Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
213 results about "Injections sites" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
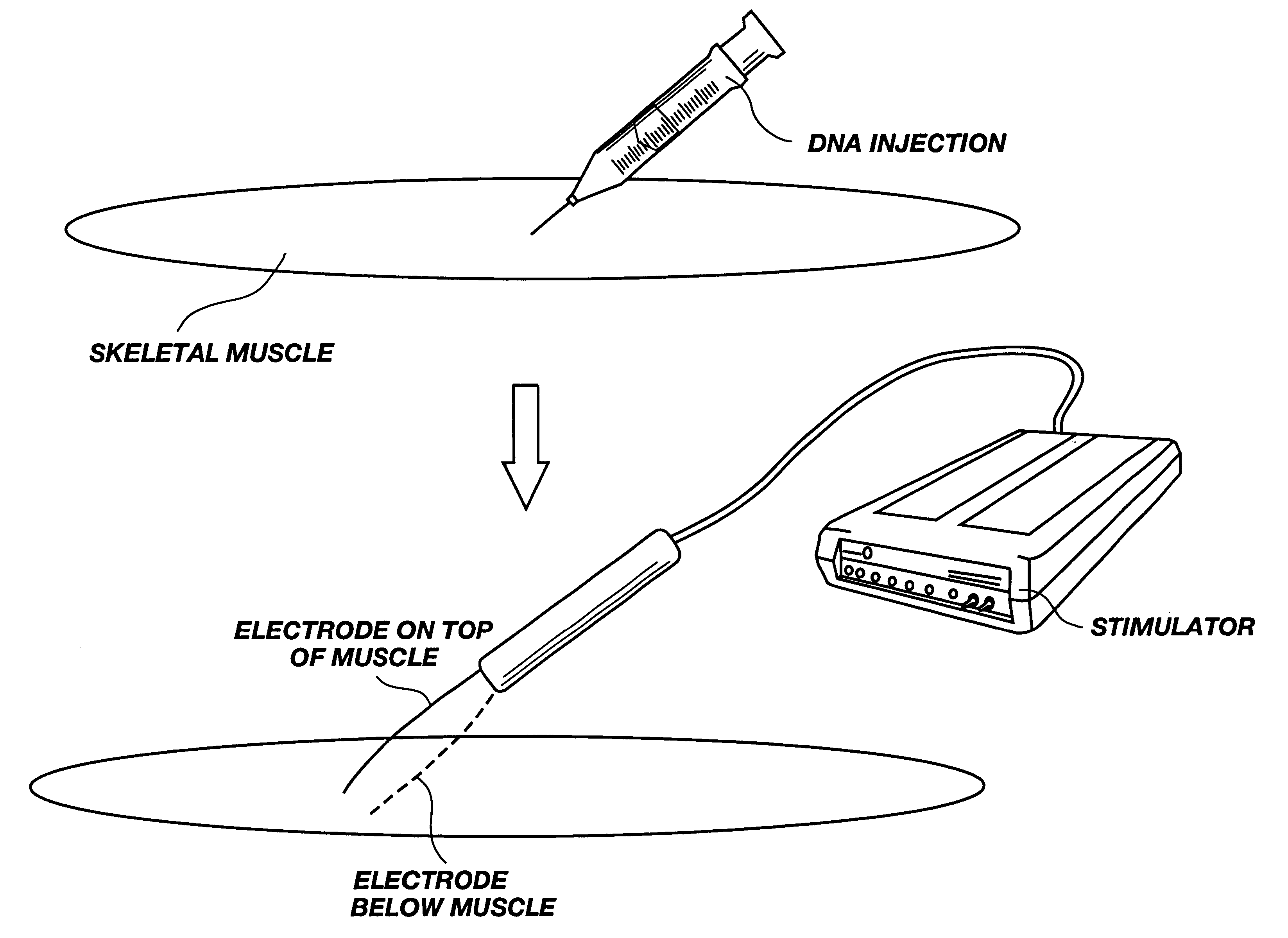
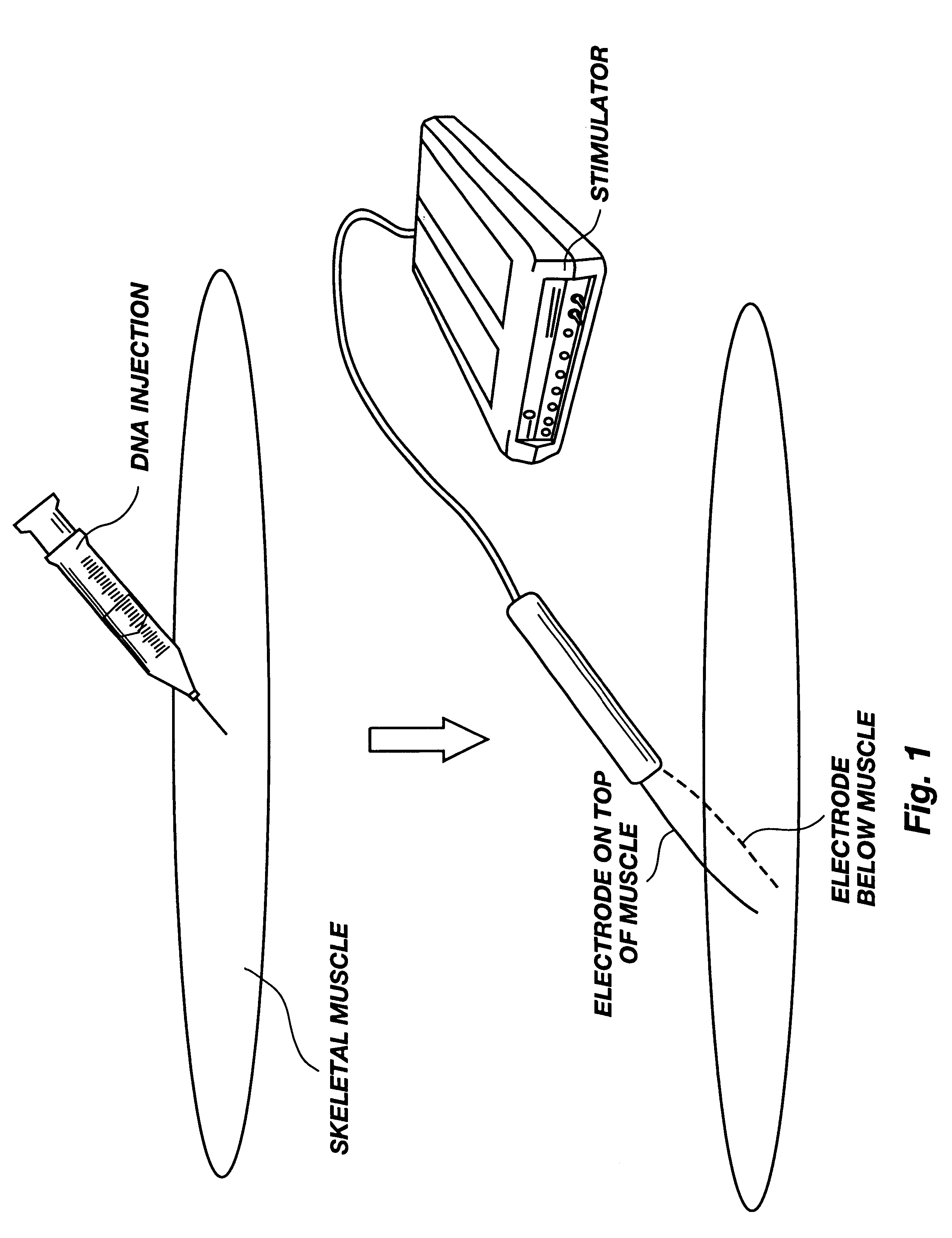
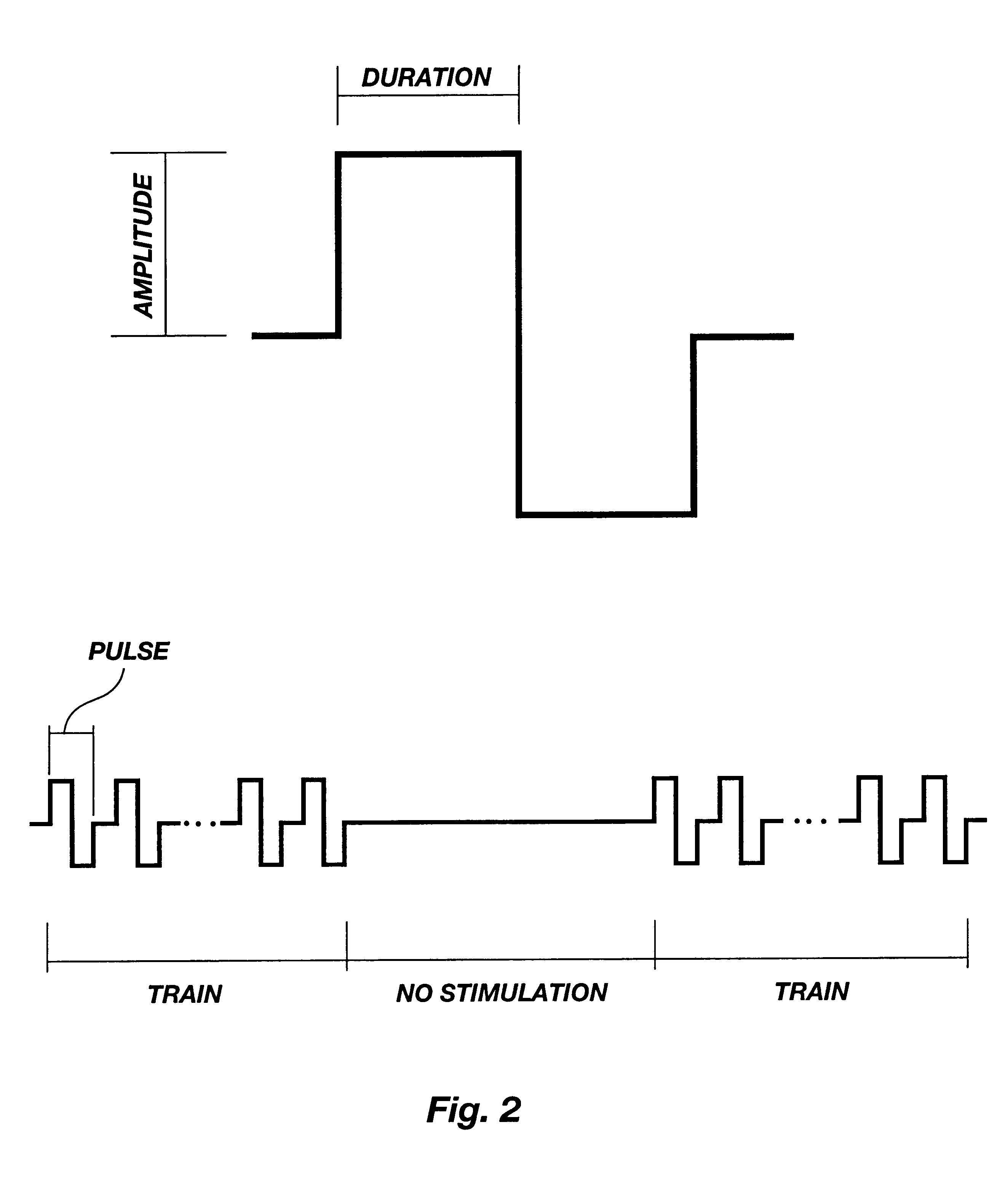
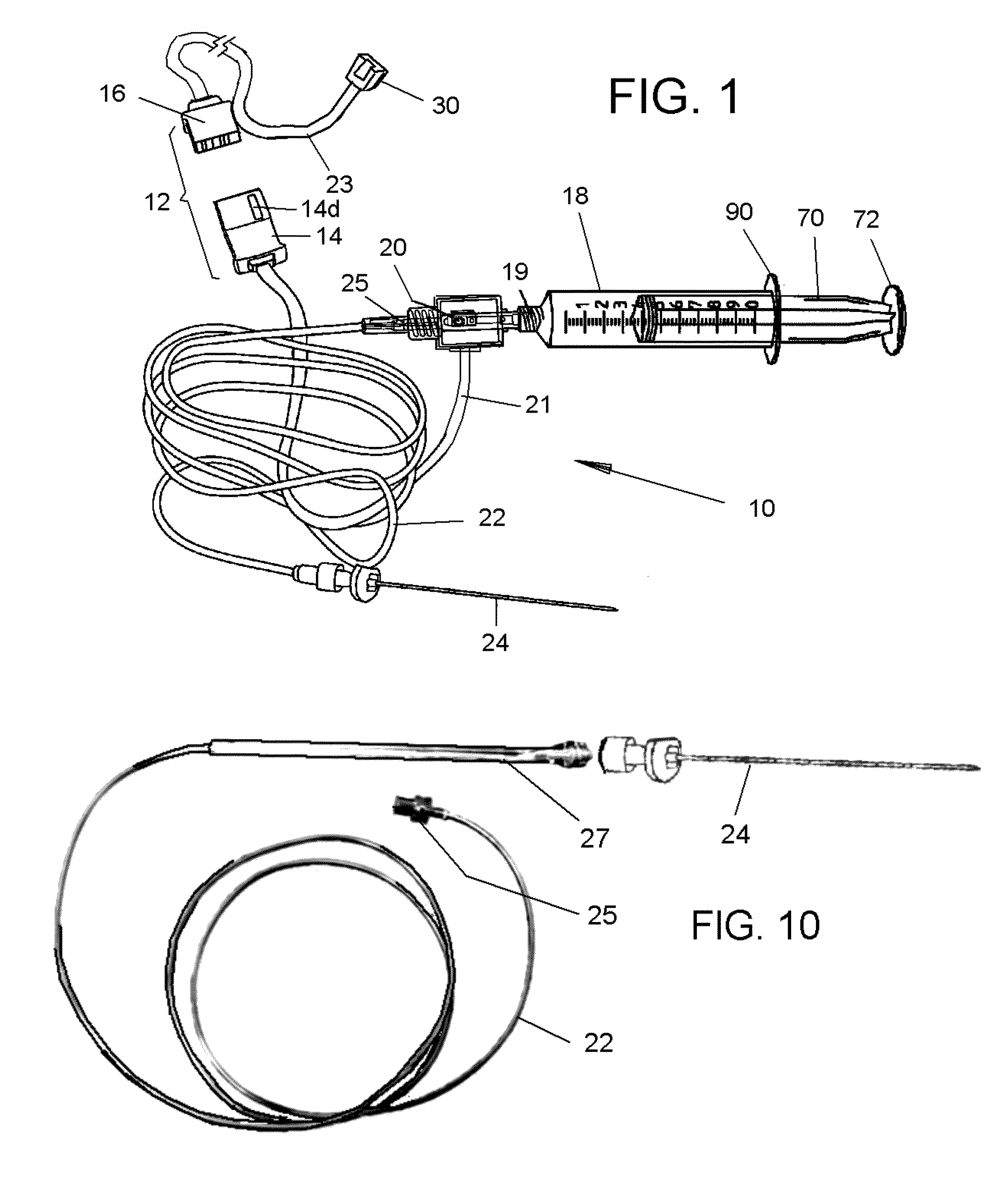
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
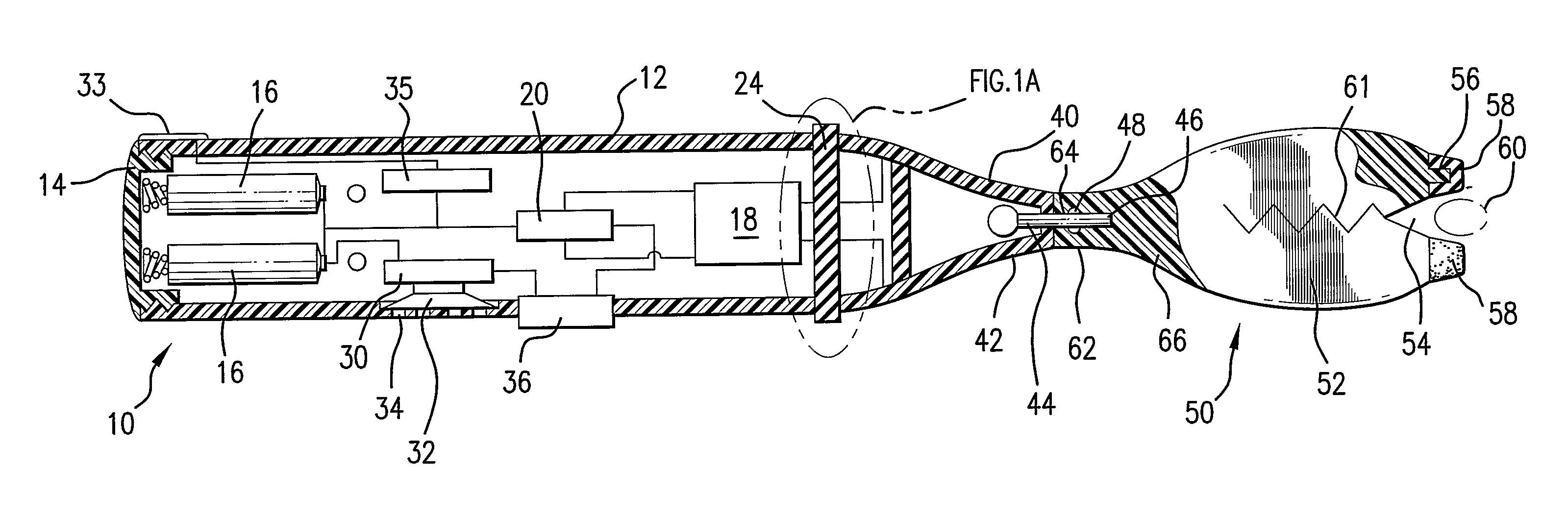
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
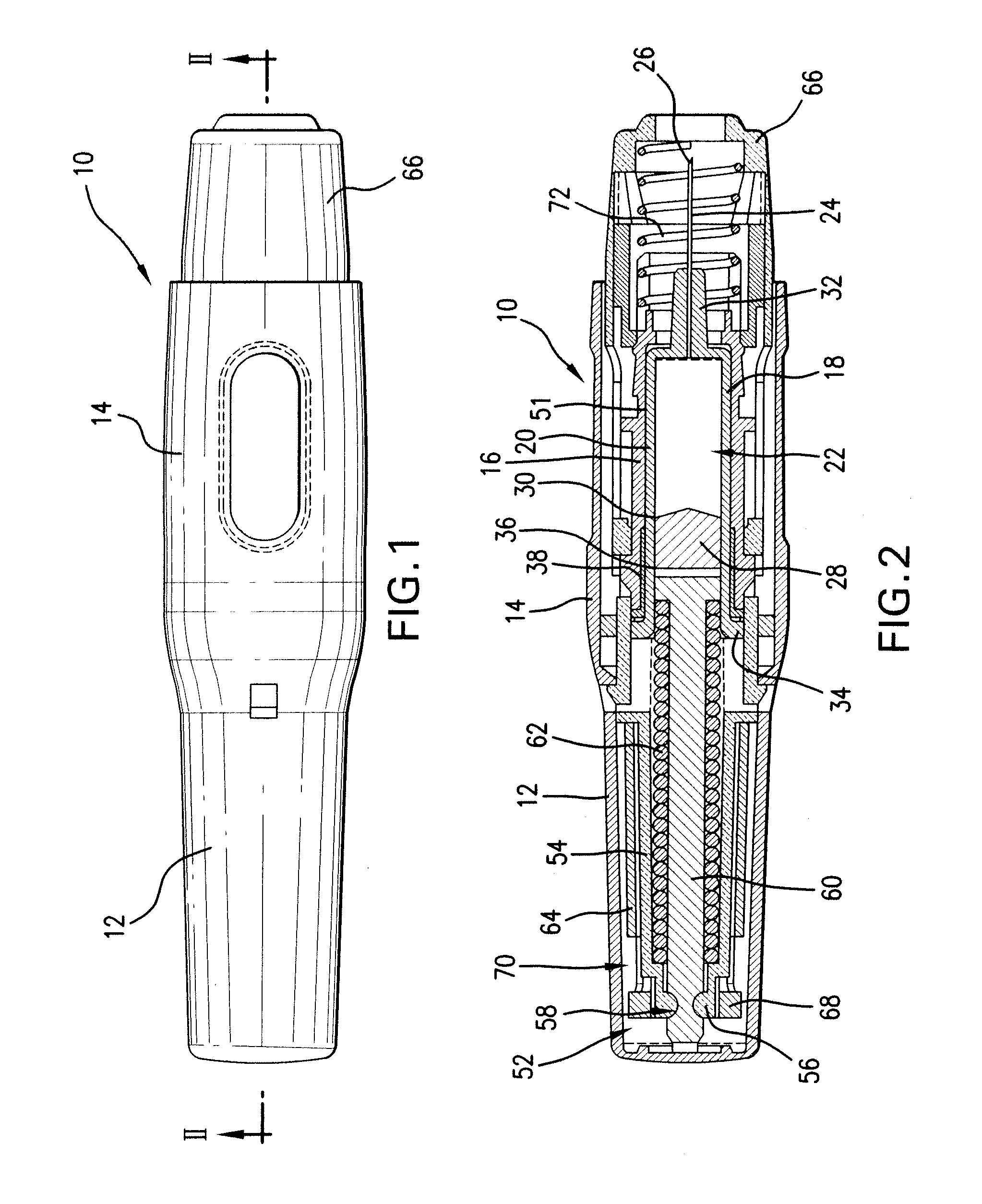
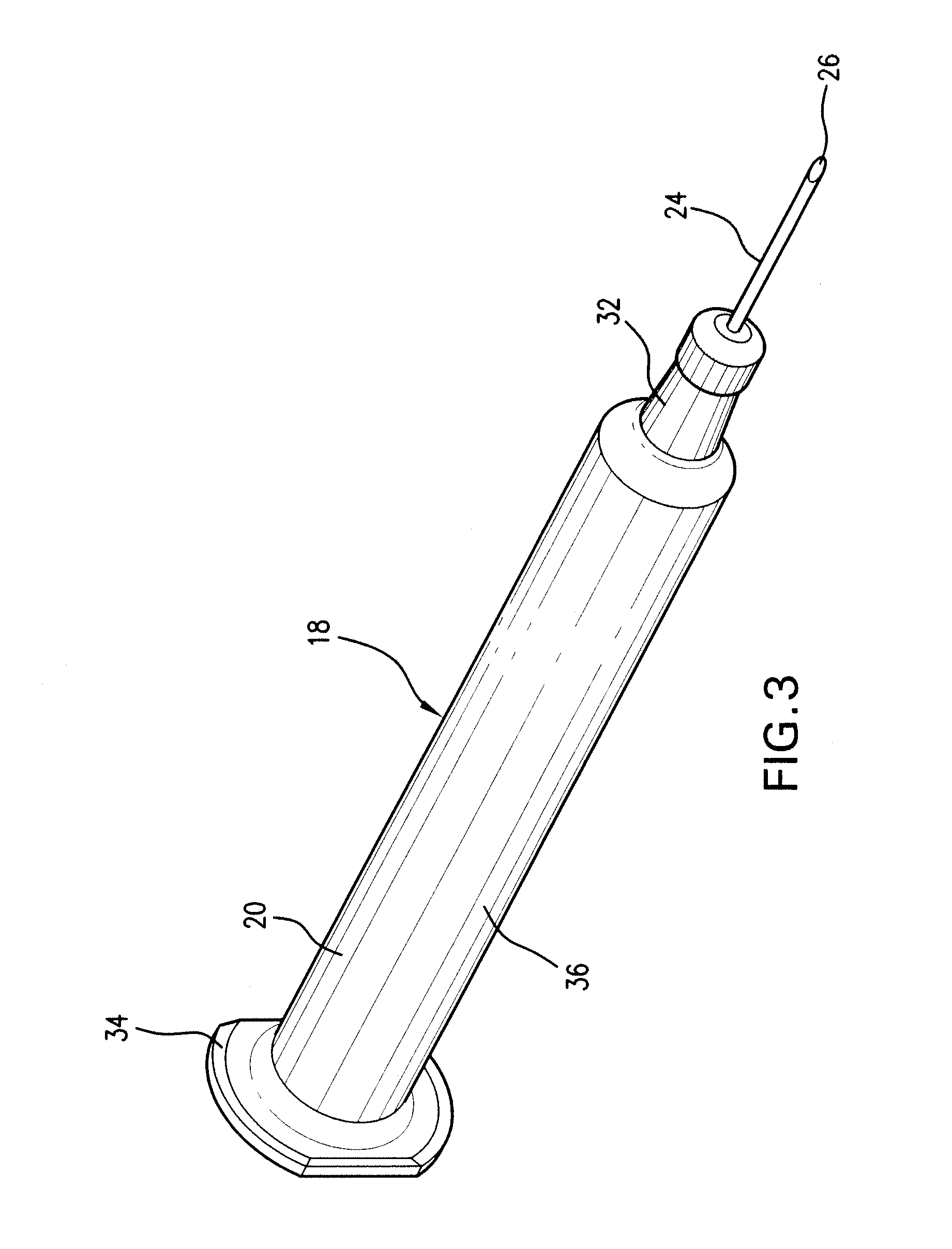
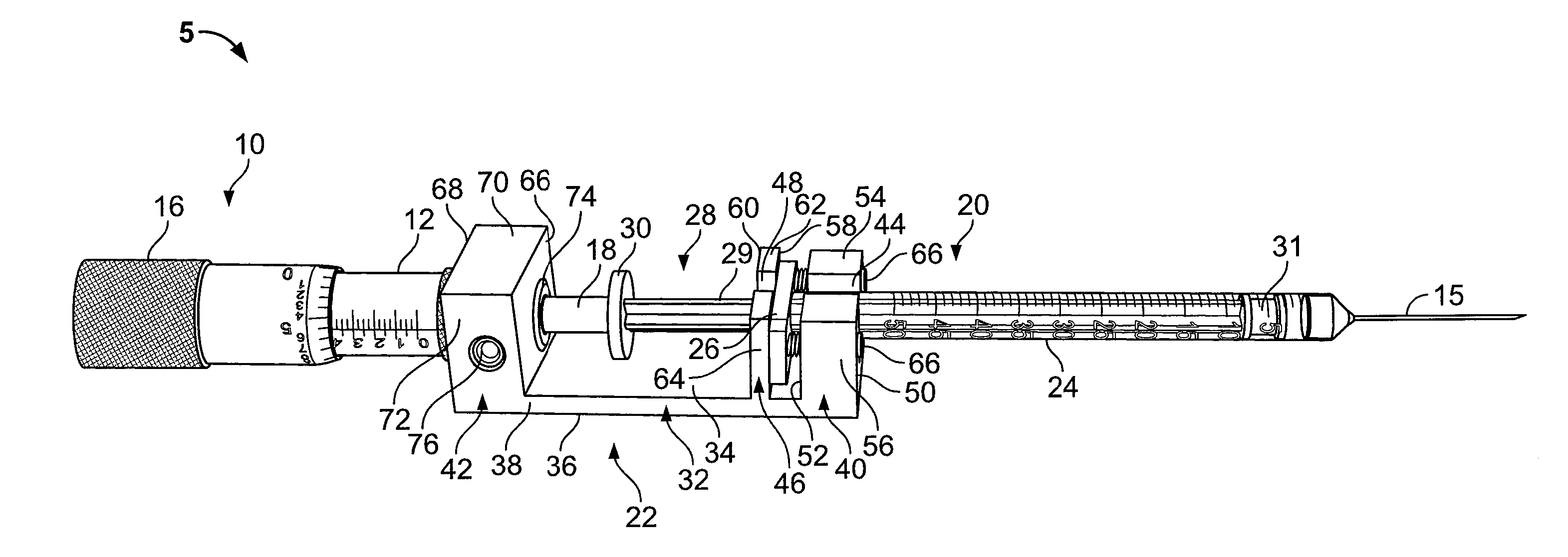
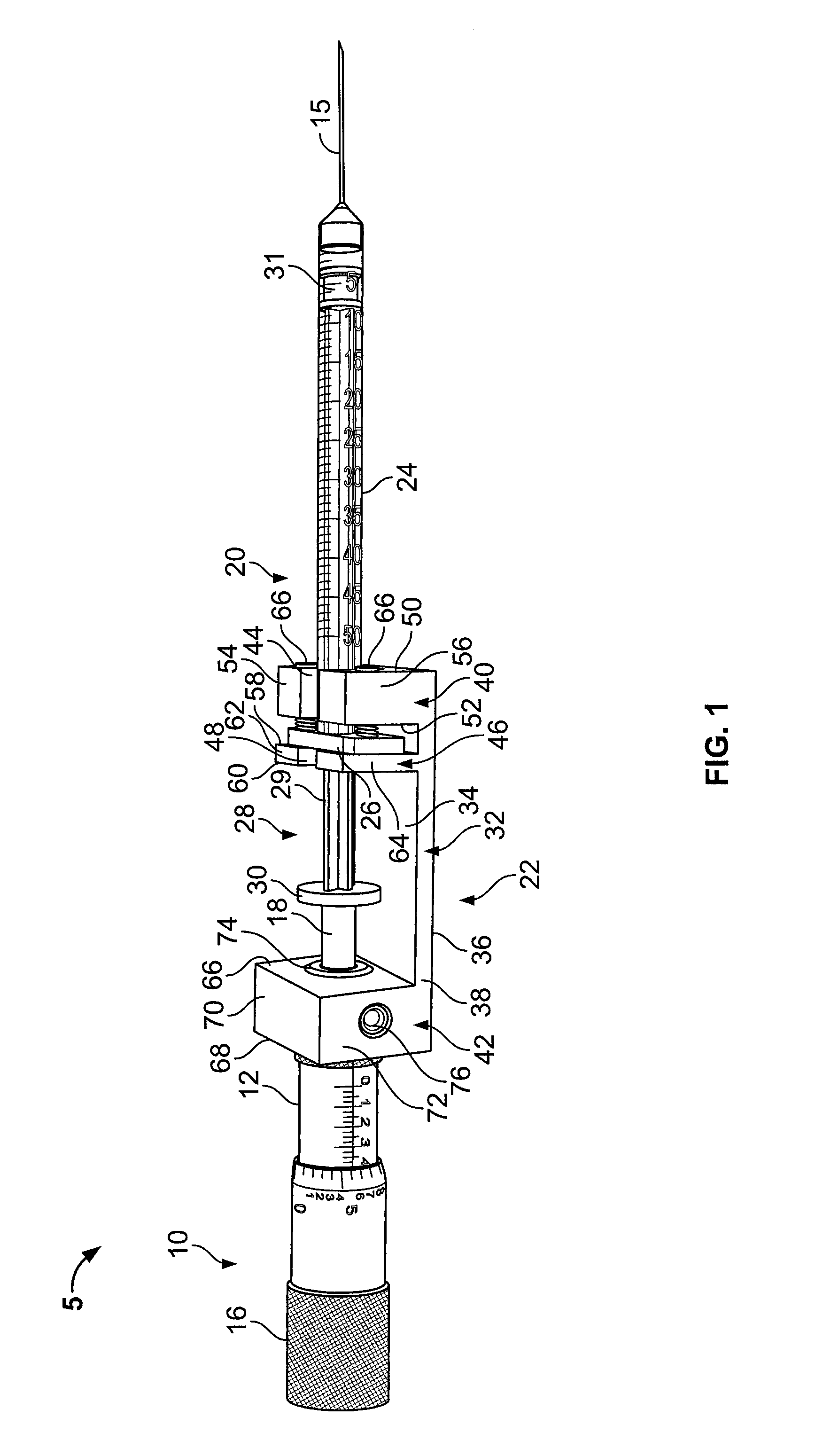
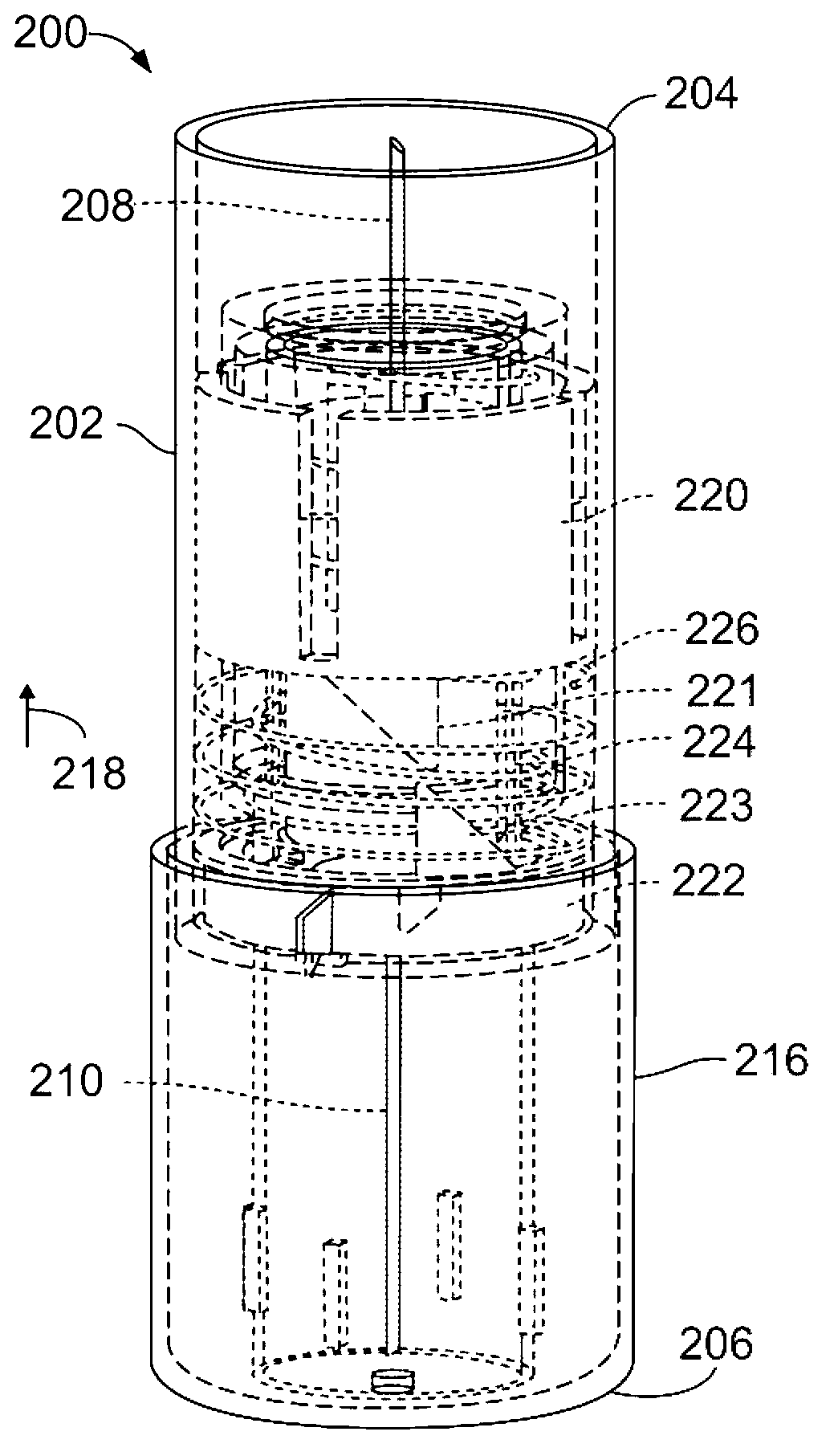
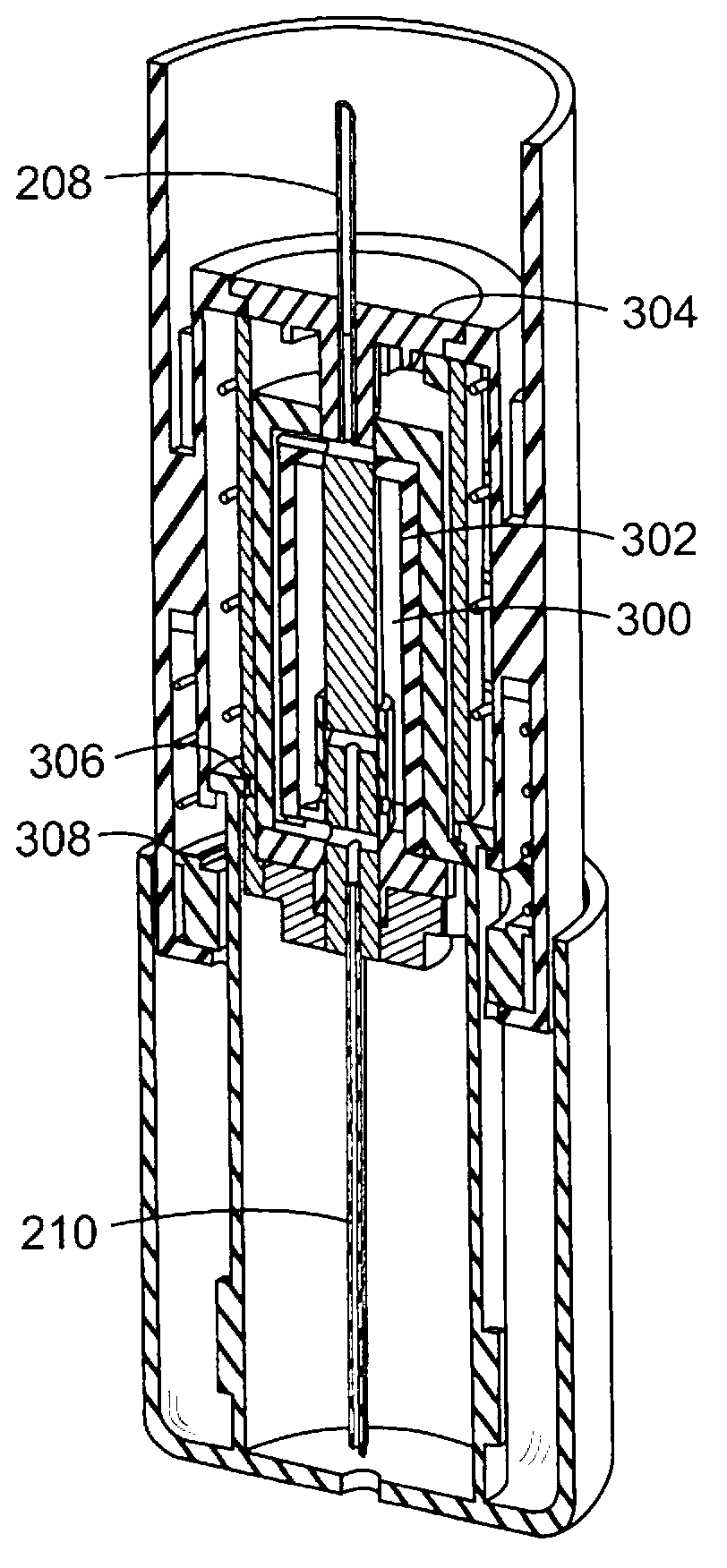
Prefilled syringe jet injector
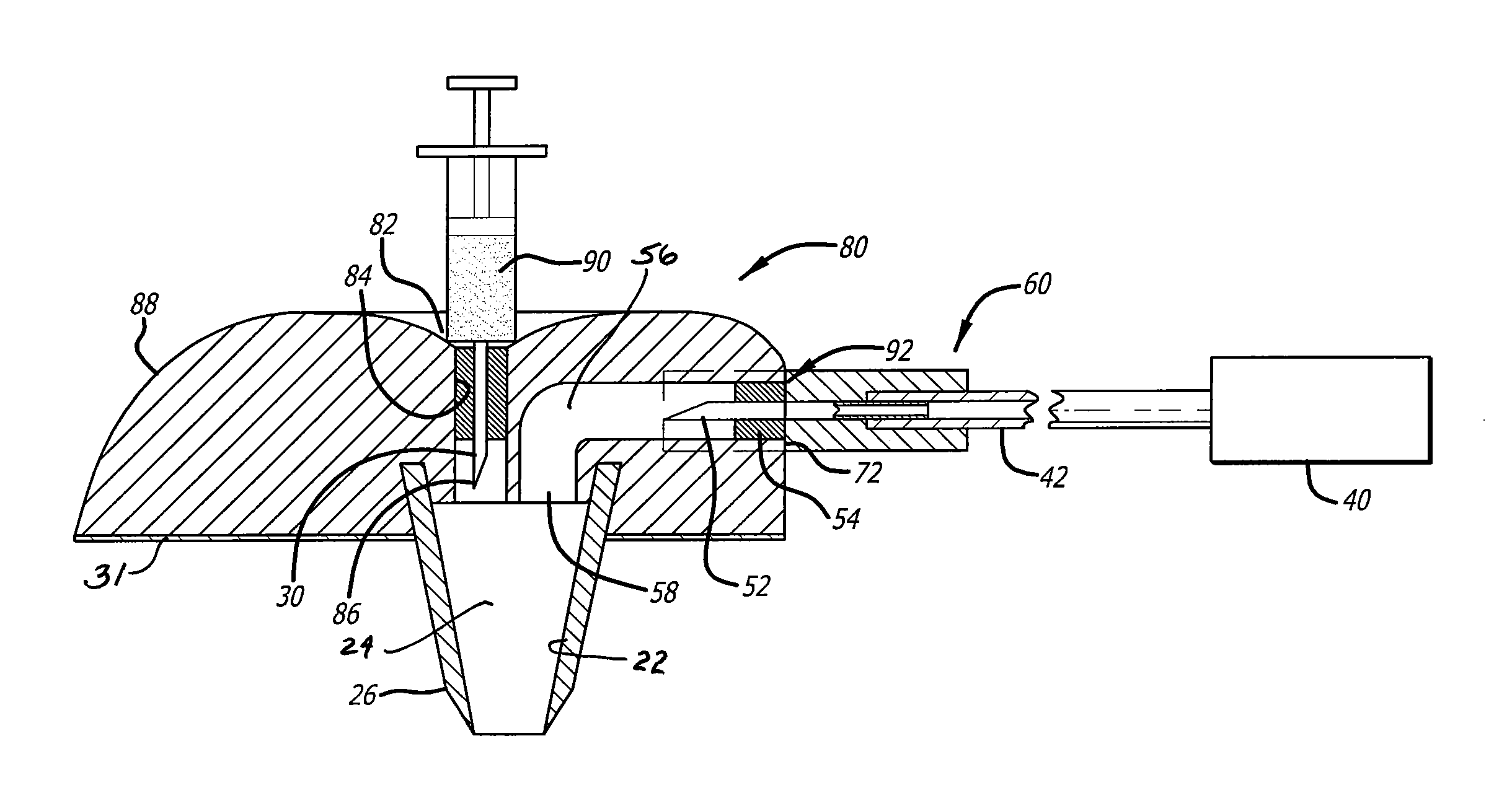
ActiveUS20080154200A1Prevent backflowImprove distributionAmpoule syringesJet injection syringesEngineeringPrefilled Syringe
A jet injector that includes a prefilled syringe. The syringe includes a fluid chamber that contains a medicament. The syringe also has an injection-assisting needle, and a plunger is movable within the fluid chamber. A housing is configured for allowing insertion of the needle to a penetration depth. An energy source is configured for biasing the plunger to produce an injecting pressure in the medicament in the fluid chamber of between about 80 and 1000 p.s.i. to jet inject the medicament from the fluid chamber through the needle to an injection site.
Owner:ANTARES PHARMA INC
Subcutaneous and intradermal patch infusers
The device infuses a compound subcutaneously or intradermally over an extended period of time. The delivery is from a conventional prefilled syringe with a staked cannula with the device adhered to the injection site and the syringe positioned substantially parallel to the injection site. The cannula is formed by a mechanism integral to the device to provide the cannula distal section substantially perpendicular to the syringe axis. The compound delivery is based on springs or other mechanisms. The approach offers a low cost mechanical system for the infusion of high volume, viscous compounds. The activation of the patch infuser required a few simple steps by the user. A single lever is used to trigger all device functions.
Owner:SID TECH LLC
Apparatus and methods for delivering fluid and material to a subject
The present invention provides fluid and material delivery methods and devices for practicing the methods. The invention provides a method of delivering cellular material comprising injecting the cellular material into a subject such that the injected cells retain their inherent morphologic characteristics upon injection. The method comprises the steps of aspirating the cellular material into a fluid delivery device which incorporates a syringe arrangement. The cellular material is aspirated into the main body of the syringe until the desired amount of a material has filled the syringe body. The needle of the fluid delivery device is then inserted into the skin of a subject at an angle about parallel to the skin until a desired depth has been reached. The cellular material is then injected in the subject until the desired volume of material has been injected. The needle of the device is then rotated approximately 45 to 90 degrees and the needle is removed from the injection site.
