Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Implantable Catheters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
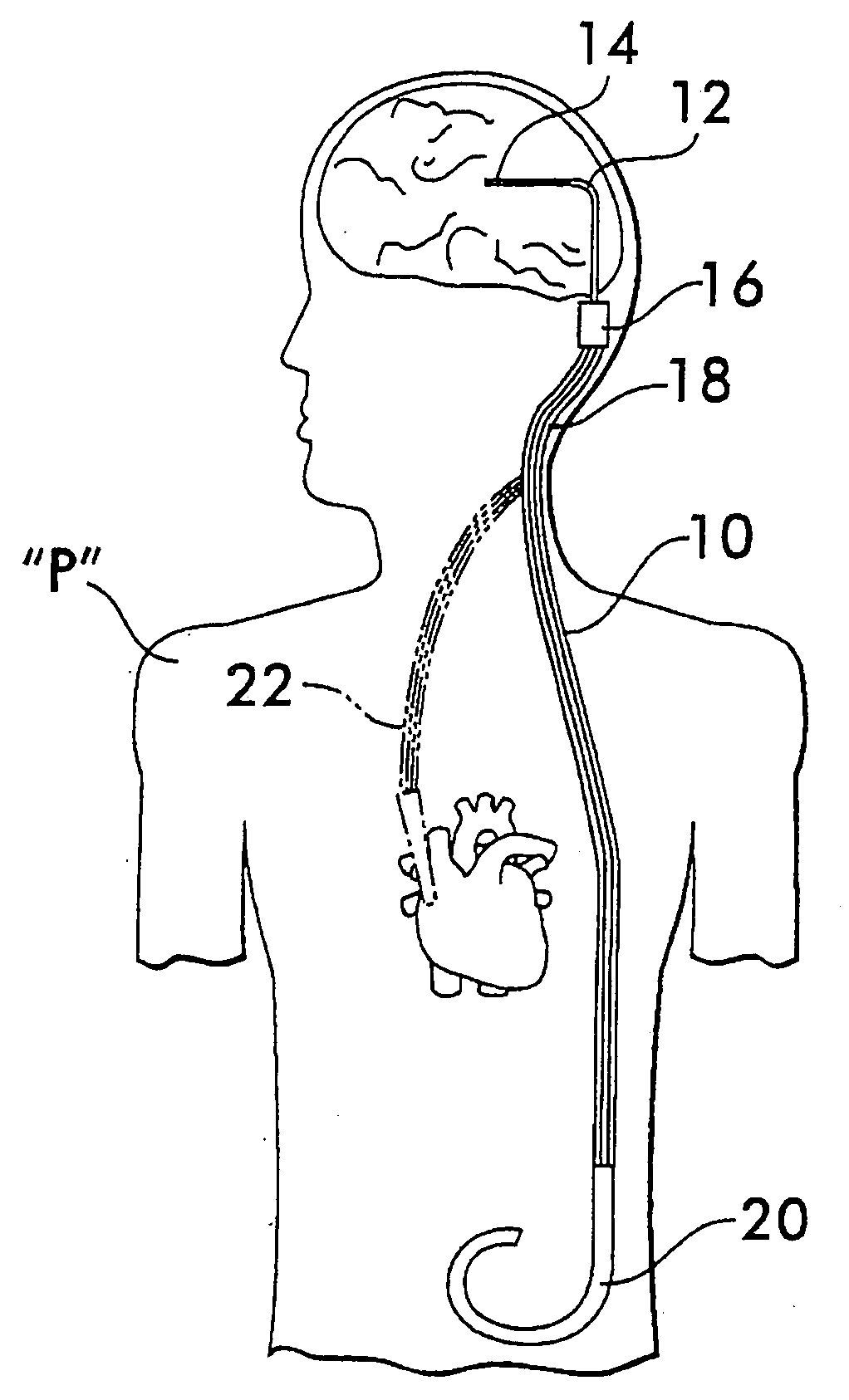
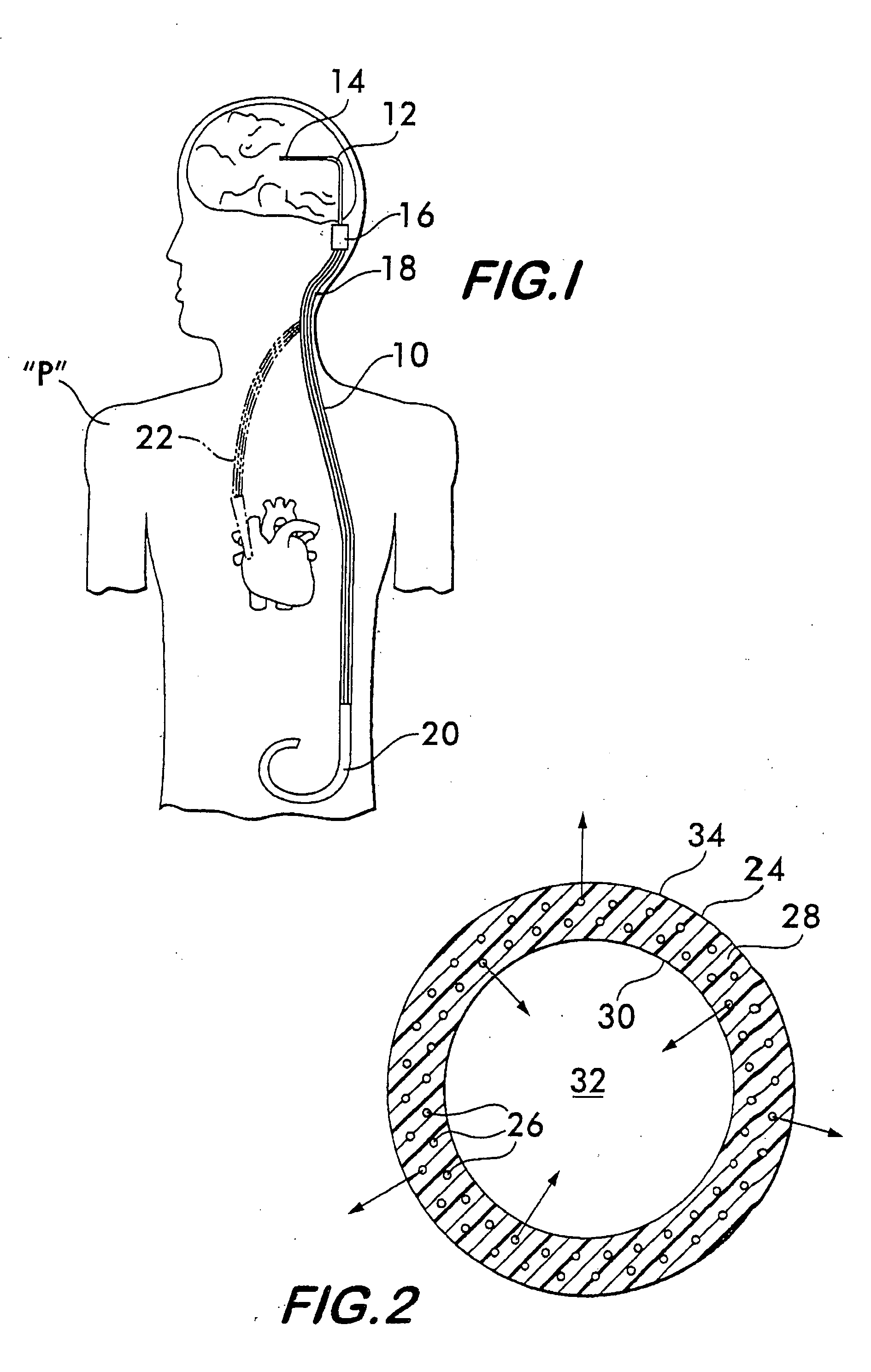
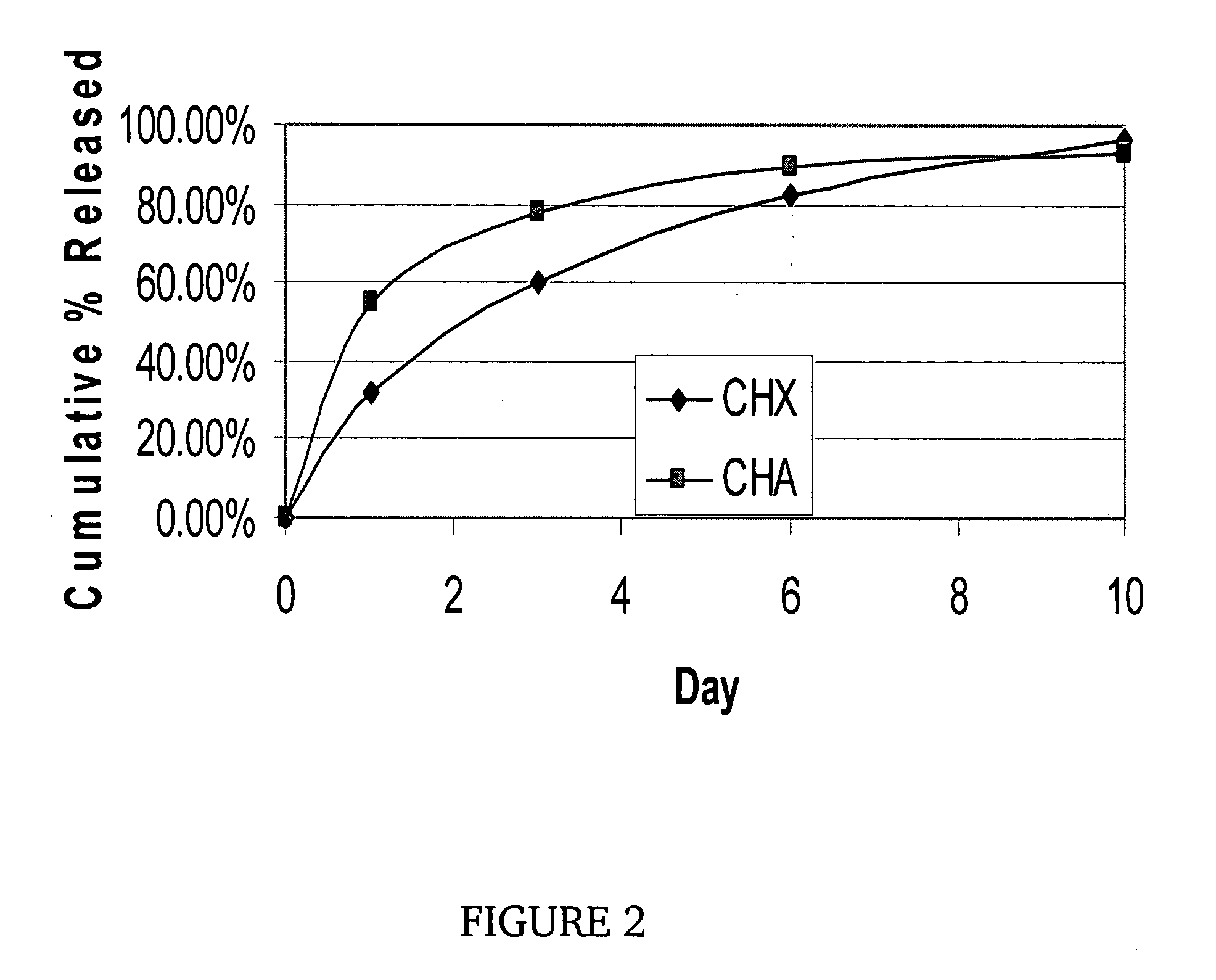
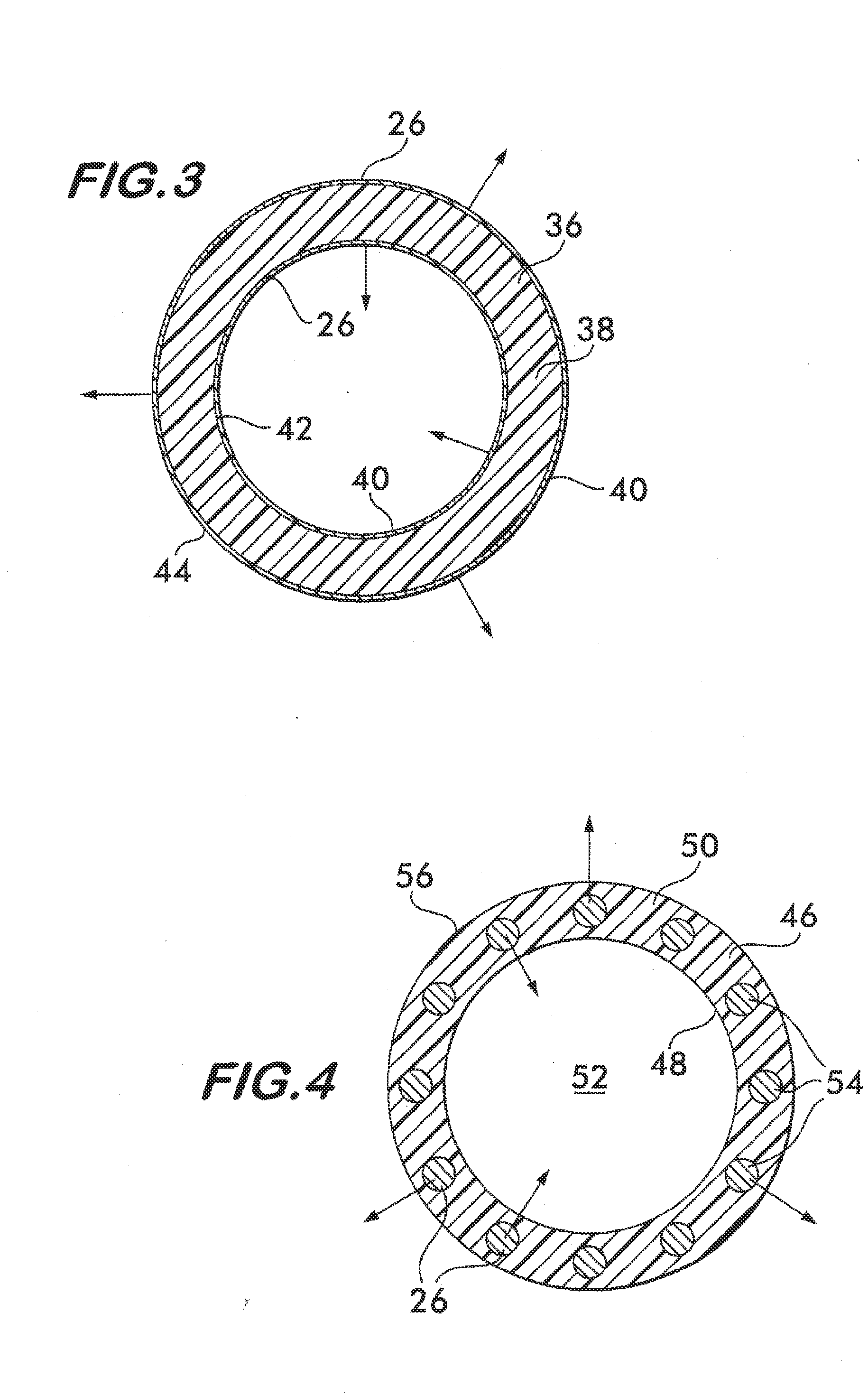
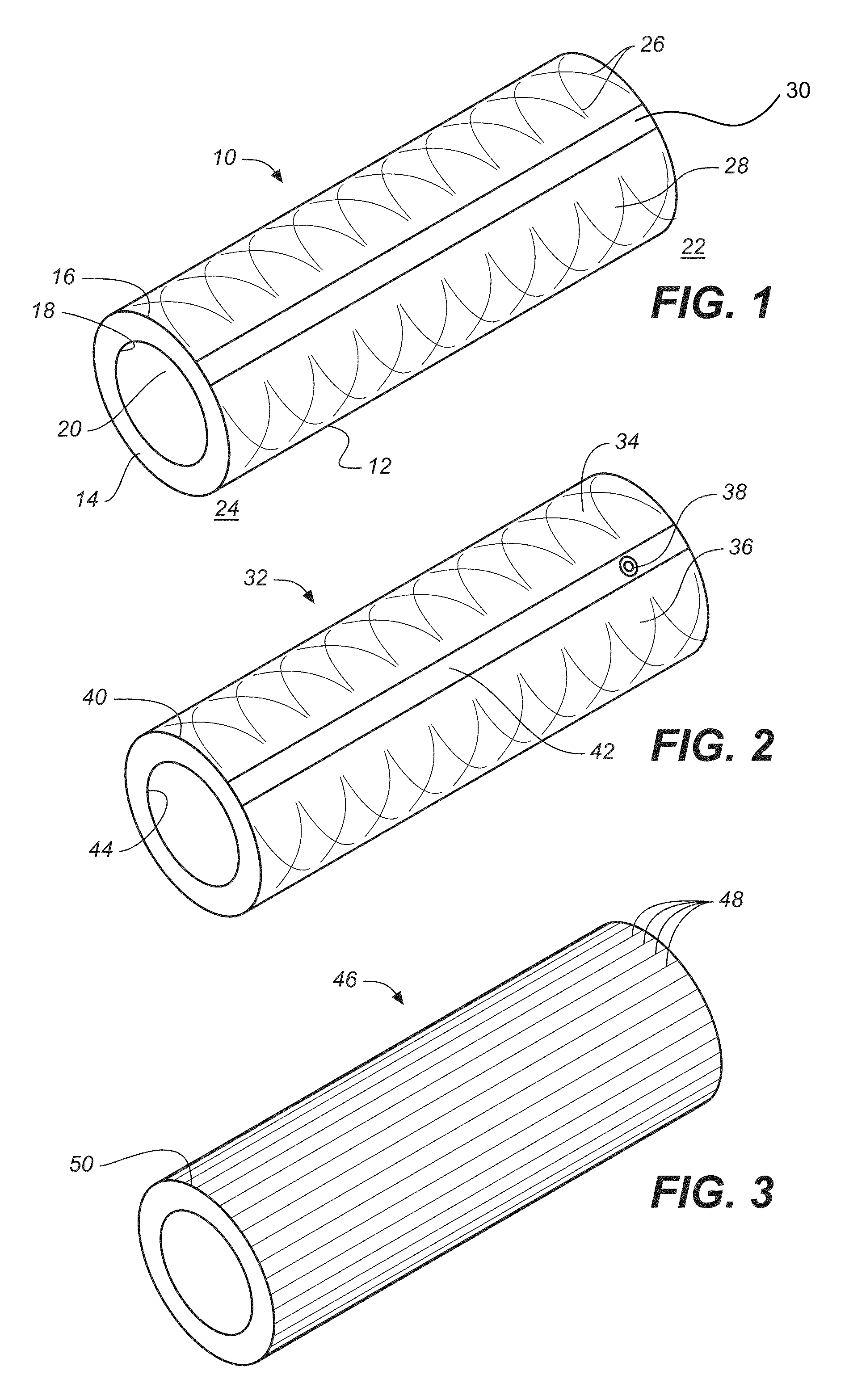
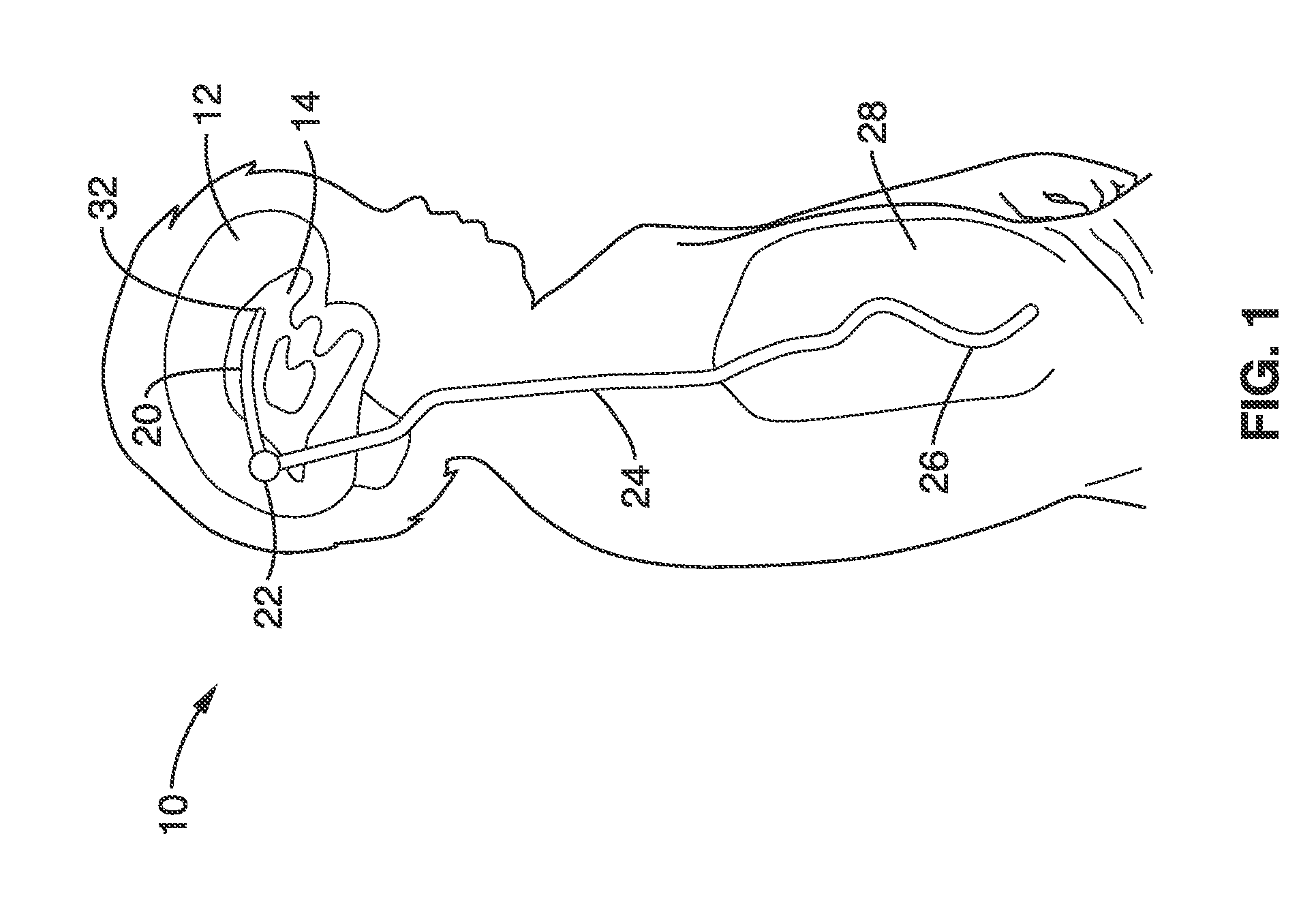
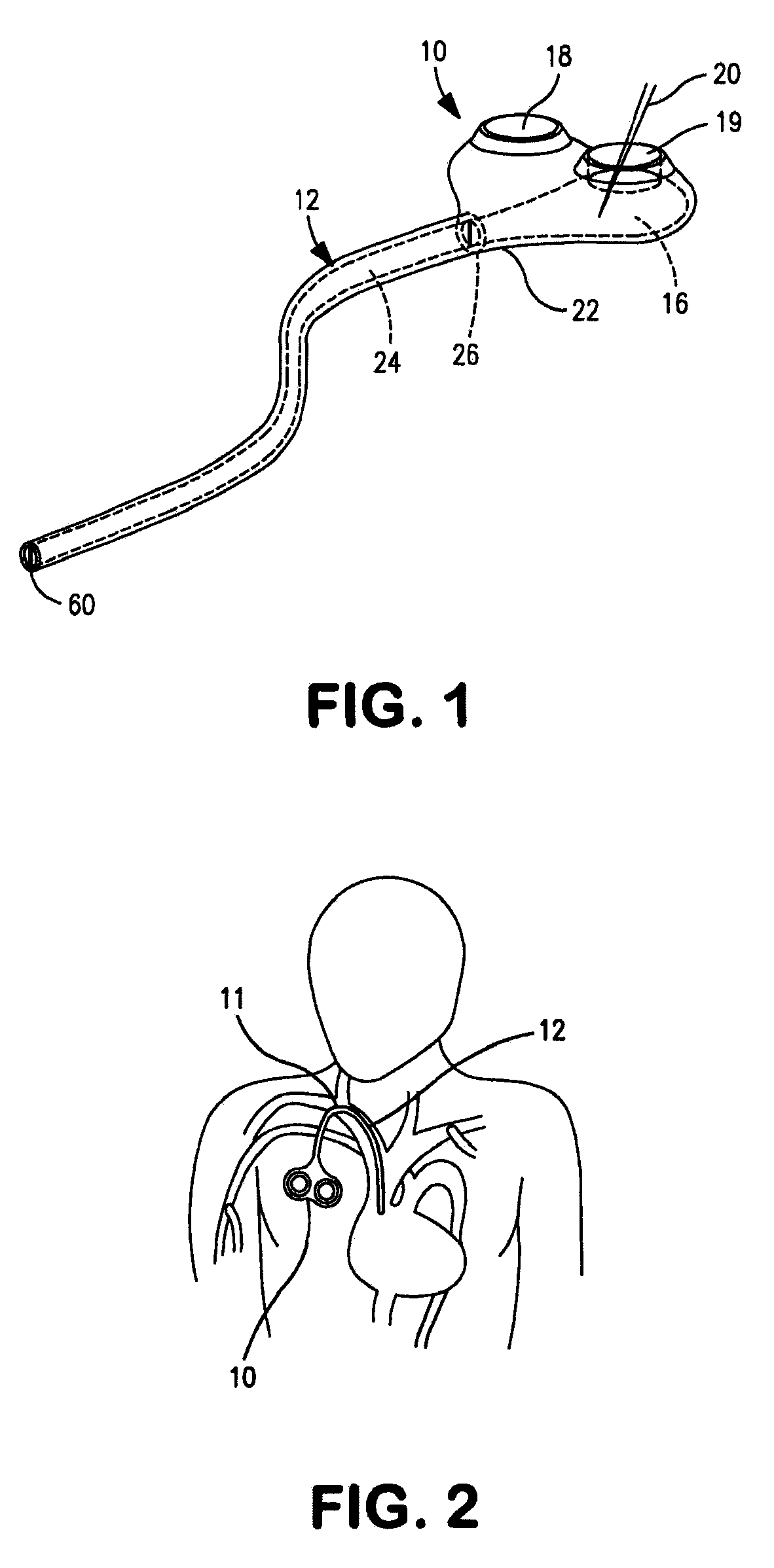
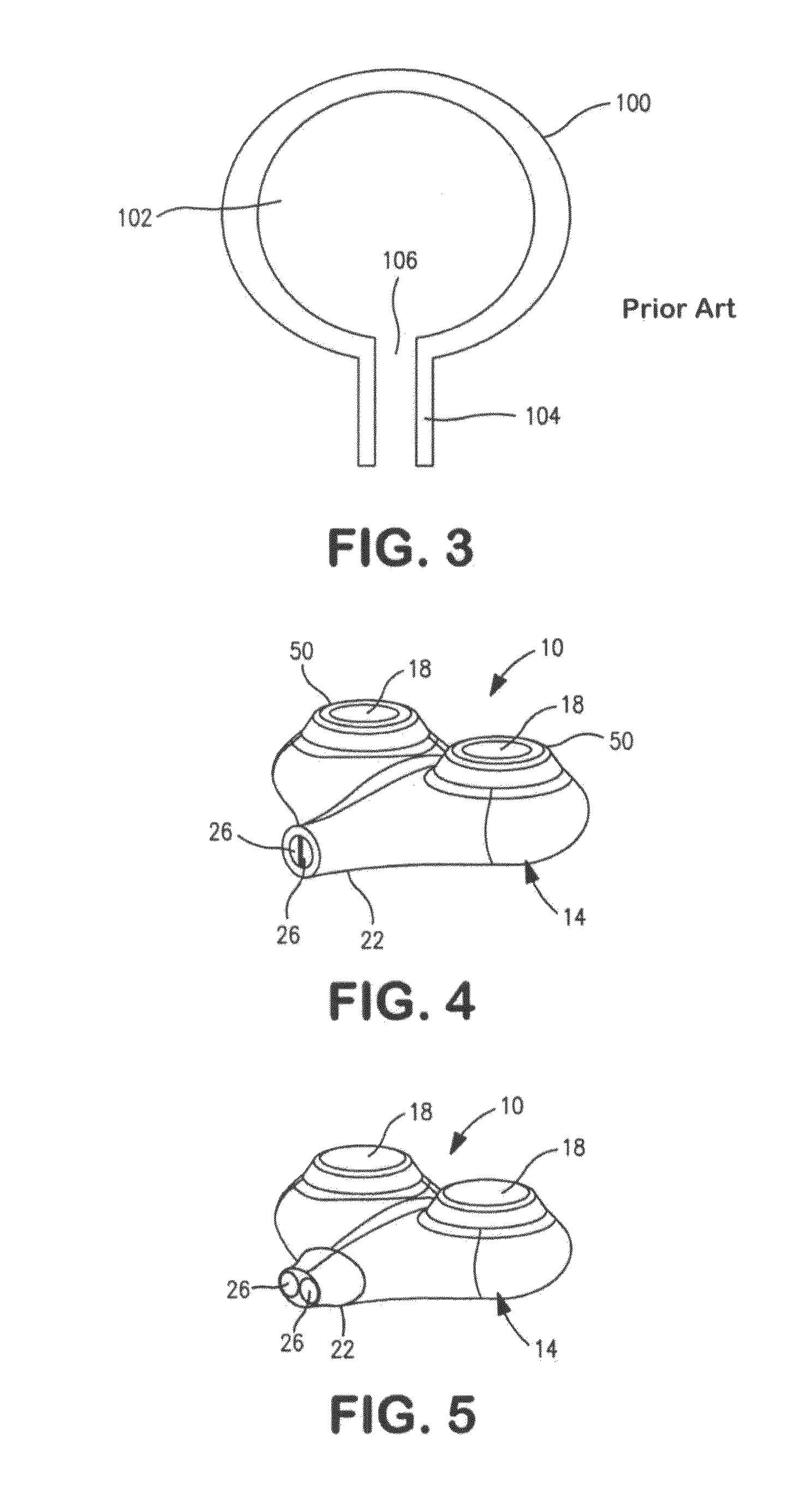
Implantable shunt or catheter enabling gradual delivery of therapeutic agents
InactiveUS20080051691A1Reduce deliveryAvoid infectionBiocideNervous disorderTherapeutic effectFlow diverter
An implantable catheter or shunt for draining fluid from a body cavity. The catheter or shunt body has a wall structure that carries one or more therapeutic agents in a manner enabling release of the therapeutic agent from the wall structure in situ after surgical implantation of the catheter or shunt body. The therapeutic agent can be gradually released over time to prevent infection, inhibit tissue ingrowths, and / or provide some other desired medicinal purpose. As an example, the therapeutic agent can be rapamycin or an mTOR inhibitor. According to some contemplated embodiments of the present invention, the therapeutic agent carried by the catheter / shunt is rechargeable or refillable in situ so that the therapeutic agent can be gradually released from the catheter / shunt over the expected useful life of the catheter / shunt.
Owner:WYETH LLC
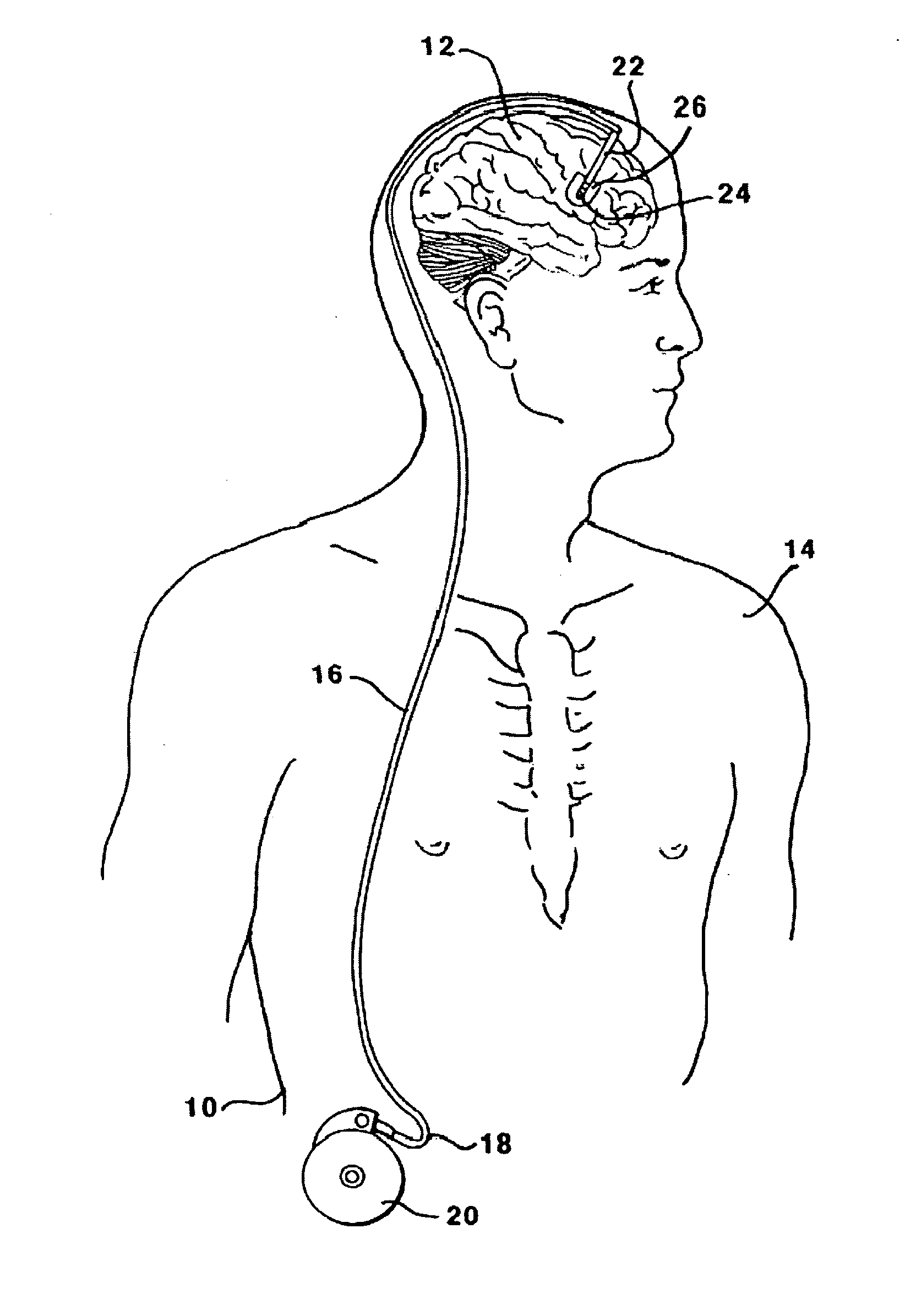
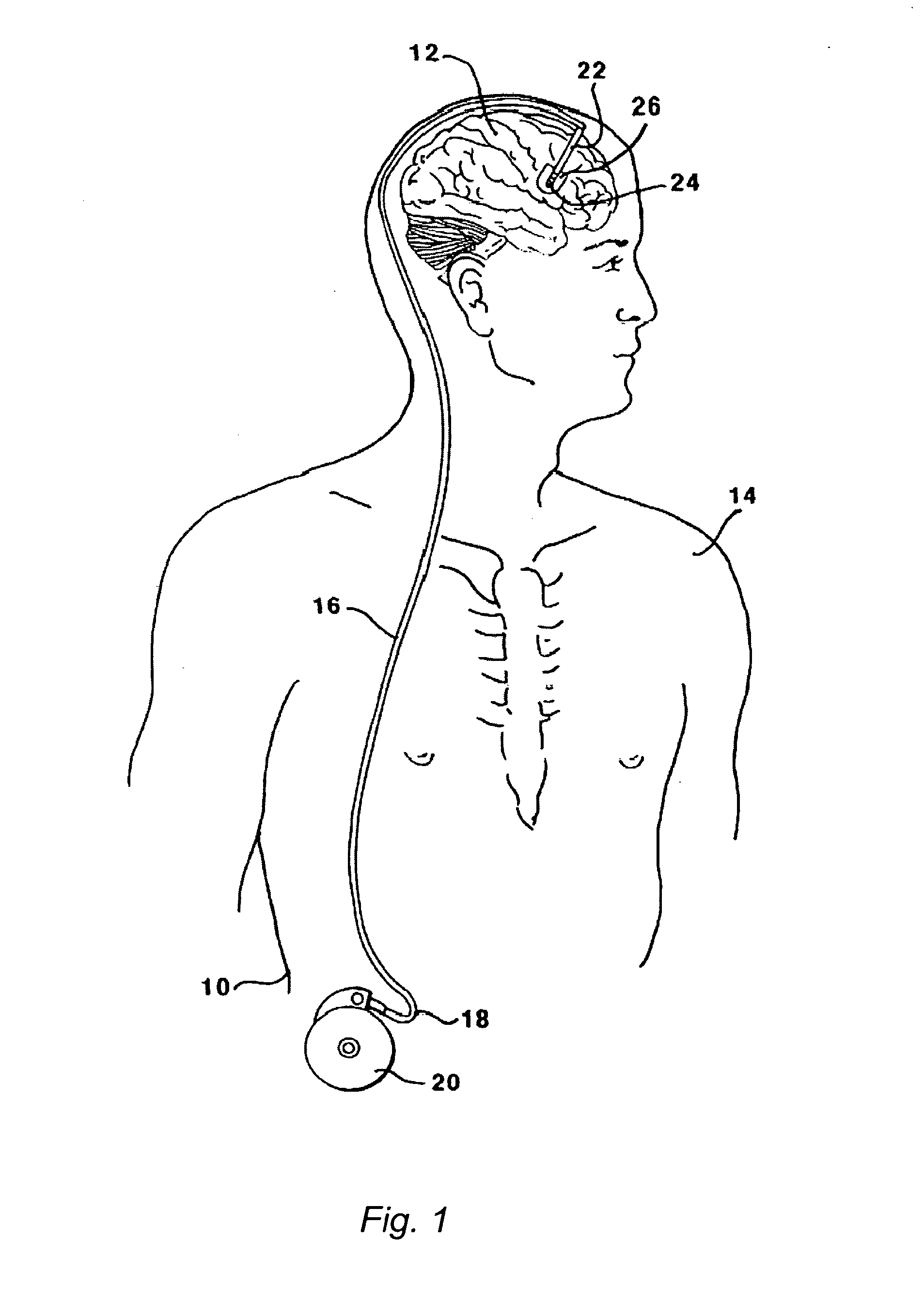
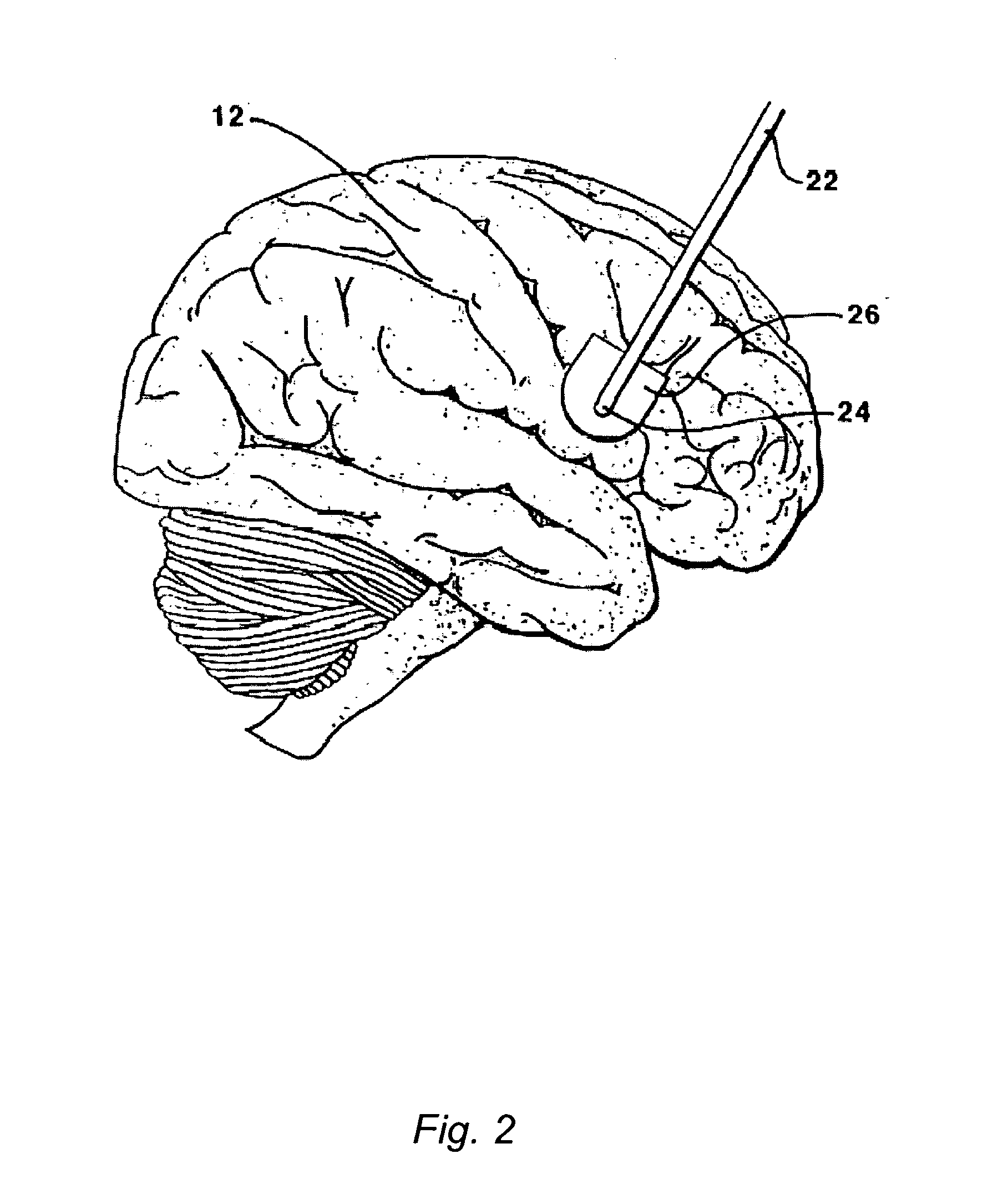
Methods and systems for treatment of neurological diseases of the central nervous system
InactiveUS20050208090A1Reduce degradationAdequate transportNervous disorderPeptide/protein ingredientsSystems designActive enzyme
