Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Phosphodiesterase I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
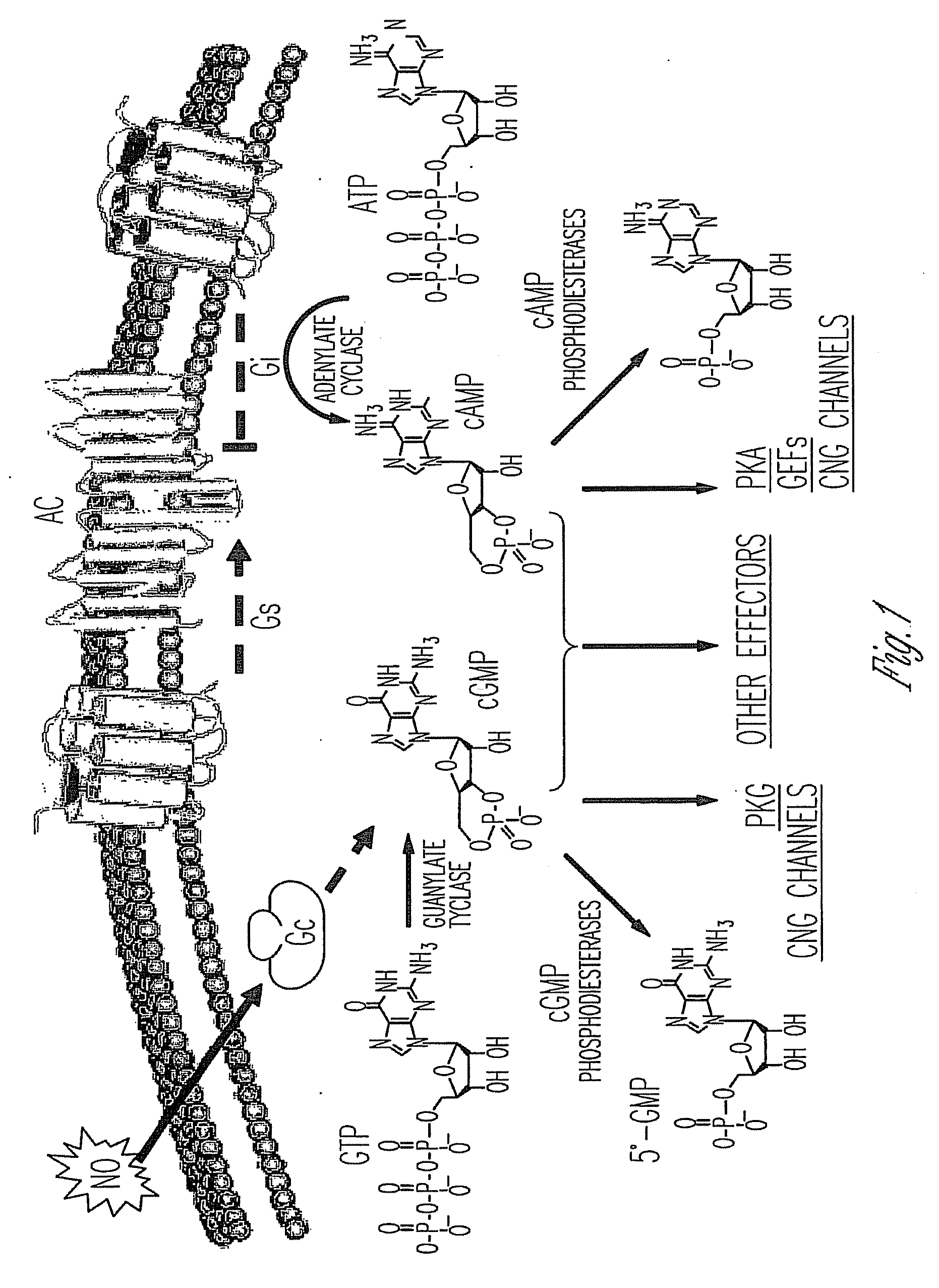
Phosphodiesterase I is an enzyme with system name oligonucleotide 5'-nucleotidohydrolase. This enzyme catalyses the following chemical reaction Hydrolytically removes 5'-nucleotides successively from the 3'-hydroxy termini of 3'-hydroxy-terminated oligonucleotides Hydrolyses both ribonucleotides and deoxyribonucleotides. Has low activity towards polynucleotides.
Phosphodiesterase inhibitors
Owner:THE GOVT OF THE US REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Phosphodiesterase-4 inhibitors belonging to the tertiary amine class
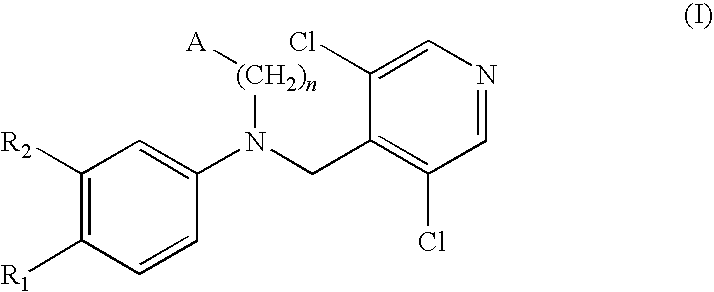
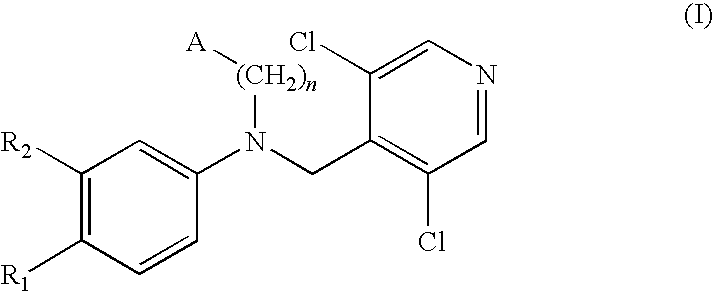
Compounds of formula (I):wherein n, A, R1, and R2 are defined in the specification, are useful as inhibitors of the phosphodiesterase 4 (PDE4) enzyme and for treating certain conditions.
Owner:CHIESI FARM SPA
Phosphodiesterase 4 inhibitor capable of avoiding vomiting and preparation method thereof
InactiveCN102716110APromote learning and memoryImprove cognitive abilityOrganic active ingredientsNervous disorderDiseasePhosphodiesterase I
The present invention relates to a phosphodiesterase 4 inhibitor capable of avoiding vomiting. The phosphodiesterase 4 inhibitor is a compound, a prodrug or a solvate represented by DTPM; and the DTPM is named as 1-(4-difluoro methoxy-3-(tetrahydrofuran-3-oxyl) phenyl)-3-dimethyl-1-ketone, and has a chemical formula shown as below. A plurality of experiments have proved that the DTPM has better effect on improvement of learning and memory compared with an existing antidepressant PDE4 inhibitor; in a Beagle dog vomiting effect observation experiment, no obvious vomiting inducing reaction is observed; and the DTPM has an inhibitor intensity 5000 times of that of other PDE4D families. The inhibitor provided by the invention can become a drug for treating depression and Alzheimer's disease and improving the cognitive ability, and capable of effectively reducing and even avoiding adverse reactions like vomiting.
Owner:徐江平
Combination of HMG-COA Reductase Inhibitors with Phosphodiesterase 4 Inhibitors for the Treatment of Inflammatory Pulmonary Disease
InactiveUS20100069392A1Inhibition is effectiveBiocidePeptide/protein ingredientsHMG-CoA reductasePhosphodiesterase I
The invention relates to the combined use of a PDE4 inhibitor with a HMG-CoA reductase inhibitor for the preventive and curative treatment of an inflammatory pulmonary disease.
Owner:TAKEDA GMBH
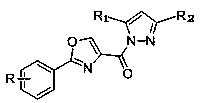
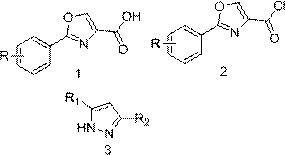
Oxazole ring containing 2,4-disubstituted pyrazole compound, preparation method therefor and application of oxazole ring containing 2,4-disubstituted pyrazole compound
ActiveCN106565695AImprove biological activityEasy to prepareOrganic active ingredientsNervous disorderPhosphodiesterase IHalogen
The invention discloses an oxazole ring containing 2,4-disubstituted pyrazole compound. The structural formula of the compound is represented by a formula I shown in the description, wherein the quantity of R is 1, R is H, halogen, nitro, hydroxyl or C1-4 alkyl or alkoxy, and R1 and R2 are the same or different and are independently selected from H and C1-4 alkyl or alkoxy separately. The invention simultaneously provides a preparation method for the compound and an application of the compound. The oxazole ring containing 2,4-disubstituted pyrazole compound provided by the invention has good bioactivity and can serve as a phosphodiesterase (PDE4) and TNF[alpha] inhibitor; and the preparation method is simple and is high in yield, and the application of oxazole and pyrazole structures in the aspect of serving as phosphodiesterase (PDE4) and TNF[alpha] inhibitors is extended.