Owner:ADERANS RES INST
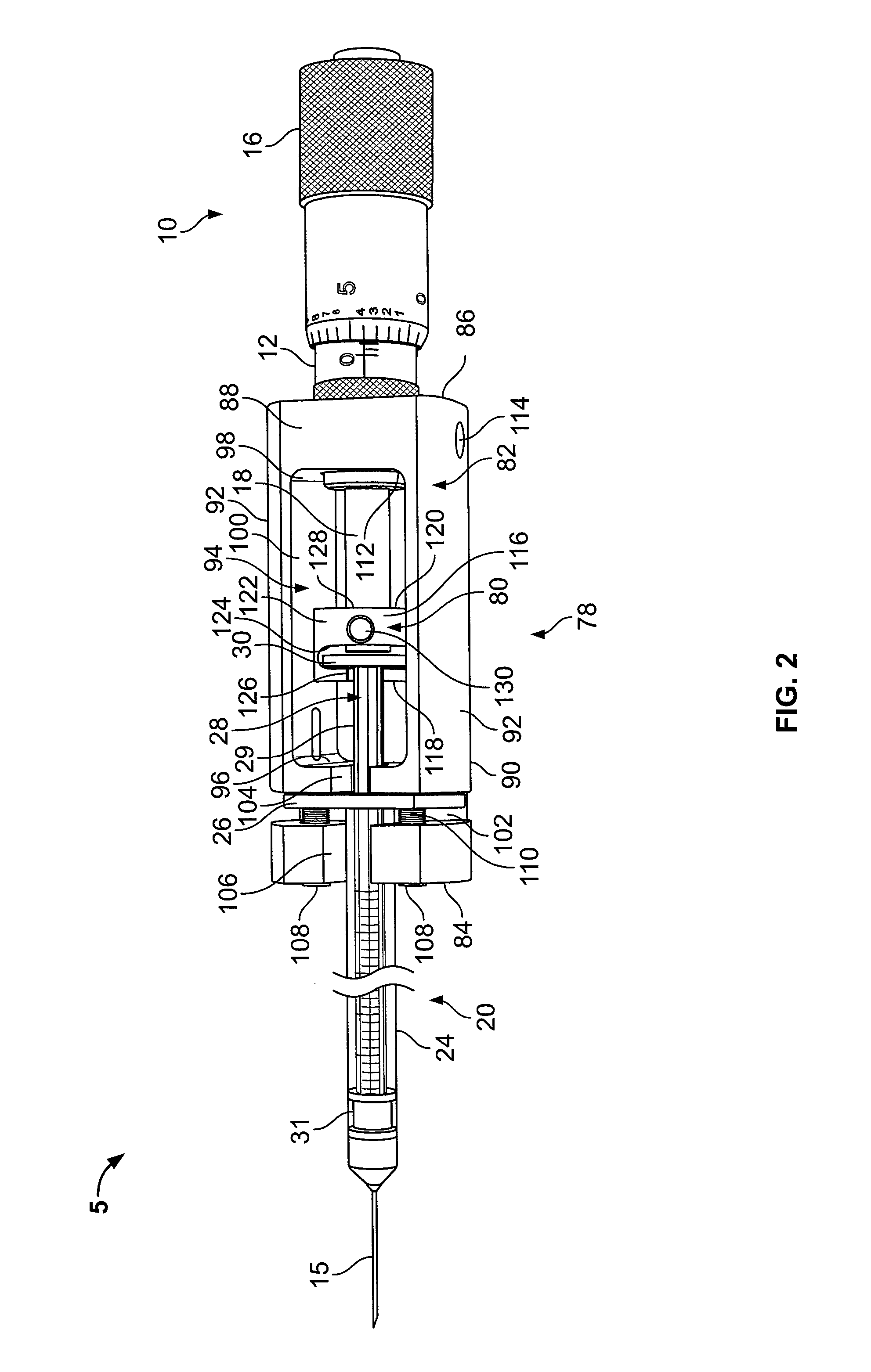
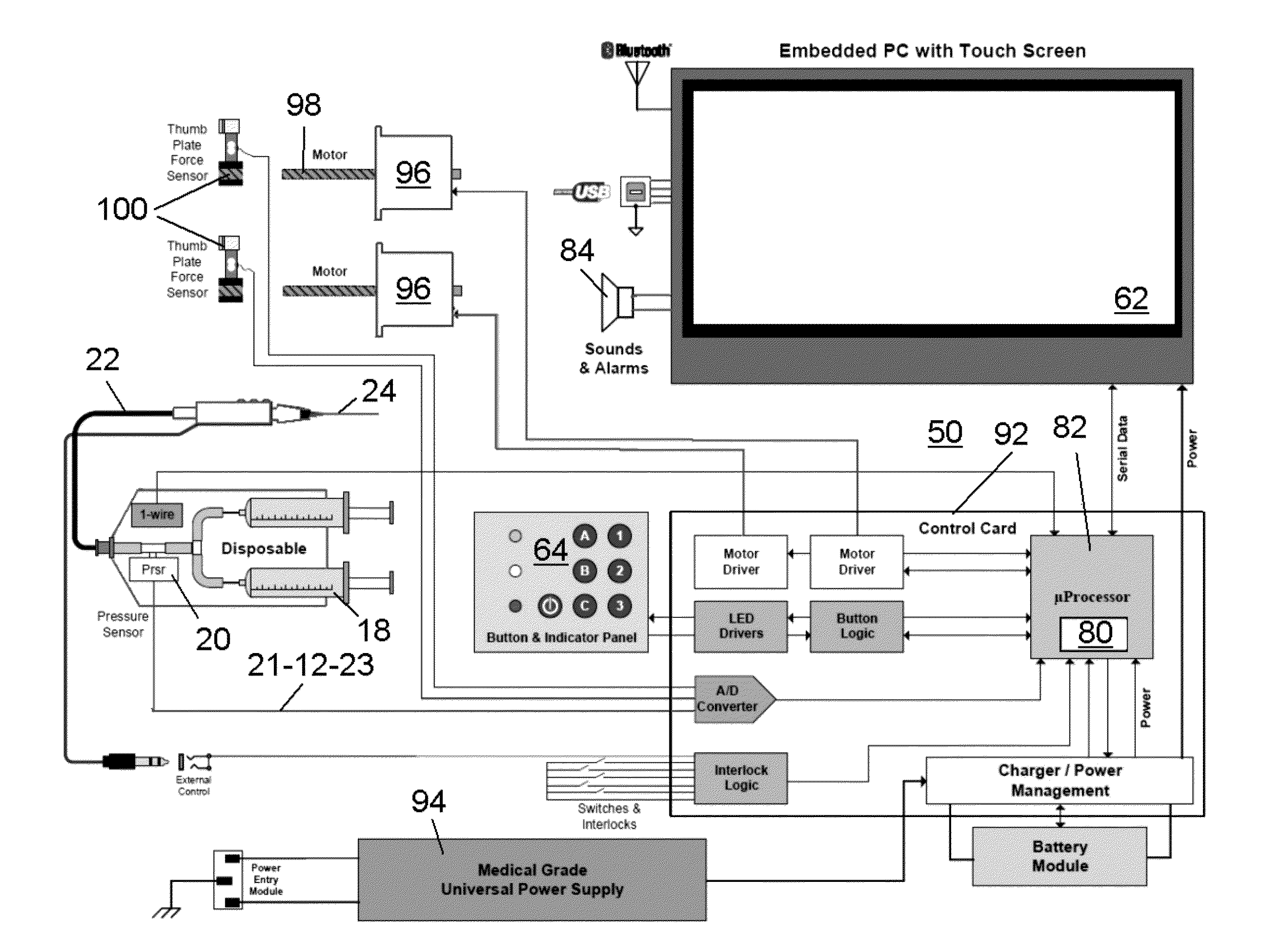
Drug infusion with pressure sensing and non-continuous flow for identification of and injection into fluid-filled anatomic spaces
ActiveUS20140012226A1Accurately and reproducibly administerLimit amount of pain and tissue damageAnaesthesiaInfusion syringesMedicinePharmaceutical drug
An automatic injection apparatus uses non-continuous fluid-flow of drugs to identify an intended injection site and includes a drive mechanism, a sensor and a controller for establishing fluid flow and pressure and preventing fluid flow until the pressure drops below a predetermined threshold. The pressure threshold is determined based on an internal pressure generated during an injection and more fluid will not flow until it drops below a predetermined pressure. An injection is performed to establish an initial pressure threshhold and then to stop the fluid flow into a patient until the pressure drops below a predetermined pressure which allows fluid flow to resume, thus identifying a fluid filled tissue space. The initial pressure threshold is used as a control parameter to a microprocessor below which controls the rate of injection. Fluid flows below certain pressures are also used to identify a specific location within the body during injections.
Owner:MILESTONE SCIENTIFIC INC
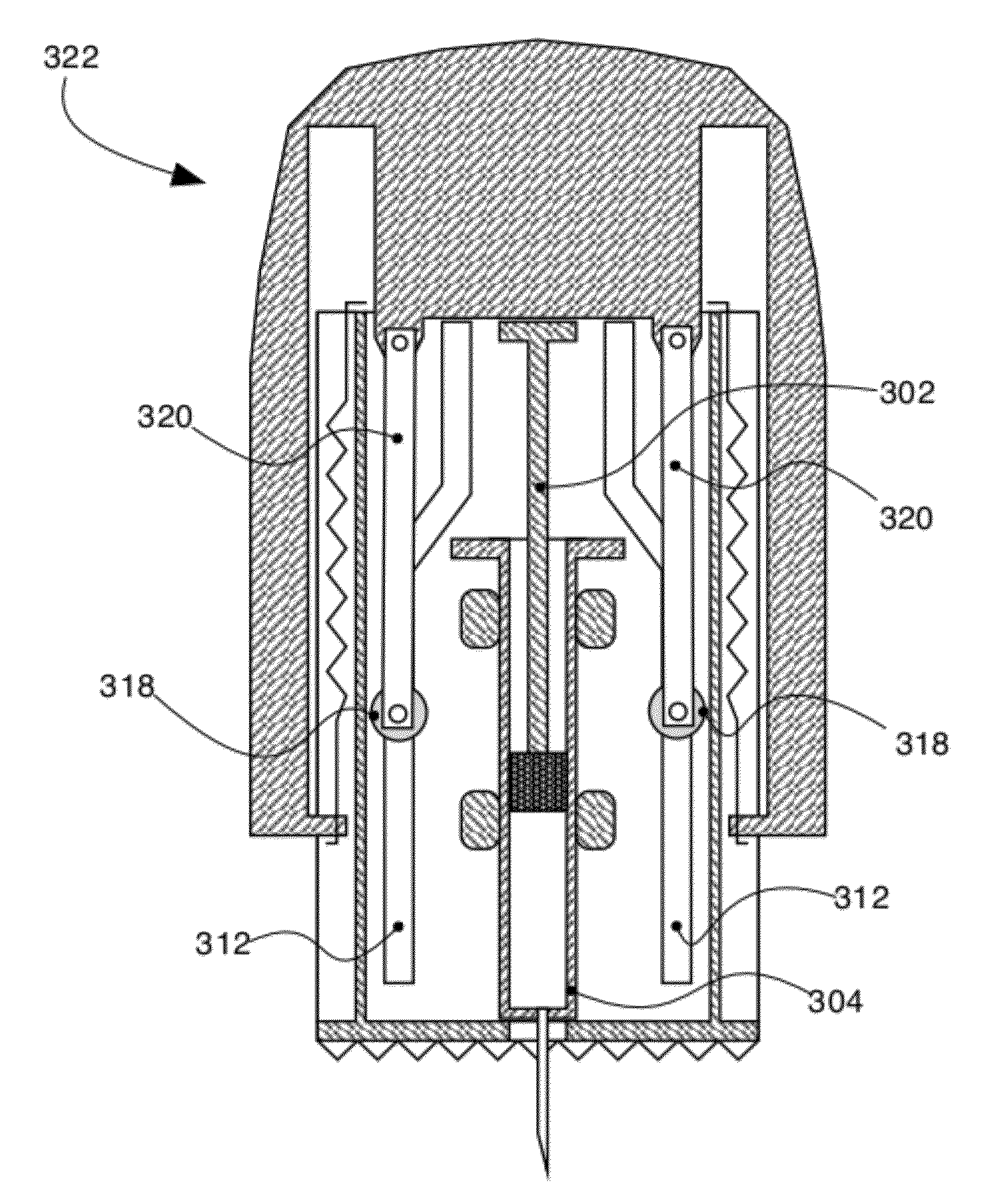
Palm-controlled injectors
InactiveUS20120253314A1Easy to carryReduce the possibilityAutomatic syringesMedical devicesPrefilled SyringeBiomedical engineering
A palm-controlled device is disclosed for injection of a substance into an organism. The device includes a palm-receiving surface for receiving a palm of a hand, the palm receiving surface being shaped so that the palm is substantially parallel to a surface of an injection site of the organism while operating the device. The palm receiving surface can also be shaped so that the palm is substantially perpendicular to a surface of the injection site. The device also includes an injector having at least one pre-filled syringe, the palm-receiving surface being cooperative with the injector such that when pressure is applied to the palm-receiving surface, the injector is actuated so as to inject contents of the at least one pre-filled syringe. Actuation of the injector is by gradual pressure; not sudden motion. Single or multiple doses of same or different medications can be included in a single injector.
