Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Preterm Births" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
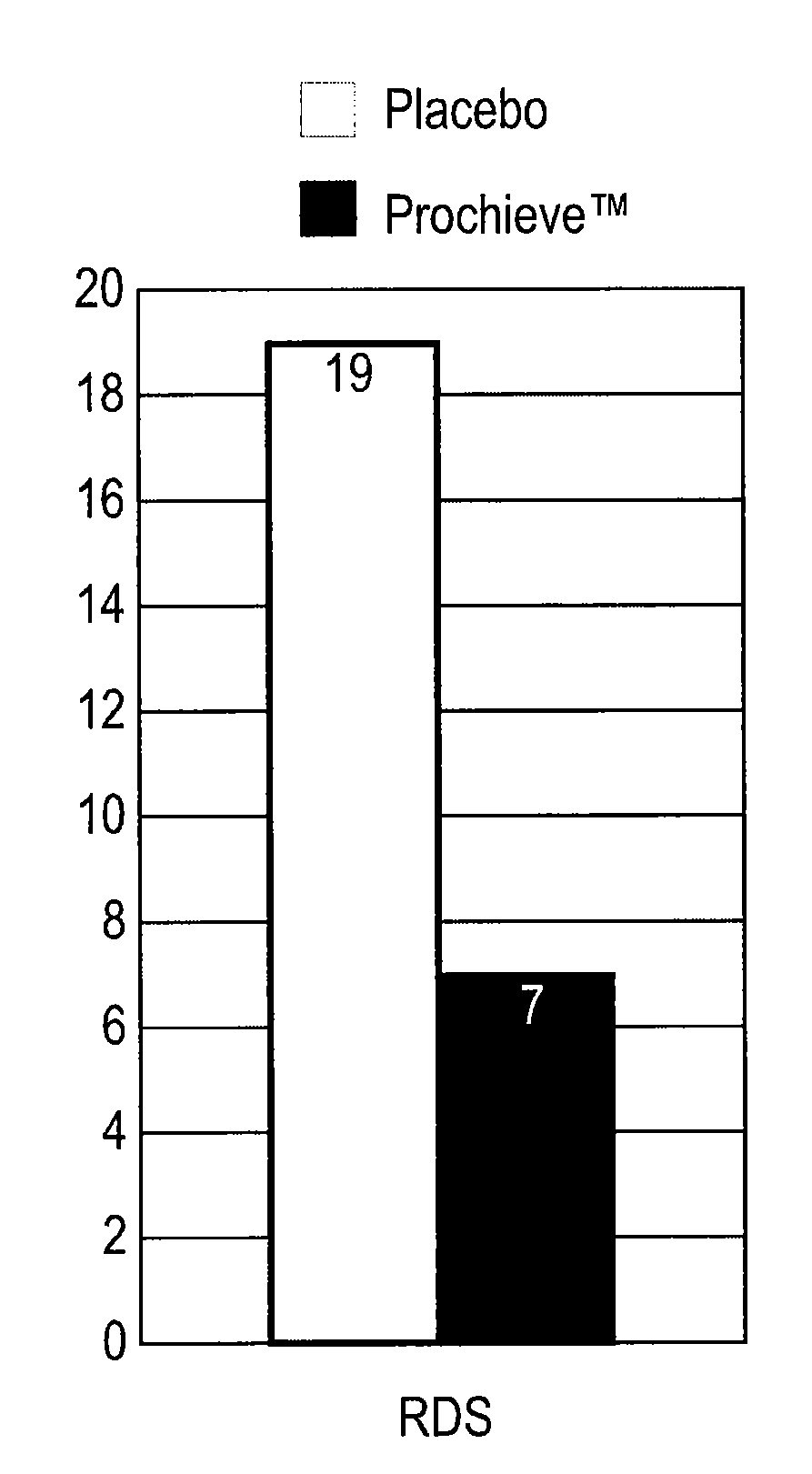
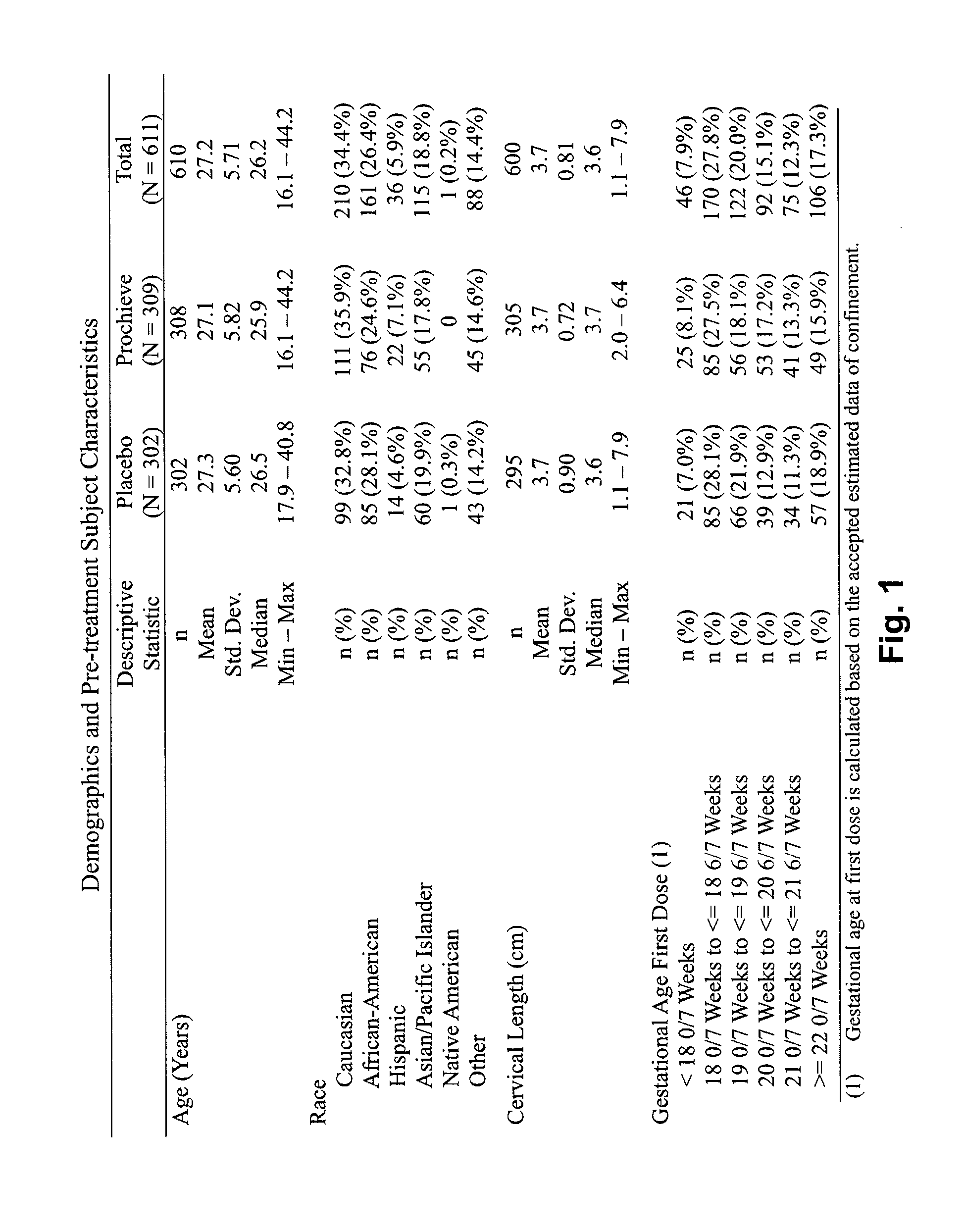
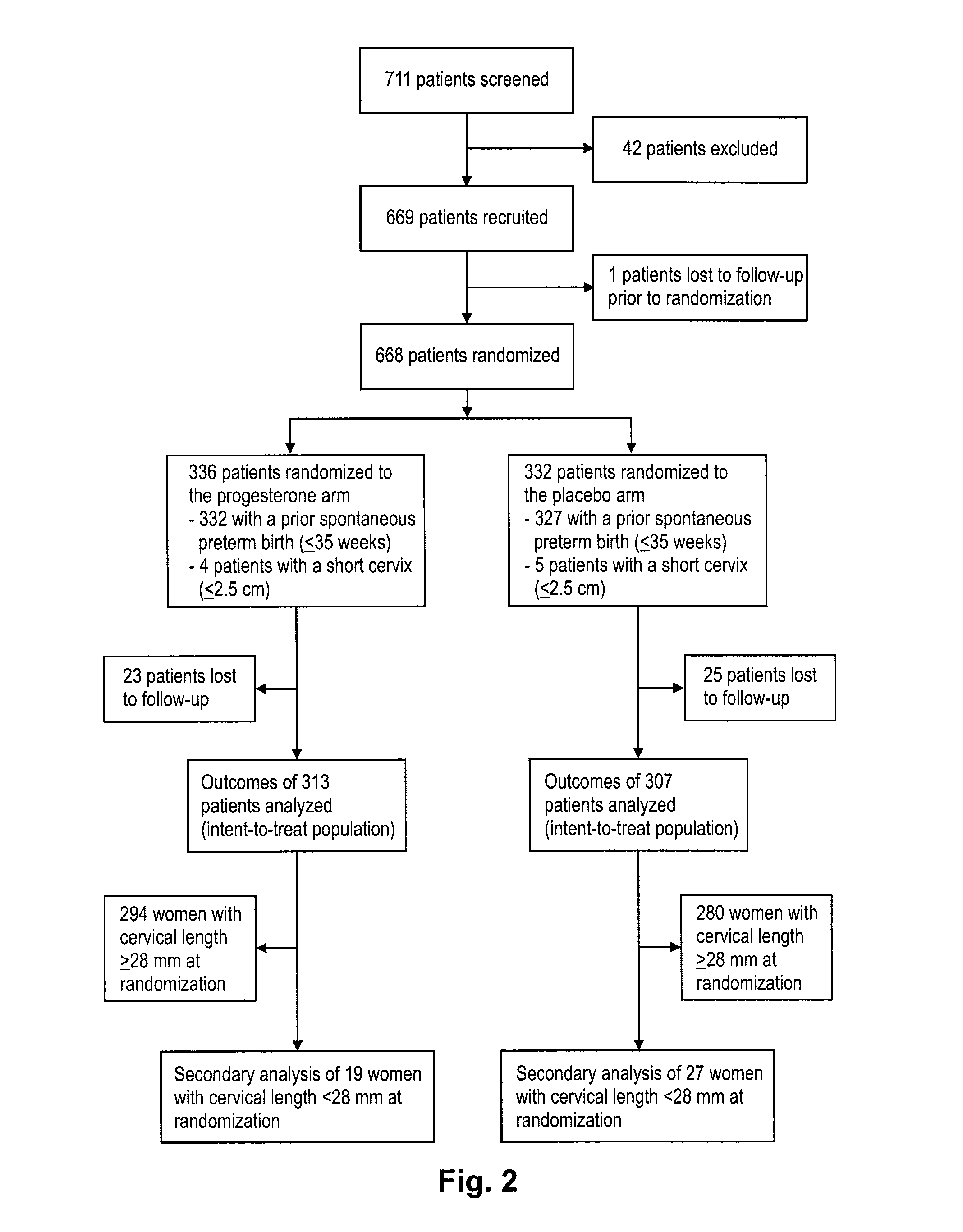
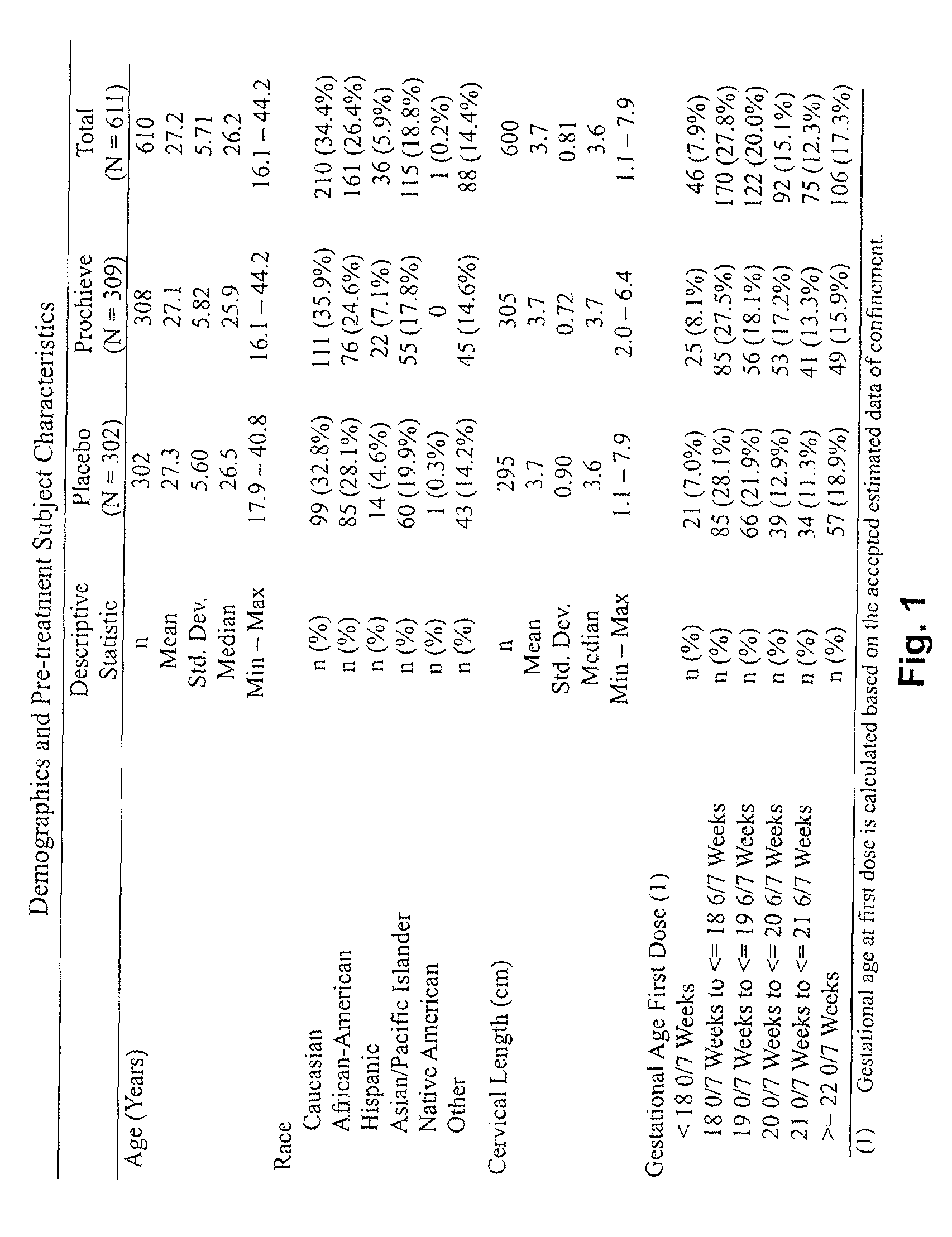
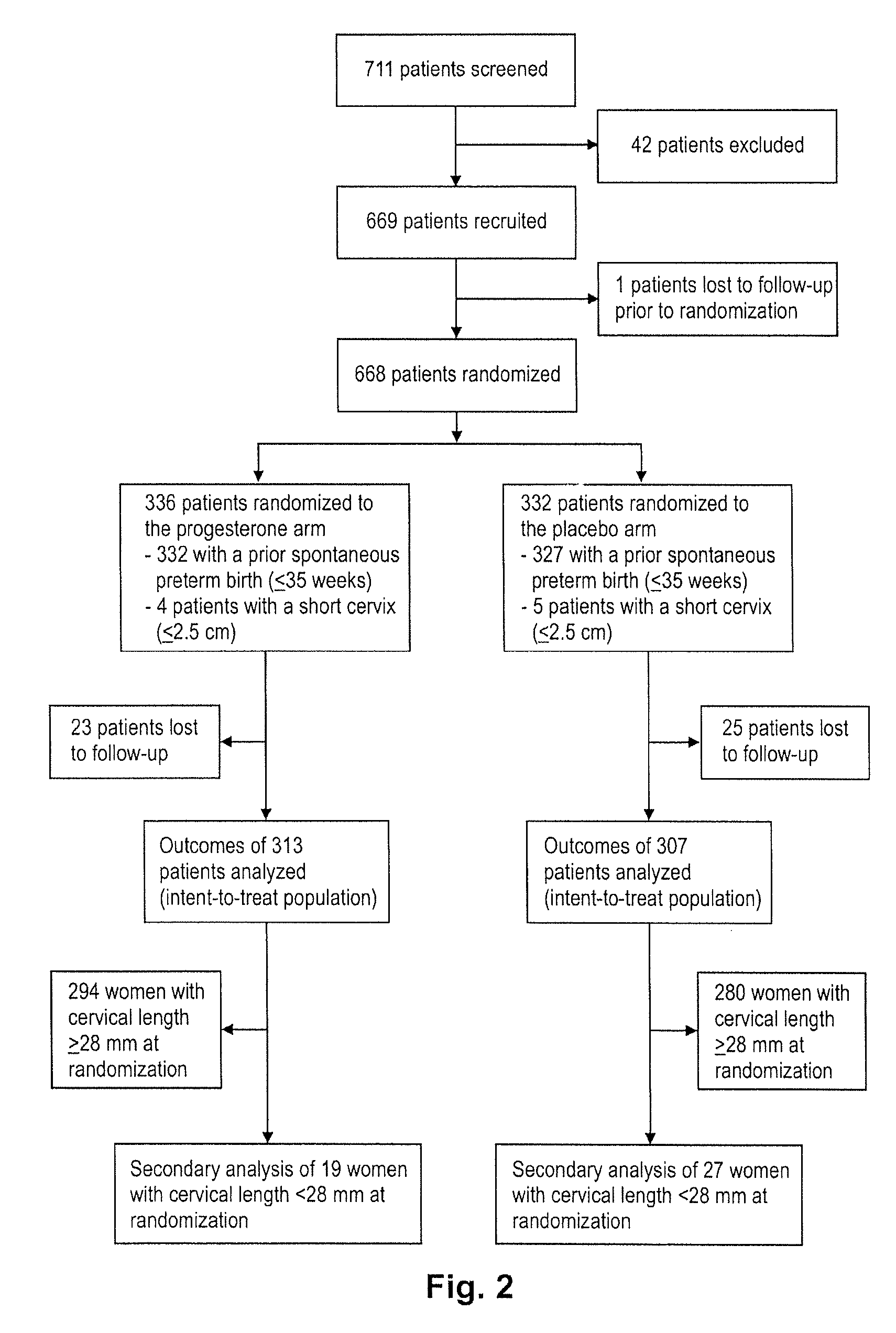
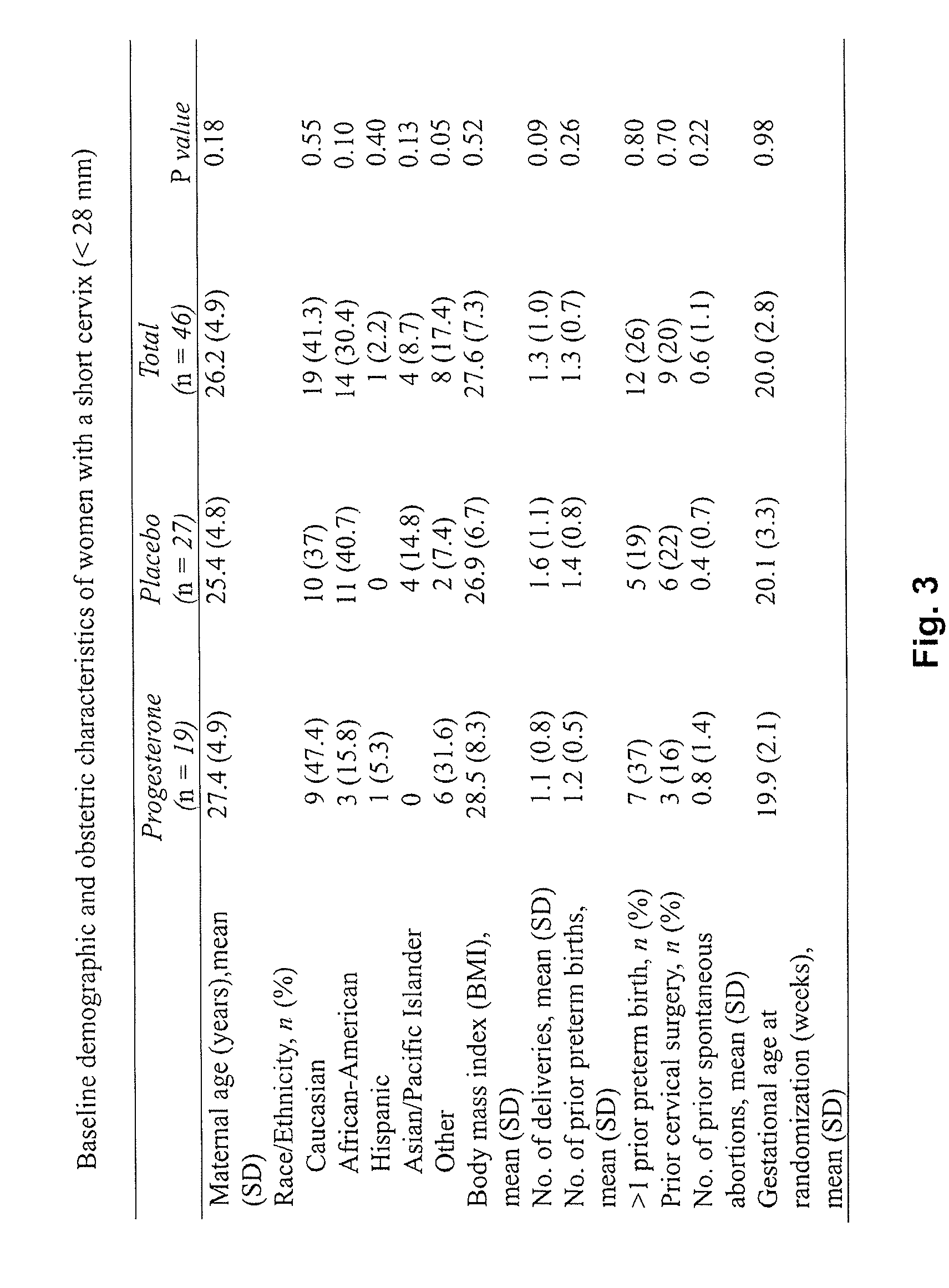
Progesterone for the treatment or prevention of spontaneous preterm birth
ActiveUS20080188829A1Minimize and delay shorteningMinimize and delay and effacingOrganic active ingredientsMedical devicesNeonatal morbidityPreterm Births
A method for treating or preventing spontaneous preterm birth in pregnant women and improving neonatal morbidity and mortality. The method includes administering to a pregnant woman in need thereof an effective amount of progesterone sufficient to prolong gestation by minimizing the shortening or effacing of her cervix. Treatment and prophylaxis with progesterone in pregnant women having symptoms of short cervix has been clinically shown to increase neonatal health.
Owner:ALLERGAN SALES LLC +3
Biomarkers of preterm birth
InactiveUS20140186332A1Reduce riskIncreased riskPeptide/protein ingredientsMicrobiological testing/measurementGynecologyObstetrics
The present disclosure relates to biomarkers of preterm birth, biomarkers of term birth, and methods of use thereof. In particular, the present disclosure provides methods of determining whether a pregnant woman is at an increased risk for premature delivery. The present disclosure further provides methods for decreasing a pregnant woman's risk for premature delivery.
Owner:NX PRENATAL INC
Methods for inhibiting preterm labor and uterine contractility disorders and preventing cervical ripening
InactiveUS20130023505A1Inhibit uterine contractilityInhibit cervical ripeningOrganic active ingredientsPharmaceutical delivery mechanismMyometrial contractilityGynecology
The invention relates to methods and pharmaceutical compositions for inhibiting or preventing preterm birth, inhibiting or delaying cervical ripening, inhibiting myometrial contractility and treating or inhibiting uterine contractility disorders. The methods comprise administering an effective amount of a composition comprising steroid hormones such as soluble progesterone.
Owner:DIGNITY HEALTH
Progesterone for the treatment or prevention of spontaneous preterm birth
InactiveUS20090264395A1Reduce morbidityReduce mortalityOrganic active ingredientsOrganic chemistryNeonatal morbidityPreterm Births
A method for treating or preventing spontaneous preterm birth in pregnant women and improving neonatal morbidity and mortality. The method includes administering to a pregnant woman in need thereof an effective amount of progesterone sufficient to prolong gestation by minimizing the shortening or effacing of her cervix. Treatment and prophylaxis with progesterone in pregnant women having symptoms of short cervix has been clinically shown to increase neonatal health.
Owner:WATSON LAB INC +1
Method and apparatus for reducing preterm labor using neuromodulation
The present invention includes an apparatus, kit and method for providing neural stimulation to reduce preterm labor contractions and thereby reduce subsequent preterm births. The present invention includes one or more implantable electrodes adapted for electrical communication with one or more sacral nerve roots and an electrical energy generator to produce one or more electrical signals in electrical communication with the one or more implantable electrodes.
