Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Bone transplant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
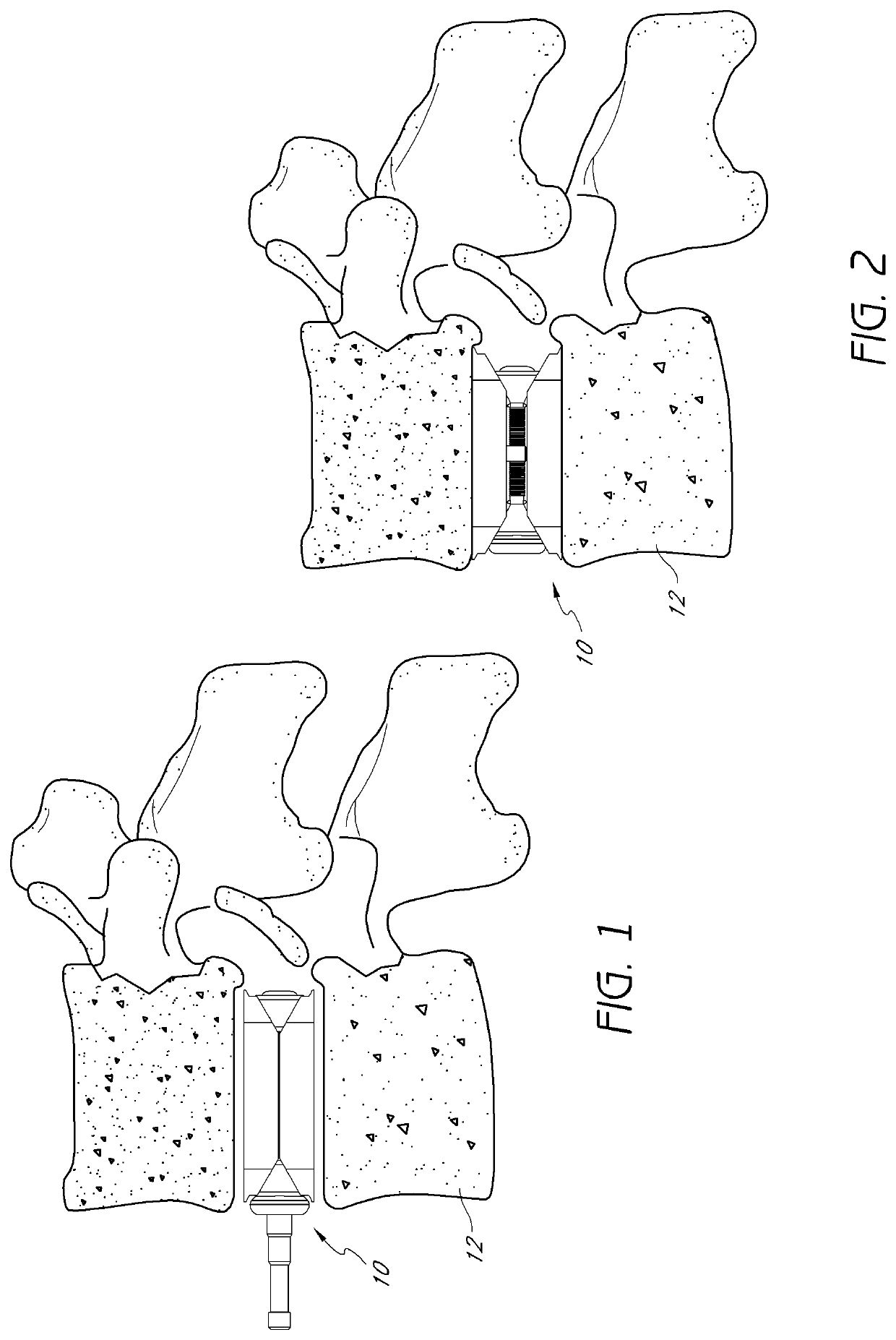
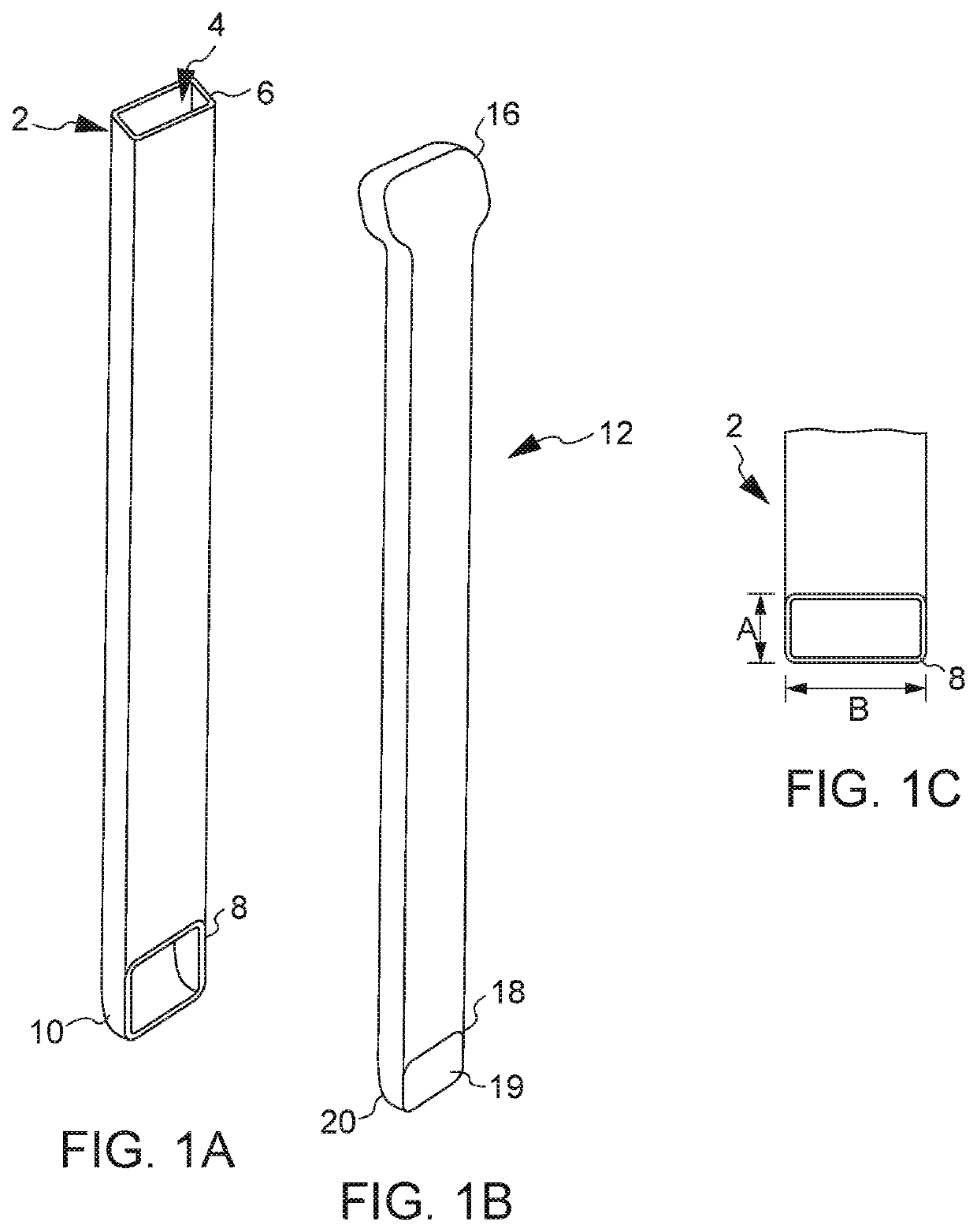
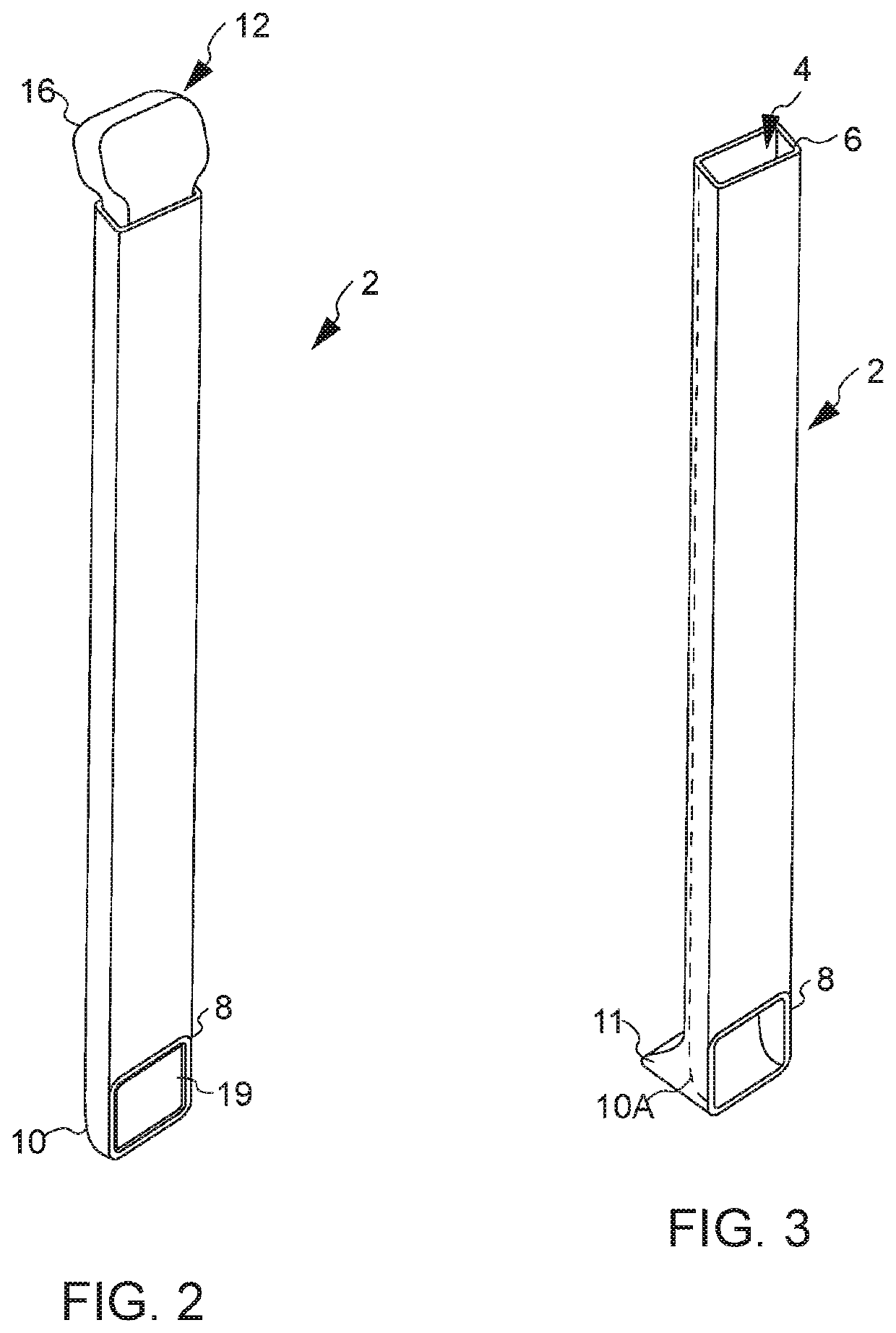
All bone requires a blood supply in the transplanted site. Depending on where the transplant site is and the size of the graft, an additional blood supply may be required. For these types of grafts, extraction of the part of the periosteum and accompanying blood vessels along with donor bone is required.
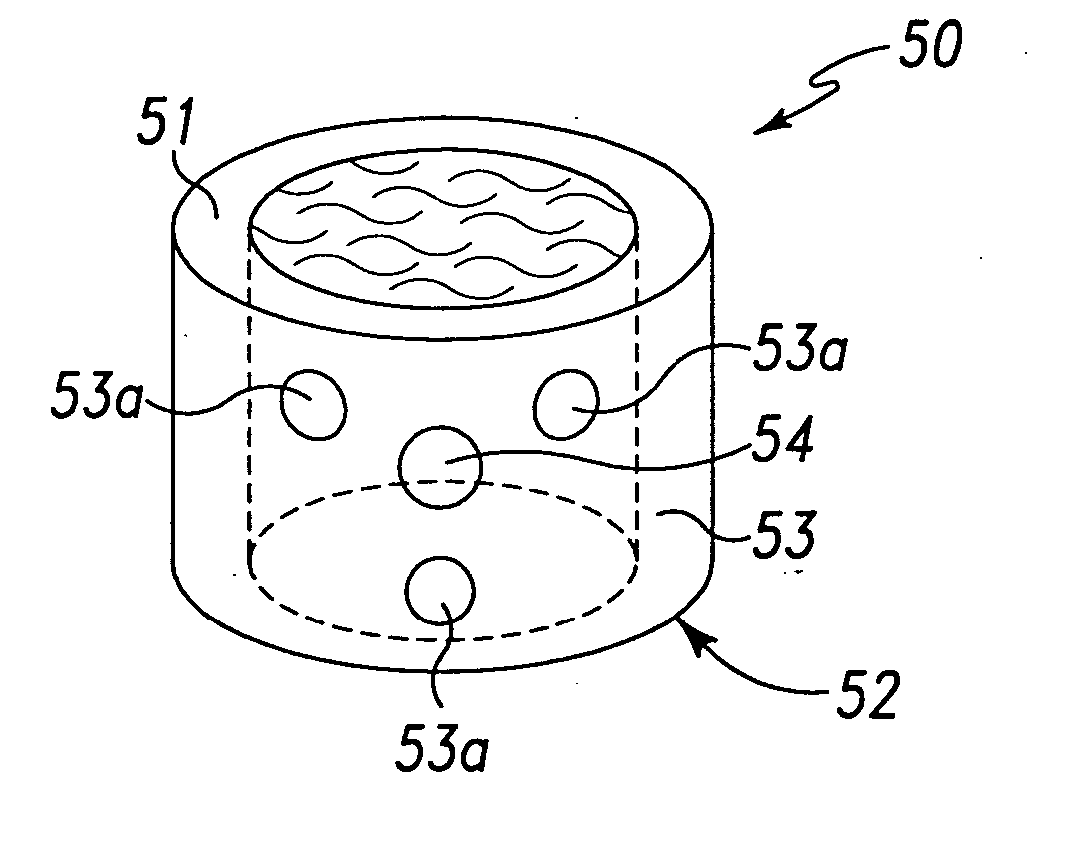
Calcium based neutral and bioresorbable bone graft
InactiveUS20030055512A1Improve mechanical propertiesReduce decreaseSurgical adhesivesBone implantSulfateBone implant
An injectable and moldable putty comprising biodegradable calcium-based compounds including calcium sulfate, hydroxyapatite, and tricalcium phosphate is invented. The putty hardens into a solid body when mixed with water, saline, serum, or other neutral aqueous solutions. The hardening time of the putty can be tailored in order to meet the specific requirements of various dental or orthopedic applications. The pH of the putty is neutral during and after mixing. The invented putty may be used as bone graft, bone implant, or implantable drug delivery device.
Owner:BERKELEY ADVANCED BIOMATERIALS
Bone grafts
InactiveUS20050004672A1Encourages bone ingrowthAvoid stress shieldingDiagnosticsBone implantBiomedical engineeringVertebra
Spinal spacers 20 are provided for fusion of a motion segment. The spacers include a load bearing member 21 having a wall 22 sized for engagement within a space between adjacent vertebrae to maintain the space and an effective amount of an osteogenic composition to stimulate osteoinduction. The osteogenic composition includes a substantially pure osteogenic factor in a pharmaceutically acceptable carrier. In one embodiment the load bearing member includes a bone graft impregnated in an osteogenic composition. In another embodiment, the osteogenic composition 30 is packed within a chamber 25 defined in the graft. Any suitable configuration of a bone graft is contemplated, including bone dowels, D-shaped spacers and cortical rings.