The present invention is directed to methods and systems for the treatment of inborn genetic errors or other defects that cause deficiencies of active enzymes or proteins within the cells of the central nervous system. Such methods and systems generally comprise an implantable catheter system designed for the chronic delivery of specially formulated proteins to intrathecal, intracerebroventricular, and / or intraparenchymal regions of the central nervous system. The invention has application in the neuropathic aspects of the broad category of lysosomal storage diseases. These genetic based diseases are the result of insufficient enzyme activity to catabolize specific substances, which thereby accumulate in the cellular lysosomes.
Owner:MEDTRONIC INC
Combination of alcohol lock and gentian violet catheter
Implantable catheters treated with gentian violet and methods for disinfecting the catheters with alcohol are provided.
Owner:TELEFLEX LIFE SCI LTD
Photocatalytic disinfection of implanted catheters
An implantable catheter is provided that may be disinfected without removal from the body of a patient, using a photocatalytic method to activate a reaction on the catheter surface that generates oxidizing agents in the form of Reactive Oxygen Species (“ROS”) and thus destroy microorganisms in a biofilm that is present or forming. A catheter system includes the implantable catheter, a light source, and a source of power operably connected to the light source. Methods are also provided for disinfecting the implantable catheter in vivo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Hemostasis cuff for catheter securement
InactiveUS20060135946A1Good hemostasisAccelerate tissue ingrowthMedical devicesCatheterCuffImplantable Catheters
Implantable catheters including one or more cuffs are described herein. At least one of the cuffs may include a collagen material, such as Avitene® collagen. The catheter may include a combination of collagen cuffs and collagen-free polymeric cuffs. Various methods for the fabrication of collagen cuffs are also disclosed herein.
