[0009]In one aspect of the invention, new therapeutic methods and therapeutic conjugates are provided for inhibiting
vascular smooth muscle cells in a mammalian host. The therapeutic conjugates contain a
vascular smooth muscle binding protein or
peptide that binds in a specific manner to the cell membranes of a
vascular smooth muscle cell or an interstitial matrix
binding protein /
peptide that binds in a specific manner to interstitial matrix (e.g., collagen) of the
artery wall, coupled to a therapeutic agent that inhibits the activity of the cell. In one embodiment, inhibition of
cellular activity results in reducing, delaying, or eliminating stenosis after
angioplasty or other vascular
surgical procedures. The therapeutic conjugates of the invention achieve these advantageous effects by associating with vascular smooth muscle cells and pericytes, which may transform into smooth muscle cells. The therapeutic conjugate may contain: (1) therapeutic agents that alter
cellular metabolism or are inhibitors of
protein synthesis, cellular proliferation, or
cell migration; (2)
microtubule and
microfilament inhibitors that affect morphology or increases in
cell volume; and / or (3) inhibitors of
extracellular matrix synthesis or
secretion. In one representative embodiment, the conjugates include a cytotoxic therapeutic agent that is a sesquiterpenoid
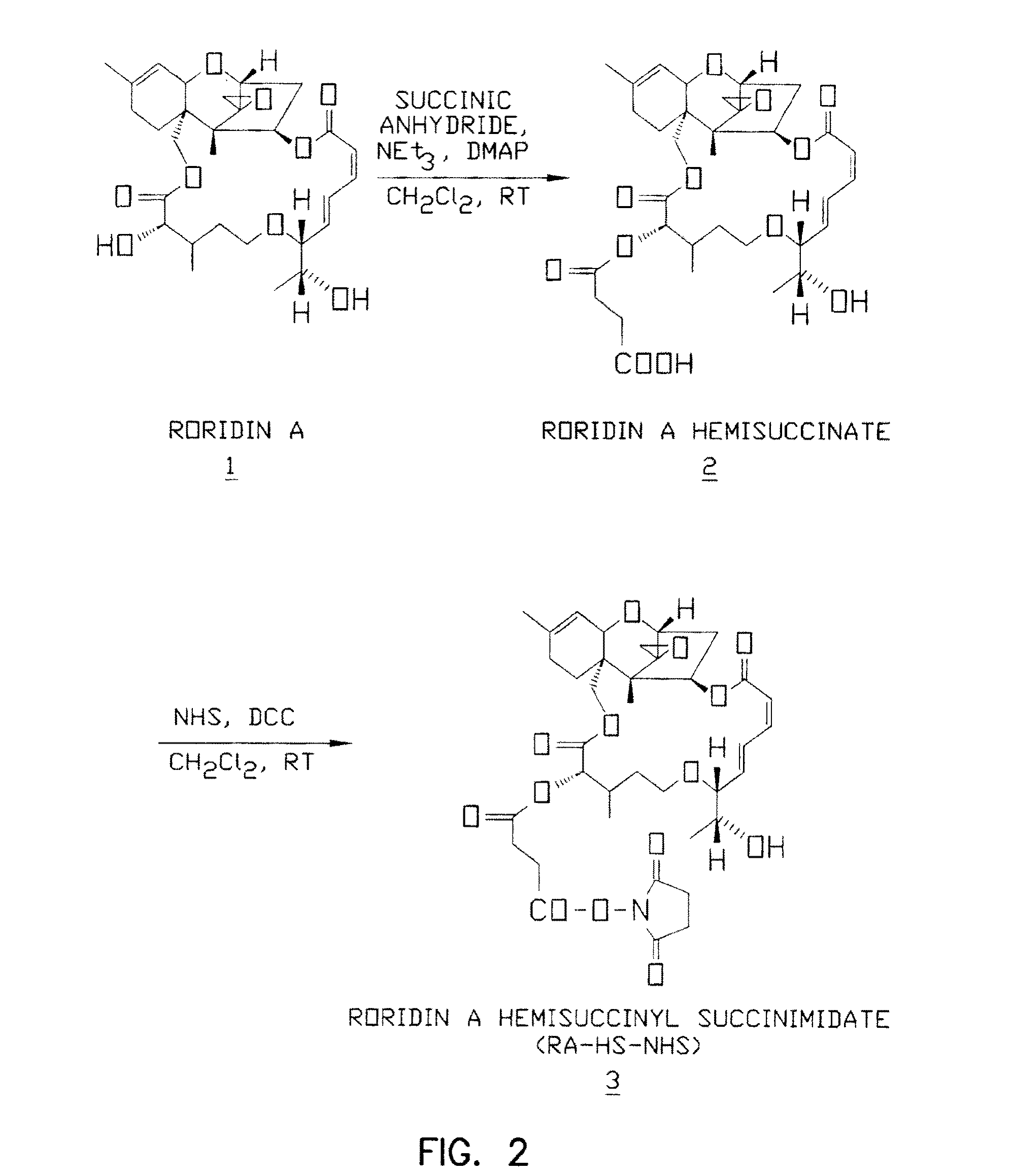
mycotoxin such as a verrucarin or a roridin. Other embodiments involve cytostatic therapeutic agents that inhibit
DNA synthesis and proliferation at doses that have a
minimal effect on
protein synthesis such as
protein kinase inhibitors (e.g., staurosporin), suramin,
transforming growth factor-beta (TGF-beta) activators or production stimulators such as trans-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine (
tamoxifen), TGF-beta itself, and
nitric oxide releasing compounds (e.g., nitroglycerin) or analogs or functional equivalents thereof. Other moieties that inhibit
cell division and are, therefore, useful in the practice of the present invention, include, for example, taxol and analogs thereof such as taxotere. In addition, therapeutic agents that inhibit the contraction or migration of smooth muscle cells and maintain an enlarged luminal area following, for example,
angioplasty trauma (e.g., the cytochalasins, such as
cytochalasin B,
cytochalasin C,
cytochalasin D, taxol or analogs thereof such as taxotere or the like) are also contemplated for use in accordance with the present invention. Other aspects of the invention relate to vascular smooth muscle binding proteins that specifically associate with a
