Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Induction immunosuppression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Induction immunosuppression is intense, prophylactic therapy used at the time of transplantation based on the empiric observation that more powerful immunosuppression is required to prevent acute rejection early.
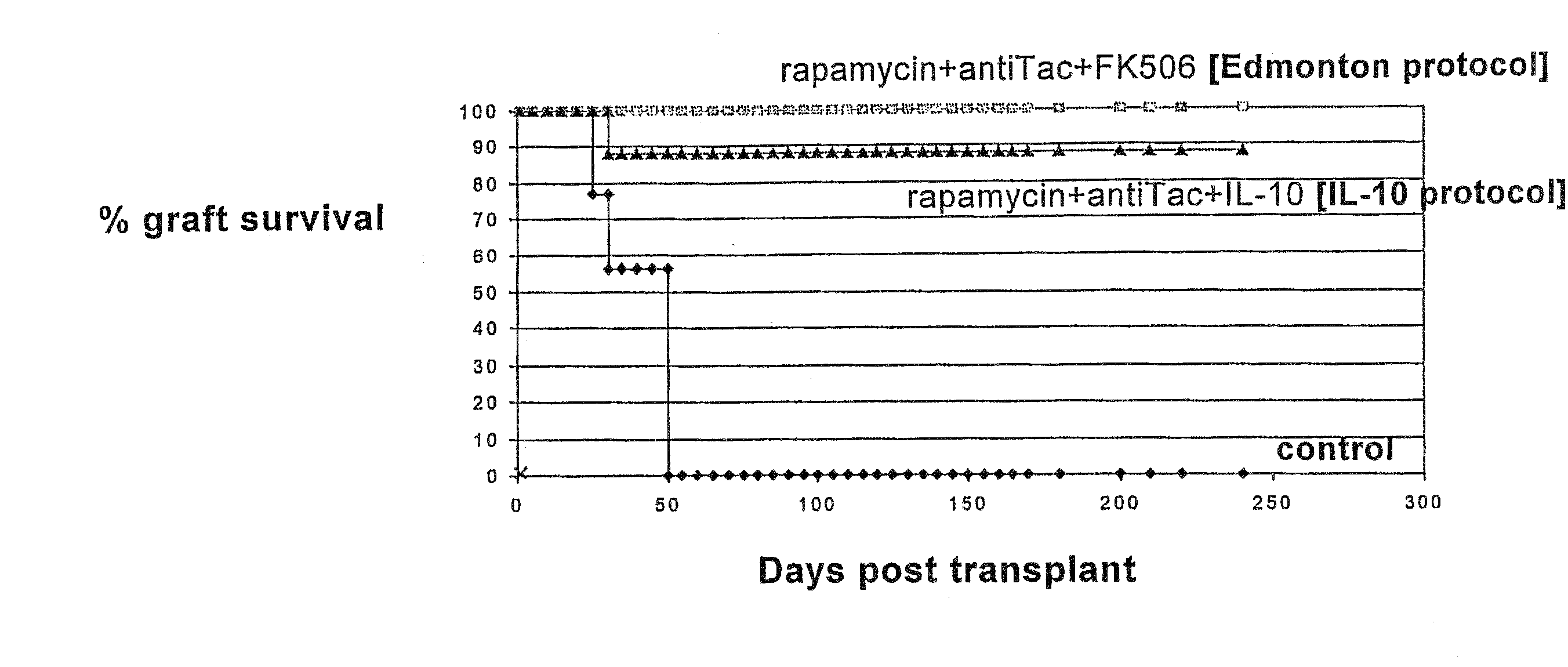
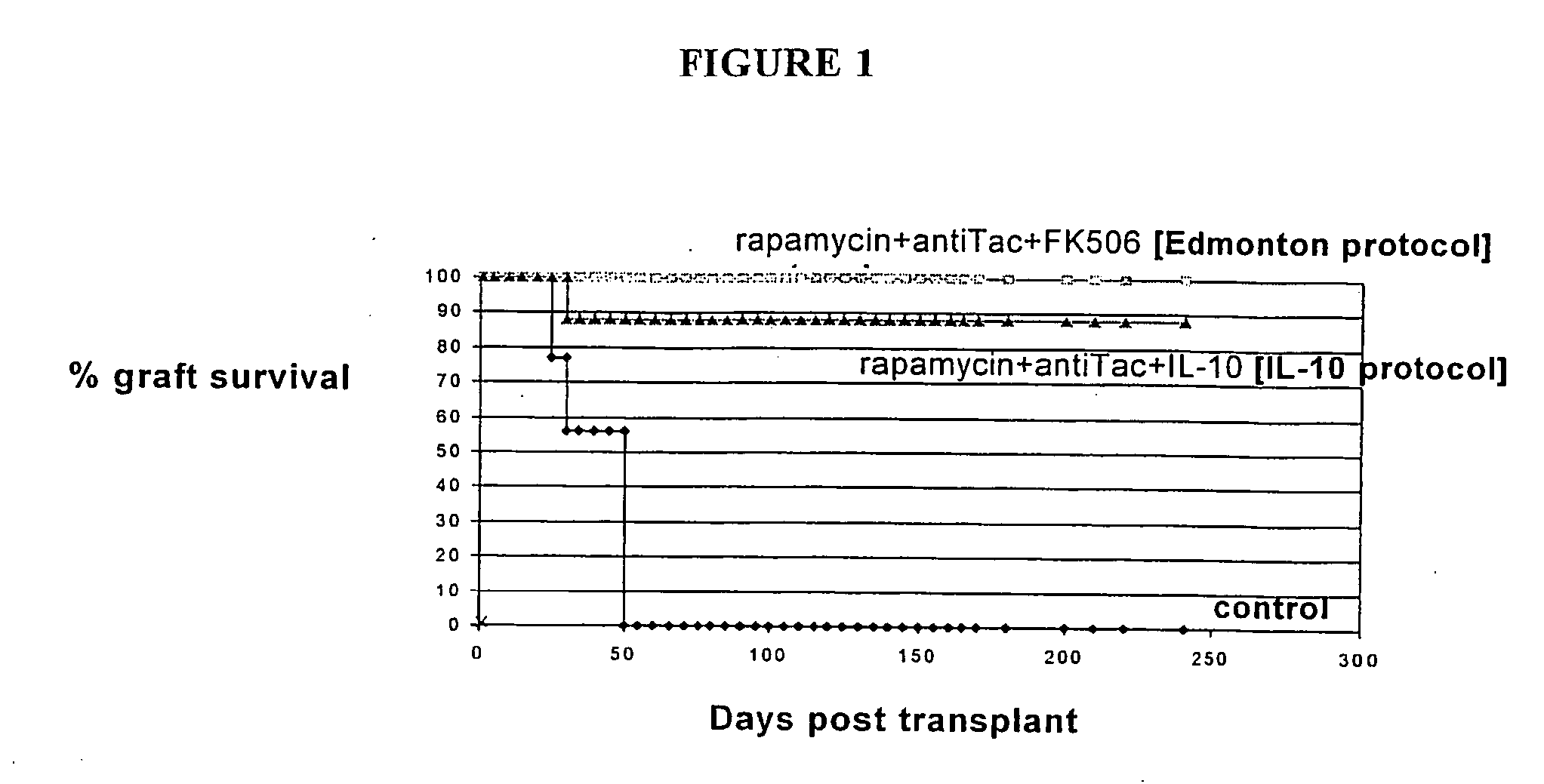
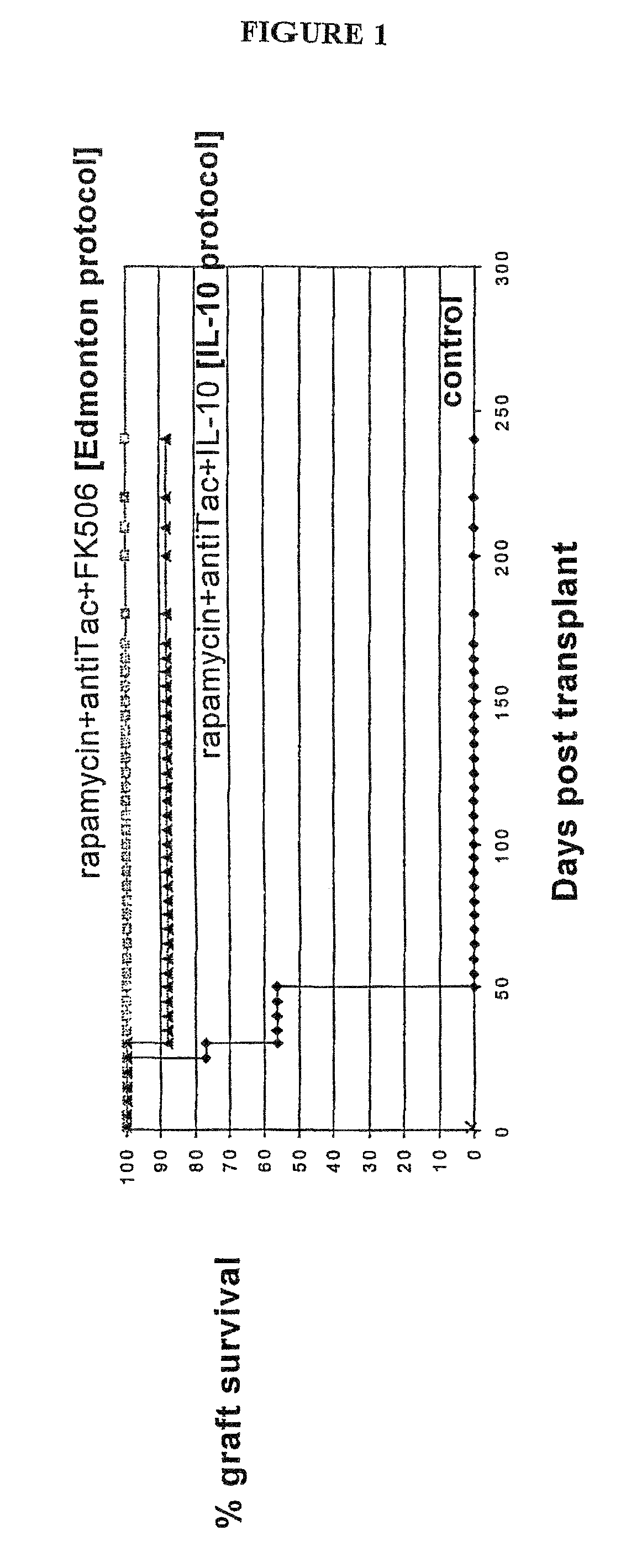
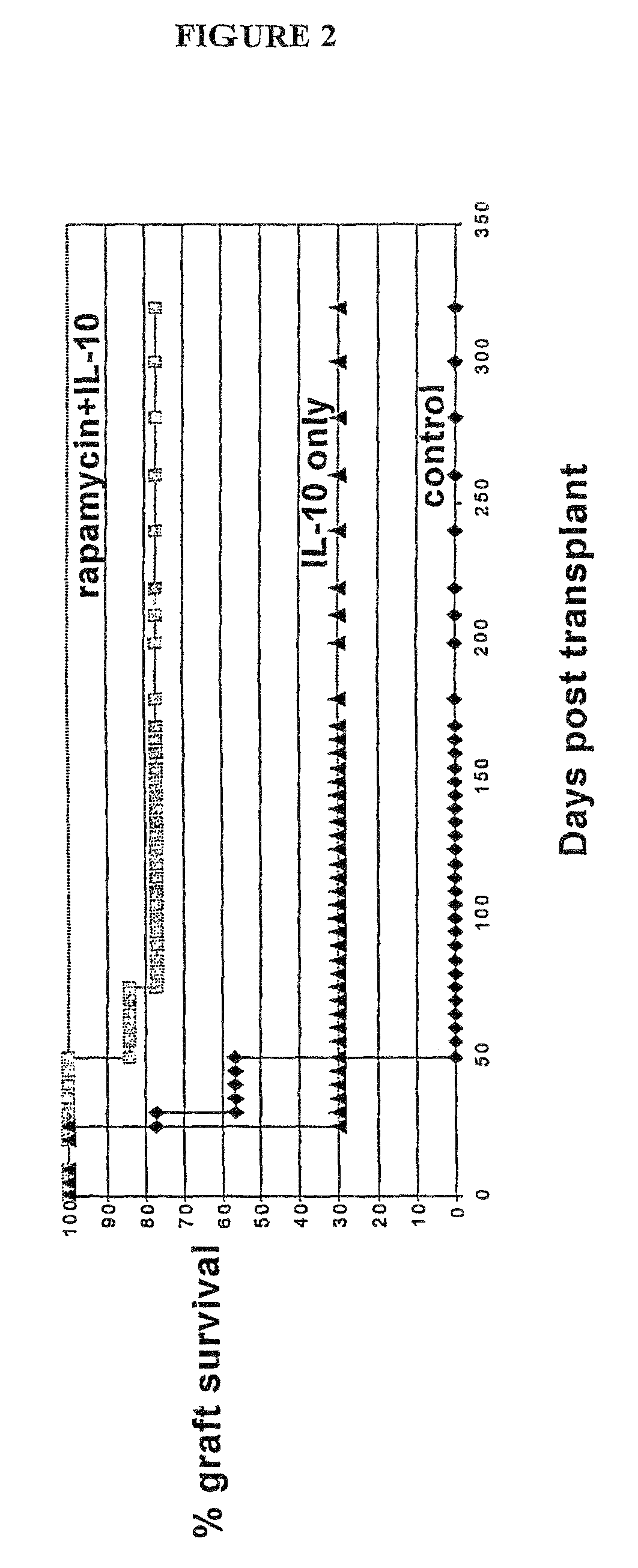
Rapamycin and il-10 for the treatment of immune diseases
ActiveUS20080069797A1Improve bioavailabilityImprove efficacyBiocideNervous disorderT cellImmune tolerance
The invention discloses a combined preparation containing IL-10 and rapamycin, able to induce immunosuppression and antigen-specific immune tolerance, and the use thereof in the treatment of diseases involving an excessive, dysfunctional or uncontrolled immune responses mediated by T cells.
