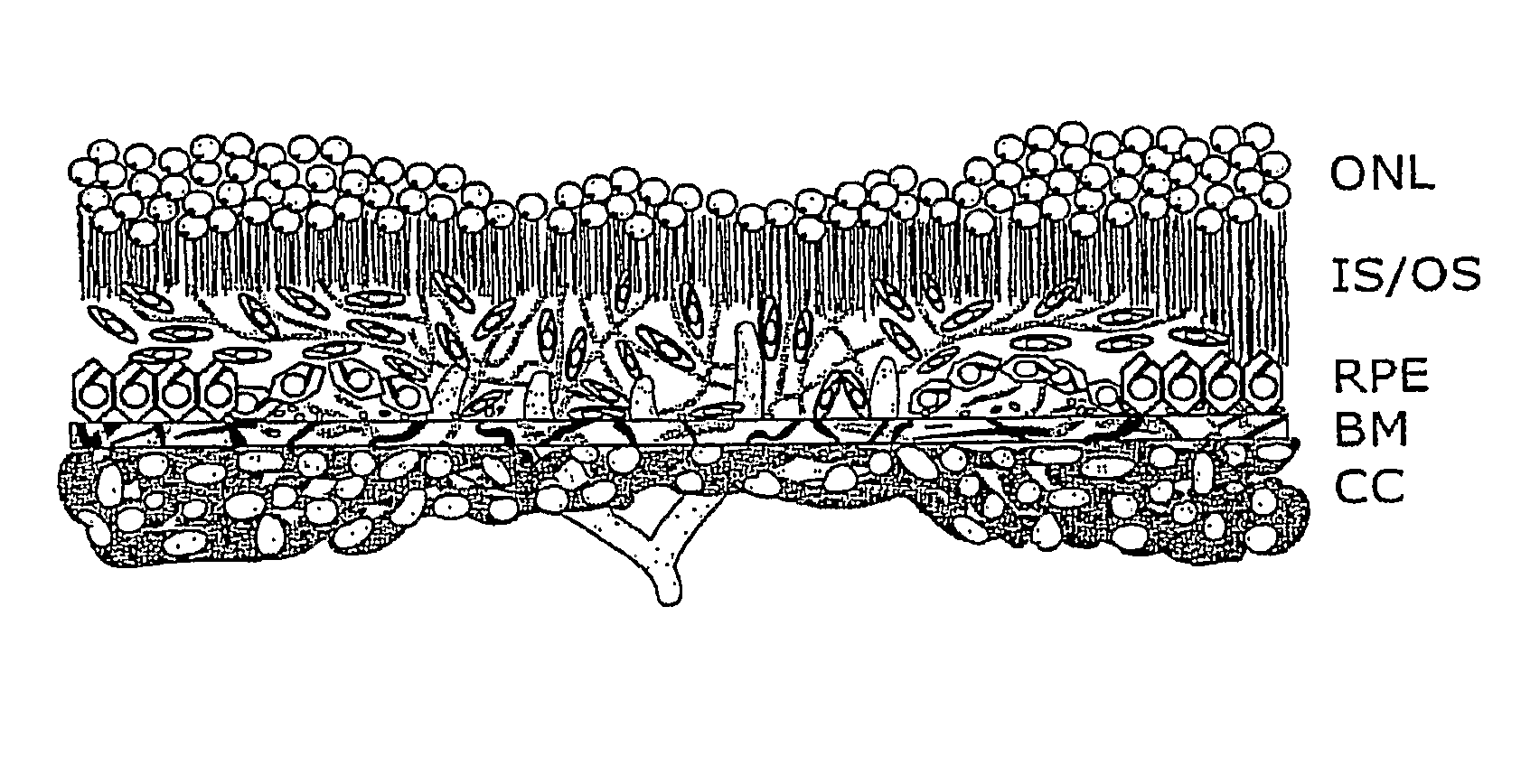
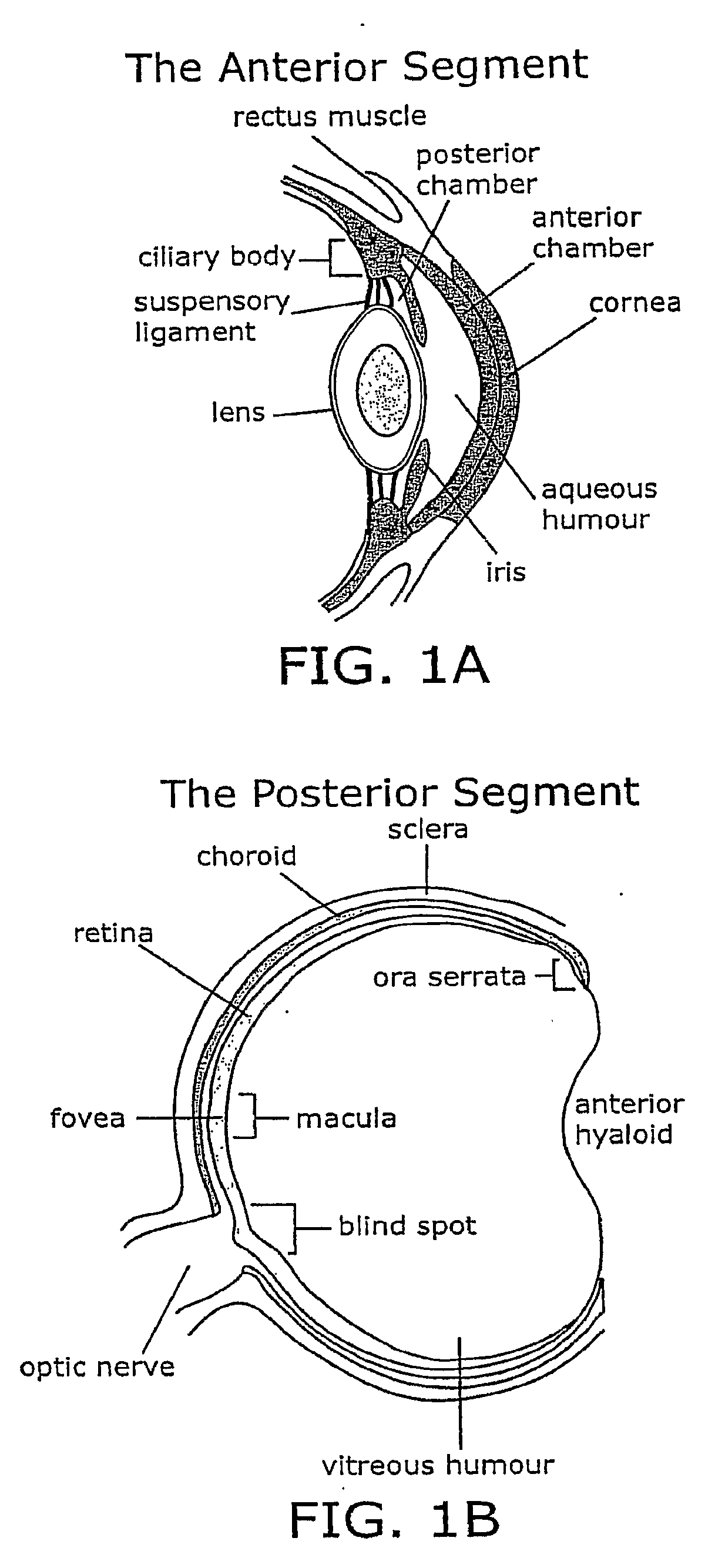
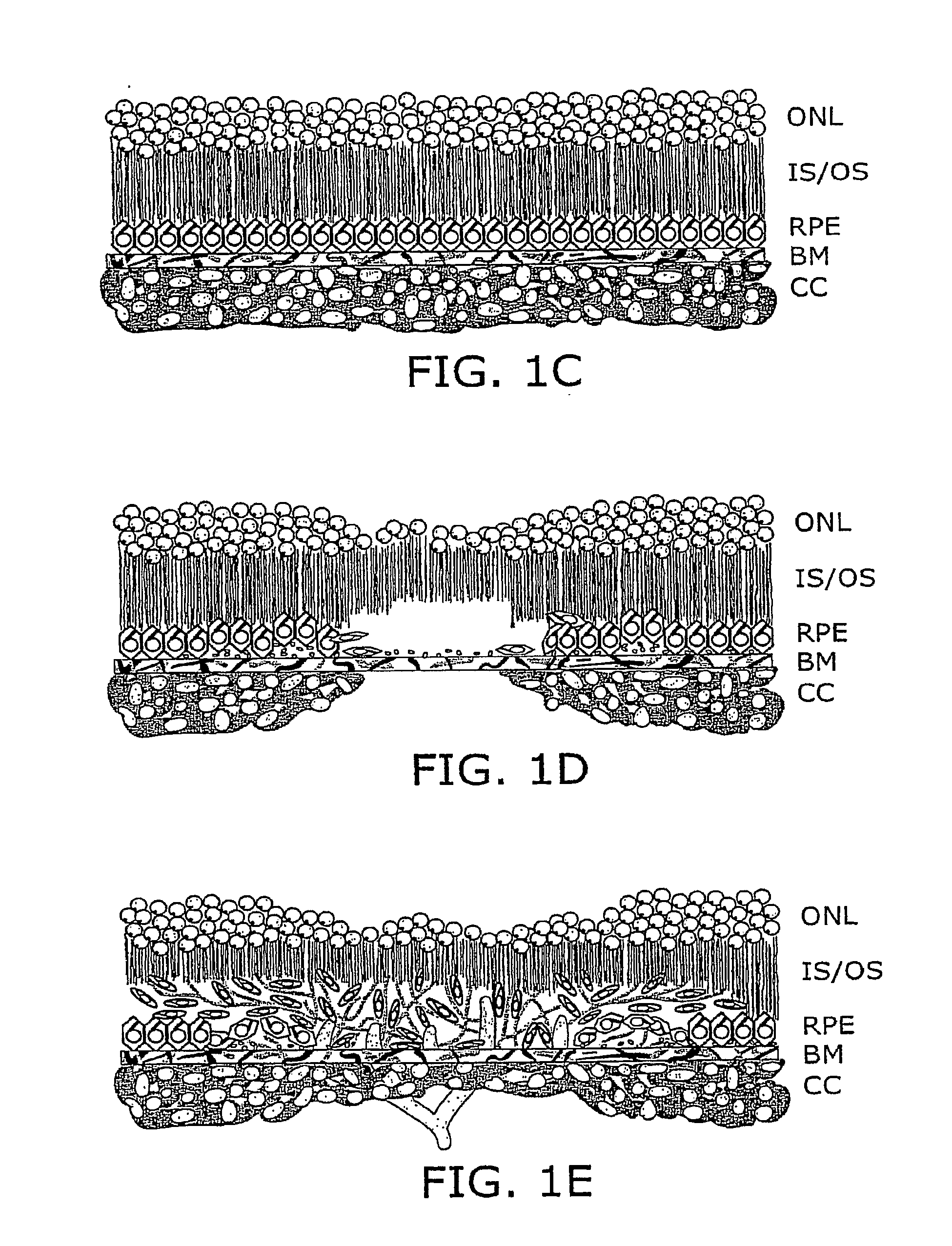
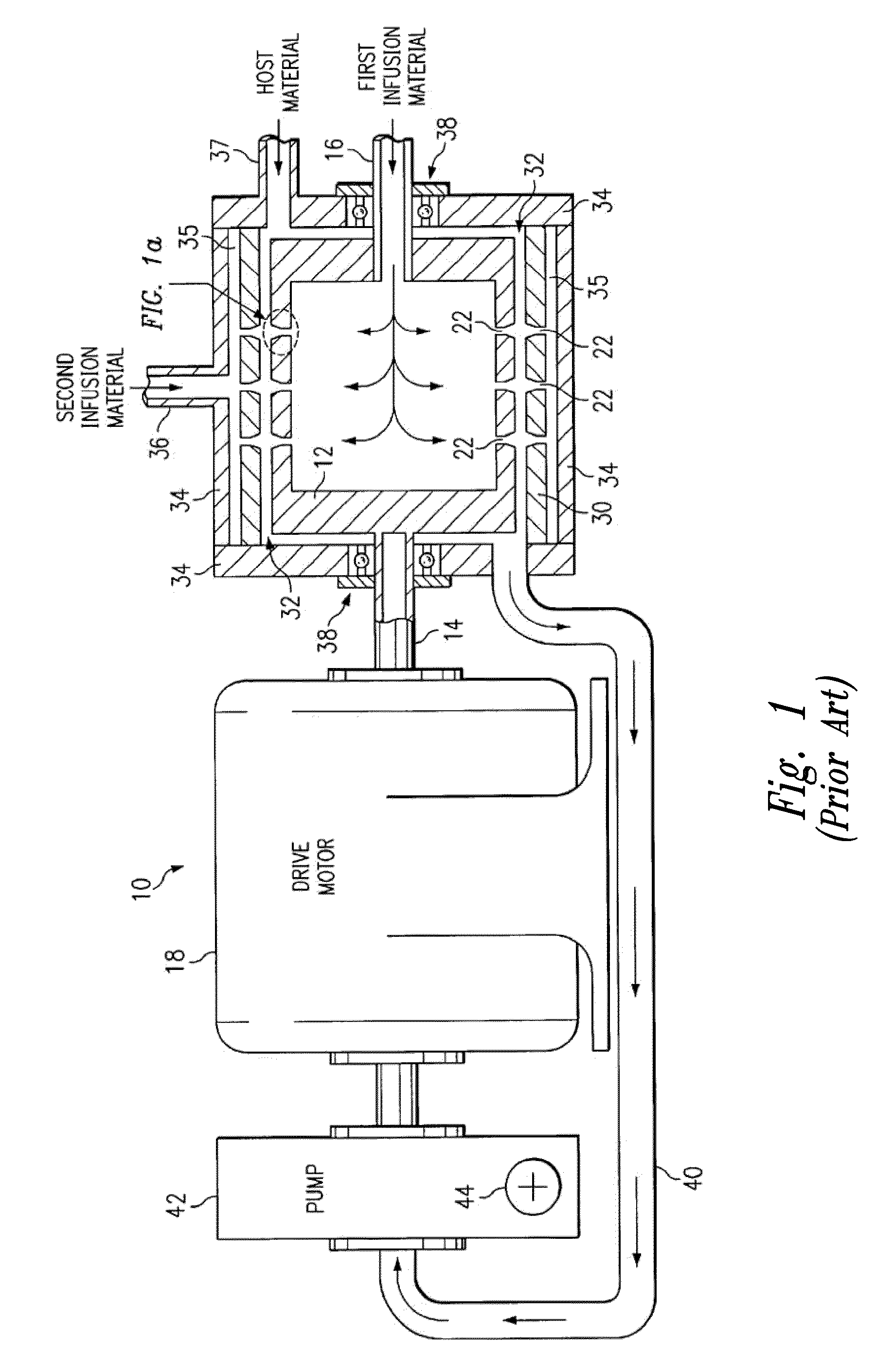
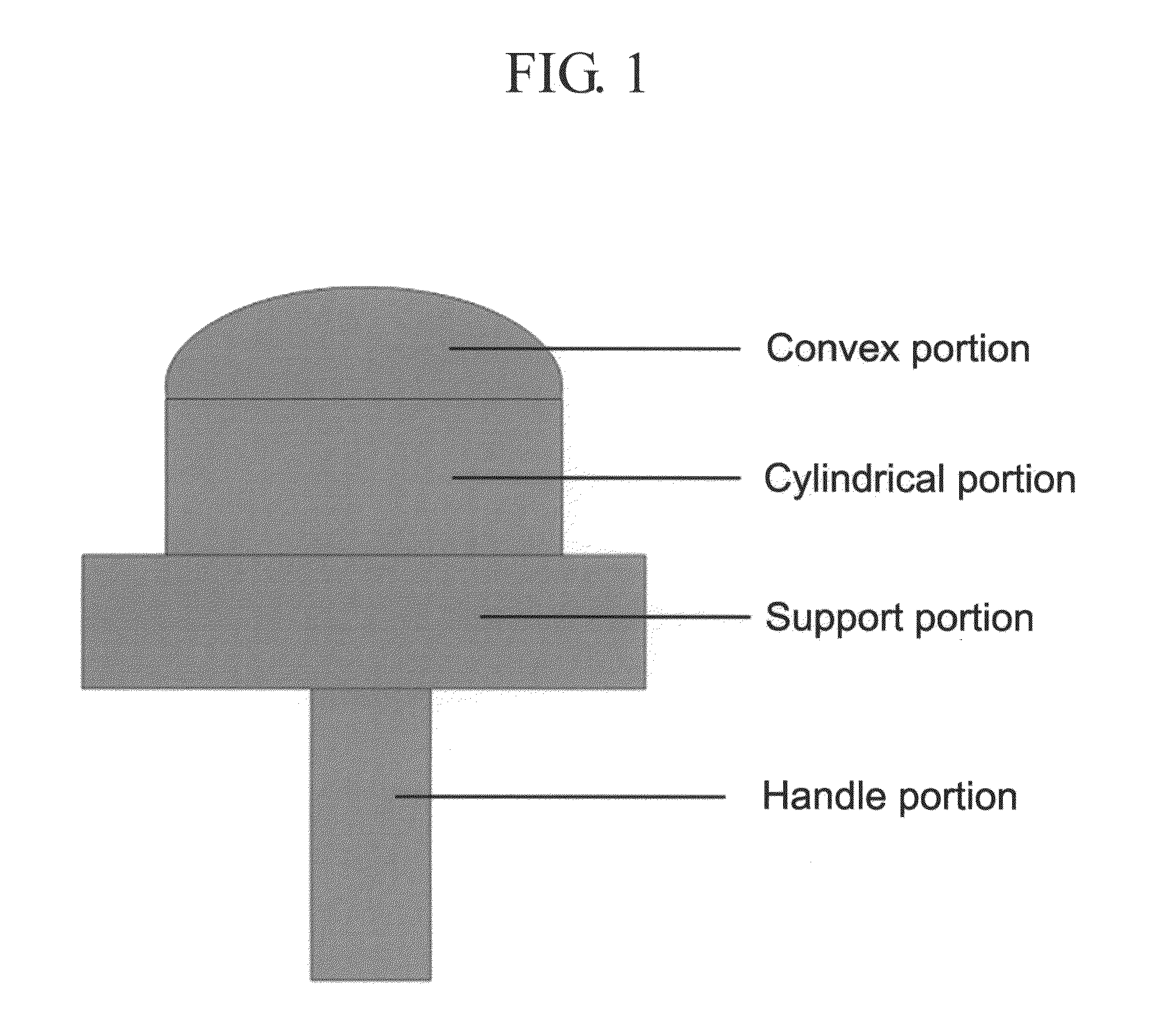
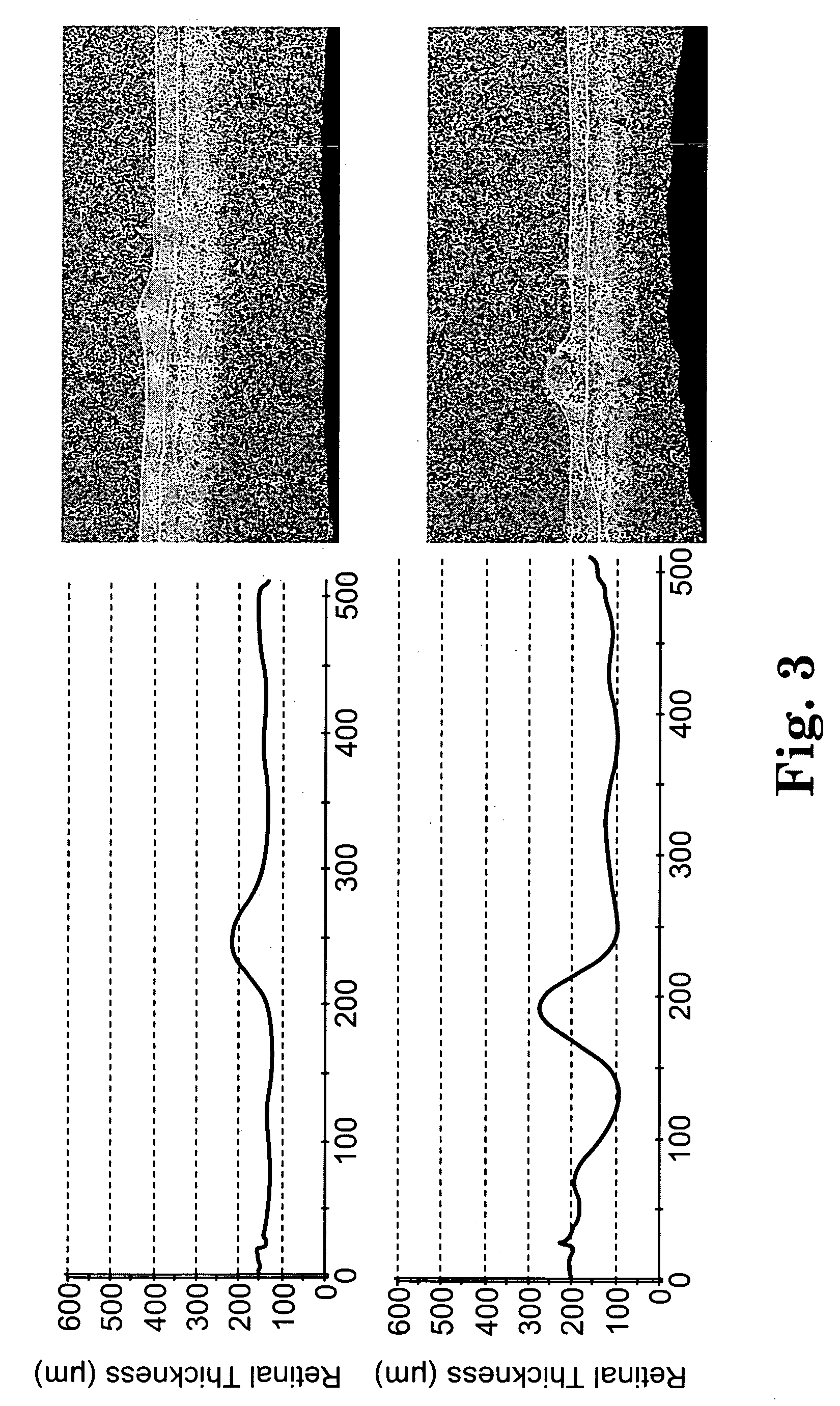
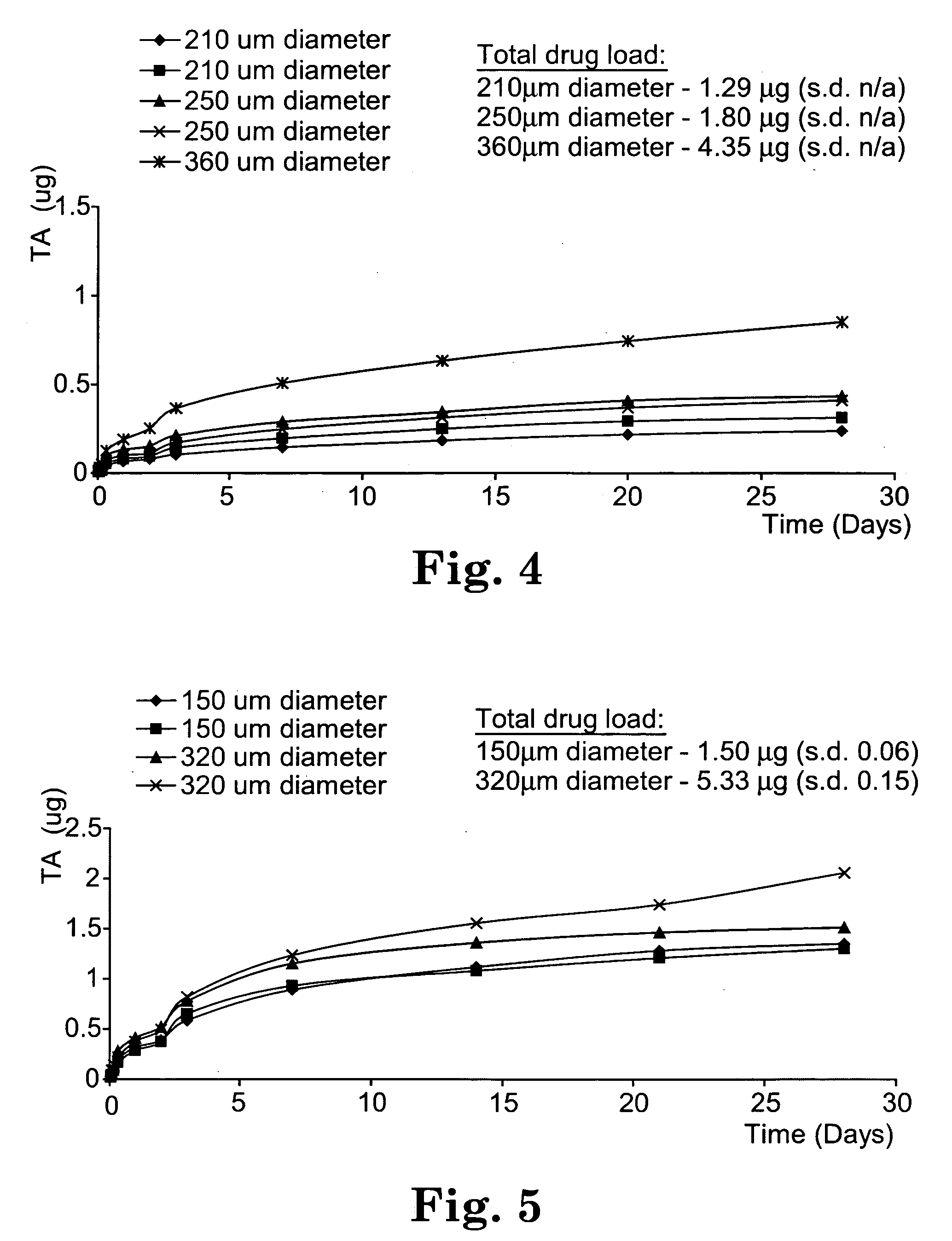
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
317 results about "Therapy eye" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
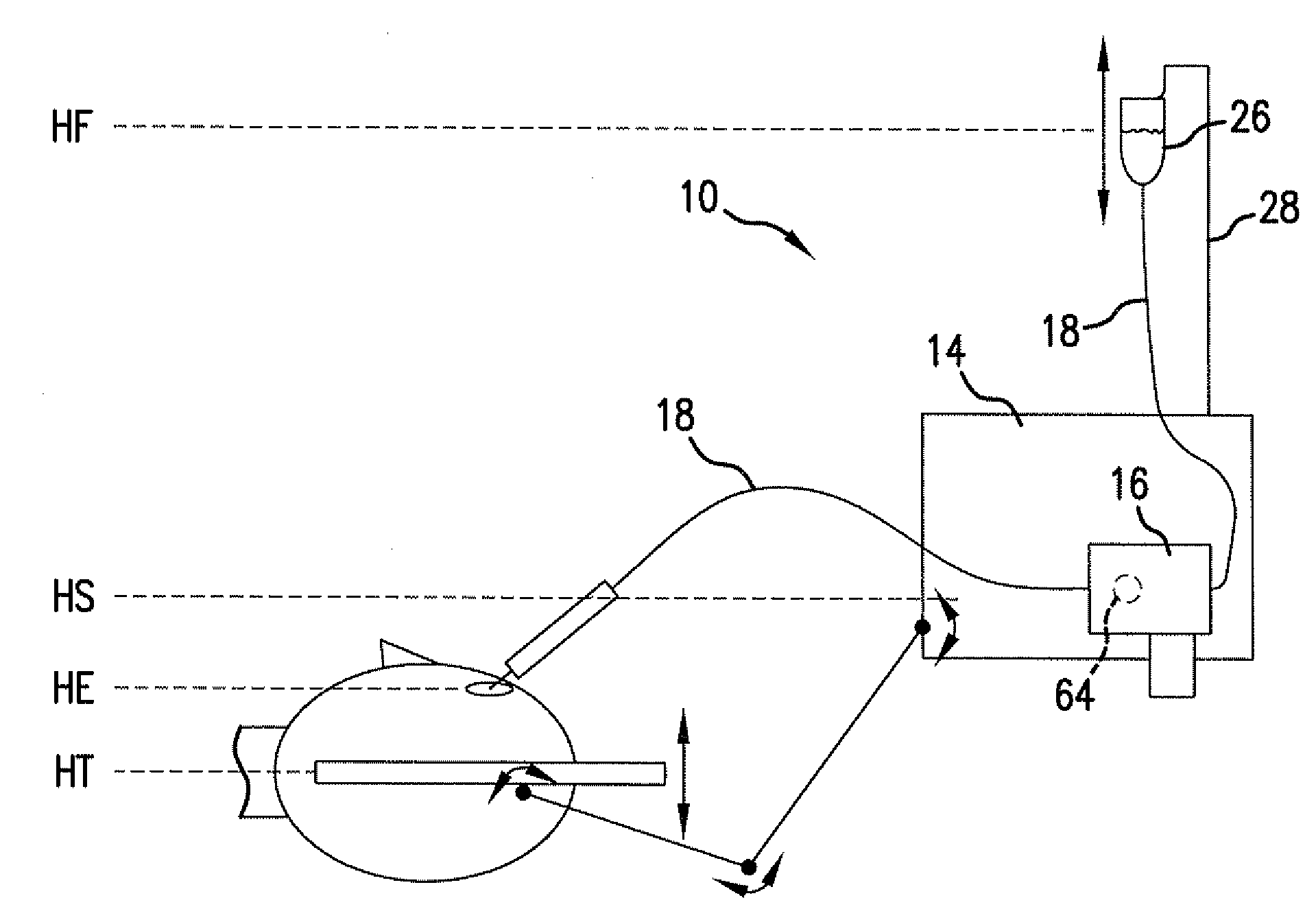
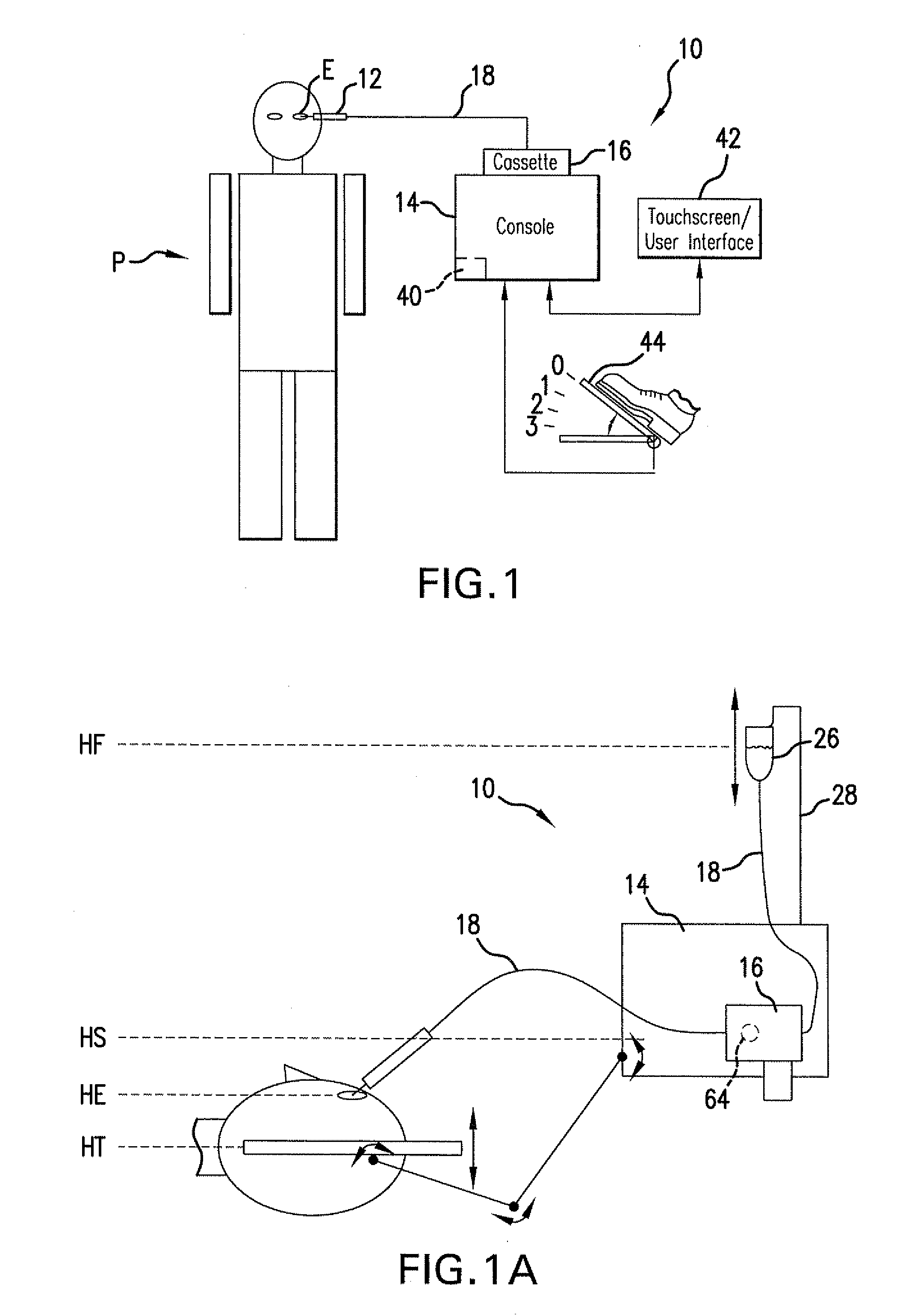
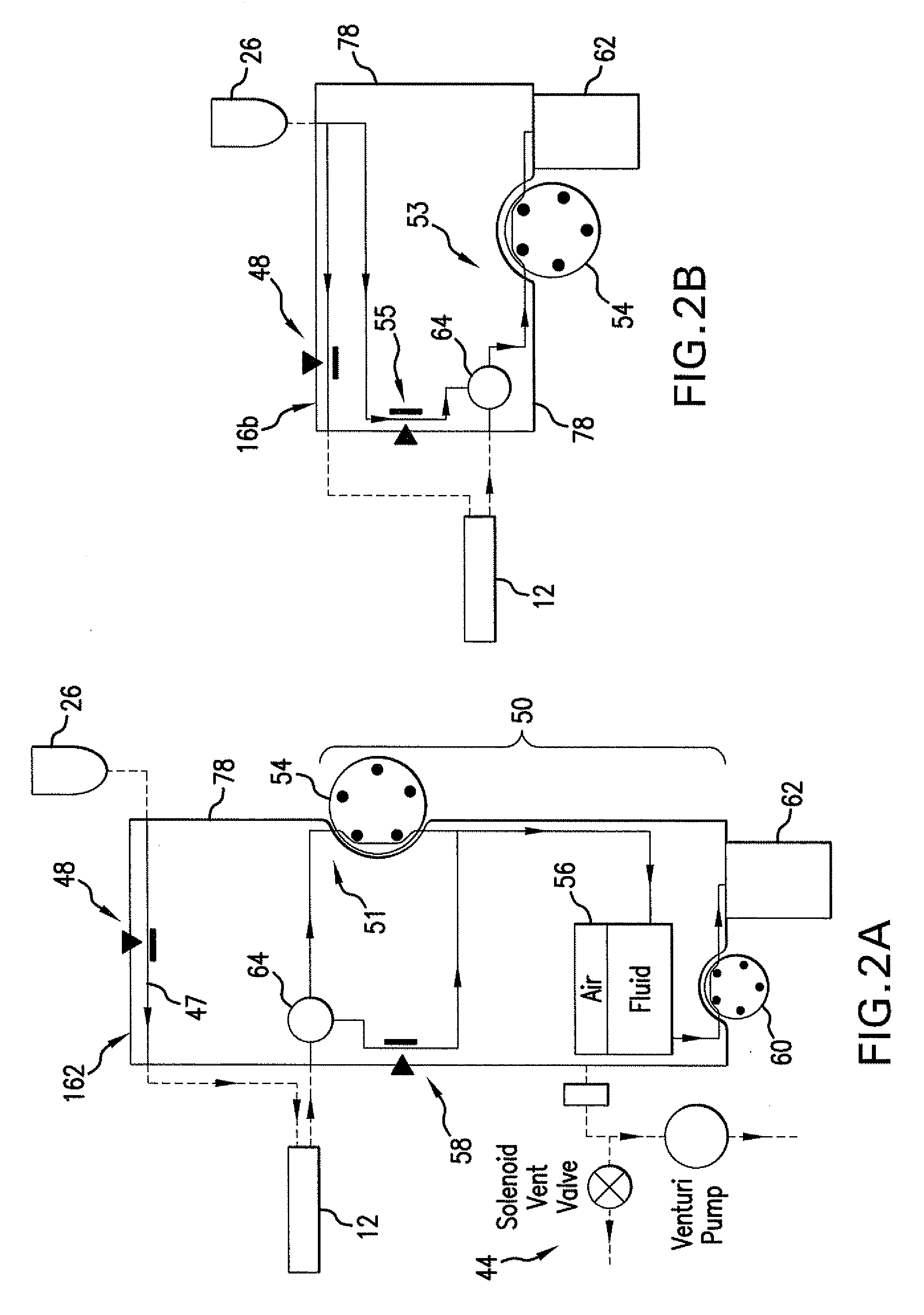
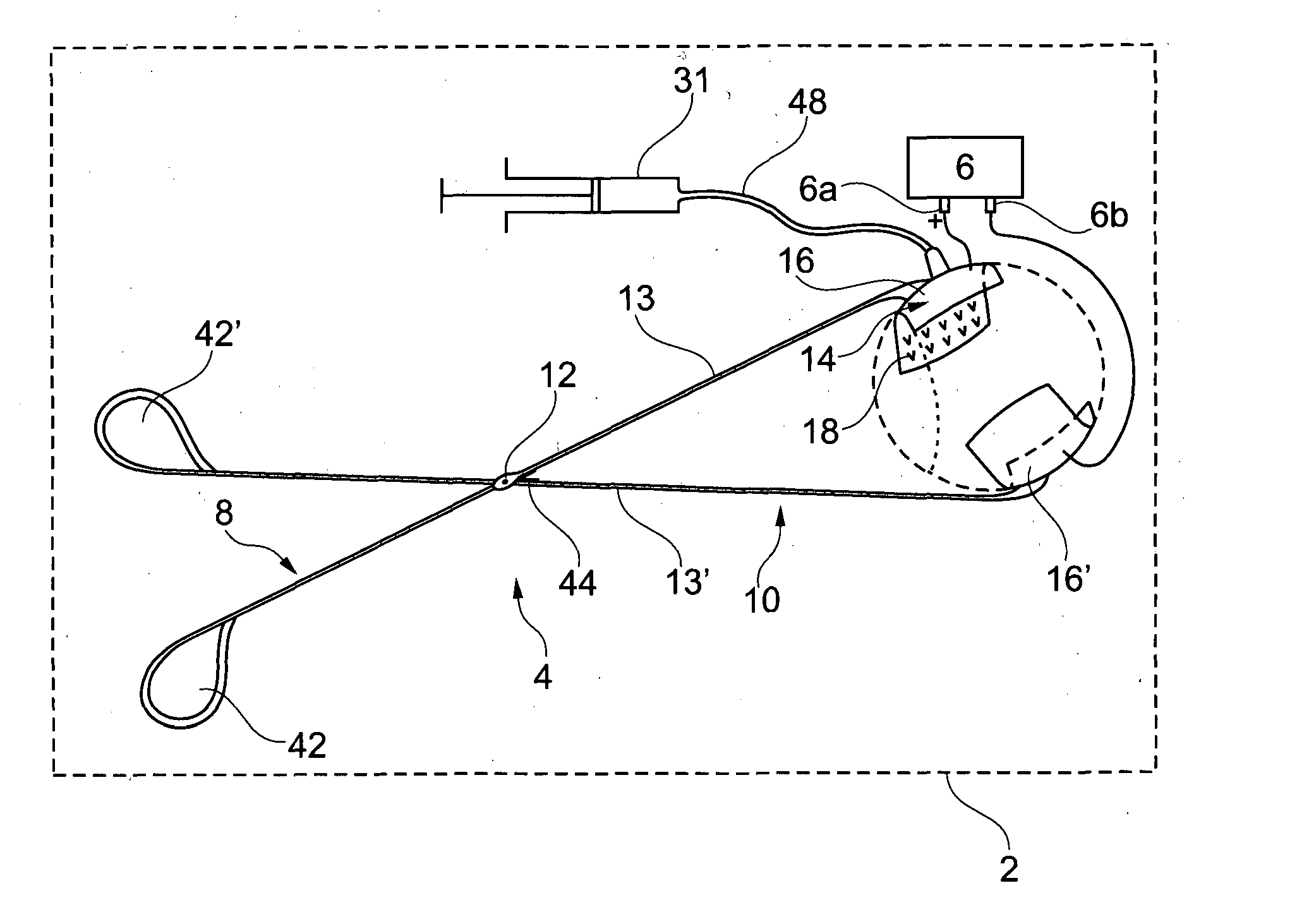
Reversible peristaltic pump and other structures for reflux in eye surgery
Devices, systems, and methods for treatment of an eye alter aspiration flow from the eye in response to an occlusion of the aspiration conduit pathway. Where aspiration is drawn from the eye using a volumetric pump, the pump can be reversed so as to induce fluid reflux from the aspiration conduit pathway into the eye to help clear the occlusion. The pump may vary the reverse flow in response to sensed aspiration pressure or the like, and the reverse flow may be halted before the pressure within the aspiration conduit pathway adjacent the eye significantly exceeds the irrigation fluid pressure and / or the pressure within the eye. Reflux may alternatively be generated by modulating a vent valve disposed between an irrigation conduit pathway and the aspiration conduit pathway.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Particle beam irradiation system
A system for treating an ocular tumor having a cone, a collimator, a light source and a camera is disclosed. The cone includes a cone input and a cone output and is capable of being mounted and un-mounted to a treatment nozzle. The cone input receives radiation from the treatment nozzle for the treatment of the ocular tumor. The collimator is coupled to the cone output and configured to collimate radiation received by the cone output. This collimated radiation is directed to the patient having the ocular tumor. The light source coupled to the cone. The light source is configured to provide a focusing point of the eye of the patient. A camera coupled to the cone monitors the position of the eye of the patient.
Owner:SIEMENS AG
Injectable Combination Therapy for Eye Disorders
InactiveUS20090220572A1Compounds screening/testingPowder deliveryLiquid mediumRetinal neovascularization
