Injectable Combination Therapy for Eye Disorders
a combination therapy and eye disorder technology, applied in the field of eye disorders, can solve the problems of armd, patients with this form of armd, slow and progressive deterioration of central vision,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
I. Overview
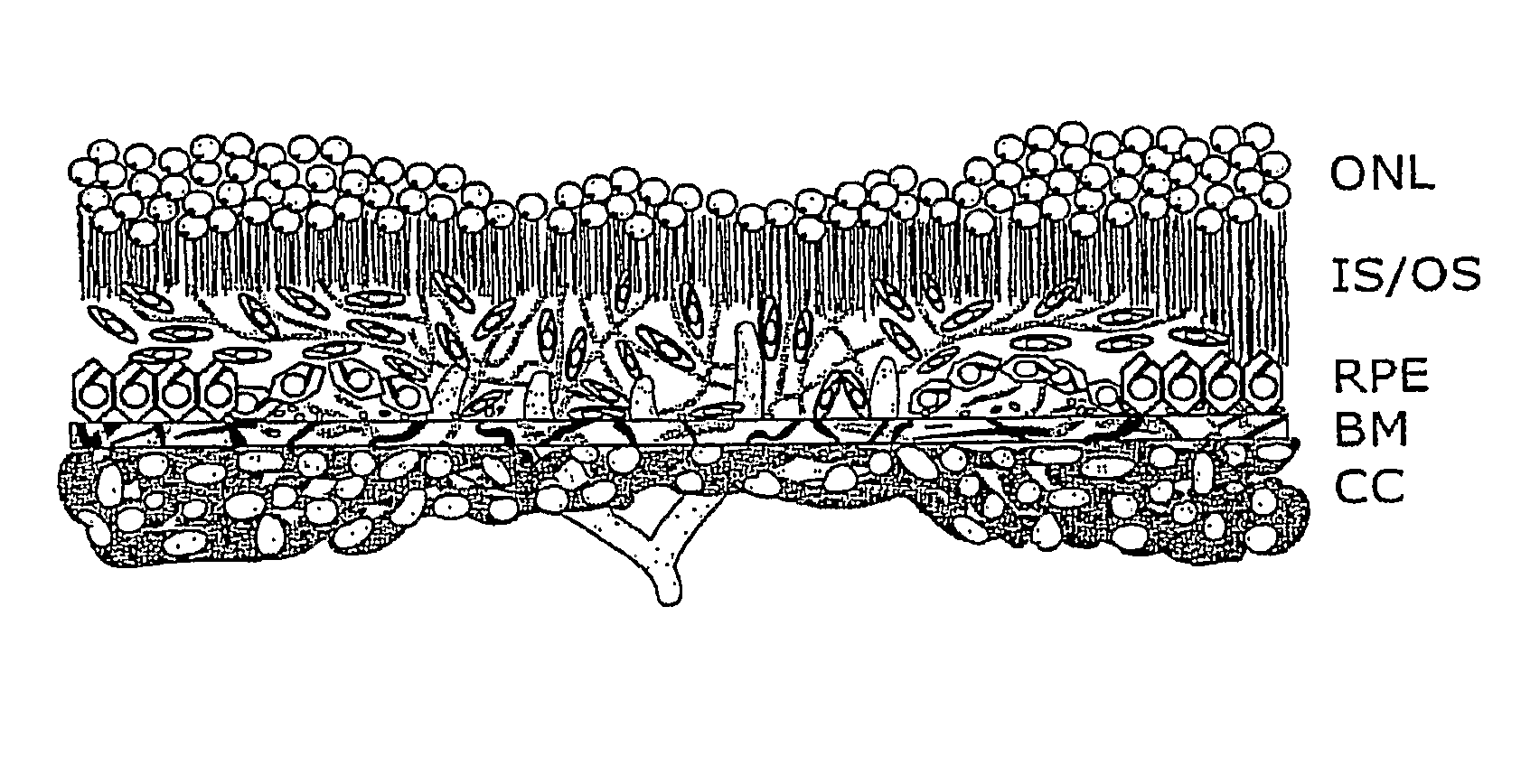
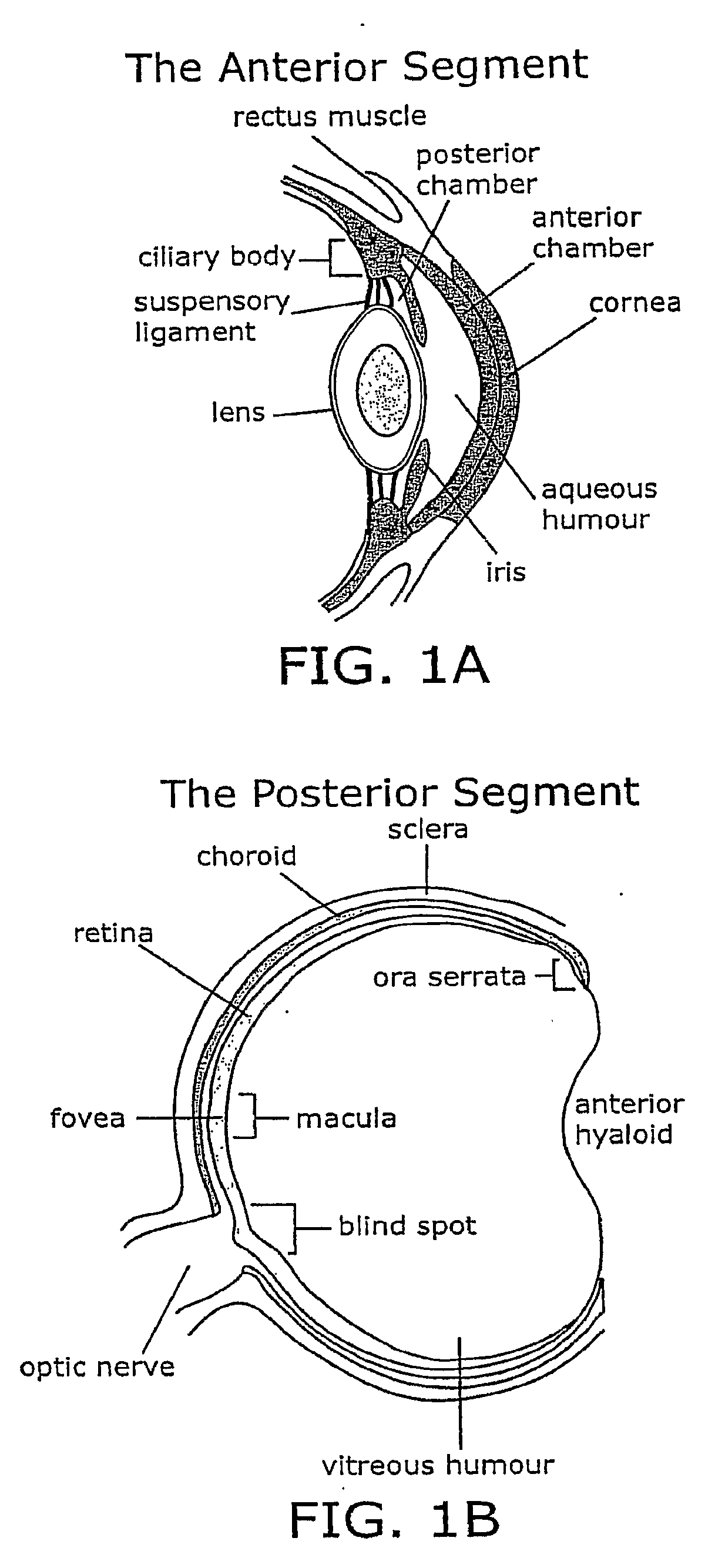
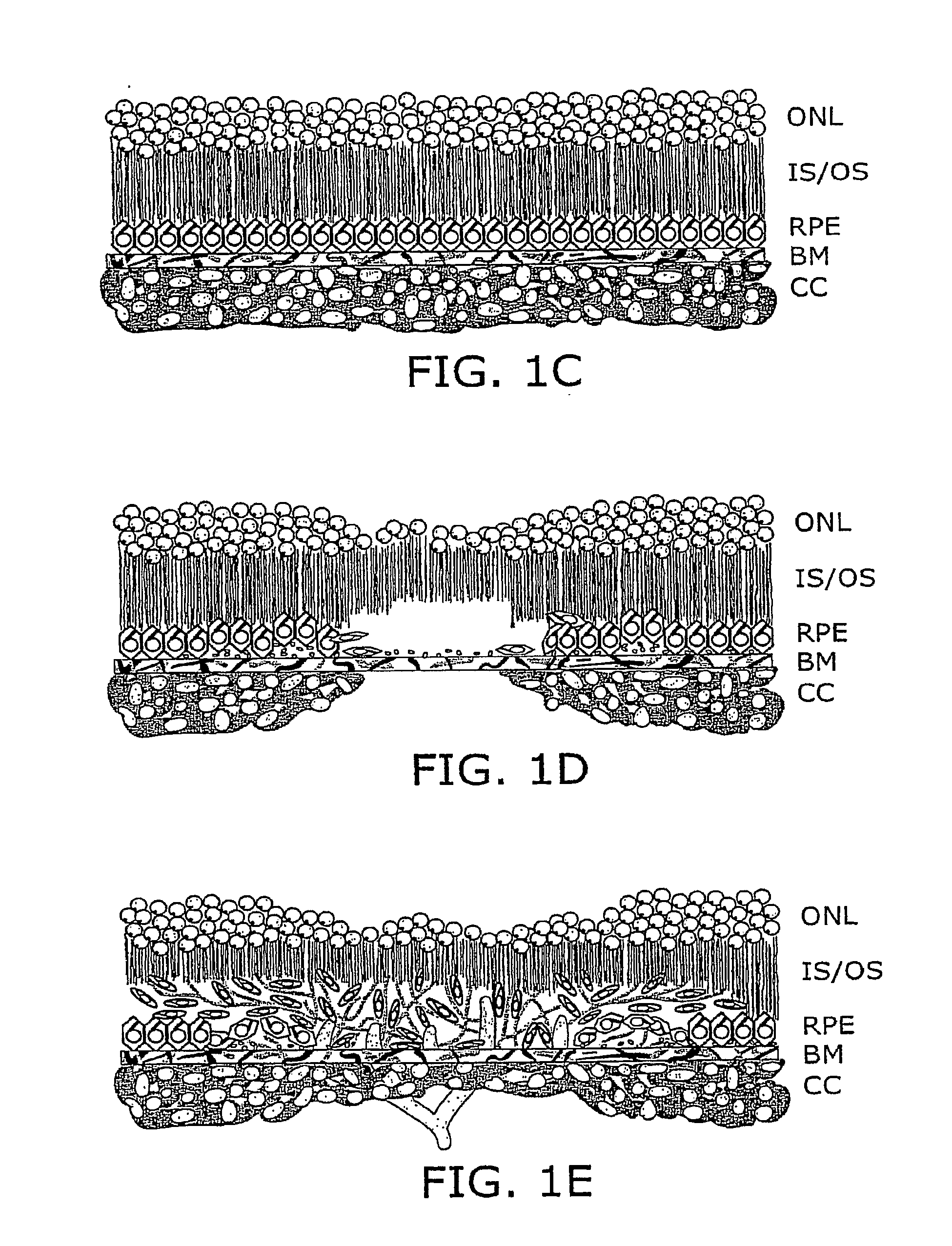
[0074]The present invention provides compositions, methods, articles of manufacture, and pharmaceutical packs or kits for the treatment of an eye disorder. In certain embodiments of the invention the eye disorder is characterized by macular degeneration, CNV, or RNV. Exemplary disorders that can be treated according to the invention include, but are not limited to, macular degeneration related conditions, diabetic retinopathy, and retinopathy of prematurity. While the concepts underlying the invention are described herein with particular reference to treatment of wet ARMD or other conditions characterized by CNV and / or RNV, they apply to a range of different ocular (and other) disorders.
[0075]The invention encompasses the recognition that eye care providers, e.g., ophthalmologists, are often reluctant to administer a therapeutic agent, e.g., an angiogenesis inhibitor, using an invasive procedure such as intravitreal injection that is associated with the risk of a severe c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com