Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "Sintering resistant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nickel-base catalyst used for autothermal reforming of ethanol for producing hydrogen and preparation method thereof
InactiveCN101972656AEasy to introduceLarge specific surface areaHydrogenMetal/metal-oxides/metal-hydroxide catalystsManganeseCarbon deposit
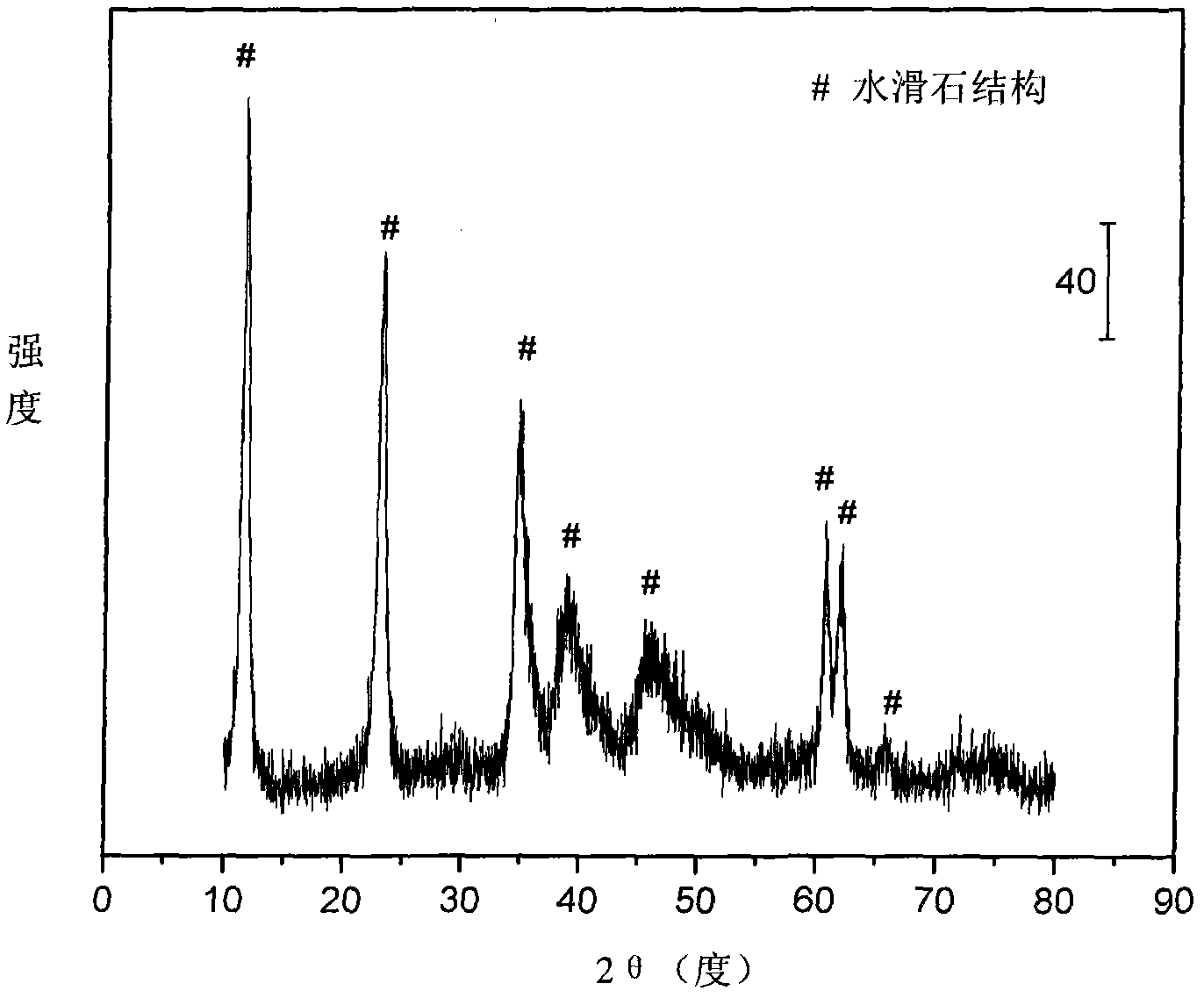
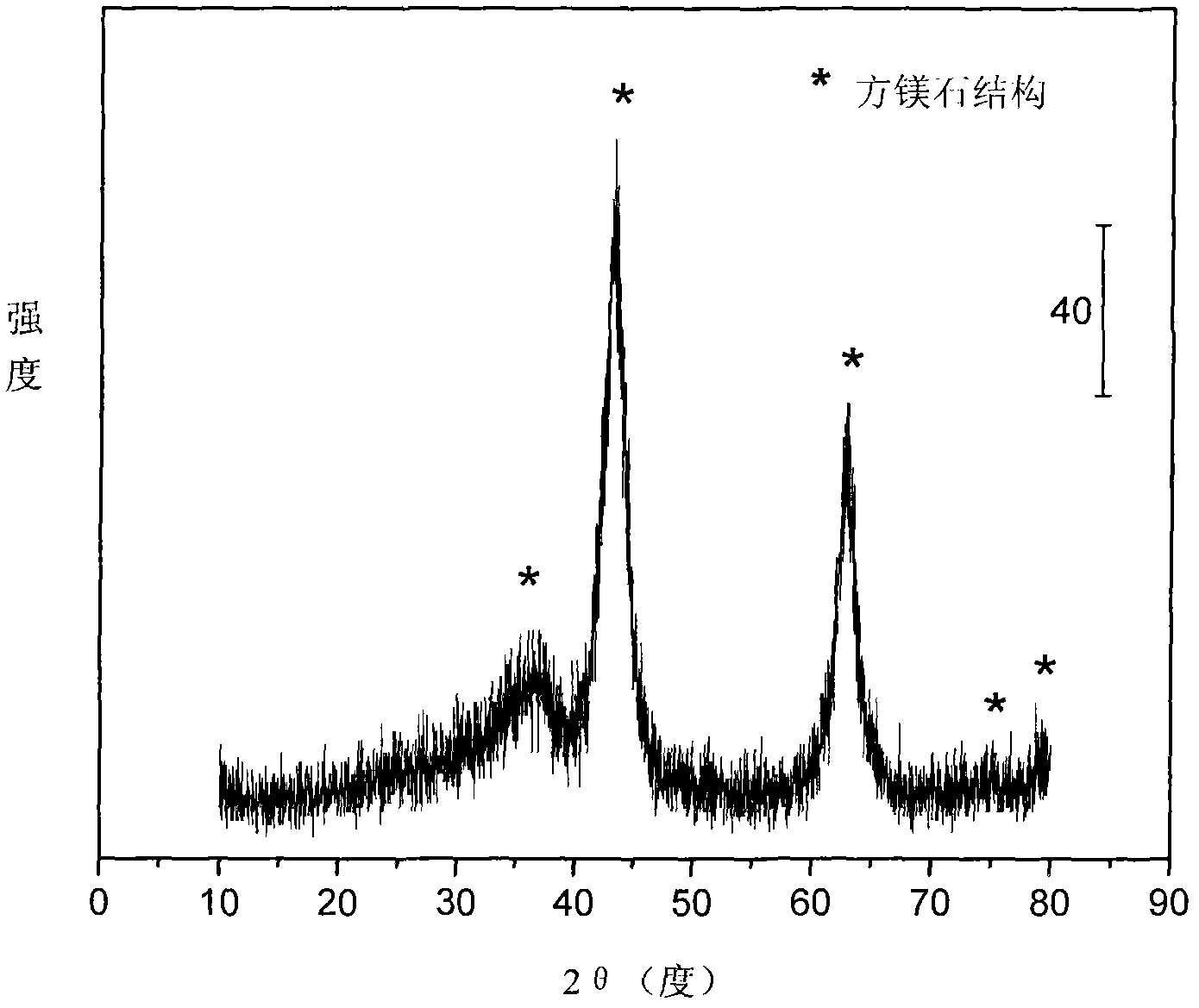
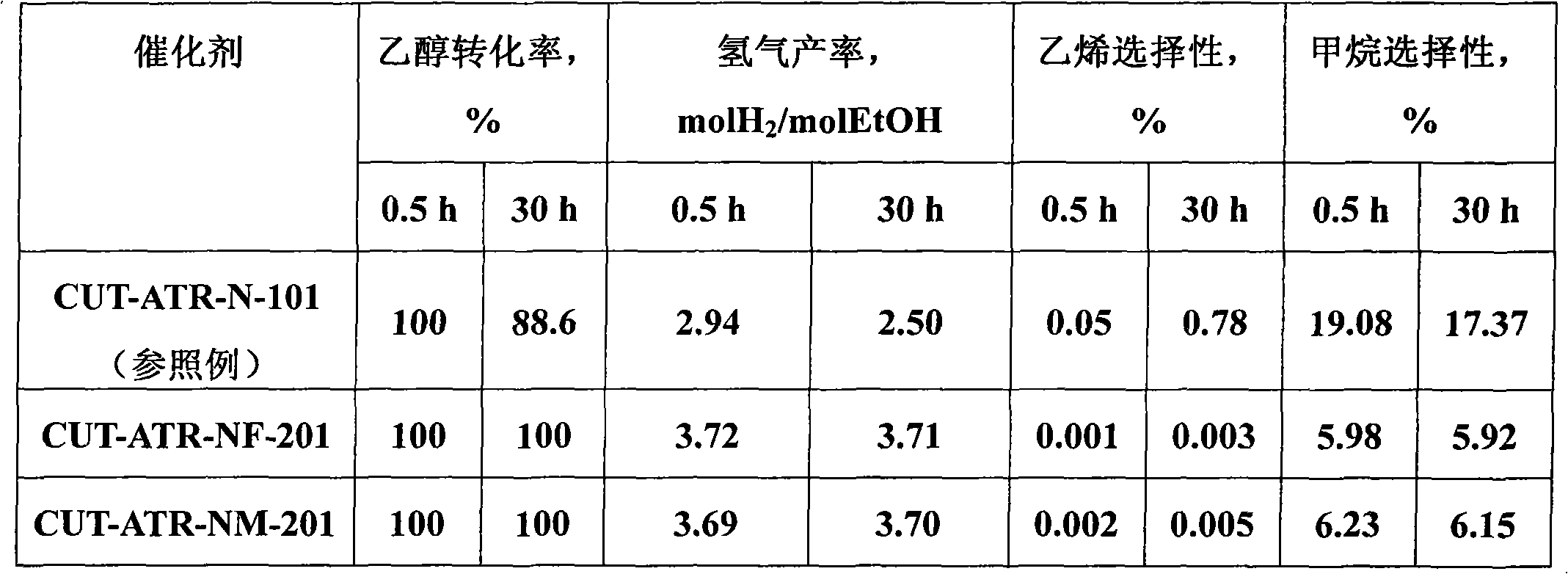
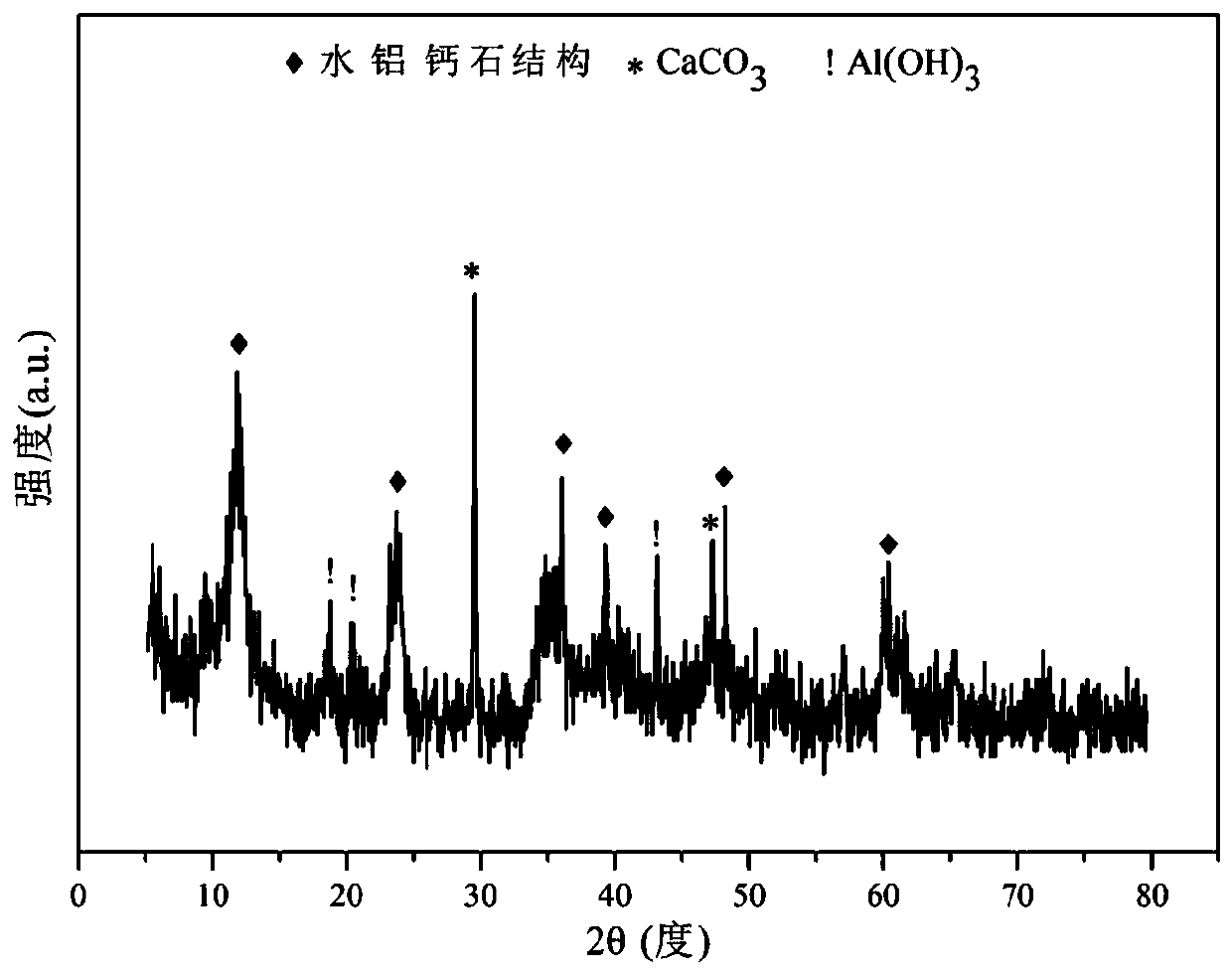
The invention relates to a nickel-based catalyst used for the autothermal reforming of ethanol for producing hydrogen and a preparation method thereof. Aiming at the problems of structural change, oxidation and sintering of active ingredients and deactivation of the conventional catalyst during the autothermal reforming of the ethanol, the invention provides a novel catalyst with a stable structure, sintering resistance, carbon deposit resistance, oxidation resistance and high activity. The chemical formula of the catalyst is NiaMgbAlcXdO4.5+ / -delta, wherein X is an auxiliary agent Fe or Mn, a is 0.25 to 0.40, b is 2.6 to 2.75, c is 0.1 to 0.8 and d is 0.2 to 0.9. A brucite-based (Mg(OH)2.mH2O) hydrotalcite structure is taken as a precursor, so that the active ingredient nickel and the auxiliary agent heteroion component are introduced into a laminated structure and a position between laminated structures; meanwhile, the auxiliary agent iron or magnesium is introduced, so the reducibility and stability of the active ingredients of the catalyst are improved, and the yield of the hydrogen is obviously improved and remains stable.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Perovskite catalyst used for autothermal reforming of ethanol for producing hydrogen and preparation method thereof
InactiveCN101972659AChange the adsorption and desorption propertiesEnhanced autothermal reforming activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsManganeseOxygen
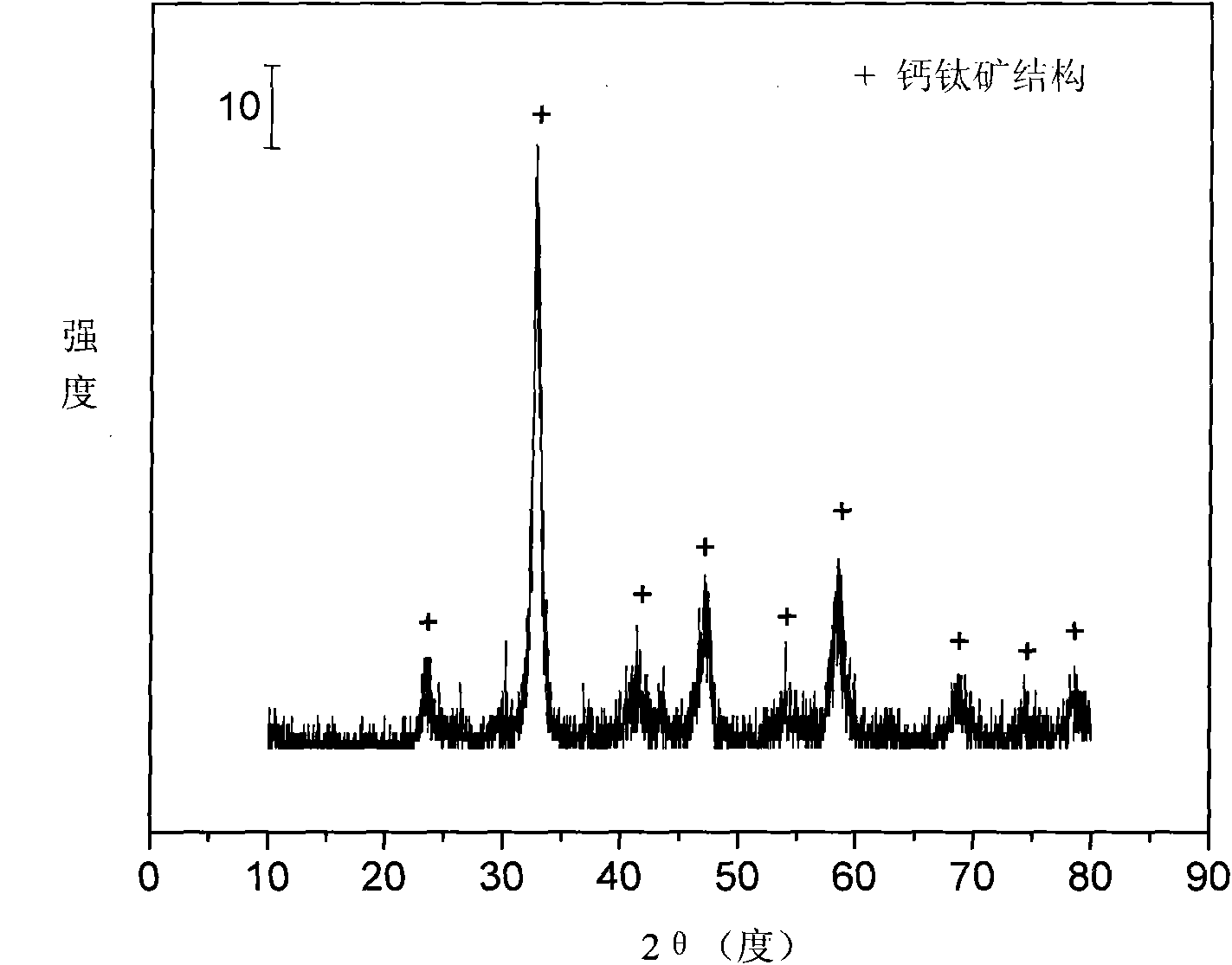
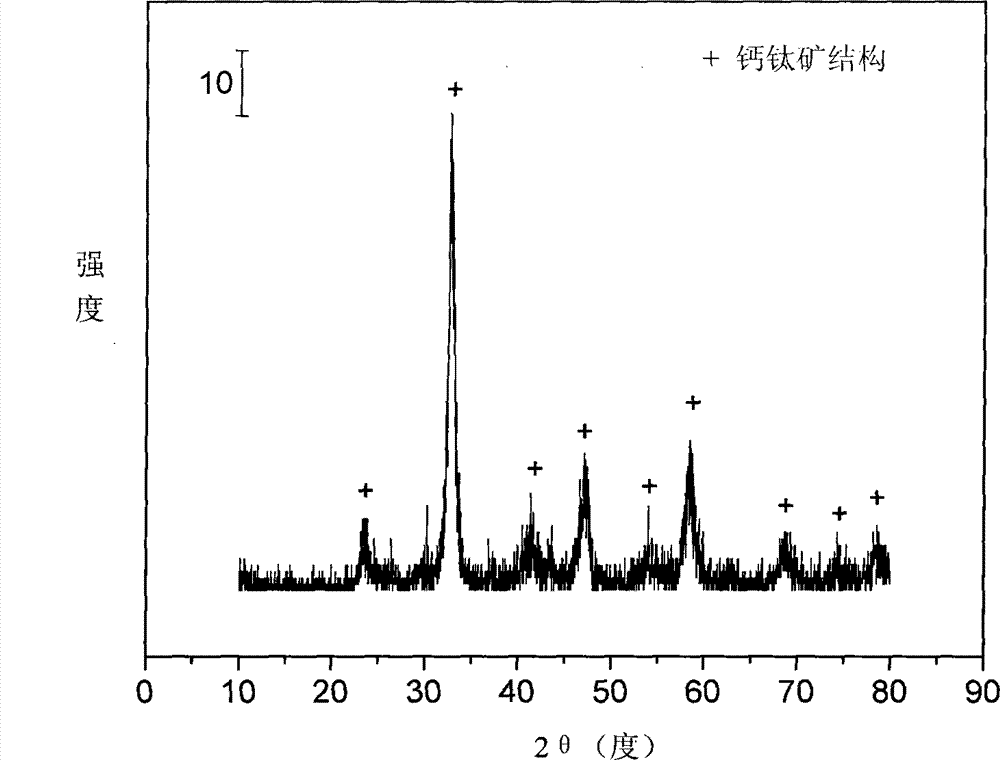
The invention relates to a perovskite catalyst used for the autothermal reforming of ethanol for producing hydrogen and a preparation method thereof. Aiming at the problems of structural change, oxidation and sintering of active ingredients and deactivation of the conventional catalyst during the autothermal reforming of the ethanol, the invention provides a novel catalyst with sintering resistance, carbon deposit resistance, oxidation resistance and high activity. The chemical formula of the catalyst is LaaSrbNicXdO3, wherein X is one of Fe, Mn and Cr, a is 0.7 to 1.0, b is 0 to 0.3, c is 0.7 to 0.95 and d is 0.05 to 0.3. The nickel-based catalyst with an ABO3 perovskite structure is prepared by a glycine complexation method, lanthanum