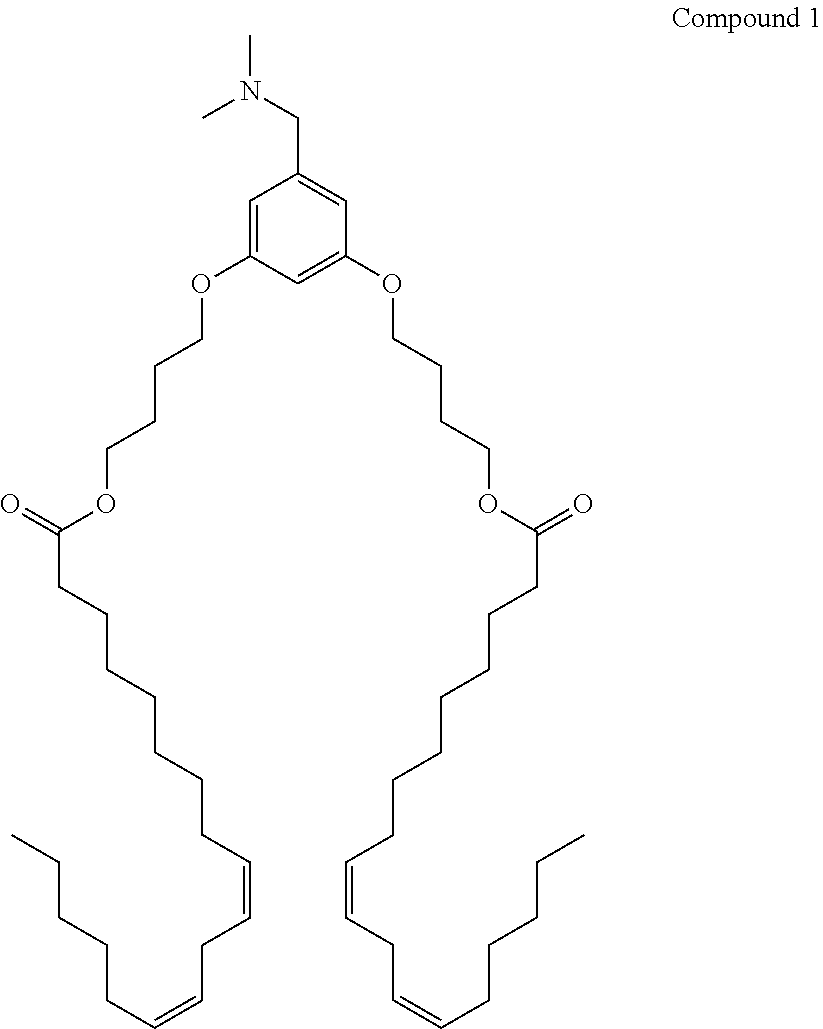
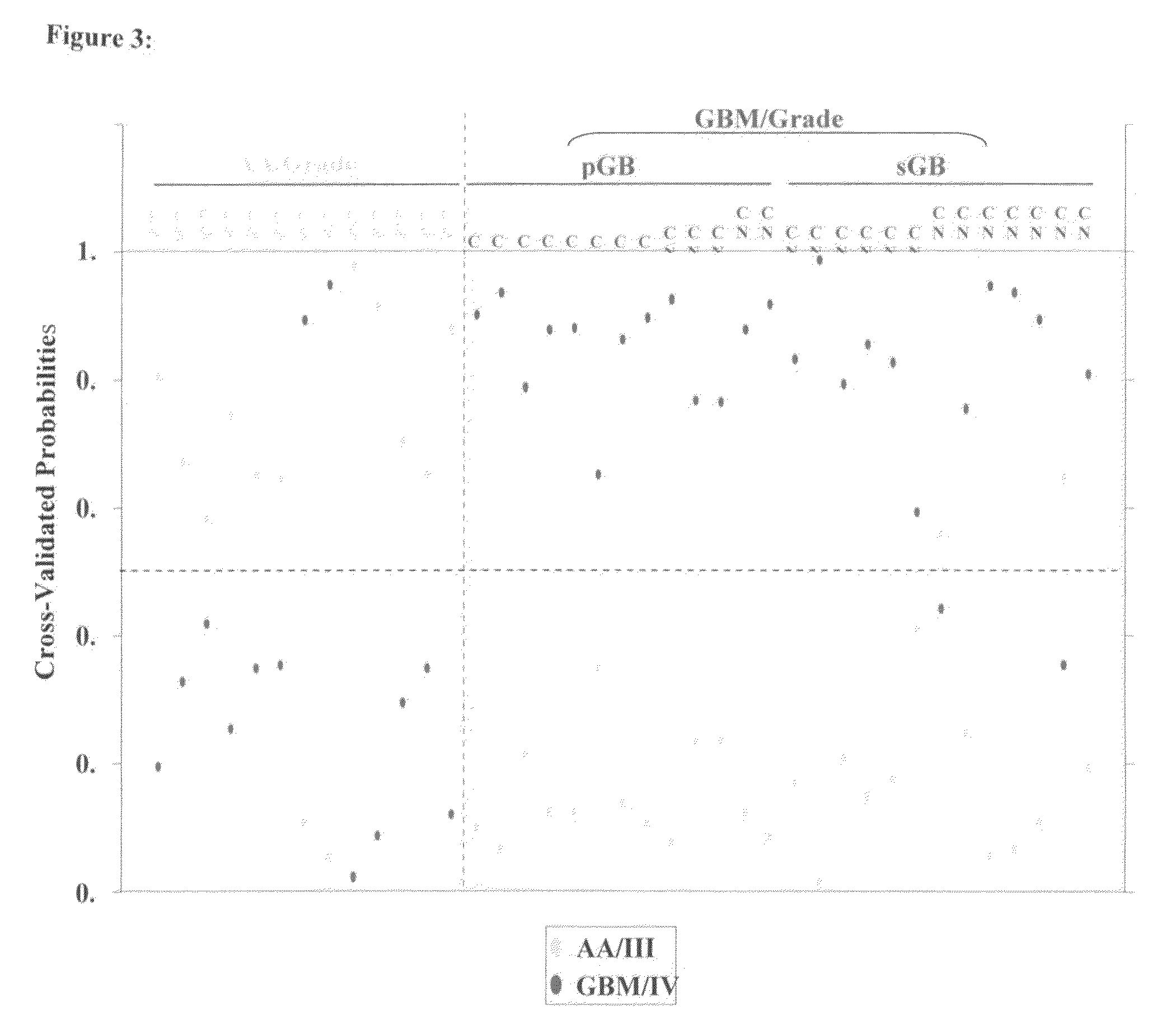Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Astrocytoma resection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
To date, complete resection of high grade astrocytomas is impossible because of the diffuse infiltration of tumor cells into normal parenchyma. Thus, high grade astrocytomas inevitably recur after initial surgery or therapy, and are usually treated similarly as the initial tumor.
Method and nucleic acids for the analysis of astrocytomas
InactiveUS20040115630A1Preferred pairing propertyEasy to detectBioreactor/fermenter combinationsBiological substance pretreatmentsCytosineOligomer
Owner:EPIGENOMICS AG
Methods for diagnosis of low grade astrocytoma
InactiveUS7279292B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsBiologyAntibody
A method for identifying low grade astrocytoma cells in a sample is provided, wherein the method distinguishes between low grade astrocytoma cells and normal astrocytes, thus permitting early diagnosis of astrocytoma. The method uses antibody directed against J1-31 to test astrocytes in the sample for the presence or absence of J1-31 polypeptide, the low grade astrocytoma cells being characterized by the absence of J1-31 while normal astrocytes are characterized by the presence of J1-31.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA +1
Method of diagnosing and treating gliomas
InactiveUS6870029B2Reduce current amplitudeSmall sizePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsGlioblastomaMonoclonal antibody
The present invention provides a recombinant toxin and monoclonal antibody which specifically binds to glial-derived or meningioma-derived tumor cells. Also provided are various methods of screening for malignant gliomas and meningiomas. Further provided are methods of treating malignant gliomas, including glioblastoma multiforme and astrocytomas.
Owner:UAB RES FOUND
Transgenic animals and cell lines for screening drugs effective for the treatment or prevention of Alzheimer's Disease
InactiveUS7226730B1Improve dementiaMicrobiological testing/measurementDisease diagnosisGerm layerMalignant astrocytoma
Disclosed are transgenic animals and transfected cell lines expressing a protein associated with Alzheimer's Disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas. Also disclosed is the use of such transgenic animals and transfected cell lines to screen potential drug candidates for treating or preventing Alzheimer's disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas. The invention also relates to new antisense oligonucleotides, ribozymes, triplex forming DNA and external guide sequences that can be used to treat or prevent Alzheimer's disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas.
Owner:THE GENERAL HOSPITAL CORP
Genetic alterations in isocitrate dehydrogenase and other genes in malignant glioma
InactiveUS20120202207A1Sugar derivativesMicrobiological testing/measurementLimonoate dehydrogenaseOligodendroglioma
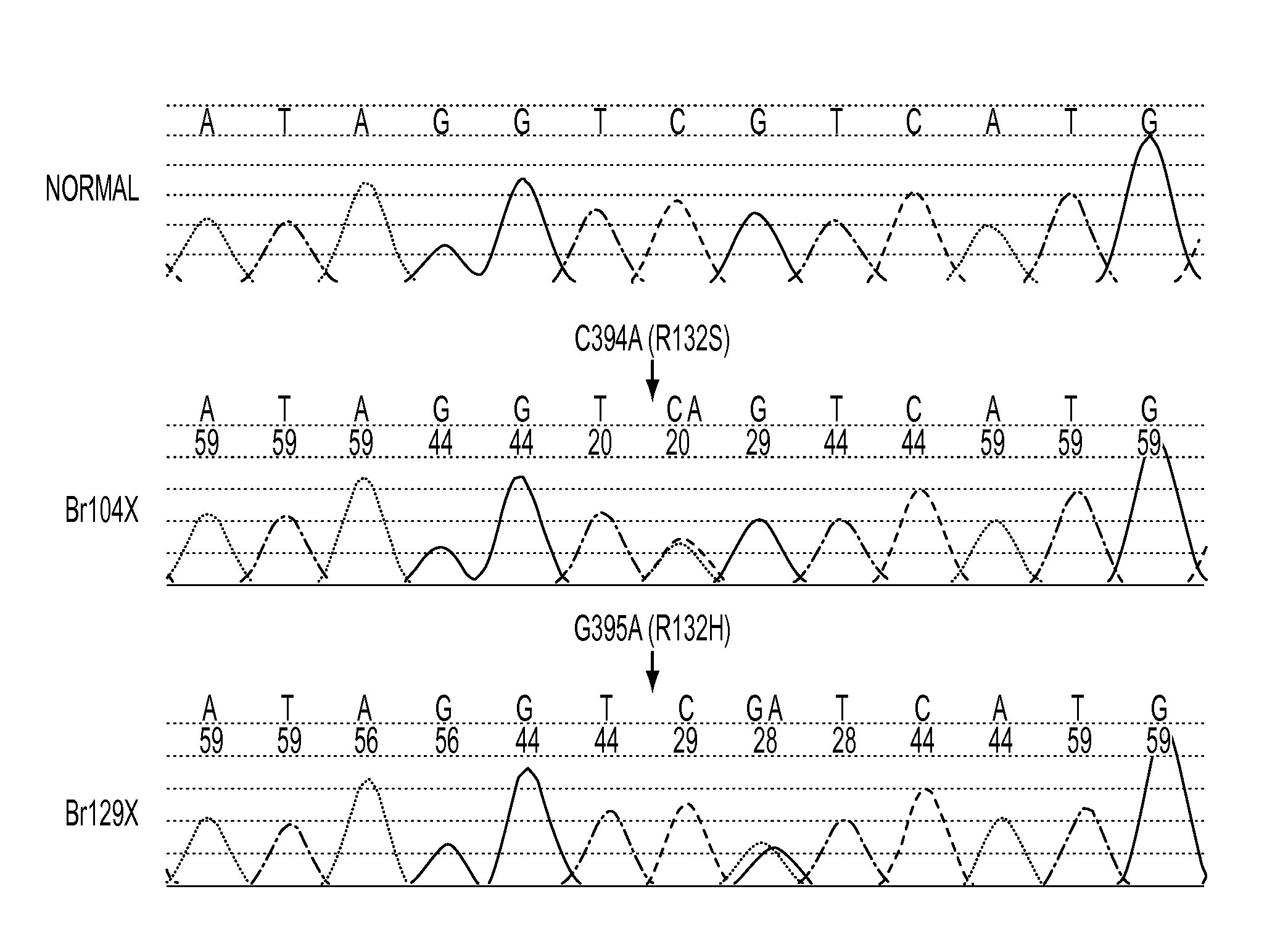
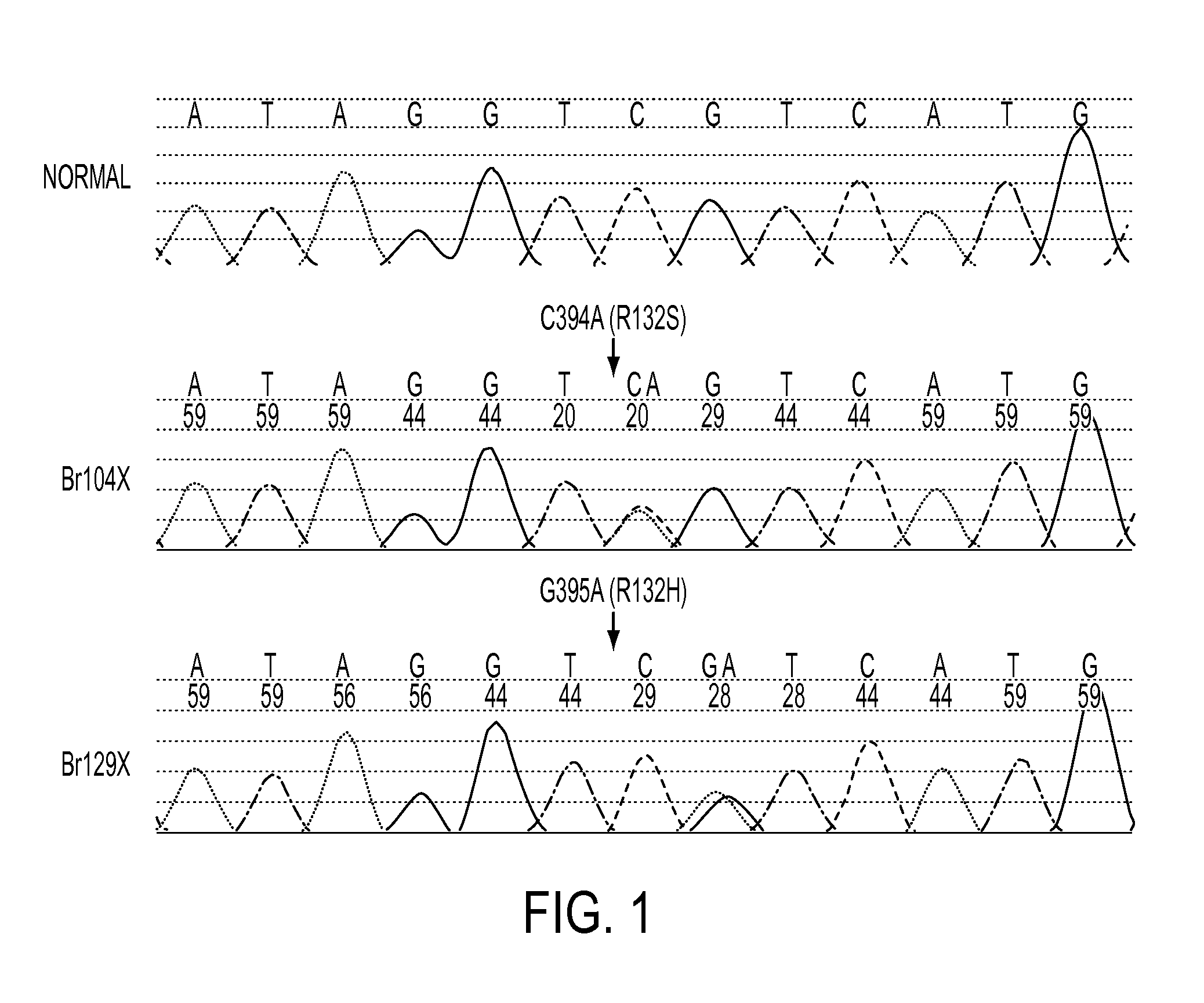
We found mutations of the R132 residue of isocitrate dehydrogenase 1 (IDH1) in the majority of grade II and III astrocytomas and oligodendrogliomas as well as in glioblastomas that develop from these lower grade lesions. Those tumors without mutations in IDH1 often had mutations at the analogous R172 residue of the closely related IDH2 gene. These findings have important implications for the pathogenesis and diagnosis of malignant gliomas.
Owner:DUKE UNIV +1
Glioma typing system based on IDH1-R132H and ATRX expression
The invention relates to a glioma typing system based on IDH1-R132H and ATRX expression. The system comprises: (1) glioma patients' tumor paraffin embedding kit and optional kit application instruction; (2) a kit for immunohistochemical detection of ATRX protein expression level of tumor of glioma patients and optional kit application instruction; and (3) a kit for immunohistochemical detection of IDH1-R132H protein expression in tumor of glioma patients and optional kit application instruction. When IDH1-R132H and ATRX losses are combined as diagnostic markers for distinguishing primary glioblastoma, WHO grading II / III oligodendroglioma, WHO grading II / III astrocytoma and secondary glioblastoma, diagnostic specificity is obviously higher than diagnostic specificity of the two single markers of IDH1-R132H or ATRX losses.
