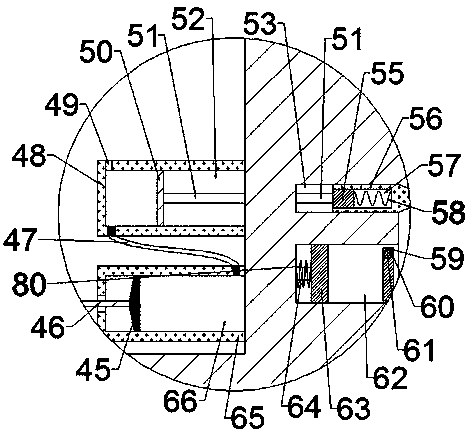
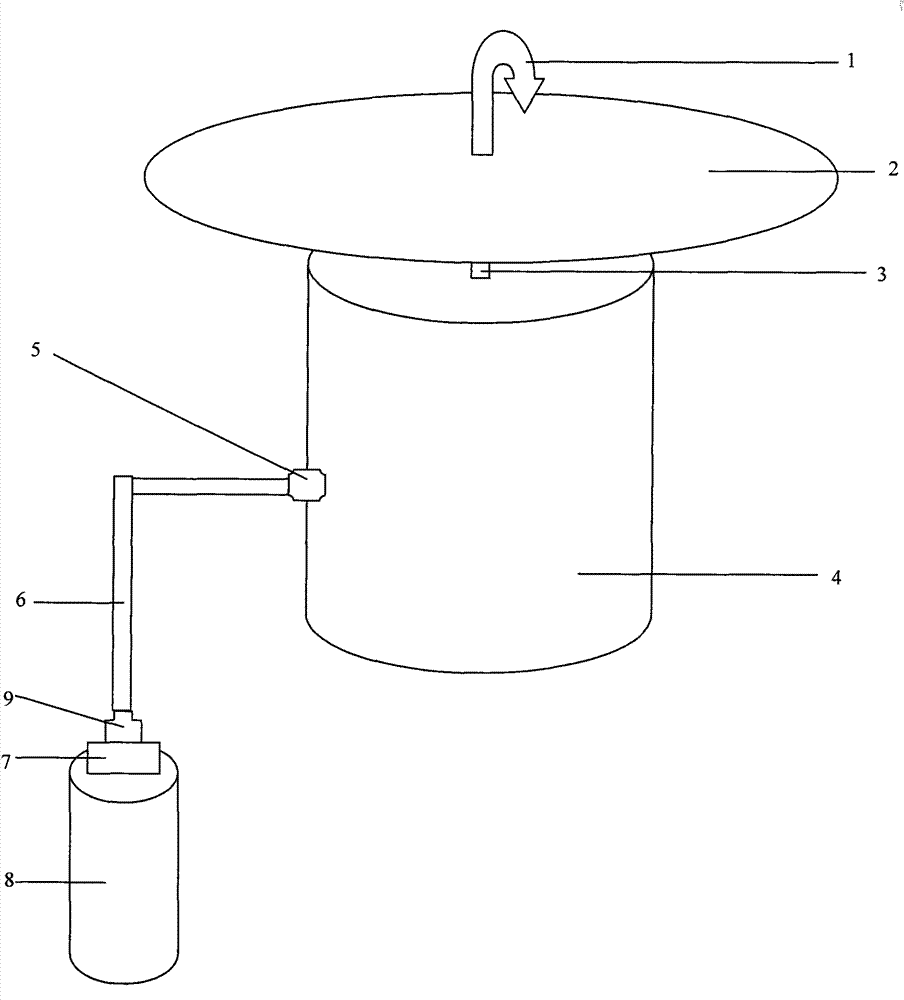
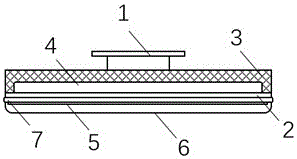
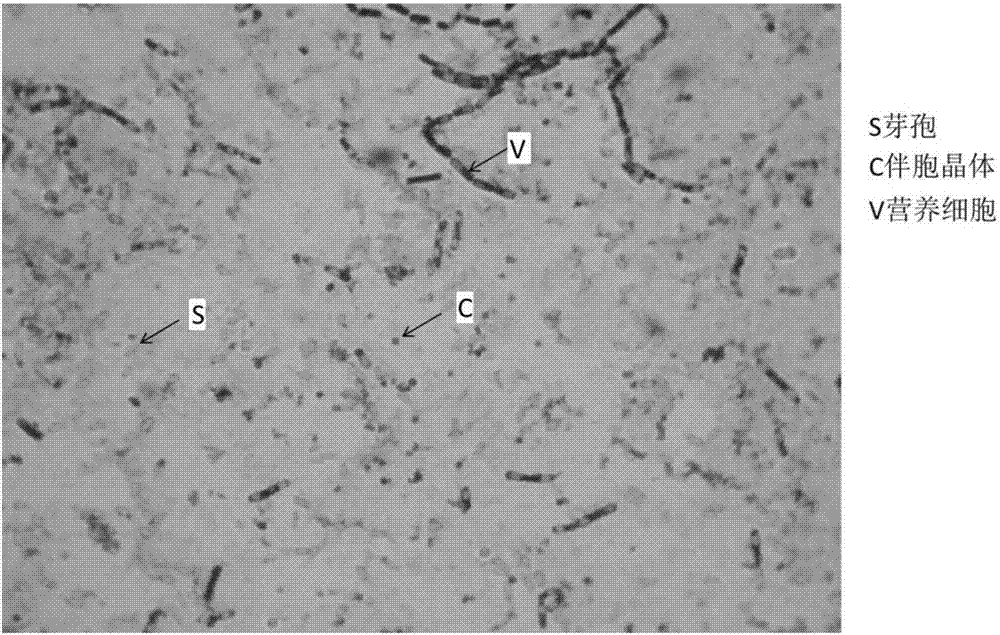
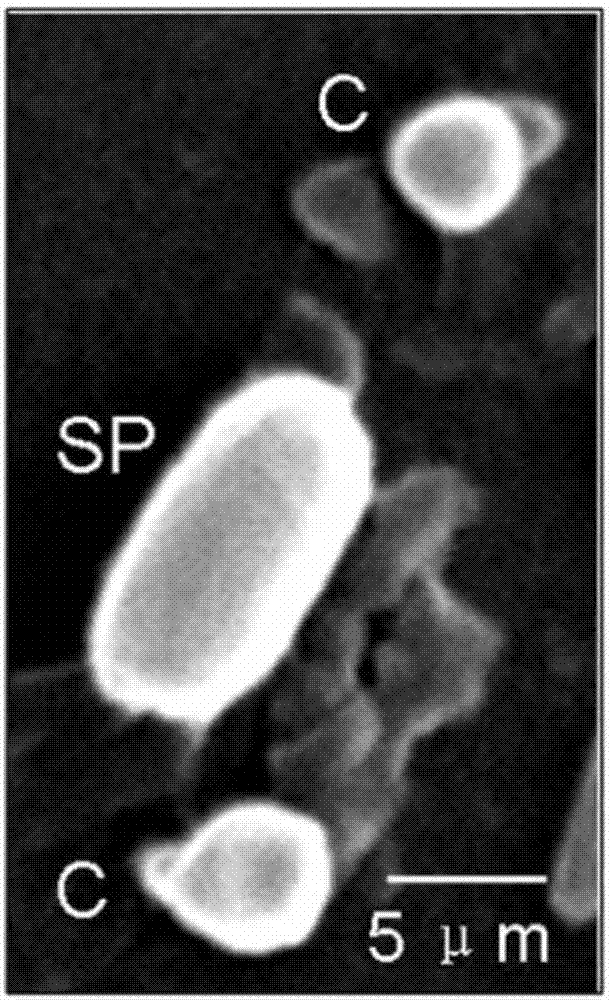
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
139 results about "Mosquito control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mosquito control manages the population of mosquitoes to reduce their damage to human health, economies, and enjoyment. Mosquito control is a vital public-health practice throughout the world and