is partially substituted by strontium at a position A and nickel is partially substituted by an auxiliary agent at a position B, so oxygen defects and lattice structure defects on the surface of the perovskite catalyst are increased; meanwhile, the auxiliary agent, namely iron, magnesium or chromium is introduced, so the acidity of the catalyst is inhibited, the reducibility and stability of the active ingredients of the catalyst are improved, and the yield of the hydrogen is obviously improved and remains stable.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
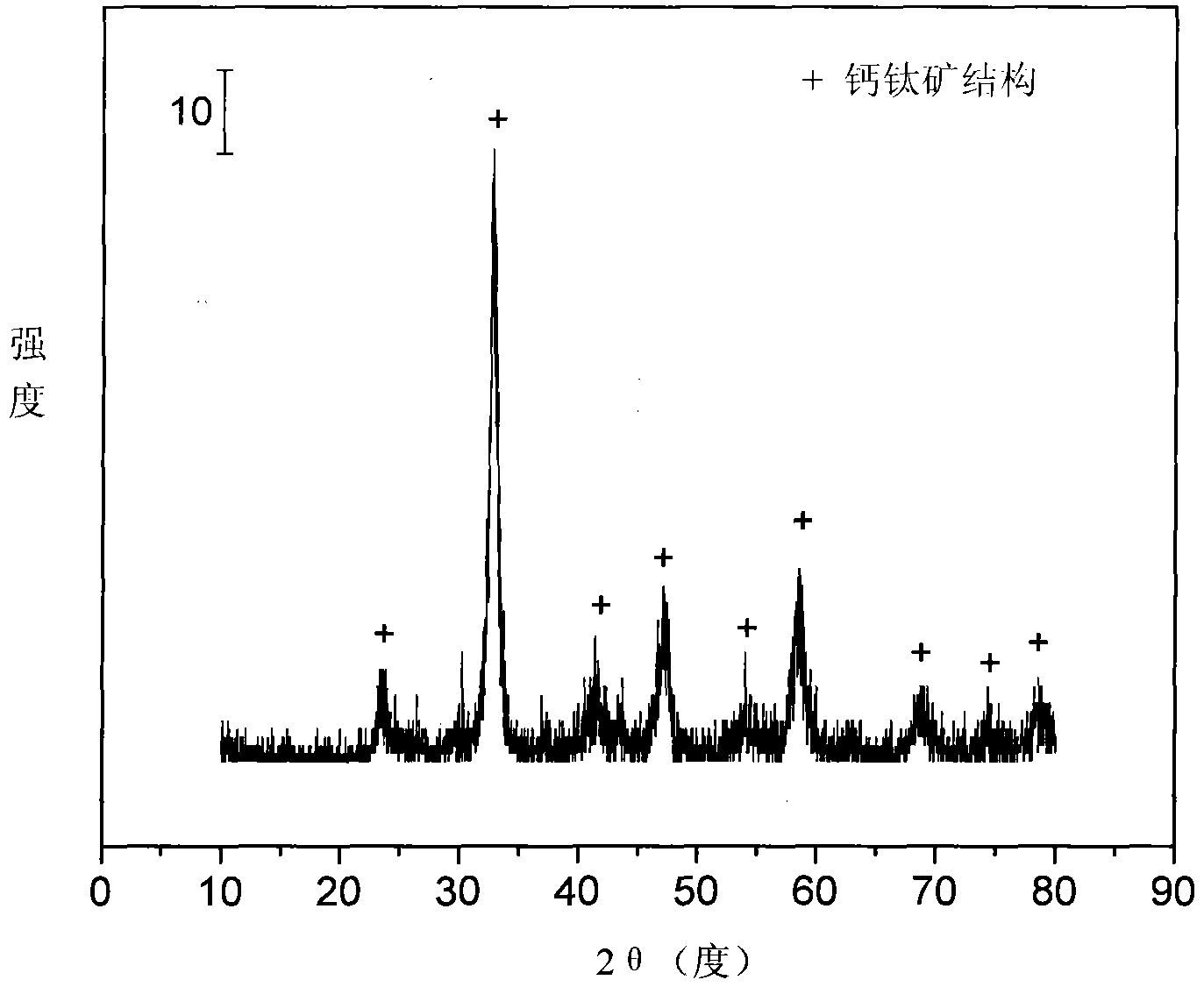
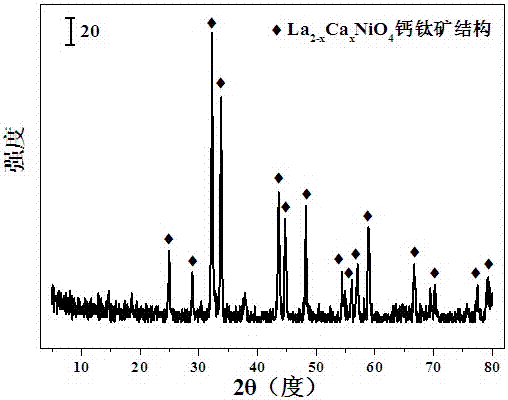
Layered perovskite catalyst for hydrogen generation from acetic acid autothermal reforming and preparation method
ActiveCN107042111AImprove stabilityAdd Surface Defect BitsHydrogenHeterogenous catalyst chemical elementsLanthanumHYDROSOL
The invention relates to a layered perovskite catalyst for hydrogen generation from acetic acid autothermal reforming and a preparation method. A novel catalyst which is sintering resistant, carbon deposition resistant, antioxidant and high in activity is provided for the problem of catalyst deactivation due to sintering, oxidation and carbon deposition of an existing catalyst in the acetic acid autothermal reforming process. A nickel-based catalyst with an A2BO4 layered perovskite structure is prepared by employing a sol-gel method; and the chemical component is La<2-x>Ca<x>NiO<4>, wherein x is equal to 0-1.5. An oxygen defect and a lattice structure defect of the surface of the perovskite catalyst are increased through replacing lanthanum with a calcium part, and the reducibility, the heat stability and the oxidation resistance of the active component nickel are improved, so that the activity and the stability of the catalyst are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
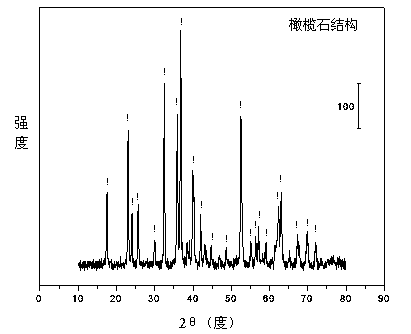
Olivine nickel-based catalyst for preparing hydrogen through autothermal reforming of acetic acid
InactiveCN103657654AIncrease lattice defectsEnhanced autothermal reforming activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsNickel catalystAcetic acid
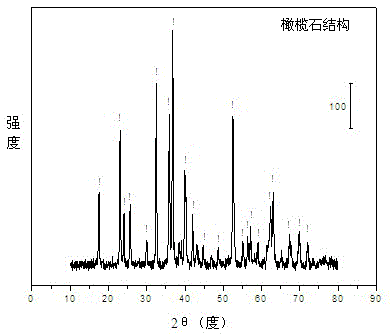
The invention relates to an olivine nickel-based catalyst for preparing hydrogen through autothermal reforming of acetic acid. Aiming to solve the problems of low hydrogen yield and oxidation / sintering inactivation of active constituents of the conventional supported nickel-based catalyst in an autothermal reforming process of acetic acid, the invention provides a novel catalyst with the effects of large specific surface area, stable structure, sintering resistance, oxidation resistance and stable activity. The catalyst provided by the invention has a chemical component of MgaFebNicSiO4, wherein a is 1.0-1.7, b