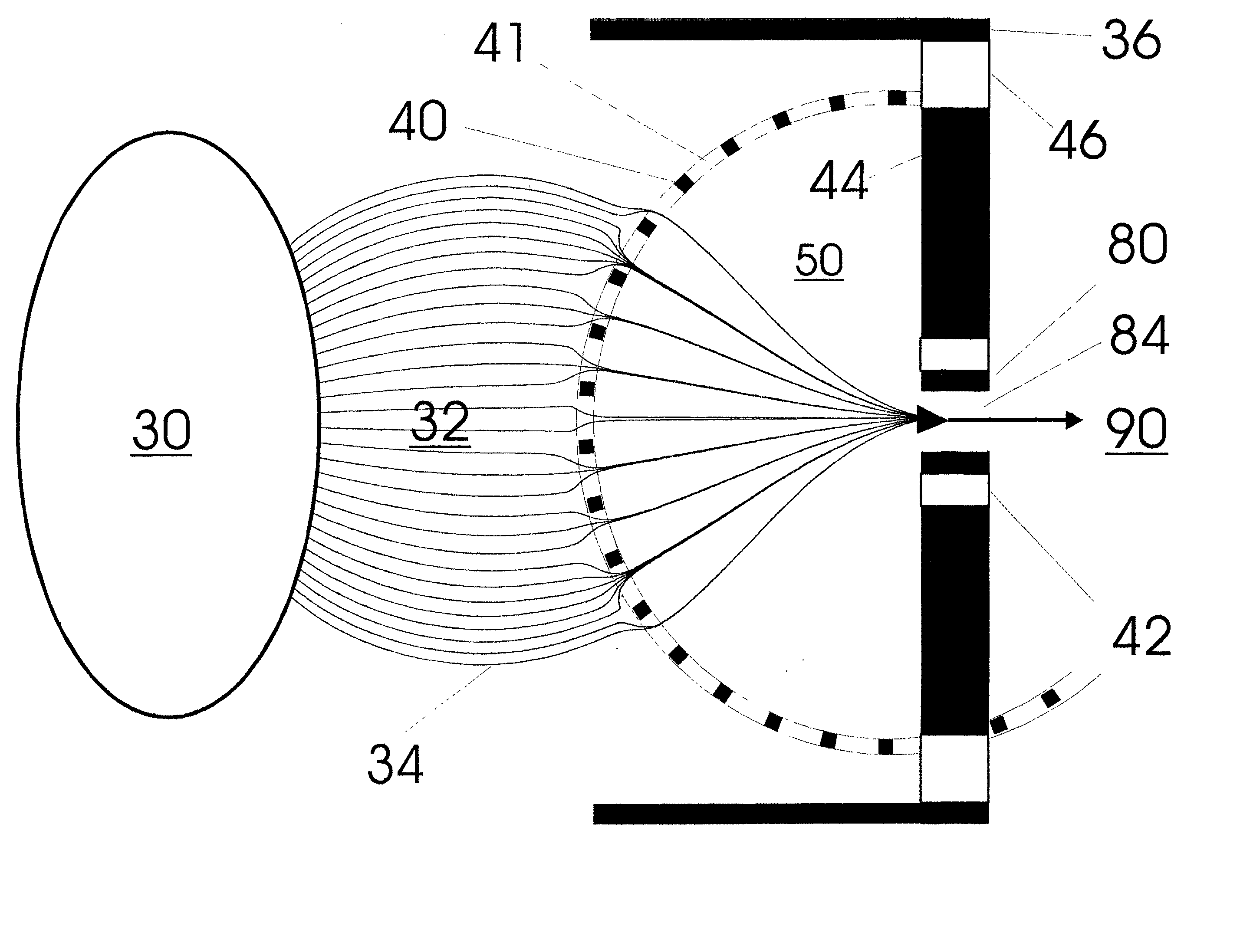
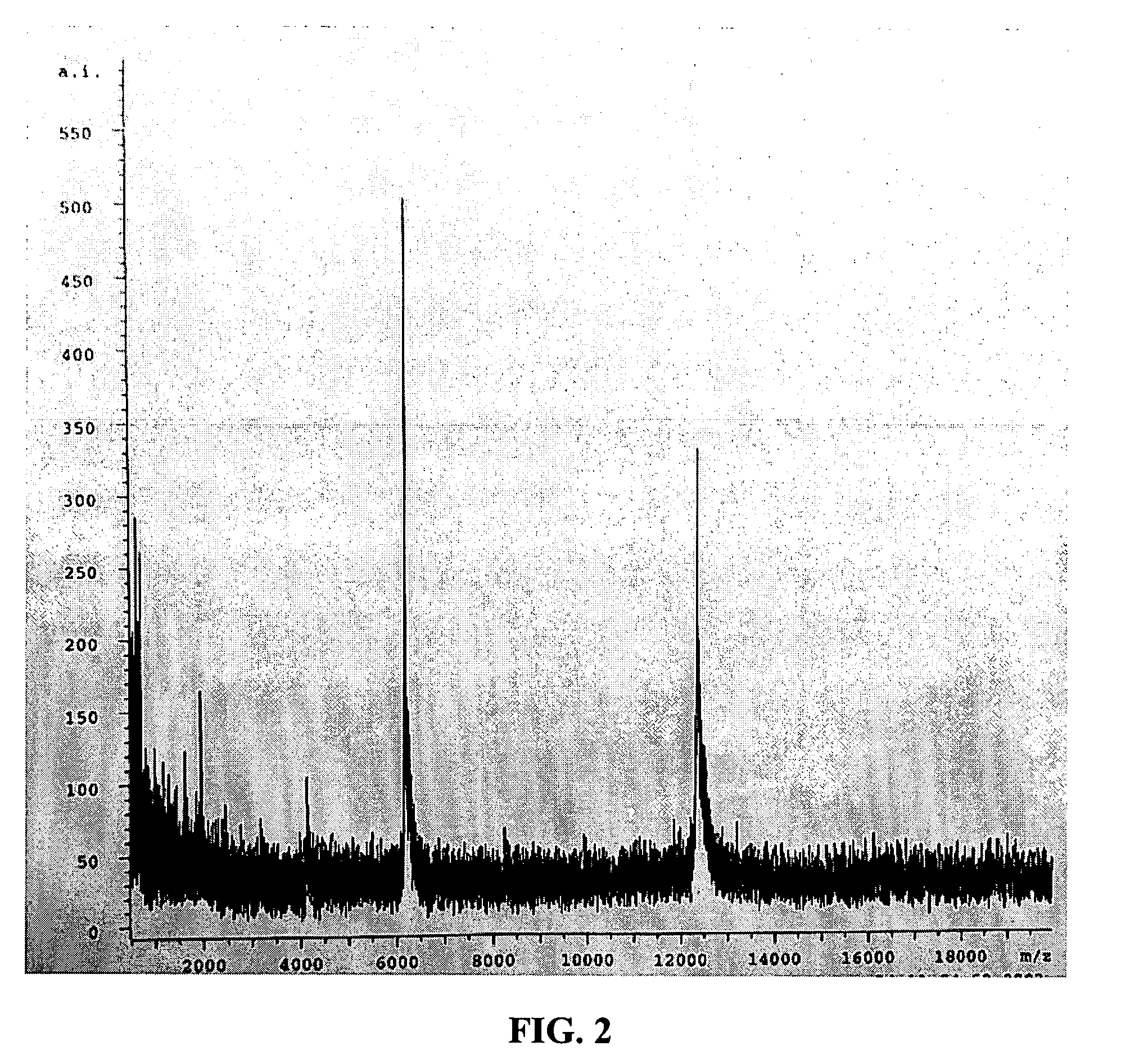
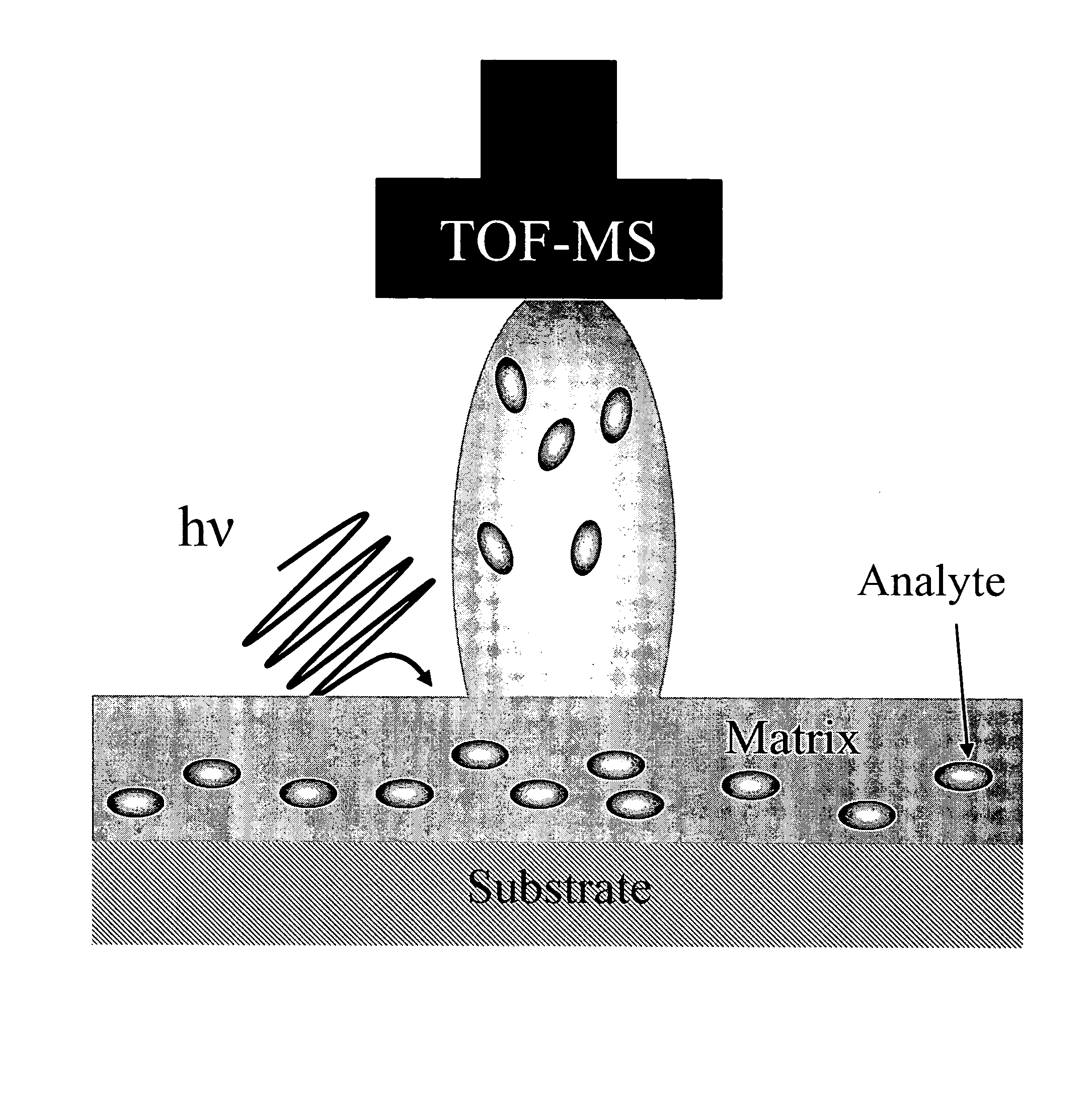
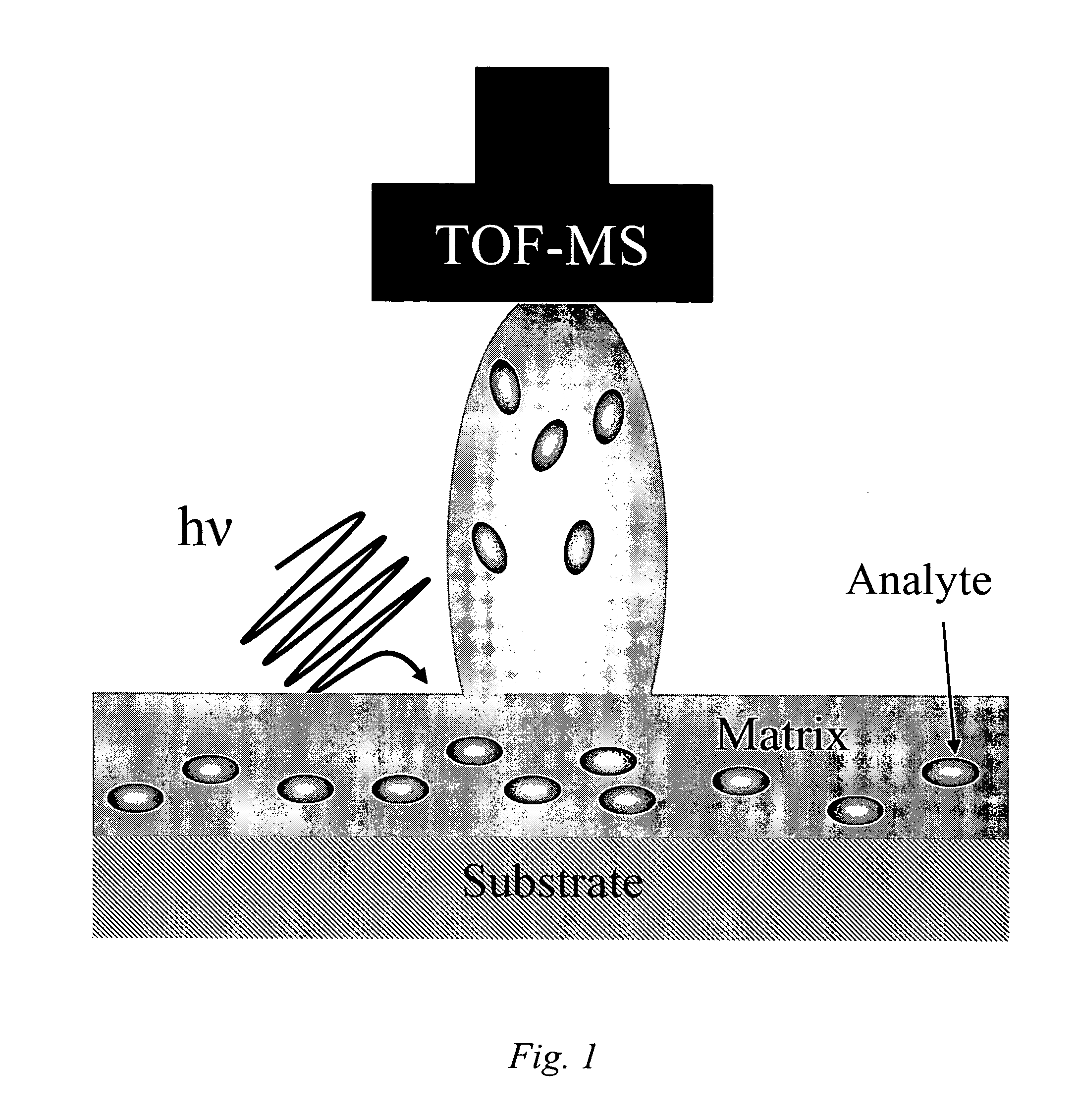
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
196 results about "Matrix-assisted laser desorption/ionization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
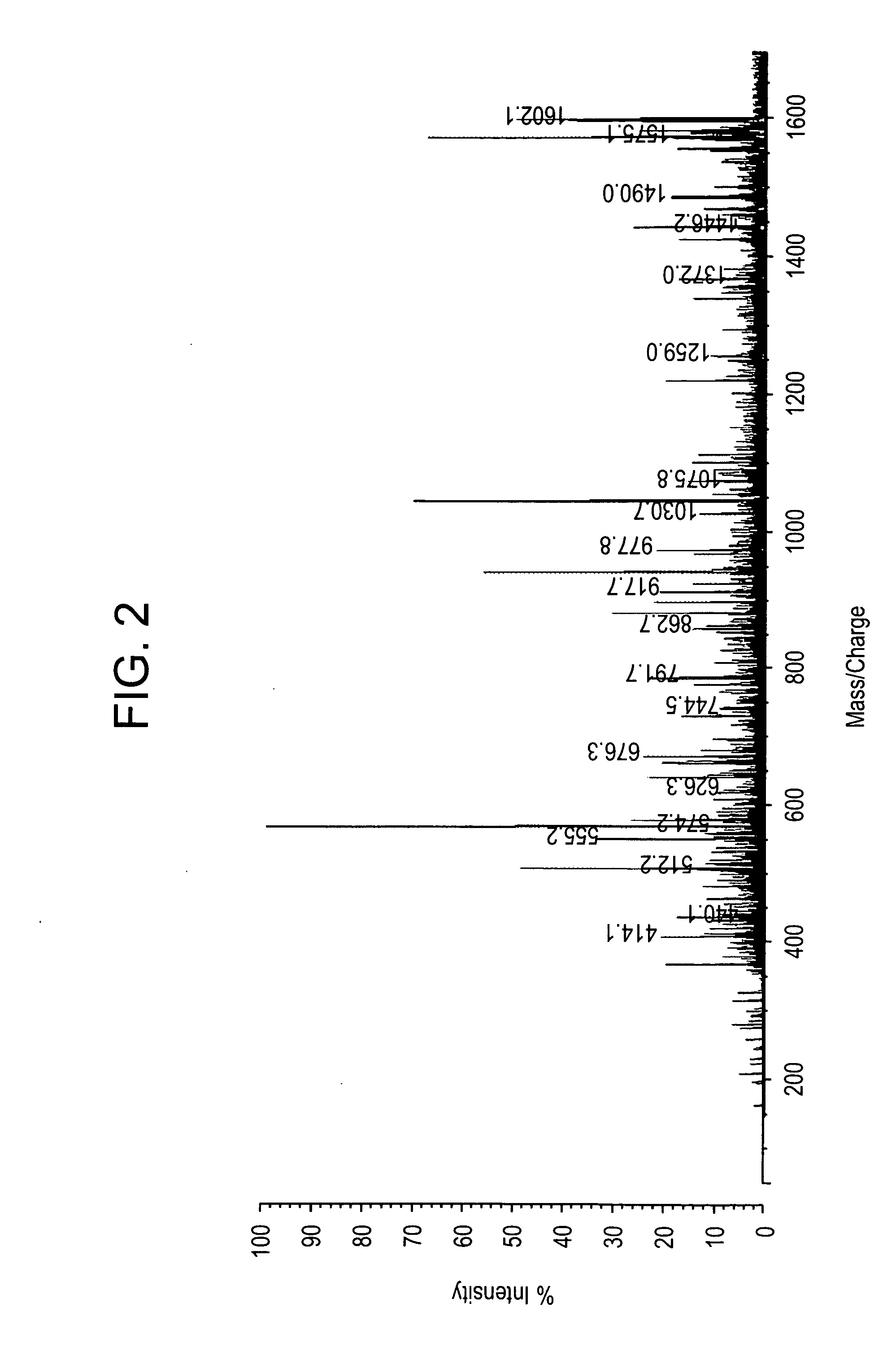
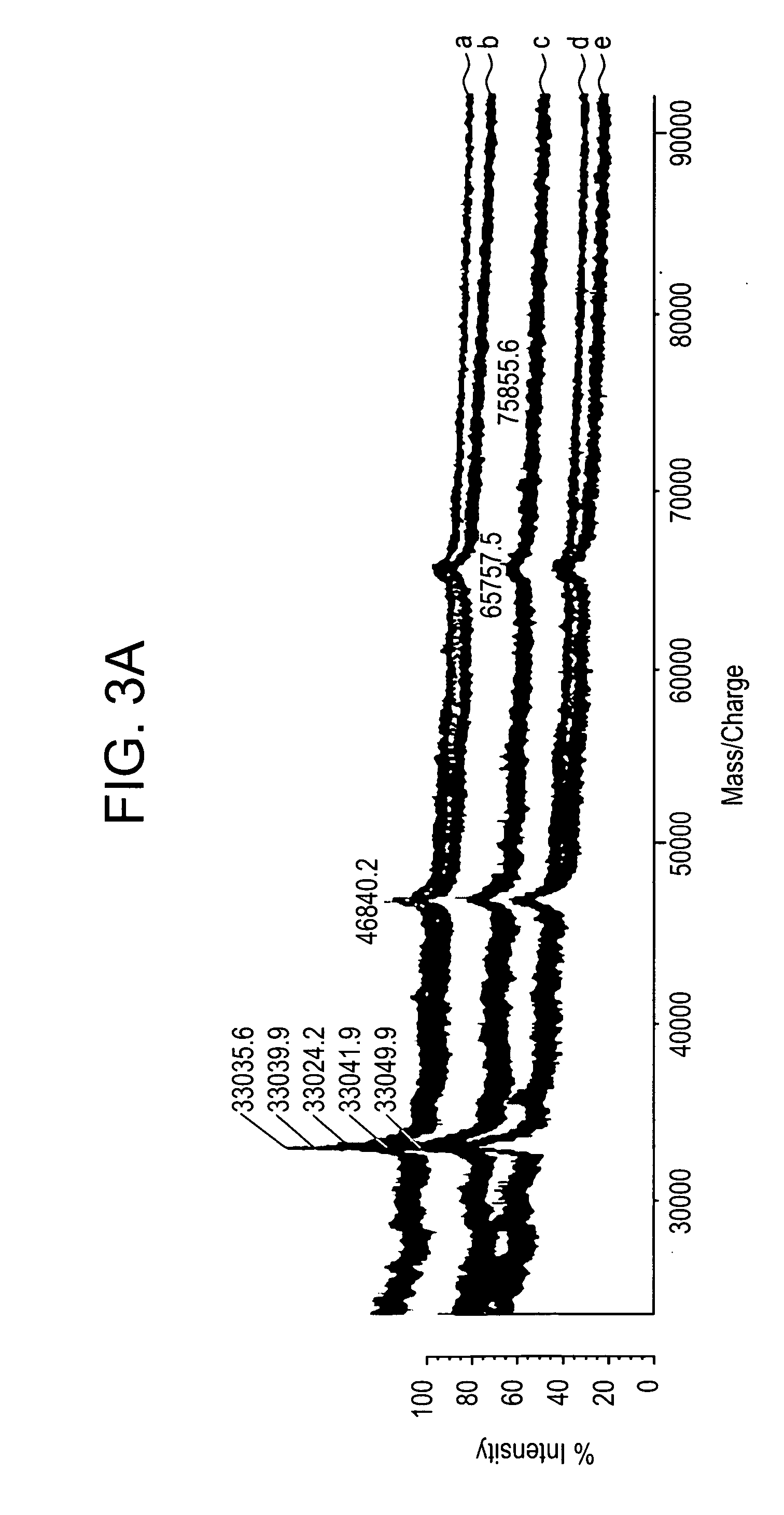
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules (biopolymers such as DNA, proteins, peptides and sugars) and large organic molecules (such as polymers, dendrimers and other macromolecules), which tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar in character to electrospray ionization (ESI) in that both techniques are relatively soft (low fragmentation) ways of obtaining ions of large molecules in the gas phase, though MALDI typically produces far fewer multi-charged ions.
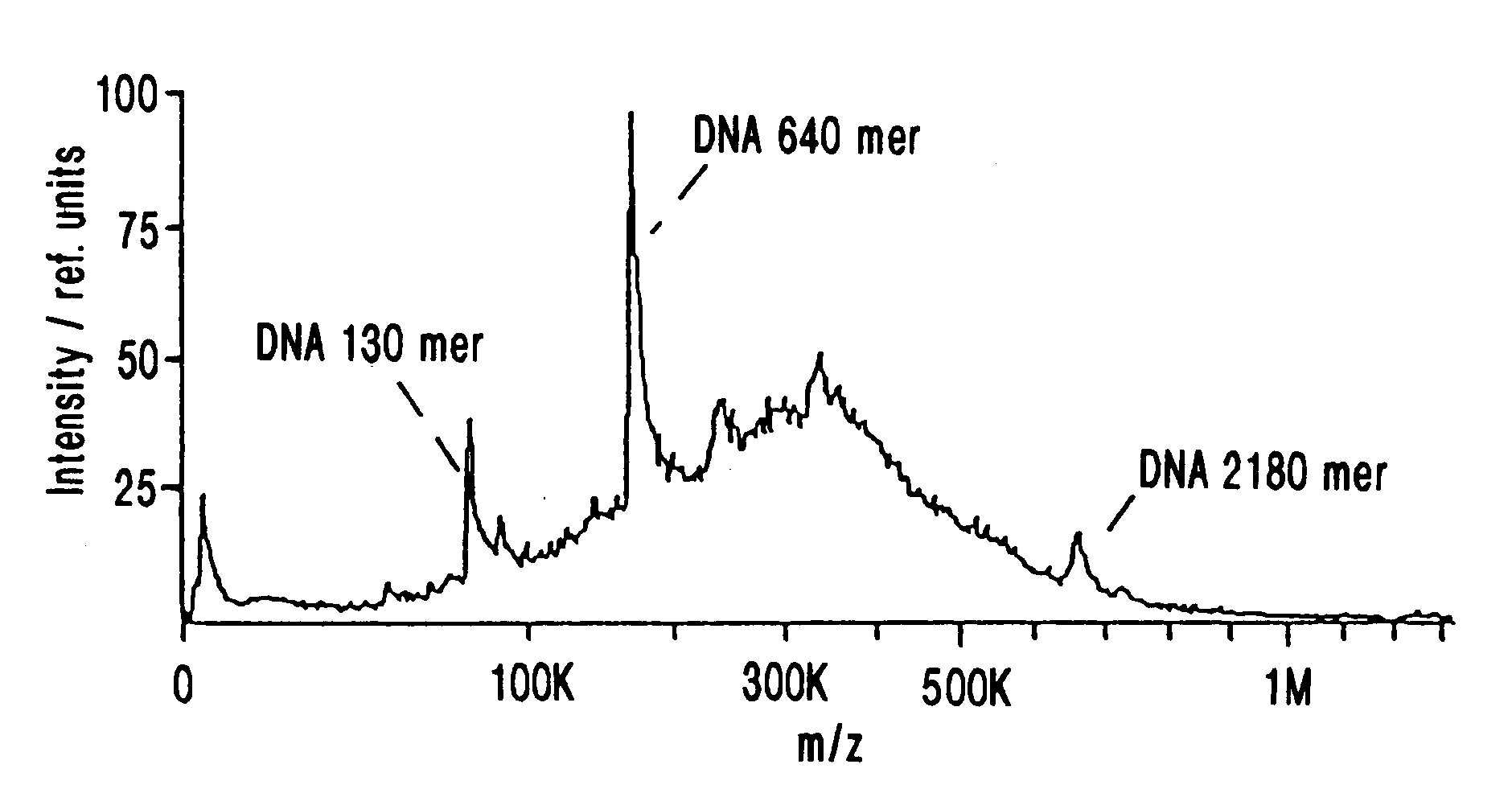
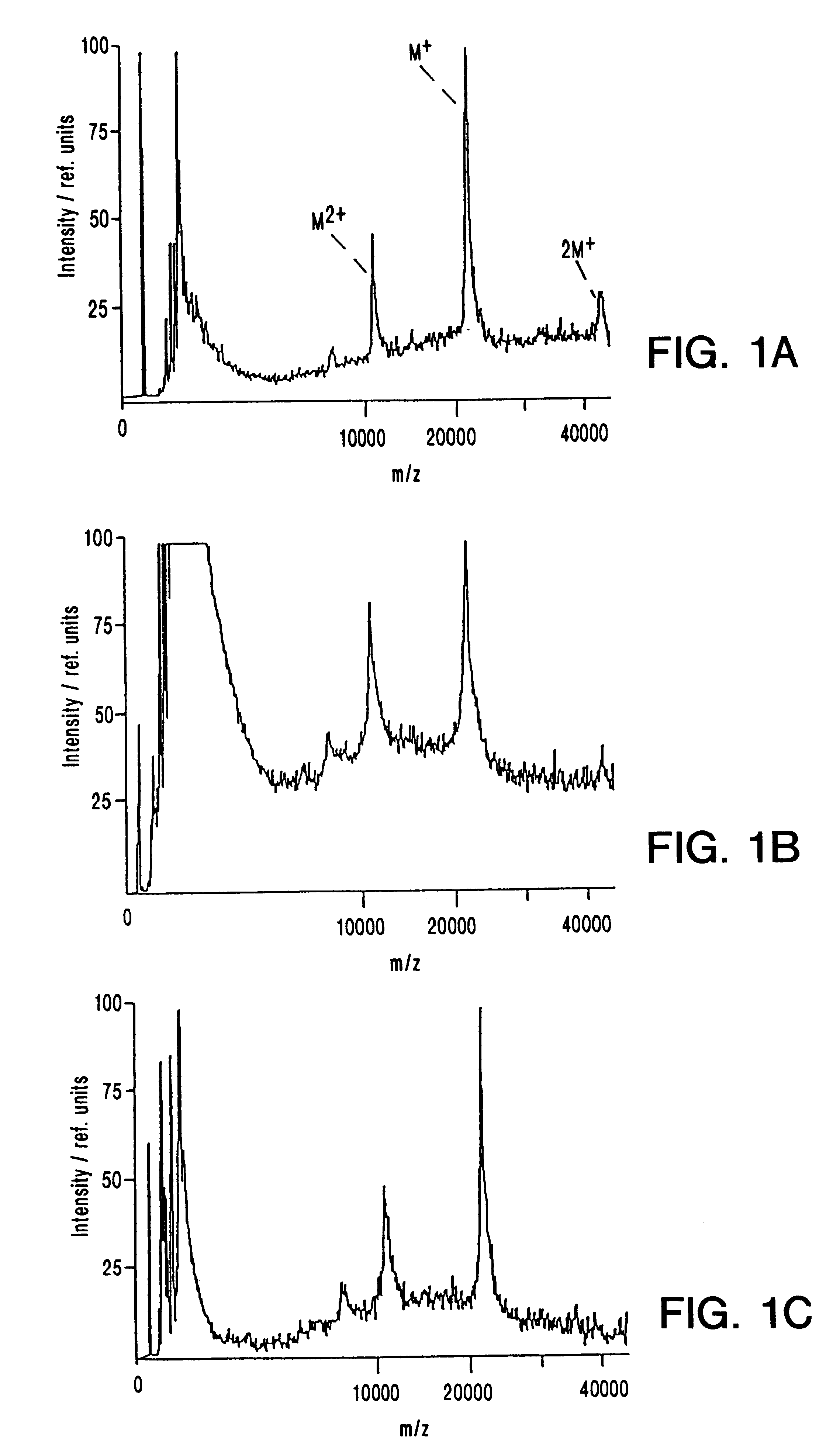
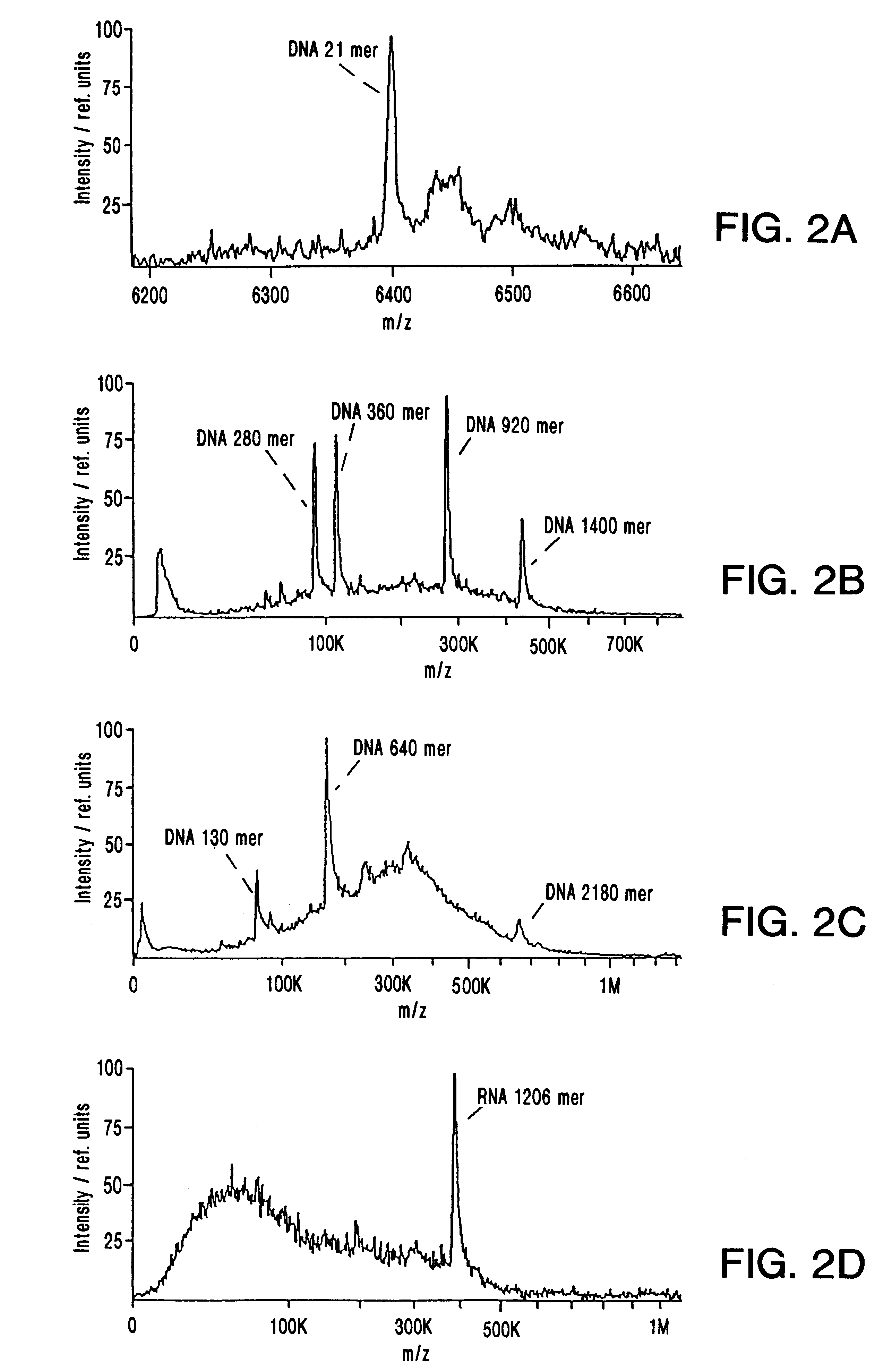
IR-MALDI mass spectrometry of nucleic acids using liquid matrices
InactiveUS6706530B2Rapidly and accurately massOptimize allocationNanostructure manufactureSamples introduction/extractionMass Spectrometry-Mass SpectrometryMatrix-assisted laser desorption/ionization
Mass spectrometry of large nucleic acids by infrared Matrix-Assisted Laser Desorption / Ionization (MALDI) using a liquid matrix is reported.
