Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
280results about "Genetic material administration regime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical composition containing a stabilised mRNA optimised for translation in its coding regions
ActiveUS20050032730A1Overcome disadvantagesImprove efficiencyAntibacterial agentsVirusesTranslational efficiencyCoding region
The present invention relates to a pharmaceutical composition comprising a modified mRNA that is stabilised by sequence modifications and optimised for translation. The pharmaceutical composition according to the invention is particularly well suited for use as an inoculating agent, as well as a therapeutic agent for tissue regeneration. In addition, a process is described for determining sequence modifications that promote stabilisation and translational efficiency of modified mRNA of the invention.
Owner:CUREVAC SE
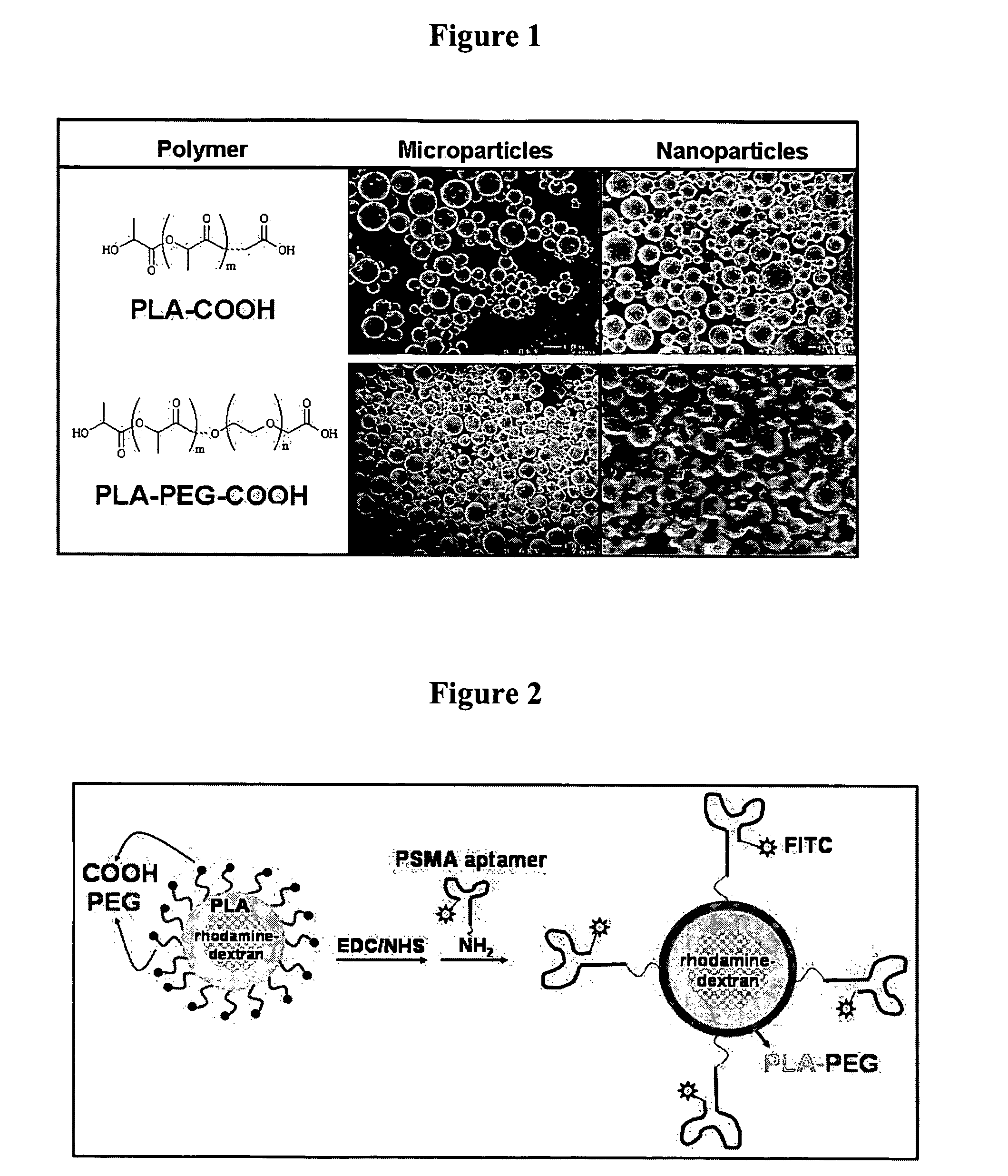
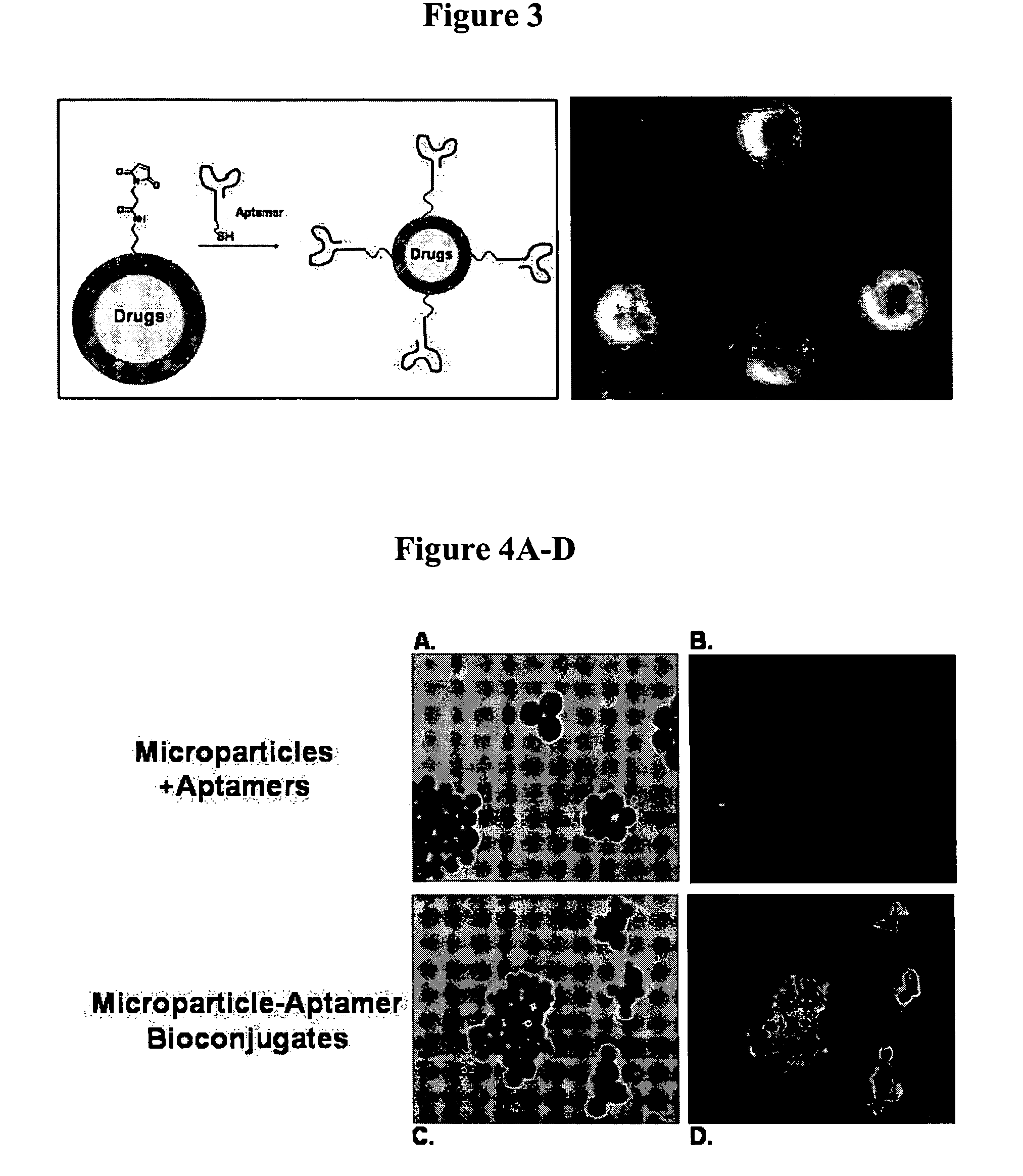
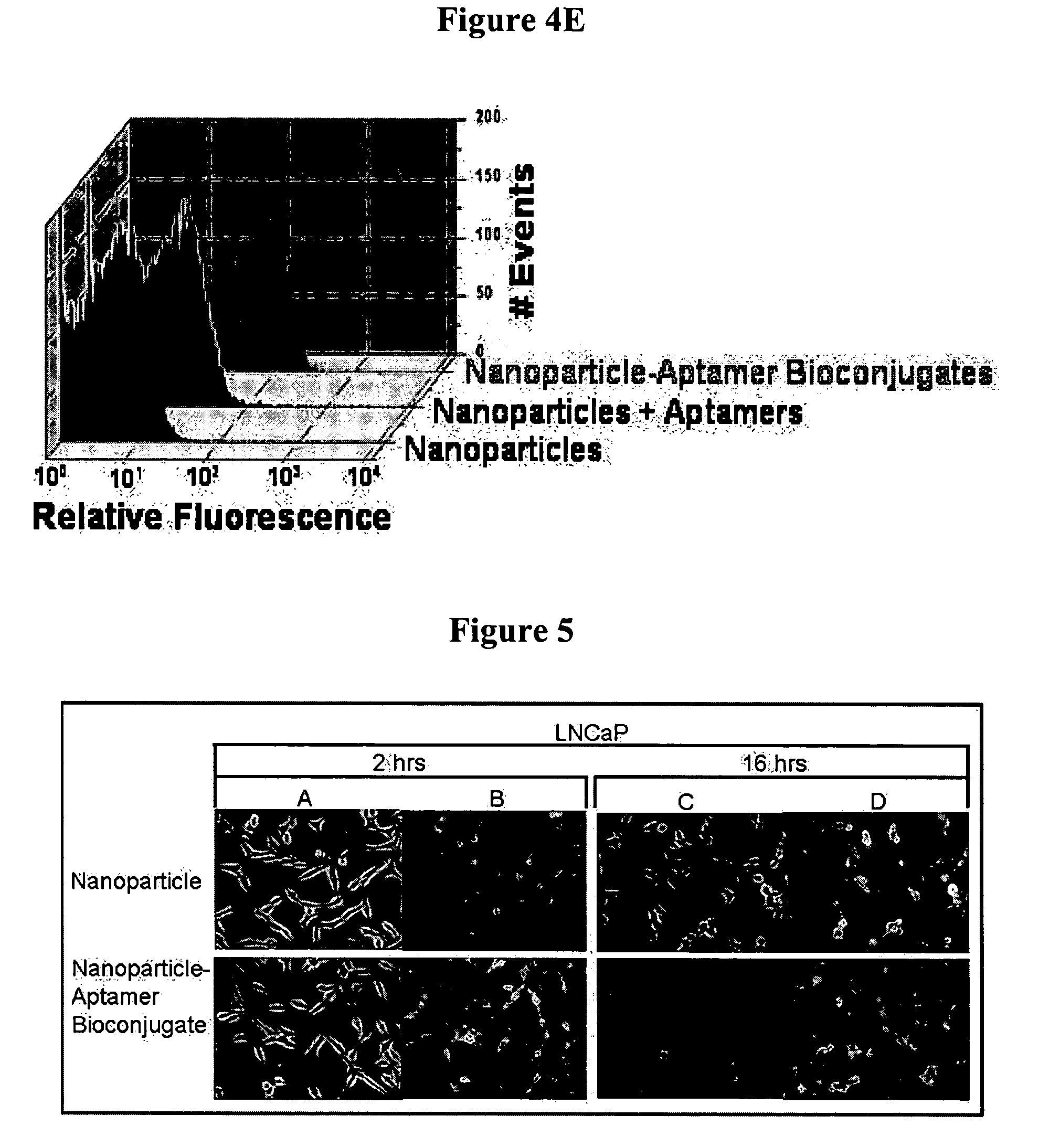
Targeted delivery of controlled release polymer systems
ActiveUS20050037075A1Strong specificityHigh affinityPowder deliveryPeptide/protein ingredientsControlled releaseCell Surface Antigens
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
Engineered nucleic acids and methods of use thereof for non-human vertebrates
InactiveUS20140206752A1Sugar derivativesVector-based foreign material introductionCell biologyVertebrate
Provided are formulations, compositions, kits and methods for delivering biological moieties such as modified nucleic acids into cells to induce, reduce or modulate protein expression in non-human vertebrates.
