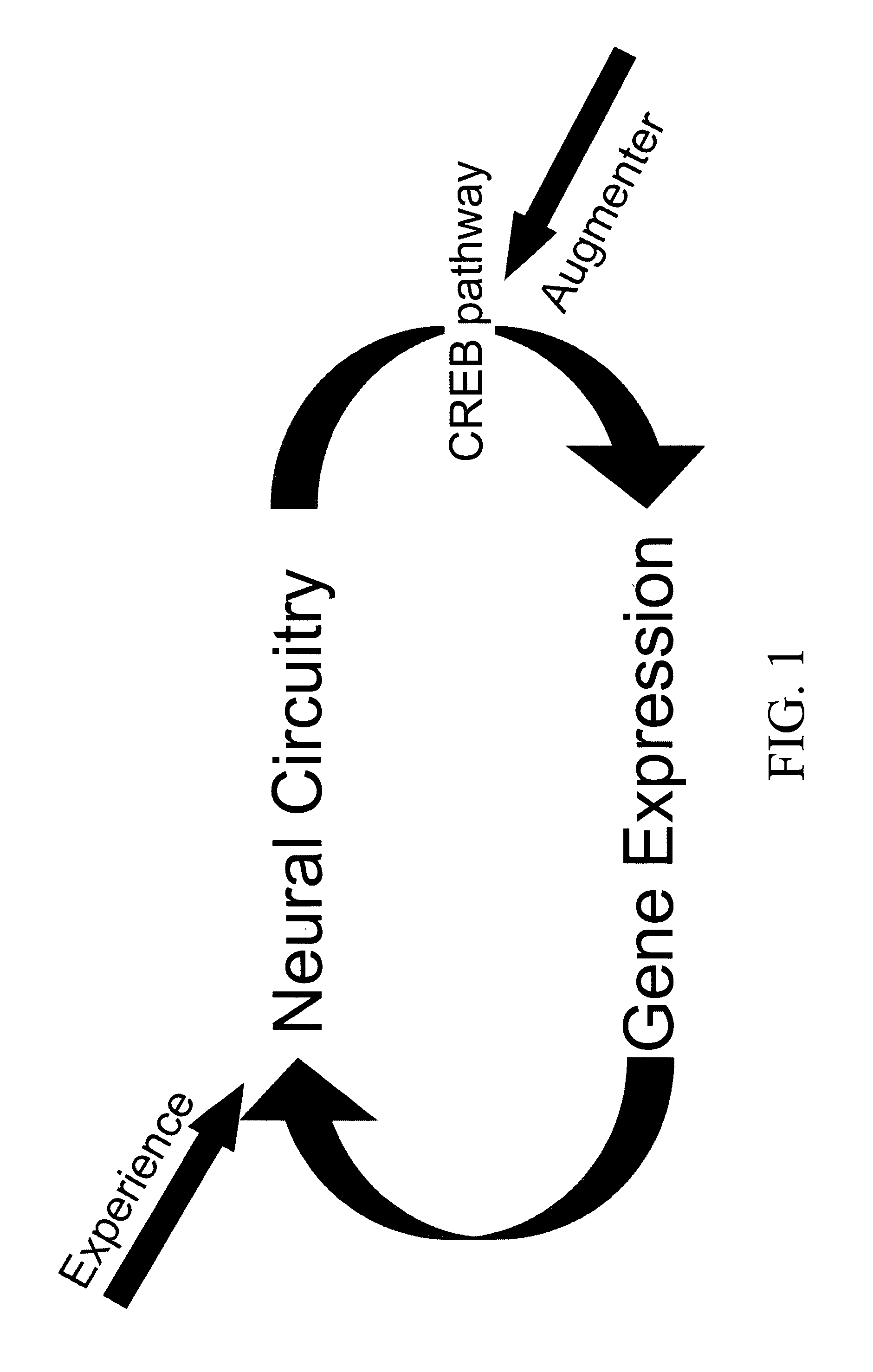
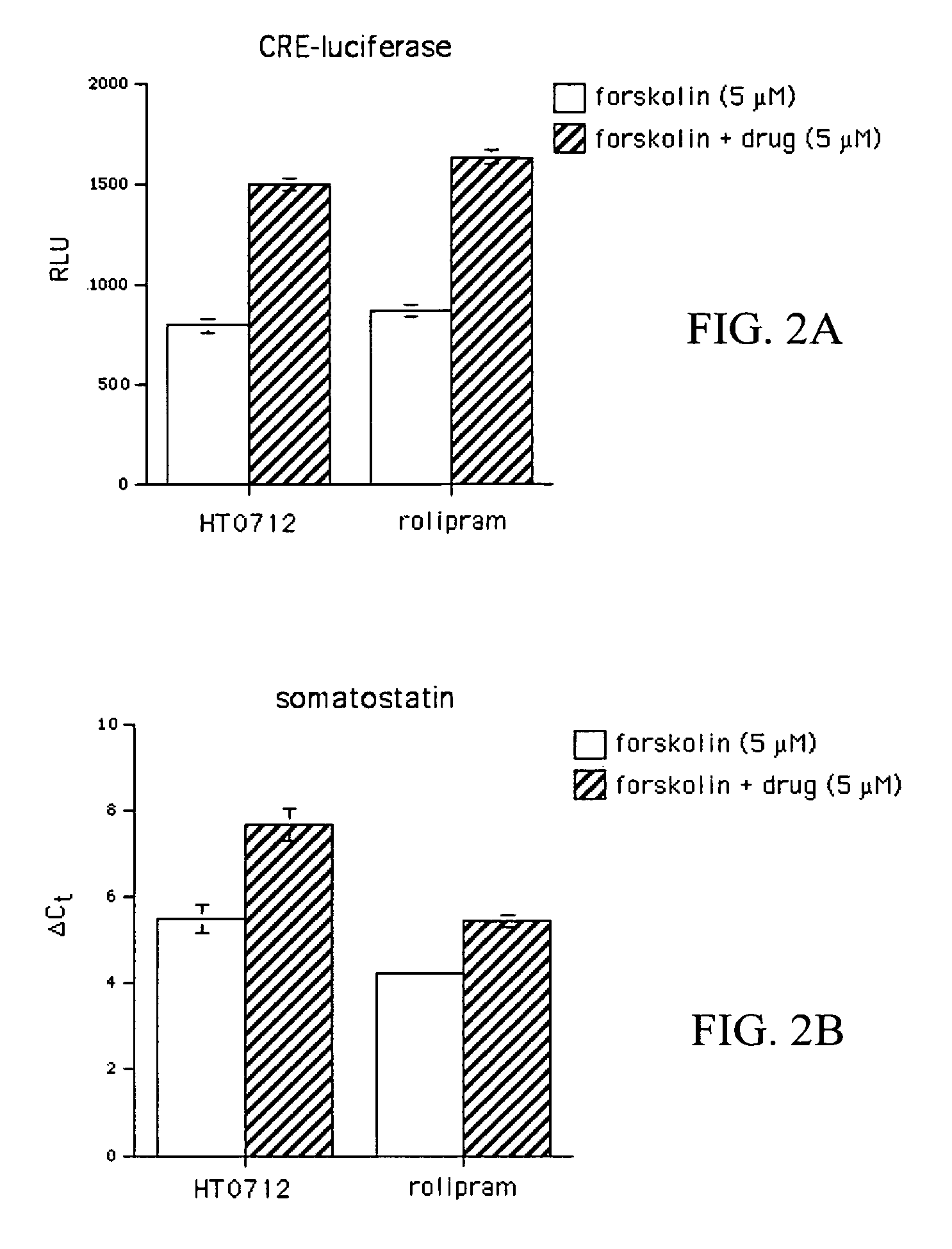
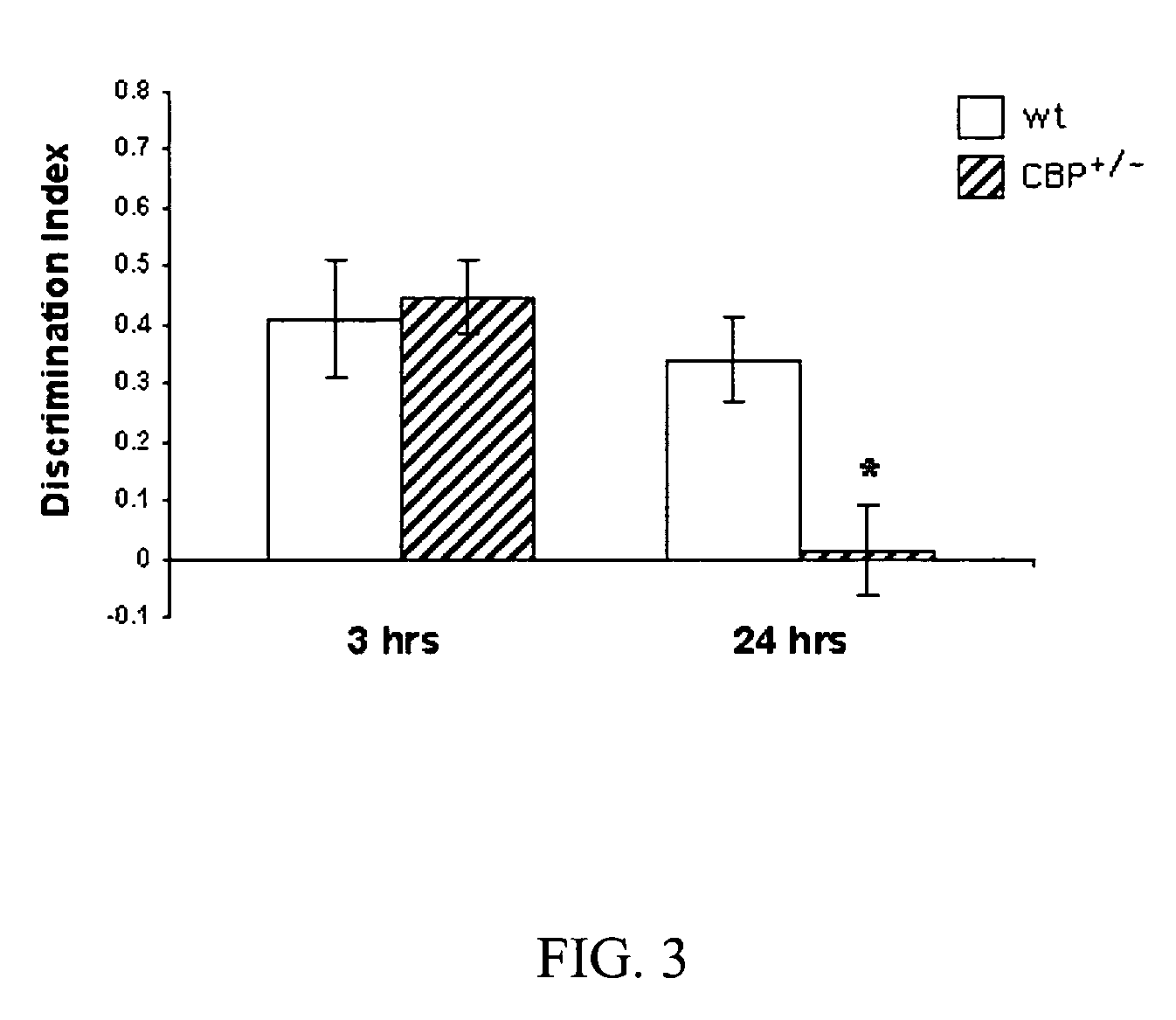
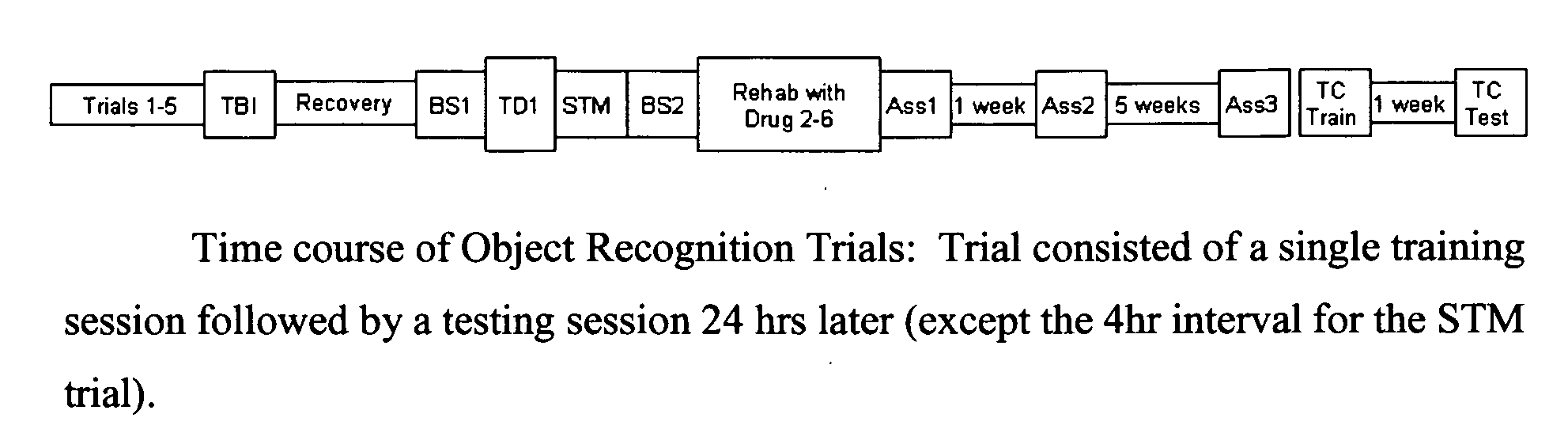
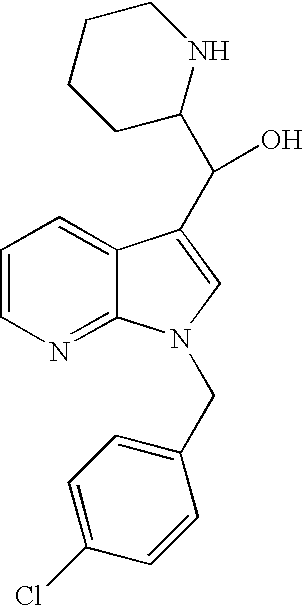
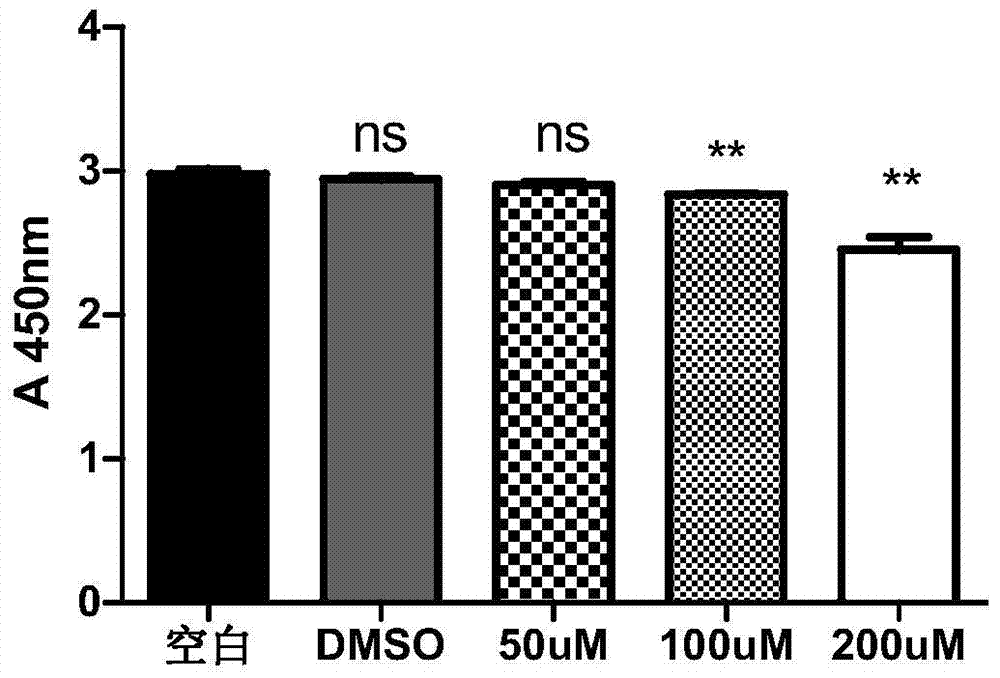
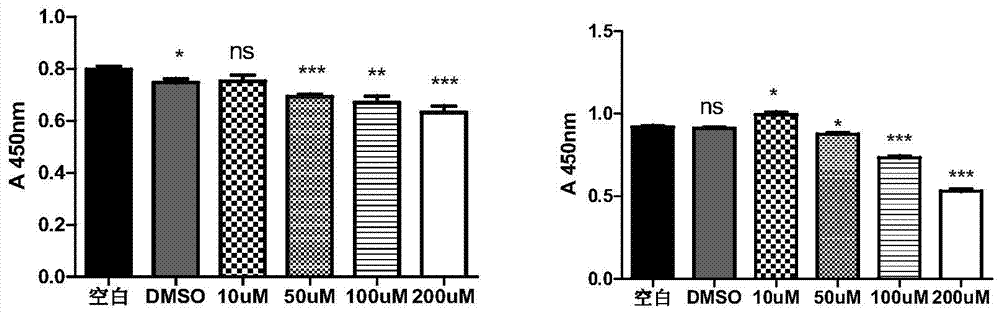
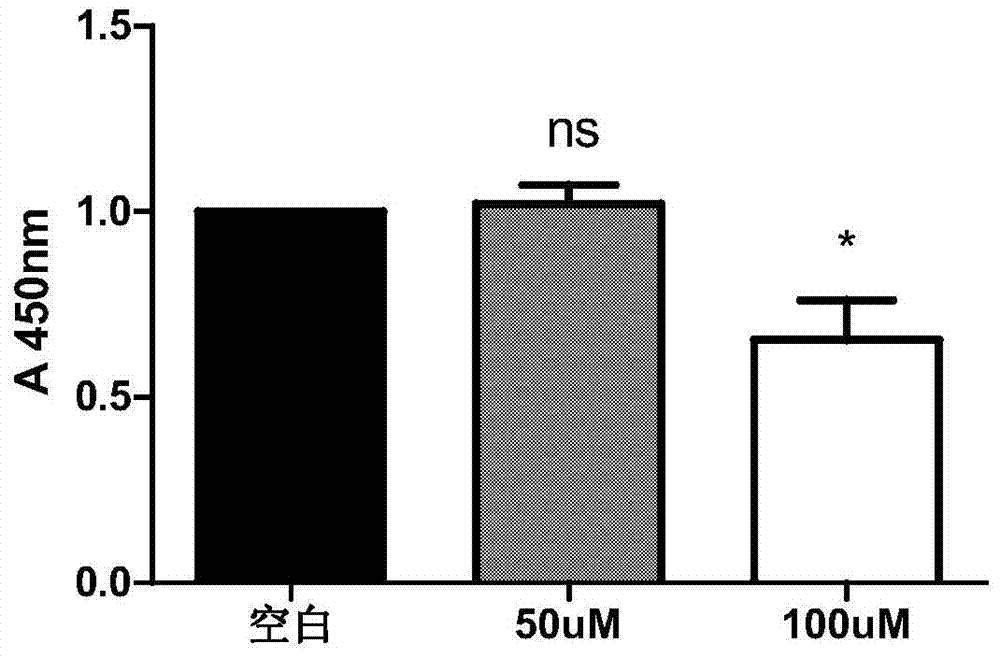
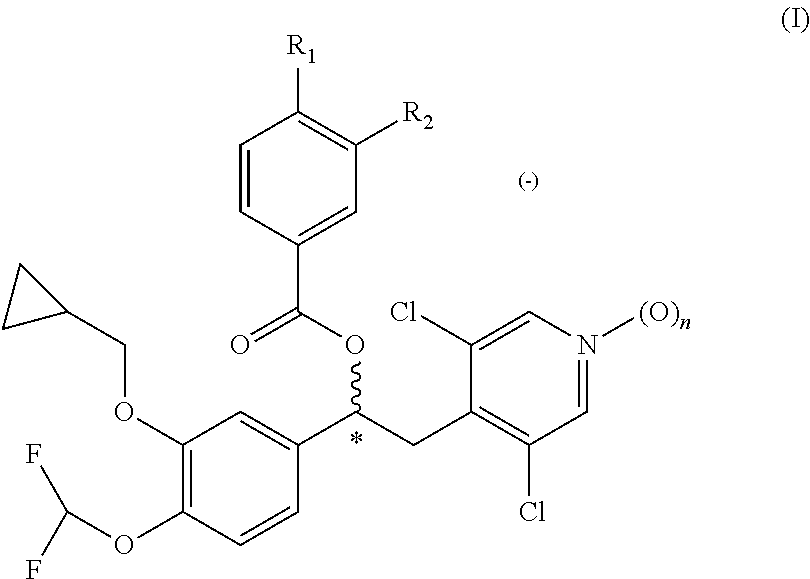
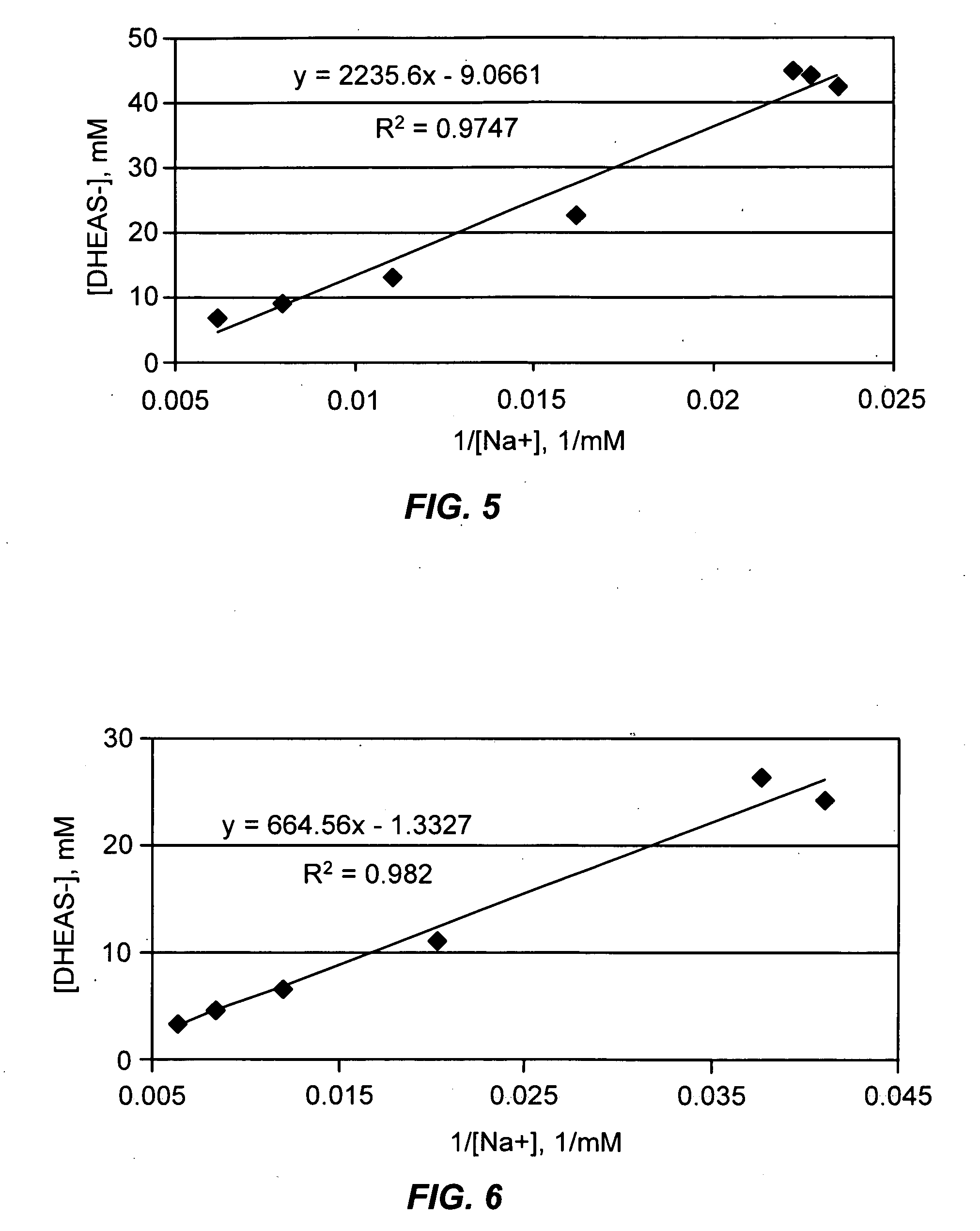
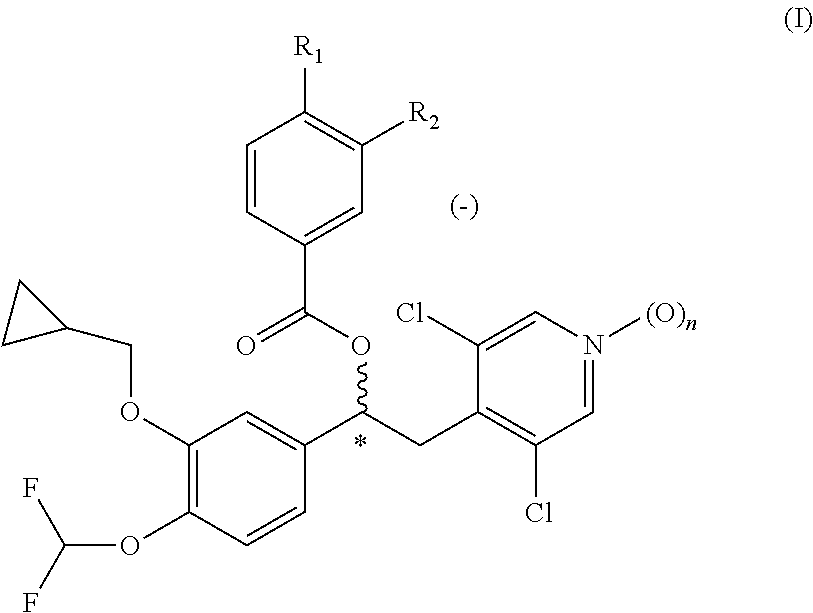
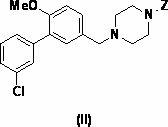
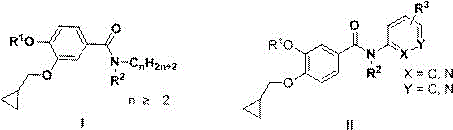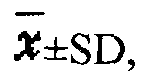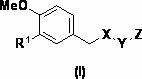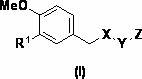Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
64 results about "Phosphodiesterase 4 Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
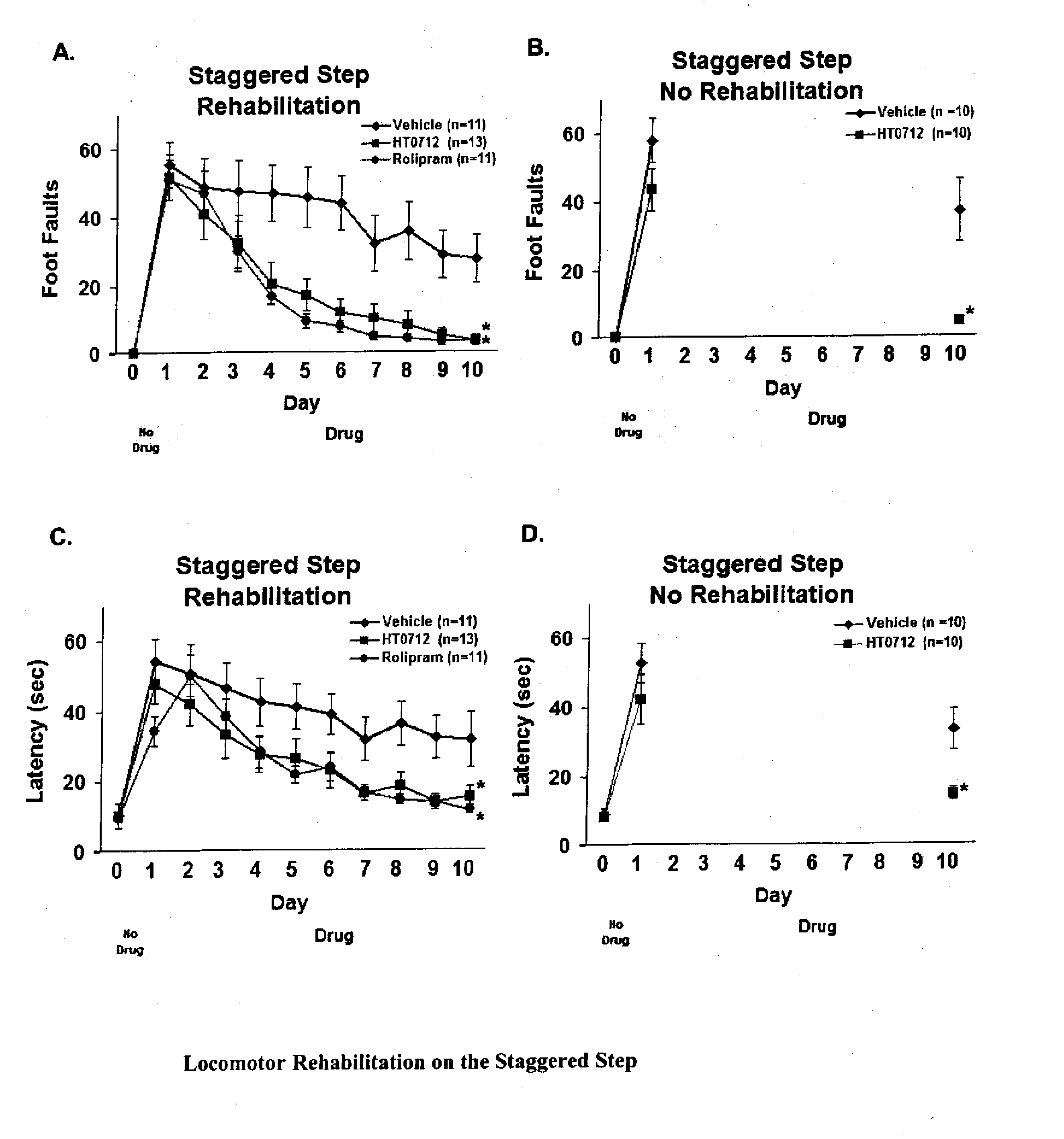
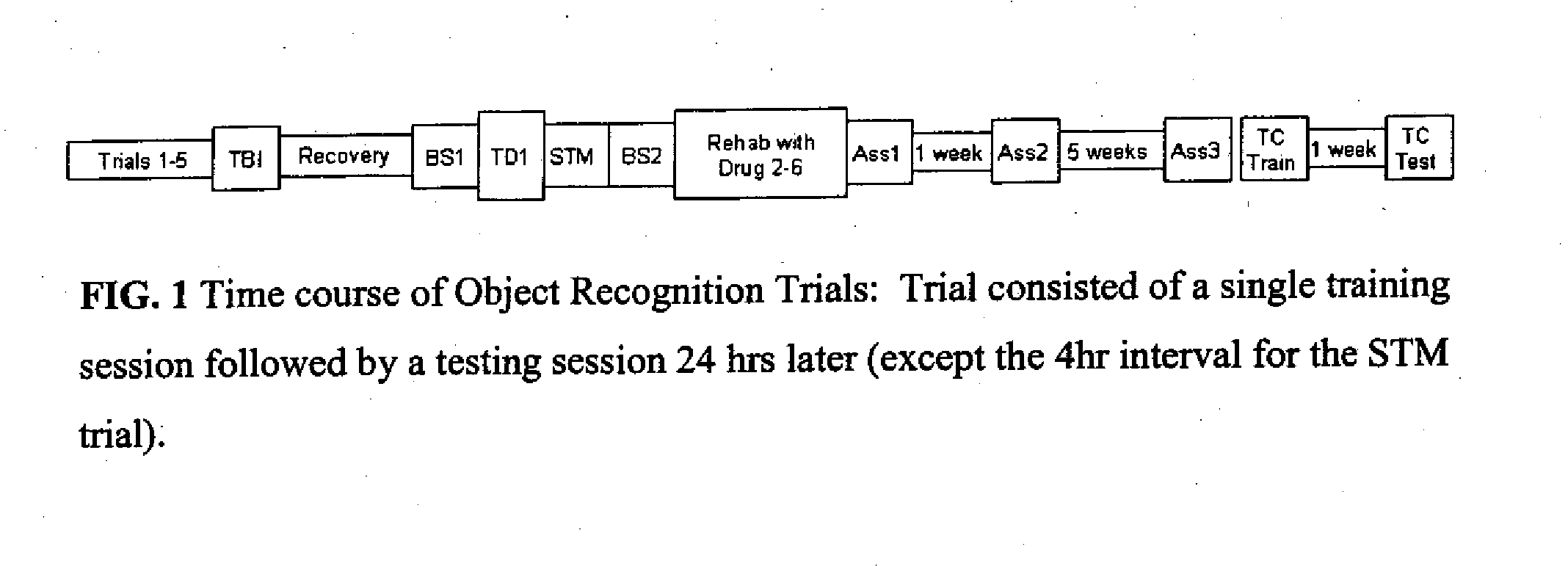