Owner:AGENA BIOSCI
Volatile matrices for matrix-assisted laser desorption/ionization mass spectrometry
InactiveUS6104028AEasy to spreadReduce formationSamples introduction/extractionWithdrawing sample devicesThermal ionization mass spectrometryRoom temperature
A sample preparation method is disclosed for volatilization and mass spectrometric analysis of nonvolatile high molecular weight molecules. Photoabsorbing molecules having significant sublimation rates at room temperature under vacuum, and preferably containing hydroxy functionalities, are disclosed for use as matrices in matrix-assisted laser desorption / ionization mass spectrometry. The samples are typically cooled in the mass spectrometer to temperatures significantly below room temperature.
Owner:AGENA BIOSCI
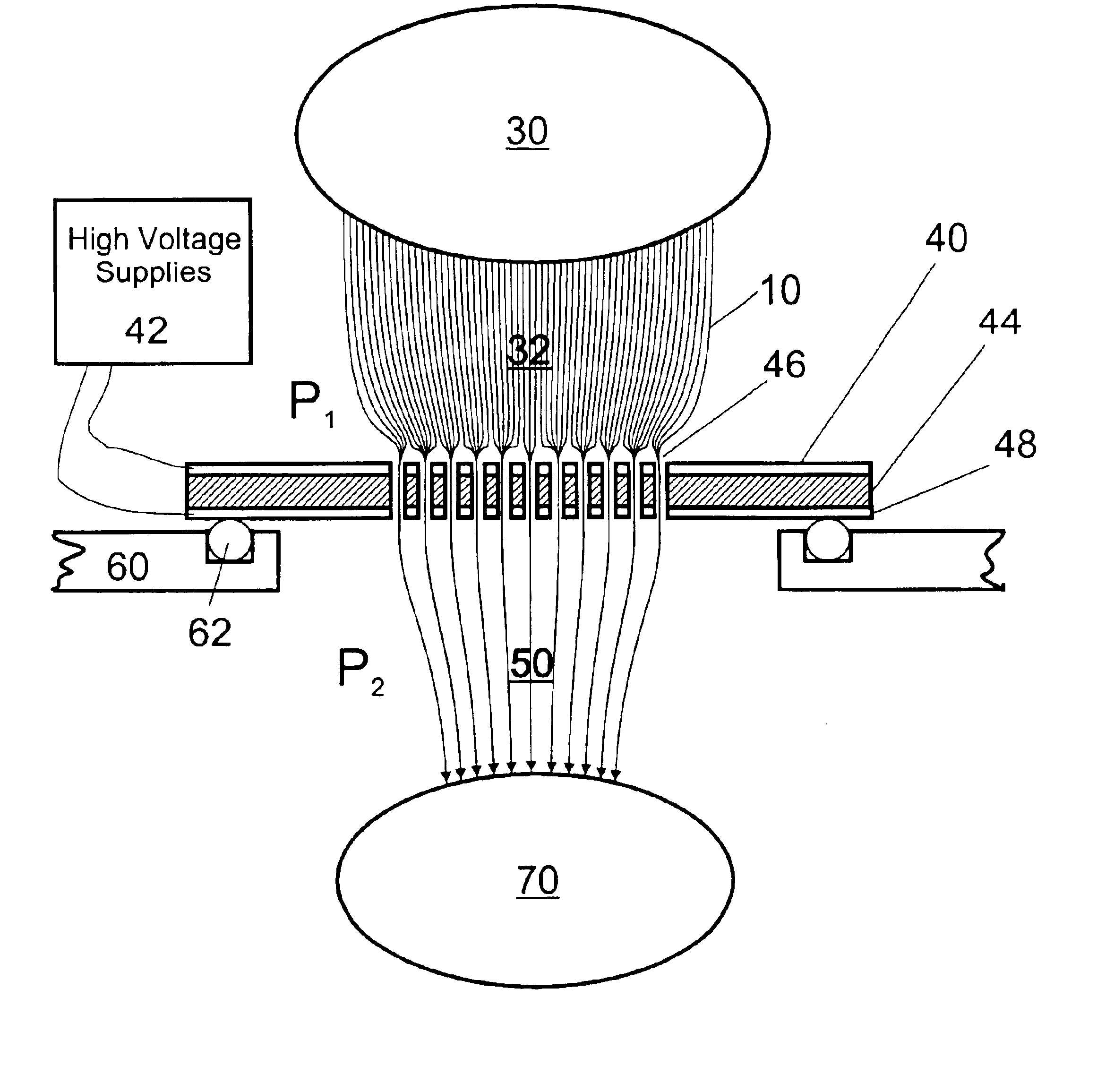
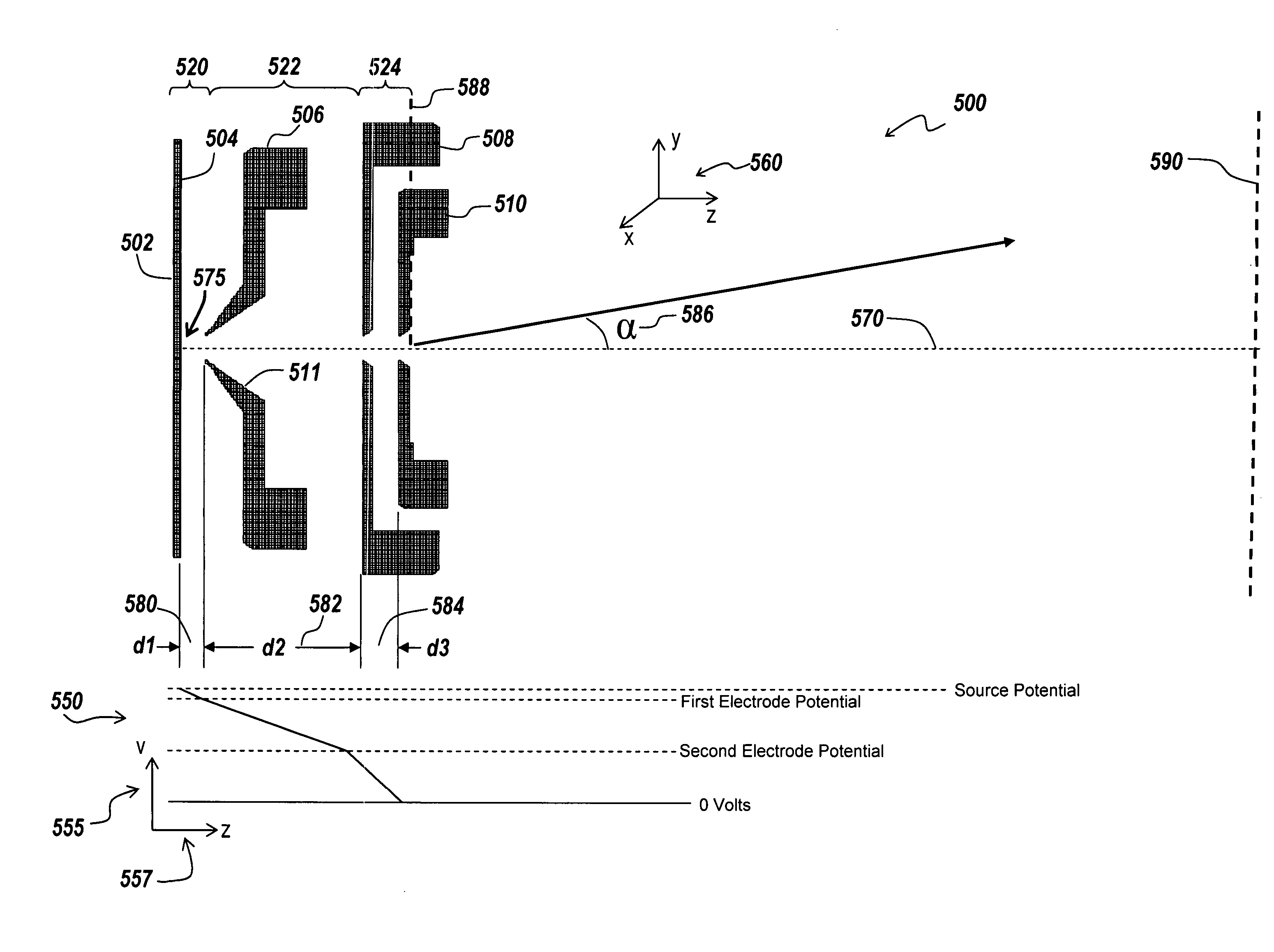
Apparatus and method for focusing ions and charged particles at atmospheric pressure
InactiveUS6744041B2Samples introduction/extractionIsotope separationInductively coupled plasmaElectrospray
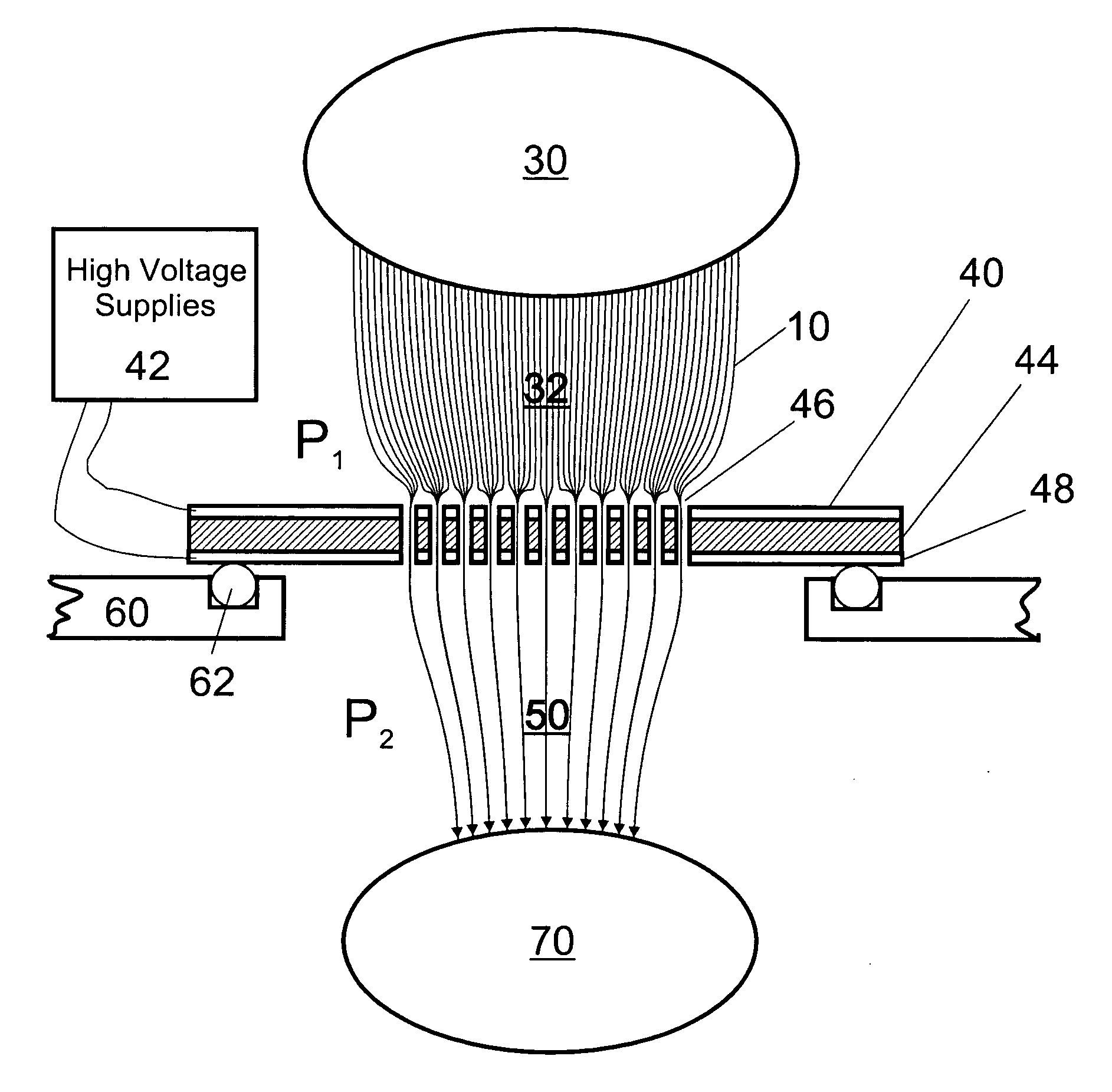
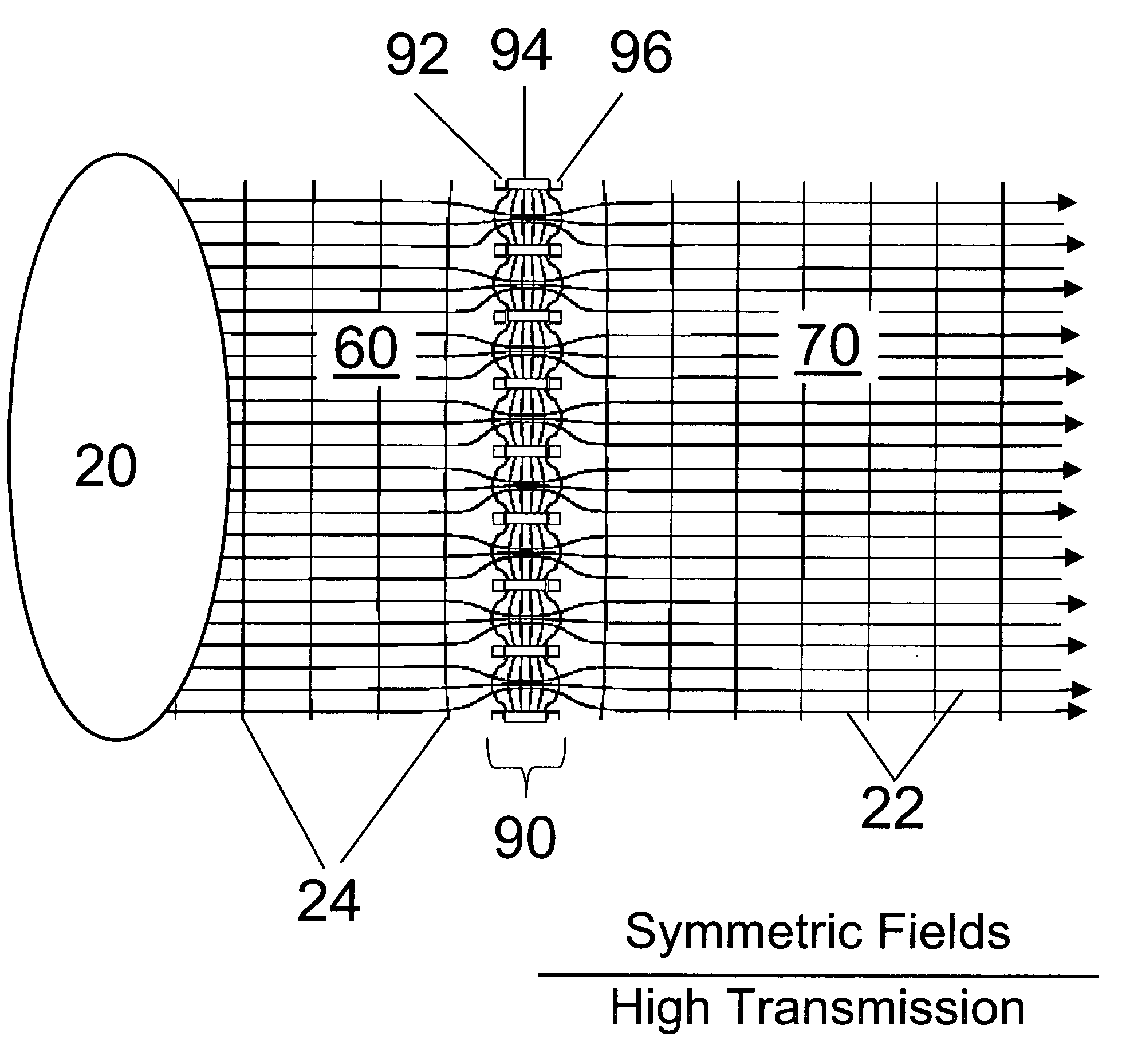
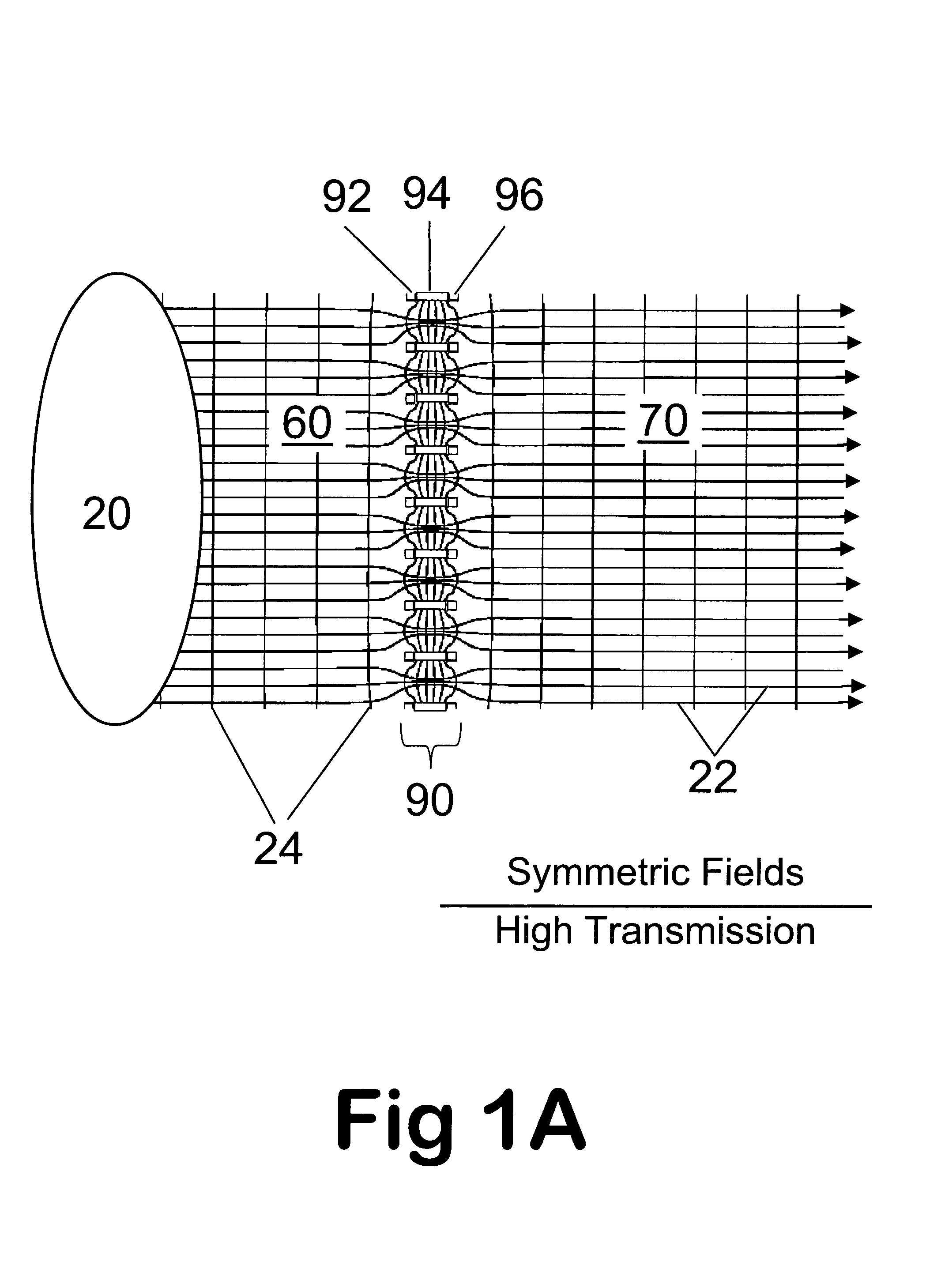
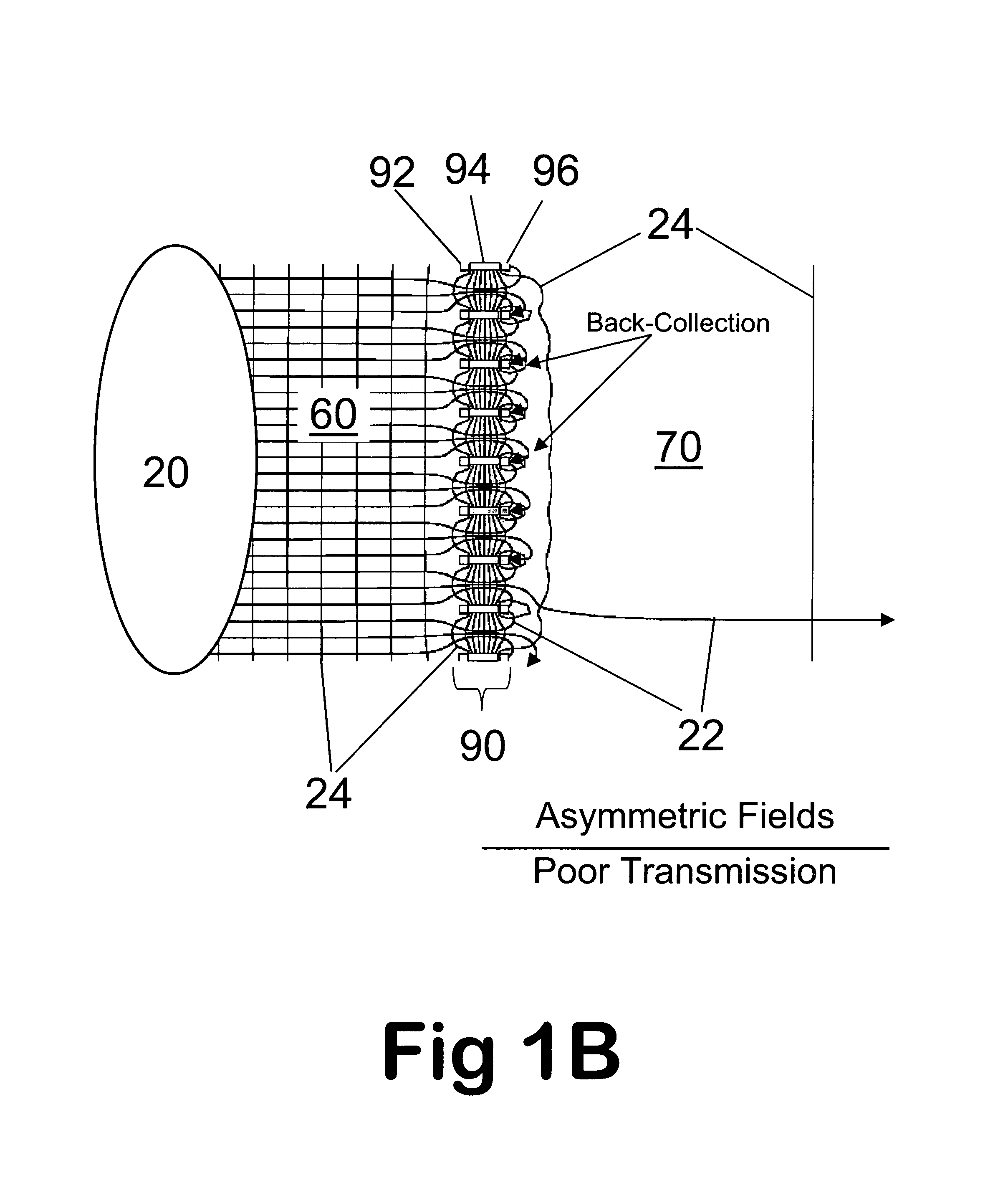
Improvements have been made for collection and focusing of ions generated from atmospheric pressure sources such as electrospray, atmospheric pressure chemical ionization, inductively coupled plasma, discharge, photoionization and atmospheric pressure matrix assisted laser desorption ionization. A high transmission electro-optical surface is placed between the source regions and the focusing regions to optimize the field geometries and strengths in each respective region. Compression ratios of greater than 5000 are capable of transferring virtually all ions from large volume dispersive ion regions into ion beam cross-sections of less than 1 mm. Embodiments of this invention are methods and devices for improving sensitivity of mass spectrometry when coupled to atmospheric pressure ionization sources.
Owner:SHEEHAN EDWARD W +1
Methods of identification of biomarkers with mass spectrometry techniques
InactiveUS20060172429A1Avoid problemsReduce the impactDisease diagnosisBiological testingMass Spectrometry-Mass SpectrometryDiagnoses diseases
The present invention provides methods for identifying various biological states. Methods for diagnosis of diseases, in particular cardiovascular and brain diseases, are provided herein. One aspect of the invention is the analysis of lipoprotein complexes with summary survey scan mass spectrum for the analysis of biological states. Another aspect of the invention is the use of matrix assisted laser desorption ionization (MALDI) mass spectrometer to analysis lipoprotein complexes for the diagnosis of cardiovascular and brain diseases. Yet another aspect of the invention is a method of diagnosis of brain diseases by evaluating the characteristics of lipoprotein complexes.
Owner:INSILICOS
Ion enrichment aperture arrays
InactiveUS6914243B2Time-of-flight spectrometersElectron/ion optical arrangementsAtmospheric pressure dischargeInductively coupled plasma
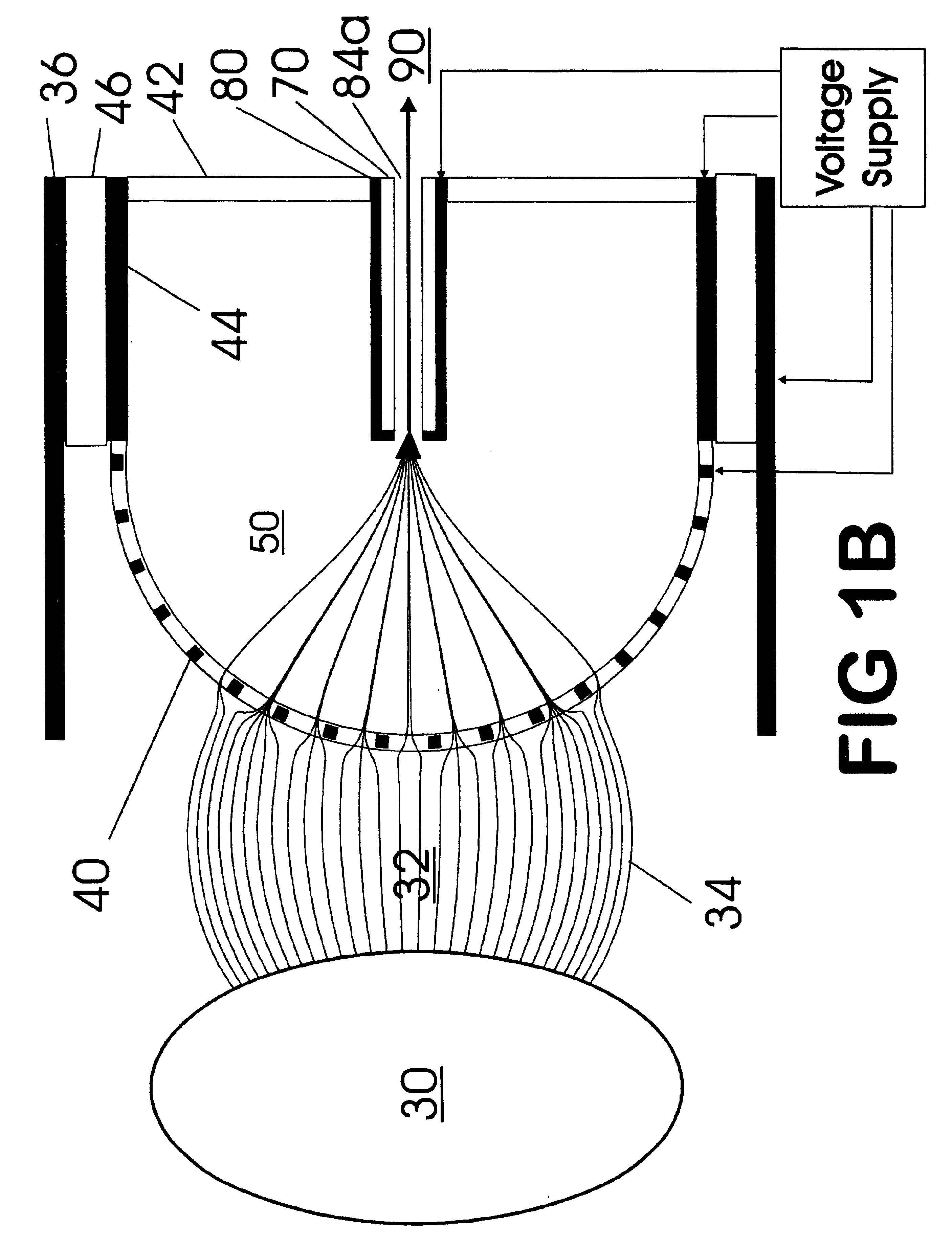
Improvements have been made for collecting, focusing, and directing of ions and / or charged particles generated at atmospheric or near atmospheric pressure sources, such as but not limited to, electrospray; atmospheric pressure discharge ionization, chemical ionization, photoionization, and matrix assisted laser desorption ionization; and inductively coupled plasma ionization. A multiple-aperture laminated structure is place at the interface of two pressure regions. Electric fields geometries and strengths across the laminated structure and diameters of the apertures; all of which act to optimize the transfer of the ions from the higher pressure region into the lower pressure region while reducing the gas-load on the lower pressure region. Embodiments of this invention are methods and devices for improving sensitivity of mass spectrometry when coupled to atmospheric, near atmospheric, or higher pressure ionization sources by reducing the gas-load on the vacuum system.
