Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Cartilage degradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
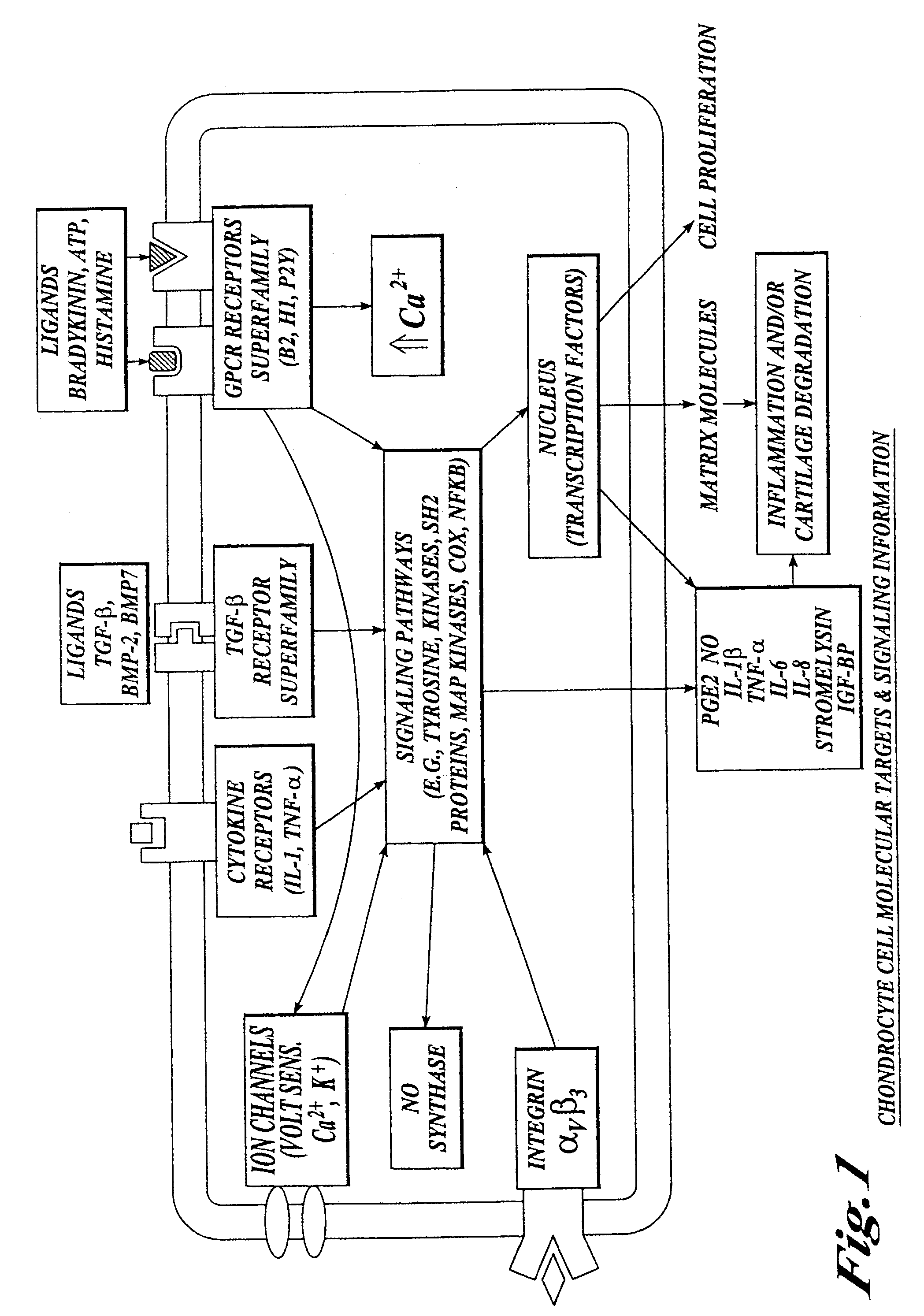
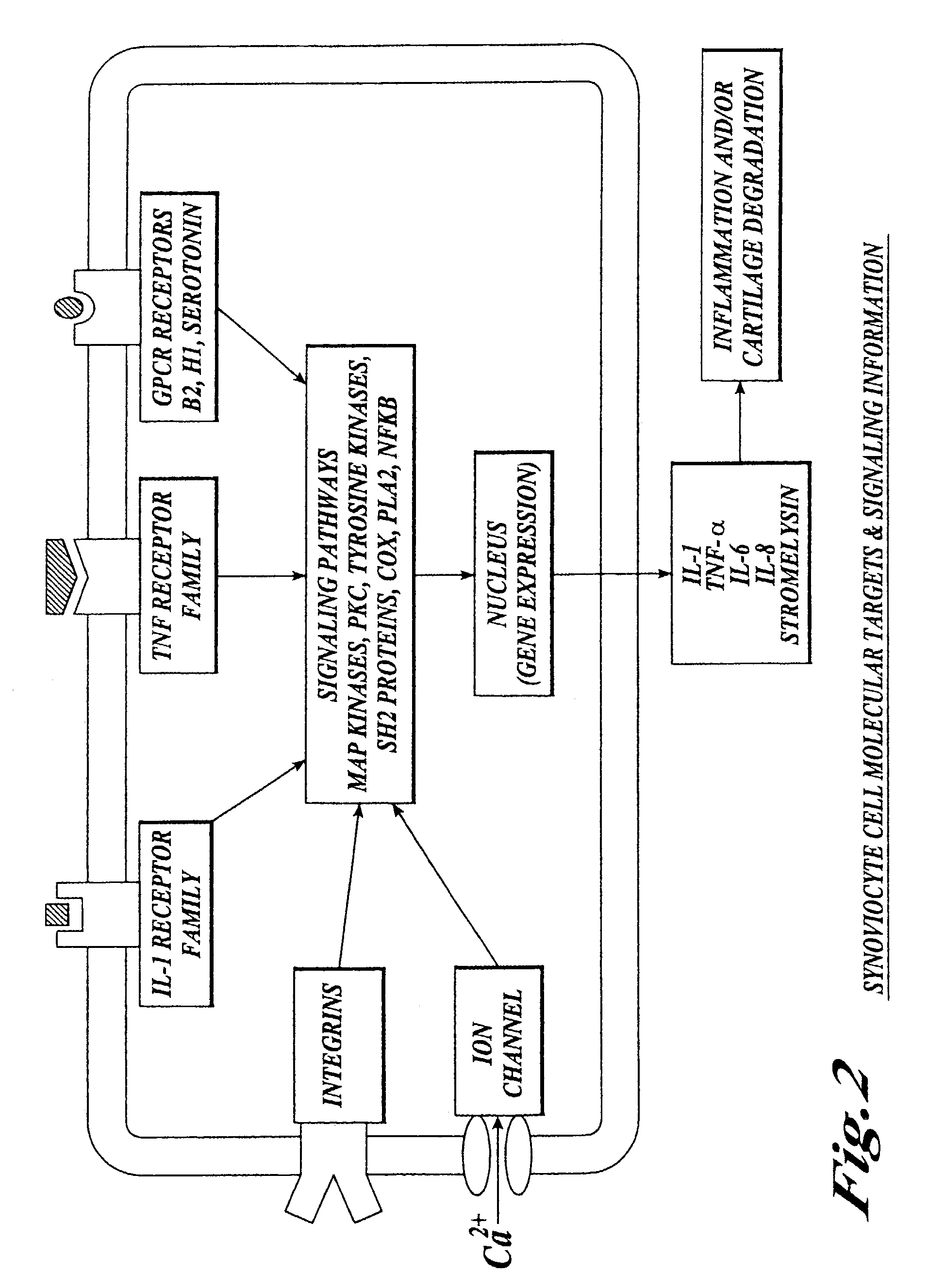
