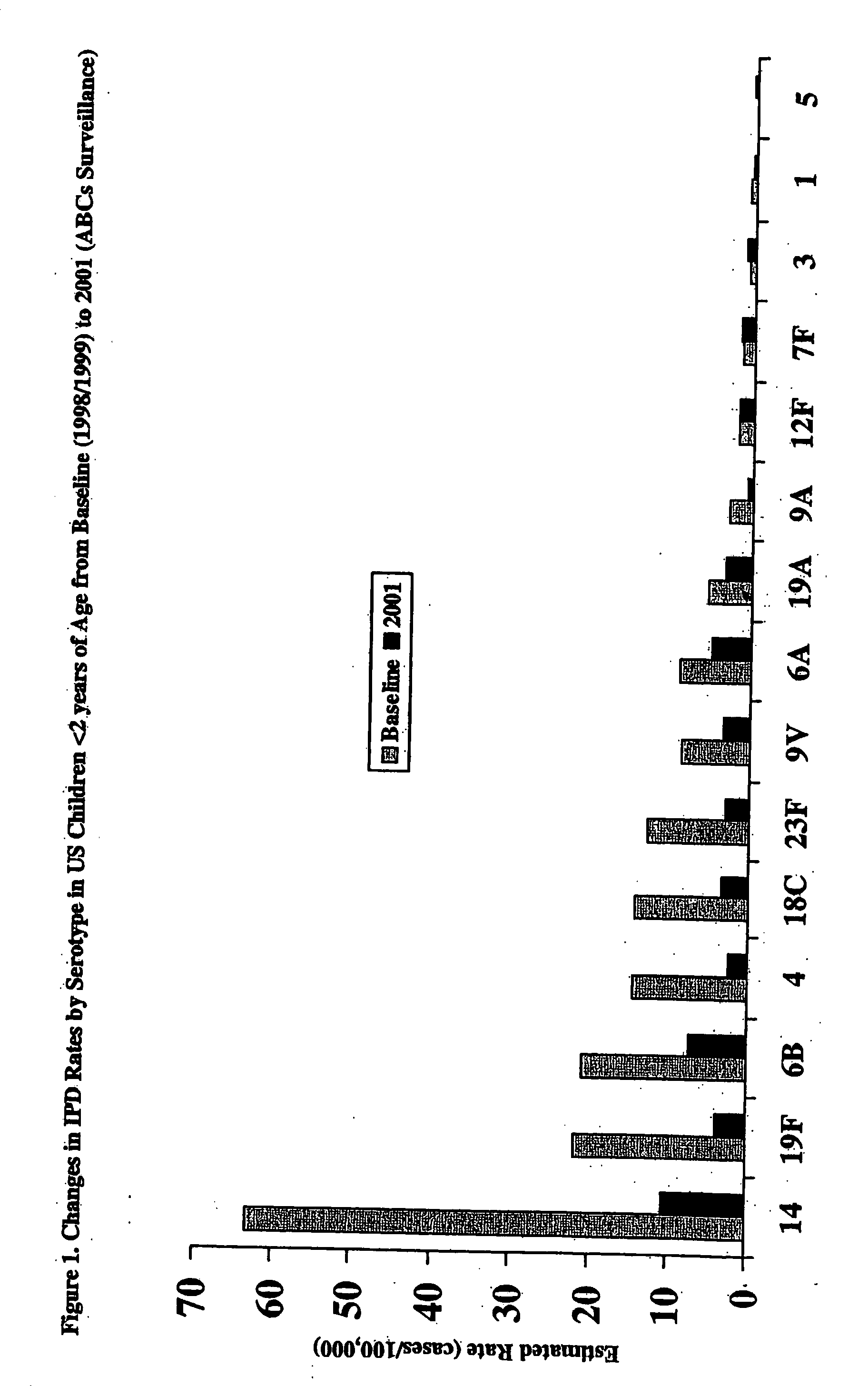
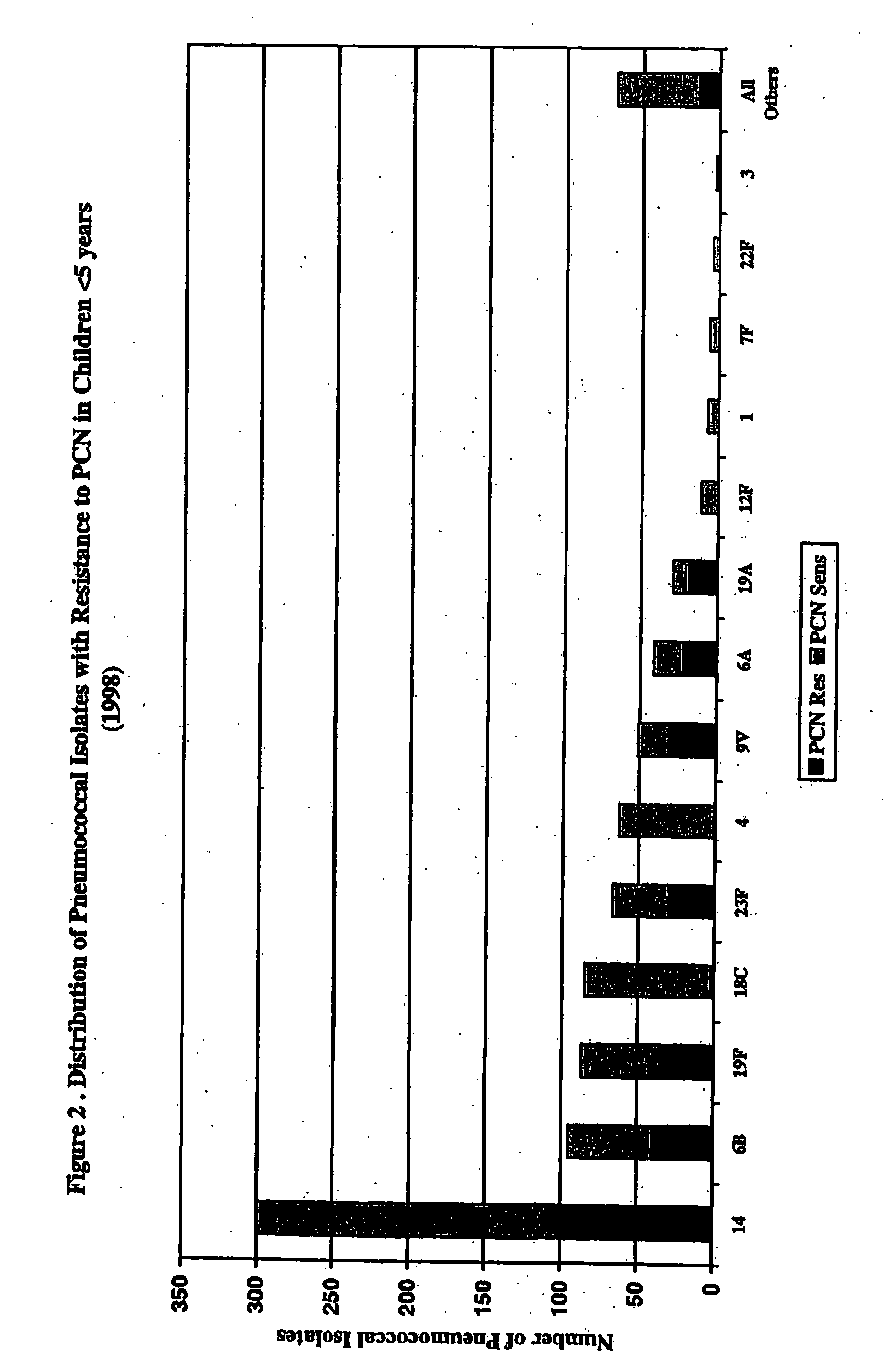
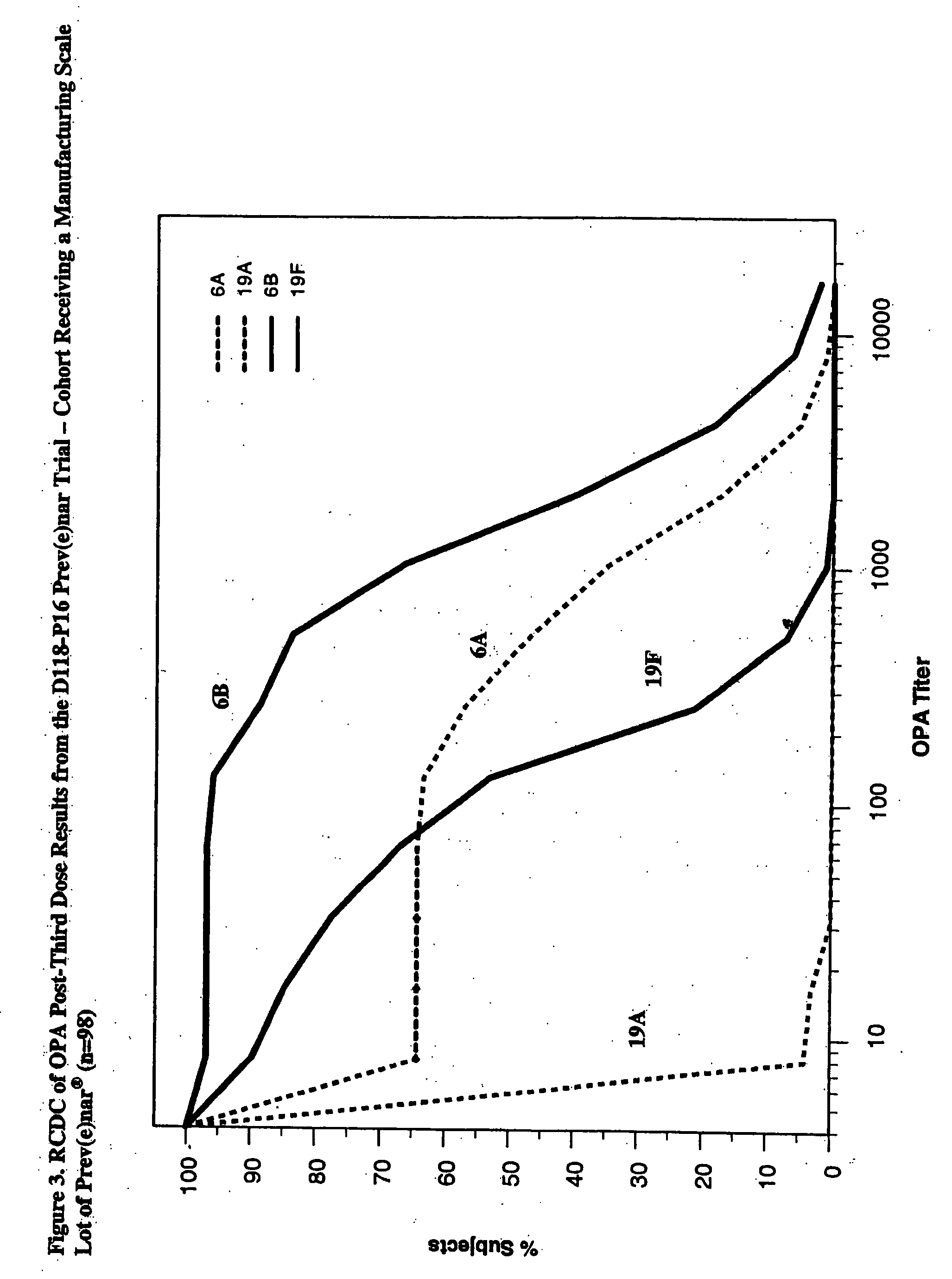
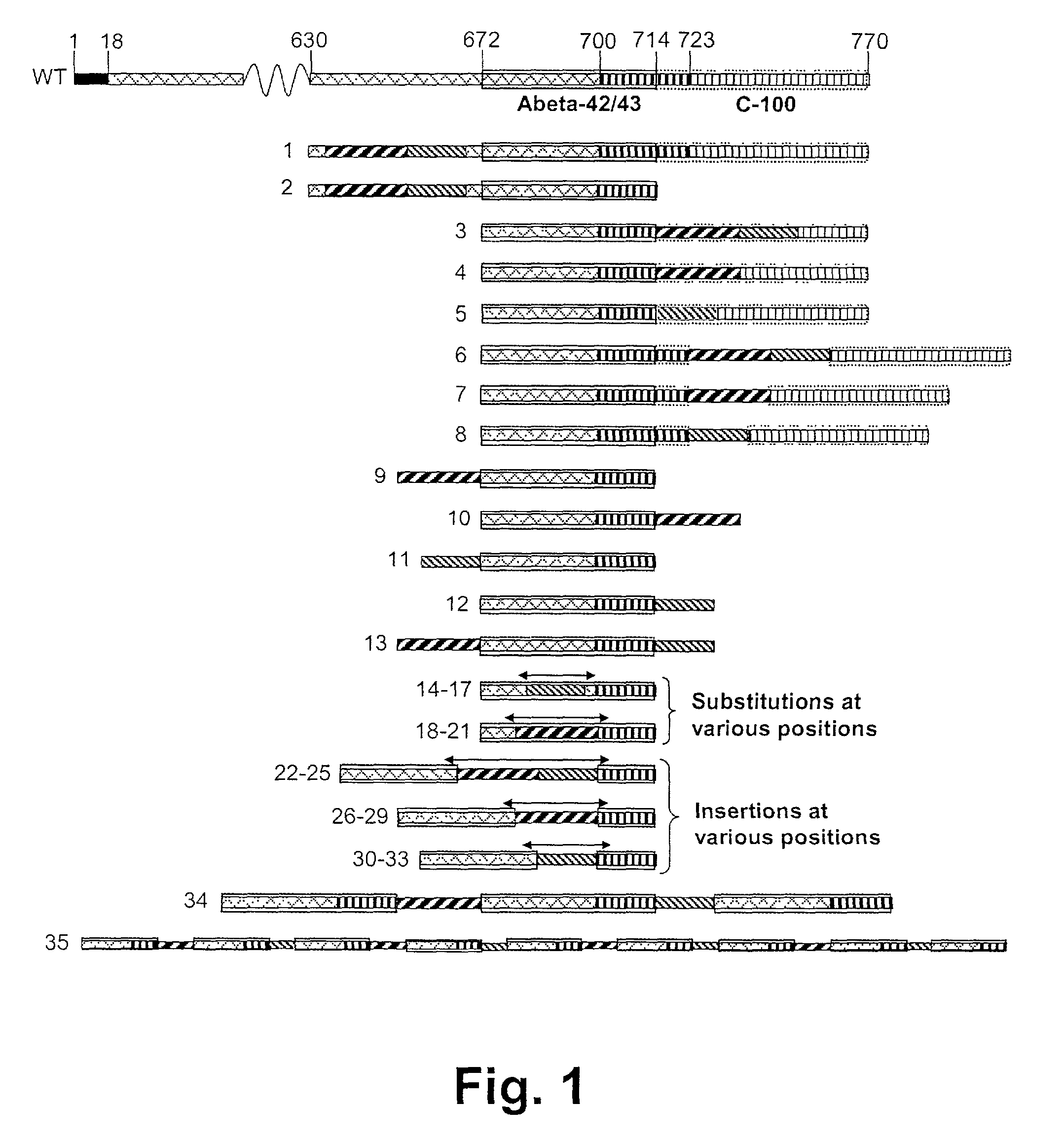
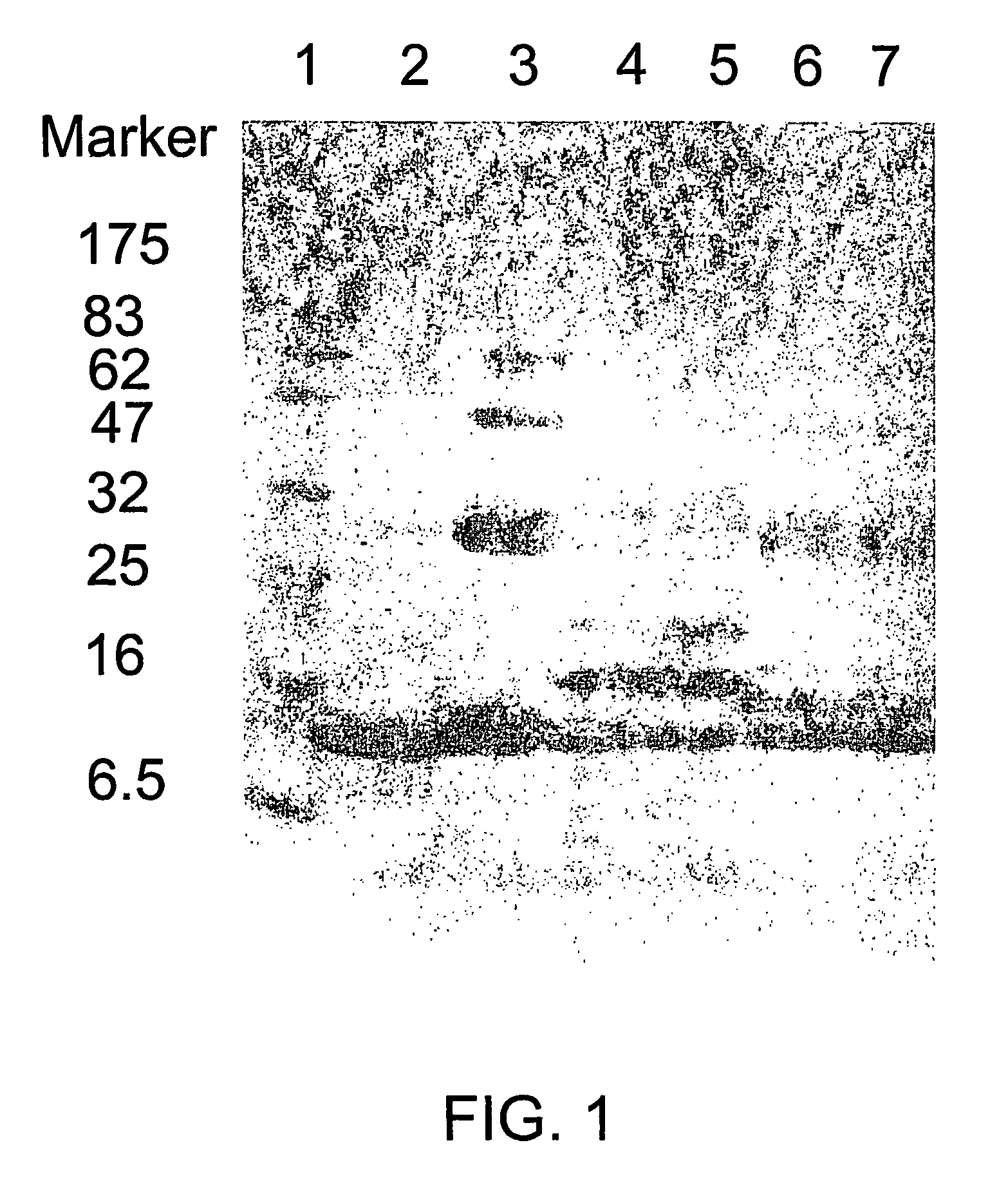
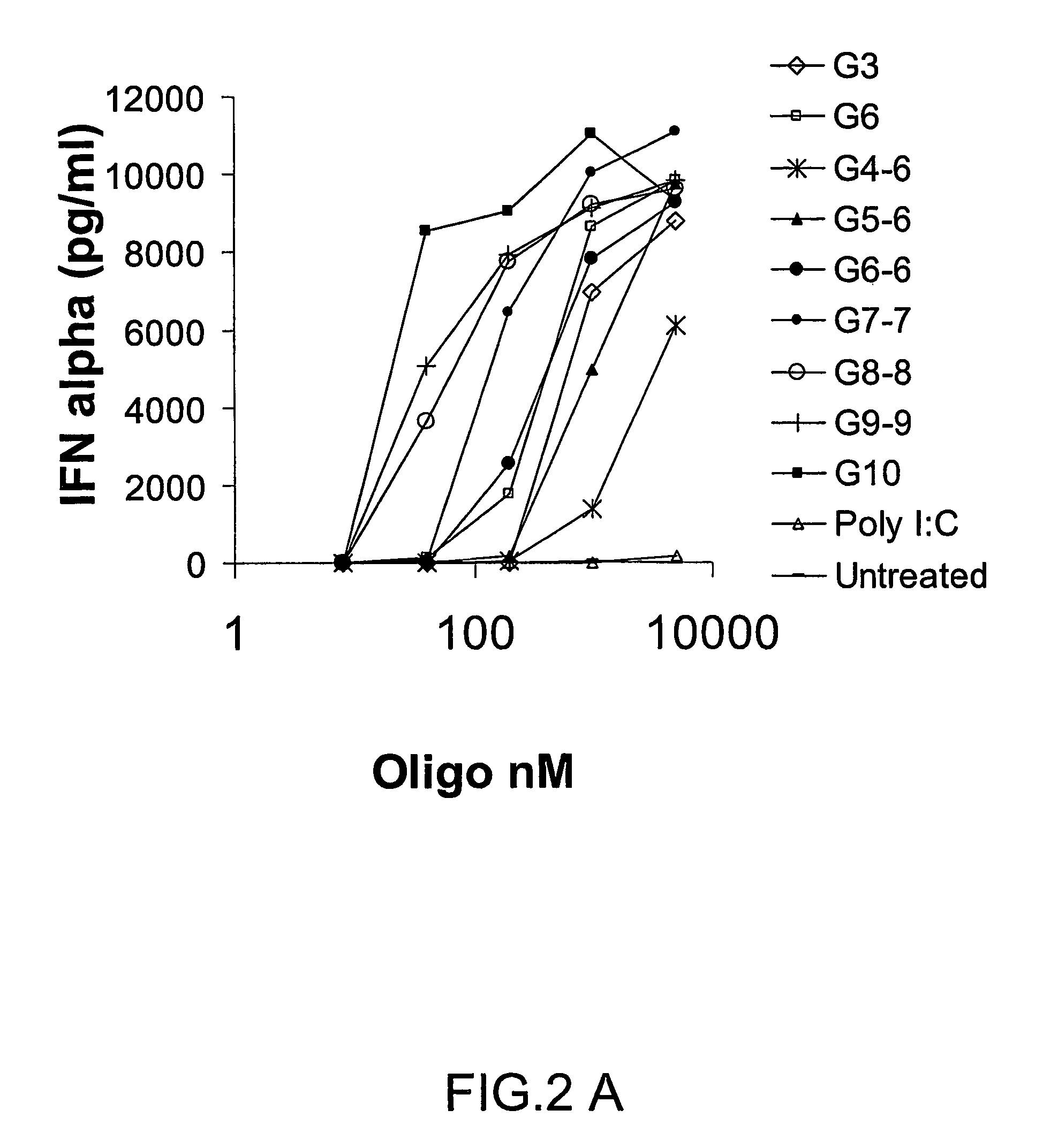
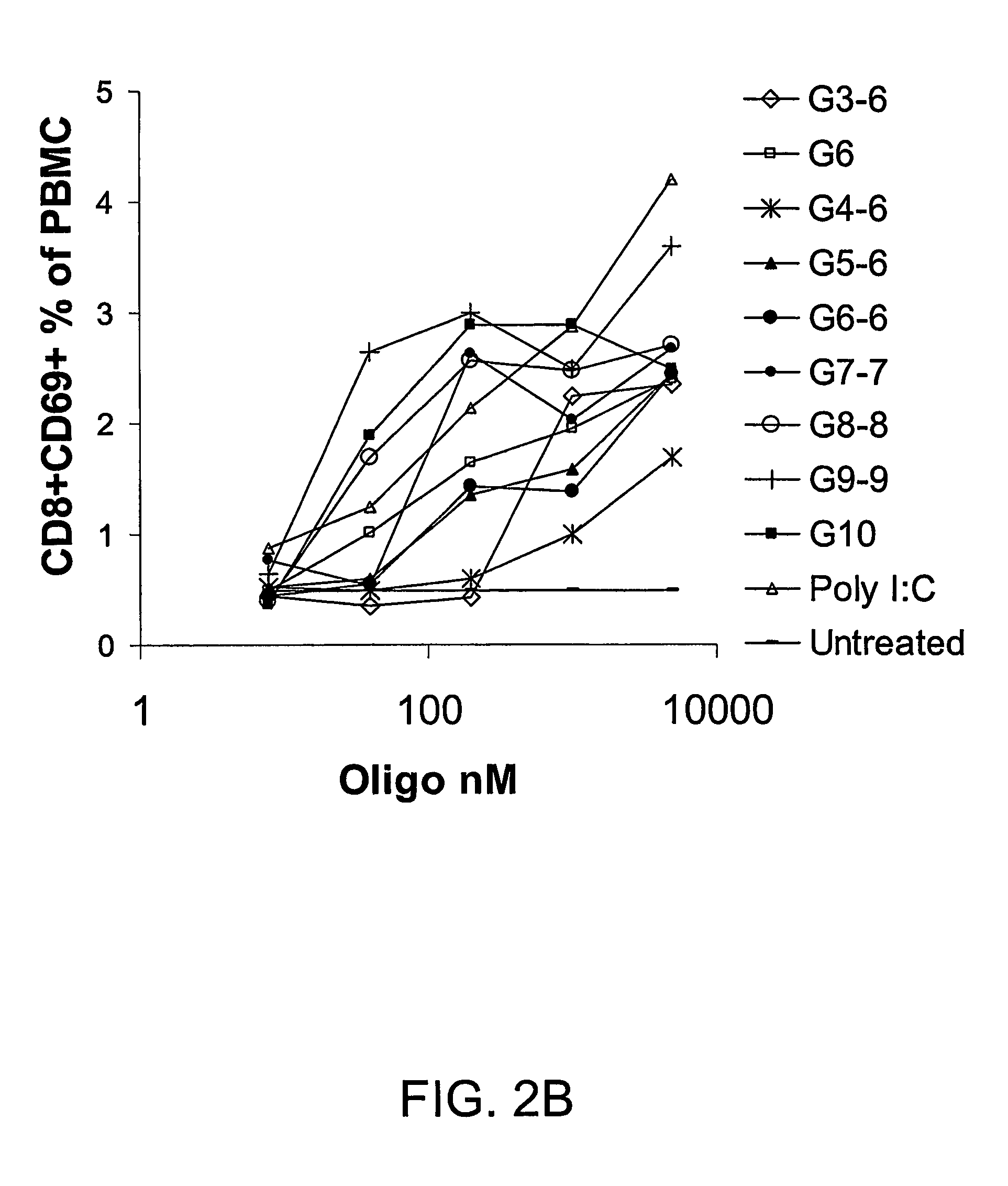
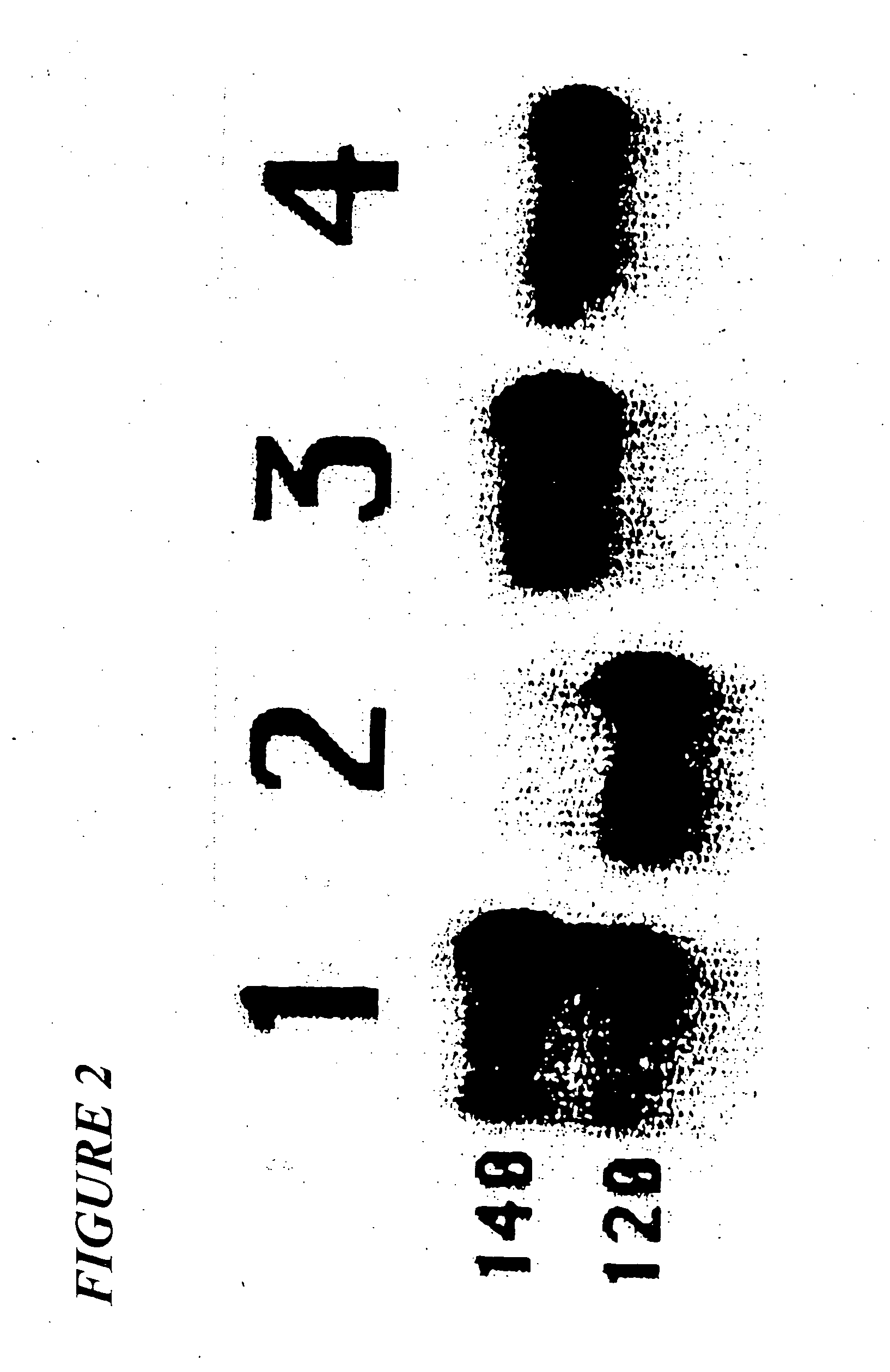
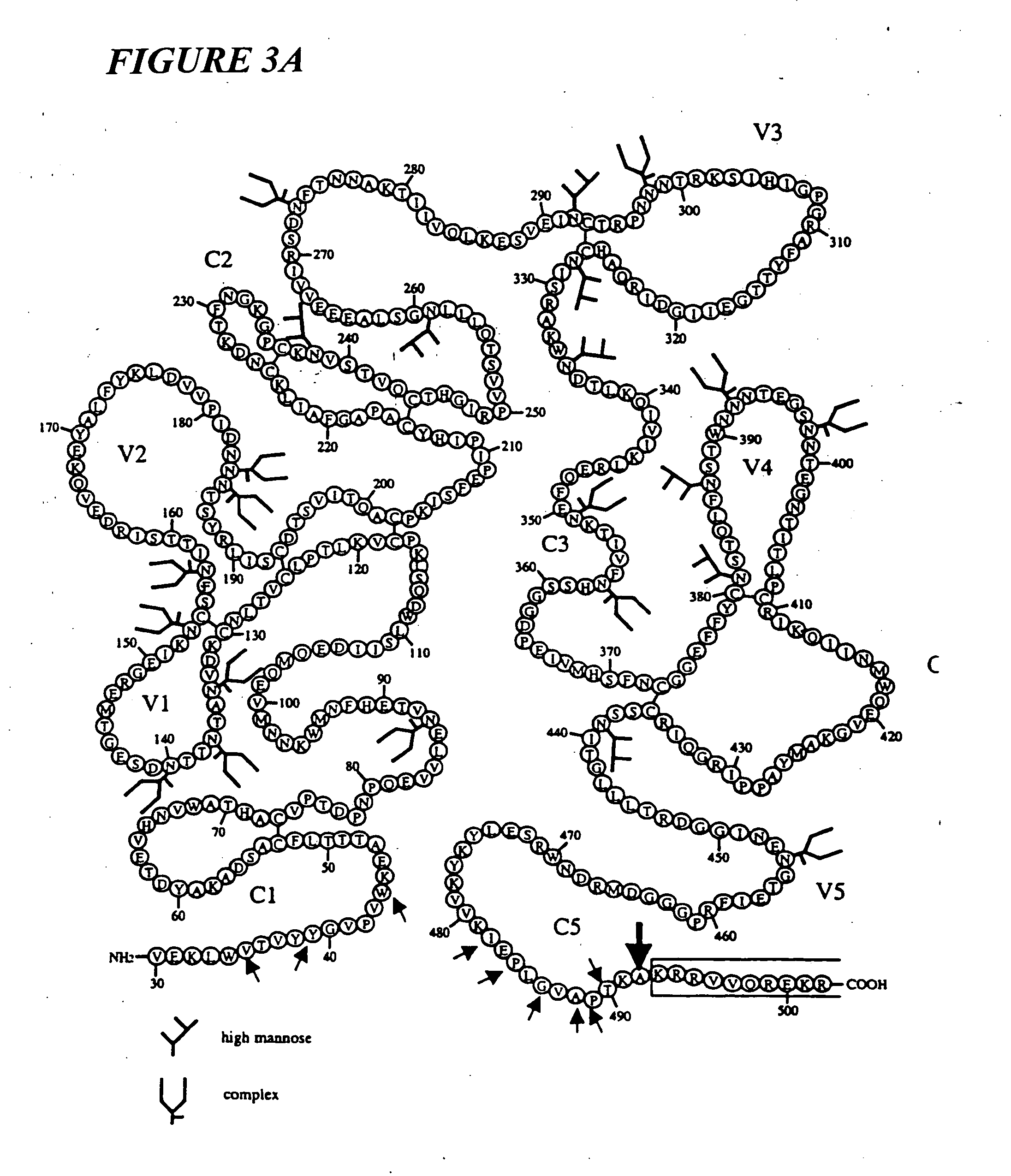
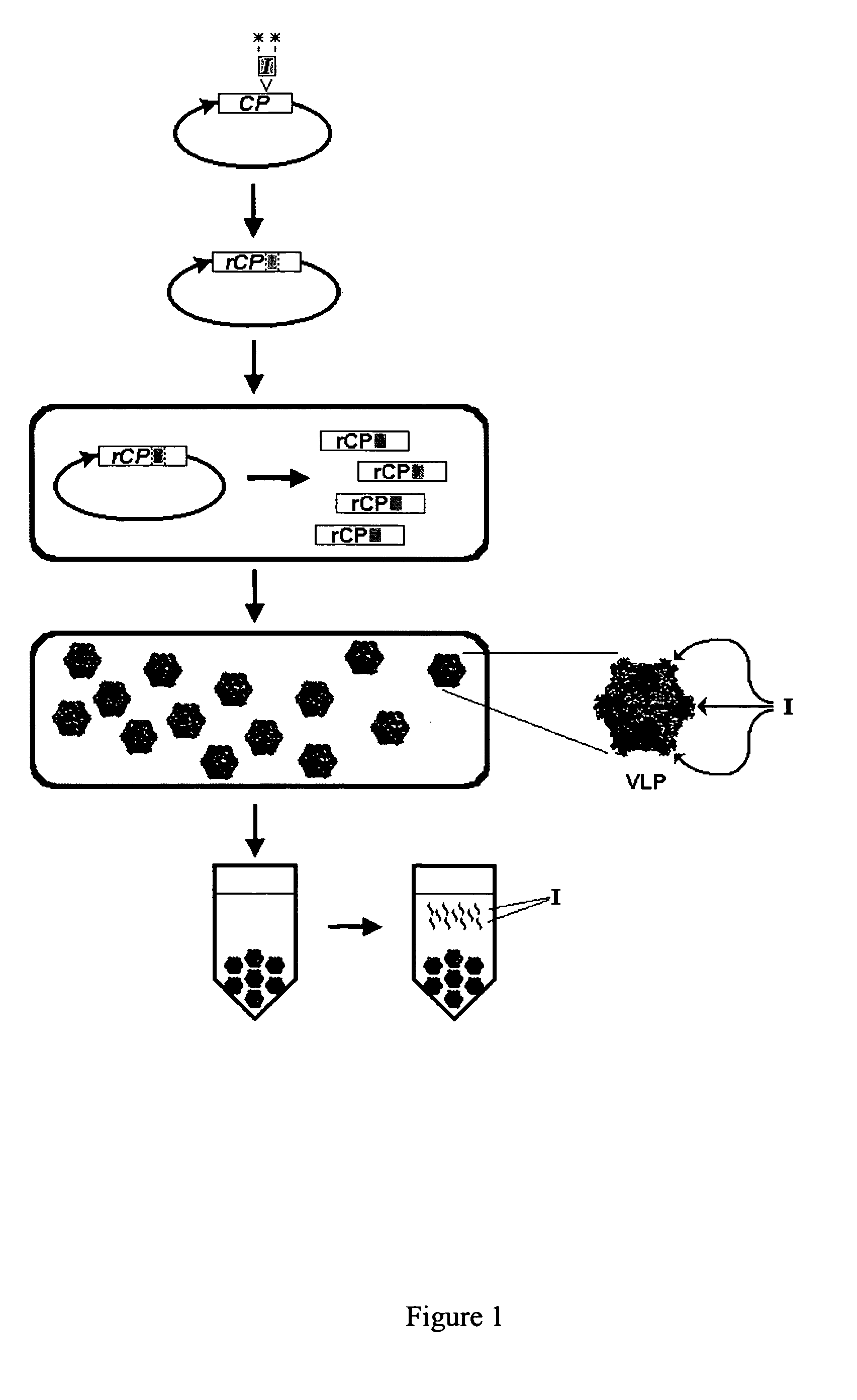
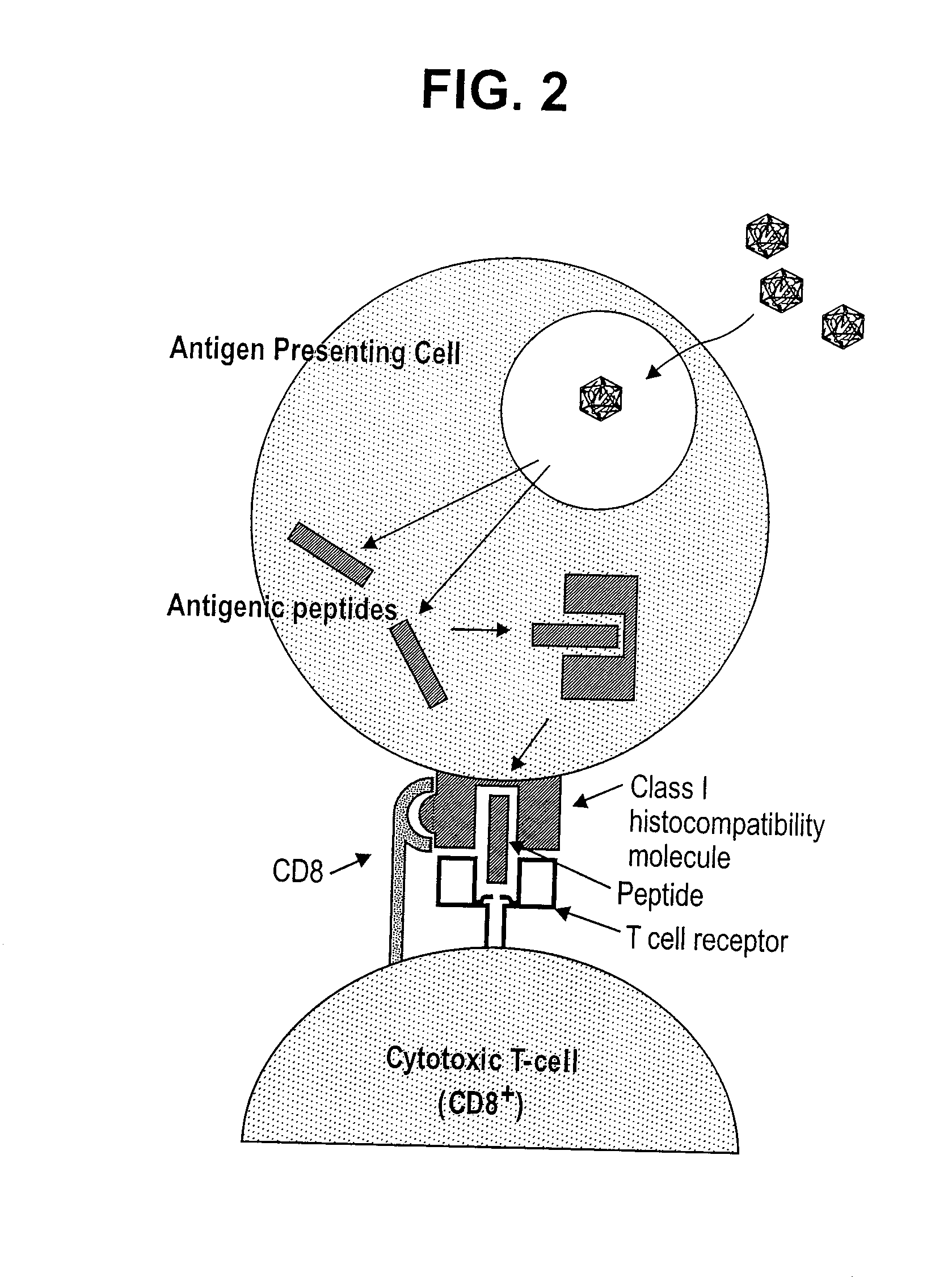
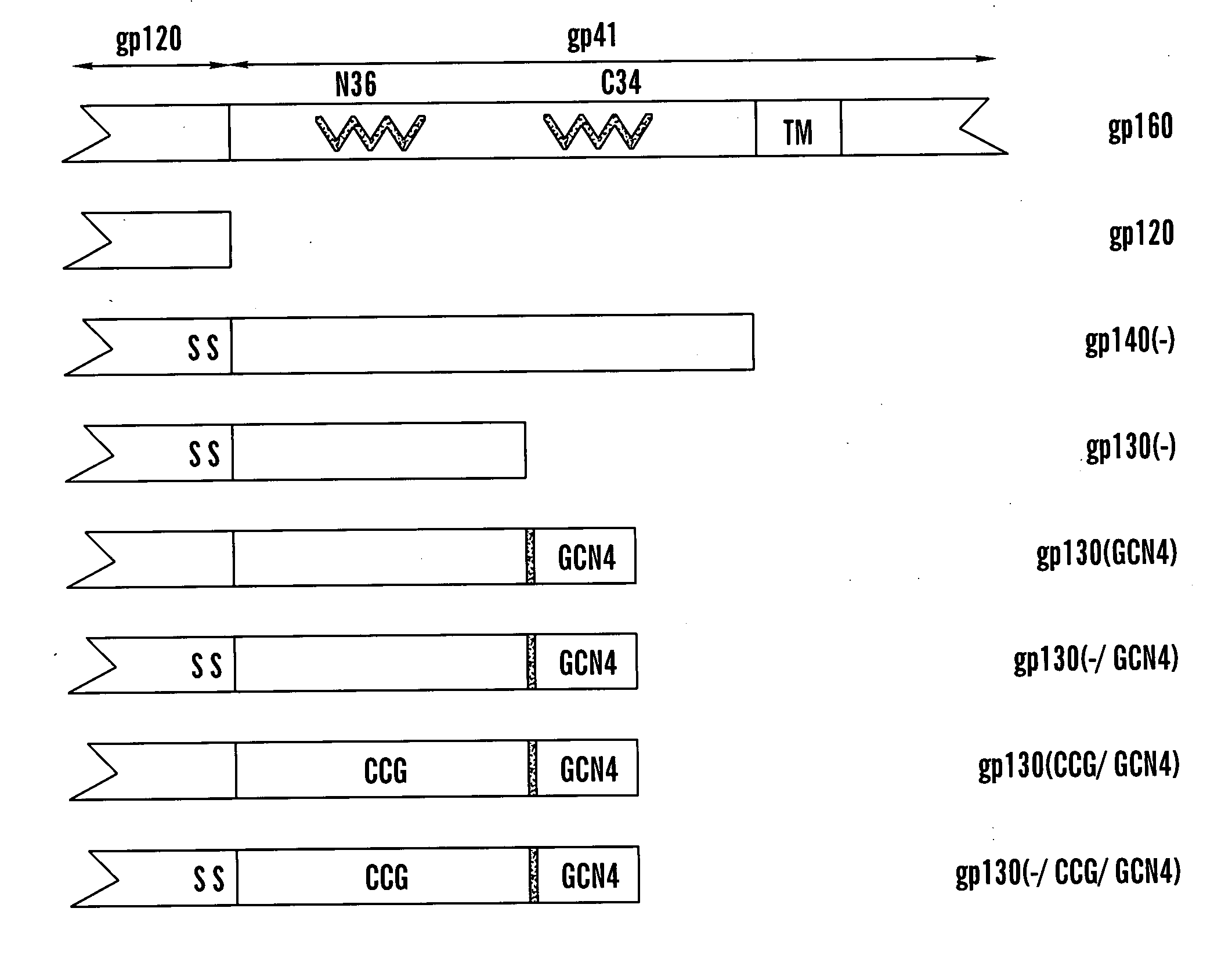
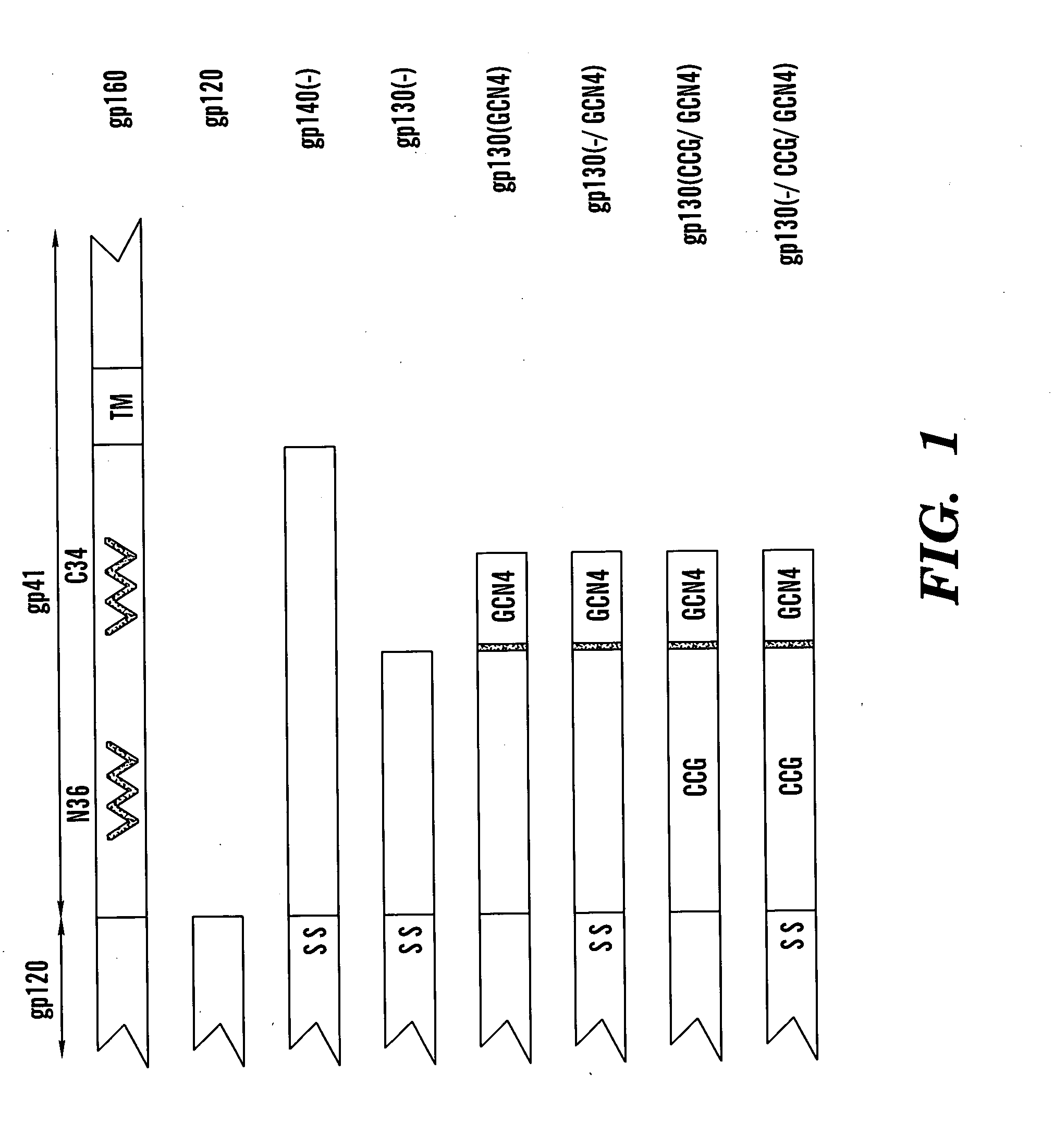
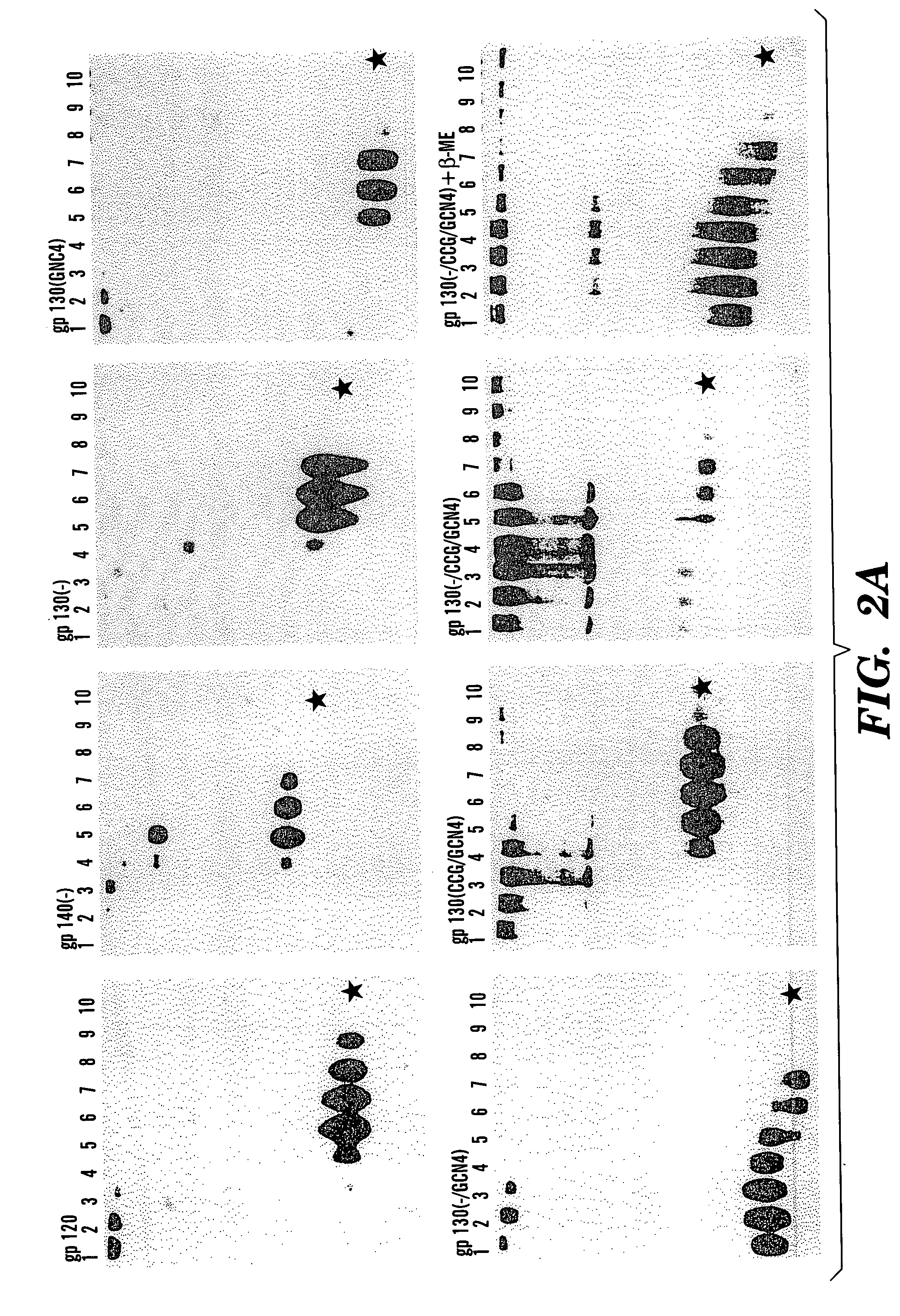
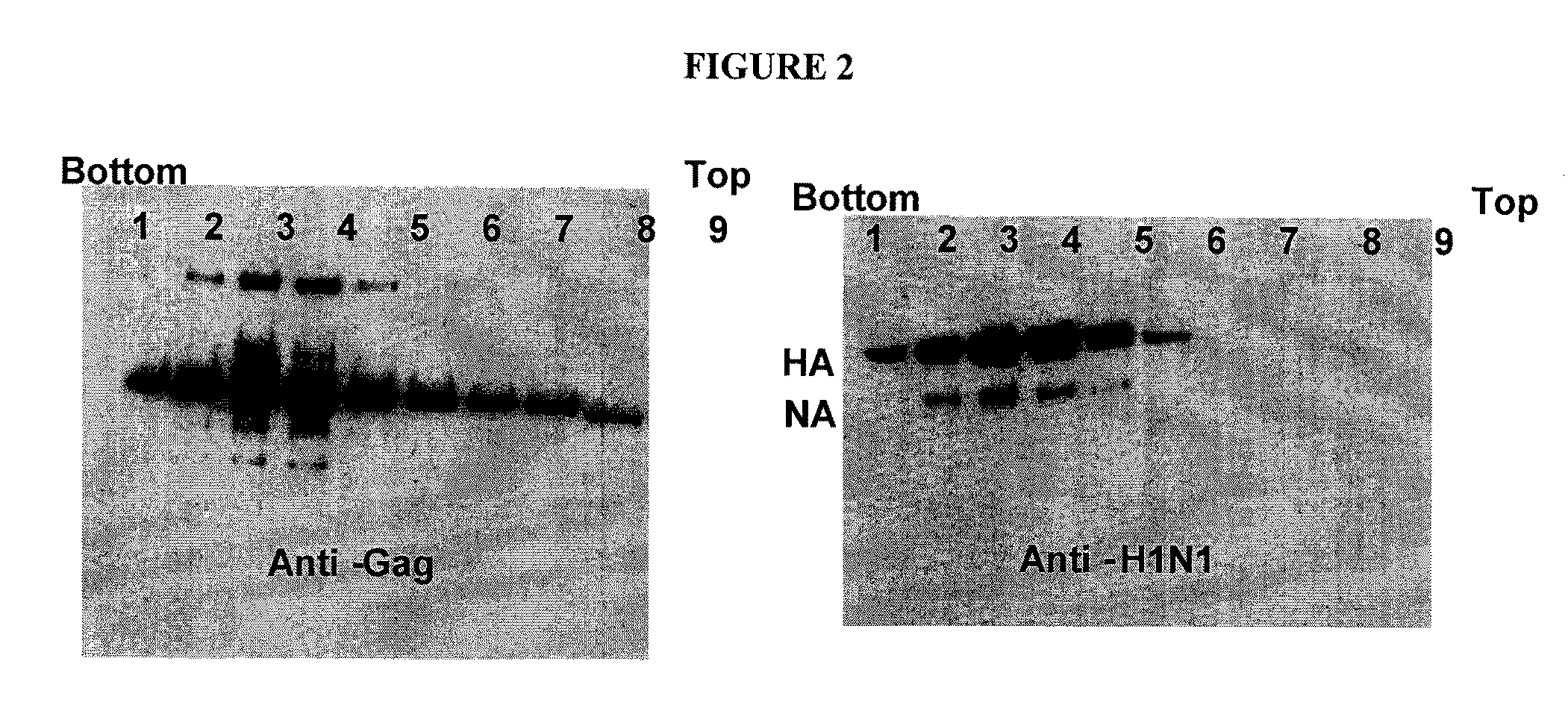
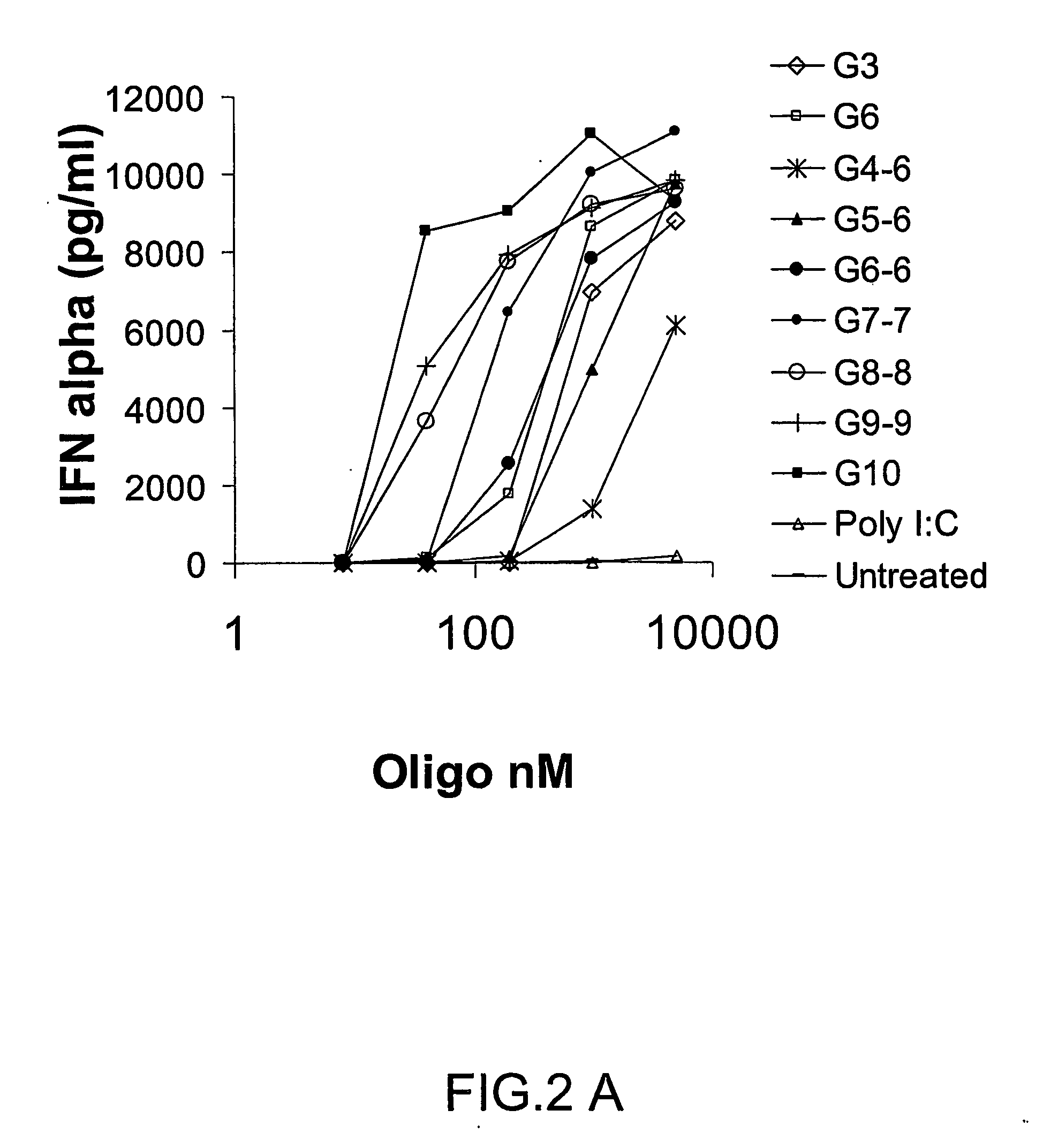
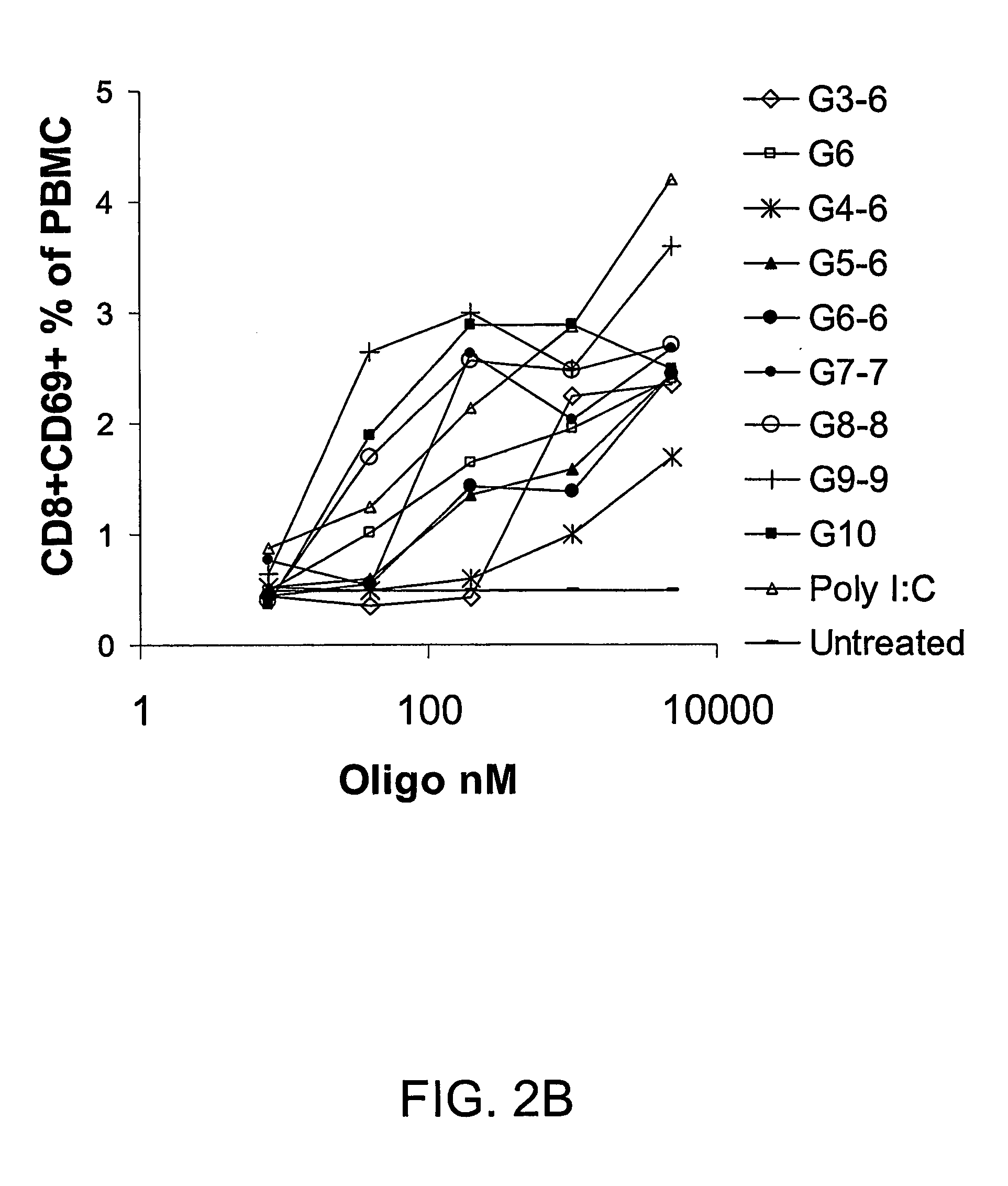
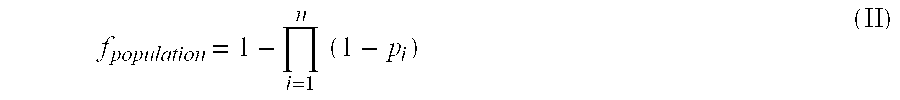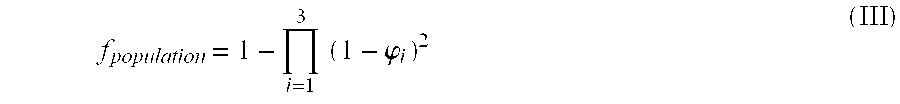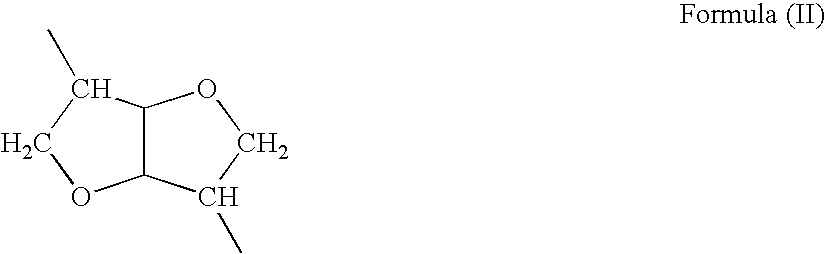Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
210results about "Carrier-antigen complex architecture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
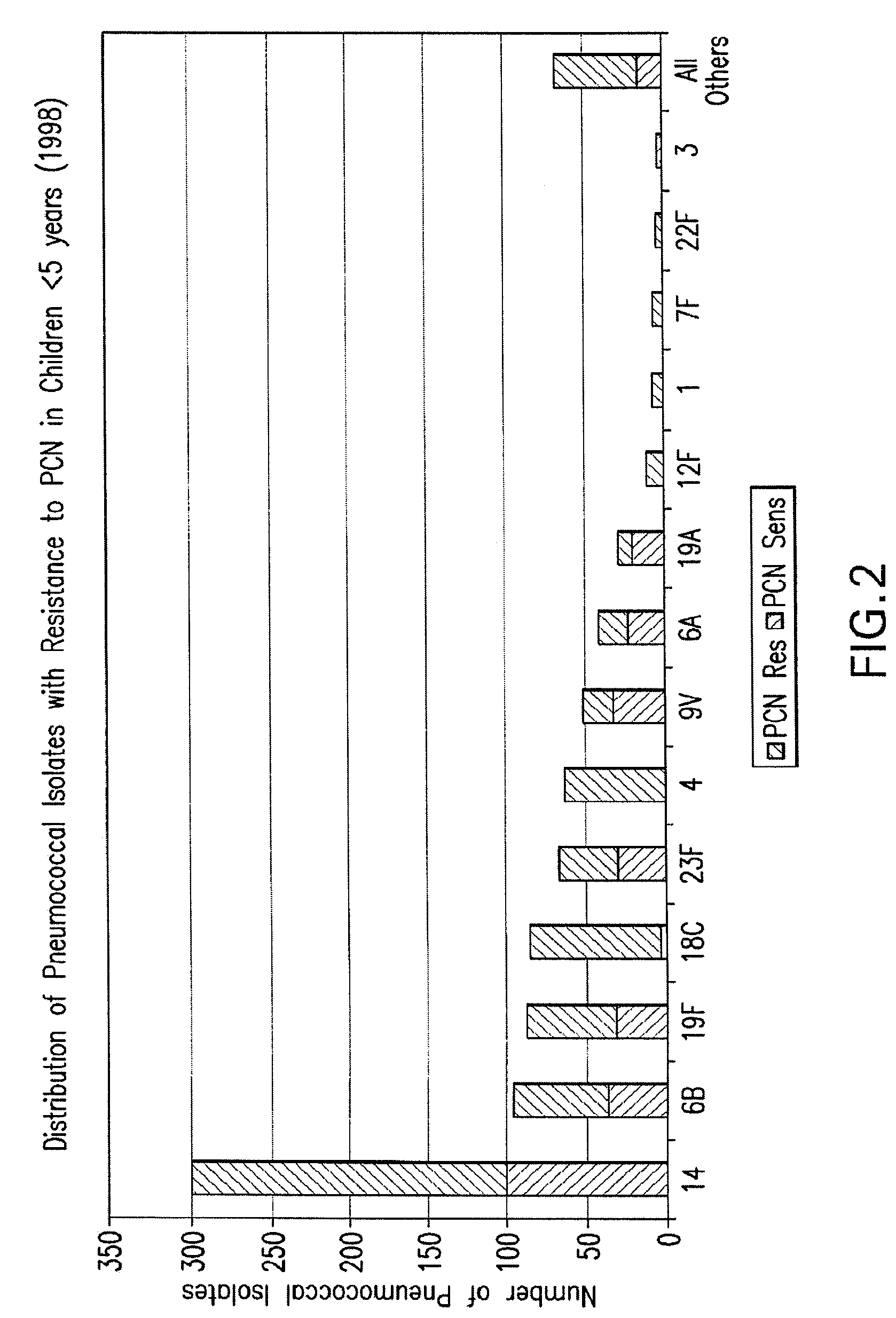
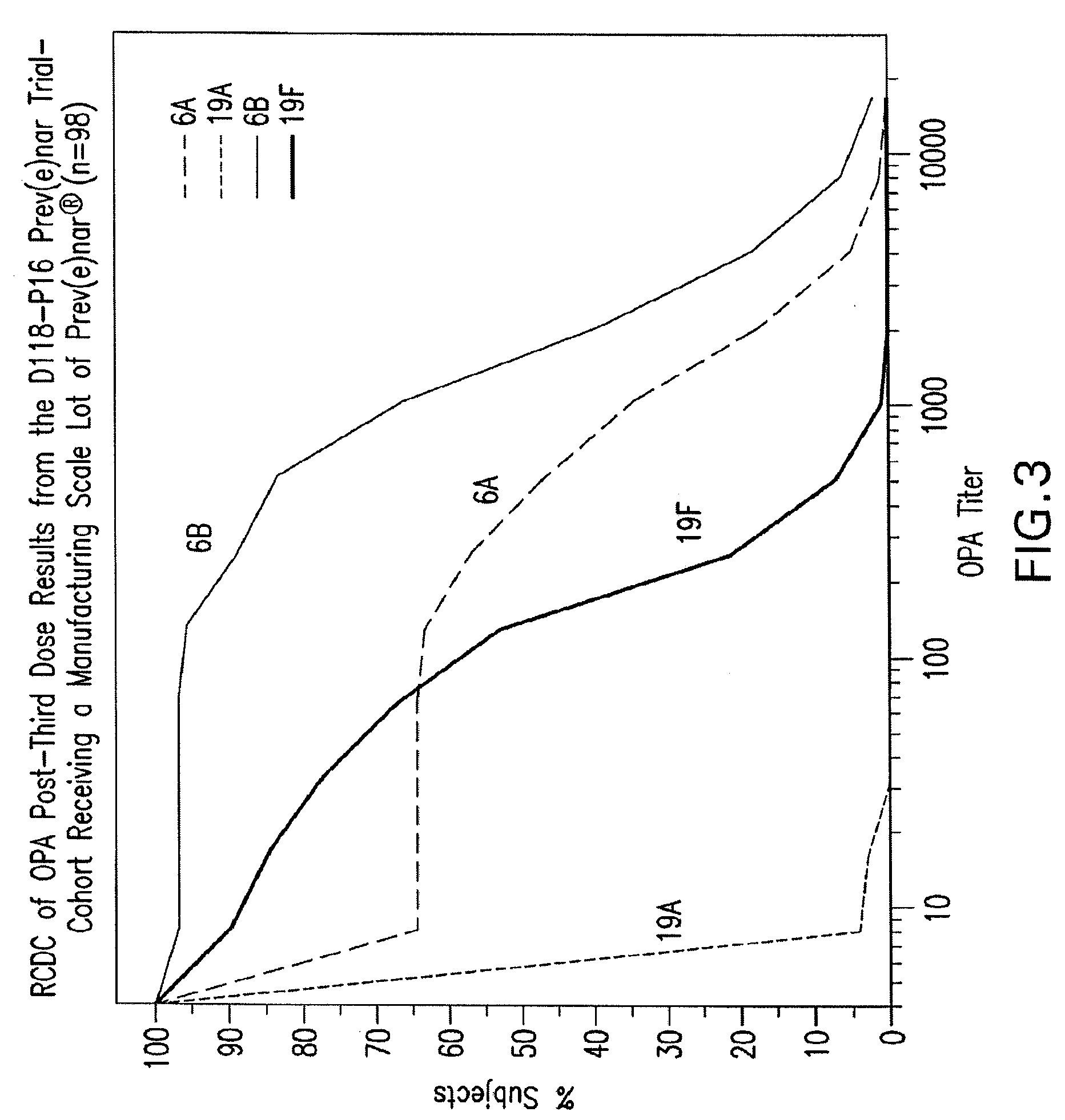
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Novel method for down-regulation of amyloid
