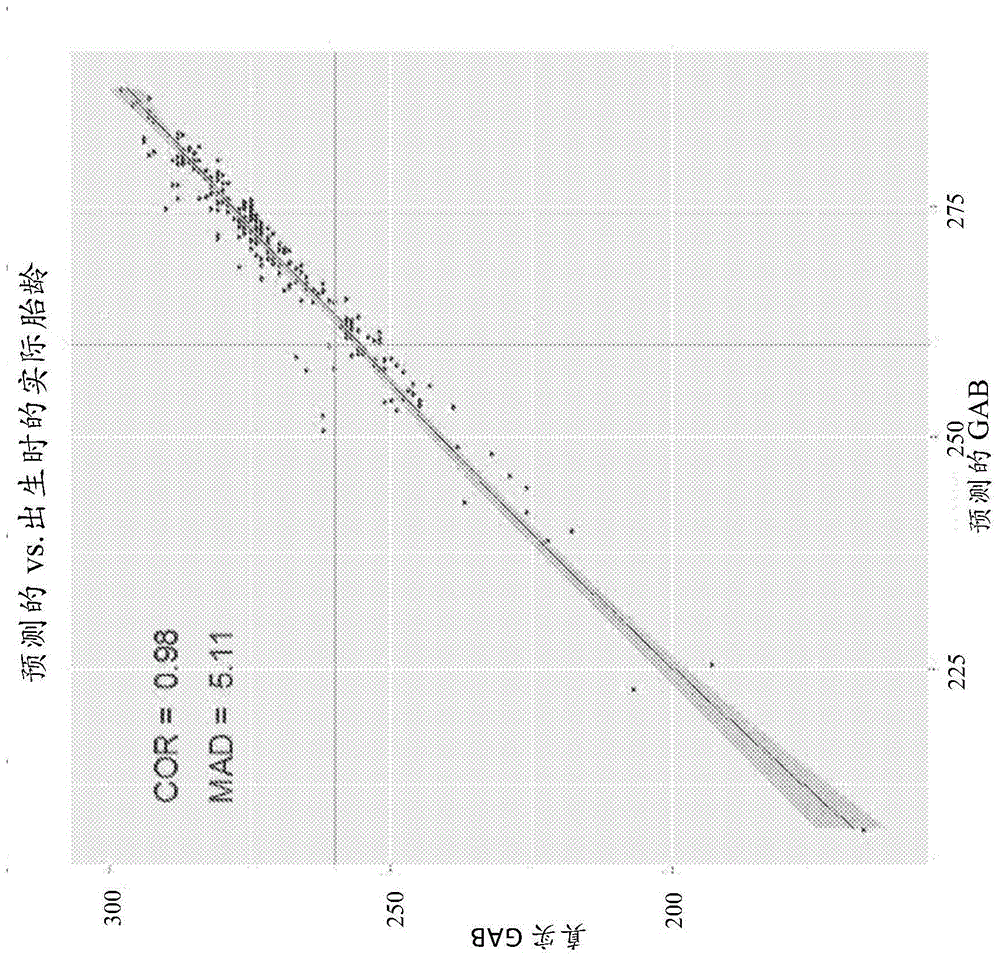
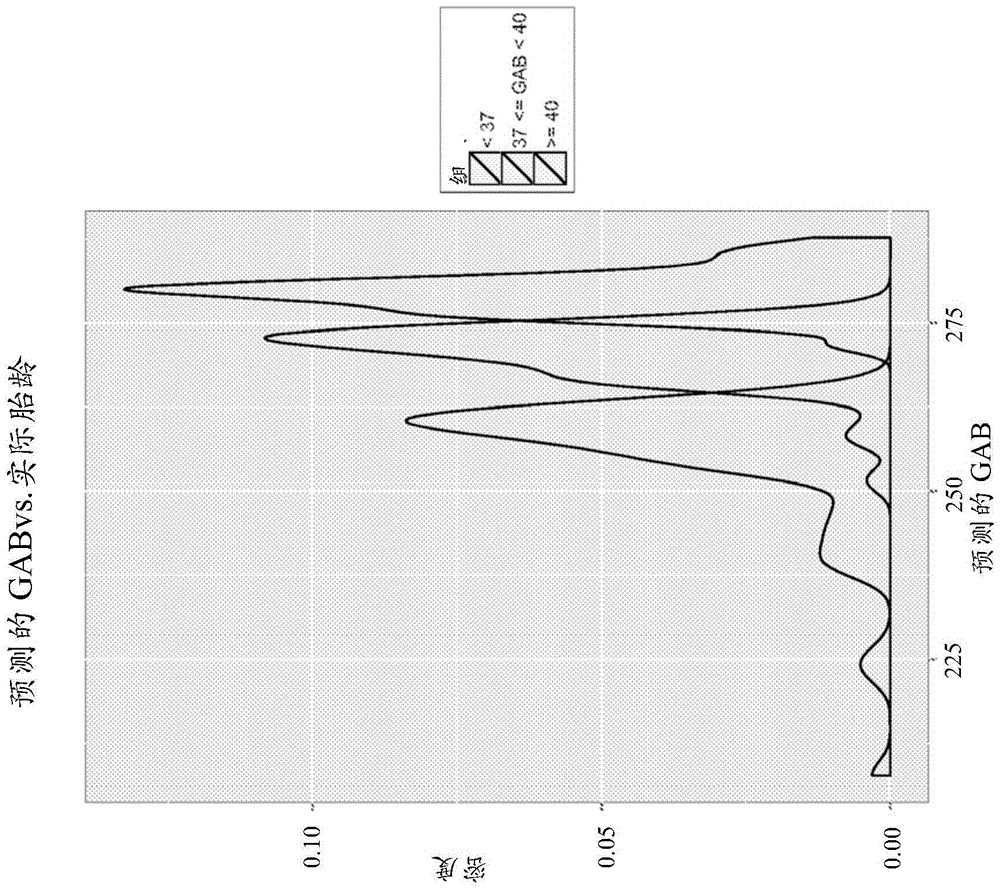
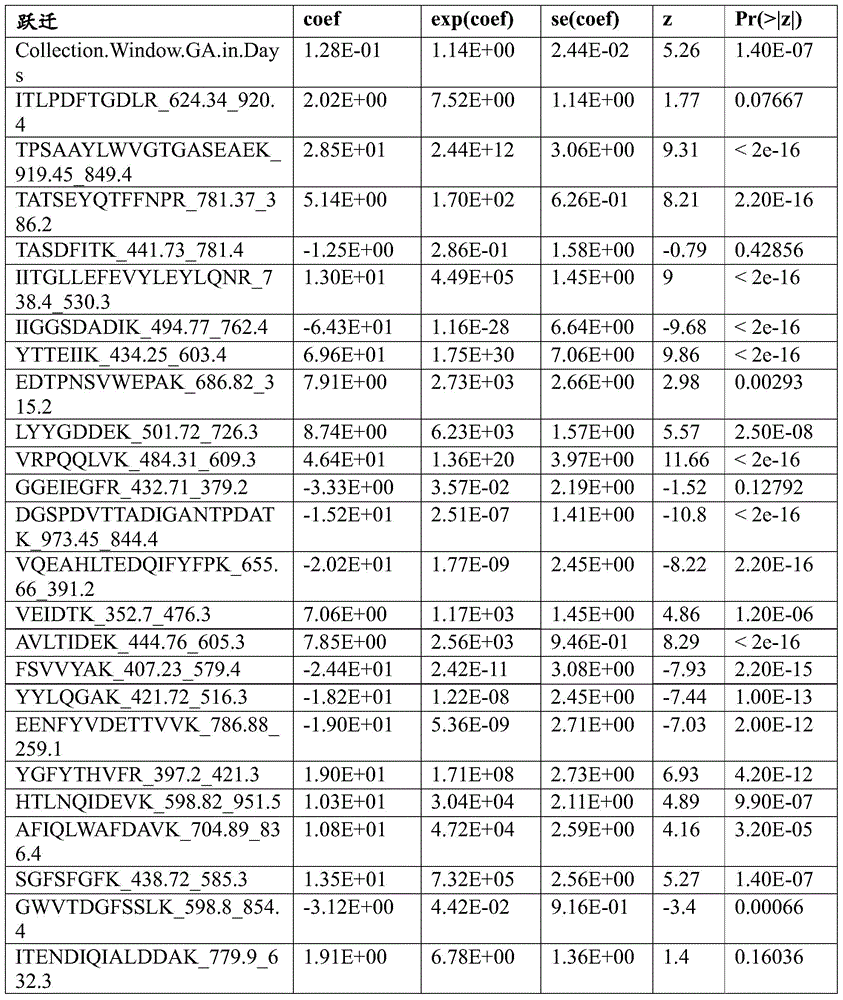
Biomarkers and methods for predicting preterm birth
A technology of biomarkers and derivatives, applied in the field of personalized medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1. Establishment of a sample set for the discovery and validation of preterm biomarkers
[0168] Establishment of standard protocols governing the conduct of proteomic evaluation of preterm birth risk (PAPR) clinical studies. The protocol also stipulates that samples and clinical information may be used to study other pregnancy complications in some subjects. Samples were obtained from women at 11 Internal Review Board (IRB)-approved sites throughout the United States. After providing informed consent, serum and plasma samples were obtained, along with relevant information on patient demographics, past medical and pregnancy history, current pregnancy history, and concurrent drug therapy. After delivery, data are collected on maternal and infant conditions and complications. Serum and plasma samples were processed according to protocols requiring standardized refrigerated centrifugation, aliquoting of samples into 0.5 mL 2-D barcoded freezing vials and subseque...
Embodiment 2
[0175] Example 2. Analysis of transitions to identify biomarkers of preterm birth I
[0176] The goal of these analyzes was to examine the data collected in Example 1 to identify transitions and proteins that are predictive of preterm birth. The specific analyzes used were (i) Cox time-event analysis and (ii) a model with preterm birth as the binary categorical dependent