is 0-0.7 and c is 0.3-0.5. The nickeliferous catalyst taking an olivine structure as a main body is prepared by a hydro-thermal synthesis method; the catalyst has rich hole structures so that the specific surface area and the dispersity of active constituents of the catalyst are effectively improved; meanwhile, the active constituents, such as nickel and iron, and additives substitute for bivalent magnesium to enter an olivine skeletal structure to effectively refrain the phenomenon of oxidation / sintering inactivation of the catalyst, so that the stability and the hydrogen yield of the catalyst in the autothermal reforming process of acetic acid are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
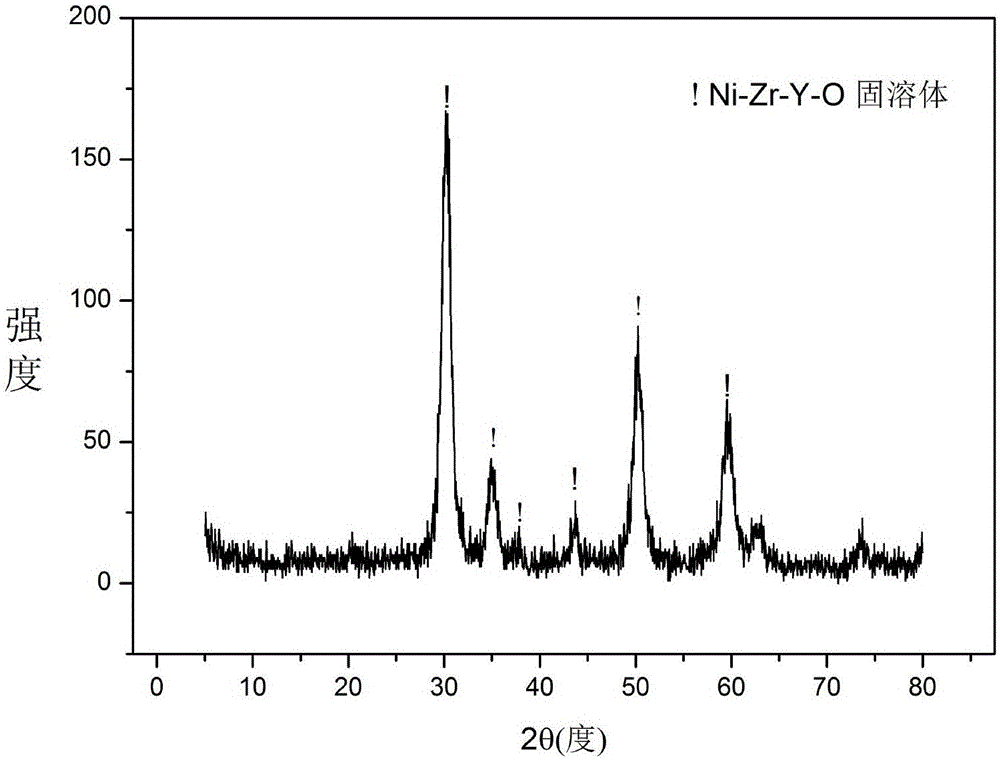
Solid solution catalyst for acetic acid self-heating hydrogen production by reforming and preparation method
ActiveCN106391036AGood dispersionImprove thermal stabilityHydrogenHeterogenous catalyst chemical elementsPolyethylene oxideEvaporation
The invention relates to a solid solution catalyst for acetic acid self-heating hydrogen production by reforming. Aiming at the problem that in an acetic acid self-heating reforming reaction of an existing catalyst, by means of oxidizing, sintering, carbon depositing and other factors of the active components of the catalyst, the catalyst is inactivated, the novel catalyst is stable in structure, resistant to sintering, resistant to carbon depositing and oxidization and stable in activity, and the composition by weight of the catalyst is (NiO)a(YO1.5)b(ZrO2)c, wherein a ranges from 0.07 to 0.20, b ranges from 0.02 to 0.35, and c ranges from 0.58 to 0.91. According to the catalyst, zirconium oxide is adopted as a carrier, an aid yttrium oxide is introduced, a polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer is adopted as a template, a mesoporous structure is formed through evaporation self-assembly, and the nickel-based catalyst with the stable ZrxY1-xOy solid solution as the main structure is obtained. The hydrogen productivity, stability and carbon depositing resisting capacity of the acetic acid self-heating reforming process are effectively improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Perovskite-type titanium-strontium-cobalt catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN109759070ALower activation energyStable activityMetal/metal-oxides/metal-hydroxide catalystsComposite oxideSol-gel
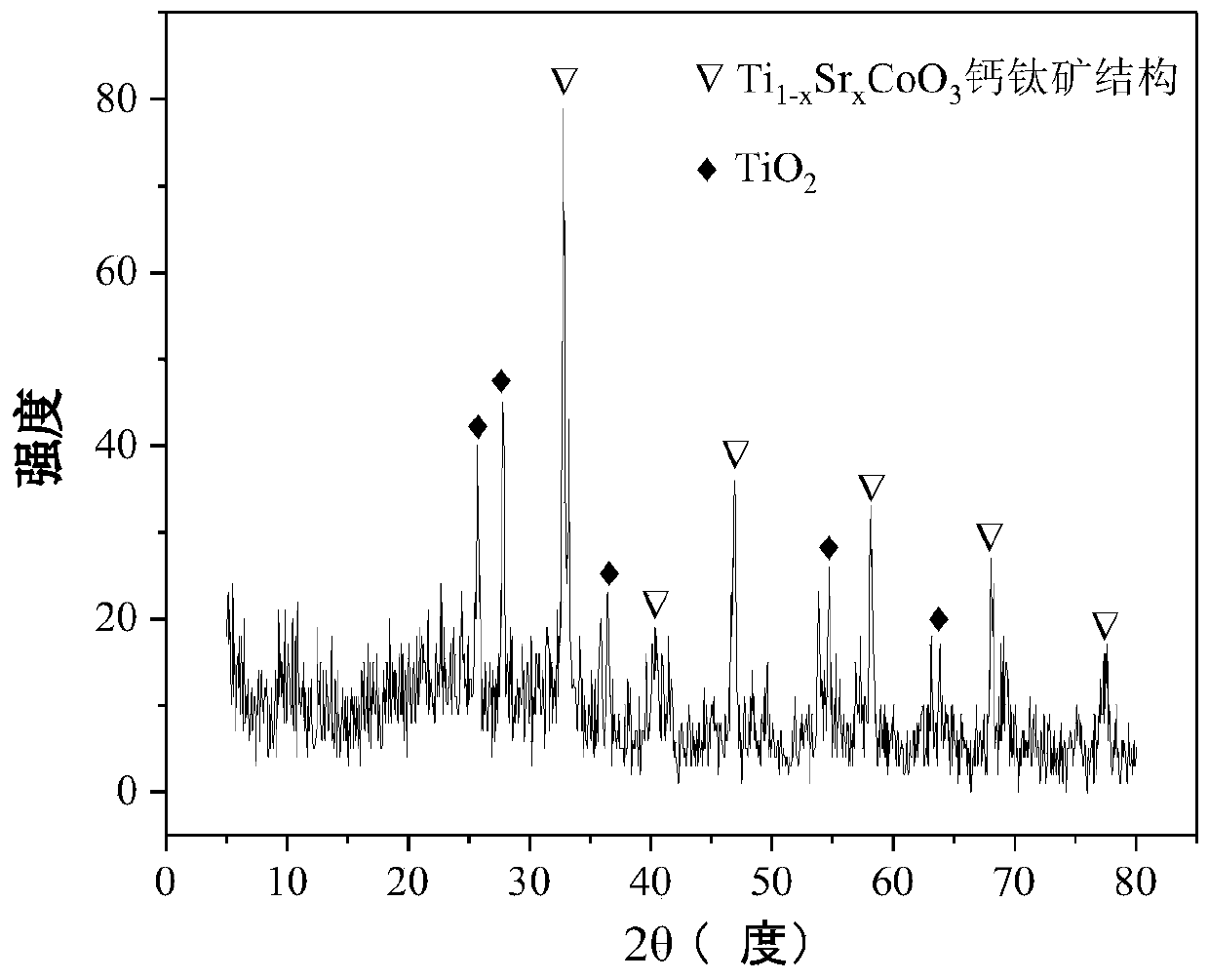
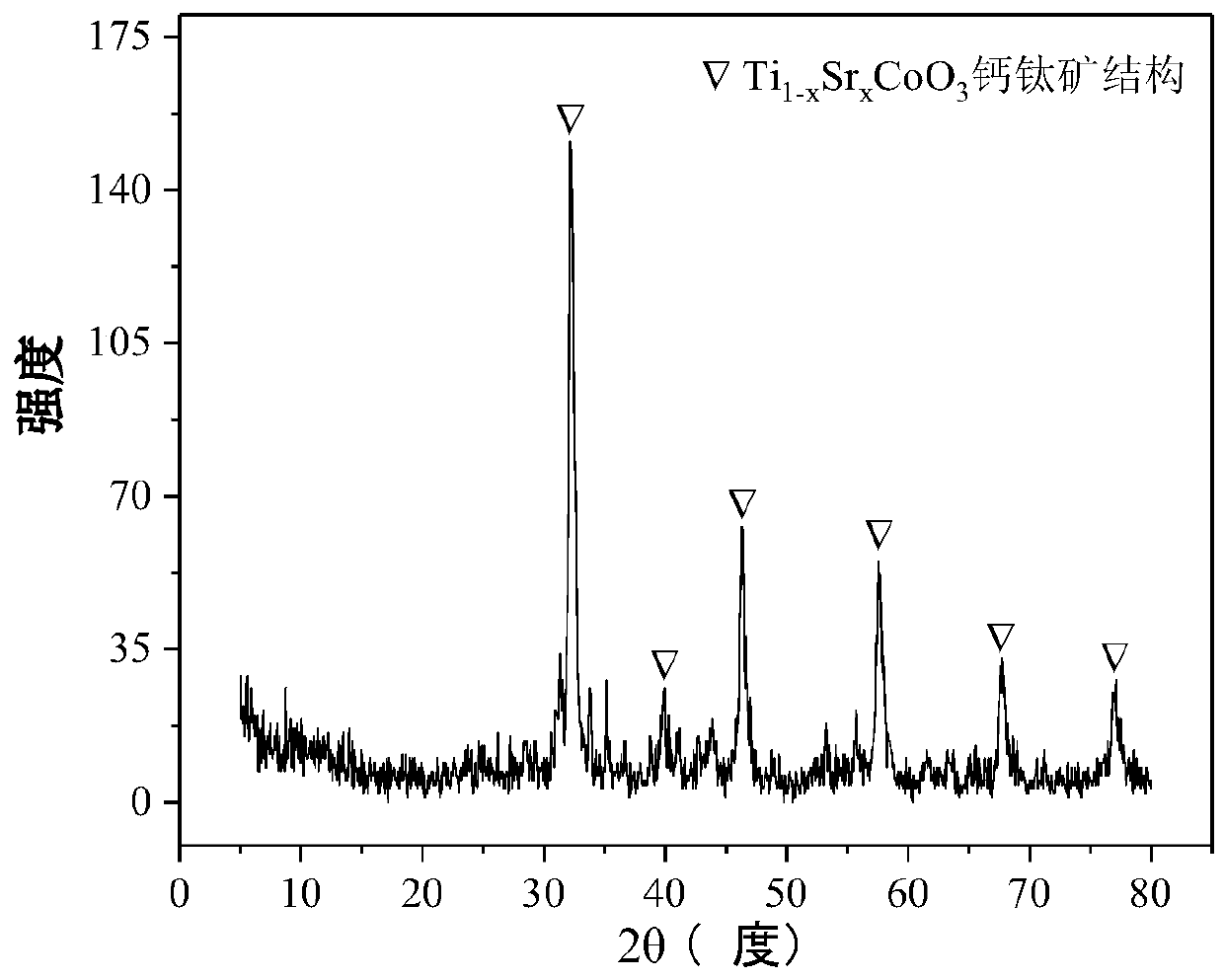
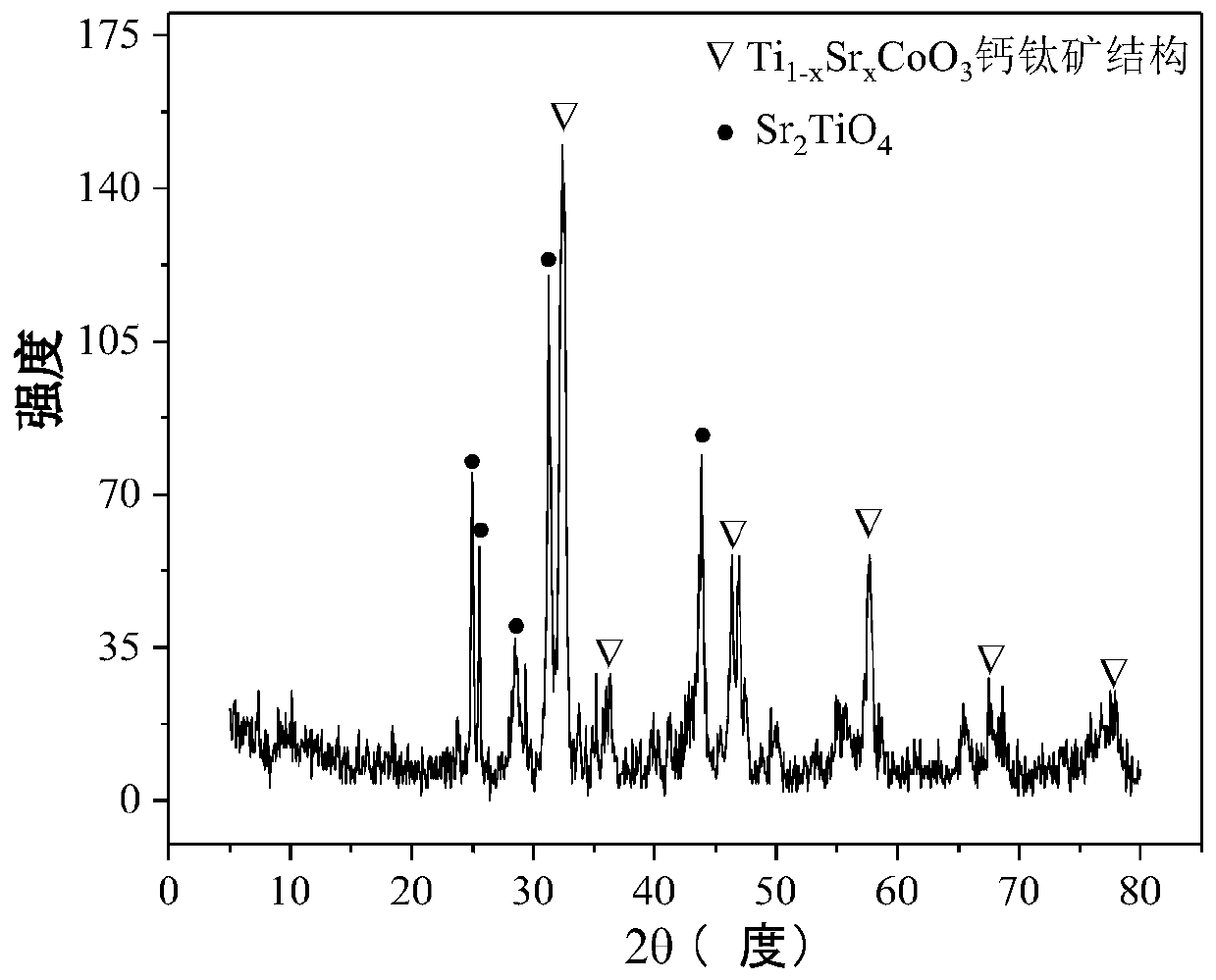
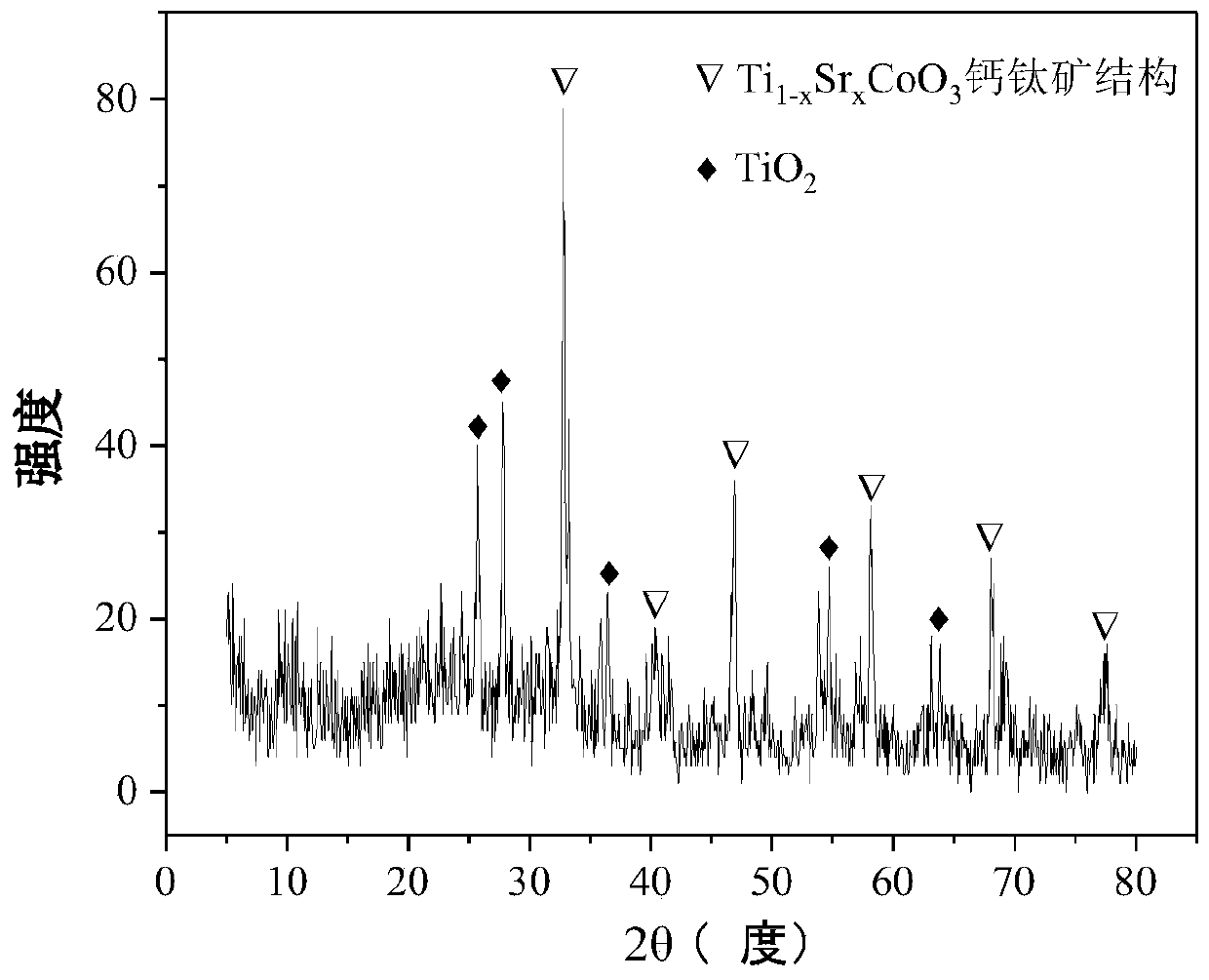
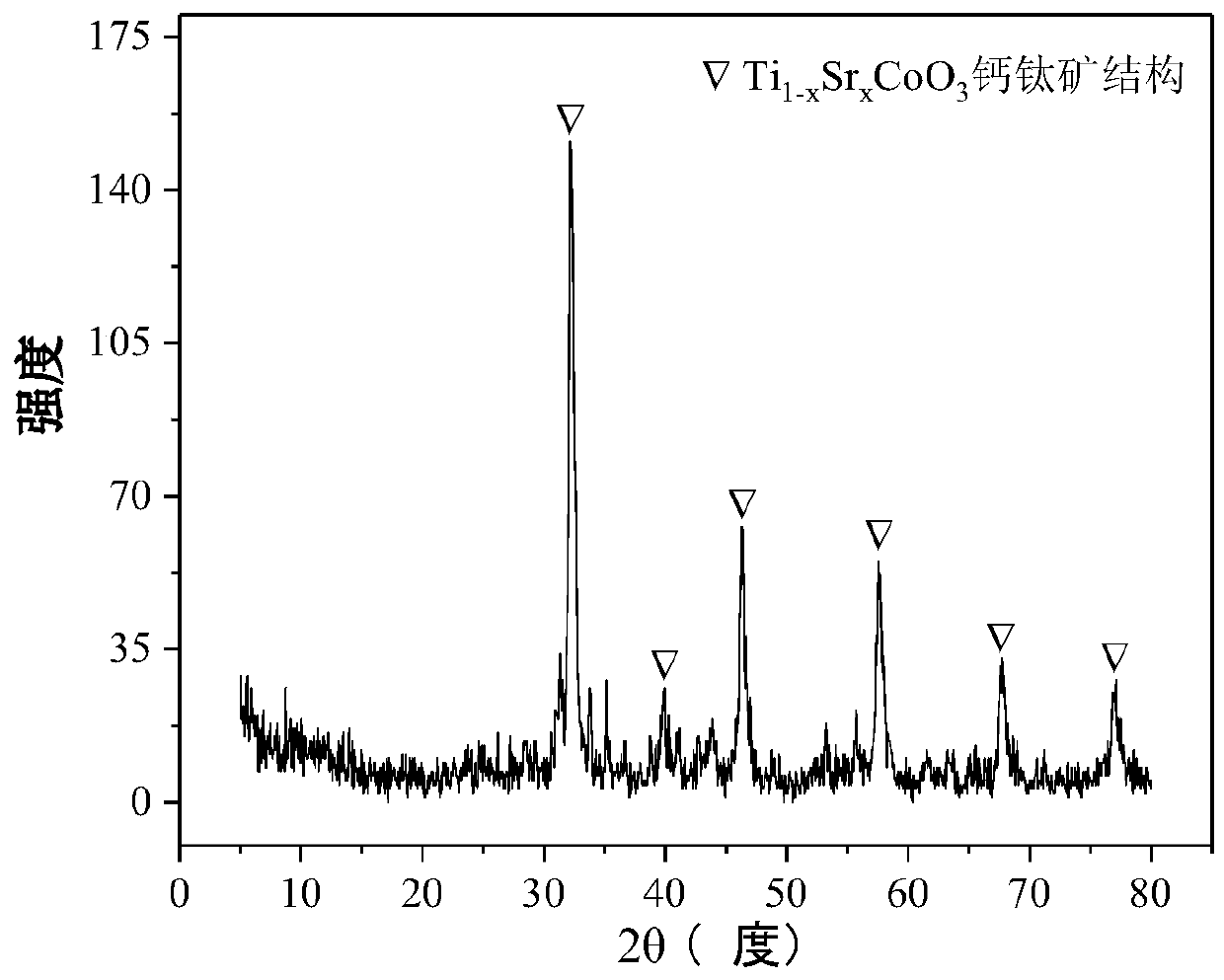
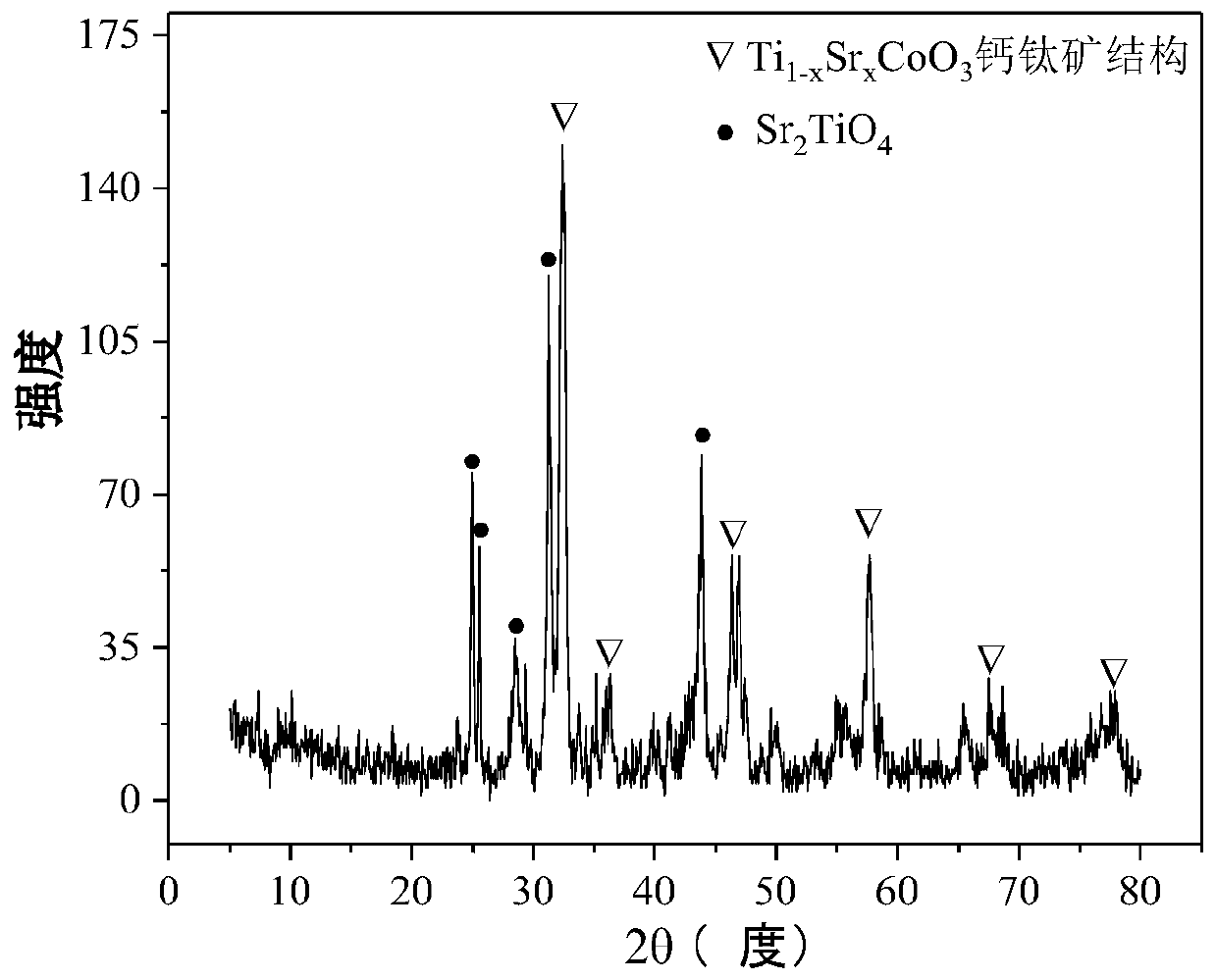