Owner:HARISH ZIV
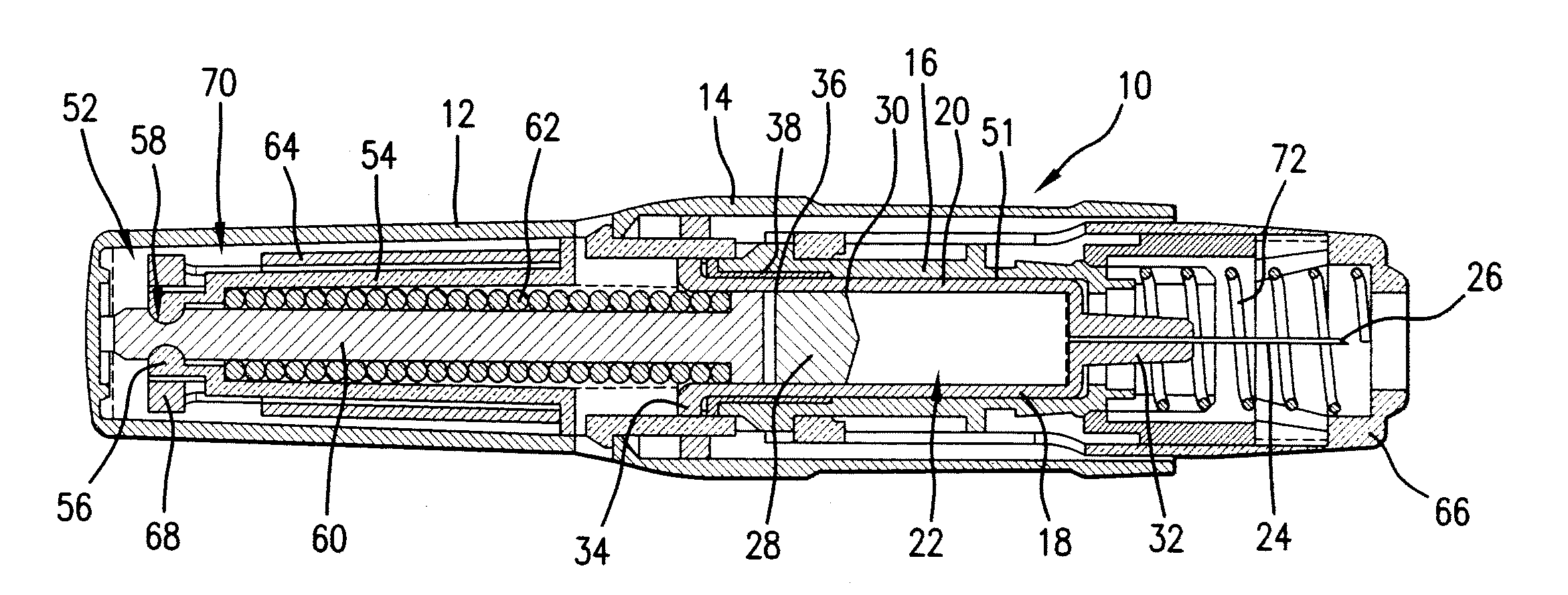
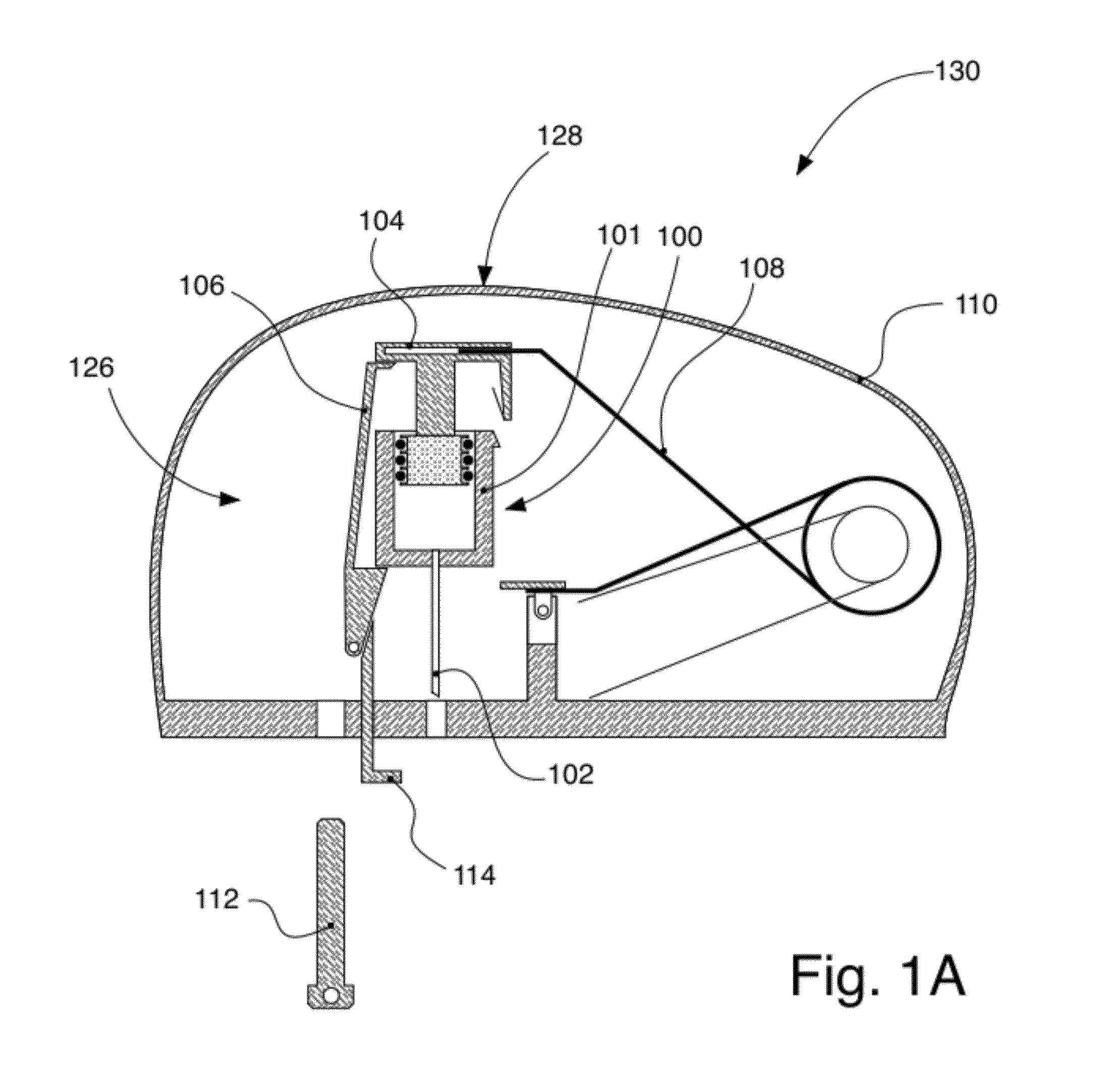
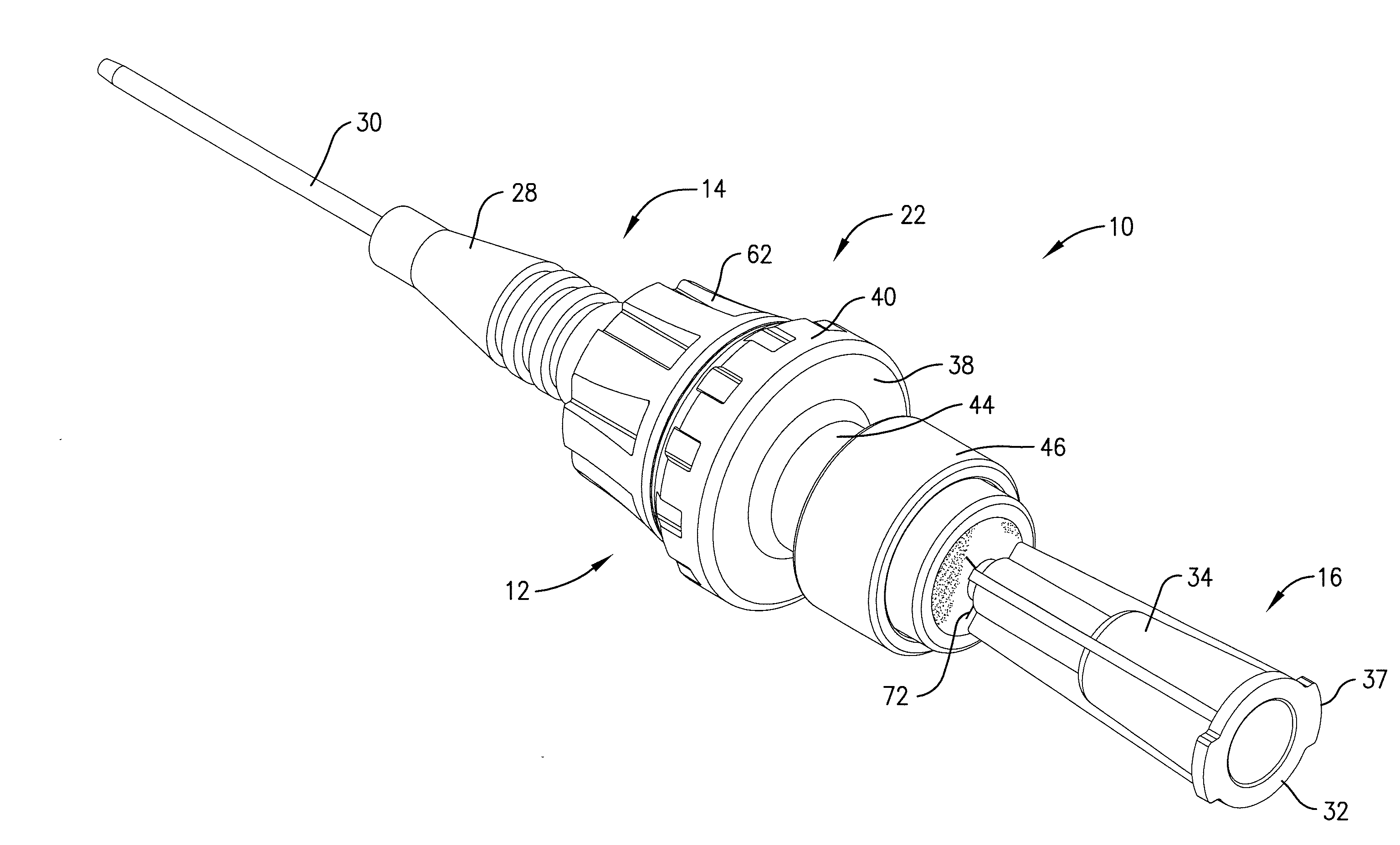
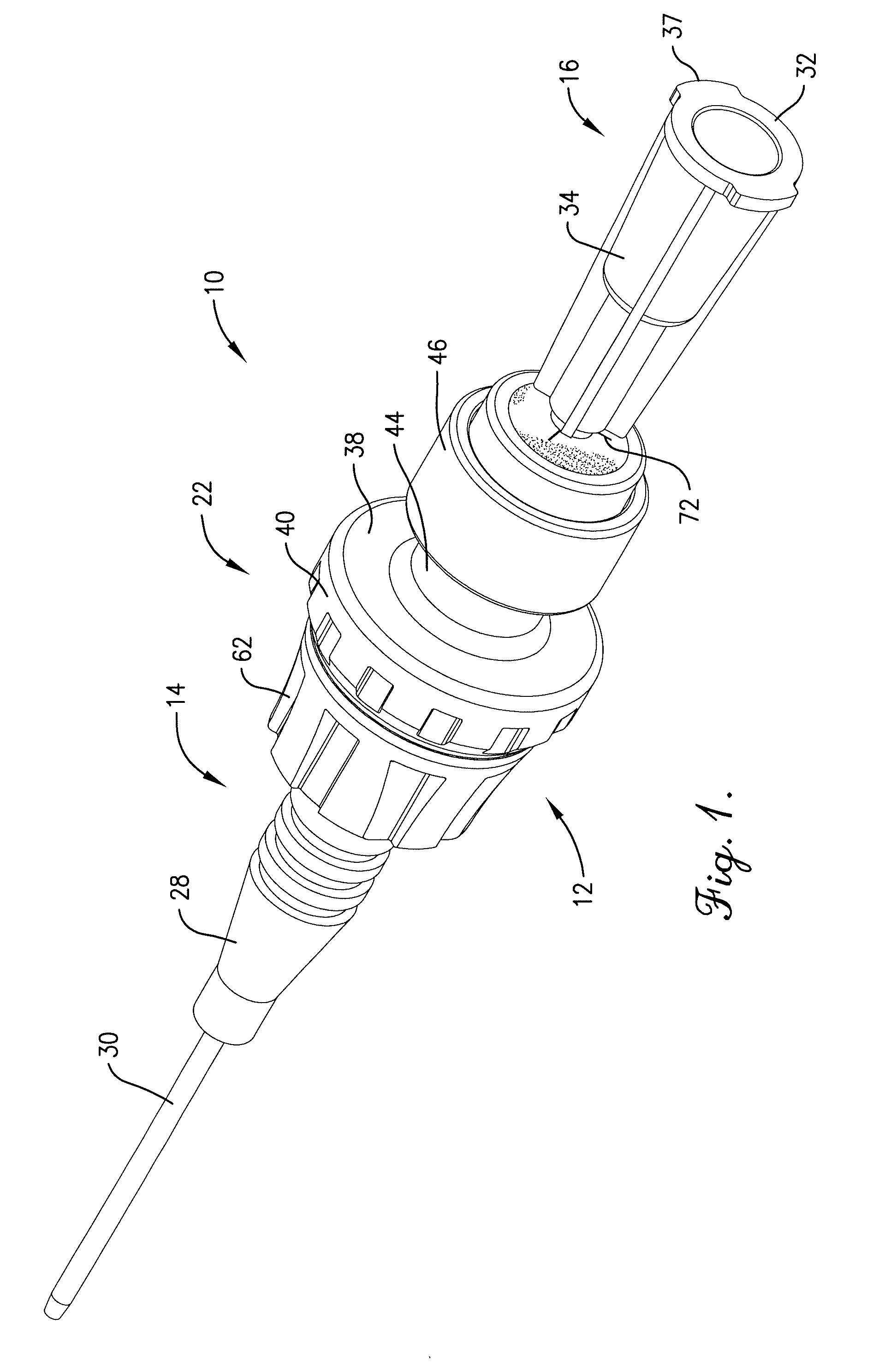
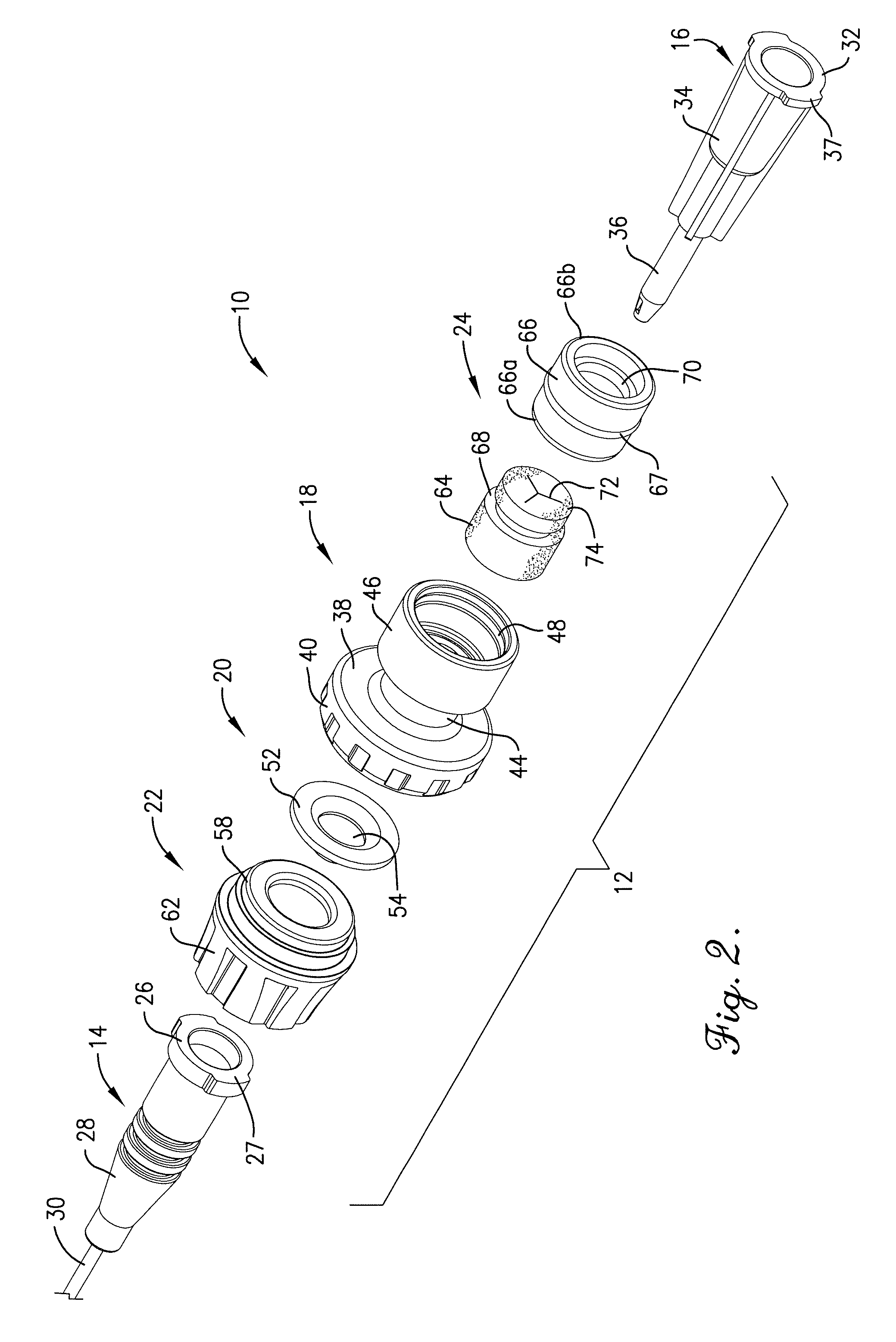
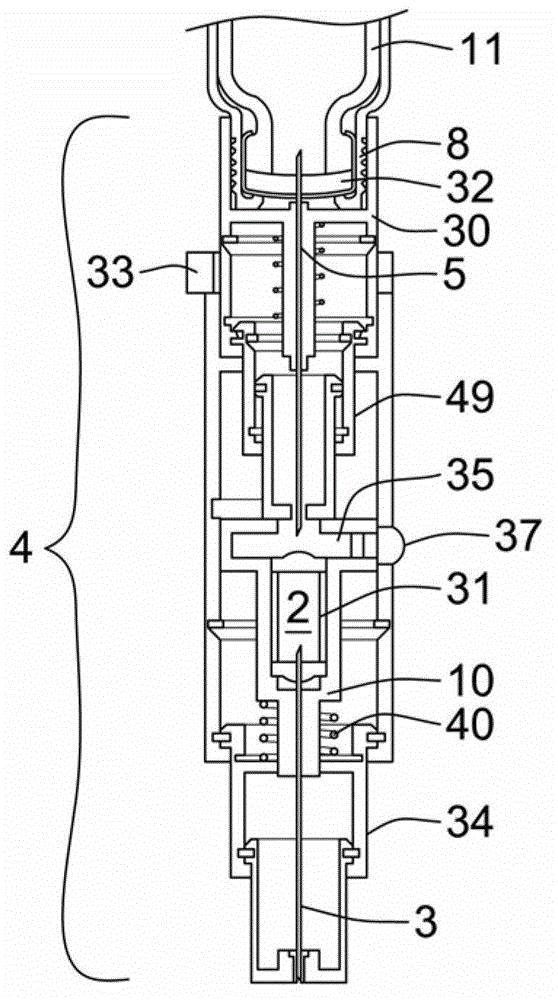
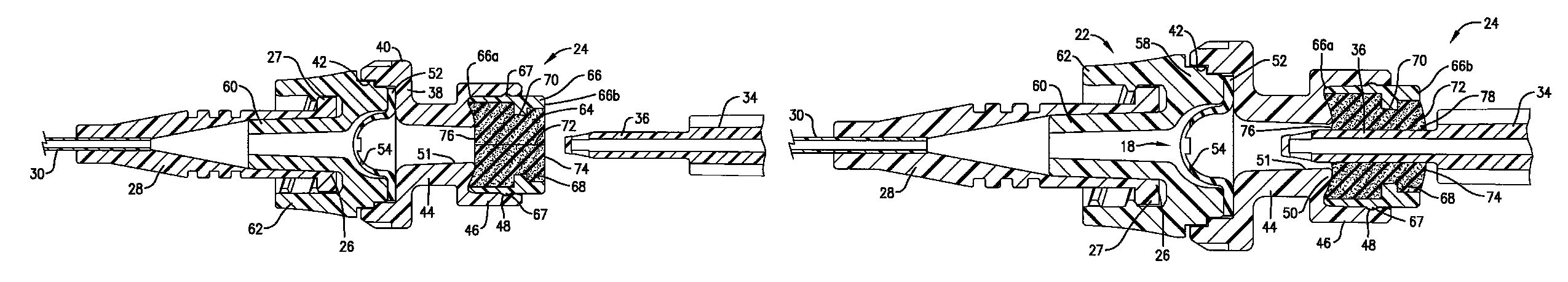
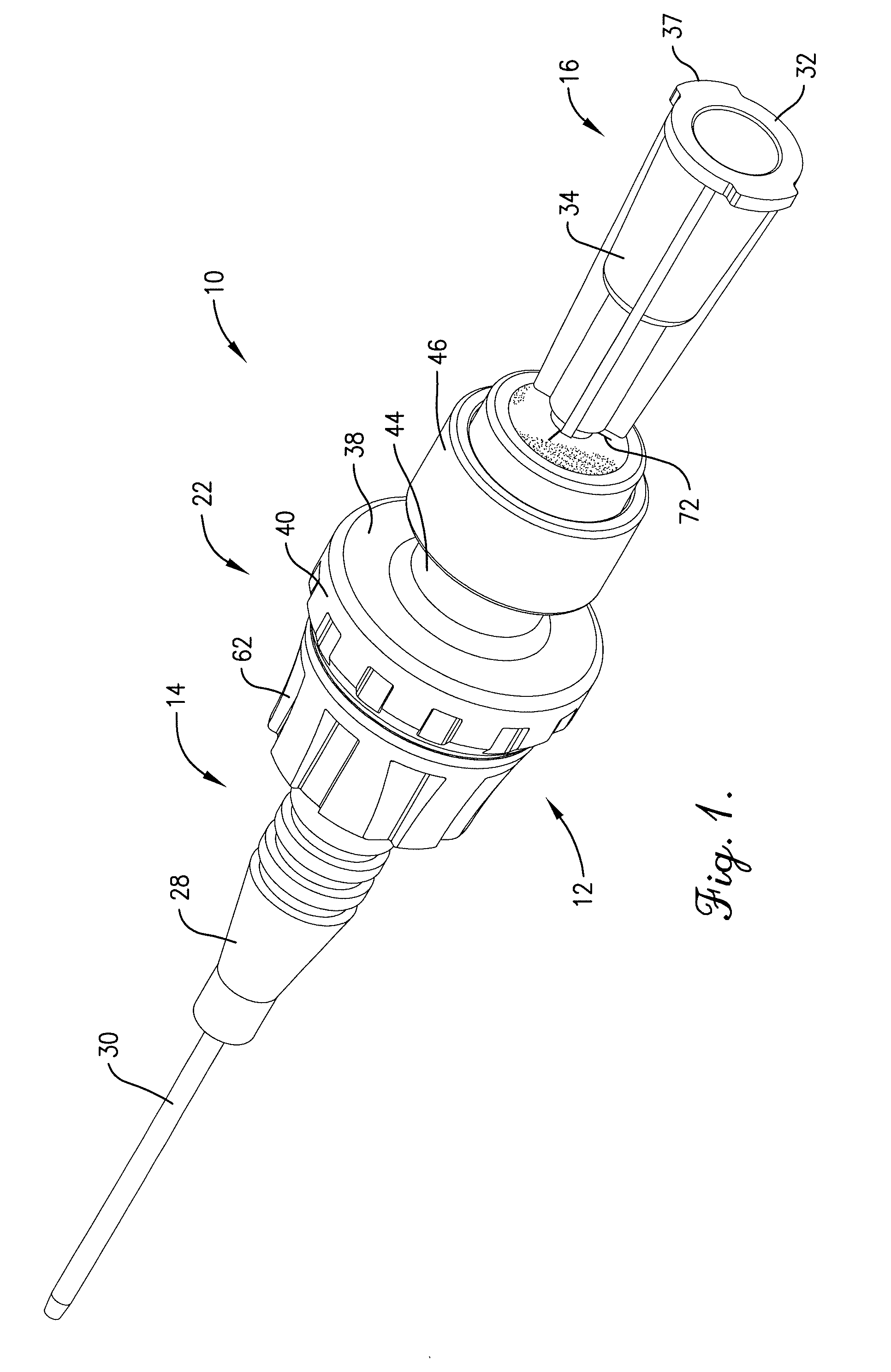
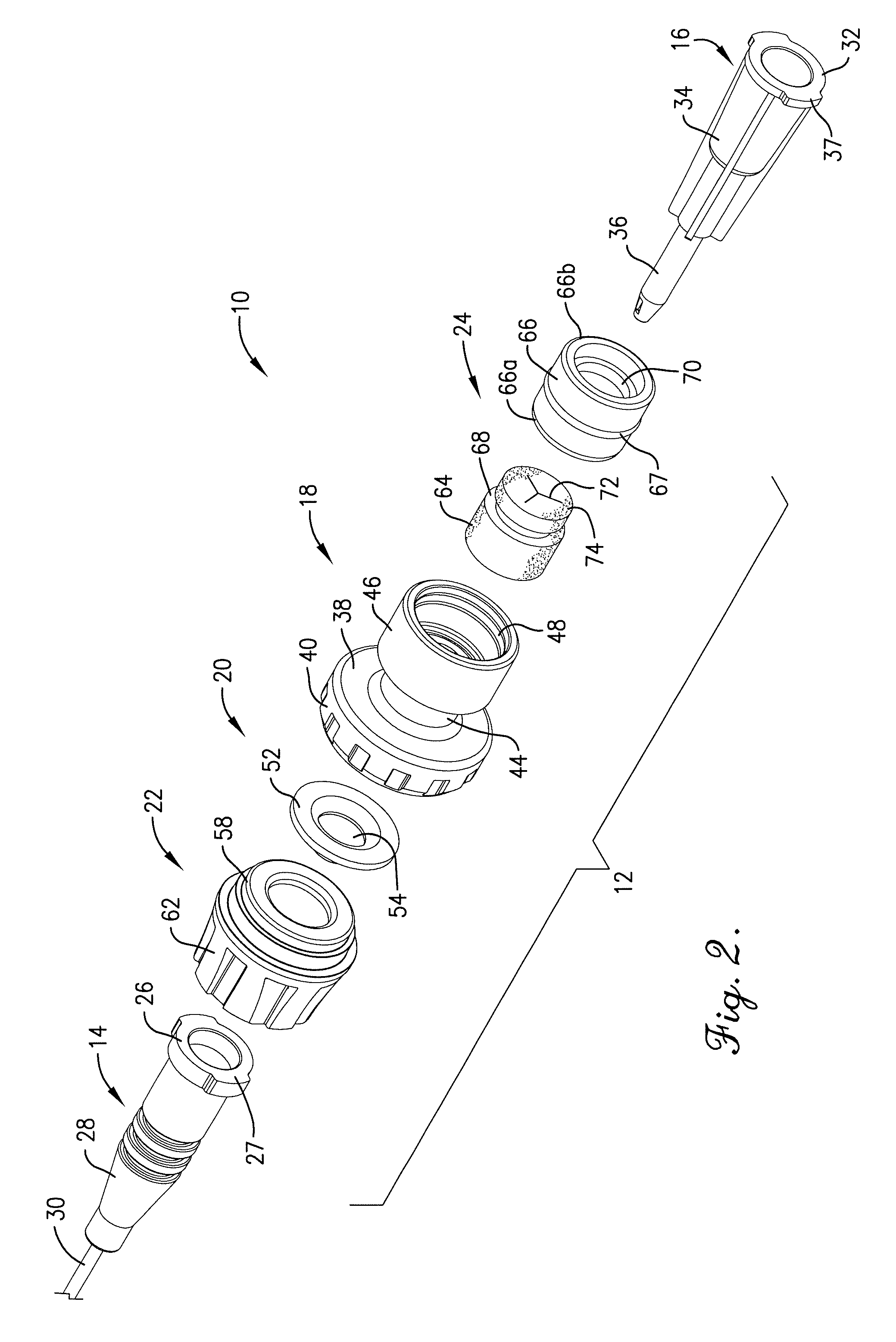
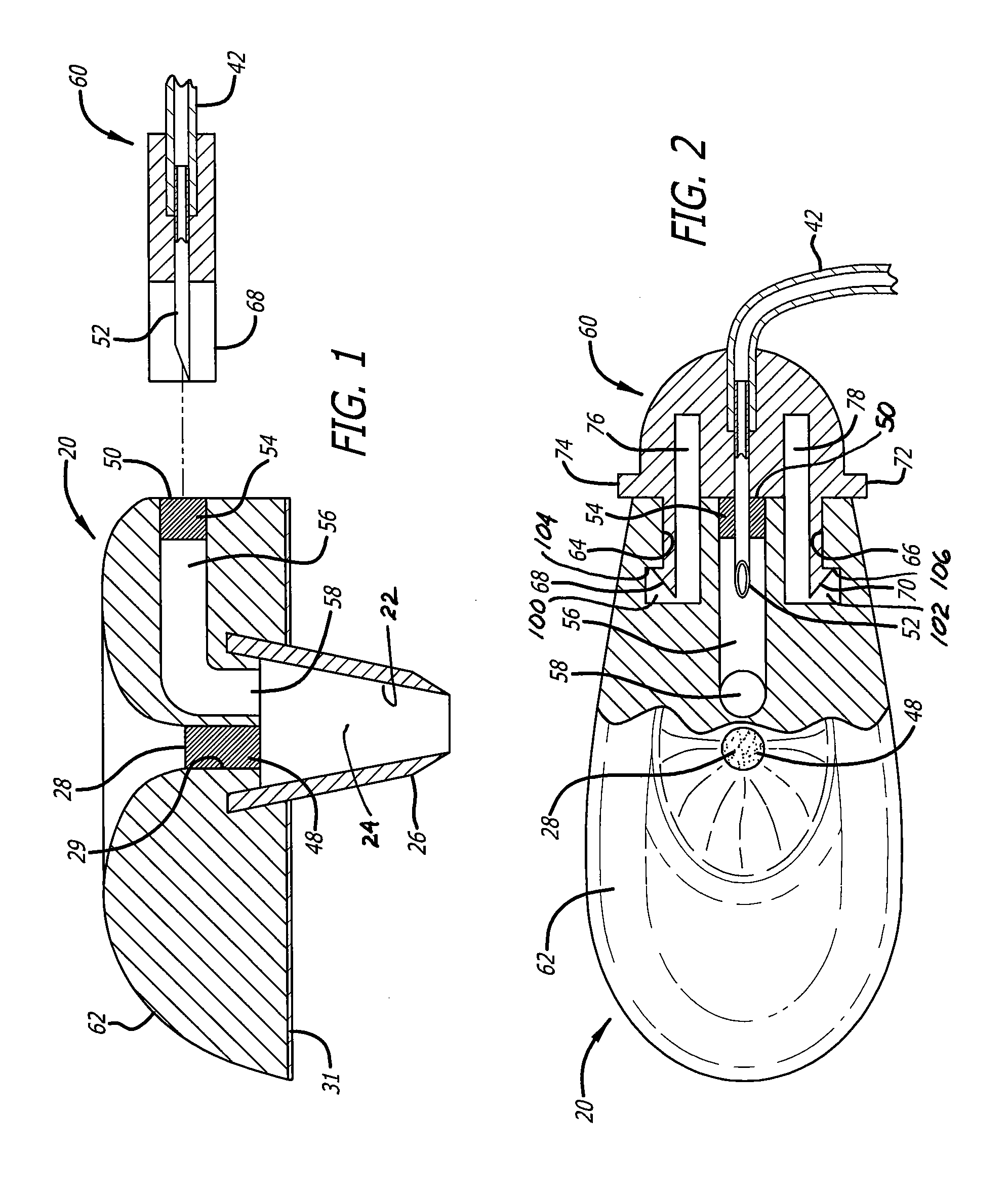
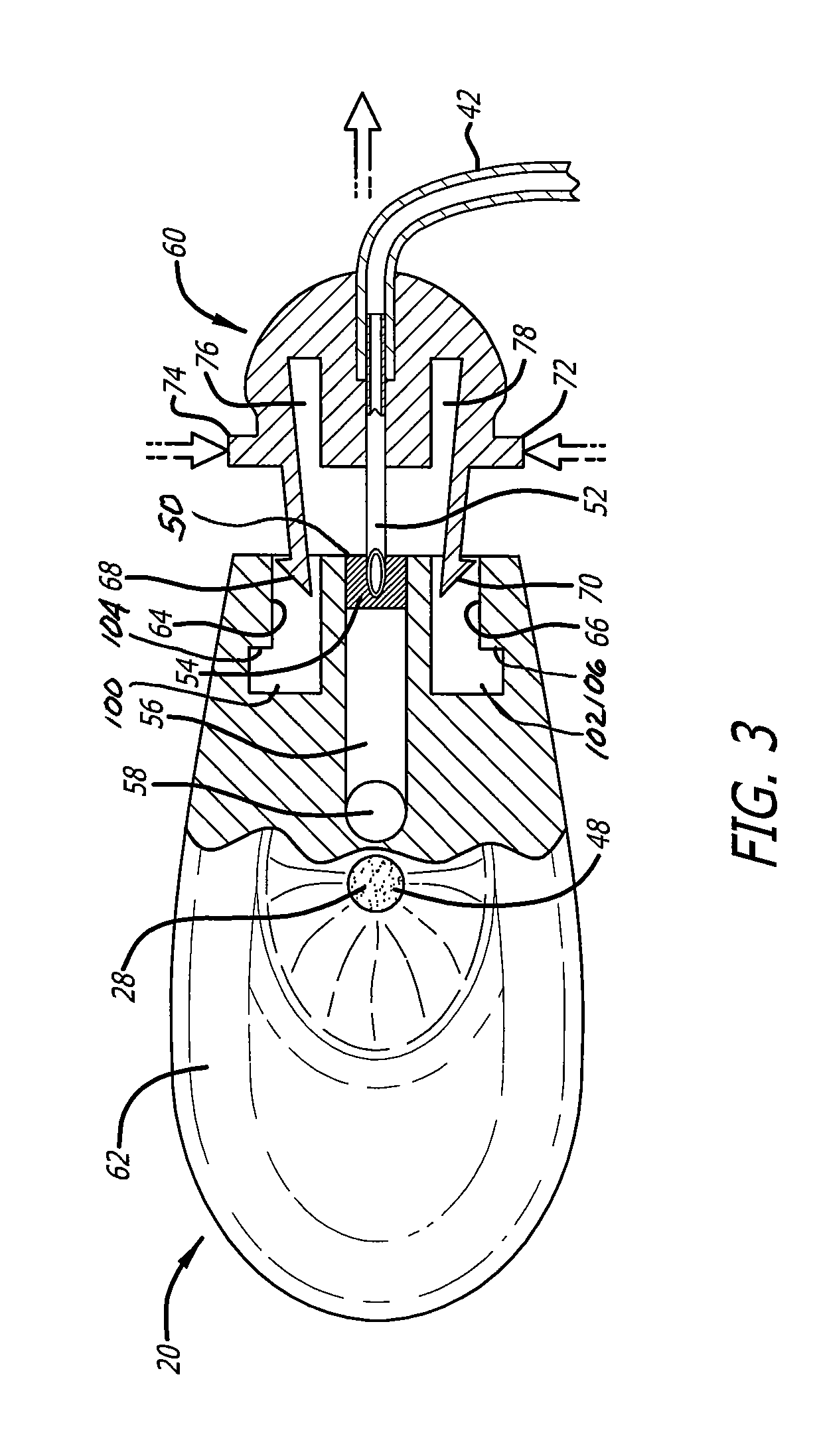
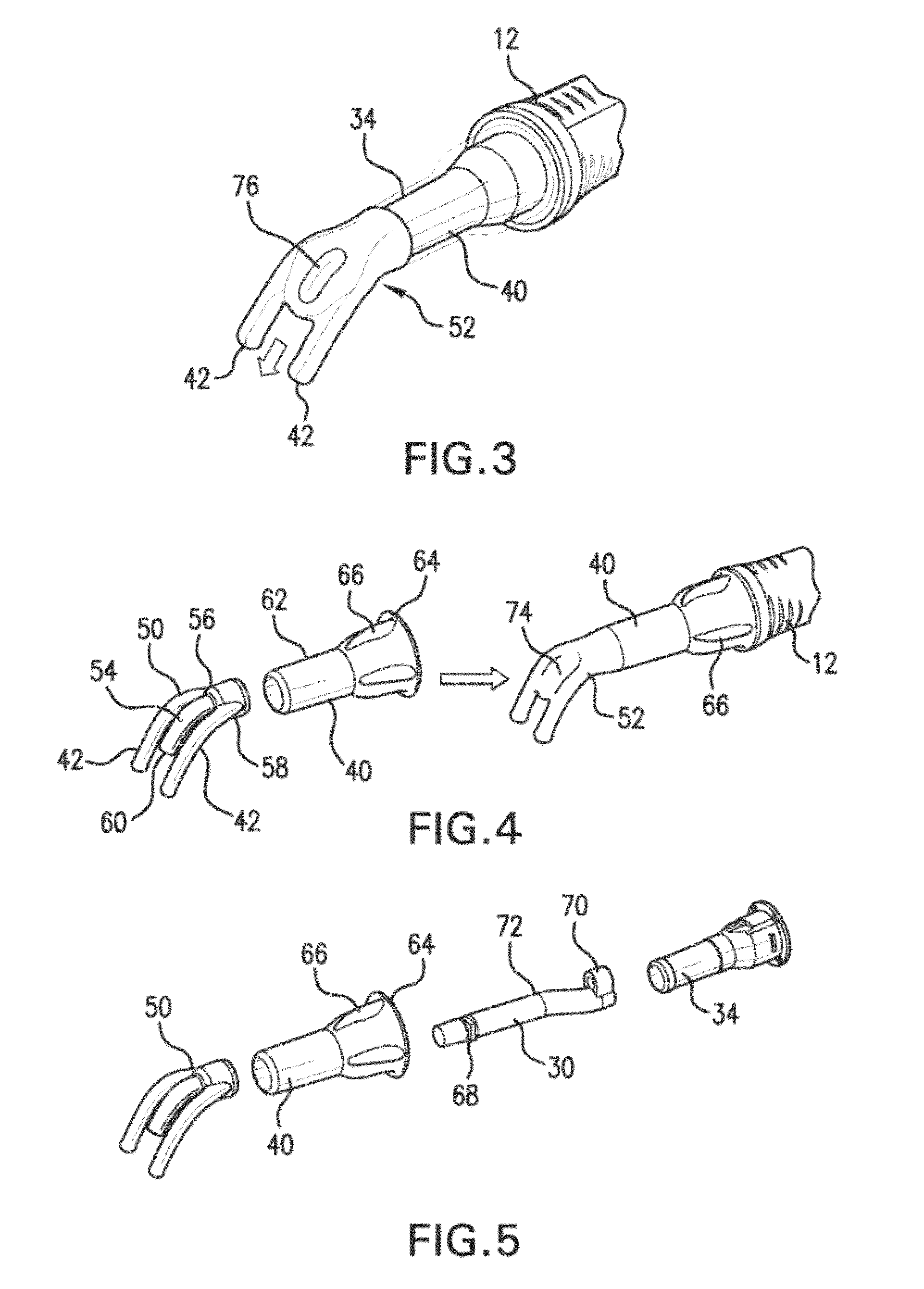
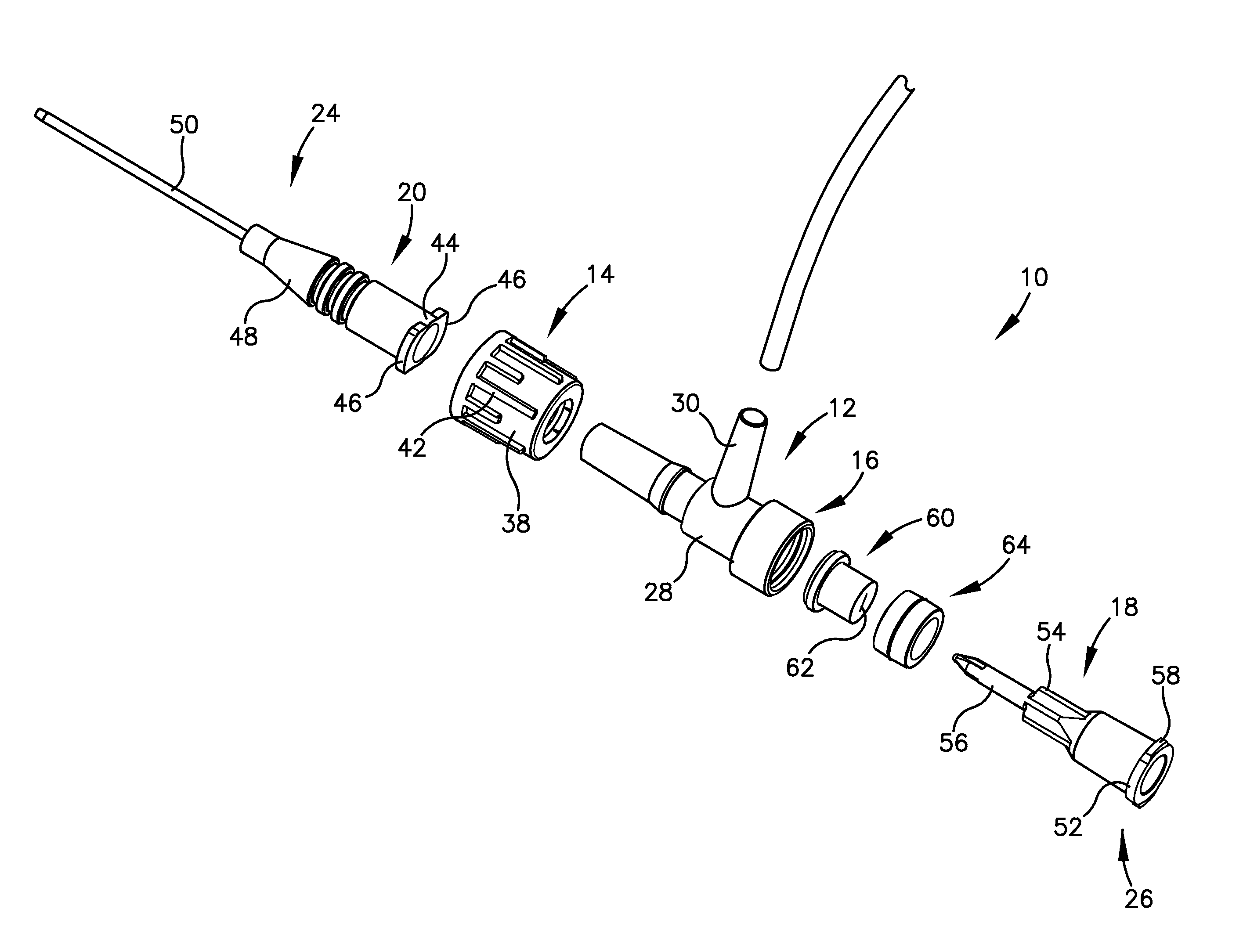
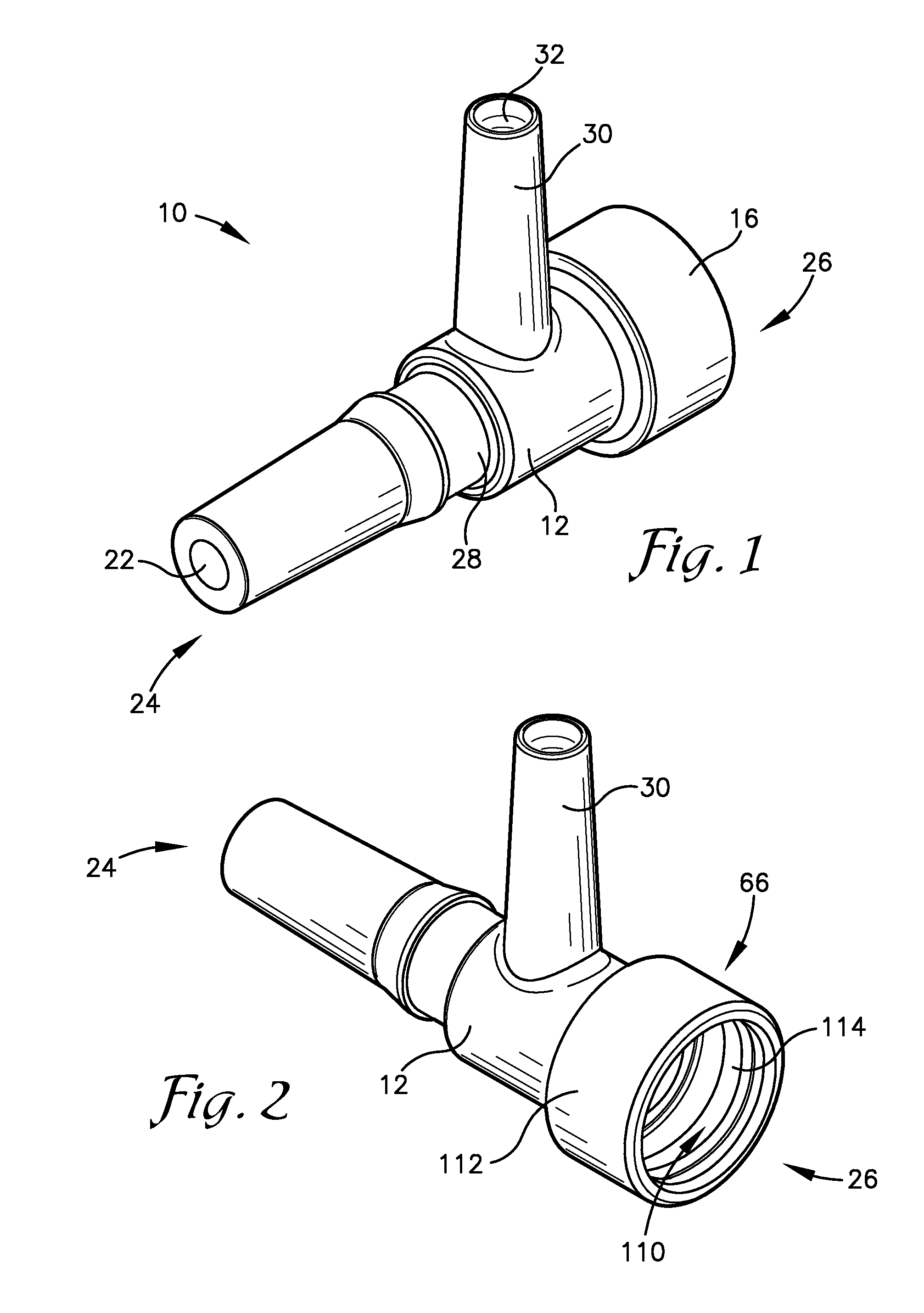
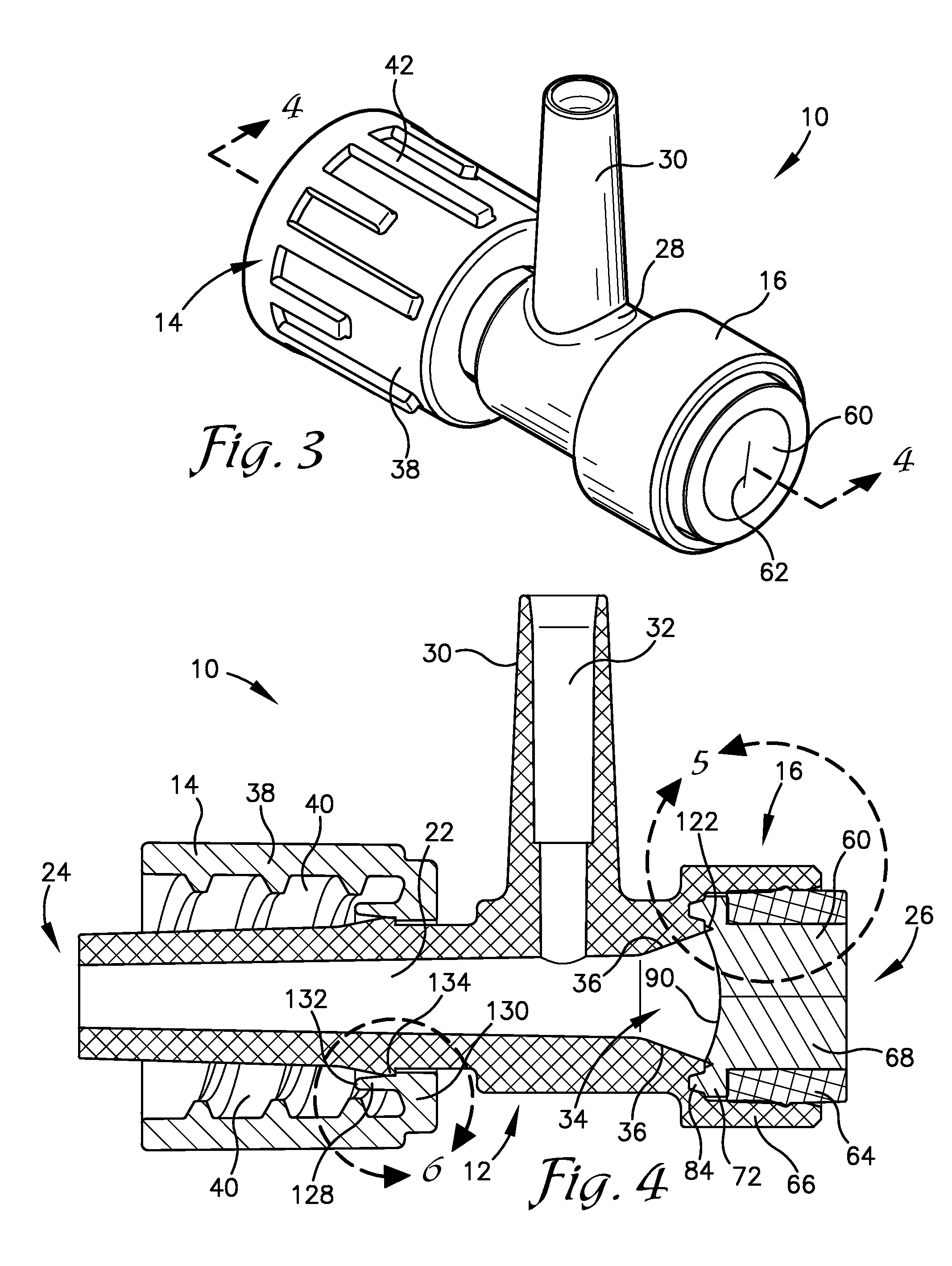
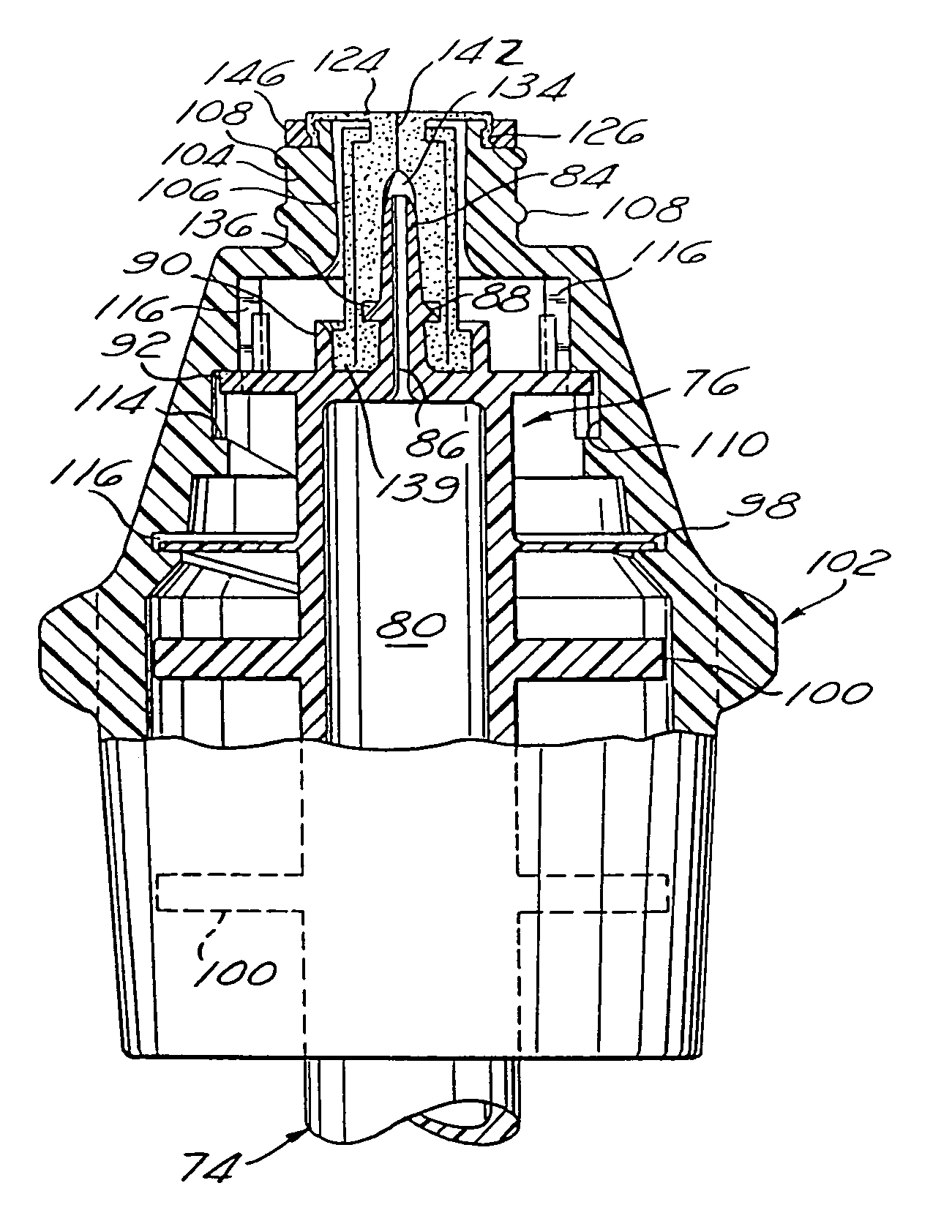
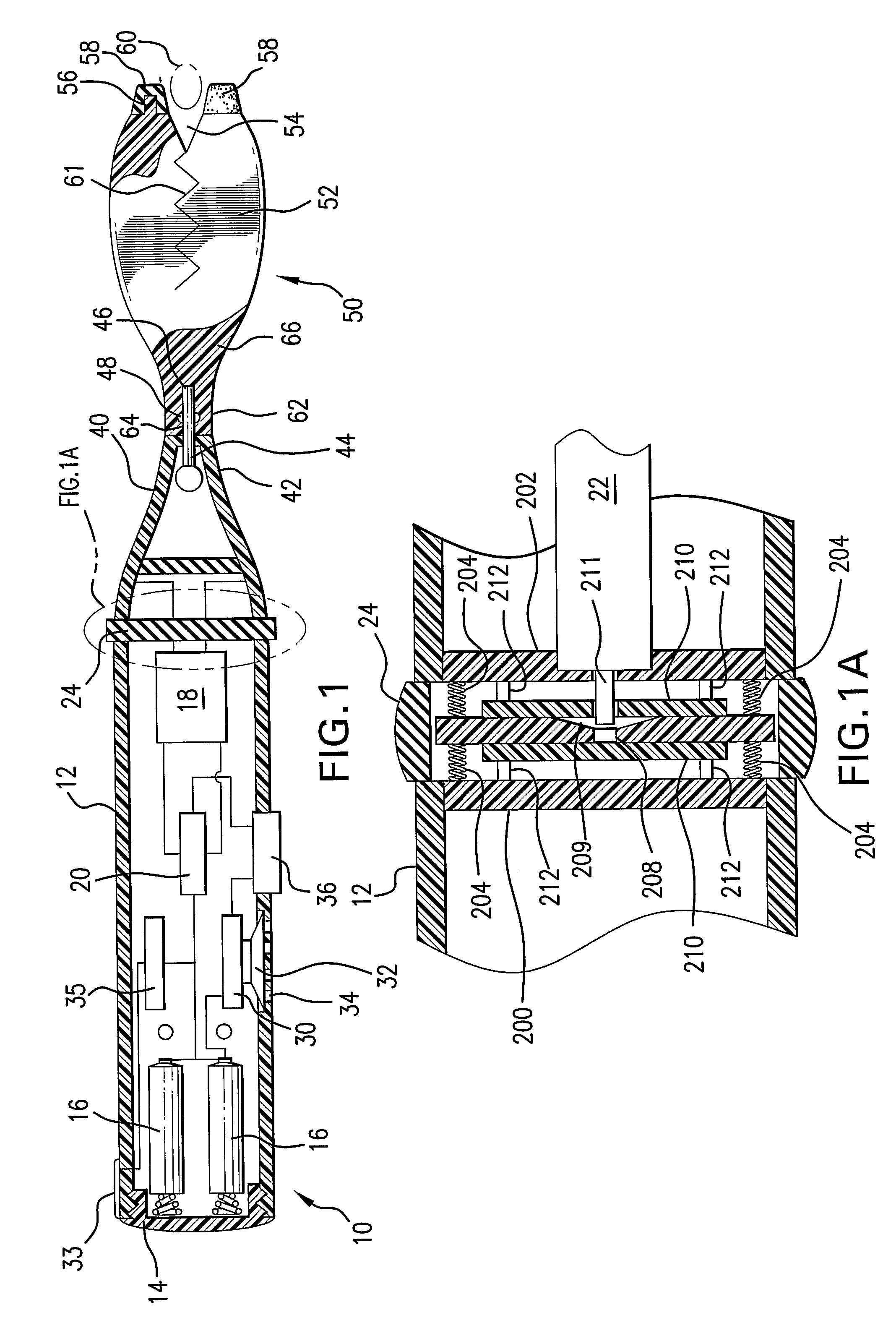
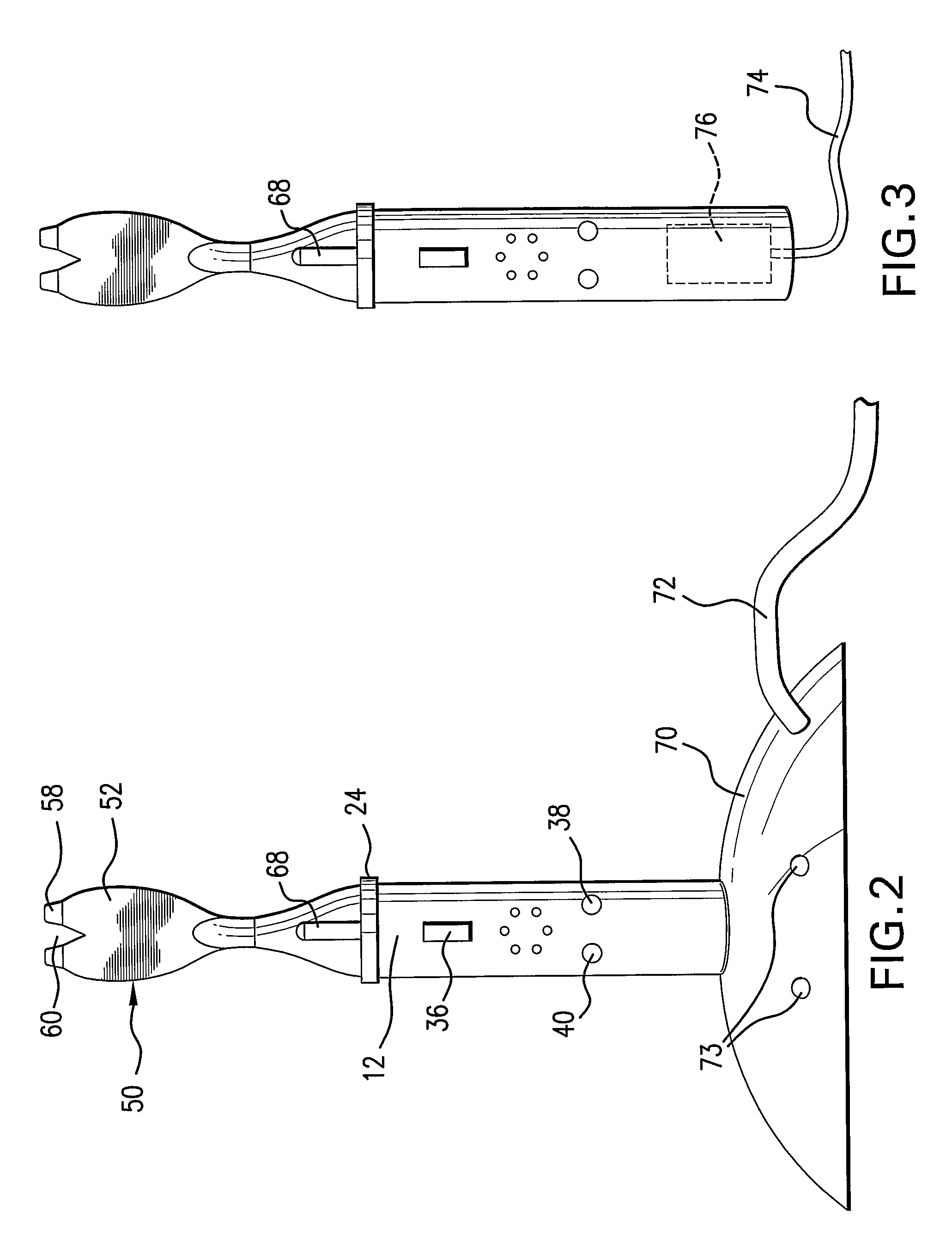
Intravenous injection site with split septum and pressure activated flow control valve
ActiveUS20070225648A1Safety managementRule out the possibilityInfusion syringesCatheterRefluxControl valves
An improved injection site (12) for infusion of parenteral fluids and the like is provided, having a pressure-actuated valve (20) and a novel split septum unit (24), which effectively prevent reflux of blood into the assembly (10). The septum unit (24) includes a resilient split septum body (64) which is precompressed so that the septum body (64) is caused to protrude proximally (78) upon insertion of a cannula (16). Consequently, upon removal of the cannula (16), there is essentially no “drumming” or creation of friction-induced negative pressures sufficient to generate blood reflux. The preloaded septum body (64) also has its proximal surface (74) essentially flush and coplanar with the adjacent proximal end (66b) of