Owner:TR1X INC
Mesenchymal stem cell-mediated autologous dendritic cells with increased immunosuppression
InactiveUS20100055076A1Inhibition of secretionPromote secretionBiocideMammal material medical ingredientsDiseaseMesenchymal stem cell
Owner:JW CREAGENE
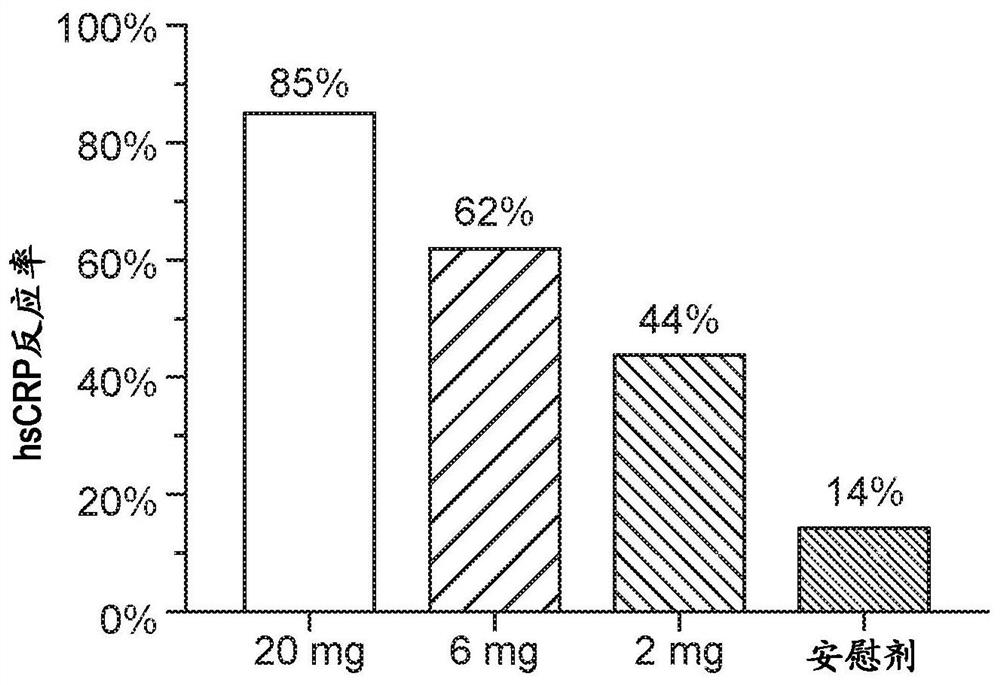
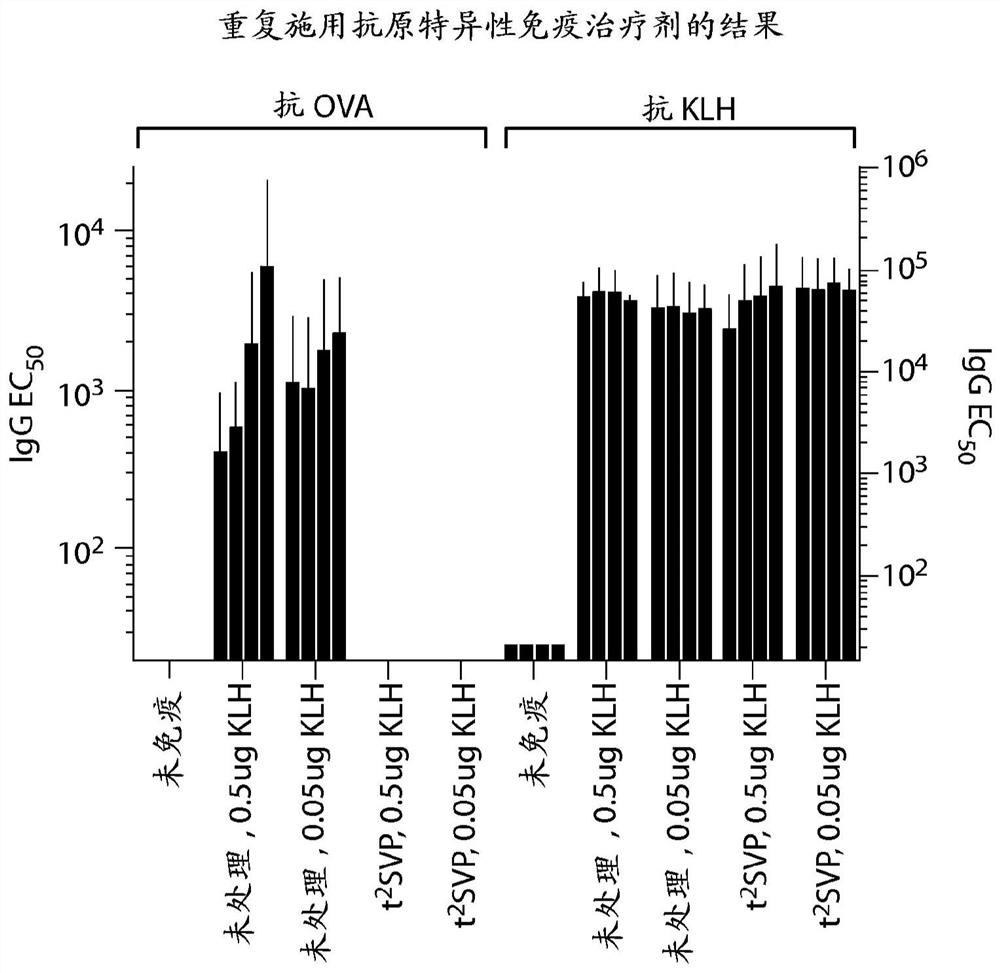
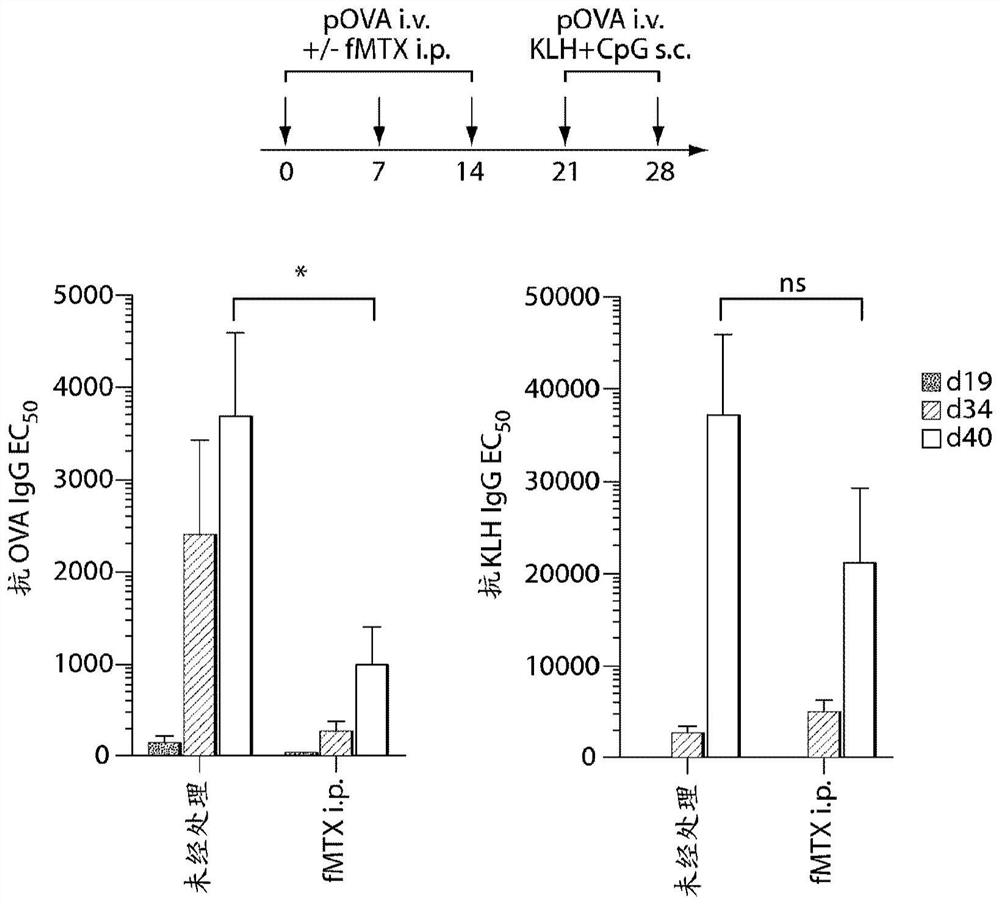
Repeated administration of non-immunosuppressive antigen specific immunotherapeutics
PendingUS20140356361A1Organic active ingredientsPowder deliveryInduction immunosuppressionAntigen specific
This invention relates to repeated administration of antigen-specific immunotherapeutics using protocols, or elements thereof, that do not induce immunosuppression. In some embodiments, the protocol has been previously shown not to induce immunosuppression in a subject.