The invention relates to a perovskite-type titanium-strontium-cobalt catalyst for hydrogen production by autothermal reforming of acetic acid and a preparation method. The catalyst with resistance tosintering, anti-carbon deposit, oxidation resistance and high activity is provided to aim at solving the problems that an existing catalyst has carbon deposits, sintering and oxidation of active components in the autothermal reforming process of acetic acid, and deactivation of the catalyst is caused. According to the perovskite-type titanium-strontium-cobalt catalyst for the hydrogen production by autothermal reforming of acetic acid and the preparation method, a sol-gel method is adopted to prepare the perovskite-type titanium-strontium-cobalt catalyst, and after calcination, a perovskite composite oxide catalyst Ti<1-x>Sr<x>CoO3 is obtained, wherein x=0-0.8. The perovskite structure facilitates the dispersion of an active component Co, strengthens the synergistic effect between the active component and a carrier, and inhibits the aggregation and growth of Co, thereby obtaining stable small-particle-size Co particles. In addition, Ti is partially replaced with Sr to increase the surface defect position and lattice defect structure of the perovskite-type catalyst, so that the carbon deposit resistance, oxidation resistance and thermal stability of the active component cobalt are improved, the diffusion of acetic acid, water vapor and oxygen is further facilitated, and the catalytic activity is increased.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
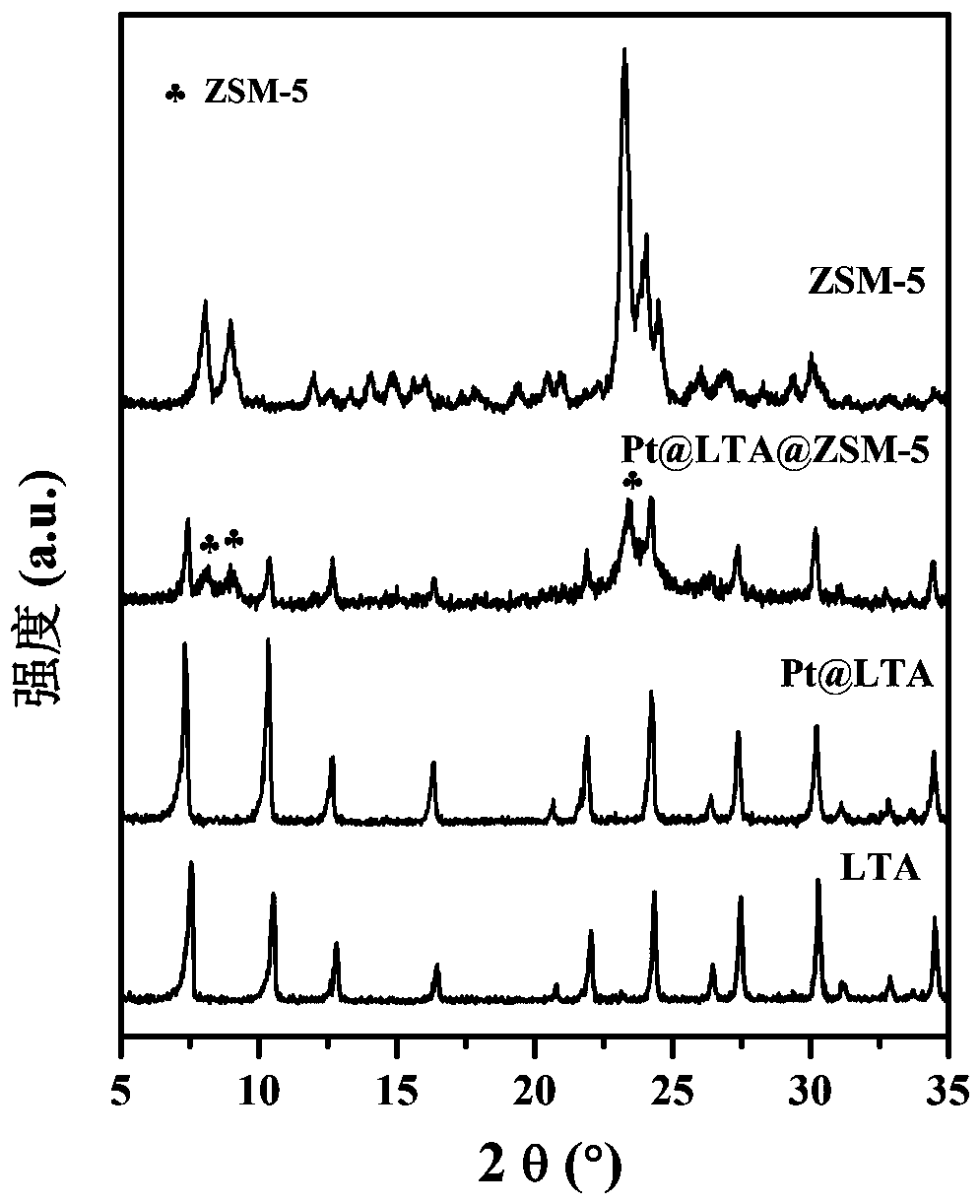
Core-shell type hydrogenation catalyst and preparation method thereof
ActiveCN109261201AHigh catalytic activityImprove sulfur resistanceMolecular sieve catalystsMolecular sieve catalystMolecular sievePolycyclic aromatic hydrocarbon
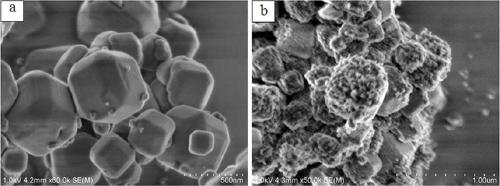
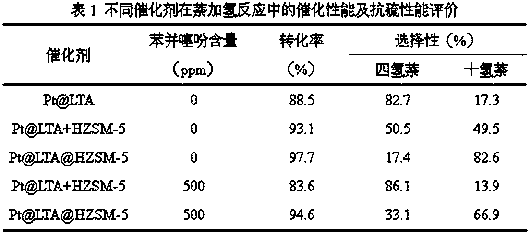
The invention discloses a core-shell type hydrogenation catalyst and a preparation method thereof. A core-layer structure of the core-shell type hydrogenation catalyst is an LTA molecular sieve, and anoble metal cluster is packaged into a hole cage; the noble metal cluster is formed by introducing a noble metal precursor into a synthesis system of the LTA molecular sieve, hydrothermally crystallizing in situ, roasting and reducing, and forming in the hole cage of the LTA molecular sieve; a shell-layer structure is a ZSM-5 zeolite molecular sieve, the LTA molecular sieve with the packaged noble metal cluster is added into a gel system for synthesis of the ZSM-5 zeolite molecular sieve, dried, and subjected to steam phase conversion reaction, and a ZSM-5 zeolite molecular sieve shell layeris formed on the surface of the LTA molecular sieve with the packaged noble metal cluster. The prepared core-shell type hydrogenation catalyst can be applied into the hydrogenation reaction of polycyclic aromatic hydrocarbon, the catalysis activity is high, and the anti-sulfur property is good.
