Cross-linked polymer gel electrolyte membrane supported by hydrophilic polytetrafluoroethylene microporous membrane
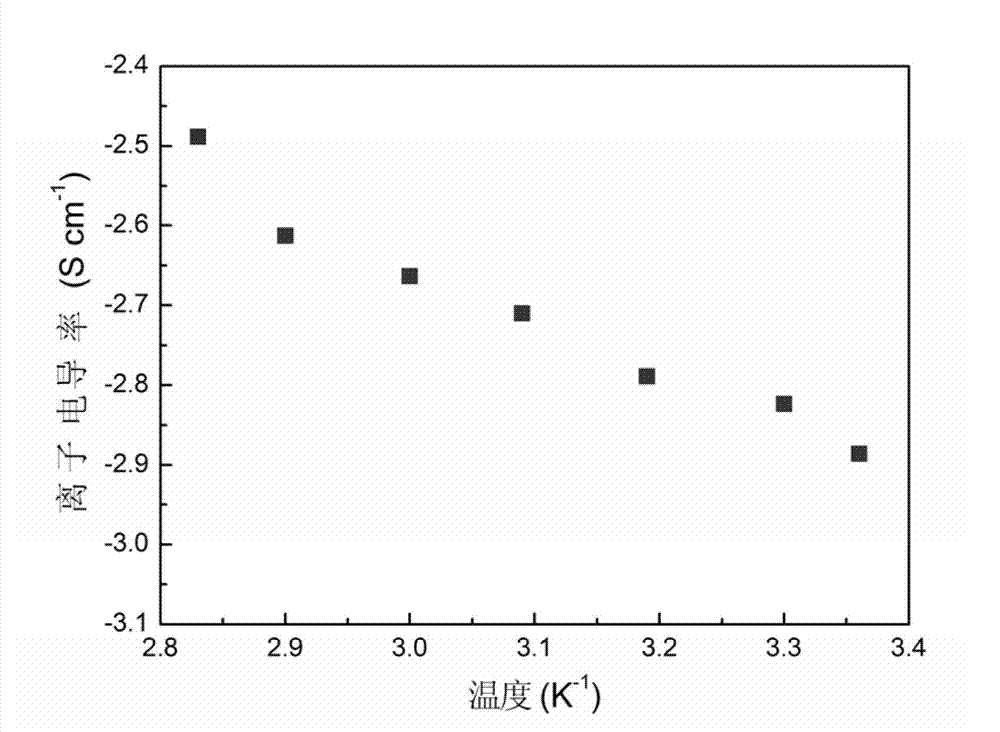
A technology of polytetrafluoroethylene and cross-linked gel, which is applied in the direction of circuits, electrical components, secondary batteries, etc., can solve the problem of the degradation of the mechanical properties of the gel polymer electrolyte, the poor affinity of the liquid electrolyte, and the safety of the battery performance degradation and other problems, to achieve excellent thermal stability and chemical stability, good interfacial compatibility, and prevent electrolyte leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
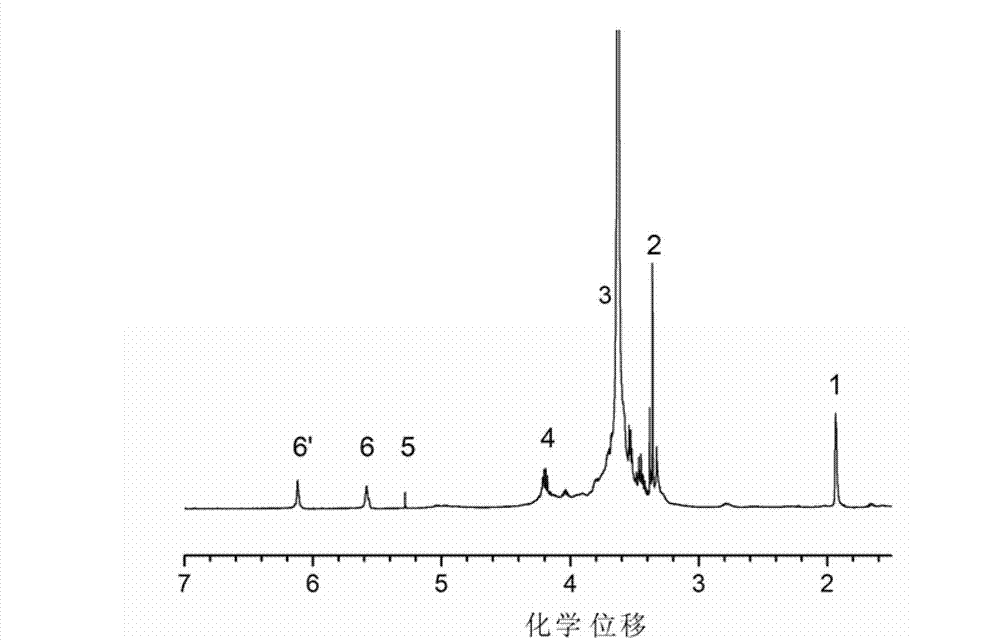
[0030] (1) Add 15g (0.015mol) of polyethylene glycol monomethyl ether (Mn=10 3 ) and 14.2g (0.1mol) of glycidyl methacrylate as a reaction monomer, then add 80ml of dichloromethane solvent, then stir until completely dissolved, under the protection of nitrogen, put it in an ice-salt bath, wait for ice The temperature of the salt bath was lowered to -12°C, and then 1.9ml (0.015mol) of boron trifluoride ether catalyst was added with a syringe to initiate the cationic ring-opening polymerization of polyethylene glycol monomethyl ether and glycidyl methacrylate, and the reaction was 50 Minutes later, a small amount of methanol was added dropwise to terminate the reaction. After the reaction was completed, the product was purified by filtration, dissolved in dichloromethane and precipitated with anhydrous ether, and repeated 3 times to obtain a transparent viscous liquid, which was vacuum-dried at room temperature for 12 hours to obtain polyethylene glycol monomethyl ether and meth...
Embodiment 2