Owner:MODERNA THERAPEUTICS INC
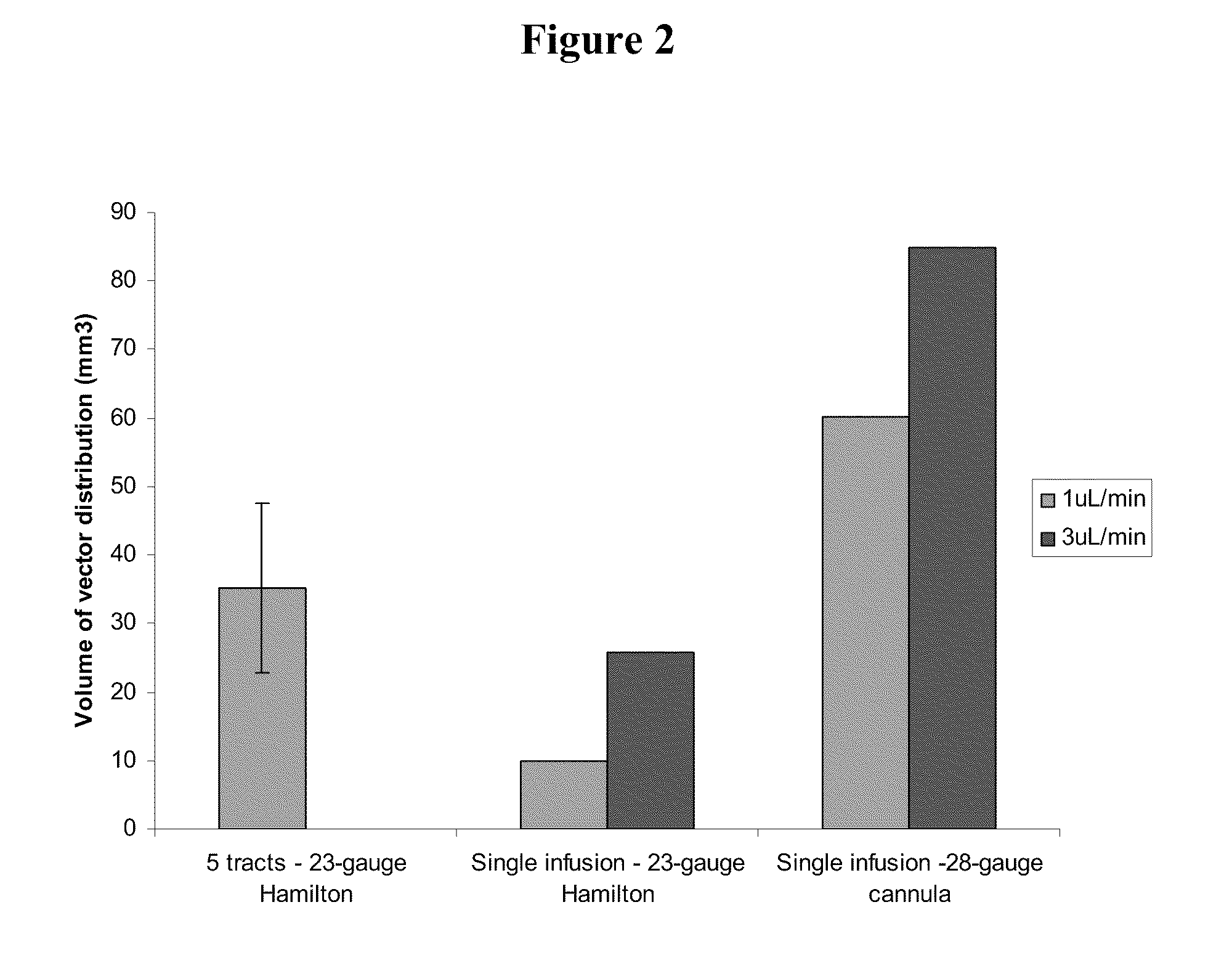
Method for vector delivery
InactiveUS20110293571A1Optimize volumeTotal volume of vector distribution in the brain wasBiocideOrganic active ingredientsNeuroscience
Owner:OXFORD BIOMEDICA (UK) LTD
PHARMACEUTICAL COMPOSITION CONTAINING A STABILISED mRNA OPTIMISED FOR TRANSLATION IN ITS CODING REGIONS
InactiveUS20100239608A1Improve efficiencyOvercome disadvantagesAntibacterial agentsOrganic active ingredientsCoding regionBiology
Owner:CUREVAC AG
Controlled release polymer nanoparticle containing bound nucleic acid ligand for targeting
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
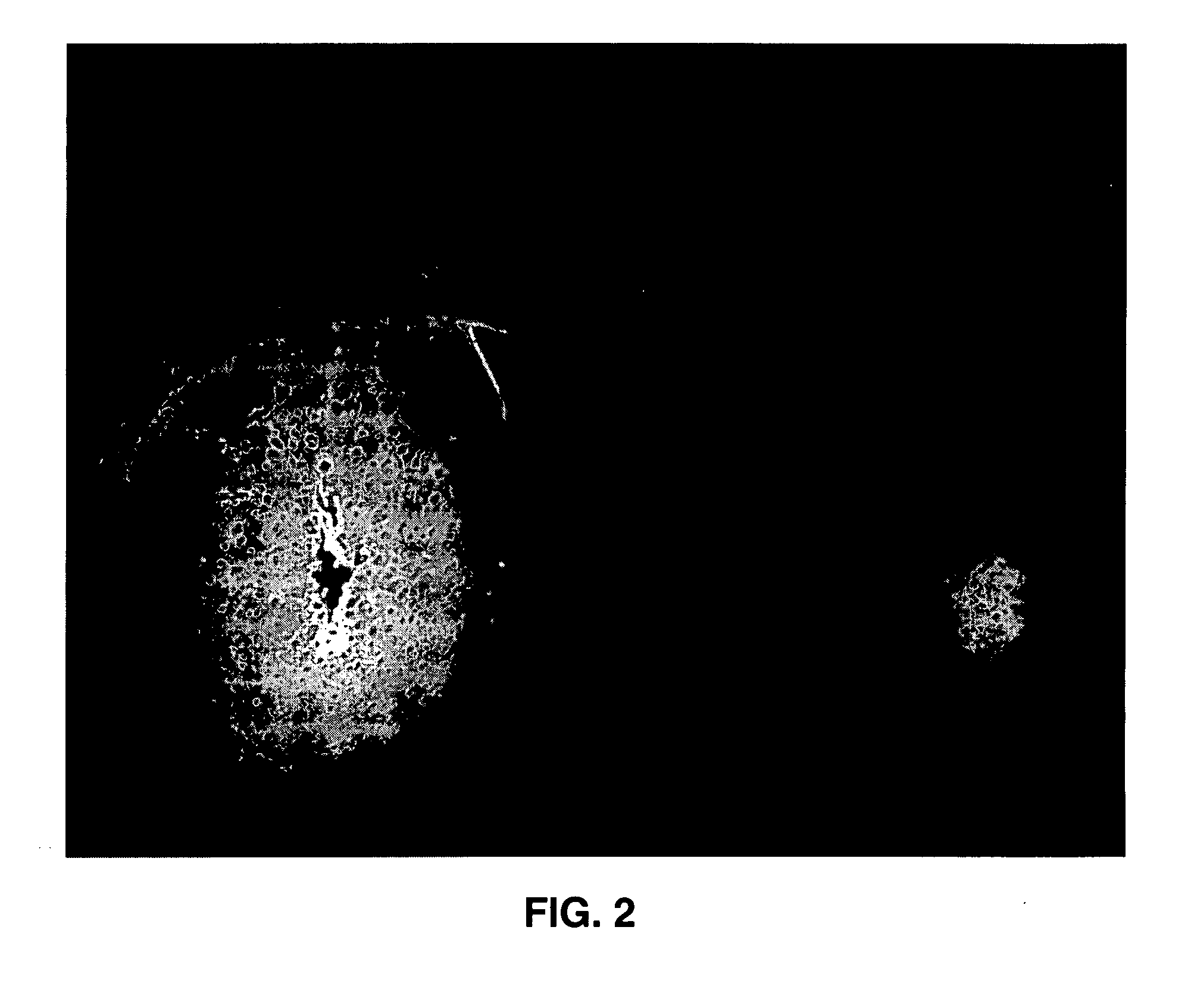
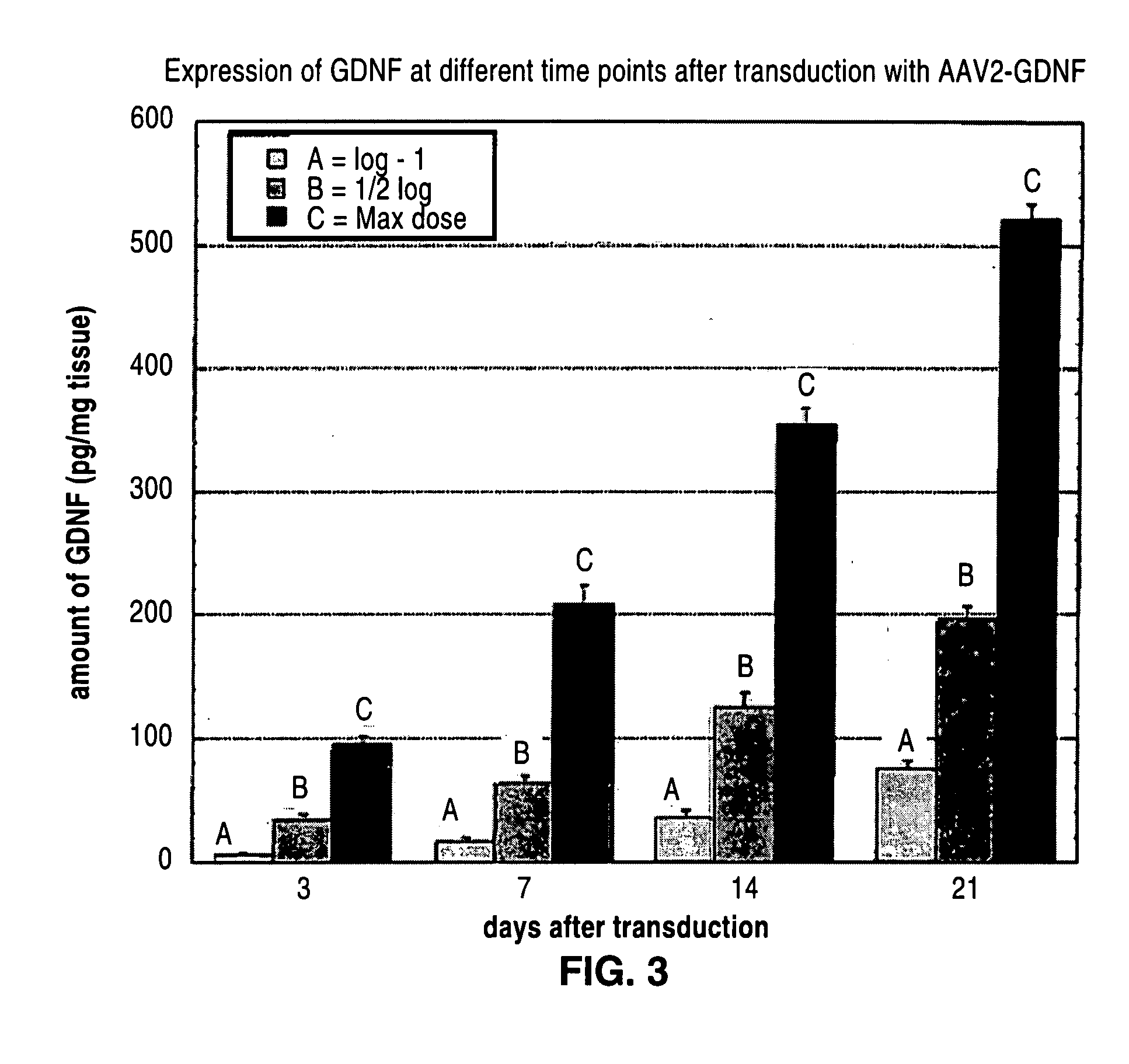
Administration of growth factors for the treatment of CNS disorders
ActiveUS20070254842A1Prevents and delay onsetReduce severityHeavy metal active ingredientsSenses disorderDiseaseNervous system
A method and system that is directed to the local delivery of growth factors to the mammalian CNS to treat CNS disorders associated with neuronal death and / or dysfunction is described.
Owner:RGT UNIV OF CALIFORNIA
Immunisation of large mammals with low doses of RNA
ActiveUS20130149375A1Conveniently preparedImprove stabilityAntibacterial agentsSsRNA viruses negative-senseMammalImmunity response
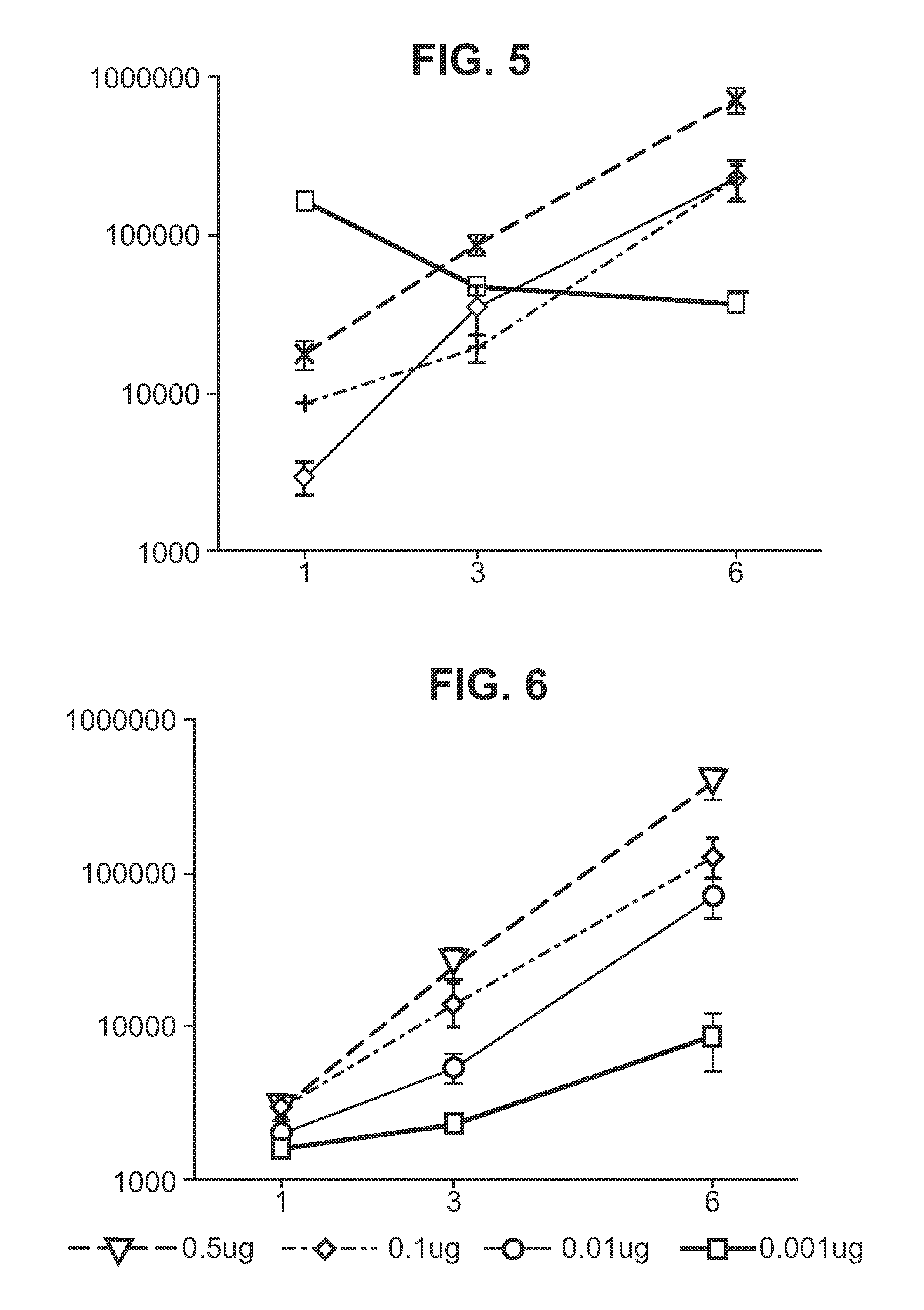
RNA encoding an immunogen is delivered to a large mammal at a dose of between 2 μg and 100 μg. Thus the invention provides a method of raising an immune response in a large mammal, comprising administering to the mammal a dose of between 2 μg and 100 μg of immunogen-encoding RNA. Similarly, RNA encoding an immunogen can be delivered to a large mammal at a dose of 3 ng / kg to 150 ng / kg. The delivered RNA can elicit an immune response in the large mammal
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Controlled release nanoparticle having bound oligonucleotide for targeted delivery
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
Parvoviral capsid with incorporated gly-ala repeat region
InactiveUS20110171262A1Improve stabilityReduced expression levelBiocideAntibody mimetics/scaffoldsNucleic acid sequencingCapsid
Parvoviral capsid with incorporated Gly-Ala repeat region The present invention provides a nucleic acid construct comprising a nucleic acid sequence encoding a parvoviral VP1, VP2 and VP3 capsid proteins comprising an immuno evasion repeat sequence. In addition, the present invention provides a cell comprising such construct, a parvoviral virion comprising a capsid protein that comprises an immune evasion repeat sequence, use of that parvoviral virion in gene therapy and a pharmaceutical composition comprising such parvoviral virion.
