Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Atovaquone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
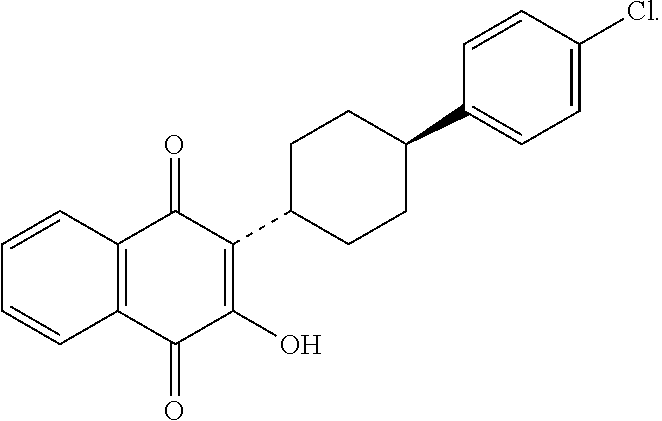
Atovaquone is used to prevent or treat a serious lung infection called Pneumocystis pneumonia (PCP).
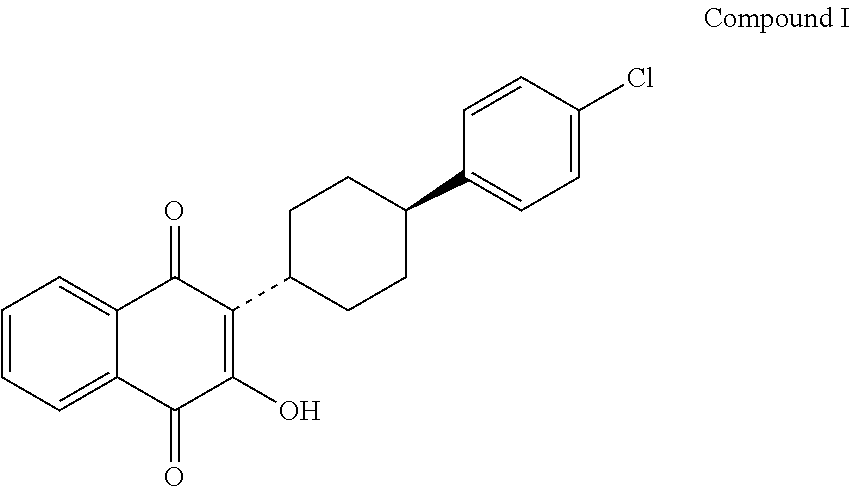
Noval polymorphs of atovaquone and process of
InactiveUS20060241311A1Increase production capacityImprove performanceBiocideQuinone separation/purificationAtovaquoneNaphthoquinone
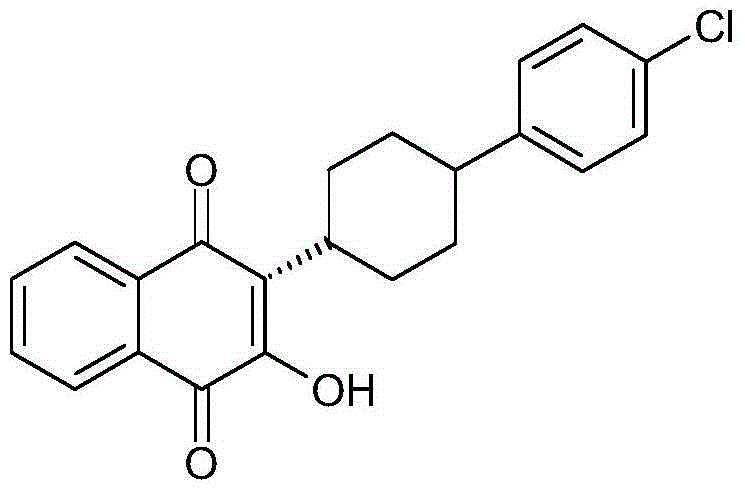
Novel crystalline forms of anti Pneumocystis carinii compound (2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone) commonly known as Atovaquone and methods for producing the same is disclosed herein. This also provides pharmaceutical compositions comprising the said polymorphs of Atovaquone and method of treating Pneumocystis carinii pneumonia, the method comprising administering to a warm blooded animal an effective amount of a product-by-process composition of matter comprising polymorphic forms of Atovaquone.
Owner:USV LTD
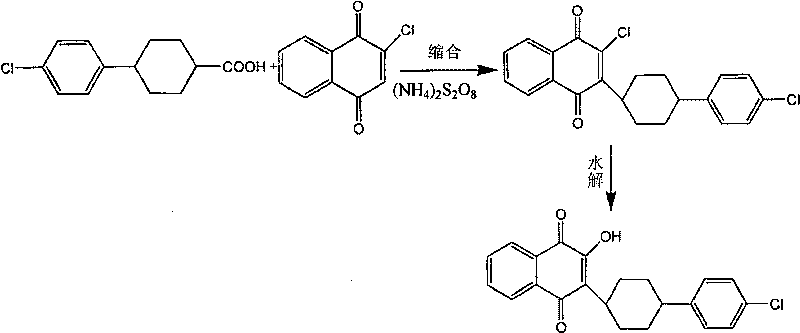
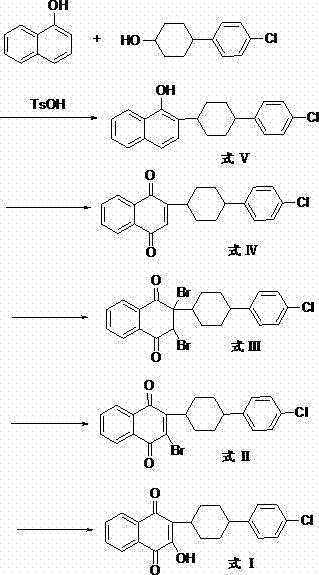
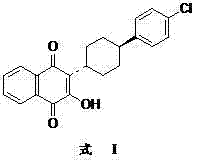
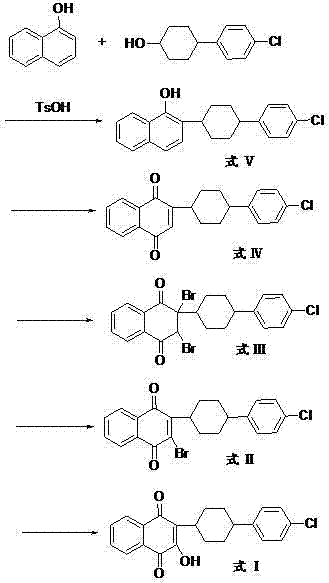
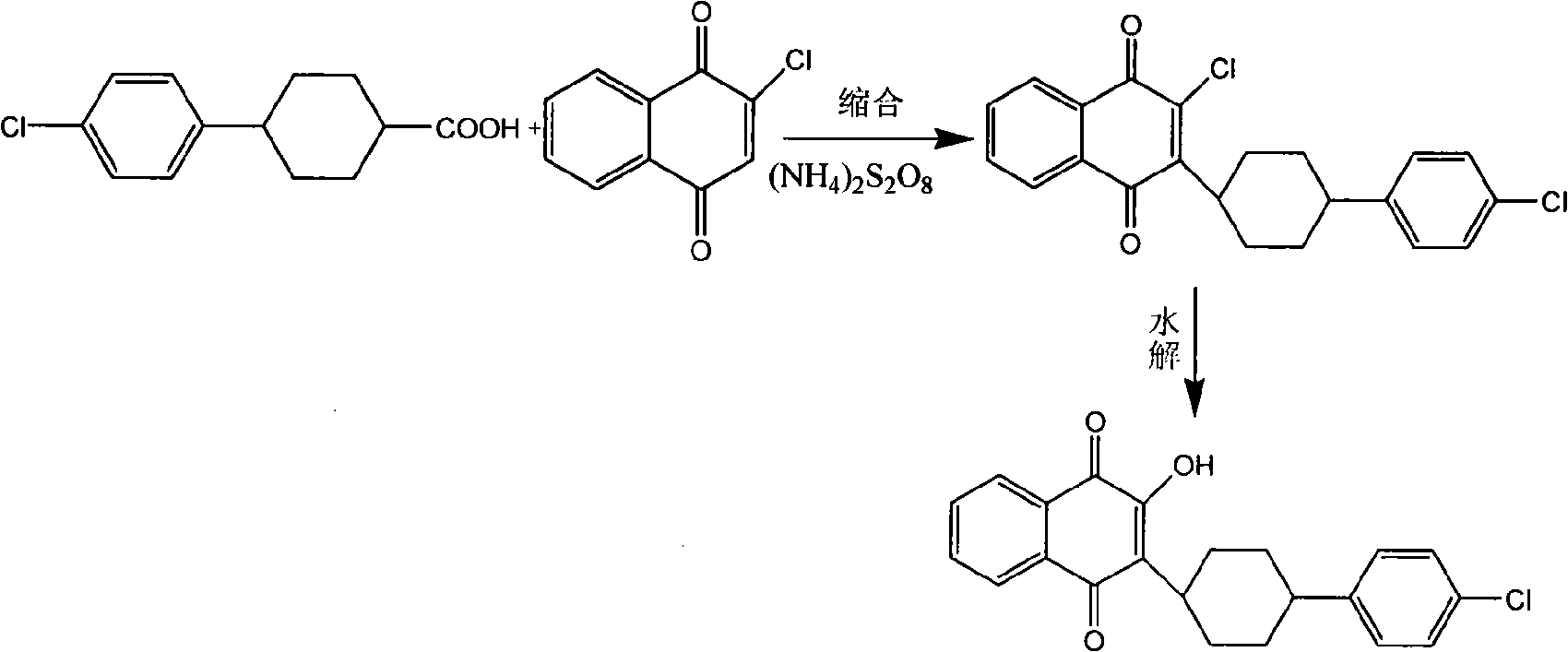
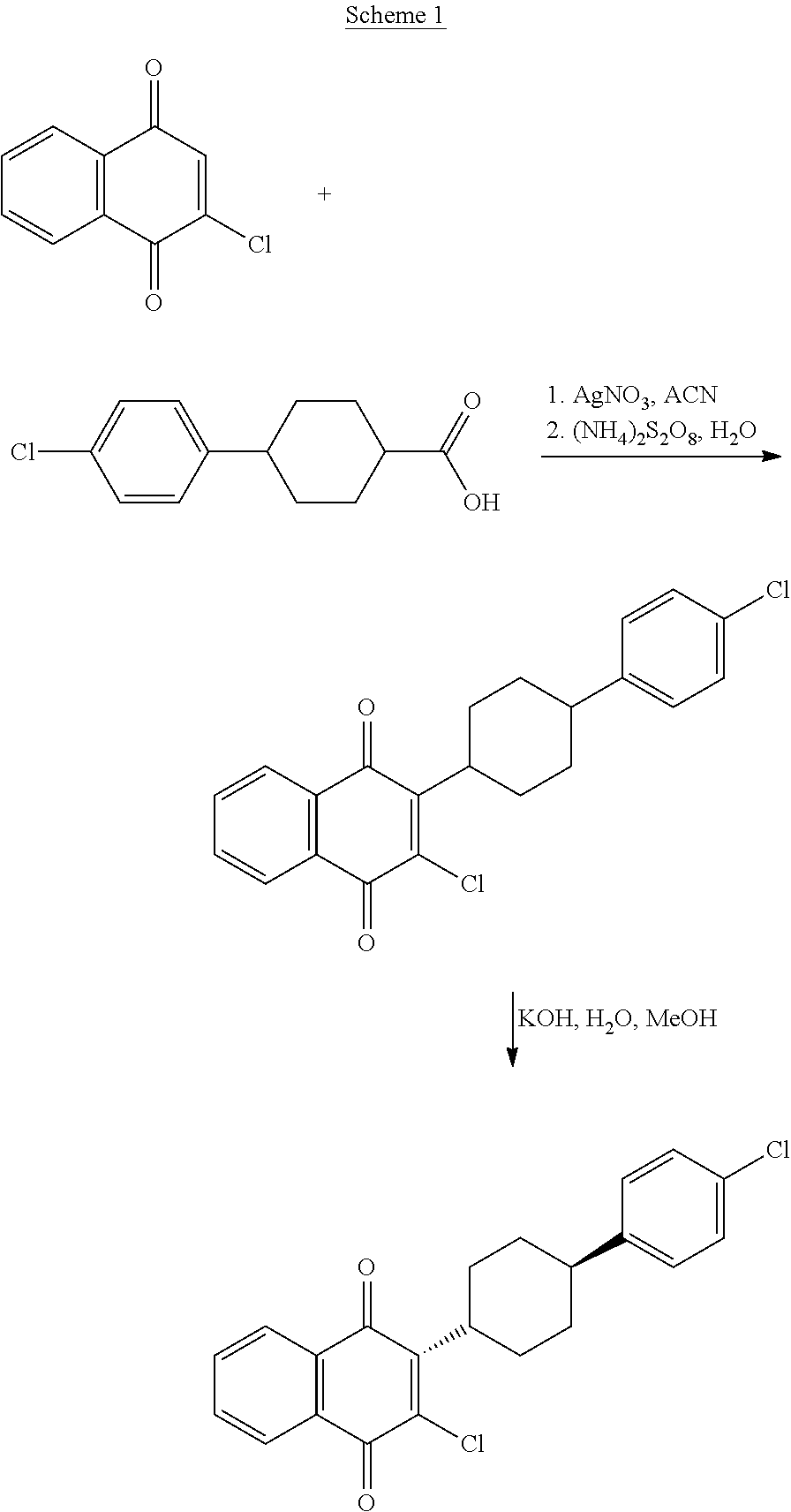
Preparation method of atovaquone
InactiveCN103570521AReduce usageReduce adverse effectsOrganic compound preparationQuinone preparation by oxidationAtovaquoneAcid catalysis
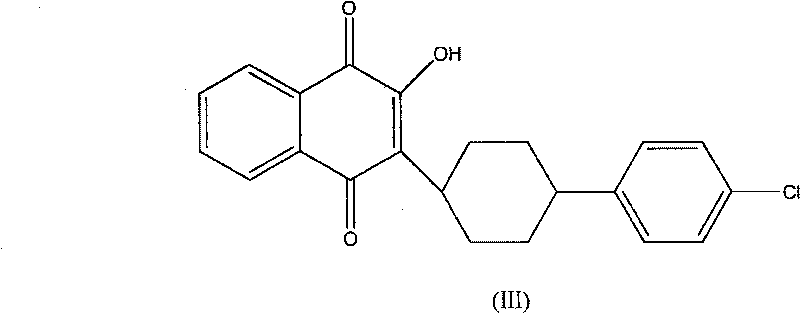
The invention discloses a preparation method of atovaquone, and belongs to the drug synthesis field. The method comprises the following steps: condensing alpha-naphthol and 4-(4-chlorophenyl)cyclohexanol under acid catalysis to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol (formula V), oxidizing the compound represented by formula V to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula IV), enabling the compound represented