Owner:DANEK MEDICAL
Plasticized bone grafts and methods of making and using same
InactiveUS7063726B2Adequate material propertyEasy to disassembleJoint implantsTissue regenerationDiseasePlasticizer
The invention provides a plasticized bone and / or soft tissue product that does not require special conditions of storage, for example refrigeration or freezing, exhibits materials properties that approximate those properties present in natural tissue, is not brittle, does not necessitate rehydration prior to clinical implantation and is not a potential source for disease transmission. Replacement of the chemical plasticizers by water prior to implantation is not required and thus, the plasticized bone or soft tissue product can be placed directly into an implant site without significant preparation in the operating room.
Owner:LIFENET HEALTH
Bone graft and scaffolding materials immobilized with osteogenesis enhancing peptides on the surface
ActiveUS20070160681A1Improve efficiencyEasy to fixCoffee millsAnimal cellsSurgical operationCell adhesion
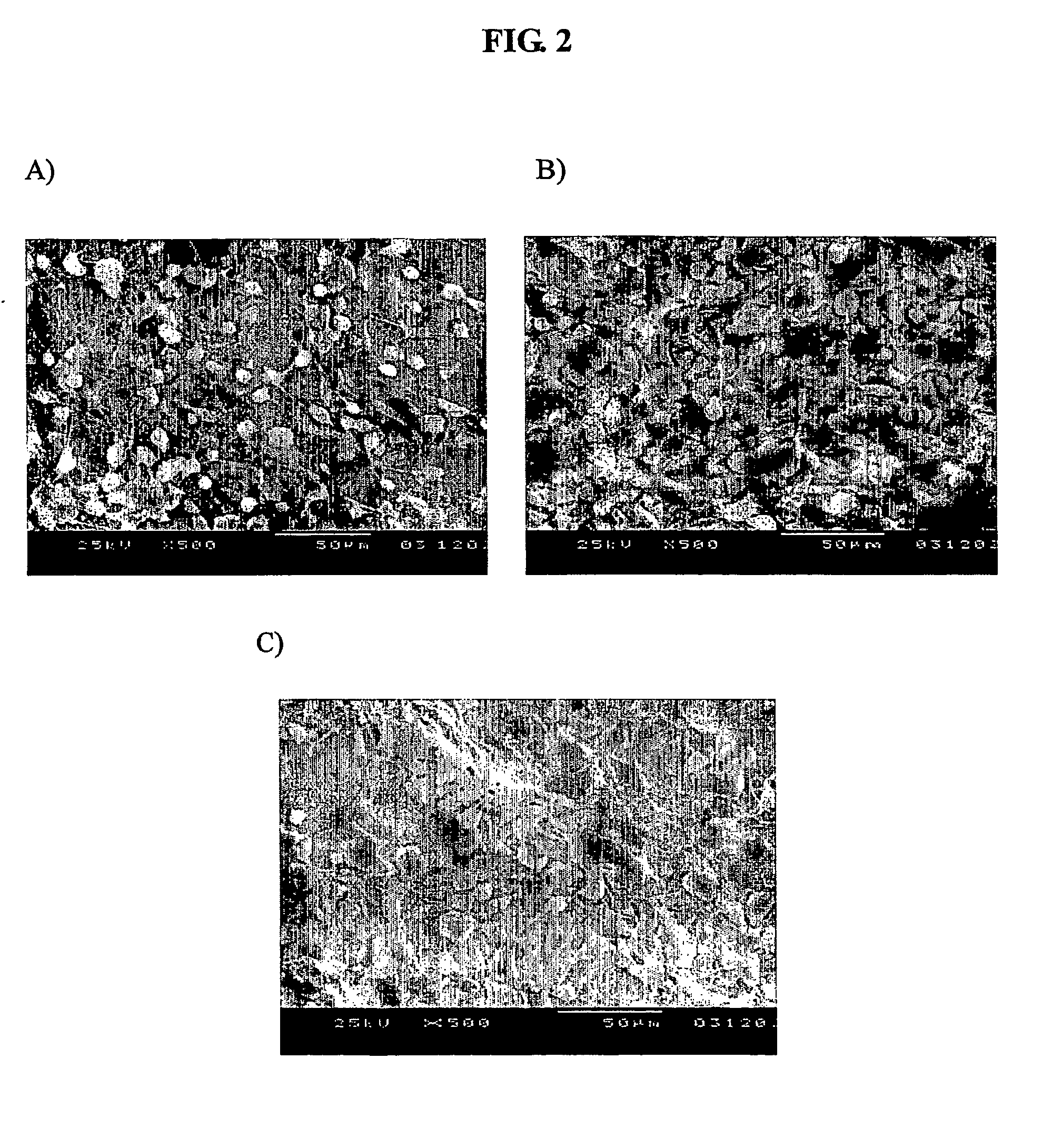
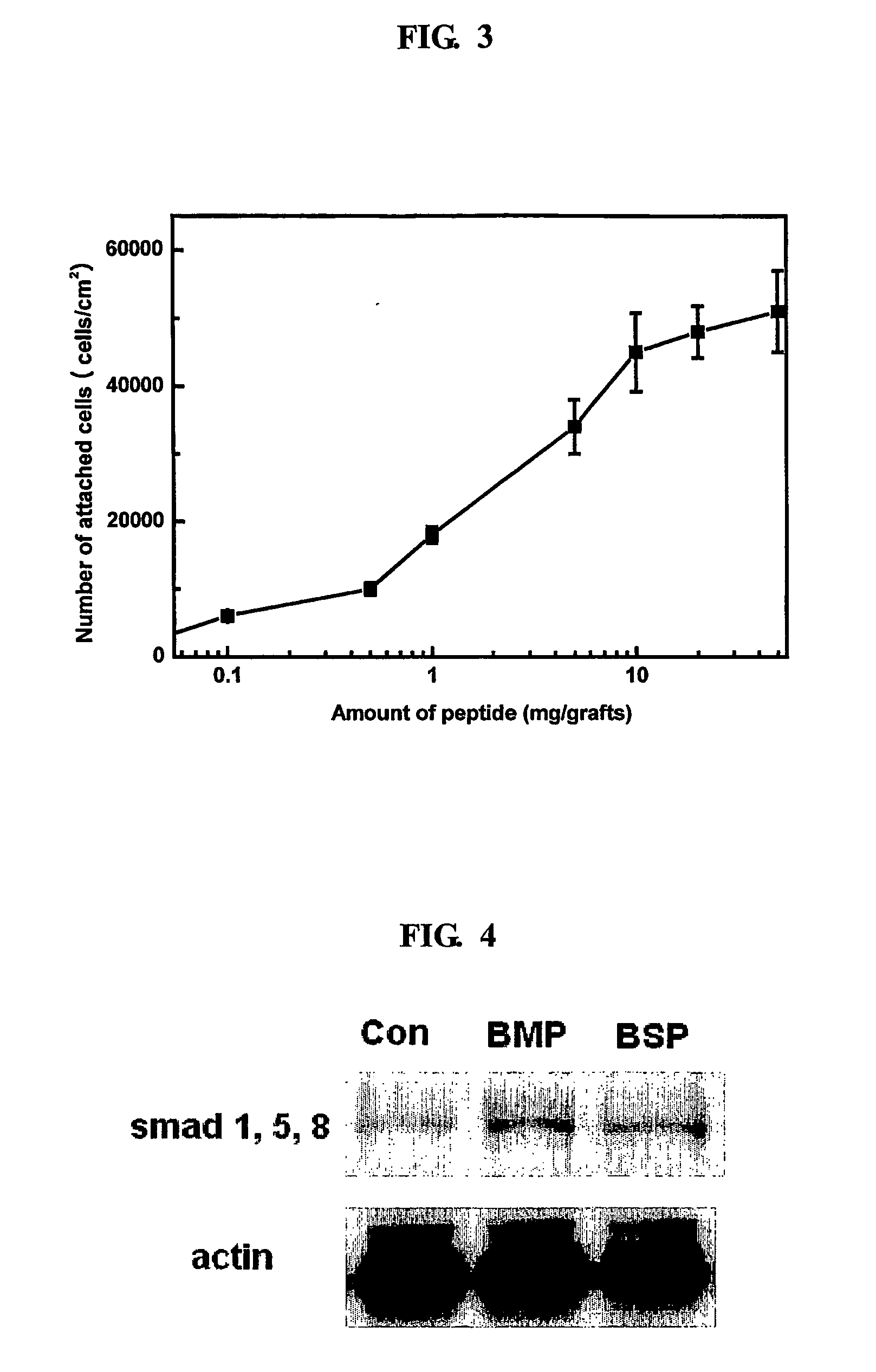
The present invention relates to a bone graft material and a scaffold for tissue engineering applications, which have an osteogenesis-promoting peptide immobilized on the surface. More particularly, the invention relates to a bone graft material and a scaffold for tissue engineering applications, which have a cell adhesion-inducing peptide and / or tissue growth factor-derived peptide immobilized on the surface. By the osteogenesis-promoting peptide immobilized on the surface, the inventive bone graft material and scaffold for tissue engineering applications can promote the transition, proliferation and differentiation of cells associated with regeneration, and eventually maximize the regeneration of tissue. Moreover, the peptide immobilized on the surface has low molecular weight, indicating a reduced risk of immune responses upon its application in the body, and can be present in a stable form within the body, thus showing lasting effects. Accordingly, the peptide makes it expedient to perform surgical operations for the regeneration of periodontal tissue, alveolar bone and other bone tissues, and will show high therapeutic effect.
Owner:SEOUL NAT UNIV R&DB FOUND
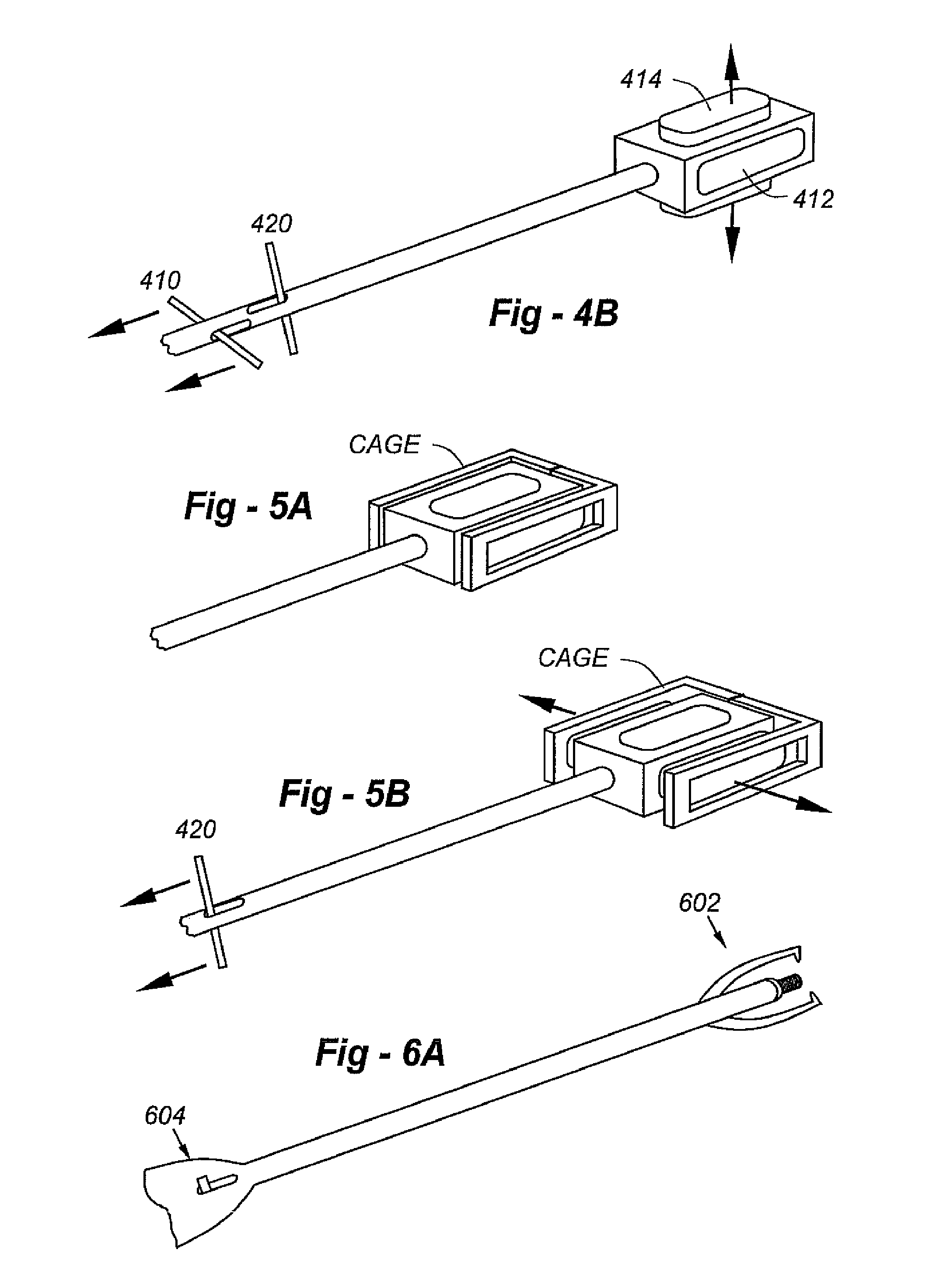
Fusion Cage With Combined Biological Delivery System
ActiveUS20130184822A1Good accommodationPreventing and mitigate risk of injuryBone implantSpinal implantsBone graft materialsBiological materials
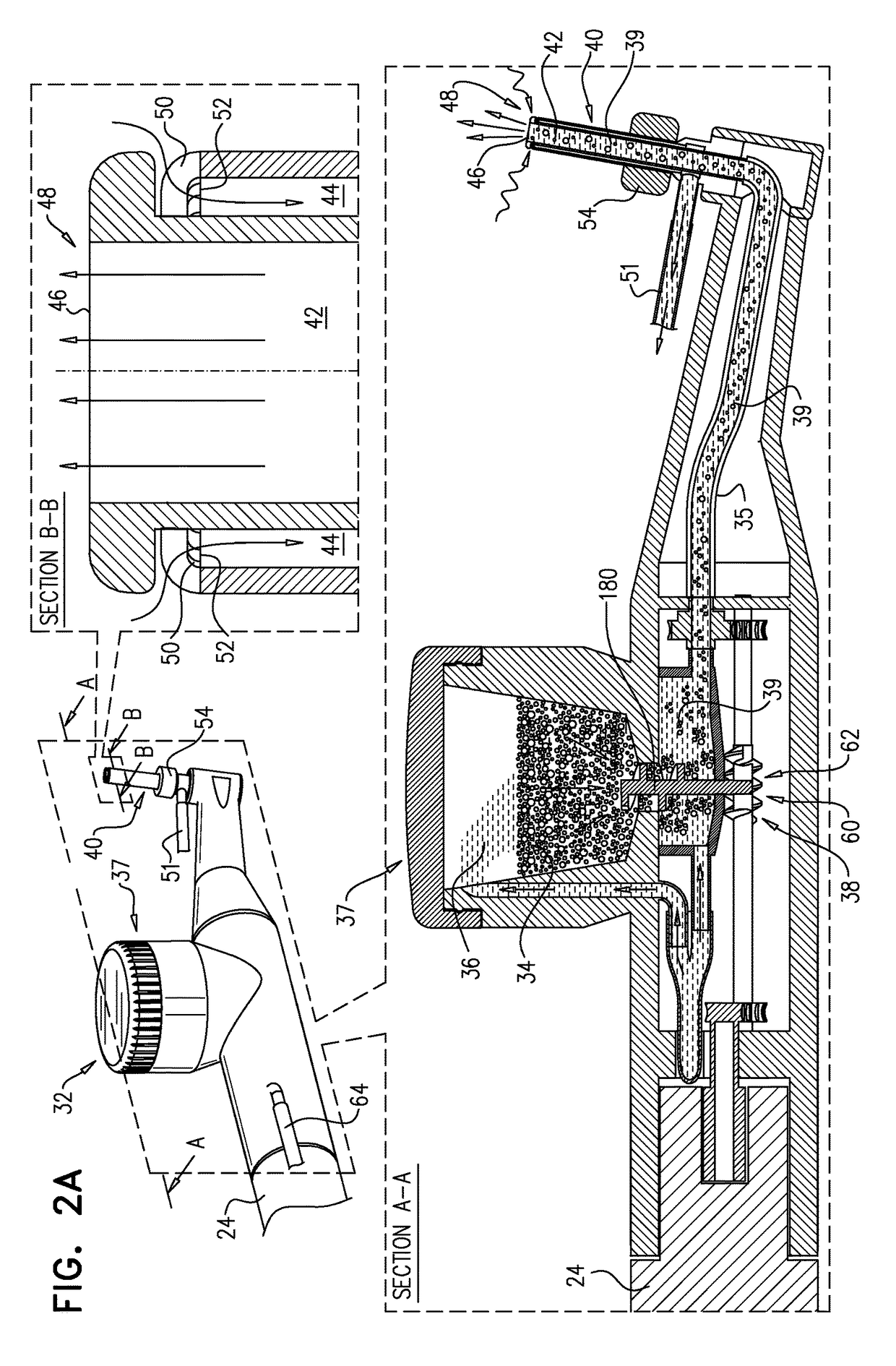
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area.
