Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Bacillus anthracis spore" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anthrax is an acute infectious disease caused by the spore-forming bacterium Bacillus anthracis (B. anthracis). B. anthracis spores are highly infective and can cause inhalation, cutaneous, or gastrointestinal anthrax.
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis
ActiveUS7201912B2Reduce in quantityLow costBacterial antigen ingredientsSnake antigen ingredientsProtective antigenFusion Protein Expression
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis having a variant of recombinant Bacillus anthracis protective antigen (rPA) and a variant of recombinant Bacillus anthracis lethal factor (rLF). These proteins may be expressed separately or as a fusion protein. The recombinant proteins are produced in an avirulent strain of Bacillus anthracis that overproduces the desired antigens. The compositions and methods induce the animal host to produce antibodies against a virulent strain of Bacillus anthracis.
Owner:EMERGENT BIODEFENSE OPERATIONS LANSING
Method for cell surface displaying of target proteins using bacillus anthracis exosporium
The present invention relates to a method for expressing a target protein on the surface of a microorganism using Bacillus anthracis exosporium protein. More particularly, to an expression vector constructed such that it comprises bclA gene encoding Bacillus anthracis exosporium protein BclA or fragments thereof as a cell surface anchoring motif and the target protein can be expressed on the surface of a cell in a form fused with BclA or a fragment thereof when the gene encoding the target protein is expressed in a host cell, as well as, a method for expressing a target protein on the surface of a microorganism using the vector. The expression vector according to the present invention is capable of effectively expressing a target protein or a peptide on the cell surface using BclA, Bacillus anthracis exosporium protein as a cell surface anchoring motif, and since a target protein can be stably expressed on the cell surface in large amounts by culturing a microorganism transformed with the expression vector, thus making it possible to effectively use for the various purposes of recombinant live vaccines, whole cells absorbents, whole cell bioconversion and the like.
Owner:KOREA ADVANCED INST OF SCI & TECH
Expression of protective antigens in transgenic chloroplasts
InactiveUS7354760B2Bacterial antigen ingredientsAntibody mimetics/scaffoldsAntigenProtective antigen
Vectors and methods for plastid transformation of plants to produce protective antigens and vaccines for oral delivery are provided. The invention provides edible vaccines for conferring immunity to a mammal against Bacillus anthracis, as well as Yersina pestis.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
B. anthracis prevention and treatment: mutant B. anthracis lacking luxS activity and furanone inhibition of growth, AI-2 quorum sensing, and toxin production
The present invention pertains to the discovery that B. anthracis possesses a luxS gene that encodes a functional LuxS polypeptide, and that B. anthracis synthesizes a functional AI-2 quorum-sensing molecule. The invention provides mutant B. anthracis bacteria lacking the function of the luxS gene, which do not produce a functional AI-2 molecule and have growth defects compared to wild-type B. anthracis. The invention also concerns methods for inhibiting the growth of B. anthracis, or for preventing or treating B. anthracis infection, by inhibiting the activity of the B. anthracis LuxS polypeptide, or by exposure of the B. anthracis to furanone. In particular, the invention concerns the use of furanone, a compound that inhibits AI-2-mediated quorum-sensing, to inhibit the growth of B. anthracis, to inhibit B. anthracis toxin production, particularly that of protective antigen, and to prevent or treat B. anthracis infection. The invention also provides methods to prevent B. anthracis infection, or enhance an immune response to B. anthracis infection, by administering a vaccine comprising a B. anthracis cell in which the luxS gene is mutated.
Owner:NEW YORK UNIV +1
Antibodies that bind B. anthracis exotoxin, formulations thereof, and methods of use
Owner:ELUSYS THERAPEUTICS
Bacillus anthracis antigens, vaccine compositions, and related methods
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by Bacillus anthracis. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:IBIO
Compositions and methods for detection, prevention, and treatment of anthrax and other infectious diseases
ActiveUS7682796B2High molecular weightEasy to produceAntibacterial agentsBacterial antigen ingredientsSerum igeAgonist
Compositions and methods for the detection, prevention, or treatment of anthrax or other infectious diseases. In one aspect, the present invention provides methods for immunizing humans or animals against Bacillus anthracis or other capsulated pathogens. The methods include administering a capsular polypeptide of a pathogen of interest and a CD40 agonist to a human or animal. The capsular polypeptide or the CD40 agonist is administered in such an amount or frequency that an immunoprotective response can be elicited in the human or animal against the pathogen of interest. In another aspect, the present invention provides methods of using passive immunization with anti-capsular polypeptide antibodies to prevent or treat infections caused by Bacillus anthracis or other pathogens. In yet another aspect, the present invention provides methods useful for diagnosis of anthrax by detection of capsular polypeptide in serum or other biological samples.
