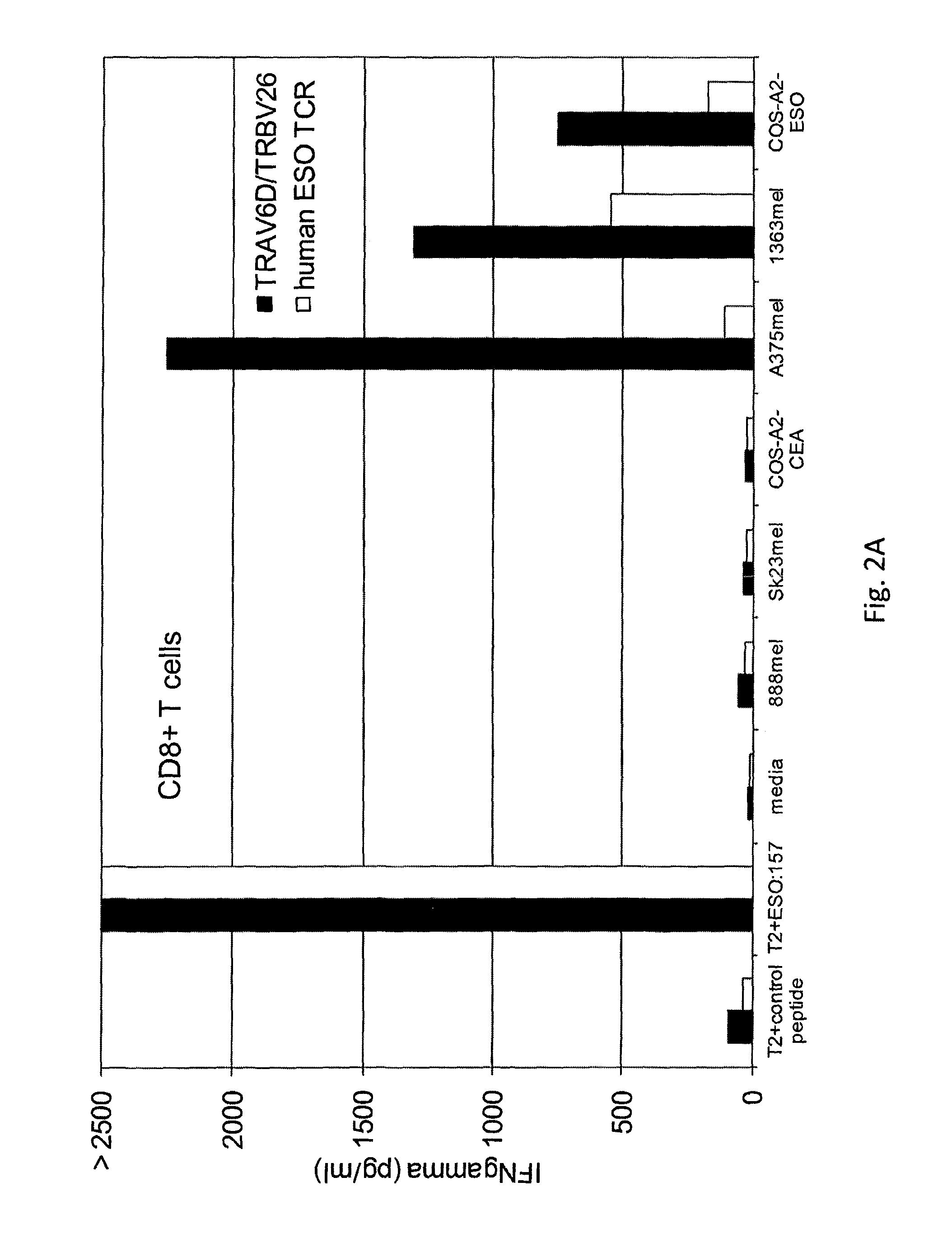
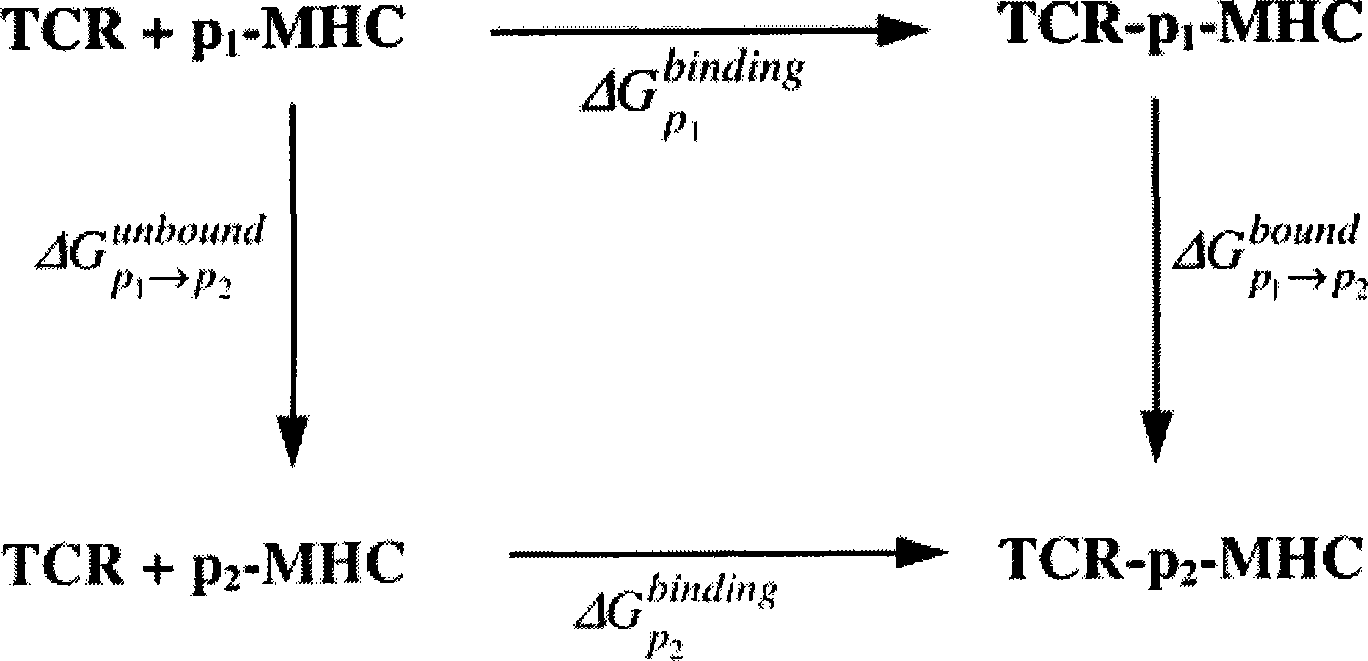
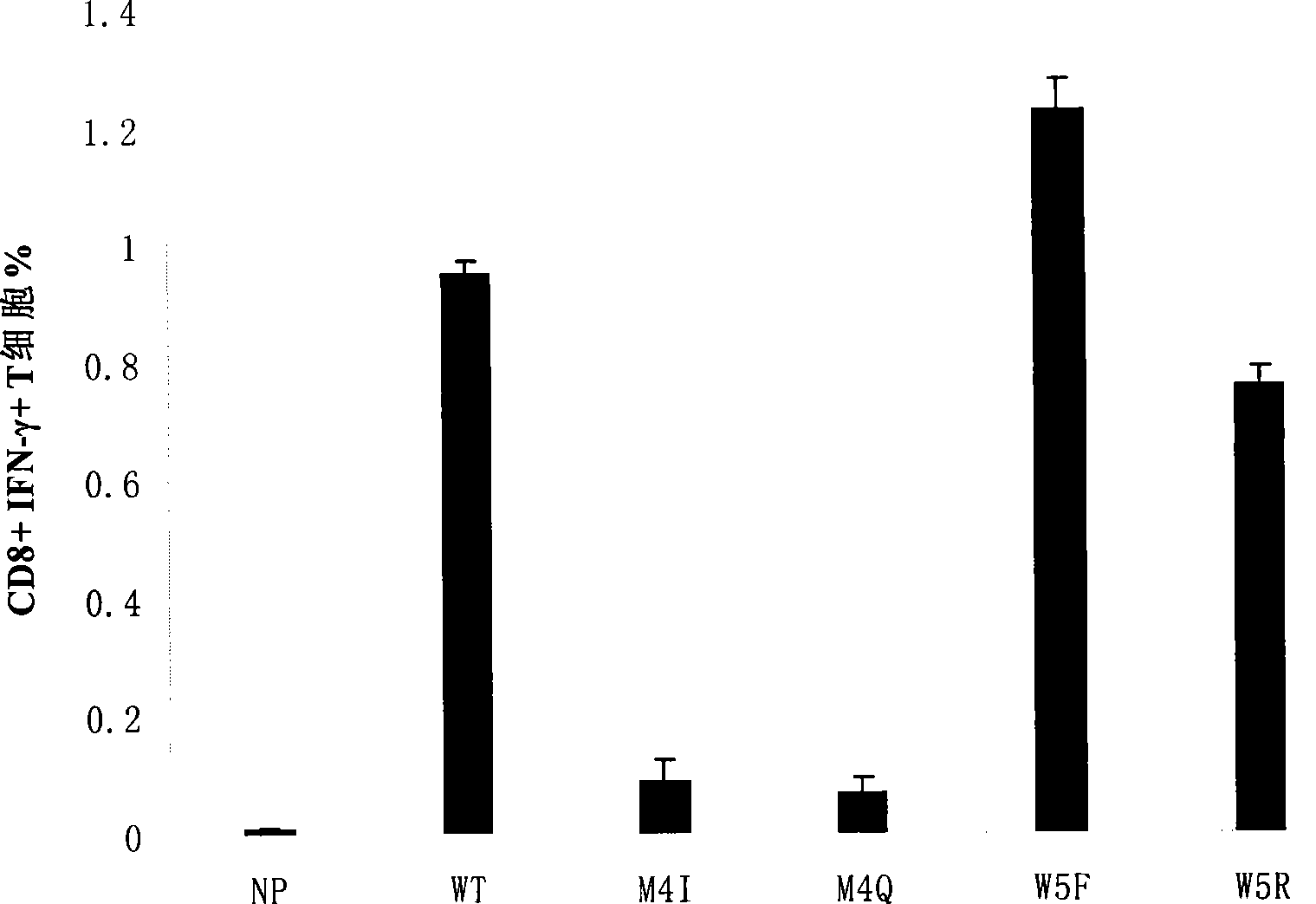
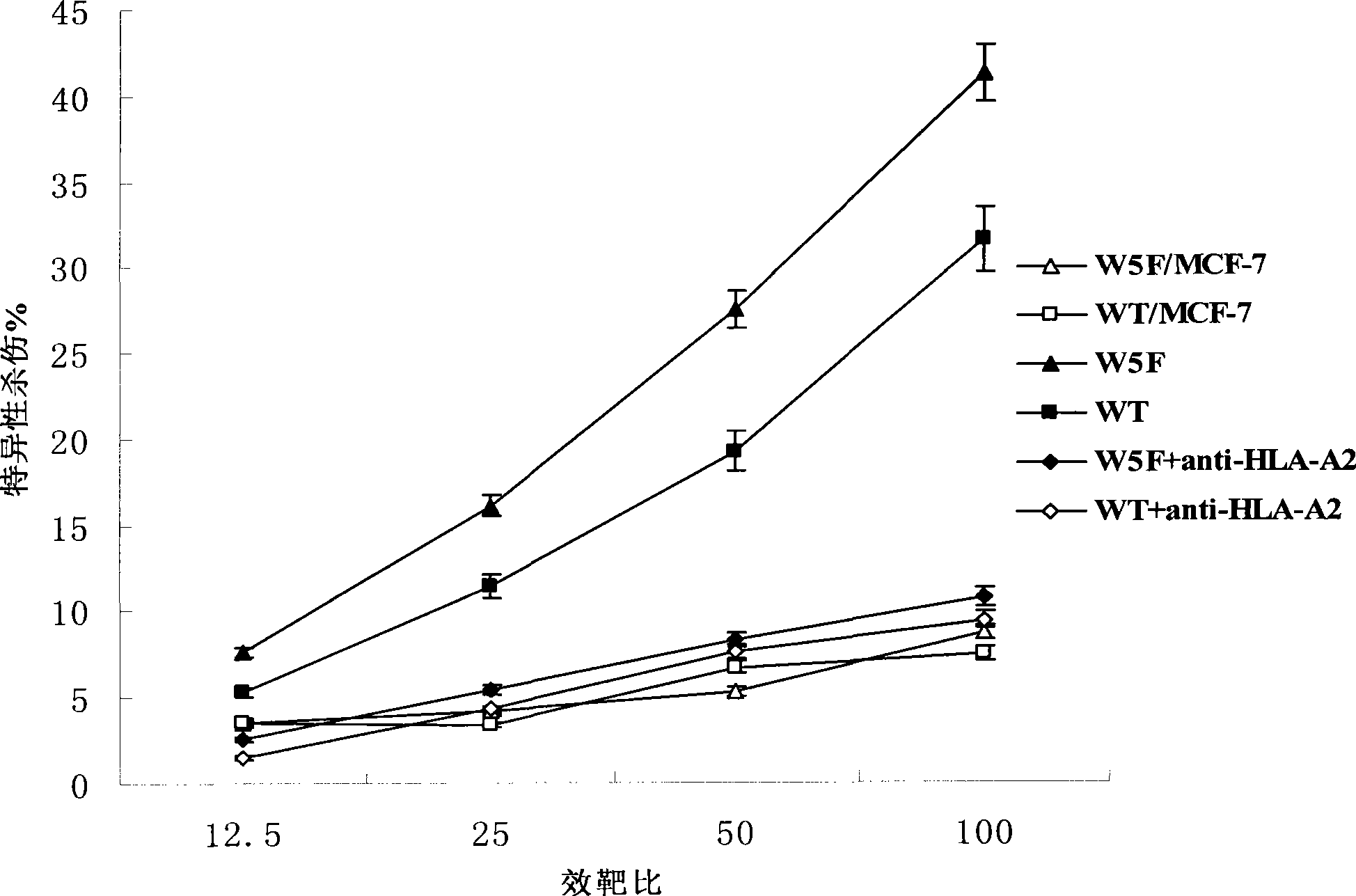
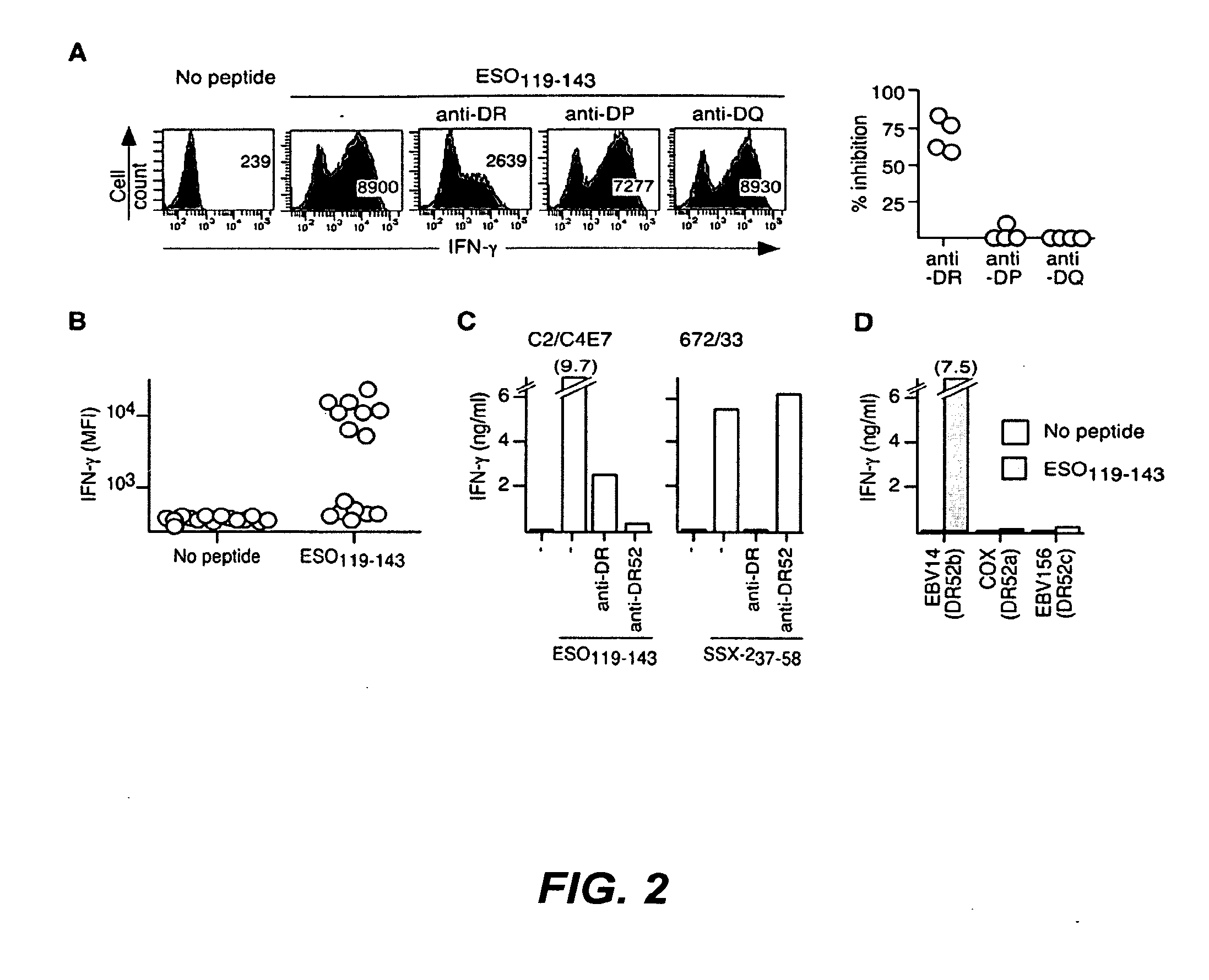
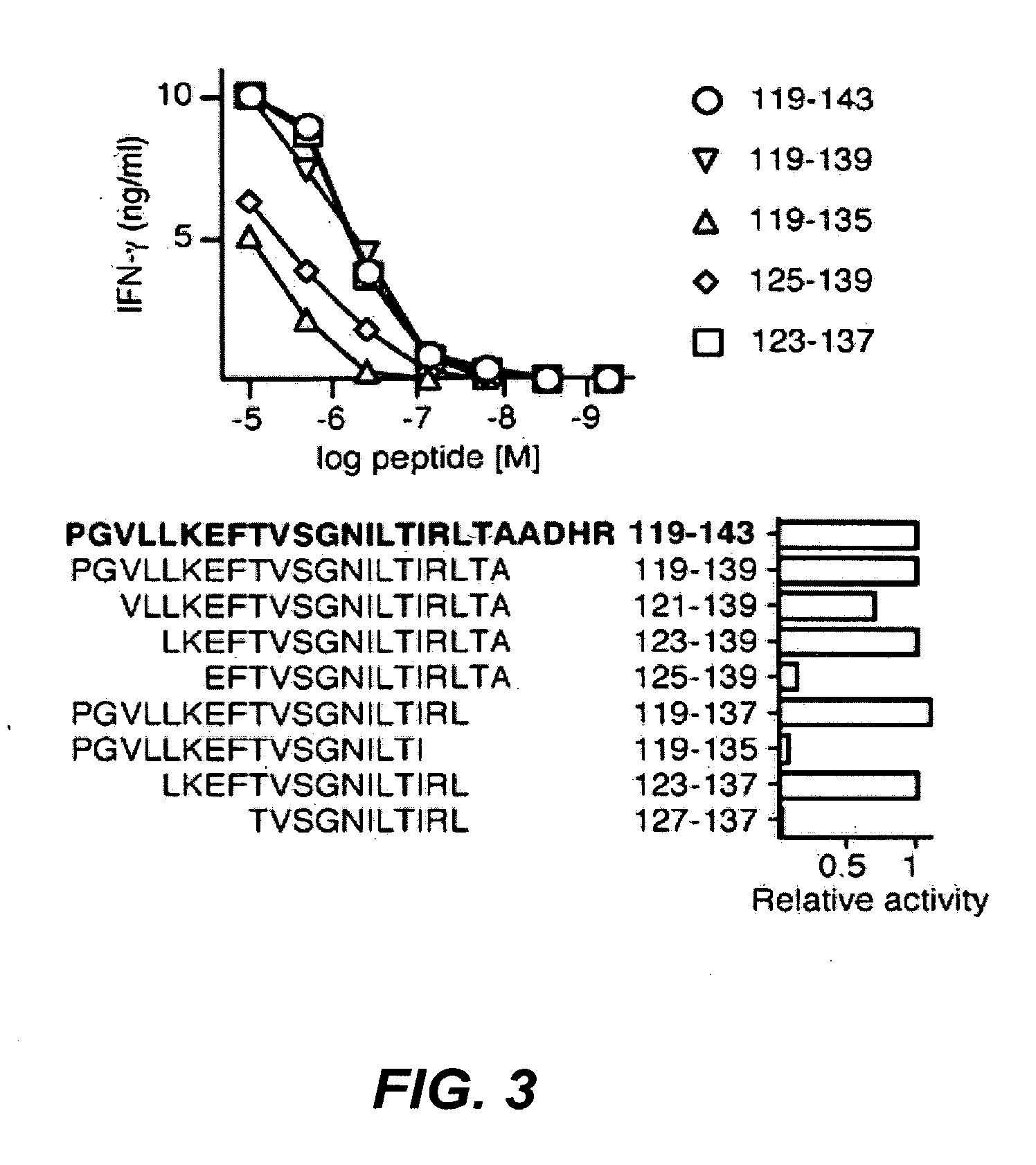
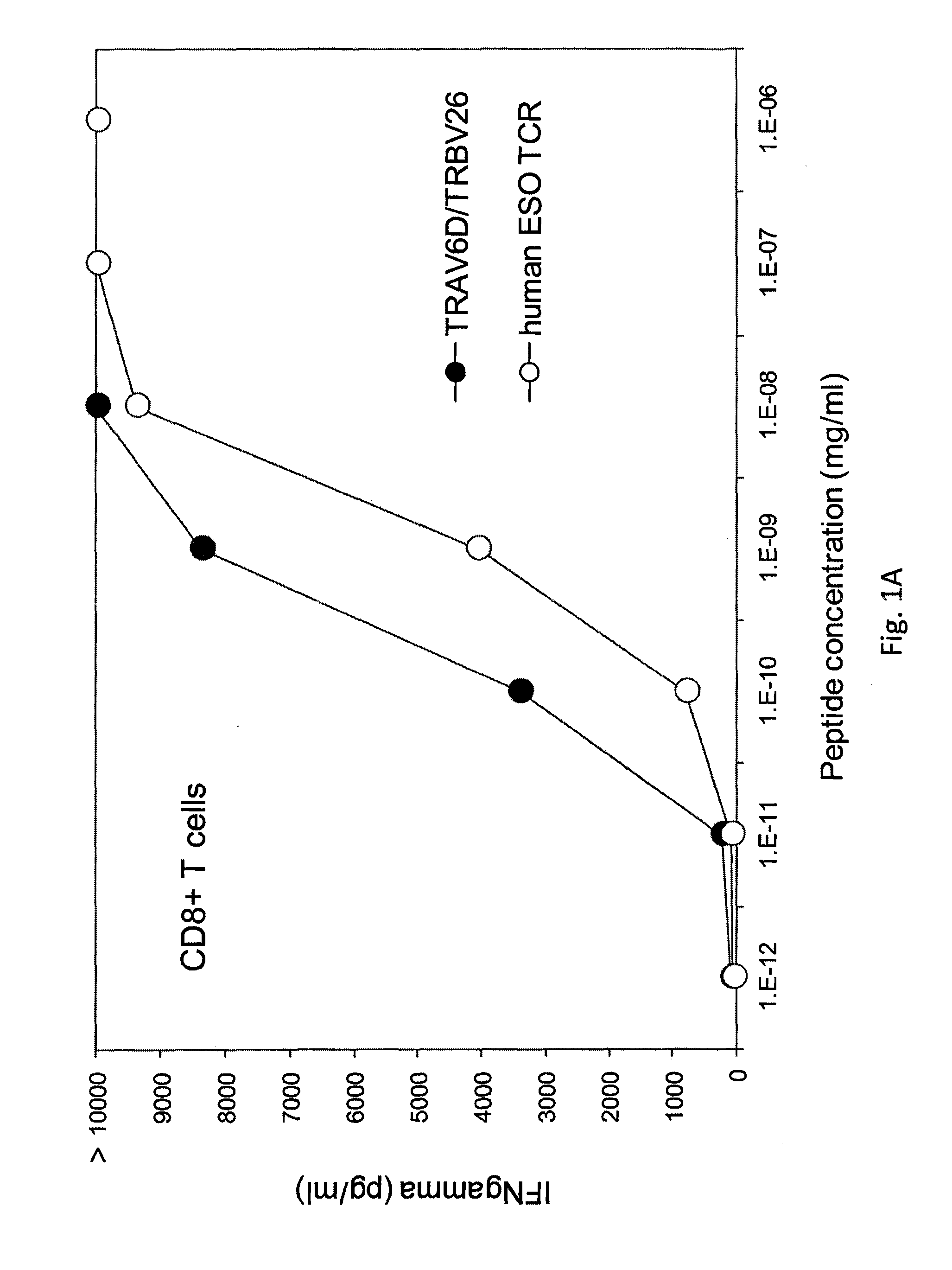
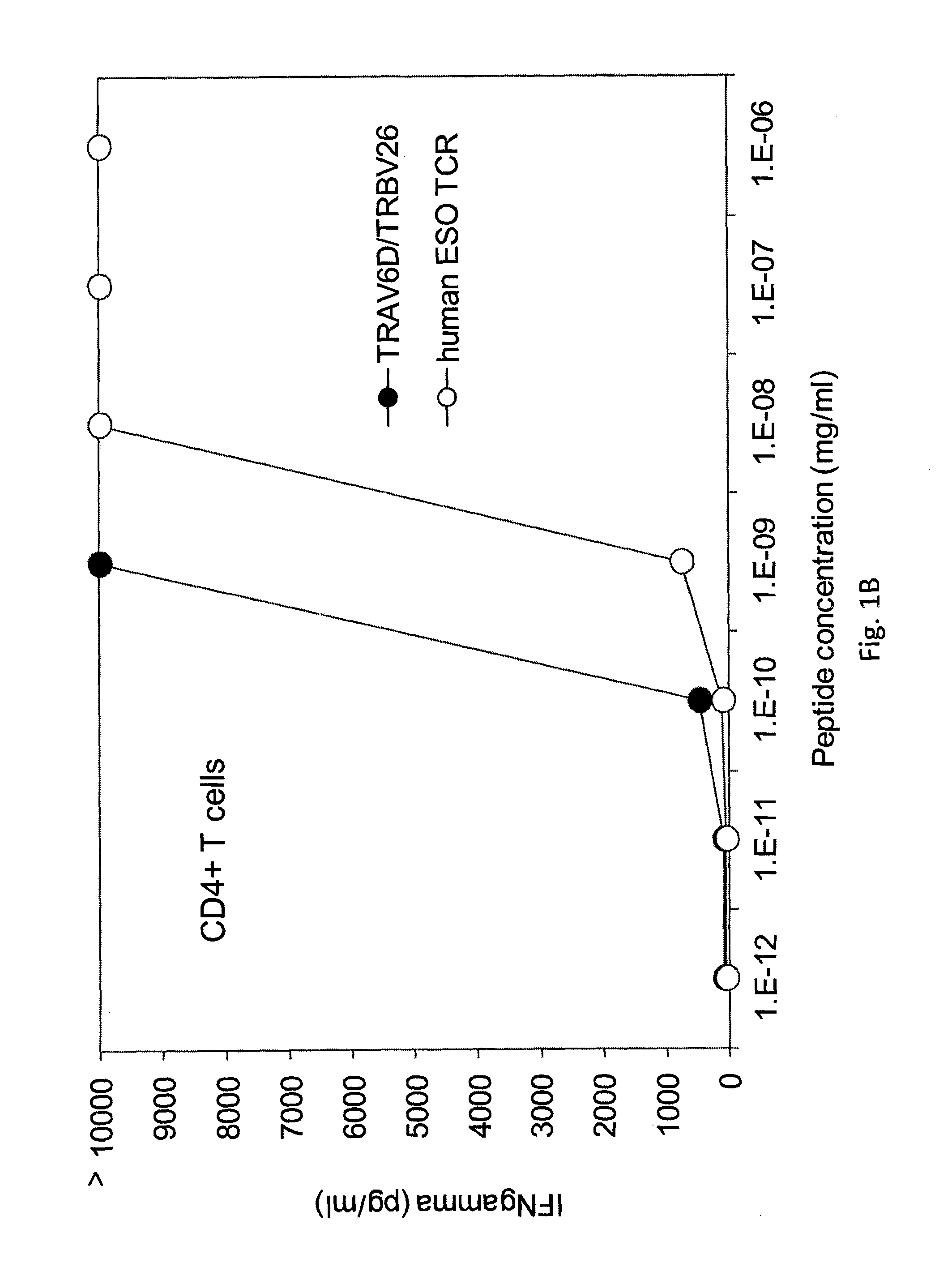
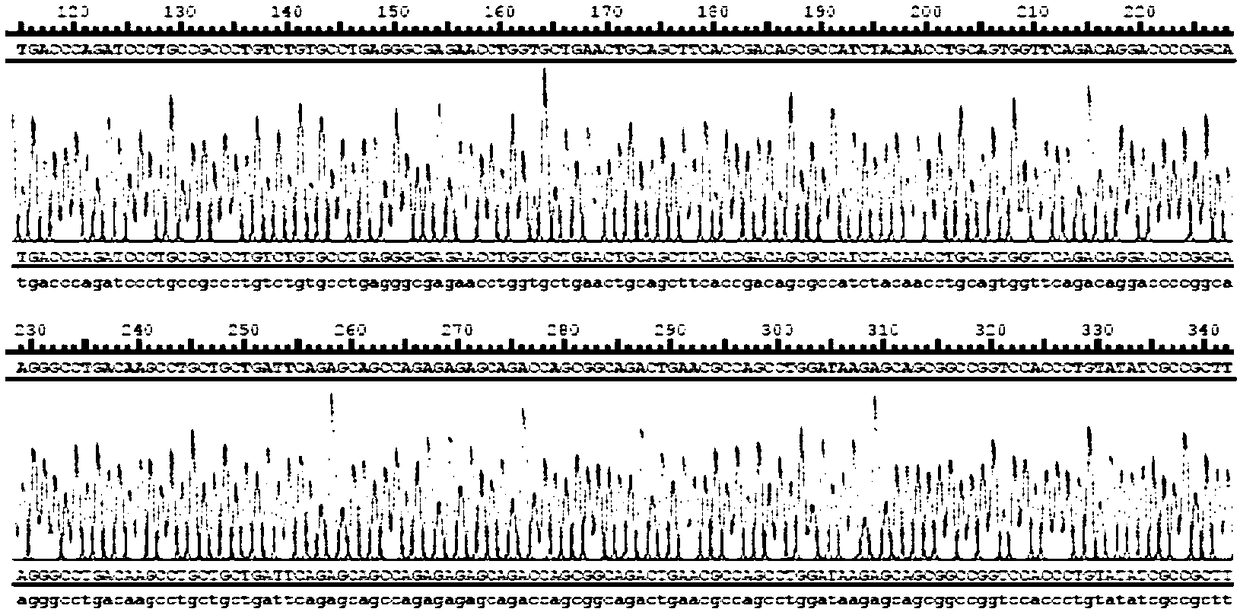