Owner:SPINAL SURGICAL STRATEGIES INC
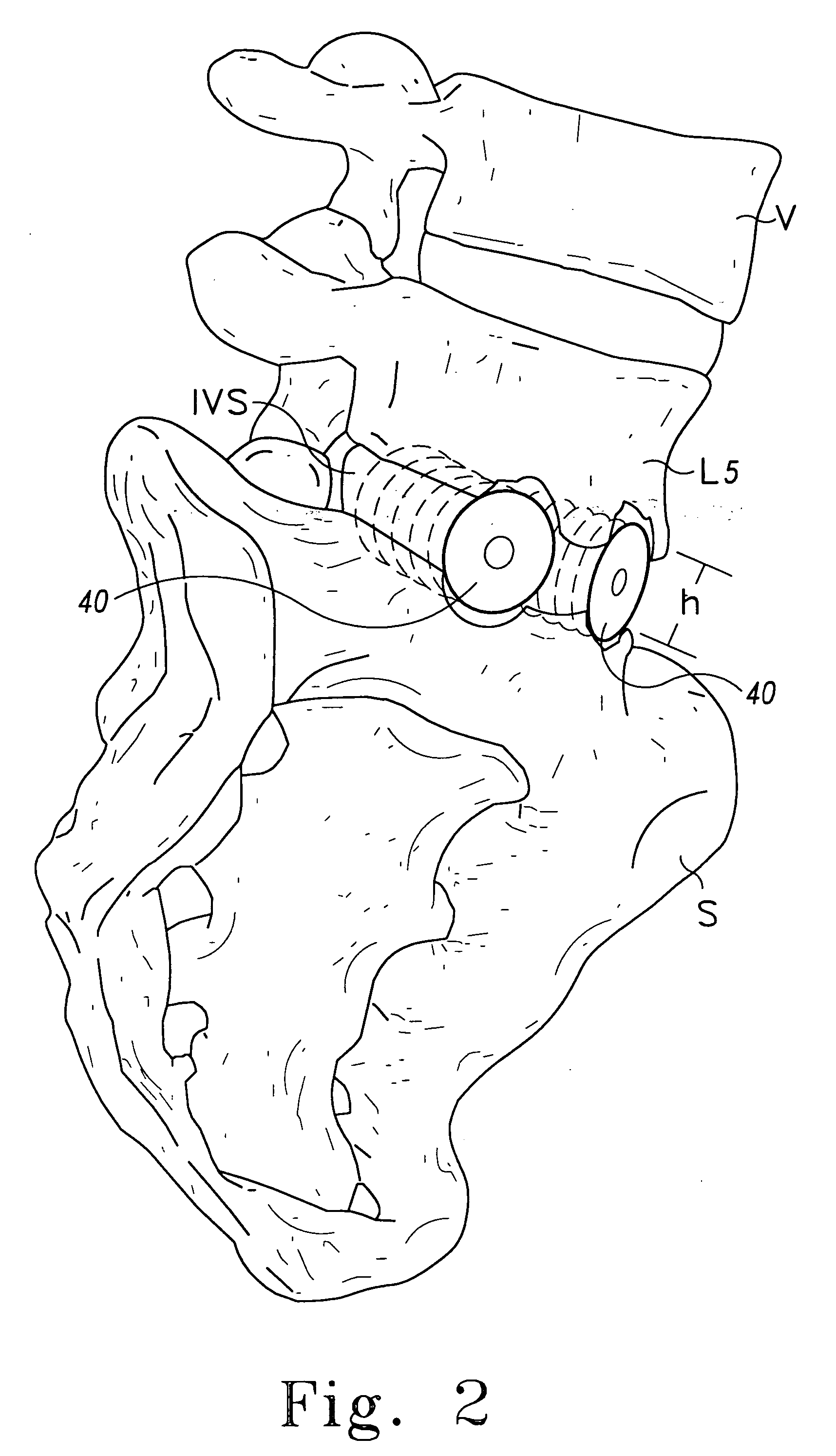
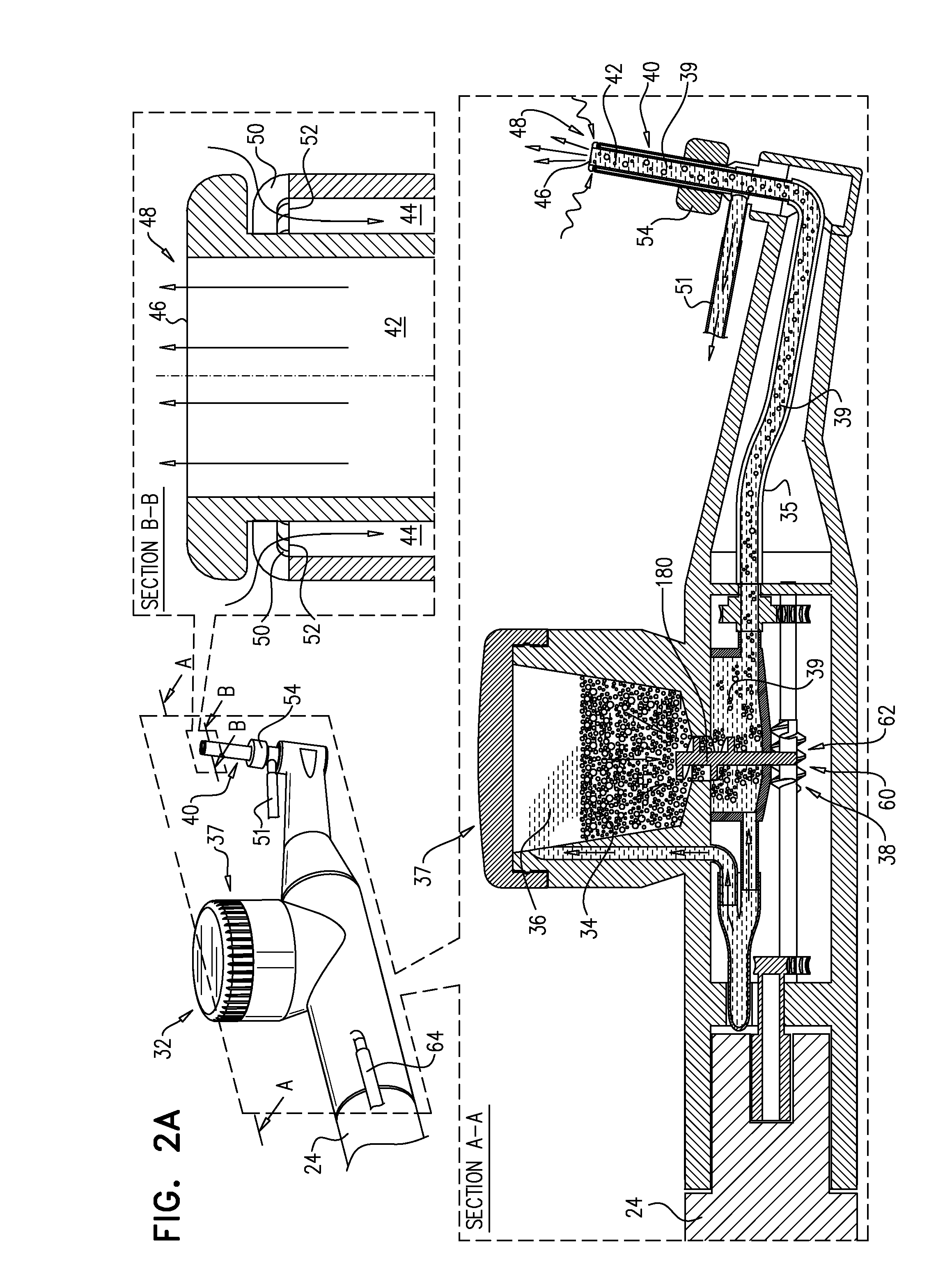
Anterior cervical spine instrumentation and related surgical method
InactiveUS20080046084A1Avoid expulsionShorten the timeInternal osteosythesisSpinal implantsIntervertebral spaceGraft size
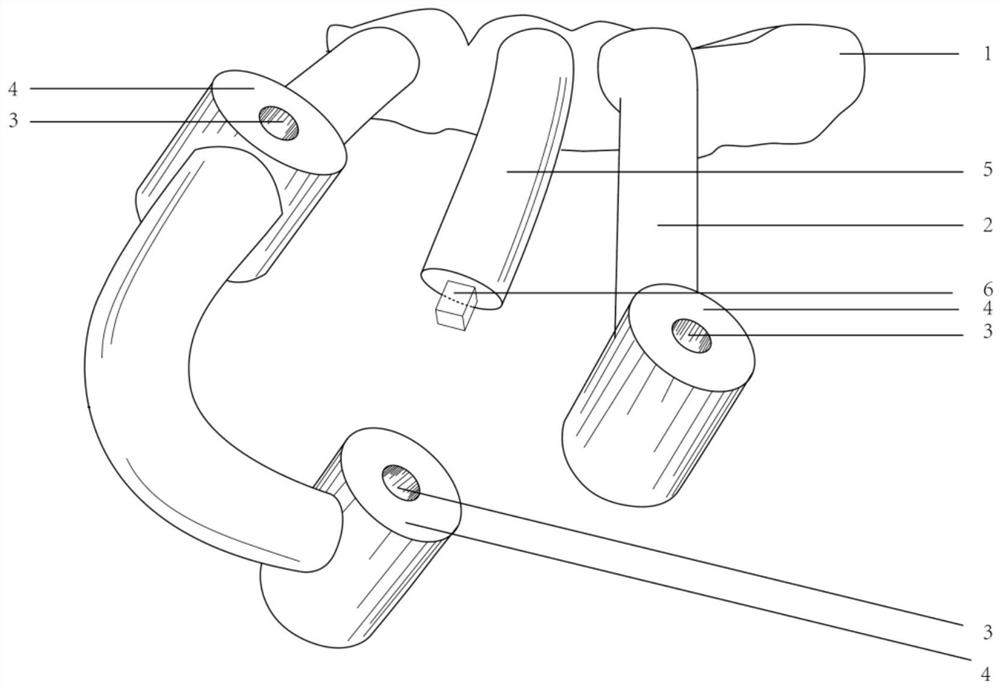
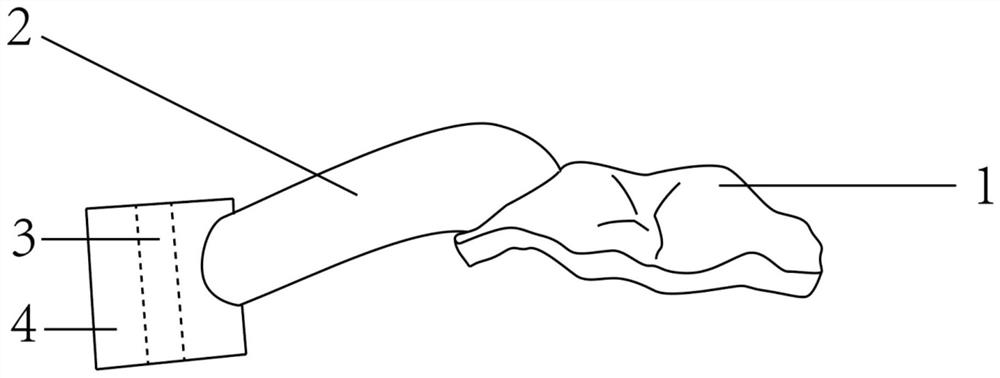
In various exemplary embodiments, the present invention provides a set of less invasive cervical spine instruments that are used to achieve cervical disc decompression, bone preparation, and the alignment of one or more matched sized bone grafts prior to cervical plate placement. This set of less invasive cervical spine instruments, and the related surgical method, result in reduced surgical time, the preparation of a precise machined bone surface while simultaneously maintaining the cervical disc decompression height of the intervertebral endplates, the selection of one or more prefabricated bone dowel grafts sized to match the machined bone surface and maximizing the surface contact required for cervical spine fusion, the placement of the one or more prefabricated bone dowel grafts (e.g. side by side) that can be of different diameters in order to fully exploit the intervertebral space available, and the alignment of the cervical plate using a cervical spine instrument that is matched to align with the one or more prefabricated bone dowel grafts beneath the cervical plate.
Owner:US SPINE INC
Flowable bone grafts
Owner:DEPUY SPINE INC (US) +1
Percutaneous posterior lateral in-situ cage
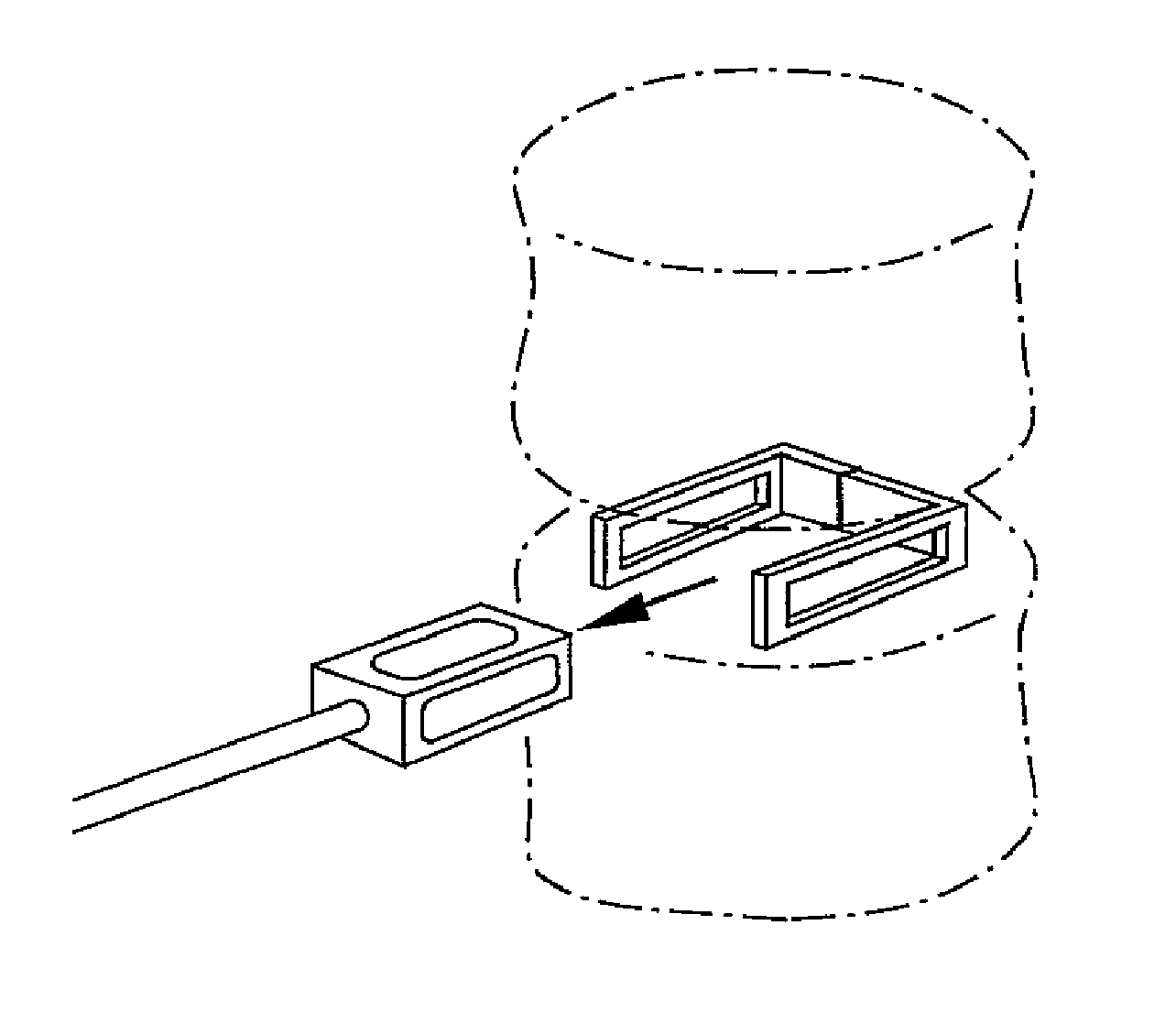
Implants, tools and techniques facilitate a percutaneous posterior lateral approach to the placement of an in-situ cage, and an inventive cage design to meet this objective. In terms of apparatus, the invention includes a laterally expandable cage, including a locking gate, enabling the system to be introduced into an intradiscal space through a minimally invasive percutaneous posteo-lateral approach. In addition to the cage designs, adapted to hold bone graft and / or other biologic materials, the invention includes other novel instruments, including an introducer associated with cage placement, deployment and closure.
