Novel amino pyridine compound, its preparation method, pharmaceutical composition containing compound and application thereof
A compound and mixture technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problem of low correlation of tumor incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
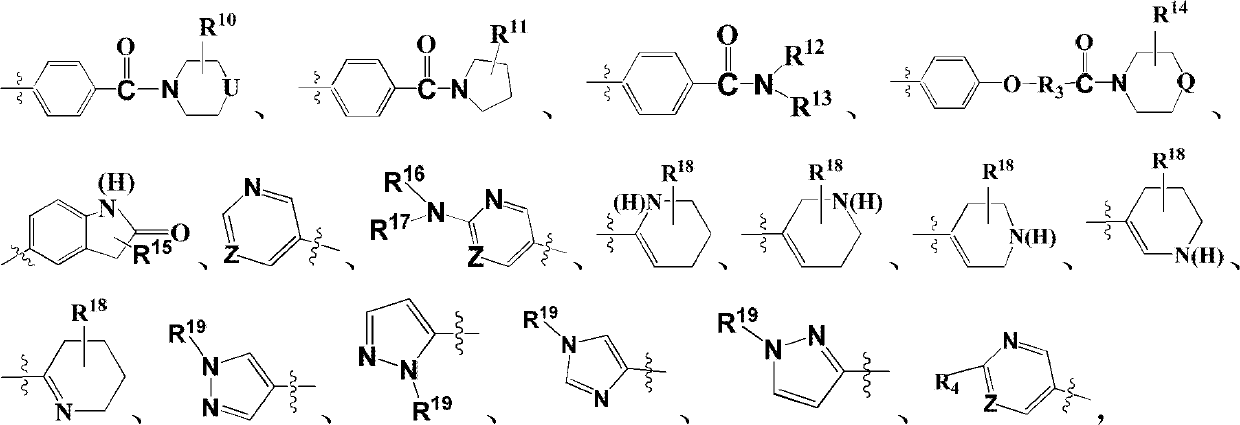
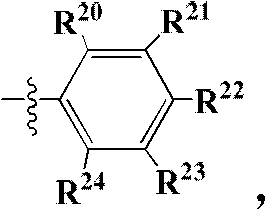
Image
Examples
Embodiment 1
[0097] Step 1: Preparation of N,N-dimethyl-1-[(2-nitropyridine-3)-oxyl]carbothioamide
[0098] Dissolve 2-nitro-3-hydroxypyridine (10.00g, 71.38mmol), N,N-dimethylaminothioformyl chloride (10.59g, 85.66mmol) in 50mL DMF, add DABCO (triethylenedi Amine) (9.61g, 85.66mmol), after stirring at room temperature for 24h, 150mL of water was added to the reaction liquid, extracted with 600mL of ethyl acetate, the organic phase was washed with 3×100mL of water and saturated brine successively, and anhydrous Na 2 SO 4 Dry, filter, and distill off the solvent under reduced pressure to obtain a yellow solid. It was directly used in the next step without purification. 1 H NMR (400MHz, CDCl 3 )δ8.47(dd, J=2.4, 4.4Hz, 1H), 7.77(dd, J=2.4, 8.0Hz, 1H), 7.70(dd, J=4.4, 8.0Hz, 1H), 3.45(s, 3 ), 3.40(s, 3H).
[0099] Step 2: Preparation of N,N-dimethyl-1-[(2-nitropyridine-3)-thio]formamide (1)
[0100] Disperse the crude N,N-dimethyl-1-[(2-nitropyridine-3)-oxyl]carbothioamide obtained in th...
Embodiment 2
[0104] Step 1: Preparation of 1-(3-fluoro-2,6-dichlorophenyl)ethanol
[0105] Dissolve 1-(3-fluoro-2,6-dichlorophenyl)ethanone (20.00 g, 96.60 mmol) in 100 mL MeOH, add NaBH in portions 4 (7.31g, 193.21mmol), after stirring at room temperature for 2h, 20mL of water was added to the reaction solution, the organic solvent was evaporated under reduced pressure, the residue was extracted with 150mL of ethyl acetate, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent under reduced pressure, a colorless transparent oily liquid was obtained. It was directly used in the next step without purification.
[0106] Step 2: Preparation of ethyl 1-(3-fluoro-2,6-dichlorophenyl)methanesulfonyl ester (2)
[0107] Dissolve the crude 1-(3-fluoro-2,6-dichlorophenyl)ethanol obtained in the previous step in 150 mL CH 2 Cl 2 In, add Et 3 N (13.43mL, 96.60mmol) catalytic amount of DMAP, cooled to 0°C, added dropwise MsCl (7...
Embodiment 3
[0111] Step 1: Preparation of 3-{[1-(3-fluoro-2,6-dichlorophenyl)ethyl]thio}-2-nitropyridine
[0112] N,N-Dimethyl-1-[(2-nitropyridine-3)-thio]formamide (10.00g, 44.01mmol), KOH (5.43g, 96.81mmol) were placed in a 250mL eggplant-shaped flask , under argon protection, place the flask in an ice-water bath, and add MeOH / THF / H with a syringe 2 O(2 / 2 / 1, v / v / v) 100mL, after adding, remove the ice-water bath, stir at room temperature, and no N,N-dimethyl-1-[(2-nitropyridine-3 )-sulfanyl] formamide, the organic solvent was evaporated under reduced pressure, and under argon protection, 1-(3-fluoro-2,6-dichlorophenyl) methanesulfonyl ethyl ester (12.64g , 44.01mmol) THF solution 120mL, after stirring at room temperature for 24h, the organic solvent was evaporated under reduced pressure, the reaction solution was extracted with ethyl acetate 300mL, the organic phase was washed with water 3×40mL, saturated brine, and anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com