Owner:BAYLOR RES INST
Compositions and methods for diagnosing and preventing spontaneous preterm birth
InactiveUS20120046261A1Abundant resourcesIncrease expensesOrganic active ingredientsNucleotide librariesPreterm BirthsObstetrics
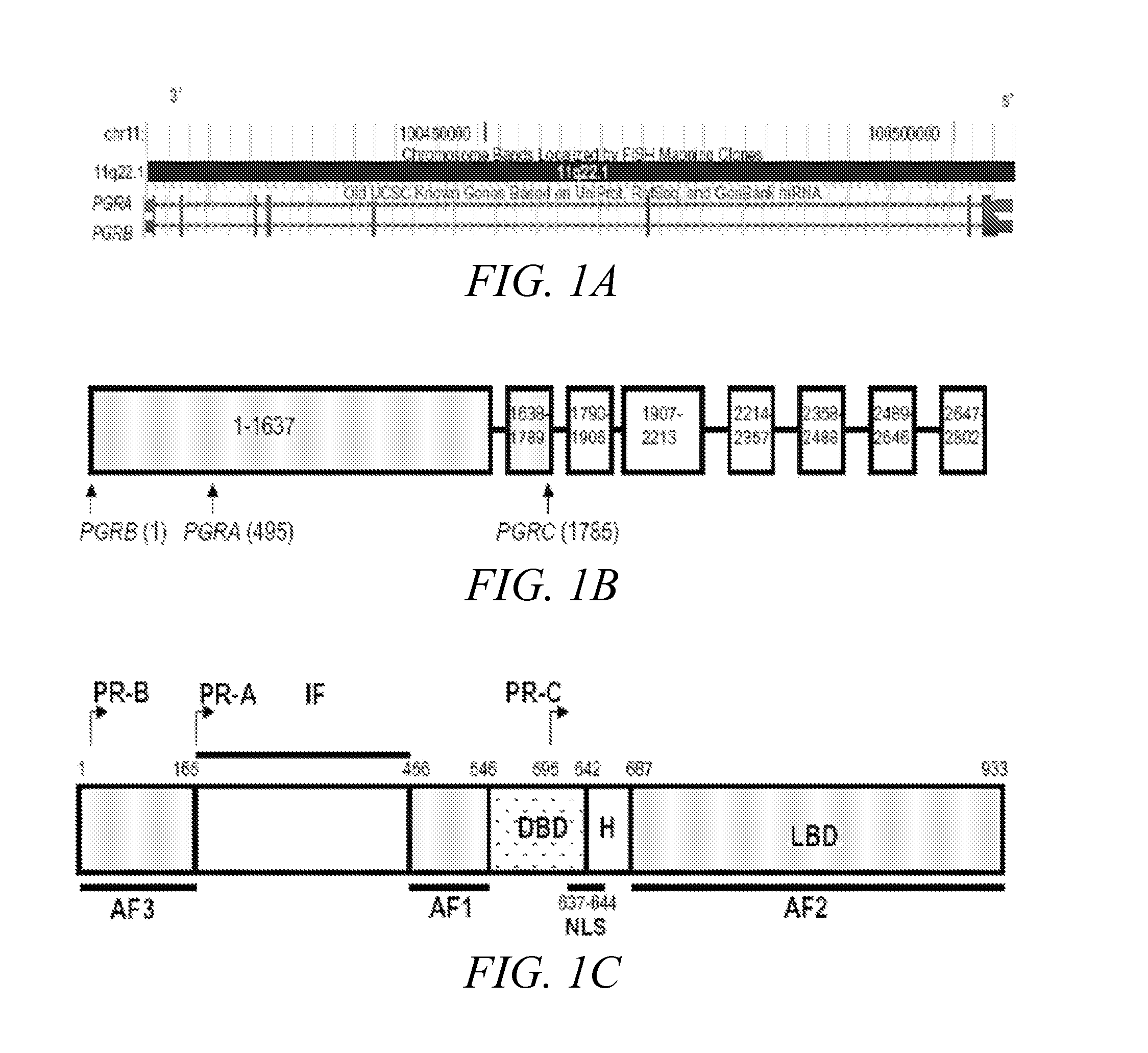
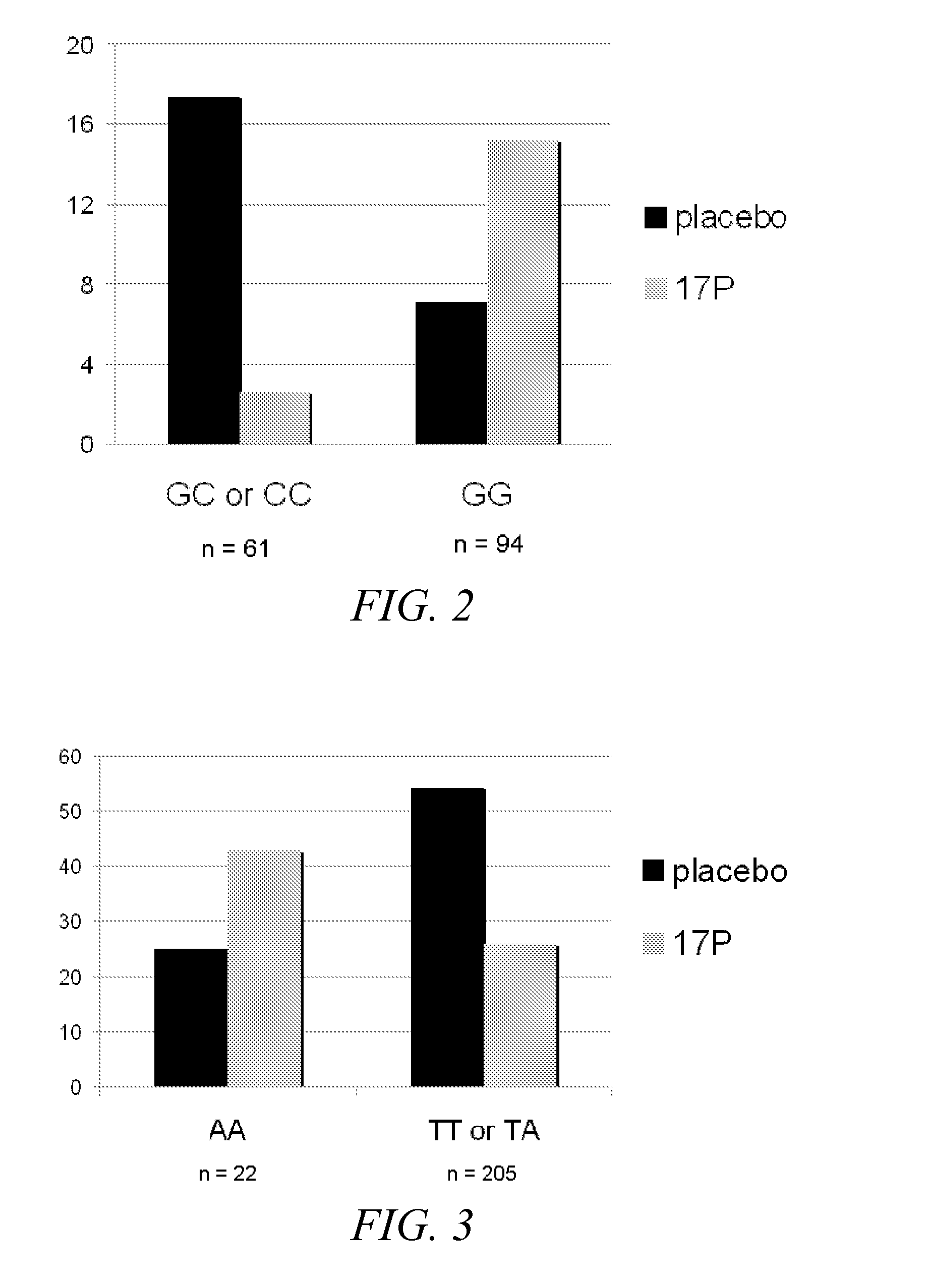
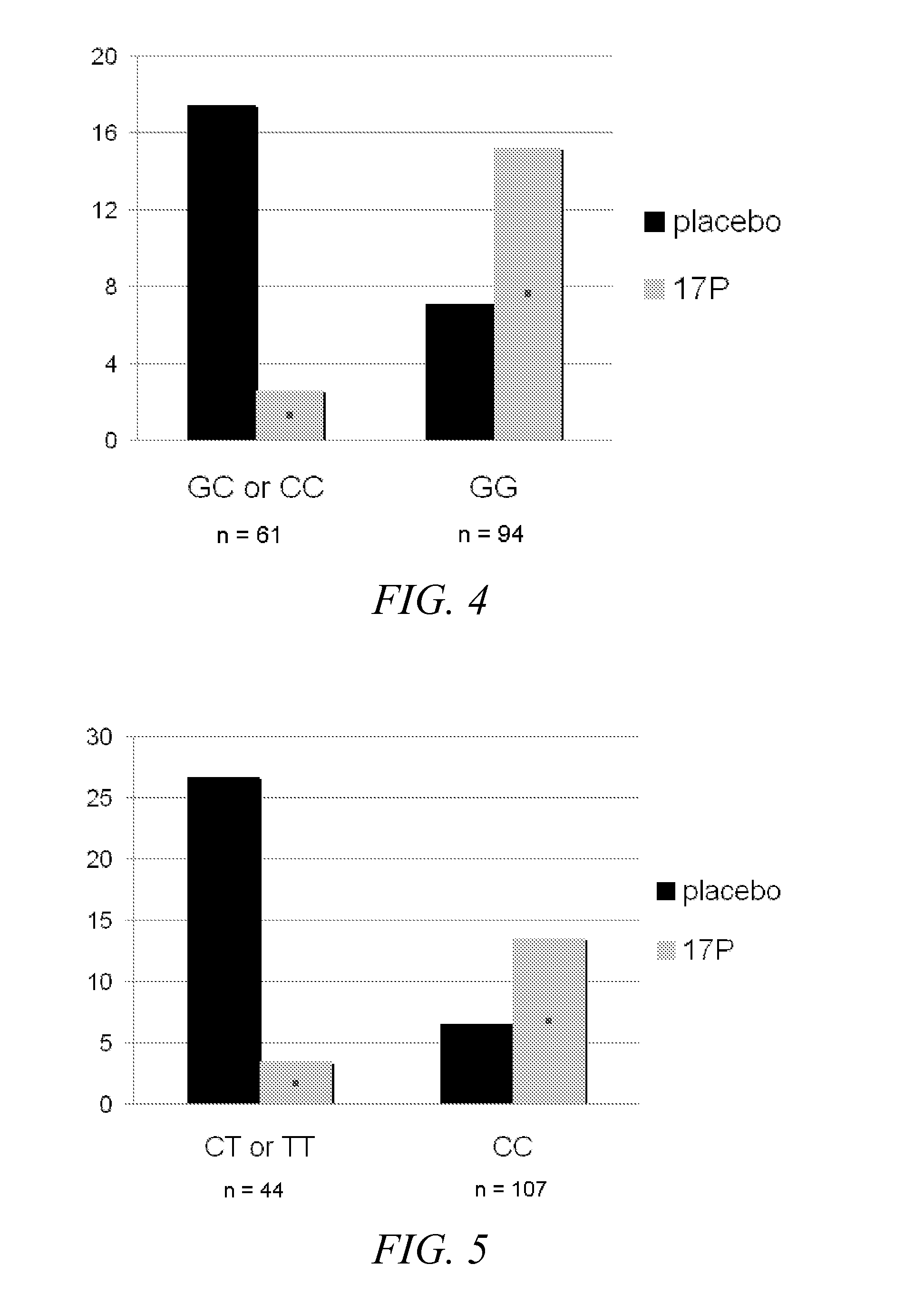
Disclosed herein are compositions and methods of identifying a subject at risk for preterm birth and selecting effective therapies for preventing preterm birth in the subject. The disclosed methods generally involve determining the identity of one or more nucleotides in the progesterone receptor (PR) gene of the subject.
Owner:UNIV OF UTAH RES FOUND
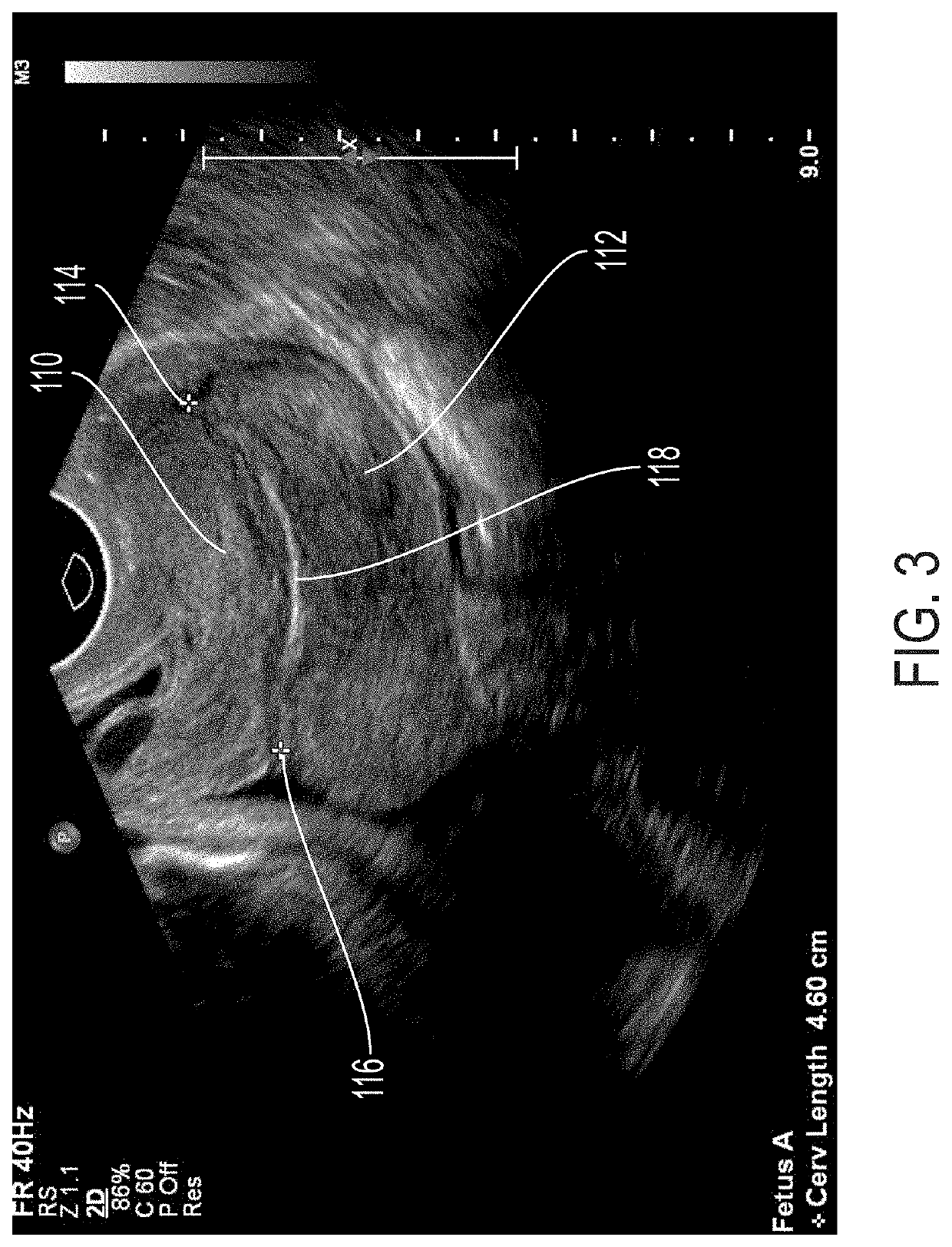
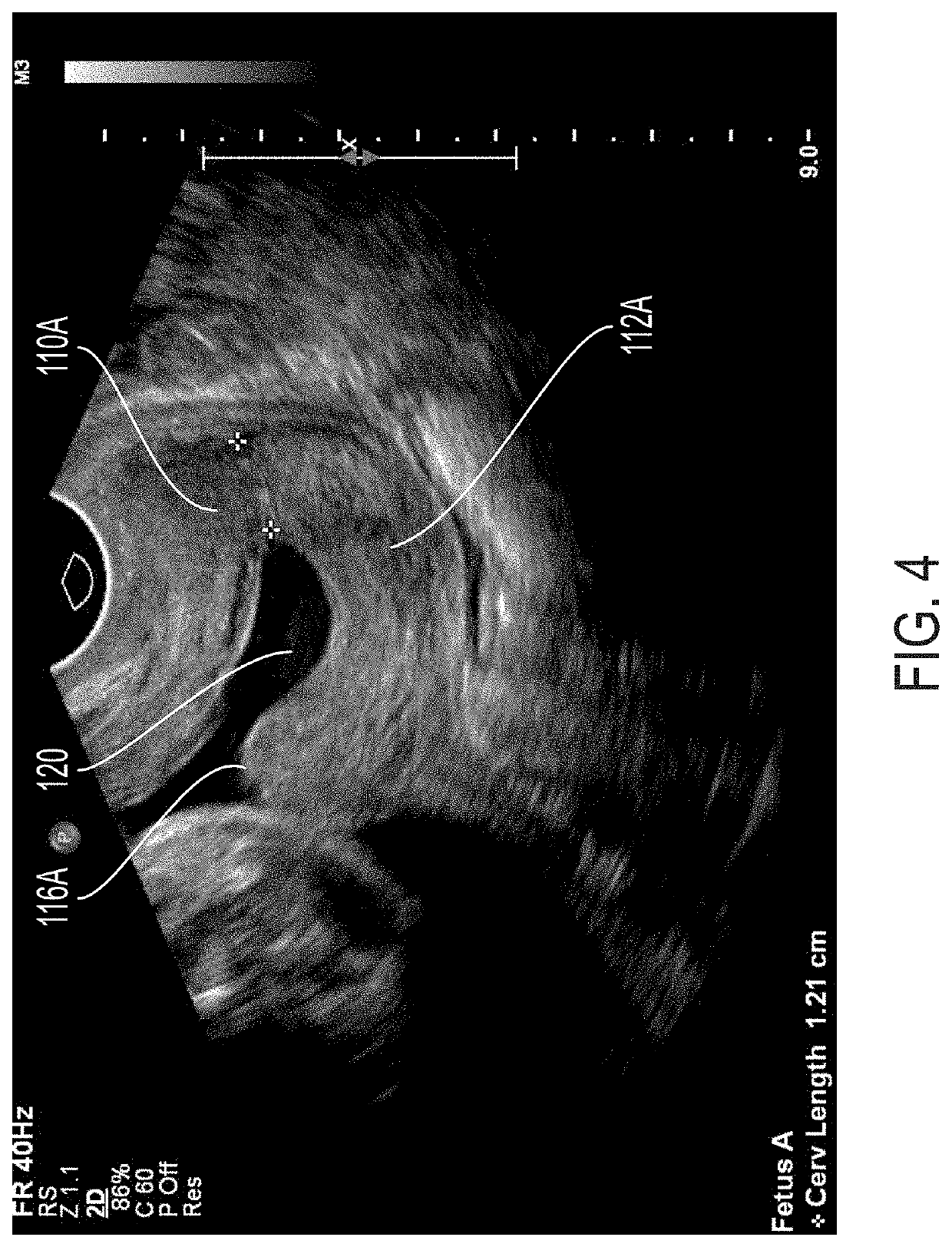
Optical imaging for preterm birth assessment
ActiveUS20180271430A1Quick snapEffective toolDiagnostic recording/measuringSensorsPreterm BirthsCervical tissue
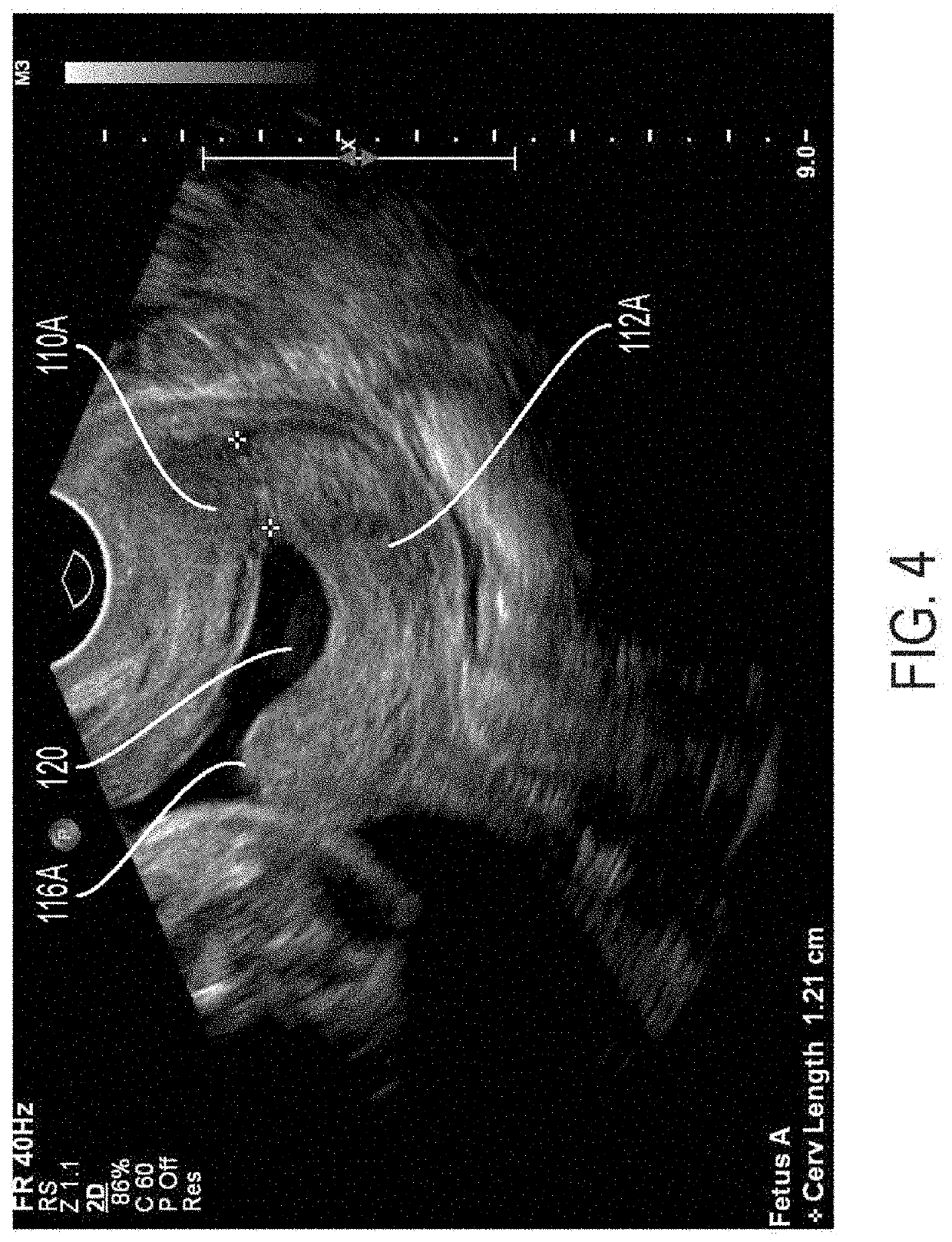
Methods, apparatuses, and systems for measuring collagen organization in the cervix, assessing the health of a woman's cervix (including a pregnant woman's cervix), characterizing the composition and structure of cervical tissue, and measuring preterm labor risk are provided. Polarization sensitive techniques and properties of cervical tissue, including birefringence, can be used. A method can include acquiring in vivo images of cervical tissue, applying Mueller matrix (MM) polarimetry (including 4×4 Mueller matrix polarimetry), and determining one or more parameters of the cervical tissue using the Mueller matrix (MM) polarimetry. The in vivo images can be analyzed and various parameters that characterize the cervical tissue can be determined. Graphs and maps of the cervical tissue can be generated for use as care provider tools.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Metabolomic markers for preterm birth
InactiveUS20140221236A1Increased riskReduce concentrationPeptide librariesLibrary screeningObstetricsPreterm Births
Panels, kits, and methods for diagnosing or predicting the likelihood of occurrence of preterm birth (PTB) in a subject are disclosed. An exemplary panel includes at least one of a first marker from a first group of markers and a second marker from a second group of markers. A significant decrease in the first marker and a significant increase in the second marker measured in a sample taken from a subject during the second trimester of pregnancy are diagnostic or predictive of an increased risk of preterm birth for the subject.