the tubular septum holder (66) to enhance the cleanliness of the unit (24). The specialized well (46) and septum unit (24) afford a resilient seal between the periphery of the septum body (64) and the surface (50), and a separate hard-surface seal between the outer margin of the surface (50) and the septum holder (66).
Owner:NEXUS MEDICAL LLC
Reparative cell delivery via hyaluronic acid vehicles
Methods are described for generating autologous tissue grafts, including generating grafts at the point of case, which include isolated cell populations that are enriched with stem cells and are mixed with hyaluronic acid and derivatives thereof. The hyaluronic acid localizes the cells to a desired injection site and stimulates collagen production thus enhancing the viability and the longevity of the graft.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
System and Method for Visualizing Catheter Placement in a Vasculature
A system for advancing a needle through a vasculature to an injection site at the heart of a patient includes a guide catheter with a reflective distal tip. Also included is an imaging unit that is mounted on the catheter to radiate an energy field. Structurally, a distal portion of the catheter is biased to bend into a predetermined configuration that will position the distal end of the catheter for interception by the energy field. If necessary, coincidence of the reflective tip with the energy field is established by moving the energy field along the length of the guide catheter. With coincidence, the reflective tip reflects a signal that is useful for advancement of the needle 34b from the guide catheter and into the injection site.