Owner:SOUTH CHINA AGRI UNIV
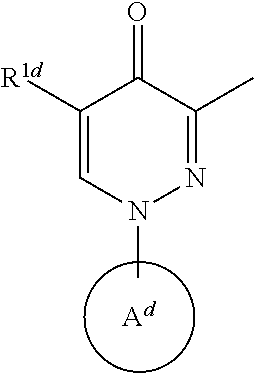
Substituted pyridazin-4(1H)-ones as phosphodiesterase 10A inhibitors
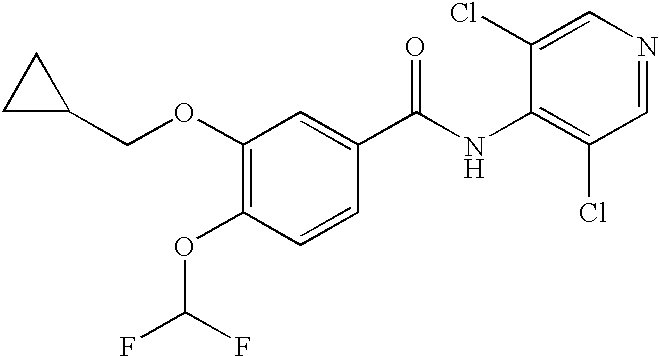
A compound having PDE inhibitory represented by formula (1), W1-W2 (1), wherein (i) W1 isand W2 is(ii) W1 isand W2 isor (iii) W1 isand W2 isor a pharmaceutically acceptable salt thereof; and a method of treating or preventing schizophrenia.
Owner:TAKEDA PHARMA CO LTD
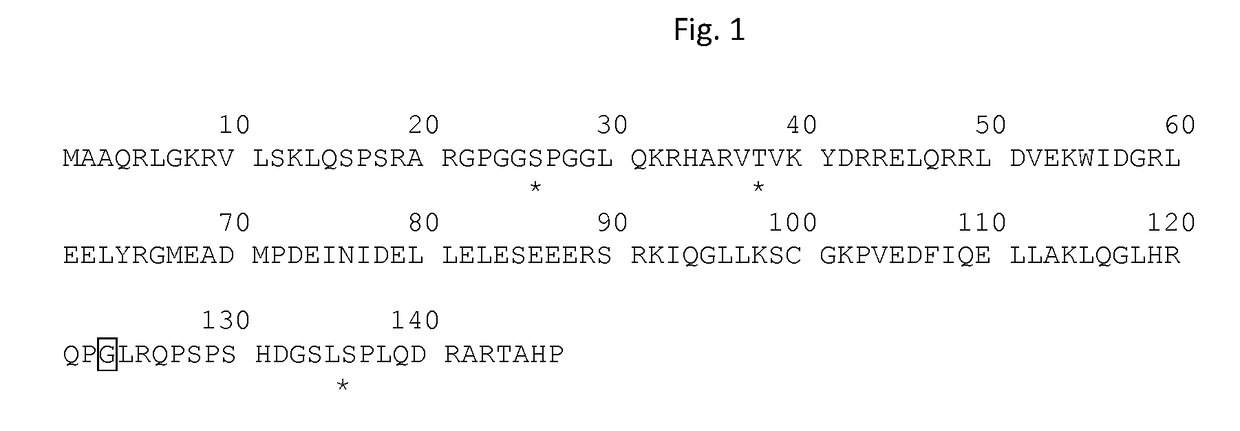
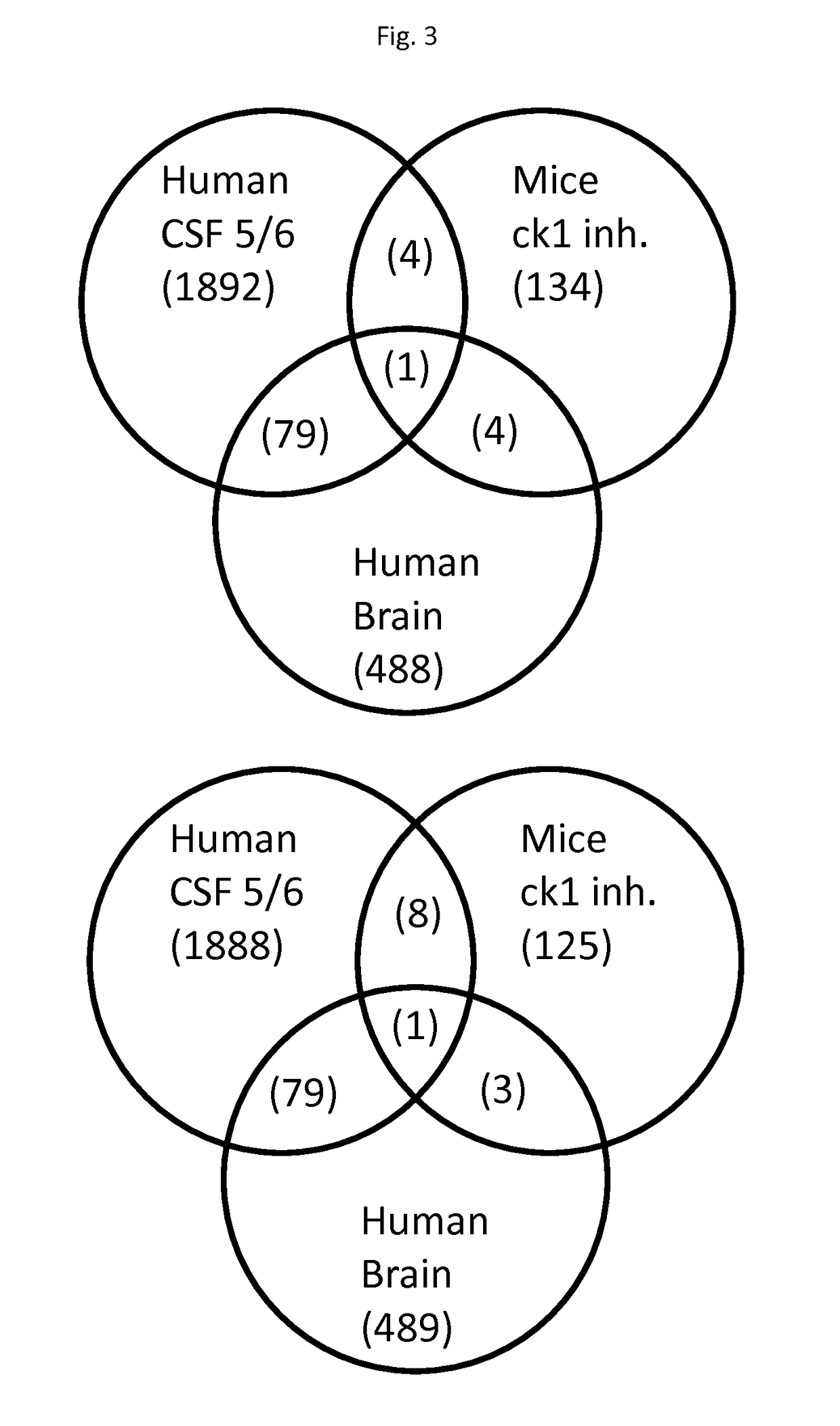
Biomolecules involved in alzheimer's disease
The invention relates to panels of biomarkers including proteins phosphatase 1 regulatory subunit 14A and / or 2′,3′-cyclic-nucleotide 3′-phosphodiesterase and / or phosphorylated tau or fragments thereof and methods using thereof for diagnosing, staging, treating and assessing the response of a treatment for a neurocognitive disorder characterised by tau toxicity, in particular for Alzheimer's disease. The present invention shows that the biomarkers disclosed herein are elevated in the brain of subjects with an advanced stage of a neurocognitive disorder (Braak stage V / VI) and / or are regulated in the CSF of AD subjects in comparison to cognitively affected non-AD controls; and / or regulated in response to two casein kinase 1 delta inhibitors.
