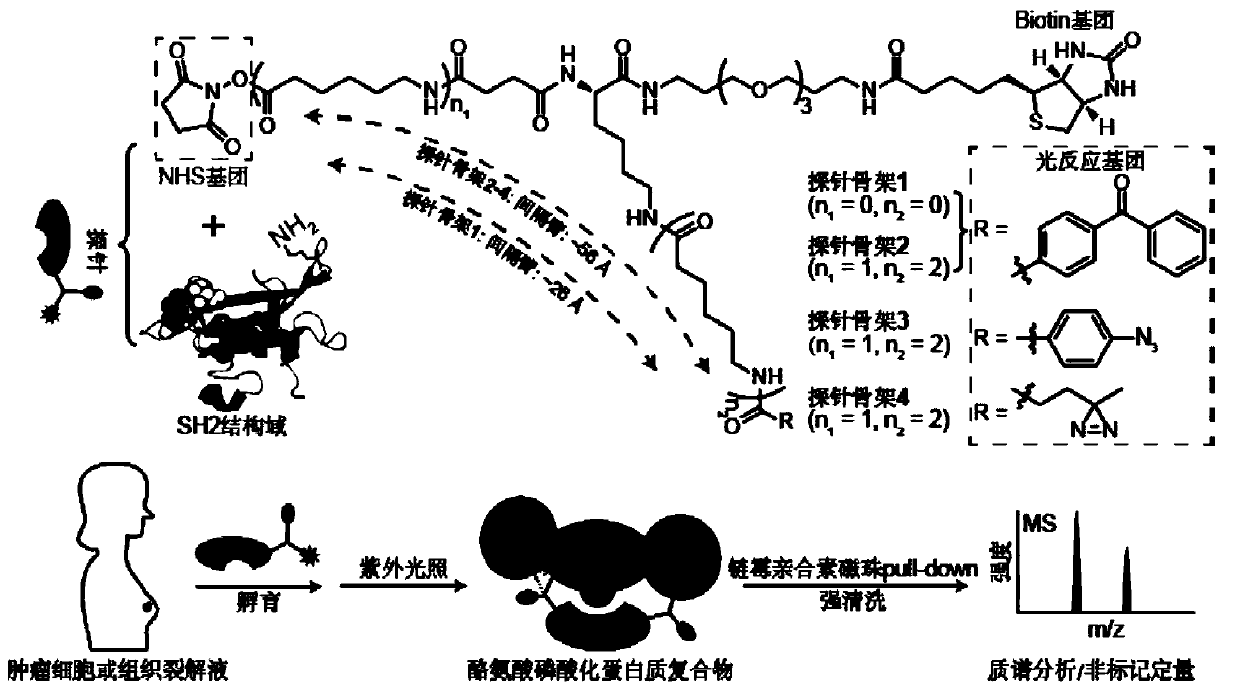
Probe and application thereof
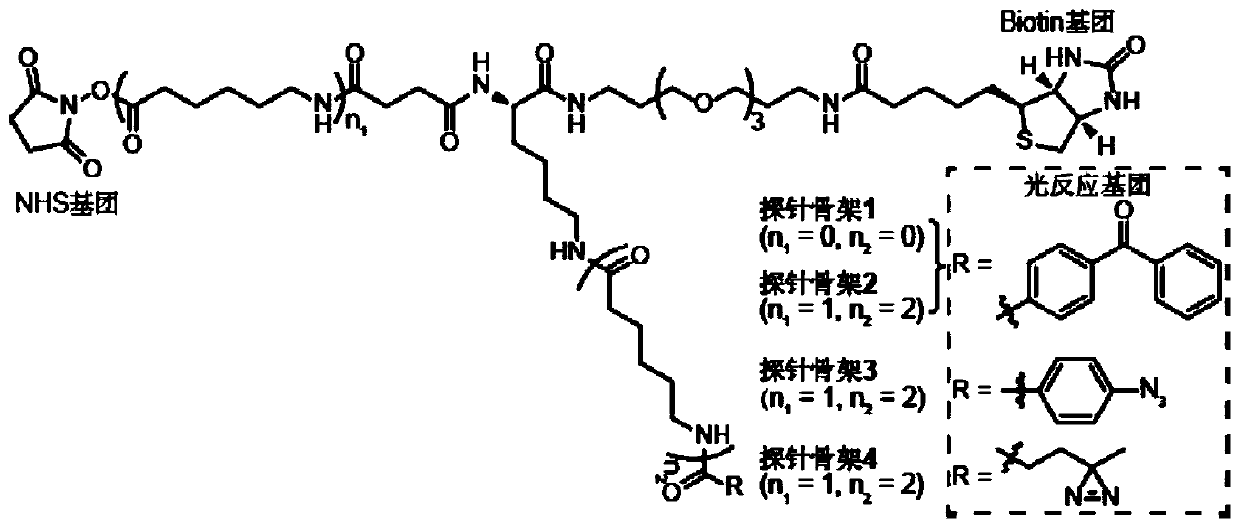
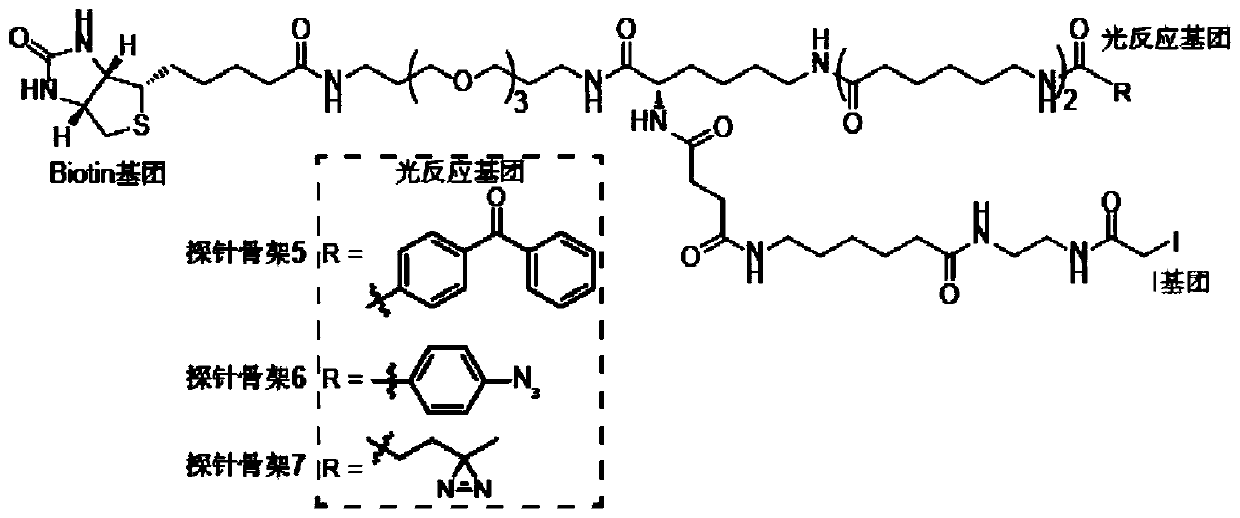
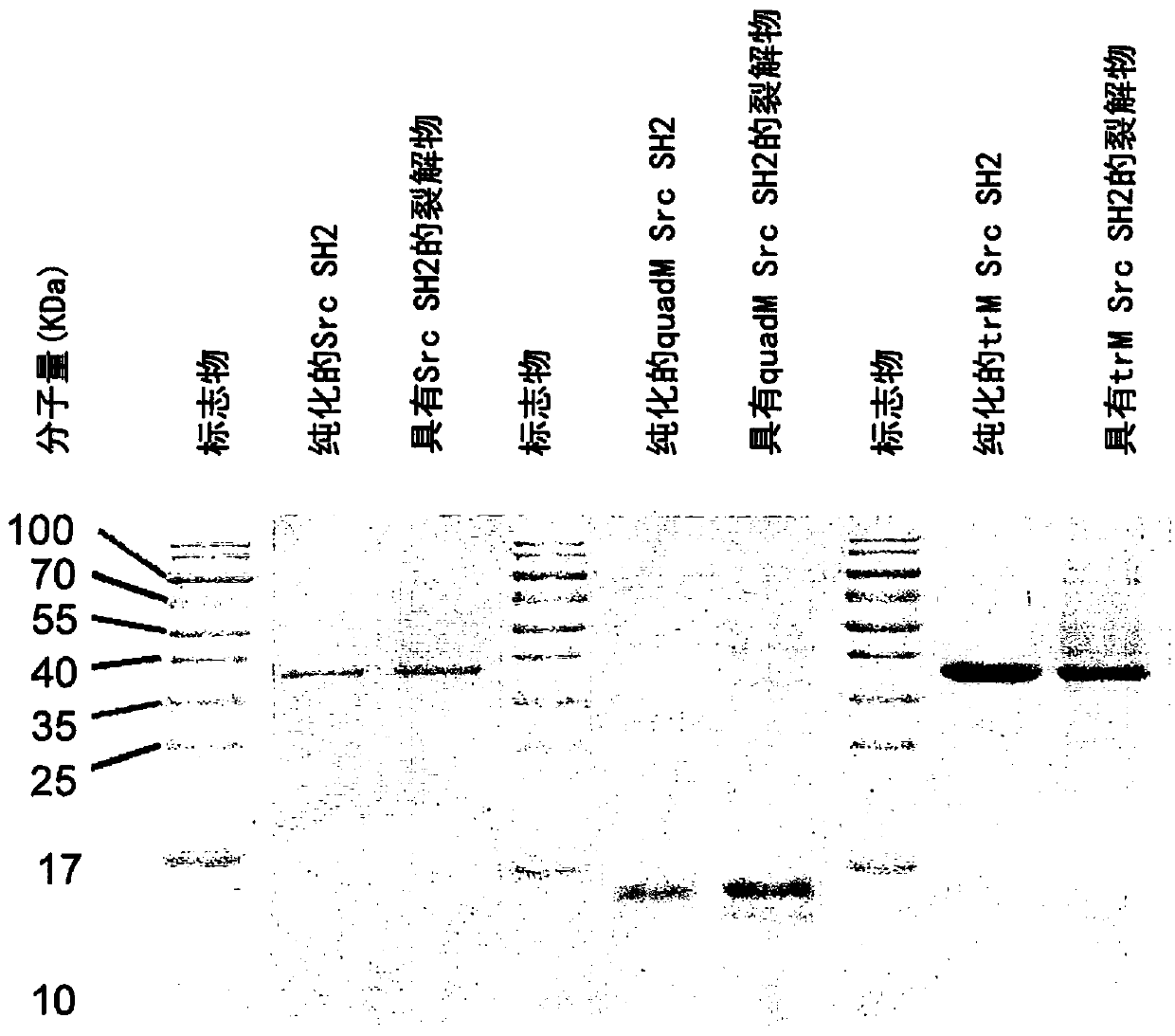
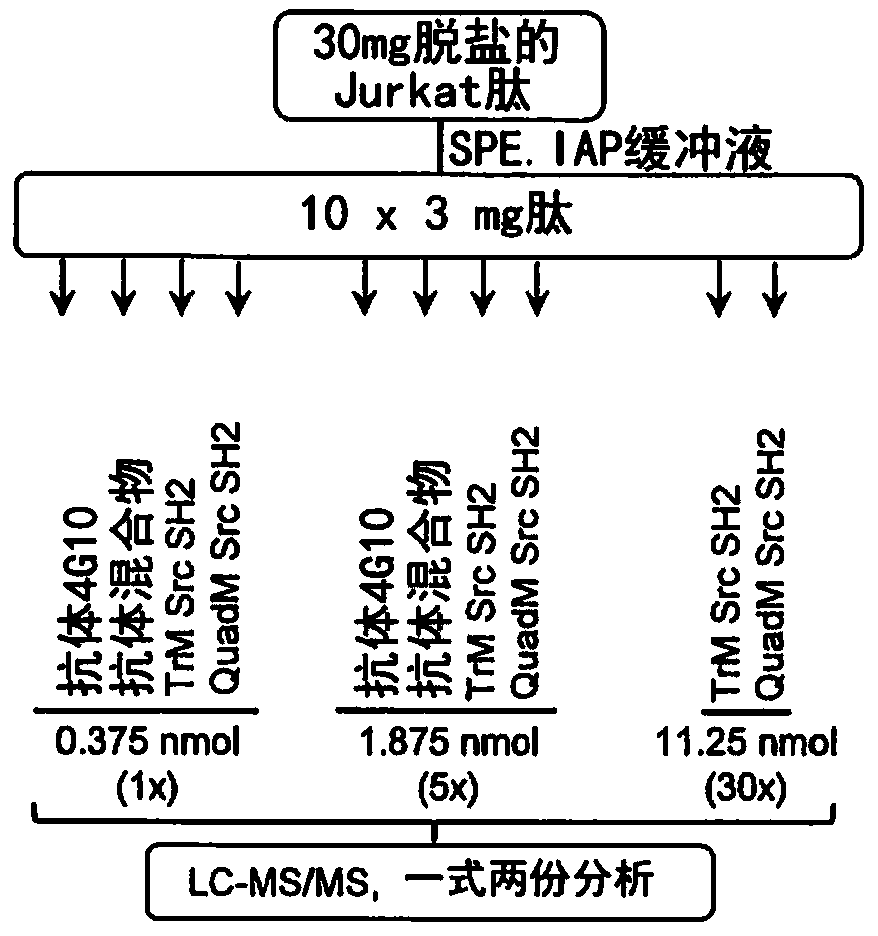
The present invention provides a probe and the application thereof. The probe comprises a spacer arm, and bait protein, a photoreactive group and an enriched group attached to the spacer arm. The baitprotein comprises a protein structure domain for identifying specific protein after translational modification, such as an SH2 domain for identifying tyrosine phosphorylation. According to the probeprovided by the present invention, the activity of the target protein cannot be affected by the probe, when the bait protein interacts with the target protein, ultraviolet radiation promotes covalentbinding of the photoreactive group and the target protein, and protein complexes are effectively enriched and obtained through the enriched group, so that analysis on weak interactions and transient interactions between proteins can be facilitated, enrichment and identification abilities of target proteins and complexes thereof in the human or animal tissue samples and near the cell membranes aresignificantly improved, enrichment and identification of tyrosine phosphorylation protein complexes in different breast cancer cell lines and breast cancer tissues are successfully realized, and the probe has broad application prospects and market value.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