Owner:江涛 +2
Tumor diagnosis reagent or kit based on detection of IGFBP-2 (insulin-like growth factor binding protein 2) autoantibody or combined detection of IGFBP-2 antoantibody and IGFBP-2, as well as application thereof
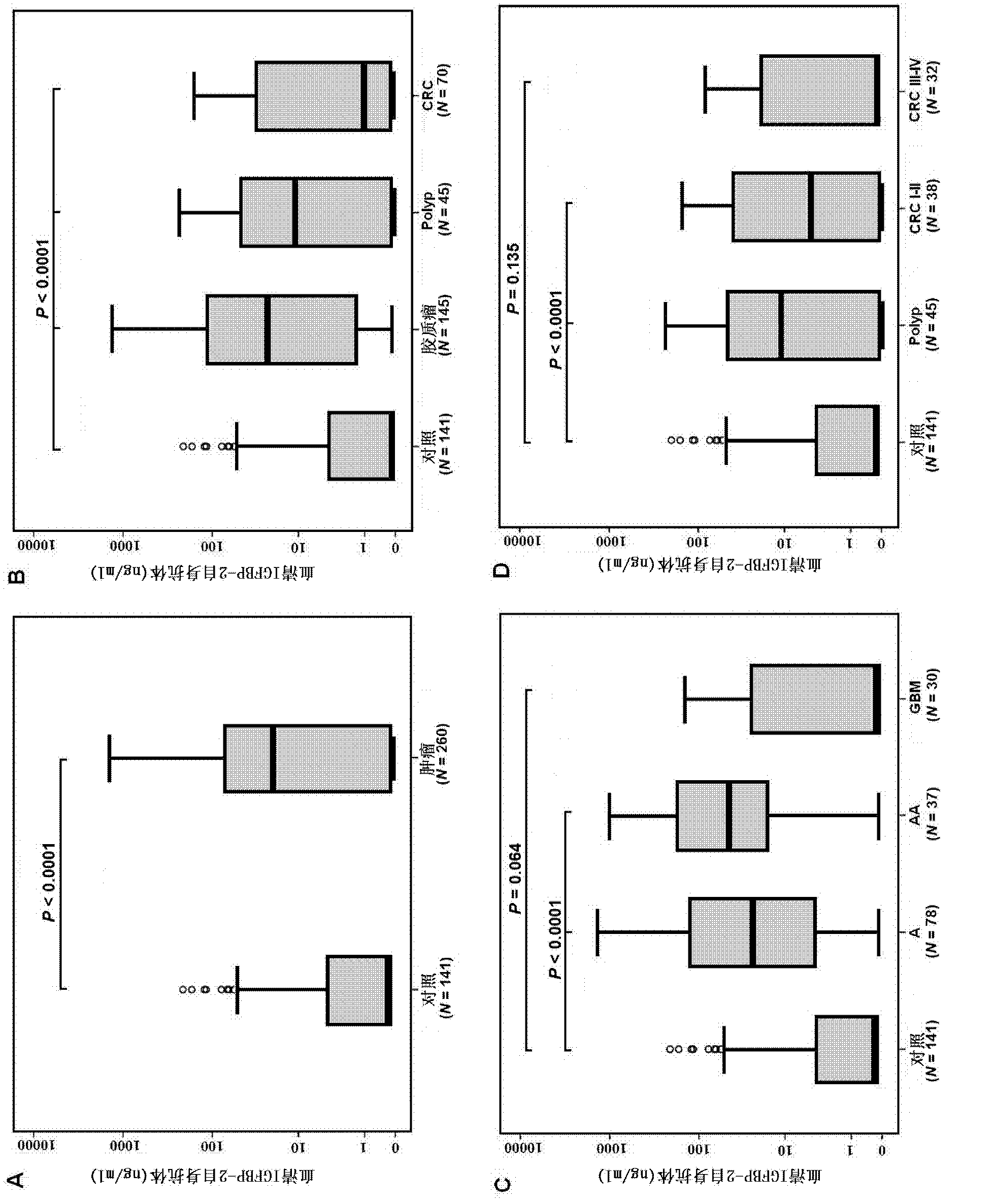
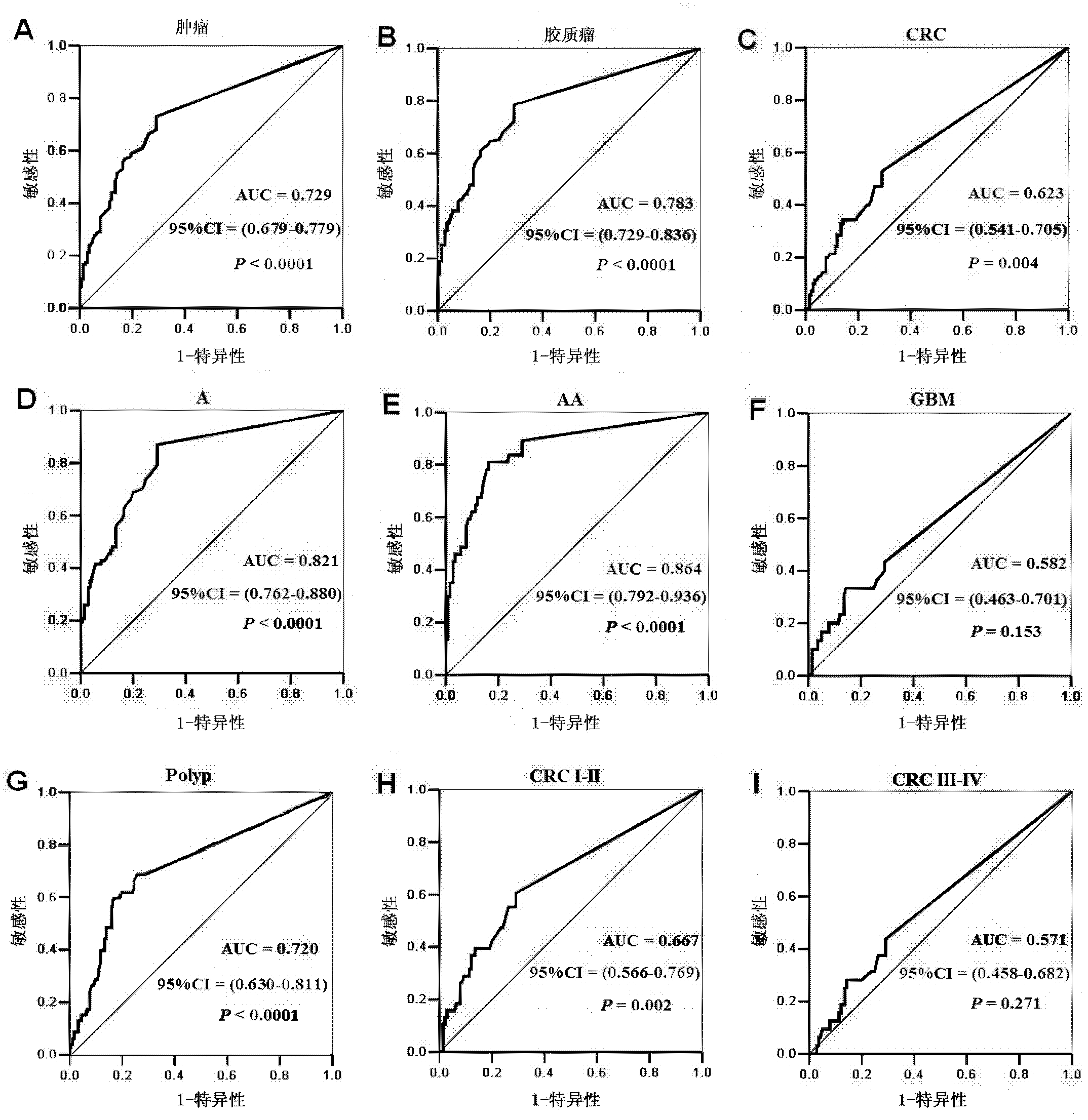
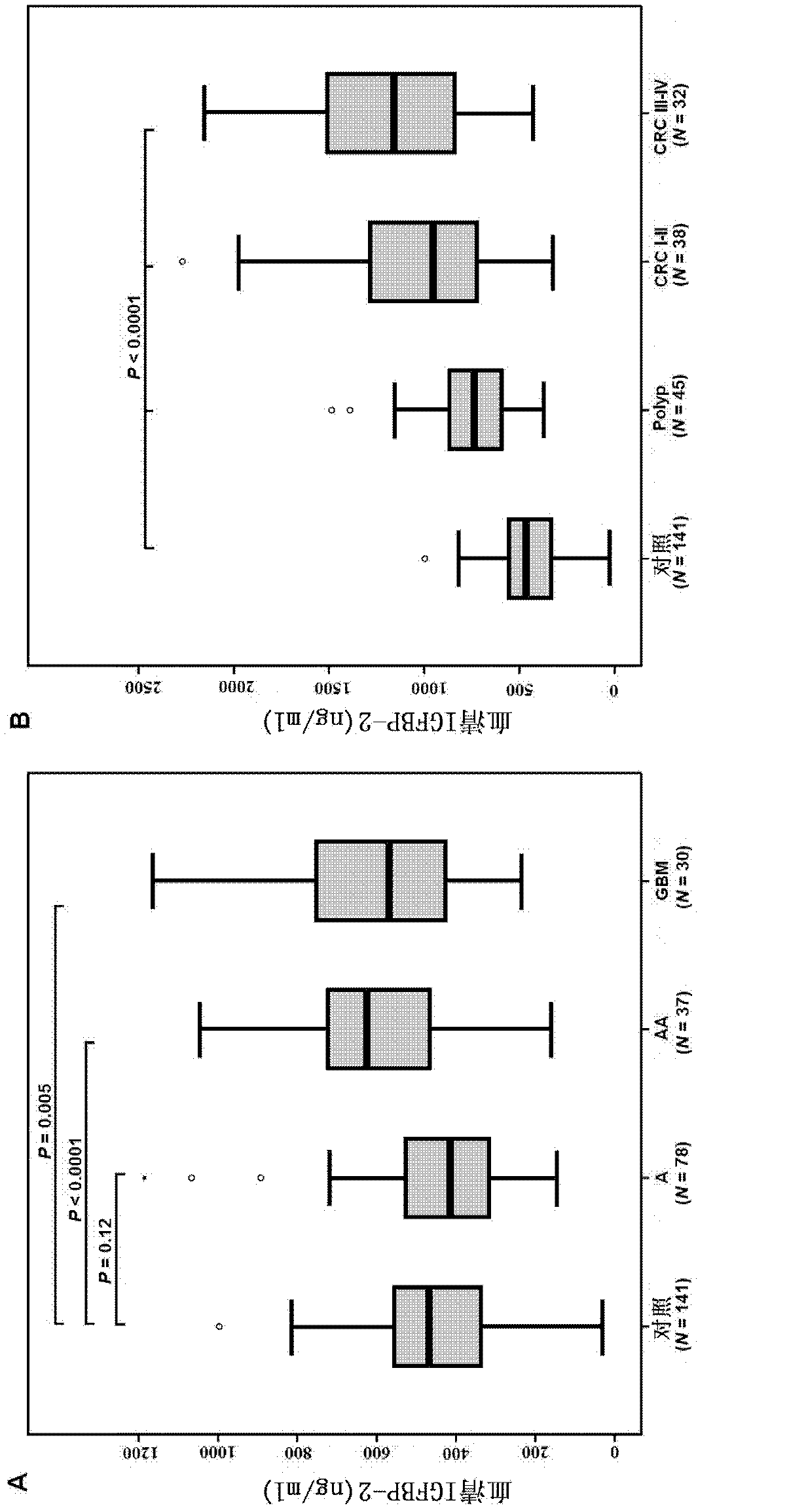
The invention provides a tumor diagnosis reagent or kit based on detection of an IGFBP-2 (insulin-like growth factor binding protein 2) autoantibody or combined detection of the IGFBP-2 autoantibody IGFBP-2. The tumor diagnosis reagent or kit comprises a first reagent group capable of detecting the IGFBP-2 autoantibody in a detected sample, or a first reagent group capable of detecting the IGFBP-2 autoantibody in the detected sample and a second reagent group capable of detecting IGFBP-2 in the detected sample. The invention also provides application of IGFBP-2 autoantibody detection or combined detection of the IGFBP-2 autoantibody and the IGFBP-2 in preparing the tumor diagnosis reagent or kit. Detection of the IGFBP-2 autoantibody can be used for diagnosing early-phase tumor or high-grade adenoma with cancerization risk, in particular for diagnosing prometaphase glioma and high-grade colorectal polyp and / or colorectal neoplasms of I-II grades with cancerization risk; and the combined detection of the IGFBP-2 autoantibody and the IGFBP-2 can be used for diagnosing tumors of different grades and monitoring each phase of a tumor from low grade to high grade, such as diagnosing and identifying astrocytoma, anaplastic astrocytoma and / or spongioblastoma, as well as I-IV grades of colorectal neoplasms.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL +1
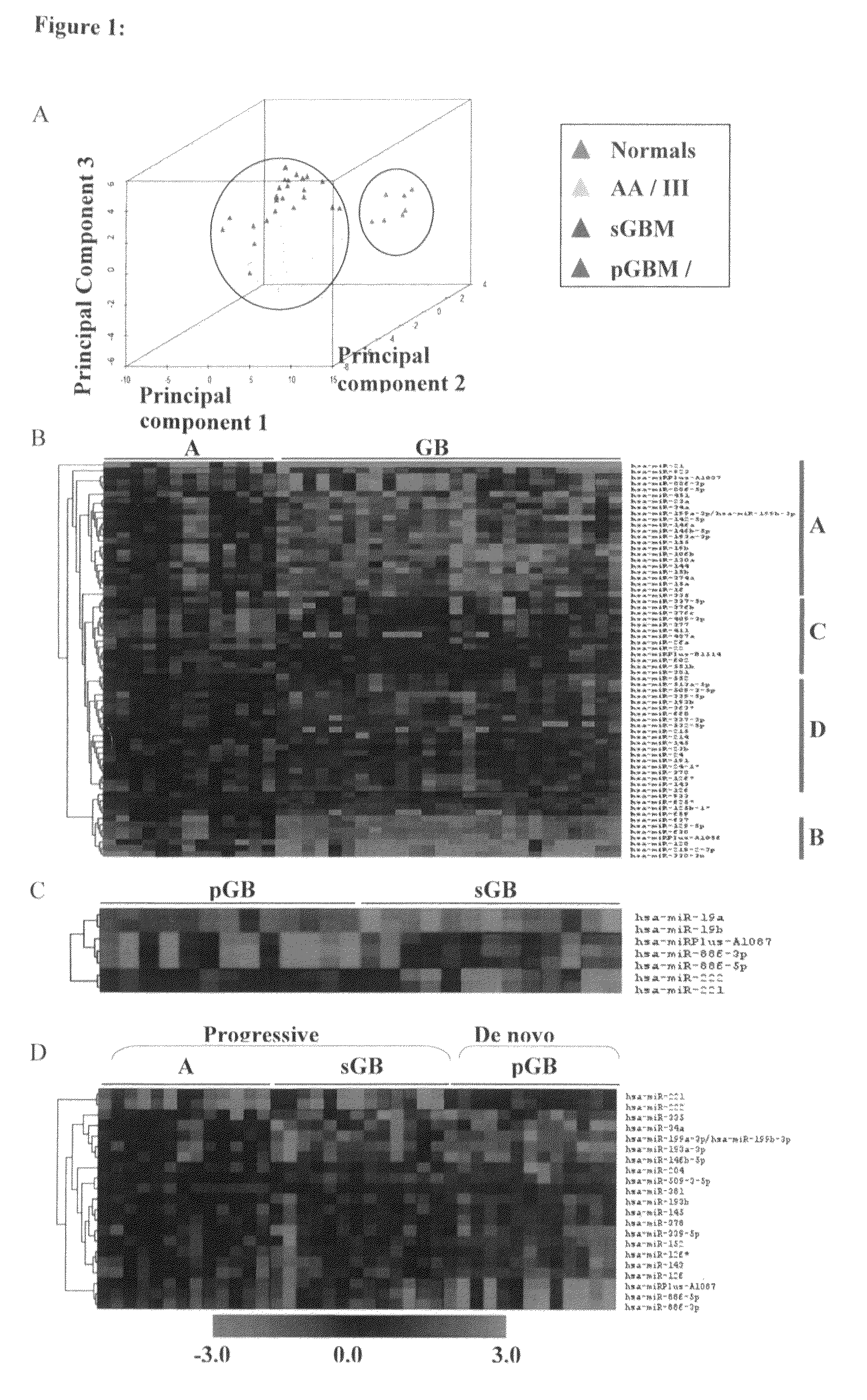
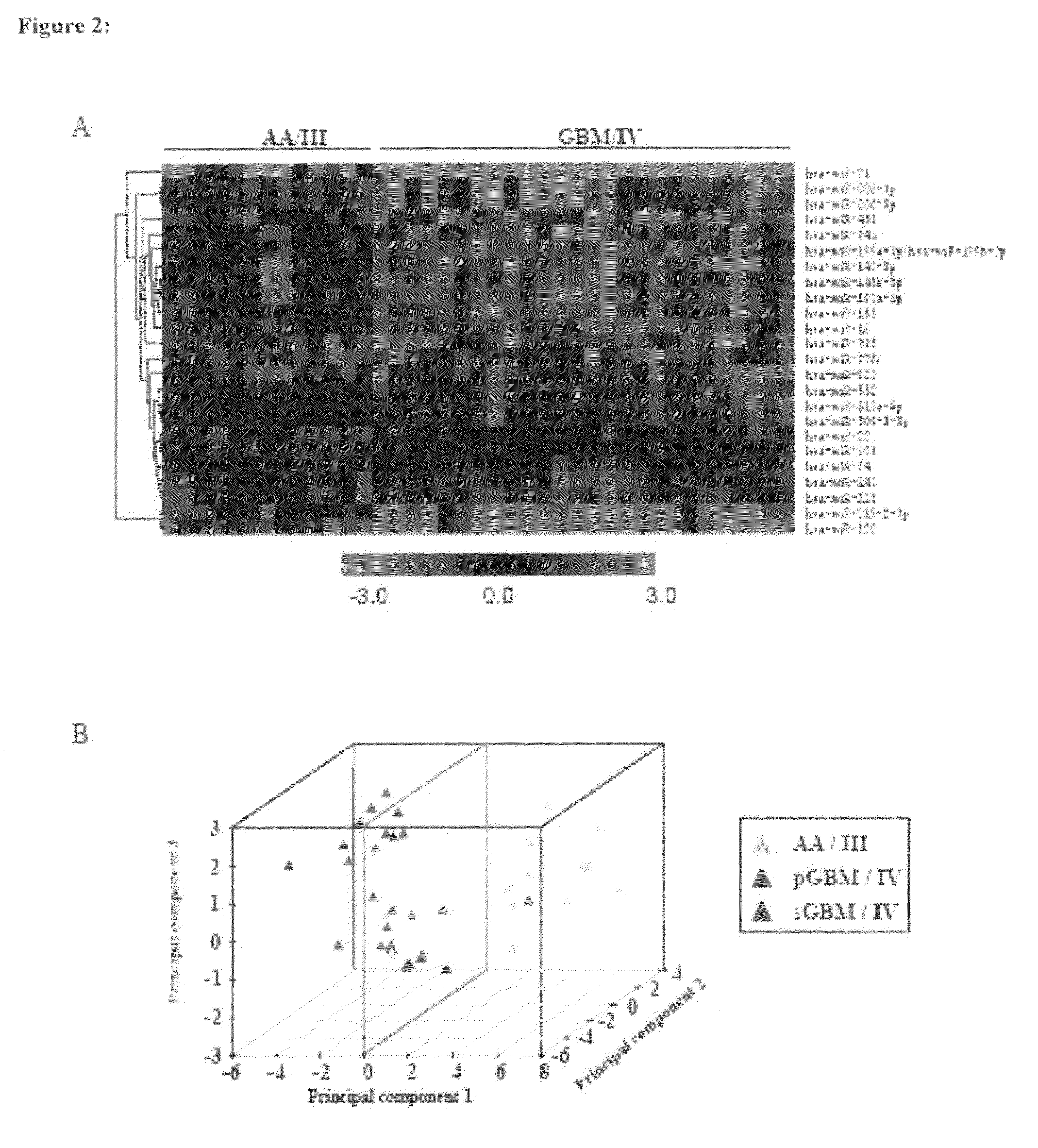
MicroRNAs (miRNA) as biomarkers for diagnosing different grades of gliomas and pathways of glioma progression
InactiveUS8637241B2Accurate classificationSugar derivativesMicrobiological testing/measurementPrimary GlioblastomaAnaplastic astrocytoma
This invention relates to a method for detecting, (i) malignant astrocytoma from normal brain tissue (ii) glioblastoma and anaplastic astrocytoma (iii) primary glioblastoma and secondary glioblastoma (iv) progressive pathway and denovo pathway comprises determining the level of expression of miRNAs listed in table 2, 3, 4, 5, wherein a higher or lower level of expression of miRNAs in the test sample as compared to the control sample differentiates and kit for characterizing a) malignant astrocytoma from normal brain tissue cell comprising reagent capable of specifically detecting the level of expression of the genes of miRNAs and instructions for using said kit for characterizing malignant astrocyoma from normal brain tissue cells b) glioblastoma from anaplastic astrocytoma comprising reagent capable of specifically detecting the level of expression of the genes of miRNAs and instructions for using said kit for characterizing glioblastoma from anaplastic astrocyoma c) primary glioblastoma from secondary glioblastoma comprising reagent capable of specifically detecting the level of expression of the genes of miRNAs and instructions for using said kit for characterizing primary glioblastoma from secondary glioldastoma, d) progressive pathway from denovo pathway comprising reagent capable of specifically detecting the level of expression of the genes of miRNAs and instructions for using said kit for characterizing progressive pathway from denovo pathway.
