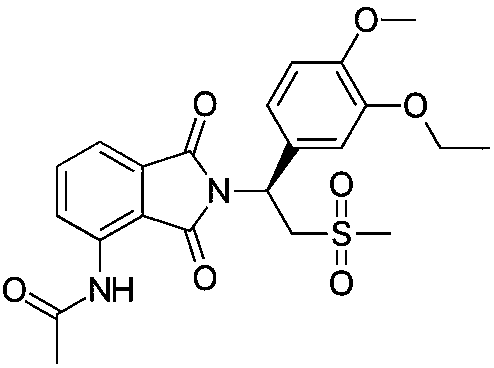
Topical pharmaceutical composition of phosphodiesterase-4 inhibitor and preparation method thereof
A phosphodiesterase and local drug technology, applied in the field of medicine, can solve the problems of easy recurrence, easy recurrence after recovery, low cure rate, etc., and achieve the effects of reducing discomfort and trouble, clear mechanism of action, and good patient tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Table 1 Aqueous base ointments
[0052] prescription composition
%(w / w)
Apster
0.5
Vitamin C
0.05
PEG4000
30
PEG400
60
6.2
2
Dimethylsulfoxide (DMSO)
1
0.05
hydrochloric acid / sodium hydroxide
Adjust pH to 5.0~6.0
[0053] Preparation Process:
[0054] a) Add DMSO into a stainless steel container, and gradually add Apremilast and mix until completely dissolved, then add propylene glycol and stir continuously with a spiral stirrer, set aside;
[0055] b) Dissolve anhydrous sodium sulfite in distilled water in addition, and set aside;
[0056] c) Heat PEG4000 and PEG400 on a water bath to 85°C to melt, and when cooled to below 40°C, add the solutions of a) and b) and stir well.
[0057] d) Adjust the pH to 5.0-6.0 with hydrochloric acid / sodium hydroxide to obtain the aqueous matrix apremilast ointment.
Embodiment 2
[0059] Table 2 Oily base ointments
[0060] prescription composition
Proportion in prescription (%)
Apster
0.5
DMSO
1.5
25
30
White Vaseline
30
3
10
[0061] Preparation Process:
[0062] a) Heat liquid paraffin, white vaseline and lanolin on a water bath to 85°C to melt to remove moisture, filter, and let cool to 70°C.
[0063] b) Dissolve Apremilast in DMSO, add vegetable oil and propylene glycol and stir evenly.
[0064] c) Add b) to a) and stir, and mix evenly to obtain an oily matrix apremilast ointment.
Embodiment 3
[0066] Table 3 cream (O / W type)
[0067] prescription composition
Proportion in prescription (%)
Apster
0.5
DMSO
25
24
White Vaseline
24
0.1
10.39
0.01
15.16
[0068] Preparation Process:
[0069] a) Weigh stearyl alcohol and white petrolatum into a stainless steel container, heat to 75°C on a water bath to melt, stir with a spiral stirring paddle, gradually add DMSO and apremilast mixture, and mix evenly (oil phase);
[0070] b) Separately weigh methylparaben and sodium lauryl sulfate into a stainless steel container, add distilled water to dissolve, and heat to 75° C. (water phase) on a water bath;
[0071] c) Gradually add the water phase to the oil phase, and stir while adding, so as to disperse evenly until it condenses, and then the O / W type apremilast cream is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com