especially in the tropics because mosquitoes spread many diseases, such as malaria and the Zika virus.
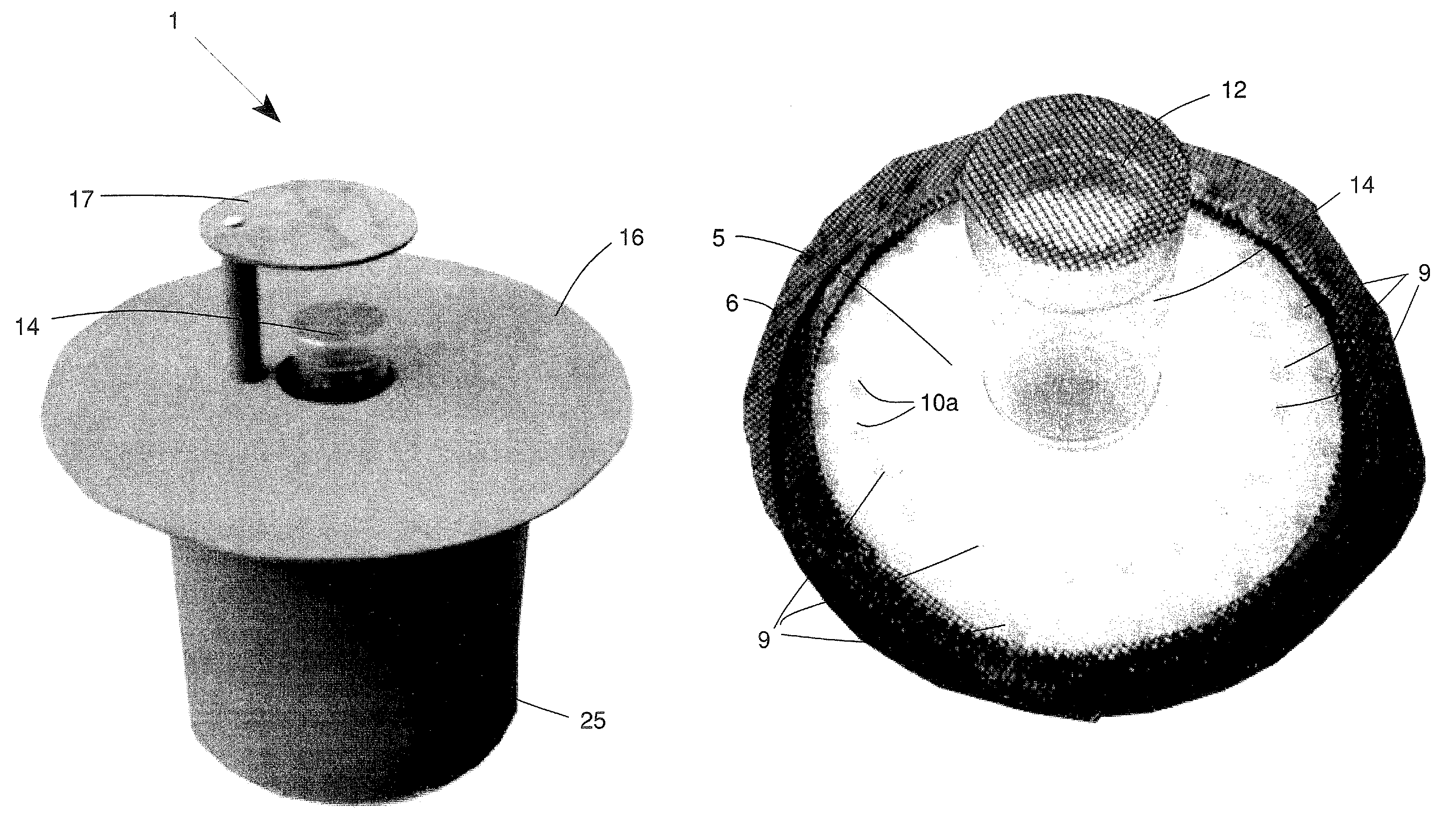
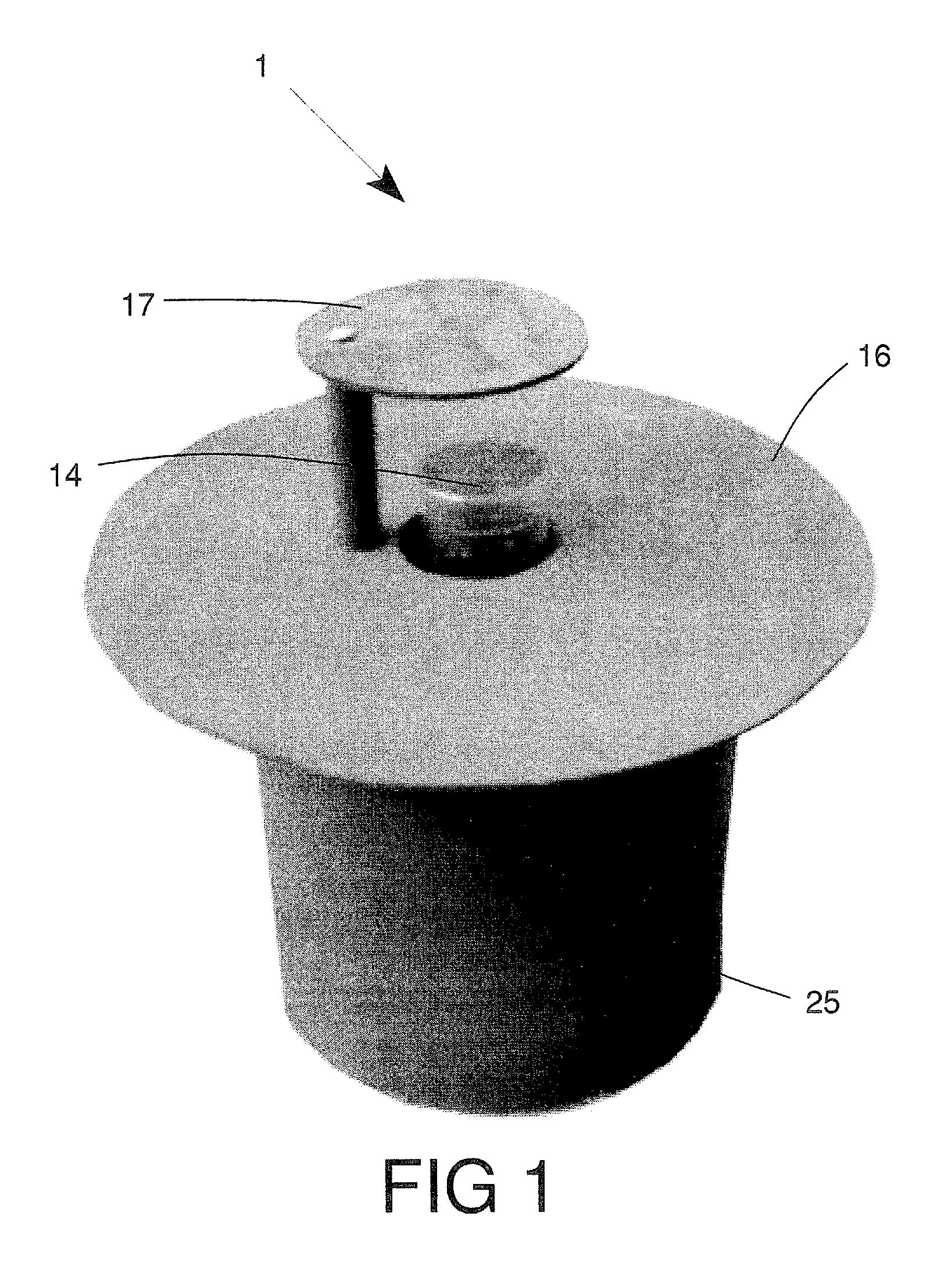
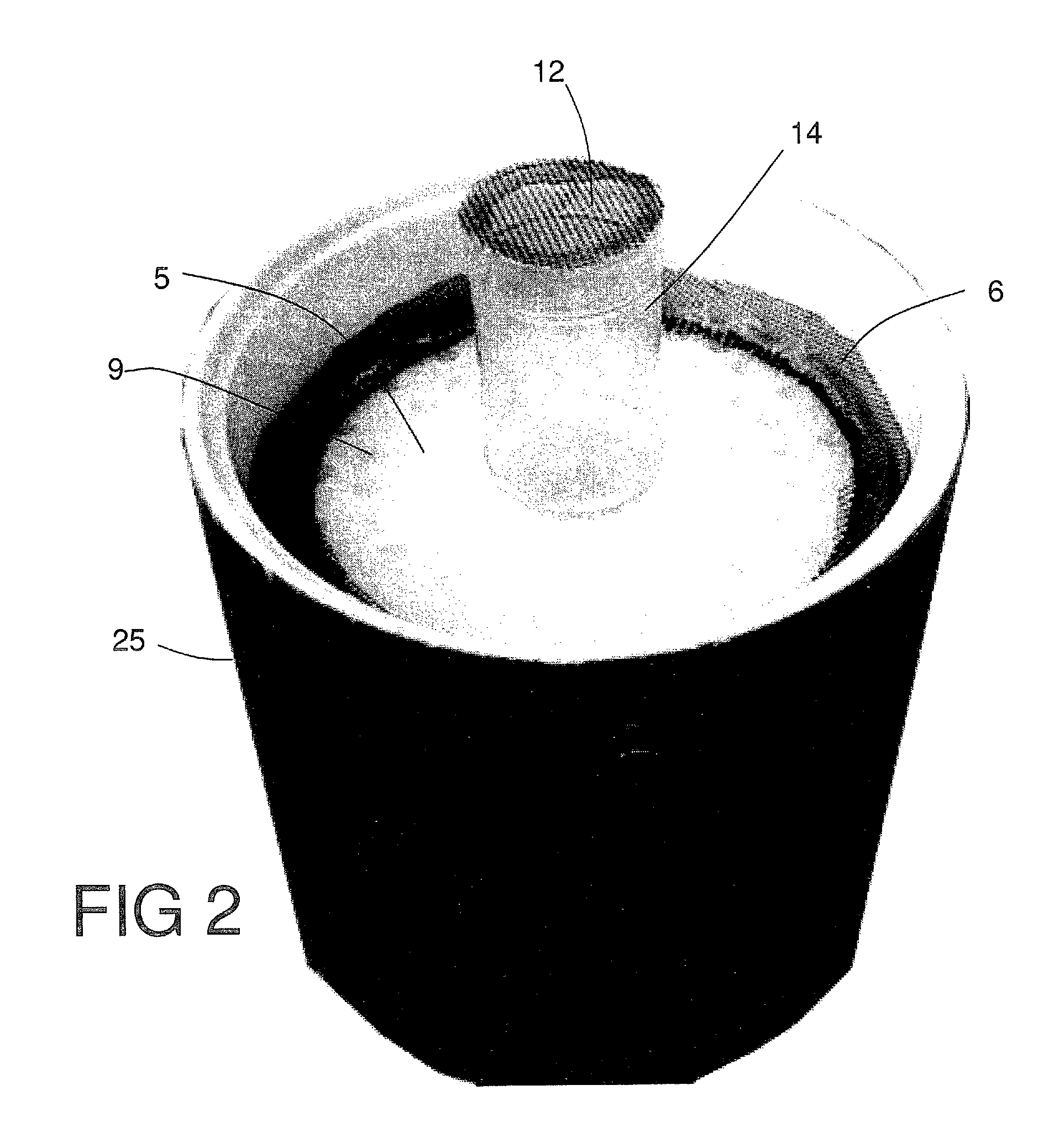
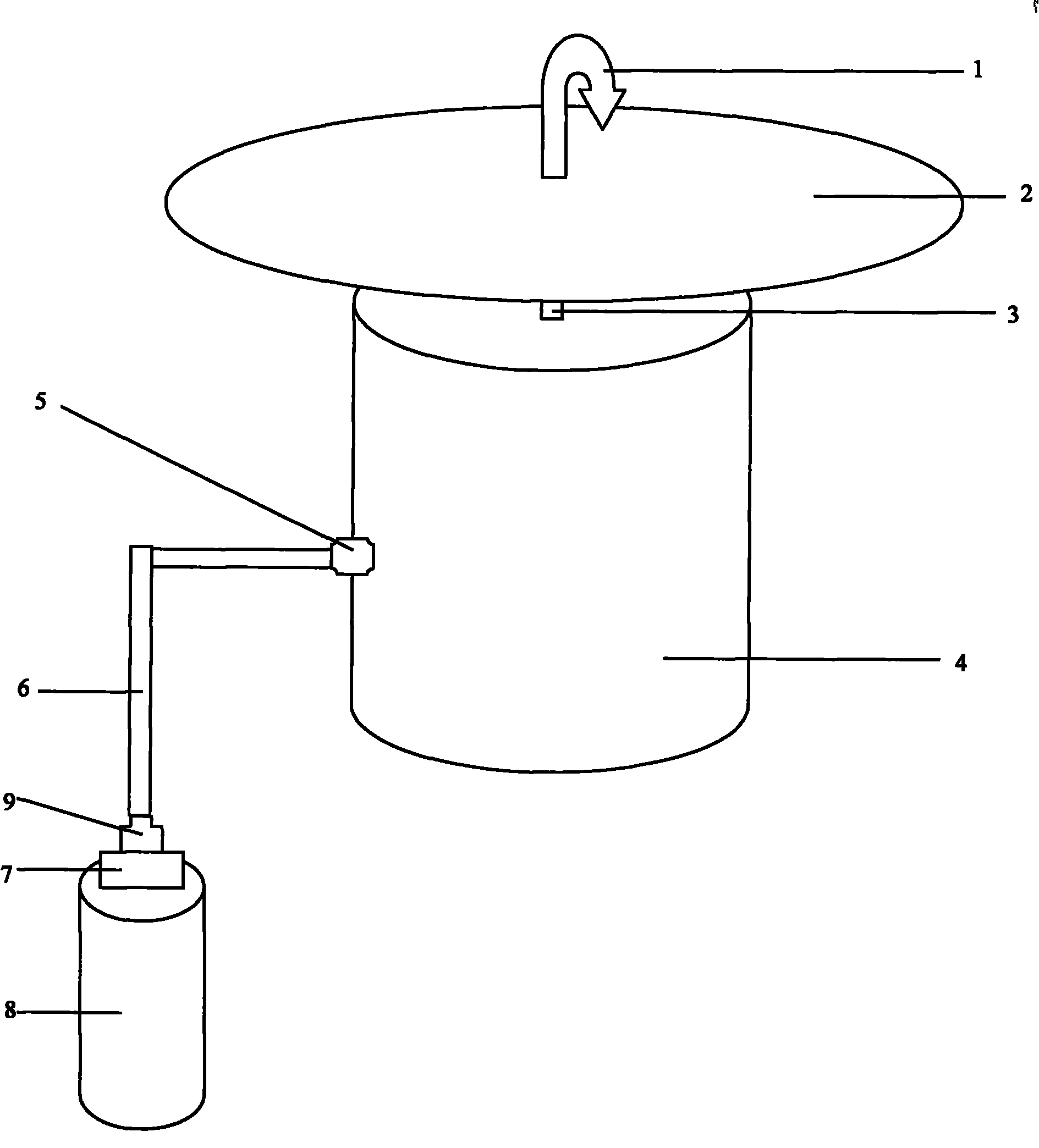
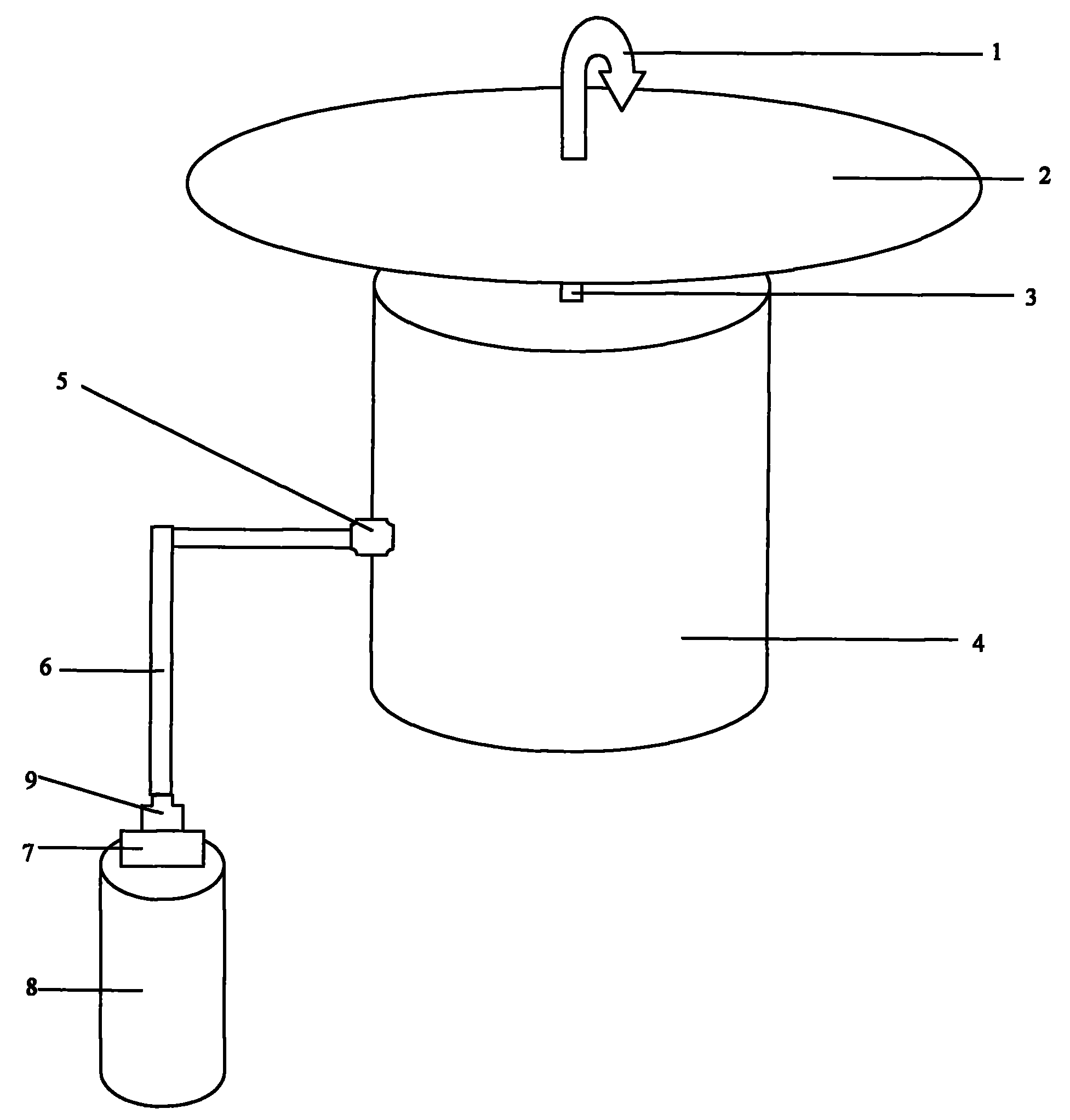
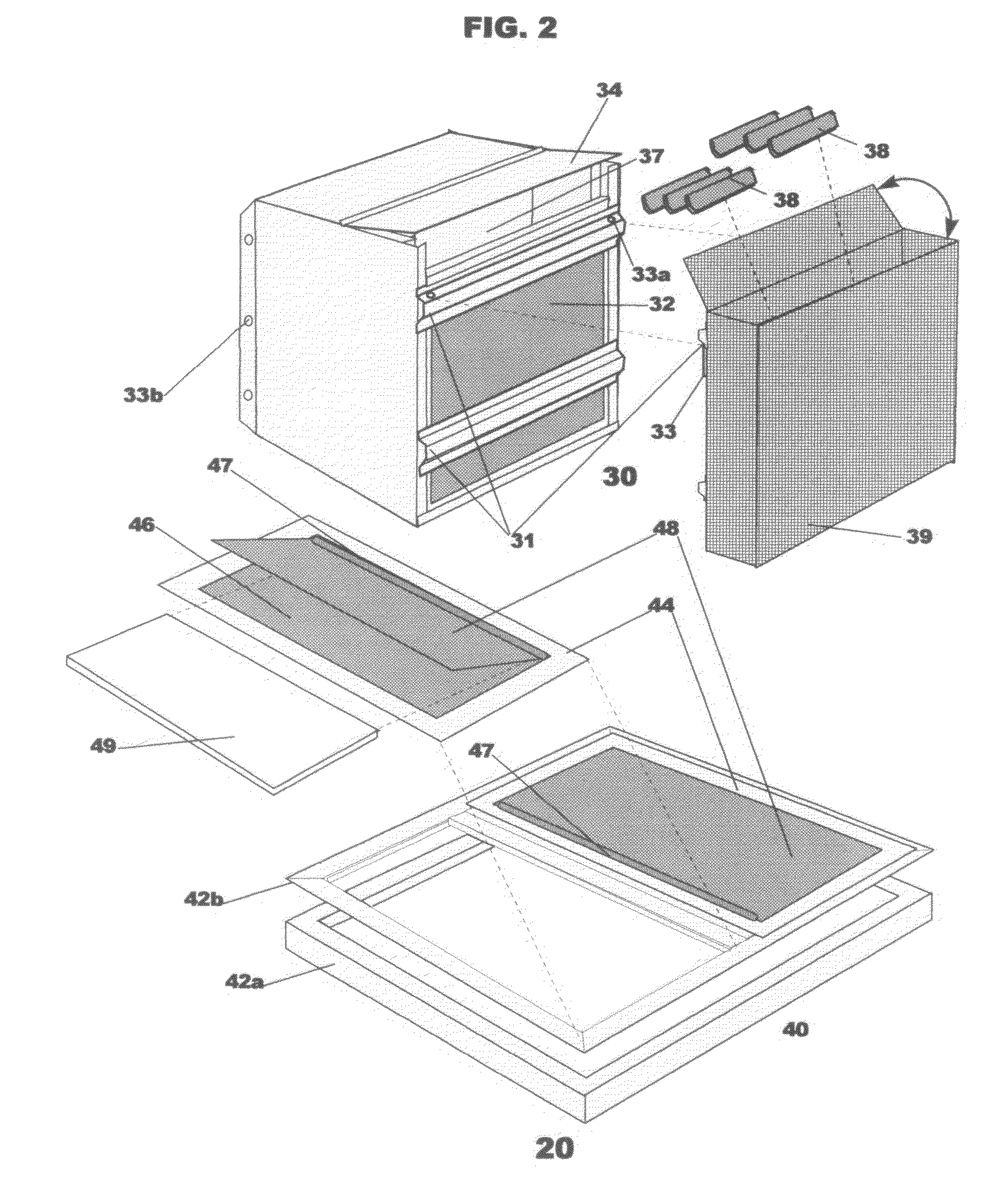
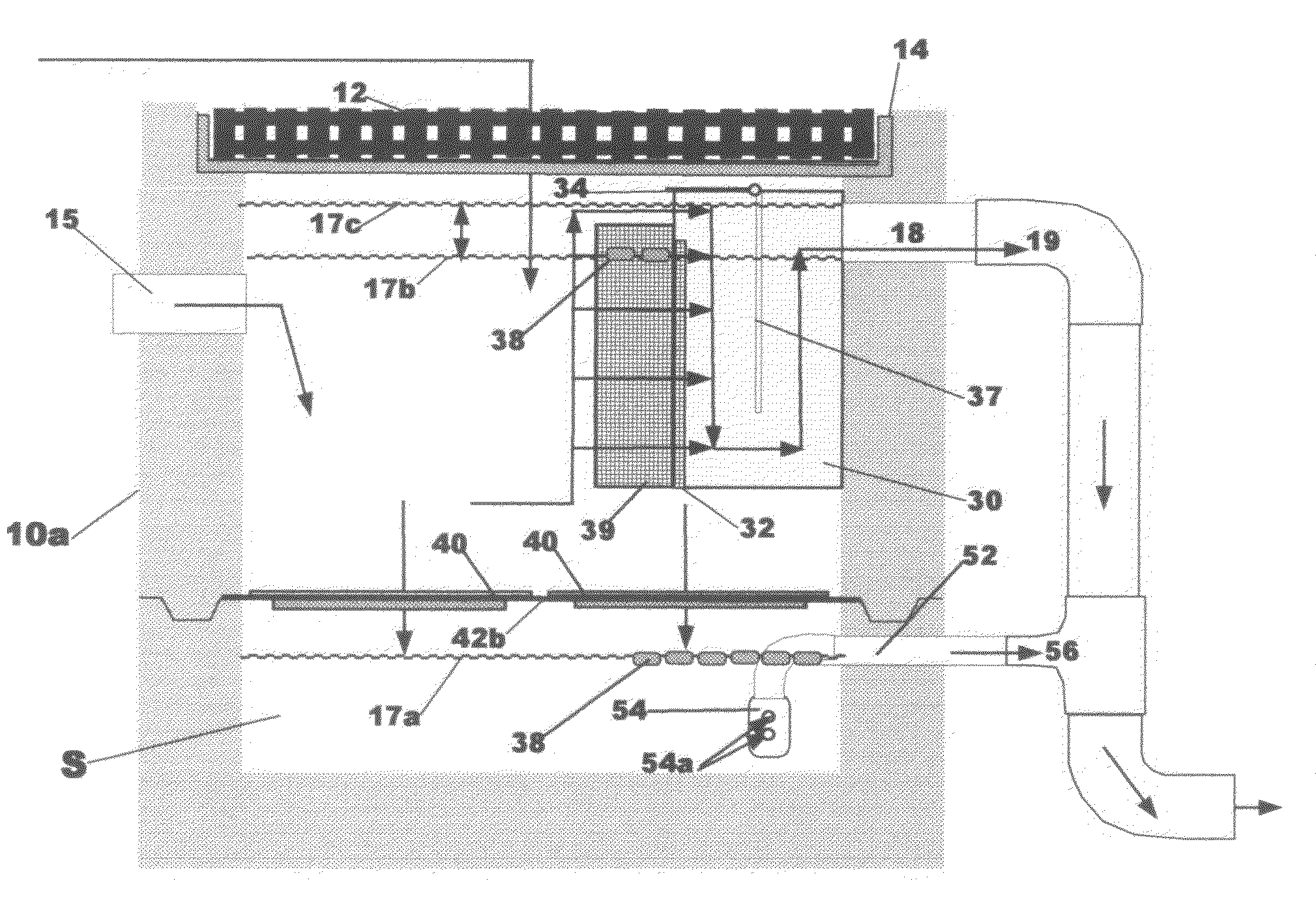
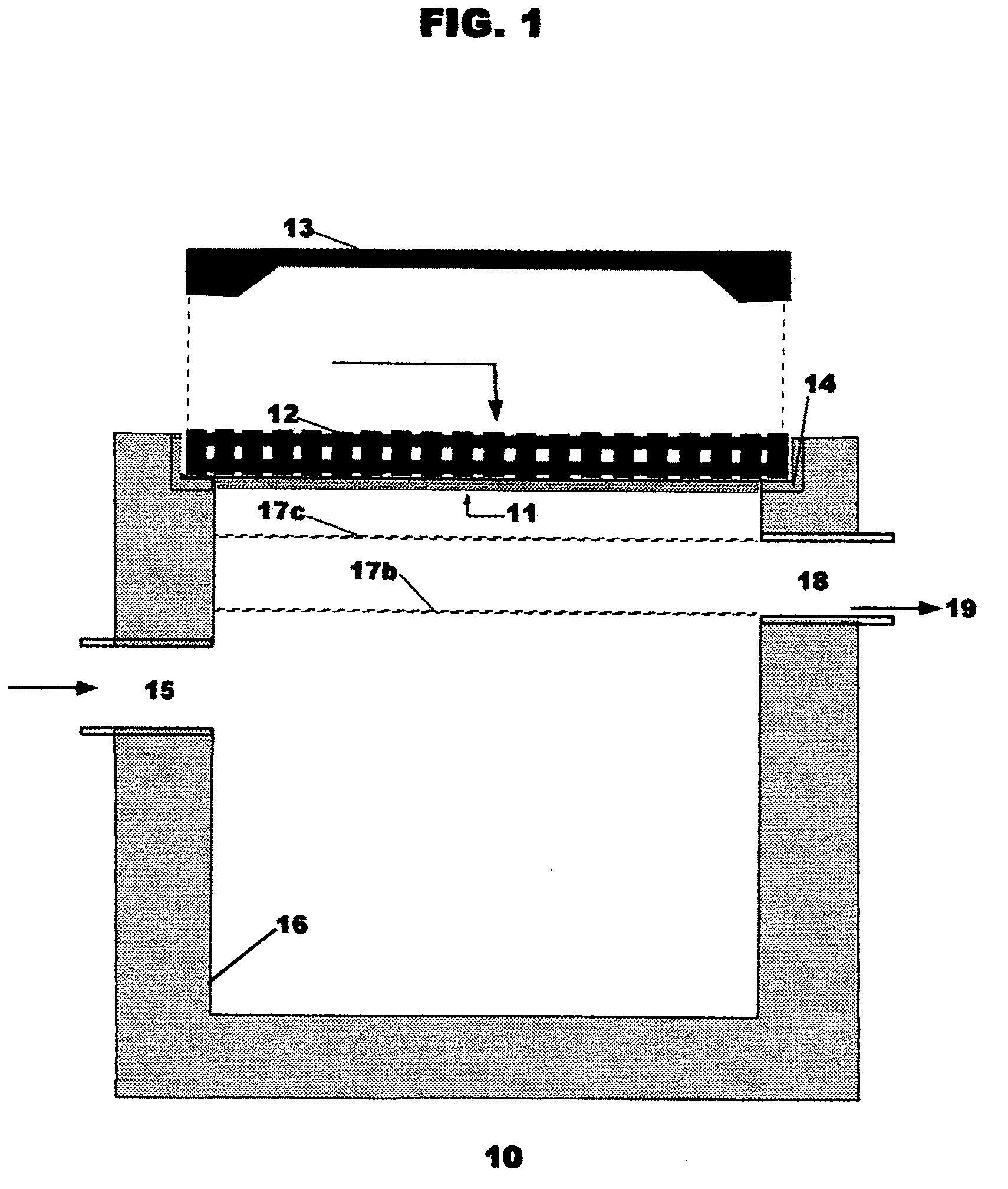
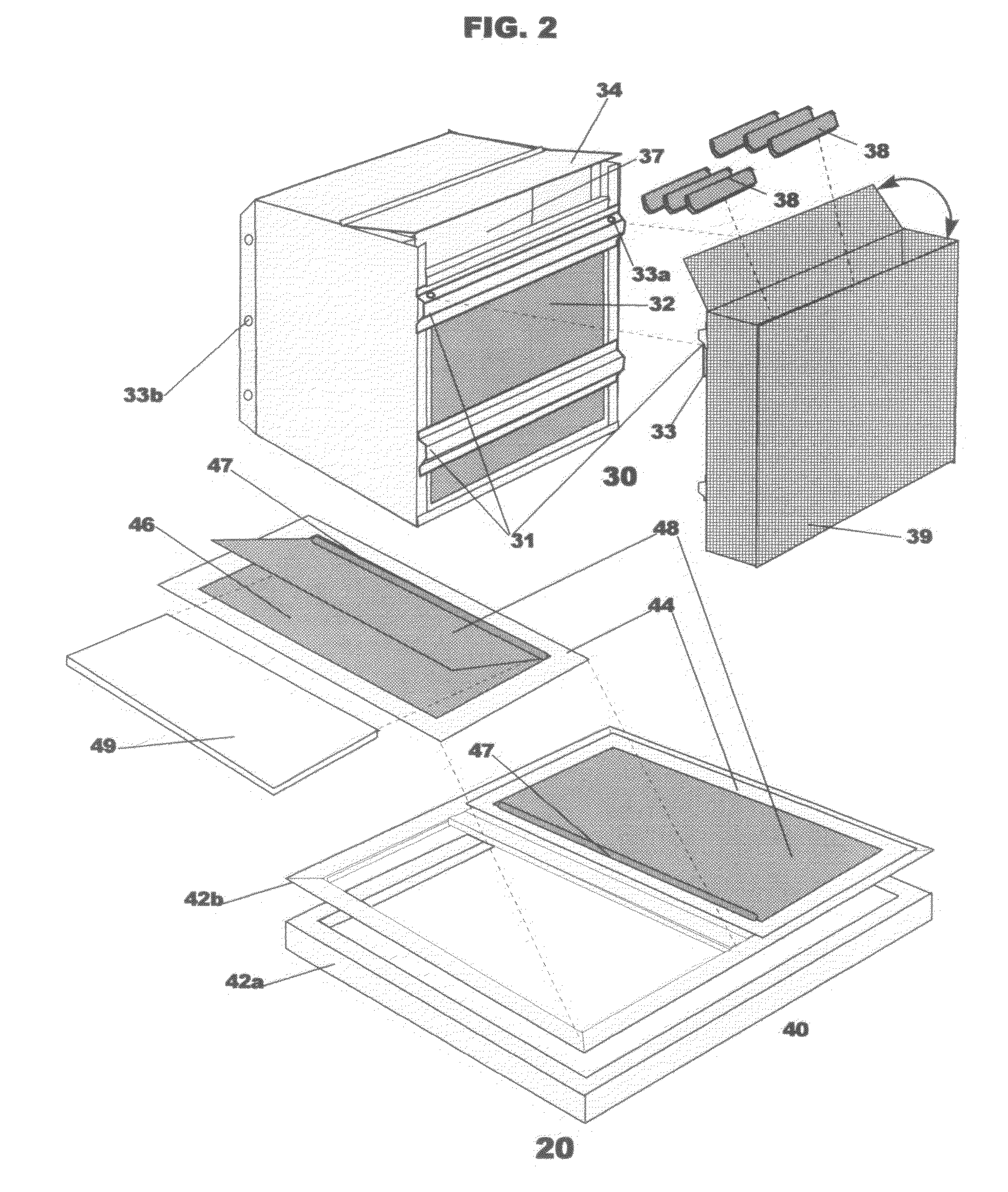
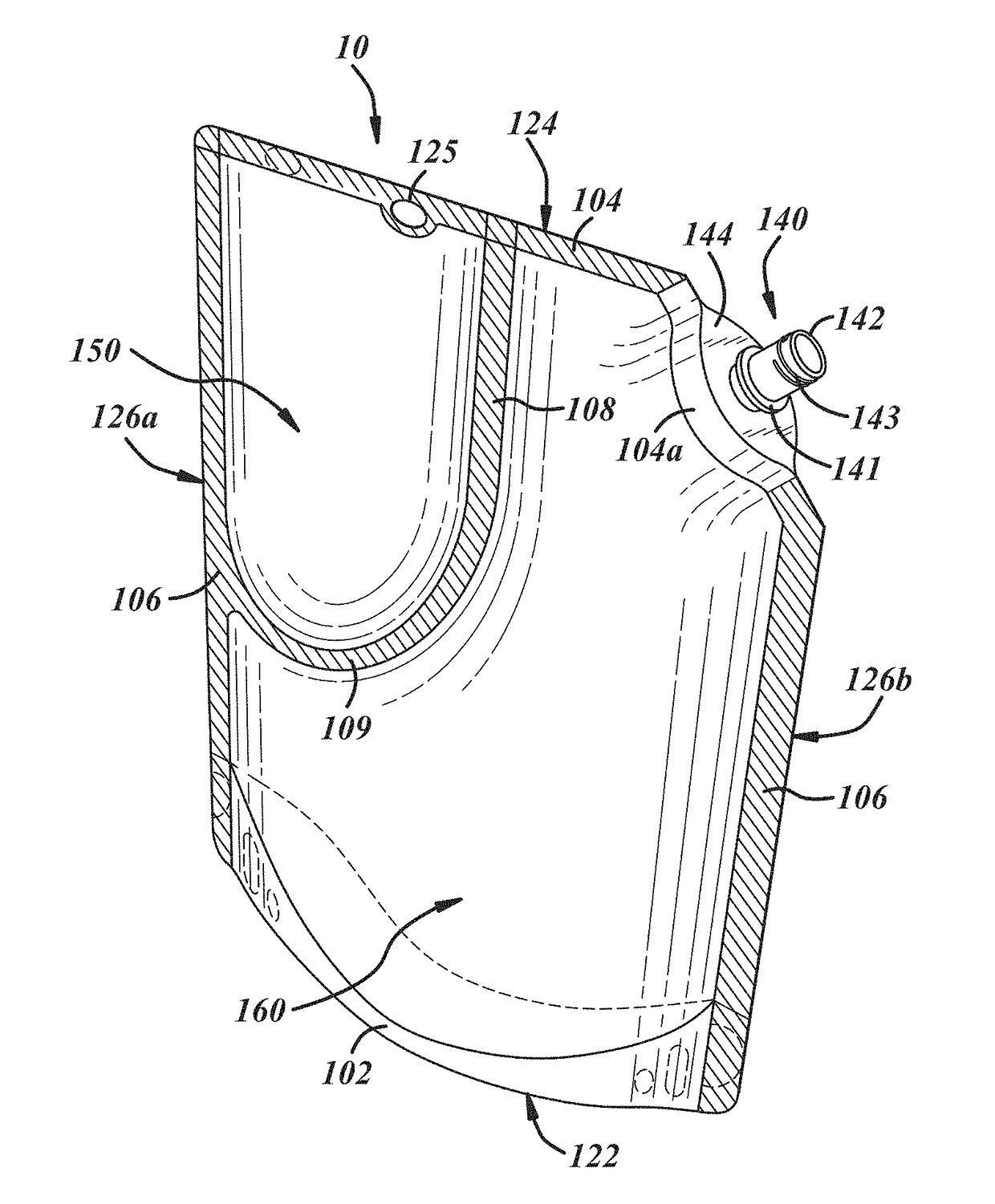
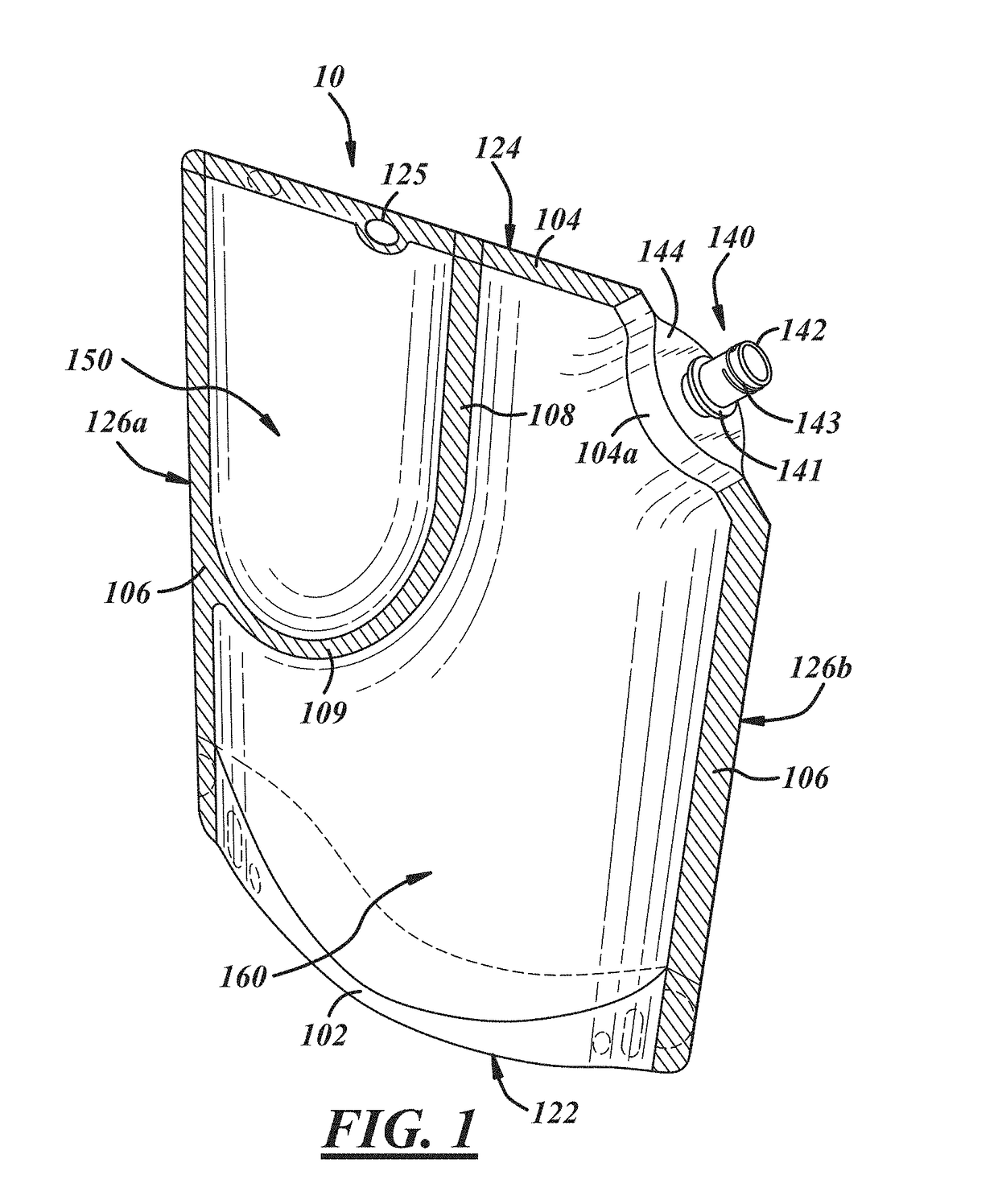