by the formula IV to react with bromine in additive reaction to obtain 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III), releasing a molecule of hydrogen bromide to obtain 3-bromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula II), hydrolyzing to obtain the atovaquone (formula I). Compared with the prior art, the method disclosed by the invention is simple in process, the expensive silver nitrate is prevented from using in the preparation process; and meanwhile, the yield is improved, the pollution to the environment is reduced, and the method has good popularization and application value.
Owner:SHANDONG LUKANG SHELILE PHARMA
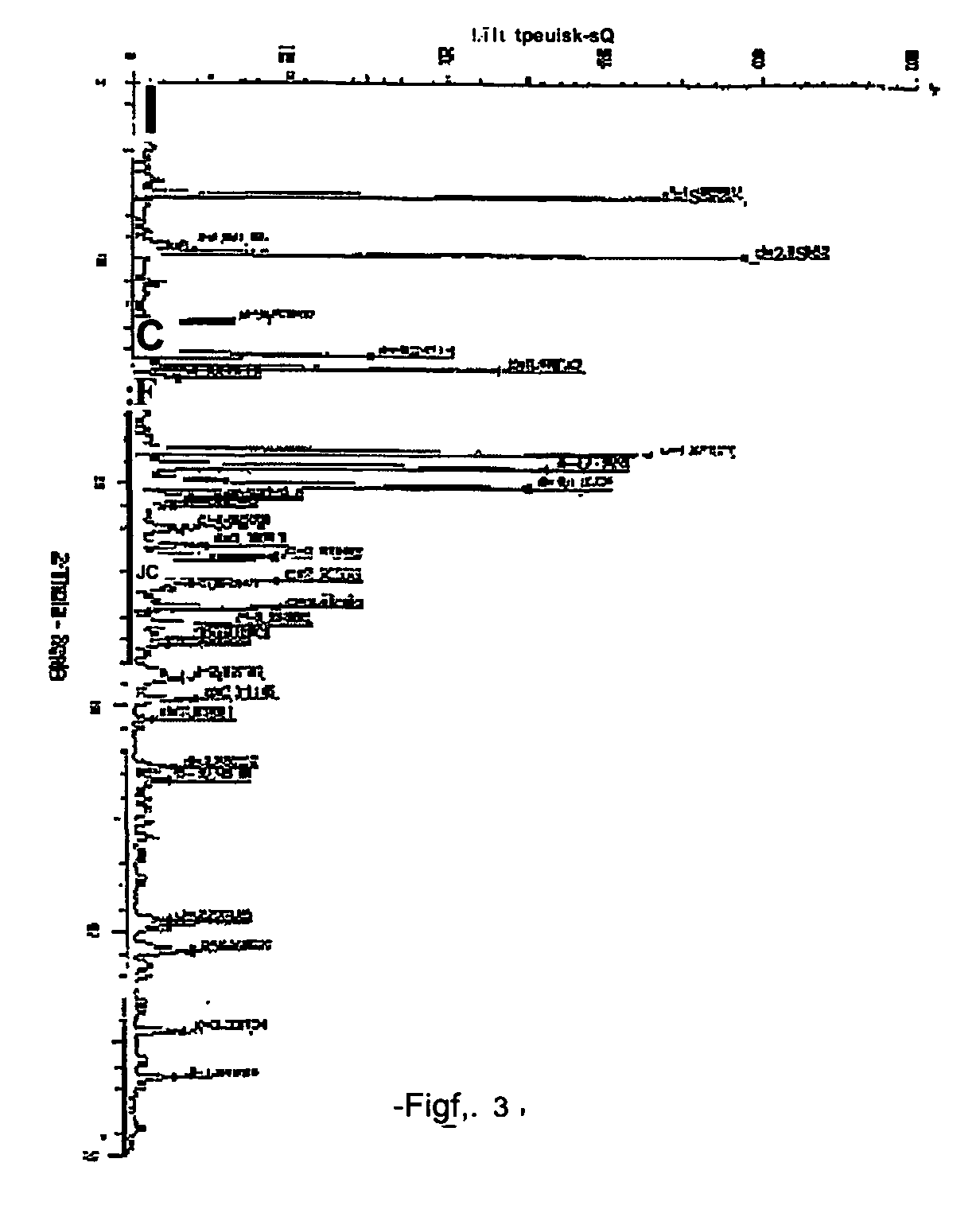
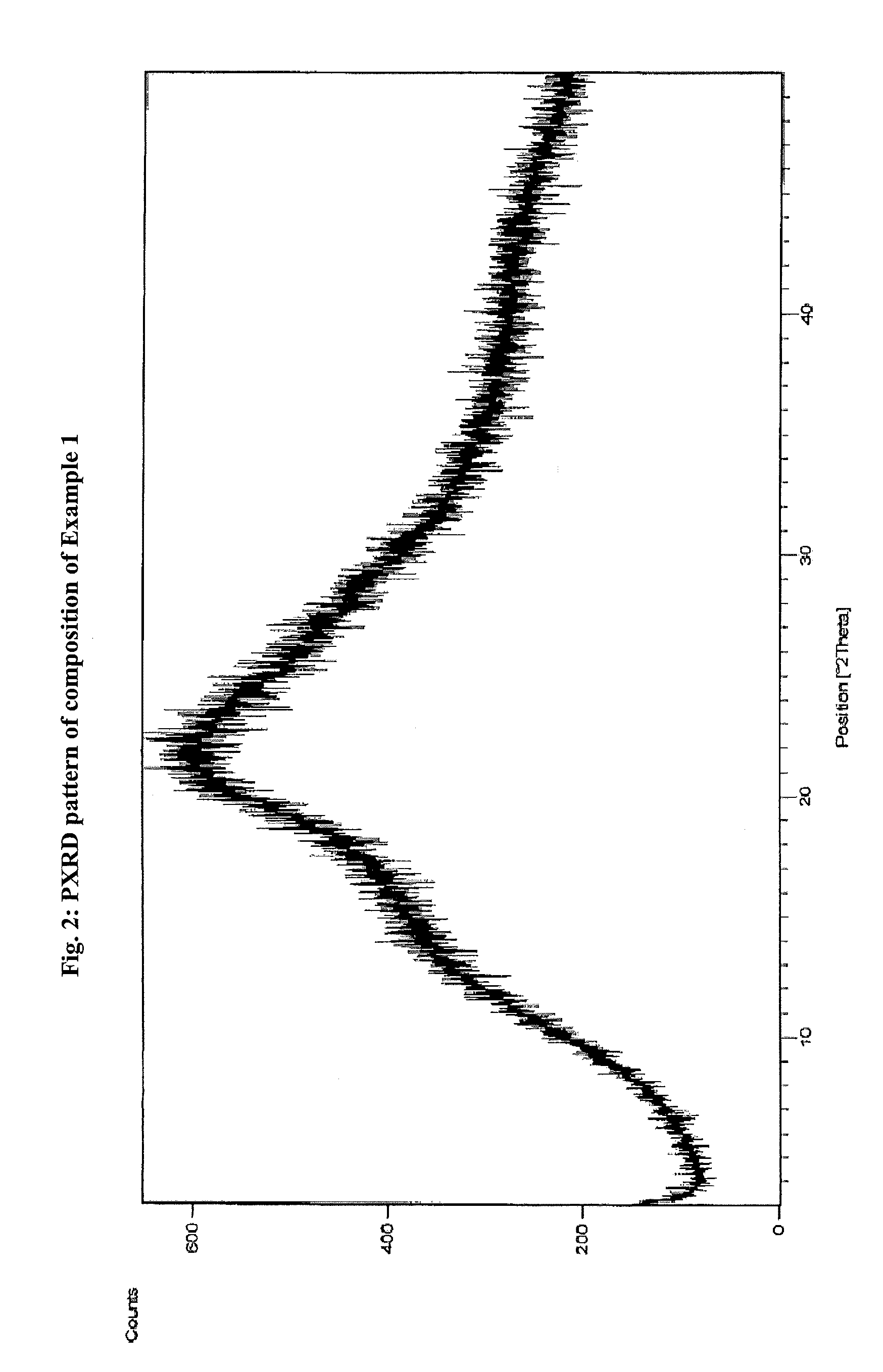
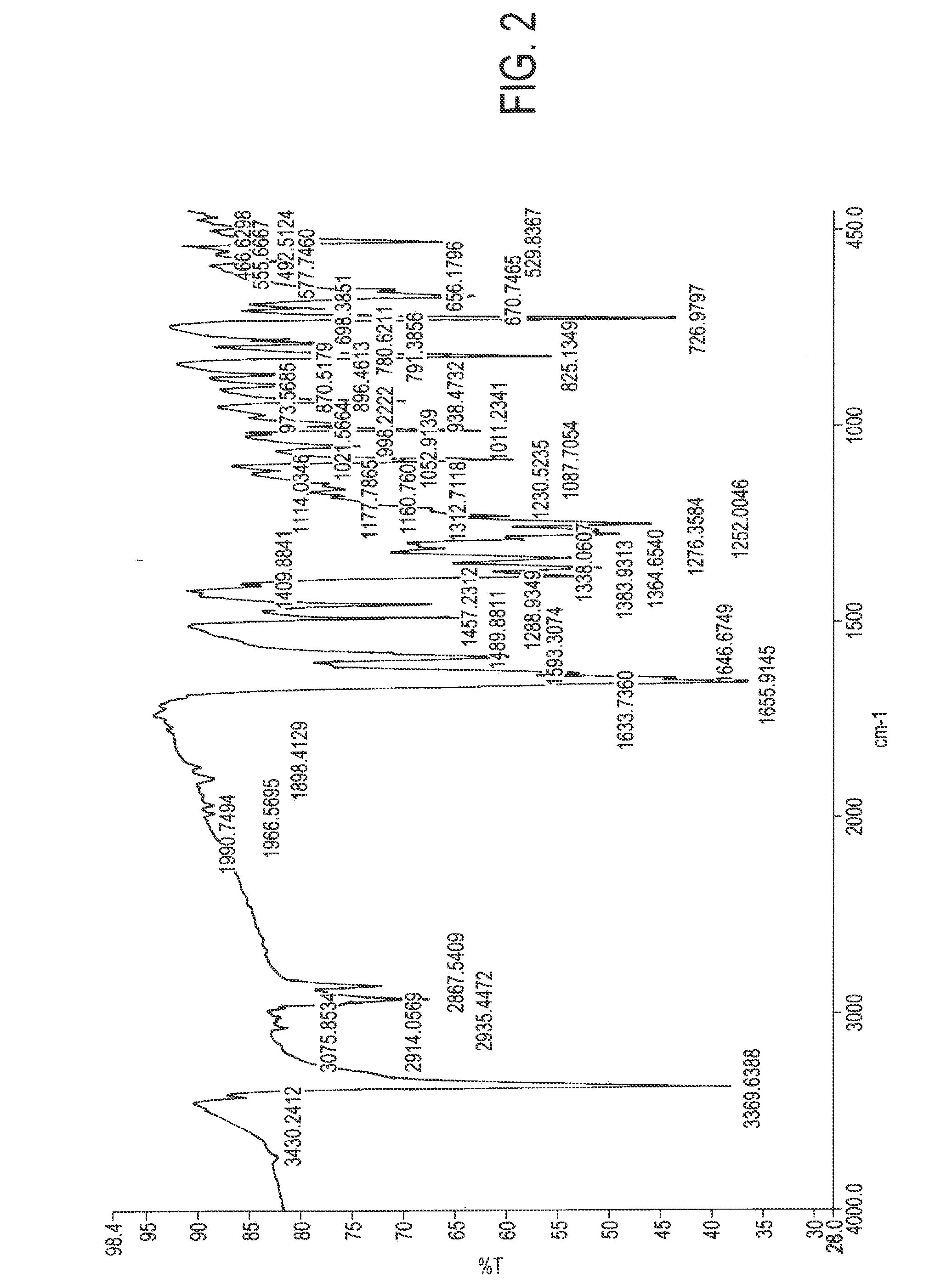
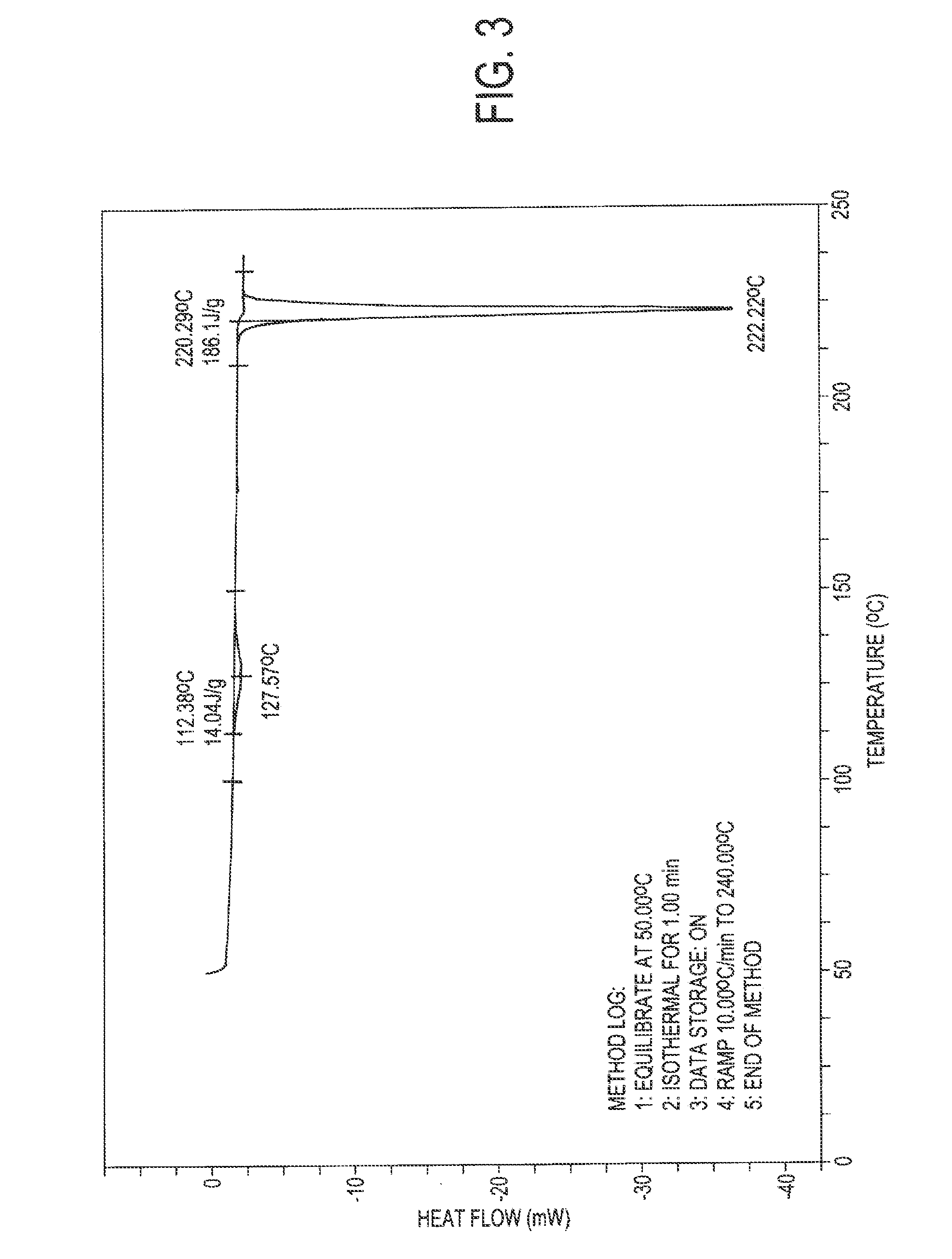
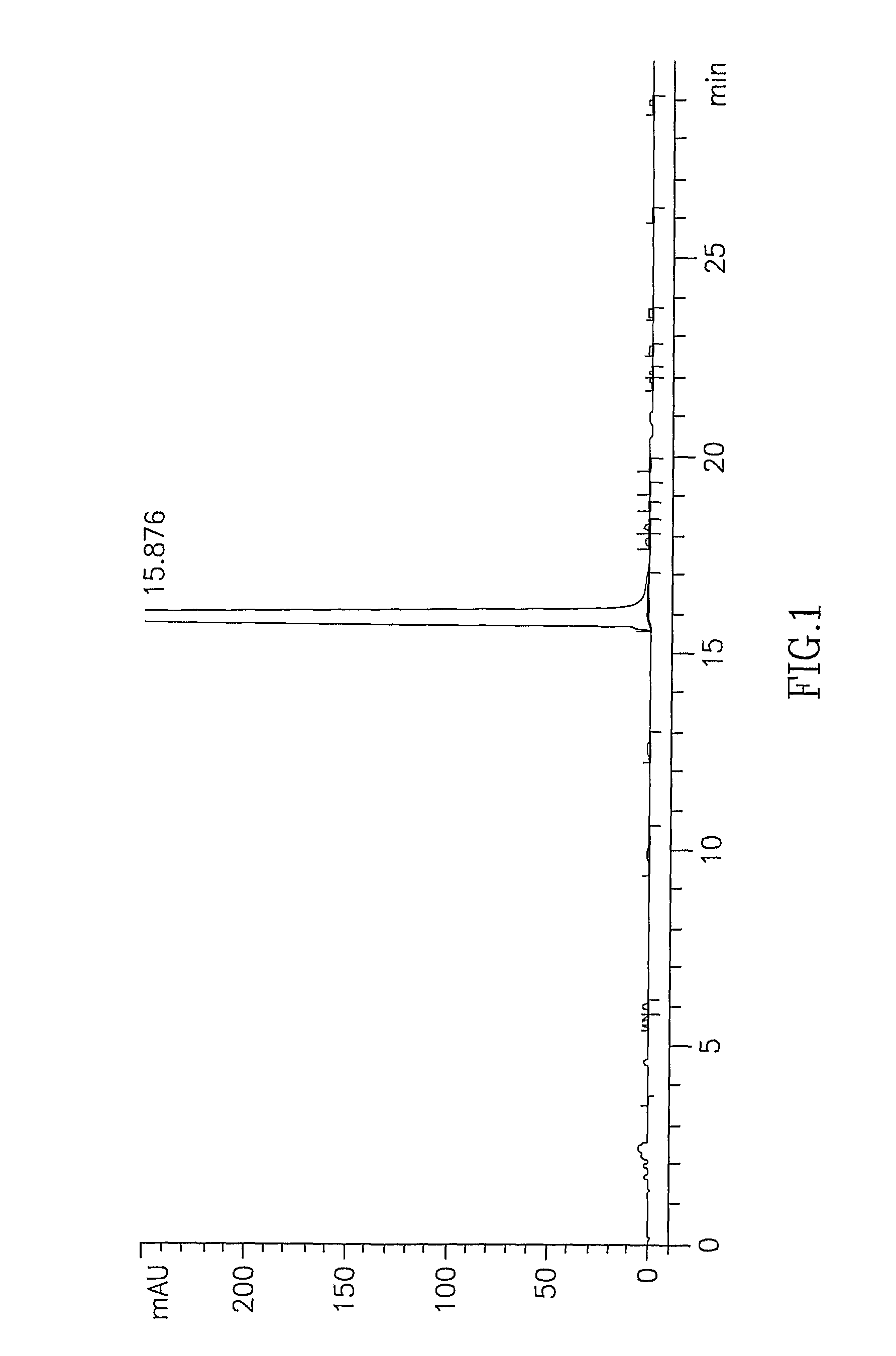
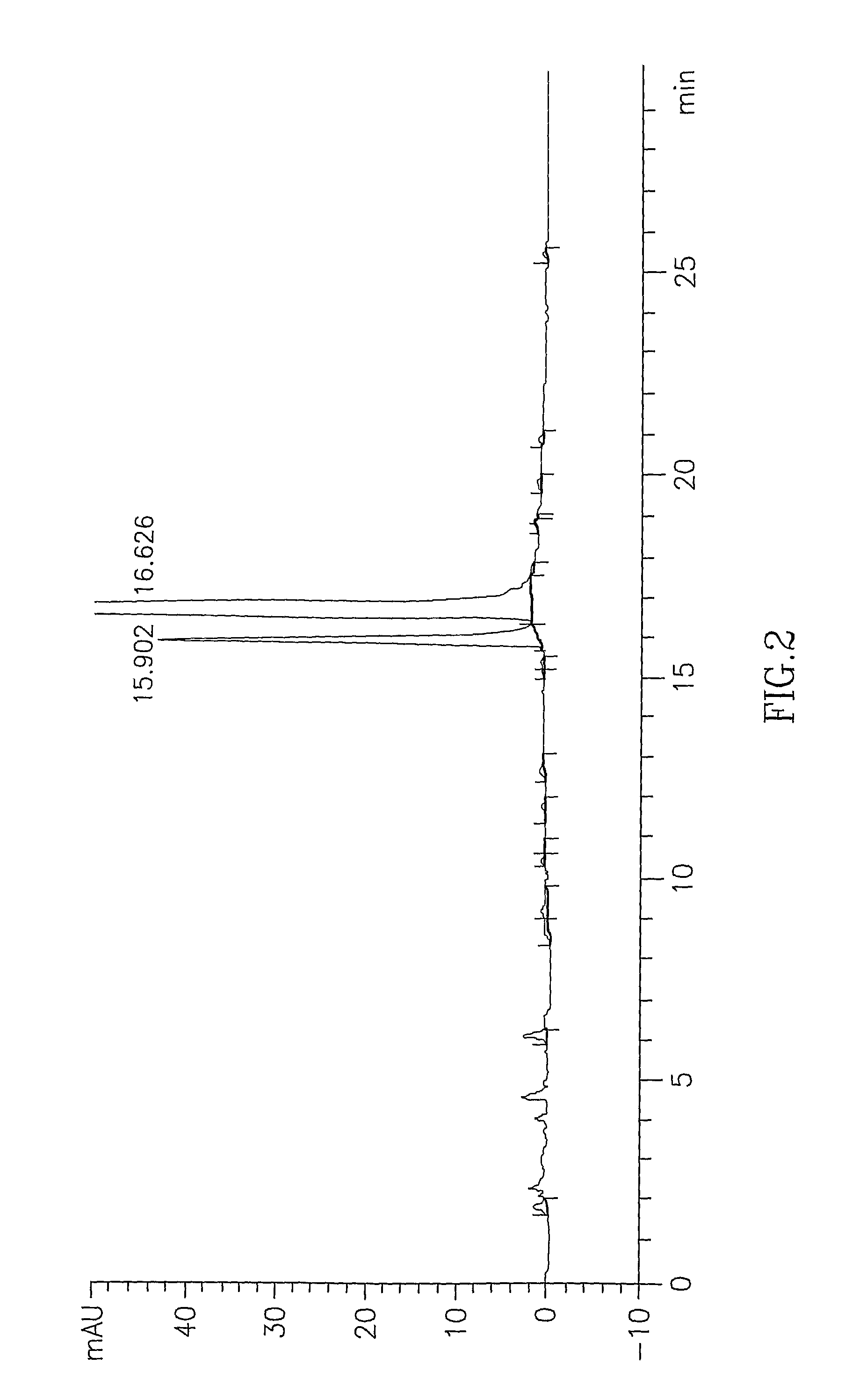
Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m
Atovaquone or a pharmaceutically acceptable salt thereof having a particle size diameter range with a D90 of between greater than 3 to about 10[mu]m.
Owner:ALPHAPHARM PTY LTD
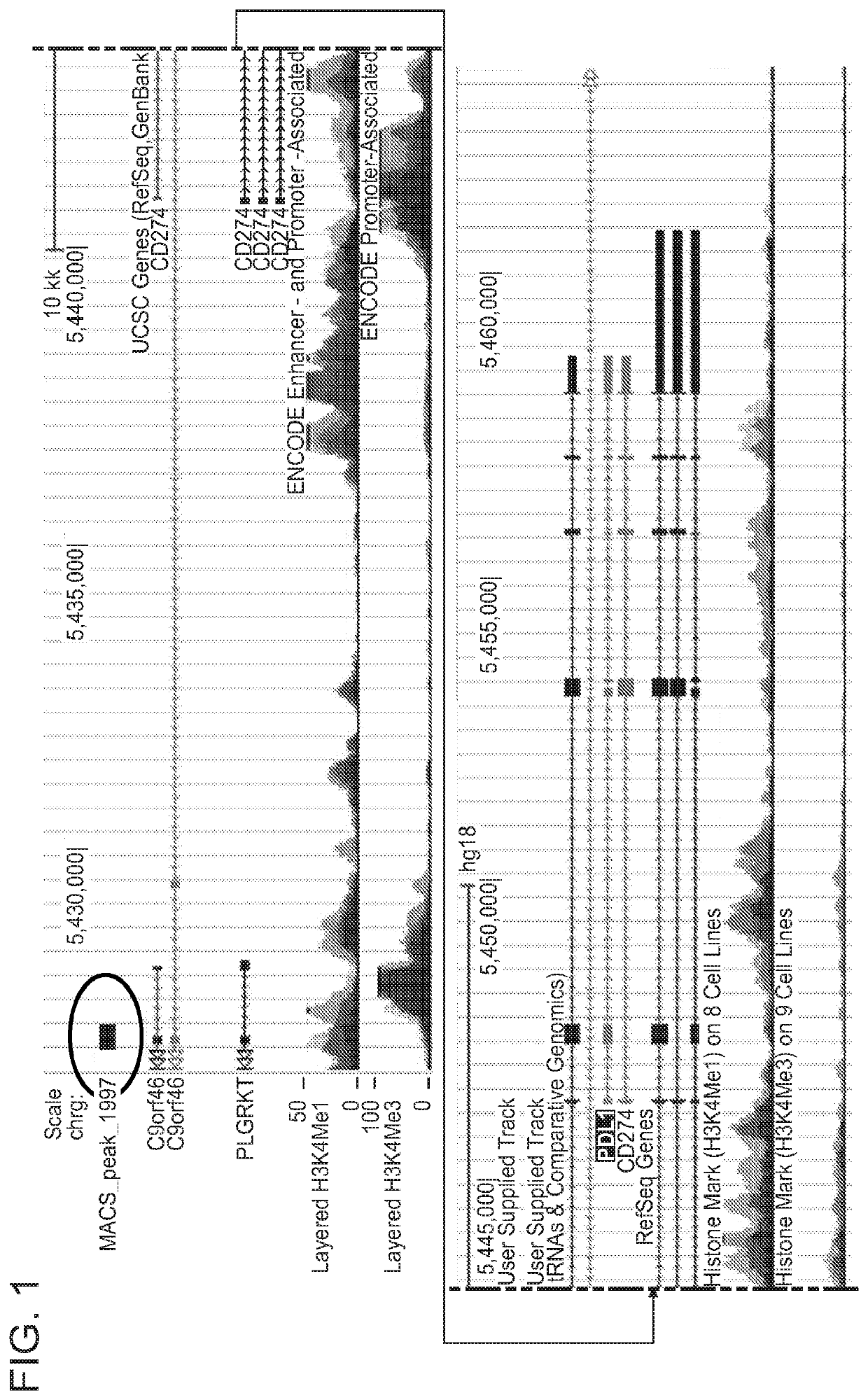
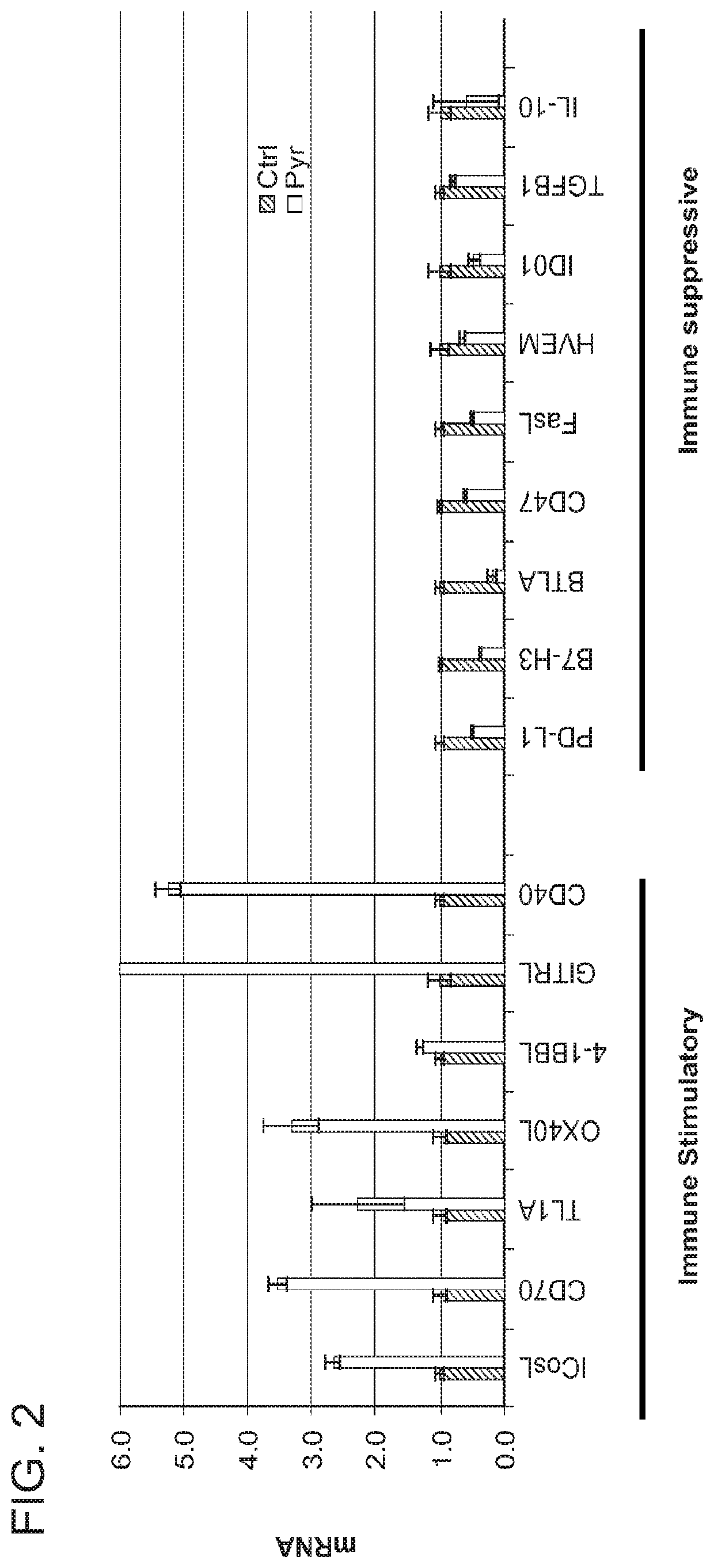
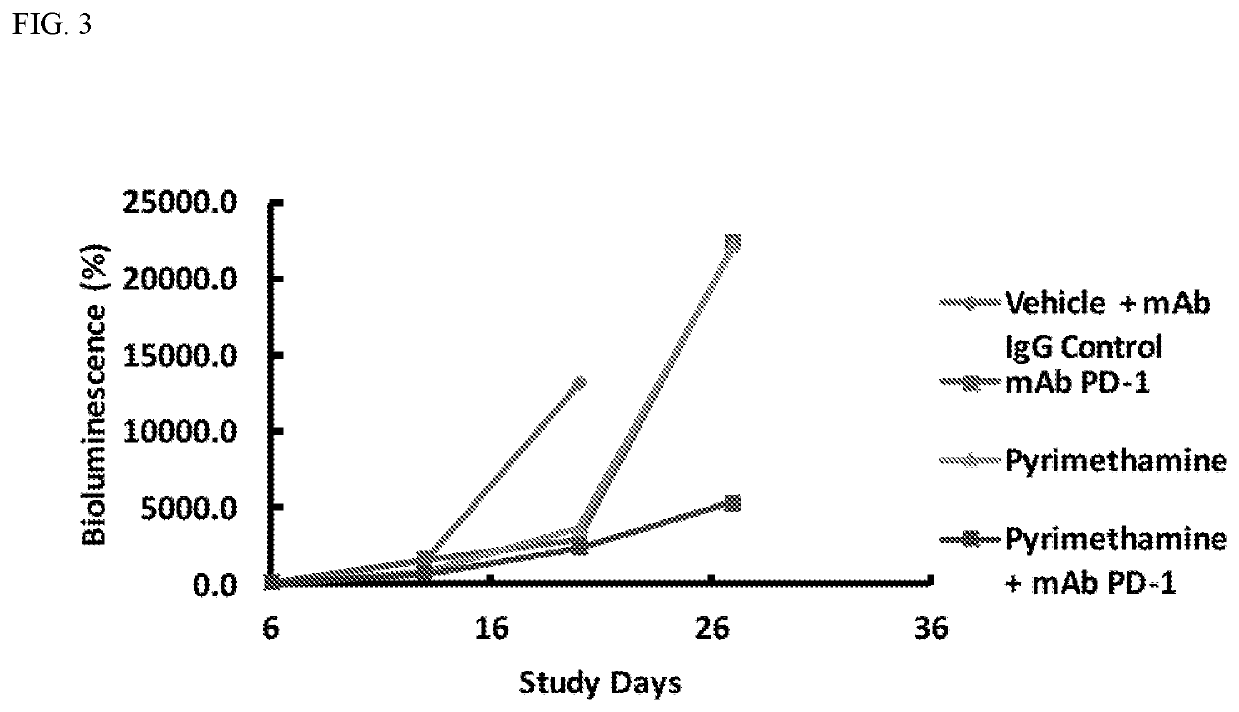
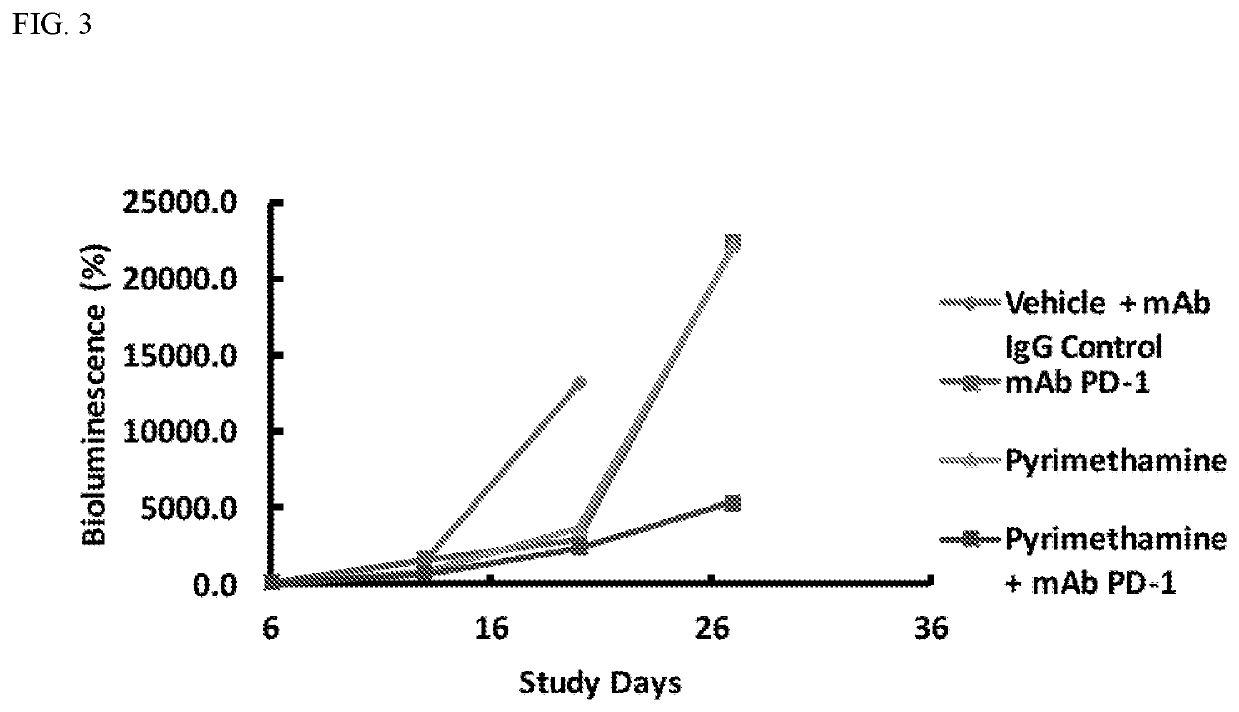
Enhanced immunotherapy of cancer using targeted transcriptional modulators
ActiveUS20200230135A1Improving immunogenicityImprove efficiencyOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAntiendomysial antibodies
The present invention relates to a combination of a signal transducer and activator of transcription 3 (STAT3) activity inhibitor; and an immune checkpoint inhibitor for use in treating or preventing the hyperproliferative disorder in a subject. Preferably, the STA3 activity inhibitor is pyrimethamine or atovaquone, and the immune checkpoint inhibitor is an anti-PD1 antibody. Preferably, the hyperproliferative disorder is a glioma or a glioblastoma.