Owner:UNIV OF IOWA RES FOUND
Biomarker pairs for predicting preterm birth
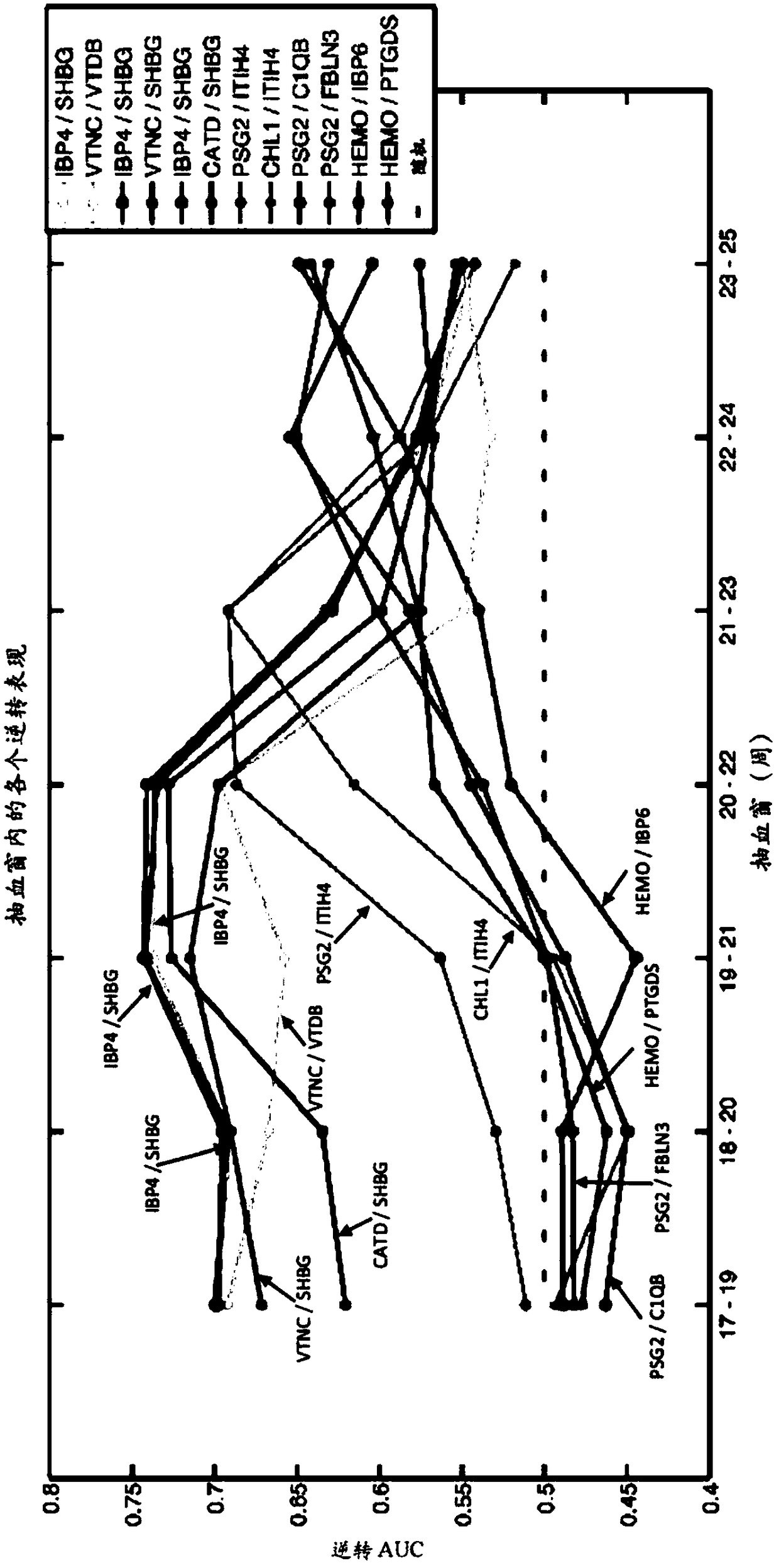
The disclosure provides a pair of isolated biomarkers selected from the group consisting of IBP4 / SHBG, IBP4 / PSG3, IBP4 / LYAM1, IBP4 / IGF2, CLUS / IBP3, CLUS / IGF2, CLUS / LYAM1, INHBC / PSG3, INHBC / IGF2, PSG2 / LYAM1, PSG2 / IGF2, PSG2 / LYAM1, PEDF / PSG3, PEDF / SHBG, PEDF / LYAMl, CD14 / LYAM1, and APOC3 / LYAM1, wherein the pair of biomarkers exhibits a change in reversal value between pregnant females at risk for pre-term birth and term controls. Also provided is a method of determining probability for preterm birth in a pregnant female, the method comprising measuring in a biological sample obtained from the pregnant female a reversal value for at least one pair of biomarkers selected from the group consisting of IBP4 / SFIBG, IBP4 / PSG3, IBP4 / LYAM1, IBP4 / IGF2, CLUS / IBP3, CLUS / IGF2, CLUS / LYAM1, INHBC / PSG3, INHBC / IGF2, PSG2 / LYAM1, PSG2 / IGF2, PSG2 / LYAM1, PEDF / PSG3, PEDF / SHBG, PEDF / LYAMl, CD14 / LYAM1, and APOC3 / LYAM1 to determine the probability for preterm birth in the pregnant female.
Owner:SERA PROGNOSTICS
Biomarkers and methods for predicting preterm birth
The disclosure provides biomarker panels, methods and kits for determining the probability for preterm birth in a pregnant female. The present disclosure is based, in part, on the discovery that certain proteins and peptides in biological samples obtained from a pregnant female are differentially expressed in pregnant females that have an increased risk of developing in the future or presently suffering from preterm birth relative to matched controls. The present disclosure is further based, in part, on the unexpected discovery that panels combining one or more of these proteins and peptides can be utilized in methods of determining the probability for preterm birth in a pregnant female with relatively high sensitivity and specificity. These proteins and peptides disclosed herein serve as biomarkers for classifying test samples, predicting a probability of preterm birth, monitoring of progress of preterm birth in a pregnant female, either individually or in a panel of biomarkers.
Owner:SERA PROGNOSTICS
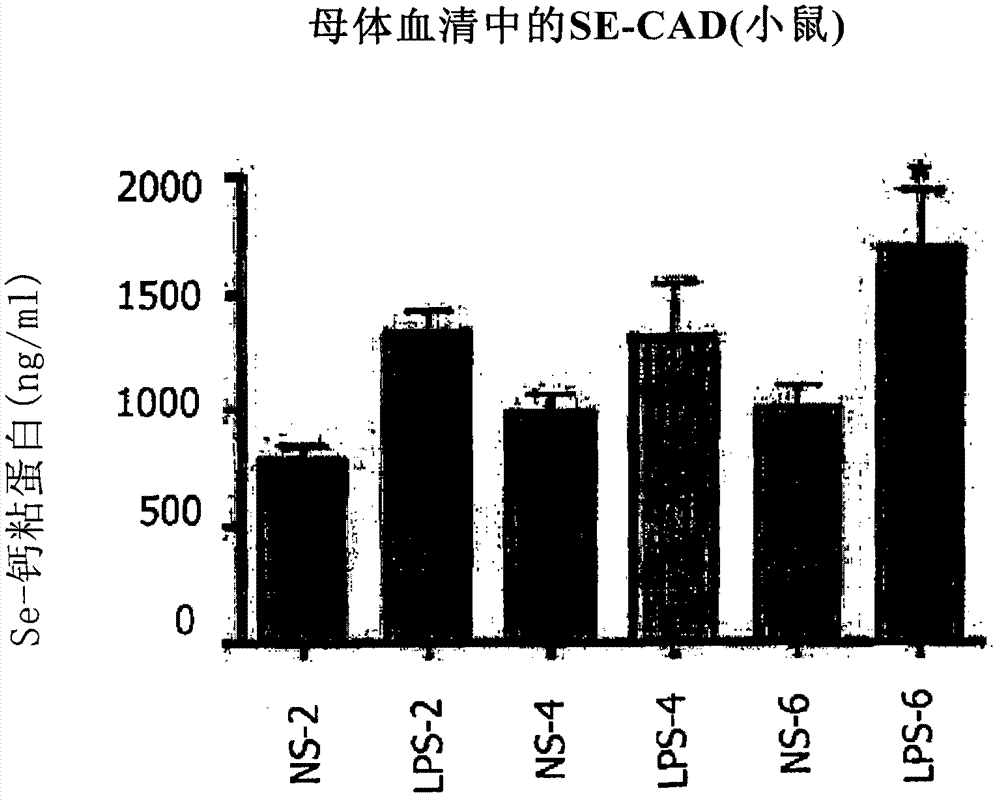
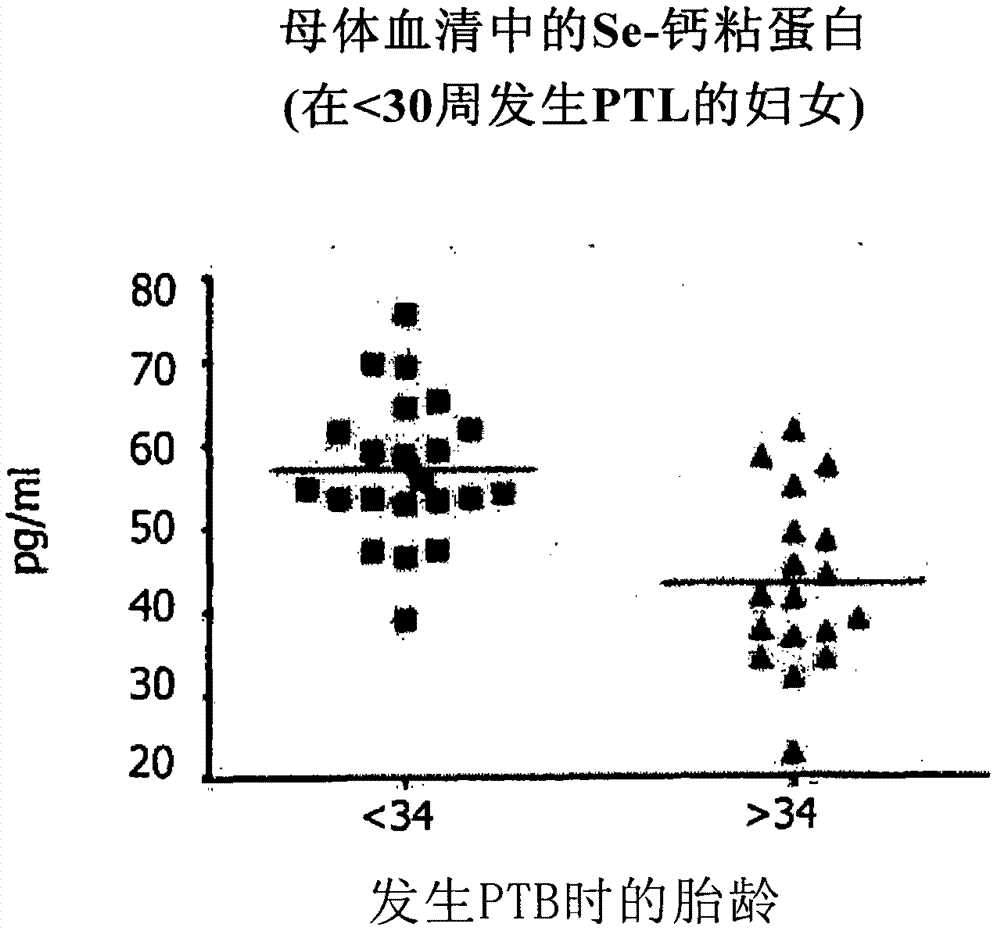
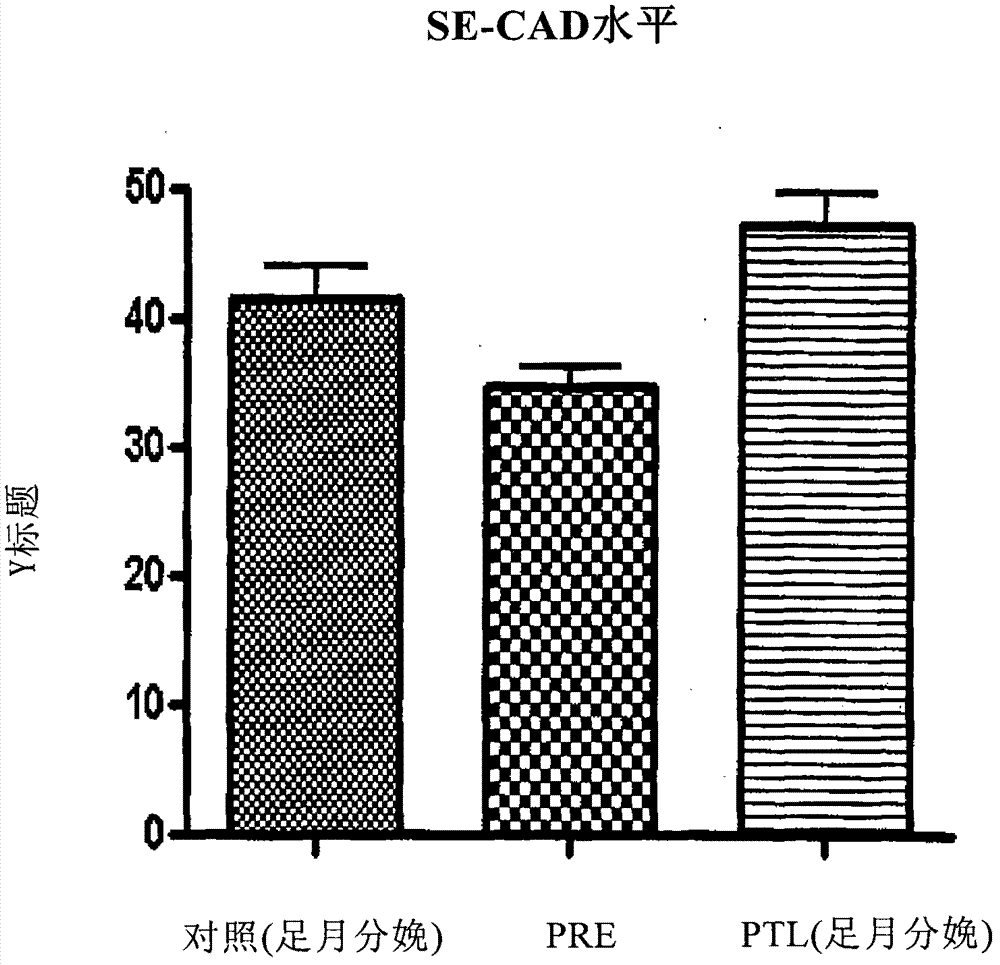
Method of predicting risk of preterm birth
A method for diagnosing, or differentially diagnosing, an increased risk of pre-term birth (PTB) involves detecting or measuring increased expression of a biomarker Soluble E-cadherin (SE-CAD) in a biological sample from a mammalian subject, particularly in the urine, cervicovaginal fluid or blood. An increased level of expression of SE-CAD above the level of expression in the same sample of a healthy mammalian subject is an indication of a diagnosis of increased risk of PTB. Such diagnosis may further involve identify other clinical symptoms of PTB or PTL. Additionally the method may use additional biomarkers, such as fetal fibronectin.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