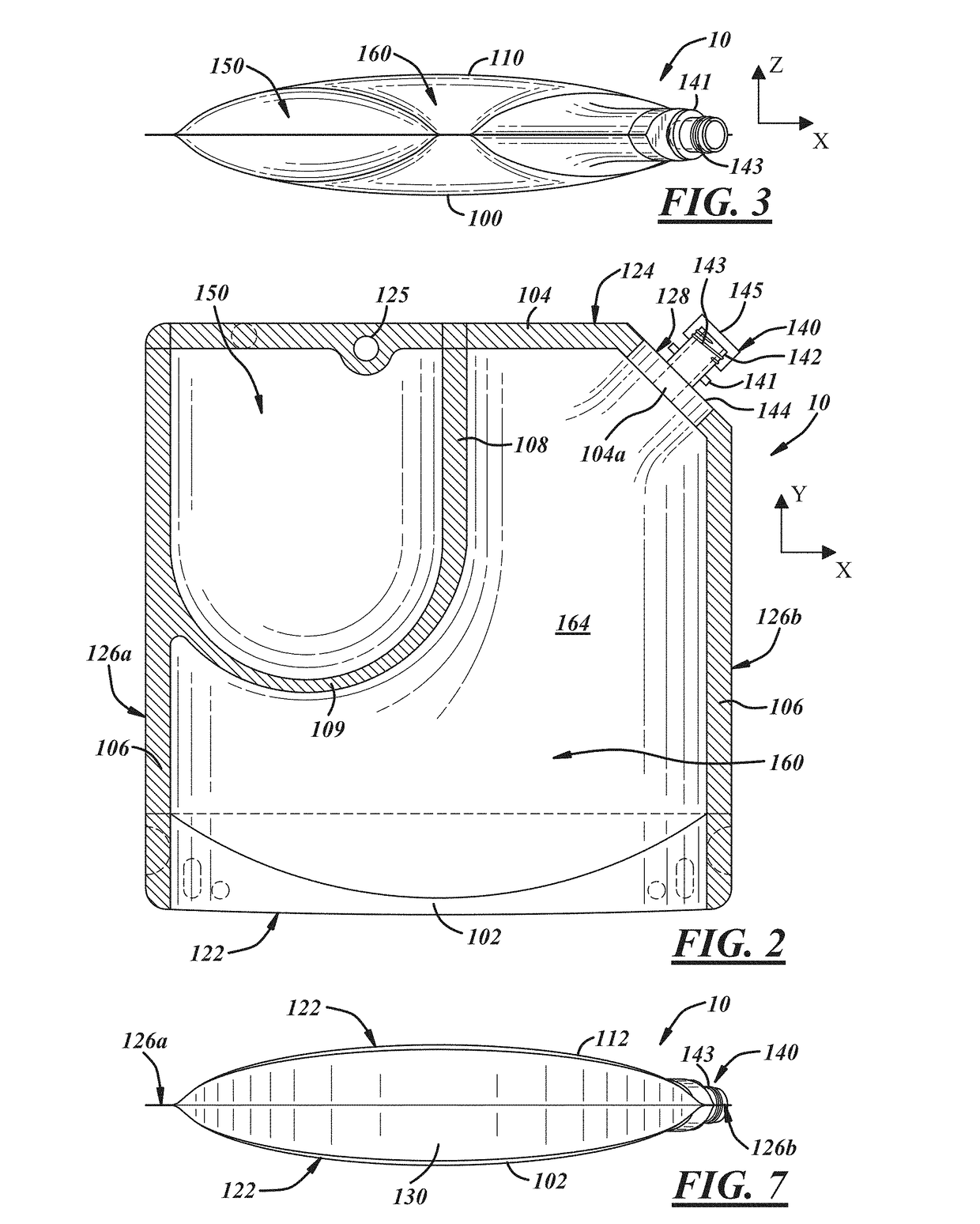
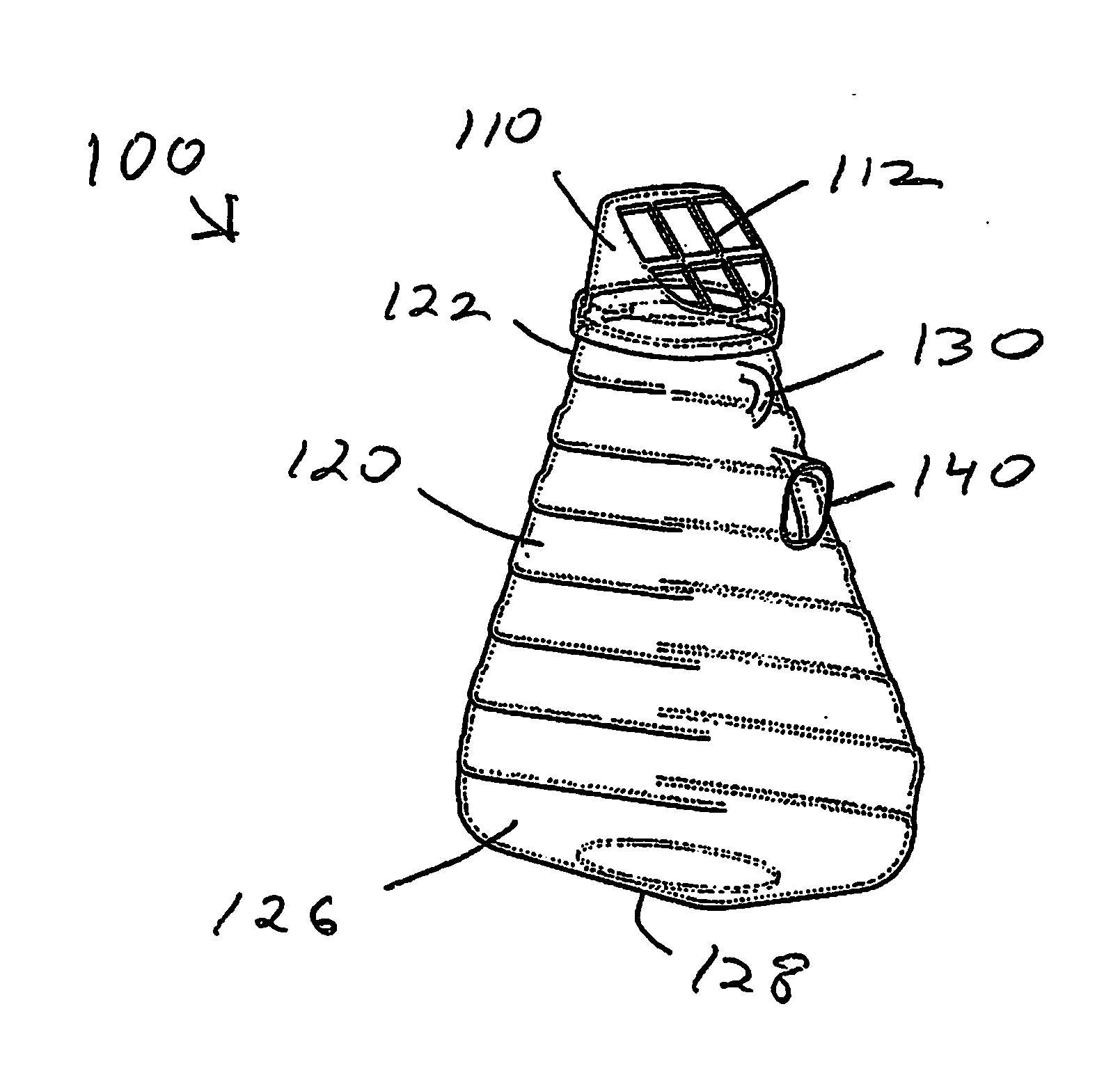
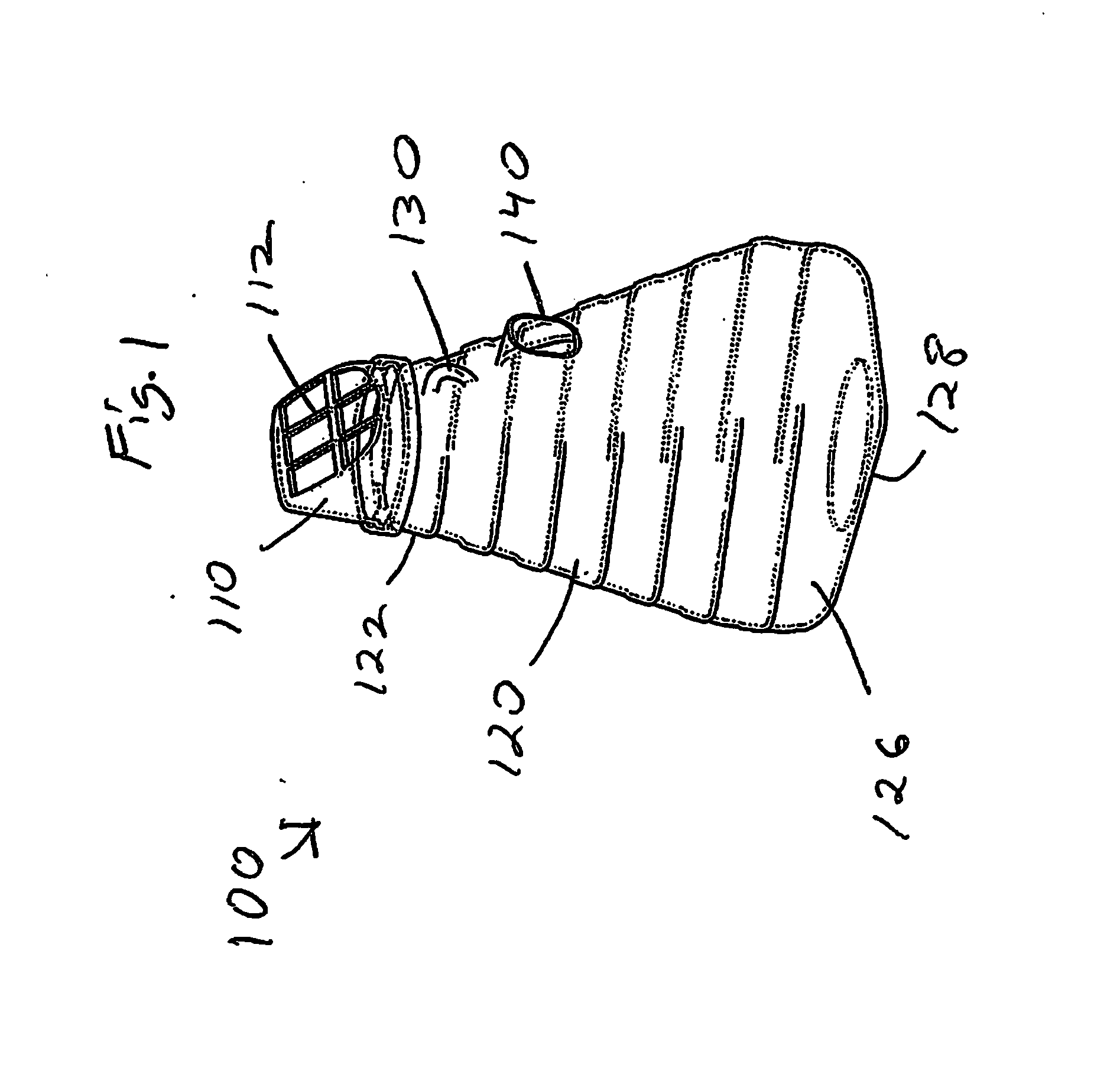
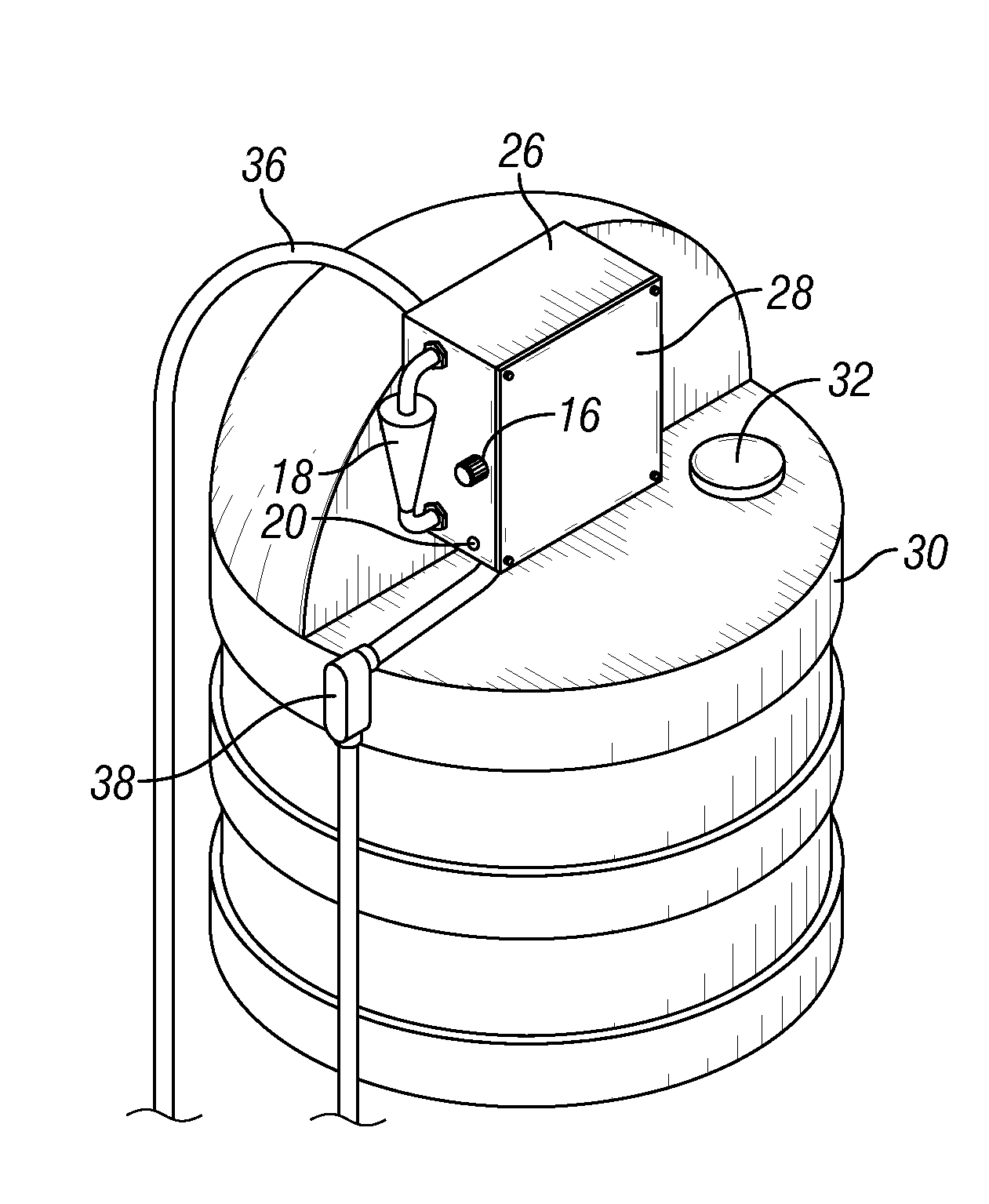
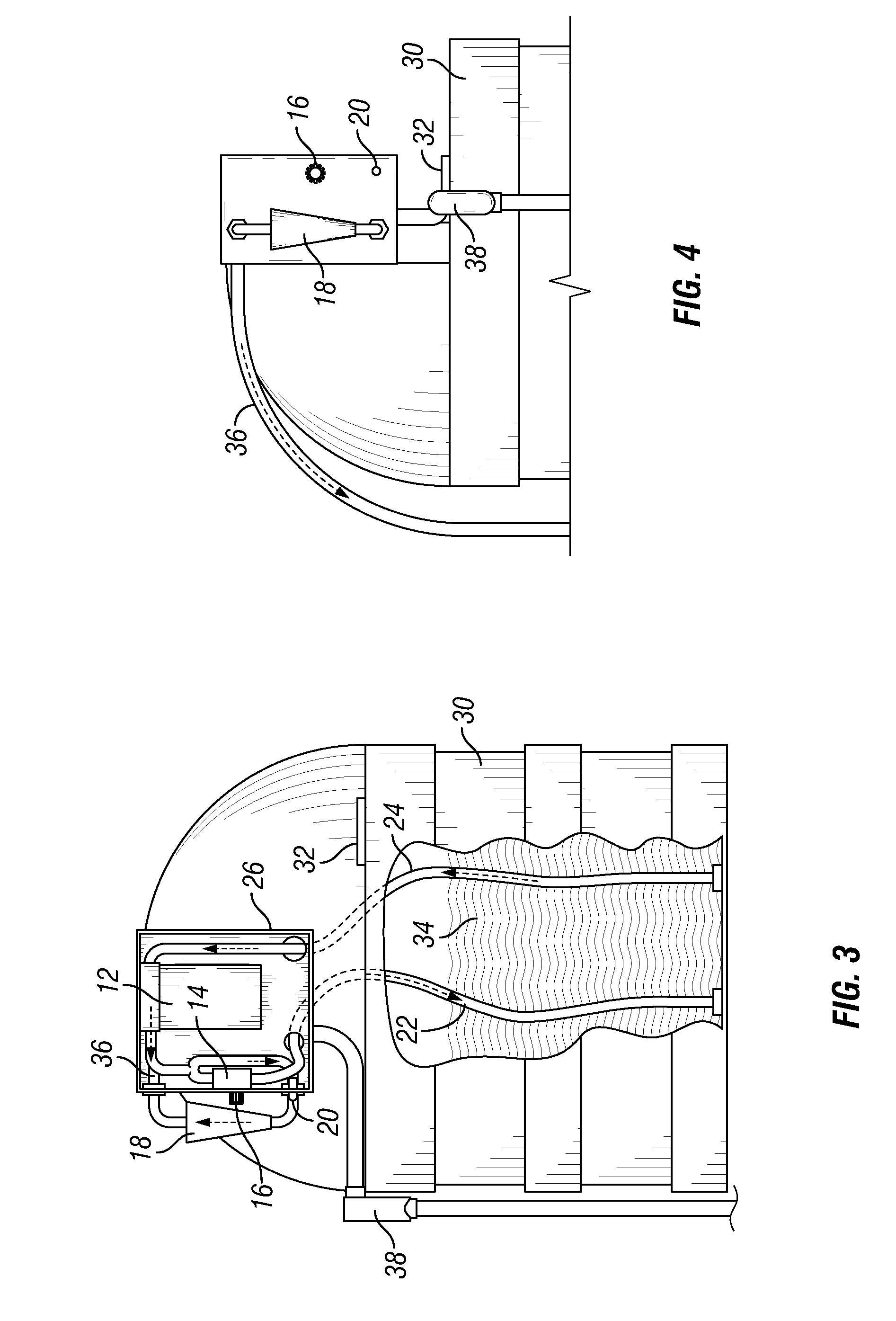
Insect repellent and attractant and auto-thermostatic membrane vapor control delivery system
InactiveUS7988984B2Easily contaminatedEfficient outputBiocideElectric shock equipmentsEngineeringInsect repellent
A membrane enclosed fluid diffusion system for insect attractants and repellents, auto thermostatic heaters, and chemical delivery using an additive and / or selectively permeable membrane that interacts with the enclosed fluid to maintain steady delivery rates over a range of temperature and humidity. Systems can be formed with permeable membranes, impermeable membranes, chemical hydrates, wicks, scent fluids, fuel fluids, catalytic heaters, energy conversion devices, visible images, infrared images, trapping systems, sound systems, electronics, and apparel. The device results in efficient and effective devices for mosquito control drug delivery, and portable heaters.