Owner:ELECTROPHORETICS LTD
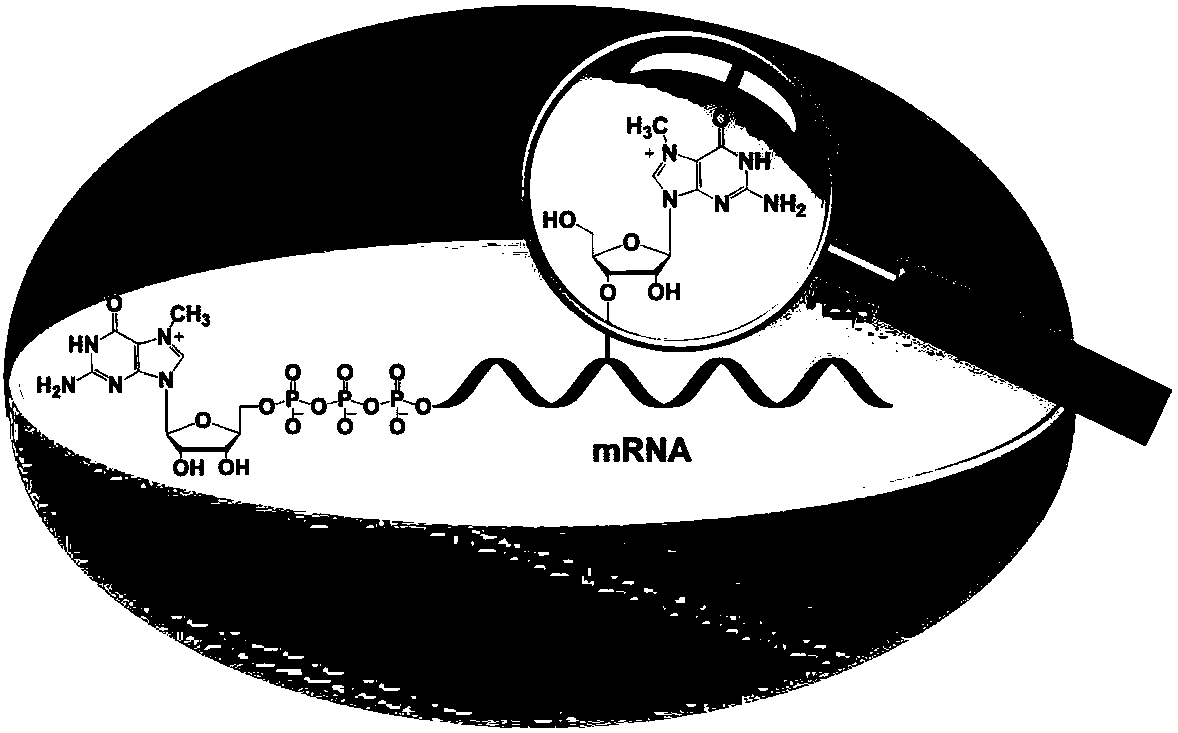
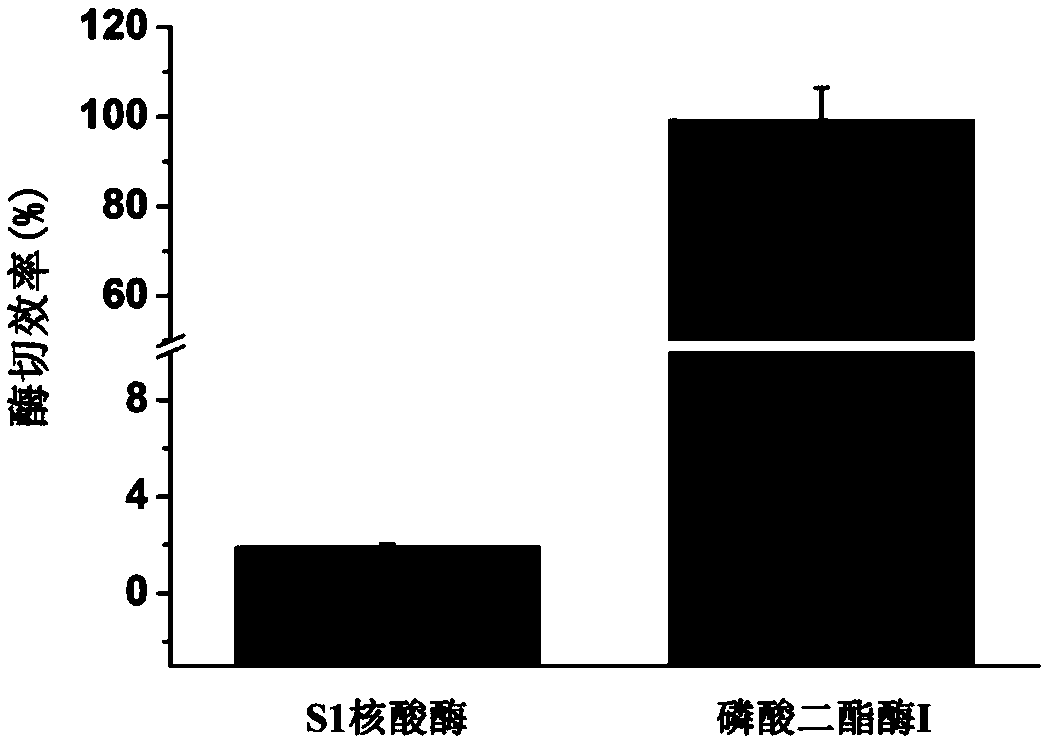
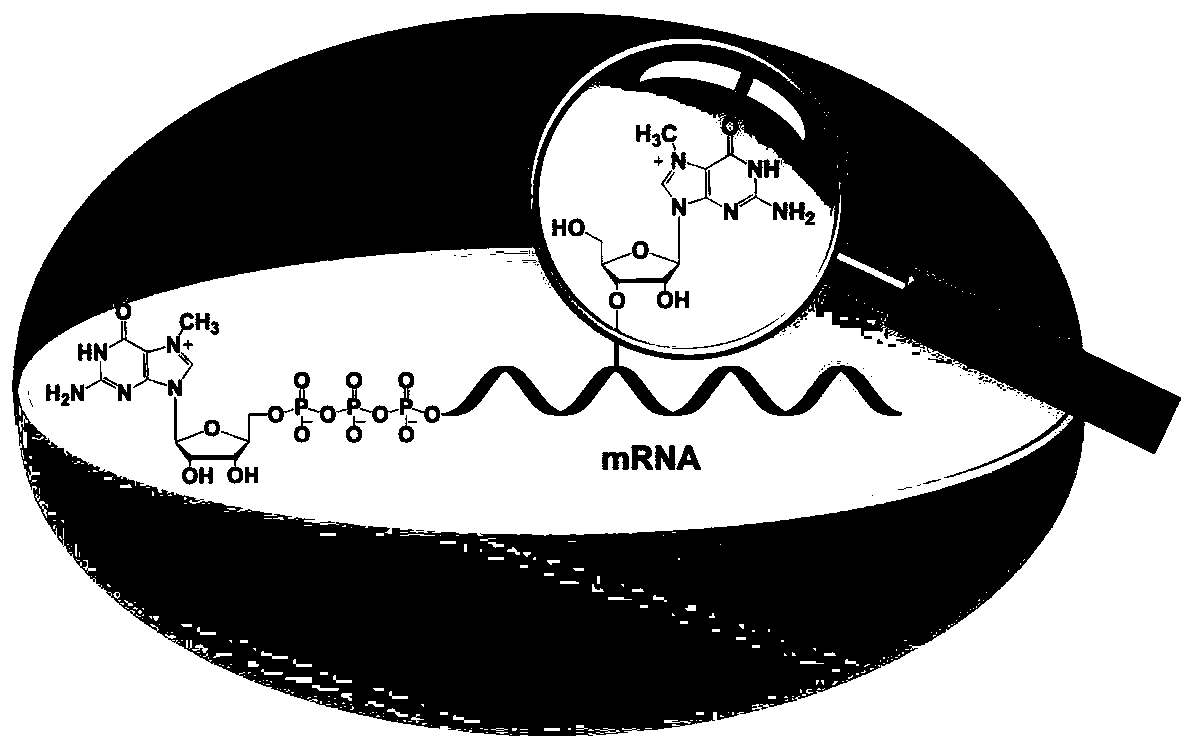
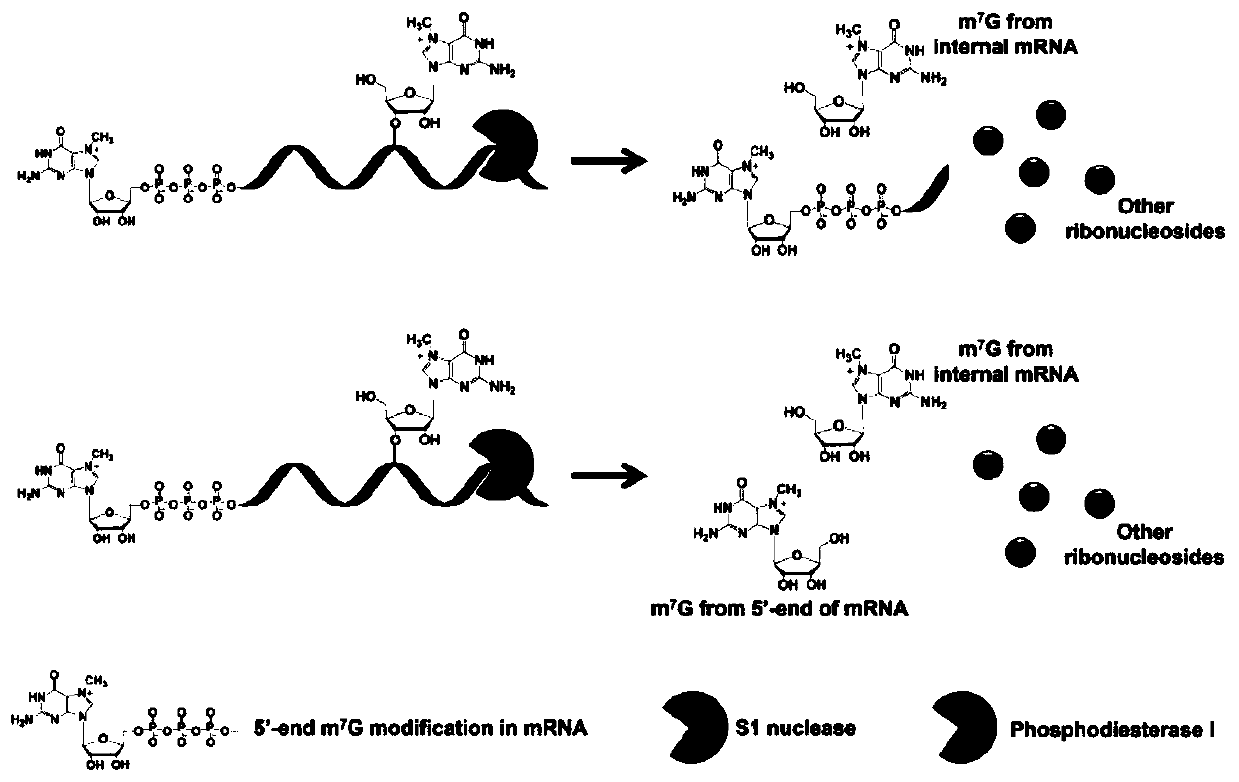
Differential nuclease digestion method and application thereof to detecting mRNA (messenger ribonucleic acid) internal modification through LC-MS (liquid chromatography-mass spectrometry)
InactiveCN107460219ASimple and fast operationRealize detection and analysisMicrobiological testing/measurementChemical recyclingPhosphodiesterase IEnzyme digestion
The invention discloses a differential nuclease digestion method and application thereof to detecting mRNA internal modification through LC-MS. The differential nuclease enzyme-digestion method comprises, according to the characteristic of difference of S1 nuclease and phosphodiesterase I in digestion of the 5'-end cap structure of mRNA, digesting the mRNA into nucleosides through the S1 nuclease and the phosphodiesterase I, then performing LC-MS detecting analysis, and combining the detecting results of digestion through the two nucleases to differentiate N7-methyguanosine (m7G) in the cap and the inside of the mRNA and according to the achieve qualitative and quantitative analysis on the m7G inside the mRNA. The differential nuclease enzyme-digestion method is high in sensitivity and selectivity, can quantitatively detect the content of m7G inside encaryotic mRNA and further can be applied to study of related functions of the m7G inside the mRNA.
Owner:WUHAN UNIV
Novel Solid Forms of Phosphodiesterase Type 5 Inhibitors
ActiveUS20150152107A1Improved physicochemicalImproved biopharmaceutical propertyBiocideOrganic chemistryPhosphodiesterase ICombinatorial chemistry
The present invention relates to novel solid forms of phosphodiesterase type 5 (PDE5) inhibitors, especially to complex co-crystals, and to the solvates and / or the polymorphs of same. Said substances can be used to produce a pharmaceutical composition containing same as the useful active ingredient. Said compounds can exhibit a constant storage stability.
Owner:LAB SENOSIAIN DE C V
Treatment of Parkinson's disease and enhancement of dopamine signal using PDE 10 inhibitor
InactiveUS8338420B1Avoid decompositionRelieve symptomsBiocideOrganic chemistryPhosphodiesterase IBULK ACTIVE INGREDIENT
The present invention relates to a therapeutic or prophylactic method for treating Parkinson's disease by administering an effective amount of a compound having a phosphodiesterase 10 inhibitory activity; and also relates to a pharmaceutical composition for treatment or prophylaxis of Parkinson's disease comprising as an active ingredient a compound having a phosphodiesterase 10 inhibitory activity. Moreover, the present invention relates to a method for enhancing dopamine signal in the brain, which comprises administering an effective amount of a compound having a phosphodiesterase 10 inhibitory activity; and also relates to pharmaceutical composition for enhancing dopamine signal in brain comprising as an active ingredient a compound having a phosphodiesterase 10 inhibitory activity.
Owner:MITSUBISHI TANABE PHARMA CORP