The present invention provides composition, methods, and articles of manufacture for treating an eye disorder, e.g., a disorder characterized by macular degeneration, choroidal neovascularization, or retinal neovascularization. One method of the invention comprises the step of: administering first and second therapeutic agents to the subject's eye in a single procedure, wherein the first therapeutic agent provides rapid improvement in the condition of the subject's eye and the second therapeutic agent is administered as a sustained release formulation of the second therapeutic agent. For example, the first and second therapeutic agents are administered by intravitreal injection. The first therapeutic agent may be dissolved in a liquid medium located in the syringe and the sustained formulation of the second therapeutic agent may comprise an ocular implant or plurality of particles located in the needle. The therapeutic agents may be selected from the group consisting of angiogenesis inhibitors and complement inhibitors.
Owner:POTENTIA PHARMA INC
Methods of therapeutic treatment of eyes
InactiveUS20100028442A1Reduce stainingIncrease in tear volume and tear film heightBiocideOrganic active ingredientsTherapeutic treatmentIrritation
Provided are electrokinetically-altered aqueous fluids (e.g., gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating an irritation, infection or inflammatory eye condition, comprising administering to, by contacting the eye of a subject in need thereof a therapeutically effective amount of an electrokinetically-altered aqueous fluid. The electrokinetically-altered fluids or therapeutic compositions and methods include electrokinetically-altered ioinic aqueous fluids optionally in combination with other therapeutic agents. Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids) and therapeutic compositions for use in treating eye conditions. Certain embodiments relate to cosmetic and / or therapeutic fluids and / or methods of treatment utilizing the fluids to treat a cosmetic and / or therapeutic symptom related to eye conditions and / or diseases.
Owner:REVALESIO CORP
Pharmaceutical Preparations For And Treatment Of Ocular Surface and Other Disorders
InactiveUS20070280924A1Significant positive effectOrganic active ingredientsBiocidePharmaceutical formulationDisease
A pharmaceutical preparation suitable for use in the eye, which comprises: (i) a pharmaceutically acceptable carrier suitable for use in the eye; (ii) one or more ingredients selected from factors and agents that promote any one or more of survival, health, cell attachment and normal differentiation of ocular surface epithelial cells and optionally factors and agents to prevent squamous metaplasia; (iii) one or more agents capable of altering the fluid properties of a tear film including at least one agent capable of establishing and / or maintaining a stable tear film and optionally one or more agents selected from opthalmological lubricating agents, viscosity enhancing agents and agents capable of reducing tear film evaporation; the factors and agents in component (ii) and (iii) being synthetic or recombinant or licensed for pharmaceutical use.