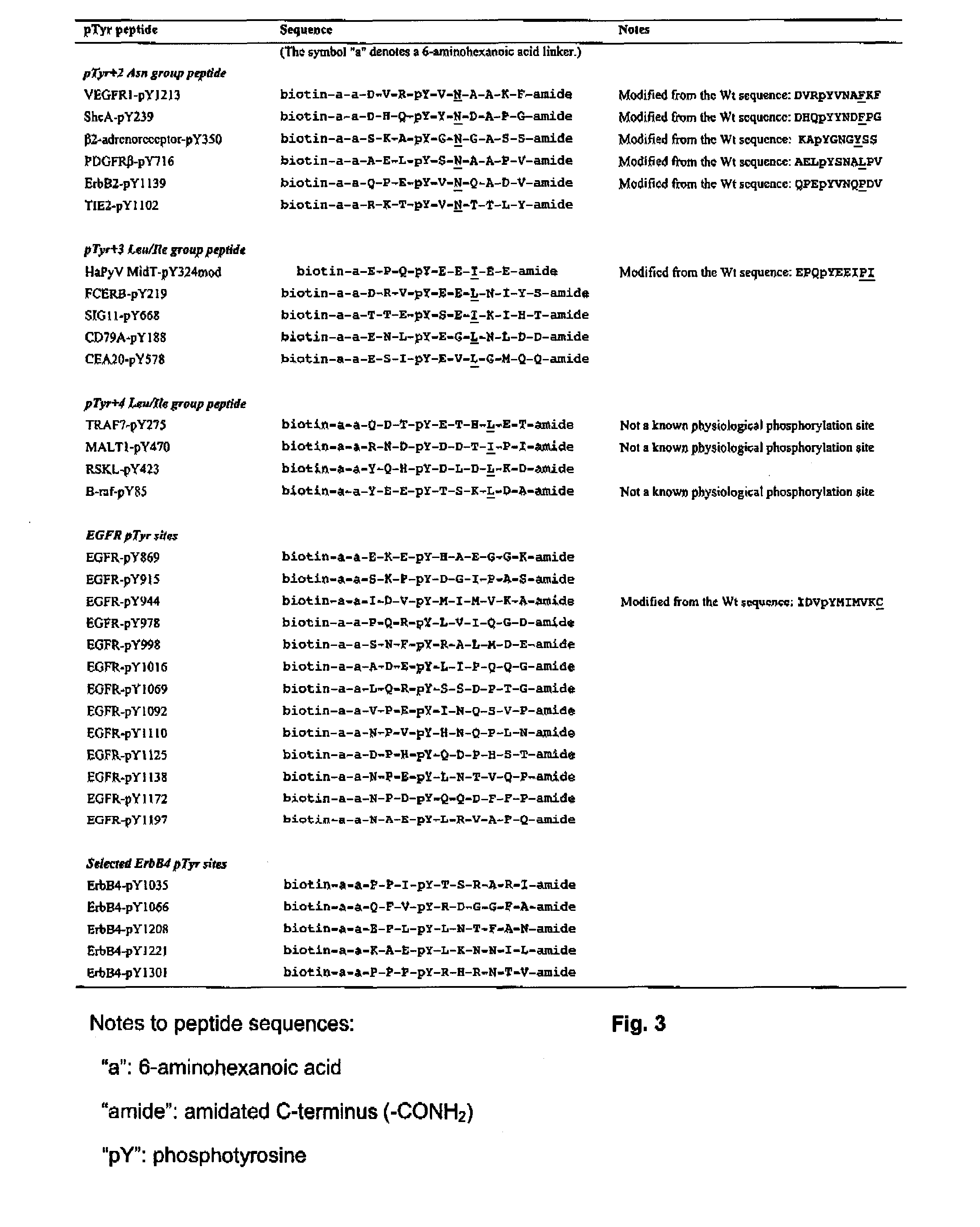
Methods for protein tyrosine phosphorylation profiling with variant sh2 domains
There is provided method of profiling protein tyrosine phosphorylation of a sample, the method comprising: contacting the sample with an SH2 Superbinder in order to bind pTyr-including peptides contained in the sample with the SH2 Superbinder; isolating the bound pTyr-including peptides from the sample; and identifying the isolated pTyr-including peptides.
Owner:李顺成
SH2 domain binding inhibitors
InactiveUS6977241B2High affinityDipeptide ingredientsTetrapeptide ingredientsHydrogenPhosphoprotein binding
Disclosed are compounds for SH2 domain binding inhibition. For example, disclosed is a compound of formula (I) wherein R1 is a lipophile; R2, in combination with the phenyl ring, forms a phenylphosphate mimic group or a protected phenylphosphate mimic group; R3 is hydrogen, azido, amino, carboxyalkyl, alkoxycarbonylalkyl, aminocarbonylalkyl, or alkylcarbonylamino, wherein the alkyl portion of R3 may be optionally substituted with a substituent selected from the group consisting of halo, hydroxy, carboxyl, amino, aminoalkyl, alkyl, alkoxy, and keto; R6 is a linker; AA is an amino acid; and n is 1 to 6; or a salt thereof. Also disclosed are a pharmaceutical composition, a method for inhibiting an SH2 domain from binding with a phosphoprotein and a method of treating breast cancer.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC OF THE