Owner:DEPT OF BIOTECHNOLOGY MINIST OF SCI & TECH GOVERNMENT OF INDIA +1
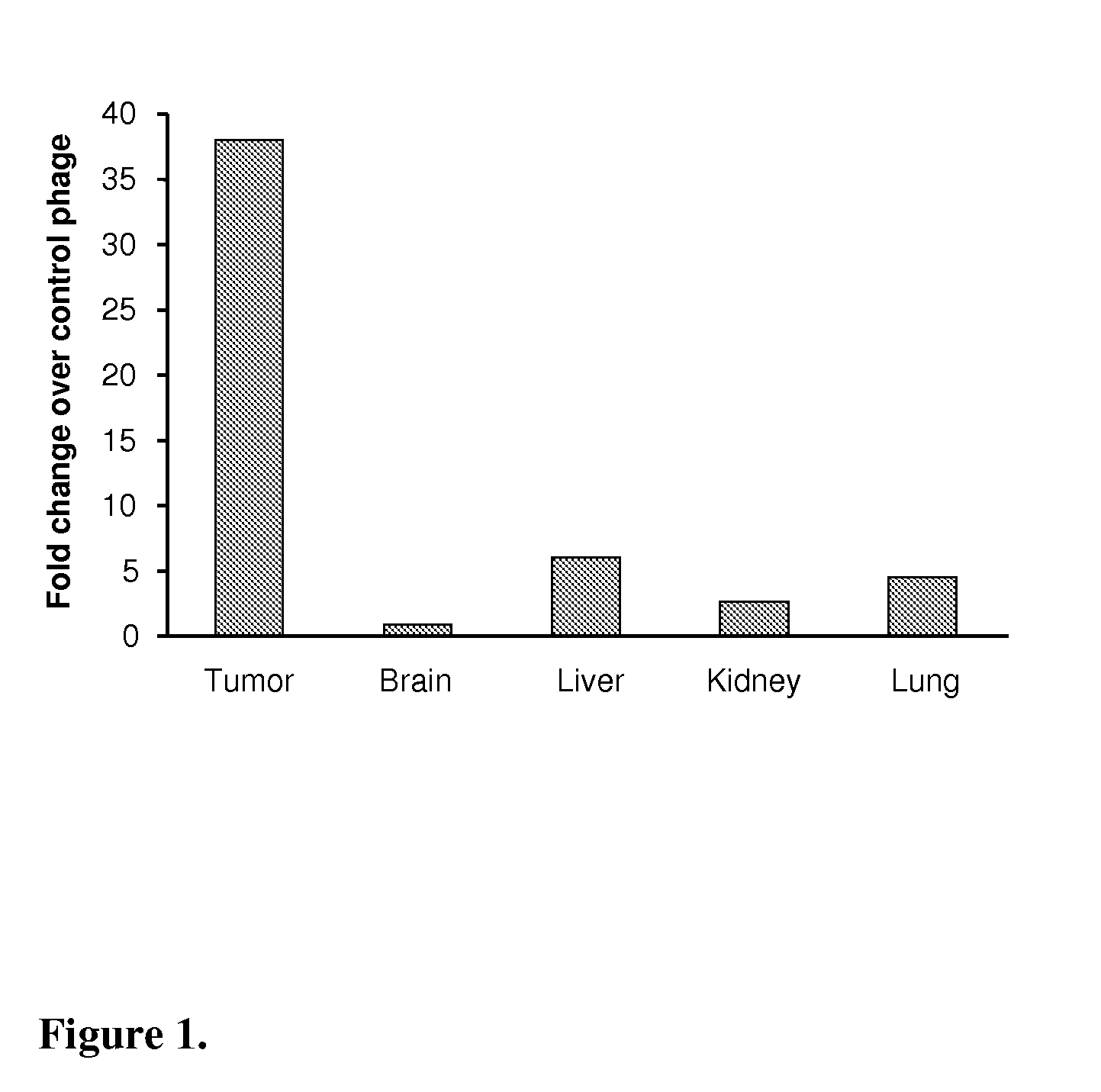
Peptide homing to brain tumors
The present invention relates to a peptide, which specifically homes to the intracranial, early stage astrocytoma model that grows as islets and harbors co-opted tumor vessels in the brain. The peptide finds its use in targeted delivery of therapeutic substances to invasive brain cancer or metastatic brain lesions as such and in combination with conventional therapies, such as surgery and radiation, and anti-angiogenic therapies, and as a tool in diagnosis of, e.g., invasive brain cancer or metastatic brain lesions.
Owner:BURNHAM INST FOR MEDICAL RES +1
Methods for diagnosing and treating astrocytomas
InactiveUS20100322949A1Reduced activityReduce functionOrganic active ingredientsPeptide/protein ingredientsAstrocytomaBiology
Owner:LUDWIG INST FOR CANCER RES
Compositions and Methods for Treating Malignant Astrocytomas
InactiveUS20150018369A1Convenient treatmentImprove efficiencyBiocideOrganic chemistryBlastomaGlioblastoma
The disclosure provides methods of treating glioblastoma, methods of screening for compounds that treat glioblastoma, and pharmaceutical compositions useful in the treatment of glioblastoma.
Owner:UNIVERSITY OF MONTANA +1
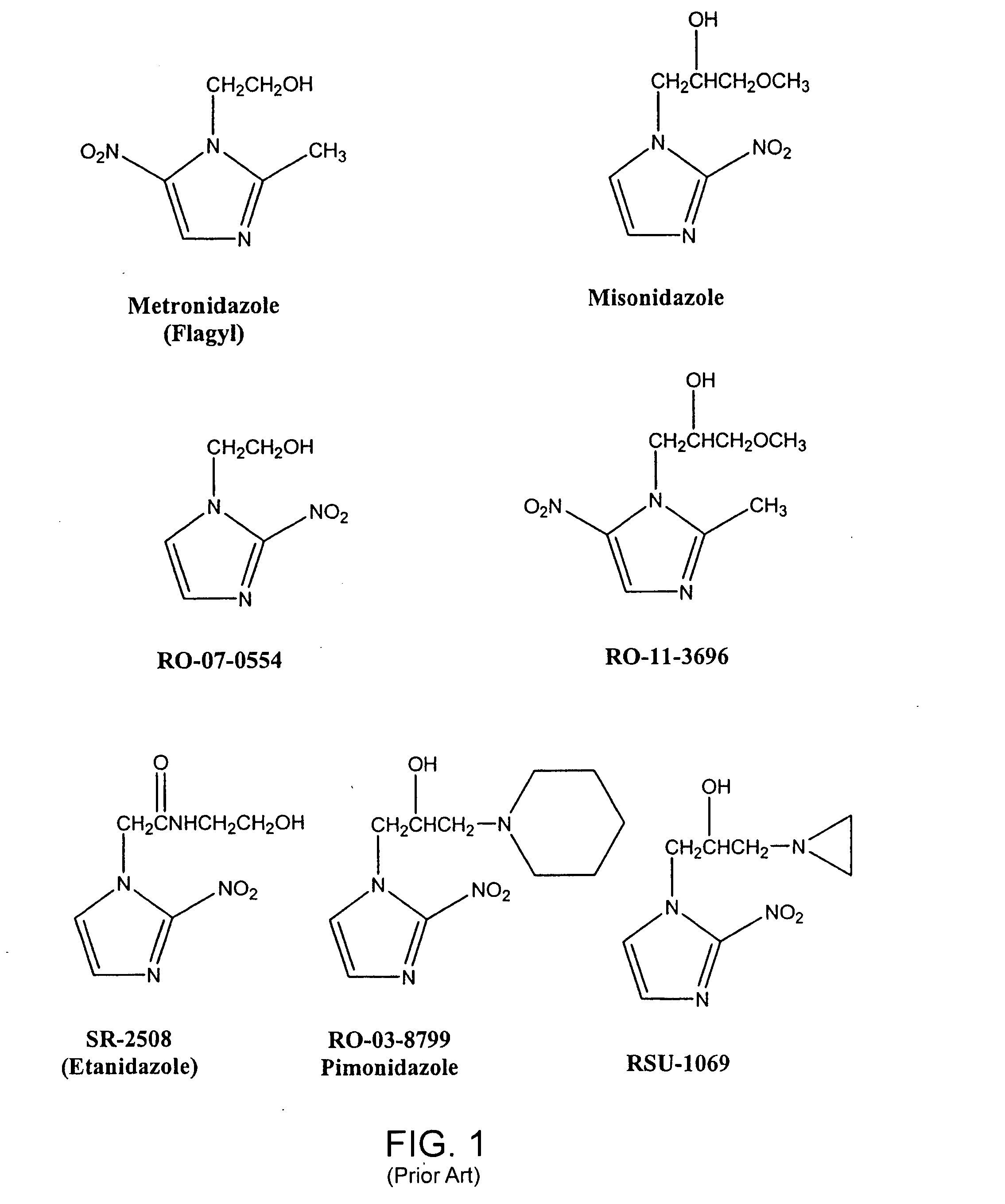
Radiosensitizer formulations and methods for use
Owner:MATTHEWS RICHARD H DR
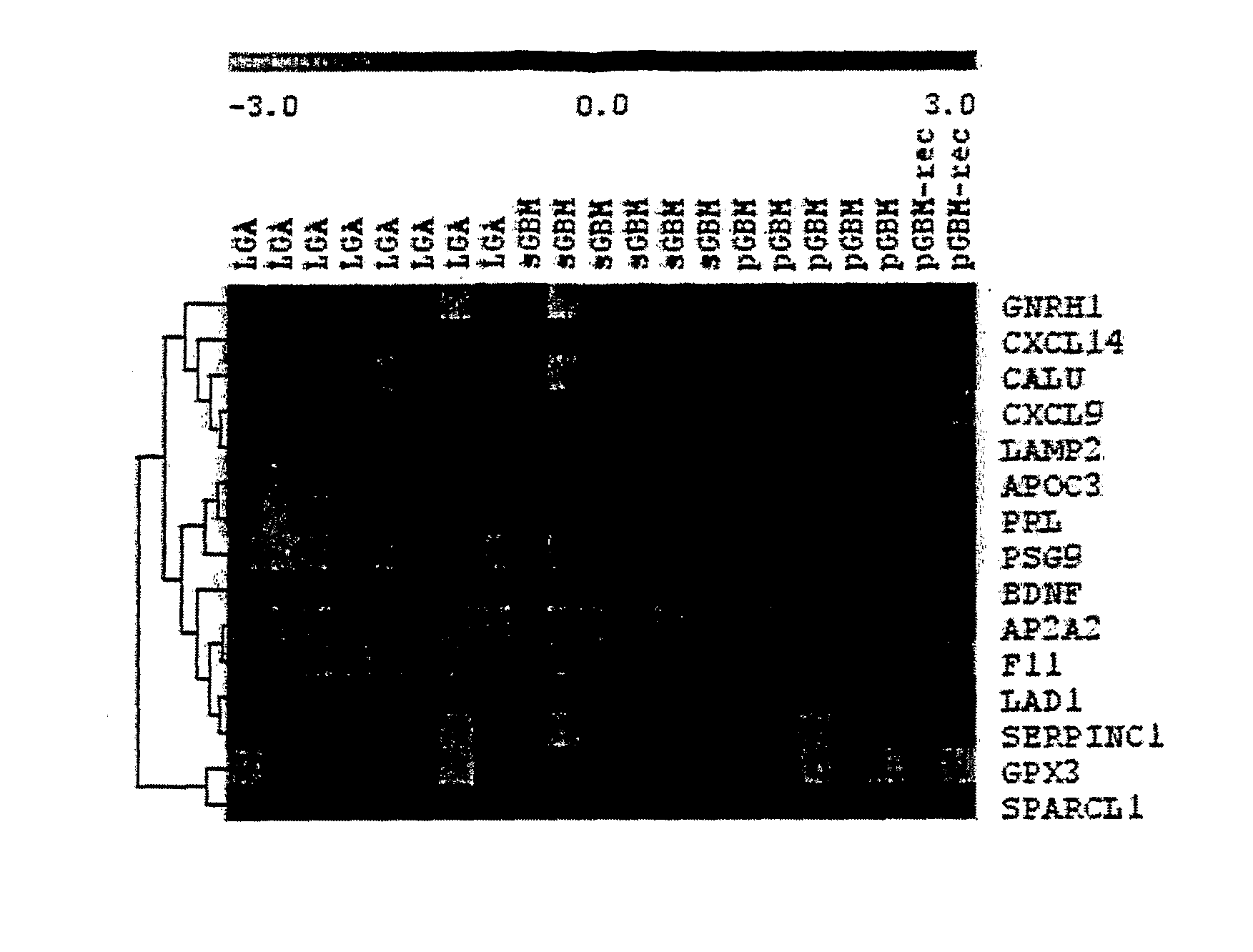
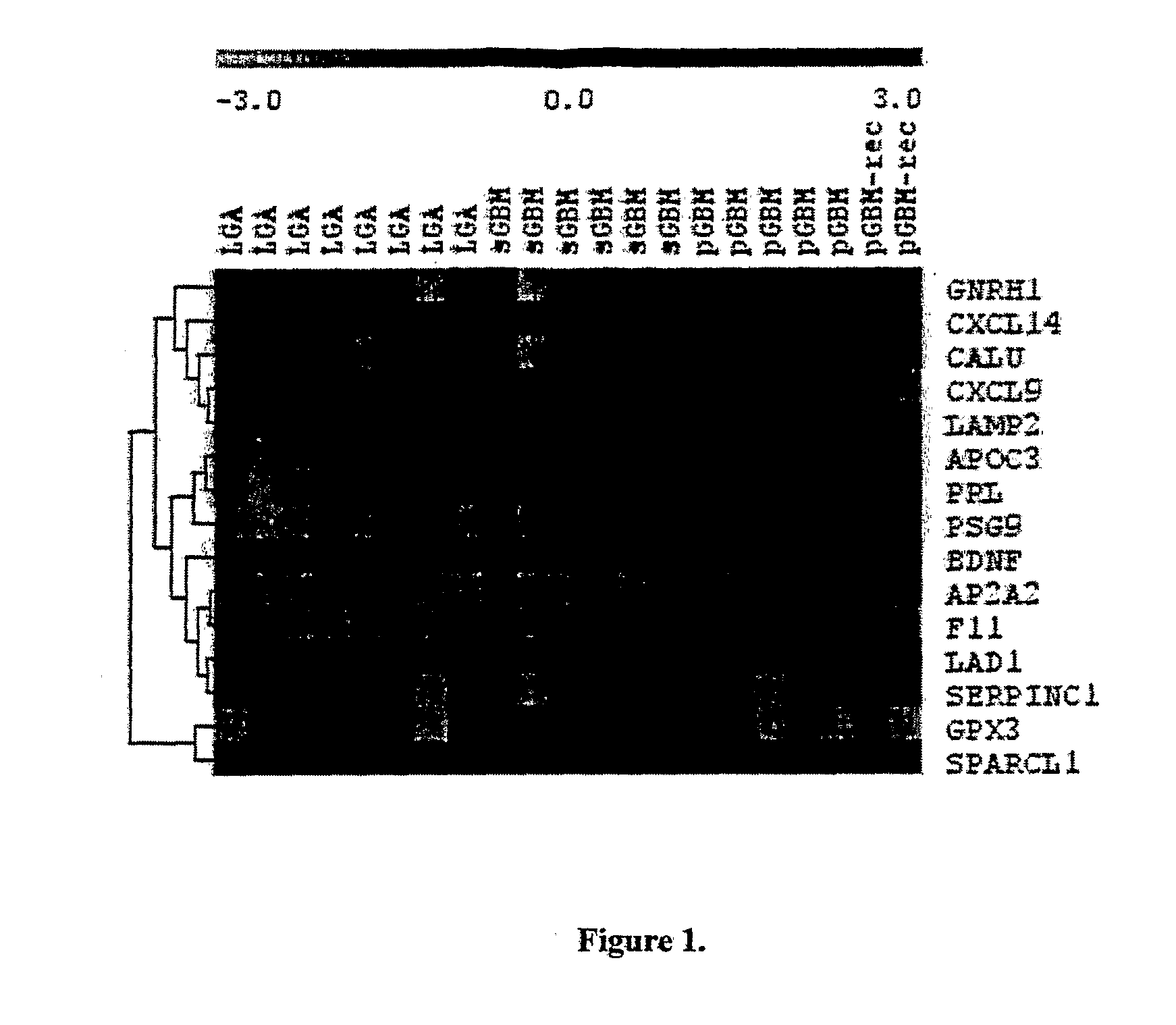
Method for the Diagnosis of Higher- and Lower-Grade Astrocytoma Using Biomarkers and Diagnostic Kit Thereof
Disclosed is a method of diagnosing the presence of higher grade astrocytoma / glioblastoma (GBM) or lower-grade grade astrocytoma (DA or AA) in a human subject using secreted or plasma membrane associated biomarkers, which involves the detection of the expression levels of said genes, alone or in combination, in either tumor tissue samples or body fluids and a diagnostic kit thereof.