A method for in vivo down-regulation of amyloid protein in an animal, including a human being, the method comprising effecting presentation to the animal's immune system of an immunogenically effective amount of at least one amyloidogenic polypeptide or subsequence thereof which has been formulated so that immunization of the animal with the amyloidgenic polypeptide or subsequence thereof induces production of antibodies against the amyloidogenic polypeptide, and / or at least one analogue of the amyloidogenic polypeptide wherein is introduced at least one modification in the amino acid sequence of the amyloidogenic polypeptide which has as a result the immunization of the animal with the analogue induces production of antibodies against the amyloidogenic polypeptide.
Owner:H LUNDBECK AS
Polypeptides and polynucleotides for enhancing immune reactivity to HER-2 protein
Compositions for stimulating the immune system and for treating malignancies associated with overexpression of the HER-2 protein are provided. Such compositions include immunogenic epitopes of the HER-2 proteins and chimeric and multivalent peptides which comprise such epitopes. The present invention also relates to polynucleotides which encode the chimeric peptides. Also provided are pharmaceutical compositions comprising such immunogenic compositions. Methods for stimulating an immune response to HER-2 protein are provided. Methods for treating breast cancer, ovarian cancer, prostate cancer, colon cancer and lung cancer are provided.
Owner:OHIO STATE INNOVATION FOUND
Multilayer films, coatings, and microcapsules comprising polypeptides
ActiveUS20070077276A1Great control over mechanical stability and diffusive propertyIncrease profitSsRNA viruses positive-sensePeptide/protein ingredientsCrystallographyPolyelectrolyte
Disclosed herein is a multilayer film comprising two or more layers of polyelectrolytes, wherein adjacent layers comprise oppositely charged polyelectrolytes. A first layer polyelectrolye comprises a composite polypeptide comprising one or more surface adsorption regions covalently linked to one or more functional regions forming a single polypeptide chain. The surface adsorption regions comprise one or more amino acid sequence motifs consisting of 5 to 15 amino acid residues. The one or more functional regions comprise 3 to about 250 amino acid residues.
Owner:LOUISIANA TECH UNIV RES FOUND A DIV OF LOUISIANA TECH UNIV FOUND
Novel method for down-regulation of amyloid
Disclosed are novel methods for combatting diseases characterized by deposition of amyloid. The methods generally rely on immunization against amyloid precursor protien (APP) or beta amyloid (Abeta). Immunization is preferably effected by administration of analogues of autologous APP or Abeta, said analogues being capable of inducing antibody production against the autologous amyloidogenic polypeptides. Especially preferred as an immunogen is autologous Abeta which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes. Also disclosed are nucleic acid vaccination against APP or Abeta and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:H LUNDBECK AS
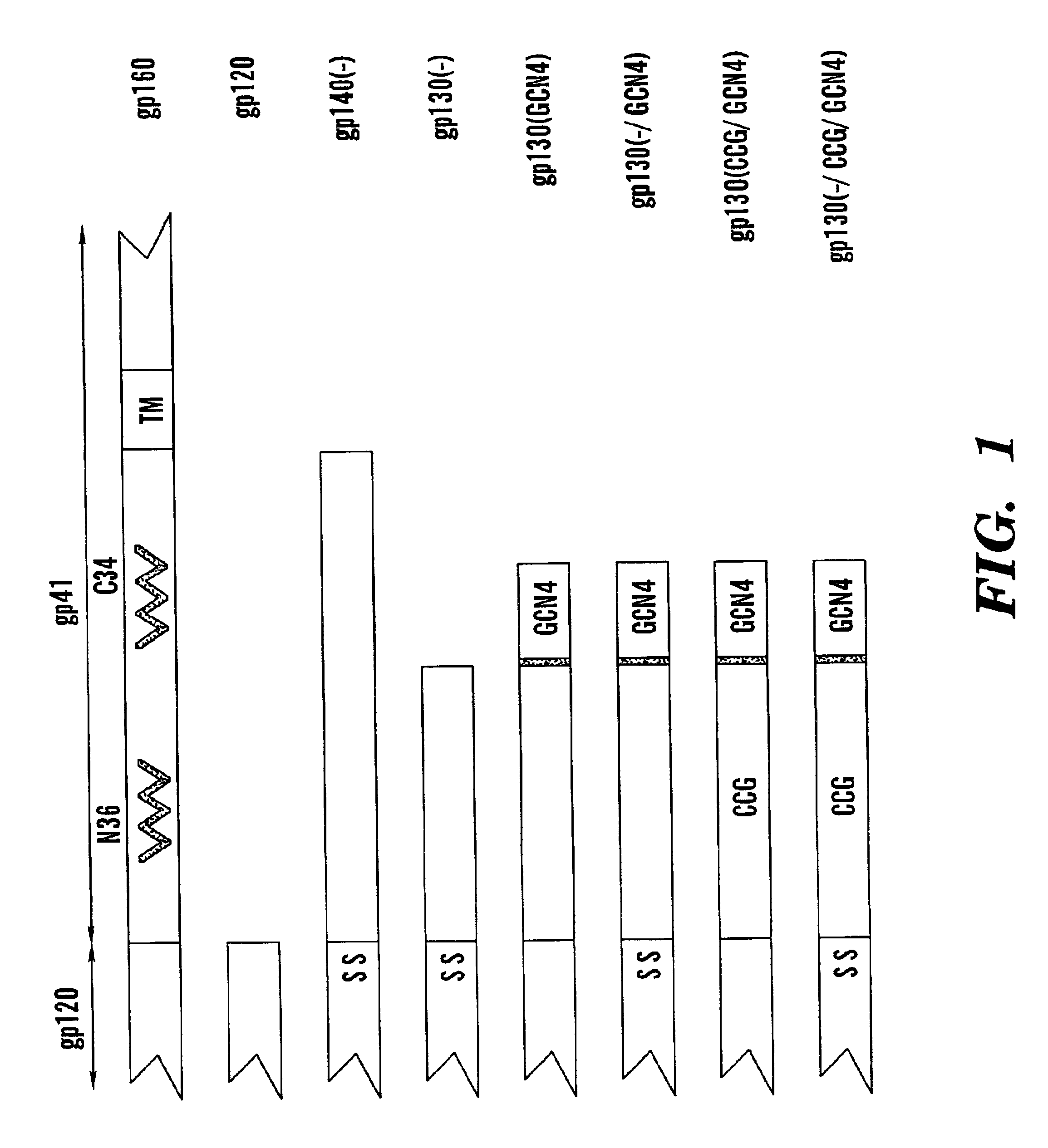
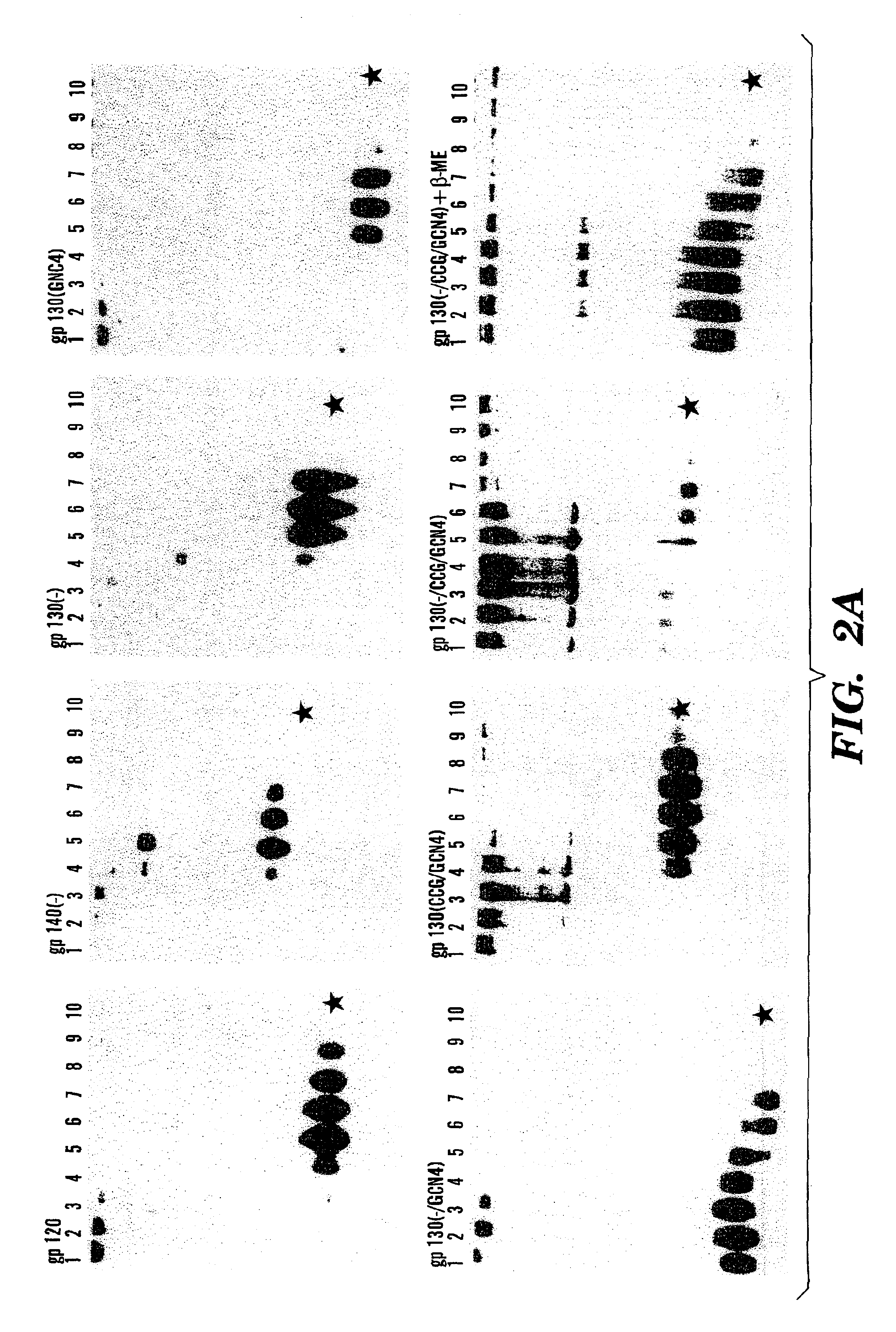
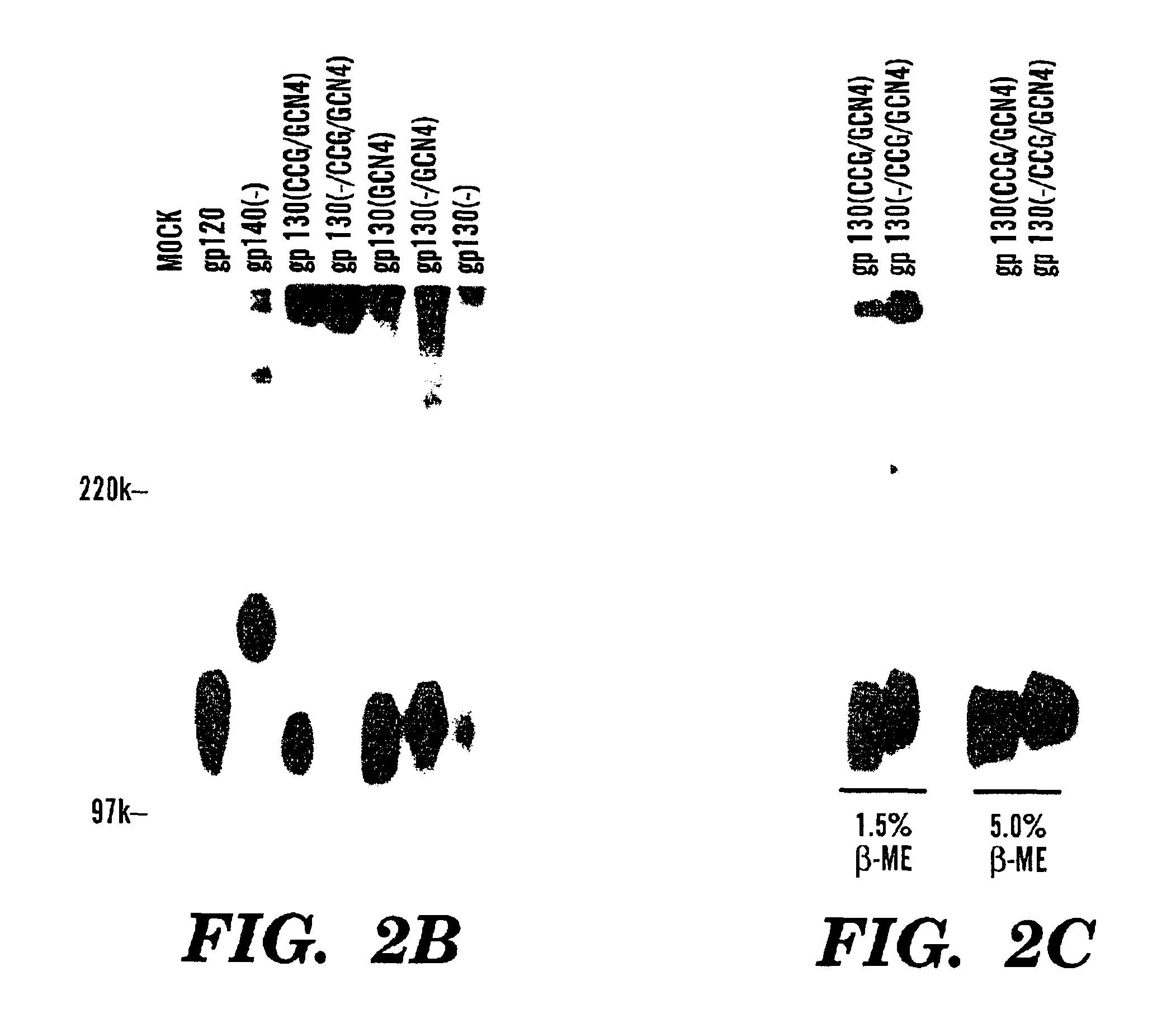
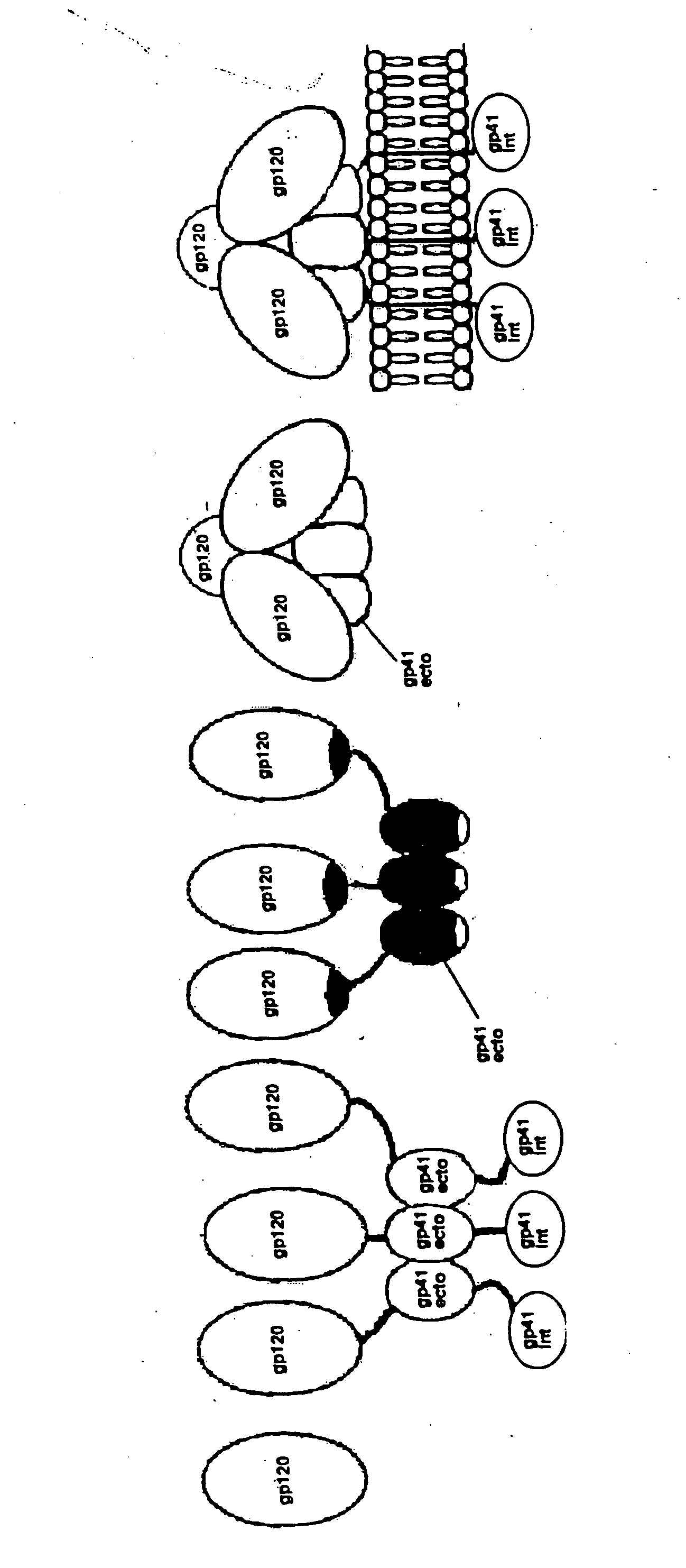
Stabilized soluble glycoprotein trimers
InactiveUS6911205B2Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
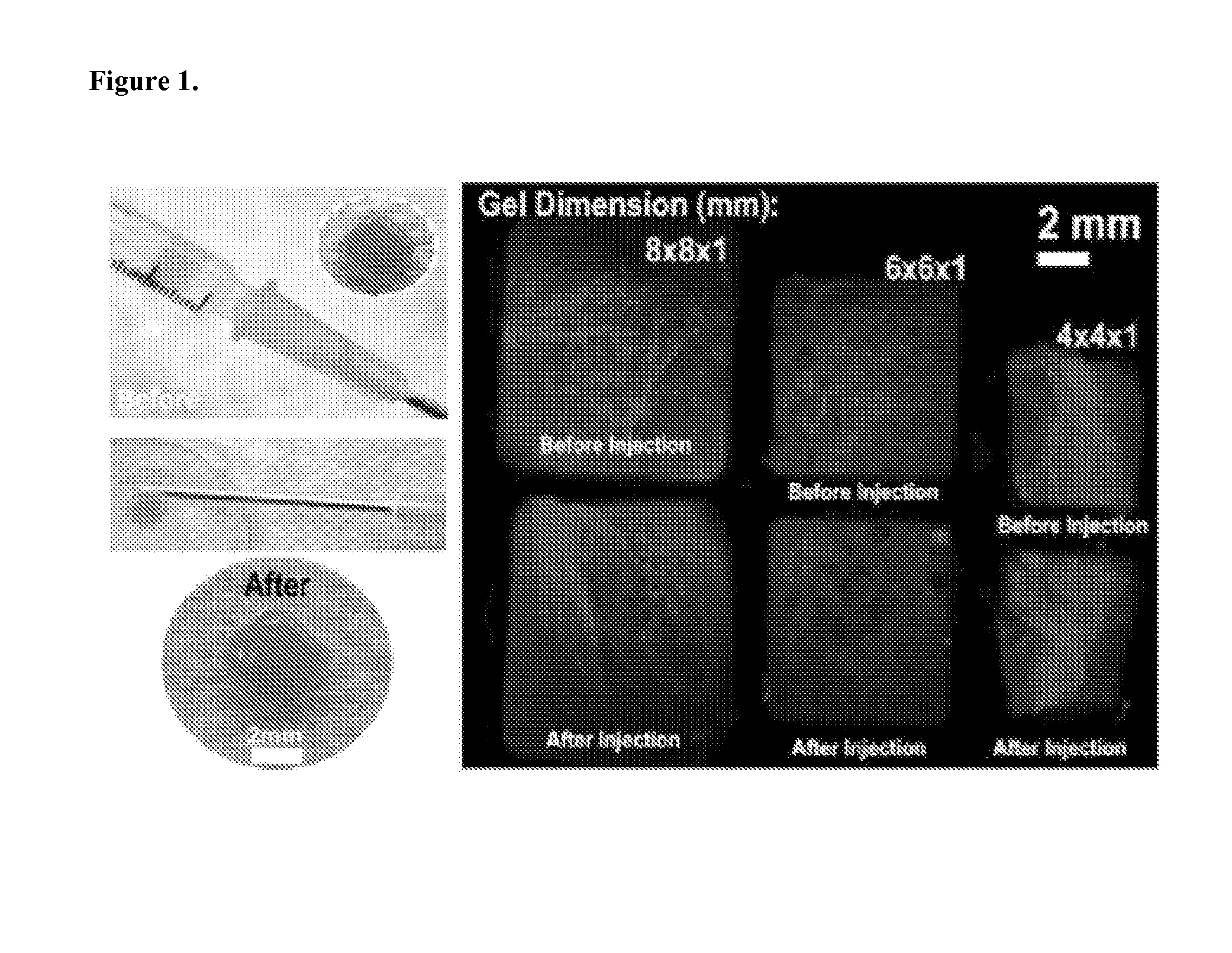
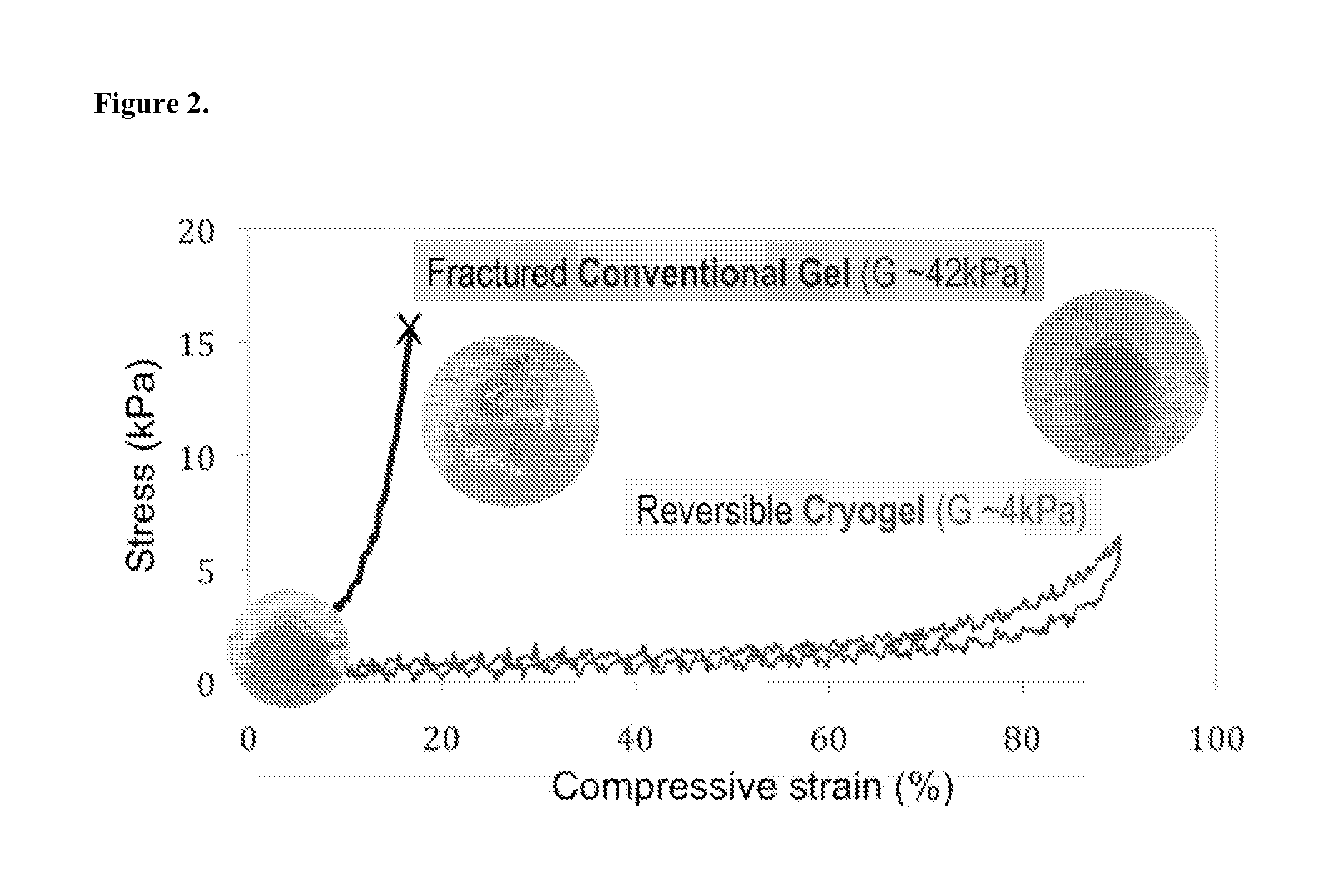
Injectable Preformed Macroscopic 3-Dimensional Scaffolds for Minimally Invasive Administration
ActiveUS20140112990A1Improve accuracyDegree of flexibilityPowder deliveryBiocidePolymer compositionDrug delivery
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for down-regulation of amyloid