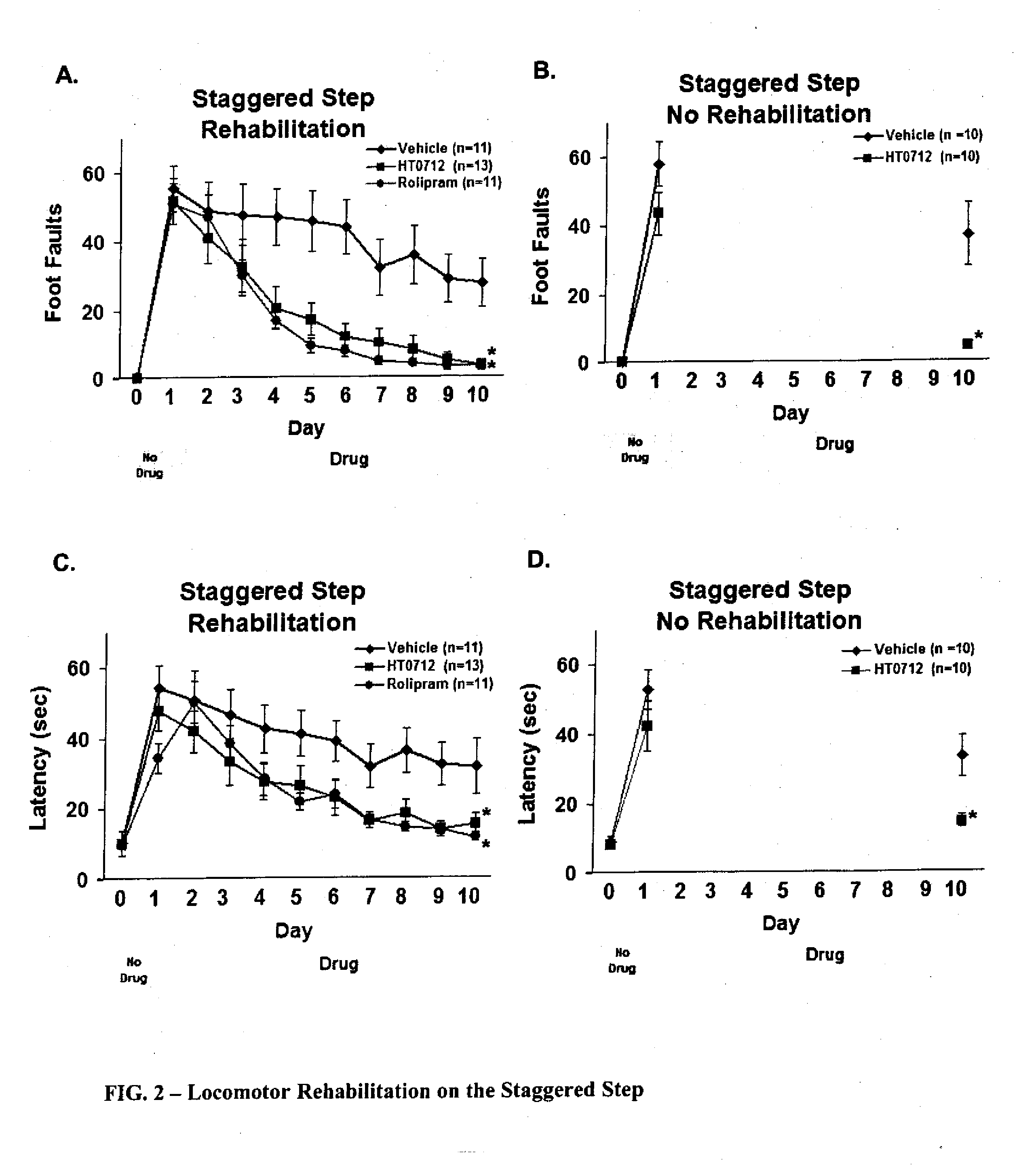
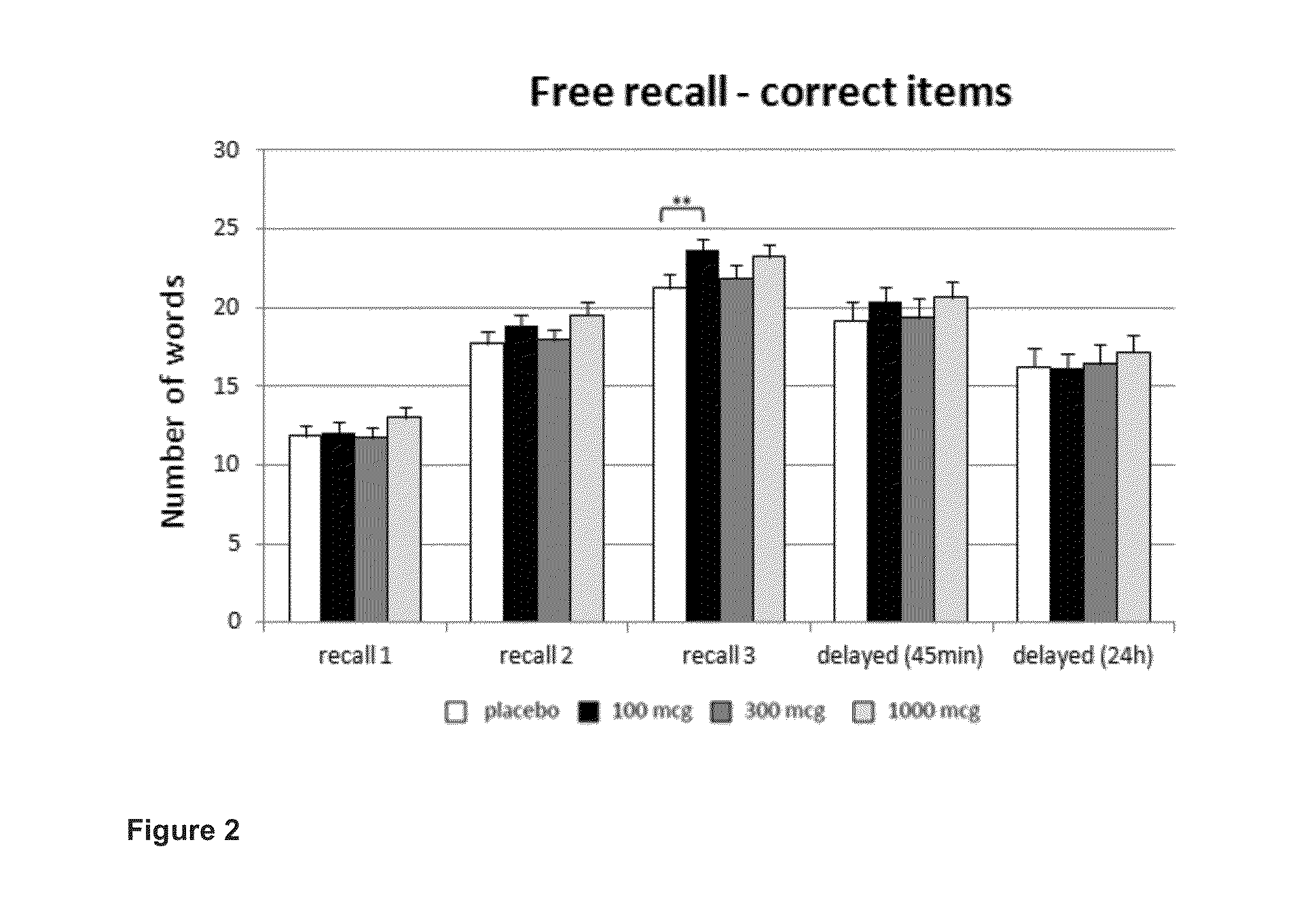
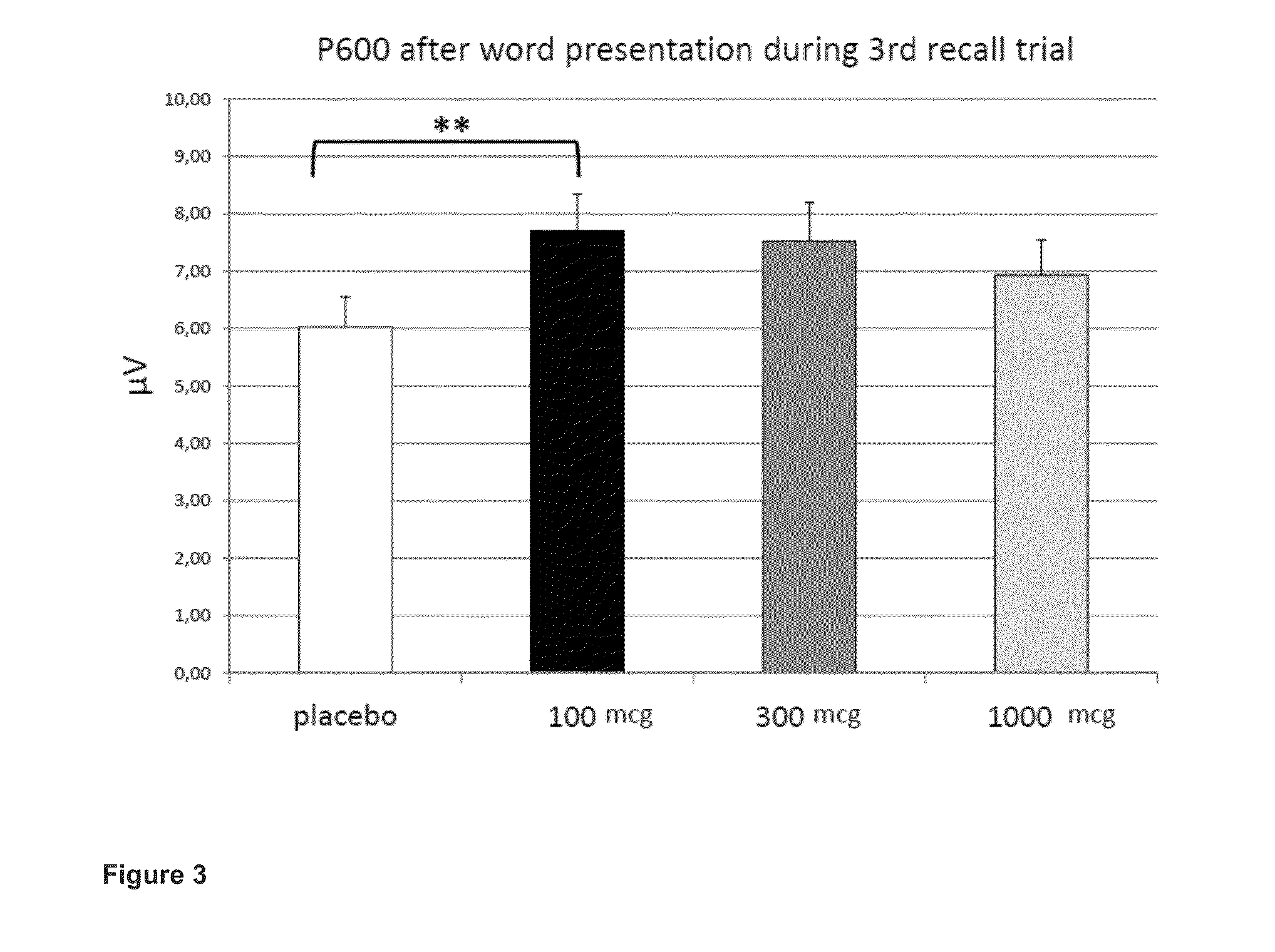
Phosphodiesesterase 4 inhibitors for the treatment of a cognitive deficit
InactiveUS7868015B2Normally performanceVarious formsPhysical therapies and activitiesBiocidePhosphodiesterase-4Psychiatry
The present invention provides methods of treating cognitive deficits associated with mental retardation. The methods comprise combining cognitive training protocols and a general administration of phosphodiesterase 4 inhibitors.
Owner:COLD SPRING HARBOR LAB INC
Phosphodiesterase 4 inhibitors for cognitive and motor rehabilitation
InactiveUS20080188525A1Function increaseImprove performanceBiocideNervous disorderMotor rehabilitationMedicine