Owner:ENERGY RELATED DEVICES
Apparatus and method of mosquito control
InactiveUS7694455B1Insect catchers and killersAgainst vector-borne diseasesMosquito controlWater trap
A mosquito control trap taking advantage of the normal stages of development for a mosquito. The trap including stagnant water trapped within a container by a weeping platform floating on top of the water. A series of holes in the weeping platform provide exposure of the water to form brood cups in which female mosquitoes lay eggs. In order to develop through the larva and pupa stage the developing insects must enter the larger reservoir of stagnant water, since there is insufficient room in the brood cups. However, the holes are too small to permit escape of the adult mosquito, thus preventing any of the eggs from developing. This device provides a means for controlling mosquitoes without the use of pesticides and is especially suitable for use in lesser developing countries.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Pest control agent, method for manufacture of pest control agent, and method for pest control
Disclosed are a pest control agent, a method for preparing a pest control agent, and a method for controlling a pest. Generally, the pest control agent is formed by providing a porous starch and an active control agent absorbed within the porous starch, and compressing the porous starch in the presence of heat to form discrete plural particles. Preferably, one or more binders are employed, as are one or more secondary absorbents / fillers. In highly preferred embodiments of the invention, the pest control agent is prepared via pelletizing in a commercial pellet mill. The particles so prepared should be sufficiently durable to withstand bulk transport, such as by rail car or bag shipment. The particles should, however, release the control agent quickly upon contact with water, such that, for instance, the control agent may be released when the pest control agent is introduced to standing water. It is contemplated that the control agent is preferably a mosquito control agent.
Owner:GRAIN PROCESSING CORP
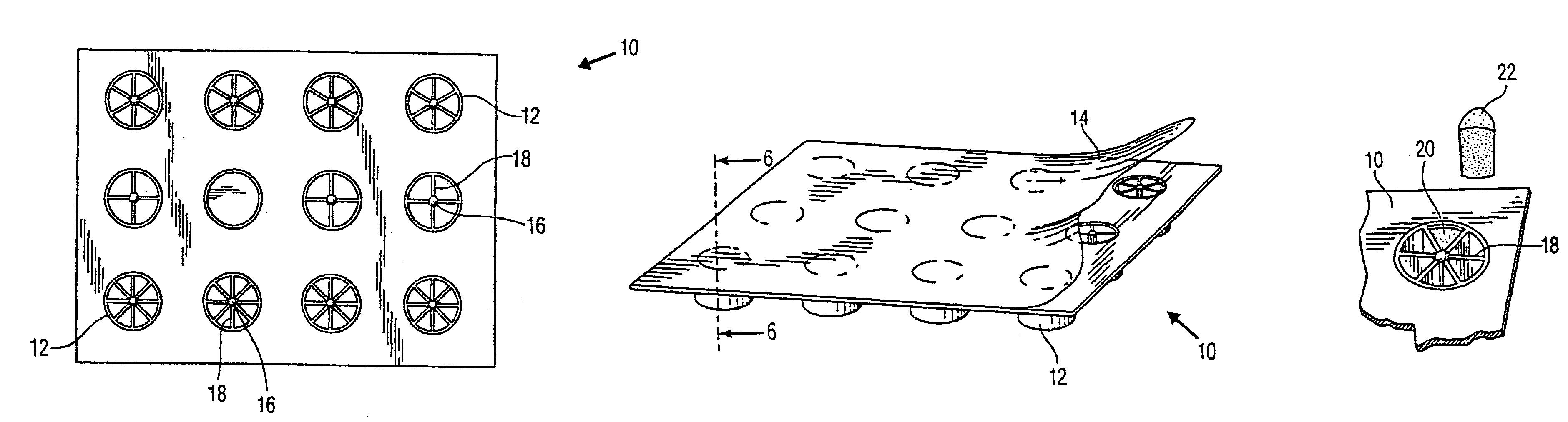
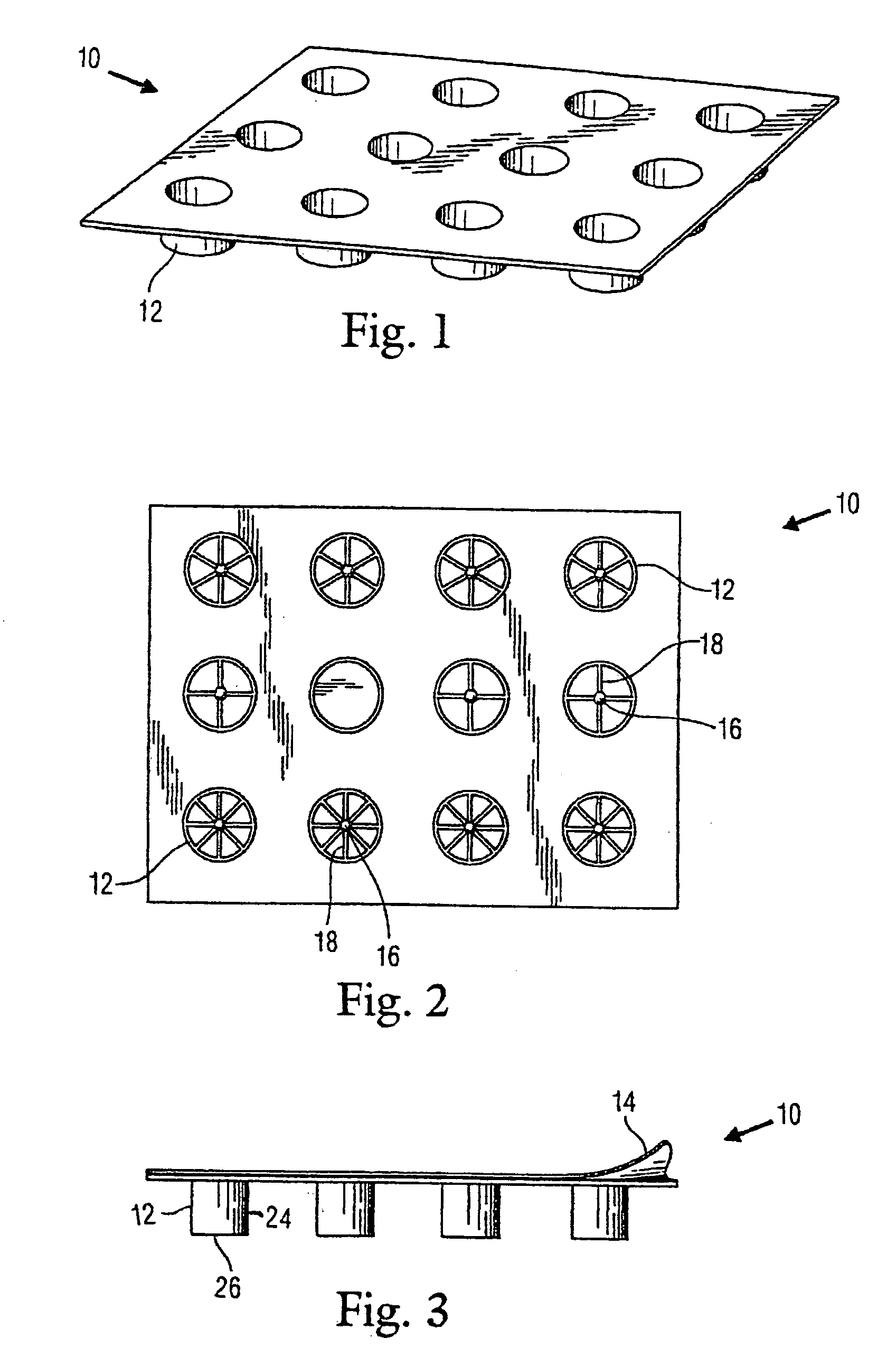
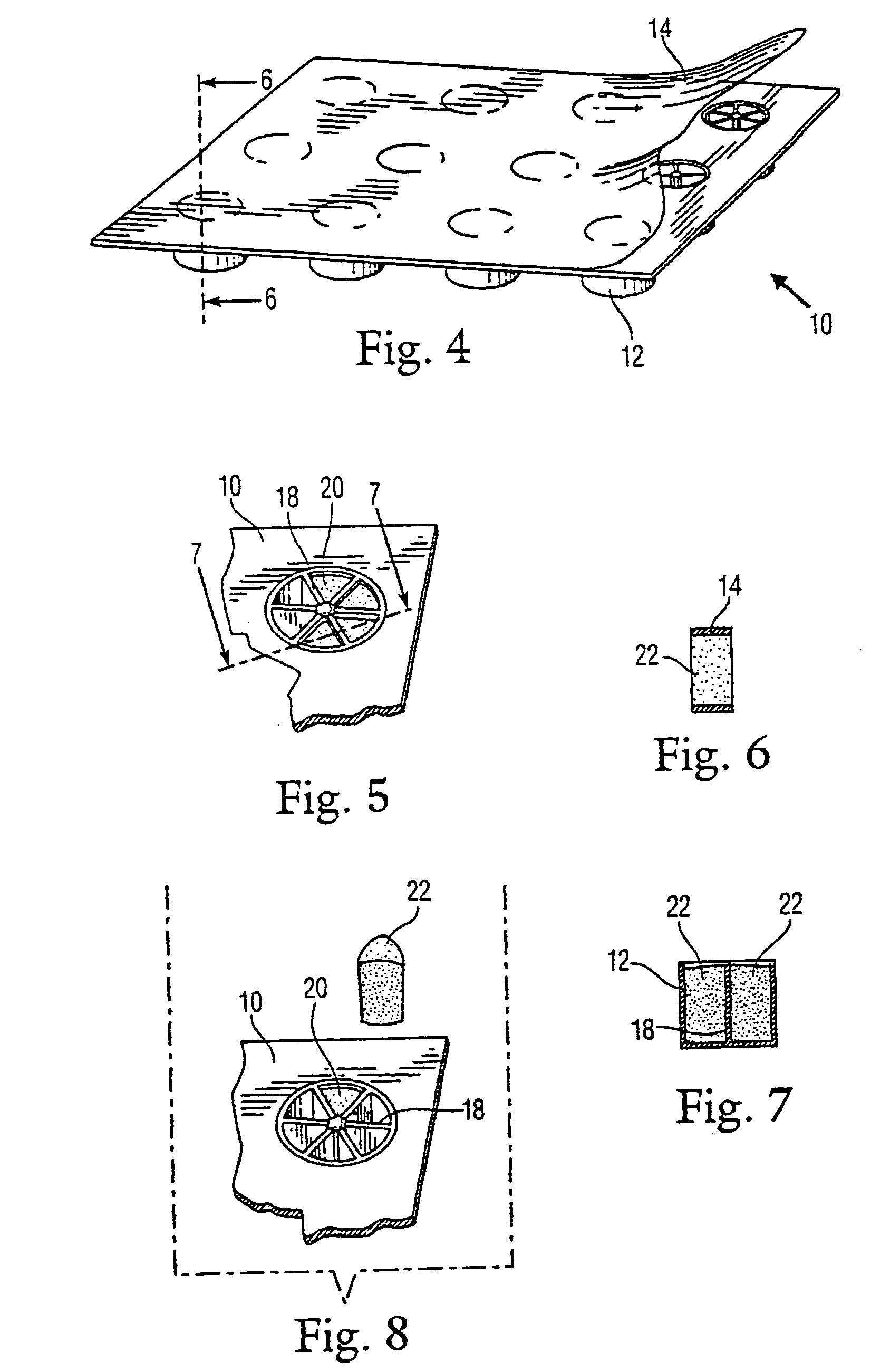
Sectioned article for mosquito control and package thereof
A package for mosquito control articles having a plurality of wells within a tray. At least one of the wells has a center post with spokes radiating from the post and forming segments within the at least one well. The mosquito control article is formed in sections with one section in each segment of the at least one well. The number of sections used to treat a body of water is determined by the surface area of the body of water and the predetermined amount of active ingredient in each section of the mosquito control article. A method of use is disclosed.