Owner:CHEM SPACE ASSOIATES
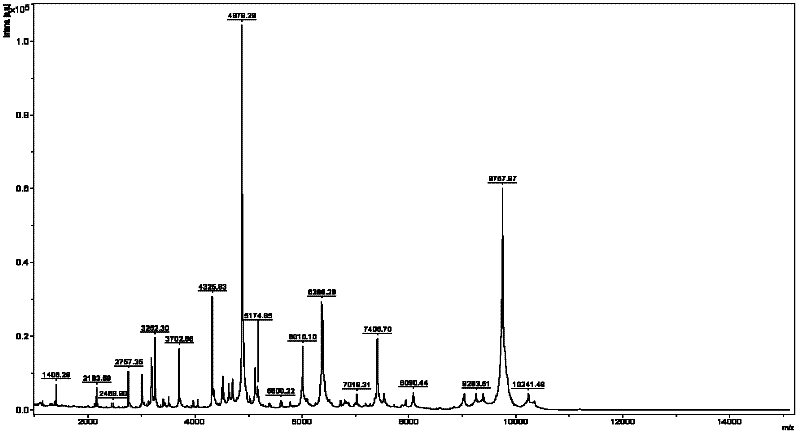
MALDI-TOF MS (Matrix-assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry)-assisted identification method for listeria monocytogenes
InactiveCN102253111AAccurate identificationSimple sample preparation in operationMaterial analysis by electric/magnetic meansTime-of-flight mass spectrometryMicroorganism
This invention relates to an MALDI-TOF MS (Matrix-assisted Laser Desorption / Ionization Time of Flight Mass Spectrometry)-assisted identification method for listeria monocytogenes, comprising the following steps of: (1) selecting 35 to 40 strains of fresh cultures of listeria monocytogenes, pre-treating the samples; (2) collecting maps of all bacterial strain samples; (3) analyzing and unifying the obtained maps by using BioTyper software to obtain a standard map of listeria monocytogenes; (4) preparing a sample of a microorganism to be detected by adopting the method in the step (1); (5) collecting the MALDI-TOF-MS map of the sample to be detected according to the method in the step (2); comparing the obtained MALDI-TOF-MS map of the sample to be detected in step (5) with the obtained standard map of listeria monocytogenes in the step (3), judging the detection results according to matching fractions. According to the invention, a mass spectrogram database and the standard map in listeria monocytogenes identification are created successfully, and the accurate identification with convenience, microscale and high automation for listeria monocytogenes can be realized.
Owner:曹际娟 +1
Ion enrichment aperture arrays
InactiveUS20040245458A1Maximizes transmission of ionImprove transmittanceTime-of-flight spectrometersElectron/ion optical arrangementsAtmospheric pressure dischargeInductively coupled plasma
Improvements have been made for collecting, focusing, and directing of ions and / or charged particles generated at atmospheric or near atmospheric pressure sources, such as but not limited to, electrospray; atmospheric pressure discharge ionization, chemical ionization, photoionization, and matrix assisted laser desorption ionization; and inductively coupled plasma ionization. A multiple-aperture laminated structure is place at the interface of two pressure regions. Electric fields geometries and strengths across the laminated structure and diameters of the apertures; all of which act to optimize the transfer of the ions from the higher pressure region into the lower pressure region while reducing the gas-load on the lower pressure region. Embodiments of this invention are methods and devices for improving sensitivity of mass spectrometry when coupled to atmospheric, near atmospheric, or higher pressure ionization sources by reducing the gas-load on the vacuum system.
Owner:CHEM SPACE ASSOIATES
Laminated lens for focusing ions from atmospheric pressure
InactiveUS7081621B1Effective independenceUniform collectionThermometer detailsMaterial analysis using wave/particle radiationCurve shapeInductively coupled plasma
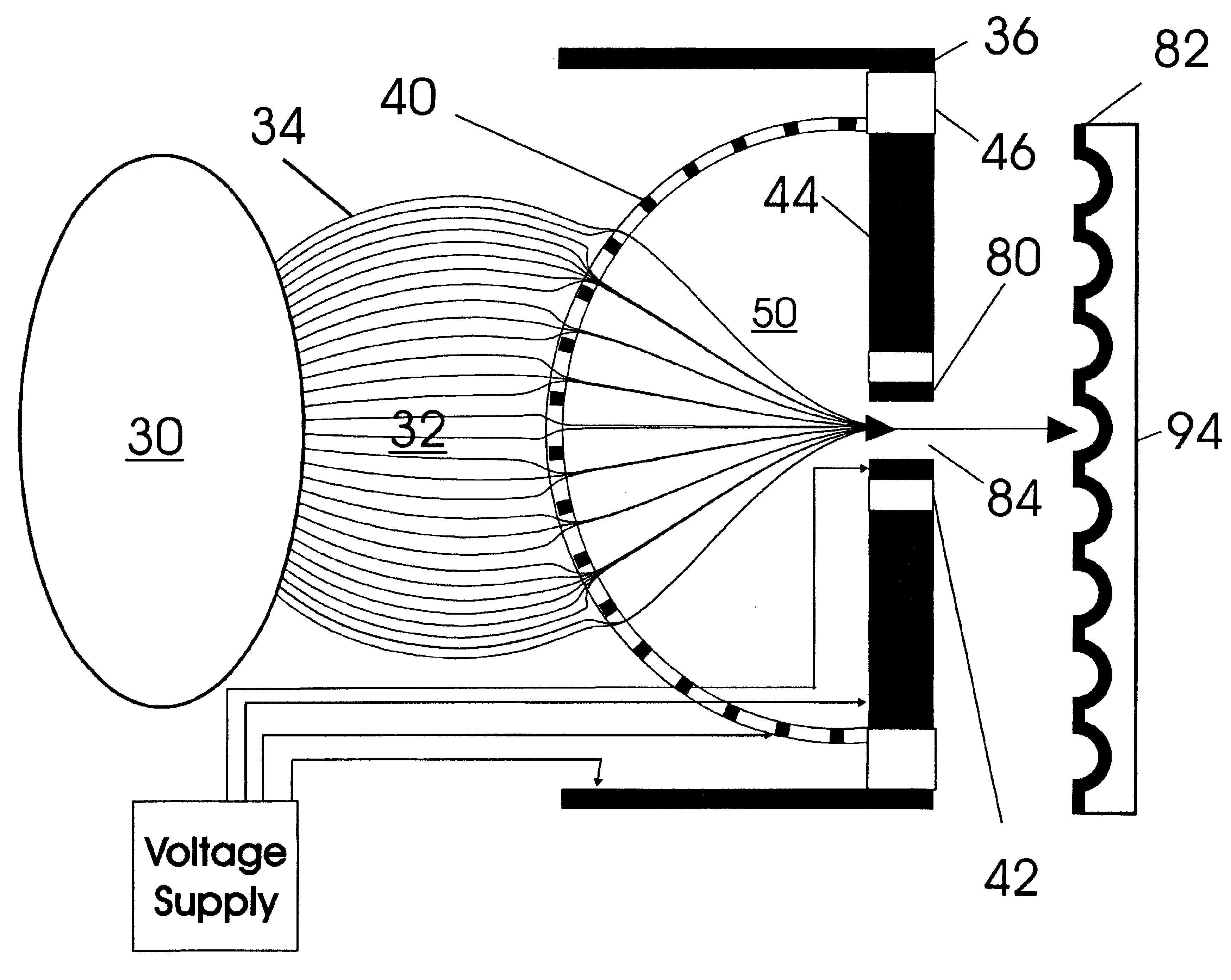
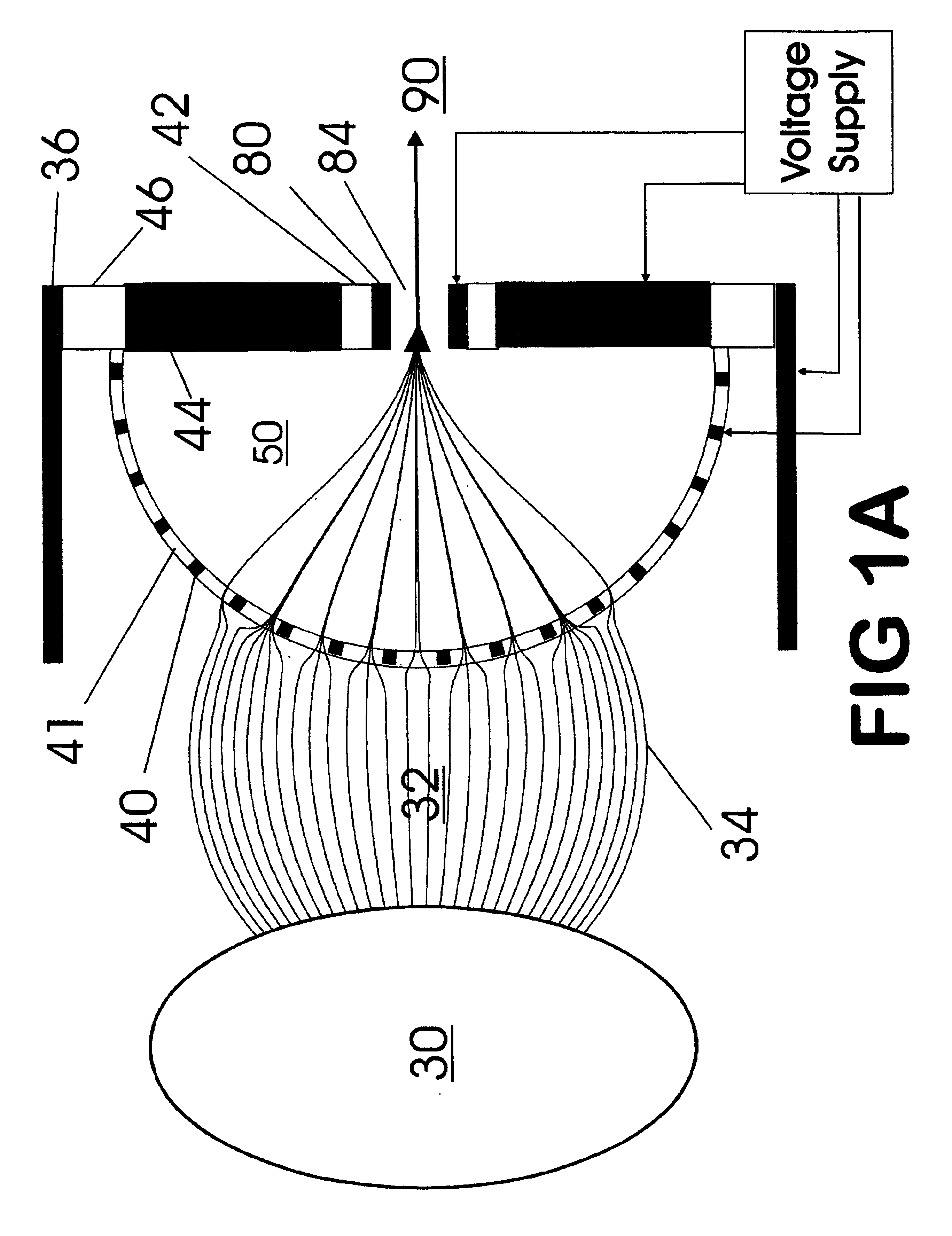
A thin laminated high transmission electro-optical lens populated with a plurality of apertures in communication with its laminates used to improve the collection, focusing, and selection of ions generated from atmospheric pressure sources, such as electrospray, atmospheric pressure chemical ionization, inductively coupled plasma, discharge, photoionization and atmospheric pressure matrix assisted laser desorption ionization. The laminated lens is made of alternating layers of electrically insulating and metal laminates. The geometry of the lens may be planar or shaped into various curve shapes, any of which act to optimize both the direct current (DC) and alternate current (AC) electric field geometries and strengths across the lens for transferring virtually all the ions from the ion source into an ion-focusing region adjacent and upstream of a high pressure or atmospheric pressure interface to a mass spectrometer, ion mobility analyzer, or combination thereof. Embodiments of this invention are methods and devices for improving sensitivity of mass spectrometry when coupled to high pressure or atmospheric pressure ionization sources.
Owner:WILLOUGHBY ROSS CLARK +1
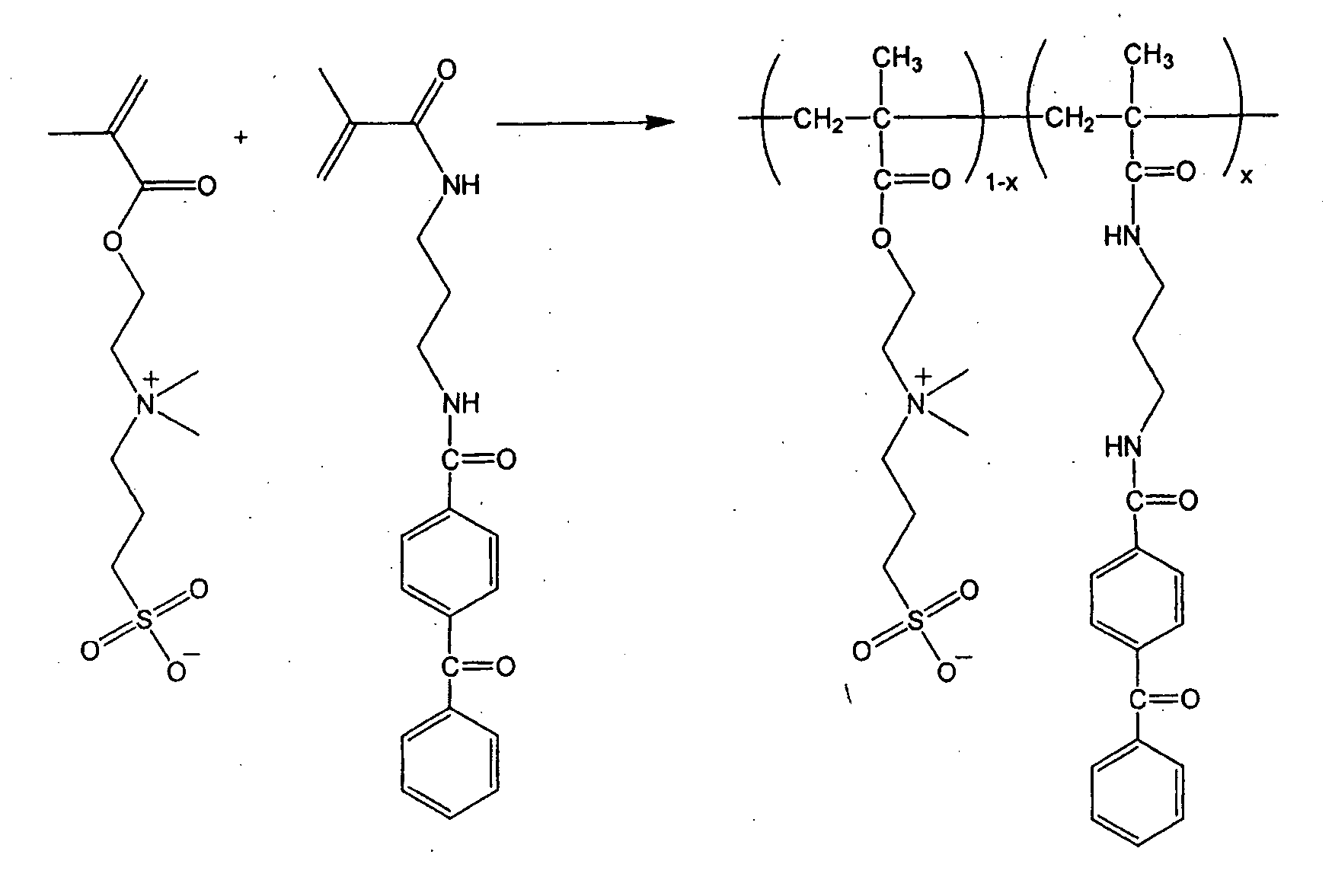
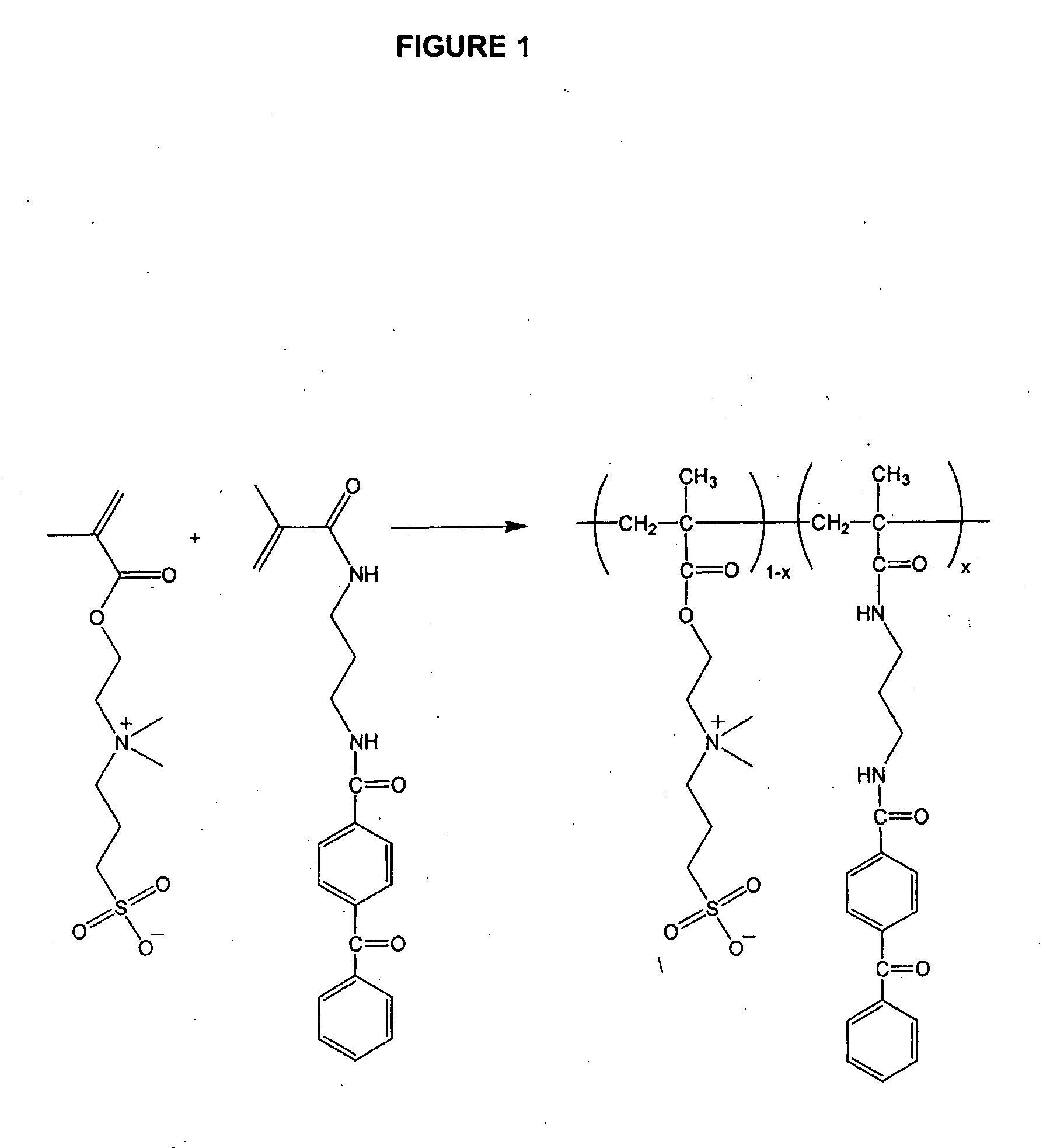
Zwitterionic polymers
InactiveUS20060183863A1Improve utilizationImprove versatilitySolid sorbent liquid separationCross-linkLaser desorption ionization mass spectrometry
Zwitterionic polymers bearing positive and negative charges are readily prepared from easily accessible precursors. The polymers show enhanced binding affinities for analytes under high salt conditions, compared to similar polymers bearing a charge of a single polarity. The polymers can also include an energy absorbing moiety for use in matrix assisted laser desorption / ionization mass spectrometry. The polymer can also include a photo-curable group, which can be used to form cross-links within the bulk polymer or between the polymer and a surface functionalized with a polymerizable moiety. The polymers are incorporated into devices of use for the analysis, capture, separation, or purification of an analyte. In an exemplary embodiment, the invention provides a substrate coated with a polymer of the invention, the substrate being adapted for use as a probe for a mass spectrometer.
Owner:BIO RAD LAB INC
Method and apparatus for maldi analysis
InactiveUS20020005478A1Extended stayBroaden TOF peak widthSamples introduction/extractionMaterial analysis by electric/magnetic meansFiberPeak value
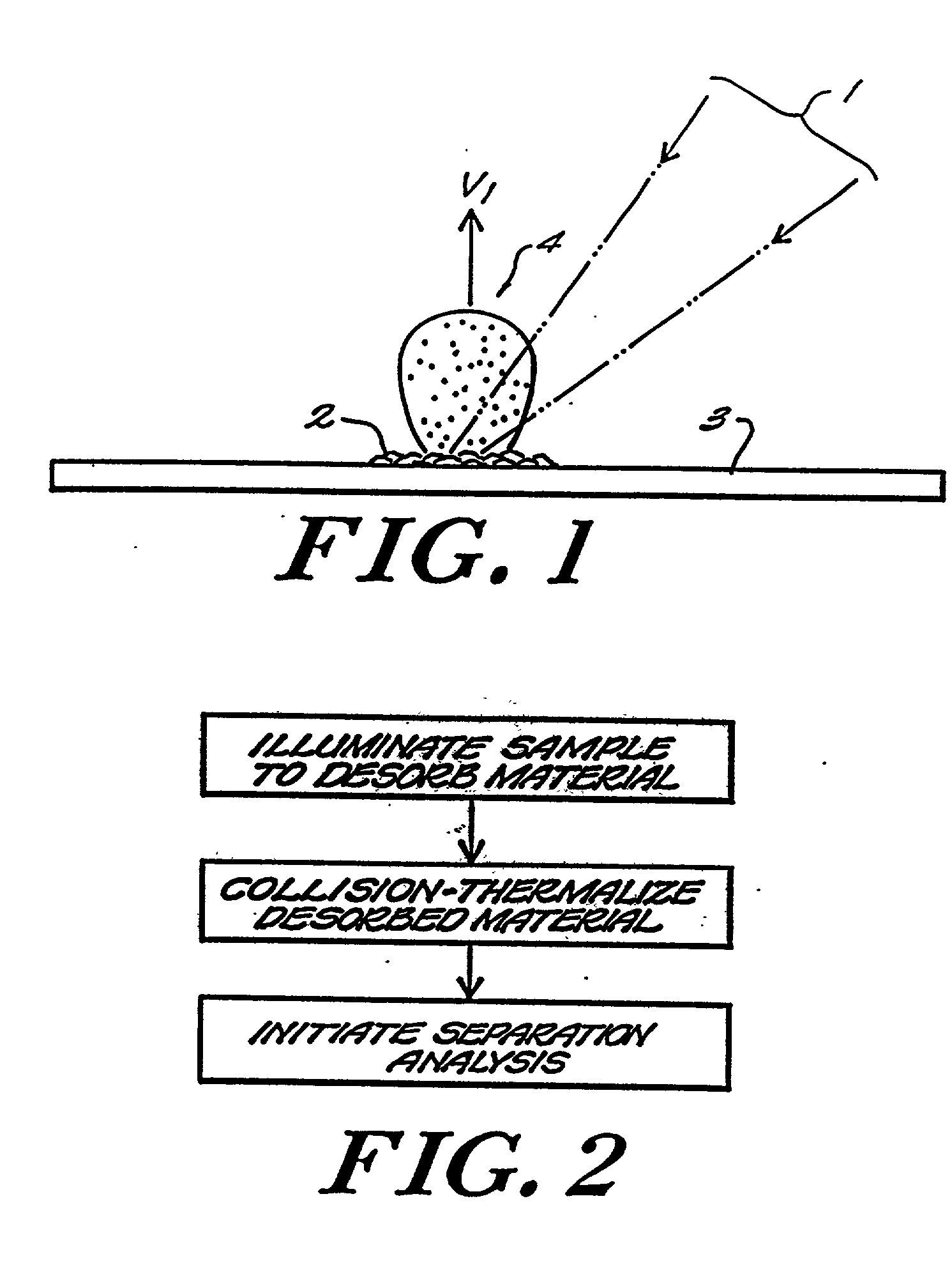
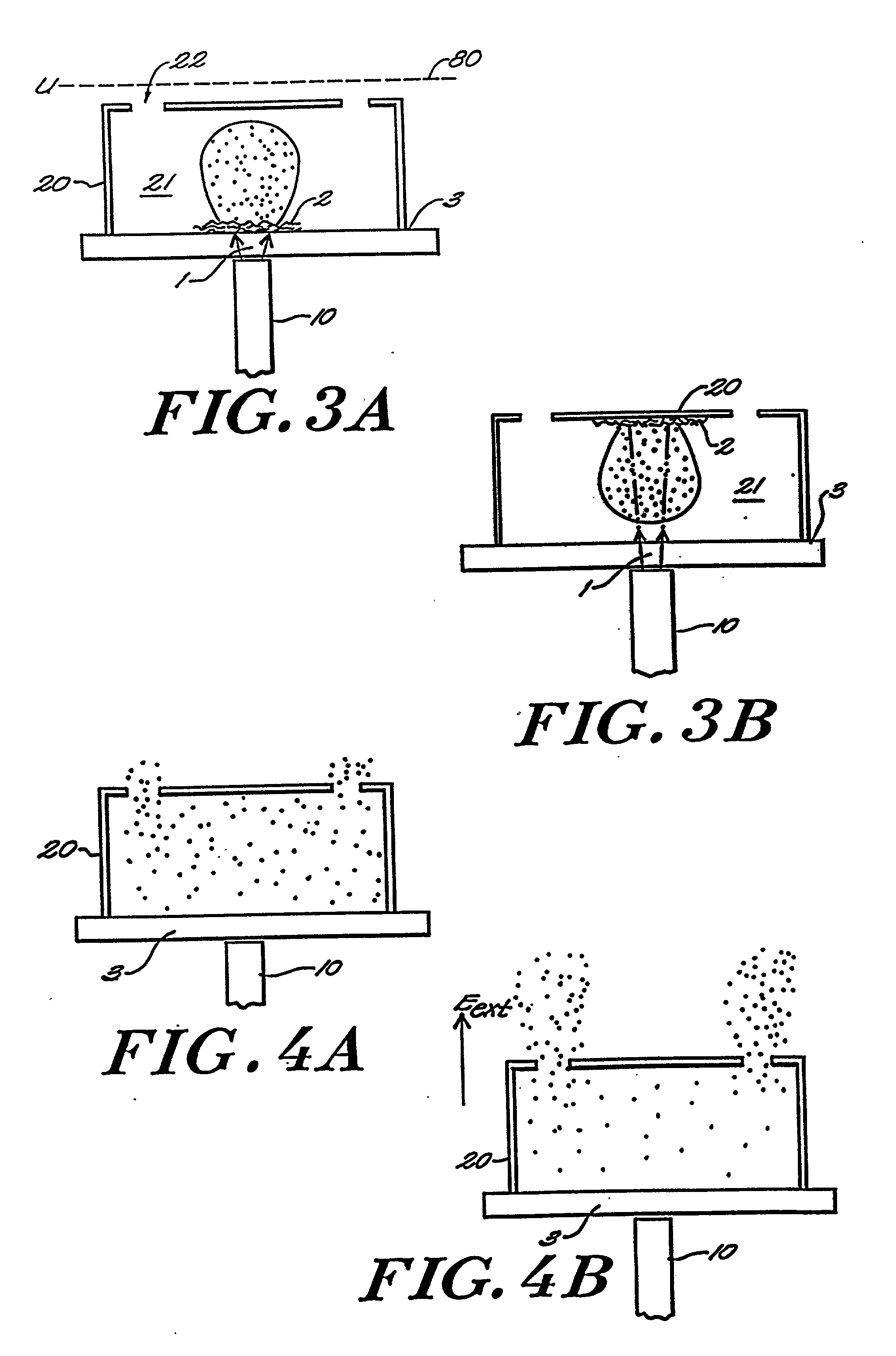
Matrix assisted laser desorption / ionization is performed in a manner to thermalize large analyte ions in a plume of desorbed material for spectroscopic analysis. The thermalized ions have a low or zero mean velocity and are presented at a well-defined instant in time, reducing artifacts and sharpening the spectral peaks. In one embodiment the light is delivered to a matrix or sample holder having a cover, baffle or compartment. The baffle or compartment impedes or contains a plume of desorbed material and the analyte undergoes collisions to lower its mean velocity and directionality. Thus "thermalized" the analyte ions are passed to a mass analysis instrument. In a preferred embodiment an optical fiber butts up against a thin transparent plate on which the specimen resides, with the matrix side in a vacuum acceleration chamber. A mechanical stage moves the specimen in both the x- and y- directions to select a point on the specimen which is to receive the radiation. The use of a fiber optic illuminator allows the entire stage assembly to be subsumed essentially within the dimensions of a conventional stage. In other embodiments, a thermalizing compartment is provided in a capillary tube about the end of the illumination fiber and the sample matrix is deposited along the inner cylindrical wall of the tube, so the capillary forms a migration path to the outlet for thermalization of the desorbed analyte. In other embodiments microstructures having the shape of a small lean-to, overhang or perforated cover plate, or providing a high aspect surface texture, provide the necessary containment to promote thermalization of the released analyte. A thin layer or cover of fibrous or permeable material may also be used to thermalize the analyte before mass analysis, and in other embodiment this material may also act as the substrate. An automated instrument may include a fixed array of illumination fibers which are illuminated at different times to eject samples from a corresponding array of points on the specimen.