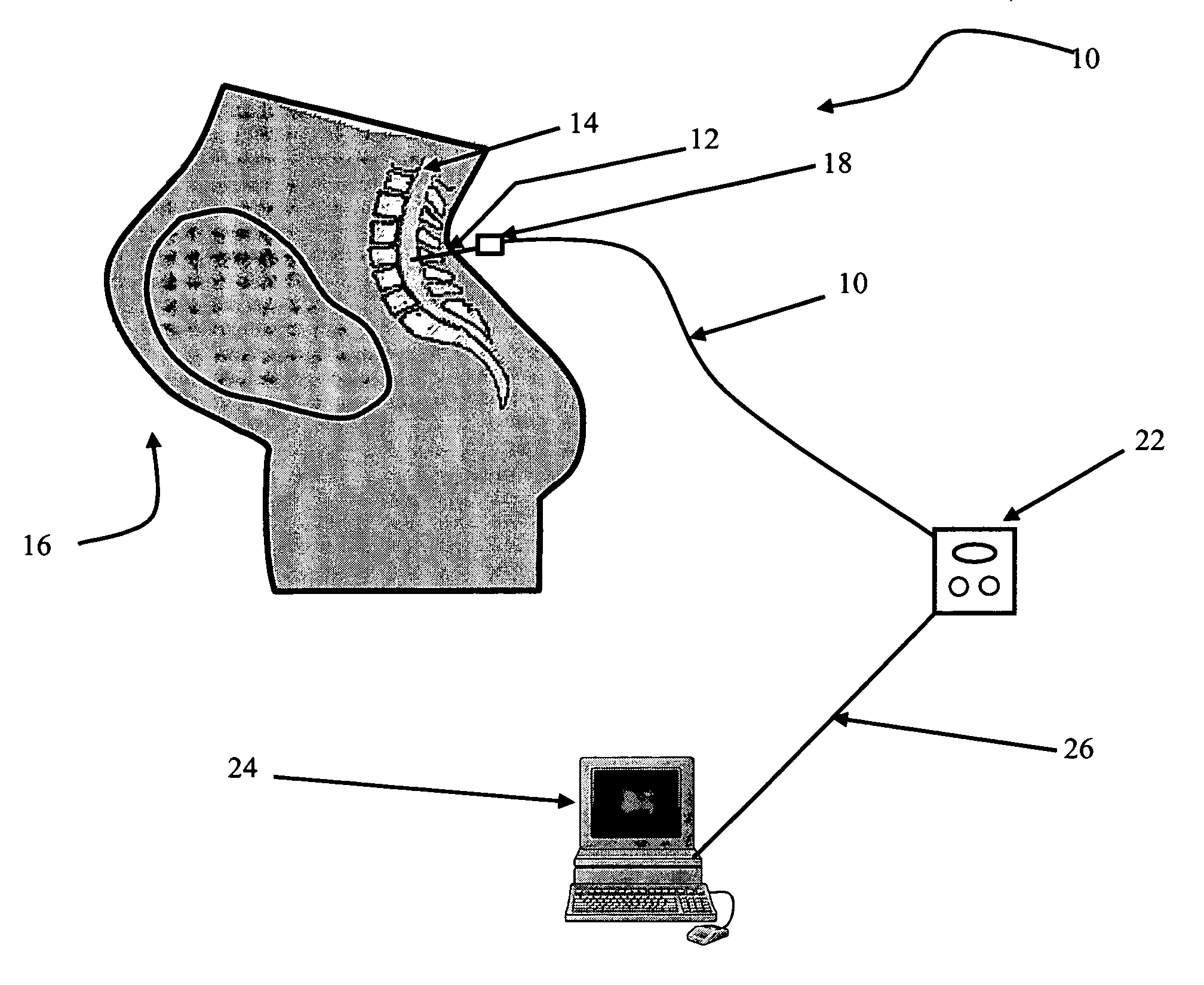
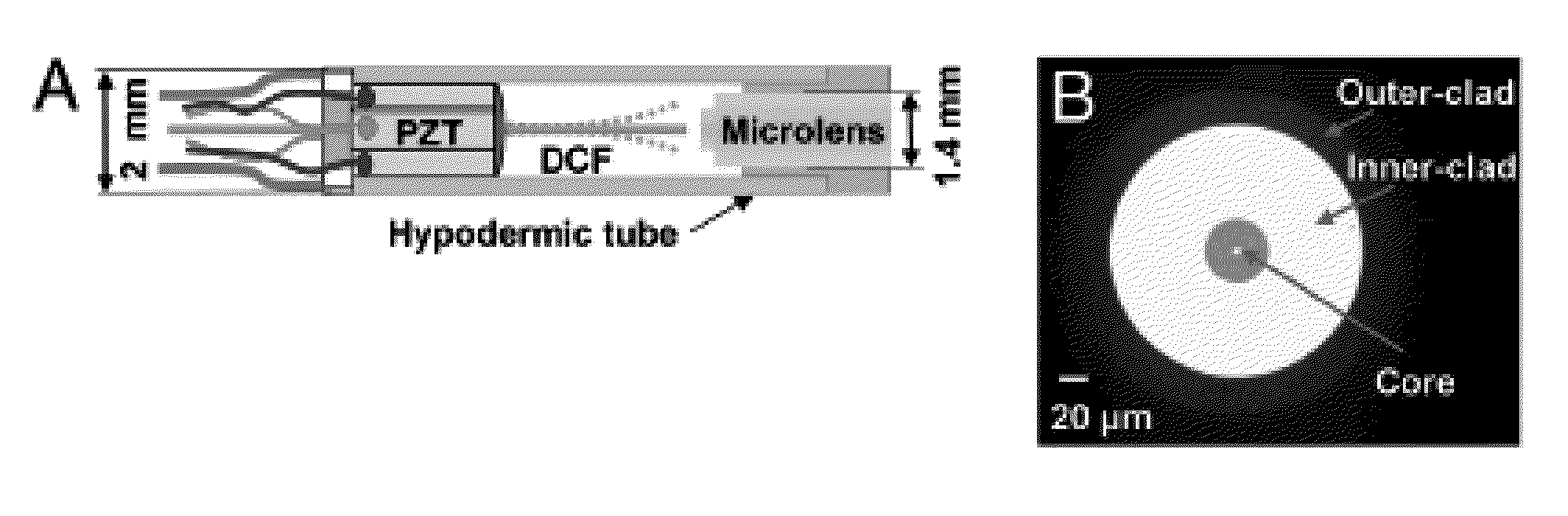
Method and system for assessing preterm birth and other pathologies
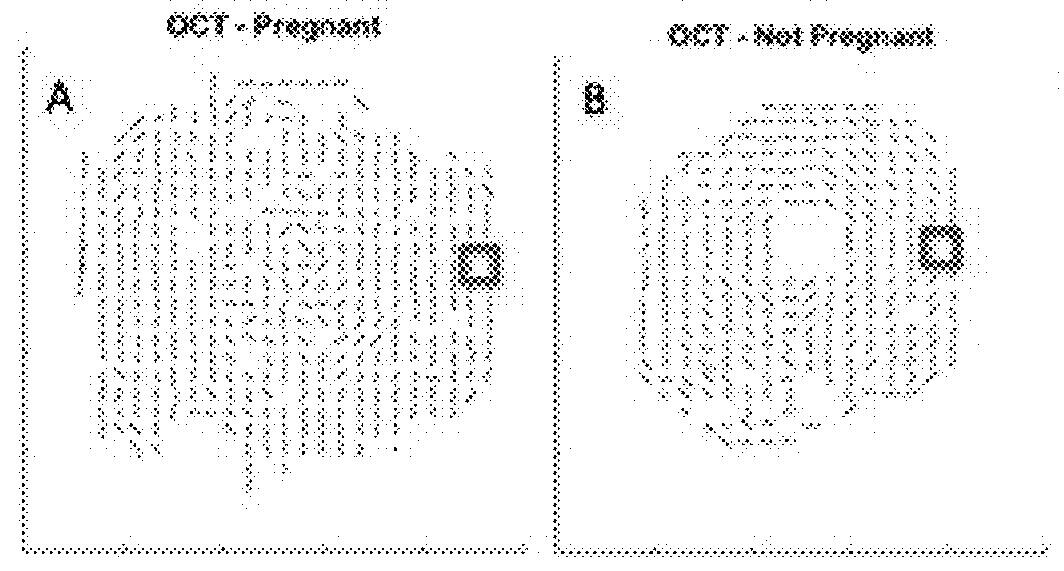
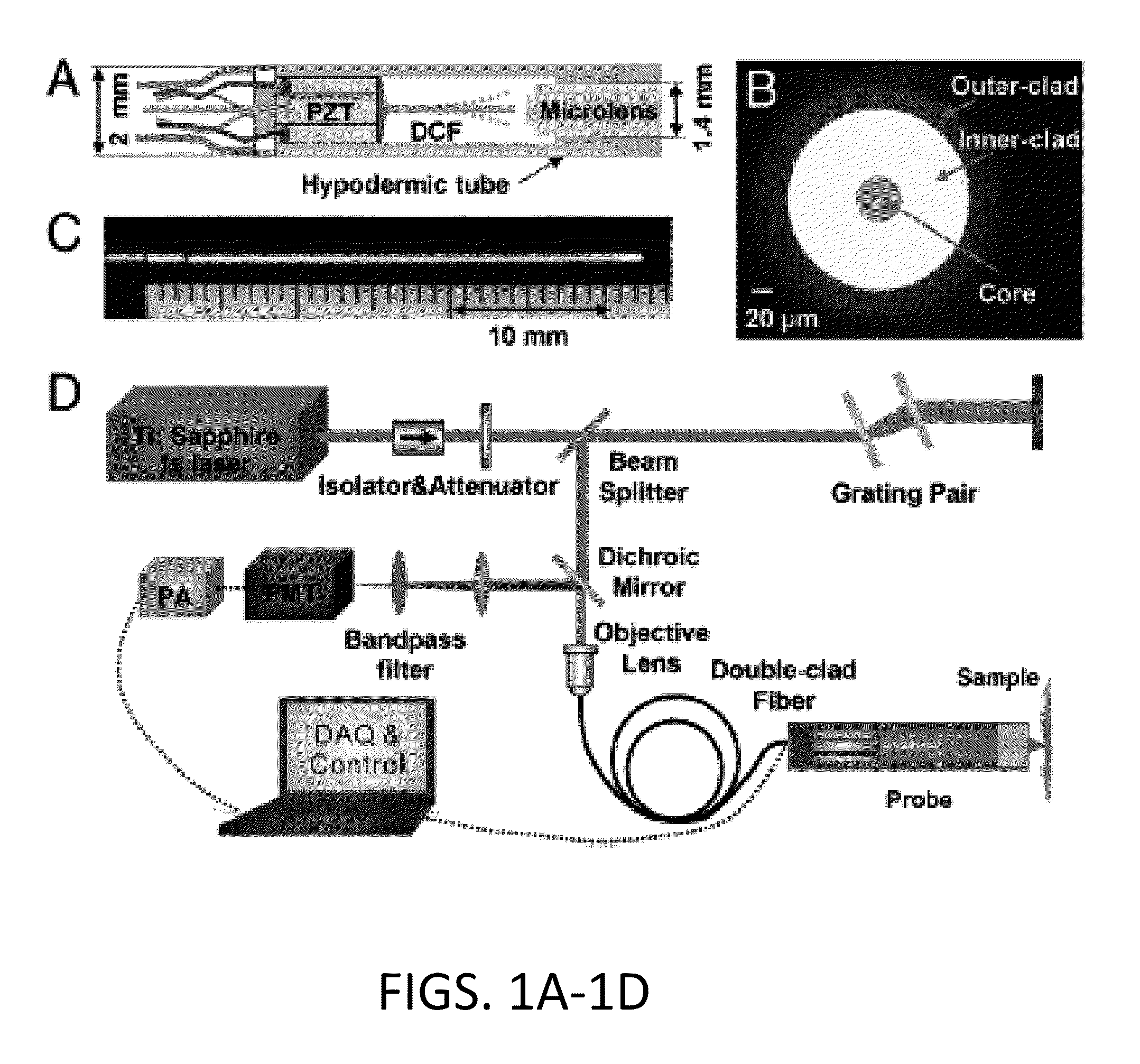
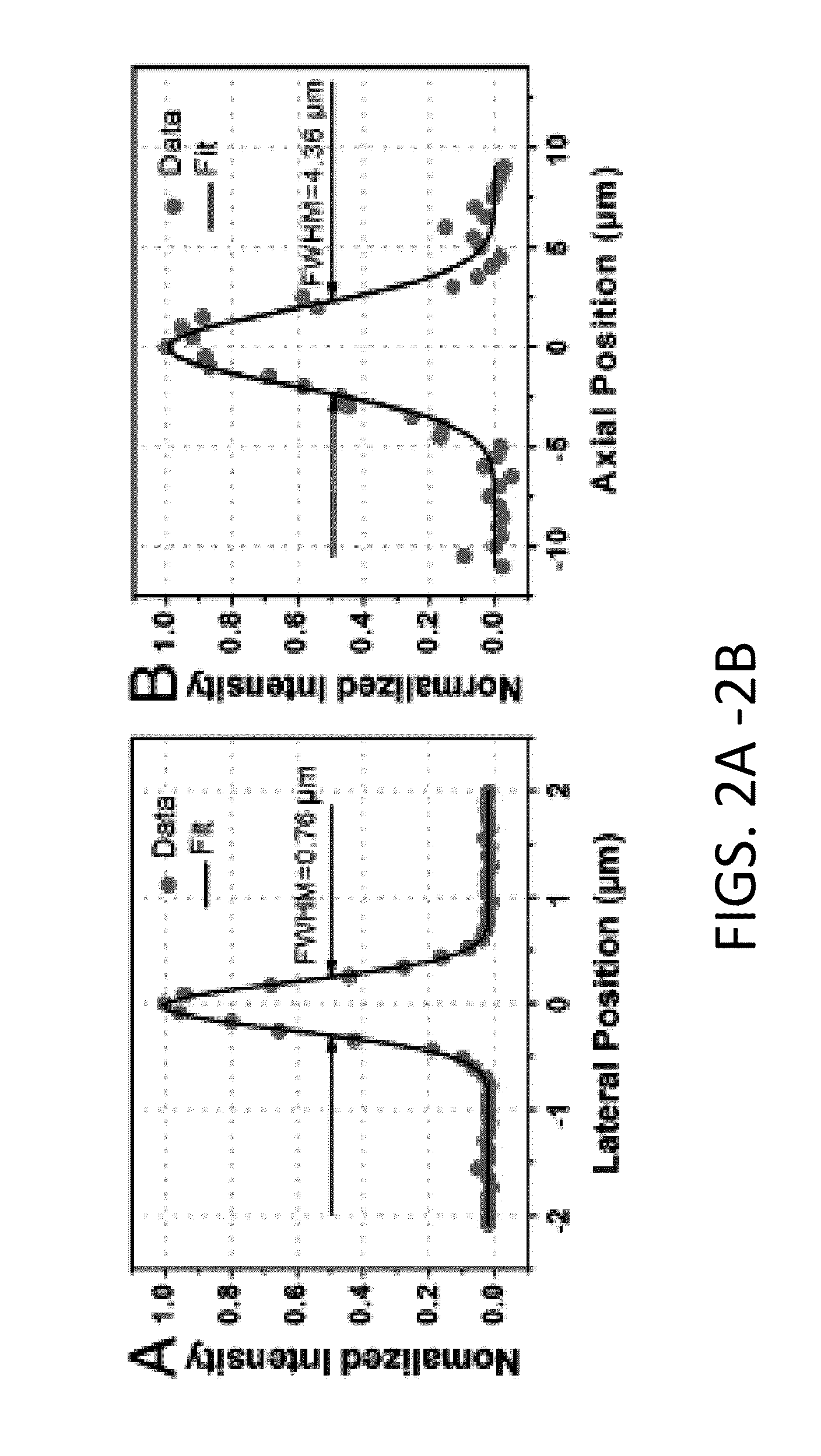
The present invention is directed to an all-fiber-optic scanning endomicroscope capable of high-resolution second harmonic generation (SHG) imaging of biological tissues. The endomicroscope has an overall 2.0 mm diameter and consists of a single customized double-clad fiber, a compact rapid two-dimensional beam scanner, and a miniature compound objective lens for excitation beam delivery, scanning, focusing, and efficient SHG signal collection. Endomicroscopic SHG images of murine cervical tissue sections at different stages of normal pregnancy reveal progressive, quantifiable changes in cervical collagen morphology with resolution similar to that of bench-top SHG microscopy. A device according to an embodiment of the present invention can also be used to assess other pathologies, such as cancer. fibrosis, and inflammation. The present invention allows for diagnosis, monitoring of the effect of therapeutics, and surgical or interventional guidance. The present invention enables visualization of histology in-vivo, in the patient, and is label-free.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Systems and methods of using machine learning analysis to stratify risk of spontaneous preterm birth
PendingUS20210057039A1Subject is at riskReduce riskOrganic active ingredientsComponent separationPattern recognitionPreterm Births
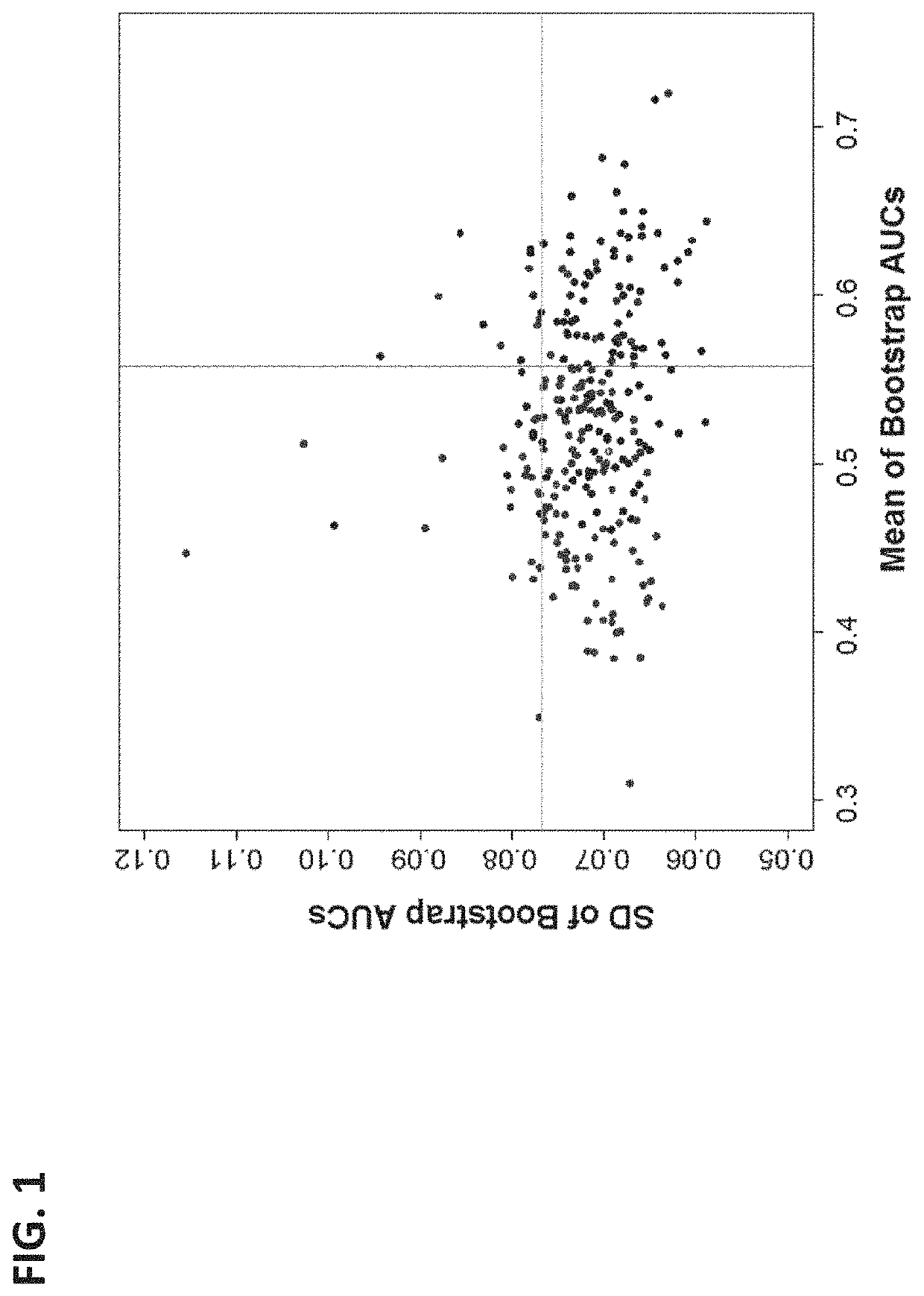
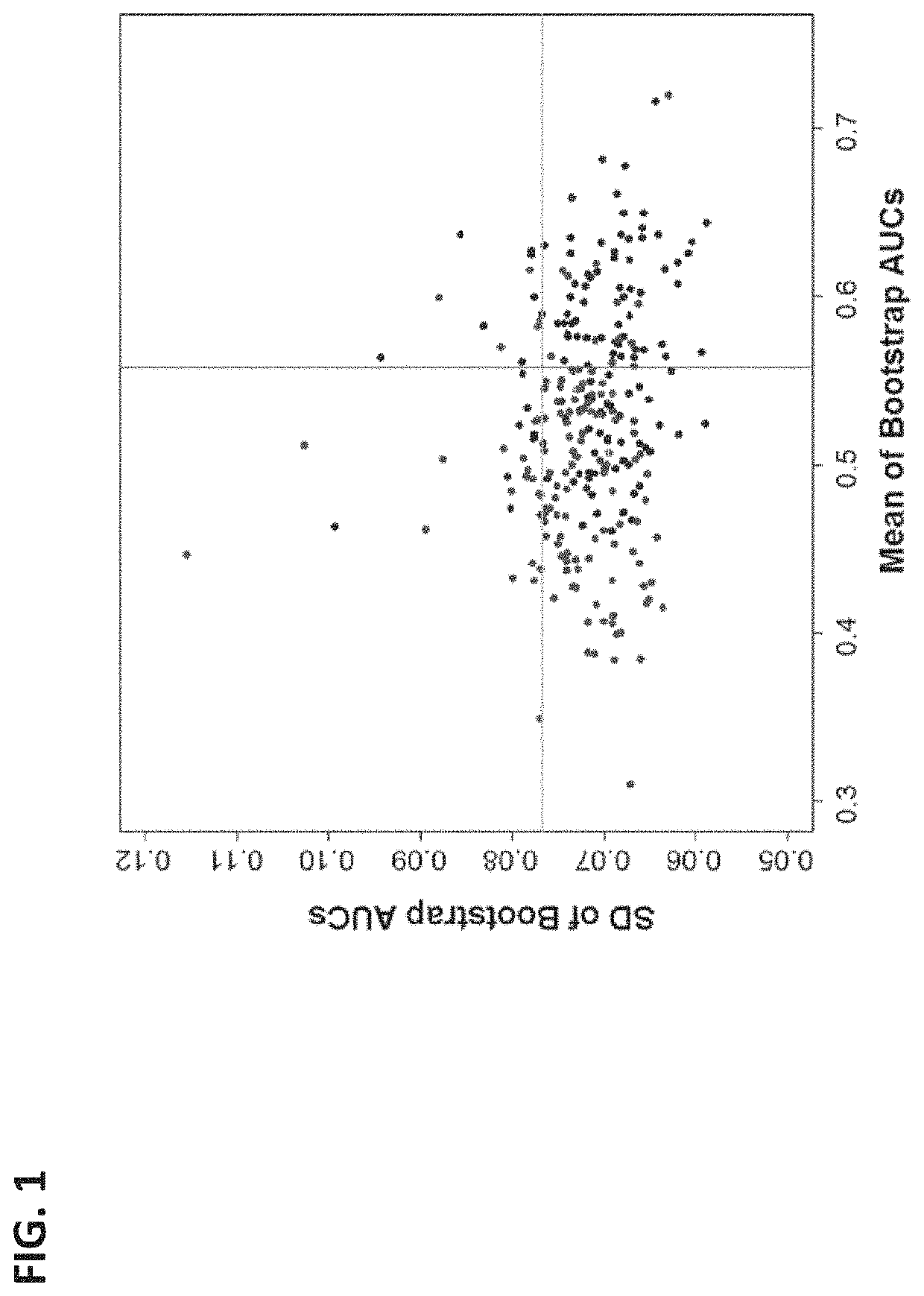
The present disclosure relates to systems and methods of using machine learning analysis to stratify the risk of spontaneous preterm birth (SPTB). In some variations, to select informative markers that differentiate SPTB from term deliveries, a processed quantification data of the markers can be subjected to univariate receiver operating characteristic (ROC) curve analysis. A Differential Dependency Network (DDN) can then applied in order to extract co-expression patterns among the markers. In order to assess the complementary values among selected markers and the range of their relevant performance, multivariate linear models can be derived and evaluated using bootstrap resampling.
Owner:NX PRENATAL INC
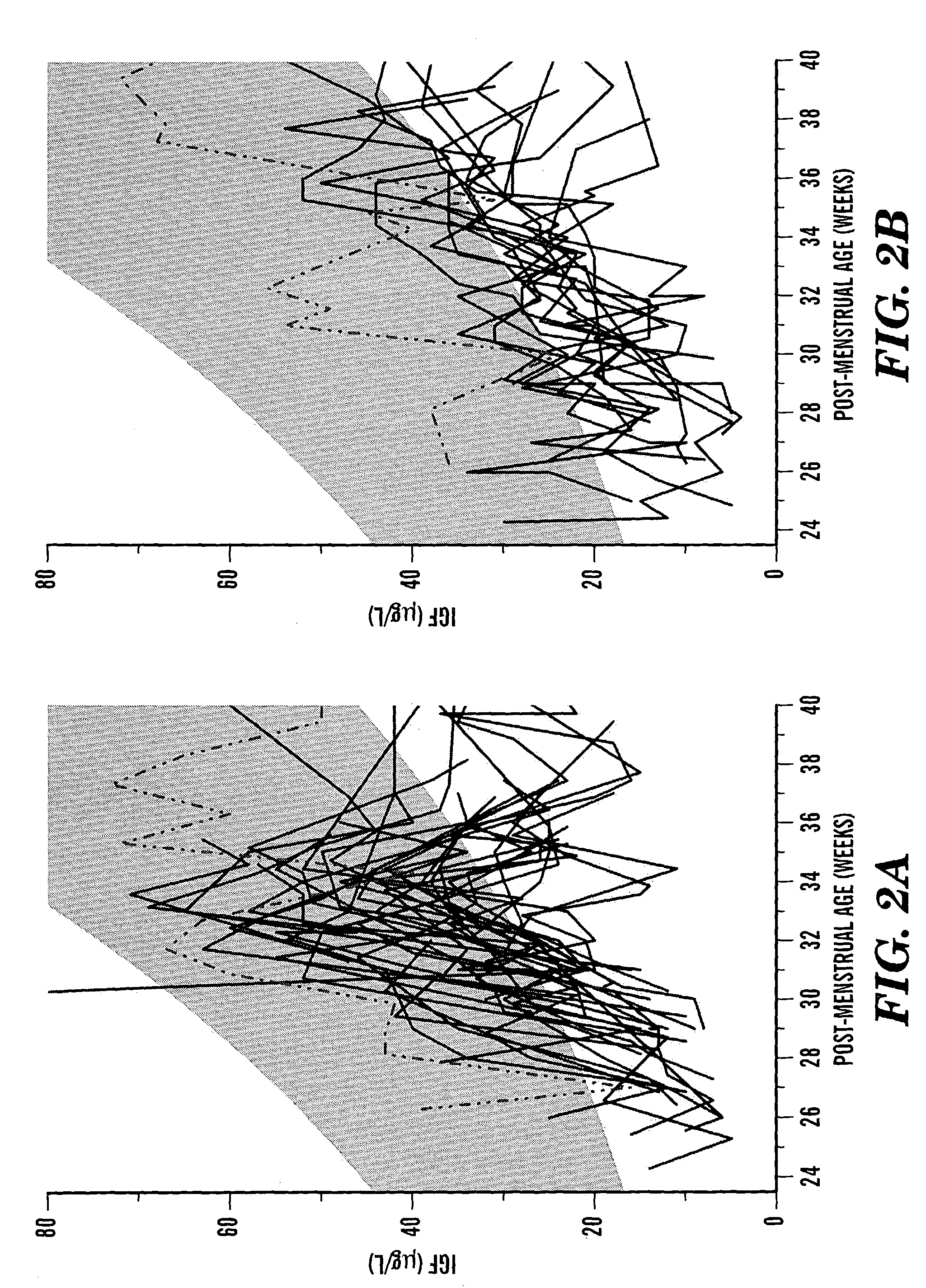
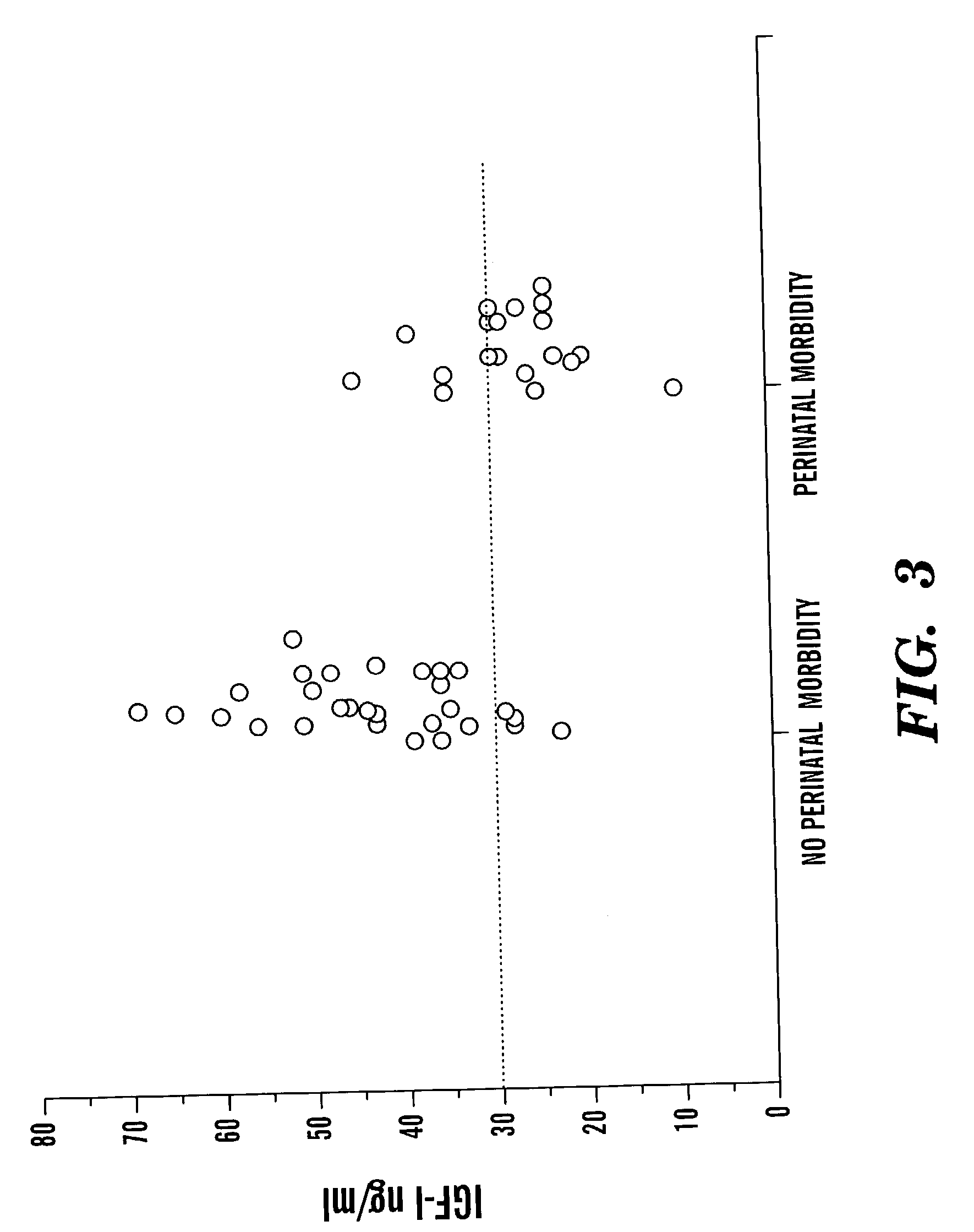
Determination of risk and treatment of complications of prematurity
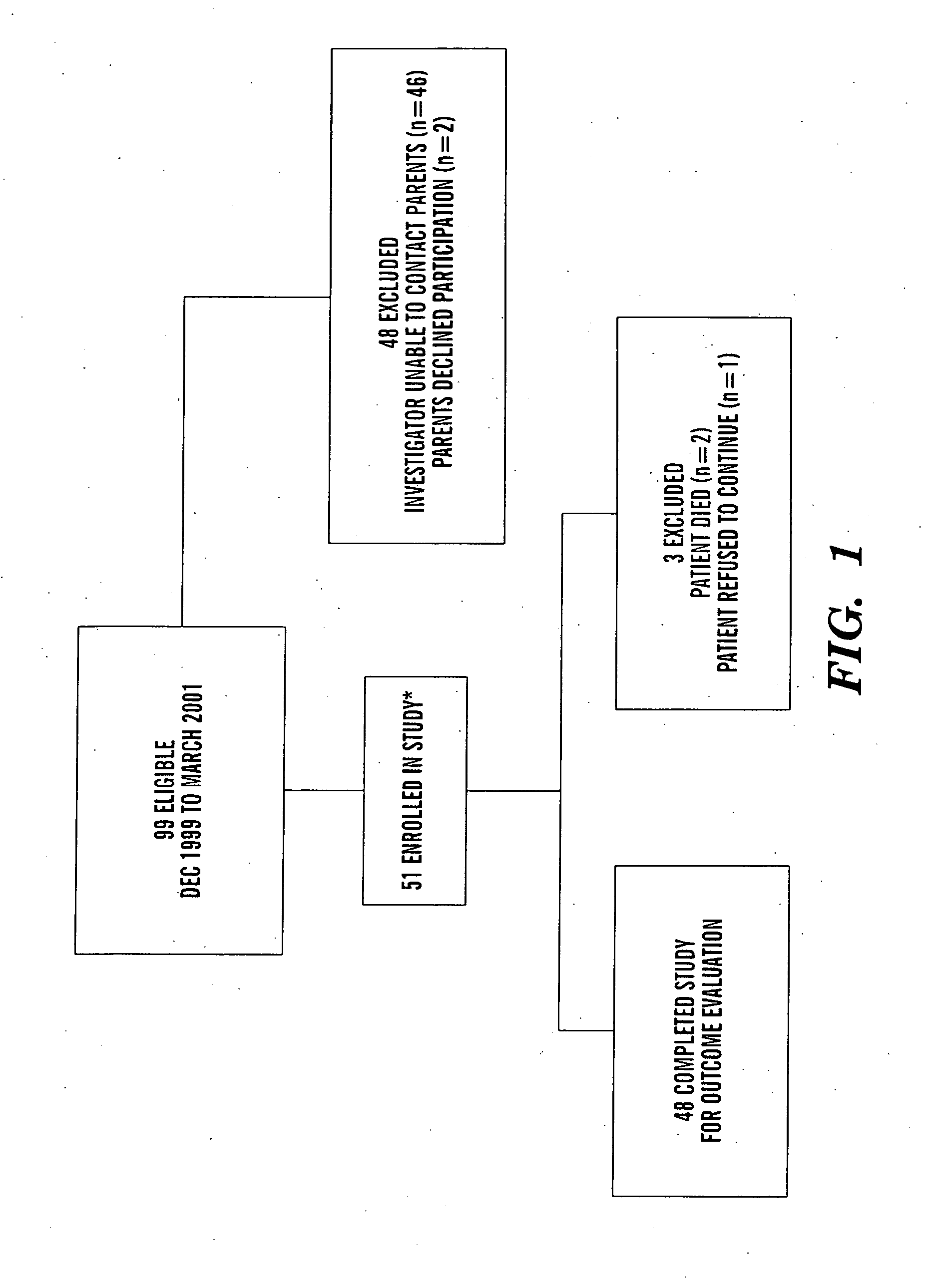
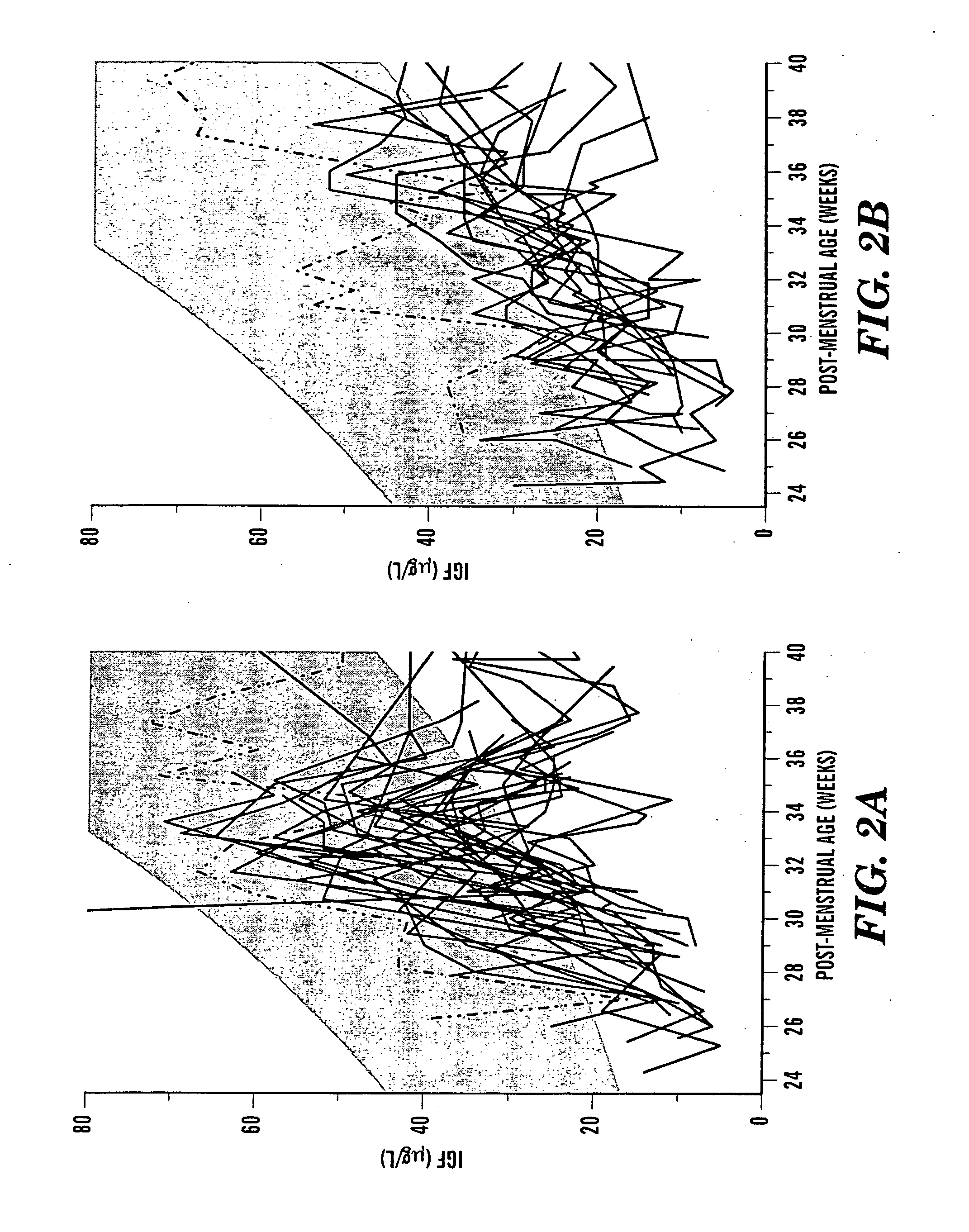
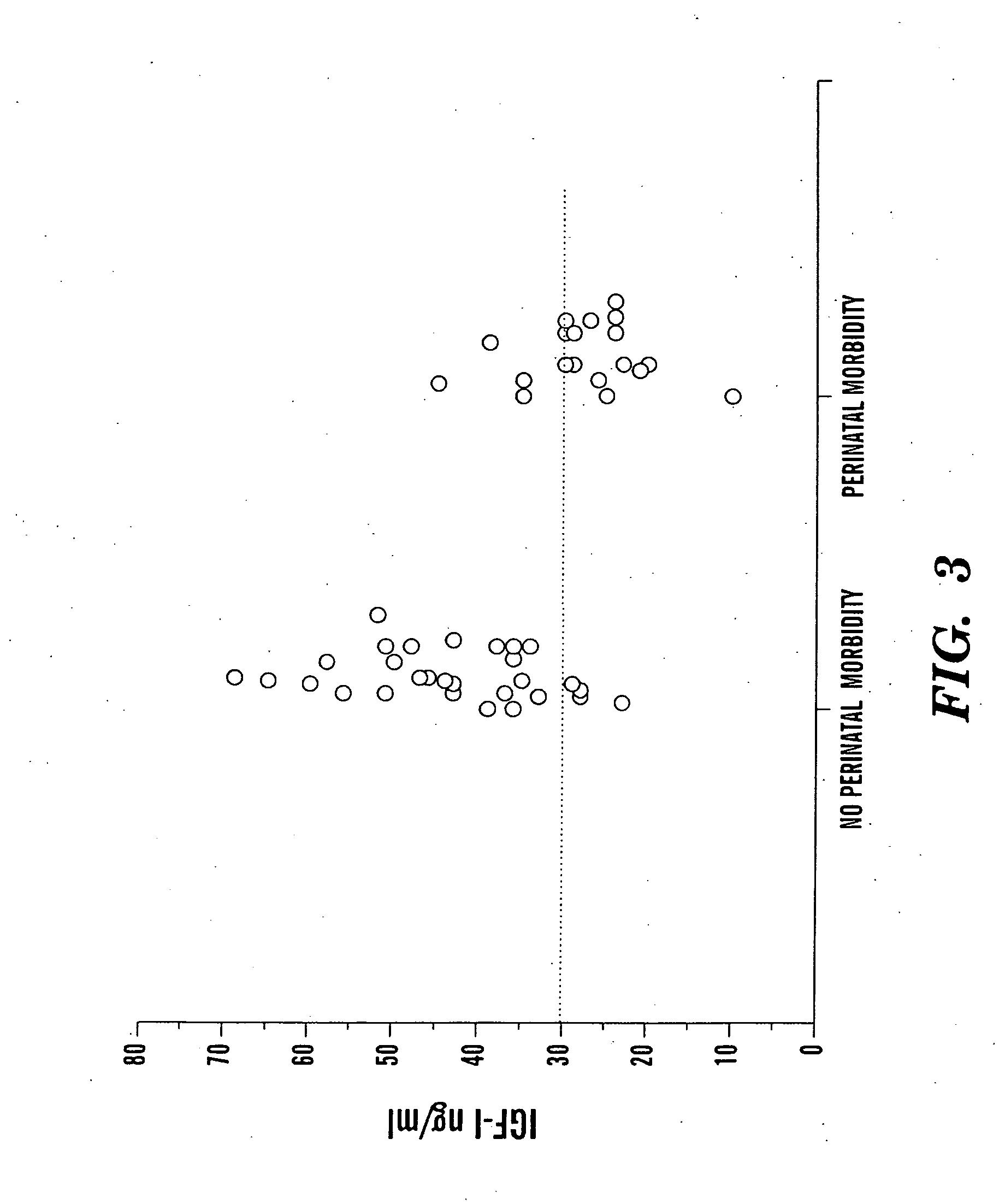
