Solid solution catalyst for acetic acid self-heating hydrogen production by reforming and preparation method
An autothermal reforming and catalyst technology, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, heterogeneous catalyst chemical elements, etc., can solve problems such as catalyst deactivation, and achieve higher ratio Surface area, enhanced activity and stability, inhibition of formation and desorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
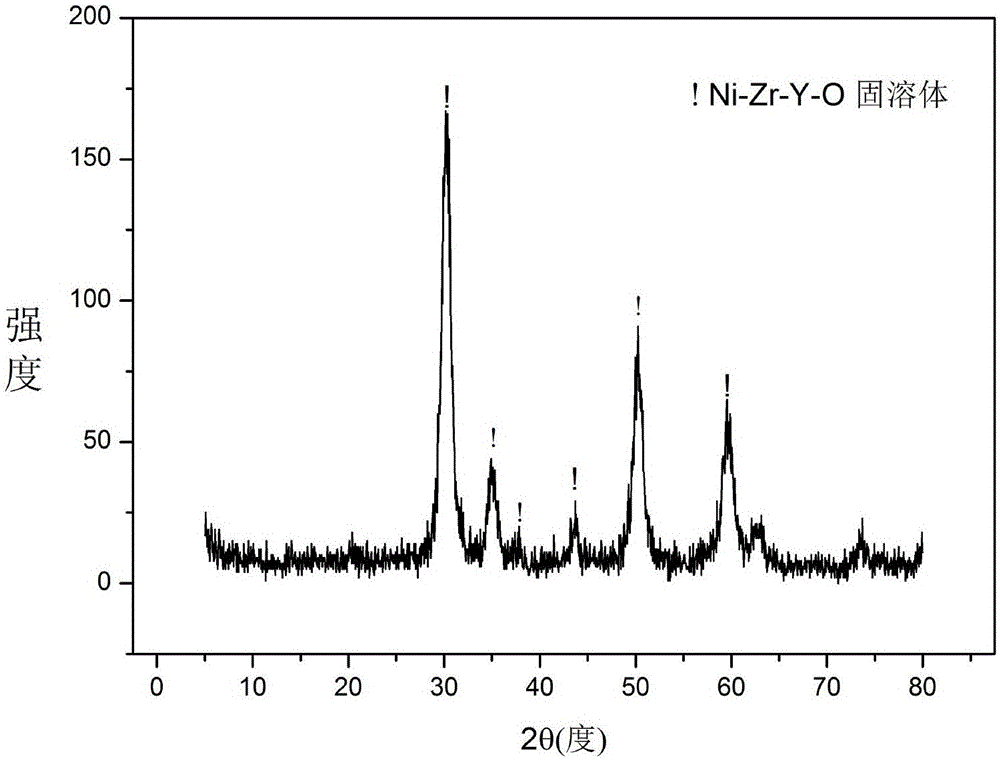
[0028] Weigh 1.0000g of P123, add it to 20ml of absolute ethanol, cover with plastic wrap, and stir magnetically at room temperature for 1h to obtain solution #1. Weigh 0.4362g of Ni(NO 3 ) 2 ·6H 2 O, 2.0045g of ZrO(NO 3 ) 2 2H 2 O and 0.3830g of Y (NO 3 ) 3 ·6H 2 O, join solution #1, continue to stir at room temperature for 5h, follow-up steps are the same as reference example 1, obtain the figure 1 Catalyst CUT-NZY10 with the solid solution structure shown. The weight percent composition of the catalyst is as follows: 9.7% of nickel oxide, 80.5% of zirconia, and 9.8% of yttrium oxide.
[0029] The catalyst CUT-NZY10 was investigated by the autothermal reforming activity of acetic acid. The reaction conditions were normal pressure, space velocity 30000mL / (g-catalyst.h), reaction temperature 700°C, raw material gas acetic acid / water / oxygen = 1 / 4.0 / 0.28 , the acetic acid conversion rate of the catalyst was stable at 100%, and the hydrogen production rate was initially...
Embodiment 2
[0031] Weigh 1.0000g of P123, add it to 20ml of absolute ethanol, cover with plastic wrap, and stir magnetically at room temperature for 1h to obtain solution #1. Weigh 0.4362g of Ni(NO 3 ) 2 ·6H 2 O, 1.7373g of ZrO(NO 3 ) 2 2H 2 O and 0.7661g of Y (NO 3 ) 3 ·6H 2 O, join solution #1, continue to stir at room temperature for 5h, follow-up steps are the same as reference example 1, obtain the figure 1 The catalyst CUT-NZY20 with the solid solution structure shown. The weight percent composition of the catalyst is as follows: 9.8% of nickel oxide, 70.4% of zirconium oxide and 19.8% of yttrium oxide.
[0032] The catalyst CUT-NZY20 was investigated by the autothermal reforming activity of acetic acid. The space velocity was 30000mL / (gcatalyst·h), the reaction temperature was 700℃, and the feed ratio was AC / H 2 O / O 2 / N 2 Under the condition of =1 / 4 / 0.28 / 4.2, the acetic acid conversion rate of this catalyst is 100%, and the productive rate of hydrogen is 3.05mol-H 2 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com