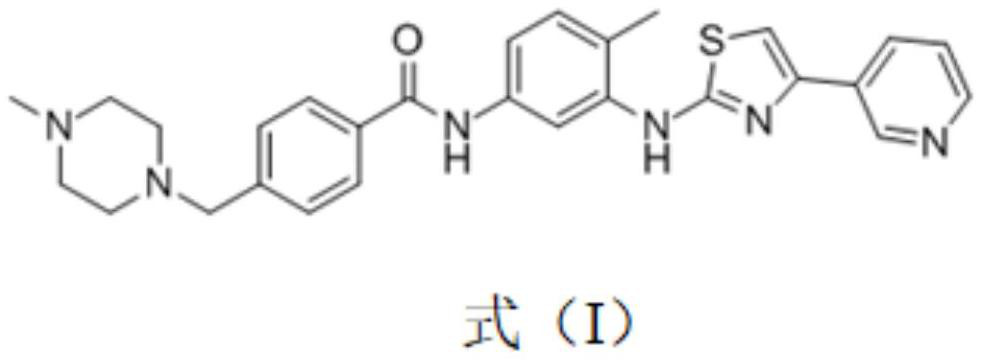
Application of receptor tyrosine kinase inhibitor in preparation of medicine for preventing and/or treating novel coronavirus infection
A technology of tyrosine kinase and coronavirus, which is applied in the field of antiviral drugs, can solve the problem of lack of effective prevention and treatment drugs for infection, and achieve the effects of enhancing human immunity, strengthening curative effect, and inhibiting replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
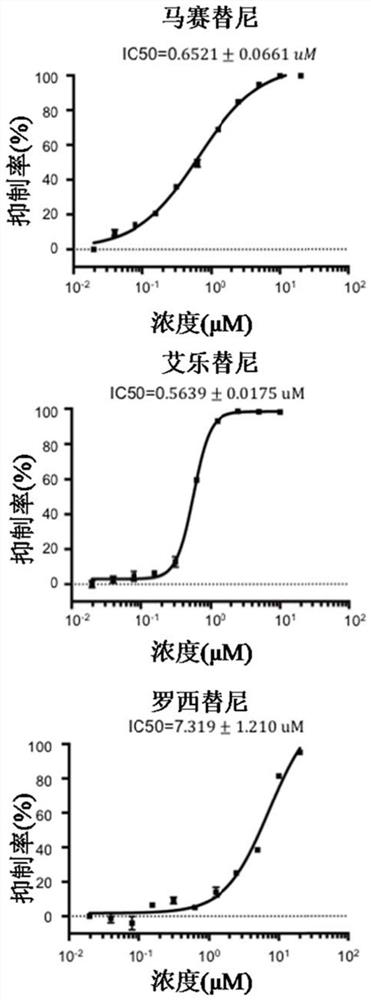
[0041] The SARS-CoV-2 safe replicon can simulate the RNA transcription and replication of SARS-CoV-2 in cells, and the luciferase activity in cells can directly reflect the degree of RNA transcription and replication of SARS-CoV-2, while not The infectious novel coronavirus SARS-CoV-2 was produced. Therefore, the present invention utilizes the novel coronavirus SARS-CoV-2 safe replicon to verify the inhibitory effect of Masitinib, Alectinib and Roxitinib on the RNA synthesis of SARS-CoV-2.
[0042] Relevant experiments can be carried out in the P2 laboratory, and the specific experimental steps are as follows:
[0043] The SARS-CoV-2 safe replicon constitutes plasmid ps2V (SEQ ID NO: 1), ps2AN (SEQ ID NO: 2), ps2AC (SEQ ID NO: 3), ps2B (SEQ ID NO: 4), according to certain Ratio Use Thermo Fisher’s Lipo2000 transfection reagent and Opti-MEM to transfect into HEK293T cells (cell density is about 6.5×10 4 / cm 2 ), see Table 1 below for reagent consumption. Specifically, 100 μ...
Embodiment 2
[0052] The present invention utilizes the isolated wild-type novel coronavirus SARS-CoV-2 to verify the inhibitory effect of masitinib, alectinib, and rositinib on virus replication. The specific experimental methods are as follows:
[0053] HEK293T cells in good growth state were evenly spread in 48-well culture plates treated with poly-lysine (cell density was about 6.5×10 4 / cm 2 ). After 16 hours of cell growth (the cell density is about 1.6×10 5 / mL), transfected with 0.1 μg of pCMV-ACE2-FLAG plasmid expressing SARS-CoV-2 receptor ACE2 purchased from Beijing Yiqiao Shenzhou Company. 24 hours after transfection, the cells were washed with PBS, and then infected with wild-type SARS-CoV-2 (MOI=0.1, 37° C., 1 hour). Subsequently, DMEM (2% FBS) containing masitinib or alectinib or roxitinib drug at different concentration gradients (20 μM, 5 μM, 1.25 μM, 0.3125 μM, 0.078125 μM, 0.01953125 μM) was replaced. After 24 hours of drug treatment, cellular RNA was extracted using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com