Owner:TAIYUAN UNIV OF TECH
Hydrotalcite-like type iron-promoted nickel-based catalyst for hydrogen preparation through autothermal reforming of acetic acid and preparation method
ActiveCN107282050AStable performanceEnhanced interactionHydrogenCatalyst activation/preparationAluminiumHydrotalcite
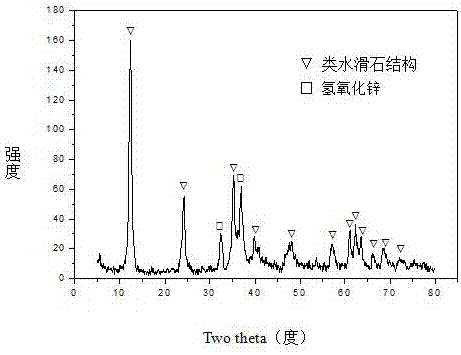
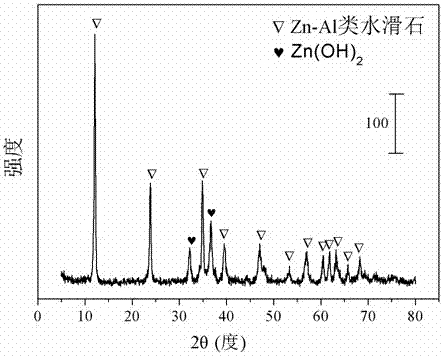
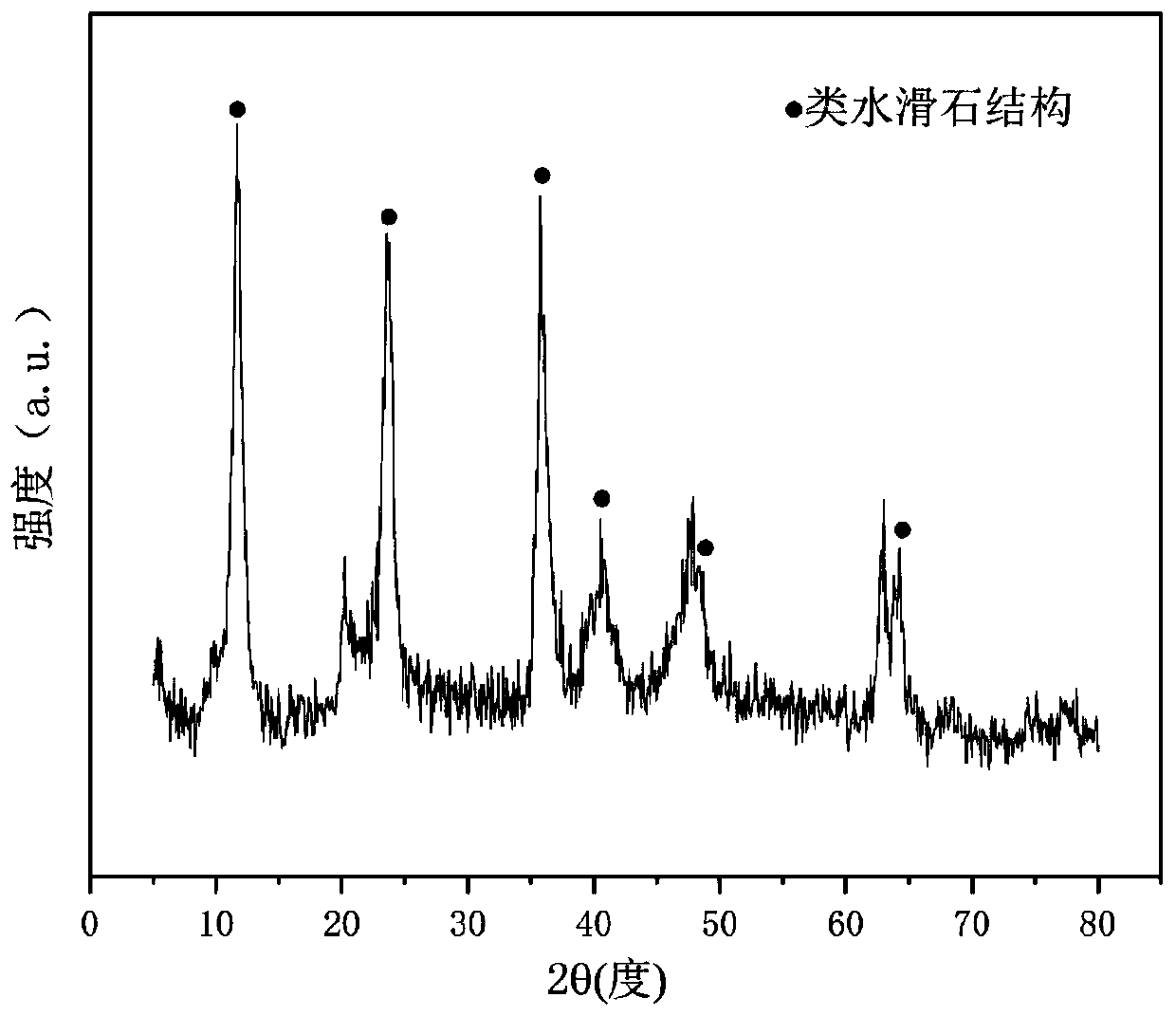
The invention relates to a hydrotalcite-like type iron-promoted nickel-based catalyst for hydrogen preparation through autothermal reforming of acetic acid and a preparation method and provides a high-activity novel catalyst resisting oxidation, sintering and carbon deposition to solve the problem that existing catalysts are inactivated due to the structure change as well as oxidation and sintering of active components of the catalysts in the autothermal reforming process of acetic acid. The chemical component of the catalyst is (ZnO)a(NiO)b(AlO1.5)c(FeO1.5)d, wherein a ranges from 0.75 to 3.25, b ranges from 0.25 to 0.75, c ranges from 0 to 1.0 and d ranges from 0 to 1.0. The Zn-Al type carbonate hydrotalcite-like structure is prepared with a co-precipitation method to serve as a precursor, and an active component nickel and an auxiliary iron are introduced and enter the position of the hydrotalcite-like structure through isomorphous substitution of zinc for nickel and isomorphous substitution of aluminum for iron; a compound oxide obtained through sintering effectively inhibits possible migration, accumulation, oxidation and sintering of the active component nickel under the high-temperature reaction condition, and the activity and the stability of the catalyst are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Cobalt-based catalyst for preparing hydrogen through acetic acid autothermal reforming and preparation method
ActiveCN107159219AHigh activityImprove thermal stabilityHydrogenHeterogenous catalyst chemical elementsOxidation resistantCobalt
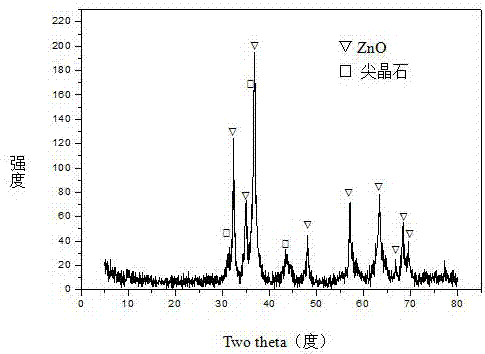
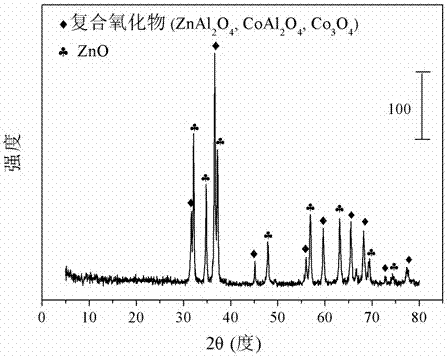
The invention relates to a cobalt-based catalyst for preparing hydrogen through acetic acid autothermal reforming and a preparation method. The novel catalyst which is sintering resistant, resistant to carbon deposition, oxidation resistant and high in activity aims at solving the problem of catalyst deactivation caused by structure change and active component oxidation and sintering of an existing catalyst in the acetic acid autothermal reforming process. The chemical component of the catalyst is CoZnAlO<7.5+ / -delta>, wherein a is 0.25-1.00 and b is 0.75-5.00. A Zn-Al layered hydrotalcite-like structure is prepared by adopting a coprecipitation method as a precursor, an active component cobalt is introduced, and enters a laminate structure of the hydrotalcite-like structure through isomorphous replacement of zinc with cobalt; a composite oxide obtained by roasting can effectively restrain the probable migration, aggregation and sintering of the active component cobalt at high temperature, so as to enhance the thermal stability of the catalyst; and meanwhile, the reductibility, the stability and the oxidation resistance of the active component cobalt are improved through the carrier zinc oxide, so that the activity and the stability of the catalyst are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Hydrocalumite derived cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN109718785AImprove anti-sintering performanceImprove thermal stabilityHydrogenMetal/metal-oxides/metal-hydroxide catalystsAcetic acidActive component
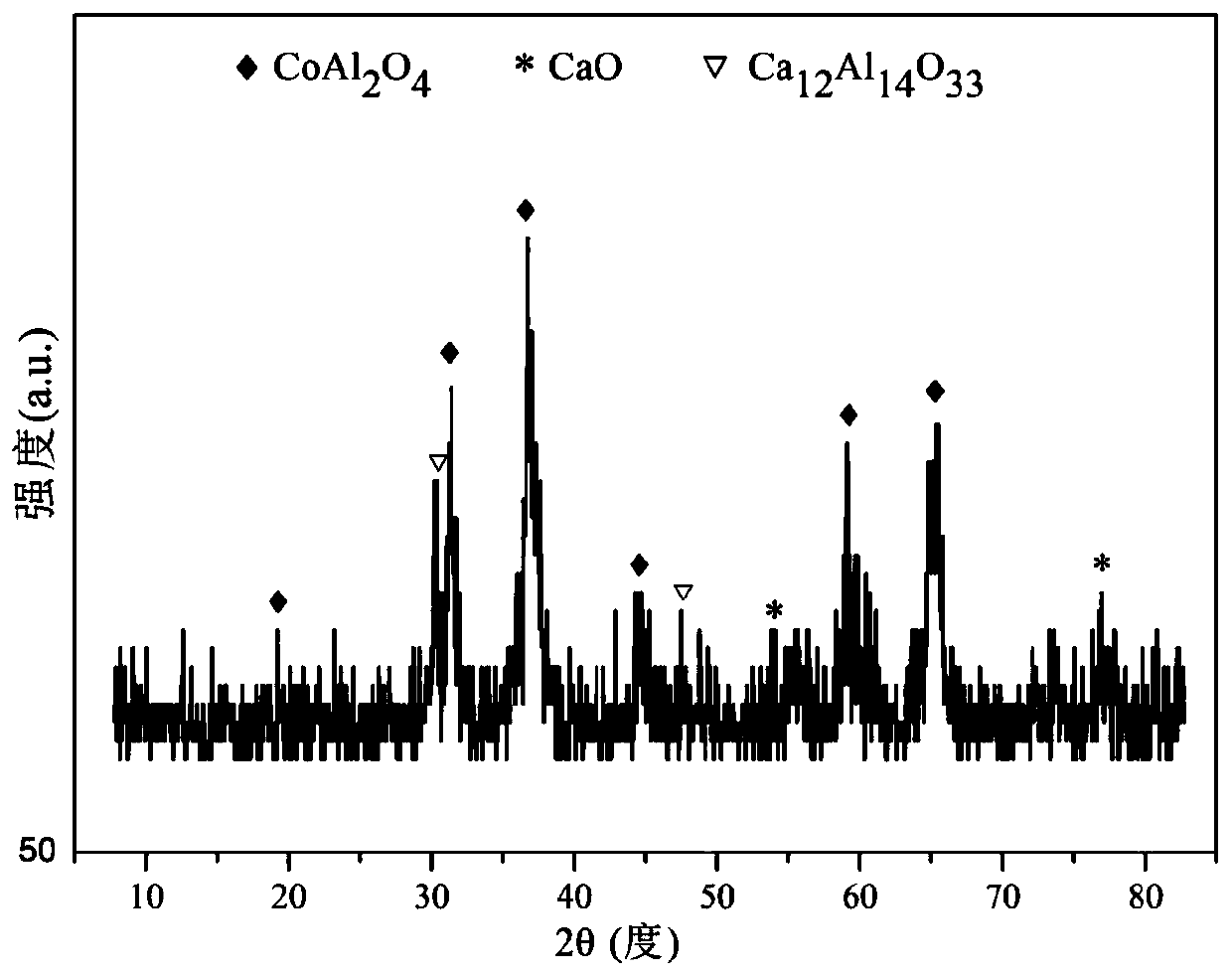
The invention relates to a hydrocalumite derived cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid. Directed at the problem that catalyst structure change and the oxidation and sintering of active components can cause catalyst deactivation in existing catalysts during the autothermal reforming reaction of acetic acid, the invention provides a new catalyst characterized by stable structure, sintering resistance, oxidation resistance, carbon deposition resistance, and high activity. The molar composition of the catalyst is (CaO)a(CoO)b(AlO1.5) c, wherein a is1.66-5.19, b is 0.34-0.81 and c is 1.0. The invention adopts coprecipitation method to prepare a catalyst precursor, and then roasting is carried out to obtain the Ca-Co-Al-O mesoporous composite oxide. The catalyst takes calcium oxide as the framework, contains cobalt-alumina spinel phase and a small amount of Ca12Al14O33, inhibits the acidity of the catalyst, improves the carbon deposition resistance and sintering resistance of the catalyst, and improves the activity of hydrogen production by autothermal reforming of acetic acid.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Mullite-loaded W-promoted Co-based catalyst for hydrogen production by auto-thermal reforming of acetic acid
ActiveCN112742412AImprove thermal stabilityImprove anti-sintering performanceHydrogenHeterogenous catalyst chemical elementsAcetic acidPtru catalyst
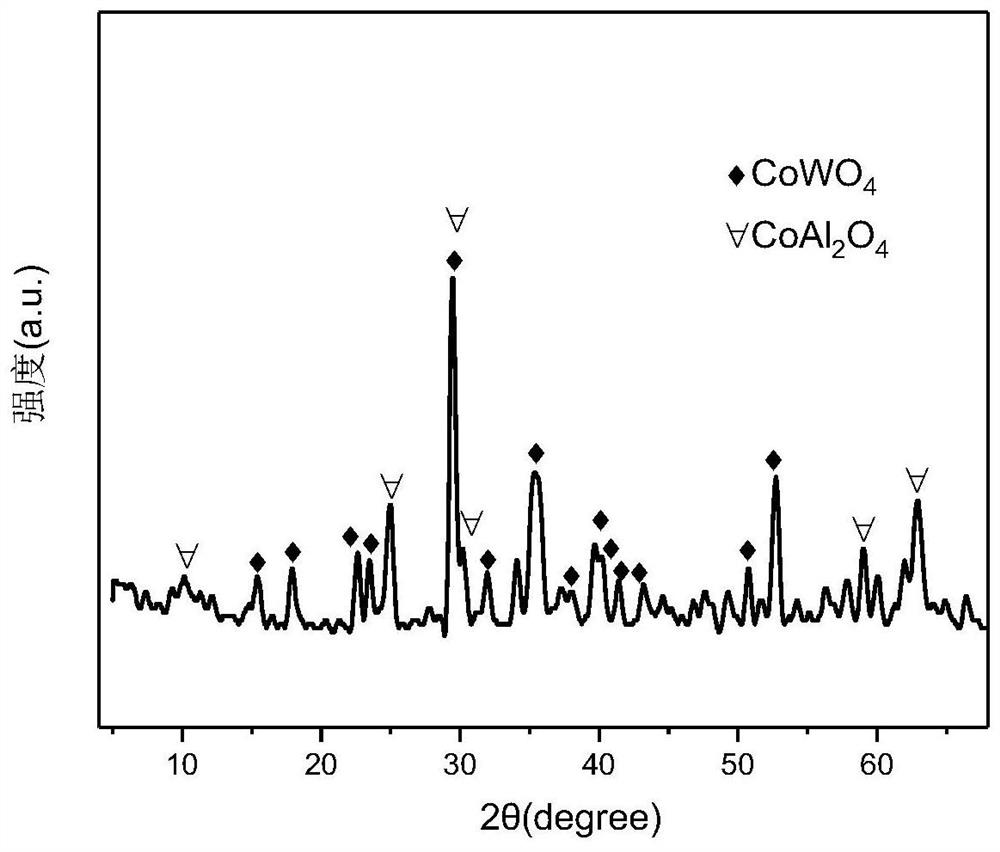
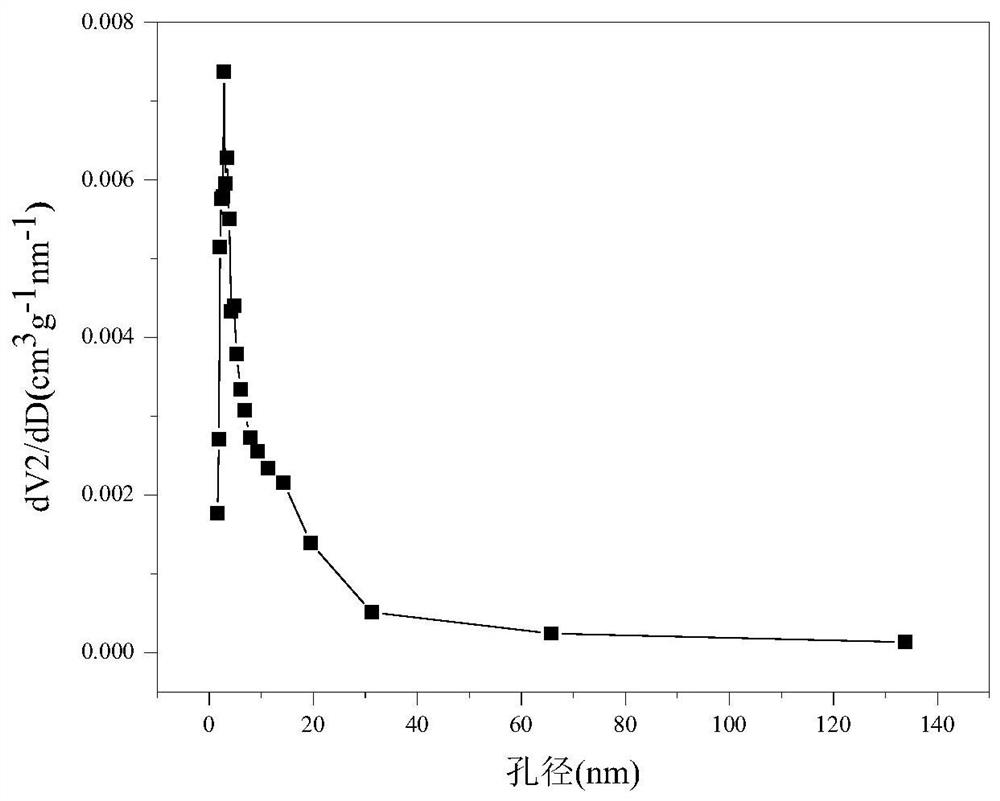