Owner:SELECTA BIOSCI
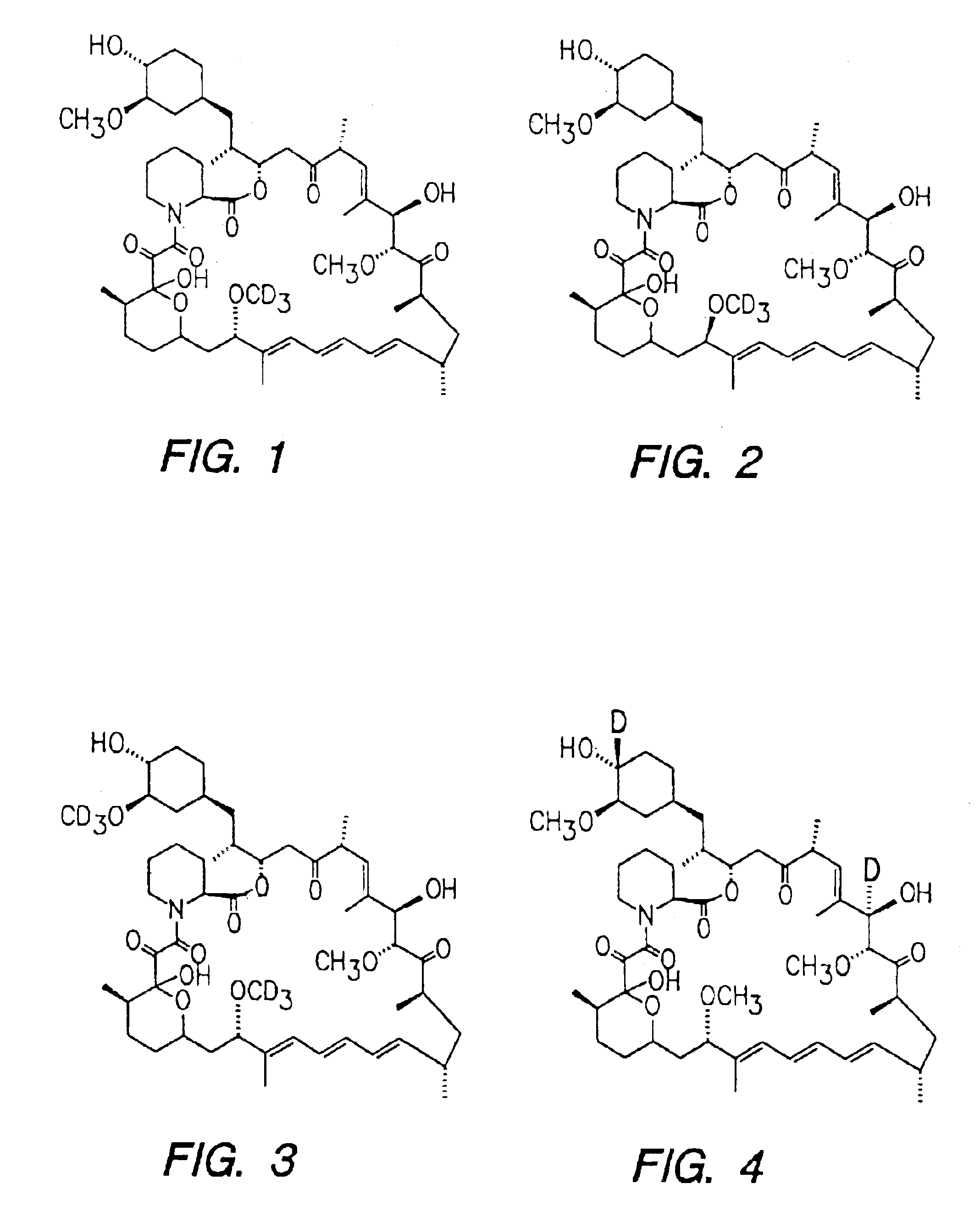
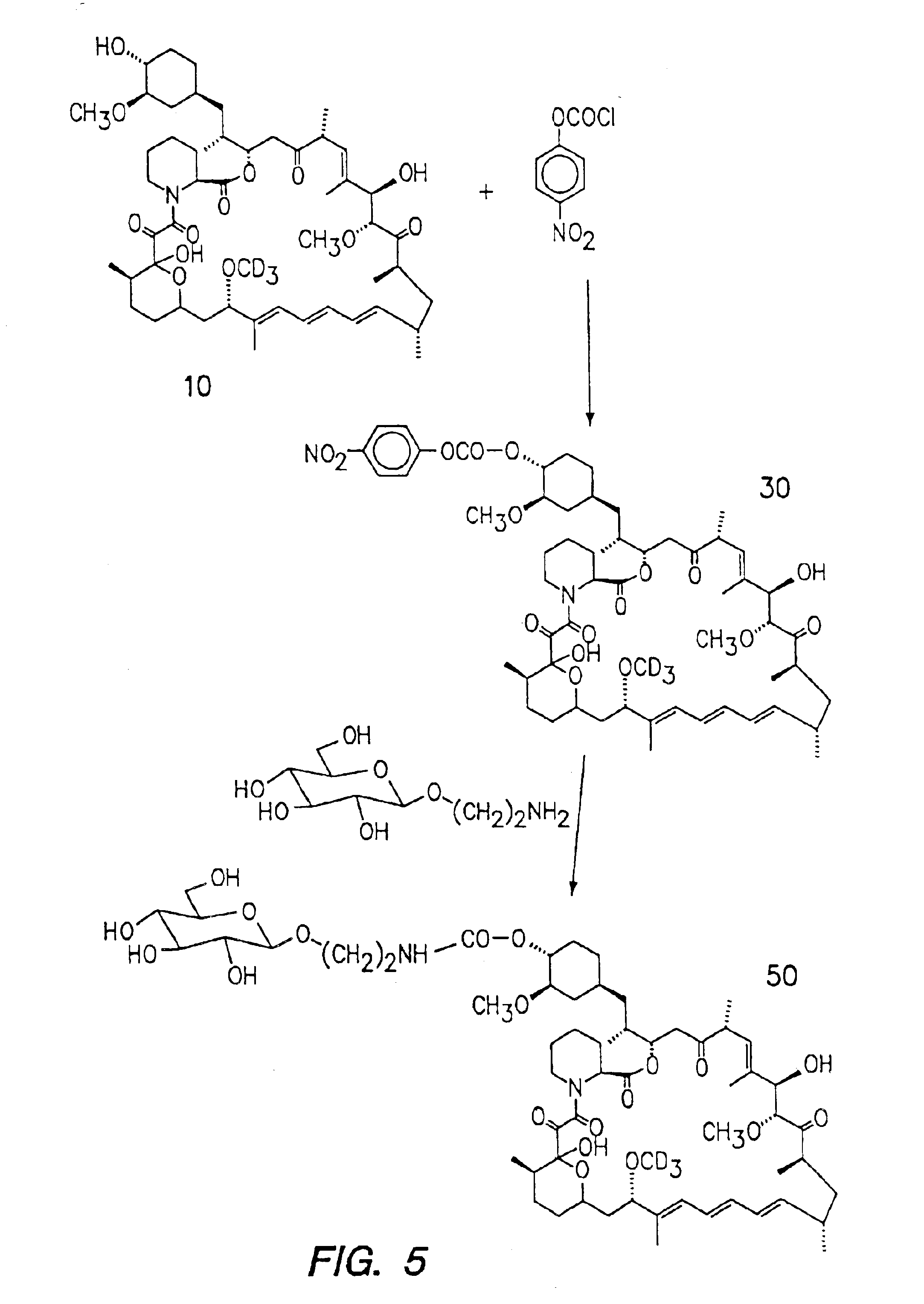
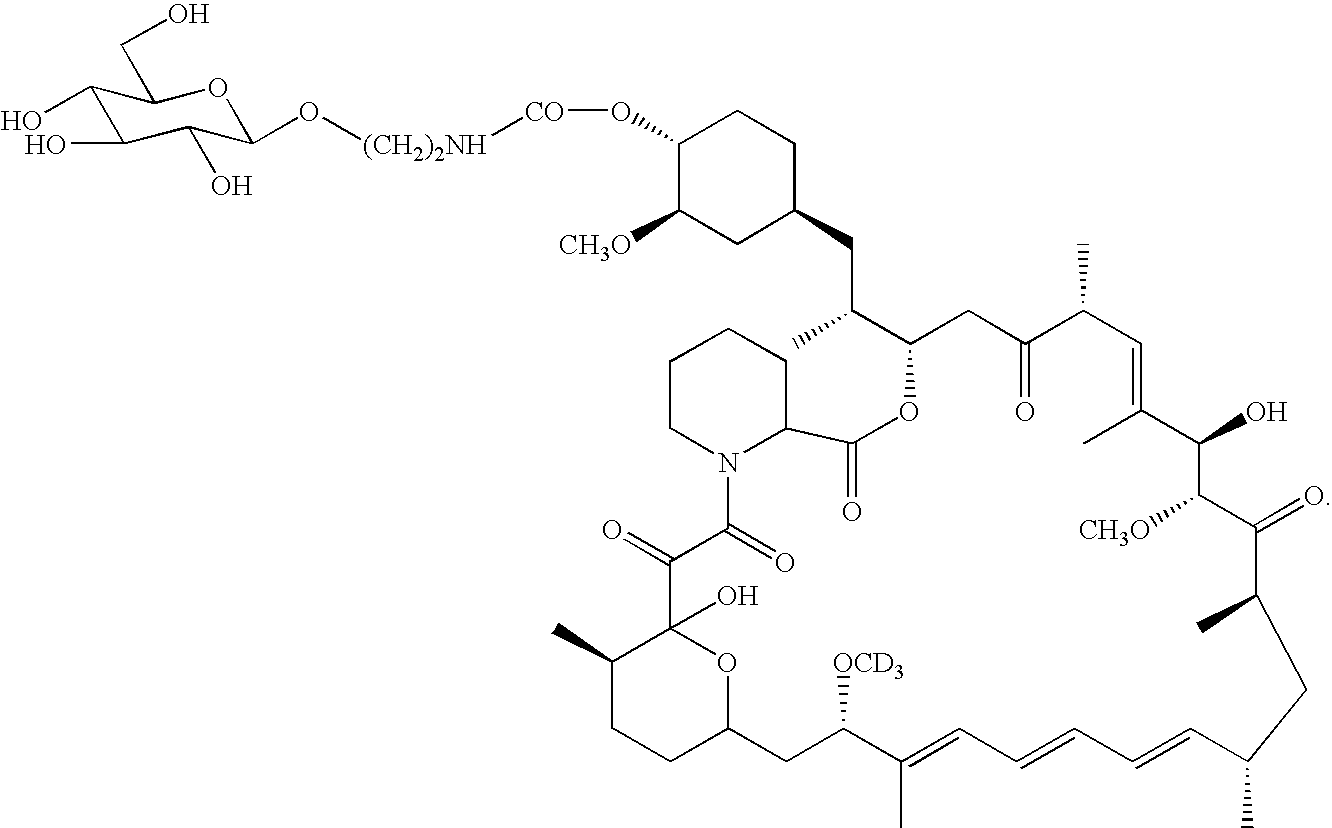
Deuterated rapamycin compounds, method and uses thereof
InactiveUS6939878B2Reduce oxidation rateReduce formationBiocideOrganic chemistryAutoimmune diseaseHost disease
The synthesis of deuterated analogues of rapamycin is disclosed together with a method for use for inducing immunosupression and in the treatment of transplantation rejection, graft vs host disease, autoimmune diseases, diseases of inflammation leukemia / lymphoma, solid tumors, fungal infections, hyperproliferative vascular disorders. Also described is a method for the synthesis of water soluble deuteratred rapamycin compounds and their use as described above.
Owner:ISOTECHNIKA INC
Methods and compositions for modulating immunity
InactiveUS7902151B2Avoid developmentAvoid survivalOrganic active ingredientsAntimycoticsDiseaseAutoimmune disease
Owner:TRILLIUM THERAPEUTICS
Non-immunosuppressive immunogenic or vaccine composition comprising a mutated E7 protein of the HPV-16 virus
InactiveUS7314629B2Inhibitory activityQuickly block immunosuppressive effectAntibody mimetics/scaffoldsVirus peptidesAmino acid substitutionBULK ACTIVE INGREDIENT
Owner:NEOVACS SA
Polyethylene glycol-cactus oligopeptide bonded rapamycin derivative
ActiveCN104448295AHigh drug loadIncrease diversityOrganic active ingredientsNervous disorderImmunologic disordersDisease
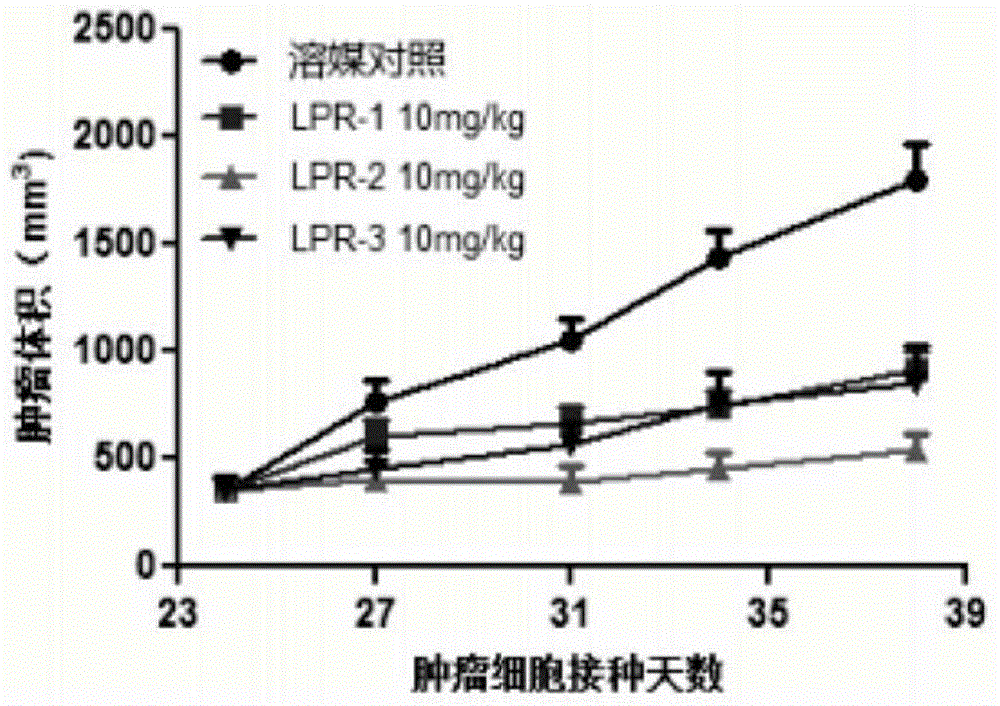
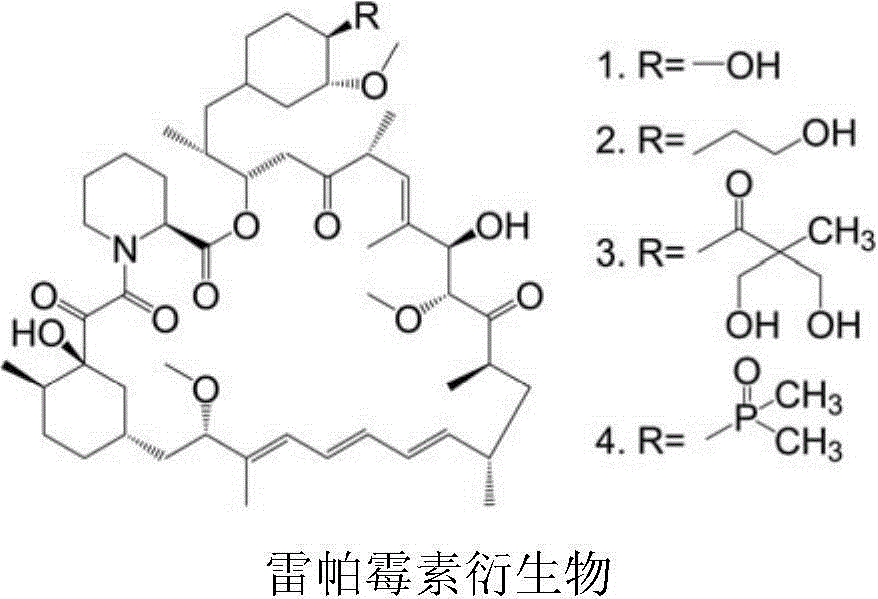
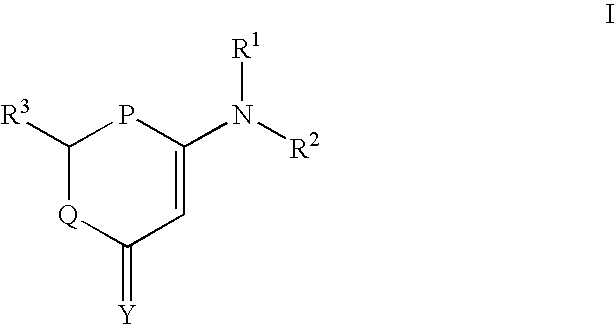
The invention provides a compound shown in the formula I in the specification and pharmaceutically acceptable salts of the compound, the preparation method of the compound and pharmaceutically acceptable salts of the compound and a medicine composition containing the compound shown in the formula I or the pharmaceutically acceptable salts of the compound. In the compound provided by the invention, each end group of the polyethylene glycol molecule is capable of bonding with a plurality of rapamycin molecules through cactus oligopeptide, and the medicine loading rate is improved. The compound can be used for inducing immunosuppression and treating transplant rejection, self-immune diseases, solid tumors, fungal infection and cardiovascular and cerebrovascular diseases.
