Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Receptor Tyrosine Kinase Inhibitors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Receptor tyrosine kinase inhibitors comprising pyridine and pyrimidine derivatives
InactiveUS20090227556A1Excellent kinase inhibitory actionBiocideSenses disorderReceptor tyrosine kinase inhibitorHalogen
Owner:EISIA R&D MANAGEMENT CO LTD
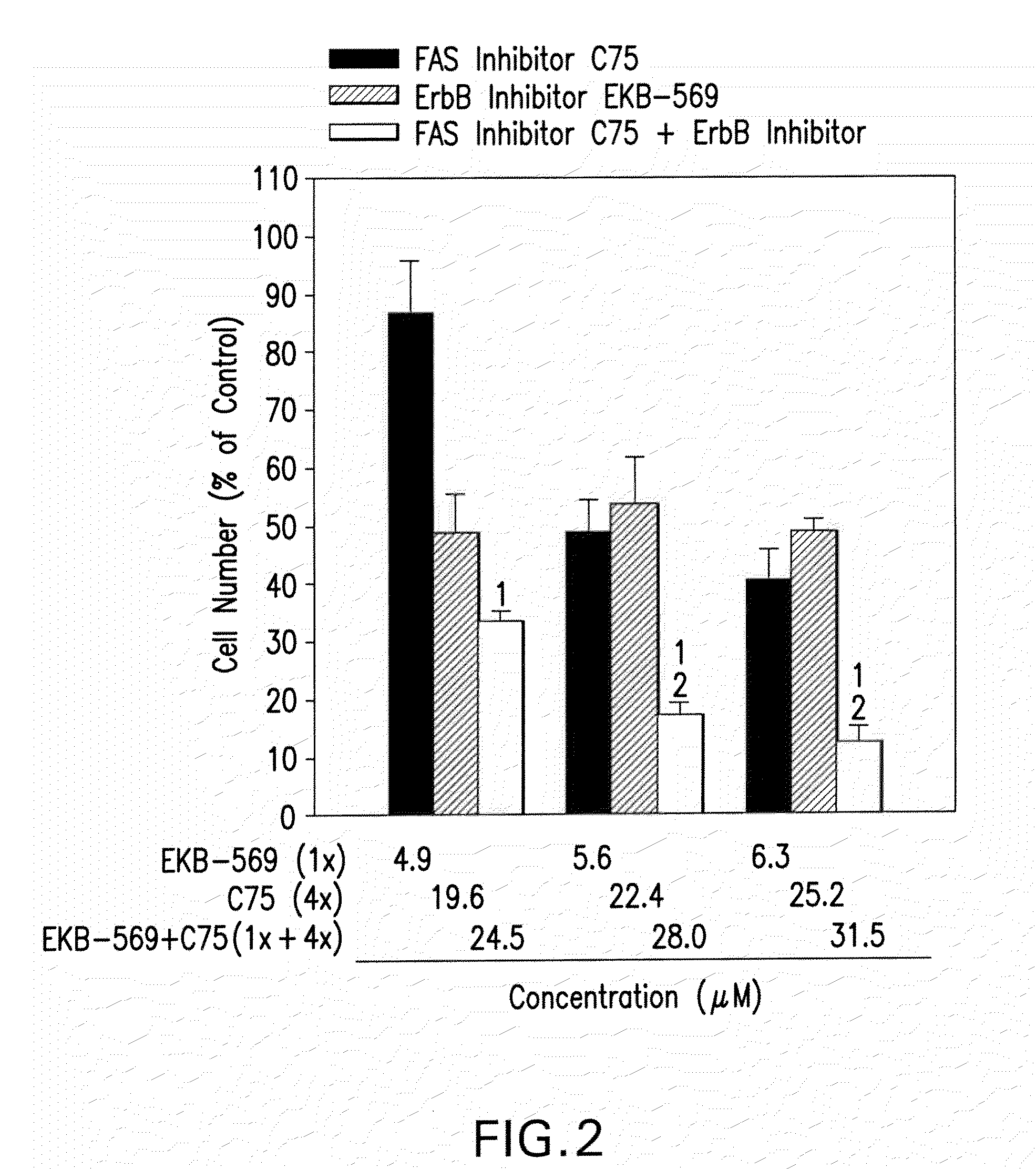
Combination Product of Receptor Tyrosine Kinase Inhibitor and Fatty Acid Synthase Inhibitor for Treating Cancer
InactiveUS20090325877A1Reducing FASN activityInhibitory activityBiocidePeptide/protein ingredientsReceptor tyrosine kinase inhibitorFatty acid
A pharmaceutical combination product is disclosed that comprises a receptor tyrosine kinase inhibitor and a fatty acid synthase inhibitor, and to the use thereof in the manufacture of a medicament for use in the treatment or prophylaxis of cancer.
Owner:WYETH
Treatment of human tumors with radiation and inhibitors of growth factor receptor tyrosine kinases
InactiveUS20040057950A1Inhibit tumor growthPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthHuman tumor
Owner:WAKSAL HARLAN W +3
Anti-CTLA4 Antibody and Indolinone Combination Therapy for Treatment of Cancer
InactiveUS20090074787A1Organic active ingredientsAntibody ingredientsDiseaseReceptor tyrosine kinase inhibitor
The invention relates to administration of an anti-CTLA4 antibody, particularly human antibodies to human CTLA4, such as those having amino acid sequences of antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also referred to as 11.2.1 or CP-675,206), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also referred to as 10D1 or MDX-010), in combination with an indolinone receptor tyrosine kinase inhibitor (RTKI), e.g., N-[2-diethylamino]ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 1), N-[2-(ethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 2), and 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 3), for treatment of cancer. The invention relates to administering a combination of an anti-CTLA4 antibody and an indolinone RTKI such as, inter alia, compound 1. The invention relates to neoadjuvant, adjuvant, first-line, second-line, and third-line therapy of cancer, whether localized or metastasized, and at any point(s) along the disease continuum (e.g., at any stage of the cancer).
Owner:PFIZER PFIZER PRODS
Assays to Identify Irreversibly Binding Inhibitors of Receptor Tyrosine Kinases
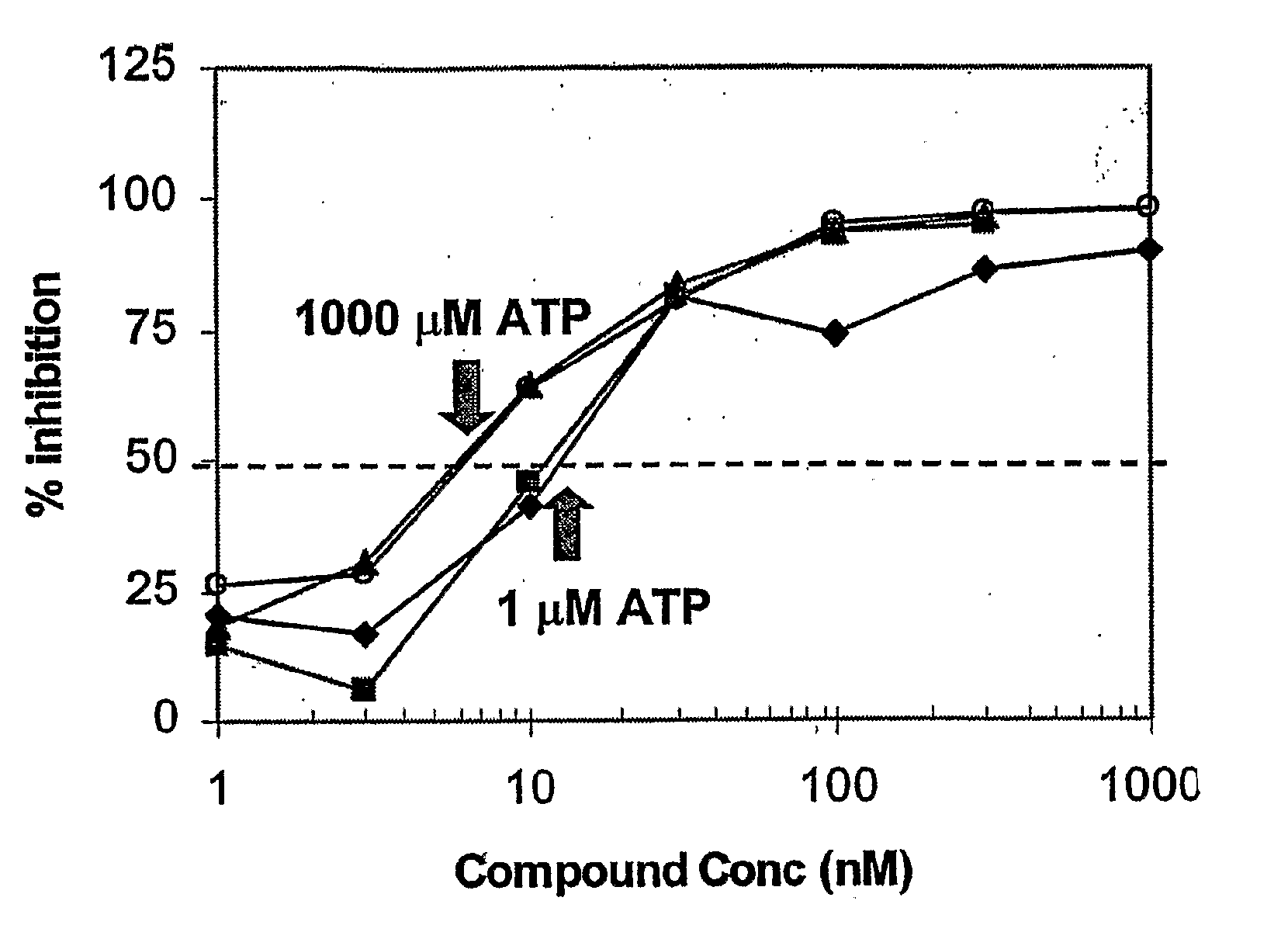
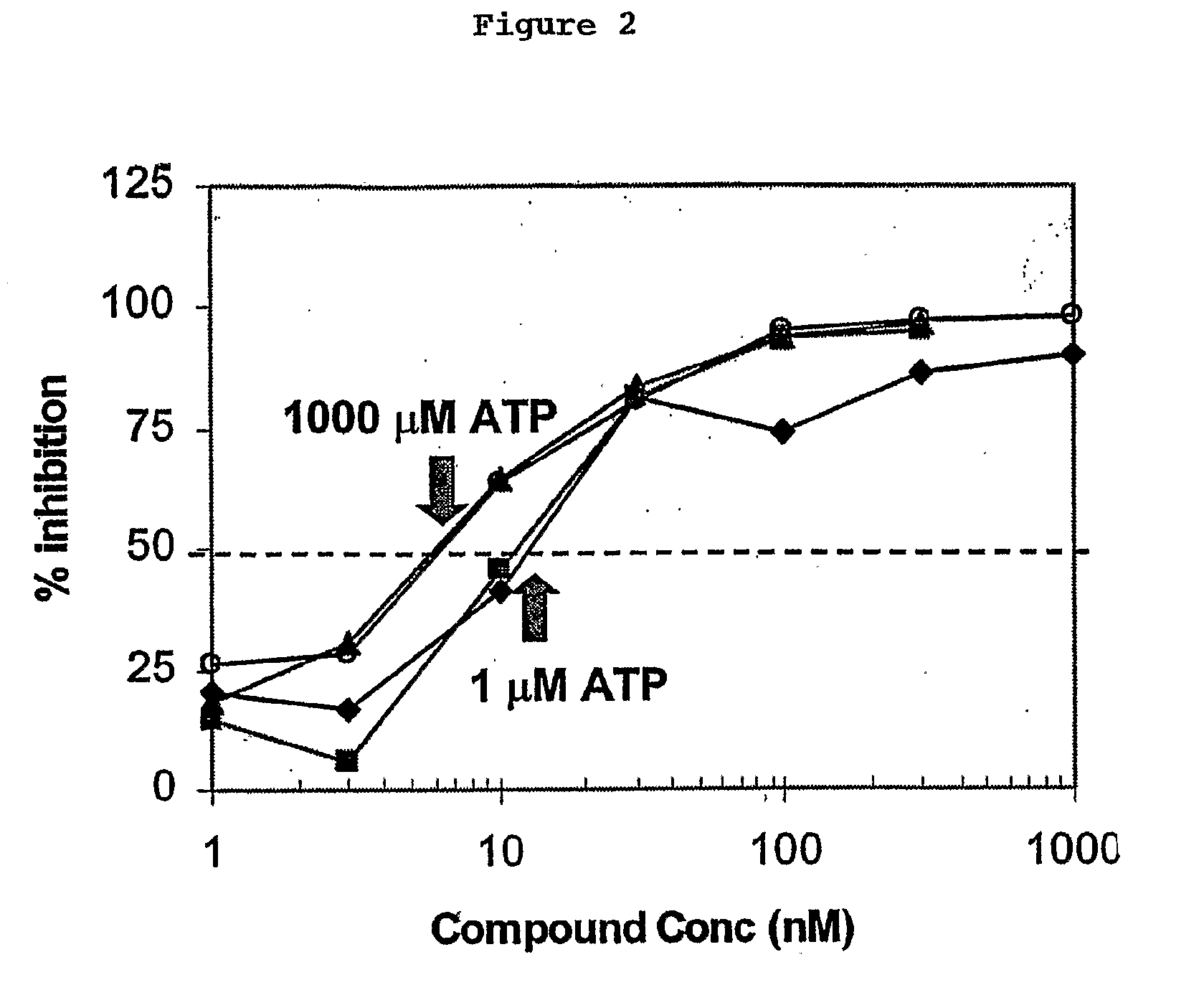
The present invention relates to a method of identifying an inhibitor of a receptor tyrosine kinase that irreversibly binds to the kinase. Specifically, the method comprises using a variety of assays, either alone or in combination, to identify compounds that irreversibly bind to tyrosine kinases. More specifically, there are four assays, which are novel variations of a basic enzyme assay and identify irreversible binding inhibitors.
Owner:WYETH LLC
Quinazoline analogs as receptor tyrosine kinase inhibitors
This invention provides quinazoline analogs of Formula I:where A is bonded to at least one of the carbons at the 5, 6, 7 or 8 position of the bicyclic ring, and the ring is substituted by up to two independent R3 groups. The invention also includes methods of using compounds of Formula I as type I receptor tyrosine kinase inhibitors and for the treatment of hyperproliferative diseases such as cancer.
