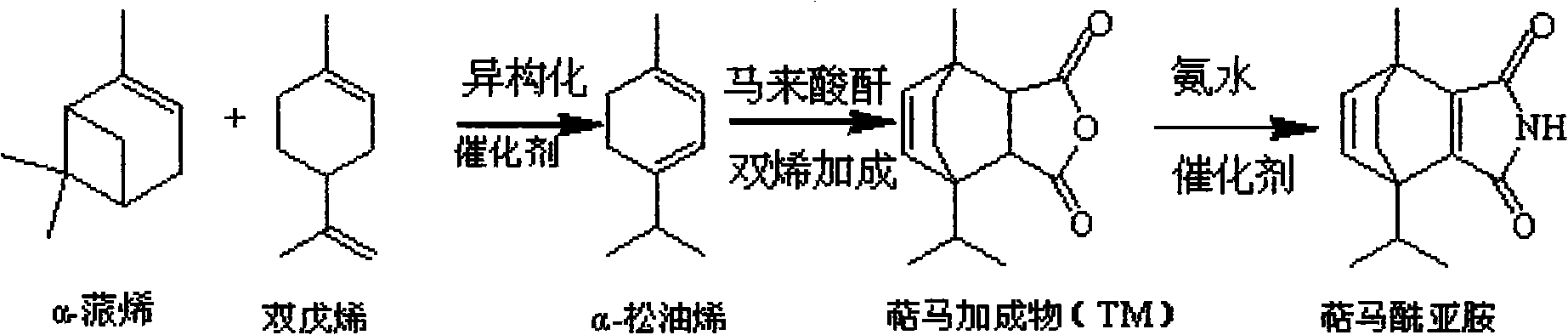
Preparation method for terpene maleimide
A technology of terpenes and terpenes, which is applied in the field of synthesizing terpenes, can solve the problems of short reaction time and long reaction time, and achieve the effects of less side reactions, high catalyst activity and low requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Catalyst preparation
[0030] Put D002 high-temperature-resistant hydrogen-type macroporous strongly acidic styrene-based cation-exchange resin into a zirconium nitrate solution with a weight content of 1.0%, the volume ratio of the ion-exchange resin to the solution is 1:3, and put it at a temperature of 50-55°C and - Under the vacuum degree of 0.05~-0.06MPa, soak for 8 hours, filter off the zirconium nitrate solution, and repeat the treatment 3 times under the same conditions. In the same way, the ion exchange resin is treated with 1.2% by weight titanium tetrachloride solution and 0.8% by weight cadmium iodate solution successively, and then washed with deionized water until there is no chloride ion. Drying at a temperature of 120°C and a vacuum of -0.08 to -0.095 MPa prepares a modified D002 high-temperature-resistant hydrogen-type macroporous strongly acidic styrene-based cation exchange resin catalyst.
Embodiment 2
[0032] Catalyst preparation
[0033] Put D002 dry hydrogen type macroporous strongly acidic styrene-based cation exchange resin into a zirconium nitrate solution with a weight content of 0.5%, the volume ratio of the ion exchange resin to the solution is 1:3, at a temperature of 50 to 55°C and -0.05 Under the vacuum degree of ~-0.06MPa, soak for 12 hours, filter out the zirconium nitrate solution, and repeat the treatment 3 times under the same conditions. In the same way, the ion exchange resin is treated with 1.5% by weight titanium tetrachloride solution and 1.2% by weight cadmium iodate solution successively, and then washed with deionized water until there is no chloride ion. Dry at a temperature of 120°C and a vacuum of -0.08 to -0.095 MPa to prepare a modified D002 dry hydrogen macroporous strongly acidic styrene-based cation exchange resin catalyst.
Embodiment 3
[0035] Catalyst preparation
[0036] Use the prepared 12.5% by weight copper nitrate, 12.5% by weight tungsten nitrate mixed solution with a volume ratio of 1:1 and 20% by weight sodium carbonate solution to stir and react, adjust the pH to about 7.5, and react for 4 hours to prepare Co-precipitates of copper oxide and tungsten trioxide were obtained, filtered by suction, washed with deionized water, dried at a temperature of 100-120°C and a vacuum of -0.08-0.095MPa, and calcined at 360-400°C for 5 hours. Then grind it evenly with activated carbon of the same quality to obtain a copper-tungsten catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com