Owner:DANA FARBER CANCER INST INC
Method for preparing atovaquone
InactiveCN101774901AImprove dissolutionReduce generationOrganic compound preparationQuinone preparationPotassium persulfateAtovaquone
The invention discloses a method for preparing atovaquone, which comprises the following steps: taking 4-(4-chlorophenyl)-cyclohexyl-1-methanoic acid and 2-chlorine-1, 4-naphthoquinone as raw materials, generating (3S)-2-chlorine-3-(4-(4-chlorophenyl) cyclohexyl)-1, 4-naphthalenedione by oxidative decarboxylation through a peroxide in the action of a catalyst of silver nitrate, and then obtaining the atovaquone through hydrolysis with alkaline. The method is characterized in that the mixed solvent of acetonitrile and choromethane is used as the solvent for the oxidative decarboxylation and the peroxide is one of the four substances, i.e., sodium persulfate, potassium persulfate, sodium percarbonate and potassium peroxycarbonate. The improved preparation method considerably improves the dissolution of the raw materials in the solvent, raises the rate of conversion, increases the yield, reduces impurities, and significantly enhances the product quality. Therefore, the method is more suitable for industrial production.
Owner:WUHAN TITON BIOTECH
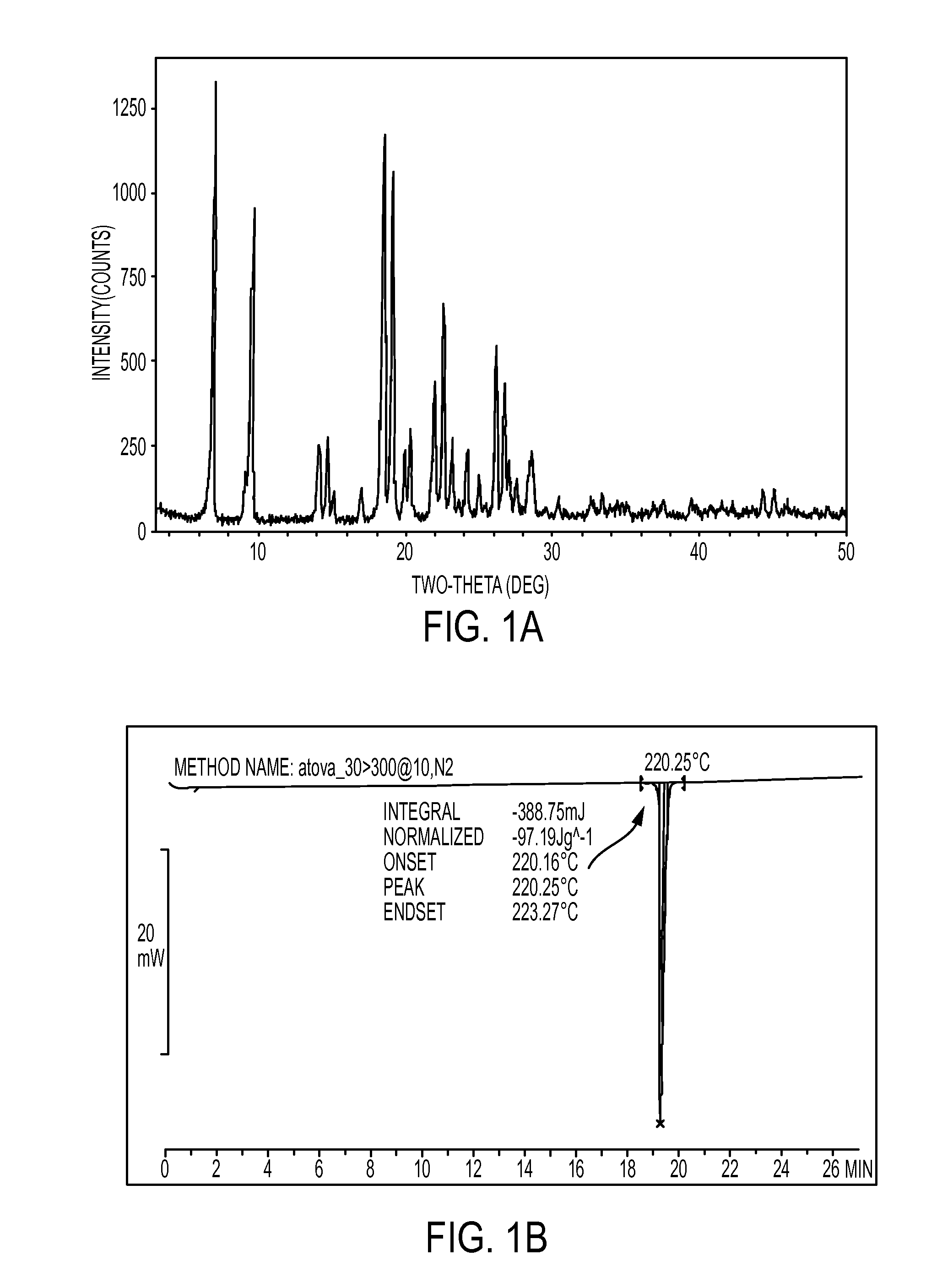
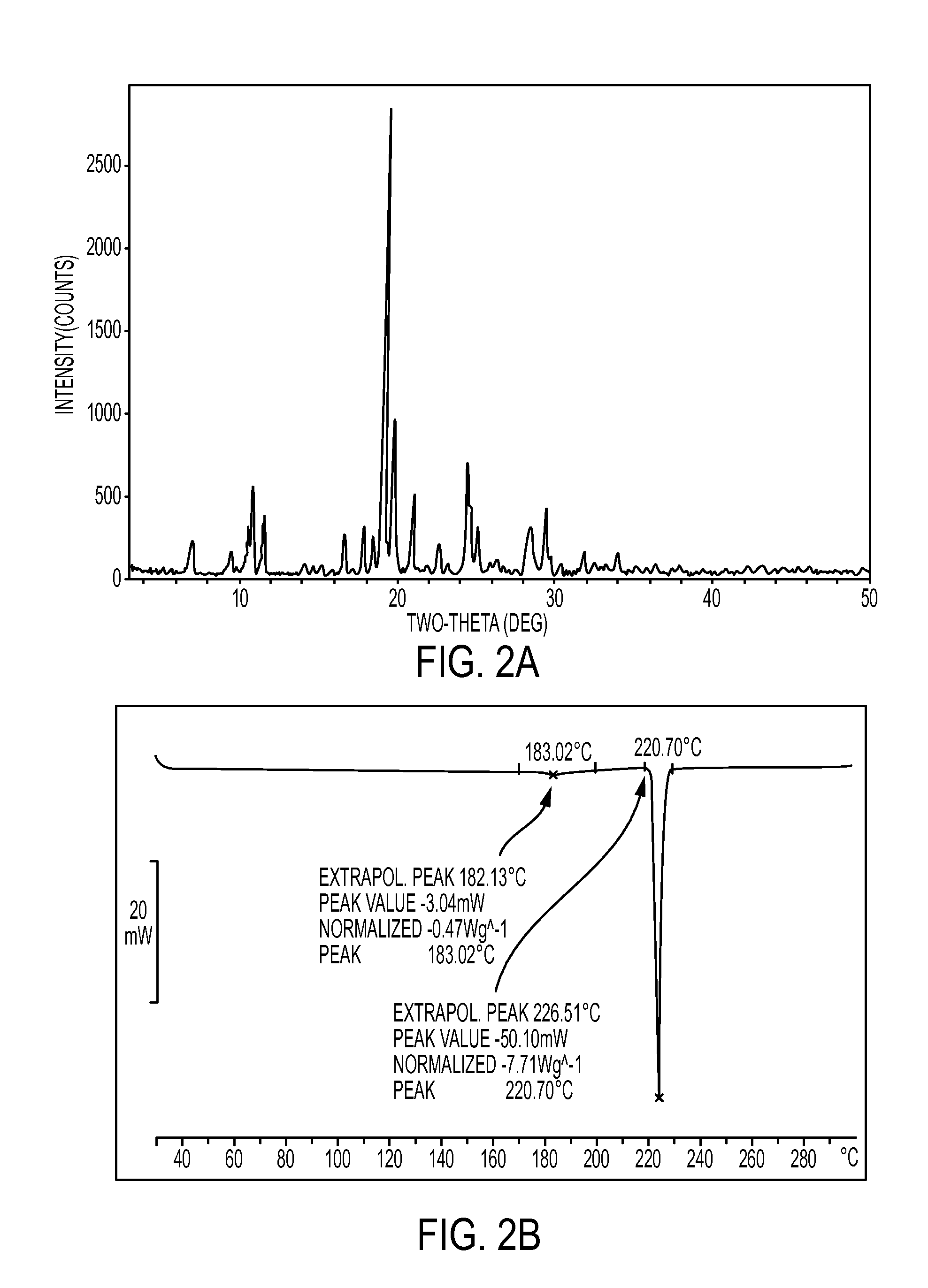
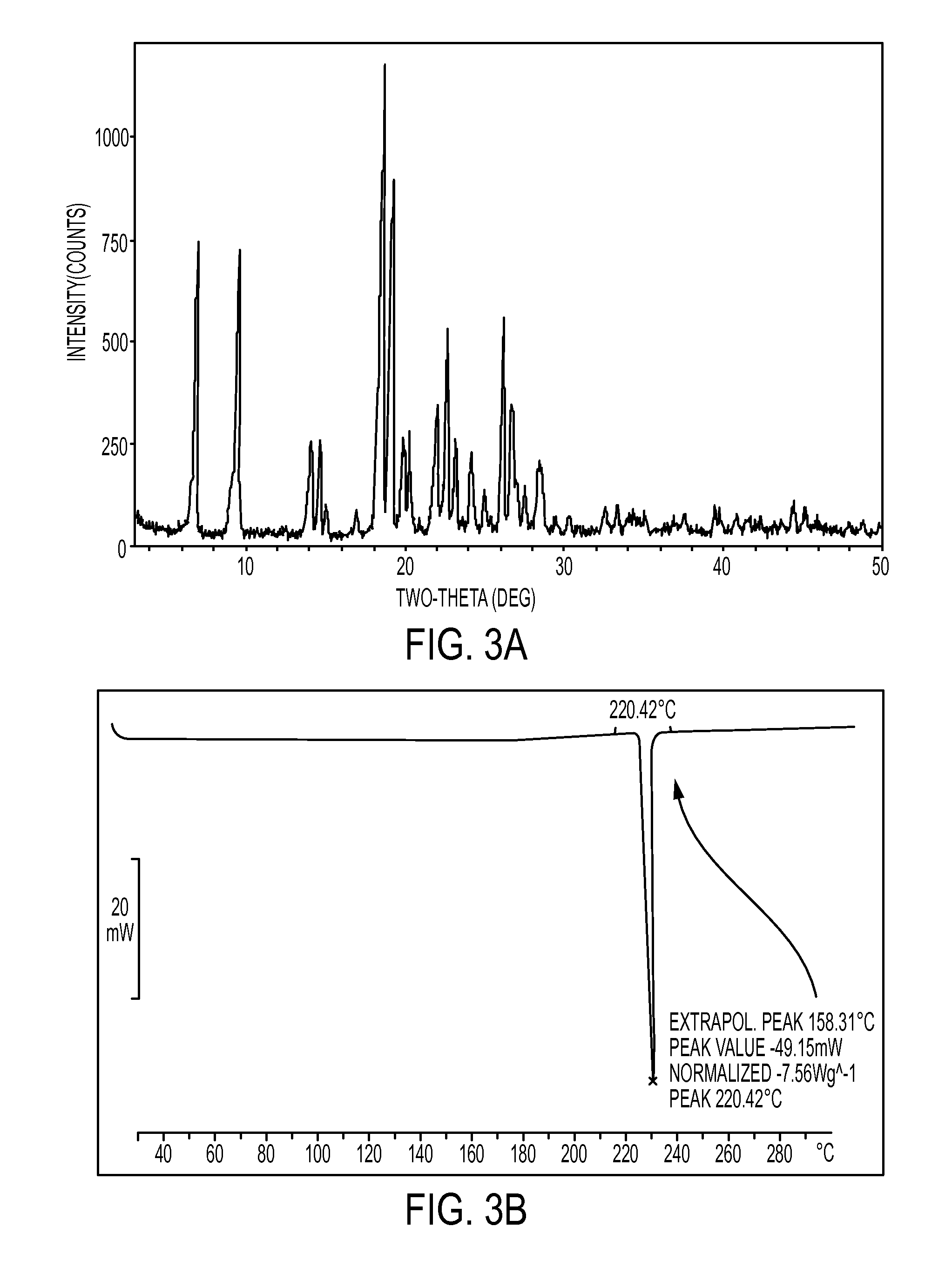
Novel crystalline forms of atovaquone
The present invention relates to two novel and stable crystalline forms of atovaquone, to processes for their preparation and to pharmaceutical compositions comprising them. The present invention also provides crystalline particles of atovaquone having a specific surface area of from about 0.7 m2 / g to about 4 m2 / g, methods for the manufacture of said crystalline particles and pharmaceutical compositions comprising said crystalline particles. The present invention further provides an improved and commercially viable process for preparation of atovaquone substantially free of its undesired isomeric impurity, namely cis-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone.