In one aspect of the present invention there is provided a method for determining the risk of developing a complication of preterm birth in a patient born before 40 weeks of gestation or weighing 10% less than the average for the patient's gestational age. The method involves measuring serum IGF-I and / or IGF-I binding protein levels after birth of the patient to obtain an IGF-I or IGF-I binding protein level; and correlating said IGF-I or IGF-I binding protein level with an in utero baseline level of IGF-I or IGF-I binding protein based on gestational age matched mean levels in utero, wherein an IGF-I or IGF-I binding protein level below the mean gestational age in utero level indicates the patient is at an increased risk of developing a complication of preterm birth. The complications of preterm birth include retinopathy of prematurity, developmental delay, mental retardation, bronchopulmonary dysplasia, and intraventricular hemorrhage. Methods for treating / preventing complications of preterm birth are also provided.
Owner:CHILDRENS MEDICAL CENT CORP
Method and Kit for Determining Risk of Preterm Birth and/or Birth of Low-Birth-Weight Baby
InactiveUS20170363637A1Birth low birth weightReduce riskOrganic active ingredientsDisease diagnosisObstetricsPreterm Births
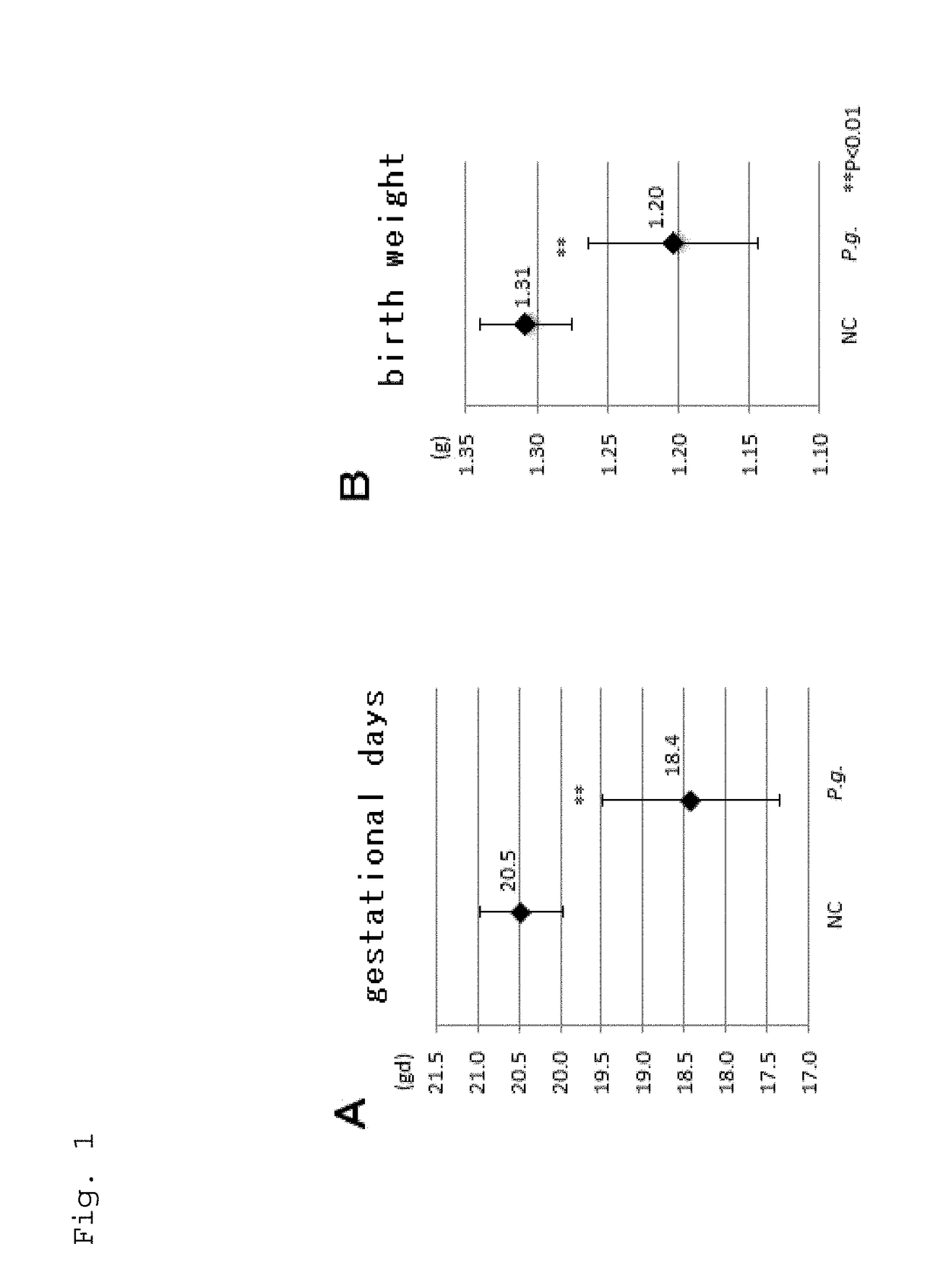
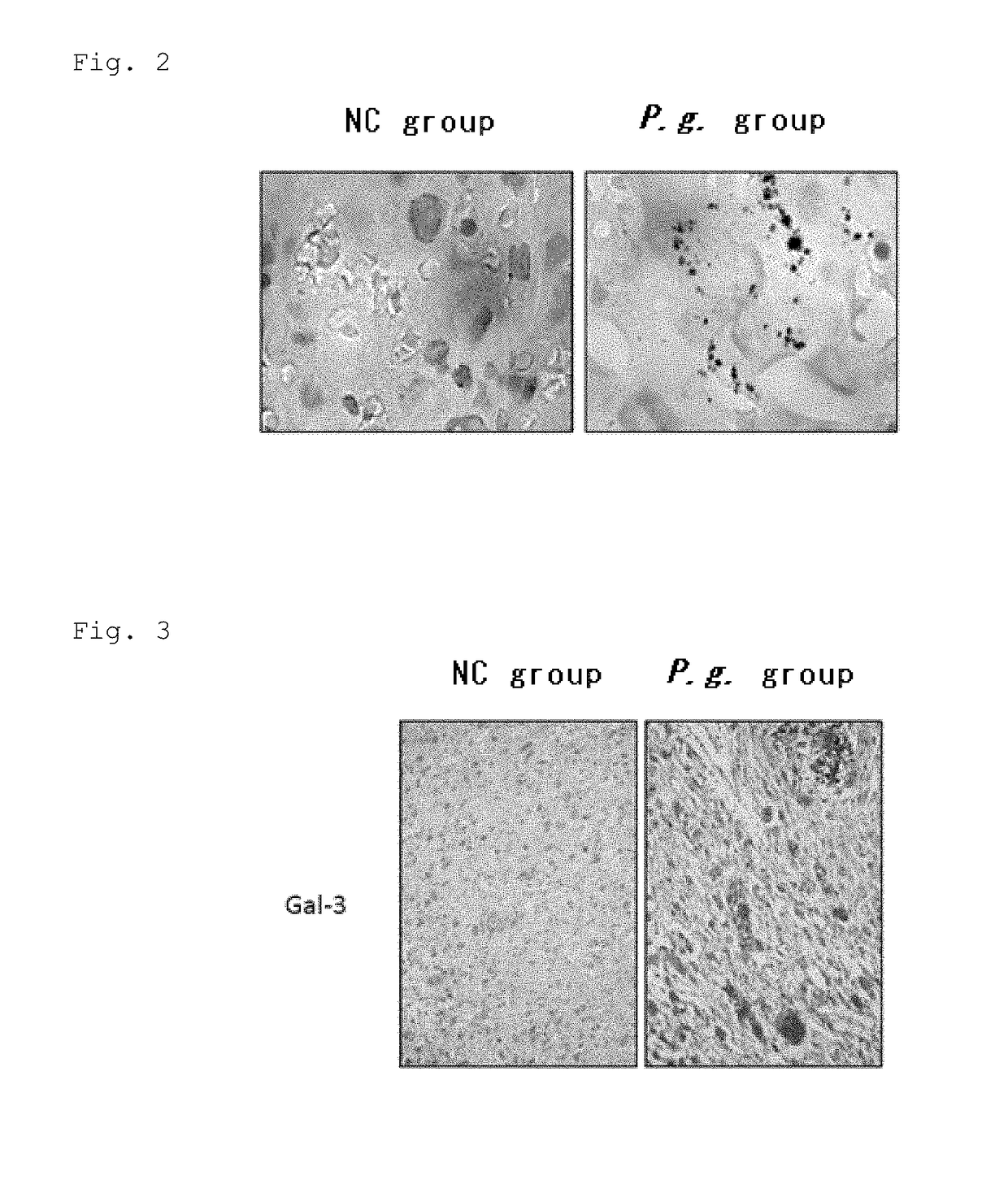
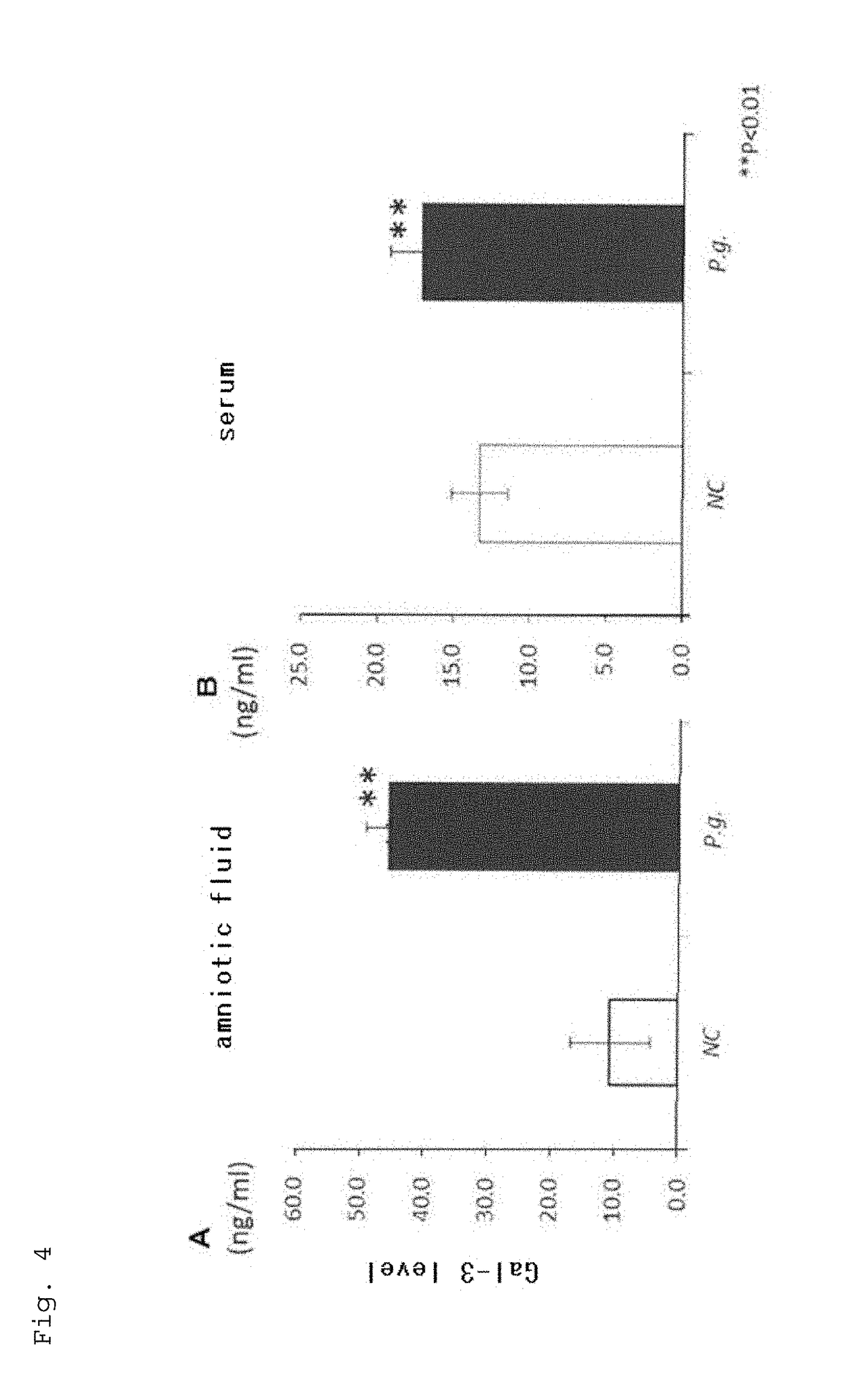
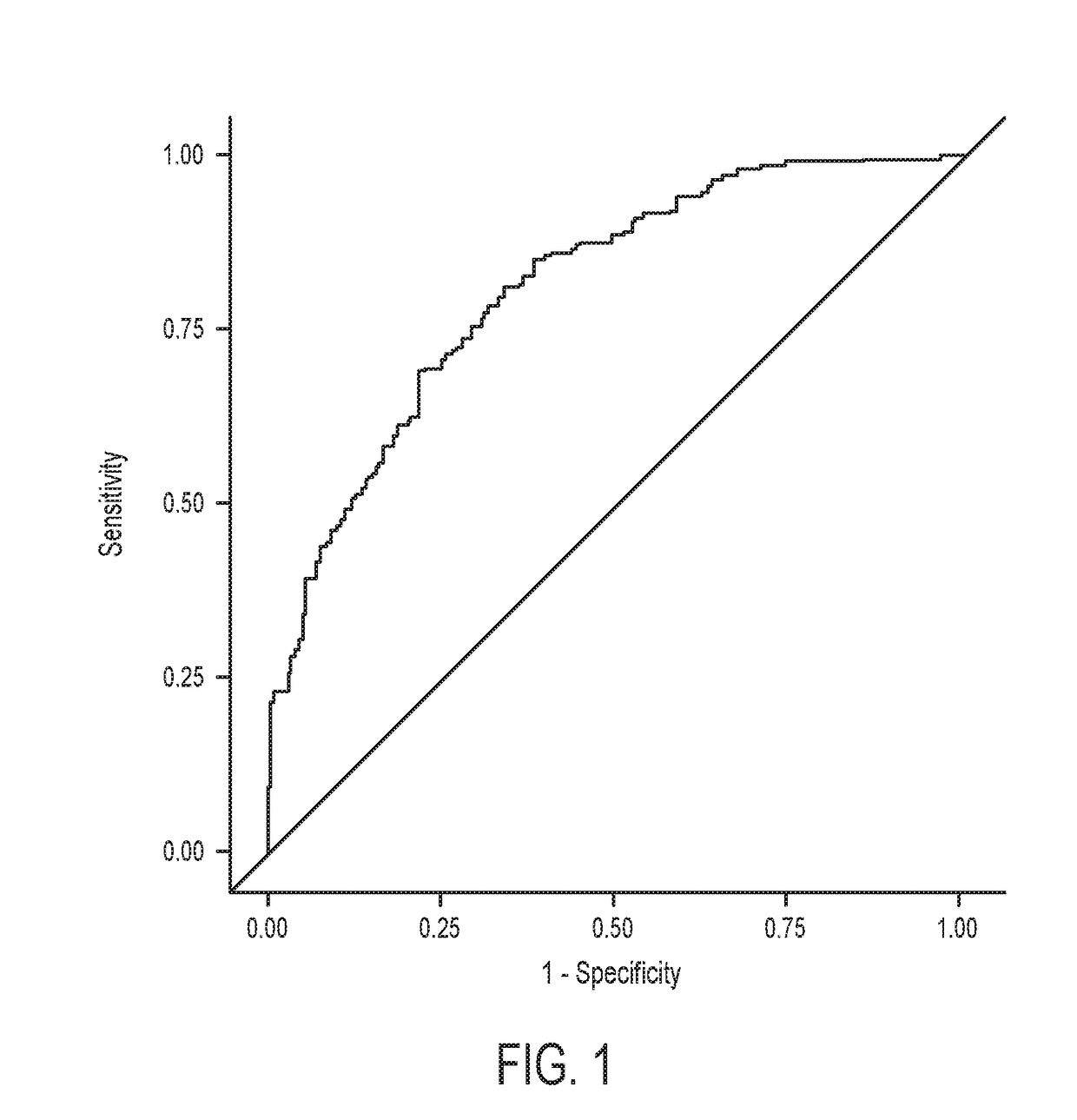
The present application relates to a method for determining a risk of preterm birth and / or low birth weight of a pregnant female comprising measuring a galectin-3 (Gal-3) level in a blood, plasma, or serum sample obtained from the pregnant female, or a kit or marker which may be used in the method. The present application also relates to a medicament effective to reduce a risk of preterm birth and / or low birth weight, a pharmaceutical composition comprising the medicament, or a method for reducing a risk of preterm birth and / or low birth weight of a pregnant female comprising administering the medicament or the pharmaceutical composition to the pregnant female.
Owner:HIROSHIMA UNIVERSITY
Tools for Predicting the Risk of Preterm Birth
PendingUS20190072564A1Reduce riskNot at risk of errorMedical simulationMedical data miningPreterm BirthsAssay
The invention is directed to methods and compositions of matter for predicting the risk of preterm birth (PTB) in a subject and administering interventions to subjects at elevated risk of PTB. The inventions provide a convenient, non-invasive, and accurate means of assessing PTB risk in a subject, and further provide a means of treating subjects in need of treatment and selecting appropriate interventions to reduce such risk. The diagnostic tools include novel panels of biomarkers and other factors which can be used to accurately predict risk of PTB across a population, wherein elevated risk is due to a variety of underlying physiological pathways and processes. In another aspect, the scope of the invention encompasses assay kits which are useful in the fast, accurate, and inexpensive prediction of PTB risk by multiplexed measurement of PTB risk factors.