Owner:AGENA BIOSCI
Direct mass spectrometric analysis of drug candidates targeting protein complexes
ActiveUS20100099200A1Improve throughputEasy to detectBioreactor/fermenter combinationsBiological substance pretreatmentsProtein targetProtein-protein complex
The invention relates to a method of using high mass matrix assisted laser desorption-ionization (MALDI) mass spectrometry for the qualitative and quantitative analysis of the effect of drug candidates on protein complexes such as protein-protein interactions in purified samples or complex biological matrices, as well as to the use of this method for lead compound optimization, drug characterization, drug manufacturing processes, and drug quality control processes, including automated high throughput applications.
Owner:COVALX
Ionization source for mass spectrometry analysis
ActiveUS20060145089A1Samples introduction/extractionMaterial analysis by optical meansGas phaseMass analyzer
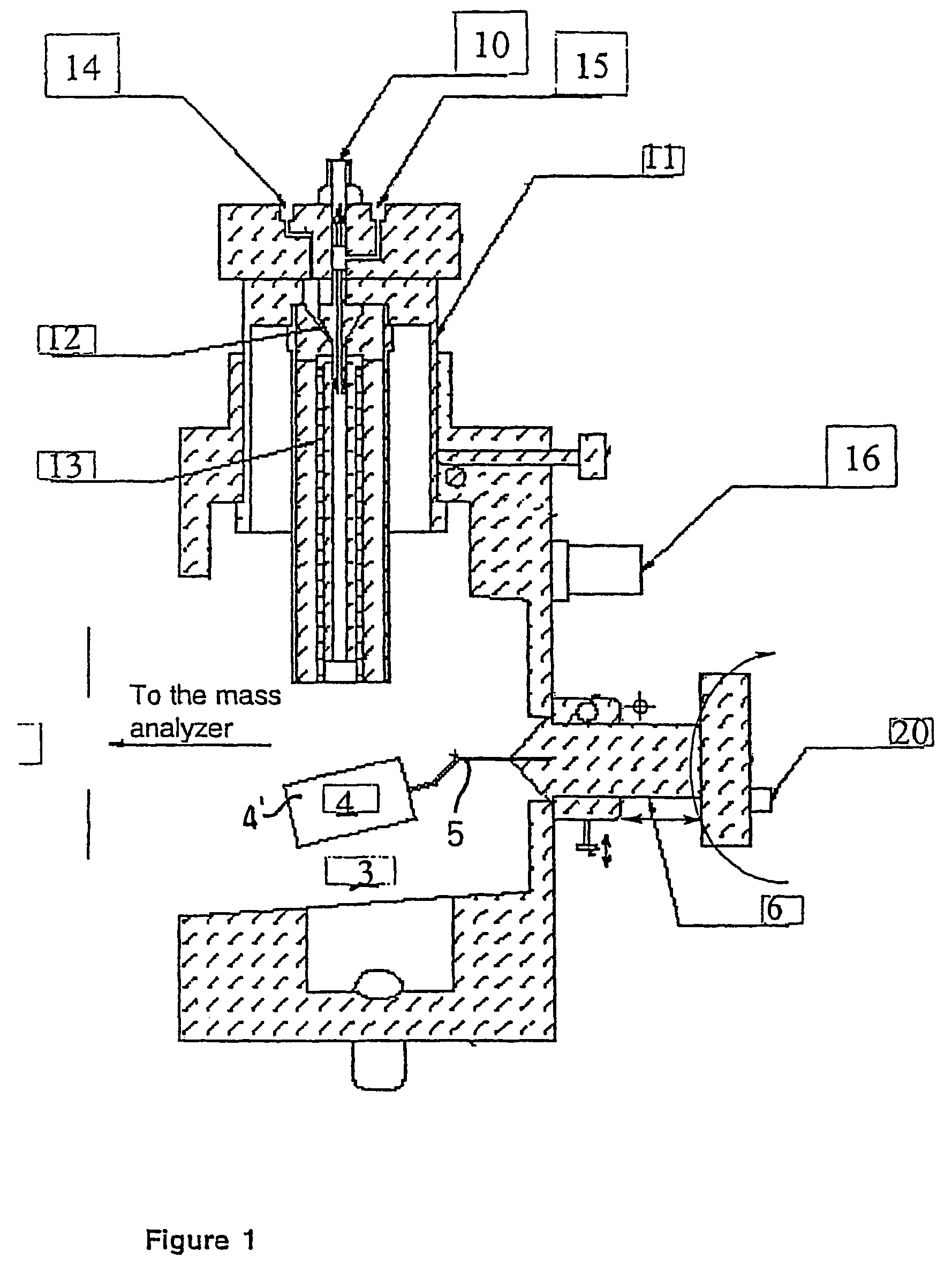
A new ionization source named Surface Activated Chemical Ionization (SACI) has been discovered and used to improve the sensitivity of the mass spectrometer. According to this invention the ionization chamber of a mass spectrometer is heated and contains a physical new surface to improve the ionization process. The analyte neutral molecules that are present in gas phase are ionized on this surface. The surface can be made of various materials and may also chemically modified so to bind different molecules. This new ionization source is able to generate ions with high molecular weight and low charge, an essential new key feature of the invention so to improve sensitivity and reduce noise. The new device can be especially used for the analysis of proteins, peptides and other macromolecules. The new invention overcomes some of the well known and critical limitations of the Electrospray (ESI) and Matrix Assisted Laser Desorption Ionization (MALDI) mass spectrometric techniques.
Owner:UNIV DELGI STUDI DI MILANO
Methods and devices for concentration and purification of analytes for chemical analysis including matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS)
InactiveUS20050116161A1High sample throughputReduce analysis costsBioreactor/fermenter combinationsElectrolysis componentsLaser desorption ionization mass spectrometryAnalyte
Owner:PROTEIN DISCOVERY
Apparatus and method for focusing ions and charged particles at atmospheric pressure
InactiveUS20020011560A1Samples introduction/extractionIsotope separationInductively coupled plasmaElectrospray
Improvements have been made for collection and focusing of ions generated from atmospheric pressure sources such as electrospray, atmospheric pressure chemical ionization, inductively coupled plasma, discharge, photoionization and atmospheric pressure matrix assisted laser desorption ionization. A high transmission electro-optical surface is placed between the source regions and the focusing regions to optimize the field geometries and strengths in each respective region. Compression ratios of greater than 5000 are capable of transferring virtually all ions from large volume dispersive ion regions into ion beam cross-sections of less than 1 mm. Embodiments of this invention are methods and devices for improving sensitivity of mass spectrometry when coupled to atmospheric pressure ionization sources.
Owner:SHEEHAN EDWARD W +1
Small volume liquid handling system
InactiveUS20080006653A1Avoid cloggingLaboratory glasswaresLiquid transferring devicesLaser desorption ionization mass spectrometrySolenoid valve
The present invention relates to non contact, vision (imaging device / camera) guided, vision-enabled, solenoid valve liquid dispensing systems and methods of using same for automation of complex laboratory workflows and especially useful for a variety of biomedical and other applications including automated front-end sample preparation for matrix assisted laser desorption / ionization (MALDI) mass spectrometry analysis.
Owner:BIOMACHINES
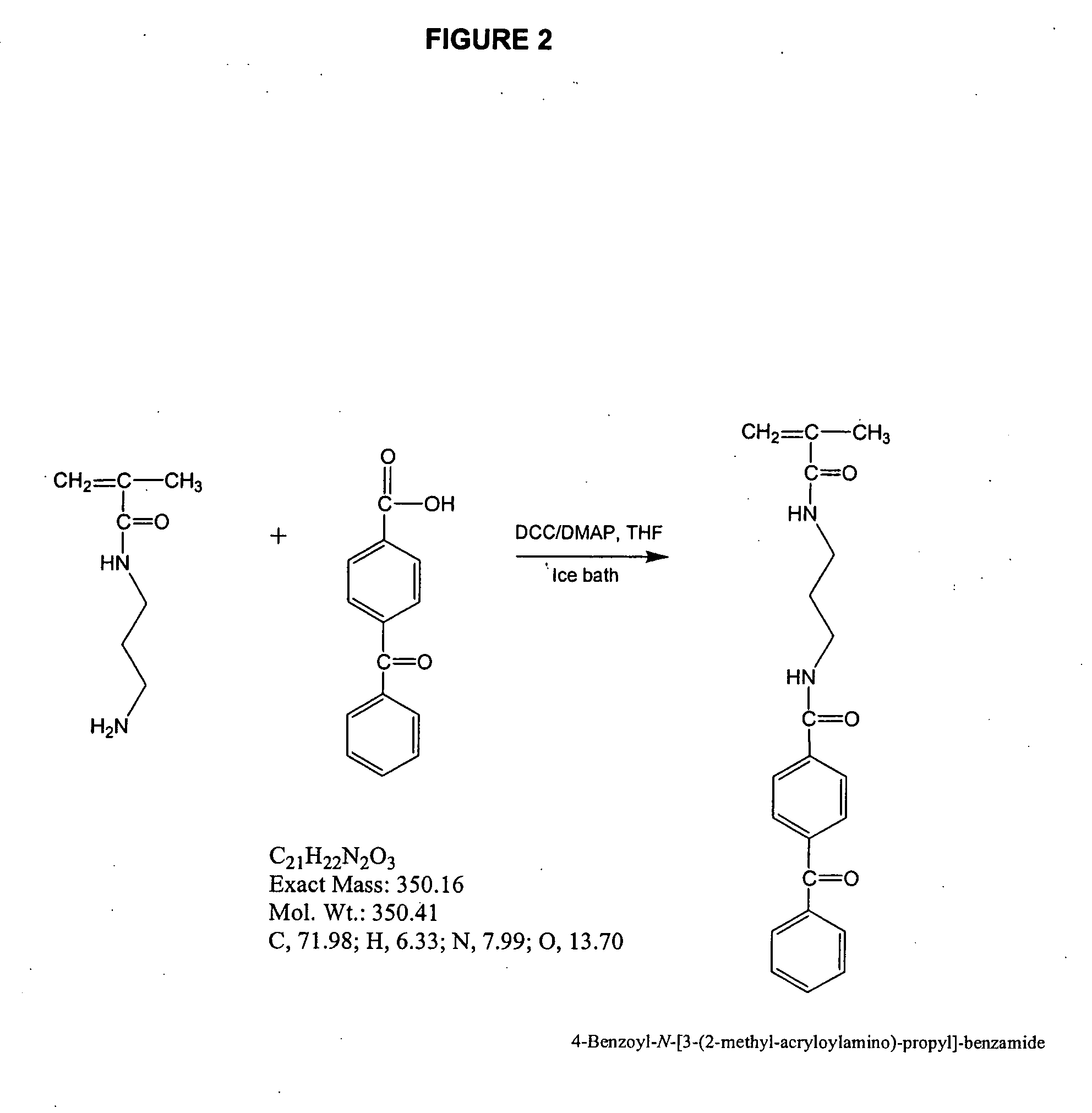
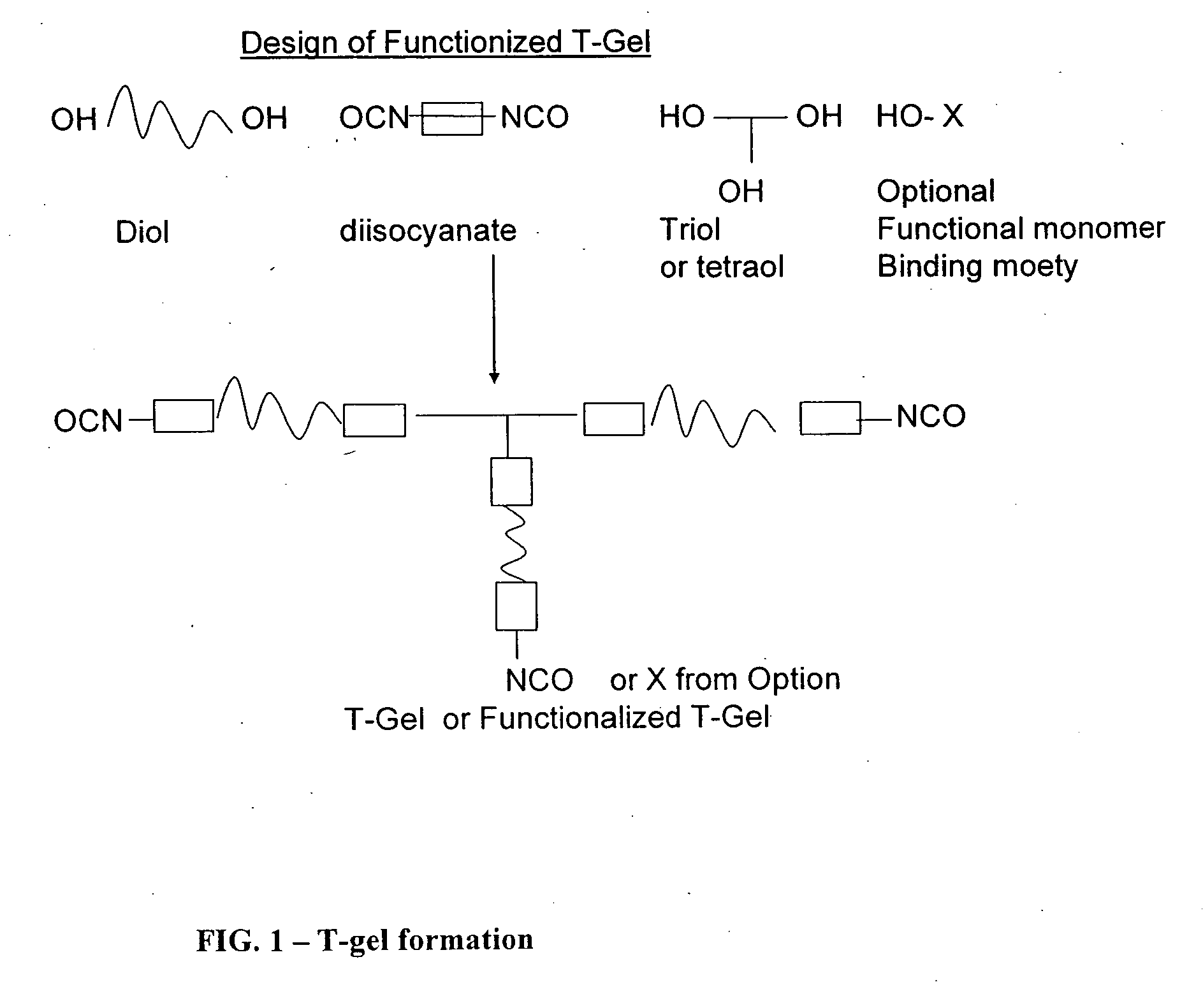
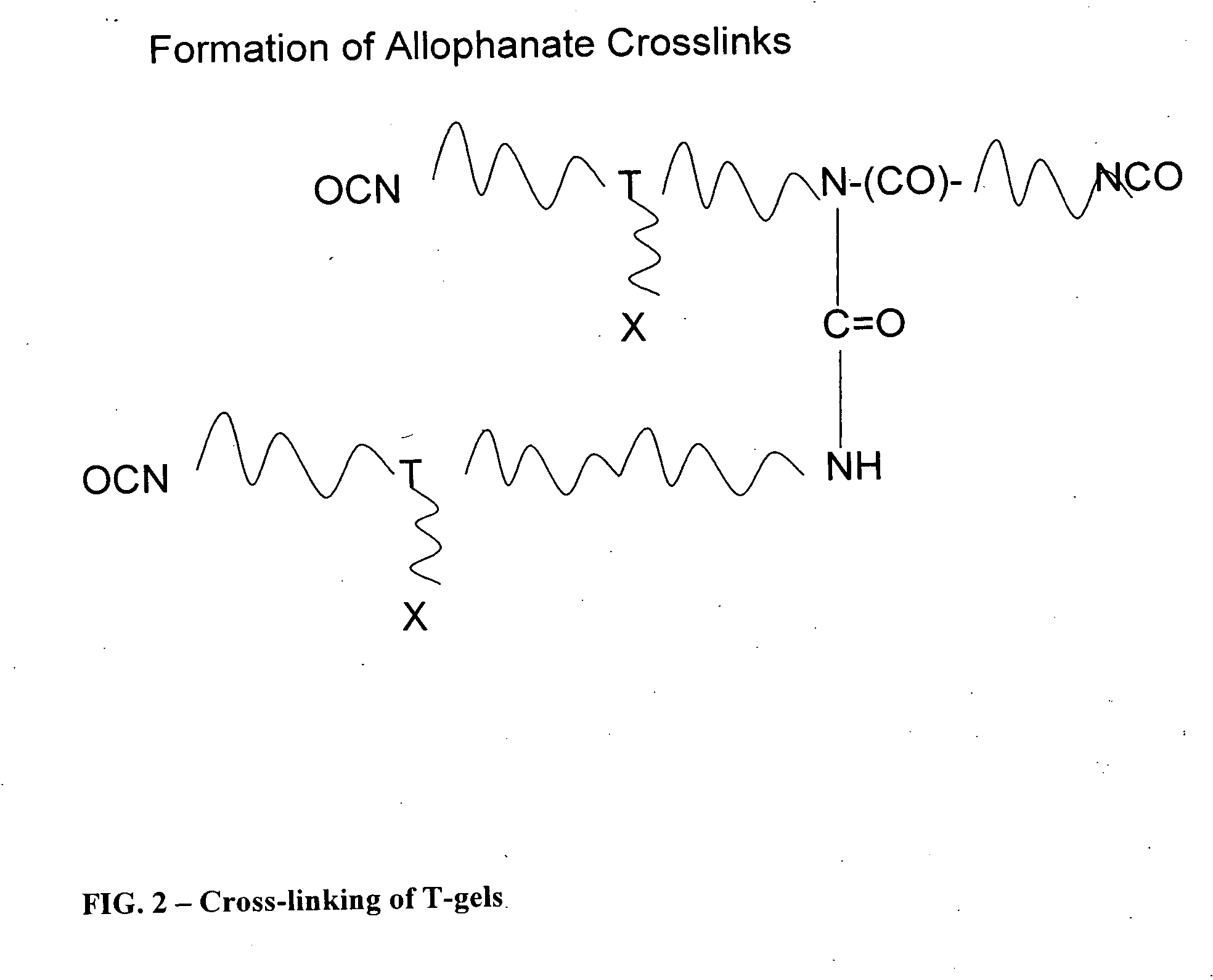
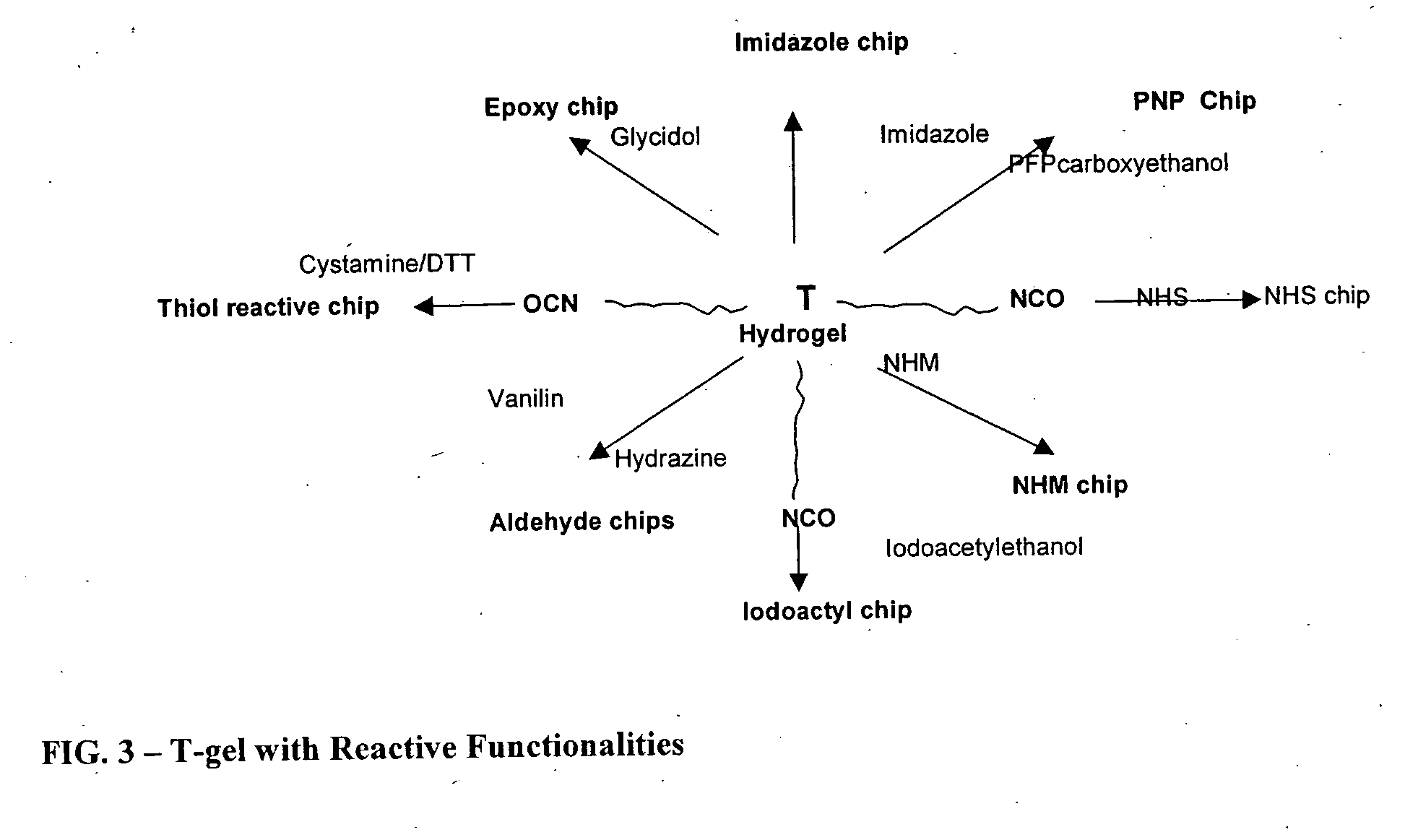
Reactive polyurethane-based polymers
InactiveUS20050112650A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLaser desorption ionization mass spectrometryFluorescence
Polyurethane polymers bearing multiple reactive groups are readily prepared from easily accessible precursors. The reactive groups of the polymers are then derivatized with binding functionalities for analytes, energy absorbing molecules for matrix assisted laser desorption / ionization mass spectrometry, fluorescent moieties and the like. The reactive groups can also be converted to different reactive groups having a desired avidity or specificity for a selected reaction partner. The polymers are incorporated into devices of use for the analysis, capture, separation, or purification of an analyte. In an exemplary embodiment, the invention provides a substrate coated with a polymer of the invention, the substrate being adapted for use as a probe for a mass spectrometer.