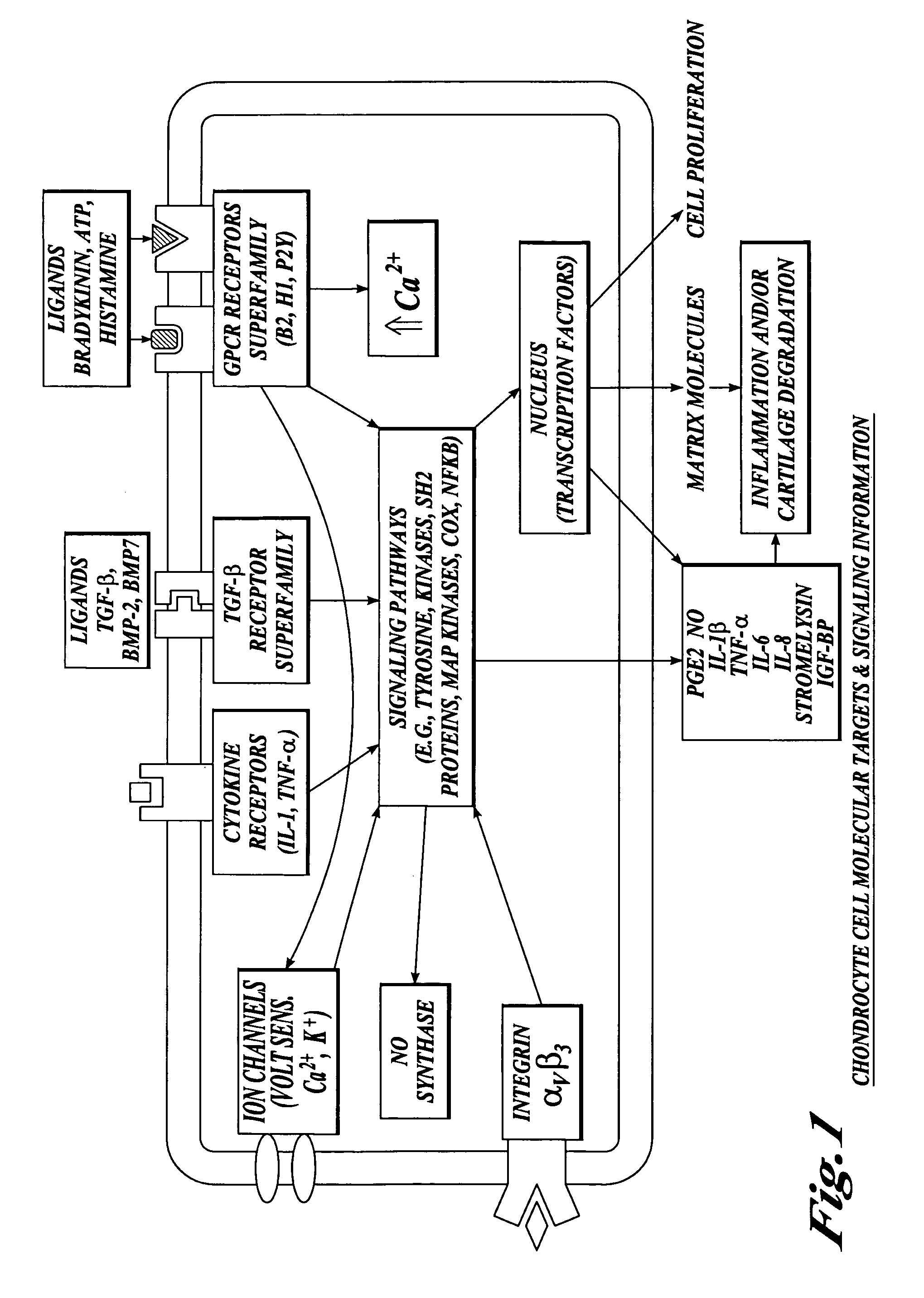
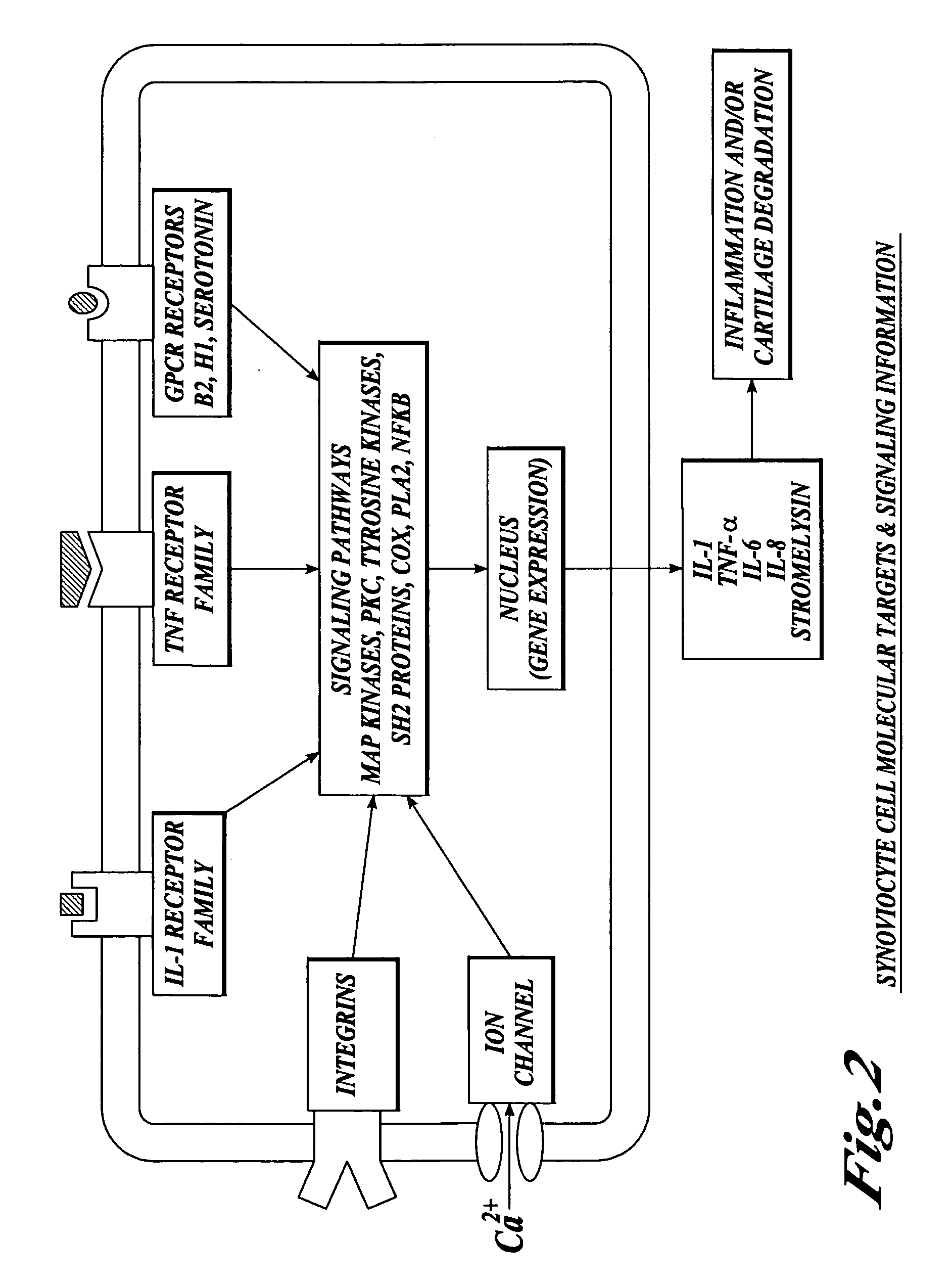
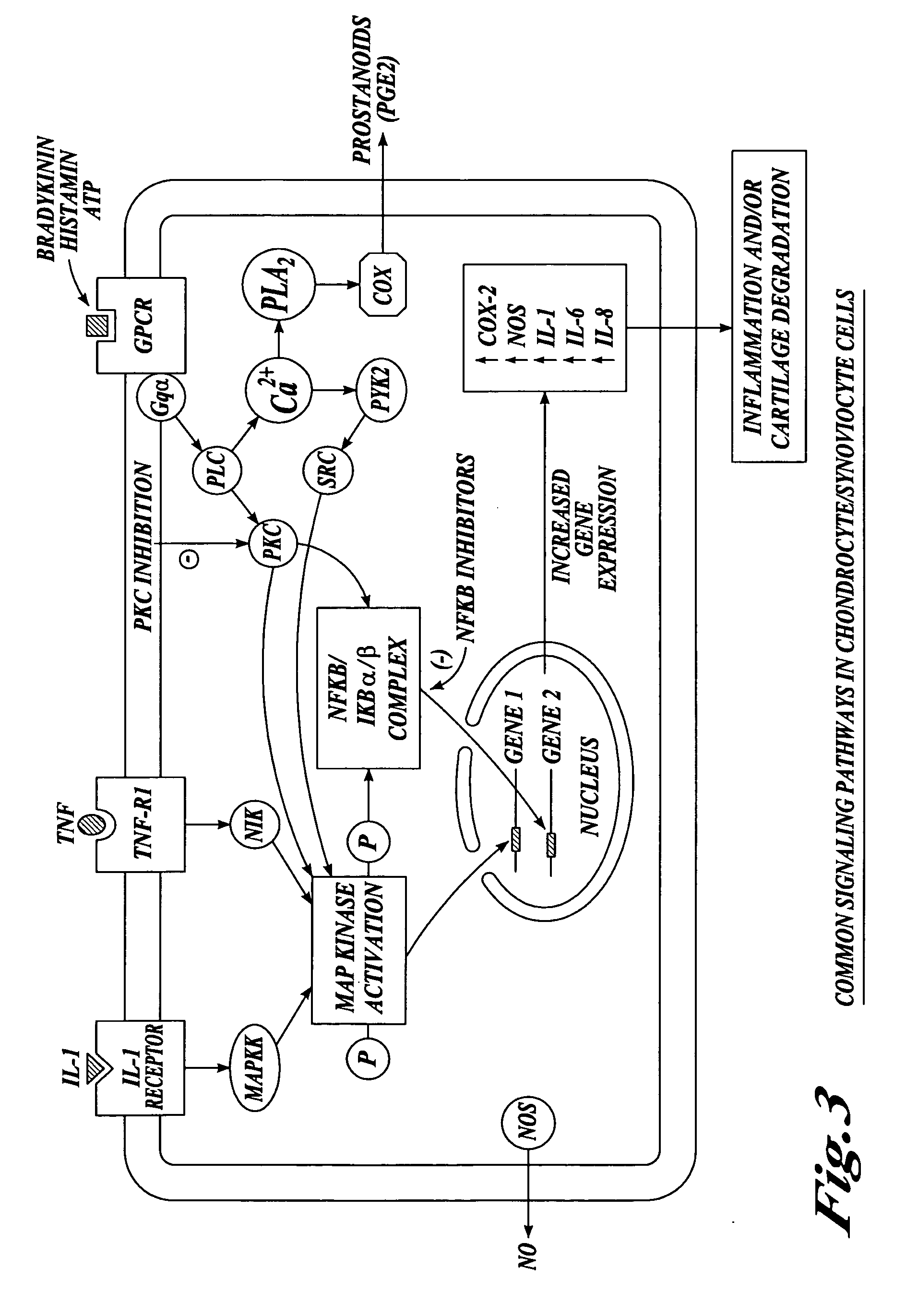
Cartilage degradation is attributed to different classes of catabolic factors, including proinflammatory cytokines, aggrecanases, matrix metalloproteinases, and nitric oxide. Recently, matrix degradation products generated by excessive proteolysis in arthritis have been found to mediate cartilage destruction.