Owner:ARRAY BIOPHARMA INC
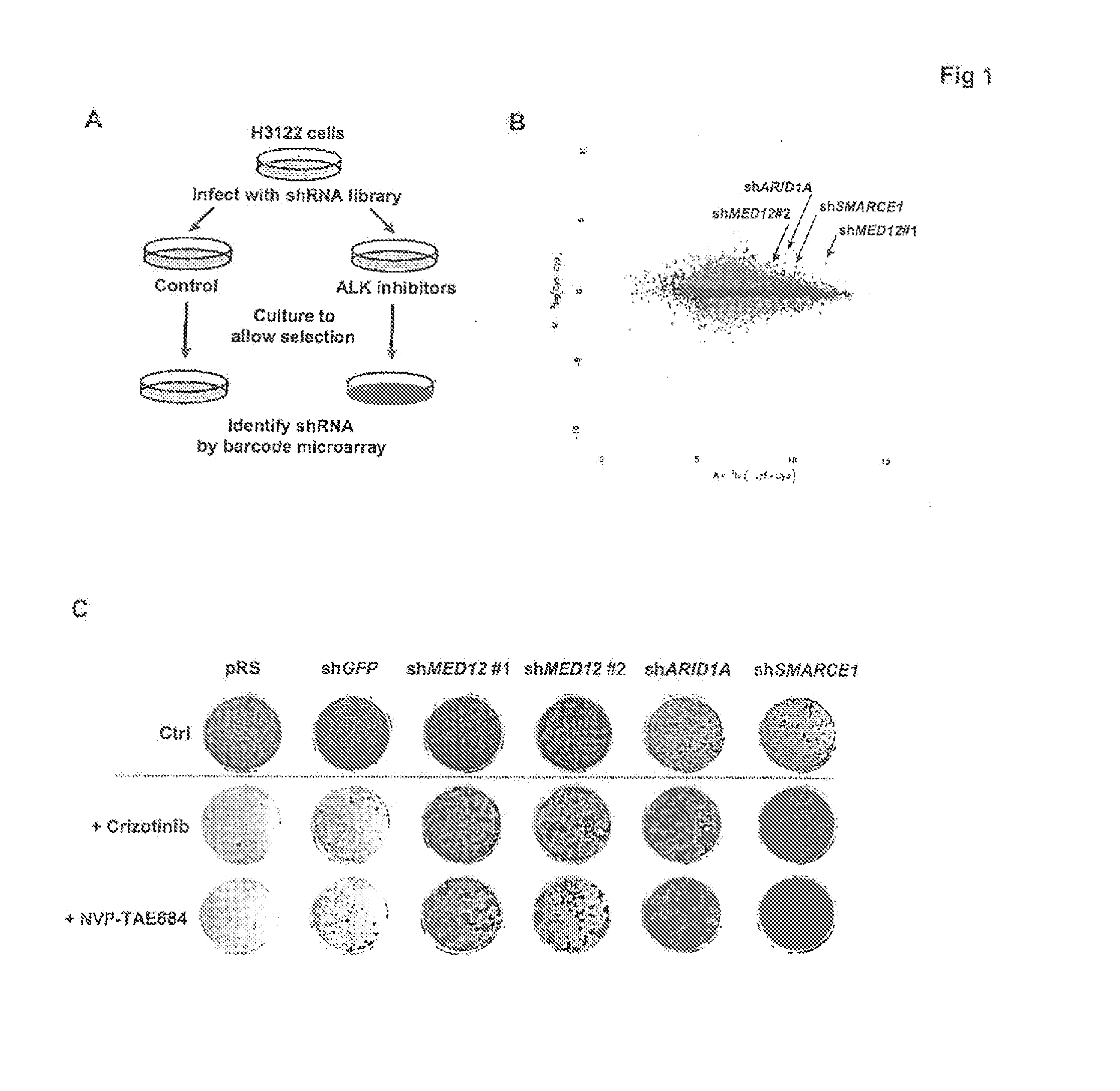
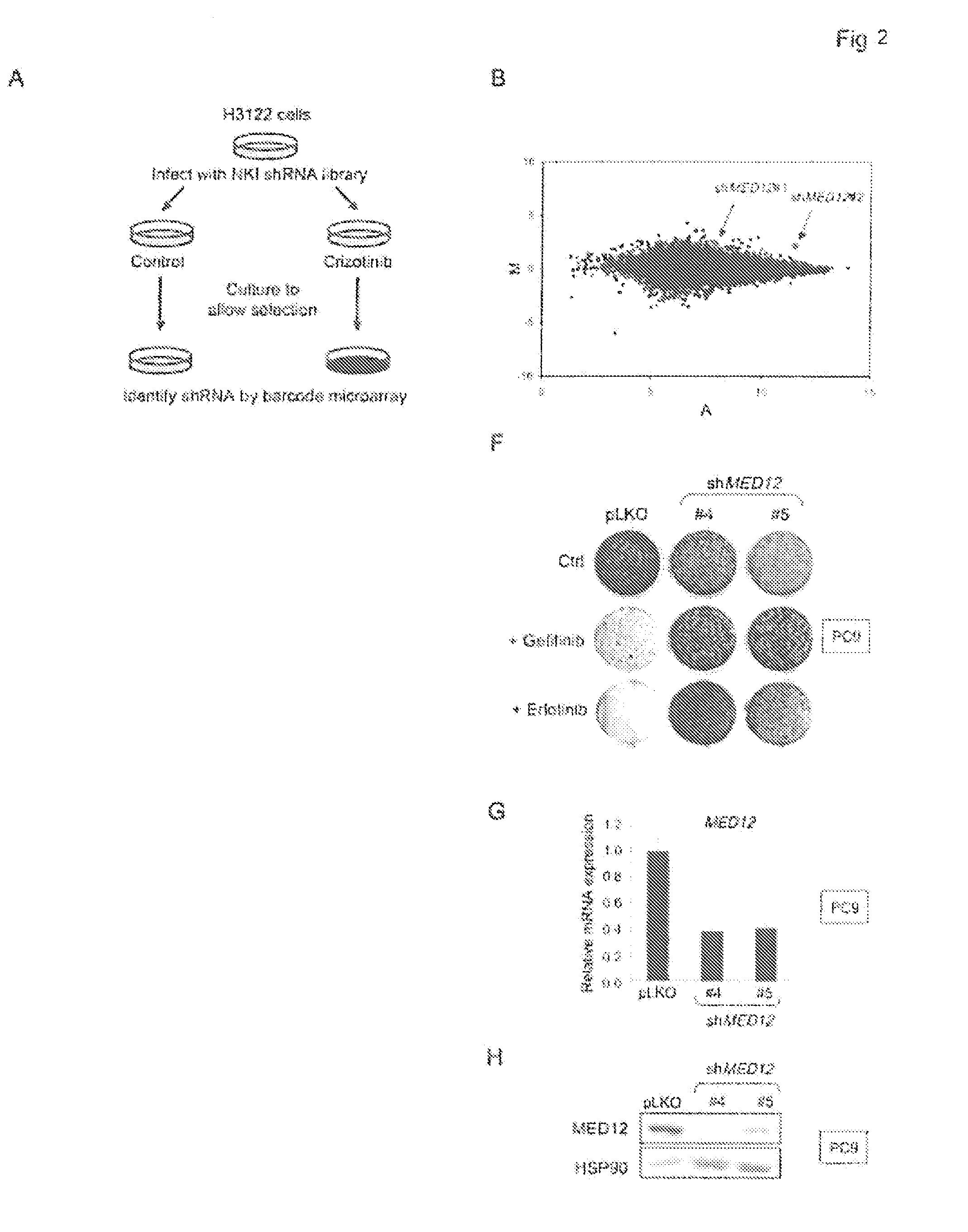
Methods and compositions for predicting resistance to anticancer treatment
InactiveUS20140296248A1Reduce expressionBiocideMicrobiological testing/measurementCancer cellReceptor tyrosine kinase inhibitor
The instant application provides methods and related compositions pertaining to the identification of resistance to anticancer treatment in a patient. In a particular embodiment, the invention provides biomarkers for the identification of resistance to anticancer treatment in a lung cancer patient, wherein a reduced expression of a MEDIATOR and / or SW1 / SNF complex gene in the lung cancer cells of the patient indicates that the lung cancer cells in the patient may be resistant to treatment with a receptor tyrosine kinase inhibitor, such as gefitinib and / or erlotinib. In some embodiments, the invention relates to methods and related compositions for predicting resistance to anticancer treatment by detecting the expression levels of one or more TGF-beta pathway nucleic acids and / or proteins.
Owner:STICHTING HET NEDERLANDS KANKER INSTIUUT ANTONI VAN LEEUWENHOEK ZIEKENHUIS
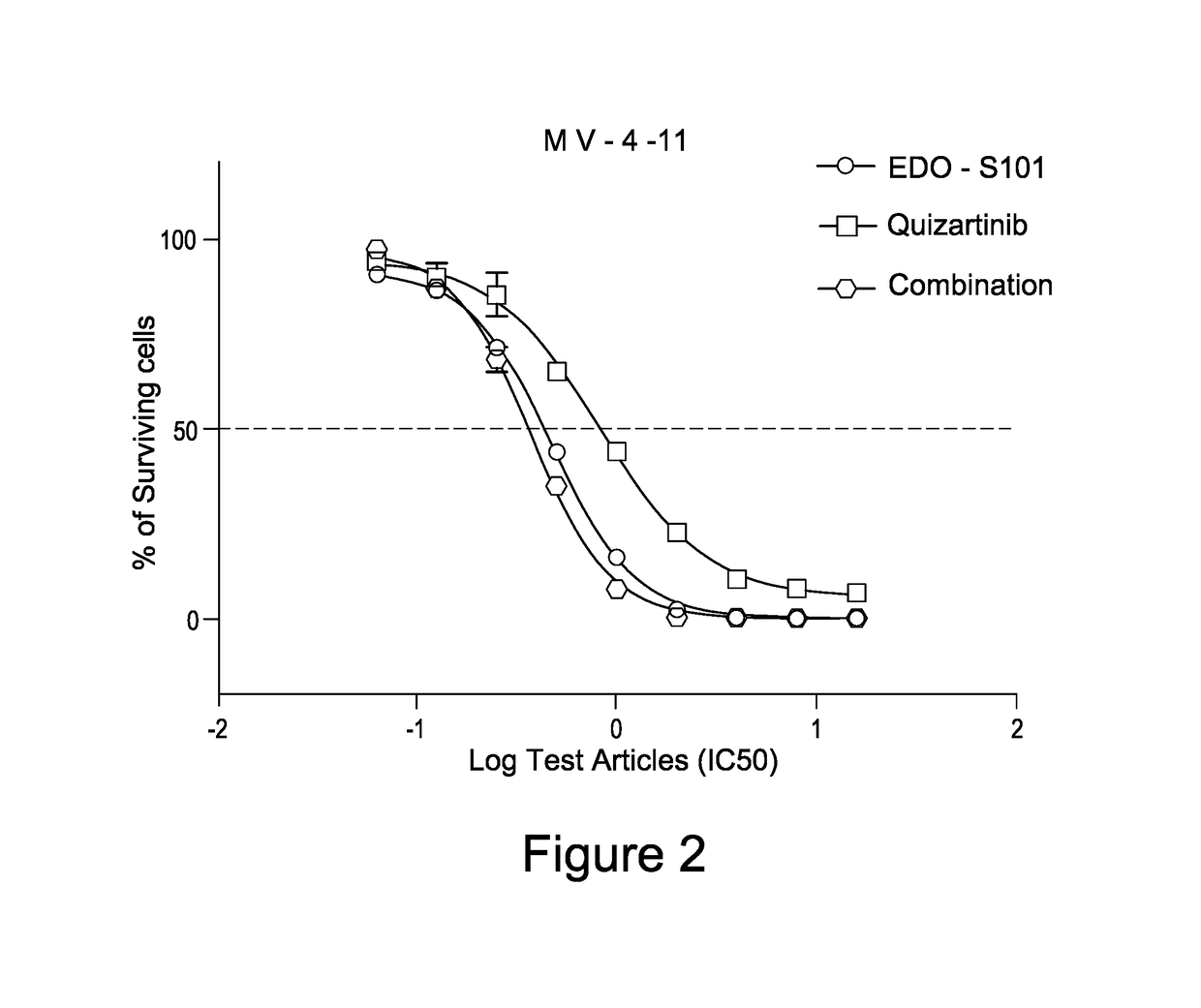
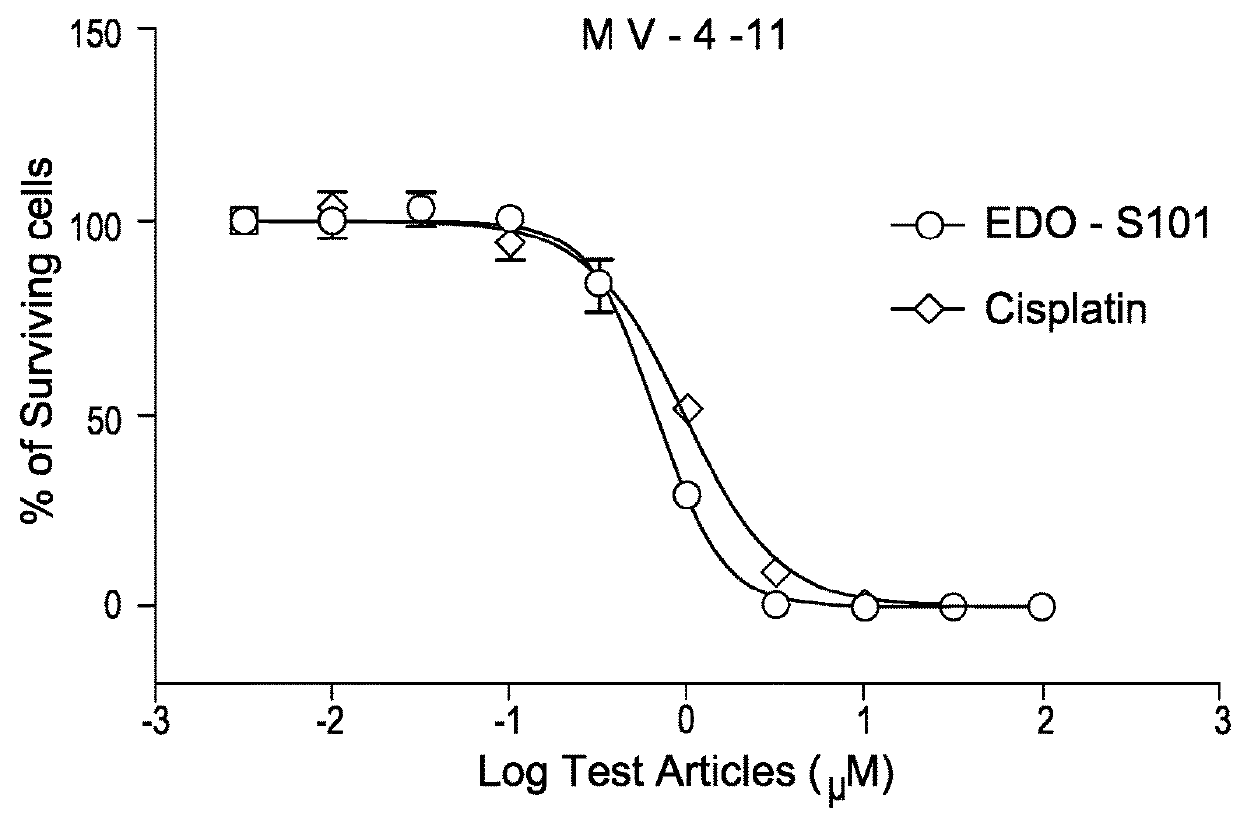
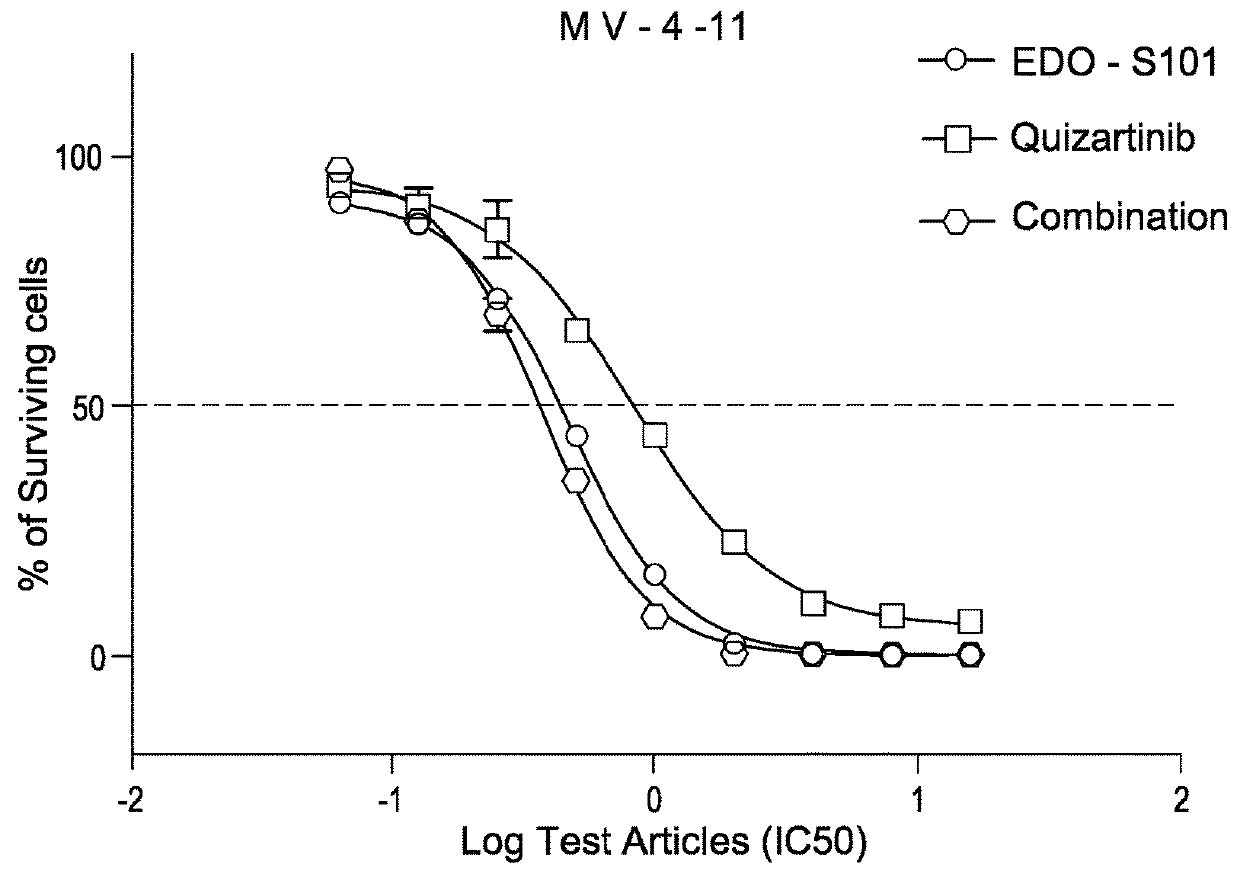
Pharmaceutical combination comprising a class iii receptor tyrosine kinase inhibitor and the alkylating histone-deacetylase inhibitor fusion molecule edo-s101 together with its use in the treatment of cancer
The present invention is directed to a combination comprising a class III receptor tyrosine kinase inhibitor and a compound of formula I or a pharmaceutically acceptable salt thereof:to a pharmaceutical composition and to a kit both comprising said combination, to the combination, composition or kit for use in the treatment of cancer, and to a method of treatment of cancer in a patient in need thereof comprising administering to said patient an effective amount of said combination, composition or kit.