Owner:INST OF OPTHALMOLOGY OF UNIV COLLEGE LONDON
Method for preparing contact lens-shaped amniotic dressing
The present invention relates to a method for preparing a contact lens-shaped amniotic dressing and a contact lens-shaped amniotic dressing prepared therefrom for treating ocular surface diseases, which does not require the use of sutures or an adhesion material. The inventive contact lens-shaped amniotic dressing is capable of solving the problems associated with suturing an amniotic membrane, e.g., highly delicate surgical techniques of suturing, long surgery time, stitch abscess, granuloma formation, tissue necrosis, and discomfort of patients; and the problems associated with the use of a support, e.g., the elimination of the support by eye blinking, breaking of the support, and discomfort.
Owner:SK BIOLAND CO LTD
Sustained release implants and methods for subretinal delivery of bioactive agents to treat or prevent retinal disease
InactiveUS20060257451A1Inhibit progressQuantity maximizationSenses disorderEye implantsChoroid membraneOphthalmology
The invention relates to sustained release implants and to methods for treating eyes, particularly the eyes of mammals having eye disorders or diseases. By using the implants and methods described herein, the delivery of the one or more bioactive agents can be localized at a desired treatment site, particularly the choroid and the retina.
Owner:SURMODICS INC
Drug eluting ocular implant
ActiveUS20130289467A1Limited abilityLimits treatment-associated side-effectsEye surgeryMedical applicatorsDrug deliveryDrug
Disclosed herein are drug delivery devices and methods for the treatment of ocular disorders requiring targeted and controlled administration of a drug to an interior portion of the eye for reduction or prevention of symptoms of the disorder. The devices are capable of controlled release of one or more drugs and may also include structures which allow for treatment of increased intraocular pressure by permitting aqueous humor to flow out of the anterior chamber of the eye through the device.
Owner:DOSE MEDICAL CORP
Ophthalmic compositions and methods for treating eyes
ActiveUS20060106104A1Harm reductionEasy and cost-effective to manufactureBiocideSenses disorderOcular surfaceAdverse effect
Ophthalmic compositions including compatible solute components and / or polyanionic components are useful in treating eyes, for example, to relieve dry eye syndrome, to protect the eyes against hypertonic insult and / or the adverse effects of cationic species on the ocular surfaces of eyes and / or to facilitate recovery from eye surgery.
Owner:ALLERGAN INC
Composition for the treatment and/or prevention of macular degeneration, method for its manufacture, and its use for treating the eye
Negatively charged phospholipids, as well as compositions including negatively charged phospholipids and possibly carotenoids and / or antioxidants, for treating the eye are disclosed. In a preferred embodiment, a composition comprising at least one negatively charged phospholipid except cardiolipin is used to treat age-related macular degeneration. Methods for producing the negatively charged phospholipids, as well as methods for producing the compositions including negatively charged phospholipids and possibly carotenoids and / or antioxidants for treating age-related macular degeneration, are also disclosed.
Owner:MULTIGENE BIOTECH
Methods and compositions for treating conditions of the eye
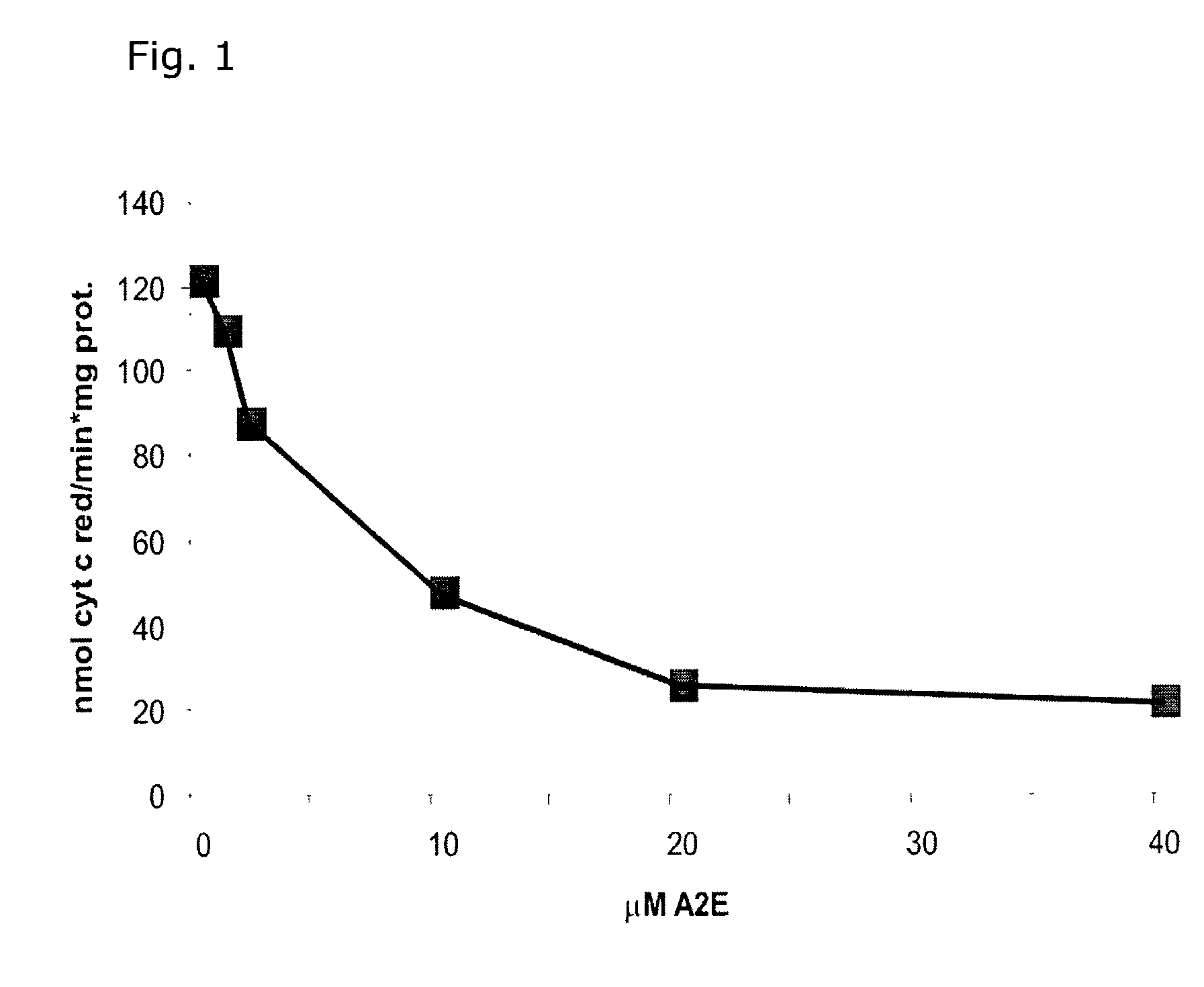
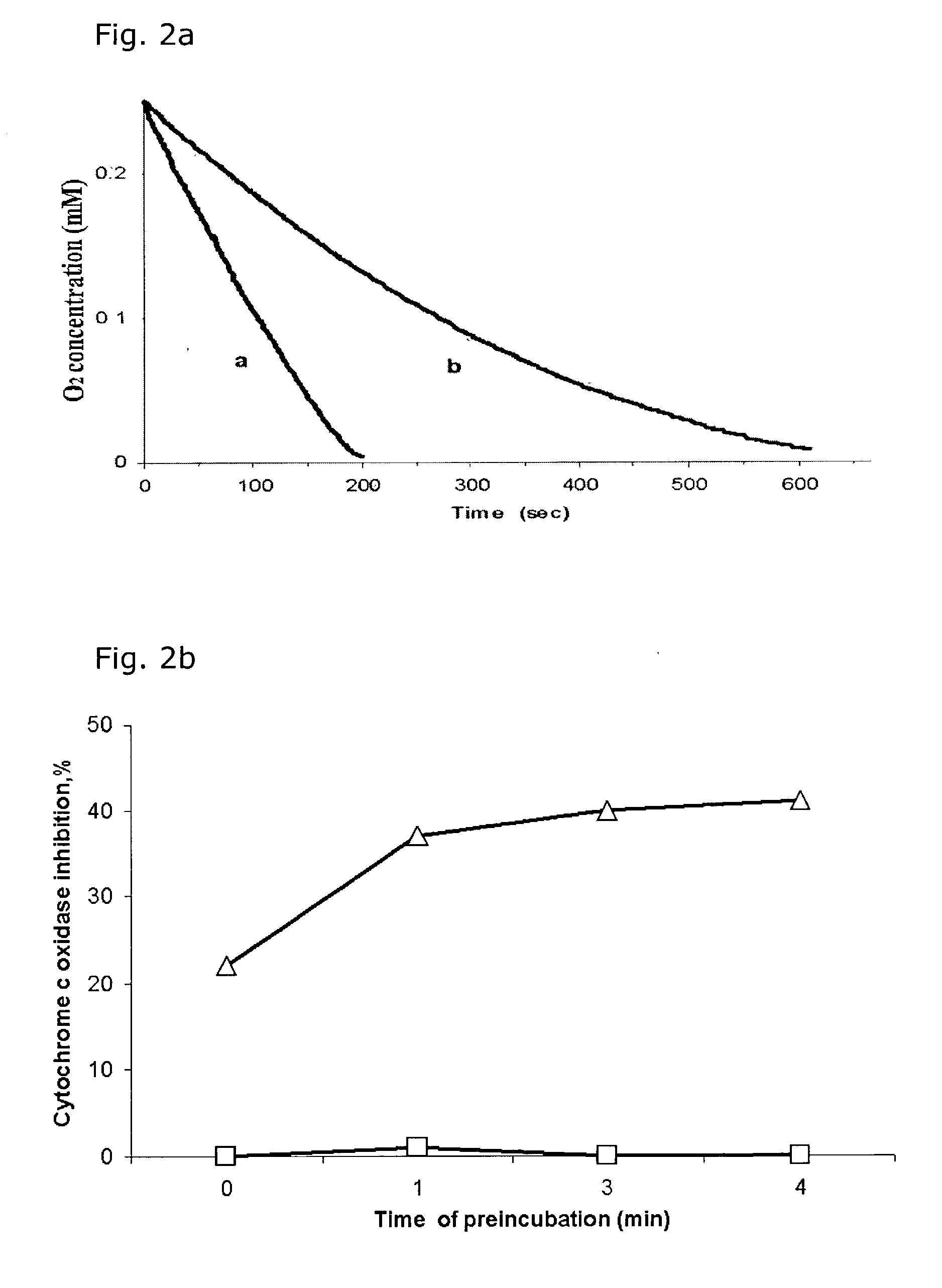
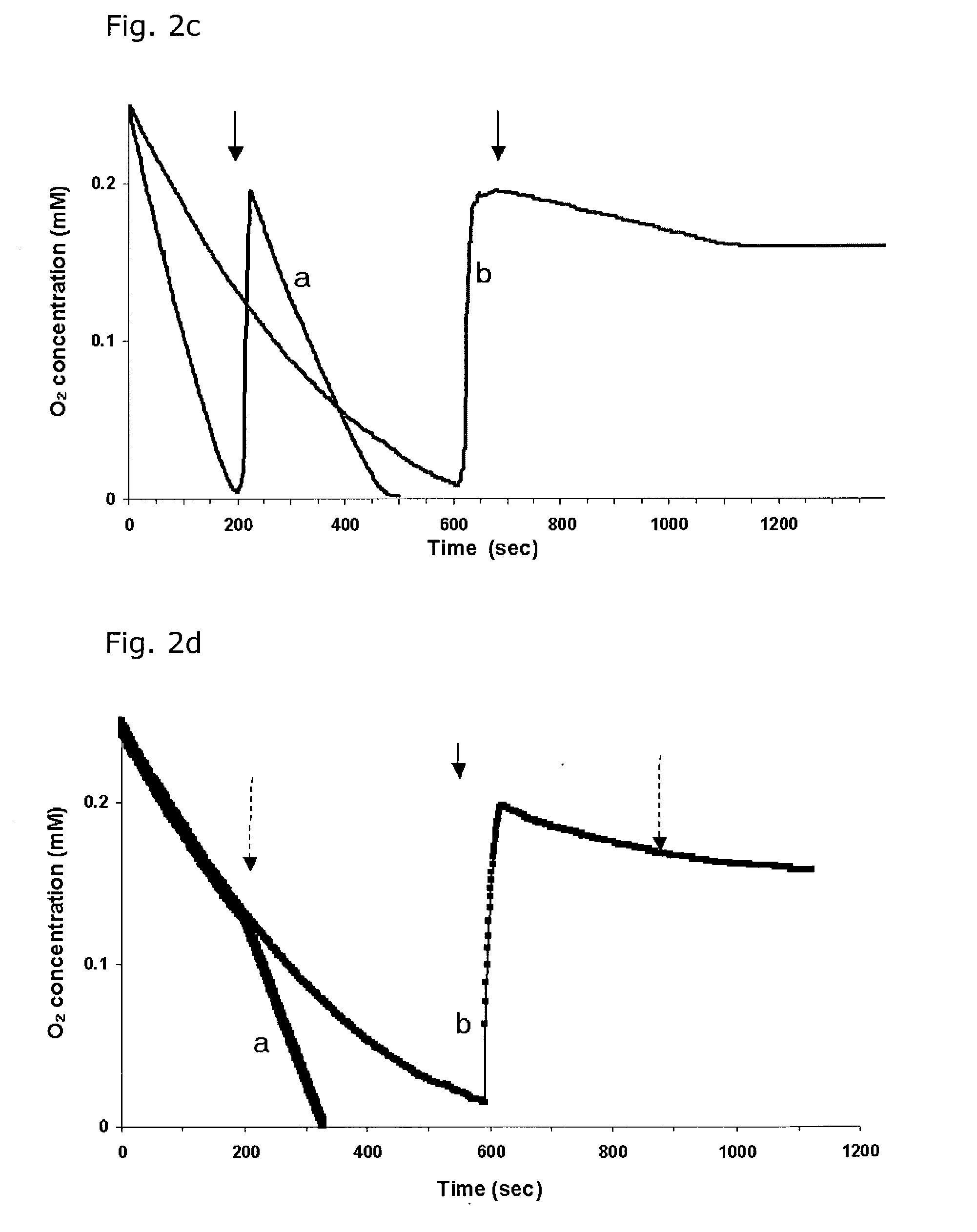
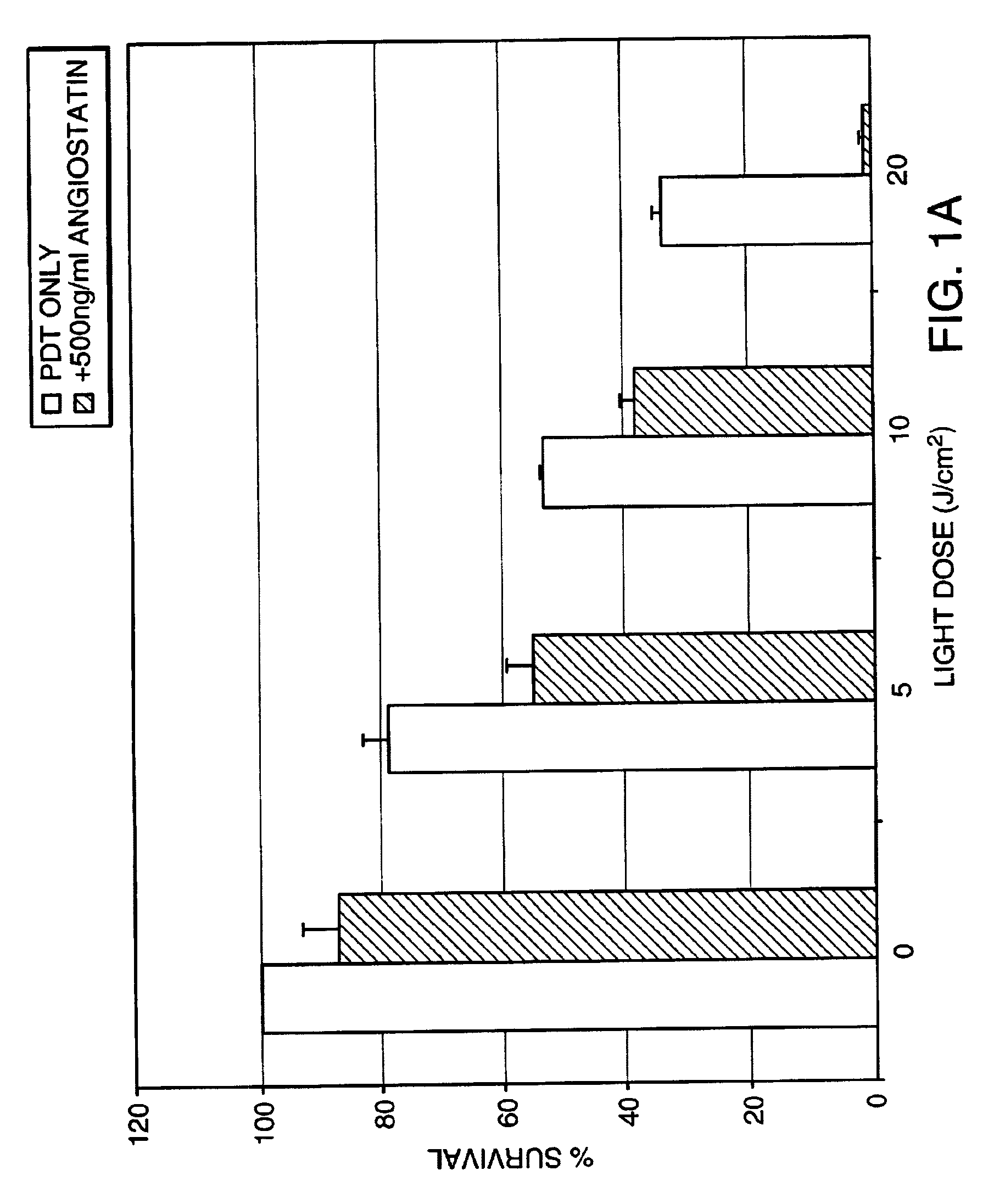
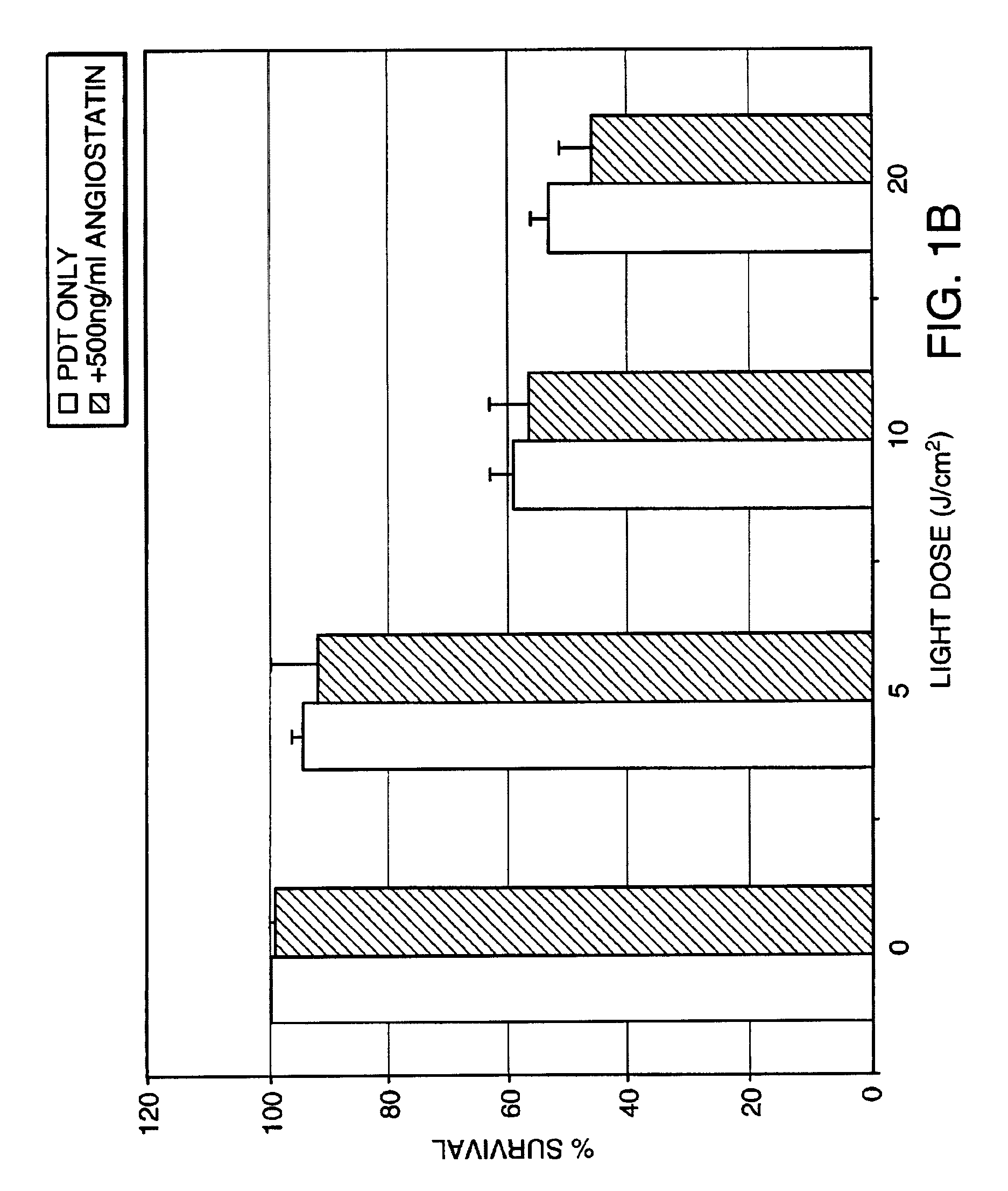
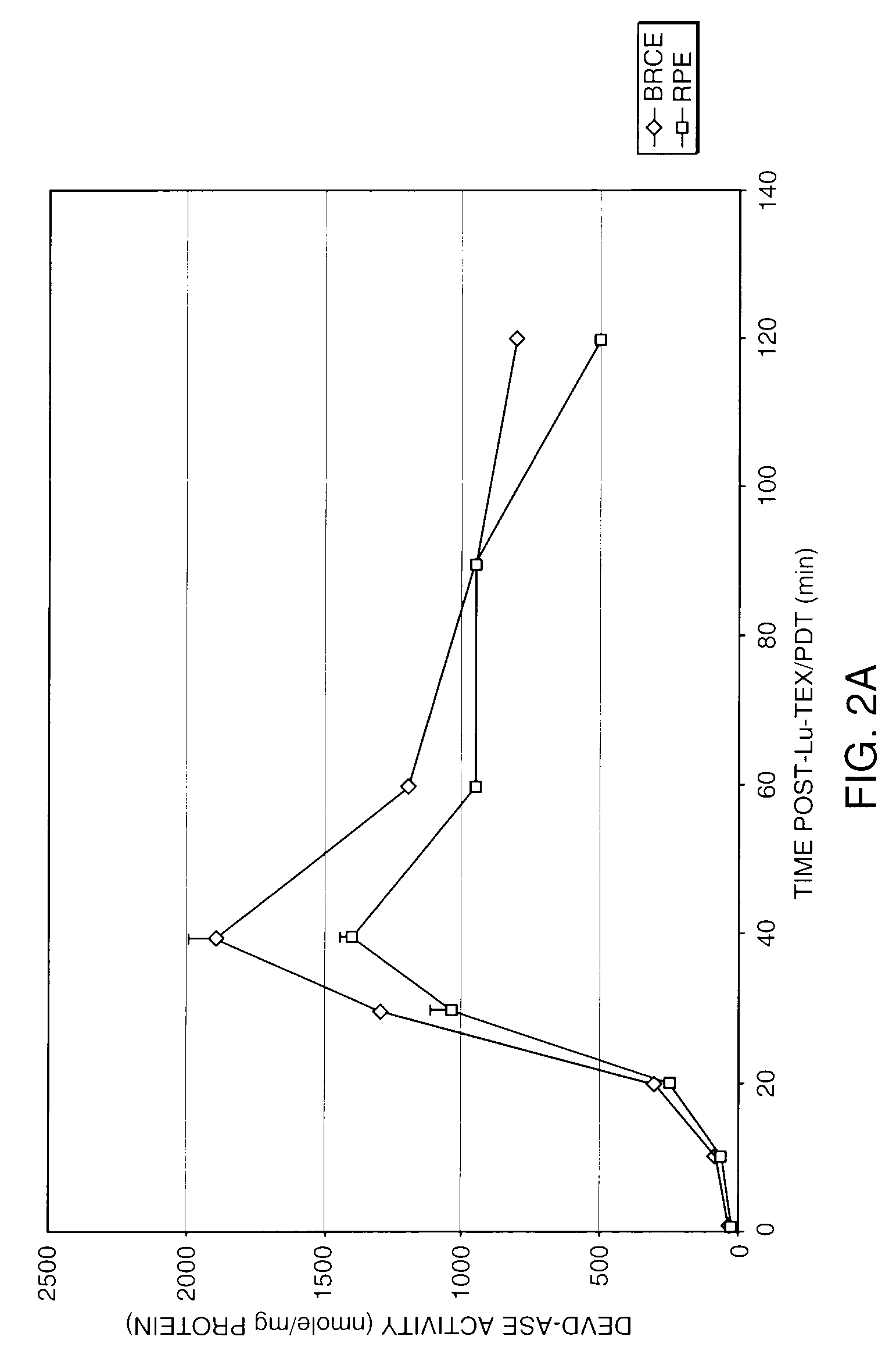
InactiveUS7125542B2Improve treatment efficacyReduced and delayed recurrenceUltrasonic/sonic/infrasonic diagnosticsBiocideAnti angiogenesisPhotosensitizer
Provided are methods and compositions for the photodynamic therapy (PDT) of ocular conditions characterized by the presence of unwanted choroidal neovasculature, for example, neovascular age-related macular degeneration. The selectivity and sensitivity of the PDT method can be enhanced by combining the PDT with an anti-angiogenesis factor, for example, angiostatin or endostatin, or with an apoptosis-modulating factor. Furthermore, the selectivity and sensitivity of the PDT may be further enhanced by coupling a targeting moiety to the photosensitizer so as to target the photosensitizer to choroidal neovasculature.
