Progesterone for the prevention of spontaneous preterm birth
A technology for hydroxyprogesterone, pregnant women, in the field of progesterone for the prevention of preterm birth, which can solve the problem of unproven newborn health benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0067] Example 1a. A randomized, double-blind, placebo-controlled trial of the effect of progesterone on preterm birth
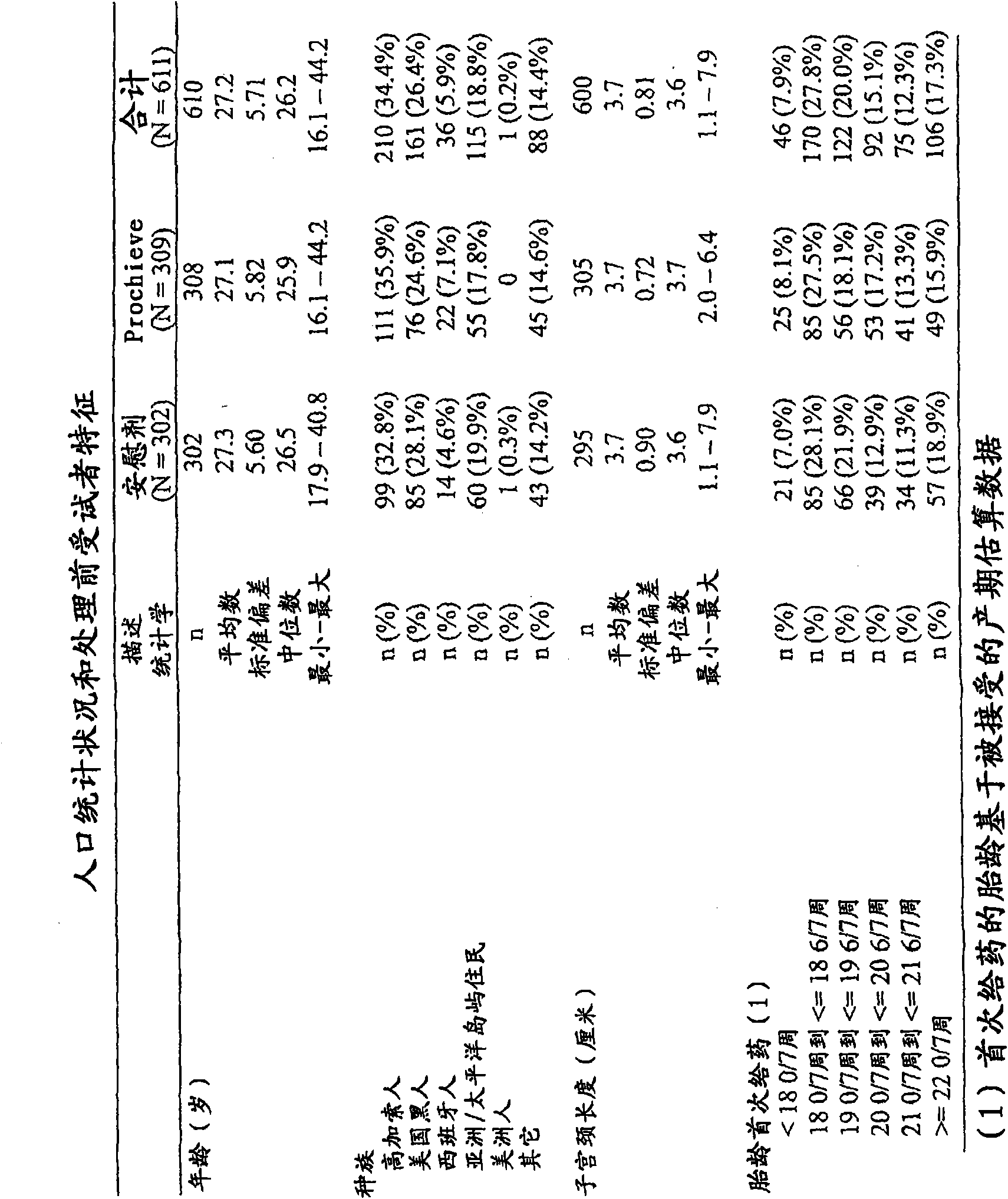
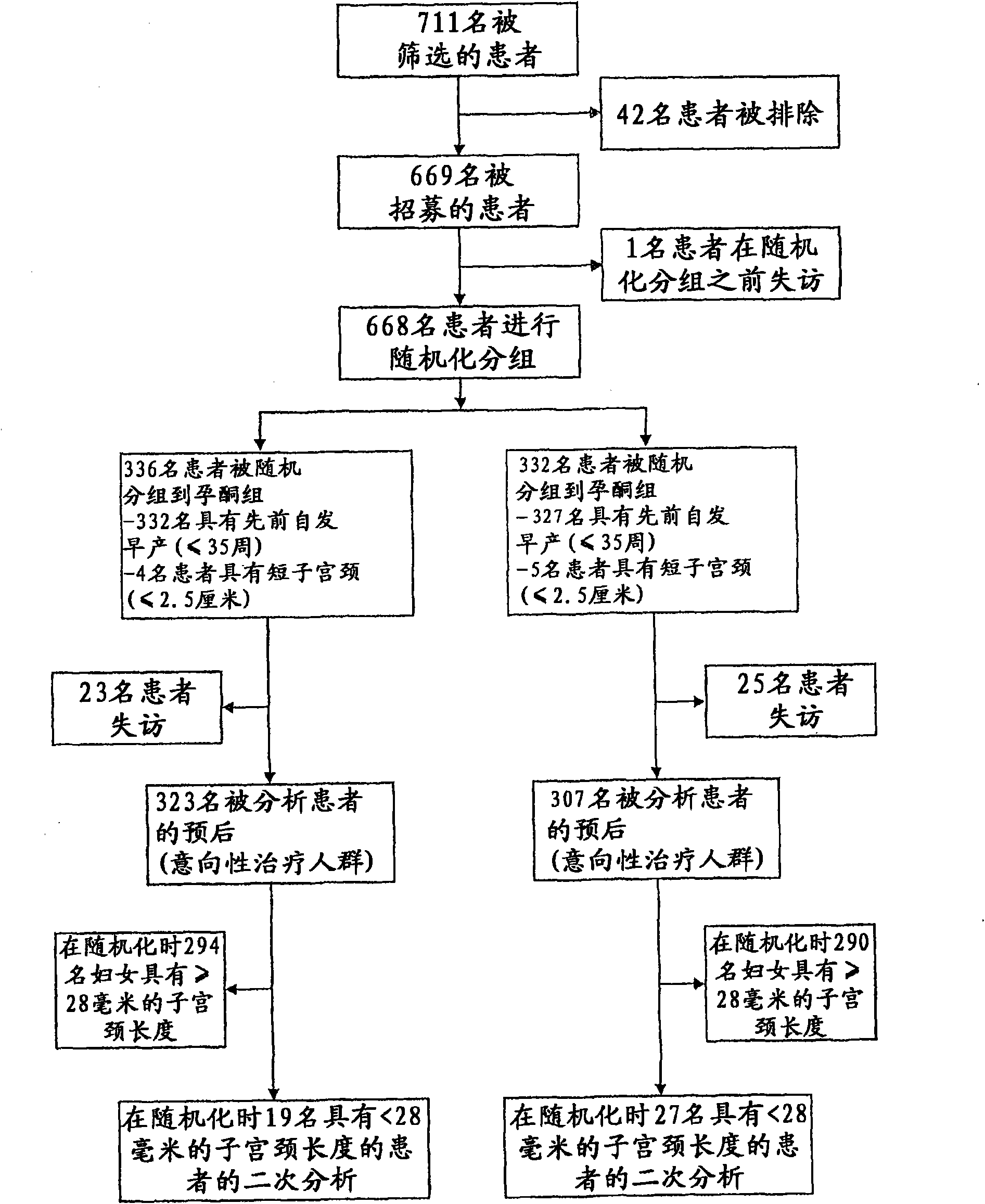
[0068] Based on the current state of the art, a study was conducted to examine the effect of progesterone on preterm labor and preterm birth in women with a previous history of preterm birth. The baseline data of the study are figure 1 shown. Participants in the study included 611 evaluable pregnant women, of whom 308 were selected for the treatment group and 302 were selected for the placebo group. The selection, classification and subdivision of the two groups are figure 2 outlined in the flowchart. The study is a prospective, randomized, placebo-controlled, double-blind, multicenter trial in pregnant subjects at high risk of spontaneous preterm birth. Study participants were screened from 160 / 7 and 226 / 7 gestational weeks. Subjects were randomized from 180 / 7 to 226 / 7 gestational weeks to receive drug or placebo.
[0069] Subjects meeting the study ...
Embodiment 1b
[0071] Example 1b. Follow-up of a randomized, double-blind, placebo-controlled trial on the effect of progesterone on preterm birth
[0072] The data presented in this example provides a further analysis of the data presented in Example 1a. The purpose of this trial was to determine whether prophylactic administration of vaginal progesterone reduces the risk of preterm birth in women with a history of spontaneous preterm birth.
[0073] The trial was a randomized, double-blind, placebo-controlled multinational trial that enrolled and randomized 659 pregnant women with only a history of spontaneous preterm birth.
[0074] Between 180 / 7 and 226 / 7 gestational weeks, patients were randomized to once-daily treatment with 8% progesterone vaginal gel or placebo until delivery, 37 weeks of gestational age, or the occurrence of premature rupture of membranes ( PROM). The primary outcome was preterm birth at ≤32 gestational weeks. Statistical analysis was based on the intent-to-treat...
