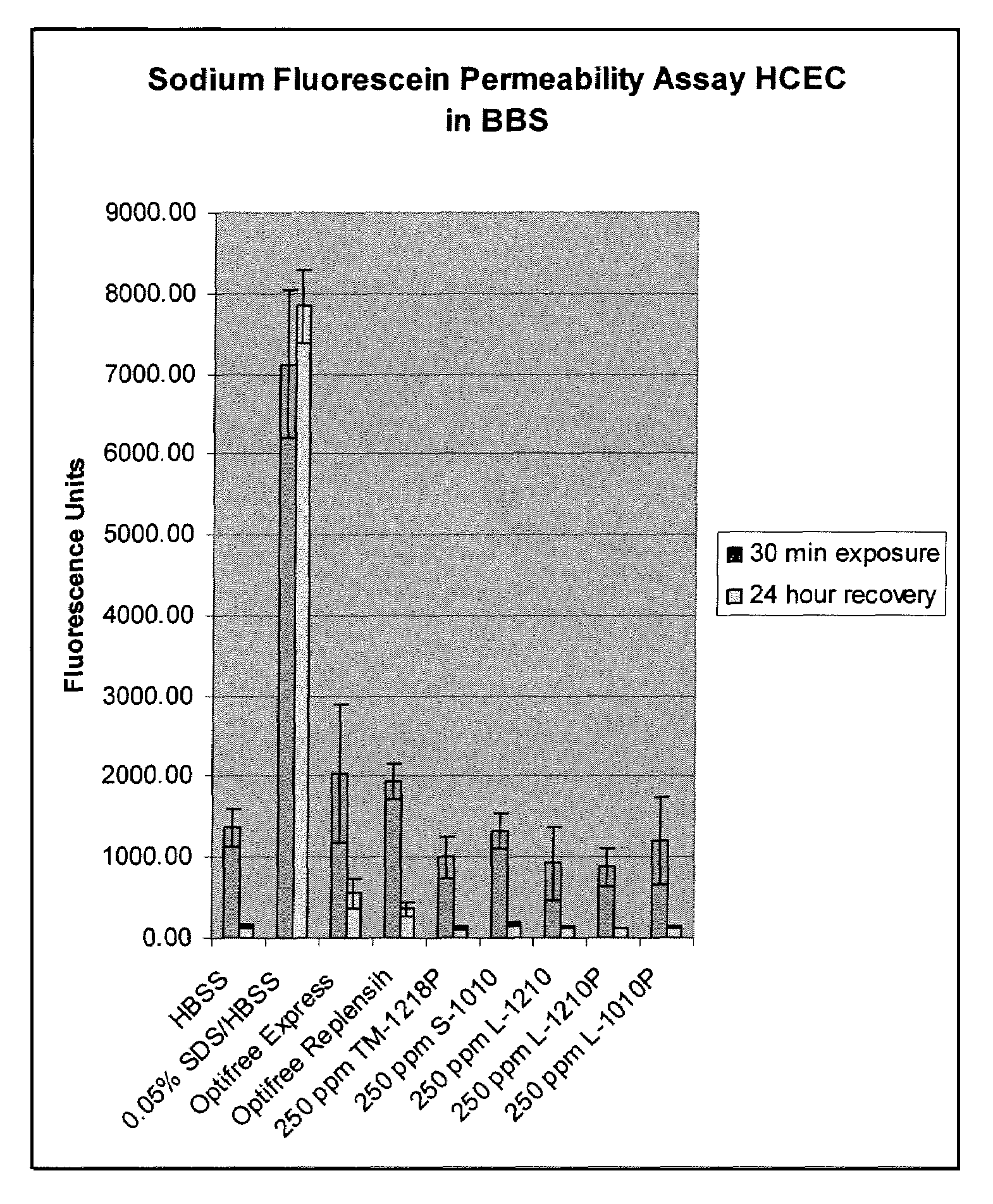
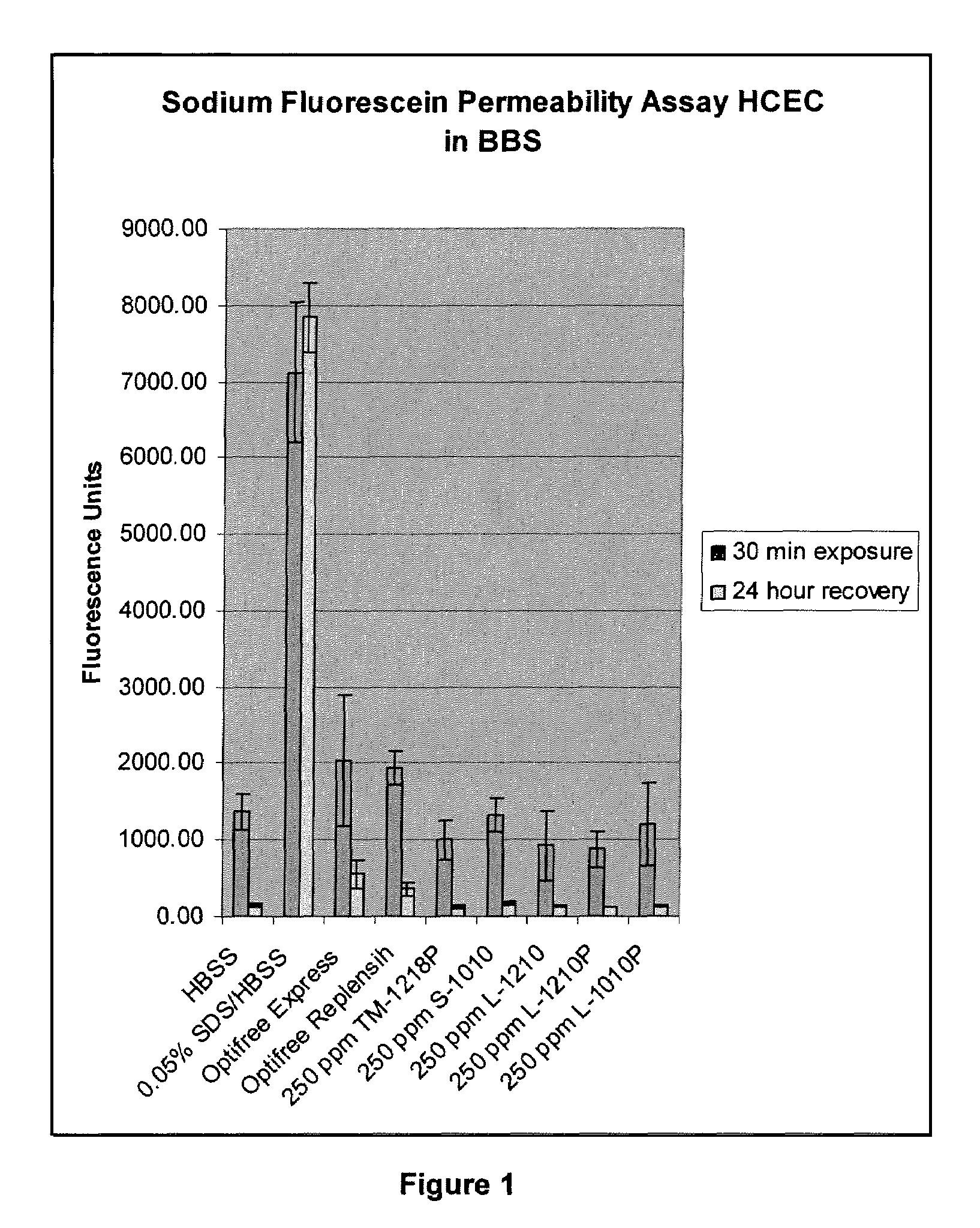
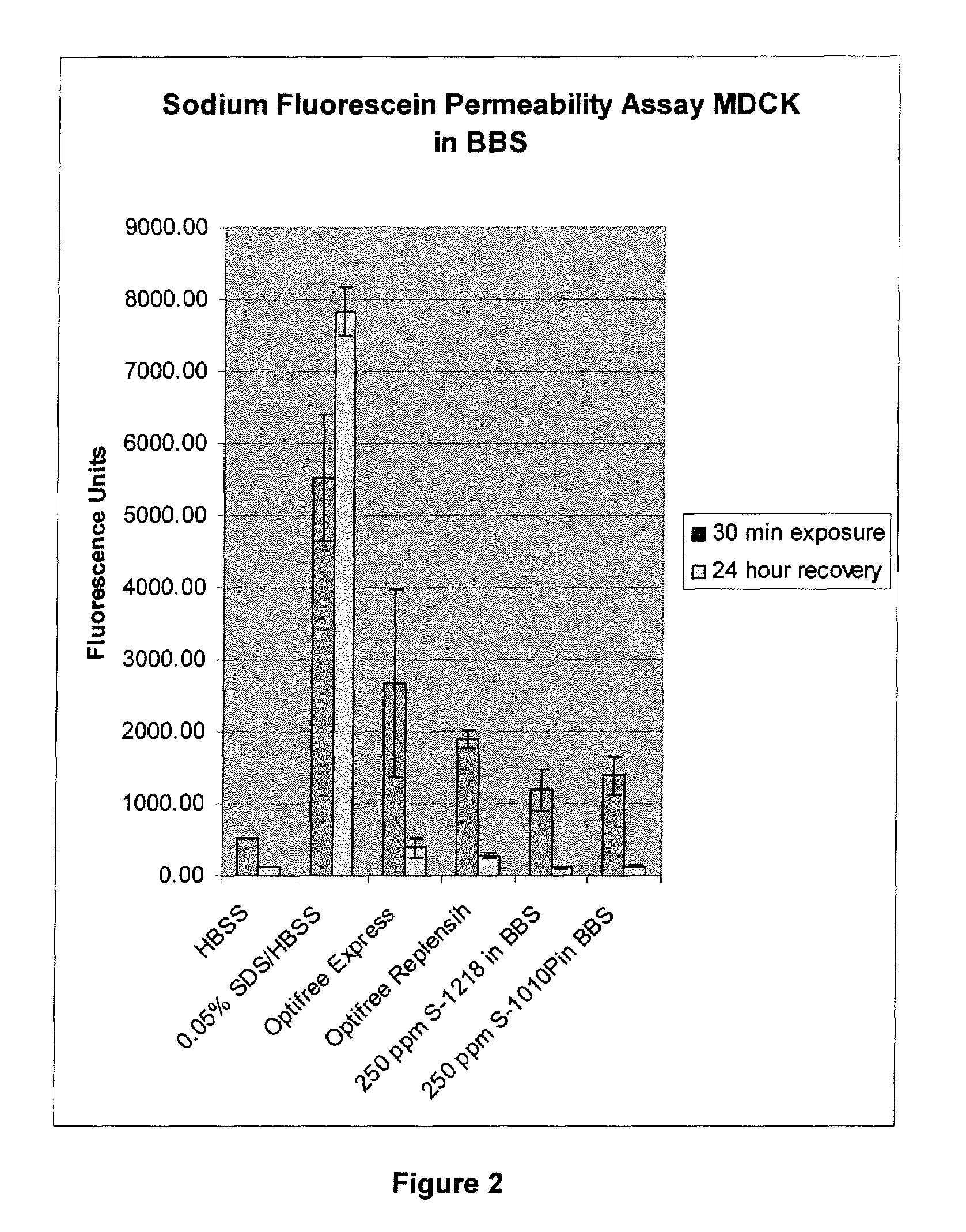
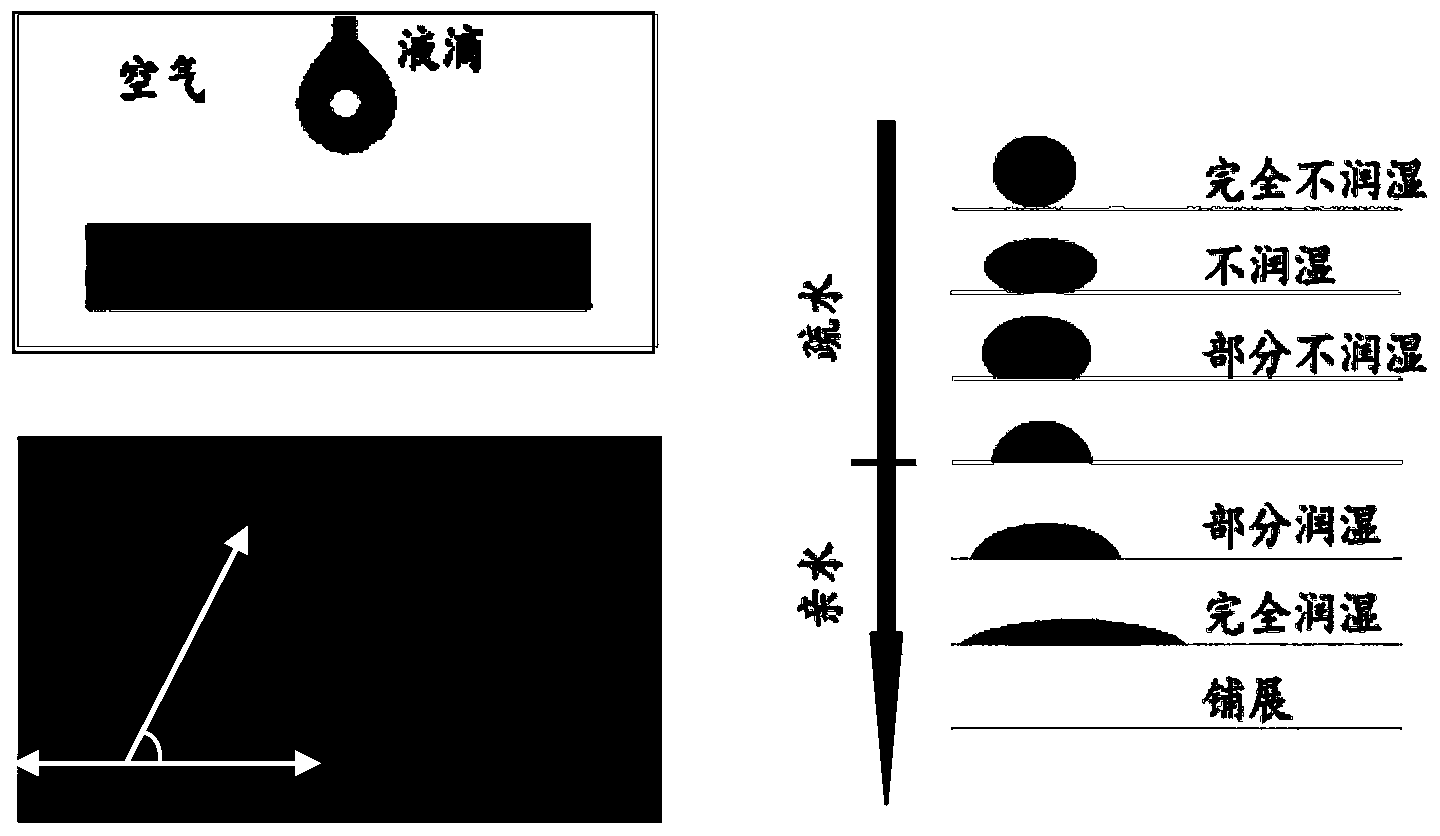
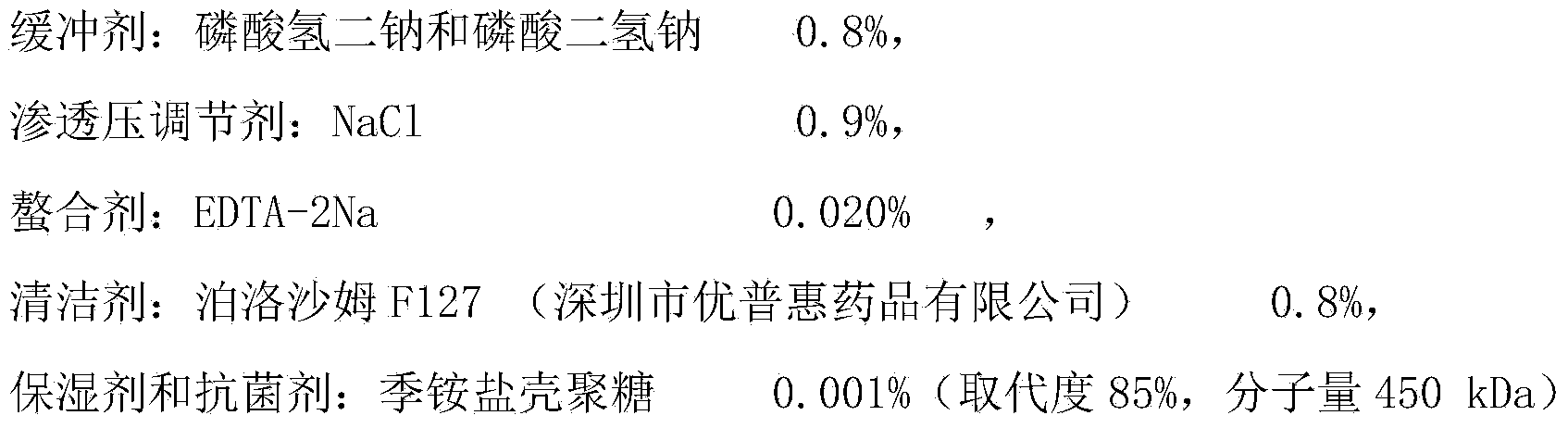
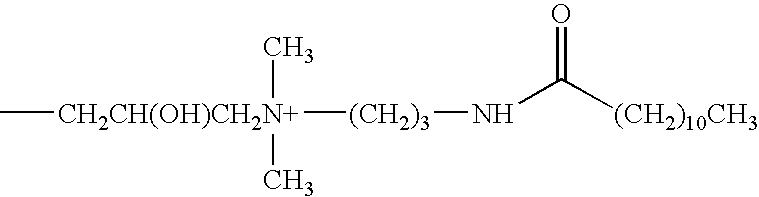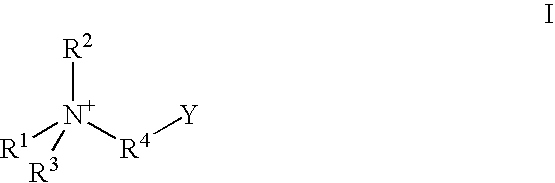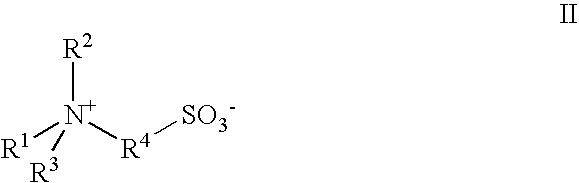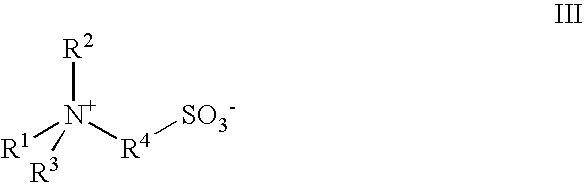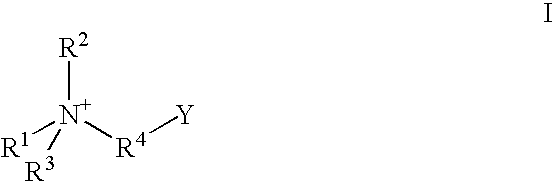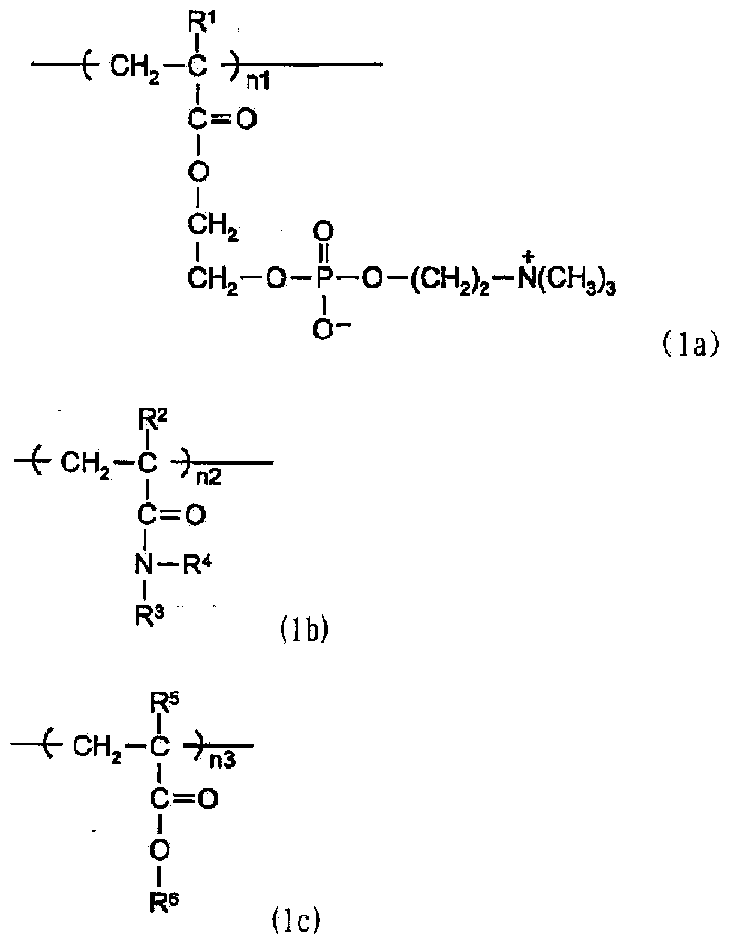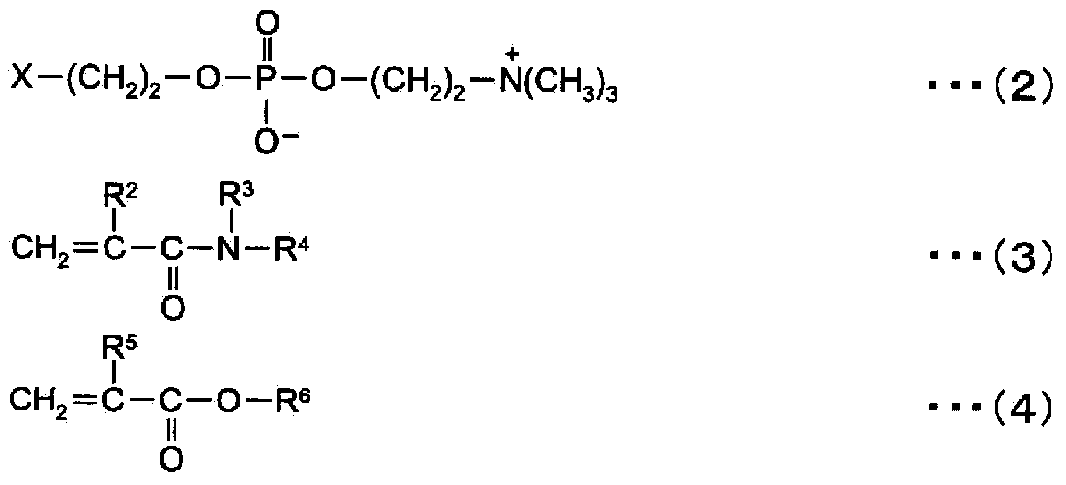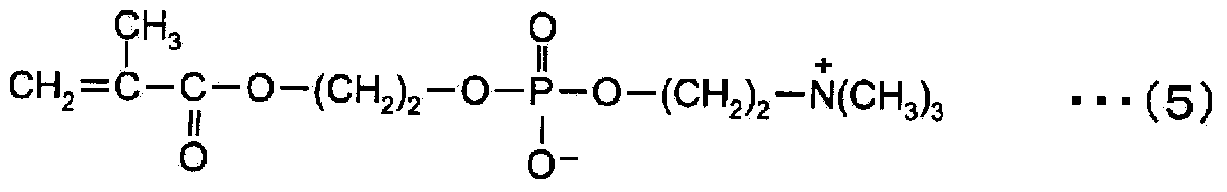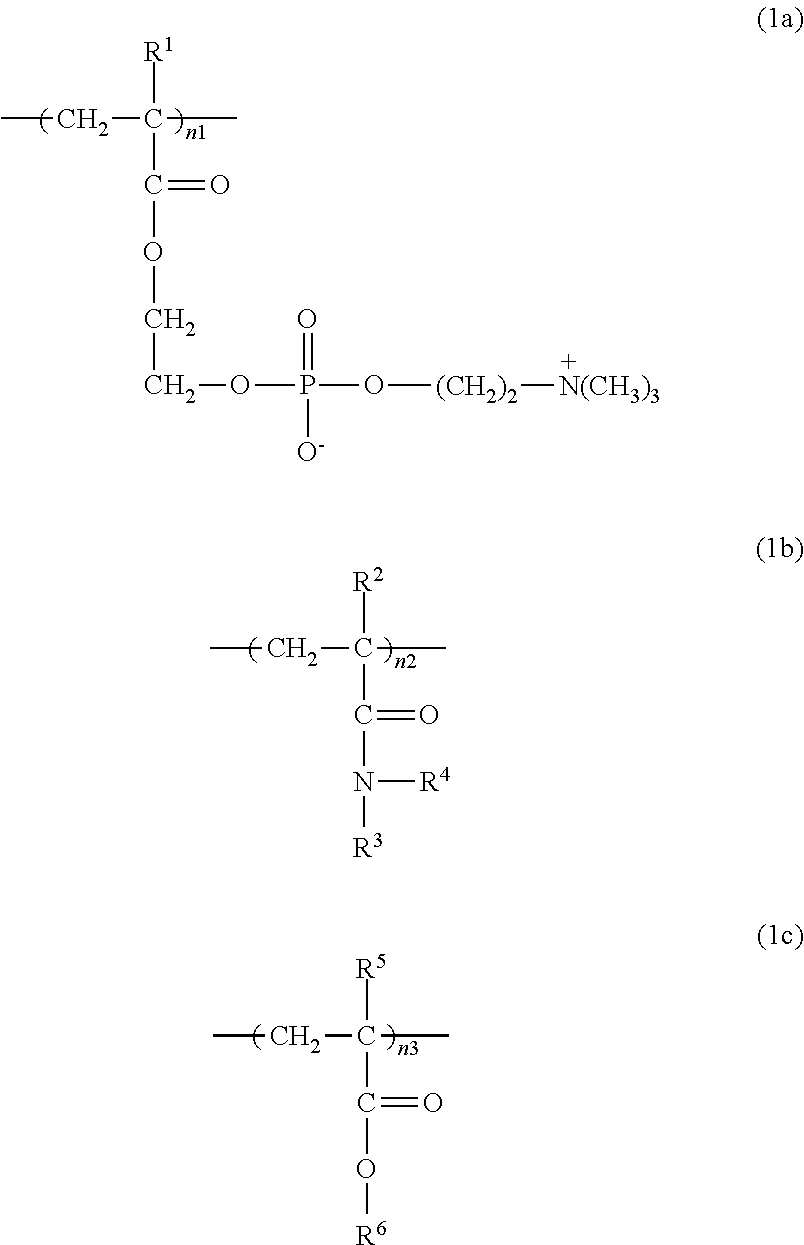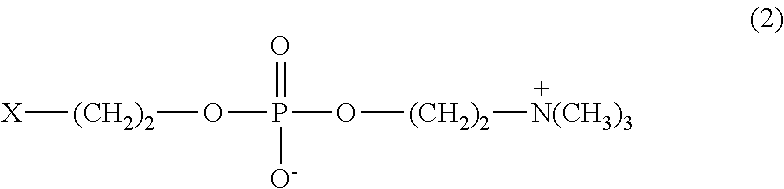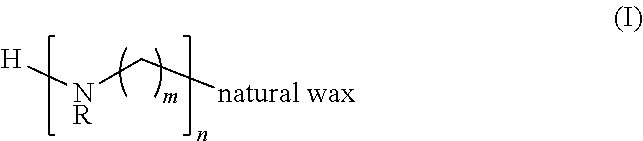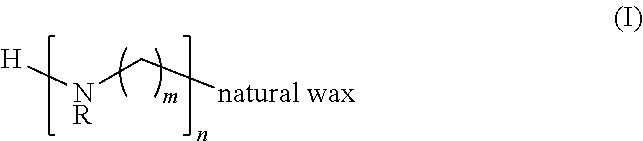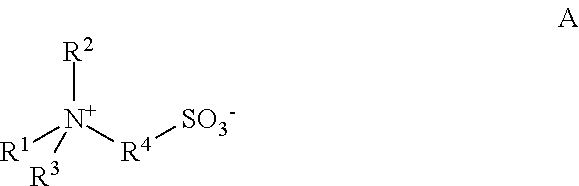Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Contact lens care" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
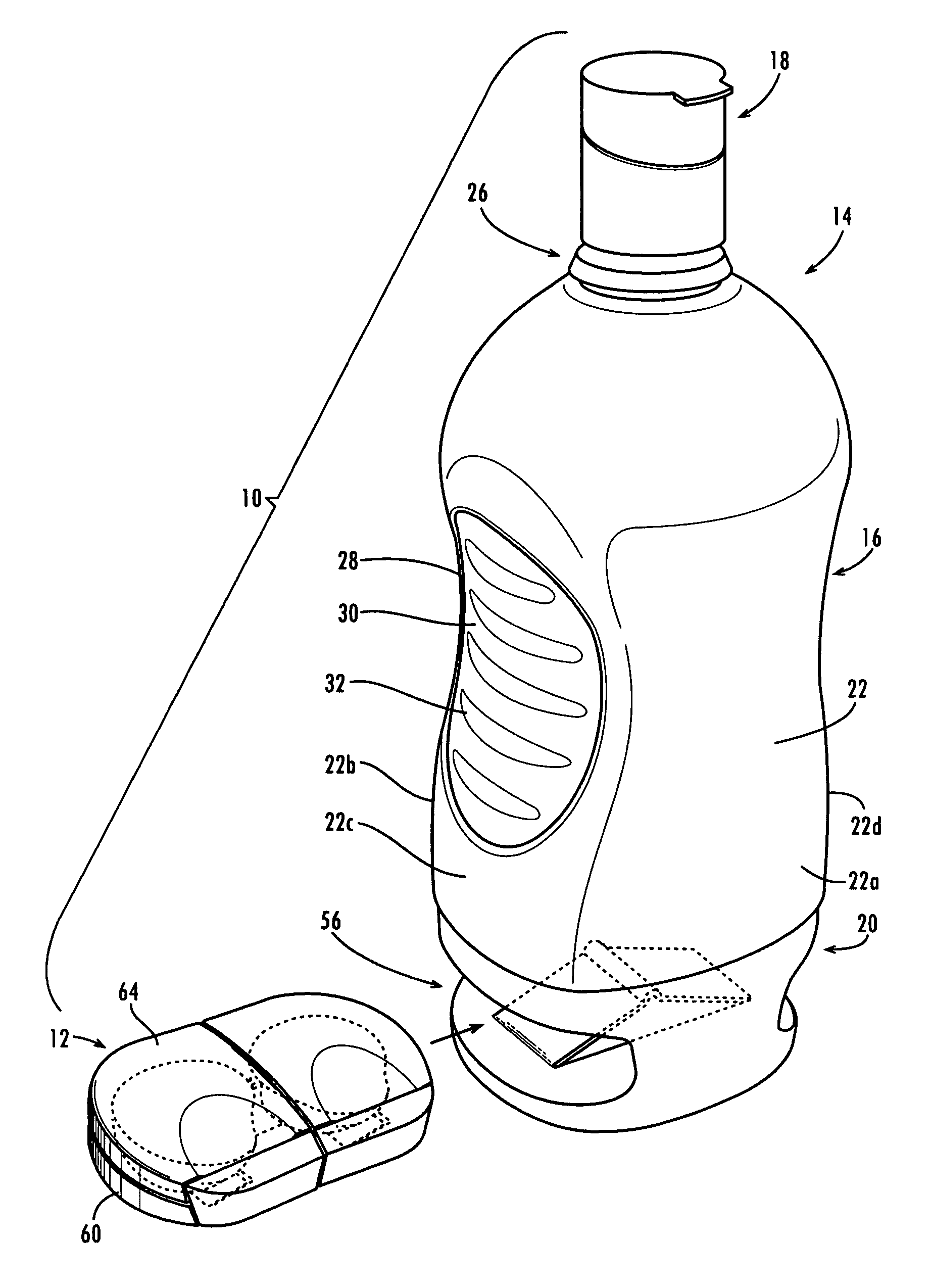
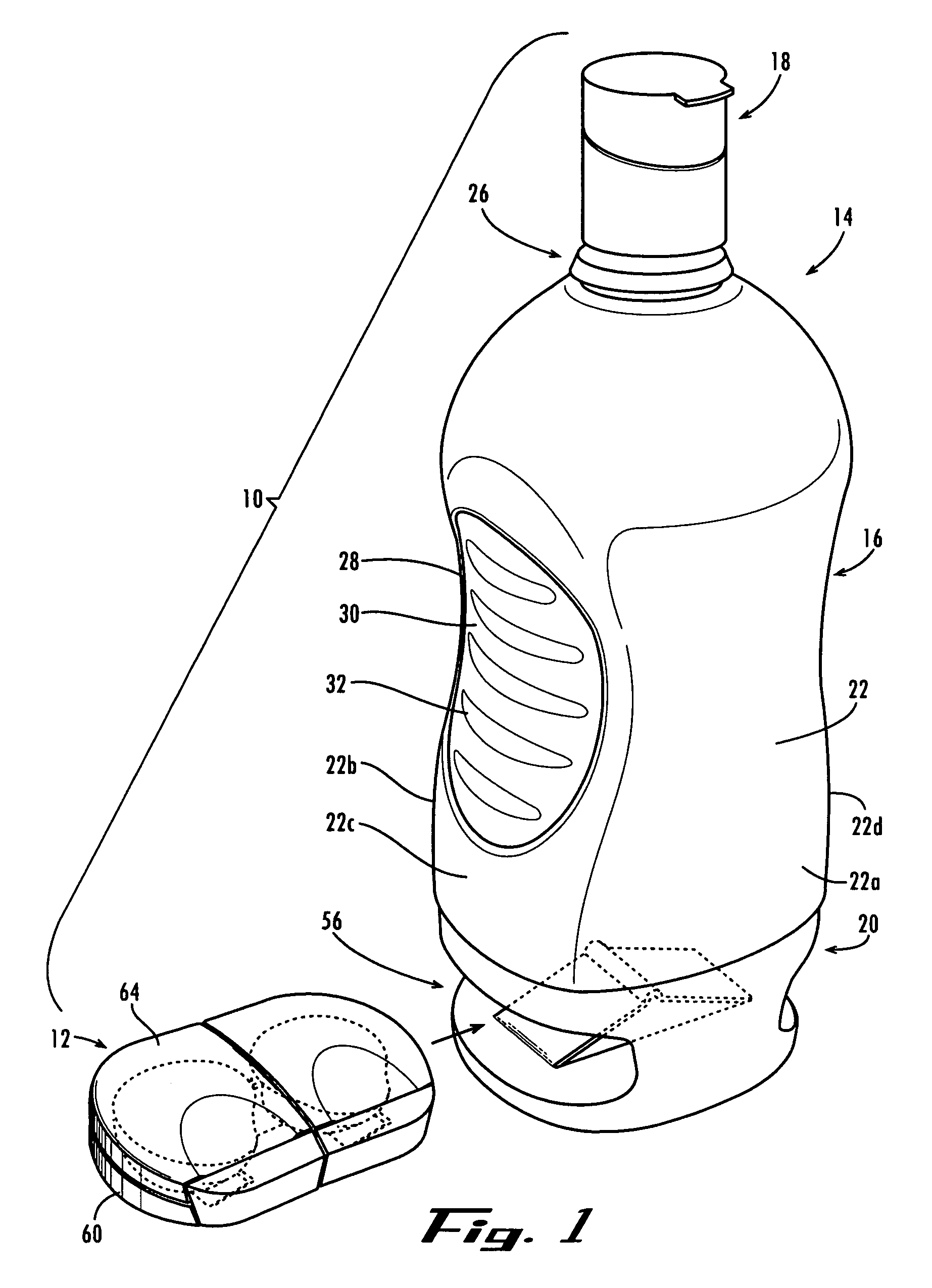
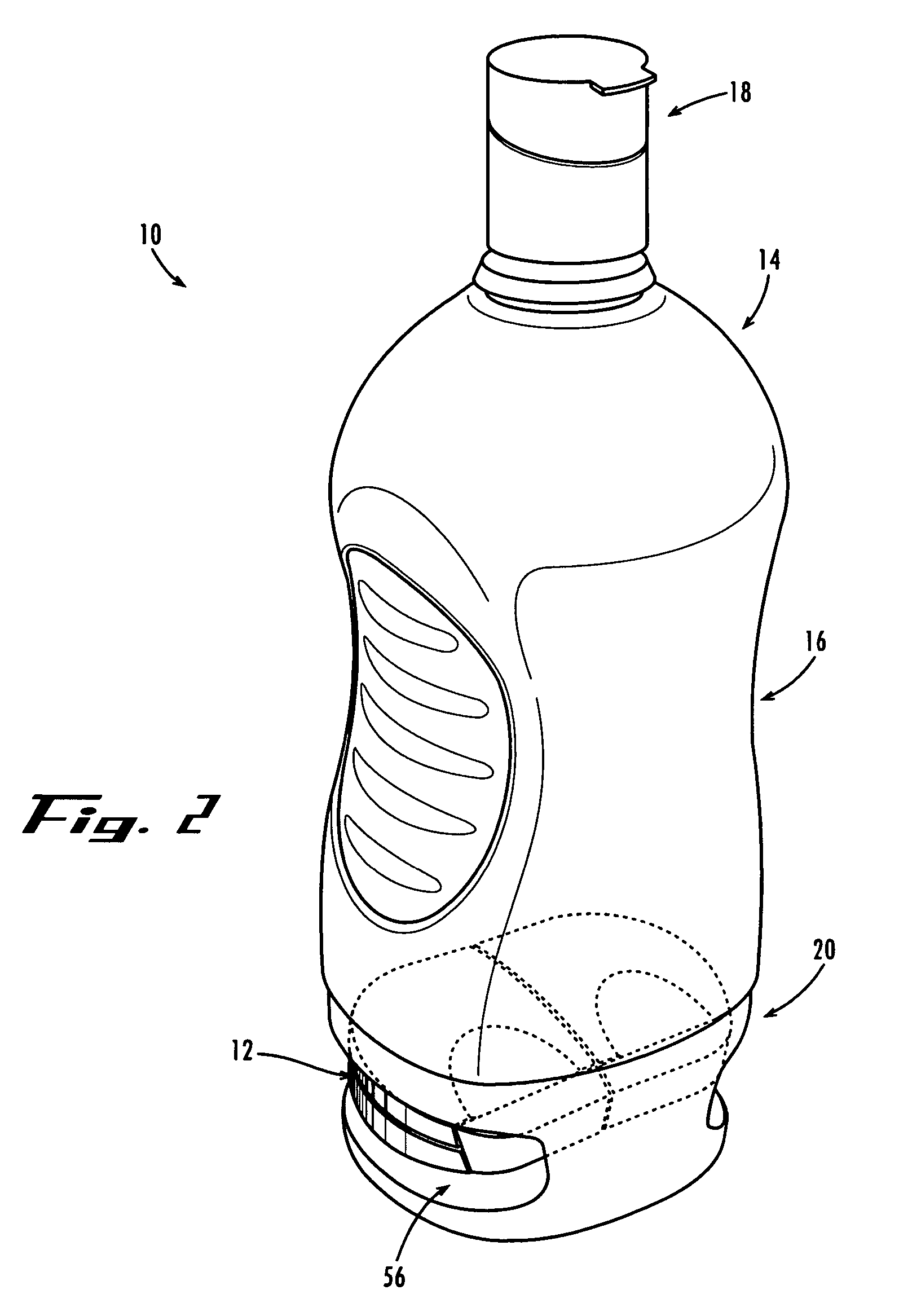
