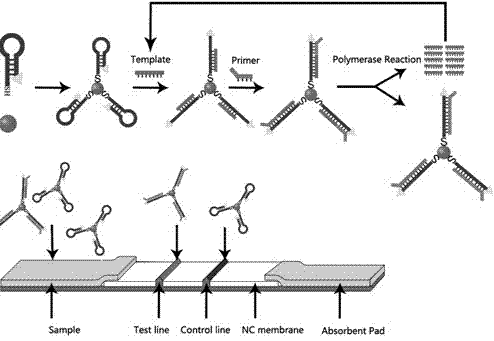
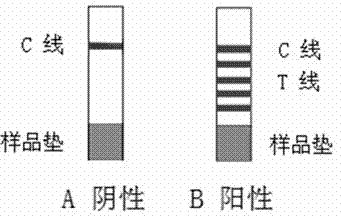
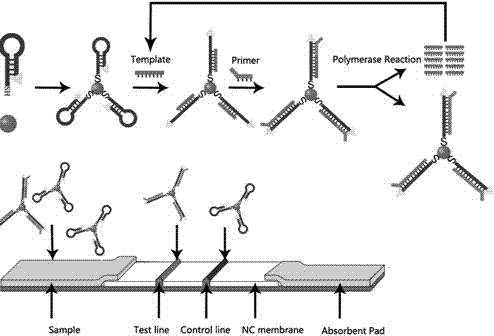
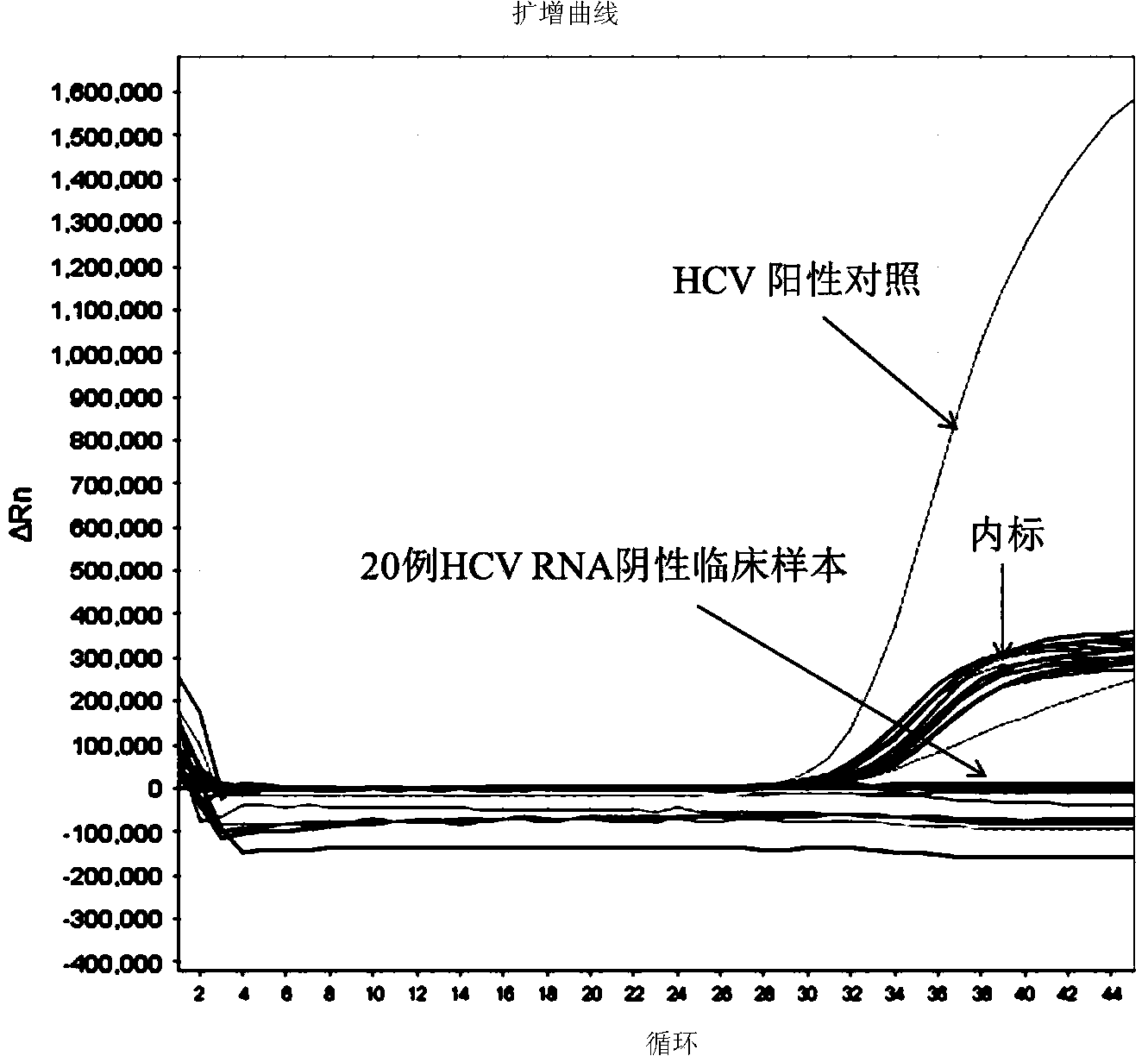
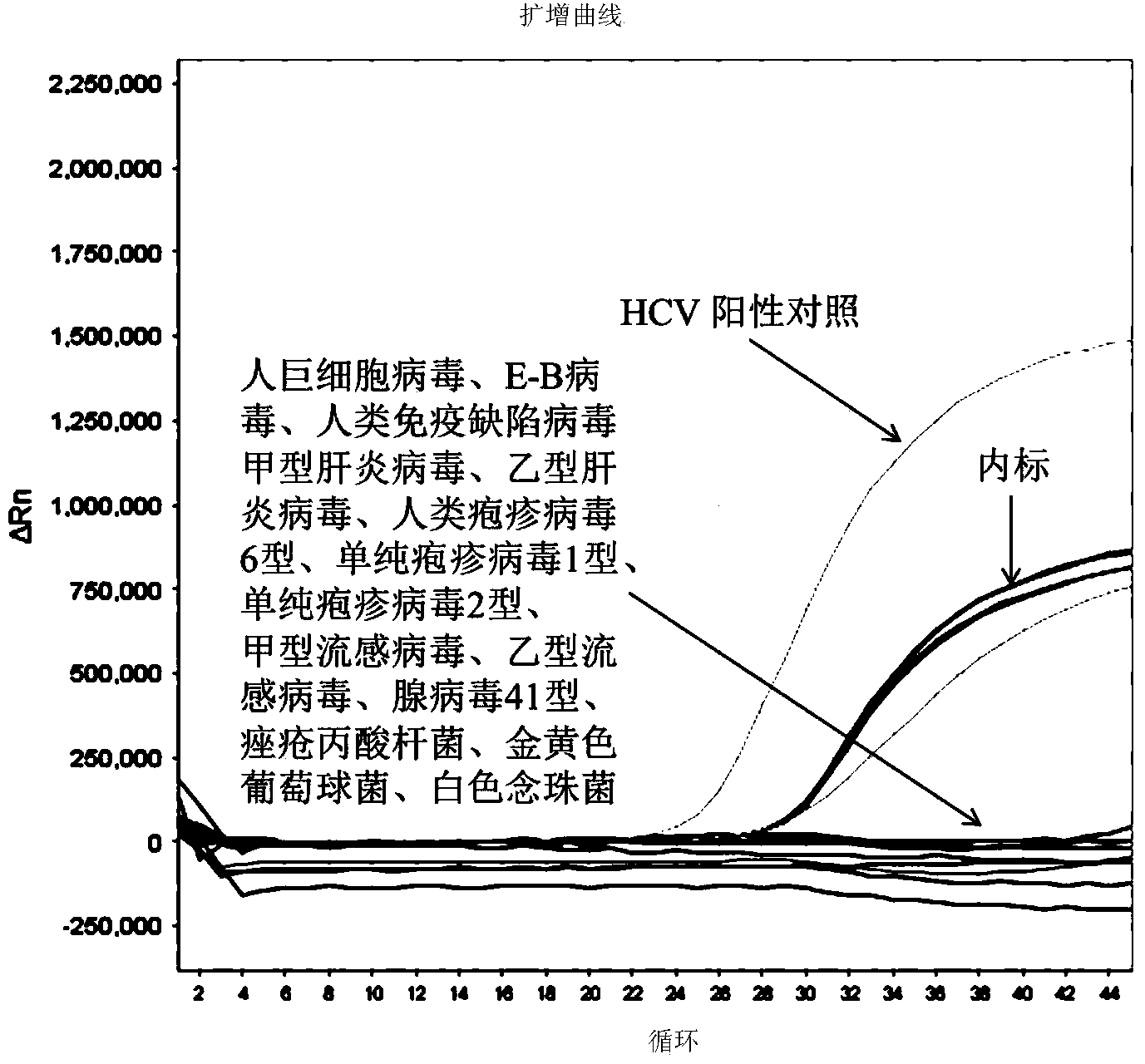
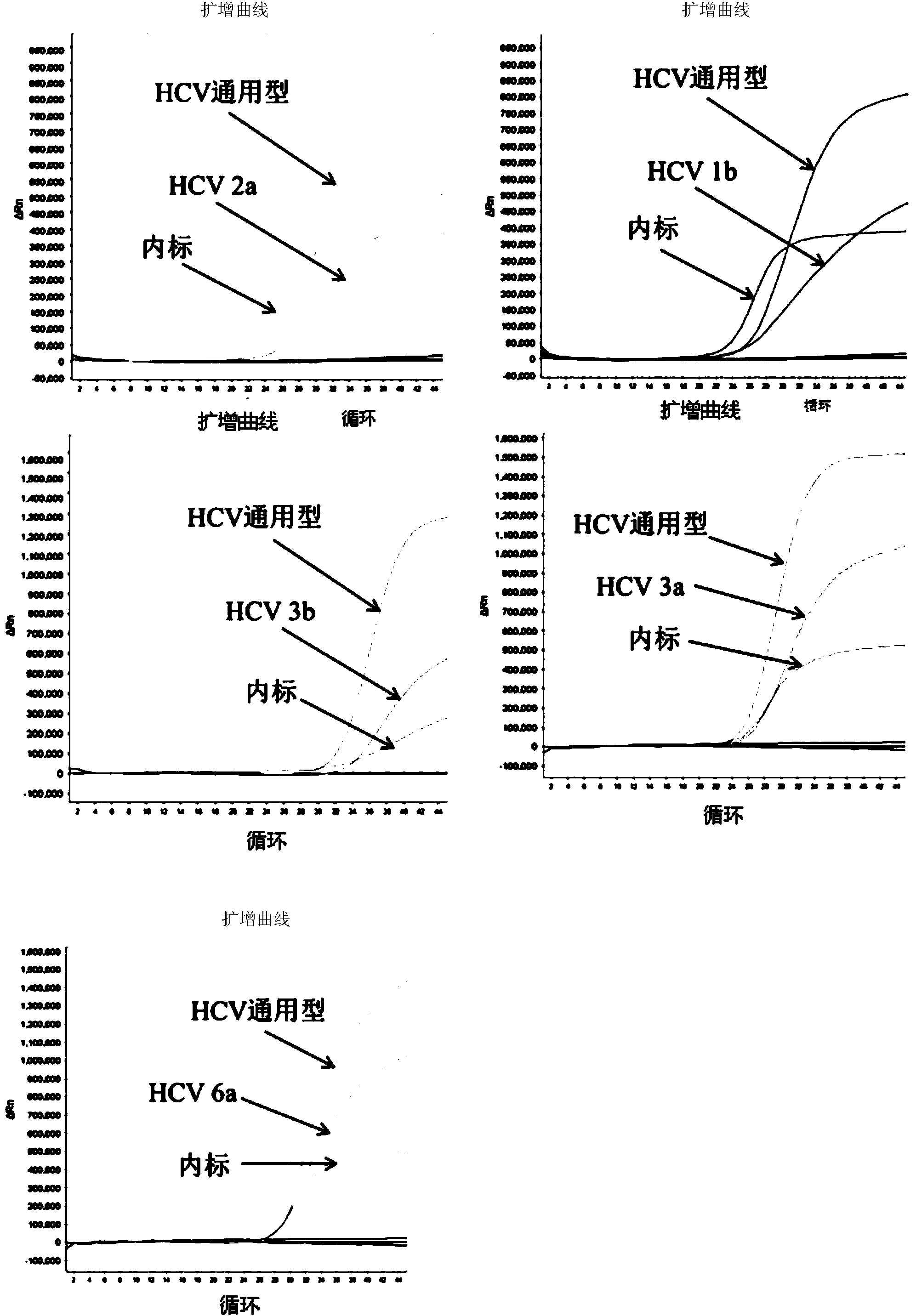
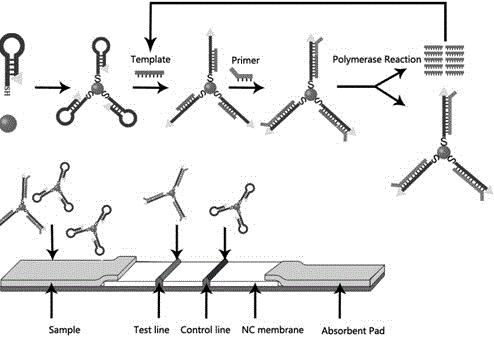
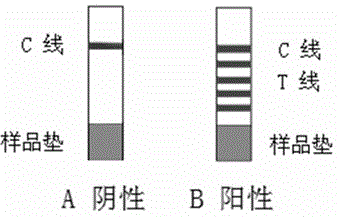
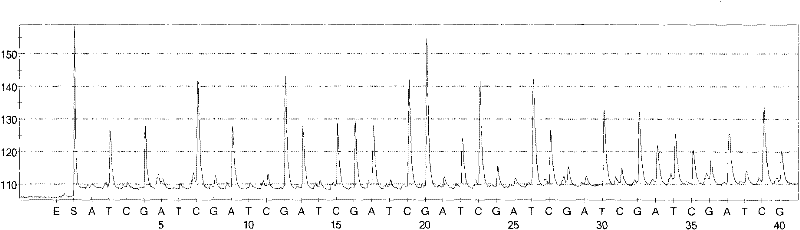
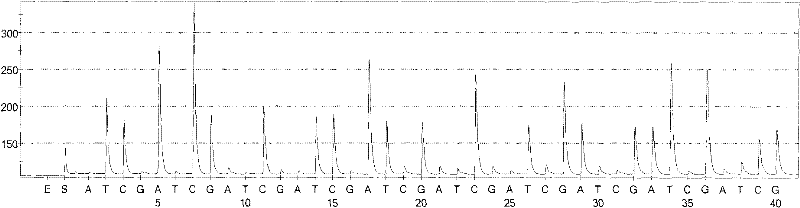
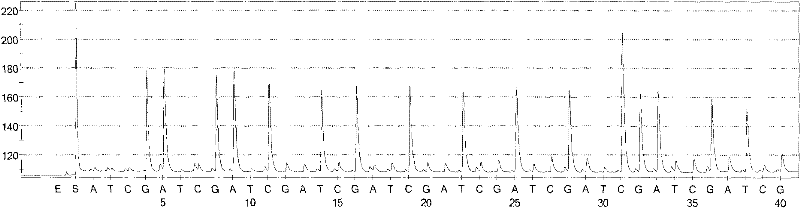
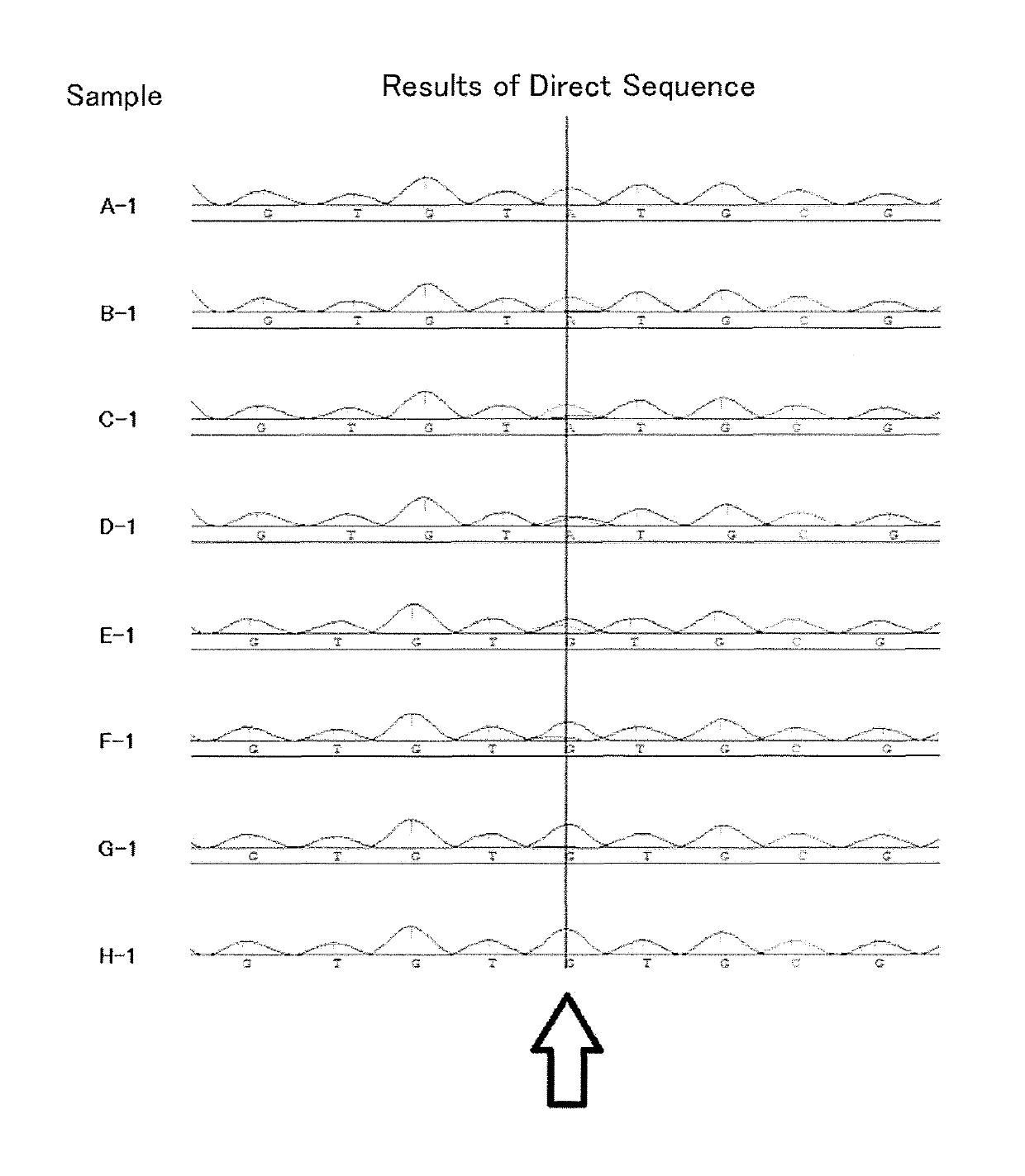
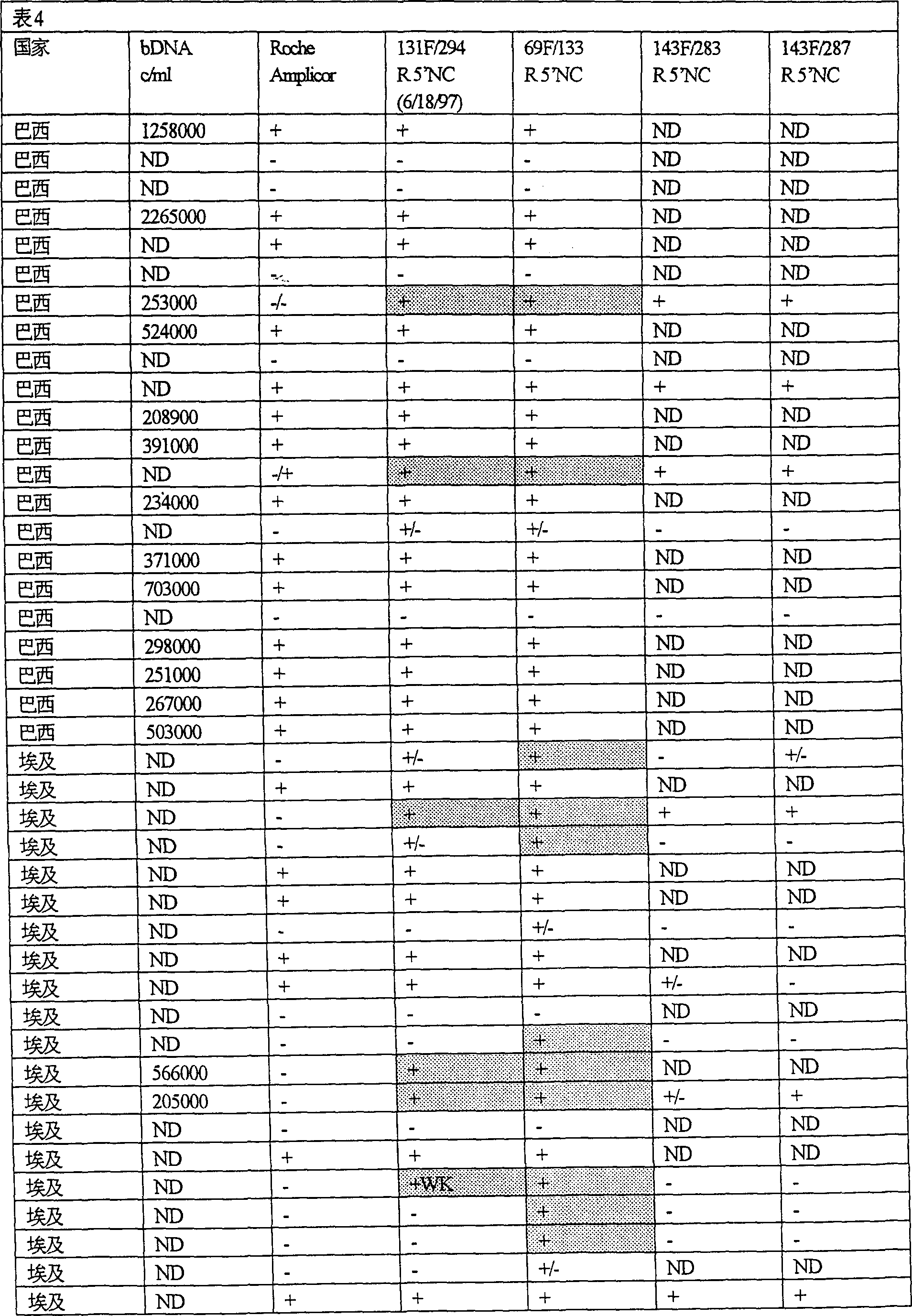
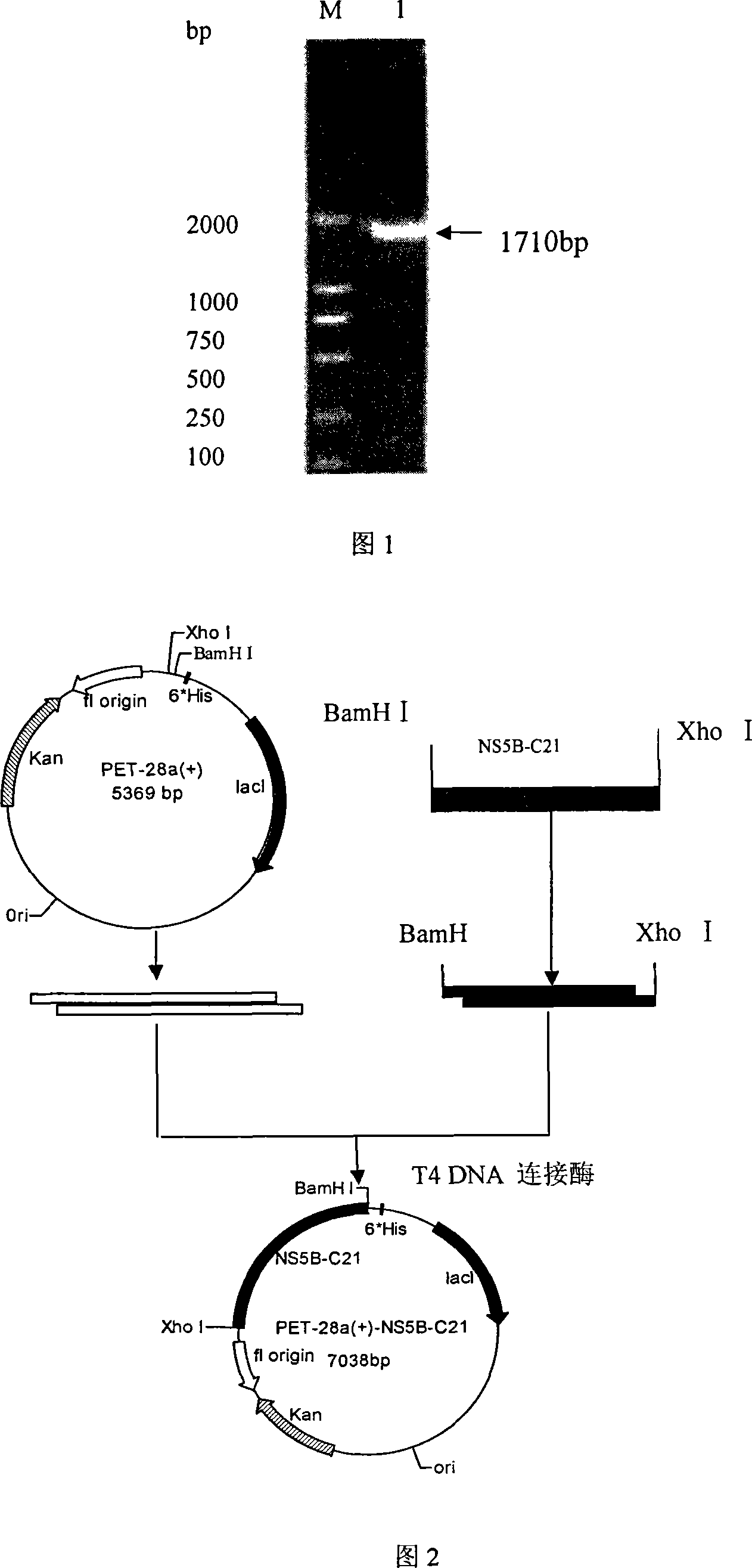
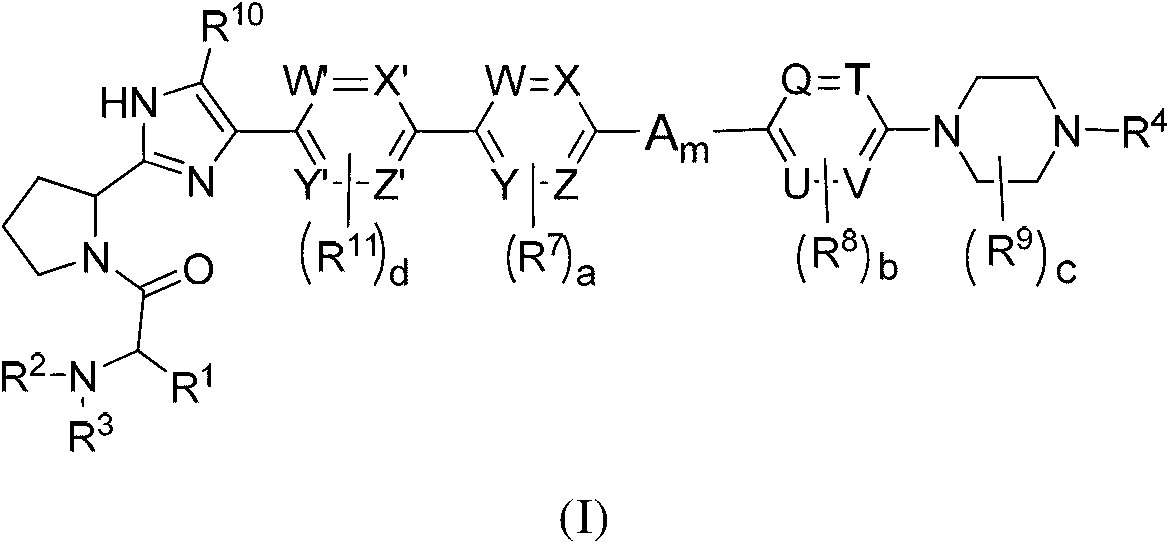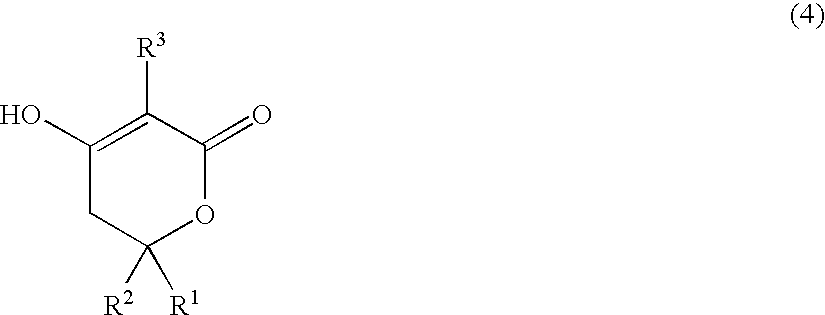Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Hepatitis C virus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhibitors of hepatitis C virus RNA-dependent RNA polymerase, and compositions and treatments using the same
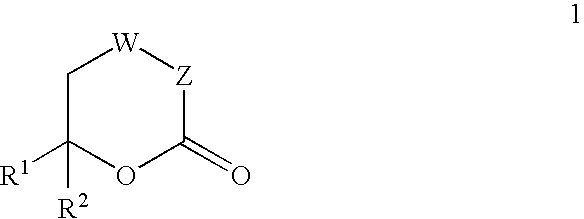
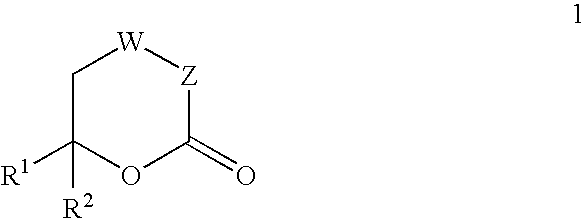
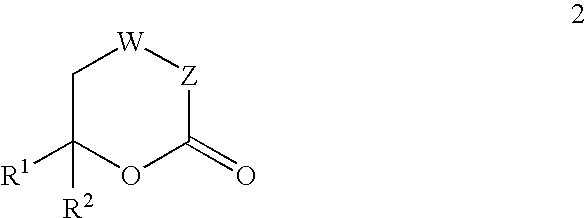
The present invention provides compounds of formula (4), and their pharmaceutically acceptable salts and solvates, which are useful as inhibitors of the Hepatitis C virus (HCV) polymerase enzyme and are also useful for the treatment of HCV infections in HCV-infected mammals. The present invention also provides pharmaceutical compositions comprising compounds of formula (4), their pharmaceutically acceptable salts and solvates. Furthermore, the present invention provides intermediate compounds and methods useful in the preparation of compounds of formula (4).
Owner:AGOURON PHARMA INC +1