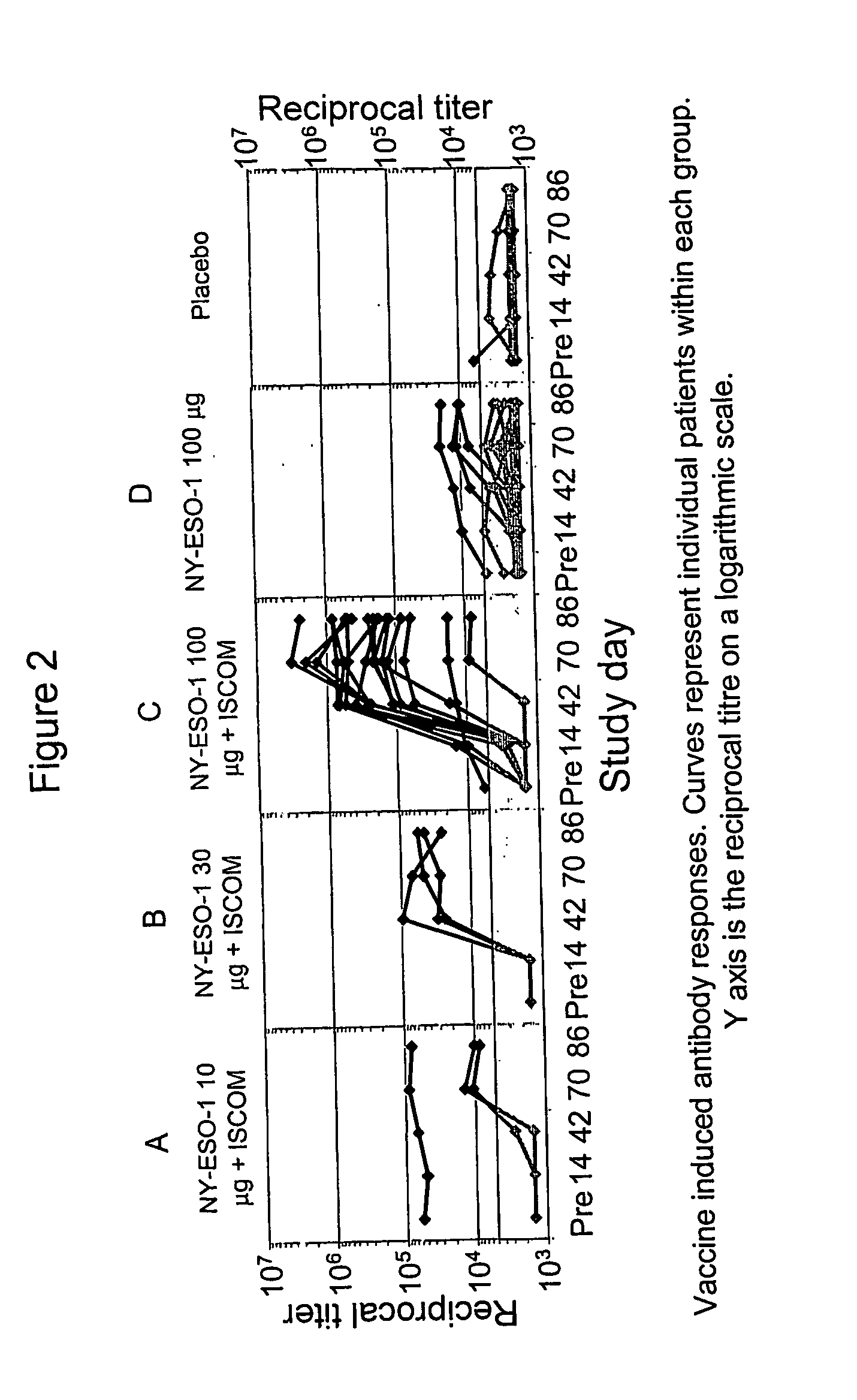
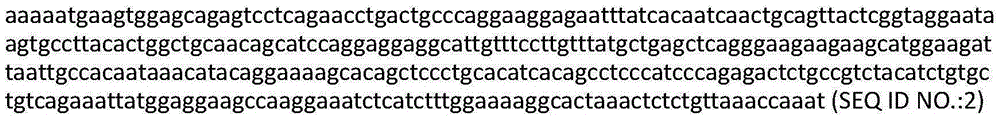Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "NY-ESO-1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
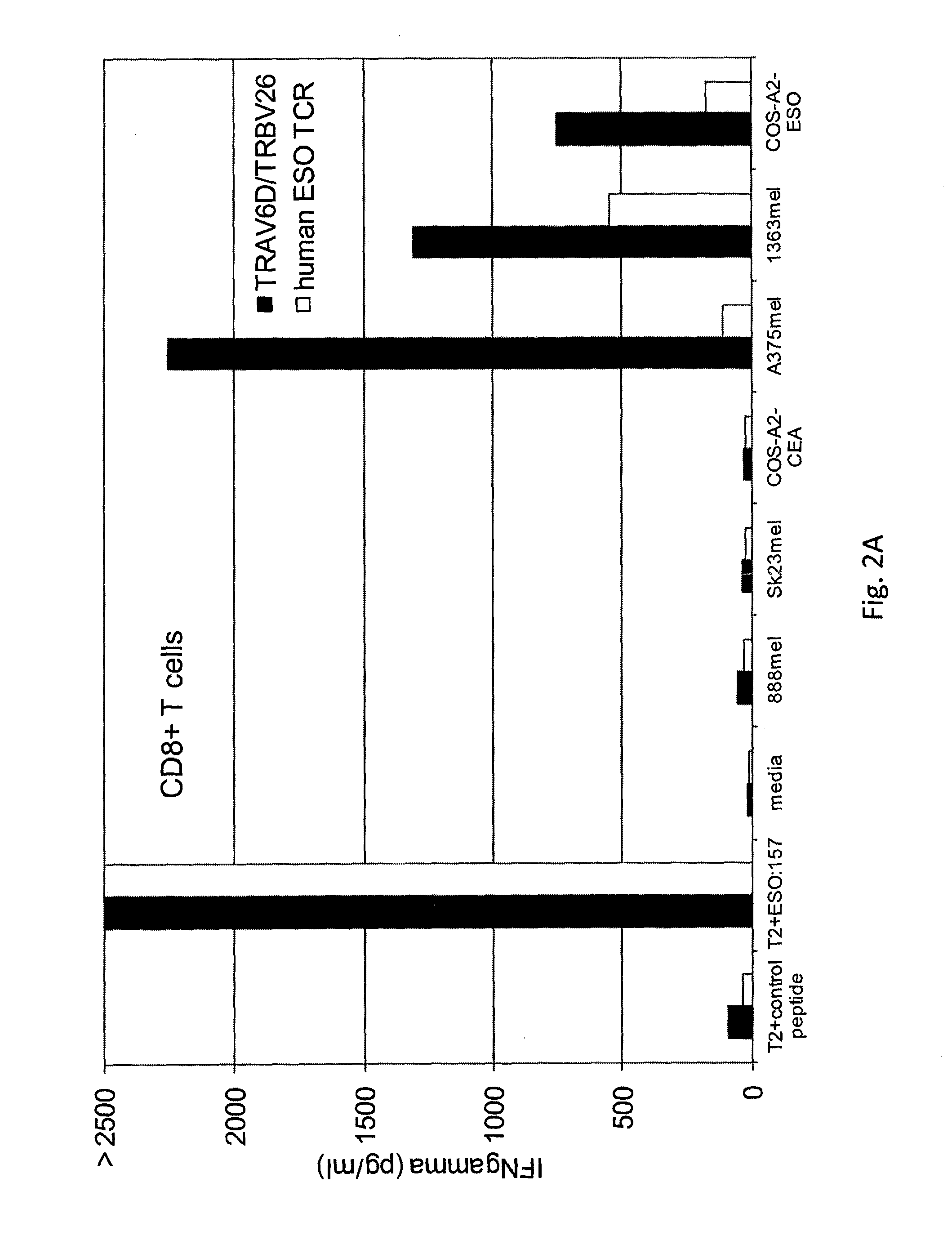
Compositions comprising t cell receptors and methods of use thereof
InactiveUS20090053184A1High affinityEfficient killingOrganic active ingredientsBiocideMelanomaBiology
Nucleic acids encoding antitumor TCRs recognizing MART-1, NY-ESO-1, and melanoma gp100 peptides; vectors and cells comprising the same; and methods of using the foregoing.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Isolated peptides consisting of amino acid sequences found in SSX or NY-ESO-1 molecules, which bind to HLA molecules
The invention relates to members of the SSX family of genes, as well as their uses. Also a part of the invention are peptides derived from SSX molecules and the NY-ESO-1 molecule, which form complexes with HLA molecules, leading to lysis of cells presenting these complexes, by cytolytic T cells.