Owner:COUNCIL OF SCI & IND RES
Preparation of monoclonal antibody for mouse anti-human type BRAF V600E mutant protein and immunohistochemical application of monoclonal antibody
InactiveCN110183535AStrong specificityHigh potencyDisease diagnosisImmunoglobulinsMutated proteinHuman type
The invention discloses a monoclonal antibody for mouse anti-human type BRAF V600E mutant protein. The monoclonal antibody is high in specificity, stable in expression and high in potency, can be applied to BRAF V600E mutant protein in malignant tumors, and has a certain medicine-based research value. The invention also relates to application of the monoclonal antibody to preparation of an immunohistochemical detecting tool for detecting the BRAF V600E mutant protein. According to the monoclonal antibody and the application of the monoclonal antibody disclosed by the invention, an immunohistochemical technology based on the BRAF V600E monoclonal antibody is built and is used in auxiliary diagnosis, selection of treatment schemes and prognosis of the malignant tumors, wherein the malignanttumors mainly comprise malignant melanoma, lung adenocarcinoma, colorectal carcinoma, gastrointestinal stromal tumors, papillary thyroid carcinoma, astrocytoma, hairy cell leukemia, lynch syndrome andthe like. Based on simple operation and less charging of the immunohistochemical method, the method can be used as a first-choice detection method of BRAF V600E gene mutation in clinic work.
Owner:南京基诺米医疗科技有限公司
Organic compositions to treat EPAS1-related diseases
ActiveUS9868949B2Low immunogenicityImprove efficacyOrganic active ingredientsNervous disorderDiseaseHepatocellular carcinoma
Owner:NOVARTIS AG
Methods of treating epidermal growth factor deletion mutant VIII related disorders
InactiveCN104168922AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAbnormal tissue growth