Composition containing phosphodiesterase inhibitor nanoparticle and protein kinase inhibitor
The invention provides a composition containing a phosphodiesterase inhibitor nanoparticle and a protein kinase inhibitor. The composition is characterized in that the nanoparticles contain the phosphodiesterase inhibitor and polylactose glycolic acid, the phosphodiesterase inhibitor and the protein kinase inhibitor are both products available in the market in China and other countries, and the composition has a synergetic antibacterial function (the drug combination index is less than 1) on multiple bacteria.
Owner:黄泳华
Treatment of parkinson's disease and enhancement of dopamine signal using PDE 10 inhibitor
InactiveUS20130072477A1High activityEnhanced signalBiocideOrganic chemistryPhosphodiesterase IBULK ACTIVE INGREDIENT
The present invention relates to a therapeutic or prophylactic method for treating Parkinson's disease by administering an effective amount of a compound having a phosphodiesterase 10 inhibitory activity; and also relates to a pharmaceutical composition for treatment or prophylaxis of Parkinson's disease comprising as an active ingredient a compound having a phosphodiesterase 10 inhibitory activity. Moreover, the present invention relates to a method for enhancing dopamine signal in the brain, which comprises administering an effective amount of a compound having a phosphodiesterase 10 inhibitory activity; and also relates to pharmaceutical composition for enhancing dopamine signal in brain comprising as an active ingredient a compound having a phosphodiesterase 10 inhibitory activity.
Owner:MITSUBISHI TANABE PHARMA CORP
Compounds and Methods for Inhibition of Hedgehog Signaling and Phosphodiesterase
ActiveUS20170190717A1Avoid signalingImprove ejection fractionOrganic active ingredientsOrganic chemistryPhosphodiesterase IMedicine
Compounds and compositions, and methods of use thereof, are provided and have utility in inhibiting hedgehog signaling and / or phosphodiesterase-4 activity.
Owner:VANDERBILT UNIV
Pyrazolopyrimidine PDE 10 inhibitors
The present invention is directed to pyrazolopyrimidine compounds which are useful as therapeutic agents for the treatment of central nervous system disorders associated with phosphodiesterase 10 (PDE10). The present invention also relates to the use of such compounds for treating neurological and psychiatric disorders, such as schizophrenia, psychosis or Huntington's disease, and those associated with striatal hypofunction or basal ganglia dysfunction.
Owner:MERCK SHARP & DOHME LLC
Co-crystals of tadalafil and a hydroxy-substituted benzoic acid coformer as phosphodiesterase type 5 inhibitors
ActiveUS9278970B2Improve propertiesImprove bioavailabilityOrganic chemistrySexual disorderPhosphodiesterase IBenzoic acid
The present invention relates to co-crystals of tadalafil with hydroxyl-substituted benzoic acid coformers having the following structure.Said co-crystals can be used to produce a pharmaceutical composition containing the same as the useful active ingredient. Said co-crystals can exhibit a constant storage stability.