Owner:LUDWIG INST FOR CANCER RES
Methods for determining breast cancer and melanoma by assaying for a plurality of antigens associated therewith
InactiveUS6338947B1Tumor rejection antigen precursorsPeptide/protein ingredientsMelanomaPolymerase L
The invention relates to assays for determining breast cancer or melanoma. It has been found that the accuracy of such assays can be improved by assaying samples for three or more known tumor rejection antigen precursors. For breast cancer, the tumor rejection antigen precursors known as SCP-1, NY-ESO-1, and SSX-2 are assayed. For melanoma, SSX-2, NY-ESO-1, and MAGE-3 are assayed. Additional known tumor rejection antigen precursors can also be determined to confirm the assays. It is preferred to carry these out via polymerase chain reactions.
Owner:LUDWIG INST FOR CANCER RES
Methods for determining breast cancer and melanoma by assaying for a plurality of antigens associated therewith
The invention relates to assays for determining breast cancer or melanoma. It has been found that the accuracy of such assays can be improved by assaying samples for three or more known tumor rejection antigen precursors. For breast cancer, the tumor rejection antigen precursors known as SCP-1, NY-ESO-1, and SSX-2 are assayed. For melanoma, SSX-2, NY-ESO-1, and MAGE-3 are assayed. Additional known tumor rejection antigen precursors can also be determined to confirm the assays. It is preferred to carry these out via polymerase chain reactions.
Owner:LUDWIG INST FOR CANCER RES +1
NY-ESO-1 peptide derivatives, and uses thereof
The invention relates to variant peptides which bind to HLA molecules, leading to lysis of cells via cytolytic T cell lines. The variants are based upon NY-ESO-1 peptides. The peptides can be incorporated into immune tetramers, which are useful as T cell sorters.
Owner:LUDWIG INST FOR CANCER RES
NY-ESO-1-peptide derivatives, and uses thereof
InactiveUS6417165B1Tumor rejection antigen precursorsPeptide/protein ingredientsNY-ESO-1 peptideLysis
The invention relates to variant peptides which bind to HLA molecules, leading to lysis of cells via cytolytic T cell lines. The variants are based upon NY-ESO-1 peptides. The peptides can be incorporated into immune tetramers, which are useful as T cell sorters.
Owner:LUDWIG INST FOR CANCER RES
Method for determining status of a cancerous condition by determining antibodies to NY-ESO-1 in a patient sample
InactiveUS6251603B1Bioreactor/fermenter combinationsTumor rejection antigen precursorsAbnormal tissue growthWilms' tumor
The invention relates to methods for determining tumor status by determining antibodies specific to NY-ESO-1 in patient samples. One can determine whether a cancerous condition is progressing, regressing, or remaining stable by determining antibodies against NY-ESO-1 in a patient sample, and comparing the value obtained to a prior value. When the tumor in question expresses NY-ESO-1, a change in this value is indicative of a change in status of the cancerous condition.
Owner:LUDWIG INST FOR CANCER RES +2
Antibodies which bind to NY-ESO-1 cancer associated proteins, and hybridomas which produce these antibodies
InactiveUS6252052B1Tumor rejection antigen precursorsPeptide/protein ingredientsAntigenMonoclonal antibody
The invention relates to antibodies which bind to the cancer associated antigen NY-ESO-1. Both polyclonal and monoclonal antibodies are part of the invention, as are chimeric forms of the antibodies, and binding portions of antibodies. Uses of these antibodies are described. Also described are truncated, recombinant forms of the cancer associated antigen.
Owner:CORNELL RES FOUNDATION INC +2
Serum autoantibody detection kit
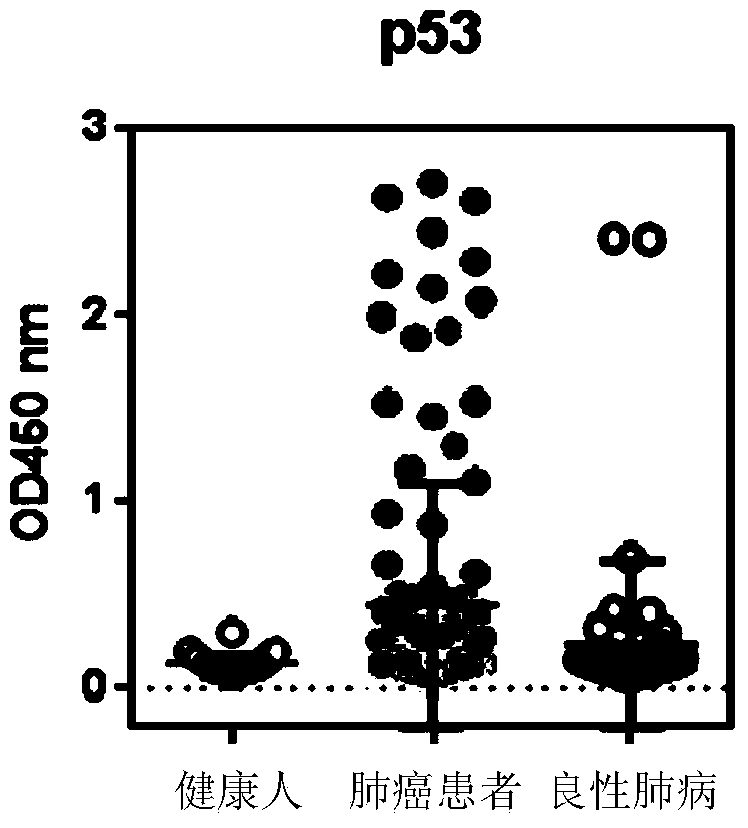
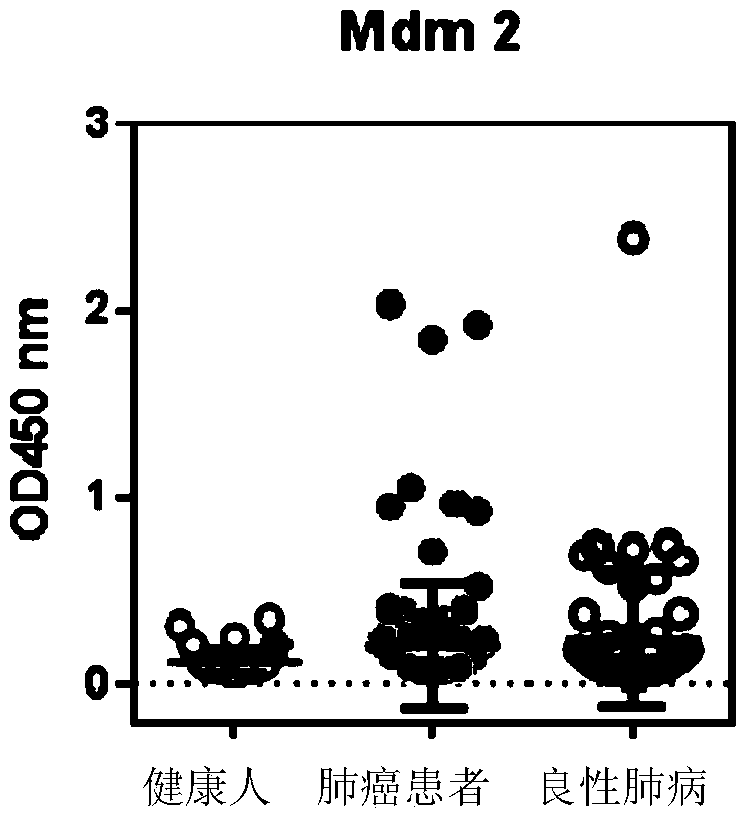
ActiveCN103869086AEfficient identificationIncreased sensitivityDisease diagnosisBiological testingCancers diagnosisSOX2
The invention discloses a detection kit used for detecting the serum autoantibody of mammals. The detection kit comprises an antigen protein, wherein the antigen protein is a combination of five or more out of p53, Annexin1, CAGE, NY-ESO-1, HuD, Cyclin D, PGP9.5, GBU4-5, MDM2, GAGE7, XAGE1b, SOX2, MAGE A1 and MAGE A4. The detection kit adopts a group of novel antigen combination corresponding to the autoantibody of a biomarker related to cancers, utilizes biotins to form characteristics of a polymer to envelope the antigen protein, and uses an anti-human label peptide as a standard of quantitative detection, thus increasing the detection sensibility and accuracy of the serum autoantibody, and providing an optimized detection method for using the autoantibody to carry out cancer diagnosis.