Owner:HETERO DRUGS LTD
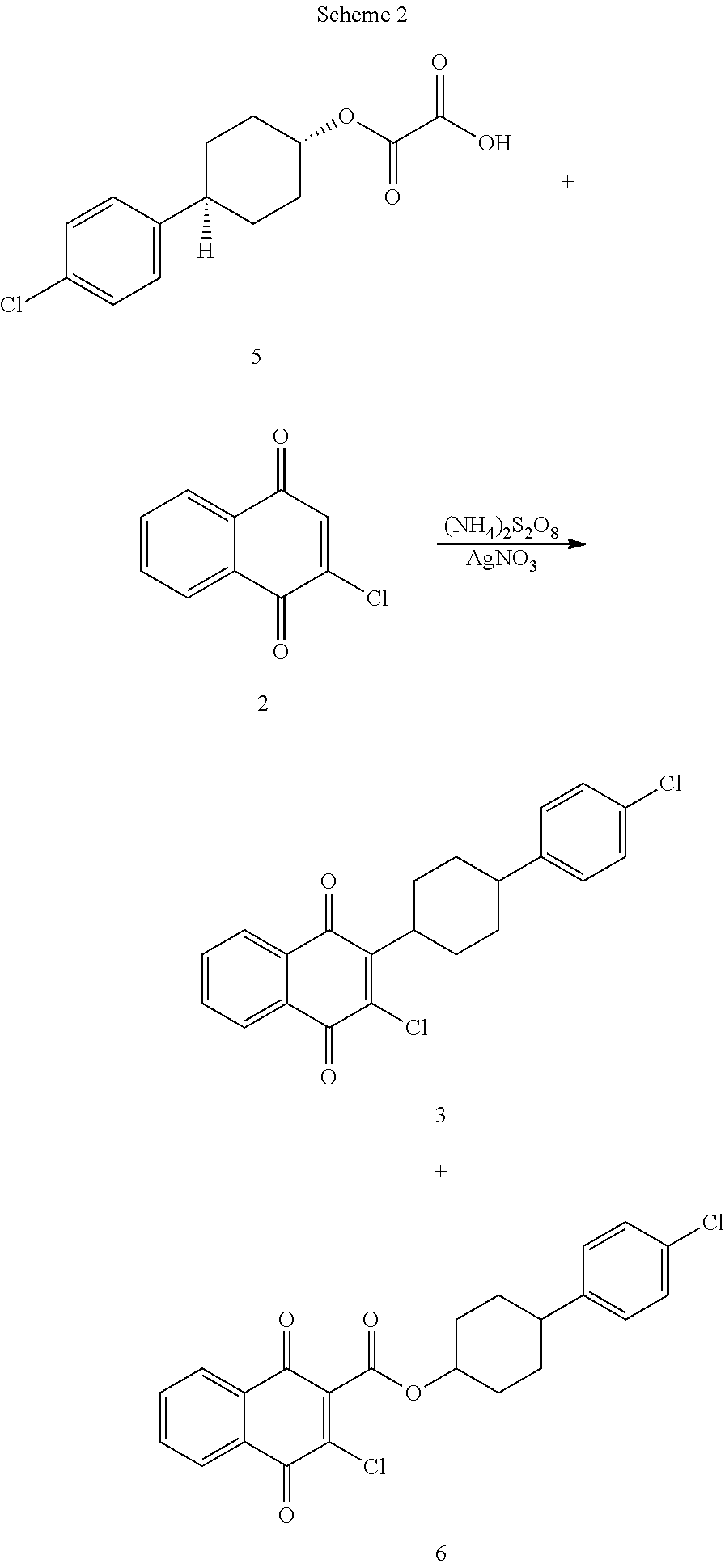
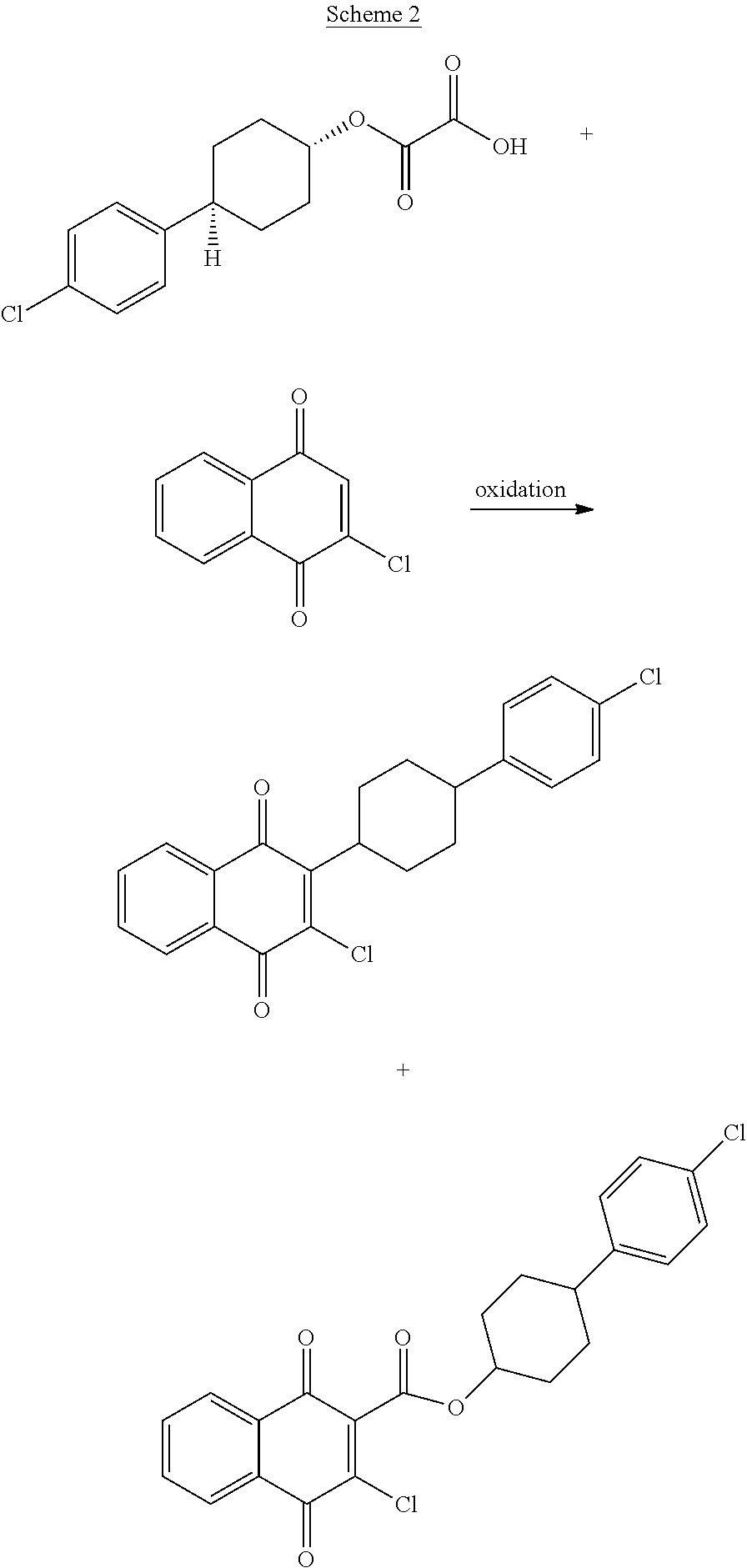
A kind of 2,3-dibromo-2,3-dihydro-1,4-naphthoquinone compound, its preparation method and application
ActiveCN103570520BLow costReduce usageOrganic compound preparationQuinone preparation by oxidationAtovaquoneAcid catalysis
The invention discloses a benzoquinones compound, a preparation method and application thereof, and belongs to the drug synthesis field. The method comprises the following steps: condensing alpha-naphthol and 4-(4-chlorophenyl)cyclohexanol under acid catalysis to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol (formula IV), oxidizing the compound represented by formula IV to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III), enabling the compound represented by the formula III to react with bromine in additive reaction to obtain 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula I), releasing a molecule of hydrogen bromide to obtain 3-bromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula II). The method further discloses a method for preparing atovaquone by using the compound, the synthesis method is simple, the AgNO3 can be prevented from using, and the total yield is high.
Owner:SHANDONG LUKANG SHELILE PHARMA
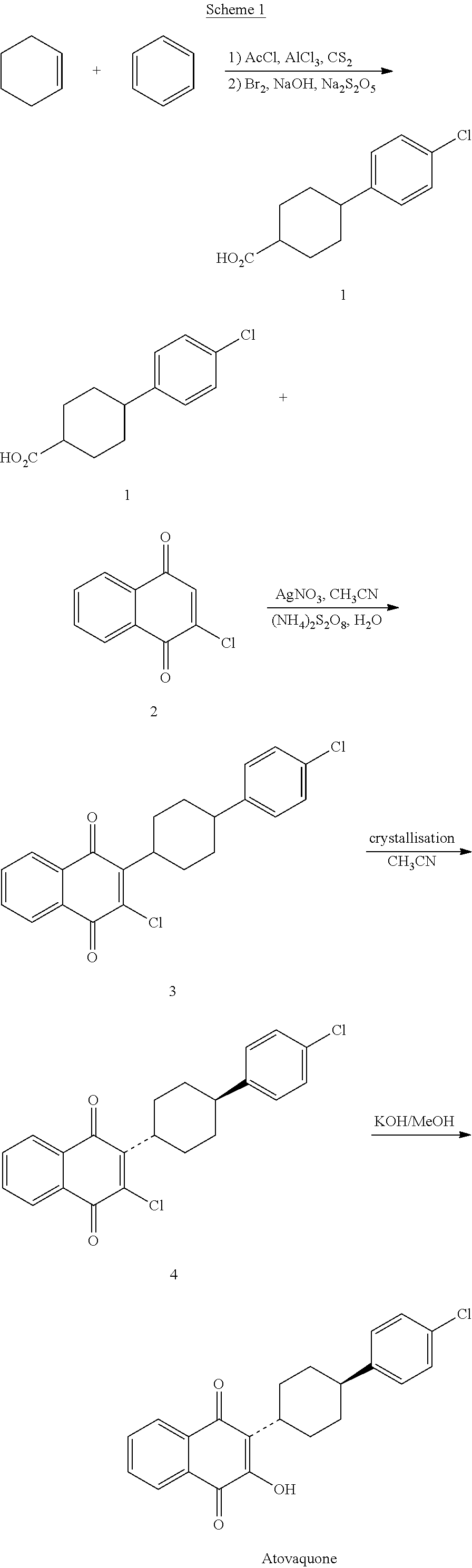
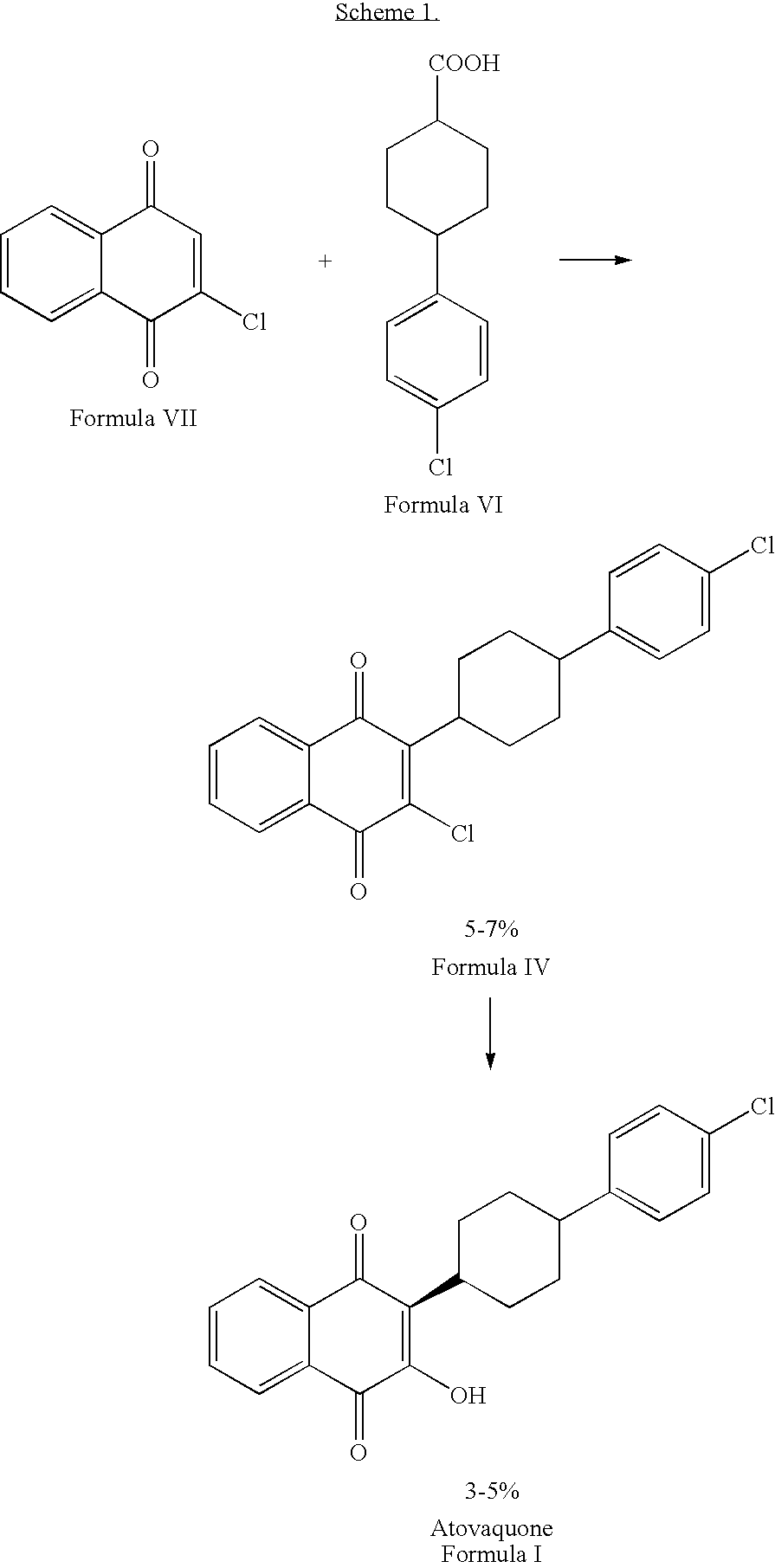
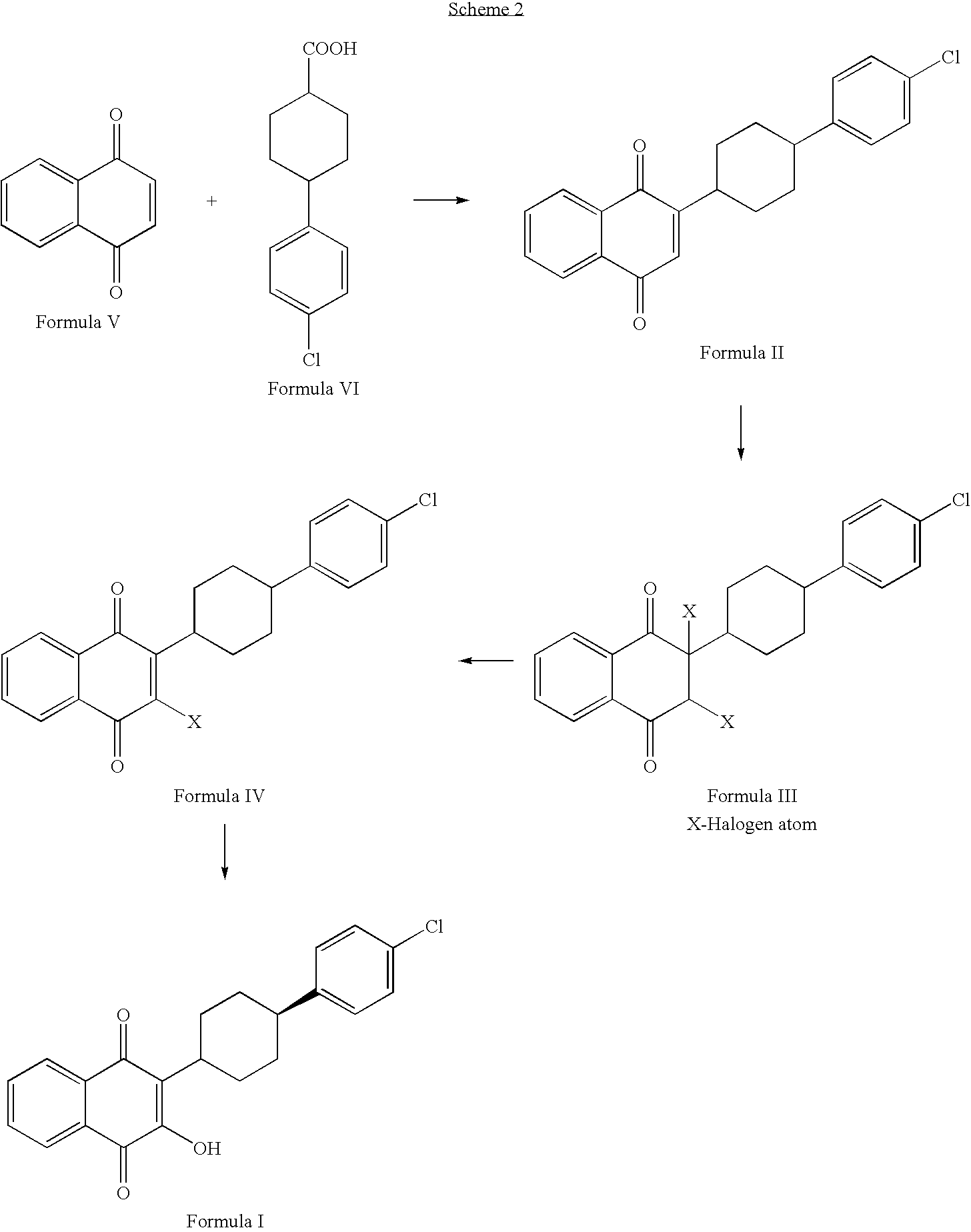
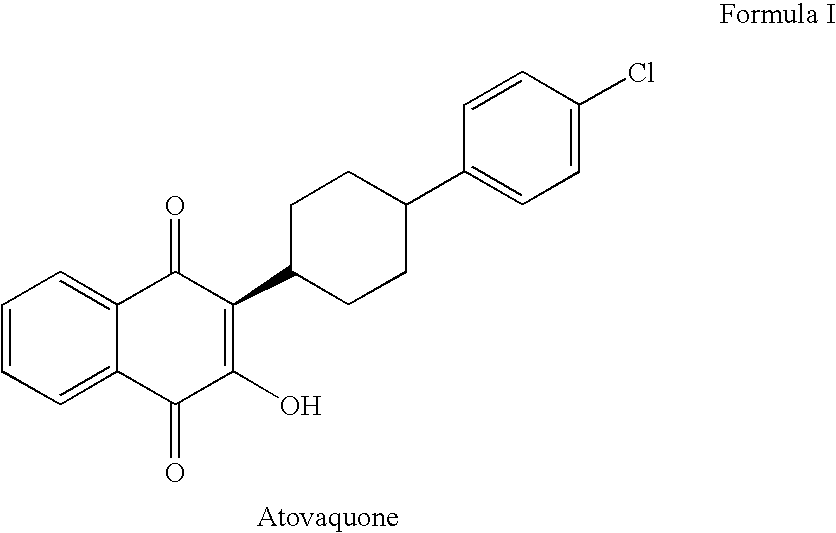
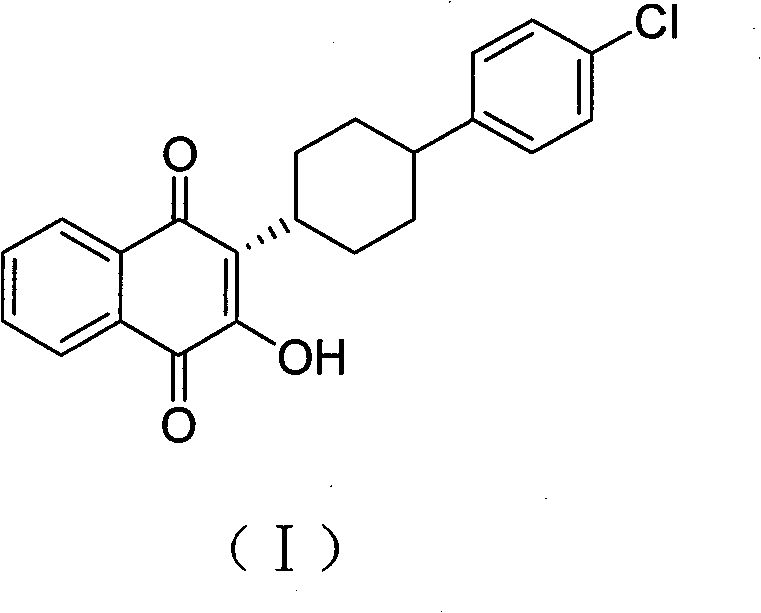
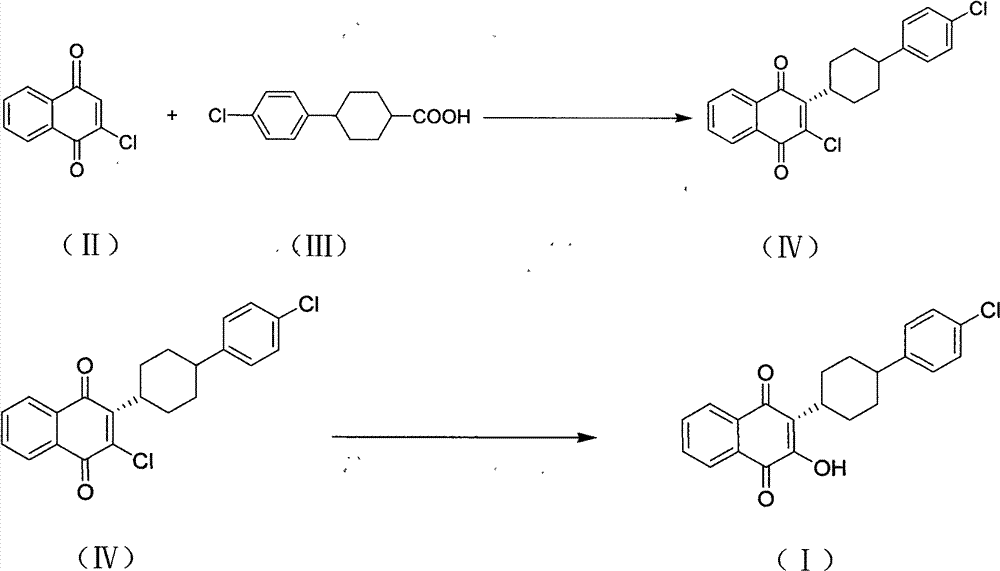
Process for preparation of atovaquone and novel intermediates thereof
Disclosed herein is a novel process for preparation of atovaquone. The process includes reacting 1,4-naphthoquinone with trans-4-(4-chlorophenyl) cyclohexane carboxylic acid followed by halogenation to obtain a dihalo-compound. Further, dehydrohalogenation of the dihalo-compound produces a monohalogeno-compound which under goes hydrolysis to produce atovaquone. The invention also discloses atovaquone in a substantially pure and well defined polymorphic form designated as “Form IPCA-ATO,” and the preparation thereof.