Owner:BIO RAD LAB INC
Method for detecting low concentrations of a target bacterium that uses phages to infect target bacterial cells
InactiveUS7166425B2Reduce concentrationBiocideComponent separationTime-of-flight mass spectrometryBacteriophage
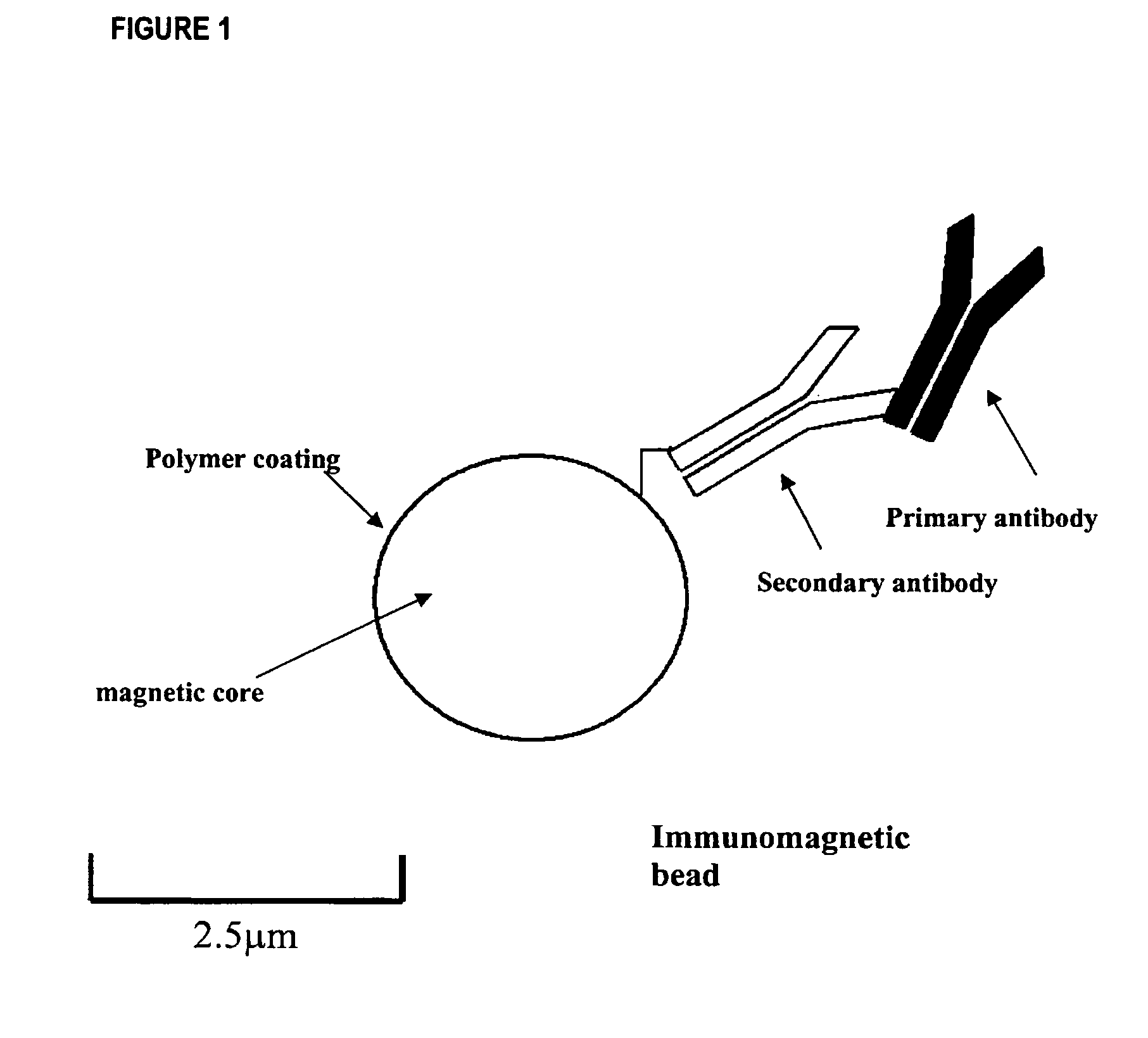
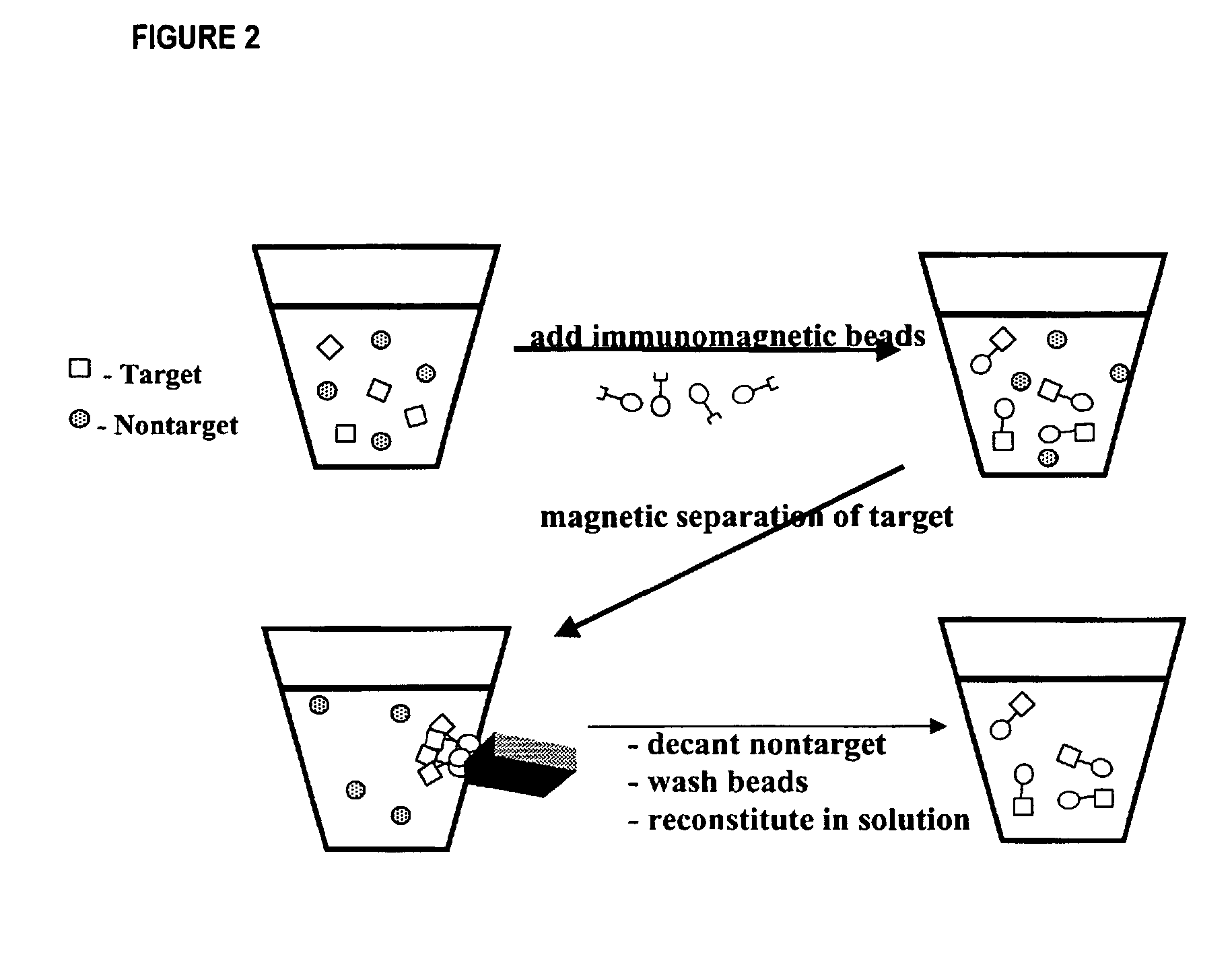
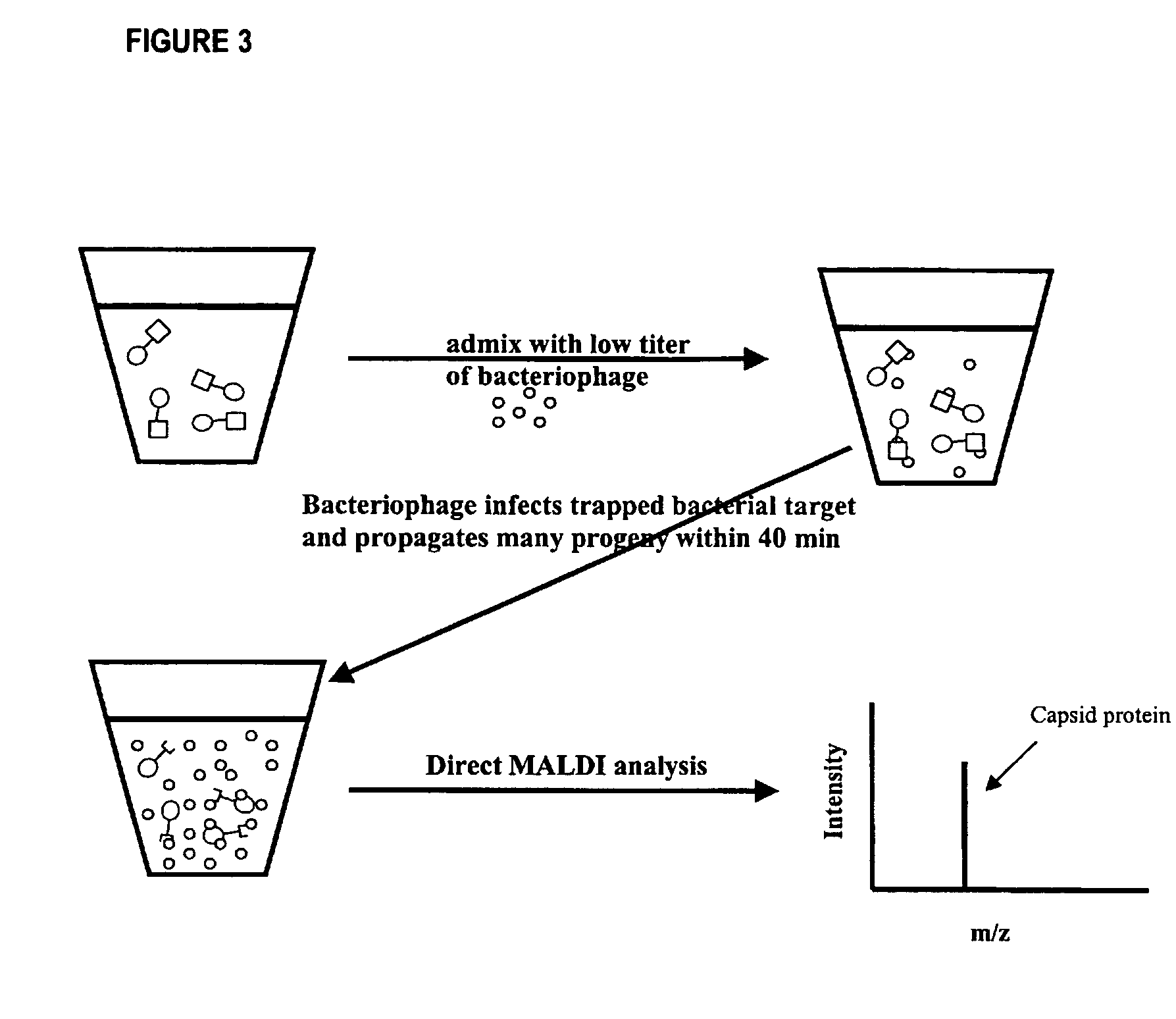
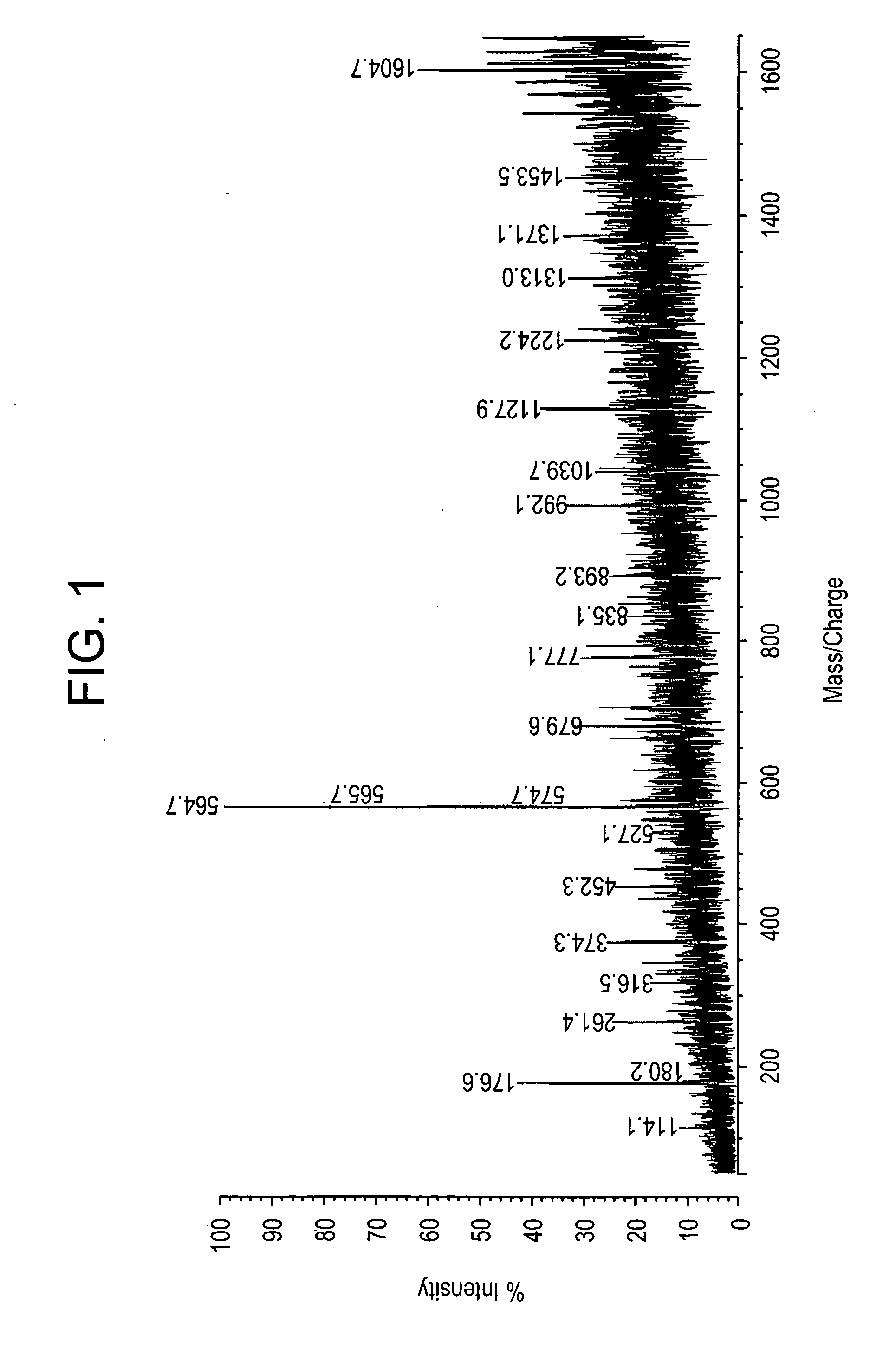
The invention is directed to a method for detecting low concentrations of bacteria in liquid solution that may or may not be complex liquid solutions. In one embodiment, immunomagnetic separation (IMS) is used to separate target bacterium that may be in a liquid mixture from other constituents in the mixture. A low concentration of a bacteriophage for the target bacteria is subsequently used to infect target bacterial cells that have been captured using the IMS technique. If at least a certain concentration of target bacterium are present, the bacteriophage will multiply to a point that is detectable. Matrix assisted laser desorption ionization / time-of-flight-mass spectrometry (MALDI / TOF-MS) is then used to produce a mass spectrum that is analyzed to determine if one or more proteins associated with the bacteriophage are present, thereby indirectly indicating that target bacterium were present in the liquid mixture.
Owner:COLORADO SCHOOL OF MINES
Methods for direct biomolecule identification by matrix-assisted laser desorption ionization (MALDI) mass spectrometry
InactiveUS20070114375A1Particle separator tubesIsotope separationLaser desorption ionization mass spectrometryMass Spectrometry-Mass Spectrometry
The present invention relates to the use of post source decay (PSD) or collision induced dissociation (CID) direct tissue (DT) MALDI-TOF or DT-MALDI-TOF-TOF mass spectrographic identification of biological molecules in a tissue or cellular sample without the need for further protein extraction. This method provides for studying cells or tissues by direct tissue MALDI (DT-MALDI), thereby substituting in situ protein release for further protein extraction. Mass / intensity data was processed with Mascot© software interrogation of the NCBI database. These results are proof of principle that DT-MALDI, combined with bioinformatics, can directly identify proteins in cells and tissues from their mass spectra.
Owner:NEW YORK UNIV
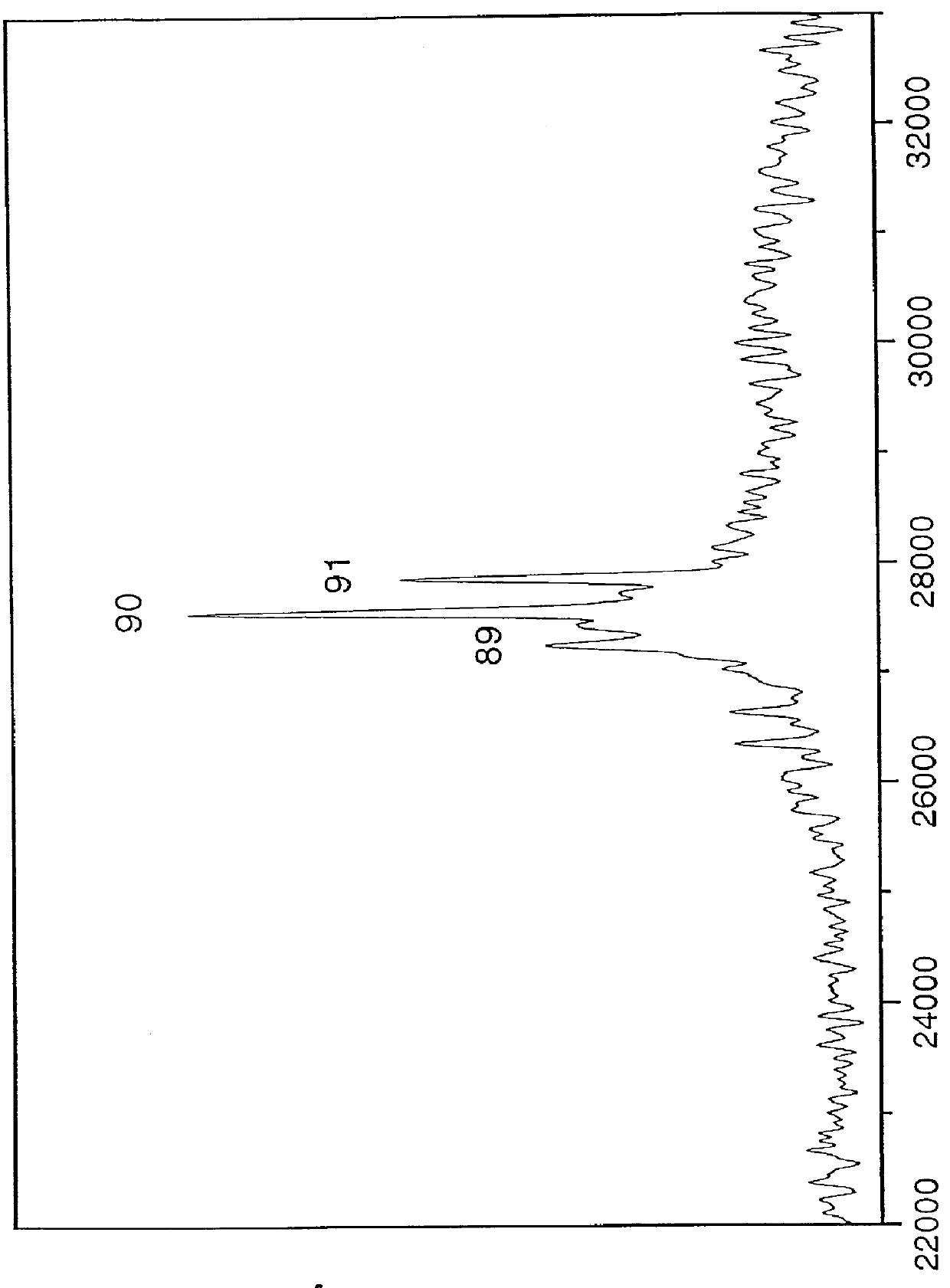
Ion sources for mass spectrometry
ActiveUS7176454B2Increase rangeParticle separator tubesCalibration apparatusMass Spectrometry-Mass SpectrometryMass analyzer
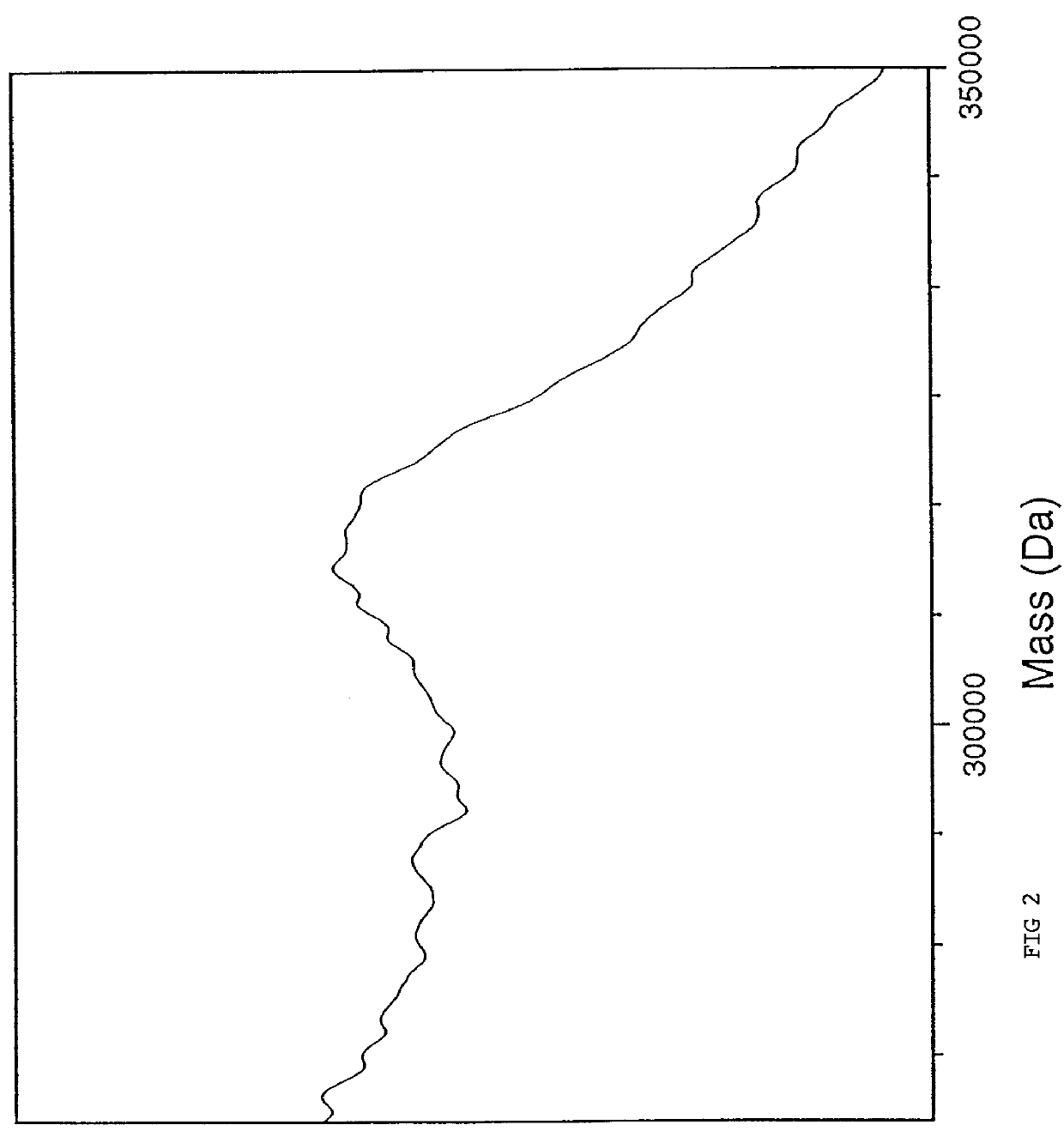
Provided are ion sources, methods of forming ions and mass analyzer systems. In various embodiments, the present teachings provide ion sources, methods for focusing ions from an ion source, and methods for operating a time-of-flight mass analyzer. In various embodiments, the present teachings relate to matrix-assisted laser desorption / ionization (MALDI) ion sources and methods of MALDI ion source operation, for use with mass analyzers. In various aspects, provided are ion sources and methods of operation thereof that facilitate increasing one or more of sensitivity and resolution of a TOF mass analyzer configured for multiple modes of operation.
Owner:APPL BIOSYSTEMS INC +1
Method and apparatus for desorption and ionization of analytes
InactiveUS20070065949A1Samples introduction/extractionWithdrawing sample devicesTime-of-flight mass spectrometryAnalyte
Owner:BAYLOR COLLEGE OF MEDICINE
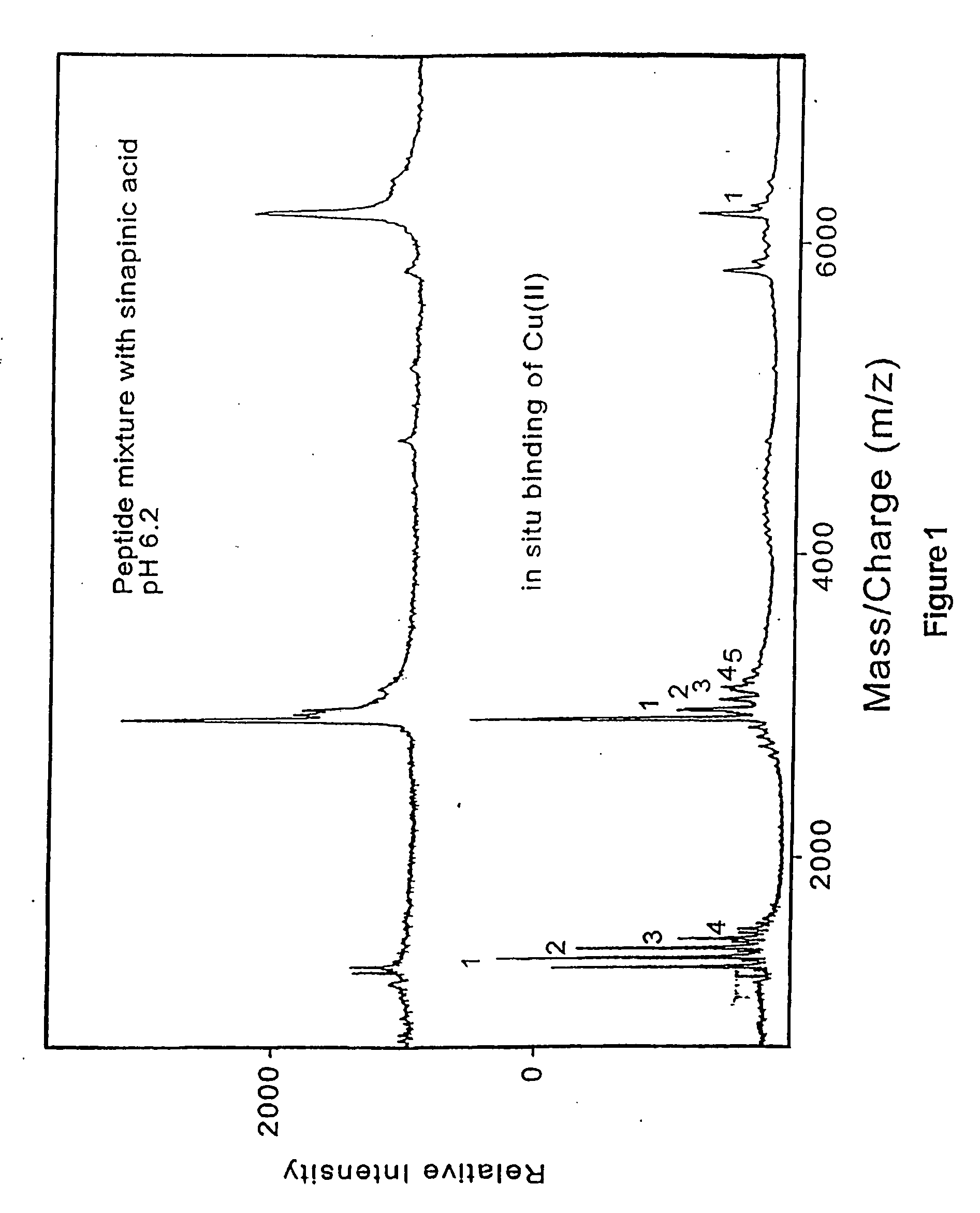
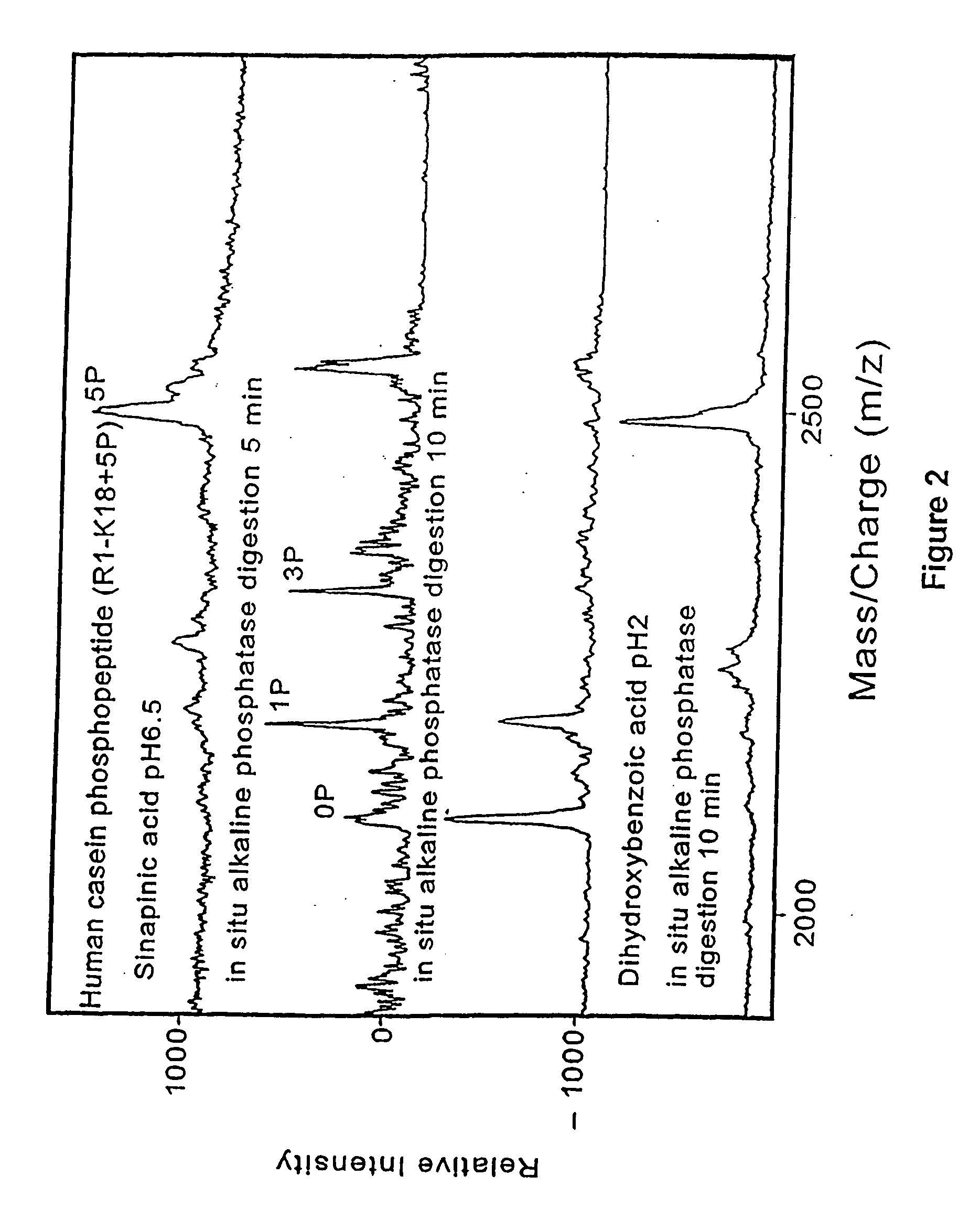
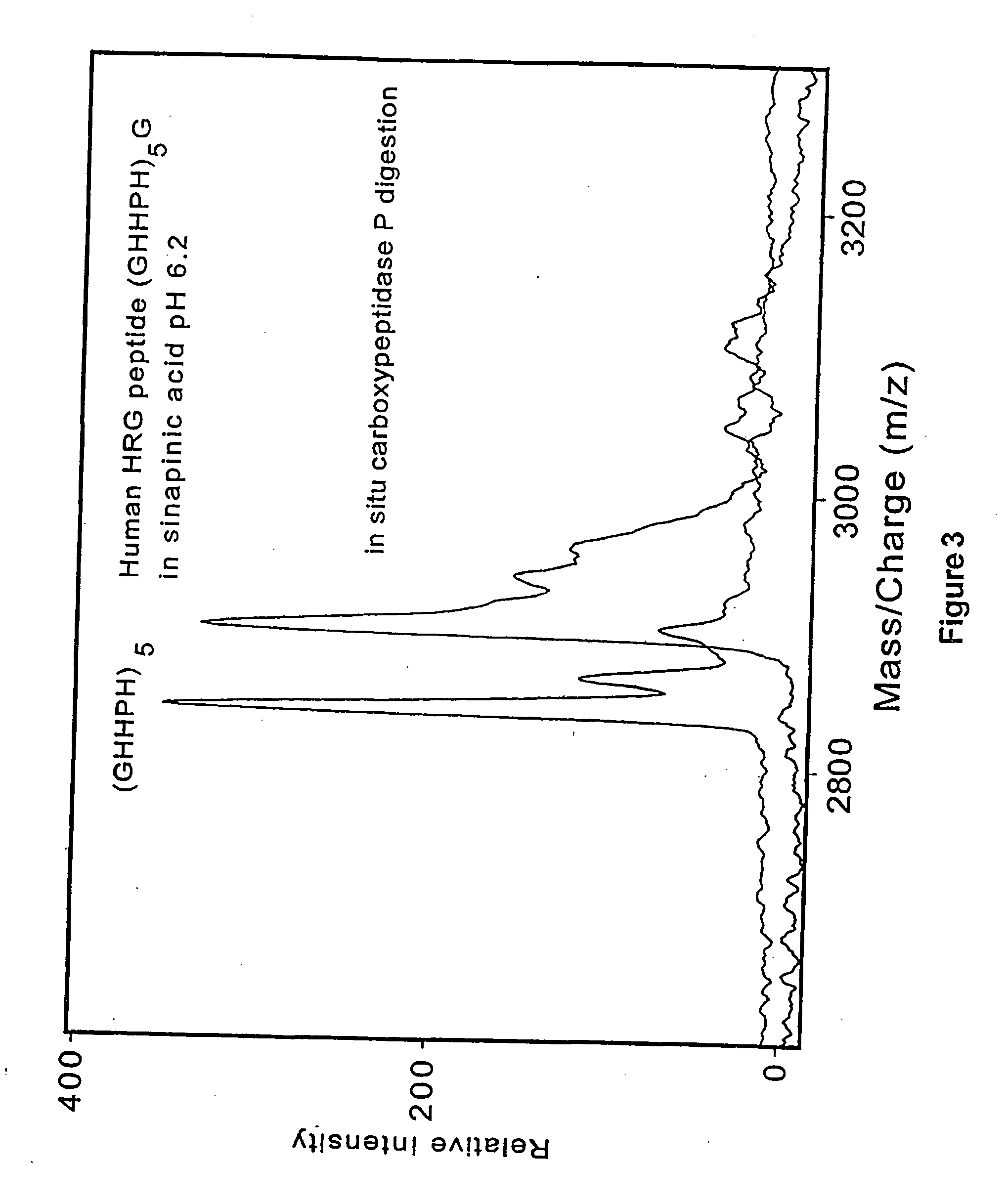
Methods and compositions for assessing a sample by maldi mass spectrometry
The invention provides methods for preparing a sample for matrix-assisted laser desorption ionization (MALDI). In general, the methods involve: binding analytes in a sample to capture agents that are bound to matrix that is present in a plurality of wells of a multi-well sample plate, washing any unbound analytes from the matrix, cleaving any bound analyte / capture agent complexes with a MALDI cleavage agent, and depositing the cleavage products on a multi-sample MALDI sample plate. Kits and other compositions are provided for performing the subject methods. The subject invention finds use in methods of simultaneously assessing the presence of several analytes in a single sample, and, as such, the invention finds use in a variety of different medical, research and proteomics applications.
Owner:AGILENT TECH INC
Ionization source for mass spectrometry analysis
ActiveUS7368728B2Samples introduction/extractionMaterial analysis by optical meansLaser desorption ionization mass spectrometryGas phase
A new ionization source named Surface Activated Chemical Ionization (SACI) has been discovered and used to improve the sensitivity of the mass spectrometer. According to this invention the ionization chamber of a mass spectrometer is heated and contains a physical new surface to improve the ionization process. The analyte neutral molecules that are present in gas phase are ionized on this surface. The surface can be made of various materials and may also chemically modified so to bind different molecules. This new ionization source is able to generate ions with high molecular weight and low charge, an essential new key feature of the invention so to improve sensitivity and reduce noise. The new device can be especially used for the analysis of proteins, peptides and other macromolecules. The new invention overcomes some of the well known and critical limitations of the Electrospray (ESI) and Matrix Assisted Laser Desorption Ionization (MALDI) mass spectrometric techniques.