Owner:CR BARD INC
Photocatalytic disinfection of implanted catheters
An implantable catheter is provided that may be disinfected without removal from the body of a patient, using a photocatalytic method to activate a reaction on the catheter surface that generates oxidizing agents in the form of Reactive Oxygen Species (“ROS”) and thus destroy microorganisms in a biofilm that is present or forming. A catheter system includes the implantable catheter, a light source, and a source of power operably connected to the light source. Methods are also provided for disinfecting the implantable catheter in vivo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
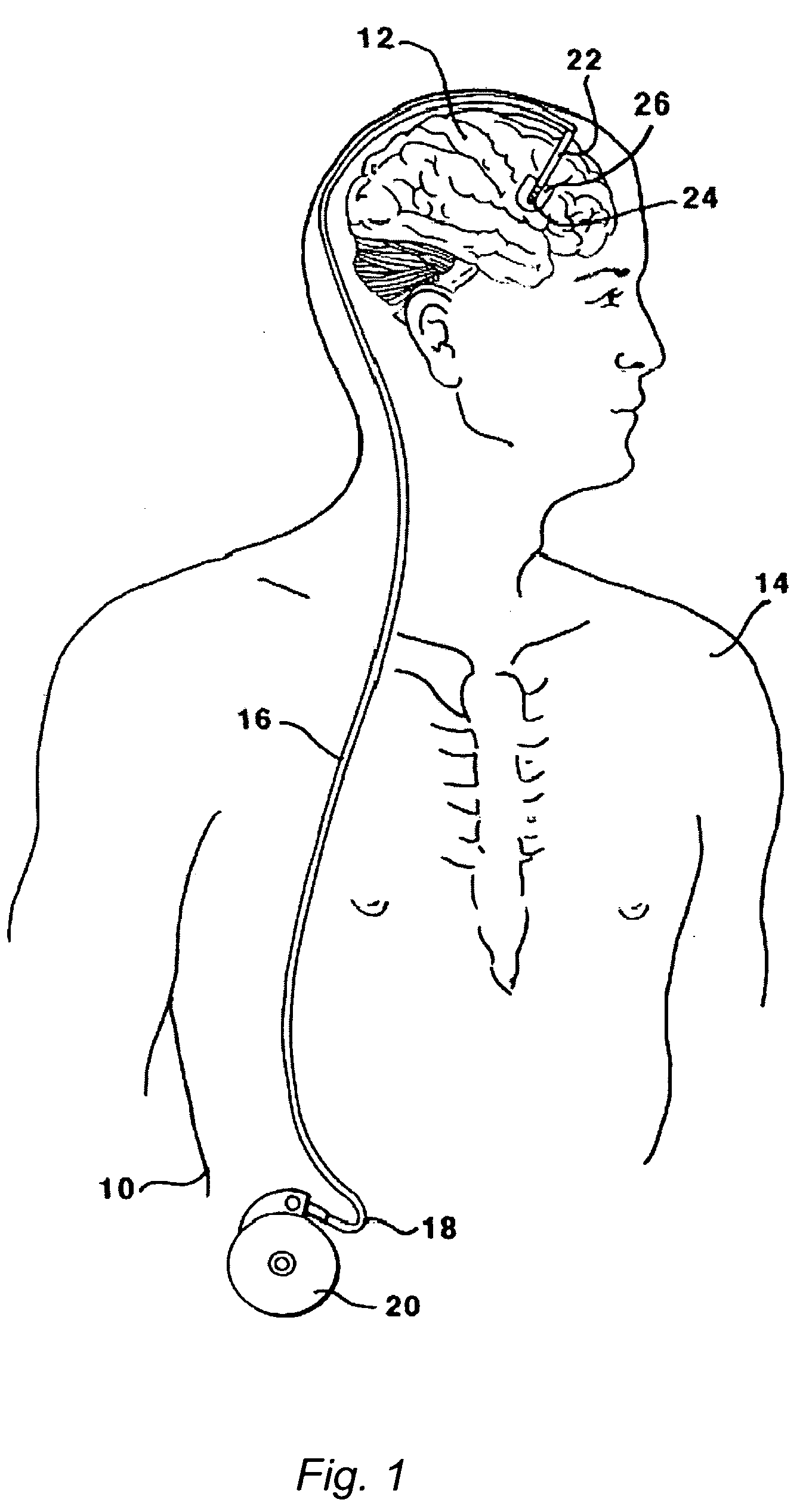
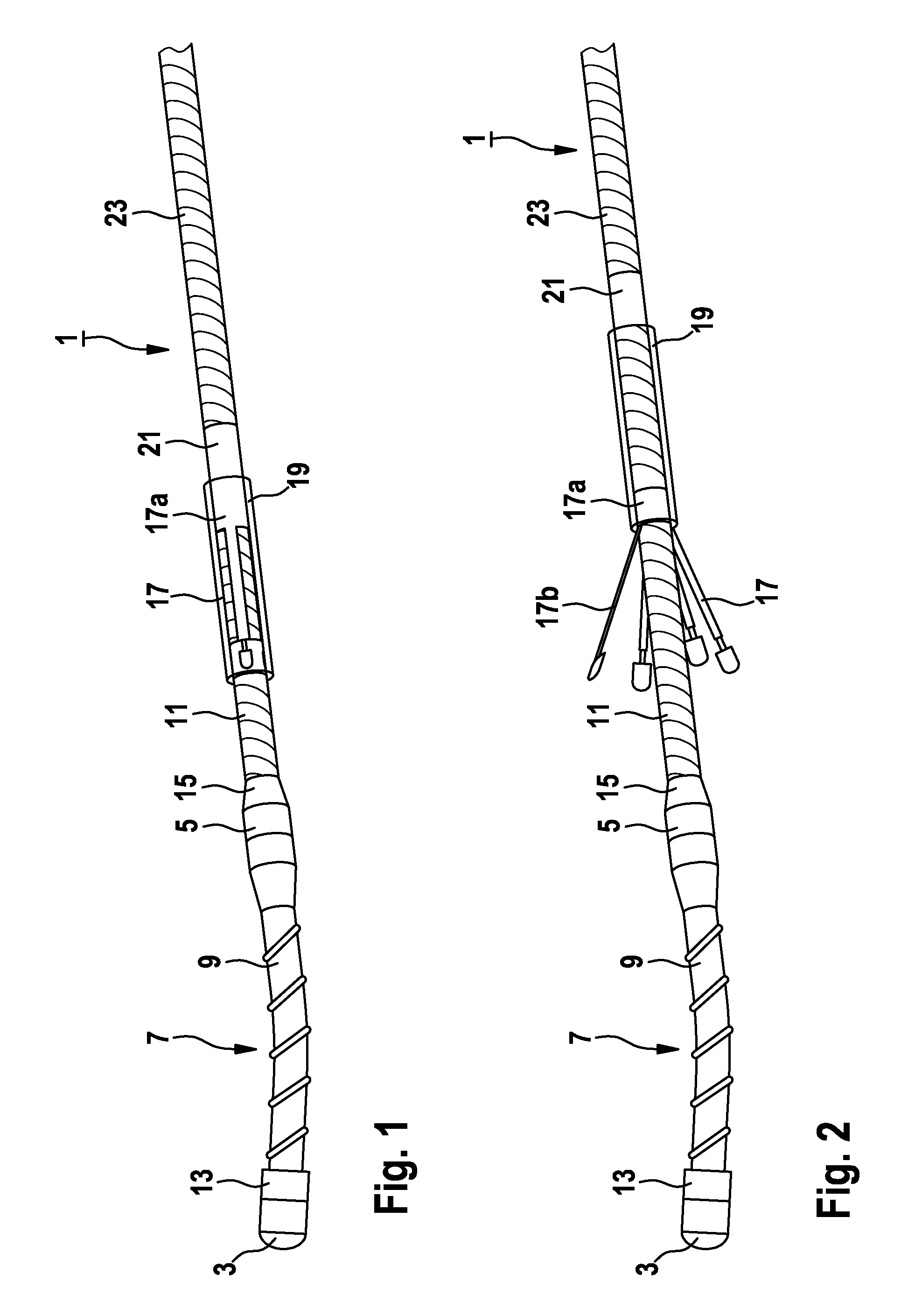
Implantable catheter lead or electrode lead
InactiveUS8452420B2Easy to disassembleSimple and inexpensive to fabricateTransvascular endocardial electrodesCatheterBlood vesselImplantable Catheters
An implantable catheter lead or electrode lead includes an elongated flexible lead body with the fixation means attached to the lead body, for the purpose of effecting fixation of the catheter lead or electrode lead in a predetermined position within a vessel or a bodily cavity of a patient. A releasable attachment is provided between the lead body and the fixation means such that an explantation of the catheter lead or electrode lead is possible after the attachment is released while the fixation means remains in place within the body of the patient.
Owner:BIOTRONIK SE & CO KG
Implantable catheter and method of using same
A catheter for implantation into a patient having a catheter tube having a distal end, a means for trimming the distal end of the catheter tube after subcutaneous insertion of the tip of the catheter lumen into a desired position within the patient to form a trimmed end portion, and a means for selectively positioning each respective lumen of the trimmed end portion of the catheter tube into fluid communication with the respective first end of one attachment tube. The attachment tube is select fluid communication with a desired medical device.
Owner:ANGIODYNAMICS INC
Implantable vascular access device
ActiveUS7101356B2Other blood circulation devicesDialysis systemsVascular Access DevicesBiomedical engineering
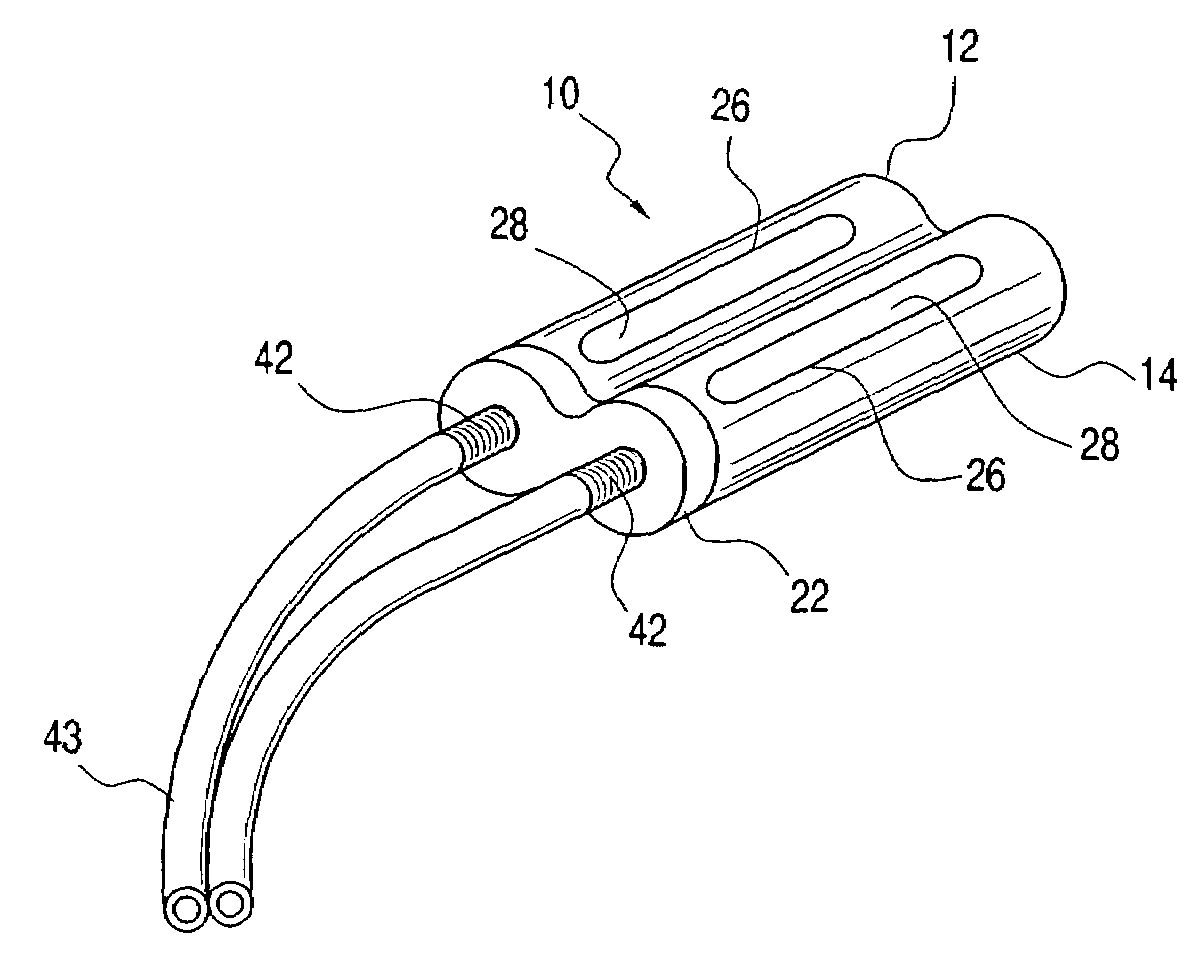
An implantable dual chambered catheter device includes a first chamber and a second chamber integrally joined. The first chamber includes a hollow first elastomeric member contained within a first slotted outer housing. A first end of the first elastomeric member is fitted with a first end cap shaped and dimensioned to accept conventional catheter tubes and the second end of the first elastomeric member is closed. The second chamber includes a hollow second elastomeric member contained within a second slotted outer housing. A first end of the second elastomeric member is fitted with a second end cap shaped and dimensioned to accept conventional catheter tubes and the second end of the second elastomeric member is closed. Needle access to the first and second chambers of the implantable catheter device is through respective slots formed within the first and second outer housings.
Owner:MILLER MEDICAL LLC
Electrochemical disinfection of implanted catheters
ActiveUS20130041238A1Inhibition formationInhibits biofilm formationElectrotherapyMedical devicesBiofilmMicroorganism
An implantable catheter is provided that may be disinfected without removal from the body of a patient, using an electrochemical method to generate an electric field on the catheter surface and thus destroy microorganisms in a biofilm that is present or forming. A catheter system includes the implantable catheter and a voltage source that is operably connected to electrodes on or embedded in the exterior and optionally the interior catheter surface. Methods are also provided for disinfecting the implantable catheter in vivo and for detecting or confirming the presence of a pathogenic biofilm thereon.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Methods and Systems for Treatment of Neurological Diseases of the Central Nervous System
InactiveUS20110213328A1Convenient treatmentReduce degradationNervous disorderPeptide/protein ingredientsActive enzymeNervous system
The present invention is directed to methods and systems for the treatment of inborn genetic errors or other defects that cause deficiencies of active enzymes or proteins within the cells of the central nervous system. Such methods and systems generally comprise an implantable catheter system designed for the chronic delivery of specially formulated proteins to intrathecal, intracerebroventricular, and / or intraparenchymal regions of the central nervous system. The invention has application in the neuropathic aspects of the broad category of lysosomal storage diseases. These genetic based diseases are the result of insufficient enzyme activity to catabolize specific substances, which thereby accumulate in the cellular lysosomes.
Owner:MEDTRONIC INC
Combined fibnrinolytic and antimicrobial cathether and uses thetherof
ActiveUS20100196434A1Decrease fibrinolytic activityAnti-microbial activity can be reducedBiocidePeptide/protein ingredientsMedicineFibrinolysis
Implantable catheters are provided which comprise an antimicrobial agent incorporated in a coating or bulk distributed, in combination with a fibrinolytic agent incorporated in a top coating.
Owner:TELEFLEX LIFE SCI LTD
Anti-infective alcohol catheter solution with chlorhexidine treated catheter
Implantable catheters treated with chlorhexidine and methods for disinfecting the catheters with alcohol are provided.
Owner:TELEFLEX LIFE SCI LTD
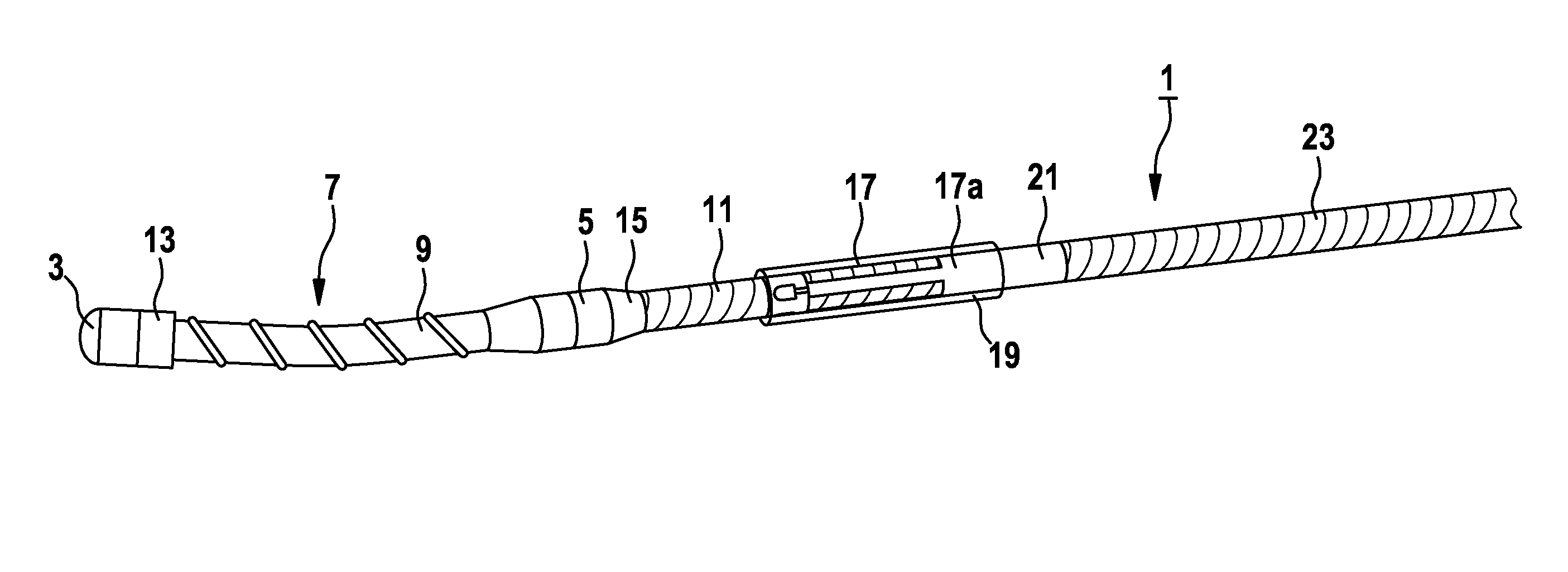
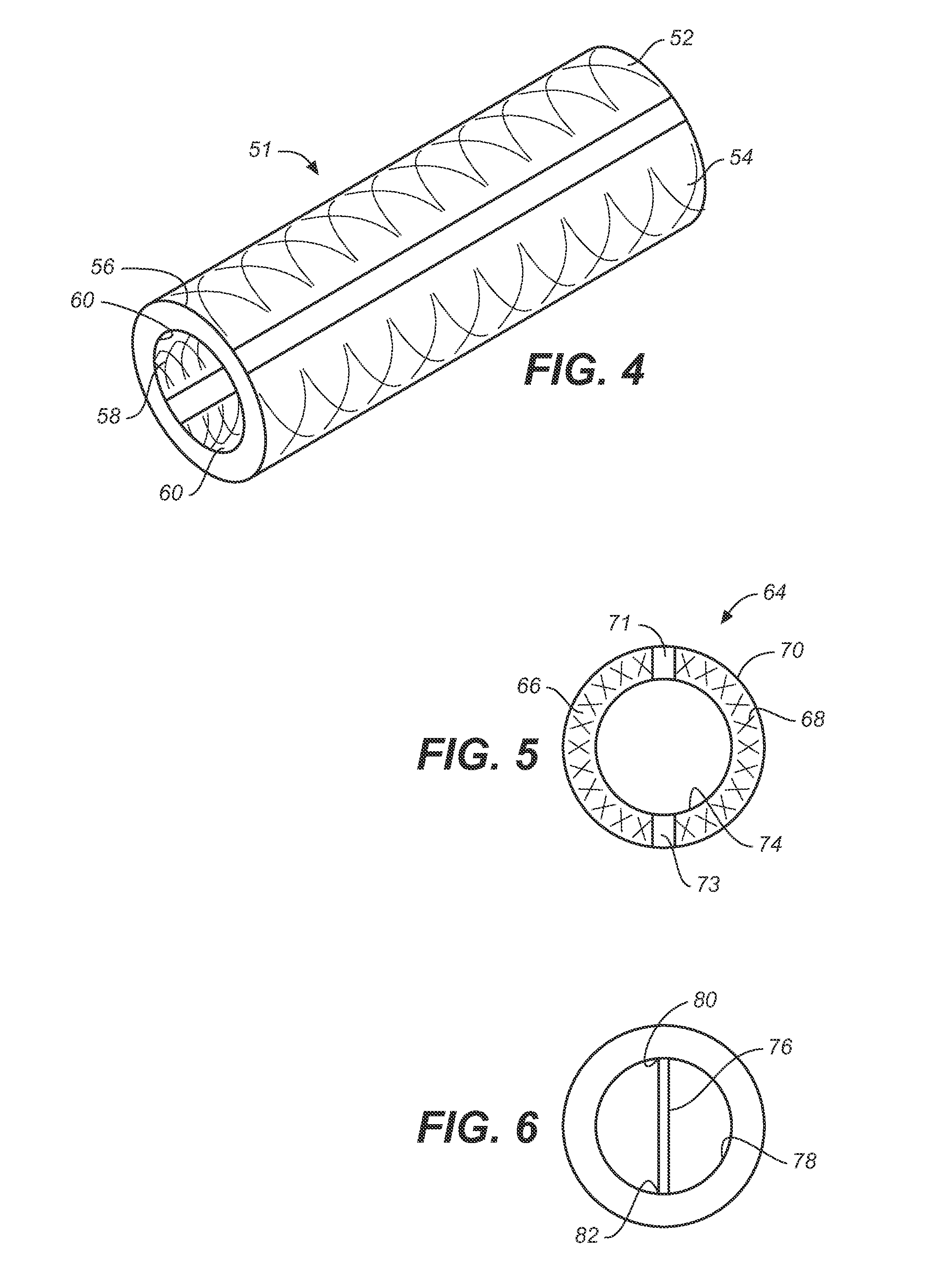
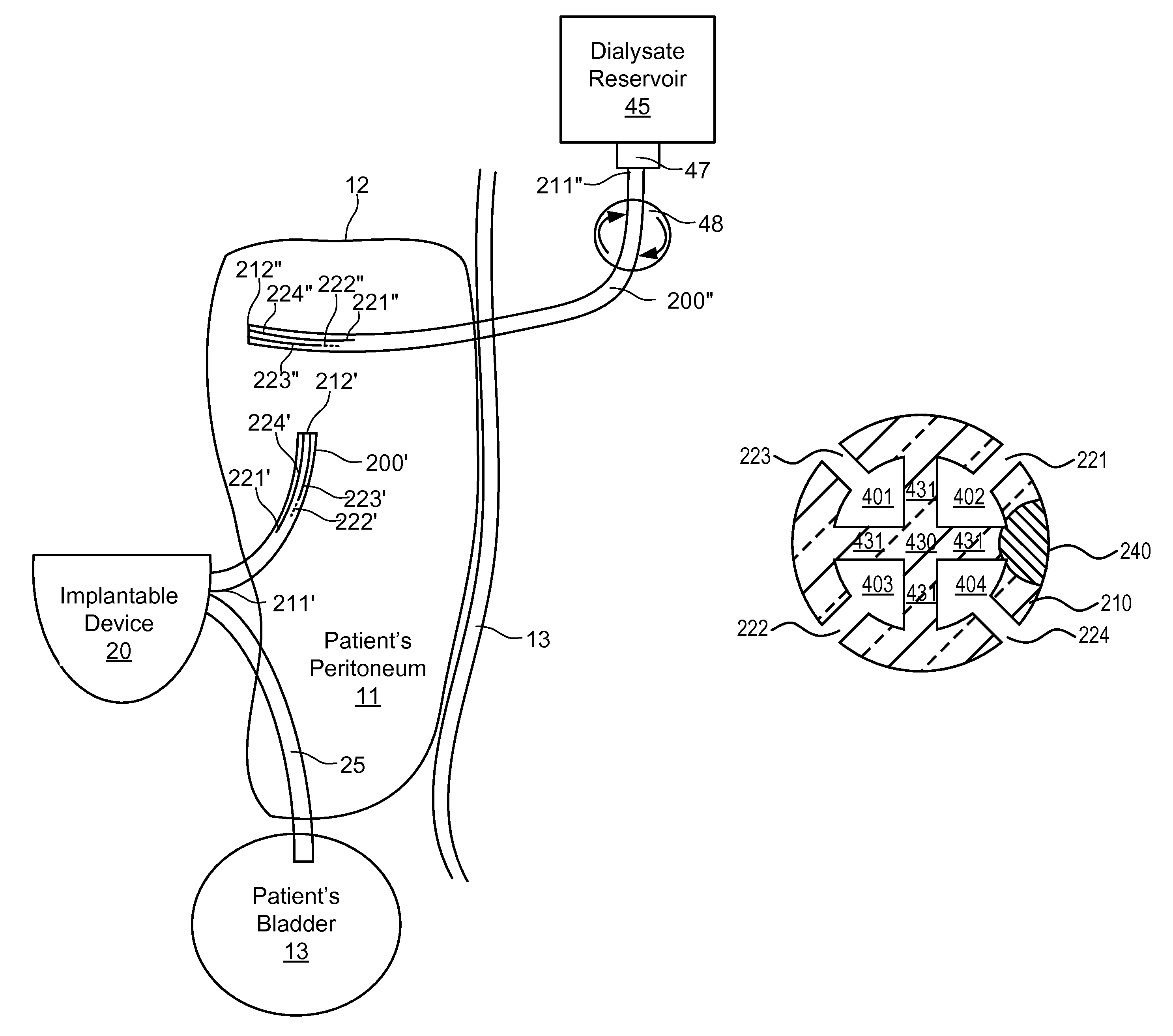
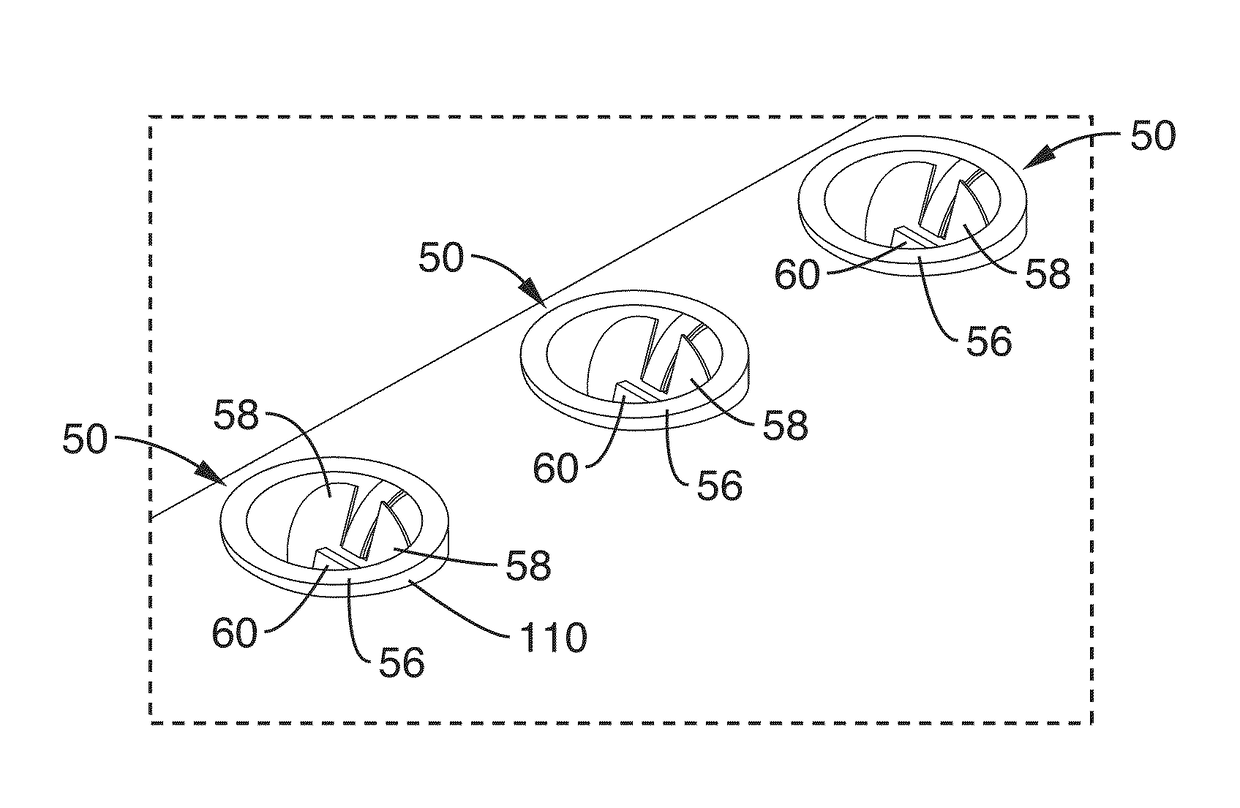
Implantable catheters with staggered slits, and methods of using same
ActiveUS9144660B2Raise the possibilityMulti-lumen catheterPeritoneal dialysisMedicineBiomedical engineering
Under one aspect of the present invention, a catheter includes an elongate member; a plurality of septa configured to define a plurality of lumens along the elongate member; and a plurality of slits defined through the elongate member, each slit configured to provide fluidic communication between an environment about the catheter and a corresponding lumen of the plurality of lumens, at least one slit having a different length than at least one other slit.