Owner:IPCA LAB LTD
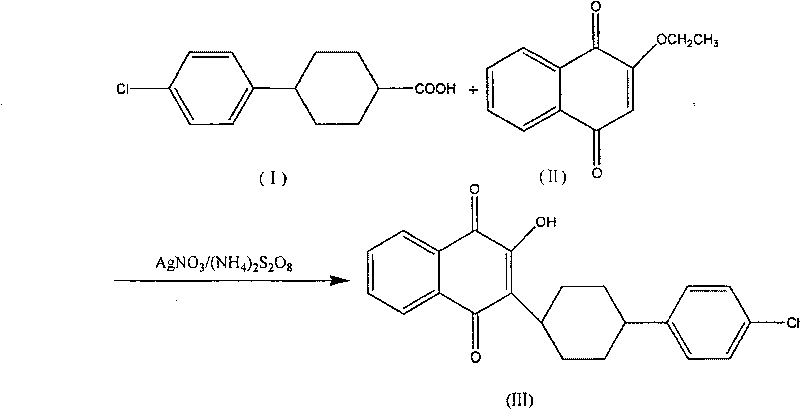
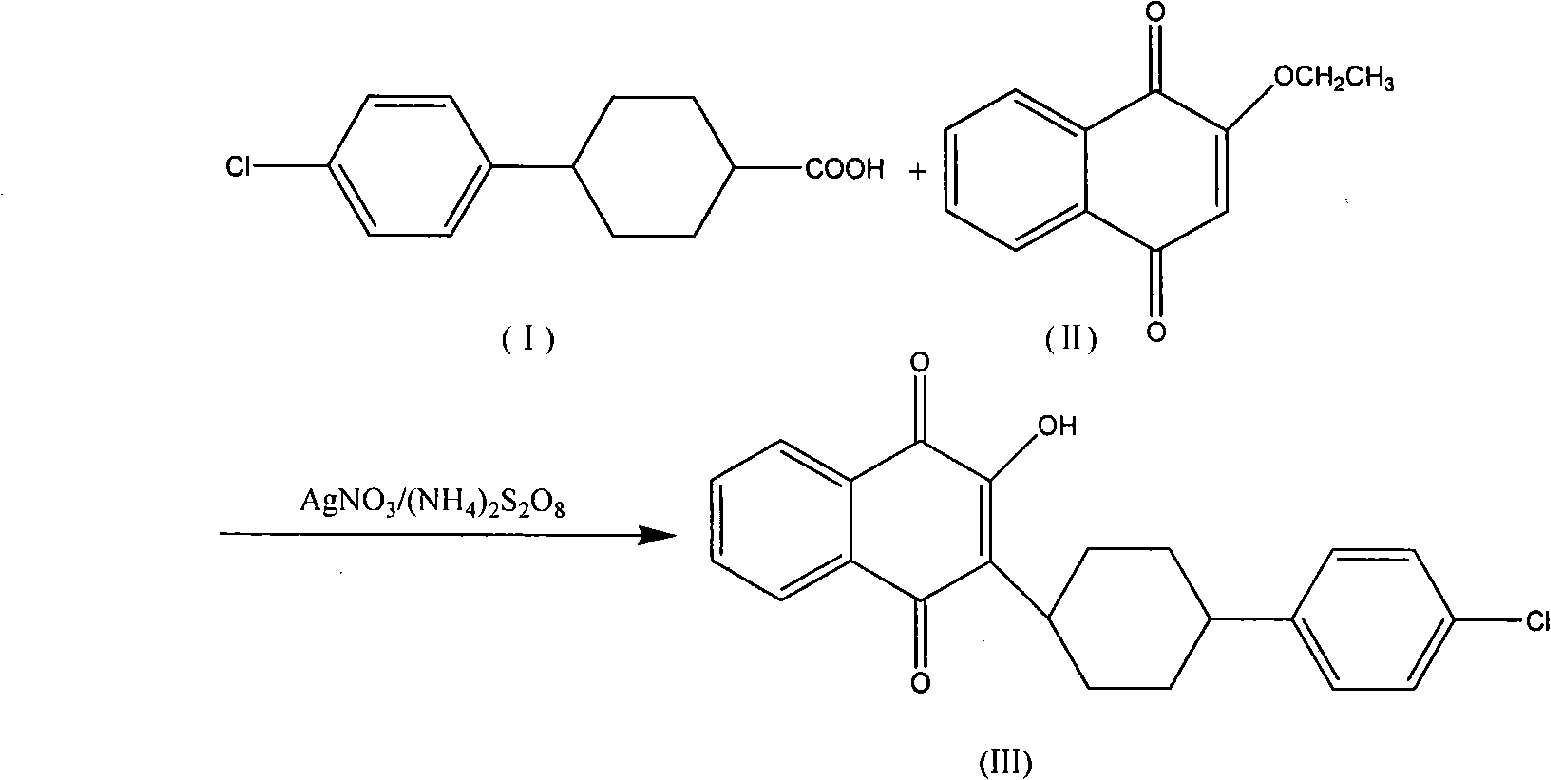
Atovaquone synthesis technology
InactiveCN105622381AIncrease conversion rate per passHigh yieldOrganic compound preparationQuinone preparationDistillationAtovaquone
The invention discloses an atovaquone synthesis technology. The atovaquone synthesis technology comprises that in the presence of a silver nitrate catalyst, 2-ethoxy-1, 4-naphthoquinone and 4-(4-chlorophenyl)cyclohexyl-1-formic acid as raw materials are dissolved in an acetonitrile solvent, then the solution is added into a reactor and is heated along with stirring until reflux so that the reaction system undergoes a reaction, wherein in reflux, an ammonium persulfate aqueous solution is dropwisely added into the reaction system and the mole amount of the ammonium persulfate is 3-5 times that of 2-ethoxy-1, 4-naphthoquinone, after the reaction, the product is cooled and forms crystals, the crystals are filtered and are dissolved through trichloromethane, the solution is filtered, the filtrate is collected and is subjected to reduced pressure distillation so that trichloromethane is removed, and the solution is subjected to acetonitrile recrystallization so that atovaquone yellow acicular crystals are obtained. The atovaquone synthesis technology only needs one step, saves a synthesis cost, has a high yield and can produce high-purity atovaquone.
Owner:QINGDAO SHOUTAI AGRI SCI & TECH CO LTD
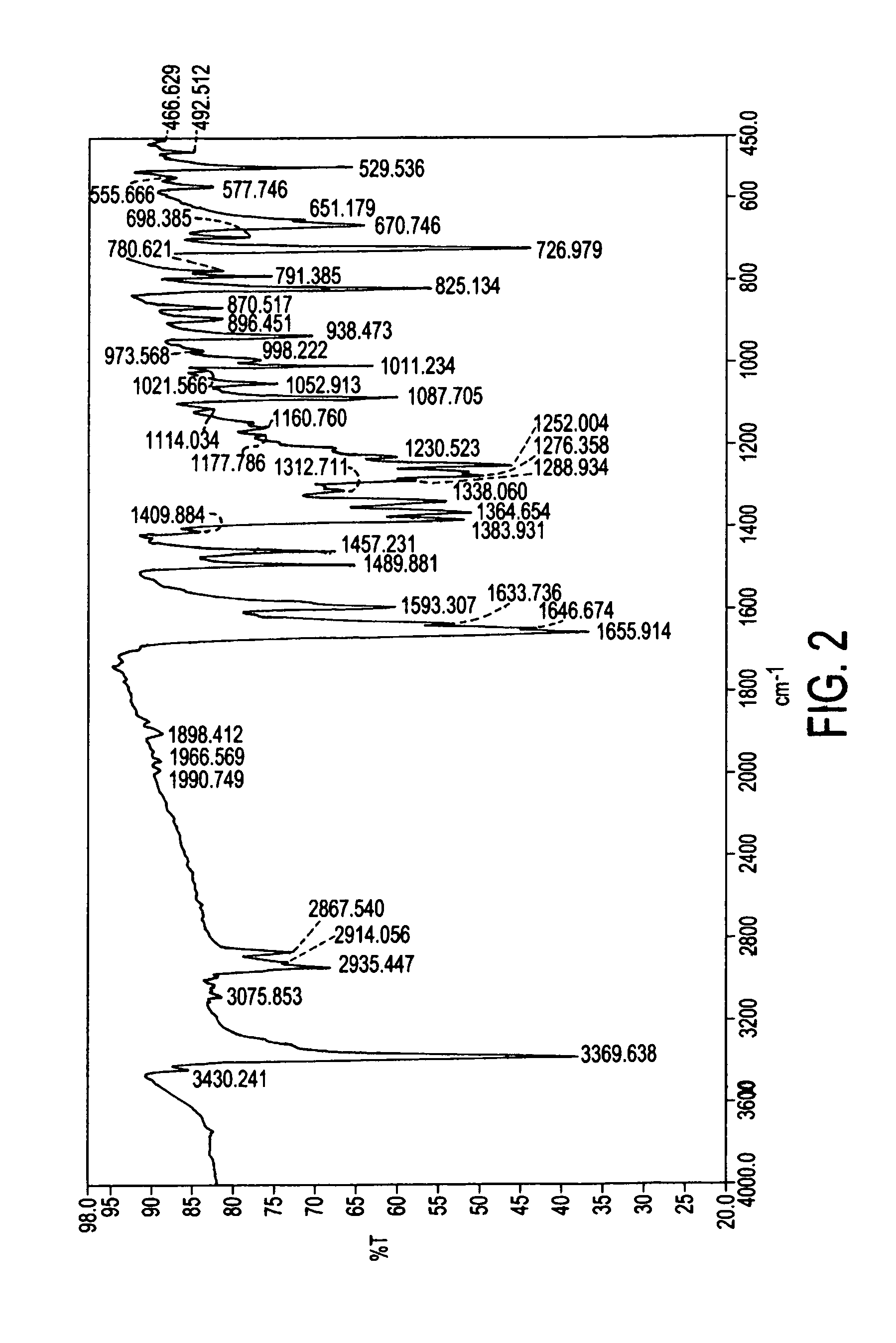
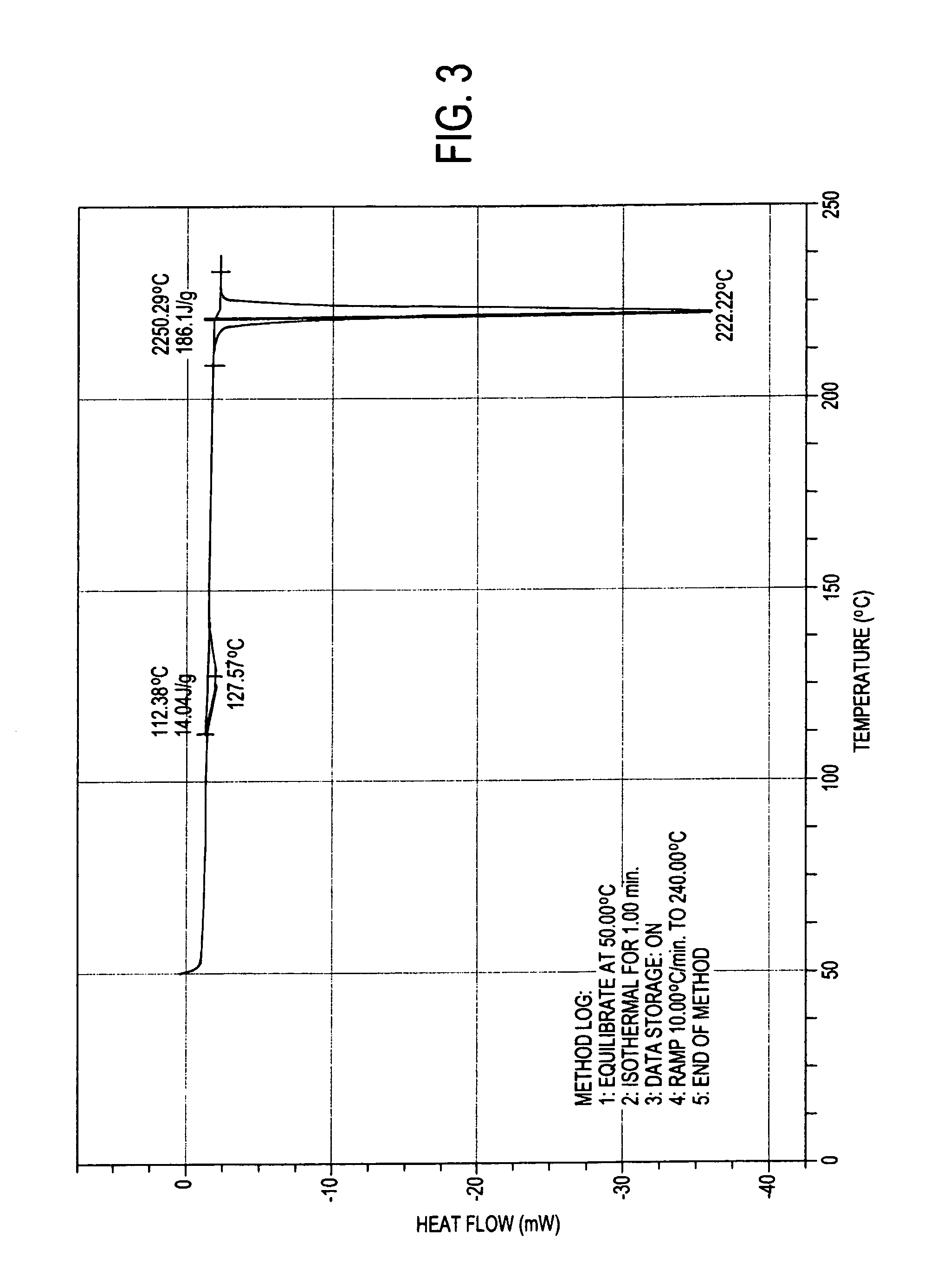
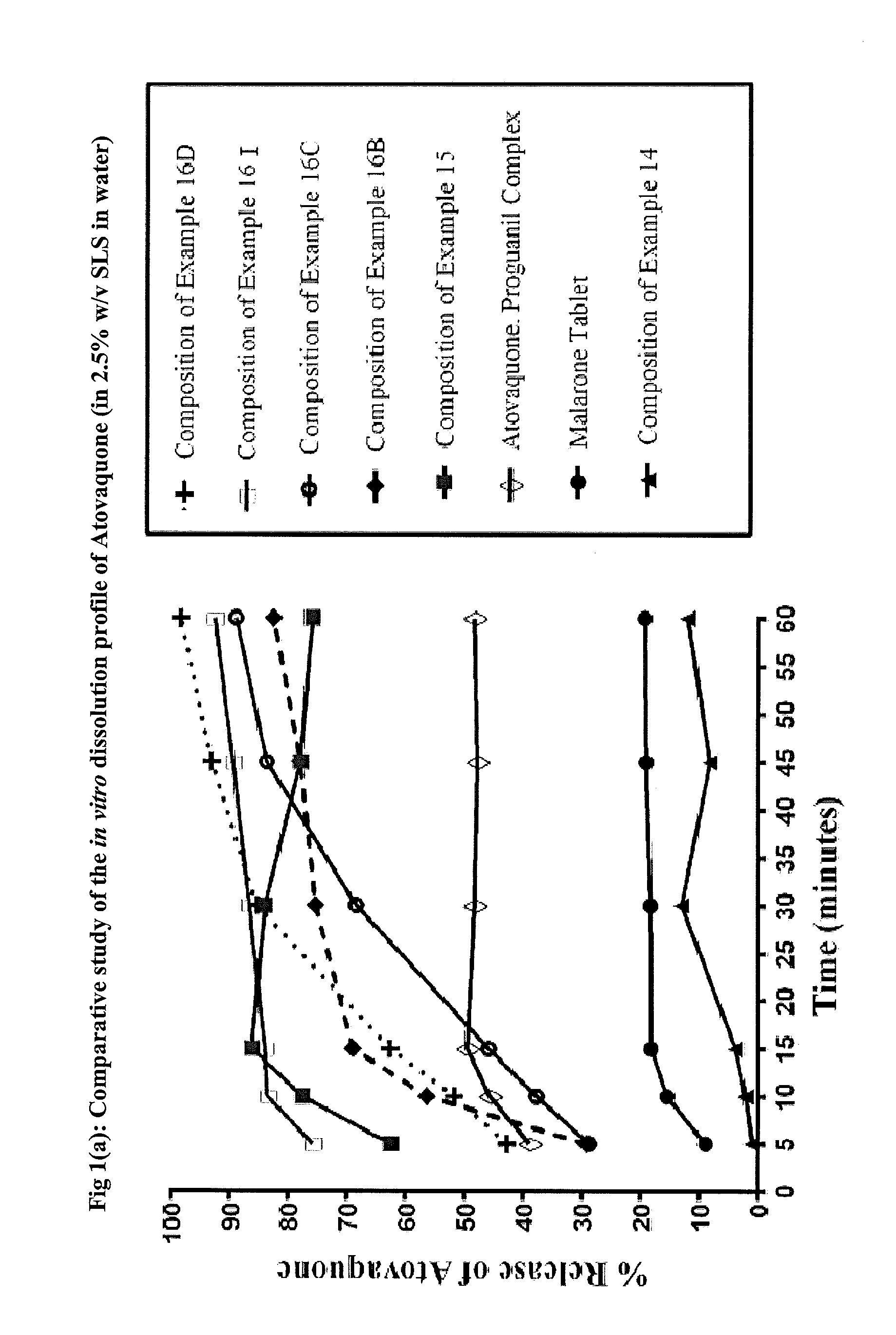
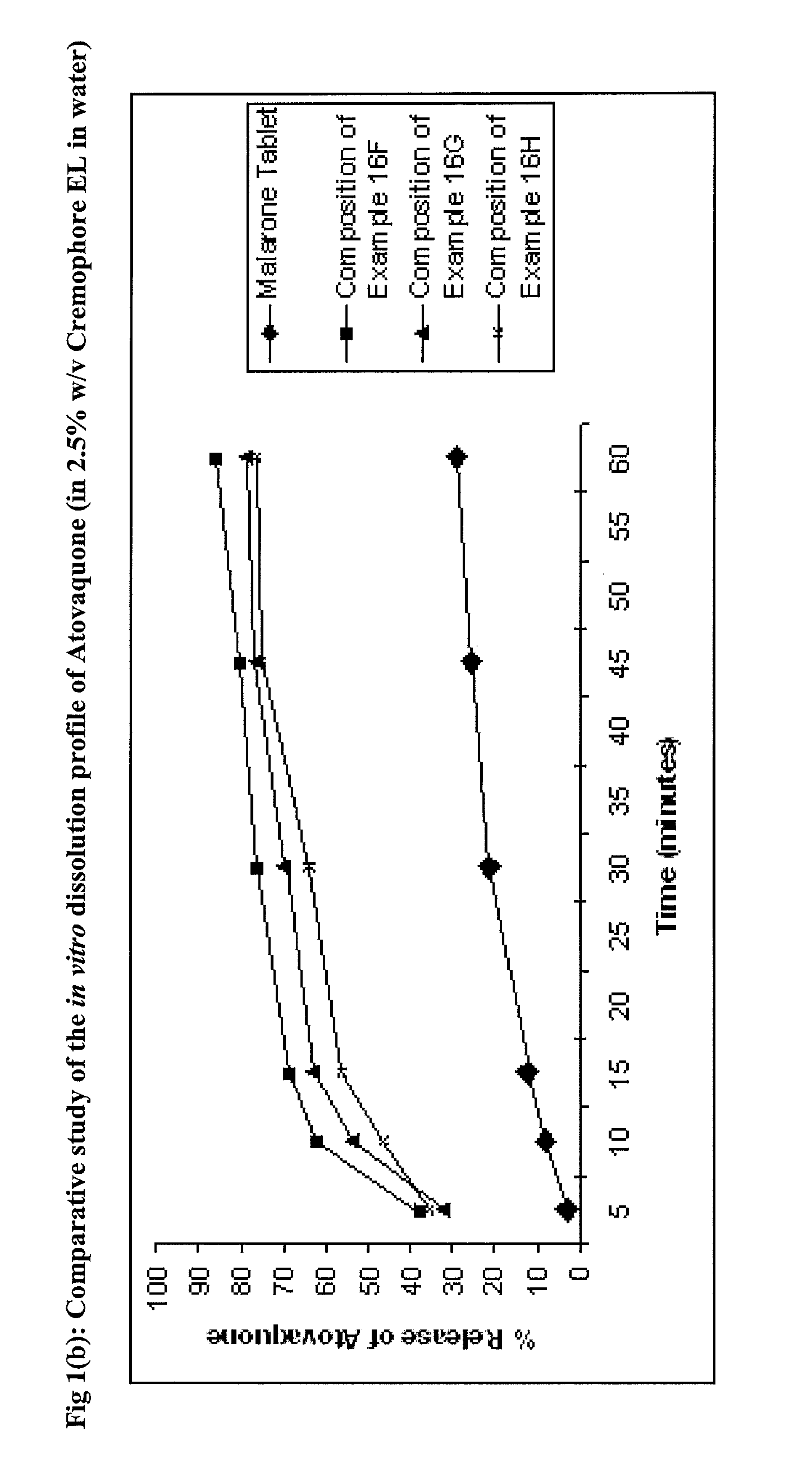
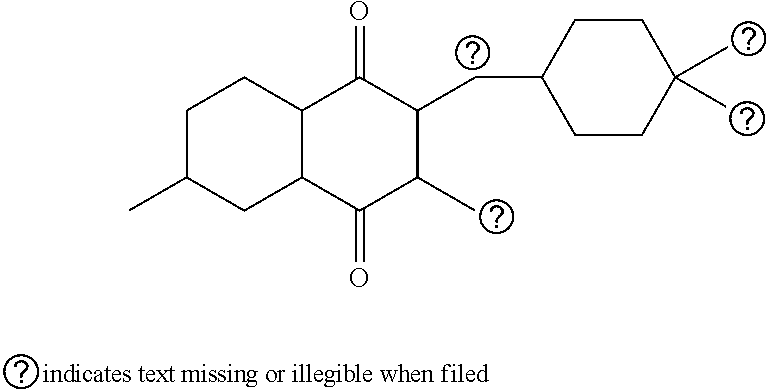
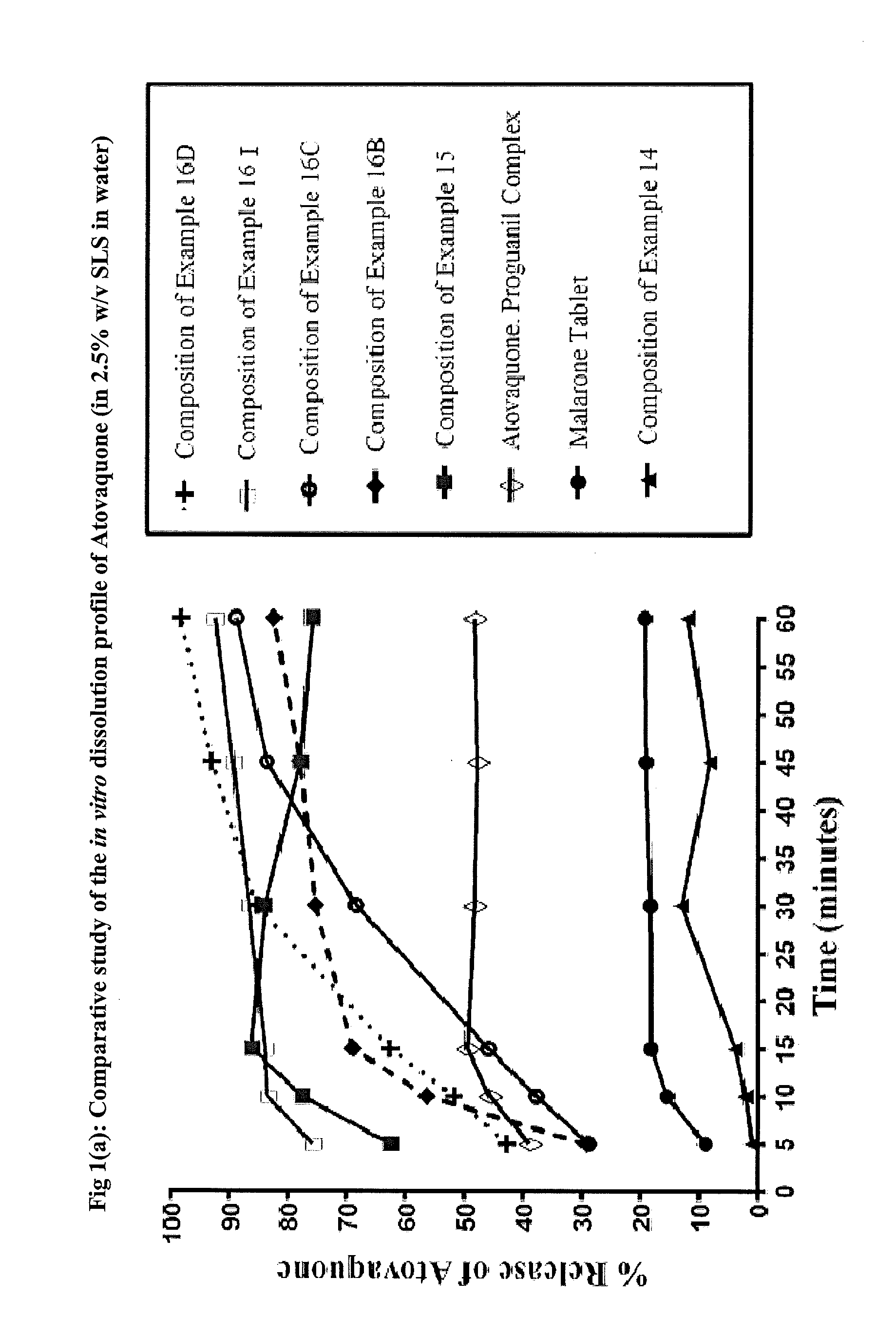
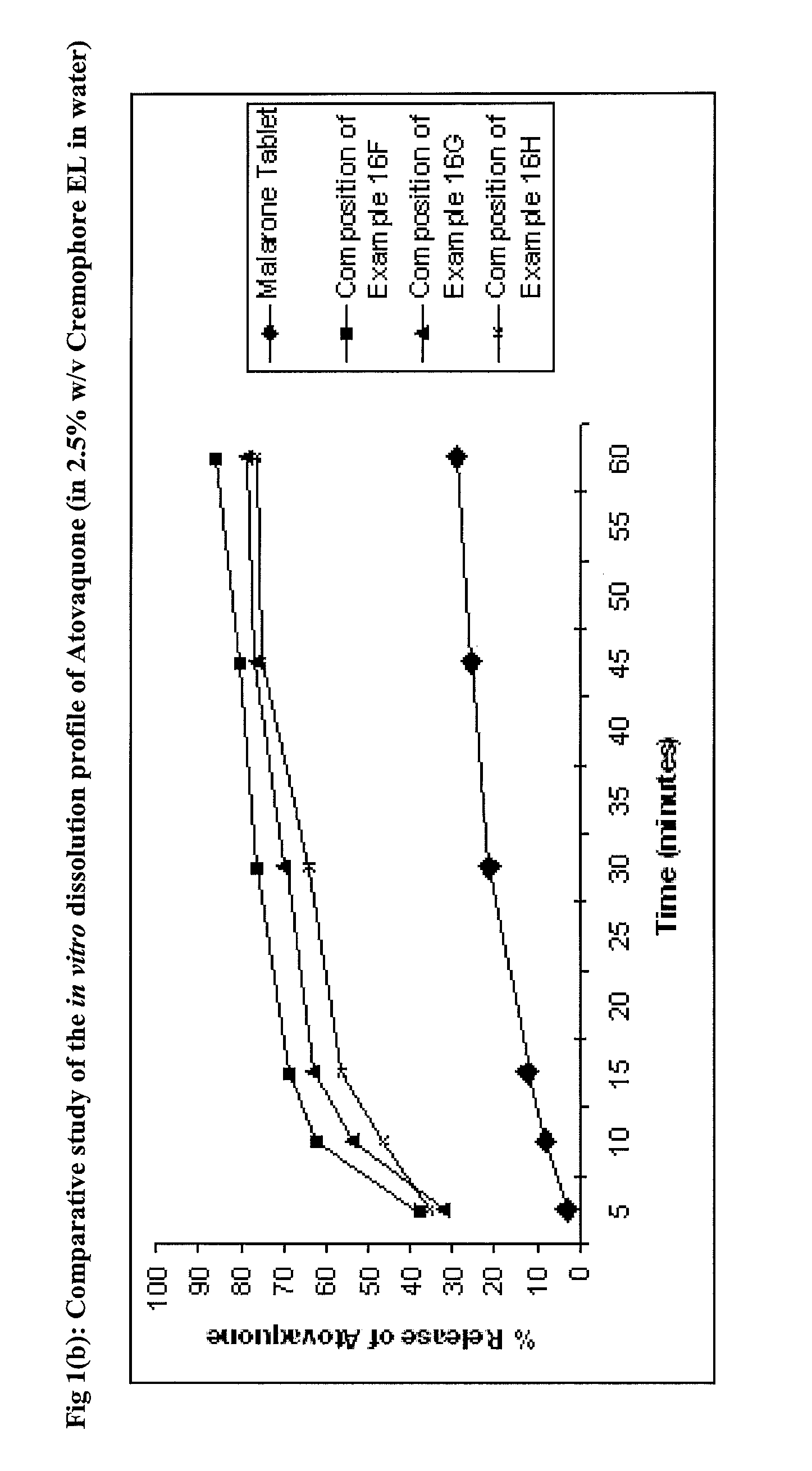
Pharmaceutical composition
ActiveUS9492406B2Good water solubilityImprove bioavailabilityPowder deliveryPill deliveryAtovaquoneProguanil
The present invention relates to a novel pharmaceutical composition having enhanced bioavailability through improved aqueous dissolution of poorly water soluble drugs, and to a method for preparing it. The invention more particularly relates to an oral pharmaceutical composition containing active ingredients of poor aqueous solubility, more specifically, antiparasitic and antipneumocystic drug Atovaquone alone or in combination with Proguanil.