Owner:AMSTERDAM MOLECULAR THERAPEUTICS
Method for increasing expression of rna-encoded proteins
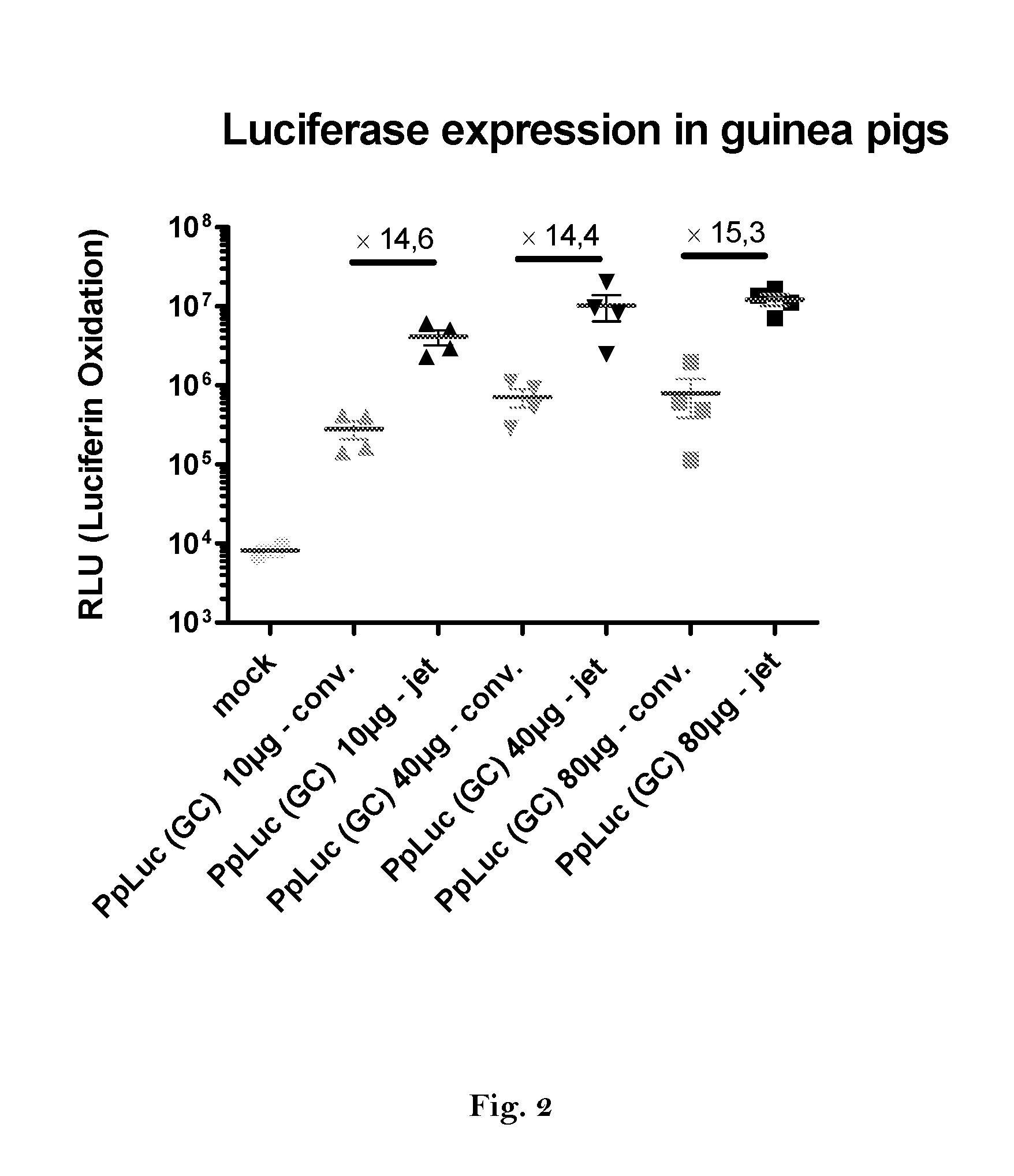
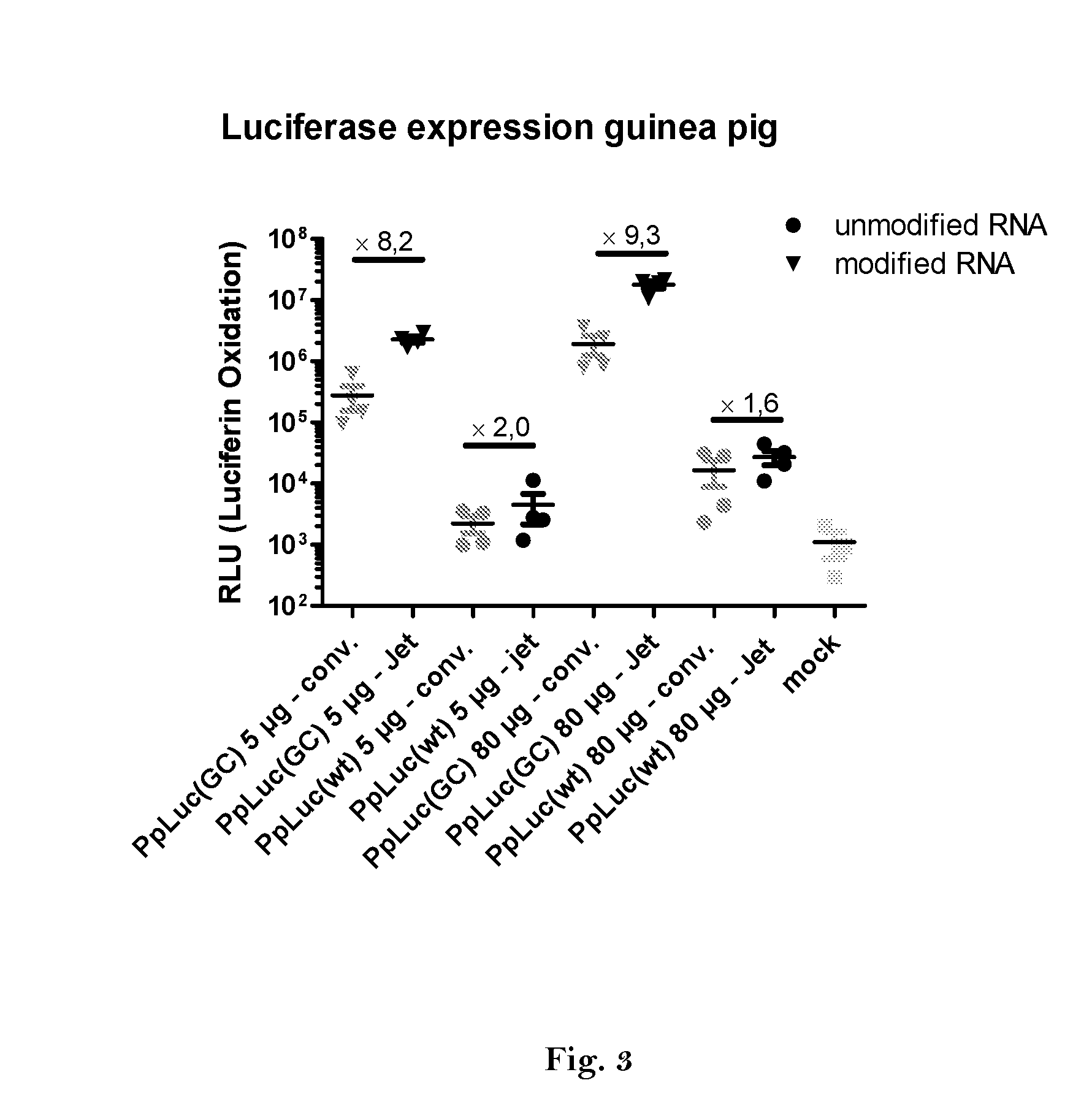
ActiveUS20160166710A1High expressionImprove securitySsRNA viruses negative-sensePeptide/protein ingredientsOpen reading frameJet injection
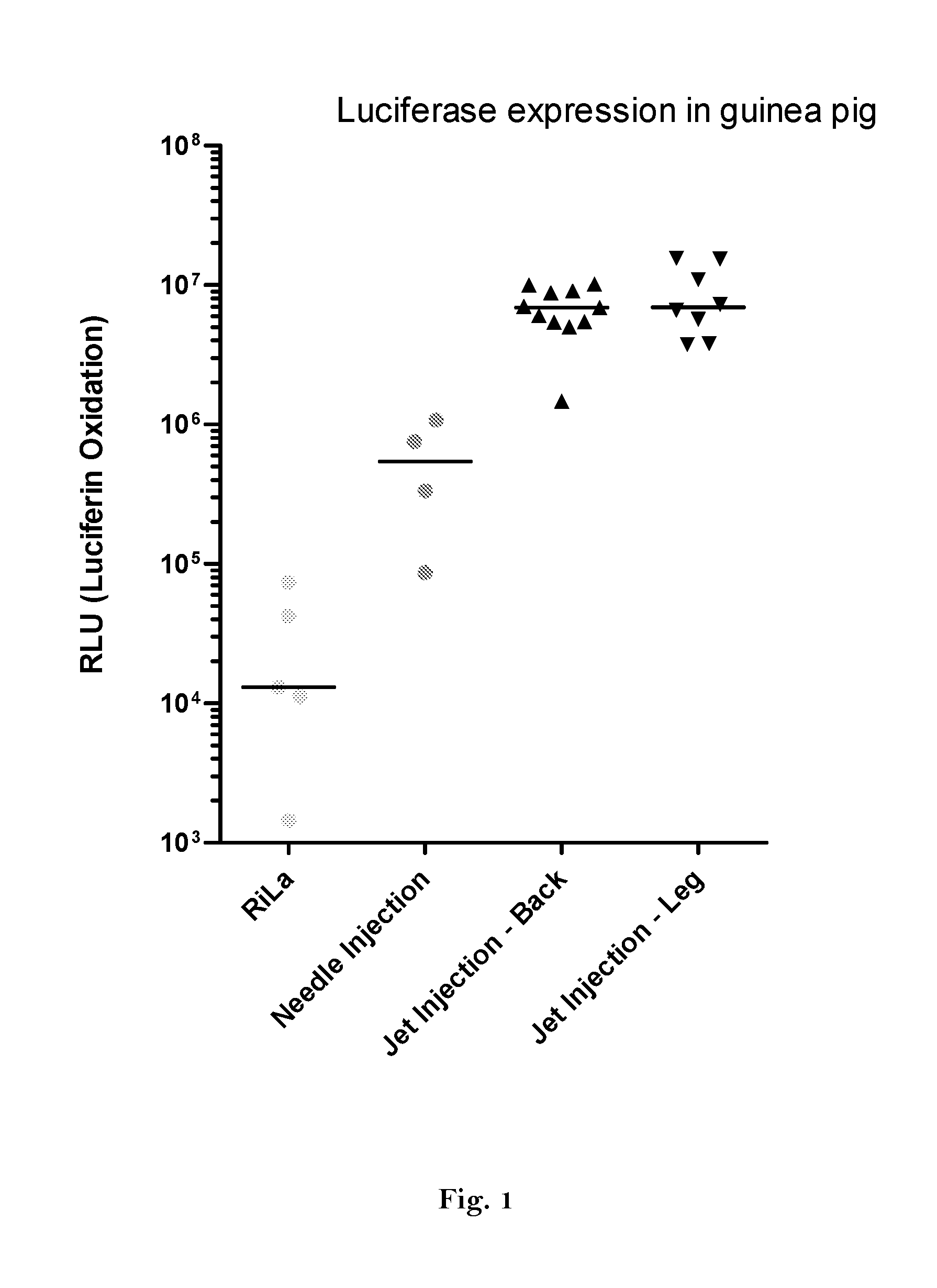
The invention relates to an RNA comprising at least one open reading frame (ORF) and comprising at least one modification, which increases the expression of the encoded peptide or protein. Furthermore, the invention relates to the medical use of such a modified RNA administered to a subject by jet injection. The invention relates further to a pharmaceutical composition and to a kit of parts comprising said modified RNA for administration by jet injection, preferably for use in the field of gene therapy and / or genetic vaccination. Additionally, the invention relates to a method for enhancing the (localized) expression of RNA-encoded peptides or proteins in the dermis or muscle (of a mammal) comprising administering the modified RNA by jet injection. And finally, the invention relates to a method of treatment comprising administering the modified RNA by jet injection to a subject in need thereof.
Owner:CUREVAC SE
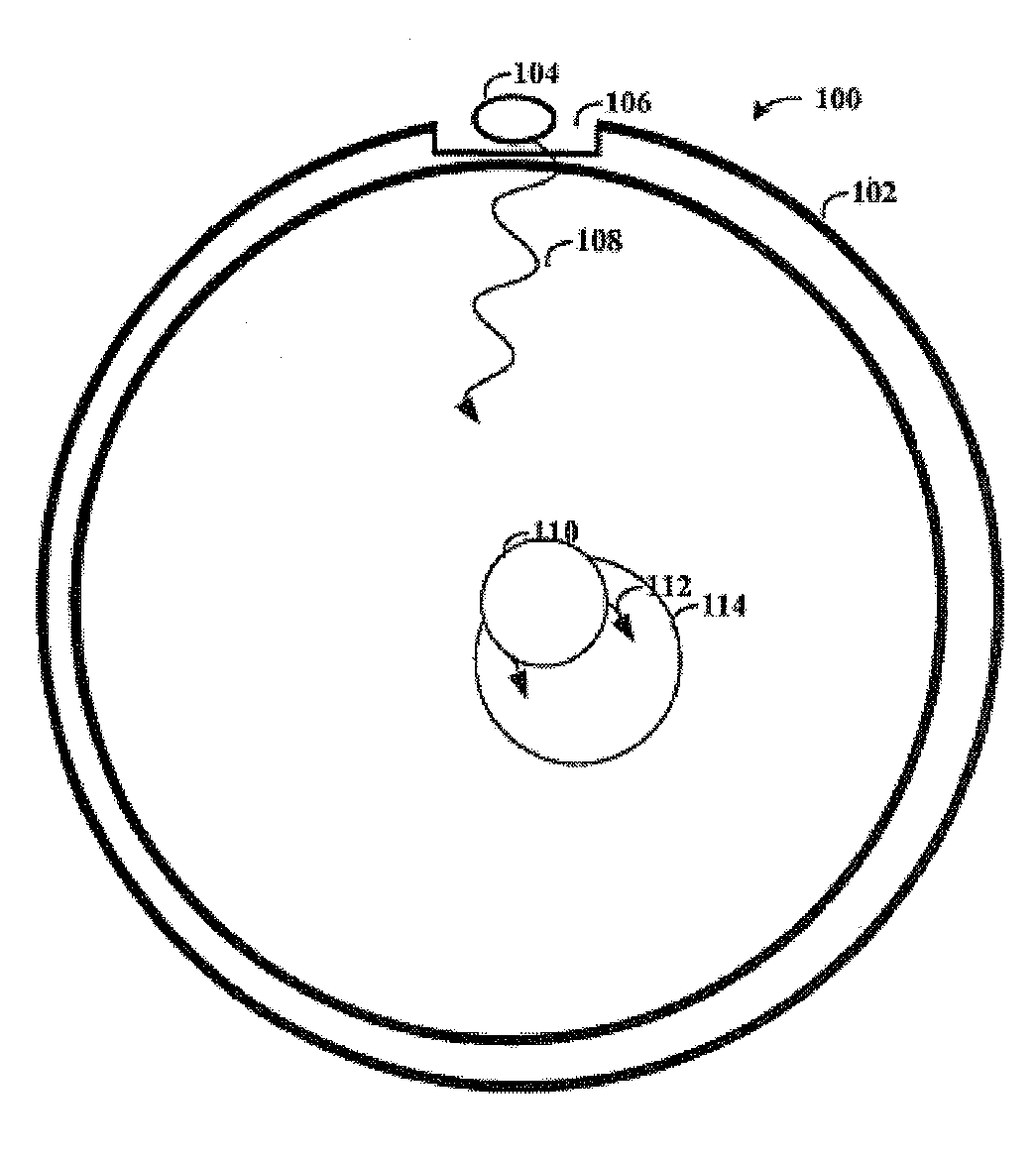
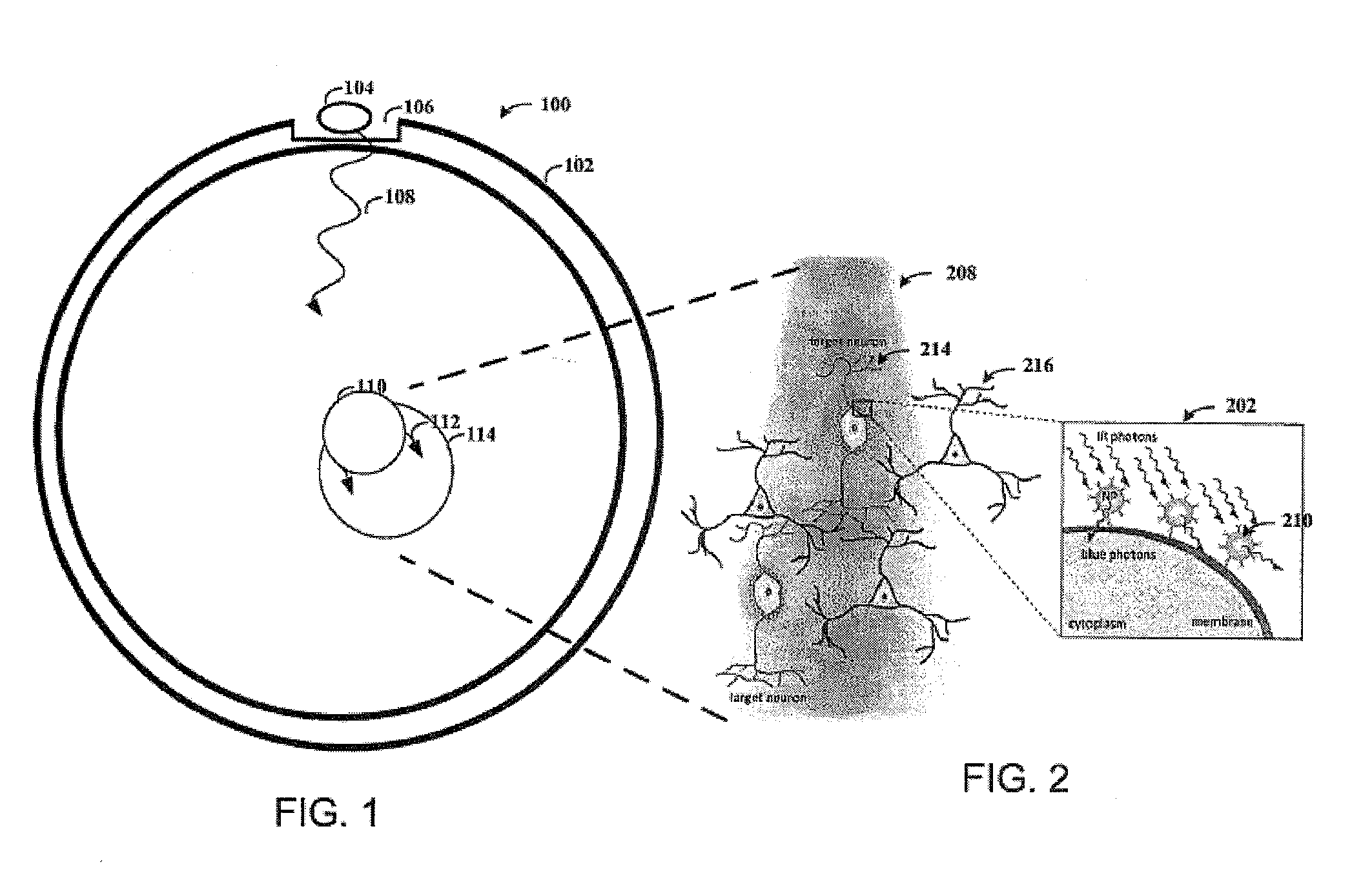
Upconversion of Light for Use in Optogenetic Methods
ActiveUS20140148880A1Easy to useReduce absorptionPeptide/protein ingredientsPhotodynamic therapyOptogeneticsNanoparticle