Owner:SEQUANA MEDICAL NV
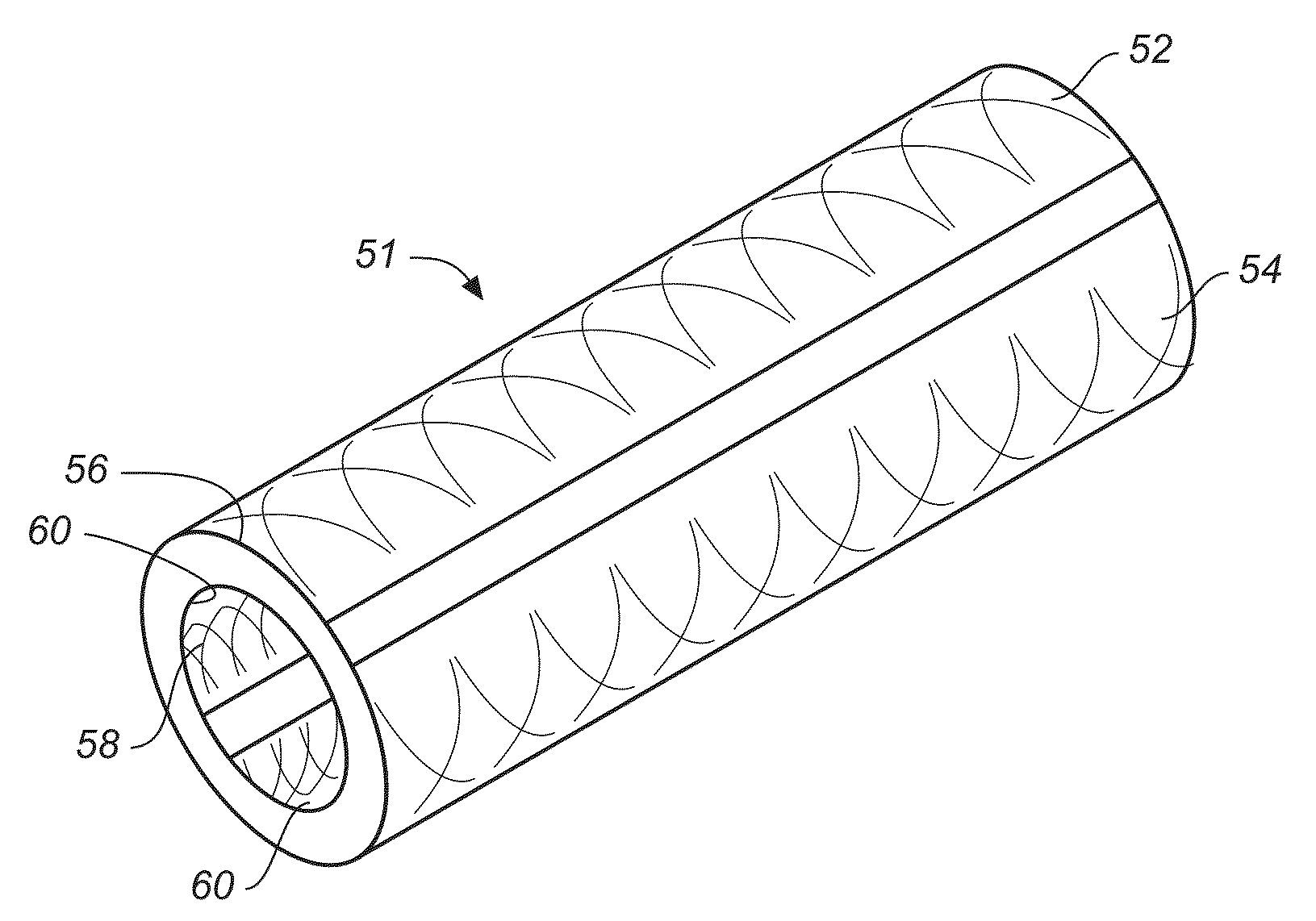
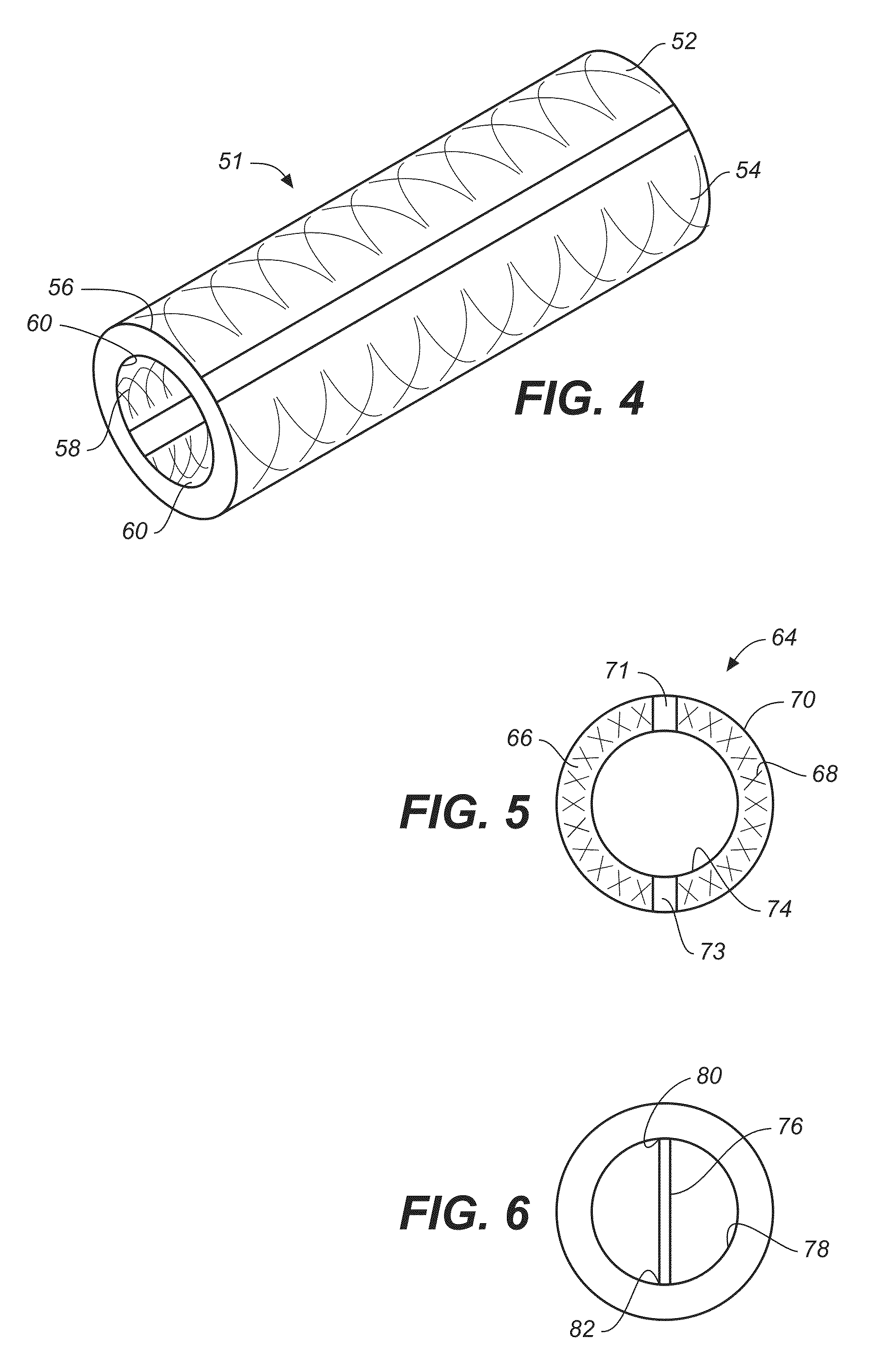
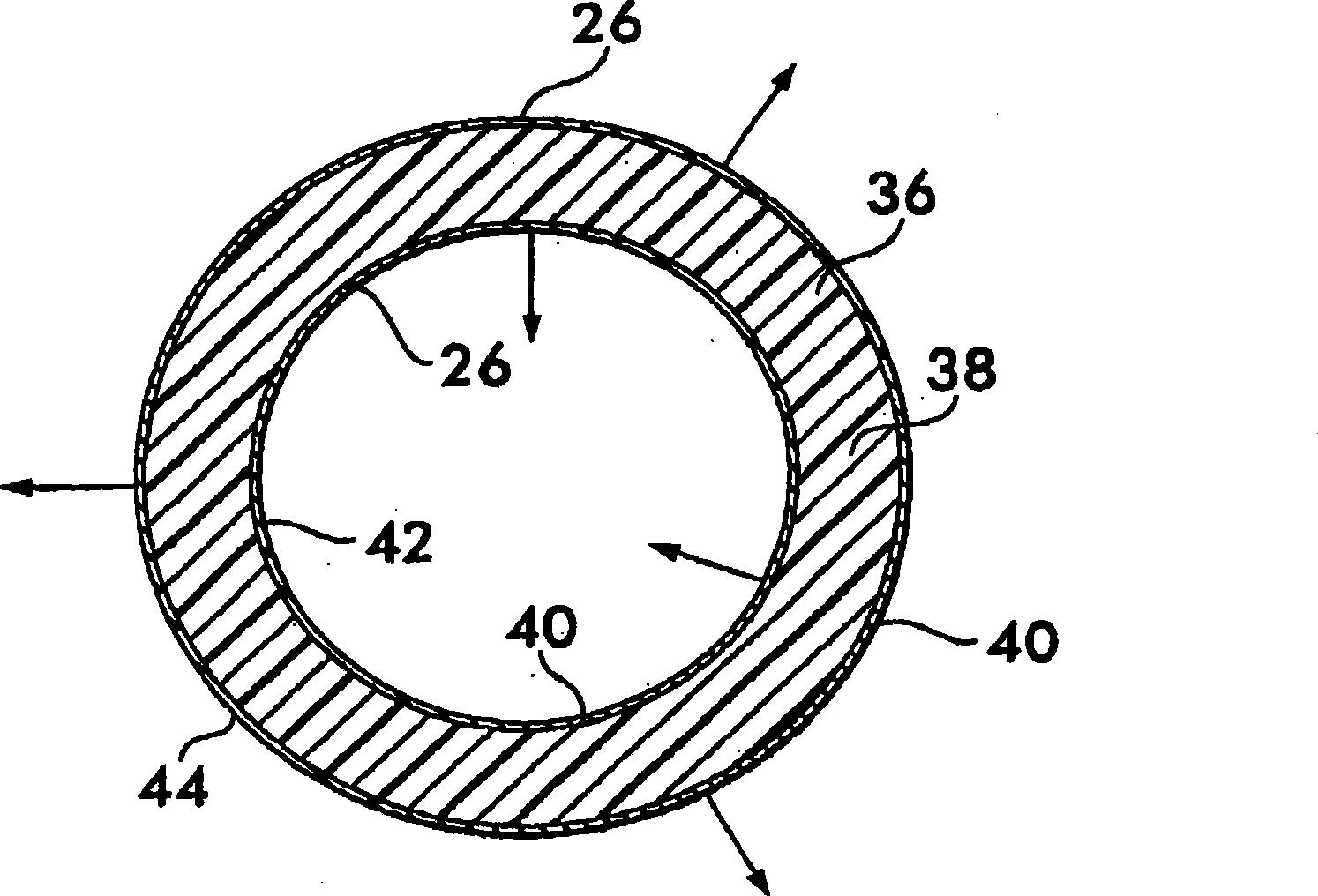
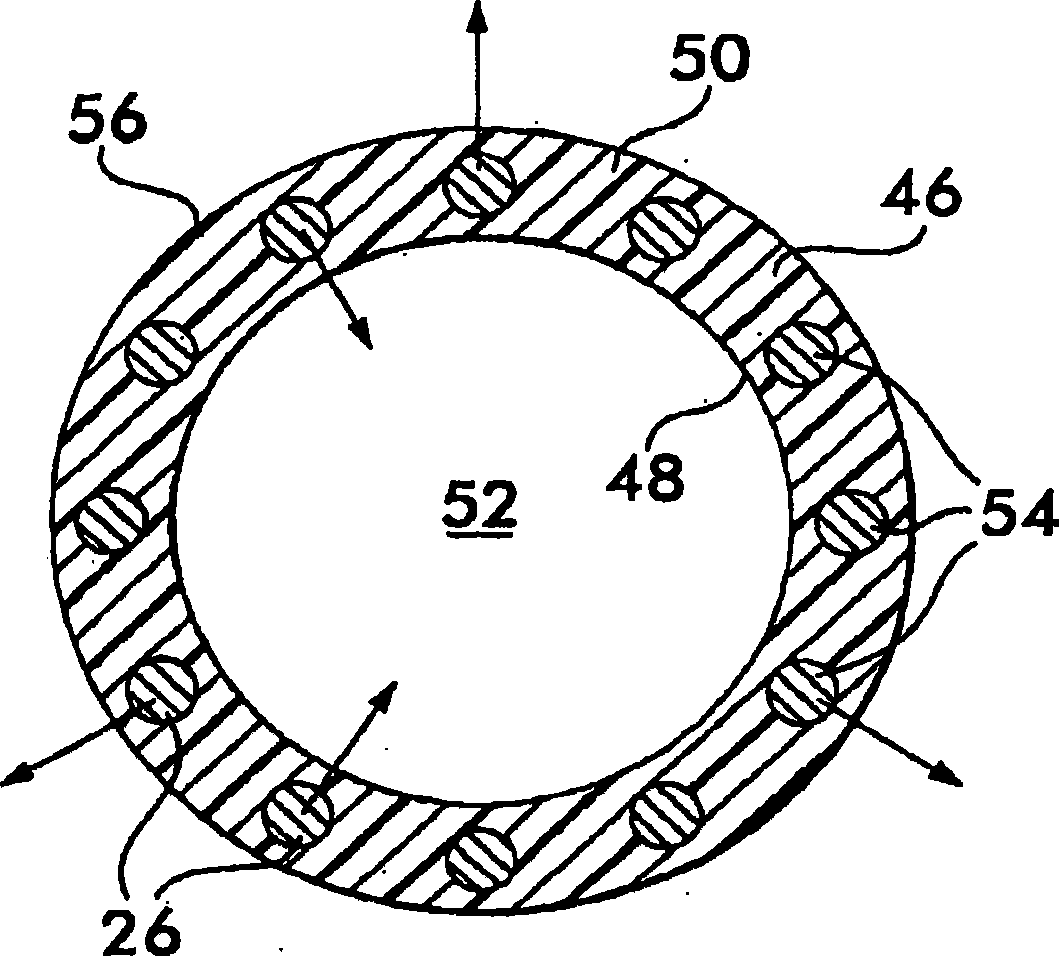
Implantable shunt or catheter enabling gradual delivery of therapeutic agents
InactiveUS20110060265A1Reduce deliveryOrganic active ingredientsNervous disorderTherapeutic effectCatheter
An implantable catheter or shunt for draining fluid from a body cavity. The catheter or shunt body has a wall structure that carries one or more therapeutic agents in a manner enabling release of the therapeutic agent from the wall structure in situ after surgical implantation of the catheter or shunt body. The therapeutic agent can be gradually released over time to prevent infection, inhibit tissue ingrowths, and / or provide some other desired medicinal purpose. As an example, the therapeutic agent can be rapamycin or an mTOR inhibitor. According to some contemplated embodiments of the present invention, the therapeutic agent carried by the catheter / shunt is rechargeable or refillable in situ so that the therapeutic agent can be gradually released from the catheter / shunt over the expected useful life of the catheter / shunt.