Owner:LAB SENOSIAIN DE C V
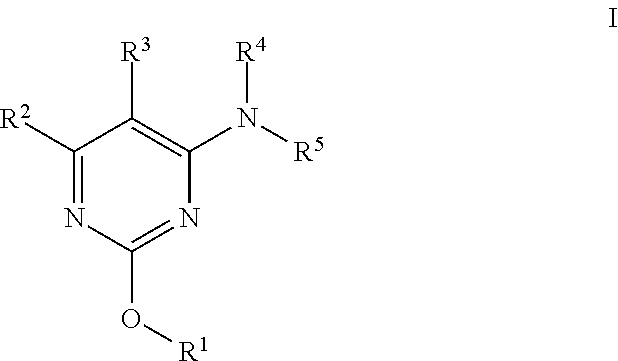
Alkoxy pyrimidine pde10 inhibitors
The present invention is directed to 2-alkoxy pyrimidine compounds which are useful as therapeutic agents for the treatment of central nervous system disorders associated with phosphodiesterase 10 (PDE10). The present invention also relates to the use of such compounds for treating neurological and psychiatric disorders, such as schizophrenia, psychosis or Huntington's disease, and those associated with striatal hypofunction or basal ganglia dysfunction.
Owner:MERCK SHARP & DOHME LLC
A kind of 2,4-disubstituted pyrazole compound containing oxazole ring and its preparation method and application
ActiveCN106565695BImprove biological activityEasy to prepareOrganic active ingredientsNervous disorderPhosphodiesterase IHalogen
The invention discloses an oxazole ring containing 2,4-disubstituted pyrazole compound. The structural formula of the compound is represented by a formula I shown in the description, wherein the quantity of R is 1, R is H, halogen, nitro, hydroxyl or C1-4 alkyl or alkoxy, and R1 and R2 are the same or different and are independently selected from H and C1-4 alkyl or alkoxy separately. The invention simultaneously provides a preparation method for the compound and an application of the compound. The oxazole ring containing 2,4-disubstituted pyrazole compound provided by the invention has good bioactivity and can serve as a phosphodiesterase (PDE4) and TNF[alpha] inhibitor; and the preparation method is simple and is high in yield, and the application of oxazole and pyrazole structures in the aspect of serving as phosphodiesterase (PDE4) and TNF[alpha] inhibitors is extended.
Owner:SOUTH CHINA AGRI UNIV
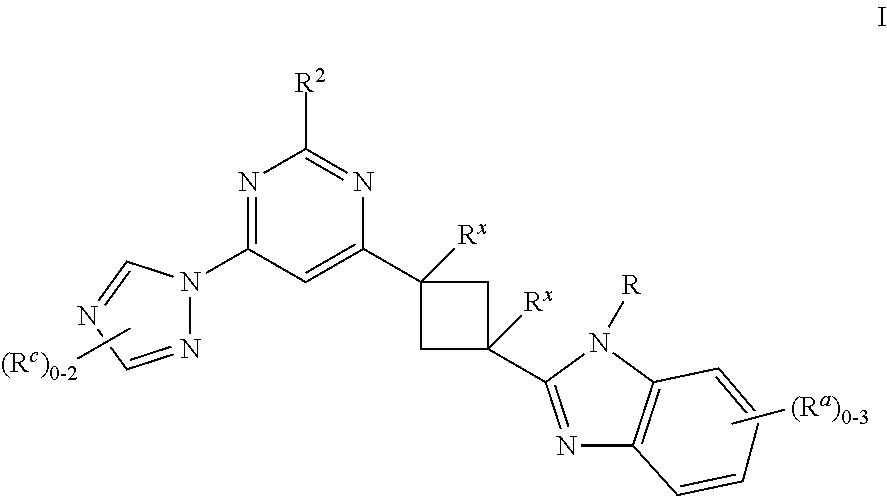
Cyclobutyl benzimidazoles as PDE 10 inhibitors
ActiveUS9284302B2Organic active ingredientsNervous disorderPhosphodiesterase IBasal ganglia dysfunction
The present invention is directed to substituted cyclobutyl benzimidazole compounds which are useful as therapeutic agents for the treatment of central nervous system disorders associated with phosphodiesterase 10 (PDE10). The present invention also relates to the use of such compounds for treating neurological and psychiatric disorders, such as schizophrenia, psychosis or Huntington's disease, and those associated with striatal hypofunction or basal ganglia dysfunction.
Owner:MERCK SHARP & DOHME LLC
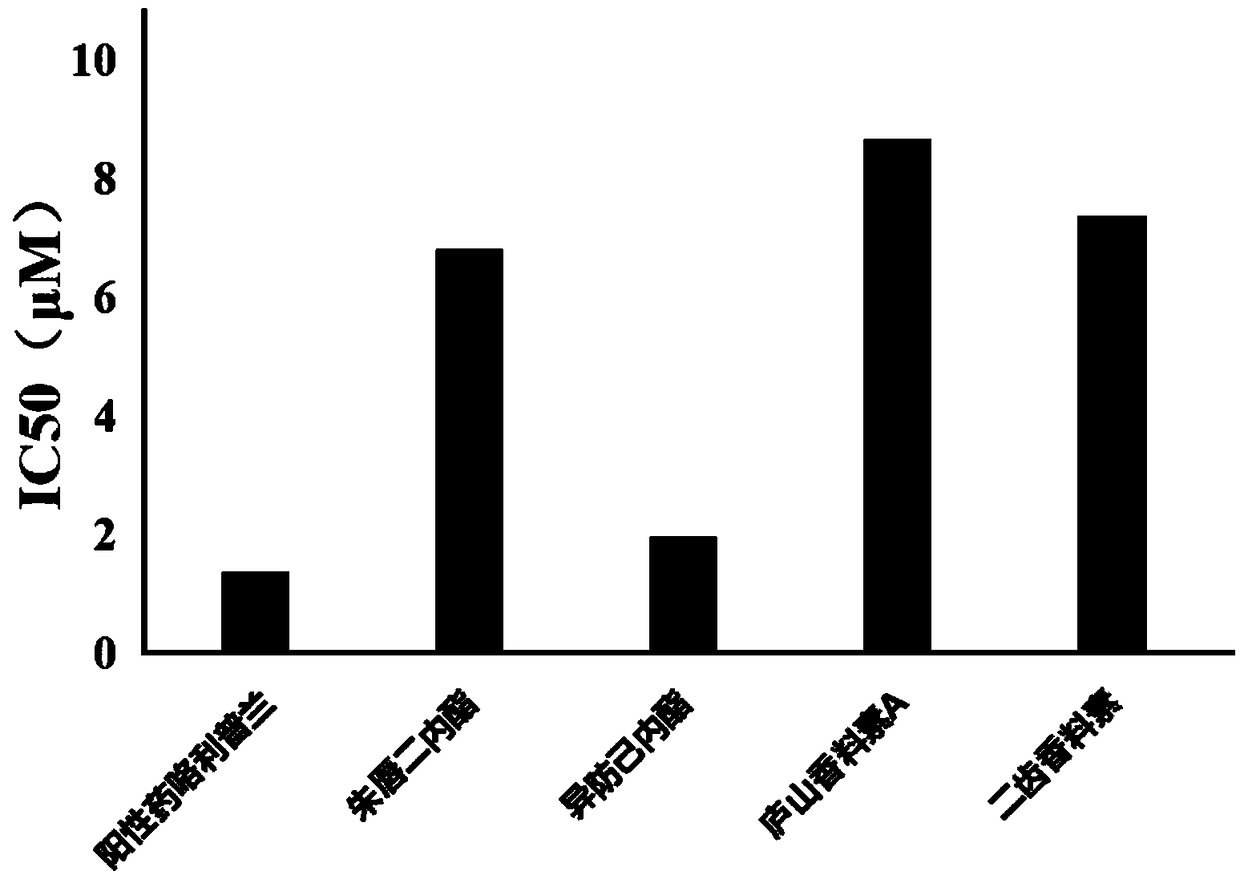
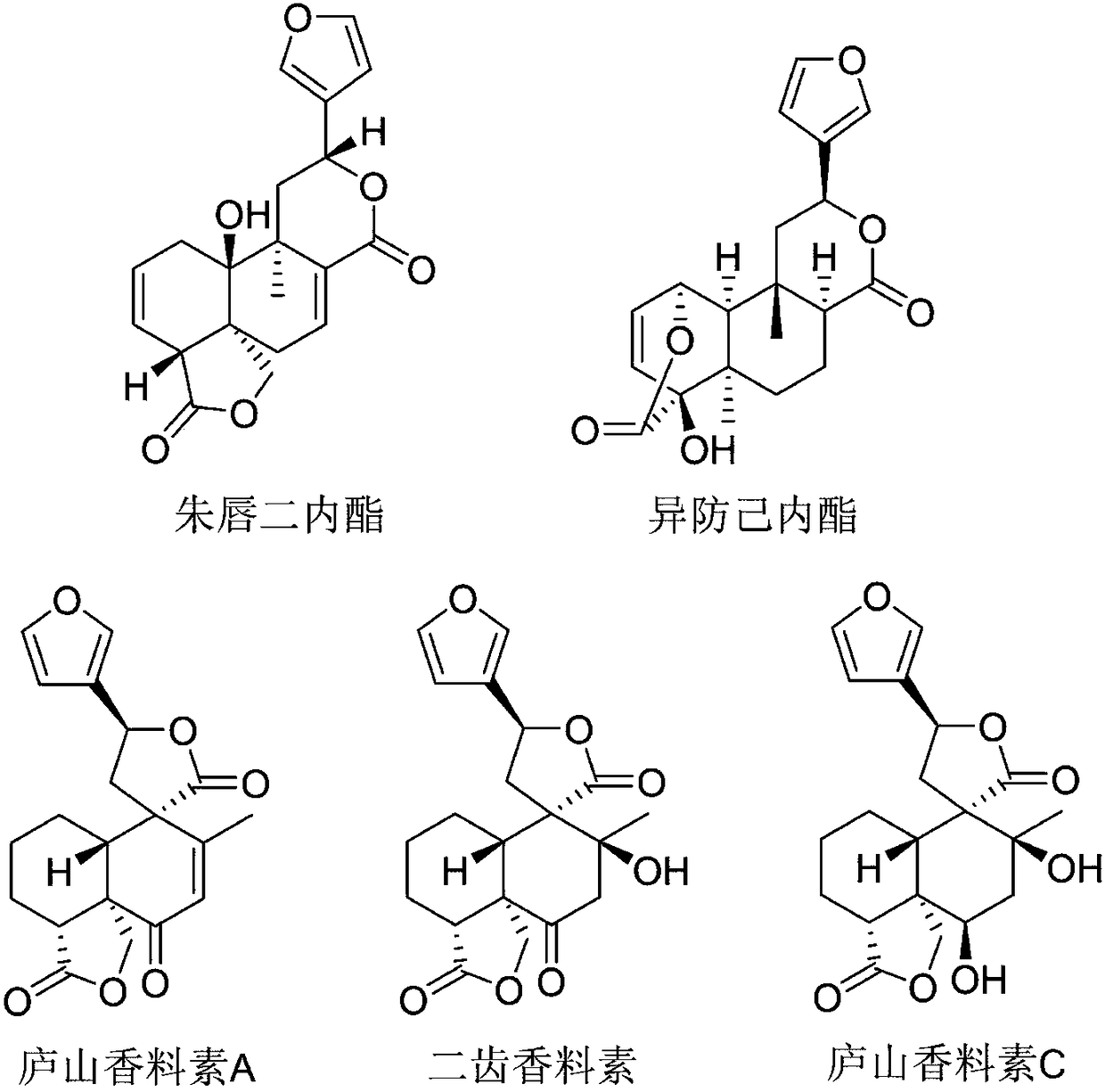
Applications of phosphodiesterase type 4 inhibitors in preparation of novel anti-inflammatory drugs