Owner:STC UNM +1
Bacillus anthracis antigens, vaccine compositions, and related methods
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by Bacillus anthracis. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:IBIO
Thymine auxotrophic bacillus anthracis and application thereof
The invention discloses a thymine auxotrophic strain and application thereof. The strain is obtained by inactivating the thyA gene and the thyX gene of bacillus anthracis, which is specifically bacillus anthracis CGMCC No.3405. The strain can serve as a host to build a balanced lethal expression system for studying relative bacillus anthracis vaccines and laying a foundation for the study of novel vaccines.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Curlicue vaccine strain of Bacillus anthracis
A new strain of Bacillus anthracis derived from the Sterne vaccine strain of Bacillus anthracis by growth on a high-nitrate-concentration, 3-amino-L-tyrosine growth medium.
Owner:AIR FORCE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE
Peptide ligands for Bacillus anthracis spore detection
InactiveUS20070281302A1Microbiological testing/measurementPeptide preparation methodsSporePaenibacillus lactis
The present invention relates to peptides that bind to the bacterial spores, such as B. anthracis, B. subtilis and B. cereus spores. The present invention also relates to method of identifying such peptides using phage display ligand screening system. The present invention further relates to the use of such peptides for the detection of bacterial spores, such as Bacillus anthracis spores.
Owner:UAB RES FOUND
Diagnosis of Bacillus anthracis infection based on detection of bacterial secreted biomarkers
InactiveUS8591899B2Microbiological testing/measurementBiological material analysisBacteroidesGene product
Owner:STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG (A R O) (VOLCANI CENT)
B. anthracis prevention and treatment: mutant b. anthracis lacking luxs activity and furanone inhibition of growth, ai-2 quorum sensing, and toxin production
The present invention pertains to the discovery that B. anthracis possesses a luxS gene that encodes a functional LuxS polypeptide, and that B. anthracis synthesizes a functional AI-2 quorum-sensing molecule. The invention provides mutant B. anthracis bacteria lacking the function of the luxS gene, which do not produce a functional AI-2 molecule and have growth defects compared to wild-type B. anthracis. The invention also concerns methods for inhibiting the growth of B. anthracis, or for preventing or treating B. anthracis infection, by inhibiting the activity of the B. anthracis LuxS polypeptide, or by exposure of the B. anthracis to furanone. In particular, the invention concerns the use of furanone, a compound that inhibits AI-2-mediated quorum-sensing, to inhibit the growth of B. anthracis, to inhibit B. anthracis toxin production, particularly that of protective antigen, and to prevent or treat B. anthracis infection. The invention also provides methods to prevent B. anthracis infection, or enhance an immune response to B. anthracis infection, by administering a vaccine comprising a B. anthracis cell in which the luxS gene is mutated.
Owner:NEW YORK UNIV +1
Spore specific antibodies
InactiveUS20060286612A1Quick checkQuick identificationBiological material analysisImmunoglobulins against bacteriaPaenibacillus lactisSpecific antibody
The invention relates to spore specific antibodies. Compositions and methods relating to the antibodies are provided along with the hybridomas that produce the antibodies. The antibodies are specific for the spores of B. anthracis relative to the vegetative form of the cells. The antibodies are also specific for the spores relative to other Bacillus spores and cells. The antibodies may be used to detect the presence of B. anthracis spores by use of methods provided herein. The invention also relates to articles of manufacture as well as kits comprising these antibodies which may be used in the detection methods of the invention.