Provided herein are compositions comprising lanthanide-doped nanoparticles which upconvert electromagnetic radiation from infrared or near infrared wavelengths into the visible light spectrum. Also provided herein are methods activating light-responsive opsin proteins expressed on plasma membranes of neurons and selectively altering the membrane polarization state of the neurons using the light delivered by the lanthanide-doped nanoparticles.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Viral vectors and methods for producing and using the same
InactiveUS20050220766A1Improve AAV production titerReduce and even essentially eliminate contaminationBiocidePeptide/protein ingredientsPolymerase LNucleic acid sequencing
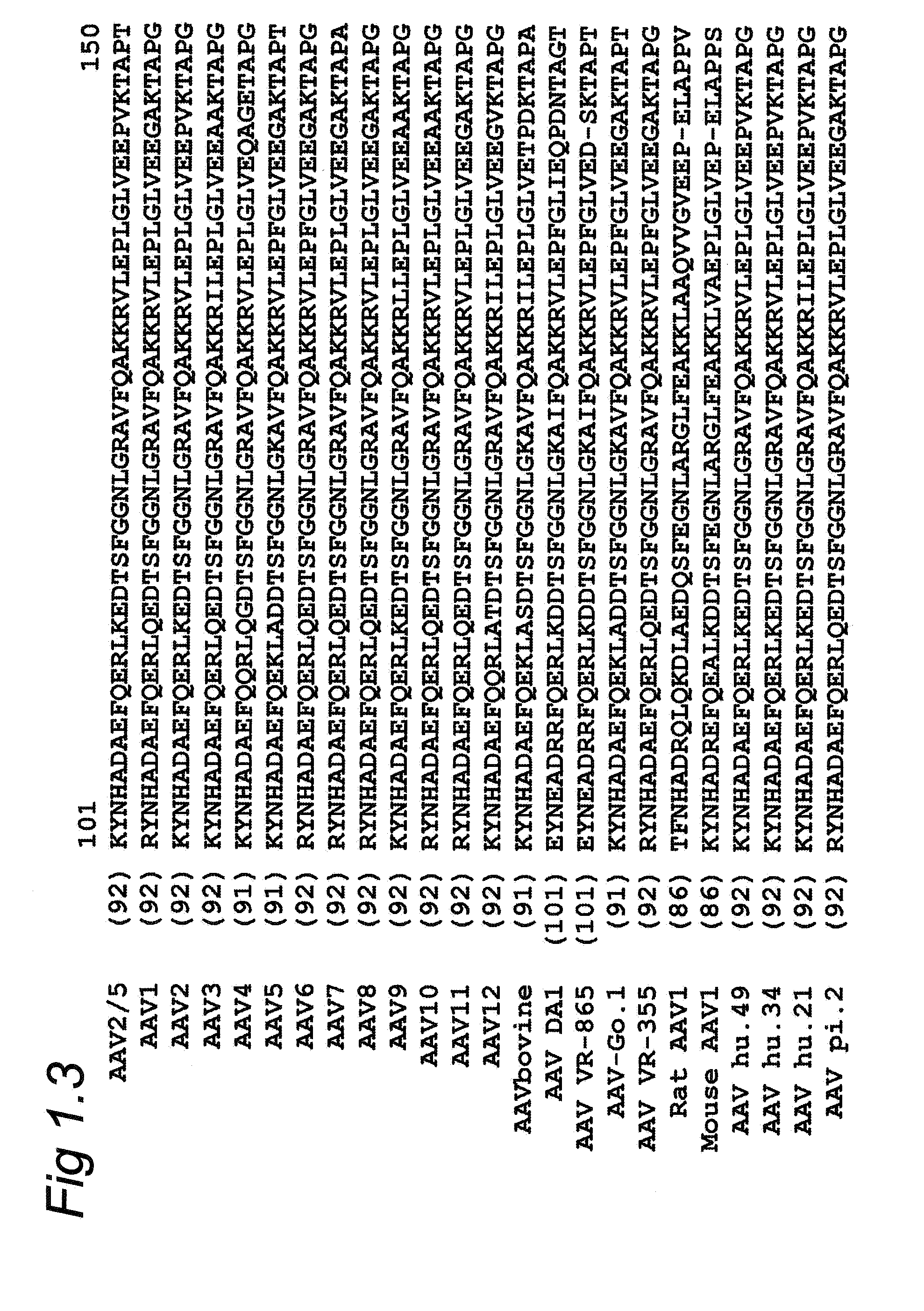
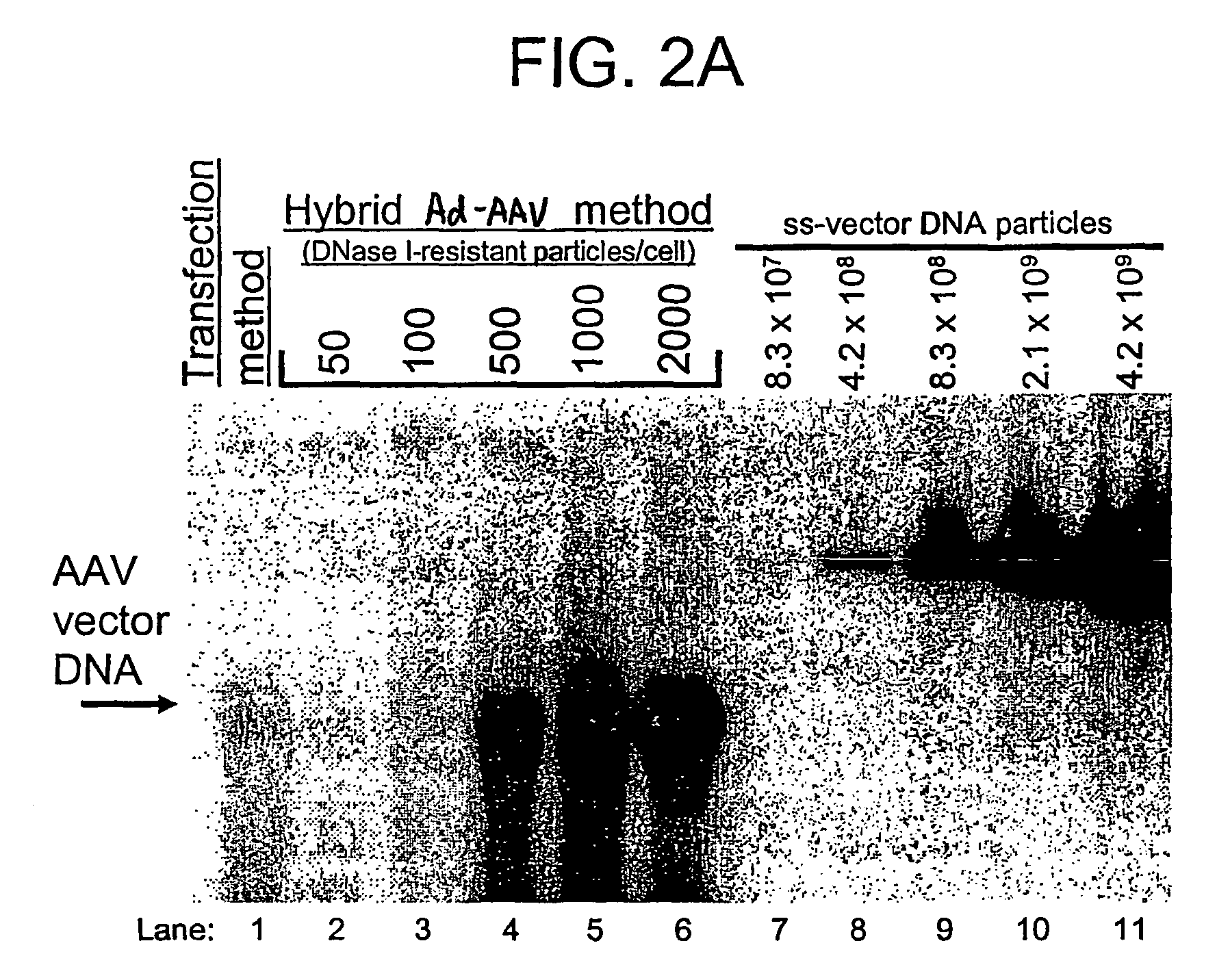
A recombinant hybrid virus, including: (a) a deleted adenovirus vector genome comprising the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation, and further comprising a deletion in an adenovirus genomic region selected from the group consisting of: (i) the polymerase region, wherein said deletion essentially prevents the expression of a functional polymerase protein from said deleted region and said hybrid virus does not otherwise express a functional polymerase protein, (ii) the preterminal protein region, wherein said deletion essentially prevents the expression of a functional preterminal protein from said deleted region, and said hybrid virus does not otherwise express a functional preterminal protein, and (iii) both the regions of (i) and (ii); and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), said recombinant AAV vector genome comprising (i) AAV 5′ and 3′ inverted terminal repeats, (ii) an AAV packaging sequence, and (iii) a heterologous nucleic acid sequence, wherein said heterologous nucleic acid sequence is flanked by the 5′ and the 3′ AAV inverted terminal repeats of (i). Methods of making and using the recombinant hybrid virus are also disclosed.