Owner:MASSACHUSETTS EYE & EAR INFARY
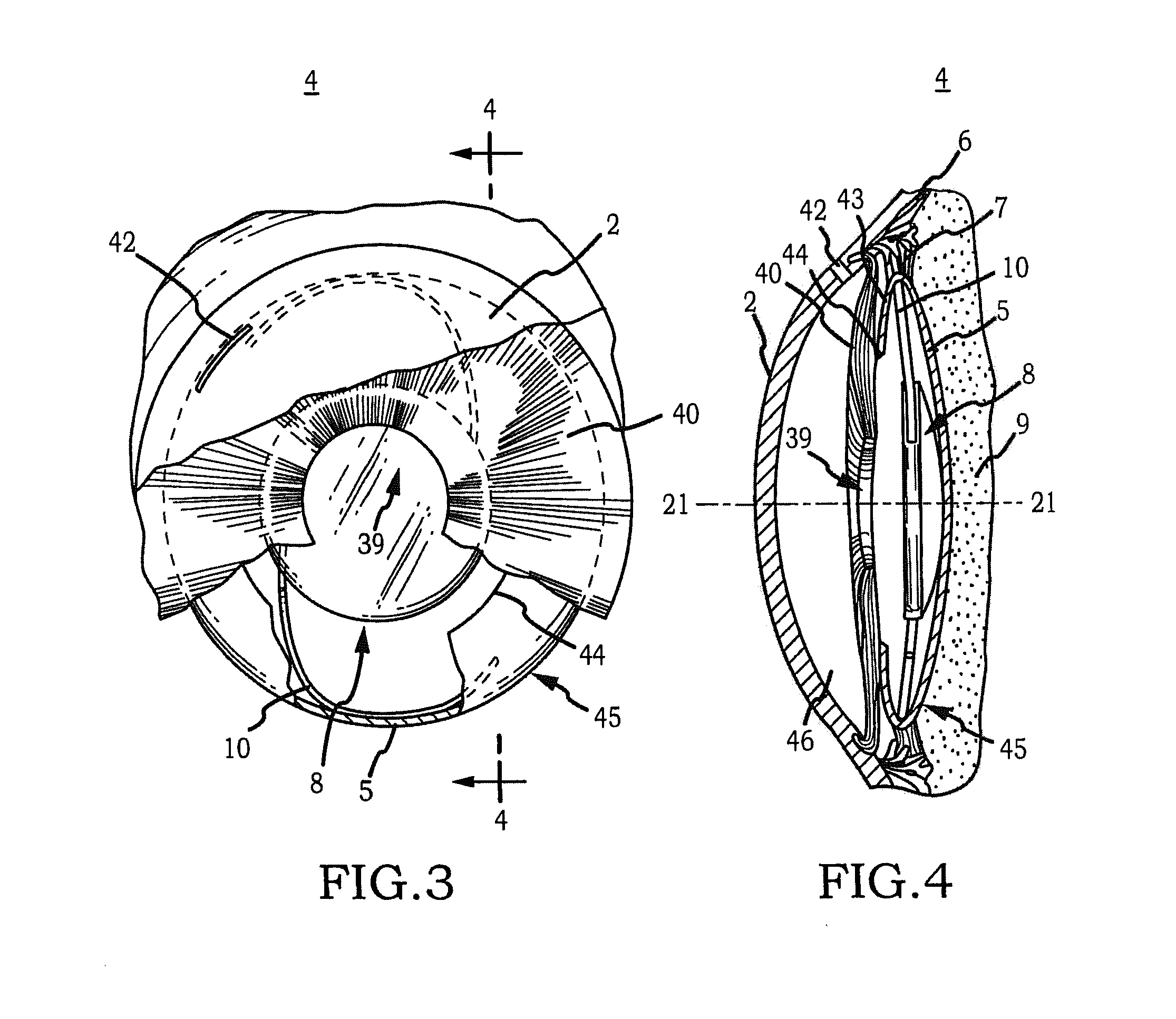
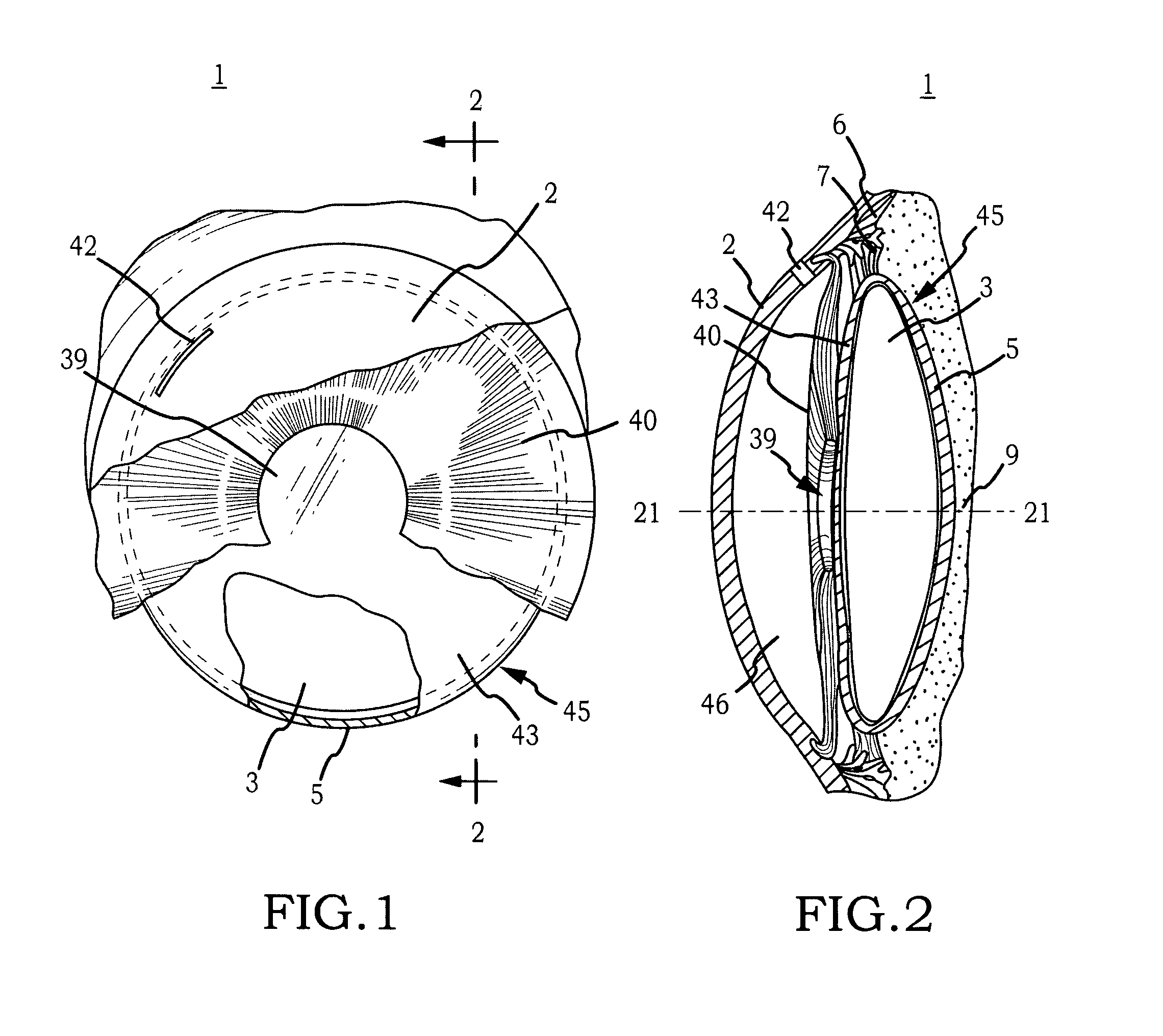
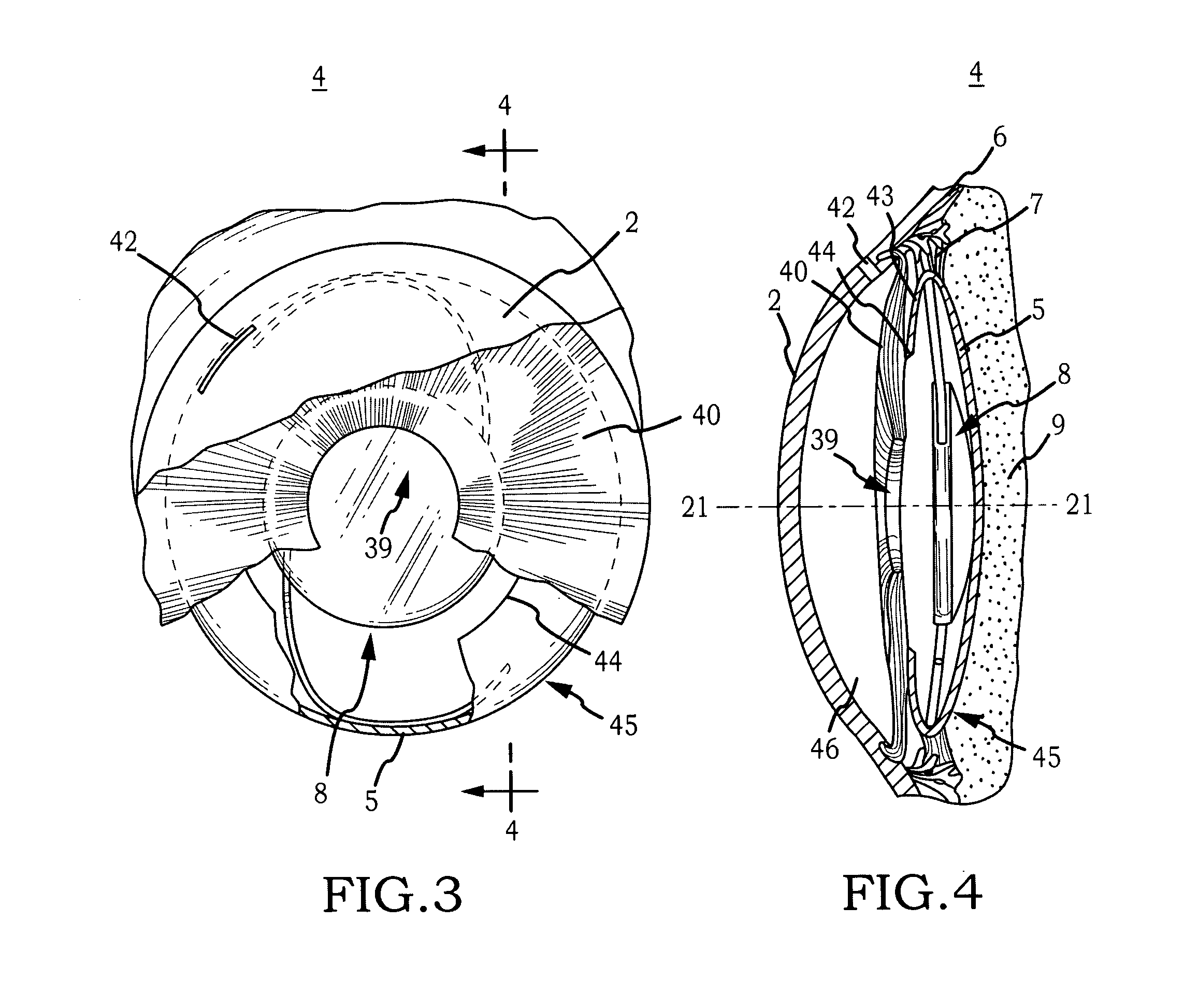
Intraocular Lens Cell Migration Inhibition System
InactiveUS20120232649A1Inhibit migrationReduce posterior capsule opacificationEye treatmentTissue regenerationPosterior capsular opacificationLens epithelial cell
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
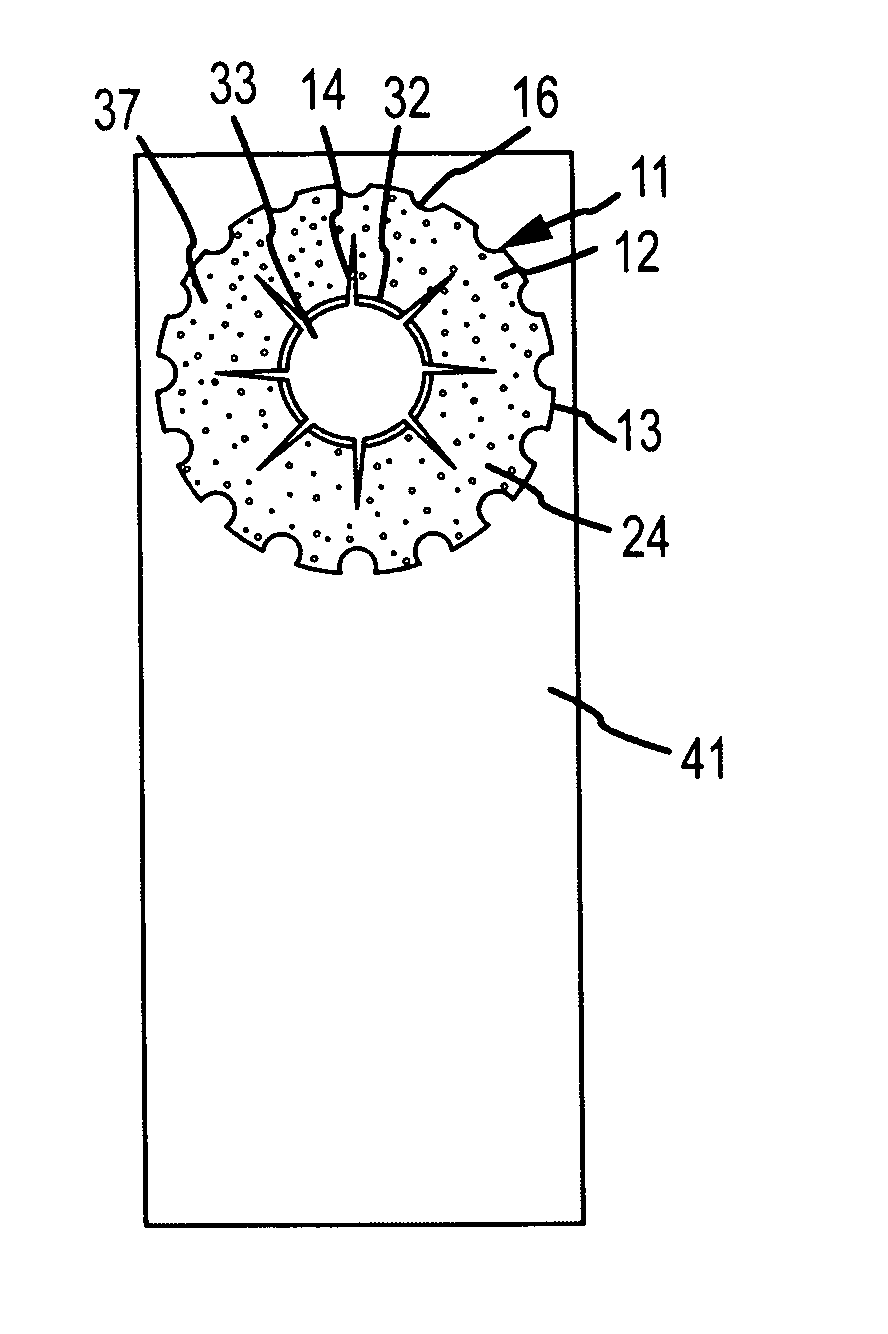
Biocompatible biodegradable intraocular implant system
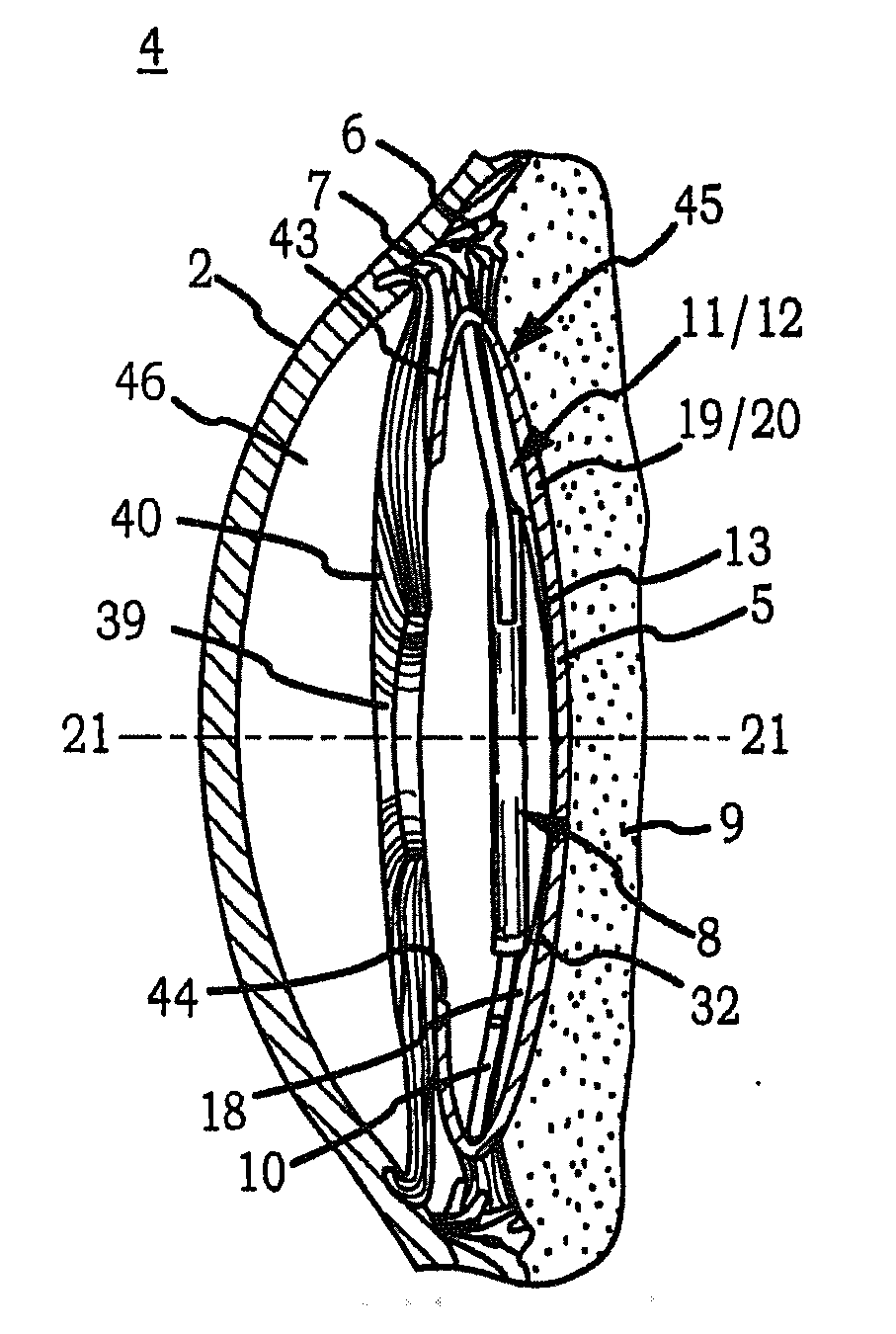
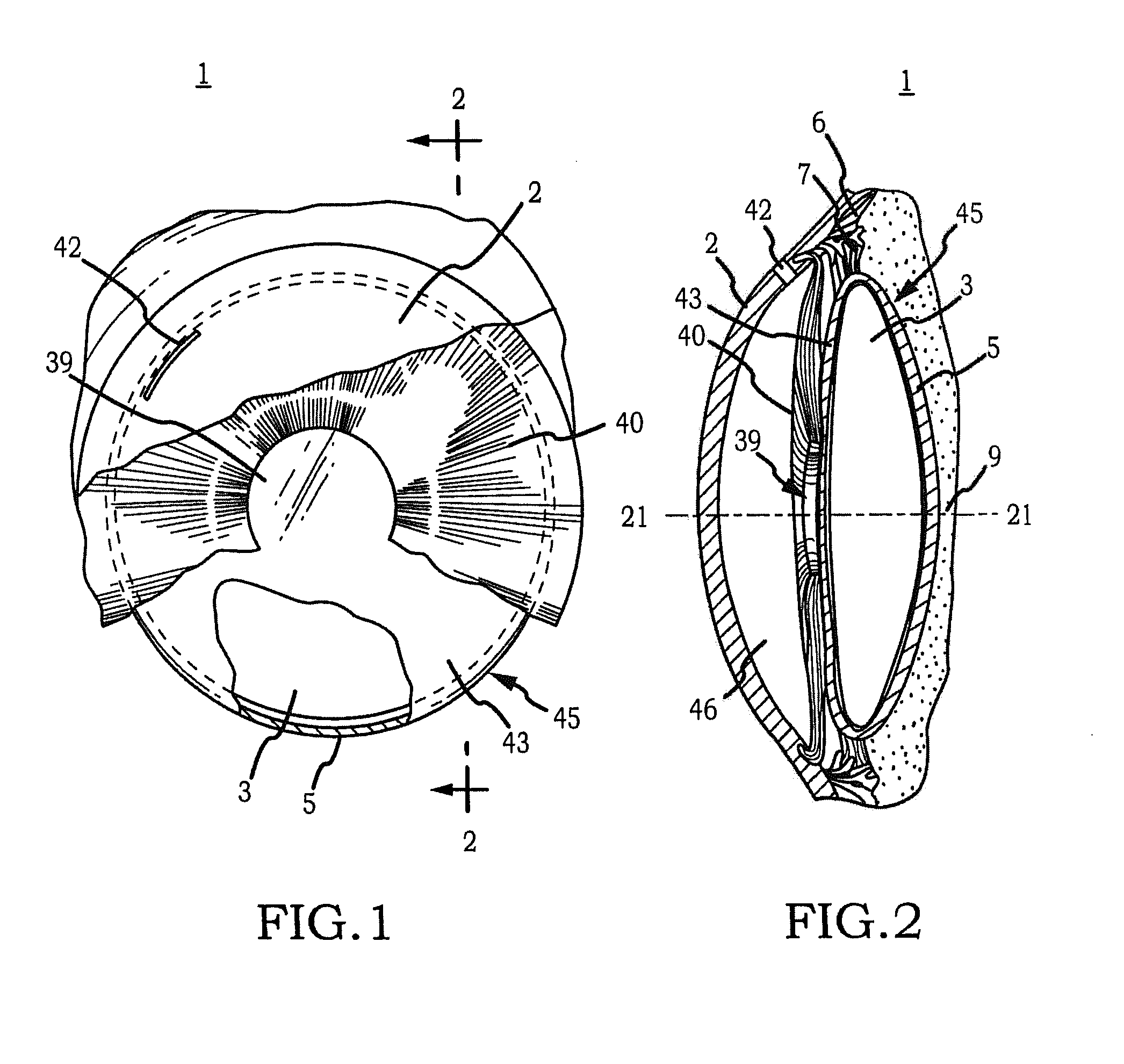
InactiveUS20110230963A1Prevent proliferationPharmaceutical delivery mechanismEye treatmentIntraocular lensActive agent
Generally, an intraocular implant and methods for treating an ocular condition. As to certain embodiments, an intraocular biocompatible biodegradable implant (11) which can provide a biocompatible biodegradable material in the form of a flexible membrane (12) containing an active agent (24) which implanted between an intraocular lens (8) and the surface of the posterior capsule (5) of the eye (1)(4) inhibits migration of residual lens epithelial cells after cataract surgery by providing structural or pharmaceutical barriers to reduce posterior capsule (5) opacification of the eye (1)(4).