Owner:UNIV DELGI STUDI DI MILANO
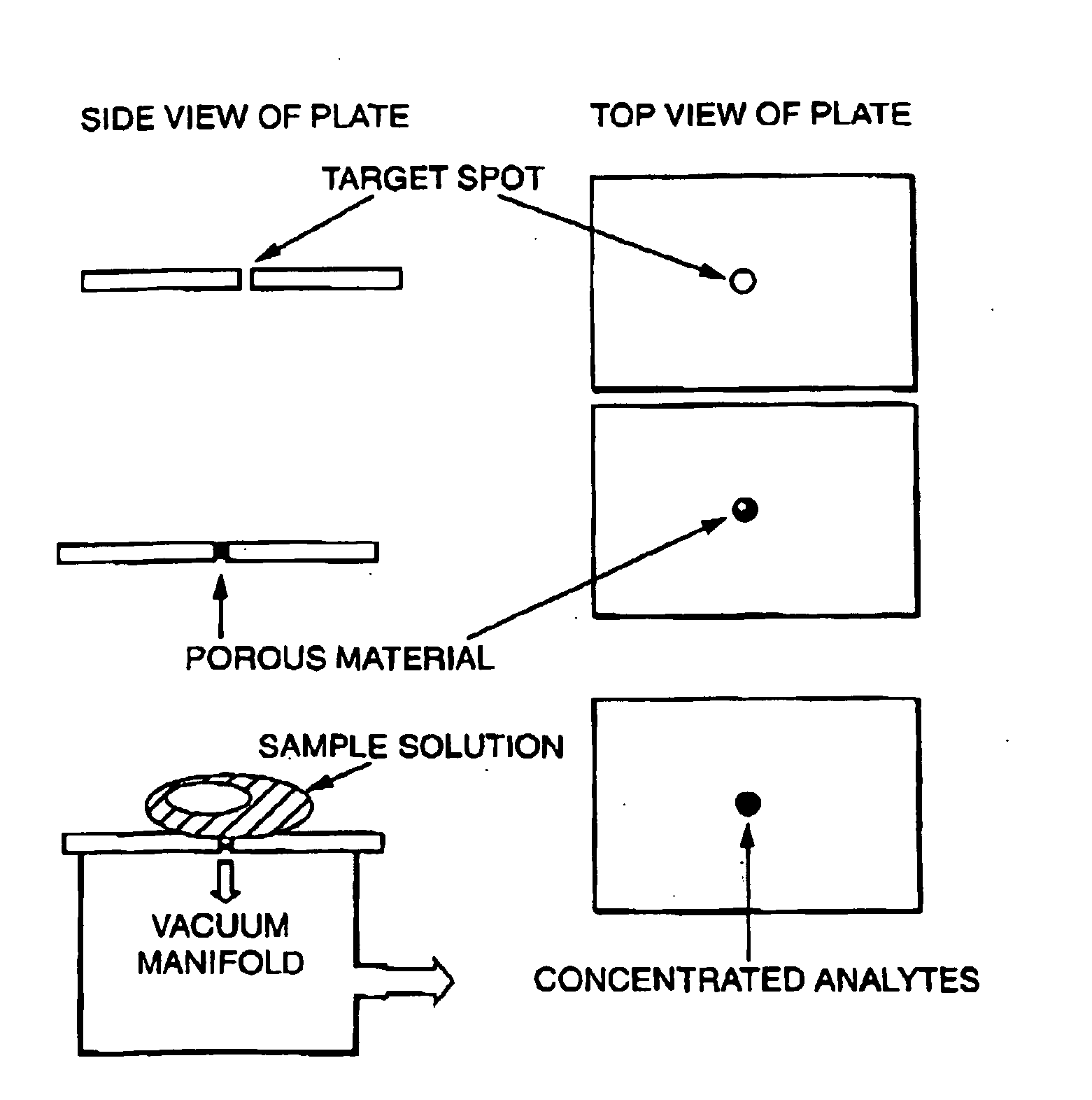
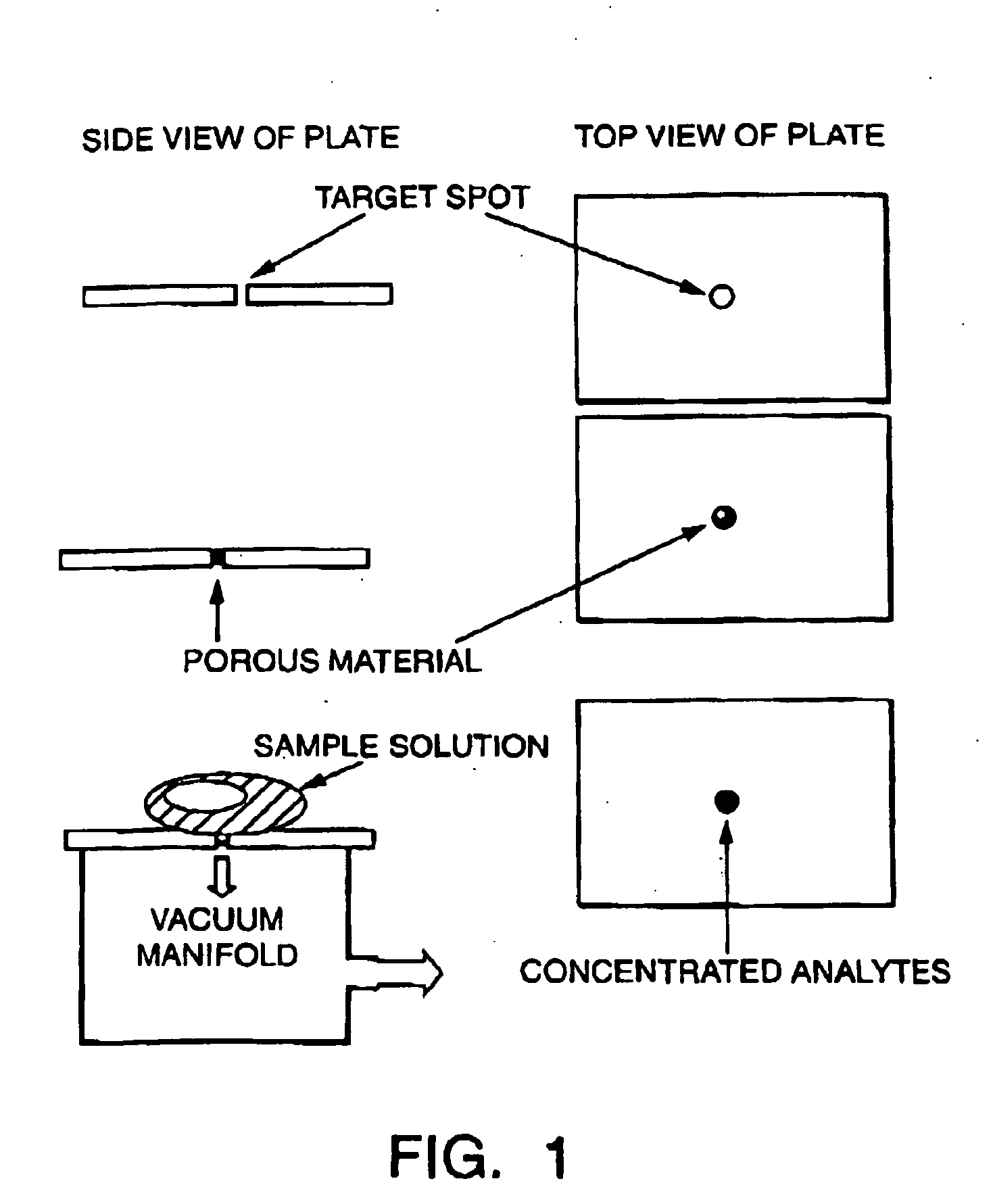
Sample concentration maldi plates for maldi mass spectrometry
InactiveUS20050130222A1Simple methodWide rangeBioreactor/fermenter combinationsBiological substance pretreatmentsLaser desorption ionization mass spectrometryAnalyte
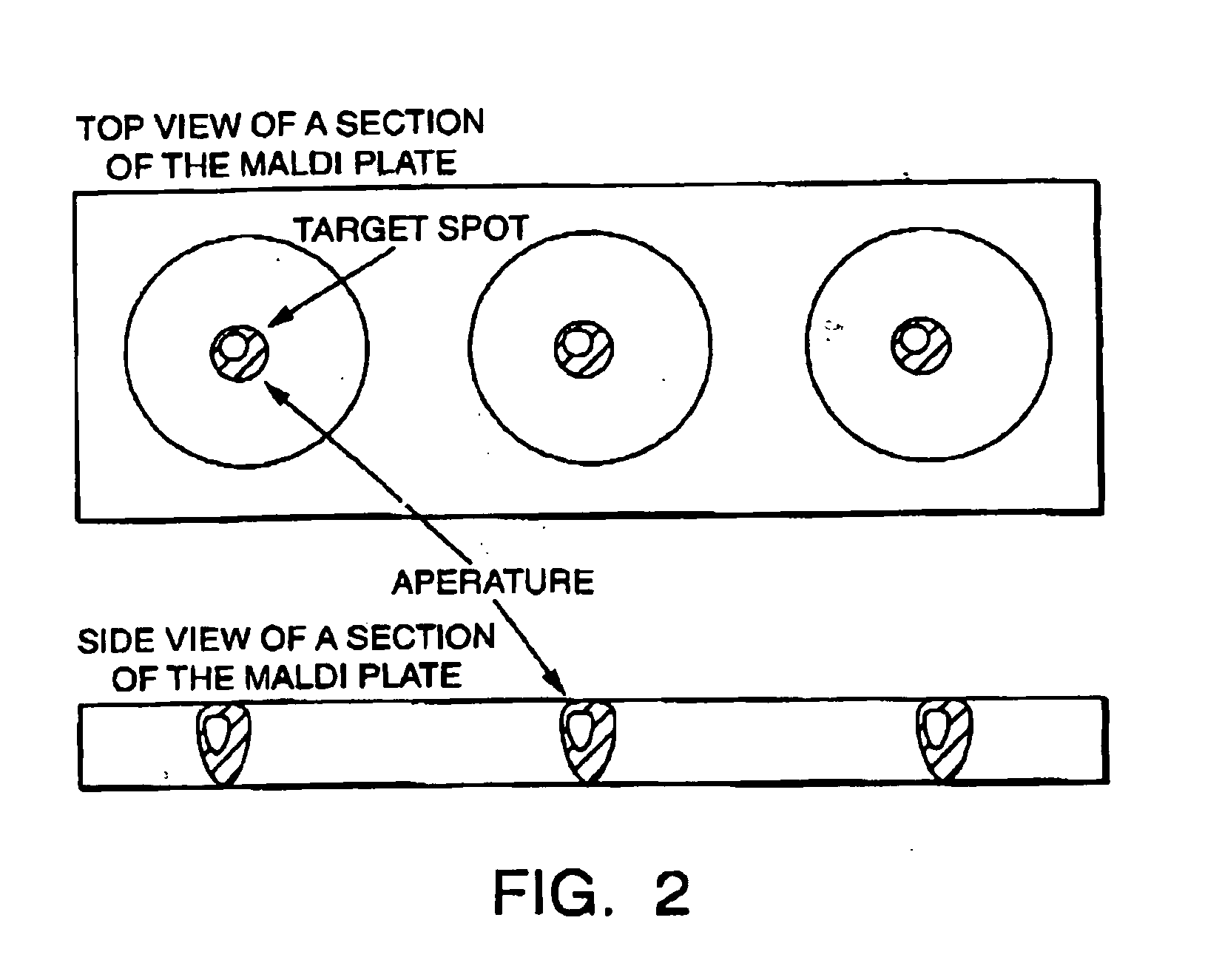
A novel Matrix-Assisted Laser Desorption / Ionization Mass Spectrometry (MALDI-MS) sample support plate is described. The plate comprises a top sample presentation surface and a lower surface, wherein the top sample presentation surface comprises at least one aperture for receiving a sample. The aperture extends through and between the top sample presentation surface and the bottom surface, and contains a porous material that retains and concentrates at the target spot analyte and matrix molecules contained in the sample on the surface of the aperture. Methods for making and using the sample support plate in conventional and automated MALDI-MS are also described.
Owner:WATERS TECH CORP
Application for analyzing micromolecules with irradiating nanometer carbon spots serving as matrix assisted laser desorption ionization (MALDI) matrix
InactiveCN102645481AOvercoming the inability to efficiently analyze small molecule samplesReduce processing requirementsMaterial analysis by electric/magnetic meansSpectroscopyBiomarker discovery
The invention discloses application for analyzing micromolecules with irradiating nanometer carbon spots serving as a matrix assisted laser desorption ionization (MALDI) matrix. The carbon spots serve as an analysis method of the MALDI matrix and are suitable to mass spectrum analysis for the micromolecules with the m / z between 150-1000. Suitable molecule types comprise amino acid, polypeptide, beta-exhilarant, fatty acid, polymer and the like. When the method is used for detecting the molecule with the m / z between 150-1000, background peaks hardly exist to produce background interference. The method can be effectively used in the fields of organic and biomass spectrometry, spectrometry imaging, protein spectroscopy, metabonomics, biomarker discovery, environment analysis and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
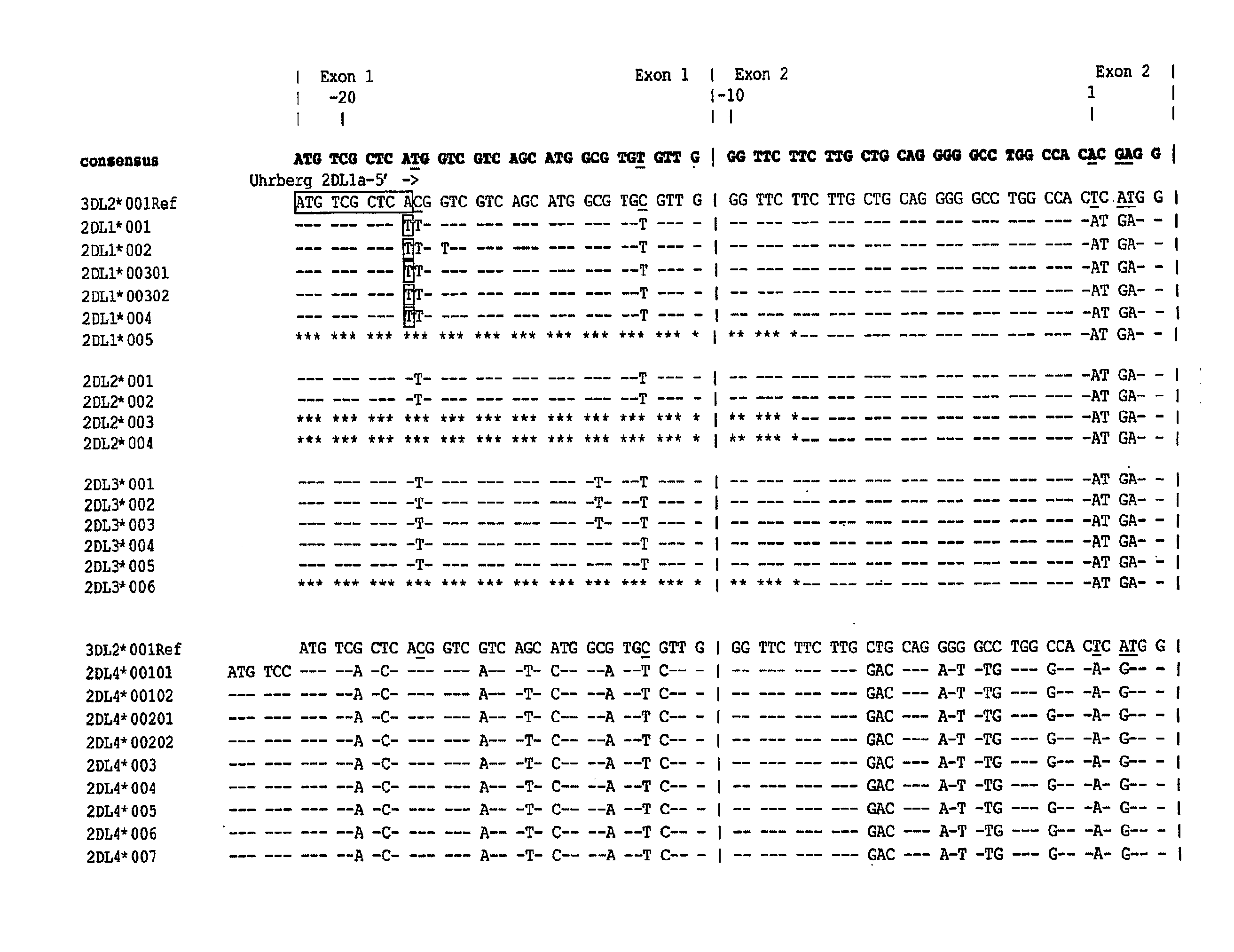
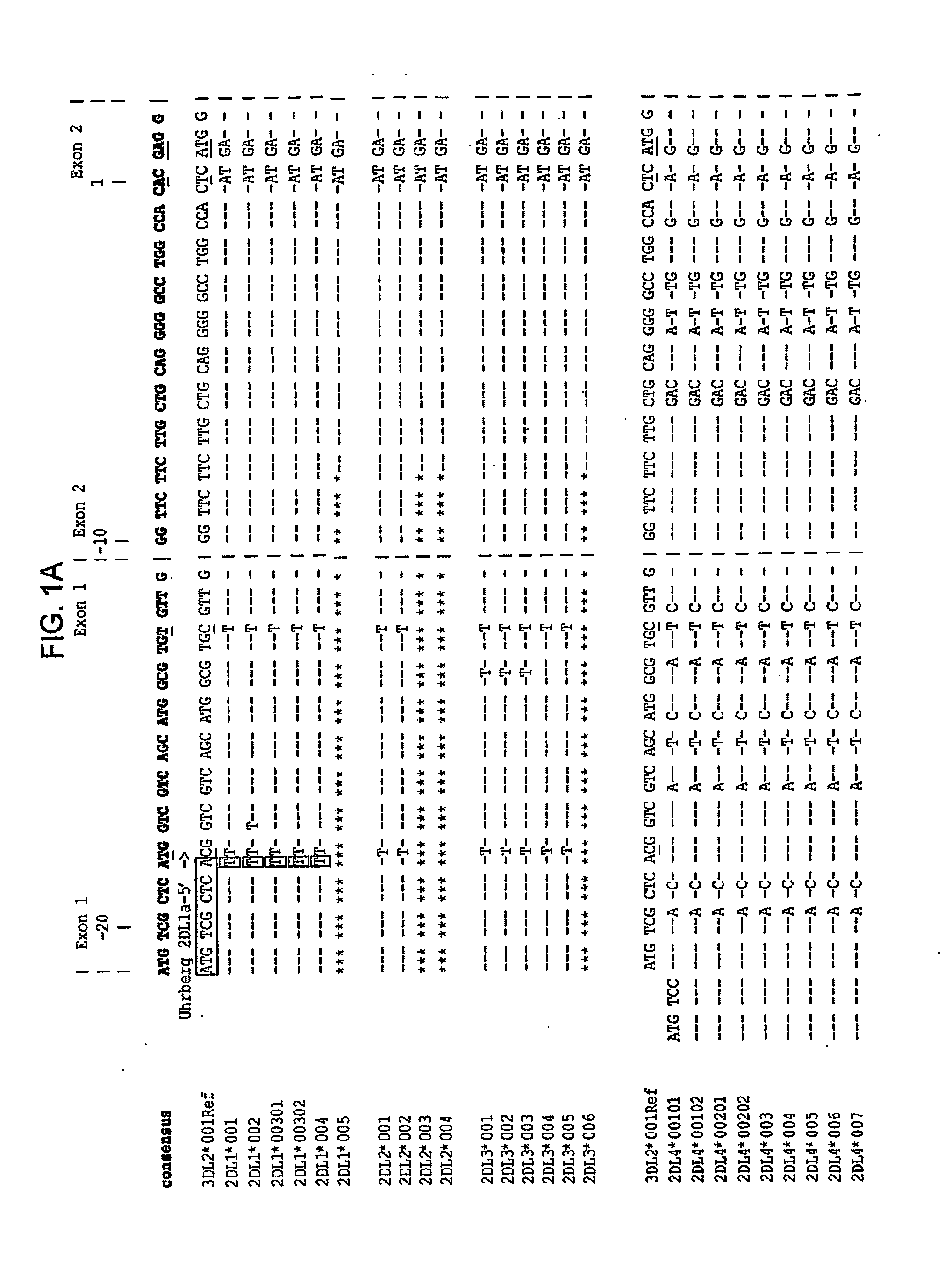