Owner:DUKE UNIV
Therapeutic cell preparation grafts and methods of use thereof
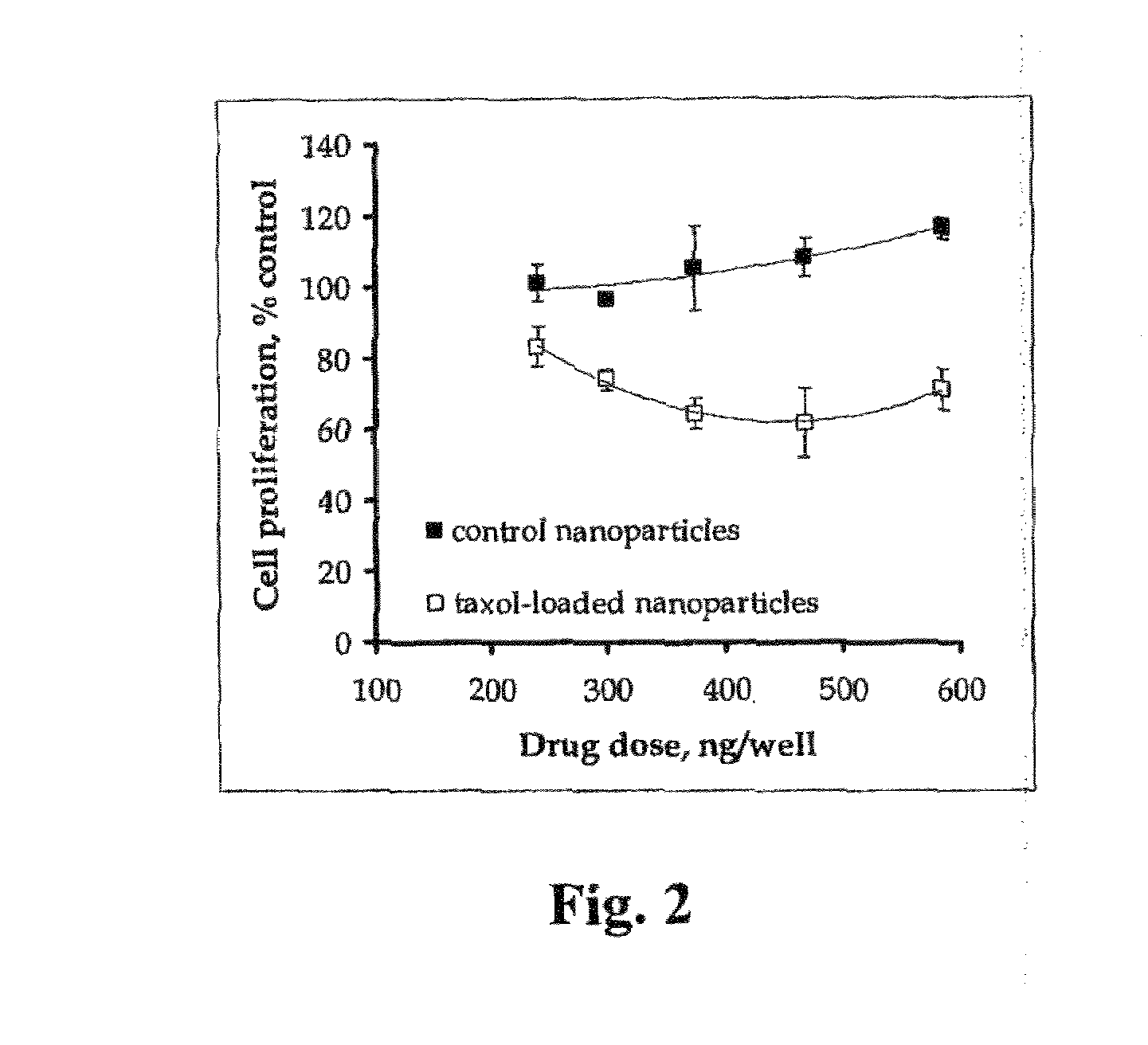
A biological preparation including genetically modified cells together with biocompatible matrices and methods of use thereof are provided. The biological preparation is useful in treating a subject at risk for or suffering from a disease in a controllable dosage and time-dependent manner, and for in vitro and in vivo screening of candidate drug therapies
Owner:KLEIN MATTHEW B +1
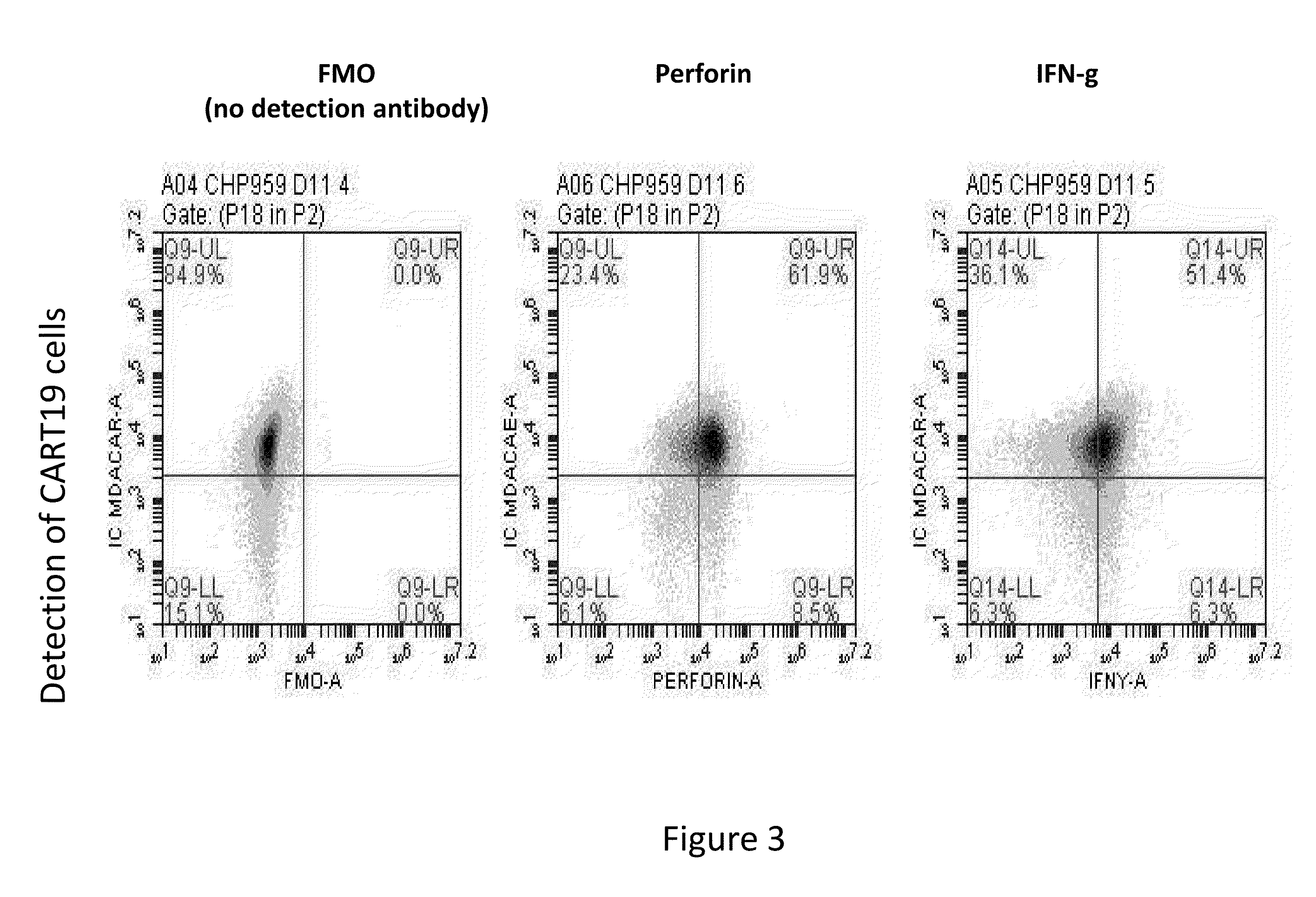
Toxicity Management for Anti-Tumor Activity of CARs
InactiveUS20150202286A1Reducing and avoiding adverse effectReducing and avoiding adverseOrganic active ingredientsBiocideAbnormal tissue growthAntigen binding
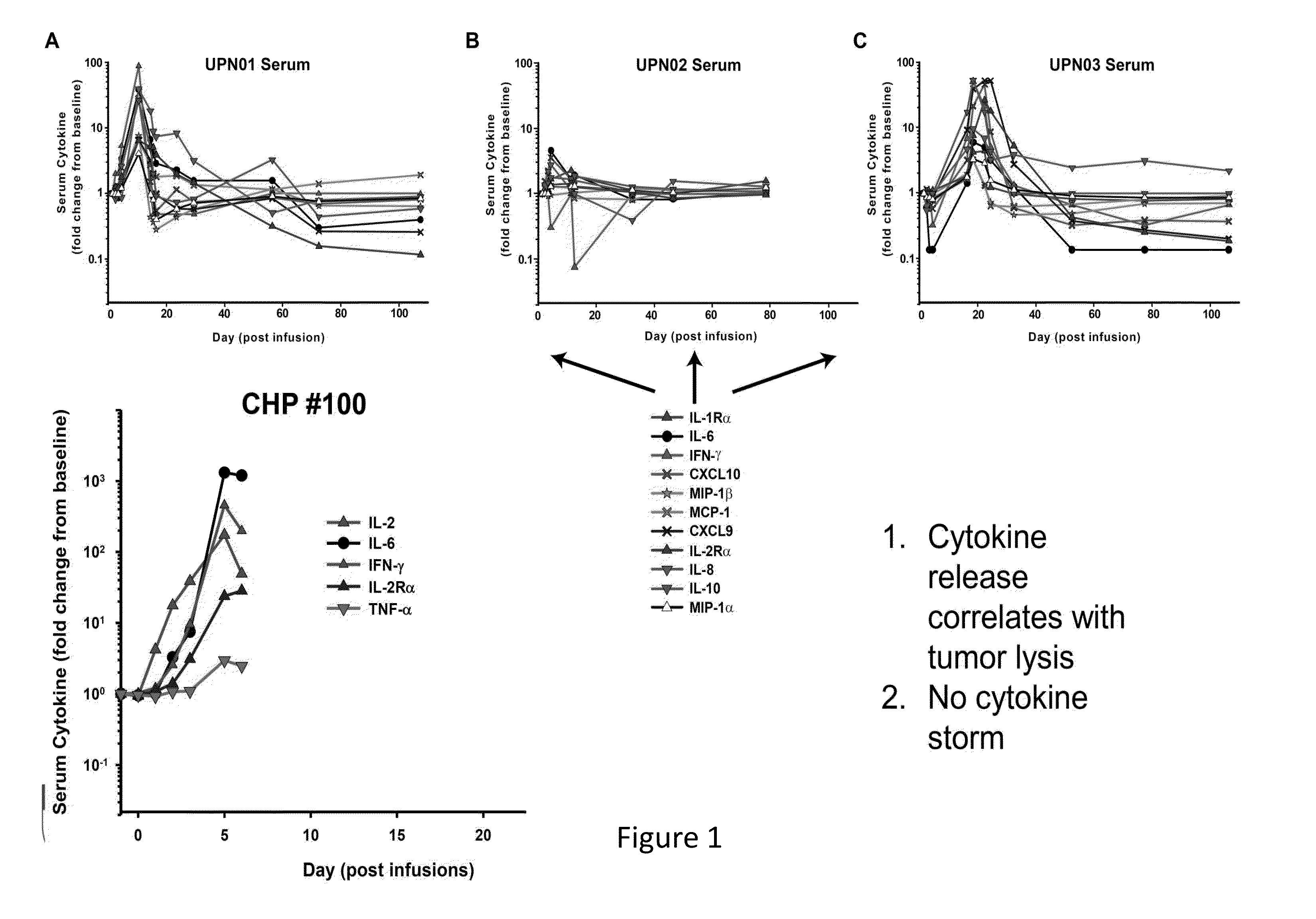
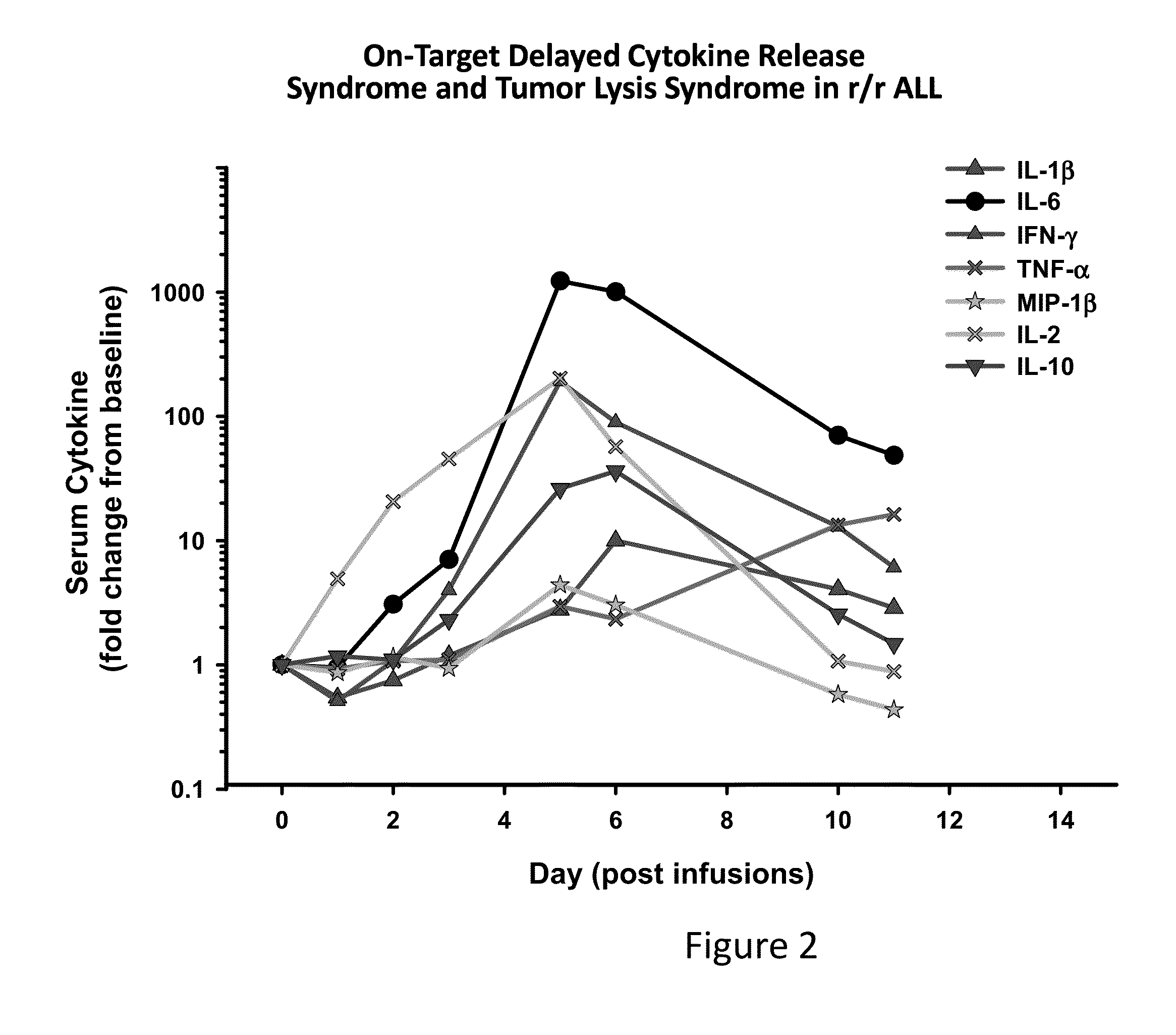
The present invention provides compositions and methods for treating cancer in a patient. In one embodiment, the method comprises a first-line therapy comprising administering to a patient in need thereof a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain and monitoring the levels of cytokines in the patient post T cell infusion to determine the type of second-line of therapy appropriate for treating the patient as a consequence of the presence of the CAR T cell in the patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
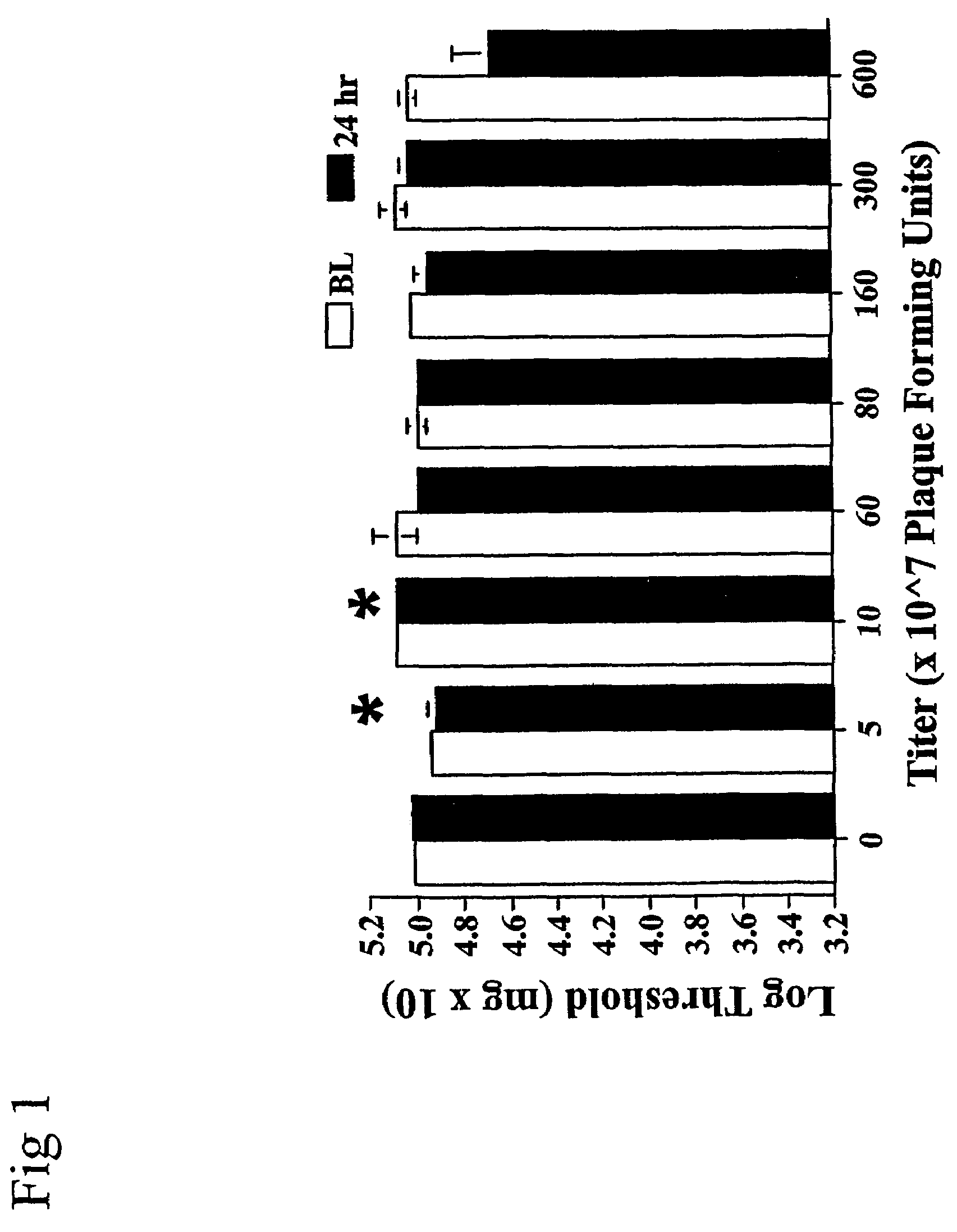
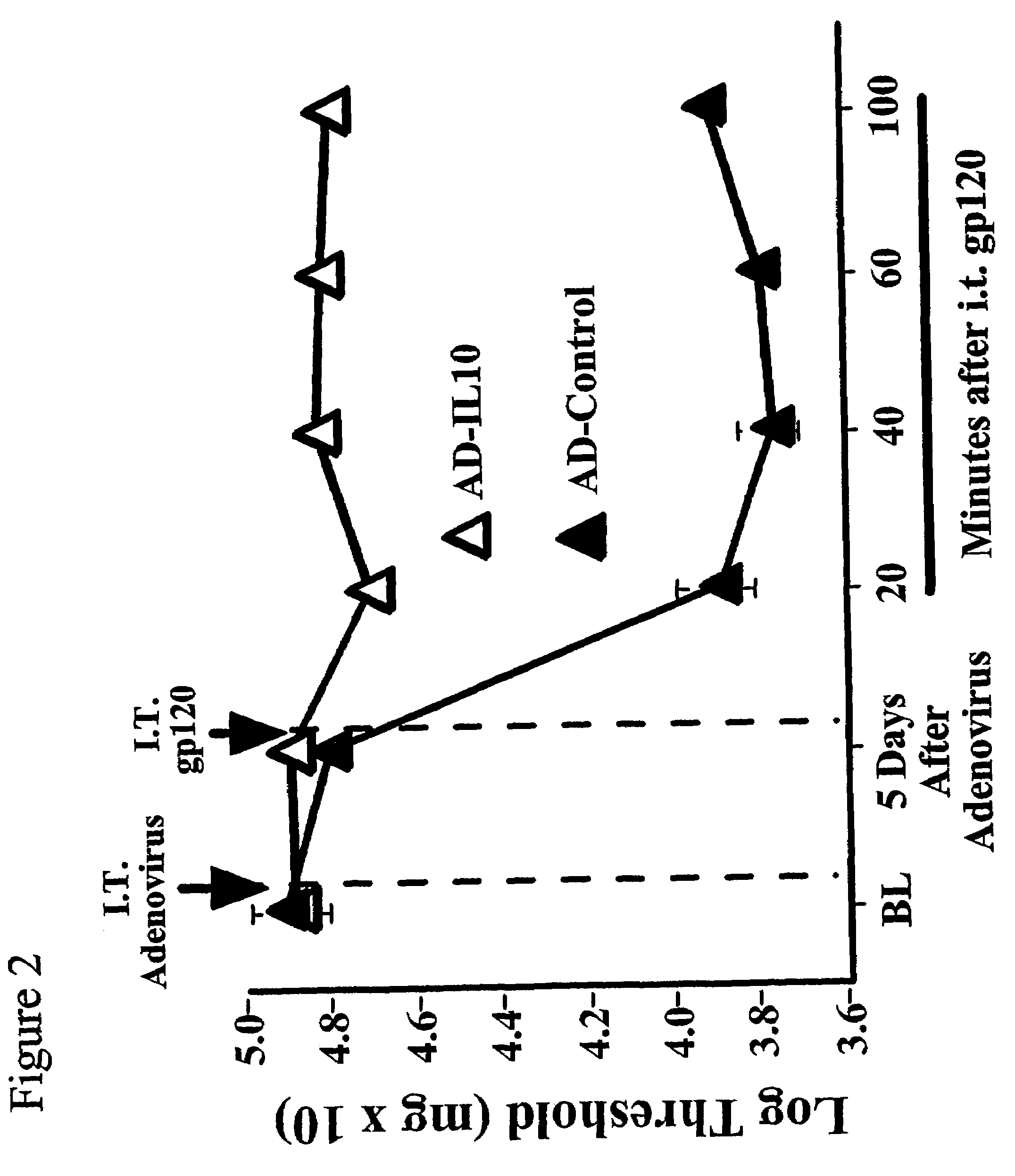
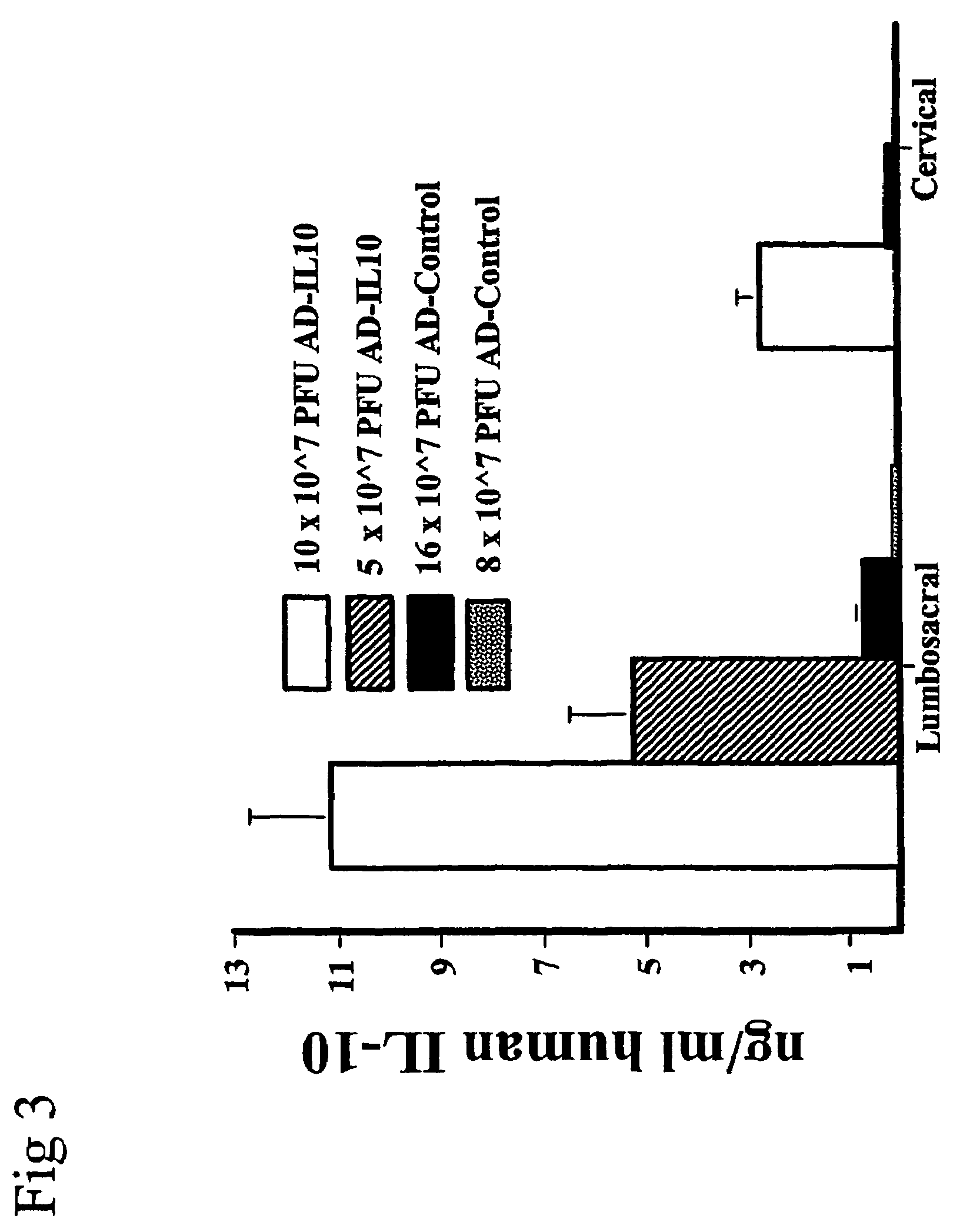
Methods for treating neuropathic pain by administering IL-10 polypeptides