The present invention relates to methods of treating treating epidermal growth factor deletion mutant vIII (EGFRvIII) related disorders, such as glioblastoma or anaplastic astrocyte tumors, using antigen binding proteins, including antibodies against EGFRvIII conjugated to a drug. Diagnostic and therapeutic formulations of such antibodies and drug conjugates thereof are also provided.
Owner:AMGEN INC
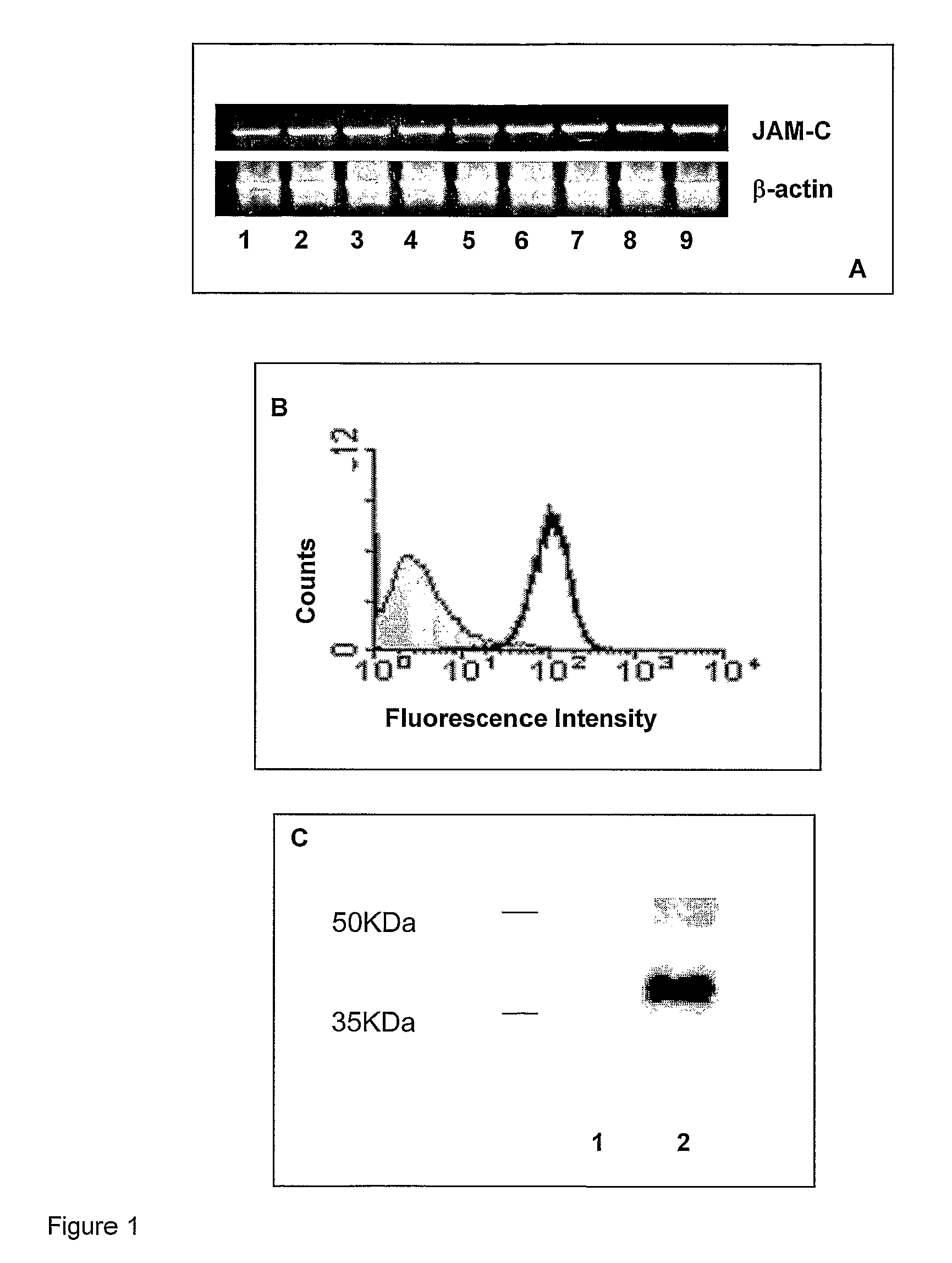
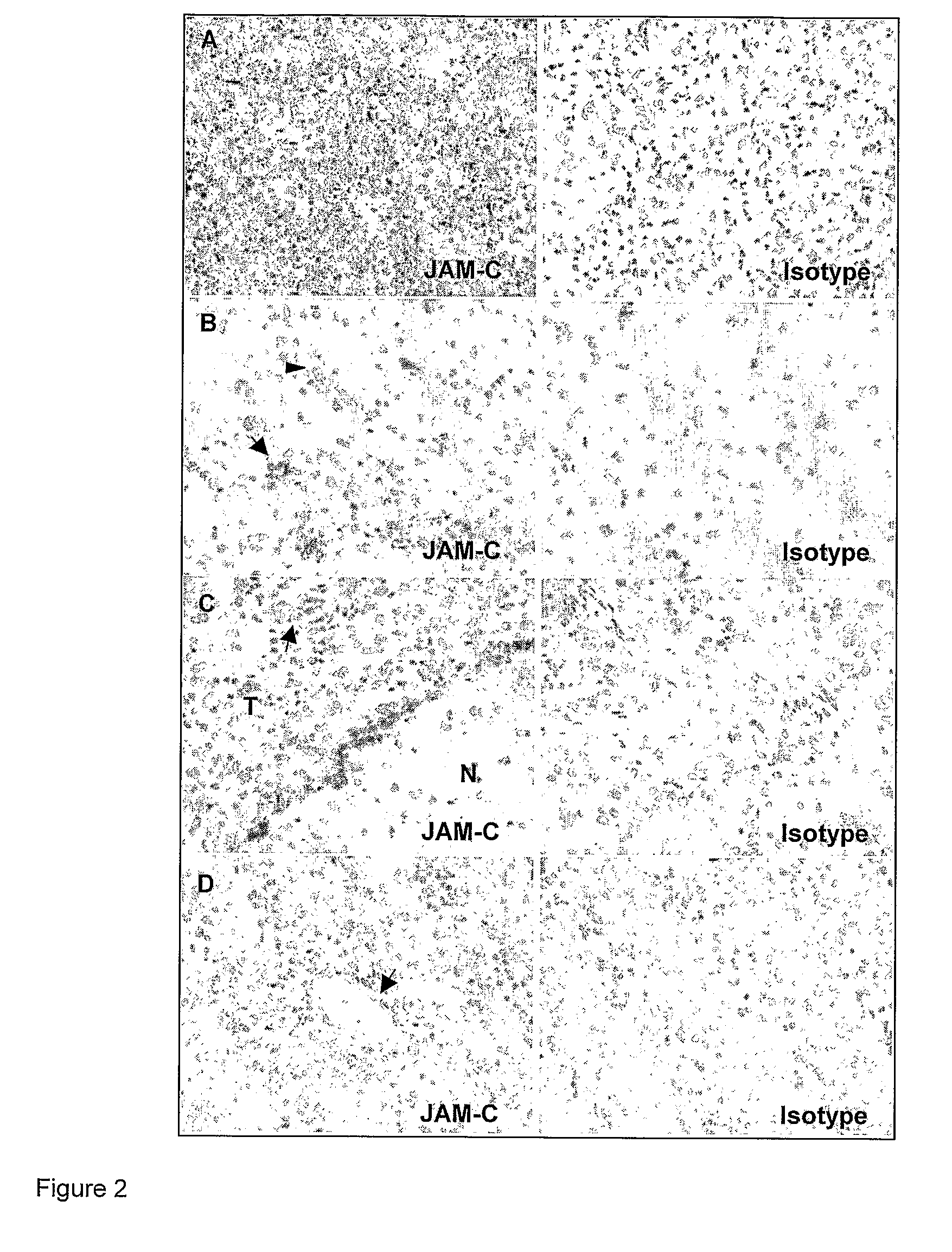
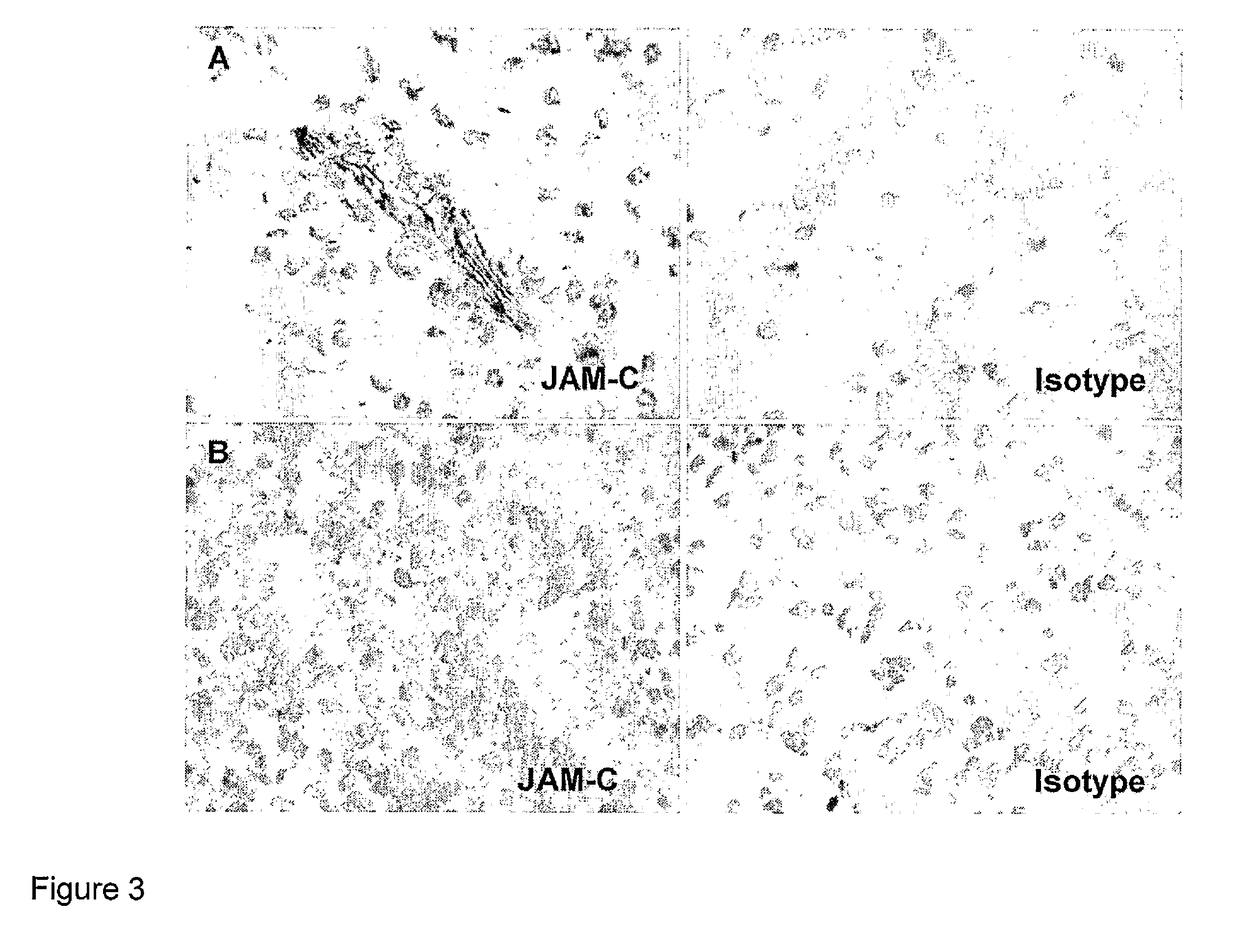
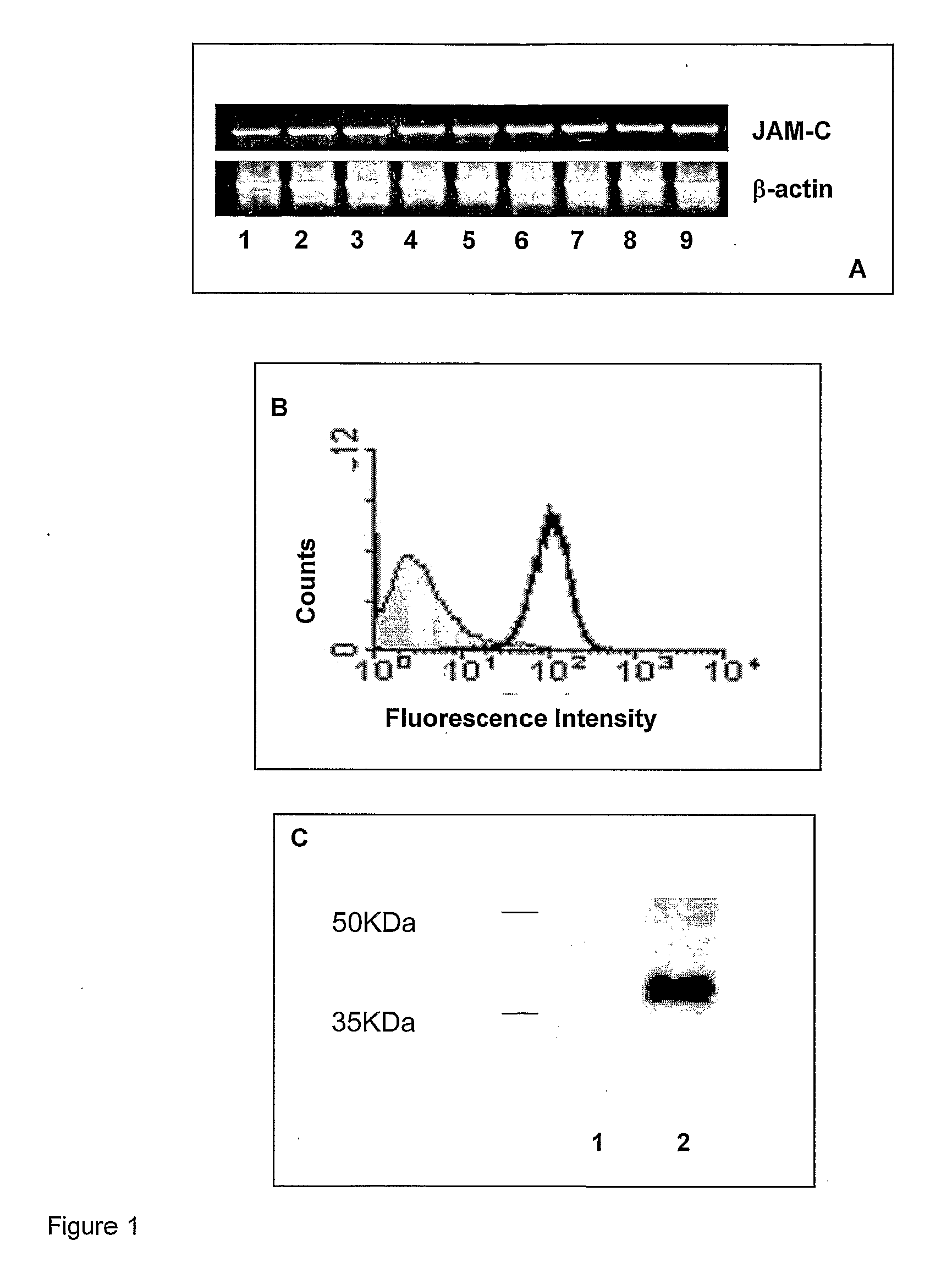
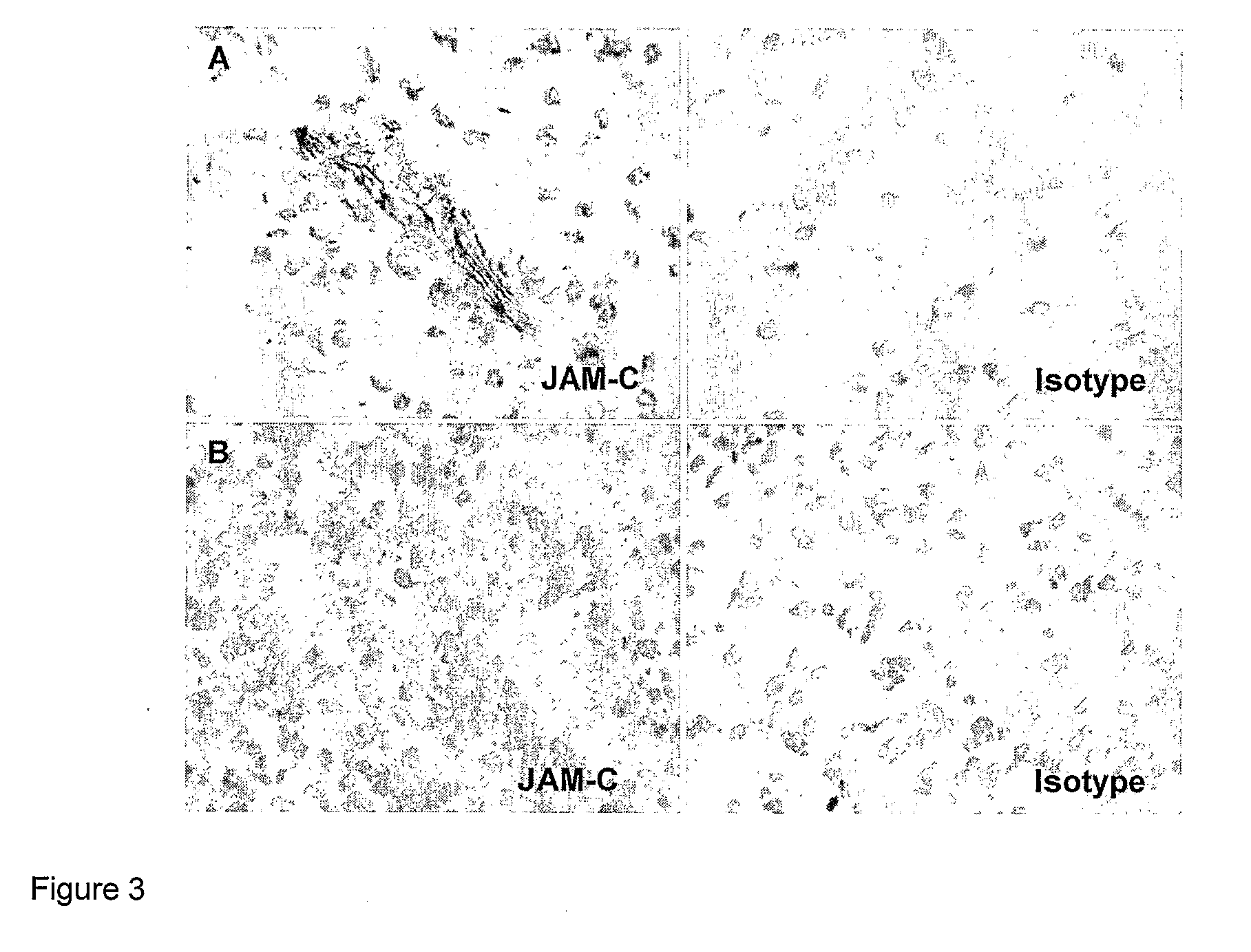
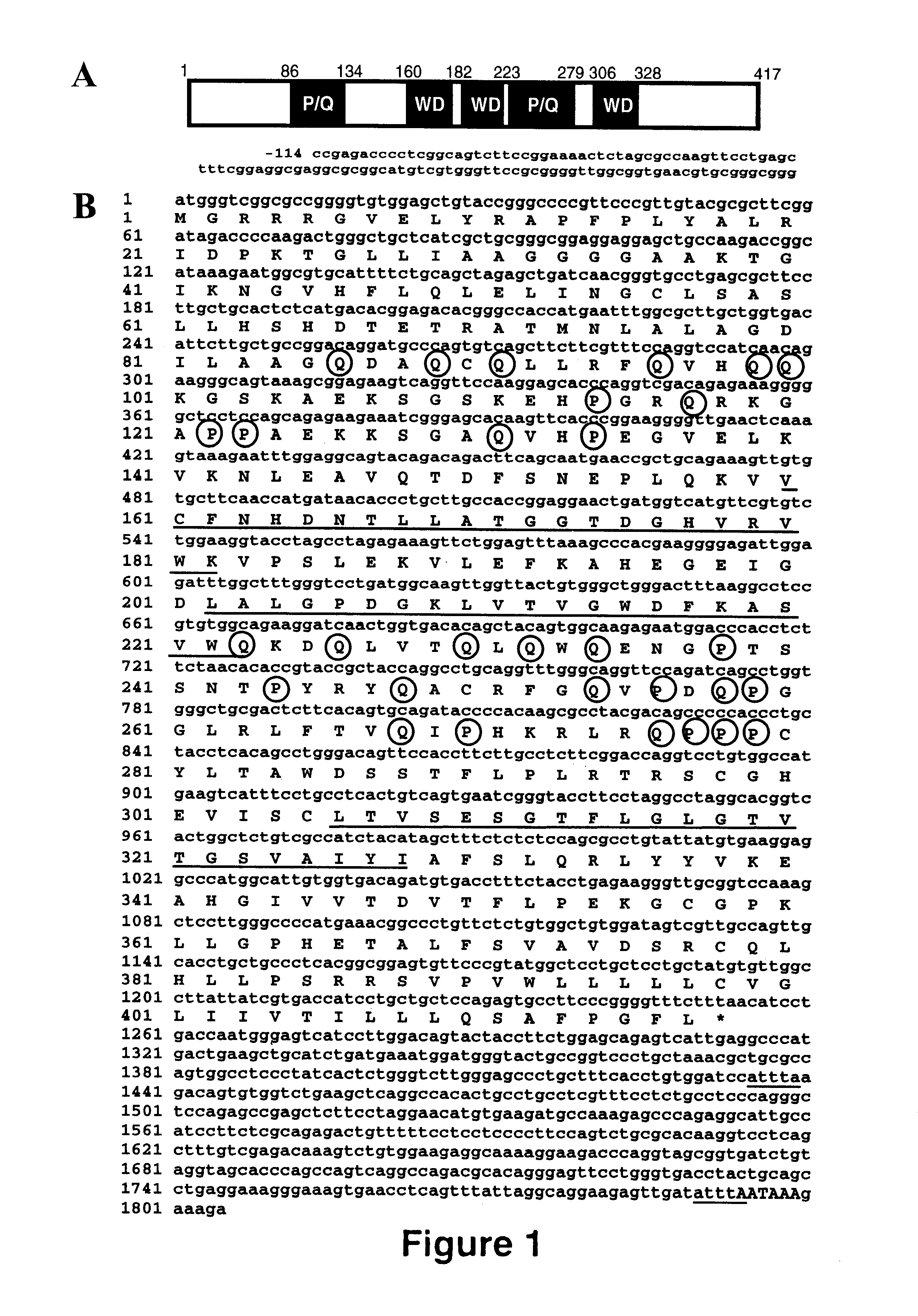
Junctional adhesion molecule-C (JAM-C) binding compounds and methods of their use
InactiveUS8007797B2In-vivo radioactive preparationsAntibody ingredientsAstrocytomaJunctional Adhesion Molecule C
The present invention relates to the use of a compound that specifically binds to JAM-C or JAM-B for the treatment of gliomas. More specifically the invention relates to the use of an antagonist of JAM-B or JAM-C for the treatment of glioma, in particular astrocytoma.
Owner:MERCK SERONO SA
Junctional adhesion molecule-c (jam-c) binding compounds and methods of their use
InactiveUS20100034737A1Growth inhibitionNegative side effectRadioactive preparation carriersAntibody ingredientsAstrocytomaAstrocytoma resection
The present invention relates to the use of a compound that specifically binds to JAM-C or JAM-B for the treatment of gliomas. More specifically the invention relates to the use of an antagonist of JAM-B or JAM-C for the treatment of glioma, in particular astrocytoma.
Owner:MERCK SERONO SA
Prolactin regulatory element binding protein and uses thereof
InactiveUS6586581B1Enhance immune responseIncrease gene expressionSugar derivativesPeptide/protein ingredientsTrisomyNucleic acid sequencing