Owner:RGT UNIV OF CALIFORNIA +1
Wireless power for pessary device
PendingUS20210016098A1Increased riskLengthen cervixOrganic active ingredientsStrain gaugeCervical shorteningPelvic region
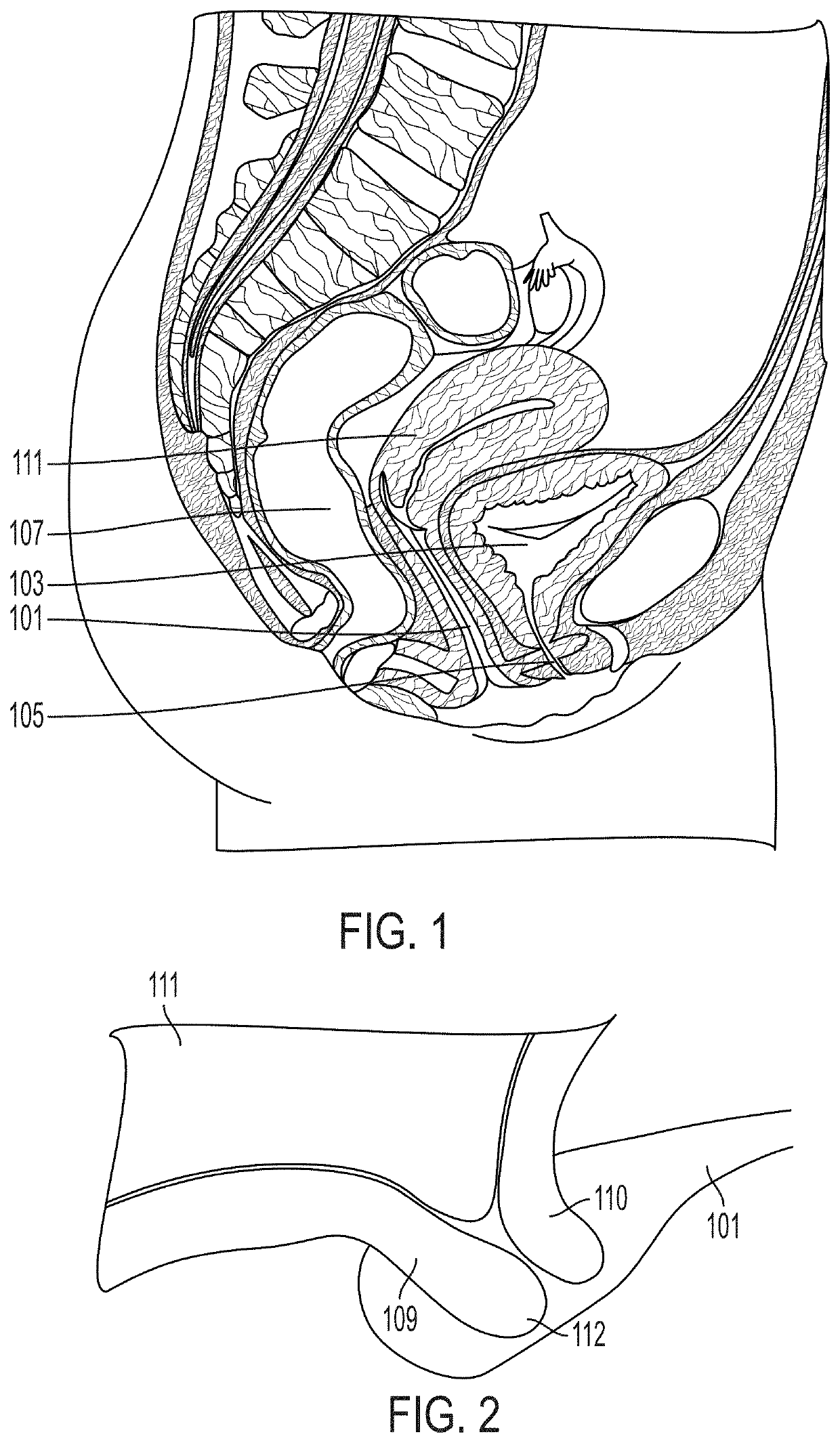
A pessary for the prevention of preterm birth, and in particular two pathological conditions of pregnancy known as isthmico-cervical incontinence and cervical shortening, both of which are associated with increased risks for pregnancy loss and / or premature deliveries of babies. The pessary includes a sleeve supported within a ring by an annular member. The sleeve is intended to contact the cervix and maximize the length of the cervix while the annular member and ring contact the vagina. The pessary may be fabricated from a pliable medical-grade silicon, and the pessary may include one or more sensors to measure various patient parameters indicative of a premature cervical contraction. The sleeve may be wirelessly powered from a charging device, such as a pillow, a table-top device, or a pelvic belt.
Owner:OBSTETRIC SOLUTIONS LLC A LLC
Electrical inhibition (EI) uterine pacemaker for controlling preterm uterine contractions and preterm birth
In an aspect of the present disclosure, a system and method for controlling uterine contractions is disclosed including receiving data from at least one sensor by a wireless apparatus inserted into the patient's vagina adjacent the cervix. The data includes an indication that a contraction of the uterus is imminent. The method further includes in response to receiving the data, causing a generator circuit of the wireless apparatus to supply electrical energy to an energy applicator of the wireless apparatus that is configured to apply the supplied electrical energy to the uterus of the patient via the cervix of the patient to control contractions of the patient's uterus.
Owner:NEW YORK UNIV
Determination of risk and treatment of complications of prematurity
In one aspect of the present invention there is provided a method for determining the risk of developing a complication of preterm birth in a patient born before 40 weeks of gestation or weighing 10% less than the average for the patient's gestational age. The method involves measuring serum IGF-I and / or IGF-I binding protein levels after birth of the patient to obtain an IGF-I or IGF-I binding protein level; and correlating said IGF-I or IGF-I binding protein level with an in utero baseline level of IGF-I or IGF-I binding protein based on gestational age matched mean levels in utero, wherein an IGF-I or IGF-I binding protein level below the mean gestational age in utero level indicates the patient is at an increased risk of developing a complication of preterm birth. The complications of preterm birth include retinopathy of prematurity, developmental delay, mental retardation, bronchopulmonary dysplasia, and intraventricular hemorrhage. Methods for treating / preventing complications of preterm birth are also provided.