Owner:INSIGHT INNOVATIONS
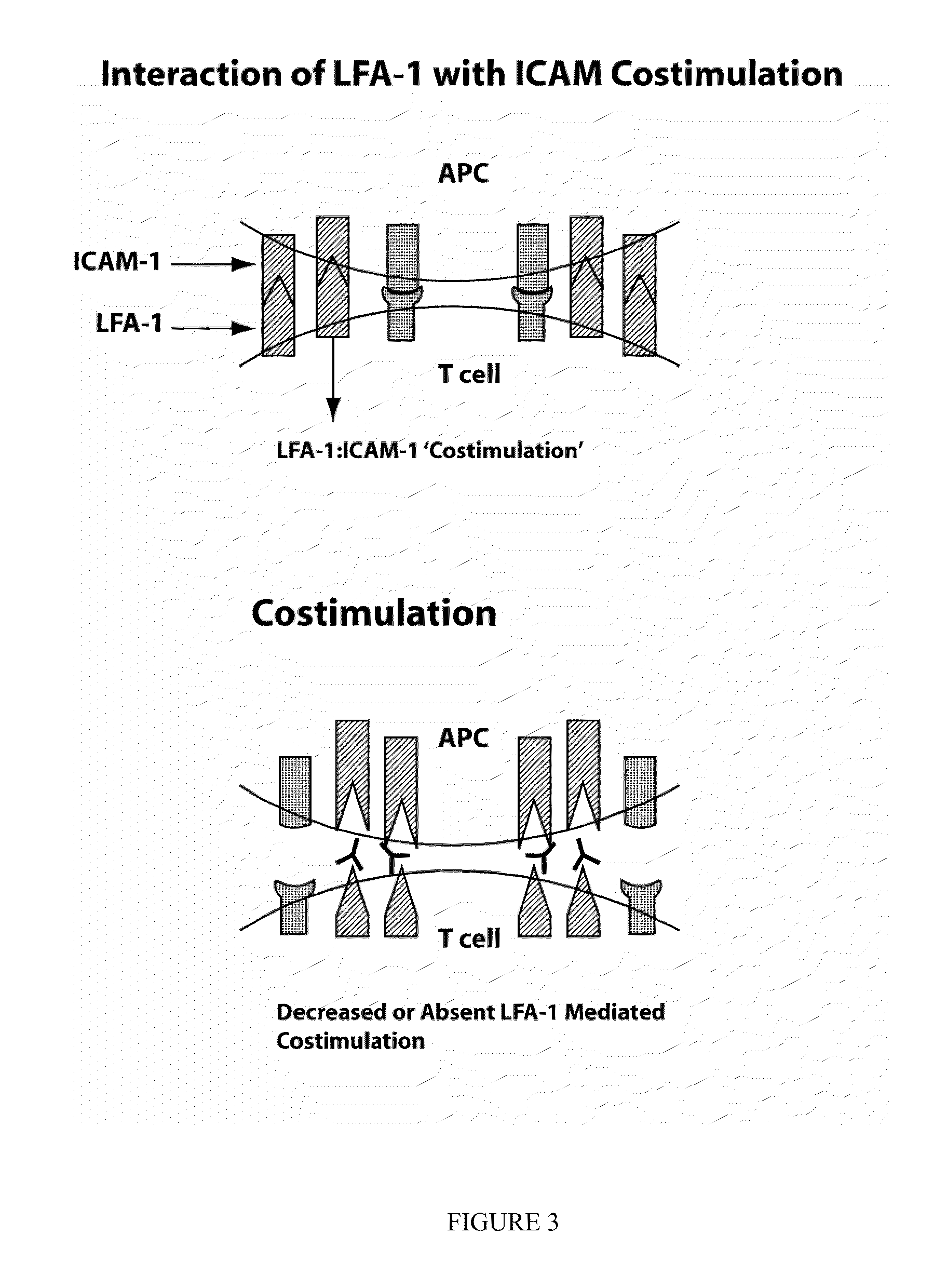
Compositions and methods for treatment of eye disorders
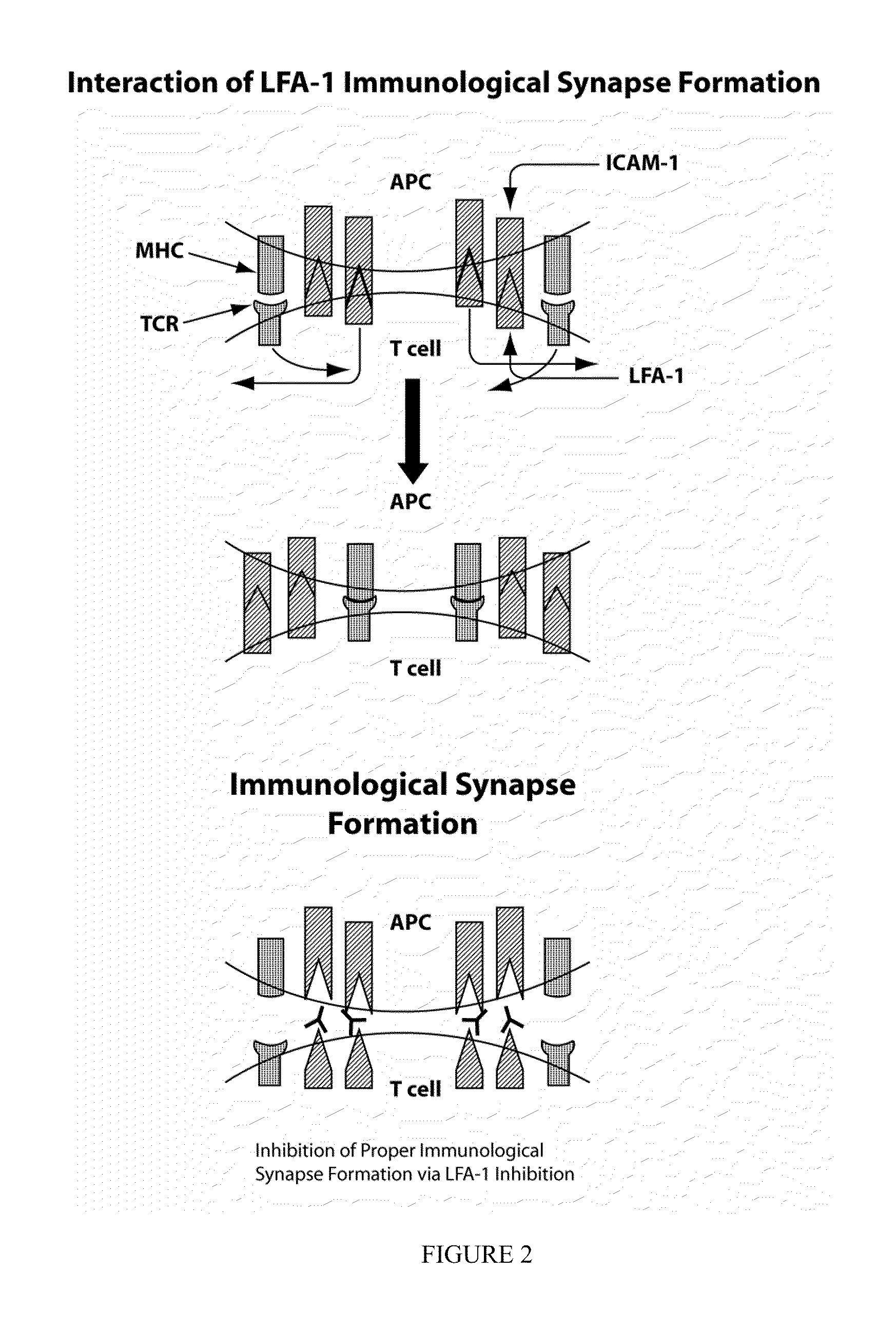
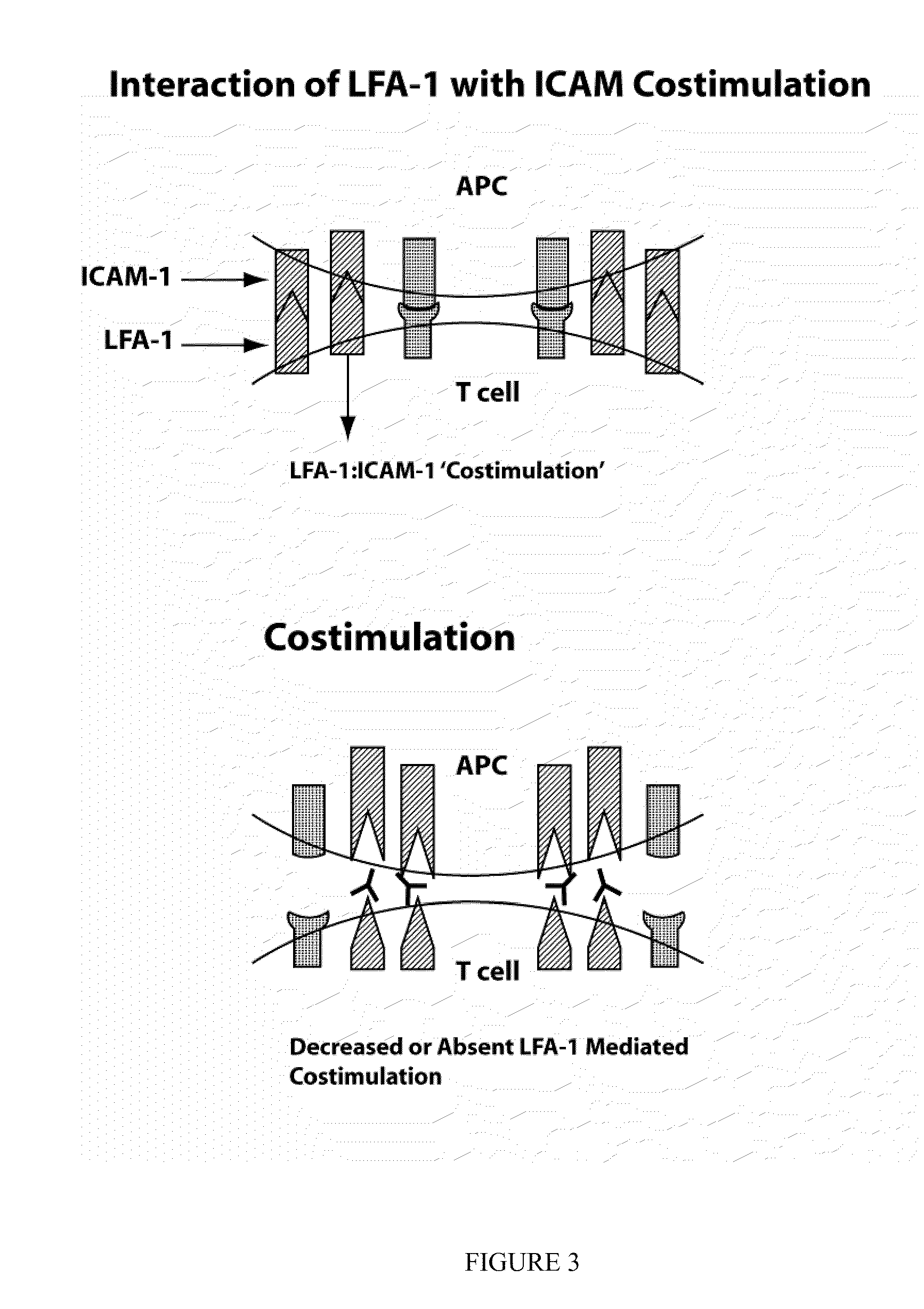
The present invention provides compounds and methods for the treatment of LFA-1 mediated diseases. In particular, LFA-1 antagonists are described herein and these antagonists are used in the treatment of LFA-1 mediated diseases. One aspect of the invention provides for diagnosis of an LFA-1 mediated disease and administration of a LFA-1 antagonist, after the patient is diagnosed with a LFA-1 mediated disease. In some embodiments, the LFA-1 mediated diseases treated are dry eye disorders. Also provided herein are methods for identifying compounds which are LFA-1 antagonists.
Owner:BAUSCH LOMB IRELAND LTD
Use Of A Viscoelastic Composition For Treating Increased Intraocular Pressure
ActiveUS20080058760A1Increased intraocular pressureRapid and cost-effectiveSenses disorderPharmaceutical delivery mechanismFistulaMedical device
A viscoelastic medium is useful for the manufacture of a medicament, such as a medical device, for treatment of increased intraocular pressure in the eye of a human or animal. The medicament is administerable into at least one sclerally penetrating fistula of the eye such that the fistula is filled with the medicament. The medium is also useful in a method of treating increased intraocular pressure in the eye of a human or an animal in need thereof, comprising the step of injecting the viscoelastic medium into at least one sclerally penetrating fistula in the eye such that the fistula is filled with the medium.
Owner:Q MED AB
Compositions and methods for treatment of eye disorders
The present invention provides compounds and methods for the treatment of LFA-1 mediated diseases. In particular, LFA-1 antagonists are described herein and these antagonists are used in the treatment of LFA-1 mediated diseases. One aspect of the invention provides for diagnosis of an LFA-1 mediated disease and administration of a LFA-1 antagonist, after the patient is diagnosed with a LFA-1 mediated disease. In some embodiments, the LFA-1 mediated diseases treated are dry eye disorders. Also provided herein are methods for identifying compounds which are LFA-1 antagonists.
Owner:NOVARTIS PHARM CORP
Under eye cream
Owner:A2 BIOSCI PTE
Method for treating an eye
In a method for treating at least one eye of a patient in need of such treatment with a pulsed electrical stimulation signal, first an individual parameter of the patient is determined, thereafter at least one stimulation parameter of the pulsed electrical stimulation signal is set depending on the at least one individual parameter, and then the pulsed electrical stimulation signal is applied to the at least one eye.