ActiveUS7261882B2Avoid painSuccessful treatmentVirusesPeptide/protein ingredientsNervous systemProtein composition
Owner:UNIV OF COLORADO THE REGENTS OF
Immunisation of large mammals with low doses of RNA
ActiveUS10487332B2Improve stabilityAccelerate phosphodiester hydrolysisSsRNA viruses negative-senseAntibacterial agentsMammalLow dose
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions and methods for enhancing receptor-mediated cellular internalization
InactiveUS6908623B2Promote absorptionMaximizing expressionPowder deliveryOrganic active ingredientsDiagnostic agentWhole body
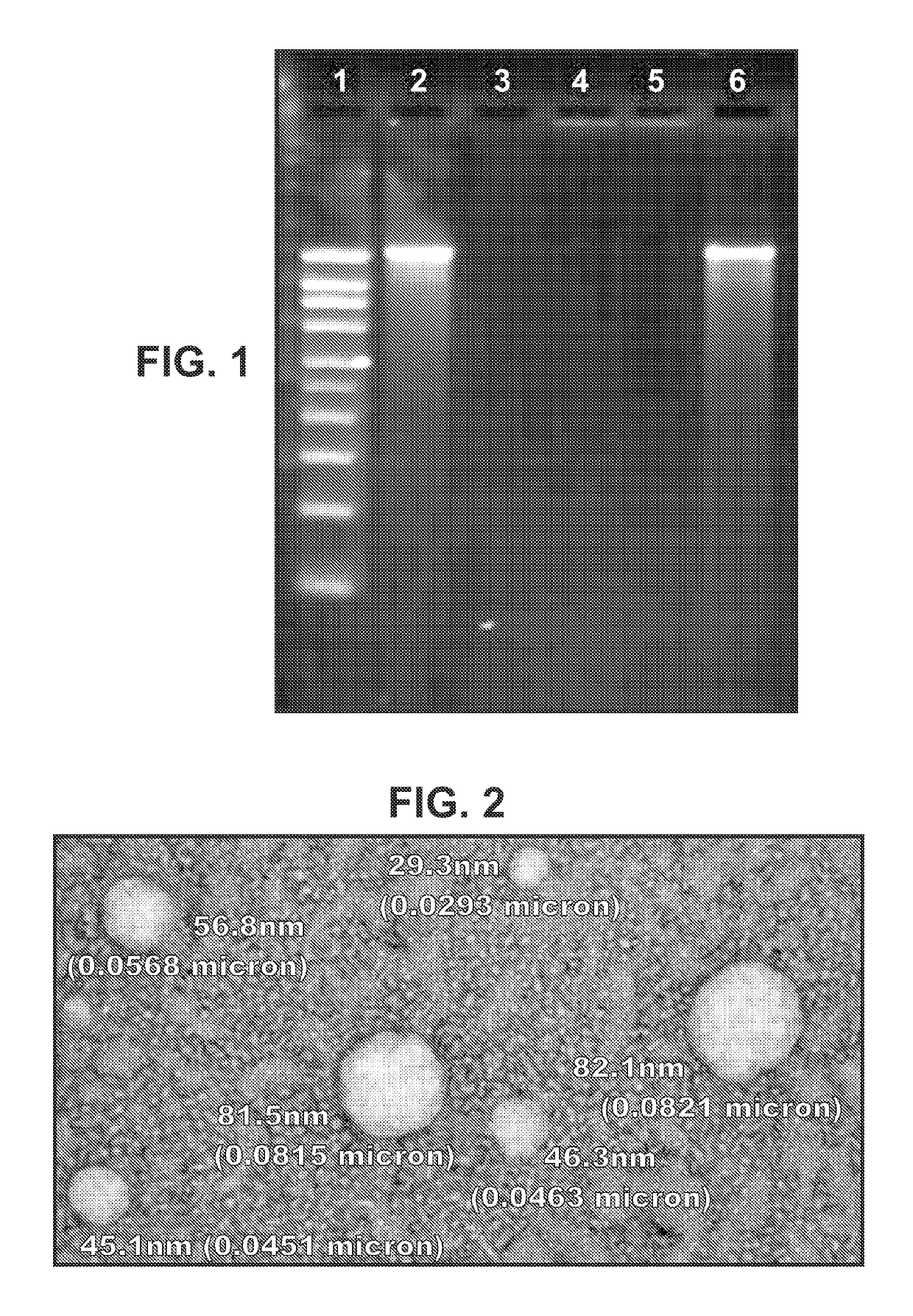
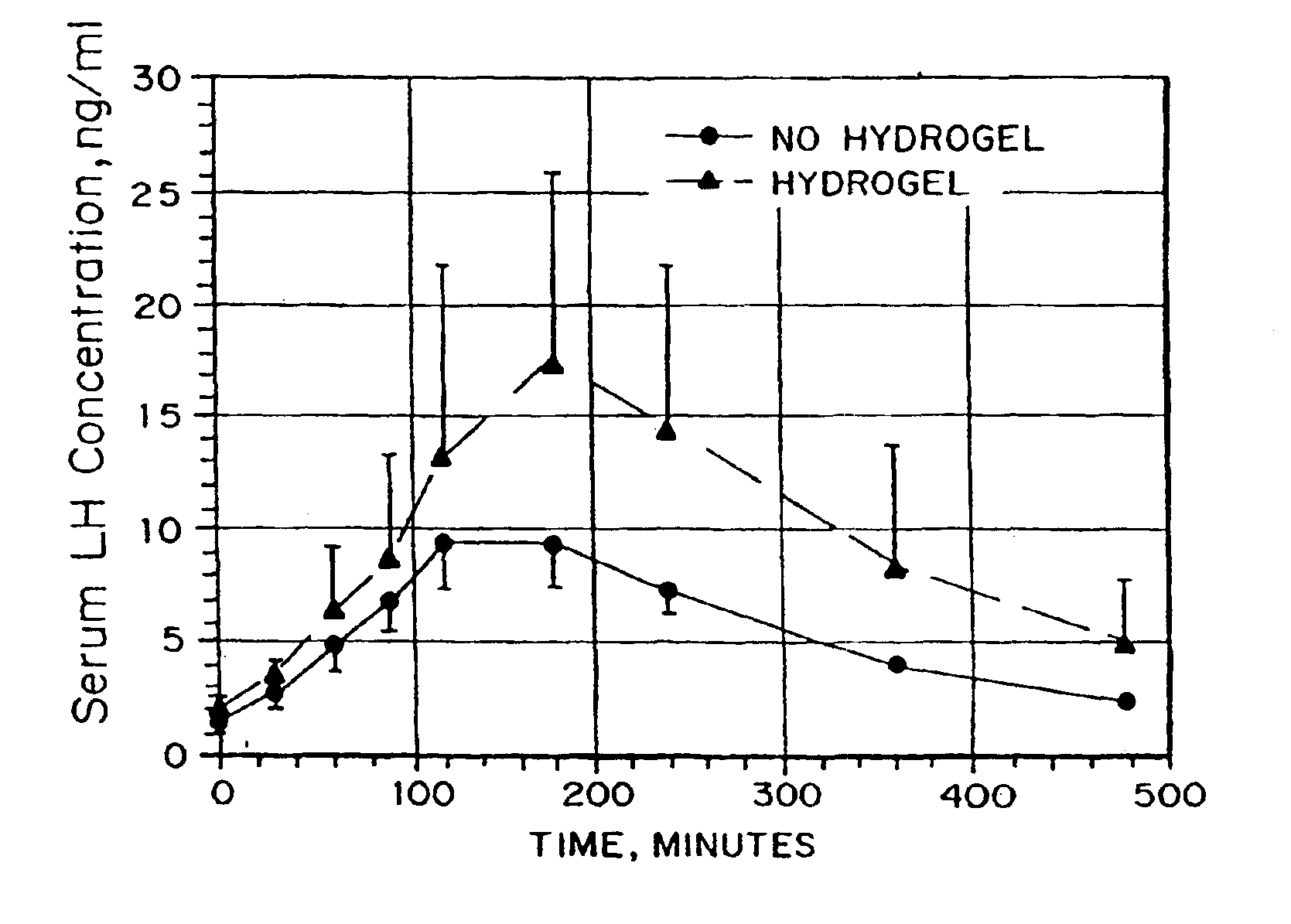
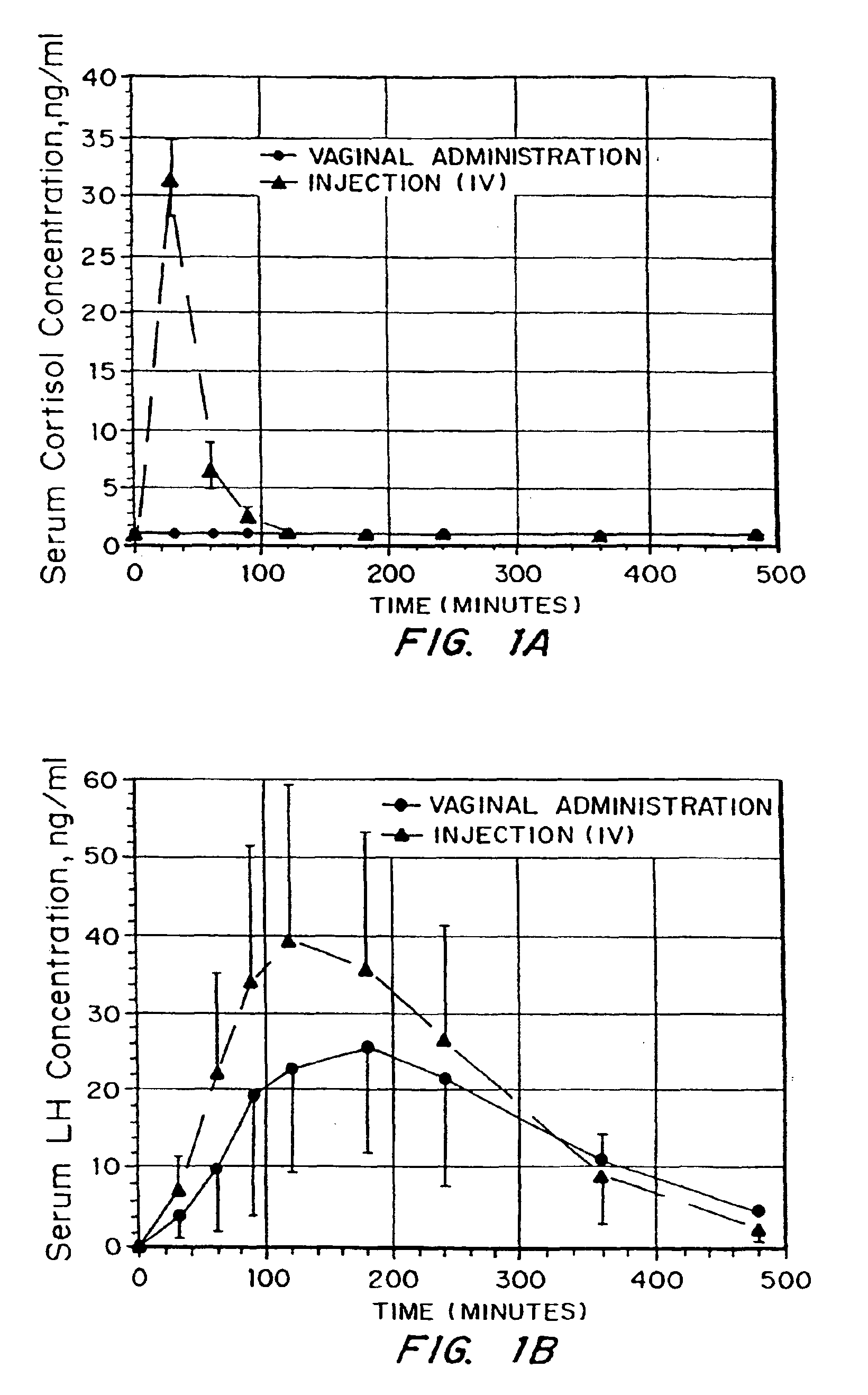
Compositions and methods for improving cellular internalization of one or more compounds are disclosed. The compositions include a compound to be delivered and a biocompatible viscous material, such as a hydrogel, lipogel, or highly viscous sol. The composition also include, or are administered in conjunction with, an enhancer in an amount effective to maximize expression of or binding to receptors and enhance RME of the compound into the cells. This leads to high transport rates of compounds to be delivered across cell membranes, facilitating more efficient delivery of drugs and diagnostic agents. Compositions are applied topically orally, nasally, vaginally, rectally, and ocularly. The enhancer is administered with the composition or separately, either systemically or preferably locally. The compound to be delivered can also be the enhancer.
