Implantable system for increasing intrathecal drug dispersion
An implantable, decentralized technology, applied in the direction of drug devices, devices introduced into the body, applications, etc., can solve problems such as reducing drug efficacy and therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
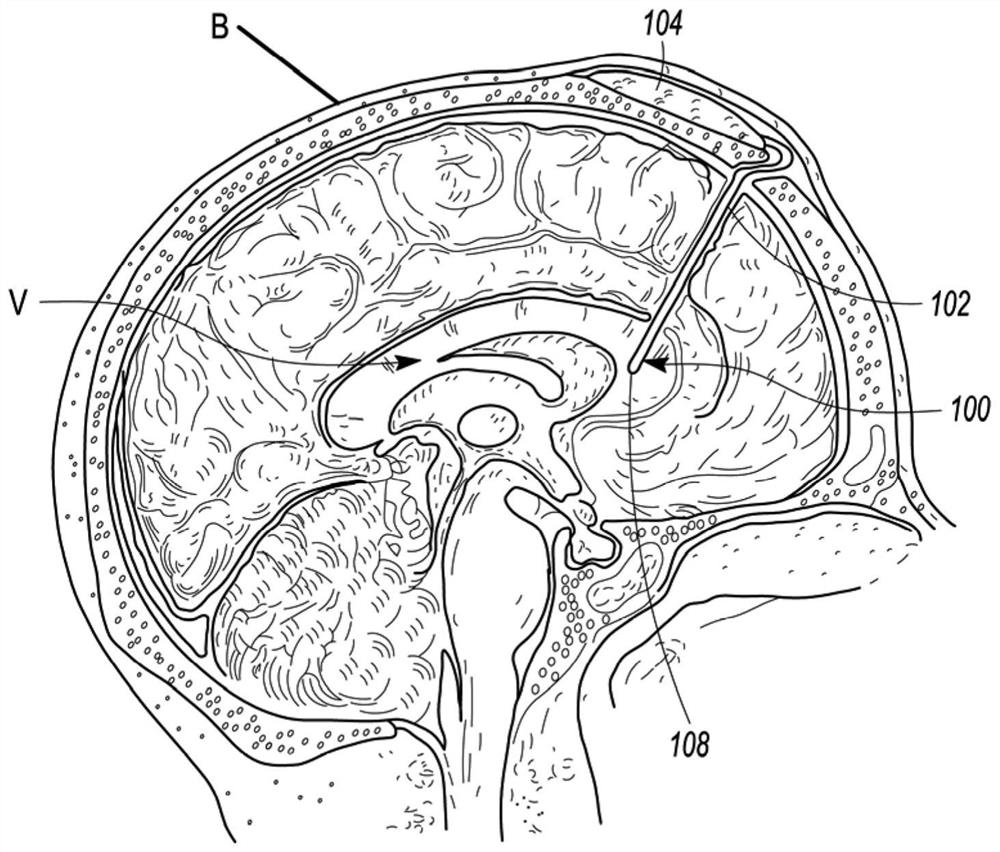
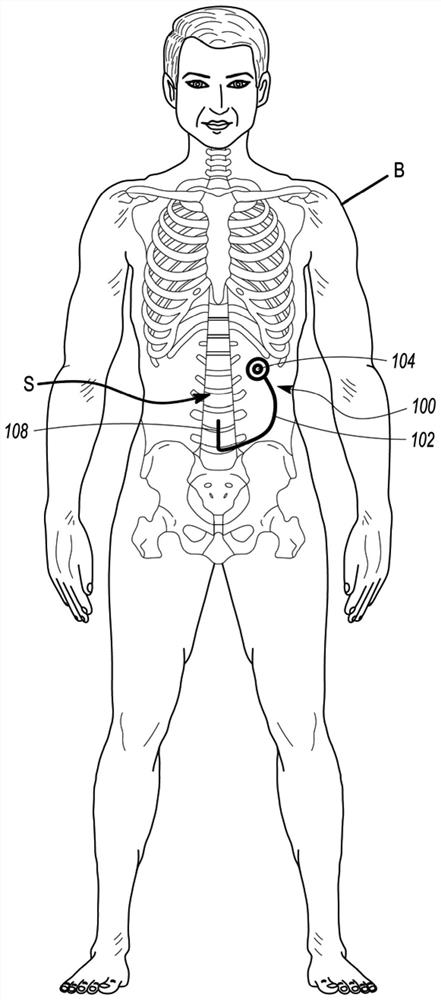
[0036] refer to Figure 1A and 1B , depicts a medical device 100 configured to improve medicament dispersion in accordance with an embodiment of the disclosure. The medical device 100 can include an implantable catheter 102 which, in some embodiments, can be connected to an implantable port 104 (such as Figure 1A -B depicted) or implantable pump 106 (as Figure 7 Depicted) fluid communication. As depicted, medical device 100 may be implanted within body B of a patient. In some embodiments, the distal tip 108 of the implantable catheter 102 may optionally be surgically implanted into a ventricle V in the patient's brain (eg, Figure 1A depicted) or the patient's intraspinal space (eg Figure 1B depicted). Implantable port 104 or implantable pump 106 can be placed cranially (e.g. Figure 1A as depicted in ), or placed inside the dry cavity or close to the patient's ribs (eg Figure 1B depicted in ). In either case, the implanted port 104 or implanted pump 106 is typically ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com