Embodiment 2
[0087] Example 2. A randomized trial investigating the efficacy of vaginal progesterone for preventing premature preterm birth in women with a shortened cervix in the second trimester.
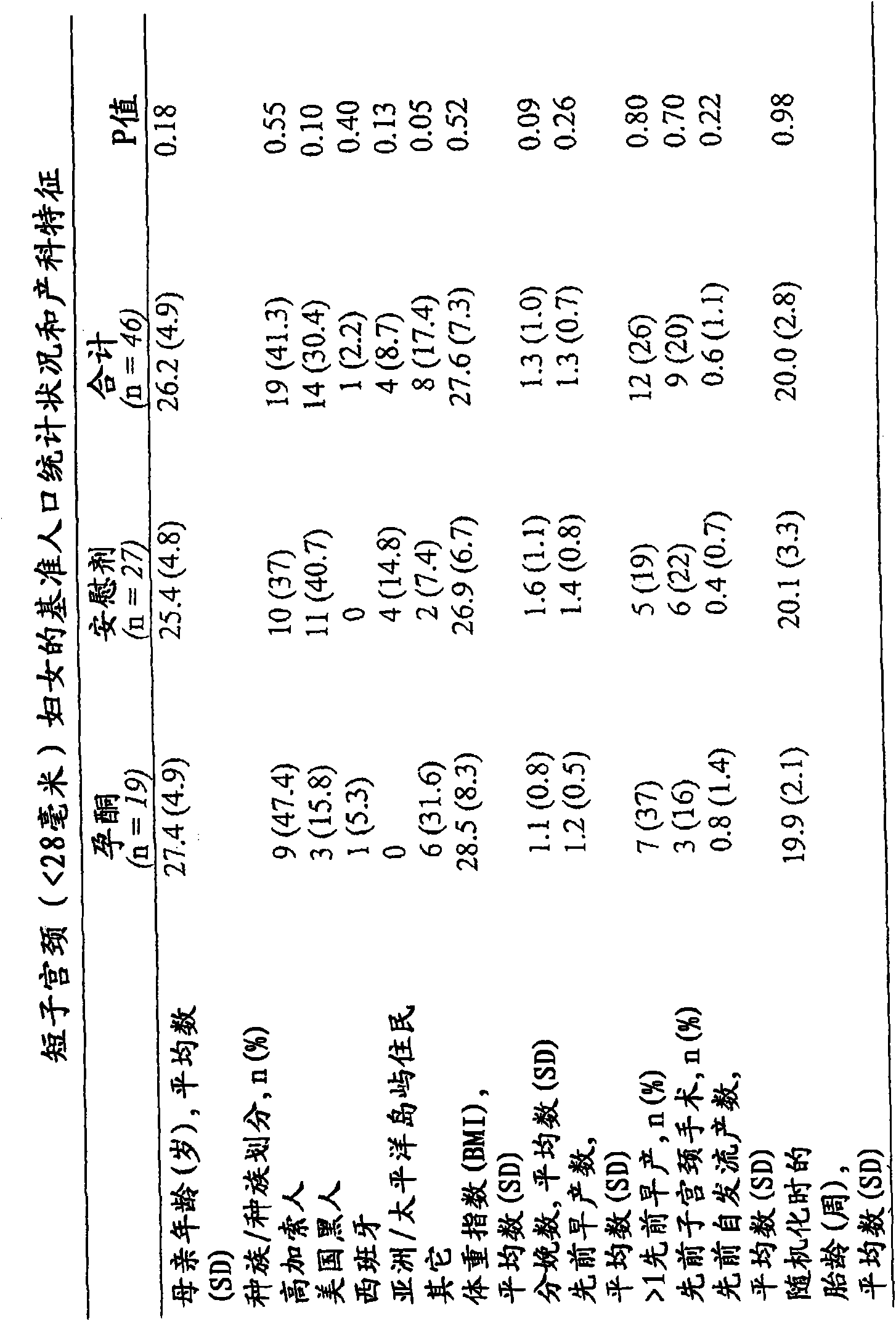
[0088] The study further included women with no history of preterm birth but with a short cervix at enrollment. Although the population with only a short cervix included 9 patients, the results suggest a possible effect of progesterone: 40% (2 / 5) delivery rates at ≤32 weeks with placebo and ≤32 weeks with progesterone The delivery rate at 32 weeks was 0 (0 / 4). Because some participants with a prior history of preterm birth also had a short cervix, the preterm study population was divided into quartiles based on cervical length. The lowest quartile (≤3.2cm) and patients with only short cervix were pooled and subdivided sequentially, primary outcomes and secondary outcomes for women with cervical length ≤3.0cm and image 3 shown.
[0089] Generally, cervical length <2.8 cm was determined in 46...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com