Owner:CTL MEDICAL CORP
Fusion cage with combined biological delivery system
ActiveUS9173694B2Reduce the risk of infectionReduce the possibilityBone implantJoint implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area.
Owner:SPINAL SURGICAL STRATEGIES INC
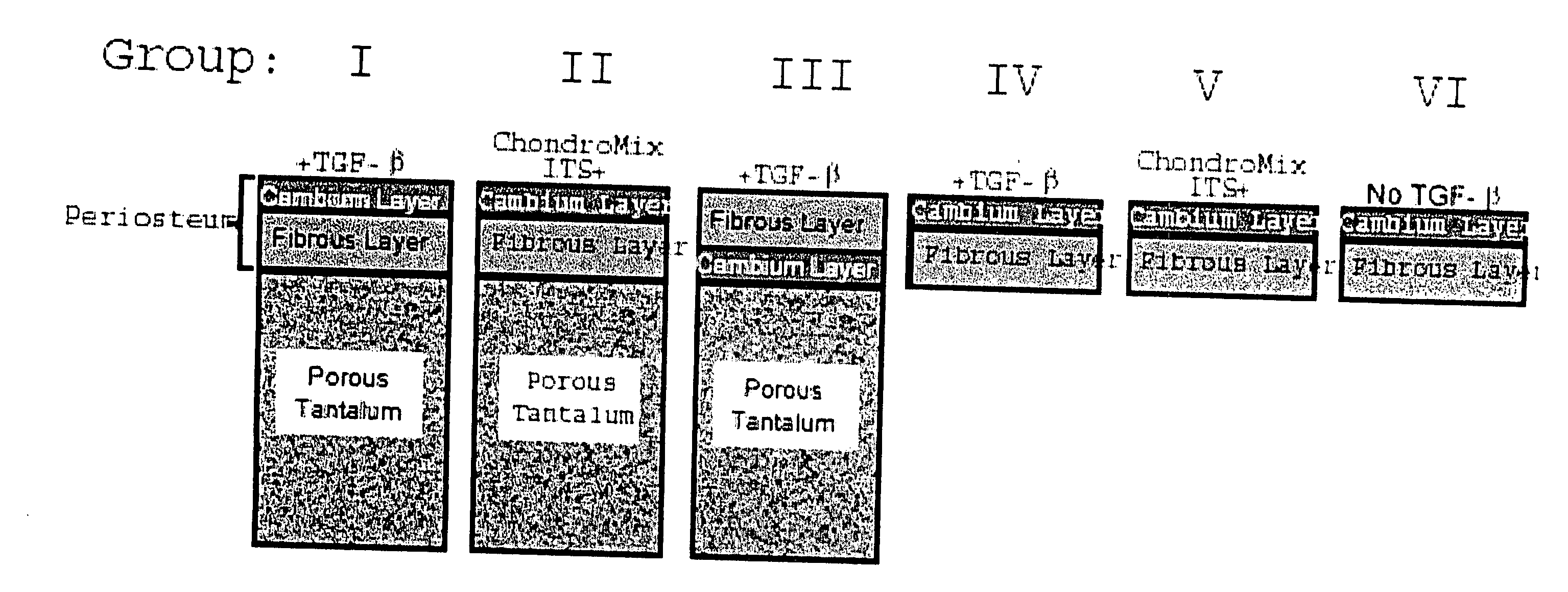
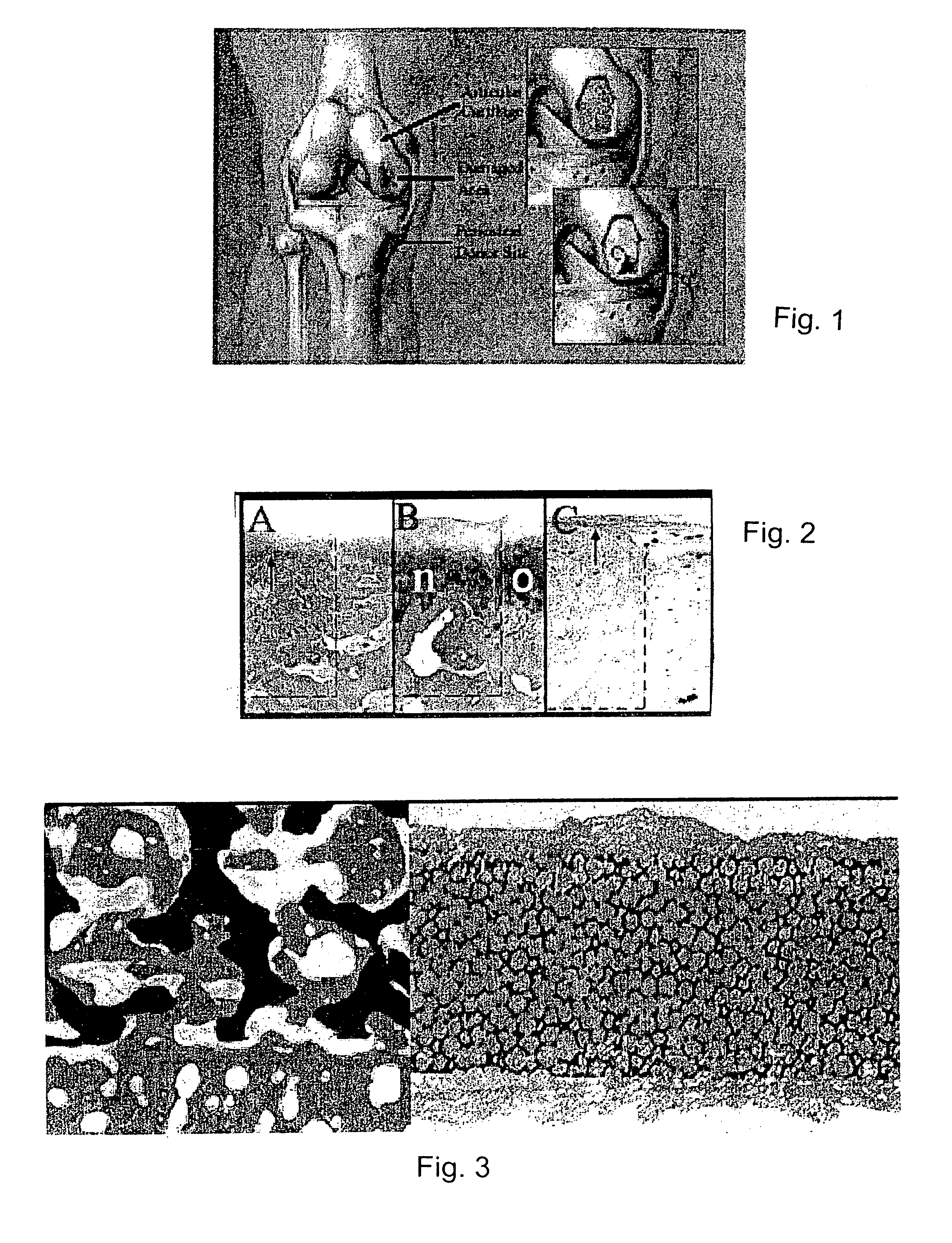
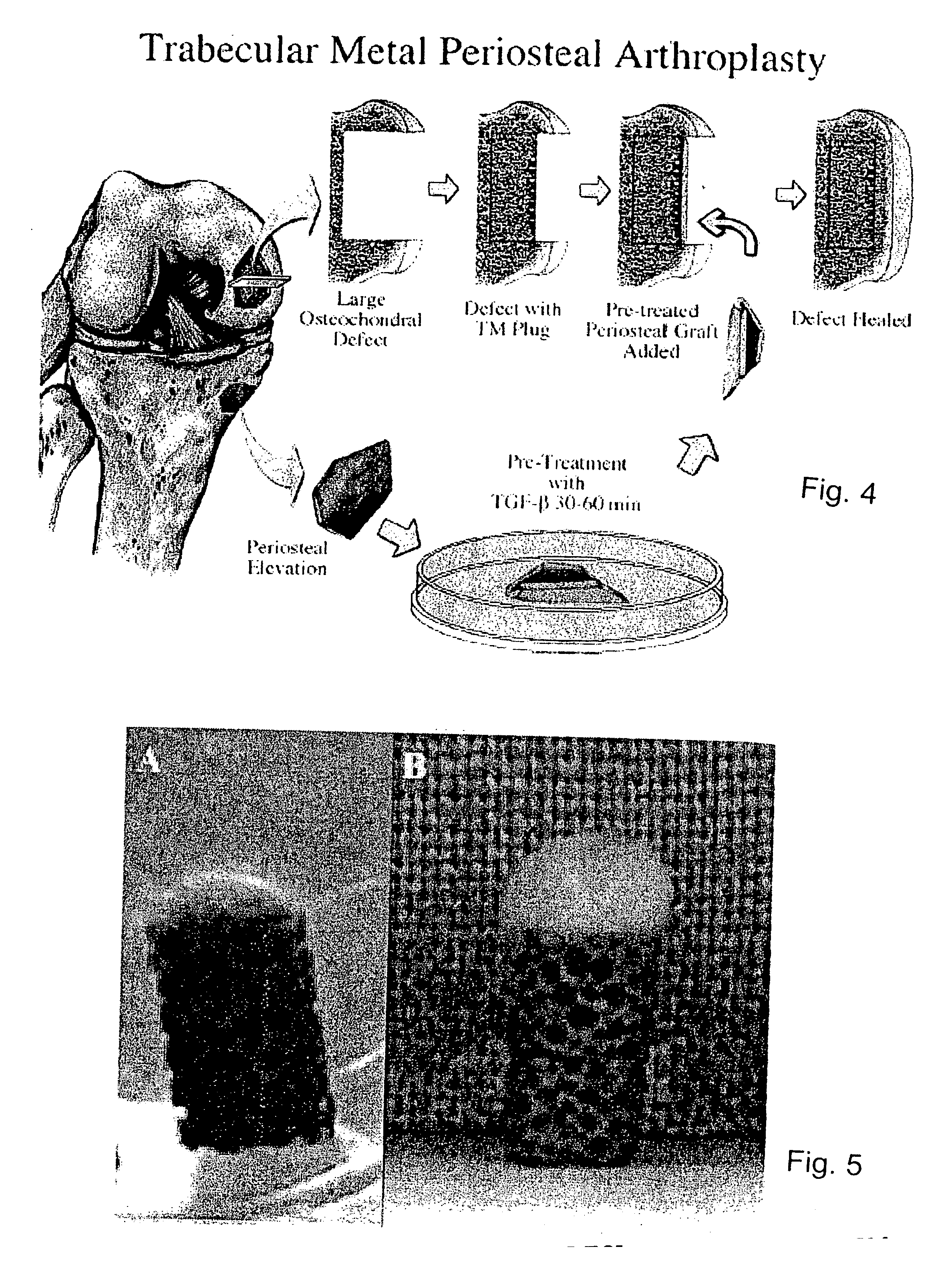
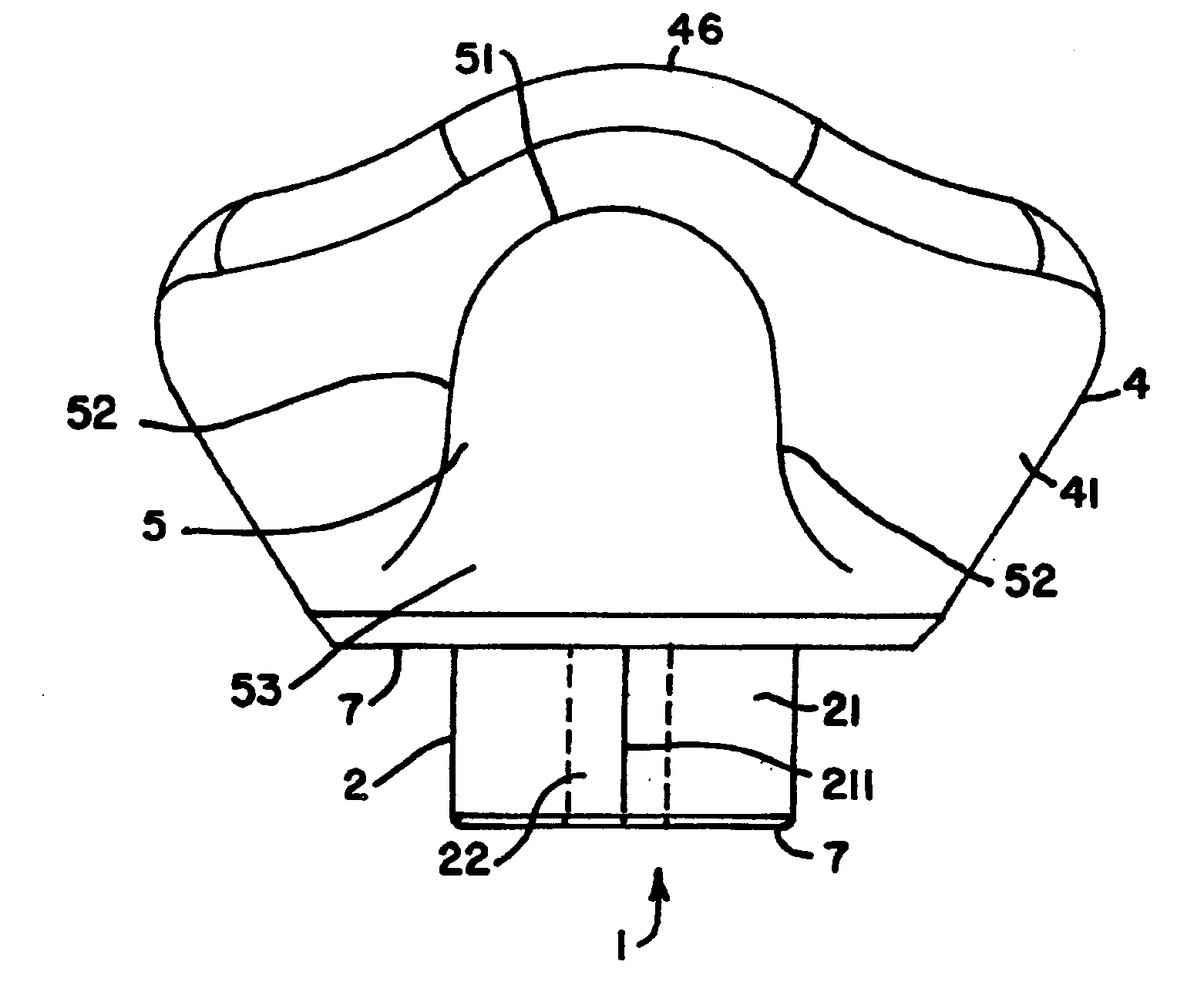
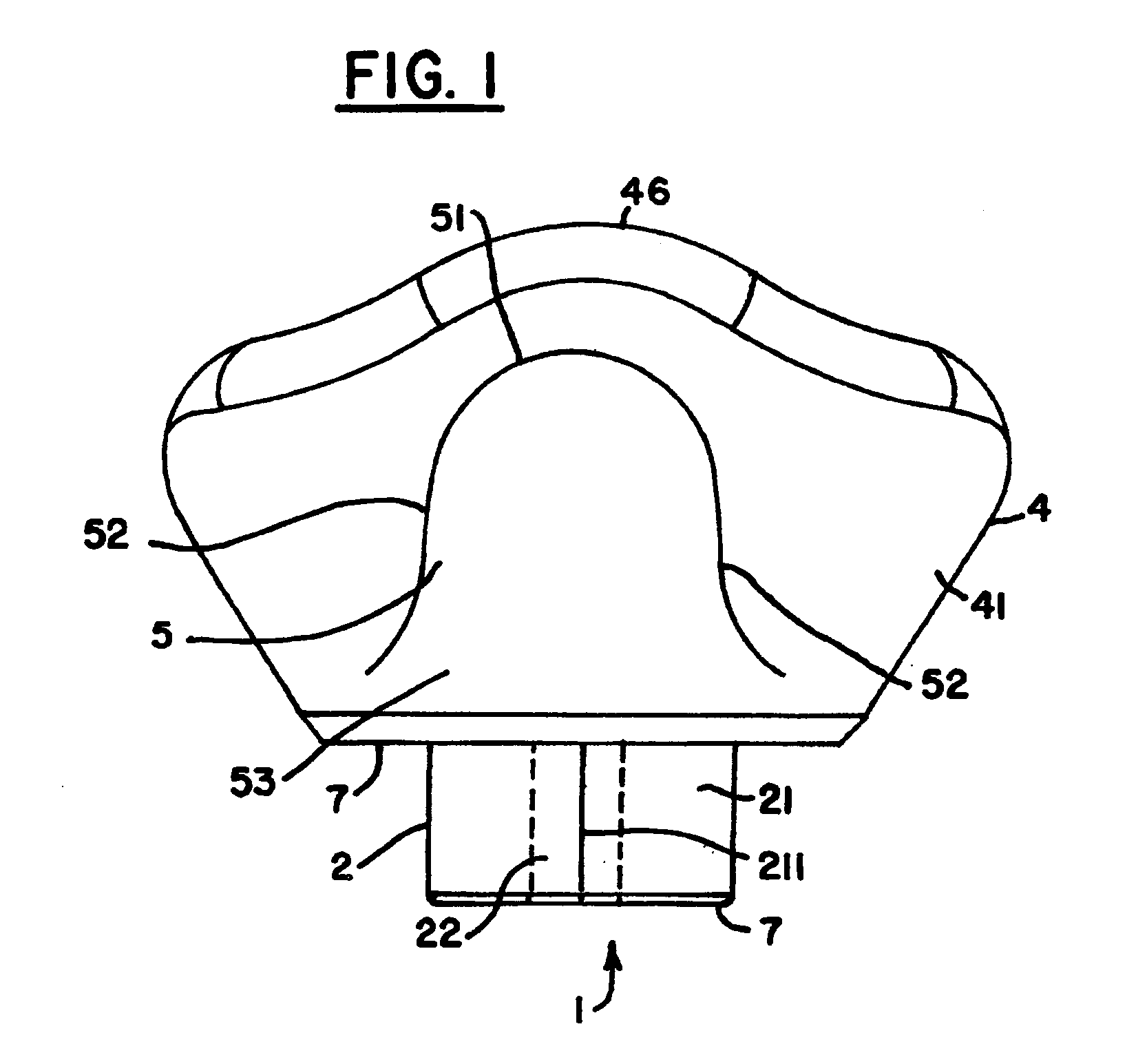
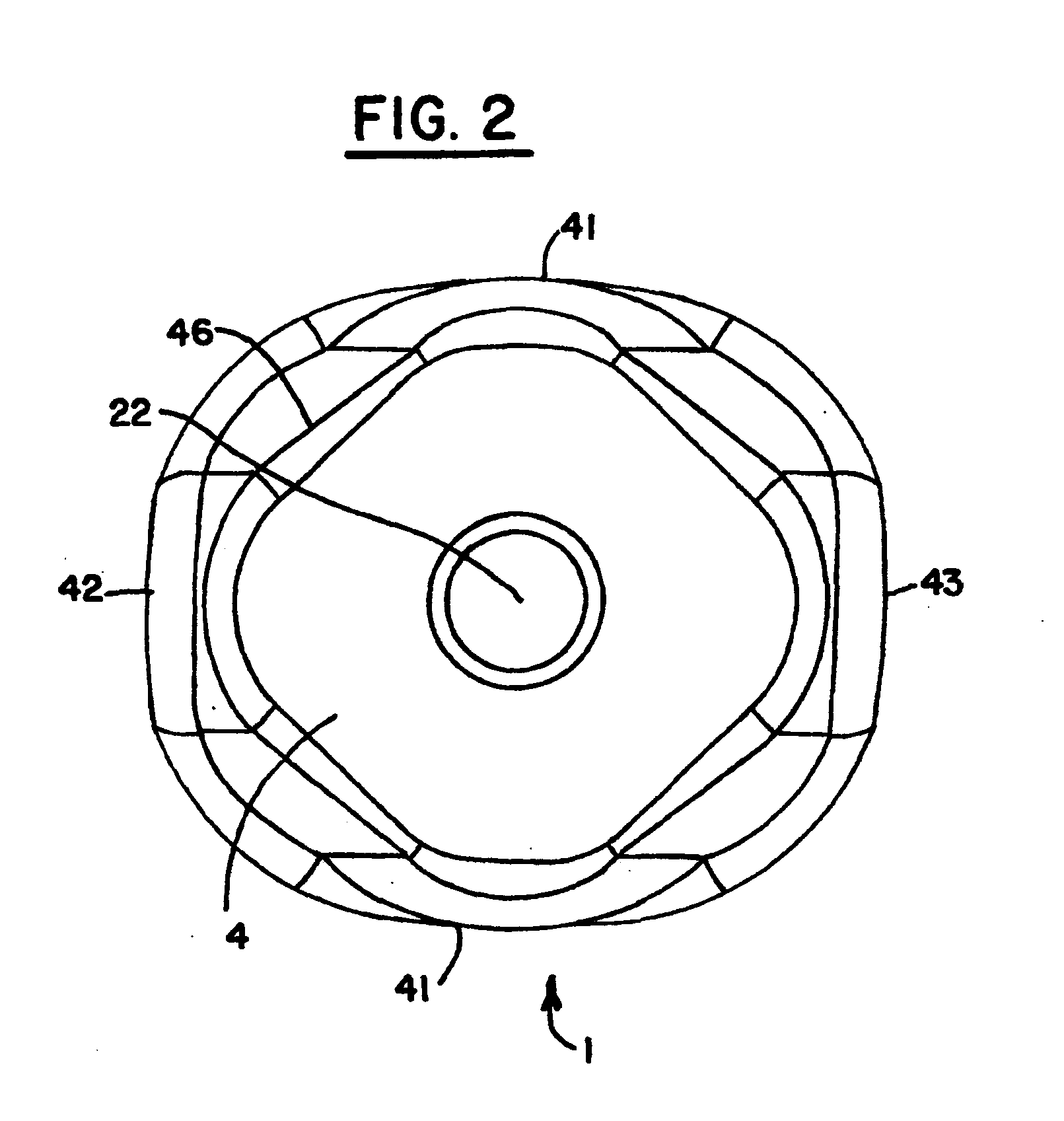
Biosynthetic composite for osteochondral defect repair
A composite for osteochondral defect repair includes a porous scaffold and a periosteal graft secured to a surface of the scaffold. The composite provides cartilage growth from autologous periosteum chondrogenesis. Biological resurfacing of large osteochondral defects, or a complete joint is feasible using the porous scaffold / autologous periosteal composite. The use of this composite eliminates the necessity of using normal cartilage