Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Homosteroids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Steroids whose structure has been expanded by the addition of one or more carbon atoms to the ring skeleton in any of the four rings.
Inhibitors of 11-beta hydroxysteroid dehydrogenase type I
InactiveUS20060235028A1Inhibitory activityBiocideNervous disorder11-beta-Hydroxysteroid DehydrogenasesCompound (substance)
Novel compounds are provided which are 11-beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds have the structure: or stereoisomers or prodrugs or pharmaceutically acceptable salts thereof, wherein G, L, Q, Z, R6, R7, and R8 are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
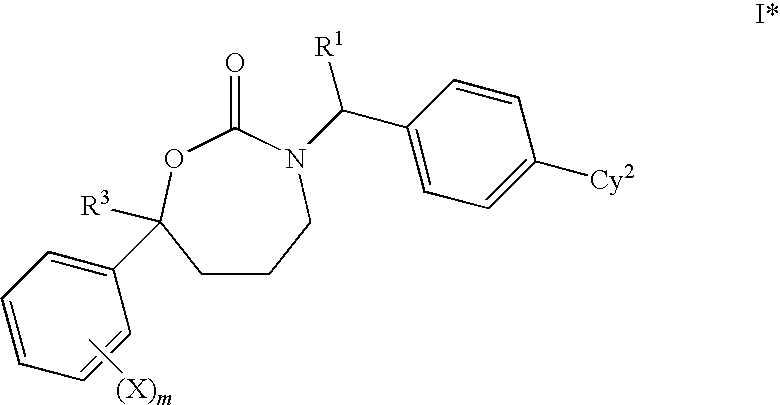
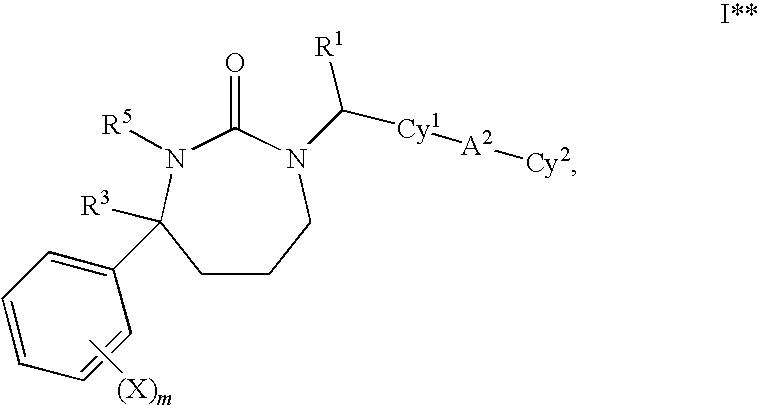
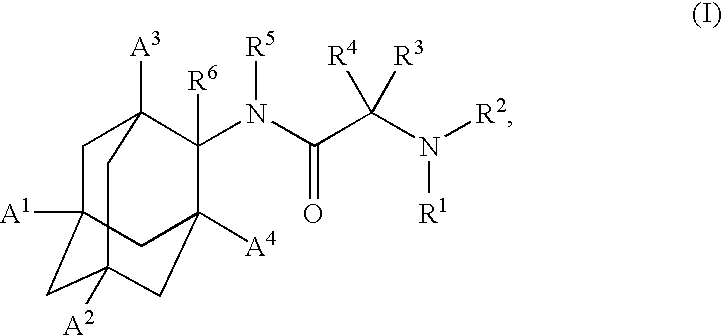
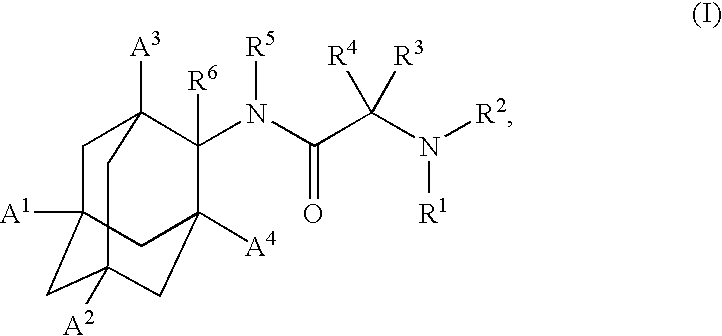
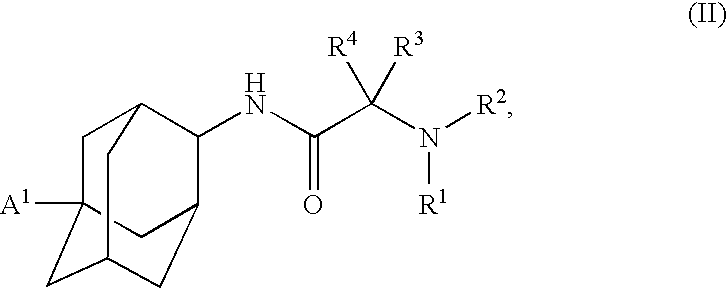
1,3-Oxazepan-2-one and 1,3-diazepan-2-one inhibitors of 11ß-hydroxysteroid dehydrogenase 1
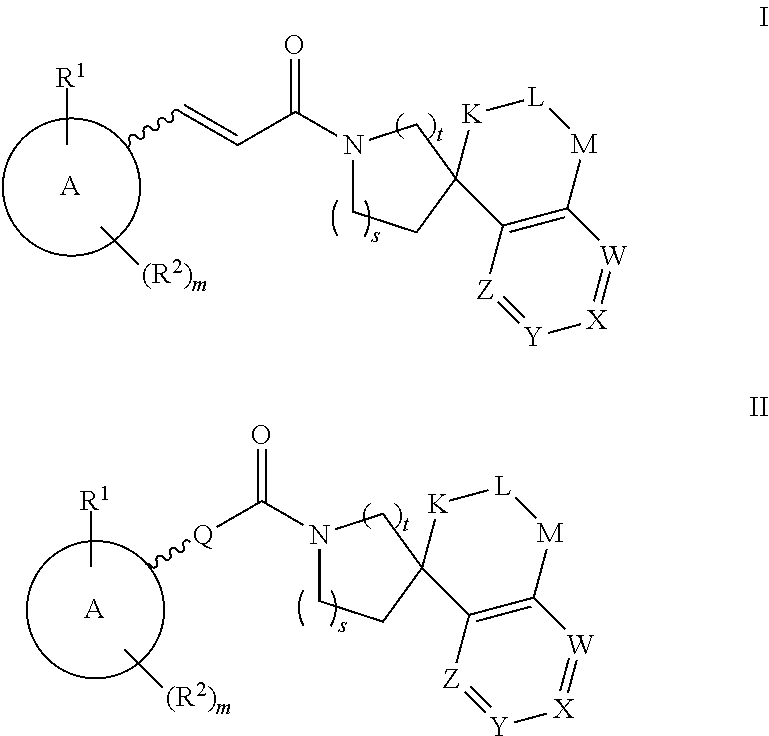
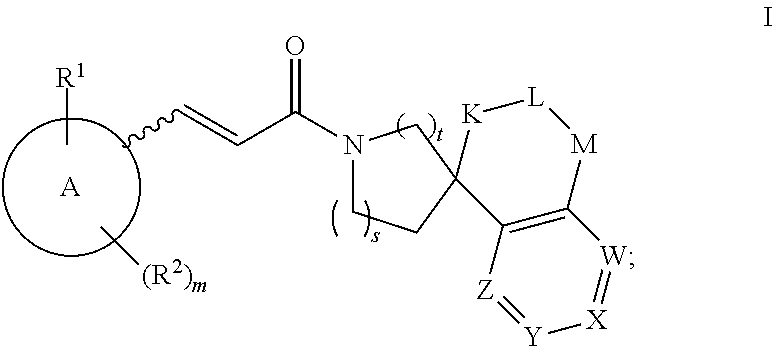
This invention relates to novel compounds of the Formula (I), (I*), (I**), I, Ia, Ib, Ic, Id, Ie, If, Ig, Il1-3, Im1-3, In1-3, or Io1-2, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC
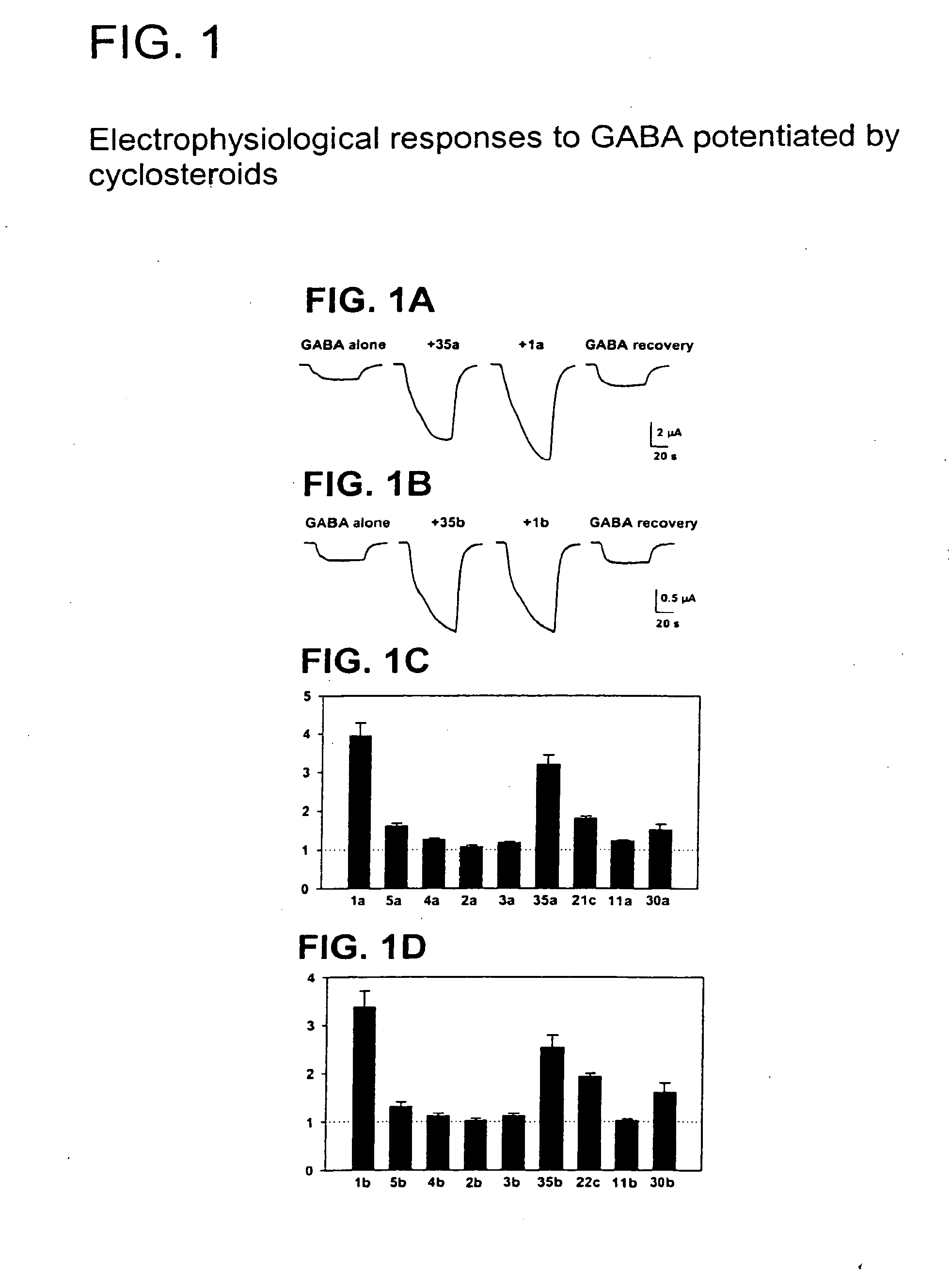
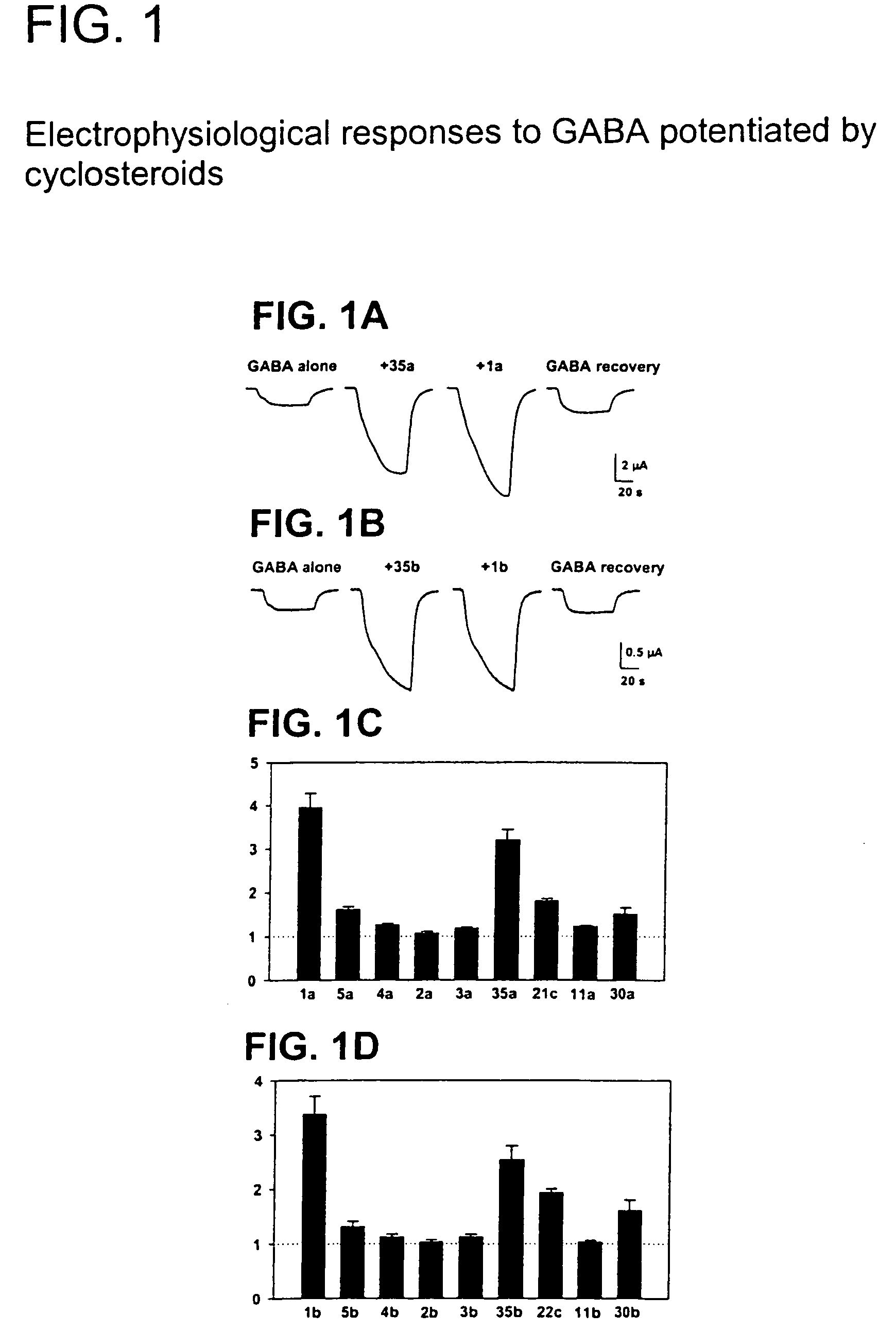
Neuroactive 13,24-Cyclo-18,21-Dinorcholanes and Structurally Related Pentacylic Steroids
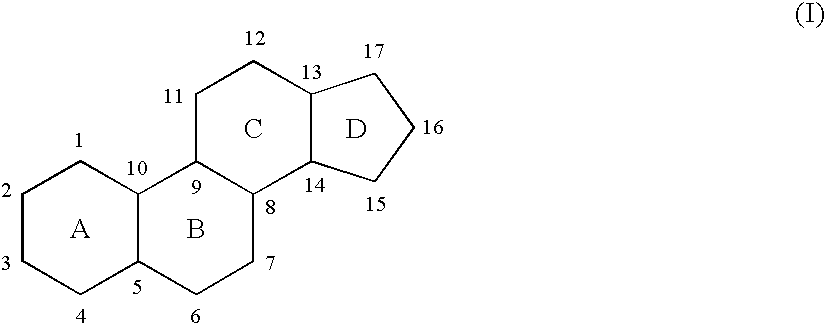
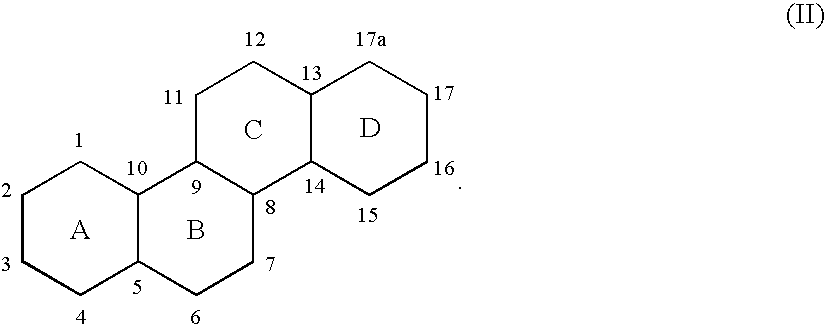
Novel pentacyclic steroids and pentacyclic D-homosteroids comprising: (i) the tetracyclic steroid ring system or tetracyclic D-homosteroid ring system, respectively; (ii) a C(3) substituent selected from the group consisting of (a) a hydroxyl or carboxyl in the α-configuration and (b) a sulfate or other negatively charged moiety; and (iii) a fused fifth ring, the fused fifth ring comprising a hydrogen bond acceptor, and (a) in the case of the pentacyclic steroid the C(13) and C(17) carbons, or (b) in the case of the pentacyclic D-homosteroid the C(13) and C(17a) carbons, having utility as anesthetics and in the treatment of disorders relating to GABA function and activity.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Neuroactive 13, 24-cyclo-18, 21-dinorcholanes and structurally related pentacyclic steriods