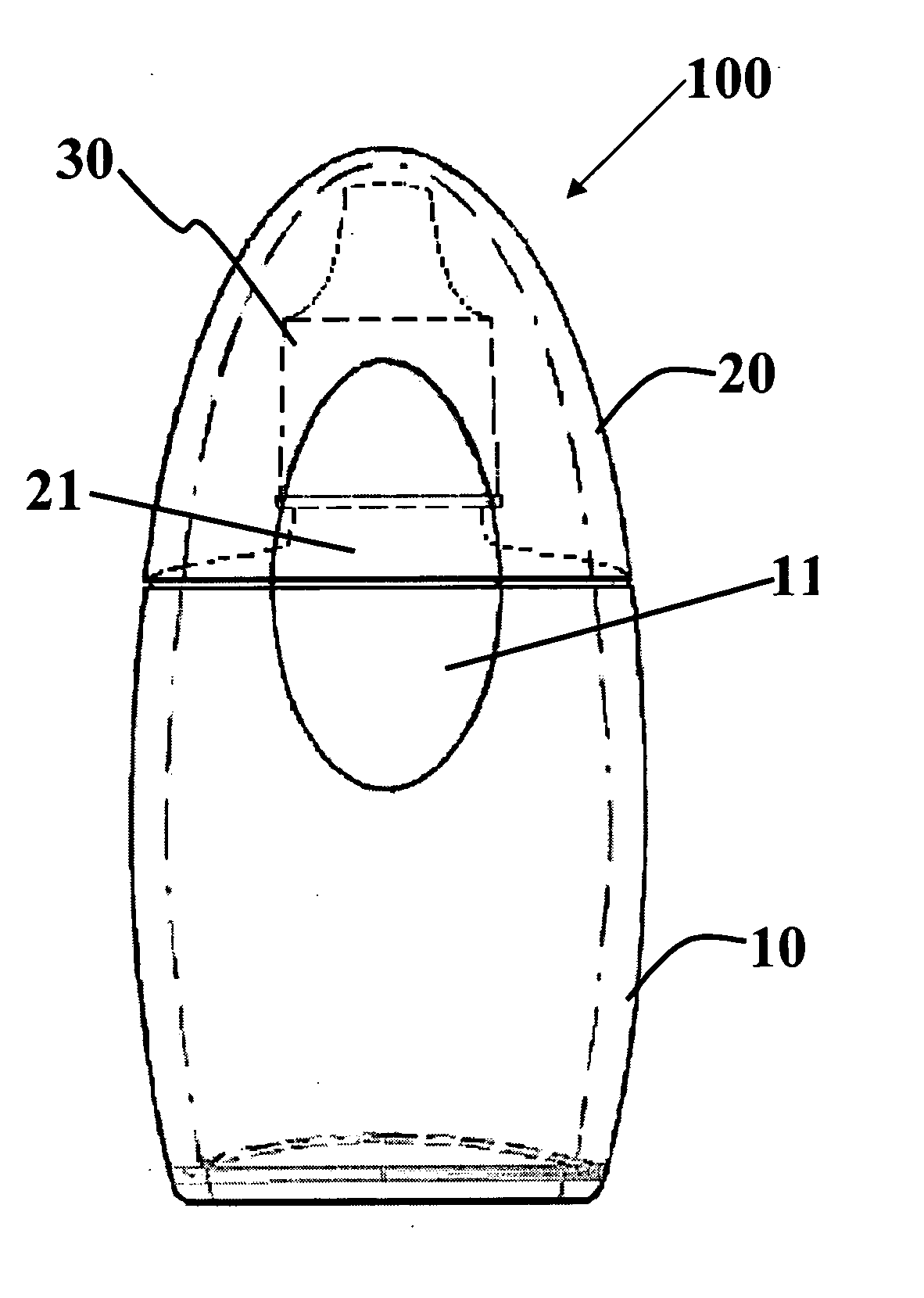
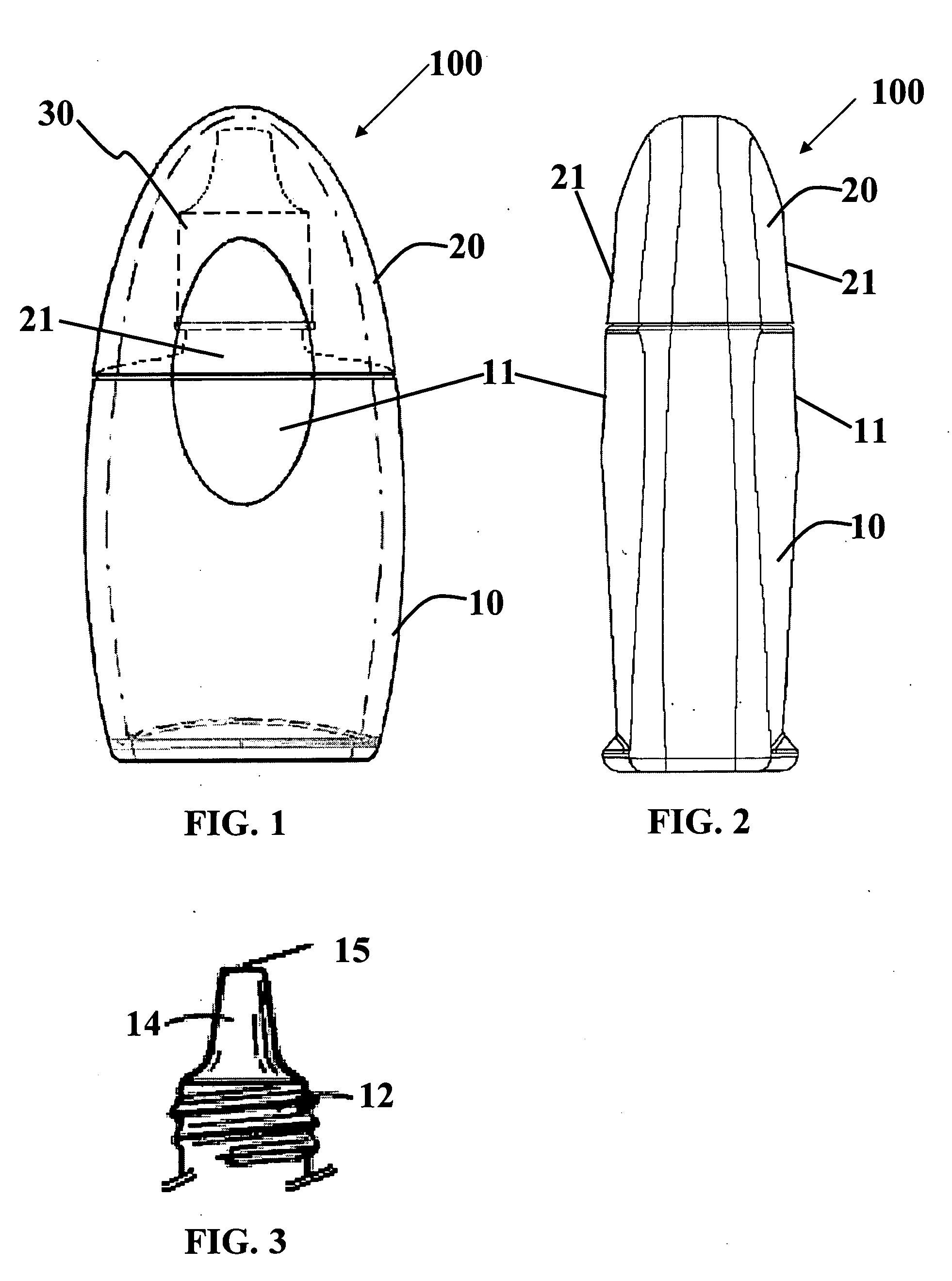
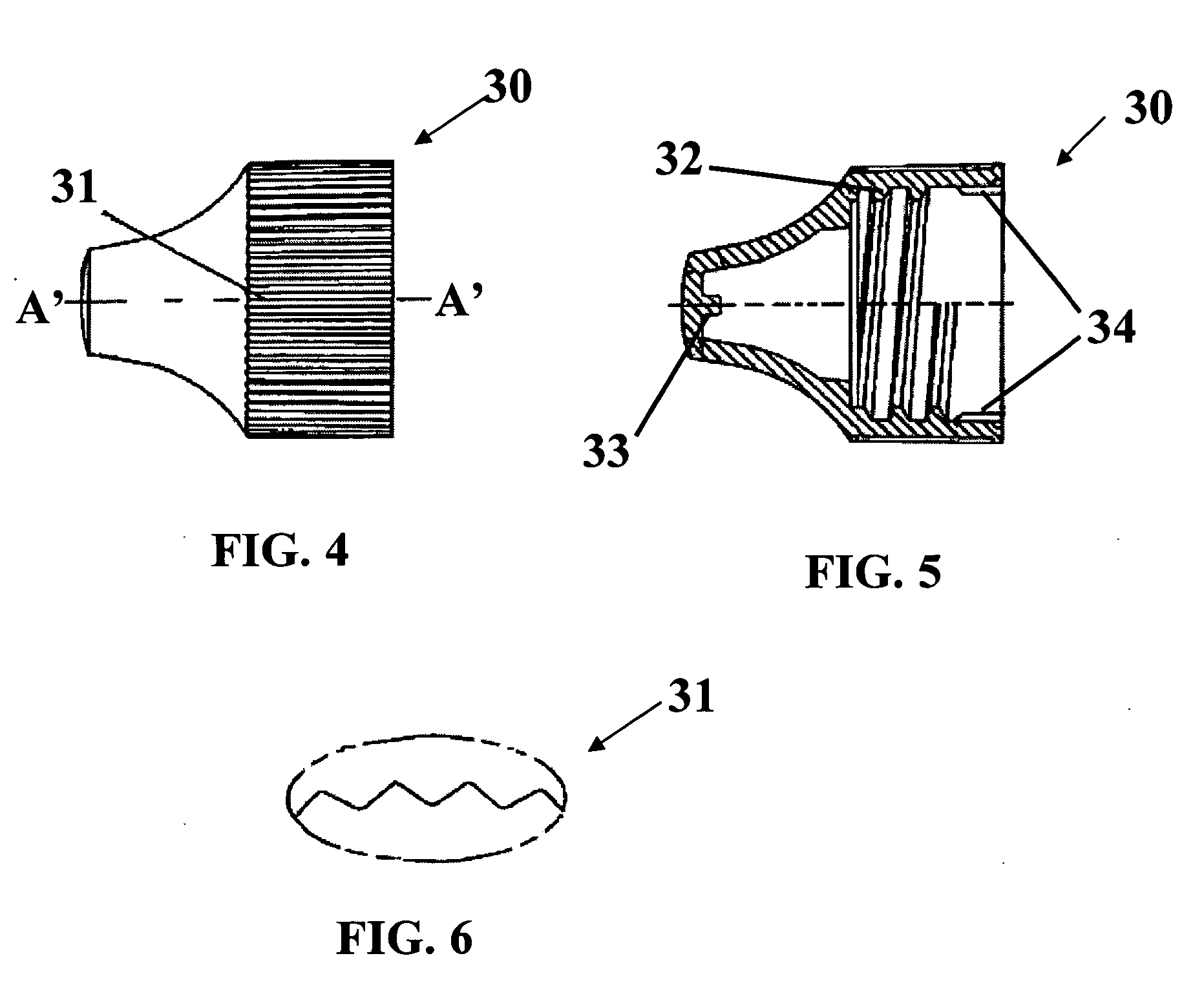
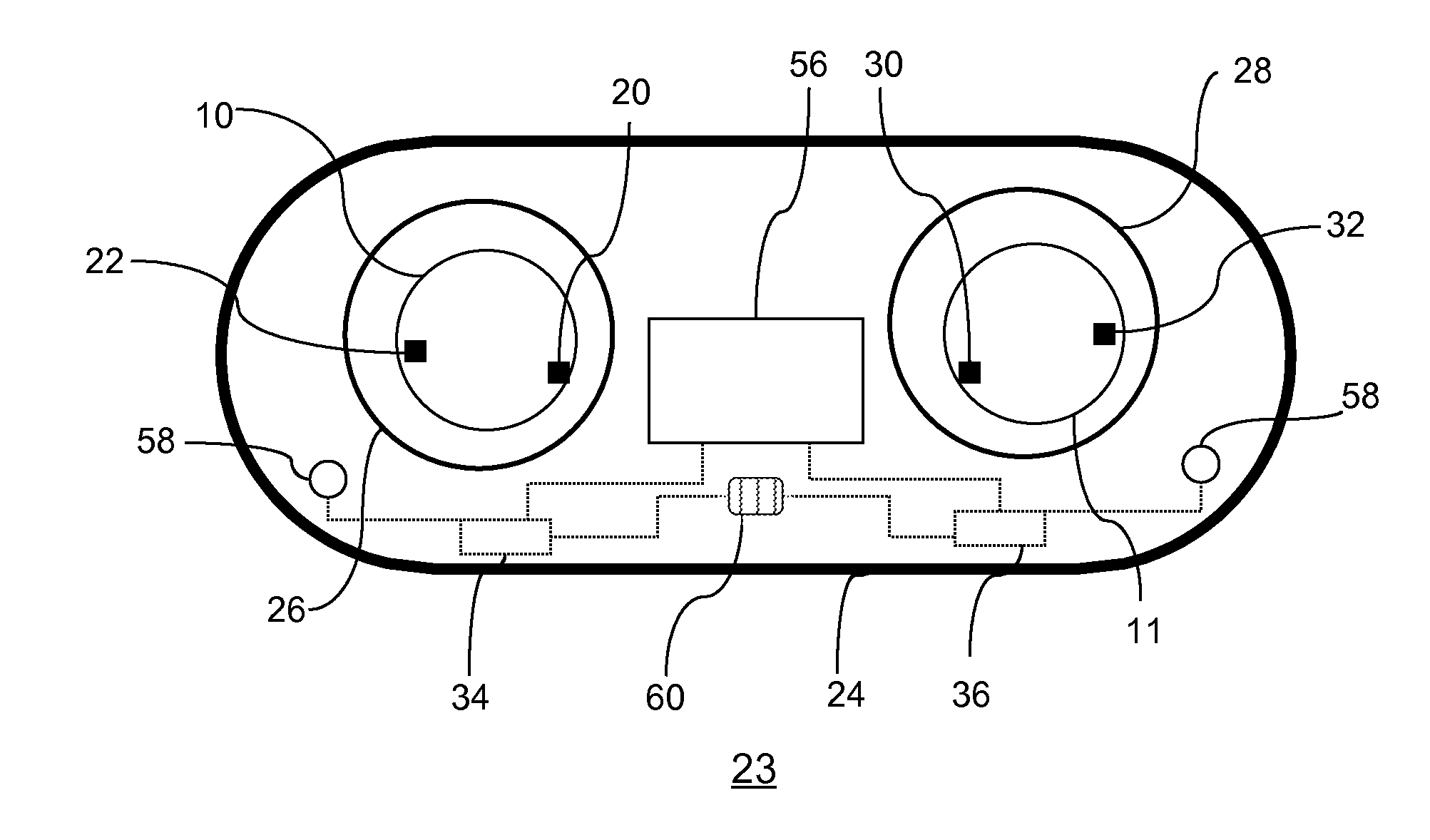
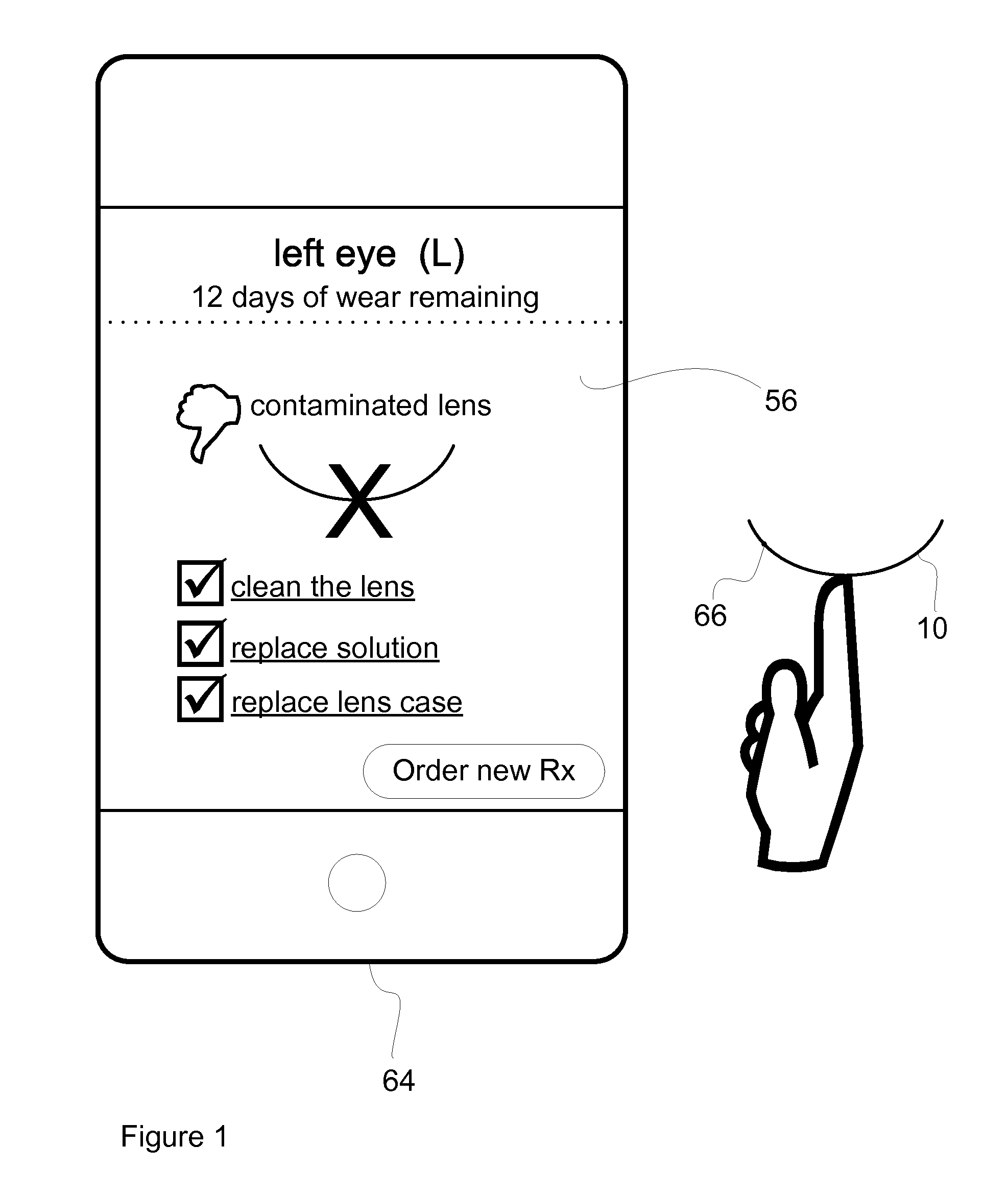
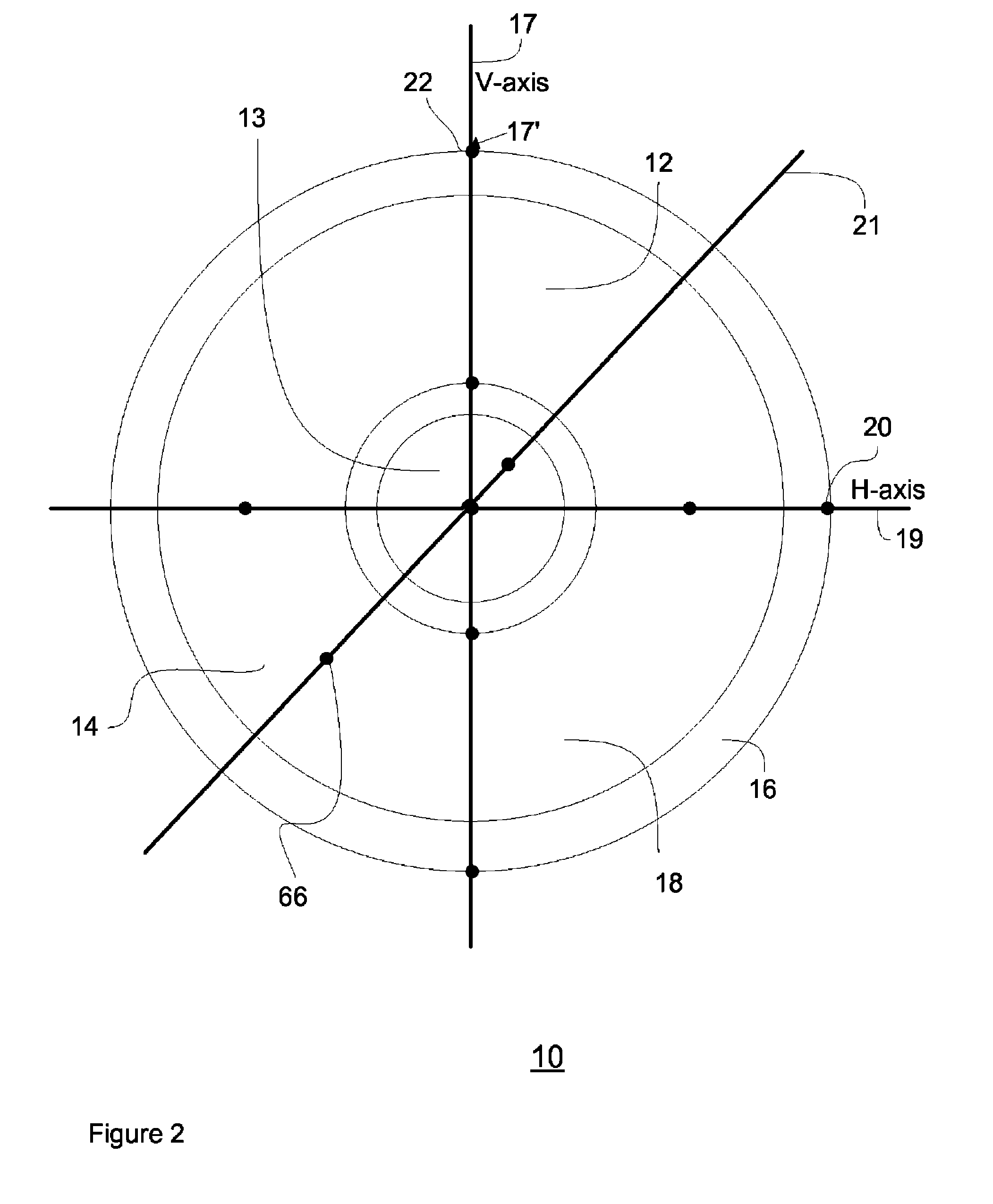
Method and System for Contact Lens Care and Compliance
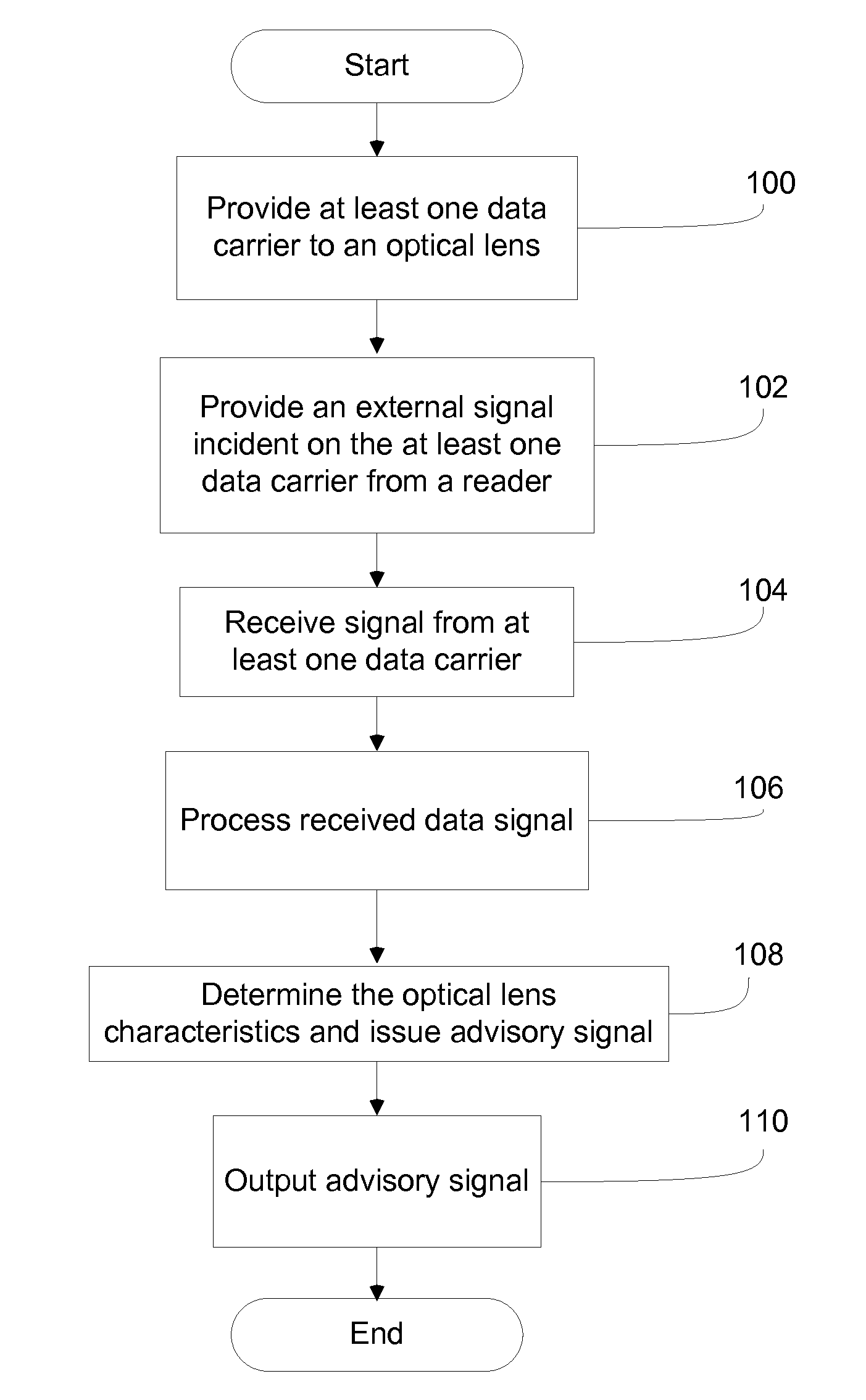
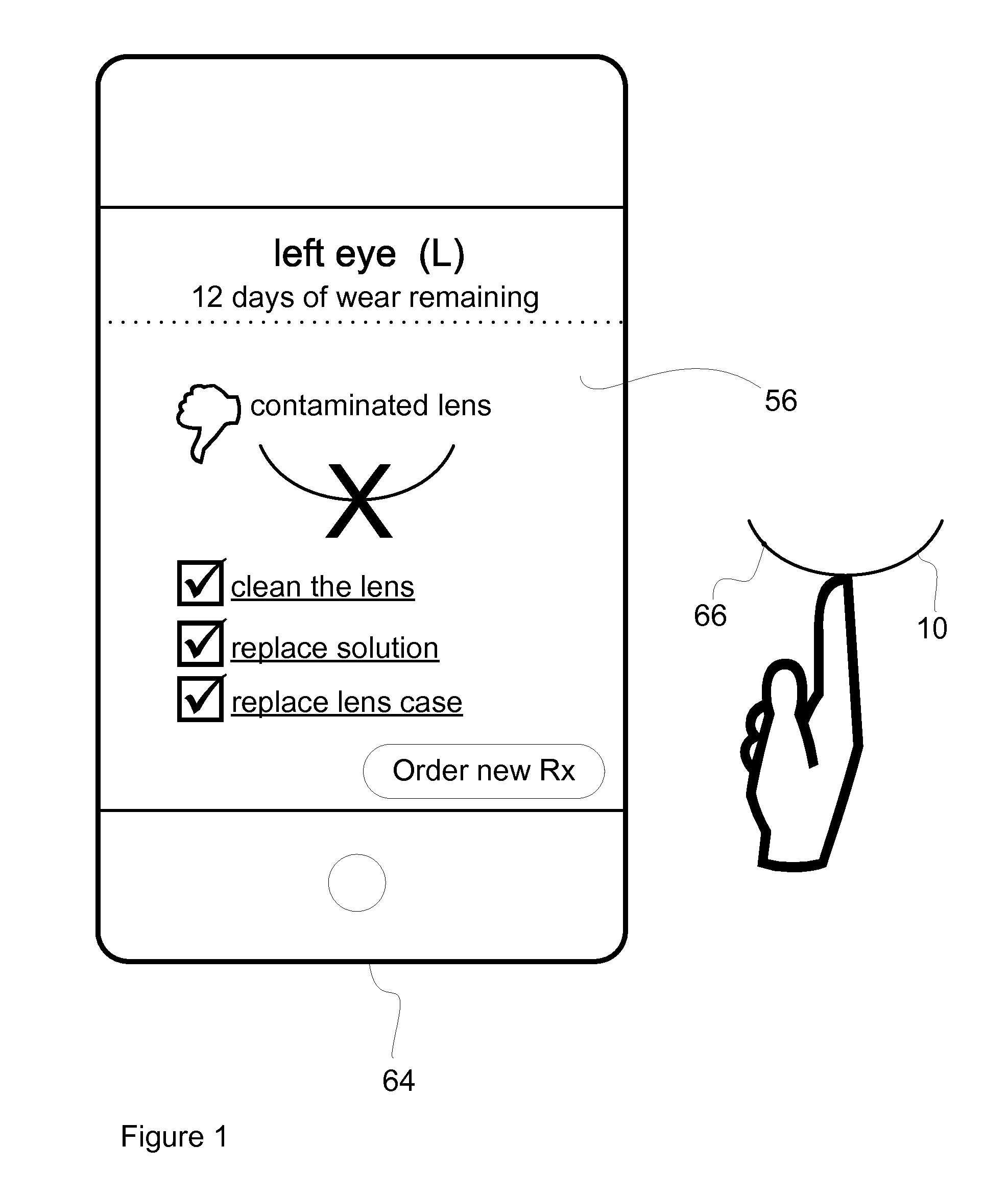
InactiveUS20110084834A1Reduce complicationsFacilitate complianceChecking apparatusOther accessoriesOphthalmic ProductComputer science
A method for tracking ophthalmic lens care compliance, said method comprising the steps of: including at least one sensor with said ophthalmic product for monitoring at least one ambient condition and for logging and recording at least one reading associated said at least one ambient condition, following a predetermined event; determining whether said at least one reading exceeds at least one predetermined threshold, and issuing an alert when said at least one reading exceeds at least one predetermined threshold.