Owner:SUMMIT CHEM
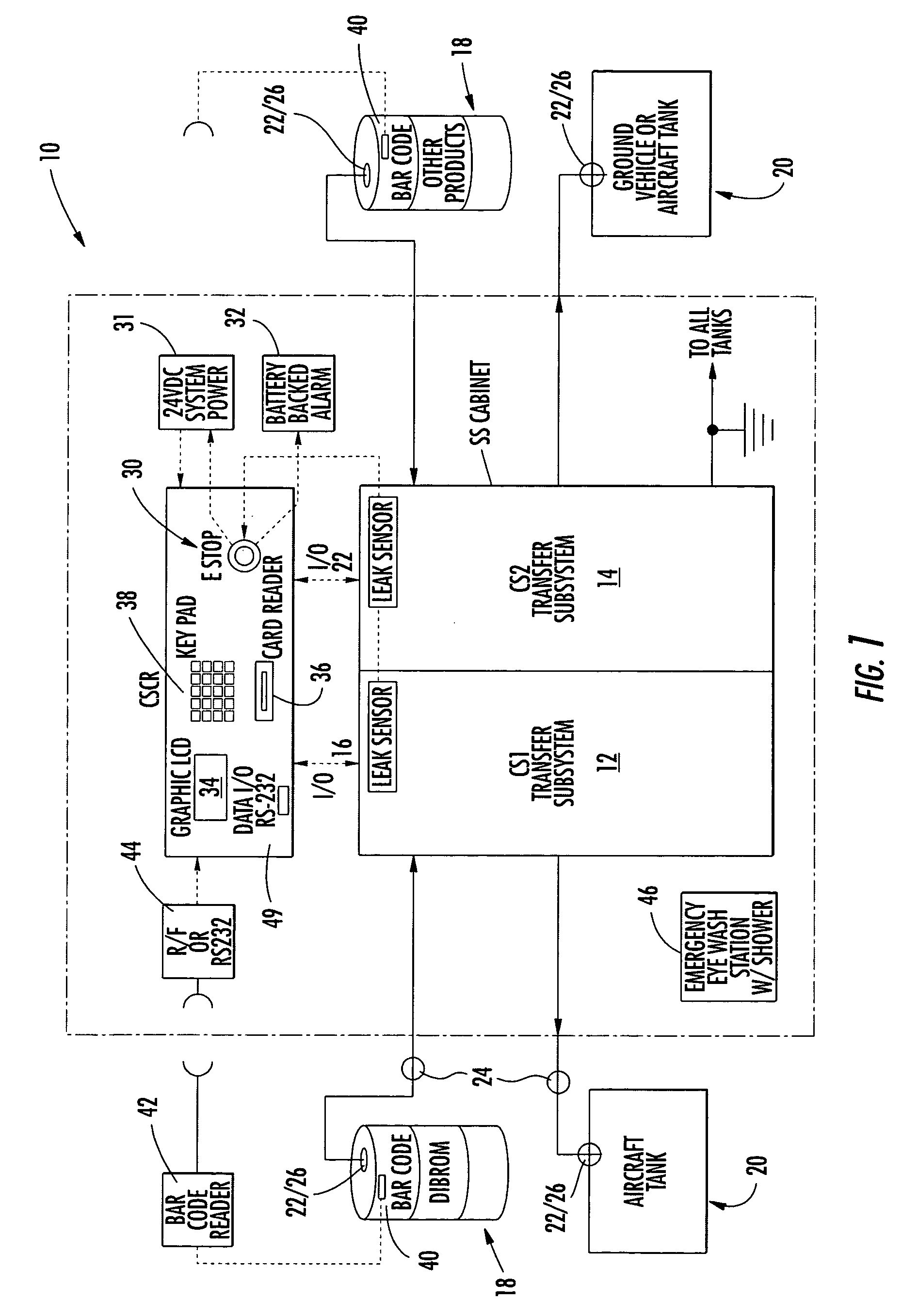
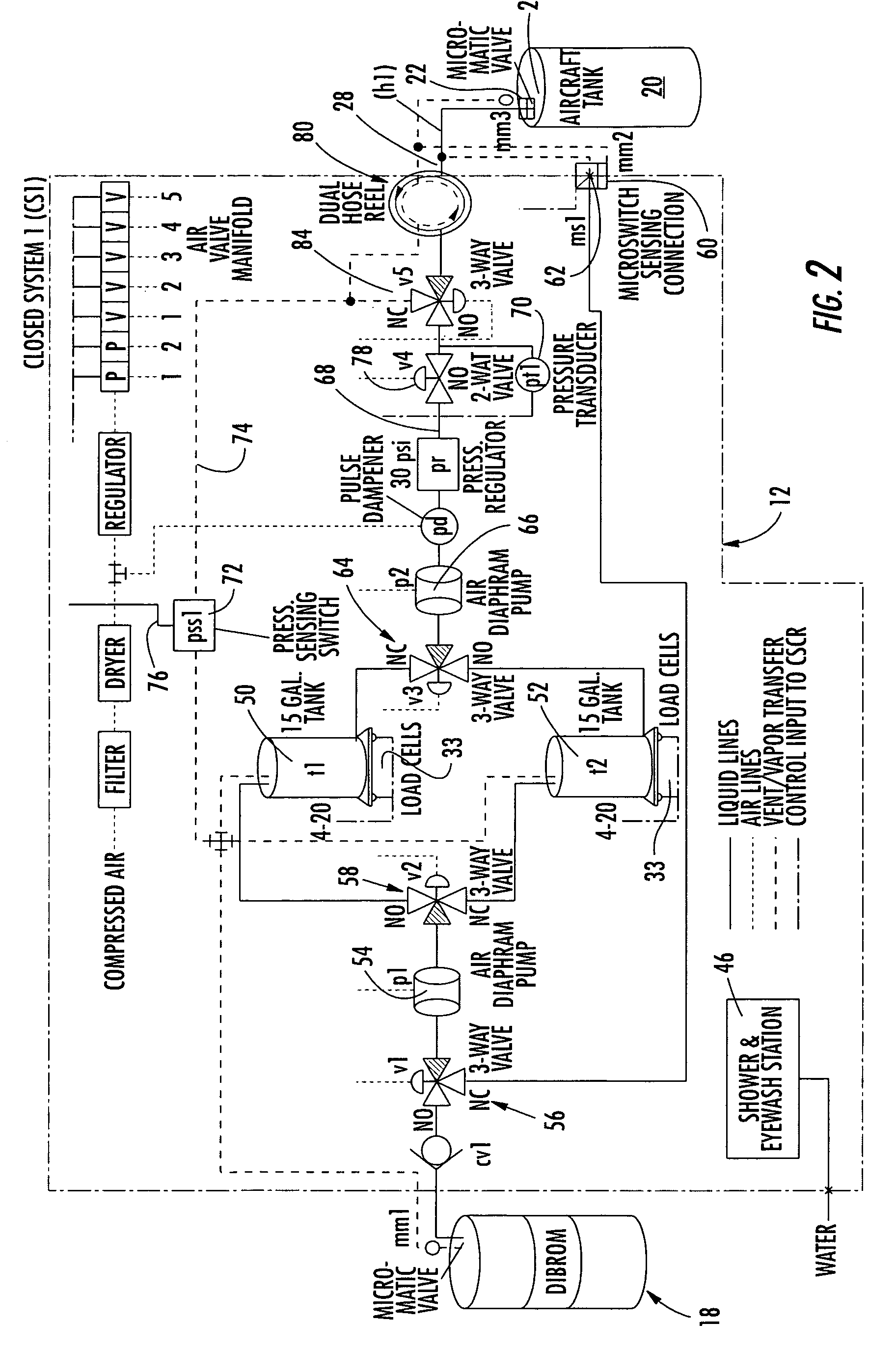
Hazardous materials transfer system and method
InactiveUS6968871B2Maximum protectionSafe transferLiquid fillingLiquid transferring devicesMosquito controlTransfer system
A hazardous fluid materials transfer system is automated to control the transfer of the hazardous fluid while maintaining the fluid within a closed environment for providing maximum personal protection to the operators handling the hazardous materials during the transfer, such as those operations in the mosquito control industry. The system includes the transfer of the fluid to storage tanks intermediate the source and target tanks between the transfer is desired. A pre-programmed processor receiving pressure, weights, and connection signals from transducers, such as pressure sensors and load cells, located throughout the system controls the operation of pumps and valves to allow the fluid being transferred to remain within a closed environment.
Owner:ADAPCO
Alluring and tagging mosquito killer
ActiveCN101946755AImprove the effect of prevention and controlAvoid pollutionBiocidePest attractantsMosquito controlVegetation
The invention provides an alluring and tagging mosquito killer, which comprises an air-permeability cloth bag of which the surface is coated with a pesticide, and a carbon dioxide generator, wherein the cloth bag is provided with a carbon dioxide filling port; and the carbon dioxide generator is communicated with the carbon dioxide filling port of the cloth bag through a filling pipeline. In the mosquito killer, a method of combining the carbon dioxide alluring and long-acting pesticide tagging is adopted, so the mosquito control effect in a special environment with better vegetation is improved, the control cost is reduced, the environmental pollution is avoided, and the alluring and tagging mosquito killer can be applied to other places not suitable for spraying pesticides. Besides, thealluring and tagging mosquito killer has the advantages of simple using method and easy operation.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
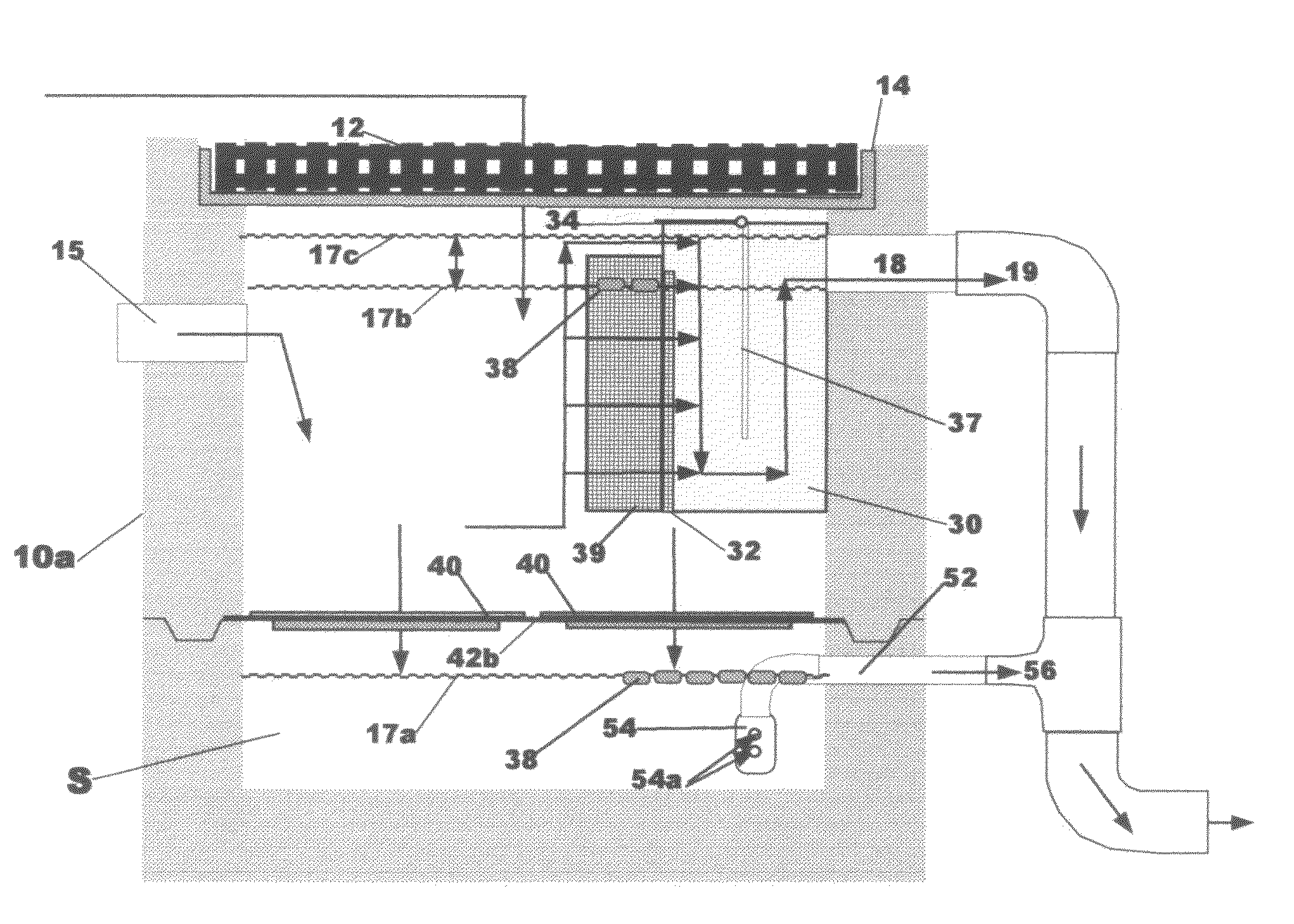
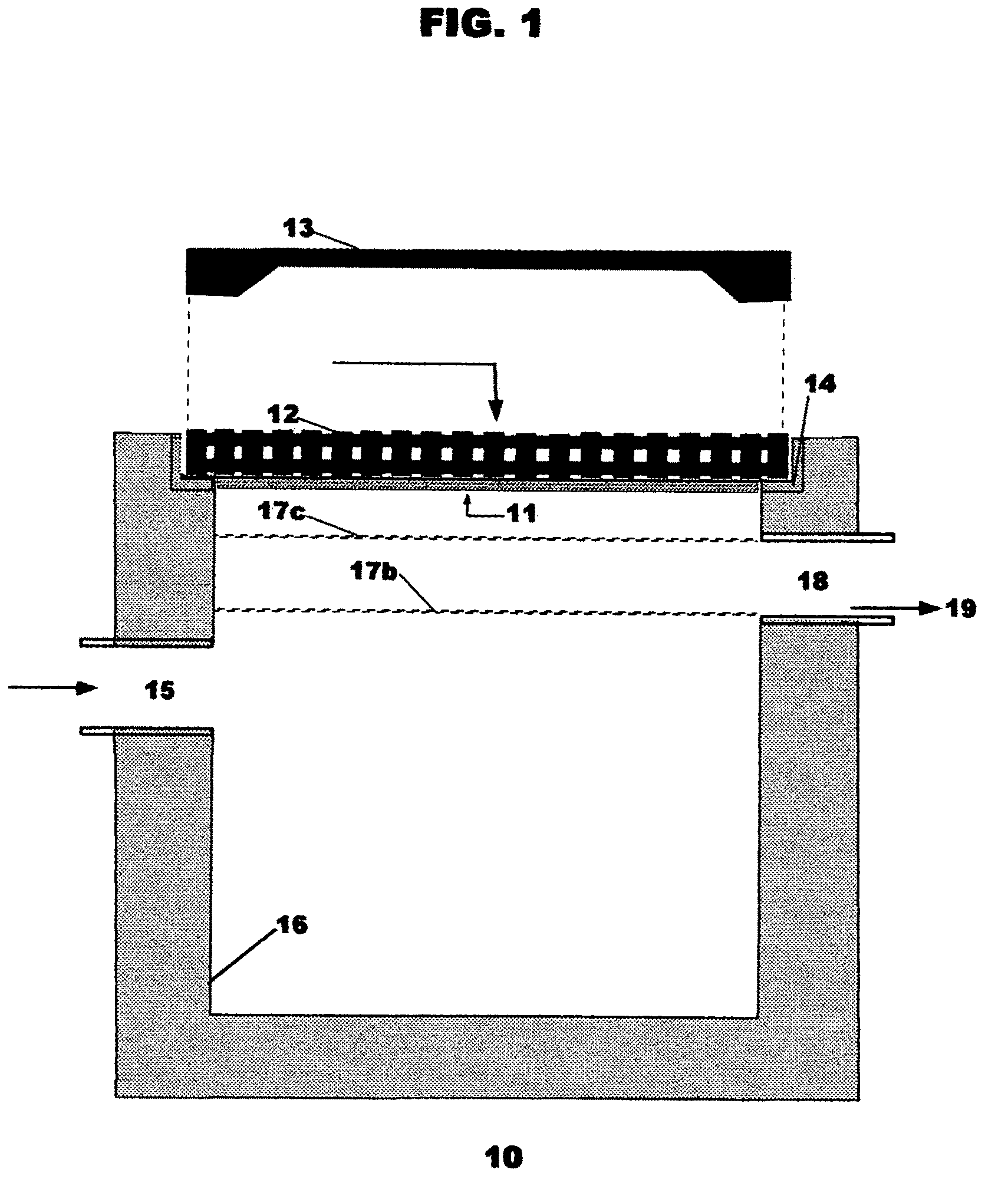
Watershed runoff treatment device & method
InactiveUS7988870B2Easy alignmentEfficient separationLoose filtering material filtersRunoff/storm water treatmentSites treatmentGravity flow
Owner:STORMWATER FILTERS
Watershed runoff treatment device & method
InactiveUS20090039022A1Easy alignmentEfficient separationLoose filtering material filtersRunoff/storm water treatmentSites treatmentMosquito control
A watershed runoff treatment device is adapted to be used to convert a single-stage drain inlet vault (catch basin) into a two-stage clarifier in which treatment can be accomplished before discharging the water to further on-site treatment or detention or to storm drain. It includes a flow control box member to be mounted on the interior of the vault covering the discharge pipe(s) so that substantially all water runoff entering the vault must flow therethrough prior to exiting the vault. Floating, disposable hydrocarbon collection pads are deployed inside an open mesh container removably attached and covering the upstream face of the flow control box, as well as below the horizontal filter array described next. A horizontal filter array with removable, cleanable filter media is deployed below the flow control box for collection of suspended solids gravity-settling within the vault space, and enabling standing water in the vault interior to gravity flow through the permeable filter and suspended solids and automatically discharge from the vault, thus providing mosquito control and de-watering of solids for easy removal.
Owner:STORMWATER FILTERS
Mosquito control product
A mosquito control product includes a two part composition including separated wet and dry ingredients. The wet and dry ingredients include active and inert ingredients. The active ingredients include thyme, rosemary, cinnamon, and citronella oil and the inert ingredients include water, sugar, yeast, wheat flour, sodium bicarbonate, and licorice extract. When the wet and dry ingredients are combined they form a carrier agent contacting a mosquito with the active ingredients wherein the mosquito is discouraged from seeking a blood meal.