Owner:DIB ULTRASOUND CATHETER
Prefilled syringe jet injector
ActiveUS20120004608A1Prevent backflowImprove distributionAmpoule syringesJet injection syringesEngineeringPrefilled Syringe
Owner:ANTARES PHARMA INC
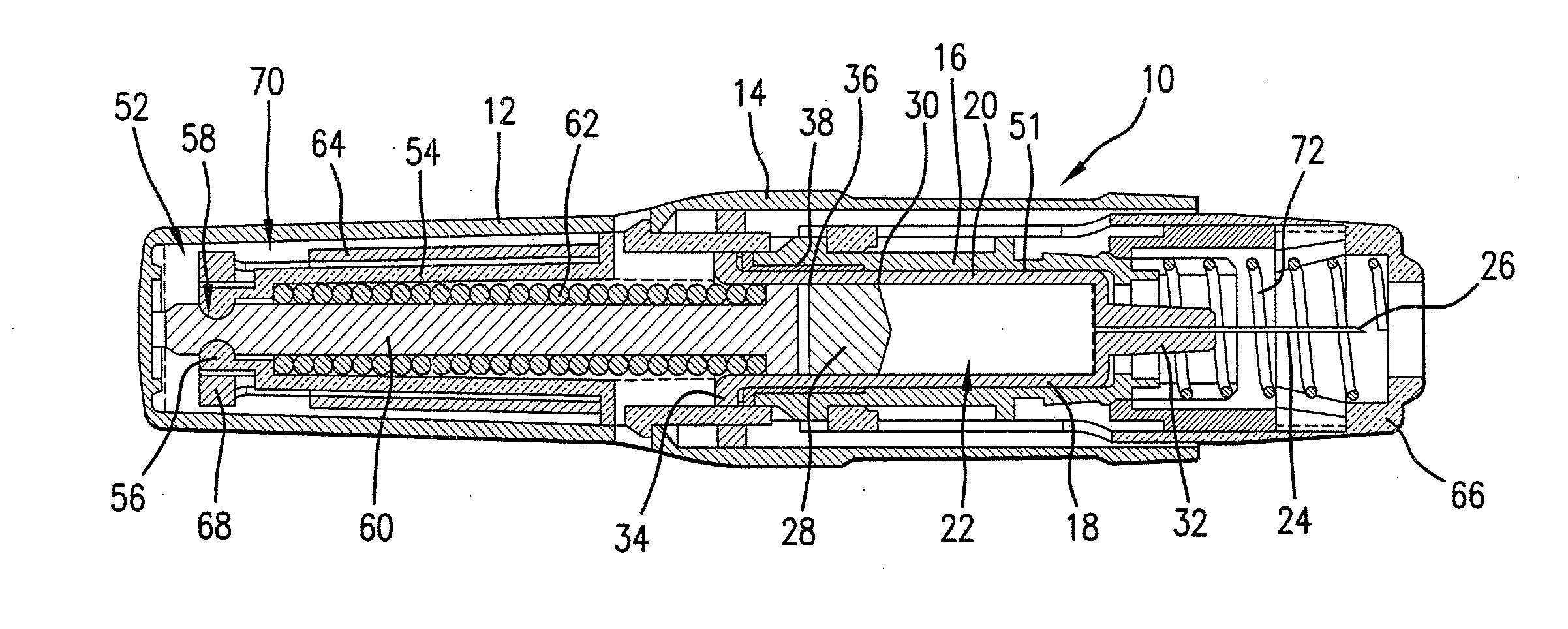
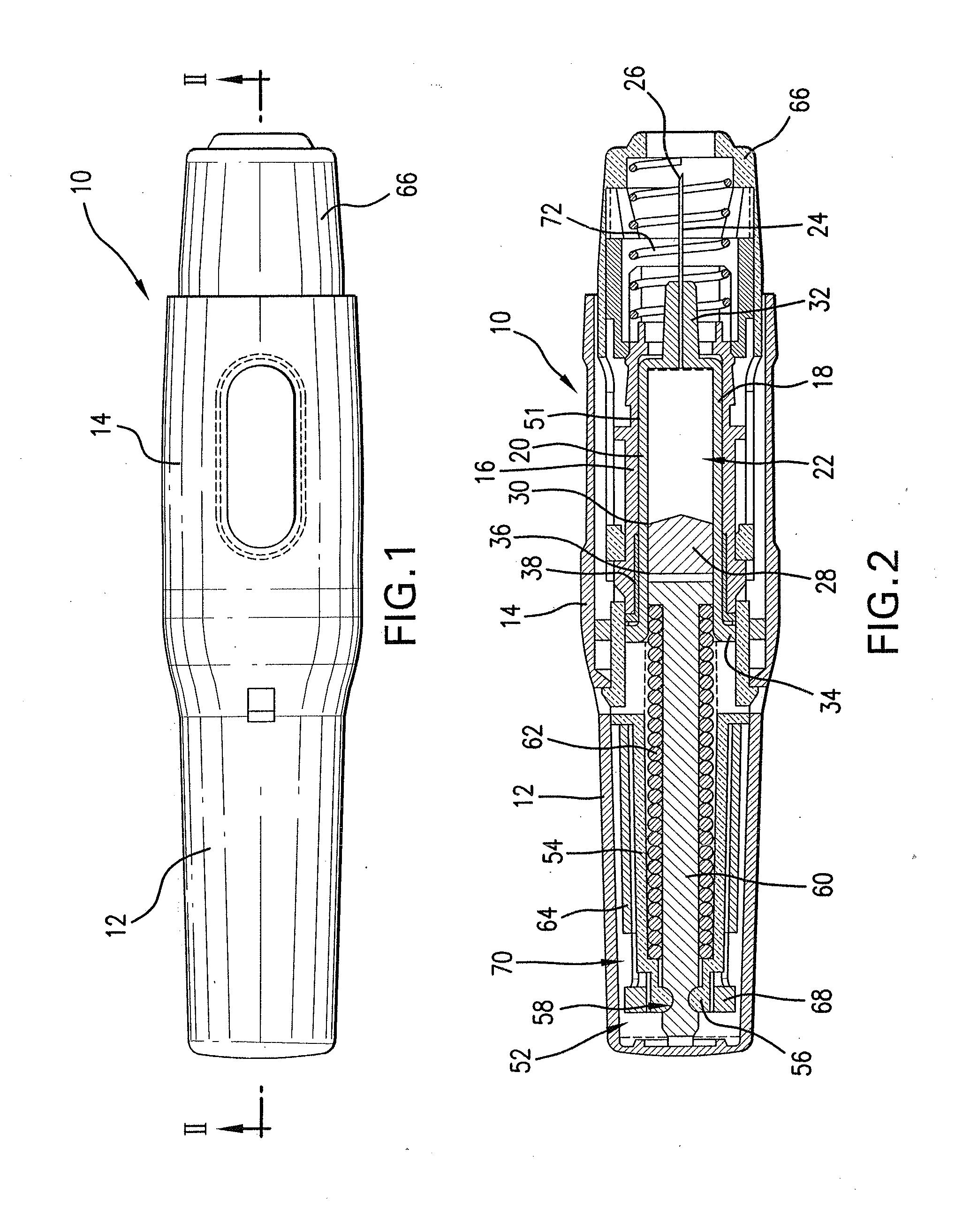
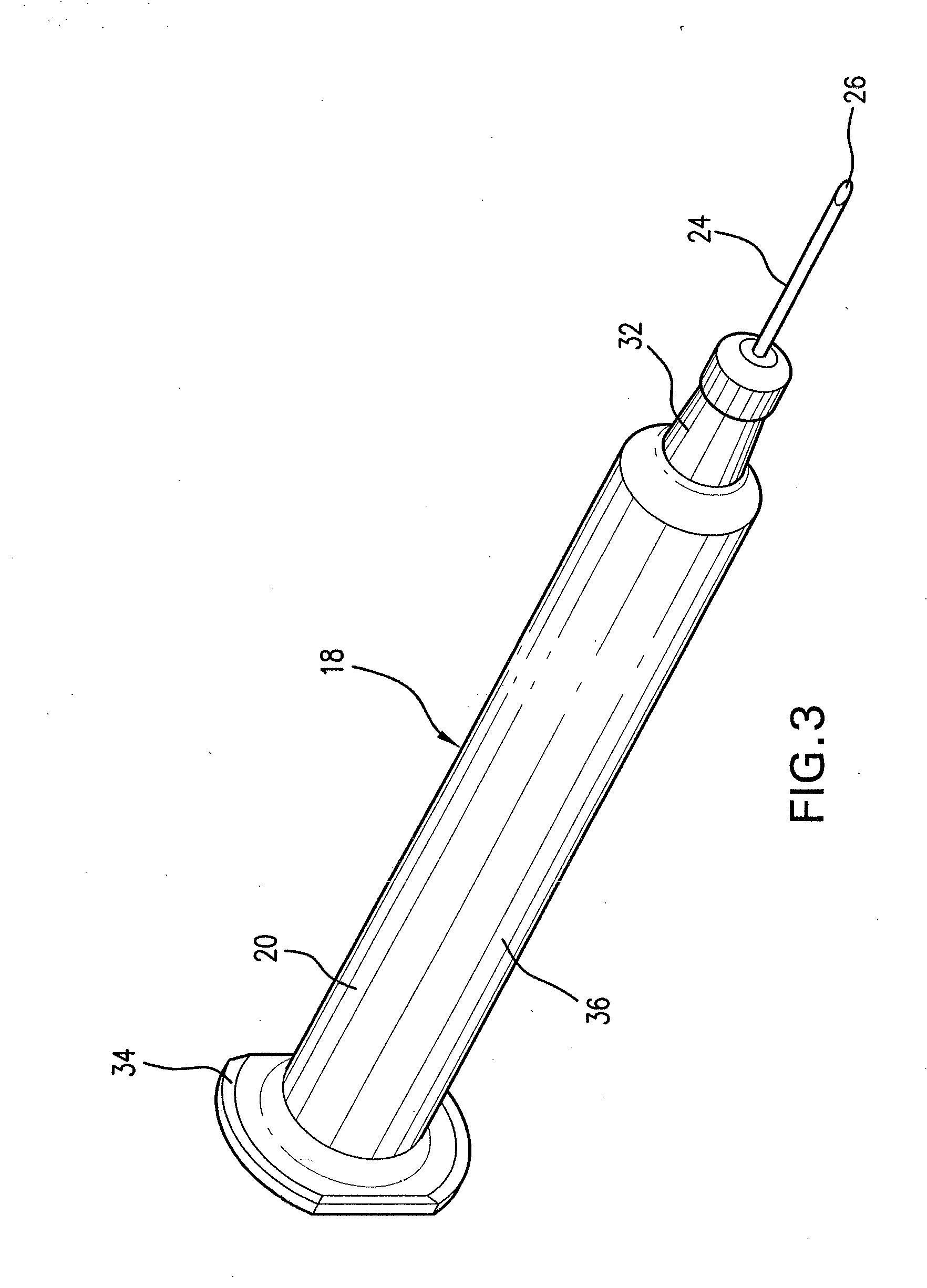
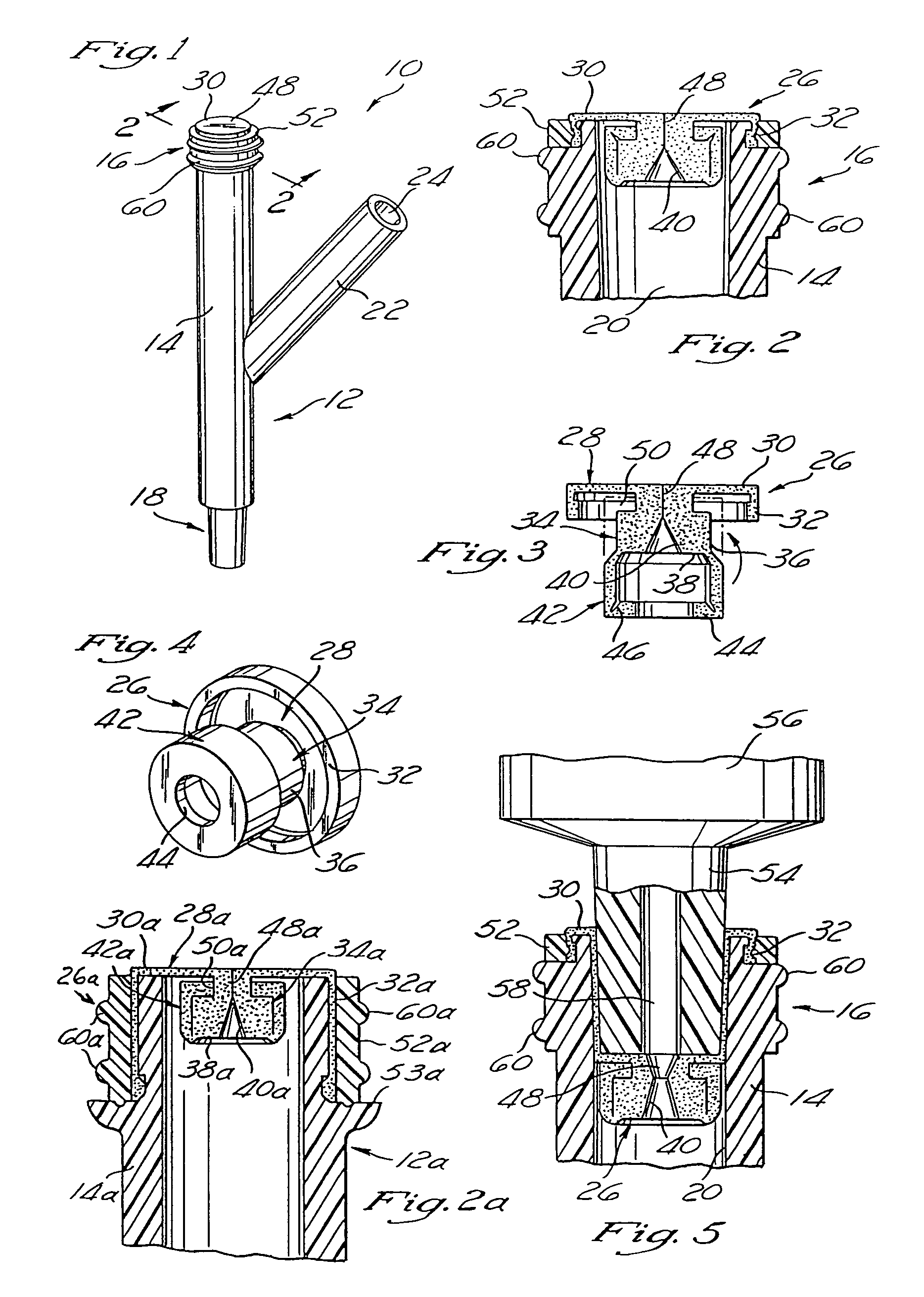
Medicated module having a double needle guard
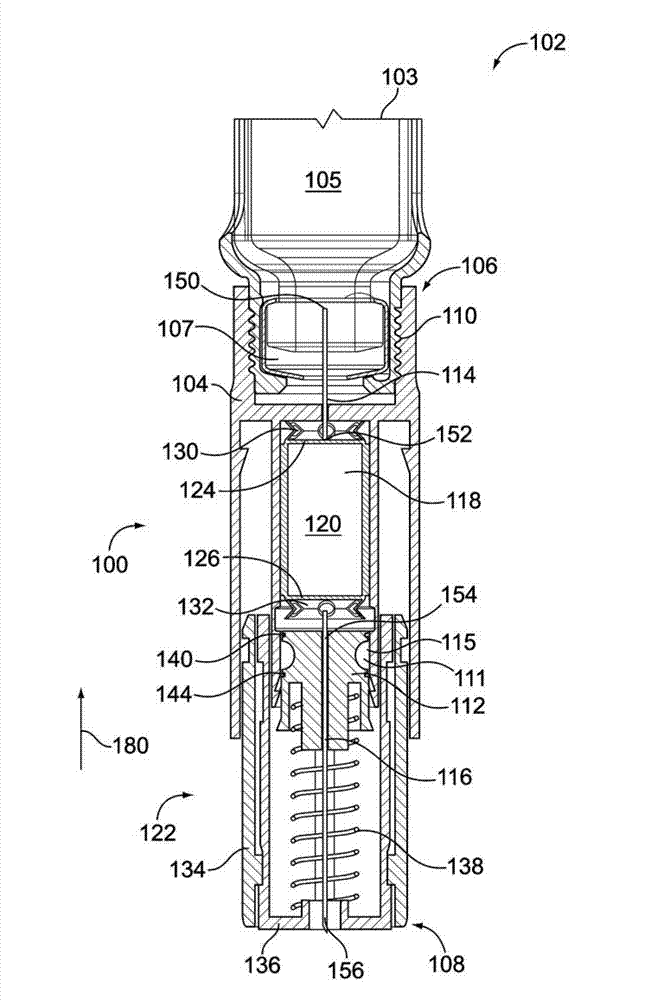
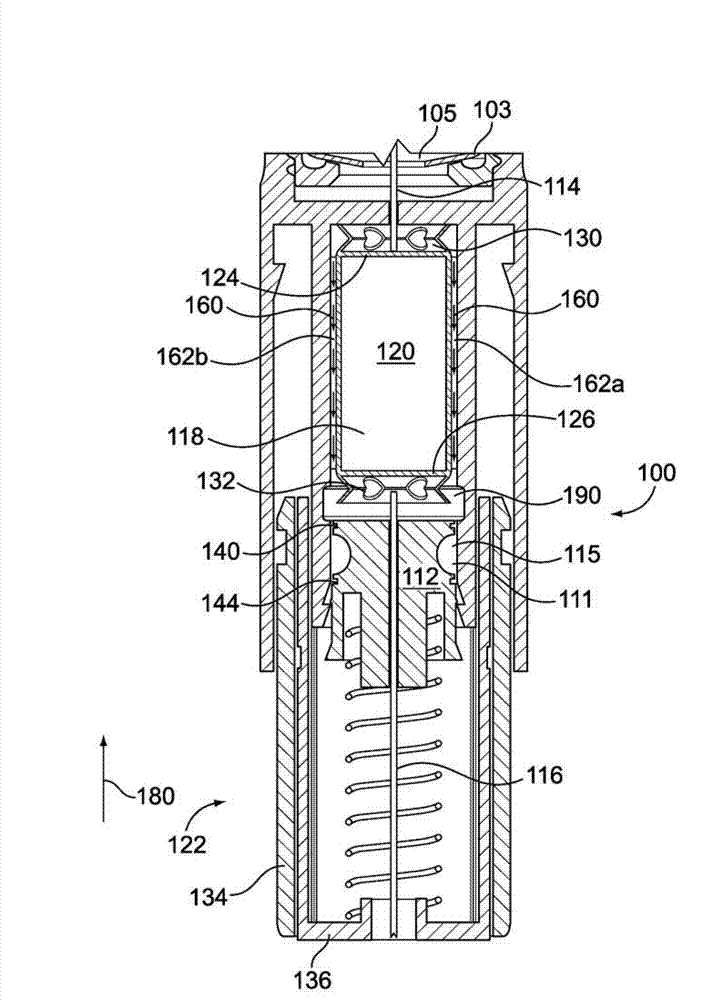
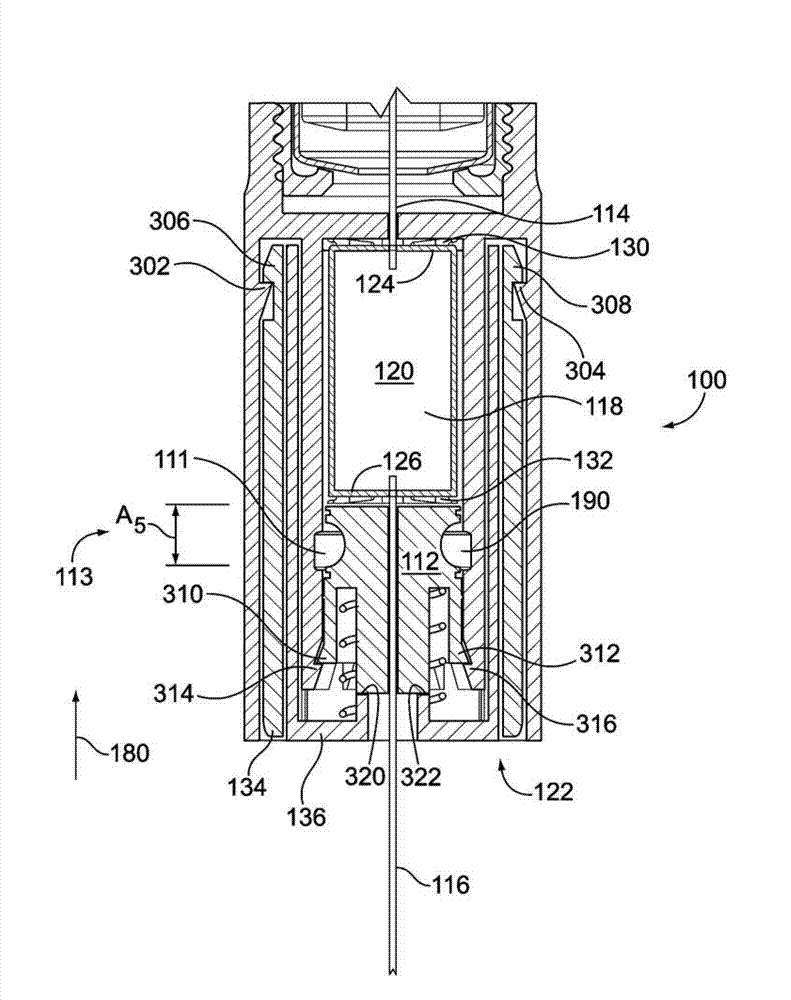
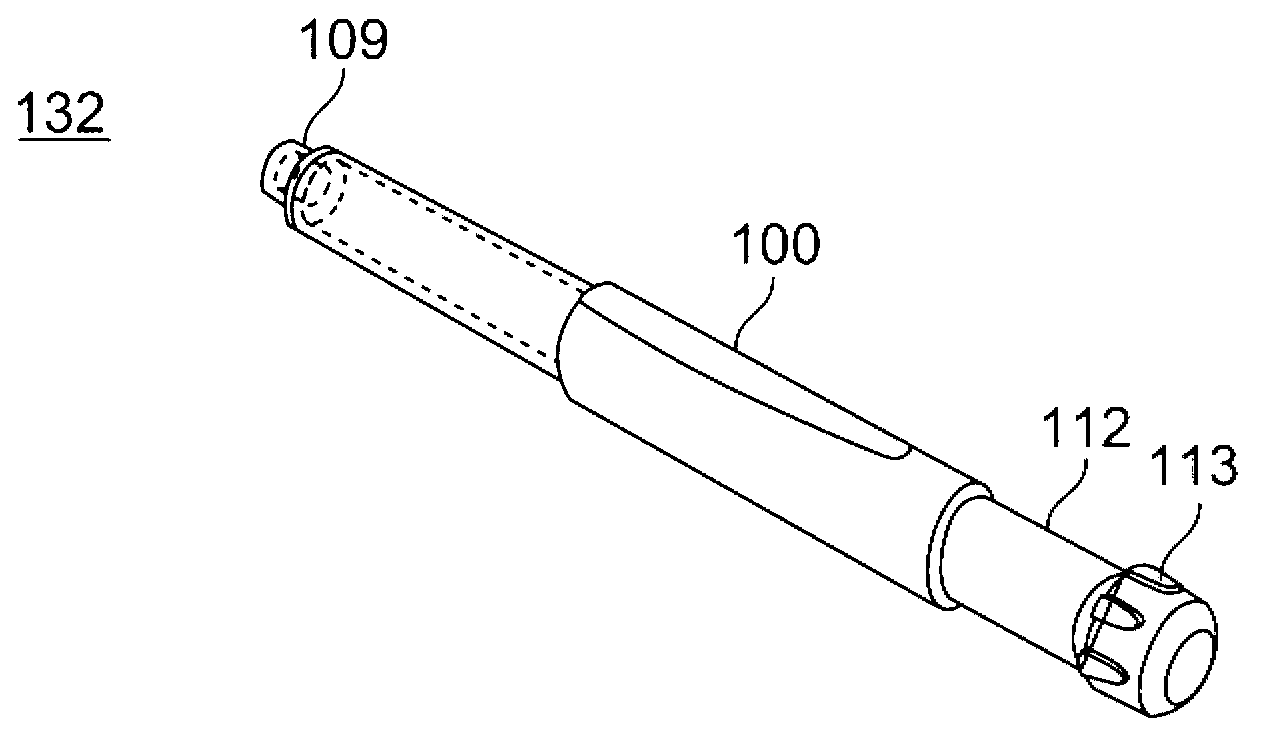
A medicated module (100) is attachable to a drug delivery device (102). The medicated module (100) includes a housing (104) having a proximal end and a distal end, the proximal end has a connector (110) configured for attachment to a drug delivery device (102). The distal end contains a shuttle (112). A first needle (114) is fixed in the housing (104) and a second needle (116) is fixed in the shuttle (112). The medicated module (100) also includes a reservoir (118) in the housing (104) comprising a medicament (12). Further, the medicated module (100) includes a guard assembly (122) positioned in the housing (104) and configured to move in an axial direction during application to an injection site. Preferably, the medicated module (100) is locked out after at least two injections of the medicament (105) contained in the drug delivery device (102).
Owner:SANOFI AVENTIS DEUTSCHLAND GMBH
Push rod activated medicated module
InactiveCN102834133AEasy to setEasy ShippingAmpoule syringesMedical devicesInjection siteNeedle guard
A medicated module for an injection system to co-deliver at least two medicaments is disclosed. The drug delivery device includes a push rod operably connected to a dose dial button. The medicated module includes a housing having a connector configured for attachment to the drug delivery device, and the housing is configured to receive a portion of the push rod when the medicated module is attached to the drug delivery device. The medicated module further includes a reservoir in the housing comprising a single dose of a medicament and a needle guard operably connected to the housing and configured to move in an axial direction during application to an injection site. Still further, the medicated module includes a sleeve in the housing configured to move axially in the housing and a locking collar in the housing configured to move axially and rotationally in the housing.
Owner:SANOFI AVENTIS DEUT GMBH
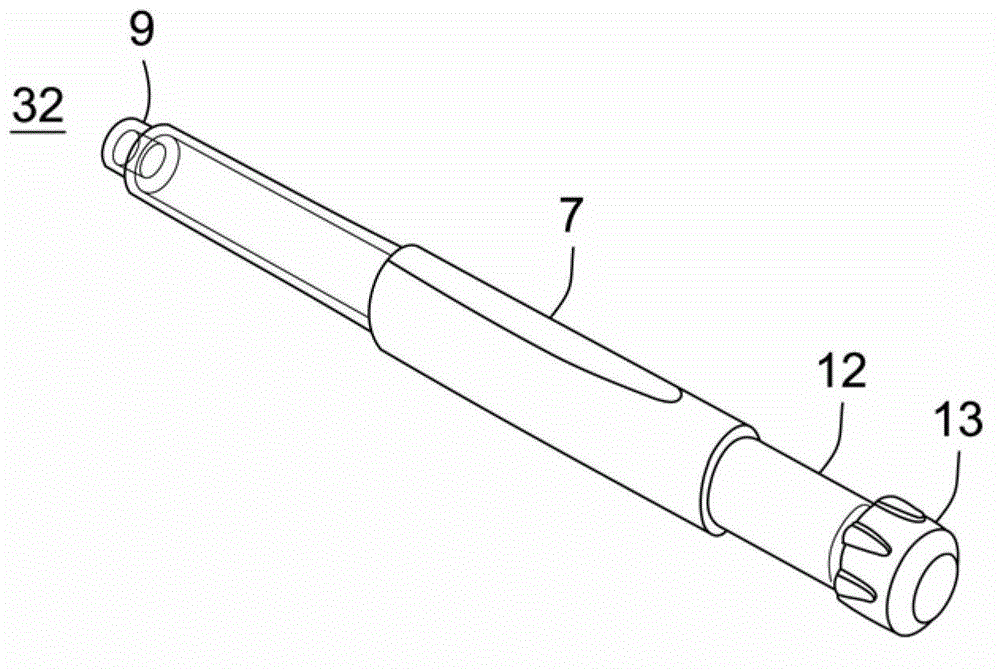
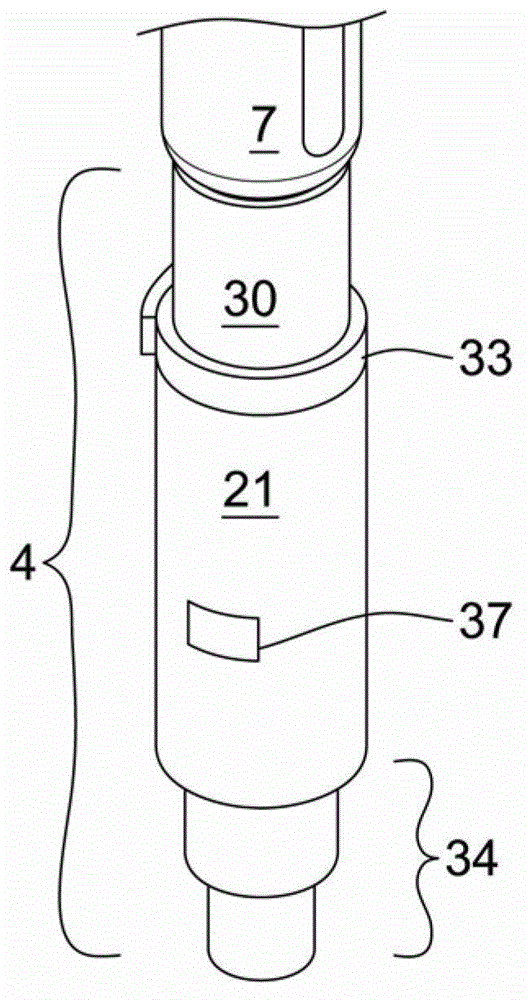
Medicated module with dual safety guards
InactiveCN102753224AEasy to setEasy to distributeAmpoule syringesMedical devicesNeedle guardInjections sites
A medicated module (4) for an injection system to co-deliver at least two medicaments is disclosed, where a drug delivery device (7) containing a primary medicament accepts a medicated module (4) containing a single dose of a second medicament (2) and where both medicaments are delivered through a single hollow needle (3). The medicated module (4) comprises two housings (21, 30) removably connected to each other, where each housing (21, 30) contains a sterile needle (3, 5) and needle needle guard assembly (34, 49) that locks out after the needle (3, 5) is inserted in an injection site.
Owner:SANOFI AVENTIS DEUT GMBH
Intravenous injection site with split septum and pressure activated flow control valve
ActiveUS8211089B2Safety managementRule out the possibilityMedical devicesCatheterRefluxVein injection
An improved injection site (12) for infusion of parenteral fluids and the like is provided, having a pressure-actuated valve (20) and a novel split septum unit (24), which effectively prevent reflux of blood into the assembly (10). The septum unit (24) includes a resilient split septum body (64) which is precompressed so that the septum body (64) is caused to protrude proximally (78) upon insertion of a cannula (16). Consequently, upon removal of the cannula (16), there is essentially no “drumming” or creation of friction-induced negative pressures sufficient to generate blood reflux. The preloaded septum body (64) also has its proximal surface (74) essentially flush and coplanar with the adjacent proximal end (66b) of the tubular septum holder (66) to enhance the cleanliness of the unit (24). The specialized well (46) and septum unit (24) afford a resilient seal between the periphery of the septum body (64) and the surface (50), and a separate hard-surface seal between the outer margin of the surface (50) and the septum holder (66).
Owner:NEXUS MEDICAL LLC
Injection port device adapted for use with insulin pump
An injection port comprises a body having an outlet delivery cannula configured to pierce subcutaneous tissue of a patient to deliver medication. The port includes multiple injection sites, each of which can be used multiple times, and each of which is sealed. Each injection port is configured to temporarily receive a delivery device and reseal itself when the delivery device is removed. In one embodiment, a seal includes a pierceable stationary septum that reseals itself when a sharp cannula is removed. One injection site also includes locking features to receive a locking device of a delivery device and temporarily lock the delivery device in place at the injection site. The locking device may later be unlocked when delivery is complete and removed. In one application, a diabetic may use one injection site for syringes during the day and the locking injection site for a insulin pump during sleeping hours.
Owner:ABBOTT DIABETES CARE INC
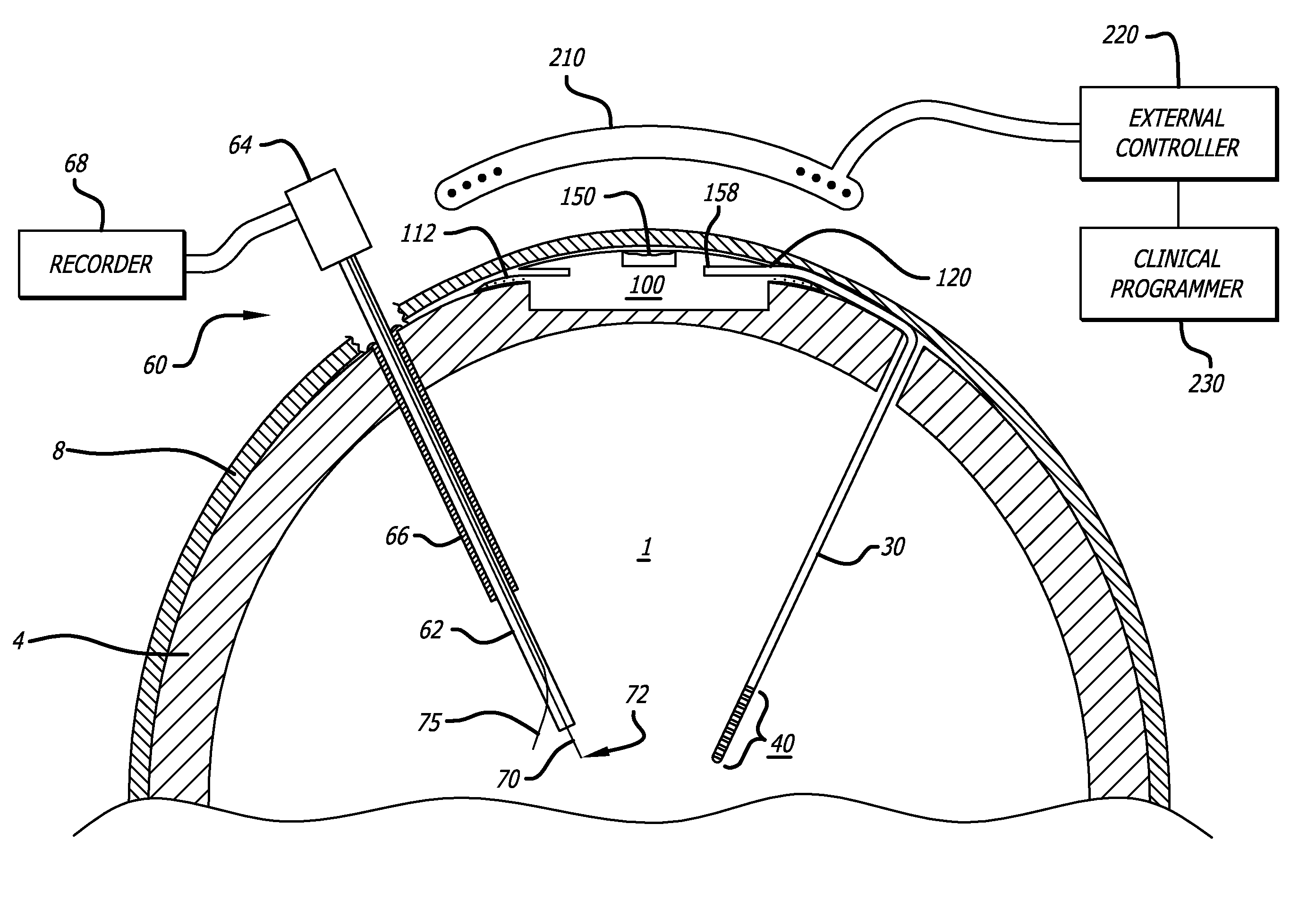
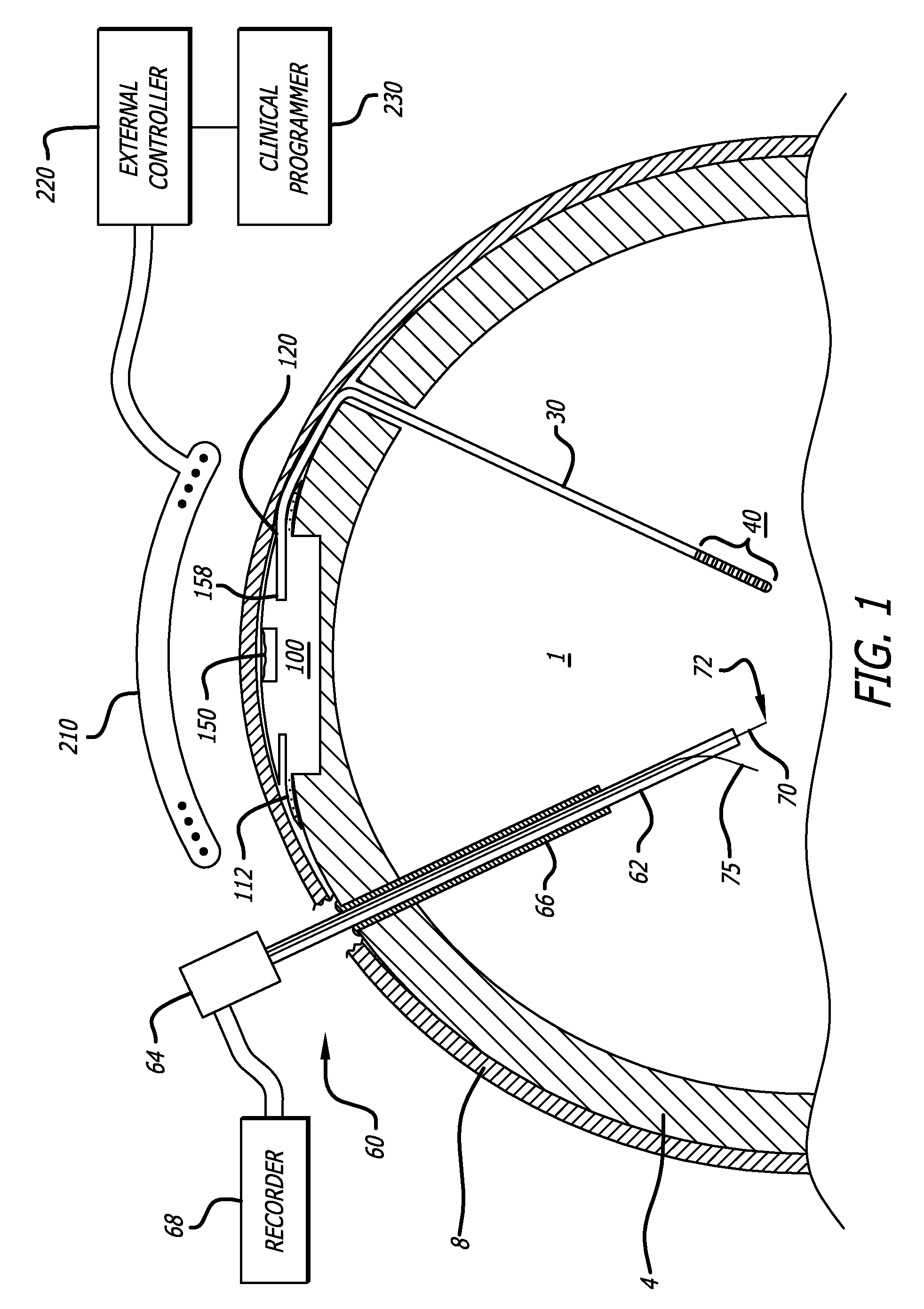
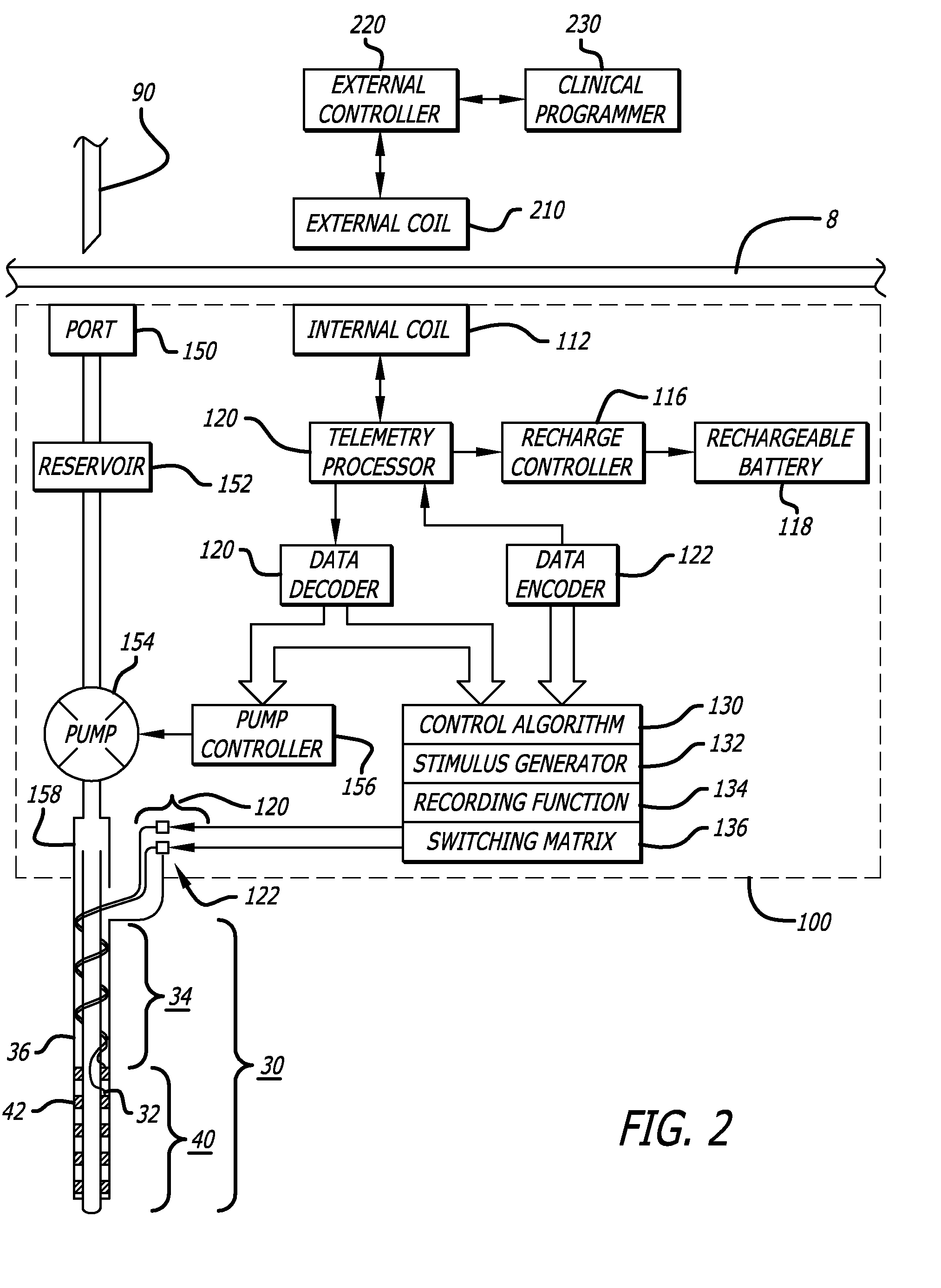
Probe for Identifying Injection Site for Deep Brain Neural Prostheses
InactiveUS20070118197A1Flexible programmingLong-term useHead electrodesExternal electrodesChemical treatmentDisease
Devices and systems for recording and / or stimulating electrical signals in order to identify a target site within a patient's brain for further electrical stimulation and chemical treatments of the brain. The deep brain stimulation devices and methods include implantable devices having various microelectrode configurations and drug delivery mechanisms. The devices can be used to treat a variety of neurological conditions.