Pseudovirion containing hepatitis c virus RNA (Ribonucleic Acid) fragment and preparation method thereof
InactiveCN104845993ANo dangerImprove the simulation effectMicrobiological testing/measurementInactivation/attenuationEscherichia coliPHA granule
The invention provides a pseudovirion containing hepatitis c virus (HCV) RNA (Ribonucleic Acid) fragment and preparation method thereof which are applied in the field of biomedical clinical vitro diagnostic. The pseudovirion is a RNA-protein complexes that MS2 bacteriophage capsid protein wraps the hepatitis c virus, and the complexes is icosahedral; the method comprises the following steps of designing and synthesizing primer to obtain target gene MS2 by overlapping and splicing PCR (Polymerase Chain Reaction) method; connecting the target gene MS2 to plasmid pET-32a (+) to obtain recombinant plasmid pET-MS2; connecting the recombinant plasmid pET-MS2 and the HCV fragment to obtain pET-MS2-HCV recombinant plasmid; the obtained pET-MS2-HCV recombinant plasmid is introduced into escherichia coli for prokaryotic expression; releasing virus-like particles by adopting ultrasonication and the obtained virus-like particles are the pseudovirion containing the hepatitis c virus RNA fragment. The pseudovirion preparation method provided by the invention has the advantages that the preparation method is simple, the operation is easy, the obtained pseudovirus is high in purity, and the pseudovirion can be used as standard and quality control material of detection of RT-PCR (Reverse Transcription-Polymerase Chain Reaction), is good in stability, has no infectivity, has RNase-resistant, and the like.