Owner:JENKEM TECH
Methods and Compositions For Modulating Immunity
InactiveUS20090232825A1Avoid developmentAvoid survivalOrganic active ingredientsNervous disorderDiseaseAllergy
Methods and compositions for inducing immune suppression are disclosed. The methods involve administering an effective amount of a CD200 protein or a nucleic acid encoding a CD200 protein. The methods are useful in preventing graft rejection, fetal loss, autoimmune disease, and allergies. Methods and compositions for preventing immune suppression are also disclosed. The methods involve administering an effective amount of an agent that inhibits CD200. Such methods are useful in treating cancer.
Owner:TRILLIUM THERAPEUTICS
Antigen capable of increasing CD4 + CD25 + Foxp3 + regulatory T cells and application thereof
InactiveCN101921325ASuppress inflammatory symptomsInhibitory reactivityPeptide/protein ingredientsAntipyreticAntigenDisease
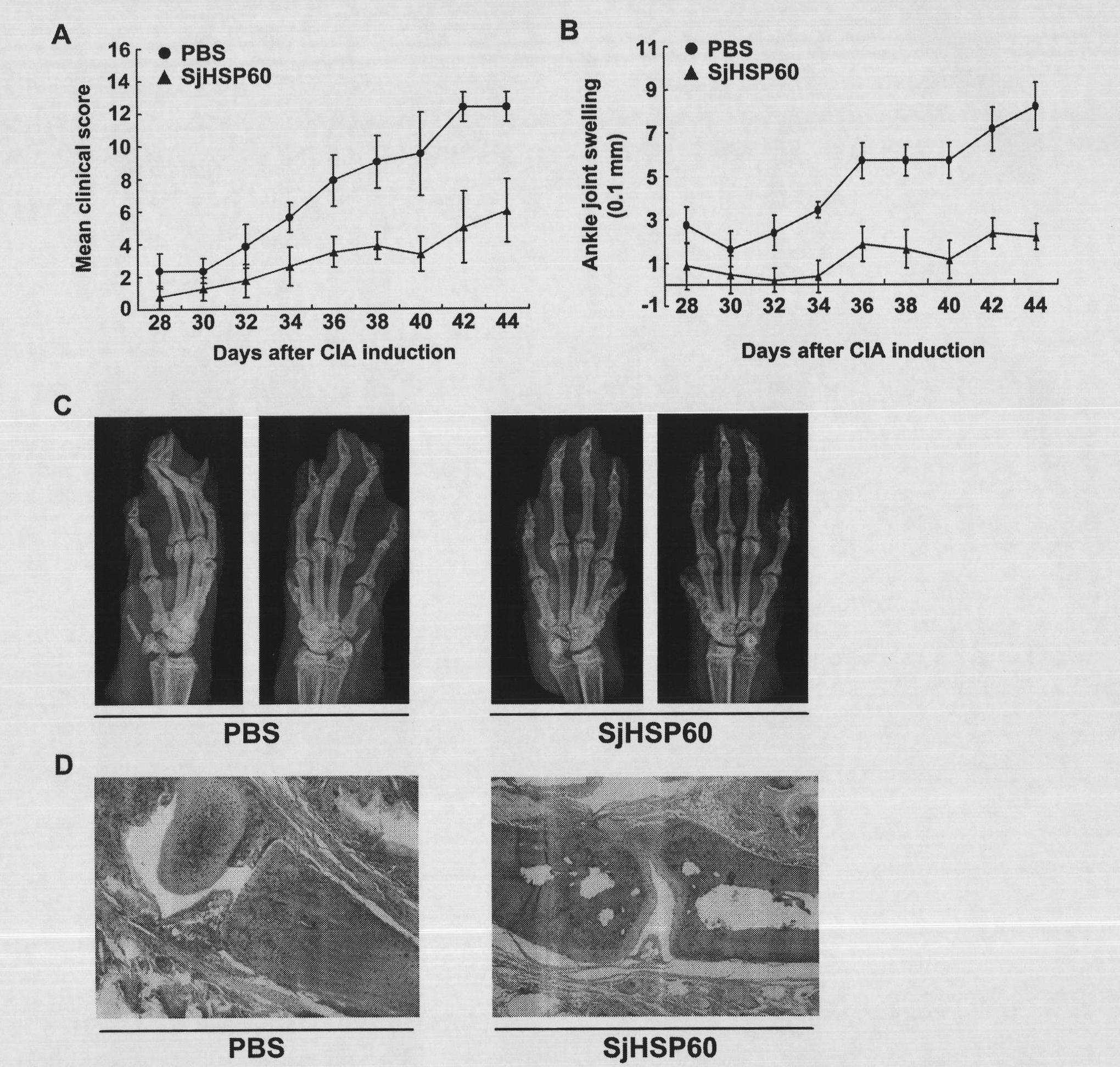
The invention belongs to the immunology field, in particular to a proteantigen molecule-Japanese blood fluke heat shock protein 60KDa (SjHSP60) which is derived from a blood fluke and is capable of increasing CD4 + CD25 + Foxp3 + regulatory T cells and application thereof. The SjHSP60 has a full-length amino acid sequence as shown in SEQ.ID.NO.1, has a series of identical or highly similar cross-reactive T cell epitopes with HSP60 infected by a host. After being used for mouse in vivo immunization or in vitro stimulus to mouse spleen and lymph gland cells, the SjHSP60 can obviously increase CD4 + CD25 + Foxp3 + Tregs. In practical application, the SjHSP60 can effectively relieve inflammatory symptoms and immunopathological effects caused by arthritis, thereby having wide prospects in the aspects of immunological suppression inducement and treatment of immunological diseases.
Owner:NANJING MEDICAL UNIV
Method for inducing immunosuppressive b-lymphocytes from human body peripheral blood
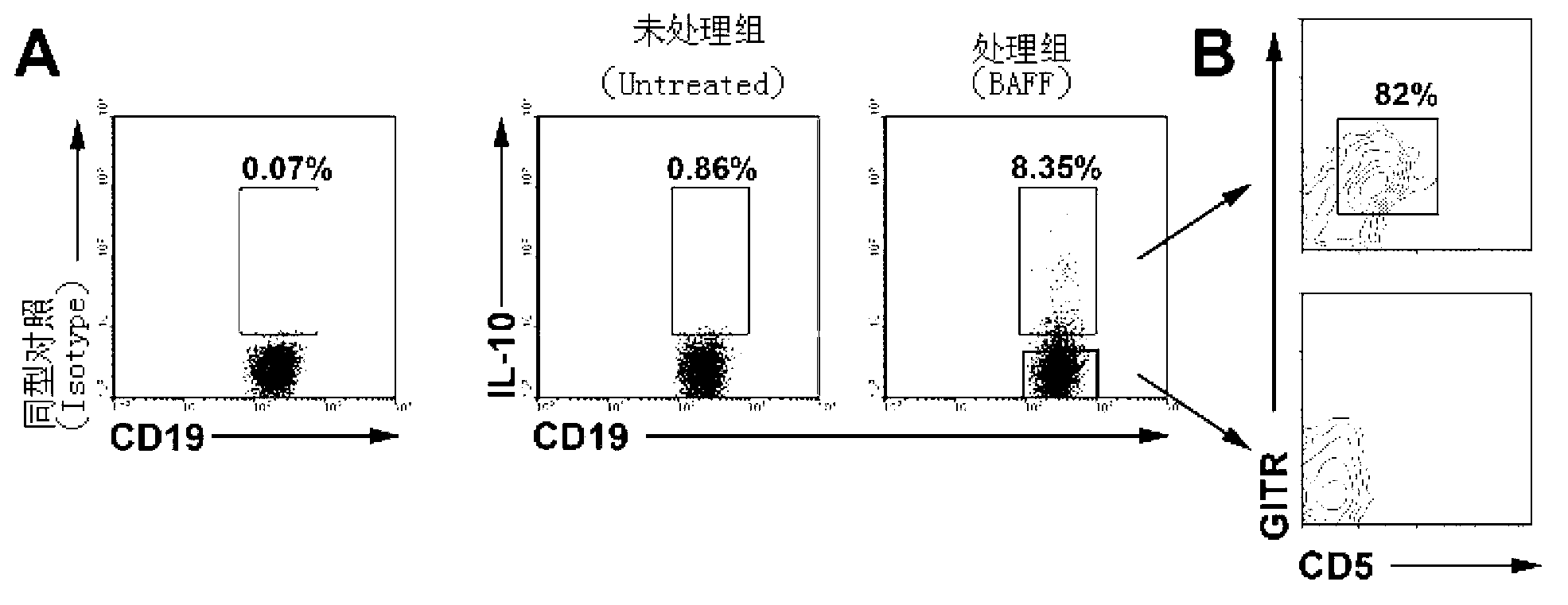
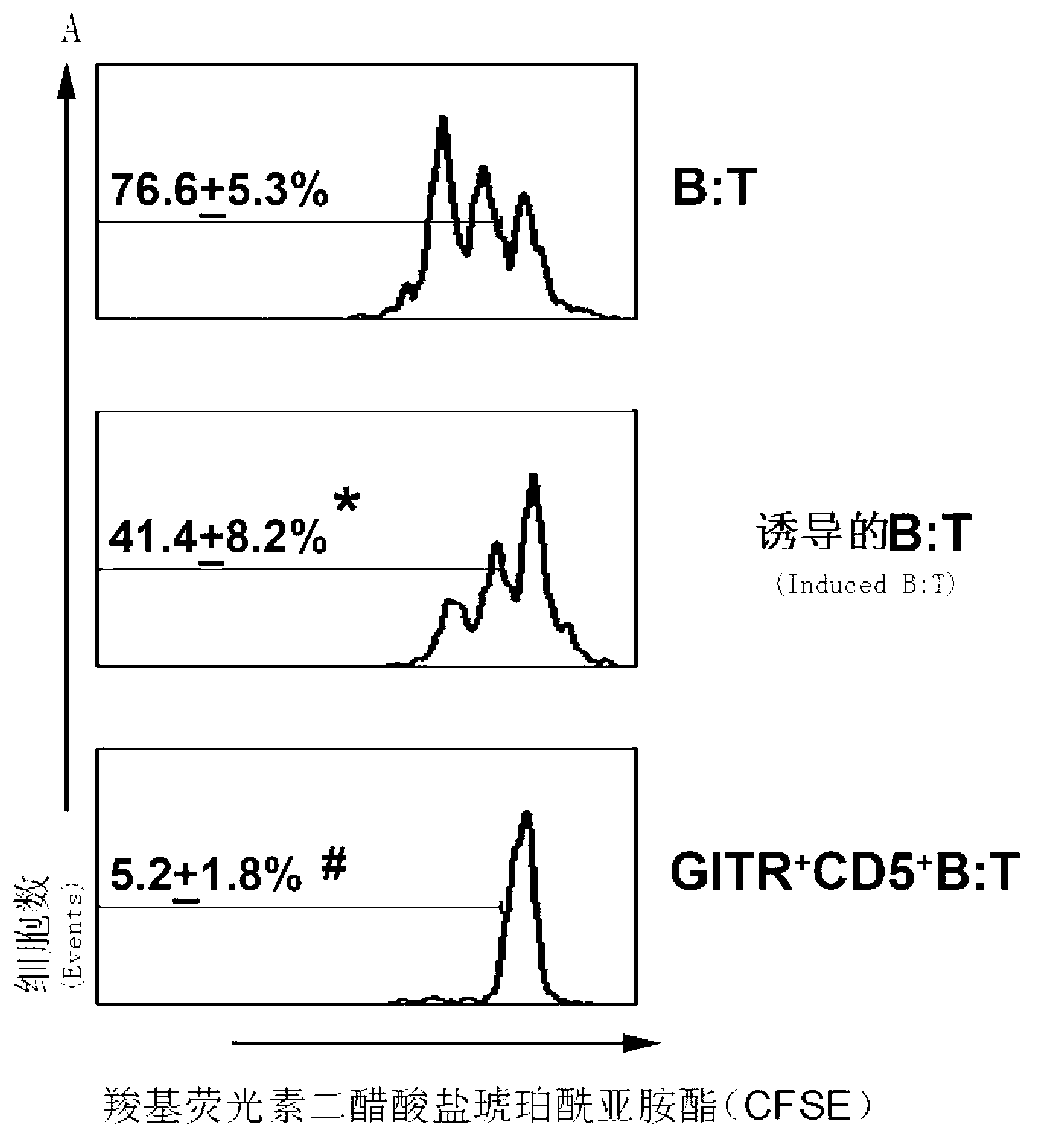
The present invention relates to the technical field of biology, specifically to induced culture and purification of human suppressive b-lymphocytes and applications of a potential cell therapy method in autoimmune diseases. According to the method, human peripheral blood is sterilely sampled, LymphoprepTM (Axis-Shield) is adopted to separate human mononuclearcell, the separated cell is placed in a 10% FBS-containing RPMI1640 culture medium to culture, and concurrently 50 ng / ml of human recombinant BAFF cytokine is added at a first day, a third day and a fifth day to induce suppressive b-lymphocyte production. According to the present invention, the BAFF is adopted for differentiation of the human peripheral blood B cells into the suppressive b-lymphocytes through in vitro induction, wherein the suppressive b-lymphocytes express IL-10, and have T lymphocyte proliferation inhibition capability.