Owner:UNIV OF SOUTHERN CALIFORNIA
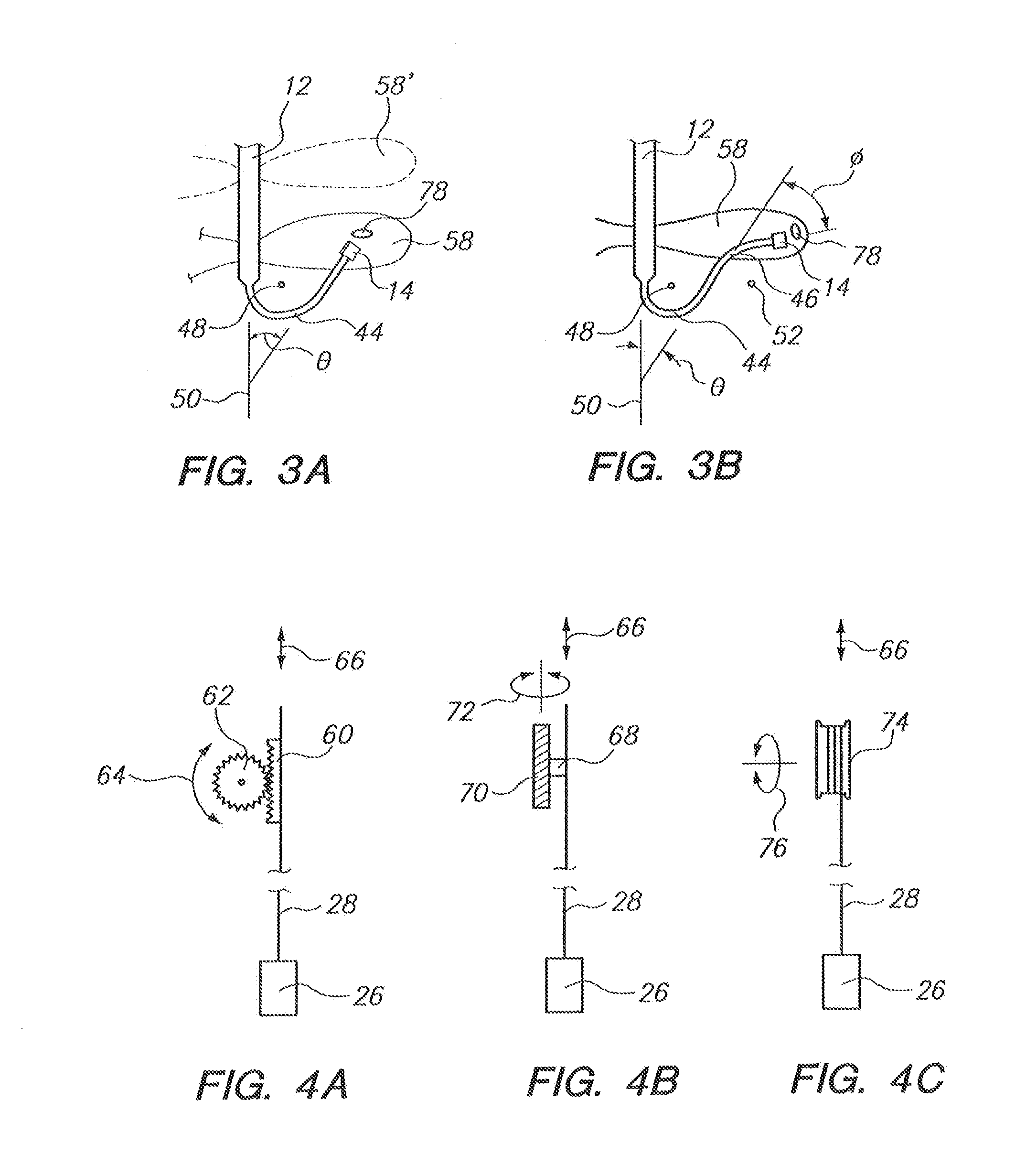
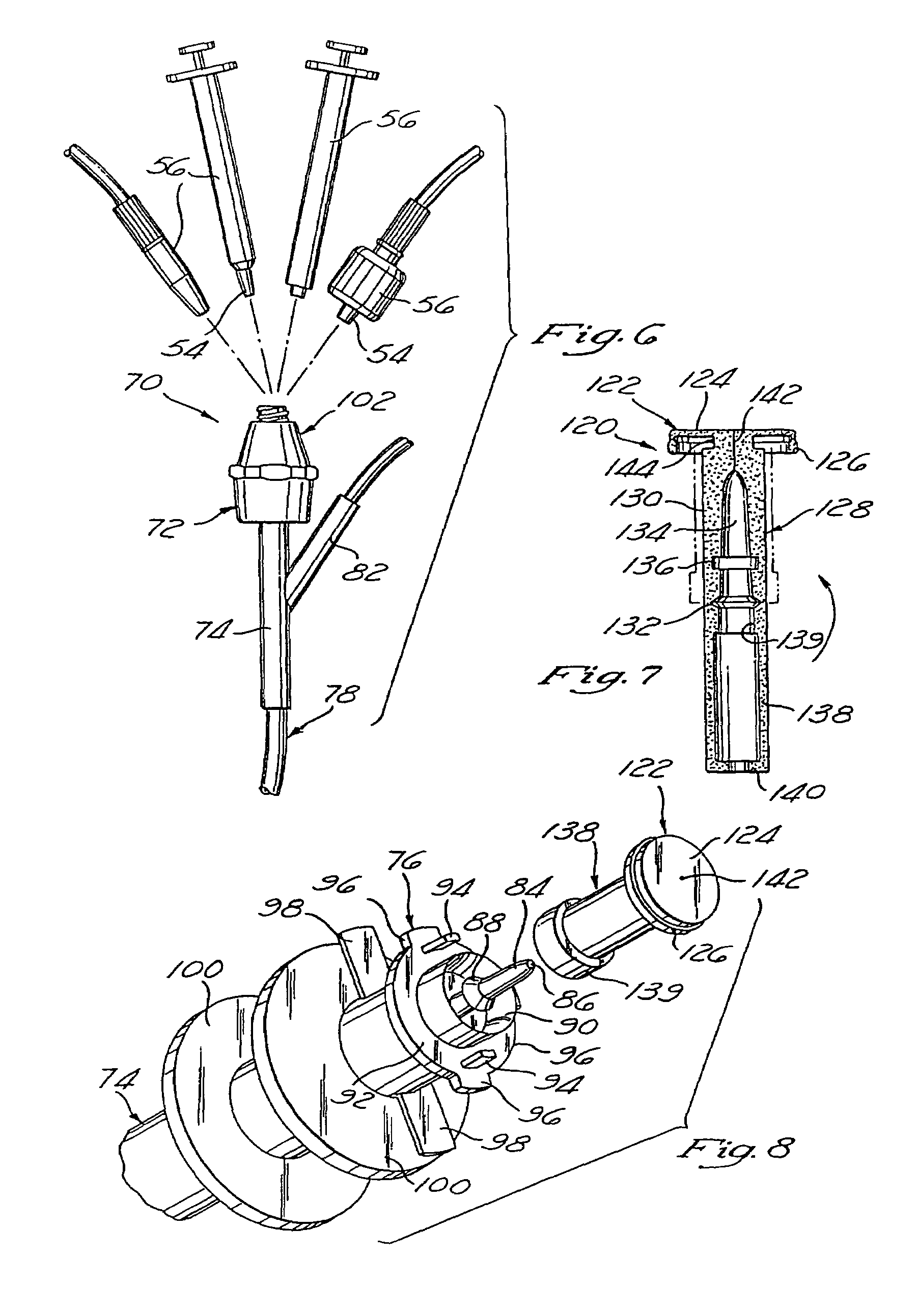
Apparatus, article and method for reducing pain during skin puncturing procedures
ActiveUS20120016292A1Easily and inexpensively utilizedOvercome disadvantagesGum massageAnaesthesiaInjections sitesSingle use
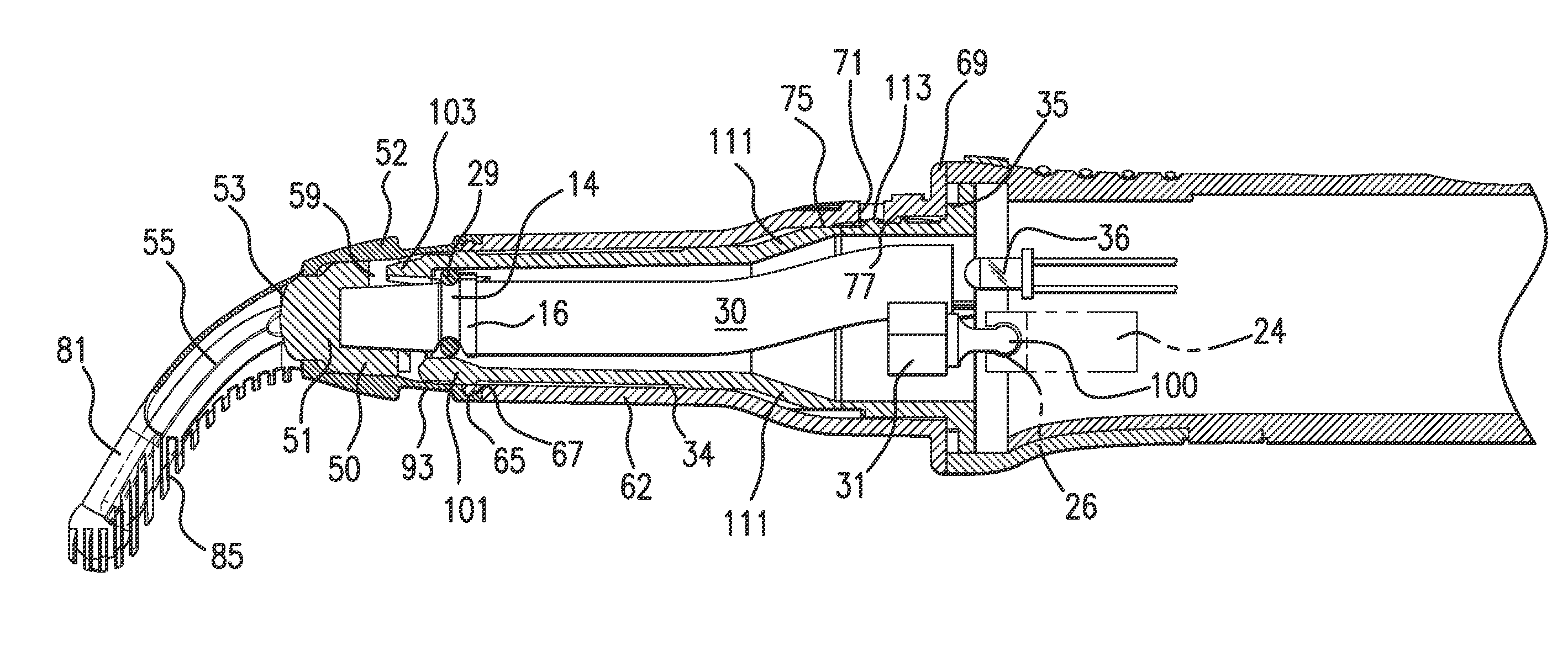
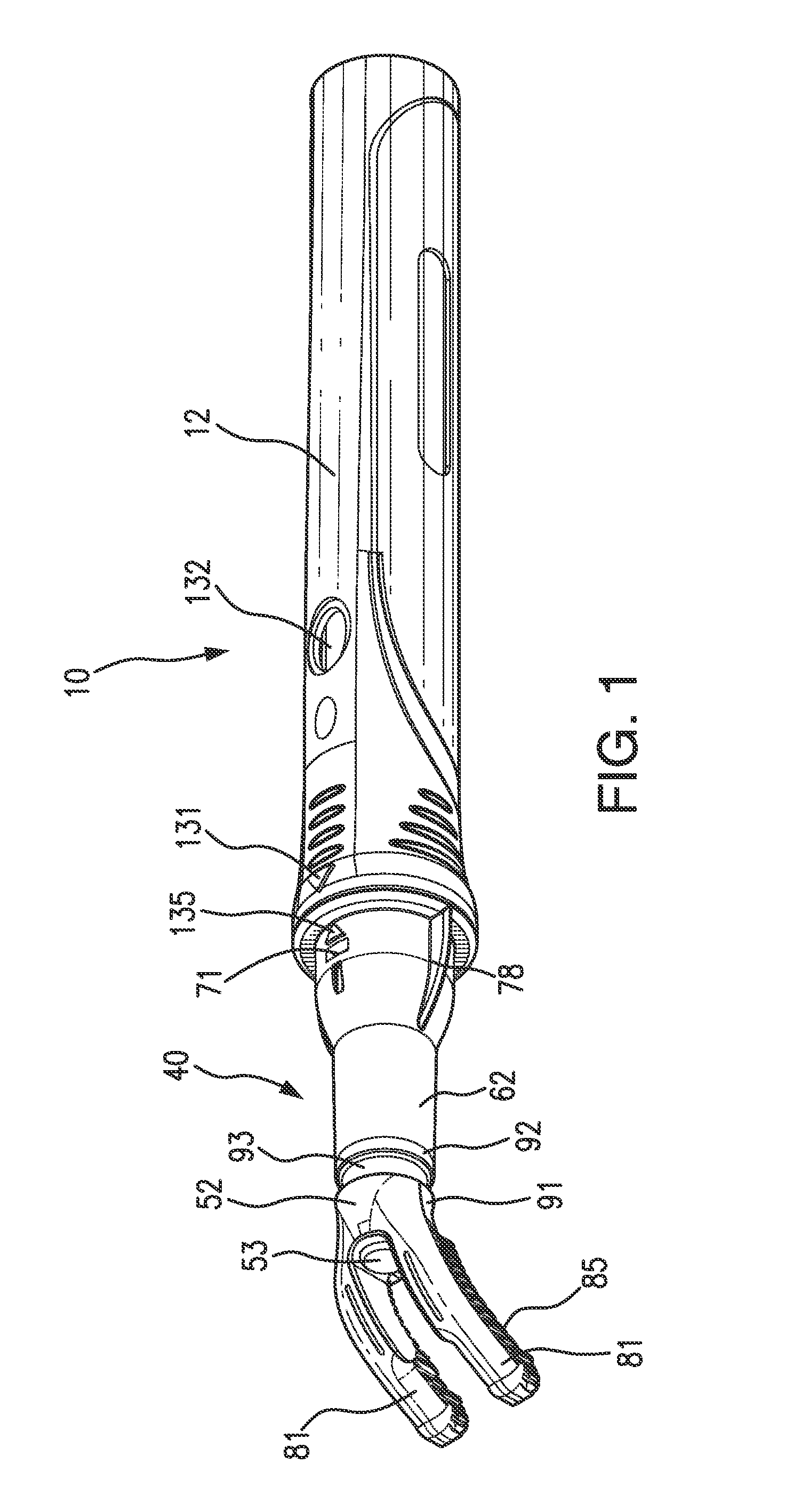
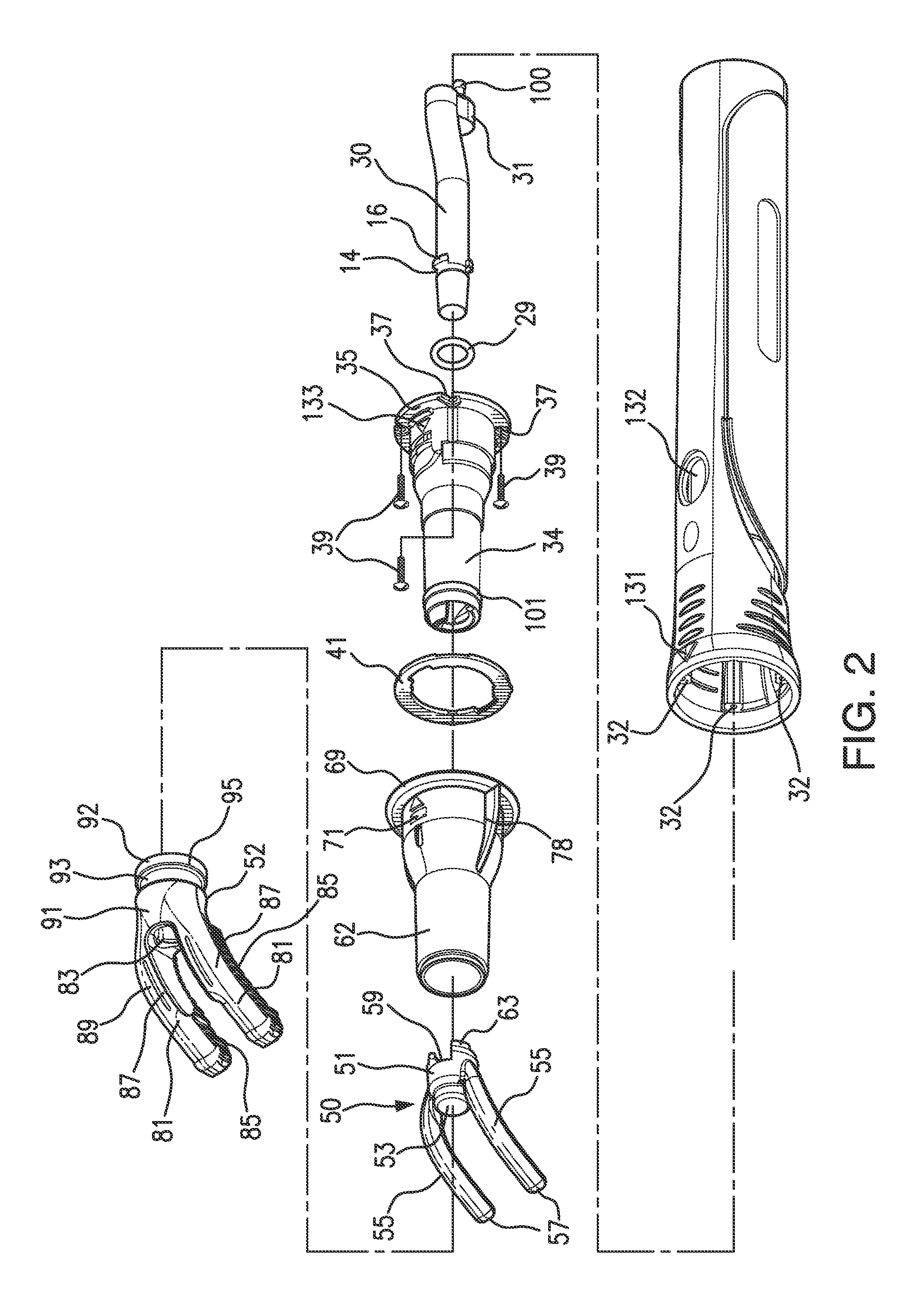
An instrument, article and method are provided for minimizing pain during administration by injection of a liquid, such as, an anesthetic. The instrument has a forward end. A lightpipe mounted freely for vibration projects out of the forward end. The article, a single use tip, is composed of a tip sleeve removably mounted on the forward end of the instrument and a tip member removably mounted on the projecting lightpipe to vibrate a preselected injection site on a human or animal. The tip sleeve and tip member are covered by an elastic overmold that enables the tip member to vibrate freely with respect to the tip sleeve and light from the lightpipe to illuminate the injection site. A vibration unit mounted in the instrument is coupled to the lightpipe. The method is carried out by imparting vibrations and illumination via the lightpipe to the tip member.