Compositions and methods for systemic inhibition of cartilage degradation
InactiveUS7067144B2Limit their usefulnessLow costPowder deliveryOrganic active ingredientsMedicineWhole body
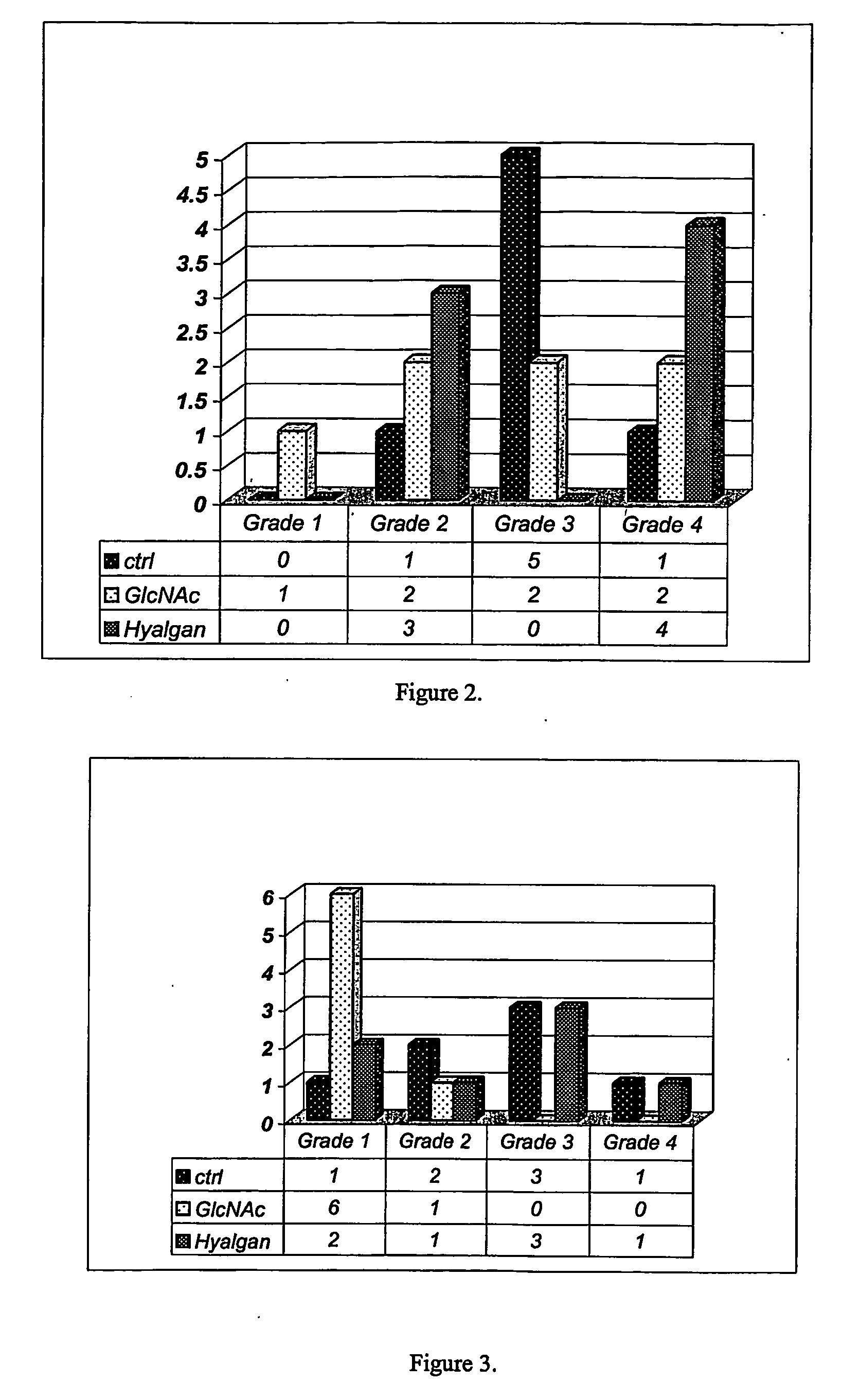
Methods and compositions for inhibiting articular cartilage degradation. The compositions preferably include multiple chondroprotective agents, including at least one agent that promotes cartilage anabolic activity and at least one agent that inhibits cartilage catabolism. The compositions may also include one or more pain and inflammation inhibitory agents. The compositions may be administered systemically, such as to treat patients at risk of cartilage degradation at multiple joints, and suitably may be formulated in a carrier or delivery vehicle that is targeted to the joints. Alternatively the compositions may be injected or infused directly into the joint.
Owner:OMEROS CORP
Compositions and methods for systemic inhibition of cartilage degradation
InactiveUS20060210552A1Control degradationControl inflammationOrganic active ingredientsPowder deliveryMedicineWhole body
Methods and compositions for inhibiting articular cartilage degradation. The compositions preferably include multiple chondroprotective agents, including at least one agent that promotes cartilage anabolic activity and at least one agent that inhibits cartilage catabolism. The compositions may also include one or more pain and inflammation inhibitory agents. The compositions may be administered systemically, such as to treat patients at risk of cartilage degradation at multiple joints, and suitably may be formulated in a carrier or delivery vehicle that is targeted to the joints. Alternatively the compositions may be injected or infused directly into the joint.