Owner:TETRACORE
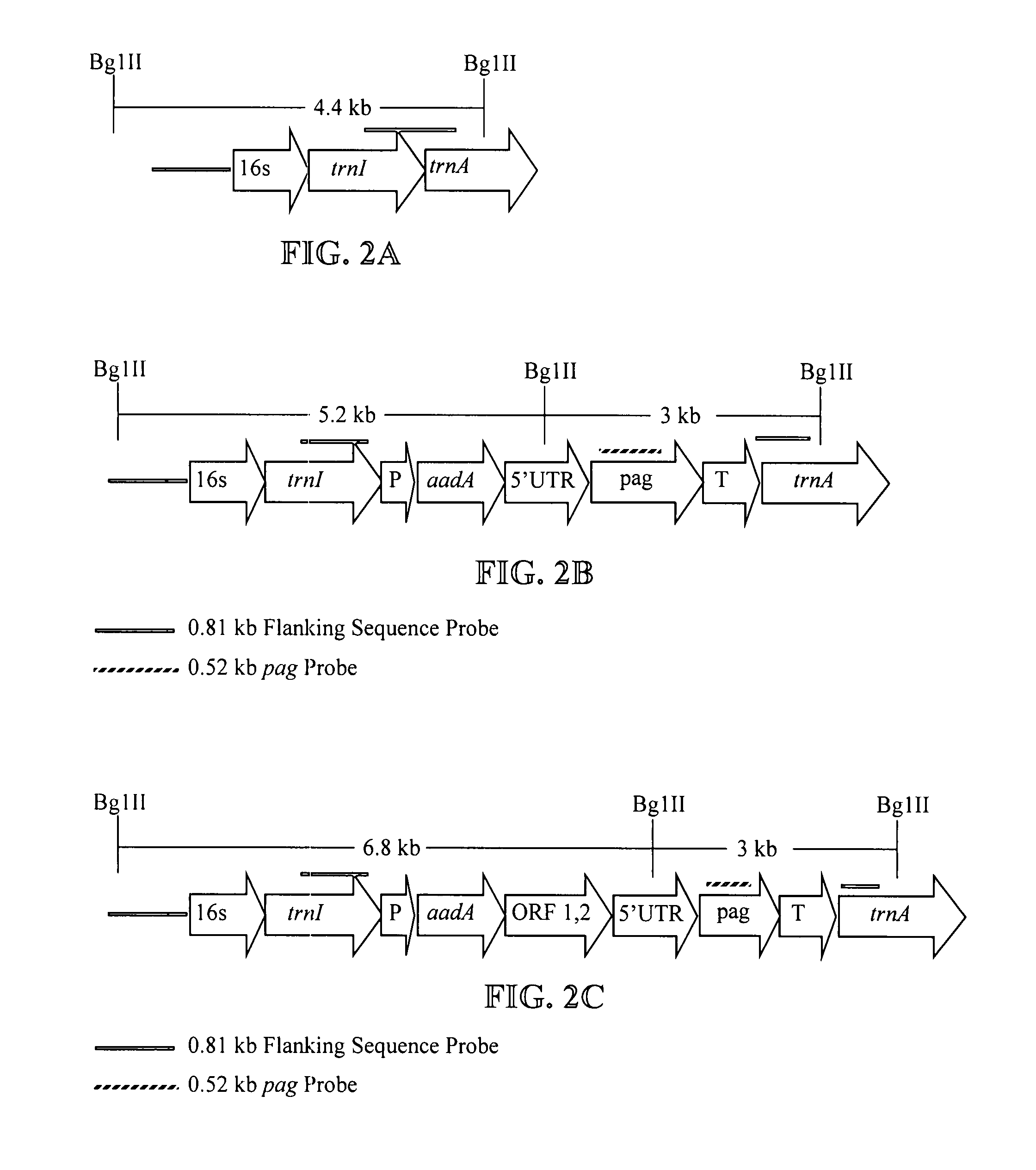
Recombinant modified bacillus anthracis protective antigen for use in vaccines
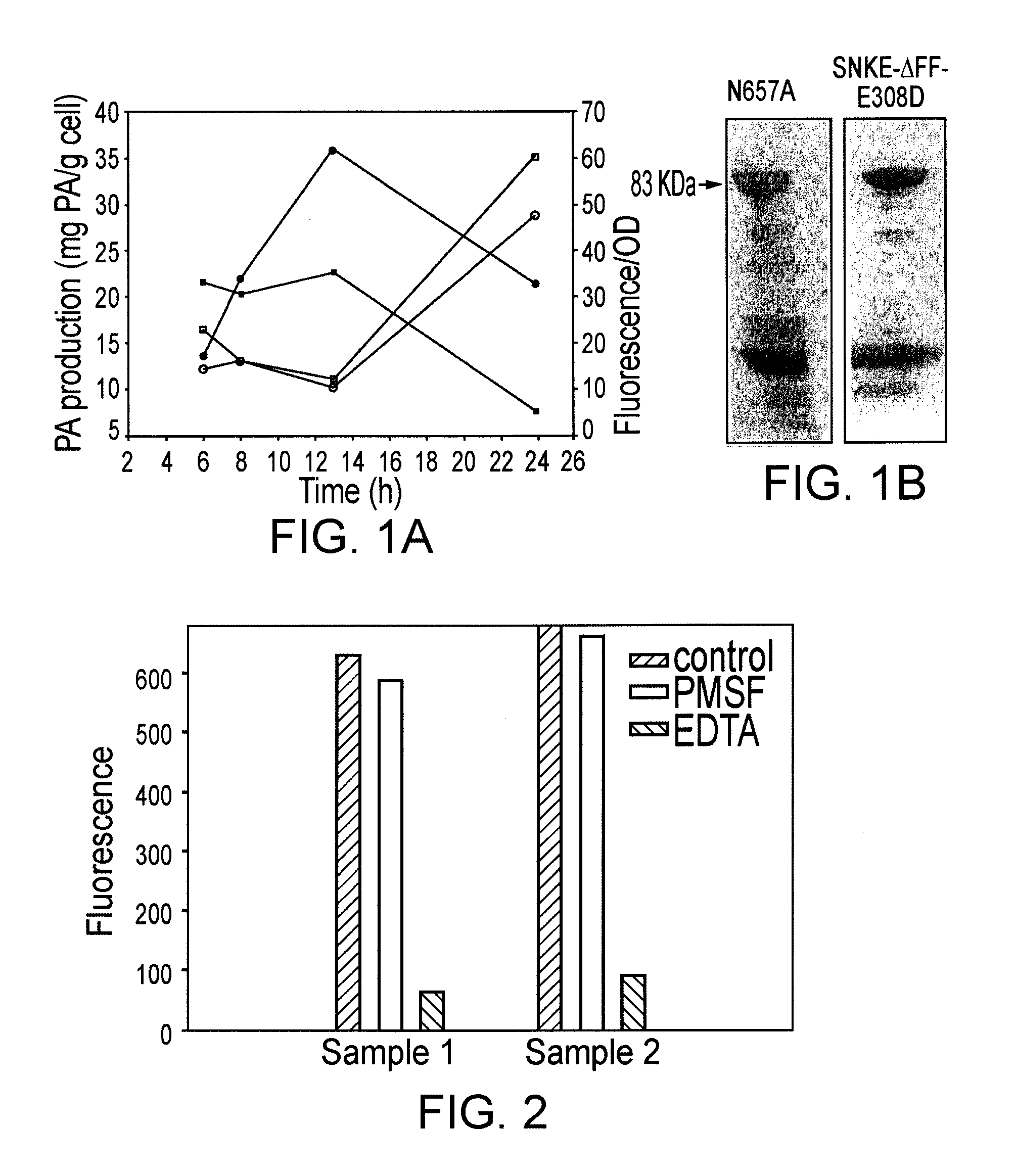
The invention relates to improved methods of producing and recovering sporulation-deficient B. anthracis mutant stains, and for producing and recovering recombinant B. anthracis protective antigen (PA), especially modified PA which is protease resistant, and to methods of using of these PAs or nucleic acids encoding these PAs for eliciting an immunogenic response in humans, including responses which provide protection against, or reduce the severity of, B. anthracis bacterial infections and which are useful to prevent and / or treat illnesses caused by B. anthracis, such as inhalation anthrax, cutaneous anthrax and gastrointestinal anthrax.
Owner:UNITED STATES OF AMERICA
Assay and compositions for detection of Bacillus anthracis nucleic acid
InactiveUS7494774B2Bacterial antigen ingredientsSugar derivativesBacteroidesNucleic acid hybridisation
The invention includes compositions and methods of detection of Bacillus anthracis that use oligonucleotide probes specific for genetic material contained in the pXO1 and pXO2 plasmids in nucleic acid hybridization reactions. Embodiments of the method may include additional probes specific for other gene sequences to distinguish B. anthracis from other bacterial species present in a sample or to provide an indication that the assay was performed properly even when no Bacillus sequence is detected.
Owner:GEN PROBE INC
Genetic vaccines directed against bacterial exotoxins
The invention provides a gene transfer vector comprising a humanized nucleic acid sequence encoding an immunogenic portion of one or more exotoxins of Bacillus anthracis and a heterologous sorting signal. The invention also provides a method of producing an immune response against Bacillus anthracis in a host comprising administering to the host the gene transfer vector.