Owner:PURDUE PHARMA PRODS
Pharmaceutical combination comprising a class III receptor tyrosine kinase inhibitor and the alkylating histone-deacetylase inhibitor fusion molecule EDO-S101 together with its use in the treatment of cancer
The present invention is directed to a combination comprising a class III receptor tyrosine kinase inhibitor and a compound of formula I or a pharmaceutically acceptable salt thereof:to a pharmaceutical composition and to a kit both comprising said combination, to the combination, composition or kit for use in the treatment of cancer, and to a method of treatment of cancer in a patient in need thereof comprising administering to said patient an effective amount of said combination, composition or kit.
Owner:PURDUE PHARMA PRODS
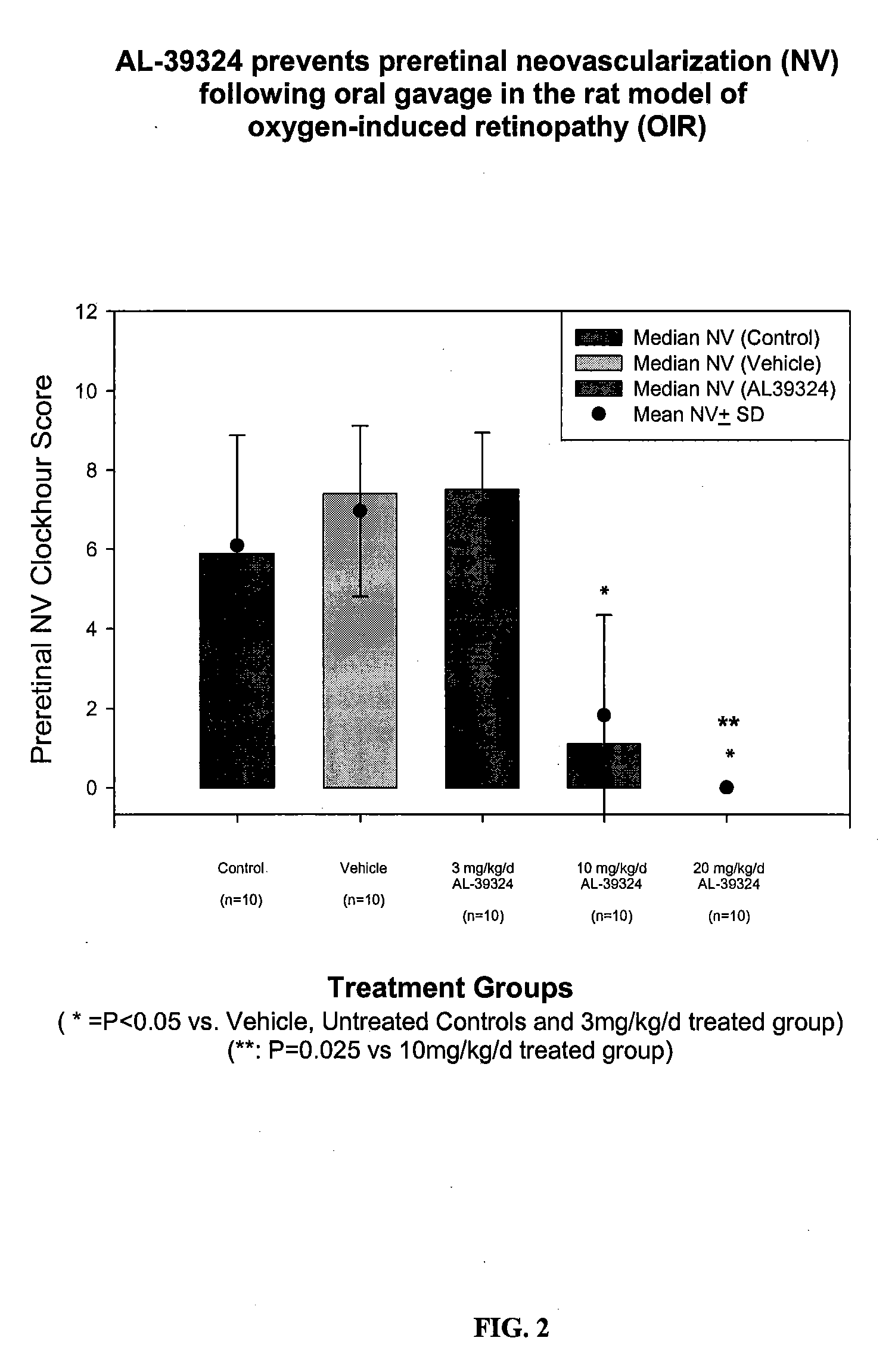
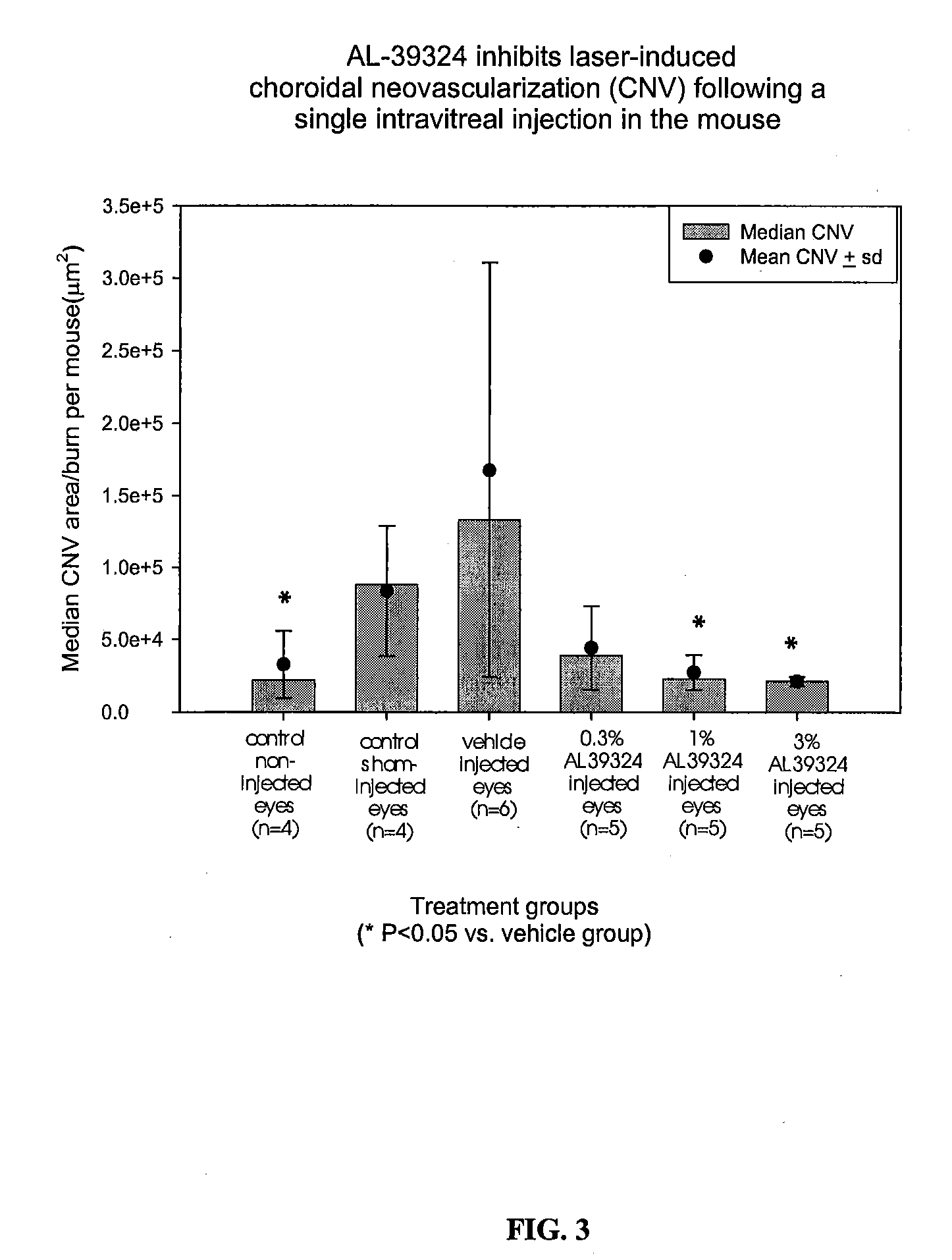
Methods for treating macular edema and ocular angiogenesis using an Anti-inflammatory agent and a receptor tyrosine kinase inhibitor
The present invention provides methods for inhibiting increased vascular permeability and / or pathologic ocular angiogenesis via administration of a combination of one or more molecules that potently inhibit select receptor tyrosine kinases (RTKs) or vascular endothelial growth factor (VEGF) and one or more anti-inflammatory agents.
Owner:NOVARTIS AG
Detection of extracellular tumor-associated nucleic acid in blood plasma or serum
InactiveUS8048629B2Enabling detectionFacilitates selection and monitoringMicrobiological testing/measurementGenetic material ingredientsReceptor tyrosine kinase inhibitorBlood plasma
This invention relates to detection of specific extracellular DNA in plasma or serum fractions of human or animal blood associated with neoplastic, pre-malignant or proliferative disease. Specifically, the invention relates to detection tumor-associated DNA, and to those methods of detecting and monitoring tumor-associated DNA found in the plasma or serum fraction of blood by using DNA extraction and amplification with or without enrichment for DNA. The invention allows the selection and monitoring of patients for various cancer therapies including receptor tyrosine kinase inhibitor therapies.
Owner:PENN STATE RES FOUND
Quinoline derivatives as axl kinase inhibitors
Novel compounds which are inhibitors of receptor tyrosine kinases of the AXL receptor family are described herein. These compounds are suitable for the treatment or prevention of disorders associated with, accompanied by or caused by hyperfunction of a receptor of the AXL family. The compounds are suitable for the treatment of hyperproliferative disorders, such as cancer, particularly cancer metastases.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
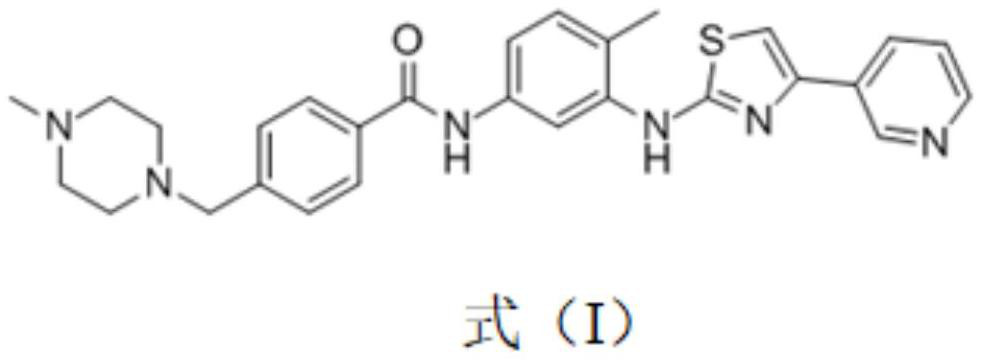
Novel amino pyridine compound, its preparation method, pharmaceutical composition containing compound and application thereof
The present invention relates to the field of medicinal chemistry and pharmacotherapeutics, specifically to a compound which is used as a receptor tyrosine kinase MET inhibitor and is shown in the formula I, its enantiomer, diastereomer, racemate and their mixture or its pharmaceutically acceptable salt, a preparation method thereof, a pharmaceutical composition containing the compound and an application thereof.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
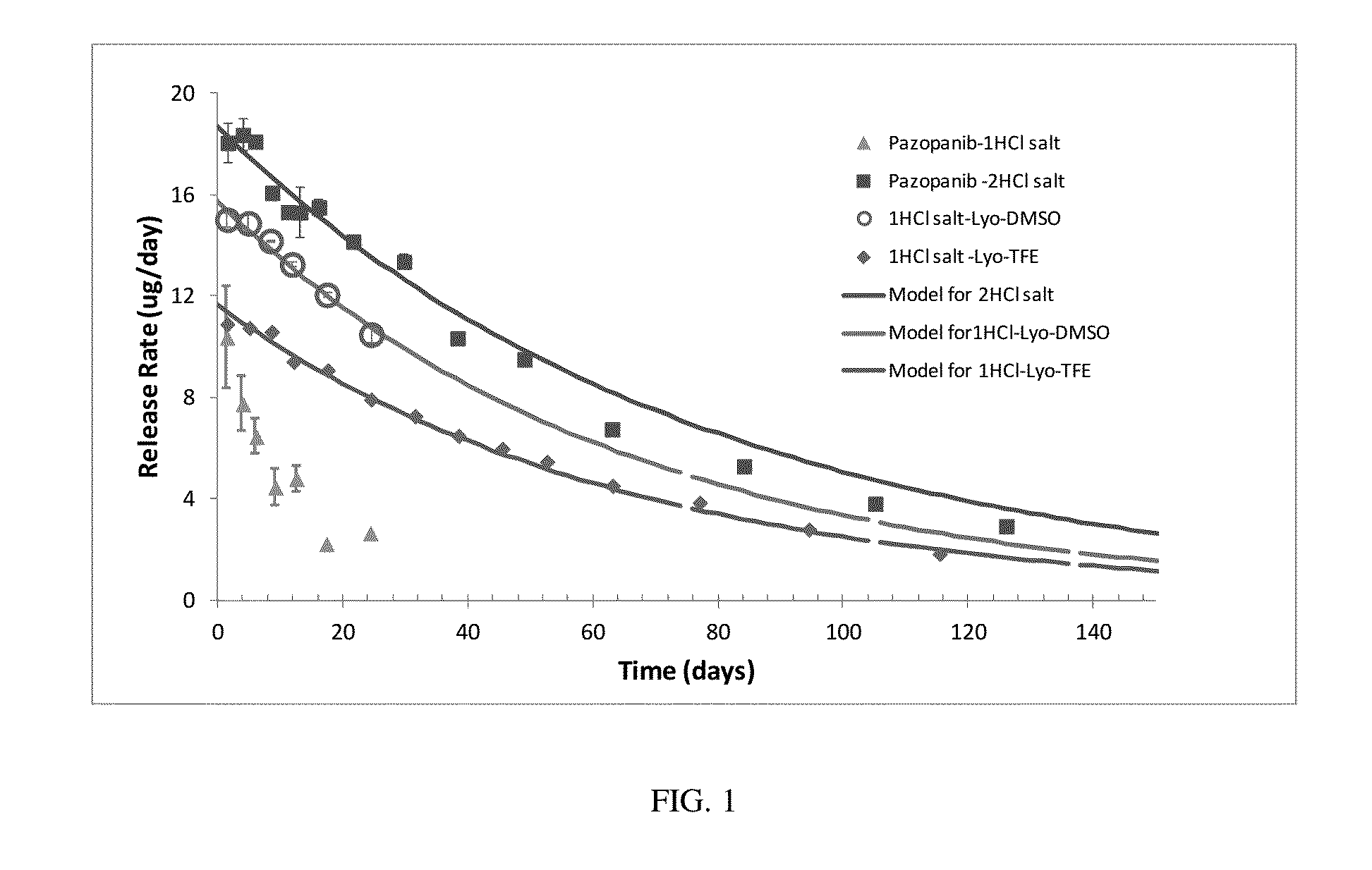
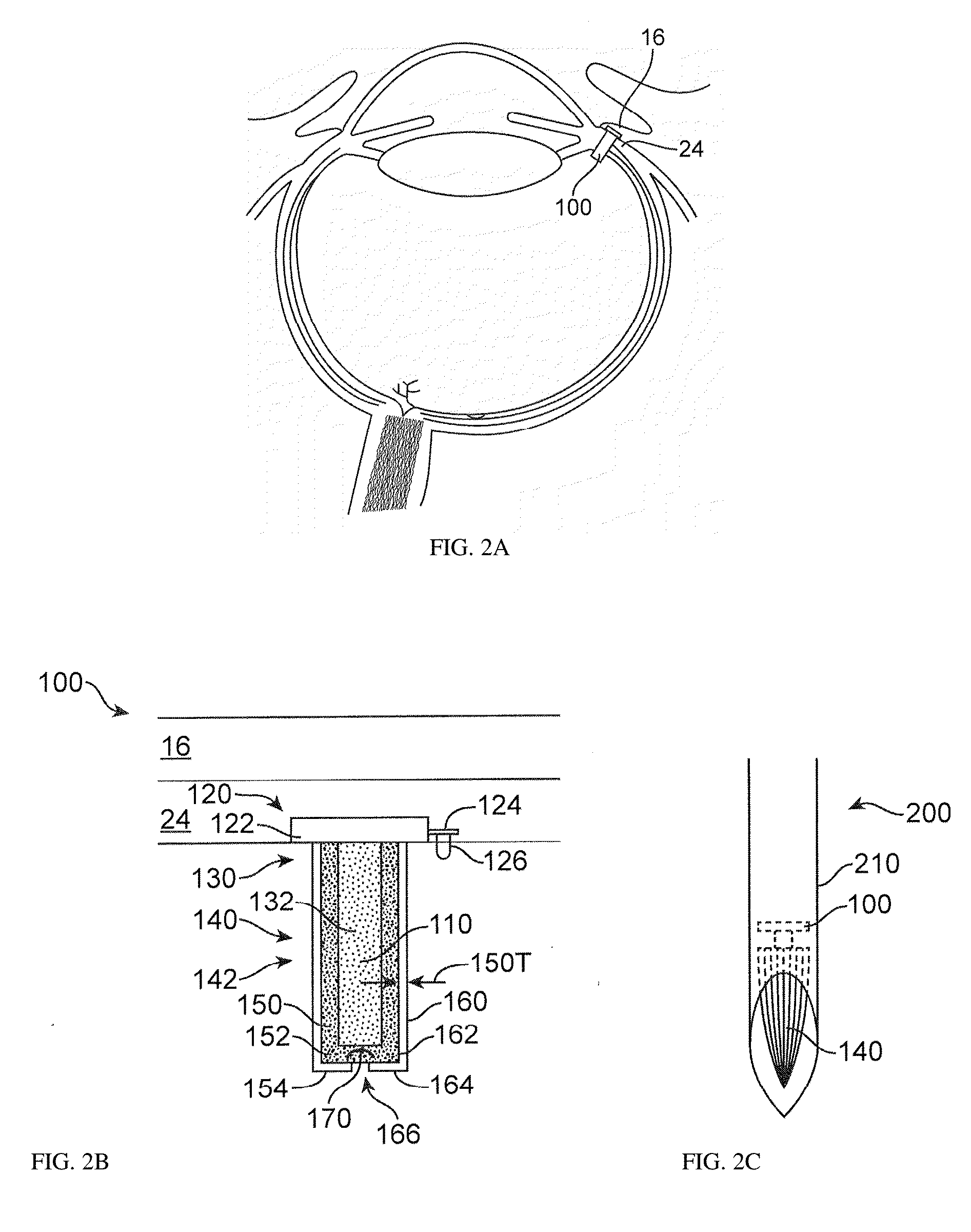
Stable and Soluble Formulations of Receptor Tyrosine Kinase Inhibitors, and Methods of Preparation Thereof
ActiveUS20160038488A1Low water solubilityImprove stabilityBiocideOrganic active ingredientsReceptor tyrosine kinase inhibitorActive agent
The present disclosure relates to stable formulations of receptor tyrosine kinase inhibitors (TKI), e.g., pazopanib; methods of preparation thereof; and use of the disclosed formulations in sustained delivery of the active agent to a target site. The disclosure further relates to methods of converting one polymorphic Form of a TKI to another polymorphic Form and / or an amorphous form.