The present invention provides methods of improving cognitive and motor deficits associated with central nervous system (CNS) disorder or condition in an animal. The methods comprise a general administration of phosphodiesterase 4 inhibitors and optionally training the animal under conditions sufficient to produce an improvement in performance.
Owner:DART NEUROSCIENCE CAYMAN LTD
Nitrogenous heterocyclic derivatives and medicine thereof
InactiveUS6352989B1High activityInhibitor of production of TNF.alphaAntibacterial agentsBiocidePhosphodiesteraseNitrogenous heterocyclic compound
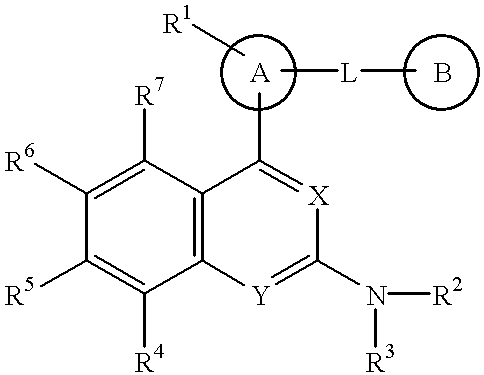
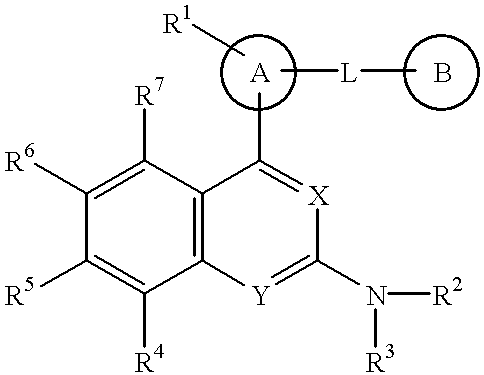
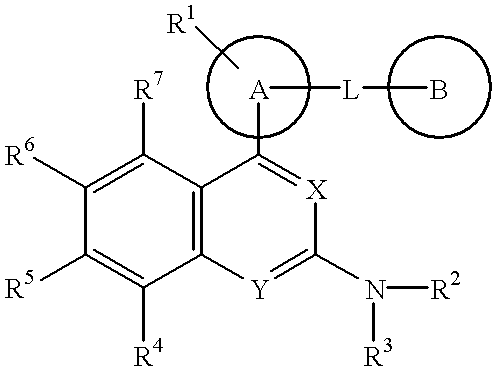
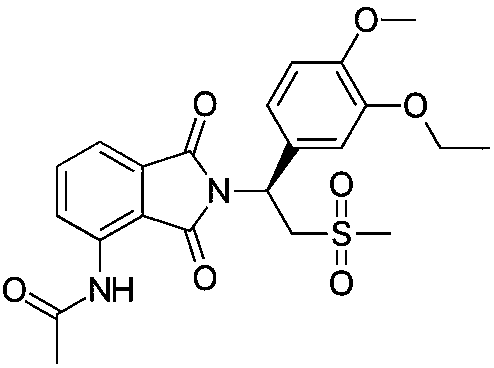
The present invention provides a novel nitrogen-containing heterocyclic compound useful as a phosphodiesterase-4 inhibitor, and a medicament comprising the same. Further, the present invention