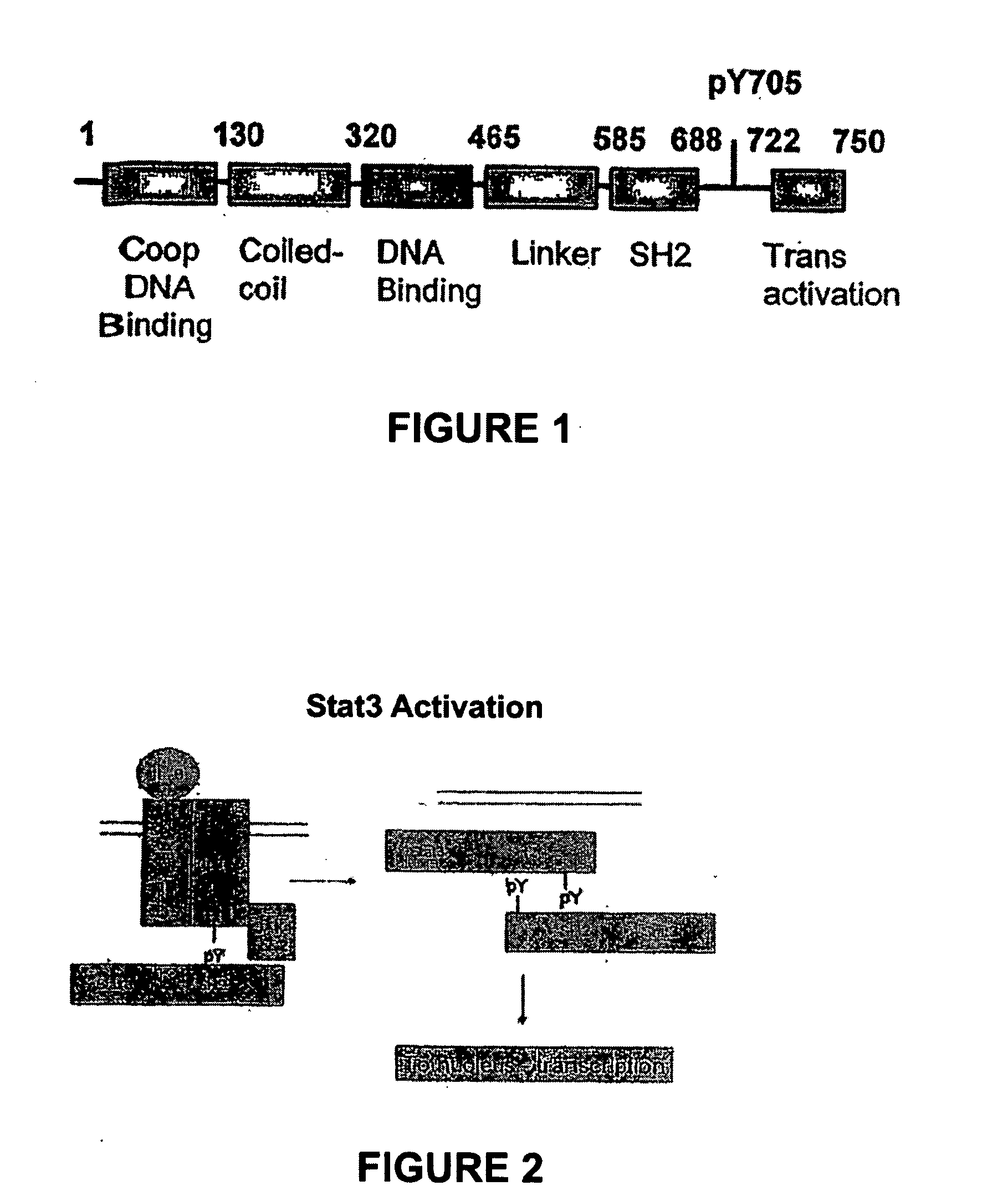
Inhibitors of STAT3 and uses thereof
InactiveUS8841257B2Group 4/14 element organic compoundsDipeptide ingredientsInterleukin 6Signalling molecules
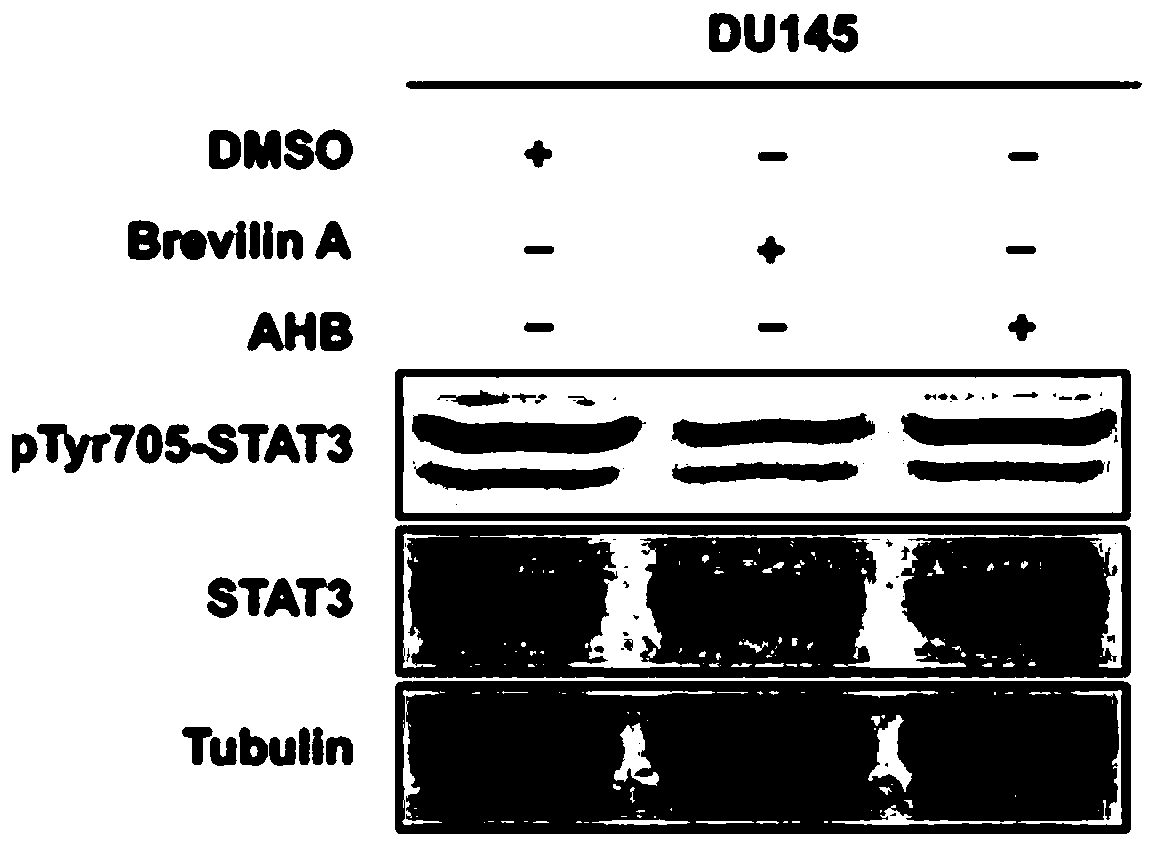
Compounds which inhibit the activity of signal transducer and activator of transcription 3 (STAT3) are provided together with methods of making and using the same. The compounds are designed to bind to the SH2 domain of STAT3, preventing STAT3 from binding to receptors for interleukin-6 family cytokines, growth factors such as the platelet-derived growth factor, the epidermal growth factor, vascular endothelial growth factor, and other signaling molecules such as leptin. Blocking these interactions prevents STAT3 from being phosphorylated on Tyr705, which is required for the dimerization of STAT3, translocation to the nucleus, binding to STAT3 response elements on promotors, and transcription of genes. In addition to these activities, binding to the SH2 domain of STAT3 breaks up pre-formed dimmers, thereby preventing the transcriptional activity of the inhibitor.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
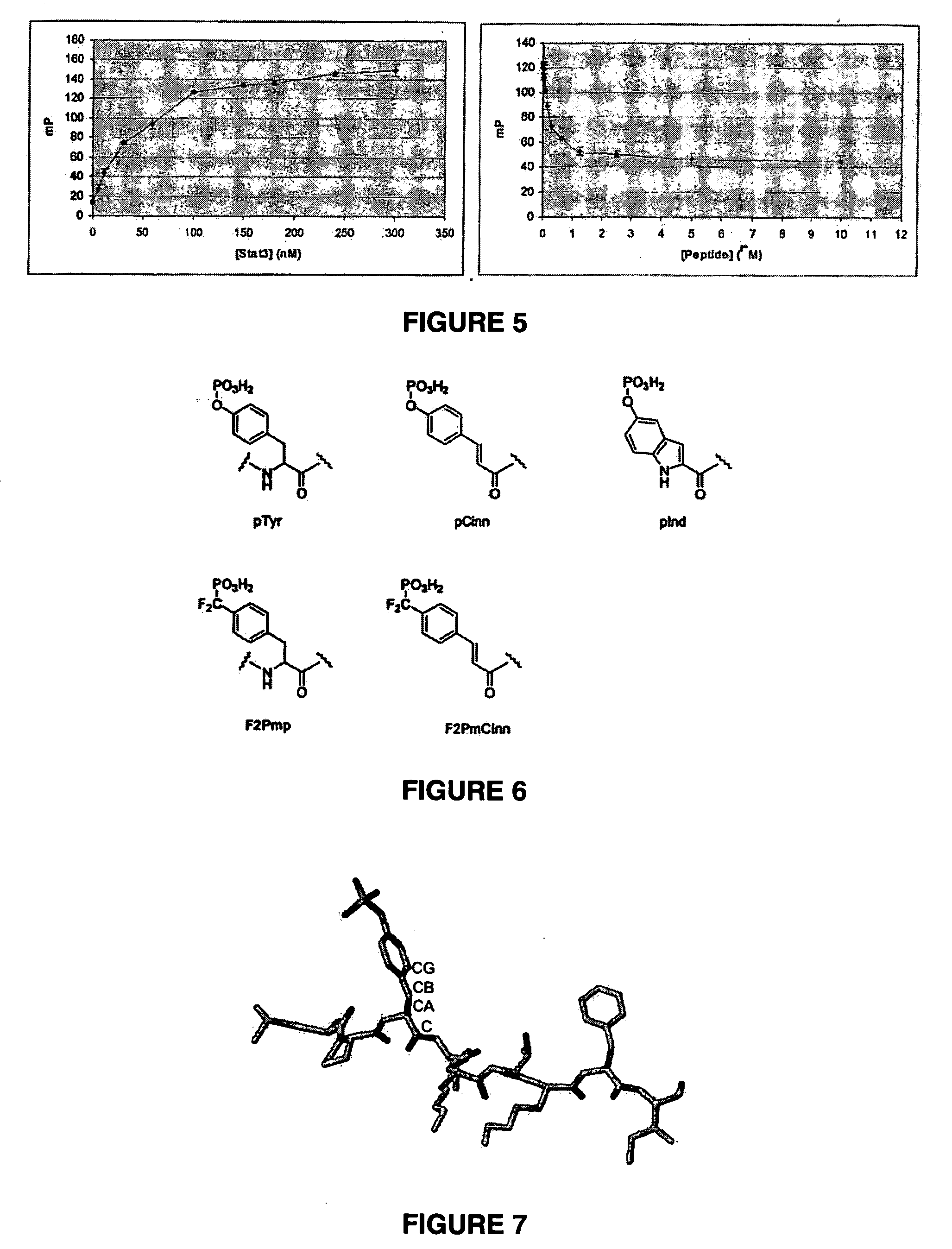
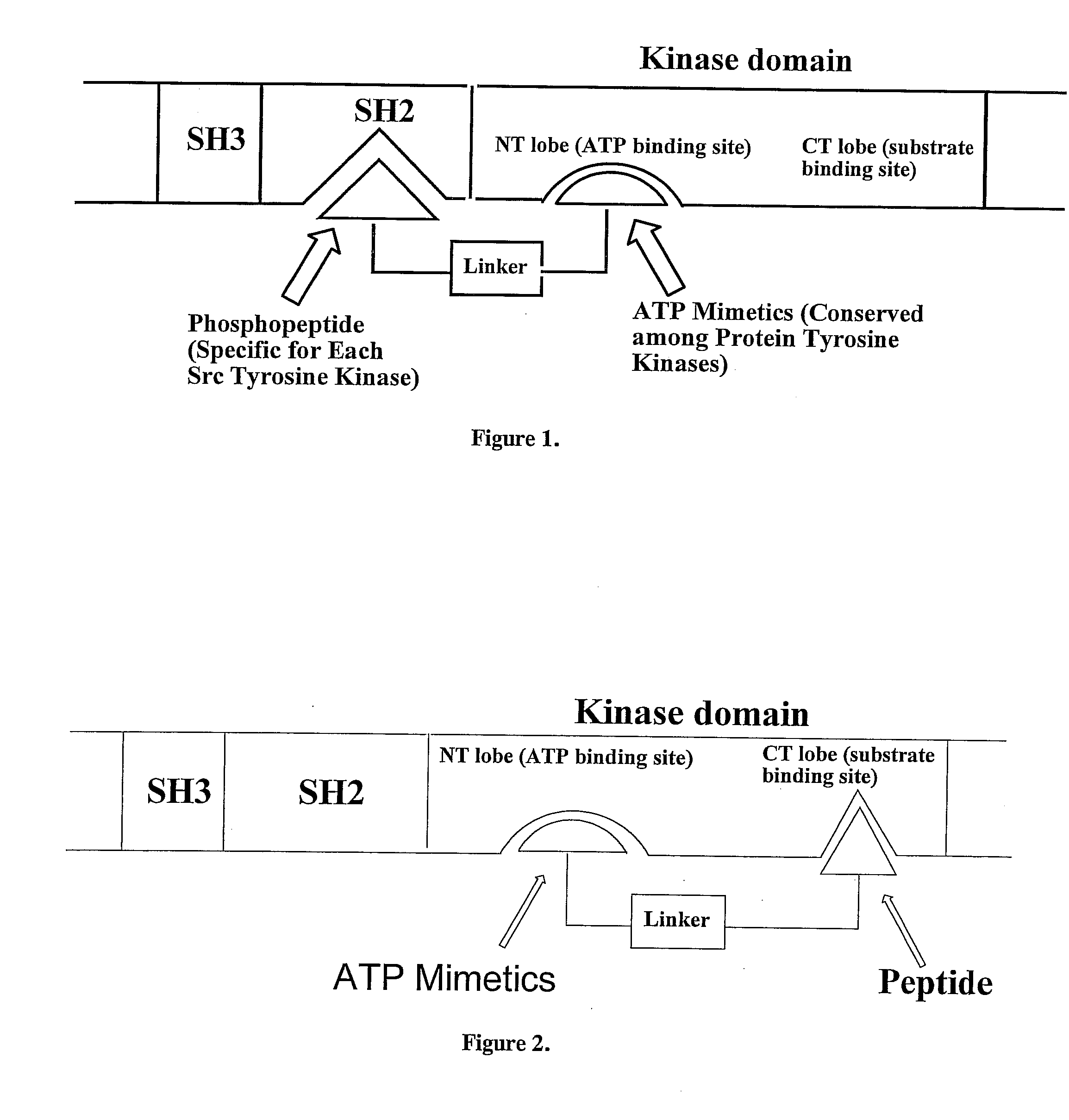
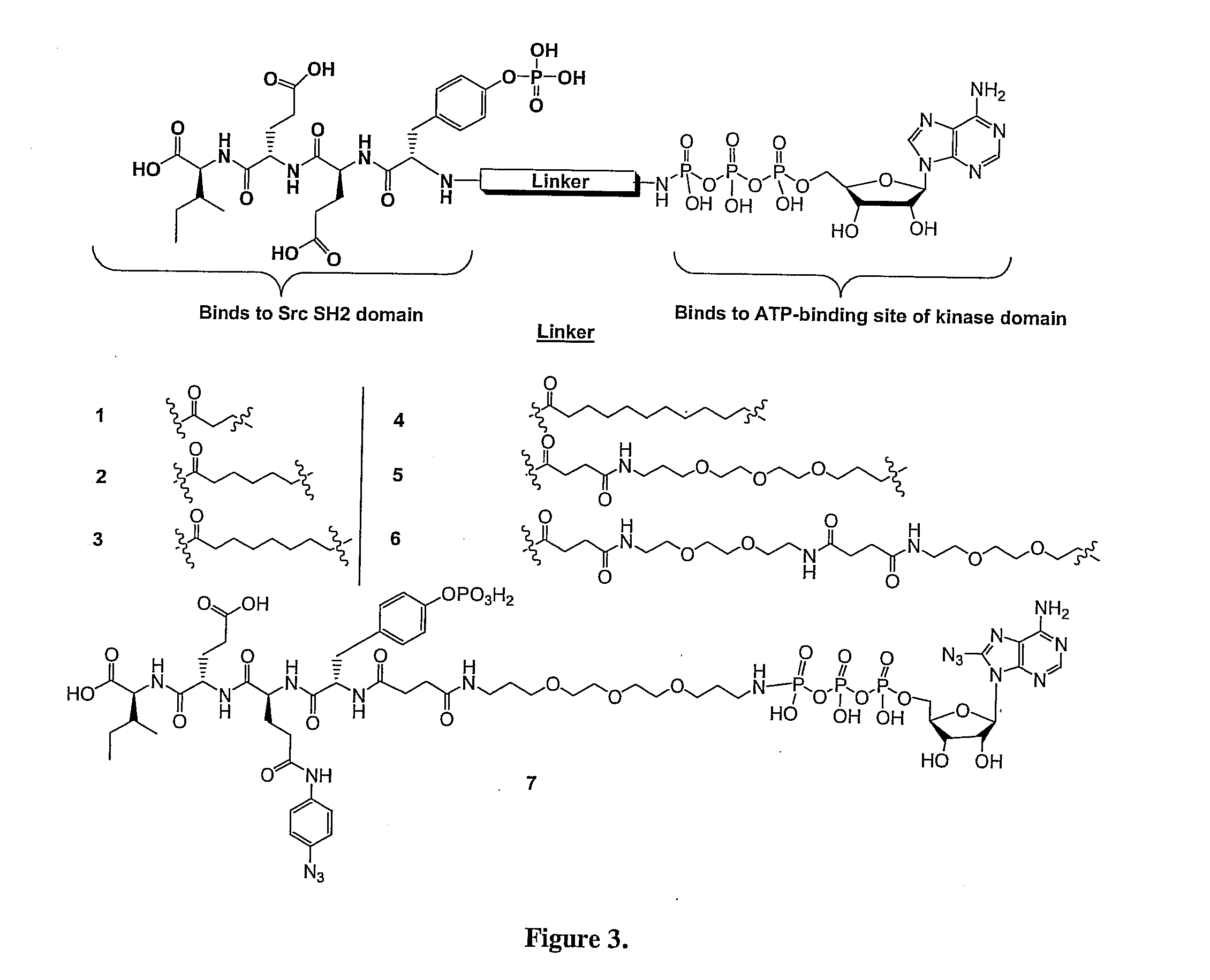
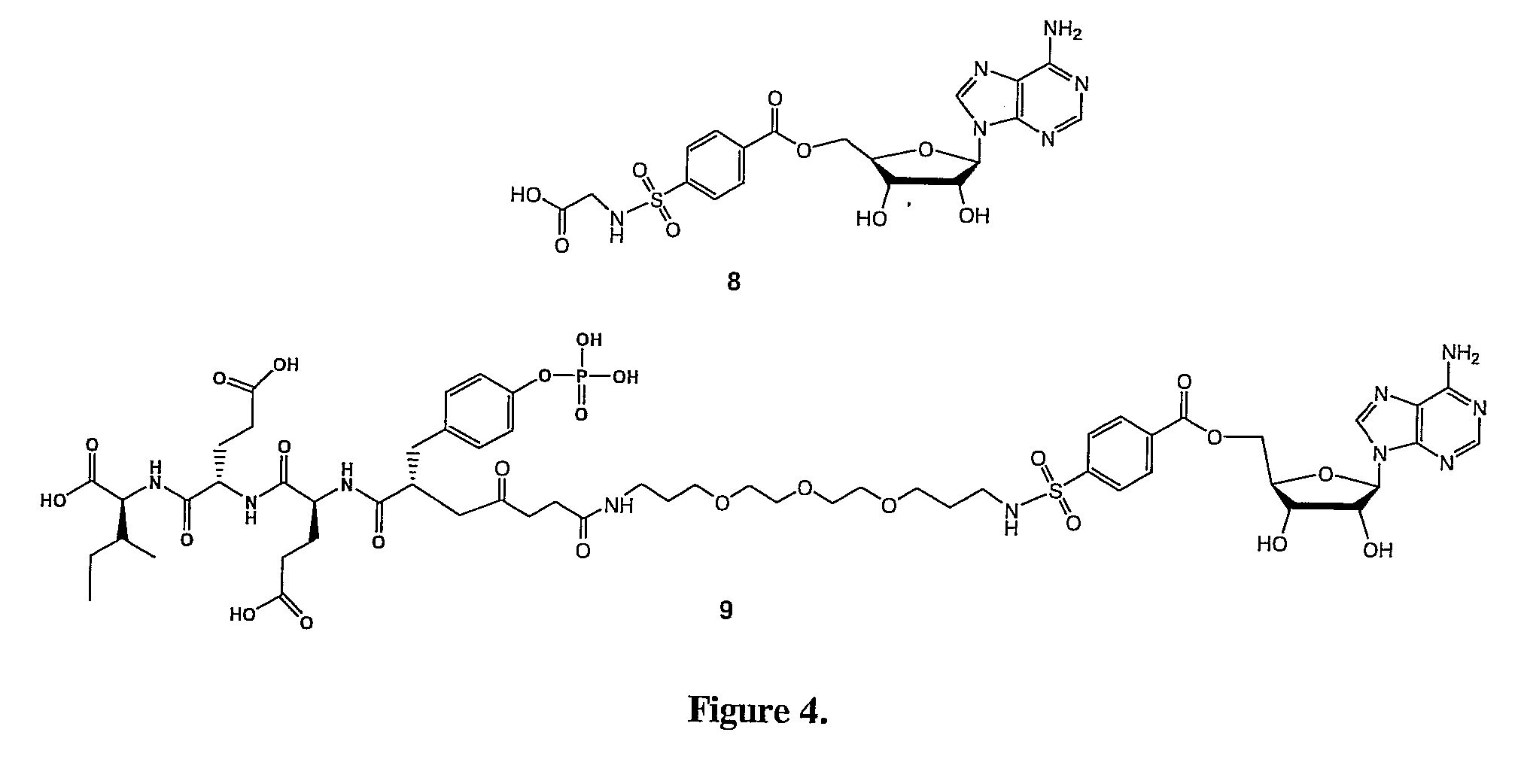
Bisubstrate inhibitors of protein tyrosine kinases as therapeutic agents