Owner:FORSIGHT VISION5 INC
Treatment of human tumors with radiation and inhibitors of growth factor receptor tyrosine kinases
InactiveUS20090297509A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman tumorReceptor tyrosine kinase inhibitor
Owner:IMCLONE SYSTEMS
Targeting medicament curative effect related gene mRNA expressive level detection Luminex and detection method
InactiveCN101705283AStrong specificityImprove signal-to-noise ratioMicrobiological testing/measurementFluorescence/phosphorescenceRNA extractionTyrosine
The invention discloses a receptor tyrosine kinases inhibiting type targeting medicament curative effect related gene mRNA expressive level detection Luminex. The detection Luminex mainly comprises a micro-ball, supporting extending probes, and amplification extension probes. The micro-ball is coupled with a supporting probe modified by amino; the supporting extending probes are used for connecting the supporting probe and a target gene mRNA, and each supporting extending probe comprises a specific sequence the terminal 5' of which can combine with a corresponding target gene mRNA, a spacer arm sequence, and a sequence the terminal 3' of which can complementarily pair with the corresponding specific sequence of the supporting probe at; and the amplification extension probes are used for connecting the target gene mRNA and information detection components, and each amplification extension probe comprises a specific sequence the terminal 5' of which can combine with the corresponding target gene mRNA, a spacer arm sequence, and a sequence the terminal 3' of which can complementarily pair with a markup probe. The invention also discloses a detection method of the receptor tyrosine kinases inhibiting type targeting medicament curative effect related gene mRNA expressive level by using the liminex. In the invention, the process can be carried out without the steps of RNA extraction, reverse transcription, PCR and the like and the detection result is influenced less by the quality of RNA in the sample, thereby the accuracy of the detection result is guaranteed; on the other hand, because the liminex technology enables a parallel multi-index detection to be realized, multiple inside genes can be set in one detection, thereby the detection result is more reliable.
Owner:SUREXAM BIO TECH
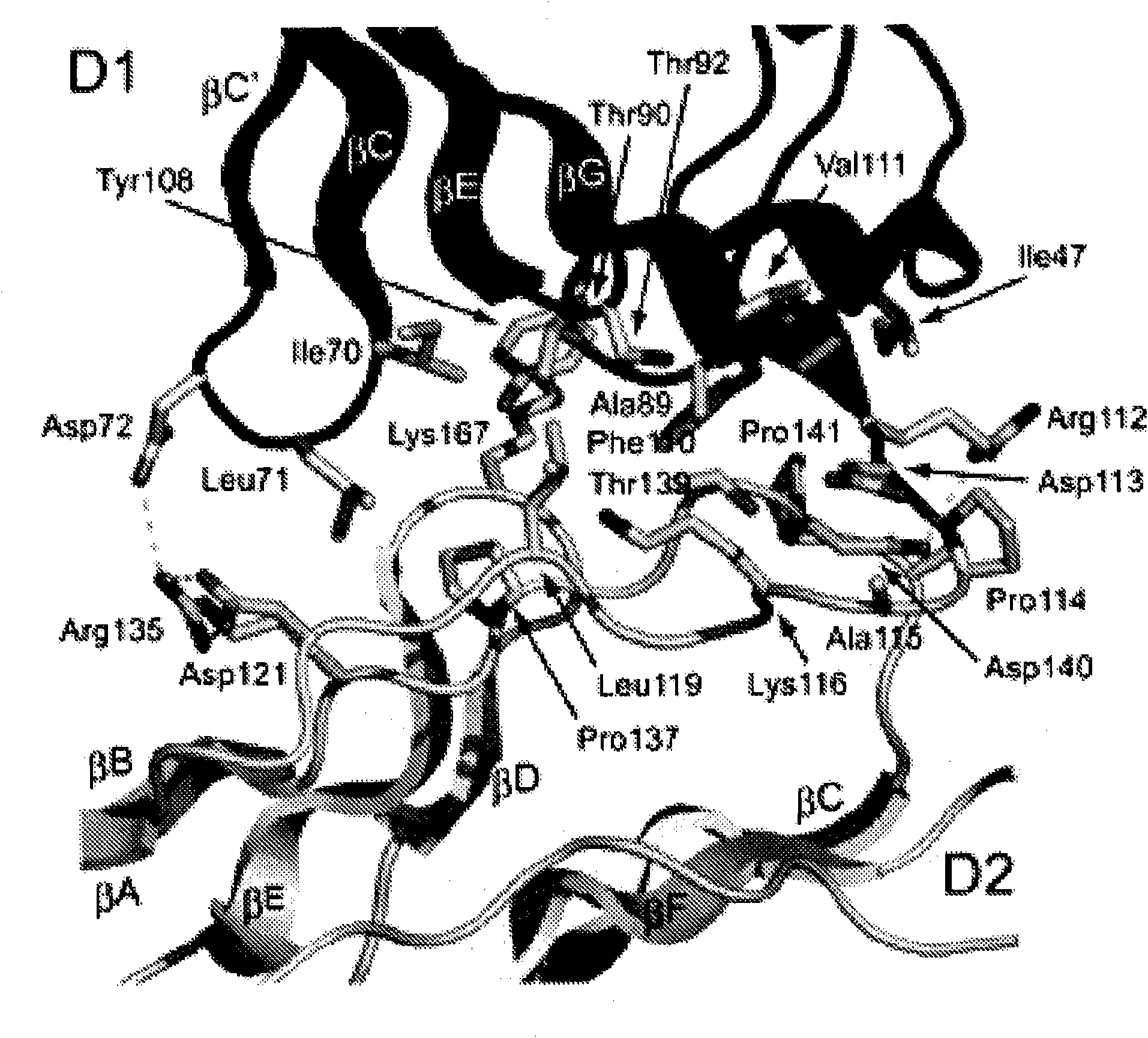
Inhibitors of receptor tyrosine kinases and methods of use thereof
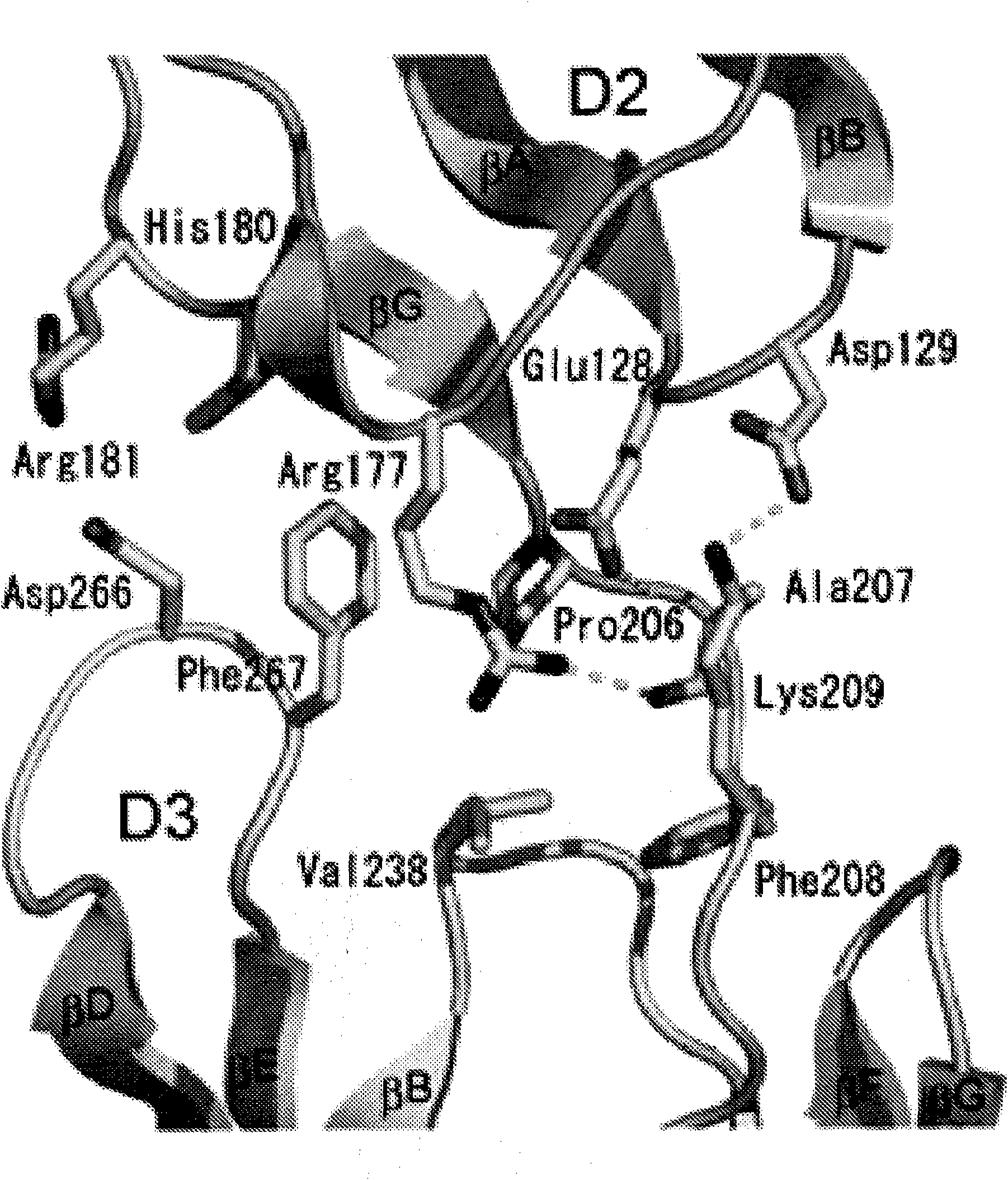
The present invention provides moieties that bind to an Ig-like domain, e.g., D4 or D5, of a human receptor tyrosine kinase, e.g., the human Kit RTK or the PDGFR RTK, or the D7 domain of a type V receptor tyrosine kinase wherein the moieties lock the ectodomain of the receptor tyrosine kinase in an inactive state thereby antagonizing the activity of the receptor tyrosine kinase.
Owner:YALE UNIV
Application of 4-benzothiopheneaminoquinazoline derivative in preparing tumor treatment medicine
InactiveCN101836992AGrowth inhibitionMany examplesOrganic active ingredientsAntineoplastic agentsProstate cancerAutophagic death
The invention discloses application of 4-benzothiopheneaminoquinazoline derivative and salt thereof in preparing tumor treatment medicine. The in vitro and in vivo activities of the 4-benzothiopheneaminoquinazoline derivative are evaluated; and the derivative kills tumor cells by means of inducing tumor cell apoptosis and autophagy to inhibit the growth of tumor cells and provide the application in preparing medicaments for treating cancers, especially pancreatic cancer, prostate cancer, lung cancer or breast cancer. In the invention, the action mechanism and the action target of the 4-benzothiopheneaminoquinazoline derivative are studied, and the derivative has stronger inhibitory activity to a plurality of receptortyrosinekinases and provides the application of acting as an inhibitor of receptortyrosinekinase, especially polyreceptortyrosinekinase.