Owner:OKUVISION
Methods and apparatuses for the treatment of glaucoma using visible and infrared ultrashort laser pulses
Owner:BERLIN MICHAEL S
Methods for treating eye conditions
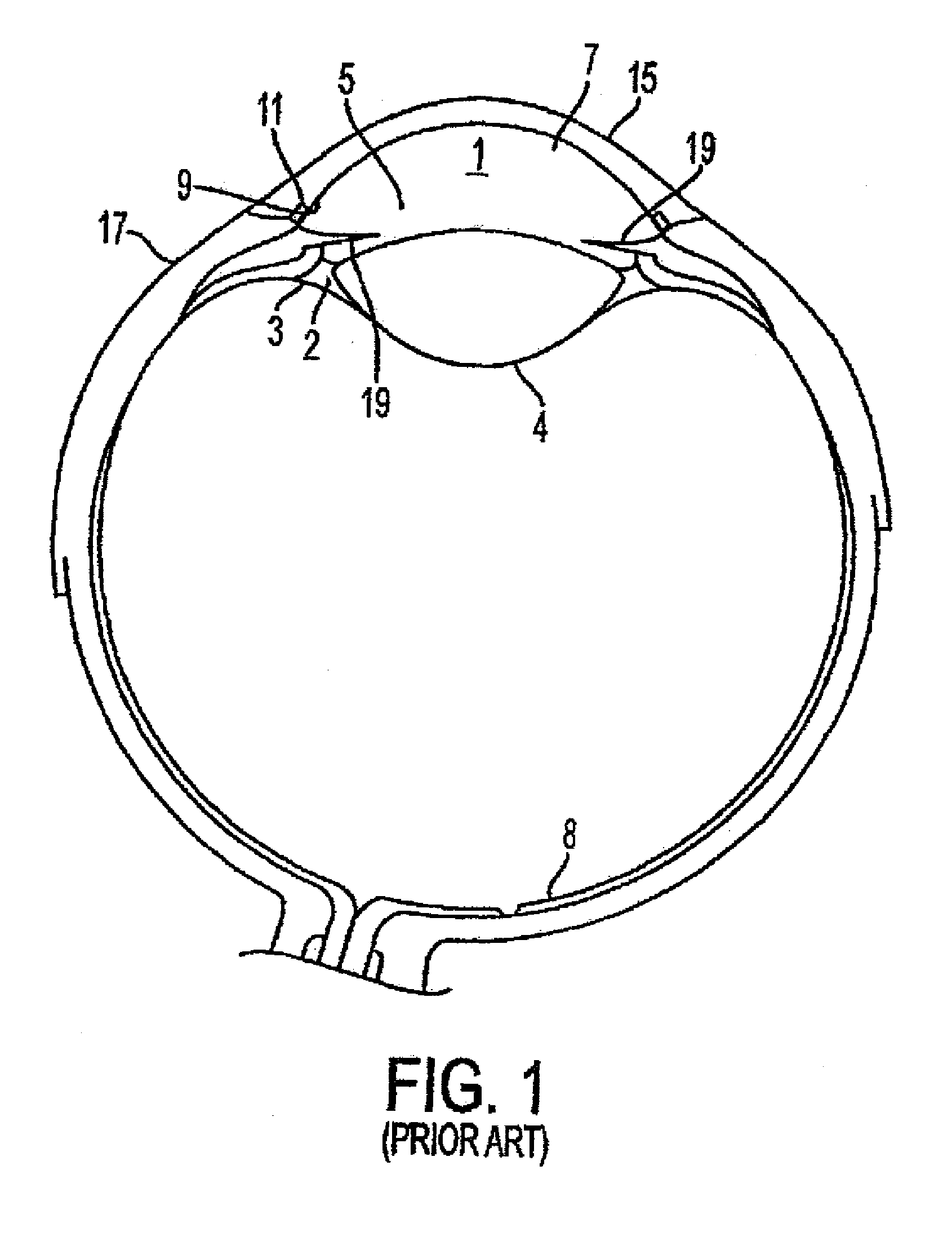
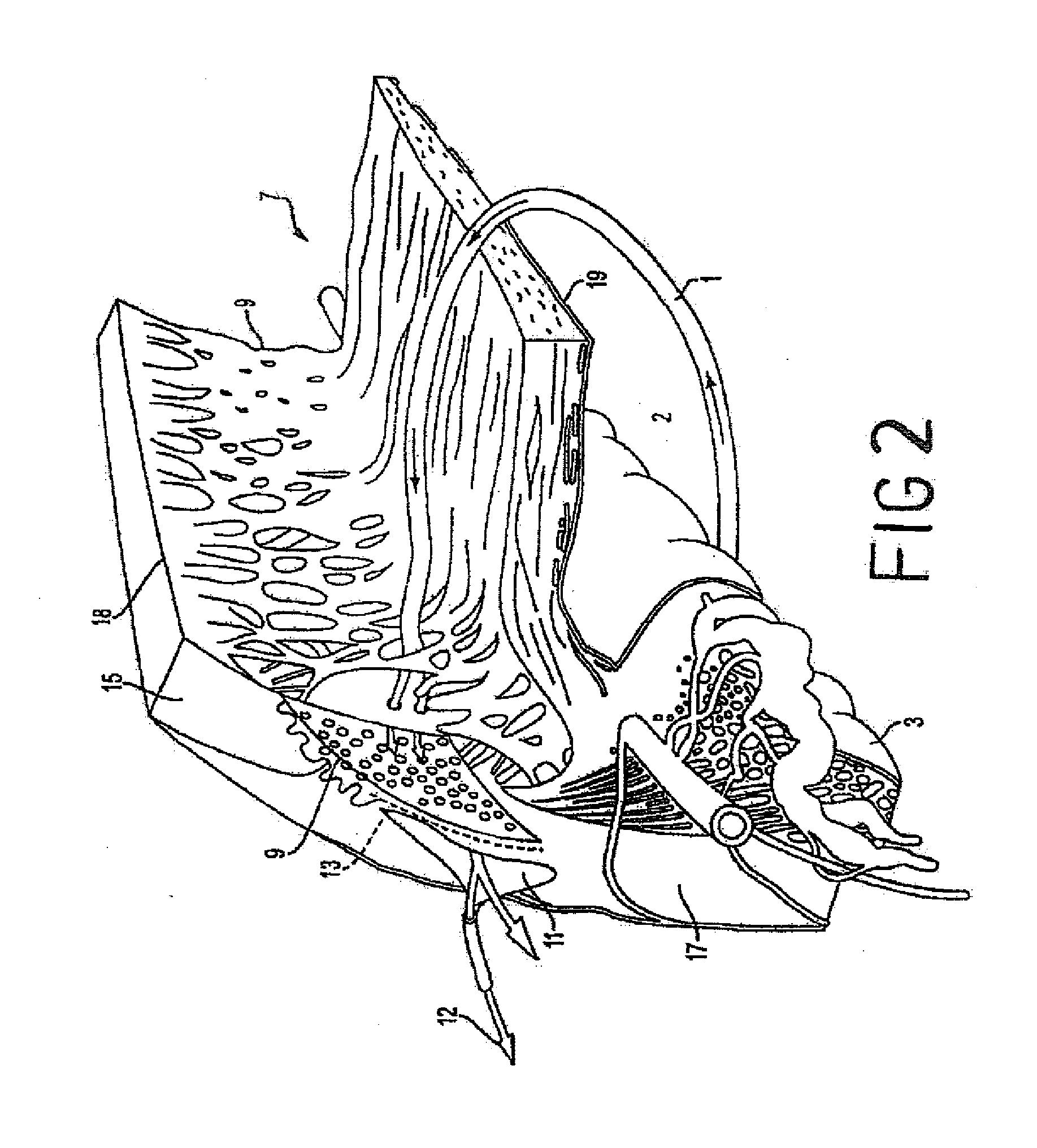
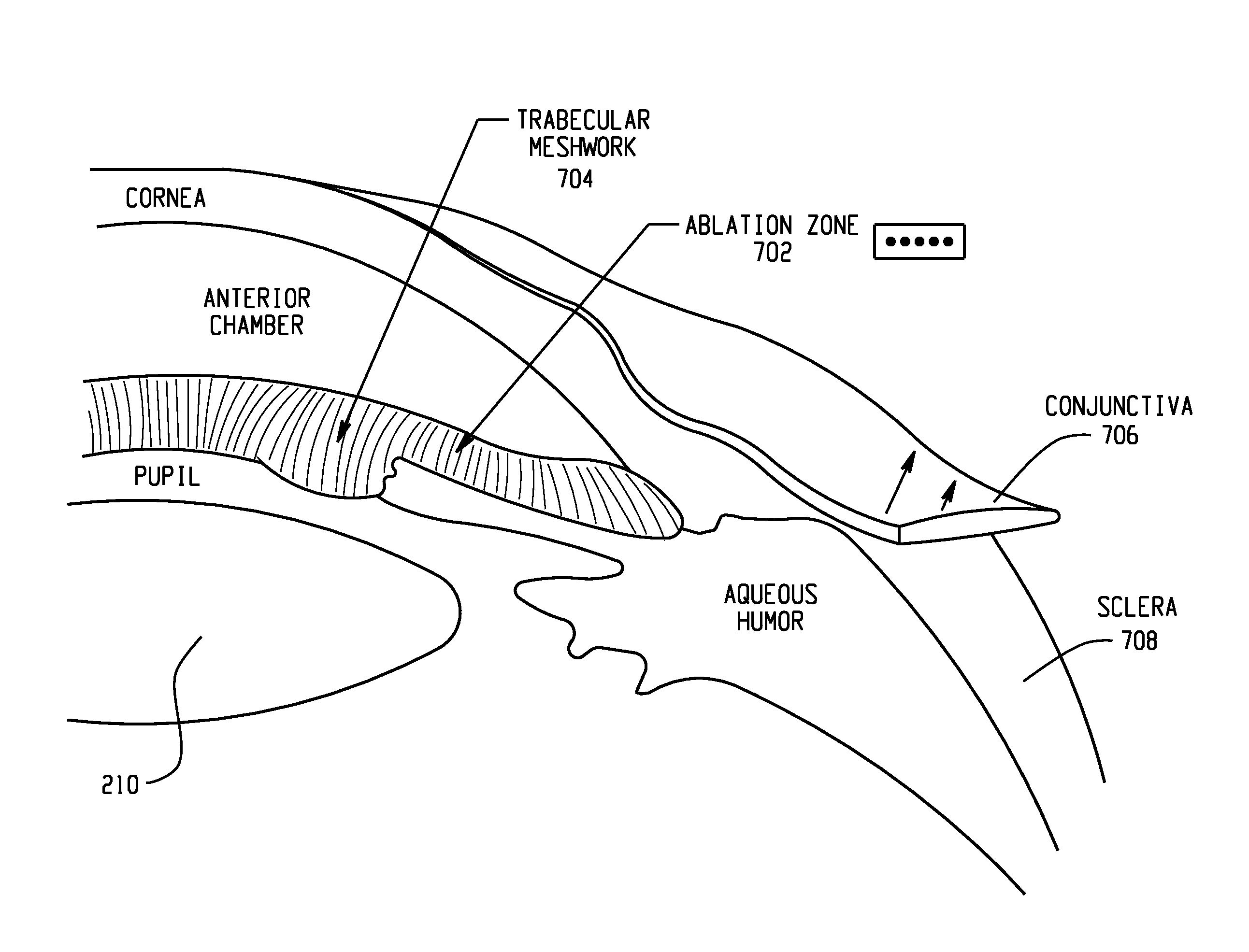
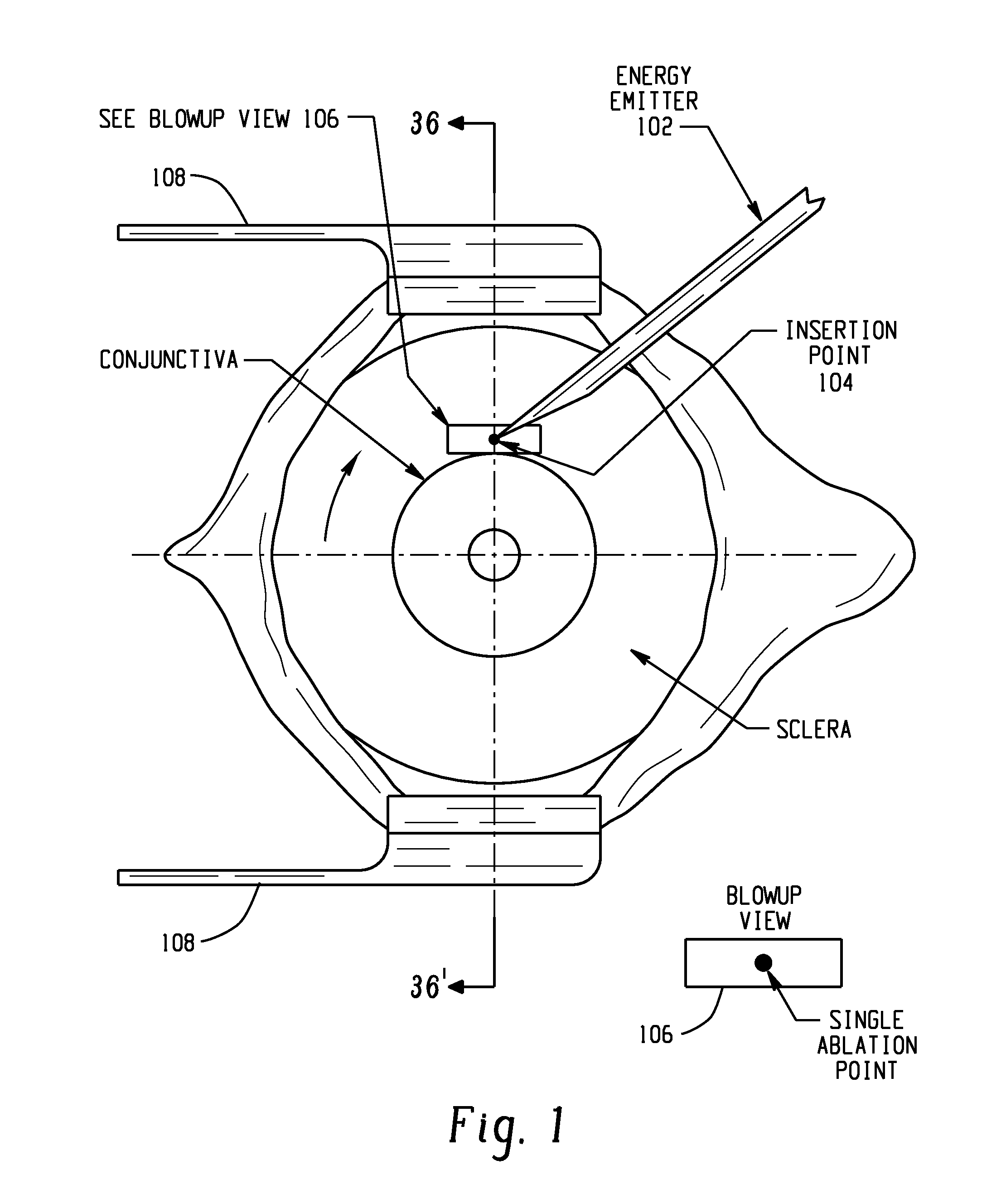
InactiveUS8827990B2Lower eye pressureReduce pressureLaser surgeryDiagnosticsConjunctivaSinus venosus sclerae
Systems and methods are provided for reducing intraocular pressure in an eye. A perpendicular incision is made through a conjunctiva of the eye to access a trabecular meshwork of the eye. Electromagnetic energy is focused through the perpendicular incision to ablate a portion of the trabecular network, where said ablation creates a channel for outflow flow of fluid through a sclera venous sinus to reduce pressure within the eye.
Owner:BIOLASE INC
Ophthalmic and contact lens solutions containing forms of vitamin b
InactiveUS20070104744A1Discomfort to userDegree of reductionOrganic detergent compounding agentsLens cleaning compositionsOphthalmic solutionsBiology
The present invention relates to improved ophthalmic solutions that employ select B vitamins; pyridoxine and its salts; and thiamine and its salts in order to more effectively preserve solutions and to reduce the degree to which cationic preservatives will deposit on contact lenses. Ophthalmic solutions are here understood to include contact lens treatment solutions, such as cleaners, soaking solutions, conditioning solutions and lens storage solutions, as well as wetting solutions and in-eye solutions for treatment of eye conditions.
Owner:FXS VENTURES LLC
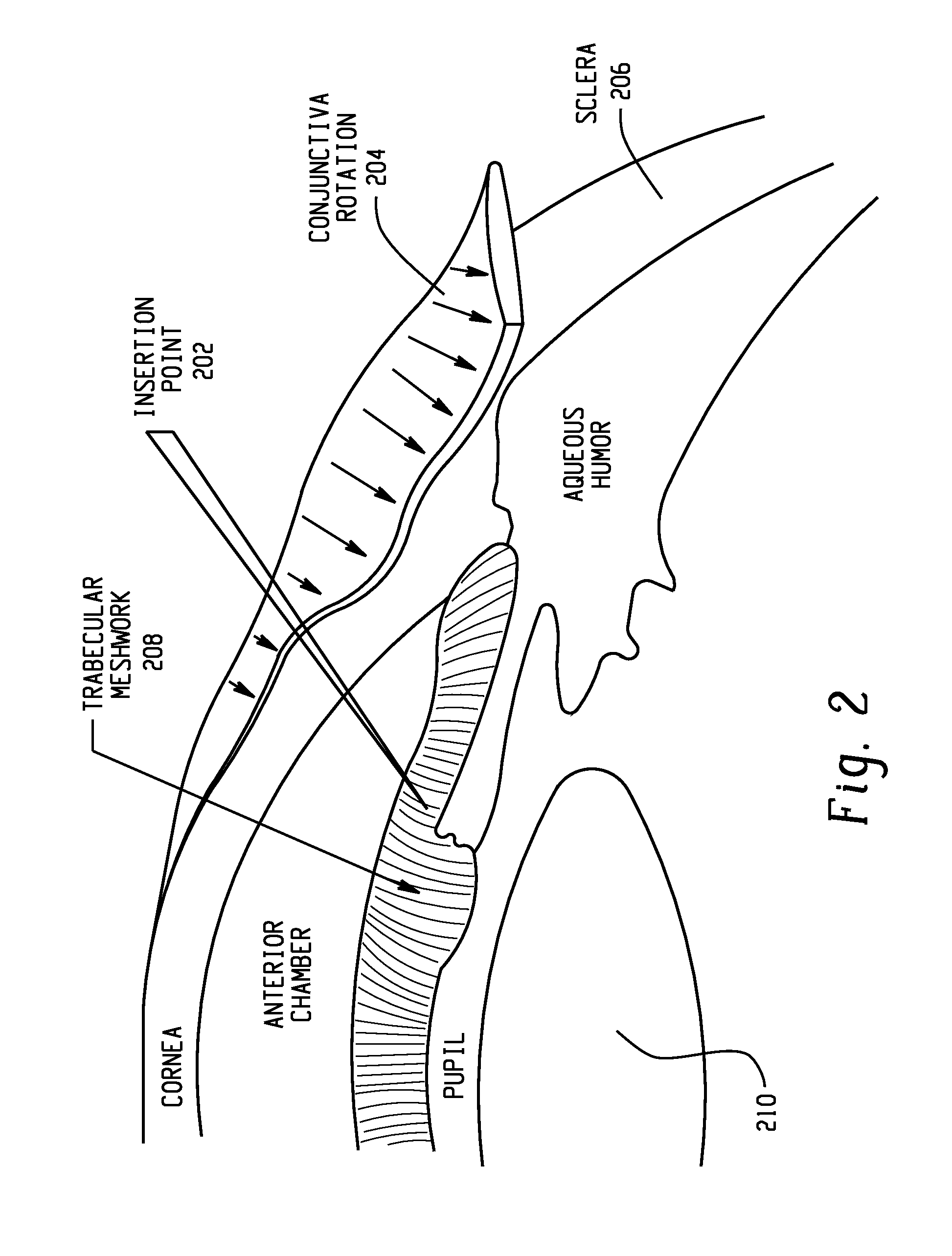
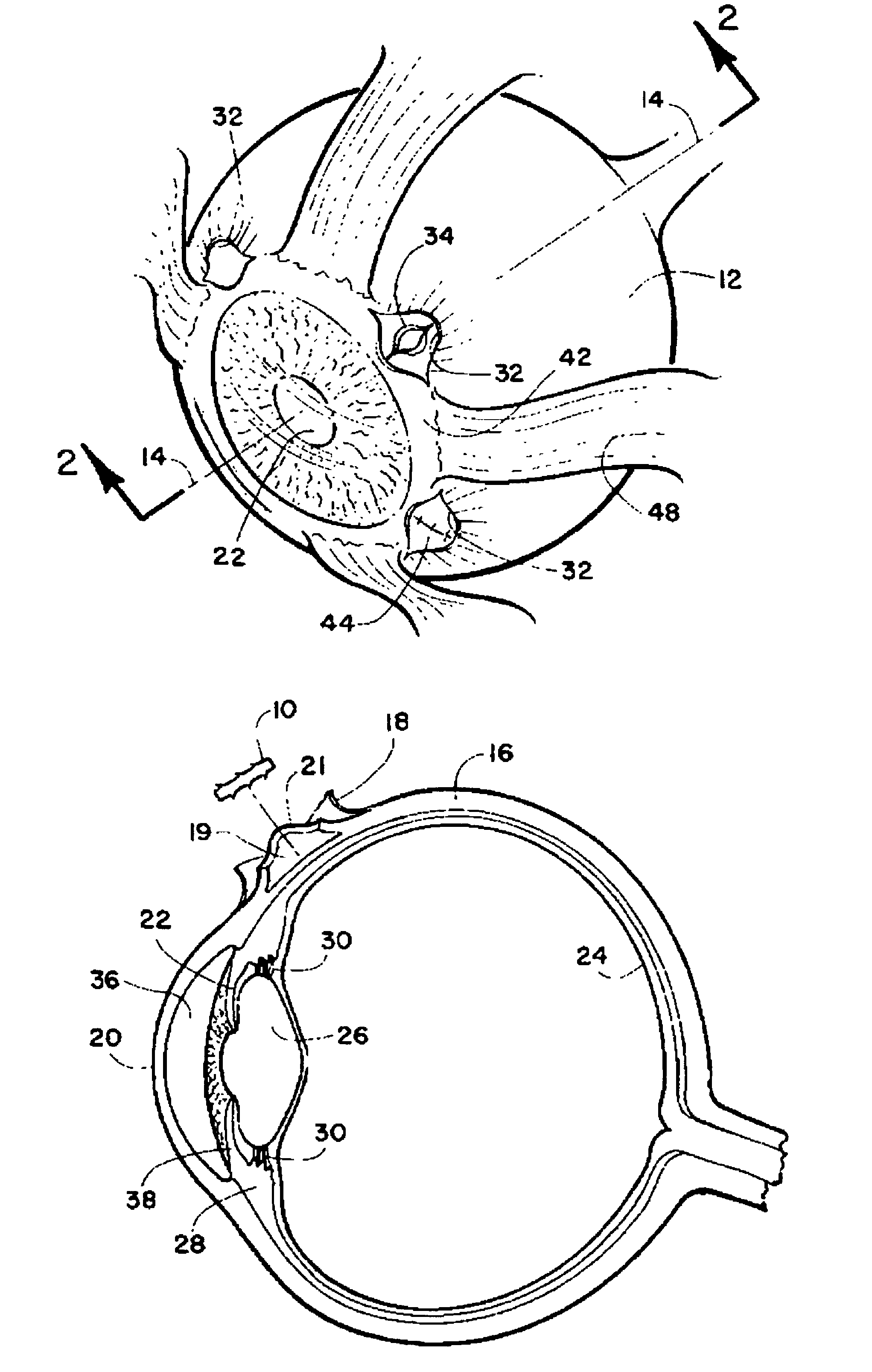
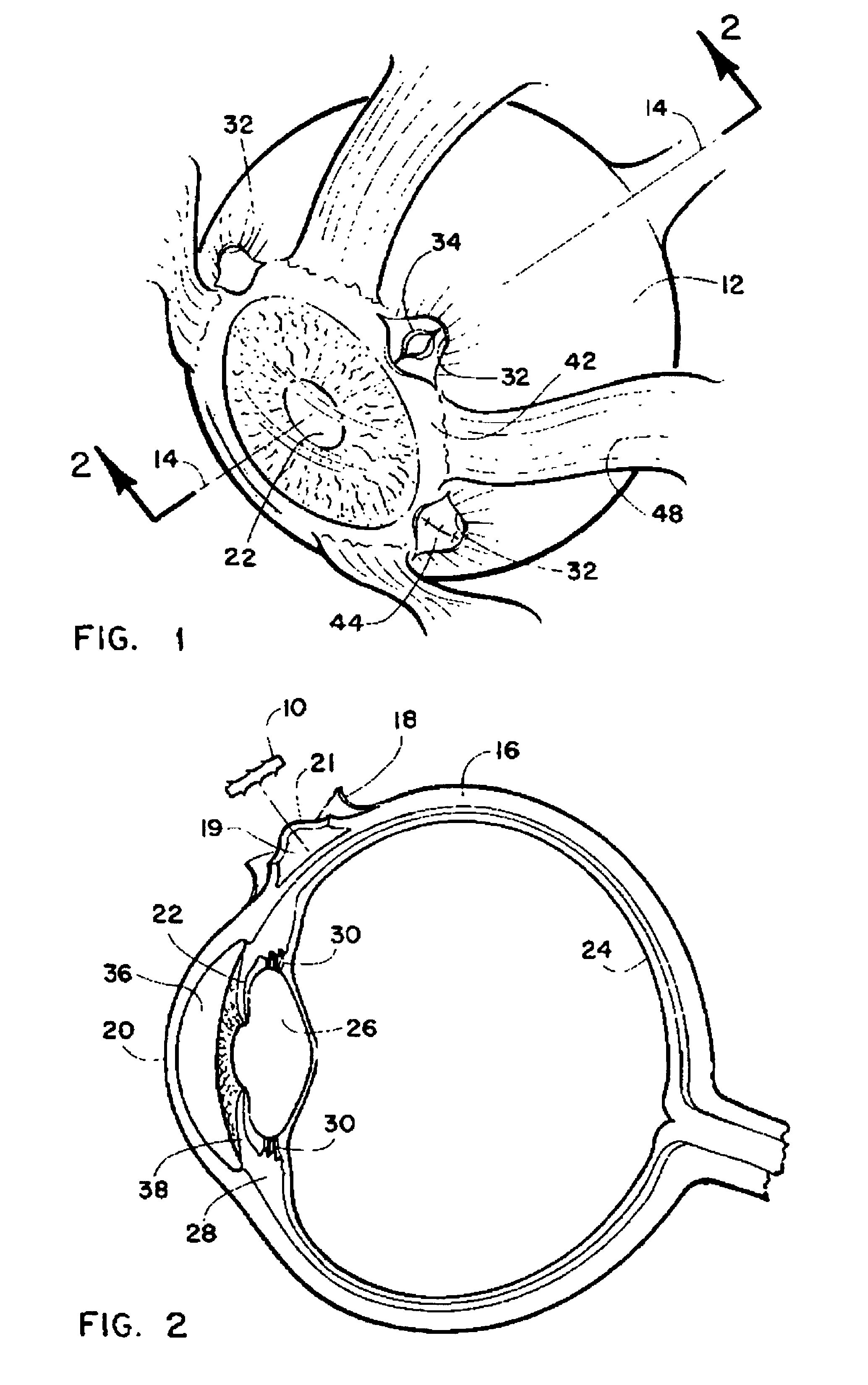
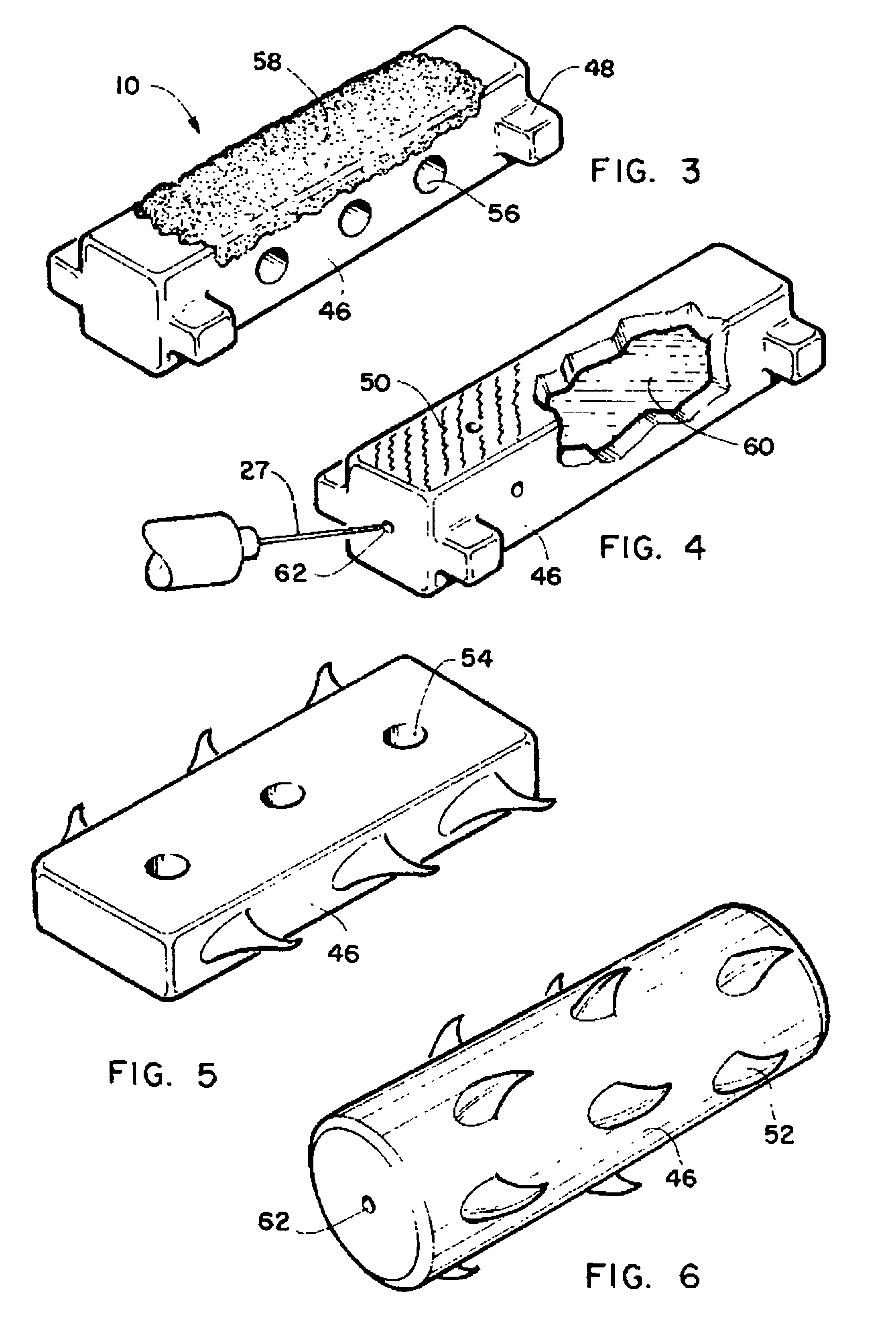
Method and intra sclera implant for treatment of glaucoma and presbyopia
An intra scleral implant and method of implantation for use in the treatment of intraocular pressure and presbyopia. The implant features a body portion and protrusions from the body portion to anchor the device in a cavity formed in the scleral wall of the eye. Optionally a drug delivery function is provided to allow long term communication of drugs to tissue surrounding the implant.