InactiveUS20070173437A1Application for drug developmentSlow downPeptide/protein ingredientsAntipyreticProtein-Tyrosine KinasesPurine
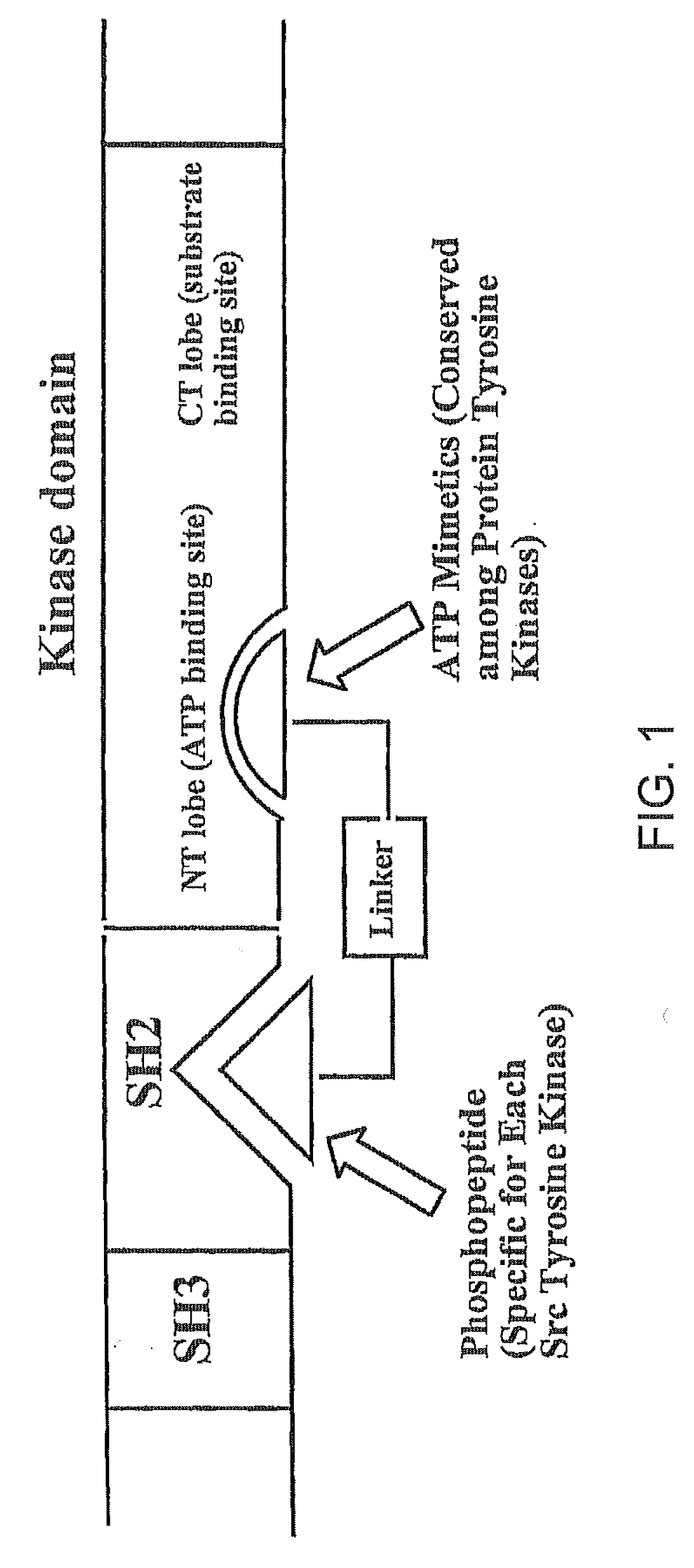
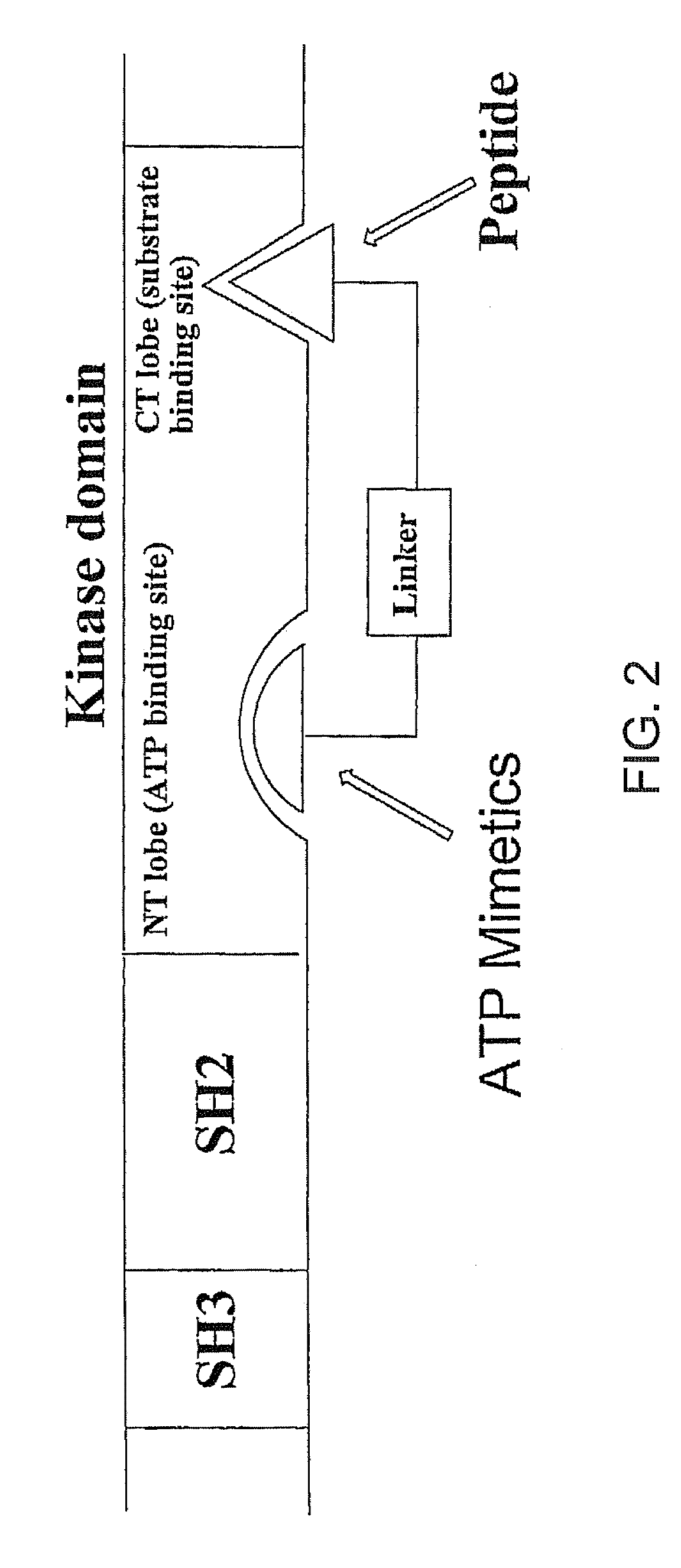
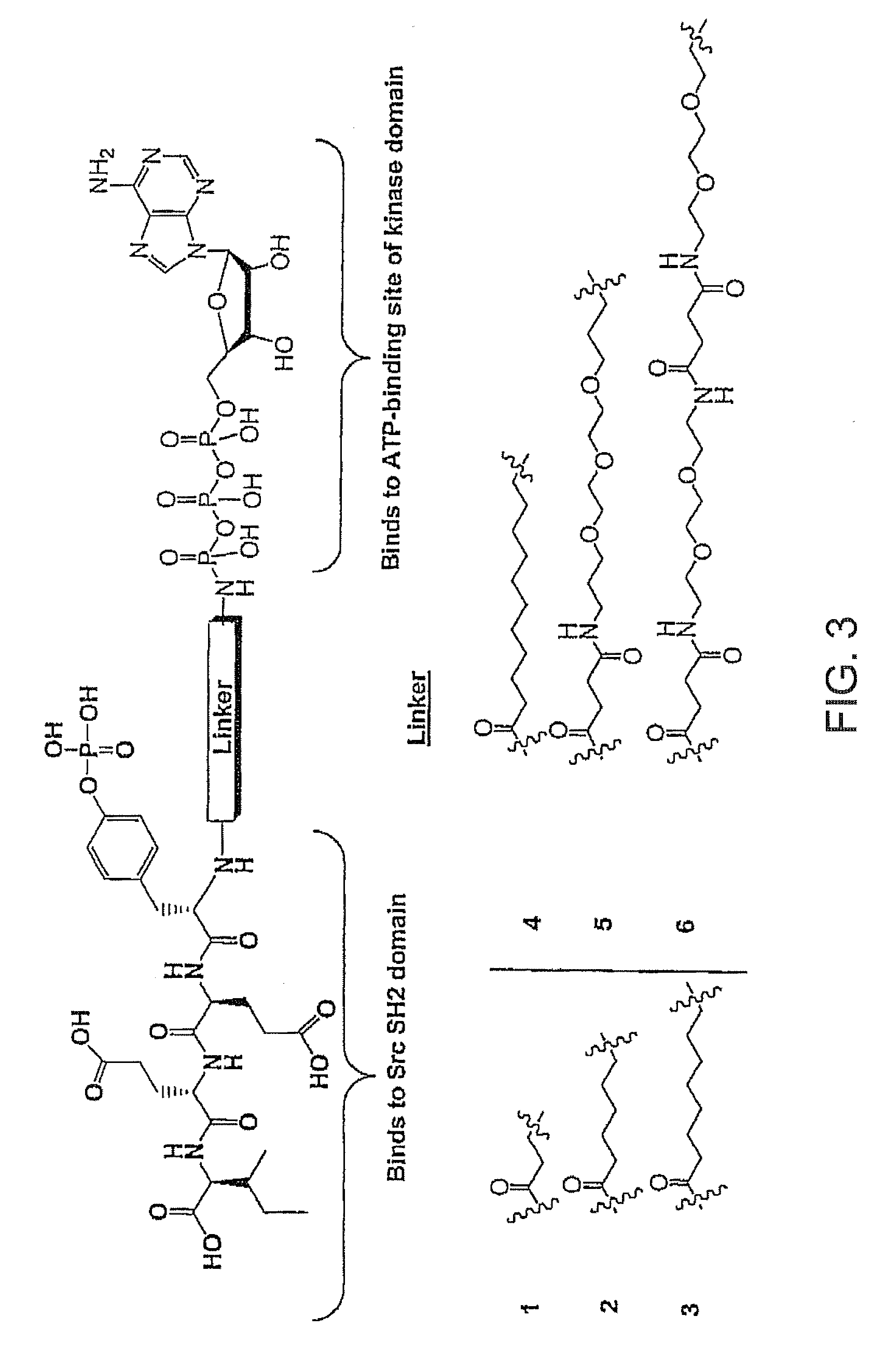
A bisubstrate inhibitor of Src kinases, having a nucleotide or N-heteroaromatic moiety; and a peptide / phosphopeptide, peptidomimetic, or phosphopeptide mimic moiety. The moieties are linked by a rigid or a flexible linker. The nucleotide or N-heteroaromatic moiety is ATP, ATP-mimics, N-heteroaromatics including purine-based derivatives, pyrimidine-based derivatives such as 2,4-diamino-5-substituted pyrimidine derivatives, pyrazole[3,4-d]pyrimidine derivatives, pyrrolo[2,3-d]pyrimidine derivatives, pyrido[2,3-d]pyrimidine derivatives, amino-substituted dihydropyrimido[4,5-d]pyrimidinone derivatives, thieno- and furo-substituted derivatives, quinazoline derivatives, and quinoline derivatives, and several natural products such as aminogenistein. The phosphopeptide mimics comprise phosphonate-based phosphotyrosine mimetics such as phosphonomethylphenylalanine (Pmp) and its analogues, carboxylic acid-based phosphotyrosine mimetics such as malonyltyrosine or phenylalanine analogues and their derivatives such as carboxymethyl phenylalanine, uncharged pTyr mimetics, and confonnationally constrained peptides. The phosphopeptide or phosphopeptide mimics inhibits the Src kinases SH2 domain.