surface as a donor site and its respective associated morbidity. In one form, the strong bone integration capacity of a porous metal (e.g., tantalum) scaffold and the high grade of integration observed from periosteal chondrogenesis into the normal cartilage eliminates the lack of chondral-chondral integration observed in the autologous osteochondral graft technique.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
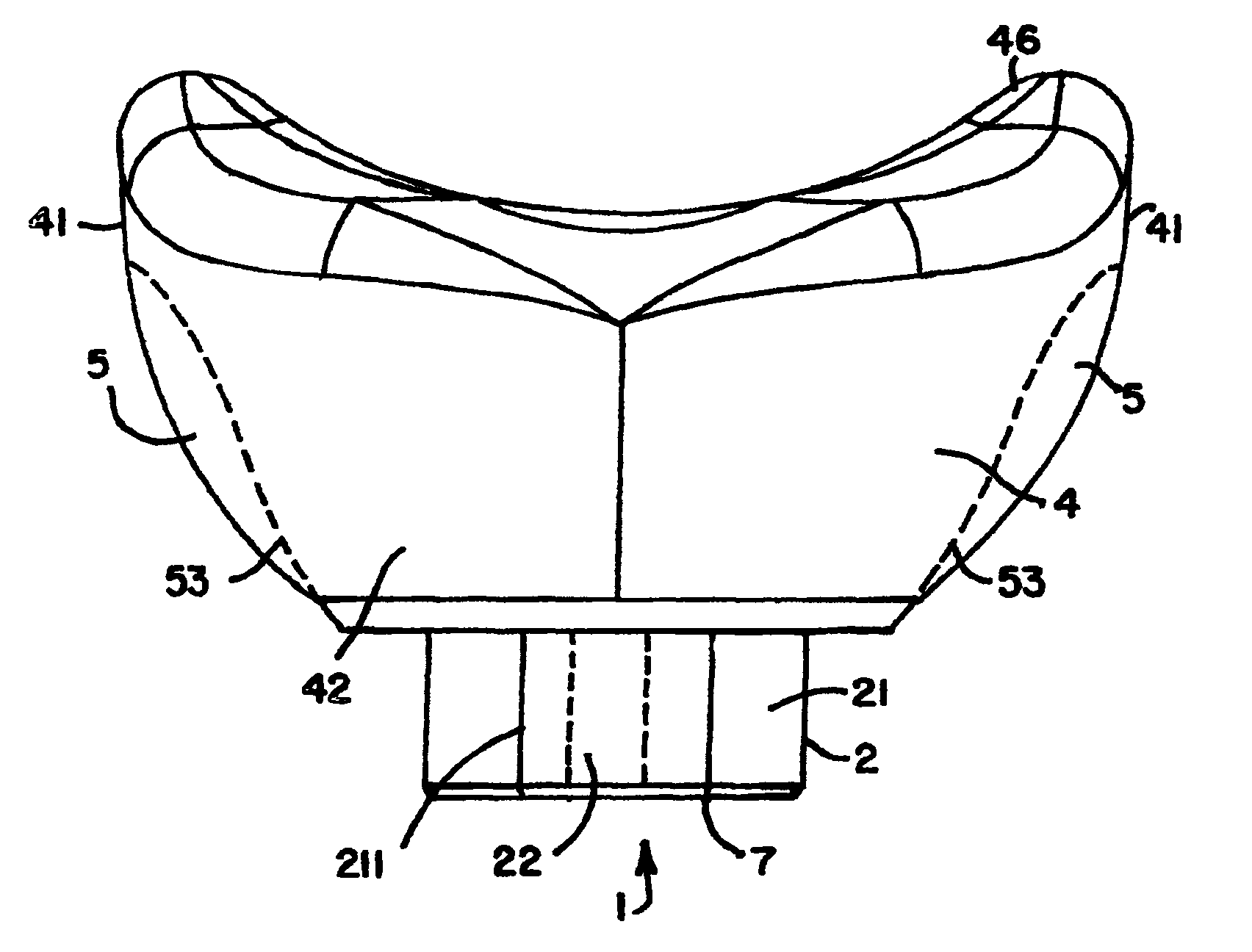
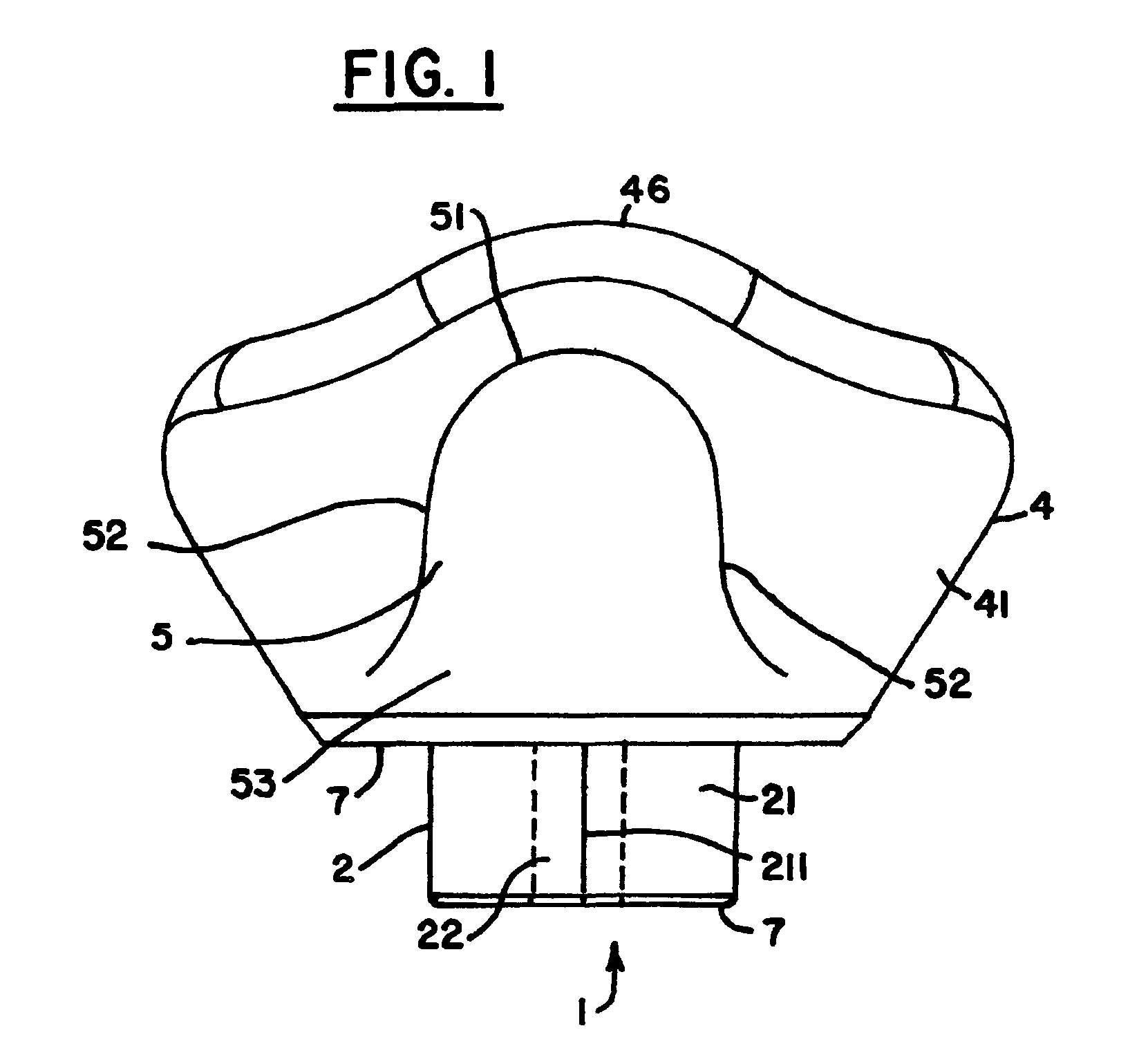
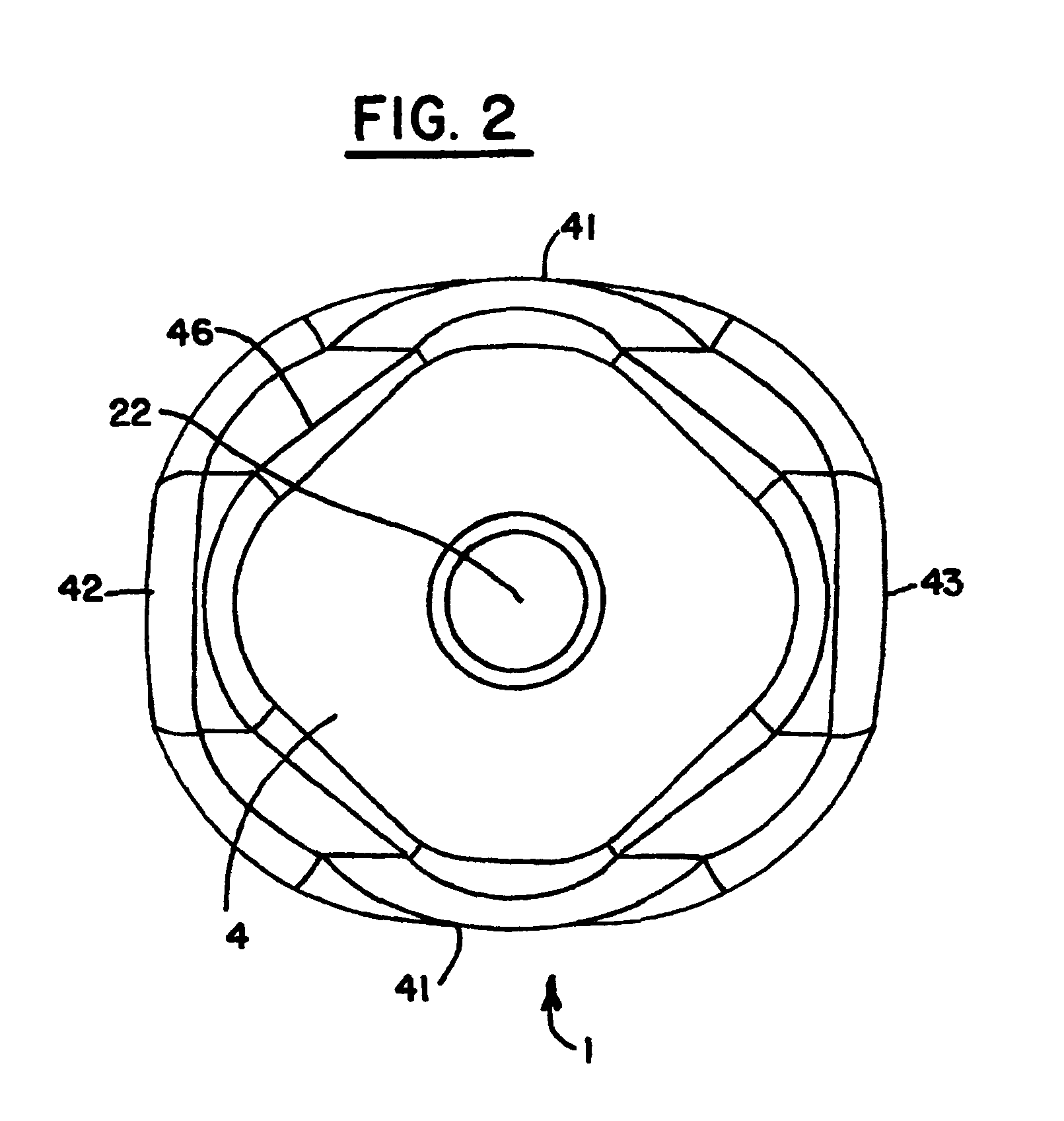
Healing abutment system for bone contouring
ActiveUS20120264081A1Improve aesthetic resultAvoid poor resultsDental implantsExtraction siteBone profile
The present invention uses an anatomically shaped bone graft contouring abutment in place of a conventional healing abutment to control the bone graft for optimal height. The extraction site aesthetics, in the form of enhanced gingival growth, are facilitated by the optimal bone graft placement.