Owner:WASHINGTON UNIV IN SAINT LOUIS
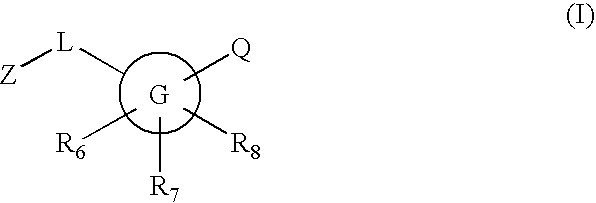
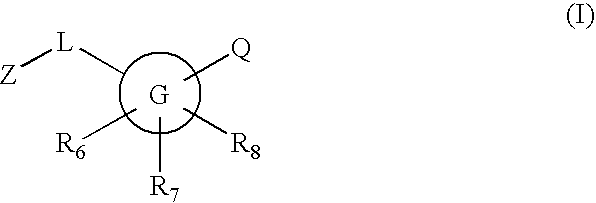
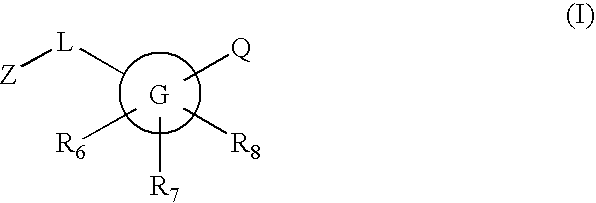
Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors
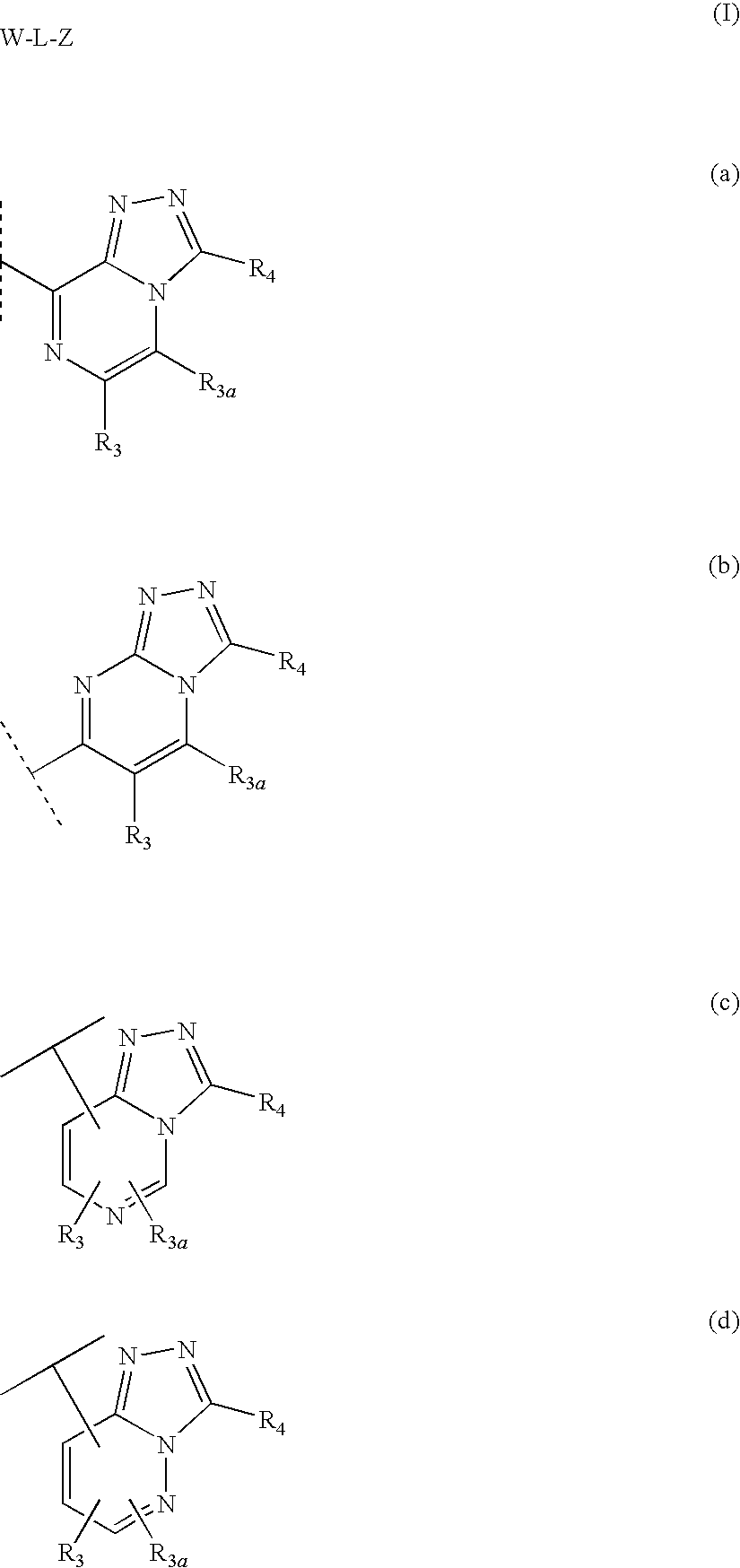
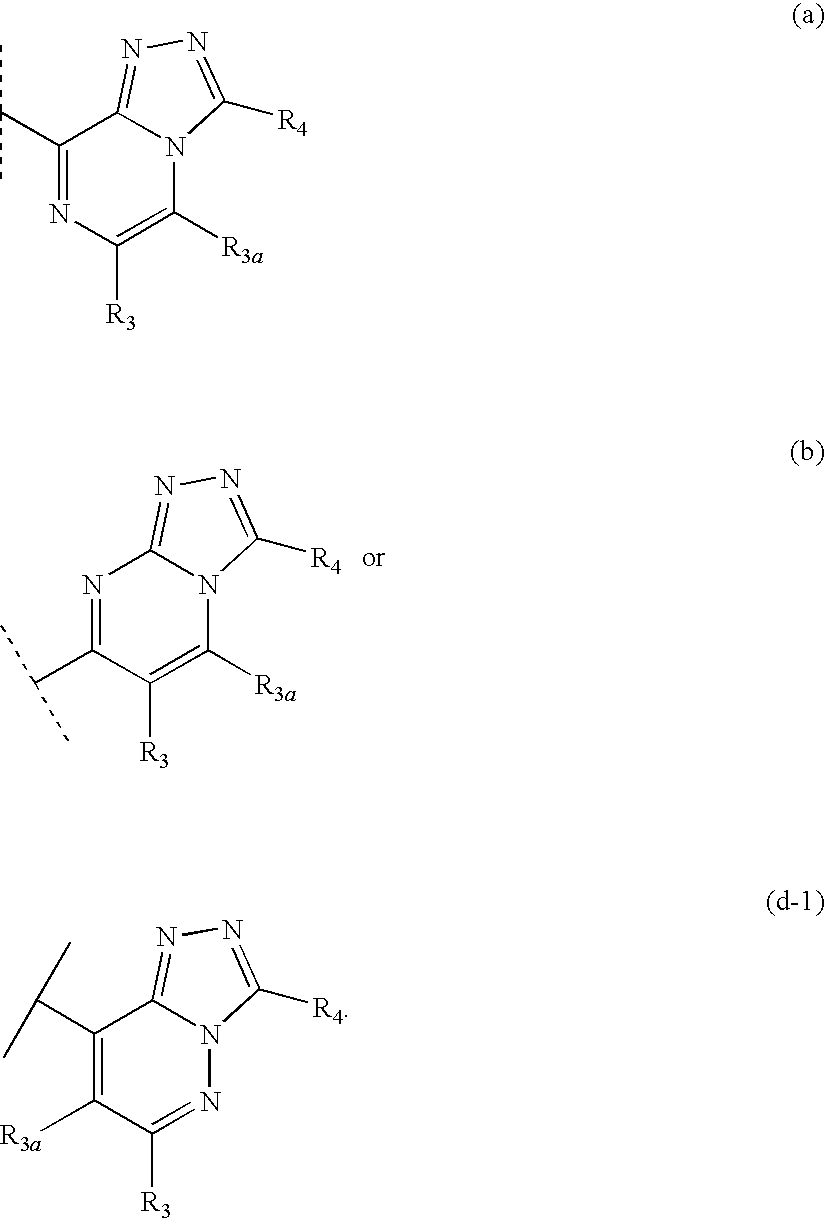
Novel compounds are provided which are 1 1-beta-hydroxysteroid dehydrogenase type I inhibitors. 1 1-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 1 1-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds have the structure: W-L-Z or stereoisomers or prodrugs or pharmaceutically acceptable salts thereof, wherein W, L are defined herein and Z is selected from the following bicyclic heteroaryl groups: (a), (b), (c), (d).
Owner:BRISTOL MYERS SQUIBB CO
Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase Type 1
This invention relates to novel compounds of the Formulae I or II and pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11 β-HSD 1 in mammals. Formula (I).
Owner:VITAE PHARMA INC
11beta-hydroxysteroid dehydrogenase inhibitors
InactiveUS20050227987A1Avoid conversionGood effectBiocideOrganic chemistry11beta hydroxysteroid dehydrogenaseMedicinal chemistry
A compound having Formula I R1-Z-R2 Formula I wherein R1 is an optionally substituted phenyl ring; R2 is or comprises an optionally substituted-aromatic ring; and Z is -X-Y-L- or -Y-X-L- wherein either X is selected from —S(═O)(═O)— and —C(═O)—, and Y is —NR3—; or X is selected from —S(═O)(═O)— and —S—, and Y is —C(R4)(R5)—; L is an optional linker; and R3, R4 and R5 are each independently selected from H and hydrocarbyl; and wherein when R2 comprises the following structural moiety wherein Q is an atom selected from the group consisting of S, O, N and C; the compound is selected from compounds of the formulae R1C(═O)—NR3-L-R2; R1—S(═O)(═O)—C(R4)(R5)-L-R2; R1—S—C(R4)(R5)-L-R2; R1—NR3—S(═O)(═O)-L-R2; R1—NR3—C(═O)-L-R2; R1—C(R4)(R5)—S(═O)(═O)-L-R2; and R1—C(R4)(R5)—S-L-R2.