The present invention relates to isolated nucleic acid molecules encoding Prolactin Regulatory Element Binding protein (PREB) and recombinant proteins encoded thereby. The nucleic acid sequences are useful in the production of recombinant PREB, as probes, and in the control of gene expression, and in particular, in the control of prolactin gene expression. In particular embodiments of the invention, PREB nucleic acid sequences are used to detect transcripts of the gene in astrocytomas, to detect trisomy and to detect a propensity of a subject to develop osteoporosis. In other embodiments of the invention, the PREB nucleic acid sequences, or the products thereof, are used for preventing or controlling osteoporosis in a subject.
Owner:MT SINAI SCHOOL OF MEDICINE
Radiosensitizer formulations and methods for use
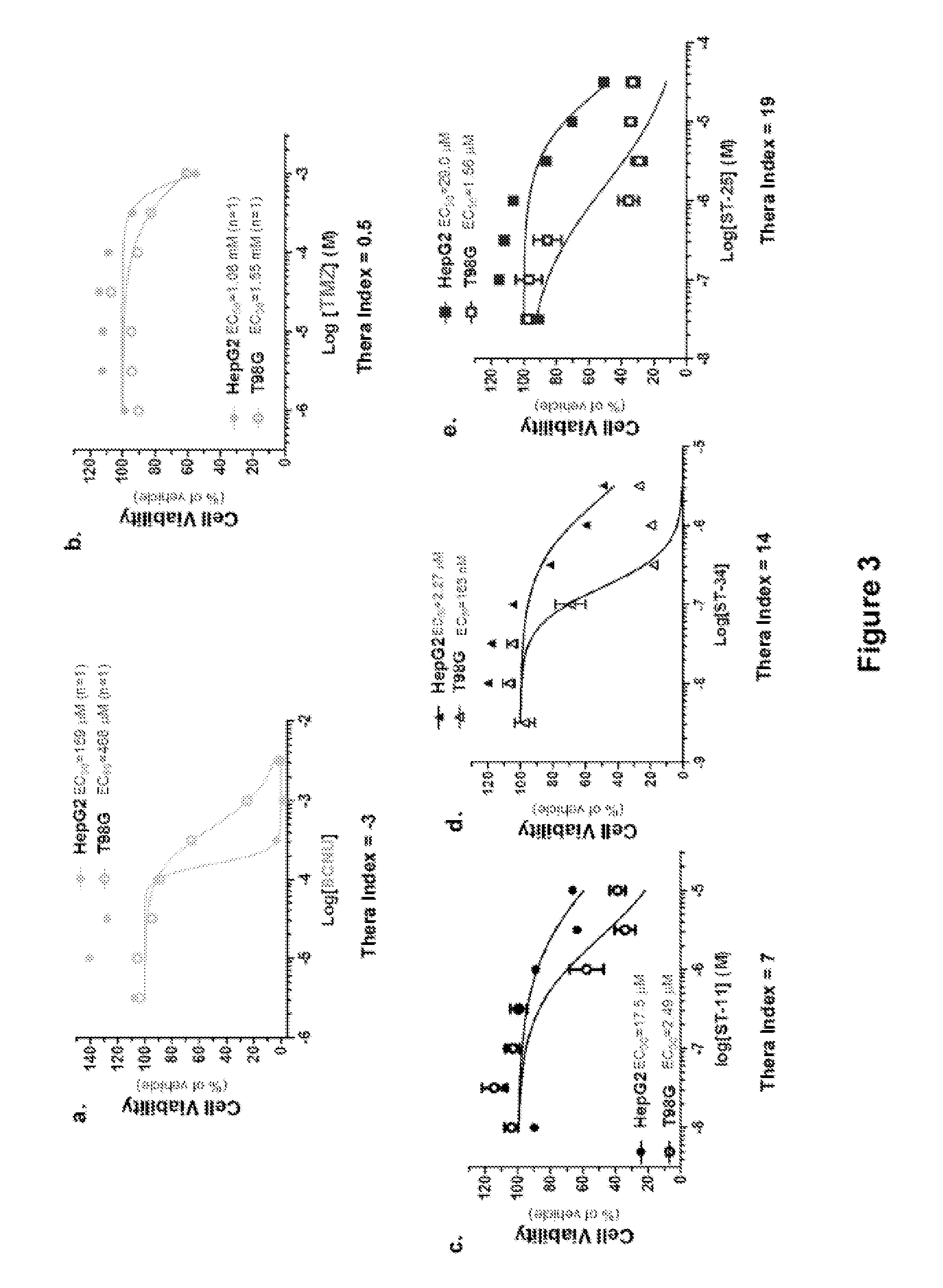
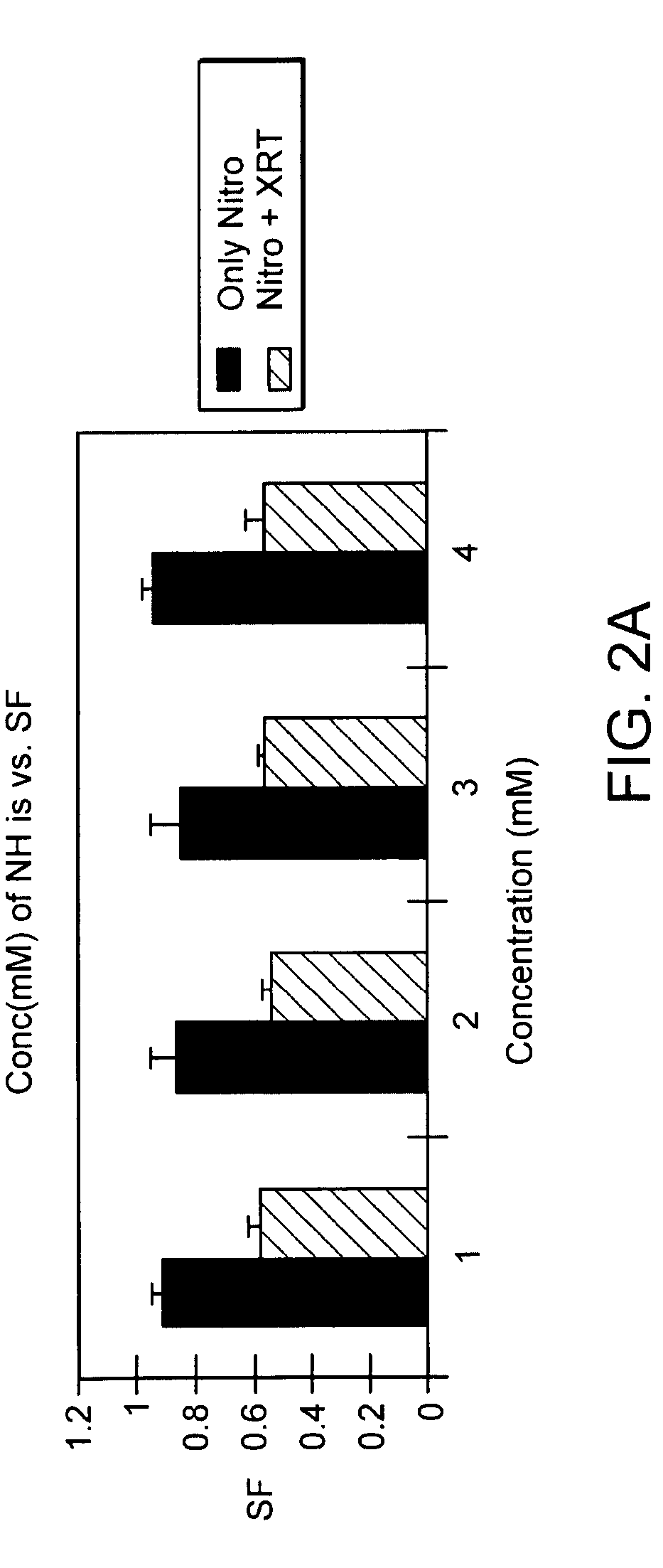
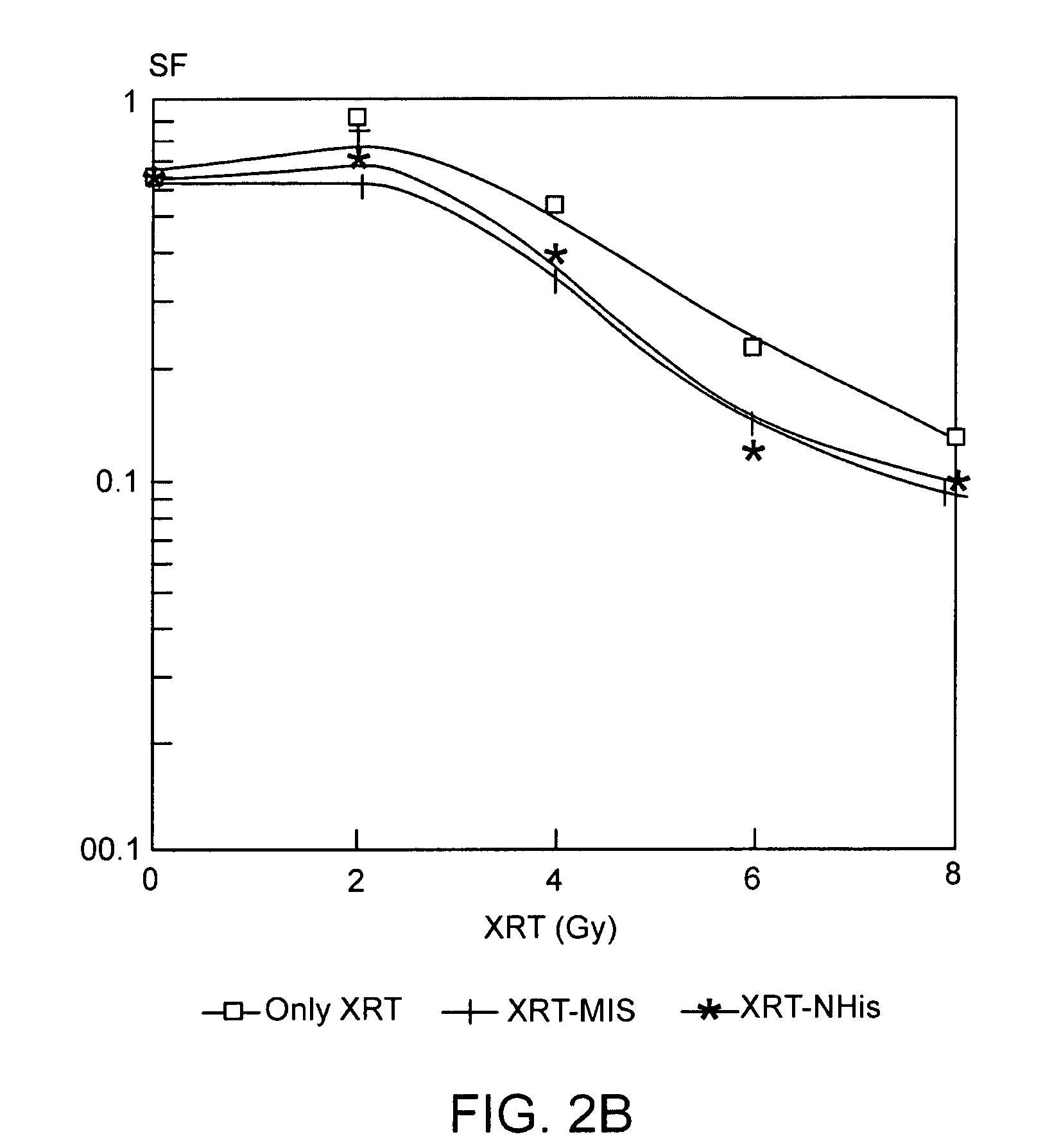
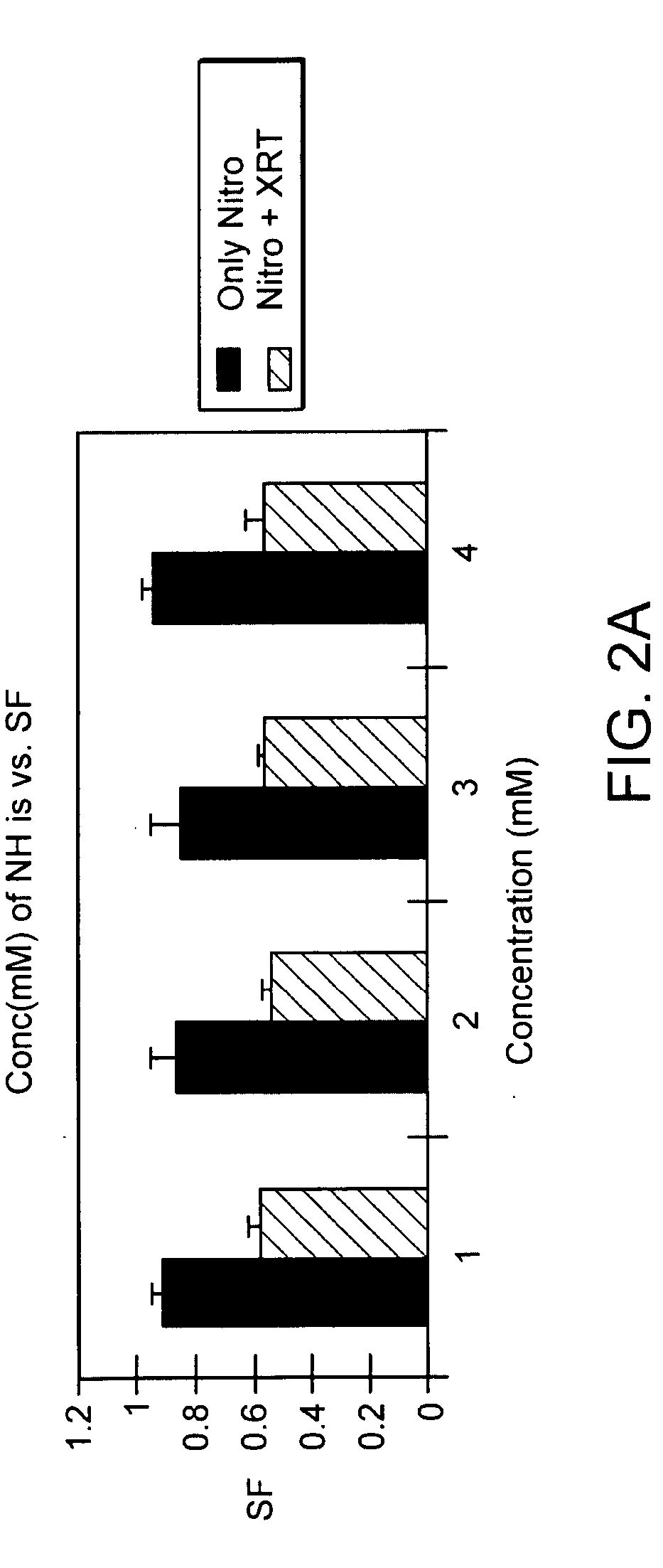
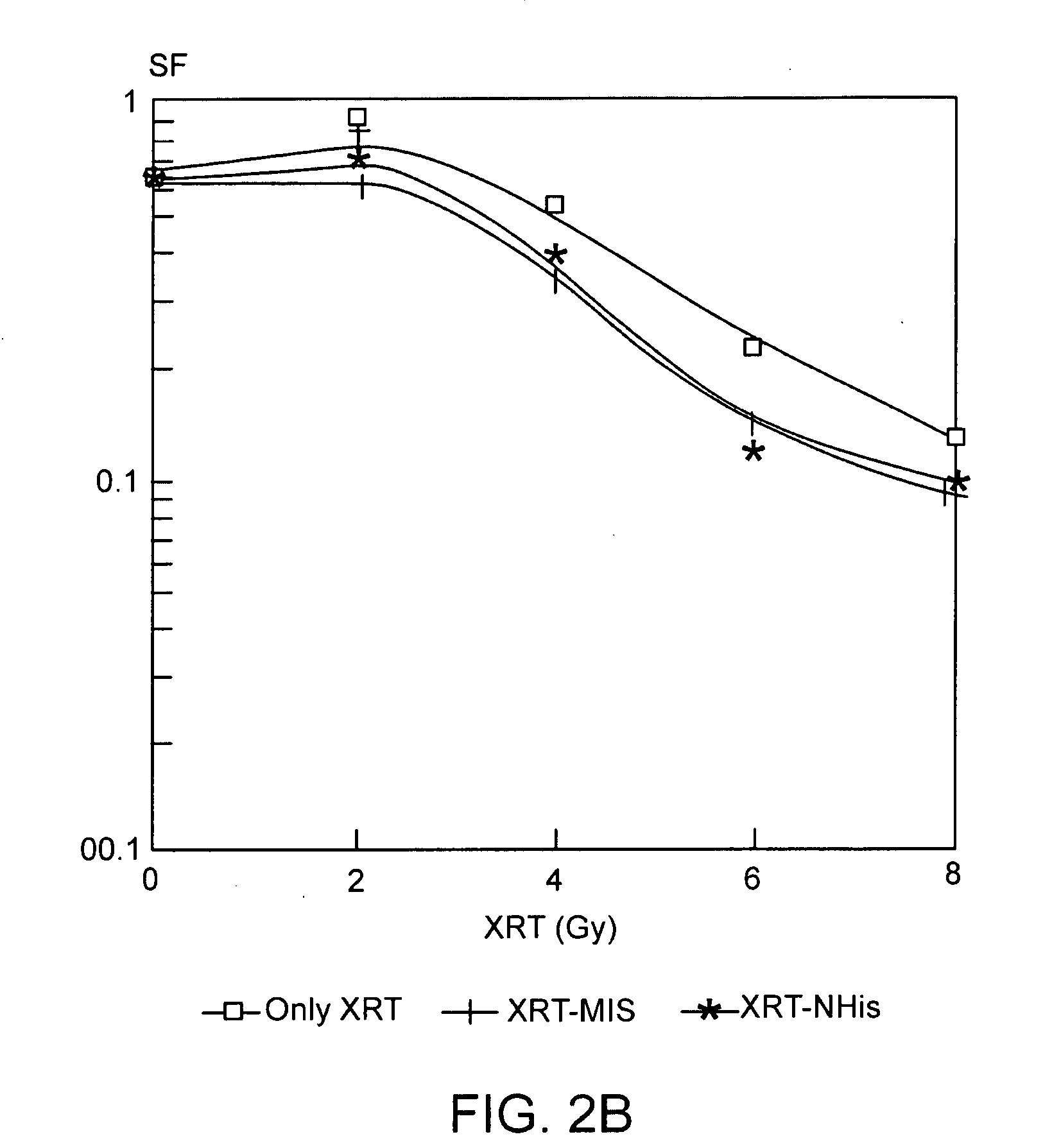
The present invention provides radiosensitizer compositions, in controlled-release formulations or other acceptable formulations, particularly nitrohistidine radiosensitizer compositions, which may be administered by any suitable means including oral, intravenous, arterial infusion, intraperitoneal, intramuscular, subcutaneous, surgical, and topical. Optionally, radiosensitizer compositions may be formulated with other agents, including chemotherapy agents and agents that provide a synergistic radiosensitizing effect. Methods of potentiating radiotherapy cancer treatment of cancers in humans, particularly of astrocytomas, are also presented, wherein a radiosensitizer composition is administered and radiotherapy is directed to the site of the tumor. Chemotherapy regimens may also be used as adjuvant therapy.