Owner:SOUTHEAST UNIV
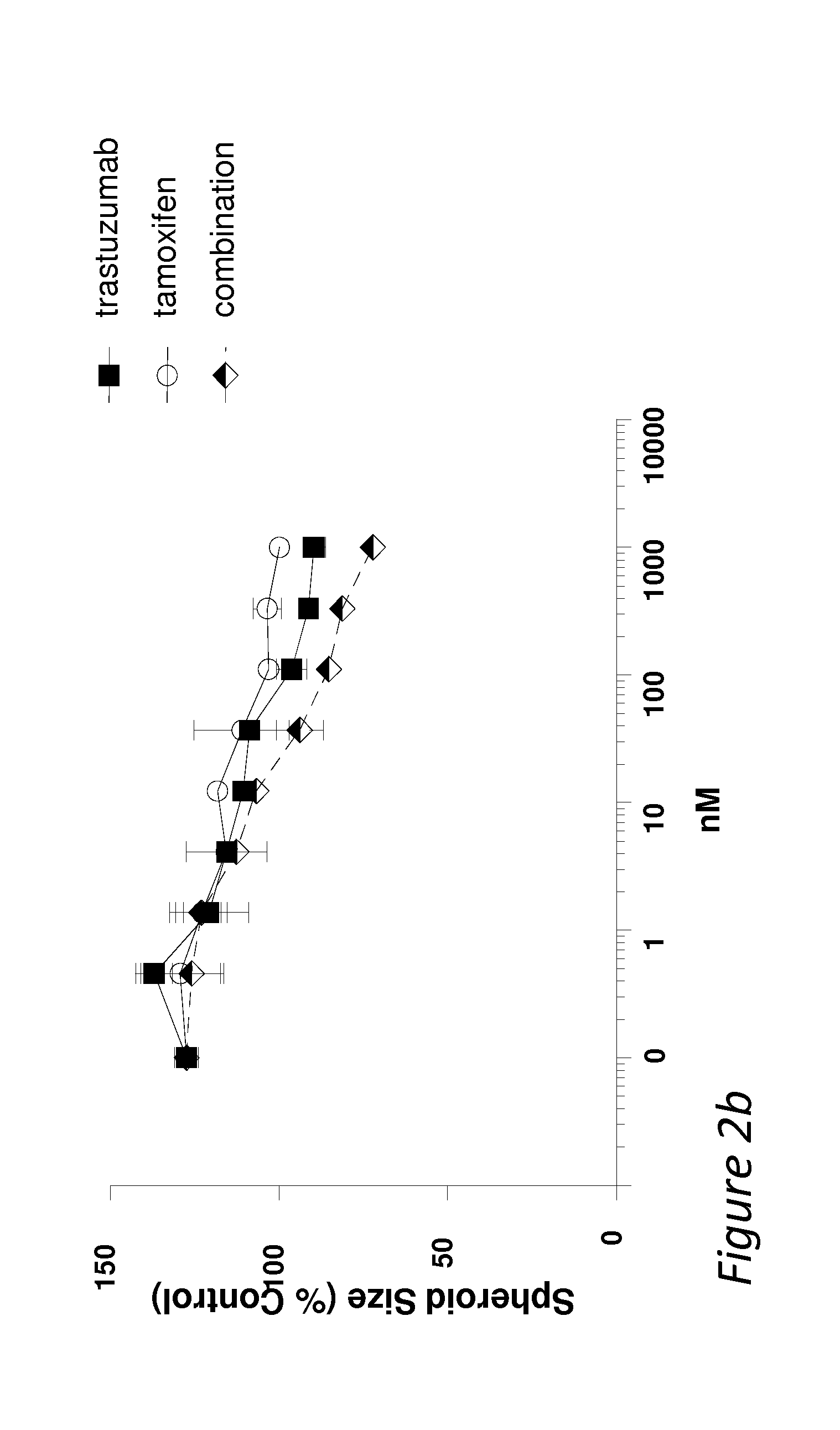
Combination therapies comprising Anti-erbb3 agents
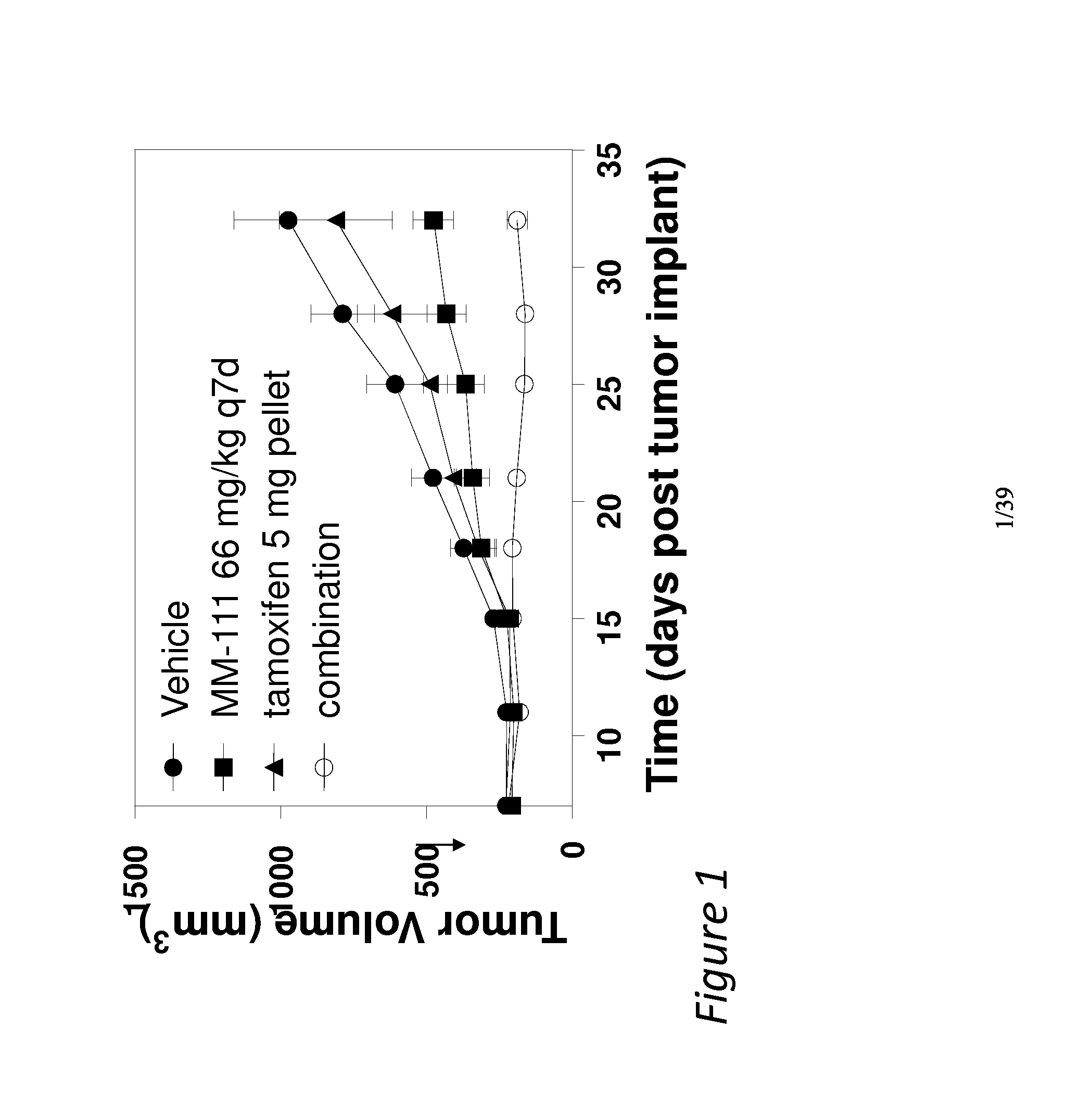
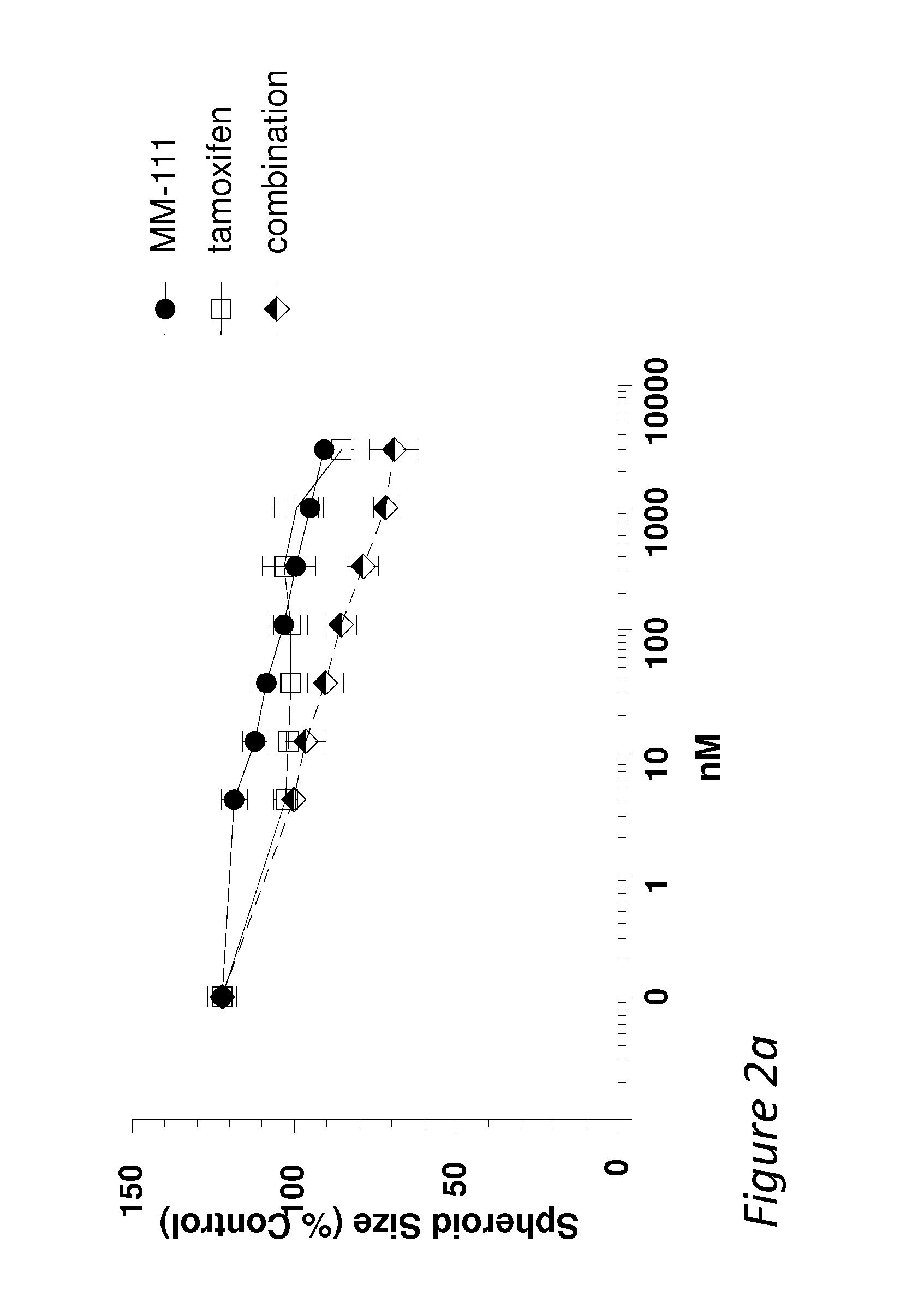
Disclosed are methods and compositions for inhibiting the growth of a tumor (e.g., a malignant tumor) in a subject. In particular, combination therapies for treating a tumor in a subject by co-administering an agent selected from i) an effective amount of an anti-estrogen agent; ii) an effective amount of a receptor tyrosine kinase inhibitor; iii) an effective amount of a MEK / PI3 kinase / AKT inhibitor; iv) an effective amount of MM-151; v) an effective amount of an mTOR inhibitor; and / or vi) an effective amount of trastuzumab or TMD1, and / or combinations thereof; and an effective amount of a bispecific anti-ErbB2 / anti-ErbB3 antibody. Also disclosed is a bispecific anti-ErbB2 / anti-ErbB3 antibody for use in the therapy of a tumor in combination with an agent selected from i) an effective amount of an anti-estrogen agent; ii) an effective amount of a receptor tyrosine kinase inhibitor; iii) an effective amount of a MEK / PI3 kinase / AKT inhibitor; iv) an effective amount of MM-151; v) an effective amount of an mTOR inhibitor; and / or vi) an effective amount of trastuzumab or TMD1, and / or combinations thereof.
Owner:14NER ONCOLOGY INC
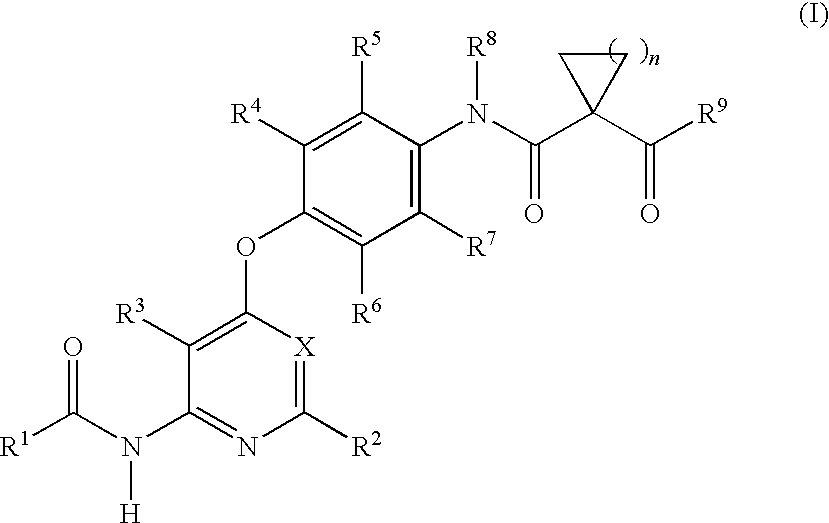
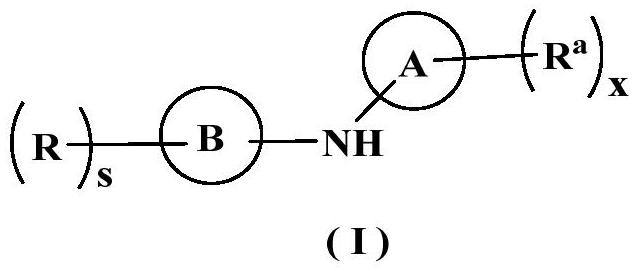
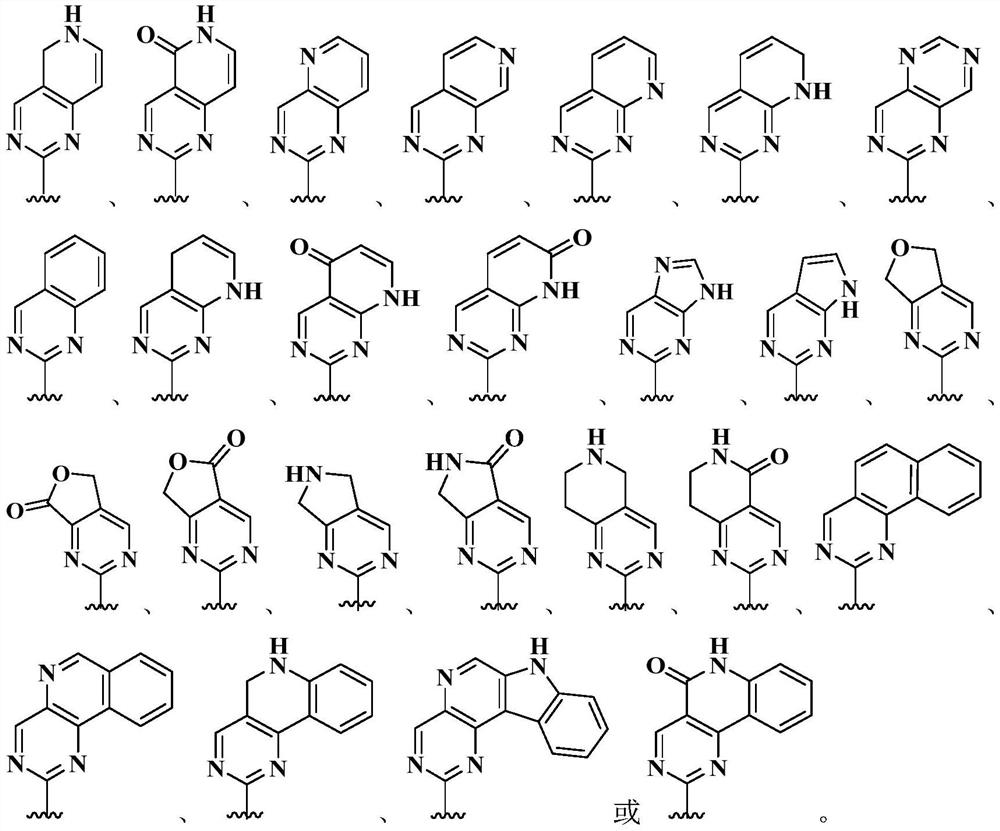
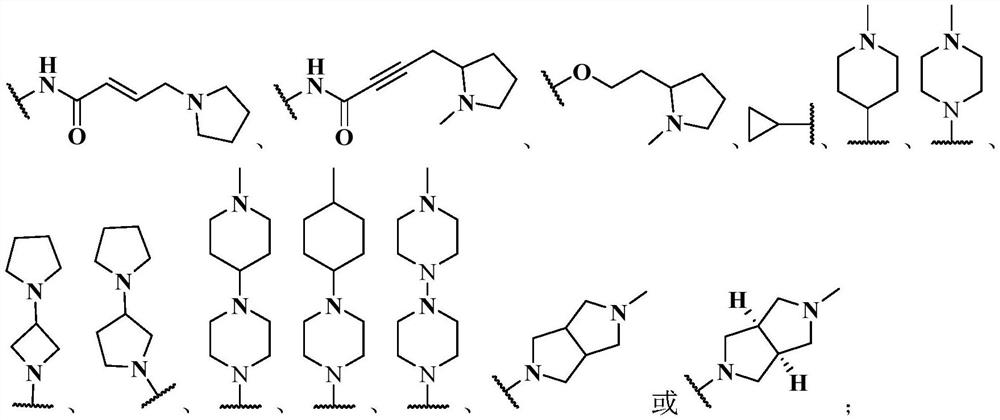
Inhibitor containing fused ring derivative as well as preparation method and application thereof
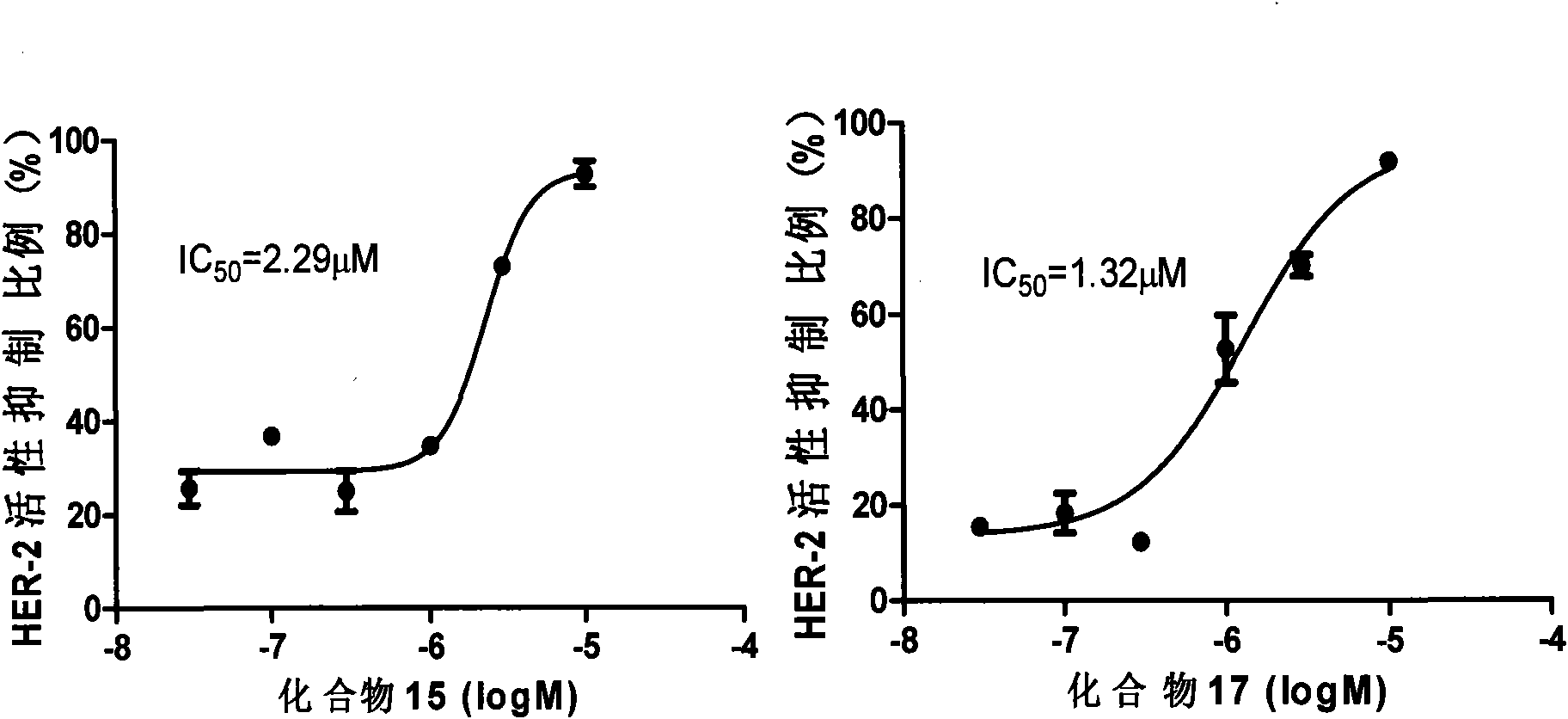
The invention relates to an inhibitor containing fused ring derivative as well as a preparation method and application thereof. Particularly, the invention relates to a compound as shown in a generalformula (I), a preparation method thereof, a pharmaceutical composition containing the compound, and application of the compound as a kinase inhibitor, particularly as a receptor tyrosine kinase inhibitor (TKI), more particularly as an EGFR (epidermal growth factor receptor) or HER2 (human epidermal growth factor receptor 2) inhibitor to treatment of cancer, inflammation, chronic liver diseases, diabetes, cardiovascular diseases, AIDS and other related diseases. Each substituent in the general formula (I) is the same as the definition in the specification.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
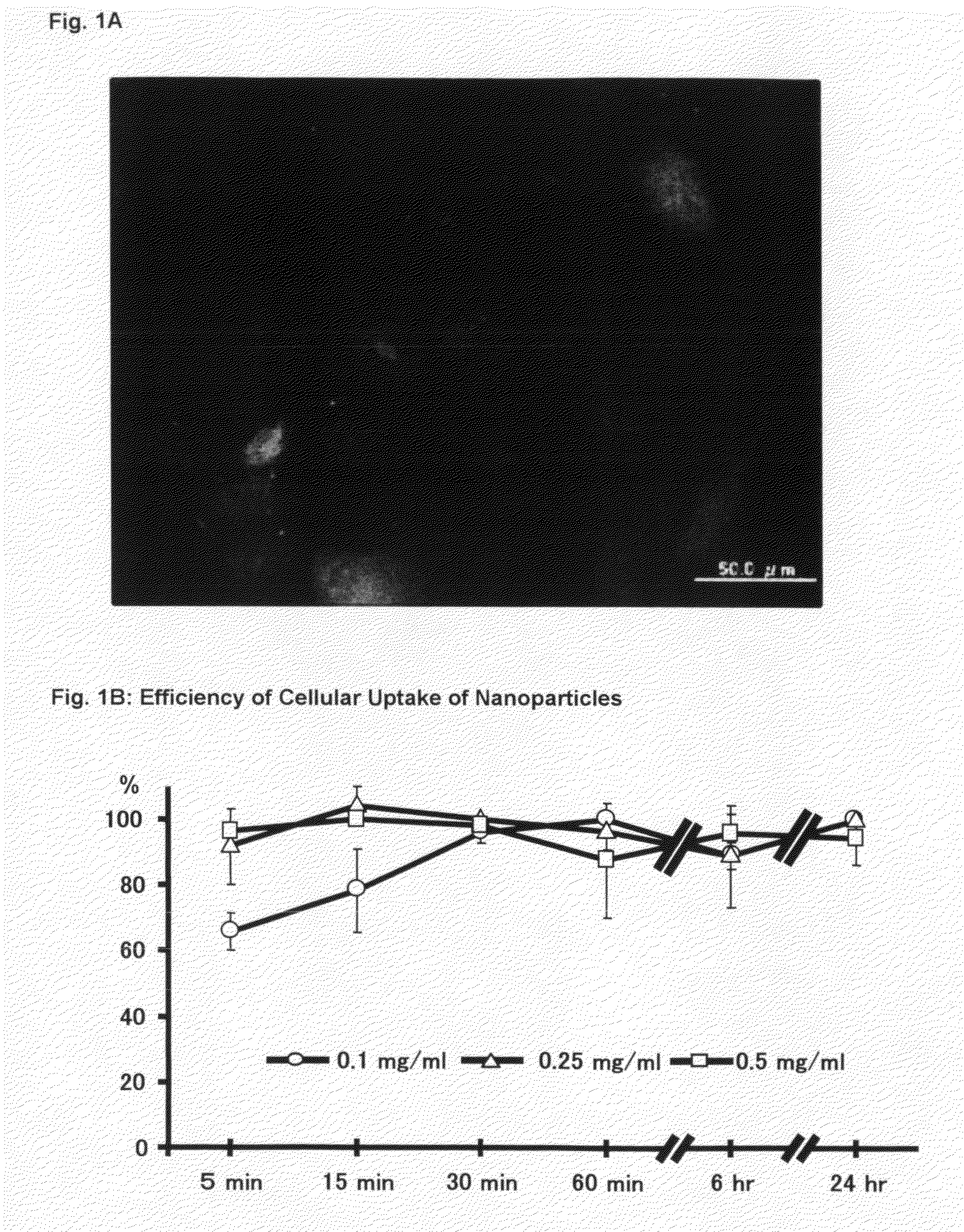
Nanoparticles Comprising a PDGF Receptor Tyrosine Kinase Inhibitor
InactiveUS20090136579A1Excellent capability of passingSuppresses proliferationPowder deliveryOrganic active ingredientsDiseaseSolubility