The invention relates to a Mullite carrier-loaded W-promoted Co-based catalyst for hydrogen production by auto-thermal reforming of acetic acid. The invention provides a novel catalyst which is high in activity, resistant to carbon deposition, resistant to sintering and resistant to oxidation, aiming at the problem of catalytic inactivation of an existing catalyst in the auto-thermal reforming process of acetic acid. The molar composition of the catalyst provided by the invention is (CoO)a(WO3)b(3Al2O3-2SiO2)c, a is in a range of 0.469 to 0.517, b is in a range of 0.010 to 0.054, and c is in a range of 0.449 to 0.502. According to the preparation method, Co and W components are impregnated on a Mullite carrier by adopting an impregnation method, a mesoporous composite oxide Co-W / Mullite catalyst containing spinel CoAl2O4 and a composite oxide CoWO4 is formed after calcination, and a Co-W-Al-O active center loaded on Mullite is obtained. The catalyst provided by the invention efficiently promotes the adsorption activation of acetic acid molecules and the conversion of intermediate products, inhibits the generation of by-products, and further improves the hydrogen yield and the stability of the catalyst.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Nickel-magnesium-chromium composite oxide catalyst for auto-thermal reforming of acetic acid to produce hydrogen
ActiveCN108927164AIncrease dispersionImprove thermal stabilityHydrogenHeterogenous catalyst chemical elementsComposite oxideMagnesium
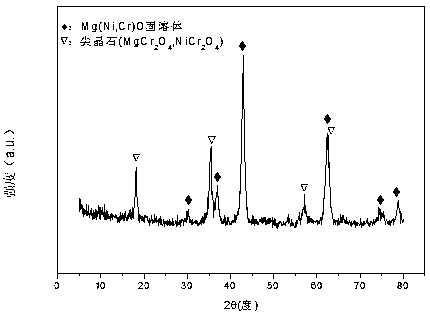
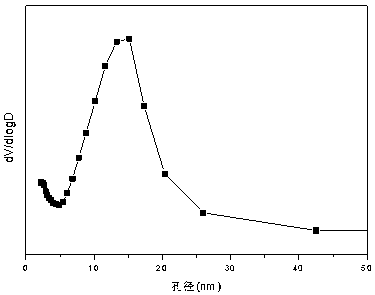
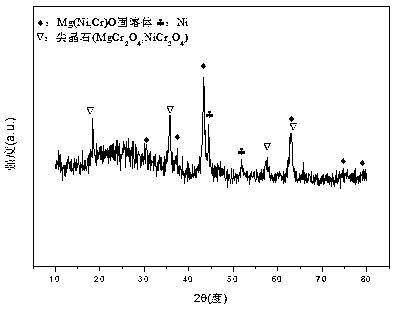
The invention relates to a nickel-magnesium-chromium mesoporous composite oxide catalyst for auto-thermal reforming of acetic acid to produce hydrogen. The invention aims to solve the problem that inthe prior art, during the acetic acid auto-thermal reforming process, the structure of a conventional catalyst changes, the active components are oxidized and sintered, and thus the catalyst is deactivated, and provides a novel catalyst having the advantages of stable structure, sintering resistance, carbon deposition resistance, oxidation resistance and high activity. The chemical formula of thecatalyst is (NiO)a(MgO)b(CrO1.5)c; wherein a is 0.08-0.12, b is 0.55-0.92, c is 0-0.33, and nickel oxide accounts for 12.0 to 20.0 wt.% of the catalyst, magnesium oxide accounts for 40.0 to 88.0 wt.%of the catalyst, and chromium oxide accounts for 0 to 40.0 wt.% of the catalyst. A catalyst precursor is prepared by a co-precipitation method, chromium is introduced into the catalyst and is taken asan auxiliary agent; after burning, a stable mesoporous composite oxide catalyst, which comprises a MgCr2O4 and NiCr2O4 spinel structure and a Mg-Ni-Cr-O solid solution, is formed; the reducing performance and stability of the active components are enhanced; at the same time, the hydrogen yield of acetic acid auto-thermal reforming, sintering resistant performance, and carbon deposition resistantperformance are all strengthened.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Lithium aluminum hydrotalcite-derived nickel-based catalysts for autothermal reforming of acetic acid to hydrogen
ActiveCN109847757BGood dispersionHigh catalytic activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsAluminateOxidation resistant
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Zinc-nickel-zirconium mesoporous composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN109718784AGood dispersionImprove thermal stabilityHydrogenMetal/metal-oxides/metal-hydroxide catalystsHigh activityComposite oxide
The invention relates to a zinc-nickel-zirconium mesoporous composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid. Directed at the problem that catalyst structure change and the oxidation and sintering of active components can cause catalyst deactivation in existing catalysts during the autothermal reforming reaction of acetic acid, the invention provides a new catalyst characterized by stable structure, sintering resistance, carbon deposition resistance, oxidation resistance, and high activity. The chemical composition of the catalyst is (ZnO)a(NiO)b(ZrO2)c,wherein a is 0.60-2.25, b is 0.40-0.75 and c is 1.00. The invention adopts Ni as the active component, uses ZnO and ZrO2 as the carrier, and adopts coprecipitation method to prepare the Ni / ZnO-ZrO 2composite oxide catalyst, which effectively inhibits the formation of ketene, acetone and other by-products in the reaction process, improves the yield and selectivity of hydrogen, and improves the carbon deposition resistance, sintering resistance and oxidation resistance of the catalyst.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
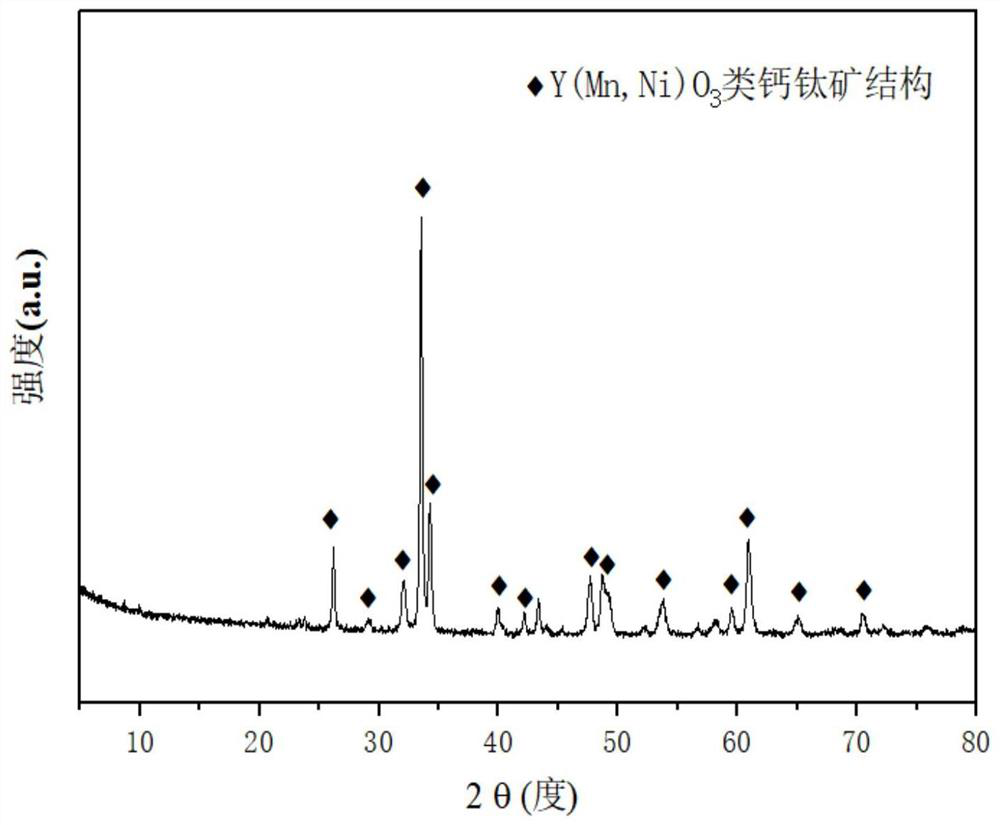
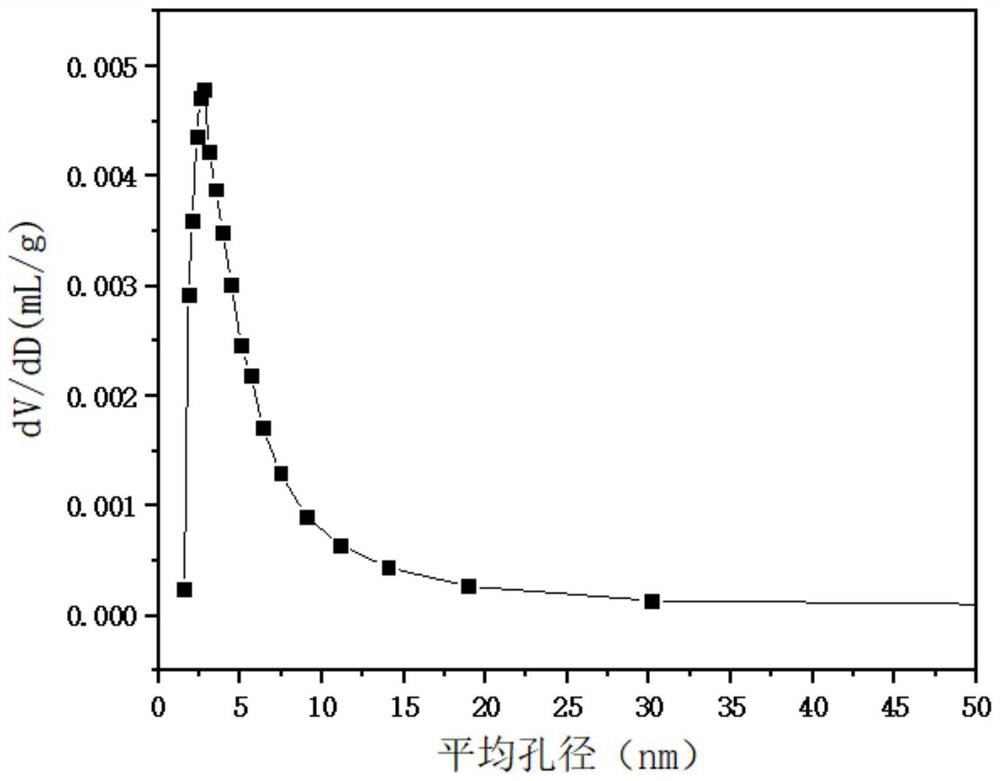
A yttrium-manganese-nickel-based perovskite catalyst for autothermal reforming of acetic acid for hydrogen production
ActiveCN112844403BHigh catalytic activityGood chemical stabilityHydrogenMetal/metal-oxides/metal-hydroxide catalystsAcetic acidPtru catalyst
The invention relates to a yttrium-manganese-nickel perovskite type catalyst for producing hydrogen by autothermal reforming of acetic acid. Aiming at the problem of catalyst deactivation in the autothermal reforming reaction of acetic acid in the existing catalyst, the present invention adopts a hydrothermal method to use Ni as an active component, introduces Y element, and partially replaces Ni by Mn element, forming mesoporous Y (Mn ,Ni)O 3 The perovskite-like structure catalyst improves the dispersion and stability of the active component nickel, and induces the efficient conversion of acetic acid, obtaining a new catalyst with high sintering resistance, coke resistance and high activity. The chemical composition of the catalyst of the present invention is (NiO) a (MnO 2 ) b (YO 1.5 ) c , where a is 0.39, b is 0.61, and c is 0.5.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