Owner:MATTHEWS RICHARD H DR
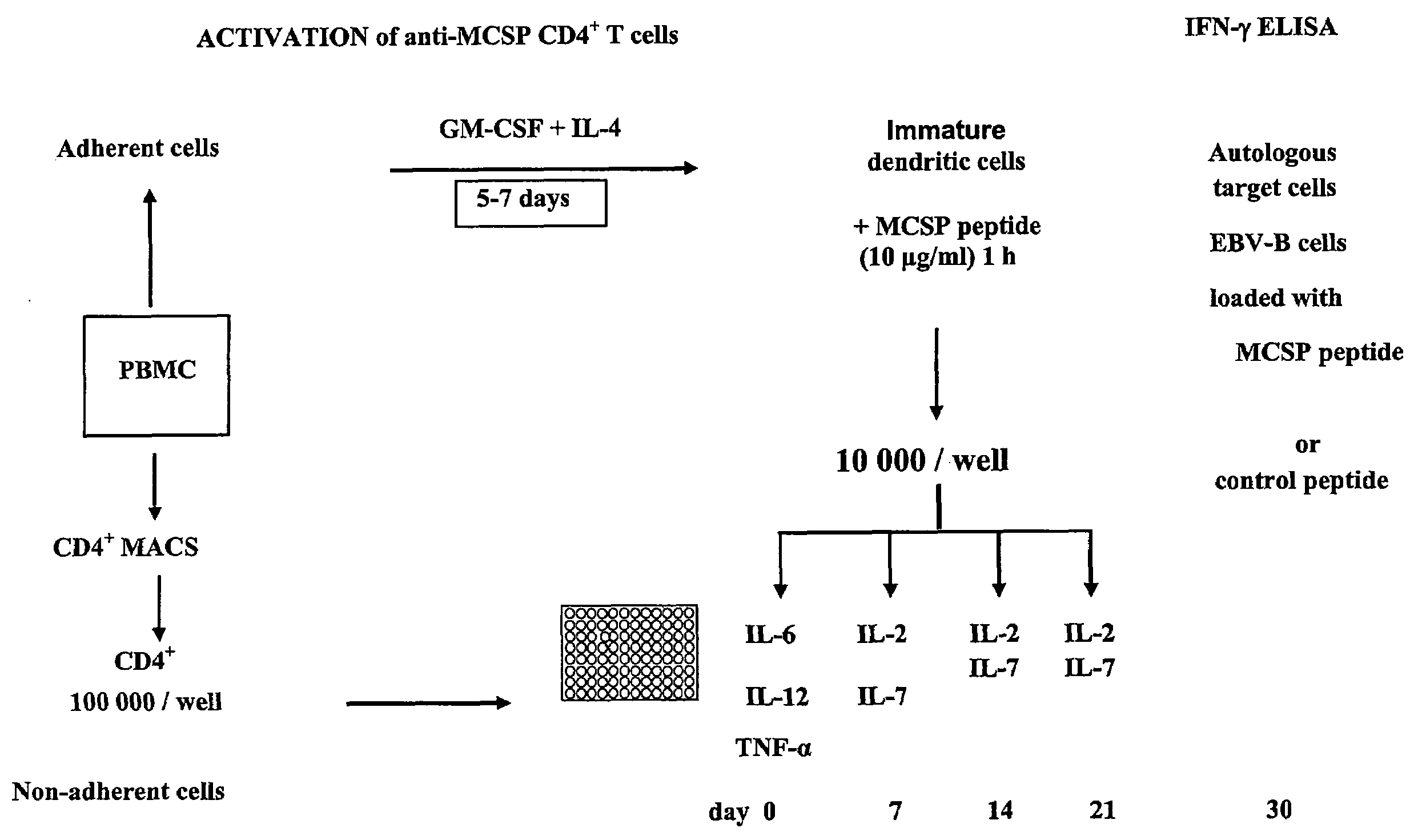
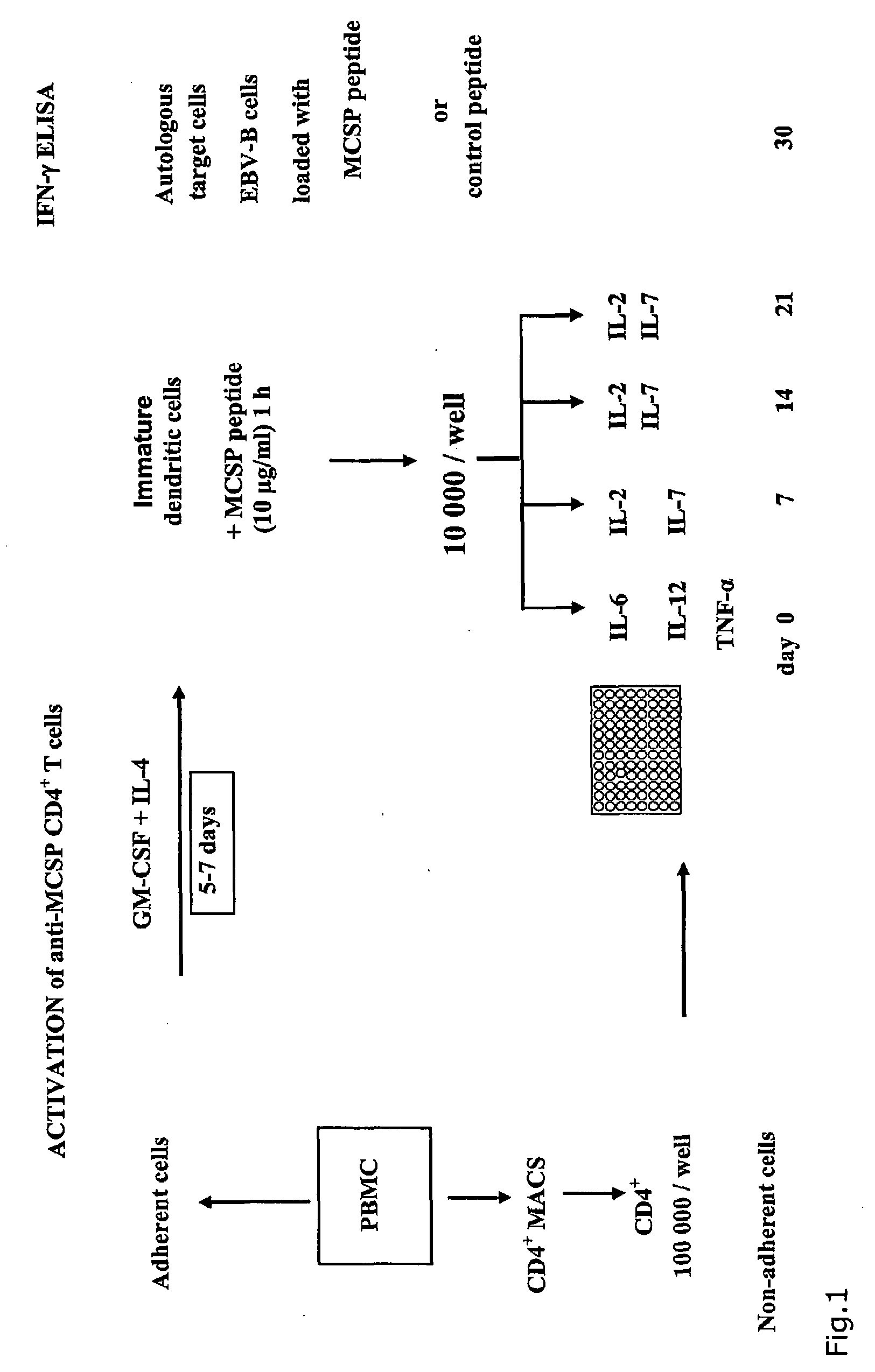
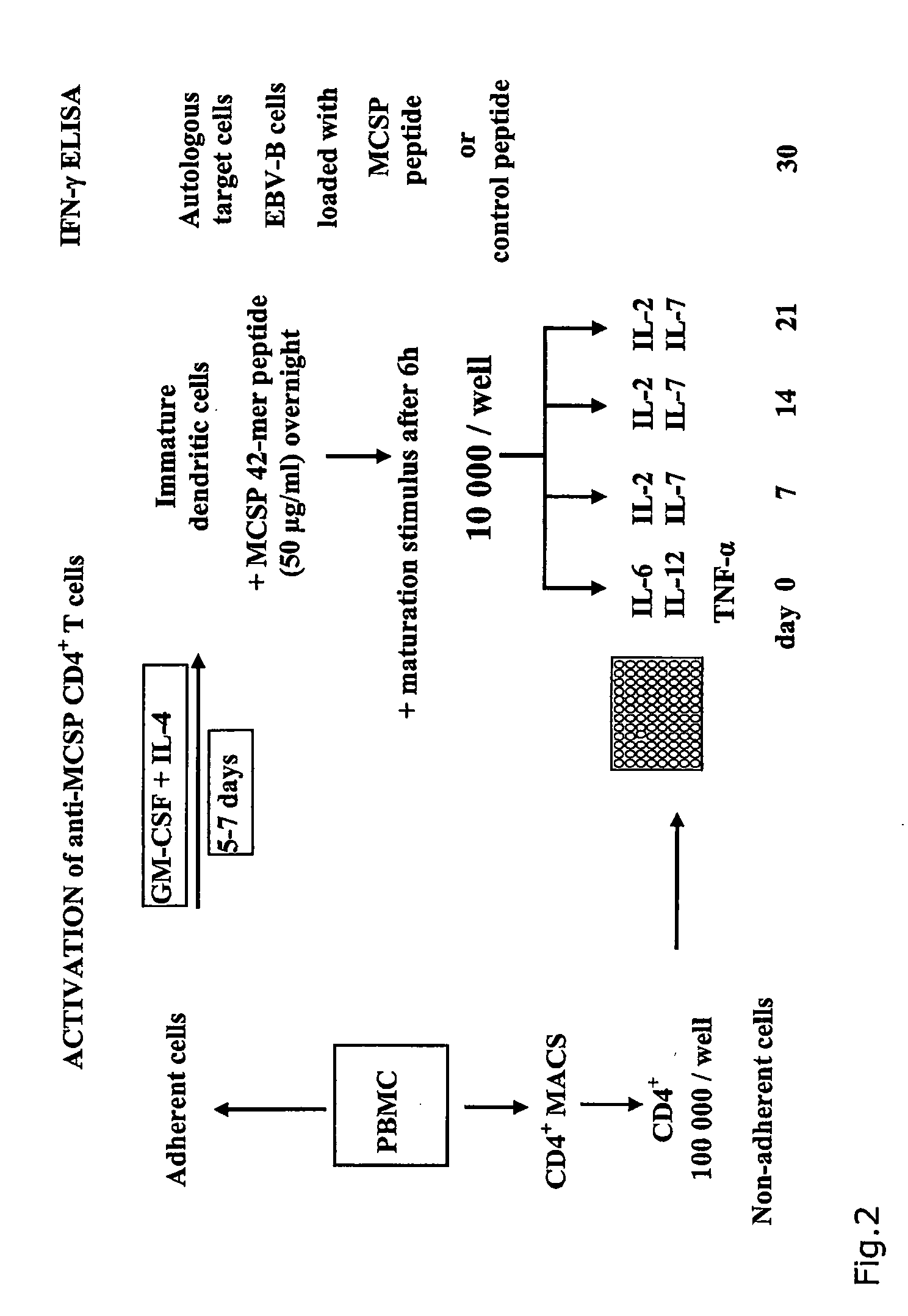
T-Cell Stimulatory Peptides From The Melanoma-Associated Chondroitin Sulfate Proteoglycan And Their Use
The present invention relates to melanoma-associated chondroitin sulfate proteoglycan (MCSP) epitopes recognized by T cells, especially by CD4+ T lymphocytes (short T-cells) and CD8+ T cells, on human melanoma cells. In more detail, the present invention relates to novel T-cell stimulatory tumour antigenic peptides corresponding to said epitopes (MCSP peptides); to fusion proteins comprising said MCSP peptides; to the use of said MCSP peptides, fusion proteins or of the full length MCSP protein itself or fragments thereof to induce an immune response, especially a T-cell response; to the use of said MCSP peptides, fusion proteins or full length MCSP protein itself or fragments thereof to prepare immune cells, such as mature dendritic cells (DCs) loaded with anyone of the peptides according to the invention, or peptide-specific T-cell clones, especially CD4+ or CD8+ T cell clones; to the use of said MCSP peptides, fusion proteins or MCSP itself or fragments thereof for research and development on / of a cancer treatment; to the use of said MCSP peptides, fusion proteins or MCSP itself or fragments thereof for preparing a medicament for inducing a T cell response in a patient, preferably for the treatment of cancer, more preferably for the treatment of melanoma, including cutaneous and ocular melanoma, and other MCSP expressing tumours such as breast cancer, notably lobular breast carcinoma, astrocytoma, glioma, glioblastoma, neuroblastoma, sarcoma and certain types of leukaemia; to the use of said MCSP peptides, fusion proteins or full length MCSP protein or fragments thereof for the preparation of a medicament, and a diagnostic agent for the treatment and prophylaxis as well the diagnosis of an immune response against tumours; and to the use of said peptide-specific T-cell clones for diagnosing or treating cancer.
Owner:SCHULTZ ERWIN +1
Organic compositions to treat epasi-related diseases
ActiveUS20160010089A1Lower Level RequirementsRestore balanceOrganic active ingredientsNervous disorderDiseaseHepatocellular carcinoma
The present disclosure relates to methods of treating EPAS1-related diseases such as cancer, metastases, astrocytoma, bladder cancer, breast cancer, chondrosarcoma, colorectal carcinoma, gastric carcinoma, glioblastoma, head and neck squamous cell carcinoma, hepatocellular carcinoma, lung adenocarcinoma, neuroblastoma, non-small cell lung cancer, melanoma, multiple myeloma, ovarian cancer, rectal cancer, renal cancer, clear cell renal cell carcinoma (and metastases of this and other cancers), gingivitis, psoriasis, Kaposi's sarcoma-associated herpesvirus, preemclampsia, inflammation, chronic inflammation, neovascular diseases, and rheumatoid arthritis, using a therapeutically effective amount of a RNAi agent to EPAS1.
Owner:NOVARTIS AG
Vector containing MTS1E1.beta. gene
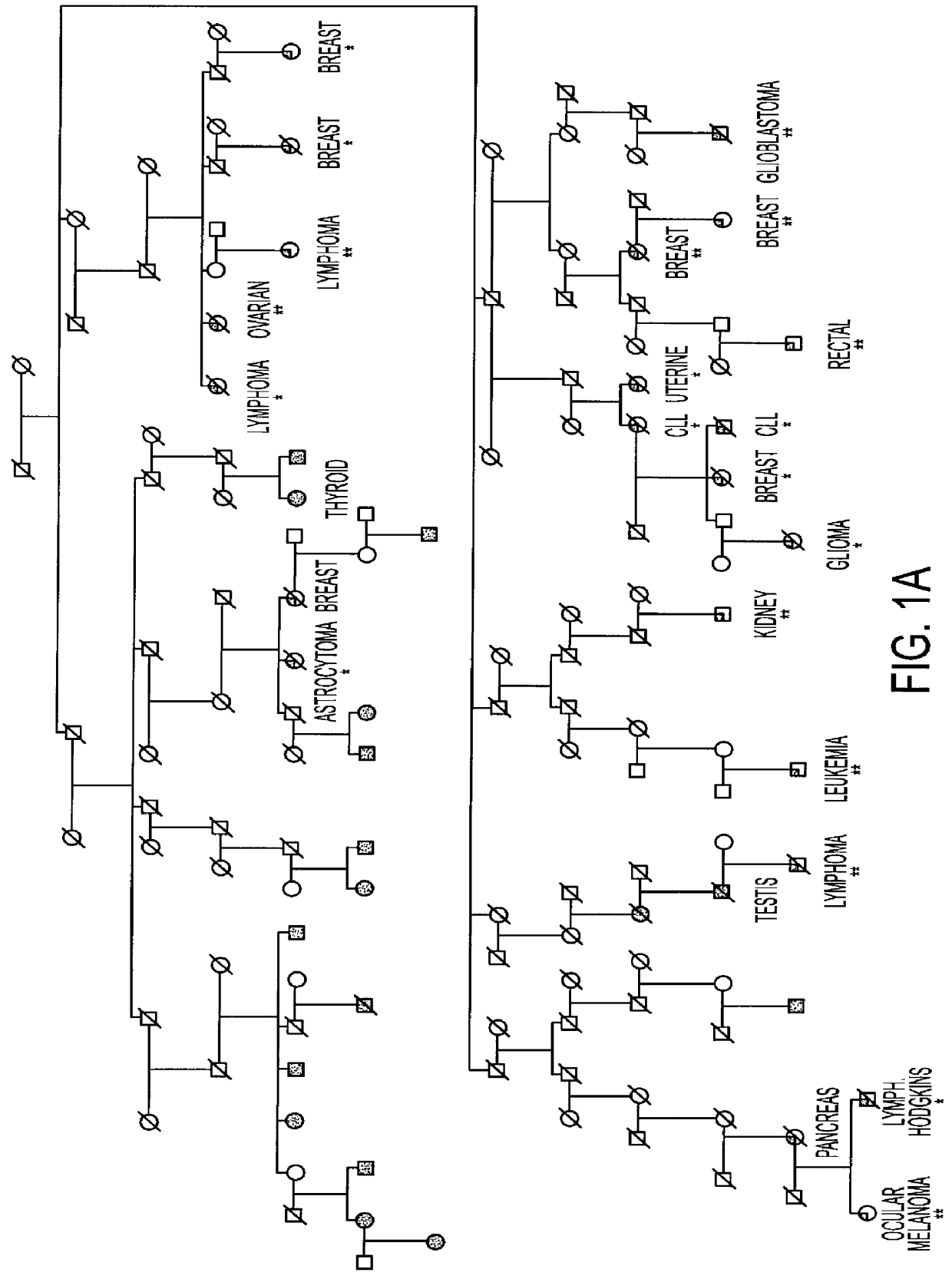
The present invention relates to somatic mutations in the Multiple Tumor Suppressor (MTS) gene in human cancers and their use in the diagnosis and prognosis of human cancer. The invention further relates to germ line mutations in the MTS gene and their use in the diagnosis of predisposition to melanoma, leukemia, astrocytoma, glioblastoma, lymphoma, glioma, Hodgkin's lymphoma, CLL, and cancers of the pancreas, breast, thyroid, ovary, uterus, testis, kidney, stomach and rectum. The invention also relates to the therapy of human cancers which have a mutation in the MTS gene, including gene therapy, protein replacement therapy and protein mimetics. Finally, the invention relates to the screening of drugs for cancer therapy.