Owner:THE BOARD FOR GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS +1
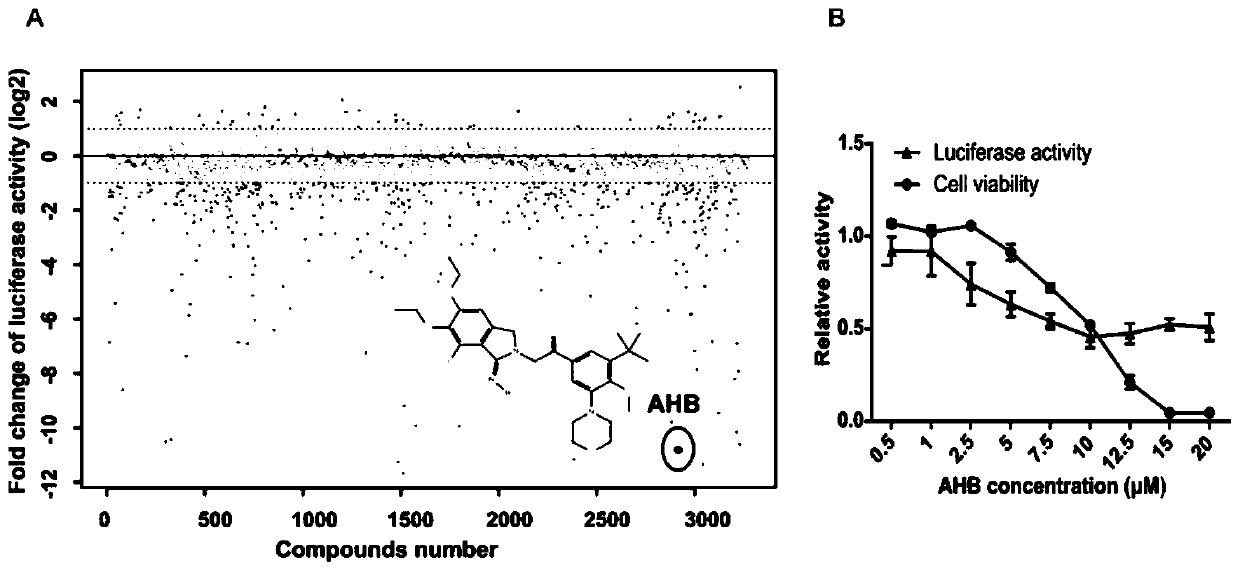
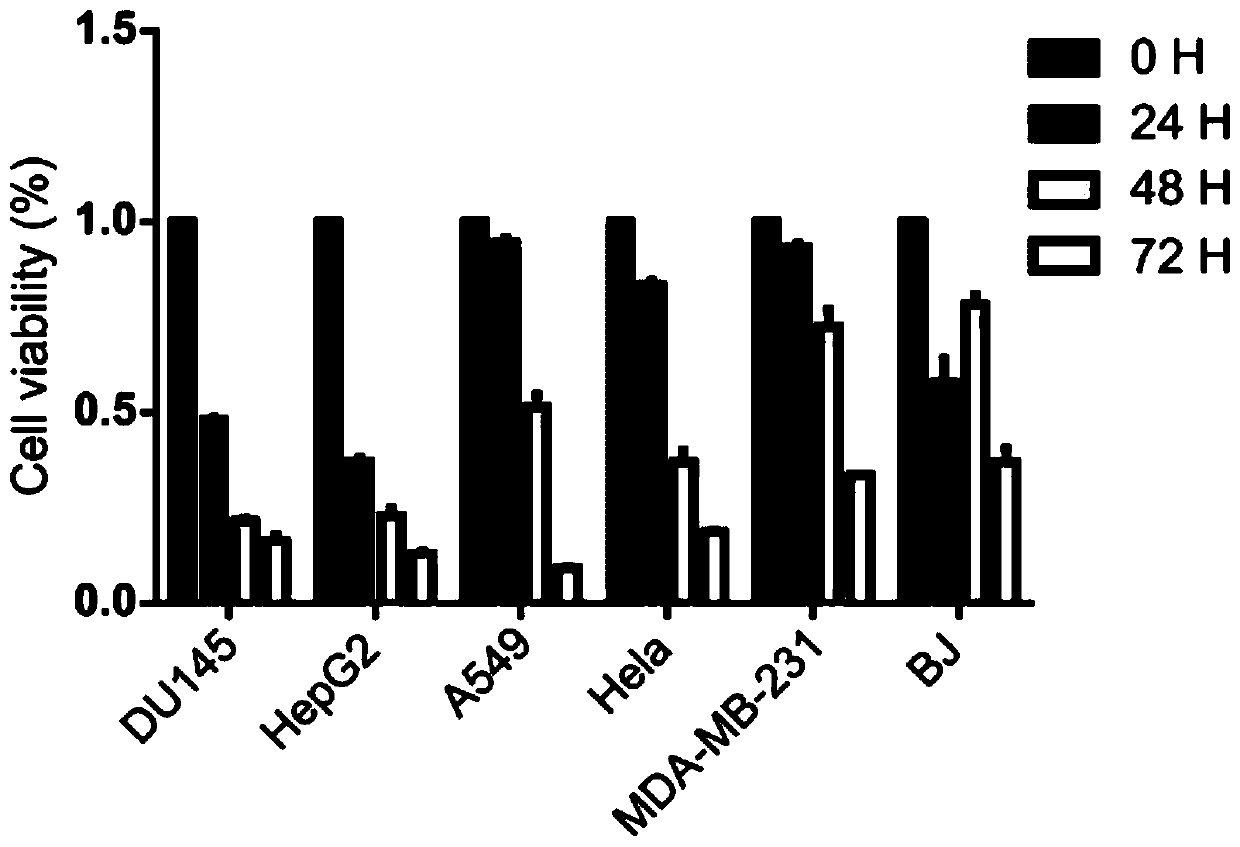
Application and research method of atopaxar hydrobromide as novel JAK-STAT3 signal path inhibitor
ActiveCN110393718ANo side effectsOrganic active ingredientsMicrobiological testing/measurementHydrobromideRNA extraction
The invention relates to an application of atopaxar hydrobromide as a novel JAK-STAT3 signal channel inhibitor. The atopaxar hydrobromide can be used for inhibiting STAT3 activity and STAT3 downstreamgene expression to control cell proliferation; the atopaxar hydrobromide has an inhibition effect on STAT3 activation induced by cytokines and JAKs self-phosphorylation; in computer molecular simulation, the atopaxar hydrobromide can be combined with an SH2 structural domain of STAT3 and has very high affinity with a JAK kinase JH1 structural domain; and the atopaxar hydrobromide can horizontallyinhibit the tumor cell growth. A research method of the atopaxar hydrobromide as a novel JAK-STAT3 signal pathway inhibitor comprises the following steps: cell culturing; protein extraction; western-Blot; RNA extraction; RT-PCR (Reverse Transcription-Polymerase Chain Reaction) and fluorescent quantitative PCR; luciferase activity analysis; cell viability analysis; cell cycle detection by flow cytometry; cell apoptosis detection by flow cytometry; nude mouse transplantation tumor experiments; computer molecular dynamics simulation docking analysis; and data calculation and processing. The invention lays a foundation for further cell cycle detection and subsequent research and development of novel small molecule drugs.
Owner:QINGDAO MARINE BIOPHARMACEUTICAL RES INST +1
Bisubstrate inhibitors of protein kinases as therapeutic agents