Owner:BING INNOVATIONS
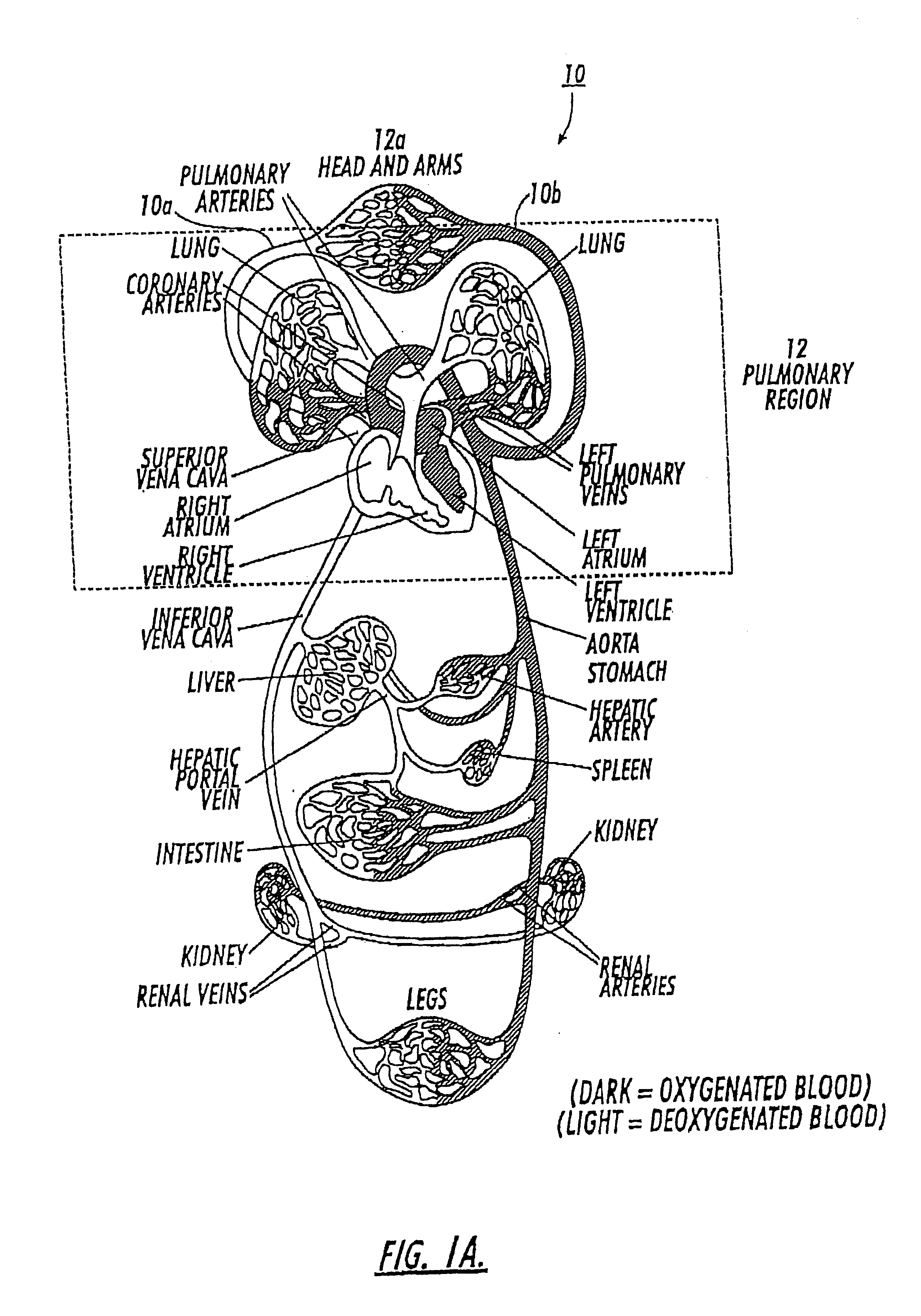
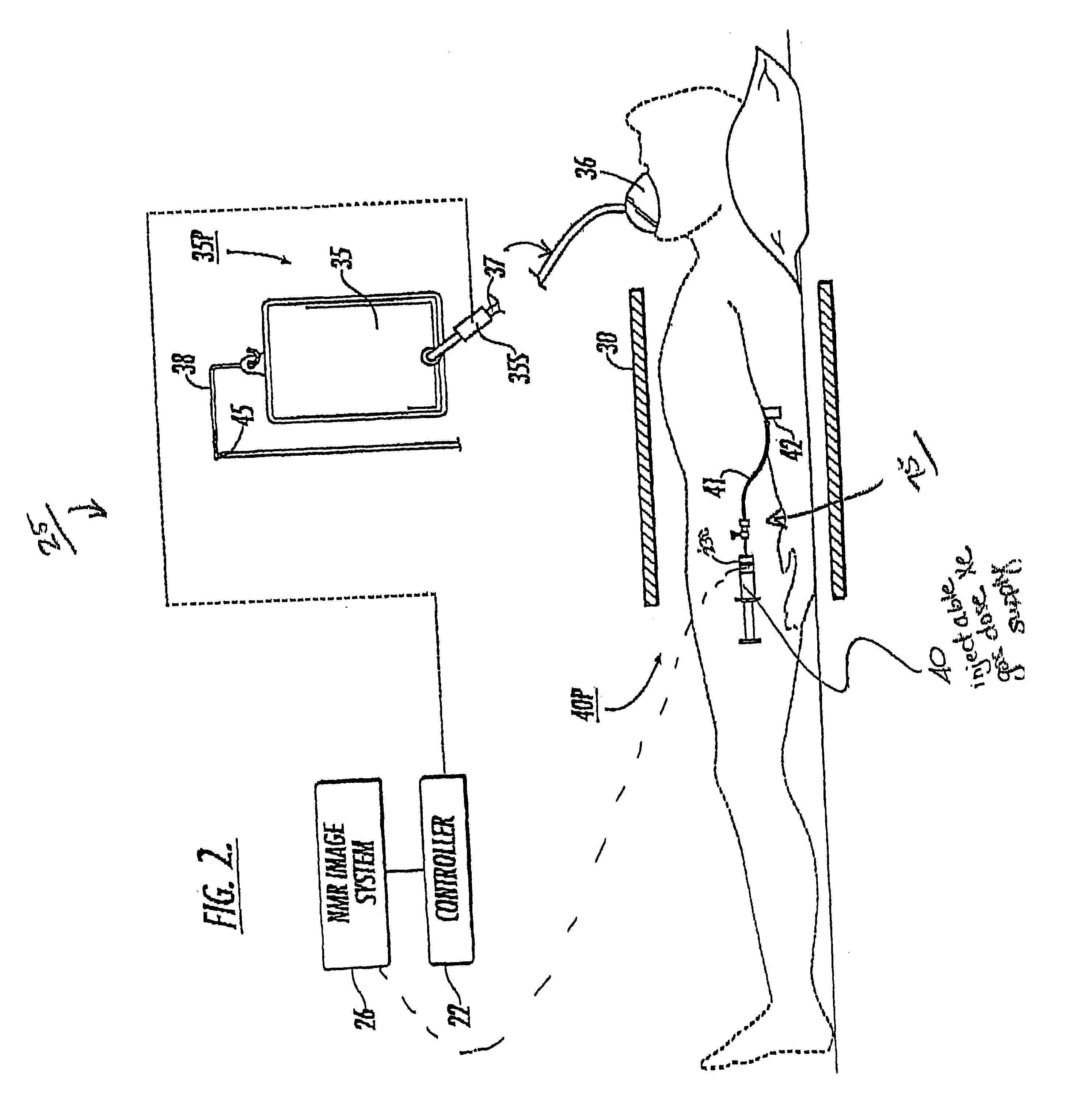
Diagnostic procedures using direct injection of gaseous hyperpolarized 129Xe and associated systems and products
InactiveUS6630126B2Prolong lifeConvenient lengthDispersion deliveryPressure infusionPulmonary vasculatureDual delivery
A method of screening for pulmonary embolism uses gaseous phase polarized <129>Xe which is injected directly into the vasculature of a subject. The gaseous <129>Xe can be delivered in a controlled manner such that the gas substantially dissolves into the vasculature proximate to the injection site. Alternatively, the gas can be injected such that it remains as a gas in the bloodstream for a period of time (such as about 8-29 seconds). The injectable formulation of polarized <129>Xe gas is presented in small quantities of (preferably isotopically enriched) hyperpolarized <129>Xe and can provide high-quality vasculature MRI images or NMR spectroscopic signals with clinically useful signal resolution or intensity. One method injects the polarized <129>Xe as a gas into a vein and also directs another quantity of polarized gas into the subject via inhalation. In this embodiment, the perfusion uptake allows arterial signal information and the injection (venous side) allows venous signal information. The dual delivery is used to generate a combined introduction path with a more complete image signal of both the arterial and venous side of the pulmonary vasculature. In this NMR imaging method, the pulmonary embolism screening method can use the same NMR chest coil for the excitation and detection of the <129>Xe signals. The direct injection of small quantities of gas at particular sites along the vasculature targets specific target regions to provide increased signal intensity NMR images. The disclosure also includes related methods directed to other diagnostic vasculature regions physiological and conditions. Associated delivery and dispensing systems and methods, containers, and quantitative formulations of the polarized gas are also described.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
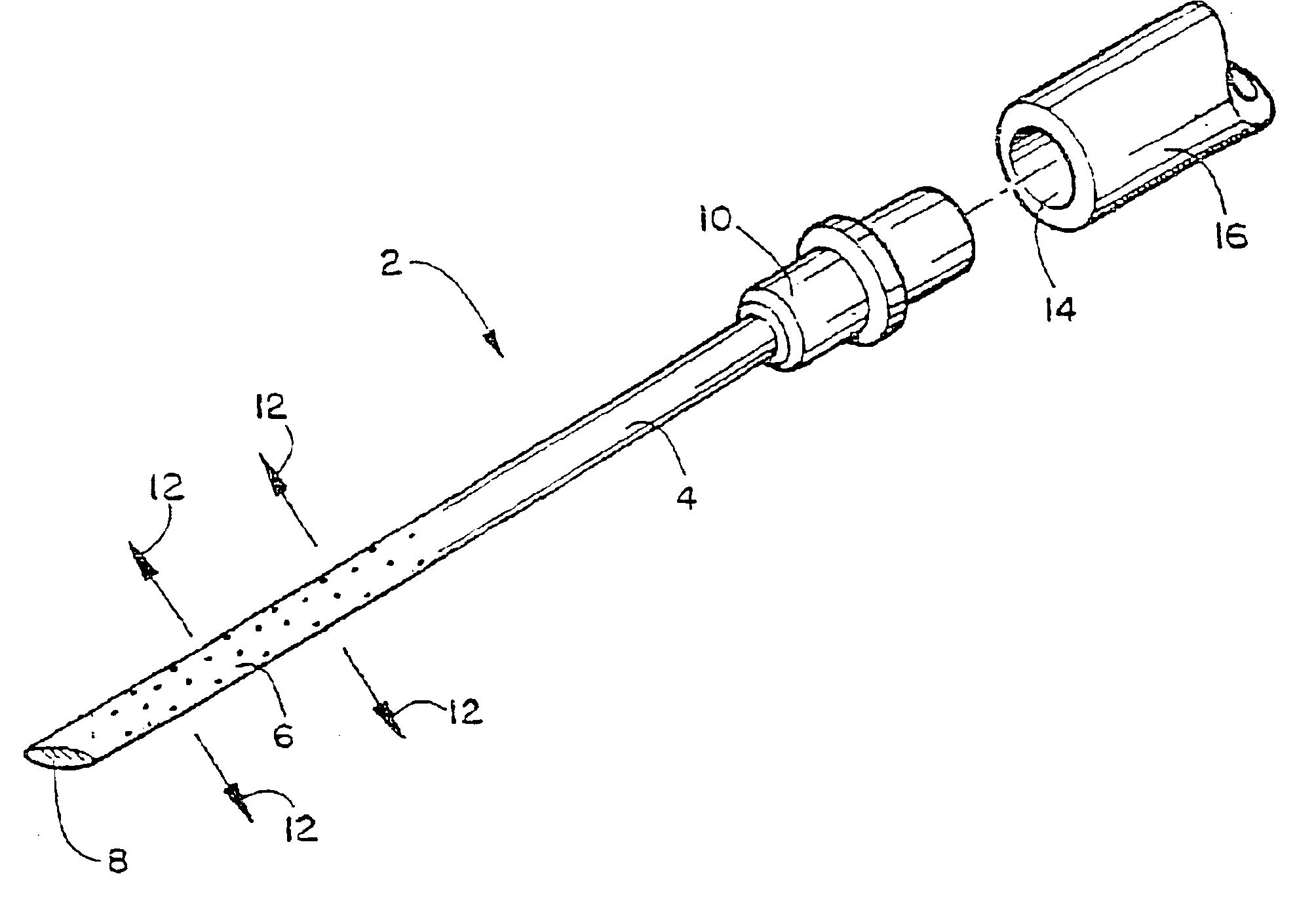
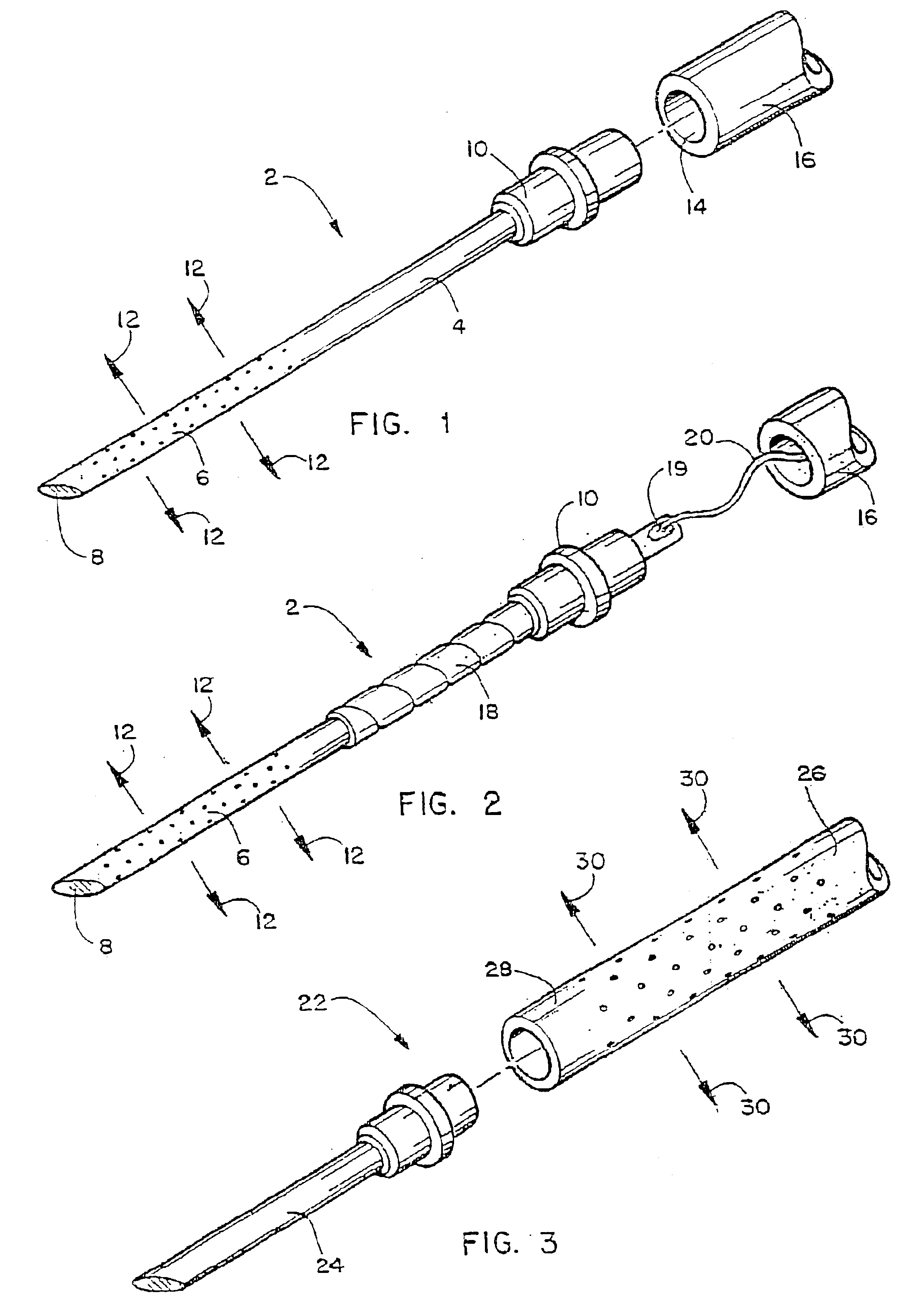
Apparatus with weeping tip and method of use
InactiveUS6855132B2Organic active ingredientsGenetic material ingredientsDistal portionBiomedical engineering
The invention provides surgical needles with a porous distal portion from which a liquid injectate will weep or ooze multidirectionally under injection pressure while the porous distal portion of the needle is inserted into a body surface. The porous distal portion of the needle can be fabricated from a porous carbon, metal, ceramic or polymer and preferably has a decreasing gradient of impedance to fluid flowing to the point of the needle to compensate for the falling off of injection pressure as fluid moves towards the point, thereby ensuring uniform weeping of the injectate along the injection course. The needle is adapted for attachment to a catheter or syringe. In another embodiment, a surgical assemblage is provided wherein a porous distal portion having similar fluid flow characteristics is located along the distal end of a catheter, and a needle point is attached to the distal end of the catheter (e.g., a steerable catheter) for piercing tissue. A guidance catheter can be used to direct the invention devices to a remote internal injection site. The invention devices and methods can be used to inject fluids (including those containing nucleic acids for gene therapy) into interior body walls or tissue, such as a beating heart, without substantial loss of fluid and without substantial damage to tissue caused by injectate.
Owner:TRICARDIA
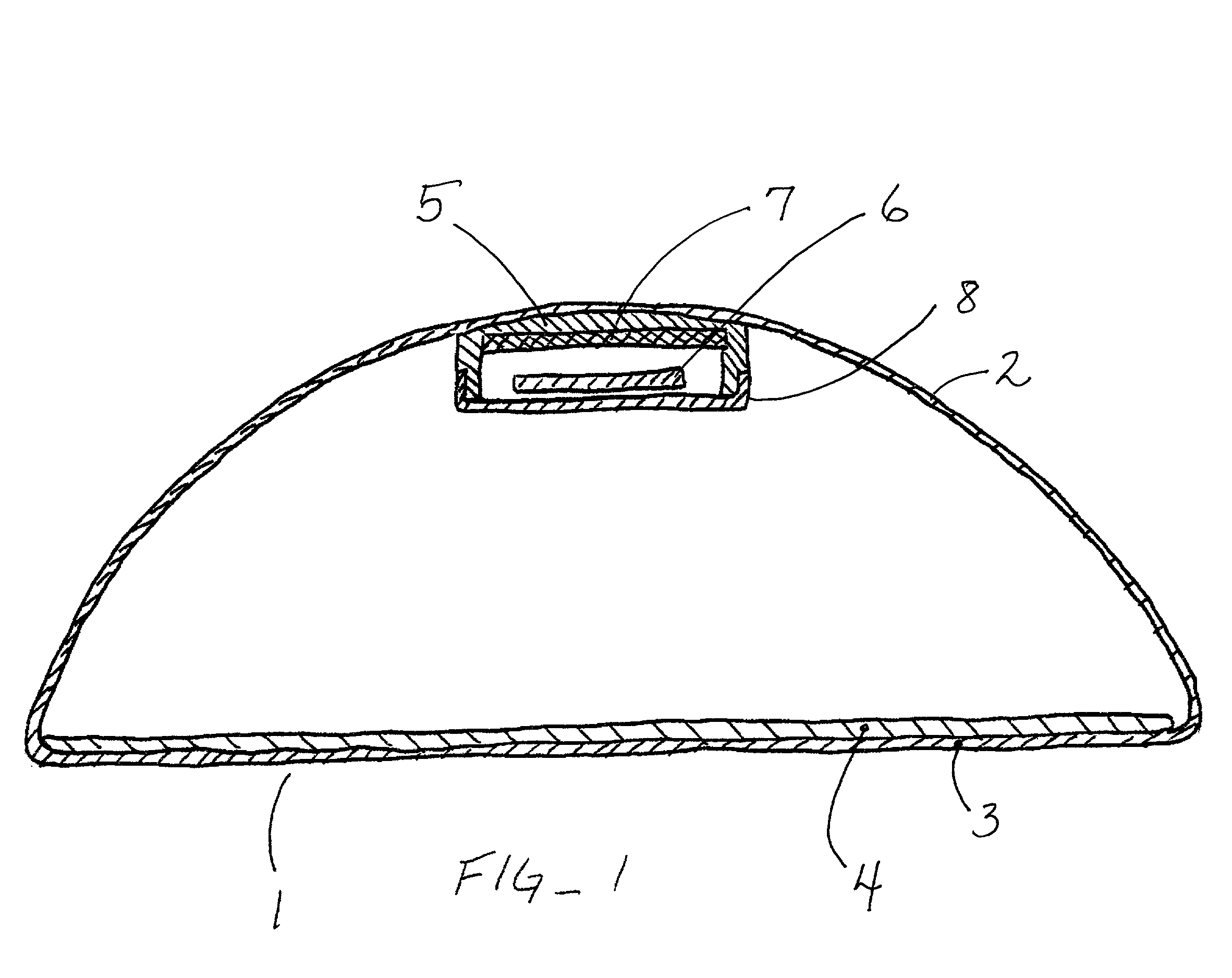
Tissue expander apparatus with magnetically locatable injection site and methods for use
InactiveUS20010004709A1Precise positioningMinimize the potential for the thinnerMammary implantsDiagnosticsMedicineTissue expander
Owner:DUBRUL WILLIAM R
Split septum assembly for an intravenous injection site
ActiveUS20150224296A1Prevent proximal leakage of fluidMedical devicesCatheterAxial compressionEngineering
An intravenous injection site having a split septum assembly interfit with the site for the intravenous administration of fluids to a patient. The split septum assembly has a resilient and compressible split septum having an axially-formed slit for receipt of a blunt cannula, needle, or other medical device through said slit, a septum holder for receipt of the split septum, and a septum housing for receipt of the combined septum holder and split septum. The septum includes a body and a flange extending radially from the body. A projection extends from the flange. The septum is mounted within the septum holder, and the septum holder is mounted within the septum housing to provide axial compression of the flange. This axial compression presents a double hermetic seal that significantly minimizes or prevents proximal leakage of fluid through the slit or around the septum holder when the cannula is removed.
Owner:NEXUS MEDICAL LLC
Needleless injection site
InactiveUS7008406B2Increase contactFacilitating frictional retentionInfusion syringesInfusion devicesNeedle Free InjectionNeedle free
A needleless injection site comprising a housing defining proximal and distal ends and including a reseal member disposed therein. The reseal member has an elastically openable and closable aperture formed therein, and normally resides within the housing in a closed position wherein the aperture is in a closed configuration. The reseal member is deformable such that the application of distally directed pressure thereto will cause the reseal member to distally advance within the housing to an open position wherein the aperture assumes an open configuration. The removal of the distally directed pressure from the reseal member will cause it to resiliently return to the closed position wherein the aperture assumes the closed configuration.
Owner:BECTON DICKINSON & CO
Helicid powder injection preparation and its preparation method
InactiveCN1535691AImprove stabilityIndicator qualifiedOrganic active ingredientsPowder deliveryConvulsionSedation
The present invention provides a helicid powder injection and its preparation method. Every powder injection contains 25-600 mg of helicid, and the rest is medicinal auxiliary material for injection. It has good stability, can be stored for 3 years at normal temperature, its irritation is small, and its injection site has no clear change or light hyperemia, and its sedation effect is good, and its convulsion-resisting action is strong, it can obviously reduce coriamyrtin convulsion incidence rate, prolong generative latent period, reduce frequency of attack and shorten time and process of attack. The tests show that said helicid powder injection has the strong action of stopping pain and relieving inflammation.
Owner:KUNMING ZIJIAN BIOTECH
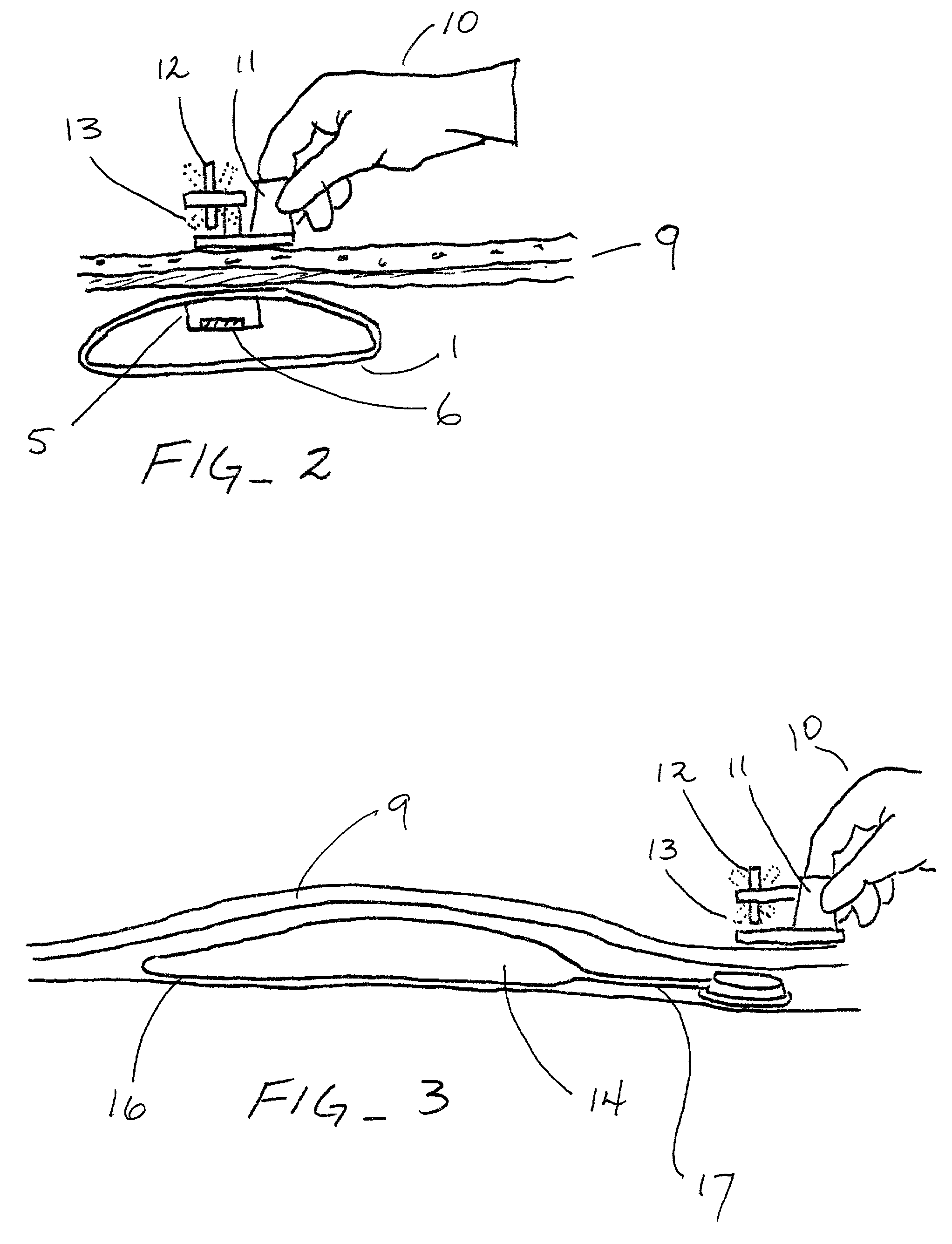
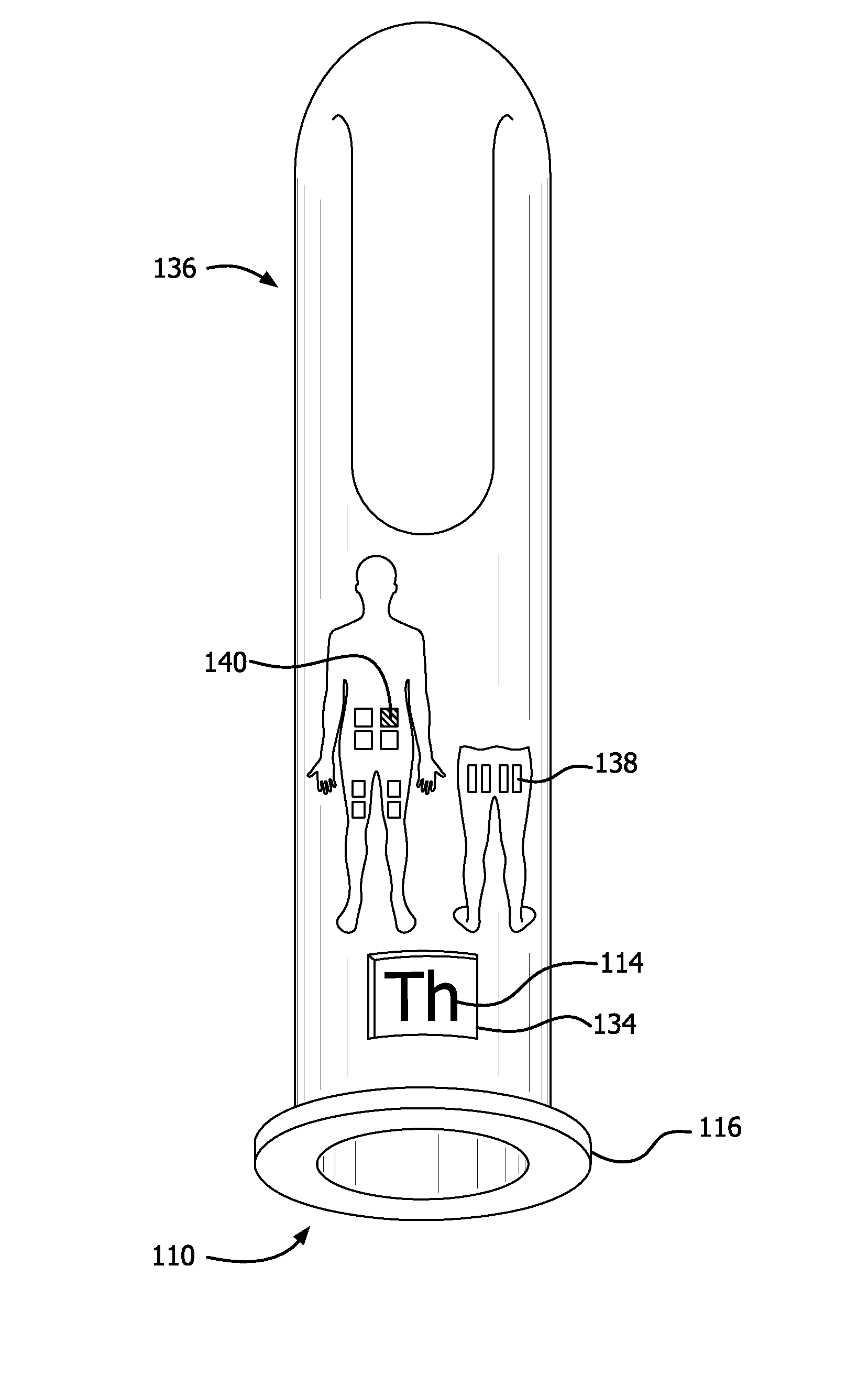
Guiding and reminding device for determining the appropriate injection site, particularly for regularly performed medication injections and/or taking blood for testing
InactiveUS20100015590A1Eliminate deficienciesSimple, easy-to-manufactureIntravenous devicesEducational modelsMedication injectionBody region
A device for determining appropriate injection or testing sites. At least two movable adjacent plates are each provided with holes arranged in a certain system that can be brought in coincidence. A date carrier plate has numbers corresponding to the days of a month arranged in a certain system. A time carrier plate has numbers corresponding to the various times, mostly the hours, or parts of a day. After moving the plates a coincidence of holes in each determines the appropriate date and corresponding time for the optimal needle insertion point for the given date and time. Site determination signs defining a more exact needle insertion point on the various body parts are also provided.