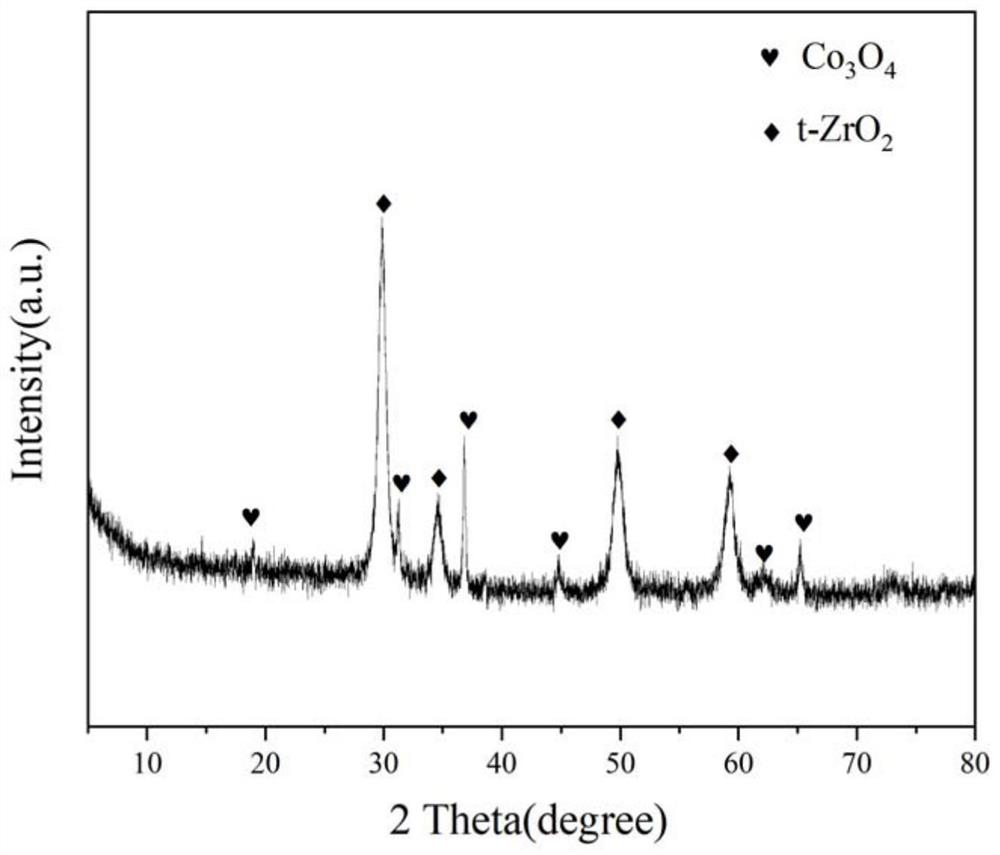
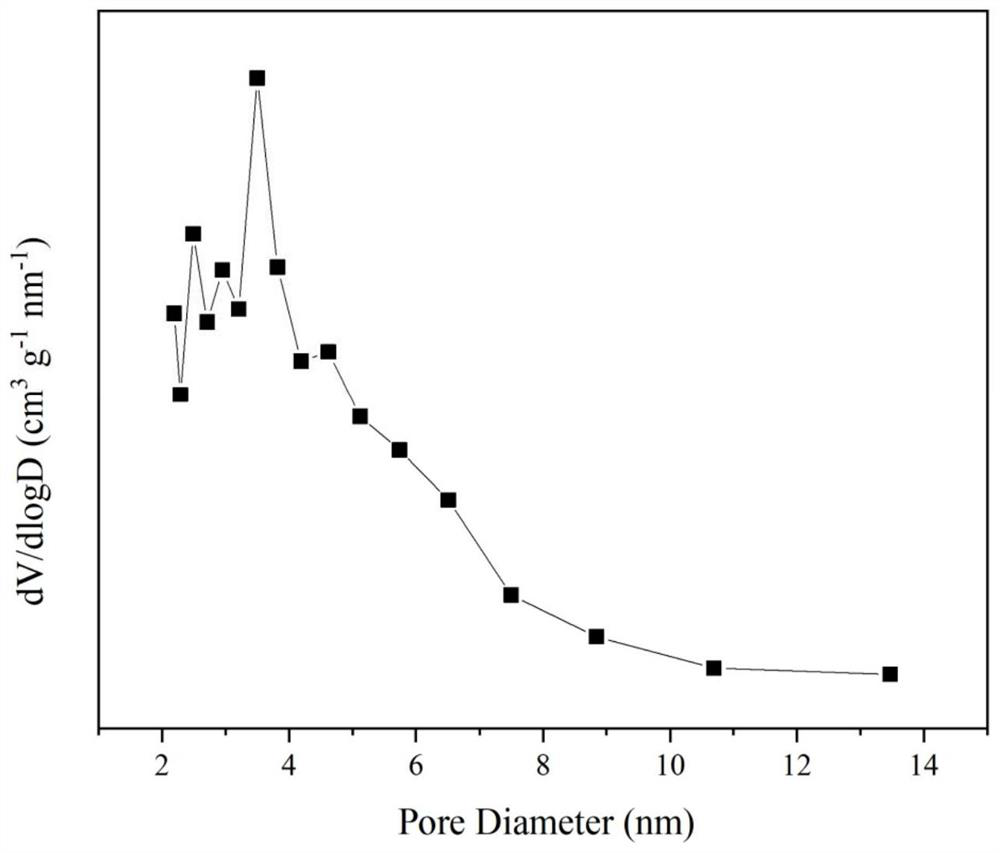
A praseodymium-zirconium composite oxide cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN112916018BEnhanced interactionEasy to passHydrogenHydrogen/synthetic gas productionAcetic acidPtru catalyst
The invention relates to a praseodymium-zirconium composite oxide cobalt-based catalyst for producing hydrogen by autothermal reforming of acetic acid. Aiming at the deactivation problems such as the change of catalyst structure, sintering and carbon deposition in the existing catalyst in the autothermal reforming reaction of acetic acid, the present invention adopts the sol-gel method to prepare Co 3 o 4 and Pr-doped t‑ZrO 2 The Zr-Pr-O composite oxide cobalt-based catalyst limits the aggregation of active components and the growth of grains, and significantly improves the catalyst's anti-coking, anti-sintering ability and hydrogen production rate. Catalyst chemical composition of the present invention is (PrO 1.5 ) a (ZrO 2 ) b (CoO 1.5 ) c , where a is 0‑0.19 and not 0, b is 0.43‑0.69, and c is 0.17‑0.21.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Hydrocalumite-derived cobalt-based catalysts for autothermal reforming of acetic acid to produce hydrogen
ActiveCN109718785BImprove anti-sintering performanceImprove thermal stabilityHydrogenMetal/metal-oxides/metal-hydroxide catalystsHigh activityCobalt
The invention relates to a hydrocalumite derived cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid. Directed at the problem that catalyst structure change and the oxidation and sintering of active components can cause catalyst deactivation in existing catalysts during the autothermal reforming reaction of acetic acid, the invention provides a new catalyst characterized by stable structure, sintering resistance, oxidation resistance, carbon deposition resistance, and high activity. The molar composition of the catalyst is (CaO)a(CoO)b(AlO1.5) c, wherein a is1.66-5.19, b is 0.34-0.81 and c is 1.0. The invention adopts coprecipitation method to prepare a catalyst precursor, and then roasting is carried out to obtain the Ca-Co-Al-O mesoporous composite oxide. The catalyst takes calcium oxide as the framework, contains cobalt-alumina spinel phase and a small amount of Ca12Al14O33, inhibits the acidity of the catalyst, improves the carbon deposition resistance and sintering resistance of the catalyst, and improves the activity of hydrogen production by autothermal reforming of acetic acid.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
A nickel-chromium-manganese mesoporous composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN109225250BGood dispersionImprove thermal stabilityHydrogenHeterogenous catalyst chemical elementsManganeseReduced properties
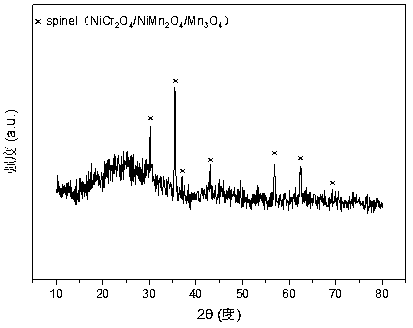
The invention relates to a nickel, chromium and manganese mesoporous compound oxide catalyst for acetic acid self-heating hydrogen production by reforming. In order to solve the problems that existingcatalysts are oxidized, sintered and deposited in carbon in the process of acetic acid self-heating by reforming, the invention provides a novel catalyst which is stable in structure, sintering-resistant, carbon deposition-resistant and oxidiation-resistant. The catalyst is as shown in a formula by mole: (NiO)a(MnO)b(CrO1.5)c, wherein a is 0.12-0.18, b is 0.7-0.33 and c is 0.14-0.5. The catalystis prepared by means of a coprecipitation method, and the Ni-Cr-Mn-O mesoporous compound oxide catalyst which is stable and contains spinels such as NiMn2O4, NiCr2O4 and Mn3O4 is obtained after sintering. The reducing property of active components and the stability of the catalyst are improved. In the process of acetic acid self-heating by reforming, generation of byproducts such as methane and acetone is inhibited, and the hydrogen yield is improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
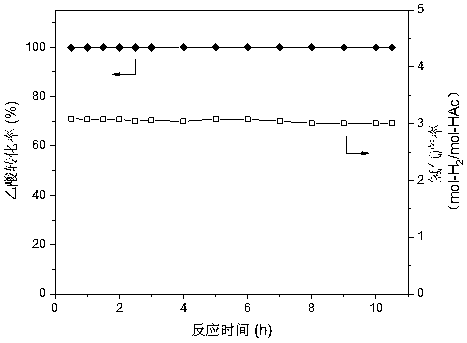
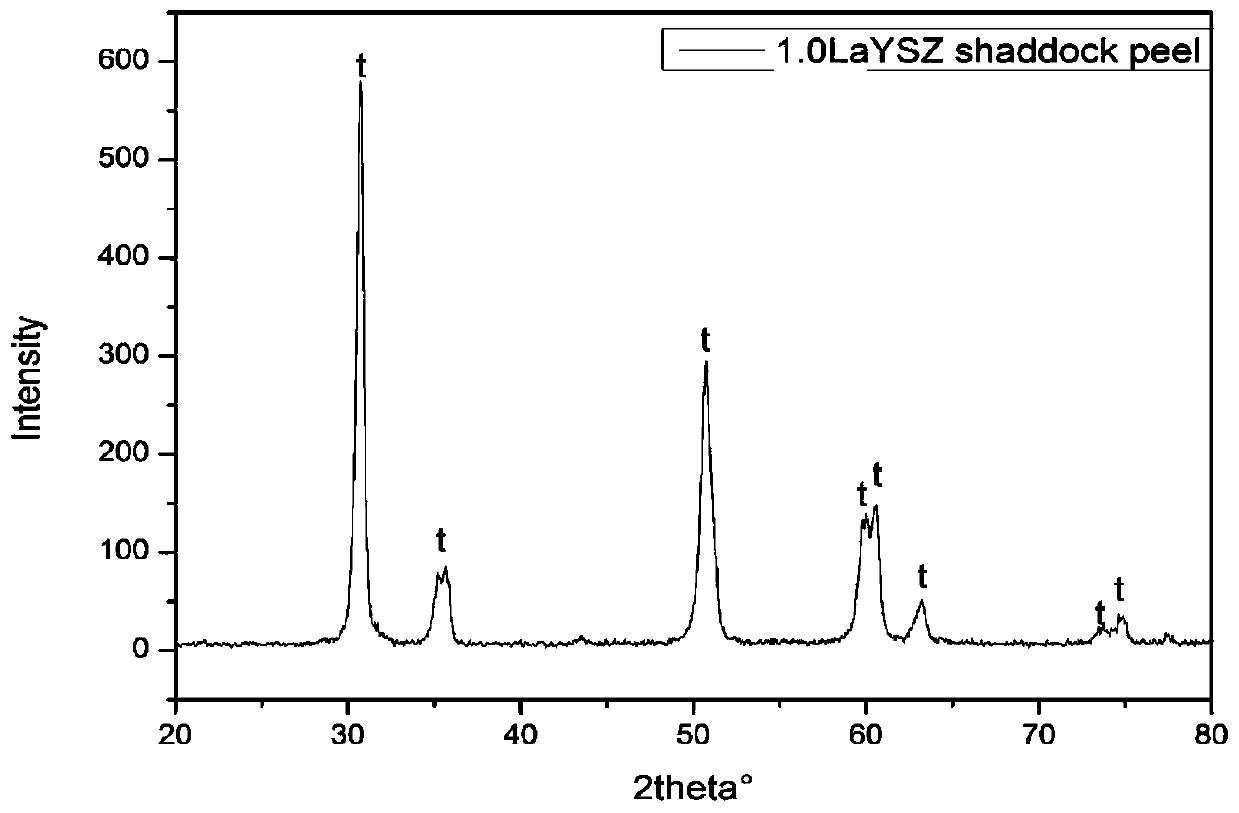
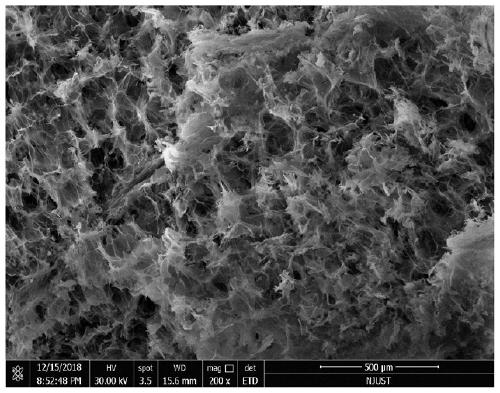
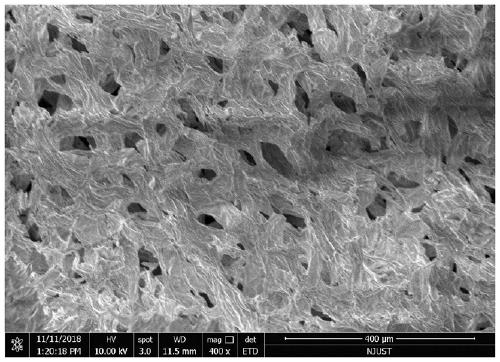
Method for obtaining porous zirconium dioxide thermal insulation ceramic through shaddock peel
The invention discloses a method for obtaining porous zirconium dioxide thermal insulation ceramic through shaddock peel, and belongs to the field of thermal insulation materials. The method includesthe steps of dissolving zircon salt and other solutes in 50 vol% ethyl alcohol to prepare a solution, soaking the classified porous shaddock peel in the solution, taking the shaddock peel out to be squeezed and dried, and placing the dried shaddock peel in an aerobic atmosphere to be sintered so as to obtain the porous zirconium dioxide ceramic. The method is simple, stable and practical, the prepared porous zirconium dioxide ceramic is high in porosity and good in thermal insulation and fire resistant performance, and the high-temperature phase is high in stability.