Owner:JIANGSU UNIV
Polyethylene glycol-cactus oligopeptide bonding rapamycin derivatives
ActiveUS20160271116A1High load rateReduced preparation specificationOrganic active ingredientsAntimycoticsImmunologic disordersAutoimmune condition
The present invention provides compounds represented by formula (I) and pharmaceutical acceptable salts thereof, preparation method therefor and pharmaceutical composition containing the compounds represented by formula (I) and pharmaceutical acceptable salts thereof. In the compounds of the present invention, each terminal group of polyethylene glycol molecule can bond with a plurality of rapamycin molecules by cactus oligopeptide, with the loading rate of the pharmaceutical being increased. The compounds can be used to induce immunosuppression and treat graft rejection, autoimmune disease, solid tumors, fungal infection, and cardiovascular and cerebrovascular disease.
Owner:JENKEM TECH
Drug product, a method of administrating a vaccine using the drug product, and an apparatus for iontophoresis
InactiveUS20100324469A1Good effectImprove complianceNervous disorderElectrotherapyAntigenInduction immunosuppression
It is an object of the present invention to provide a method and an apparatus capable of noninvasively administrating vaccine through the skin, and thereby making the immune response activate. A drug product according to the present invention is characterized in that the drug product has a support medium, an electrode formed on the support medium and protected partly by an insulating material for avoiding a direct cutaneous contact, a vaccine containing layer for containing a vaccine, wherein the vaccine is an antigen for inducing an immune suppression protein. A method of administrating a vaccine according to the present invention is characterized in that the vaccine is administrated by the iontophoresis using the drug product according to the drug product of the present invention as an electrode. An apparatus for the iontophoresis according to the present invention is characterized in that the drug product according to the present invention is used as an electrode.
Owner:JOSAI UNIV CORP
Methods of treating allergy by administering a CD200 protein
InactiveUS7223729B2Inhibited graft survivalReduce miscarriage rateNervous disorderPeptide/protein ingredientsDiseaseAutoimmune disease
Methods and compositions for inducing immune suppression are disclosed. The methods involve administering an effective amount of a CD200 protein or a nucleic acid encoding a CD200 protein. The methods are useful in preventing graft rejection, fetal loss, autoimmune disease, and allergies. Methods and compositions for preventing immune suppression are also disclosed. The methods involve administering an effective amount of an agent that inhibits CD200. Such methods are useful in treating cancer.
Owner:TRILLIUM THERAPEUTICS
Rapamycin and il-10 for the treatment of immune diseases
InactiveUS20060127357A1Improve bioavailabilityImprove efficacyBiocideNervous disorderImmune toleranceT cell
The invention discloses a combined preparation containing IL-10 and rapamucin able to induce immunosuppression and antigen-specific immune tolerance, and the use thereof in the treatment of diseases involving an excessive, dysfunctional or uncontrolled immune response mediated by T cells.
Owner:RONCAROLO MARIA GRAZIA
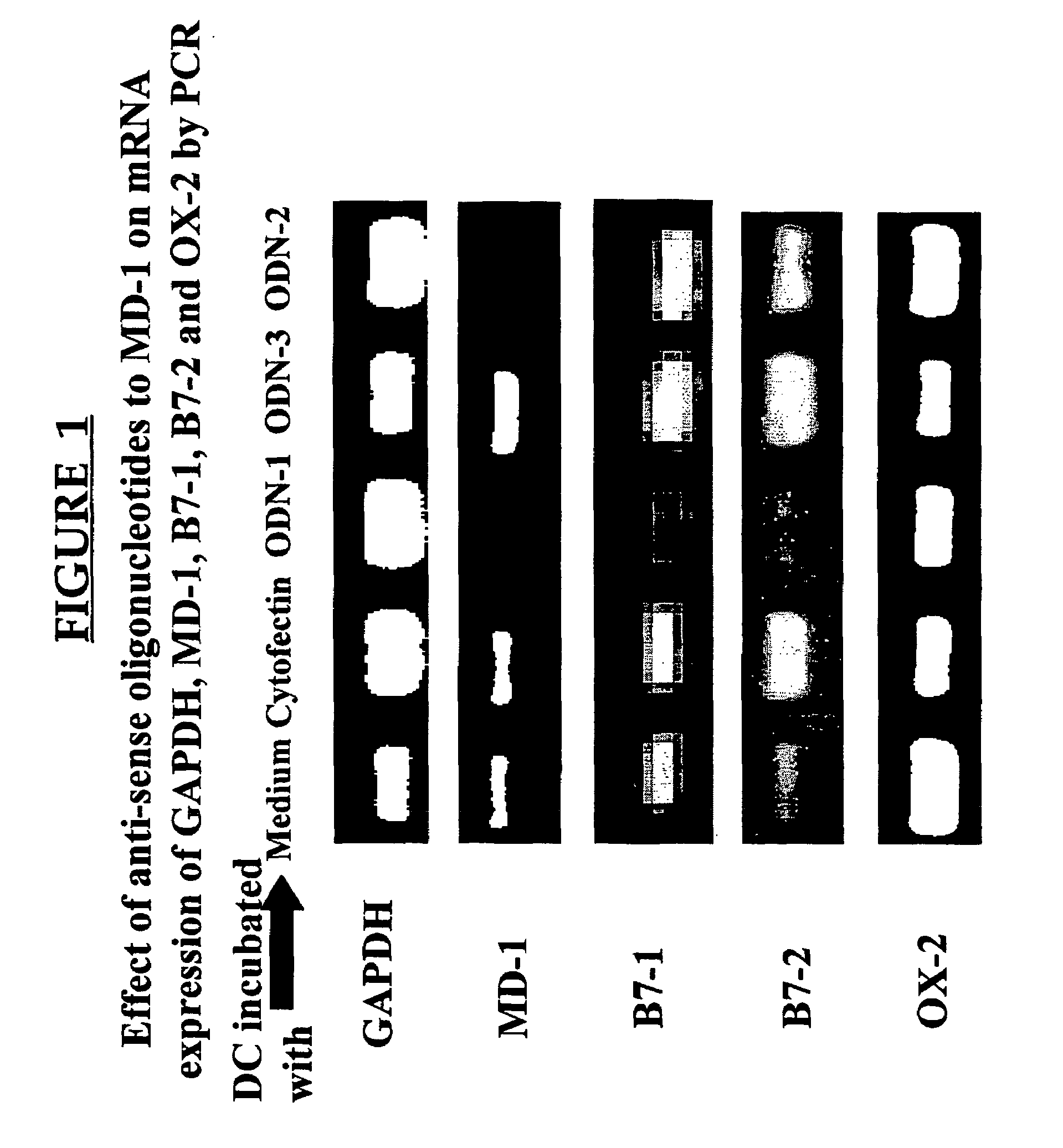
MD-1 inhibitors as immune suppressants
InactiveUS7291330B2Improve graft survivalInhibit productionAntibacterial agentsOrganic active ingredientsIMMUNE SUPPRESSANTSAutoimmune responses
Methods and compositions for inducing immune suppression are disclosed. The methods involve administering an effective amount of an agent that inhibits MD-1 with or without an OX-2 protein or a nucleic acid encoding an OX-2 protein. The methods are useful in preventing graft rejection, fetal loss, autoimmune disease, and allergies. Methods and compositions for preventing immune suppression are also disclosed. The methods involve administering an effective amount of MD-1 or an agent that activates or stimulates MD-1.
Owner:TRILLIUM THERAPEUTICS
Method for screening a test compound for potential as an immunosuppressive drug candidate
InactiveUS6436656B1Avoid undesirable side effectPrevent adverse side effectsVectorsMicrobiological testing/measurementDrugImmunosuppressive drug
The present invention relates, in general, to immunosuppression and, in particular, to a method of inducing an immunosuppressive effect. The invention further relates to a method of screening compounds for immunosuppressive activity.
Owner:DUKE UNIV
Methods and Compositions for Modulating Immunity
InactiveUS20070197443A1Avoid developmentAvoid survivalSenses disorderPeptide/protein ingredientsAutoimmune conditionAutoimmune disease
Methods and compositions for inducing immune suppression are disclosed. The methods involve administering an effective amount of a CD200 protein or a nucleic acid encoding a CD200 protein. The methods are useful in preventing graft rejection, fetal loss, autoimmune disease, and allergies. Methods and compositions for preventing immune suppression are also disclosed. The methods involve administering an effective amount of an agent that inhibits CD200. Such methods are useful in treating cancer.
Owner:TRILLIUM THERAPEUTICS
Induction of immunosuppression by inhibition of ATM
InactiveUS20080279866A1Prevent rejectionImpaired to respondOrganic active ingredientsMetabolism disorderAutoimmune responsesAllergy
The present invention is directed to methods of inducing immunosuppression in a patient by administering an inhibitor of the enzyme Ataxia telangiectasia mutated (Atm). The method may be used as a treatment for allergies, autoimmune diseases or lymphomas. It may also be used to prevent organ rejection in transplant patients and to treat or prevent graft versus host disease.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Method for the treatment of automimmune diseases comprising administering rapamycin and IL-10
ActiveUS8329637B2Improve bioavailabilityImprove efficacyOrganic active ingredientsNervous disorderT cellImmune tolerance
The invention discloses a combined preparation containing IL-10 and rapamycin, able to induce immunosuppression and antigen-specific immune tolerance, and the use thereof in the treatment of diseases involving an excessive, dysfunctional or uncontrolled immune responses mediated by T cells.