A method for in vivo down-regulation of amyloid protein in an animal, including a human being, the method comprising effecting presentation to the animal's immune system of an immunogenically effective amount of at least one amyloidogenic polypeptide or subsequence thereof which has been formulated so that immunization of the animal with the amyloidgenic polypeptide or subsequence thereof induces production of antibodies against the amyloidogenic polypeptide, and / or at least one analogue of the amyloidogenic polypeptide wherein is introduced at least one modification in the amino acid sequence of the amyloidogenic polypeptide which has as a result the immunization of the animal with the analogue induces production of antibodies against the amyloidogenic polypeptide.
Owner:H LUNDBECK AS
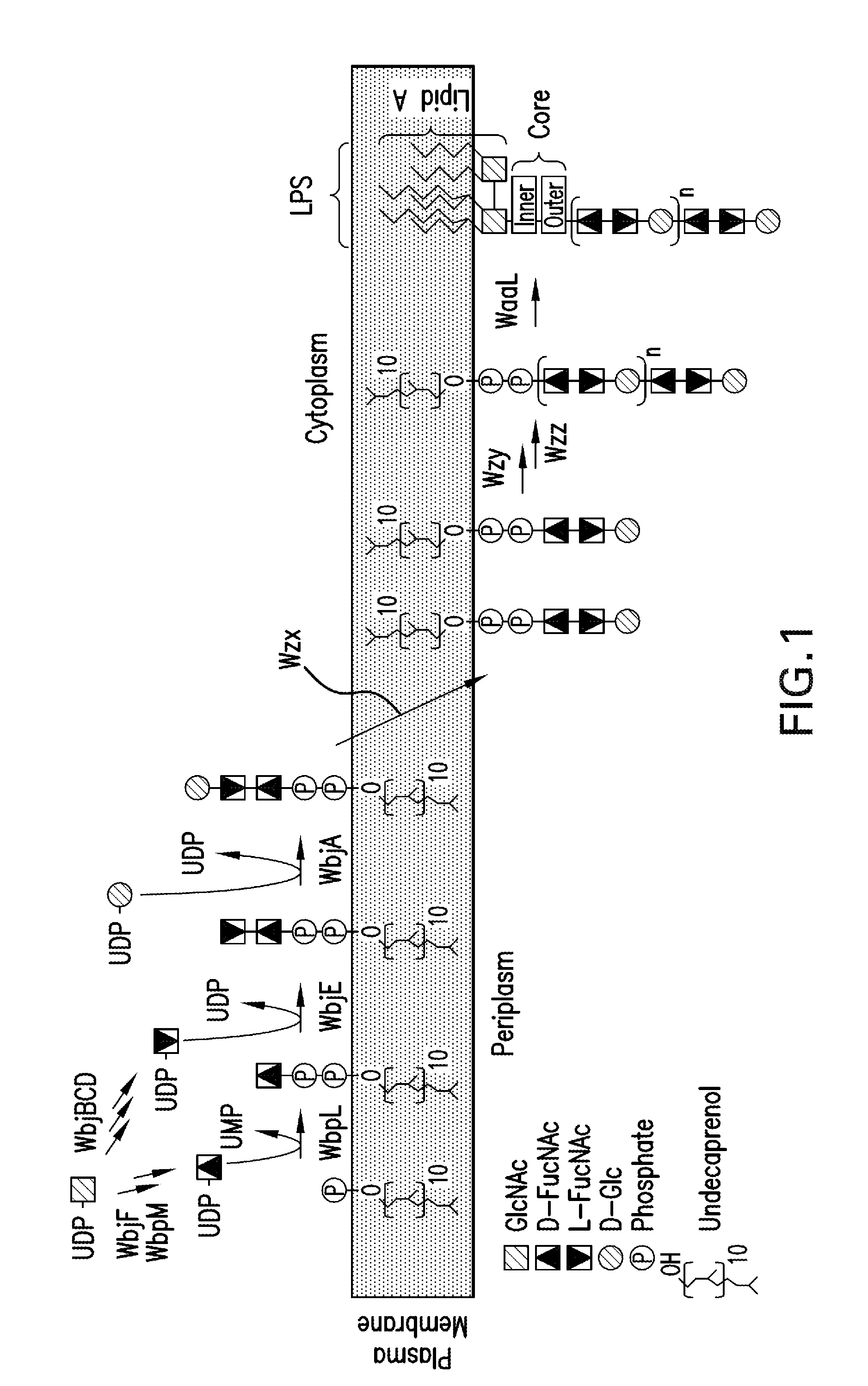
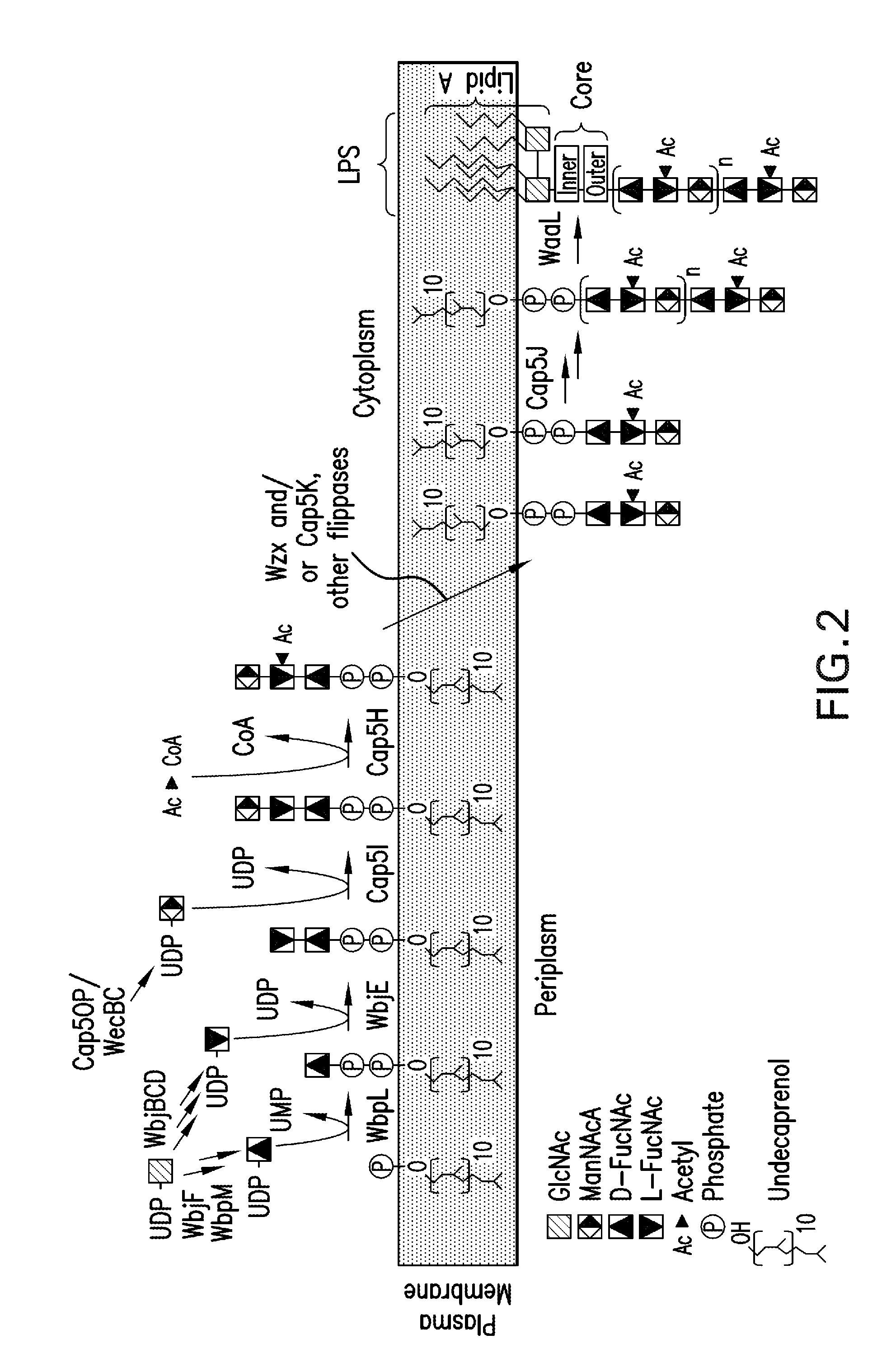
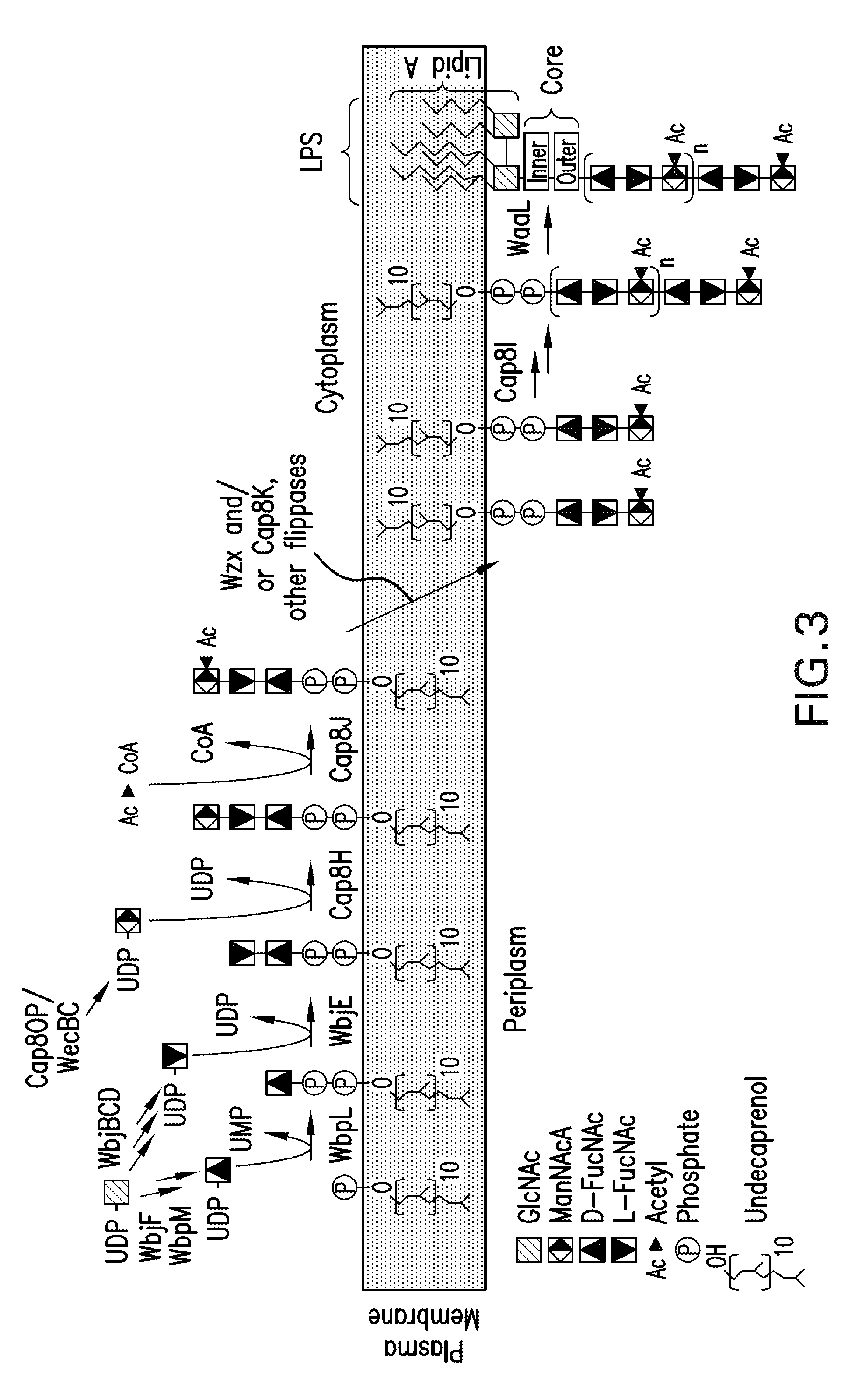
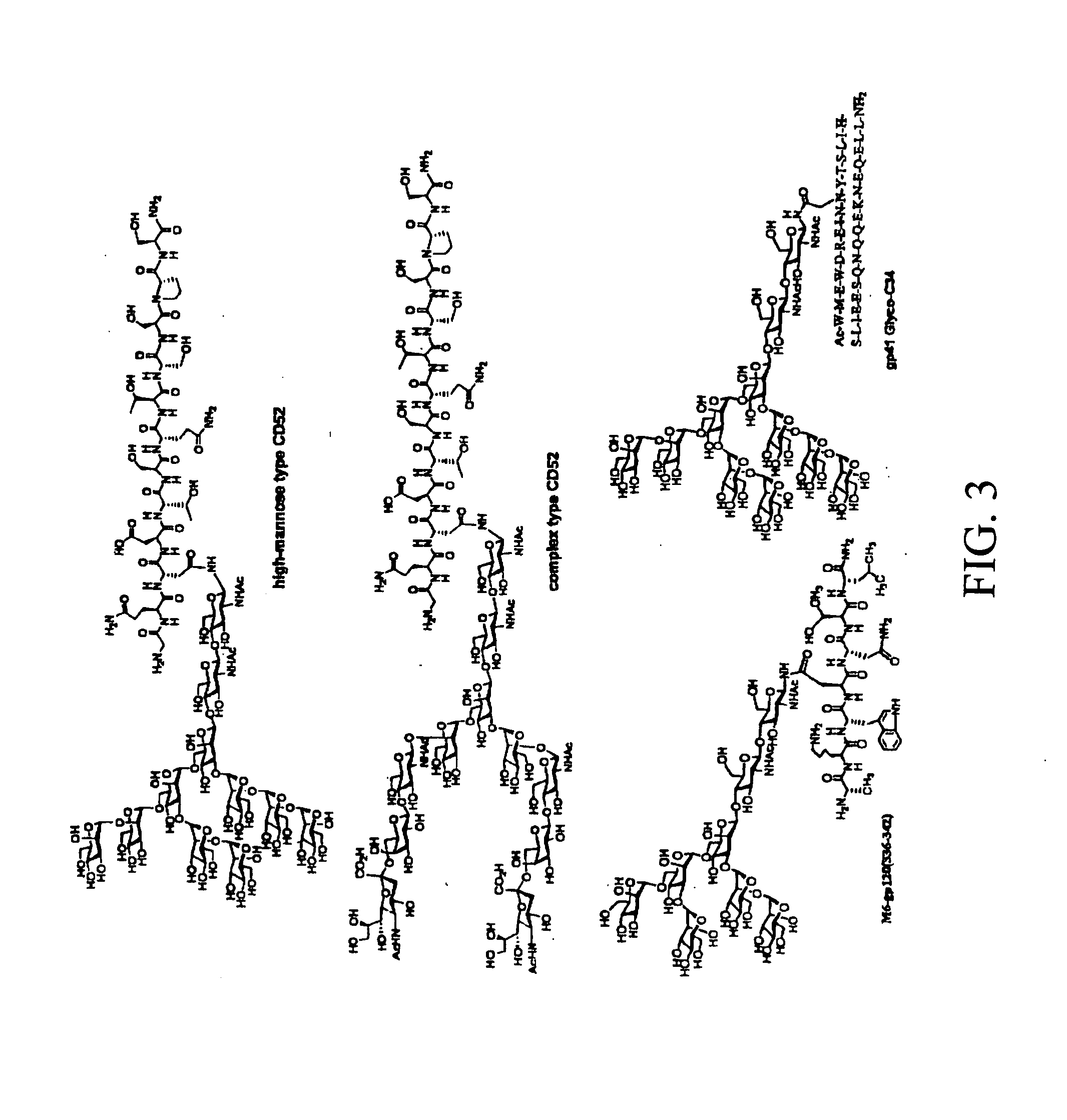
Capsular gram-positive bacteria bioconjugate vaccines
The present invention encompasses a novel S. aureus bioconjugate vaccine. More generally, the invention is directed to Gram-positive and other bioconjugate vaccines containing a protein carrier, at least one polysaccharide such as a capsular Gram-positive polysaccharide, and, optionally, an adjuvant or pharmaceutically acceptable carrier. The instant invention also includes methods of producing Gram-positive and other bioconjugate