Owner:CHILDRENS MEDICAL CENT CORP
Pessary device and methods for preventing premature births
PendingUS20200398052A1Increased riskLengthen cervixOrganic active ingredientsStrain gaugeCervical shorteningPreterm Births
A pessary for the prevention of preterm birth, and in particular two pathological conditions of pregnancy known as isthmico-cervical incontinence and cervical shortening, both of which are associated with increased risks for pregnancy loss and / or premature deliveries of babies. The pessary includes a sleeve supported within a ring by an annular member. The sleeve is intended to contact the cervix and maximize the length of the cervix while the annular member and ring contact the vagina. The pessary may be fabricated from a pliable medical-grade silicon, and the pessary may include one or more sensors to measure various patient parameters indicative of a premature cervical contraction.
Owner:OBSTETRIC SOLUTIONS LLC
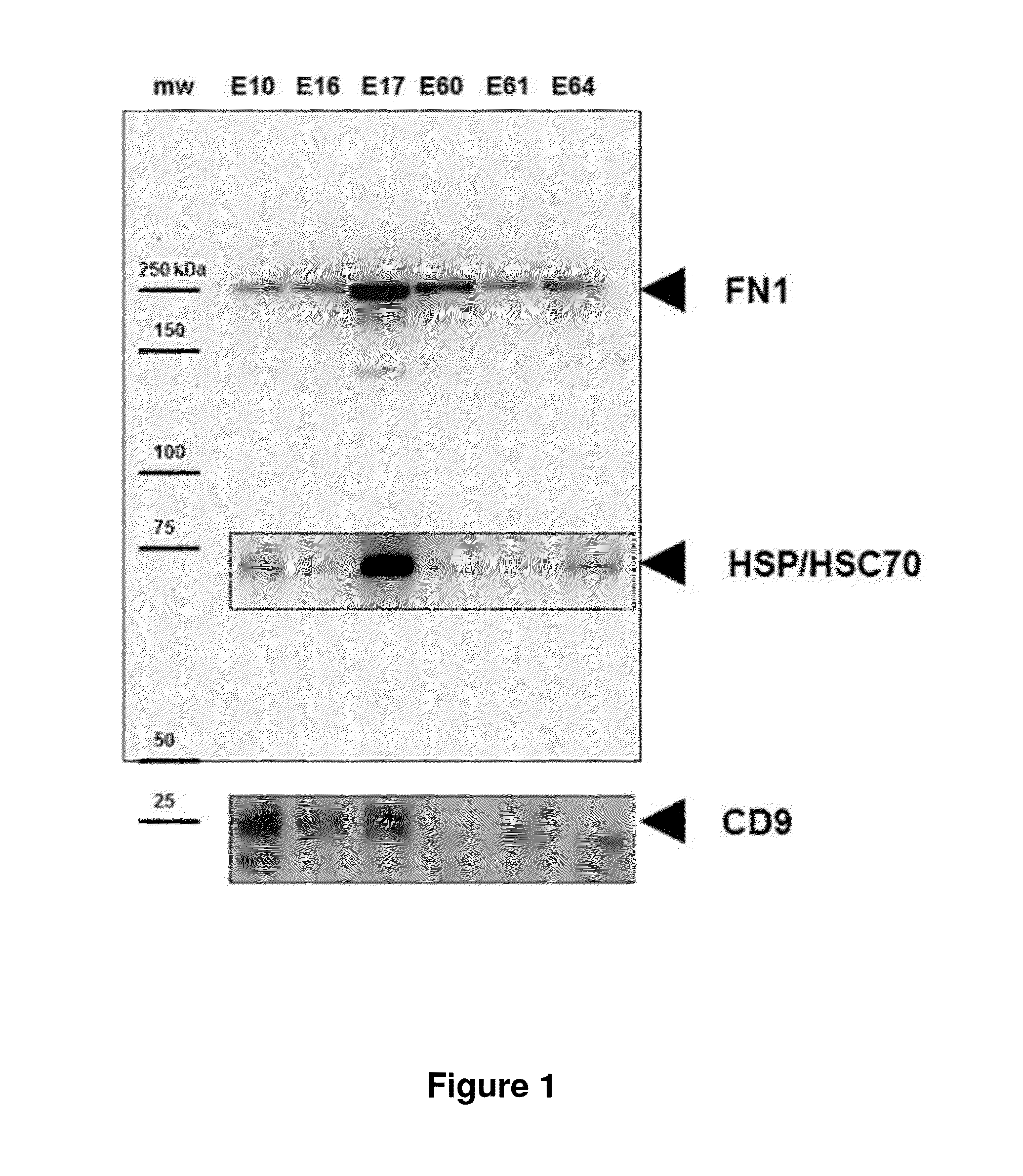
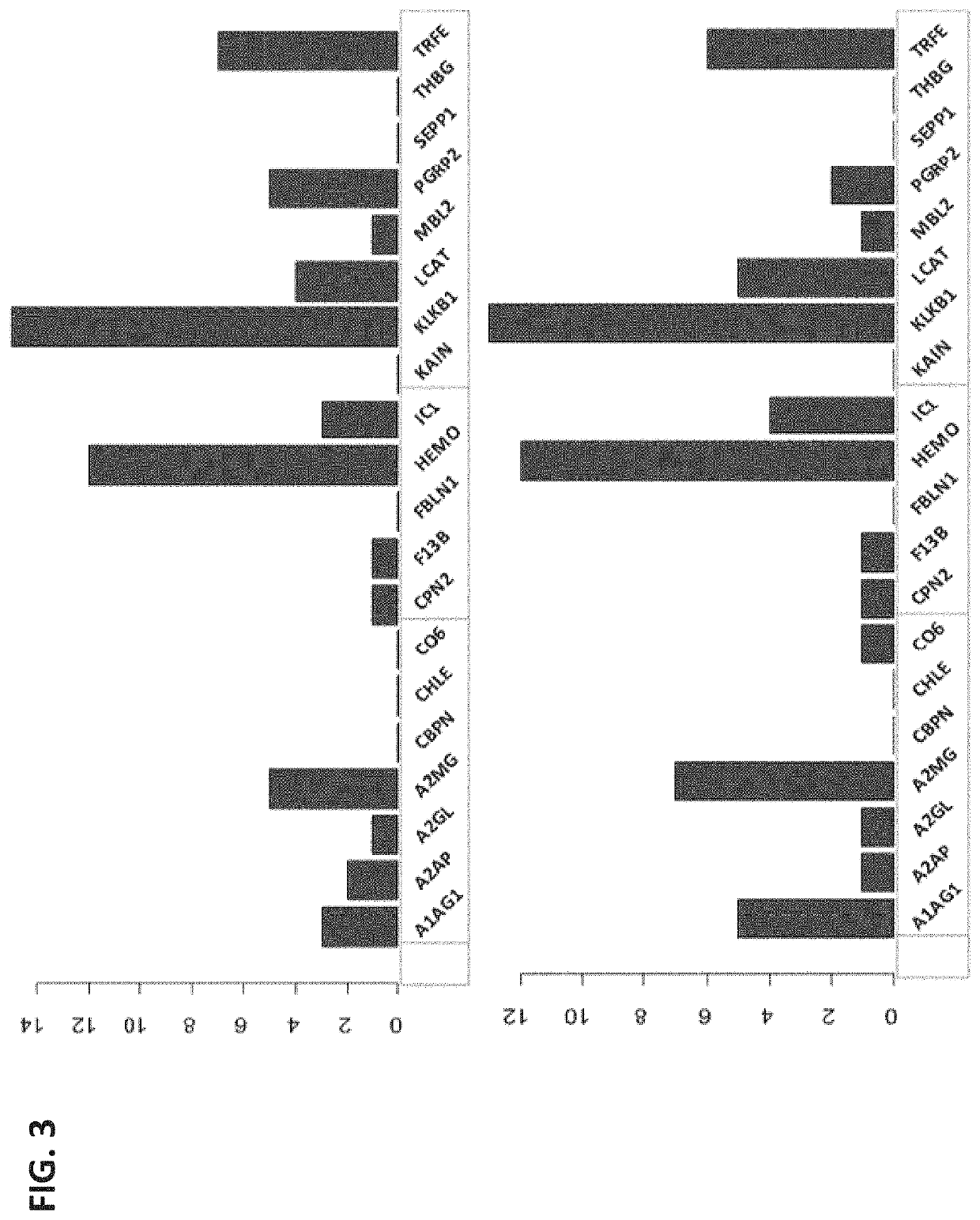
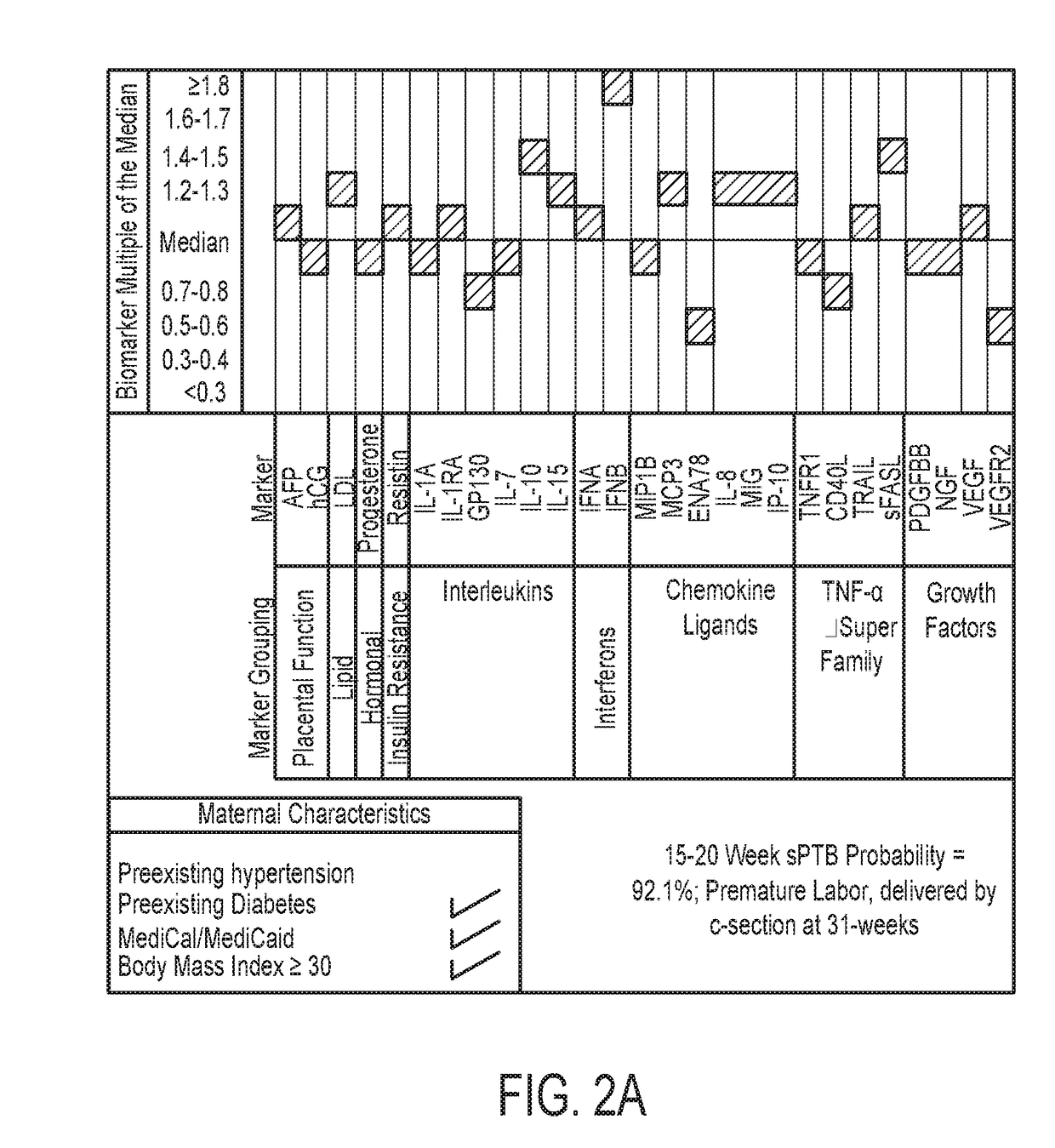
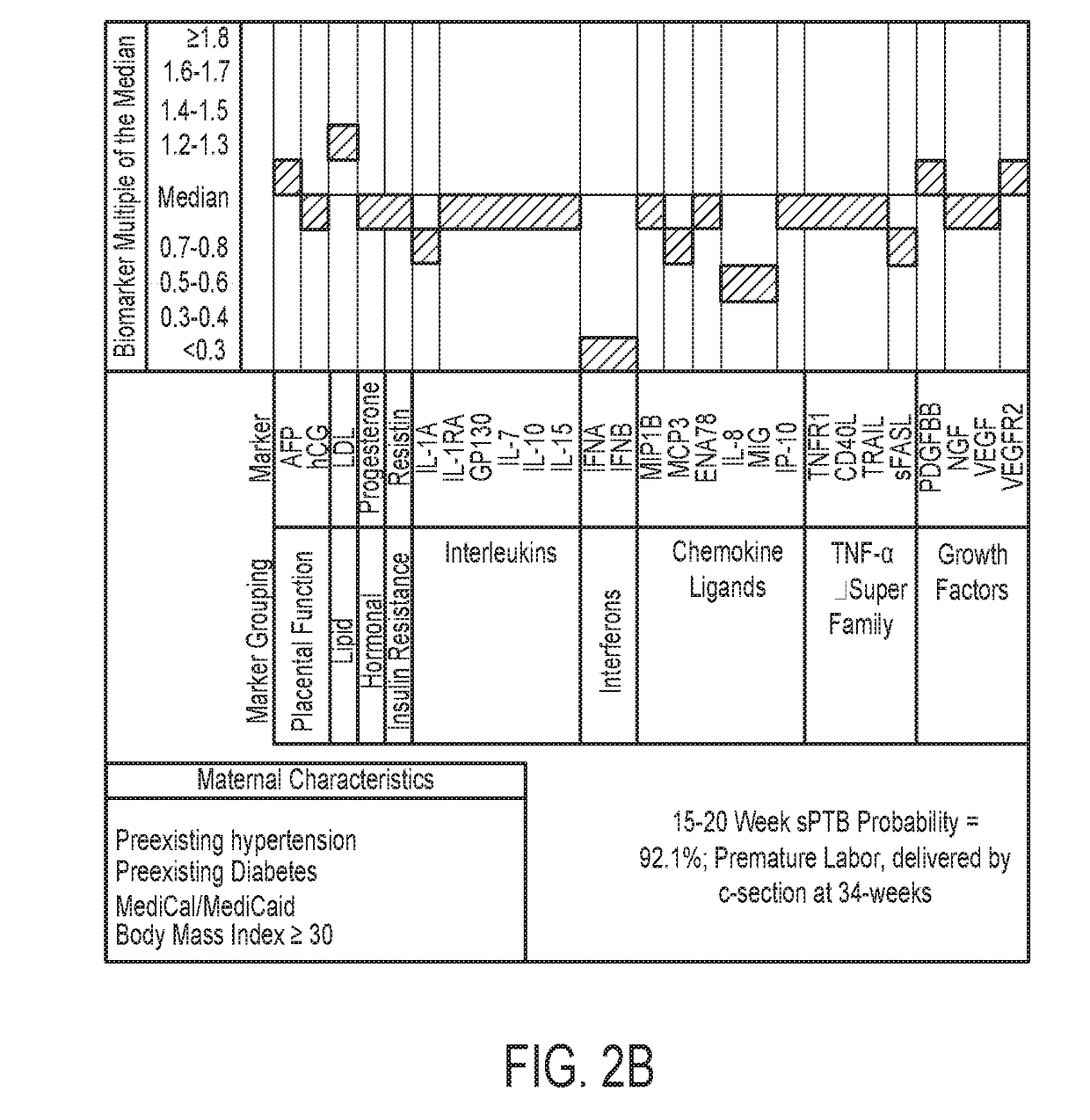
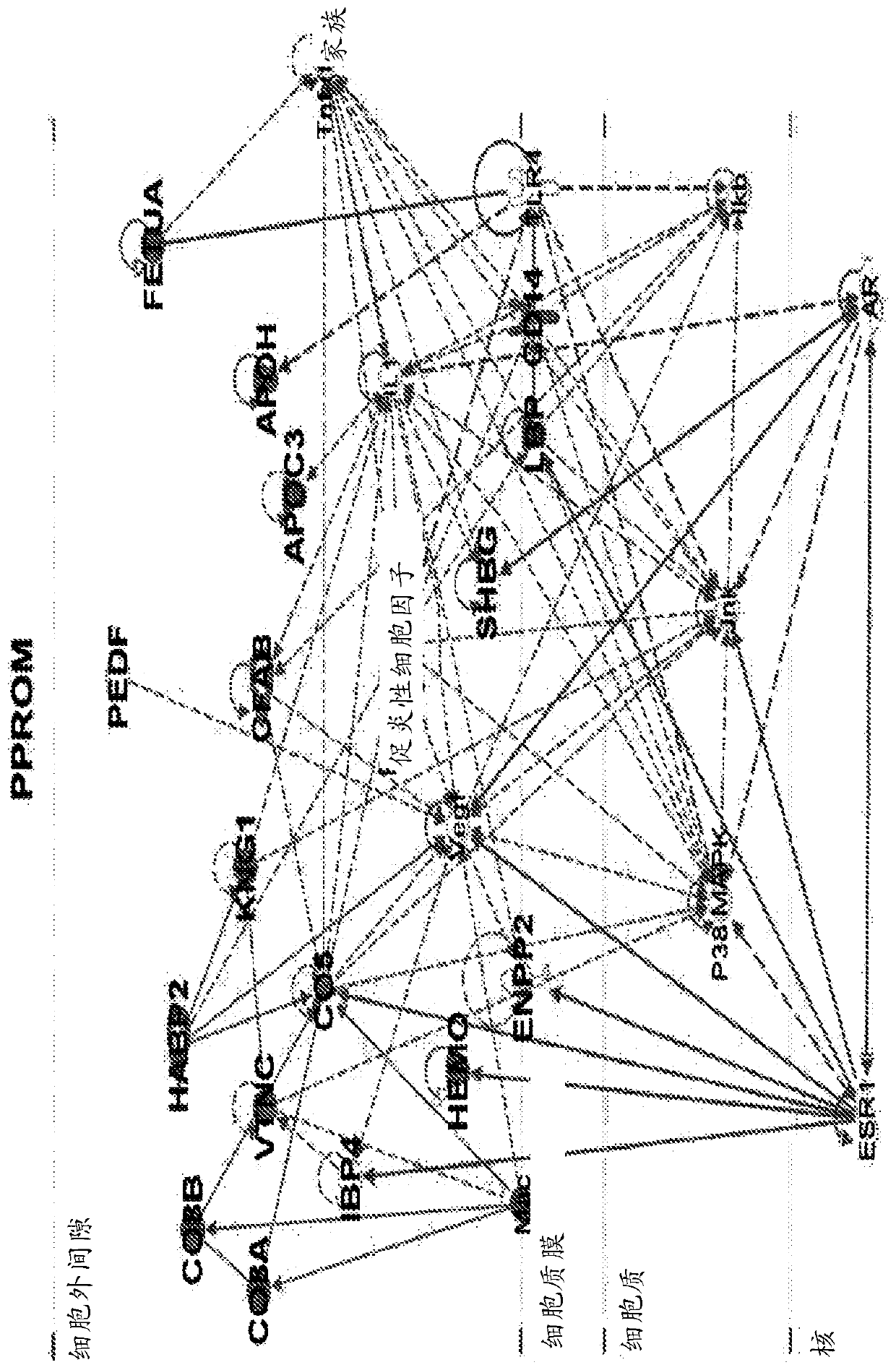
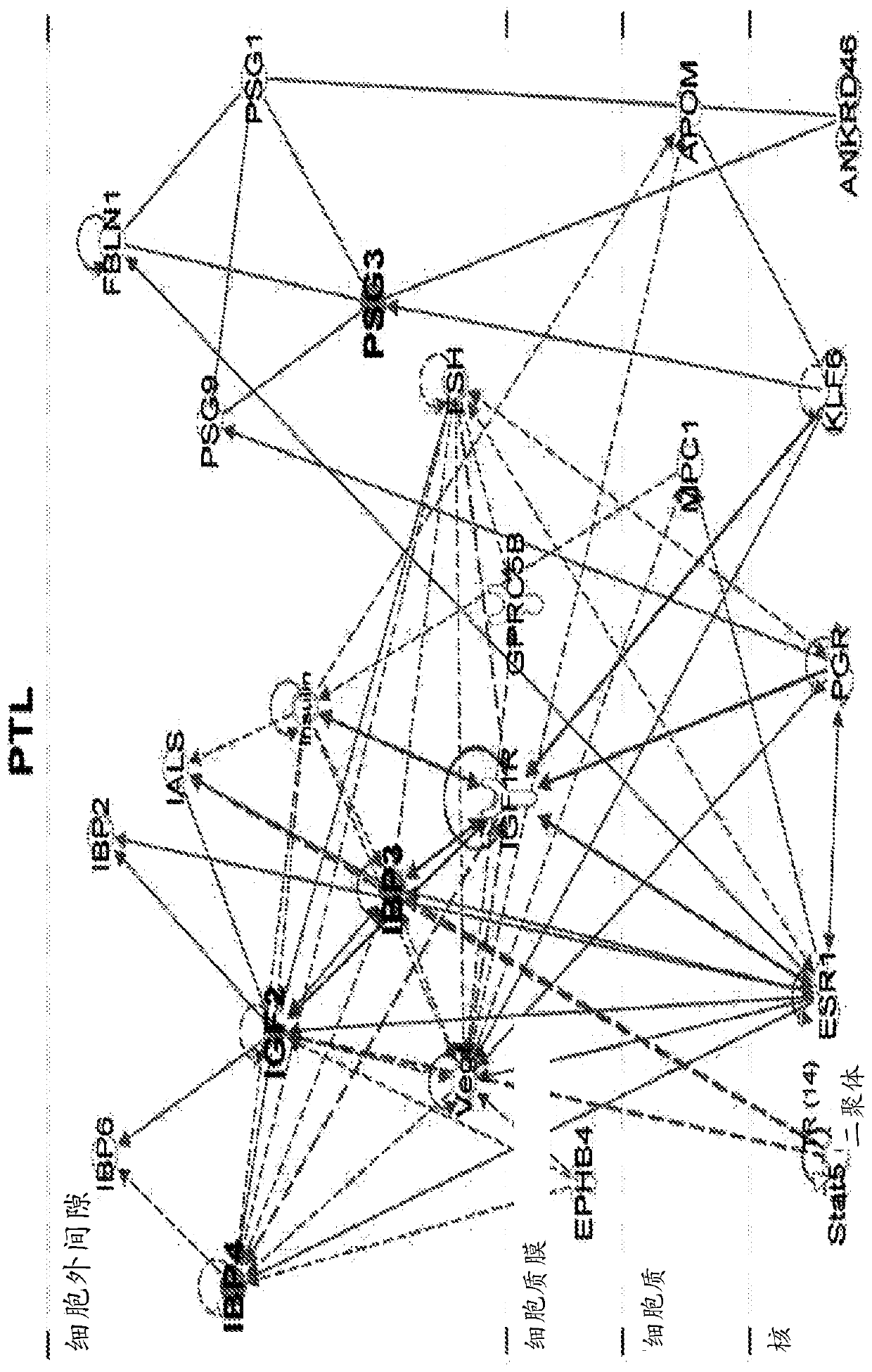
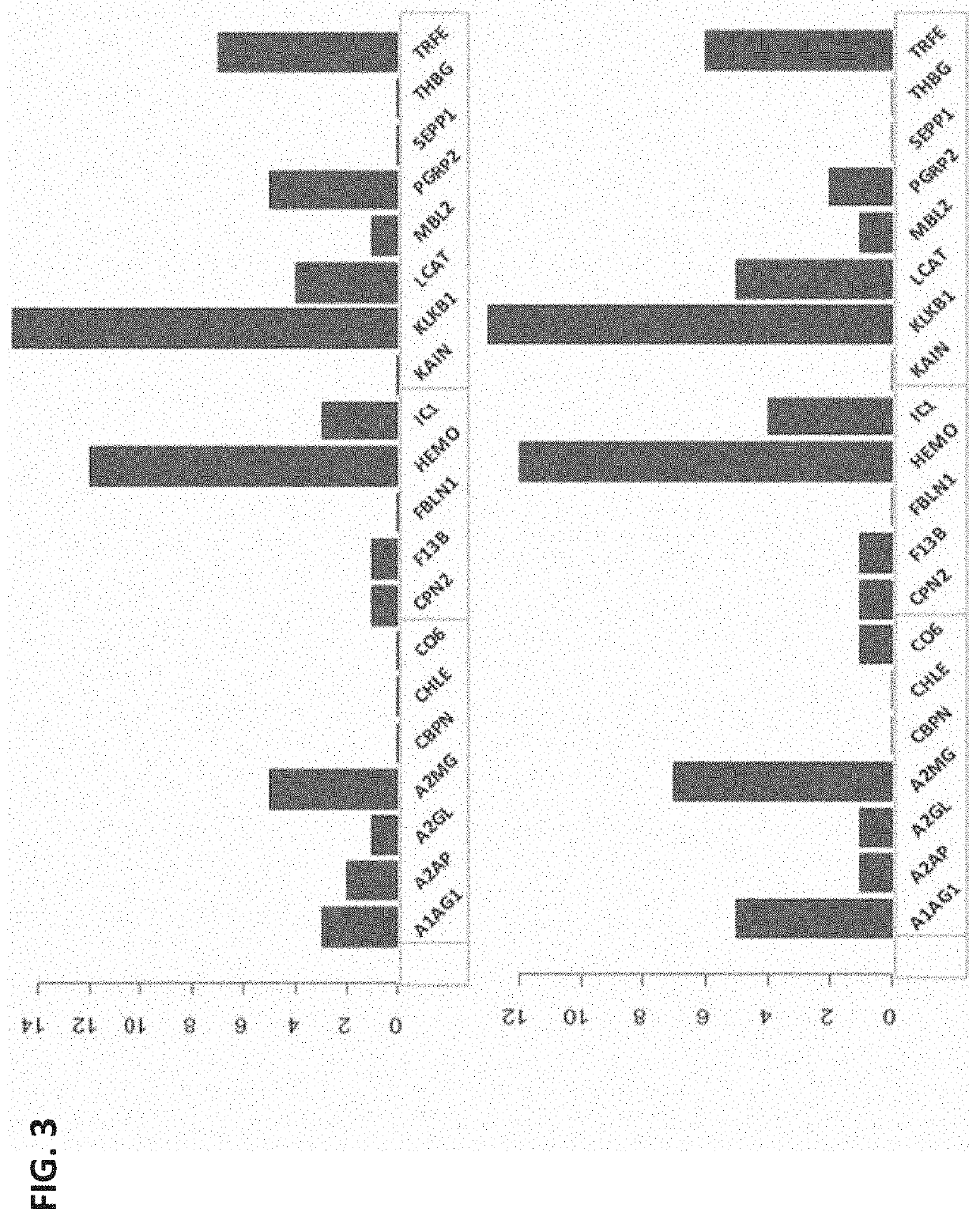
Biomarkers for predicting preterm birth due to preterm premature rupture of membranes versus idiopathic spontaneous labor
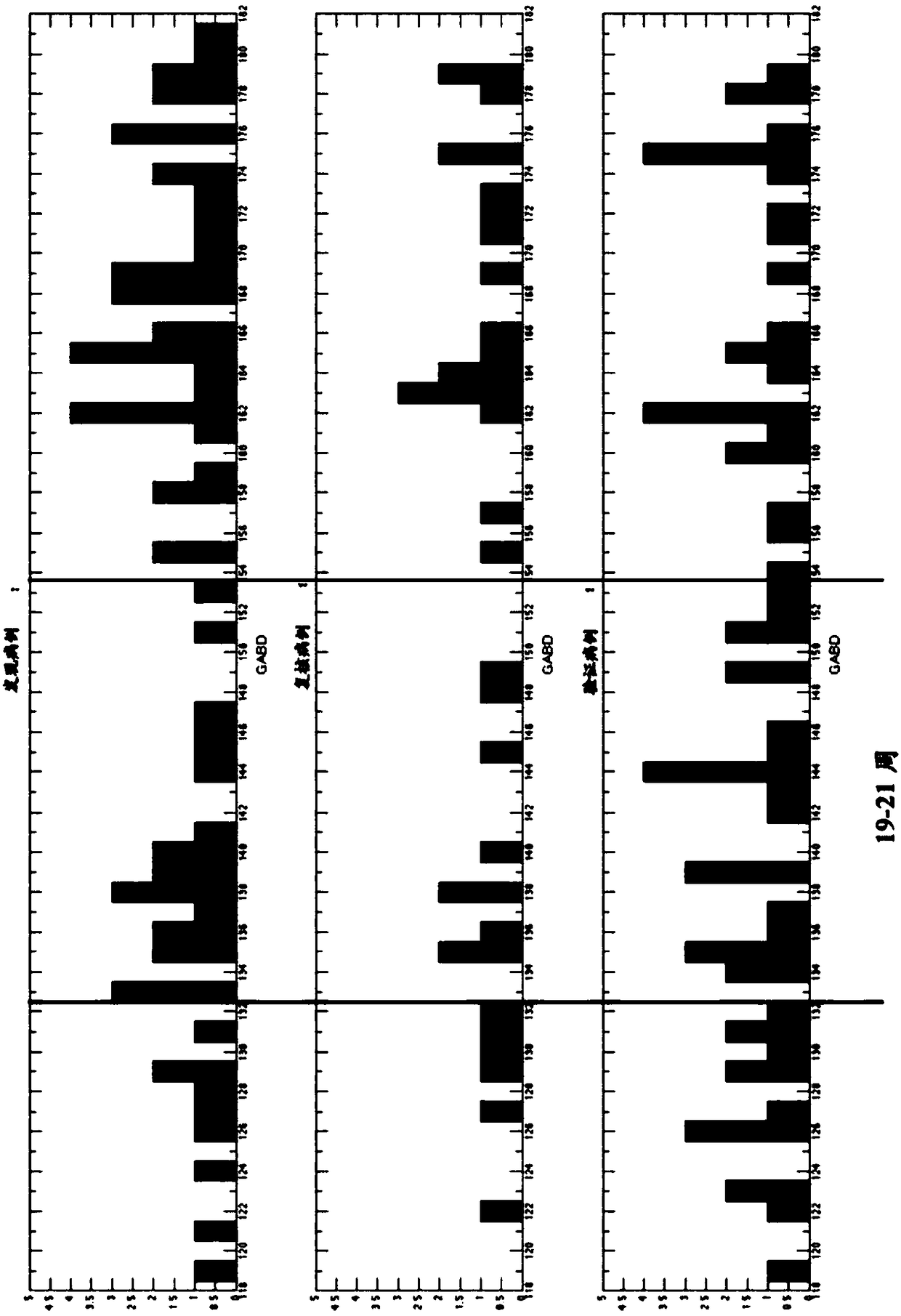
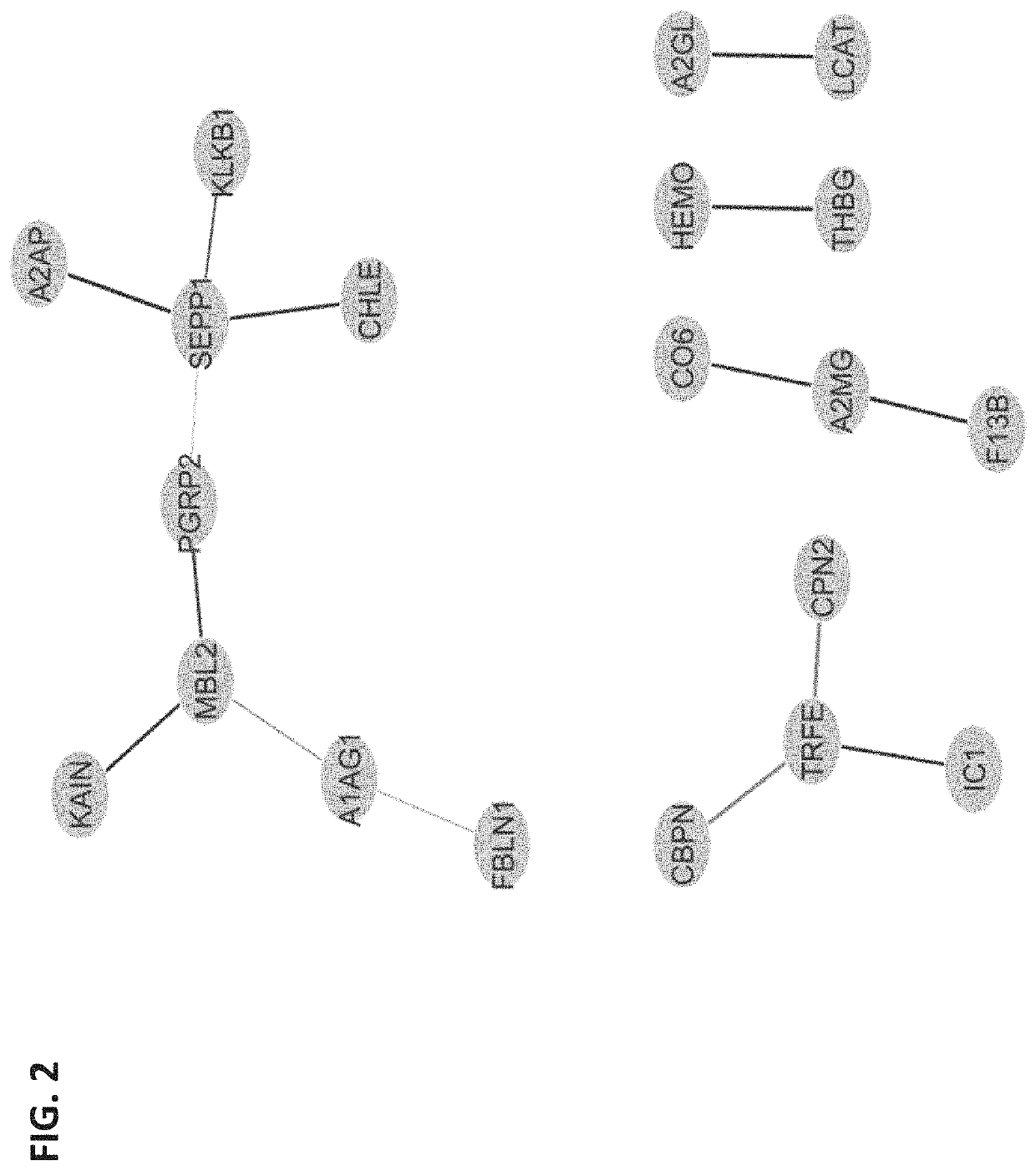
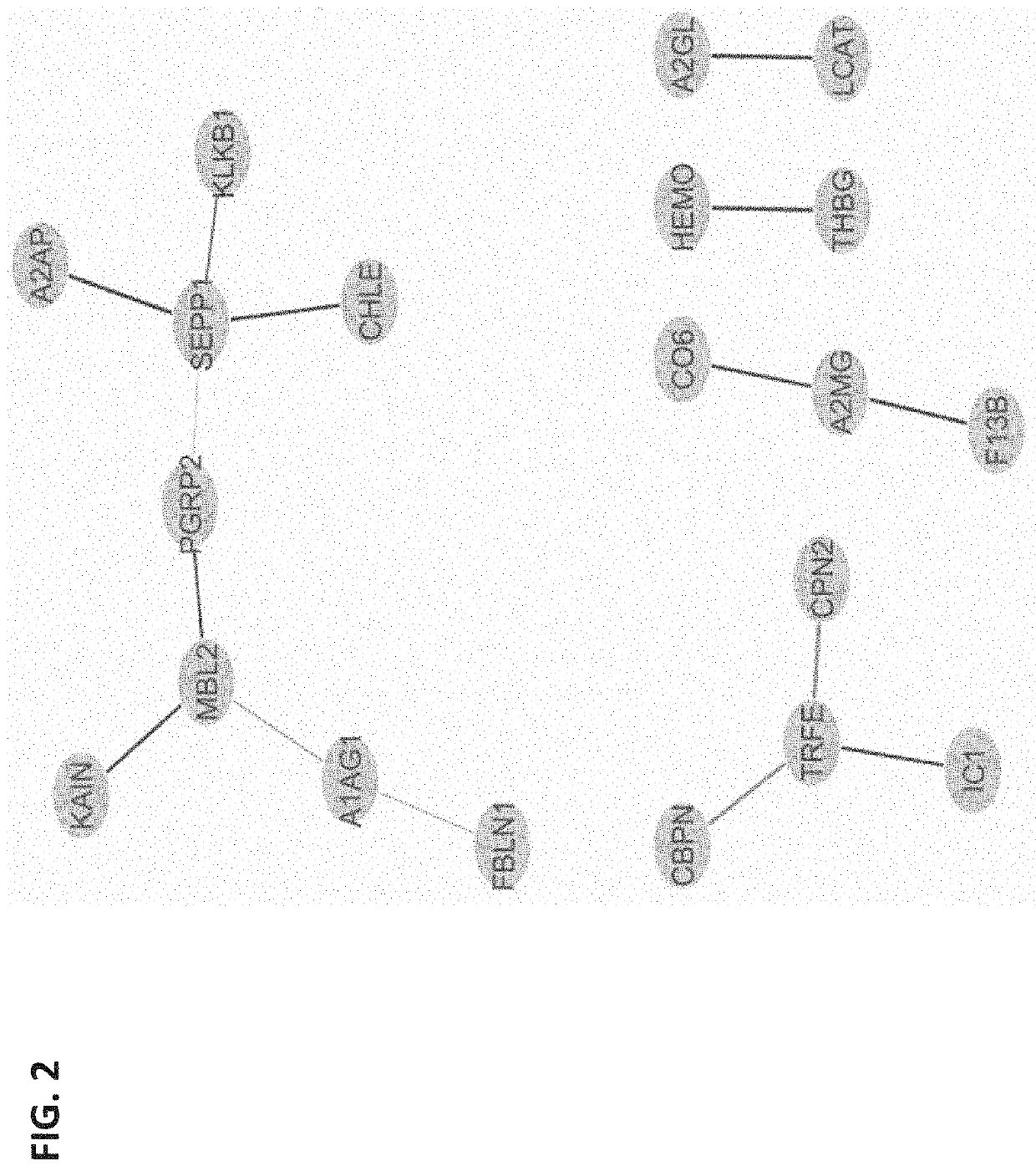
The present invention provides compositions and methods for predicting the probability of preterm birth in a pregnant female. The present invention provides a composition comprising one or more biomarkers selected from the group consisting of the biomarkers set forth in Figures 1 and 2 and Tables 1 through (3), (6) through (38), and (44) through (68). In one embodiment, the invention provides a method of determining probability for preterm birth in a pregnant female, optionally preterm birth associated with preterm premature rupture of membranes (PPROM) or preterm birth associated idiopathic spontaneous labor (PTL), the method comprising measuring in a biological sample obtained from the pregnant female one or biomarkers selected from one or more of the biomarkers set forth in Figures 1 and 2 and Tables 1 through (3), (6) through (38), and (44) through (68) to determine the probability for preterm birth in said pregnant female.