Owner:PHILIBIN TERRY B
Healing abutment system for bone contouring
The present invention uses an anatomically shaped bone graft contouring abutment in place of a conventional healing abutment to control the bone graft for optimal height. The extraction site aesthetics, in the form of enhanced gingival growth, are facilitated by the optimal bone graft placement.
Owner:PHILIBIN TERRY B
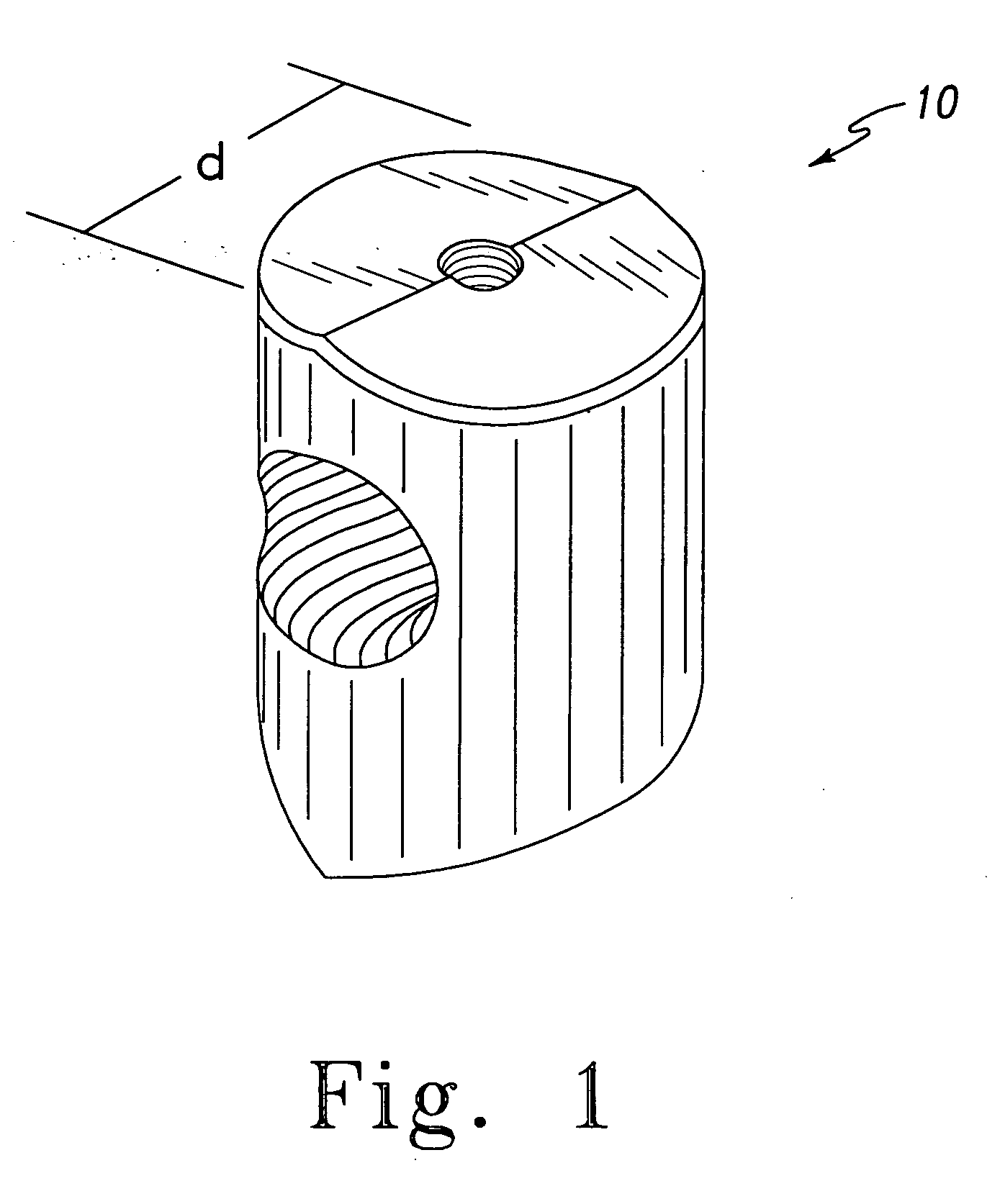
Injectable-porous-drug loaded polymethyl methacrylate-based composite scaffold bone transplant material and preparation method thereof
ActiveCN104906637AEasy to prepareGood biocompatibilityProsthesisReaction temperaturePolymethyl methacrylate
The present invention discloses an injectable-porous-drug loaded polymethyl methacrylate-based composite scaffold bone transplant material and a preparation method thereof, and belongs to the field of organic functional materials preparation. The polymethyl methacrylate-based composite scaffold bone transplant material uses polymethyl methacrylate (PMMA) as a scaffold for providing mechanical supporting, and a chitosan-based thermosensitive hydrogel as a pore forming agent and an osteoconductive material and a drug carrier, and the polymethyl methacrylate (PMMA) and the chitosan-based thermosensitive hydrogel are mixed with each other to form an injectable-porous three-dimensional structural bone cement composite. The scaffold bone transplant material is simple in preparation method, suitable in reaction temperature, and good in biocompatibility, has matched mechanical properties, good biological mineralization and corresponding anti-bacterial, anti-inflammatory or anti-tumor capabilities, and has broad prospects in clinical application of reconstruction of bone tissues in future.
Owner:WUHAN UNIV
Methods of injecting calcium based neutral and bioresorbable bone grafts
InactiveUS20090318982A1Reduce decreaseImprove mechanical propertiesSurgical adhesivesBone implantOrthopedic departmentTri calcium phosphate
Owner:BERKELEY ADVANCED BIOMATERIALS
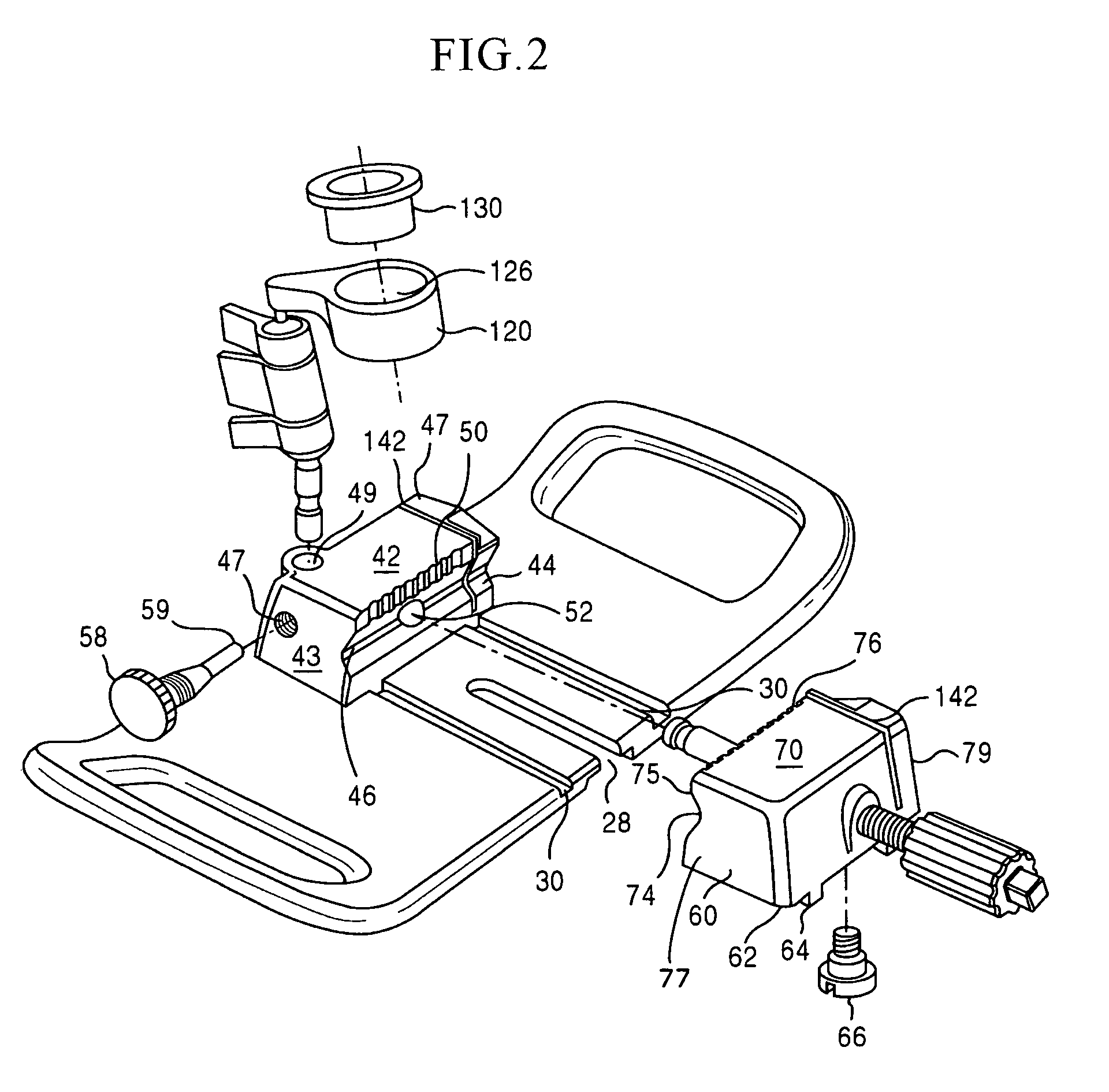
Osteochondral allograft cartilage transplant workstation
ActiveUS7780668B2Easy to useSmall sizeSurgeryDrawing boardsReoperative surgeryBiomedical engineering
A portable surgical workstation for implant formation comprising a base with a central planar section. The central planar section has a plurality of tracks and a throughgoing slot with a recessed stepped surrounding surface formed on a bottom surface of the central planar section. A vise assembly mounted to the base comprises a fixed jaw member secured to the base, a traveling jaw member moveably mounted to the base and a fixed drive housing mounted to the base. The traveling jaw member has a plurality of rail members adapted to be slidably mounted in the central planar section tracks. The fixed drive housing has a threaded longitudinal bore which receives a threaded drive shaft, one end of the drive shaft being secured in the traveling jaw member to transport the traveling jaw member.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Percutaneous posterior lateral in-situ cage
Implants, tools and techniques facilitate a percutaneous posterior lateral approach to the placement of an in-situ cage, and an inventive cage design to meet this objective. In terms of apparatus, the invention includes a laterally expandable cage, including a locking gate, enabling the system to be introduced into an intradiscal space through a minimally invasive percutaneous posteo-lateral approach. In addition to the cage designs, adapted to hold bone graft and / or other biologic materials, the invention includes other novel instruments, including an introducer associated with cage placement, deployment and closure.