Owner:TR1X INC
Non-immunosuppressive imunogenic or vaccine composition comprising a mutated e7 protein of the hpv-16 virus
InactiveUS20060233820A1Inhibitory activityQuickly block immunosuppressive effectAntibody mimetics/scaffoldsVirus peptidesBULK ACTIVE INGREDIENTActive ingredient
The invention relates to an immunogenic or vaccine composition inducing an immune response towards the HPV-16 Papillomavirus native E7 protein, without simultaneously inducing an immunosuppression, said composition comprising, as the active ingredient, a non immunosuppressive mutated E7 protein, comprising the amino acid sequence consisting, from the N-terminal end to the C-terminal end, in: i. the 1-19 amino acid sequence of sequence SEQ ID No. 3; ii. an amino acid sequence possessing (a) the substitution of at least one amino acid, compared to the 20-29 corresponding amino acid sequence of sequence SEQ ID No. 3 or (b) the deletion of at least four consecutive amino acids, compared to the 20-29 corresponding amino acid sequence of sequence SEQ ID No. 3; and iii. the 30-98 amino acid sequence of sequence SEQ ID No.;
Owner:NEOVACS SA
T cell-bound cytokine assay for antigen-specific tolerance
In vitro methods for detecting and measuring an antigen-specific regulatory T cell response are described. In particular, there is provided, for example, a method of detecting a change in surface expression of particular T cell markers in T cells obtained from the subject as a way to detect induced immune suppression in response to exposure to a particular antigen or plurality of antigens.
Owner:UNIVERSITY OF PITTSBURGH +2
Vaccine for treating and/or preventing type I diabetes and application thereof
ActiveCN106668852AEffective treatmentRaise the ratioMetabolism disorderAntibody ingredientsIMMUNE SUPPRESSANTSCD8
The invention discloses a vaccine for treating and / or preventing type I diabetes and an application thereof. The active ingredients of the vaccine are as follows: 1) or 2) or 3): 1) proteantigen of type I diabetes and an immunosuppressor; 2) an epitope peptide of the proteantigen of type I diabetes and the immunosuppressor; and 3) the proteantigen of type I diabetes, the epitope peptide of the proteantigen of type I diabetes and the immunosuppressor, wherein the proteantigen of type I diabetes is at least one of insulin, glutamate decarboxylase and islet amyloid polypeptide; and the immunosuppressor is at least one of dexamethasone, cyclosporin A, tacrolimus, mycophenolate mofetil, azathioprine, prednisone, early-group prednisolone, anti-CD4 monoclonal antibody and anti-C3 monoclonal antibody. The composition can promote Treg proliferation, accelerate secretion of IL-10 and TGF-beta, control the blood glucose level, inhibit the killing action of autoimmune responsive CD8 T cells, inhibit DC cell maturation, and induce immunosuppression so as to effectively treat type I diabetes.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD
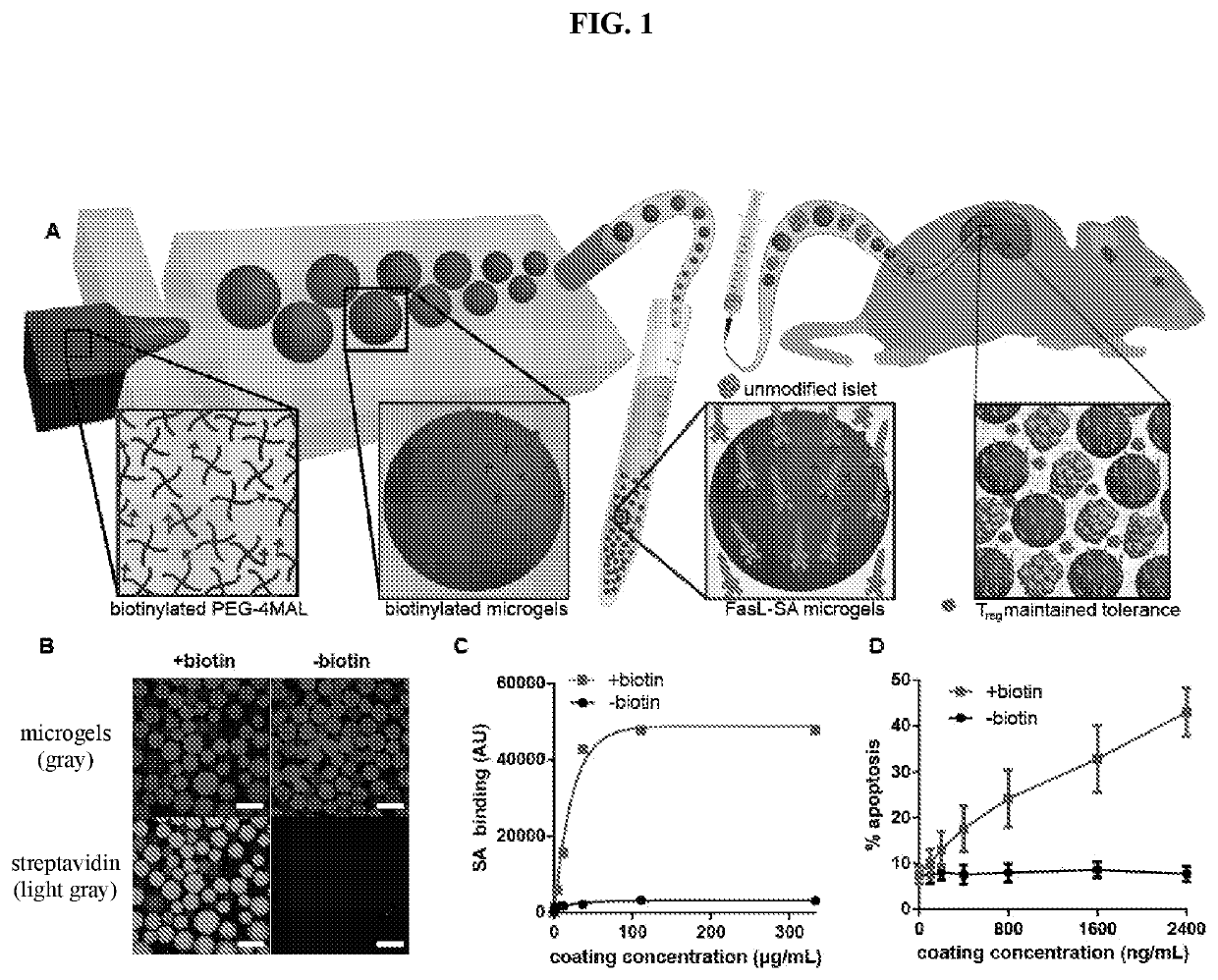
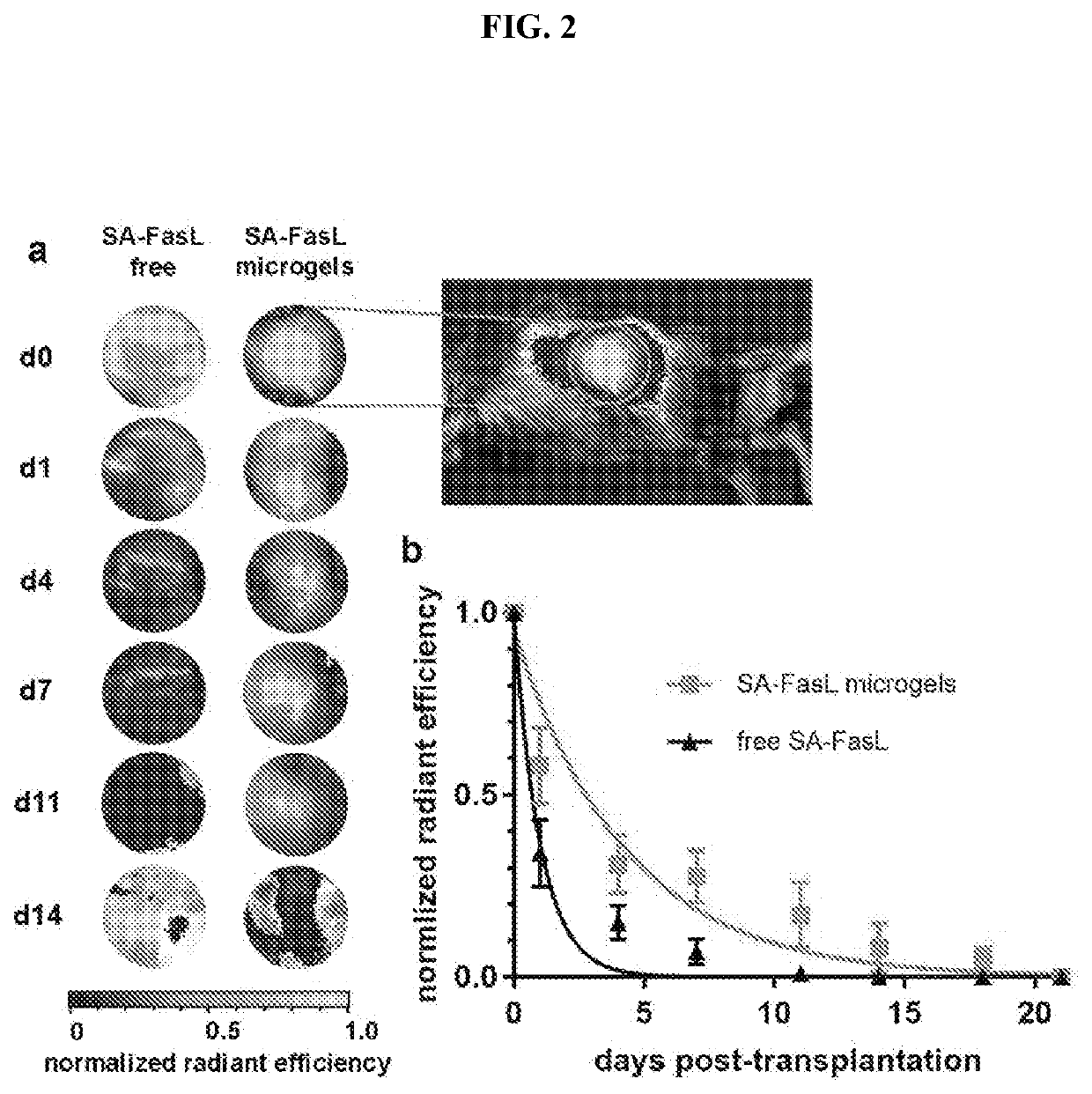
Fasl-engineered biomaterials with immunomodulatory function
ActiveUS20200046780A1Prevent and reduce riskOrganic active ingredientsPeptide/protein ingredientsImmunologic disordersDisease
Described herein are FasL-engineered biomaterials, as well as methods of making and using such FasL-engineered biomaterials, such as for immunomodulation, such as for inducing immunosuppression and specific immune tolerance, such as for preventing or reducing the risks of rejection of cellular or tissue grafts and / or the treatment of autoimmune disorders such as Type I diabetes. In specific embodiments, the FasL-engineered biomaterials are biotinylated microgels bound to SA-FasL.
Owner:GEORGIA TECH RES CORP +1
Immune tolerance inducer
ActiveUS20140294869A1Efficient inductionGood effectOrganic active ingredientsSugar derivativesSpecific immunityImmunosuppressive effect
An object of the present invention is to provide a drug and a method for effectively inducing donor-specific immune tolerance in a recipient in transplantation therapy. A complex of an siRNA for a costimulatory factor and schizophyllan is delivered to a Dectin-1 expressing cell which specifically recognizes schizophyllan to regulate the function of the Dectin-1 expressing cell, and therefore can induce immunosuppression effect as well as induce immune tolerance effectively.