The present invention relates to nanoparticles comprising a platelet-derived growth factor (PDGF) receptor tyrosine kinase inhibitor, especially a PDGF receptor tyrosine kinase inhibitor having a water-solubility at 20° C. between about 2.5 g / 100 ml and 250 g / 100 ml, more specifically nanoparticles comprising an N-phenyl-2-pyrimidine-amine derivative of formula I,in which the symbols and substituents have the meanings as given herein above, in free form or in pharmaceutically acceptable salt form; to the intracellular delivery of PDGF receptor tyrosine kinase inhibitors such as Imatinib with bio-absorbable polymeric nanoparticles; the use of such nanoparticles in the manufacture of a pharmaceutical composition for the treatment of vascular smooth muscle cells growth diseases; to a method of treatment of warm-blooded animals suffering from vascular smooth muscle cells growth diseases; to a process to prepare such nanoparticles; to pharmaceutical compositions comprising such nanoparticles; and to drug delivery systems incorporating such nanoparticles for the prevention and treatment of vascular smooth muscle cells growth diseases.
Owner:KYUSHU UNIV +1
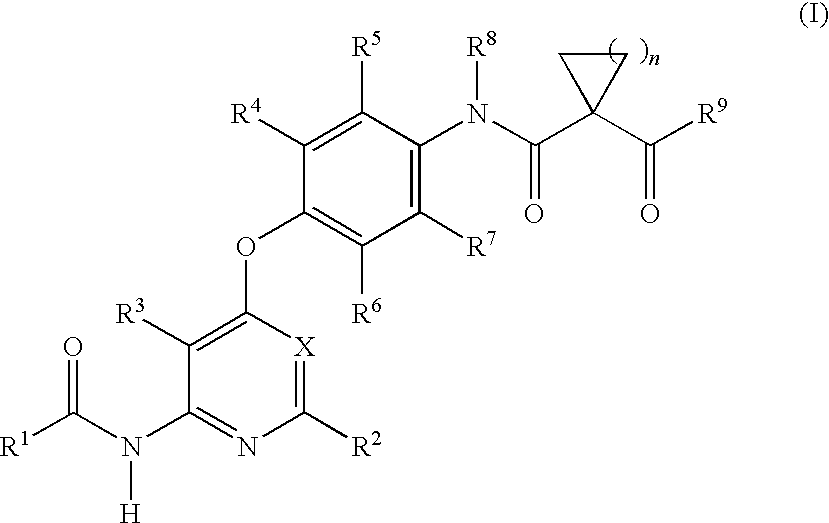
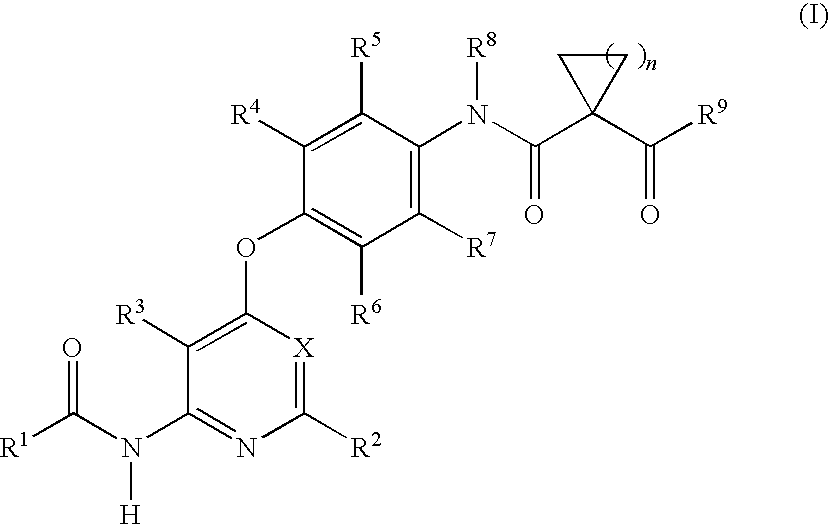
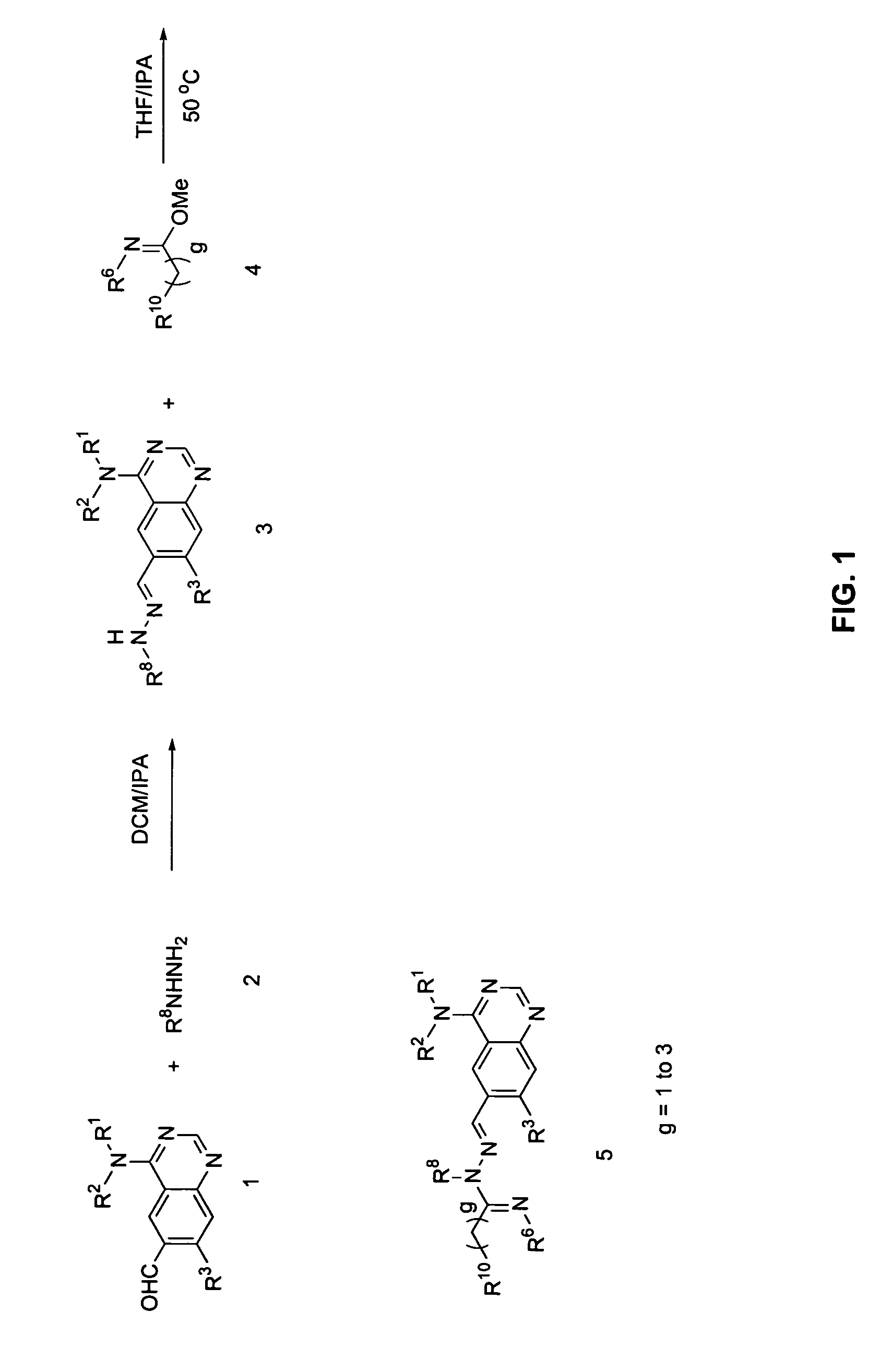
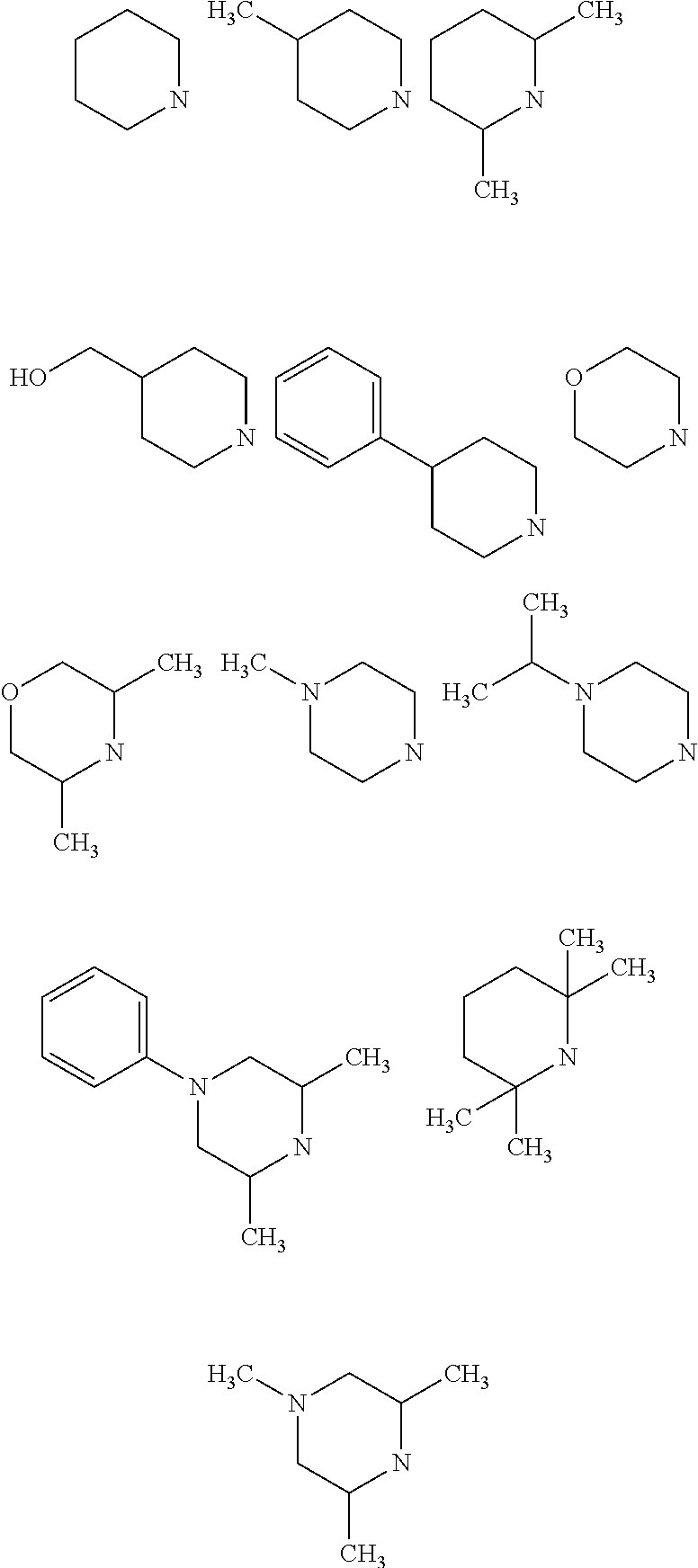
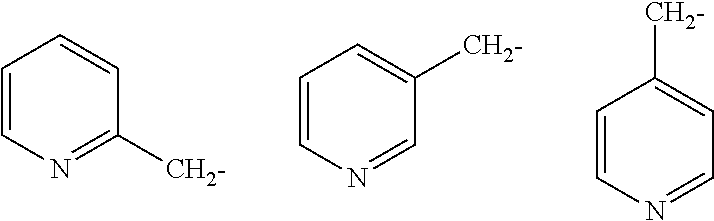
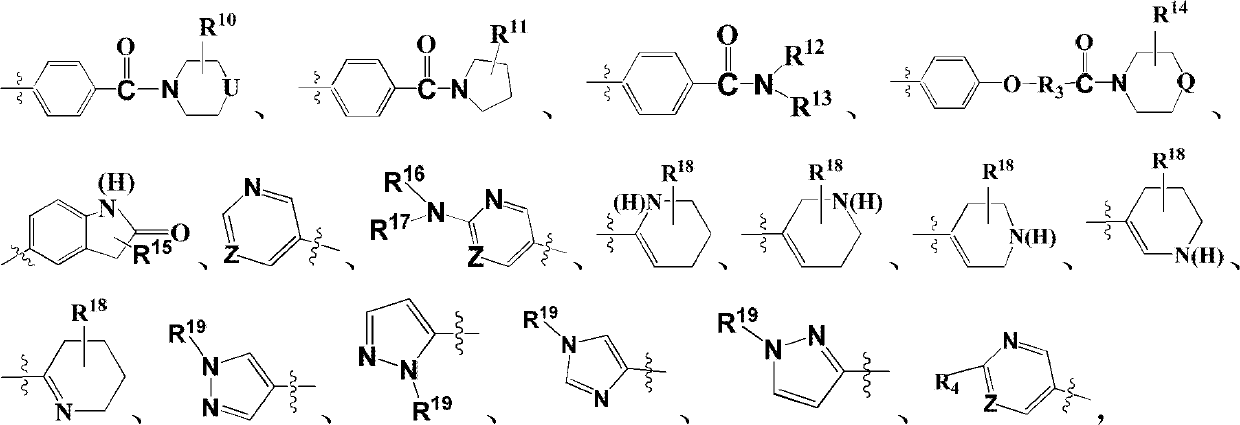
Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors
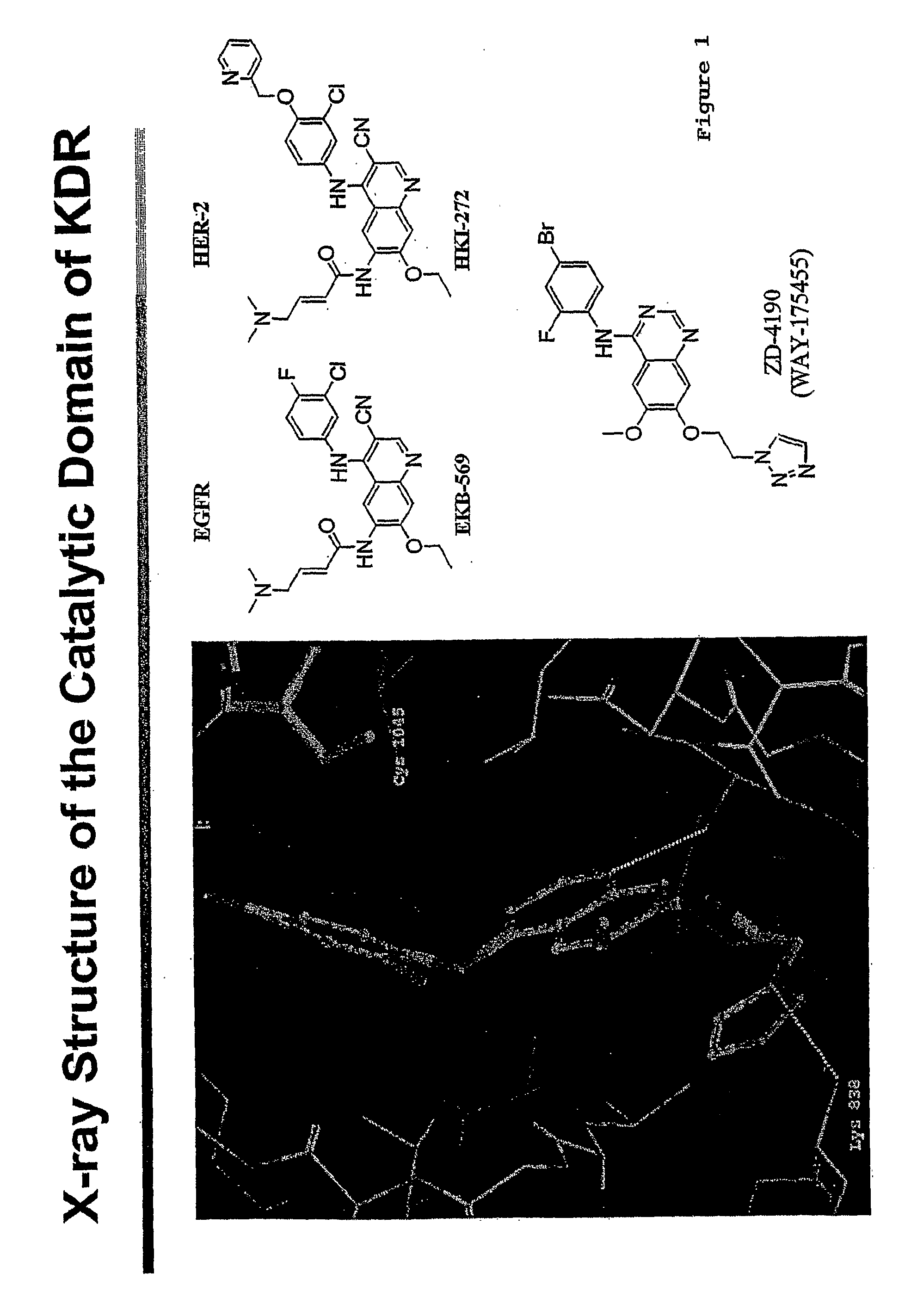
Compounds of Formula I: in which A, B, R<1>, R<1a>, R<2>, R<3>, R<4>, R<5> R<6>, R<7> and R<8> have the meanings given in the specification, are receptor tyrosine inhibitors useful in the treatment ofdiseases mediated by class 3 and class 5 receptor tyrosine kinases. Particular compounds of this invention have also been found to be inhibitors of Pim-1.
Owner:ARRAY BIOPHARMA
Compositions of protein receptor tyrosine kinase inhibitors
The present invention relates to novel synthetic substituted heterocyclic compounds and pharmaceutical compositions containing the same that are capable of inhibiting or antagonizing a family of receptor tyrosine kinases, Tropomysosin Related Kinases (Trk), in particular the nerve growth factor (NGF) receptor, TrkA. The invention further concerns the use of such compounds in the treatment and / or prevention of pain, cancer, restenosis, atherosclerosis, psoriasis, thrombosis, or a disease, disorder or injury relating to dysmyelination or demyelination or the disease or disorder associated with abnormal activities of NGF receptor TrkA.
Owner:PURDUE PHARMA LP
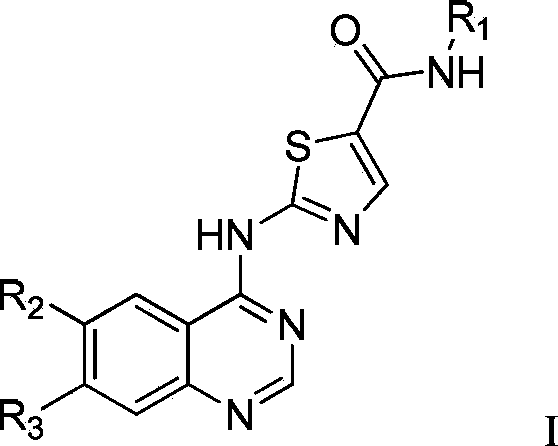
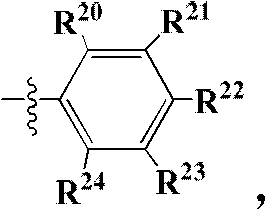