Owner:PENN STATE RES FOUND
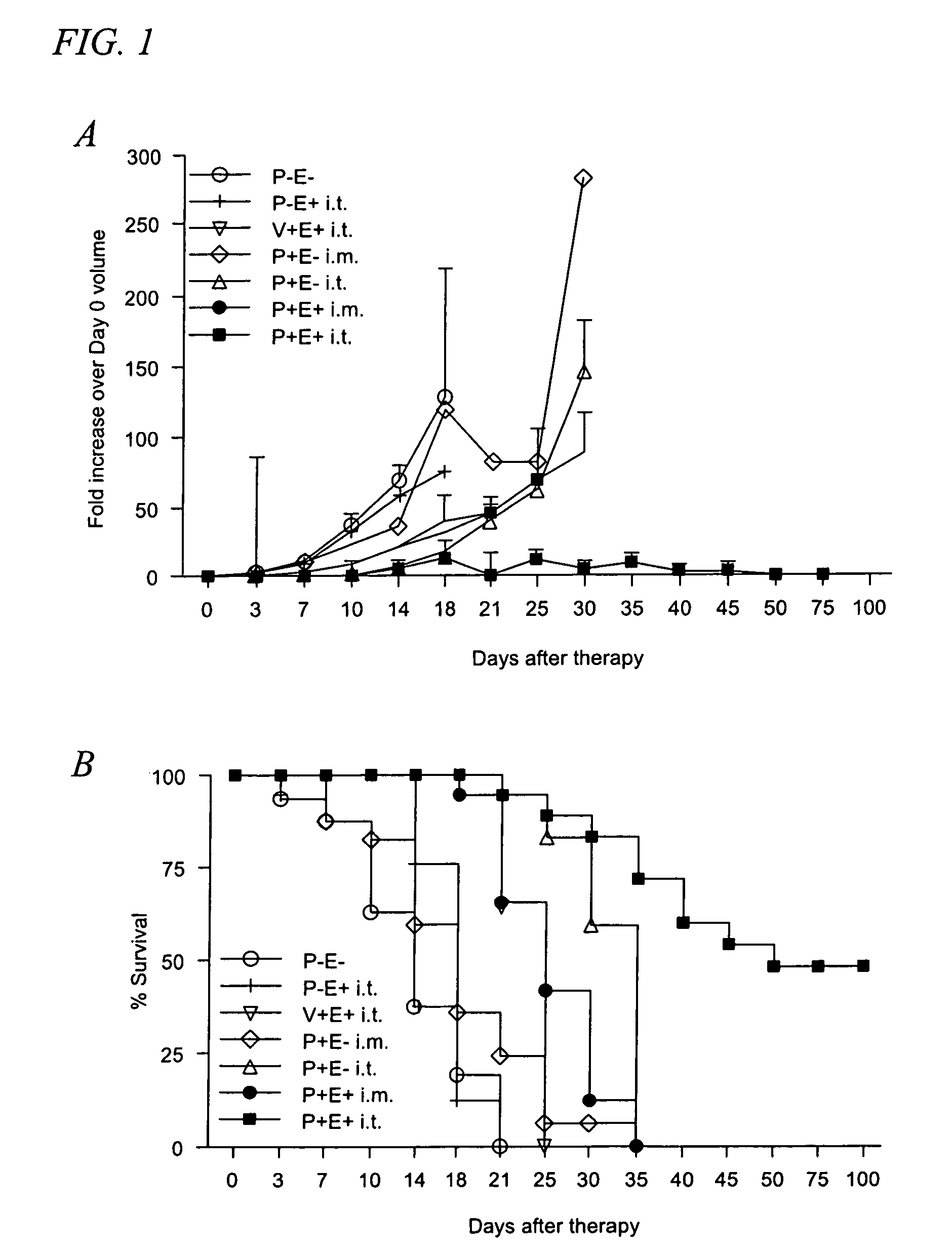
Treating malignant tumors with high field strength electroporation of plasmids encoding IL-12
ActiveUS8026223B1Improve survival rateBiocidePeptide/protein ingredientsElectroporation therapyTherapeutic protein
In accordance with the present invention is provided a method of treating a subject having a cancerous tumor. The treatment protocol methodology includes injecting the cancerous tumor with an effective dose of plasmid coding for a therapeutic protein followed by administering electroporation therapy to the tumor, the electroporation therapy includes the administration of at least one high voltage, short duration pulse to the tumor.
Owner:UNIV OF SOUTH FLORIDA
Viral vectors and methods for producing and using the same
InactiveUS7858367B2Reduce and even essentially eliminate contaminationReduce dependenceBiocidePeptide/protein ingredientsHeterologousPolymerase L
A recombinant hybrid virus which includes: (a) a deleted adenovirus vector genome having the adenovirus 5′ and 3′ cis-elements for viral replication and encapsidation and a deletion in an adenovirus genomic region selected from the polymerase region and / or the preterminal protein region, wherein the deletion essentially prevents the expression of a functional polymerase and / or preterminal protein from the deleted region and the hybrid virus does not otherwise express a functional polymerase protein; and (b) a recombinant adeno-associated virus (AAV) vector genome flanked by the adenovirus vector genome sequences of (a), wherein the recombinant AAV vector genome includes an AAV packaging sequence and a heterologous nucleic acid sequence, wherein the heterologous nucleic acid sequence is flanked by 5′ and 3′ AAV inverted terminal repeats.
Owner:DUKE UNIV
Uniform field magnetization and targeting of therapeutic formulations
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
Use of nucleic acids with reduced pressure therapy
ActiveUS20090275884A1Organic active ingredientsPeptide/protein ingredientsPharmaceutical drugWound site
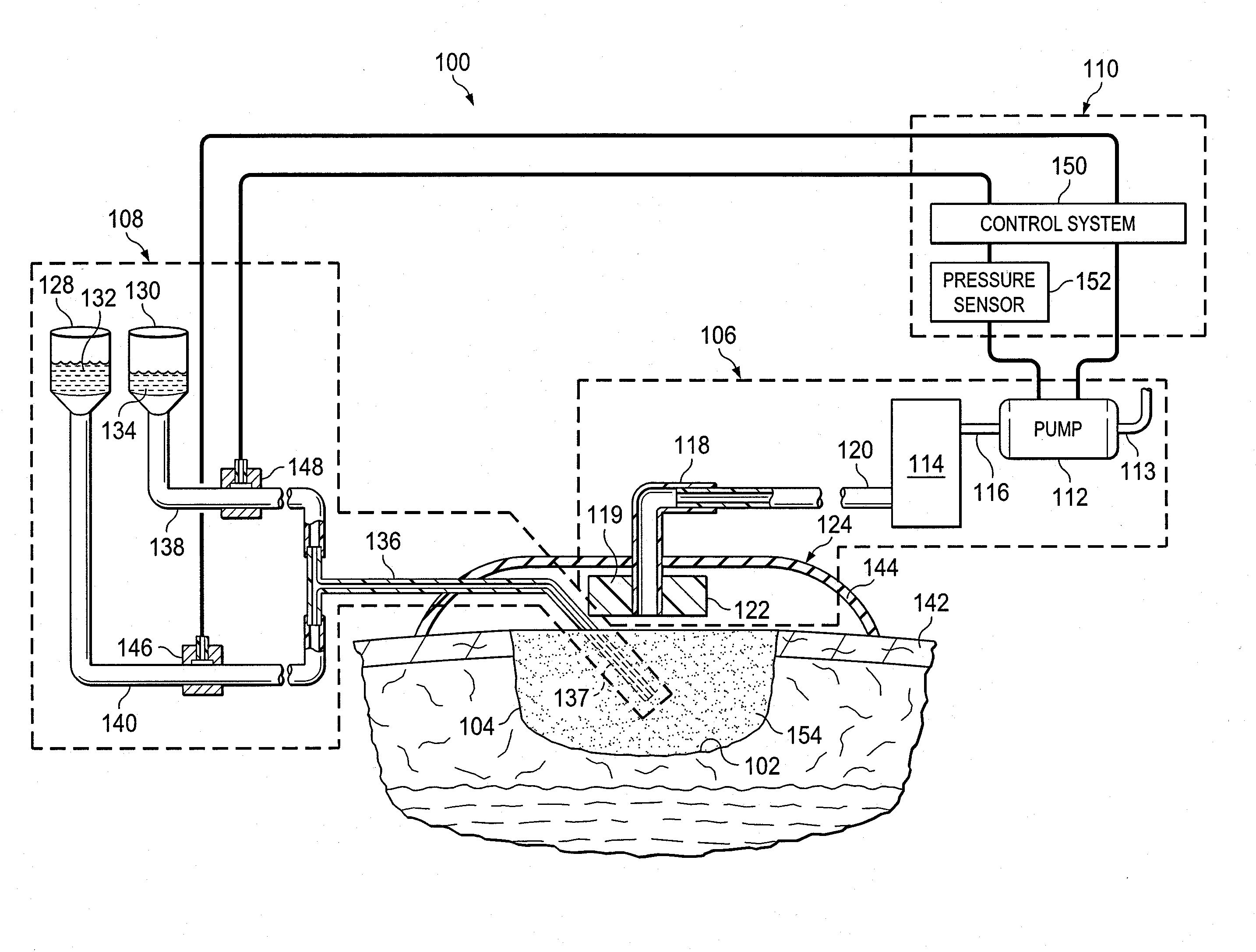
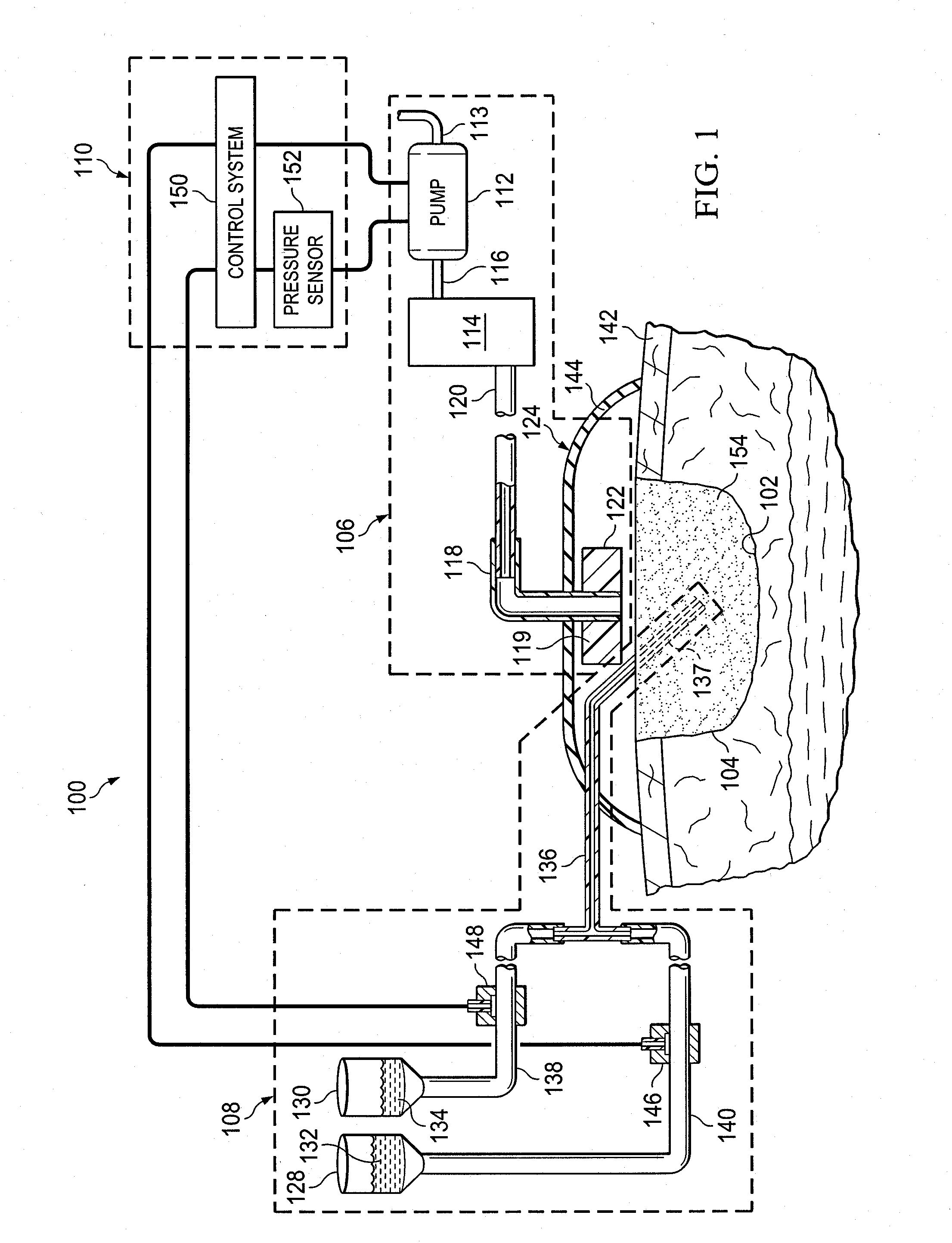
Provided is a method of treating a wound site. Also provided is a system for treating a wound site. Additionally provided is the use of reduced pressure and a nucleic acid that promotes wound healing for treatment of a wound site. Further provided is the use of a nucleic acid that promotes wound healing for the manufacture of a medicament for treating a wound site that is undergoing reduced pressure treatment.