A bisubstrate inhibitor of Src kinases, having a nucleotide or N-heteroaromatic moiety; and a peptide / phosphopeptide, peptidomimetic, or phosphopeptide mimic moiety. The moieties are linked by a rigid or a flexible linker. The nucleotide or N-heteroaromatic moiety is ATP, ATP-mimics, N-heteroaromatics including purine-based derivatives, pyrimidine-based derivatives such as 2,4-diamino-5-substituted pyrimidine derivatives, pyrazole[3,4-d]pyrimidine derivatives, pyrrolo[2,3-d]pyrimidine derivatives, pyrido[2,3-d]pyrimidine derivatives, amino-substituted dihydropyrimido[4,5-d]pyrimidinone derivatives, thieno- and furo-substituted derivatives, quinazoline derivatives, and quinoline derivatives, and several natural products such as aminogenistein. The phosphopeptide mimics comprise phosphonate-based phosphotyrosine mimetics such as phosphonomethylphenylalanine (Pmp) and its analogues, carboxylic acid-based phosphotyrosine mimetics such as malonyltyrosine or phenylalanine analogues and their derivatives such as carboxymethyl phenylalanine, uncharged pTyr mimetics, and conformationally constrained peptides. The phosphopeptide or phosphopeptide mimics inhibits the Src kinases SH2 domain.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
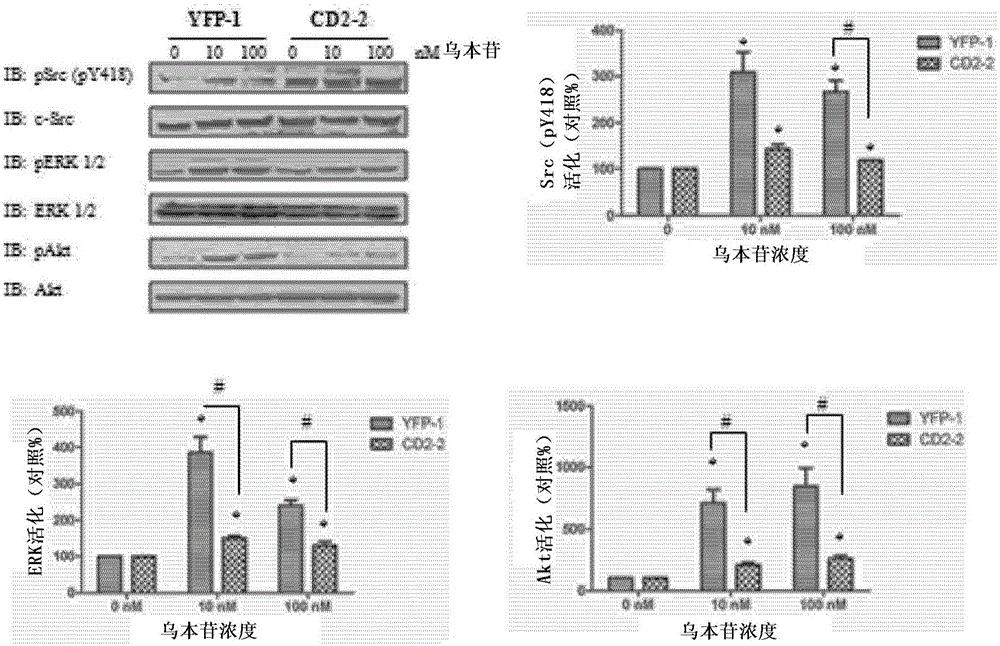
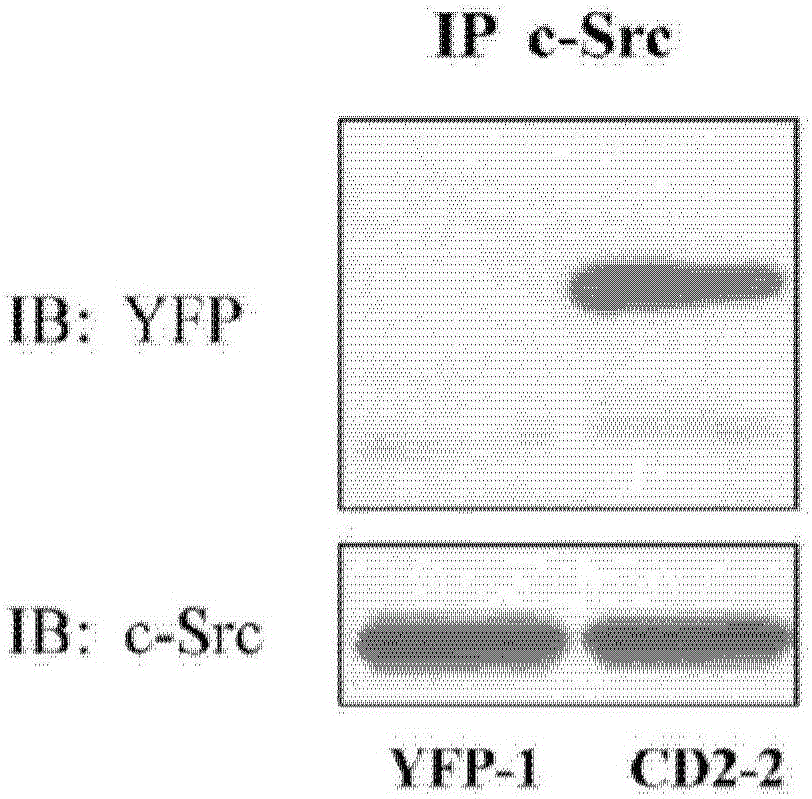
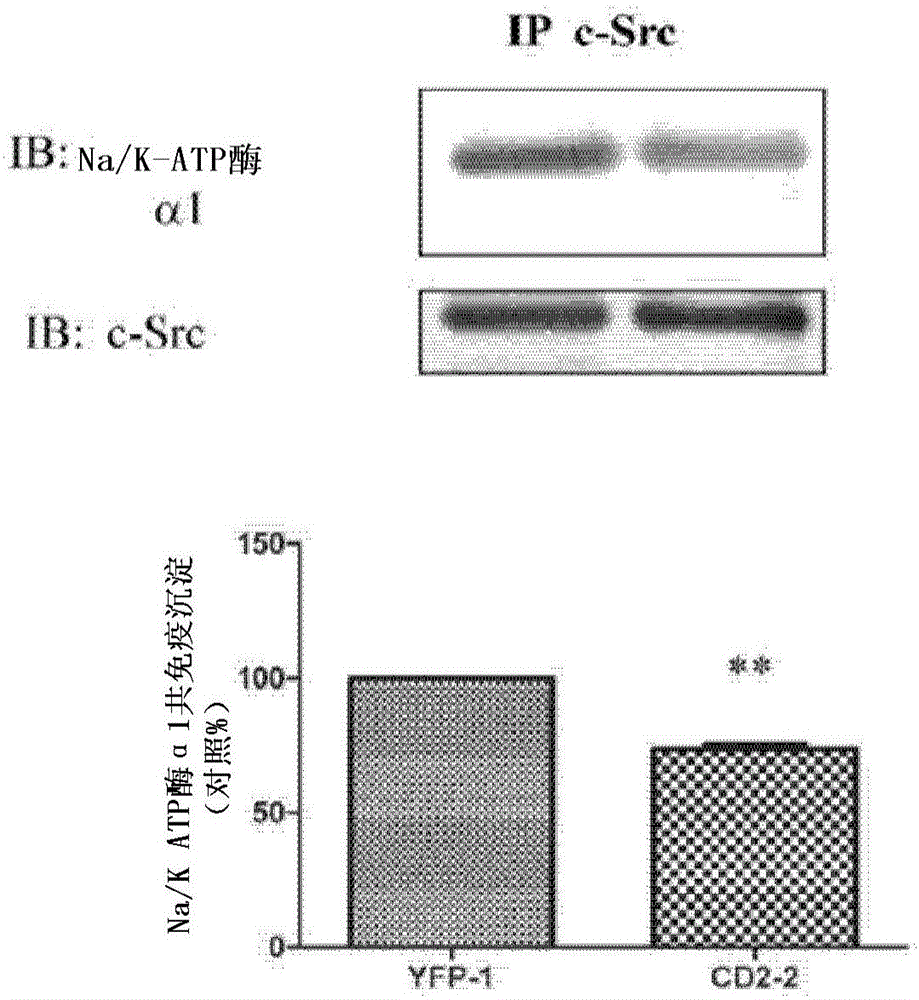
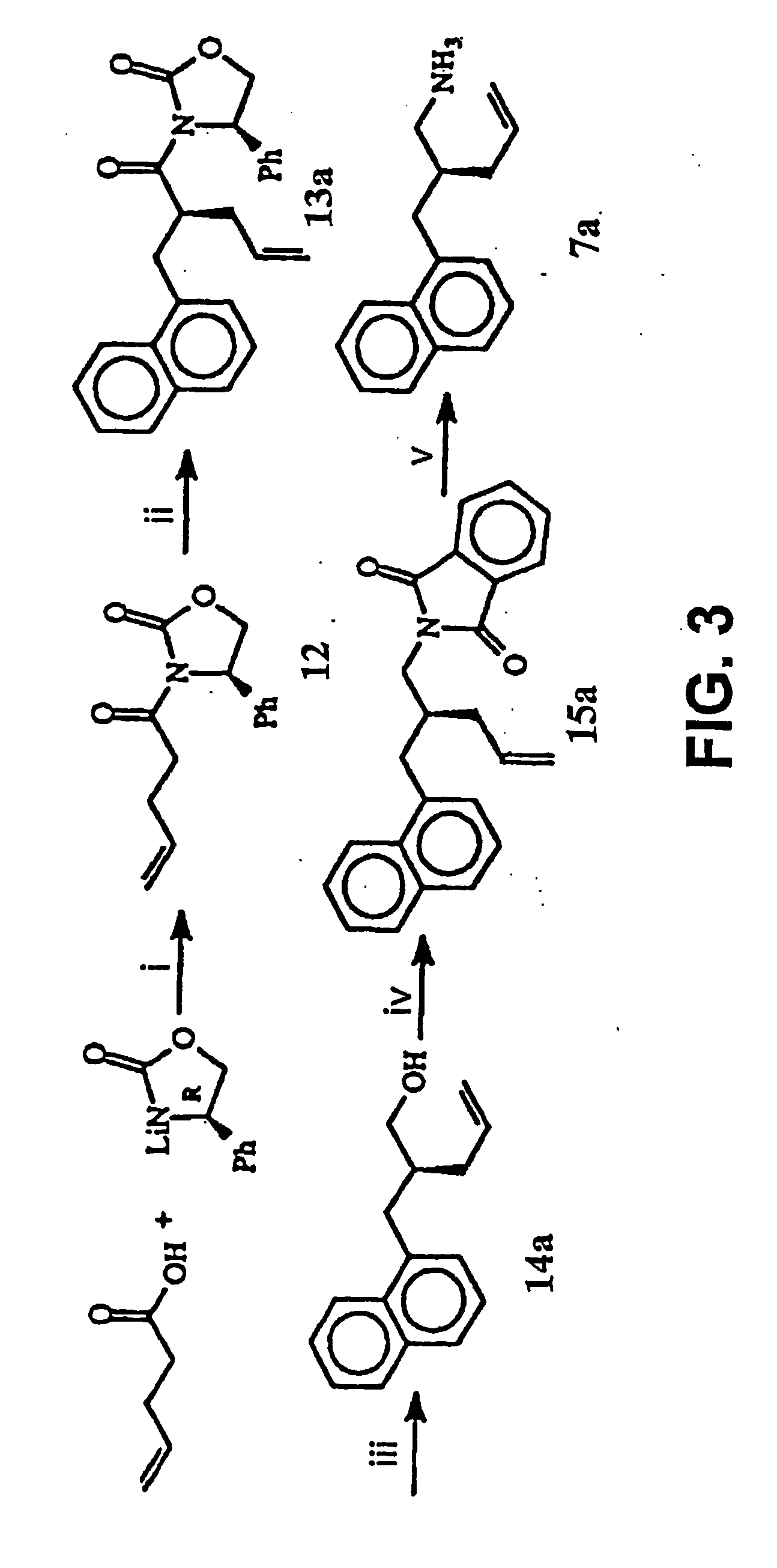
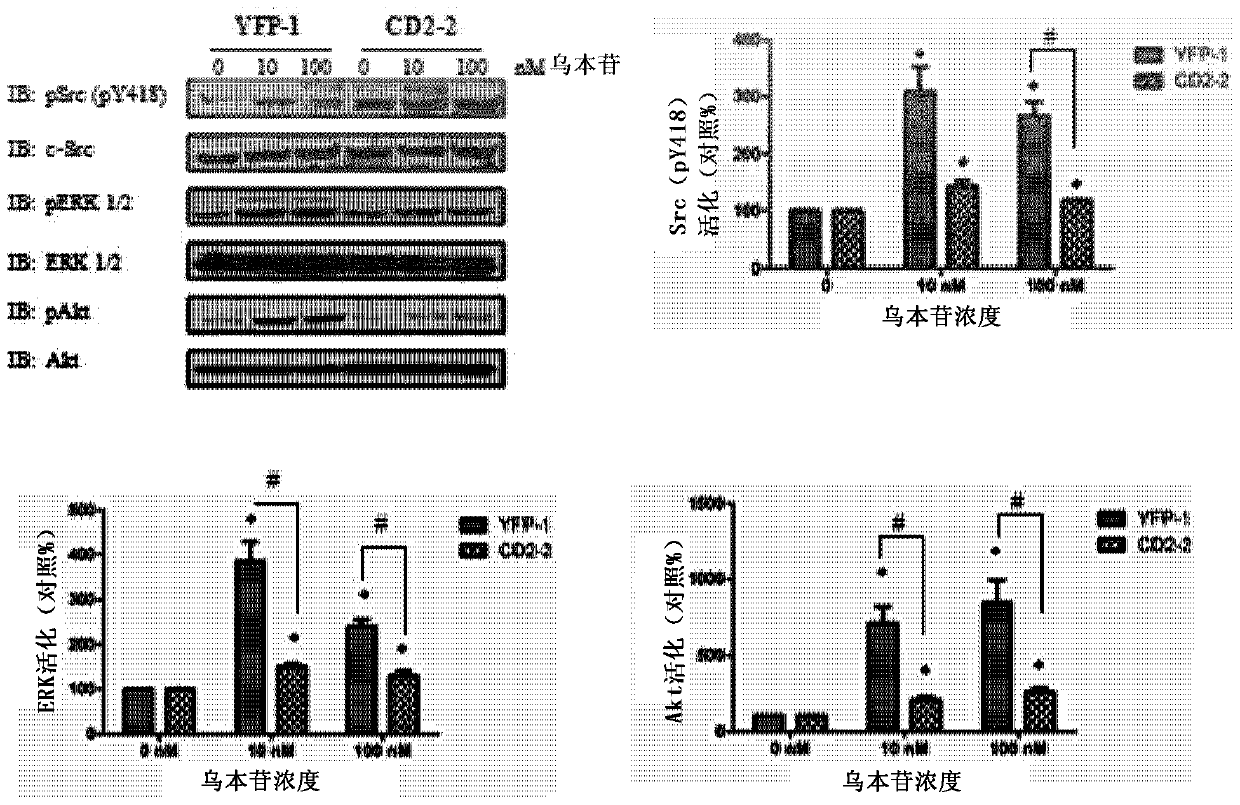
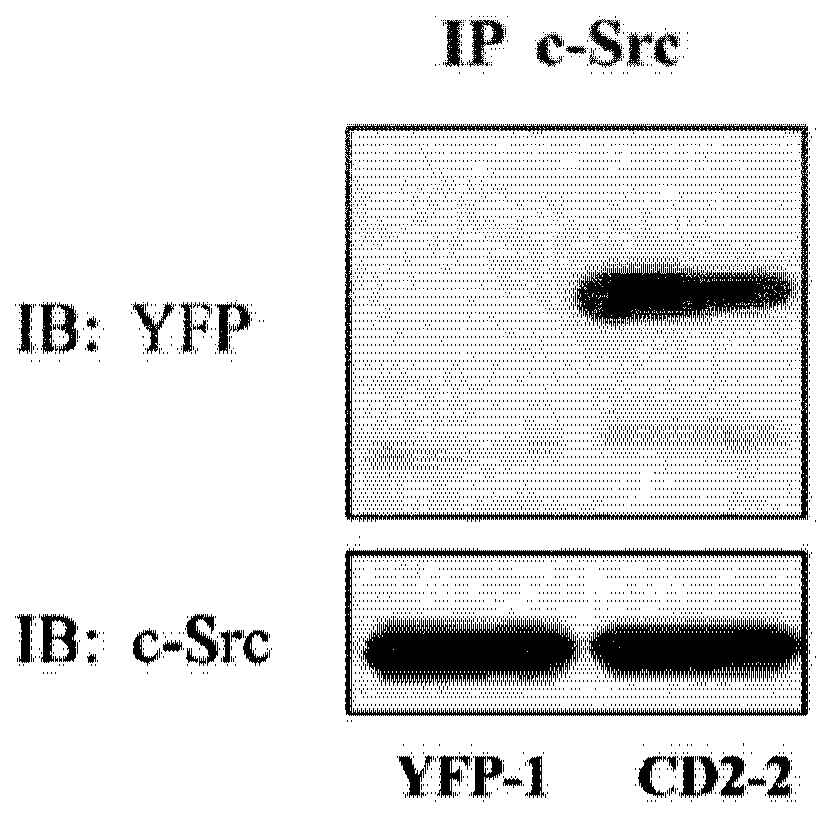
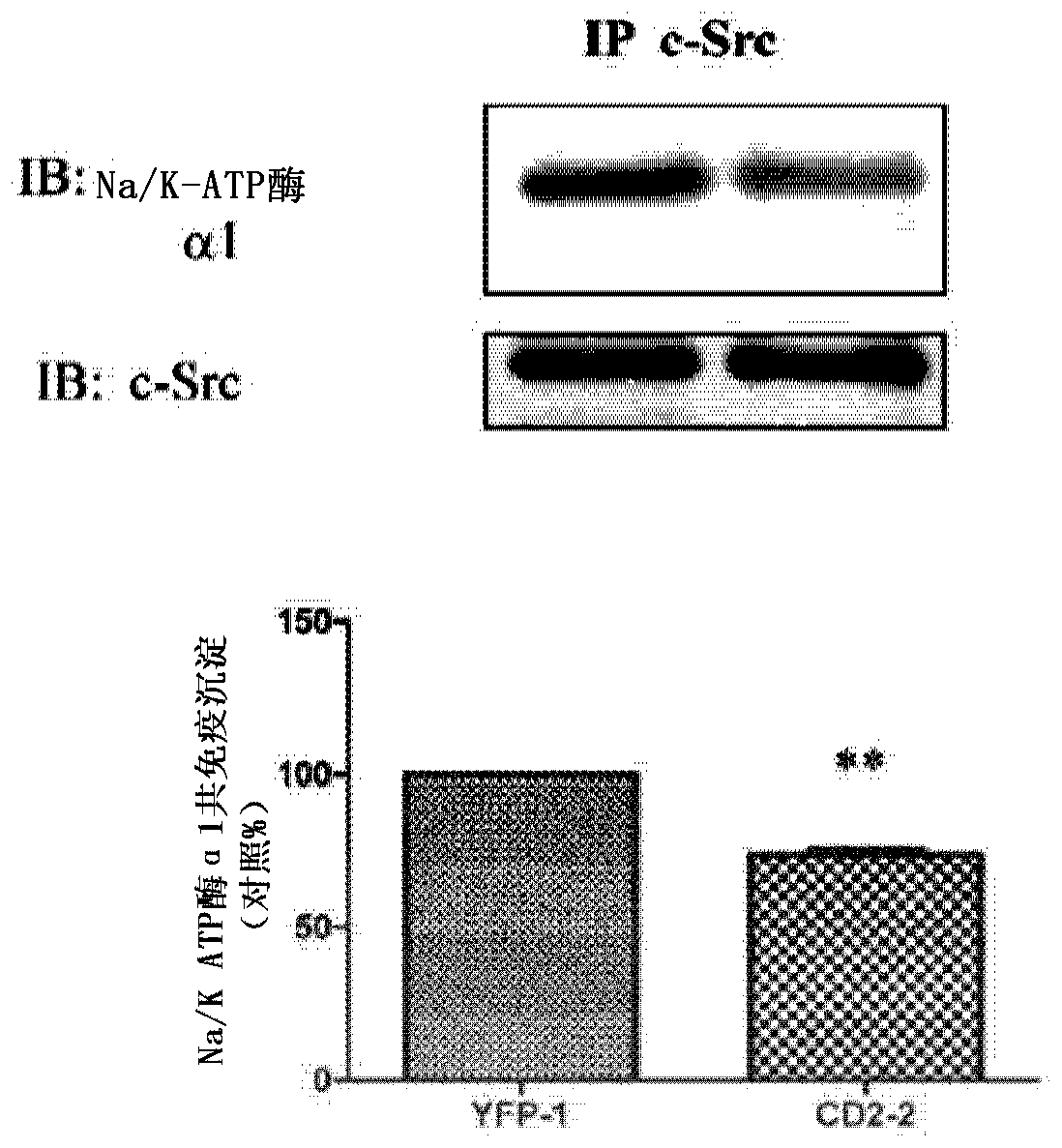
Cardiotonic steroid antagonists and related methods
ActiveCN106459168APolypeptide with localisation/targeting motifHydrolasesDiseaseCardiotonic steroids
Non-naturally occurring peptides are provided that act as a Src SH2 domain antagonist of cardiotonic steroids. Pharmaceutical compositions comprising the peptides are also provided along with vectors encoding the peptides. Methods of treating a Src-associated disease and reducing Src activity in a cell are further provided and include administering or contacting a cell with an effective amount of the peptide.