Pyrazolo[1,5-a]miazine compound and its preparation method and medical use
ActiveCN104250252AStrong antiproliferative activityEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryDiseaseReceptor tyrosine kinase inhibitor
The invention relates to a pyrazolo[1,5-a]miazine compound and its preparation method and medical use. The invention relates to the preparation method of the pyrazolo[1,5-a]miazine compound shown in the general formula (I) and its salt, a pharmaceutical composition containing the pyrazolo[1,5-a]miazine compound or its medicinal salt, the use of the pyrazolo[1,5-a]miazine compound or the pharmaceutical composition in the medicine field and especially as a receptor tyrosine kinase inhibitor and a c-Met inhibitor, and the use of the pyrazolo[1,5-a]miazine compound or the pharmaceutical composition in preparation of drugs for preventing and / or treating c-Met abnormality-related diseases.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
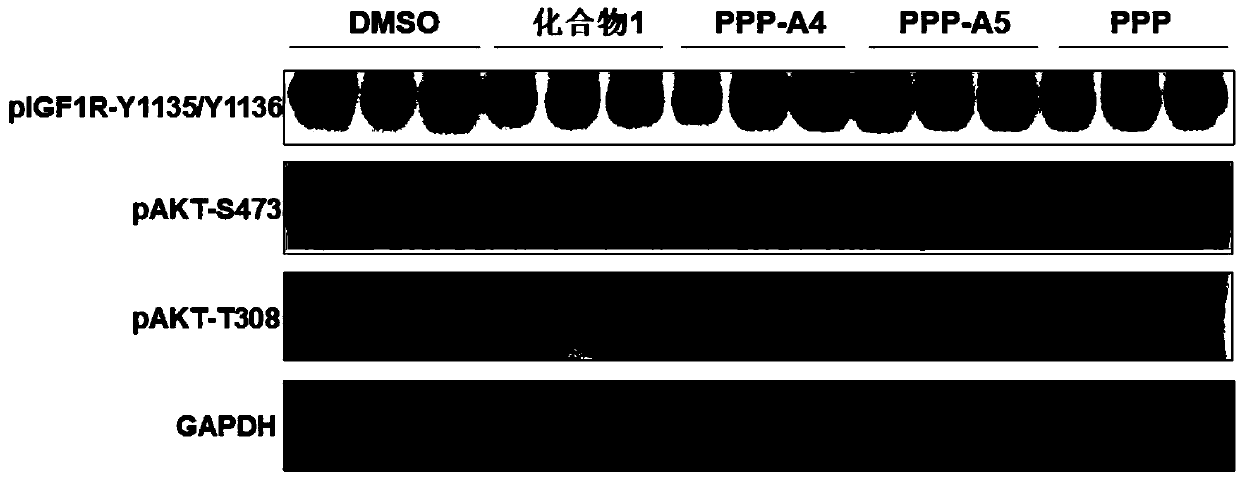
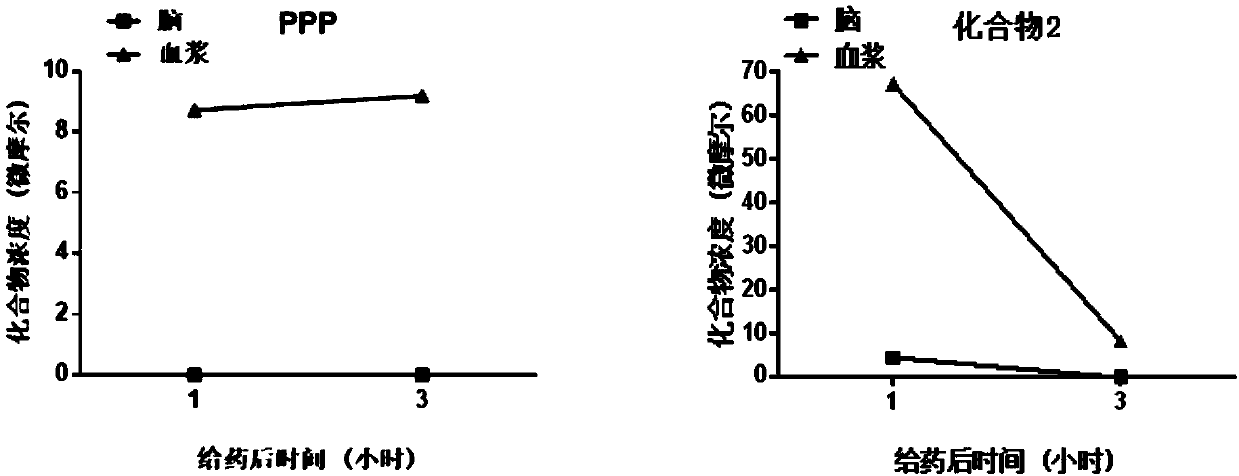
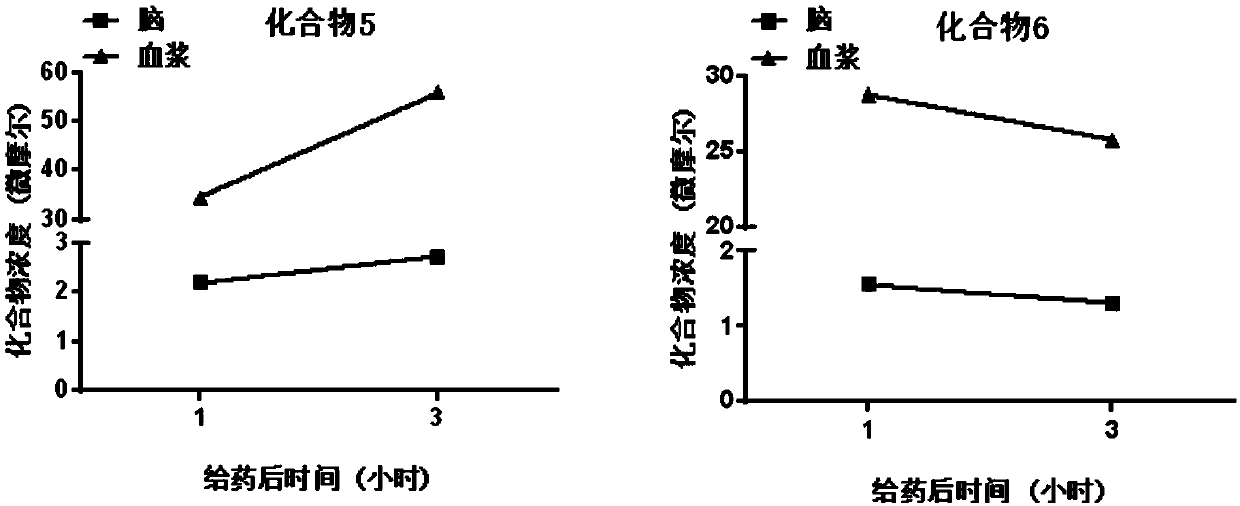
Insulin-like growth factor-1 receptor tyrosine kinase inhibitor and uses thereof
The invention discloses a class of compounds capable of being used as an insulin-like growth factor-1 receptor tyrosine kinase inhibitor, and a preparation method thereof, a pharmaceutical compositioncontaining the compound, and applications of the pharmaceutical composition in prevention or treatment of tumors, especially brain glioma.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
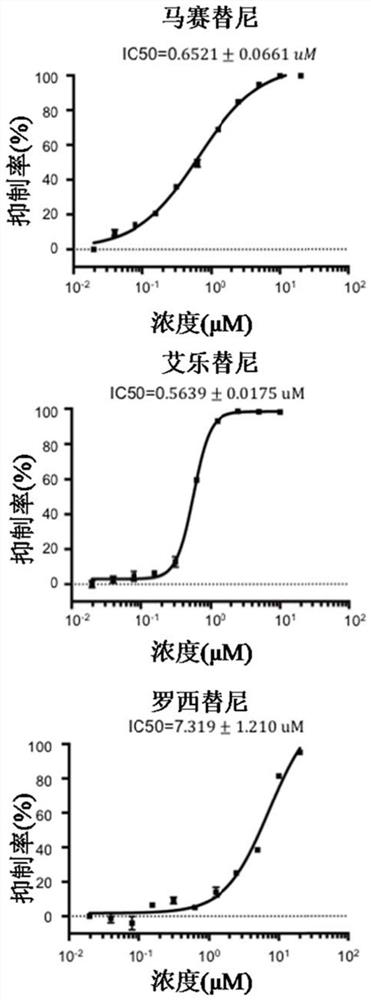
Application of receptor tyrosine kinase inhibitor in preparation of medicine for preventing and/or treating novel coronavirus infection
ActiveCN111991559ARepress transcriptionInhibition of replicationOrganic active ingredientsAntiviralsTyrosinePharmaceutical medicine
The invention belongs to the field of antiviral medicines, and discloses application of a receptor tyrosine kinase inhibitor in preparation of a medicine for preventing and / or treating novel coronavirus infection. The receptor tyrosine kinase inhibitor comprises mosatinib, alectinib, roxatidine or pharmaceutically acceptable salts of the small molecular compounds. The receptor tyrosine kinase inhibitor such as mosatinib, alectinib and roxatidine can strongly inhibit synthesis of replicon RNA of a novel coronavirus SARS-CoV-2 and reduce the RNA copy amount of a wild type novel coronavirus SARS-CoV-2 in cells, so that replication of the novel coronavirus SARS-CoV-2 is inhibited, and the receptor tyrosine kinase inhibitor has the effect of preventing and / or treating novel coronavirus SARS-CoV-2 infection.