vaccines. An N-glycosylated protein is also provided that contains one or more polysaccharides such as Gram-positive polysaccharides. The invention is additionally directed to engineered prokaryotic organisms comprising nucleotide sequences encoding a glycosyltransferase of a first prokaryotic organism and a glycosyltransferase of a second prokaryotic organism. The invention further includes plasmids and prokaryotic cells transformed with plasmids encoding polysaccharides and enzymes which produce an N-glycosylated protein and / or bioconjugate vaccine. Further, the invention is directed to methods of inducing an immune response in a mammal comprising administering said bioconjugate vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Melan-A- carrier conjugates
InactiveUS7537767B2Improve responseMore immunogenicSsRNA viruses negative-senseBiocideDiseaseAllergy
Owner:CYTOS BIOTECHNOLOGY AG
Synthetic vaccine agents
InactiveUS7097837B2Promote absorptionImprove responsePeptide/protein ingredientsAntibody mimetics/scaffoldsCtl epitopeHelper epitope
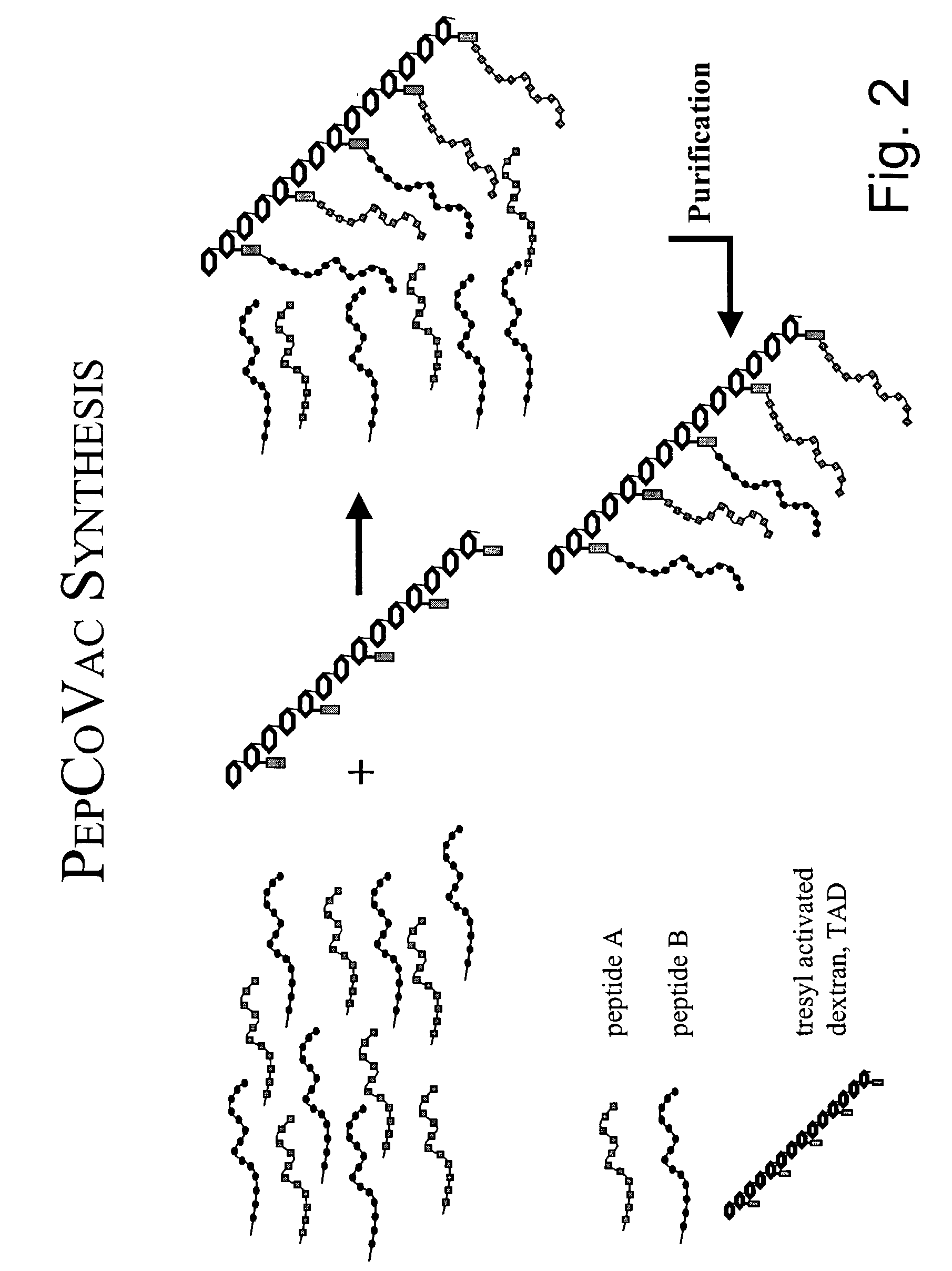
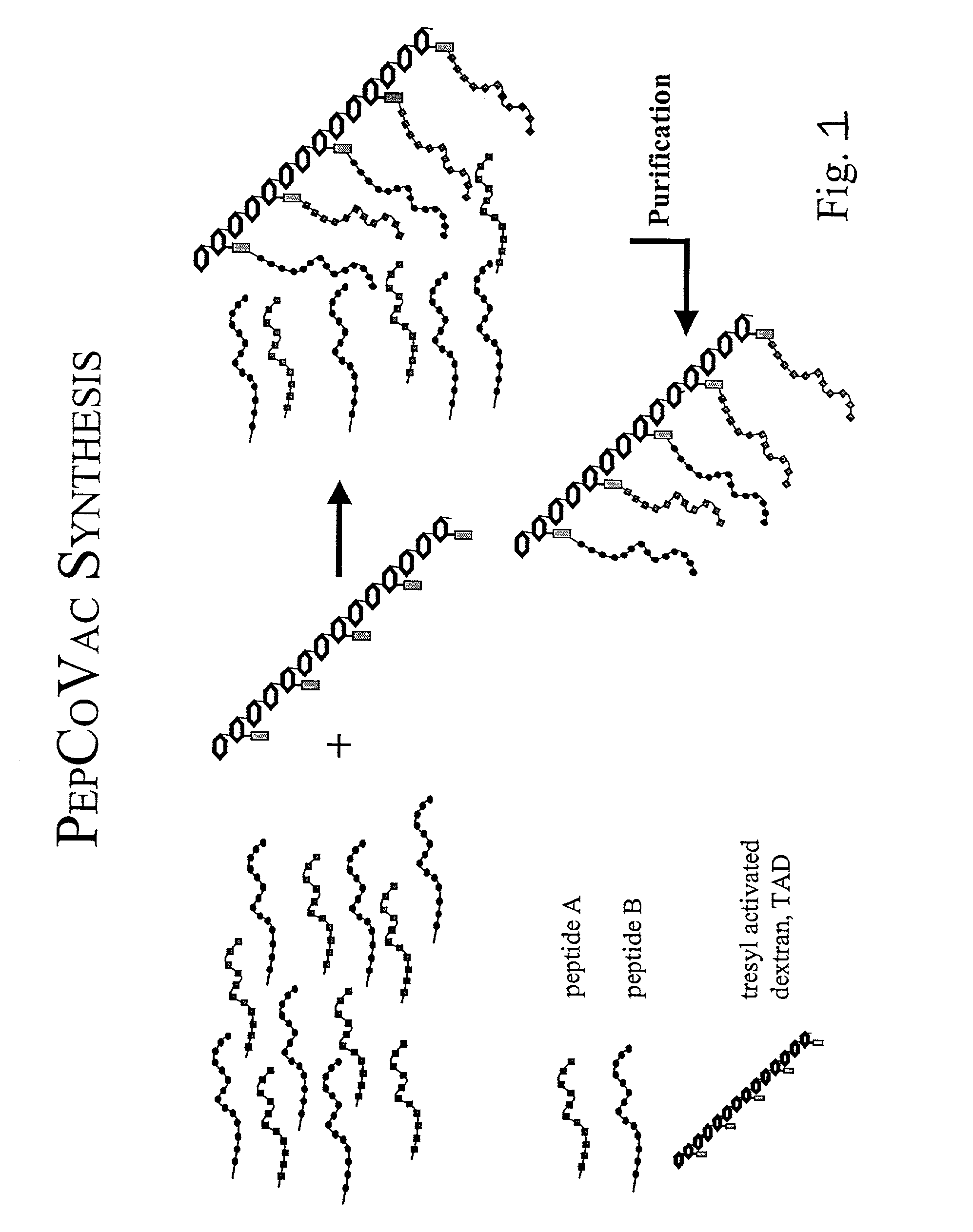
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunization and a method for identification of suitable immunogens of the invention.