Owner:宝瑞源生物技术(北京)有限公司
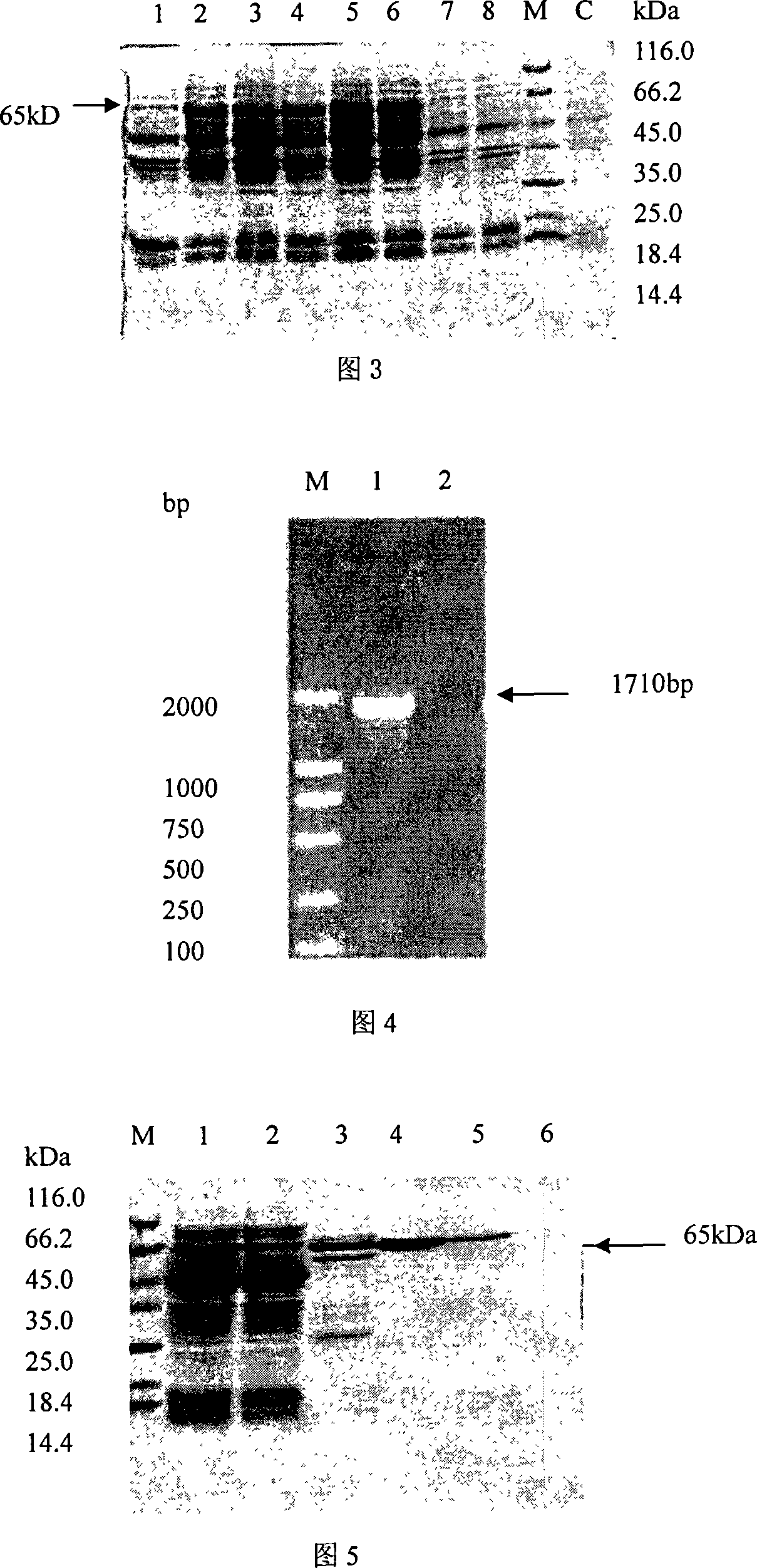
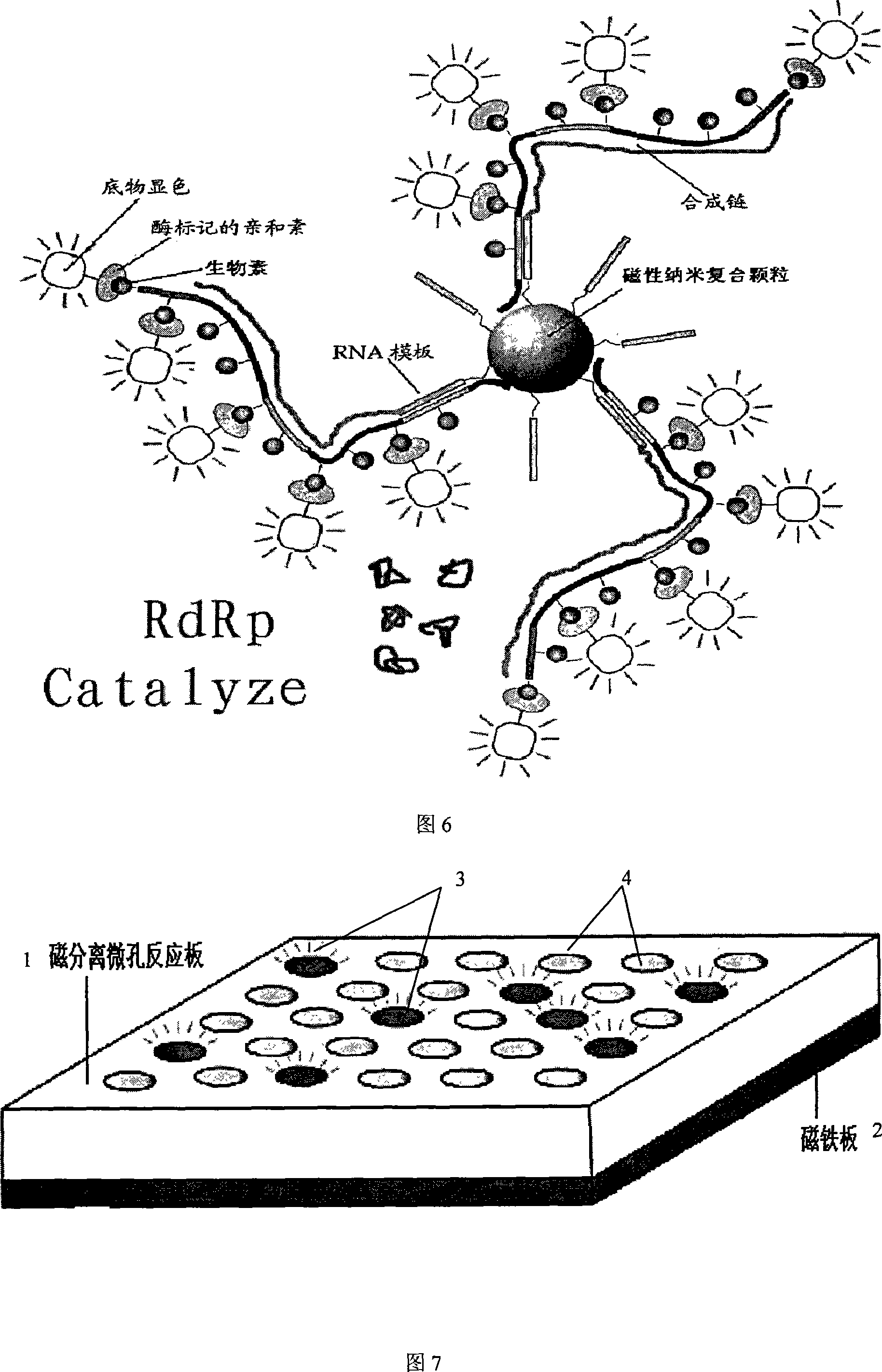
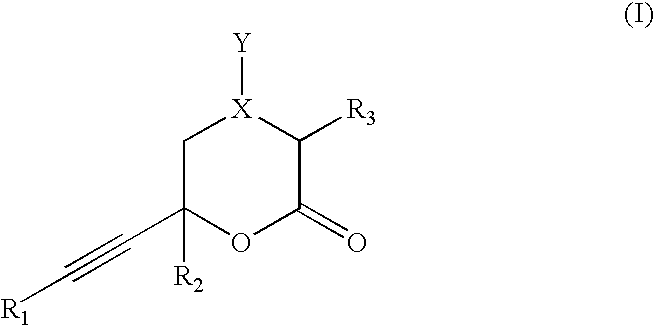
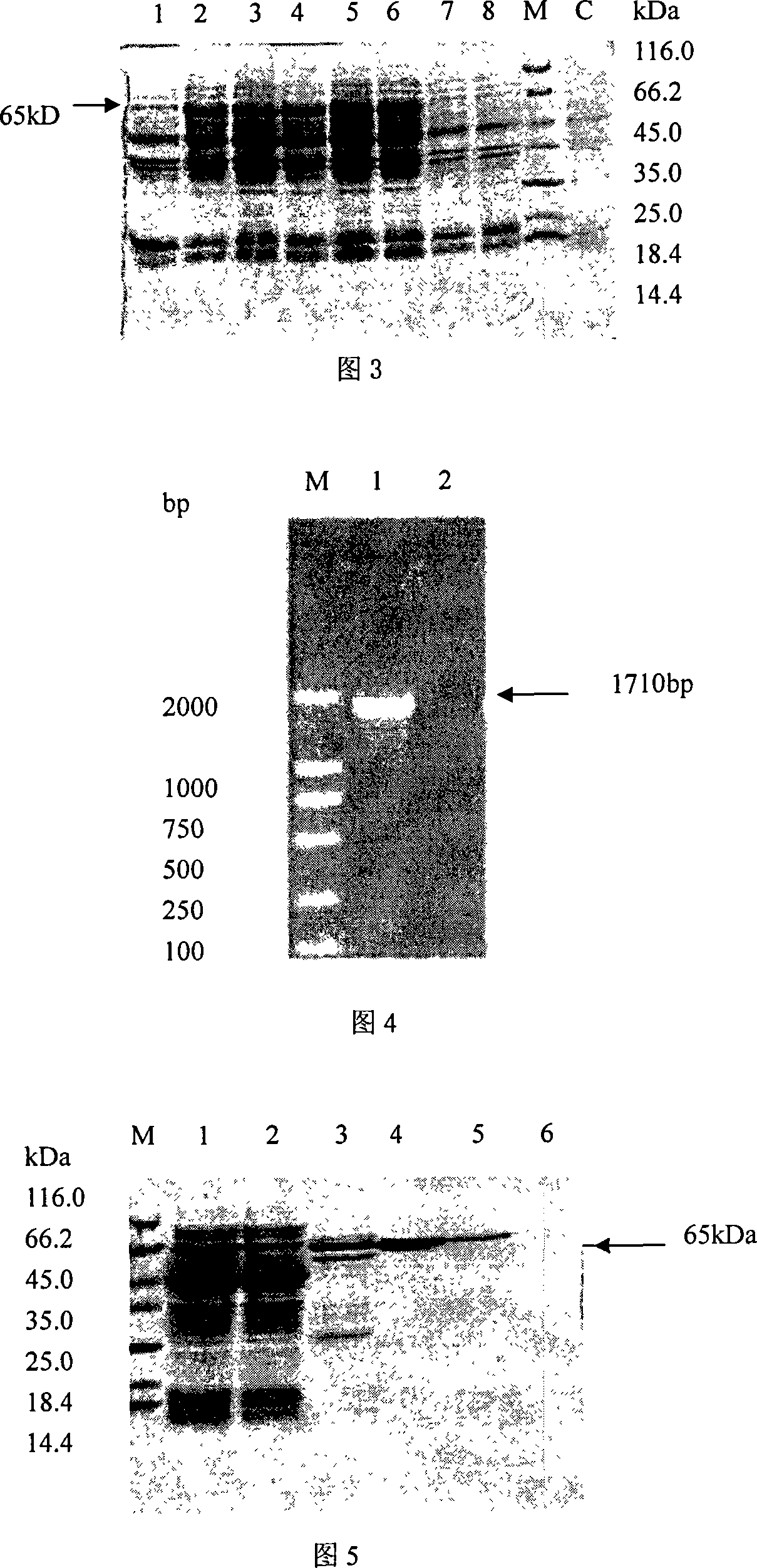
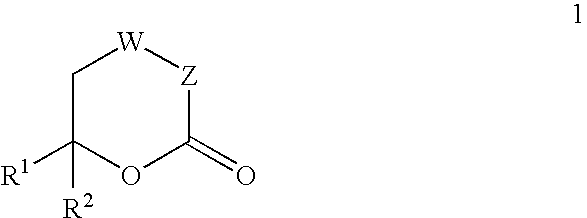
In vitro activity measuring method for hepatitis C virus RNA depending RNA polymerase and application thereof
InactiveCN101168781ASimple methodPractical methodMicrobiological testing/measurementLibrary screeningMagnetite NanoparticlesGenetic engineering
The invention provides a detecting method and an application of the extraneous activity of the RNA polymerase relied on by the hepatitis C virus RNA, which relates to an secure and practical establishing method for the detection of the activity extraneous detection of the RNA polymerase relied on by the hepatitis C virus RNA, and the application relates to the genetic engineering technology and the nanometer segregation enrichment and RNA related technology field. The invention expresses the soluble NS5B protein through the utilization of the genetic engineering technology, and prepares the nanometer magnetic particle. One end of a RNA template is fixed on the nanometer magnetic particle, and added in the 96 hole reaction plate micromesh, and added with NS5B protein to compose a RNA duplication system, and a duplicated RNA double chain on the nanometer magnetic particle is mixed with marked biological elements. After the RNA is duplicated, the nanometer magnetic particle is attached at the micromesh bottom through an external magnetic field magnet, after repeatedly cleaning and attaching, the nanometer magnetic particle is combined with the marked combining factor of the horse radish peroxidase, after attaching and cleaning, the substrate constant of the horse radish peroxidase is added to develop color. New NS5B protease activity inhibitor can be screened from a traditional Chinese storeroom and a compound storeroom by the established method.
Owner:李越希
Inhibitors of hepatitis C virus RNA-dependent RNA polymerase, and compositions and treatments using the same
InactiveUS6878727B2Inhibition of replicationBiocideOrganic chemistryRNA-dependent RNA polymeraseProphylactic treatment
Compounds of formula I are hepatitis C virus (HCV) RNA-dependent RNA polymerase (RdRp) inhibitors, and are useful in therapeutic and prophylactic treatment of persons infected with hepatitis C virus
Owner:AGOURON PHARMA INC +1
Hepatitis C sequencing and typing kit and detection method thereof
The invention discloses a hepatitis C sequencing and typing kit which comprises: (1) primers of specific amplified hepatitis C virus RNA reverse transcription product c DNA and (2) hepatitis C virus specific sequencing primers, wherein the nucleotide sequences of the primers of specific amplified hepatitis C virus RNA reverse transcription product c DNA are shown as SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4, the SEQ ID NO:1 and the SEQ ID NO:2 are a primer pair and the SEQ ID NO:3 and the SEQ ID NO:4 are another primer pair; and the nucleotide sequences of the hepatitis C virus specific sequencing primers are shown as SEQ ID NO:5, SEQ ID NO:6 and SEQ ID NO:7; the SEQ ID NO:4 is marked with a biotin at a 5' end; and all the primers are all used in a detection. The invention also discloses a detection method of the hepatitis C sequencing and typing kit. The hepatitis C sequencing and typing kit can be used for sequencing and typing hepatitis C, thereby providing more comprehensive reference information for clinical diagnosis and treatment.
Owner:CHONGQING DIAN SRAB CENT FOR CLINICAL LAB CO LTD
Isothermal chain multiple detection card of pathogen nucleic acid
ActiveCN102230032AAvoid cross contaminationAvoid false positivesMicrobiological testing/measurementSpiroplasmaQuarantine
The invention discloses an isothermal chain multiple displacement detection card of pathogen nucleic acid. The isothermal chain multiple displacement detection card of the pathogen nucleic acid utilizes the detection card which is prepared through a nucleic acid isothermal chain displacement method and a colloidal gold detection technology to carry out multiple detection on HBV-DNA, HCV-RNA, HIV-RNA, and TP-DNA. The isothermal chain multiple displacement detection card of the pathogen nucleic acid has the characteristics of simple and rapid operation, high sensitivity, and no need of professional equipment. The pathogen nucleic acid to be detected is not amplified in the detection process, so the detection card has the advantages of preventing amplified matter cross contamination in laboratories and preventing false positivity, can be widely used in high sensitivity pathogen nucleic acid detection in the fields of clinical detection, inspection and quarantine, infectious disease control, biological technology and the like, and has a wide application prospect.