Owner:HANGZHOU KAIBAOLUO BIOLOGICAL SCI & TECH
Compositions comprising T cell receptors and methods of use thereof
Nucleic acids encoding antitumor TCRs recognizing MART-1, NY-ESO-1, and melanoma gp100 peptides; vectors and cells comprising the same; and methods of using the foregoing.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
NY-ESO-1 peptide derivatives, and uses thereof
InactiveUS6689742B1Tumor rejection antigen precursorsPeptide/protein ingredientsNY-ESO-1 peptideLysis
The invention relates to variant peptides which bind to HLA molecules, leading to lysis of cells via cytolytic T cell lines. The variants are based upon NY-ESO-1 peptides. The peptides can be incorporated into immune tetramers, which are useful as T cell sorters.
Owner:THE CHANCELLOR MASTERS & SCHOLARS OF THE UNIV OF OXFORD
Isolated peptides which bind to MHC class II molecules, and uses thereof
InactiveUS6800730B1Tumor rejection antigen precursorsPeptide/protein ingredientsHuman leukocyte antigen DRCD4 antigen
Peptides which have an amino acid sequence identical to sequences found in tumor rejection antigen precursors, such as NY-ESO-1, and SSX-2, are disclosed. These peptides bind to MHC-Class II molecules, such as HLA-DR molecules, and provoke proliferation of CD4+ cells.
Owner:LUDWIG INST FOR CANCER RES
Isolated peptides corresponding to amino acid sequences of NY-ESO-1, which bind to MHC Class I and MHC Class II molecules, and uses thereof
The invention relates to peptides which bind to MHC Class I and to MHC Class II molecules. These peptides are useful in different therapeutic and diagnostic contexts.
Owner:LUDWIG INST FOR CANCER RES +3
Murine anti-NY-ESO-1 T cell receptors
The invention provides an isolated or purified T cell receptor (TCR) having antigenic specificity for NY-ESO-1. Also provided are related polypeptides, proteins, nucleic acids, recombinant expression vectors, isolated host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions. The invention further provides a method of detecting the presence of cancer in a mammal and a method of treating or preventing cancer in a mammal using the inventive TCRs or related materials.
Owner:UNITED STATES OF AMERICA
NY-ESO-1 tumour antigen mimic epitope and use thereof
InactiveCN101381402AImproving immunogenicityStrong specificityPeptidesAntibody medical ingredientsCtl epitopePredictive methods
The invention discloses epitope for an NY-ESO-1 tumor antigen. The amino acid sequence of the mimic epitope is Ser-Leu-Leu- Met-Phe-Ile-Thr-Trp-Cys, namely SLLMFITWC; the mimic epitope can raise a CTL immunity response, can undergo a cross reaction with a natural epitope, has the characteristics of strong immunogenicity, immune tolerance breaking, strong specificity, safety, low cost, and easy synthesis and storage, and can be used to prepare tumor-therapeutic polypeptide vaccine. The invention also discloses a method for predicting the mimic epitope, which comprises steps of the establishment of a structural model of a TCR-pMHC complex, the analysis of TCR binding sites of the epitope, and the analysis of amino acid replacement of the TCR binding sites of the epitope. The method is also applicable to the computer-aided modification of CTL epitopes of other antigens, and can provide a useful tool for the design and research of therapeutic polypeptide vaccine.
Owner:ARMY MEDICAL UNIV
T cell receptor (TCR) capable of identifying NY-ESO-1 antigen short-peptides
ActiveCN106632660AGood killing effectPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenNY-ESO-1
Owner:XLIFESC LTD
Immunodominant mhc dr52b restricted ny-eso-1 epitopes, mhc class ii monomers and multimers, and uses thereof
InactiveUS20130029358A1Long-term outcome is improvedPrevent relapseTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeNY-ESO-1
Owner:LUDWIG INST FOR CANCER RES
Murine Anti-ny-eso-1 t cell receptors
ActiveUS20150141347A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigen bindingB-cell receptor
The invention provides an isolated or purified T cell receptor (TCR) having antigenic specificity for NY-ESO-1. Also provided are related polypeptides, proteins, nucleic acids, recombinant expression vectors, isolated host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions. The invention further provides a method of detecting the presence of cancer in a mammal and a method of treating or preventing cancer in a mammal using the inventive TCRs or related materials.
Owner:UNITED STATES OF AMERICA
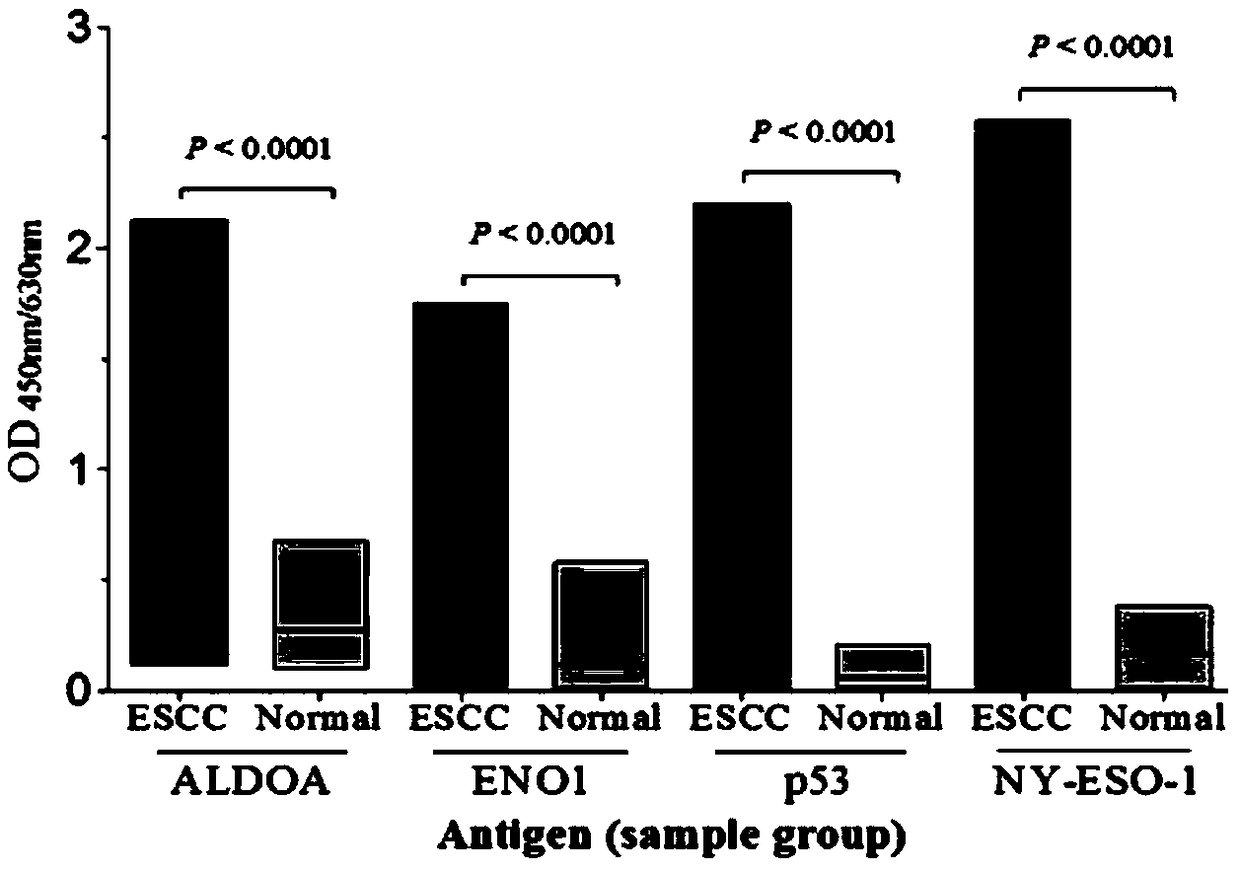
Esophageal squamous cell carcinoma autoantibody molecular marker model and application thereof
ActiveCN109342727AGood distinctionIncreased sensitivityDisease diagnosisStage I Esophageal Squamous Cell CarcinomaAutoantibody production
The invention relates to an esophageal squamous cell carcinoma autoantibody molecular marker model and an application thereof. The molecular model mainly comprises an ALDOA autoantibody, an ENO1 autoantibody, a p53 autoantibody, and an NY-ESO-1 autoantibody, and can be used for preparing a kit for distinguishing esophageal squamous carcinoma patients and healthy medical examiners. The kit for detecting esophageal squamous cell carcinoma patients mainly comprises recombinant ALDOAD protein, recombinant ENO1 protein, recombinant p53 protein, and recombinant NY-ESO-1 protein. According to the esophageal squamous cell carcinoma autoantibody molecular marker model and the application thereof, the ENO1 autoantibody is found to be elevated in serum level in the esophageal squamous carcinoma patients for the first time, and is jointly detected with the ALDOA autoantibody, the p53 autoantibody, and the NY-ESO-1 autoantibody for distinguishing the esophageal squamous cell carcinoma patients andthe healthy medical examiners, and has a better distinguishing effect than a single index detection; and in addition, the detection method adopted by the invention is an enzyme-linked immunosorbent assay indirect method, is simple and convenient to implement, has good sensitivity and specificity, and is a method which is mature and reliable and can be widely used in base layer hospitals.