Owner:BEALE STREET 143 INVEST APS
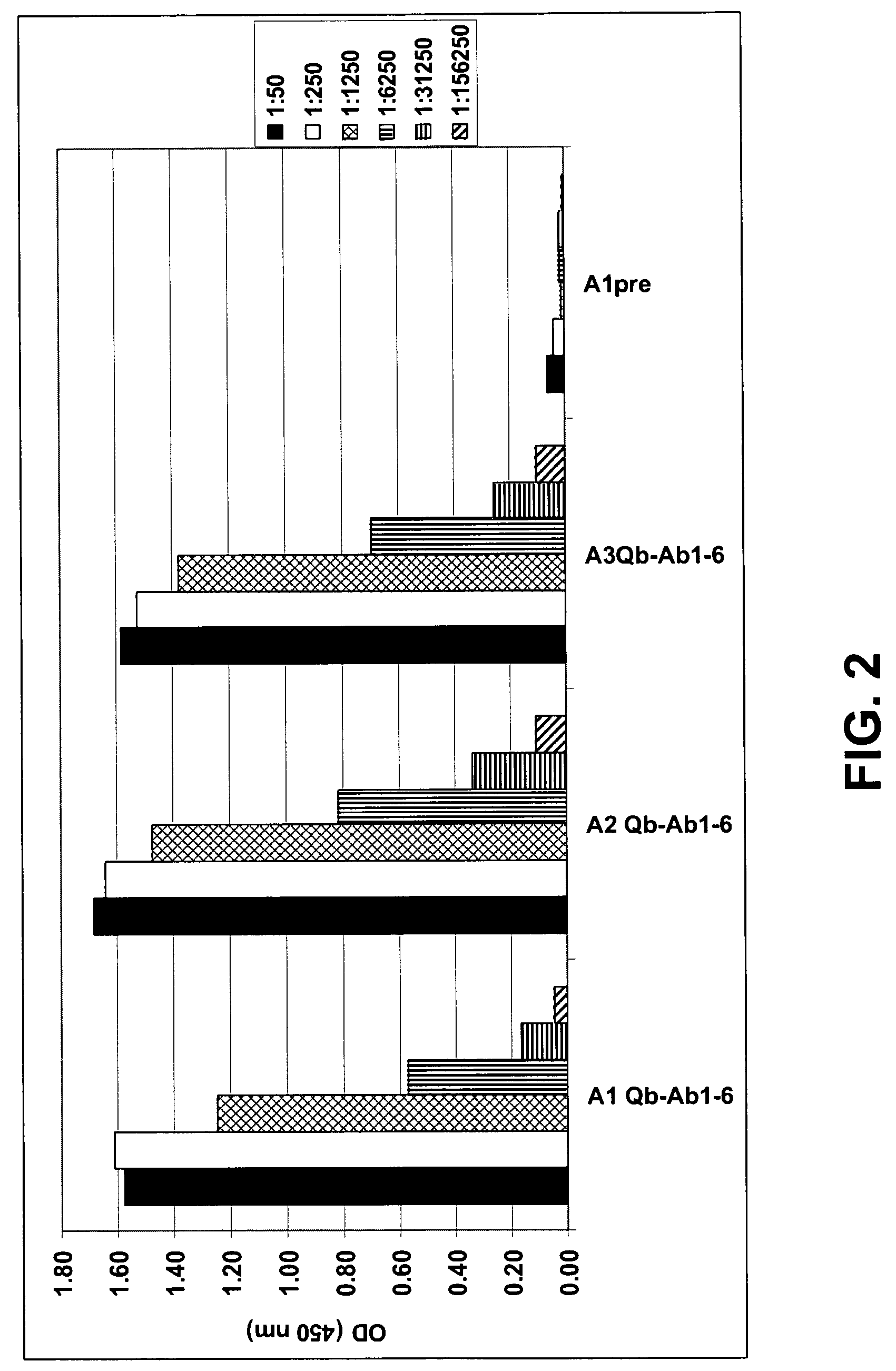
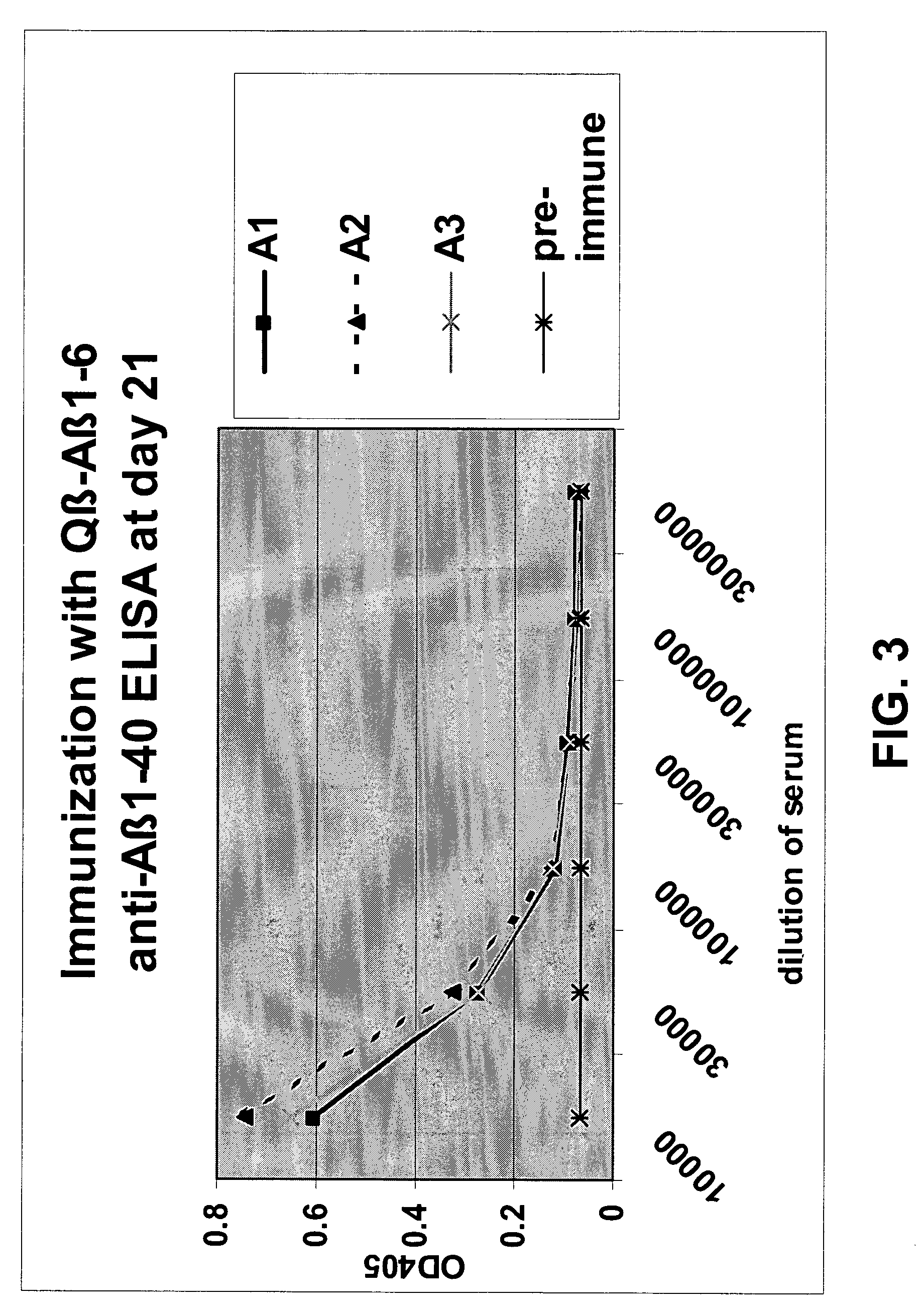
Amyloid β1-6 antigen arrays
InactiveUS7279165B2High potencyEfficient inductionNervous disorderVirus peptidesVirus-like particleSpecific immunity
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising an ordered and repetitive antigen or antigenic determinant array, and in particular an Aβ1-6 peptide-VLP-composition. More specifically, the invention provides a composition comprising a virus-like particle and at least one Aβ1-6 peptide bound thereto. The invention also provides a process for producing the conjugates and the ordered and repetitive arrays, respectively. The compositions of the invention are useful in the production of vaccines for the treatment of Alzheimer's disease and as a pharmaccine to prevent or cure Alzheimer's disease and to efficiently induce immune responses, in particular antibody responses. Furthermore, the compositions of the invention are particularly useful to efficiently induce self-specific immune responses within the indicated context.
Owner:NOVARTIS AG
Human immunodeficiency virus envelope clycoprotein mutants and uses thereof
This invention provides stable HIV-1 pre-fusion envelope glycoprotein trimeric complexes. This invention also provides related polypeptides and compositions comprising pharmaceutically acceptable particles and the trimeric complexes operably affixed thereto. This invention further provides related nucleic acids, vectors, host cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:CORNELL RES FOUNDATION INC +1
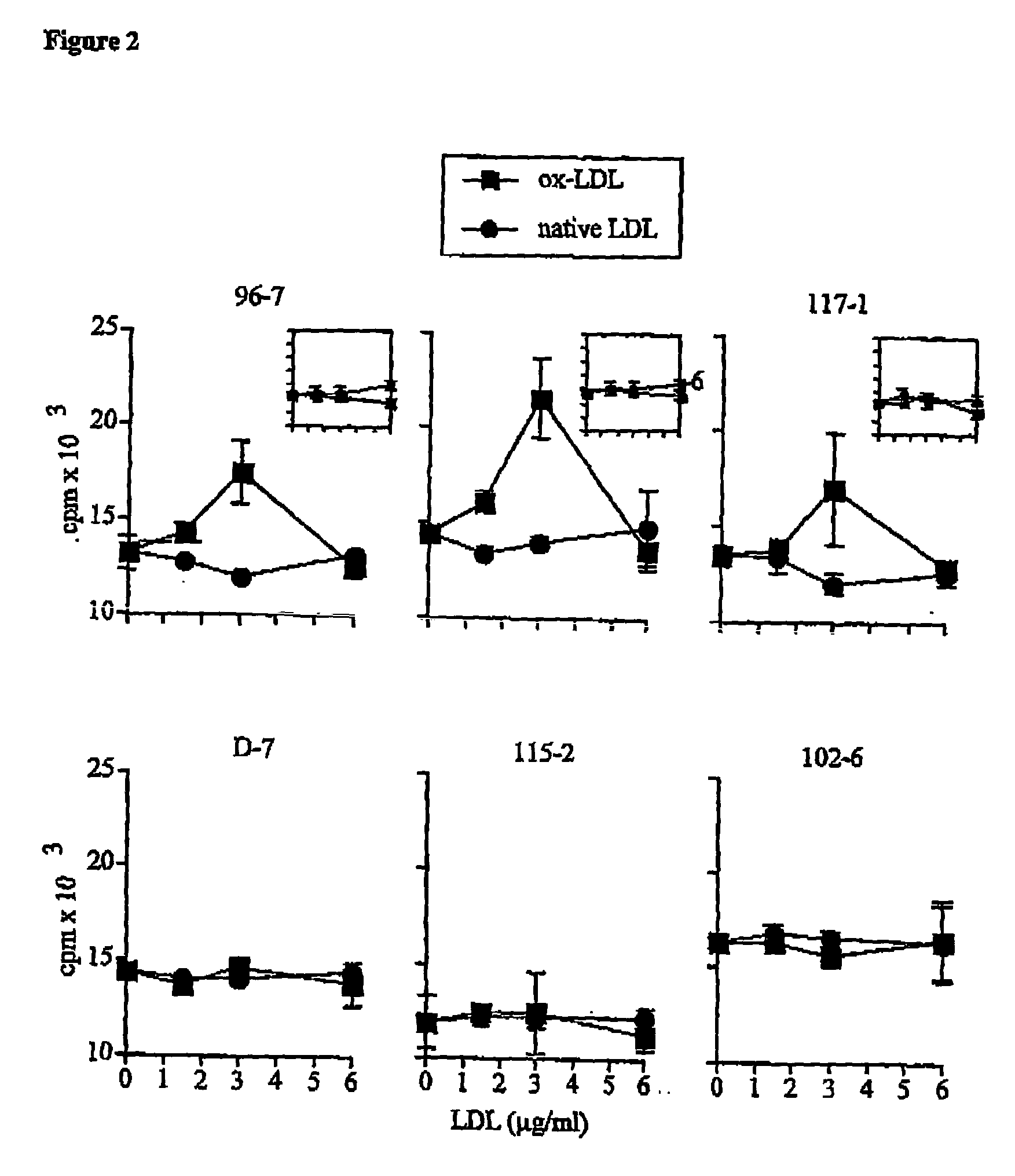
Atherosclerosis vaccine
InactiveUS20040002111A1Peptide preparation methodsLipid/lipoprotein ingredientsAntigenComplementarity determining region
The present invention relates to an antigenic composition capable of eliciting antibodies by interacting with alphabeta chains of a T cell receptor (TcR), which composition is comprised of a peptide-aldehyde conjugate. The aldehyde portion may be a dialdehyde, such as malondialdehyde (MDA), or a monoaldehyde, such as 4-hydroxynonenal (4-HNE), while the peptide portion preferably comprises at least one lysine residue. The antigenic composition according to the invention is capable of recognizing and interacting with a TcR having a complementarity-determining region 3 (CDR3) of alpha10 and beta6 chains that comprises a cluster of charged and polar amino acids. The invention also relates to a method of producing a vaccine against atherosclerosis by screening of a library of candidate compounds for their ability to bind to a conjugate of oxidized LDL and a dialdehyde as well as to such a vaccine as such.
Owner:CARDIOVAX
Multilayer films, coatings, and microcapsules comprising polypeptides
InactiveUS7544770B2Great control over mechanical stability and diffusive propertyIncrease profitSsRNA viruses positive-sensePeptide/protein ingredientsCrystallographyPolyelectrolyte
Owner:LOUISIANA TECH UNIV RES FOUND A DIV OF LOUISIANA TECH UNIV FOUND
Particle-bound human immunodeficiency virus envelope glycoproteins and related compositions and methods
InactiveUS20060051373A1Inhibition of growth rateSlow growth rateBiocideOrganic active ingredientsParticulate antigenPharmaceutical medicine
This invention provides a first composition comprising a pharmaceutically acceptable particle and a stable HIV-1 pre-fusion envelope glycoprotein trimeric complex operably affixed thereto. This invention further provides a second composition comprising (a) a pharmaceutically acceptable particle, (b) an antigen, and (c) an agent which is operably affixed to the particle and is specifically bound to the antigen, whereby the antigen is operably bound to the particle. Finally, this invention provides related nucleic acids, vectors, cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:PROGENICS PHARMA INC
Synthetic vaccine agents
InactiveUS20040191264A1Promote absorptionImprove responseBacterial antigen ingredientsSnake antigen ingredientsCtl epitopeHelper epitope
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunisation and a method for identification of suitable immunogens of the invention.
Owner:PHARMEXA
High efficiency peptide production in plant cells
ActiveUS20050260758A1Less variableMore stablyAntibacterial agentsSsRNA viruses positive-sensePlant cellEukaryotic plasmids
The present invention provides an improved process for the production of recombinant peptides. In particular, the present invention provides an improved process for the production of recombinant peptides in the form of viral capsid fusion proteins which can be assembled in vivo in plant cell suspension cultures. The invention also includes plasmids, sequences, and plant cells which allow for non-infectious viral capsid fusion peptide production.
Owner:DOW AGROSCIENCES LLC
Stabilized coronavirus spike (S) protein immunogens and related vaccines
The present invention provides redesigned soluble coronavirus S protein derived immunogens that are stabilized via specific modifications in the wildtype soluble S sequences. Also provided in the invention are nanoparticle vaccines that contain the redesigned soluble S immunogens displayed on self-assembling nanoparticles. Polynucleotide sequences encoding the redesigned immunogens and the nanoparticle vaccines are also provided in the invention. The invention further provides methods of using the vaccine compositions in various therapeutic applications, e.g., for preventing or treating coronaviral infections.
Owner:THE SCRIPPS RES INST
Protein cage immunotherapeutics
The present invention provides compositions of heat shock protein cages for use in therapeutic vaccines. The heat shock protein cages of the invention have attached antigen, located either on the interior or exterior of the protein cage, and optionally an adjuvant.
Owner:MONTANA STATE UNIVERSITY
Stabilized soluble glycoprotein trimers
InactiveUS20050106177A1Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Vaccine delivery compositions and methods of use
InactiveUS20060188469A1Easy to produceSsRNA viruses negative-senseViral antigen ingredientsPolyesterOrganism
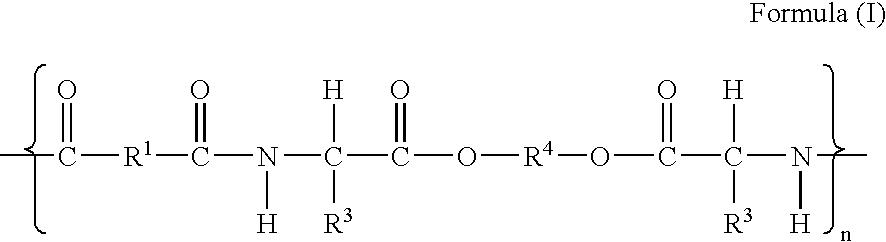
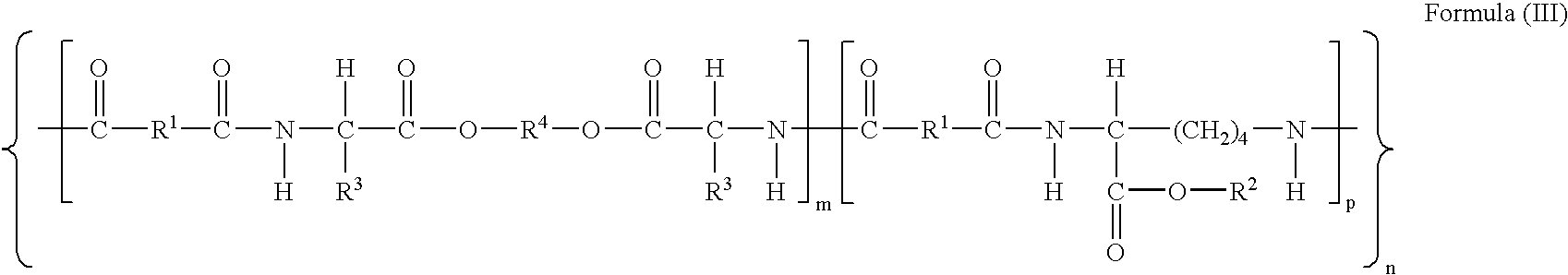
The present invention provides synthetic vaccine delivery compositions based on polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) polymers for stimulating an immune response to a variety of pathogenic organisms and tumor cells in humans and other mammals. The vaccine delivery compositions are formulated as a liquid dispersion of polymer particles or molecules including class I or class II antigen peptides derived from organism or tumor cell proteins, which are taken up by antigen presenting cells of the mammal to induce an immune response in the mammal. Methods of inducing an immune response to the pathogenic organism or tumor cells in the invention compositions are also included.
Owner:MEDIVAS LLC
Immunotherapy targeting intracellular pathogens
InactiveUS20120009678A1SsRNA viruses negative-sensePeptide/protein ingredientsOxidation-Reduction AgentRedox
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an intracellular pathogen-associated antigen and a redox motif such as C-(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of infection with an intracellular pathogen and in the manufacture of medicaments therefore.
Owner:LIFE SCI RES PARTNERS VZW
Chimeric virus-like particles
InactiveUS20100047266A1SsRNA viruses negative-senseAntibacterial agentsVirus-like particleChimeric virus
Chimeric virus-like particles including gag polypeptides are described. Virus-like particles are generated with a gag polypeptide and lipid raft-associated polypeptide linked to an antigen that is not naturally associated with a lipid raft. Preferred methods of generation include expression in insect cells.