[0043] (1) Add 15g (0.015mol) of polyethylene glycol monomethyl ether (Mn=350) and 14.2g (0.1mol) of glycidyl methacrylate as reaction monomers in a three-necked flask, then add 80ml of Dichloromethane solvent, then stir until it is completely dissolved, put it in an ice-salt bath under the protection of nitrogen, wait until the temperature of the ice-salt bath drops to -15°C, and then add 1.9ml (0.015mol) of boron trifluoride with a syringe The ether catalyst initiates the cationic ring-opening polymerization of polyethylene glycol monomethyl ether and glycidyl methacrylate, and drops a small amount of methanol to terminate the reaction after 50 minutes of reaction. After the reaction is completed, filter and purify the product, dissolve the product in dichloromethane and precipitate with anhydrous ether, repeat 3 times to obtain a transparent viscous liquid, and vacuum-dry the obtained polyethylene glycol monomethyl ether and methacrylic acid at room temperature for 12 hours ...
Embodiment 3
[0048] (1) Add 15g (0.015mol) of polyethylene glycol monomethyl ether (Mn=5×10 3 ) and 14.2g (0.1mol) of glycidyl methacrylate as a reaction monomer, then add 80ml of dichloromethane solvent, then stir until completely dissolved, under the protection of nitrogen, put it in an ice-salt bath, wait for ice The temperature of the salt bath was lowered to -10°C, and then 1.9ml (0.015mol) of boron trifluoride ether catalyst was added with a syringe to initiate the cationic ring-opening polymerization of polyethylene glycol monomethyl ether and glycidyl methacrylate, and the reaction was 50 Minutes later, a small amount of methanol was added dropwise to terminate the reaction. After the reaction is completed, filter and purify the product, dissolve the product in dichloromethane and precipitate with anhydrous ether, repeat 3 times to obtain a transparent viscous liquid, and vacuum-dry the obtained polyethylene glycol monomethyl ether and methacrylic acid at room temperature for 12 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com