provides a nitrogen-containing heterocyclic compound represented by the following formula, its salt or hydrates thereof, and a medicament comprising the same.wherein the ring A is an aromatic hydrocarbon ring which may have a heteroatom, the ring B represents (a) a saturated hydrocarbon ring, (b) an unsaturated hydrocarbon ring, (c) a saturated heterocyclic ring or (d) an unsaturated heterocyclic ring, all of which may have a substituent group.
Owner:EISIA R&D MANAGEMENT CO LTD
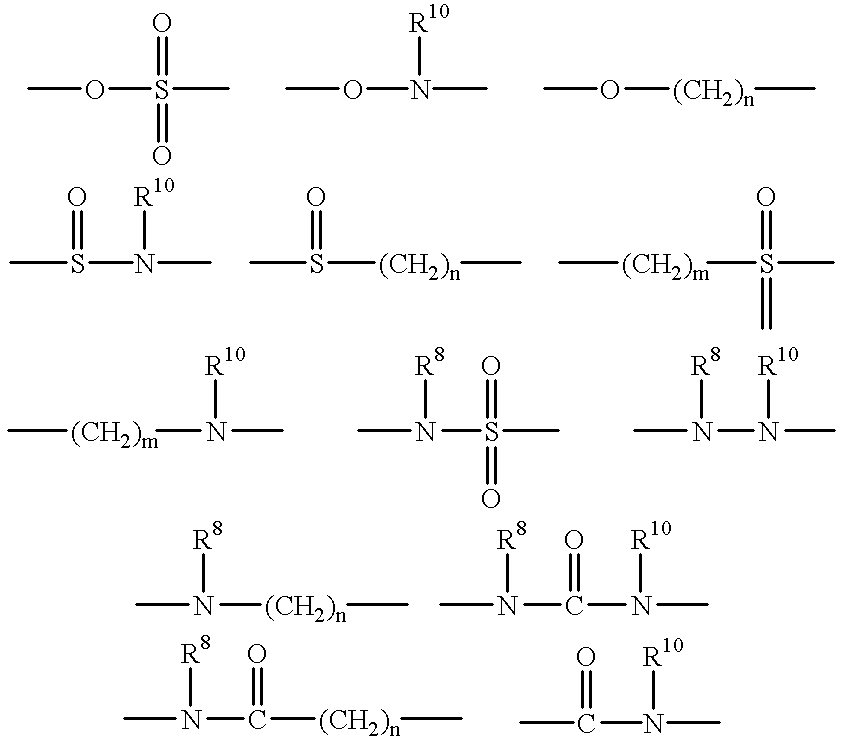
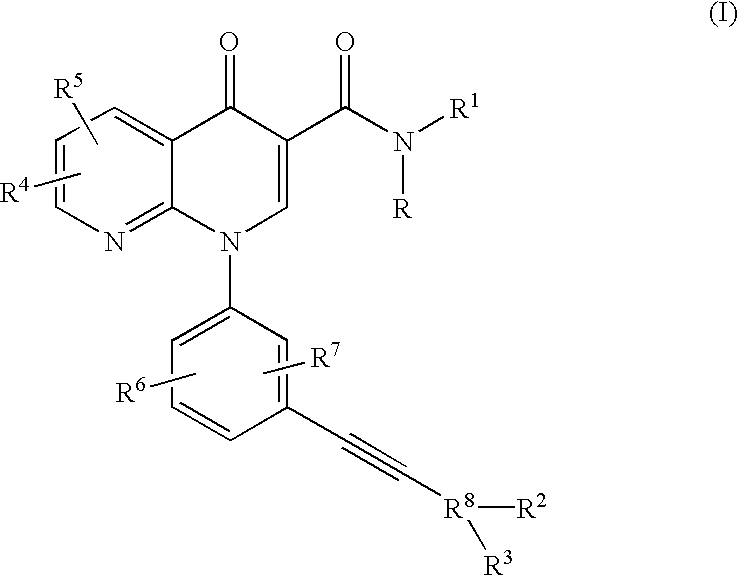
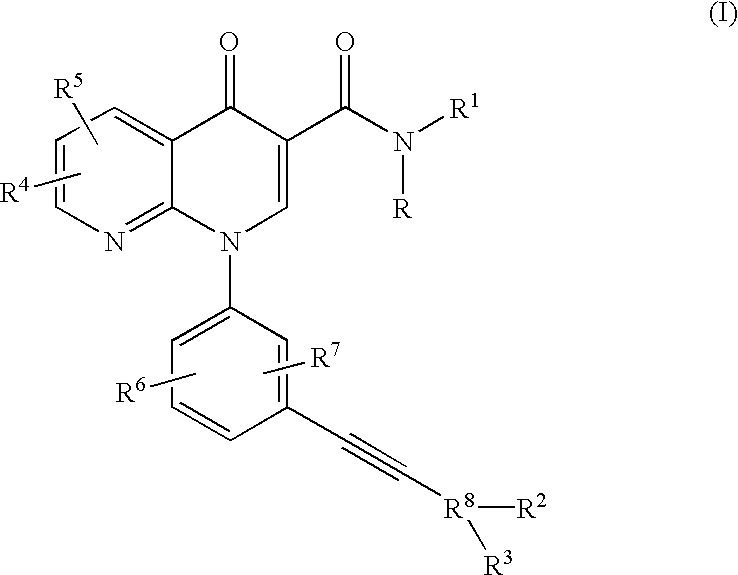
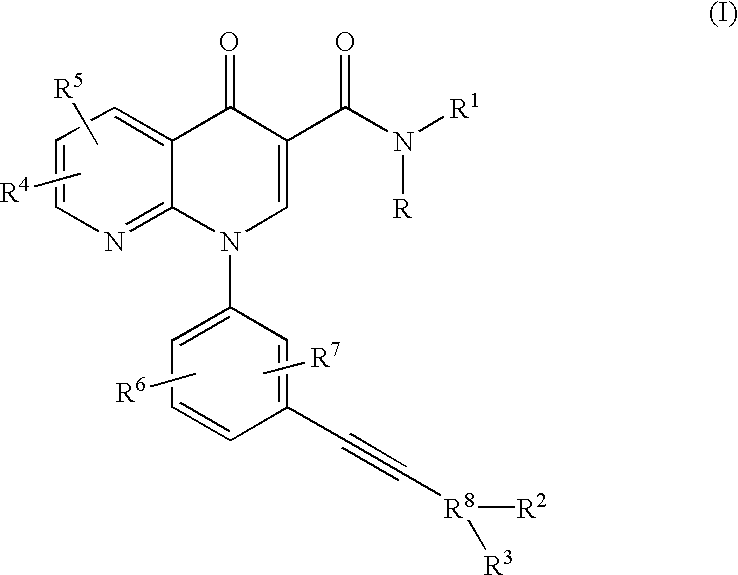
Alkyne-aryl phosphodiesterase-4 inhibitors
Compounds represented by Formula (I):or a pharmaceutically acceptable salt thereof, are phosphodiesterase 4 inhibitors useful in the treatment of asthma and inflammation.