Owner:STRIX LTD
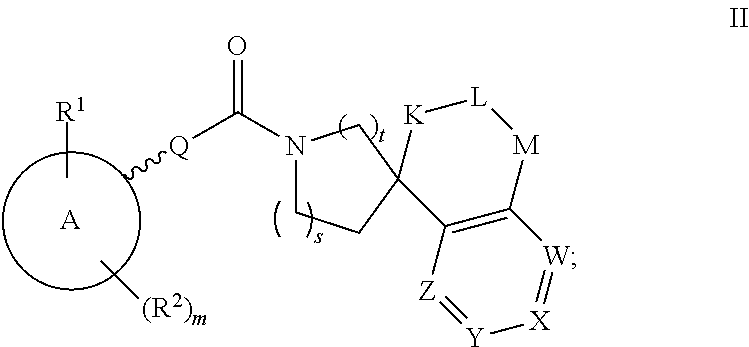
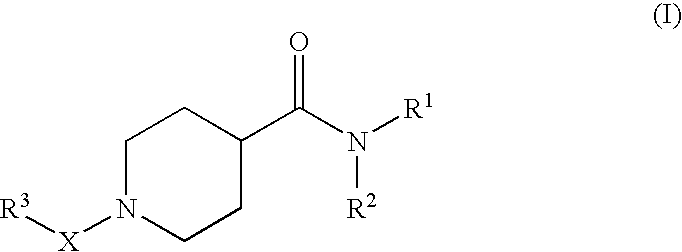
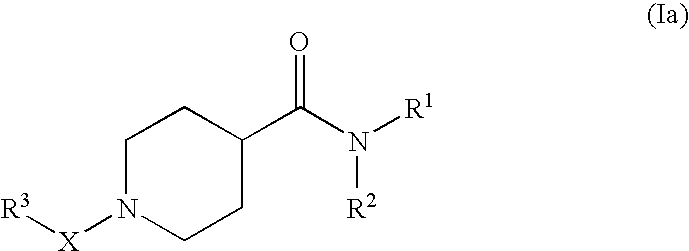
Pharmaceutical use of substituted piperidine carboxamides
A novel class of compounds of the general formula (I), their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments are described. The present compounds modulate the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and are accordingly useful in the treatment of diseases in which such a modulation is beneficial, e.g. the metabolic syndrome.
Owner:HIGH POINT PHARMA
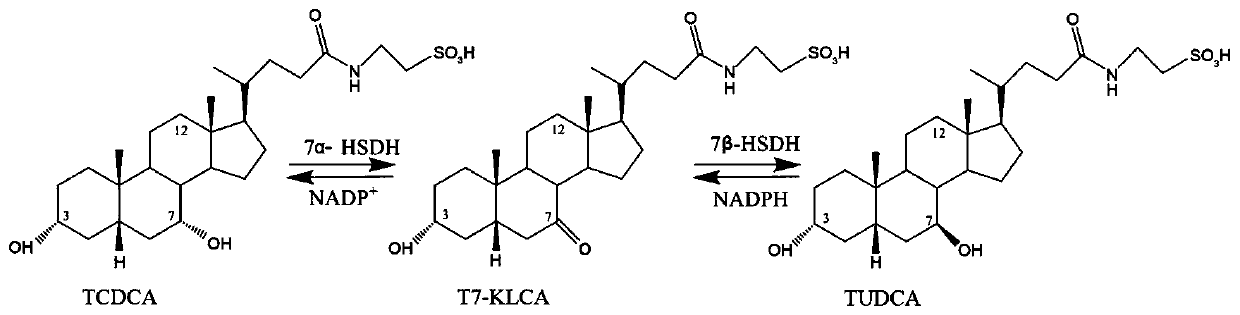
Clostridium sardiniense 7alpha-hydroxysteroid dehydrogenase mutant T145S
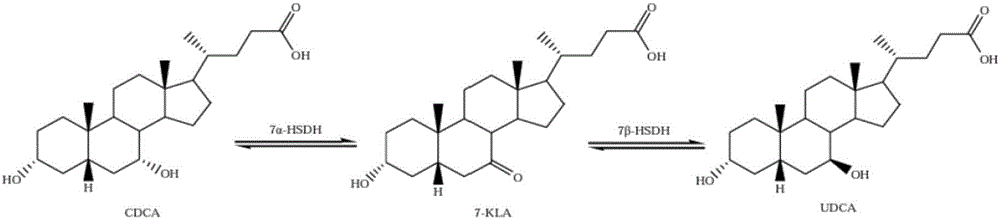
The invention relates to hydroxysteroid dehydrogenase and particularly relates to a clostridium sardiniense 7alpha-hydroxysteroid dehydrogenase mutant T145S. The amino acid sequence of the clostridium sardiniense 7alpha-hydroxysteroid dehydrogenase mutant T145S is shown by SEQ ID No:2 and obtained by changing the 145th-site amino acid of the 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence of SEQ ID No:1 from Thr into Ser. The mutant is 4.85 times of a wild type in the catalysis efficiency against the substrates TCDCA and NADP<+> and has a huge application potential in a biotransformation process of CDCA / TCDCA for obtaining UDCA / TUDCA.
Owner:CHONGQING UNIV
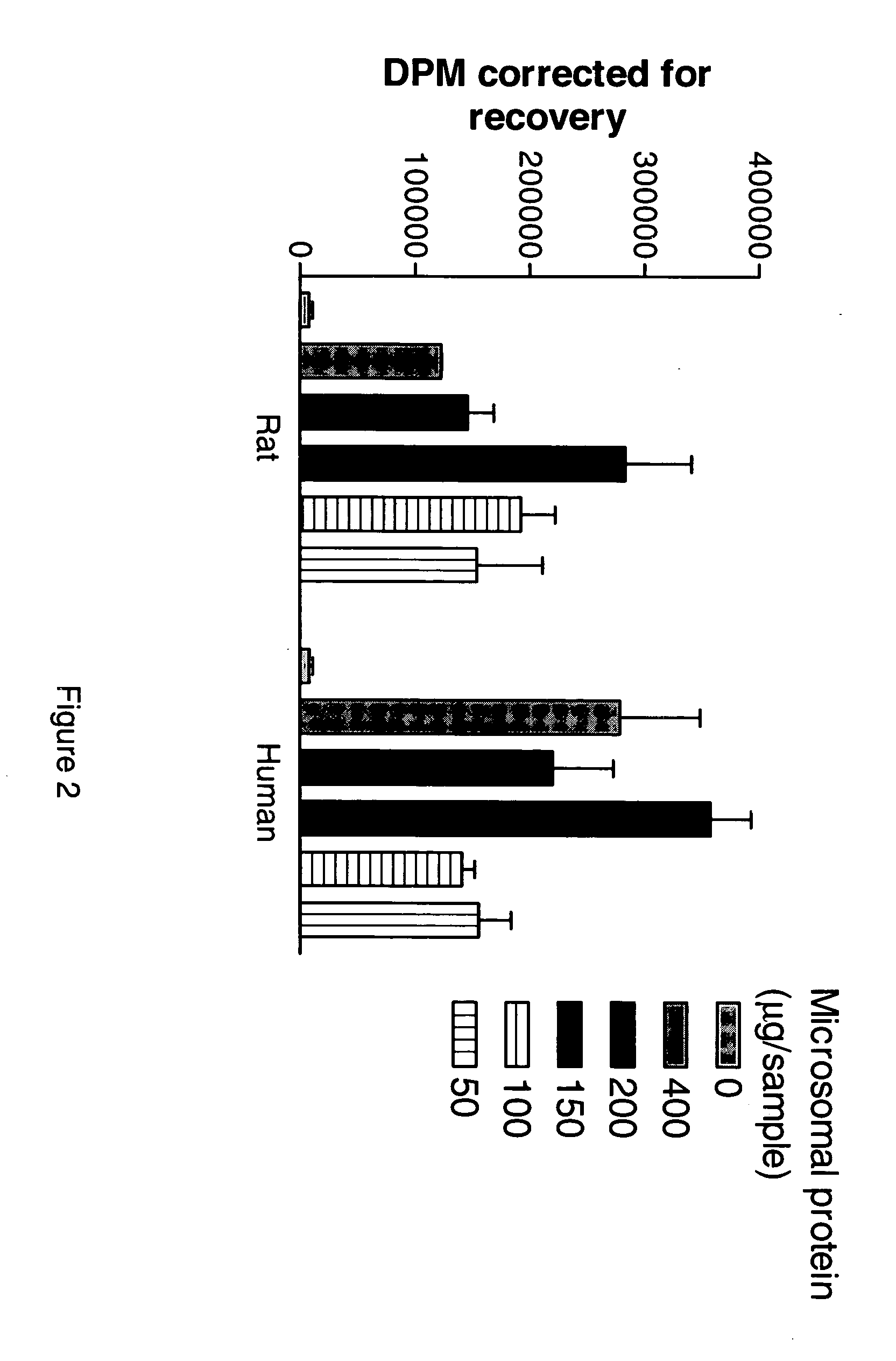
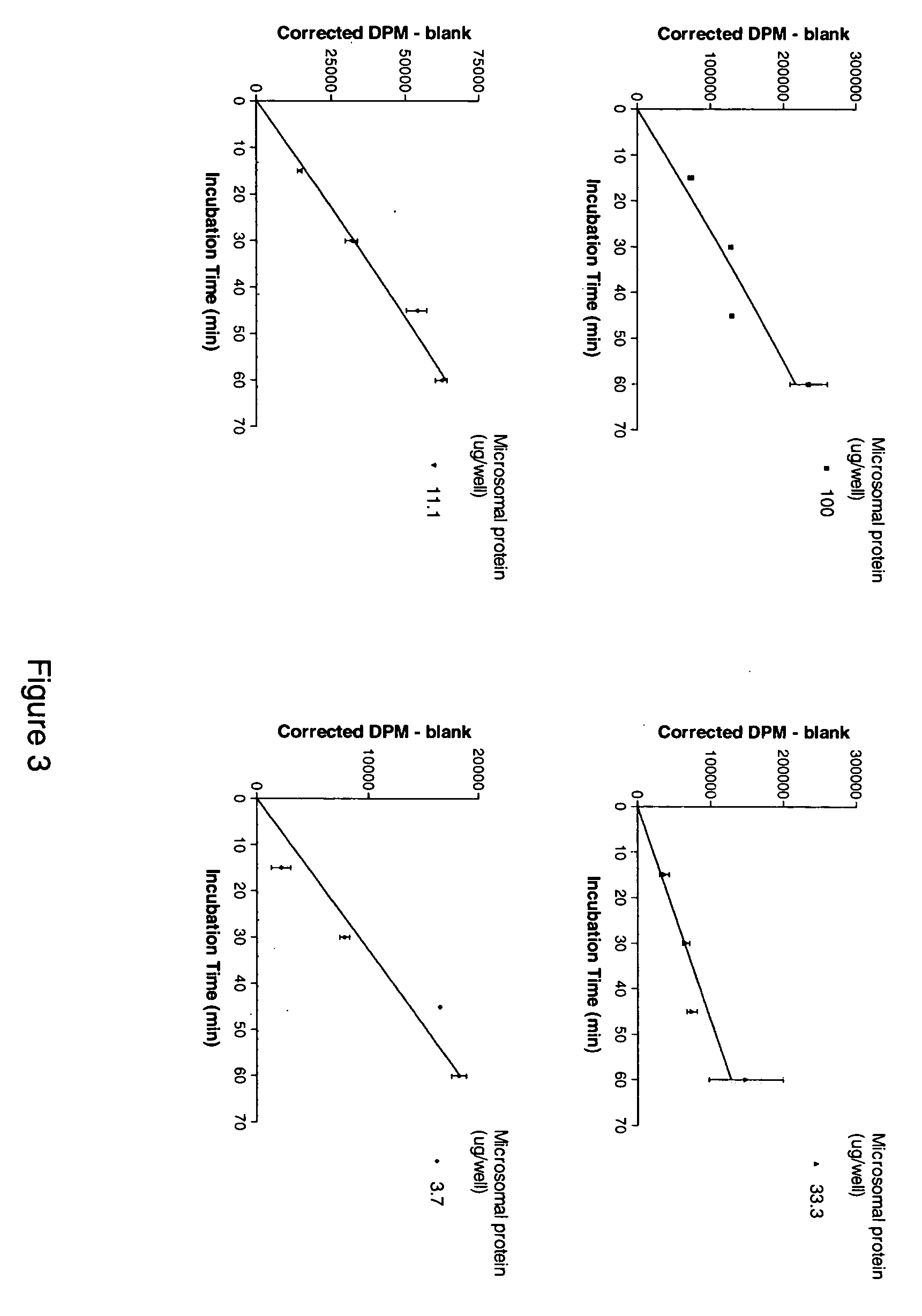
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme
The present invention relates to compounds which are inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome, and other diseases and conditions that are mediated by excessive glucocorticoid action.