variable. For all Cox analyses, the dependent variable was gestational age at time of event (where event was preterm birth). For the purposes of the Cox analysis, preterm subjects had an event on the day of delivery. Full-term subjects were examined on the day of delivery. Gestational age on the day of sample collection was a covariate in all Cox analyses.
[0177] Assay data were previously adjusted for run order and batch removal, and log transformed. Values for gestational age at sample collection were adjusted as follows. Only controls (non-preterm subjects) were used to regress the jump for gestati...
Embodiment 3
[0197] Example 3. Study II to Identify and Validate Biomarkers of Preterm Birth
[0198] Further studies were performed using essentially the same methods described in the examples above, unless noted below. In this study, 2 gestational matched controls were used for each of the 28 cases and 56 matched controls, all samples were from the early gestational window (17-22 weeks).
[0199] Samples were processed in 4 batches, each containing 7 cases, 14 matched controls and 3 HGS controls. The 14 most abundant serum samples among the serum samples were removed by MARS14 as described in Example 1. The depleted serum was then reduced with dithiothreitol, alkylated with iodoacetamide, and hydrolyzed overnight at 37°C with trypsin at a trypsin:protein ratio of 1:20. After trypsin hydrolysis, samples were desalted on Empore C18 96-well solid phase extraction plates (3M Company) and lyophilized. Desalted samples were redissolved in reconstitution solution containing 5 internal standa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com