Owner:MERCK CANADA
Phosphodiesterase 4 inhibitors for cognitive and motor rehabilitation
InactiveUS20080051437A1Function increaseImprove performanceCompounds screening/testingBiocideMotor rehabilitationMedicine
The present invention provides methods of improving cognitive and motor deficits associated with central nervous system (CNS) disorder or condition in an animal. The methods comprise a general administration of phosphodiesterase 4 inhibitors and optionally training the animal under conditions sufficient to produce an improvement in performance.
Owner:DART NEUROSCIENCE CAYMAN LTD
Nitrogen-containing heterocyclic compounds and medicaments containing the compounds
InactiveUS20020055516A1Easy to convertEasy to getAntibacterial agentsBiocideNitrogenous heterocyclic compoundNitrogen
The present invention provides a novel nitrogen-containing heterocyclic compound useful as a phosphodiesterase-4 inhibitor, and a medicament comprising the same. Further, the present invention provides a nitrogen-containing heterocyclic compound represented by the following formula, its salt or hydrates thereof, and a medicament comprising the same.
Owner:EISIA R&D MANAGEMENT CO LTD
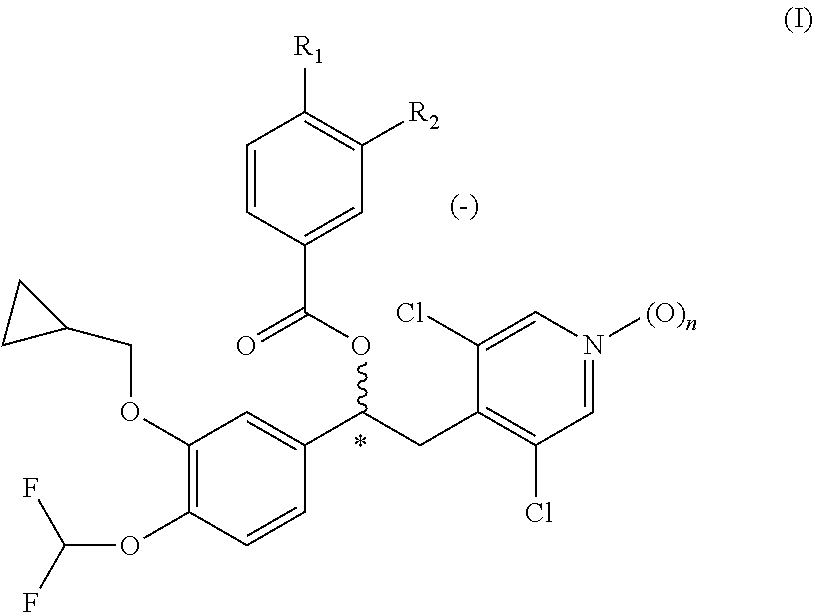
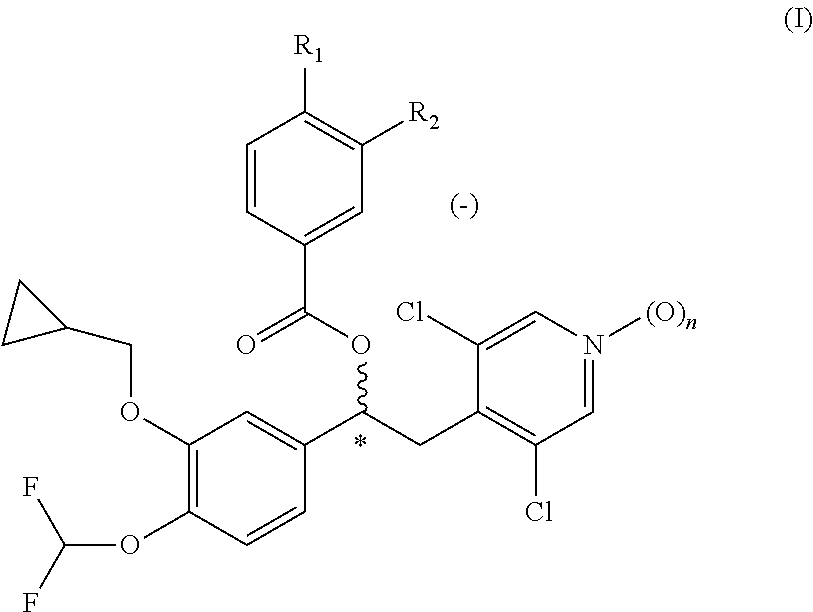
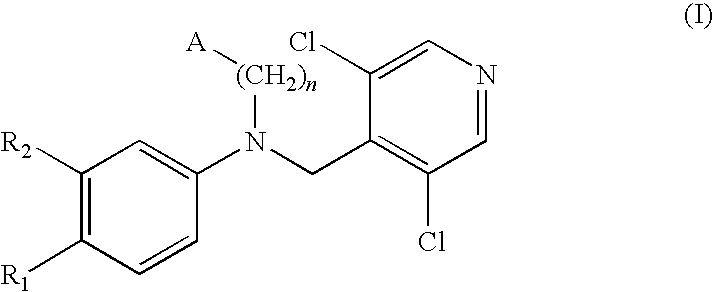
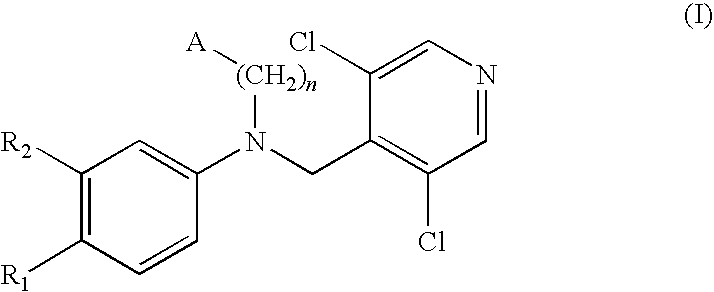
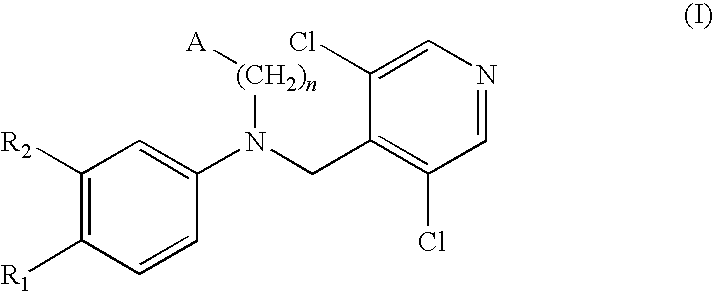
Phosphodiesterase-4 inhibitors belonging to the tertiary amine class
Compounds of formula (I):wherein n, A, R1, and R2 are defined in the specification, are useful as inhibitors of the phosphodiesterase 4 (PDE4) enzyme and for treating certain conditions.
Owner:CHIESI FARM SPA
Therapies for treating respiratory diseases
This invention relates to treating respiratory diseases by administering a phosphodiesterase 4 inhibitor in combination with a β agonist and an anti-inflammatory steroid.
Owner:GLAXO GROUP LTD
Dry powder formulation comprising a phosphodiesterase inhibitor
ActiveUS20120031403A1Improve liquidityGood chemical stabilityRespiratorsBiocideCOPDPhosphodiesterase inhibitor
Pharmaceutical formulations in the form of inhalable dry powder comprising particles of a phosphodiesterase-4 inhibitor as active ingredient are useful for the prevention and / or treatment of respiratory diseases, such as asthma and COPD.
Owner:CHIESI FARM SPA
Novel 7-azaindoles, use thereof as phosphodiesterase 4 inhibitors and method for producing the same
The invention relates to new 7-azaindoles, their use as inhibitors of phosphodiesterase 4 and to methods for their synthesis.
Owner:BIOTIE THERAPIES GMBH
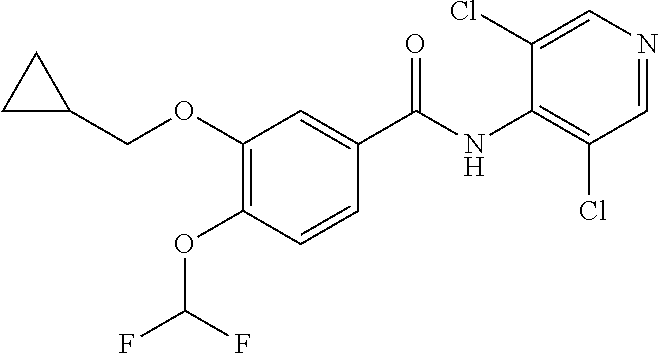
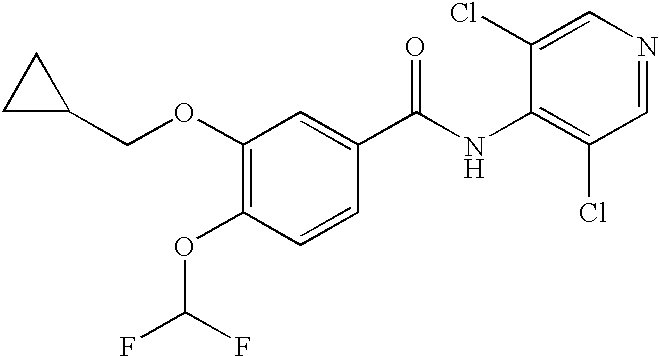
Novel 3-cyclopropylmethoxy-4-alkoxybenzamide PDE (phosphodiesterase) 4 inhibitors
ActiveCN105523954AHigh in vitro enzyme inhibitory activityGood anti-inflammatory effectNervous disorderOrganic chemistryPhosphodiesteraseEnzyme inhibition
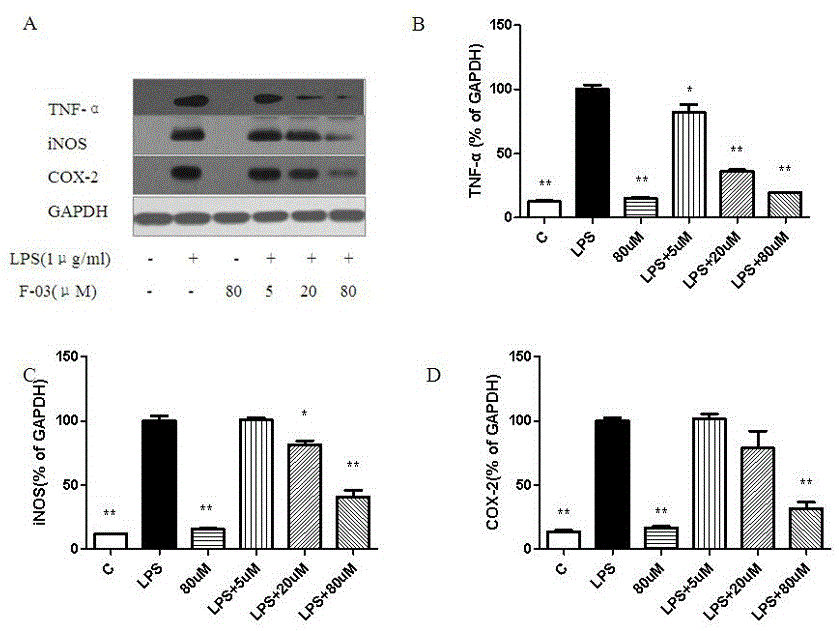
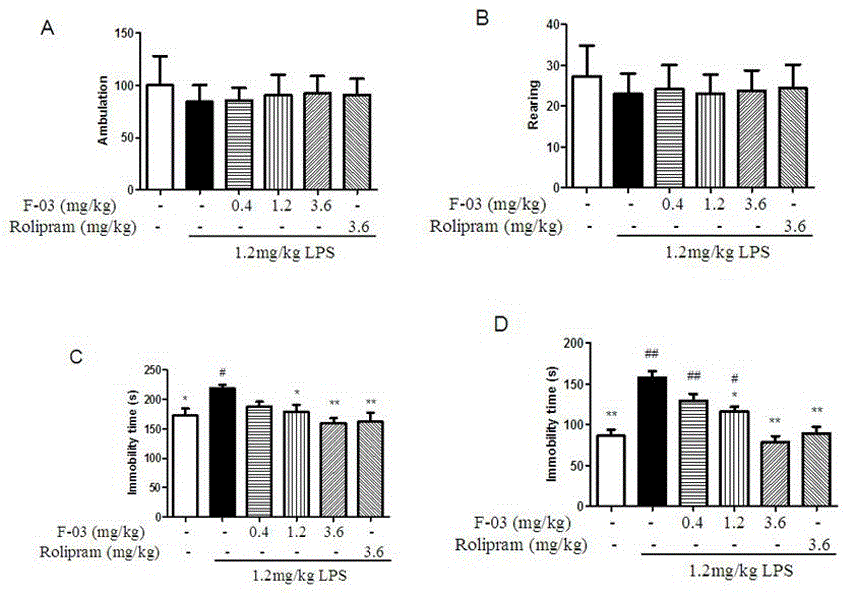
The invention discloses novel 3-cyclopropylmethoxy-4-alkoxybenzamide PDE (phosphodiesterase) 4 inhibitors. The inhibitors are compounds represented in a general formula I or a general formula II, prodrugs thereof, pharmaceutically usable salts thereof or solvates thereof, wherein R<1> is independent methyl (Me-) or difluoromethyl (CF2H-); R<2> is independent H, Me or Et; R<3> is independent H, MeO or Cl; CnH(2n+2) is alkyl, and N is larger than or equal to 2; a substance shown in the specification is a substituted benzene ring or pyridine ring; X is C or N; Y is C or N. The novel 3-cyclopropylmethoxy-4-alkoxybenzamide PDE 4 inhibitors have the advantages of high in-vitro enzyme inhibition activity, capability of selective application to PDE4 and good anti-inflammation effect and anti-depression effect.