Owner:NAPAJEN PHARMA
Pharmaceutical composition for preventing or treating neurodegenerative disease comprising nckap1 protein or gene encoding same
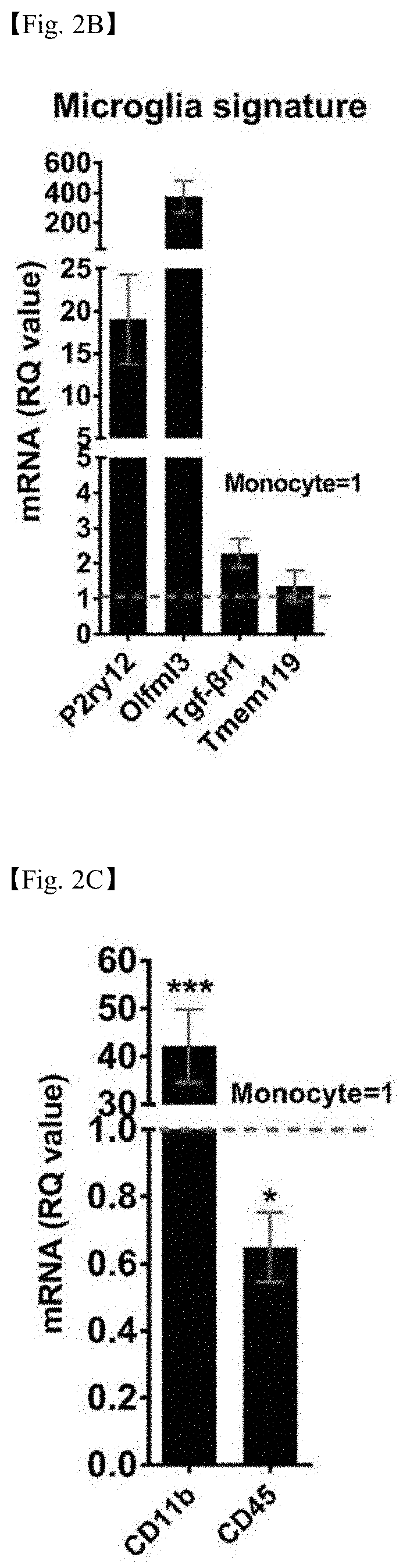
PendingUS20200247854A1Regulating phagocytic functionImprove securityNervous disorderPeptide/protein ingredientsNeuro-degenerative diseaseBULK ACTIVE INGREDIENT
The present invention relates to a novel use of NCKAP1 gene in neurodegenerative diseases. More specifically, the present invention relates to a marker composition for predicting the prognosis of a neurodegenerative disease, comprising a NCKAP1 protein or a gene encoding same, a composition and a kit for predicting the prognosis of a neurodegenerative disease, which comprises a formulation for measuring the level of the protein or an mRNA of the gene encoding same, and a pharmaceutical composition for preventing or treating neurodegenerative disease, comprising the protein or the gene encoding same as an active ingredient. The pharmaceutical composition comprising the NCKAP1 protein or the gene encoding the same, according to the present invention, can selectively control only specific signal transduction related to the phagocytosis of microglial cells unlike conventional therapeutic agents that induce immunosuppression, and thus can be usefully used for the development of therapeutic agents with high safety and efficiency, and the NCKAP1 protein or the gene encoding the same may be usefully utilized, as a marker for predicting the prognosis of a neurodegenerative disease, for predicting a disease progression rate and treatment outcome.
Owner:COLLEGE OF MEDICINE POCHON CHA UNIV IND
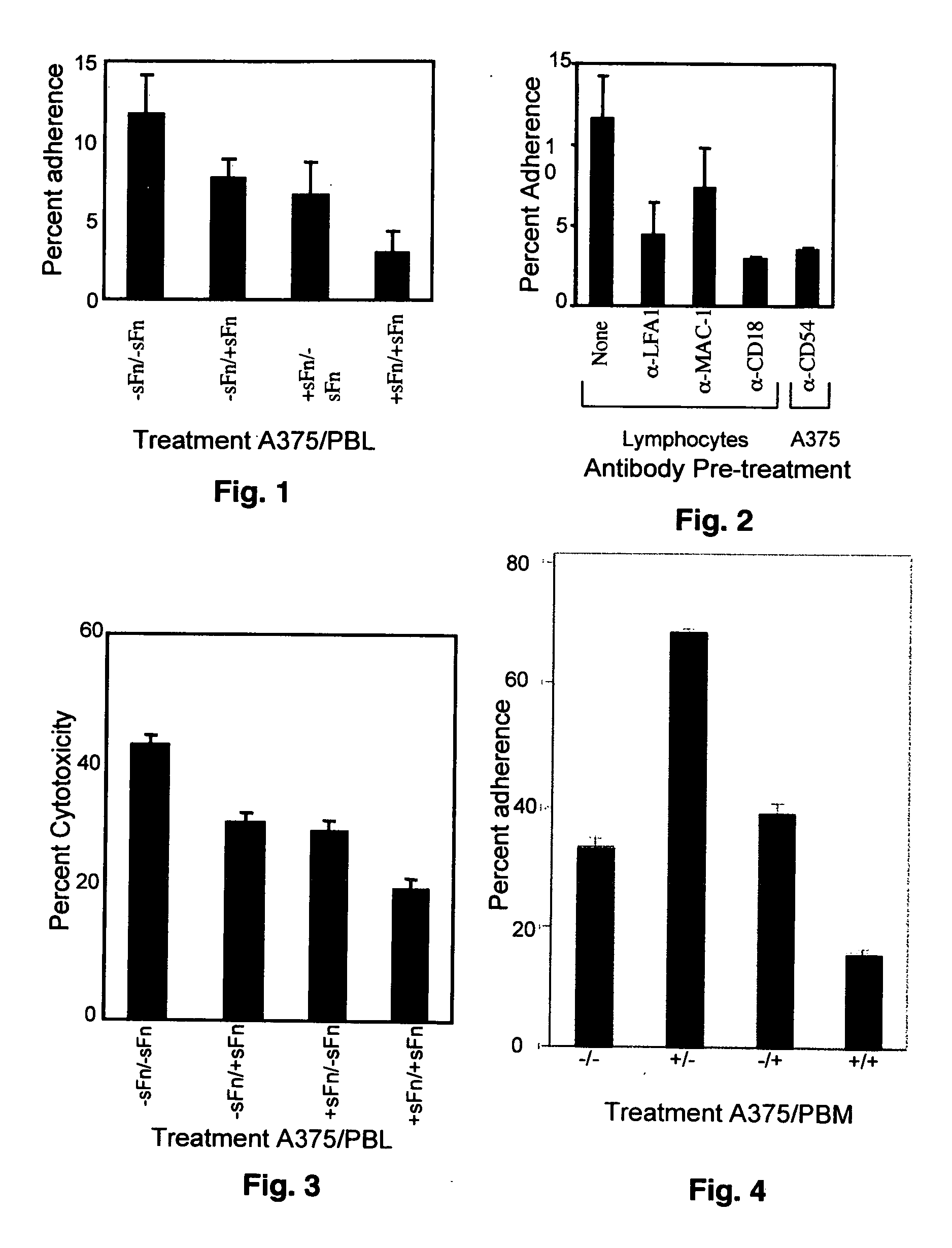
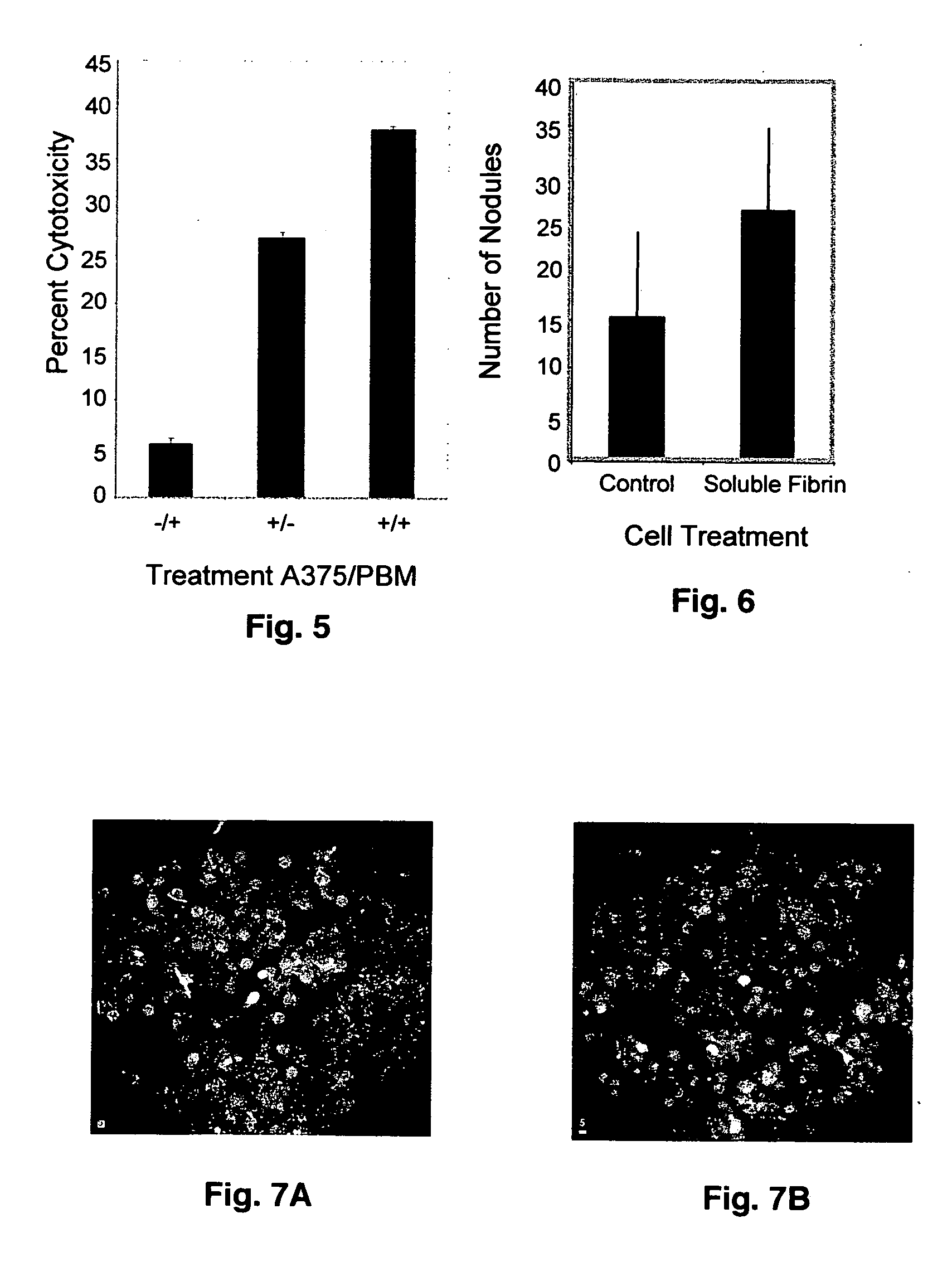
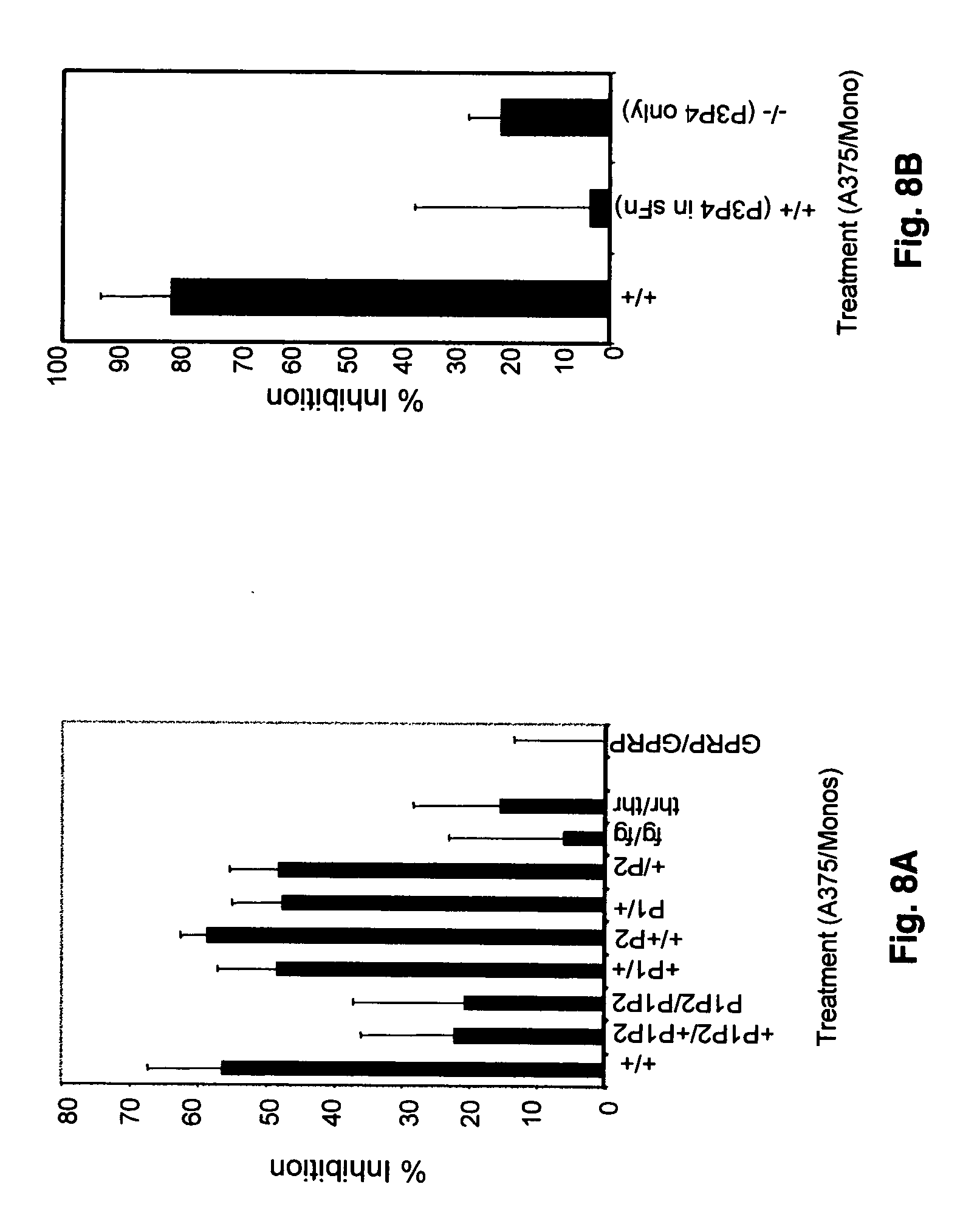
Soluble fibrin inhibitory peptides and uses thereof
ActiveUS20070060522A1Reduce inhibitionRestores immune responseFibrinogenPeptide/protein ingredientsLymphatic SpreadImmunologic Cytotoxicity
The present invention demonstrated that soluble fibrin binds to both Mac-1 and ICAM-1-expressing cells and inhibited adherence of these cells and immune cytotoxicity, thus inducing immune suppression in cancer. Additionally, the present invention also demonstrated that soluble fibrin enhanced metastasis in an in vivo model. Furthermore, the present invention demonstrated the utility of specific peptides that block binding of soluble fibrin to these cells as therapeutic agents in cancer progression and metastasis. It is further contemplated that these peptides can also be used to treat other diseases such as cardiovascular disease, arthritis and in many inflammatory responses where there is increased levels of soluble fibrin.