Owner:CORNELL RES FOUNDATION INC
Preparation for protein suspending chip of bacillus anthracis spore and its quantitative determination method
InactiveCN101504415ACoating volume improvementReduce the amount of coatingMaterial analysisBacteroidesQuantitative determination
The invention relates to a preparation method, a detection method and a quantitative method for a suspension chip of a bacillus anthracis spore protein. The methods have the advantages of good detection capability, high sensitivity, strong specificity and wide dynamic range, and establish an open detection modeling platform for bacterial spores represented by bacillus anthracis.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Diagnosis of bacillus anthracis infection based on detection of bacterial secreted biomarkers
InactiveUS20120015379A1Microbiological testing/measurementBiological material analysisBacteroidesFhit gene
A novel and rapid diagnostic method for anthrax infection is provided. Three B. anthracis gene products are described, as well as antibodies against the same, which may be used for the detection and monitoring of anthrax infection. Kits for the diagnosis of anthrax are also provided.
Owner:THE STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG ARO VOLCANI CENT
Application of dominant suppressing mutant F427D as anthrax bacillus toxin inhibitor and vaccine
InactiveCN101392232BImproving immunogenicityNo risk of latent infectionAntibacterial agentsBacterial antigen ingredientsAntigenEscherichia coli
The invention relates to the technical fields of animal bacteriology and zoonosis science, particularly to an application of a dominant inhibitory mutant F427D as an inhibitor of Bacillus anthracis toxin and an application of vaccine thereof. The invention, through cloning of Bacillus anthracis lethal toxin gene, expression and purification of proteins, site-directed saturation mutagenesis of genes and the like, obtains protective antigen (namely the dominant inhibitory mutant F427D) of the Bacillus anthracis, the mutant is successfully cloned into pronucleus expression carrier pGEX-KG and composes recomposed plasmid pGEX-KG-PA (F427D). Escherichia coli containing the recomposed plasmid is nominated as Escherichia coli DH5Alpha / pGEX-KG-PA (F427D) and collected in the China Center for TypeCulture Collection with a collection number of CCTCC-M208158. The invention further discloses the method for preparing the antigen protein, the preparation of anthrax toxin inhibitor and subunit vaccine by using the antigen protein and the application thereof.
Owner:HUAZHONG AGRI UNIV
Peptides for selectively and specifically binding Bacillus anthracis spores and use thereof in landscape phages
InactiveUS20070037230A1Produced rapidly and inexpensivelyBiological material analysisImmunoglobulins against bacteriaSporeBacillus aryabhattai
Several peptides for selectively and specifically binding Bacillus anthracis spores are disclosed herein. It is contemplated that the isolated peptides will be incorporated into diagnostic probes. The diagnostic probes may include landscape page probes and antibody probes, as well as any other probes known in the art. Inventors contemplate that the probes could be further used in real-time continuous monitoring of the environment so that detection systems for monitoring the low concentrations of Bacillus anthracis spores may be developed.
Owner:AUBURN UNIV
Bacillus anthracis capable of showing PA20 protein on surface of spore and application thereof
The invention discloses a bacillus anthracis capable of showing a PA20 protein on the surface of a spore and an application thereof. The bacillus anthracis is obtained by inserting a pagA20 gene into the tail end of a bacillus anthracis spore protein bcIA gene; and particularly the bacillus anthracis can be a bacillus anthracis CGMCC No.3403. The bacillus anthracis can serve as a host and is used for preparing, an immunomodulator which is preferably an inactivated vaccine, an attenuated live vaccine, a subunit vaccine or a gene engineering vaccine.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Chemical inactivation of bacillus anthracis spores in soil
A method for the inactivation of B. anthracis spores in soil comprising contacting the soil with an effective amount of a persulfate and an activator for a time sufficient to inactivate substantially all of the B. anthracis spores contained therein.
Owner:ENVIRONMENTAL PROTECTION AGENCY US +1
Antibodies for the detection of Bacillus anthracis and vaccine against B. anthracis infections
The present invention relates to conjugates of oligosaccharides of formula 1, wherein R is a linker to a carrier protein and optionally comprises up to three further saccharides, 5 and which are useful for vaccination, methods of synthesis of such conjugates, antibodies against this antigen, hybridoma producing monoclonal antibodies against this antigen, assays using these antibodies for the detection of B. anthracis spores and kits comprising these antibodies, and a vaccine for the prevention of B. anthracis infection comprising the conjugates of oligosaccharides of formula 1. Monoclonal antibodies 10 according to the invention selectively bind to B. anthracis, but not to related bacteria such as B. subtilis, B. cereus and other bacteria of this group such as B. thuringiensis.
