Biocompatible biodegradable intraocular implant system
a biodegradable, intraocular implant technology, applied in the direction of prosthesis, eye treatment, coating, etc., can solve the problems of irreversible amblyopia, difficult treatment, and damage to the iol layer,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
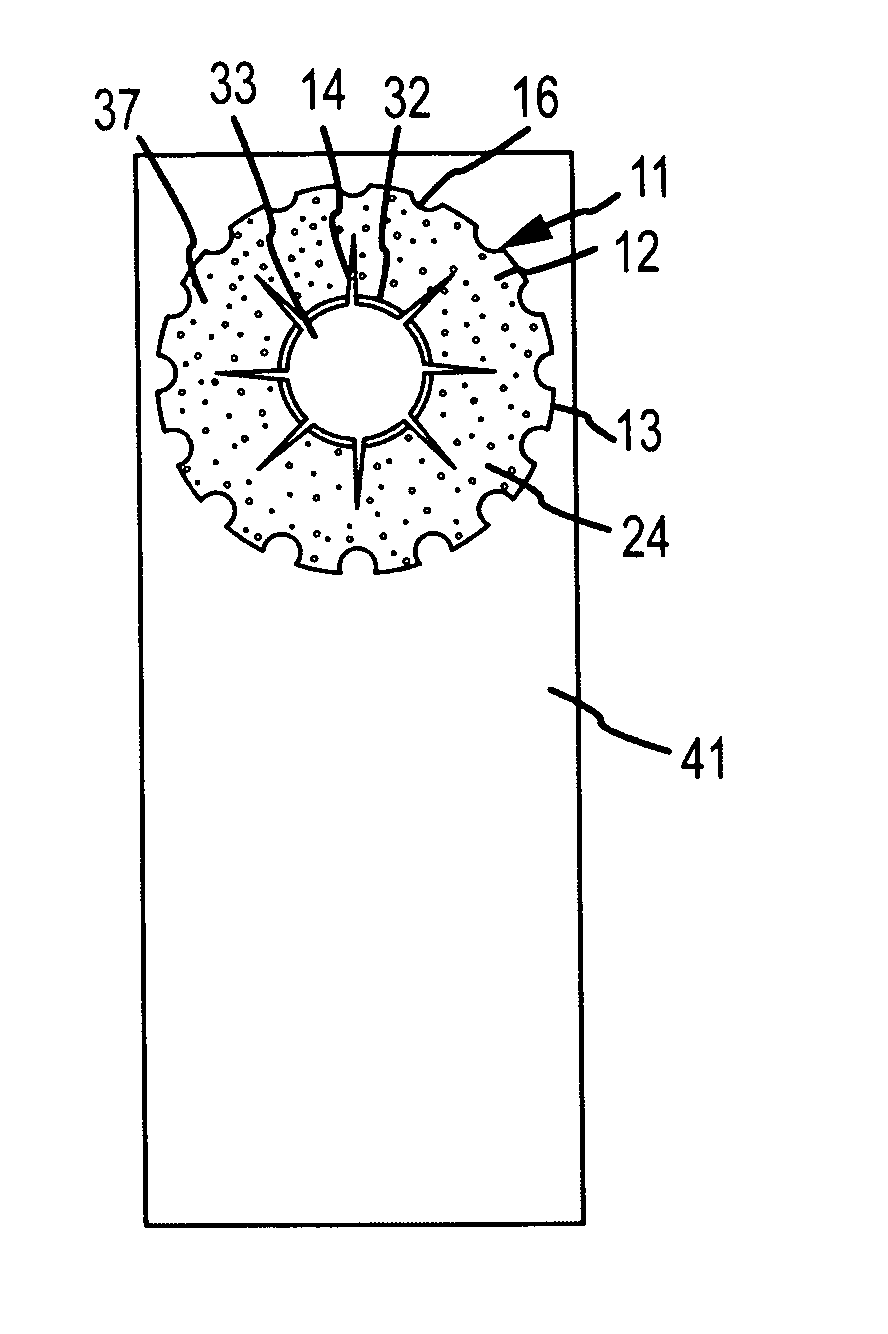
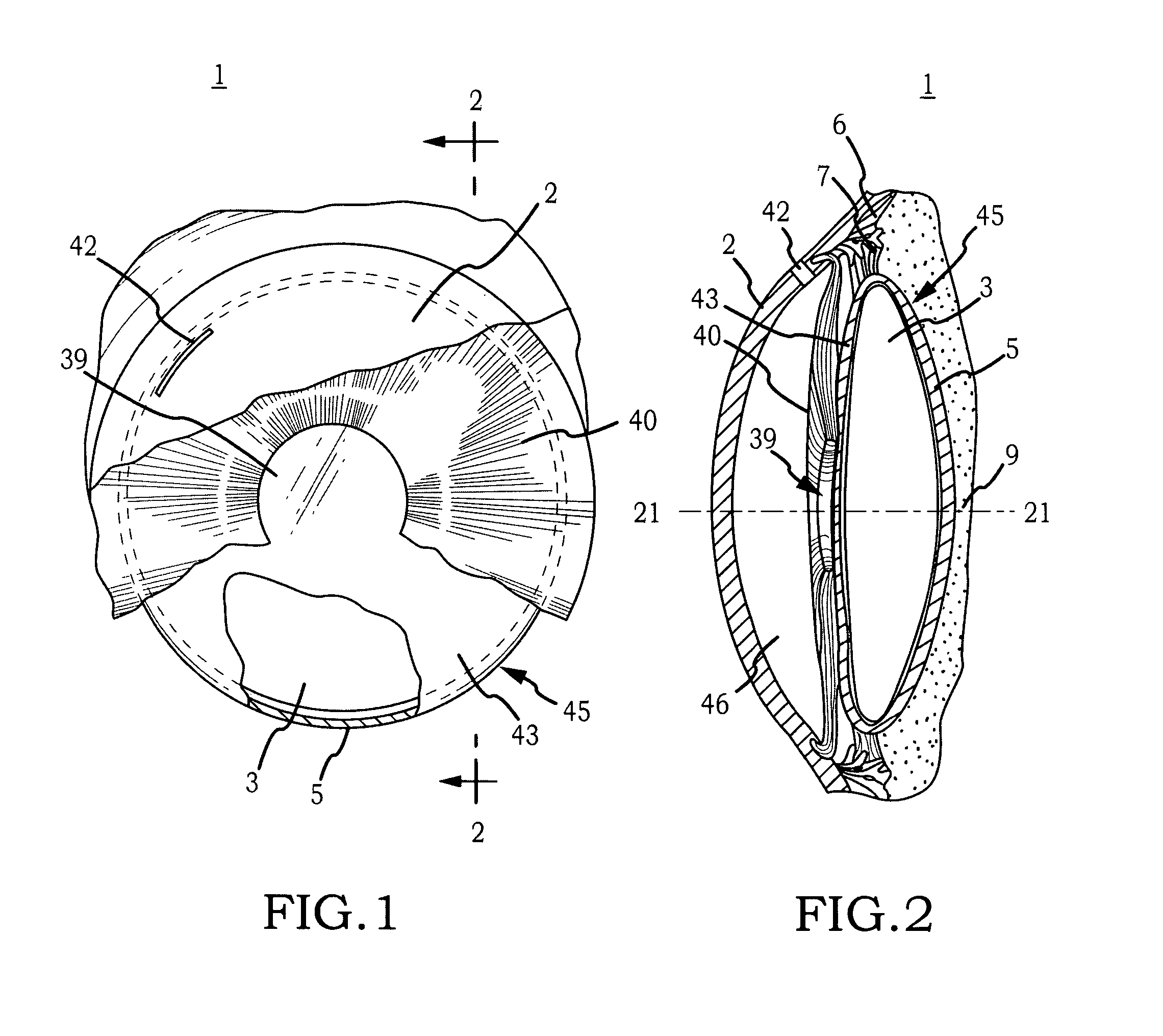
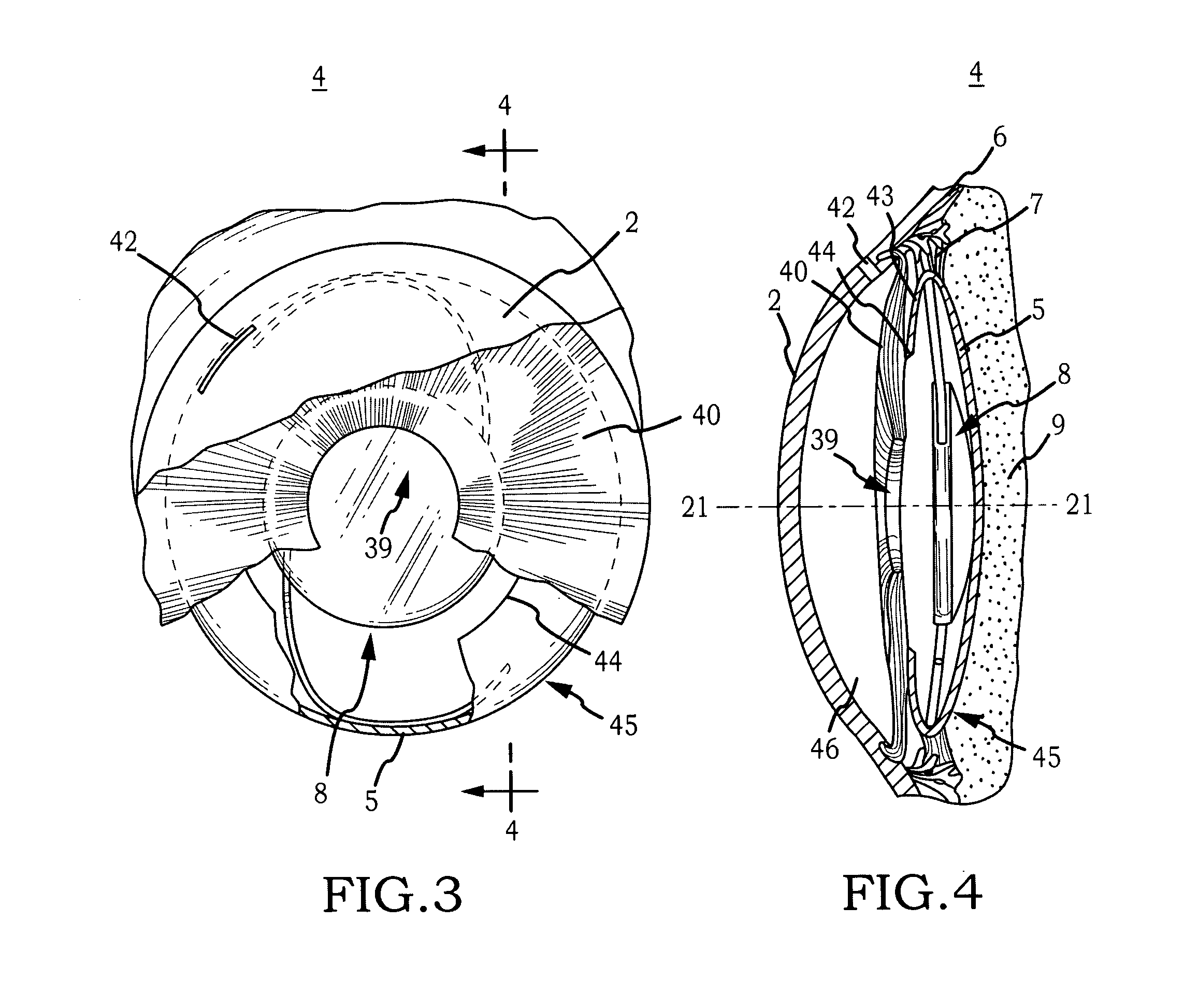
[0044]Generally, the invention comprises an intraocular implant and methods for treating an ocular condition. In particular, an embodiment of a biocompatible biodegradable intraocular implant including a biocompatible material or a biocompatible biodegradable material and an active agent which implanted between an IOL and the surface of the posterior capsule of the eye inhibits migration of residual LECs after cataract surgery by providing structural or pharmaceutical barriers to reduce posterior capsule opacification of the eye.
DEFINITIONS
[0045]“A” or “an” entity refers to one or more of that entity; for example, “a polymer” refers to one or more of those compositions or at least one composition. As such, the terms “a” or “an”, “one or more” and “at least one” can be used interchangeably herein. Furthermore, the language “selected from the group consisting of” refers to one or more of the elements in the list that follows, including combinations of two or more of the elements.
[0046...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com