chondroitin sulfate proteoglycan (CSPG) expressed on the membranes of a vascular smooth muscle cell, and in a preferred embodiment this CSPG has a molecular weight of about 250 kDaltons. In preferred embodiments the vascular smooth muscle
binding protein binds to a CSPG target on the cell surface with an association constant of at least 10−4 M. In another preferred embodiment, the vascular smooth muscle binding protein contains a sequence of amino acids found in the Fab, Fv or CDR (complementarity determining regions) of
monoclonal antibody NR-AN-01 or functional equivalents thereof.
[0010]Other aspects of the invention include methods for inhibiting stenosis, e.g., following angioplasty in a mammalian host, by administering to a human or
animal subject in need of such treatment a therapeutically effective dosage of a therapeutic conjugate of the invention. In one representative embodiment, the dosage of therapeutic conjugate may be administered with an
infusion catheter, to achieve a 10−3 M to 10−12 M concentration of said therapeutic conjugate at the site of administration in a
blood vessel.
[0011]The present invention also contemplates therapeutic methods and therapeutic dosage forms involving sustained release of therapeutic agent to target cells. Preferably, the target cells are vascular smooth muscle cells,
cancer cells, somatic cells requiring modulation to ameliorate a
disease state and cells involved in
immune system-mediated diseases that are accessible by local administration of the
dosage form. Consequently, the methods and dosage forms of this aspect of the present invention are useful for inhibiting vascular smooth muscle cells in a mammalian host, employing a therapeutic agent that inhibits the activity of the cell (e.g., proliferation, contraction, migration or the like) but does not kill the cell and, optionally, a vascular smooth muscle
cell binding protein. Also, the methods and dosage forms of this aspect of the present invention are useful for inhibiting target cell proliferation or killing such target cells, employing a therapeutic agent that inhibits proliferation or is cytotoxic to the target cells and, optionally, a target
cell binding protein. In addition, the methods and dosage forms of this aspect of the present invention are useful for delivering cytostatic, cytocidal or
metabolism modulating therapeutic agents to target cells, such as
effector cells of the
immune system, that are accessible by local administration of the
dosage form, optionally employing a target
cell binding protein. Finally, dosage forms of the present invention are useful to reduce or eliminate
pathological proliferation or hyperactivity of
normal tissue (i.e., somatic cells).