Owner:SERA PROGNOSTICS
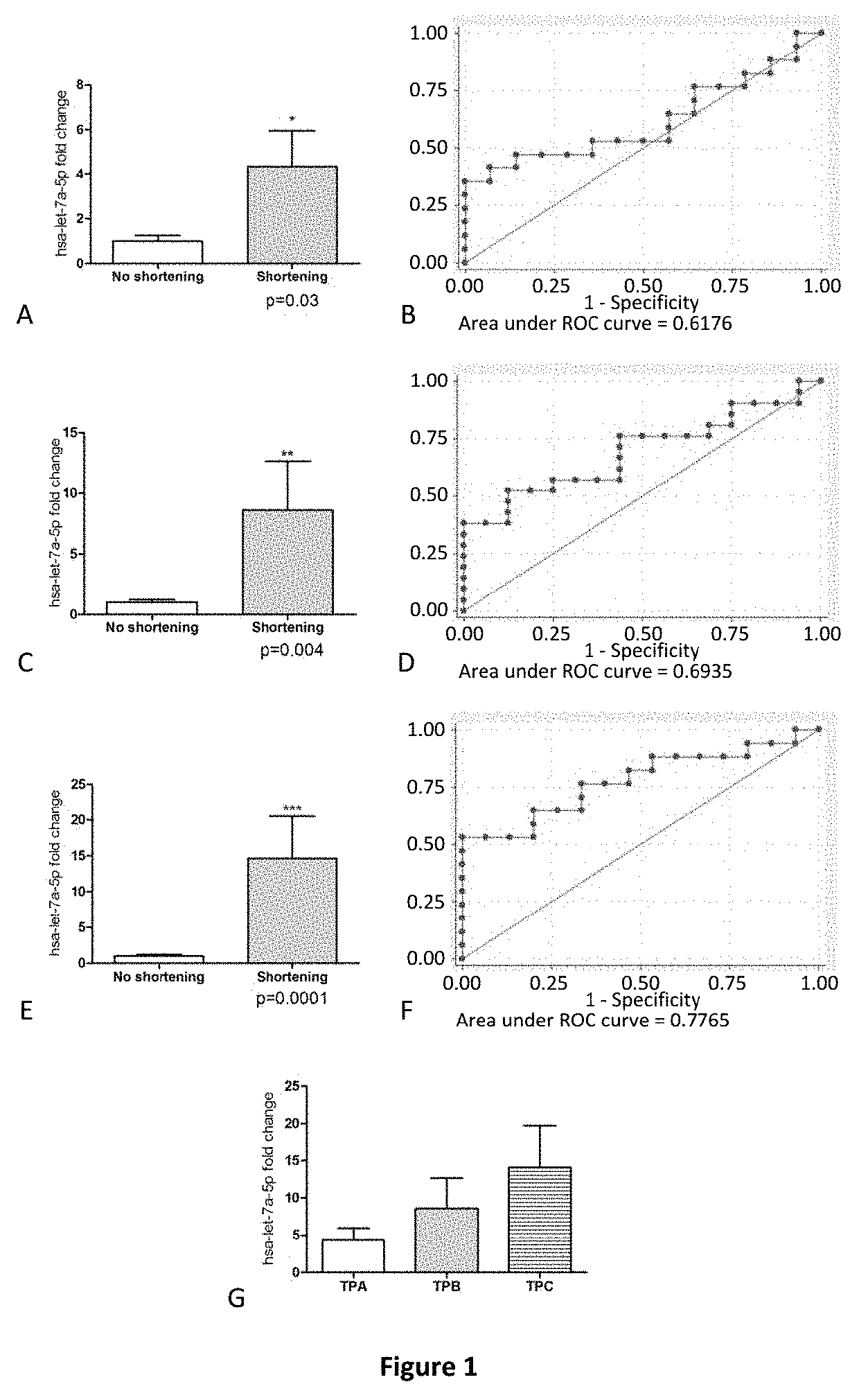
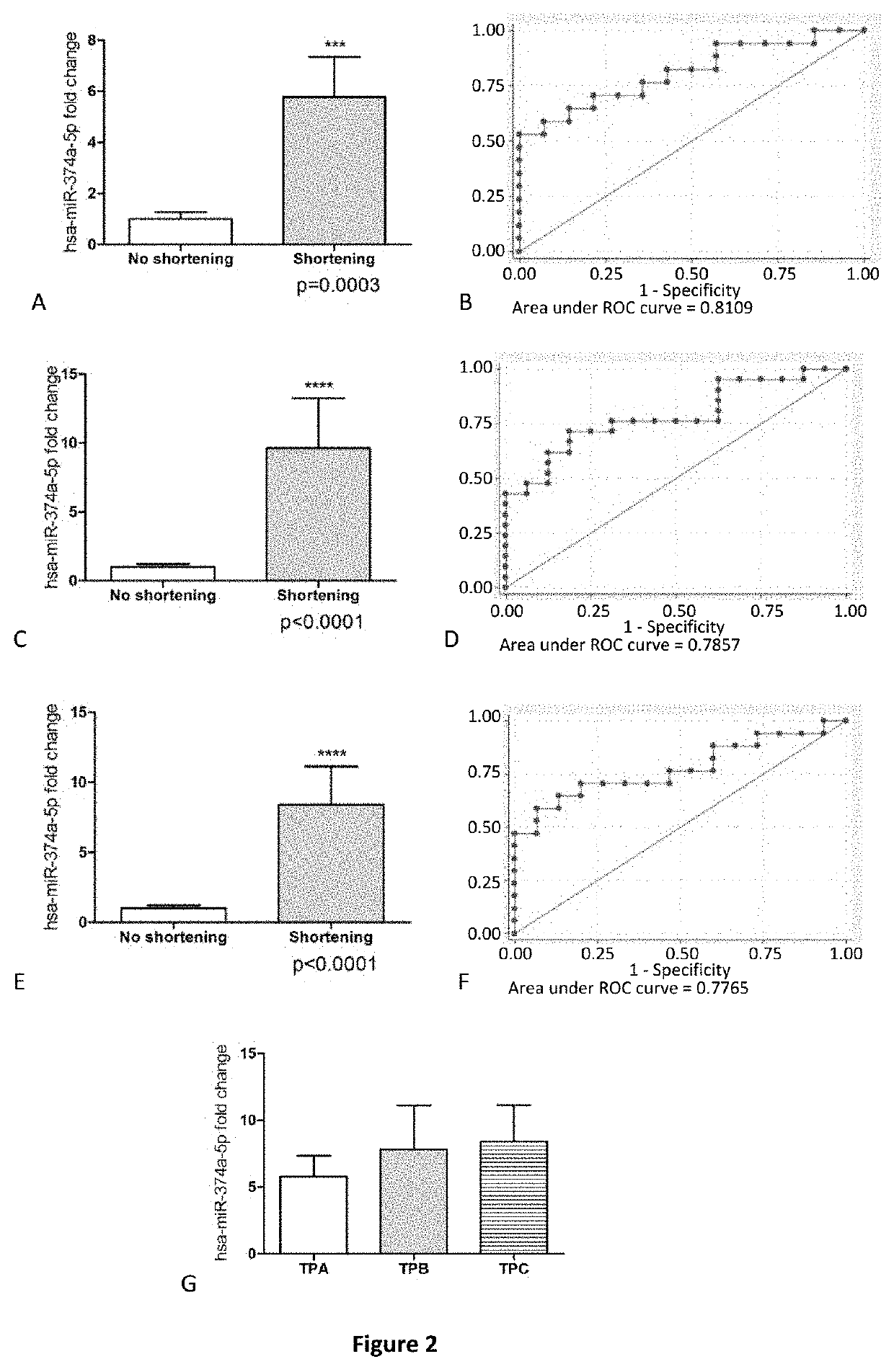
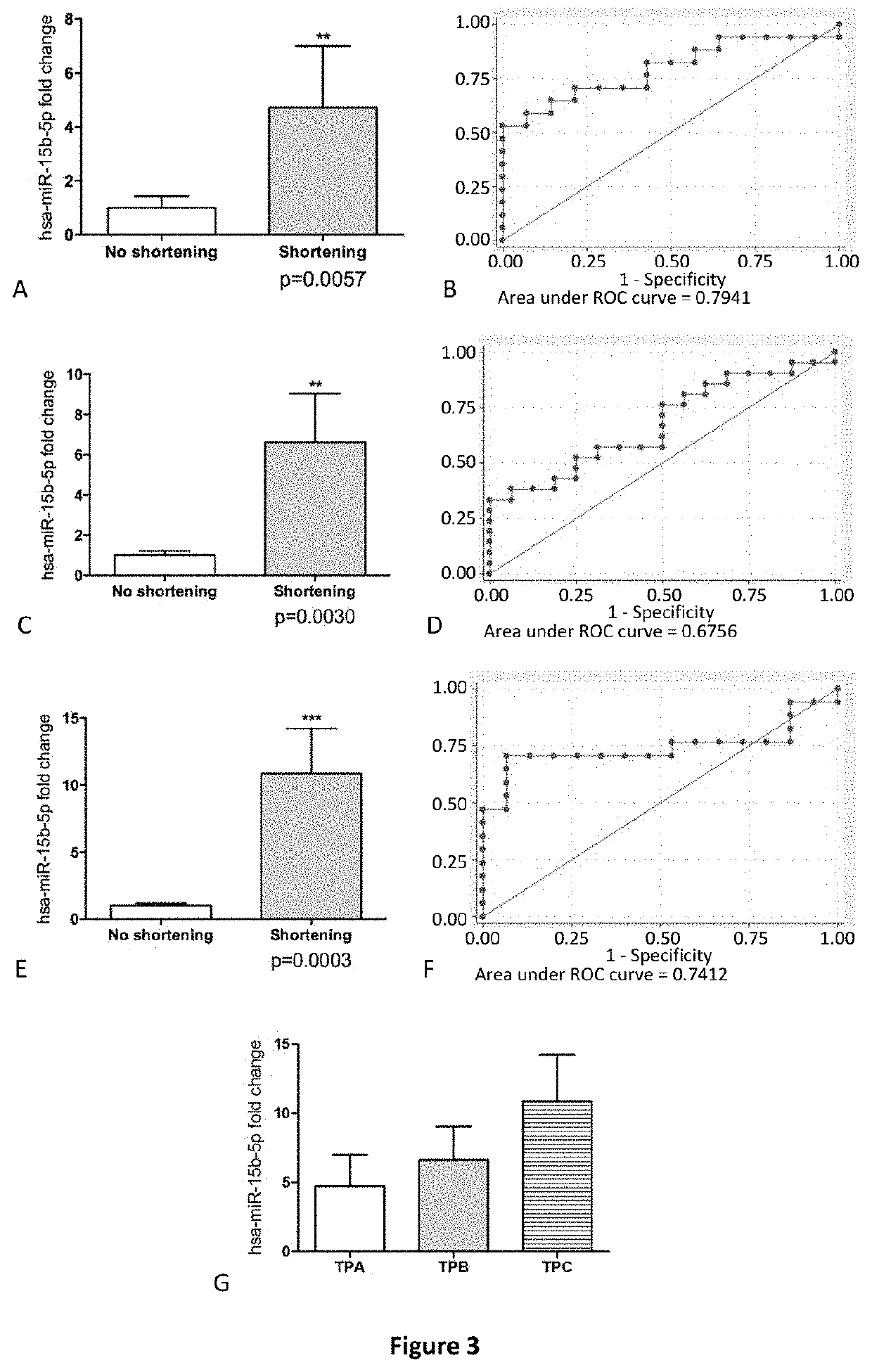
Method for predicting cervical shortening and preterm birth
The present invention provides method for predicting risk of cervical shortening, methods for predicting risk of preterm labour (PTL), and methods for characterising a pregnant subject having a history of previous PTL, mid-trimester loss or cervical cone biopsy as being in need of surveillance and / or intervention to prevent preterm labour, comprising determining the expression level of one or more of the miRNA molecules identified in Table 1 or Table 2 extracted from a biological sample obtained from said subject and comparing to a control value. Biochips and kits for use in carrying out the methods of the invention are also provided.
Owner:IMPERIAL INNOVATIONS LTD
Free radical scavengers or promoters thereof as therapeutic adjuvants in preterm parturition
InactiveUS6852698B2Prevent ROS formationInhibition formationAntibacterial agentsBiocidePremature thelarchePreterm Births
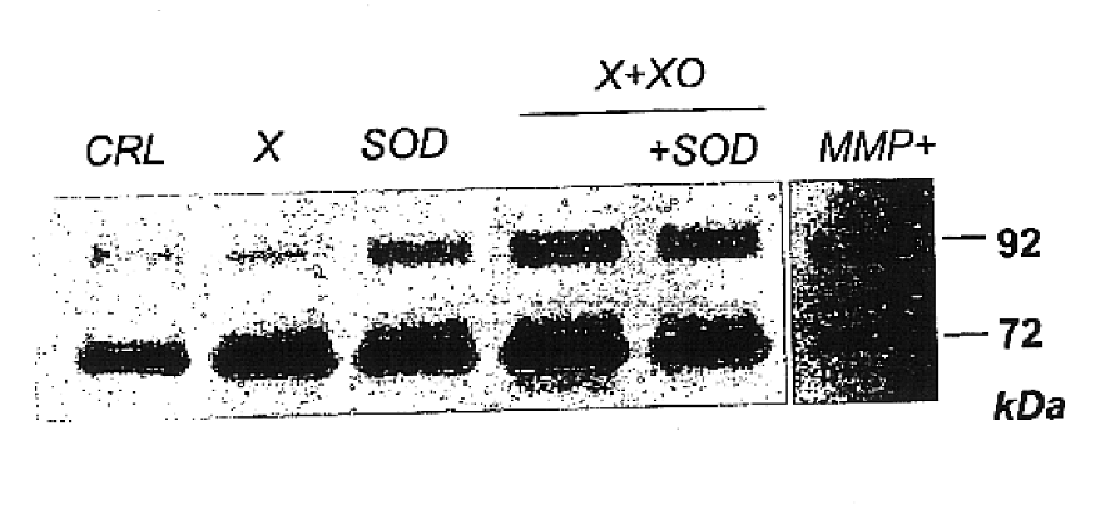
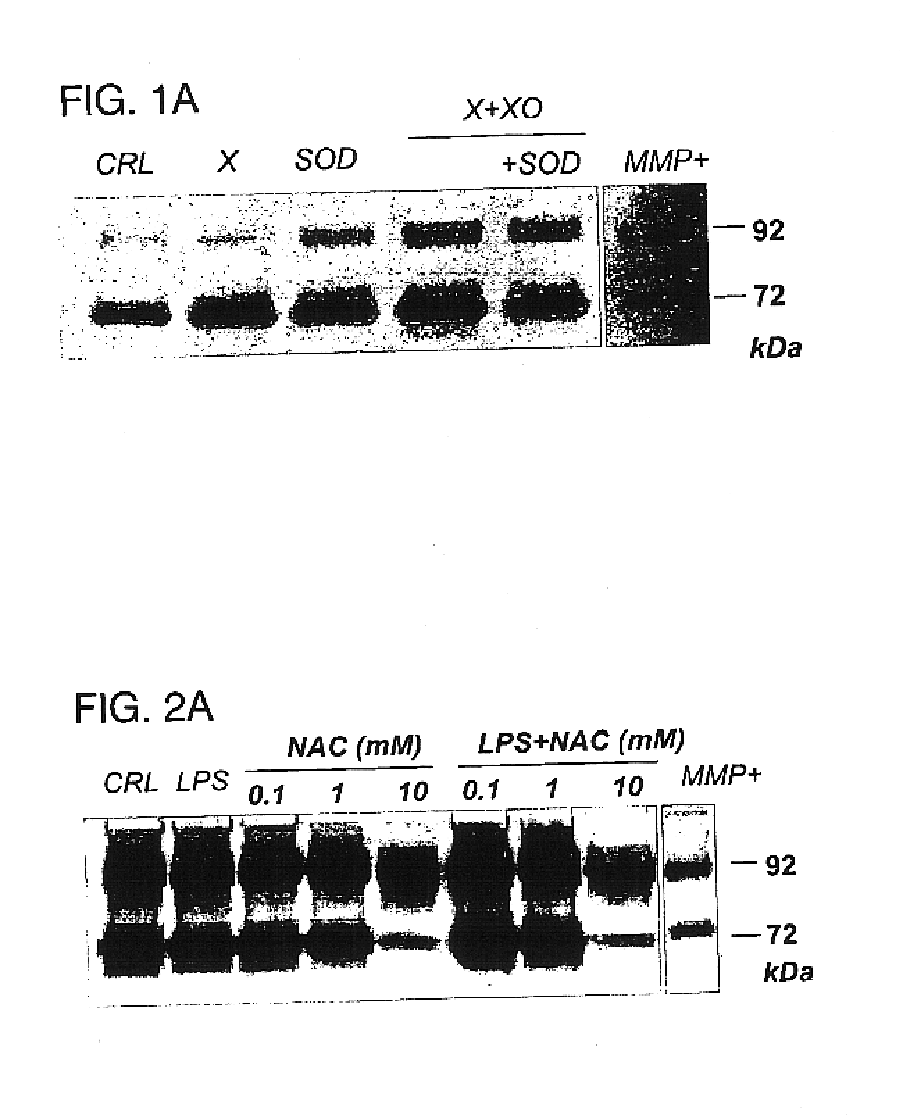
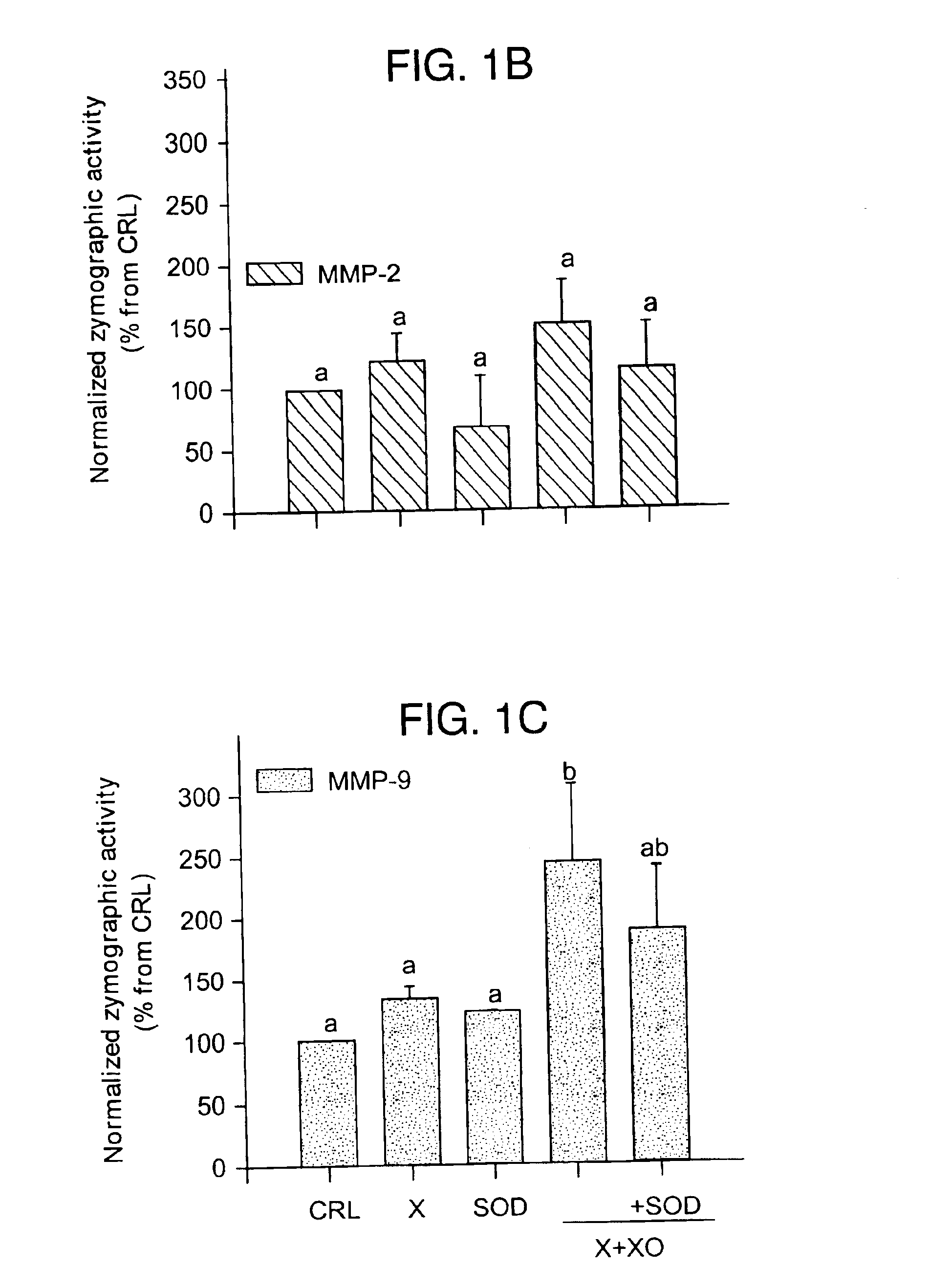
The usage of compounds that improve fetal and neonatal outcome of preterm birth is described. These compounds are scavengers of ROS, their precursors, and agents that induce production of the scavengers. Examples of these compounds are glutathione, NAC, antioxidants, and spin trapping compounds. These compounds improve fetal outcome by inhibiting a fetal inflammatory process that may affect the fetus independently of prematurity. This fetal inflammatory response is characterized by increased cytokine and matrix metalloproteases (MMP) levels both in the mother and fetus and may be modulated by ROS at different levels. Targeting ROS formation with compounds such as specific antioxidants, glutathione or spin trapping compounds, their precursors, and / or agents which induce their production will suppress both the direct effects of ROS and its indirect effects through cytokines and MMPs already circulating in the system. This therapeutical intervention would limit the pathophysiologoical chain of events that ultimately leads to PPROM, preterm birth and / or adverse fetal and neonatal outcome.
Owner:PERINET INC
Progesterone for the prevention of spontaneous preterm birth
A method for treating or preventing spontaneous preterm birth in pregnant women and improving neonatal morbidity and mortality. The method includes administering to a pregnant woman in need thereof an effective amount of a progesterone sufficient to prolong gestation by minimizing the shortening or effacing of her cervix. Treatment and prophylaxis with progesterone in pregnant women having symptoms of short cervix has been clinically shown to increase neonatal health.
Owner:COLUMBIA LAB BERMUDA
Treatment of spontaneous preterm birth
ActiveUS10877046B2Subject is at riskReduce riskComponent separationHealth-index calculationObstetricsPreterm Births
Owner:NX PRENATAL INC
Eva segmented intravaginal rings containing progesterone
PendingUS20210000641A1Avoid overgrowthOrganic active ingredientsSurgeryCervical shorteningPreterm Births
Disclosed herein are segmented EVA Rings that contain progesterone that can be used to prevent preterm birth in subjects with a shortened cervix or to treat luteal phase deficiency or as luteal phase support.
Owner:JUNIPER PHARM INC N K A CATALENT JNP INC
Compositions and methods for predicting risk of preterm birth
PendingUS20190339269A1Reduce riskMicrobiological testing/measurementDisease diagnosisPreterm BirthsSubject Biomarker
Compositions, kits and methods for diagnosing and treating an increased risk of preterm birth (PTB) involves a composition comprising at least one reagent capable of detecting, binding, specifically complexing with, or measuring the level of one of a subject bacterium in a sample. Optionally, a composition of the invention further comprises a reagent capable of detecting, binding, specifically complexing with, or measuring the expression of a subject biomarker.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Treatment of spontaneous preterm birth
ActiveUS10928402B2Reduce riskIncreased riskHydroxy compound active ingredientsDisease diagnosisGynecologyObstetrics
The present disclosure relates to biomarkers of preterm birth, biomarkers of term birth, and methods of use thereof. In particular, the present disclosure provides methods of determining whether a pregnant woman is at an increased risk for premature delivery. The present disclosure further provides methods for decreasing a pregnant woman's risk for premature delivery.
Owner:NX PRENATAL INC
Use of circulating microparticles to stratify risk of spontaneous preterm birth
ActiveUS20190041391A1Subject is at riskReduce riskComponent separationUltrafiltrationObstetricsPreterm Births
The present disclosure relates to proteomic biomarkers of spontaneous preterm birth, proteomic biomarkers of term birth, and methods of use thereof. In particular, the present disclosure provides tools for determining whether a pregnant subject is at an increased risk for premature delivery, as well as tools for decreasing a pregnant subject's risk for premature delivery.
Owner:NX PRENATAL INC
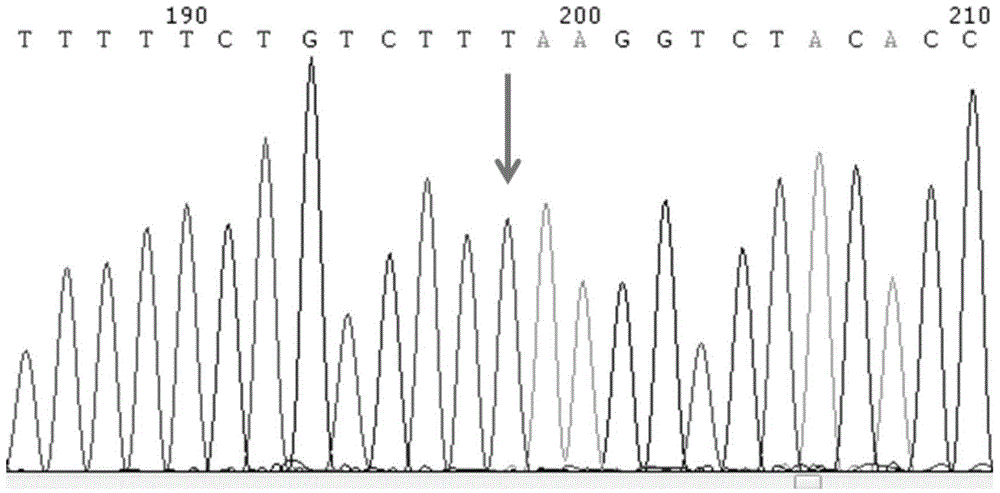
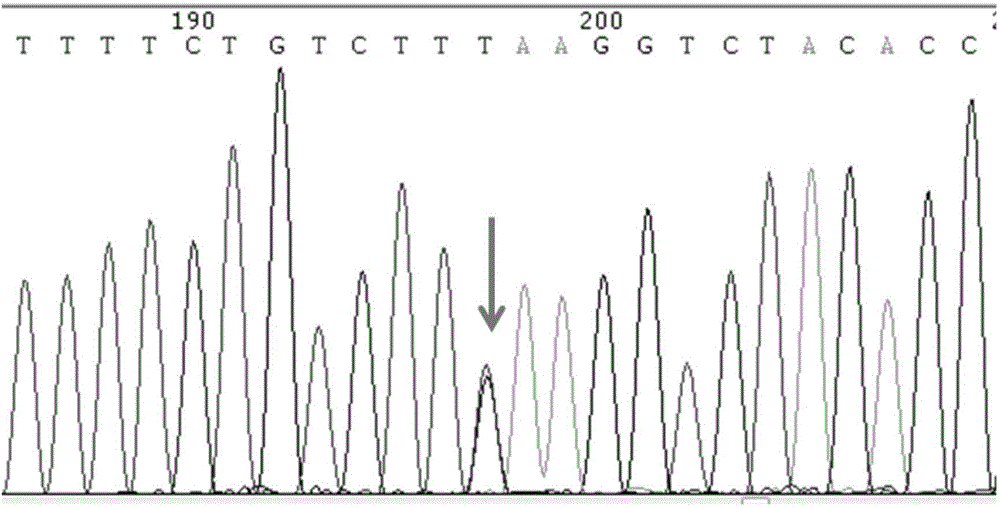
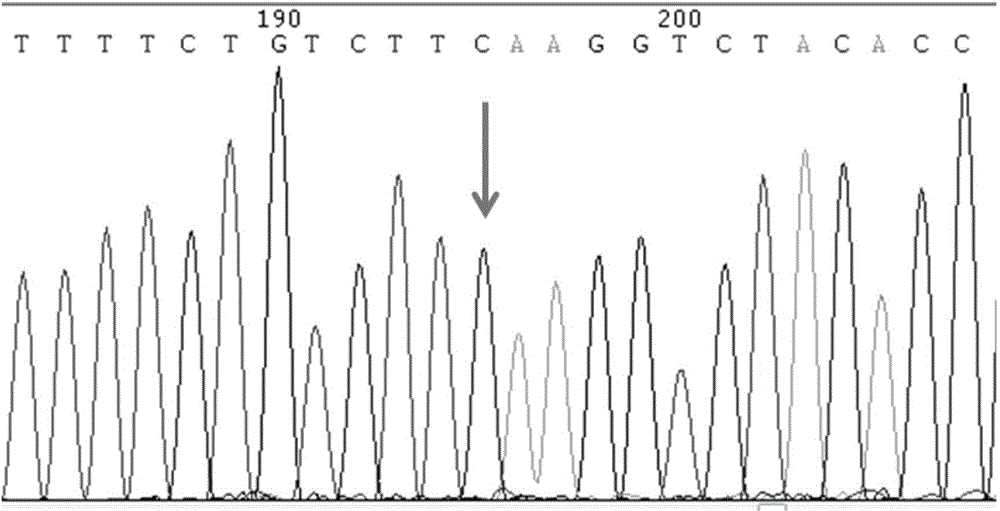
SNP molecular marker of RANTES gene related to generation of preterm birth and kit for detecting RANTES gene
InactiveCN104630217AGood clinical valueMicrobiological testing/measurementDNA/RNA fragmentationPreterm BirthsNucleotide
The invention provides a SNP molecular marker of RANTES gene related to generation of preterm birth and a kit for detecting the RANTES gene. According to the invention, statistic analysis of a large number of clinical samples discovers that polymorphic site In1.1T / C of RANTES gene is related to generation of preterm birth. The invention also provides the kit for detecting the polymorphic site In1.1T / C of the RANTES gene. Based on a PCR method, a pair of primers are designed, nucleotide sequence is shown in SEQ ID NO.1-2, PCR amplification is carried out on to-be-detected sample genome DNA, and direct sequencing is carried out on the amplified product to be used for determining genotype of the polymorphic site In1.1T / C of the RANTES gene. The kit is simple to operate, high in sensitivity, strong in accuracy and low in detection cost and has important application value.
Owner:THE MILITARY GENERAL HOSPITAL OF BEIJING PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com