Owner:汕头市颂美恩生物科技有限公司
Fusion protein
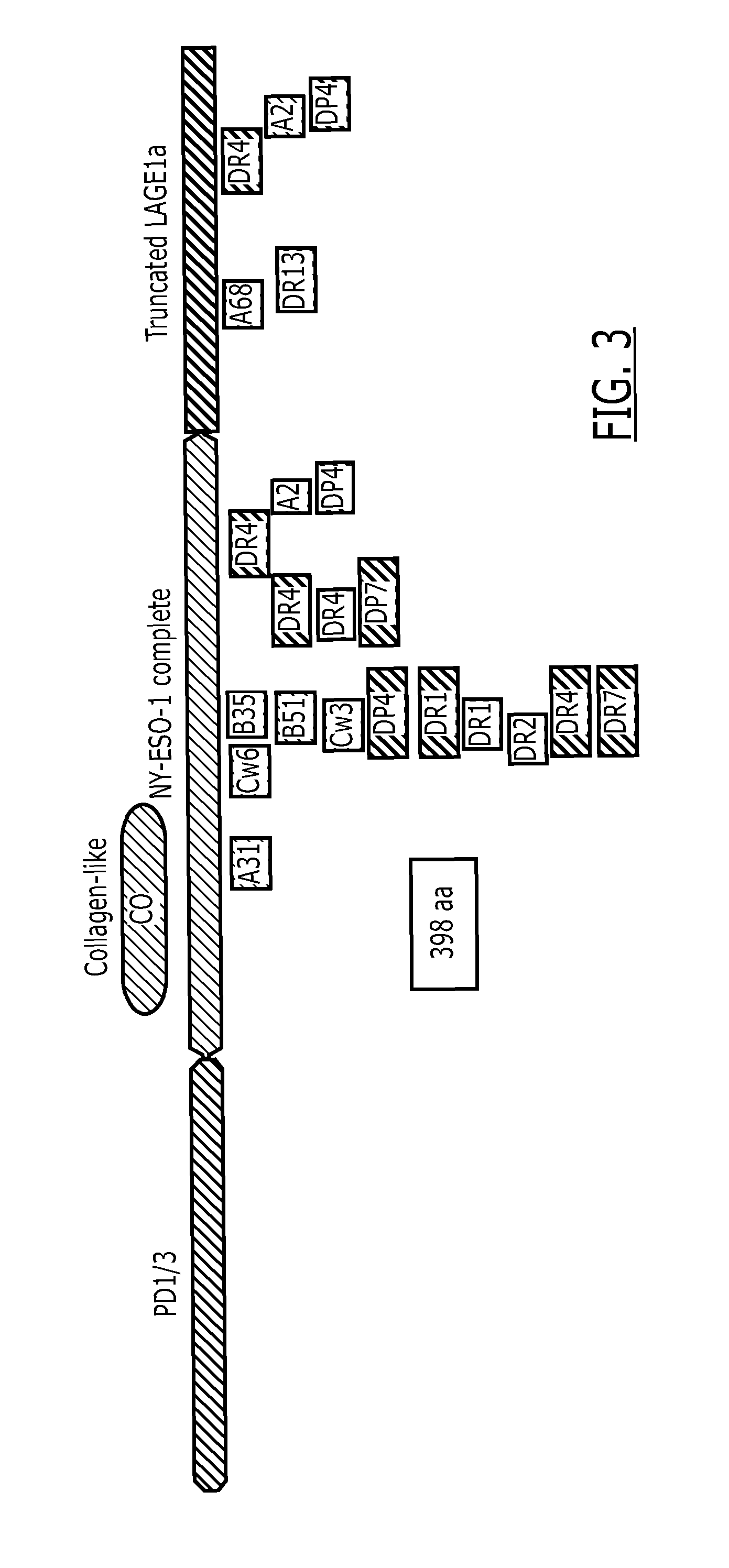
Fusion proteins comprising an antigen derived from NY-ESO-1 linked to an antigen derived from LAGE-1, which may further comprise carriers, fusion partners, or the like, are provided. Methods for preparing, formulating, and using such fusion proteins are also provided. Such proteins are useful a vaccine components for inducing an immune response against a range of cancer-antigen-bearing cells.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Mage-3 and NY-ESO-1 Based Polyvalent Vaccine for Cancer Immunotherapy
InactiveUS20070243196A1Good effectStimulate immune responseSnake antigen ingredientsCancer antigen ingredientsAntigenAdjuvant
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Liquid chip kit for detecting lung cancer autoantibody
InactiveCN103383395AImprove detection accuracyRealize joint detectionMaterial analysisBiotin-streptavidin complexAntigen
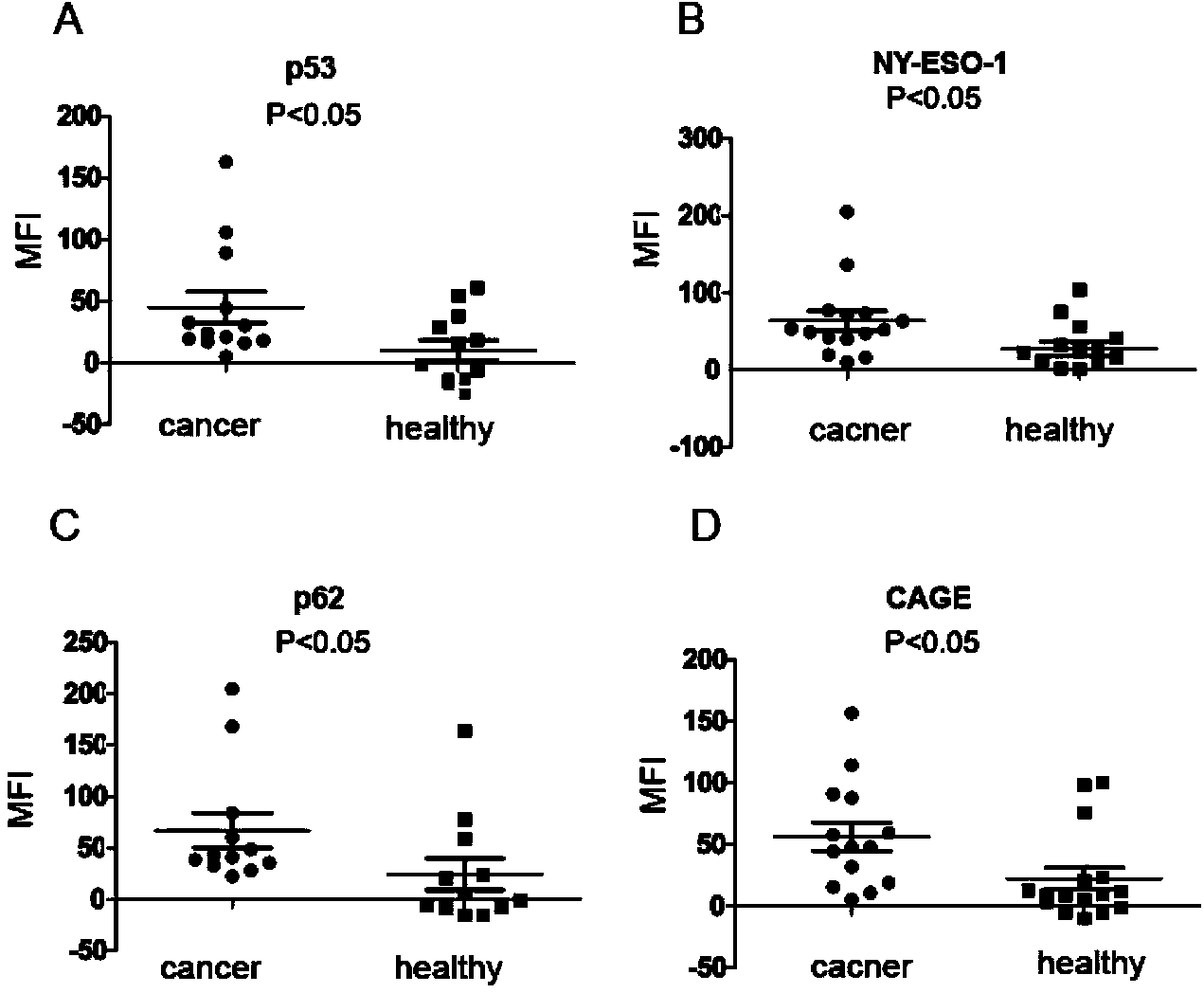
The invention discloses a liquid chip kit for detecting a lung cancer autoantibody. The kit includes: microspheres coupling antigen proteins, a biotin labeled second antibody, streptavidin-phycoerythrin, a reaction buffer and a dilution buffer solution. The antigen proteins are at least two of p62, NY-ESO-1, p53 and CAGE, wherein the microspheres coupling different antigen proteins have different color codes. The second antibody is anti-human immunoglobulin G. The liquid chip kit for detecting the lung cancer autoantibody provided in the invention realizes joint detection of four antibodies in serum on a liquid chip technology platform, and takes the p62 antibody, the NY-ESO-1 antibody, the p53 antibody and the CAGE antibody as marks, has high detection accuracy, and provides a new idea for early diagnosis of lung cancer.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL +2
Preparation process of dendritic cell targeted nanoliposome tumor vaccine
InactiveCN101690805APromote maturityAntibody medical ingredientsAntineoplastic agentsYolkDendritic cell
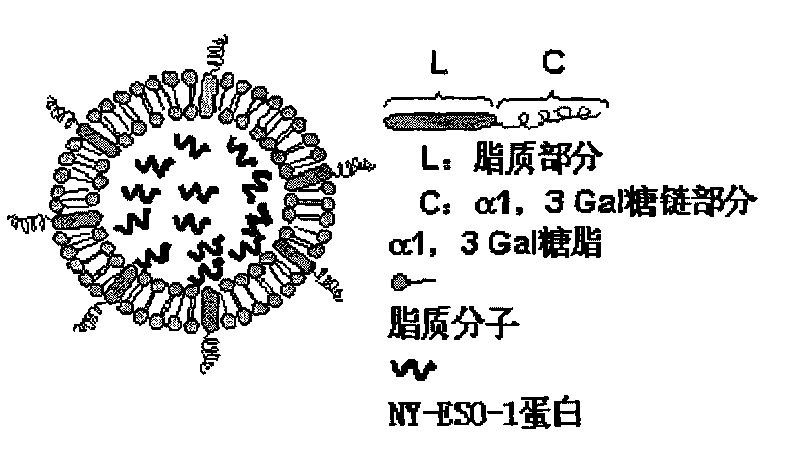
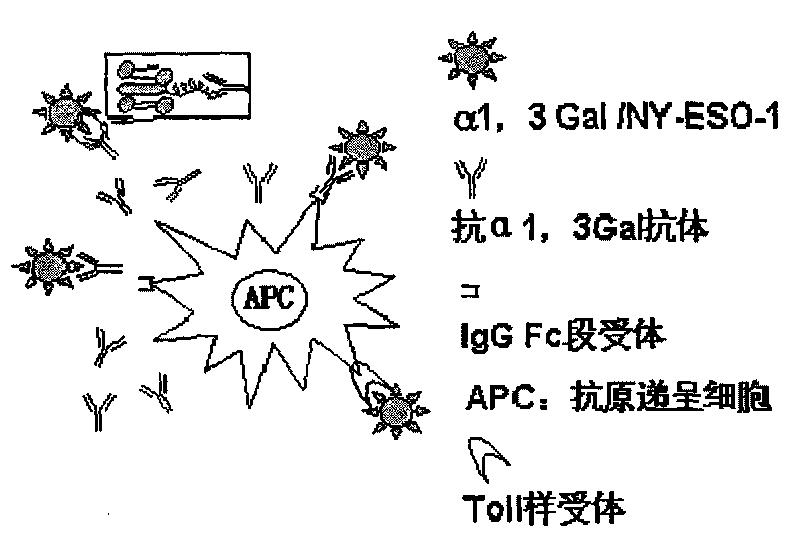
The invention relates to a preparation process of dendritic cell targeted nanoliposome tumor vaccine and the tumor vaccine can be absorbed and processed targetedly by antigen presenting cells in human body and can be used to induct the antigen presenting cells to mature. The preparation process of the invention comprises the following steps: (1) extracting alpha1 and 3Ga1 glycolipid from rabbit red cell membranes: breaking down the concentrated red cells of the rabbit, mixing cell membranes with chloroform and methanol to react, filtrating to remove granule constitutes, adding double distilled water to obtain oil phase and water phase; collecting water phase and removing methanol and trace chloroform to obtain alpha1-3Ga1-triglyceride composite for standby; and (2) adding a certain amount of alpha1 and 3Ga1 glycolipid in trichloromethane to dissolve, adding the obtained product in a certain amount of yolk lecithin, cholesterol and PEG2000-DSPE mixture, vibrating the obtained mixture for dissolving, performing rotating film evaporation to obtain liposome film, adding PBS buffer solution with 1mg / ml of NY-ESO-1 protein and performing ultrasonic emulsification to obtain the product provided by the invention.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