Owner:WYETH LLC
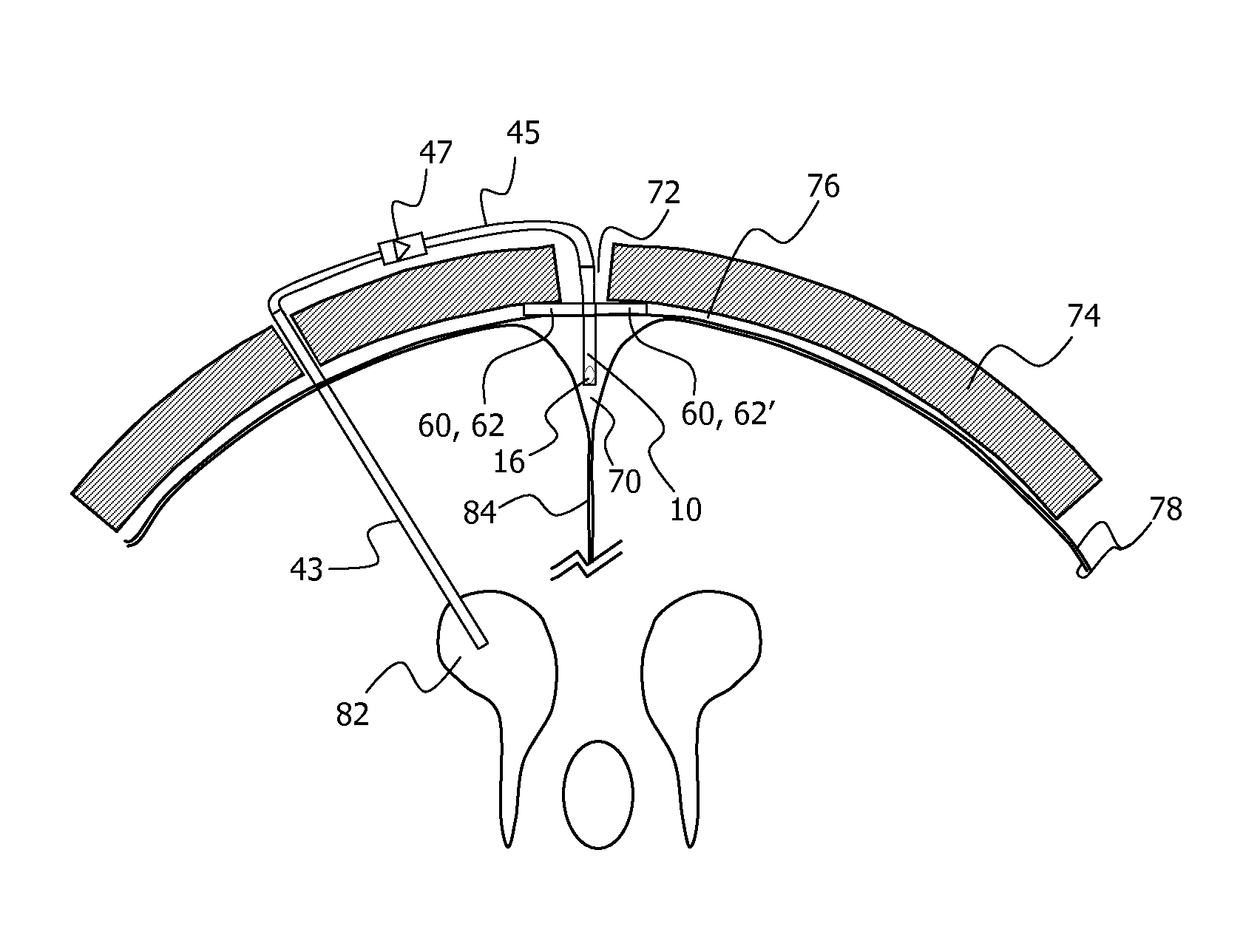
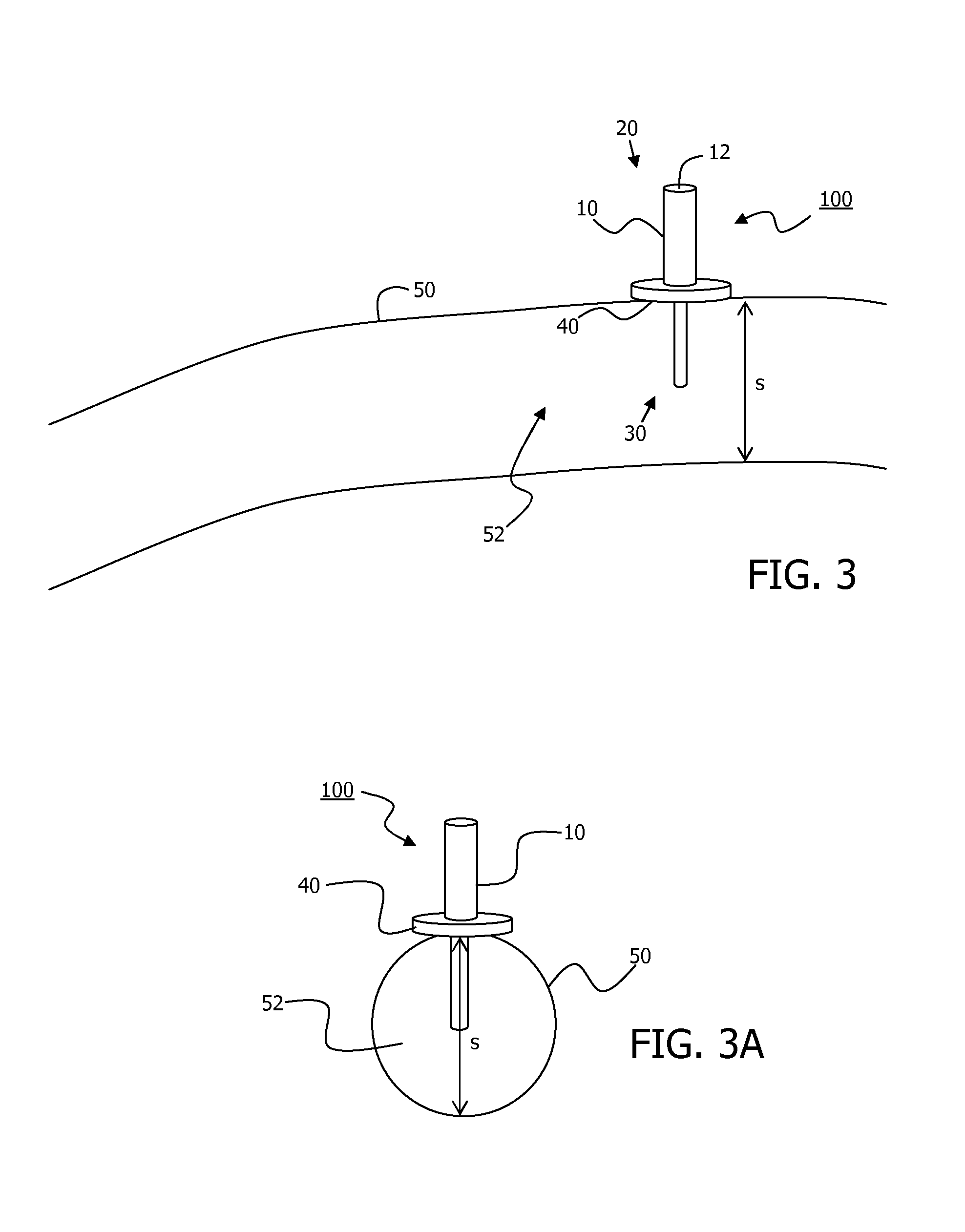
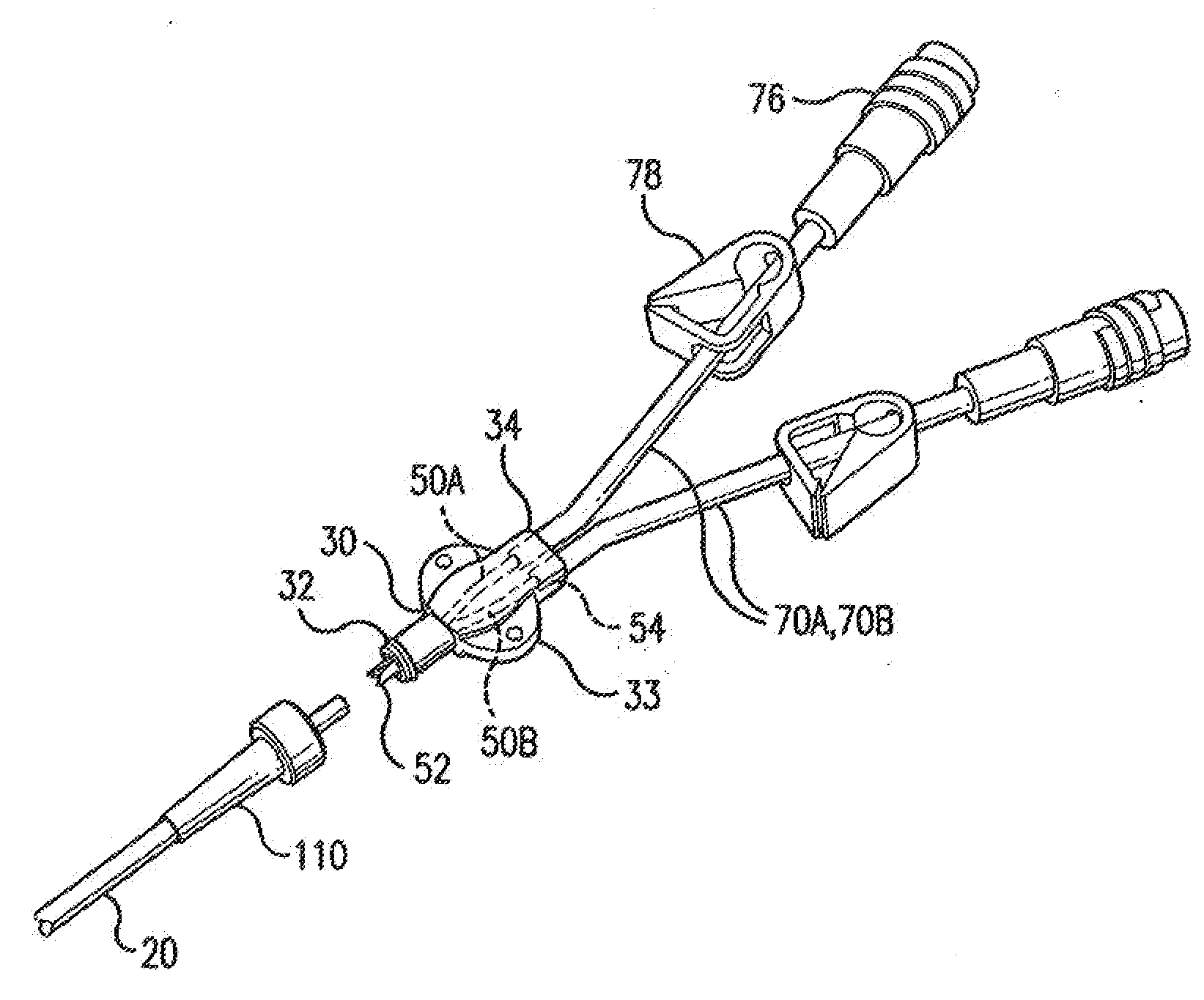
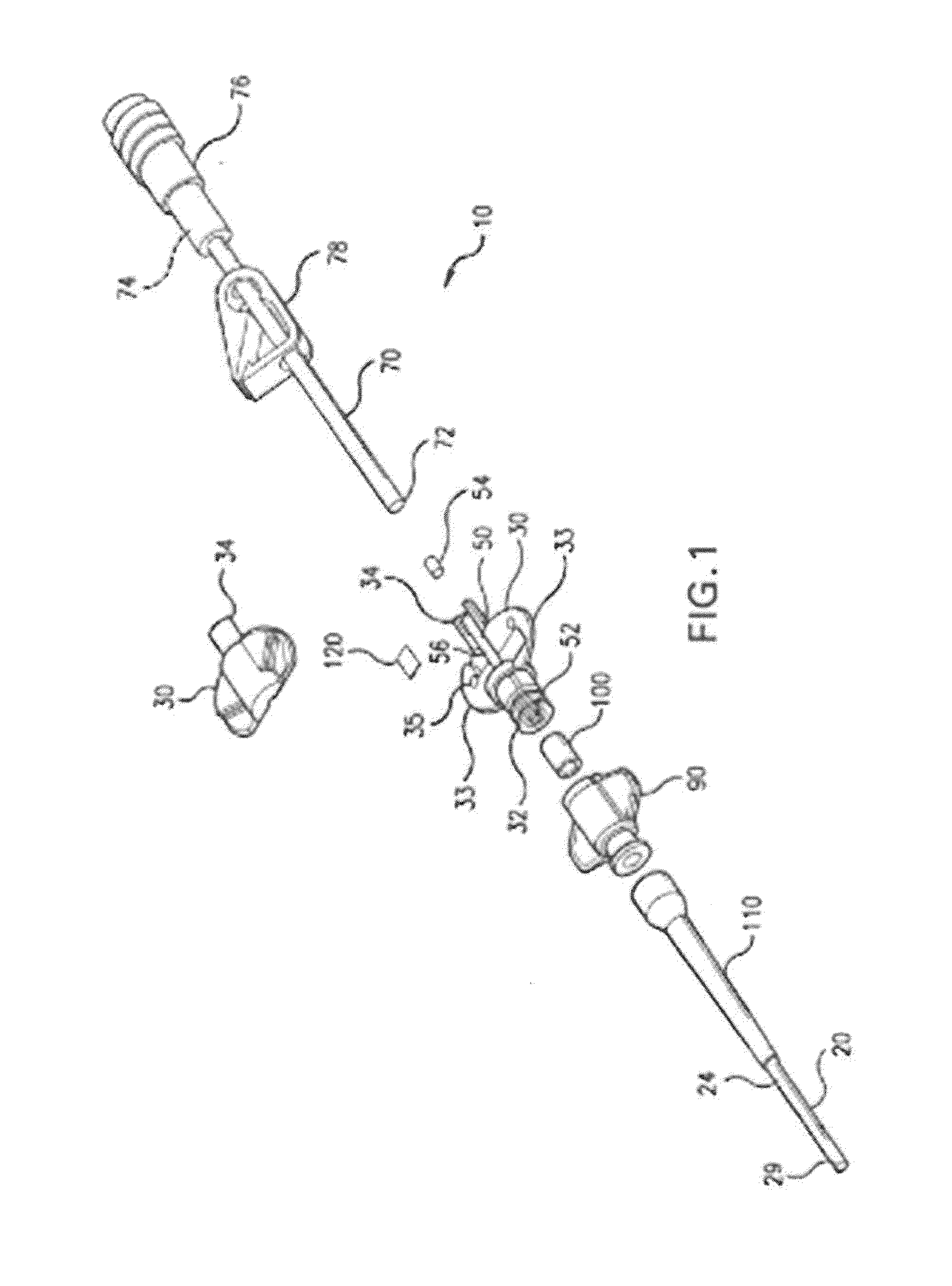
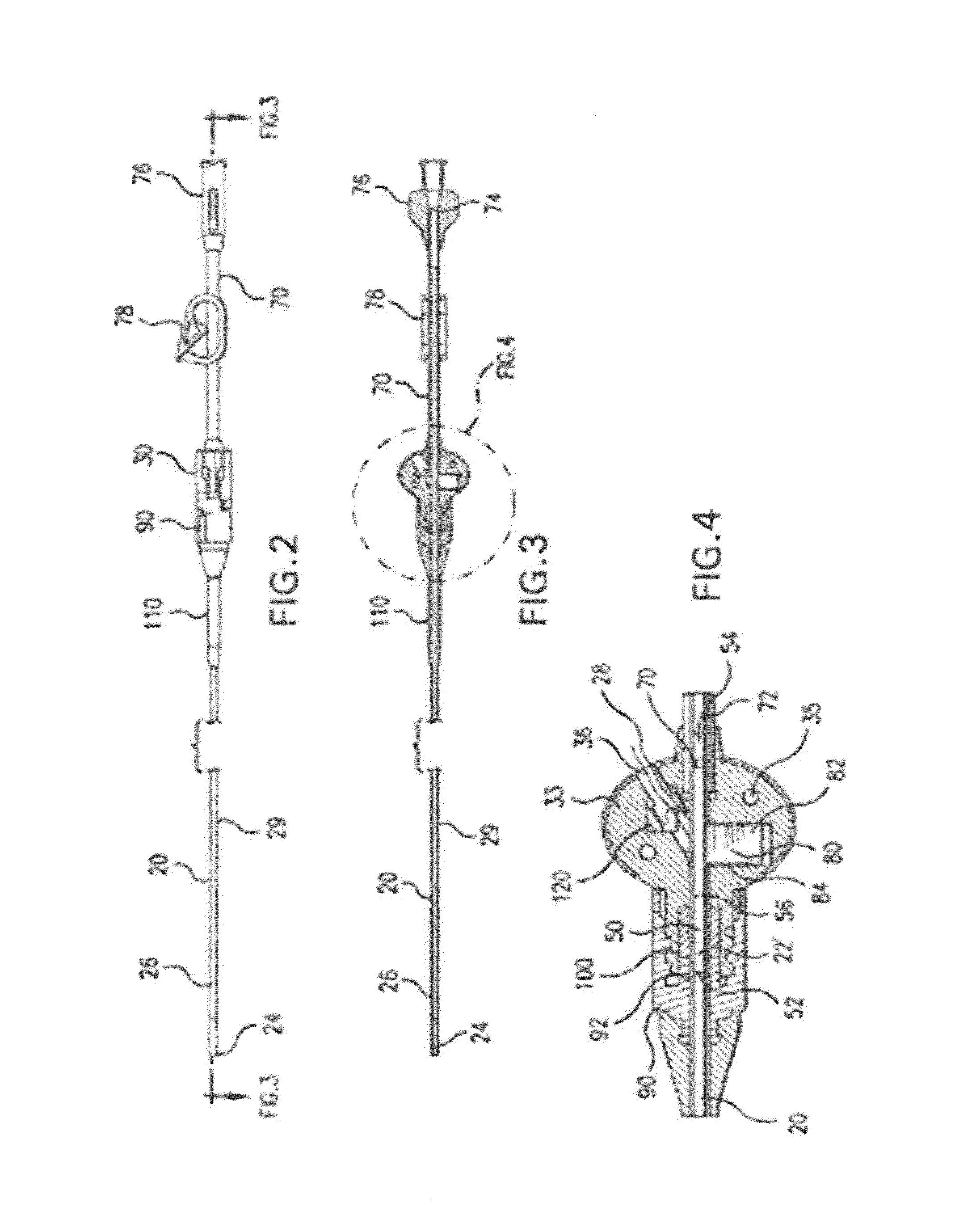
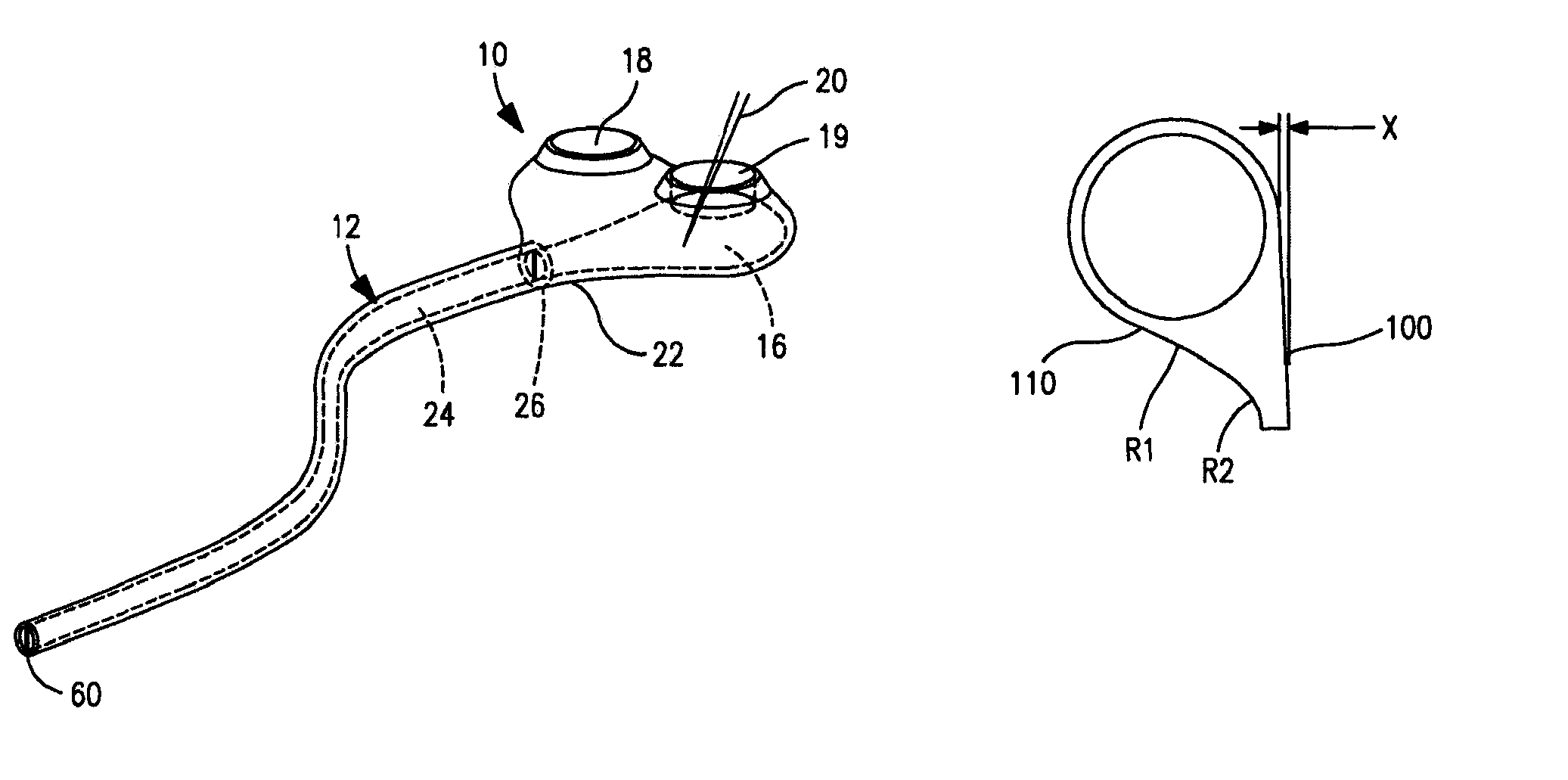
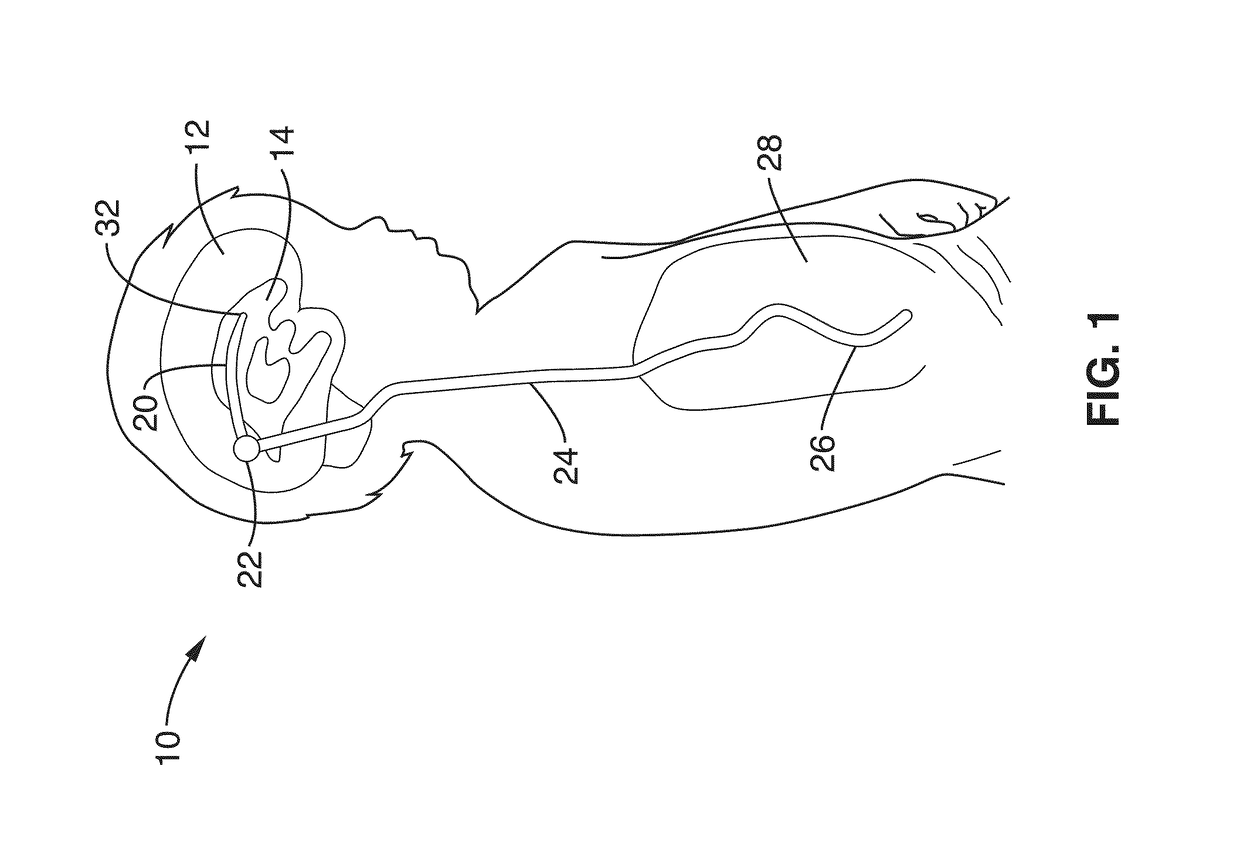
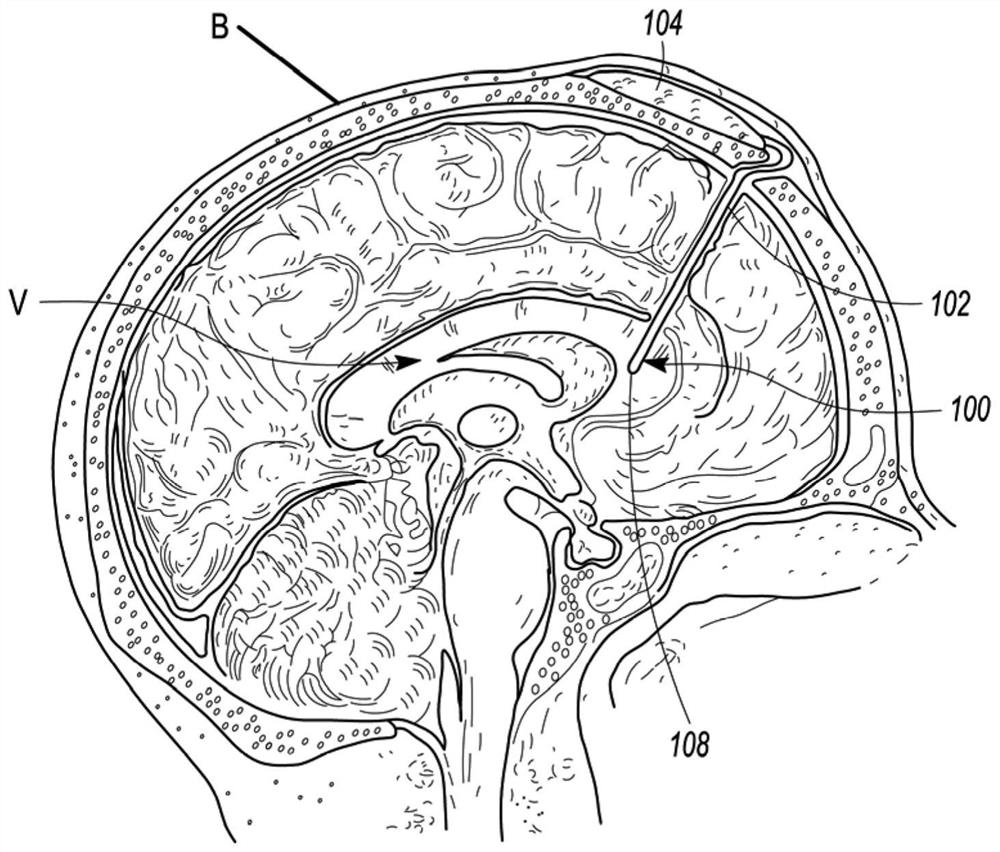
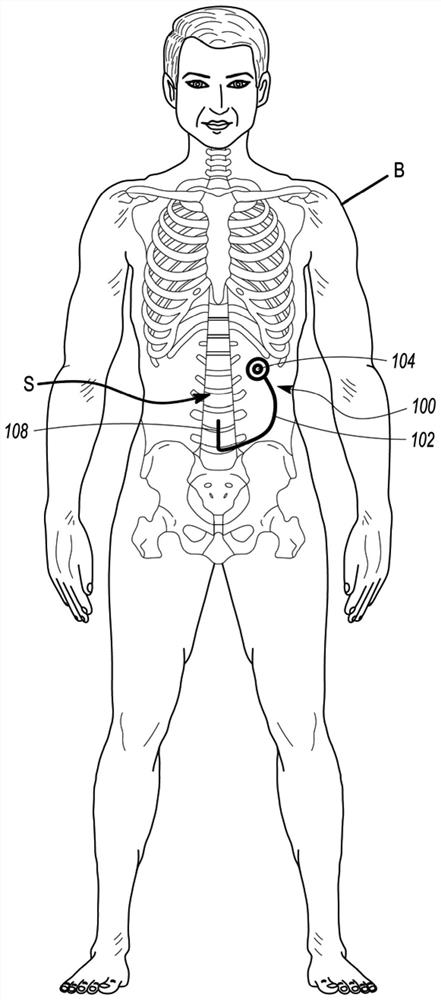
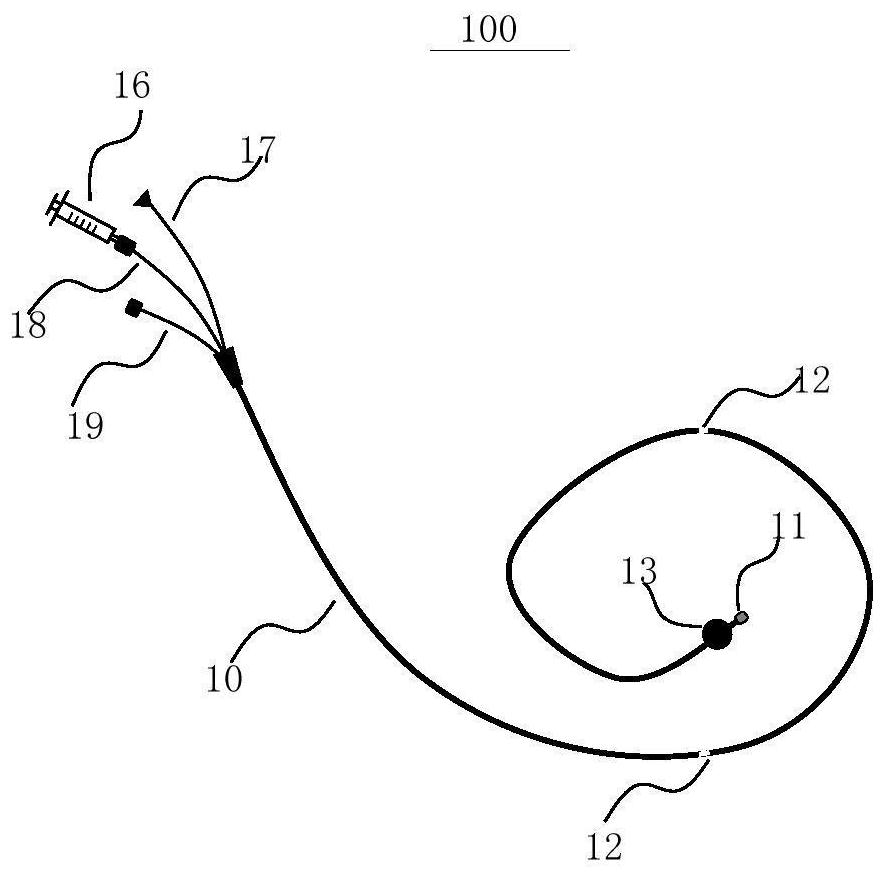
Minimally-advancing luminal catheter
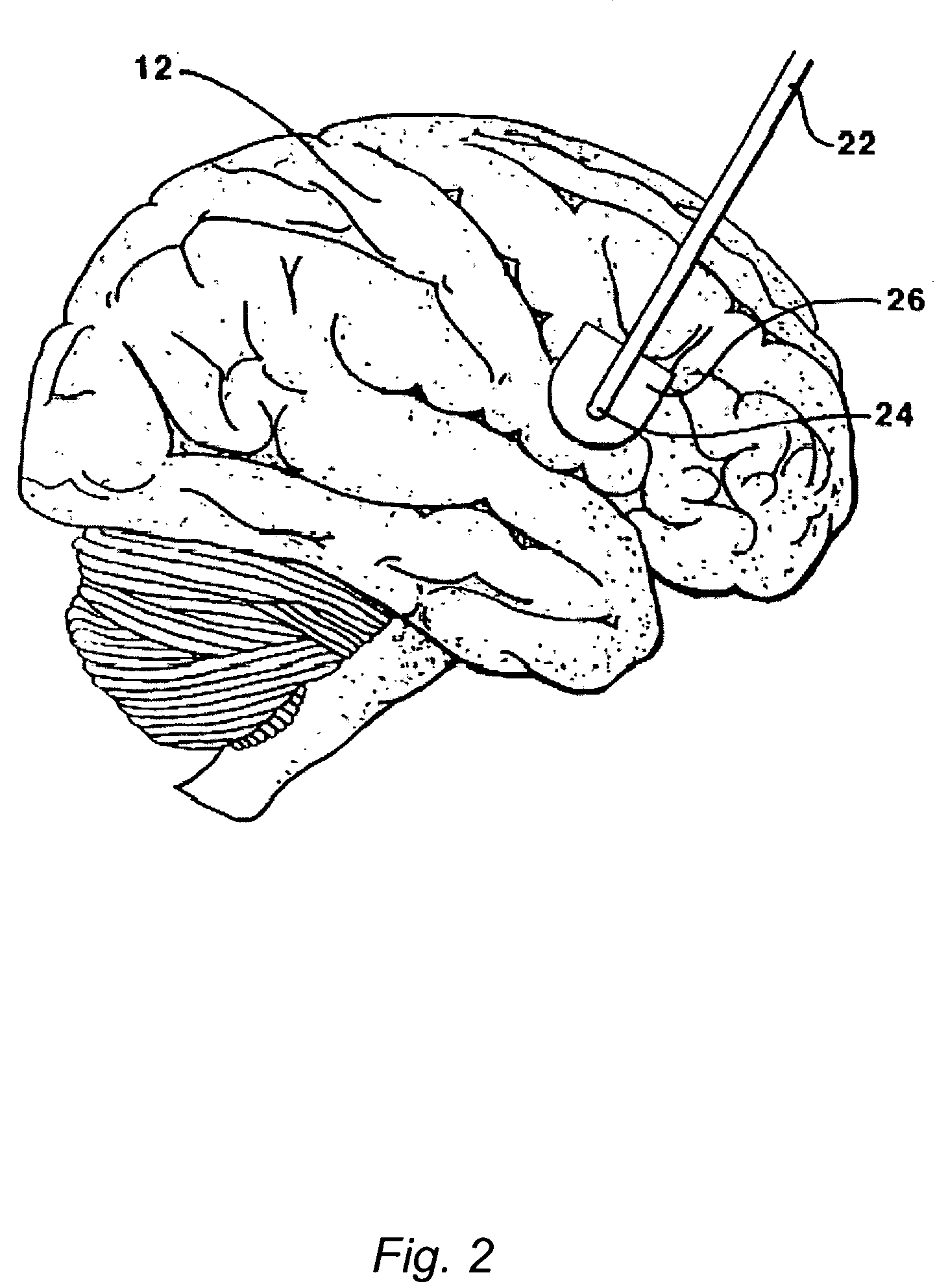
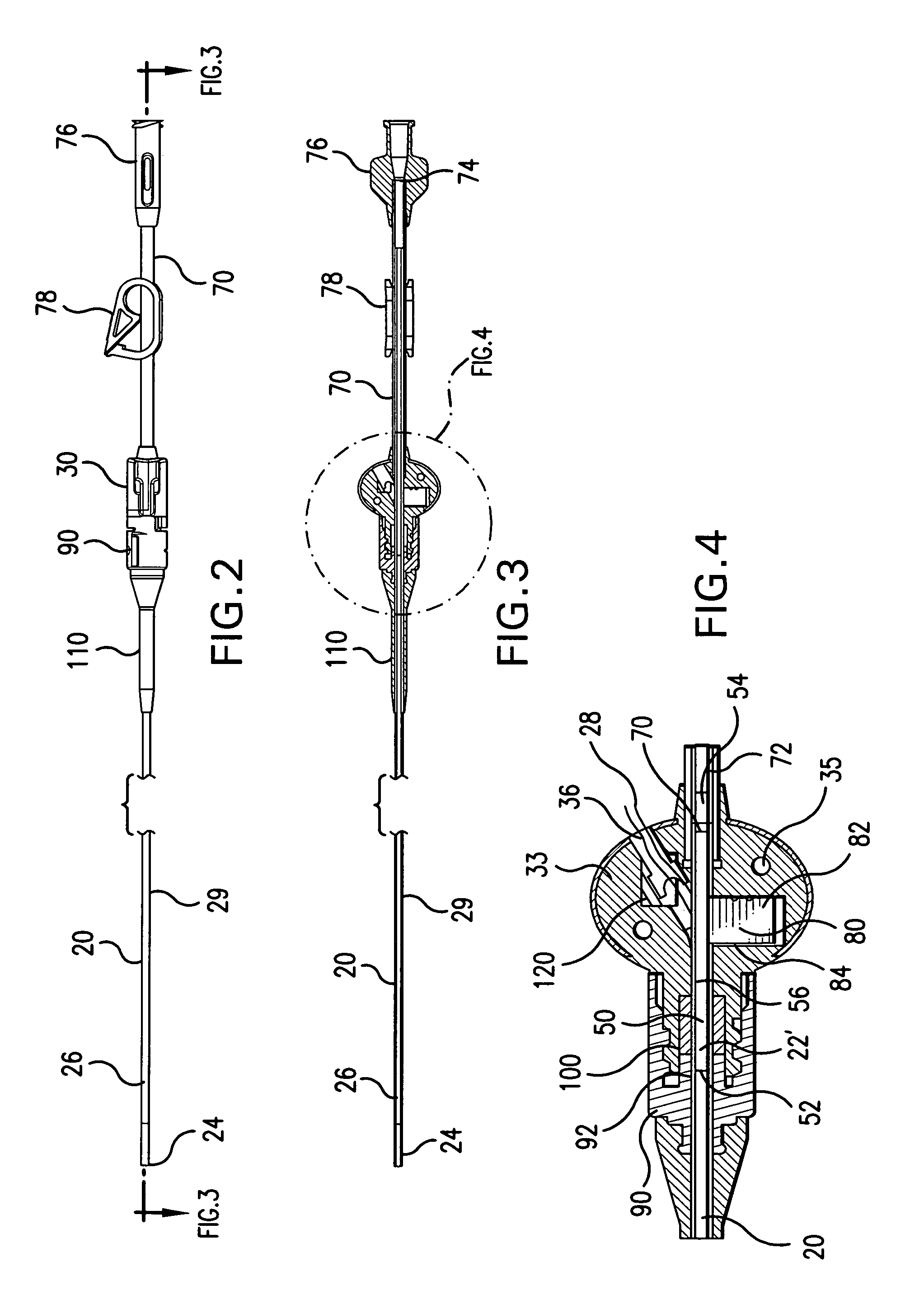
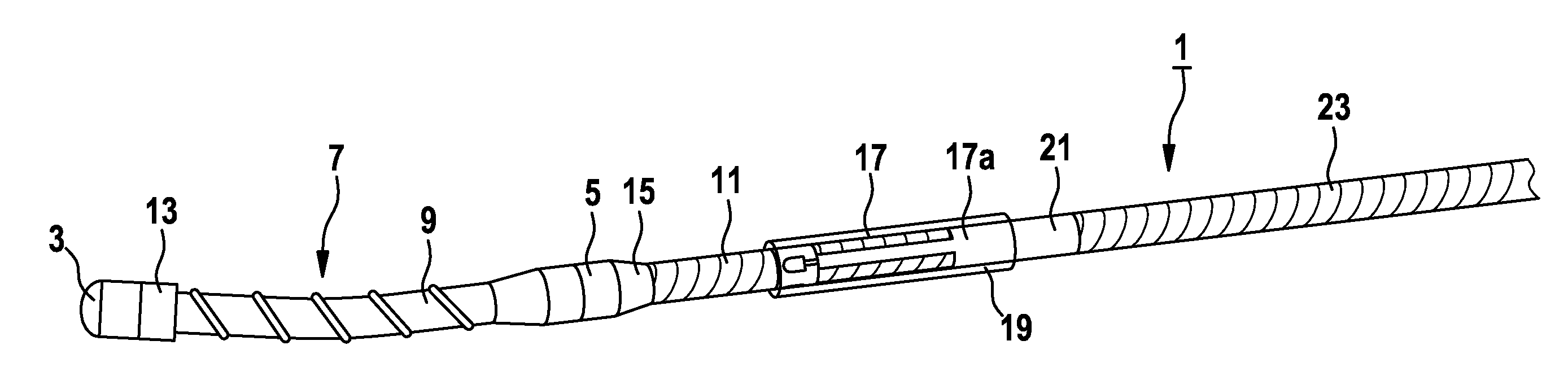
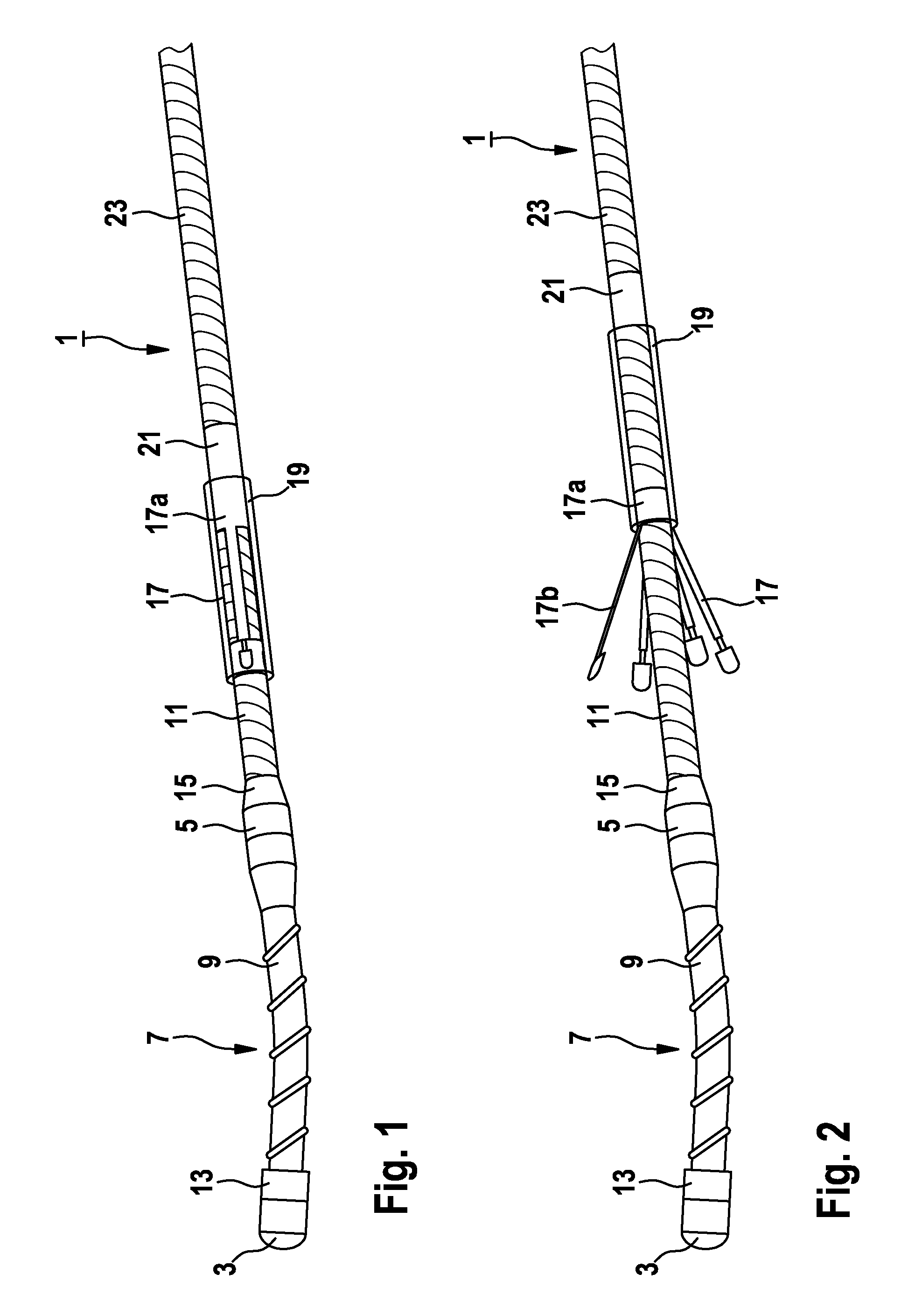
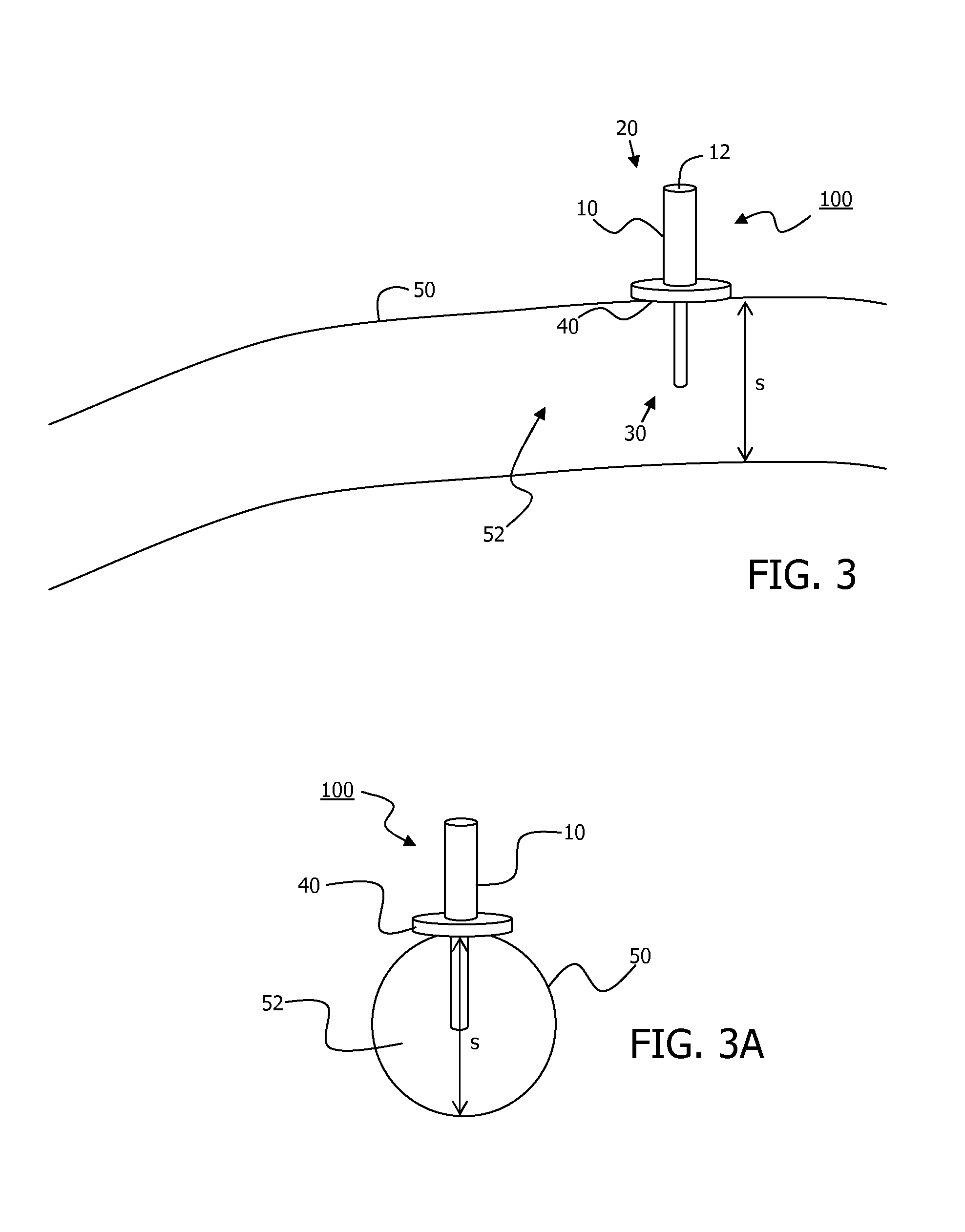
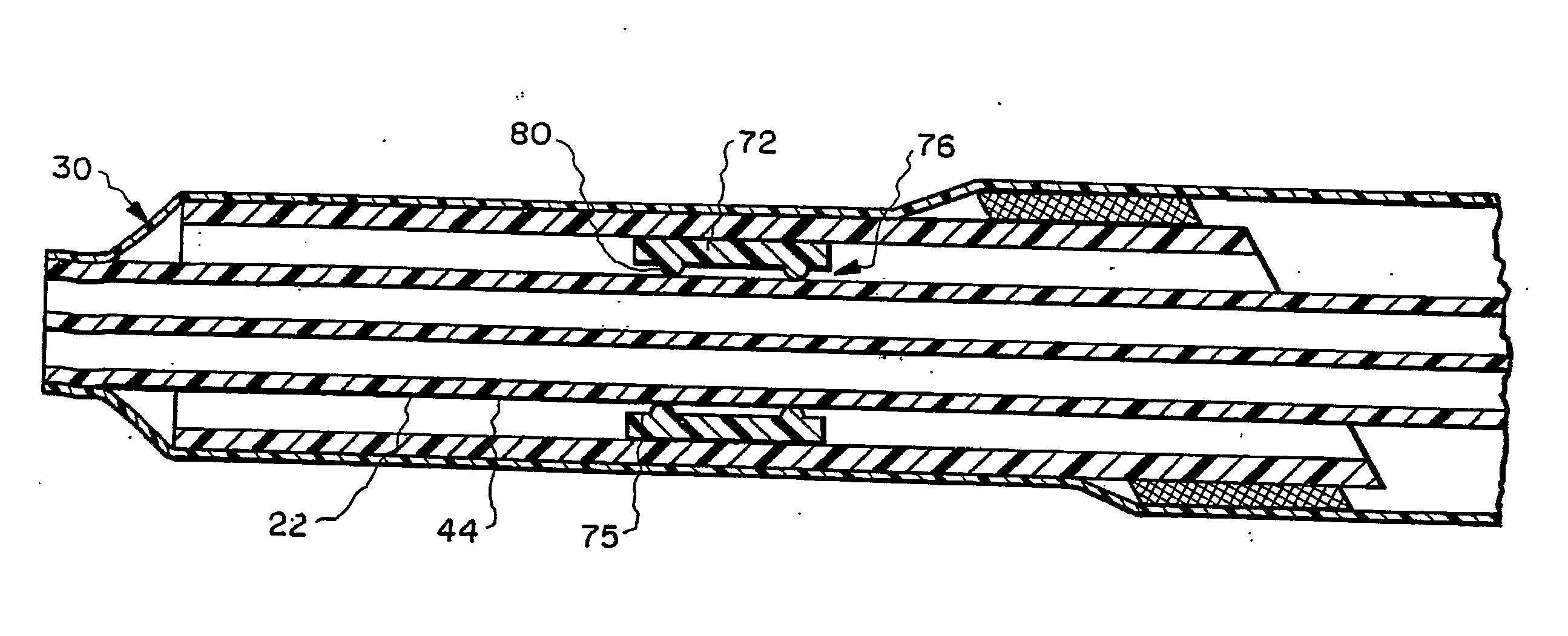
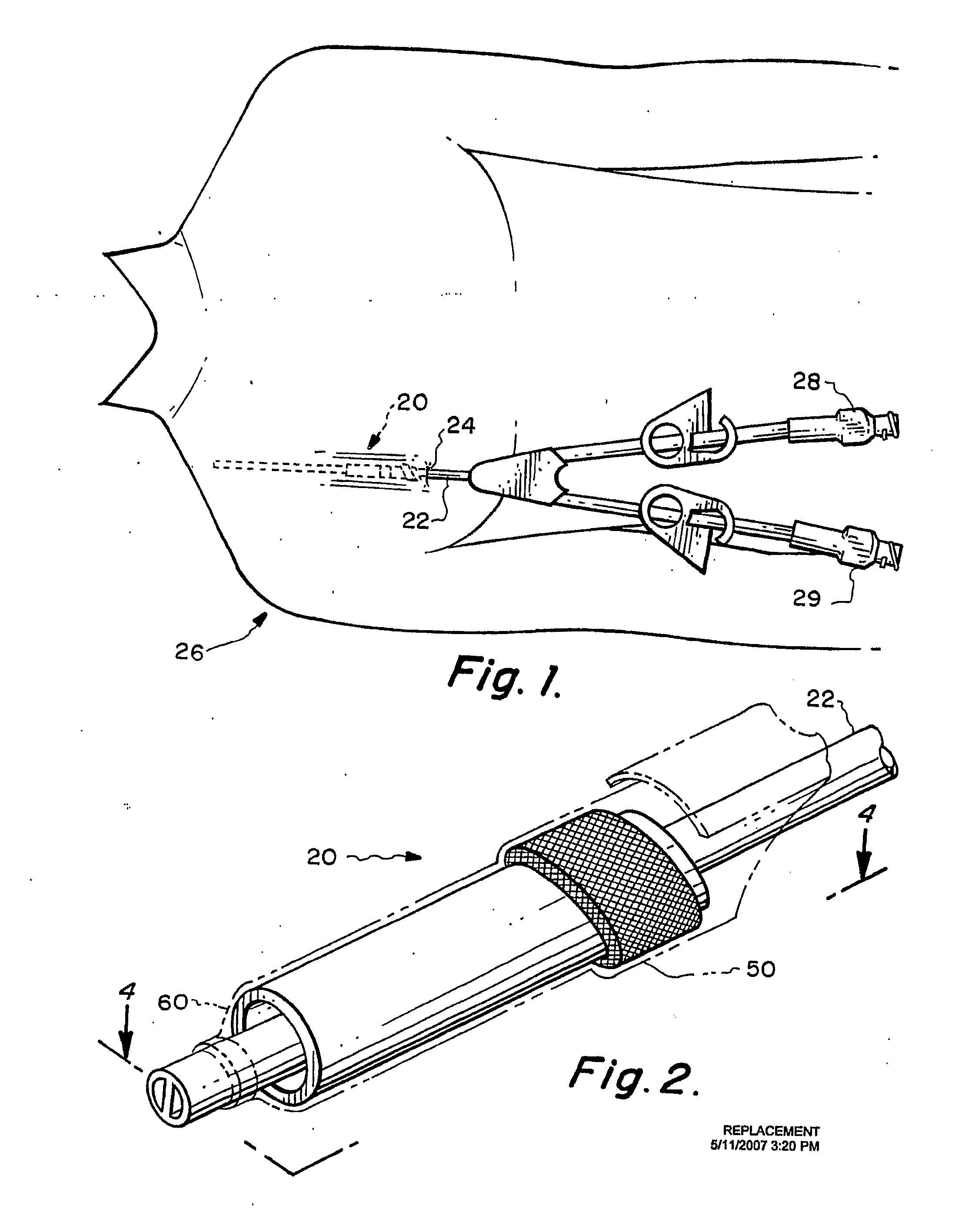
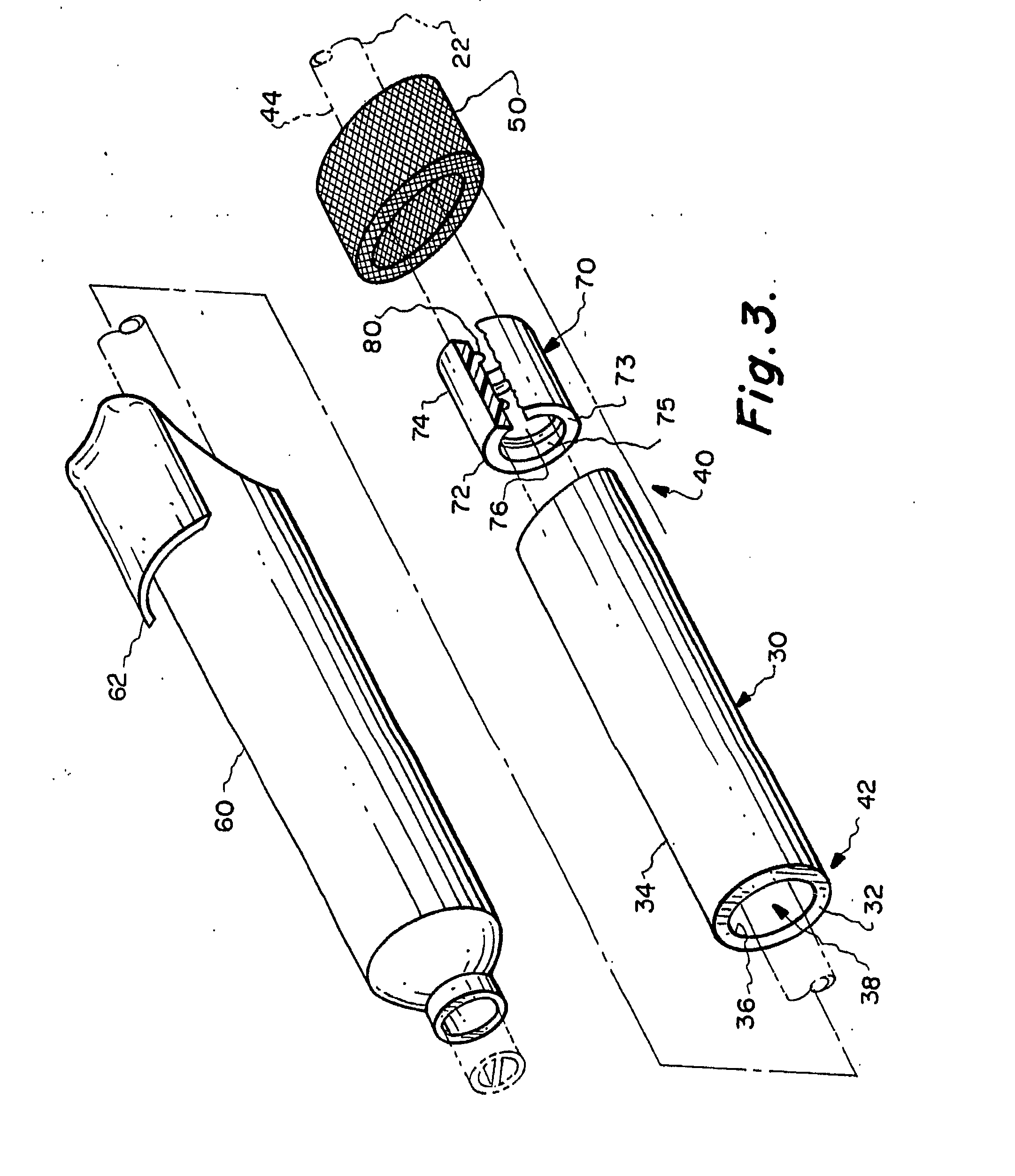
The present invention relates to an implantable catheter (100) provided for insertion through a wall (50) of a dural venous sinus (70) in a subject, having a proximal (20) and distal end (30), comprising: —a tubular shaft (10) for insertion through the wall (50) of the venous sinus (70) into the sinus (52), provided with a shaft lumen (12) in fluid connection with a proximal port (14) at the proximal end and a distal port (16) at the distal end of the shaft (10), and —a stop element (40) disposed on an outer surface of the tubular shaft (10), configured to limit the depth of insertion of the tubular shaft (10) into the sinus.
Owner:STEERABLE INSTR NV +1
Implantable catheter lead or electrode lead
InactiveUS20110029057A1Prevent blood flowLess stringent demandTransvascular endocardial electrodesCatheterCatheterImplantable Catheters
An implantable catheter lead or electrode lead includes an elongated flexible lead body with the fixation means attached to the lead body, for the purpose of effecting fixation of the catheter lead or electrode lead in a predetermined position within a vessel or a bodily cavity of a patient. A releasable attachment is provided between the lead body and the fixation means such that an explantation of the catheter lead or electrode lead is possible after the attachment is released while the fixation means remains in place within the body of the patient.
Owner:BIOTRONIK SE & CO KG
Minimally-advancing luminal catheter
The present invention relates to an implantable catheter (100) provided for insertion through a wall (50) of a dural venous sinus (70) in a subject, having a proximal (20) and distal end (30), comprising: —a tubular shaft (10) for insertion through the wall (50) of the venous sinus (70) into the sinus (52), provided with a shaft lumen (12) in fluid connection with a proximal port (14) at the proximal end and a distal port (16) at the distal end of the shaft (10), and —a stop element (40) disposed on an outer surface of the tubular shaft (10), configured to limit the depth of insertion of the tubular shaft (10) into the sinus.
Owner:STEERABLE INSTR NV +1
Electrochemical disinfection of implanted catheters
ActiveUS9320832B2Inhibits biofilm formationInhibition formationElectrotherapyPharmaceutical delivery mechanismCatheterBiological membrane
An implantable catheter is provided that may be disinfected without removal from the body of a patient, using an electrochemical method to generate an electric field on the catheter surface and thus destroy microorganisms in a biofilm that is present or forming. A catheter system includes the implantable catheter and a voltage source that is operably connected to electrodes on or embedded in the exterior and optionally the interior catheter surface. Methods are also provided for disinfecting the implantable catheter in vivo and for detecting or confirming the presence of a pathogenic biofilm thereon.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Implantable Catheter and Method of Using Same