Owner:ABBOTT LAB INC
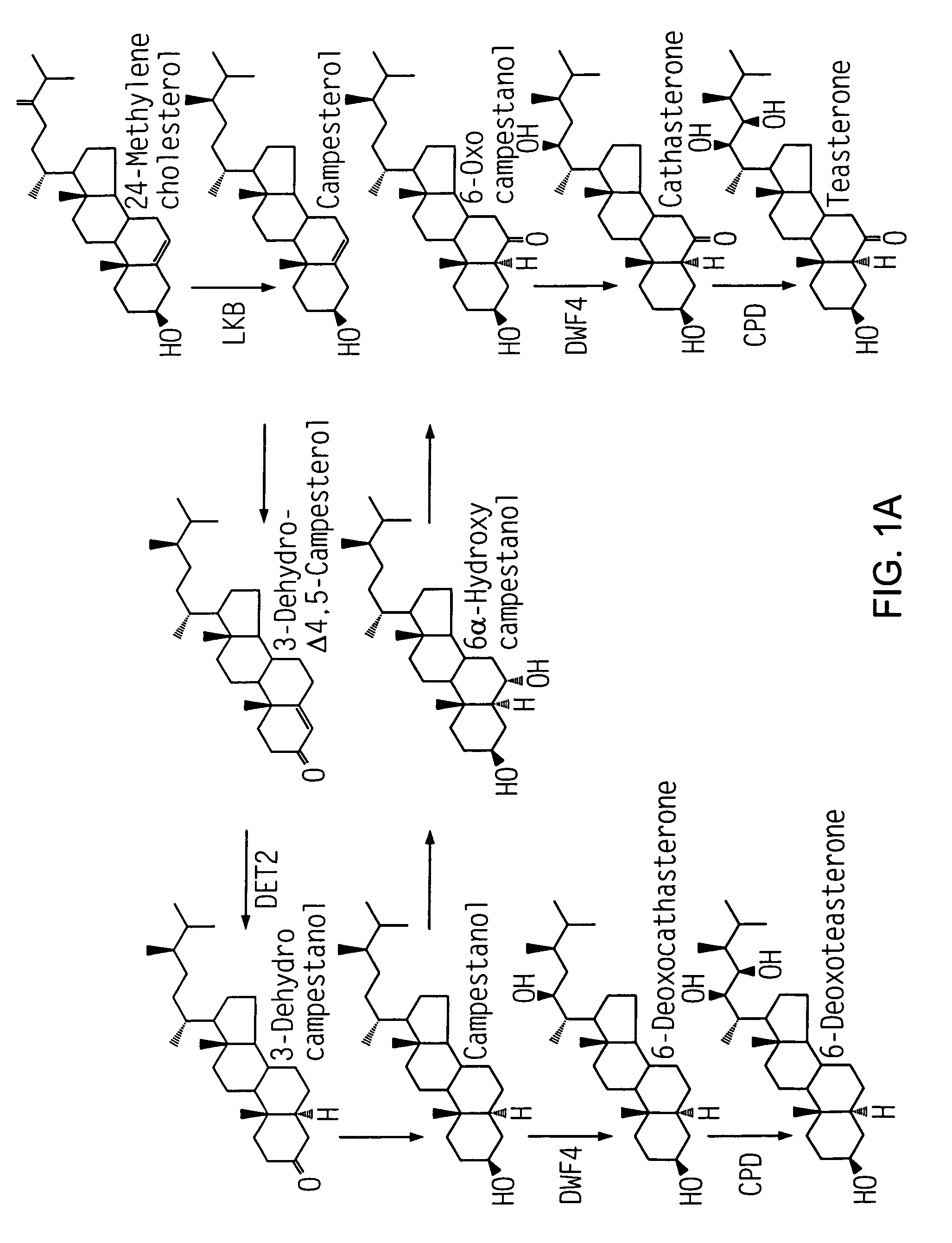
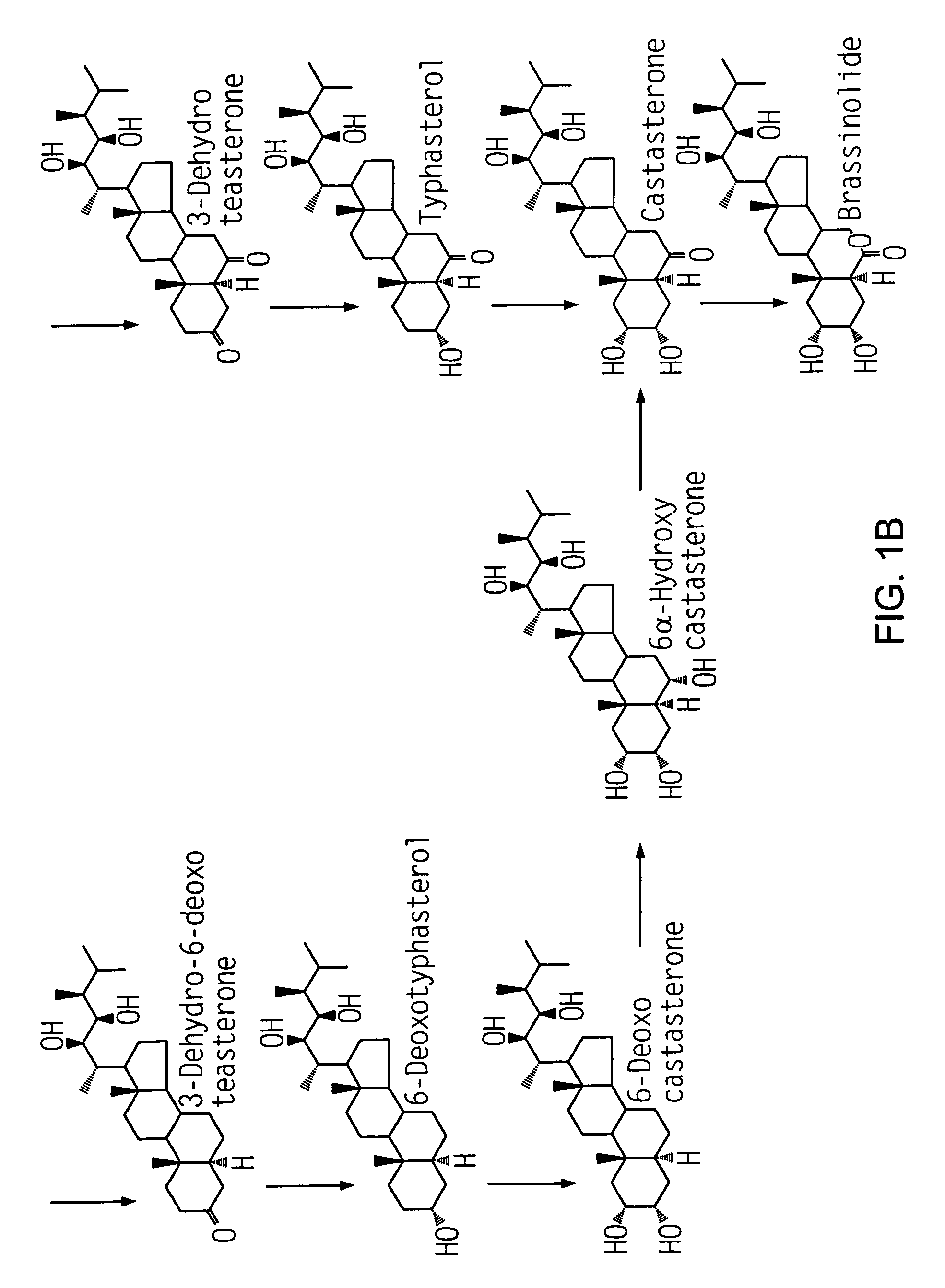
Dwf4 polynucleotides, polypeptides and uses thereof
The present invention relates to novel, polynucleotides isolated from dwarf plants. The dwf4 polynucleotides that encode all, or a portion of, a DWF4 polypeptide, a cytochrome P450 enzyme that mediates multiple steps in synthesis of brassinosteroids. The present invention also relates to isolated polynucleotides that encode regulatory regions of dwf4. Uses of the dwf4 polypeptides and polynucleotides are also disclosed.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
NOVEL 7Beta-HYDROXYSTEROID DEHYDROGENASE MUTANTS AND PROCESS FOR THE PREPARATION OF URSODEOXYCHOLIC ACID
The invention relates to novel 7β-hydroxysteroid dehydrogenase mutants, to the sequences which encode these enzyme mutants, to processes for the preparation of the enzyme mutants and to their use in enzymatic reactions of cholic acid compounds, in particular in the preparation of ursodeoxycholic acid (UDCS). The invention also relates to novel processes for the synthesis of UDCS using the enzyme mutants; and to the preparation of UDCS using recombinant, multiply-modified microorganisms.
Owner:PHARMAZELL GMBH
Novel 7alpha-hydroxysteroid dehydrogenase knockout mutants and use thereof
The invention relates to novel microbial 7alpha-hydroxysteroid dehydrogenase (7alpha-HSDH) knockout mutants and to the use thereof for producing other HSDHs having various functionalities, such as 3alpha-, 7beta- or 12alpha-HSDH, and to the use of thus-produced HSDH enzymes in enzymatic reactions of cholic acid compounds, and in particular for producing ursodeoxycholic acid (UDCS). The invention relates in particular to novel methods for synthesizing UDCS.
Owner:CELL PHARM CO LTD
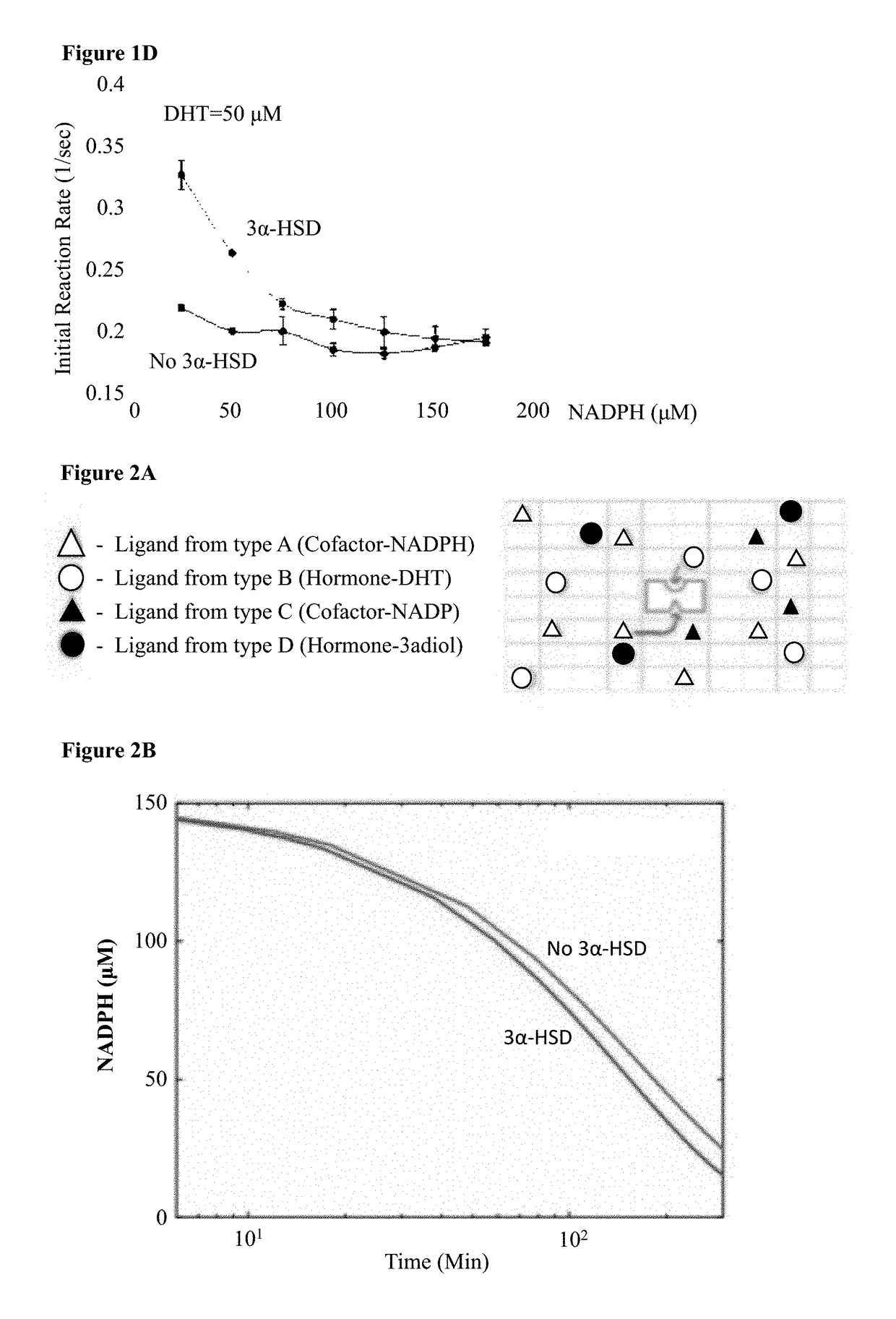
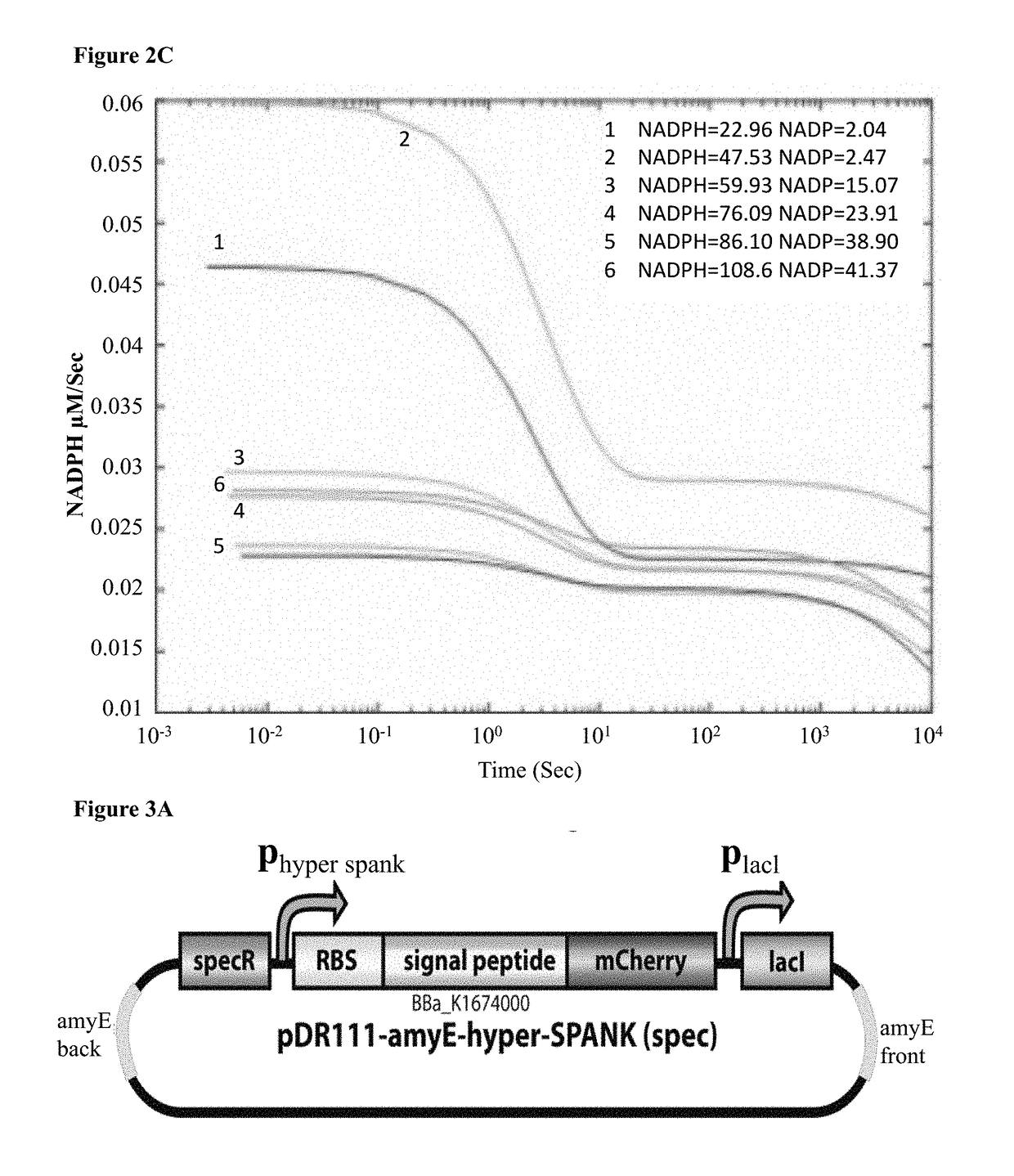
Composition and method for treating androgen-dependent disorders
ActiveUS20170275596A1Peptide/protein ingredientsPharmaceutical delivery mechanismDiseaseMicroorganism
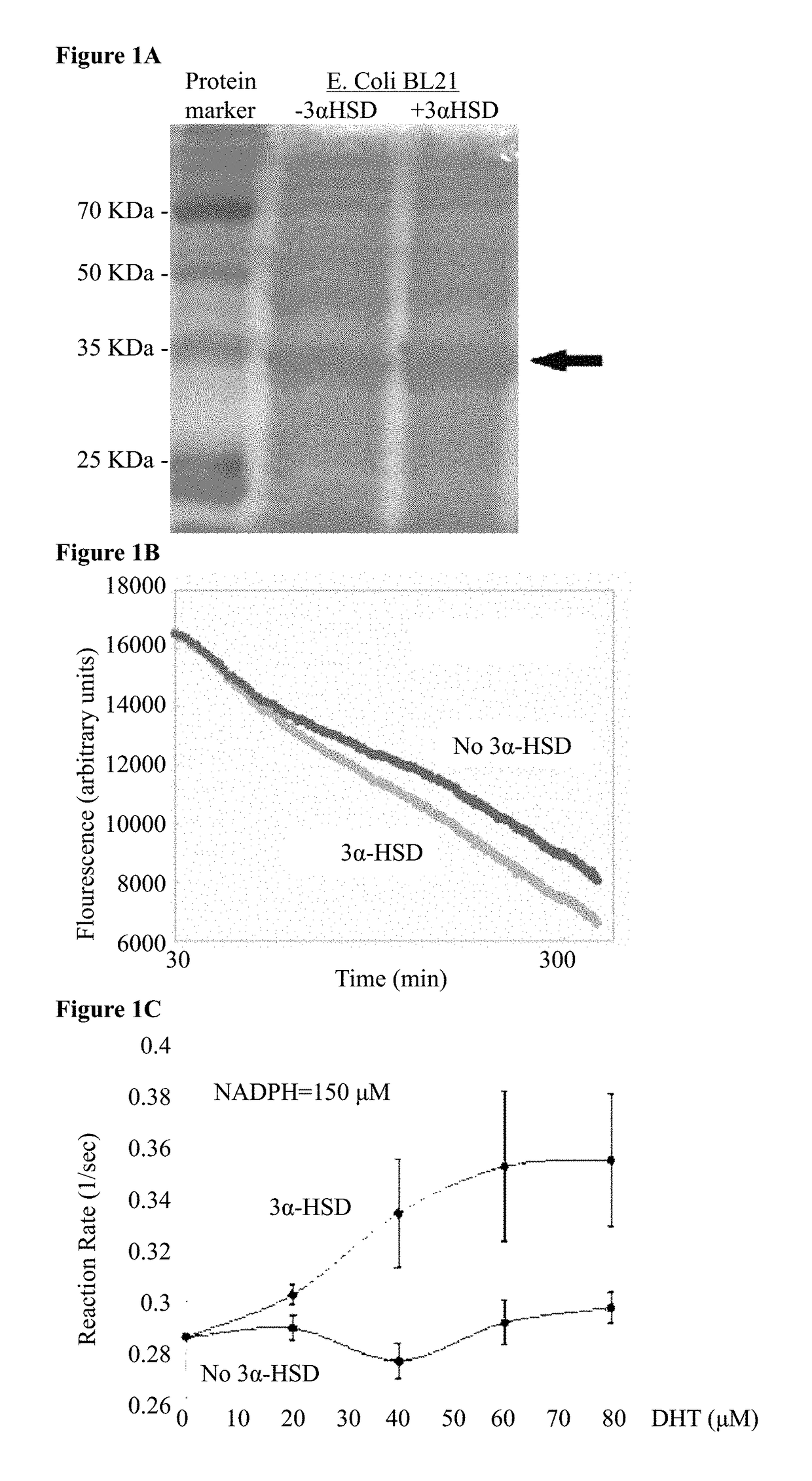
Chimeric polypeptides comprising a dihydrotestosterone (DHT) reductase moiety, such as 3 alpha-hydroxysteroid dehydrogenase (3α-HSD), fused to a signal peptide moiety, polynucleotides encoding same, and compositions comprising at least one microorganism cell capable of secreting the chimeric polypeptide, are provided. Further provided are methods and kits for treating, preventing or ameliorating androgen-dependent disorders, including but not limited to androgenic alopecia.