Owner:OMEROS CORP
Viral vectors to inhibit leukocyte infiltration or cartilage degradation of joints
Methods for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule containing the DNA sequence encoding for the product and infecting the target cell of the mammalian host using the vector. The injection can be done in vivo, by directly injecting the vector into the host, or can be done in vitro by transfecting a population of cultured target cells with the vector and transplanting them each into the host. Nonviral means can also be used to introduce the DNA sequence to the host. Administration of more than one gene of interest results in an enhanced therapeutic benefit. Also disclosed is a method for treating a connective tissue disorder by introducing at least one gene encoding a product into at least one target cell of a joint of a host for use in treating multiple joints of the host. Injection of a vector molecule containing the DNA sequence encoding for a product of interest, or non-viral introduction of such a DNA sequence, to one join of a mammalian host results in a therapeutic benefit in that joint as well as other joints in the host
Owner:UNIVERSITY OF PITTSBURGH
Treatment of a condition in a mammal with administration of aminosugar and uses thereof
InactiveUS20070142326A1Lessen and prevent and reverse pathologyPrevents and lessens and pathologyBiocidePowder deliverySubchondral boneAminosugar
The present invention relates to treating joint related conditions in mammals by administering an aminosugar, and wherein said treatment specifically prevents, lessens or reverses pathologies associated with the joint condition, said pathologies being selected from the group consisting of synovitis, subchondral bone edema, and cartilage degradation.
Owner:THE SCRIPPS RES INST +1
Composition and method for use in cartilage affecting conditions
ActiveUS20060073192A1Improving joint healthDecreasing cartilage abnormalityBiocidePeptide/protein ingredientsMedicineManganese
Owner:HILLS PET NUTRITION INC
Novel compounds useful for the treatment of degenerative and inflammatory diseases
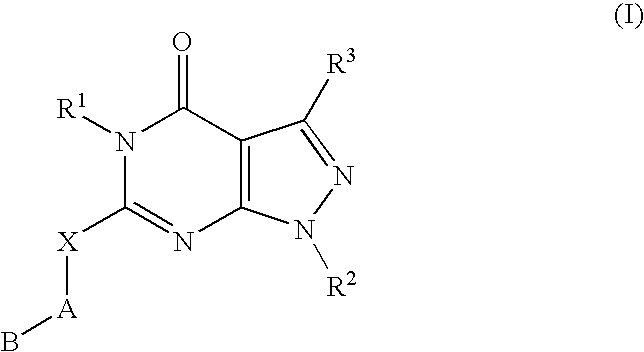
ActiveUS20100029709A1Prevent and treat any maladyModify activityBiocideOrganic chemistryMammalArthritis
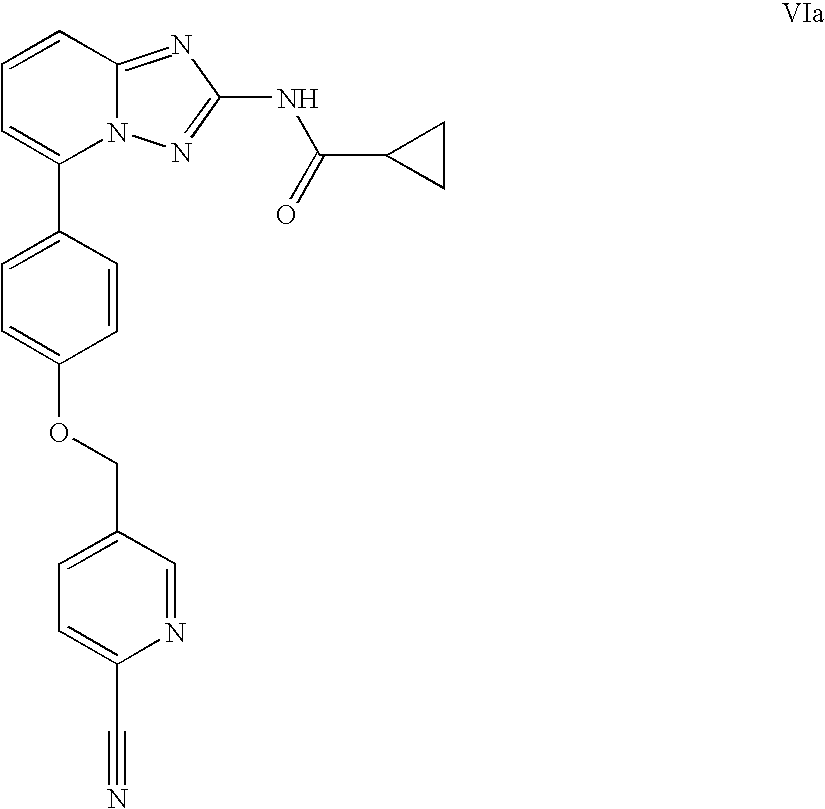
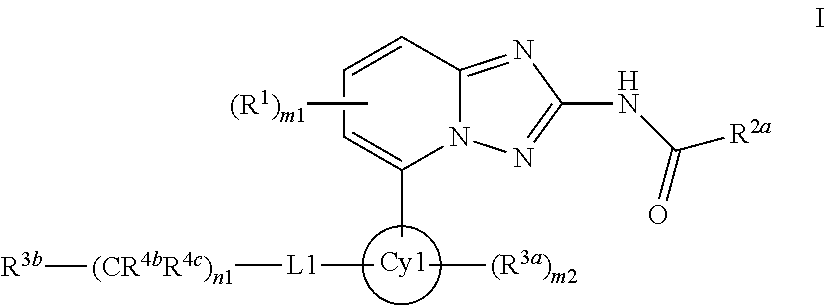
A novel [1,2,4]triazolo[1,5-a]pyridine compound is disclosed that has a formula represented by the following:This compound may be prepared as a pharmaceutical composition, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g. diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6 and transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
Treatment of degenerative cartilage conditions in a mammal with Glycosidasc Inhibitors
This invention relates to treating, preventing, and lessening the severity of conditions selected from the group consisting of osteoarthritis, rheumatoid arthritis, synovitis, subchondral bone edema, and cartilage degradation with administration of glycosidase inhibitors.