A catheter for implantation into a patient having a catheter tube having a distal end, a means for trimming the distal end of the catheter tube after subcutaneous insertion of the tip of the catheter lumen into a desired position within the patient to form a trimmed end portion, and a means for selectively positioning each respective lumen of the trimmed end portion of the catheter tube into fluid communication with the respective first end of one attachment tube. The attachment tube is select fluid communication with a desired medical device. Additionally, a repair kit and method of repairing a damaged catheter in which a fluid-tight connection is created between an existing implanted catheter and a replacement tubing.
Owner:ANGIODYNAMICS INC
Unobstructing microdevices for self-clearing implantable catheters
A self-clearing actuator configured to be positioned in a pore providing fluid communication into a central lumen of a ventricular catheter body is described. The actuator extends into a central bore via a cantilever beam having a first end emanating at the central bore and a second end terminating at the actuator, wherein the actuator is configured to reciprocate within the central bore between a first position extending downward at an angle into the central bore and a second position substantially at or above the external surface of the catheter. The cantilever beam is stressed, e.g. via a composite compress layer, such that it is preloaded to nominally curve downward to extend the actuator into the second position. The actuator is preferably a magnet responsive to magnetic field such that the magnetic field drives the actuator toward the first position.
Owner:RGT UNIV OF CALIFORNIA
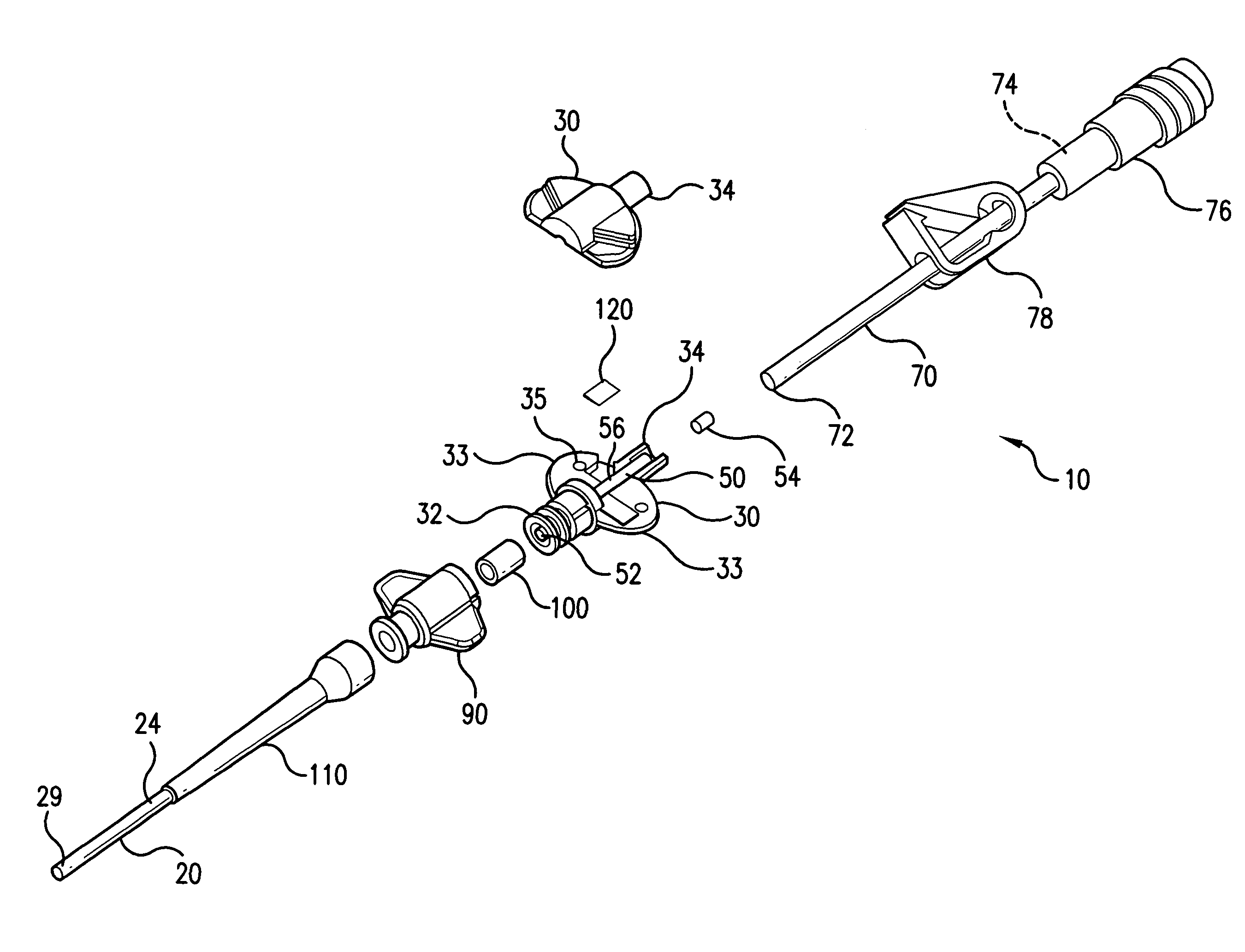
Implantable catheter port
InactiveUS8079990B2Smoother featureMinimized volumePharmaceutical delivery mechanismMedical devicesVascular Access DevicesMedicine
A single or multi-port vascular access device including one or more reservoirs each covered by a needle-penetrable, self-sealing septum. The one or more reservoirs each open to an outlet in a stem to which the catheter is attached. The reservoir may be generally circular or ellipsoid in shape and large enough so that fluid movement into and out of the needle is unimpeded. In order to maximize flow between the reservoir and catheter and to minimize any regions of impeded or low fluid flow where coagulation or cell shearing may occur, the fluid passage leading from the reservoir through the outlet into the catheter is defined by unique tapered and tangential geometries. For example, the outlet surface may be globally tangent along the entire reservoir surface, or may be globally tangent along only one side of the reservoir surface.
Owner:VECTOR CORP
Unobstructing microdevices for self-clearing implantable catheters
A self-clearing actuator configured to be positioned in a pore providing fluid communication into a central lumen of a ventricular catheter body is described. The actuator extends into a central bore via a cantilever beam having a first end emanating at the central bore and a second end terminating at the actuator, wherein the actuator is configured to reciprocate within the central bore between a first position extending downward at an angle into the central bore and a second position substantially at or above the external surface of the catheter. The cantilever beam is stressed, e.g. via a composite compress layer, such that it is preloaded to nominally curve downward to extend the actuator into the second position. The actuator is preferably a magnet responsive to magnetic field such that the magnetic field drives the actuator toward the first position.