Owner:兰晟生物医药(苏州)有限公司
Augmented cognitive training
InactiveUS20060247252A1Improve performanceNormally performanceBiocideNervous disorderClinical psychologyPhosphodiesterase-4
The present invention provides methods of treating cognitive deficits associated with mental retardation. The methods comprise combining cognitive training protocols and a general administration of phosphodiesterase 4 inhibitors.
Owner:COLD SPRING HARBOR LAB INC
Applications of phosphodiesterase 4 inhibitor ZL-n-91 in preparation of medicines preventing lung cancer proliferation and metastasis
ActiveCN107041880AStrong targetingLittle side effectsOrganic active ingredientsPharmaceutical delivery mechanismLymphatic SpreadOncology
The invention discloses applications of a novel phosphodiesterase 4 (PDE4) inhibitor ZL-n-91 in preparation of medicines preventing lung cancer proliferation and metastasis. A survivorship curve and in-vitro cell experiments of mice prove that the phosphodiesterase 4 inhibitor ZL-n-91 can significantly inhibit lung cancer cell proliferation and metastasis, indicating that the phosphodiesterase 4 inhibitor ZL-n-91 is expected to be an important target in lung cancer proliferation and metastasis preventing researches, and providing bases for preparing medicines preventing lung cancer proliferation and metastasis. The phosphodiesterase 4 inhibitor ZL-n-91 has a good application prospect.
Owner:GUANGZHOU HUAZHEN PHARM CO LTD
Dry powder formulation comprising a phosphodiesterase inhibitor
ActiveUS20150342936A1Improve liquidityImprove stabilityPowder deliveryBiocideCOPDPhosphodiesterase inhibitor
Pharmaceutical formulations in the form of inhalable dry powder comprising particles of a phosphodiesterase-4 inhibitor as active ingredient are useful for the prevention and / or treatment of respiratory diseases, such as asthma and COPD.
Owner:CHIESI FARM SPA
Topical pharmaceutical composition of phosphodiesterase-4 inhibitor and preparation method thereof
InactiveCN108283620AEffective treatmentAvoid or reduce side effectsOrganic active ingredientsAerosol deliverySide effectAdditive ingredient
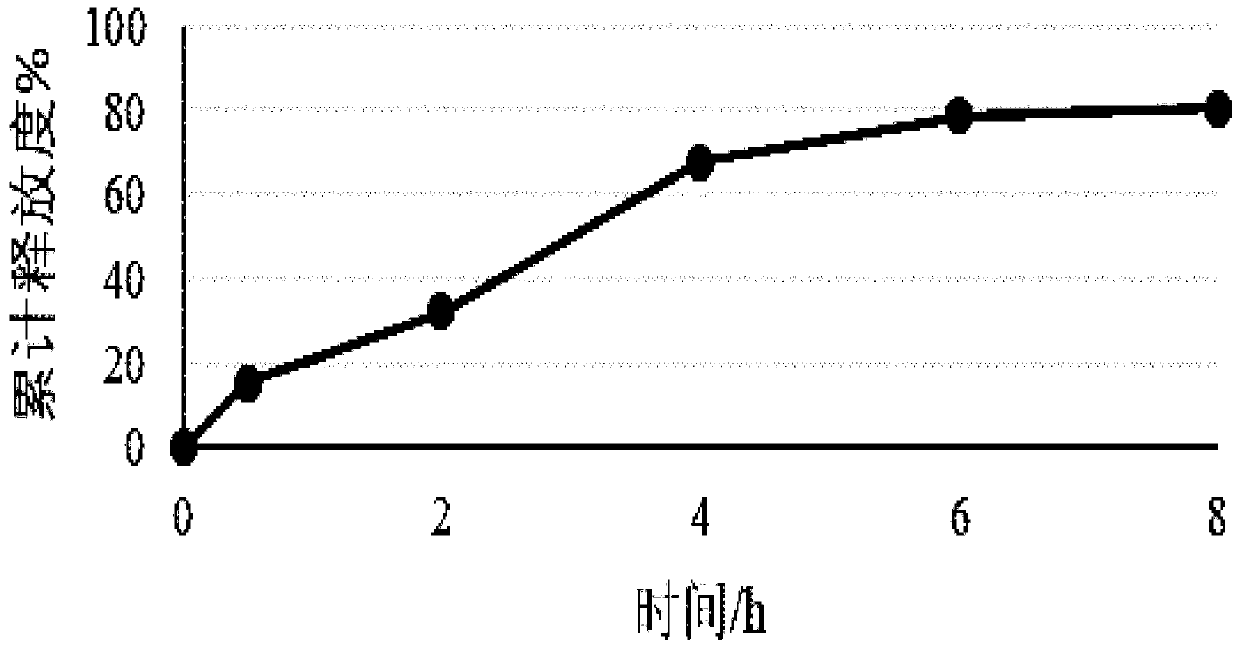
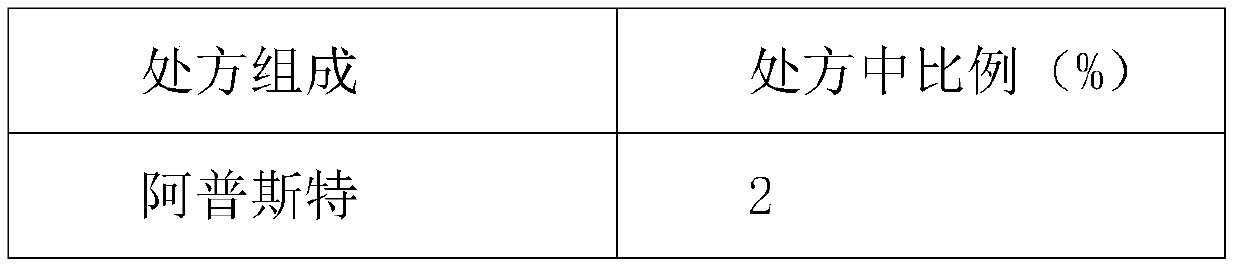
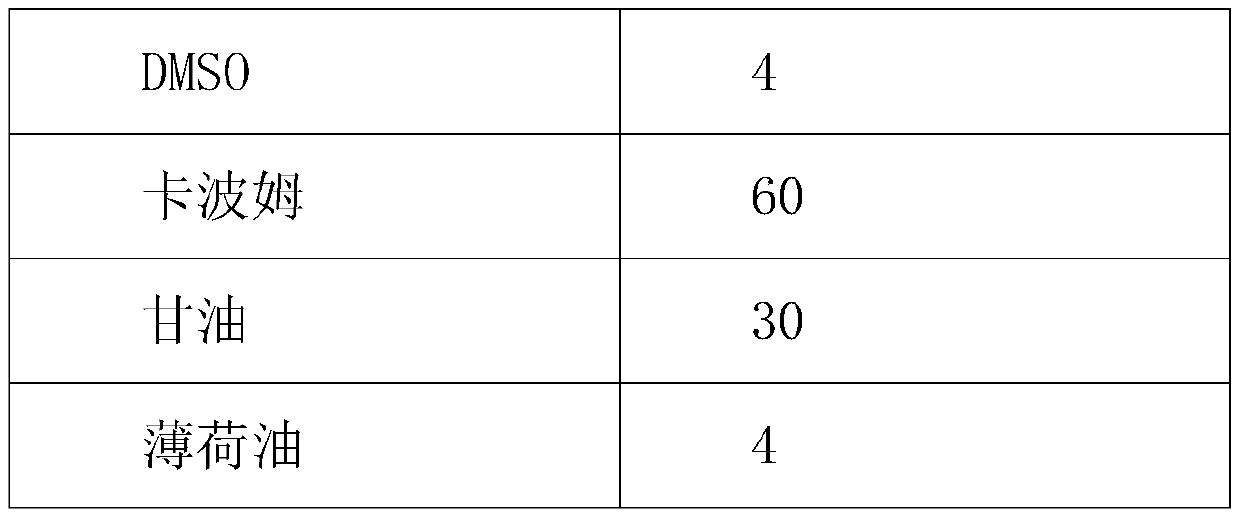
The invention discloses a topical pharmaceutical composition of a phosphodiesterase-4 inhibitor and a preparation method thereof, wherein the topical pharmaceutical composition comprises 0.5 to 10% bymass of apremilast active ingredient, 0.2 to 25% by mass of dimethyl sulfoxide and 65 to 99.3% by mass of a topical pharmaceutical acceptable carrier. The dosage forms of the topical pharmaceutical composition are ointments, creams, gels, coatings, lotions, tinctures, powders and liniments. The invention also discloses the preparation method of the topical pharmaceutical composition and use of the topical pharmaceutical composition. The pharmaceutical composition is not like hormonal drugs which have the side effects to cause adermotrophia after long time of use, and the pharmaceutical composition has no disadvantages such as recurrence after curing, has desired pharmacological activity and less side effects, is stable in quality and convenient to use, is safe and effective in treatment of eczema, and has strong practical application value.
Owner:ZHAOKE PHARMA GUANGZHOU
Phosphodiesterase 4 inhibitor capable of avoiding vomiting and preparation method thereof
InactiveCN102716110APromote learning and memoryImprove cognitive abilityOrganic active ingredientsNervous disorderDiseasePhosphodiesterase I
The present invention relates to a phosphodiesterase 4 inhibitor capable of avoiding vomiting. The phosphodiesterase 4 inhibitor is a compound, a prodrug or a solvate represented by DTPM; and the DTPM is named as 1-(4-difluoro methoxy-3-(tetrahydrofuran-3-oxyl) phenyl)-3-dimethyl-1-ketone, and has a chemical formula shown as below. A plurality of experiments have proved that the DTPM has better effect on improvement of learning and memory compared with an existing antidepressant PDE4 inhibitor; in a Beagle dog vomiting effect observation experiment, no obvious vomiting inducing reaction is observed; and the DTPM has an inhibitor intensity 5000 times of that of other PDE4D families. The inhibitor provided by the invention can become a drug for treating depression and Alzheimer's disease and improving the cognitive ability, and capable of effectively reducing and even avoiding adverse reactions like vomiting.