Owner:PENTA 5 USA LLC
Mosquito Control Device Using Durable Coating Embedded Pesticides
Dual action lethal containers, systems and methods and novel compositions and formulas which are used to kill mosquitoes and their larvae. Generally pyramid shaped containers can have combined interior larvacidal and adultacidal coatings above and below a side opening in the container. A removable inclined grate cap can also allow for mosquitoes to enter into the container. Inclined stacked walls inside the container form attractive surfaces for mosquitoes to breed. Water-holding containers, such as flower pots, water holding dishes used under plant pots, vases, bird baths, and fountains and storm water inlets, can be coated with novel larvicide and / or adulticide coatings. Small objects can be coated with larvicide or larvicide and adulticide combination, which can be dropped in water-holding containers which can leach out pesticide over time which prevents mosquitoes from breeding in the water-holding containers.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
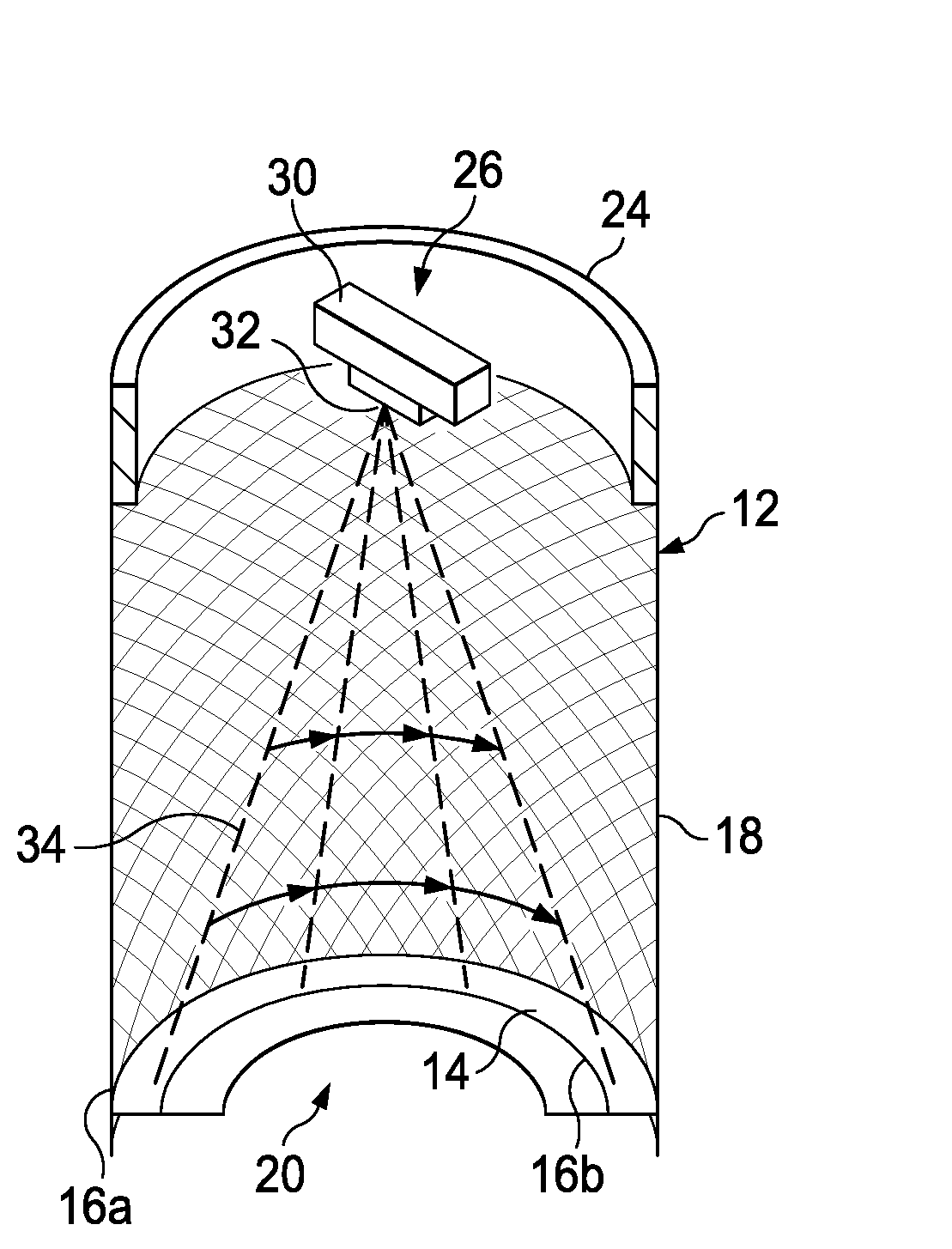
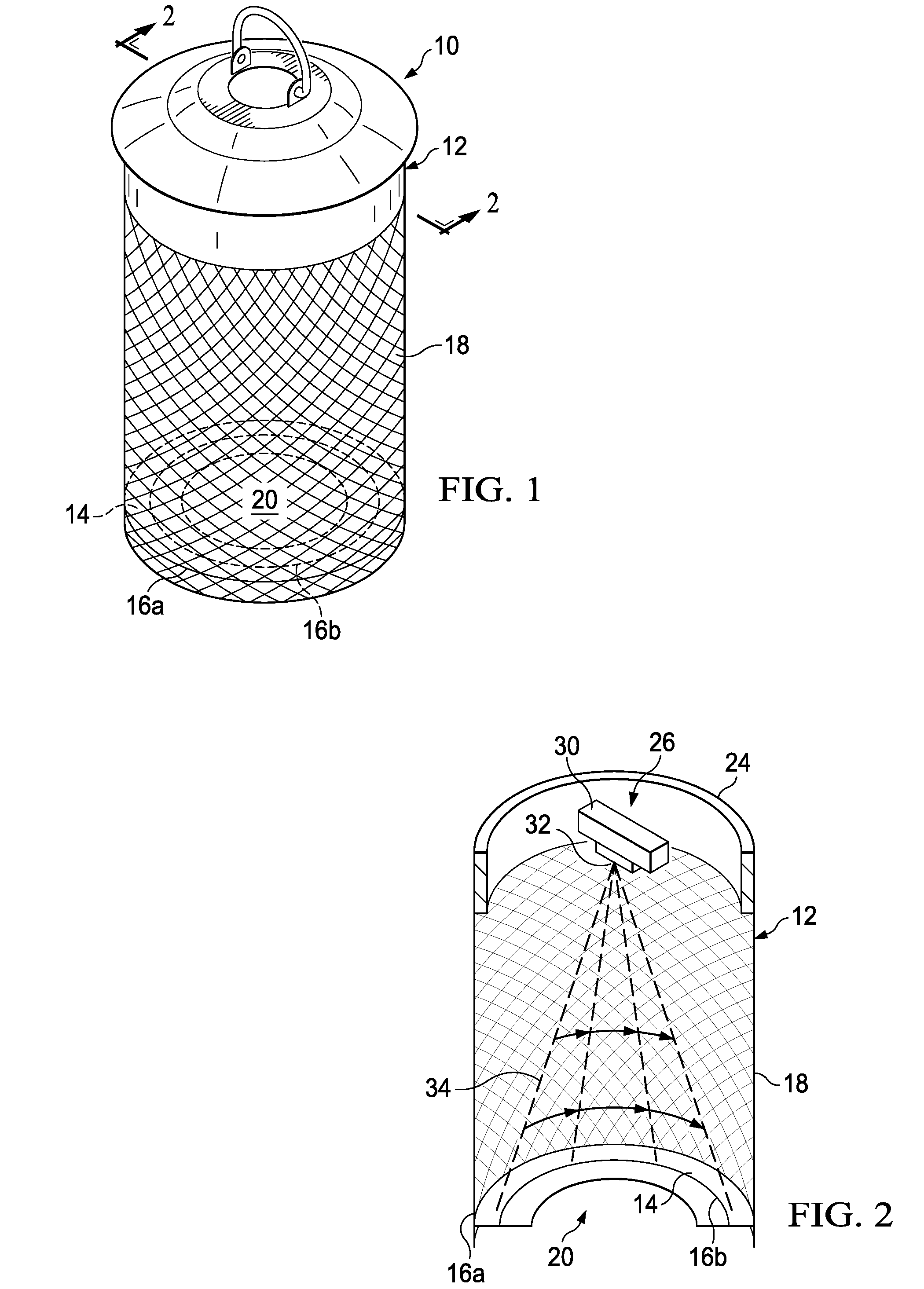
Method for laser mosquito control
InactiveUS20160270387A1Reduce transmissionMore transmissionInsect catchers and killersMosquito controlElectricity
The present invention provides an apparatus for damaging a flying pest comprising: a housing connected to a continuous perforated side wall to a laser contacting surface; a laser beam generator and a laser rotating mechanism attached to the housing and positioned to transmit a laser beam generally radially downward toward the laser contacting surface in a 360 degree pattern on the laser contacting surface, wherein the laser beam contacts and damages a flying pest; a control mechanism in communication with the laser beam generator to control the generation of the laser beam and in communication with the laser rotating mechanism to control one or more parameters relating to the pattern; and a power supply in electrical communication with the control mechanism, the laser rotating mechanism, and the laser beam generator.
Owner:INNOVATION CONSULTANTS
Method and apparatus for laser mosquito control
InactiveUS9374990B2Reduce transmissionMore transmissionInsect catchers and killersElectricityMosquito control
The present invention provides an apparatus for damaging a flying pest comprising: a housing connected to a continuous perforated side wall to a laser contacting surface; a laser beam generator and a laser rotating mechanism attached to the housing and positioned to transmit a laser beam generally radially downward toward the laser contacting surface in a 360 degree pattern on the laser contacting surface, wherein the laser beam contacts and damages a flying pest; a control mechanism in communication with the laser beam generator to control the generation of the laser beam and in communication with the laser rotating mechanism to control one or more parameters relating to the pattern; and a power supply in electrical communication with the control mechanism, the laser rotating mechanism, and the laser beam generator.
Owner:INNOVATION CONSULTANTS
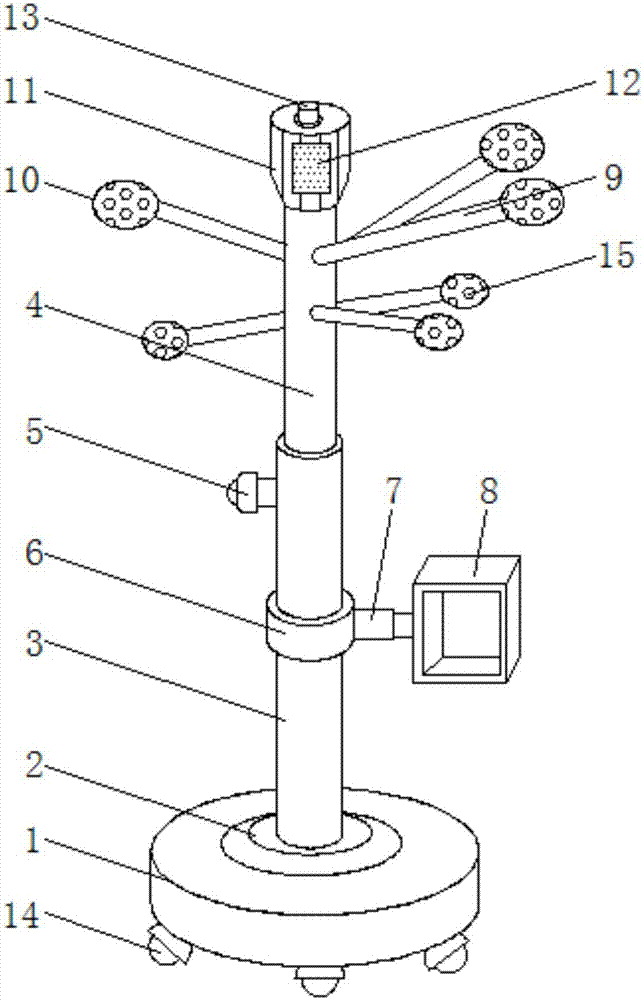
Multifunctional coat hanger for bedroom
InactiveCN107126060AEasy to moveNot easy to smellApparel holdersInsect catchers and killersMosquito controlEngineering
The invention discloses a multifunctional clothes hanger for a bedroom, which comprises a base, a storage box and an electronic mosquito killer. Rollers are installed at the bottom of the base, and a turntable is installed at the center of the top of the base, and a sleeve is installed on the turntable. , the inside of the sleeve is plugged with a support rod, the upper part of the support rod is obliquely inserted with a clothes hanger, the end of the clothes hanger is installed with a ball head, the inside of the ball head is placed with a spice bag, and the top of the support rod is installed with an electronic mosquito killer. device, a music player is installed on the other side of the top of the storage box. In the present invention, the electronic mosquito killer is installed on the top of the support rod, so that the clothes hanger has the function of killing mosquitoes, the effect of killing mosquitoes is good, the quality of people's sleep is improved, and it is environmentally friendly and pollution-free, reducing the impact on people's health. Health hazard, by placing a spice bag inside the ball head, the peculiar smell on the clothes can be removed and the fragrance of the clothes can be increased, and the structure is simple, easy to move, adjustable in height, and strong in practicability.
Owner:江苏森洋巨星机械有限公司
Combustible pesticidal products
A combustible pesticidal product is disclosed which comprises a structural element formed from a cardboard having a thickness of at least 0.75 mm, a density of 450-850 kgm<3> and consisting of 1 or more plies, the cardboard including: an alkali or alkaline earth metal nitrate or nitrite in an amount of from 0 to 1.83 % w / w, or an alkali or alkaline earth carbonate or bicarbonate in an amount of from 0.02 to 7.0 % w / w; one or more mineral silicates in an amount of from 0.01 to 8.0 % w / w; a phosphate in an amount of from 0.01 to 0.40 % w / w and selected from the group consisting of diammonium phosphate, monoammonium phosphate, triammonium phosphate and mixtures thereof; a boron compound in an amount of from 0.001 to 0.92 % w / w (as boron) and selected from the group consisting of boric acid, sodium tetraborate hydrous, sodium borate, potassium borate, calcium borate, zinc perborate, boronatrocalcite and mixtures thereof; one or more pesticides; and optionally a perfume and / or a dye, which product on combustion emanates the pesticide into the atmosphere. Typically the combustible product will be a mosquito coil which has been impregnated with one or more insecticides effective against mosquitoes. On combustion of the coil, insecticide is emanated into the atmosphere for a period of at least 4 hours. However, the coils of the invention may be active against mosquitoes for 8 hours or more.
Owner:RECKITT BENCKISER AUSTRALIA
Mosquito control device using durable coating-embedded pesticides
Dual action lethal containers, systems and methods and novel compositions and formulas which are used to kill mosquitoes and their larvae. Generally pyramid shaped containers can have combined interior larvacidal and adultacidal coatings above and below a side opening in the container. A removable inclined grate cap can also allow for mosquitoes to enter into the container. Inclined stacked walls inside the container form attractive surfaces for mosquitoes to breed. Water-holding containers, such as flower pots, water holding dishes used under plant pots, vases, bird baths, and fountains and storm water inlets, can be coated with novel larvicide and / or adulticide coatings. Small objects can be coated with larvicide or larvicide and adulticide combination, which can be dropped in water-holding containers which can leach out pesticide over time which prevents mosquitoes from breeding in the water-holding containers.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Mosquito killer
InactiveCN106386738AGuaranteed eradicationUniform temperatureInsect catchers and killersMosquito controlElectricity
The invention relates to a mosquito killer. The mosquito killer includes a mosquito trap unit and a mosquito control unit. The mosquito trap unit and the mosquito control unit are detachably connected. The mosquito trap unit includes an upper shell, a mosquito trap lamp, a fan and a switch. The mosquito trap lamp is connected with the fan and the switch. The mosquito control unit includes a mosquito storage box, a valve cover and an electric heating switch, the inner wall of the mosquito storage box is placed with an electric heating mesh that is separated into a number of zones. The switch controls the on-and-offs of the mosquito trap lamp and the fan, the electric heating switch control the on-and-offs of the mosquito storage box. The mosquito catching and killing are divided into two steps; the electric heating mesh dries the mosquito to death, odorless and without a spark, thus achieving the purpose of thorough mosquito killing and safe for environment. In addition, the layered structure of the mosquito storage box balances the temperature, eliminates the existence of a death corner for mosquito killing, and kills mosquitoes instantly.