Owner:TECHNION RES & DEV FOUND LTD
Triazolopyridine 11-beta hydroxysteroid dehydrogenase type i inhibitors
Novel compounds are provided which are 11-beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds of formula I:or stereoisomers or pharmaceutically acceptable salts thereof, wherein G, Q, X, Y, R3, R3a, and R3b are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
7alpha-hydroxysteroid dehydrogenase (St-2-2) mutants
ActiveCN111254126AHigh catalytic efficiencyBacteriaMicroorganism based processesHydroxysteroid DehydrogenasesHomosteroids
The invention relates to hydroxysteroid dehydrogenase, and in particular to 7alpha-hydroxysteroid dehydrogenase (St-2-2) mutants. The amino acid sequences of the mutants are shown as SEQ ID NO:2, 3, 4, 5, 6, 7, 8, 9 or 10, and are obtained by changing the 255th amino acid of the 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence of SEQ ID NO:1 from Ile to Tyr, Gln, Leu, Thr, Gly, Asn,Ser, Ala or Phe. In the presence of same substrates TCDCA and NADP<+>, enzyme activity of the mutants is respectively 1.49, 1.78, 1.79, 1.79, 1.93, 2.44, 2.58, 2.97 and 3.34 times of enzyme activityof a wild type hydroxysteroid dehydrogenase, and the mutant has a great application potential in a process of obtaining tauroursodeoxycholic acid (TUDCA) through biotransformation of taurochenodeoxycholic acid (TCDCA).
Owner:CHONGQING UNIV
Gene S1-a-1 of novel 7 alpha-HSDH (hydroxysteroid dehydrogenase)
ActiveCN106701707ACatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
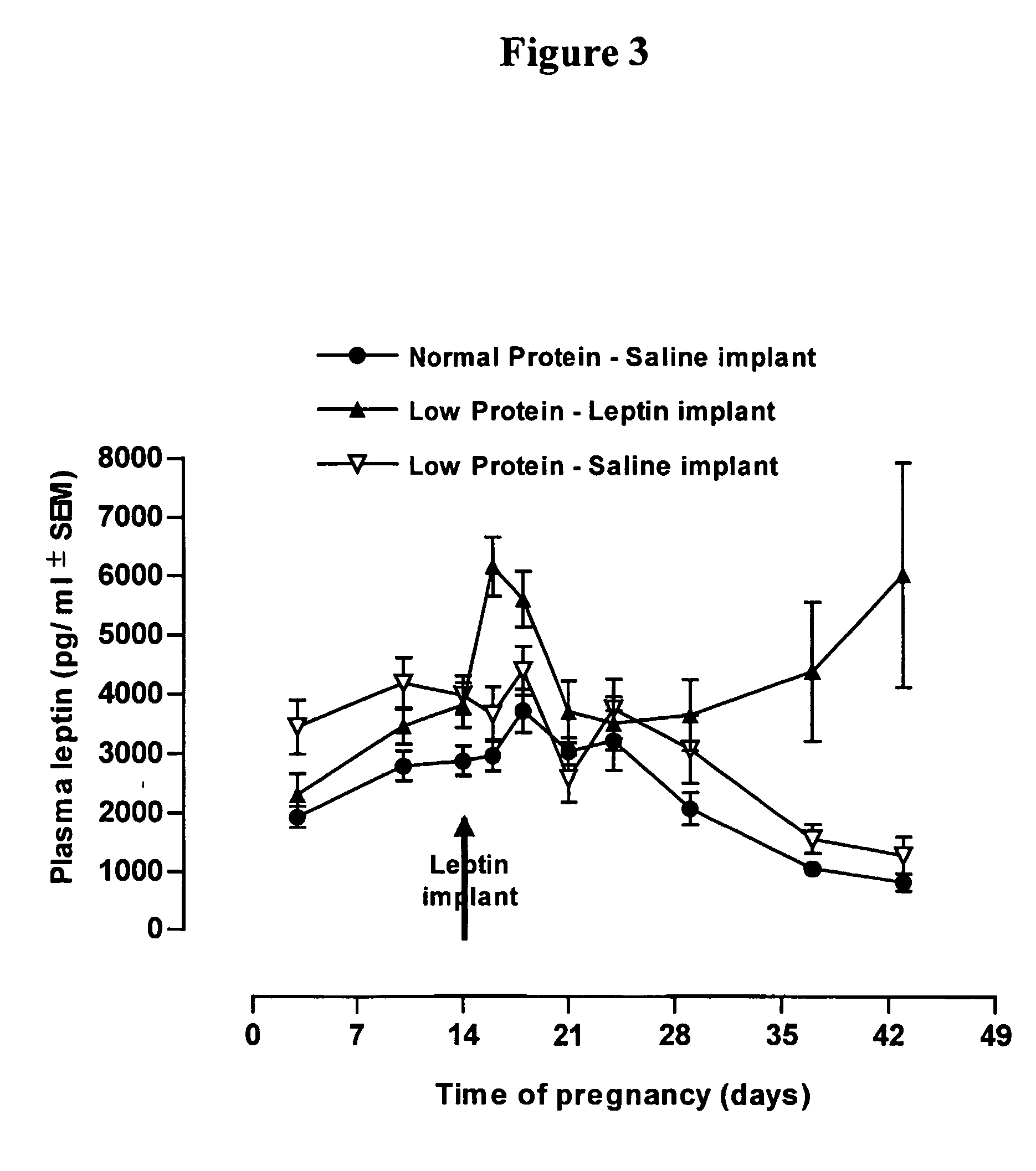
Use of leptin for infant with low birth weight for prevention of obesity
InactiveUS20050065078A1Avoid developmentEfficiently provideMilk preparationPeptide/protein ingredientsObesity preventionPediatrics
The invention discloses the therapeutic and prophylactic administration of leptin to (i) an infant of low birth weight for age; (ii) a nursing mother of an infant, the infant having low birth weight for age; or (iii) a pregnant female predisposed to giving birth to an infant of low birth weight for age; for the prevention or treatment, in later life of the infant, of a metabolic disorder or other condition associated with low birth weight, such as type 2 diabetes, obesity, cardiovascular disease, gestational diabetes, impaired glucose tolerance, insulin resistance, hypertension or syndrome X. It is thought that the beneficial properties of leptin may be due at least in part to an effect on glucocorticoid metabolism, in particular to an increase in type 2 11β-hydroxysteroid dehydrogenase activity.