Owner:NANJING UNIV OF SCI & TECH
A nickel-molybdenum carbide composite catalyst for dry reforming of methane to synthesis gas
InactiveCN105107515BImprove thermal stabilityInhibit migrationHydrogenMetal/metal-oxides/metal-hydroxide catalystsHydrogen selectivityCarbonization
The invention relates to a nickel-molybdenum carbide composite catalyst for preparing a synthesis gas through dry reforming of methane, and aims at solving the problem that catalyst deactivation is caused by the situation that active components in the conventional nickel-based catalyst and molybdenum-based catalyst are easy to oxidize and sinter during the process of preparing the synthesis gas through dry reforming of methane. The preparation method of the nickel-molybdenum carbide composite catalyst comprises the following steps: a nickel molybdenum oxide precursor is synthesized through a one-step synthesis urea combustion method by adopting the double components, namely nickel and molybdenum; the virgin gas is subjected to dry reforming through methane, and an Ni-Mo2C composite structure is compounded in situ; carbonization-oxidation circulation of Mo2C is established during the process of dry reforming of methane at the same time, so as to obtain the nickel-molybdenum carbide composite catalyst which is sintering-resistant, oxidation-resistant and stable in structure. According to the nickel-molybdenum carbide composite catalyst, the stability and hydrogen selectivity of the process of preparing the synthesis gas through dry reforming of methane are improved; the chemical composition of the catalyst provided by the invention is Nia (Mo2C)b (Al2O3 )c in which the a is 0.09-0.19, the b is 0.03-0.10 and the c is 0.75-0.88.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
A cobalt-cerium-manganese composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN111450840BHigh selectivityReduce generationHydrogenHydrogen/synthetic gas productionAcetic acidPtru catalyst
The invention relates to a cobalt-cerium-manganese composite oxide solid solution catalyst for hydrogen production by autothermal reforming of acetic acid. Aiming at the problem of catalyst deactivation of an existing catalyst in an acetic acid autothermal reforming reaction, the invention provides an efficient and stable new catalyst. The chemical component of the catalyst provided by the invention is (CoO1.5) a (CeO2) b (MnOx) c, wherein a is 0.15 to 0.19, b is 0.41 to 0.72, and c is 0.09 to 0.44. Co is used as an active component, Ce and Mn are introduced by adopting a coprecipitation method to form the Co / Ce-Mn-Ox composite oxide solid solution catalyst, generation of byproducts such as acetaldehyde and acetone in the reaction process is effectively inhibited, and the yield of hydrogenand the carbon deposition resistance, sintering resistance and oxidation resistance of the catalyst are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Perovskite TiSrCo Catalyst for Hydrogen Production by Autothermal Reforming of Acetic Acid
ActiveCN109759070BEnhanced adsorption and activationSimple structureMetal/metal-oxides/metal-hydroxide catalystsLattice defectsWater vapor
The invention relates to a perovskite-type titanium-strontium-cobalt catalyst for hydrogen production by autothermal reforming of acetic acid and a preparation method. The catalyst with resistance tosintering, anti-carbon deposit, oxidation resistance and high activity is provided to aim at solving the problems that an existing catalyst has carbon deposits, sintering and oxidation of active components in the autothermal reforming process of acetic acid, and deactivation of the catalyst is caused. According to the perovskite-type titanium-strontium-cobalt catalyst for the hydrogen production by autothermal reforming of acetic acid and the preparation method, a sol-gel method is adopted to prepare the perovskite-type titanium-strontium-cobalt catalyst, and after calcination, a perovskite composite oxide catalyst Ti<1-x>Sr<x>CoO3 is obtained, wherein x=0-0.8. The perovskite structure facilitates the dispersion of an active component Co, strengthens the synergistic effect between the active component and a carrier, and inhibits the aggregation and growth of Co, thereby obtaining stable small-particle-size Co particles. In addition, Ti is partially replaced with Sr to increase the surface defect position and lattice defect structure of the perovskite-type catalyst, so that the carbon deposit resistance, oxidation resistance and thermal stability of the active component cobalt are improved, the diffusion of acetic acid, water vapor and oxygen is further facilitated, and the catalytic activity is increased.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Perovskite catalyst used for autothermal reforming of ethanol for producing hydrogen and preparation method thereof
InactiveCN101972659BChange the adsorption and desorption propertiesEnhanced autothermal reforming activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsManganeseOxygen
The invention relates to a perovskite catalyst used for the autothermal reforming of ethanol for producing hydrogen and a preparation method thereof. Aiming at the problems of structural change, oxidation and sintering of active ingredients and deactivation of the conventional catalyst during the autothermal reforming of the ethanol, the invention provides a novel catalyst with sintering resistance, carbon deposit resistance, oxidation resistance and high activity. The chemical formula of the catalyst is LaaSrbNicXdO3, wherein X is one of Fe, Mn and Cr, a is 0.7 to 1.0, b is 0 to 0.3, c is 0.7 to 0.95 and d is 0.05 to 0.3. The nickel-based catalyst with an ABO3 perovskite structure is prepared by a glycine complexation method, lanthanum is partially substituted by strontium at a position A and nickel is partially substituted by an auxiliary agent at a position B, so oxygen defects and lattice structure defects on the surface of the perovskite catalyst are increased; meanwhile, the auxiliary agent, namely iron, magnesium or chromium is introduced, so the acidity of the catalyst is inhibited, the reducibility and stability of the active ingredients of the catalyst are improved, and the yield of the hydrogen is obviously improved and remains stable.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Strontium-promoted cobalt-based composite oxide catalysts for hydrogen production by autothermal reforming of acetic acid
ActiveCN111450833BEvenly dispersedIncrease contact areaHydrogenHeterogenous catalyst chemical elementsAcetic acidPtru catalyst
The invention relates to a cerium dioxide-loaded strontium-promoted cobalt-based composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid. The invention provides a novel catalyst which is stable in structure and high in activity in order to solve the problem that an existing catalyst is inactivated in an acetic acid autothermal reforming reaction. The molar composition of the catalyst is (SrO) a (CoO1.5) b (CeO2) c, wherein a ranges from 0.75 to 1.75, b ranges from 1.05 to 1.15, and c ranges from 1.50 to 2.50. According to the invention, Co is used as an activecomponent; the cerium dioxide-loaded strontium-promoted cobalt-based composite oxide is prepared by adopting a hydrothermal synthesis method; according to the catalyst, cerium dioxide is used as a skeleton, the catalyst contains a small amount of cobaltosic oxide spinel phase, and the Sr-Co-Ce-O mesoporous composite oxide solid solution catalyst is formed; possible migration, aggregation and sintering of an active component cobalt under a high-temperature condition are effectively inhibited, and the hydrogen yield, sintering resistance and carbon deposition resistance in the acetic acid autothermal reforming process are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
A Zn-Cr hydrotalcite-derived Ni-based catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN108940295BHigh activityEnhanced interactionHydrogenHeterogenous catalyst chemical elementsBULK ACTIVE INGREDIENTHigh activity
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Ba-Mn perovskite-type cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
ActiveCN111111686BGood for adsorption and activationGood structural adjustabilityHydrogenHeterogenous catalyst chemical elementsPtru catalystPerovskite (structure)
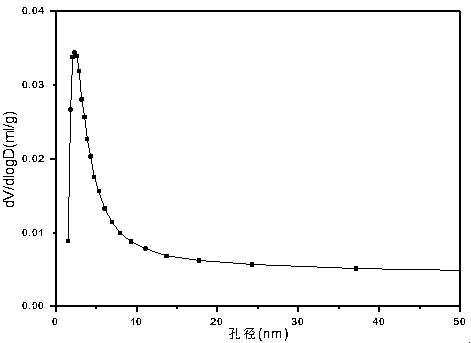
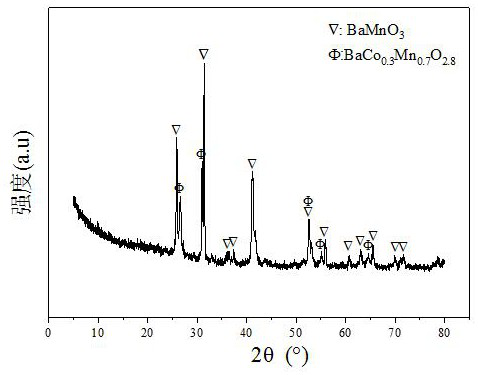
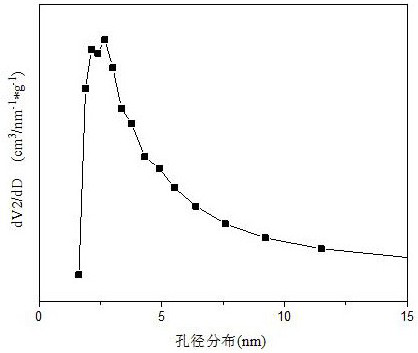
The invention relates to a Ba-Mn perovskite type cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid. Aiming at the problem that an existing catalyst is inactivated in the acetic acid autothermal reforming process, the invention provides a novel catalyst with a stable structure and high activity. The molar composition of the catalyst is (BaO)a(MnO1.5)b(CoO1.5)c interms of oxides, wherein a is 0.177-0.430, b is 0.430-0.709, and c is 0.113-0.140; the catalyst is composed of the components in percentage by weight based on oxides: 30.4 to 60.4 percent of barium oxide, 29.5 to 59.6 percent of manganese oxide and 8.0 to 11.0 percent of cobalt oxide. A sol-gel method is adopted for preparation; cobalt is used as an active component, manganese and barium are introduced, and a stable BaMn0.7Co0.3O2.8 and BaMnO3 perovskite structure composite oxide structure is formed after roasting, so that the oxidation resistance and dispersity of active components are improved, and meanwhile, the hydrogen yield, sintering resistance and carbon deposition resistance in the acetic acid autothermal reforming process are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Olivine nickel-based catalyst for preparing hydrogen through autothermal reforming of acetic acid
InactiveCN103657654BIncrease lattice defectsEnhanced autothermal reforming activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsNickel catalystAcetic acid
The invention relates to an olivine nickel-based catalyst for preparing hydrogen through autothermal reforming of acetic acid. Aiming to solve the problems of low hydrogen yield and oxidation / sintering inactivation of active constituents of the conventional supported nickel-based catalyst in an autothermal reforming process of acetic acid, the invention provides a novel catalyst with the effects of large specific surface area, stable structure, sintering resistance, oxidation resistance and stable activity. The catalyst provided by the invention has a chemical component of MgaFebNicSiO4, wherein a is 1.0-1.7, b is 0-0.7 and c is 0.3-0.5. The nickeliferous catalyst taking an olivine structure as a main body is prepared by a hydro-thermal synthesis method; the catalyst has rich hole structures so that the specific surface area and the dispersity of active constituents of the catalyst are effectively improved; meanwhile, the active constituents, such as nickel and iron, and additives substitute for bivalent magnesium to enter an olivine skeletal structure to effectively refrain the phenomenon of oxidation / sintering inactivation of the catalyst, so that the stability and the hydrogen yield of the catalyst in the autothermal reforming process of acetic acid are improved.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Natural gas distributed energy flue gas denitrification catalyst and its preparation process
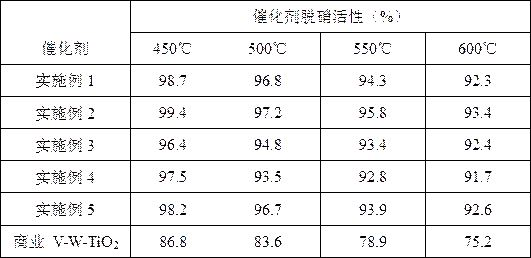
ActiveCN109046370BSintering resistantHigh activityGas treatmentHeterogenous catalyst chemical elementsPtru catalystFlue gas
Owner:JIANGSU LONGKING COALOGIX CATALYST REGENERATION CO LTD
Hydrotalcite-like iron-promoted nickel-based catalyst for hydrogen production by autothermal reforming of acetic acid and preparation method thereof
ActiveCN107282050BPrevent oxidationPrevent sinteringHydrogenCatalyst activation/preparationAcetic acidPtru catalyst
The invention relates to a hydrotalcite-like iron-promoted nickel-based catalyst for hydrogen production by autothermal reforming of acetic acid and a preparation method. The present invention aims at the problems that the existing catalyst changes in the catalyst structure and the oxidation and sintering of active components during the autothermal reforming of acetic acid, resulting in catalyst deactivation, and provides a catalyst that is resistant to oxidation, sintering, coking, and has high activity. new catalyst. The chemical component of the catalyst of the present invention is (ZnO) a (NiO) b (AlO 1.5 ) c (FeO 1.5 ) d , where a is 0.75‑3.25, b is 0.25‑0.75, c is 0‑1.0 and d is 0‑1.0. The present invention uses a co-precipitation method to prepare a Zn-Al carbonate-type hydrotalcite-like structure as a precursor, and introduces the active component nickel and the additive iron. Through the isomorphous substitution of nickel for zinc and iron for aluminum, it enters the Hydrotalcite-like structural position; the composite oxide obtained by roasting effectively inhibits the possible migration, aggregation, oxidation and sintering of the active component nickel under high-temperature reaction conditions, thus improving the activity and stability of the catalyst.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com