Owner:ENSION
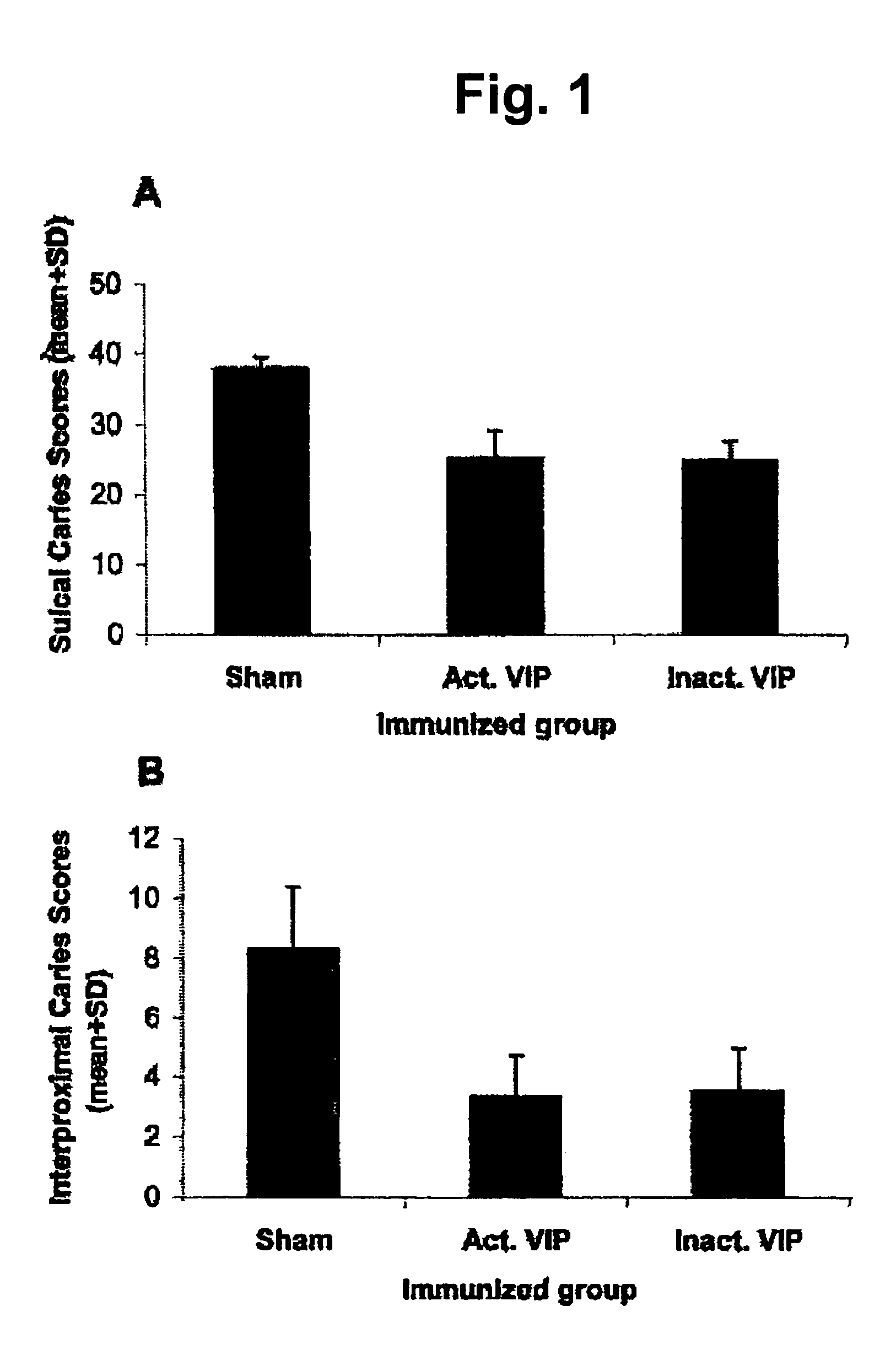
Vaccine against dental caries based on virulence-associated immunomodulatory extracellular proteins produced by the cariogenic bacteria Streptococcus sobrinus and Streptococcus mutans
InactiveUS7541041B2Cosmetic preparationsBacterial antigen ingredientsBacteroidesVirulent characteristics
This invention is concerned with a vaccine against dental caries obtained from the supernatant over cultures of the cariogenic bacteria Streptococcus sobrinus and S. mutans that consists of extracellular proteins. These proteins provide the suppression of the immune response in the host through the early production of IL-10, an anti-inflammatory cytokine and are, for that reason, virulent factors to the microorganism enhancing bacterial load in the host. These proteins were designated as virulence-associated immunomodulatory extracellular proteins (VIP). Vaccination through the host immunization with active VIP in a submitogenis dose, inactivated VIP or enolase in a dose unable to induce immunosuppression, induces the immunoneutralization of the VIP immunobiological effects.Vaccination can be used both as a preventive or therapeutic measure of dental caries as long as it is administered (intranasally, orally or subcutaneously) into mammals before or after bacterial infection, respectively.
Owner:UPIN - UNIVE DO PORTO INOVACAO
Polyethylene glycol-cactus oligopeptide bonding rapamycin derivatives
ActiveUS10098870B2High load rateReduce diversityOrganic active ingredientsNervous disorderImmunologic disordersAutoimmune condition
The present invention provides compounds represented by formula (I) and pharmaceutical acceptable salts thereof, preparation method therefor and pharmaceutical composition containing the compounds represented by formula (I) and pharmaceutical acceptable salts thereof. In the compounds of the present invention, each terminal group of polyethylene glycol molecule can bond with a plurality of rapamycin molecules by cactus oligopeptide, with the loading rate of the pharmaceutical being increased. The compounds can be used to induce immunosuppression and treat graft rejection, autoimmune disease, solid tumors, fungal infection, and cardiovascular and cerebrovascular disease.
Owner:JENKEM TECH
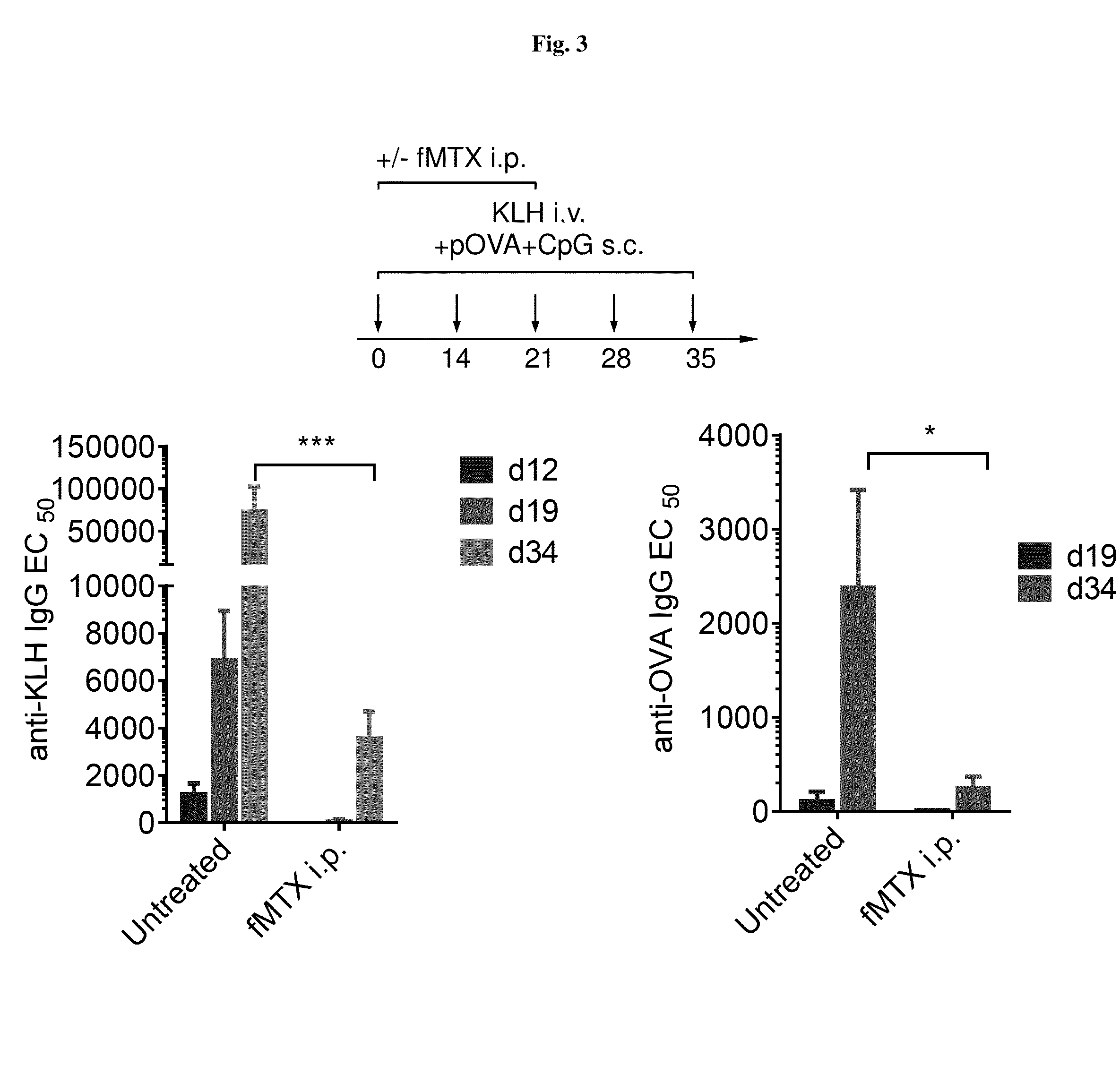
Methods for treating il-6 mediated inflammation without immunosuppression
The disclosure provides methods of treating inflammation without inducing immune suppression. The method comprises administering a therapeutically effective amount of an IL-6 antagonist at a dose sufficient to reduce inflammation without causing immune suppression.
Owner:NOVO NORDISK AS
Repeated administration of non-immunosupressive antigen specific immunotherapeutics
This invention relates to repeated administration of non-immunosupressive antigen specific immunotherapeutics, and relates to repeated administration of antigen- specific immunotherapeutics using protocols, or elements thereof, that do not induce immunosuppression. In some embodiments, the protocol has been previously shown not induce immunosuppression in a subject.
Owner:SELECTA BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com