Owner:RGT UNIV OF CALIFORNIA
Radioiodinated sulfonated phenols and process therefore
InactiveUS6861044B2High yieldHigh purityRadioactive preparation carriersMacromolecular non-active ingredientsFencholIodine
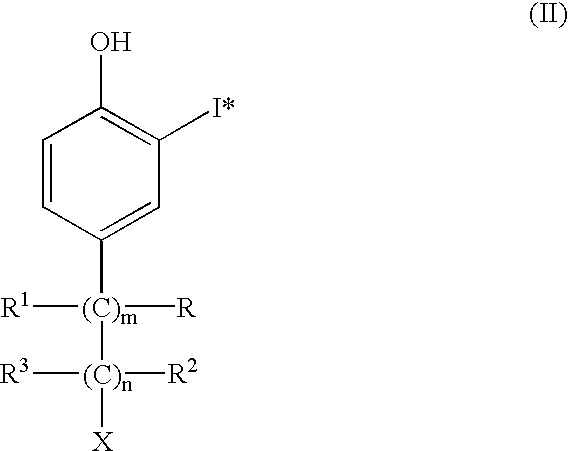
The present invention concerns the use of a radioiodinated phenolic compound of the formula wherein: m and n are independently 0, 1, 2 or 3, X is a group that is negatively or positively charged at physiological pH, R, R1, R2 and R3 are independently hydrogen, C1-C4 alkyl, or a carboxyl group, and I* is 123I, 131I or 125I, and its pharmaceutically-acceptable salts. The compound is formulated and used in vivo in an animal in brachytherapy in an implantable catheter. In addition, due to the rapid renal clearance of these compounds, they may be used to study renal function. A process to prepare these compounds is also disclosed.
Owner:THE DOW CHEM CO
Gastric diversion apparatus and implantable catheter thereof
ActiveCN107158546ASimple structureLow structural suitabilityMedical devicesCatheterLow-density polyethyleneLinear low-density polyethylene
The invention discloses a gastric diversion apparatus and an implantable catheter thereof. The implantable catheter comprises a membrane tube, wherein a stent is connected to one end of the membrane tube; and the membrane tube is prepared by mixing and blowing the following materials in parts by weight: 0.5-1.5 parts by weight of high density polyethylene, 4 parts by weight of low density polyethylene, 4 parts by weight of linear low density polyethylene and 0.5-1.5 parts by weight of barium sulfate. According to the scheme, the implantable catheter is made from a membrane tube material which is softer and more comfortable, lower in human rejection and free from injury; the implantable catheter is convenient in manufacturing process, low in cost and high in production speed; and the implantable catheter is applicable to long-term implantation in a human digestive tract.
Owner:HANGZHOU TANGJI MEDICAL TECH CO LTD
Method and Subcutaneous Apparatus for Facilitating the Replacement of an Implanted Catheter
InactiveUS20100234814A1Inhibit migrationEasy to peelGuide needlesMedical devicesSubcutaneous implantationGuide tube
A medical apparatus and method of use for implanting a catheter in a patient's body which catheter can be easily positioned, repositioned, and replaced. The apparatus includes an elongate sleeve intended to be subcutaneously implanted. The sleeve comprises a wall surrounding an interior elongate passageway which extends from a sleeve proximal end to a sleeve distal end. The sleeve outer peripheral surface carries a layer of porous material intended to be placed just under the patient's outer skin layer in contact with the dermis to promote tissue ingrowth for anchoring the sleeve and forming an infection resistant barrier. The sleeve passageway includes a sealing nib dimensioned to engage the outer surface of the catheter while permitting the catheter to slide relative to the sleeve. The sealing nib prevents deleterious material from migrating into the patient's body along the catheter outer surface.