Owner:IPCA LAB LTD
Novel polymorph of atovaquone
The present invention relates to a novel polymorphic form of atovaquone. More particularly, it relates to a novel crystalline form, that has improved solubility and other bulk characteristics suitable for pharmaceutical application. The present invention also relates to processes for preparing a new polymorphic form of atovaquone and its use in industry.
Owner:IPCA LAB LTD
Process for preparing atovaquone and associate intermediates
InactiveUS20110137041A1Organic compound preparationQuinone preparationBiochemical engineeringAtovaquone
The invention provides novel intermediates of atovaquone and use thereof for the preparation of atovaquone
Owner:CHEMAGIS
Technique for synthesizing atovaquone
ActiveCN101265171AShort processHigh purityOrganic compound preparationQuinone preparationAtovaquoneFiltration
The invention discloses a synthesis technology of Atovaquone. When a silver nitrate catalyst exists, 2-ethoxyl-1, 4-naphthoquinone and 4-(4-chlorophenyl) cyclohexyl-1-methanoic acid which are used as raw material react in acetonitrile solvent, and reaction reagent is added into a reaction vessel, the mixed solution is stirred and heated until refluxing is generated; when the refluxing is dripped down, the water solution of ammonium persulfate is added in, and the quantity of the ammonium persulfate is 2 to 3 times of the mole number of the 2-ethoxyl-1, 4-naphthoquinone; after the reaction is completed, the mixed solution is performed through cooling crystallization and filtration, and is filtered again by using the dissolving crystalization product of chloroform; filter liquor is collected, decompressed and evaporated to obtain the chloroform which is recrystallized to obtain yellow acicular crystal of the Atovaquone by using the acetonitrile. The synthesis route only comprises one-step reaction, the synthesizing cost is saved, the yield rate is high, and the prepared Atovaquone has high purity quotient.
Owner:CHONGQING KOOPPER CHEM IND
Enhanced immunotherapy of cancer using targeted transcriptional modulators
ActiveUS11285149B2Improving immunogenicityImprove efficiencyPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
Owner:DANA FARBER CANCER INST INC
Anti-malaria mosquito-repellent antibacterial composition and preparation method thereof
PendingCN114831115AResistance spreadEffective in repelling mosquitoes and malariaBiocidePest repellentsMalarial parasiteAtovaquone
The invention discloses an anti-malarial mosquito-repellent bacteriostatic composition and a preparation method thereof. The anti-malarial mosquito-repellent bacteriostatic composition comprises the following components: an in-vitro anti-malarial drug, a mosquito repellent, a solvent, a surfactant polyethylene glycol-400, a bacteriostatic agent polyhexamethylene biguanide, essence and deionized water. The preparation method of the anti-malaria mosquito-repelling antibacterial composition specifically comprises the following steps: preparation of antibacterial water, preparation of a mosquito-repelling ester antibacterial solution, preparation of an atorvaquone suspension mother solution and preparation of an atorvaquone mosquito-repelling ester antibacterial solution. The composition of atorvaquone and dimethyl phthalate can kill plasmodium in mosquito bodies so as to prevent malaria from spreading, and compared with an existing single mosquito repellent, the composition of atorvaquone and dimethyl phthalate has more remarkable mosquito-repelling and malaria-preventing effects; the composition is safe to human bodies, free of toxic and side effects, environmentally friendly and capable of being applied to the human bodies and the environment; the composition does not contain propellants in the formula, is low in alcohol content, and is beneficial to production, storage and transportation.
Owner:浙江澳岚丁生物科技有限公司
Atovaquone nanoparticulate compositions
PendingUS20220265566A1Improve bioavailabilityOrganic active ingredientsPowder deliveryNanoparticleAtovaquone
A nanoparticle ATQ composition is provided which has good stability and bioavailability. Compositions and methods of using the nanoparticle ATQ composition in treating parasitic and other infections is described.
Owner:TULEX PHARMA INC
Pharmaceutical Composition
ActiveUS20150238416A1Good water solubilityImprove bioavailabilityPowder deliveryBiocideAtovaquoneWater soluble
The present invention relates to a novel pharmaceutical composition having enhanced bioavailability through improved aqueous dissolution of poorly water soluble drugs, and to a method for preparing it. The invention more particularly relates to an oral pharmaceutical composition containing active ingredients of poor aqueous solubility, more specifically, antiparasitic and antipneumocystic drug Atovaquone alone or in combination with Proguanil.
Owner:IPCA LAB LTD
New process for preparation of atovaquone and novel intermediates thereof
Disclosed herein is a novel process for preparation of atovaquone. The process includes reacting 1,4-naphthoquinone with trans-4-(4-chlorophenyl) cyclohexane carboxylic acid followed by halogenation to obtain a dihalo-compound. Further, dehydrohalogenation of the dihalo-compound produces a monohalogeno-compound which under goes hydrolysis to produce atovaquone. The invention also discloses atovaquone in a substantially pure and well defined polymorphic form designated as “Form IPCA-ATO,” and the preparation thereof.
Owner:IPCA LAB LTD
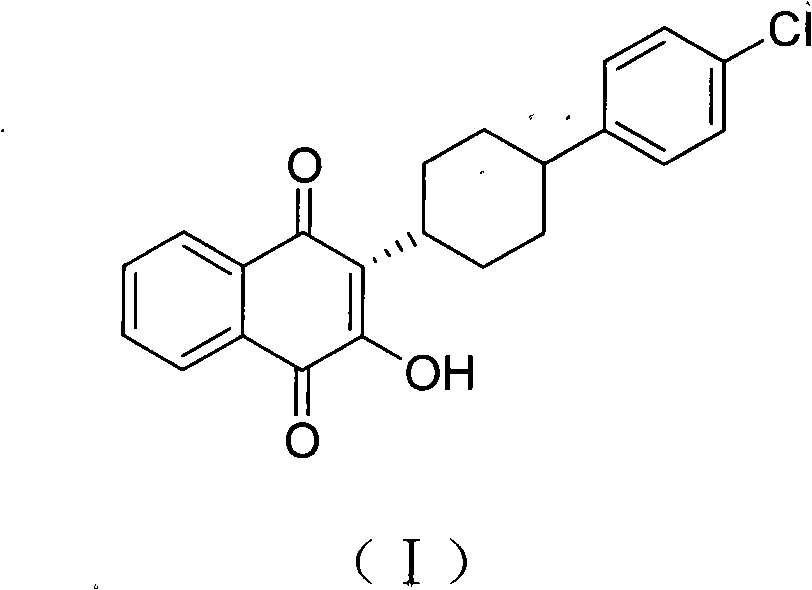
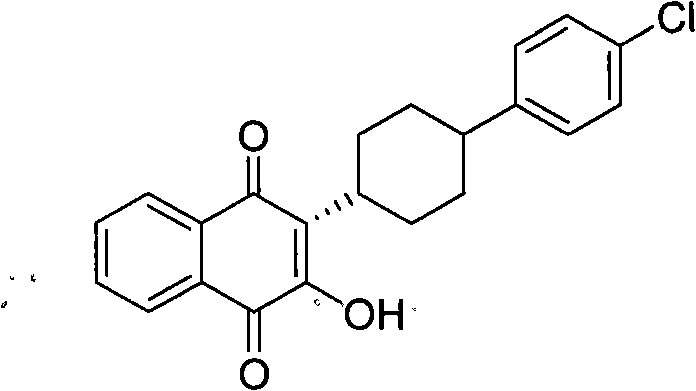
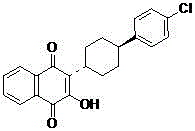
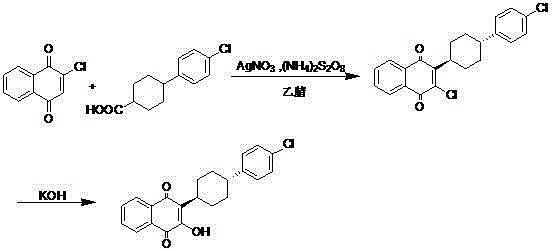
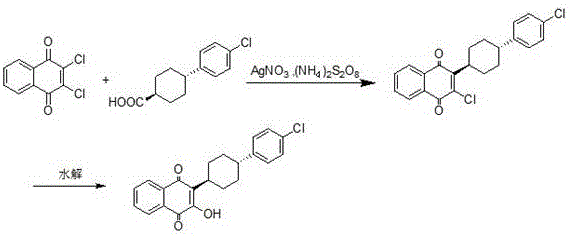
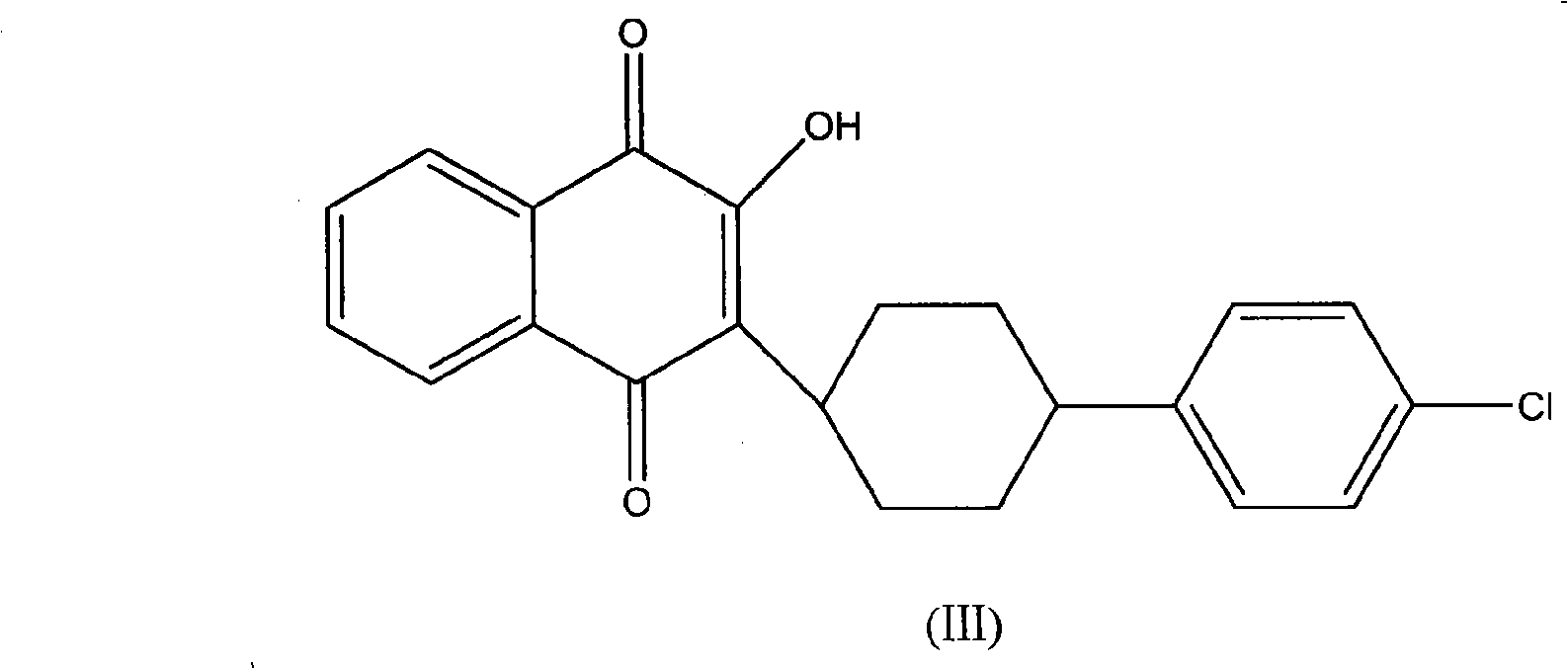
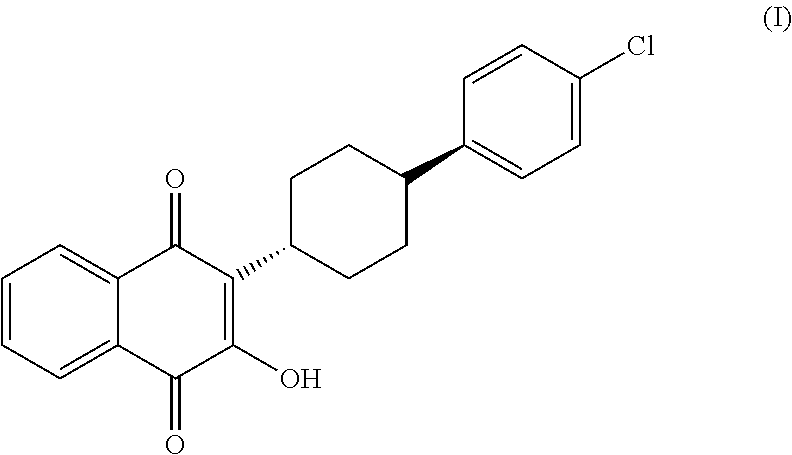
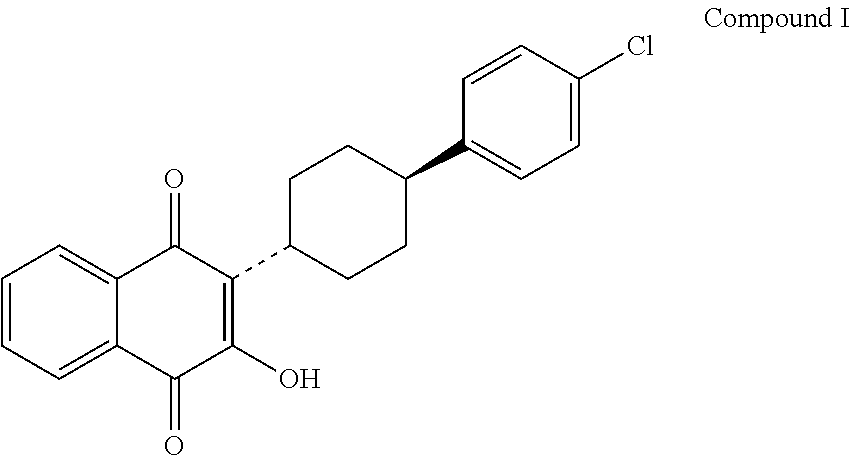
3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug
Owner:ALKEM LAB LTD
IR820 and atorvaquone carrier-free self-assembled nanoparticles and preparation method and application thereof
ActiveCN112933229ASolve problems such as poor stability and easy eliminationSolve the problems of poor water solubility and low bioavailabilityPowder deliveryOrganic active ingredientsBenign tumoursAtovaquone
The invention provides IR820 and atorvaquone carrier-free self-assembled nanoparticles and a preparation method and application thereof, and belongs to the technical field of medicines. According to the nanoparticle, IR820 and atorvaquone are taken as active ingredients, the nanoparticle not only solves the problems that IR820 is extremely easy to remove and poor in stability, but also solves the problems that atorvaquone is extremely poor in water solubility and low in bioavailability, and treatment resistance of IR820 is caused by rising of heat shock protein in photo-thermal therapy. The nanoparticle is high in drug loading capacity, uniform in particle size, high in stability and good in biocompatibility, provides more efficient photothermal therapy, can be used for treating or relieving benign tumors or malignant tumors, and therefore has good practical application value.
Owner:SHANDONG UNIV
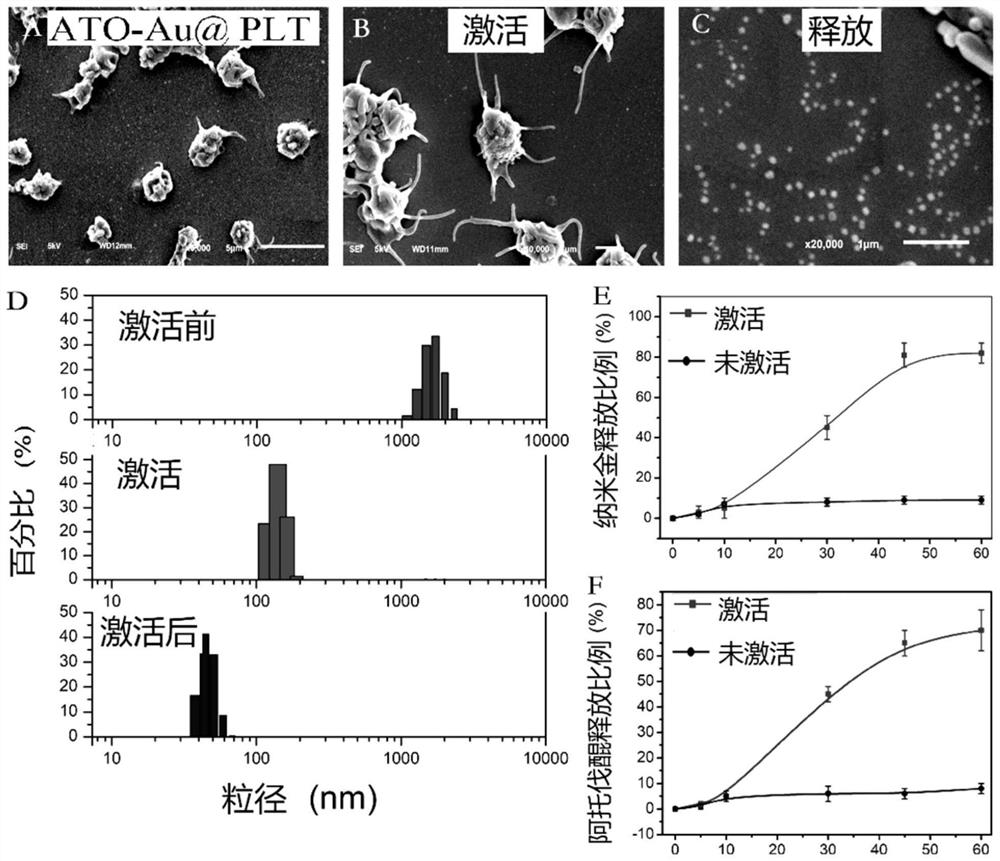
A platelet drug-carrying system targeting tumors and improving radiotherapy sensitivity and preparation method thereof
ActiveCN111053900BInhibition of oxidative phosphorylationImprove radiosensitization effectOrganic active ingredientsPharmaceutical non-active ingredientsAtovaquonePhosphorylation
A platelet drug-loading system that targets tumors and improves radiotherapy sensitivity. The platelet drug-loading system refers to a rat platelet drug-loading system, which is a mixture of mPEG-PCL-modified atovaquone, PEG-modified nano-gold and rat platelets, namely Ato ‑Au@Plt; said targeting tumor, improving radiotherapy sensitivity The platelet drug-loading system uses platelets as a carrier to achieve long circulation in vivo and targeted release of drugs in the tumor microenvironment, and improve the radiotherapy sensitivity of tumors. The invention activates the platelet carrier through the tumor microenvironment, deforms, aggregates, and targets the tumor to release the drug; the released drug can inhibit the oxidative phosphorylation of tumor cells, solve hypoxia, and at the same time synergize with the nano-gold to improve the sensitivity of the tumor to radiotherapy , and then improve the effect of radiotherapy.