Owner:SUN YAT SEN UNIV
Anti-CTLA4 antibody and indolinone combination therapy for treatment of cancer
InactiveCN101146553AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsReceptor tyrosine kinase inhibitorMethyl group
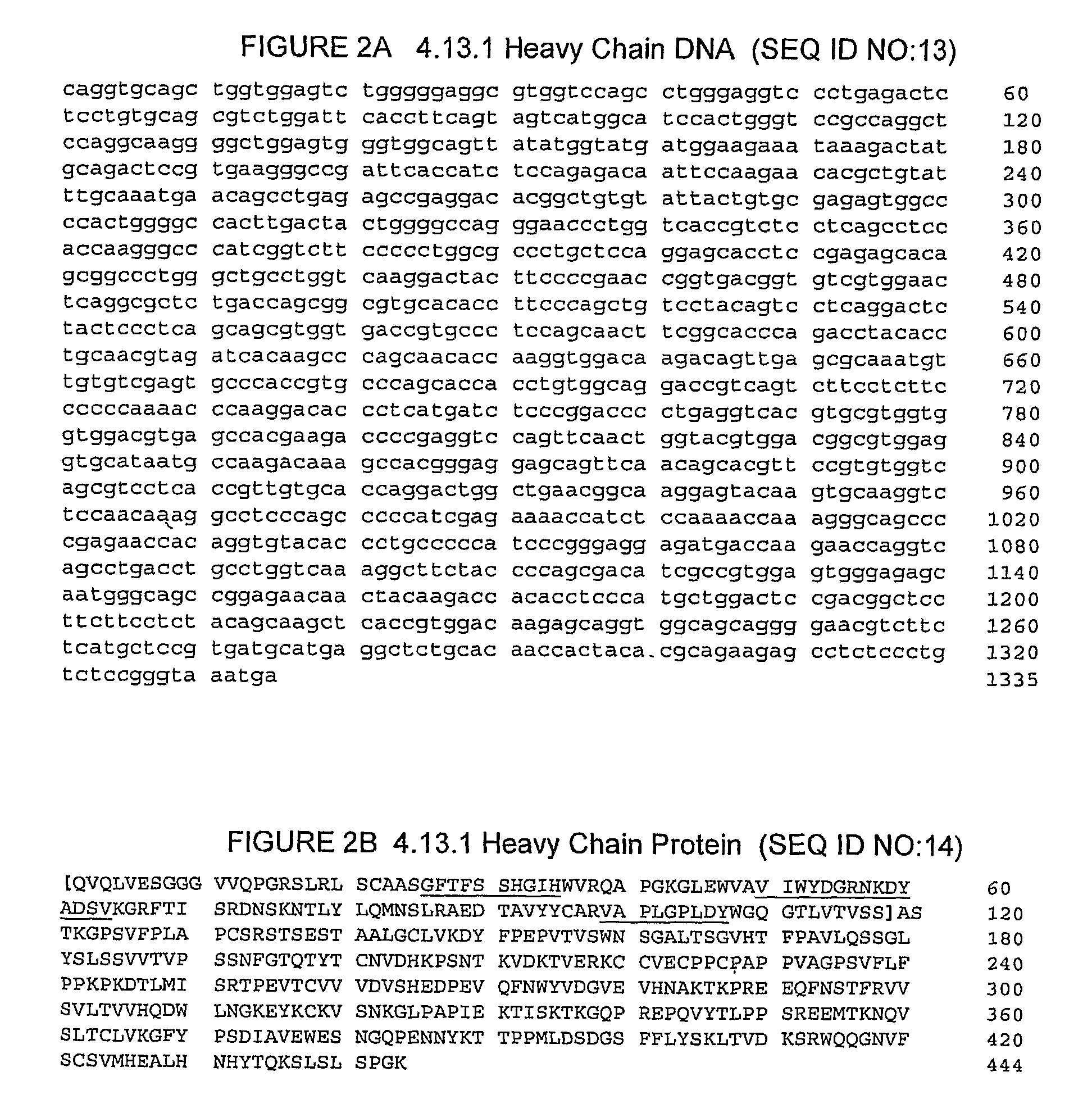
The present invention relates to the administration of anti-CTLA4 antibodies, particularly human antibodies against human CTLA4, such as with antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1 or CP-675, 206), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and antibodies to the amino acid sequences of ipilimumab (also known as 10D1 or MDX-010) , with an indolinone receptor tyrosine kinase inhibitor (RTKI), for example, N-[2-diethylamino]ethyl]-5-[(Z)-(5-fluoro-2-oxo-1, 2-dihydro-3H-indole-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carbamoyl (compound 1), N-[2-(ethylamino) Ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H -pyrrole-3-carbamoyl (compound 2), and 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene)methyl] -N-[(2S)-2-Hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1H-pyrrole-3-carbamoyl (compound 3) combination for the treatment of cancer. The present invention relates to the administration of an anti-CTLA4 antibody in combination with an indolinone RTKI, such as, in particular, Compound 1. The present invention relates to neoadjuvant, adjuvant, first-line, second-line, and third-line treatments of cancer, whether localized or metastatic, and at any point in disease progression (eg, at any stage of the cancer).
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Stable and soluble formulations of receptor tyrosine kinase inhibitors, and methods of preparation thereof
ActiveUS9474756B2Low water solubilityImprove stabilityPowder deliveryOrganic active ingredientsReceptor tyrosine kinase inhibitorMedicine
The present disclosure relates to stable formulations of receptor tyrosine kinase inhibitors (TKI), e.g., pazopanib; methods of preparation thereof; and use of the disclosed formulations in sustained delivery of the active agent to a target site. The disclosure further relates to methods of converting one polymorphic Form of a TKI to another polymorphic Form and / or an amorphous form.
Owner:FORSIGHT VISION5 INC
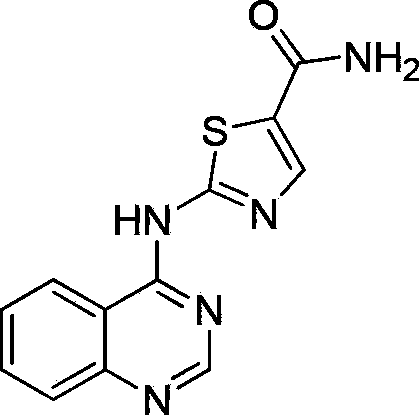
2-(quinazoline-4-amino)-5-thiazole carboxamide derivatives and biological medicine use thereof
The invention belongs to the field of biopharmacy and relates to 2-(quinazoline-4-amino)-5-thiazole carboxamide derivatives and a biological medicine use thereof. Based on a crystal structure of a compound of high-quality Src receptor tyrosine kinase and a Src receptor tyrosine kinase inhibitor saracatinib, a Src active pocket is defined, and a searching process is carried out on a known chemical storage by high-speed virtual screening software DOCK and an accurate scoring function so that the 2-(quinazoline-4-amino)-5-thiazole carboxamide small-molecular compounds having high inhibition activity to Src are obtained. A biological activity test proves that the 2-(quinazoline-4-amino)-5-thiazole carboxamide small-molecular compounds have strong inhibition activity to Src receptor tyrosine kinase, can be used for preparing antitumor drugs especially such as a drug for resisting Src kinase signal transduction system adjustment disorder-caused tumors, can be used as drug lead compounds for synthesis of a novel Src kinases inhibitor and especially can be used for treating Src-related tumor diseases.
Owner:FUDAN UNIV
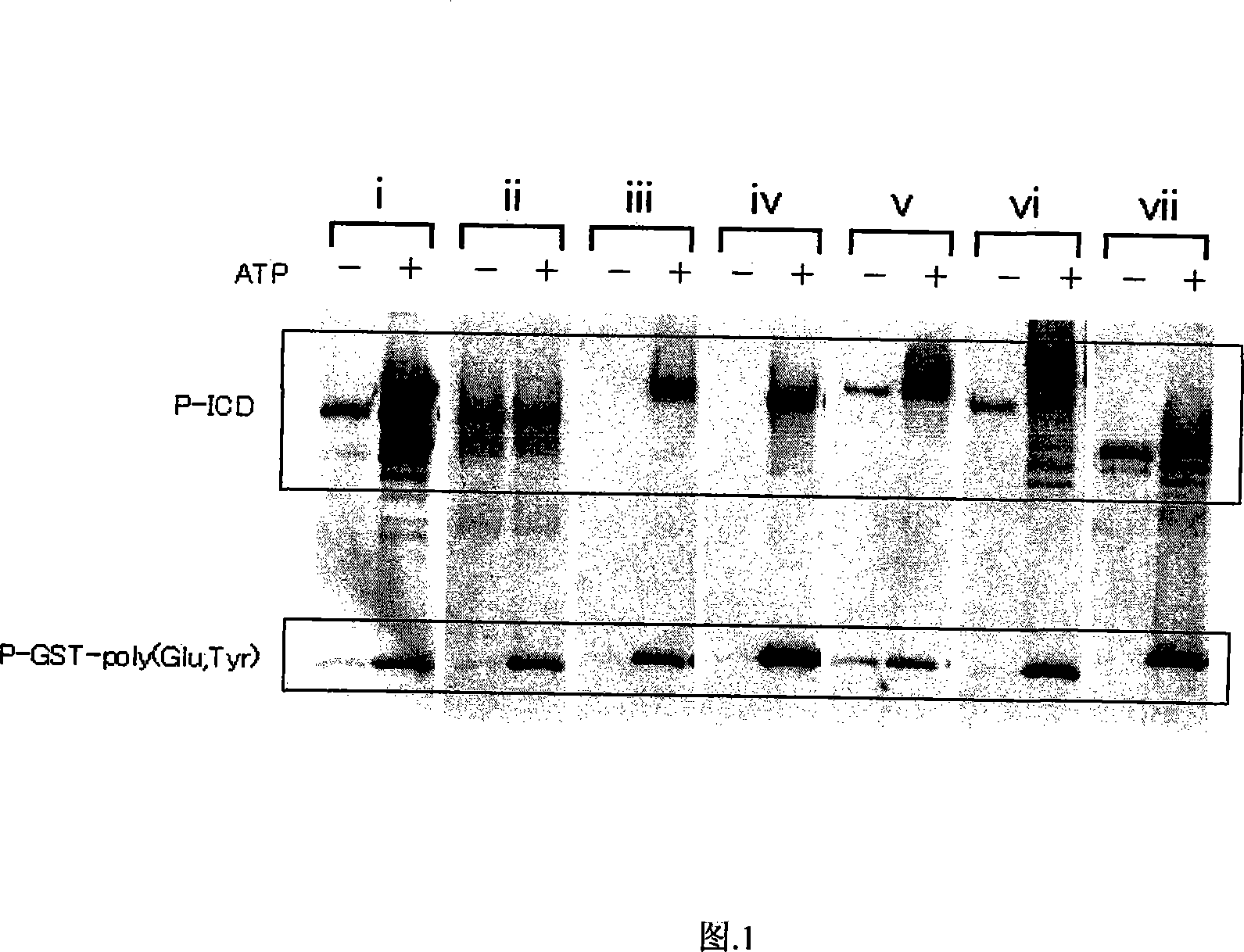
Method for assessing proliferation inhibiting effect of inhibitor, and method for determining sensitivity of tumor cell to inhibitor
InactiveCN101157951APrevent proliferationCompound screeningApoptosis detectionPhosphorylationTyrosine-kinase inhibitor
A method for assessing the proliferation inhibiting effect of a receptor tyrosine kinase inhibitor is described. In the method, a cytoplasm is separated from a tumor cell to prepare a sample containing various receptor tyrosine kinases. The prepared sample is treated with the inhibitor. The receptor tyrosine kinases in the treated sample, and a substrate for at least two kinds of receptor tyrosine kinases are contacted. The phosphorylated substrate is detected, and the activity value of the receptor tyrosine kinases is measured based on the detection result. The proliferation inhibiting effect is assessed based on the resulting activity value.
Owner:SYSMEX CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




















![Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/293e589d-5fa6-4ecd-9ea1-d6a7c43509e8/A2008800187020002C1.PNG)
![Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/293e589d-5fa6-4ecd-9ea1-d6a7c43509e8/A2008800187020004C1.PNG)
![Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors Imidazo[1,2-a]pyridine compounds as receptor tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/293e589d-5fa6-4ecd-9ea1-d6a7c43509e8/A2008800187020004C2.PNG)
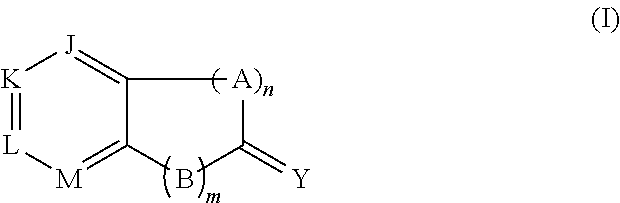
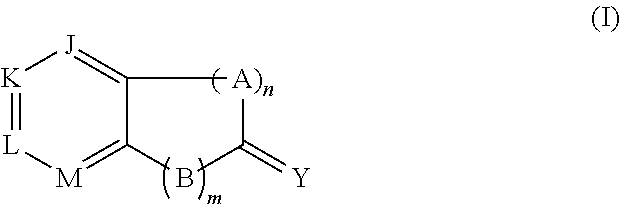
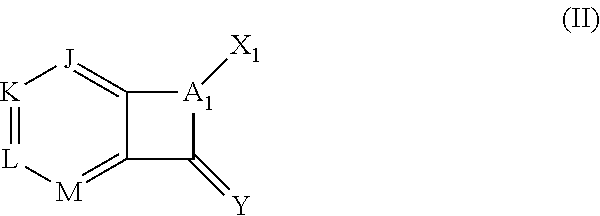
![Pyrazolo[1,5-a]miazine compound and its preparation method and medical use Pyrazolo[1,5-a]miazine compound and its preparation method and medical use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87c565b9-7348-4f70-958b-669bd83453f2/BDA00003428167000031.PNG)
![Pyrazolo[1,5-a]miazine compound and its preparation method and medical use Pyrazolo[1,5-a]miazine compound and its preparation method and medical use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87c565b9-7348-4f70-958b-669bd83453f2/BDA00003428167000061.PNG)
![Pyrazolo[1,5-a]miazine compound and its preparation method and medical use Pyrazolo[1,5-a]miazine compound and its preparation method and medical use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87c565b9-7348-4f70-958b-669bd83453f2/BDA00003428167000062.PNG)