Owner:SABETA ANTON
Phospholipid Compositions for Contact Lens Care and Preservation of Pharmaceutical Compositions
InactiveUS20080287395A1Disinfect lensReduce in quantityAntibacterial agentsBiocideSolubilityPhosphate
The use of certain synthetic phospholipids to preserve pharmaceutical compositions from microbial contamination is described. The synthetic phospholipids have unique molecular arrangements wherein a phosphate group is linked to a quaternary ammonium functionality via a substituted-propenyl group, and the quaternary ammonium functionality is further linked to at least one long hydrocarbon chain. Such molecular arrangements are what make the phospholipids of formula (I) highly water soluble, e.g., the length of the hydrocarbon chain assists to maintain solubility and efficacy of the molecules for the uses described herein. The synthetic phospholipids described herein have been found to be particularly useful as antimicrobial preservatives for ophthalmic, otic and nasal pharmaceutical compositions, especially ophthalmic compositions. These compounds may also be utilized to disinfect contact lenses. The invention is based in-part upon a finding that the antimicrobial activity of the synthetic phospholipids is affected by the ionic strength of the compositions in which the compounds are contained. The provision of compounds having limited ionic strengths is therefore preferred.
Owner:ALCON RES LTD
Contact Lens Care Solutions with a Low Molecular Weight Oligomer
InactiveUS20100086514A1Minimize uptake of antimicrobialExtension of timeBiocidePharmaceutical non-active ingredientsOligomerNitrogen
A contact lens care solution comprising: a cationic antimicrobial component having an average molecular weight (MNA), and a cationic oligomer or nitrogen / amine oligomer having a number average molecular weight (MNO) from 500 daltons to 15,000 daltons. The lens care solutions are used to clean and disinfect contact lenses, and in particular, soft, silicone hydrogel contact lenses.
Owner:BAUSCH & LOMB INC
Contact lens care system
An integrated lens care system including a lens case for contact lenses and a container for dispensing a lens care solution. The container includes a docking site where the lens case can be stored and then removed for use, and a retainer mechanism for releasably securing the lens case in the docked position. In example embodiments, the docking site is a chamber into which the lens case is inserted, and the retainer mechanism includes at least one protrusion that extends into the docking chamber and releasably couples to at least one recess in the lens case. In another example embodiment, the docking site is a peripheral edge of the container body where a combination lens case / closure cap mounts, and the retainer mechanism includes a snap-fit coupling at the peripheral edge.
