Alkyne-aryl phosphodiesterase-4 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
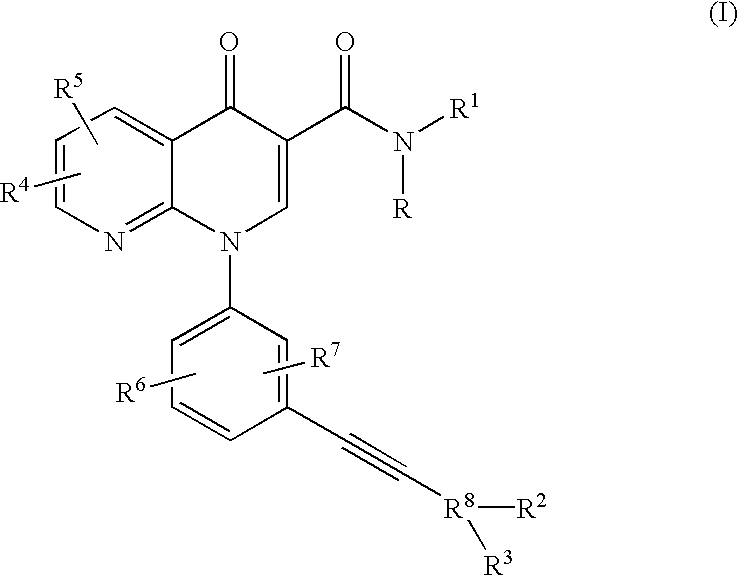
N-Isopropyl-1-[3-(phenylethynyl)phenyl]-1,4-dihydro[1,8]naphthyridin-4-one-3-carboxamide
Step 1: Ethyl 3-(3-bromoanilino)-2-(2-chloronicotinoyl)acrylate.
A mixture of ethyl 2-chloronicotinoyl acetate (41.1 g, 180.5 mmol), triethyl orthoformate (40.12 g, 271 mmol) and acetic anhydride (92.05 g, 902.5 mmol) was heated at 130.degree. C. for 2.5 hours. The volatile components were distilled off and the resulting residue was co-evaporated twice with xylene. The oily residue was dissolved in methylene chloride (250 mL) and 3-bromoaniline (37.25 g, 216.6 mmol) was added slowly. The resulting solution was stirred at room temperature for 18 hours, and the solvent evaporated away. The resulting crude compound was used as such in the next step.
Step 2: Ethyl 1-(3-bromophenyl)-1,4-dihydro[1,8]naphthyridin-4-one-3-carboxylate.
The crude compound from Step 1 was dissolved in tetrahydrofuran (500 mL), the solution was cooled to 0.degree. C., and sodium hydride (as a 60% dispersion in oil, 9.4 g, 235 m...
example 2
N-Isopropyl-1-[3-(2-pyridinylethynyl)phenyl]-1,4-dihydro[1,8]naphthyridin-4-one-3-carboxamide
Following the procedure of Step 5 of EXAMPLE 1, but substituting 2-ethynylpyridine for phenylacetylene, the title compound was obtained as a brown solid.
.sup.1 H NMR (Acetone-d.sub.6) .delta. 1.25 (d, 6H), 4.18 (m, 1H), 7.38 (m, 1H), 7.59-7.64 (m, 2H), 7.71-7.76 (m, 2H), 7.81-7.85 (m, 2H), 7.92 (s, 1H), 8.61 (m, 1H), 8.74 (m, 1H), 8.78 (dd, 1H), 8.89 (s, 1H), 9.62 (br, NH).
example 3
N-Isopropyl-1-[3-(4-pyridinylethynyl)phenyl]-1,4-dihydro[1,8]naphthyridin-4-one-3-carboxamide
Following the procedure of Step 5 of EXAMPLE 1, but substituting 4-ethynylpyridine (J.Org.Chem. 1996, 61, 6535) for phenylacetylene, the title compound was obtained as a solid.
.sup.1 H NMR (Acetone-d.sub.6) .delta. 1.24 (d, 6H), 4.18 (m, 1H), 7.49 (m, 2H), 7.61 (m, 1H), 7.71-7.78 (m, 2H), 7.81 (m, 1H), 7.92 (s, 1H), 8.62 (m, 2H), 8.73 (m, 8.78 (dd, 1H), 8.87 (s, 1H), 9.62 (br, NH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com