Owner:武汉中科志康生物科技有限公司
Multi-color fluorescent PCR detection kit for nucleic acid testing and genetic typing of hepatitis c virus and application
ActiveCN103773897AHigh sensitivityFast detection methodMicrobiological testing/measurementPositive controlFluorescence
The invention discloses a multi-color fluorescent PCR kit for nucleic acid testing and genetic typing of hepatitis c virus and an application thereof. The kit comprises RT-PCR reaction liquid, enzyme mixed liquid, HCV reaction liquid I, HCV reaction liquid II and HCV reaction liquid III, and the kit preferably also comprises HCV typing negative control, HCV typing positive control and application instruction. For the extracted HCV RNA, the hepatitis c virus RNA and the subtype of the hepatitis c virus in a test sample can be rapidly detected by adopting three-tube multi-color fluorescent PCR through one-step RT-PCR. The amplification method of the kit is simple, simplicity in operation is realized, the testing result is high in sensitivity, the specificity is good, the kit can be used for testing and genetic typing of HCV RNA in human serum or plasma, the judgment on the treatment difficulty can be favored, the establishment of an individual antivirus treatment scheme is favored, and the kit has the clinical popularization value.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Method for HCV RNA real-time fluorescent detection with two probes
InactiveCN1908627AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceTrue positive rate
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
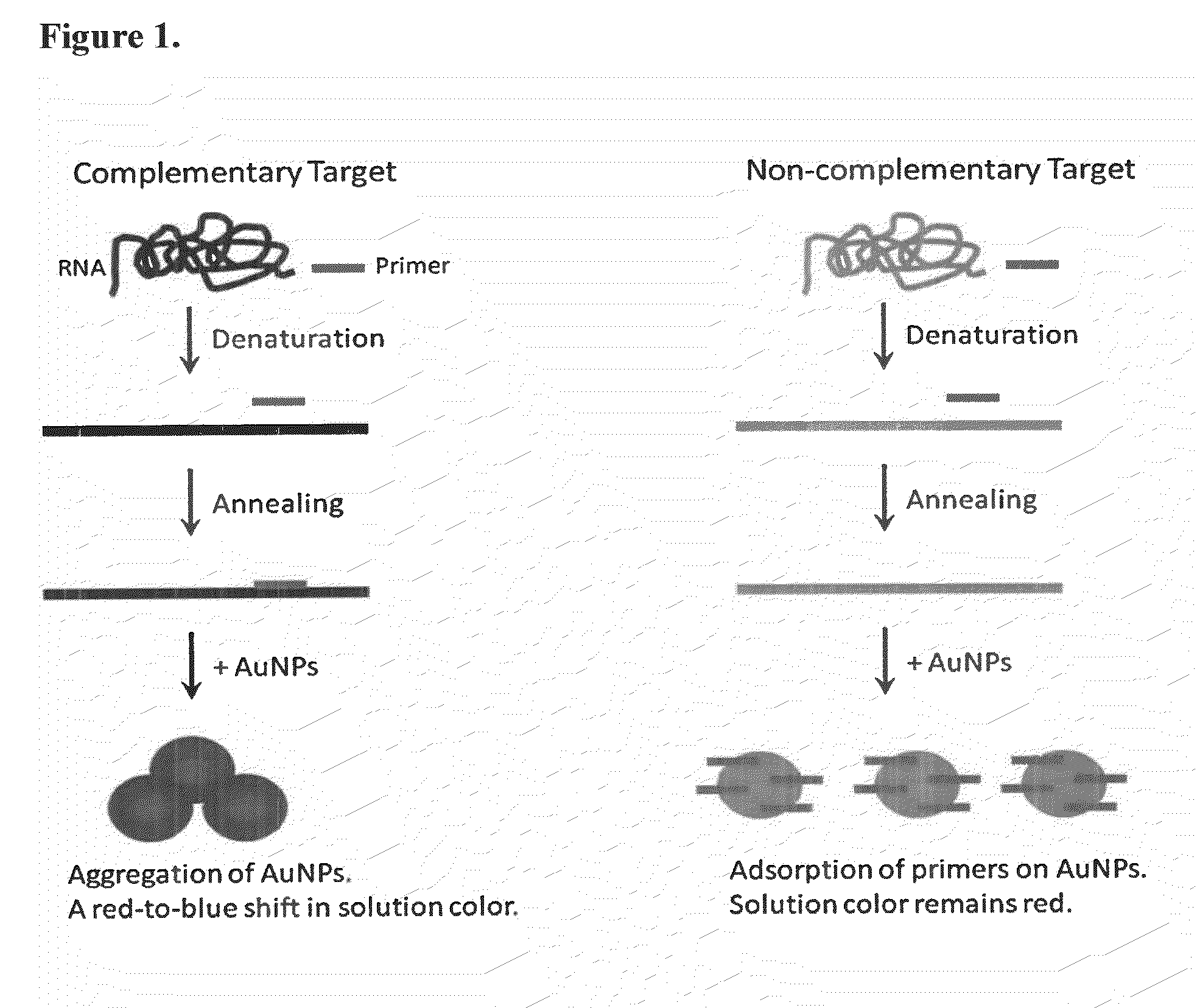
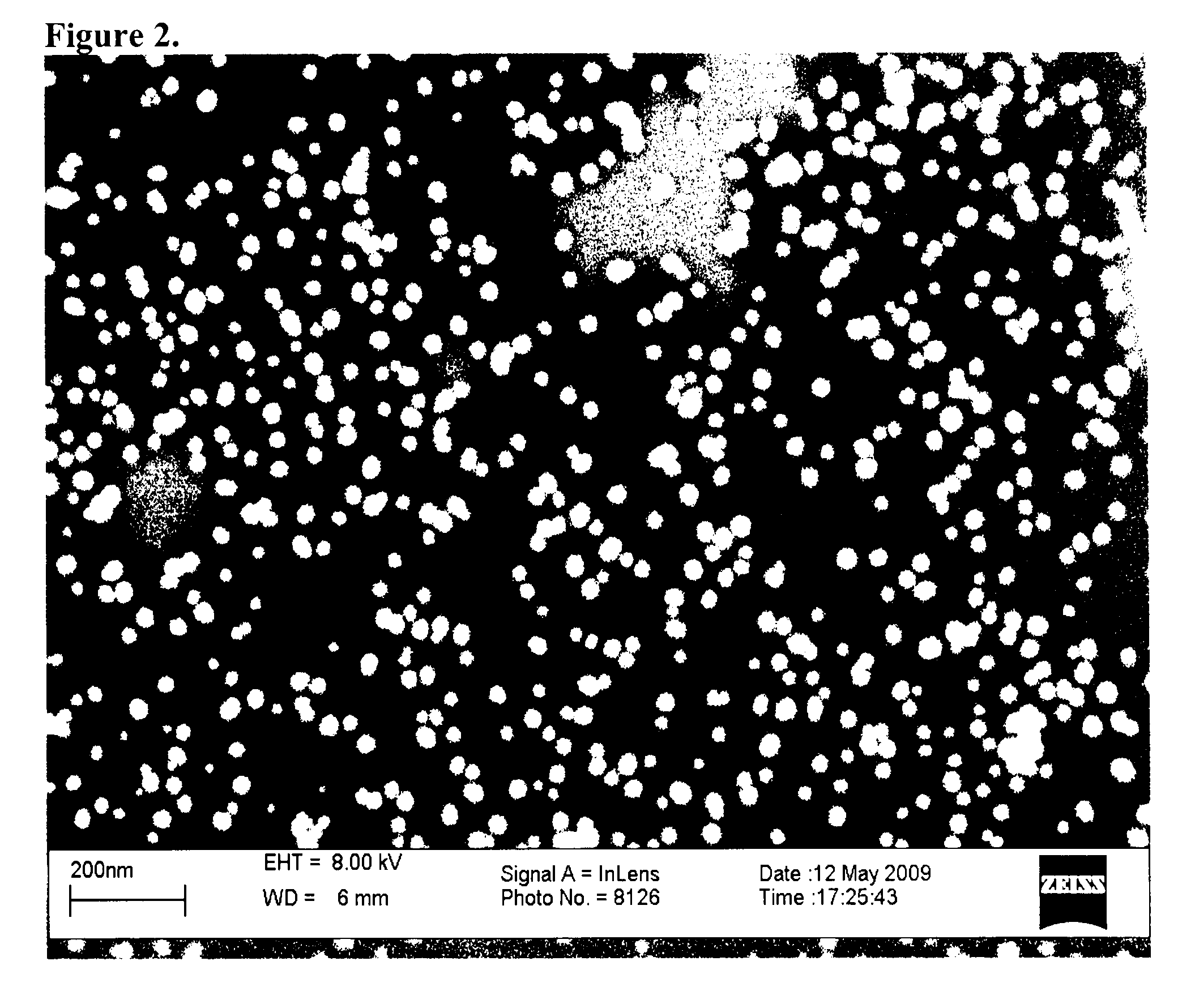
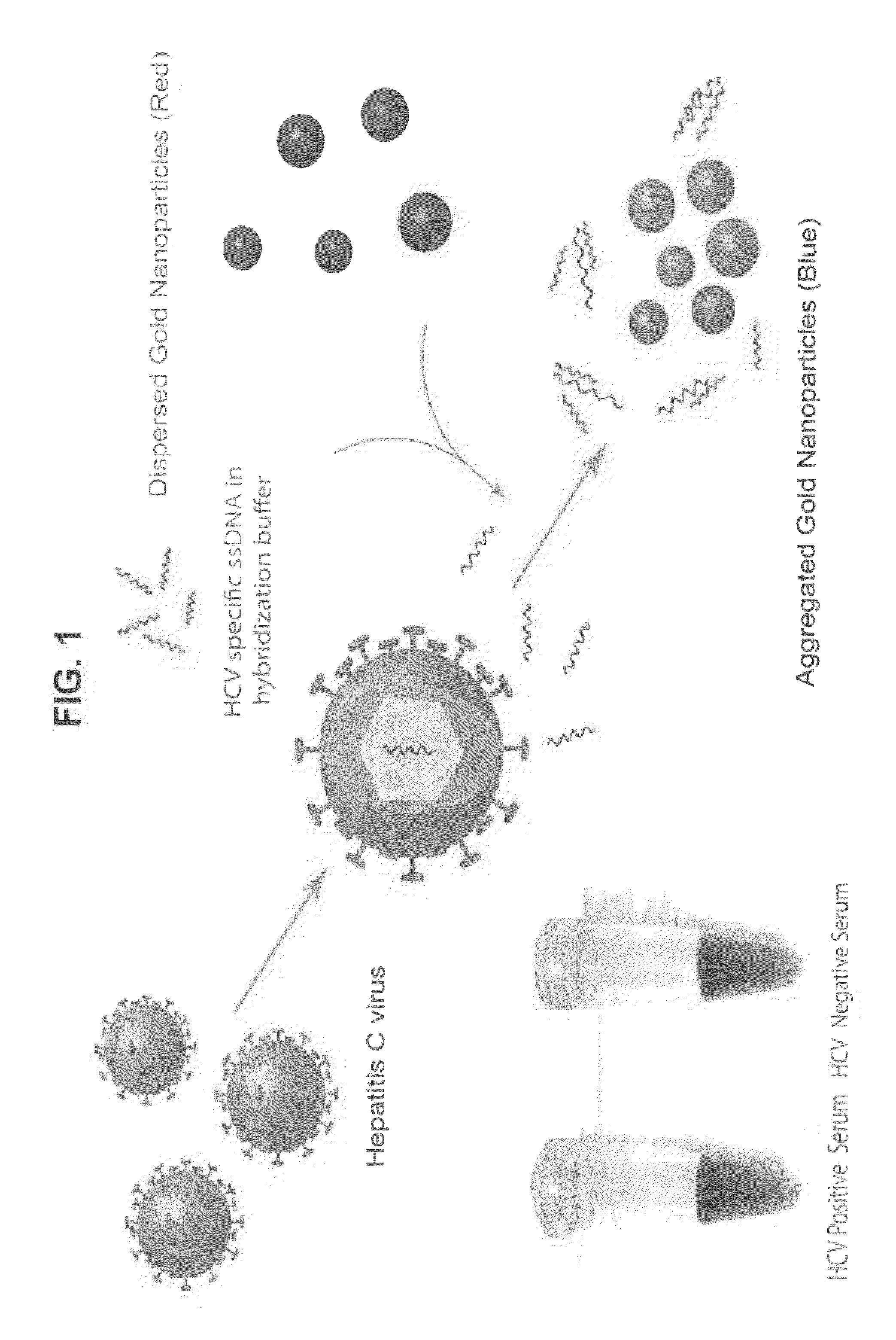
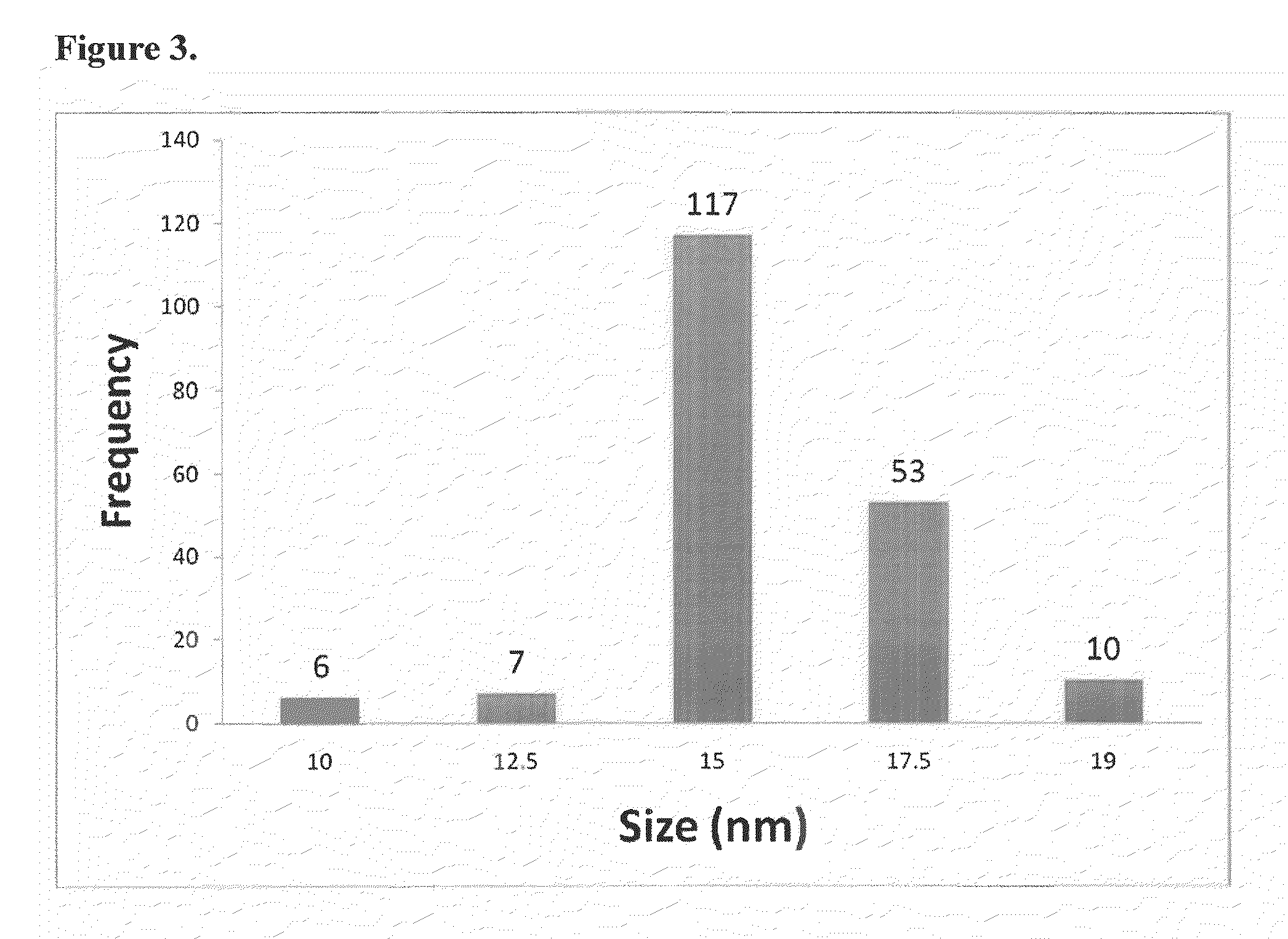
Direct detection of unamplified hepatitis c virus RNA using unmodified gold nanoparticles
InactiveUS20130236880A1Simple, rapid, and sensitive colorimetric assaySensitively detect HCVMicrobiological testing/measurementNanosensorsNanoparticleHepatitis C virus RNA
A gold nanoparticle-based colorimetric assay kit for hepatitis C virus RNA that detects unamplified HCV RNA in clinical specimens using unmodified AuNPs and oligotargeter polynucleotides that bind to HCV RNA. A method for detecting hepatitis C virus comprising contacting a sample suspected of containing hepatitis C virus with a polynucleotide that binds to hepatitis C virus RNA and with gold nanoparticles, detecting the aggregation of nanoparticles, and detecting hepatitis C virus in the sample when the nanoparticles aggregate (solution color becomes blue) in comparison with a control or a negative sample not containing the virus when nanoparticles do not aggregate (solution color remains red).
Owner:THE AMERICAN UNIV OF CAIRO
Composition for treating hepatitis C
ActiveUS7381435B2Useful in treatmentControl progressBiocideDigestive systemChronic viral hepatitis CPumpkin seed
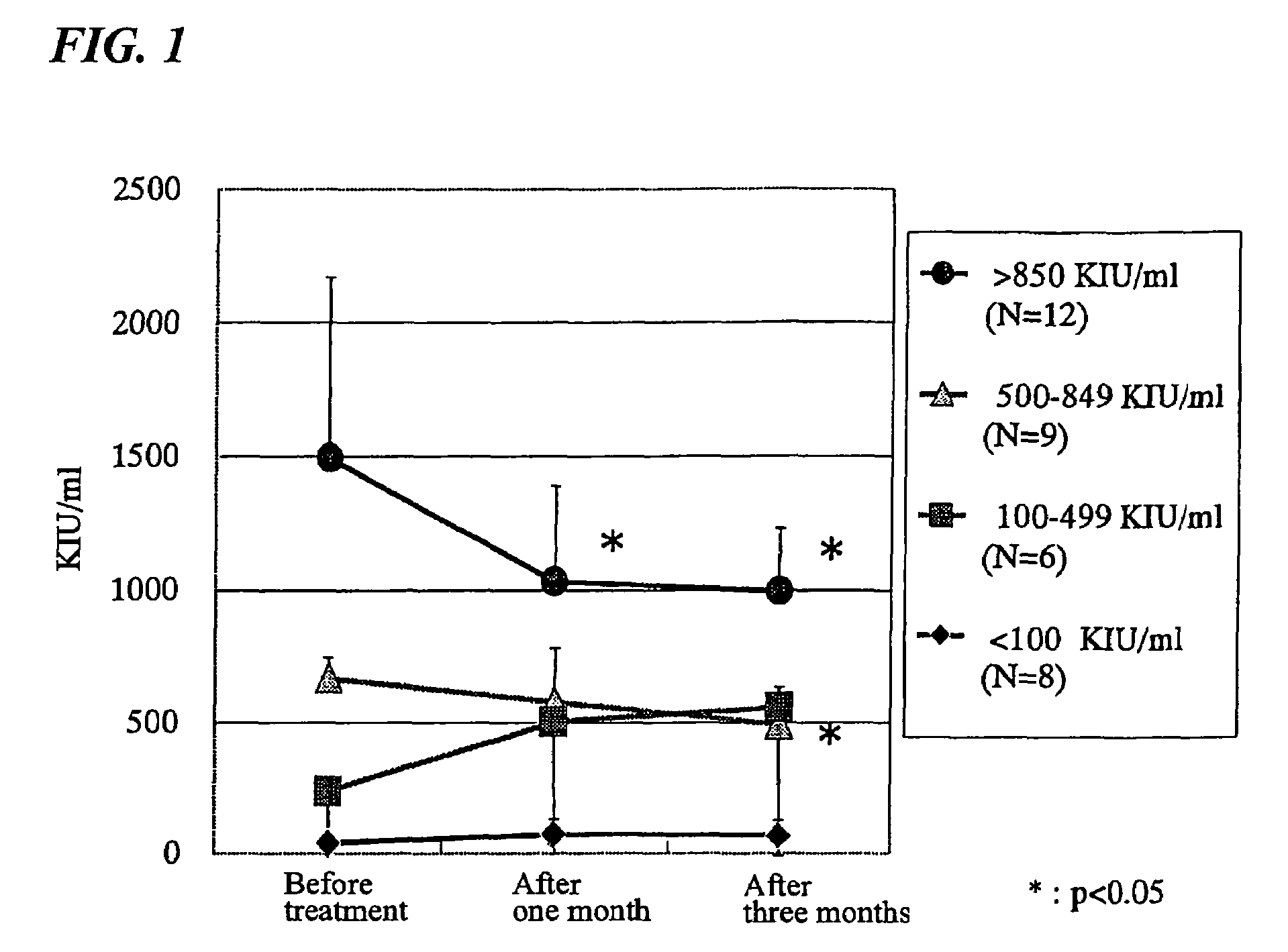
By administering a composition comprising pumpkin seed, safflower, plantain and honeysuckle, subjective symptoms (for example, general malaise and abdominal swelling) of a patient with chronic hepatitis C can be eliminated and, moreover, objective symptoms diagnosed by a medical doctor (for example, liver enlargement and palm erythema) can be relieved or eliminated. From 1 to 3 months after the administration of the composition, a significant decrease in hepatitis C virus RNA level is gradually observed. Therefore, the above composition is useful at least as a composition for treating chronic hepatitis C. In particular, it is advantageous in treating a chronic hepatitis C patient showing a high chronic hepatitis C virus RNA level.