Owner:ETH ZZURICH +1
Dual anthrax-plague vaccines that can protect against two tier-1 bioterror pathogens, bacillus anthracis and yersinia pestis
ActiveUS20180264102A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsPlague vaccineAnaplasma phagocytophilum
Bivalent immunogenic compositions against anthrax and plague are disclosed herein. One bivalent immunogenic composition comprises a triple fusion protein containing three antigens, F1 and V from Yersinia pestis and PA antigen from Bacillus anthracia fused in-frame and retaining structural and functional integrity of all three antigens. Another bivalent immunogenic composition comprises bacteriophage nanoparticles arrayed with these three antigens on the capsid surface of the bacteriophage nanoparticles. These bivalent immunogenic compositions are able to elicit robust immune response in a subject administered said the bivalent immunogenic compositions and provide protection to the subject against sequential or simultaneous challenge with both anthrax and plague pathogens.
Owner:CATHOLIC UNIV OF AMERICA
Chemical inactivation of bacillus anthracis spores in soil
A method of inactivating B antracis spores in a contaminated target environment by: exposing the environment containing said spores to an effective amount of persulfate in solution and an oxidation agent, and allowing the persulfate solution and oxidation agent to remain in contact with the environment containing said spores for sufficient time to inactivate the spores.
Owner:ENVIRONMENTAL PROTECTION AGENCY US +1
Detection, prevention, and treatment of anthrax and other infectious diseases
ActiveUS9435804B2High molecular weightEasy to produceBacterial antigen ingredientsBiological material analysisAgonistOrganism
Compositions and methods for the detection, prevention, or treatment of anthrax or other infectious diseases. In one aspect, the present invention provides methods for immunizing humans or animals against Bacillus anthracis or other capsulated pathogens. The methods include administering a capsular polypeptide of a pathogen of interest and a CD40 agonist to a human or animal. The capsular polypeptide or the CD40 agonist is administered in such an amount or frequency that an immunoprotective response can be elicited in the human or animal against the pathogen of interest. In another aspect, the present invention provides methods of using passive immunization with anti-capsular polypeptide antibodies to prevent or treat infections caused by Bacillus anthracis or other pathogens. In yet another aspect, the present invention provides methods useful for diagnosis of anthrax by detection of capsular polypeptide in serum or other biological samples.
Owner:RGT UNIV OF MINNESOTA +2
Combination Antibodies For The Treatment And Prevention Of Disease Caused By Bacillus Anthracis And Related Bacteria And Their Toxins
InactiveUS20110129460A1Enhanced protection against infectionImprove survival rateAntibacterial agentsBacterial antigen ingredientsDiseaseBacteroides
The invention relates to methods and compositions for the prevention and treatment of disease caused by B. anthracis or a bacterium which produces toxins or toxin components homologous to the virulence factors produced by B. anthracis or to the toxins or toxin components themselves, in the absence of bacteria. The methods and compositions of the invention comprise a combination of at least two antibodies, preferably monoclonal antibodies, most preferably human monoclonal antibodies, each of which binds with high affinity to a different epitope of one or more bacterial antigens.
Owner:IQ THERAPEUTICS
Novel protein capable of inhibiting anthrax toxin activity
The invention particularly relates to inhibition of the cleavage of protective antigen (PA) of Bacillus anthracis, which subsequently leads to inhibition of activity of anthrax toxin.
Owner:COUNCIL OF SCI & IND RES
Agent exhibiting bactericidal action with respect to vegetative and spore cells bacillus anthracis, anthrax preventing and treating method
InactiveUS20140248250A1Peptide/protein ingredientsBacteria material medical ingredientsSporeLysobacter sp.
The drug has a bactericidal effect on vegetative and spore cells of Bacillus anthracis and comprises a bacteriolytic complex produced by Lysobacter sp. XL 1. The bacteriolytic complex produced by is in a method of anthrax prevention and treatment.
Owner:FISIOLOGII MIKROORGANISMOV IM G K SKRJABINA RAN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com