Owner:MYRIAD GENETICS
Compositions and methods for diagnosing tumors using LEDGF/p75
InactiveUS8168393B2Microbiological testing/measurementPeptide preparation methodsAbnormal tissue growthMammal
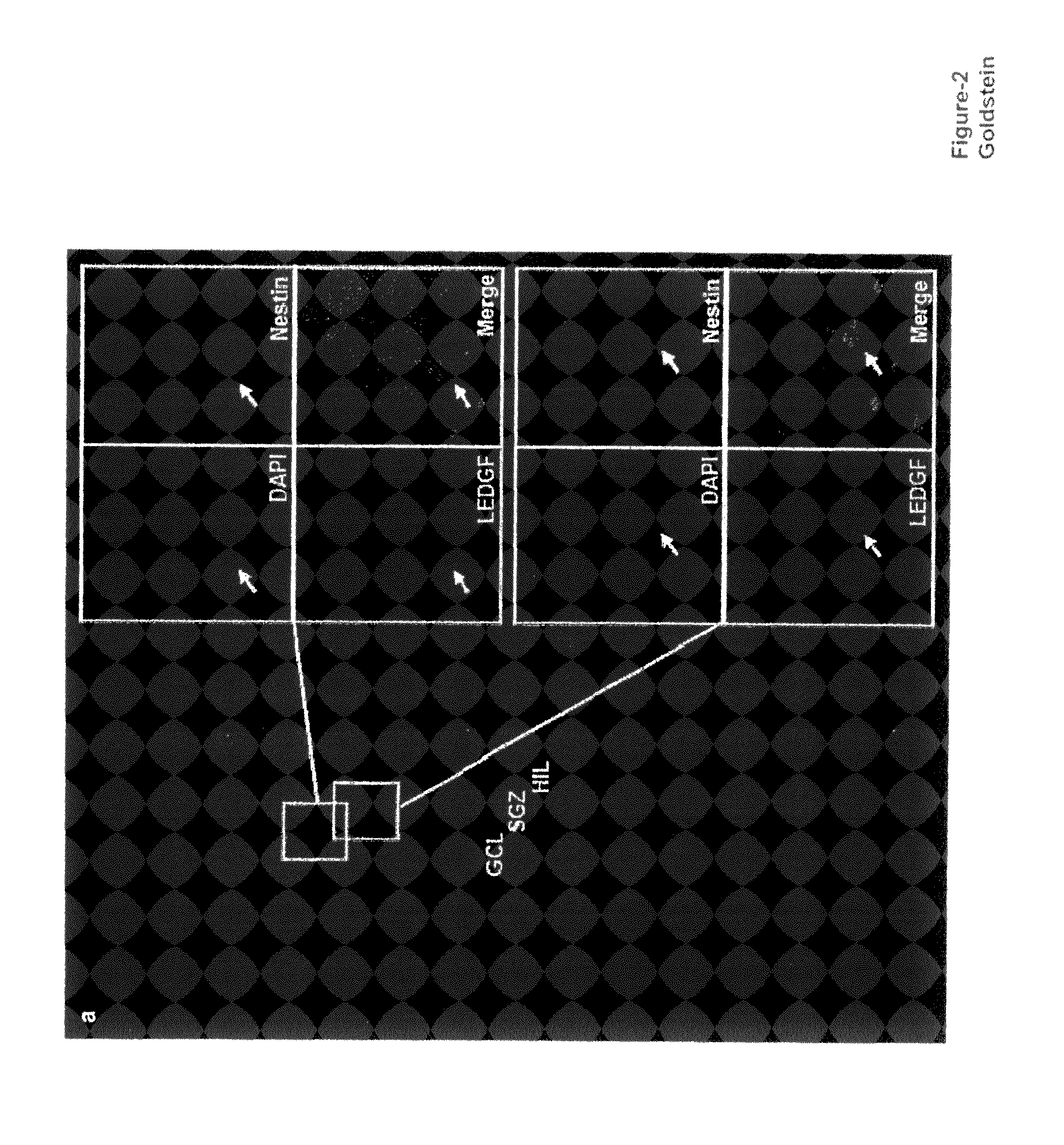
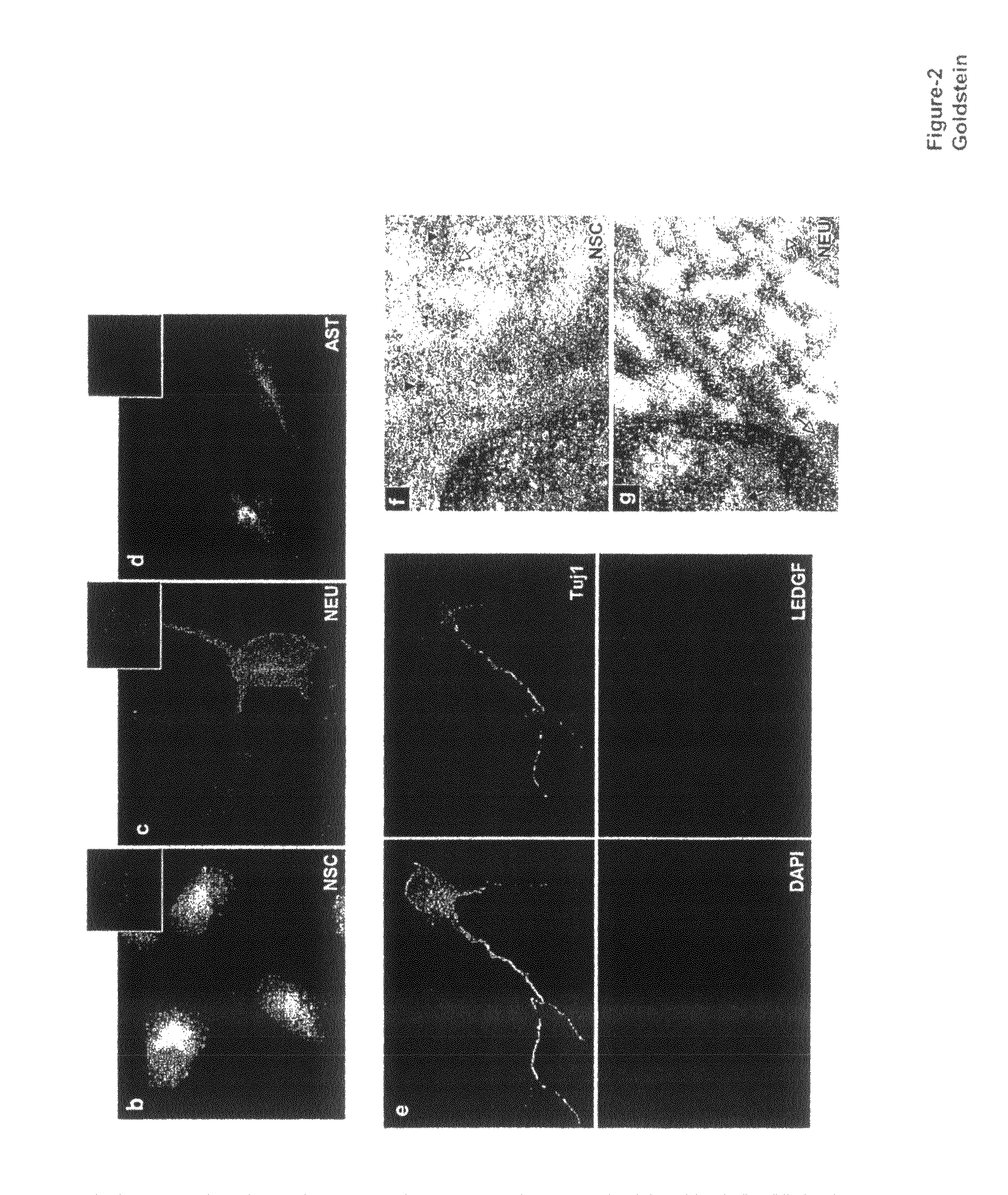
The invention provides methods for diagnosing tumors in mammals using a reagent that bind to LEDGF / p75 or to a nucleic acid encoding LEDGF / p75. For example, the tumor may be located in the CNS, the prostate, the skin, the bone marrow, or the gut of the mammal. Also provided are methods for diagnosing brain tumors such as medulloblastomas, meningiomas, astrocytomas, glioblastomas multiforme, and ependymomas by examining LEDGF / p75 or a nucleic acid encoding LEDGF / p75 localization. The invention also involves methods for diagnosing cancers involving cancerous epithelial cells such as colon cancer. The instant invention also provides methods for isolating stem cells from a heterogeneous population of cells, as well as methods for identifying neuroepithelial stem cells, newly differentiated neurons, and astrocytes in a subject. Also provided are methods for inducing the differentiation of neuroepithelial stem cells into astrocytes or neurons and methods for screening candidate compounds that regulate the differentiation of neuroepithelial stem cells.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Novel Primers for Identification of Astrocytoma, Its Grades and Glioblastoma Prognosis
ActiveUS20110027784A1High expressionPoor survivalSugar derivativesMicrobiological testing/measurementGlioblastomaDiffuse Astrocytoma
The present invention relates to novel primers for identification of astrocytoma, it's grades and glioblastoma prognosis. Further, disclosed is a method of diagnosing the presence of different grades of diffuse astrocytoma and glioblastoma, in a human subject, which involves detection of the expression levels of said genes in tumor tissue samples in comparison to normal brain. Also disclosed is a method of distinguishing between the two types of Glioblastoma—the progressive and de novo types. Also disclosed is a method of prognosis of glioblastoma based on the expression of the gene PBEF1, wherein the higher level of expression of the gene in the tumor sample, indicates poorer survival of the human subject. The disclosed compositions are useful, for example, in the diagnosis, prevention, treatment and / or prognosis of astrocytoma. The invention further provides kits for the detection and prognosis of the said diseases.
Owner:COUNCIL OF SCI & IND RES
Transgenic animals and cell lines for screening drugs effective for the treatment or prevention of Alzheimer's disease
Disclosed are transgenic animals and transfected cell lines expressing a protein associated with Alzheimer's Disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas. Also disclosed is the use of such transgenic animals and transfected cell lines to screen potential drug candidates for treating or preventing Alzheimer's disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas. The invention also relates to new antisense oligonucleotides, ribozymes, triplex forming DNA and external guide sequences that can be used to treat or prevent Alzheimer's disease, neuroectodermal tumors, malignant astrocytomas, and glioblastomas.
Owner:THE GENERAL HOSPITAL CORP
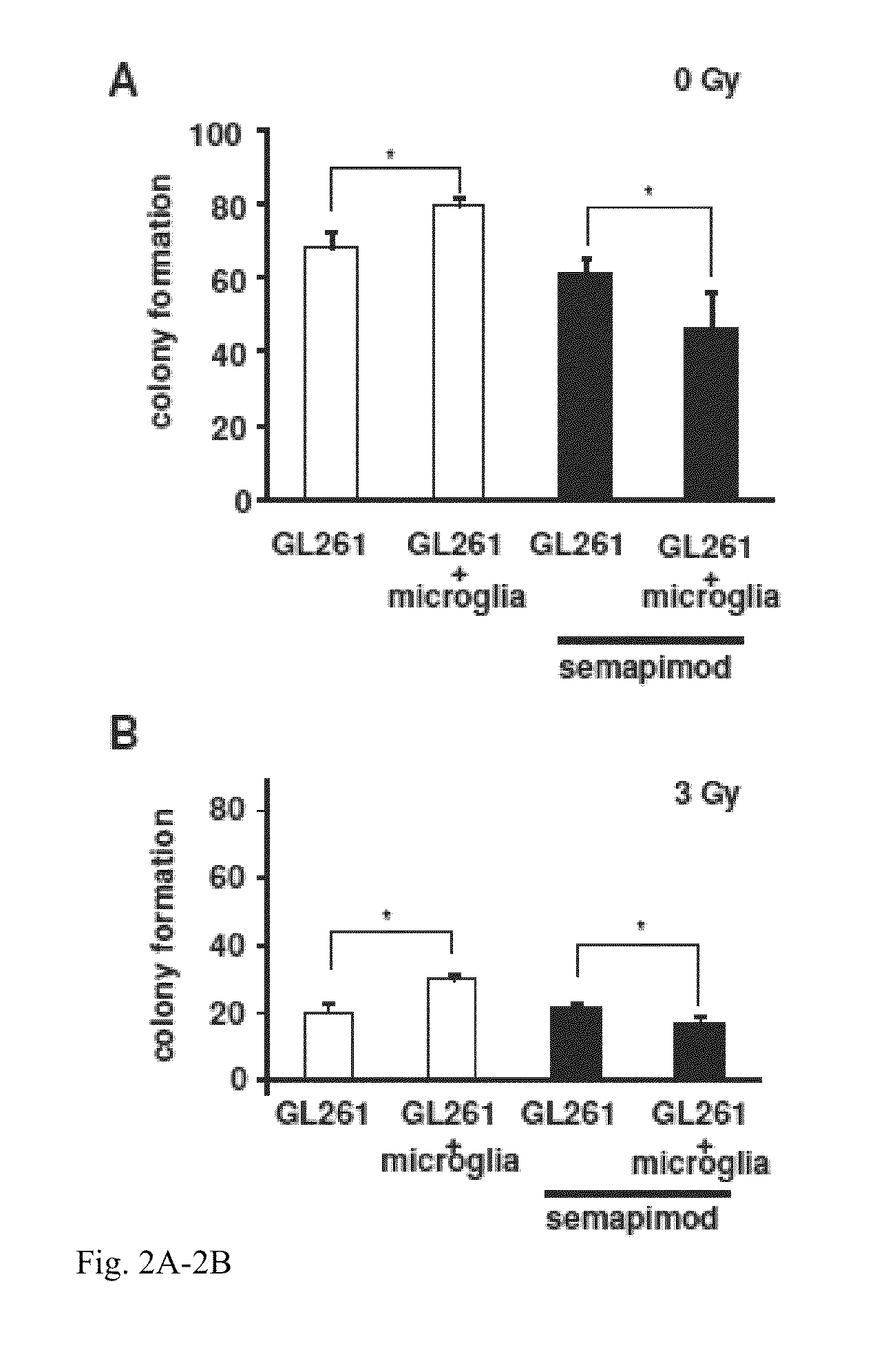
Method for treating glioblastomas and other tumors
InactiveUS9440914B2Good curative effectInhibit functioningOrganic active ingredientsOrganic chemistryMISCELLANEOUS TUMORSGlioblastoma
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Methods of using LEDGF/p75
The invention provides methods for diagnosing tumors in mammals using a reagent that bind to LEDGF / p75 or to a nucleic acid encoding LEDGF / p75. For example, the tumor may be located in the CNS, the prostate, the skin, the bone marrow, or the gut of the mammal. Also provided are methods for diagnosing brain tumors such as medulloblastomas, meningiomas, astrocytomas, glioblastomas multiforme, and ependymomas by examining LEDGF / p75 or a nucleic acid encoding LEDGF / p75 localization. The invention also involves methods for diagnosing cancers involving cancerous epithelial cells such as colon cancer. The instant invention also provides methods for isolating stem cells from a heterogeneous population of cells, as well as methods for identifying neuroepithelial stem cells, newly differentiated neurons, and astrocytes in a subject. Also provided are methods for inducing the differentiation of neuroepithelial stem cells into astrocytes or neurons and methods for screening candidate compounds that regulate the differentiation of neuroepithelial stem cells.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
(s,r.)-3-phenyl-4,5 dihydro-5-isoxazole acetic acid-nitric oxide and use thereof as Anti-cancer and antiviral agent
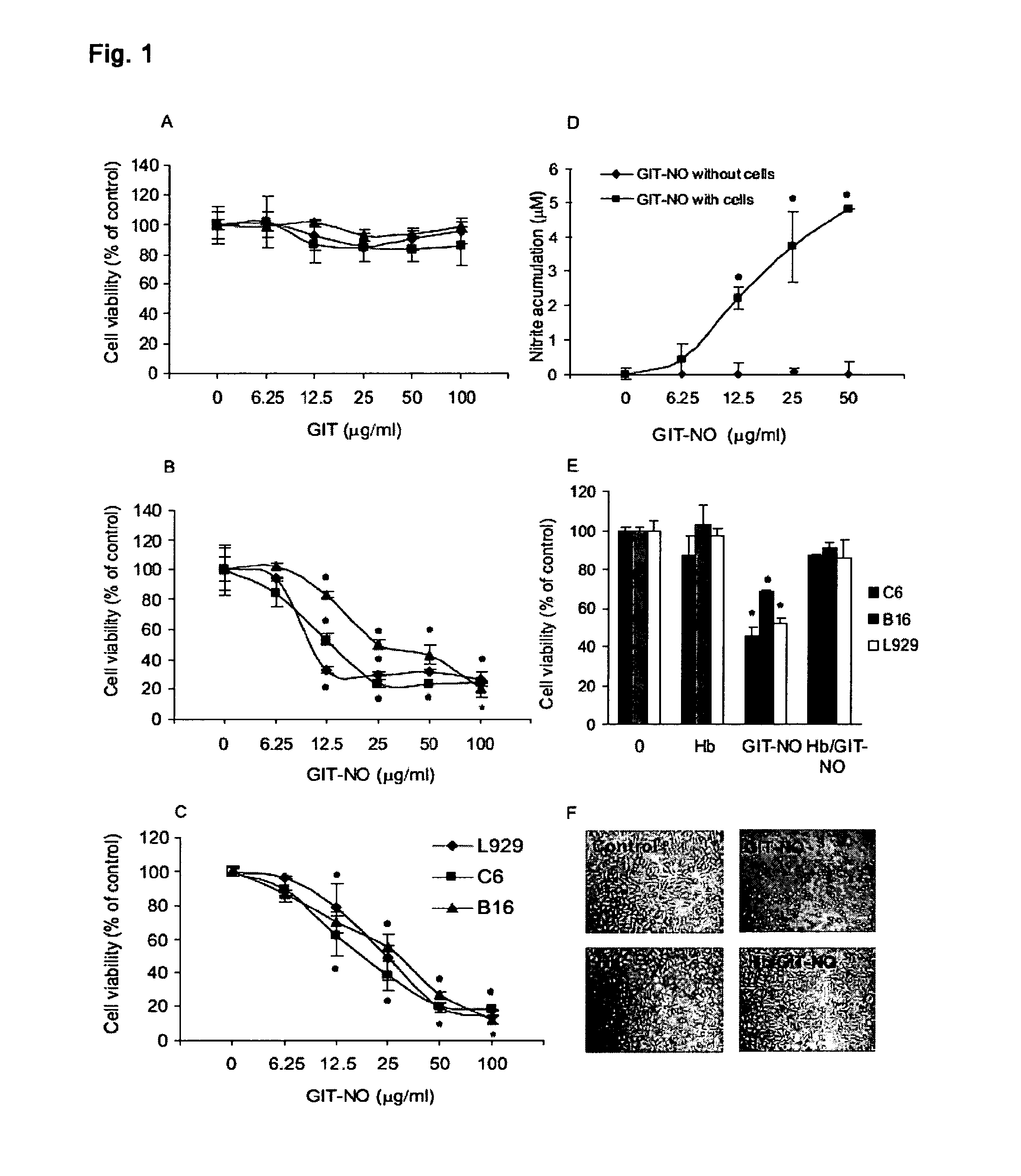
The present invention relates to an isoxazole derivative, the compound of formula (I)herein after referred to as GIT27-NO, which is the NO-donating structurally modified form of (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid, herein after referred to as VGX-1027.Treatment of three tumor cell lines, rat astrocytoma C6, mouse fibrosarcoma L929, and mouse melanoma B16 cells with GIT27-NO resulted in a significant reduction of cell respiration and of number of viable cells, while VGX-1027 was completely ineffective. Hemoglobin, which act as NO-scavenger, restored cell viability, thus indicating the NO-mediated tumoricidal effect of compound (I). GIT27-NO triggered apoptotic cell death in L929 cell cultures, while autophagic cell death is mainly responsible for the diminished viability of C6 and B16 cells. Moreover, GIT27-NO induced the production of reactive oxygen species which can be neutralized by antioxidant N-acetyl cysteine (NAC), indicating that reactive oxygen species (ROS) are at least partly involved in the reduction of cell viability. The anti-tumor activity of GIT27-NO is mediated through activation of MAP kinases (ERK1 / 2, p38 and JNK) in cell-specific manner. The role of MAP kinases was further confirmed by specific inhibitors of these molecules, PD98059, SB202190, and SP600125. Finally, in vivo treatment with GIT27-NO significantly reduced tumor growth in syngeneic C57BL / 6 mice implanted with B16 melanoma.
Owner:ONCONOX
Methods for diagnosis of low grade astrocytoma
InactiveUS20060281138A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsBiologyAntibody
A method for identifying low grade astrocytoma cells in a sample is provided, wherein the method distinguishes between low grade astrocytoma cells and normal astrocytes, thus permitting early diagnosis of astrocytoma. The method uses antibody directed against JI-31 to test astrocytes in the sample for the presence or absence of JI-31 polypeptide, the low grade astrocytoma cells being characterized by the absence of JI-31 while normal astrocytes are characterized by the presence of JI-31.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com