Owner:ORIGINAL IMAGE
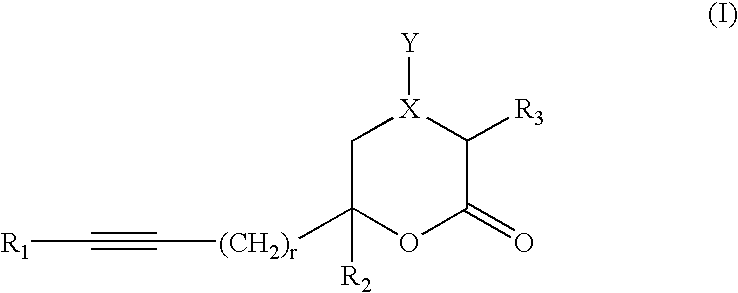
Novel inhibitors of hepatitis C virus
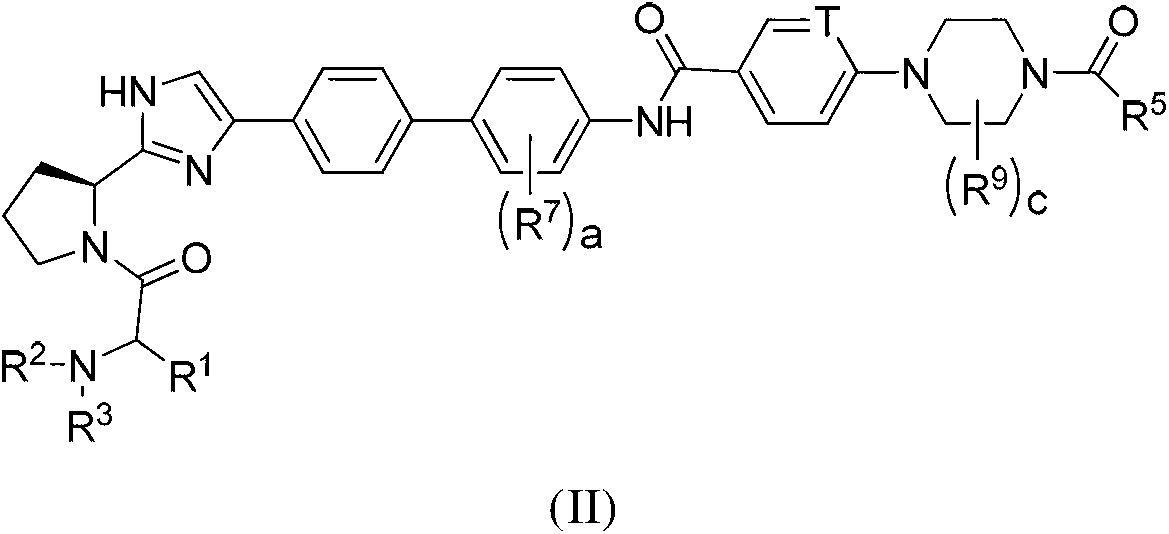
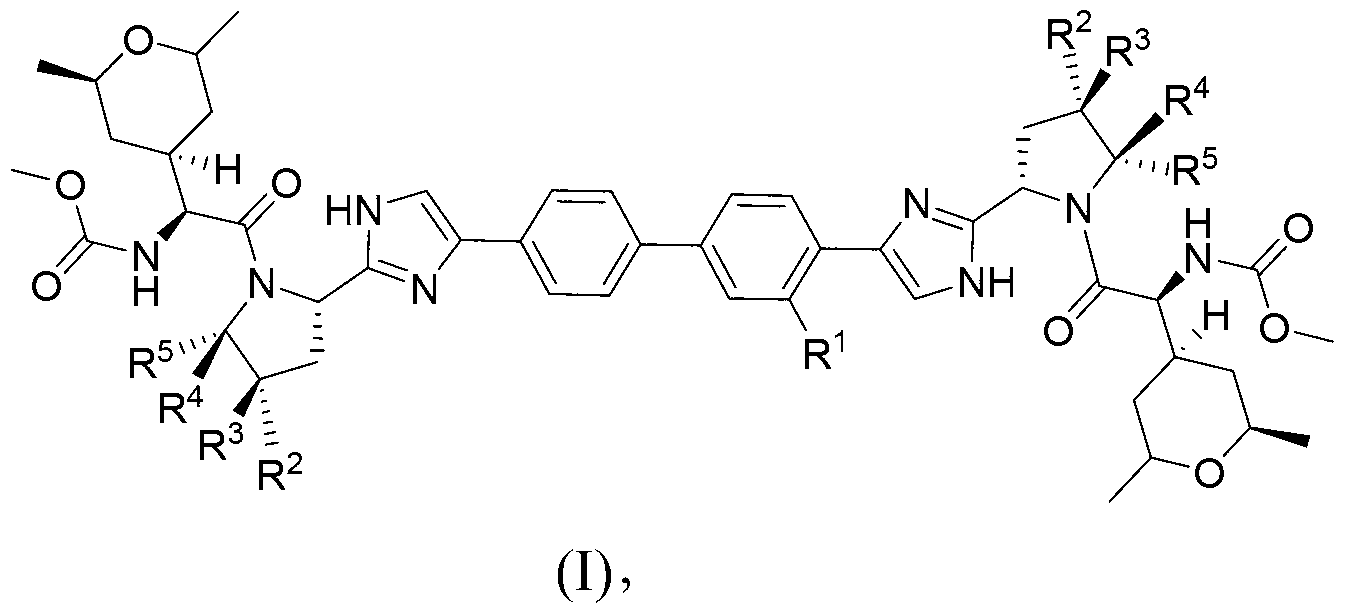
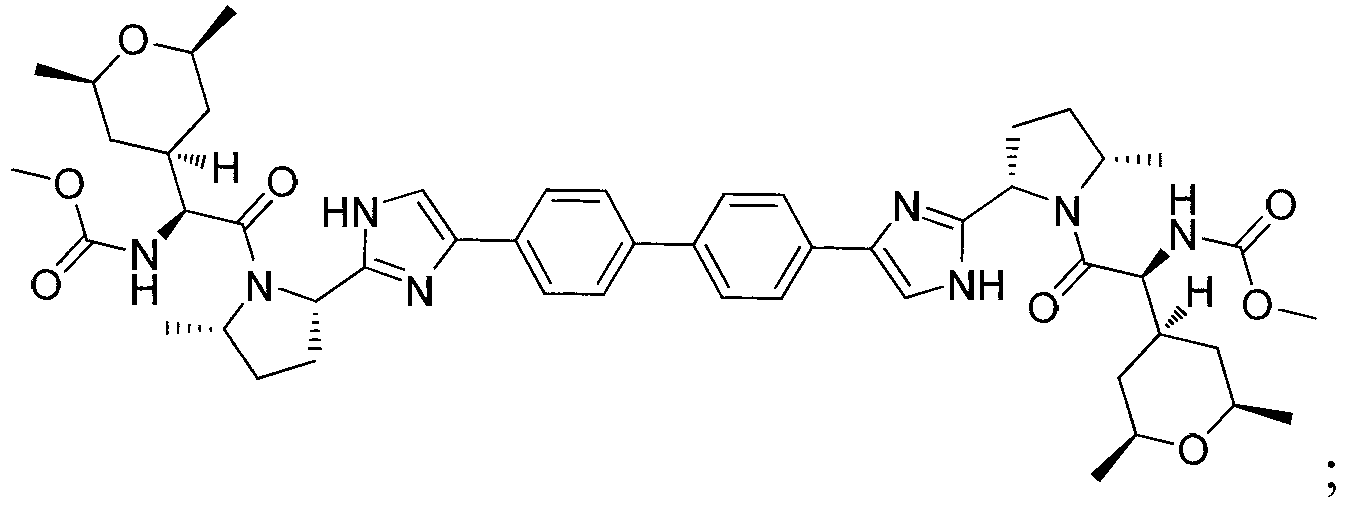
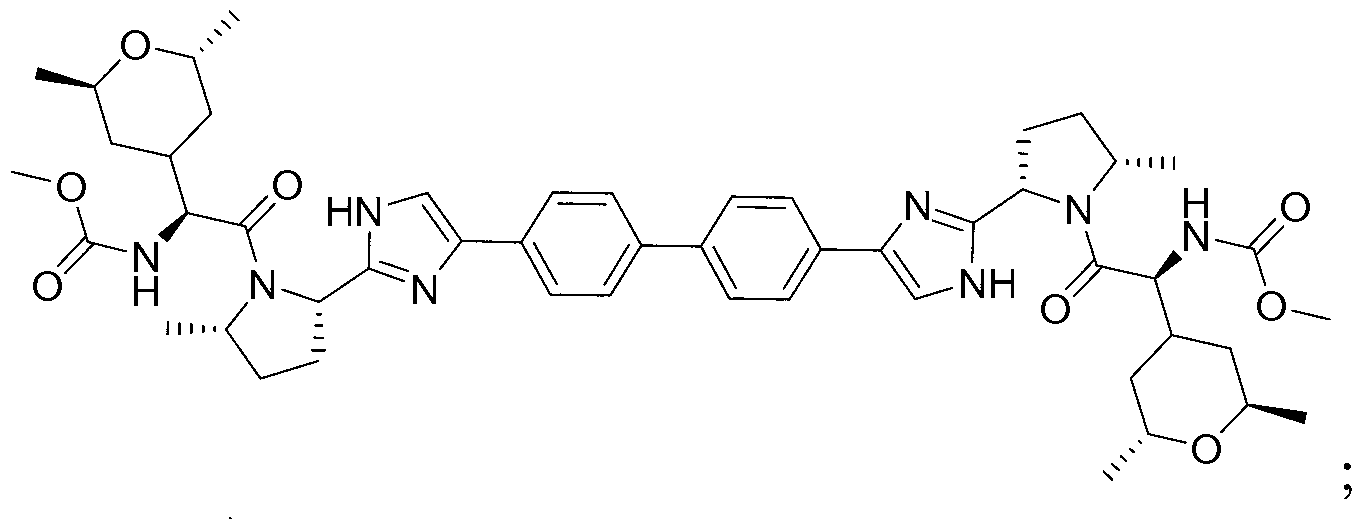
The invention provides compounds of formula (I) wherein the variables are defined in the specification, or a pharmaceutically-acceptable salt thereof, that are inhibitors of replication of the hepatitis C virus. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat hepatitis C viral infections, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Hepatitis C virus inhibitors
InactiveCN103347878APollution controlReduce the risk of infectionOrganic active ingredientsOrganic chemistryHepatitis C virus RNAVirology
Owner:BRISTOL MYERS SQUIBB CO
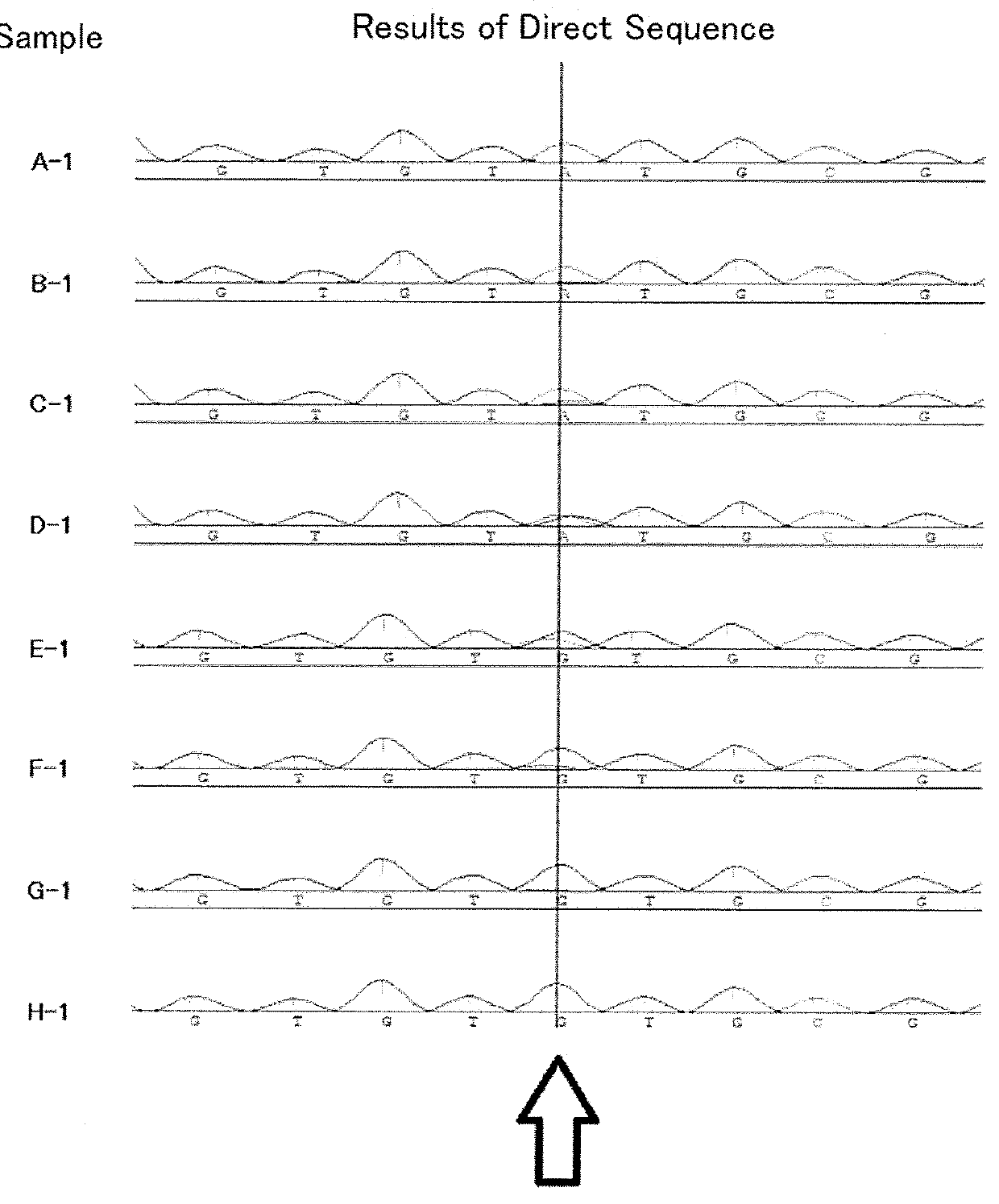
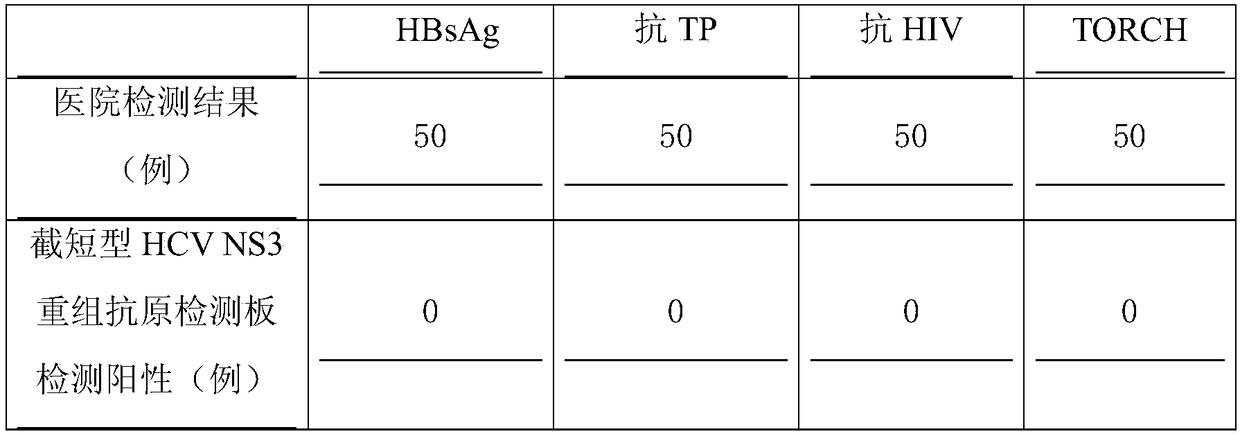
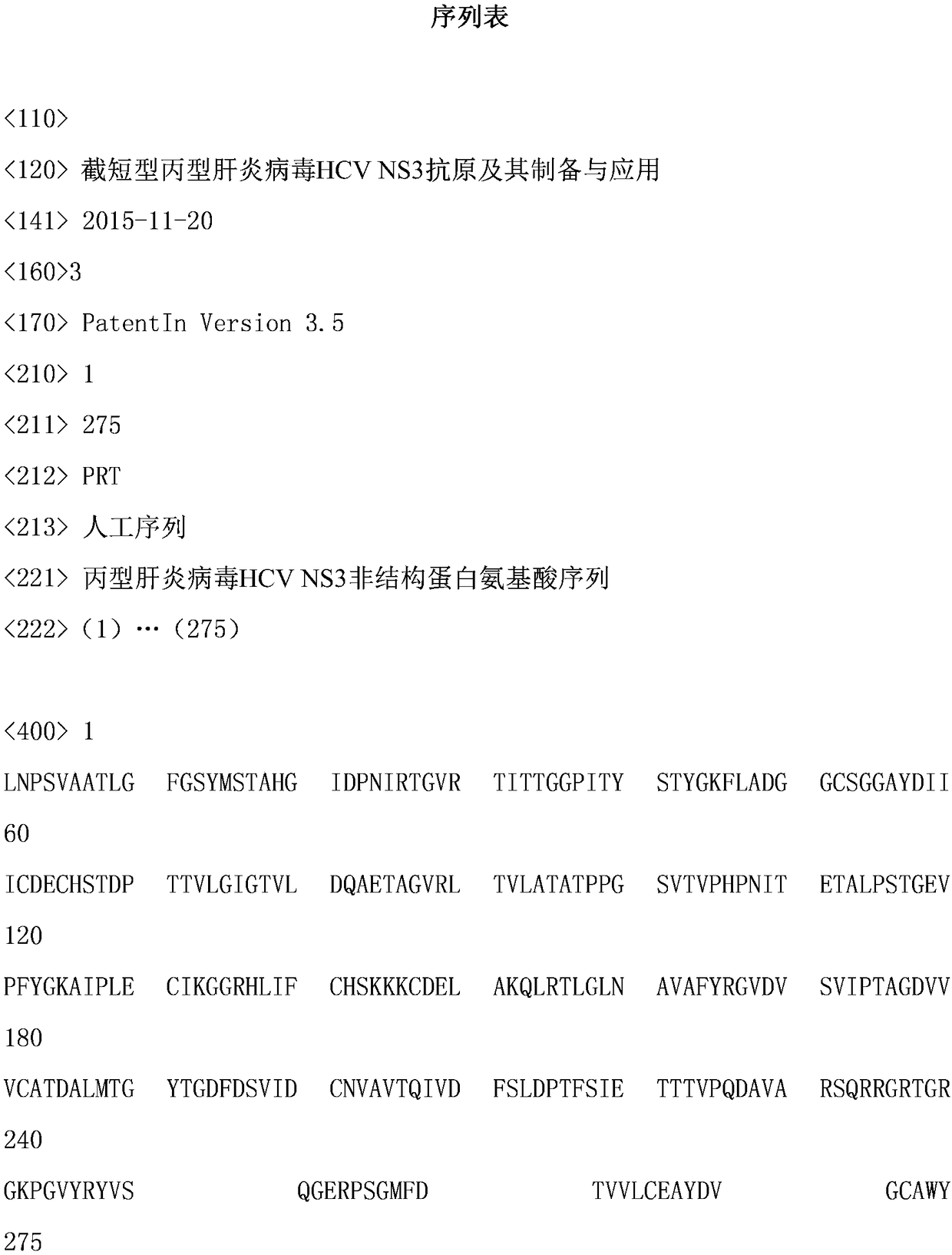
Truncated type hepatitis c virus HCV NS3 antigen and preparation method and application thereof
ActiveCN105254724AEfficient developmentHigh positive serum coverageBacteriaVirus peptidesAntigenEscherichia coli
The invention discloses a truncated type hepatitis c virus HCV NS3 antigen which is a nonstructural protein amino acid sequence of the NS3 full-length gene encoded 1252th-1526aath segment in an HCV genome. The amino acid sequence is shown as SEQ ID NO.1, and a corresponding nucleotide sequence is shown as SEQ ID NO.2. A preparation method of HCV NS3 recombinant antigen protein comprises the steps that an optimized escherichia coli codon for expressing the truncated type hepatitis c virus HCV NS3 antigen are established, the nucleotide sequence of the codon is shown as SEQ ID NO.3, the gene segment of the codon undergoes double enzyme digestion and then is connected with an expression vector undergoing the enzyme digestion as well to obtain a recombinant expression plasmid, the antigen can perform expression by adopting a conventional method, and the antigen protein is obtained through purification. The antigen protein has high sensitivity and good specificity and is used for detecting an HCV antibody, and the coincidence rate of a detection result of a patient with positive hepatitis c virus RNA is as high as 97%. It is indicated that the antigen can be applied to polyclonal or monoclonal antibodies and preparation of hepatitis c virus detection kits and has good prospect of clinical application.