Owner:CTL MEDICAL CORP
Intervertebral implant and bone graft inserter
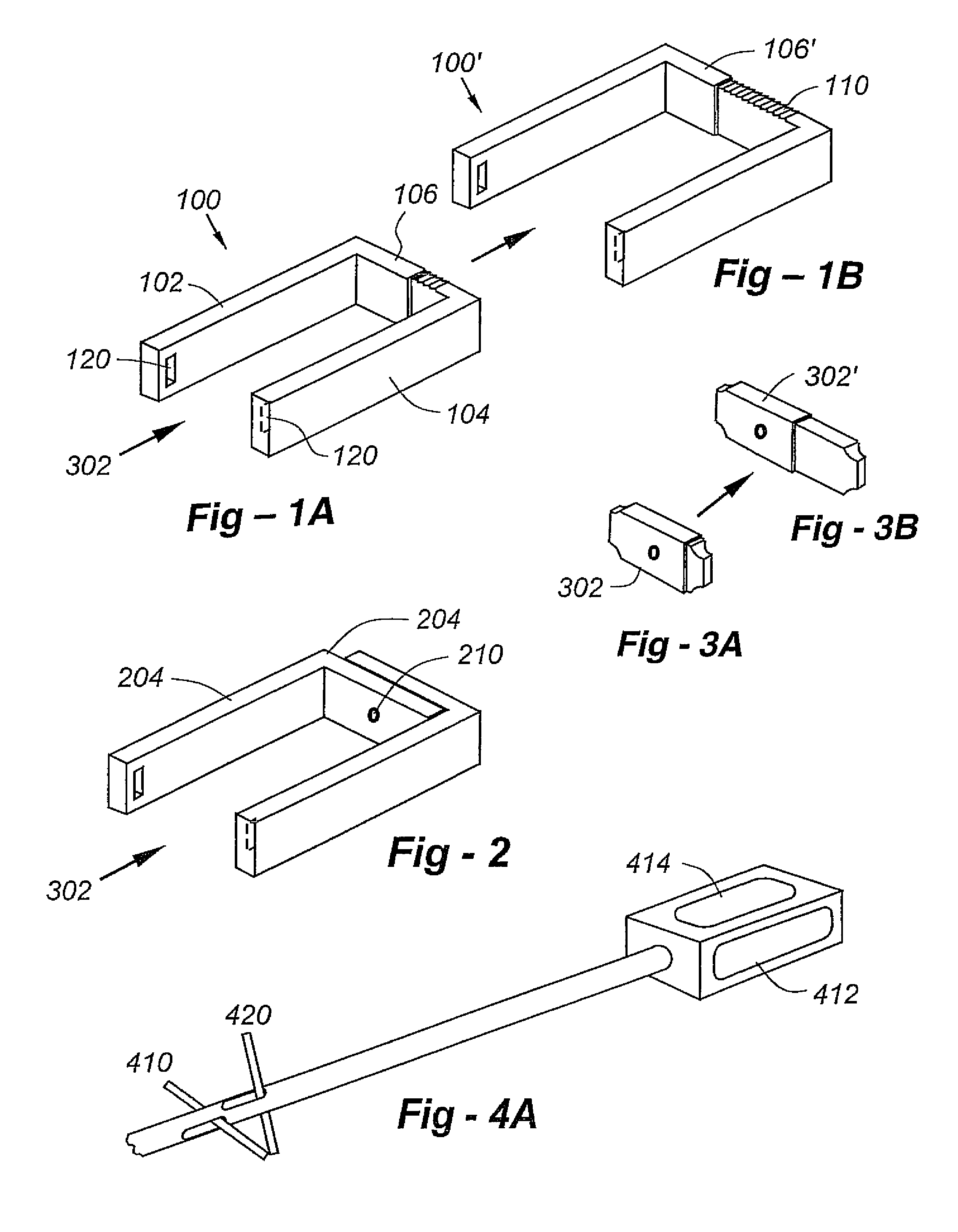
An adjustable spinal fusion intervertebral implant is provided that can comprise upper and lower body portions, and proximal and distal wedges. An actuator shaft disposed intermediate the upper and lower body portions can be actuated to cause proximal and distal wedges to converge towards each other. The implant comprises one or more channels that interact with a bone graft inserter to direct the flow of material through the implant. The bone graft inserter and methods of use are also provided.
Owner:DEPUY SYNTHES PROD INC
Multi-phase large-aperture bone regeneration bracket material transformed from cuttlebone and preparation method thereof
InactiveCN101987208AGood biocompatibilityWide variety of sourcesProsthesisSurgical operationBone conduction hearing
The invention discloses a large-aperture bone regeneration bracket material and a preparation method thereof, and belongs to the technical field of medical biomimetic material engineering. A natural porous inorganic biological material (cuttlebone) and ammonium dihydrogen phosphate undergo a full reaction at high temperature under high pressure so as to be transformed into a multi-phase biological ceramic (consisting of calcium carbonate, hydroxyapatite and calcium phosphate). The preparation method for the material is simple in process, the material source is abundant, and the cost is low. In addition, the prepared material has good biological compatibility and high compression strength, and is environmentally-friendly. The bone cell experiment shows that the material has bone conduction activity, can promote and induce bone regeneration self-repair, can be degraded step by step in vivo, is an ideal bone transplant replacement material, and has wide application range and obvious social and economic benefits in bone surgical operation.
Owner:NINGBO UNIV
Controlled-release antibiotic nanoparticles for implants and bone grafts
InactiveUS20130209537A1Good medicineEasily integrated onto surfaceBiocideSolar heating energyControlled releaseNanoparticle
The present invention relates to the preparation and use of antibiotic-containing nanoparticles for coating an implant including cranial implants and bone graft sites to provide for the extended release of antibiotics to treat infection.
Owner:GOVERNORS STATE UNIVERSITY
Single tunnel, double bundle anterior cruciate ligament reconstruction using bone-patellar tendon-bone grafts
ActiveUS9011533B2Accurate anatomical reconstructionGreat easeSuture equipmentsInternal osteosythesisTibial bonePosterolateral bundle
Anterior cruciate ligament reconstruction methods and devices are designed to achieve an anatomically accurate double bundle anterior cruciate ligament reconstruction by using a single femoral and tibial tunnel. The method and devices reconstruct the two bundles of the anterior cruciate ligament in a single femoral and tibial tunnel using a bone-patellar tendon-bone graft. The methods and devices enable an accurate anatomical reconstruction of the anteromedial and posterolateral bundles by creating a single femoral and tibial tunnel as opposed to creating two tunnels in the tibia and femur.
Owner:THE GENERAL HOSPITAL CORP
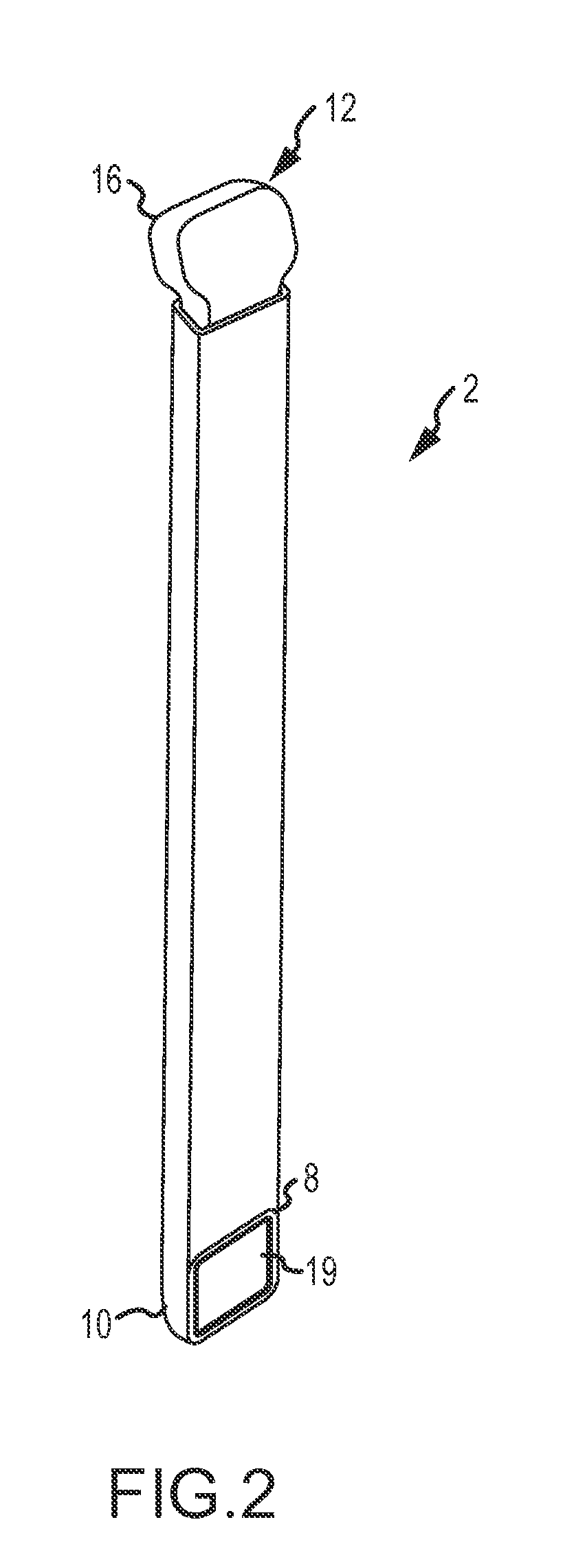
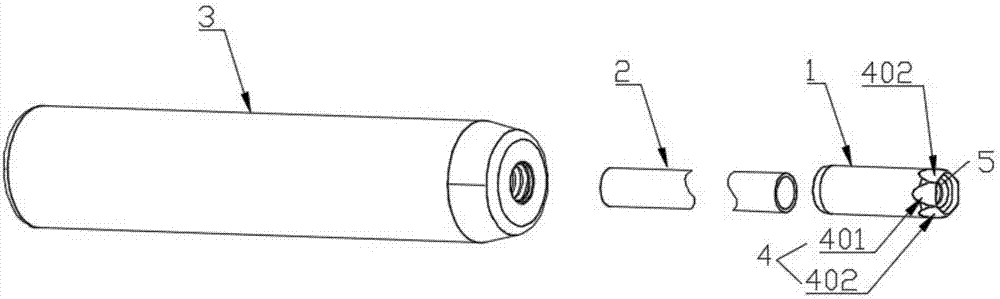
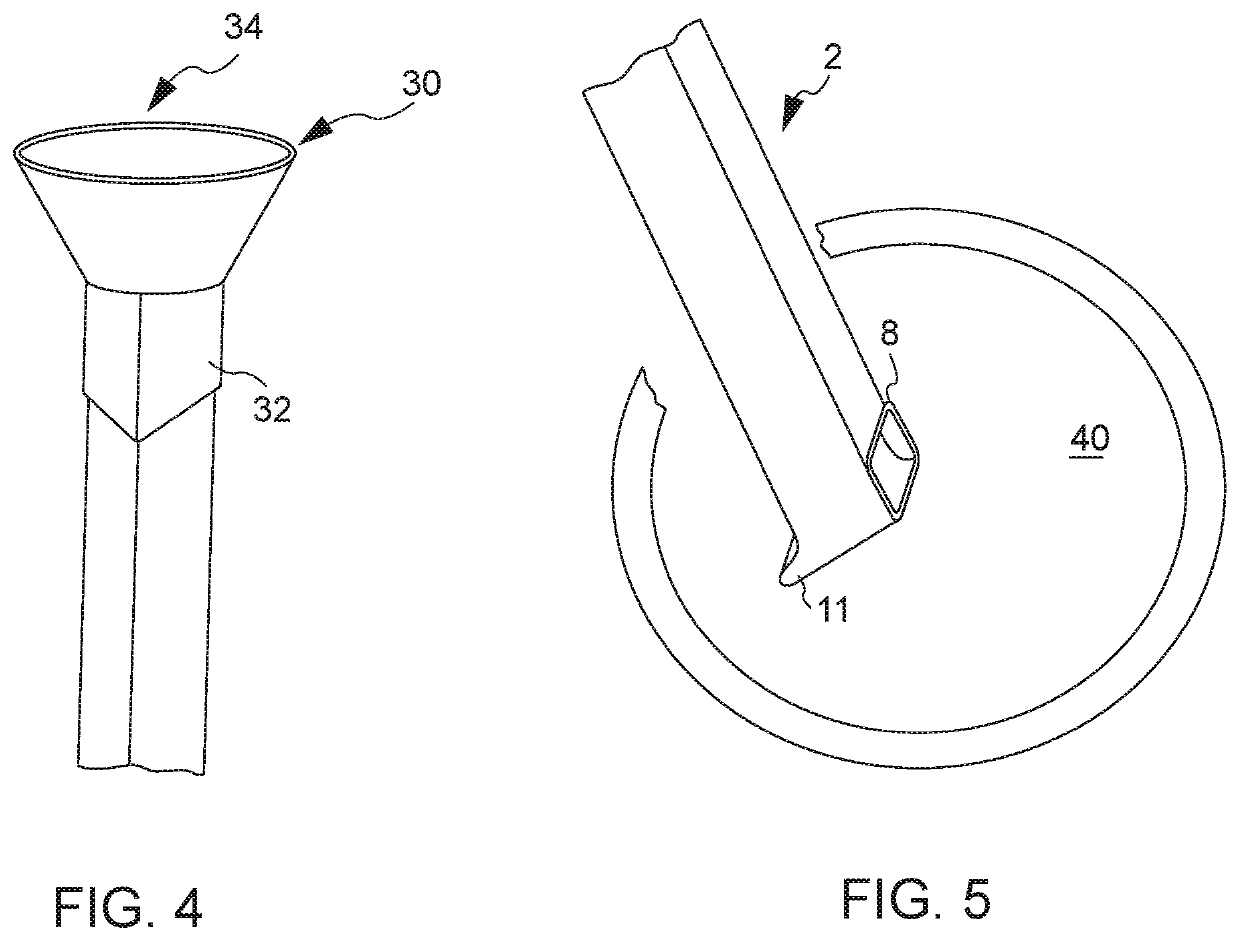
Tubular tool for acquiring human vein transplant in minimally invasive manner
The invention provides a tubular tool for acquiring a human vein transplant in a minimally invasive manner. The tubular tool comprises a tubular tool bit, a tunnel tube and a handle which are sequentially connected and is characterized in that one end of the tool bit is an edge; the inner wall of the tool bit is provided with an annular protruding part which is close to the side, with the edge, ofthe tool bit. The tubular tool has the advantages that the vein transplant can be acquired in an minimally invasive manner, a large skin cut is avoided, and the tubular tool is fast to operate, simple, practicable, capable of saving time and labor and capable of greatly reducing medical treatment cost and the wound of a patient; a long skin cut and a large subcutaneous tissue wound are avoided, the tubular tool rotates and moves forwardly to separate the vein from peripheral tissue and cut off vein peripheral collateral branches while the main part of the vein transplant is kept intact, the main part of the vein transplant is guided into the tunnel tube, the vein is cut off and the transplant is taken out after required acquiring length is reached, and a minimally invasive effect is guaranteed.