Owner:ALCON INC
Contact lens care product
InactiveUS20070045354A1Improve comfortEasy to holdLens cleaning compositionsClosuresContact lens careOptometry
Owner:NOVARTIS AG
Composition for treaitng contact lens and its application
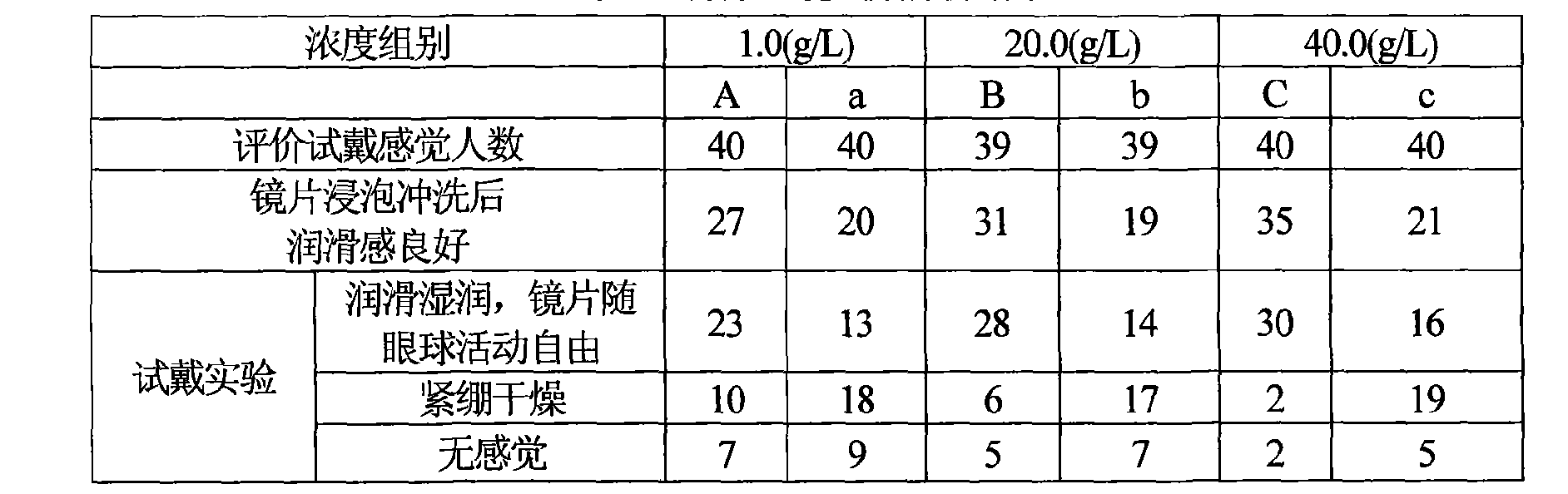
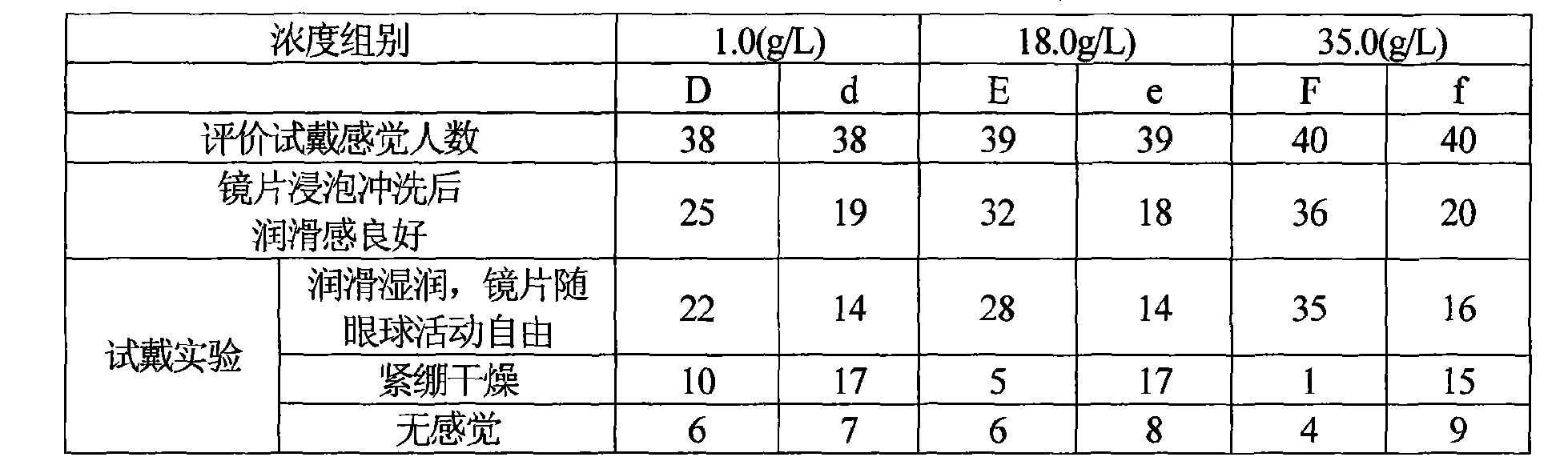
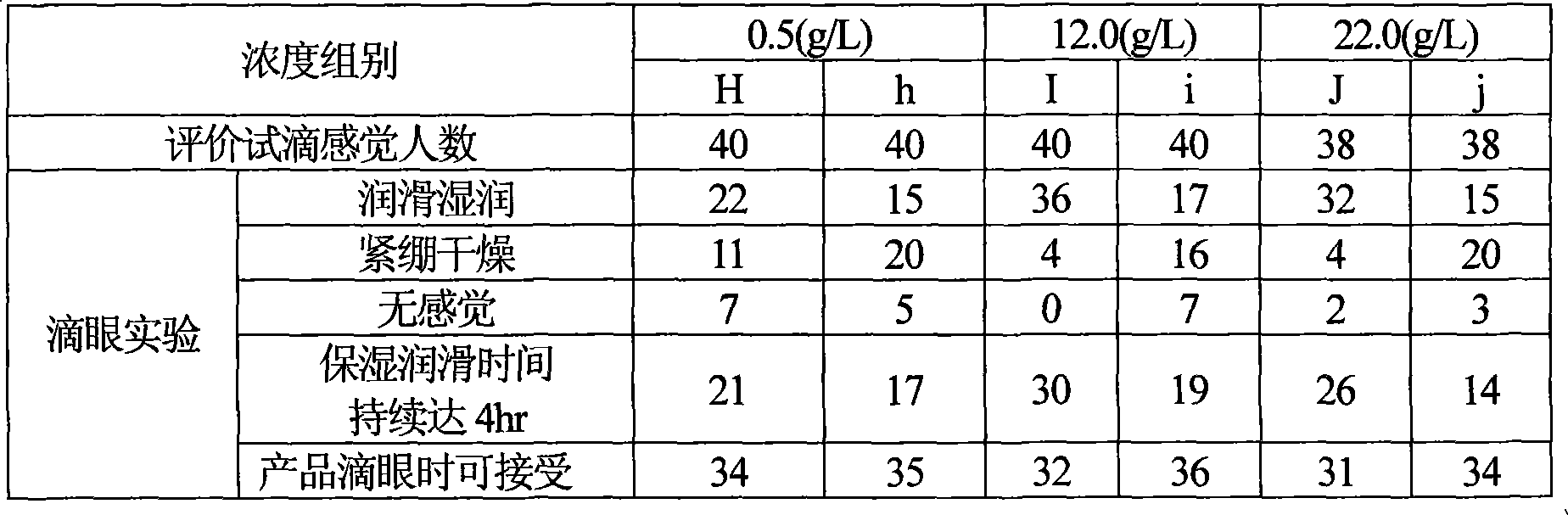
InactiveCN1390606AImprove the lubrication effectGood moisturizing effectChemicalsIrritationDesiccation
A composition for treating contact lens is a nursing solution containing sodium hyaluronate, and can be used for cleaning, sterilizing and storing contact lens, and wetting eye and lens, preventing eye's desiccation and irritation.
Owner:上海卫康光学眼镜有限公司
Ophthalmic compositions comprising a carboxyl-modified fructan or a salt thereof
Ophthalmic composition including a carboxyl-modified fructan or a salt thereof. The ophthalmic composition can be used in an eye care product or a contact lens care product such as a contact lens packaging solution or contact lens disinfecting solution.
Owner:BAUSCH & LOMB INC
Packaging materials for formulations containing 2-pyrrolidone derivatives
Packaging materials substantially lacking adsorptive and absorptive properties for 2-pyrrolidone derivatives are provided. The packaged products described herein provide for retention of the 2-pyrrolidone derivative within the formulation thereby maintaining the integrity of such formulations. Packaged products are useful for ophthalmic, otic, and nasal applications. Ophthalmic application includes therapeutic uses and contact lens care uses.
Owner:ALCON RES LTD
Contact lens care system
An integrated lens care system including a lens case for contact lenses and a container for dispensing a lens care solution. The container includes a docking site where the lens case can be stored and then removed for use, and a retainer mechanism for releasably securing the lens case in the docked position. In example embodiments, the docking site is a chamber into which the lens case is inserted, and the retainer mechanism includes at least one protrusion that extends into the docking chamber and releasably couples to at least one recess in the lens case. In another example embodiment, the docking site is a peripheral edge of the container body where a combination lens case / closure cap mounts, and the retainer mechanism includes a snap-fit coupling at the peripheral edge.
Owner:ALCON INC
Contact lens nursing liquid or moisture retention eye drop containing polyethylene glycol hydrogenated castor oil
InactiveCN101391111AGood moisturizing effectImprove the lubrication effectChemicalsViscous liquidPolyethylene glycol
The invention discloses contact lens nursing liquid or moisture eye moistening liquid containing polyoxyethylene hydrogen castor oil. The contact lens nursing liquid is composed of the polyoxyethylene hydrogen castor oil, pH regulator, osmotic pressure regulator, metal complex, surface active agent, thickening agent, bactericide and water, and the contact lens lubricating moist liquid is composed of the polyoxyethylene hydrogen castor oil, the pH regulator, the osmotic pressure regulator, the metal complex, the thickening agent, preservative and water. The polyoxyethylene hydrogen castor oil selected by the invention has a plurality of branched chains and good lubricating performance; and the existence of the polyoxyethylene end enable the product to have good moist performance. The polyoxyethylene hydrogen castor oil is viscous liquid, is easily dissolved in water, has even solution performance, and does not have the condition that fine granules or macromolecules curl and enwind in aqueous solution because solid macromolecule raw materials can not completely dissolved to further cause the problem that eyes feel uncomfortable. The obtained product is achromatous and flavourless, and has good user reception, low production cost, and wide source of the main material of the castor oil.
Owner:GUANGZHOU BAOSHINING OPTICAL PROD
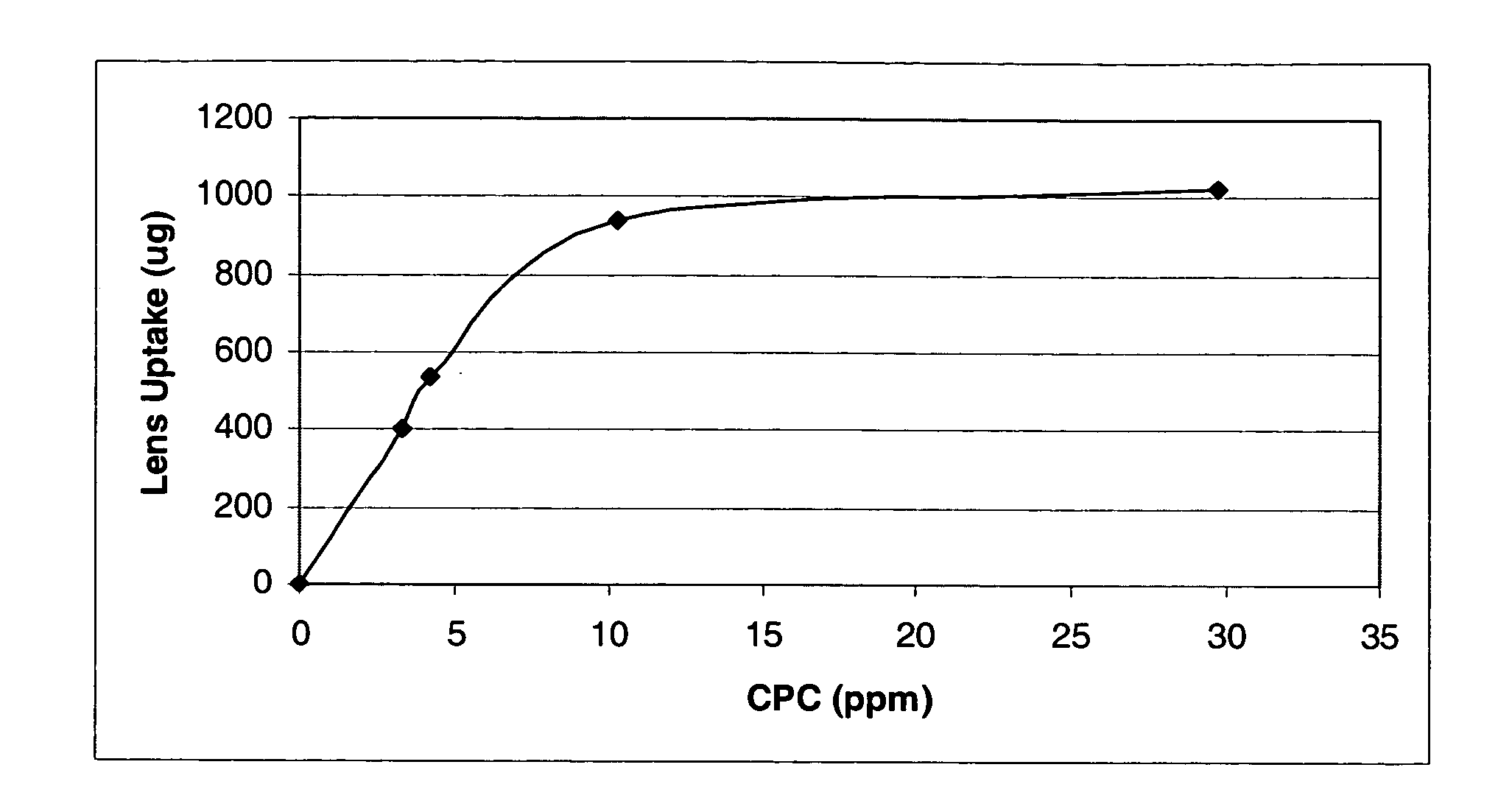
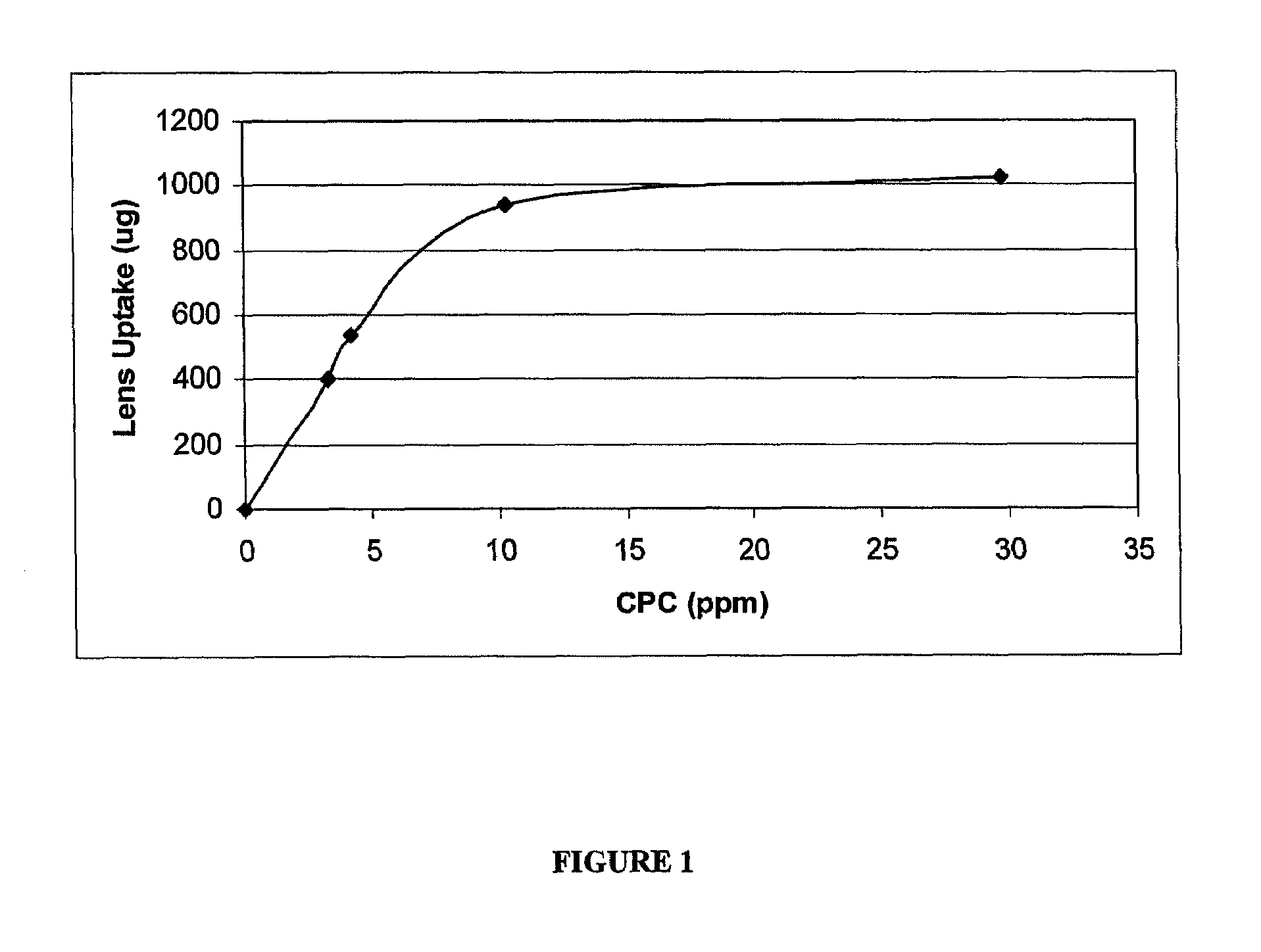
Cetylpyridinium chloride as an antimicrobial agent in ophthalmic compositions
A multi-purpose contact lens care solution having high antimicrobial activity comprising, in an aqueous liquid medium, cetylpyridinium chloride and a non-ionic surfactant. In one embodiment of the invention, the non-ionic surfactant is a poly(oxypropylene)-poly(oxyethylene) block copolymer. The solution may optionally also include additional antimicrobial components, a buffer component, a viscosity inducing component, a surfactant, taurine, propylene glycol and / or a tonicity component. This solution additionally prevents losses in ocular tissue membrane integrity during contact lens wear.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Lens care solutions comprising alkyldimonium hydroxypropyl alkylglucosides
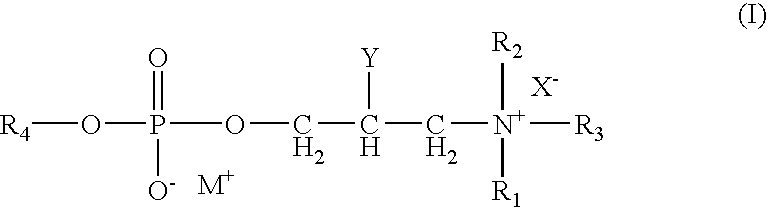
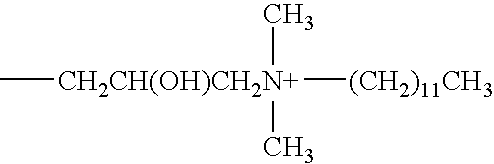
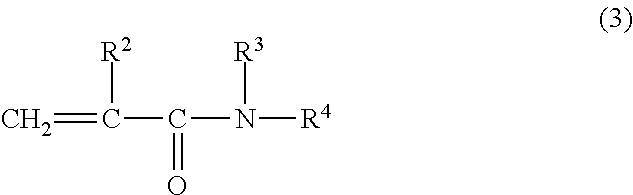
A contact lens care solution comprising one or more alkyldimonium hydroxypropyl alkylglucosides of general formula I or general formula IIwherein R is a straight or branched C8-C24alkyl;A is —CH2CH(OH)CH2N+(CH3)2R1X−, wherein R1 is a C8-C24alkyl and X− is a common counteranion, n is an average value from 1 to 6 and m is an average value from 1 to 2. The solution is formulated to clean and disinfect contact lenses or the solution is formulated as a rewet eye drop for use with contact lenses.