InactiveCN108619135AEnhanced inhibitory effectAntipyreticAnalgesicsPhosphodiesterase IPhosphodiesterase Type 4
Applications of phosphodiesterase type 4 inhibitors in preparation of novel anti-inflammatory drugs are disclosed. Salviacoccin, isocolumbin, teupernin A and bidentatin are effective phosphodiesterasetype 4 inhibitors, and teupernin C does not have obvious inhibition effect. In the salviacoccin, the isocolumbin, the teupernin A and the bidentatin, the isocolumbin has highest inhibition effect close to that of a positive drug that is rolipram.
Owner:胡艳
A Differential Nuclease Digestion Method and Its Application in Detection of Internal Modifications of mRNA by LC-MS Method
InactiveCN107460219BSimple and fast operationRealize detection and analysisMicrobiological testing/measurementChemical recyclingPhosphodiesterase IS1 nuclease
The invention discloses a differential nuclease digestion method and application thereof to detecting mRNA internal modification through LC-MS. The differential nuclease enzyme-digestion method comprises, according to the characteristic of difference of S1 nuclease and phosphodiesterase I in digestion of the 5'-end cap structure of mRNA, digesting the mRNA into nucleosides through the S1 nuclease and the phosphodiesterase I, then performing LC-MS detecting analysis, and combining the detecting results of digestion through the two nucleases to differentiate N7-methyguanosine (m7G) in the cap and the inside of the mRNA and according to the achieve qualitative and quantitative analysis on the m7G inside the mRNA. The differential nuclease enzyme-digestion method is high in sensitivity and selectivity, can quantitatively detect the content of m7G inside encaryotic mRNA and further can be applied to study of related functions of the m7G inside the mRNA.
Owner:WUHAN UNIV
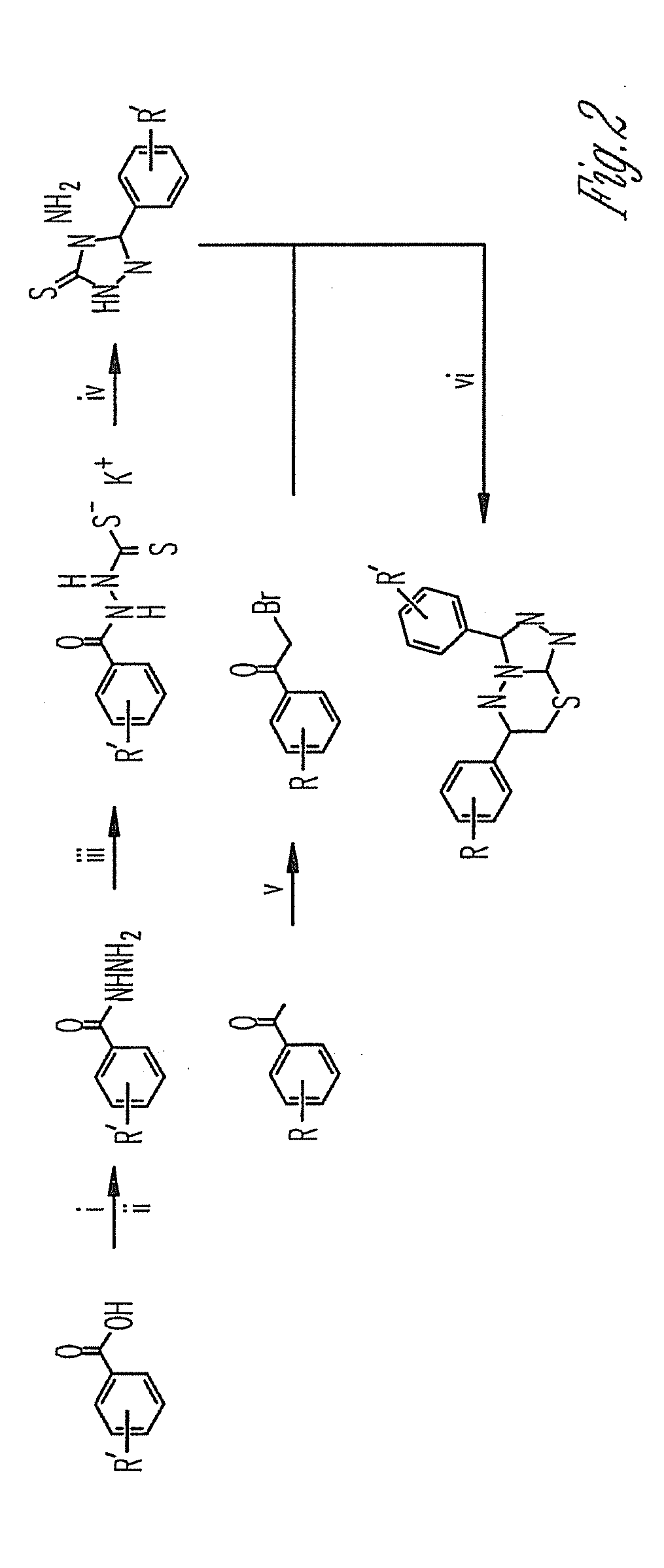
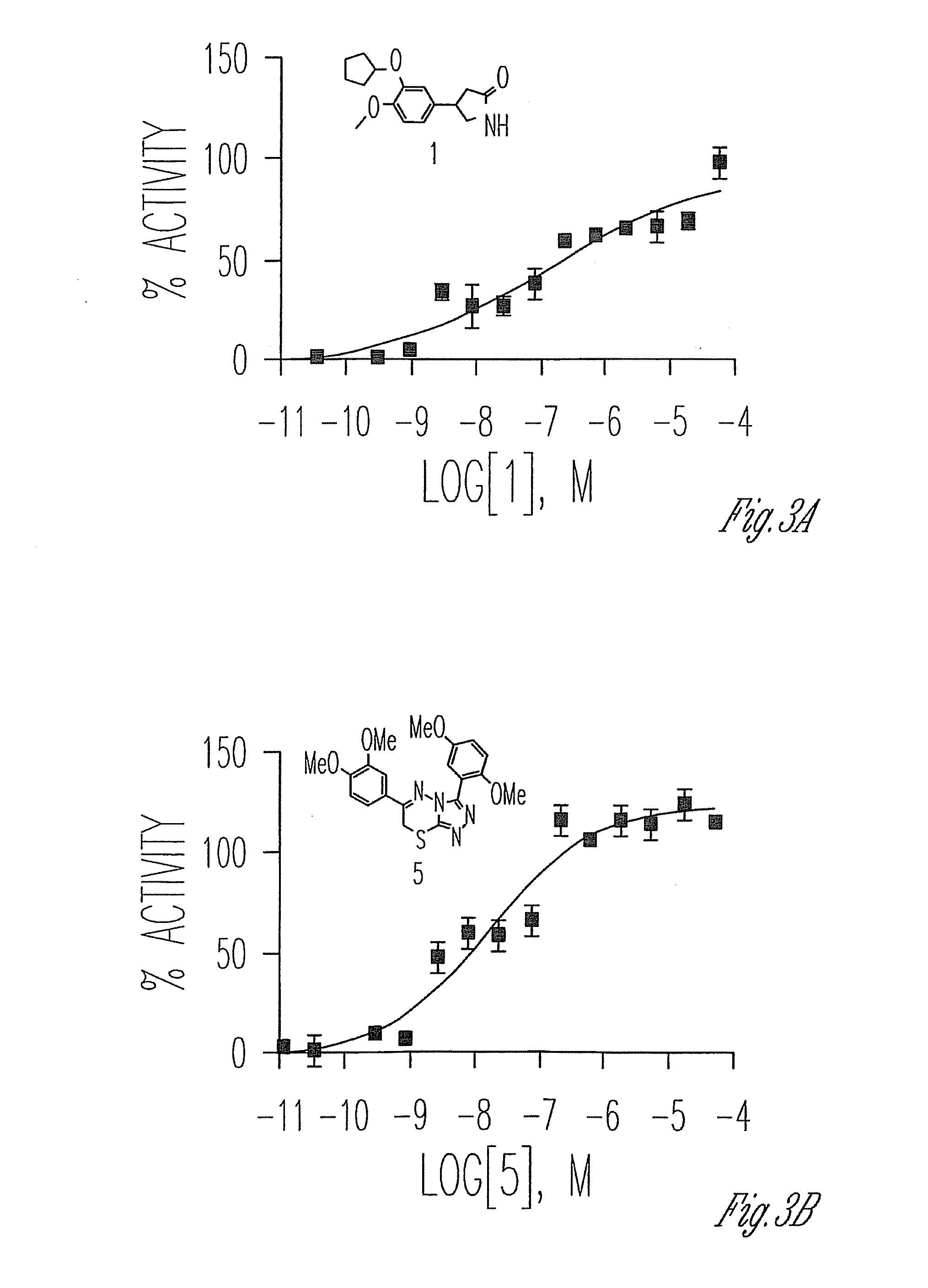
2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme
The present invention relates to the field of molecular biology, biochemistry and biotechnology, namely, to compound 2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl) thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9bh)-one, which is a hydrazine-thiazole derivative of usnic acid of formula I and which is able to inhibit the action of human tyrosyl-DNA-phosphodiesterase 1 enzyme. Technical result: increase in inhibitory action towards human tyrosyl-DNA-phosphodiesterase 1 enzyme (Tdp1) and increase in the number of inhibitors of this enzyme.
Owner:INNOVATIVE PHARMACOLOGY RES OOO
Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp
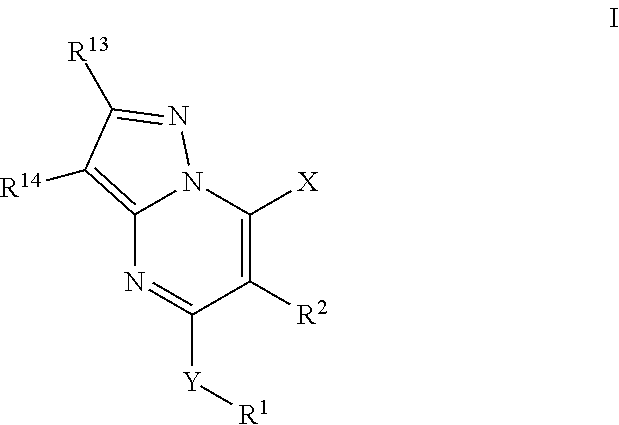
PendingUS20180185347A1Reduction in PSA levelSmall sizeUrinary disorderCapsule deliveryPhosphodiesterase IMedicine