Owner:MARSHALL UNIV RES
Inhibition of cell motility and angiogenesis by inhibitors of Grb2-SH2-domain
InactiveUS7132392B1Inhibiting cellular motilityInhibit angiogenesisDipeptide ingredientsTetrapeptide ingredientsDiseaseMammal
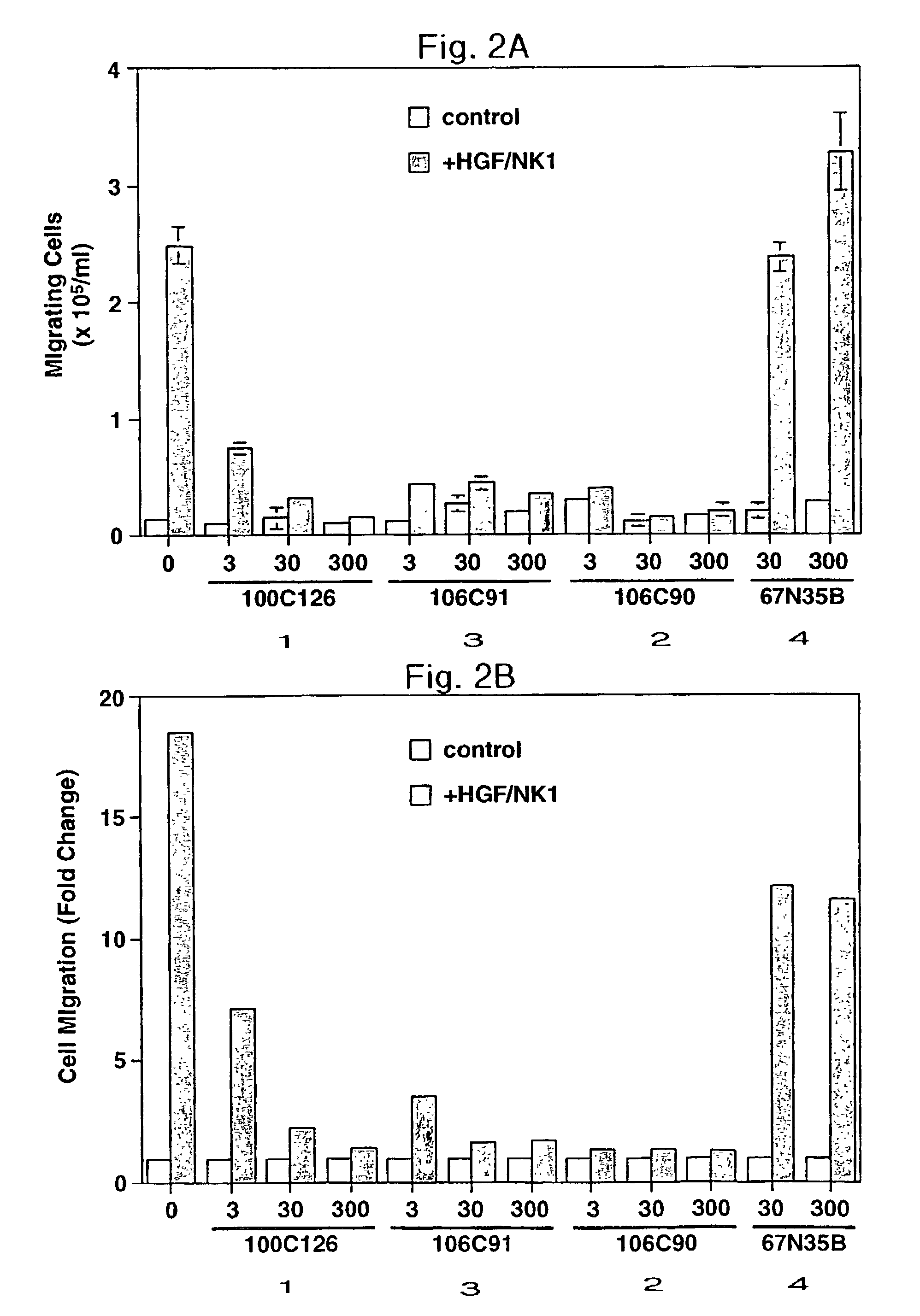
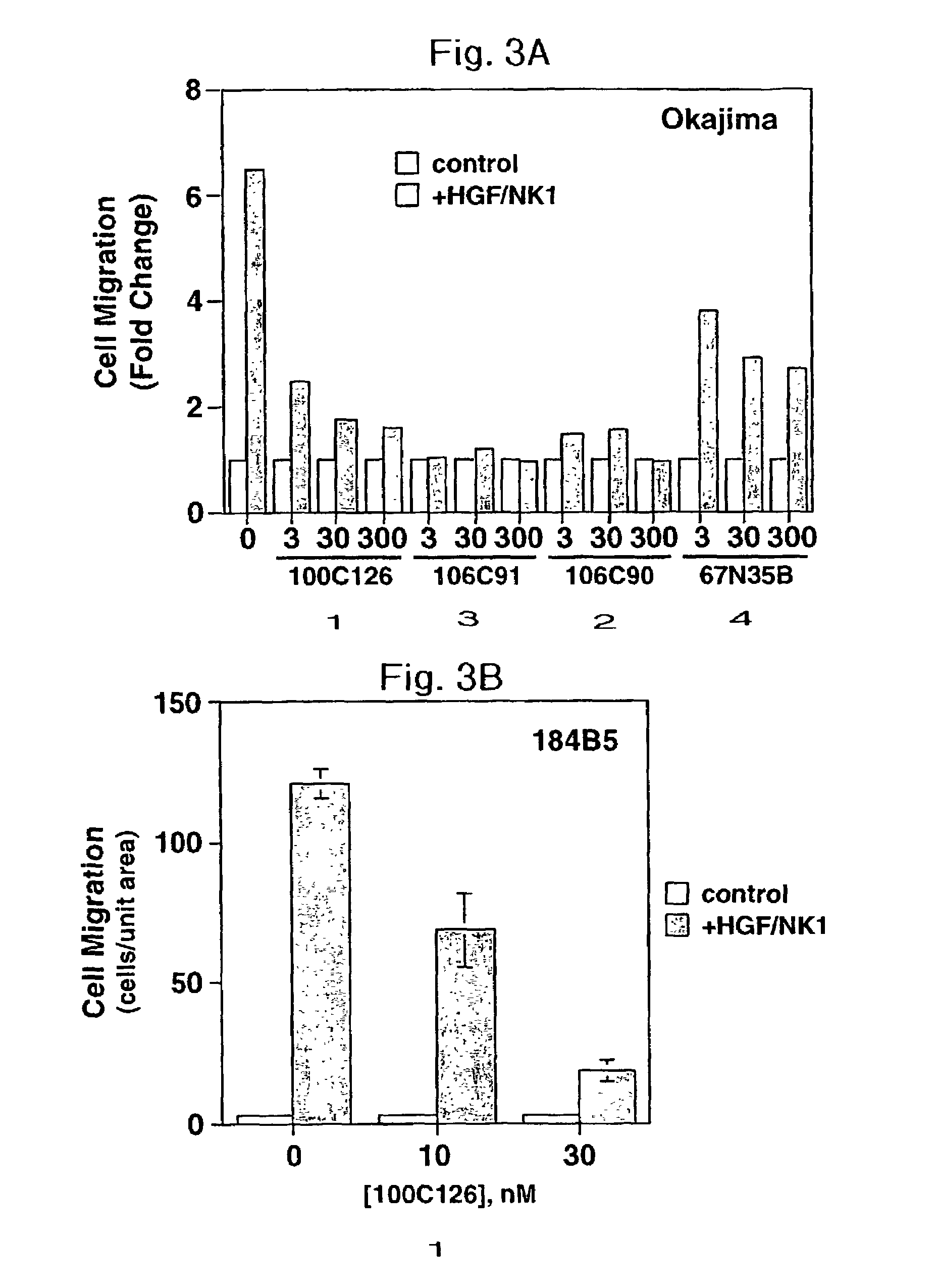
Disclosed are methods of inhibiting cell motility, for example, by inhibiting the binding between an intracellular transducer and a receptor protein tyrosine kinase, and more particularly by inhibiting hepatocyte growth factor (HGF) induced cell motility. The present invention also provides a method of inhibiting angiogenesis. The methods of the present invention employ peptides such as phosphotyrosyl mimetics. The present invention further provides methods of preventing and / or treating diseases, disorders, states, or conditions such as cancer, particularly metastatic cancer comprising administering to a mammal of interest one or more peptides of the present invention. Also disclosed are methods of blocking HGF, VEGF, or bFGF-stimulated migration, cell proliferation, and formation of capillary-like structures
Owner:HEALTH & HUMAN SERVICES DEPT OF GOVERNMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY SEC THE
Variant SH2 structural domain having high affinity with tyrosine-containing phosphorylation modification peptide
ActiveCN112250750AComprehensive screeningPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein-Tyrosine KinasesSH2 domain
The invention belongs to the technical field of application of protein tyrosine kinase (PTKs), and relates to a variant SH2 structural domain having high affinity with tyrosine-containing phosphorylation modification peptide. The invention provides the variant SH2 structural domain having high affinity with the tyrosine-containing phosphorylation modification peptide. The variant SH2 structural domain comprises amino acid sequences selected from SEQ ID NOs: 3, 4, 5 or 6. According to the invention, a residue is used for substituting a pTyr-binding region which is introduced into an SH2 domain,and a favorable substitution for enhanced binding affinity for a pTyr-containing peptide is set forth. Different substitution combinations exhibit different degrees of impact on affinity enhancement.The variant SH2 structural domain shows that the strength of the variant SH2 structural domain is much higher than that of a wild type SH2 structural domain in in-vitro binding analysis of pTyr-containing protein.
Owner:QINGDAO CANCER INST +1
Redox-stable, non-phosphorylated cyclic peptide inhibitors of sh2 domain binding to target protein, conjugates, thereof, compositions and methods of synthesis and use
InactiveUS20080031812A1Facilitates cellular internalizationInhibit bindingIn-vivo radioactive preparationsReceptors for hormonesCyclic peptideProtein target
A compound of formula: in which (i) aa1 is Adi and aa4 is Glu or (ii) each of aa1 and aa4 is Adi, L is sulfur, sulfoxide, oxygen or methylene, which compound (and its conjugates) bind to an SH2 domain in a protein comprising an SH2 domain, is non-phosphorylated, is redox-stable in vivo, is characterized by an IC50 in vivo of less than about 4.0 μM with respect to the SH2 domain in Grb2, and, upon binding to the SH2 domain of Grb2, has a turn conformation. A conjugate comprising a compound as described above and a carrier agent, a composition comprising (i) a compound or a conjugate as described above and (ii) a carrier, a method of inhibiting binding of an SH2 domain in a protein comprising an SH2 domain to a target protein in an animal, wherein the SH2 domain is contacted with a target protein-binding inhibiting effective amount of a compound or a conjugate as described above, and a method of synthesizing such conjugates.
Owner:GOVERNMENT OF THE USA REPRESENTED BY THE SEC DEPA +1
Small molecule inhibitors of lck sh2 domain binding
The present invention provides materials and methods for modulating an immune response. The materials and methods may be used to treat diseases associated with an aberrant immune response. In some embodiments, materials and methods of the invention may be used to treat autoimmune diseases, for example, rheumatoid arthritis.