Owner:BAUSCH & LOMB INC
Multi purpose contact lens care compositions including propylene glycol or glycerin
Multi-purpose solutions for contact lens care provide substantial lens wearer / user comfort and / or acceptability. Such solutions include an aqueous liquid medium; an antimicrobial component, preferably a biguanide polymer present in an amount of 0,00001 % (w / v) to 2 % (w / v); propylene glycol or glycerin in an amount sufficient to increase antimicrobial activity; a surfactant component, preferably a poly (oxyethylene) -poly (oxypropylene) block copolymer surfactant, in an effective amount; a phosphate buffer component in an effective amount; preferably (2x) a viscosity inducing component, preferably selected from cellulosic derivatives, in an effective amount; and preferably (2x) a tonicity component in an effective amount. Such solutions have substantial performance, comfort and acceptability benefits, which, ultimately, lead to ocular health advantages and avoidance of problems caused by contact lens wear.
Owner:ADVANCED MEDICAL OPTICS
Ophthalmic solutions, including contact lens care and eye drops comprising carnosine, preferably in combination with dexpanthenol and/or hyaluronic acid
InactiveUS20110230424A1Prevent of even repair damagePrevent and reduce stainingOrganic active ingredientsSenses disorderDexpanthenolOphthalmic solutions
The current invention relates to a contact lens care solution comprising a compound suitable for treating the eye, or the contact lens, use of such compound in such contact lens care solutions, and methods for introducing such compound in the eye of a person wearing contact lenses. With the current invention damage to the eye, in particular the cornea, in particular damage to the eye, in particular the cornea, of a person wearing a contact lens, or as a consequence of wearing a contact lens is prevented or reduced. / esp.
Owner:WAGENAAR LOUIS JOHAN
Compositions and methods using sub-PPM combinations of polyquaternium-1 and high molecular weight PHMB
ActiveUS6930077B2Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsLiquid mediumCopolymer
Multi-purpose solutions for contact lens care provide substantial lens wearer / user comfort and / or acceptability, with minimal, if any, corneal epithelial punctate fluorescein staining. Such solutions may include an aqueous liquid medium; an antimicrobial component comprising polyquarternium-1 and a hexamethylene biguanide polymer having a number average molecular weight in the range of from about 4,000 to about 45,000; a surfactant component, preferably a poly(oxyethylene)-poly(oxypropylene) block copolymer surfactant, in an effective amount; a buffer component in an effective amount; a viscosity-inducing component, preferably selected from cellulosic derivatives, in an effective amount; and a tonicity component in an effective amount. Such solutions have substantial performance, comfort and acceptability benefits, which, ultimately, lead to ocular health advantages and avoidance of problems caused by contact lens wear.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Contact lens care preparation and packaging solution
ActiveCN104272174AInhibition of attachmentThe effect of inhibiting adhesionSpectales/gogglesLens cleaning compositionsPhosphorylcholinePhosphoric acid
Provided are a contact lens care preparation capable of imparting durable surface lubricity and amebic adhesion inhibitory effect to a contact lens surface through a simple treatment, and a contact lens packaging solution utilizing the same. The contact lens care preparation is composed of a solution containing 0.01 to 2 weight / volume% of a polymer having particular monomer units with a phosphorylcholine-like group, particular (meth) acrylamide derivative units, and particular monomer units with a hydrophobic group at a particular ratio, and a weight average molecular weight of 5,000 to 2,000,000, and is useful as a contact lens packaging solution.
Owner:NOF CORP
Contact lens care preparation and packaging solution
ActiveUS20150024987A1Simple treatmentIncrease surface lubricityLens cleaning compositionsChemicalsMeth-Ophthalmology
Provided are a contact lens care preparation capable of imparting durable surface lubricity and amebic adhesion inhibitory effect to a contact lens surface through a simple treatment, and a contact lens packaging solution utilizing the same. The contact lens care preparation is composed of a solution containing 0.01 to 2 weight / volume % of a polymer having particular monomer units with a phosphorylcholine-like group, particular (meth)acrylamide derivative units, and particular monomer units with a hydrophobic group at a particular ratio, and a weight average molecular weight of 5,000 to 2,000,000, and is useful as a contact lens packaging solution.
Owner:NOF CORP
Cetylpyridinium chloride as an antimicrobial agent in ophthalmic compositions
A multi-purpose contact lens care solution having high antimicrobial activity comprising, in an aqueous liquid medium, cetylpyridinium chloride and a non-ionic surfactant. In one embodiment of the invention, the non-ionic surfactant is a poly(oxypropylene)-poly(oxyethylene) block copolymer. The solution may optionally also include additional antimicrobial components, a buffer component, a viscosity inducing component, a surfactant, taurine, propylene glycol and / or a tonicity component. This solution additionally prevents losses in ocular tissue membrane integrity during contact lens wear.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Hard contact lens care solution
InactiveCN107789657AGuaranteed pHEffective pH controlBiocideOrganic detergent compounding agentsDisinfectantClearing Agent
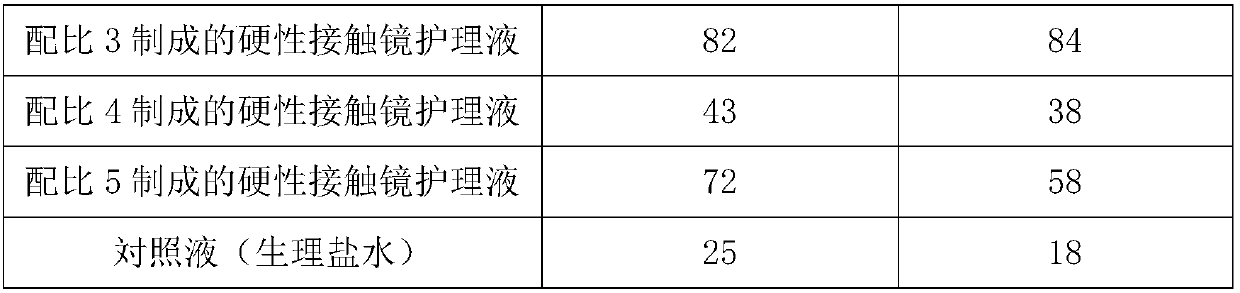
The invention discloses a hard contact lens care solution. The hard contact lens care solution comprises 0.02 to 20 parts of a buffering agent, 0.01 to 10 parts of an osmotic pressure adjusting agent,0.001 to 30 parts of a clearing agent, 0.005 to 1.0 part of a disinfectant, and 0.02 to 20 parts of a tackifier. The hard contact lens care solution can be used for cleaning, washing, disinfecting, lubricating, moisture retention, immersing, and storage of hard contact lens with high efficiency, is capable of prolonging service life of lens, maintaining the vision definition after wearing glasses, and reducing complication caused by wearing of contact lens.
Owner:AUTEK CHINA
Method and system for contact lens care and compliance
InactiveUS8628194B2Facilitate complianceReduce complicationsChecking apparatusOther accessoriesOphthalmic ProductComputer science
A method for tracking ophthalmic lens care compliance, said method comprising the steps of: including at least one sensor with said ophthalmic product for monitoring at least one ambient condition and for logging and recording at least one reading associated said at least one ambient condition, following a predetermined event; determining whether said at least one reading exceeds at least one predetermined threshold, and issuing an alert when said at least one reading exceeds at least one predetermined threshold.
Owner:SABETA ANTON
Multi purpose contact lens care compositions including propylene glycol or glycerin
InactiveUS20070059332A1Significant comprehensive benefitsIncrease user complianceBiocideLens cleaning compositionsLiquid mediumPhosphate
Multi-purpose solutions for contact lens care provide substantial lens wearer / user comfort and / or acceptability. Such solutions include an aqueous liquid medium; an antimicrobial component, preferably a biguanide polymer present in an amount of less than about 5 ppm; propylene glycol or glycerin in an amount sufficient to increase antimicrobial activity; a surfactant component, preferably a poly(oxyethylene)-poly(oxypropylene) block copolymer surfactant, in an effective amount; a phosphate buffer component in an effective amount; a viscosity inducing component, preferably selected from cellulosic derivatives, in an effective amount; and a tonicity component in an effective amount. Such solutions have substantial performance, comfort and acceptability benefits, which, ultimately, lead to ocular health advantages and avoidance of problems caused by contact lens wear.
Owner:ADVANCED MEDICAL OPTICS
Sucrose ester dual-cleaning system-containing contact lens care solution
ActiveCN106867704AGood biocompatibilityNon-cytotoxicInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsSucroseBiocompatibility Testing
The invention discloses a sucrose ester dual-cleaning system-containing contact lens care solution. The contact lens care solution comprises a cleanser, an antibacterial agent, a humectant, a buffering agent, an osmotic pressure regulator and a chelating agent, wherein the cleanser consists of a biological surfactant and other surfactants; the biological surfactant is a sucrose ester; the sucrose ester is taken as a cleaning component in the contact lens care solution and has effects of removing proteins and lipids; the content of the sucrose ester is 0.005 to 0.1 percent; and the other cleaning component is optionally selected from one of poloxamer 188, poloxamer 407, dimethylpropiothetin, Tetronic-1107 and Tetronic-1304. The sucrose ester dual-cleaning system-containing contact lens care solution is prepared. As the sucrose ester is taken as a primary cleaning component and forms a dual-cleaning system with the other surfactant, the care solution has very good detergency and decontamination property, can effectively clean the proteins and the lipids, and is safe, non-toxic and good in biocompatibility.
Owner:SOUTHEAST UNIV
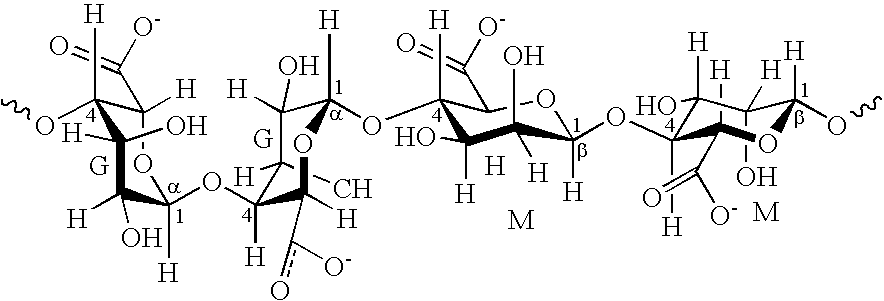
Poly(Nitrogen/Amine) Derivatives of a Natural Wax and Ophthalmic Compositions
A poly(nitrogen / amine) derivative of a natural wax of formula I, and ophthalmic compositions and contact lens care solutions that contain the poly(nitrogen / amine) derivative of a natural wax of formula I. The invention is also directed to a method of treating a patient with dry eyes, the method comprising instructing a patient to administer one or more eye drops of the ophthalmic composition that includes a poly(nitrogen / amine) derivative of a natural wax of formula I.