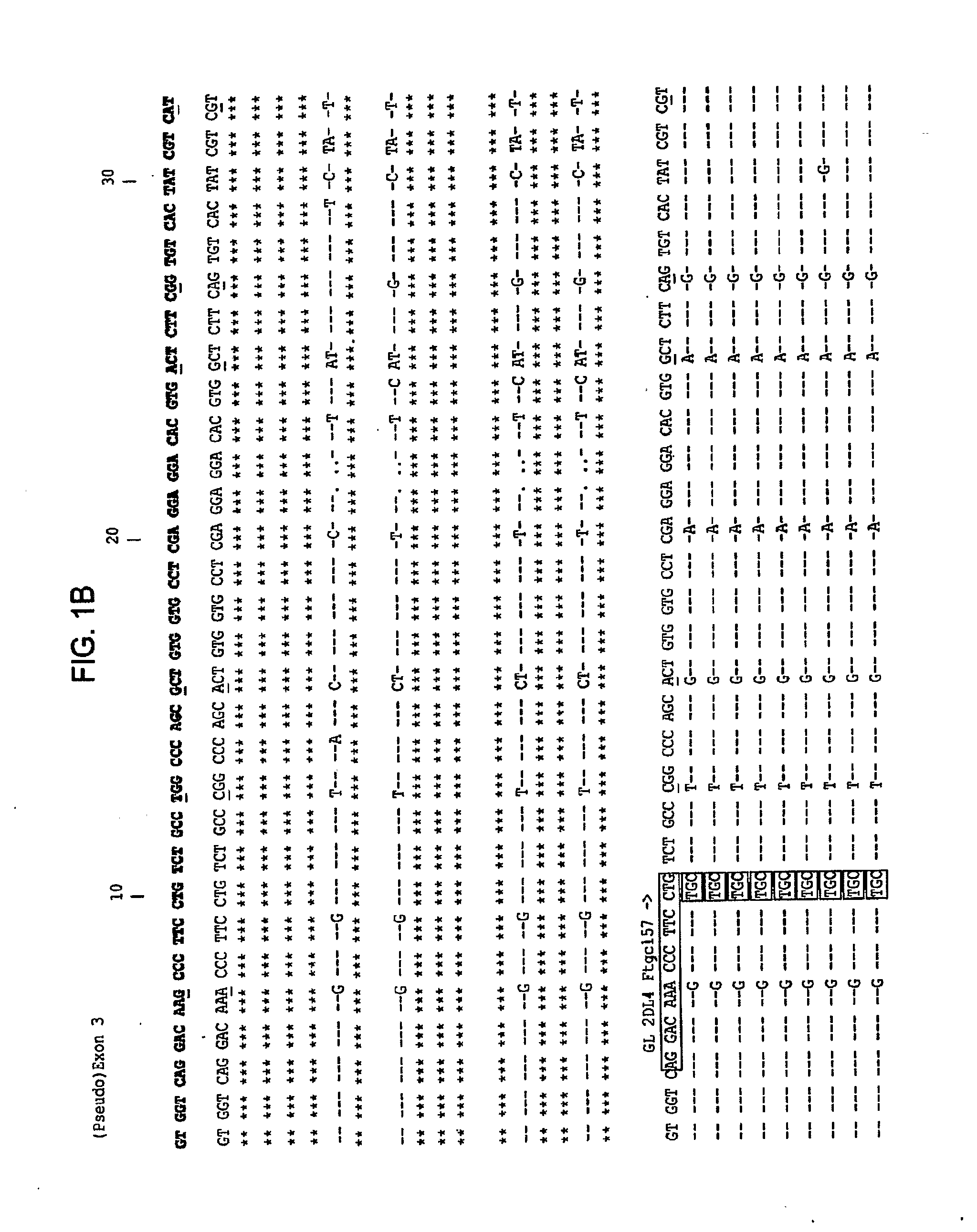
Methods and Compositions for Kir Genotyping
InactiveUS20080213787A1Sugar derivativesMicrobiological testing/measurementGene clusterIonization time of flight
The present invention provides methods for single nucleotide polymorphism (SNP)-based killer cell immunoglobulin-like receptor (KIR) gene cluster genotyping using the matrix-assisted laser desorption / ionization time-of-flight (MALDI-TOF) mass spectrometer. In general, the methods involve amplifying a plurality of target sequences of a plurality of KIR genes, and detecting the presence or absence of a plurality of single SNPs of the plurality of KIR genes by MALDI-TOF mass spectrometry. The invention also features compositions, including arrays of capture primers and optionally extension primers on a substrate surface, and kits, for use in the methods of the invention.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
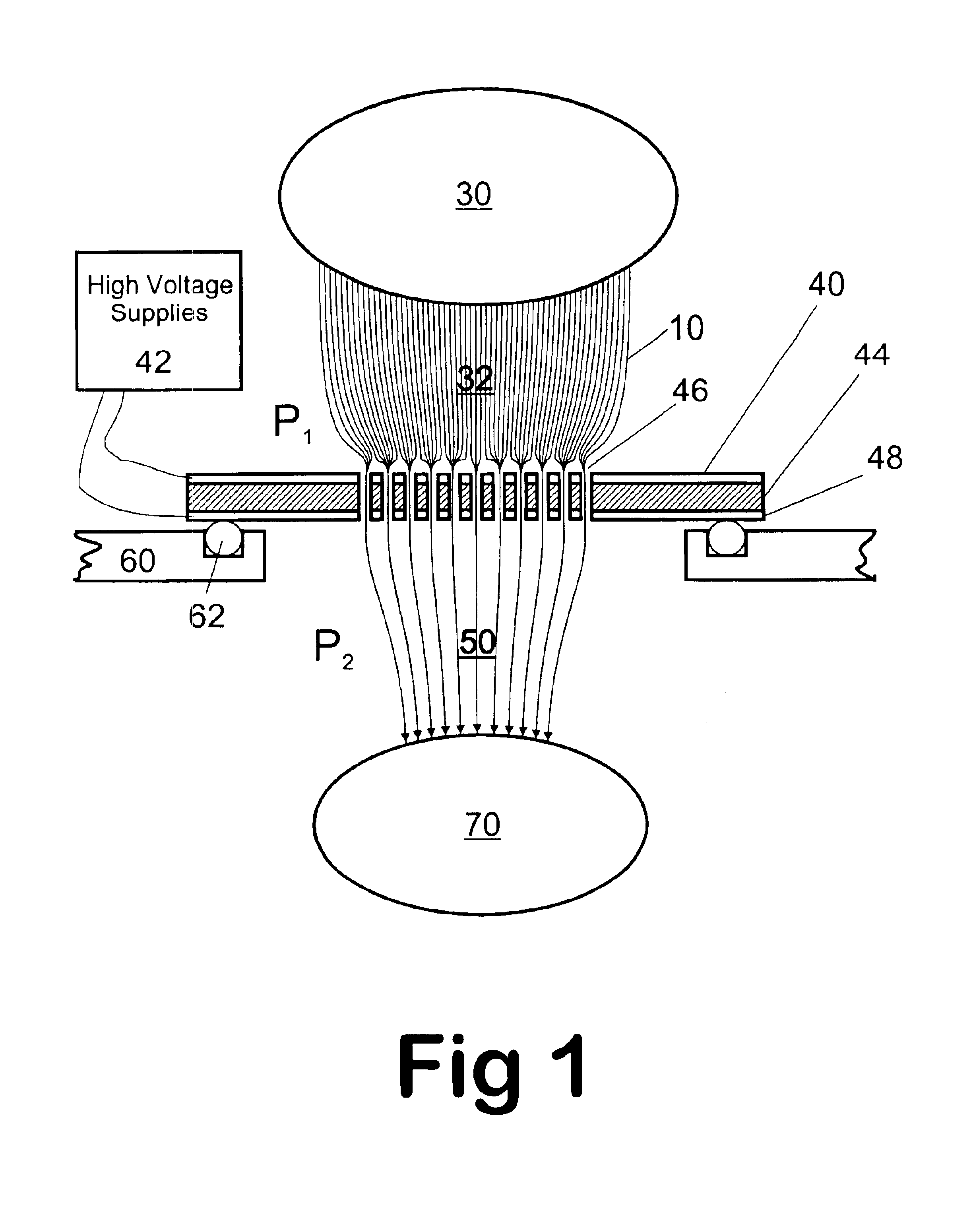
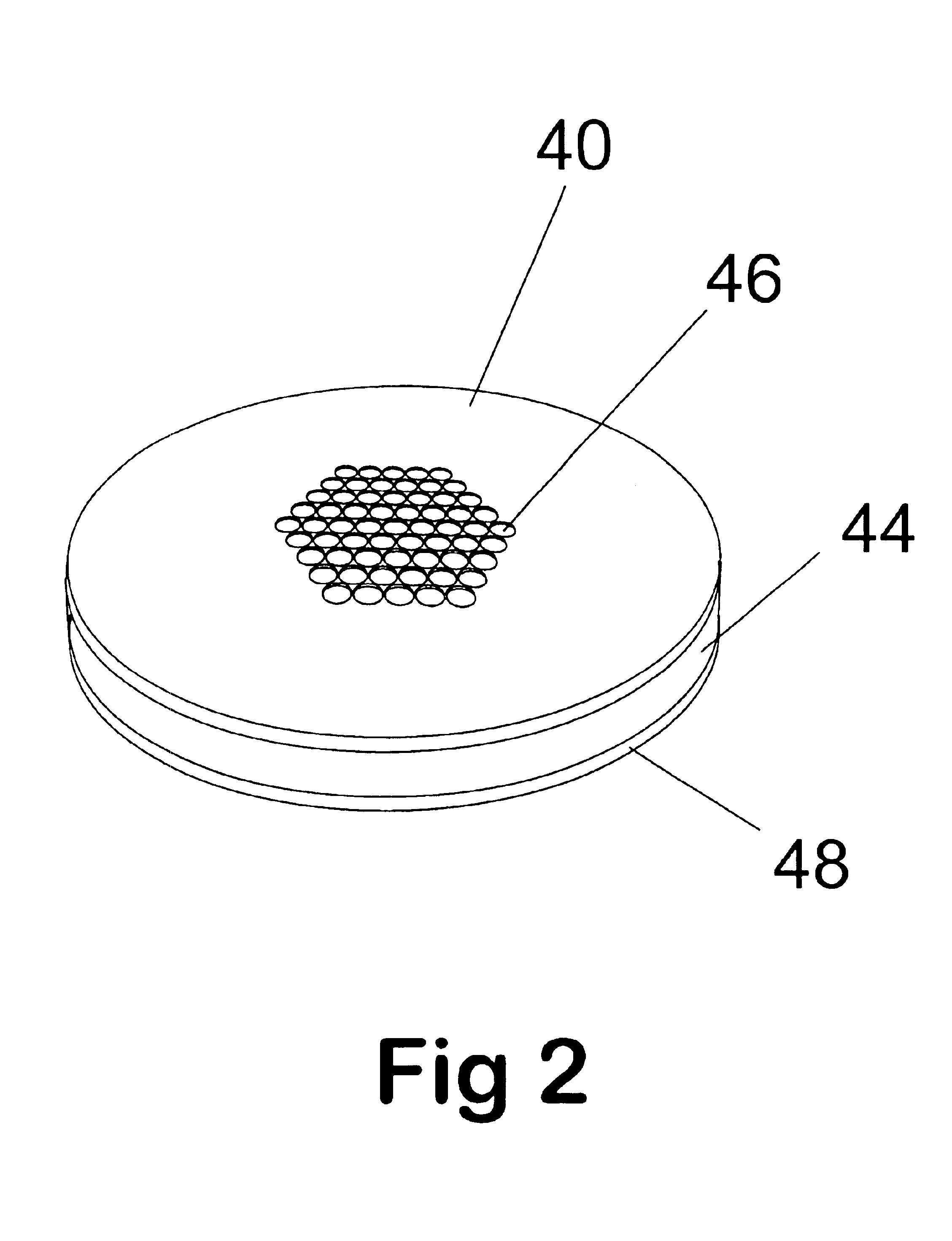
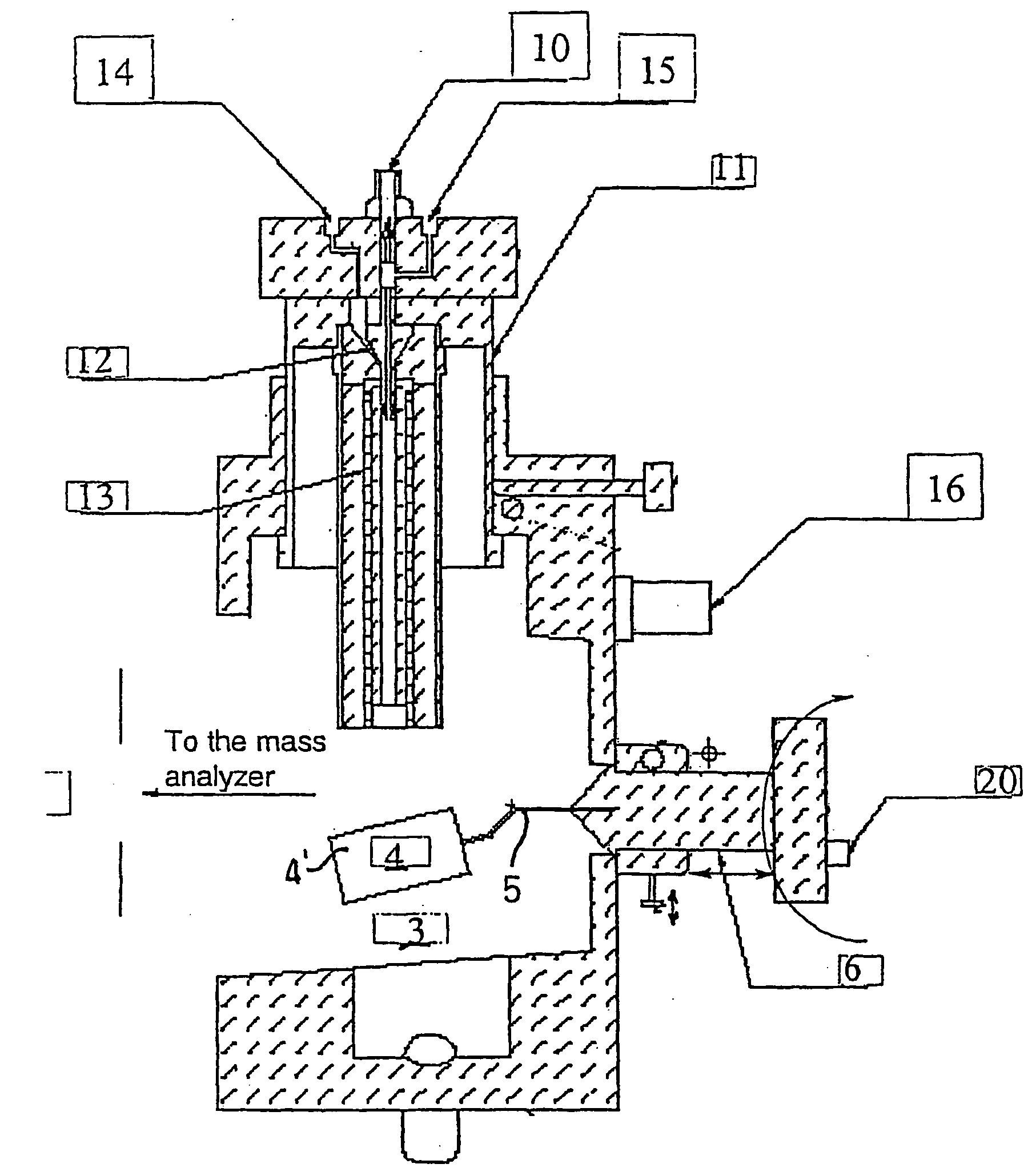
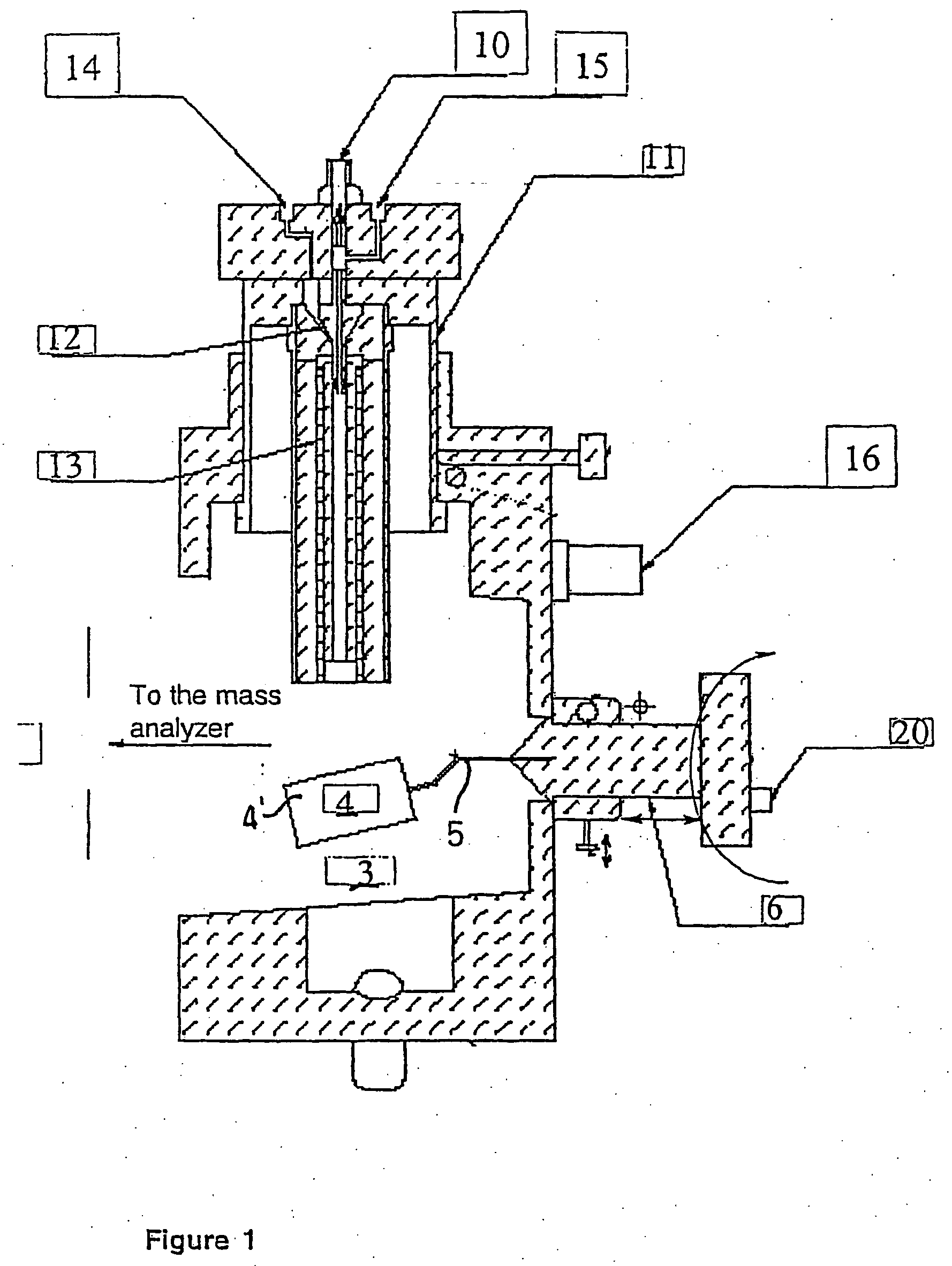
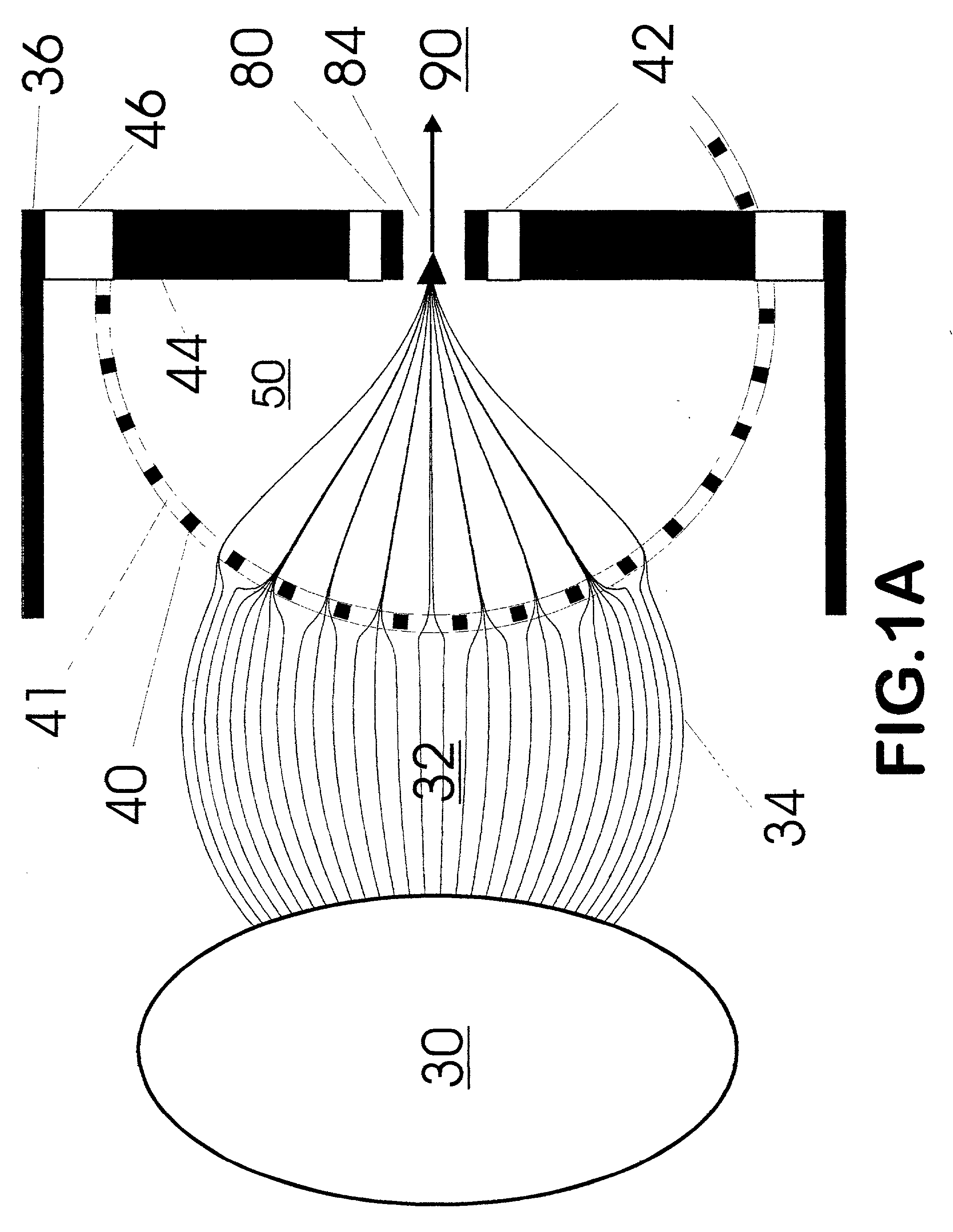
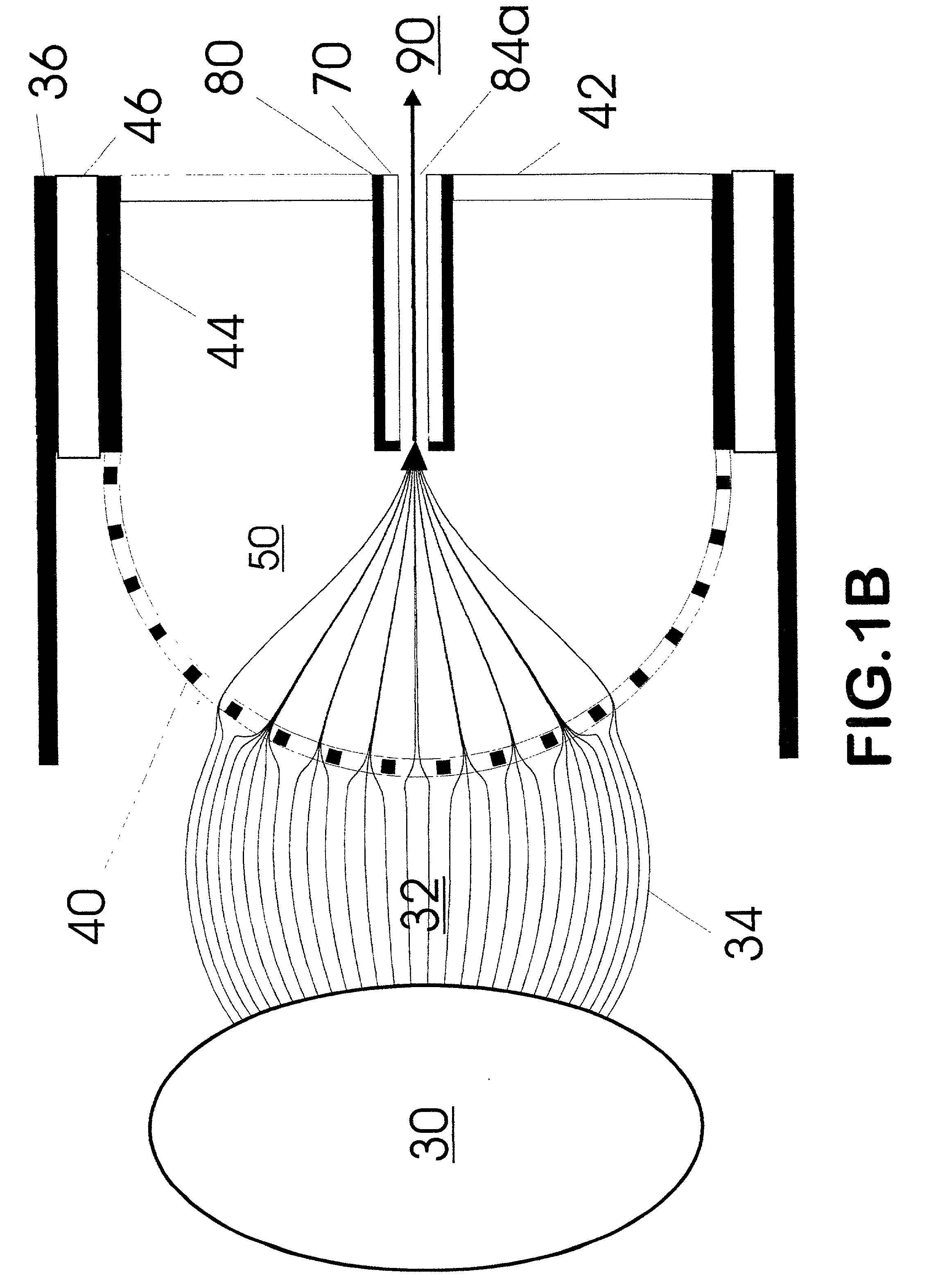
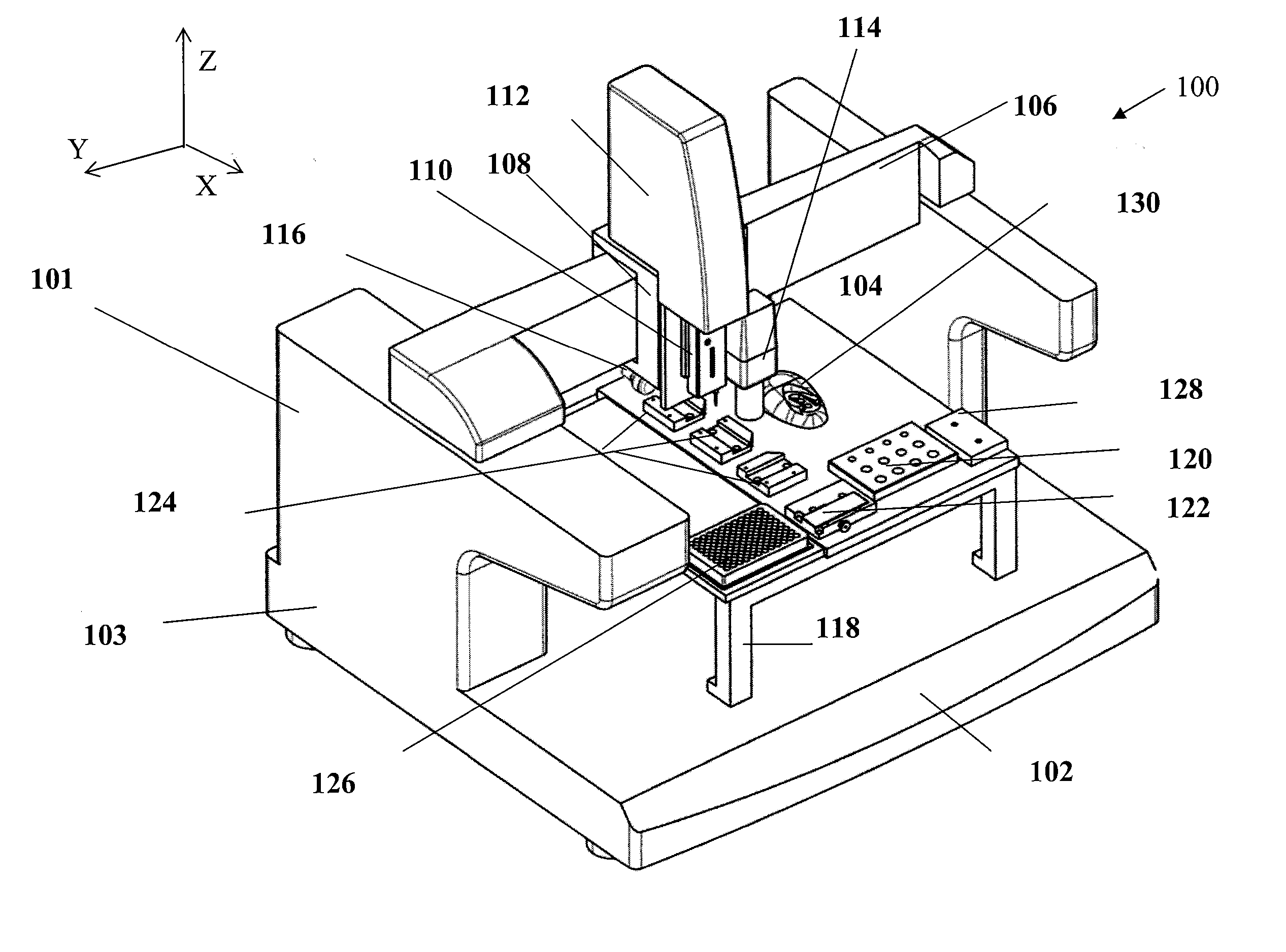
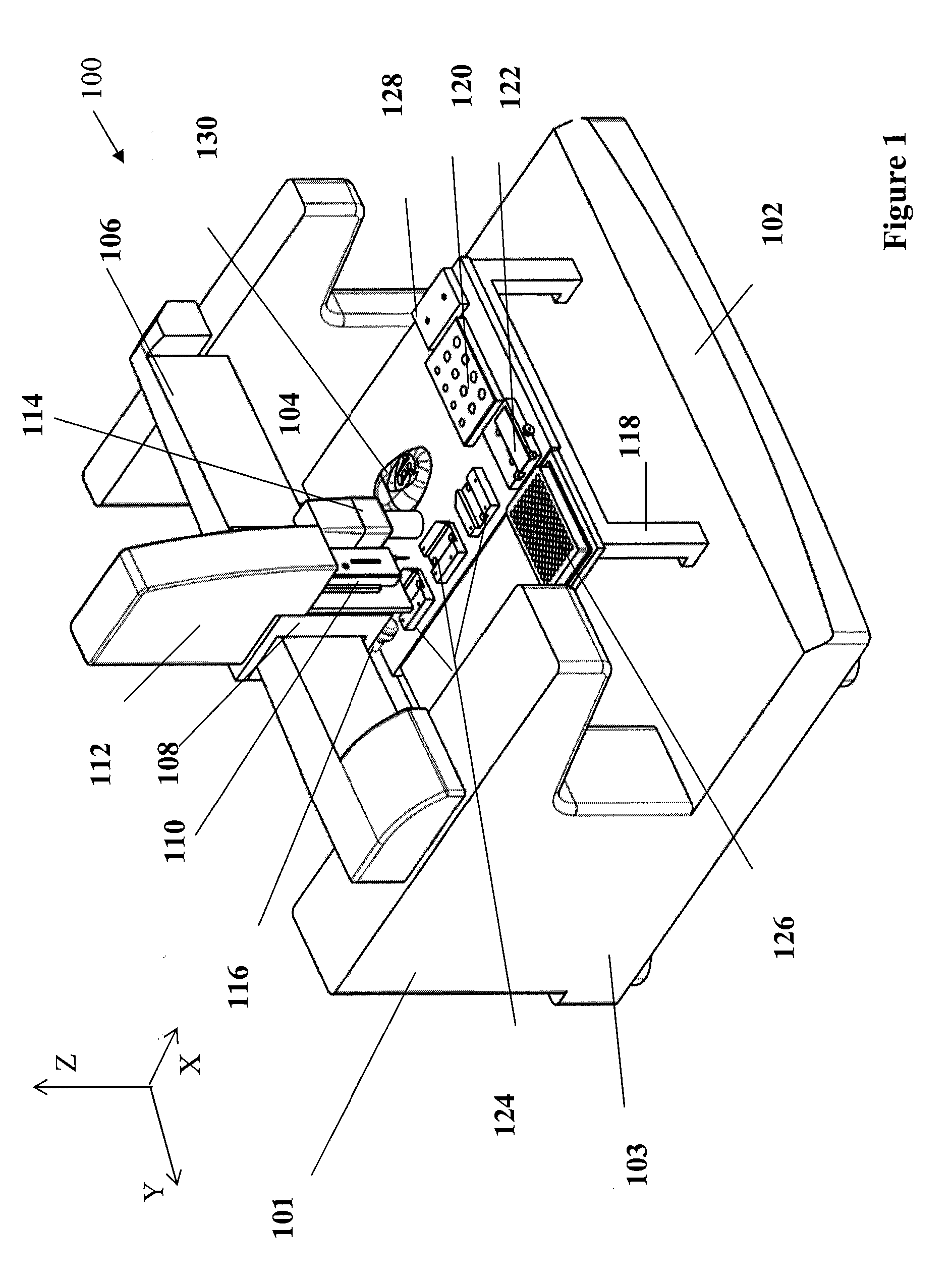
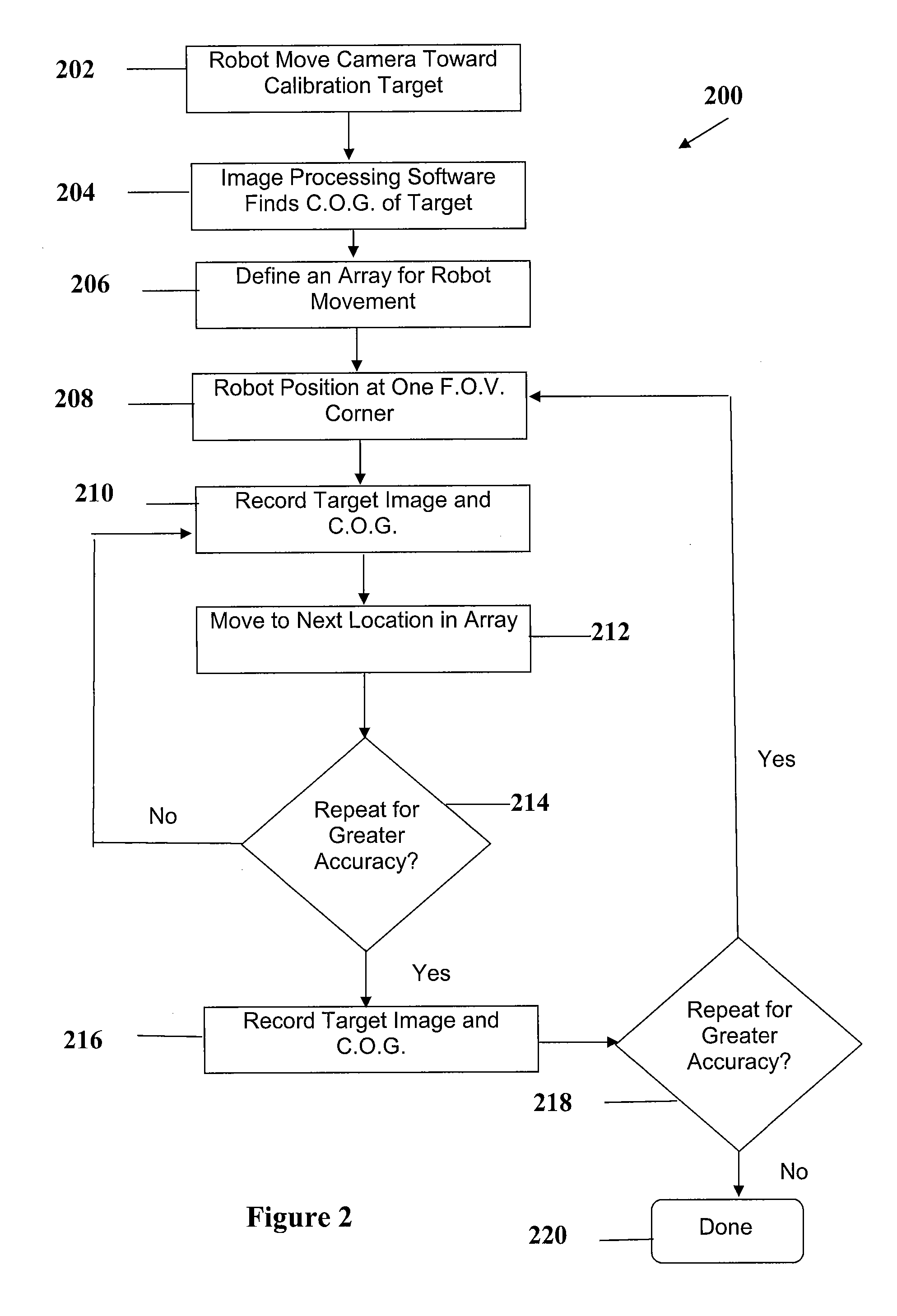
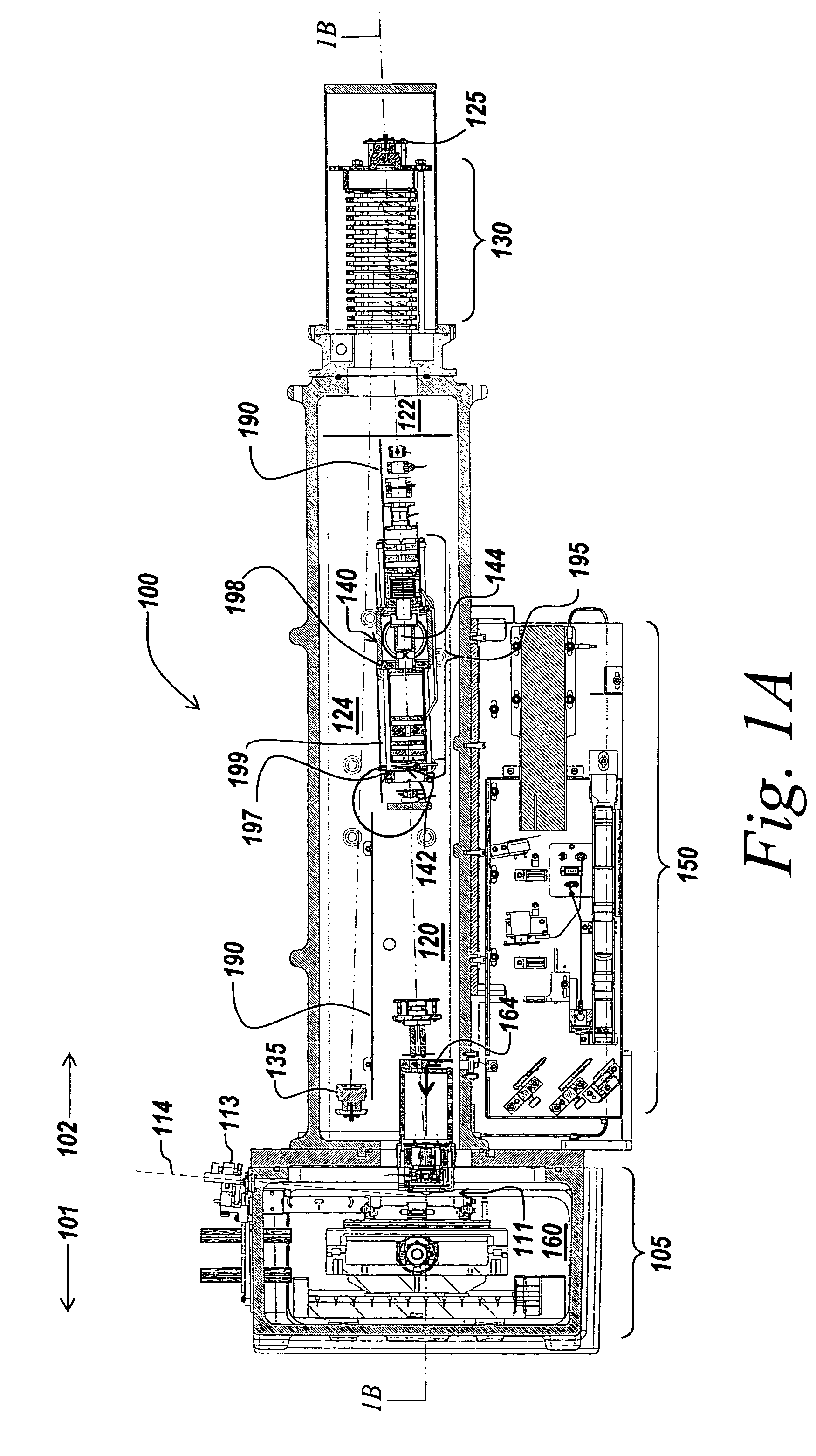
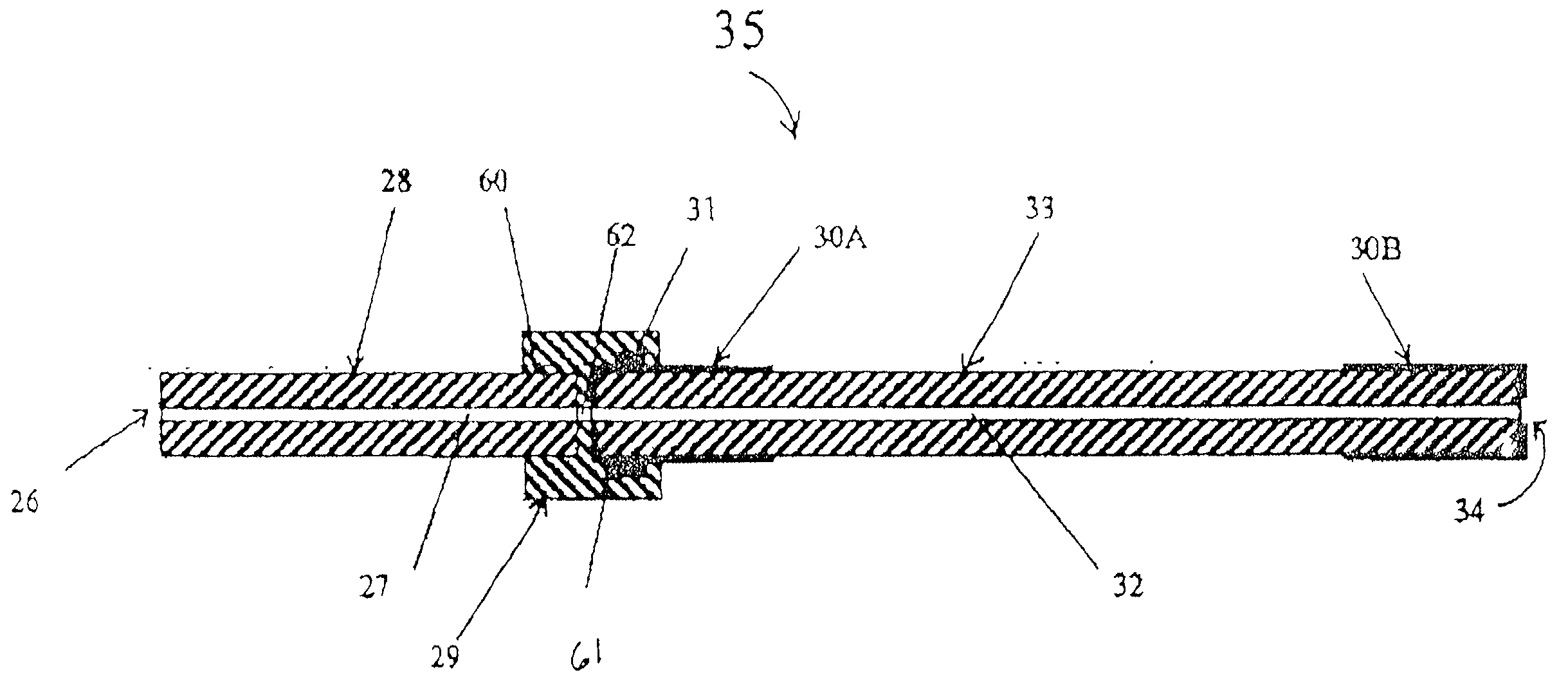
Method and apparatus for automating a matrix-assisted laser desorption/ionization (MALDI) mass spectrometer
The present invention provides an apparatus and method for automated and rapid loading of a large number of samples for mass spectrometric analysis using various ionization methods (e.g. matrix assisted desorption by laser bombardment (MALDI) and atmosperic pressure ionization (API) methods such as electrospray). The aparatus utilizes microtiter plates to hold the sample, optical elements (e.g. fiber optic) to facilitate automated transport of the ions, and a multiple part capillary comprising at least two capillary sections joined with airtight seal by a union for use in mass spectrometry (particularly with ionization sources) to transport ions between pressure regions of a mass spectrometer for analysis is described herein. Preferably, the capillary is useful to transport ions from an elevated pressure ionization source to a first vacuum region of a mass analysis system.