Owner:SHEYANG RES INST OF NANJING UNIV
Oily suspension of atovaquone
The present invention relates to an oily suspension of atovaquone comprising atovaquone particles and a combination of surfactants having HLB more than 10.
Owner:RANBAXY LAB LTD
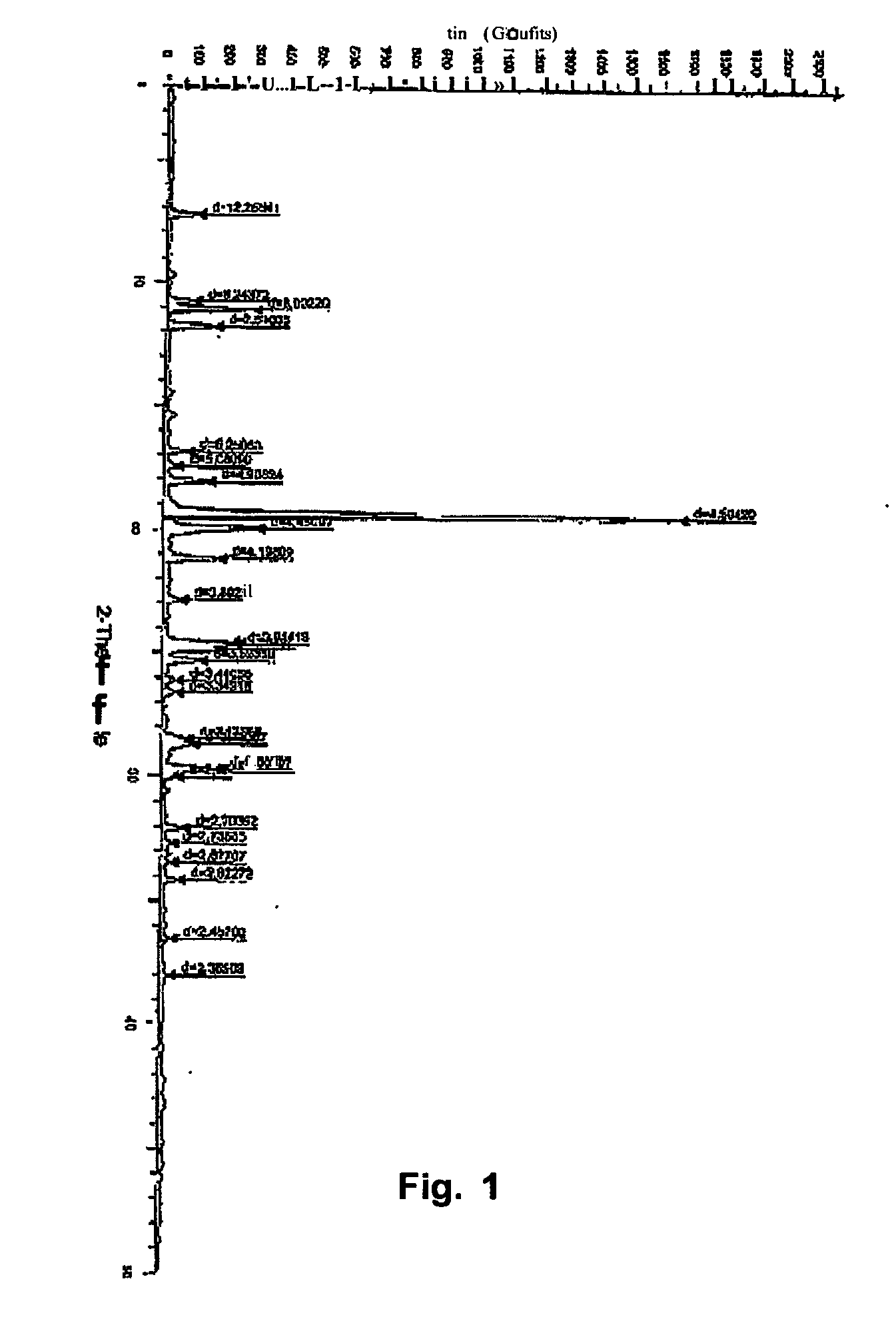
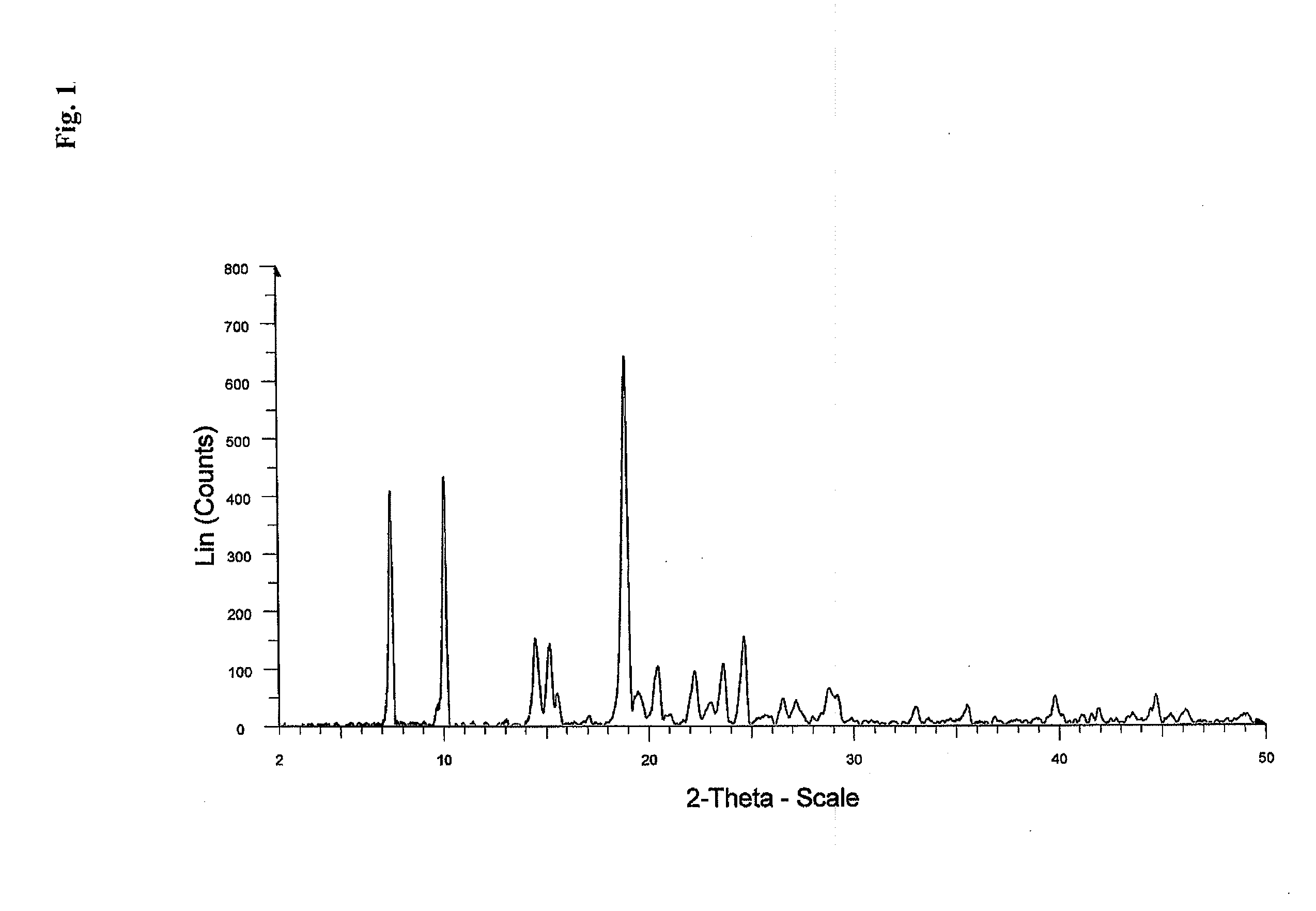
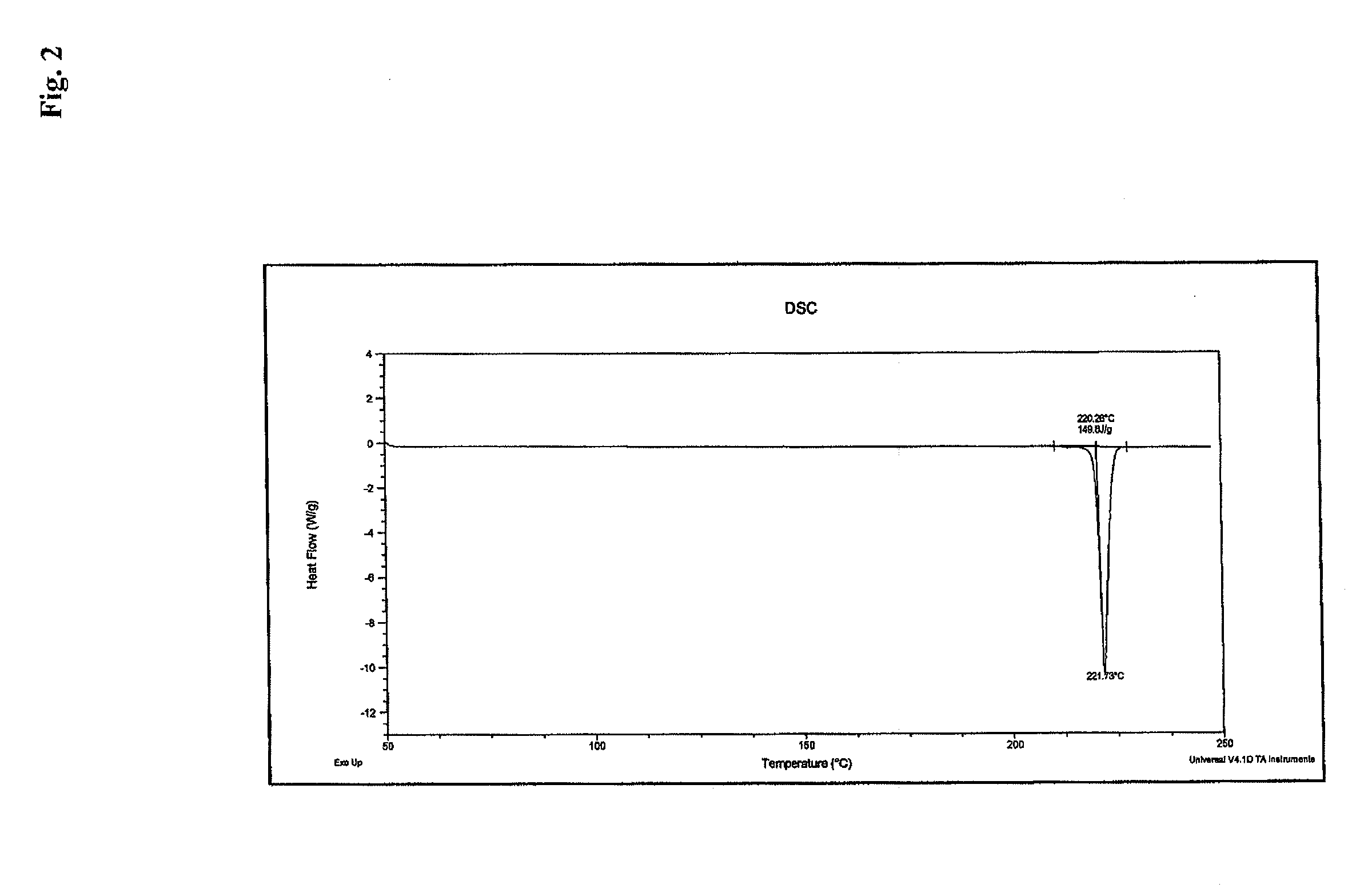
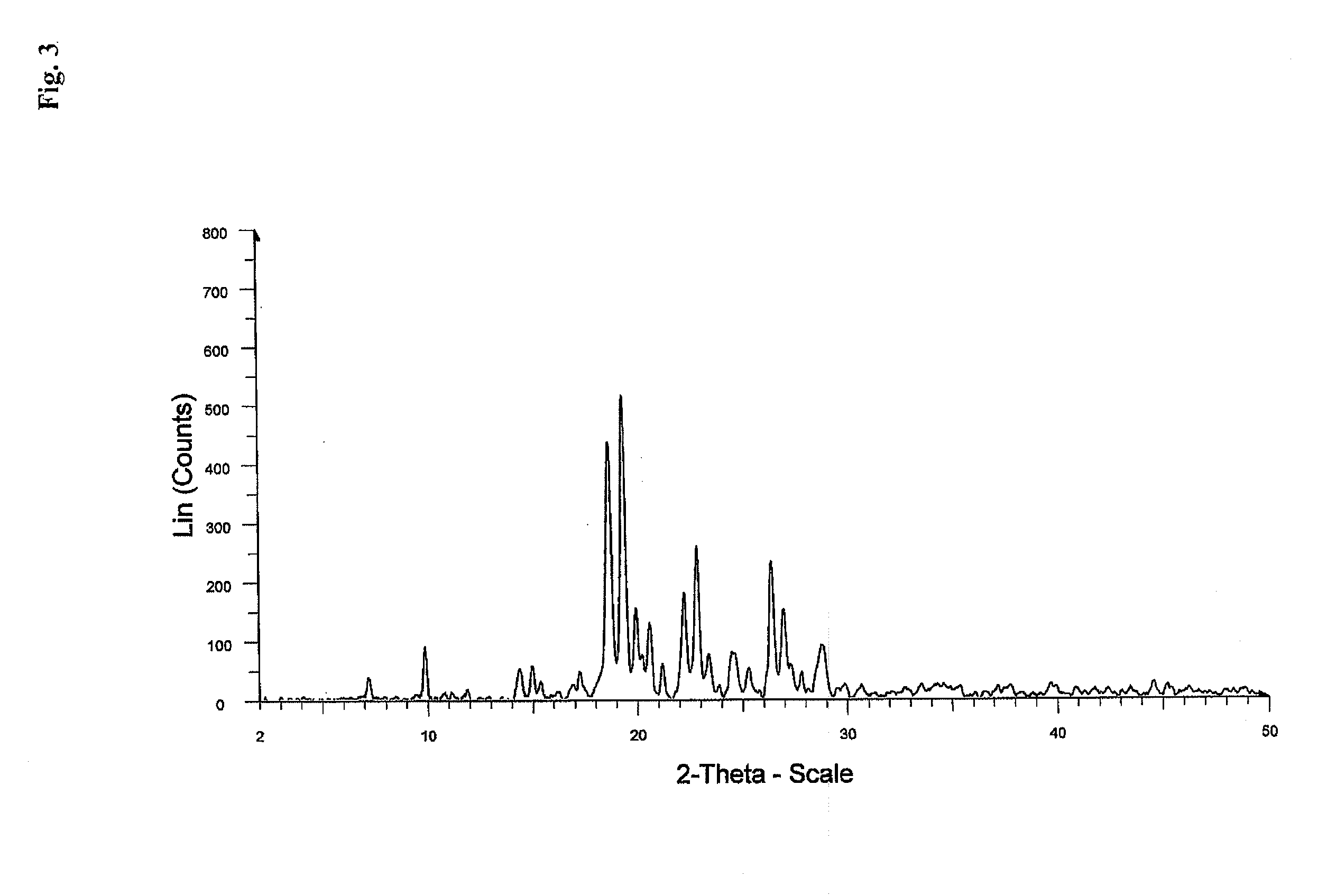
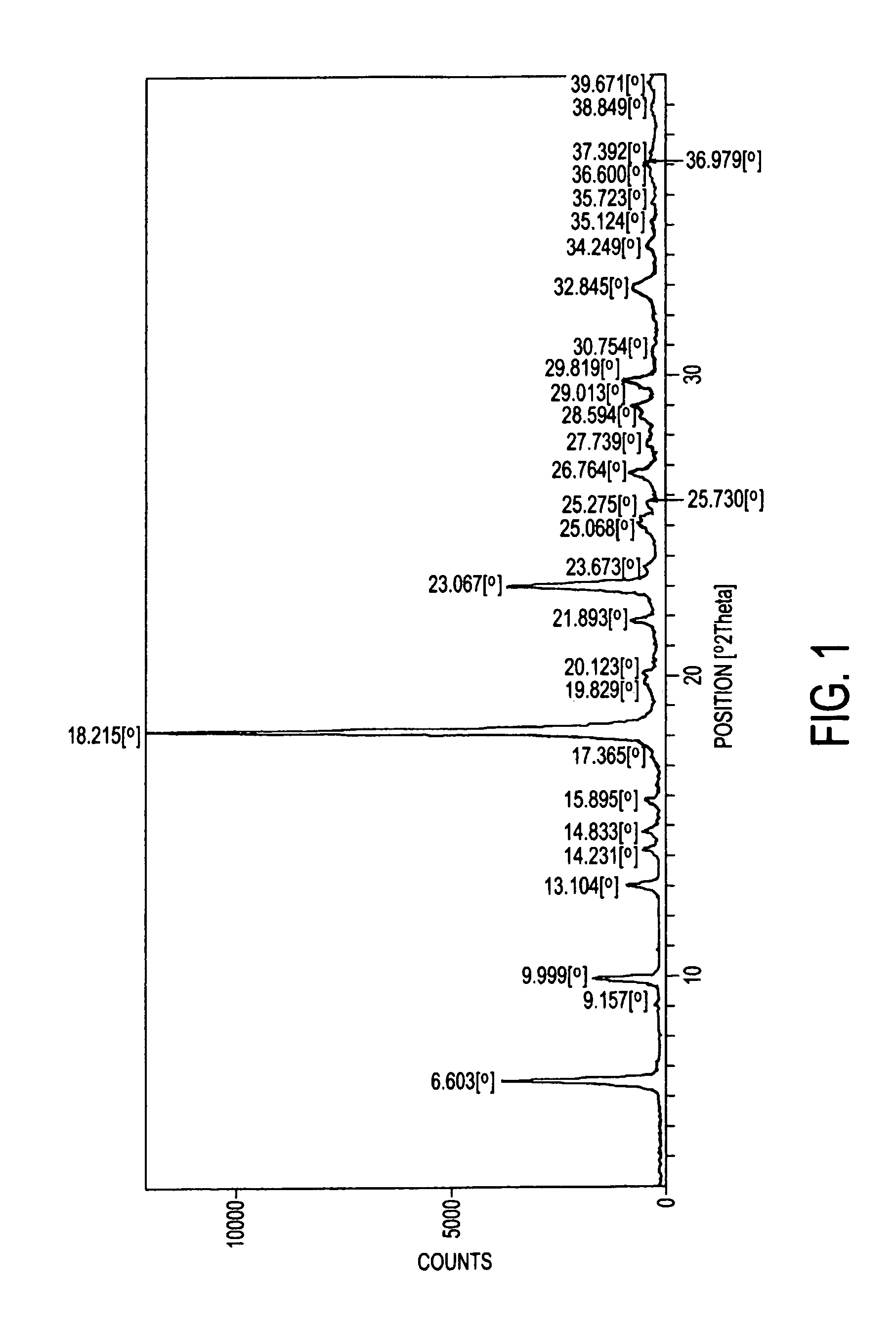
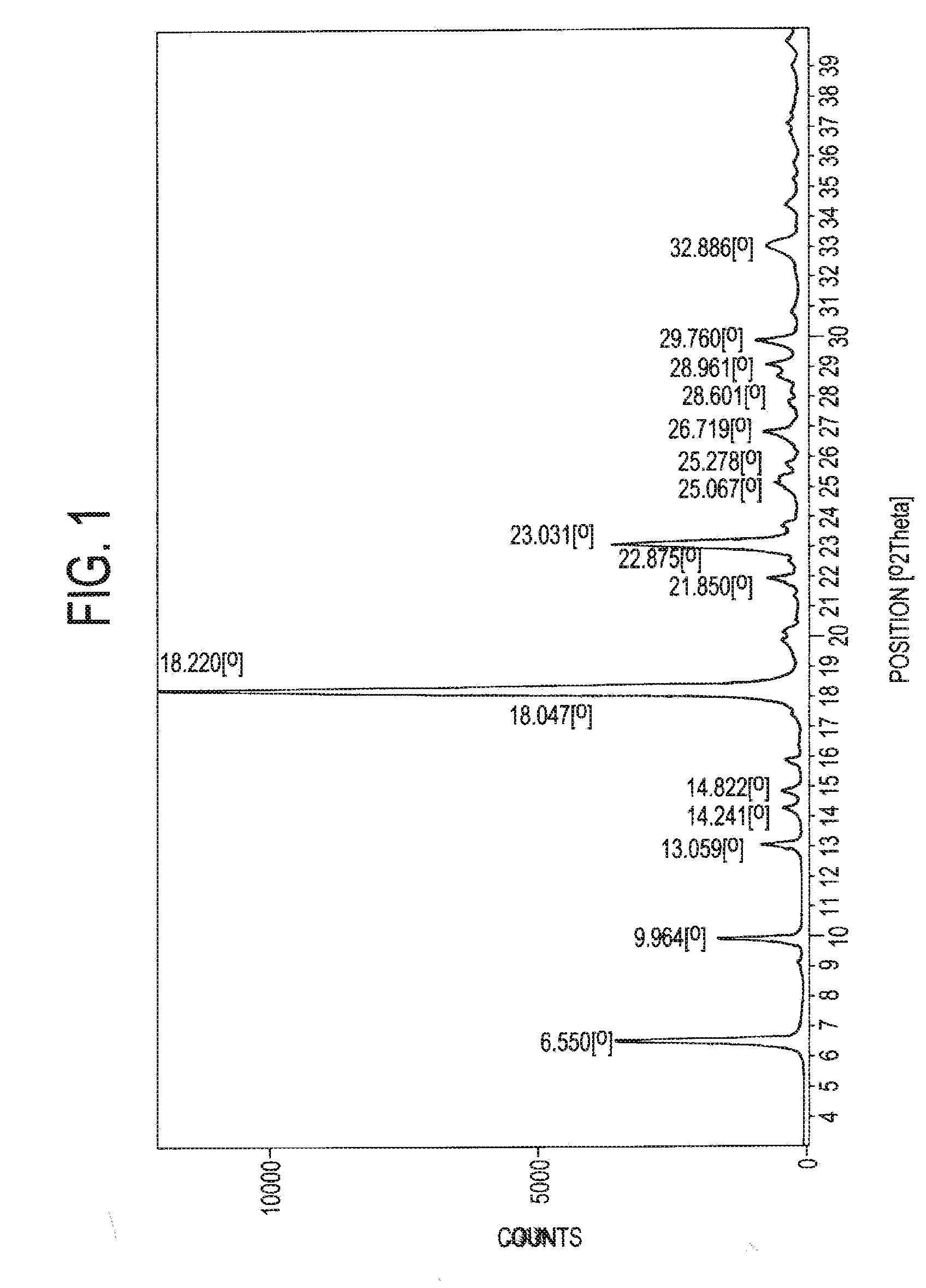
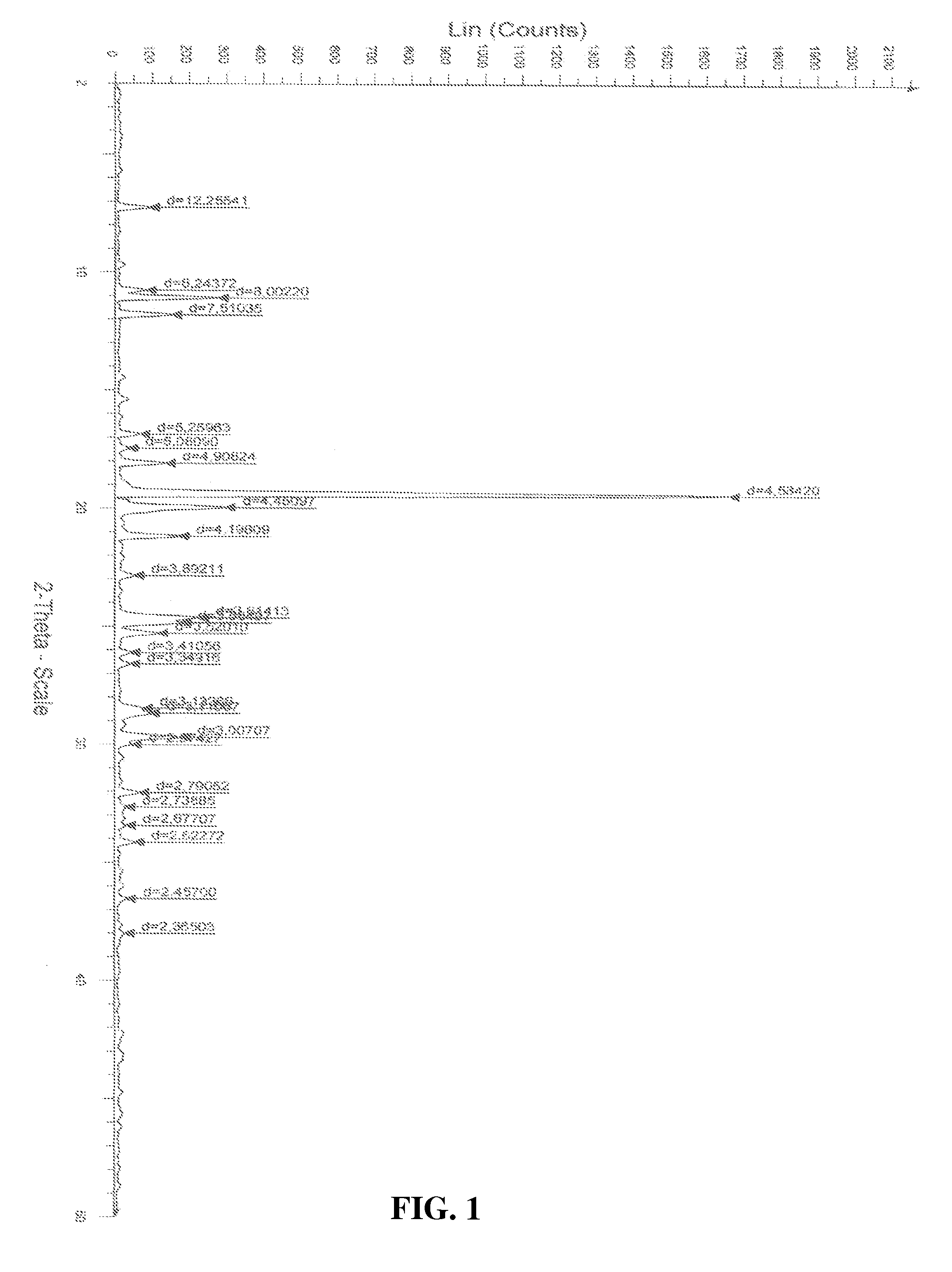
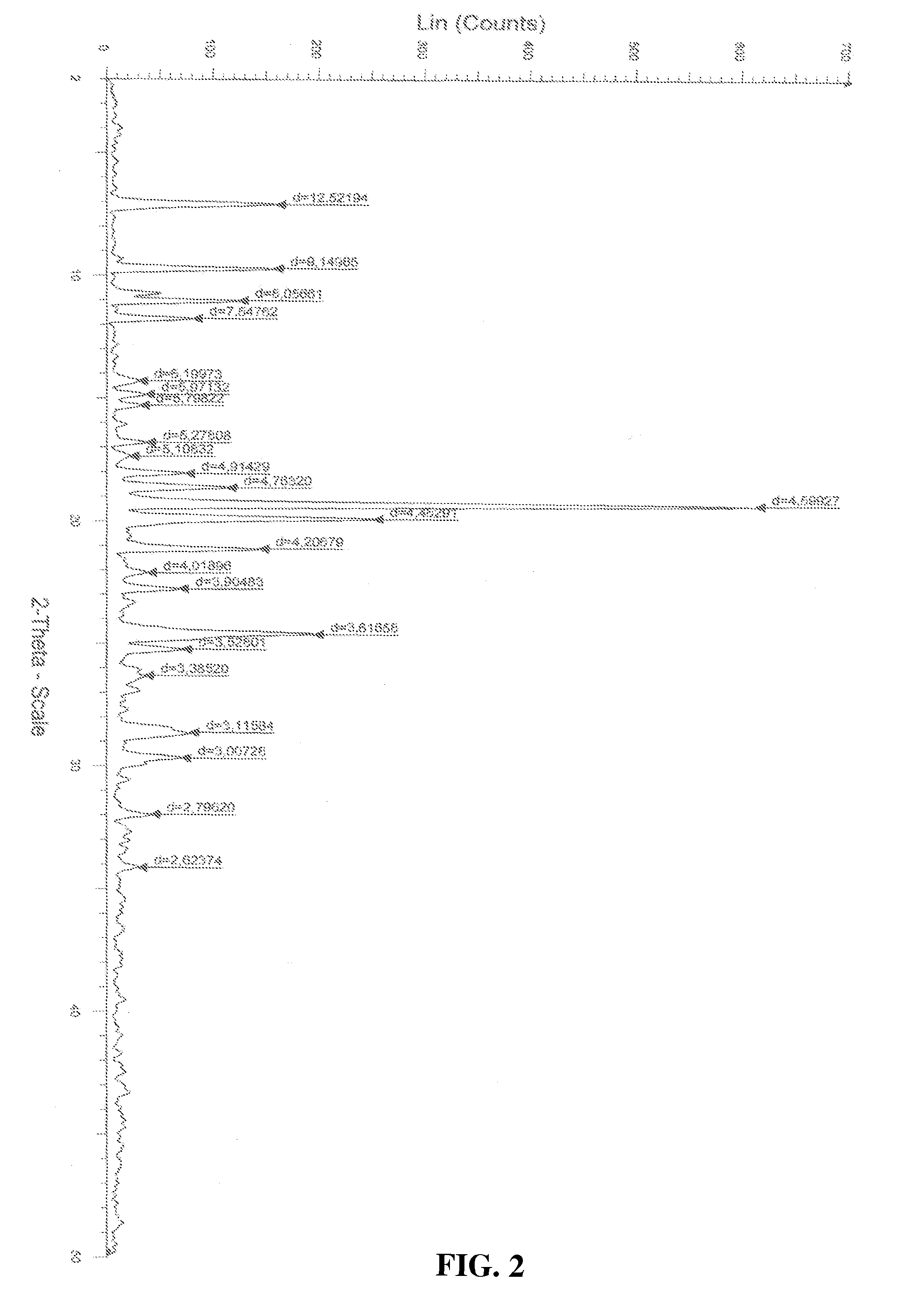
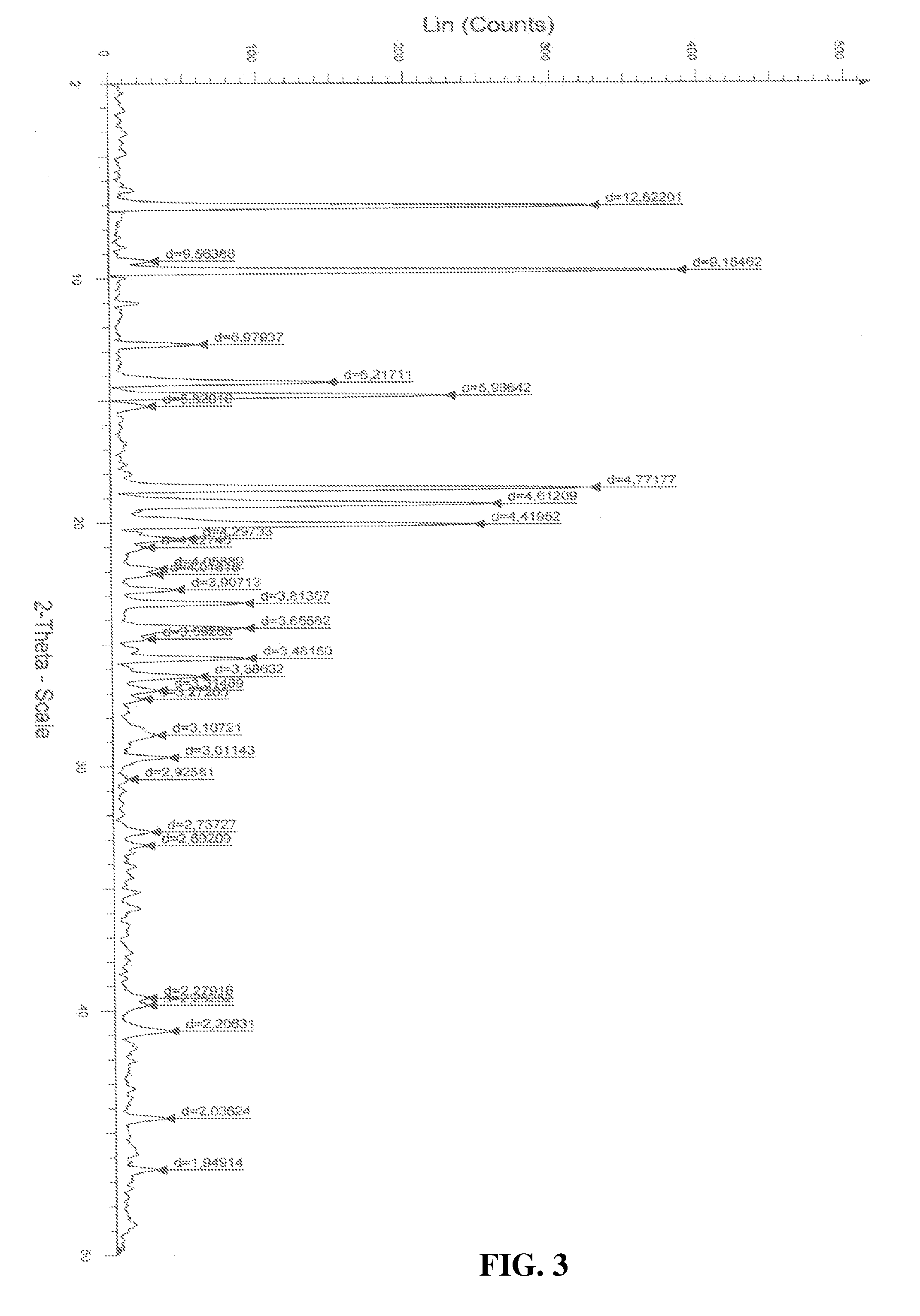
Process for the preparation of a stable polymorphic form of atovaquone
InactiveUS20150307431A1High yieldHigh purityOrganic compound preparationOrganic chemistry methodsAtovaquoneOrganic chemistry
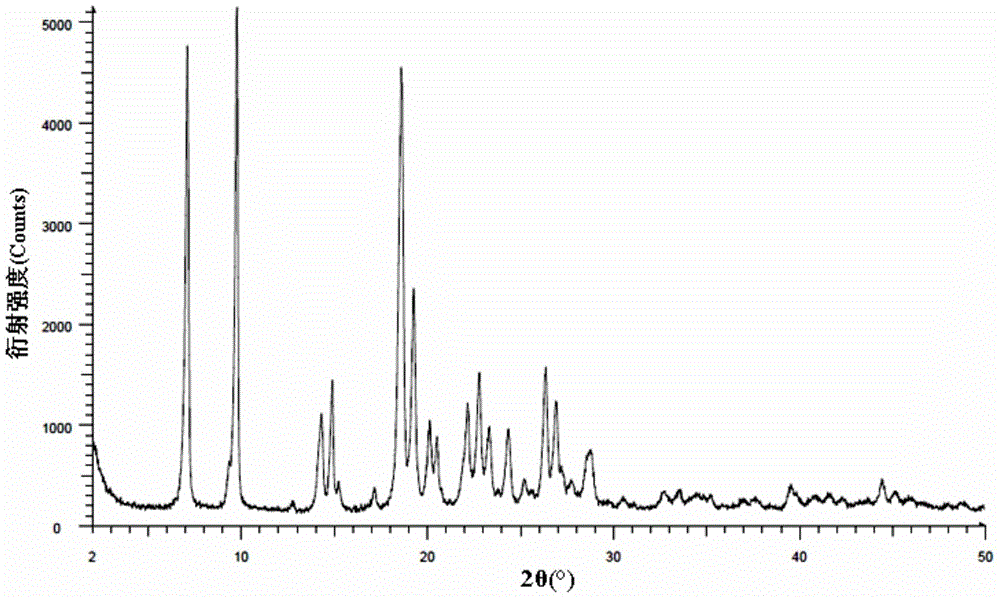
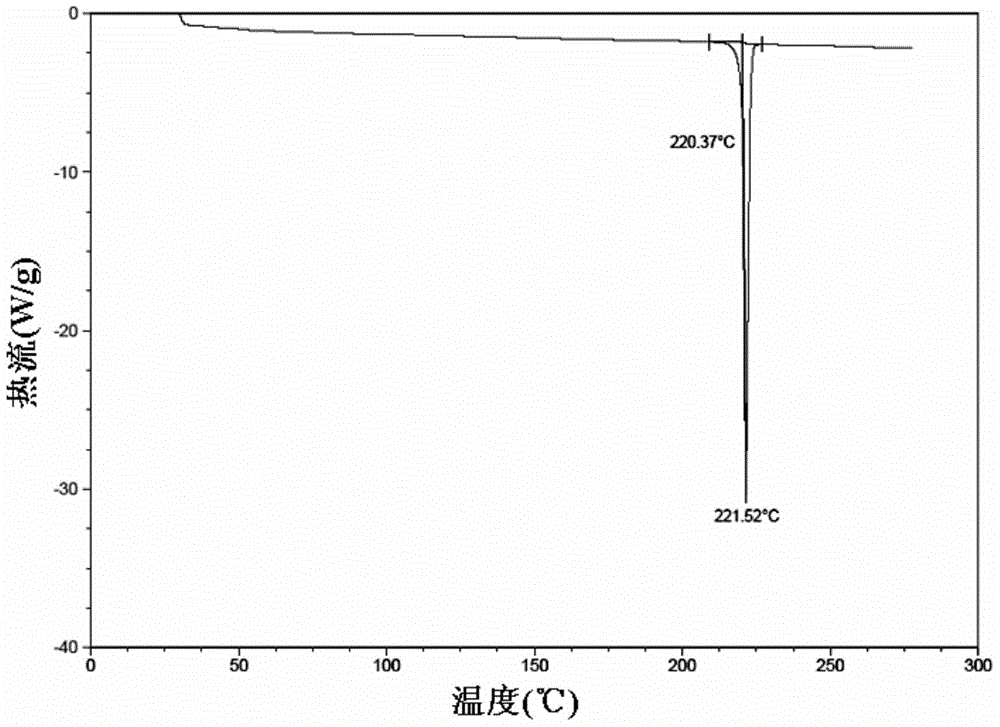
The present invention provides a process for the preparation of a stable polymorph III of Atovaquone exhibiting characteristic peaks (expressed in degrees 20±0.2°θ) at about 6.9, 9.6, 14.1, 14.7, 17.0, 18.5, 19.1, 19.9, 20.3, 22.0, 22.6, 23.2, 24.2, 26.8, and 28.5, which comprises: (a) providing a sample of Atovaquone particles; (b) heating the sample of Atovaquone particles at a minimal temperature of between 140° C. to 160° C. depending on the particle size of the sample; and (c) cooling the sample to obtain the stable polymorphic form of Atovaquone.
Owner:TARO PHARMA INDS
Process for the preparation of atovaquone
Disclosed herein is novel process for preparation of atovaquone, which process includes reacting 1H-2-benzopyran-1,4(3H)-dione with 4-(4-chlorophenyl)cyclohexanecarbaldehyde. The invention further discloses novel intermediates useful in the preparation of atovaquone.
Owner:GLAXO GRP LTD
Method for preparing atovaquone
InactiveCN101774901BImprove dissolutionReduce generationOrganic compound preparationQuinone preparationPotassium persulfateAtovaquone
The invention discloses a method for preparing atovaquone, which comprises the following steps: taking 4-(4-chlorophenyl)-cyclohexyl-1-methanoic acid and 2-chlorine-1, 4-naphthoquinone as raw materials, generating (3S)-2-chlorine-3-(4-(4-chlorophenyl) cyclohexyl)-1, 4-naphthalenedione by oxidative decarboxylation through a peroxide in the action of a catalyst of silver nitrate, and then obtaining the atovaquone through hydrolysis with alkaline. The method is characterized in that the mixed solvent of acetonitrile and choromethane is used as the solvent for the oxidative decarboxylation and the peroxide is one of the four substances, i.e., sodium persulfate, potassium persulfate, sodium percarbonate and potassium peroxycarbonate. The improved preparation method considerably improves the dissolution of the raw materials in the solvent, raises the rate of conversion, increases the yield, reduces impurities, and significantly enhances the product quality. Therefore, the method is more suitable for industrial production.
Owner:WUHAN TITON BIOTECH
Process for the epimerization of atovaquone isomer, atovaquone intermediates and mixture thereof
InactiveUS20110144347A1Speed up the processOrganic compound preparationQuinone separation/purificationAtovaquoneStereochemistry
Provided is a process for the epimerization of the cis isomer of atovaquone, atovaquone intermediates and isomeric mixtures thereof into their corresponding trans-isomers resulting in higher yield of pure atovaquone.
Owner:CHEMAGIS
Polymorphs of atovaquone and process of preparation thereof
Novel crystalline forms of anti Pneumocystis carinii compound (2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone) commonly known as Atovaquone and methods for producing the same is disclosed herein. This also provides pharmaceutical compositions comprising the said polymorphs of Atovaquone and method of treating Pneumocystis carinii pneumonia, the method comprising administering to a warm blooded animal an effective amount of a product-by-process composition of matter comprising polymorphic forms of Atovaquone.