[0012]The dosage forms of the present invention are preferably either non-degradable microparticulates or nanoparticulates or biodegradable microparticulates or nanoparticulates. More preferably, the microparticles or nanoparticles are formed of a
polymer containing matrix that biodegrades by random, nonenzymatic, hydrolytic scissioning. A particularly preferred structure is formed of a mixture of
thermoplastic polyesters (e.g., polylactide or
polyglycolide) or a
copolymer of
lactide and glycolide components. The
lactide / glycolide structure has the added
advantage that
biodegradation thereof forms
lactic acid and
glycolic acid, both normal metabolic products of mammals.
[0013]Preferable therapeutic agents dispersed within the microparticulates or nanoparticulates are those exhibiting inhibition of a therapeutically significant target
cell activity without killing the target cell, or target
cell killing activity. For treatment of restenosis of vascular smooth muscle cells, useful therapeutic agents inhibit target
cell activity (e.g., proliferation or migration) without killing the target cells. Preferred therapeutic moieties for this purpose are protein
kinase inhibitors (e.g., staurosporin or the like), TGF-beta production or activation stimulators, such as
tamoxifen or TGF-beta itself, taxol or analogs thereof (e.g., taxotere), smooth muscle migration and / or contraction inhibitors (e.g., the cytochalasins, such as cytochalasin B, cytochalasin C, cytochalasin D or the like), suramin, and
nitric oxide-releasing compounds, such as nitroglycerin, or analogs or functional equivalents thereof. In
cancer therapy, useful therapeutic agents inhibit proliferation or are cytotoxic to the target cells. Preferred therapeutic moieties for this purpose are TGF-beta production or activation stimulators, such as
tamoxifen or TGF-beta itself, taxol or analogs thereof (e.g., taxotere), Roridin A and
Pseudomonas exotoxin, or analogs or functional equivalents thereof. For treatment of
immune system-modulated diseases, such as
arthritis, useful therapeutic agents deliver cytostatic, cytocidal or
metabolism-modulating therapeutic agents to target cells that are accessible by local administration of the
dosage form. Preferred therapeutic moieties for this purpose are Roridin A,
Pseudomonas exotoxin, suramin, TGF-beta production or activation stimulators, such as tamoxifen or TGF-beta itself, taxol or analogs thereof (e.g., taxotere) and protein
kinase inhibitors (e.g., staurosporin),
sphingosine, or analogs or functional equivalents thereof. For treatment of pathologically proliferating normal tissues (e.g.,
proliferative vitreoretinopathy, corneal pannus and the like), anti-proliferative agents or antimigration agents are preferred (e.g., cytochalasins, taxol or analogs thereof,
somatostatin,
somatostatin analogs, N-ethylmaleimide,
antisense oligonucleotides, TGF-beta production or activation stimulators, such as tamoxifen or TGF-beta itself and the like).
[0014]The dosage forms of the present invention are optionally targeted to a relevant target cell
population by a binding protein or
peptide. Preferred binding proteins / peptides of the present invention are vascular smooth muscle cell binding protein, tumor cell binding protein and immune
system effector cell binding protein. Preferred vascular smooth muscle cell binding proteins specifically associate with a
chondroitin sulfate proteoglycan (CSPG) expressed on the membranes of a vascular smooth muscle cell, and in a preferred embodiment this CSPG has a molecular weight of about 250 kDaltons. In preferred embodiments, the vascular smooth muscle binding protein binds to a CSPG target on the cell surface with an association constant of at least 10−4 M. In other preferred embodiments, the vascular smooth muscle binding protein contains a sequence of amino acids found in the Fab, Fv or CDR (complementarity determining regions) of
monoclonal antibody NR-AN-01 or functional equivalents thereof. Other preferred binding peptides useful in this embodiment of the present invention include those that localize to intercellular stroma and matrix located between and among vascular smooth muscle cells. Preferred binding peptides of this type are specifically associated with collagen, reticulum fibers or other intercellular matrix compounds. Preferred tumor cell binding proteins are associated with surface cell markers expressed by the target tumor cell
population or cytoplasmic epitopes thereof. Preferred immune
system-modulated target cell binding proteins are associated with cell surface markers of the target immune
system effector cells or cytoplasmic epitopes thereof. Binding peptides / proteins of the present invention also target pathologically proliferating normal tissues.
 Login to View More
Login to View More