Owner:高峰
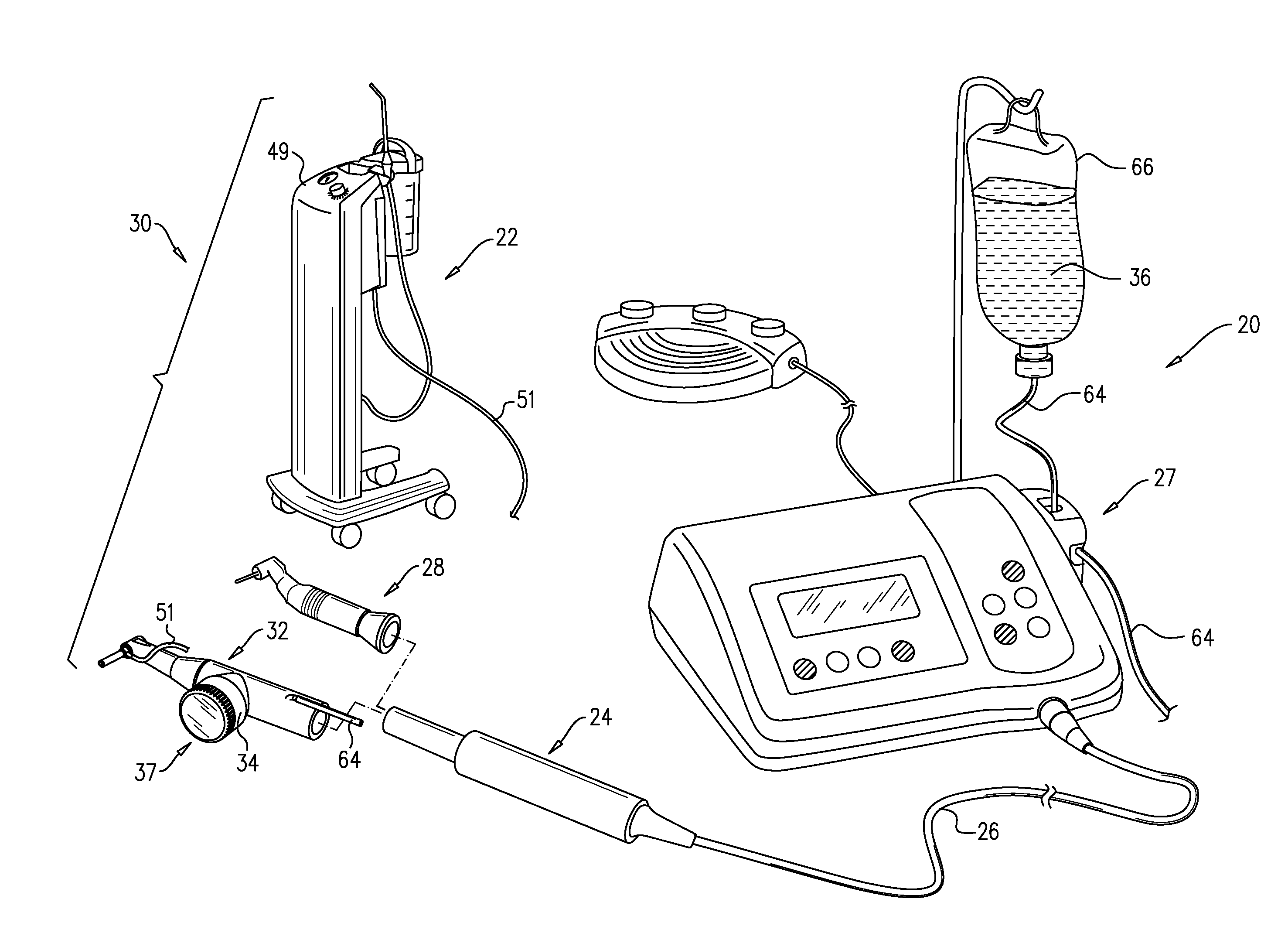
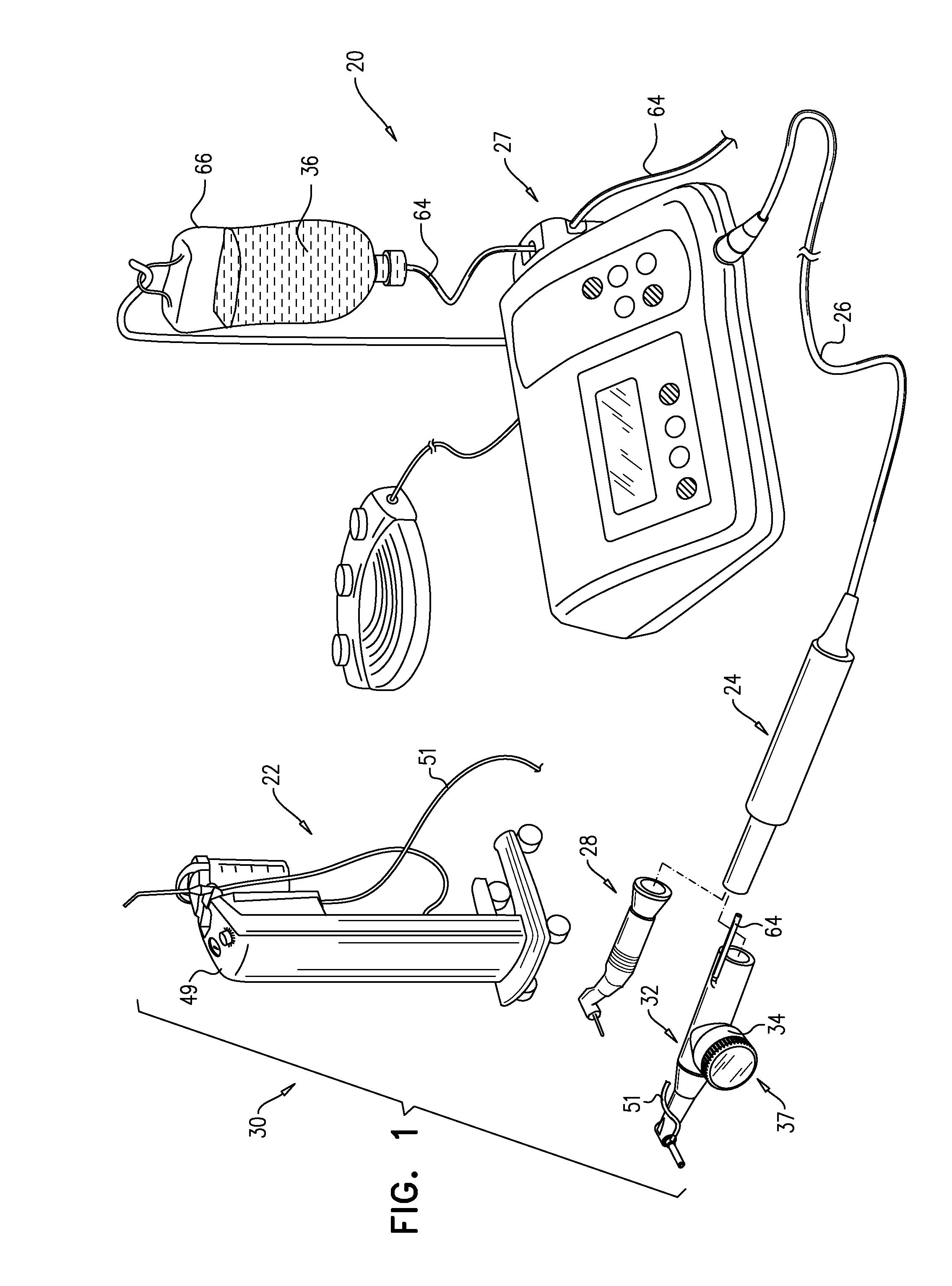
Bone graft injection methods
InactiveUS9730773B2Reduce riskAugmenting the maxillary alveolar ridgeDental implantsDiagnosticsPhysiological fluidAnatomy
A method is provided that includes injecting, from a first side of a bone, through (a) exactly one bore that passes through the bone from the first side to a second side of the bone, and (b) into a cavity adjacent to the second side of the bone, a solid-liquid composition of solid particles and a physiological liquid solution. The physiological liquid solution is drained from the cavity and through the bore while passage of the solid particles of the solid-liquid composition is inhibited, such that the solid particles accumulate in the cavity.
Owner:MAXILLENT
Bone graft delivery system and method for using same
ActiveUS20190224024A1Precise positioningEasy to apply pressureBone implantJoint implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectively detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area. In one embodiment, the integrated fusion cage is an expandable integrated fusion cage.
Owner:SPINAL SURGICAL STRATEGIES INC
Bone graft delivery system and method for using same
ActiveUS10973656B2Easy to apply pressureFacilitate controlled movementBone implantJoint implantsSpinal columnSurgical site
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectively detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area. In one embodiment, the integrated fusion cage is an expandable integrated fusion cage.
Owner:SPINAL SURGICAL STRATEGIES INC
Intraoral combined tooth positioning blocky bone extraction and transplantation whole-course guide plate and manufacturing method thereof
ActiveCN112842587AAccurate placementReduce stepsDental implantsSurgical navigation systemsReoperative surgeryTooth position
The invention relates to an intraoral combined tooth positioning blocky bone extraction and transplantation whole-course guide plate and a manufacturing method thereof, a bone supply area tooth positioning guide plate is connected with a positioning and trimming reference block, and the bone supply area tooth positioning guide plate is used for setting a plurality of positioning points for a bone supply area after being connected with the positioning and trimming reference block; a bone taking guide plate can be connected with the bone supply area tooth positioning guide plate and the positioning and trimming reference block, can be fixed to the bone supply area according to a plurality of positioning points, and is used for cutting out blocky bones; a bone receiving area tooth positioning guide plate can be connected with the positioning and trimming reference block through a slot, the positioning and trimming reference block is the same as a blocky bone in shape, a plurality of positioning points corresponding to the position of the bone supply area are arranged in the bone receiving area through the positioning and trimming reference block, and the obtained blocky bone can be transplanted to the bone receiving area; the intraoral autologous blocky bone can be accurately and safely obtained in a minimally invasive mode, guidance of the whole process of bone taking, bone grafting and implant implanting can be provided for doctors, the operation difficulty is reduced, and the doctors are assisted in completing the whole operation more accurately, efficiently and safely.
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY
Bone graft injection device
InactiveUS20160310243A1Increase heightReduce riskDental implantsMixing methodsMedicineSolid particle
Apparatus is provided that includes a surgical tool for use with solid particles and a physiological liquid solution. The surgical tool includes (a) a shaft unit, which is shaped so as to define a delivery lumen, and a distal opening, which is disposed within 10 mm of a distal end of the shaft unit, in fluid communication with the delivery lumen; (b) a composition source, which is coupled in fluid communication with the delivery lumen, and which is configured to provide a solid-liquid composition of the solid particles and the physiological liquid solution; and (c) a pump, which is configured to pump the solid-liquid composition through the distal opening via the delivery lumen.
Owner:MAXILLENT
Calcium containing chitosan stent material
The invention discloses a scaffold material of chitosan calcic liquid colloid. The scaffold material of the invention is prepared by the steps that chitosan particles precisely weighed and sub-packaged are radiated for 1000min by 10K Gy Co<60> for sterilization; 0.1 percent of acetic acid is served as a solvent to be made into 1.2 percent of water solution; 0.108g of tricalcium phosphate powder and 5 percent of gelatin granules are added and evenly blended under the constant stirring by a magnetic rotor in the water bath of 60 DEG C; 1M NaOH solution titration is added until the pH is 7 after the still standing and cross-linking for 1.5h. The scaffold material of chitosan calcic liquid colloid of the invention is used for being injected into a bone bioreactor at the subperiosteum cavity in a human body to induce osteogenesis and has good biocompatibility, strong flexibility, good quality of osteogenesis, low cost and great potential in bone transplant with simple operation without being cultured by external cells and adding cell factors or growth factors, is absorbable in the human body, and also ensures no risks of infection, immune rejection and the like. In addition, the end product of the metabolism of glucose can also provide energy for series osteoblastic responses. Therefore, the invention provides the good scaffold material for the bone bioreactor.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Bone graft composition, method and implant
ActiveUS8690957B2Improve stabilityHigh strengthMaterial nanotechnologyBone implantBone regenerationNanofiber
A bone regenerative composition includes a resorbable osteoconductive matrix and a multiplicity of substantially rigid nanofibers dispersed within structure of the matrix to impart structural integrity with nanofiber ends projecting out of a surface of the matrix to provide differential load bearing surface bristles.
Owner:WARSAW ORTHOPEDIC INC
Bone graft delivery system and method for using same
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectively detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area. In one embodiment, the integrated fusion cage is an expandable integrated fusion cage. In another embodiment, the bone graft material is loaded into a breech area in the hollow tube.
Owner:SPINAL SURGICAL STRATEGIES INC
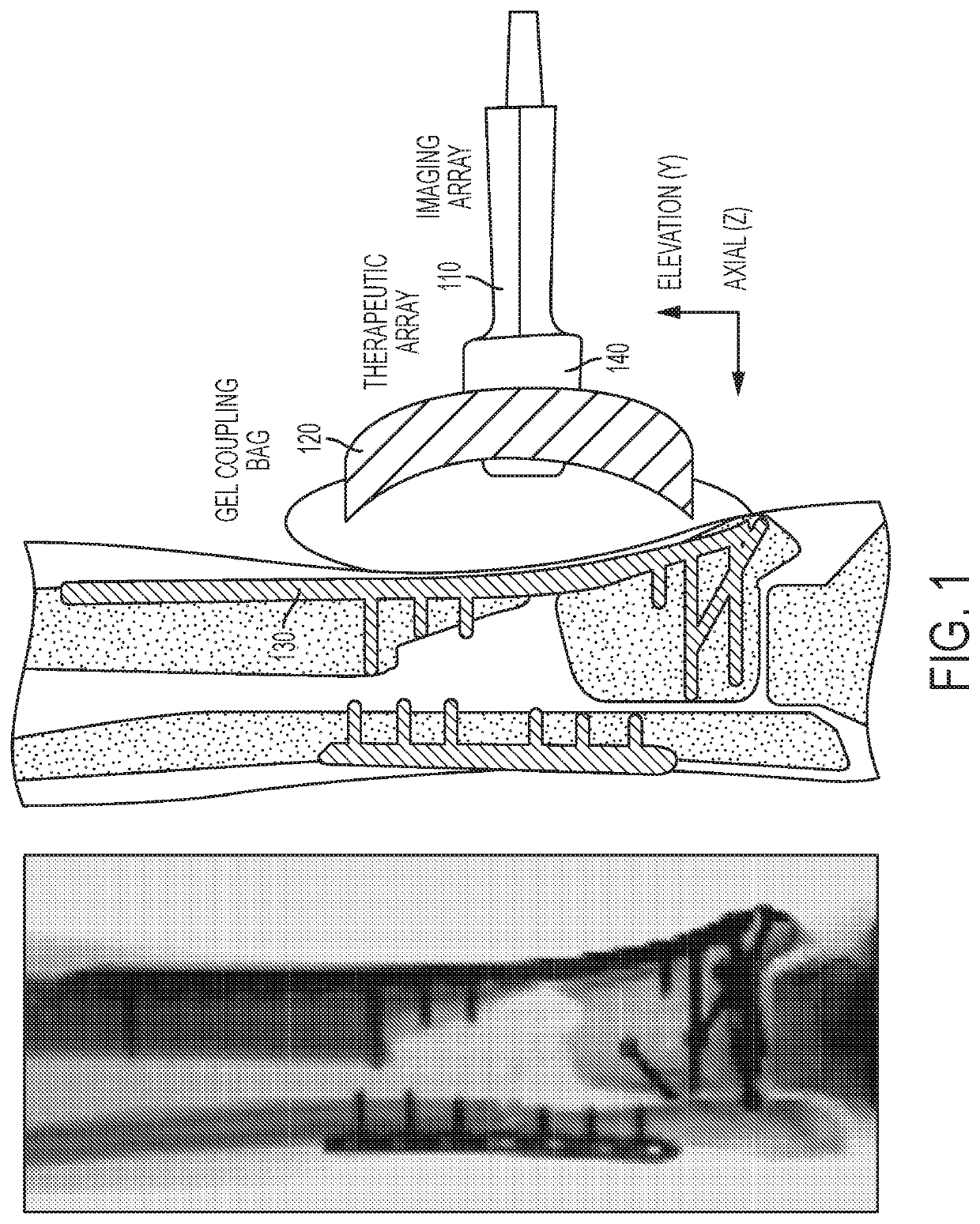
Novel transfection and drug delivery
ActiveUS20200078607A1Shorten recovery timeIncreasing patient comfortUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyUltrasonic imagingRadiology
An ultrasound transmitter device for treating a patient is provided. The ultrasound transmitter device includes an imaging probe; an imaging array; and a therapeutic ultrasound device, wherein the imaging probe is configured to guide the therapeutic ultrasound device to the patients treatment site by use of ultrasound imaging with the imaging array, wherein the therapeutic ultrasound device is configured to produce a controlled intensity of ultrasound energy for treating the patients treatment site, and wherein the imaging probe and the therapeutic ultrasound device are configured to work in conjunction with one another to apply therapeutic ultrasound to tissue or bone graft sites in the patient.
Owner:CEDARS SINAI MEDICAL CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com