Owner:CAWTHORNE ANTHONY MICHAEL +1
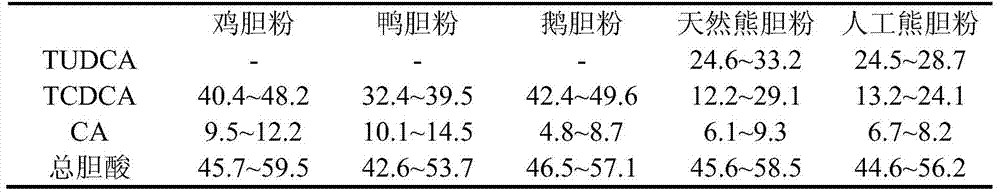
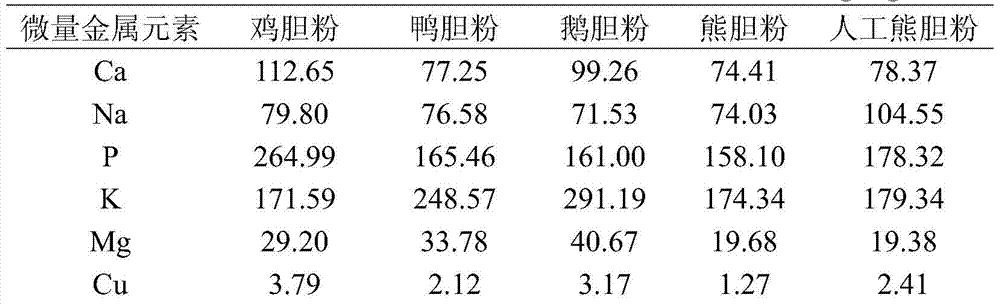
A kind of artificial bear bile powder and preparation method thereof
ActiveCN104382941BEasy to processGallbladder source is easy to getSenses disorderAntipyreticEnterohepatic circulationNatural source
Owner:SHANGHAI KAIBAO PHARMA
Novel 7 alpha-Hydroxysteroid Dehydrogenase Knockout Mutants and Use Therefor
The invention relates to novel microbial 7α-hydroxysteroid dehydrogenase (7α-HSDH) knockout mutants and to the use thereof for producing other HSDHs having various functionalities, such as 3α-, 7β- or 12α-HSDH, and to the use of thus-produced HSDH enzymes in enzymatic reactions of cholic acid compounds, and in particular for producing ursodeoxycholic acid (UDCS). The invention relates in particular to novel methods for synthesizing UDCS.
Owner:PHARMAZELL GMBH
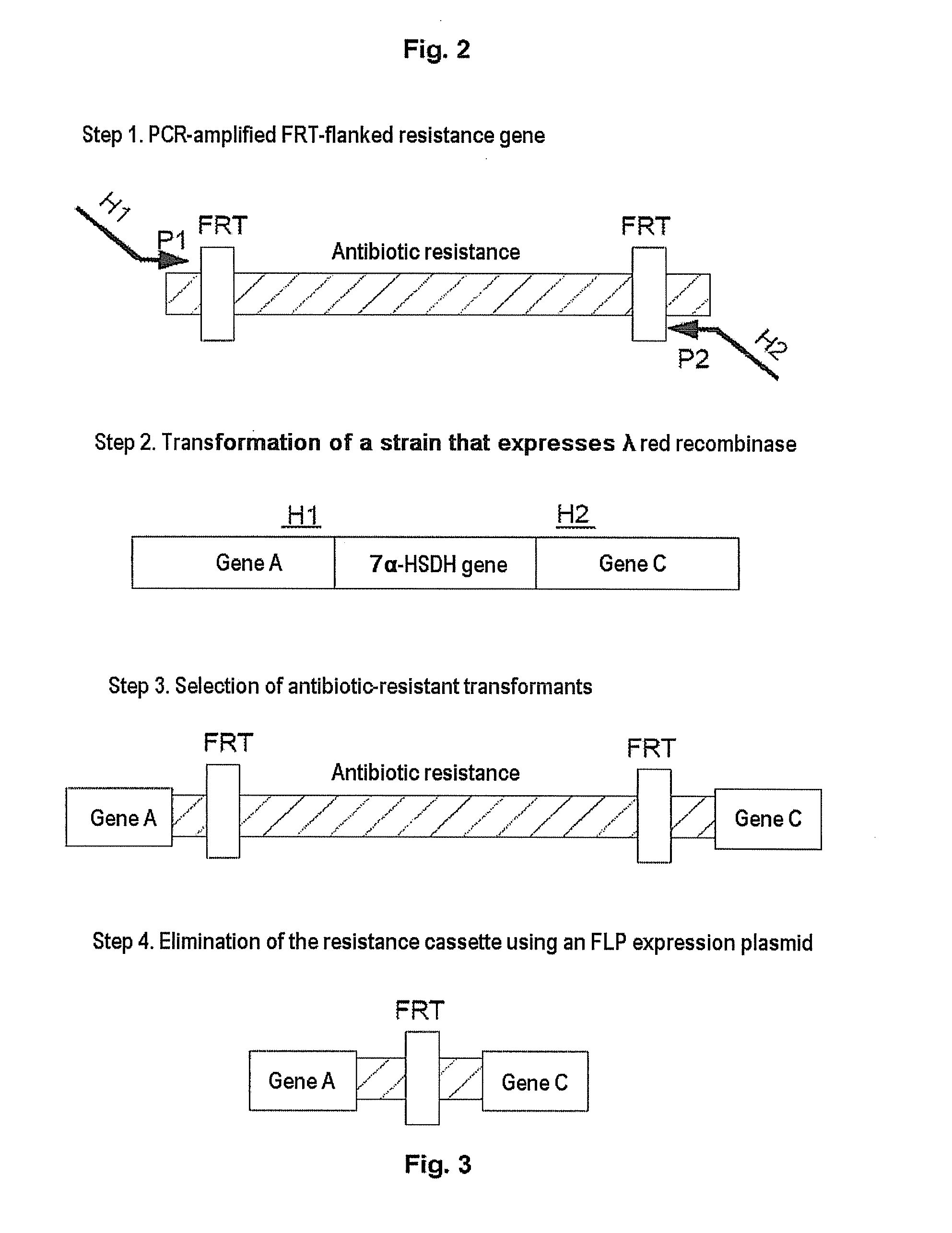
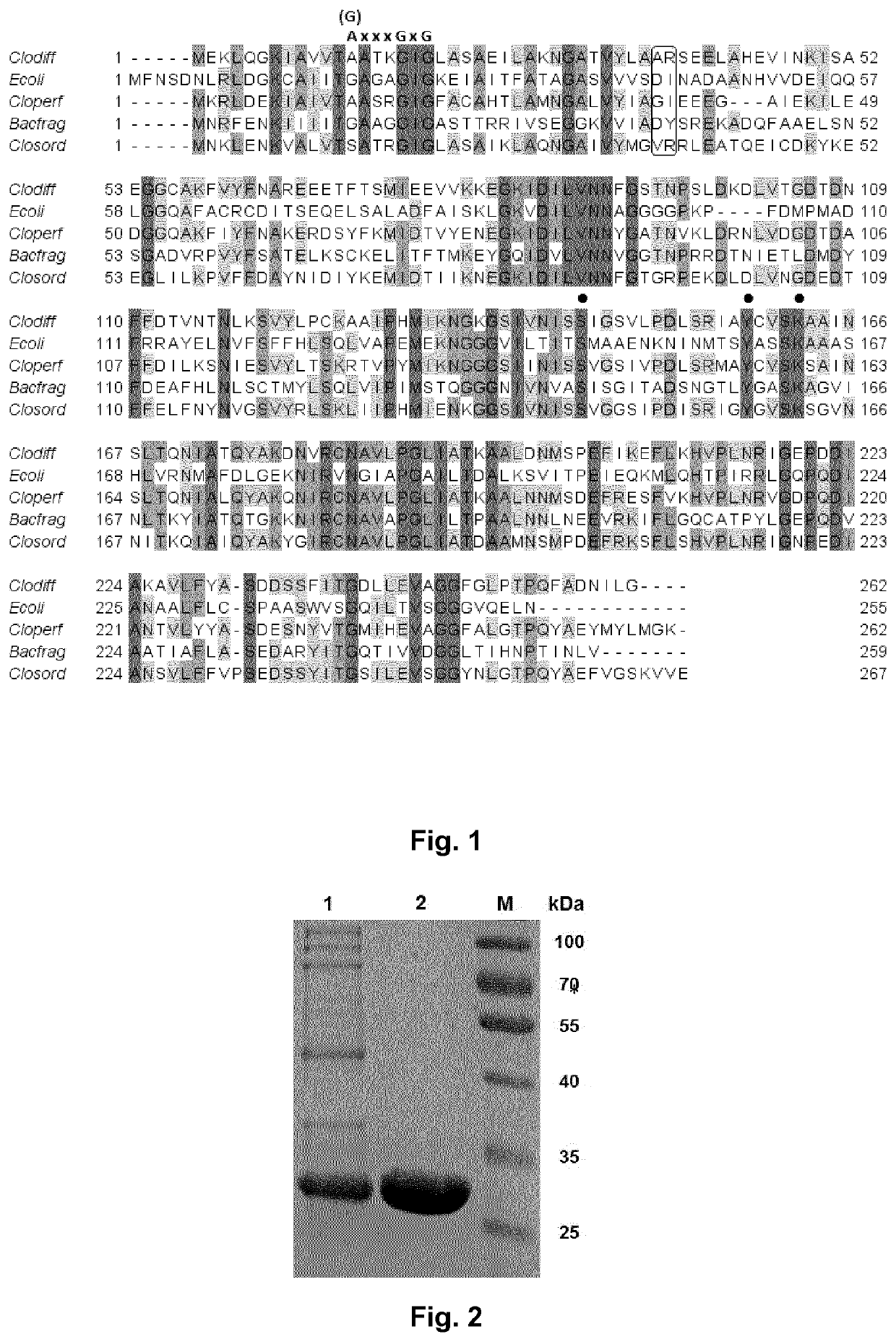
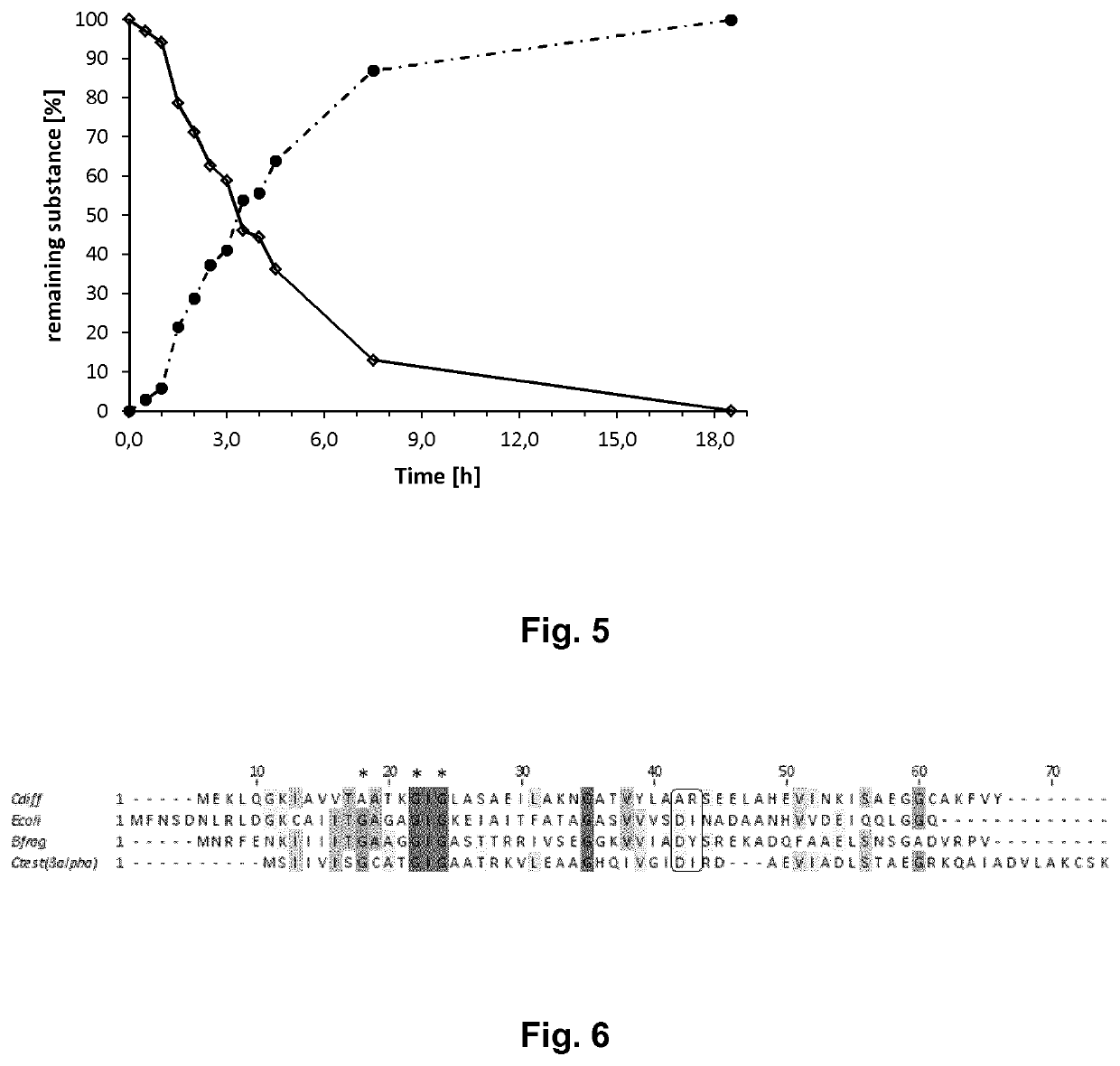
Coupled, Self-Sufficient Biotransformation of Chenodeoxcholic Acid to Ursodeoxycholic Acid and Novel Enzyme Mutants Applicable in said Process
The present invention relates to a coupled biotransformation process of converting chenodeoxycholic acid (CDCA) and related compounds to ursodeoxycholic acid (UDCA) and related compounds. It also relates to the cloning, expression, and biochemical characterization of a novel NADP+-dependent 7α-hydroxysteroid dehydrogenase (7α-HSDH) from Clostridium difficile, cofactor switch mutants thereof, and their application for the oxidation of bile acids. A further aspect of the invention relates to novel NADP-dependent cofactor switch mutants of the NADP+-dependent 7α-HSDH of E. coli and their application for the oxidation of bile acids.