Owner:GLAUCOMA RES TECH
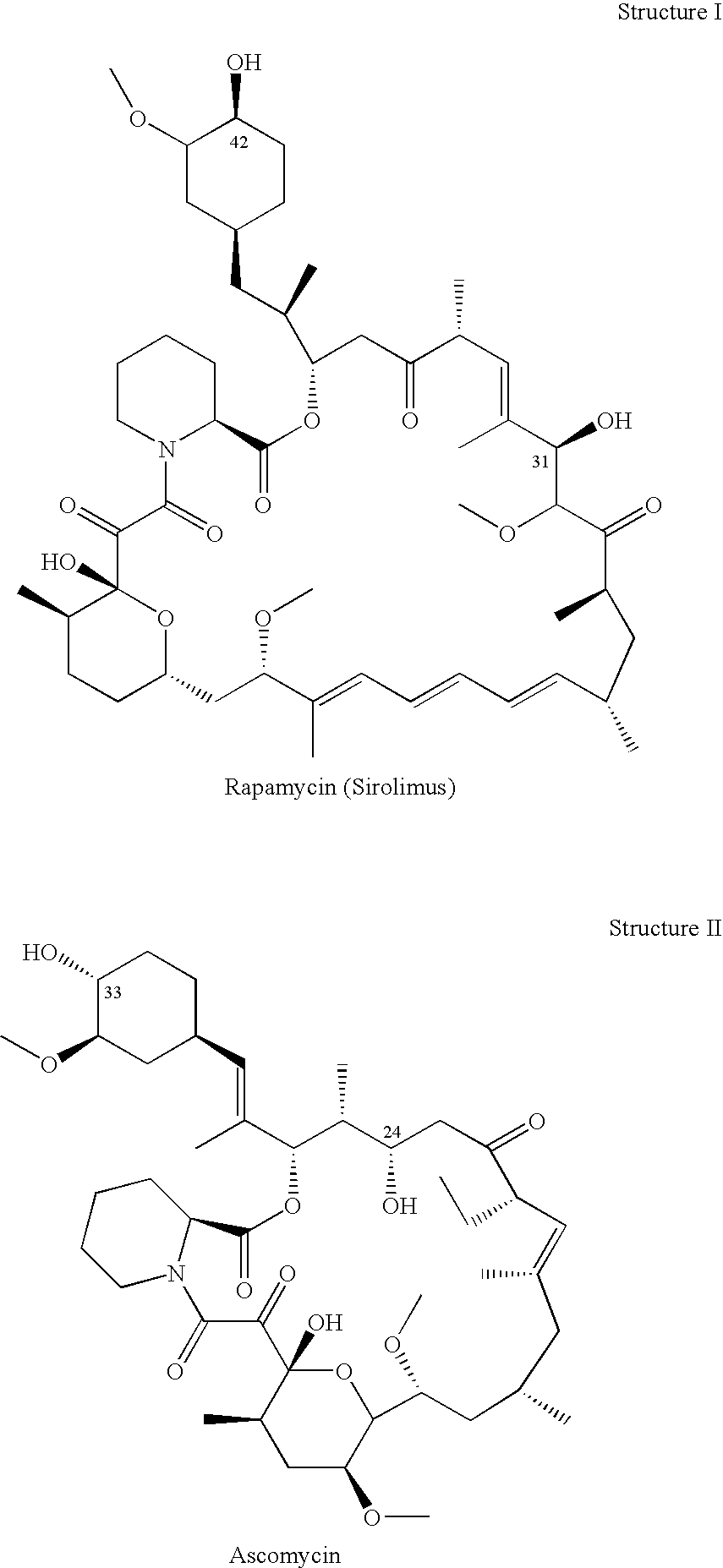
Non-Invasive Ocular Delivery of Rapamycin
Methods and systems for preventing or treating various ocular conditions are disclosed and described. In one aspect, for example, a method for noninvasively delivering a water insoluble macrolide into an eye of a subject for treatment of an ocular condition is provided. Such a method may include administering non-invasively a water soluble form of the macrolide directly into an eye of a subject having or at risk for having the ocular condition, wherein the water soluble form is converted into the water insoluble macrolide in the eye in order to treat the ocular condition.
Owner:HIGUCHI JOHN W
Method of use of carboxylated polysaccharides topically on the eyeball
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
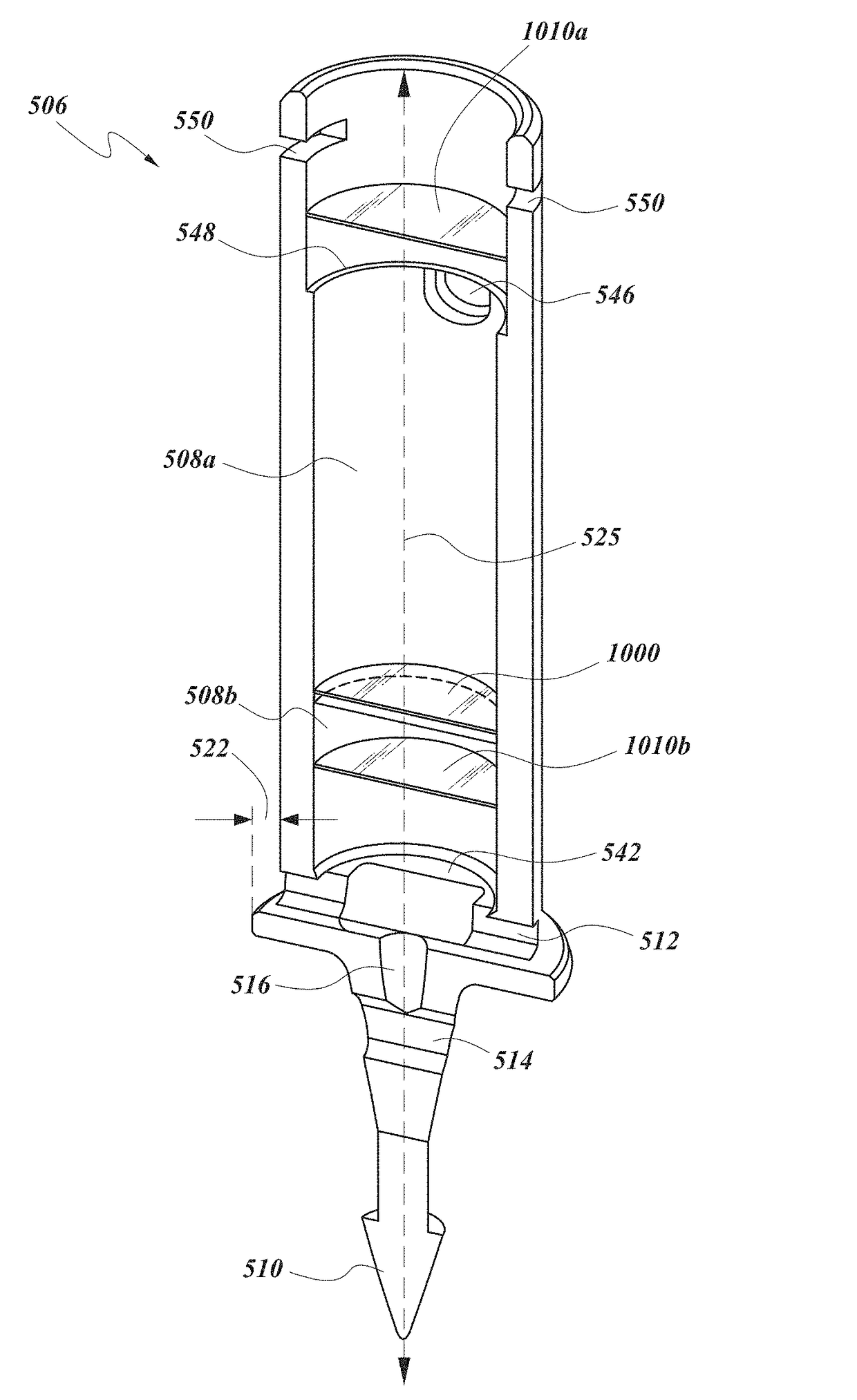
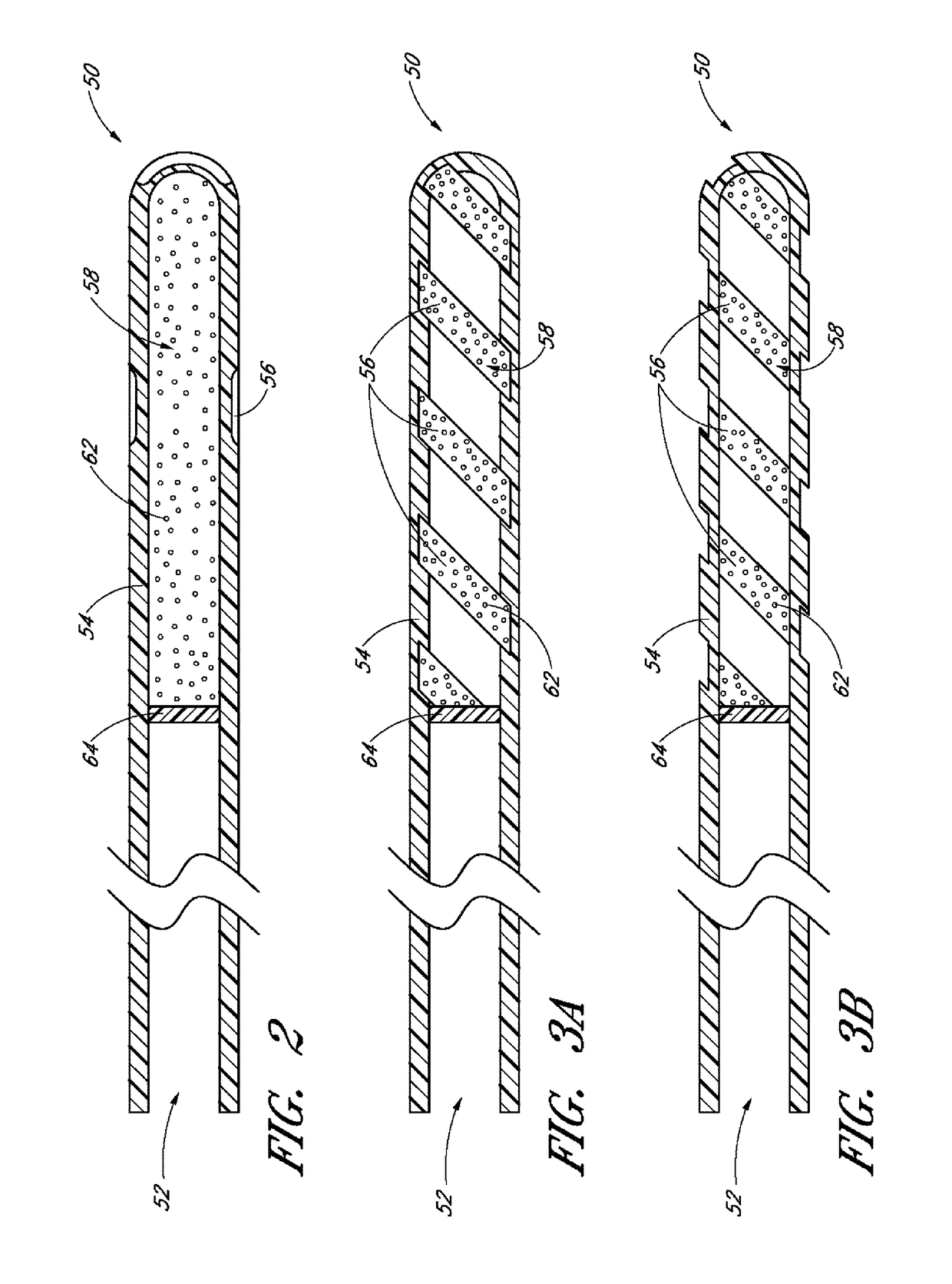
Drug delivery implants with bi-directional delivery capacity
PendingUS20180303665A1Eliminate side effectsIncrease blood flowPowder deliveryEye surgeryControl releaseOphthalmology
Disclosed herein are drug delivery devices and methods for the treatment of ocular disorders requiring targeted and controlled administration of a drug to an interior portion of the eye for reduction or prevention of symptoms of the disorder. In several embodiments, the devices are capable of controlled release of two or more drugs, the release of each drug into a different ocular space (e.g., an ocular compartment versus an ocular fluid outflow pathway).
Owner:GLAUKOS CORP
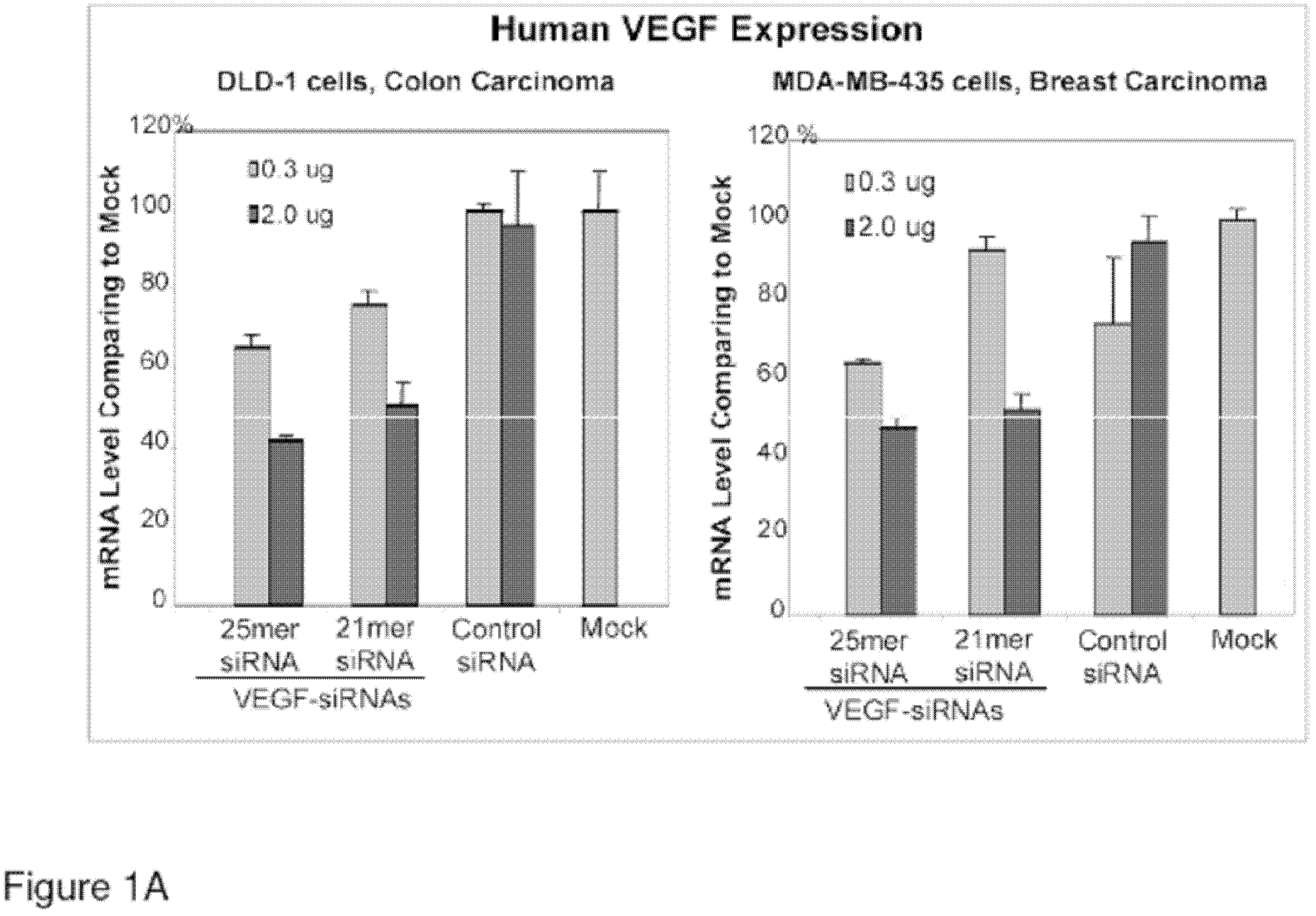
Composition for treating eye diseases by double-target/multi-target small nucleic acid and applications of composition
The invention discloses a composition for treating eye diseases by double-target / multi-target small nucleic acid and applications of the composition. The composition comprises two small nucleic acid molecules and a medicinal carrier, target genes of the two small nucleic acid molecules are selected from two of VEGF (vascular endothelial growth factor) gene, VEGFR2 (vascular endothelial growth factor receptor2) gene and TGF-b1 (transforming growth factor-beta1) gene; or the composition comprises three small nucleic acid molecules and a medicinal carrier, and target genes of the three small nucleic acid molecules are respectively VEGF gene, VEGFR2 gene and TGF-b1 gene. The composition can be used for effectively treating eye diseases by virtue of ribonucleic acid interference (RNAi) mediated inhibitor gene expression and biochemical pathway, and can be prepared to form a medicament for treating eye diseases, including proliferatived diabetic retinophathy, diabetic macular edema, herpes simplex interstitial keratitis, age-related macular degeneration, uveitis and the like.