Owner:山东莱博生物科技有限公司
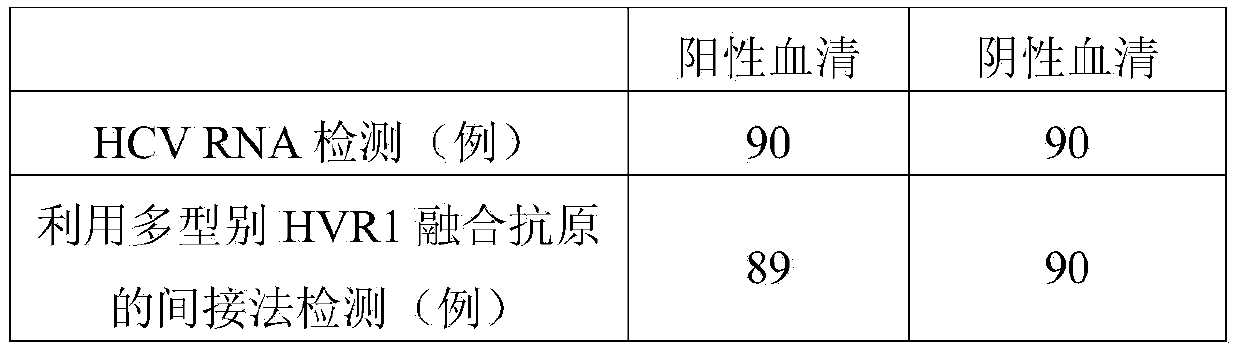
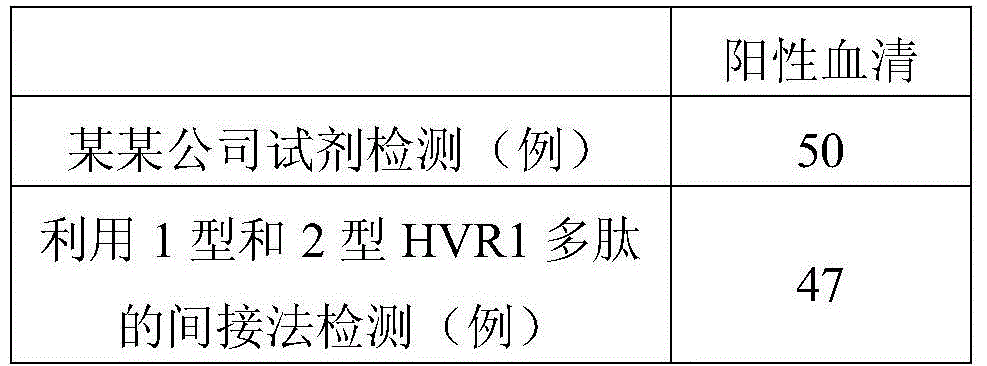
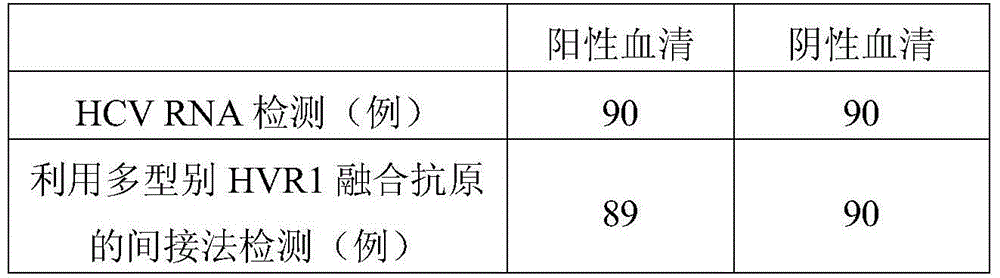
Hepatitis C virus HVR1 (Hypervariable Region 1) fusion antigen and application thereof
ActiveCN104193827AAvoid disadvantagesEliminate missed detectionBiological testingHybrid peptidesHcv hepatitisAntibody hypervariable region
The invention discloses a hepatitis C virus HVR1 (Hypervariable Region 1) fusion antigen. An amino acid sequence of the fusion antigen is shown as SEQ ID No.1. The invention also discloses application of the fusion antigen in preparation of a hepatitis C virus HVR1 antibody detection kit. The experiment proves that the cross reactivity of the fusion antigen is obviously improved compared with that of a single segment HVR1 antigen, and the detection coincidence rate of hepatitis C virus RNA positive patients is 99 percent. Because the HVR1 is a main neutralizing antigen epitope in the hepatitis C virus, the antigen can be used for achieving an aim of comprehensively detecting HCV infection for hepatitis C virus HVR1 antibody detection and effectively solving the problems such as outcome and prognosis of HCV diseases and prediction of interferon curative effect and has extremely great clinical application prospects.
Owner:山东莱博生物科技有限公司
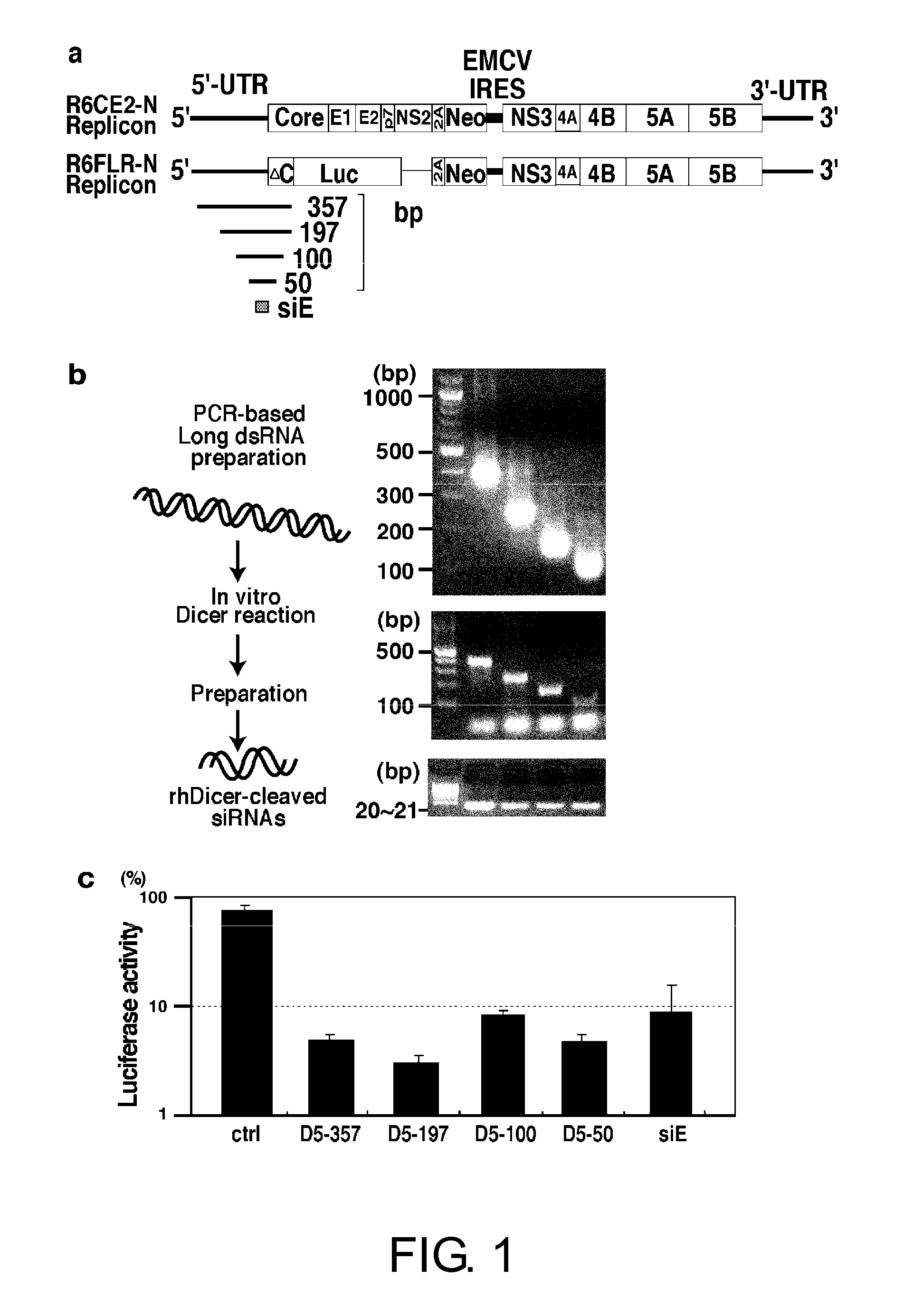
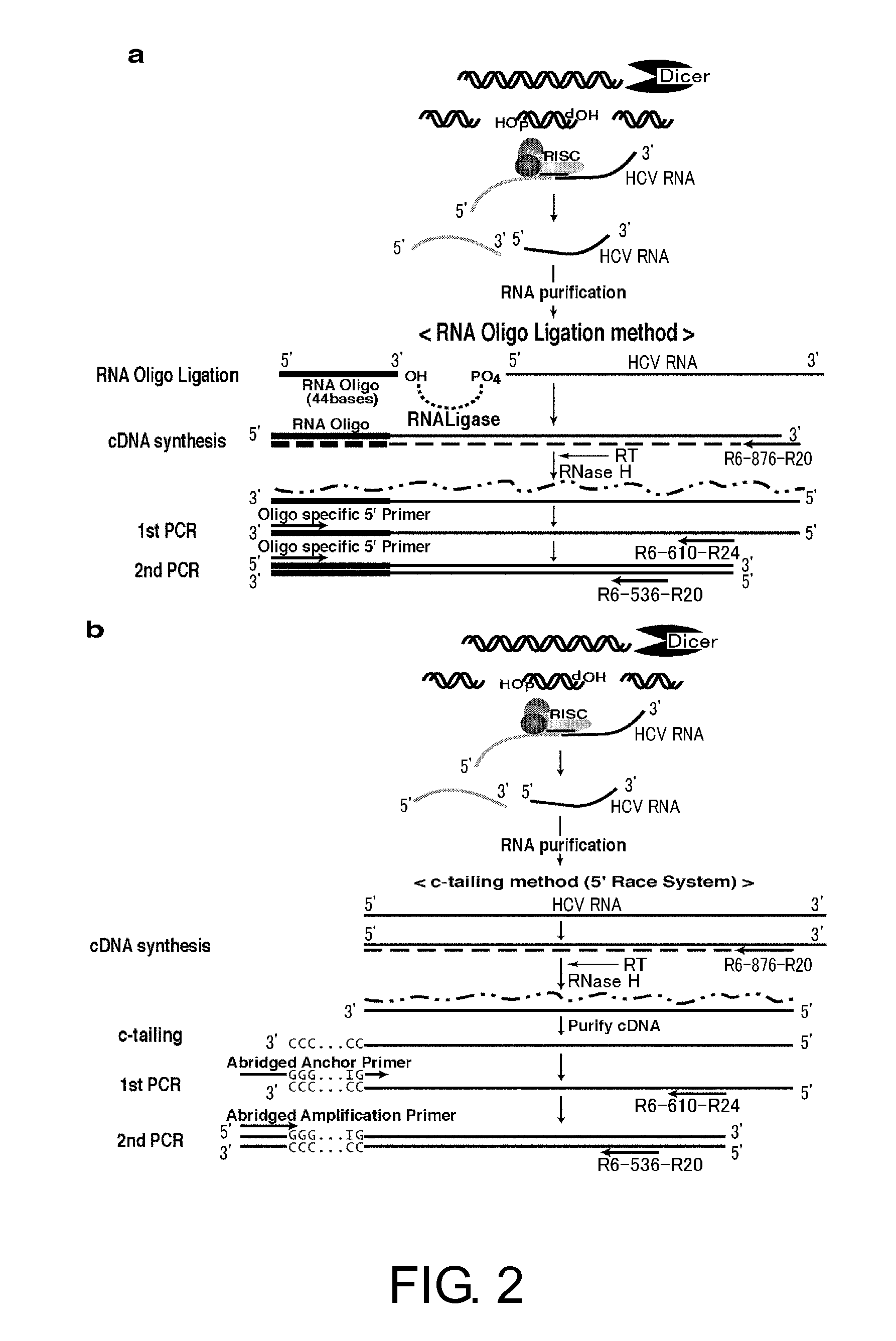
Oligoribonucleotide or Peptide Nucleic Acid Capable of Inhibiting Activity of Hepatitis C Virus
InactiveUS20110281271A1Inhibitory activityInhibiting replication abilitySugar derivativesMicrobiological testing/measurementOligoribonucleotidesIn vivo
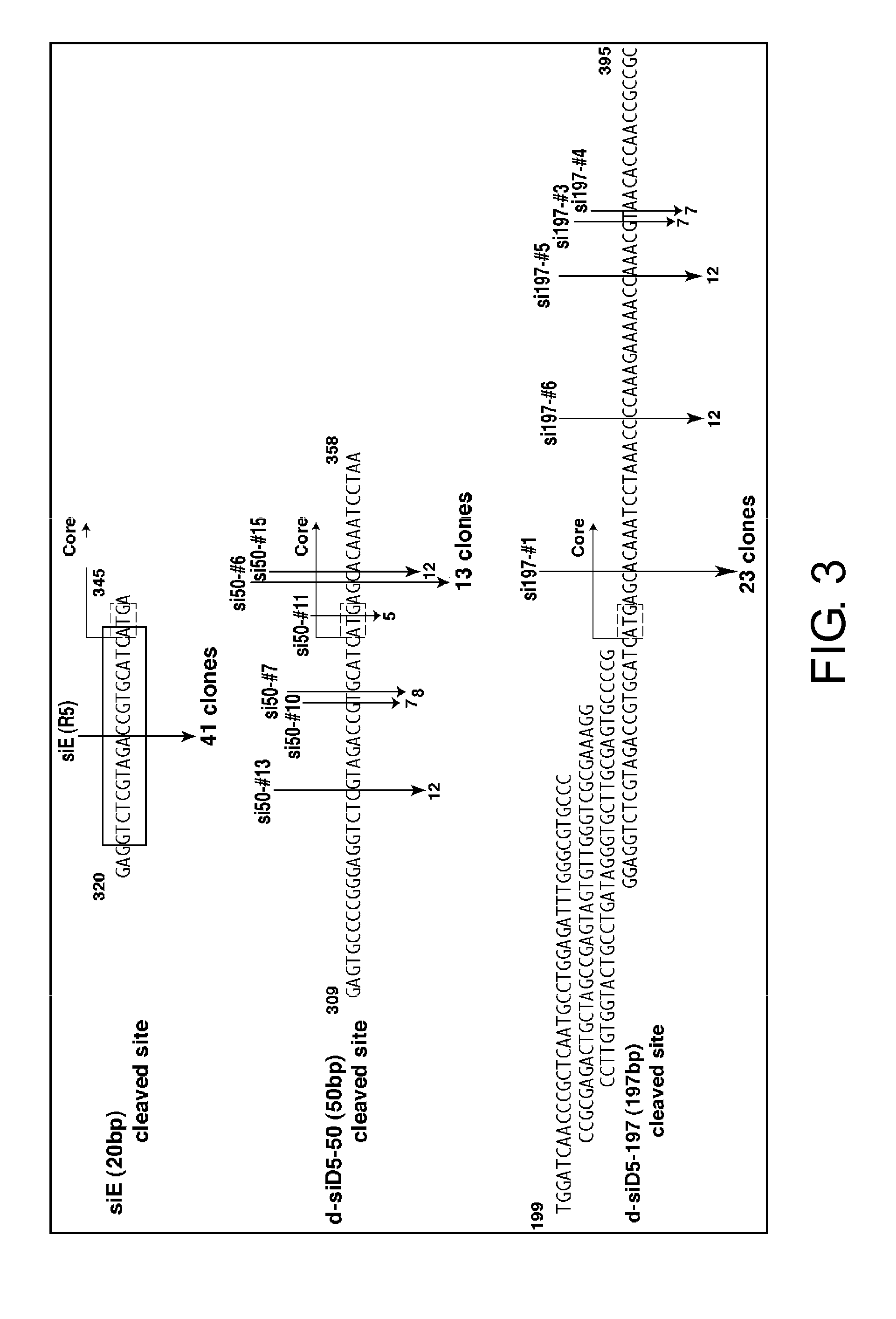
The present inventors focused on siE sequences that have been thought to show RNAi activity against HCV viral RNAs, and mainly selected the D5-50 and D5-197 regions present within the IRES region, and carried on the analysis. As a result, the present inventors successfully identified siRNA sequences that exhibit a more effective RNAi activity against hepatitis C virus RNAs. Furthermore, the siRNAs were demonstrated to have a significant inhibitory effect on HCV propagation in an in vivo system.