Owner:3M INNOVATIVE PROPERTIES CO
Enhancement of transfection of DNA into the liver
InactiveUS7211248B2High expressionFacilitating transfectionBiocideOrganic active ingredientsTransfectionDNA
A method for transfection of DNA or RNA encoding apo A1 into the liver to regulate lipid metabolism comprises injection of a solution containing the DNA or RNA gene into the liver in combination with gas microbubbles or microspheres and using ultrasound to induce the transfection of the DNA or RNA in the liver.
Owner:SONOGENE
Biologic device for regulation of gene expression and method therefor
InactiveUS20070036771A1Desirable effectAltered expressionBiocideInternal electrodesOpen reading frameRegulation of gene expression
A system and device are provided which include a gene regulatory system controlling expression of one or more expression cassettes present in or released by the device, by emitting one or more stimulations. An expression cassette includes a regulatable transcription control element that is responsive to the emitted stimulations linked to an open reading frame of interest. The system optionally includes a sensor to sense a parameter indicative of a need, a telemetry module to receive an external command, or a programmable device, for regulating gene expression of the open reading frame.
Owner:CARDIAC PACEMAKERS INC
Administration of growth factors for the treatment of CNS disorders
ActiveUS7922999B2Increased growth factor mobilityAvoid delayHeavy metal active ingredientsSenses disorderNeuron deathNeuron
A method and system that is directed to the local delivery of growth factors to the mammalian CNS to treat CNS disorders associated with neuronal death and / or dysfunction is described.
Owner:RGT UNIV OF CALIFORNIA
Gene therapy for niemann-pick disease type a
InactiveUS20090117156A1Symptoms improvedCompound screeningOrganic active ingredientsMedicineTransgene
This disclosure pertains to methods and compositions for tolerizing a mammal's brain to exogenously administered acid sphingomyelinase polypeptide by first delivering an effective amount of a transgene encoding the polypeptide to the mammal's hepatic tissue and then administering an effective amount of the transgene to the mammal's central nervous system (CNS).
Owner:GENZYME CORP
Glucocorticoid modulation of nucleic acid-mediated immune stimulation
InactiveUS20070054873A1Minimize and inhibit immune responseOrganic active ingredientsBiocideLipid formationDosing regimen
The present invention provides methods for minimizing or inhibiting immune responses to immunostimulatory nucleic acids by pretreating with one or more doses of a glucocorticoid prior to nucleic acid administration. The nucleic acids are typically administered using a lipid-based carrier system such as a nucleic acid-lipid particle or liposome. As a result, patients following a glucocorticoid dosing regimen advantageously benefit from nucleic acid therapy without suffering any of the immunostimulatory side-effects associated with such therapy.
Owner:PROTIVA BIOTHERAPEUTICS
Methods for therapy of neurodegenerative disease of the brain
InactiveUS6815431B2Improve the level ofMinimal toxicityBiocidePeptide/protein ingredientsMammalMammalian brain
Owner:RGT UNIV OF CALIFORNIA
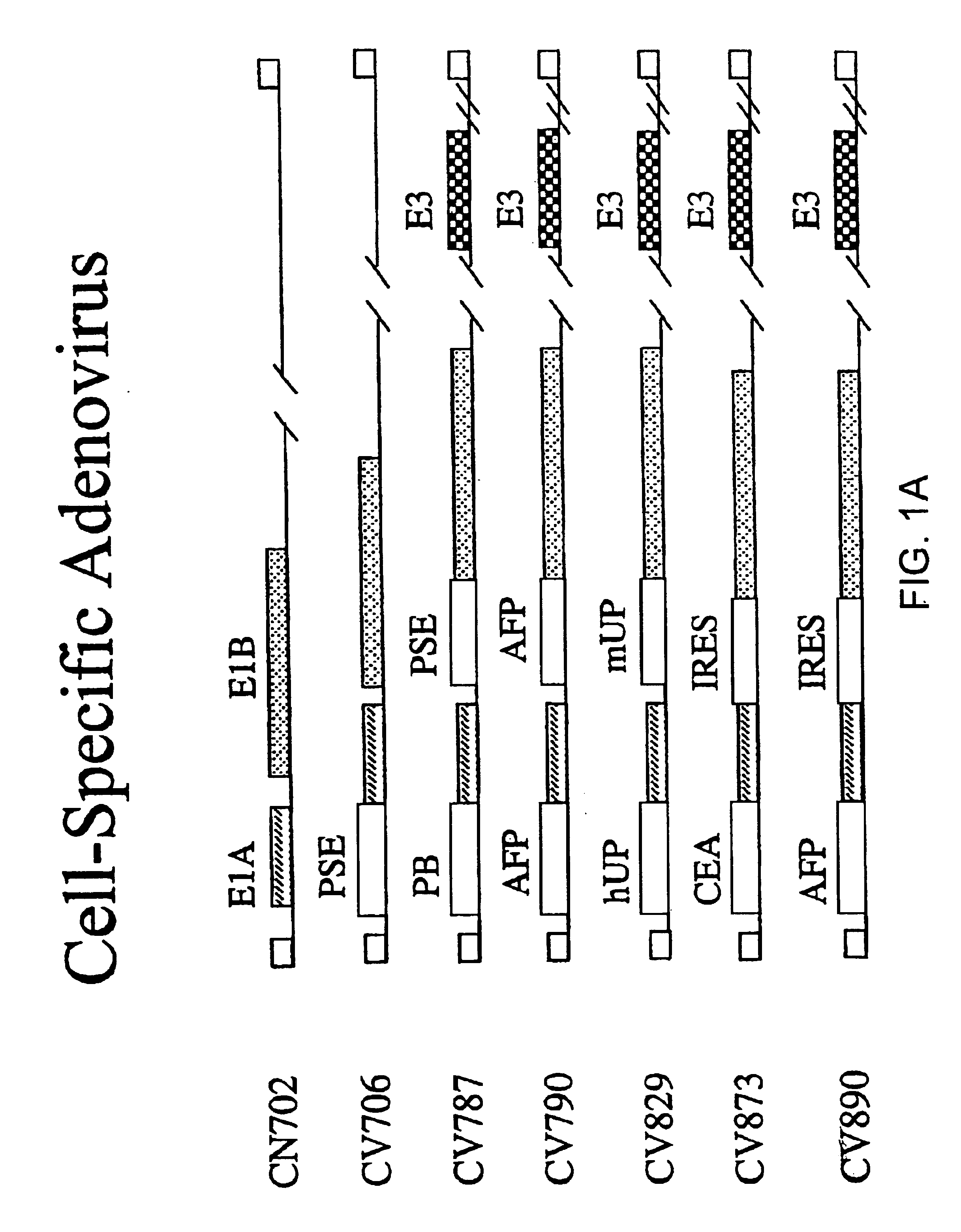
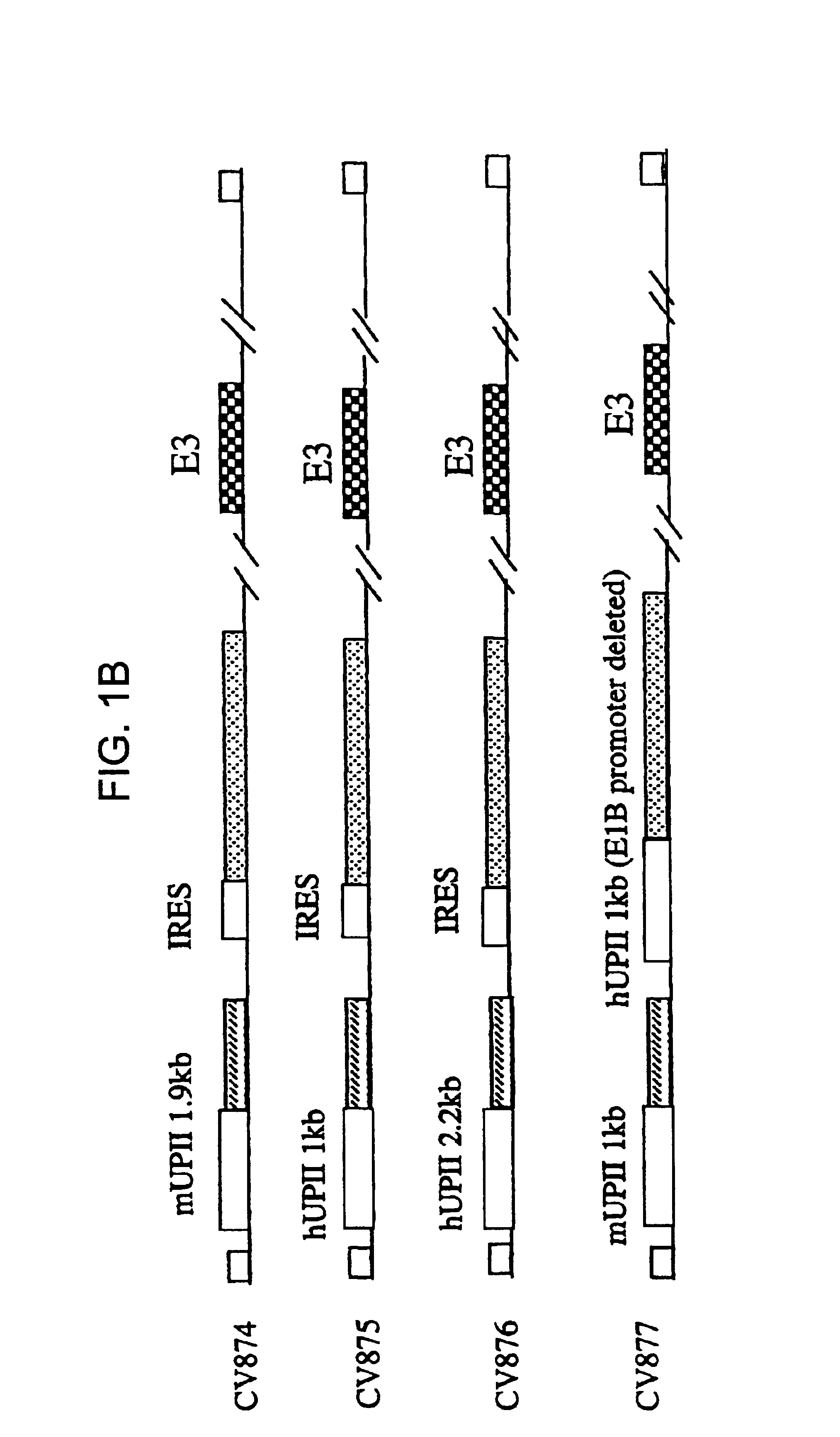
Methods of treating neoplasia with combination of target-cell specific adenovirus, chemotherapy and radiation
The invention provides methods of treating neoplasia using combinations of target cell-specific replication competent adenoviral vectors and chemotherapy, radiation therapy or combinations thereof. The adenoviral vectors are target cell-specific for the particular type of neoplasia for which treatment is necessary and the combination with the chemotherapy and / or radiation leads to synergistic treatment over existing adenoviral therapy or traditional chemotherapy and radiation therapy.
Owner:CELLS GENESYS INC
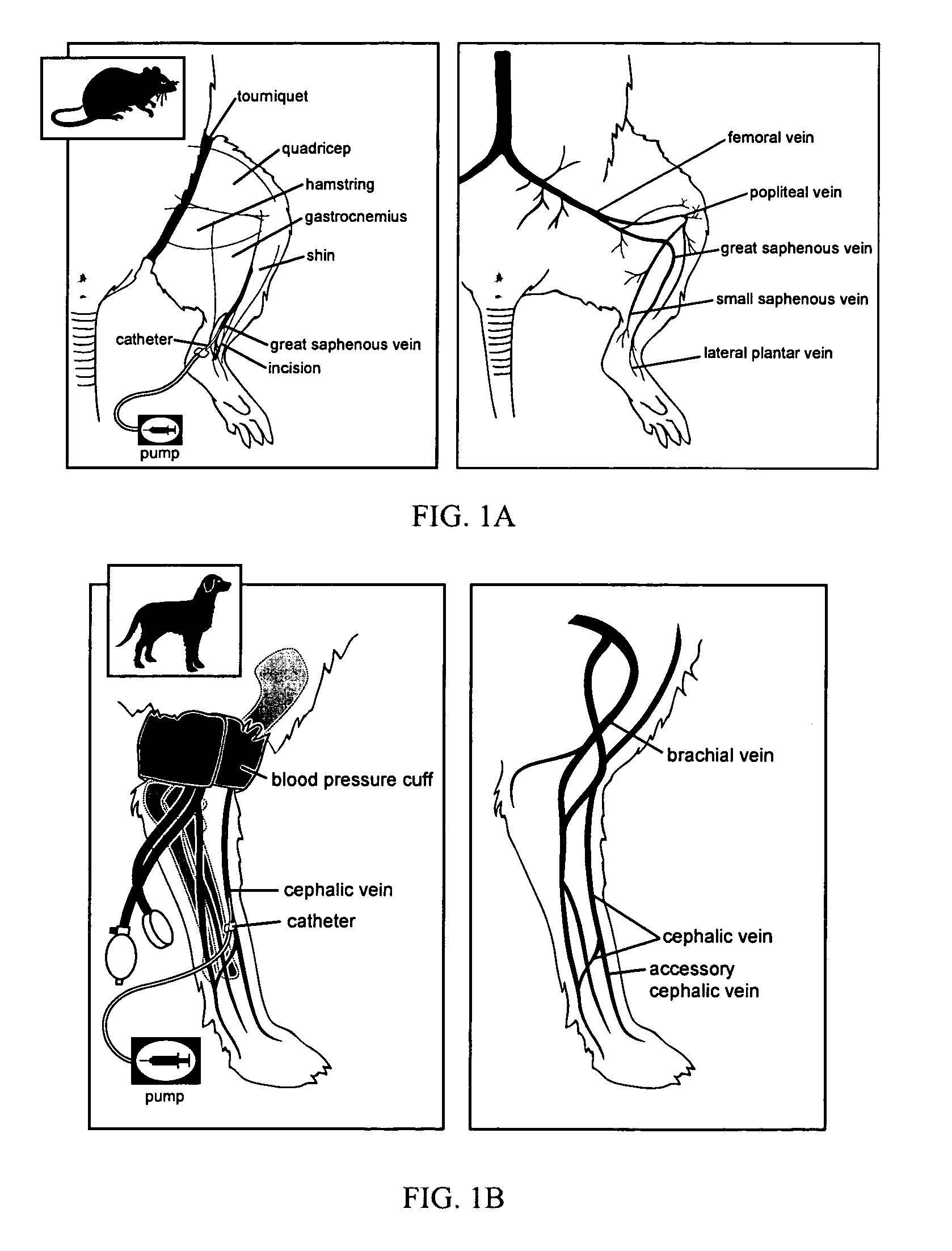
Intravascular delivery of nucleic acid
InactiveUS7015040B2Relieve painImprove breathabilitySugar derivativesMicroencapsulation basedMammalBlood vessel
Disclosed is a process for providing for expression of an exogenous nucleic acid in an extravascular parenchymal cell of a mammal. The nucleic acid is inserted into a vessel of a mammal and the permeability of the vessel is increased. Increasing permeability of the vessel allows delivery of the nucleic acid to an extravascular parenchymal cell.
Owner:ARROWHEAD MADISON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com