Methods of treating the signs and symptoms of Benign Prostatic Hyperplasia (BPH) in a subject by administering at least one tetrahydrocyclopenta[b]indole compound are disclosed. Also disclosed are methods of treating the signs and symptoms of BPH in a subject by administering at least one tetrahydrocyclopenta[b]indole compound in combination with a phosphodiesterase type-5 inhibitor.
Owner:EIRGEN PHARMA LTD
Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor
ActiveCN102260266BHas inhibitory activityOrganic active ingredientsNervous disorderPhosphodiesterase IIsrapafant
The invention discloses pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of a phosphodiesterase IX inhibitor. The structure of the compounds is shown as a formula (1); in the formula, when R' is 2-chlorophenyl, R refers to phenyl, substituted phenyl, benzyl, substituted benzyl, 3-methylpyridine, 1-phenylethyl, diphenylmethyl or CHCH3CONHR1, wherein R1 refers to phenyl ormethyl, methoxyl, ethoxyl, isopropoxy, methylthio, NHCOCH3 or NCH3CH3 substituted phenyl; when R' is phenyl, R refers to CHCH3CONHR2, wherein R2 refers to methoxyl or ethoxyl substituted phenyl; and when R' is methyl, R refers to 4-methoxy-benzyl, diphenylmethyl or N-(2-amino-cyclohexyl)-4-methoxy-benzenesulfonamide. The pyrazolo[3,4-d]pyrimidone compounds have activity of inhibiting phosphodiesterase IX, can serve as the phosphodiesterase IX inhibitor, can be used for preparing the phosphodiesterase IX inhibitor, and have wide application prospect. The compounds are shown as the formula (1).
Owner:SUN YAT SEN UNIV
Compounds and methods for inhibition of hedgehog signaling and phosphodiesterase
InactiveUS10329304B2Improve ejection fractionIncrease heart rateOrganic active ingredientsOrganic chemistryPhosphodiesterase IMedicine
Compounds and compositions, and methods of use thereof, are provided and have utility in inhibiting hedgehog signaling and / or phosphodiesterase-4 activity.
Owner:VANDERBILT UNIV
Phosphodiesterase 4 inhibitors and application thereof
InactiveCN109125319AEnhanced inhibitory effectOrganic active ingredientsAntipyreticPhosphodiesterase IProstaglandin a
The invention discloses phosphodiesterase 4 inhibitors and an application thereof, which finds that the labiacinol, isopredomyl lactone, scorpion prostaglandin A and bidentenol are effective phosphodiesterase 4 inhibitors, and the inhibitory effect of scorpion prostaglandin C is not obvious. Among the labiacinol, isopredomyl lactone, scorpion prostaglandin A and bidentenol, the isopredomyl lactonehas the strongest inhibitory effect, which is close to the positive drug rolipram.
