Injectable neurosteroid formulations containing nanoparticles
A technology of steroids and nanoparticles, applied in medical preparations containing active ingredients, nervous system diseases, wave energy or particle radiation treatment materials, etc., can solve problems such as unapproved treatment of female children with epilepsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0275] Example 1: Preparation of Ganaxolone Nanosuspension (10% WT) by Wet Bead Milling
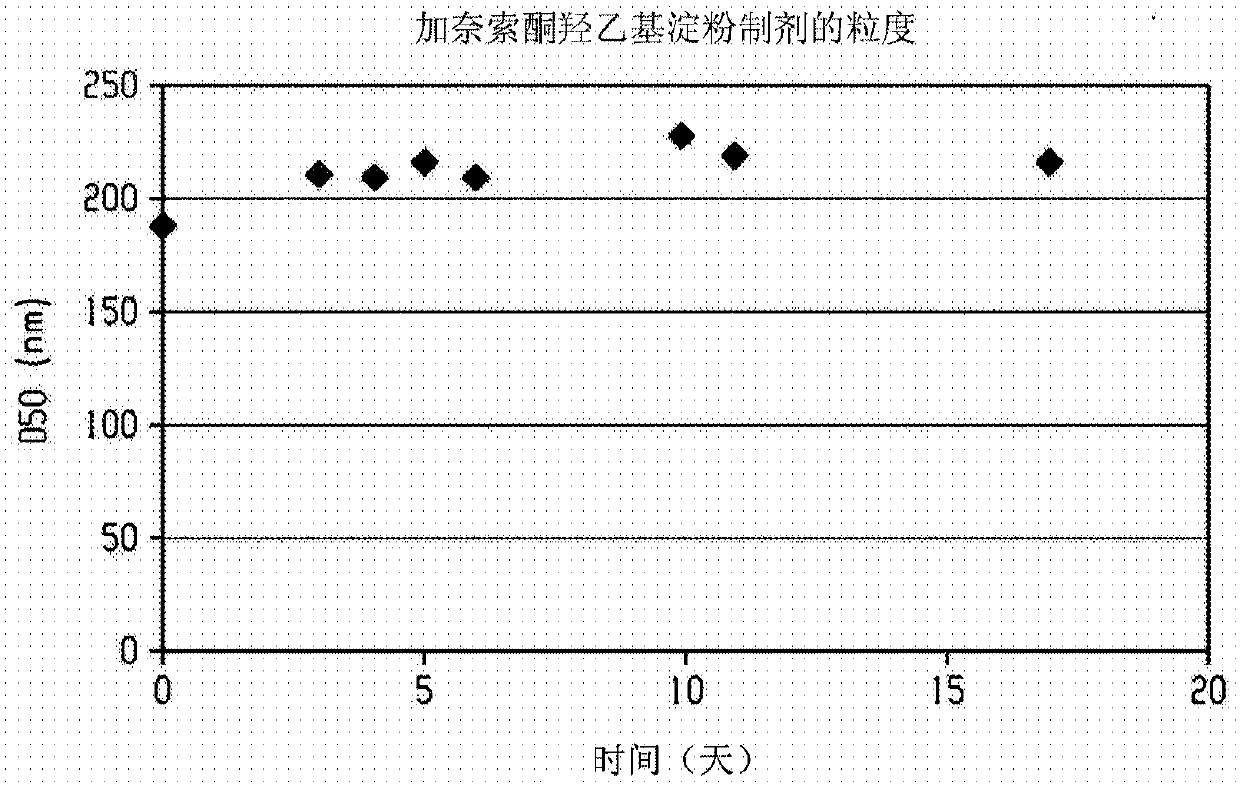
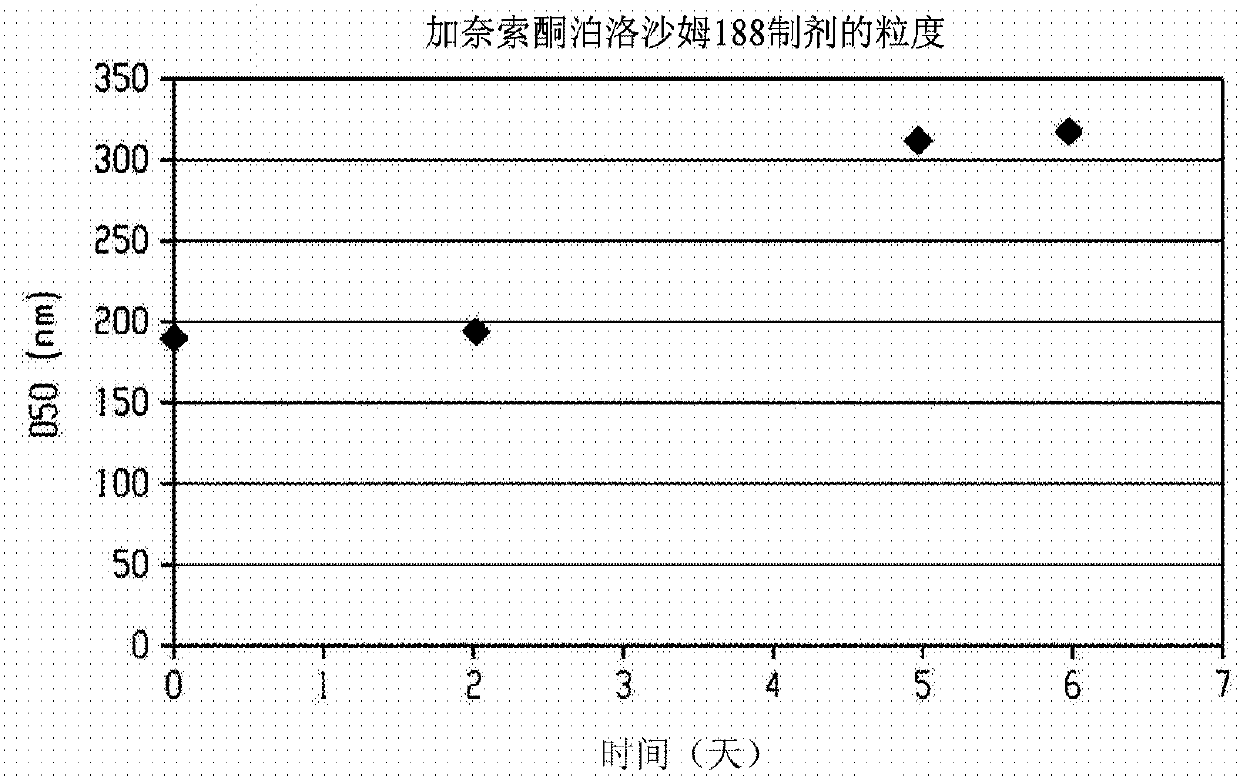
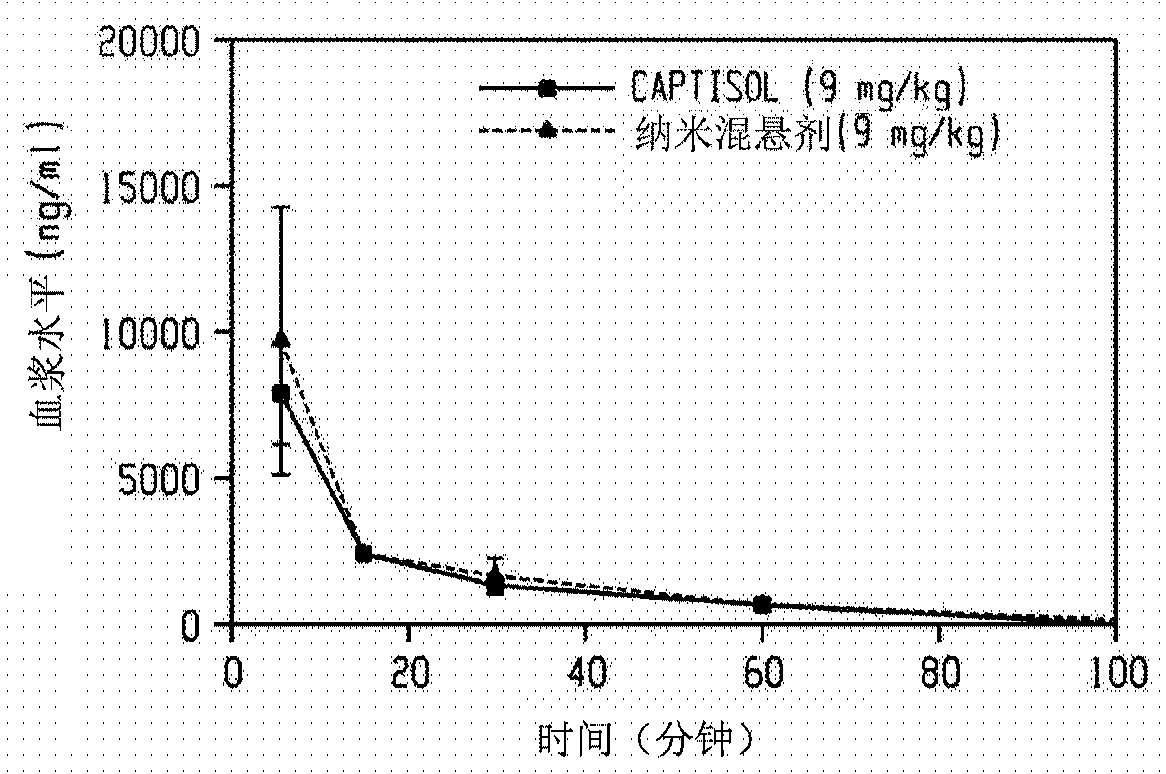
[0276] Use Netzsch Mill (Minicer) utilizing 0.3mm YTZ beads (yttrium stabilized grinding media, Tosoh Corporation, Japan, ZrO 2 +HfO 2 (95wt% (weight %)), Y 2 o 2 (5 wt %)) An aqueous slurry (250 g) containing ganaxolone (25 g), hydroxyethyl starch (7.5 g), sodium deoxycholate (0.5 g) and 30% simethicone (1 drop) was ground. Two additional portions of solid sodium deoxycholate (0.5 g each) were added 100 and 130 minutes after the start of milling. The particle size of the ground slurry was measured using a Horiba LA-910 laser diffraction particle size analyzer. After milling for 170 minutes, the D50 was 192 nm (188 nm after 1 minute sonication). At this point, grinding was stopped and the ground slurry was kept at room temperature overnight. The next morning, grinding was resumed until a total grinding time of 320 minutes was reached, at which point the D50 was 167 nm (169 nm after ...
Embodiment 2
[0277] Example 2. Preparation of Ganaxolone Nanosuspension (20% WT) by Wet Bead Milling
[0278] An aqueous slurry containing Ganaxolone (50 g), Hetastarch (15 g), Sodium Deoxycholate (3 g) and 30% Simethicone (0.15 g) was milled with 0.3 mm YTZ beads using a Netzsch Mill (Minicer) (250g) 240 minutes. The D50 of the milled slurry was 189 nm (185 nm after 1 minute sonication).
Embodiment 3
[0279] Example 3: Preparation of Ganaxolone Nanosuspension (20 wt %) by Wet Bead Milling Using 0.2mm YTZ Beads
[0280] An aqueous ganaxolone slurry having the same composition as described in Example 2 was milled for 245 minutes using a Netzsch Mill (Minicer) with 0.2 mm YTZ beads. D50 was 172nm (167nm after 1 min sonication).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com