Owner:SUZHOU SIRNAOMICS BIOPHARMACEUTICALS CO LTD +1
Device for the treatment of an ocular disease
InactiveUS20140316326A1Efficient for electroporationAccurate placementHead electrodesMachines/enginesDiseaseInsertion point
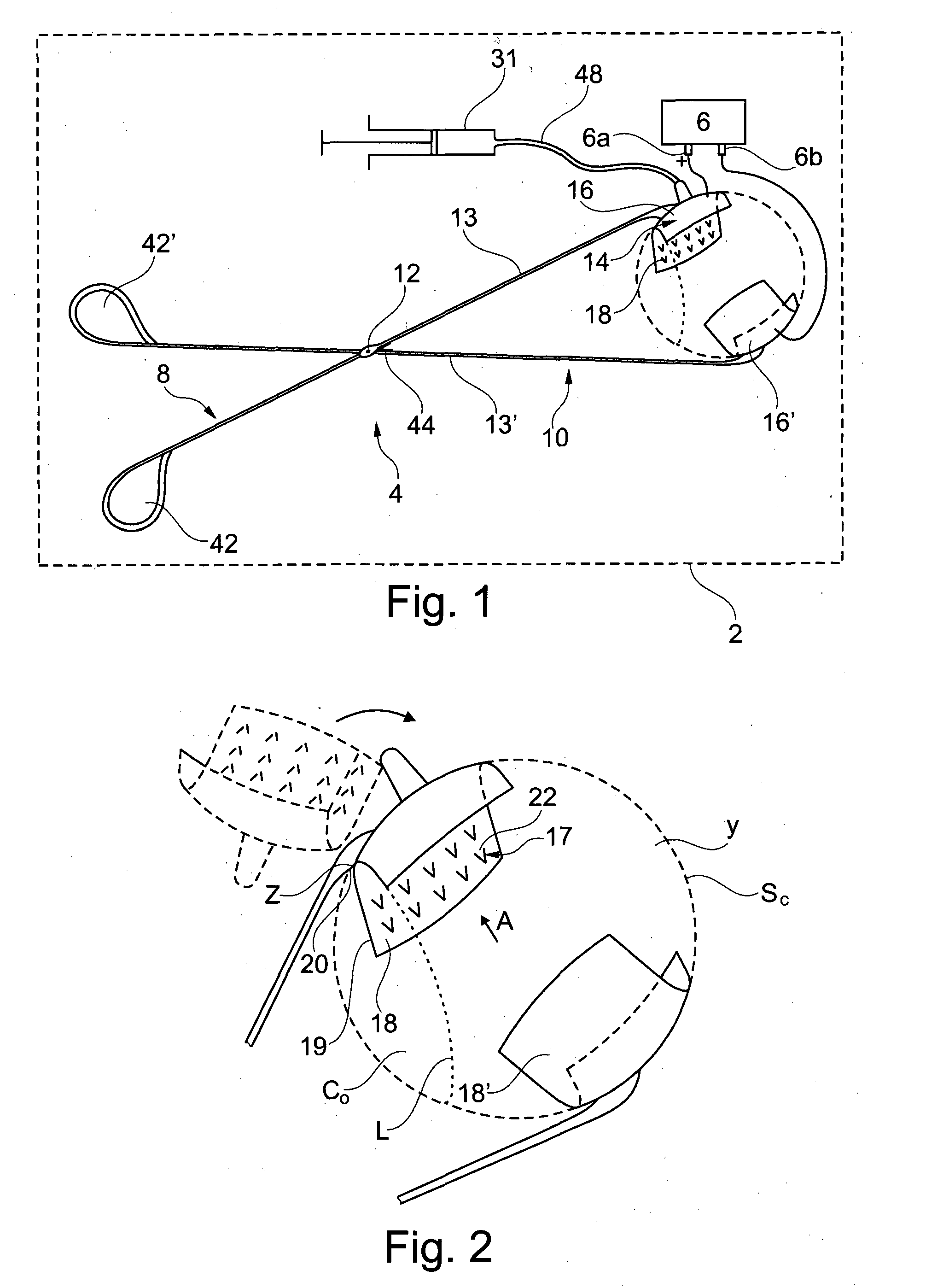
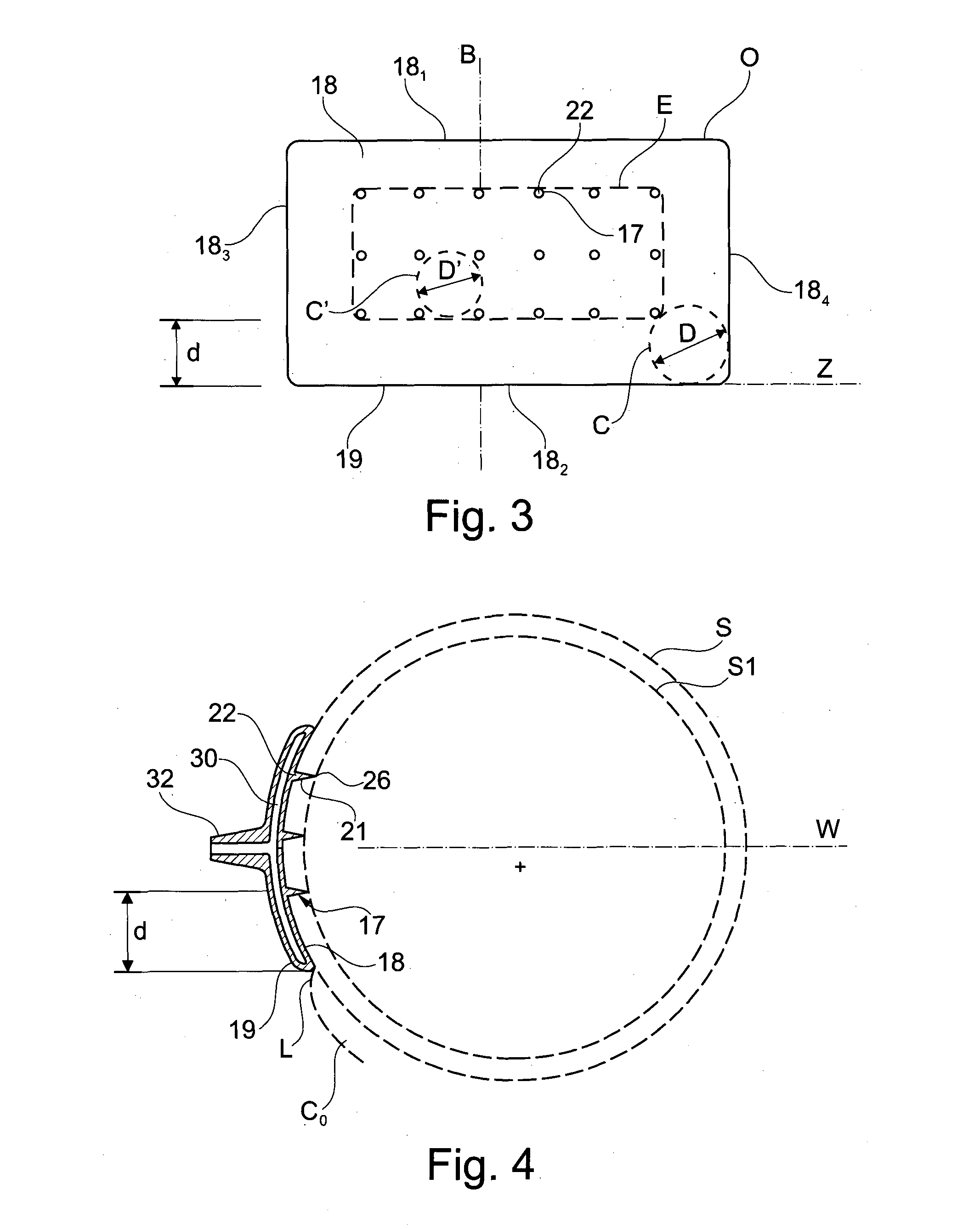
The present invention relates to an injection device comprising: —a first support (14) having a cup-shaped first contact surface (18) intented to come into contact with a first region of an outside surface of an eye, —a set of at least four injection needles (17) in fluid communication with each other and protruding from said first contact surface (18) at respective insertion points (22) so that the distance between the distal end (26) of any of said injection needles to said first contact surface is between 0.6 mm and 1.3 mm, the insertion points of the injection needles on the first contact surface being spread on said first contact surface so that the diameter (D′) of the largest circle (C′) that it is possible to include completely in the convex surface (E) defined by said insertion points on a front view of said first contact surface, without any insertion point being included in said circle, is less than 8 mm.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Compositions, methods, and devices for the treatment of eye stain
InactiveUS20140242176A1Reduce infectionReducing eye stainBiocideCarbohydrate active ingredientsCanis lupus familiarisStain
Embodiments provided herein relate to compositions, methods, and devices useful for treating eye stain. More particularly, compositions and devices are provided that include tylosin and a carrier suitable for ophthalmic application. Such compositions and devices can be used to treat eye stain in an animal, such as a dog or cat.
Owner:ADER ENTERPRISES
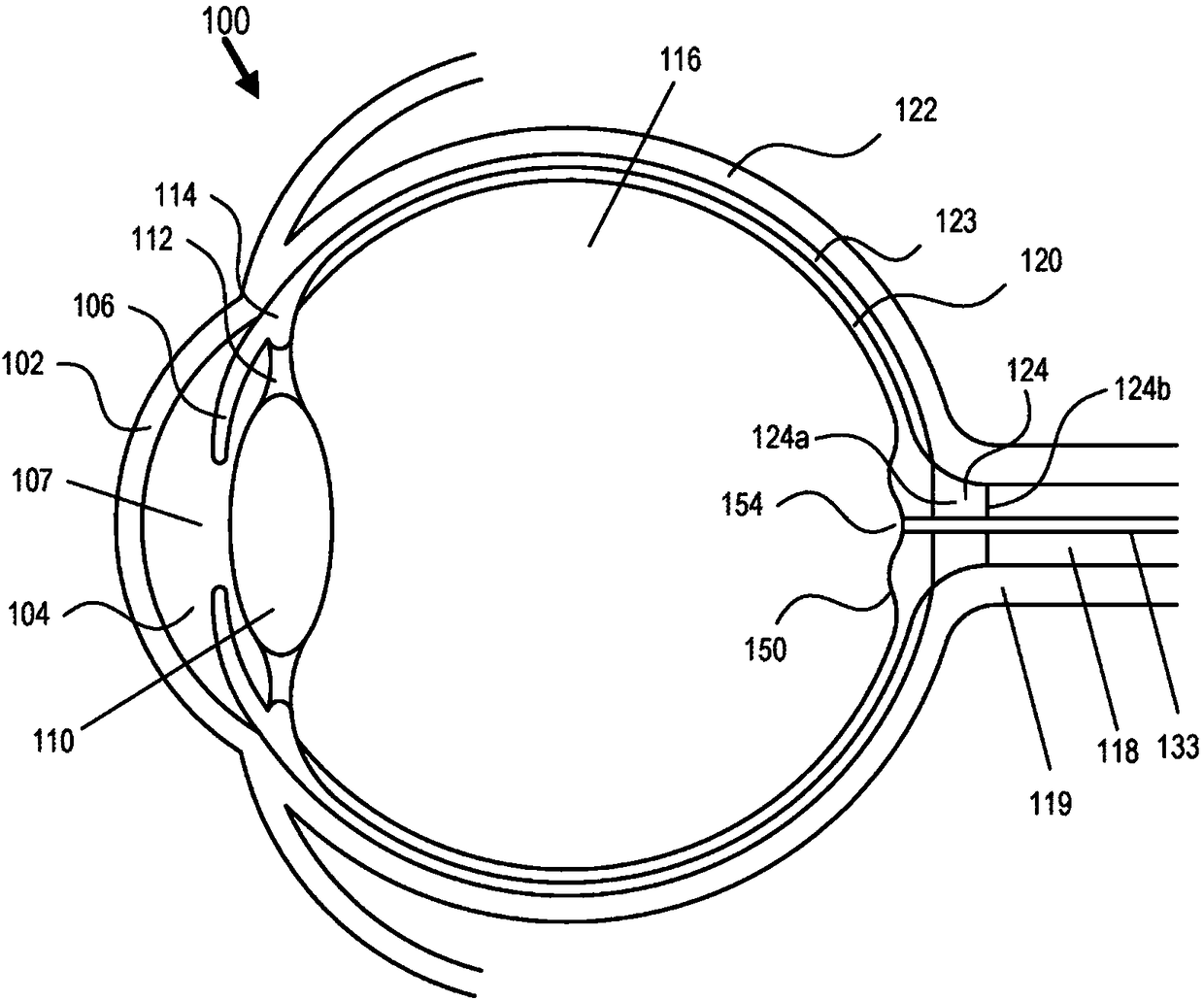
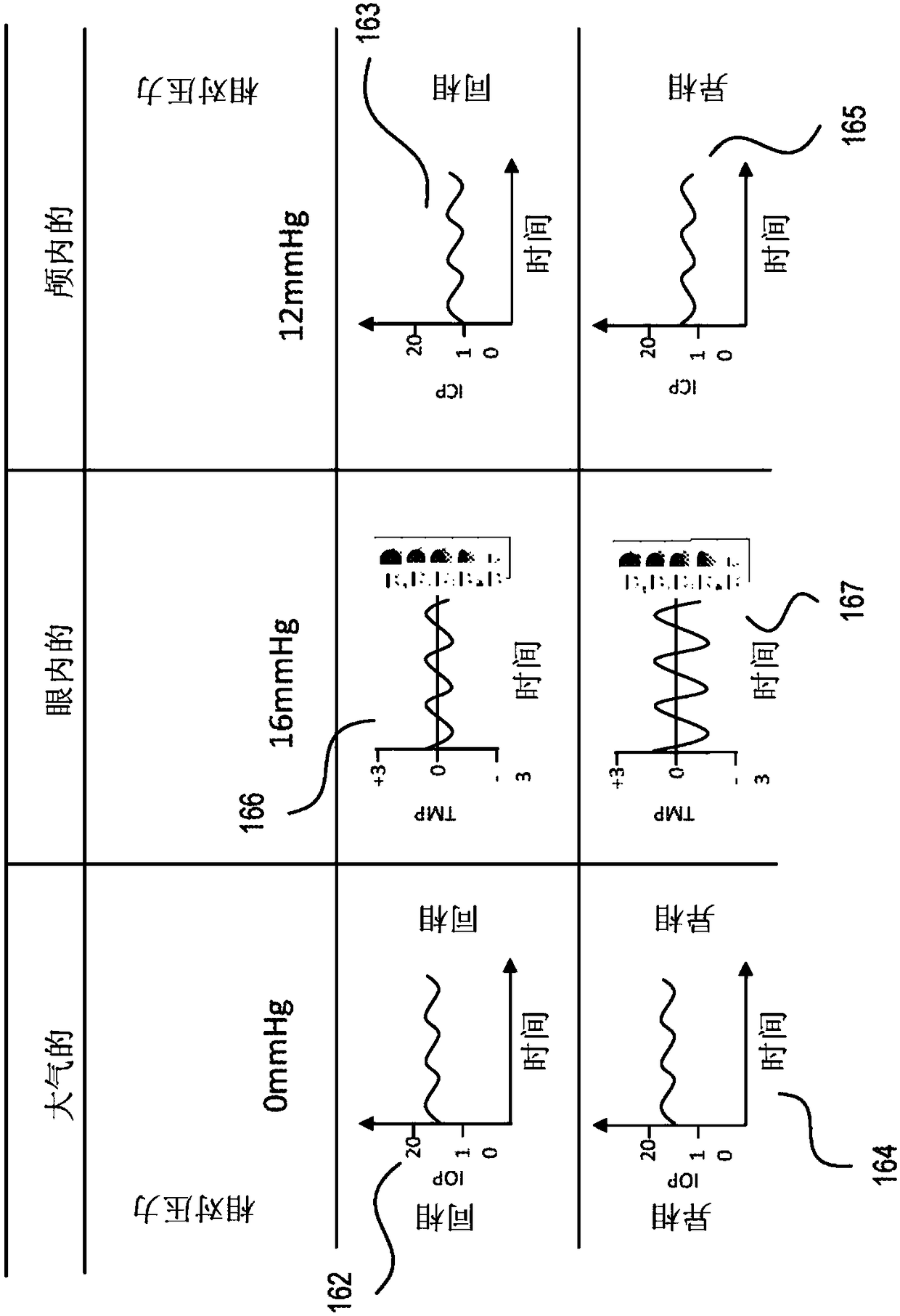
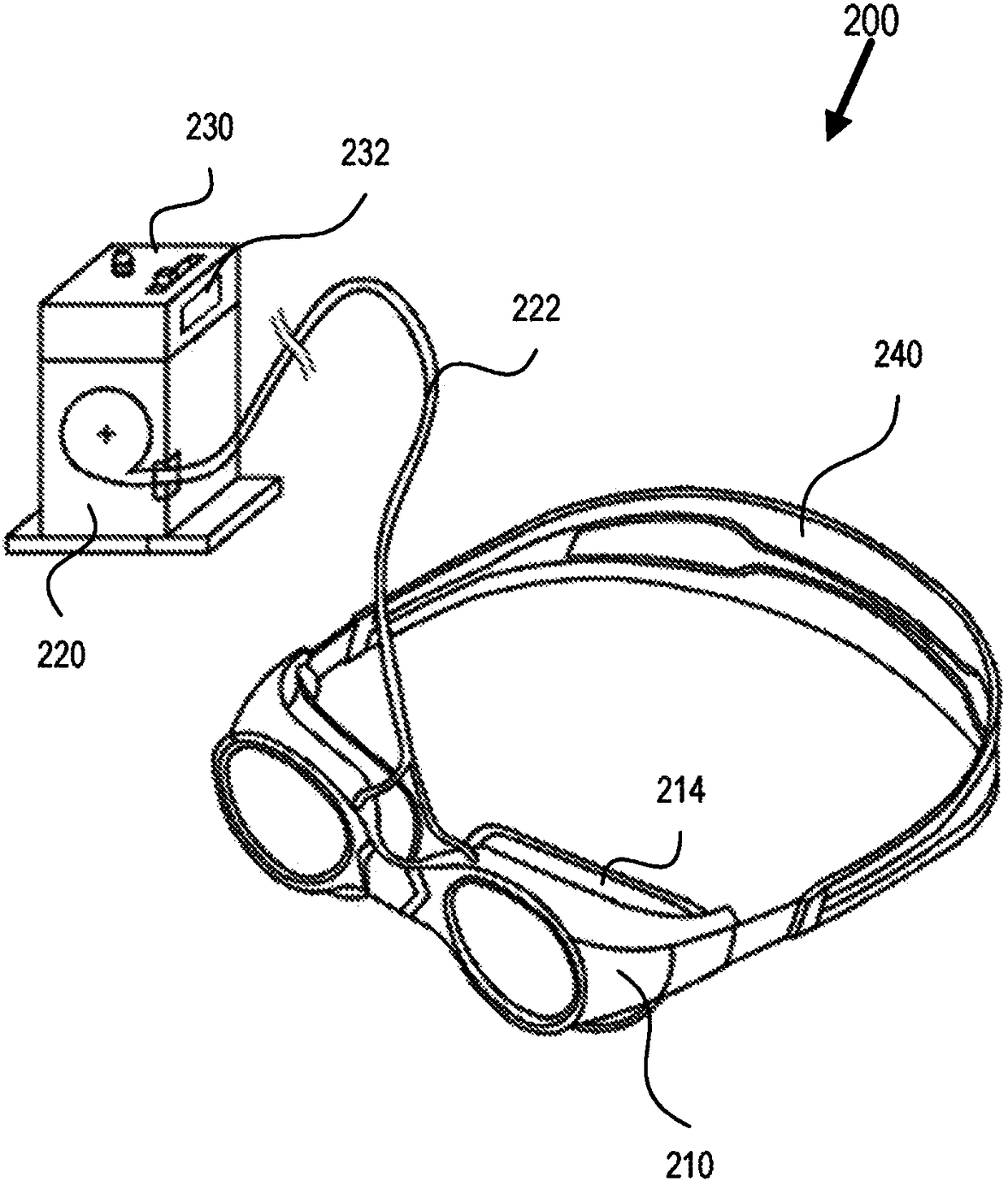
Eye-related intrabody pressure identification and modification
An apparatus for at least one of diagnosing or treating an eye condition can include a goggle enclosure, sized and shaped to be seated on an eye socket of an eye to provide one or more cavities withinthe enclosure that extend about an entire exposed anterior portion of the eye, a pump, in fluidic communication with the one or more cavities to apply a fluid pressure to t he one or more cavities, the pump configured to adjust a fluid pressure within the one or more cavities of the goggle enclosure, and a control circuit, including a data interface to receive data directly or indirectly indicating at least one of an intraorbital pressure, ICP, IOP, or a relationship between ICP and IOP, and based on processing the received data as a feedback control variable, controlling the pump to adjust the fluid pressure within the one or more cavities, the controlling including using further monitoring of the received data to control the pump.
Owner:EQUINOX OPHTHALMIC INC
Ophthalmic solutions, including contact lens care and eye drops comprising carnosine, preferably in combination with dexpanthenol and/or hyaluronic acid
InactiveUS20110230424A1Prevent of even repair damagePrevent and reduce stainingOrganic active ingredientsSenses disorderDexpanthenolOphthalmic solutions
The current invention relates to a contact lens care solution comprising a compound suitable for treating the eye, or the contact lens, use of such compound in such contact lens care solutions, and methods for introducing such compound in the eye of a person wearing contact lenses. With the current invention damage to the eye, in particular the cornea, in particular damage to the eye, in particular the cornea, of a person wearing a contact lens, or as a consequence of wearing a contact lens is prevented or reduced. / esp.
Owner:WAGENAAR LOUIS JOHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com