Owner:BRUKER SCI LLC
Deposition of samples and sample matrix for enhancing the sensitivity of matrix assisted laser desorption/ionization mass spectrometry
ActiveUS20060138319A1Better signal to noise ratioLess analyteSamplingSamples introduction/extractionLaser desorption ionization mass spectrometryAnalyte
The present invention relates to small sample spots for MALDI targets and methods for producing the same. The sample spots are composed of a layer of matrix material and a layer of analyte. In some instances, the samples spots have diameters of no more than 50 micrometers or even smaller. The sample spots are deposited onto a MALDI target substrate using ultrasonic deposition from a nozzle.
Owner:WISCONSIN ALUMNI RES FOUND
Carbon nanotubes and their derivatives as matrix elements for the matrix-assisted laser desorption mass spectrometry of biomolecules and sequencing using associated fragmentation
InactiveUS20050277201A1Analysis by subjecting material to chemical reactionBiological testingAnalyteSpectroscopy
The present invention is directed toward novel matrix elements, generally comprising functionalized carbon nanotubes, for matrix-assisted laser desorption ionization (MALDI)-mass spectroscopy (MS), methods of making such matrix elements, and to methods of using such matrix elements in MALDI-MS applications, particularly for the analysis of biological molecules. In some embodiments, by carefully tuning the absorption characteristics of the matrix element, biomolecular analytes can be sequenced.
Owner:RICE UNIV
Quantification of vitellogenin
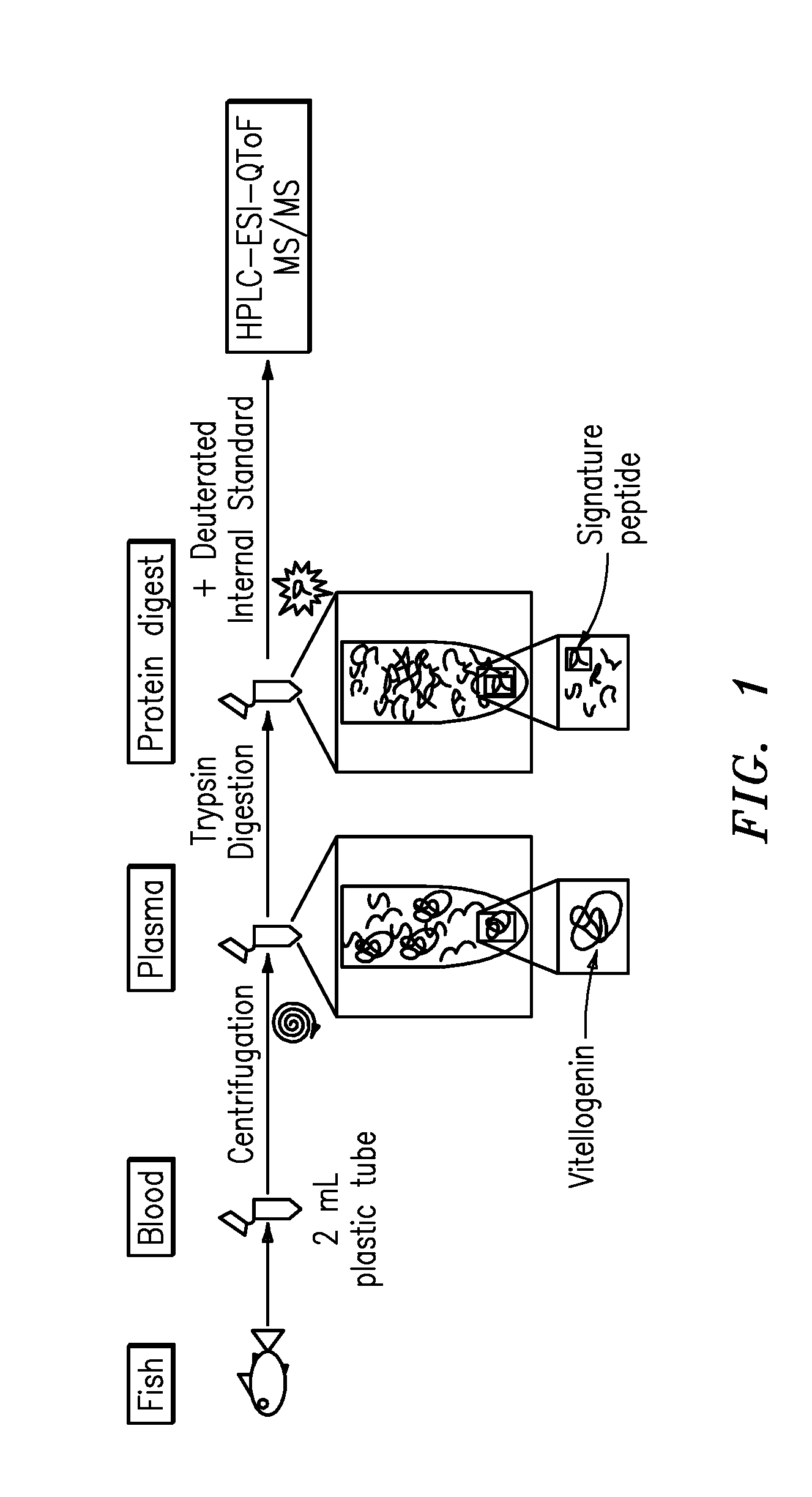
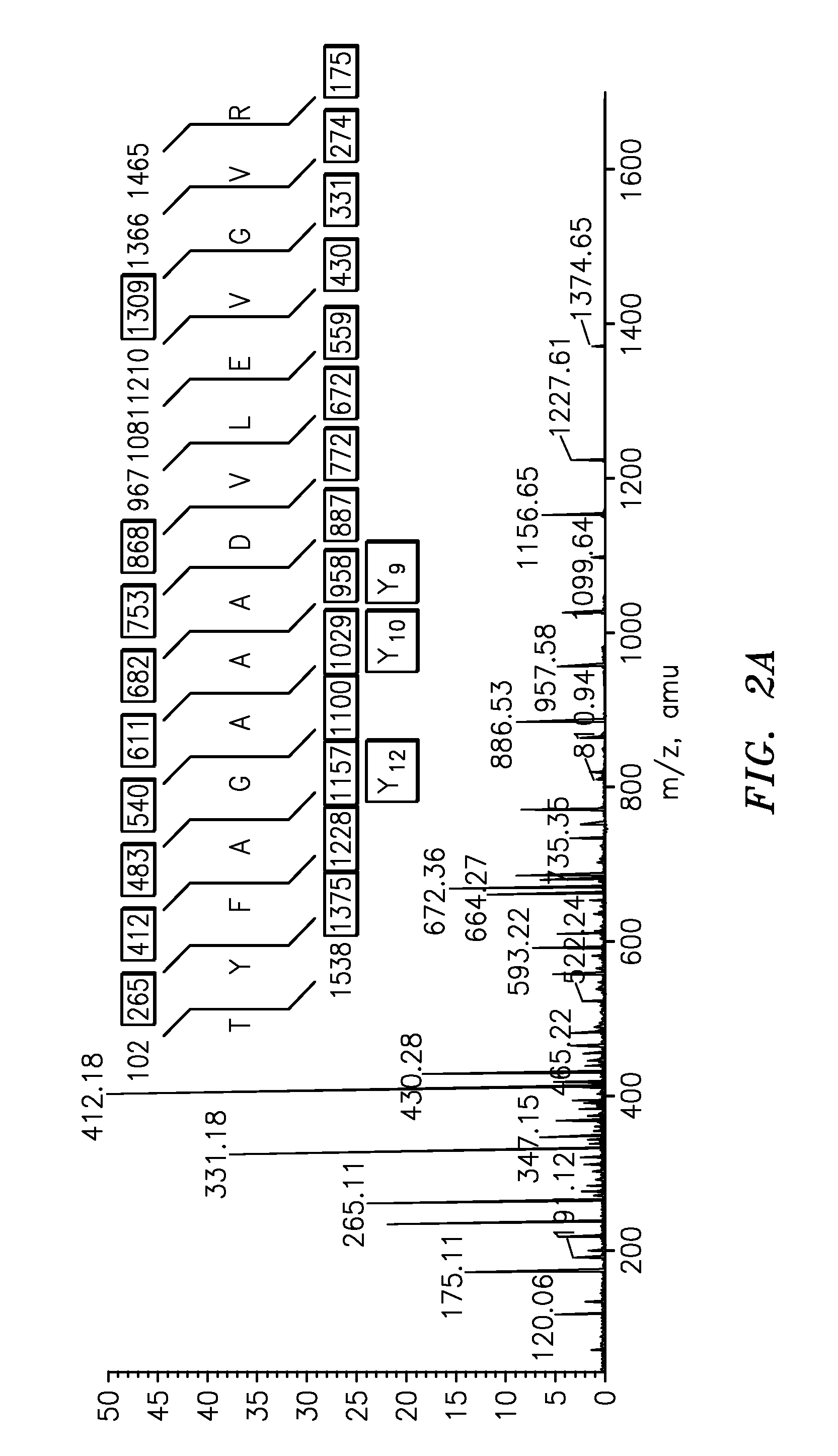
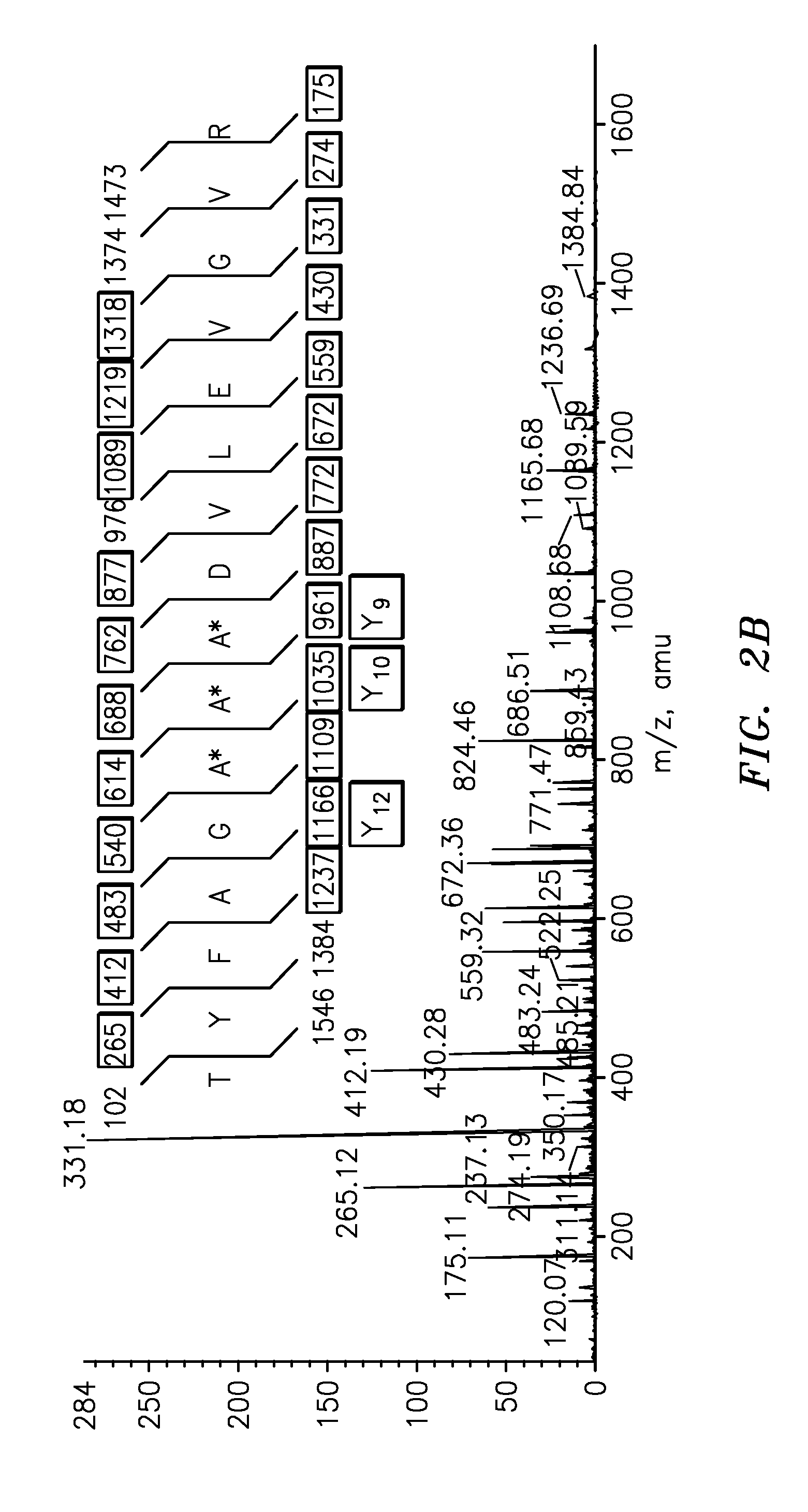
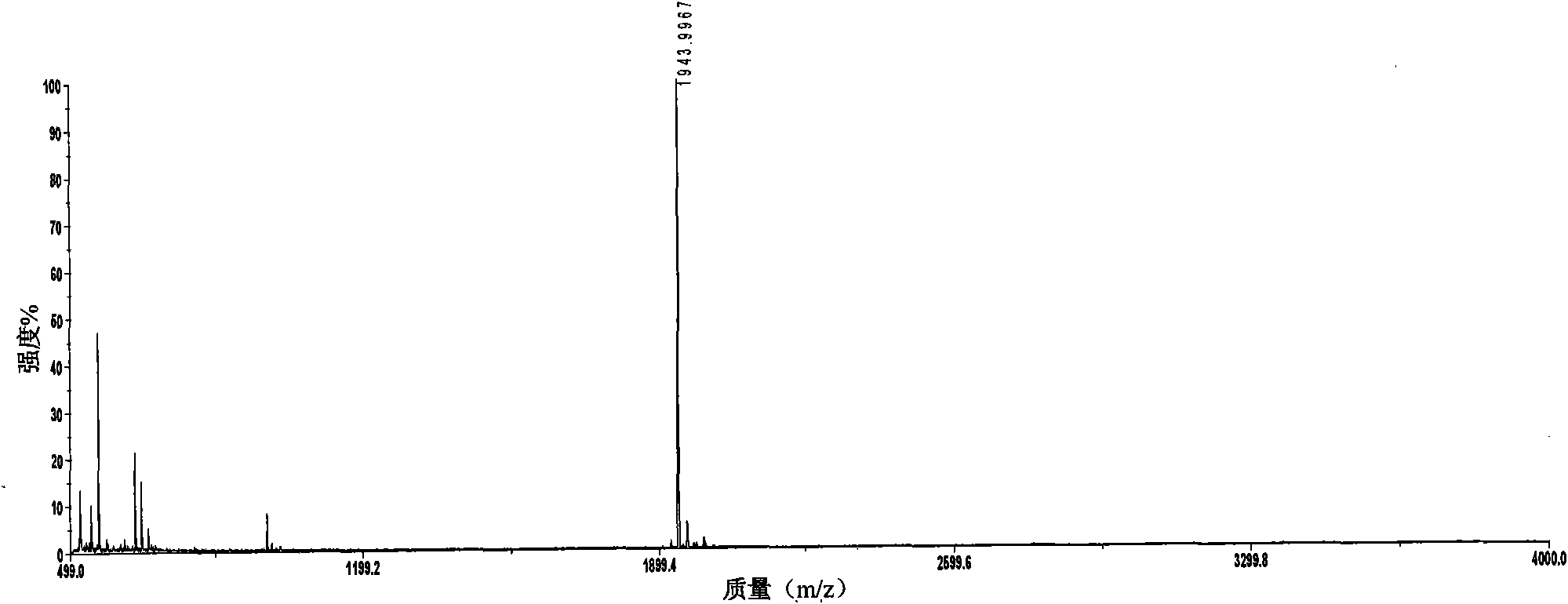
InactiveUS20090011447A1Simple methodMicrobiological testing/measurementPeptidesVitellogeninsGlu-Val-Gly
The present invention is directed to a simple method for absolute quantification of plasma vitellogenin from two or more different fish species such as Rainbow trout and Atlantic salmon, or Atlantic cod and haddock. In the case of Rainbow trout and Atlantic salmon, plasma samples obtained from control and β-estradiol induced fish were digested with trypsin. A characteristic ‘signature peptide’ was selected and analyzed by high performance liquid chromatography coupled to an electrospray quadrupole-time-of-flight tandem mass spectrometer, using a deuterated homologue peptide as an internal standard. The hybrid tandem mass spectrometer was operated in a ‘pseudo’ selected reaction monitoring mode by which three diagnostic product ions were monitored for identification and quantification purposes. The reproducibility (coefficient of variation ˜5%) and sensitivity (limit of quantification of 0.009 mg / mL) achieved by this simple assay allow it to be considered as an alternative to immunological assays. In the case of Atlantic cod and haddock, the amino acid sequence of the vitellogenin protein has not yet been determined, but, the Atlantic cod vitellogenin has been characterized using a ‘bottom-up’ mass spectrometric approach. Vitellogenin synthesis was induced ‘in vivo’ with β-Estradiol, and subjected to trypsin digestion for characterization by matrix-assisted laser desorption / ionization-Quadrupole-Time-of-flight tandem mass spectrometry. A peptide mass fingerprint was obtained and ‘de novo’ sequencing of the most abundant tryptic peptides was performed by low energy collision induced dissociation-tandem mass spectrometry. Thus, the sequences of various tryptic peptides have been elucidated. It has also been determined that Atlantic cod vitellogenin shares a series of common peptides with the two different known vitellogenin sequences of Haddock, a closely related species. There are also disclosed novel isolated signature peptides, namely Thr-Tyr-Phe-Ala-Gly-Ala-Ala-Ala-Asp-Val-Leu-Glu-Val-Gly-Val-Arg, Asp Leu Gly Leu Ala Tyr Thr Glu Lys, Phe Phe Gly Gln Glu Ile Ala Asn Ile Asp Lys, Glu Ile Val Leu Leu Gly Tyr Gly Thr Met Ile Ser Lys and Tyr Glu Ser Phe Ala Val Ala Arg.
Owner:BANOUB JOSEPH H +2
Polypeptide immunoassay kit and detection method thereof
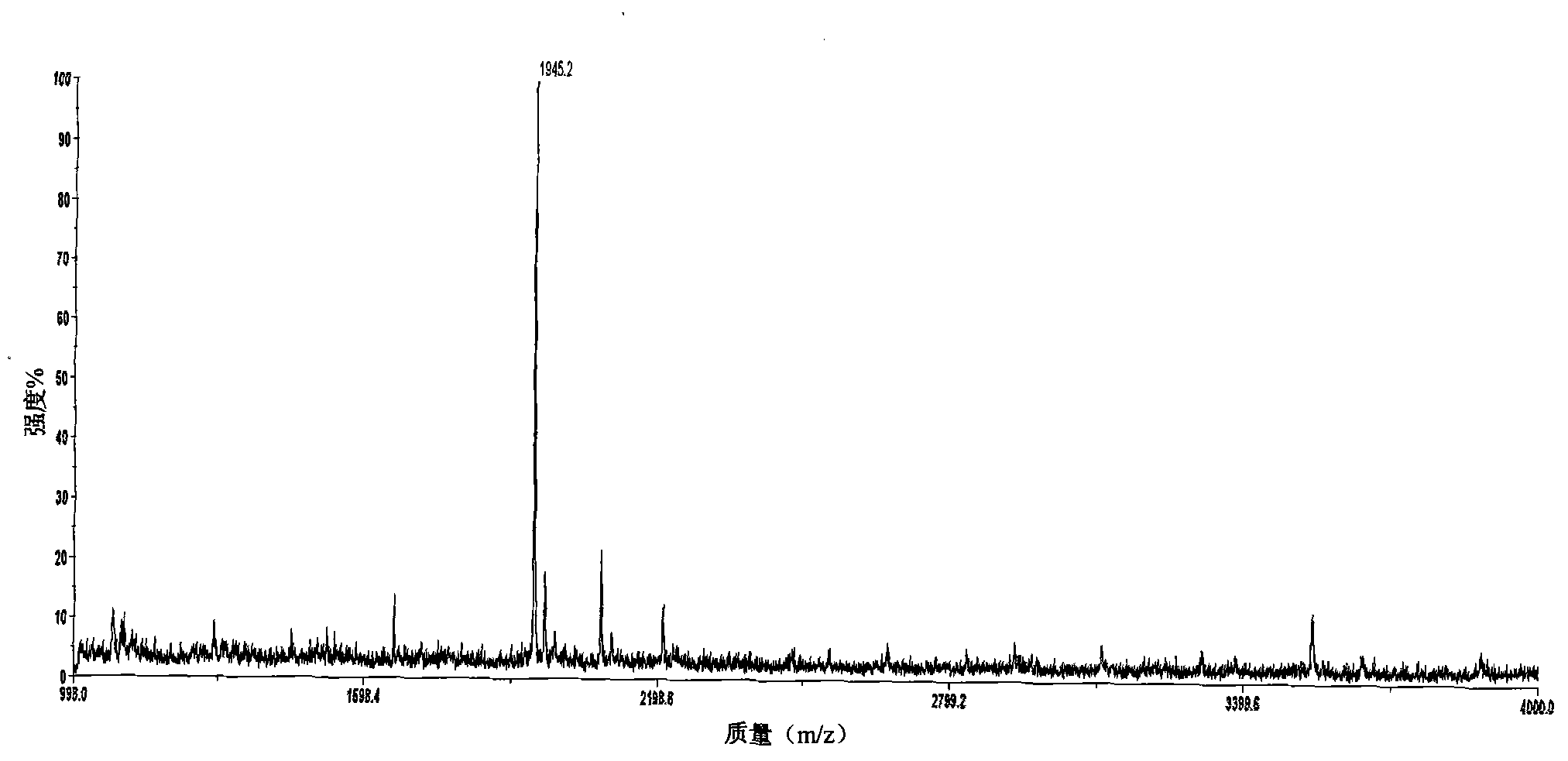
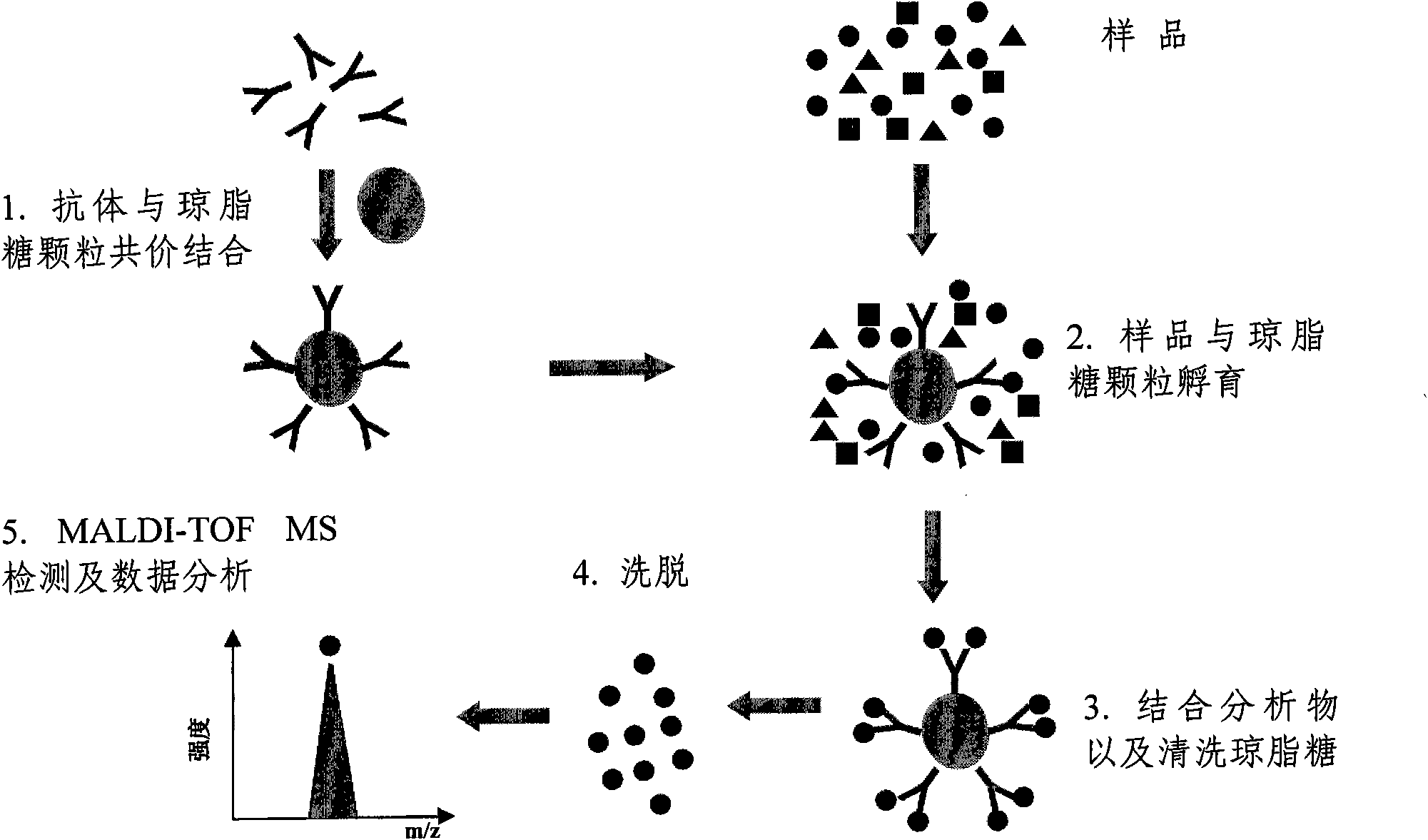
InactiveCN102062780AStrong specificityStrong elution abilityImmunoglobulins against animals/humansMaterial analysis by electric/magnetic meansAntigenSolid phases
The invention relates to a polypeptide immunoassay kit, which comprises a protein G or protein A agarose serving as a solid phase carrier, a purified serum polypeptide antibody and PBS buffer solution. The invention also relates to a polypeptide immunomic spectrometry analysis method, which comprises the following steps: coupling a purified serum antibody with a proper solid carrier; allowing the coupled product to bond with a polypeptide marker antigen specifically, and separating the antigen from the carrier by using eluent; and finally, detecting polypeptide antigen in the eluent by high-pass matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOFMS). Thus, the complete immunomic spectrometry analysis method is established.
Owner:BEIJING C & N INT SCI TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com