Owner:谢文娟
Efficient environment-friendly mosquito killing device
InactiveCN107711760AIncrease the touch areaAvoid accumulationInsect catchers and killersMosquito controlMotor drive
The invention discloses a high-efficiency and environment-friendly mosquito killing device, which comprises a mosquito killing device body, a mosquito trap lamp and a mosquito killing grid. The body of the mosquito killing device is vertically provided with side plates, and the upper part of the miniature air purifier is sequentially arranged. There are mosquito trap lamps and mosquito killing grids, the mosquito killer lamps are fixedly connected to the side panels through the lamp sockets, the end of the rotating shaft is connected to the motor through the transmission shaft, and the top of the side panels is connected to the top cover through gauze . In the present invention, under the transmission action of the motor, the mosquito-killing grid can rotate around the axis, and through the rotation of the mosquito-killing grid, the contact area between the mosquito-killing grid and the mosquito can be increased, thereby effectively killing the mosquito and preventing its Escape improves the efficiency of mosquito killing. At the same time, the rotating mosquito killing grid will guide the killed mosquito corpses into the storage box at the bottom, preventing the accumulation of mosquito corpses on the mosquito killing grid.
Owner:四川新天地环保科技有限公司
Injection system for delivering liquid into sprinkler system
InactiveUS20140238514A1Improve efficiencyDomestic plumbingPressurised distribution of liquid fertiliserMosquito controlLow voltage
An injection system for adding a liquid, such as a pesticide or mosquito control formula, to a sprinkler system is disclosed. The injection system includes a tank and a pump. The pump may be powered only by a low-voltage connection to the sprinkler system's timer. The pump may use a suction hose to draw piqued out of the tank. An outlet on the pump may be connected to a forked hose. One side of the fork may be a return hose for recirculating the contents of the tank. The other side of the fork may be a discharge connection hose for connecting to the sprinkler system. The discharge connection hose can further comprise an injector fitting.
Owner:YARBROUGH JR DONNIE WAYNE
Method for mosquito control
A formulation and method for insect control is provided in the form of insecticide carrying insects which can be introduced in a population to thereby control the insect population. The formulation may include artificially generated adult insect carriers of a larvicide in which the larvicide has minimal impact on the adult insect and which larvicide affects juvenile survival or interferes with metamorphosis of juvenile insects to adulthood. The insects may be either male or female and may include mosquitoes.
Owner:S·多布森
Control and repellency of mosquitoes
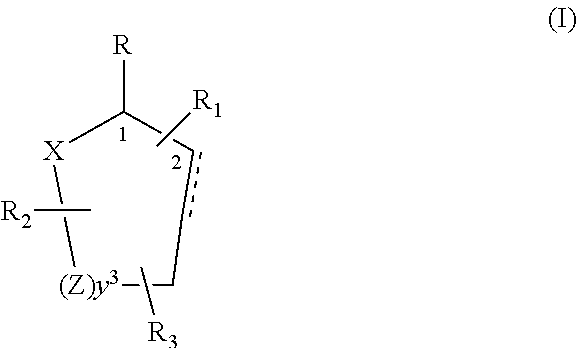
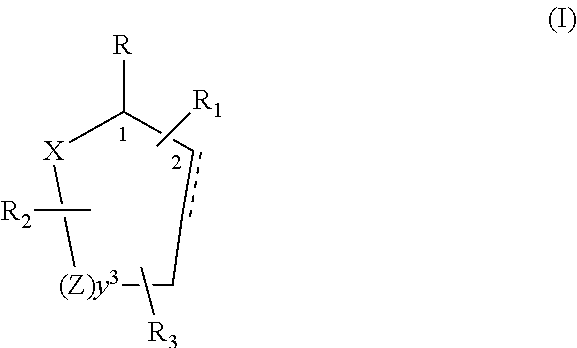
Control or repellency of mosquitoes is accomplished by bringing the insects into contact with at least one of the compounds of the structure (I)whereinR is selected from —OH, ═O, —OC(O)R4, —OR6, —(OR6)2, wherein each R6 is independently selected from an alkyl group containing from 1 to 4 carbon atoms and R4 is a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms;X is O or CH2, with the proviso that when X is O R can only be ═O;each Z is independently selected from (CH) and (CH2);y is a numeral selected from 1 and 2;R1 is selected from H or a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms;R2 is selected from H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms;R3 is selected from the group consisting of H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms, —(CH2)nOH, —C(O)OR5, —CH2C(O)OR7, —CH2C(O)R8, —C(O)NR9R10, —CH2C(O)NR11R12 where each of R5, R7, R8, R9, R10, R11 and R12 is independently selected from H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms and n is an integer of from 1 to 12;the bond between the 2 and 3 positions in the ring structure may be a single or a double bond; andwherein the compounds of structure (I) contain from 11 to 20 total carbon atoms in the compounds, with the proviso that when R is ═O, X is CH2, Z is CH2, y is 1 R2 is H, and R3 is CH2C(O)OR7 then the total number of carbon atoms in the compounds of structure (I) is from 15 to 20 carbon atoms, and when X is O and R is ═O the total number of carbon atoms in the compounds of structure (I) is from 11 to 17 carbon atoms. The invention also includes optical isomers, diastereomers and enantiomers of the named structures. Thus, at all stereocenters where stereochemistry is not explicitly defined, all possible epimers are envisioned.
Owner:BEDOUKIAN RES
Comprehensive control method for city mosquitoes
The invention discloses a comprehensive control method for city mosquitoes. Mosquito control is divided into indoor adult control, indoor larva control, outdoor adult control and outdoor larva control. Indoor adult control is carried out by adopting the methods of gauze isolation and chemical avoidance particularly, indoor larva control is carried out by adopting the methods of removing accumulated water and placing pesticide, outdoor adult control is carried out by adopting the methods of light wave trap and kill and sex attractant placement for trap and kill, and indoor larva control is carried out by adopting the methods of removing accumulated water, placing natural enemies and placing pesticide. The city mosquitoes can be controlled to below the standard of civilized city mosquito eradication, no chemical pesticide is needed, and the method has the significance in control over life quality of residents and mosquito-borne diseases and environmental protection.
Owner:ZHANGZHOU LIKANG PEST PREVENTION & CONTROL CO LTD
INSECTICIDAL CARBAMATES EXHIBITING SPECIES-SELECTIVE INHIBITION OF ACETYLCHOLINESTERASE (AChE)
ActiveUS20090068242A1Increase lethalityBiocideSilicon organic compoundsMethyl carbamateInsecticide-treated mosquito nets
The present invention includes insecticidal carbamates that are useful, for example, for the control of insects, such as mosquitoes, which can be used in applications where exposure to and / or contact with humans is likely. The insecticides of the present invention include phenyl N-methyl carbamates and compositions comprising them that exhibit species-selective inhibition of acetylcholinesterase (AChE) and are preferably toxic to mosquitoes but not humans. Of particular interest are compounds of Formula (I) and Formula (II):Compounds of Formula (I) and Formula (II) are especially suitable for insecticide treated nets and indoor residual spraying for mosquito control.
Owner:VIRGINIA TECH INTPROP INC
Lifting type lawn lamp
ActiveCN110671667AHave the effect of killing mosquitoesEasy to operateMechanical apparatusLighting support devicesMosquito controlElectric machinery
The invention discloses a lifting type lawn lamp comprising a lawn lamp body, a working cavity is formed in the lawn lamp body, a mosquito repelling mechanism is arranged in the working cavity, the mosquito repelling mechanism comprises a connecting block, a moving mechanism is arranged on the lower side of the mosquito repelling mechanism, the moving mechanism comprises an air storage frame fixedto the right end wall of the working cavity, an air storage cavity is formed in the air storage frame, a rotating mechanism is arranged on the right side of the moving mechanism, the rotating mechanism comprises a transmission shaft, and a transmission cavity is formed in the right side of the lawn lamp body. The device is simple to operate; when the lawn lamp needs to be used, a motor is started, and the lawn lamp rises from the lower portion of a lawn to provide illumination; when a certain temperature is reached, the lawn lamp can also play a role in mosquito control; and after the motor is turned off, the lawn lamp is fixed on the ground for a period of time, and after a long time when gas in the gas storage cavity is completely leaked, the lawn lamp downwards returns to a position below the lawn.
Owner:广州柏思园林景观设计有限公司
Alluring and tagging mosquito killer
ActiveCN101946755BImprove the effect of prevention and controlAvoid pollutionBiocidePest attractantsVegetationMosquito control
The invention provides an alluring and tagging mosquito killer, which comprises an air-permeability cloth bag of which the surface is coated with a pesticide, and a carbon dioxide generator, wherein the cloth bag is provided with a carbon dioxide filling port; and the carbon dioxide generator is communicated with the carbon dioxide filling port of the cloth bag through a filling pipeline. In the mosquito killer, a method of combining the carbon dioxide alluring and long-acting pesticide tagging is adopted, so the mosquito control effect in a special environment with better vegetation is improved, the control cost is reduced, the environmental pollution is avoided, and the alluring and tagging mosquito killer can be applied to other places not suitable for spraying pesticides. Besides, the alluring and tagging mosquito killer has the advantages of simple using method and easy operation.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Natural plant and medicine stone nanometer mosquito-controlling slow-release preparation
The invention discloses a natural plant and medicine stone nanometer mosquito-controlling slow-release preparation and belongs to the technical field of preparation and production of sanitary insect pest mosquito-controlling slow-release preparations applying a high-technology nanotechnology. The natural plant and medicine stone nanometer mosquito-controlling slow-release preparation comprises a cinnamomum camphora and medical stone natural microcapsule mosquito-driving and controlling slow-release preparation, a tetramethrin and zeolite organophosphorus mosquito-controlling slow-release preparation, an allethrin and seaweed charcoal oxidation exothermic nanometer mosquito-controlling slow-release preparation, a diethyltoluamide and sepiolite volatile mosquito-controlling slow-release preparation, a rhizoma atractylodis and limonite heating evaporation mosquito-controlling slow-release preparation, a propylene, pyrethrum and stalactite microcapsule slow-release mosquito-controlling preparation and a nanometer mosquito-controlling slow-release preparation. By using a nanometer microcapsule formed by coating small nanometer solid powder or liquid particles of natural plants such as pyrethrum, cinnamomum camphora, rhizoma atractylodis and folium artemisiae argyi by ores such as the medical stone, the zeolite and tourmaline with a porous honeycomb structure, the effect of controlling mosquitoes by slow release is achieved, anion far infrared rays in elements are released for a long time to promote the health of a human body, and the mosquito-controlling slow-release preparation has two mosquito-controlling health functions and is used for prolonging the service life.
Owner:NANTONG SNAKEBITE THERAPY INST
Mosquito prevention LED ceiling lamp
InactiveCN105953148AWith mosquito protectionExtended service lifeLighting support devicesLighting elementsMosquito controlEngineering
The invention discloses a mosquito prevention LED ceiling lamp. The mosquito prevention LED ceiling lamp comprises a lamp base, a base plate, a mosquito control mesh cover, a mosquito control lamp, an LED illumination module, a lamp cover and a rubber sealing washer; the lamp base is fixed on a ceiling; the base plate is fixed in the lamp base; the mosquito control mesh cover is arranged between the base plate and the lamp base; the mosquito control lamp is arranged in the mosquito control mesh cover; the LED illumination module is arranged on the lower surface of the base plate; and the lamp cover and the base plate are fixedly connected through the rubber sealing washer. Through the mode, the mosquito prevention LED ceiling lamp, provided by the invention, is additionally provided with the mosquito control mesh cover and the mosquito control lamp above the base plate for solving the problem of mosquitoes entering the lamp cover of a general ceiling lamp, so that the ceiling lamp has a mosquito prevention function for preventing the mosquitoes from entering the lamp cover to cause electric faults to reduce the illumination performance, also gives consideration to moisture prevention, and effectively prolongs the service life of the LED illumination module.
Owner:SUZHOU KUNLUN IND DESIGN
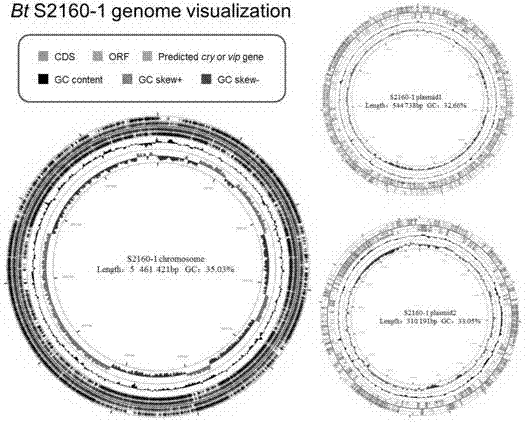
Bacillus thuringiensis for killing mosquito larvae and application of bacillus thuringiensis
InactiveCN107254426AReduce the risk of resistance developmentBiocideBacteriaBacillus thuringiensisAureobasidium sp.
The invention belongs to the technical field of biological prevention and control of sanitary insect pests, and mainly discloses a material and a method for preventing and controlling larvae of mosquitoes which are the leader of 'four pests'. The invention discloses a novel strain Bt S2160-1 of Bacillus thuringiensis, Bt for killing mosquito larvae, wherein the preservation number of the novel strain is CGMCC No.13274. The strain Bt S2160-1 has very high insecticidal activity upon larvae of culex fatigans and aedes albopictus, and compared with a Bt mosquito strain Bti recommended by WHO (World Health Organization), the Bt S2160-1 has relatively high activity upon larvae of aedes albopictus. The Bacillus thuringiensis Bt S2160-1 disclosed by the invention can be made into an insecticide for large-scale prevention and control on larva populations of culex fatigans and aedes albopictus. Therefore, the strain can be powerful supplement as a mosquito control microbial agent in addition to Bti, or even can be used as a substitution of Bti for controlling populations of aedes albopictus, and the risk that mosquitoes have resistance to a Bti crystal protein which is used for a long time to control mosquito populations can be reduced.
Owner:浙江翠溪农业开发有限公司
Integrated mosquito control sanitary device for underground garage of high-rise building
ActiveCN102888899ALow densityGood for healthPest controlInsect catchers and killersMosquito controlEngineering
The invention relates to an integrated mosquito control sanitary device for an underground garage of a high-rise building and belongs to the technical field of health-epidemic prevention. The underground garage is built below the high-rise building; a sedan entrance, a first mosquito baffle and an air-blowing airflow inlet are arranged on the left side of the underground garage; a sedan exit, a second mosquito baffle and an air exhaust airflow outlet are arranged on the right side of the underground garage; a top electric shock mosquito trap is arranged on the top of the underground garage; underground garage upright posts are built in the underground garage; a temperature sensor, an image sensor and a humidity sensor are arranged on the underground garage upright posts; an underground garage elevator entrance / exit is formed in the middle of the underground garage; a portable water suction pump is hung on the wall of the underground garage, and an ultraviolet mosquito killer lamp is arranged on the wall of the underground garage; pyrethrin volatilization pots are arranged on two sides of the elevator entrance / exit; ultraviolet mosquito killer lamps are arranged on the left and right sides of the elevator entrance / exit of each floor of the high-rise building; pyrethrum potted landscapes are arranged behind office windows; and a mosquito killer property management calculation and prevention and control center is arranged in the high-rise building.
Owner:徐州博创建设发展集团有限公司
Mosquito-control emulsion
InactiveCN105379710AGood effect of killing mosquitoesLong failureBiocidePest attractantsMosquito controlEmulsion
The invention provides mosquito-control emulsion. The mosquito-control emulsion is prepared from the following raw materials: mosquitocide, attractant, organic solvent, dispersant, thickening agent and water. By taking the total weight of the emulsion as 1, the mosquitocide contains the components with the following content: 10-45% of pyrethrum, 2-10% of marigold, 1-8% of mint, 1-6% of garlic and 1-6% of molasses. The mosquito-control emulsion provided by the invention not only has a good mosquito-control effect, but also has relatively long effect. Furthermore, compared with the prior art, the mosquito-control emulsion is neither mosquito-repellent incense nor mosquito-control liquid, but is emulsion; and the mosquito-control emulsion can be dropped into water to kill larvae of mosquitoes and also can be smeared or sprayed to carry out mosquito control.
Owner:GAOYOU FENGTIAN AGROCHEM CO LTD
Mosquito prevention and control method
InactiveCN109006737AGood attractionPrevent pestsInsect catchers and killersAgainst vector-borne diseasesMosquito controlInfrared
The invention discloses a mosquito prevention and control method. According to insect information detected via an infrared induction module, a controller is in 4g or WiFi connection with an upper computer; via the detected insect information, the controller is controlled via the upper computer to drive a mosquito luring lamp driving module, and the controller is connected with a mosquito luring lamp via the mosquito luring lamp driving module; light-emitting frequency and intensity and light color of the mosquito luring lamp are changed according to the insect information detected via an infrared induction device so as to well lure mosquitoes; high voltage power grid rotation is started, a laser device is turned on, and lured mosquitoes are killed so as to well perform mosquito control.
Owner:肇庆市高新区甜慕新能源技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com