Owner:INCUMED LLC A NEVADA LIMITED LIABILITY
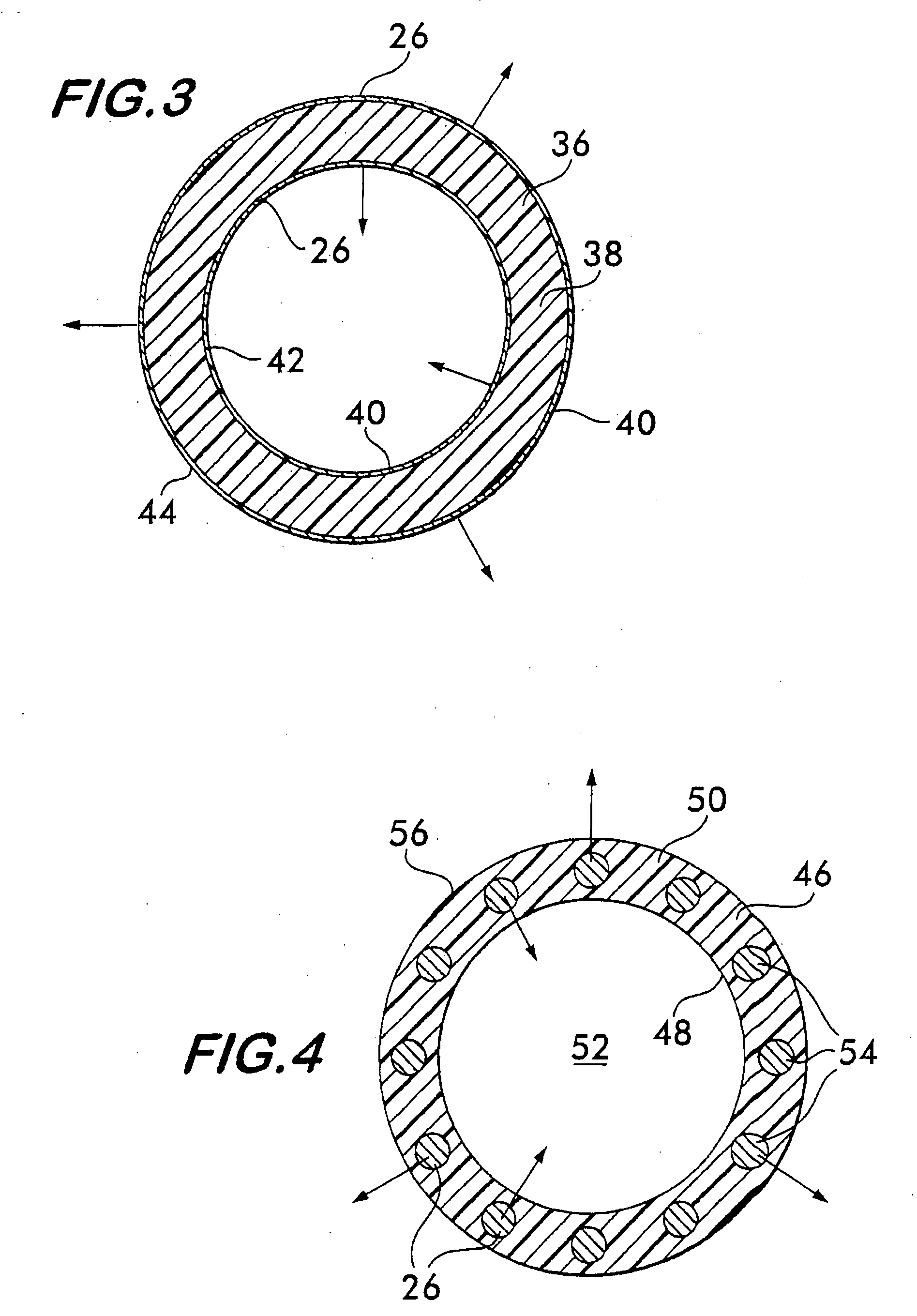
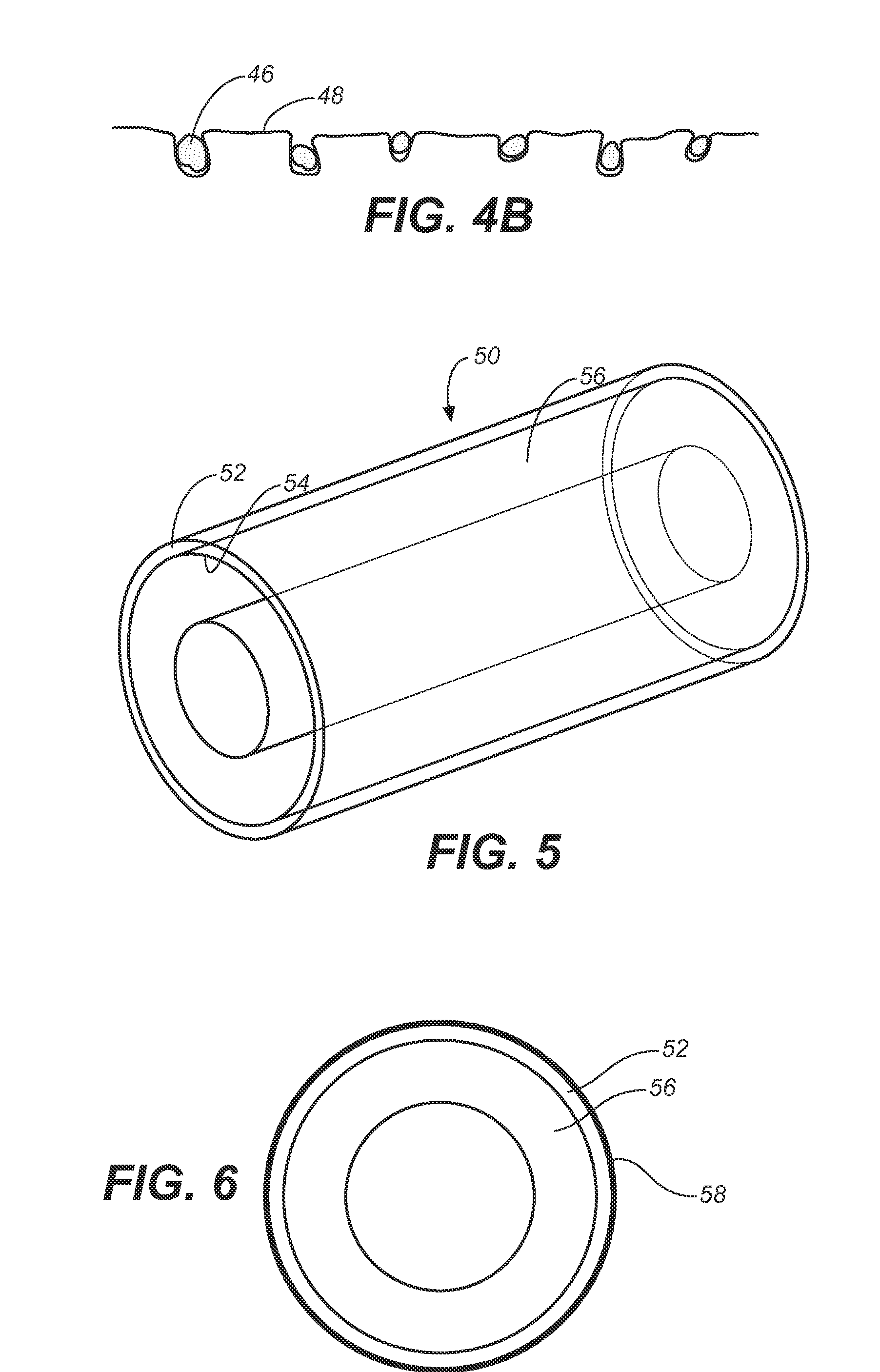
Implantable shunt or catheter enabling gradual delivery of therapeutic agents
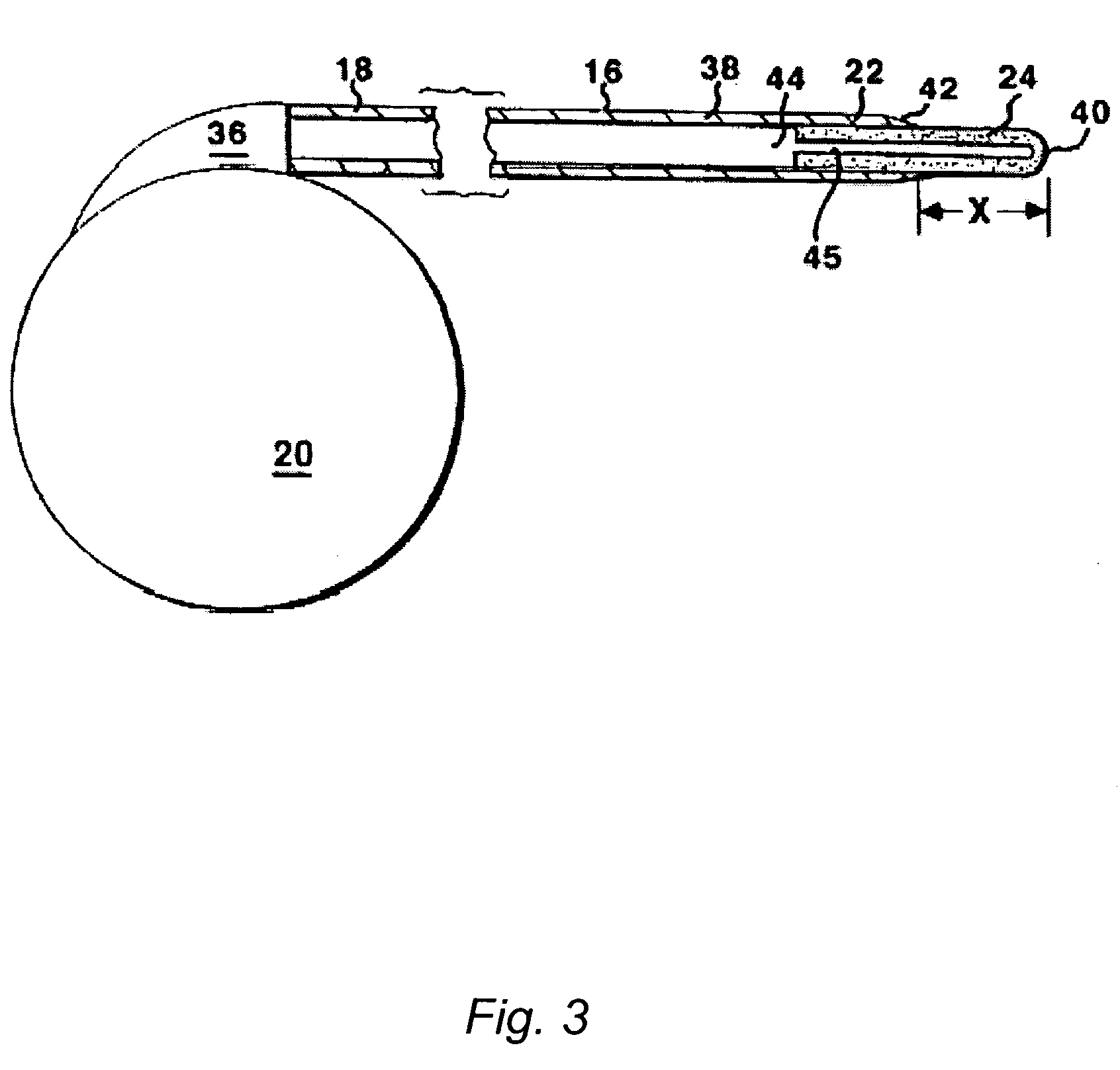
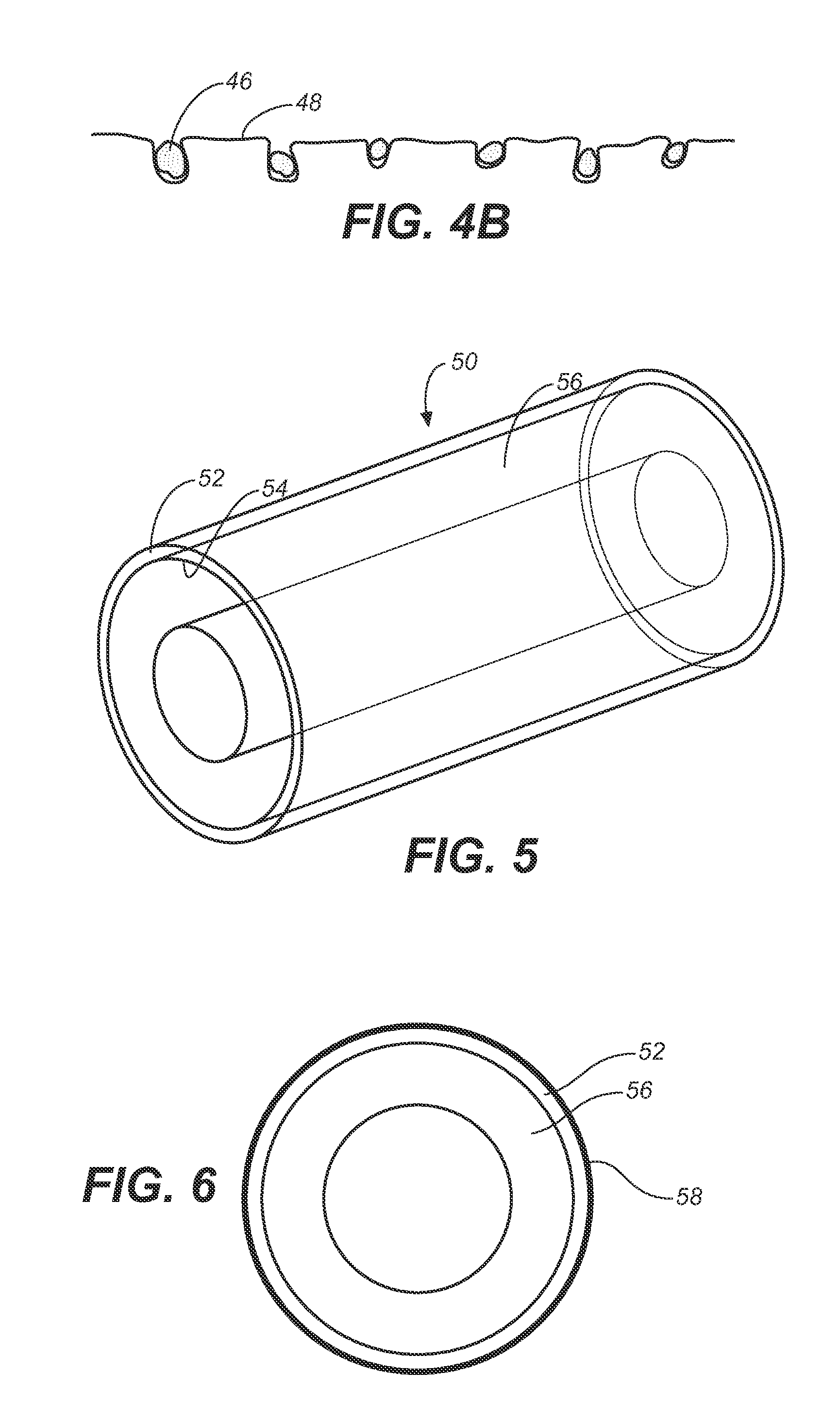
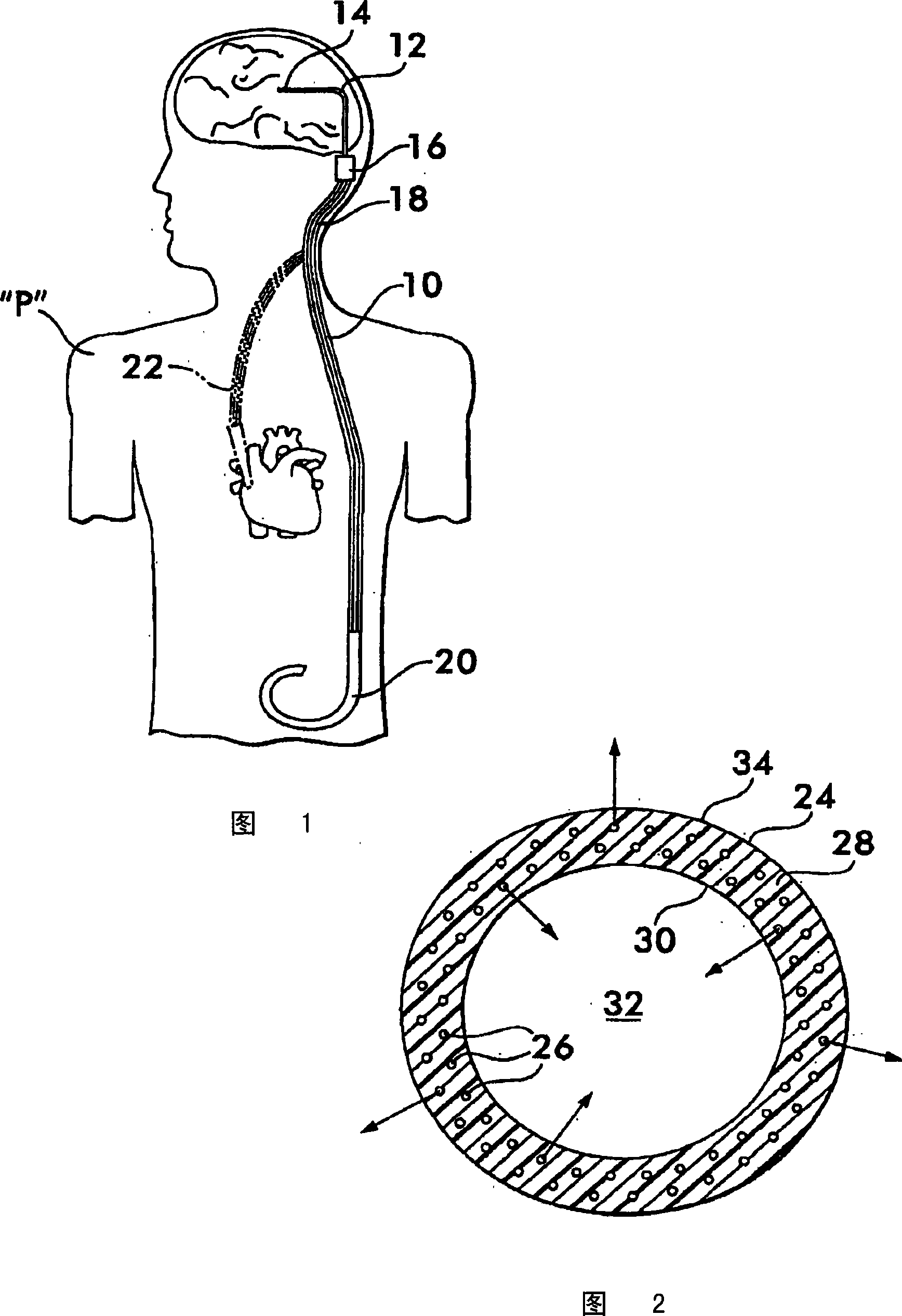
An implantable catheter or shunt (24) for draining fluid from a body cavity. The catheter or shunt body has a wall structure (28) that carries one or more therapeutic agents (26) in a manner enabling release of the therapeutic agent from the wall structure in situ after surgical implantation of the catheter or shunt body. The therapeutic agent can be gradually released over time to prevent infection, inhibit tissue ingrowths, and / or provide some other desired medicinal purpose. As an example, the therapeutic agent can be rapamycin or an mTOR inhibitor. According to some contemplated embodiments of the present invention, the therapeutic agent carried by the catheter / shunt is rechargeable or refillable in situ so that the therapeutic agent can be gradually released from the catheter / shunt over the expected useful life of the catheter / shunt.
Owner:WYETH LLC
Implantable system for increasing intrathecal drug dispersion
A medical device configured to improve dispersion of a medicament within cerebrospinal fluid of a patient is disclosed. The medical device includes an implantable catheter having: a distal tip configured to be positioned within a cerebrospinal fluid flow; a proximal tip; a body defining a lumen extending longitudinally along the implantable catheter, the lumen configured to enable a medicament to flow from the proximal tip to an infusion port positioned proximate the distal tip; and a piezoelectric element positioned proximate the infusion port, the piezoelectric element configured to selectively oscillate during administration of a medicament to improve dispersion of the medicament within the cerebrospinal fluid.
Owner:MEDTRONIC INC
An In Situ Ultraviolet Sterilization System of Intrabody Inserted Catheter
An in-situ ultraviolet sterilizing system for an in-body catheter, comprising an ultraviolet light source, an ultraviolet light transmission channel, a light source-channel coupling interface and end fittings, where the ultraviolet light source is connected to one end of the ultraviolet light transmission channel through the light source-channel coupling interface, The end fittings are connected to the other end of the ultraviolet light transmission channel; the ultraviolet light transmission channel and the end fittings extend into the in-body catheter, and the light propagates along the longitudinal direction of the ultraviolet light transmission channel and irradiates into the lumen of the in-body catheter On the surface, the end fittings can reflect or scatter the ultraviolet rays propagating axially along the ultraviolet light transmission channel to the direction of the included angle; the inner wall of the main pipe can be sterilized in situ through the ultraviolet light transmission conduit, and the inner wall of the cross branch pipe can be sterilized through the end fittings Sterilize in situ. The present invention can conform to the shape of the in-body catheter, and has the characteristics of compactness, portability, instant use, and the advantages of high efficiency, energy saving, environmental protection and long service life.
Owner:XI AN JIAOTONG UNIV
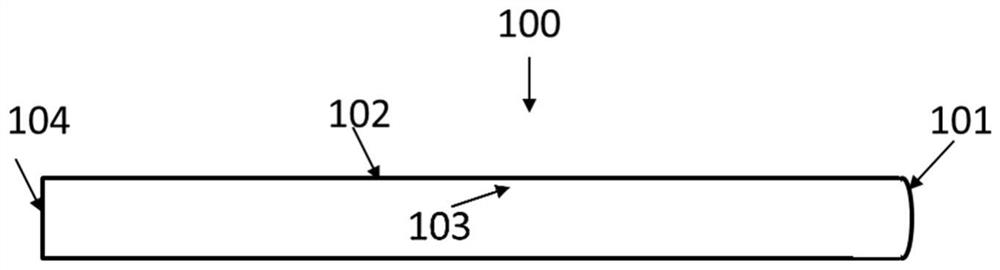
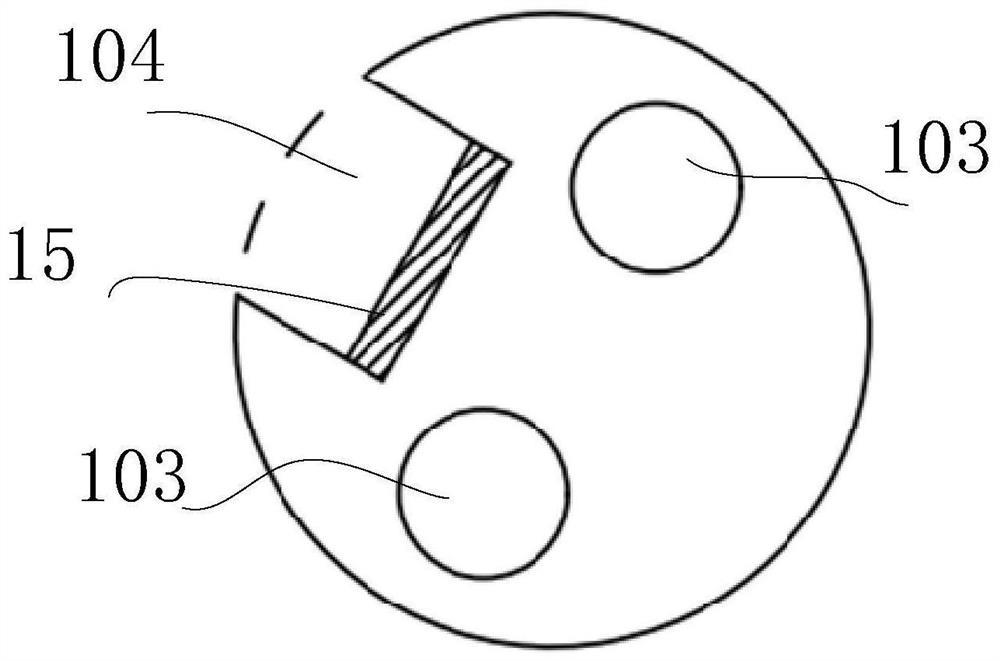
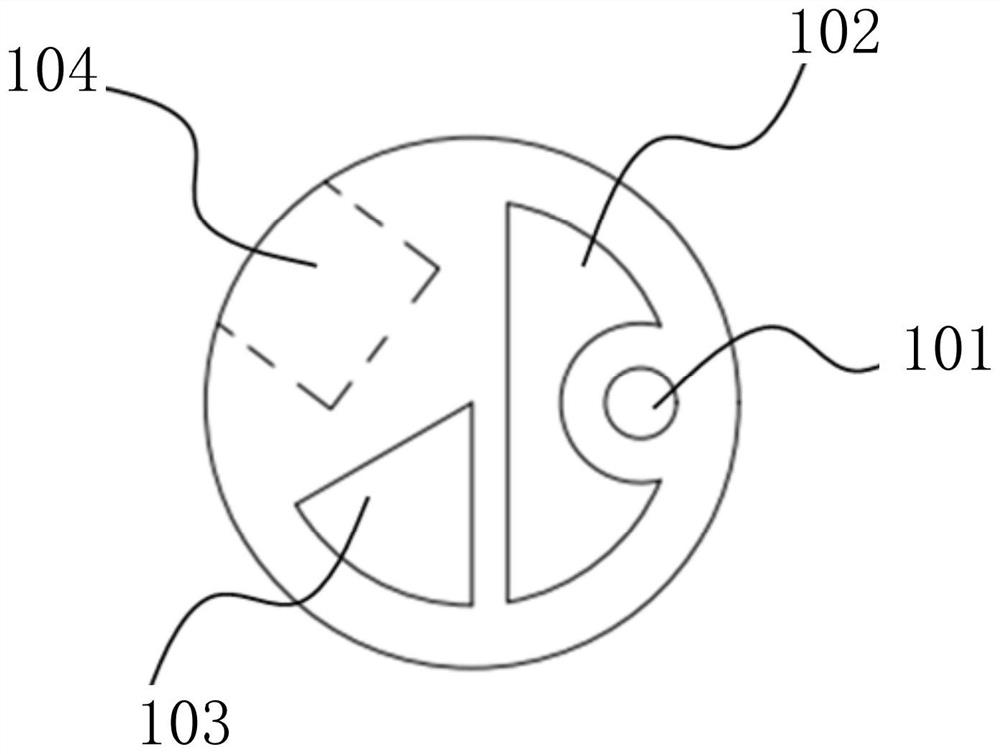
Implantable catheter, use method and preparation method thereof
InactiveCN113181514ASimple structureSmall diameterBalloon catheterMulti-lumen catheterForce sensorGuide wires
The invention relates to the field of medical instruments, and particularly discloses an implantable catheter, a use method and a preparation method thereof. The implantable catheter comprises a catheter body with a near end and a far end, wherein the catheter body is internally provided with a guide wire cavity channel, an inflation cavity channel and a guide wire cavity channel which are independent of one another, and a guide wire is arranged in the guide wire cavity channel; a pressure sensor arranged on the tube body, connected with the lead in the lead cavity and used for monitoring the pressure of the implanted tissue in real time; and a balloon arranged on the tube body and communicated with the inflation cavity channel. The pressure sensor is integrated on the catheter body, so that the pressure in the implanted tissue is directly measured, the structure of the catheter is simplified, and the diameter of the catheter is reduced.
Owner:HANGZHOU WEIMING XINKE TECH CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com