Owner:USV LTD
3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug
The present invention relates to atovaquone prodrug compound of formula (I). Accordingly, present invention provides a process involving condensation of Atovaquone (II) with 5-methyl-4-chloromethyl dioxalone (III) in suitable solvent system and optionally followed by distillation and crystallization to provide Atovaquone prodrug compound of formula (I) in high yields, purity, and suitable for large-scale manufacture.
Owner:ALKEM LAB LTD
Preparation method for atovaquone III type crystal
InactiveCN105152900AEasy to operateProcess conditions are easy to controlQuinone separation/purificationOrganic solventAtovaquone
The invention discloses a preparation method for atovaquone III type crystal. The method comprises the following steps: dissolving an atovaquone crude product in an organic solvent at room temperature; cooling to crystallize, collecting crystal, and drying. The preparation method for high purity atovaquone III type crystal has low cost and high yield, and has great industrial value for preparing atovaquone III type crystal in large-scale manner.
Owner:SHANGHAI DESANO CHEM PHARMA +1
Technique for synthesizing atovaquone
ActiveCN101265171BShort processHigh purityOrganic compound preparationQuinone preparationAtovaquoneFiltration
The invention discloses a synthesis technology of Atovaquone. When a silver nitrate catalyst exists, 2-ethoxyl-1, 4-naphthoquinone and 4-(4-chlorophenyl) cyclohexyl-1-methanoic acid which are used asraw material react in acetonitrile solvent, and reaction reagent is added into a reaction vessel, the mixed solution is stirred and heated until refluxing is generated; when the refluxing is dripped down, the water solution of ammonium persulfate is added in, and the quantity of the ammonium persulfate is 2 to 3 times of the mole number of the 2-ethoxyl-1, 4-naphthoquinone; after the reaction is completed, the mixed solution is performed through cooling crystallization and filtration, and is filtered again by using the dissolving crystalization product of chloroform; filter liquor is collected, decompressed and evaporated to obtain the chloroform which is recrystallized to obtain yellow acicular crystal of the Atovaquone by using the acetonitrile. The synthesis route only comprises one-step reaction, the synthesizing cost is saved, the yield rate is high, and the prepared Atovaquone has high purity quotient.
Owner:CHONGQING KOOPPER CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bf3912af-9184-4e58-a0ad-0eee5e3464b6/BPA00001307227700011.PNG)
![Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bf3912af-9184-4e58-a0ad-0eee5e3464b6/BPA00001307227700021.PNG)
![Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m Atovaquone with a particle size diameter range (D90) of greater than 3 [mu]m to about 10 [mu]m](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bf3912af-9184-4e58-a0ad-0eee5e3464b6/BPA00001307227700141.PNG)



![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/515227a0-7e12-432f-b6b5-2d9199b7f819/US09169232-20151027-C00001.PNG)
![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/515227a0-7e12-432f-b6b5-2d9199b7f819/US09169232-20151027-C00002.PNG)
![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2-trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/515227a0-7e12-432f-b6b5-2d9199b7f819/US09169232-20151027-C00003.PNG)






![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0b3f558-80ed-40fa-878c-2ad9d71c08e5/US20140343297A1-20141120-C00001.png)
![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0b3f558-80ed-40fa-878c-2ad9d71c08e5/US20140343297A1-20141120-C00002.png)
![3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug 3-(5-methyl-2-oxo-l, 3-dioxol-4-yl) methyloxy-2- trans-[(4-chloro phenyl) cyclohexyl][1,4]naphthaquinone-atovaquone prodrug](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0b3f558-80ed-40fa-878c-2ad9d71c08e5/US20140343297A1-20141120-C00003.png)