Owner:PHARMAZELL GMBH
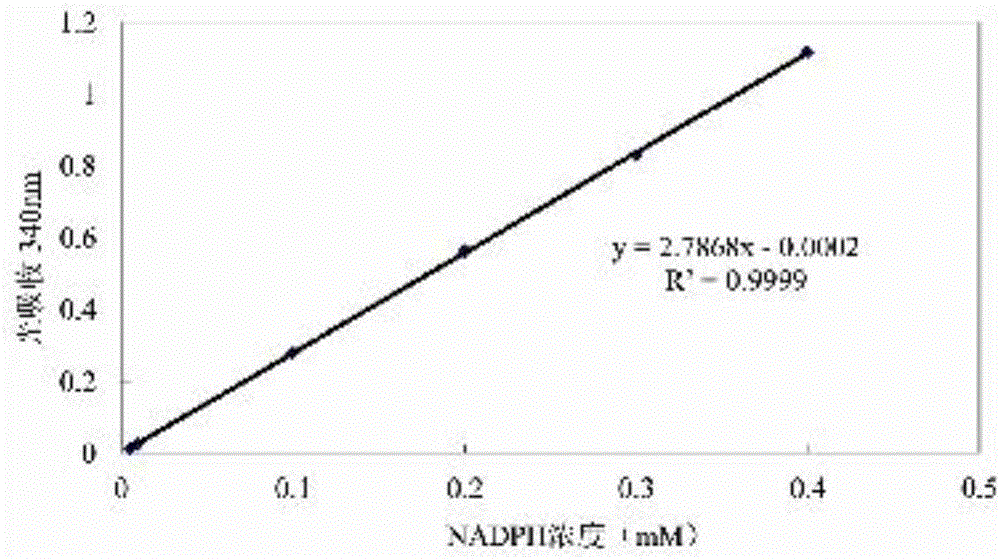
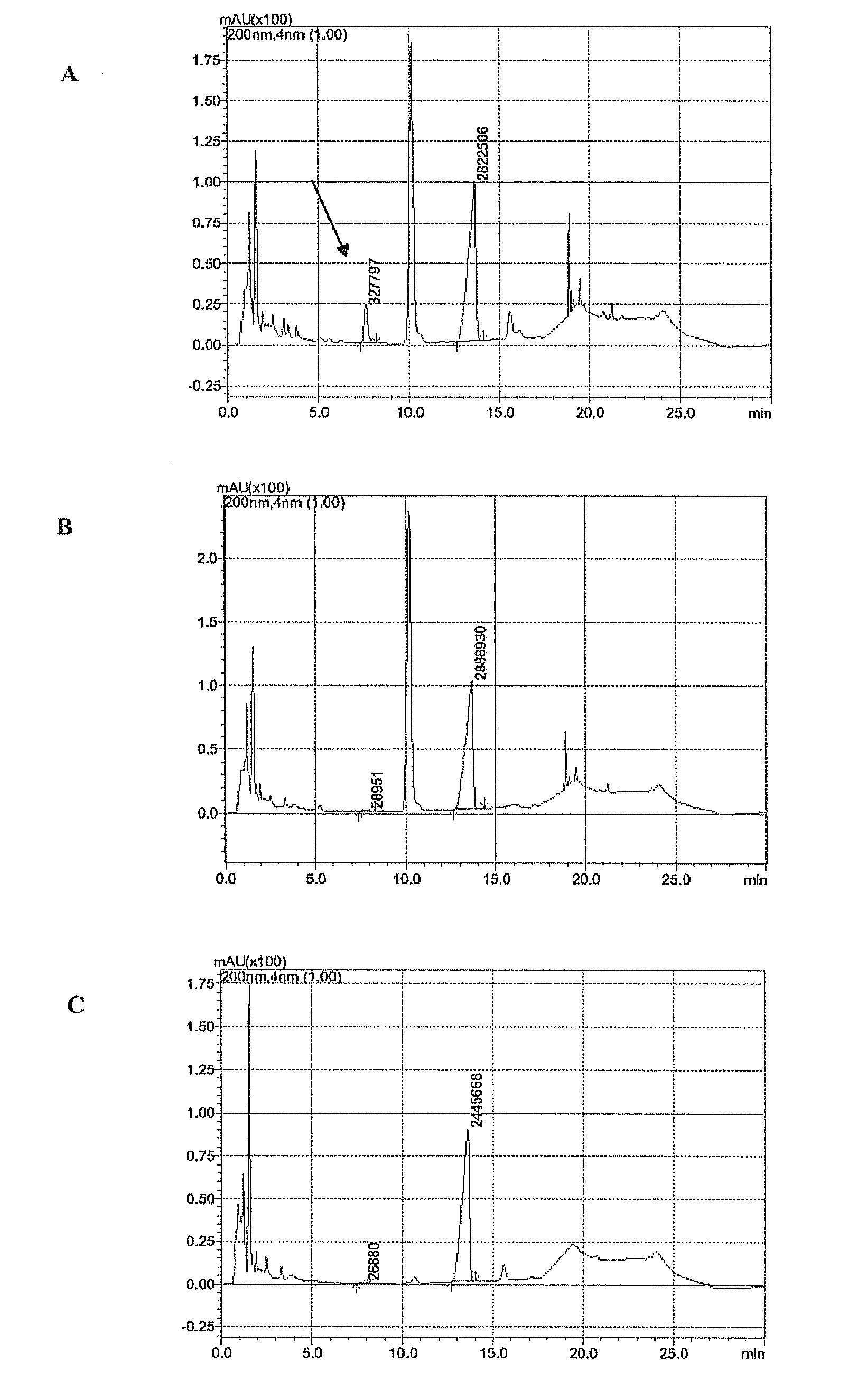
7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof
ActiveCN112175918ANo catalytic effectBacteriaMicroorganism based processesGlycineHydroxysteroid Dehydrogenases
The invention relates to hydroxysteroid dehydrogenase (HSDH), in particular to a 7[alpha]-HSDH mutant St-2-2 [delta]C10 and application thereof. The amino acid sequence of the 7[alpha]-HSDH mutant isshown as SEQ ID NO: 2, and the 7[alpha]-HSDH mutant is obtained by truncating 10 amino acids at the C end of 7[alpha]-HSDH with the amino acid sequence shown as SEQ ID NO: 1. The mutant has substrateselectivity, can specifically catalyze CDCA and taurine or glycine conjugates thereof, has no catalytic activity on CA and taurine or glycine conjugates thereof, can be used for efficiently synthesizing TUDCA from complex substrate chicken gall powder, does not generate toxic substances, namely by-product TUCA, and has huge application potential in the process of obtaining the TUDCA through specific biotransformation of the TCDCA.
Owner:CHONGQING UNIV
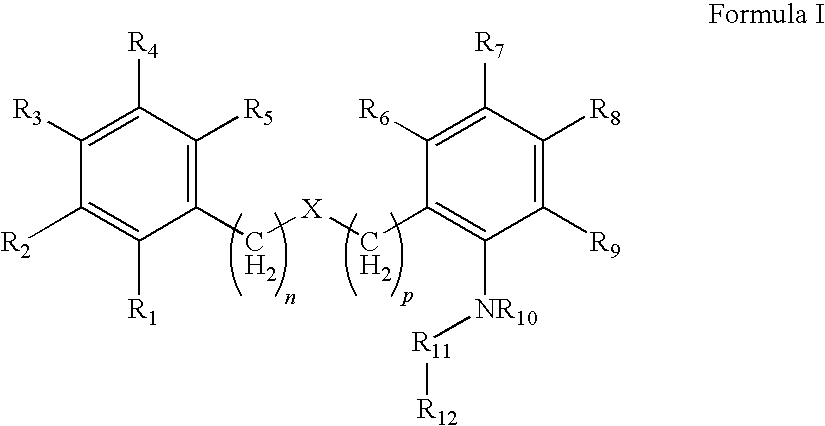
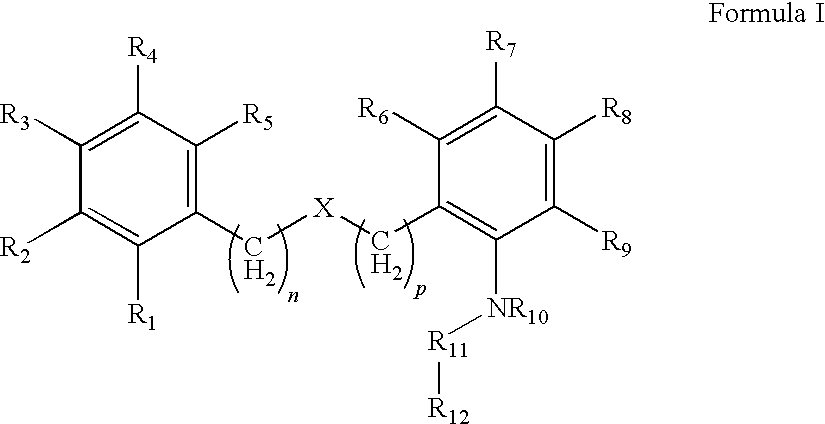
Compound capable of inhibiting 17-beta hydroxysteriod dehydrogenase
There is provided a compound having Formula Iwhereineach of R1, R2, R3, R4, R5, R6, R7, R8 and R9 are independently selected from(a) H, (b) R13, —OC(R13)3, —OCH(R13)2, —OCH2R13, C(R13)3, —CH(R13)2, or —CH2R13 wherein R13 is a halogen; (c) —CN; (d) optionally substituted alkyl, (e) optionally substituted heteroalkyl; (f) optionally substituted aryl; (g) optionally substituted heteroaryl; (h) optionally substituted arylalkyl; (i) optionally substituted heteroarylalkyl; (j) hydroxy; (k) alkoxy; (l) aryloxy; (m) —SO2-alkyl; and (n) —N(R14)C(O)R15,wherein R14 and R15 are independently selected from H and hydrocarbyl,wherein the optional substituents of (d) (e) (f) (h) and (i) are selected from the group consisting of: C1-6 alkyl, halo, cyano, nitro, haloalkyl, hydroxy, C1-6 alkoxy, carboxy, carboxyalkyl, carboxamide, mercapto, amino, alkylamino, dialkylamino, sulfonyl, sulfonamido, aryl and heteroaryl;wherein n and p are independently selected from 0 and 1;X is an optional group selected from O, S, S=0, S(═O)2, C═O, S(═O)2NR16, C═ONR17, NR18, in which R16, R17, and R18 are independently selected from H and hydrocarbyl,R10 is selected from H and hydrocarbyl,R11 is selected from CR19R20 and C═O, in which R19 and R20 are independently selected from H and hydrocarbyl,R12 is selected from a substituted five or six membered carbon rings optionally containing one or more hetero atoms selected from N, S, and O and optionally having fused thereto a further ring, and wherein the one or more substituents are selected from hydrocarbyl groups.
Owner:UNIVERSITY OF BATH
7 α-hydroxysteroid dehydrogenase knockout mutants and use therefor
The invention relates to 12 α-hydroxysteroid dehydrogenases, nucleic acid sequences coding for the same, expression cassettes and vectors, recombinant microorganisms containing the corresponding coding nucleic acid sequences, a method for producing said 12 α-hydroxysteroid dehydrogenases, a method for enzymatic oxidation of 12 α-hydroxysteroids using said enzyme, a method for enzymatic reduction of 12-ketosteroids using said enzyme, a method for qualitative or quantitative determination of 12-ketosteroids and / or 12α-hydroxysteroids using said 12α-hydroxysteroid dehydrogenases and a method for production of ursodesoxycholic acid, comprising the enzyme-catalyzed cholic acid oxidation using said 12 α-hydroxysteroid dehydrogenases.