Owner:PANASONIC CORP +2
Novel compounds useful for the treatment of degenerative & inflamatory diseases
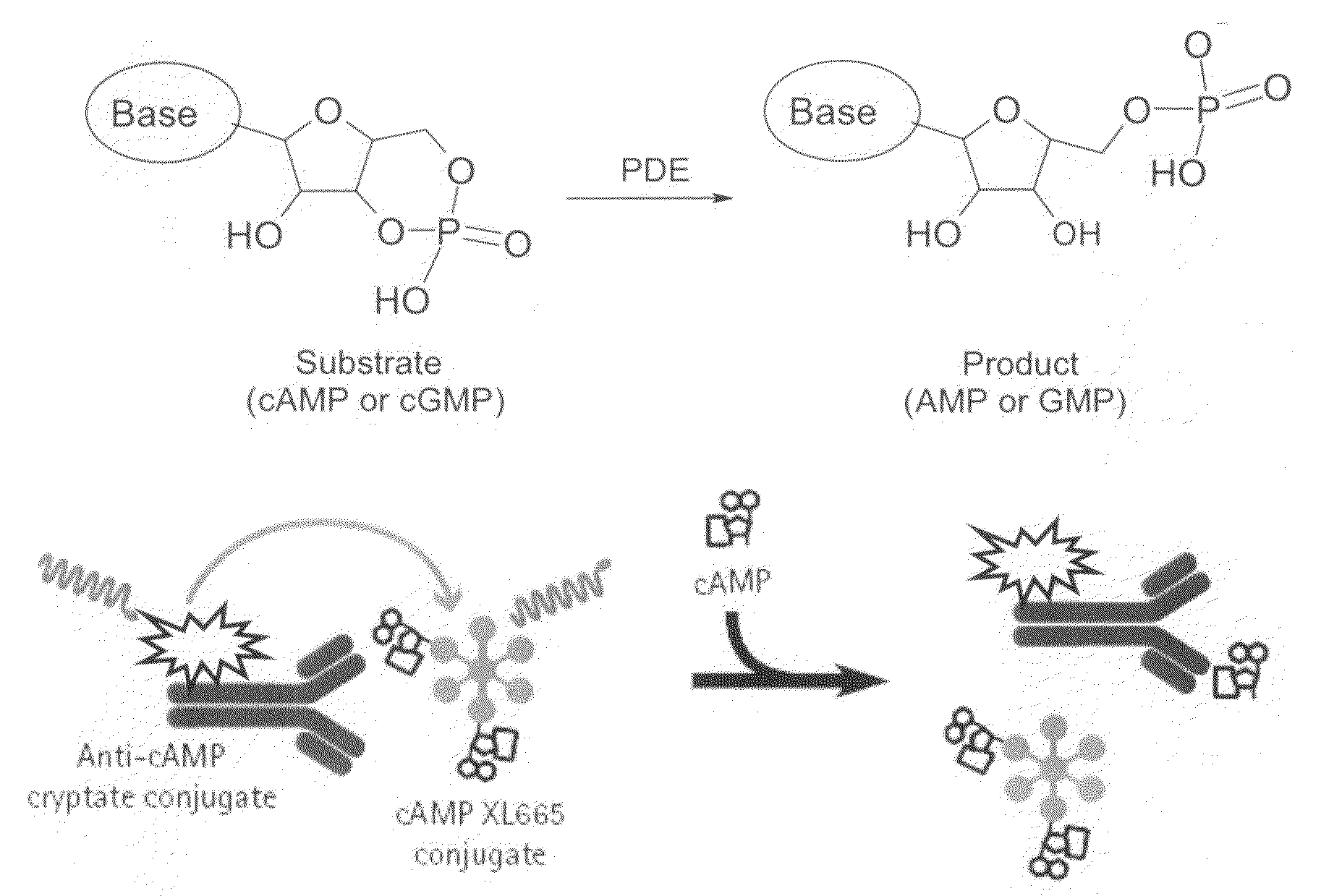
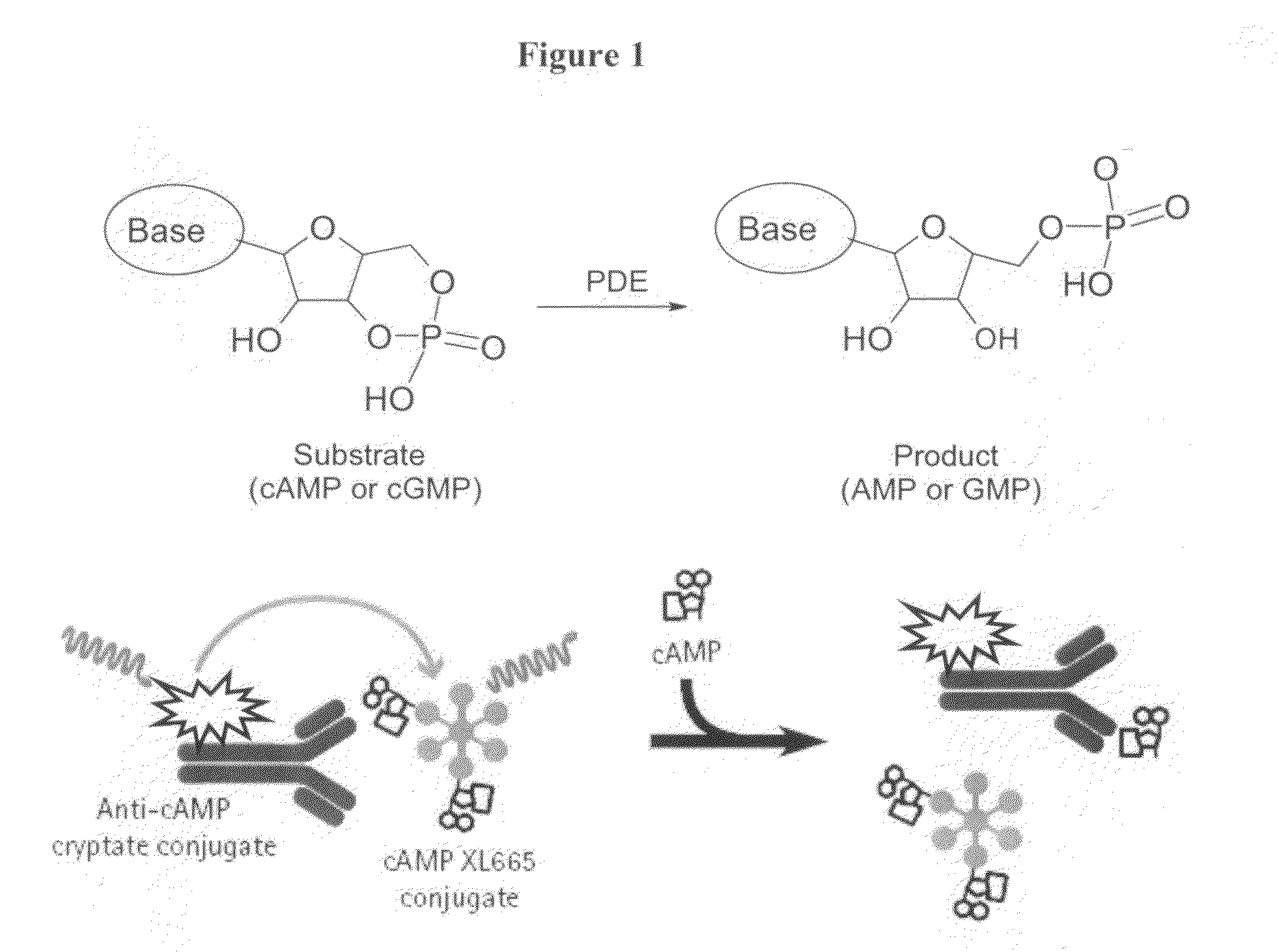
InactiveUS20090137549A1Useful in treatmentOrganic active ingredientsBiocidePhosphodiesteraseMedicine
The present invention relates to compounds that are inhibitors of PDE1A, a phosphodiesterase that is involved in the modulation of the degradation of cartilage, joint degeneration and diseases involving such degradation and / or inflammation.