T cell receptor recognizing NY-ESO-1 antigen short peptide
InactiveCN106188275AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenT-Cell Receptor Beta
The invention provides a T cell receptor (TCR) which can be specifically combined with an NY-ESO-1 antigen short peptide SLLMWITQC. The antigen short peptide SLLMWITQC and HLA A0201 can form a composite which is presented onto the surface of the cells together. The invention also provides a nucleic acid molecule encoding the TCR and a carrier containing the nucleic acid molecule, and also provides a cell that transduces the TCR.
Owner:GUANGDONG XIANGXUE LIFE SCI LTD
Isolated Ny-Eso-1 Peptides Which Bind To Hla Class II Molecules And Uses Thereof
InactiveUS20080139464A1Tumor rejection antigen precursorsPeptide/protein ingredientsNY-ESO-1 peptideHla class ii
The invention relates to peptides which consist of amino acid sequences found in the NY-ESO-1 molecule, which bind to MHC-Class II molecules. These can be used alone, or in combination with other peptides.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT +1
NY-ESO-1-targeting T cell receptor and uses thereof
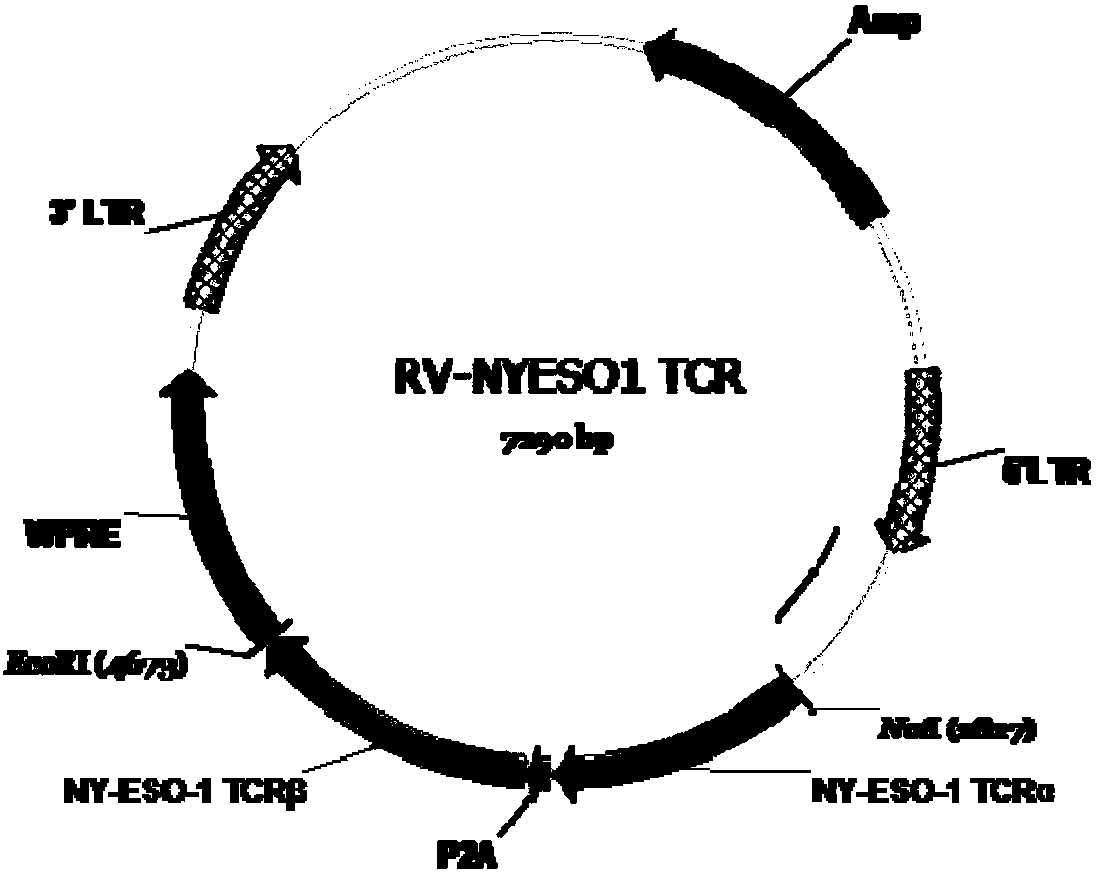
The invention discloses a NY-ESO-1-targeting T cell receptor and uses thereof, wherein the NY-ESO-1-targeting T cell receptor is formed by linking a NY-ESO-1 TCR[alpha] chain, P2A and a NY-ESO-1 TCR[beat] chain in series. According to the present invention, the T cell receptor is used for modifying human T lymphocytes, and the modified T cells (TCR-T cells) can be used for the treatment of HLA-A2+NY-ESO-1 positive tumors; and the prepared NY-ESO-1 TCR-T cells have strong functions on specific tumor cells (U266 and T2-NY-ESO-1), has high CD107a expression and high IFN[gamma] secretion, and achieves the killing efficiency of 80% in the case of an effect-to-target ratio of 5:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Preparation and application of novel enhanced antigen-binding polypeptide-induced hepatoma-specific CTL cell
ActiveCN107488235AIncrease lethalityAbility to inhibit proliferation and differentiationTumor rejection antigen precursorsAntibody mimetics/scaffoldsAntigen bindingWilms' tumor
Owner:BEIJING GENE KEY LIFE TECH CO LTD
TCR (T cell receptor) capable of identifying short peptide of NY-ESO-1 antigen
ActiveCN106478808AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenNY-ESO-1
The invention provides a TCR (T cell receptor) capable of being specifically bound with a short peptide LLMWITQCF deriving from an NY-ESO-1 antigen. The short peptide LLMWITQCF and HLA A2402 can form a compound to be transferred to the surface of a cell together. The invention further provides a nucleic acid molecule capable of encoding the TCR and a supporter containing the nucleic acid molecule. Besides, the invention further provides a cell for transducing the TCR.
Owner:XLIFESC LTD
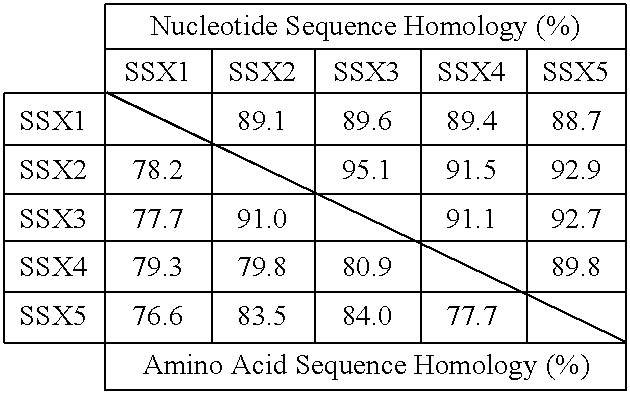
Isolated nucleic acid molecules encoding isolated peptides which correspond to contiguous amino acids of an SSX molecule or NY-ESO-1 and uses thereof
The invention relates to members of the SSX family of genes, as well as their uses. Also a part of the invention are peptides derived from SSX molecules and the NY-ESO-1 molecule, which form complexes with HLA molecules, leading to lysis of cells presenting these complexes, by cytolytic T cells.
Owner:LUDWIG INST FOR CANCER RES LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com