Owner:BAUSCH & LOMB INC
Application of chitosan quaternary ammonium to contact lens care solution
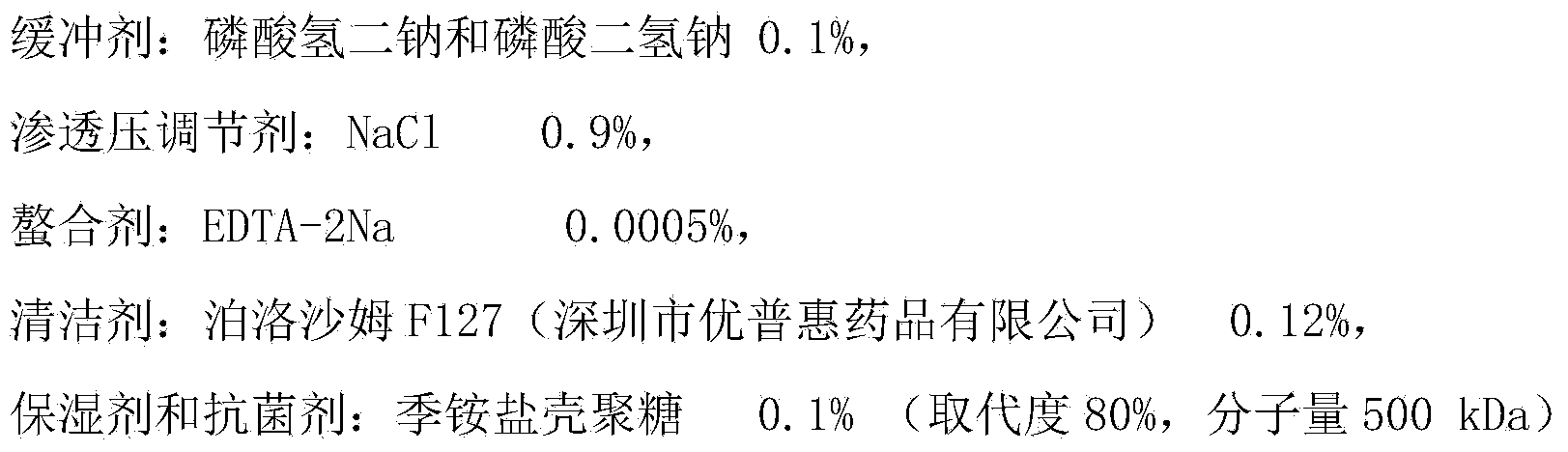
InactiveCN103656726AGood biocompatibilityGood moisturizing effectChemicalsQuaternary ammonium cationMoisture absorption
The invention discloses application of chitosan quaternary ammonium to a contact lens care solution, wherein chitosan quaternary ammonium is used as an antibacterial humectant and has the effective content of 0.001%-2% by mass of the contact lens care solution. By applying chitosan quaternary ammonium with good moisture-absorption moisture-retention performances and strong antibacterial performance to the contact lens care solution as the antibacterial humectants, the obtained care solution has the antibiosis rate on E.coil of 99.95-100% and the antibiosis rate on S.aureus of 99.91-99.99%, and the contact angle on silicon hydrogel is 20 DEG-40 DEG. The advantages comprise that the natural substance is used as the humectant and the antiseptic of the contact lens care solution, and chitosan quaternary ammonium is endowed with new application.
Owner:HAICHANG CONTACT LENSES +1
Composition for prevention and treatment of contact lens papillary conjunctivitis and allergic eye disease
ActiveUS20130102679A1Reduce generationSigns of redness of the eyeBiocideSenses disorderPapillary conjunctivitisConjunctival inflammation
The invention provides a use of panthenol or dexpanthenol in the prevention and / or treatment of contact lens papillary conjunctivitis in a subject. A contact lens care solution containing 0.001 to 10% by dry weight dexpanthenol is also provided.
Owner:BRIEN HOLDEN VISION INST (AU)
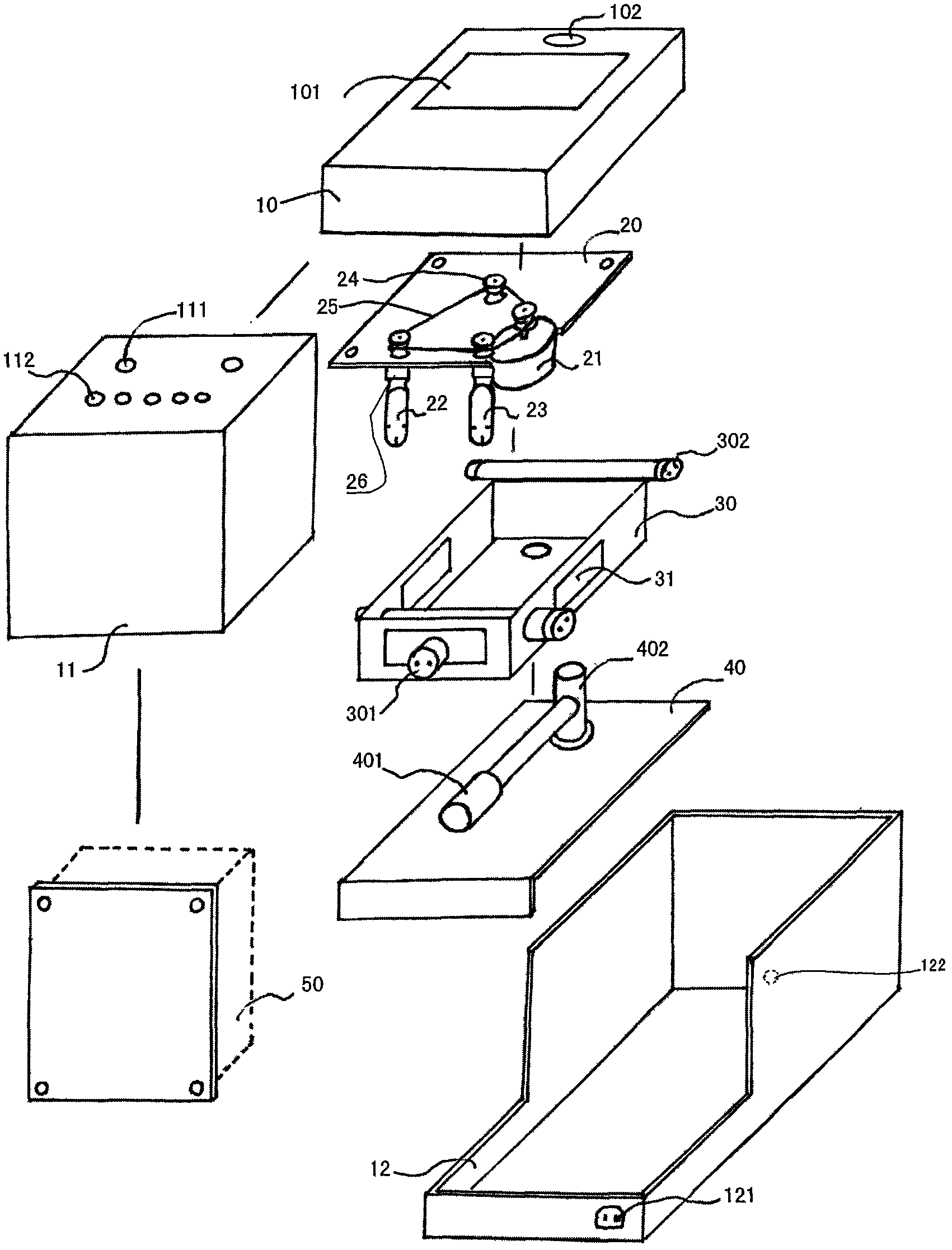
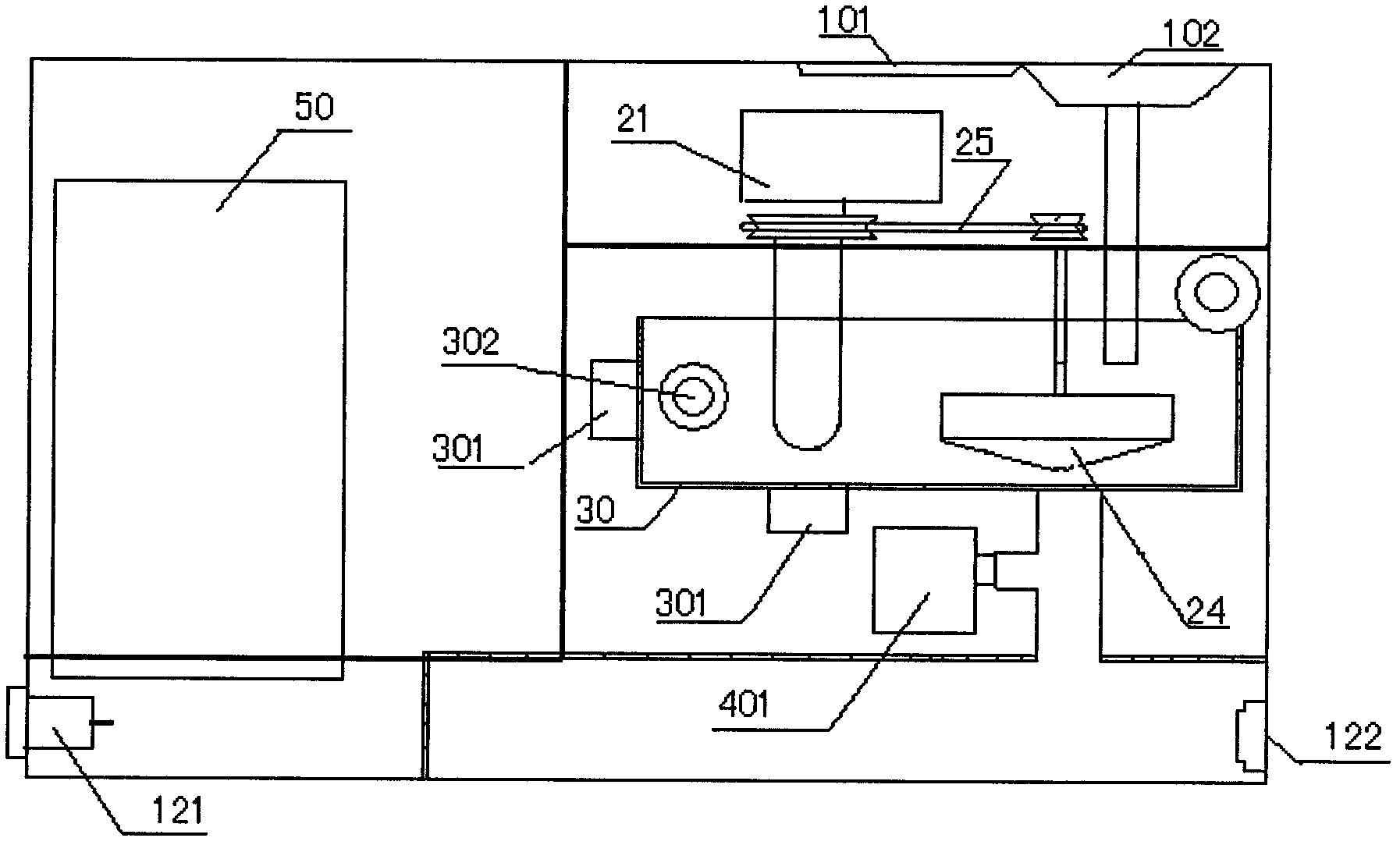
Contact lens care device
ActiveCN102274539AConvenience of professional careProfessional Care Made EasySpectales/gogglesRadiationImpellerUltraviolet lights
The invention discloses a nursing instrument for a corneal contract lens. The nursing instrument has a water tank variable-frequency ultrasonic vibrating structure with independent freedom, an automatic electromagnetic valve structure, a lens clip bracket self-rotating structure and an ultraviolet light tube sterilizing structure. Main parts of the nursing instrument include a cover, a base, a water tank, an RL (Return Loss) lens clip bracket, a motor, a motor frame, an impeller, an ultrasonic vibrator, an ultraviolet lamp and a circuit board component. In the nursing instrument for the corneal contact lens, dirt on the surface of the corneal contact lens and in a rhicnosis recess can be cleaned by vibrating, oscillating and cleaning in multiple directions at multiple frequencies, and harmful bacteria on the surface of the corneal contact lens and in the rhicnosis recess can be killed, cleaned, sterilized and nursed by using ultraviolet rays from different directions at different strengths.
Owner:杭州视亨光电有限公司
Contact lens care solution and preparation method
InactiveCN111518627AIngredient safetyGood antibacterial effectInorganic/elemental detergent compounding agentsOrganic active ingredientsOphthalmologyActive agent
The invention discloses a contact lens care solution. The contact lens care solution comprises ectoin or an eye science acceptable ectoin derivative, a surfactant, an antibacterial agent, a humectant,a buffer agent, an osmotic pressure regulator and a chelating agent. The invention further provides a preparation method of the contact lens care solution. According to the contact lens care solutionprovided by the invention, the moisturizing and lubricating performance of contact lenses can be improved, the wearing comfort is improved, a repairing effect is achieved, and the phenomena of redness, swelling, inflammation and the like caused by long-term wearing of the contact lenses can be eliminated.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Alkylamine as an antimicrobial agent in ophthalmic compositions
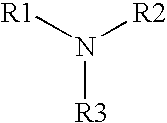
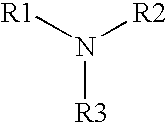
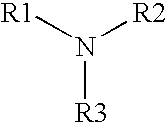
A multi-purpose contact lens care solution having high activity against fungi and certain bacteria comprising, in liquid aqueous medium, an alkylamine having the following formula, where R1 is a C13-17 alkylamine, and R2 and R3 are each independently H or —CH3, and a non-ionic surfactant. The solution may optionally also include additional antimicrobial components, a buffer component, a viscosity inducing component, a surfactant, taurine, propylene glycol and / or a tonicity component. This solution additionally prevents losses in ocular tissue membrane integrity during contact lens wear.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Contact lens care composition
InactiveCN105536026APlay the role of disinfection and sterilizationWon't hurtChemicalsInorganic saltsNon ionic
The present invention discloses a contact lens care composition, which contains, by mass, 0.005-5.0% of a hyaluronic acid and antibacterial peptide bonding substance, 0.1-5.0% of a non-ionic surfactant, 0.5-9.0% of an inorganic salt, 0.001-5.0% of a buffer, 0.001-2.0% of a chelating agent, and the balance of sterilized water. According to the present invention, the antibacterial peptide in the formula can provide the disinfection and sterilization effect, and with the hyaluronic acid, the wetting of the contact lens can be ensured, and the gas permeation of the contact lens can be improved; and the contact lens care composition has characteristics of broad-spectrum antibacterial property, cleaning property, moisturizing, mild formula, no irritating and high stability, can be used for cleaning, disinfecting and storage of the contact lens, and can further be used as the wetting agent when wearing the contact lenses.
Owner:刘山
Contact lens care solution
The invention discloses a contact lens care solution with good sterilizing effect. The contact lens care solution is composed of the following components in percentage by weight: 0.1-2% of cellulose, 0.05-0.5% of biguanide polycyclic ethane, 0.05-1% of polyhexamethylene guanidine, 0.1-2% of boric acid, 0.01-0.2% of borax, 0.1-3% of borneol, 1-3% of ethanol, and the balance of distilled water. A preparation method of the contact lens care solution comprises the following steps of: (1) heating up biguanide polycyclic ethane and polyhexamethylene guanidine to 40 DEG C according to a proportion for dissolution; (2) dissolving borneol with ethanol, then adding borneol to a solution of the step (1); (3) adding cellulose, boric acid and borax to the above solution, heating up to 85 DEG C, fully stirring and mixing, then filling to a closed container, standing at the room temperature for 24 hours, and sterilizing for 1 hour; and (4) filling the sterilized and cooled solution to a sterile eye drop bottle.
Owner:孙立伟
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com