Owner:GALAPAGOS NV
Cartilage extracellular matrix and silk fibroin composite orientation cartilage support and preparation method thereof
ActiveCN105251052AFix performance issuesStructural solutionAnimals/human peptidesProsthesisCell-Extracellular MatrixBiocompatibility Testing
The invention discloses a cartilage extracellular matrix and silk fibroin composite orientation cartilage support and a preparation method thereof and belongs to the biological tissue engineering technology. According to the cartilage extracellular matrix and silk fibroin composite orientation cartilage support and the preparation method of the cartilage support, cartilage extracellular matrixes are prepared through articular cartilage of people, pigs, cows or sheep, silk fibroin is prepared through mulberry silk, after the cartilage extracellular matrixes and the silk fibroin are evenly mixed according to a specific proportion, directed crystallization is performed under a certain temperature, ultraviolet cross linking is firstly performed after freezing and drying, and then cross linking of carbodiimide and N-hydroxysuccinimide or glutaraldehyde and genipin is performed so that the cartilage extracellular matrix and silk fibroin composite orientation cartilage support can be prepared. Due to the design, biocompatibility is good, and immunological rejective reaction is avoided; a composition structure and mechanical properties are similar to those of cartilage extracellular matrixes of people; material sources are wide, cost is low, the preparation technology is