Owner:UNIV OF MARYLAND BALTIMORE
Phenylalanine derivatives
InactiveUS20070219194A1Good treatment effectOrganic active ingredientsAntipyreticCarboxyl radicalOrtho position
The present invention provides phenylalanine derivatives that inhibit SH2 domain binding with a phosphoprotein. These derivatives include compounds of the formula: W—Y-(AA)n-Z wherein n is 0 to 15; Y is a phenylalanyl radical having a phenyl ring, an amine end, and a carboxyl end, the phenyl ring having one or more substituents, e.g., hydroxyl, carboxyl, formyl, carboxyalkyl, carboxyalkyloxy, dicarboxyalkyl, dicarboxyalkyloxy, dicarboxyhaloalkyl, dicarboxyhaloalkyloxy, and phosphonoalkyl, or phosphonohaloalkyl; W is a moiety attached to the nitrogen of Y and is, e.g., alkylcarbonyl, oxalyl, alkylaminooxalyl, arylaminooxalyl, arylalkylaminooxalyl, or alkoxyoxalyl; AA is an amino acid, the amine end of which is attached to the carboxyl end of Y; and Z is an arylalkylamino or arylheterocyclyl alkylamino; or a salt thereof; with the proviso that W is not arylalkylamino when the phenyl ring of phenylalanyl contains a phosphonoalkyl or phosphonohaloalkyl substituent at a position para to the alkylamido group and the ortho and meta positions are unsubstituted. The present invention further provides precursors suitable for preparing the phenylalanine derivatives and a method for the preparation of the precursors. The present invention further provides conjugates comprising a precursor and a conjugant that are covalently linked. These conjugates have biological and / or pharmacological properties.
Owner:UNITED STATES OF AMERICA +1
Recombination ubiquitin ligase SH2-U-box fusion gene as well as expression vectors and applications thereof
InactiveCN102517312APromote degradationPromote ubiquitinationGenetic material ingredientsFermentationCancer cellNucleotide
The invention discloses a recombination ubiquitin ligase SH2-U-box fusion gene as well as expression vectors and applications thereof. The recombination ubiquitin ligase SH2-U-box fusion gene is characterized in that an Src Homology 2 (SH2) structural domain gene of a growth factor receptor bound protein (Grb2) is connected with a ubiquitin-box (U-box) structural domain gene of a carboxy terminus of Hsc70 interacting protein (CHIP), and a concrete nucleotide sequence is shown as SEQ.ID.NO.1. The constructed SH2-U-box fusion gene can be cloned to different expression vectors, and thereby, the constructed SH2-U-box fusion gene enters cancer cells through different paths to perform actions, wherein the expression vectors comprise eukaryotic expression vectors or adenovirus expression vectors. The fusion gene is capable of knocking down carcinogenic related proteins BCR / Abl of host cancer cells after transfecting the cancer cells, thus, the cell proliferation capacity of the cancer cells is obviously reduced, and proliferation inhibition and invasion inhibition are shown on the cancer cells, thereby, an anti-cancer effect is achieved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
SH2 domain binding inhibitors
InactiveUS7425537B2High affinityGroup 5/15 element organic compoundsPeptide preparation methodsHydrogenPhosphoprotein binding
Disclosed are compounds for SH2 domain binding inhibition, for example, a compound of formula (I), wherein R1 is a lipophile; R2, in combination with the phenyl ring, is a phenylphosphate mimic group or a protected phenylphosphate mimic group; R3 is hydrogen, azido, amino, carboxyalkyl, alkoxycarbonylalkyl, aminocarbonylalkyl, or alkylcarbonylamino, wherein the alkyl portion of R3 may be optionally substituted with a substituent selected from the group consisting of halo, hydroxy, carboxyl, amino, aminoalkyl, alkyl, alkoxy, and keto; R6 is a linker; AA is an amino acid; and n is 1 to 6; or a salt thereof. The conformationally compounds provide enhanced binding affinity with SH2 domain protein. Also disclosed are a pharmaceutical compositions and a method for inhibiting an SH2 domain from binding with a phosphoprotein.
Owner:UNITED STATES OF AMERICA
Sh2 domain binding inhibitors
InactiveUS20060167222A1High affinityGroup 5/15 element organic compoundsCyclic peptide ingredientsPhosphoprotein bindingSH2 domain
Disclosed are compounds for SH2 domain binding inhibition, for example, a compound of formula (I), wherein R1 is a lipophile; R2, in combination with the phenyl ring, is a phenylphosphate mimic group or a protected phenylphosphate mimic group; R3 is hydrogen, azido, amino, carboxyalkyl, alkoxycarbonylalkyl, aminocarbonylalkyl, or alkylcarbonylamino, wherein the alkyl portion of R3 may be optionally substituted with a substituent selected from the group consisting of halo, hydroxy, carboxyl, amino, aminoalkyl, alkyl, alkoxy, and keto; R6 is a linker; AA is an amino acid; and n is 1 to 6; or a salt thereof. The conformationally compounds provide enhanced binding affinity with SH2 domain protein. Also disclosed are a pharmaceutical compositions and a method for inhibiting an SH2 domain from binding with a phosphoprotein.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Cardiac steroid antagonists and related methods
The present invention provides non-naturally occurring peptides that act as Src SH2 domain antagonists of cardiac steroids. The present invention also provides a pharmaceutical composition comprising the peptide and a vector encoding the peptide. The present invention further provides a method for treating Src-related diseases and reducing Src activity in cells, and the method comprises administering or contacting the cells with an effective amount of the peptide.
Owner:MARSHALL UNIV RES
Gamma-aa-peptide stat3/dna inhibitors and methods of use
ActiveUS20180244719A1Antineoplastic agentsPeptides with abnormal peptide linkDNA-binding domainGene expression level
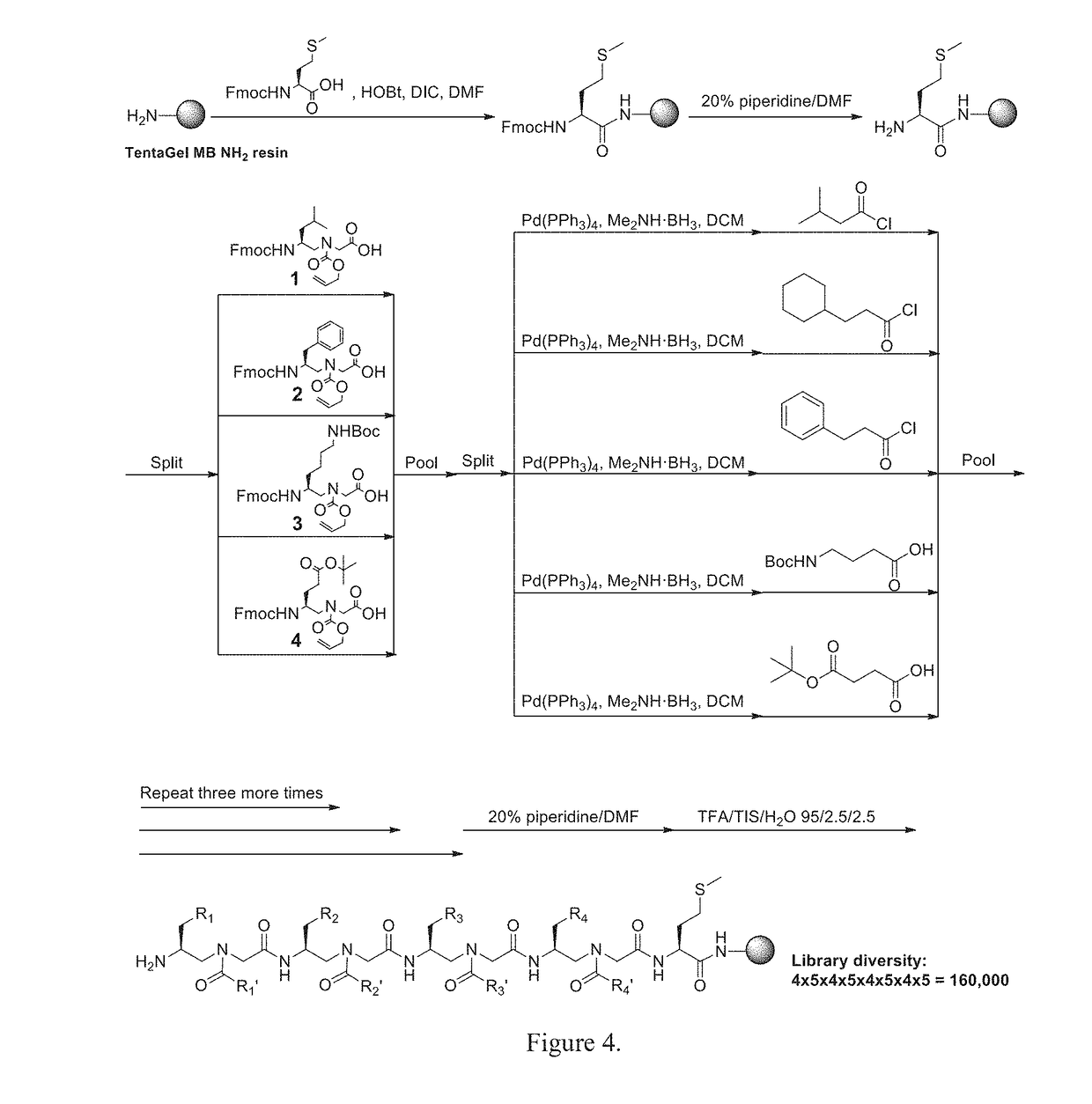
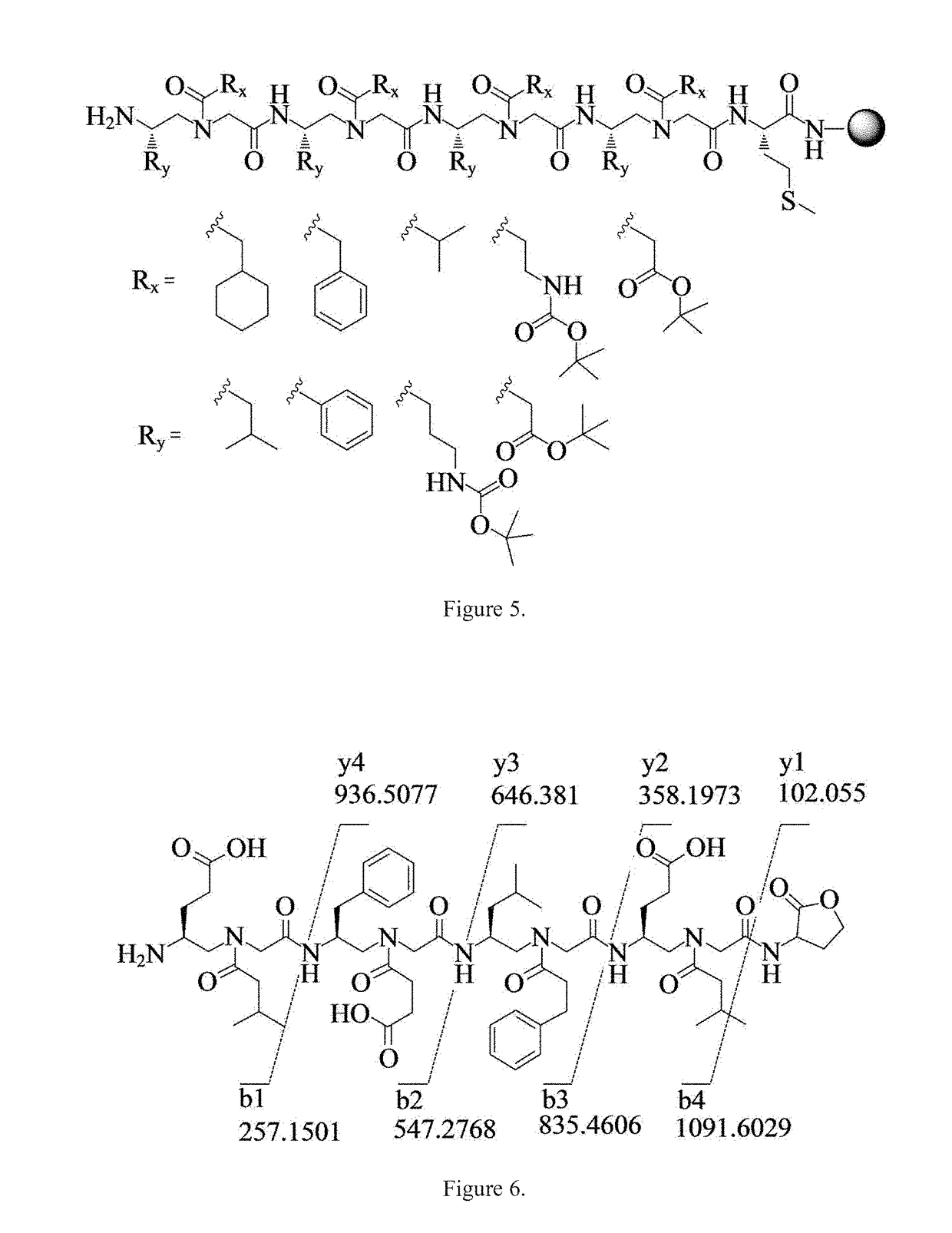
STAT3 hyperphosphorylation, dimerization and DNA binding are required for its ability to contribute to malignant transformation. As such, STAT3 has been recognized as a promising target for cancer therapy. Although a number of inhibitors of STAT3-STAT3 dimerization have been reported, molecular ligands that prevent interactions between STAT3 and DNA are very rare. The γ-AApeptide-based one-bead-one-compound (OBOC) combinatorial library was used, and identified γ-AApeptides that can selectively inhibit STAT3 / DNA interaction and suppress the expression levels of STAT3 target genes in intact cells. The results not only validate γ-AApeptides as novel inhibitors of STAT3 signaling pathway, but also demonstrate that in addition to the SH2 domain, the DNA binding domain of STAT3 is targetable for the development of new generation of anti-cancer therapeutics. This also validates the approach of OBOC combinatorial library for the identification of ligands targeting traditionally recognized “undruggable targets”.
Owner:UNIV OF SOUTH FLORIDA +1
SH2 domain variants
ActiveUS10274499B2Peptide/protein ingredientsPeptide preparation methodsAmino acid substitutionO-phospho-L-tyrosine
Owner:UNIV OF WESTERN ONTARIO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com