Owner:KISS BARNABAS
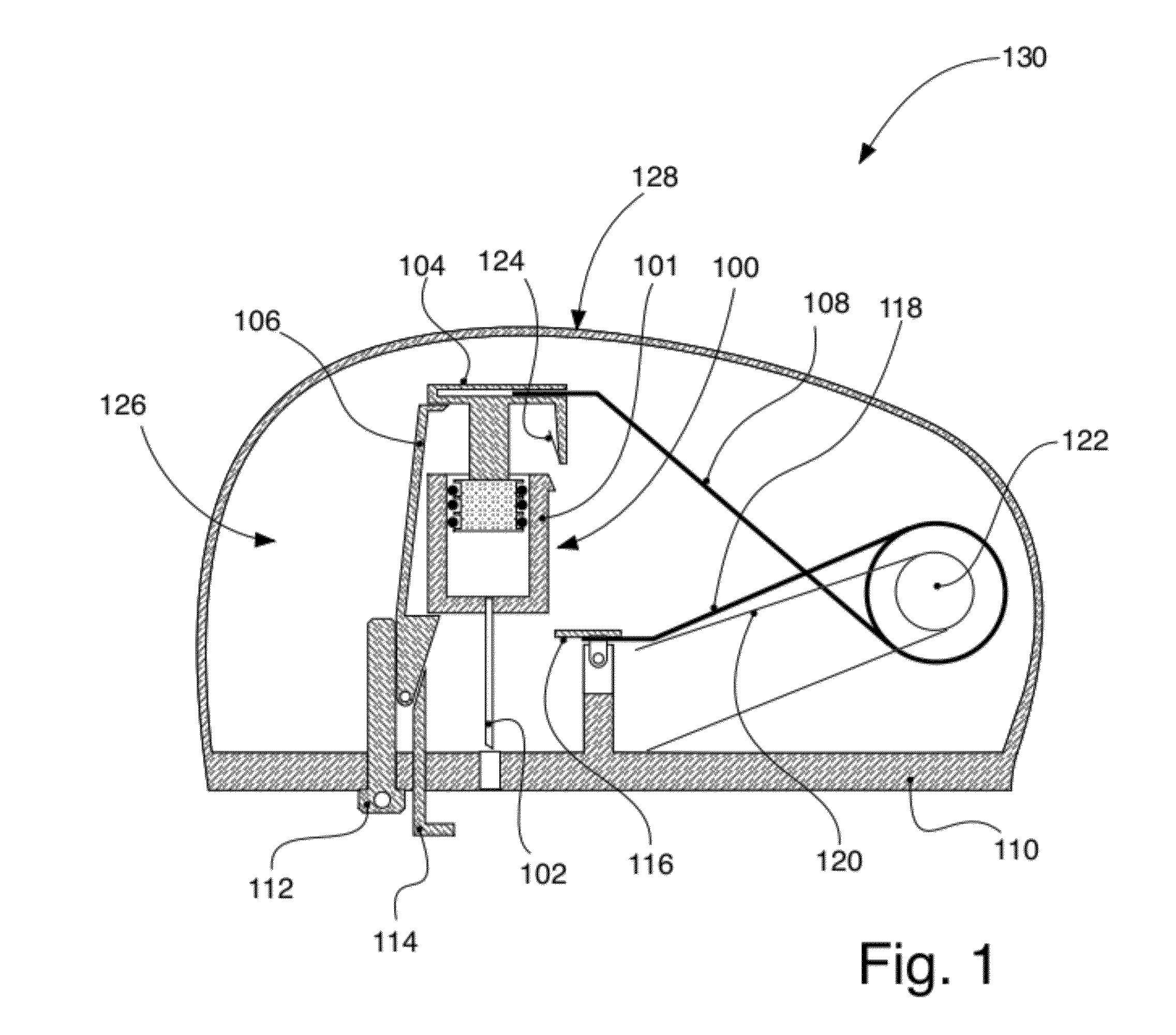
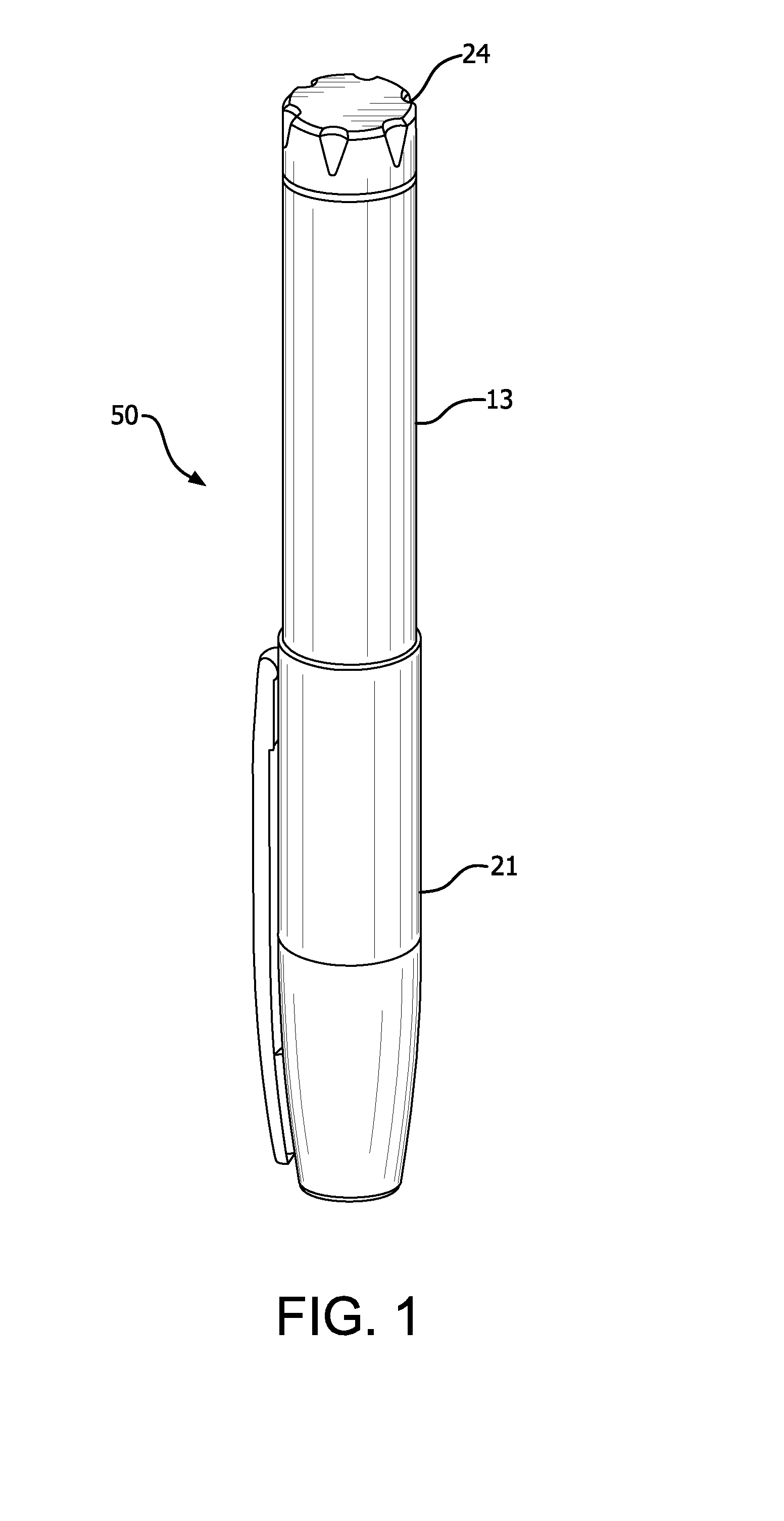
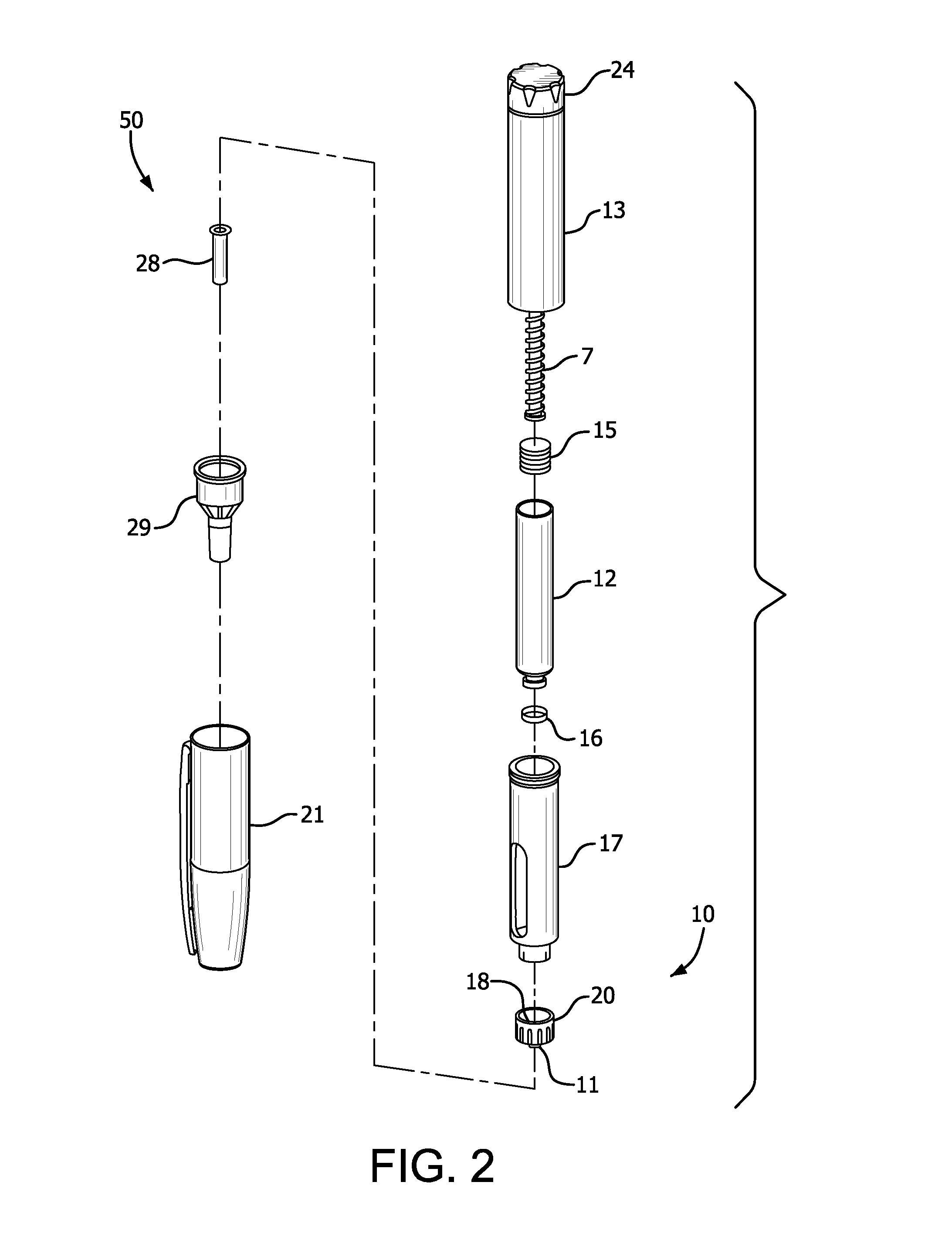
Apparatus for injecting a pharmaceutical with automatic syringe retraction following injection
ActiveUS9044553B2Convenient manual controlAmpoule syringesAutomatic syringesSyringe needleBiomedical engineering
Owner:ELI LILLY & CO
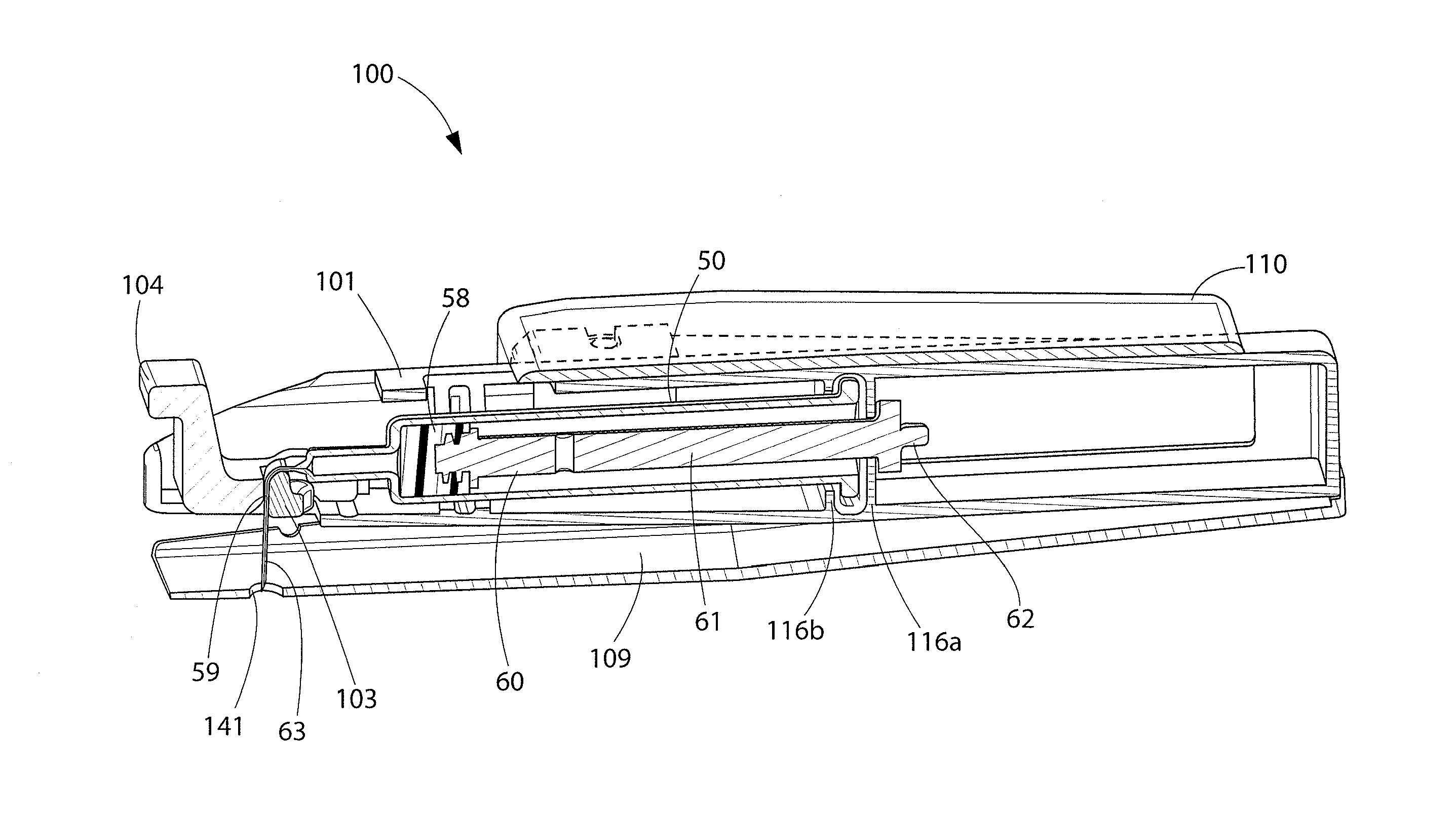
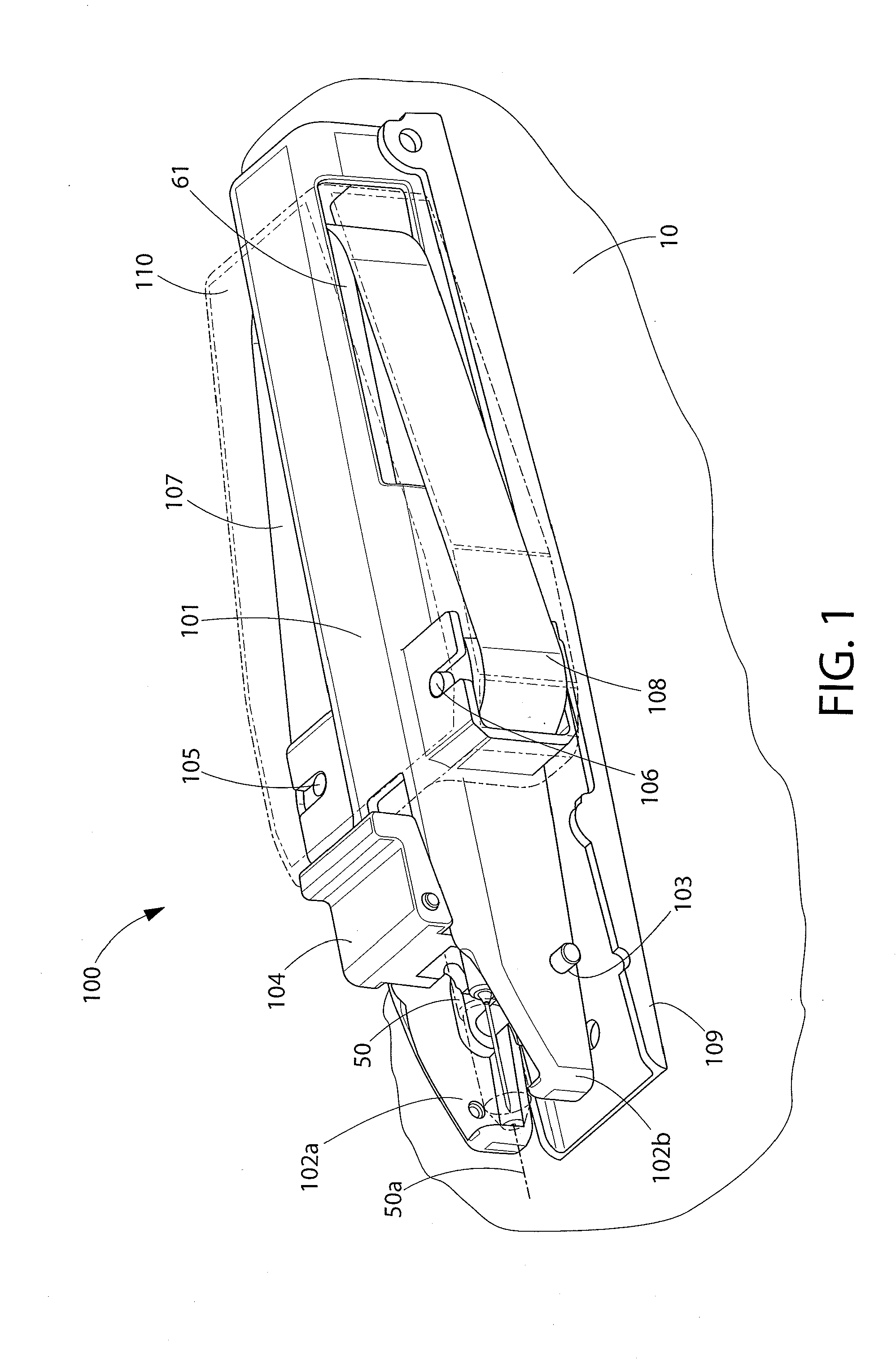
Systems, apparatuses and methods to encourage injection site rotation and prevent lipodystrophy from repeated injections to a body area
ActiveUS20170028141A1Inhibit injectionMinimize lipohypertrophyInfusion syringesDrug and medicationsPrint mediaLost tooth
Systems and methods encourage users to rotate injection sites and avoid lipodystrophy. Sleeves and / or lost-tooth gear dials and / or microswitches in or on injection pens or their caps, on vials, and on other portable devices manually adjust an indicator before or after an injection to show a current or next injection site in accordance with a site rotation plan. Injected medicine packaging and related printed indicia encourage site rotation. Optical devices employing optical mouse or projection technology help locate and / or distribute injection sites within a body area. A mobile phone app tracks injections and locations to select next injection site, and can use imaging to locate a target injection site and optionally diagnose lipodystrophic conditions and record them. Tactile and print media educational tools are presented to help users palpate and identify lipos in body areas having injection sites.
Owner:EMBECTA CORP
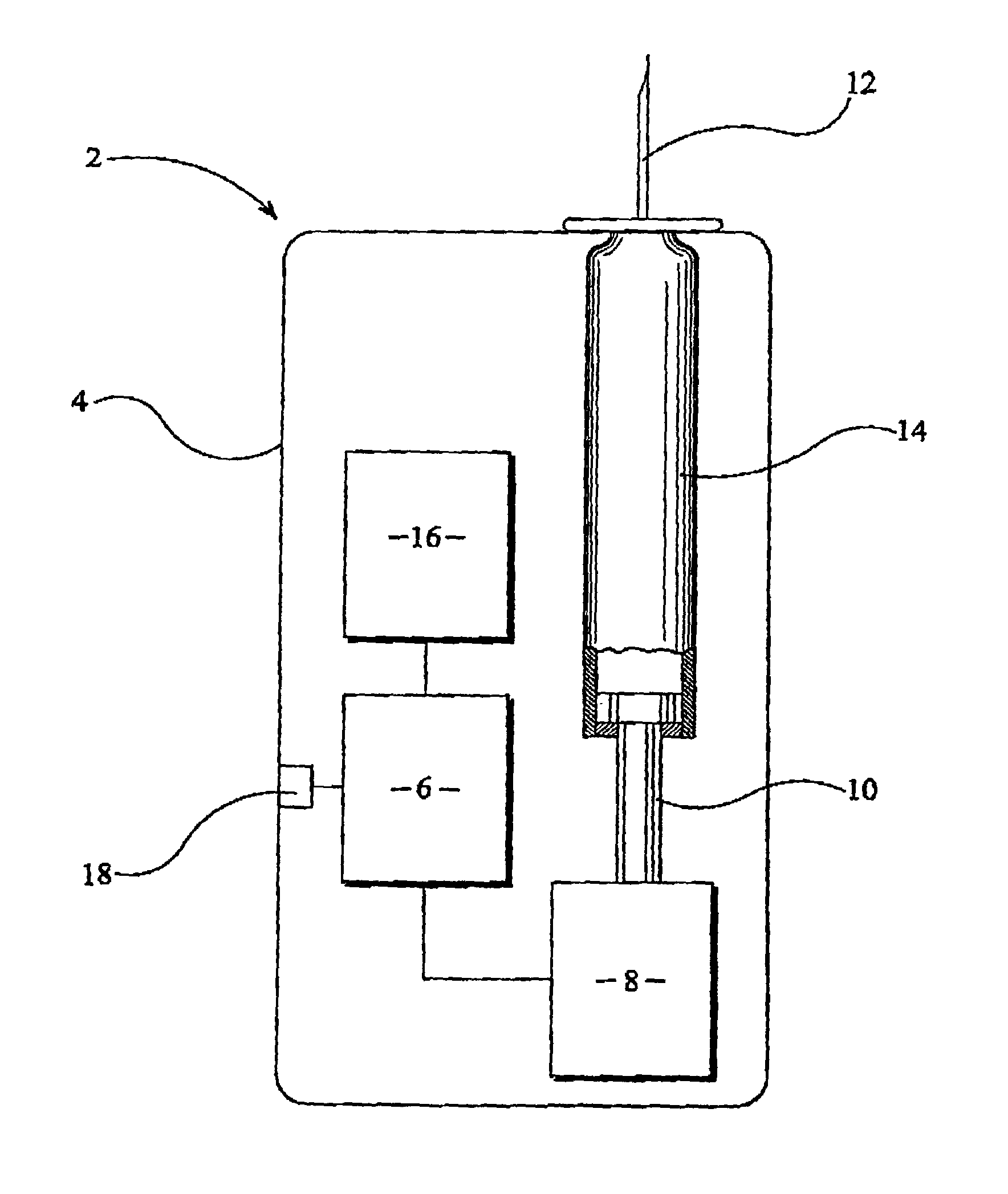
Medicament injection apparatus
A medicament injection device in which the time elapsed is calculated from the end of a predetermined period during which medicament is permitted to disperse subsequent to completion of an injection stroke. The medicament injection device is particularly suitable for use by those with diabetes in which once a desired dosage of medicament has been selected and expelled from the medicament cartridge into the body of the patient, the injected medicament is allowed to disperse locally from the injection site within the patient's body before the needle arrangement is removed from the patient's body.
Owner:SANOFI AVENTIS DEUT GMBH
Illuminated intra-oral delivery device
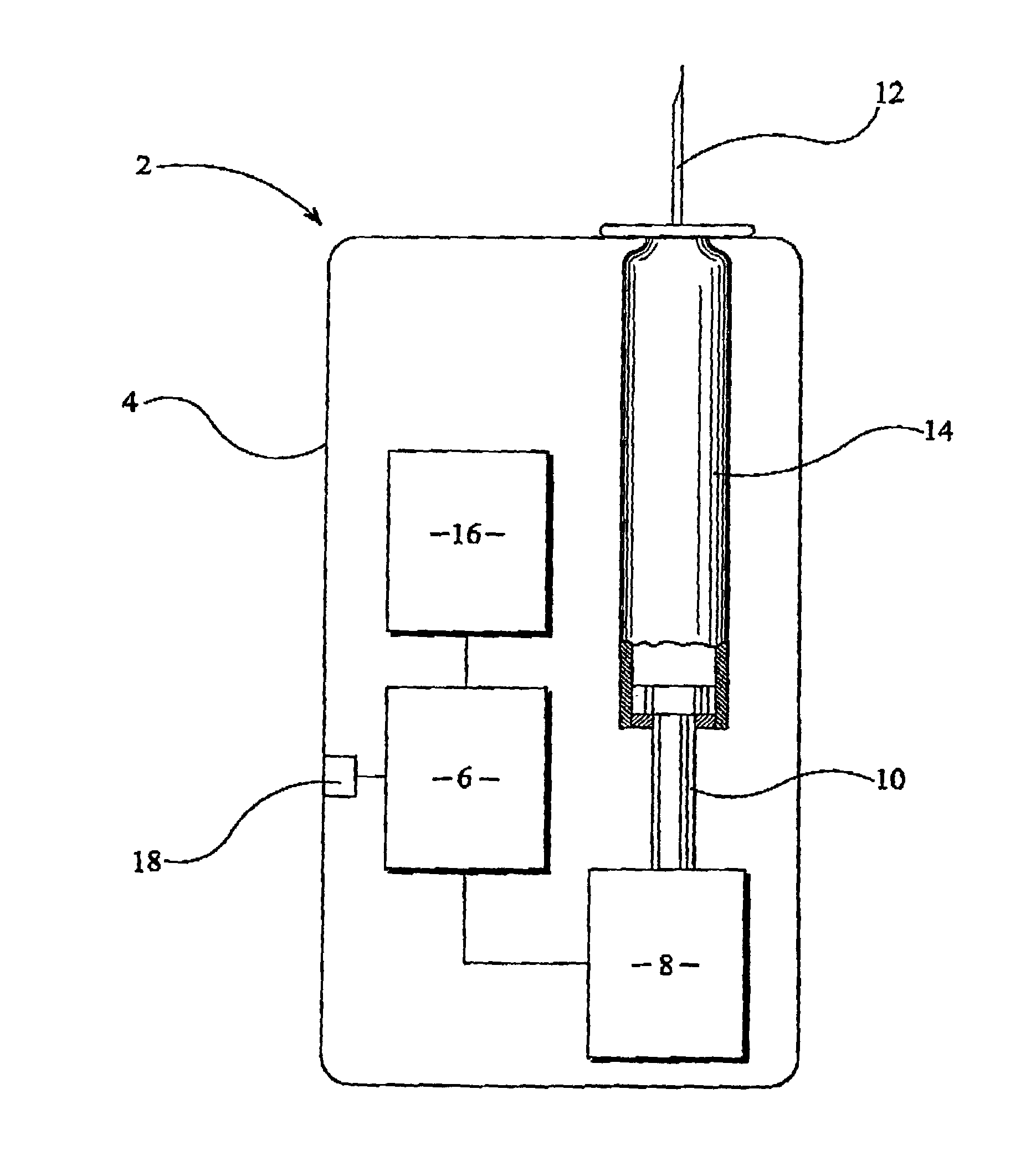
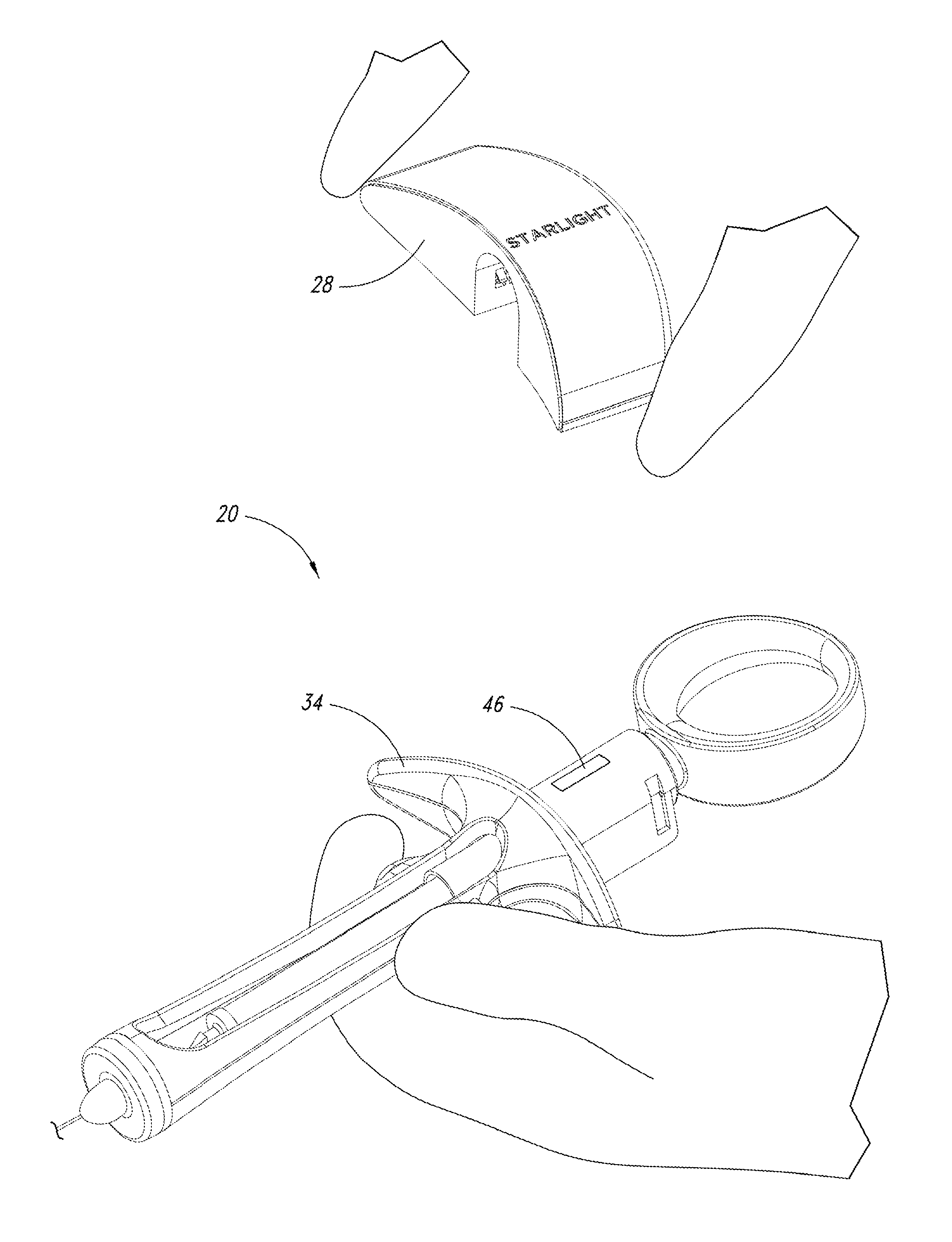
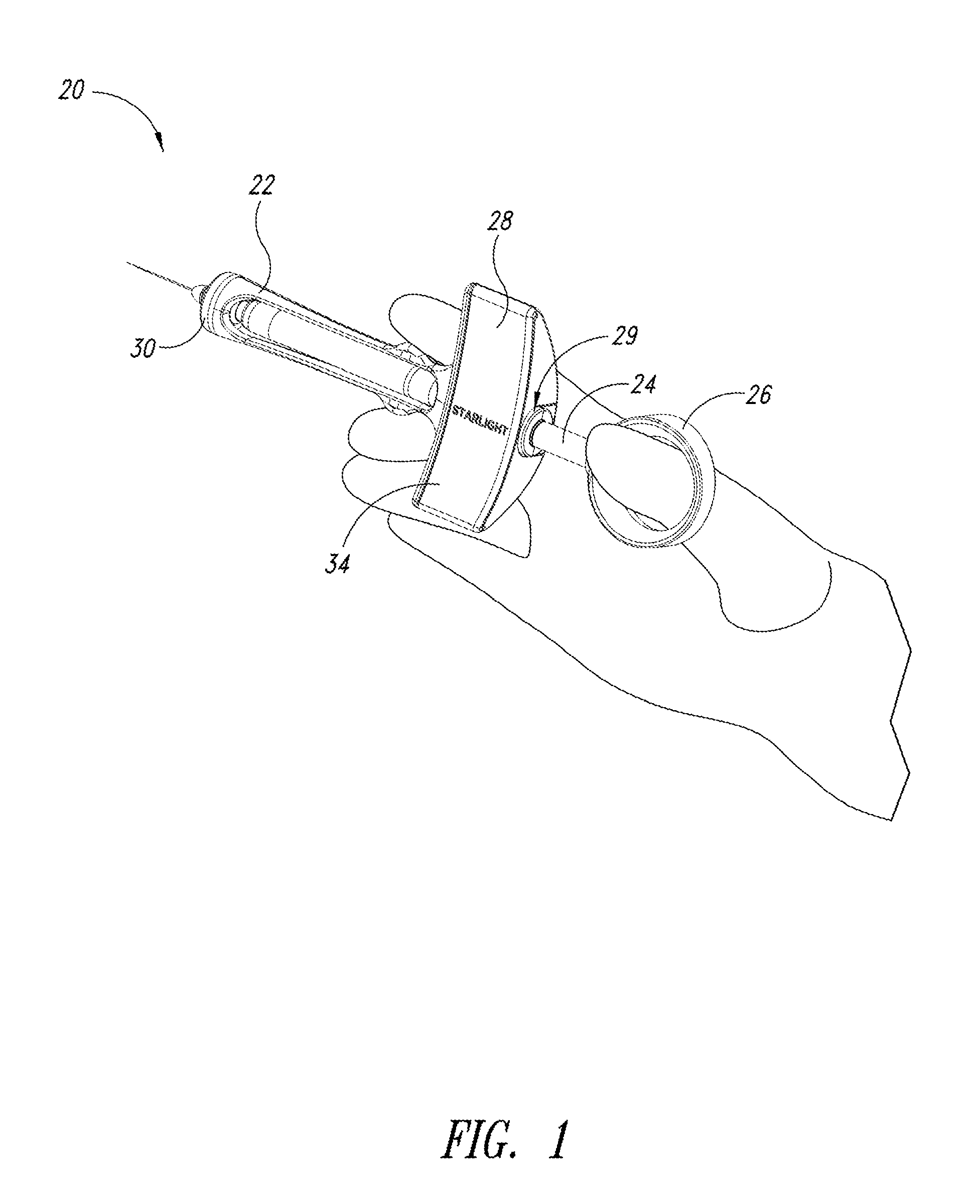
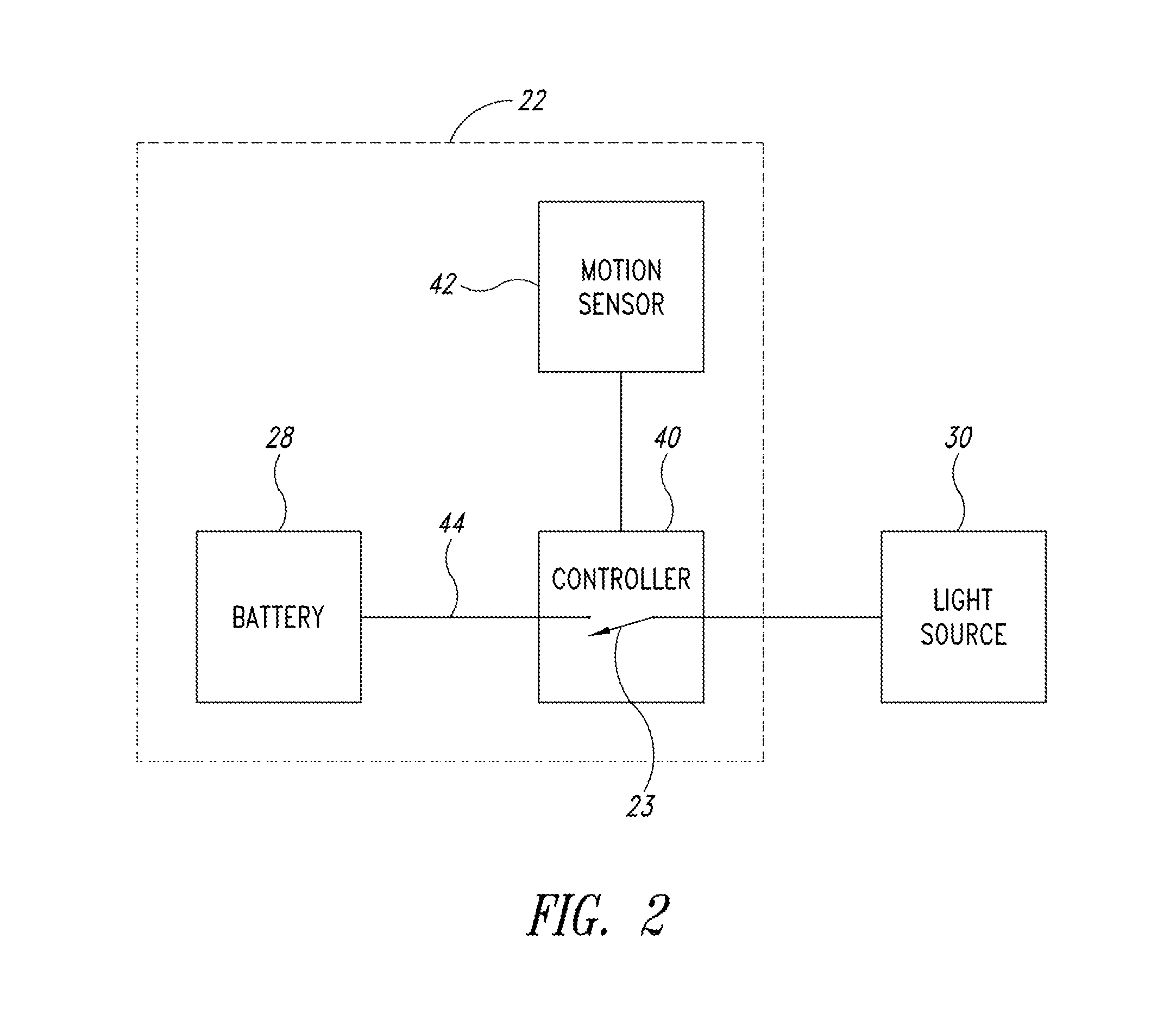
A medical syringe with a light source mounted on a distal end of the syringe to provide illumination of an injection site, a battery pack receiver configured to receive a battery pack with magnetic attraction a proximal end of the syringe, and a control circuit that is configured to illuminate the light source when the control circuit detects movement of the syringe and disconnects the battery from the light source either after a fixed period of time or after motion of the syringe ceases. Ideally the light source is a disc-shaped array of LED lights. The syringe is constructed of materials that can be sterilized.
Owner:SABOURIN CHRISTOPHER R
System and method for pain reduction during skin puncture and breakable tip therefor
ActiveUS20110319812A1Easily and inexpensively utilizedFree from painSurgeryMedical devicesInjection siteEngineering
An instrument, article and method are provided for minimizing pain during administration by injection of a liquid, such as, an anesthetic. The instrument has a forward end. A lightpipe mounted freely for vibration projects out of the forward end. The article, a single use tip, is composed of a tip sleeve removably mounted on the forward end of the instrument and a tip member removably mounted on the projecting lightpipe to vibrate a preselected injection site on a human or animal. The tip sleeve and tip member are covered by an elastic overmold that enables the tip member to vibrate freely with respect to the tip sleeve and light from the lightpipe to illuminate the injection site. The overmold of the single use tip is torn during removal of the single use tip from the instrument.
Owner:BING INNOVATIONS
Apparatus and method for reducing pain during skin puncturing procedures
ActiveUS7981071B2Easily and inexpensively utilizedFree from painTooth pluggers/hammersSurgeryCantileverMinimizing pain
Owner:BING INNOVATIONS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com