Owner:胡艳
Special compound enzyme for yeast hydrolysis and preparation method thereof
ActiveCN102115734BEfficient releaseEffective structureEnzymesFood preparationPhosphodiesterase IGeneration rate
The invention discloses a special compound enzyme for yeast hydrolysis and a preparation method thereof. The compound enzyme comprises the following enzyme components in parts by weight: 1-30 parts of beta-glucanase, 1-30 parts of mannase, 1-30 parts of saccharifying enzyme, 15-65 parts of endoenzyme, 1-10 parts of exoenzyme, 1-10 parts of flavourzyme and 1-10 parts of phosphodiesterase. The preparation method comprises the following steps: respectively fermenting, extracting, freeze-drying and pulverizing the beta-glucanase, mannase, saccharifying enzyme, endoenzyme, exoenzyme, flavourzyme and phosphodiesterase, and evenly mixing according to the proportion designed in the formula to obtain the special compound enzyme for yeast hydrolysis. Since multiple biological enzymes are coupled together for hydrolysis, the yeast hydrolysis time is shortened from 20-32 hours in the traditional way to 10-25 hours, the utilization ratio of the proteins is higher than 90%, the generation rate of amino acids is higher than 75%, the content of amino acids is higher than 0.83g / 100ml, and the total nitrogen content on dry basis is higher than 12.0%.
Owner:南宁庞博生物工程有限公司
Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders
The present invention relates to combinations of phosphodiesterase 2 (PDE2) inhibitors with inhibitors of phosphodiesterase 10 (PDE 10). In particular, the invention relates to combinations of 1-aryl-4- methyl-[1,2,4]triazolo[4,3-a]-quinoxaline derivatives which have been found to inhibit phosphodiesterase 2 (PDE2), with inhibitors of phosphodiesterase 10 (PDE10). Particular PDE10 inhibitors are selected from the group of MP-10, PQ-10, TP-10, papaverine, and the compounds disclosed in WO 2011 / 051342 and in WO 2011 / 110545. The invention is also directed to pharmaceutical compositions comprising such combinations, to processes for preparing such compositions, to the use of PDE2 inhibitors, in particular of 1- aryl-4-methyl- [1,2,4]triazolo[4,3-a]-quinoxaline derivatives for the potentiation of said PDE10 inhibitors, and to the use of said PDE10 inhibitors for the potentiation of the effect of said PDE2 inhibitors, in particular, 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]-quinoxaline derivatives, and to the use of such combinations and compositions for the prevention and treatment of disorders in which PDE2 and PDE10 are involved, such as neurological and psychiatric disorders, and endocrinological or metabolic diseases.
Owner:JANSSEN PHARMA NV
Fused triazole derivatives as phosphodiesterase 10A inhibitors
ActiveUS10138245B2Good effectEnhanced PDE1 expressionOrganic active ingredientsNervous disorderPhosphodiesterase IAryl
Compounds of the general formula (I), wherein one of X1 and X2 represents N, and the other one of X1 and X2 represents —C(CH3), A represents unsubstituted or substituted 5-, 6- or 10-membered aryl or heteroaryl, n is 0 or 1 and B is a bicyclic heteromoiety defined in the specification. Compounds are phosphodiesterase 10A inhibitors and can find use in medicine in the treatment psychotic, neurological and cognitive functions diseases and disorders. (I)
Owner:CELON PHARMA
For use in the treatment of neurological disorders or metabolic combinations used in obstacles
The present invention relates to combinations of inhibitors of phosphodiesterase 2 (PDE2) and inhibitors of phosphodiesterase 10 (PDE10). In particular, the present invention relates to 1-aryl-4-methyl-[1,2,4]triazol[4,3-a]-quinoxaline derivatives and inhibitors of phosphodiesterase 10 (PDE10) Combination of this derivative has been found to inhibit phosphodiesterase 2 (PDE2). Particular PDE10 inhibitors are selected from the group consisting of MP-10, PQ-10, TP-10, papaverine, and compounds disclosed in WO 2011 / 051342 and WO 2011 / 110545. The invention also relates to pharmaceutical compositions comprising these combinations, to processes for the preparation of these compositions, to PDE2 inhibitors, in particular 1-aryl-4-methyl-[1,2,4]triazole[ 4,3-a]-quinoxaline derivatives for the enhanced use of said PDE10 inhibitors and to said PDE10 inhibitors for said PDE2 inhibitors, in particular 1-aryl-4-methyl Use of ‑[1,2,4]triazol[4,3‑a]‑quinoxaline derivatives for enhancing the effect and to the use of these combinations and compositions for the prevention and treatment of disorders involving PDE2 and PDE10 , such disorders as neurological and psychiatric disorders, and endocrine or metabolic diseases.
Owner:JANSSEN PHARMA NV
Method for detecting karyon DNA (deoxyribonucleic acid) and mitochondrion DNA methylation levels of corn through high-performance liquid chromatography
The invention discloses a method for detecting the karyon DNA (deoxyribonucleic acid) and mitochondrion DNA methylation levels of a corn through high-performance liquid chromatography. The preparation method of a hydrolysis mixed solution for treating 100 parts of DNA of 1 mu g comprises the following step: adding 250 U of Benzonase, 300 muU of phosphodiesterase I and 200 U of alkaline phosphatase into 5 mL of Tris-HCl hydrolysis buffer solution having a pH value of 7.9, wherein the pH values of mobile phases are as follows: the pH value of mitochondrion DNA is regulated to 3.42 with phosphoric acid, and the pH value of karyon DNA is regulated to 3.0 with phosphoric acid; the chromatographic conditions are as follows: a C18 filler chromatographic column is used, the flow rate is 0.8 mL / min, the detector wavelength is 287 nm, the column temperature is 35 DEG C, and the sample size is 20 muL; and methanol, 5mM sodium pentanesulfonate and triethylamine of which the ratio is 10:90:0.2 (V / V) are used as the mobile phases, and the pH values are respectively regulated to 3.42 and 3.0 with phosphoric acid. The method is simple and easy to implement, and all the components can be completely separated within 20 minutes.
Owner:SICHUAN AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com















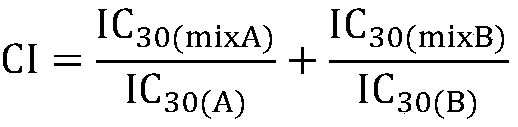




















![2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme 2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/787c7c54-7cb6-4738-931f-4e28727da92a/US20190177315A1-D00001.png)
![2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme 2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/787c7c54-7cb6-4738-931f-4e28727da92a/US20190177315A1-D00002.png)
![2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme 2-acetyl-6-(2-(2-(4-bromobenzylidene)hydrazinyl)thiazole-4-yl)-3,7,9-trihydroxy-8,9b-dimethyldibenzo[b,d]furan-1(9BH)-one exhibiting an inhibitory effect on human tyrosyl-dna-phosphodiesterase 1 enzyme](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/787c7c54-7cb6-4738-931f-4e28727da92a/US20190177315A1-C00001.png)
![Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/816abd12-c8eb-4b0a-82b8-1dfb3db24b23/US20180185347A1-20180705-D00000.png)
![Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/816abd12-c8eb-4b0a-82b8-1dfb3db24b23/US20180185347A1-20180705-D00001.png)
![Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp Tetrahydrocyclopenta[b]indole compounds and phosphodiesterase inhibitors for the treatment of the signs and symptoms of bhp](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/816abd12-c8eb-4b0a-82b8-1dfb3db24b23/US20180185347A1-20180705-D00002.png)
![Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/851111a9-687e-478e-9532-836a2ea4e117/FDA00002897384600011.PNG)
![Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/851111a9-687e-478e-9532-836a2ea4e117/FDA00002897384600012.PNG)
![Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor Pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of phosphodiesterase IX inhibitor](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/851111a9-687e-478e-9532-836a2ea4e117/FDA00002897384600013.PNG)







![Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cfbbaafa-7838-423b-86b9-db45ff3076f2/HDA0000642388400000011.PNG)
![Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cfbbaafa-7838-423b-86b9-db45ff3076f2/HDA0000642388400000012.PNG)
![Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl- [1,2,4] triazolo [4,3-a] quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological or metabolic disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cfbbaafa-7838-423b-86b9-db45ff3076f2/HDA0000642388400000013.PNG)








