Methods for treating il-6 mediated inflammation without immunosuppression
An IL-6, immunosuppressive technology, applied in immunoglobulins, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve problems such as immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
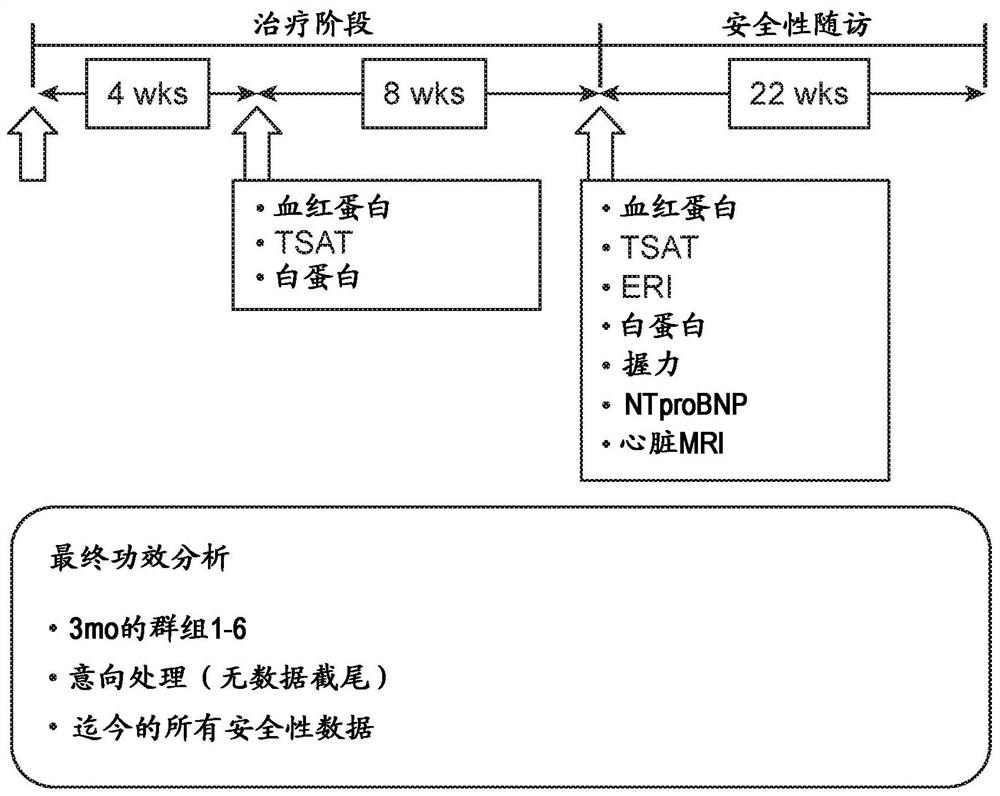
[0347] 3.1 Example 1: Phase 1 / 2 Clinical Study
[0348] A Phase 1 / 2 clinical study was conducted to evaluate the safety, pharmacokinetics and pharmacodynamics of multiple IV doses of COR-001.
[0349] 3.1.1 Drug Products (COR-001)
[0350] COR-001 is a human IgG1, kappa antibody directed against interleukin-6 (IL-6). COR-001 contains a "YTE" mutation in its Fc region. The sequence and other characteristics of COR-001 are described in Section 2.7.1.1 above.
[0351] 3.1.2 Study design
[0352] This study is a randomized, double-blind, placebo-controlled trial designed to evaluate the safety, pharmacokinetics and pharmacokinetics of multiple doses of COR-001 (MEDI5117) or placebo administered to a sequential cohort of hemodialysis patients. Efficacy.
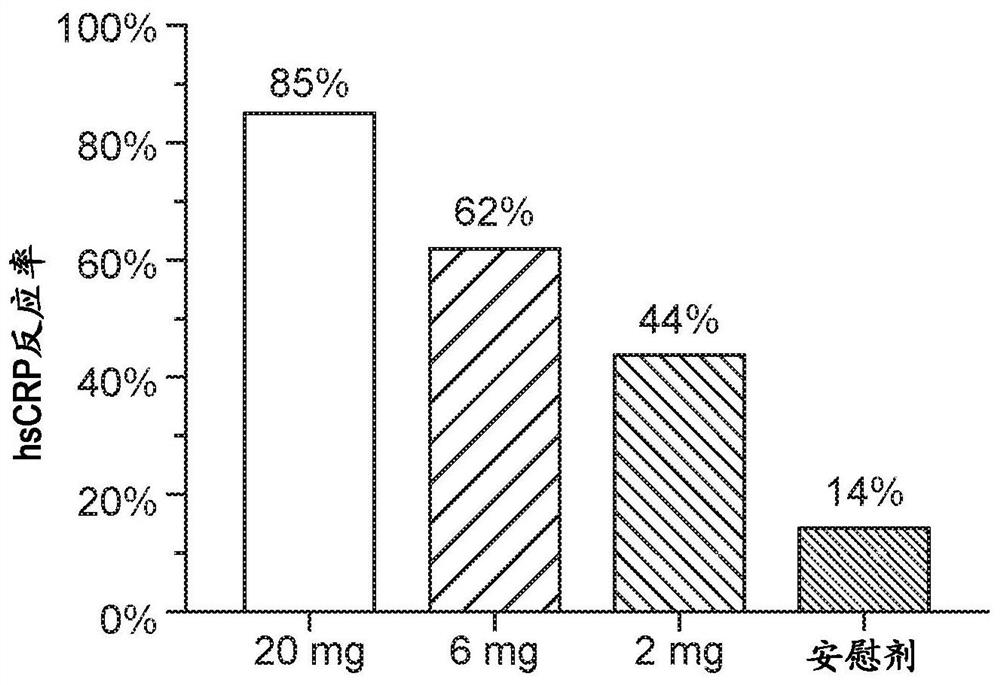
[0353] Key inclusion criteria included stage 5 chronic kidney disease (CKD-5) on hemodialysis, TMPRSS6 736A genotype (major allele) positivity, IL-6 levels above 4 pg / mL, and erythropoietic anti-inflammatory drugs above 8. s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com