Methods and compositions to enhance the immunogenicity of tumors
An immunogenic, tumor cell-based technology, applied in drug combinations, antineoplastic drugs, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Method for selecting immunoreactive peptides from C3d
[0067] The methods described herein and in Example 2 are based on the methods of Knopf, P.M. et al., Immunol. Cell Biol. (2008) 86, 221-225, and De Groot, A.S., Immunol. Cell Biol. (2014) 1-9, to Identification of immunostimulatory MHC class II binding peptides present in C3d. This method can be extended to identify other peptides suitable for use in the present invention. The purpose of these extensions is to:
[0068] 1. Identification of high-affinity class I and class II major histocompatibility complex (MHC) binding sites for C3d-derived peptides using a non-proprietary algorithm;
[0069] 2. Ensure that metalloproteases in the tumor environment can digest them to the appropriate size for binding to MHC proteins.
[0070] step
[0071] 1. Human C3d amino acid sequence: (SEQ ID NO: 5)
[0072]
[0073] Alignment with sequences from dog, pig, cow, mouse and rat to identify conserved regions. ...
Embodiment 2
[0090] Example 2: C3 MHC Peptides
[0091] Figure 6 Listed are the C3 wild-type amino acid sequences (SEQ ID NOs: 6, 8, 10, 12 and 14) and their associated mutant sequences (SEQ ID NOs: 7, 9, 11, 13 and 15), which have been Optimized to improve MHC binding and alter the proteolytic site of metalloproteases. The mutated peptides have reasonably improved MHC binding and improved immune stimulation.
Embodiment 3
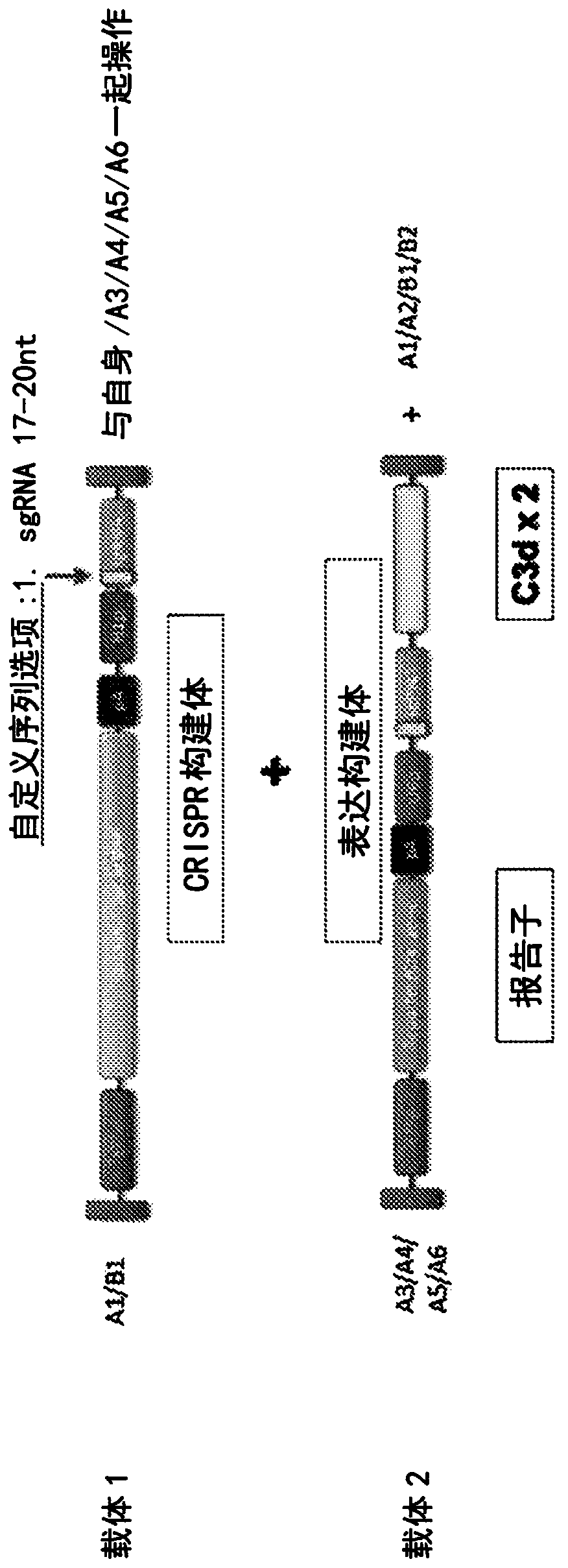
[0092] Example 3: Design of Guide RNA Sequences for CRISPR
[0093] For the method of gene editing using CRISPR technology, a guide RNA sequence is required that edits / cuts out the C3 gene but not the C3d gene sequence properly. See eg portals.broadinstitute.org / gpp / public / analysis-tools / sgrna-design. Exemplary guide RNA sequences (SEQ ID NO: 16-18) and corresponding PAM sequences are at Figure 7 shown in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com