Owner:徐江平
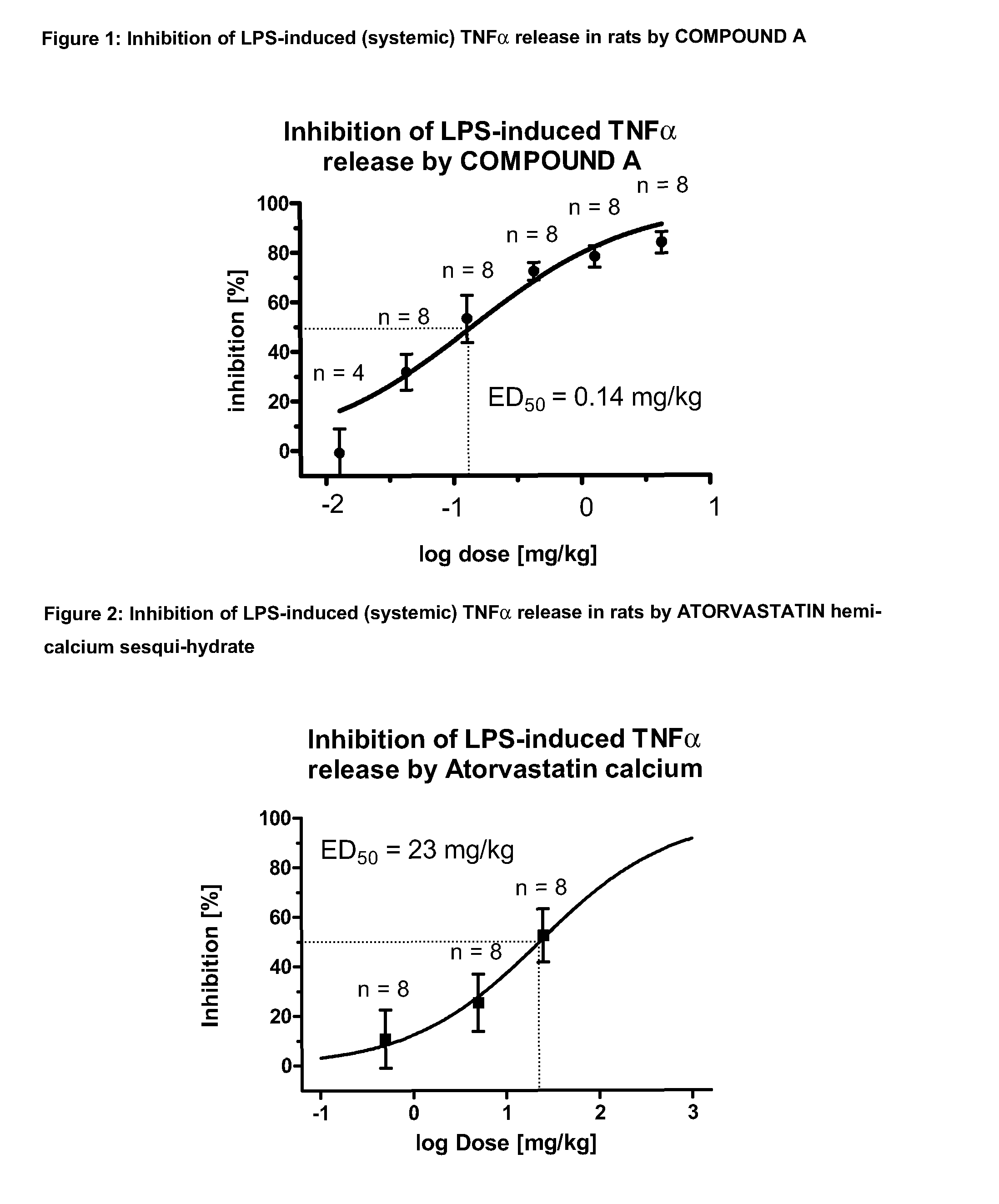
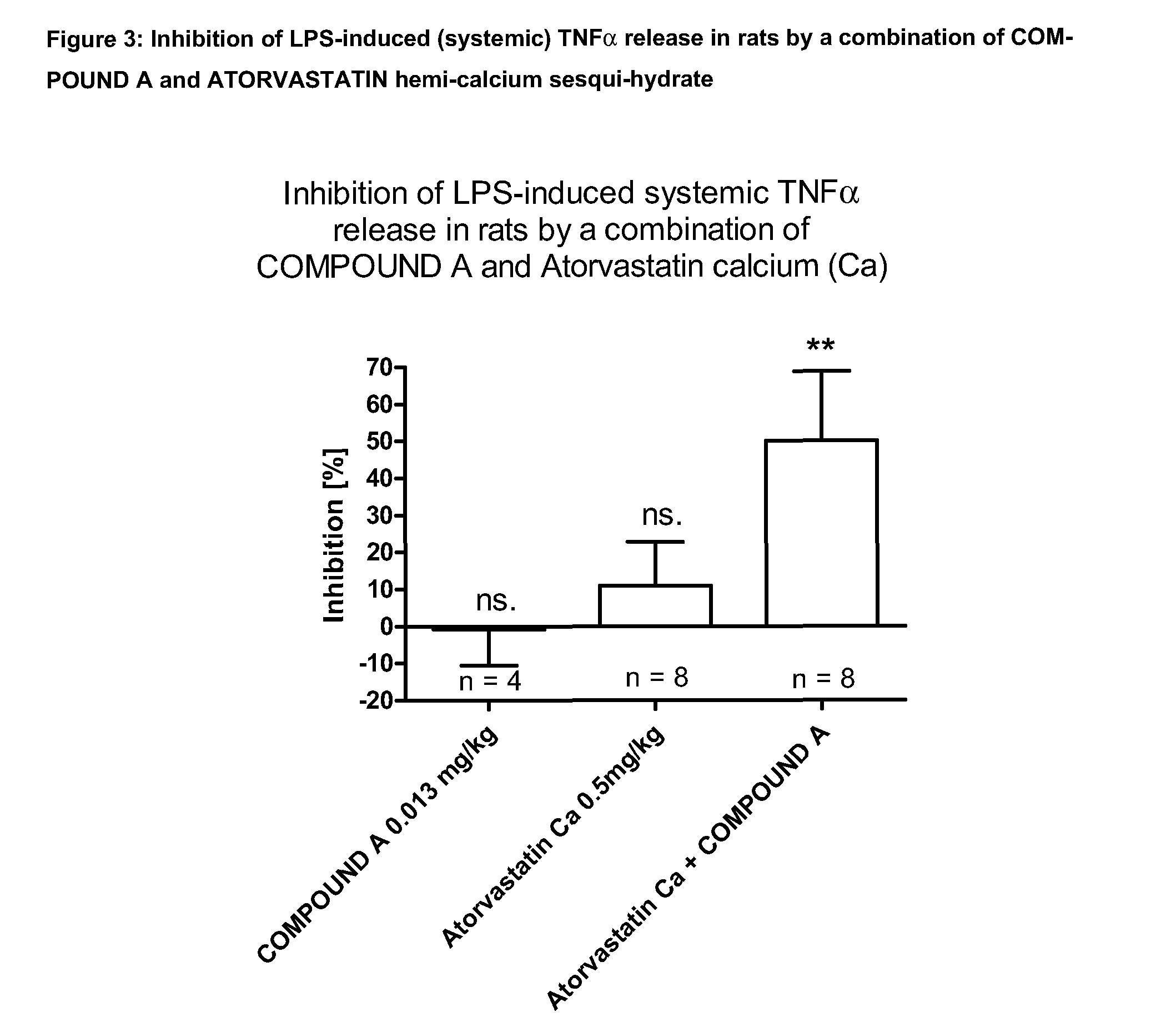
Combination of hmg-coa reductase inhibitors with phosphodiesterase 4 inhibitors for the treatment of inflammatory pulmonary diseases
InactiveUS20150272949A1Potent (synergistic) and effective inhibitionBiocideAntipyreticCurative treatmentPDE4 Inhibitors
The invention relates to the combined use of a PDE4 inhibitor with a HMG-CoA reductase inhibitor for the prevention and curative treatment of an inflammatory pulmonary disease.
Owner:ASTRAZENECA AB
Combination of HMG-COA Reductase Inhibitors with Phosphodiesterase 4 Inhibitors for the Treatment of Inflammatory Pulmonary Disease
InactiveUS20100069392A1Inhibition is effectiveBiocidePeptide/protein ingredientsHMG-CoA reductasePhosphodiesterase I
The invention relates to the combined use of a PDE4 inhibitor with a HMG-CoA reductase inhibitor for the preventive and curative treatment of an inflammatory pulmonary disease.
Owner:TAKEDA GMBH
Phosphodiesterase-4 inhibitor pharmaceutical composition for treatment of oral ulcer and preparation method thereof
ActiveCN110403935AQuality improvementEasy to useOrganic active ingredientsAerosol deliveryPhosphodiesteraseOral ulcers
The invention discloses a novel pharmaceutical composition for local drug administration with a phosphodiesterase-4 (PDE-4) inhibitor as an active component. The oral gel composition includes commonlyused additives: a gel matrix, a surfactant, a solubilizer, a pH regulator, a flavoring agent, a preservative, a solvent containing water or ethanol, and other ingredients. The composition acts directly on oral lesions, and effectively avoids the gastrointestinal adverse reactions caused by the composition. The preparation method of the local pharmaceutical composition is feasible in commercial production, and the quality of the prepared local pharmaceutical composition is stable, reliable, safe, effective, and convenient to use.
Owner:ZHAOKE PHARMA GUANGZHOU
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a PDE-4 inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050085430A1Alleviate different aspectConvenient treatmentBiocidePowder deliveryDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a phosphodiesterase-4 inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Medicinal use of lithospermi naphthoquinone compounds
ActiveCN102198120AOrganic active ingredientsDigestive systemUlcerative colitisObstructive Pulmonary Diseases
The invention discloses the use of lithospermi naphthoquinone compounds in the preparation of phosphodiesterase 4 inhibitor. The invention also discloses the use of the lithospermi naphthoquinone compounds in the preparation of medicines for treating asthma and chronic obstructive pulmonary diseases, the use in the preparation of medicines for treating inflammatory bowel diseases, the use in the preparation of medicines for treating rheumatoid arthritis, particularly the use in the preparation of medicines for treating ulcerative colitis and Crohn disease. The invention also discloses the medicinal use of one or several of lithospermi extracts.
Owner:CHIATAI QINGCHUNBAO PHARMA
Dry powder formulation comprising a phosphodiesterase inhibitor
ActiveUS9132121B2Improve liquidityImprove stabilityRespiratorsBiocideCOPDPhosphodiesterase inhibitor
Pharmaceutical formulations in the form of inhalable dry powder comprising particles of a phosphodiesterase-4 inhibitor as active ingredient are useful for the prevention and / or treatment of respiratory diseases, such as asthma and COPD.