Owner:TAKEDA VACCINES INC
Melan-a peptide analogue-virus-like-particle conjugates
InactiveUS20060204475A1More immunogenicImprove responseSsRNA viruses negative-senseBiocideAbnormal tissue growthViral disease
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a modified virus-like particle (VLP) comprising a VLP which can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs), and particular peptides derived from MelanA linked thereto. Such CpGVLPs are dramatically more immunogenic than their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against MelanA peptide analogues optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against the VLP itself. In addition, the T cell responses against the MelanA peptide analogues are especially directed to the Thl type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:CYTOS BIOTECHNOLOGY AG
HIV-1 glycopeptides and derivatives; preparation and applications thereof
InactiveUS7728106B2Peptide/protein ingredientsViral antigen ingredientsGlycopeptideCombinatorial chemistry
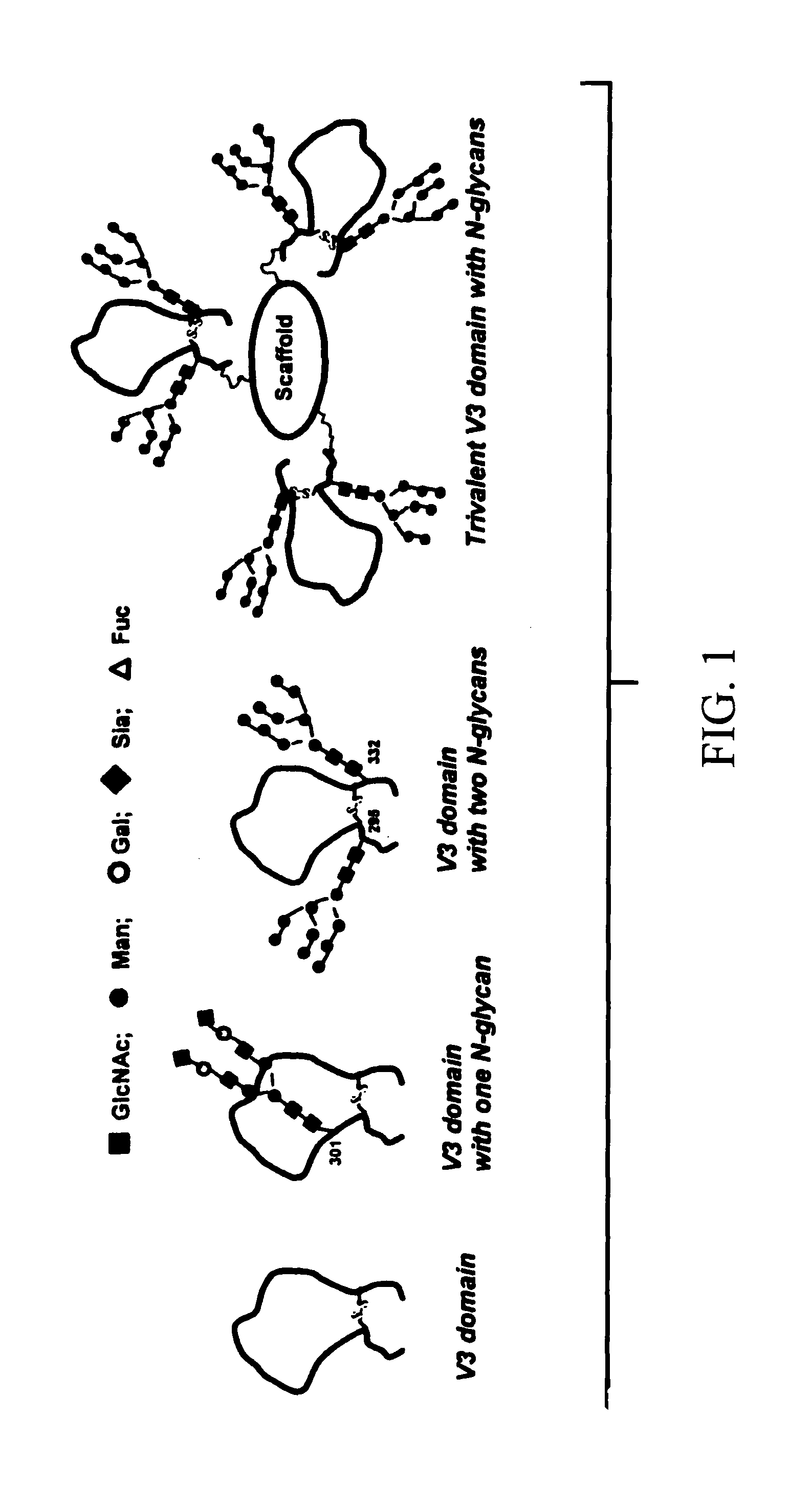
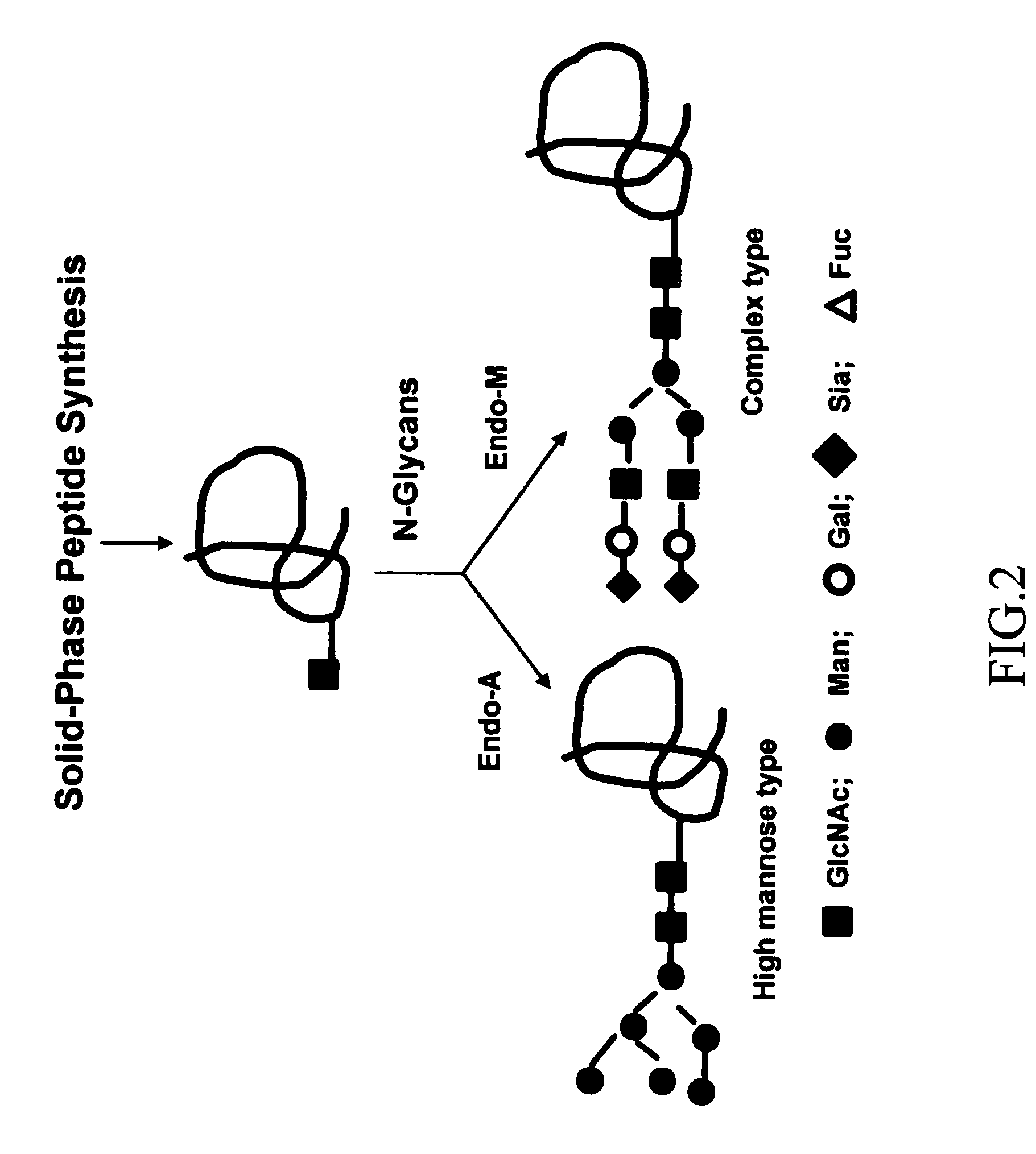
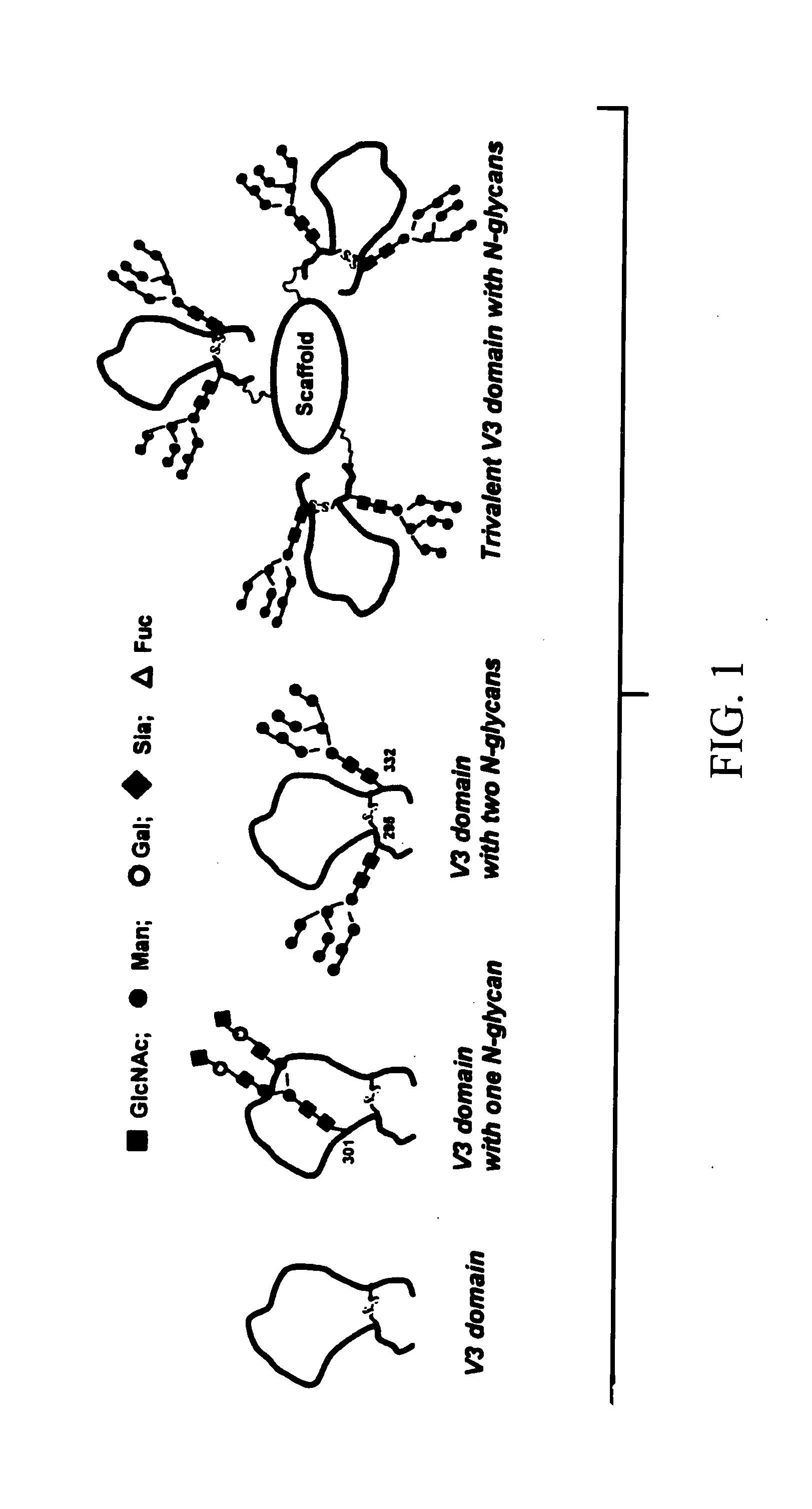
A method of making a synthetic glycopeptide, by addition of a synthetic oligosaccharide oxazoline to a GlcNAc-containing peptide precursor in the presence of an enzyme selected from among Endo-A and Endo-M. In a specific implementation, the method is utilized to synthesize a trivalent V3-domain glycopeptide including three V3-domain glycopeptides on a scaffold, wherein the three V3-domain glycopeptides are arranged to mimic the V3 domain presentation in trimeric gp120. Such trivalent V3-domain glycopeptides can be utilized in a vaccine for the treatment or prevention of HIV-1 infection.
Owner:UNIV OF MARYLAND BALTIMORE +1
HIV-1 glycopeptides and derivatives; preparation and applications thereof
InactiveUS20070224211A1Peptide/protein ingredientsViral antigen ingredientsGlycopeptideOligosaccharide
A method of making a synthetic glycopeptide, by addition of a synthetic oligosaccharide oxazoline to a GlcNAc-containing peptide precursor in the presence of an enzyme selected from among Endo-A and Endo-M. In a specific implementation, the method is utilized to synthesize a trivalent V3-domain glycopeptide including three V3-domain glycopeptides on a scaffold, wherein the three V3-domain glycopeptides are arranged to mimic the V3 domain presentation in trimeric gp120. Such trivalent V3-domain glycopeptides can be utilized in a vaccine for the treatment or prevention of HIV-1 infection.
Owner:UNIV OF MARYLAND +1
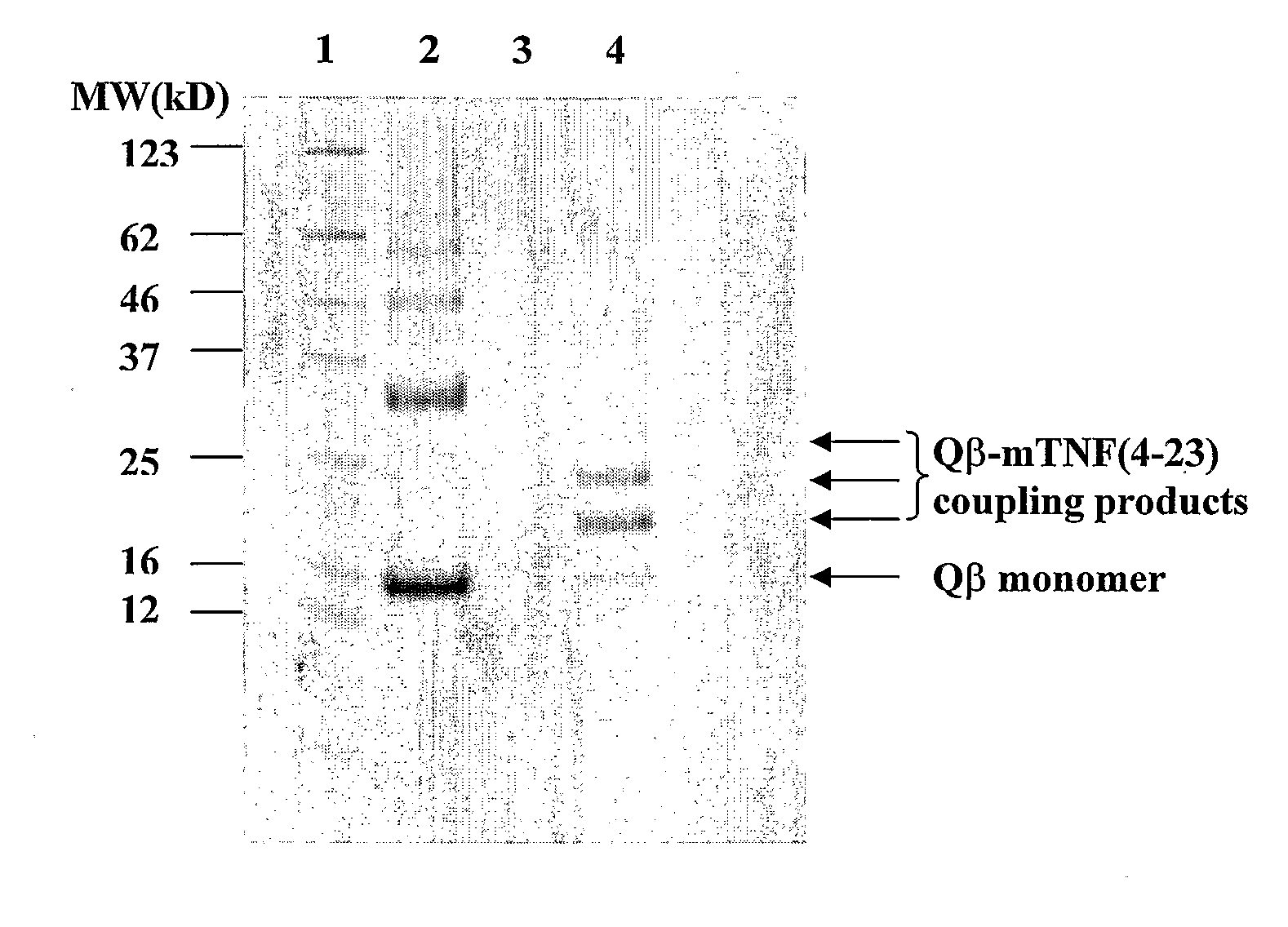
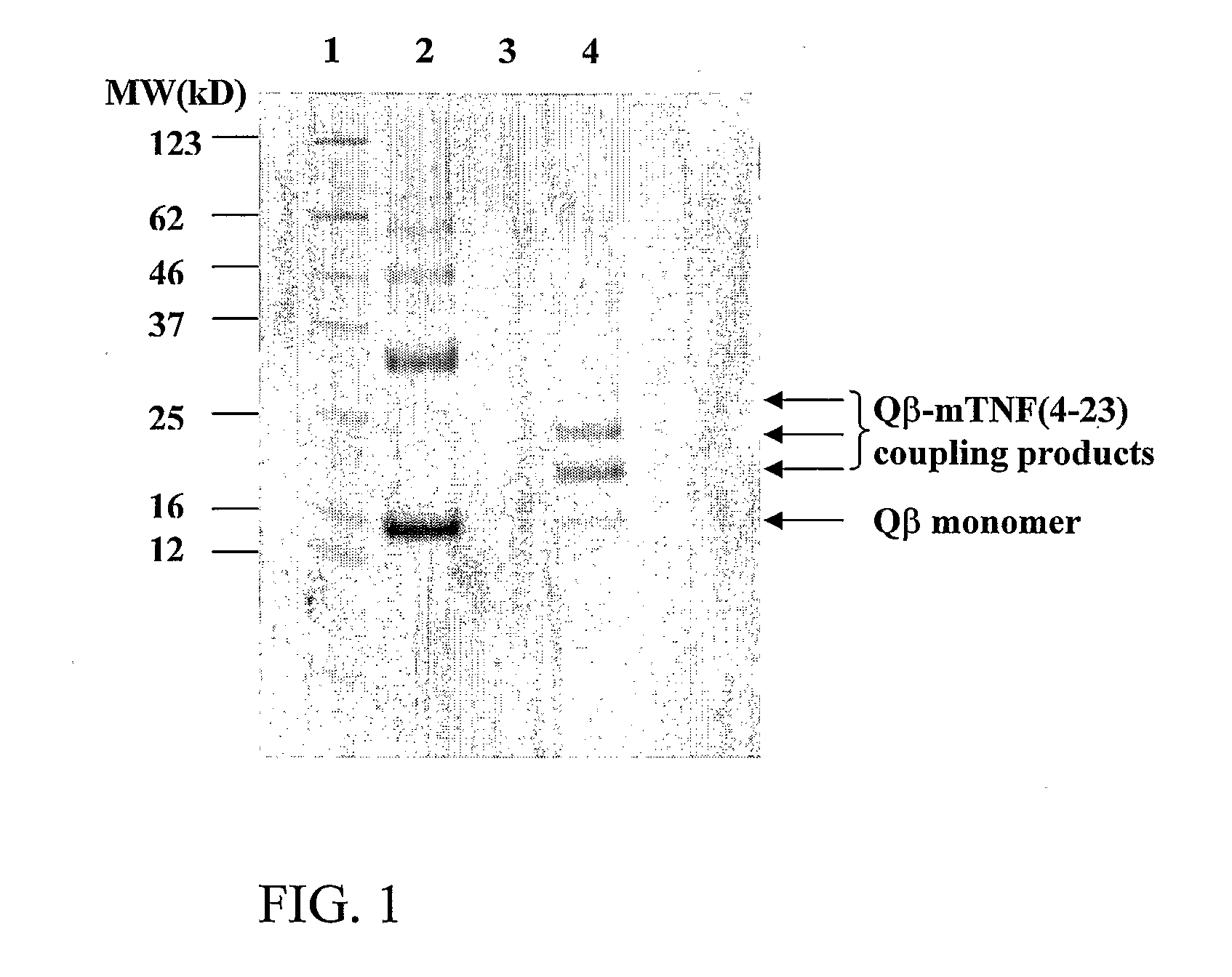
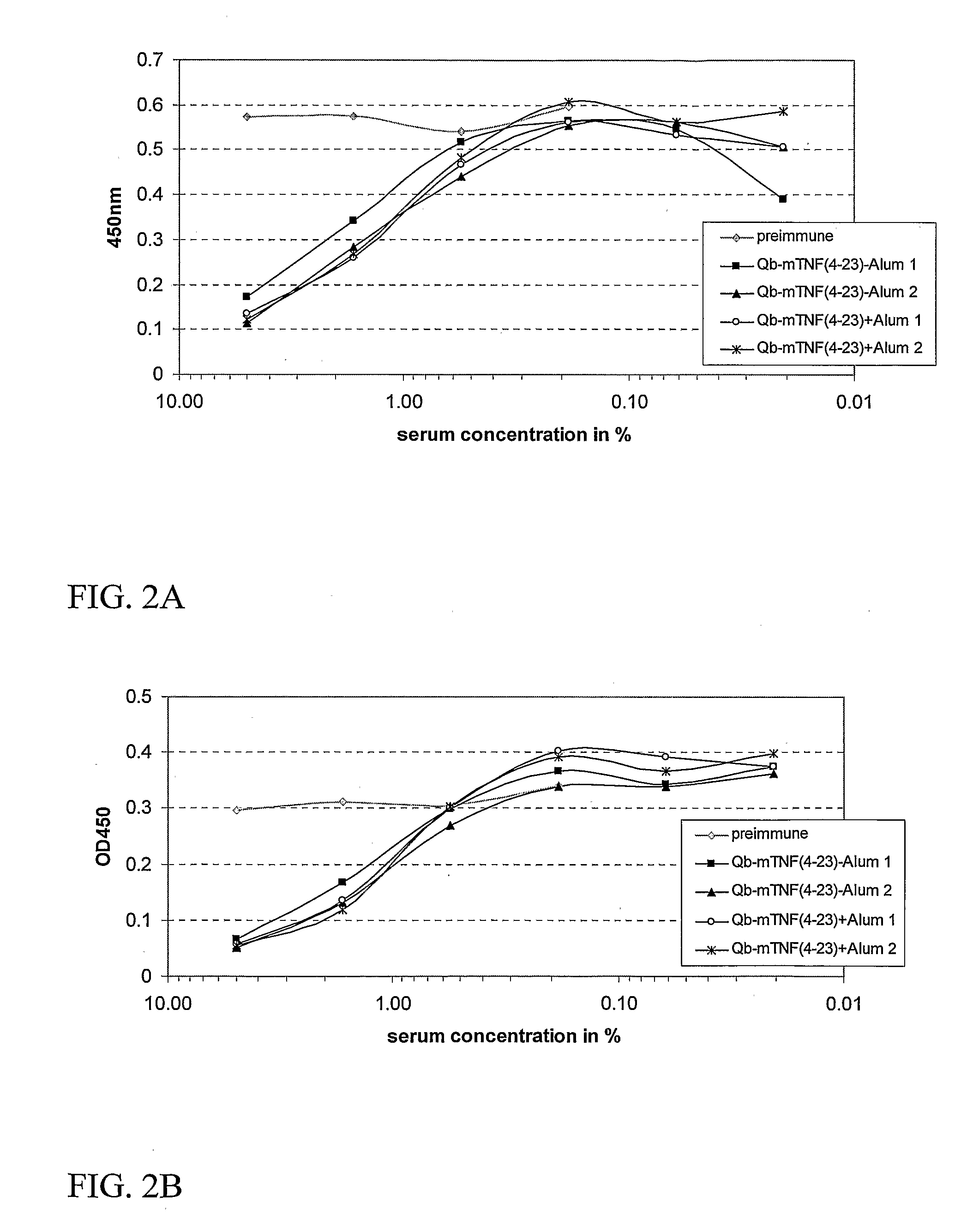
Medical Uses of Carrier Conjugates of Non-Human Tnf -Peptides
InactiveUS20070248617A1Good skin permeabilityEnhance antigen absorptionNervous disorderAntipyreticAutoimmune conditionVirus-like particle
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a modified virus-like particle (VLP) comprising—a VLP and a particular peptide derived from a polypeptide from the TNF-superfamily linked thereto for use in the production of vaccines for the treatment of autoimmune diseases and bone-related diseases and to efficiently induce immune responses, in particular antibody responses. Furthermore, the compositions of the invention are particularly useful to efficiently induce self-specific immune responses within the indicated context.
Owner:CYTOS BIOTECHNOLOGY AG
Chimeric peptides as immunogens, antibodies thereto, and methods for immunization using chimeric peptides or antibodies
The invention provides a chimeric peptide or mixture of chimeric peptides that can be formulated as an immunizing composition and used in a method for immunization of a mammal against an internal peptide cleavage product derived from a precursor or mature protein, for which the peptide cleavage product and the precursor or mature protein are self molecules. The chimeric peptide or peptides have an end-specific B cell epitope from a naturally-occurring internal peptide cleavage product of a precursor or mature protein, as a free N- or C-terminus, fused with or without spacer residues to a T helper cell epitope derived from a living source different from that of the internal peptide cleavage product.
Owner:COLLATERAL AGENTS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com