Owner:CHUGAI PHARMA CO LTD +1
Hepatitis C virus RNA (ribonucleic acid) extracting kit
ActiveCN107254463AReduce usageImprove securityMicrobiological testing/measurementDNA preparationHepatitis C virus RNAHydroxycitric acid
The invention relates to a hepatitis C virus RNA extracting kit, belongs to the field of biomedical detection and relates to the nucleic acid isolation technology, particularly to the hepatitis C virus RNA extracting technology. By combining guanidine hydrochloride with a citric acid buffer system, the hepatitis C virus RNA extracting kit achieves the technical effects of effectively extracting hepatitis C virus RNA in samples in high security, high sensitivity and high stability.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Antiviral Peptide Against Hepatitis C Virus
InactiveUS20110091966A1Improve efficiencyHigh affinityOrganic active ingredientsSugar derivativesIn vivoC-terminus
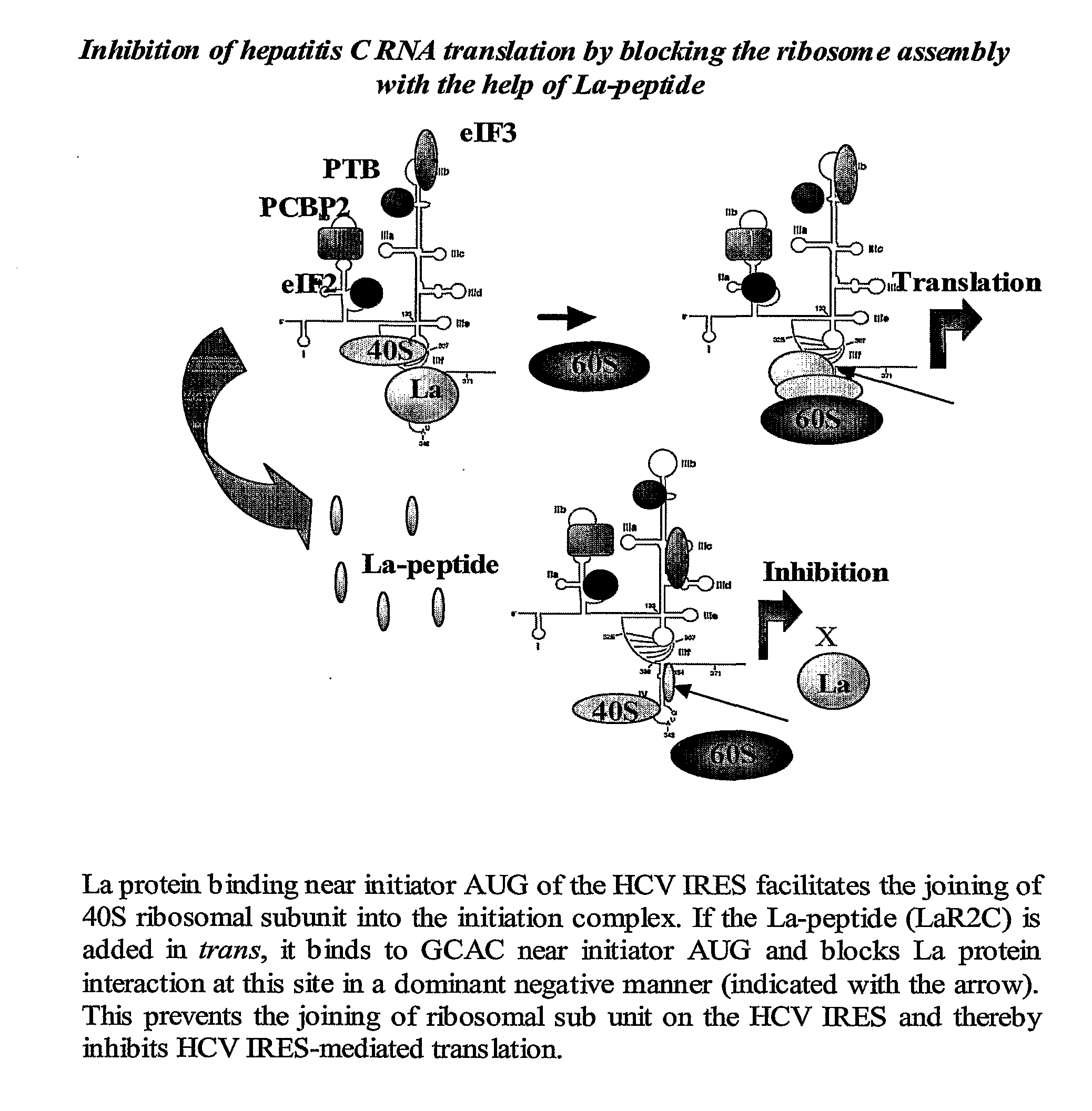
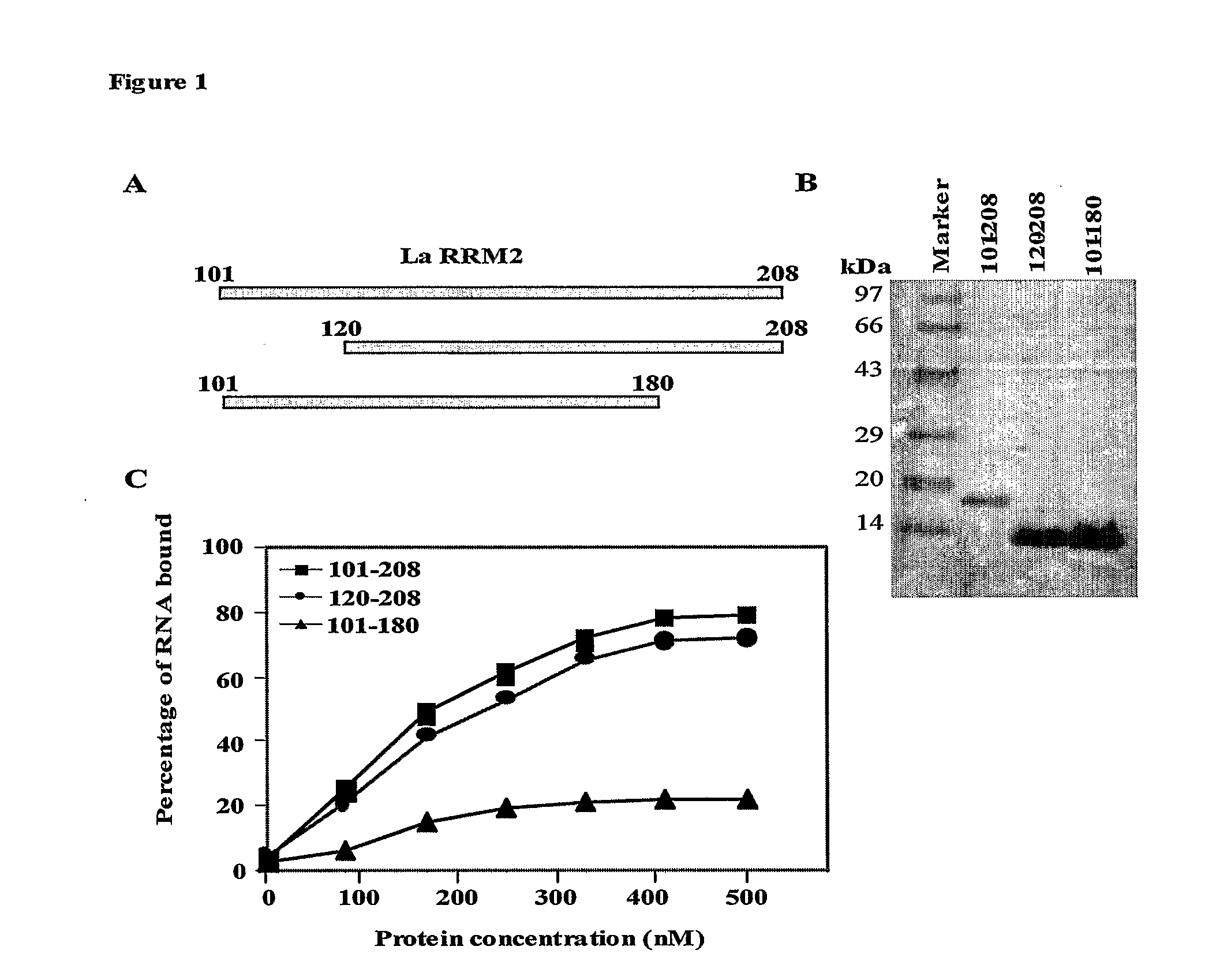
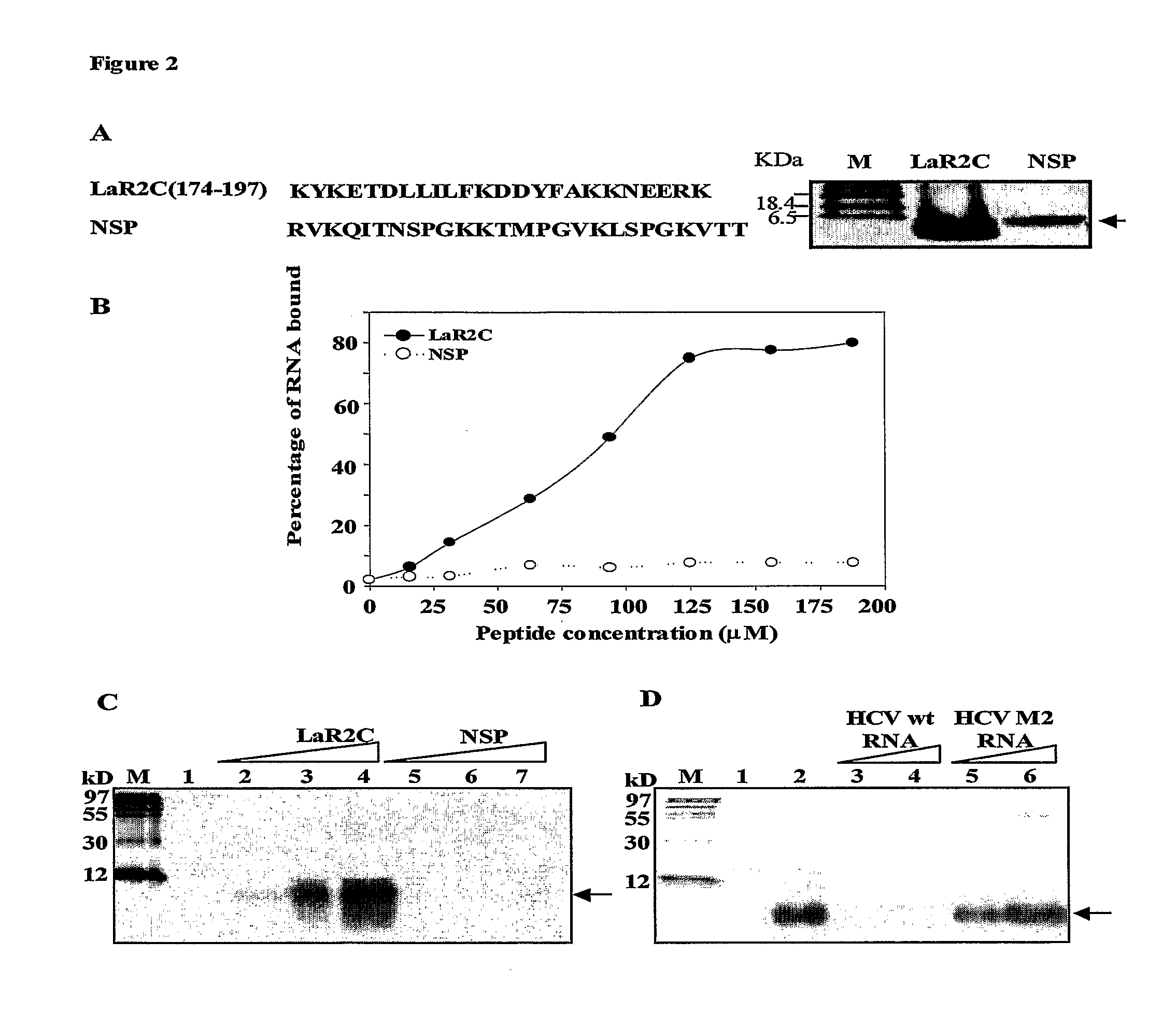
Disclosed herein is a small peptide, LaR2C, corresponding to the C terminus of RRM2 of the human La protein that binds to the IRES element of hepatitis C virus RNA and its derivatives. This invention demonstrates that human La protein interacts with the HCV IRES element both in vitro and in vivo and also shown that this interaction enhances the efficiency of viral RNA translation (Pudi et al, J of Biol Chem, 2003). La protein has three putative RNA recognition motifs (RRM1-3). It has been established that RRM2 binds with high affinity around the GCAC sequence near the initiator AUG and the binding induces a conformational change in the HCV IRES which is critical for the internal initiation of translation (Pudi et al, J of Biol Chem, 2004).