Owner:CHIESI FARM SPA
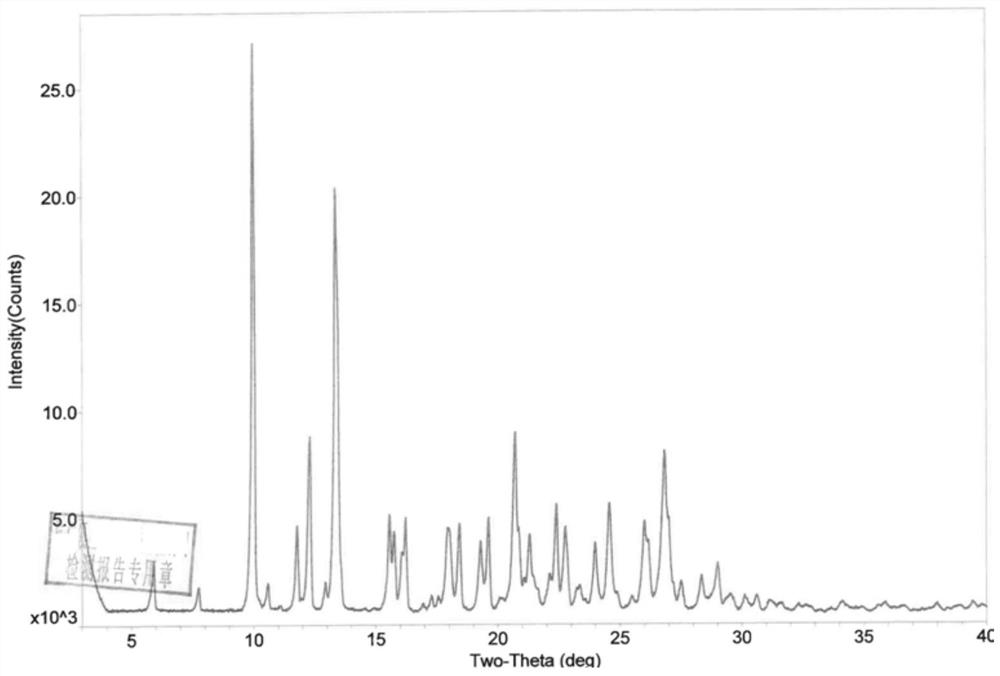
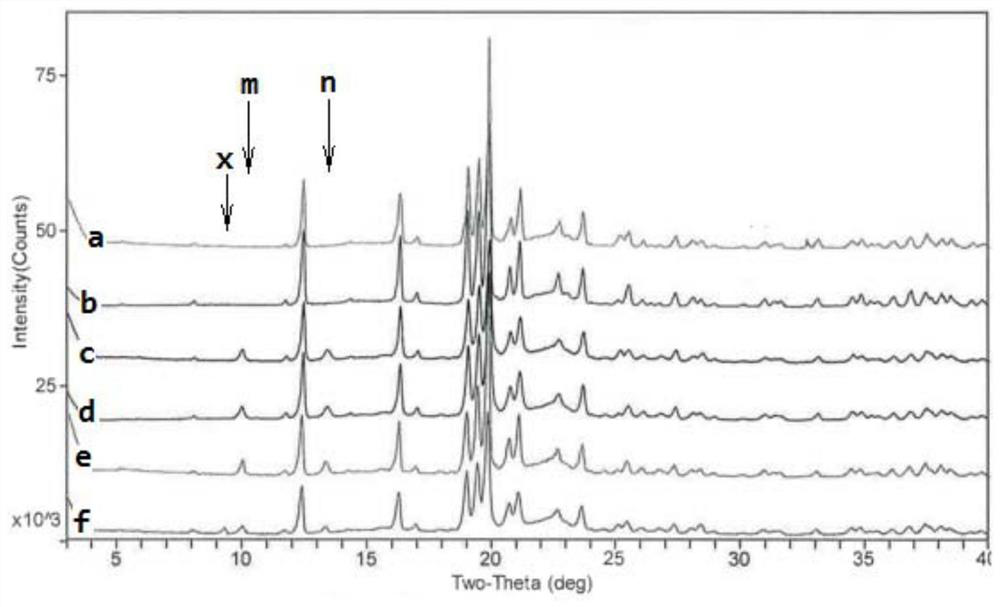
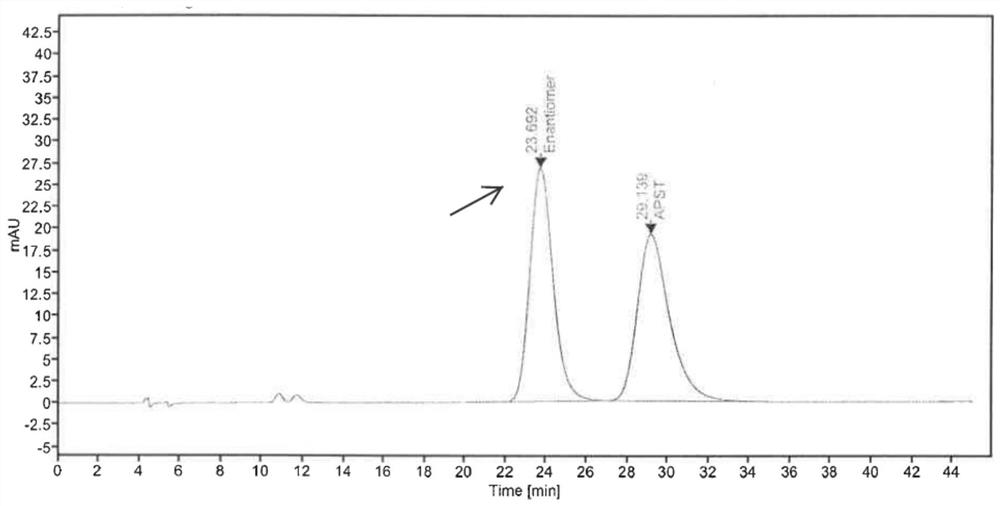
Phosphodiesterase-4 inhibitor apremilast composition and quality detection method
PendingCN112305107AGood choiceWide applicabilityOrganic active ingredientsComponent separationOral ulcersEnantiomer
The invention relates to a phosphodiesterase 4 inhibitor apremilast composition and a quality detection method. The apremilast composition is a tablet, comprises lactose, magnesium stearate and otherauxiliary materials, and is used for treating adult patients suffering from active psoriatic arthritis, adult patients suffering from moderate-to-severe plaque psoriasis and suitable for phototherapyor systemic therapy, and adult oral ulcer patients related to Bessel's disease. The quality detection method comprises the step of determining the content of the enantiomer in the tablet, and comprises the following operations: by using a chiral chromatographic column and using n-hexane, isopropanol and the like as mobile phases, in a high performance liquid chromatography system, injecting 5 [mu]l of a system applicable solution into a liquid chromatograph, wherein the peak appearance sequence is enantiomer peaks and apremilast peaks successively, and the separation degree between two peaks is not less than 1.5, and precisely measuring 5 [mu]l of the test solution, injecting the test solution into a liquid chromatograph, recording a chromatogram, and calculating the content of the enantiomer according to an area normalization method if enantiomer peaks exist in the chromatogram of the test solution. The compositions and methods of the present invention exhibit excellent technical effects as described in the specification.
Owner:HANGZHOU ZHUYANGXIN PHARMA
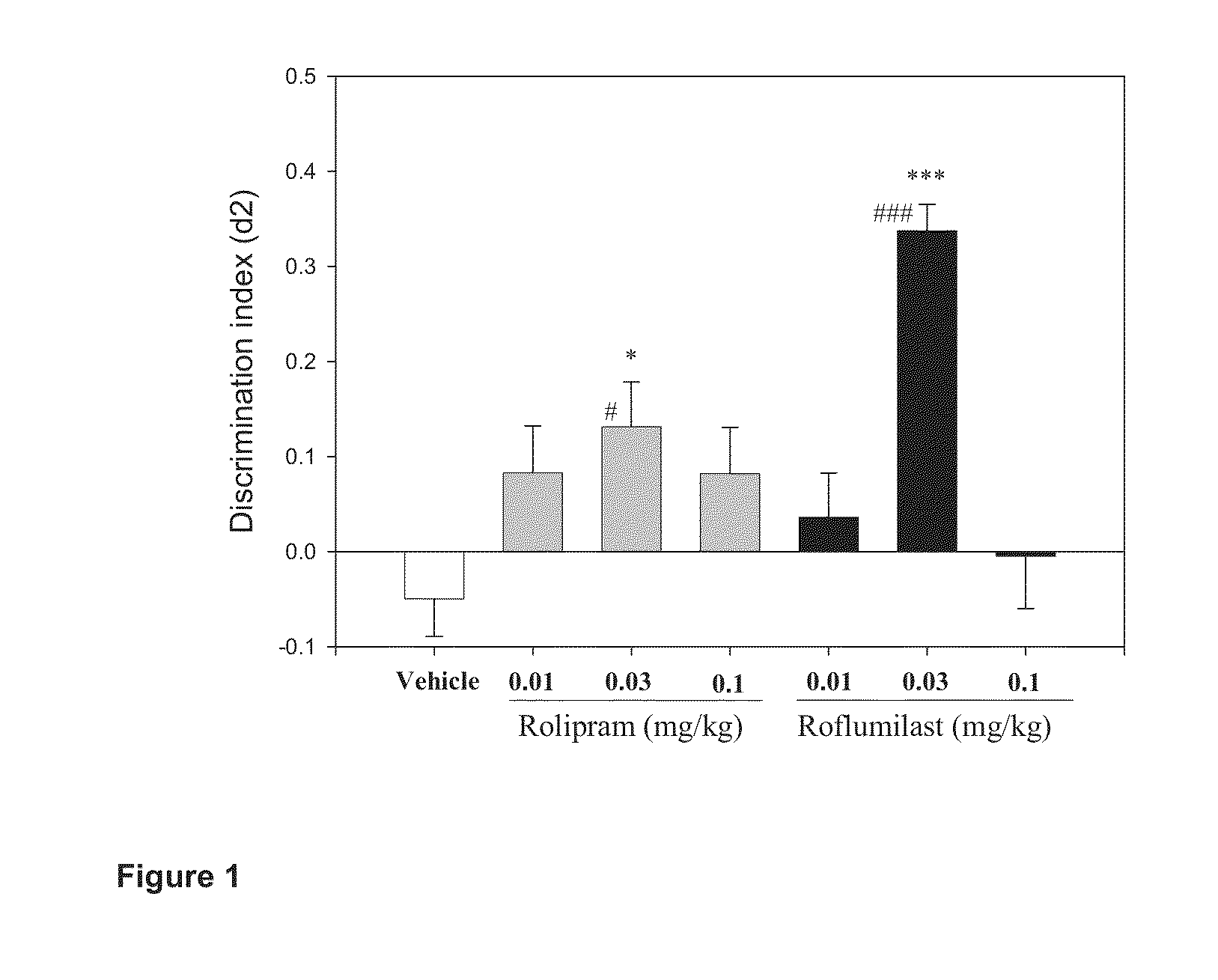
Treatment of Cognitive Impairment with Combination Therapy
Treatment of varying degrees of cognitive impairment associated with Alzheimer's disease with a combination of a phosphodiesterase 4 inhibitor and an acetylcholinestase inhibitor, including roflumilast and donepezil hydrochloride.
Owner:MAASTRICHT UNIVERSITY
Phosphodiesterase 4 inhibitor capable of avoiding vomiting reaction
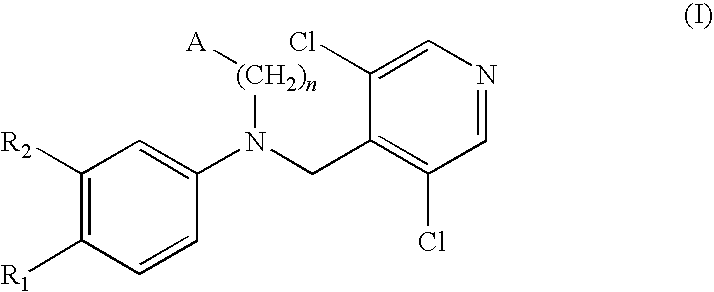
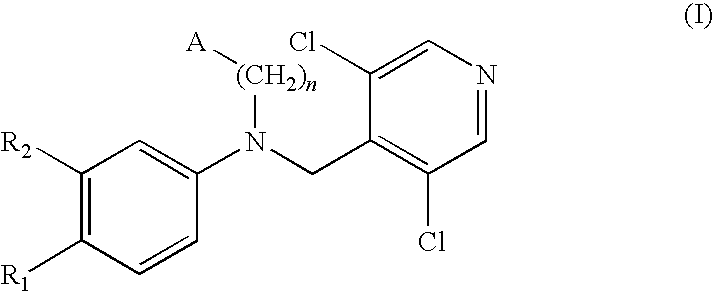
ActiveCN102603676AAvoid gag reactionStrong inhibitory activityNervous disorderOrganic chemistryDiseaseAryl
The invention relates to a phosphodiesterase 4 inhibitor capable of avoiding a vomiting reaction. The phosphodiesterase 4 inhibitor is a compound or a pro-drug or a solvate represented by formula (I), wherein R1 is independent methoxyl, bromine and substituted aryl; X is a randomly substituted six-membered heterocycle; Y is -(CH2)n-, -NH(CH2)n- and -NH(CH2)n-O-, wherein n can be any one of 0, 1, 2 or 3; and Z is a randomly substituted aromatic ring or a randomly substituted heteroaromatic ring. The phosphodiesterase 4 inhibitor capable of avoiding the vomiting reaction is a novel biphenyl PDE4D inhibitor which is applicable to treating depression and Alzheimer's disease and improving cognitive competence, and is capable of avoiding a side effect such as the vomiting.
Owner:兰晟生物医药(苏州)有限公司
Fused-polycyclic compounds
InactiveUS20040204418A1Excellent PDE inhibitory activityUseful in prophylaxisBiocideSenses disorderPolycyclic compoundPhosphodiesterase
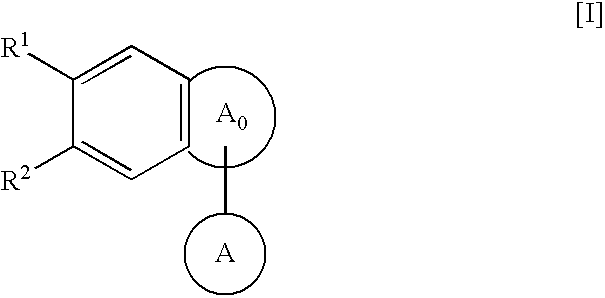
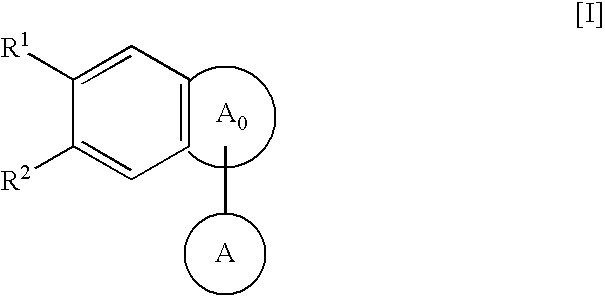
The present invention provides a novel a condensed polycyclic compound useful as a phosphodiesterase 4 inhibitor, which is shown by the formula [I]: or a pharmaceutically acceptable salt thereof and a pharmaceutical composition containing the same.
Owner:MITSUBISHI TANABE PHARMA CORP +1
Phosphodiesterase 4 inhibitors for cognitive and motor rehabilitation
InactiveCN101500559BEasy to getOrganic active ingredientsNervous disorderDiseaseMotor rehabilitation
Owner:DART NEUROSCIENCE CAYMAN LTD
Phosphodiesterase-4 inhibitors belonging to the tertiary amine class
Compounds of formula (I):wherein n, A, R1, and R2 are defined in the specification, are useful as inhibitors of the phosphodiesterase 4 (PDE4) enzyme and for treating certain conditions.
Owner:CHIESI FARM SPA
Phosphodiesterase 4 inhibitors for cognitive and motor rehabilitation
The present invention provides methods of improving cognitive and motor deficits associated with central nervous system (CNS) disorder or condition in an animal. The methods comprise a general administration of phosphodiesterase 4 inhibitors and optionally training the animal under conditions sufficient to produce an improvement in performance.
Owner:DART NEUROSCIENCE CAYMAN LTD
Augmented cognitive training
InactiveUS8097647B2Normally performanceVarious formsBiocideNervous disorderClinical psychologyPhosphodiesterase-4
The present invention provides methods of treating cognitive deficits associated with mental retardation. The methods comprise combining cognitive training protocols and a general administration of phosphodiesterase 4 inhibitors.
Owner:COLD SPRING HARBOR LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com