Owner:PHARMAZELL GMBH
DNA sequence for expressing 3 alpha-hydroxysteroid dehydrogenase in Pseudomonas aeruginosa
InactiveCN104388440AHigh activityLow costMicrobiological testing/measurementOxidoreductasesEnzyme kineticsA-DNA
Owner:SICHUAN UNIV
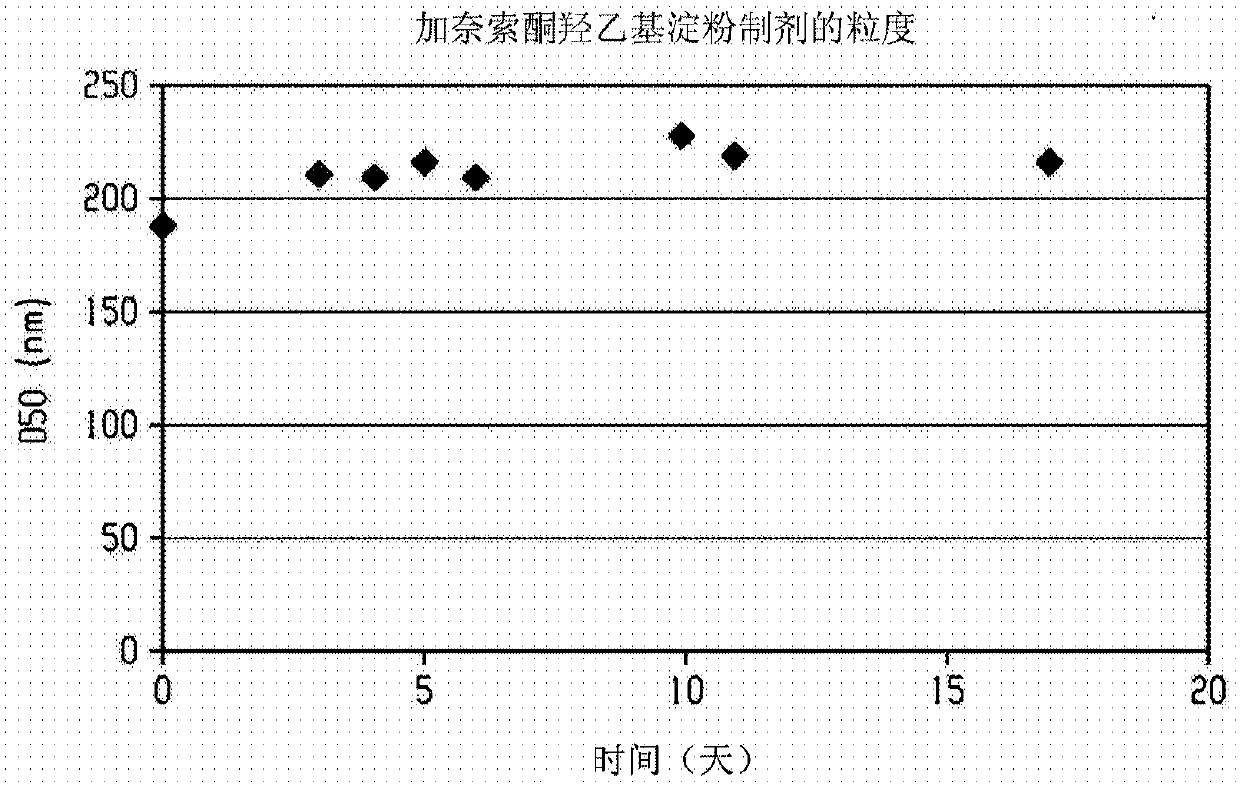
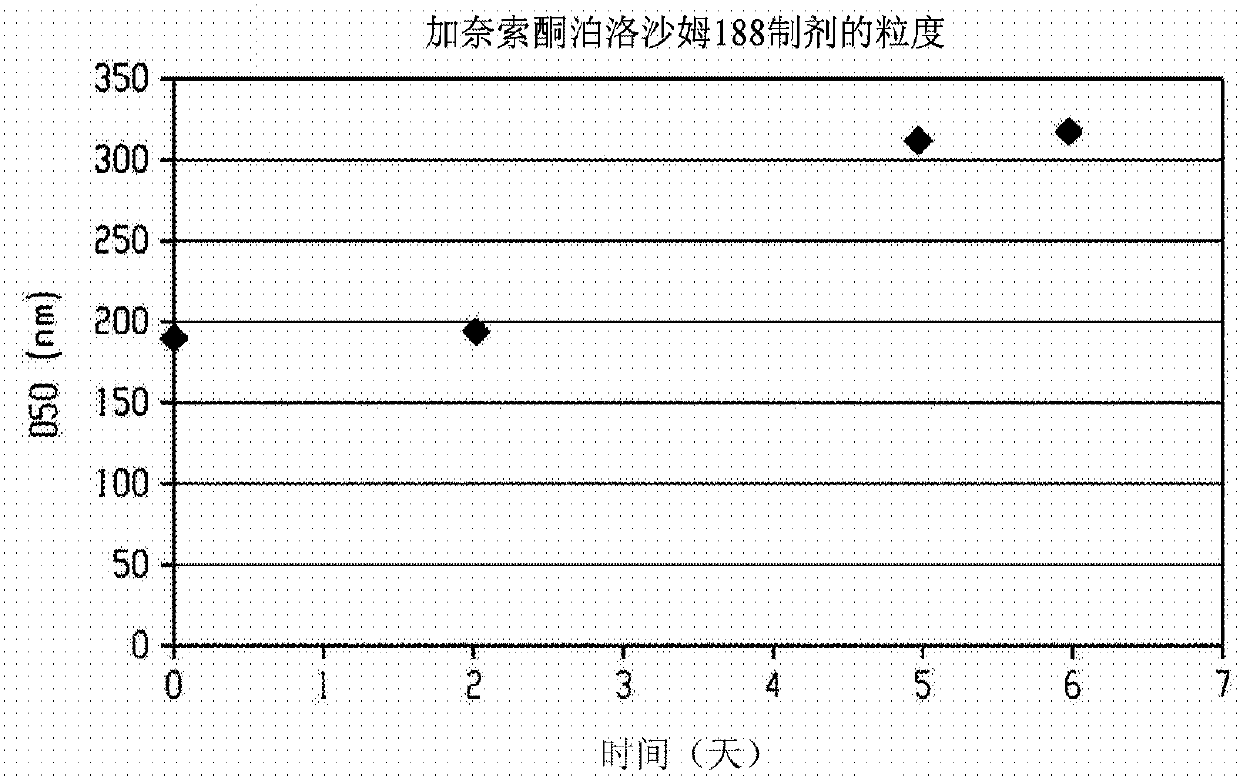
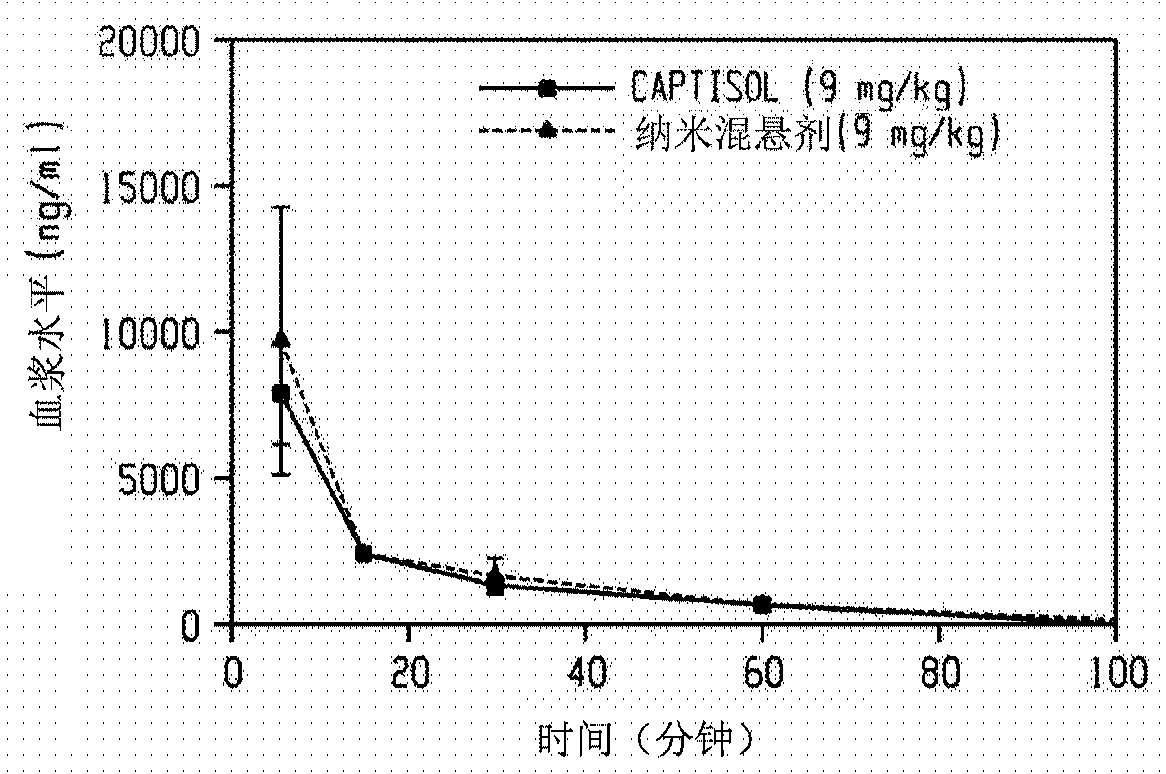
Injectable neurosteroid formulations containing nanoparticles
The disclosure provides an injectable neurosteroid nanoparticle formulation comprising nanoparticles having a D50 of less than 2000 nm the nanoparticles comprising a neurosteroid of Formula I, where the variables R1-R9 and X are defined herein and at least one surface stabilizer. The surface stabilizer can be a polymeric surface stabilizer such as hydroxyethyl starch, dextran, or povidone. The injectable neurosteroid nanoparticle formulation can be an intravenous formulation. The disclosure also provides a lyophilized powder of the injectable neurosteroid nanoparticle formulation that can be reconstituted in an aqueous solution prior to administration. The disclosure provides injectable neurosteroid nanoparticle formulations and dry powders of such formulations that have been sterilized byebeam irradiation. The disclosure provides a method of treating a patient having a seizure disorder, stroke, or traumatic brain injury, comprising administering an effective amount of the injectableneurosteroid nanoparticle formulation. The disclosure also provides combination methods in which the injectable neurosteroid nanoparticle formulation is a first active agent that is administered in combination with at least one additional active agent.
Owner:MARINUS PHARMA
Fused heterocyclic 11-beta-hydroxysteroid dehydrogenase type 1 inhibitors
ActiveUS20100144744A1Inhibitory activityBiocideSenses disorder11-beta-Hydroxysteroid DehydrogenasesDisease
Novel compounds are provided which are 1 1-beta-hydroxysteroid dehydrogenase type I inhibitors. 1 1-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 1 1-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds have the structure: W-L-Z or stereoisomers or prodrugs or pharmaceutically acceptable salts thereof, wherein W, L are defined herein and Z is selected from the following bicyclic heteroaryl groups: (a), (b), (c), (d).
Owner:BRISTOL MYERS SQUIBB CO
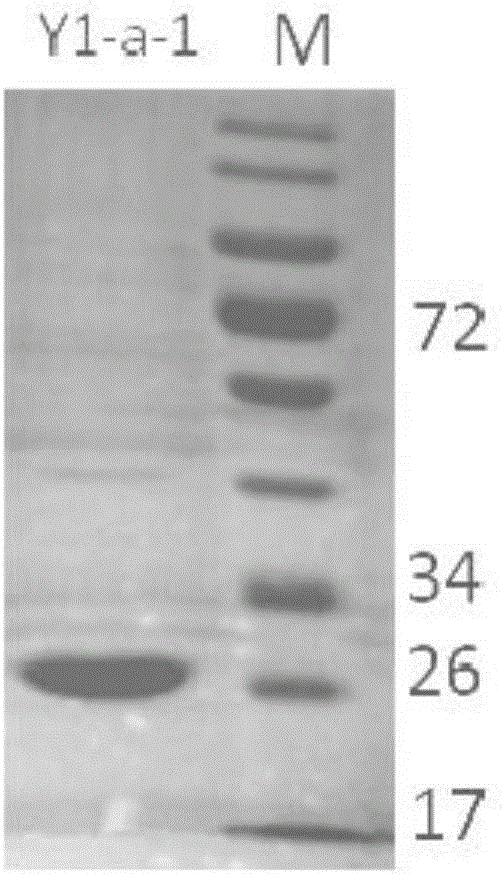
New 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1
ActiveCN106701708AHigh industrial application valueBetter than activityBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to hydroxysteroid dehydrogenase, in particular to a new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1. The nucleotide sequence of the gene is as shown in SEQ ID NO. 2. New 7alpha-hydroxysteroid dehydrogenase is coded, and the amino acid sequence of new 7alpha-hydroxysteroid dehydrogenase is as shown in SEQ ID NO.1; new 7alpha-hydroxysteroid dehydrogenase can catalyze chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA) to generate 7-ketone lithocholic acid (7K-LCA) and tauro 7-ketone lithocholic acid (T7K-LCA); the catalysis activity for CDCA or TCDCA is more than two times that of existing Sardinia clostridium 7alpha-HSDH, and the new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1 has an extremely large industrial application value.
Owner:CHONGQING UNIV
Liquid crystal aligning agent, method of producing a liquid crystal alignment film and liquid crystal display device
ActiveUS8216649B2Improve heat resistanceImprove electrical performanceLiquid crystal compositionsVacuum evaporation coatingArylLiquid-crystal display
The present invention relates to a liquid crystal aligning agent which contains a reaction product of at least one selected from the group consisting of a polysiloxane having a structure represented by the following formula (S-1), a hydrolysate thereof and a condensate of the hydrolysate and a compound represented by the following formula (1):(YI in the formula (S-1) is a hydroxyl group, an alkoxyl group having 1 to 10 carbon atoms, an alkyl group having 1 to 6 carbon atoms or an aryl group having 6 to 10 carbon atoms, R in the formula (1) is a group having an alkyl group with 4 to 20 carbon atoms, a fluoroalkyl group with 1 to 20 carbon atoms or a cyclohexyl group, or a group having 17 to 51 carbon atoms and a steroid skeleton, and XI in the formula (S-1) and Z in the formula (1) are reacted with each other to form a bond group for bonding the silicon atom in the formula (S-1) to R in the formula (1)).
Owner:JSR CORPORATIOON
7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA
PendingCN112029739AMeet the requirements of industrial productionCatalytic reaction specificityOxidoreductasesFermentationHigh-Throughput Screening MethodsHydroxysteroid Dehydrogenases
The invention relates to a 7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA, and belongs to the technical field of enzyme engineering. An amino acid sequenceshown in SEQ ID NO: 2 is obtained through mutant library construction and a high-throughput screening method. Key amino acids influencing the activity of 7[beta]-HSDH are determined through error-prone PCR, site-specific saturation mutagenesis is carried out, 200 mmol / L 7-KLCA99% can be converted into UDCA through the screened 7[beta] hydroxysteroid dehydrogenase mutant SEQ ID NO: 2, and the industrial production requirement is basically met.
Owner:JIANGXI BONTAC GREEN BIOCATALYSIS ECOIND PARK CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14c918c5-545e-4a7a-9616-6cdcaf0b3f40/US08546394-20131001-C00001.png)
![Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14c918c5-545e-4a7a-9616-6cdcaf0b3f40/US08546394-20131001-C00002.png)
![Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors Substituted [1,2,4]triazolo[4,3-A]pyrazine 11-beta-hydroxysteroid dehydrogenase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14c918c5-545e-4a7a-9616-6cdcaf0b3f40/US08546394-20131001-C00003.png)











![7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof 7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bcc3dd2-02c4-4dfb-bbcc-0a4b7627bd9d/HDA0002731273320000011.png)
![7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof 7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bcc3dd2-02c4-4dfb-bbcc-0a4b7627bd9d/HDA0002731273320000012.png)
![7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof 7[alpha]-hydroxysteroid dehydrogenase (HSDH) mutant St-2-2 [delta]C10 and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bcc3dd2-02c4-4dfb-bbcc-0a4b7627bd9d/HDA0002731273320000013.png)








![7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA 7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f48a951-25a7-4013-a59f-e02ce74504f5/HDA0002682535740000011.png)
![7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA 7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f48a951-25a7-4013-a59f-e02ce74504f5/HDA0002682535740000012.png)
![7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA 7[beta]-hydroxysteroid dehydrogenase mutant and application thereof in preparation of UDCA](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f48a951-25a7-4013-a59f-e02ce74504f5/HDA0002682535740000021.png)