Owner:DAS SAUMITRA +2
kit for extraction of hepatitis c virus rna
ActiveCN107254463BLow detection limitStable extractionMicrobiological testing/measurementDNA preparationHepatitis C virus RNACitric acid
The invention relates to a hepatitis C virus RNA extracting kit, belongs to the field of biomedical detection and relates to the nucleic acid isolation technology, particularly to the hepatitis C virus RNA extracting technology. By combining guanidine hydrochloride with a citric acid buffer system, the hepatitis C virus RNA extracting kit achieves the technical effects of effectively extracting hepatitis C virus RNA in samples in high security, high sensitivity and high stability.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Isothermal chain multiple detection card of pathogen nucleic acid
ActiveCN102230032BClear and variable resultsPrevention of Amplified Cross ContaminationMicrobiological testing/measurementSpiroplasmaQuarantine
The invention discloses an isothermal chain multiple displacement detection card of pathogen nucleic acid. The isothermal chain multiple displacement detection card of the pathogen nucleic acid utilizes the detection card which is prepared through a nucleic acid isothermal chain displacement method and a colloidal gold detection technology to carry out multiple detection on HBV-DNA, HCV-RNA, HIV-RNA, and TP-DNA. The isothermal chain multiple displacement detection card of the pathogen nucleic acid has the characteristics of simple and rapid operation, high sensitivity, and no need of professional equipment. The pathogen nucleic acid to be detected is not amplified in the detection process, so the detection card has the advantages of preventing amplified matter cross contamination in laboratories and preventing false positivity, can be widely used in high sensitivity pathogen nucleic acid detection in the fields of clinical detection, inspection and quarantine, infectious disease control, biological technology and the like, and has a wide application prospect.
Owner:武汉中科志康生物科技有限公司
Hepatitis C sequencing and typing kit and detection method thereof
ActiveCN101921871BImprove accuracyImprove throughputMicrobiological testing/measurementNucleotideTyping
The invention discloses a hepatitis C sequencing and typing kit which comprises: (1) primers of specific amplified hepatitis C virus RNA reverse transcription product c DNA and (2) hepatitis C virus specific sequencing primers, wherein the nucleotide sequences of the primers of specific amplified hepatitis C virus RNA reverse transcription product c DNA are shown as SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4, the SEQ ID NO:1 and the SEQ ID NO:2 are a primer pair and the SEQ ID NO:3 and the SEQ ID NO:4 are another primer pair; and the nucleotide sequences of the hepatitis C virus specific sequencing primers are shown as SEQ ID NO:5, SEQ ID NO:6 and SEQ ID NO:7; the SEQ ID NO:4 is marked with a biotin at a 5' end; and all the primers are all used in a detection. The invention also discloses a detection method of the hepatitis C sequencing and typing kit. The hepatitis C sequencing and typing kit can be used for sequencing and typing hepatitis C, thereby providing more comprehensive reference information for clinical diagnosis and treatment.
Owner:CHONGQING DIAN SRAB CENT FOR CLINICAL LAB CO LTD
Detection method for mutation in 93rd amino acid of hepatitis C virus NS5A protein, and detection kit for mutation in 93rd amino acid of hepatitis C virus NS54 protein
ActiveUS10370730B2Low costInhibition effectMicrobiological testing/measurementBiochemistryHepatitis C virus RNA
A method for detecting a mutation in an amino acid at position 93 of a hepatitis C virus NS5A protein, the method including:synthesizing cDNA using, as a template, hepatitis C virus RNA in a sample; andperforming a real-time PCR with a cycling probe method using, as a template, the cDNA;wherein a primer set used in the real-time PCR is a certain primer set; andwherein probes used in the real-time PCR include certain probes.
Owner:SAITAMA MEDICAL UNIVERSITY
Oligonucleotide primer for effective detection of hepatitis C virus (HCV) and its use
InactiveCN1237186CSsRNA viruses positive-senseMicrobiological testing/measurementOligonucleotide primersHepatitis C virus RNA
Described herein are methods and kits for the detection of hepatitis C virus RNA is biological samples obtained from human subjects. The invention includes novel amplification primers and probes useful in the amplification of DNA derived from hepatitis C virus RNA, and kits and methods which incorporate the novel primers.
Owner:ORTHO-CLINICAL DIAGNOSTICS
In vitro activity measuring method for hepatitis C virus RNA depending RNA polymerase and application thereof
InactiveCN101168781BSimple methodPractical methodMicrobiological testing/measurementLibrary screeningMagnetite NanoparticlesFhit gene
The invention provides a detecting method and an application of the extraneous activity of the RNA polymerase relied on by the hepatitis C virus RNA, which relates to an secure and practical establishing method for the detection of the activity extraneous detection of the RNA polymerase relied on by the hepatitis C virus RNA, and the application relates to the genetic engineering technology and the nanometer segregation enrichment and RNA related technology field. The invention expresses the soluble NS5B protein through the utilization of the genetic engineering technology, and prepares the nanometer magnetic particle. One end of a RNA template is fixed on the nanometer magnetic particle, and added in the 96 hole reaction plate micromesh, and added with NS5B protein to compose a RNA duplication system, and a duplicated RNA double chain on the nanometer magnetic particle is mixed with marked biological elements. After the RNA is duplicated, the nanometer magnetic particle is attached at the micromesh bottom through an external magnetic field magnet, after repeatedly cleaning and attaching, the nanometer magnetic particle is combined with the marked combining factor of the horse radish peroxidase, after attaching and cleaning, the substrate constant of the horse radish peroxidase is added to develop color. New NS5B protease activity inhibitor can be screened from a traditional Chinese storeroom and a compound storeroom by the established method.
Owner:李越希
Inhibitors of hepatitis C virus RNA-dependent RNA polymerase, and compositions and treatments using the same
Owner:PFIZER INC
Direct detection of unamplified hepatitis C virus RNA using unmodified gold nanoparticles
InactiveUS9303292B2Simple, rapid, and sensitive colorimetric assaySensitively detect HCVMicrobiological testing/measurementNanosensorsNanoparticleHepatitis C virus RNA
Owner:THE AMERICAN UNIV OF CAIRO
Detection method for mutation in 93rd amino acid of hepatitis c virus ns5a protein, and detection kit for mutation in 93rd amino acid of hepatitis c virus ns5a protein
ActiveUS20180163278A1Low costInhibition effectMicrobiological testing/measurementHepatitis C virus RNAAmino acid mutation
A method for detecting a mutation in an amino acid at position 93 of a hepatitis C virus NS5A protein, the method including:synthesizing cDNA using, as a template, hepatitis C virus RNA in a sample; andperforming a real-time PCR with a cycling probe method using, as a template, the cDNA;wherein a primer set used in the real-time PCR is a certain primer set; andwherein probes used in the real-time PCR include certain probes.
Owner:SAITAMA MEDICAL UNIVERSITY
Truncated hepatitis C virus hcv NS3 antigen and its preparation and application
ActiveCN105254724BHigh positive serum coverageSmall molecular weightBacteriaVirus peptidesEscherichia coliEnzyme digestion
The invention discloses a truncated type hepatitis c virus HCV NS3 antigen which is a nonstructural protein amino acid sequence of the NS3 full-length gene encoded 1252th-1526aath segment in an HCV genome. The amino acid sequence is shown as SEQ ID NO.1, and a corresponding nucleotide sequence is shown as SEQ ID NO.2. A preparation method of HCV NS3 recombinant antigen protein comprises the steps that an optimized escherichia coli codon for expressing the truncated type hepatitis c virus HCV NS3 antigen are established, the nucleotide sequence of the codon is shown as SEQ ID NO.3, the gene segment of the codon undergoes double enzyme digestion and then is connected with an expression vector undergoing the enzyme digestion as well to obtain a recombinant expression plasmid, the antigen can perform expression by adopting a conventional method, and the antigen protein is obtained through purification. The antigen protein has high sensitivity and good specificity and is used for detecting an HCV antibody, and the coincidence rate of a detection result of a patient with positive hepatitis c virus RNA is as high as 97%. It is indicated that the antigen can be applied to polyclonal or monoclonal antibodies and preparation of hepatitis c virus detection kits and has good prospect of clinical application.
Owner:山东莱博生物科技有限公司
HCV RNA having novel sequence
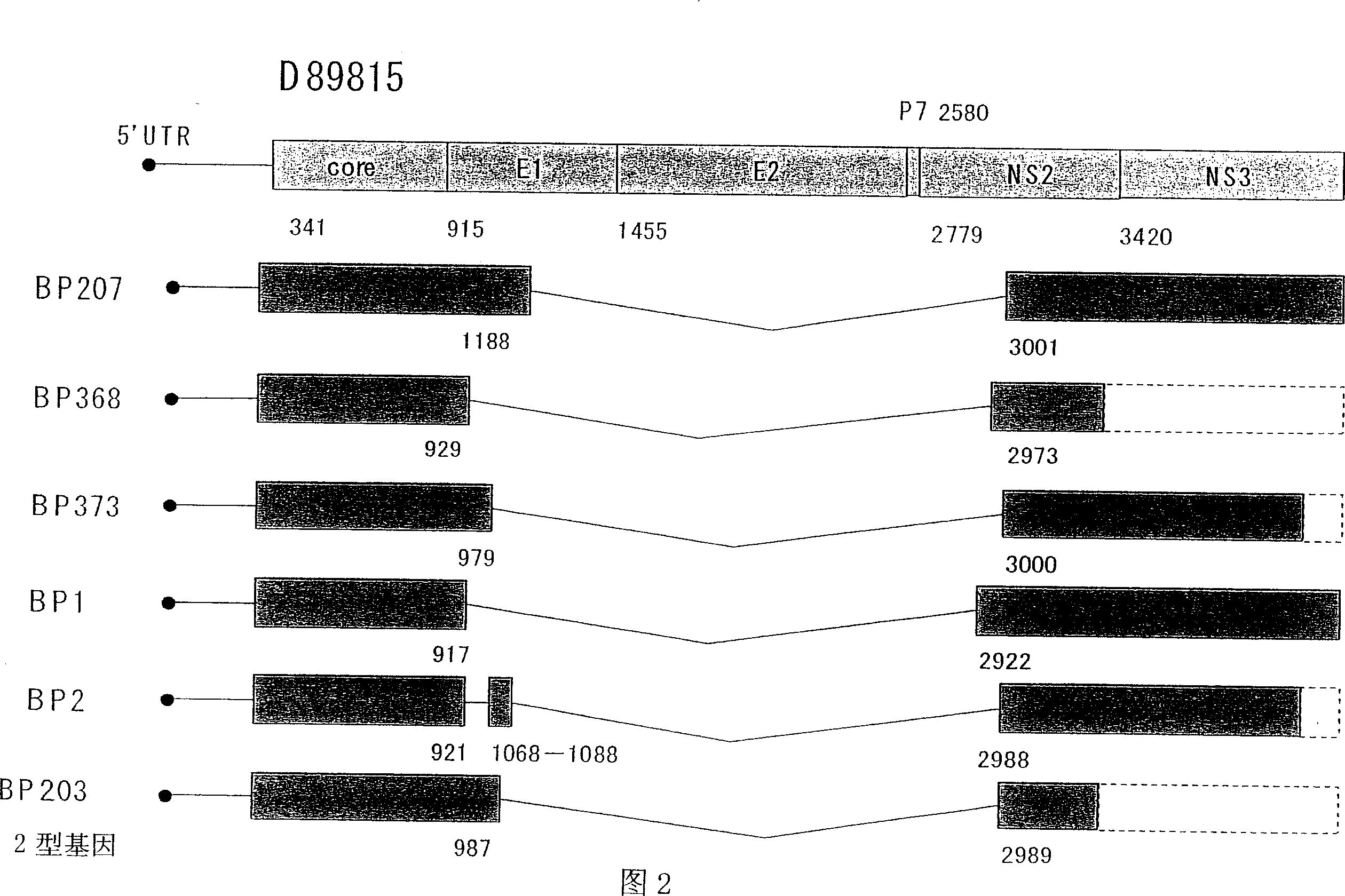
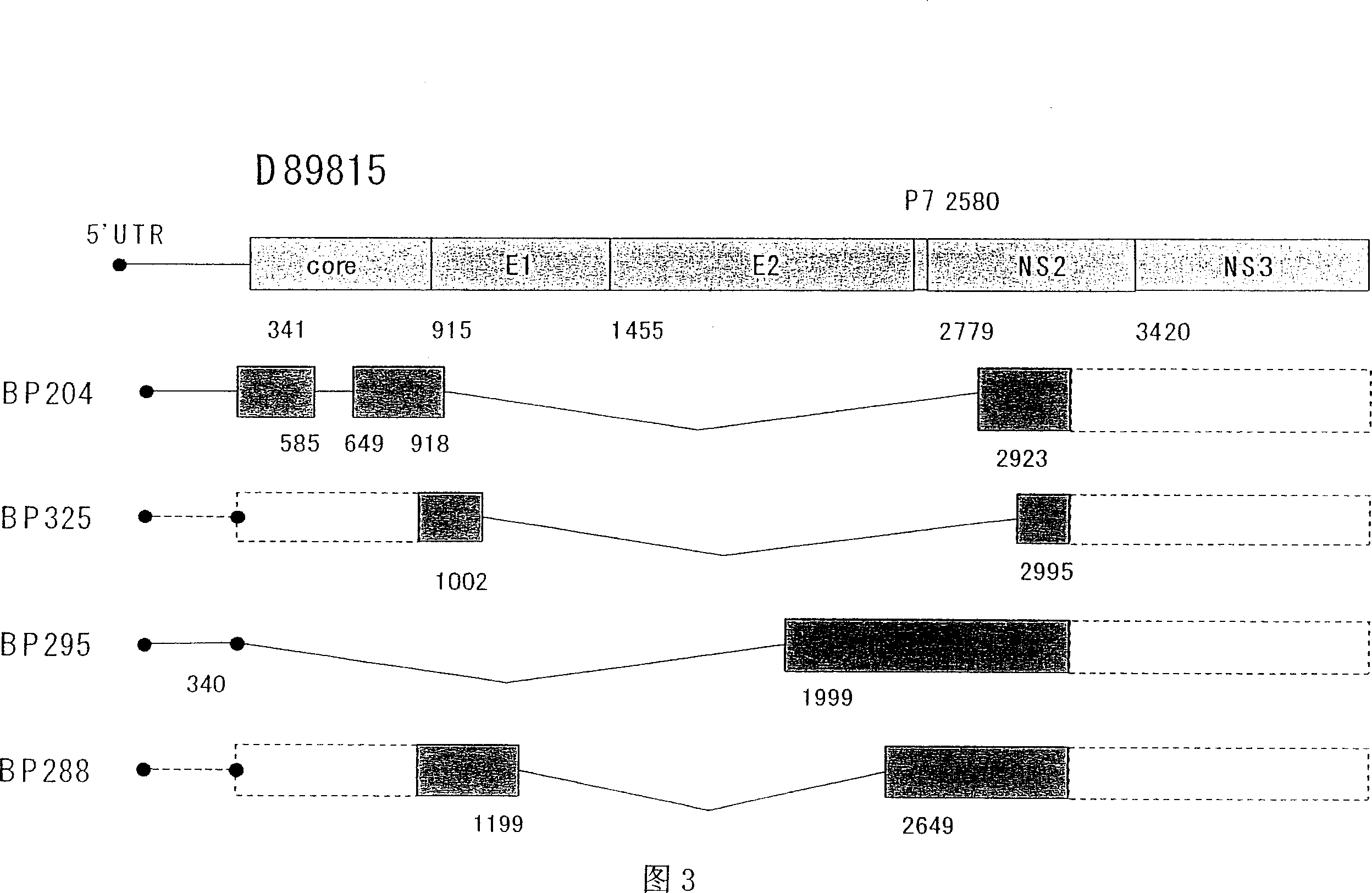
A truncated form of hepatitis C virus gene obtained by deleting a portion of a region of a gene encoding NS2 protein from the core protein of hepatitis C virus while sustaining its translation frame. In particular, a gene as described above wherein the above-described portion of a region of the gene is located in a region encoding E1 protein and E2 protein.
Owner:ADVANCED LIFE SCI INST
Inhibitors of hepatitis c virus RNA-dependent RNA polymerase, and compositions and treatments using the same
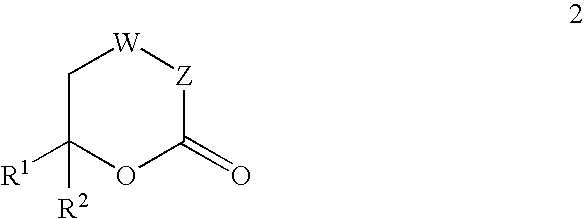
The present invention provides compounds of formula (4), and their pharmaceutically acceptable salts and solvates, which are useful as inhibitors of the Hepatitis C virus (HCV) polymerase enzyme and are also useful for the treatment of HCV infections in HCV-infected mammals. The present invention also provides pharmaceutical compositions comprising compounds of formula (4), their pharmaceutically acceptable salts and solvates. Furthermore, the present invention provides intermediate compounds and methods useful in the preparation of compounds of formula (4).
Owner:PFIZER INC
A fusion antigen of the first hypervariable region of hepatitis C virus and its application
ActiveCN104193827BHigh positive serum coverageIncreased cross-reactivityBiological testingHybrid peptidesAntibody hypervariable regionPositive patient
The invention discloses a fusion antigen of the first hypervariable region of hepatitis C virus. The amino acid sequence of the fusion antigen is shown in SEQ ID No.1. The invention also discloses the detection of the fusion antigen in the preparation of hepatitis C virus HVR1 antibody. application in the kit. It is verified by experiments that the cross-reactivity of the fusion antigen of the present invention is significantly improved compared with the single fragment HVR1 antigen, and the detection coincidence rate with hepatitis C virus RNA-positive patients is as high as 99%. Since HVR1 is the main neutralizing epitope in hepatitis C virus, through the application of the antigen of the present invention, for the detection of hepatitis C virus HVR1 antibody, the purpose of comprehensive detection of HCV infection can be realized, and it can be used for the transformation of HCV disease. Problems such as reconciliation prognosis and interferon curative effect prediction can be effectively solved, and have great clinical application prospects.
Owner:山东莱博生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com