simple, the cartilage extracellular matrix and silk fibroin composite orientation cartilage support can be used for constructing tissue engineering cartilage and repairing cartilage degradation, and clinical application prospects are good.
Owner:TIANJIN HOSPITAL
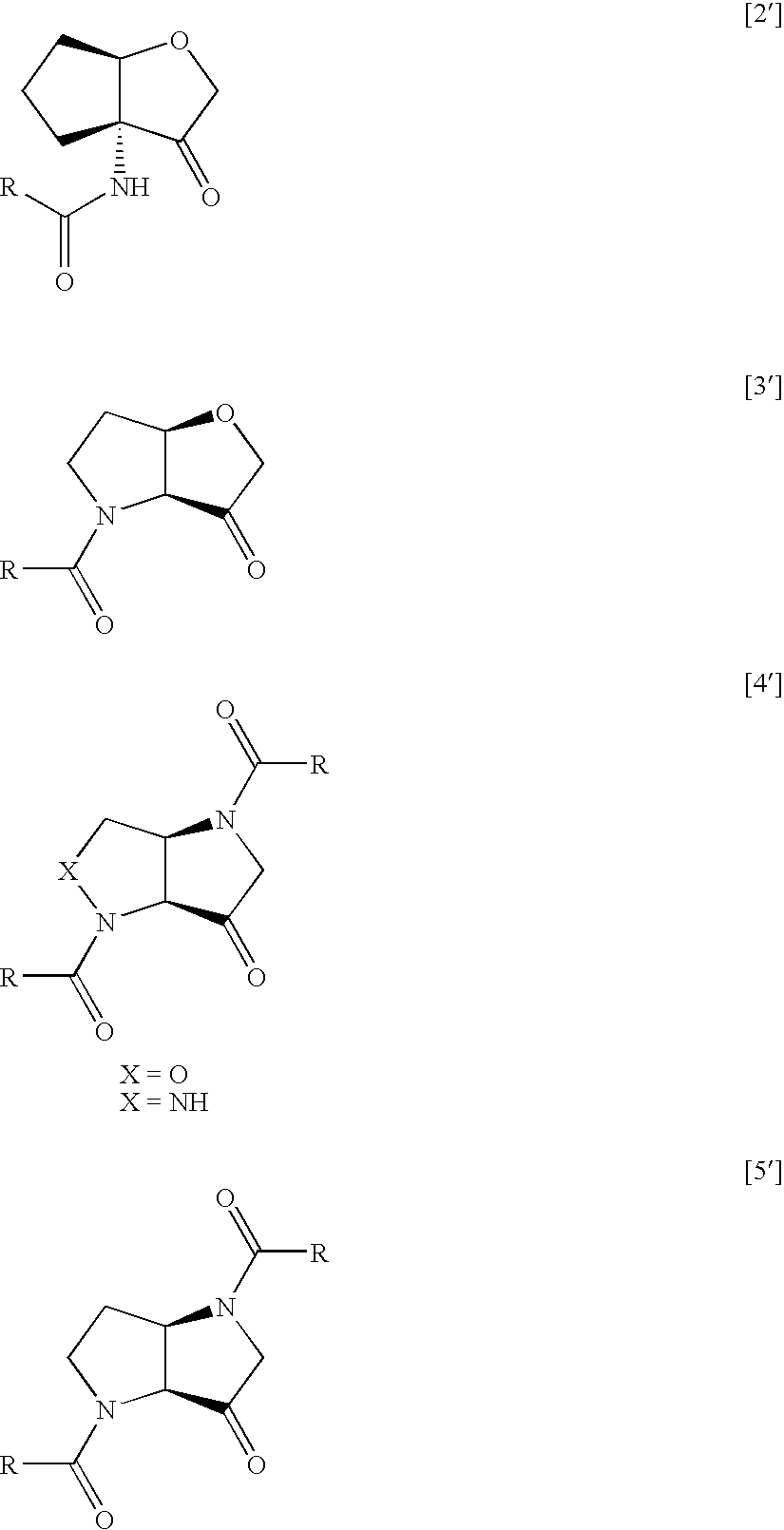
Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors
InactiveUS20100010009A1Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryGingival diseaseChagas disease
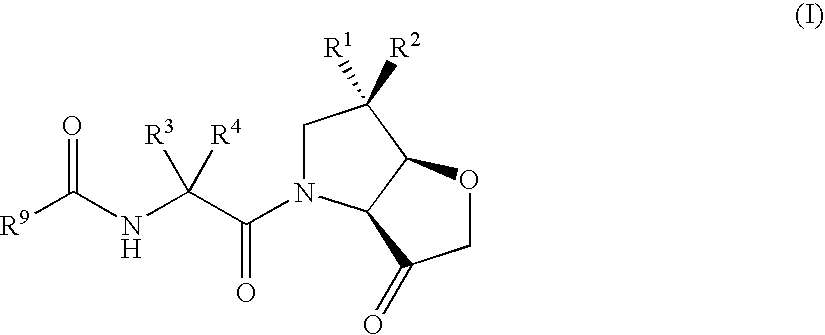
The present invention relates to compounds of formula (1), and pharmaceutically acceptable salts thereof, A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof (I), wherein: one of R1 and R2 is H, and the other is selected from F and Cl, or R1 and R2 are both F; R3 is selected from cyclopentyl and cyclohexyl; R4 is an optionally substituted 5- or 6-membered monocyclic or an 8- to 10-membered bicyclic aryl or heteroaryl ring which includes up to four heteroatoms. The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:GRUNENTHAL GMBH
Novel compounds useful for the treatment of degenerative and inflammatory diseases
ActiveUS20110190260A1Prevent and treat any maladyModify activityBiocideOrganic chemistryArthritisOsteocyte
[1,2,4]Triazolo[1,5-a]pyridine compounds are disclosed that have a formula represented by the formula (I). The compounds may be prepared as pharmaceutical compositions, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6, transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
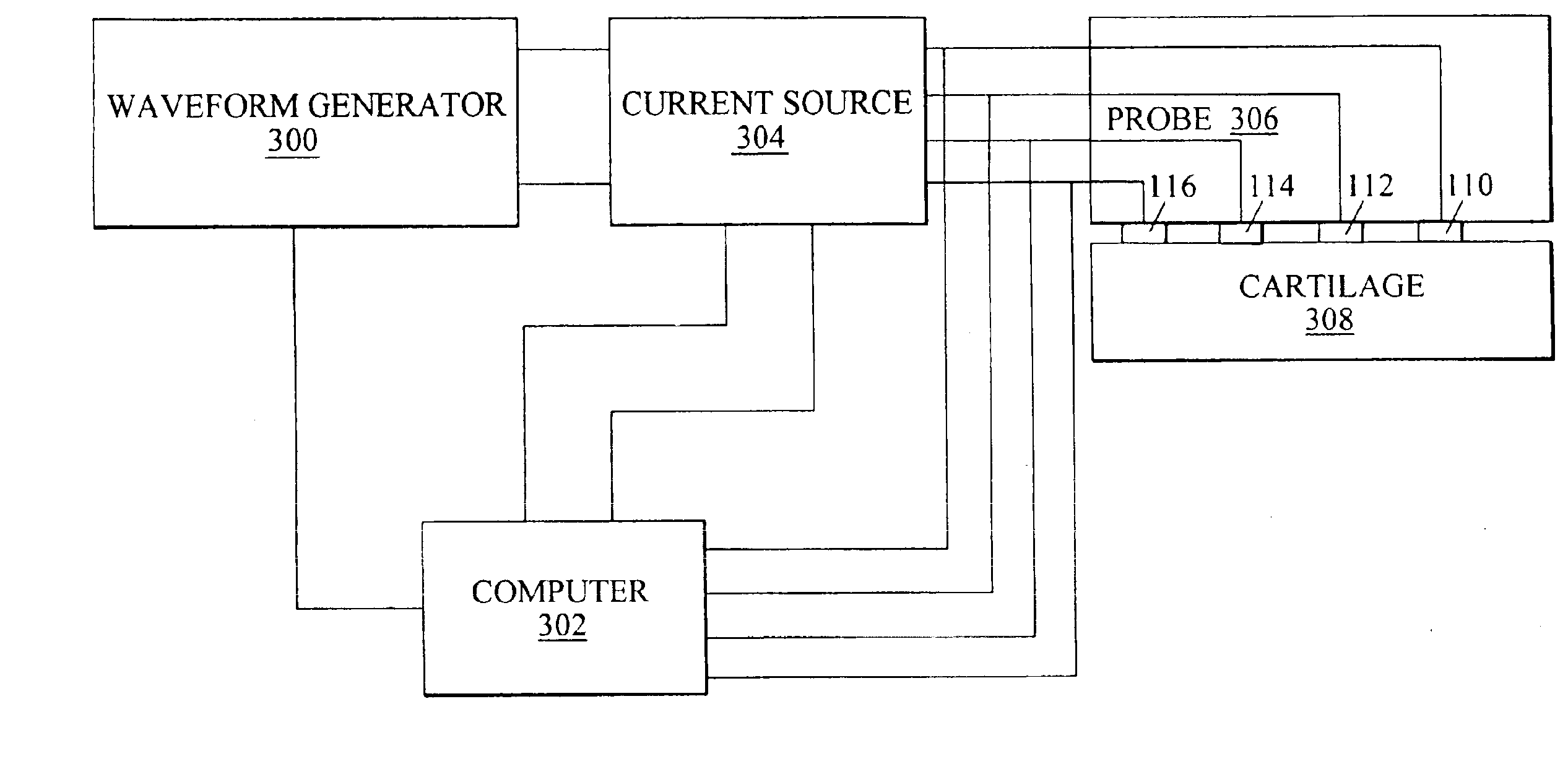
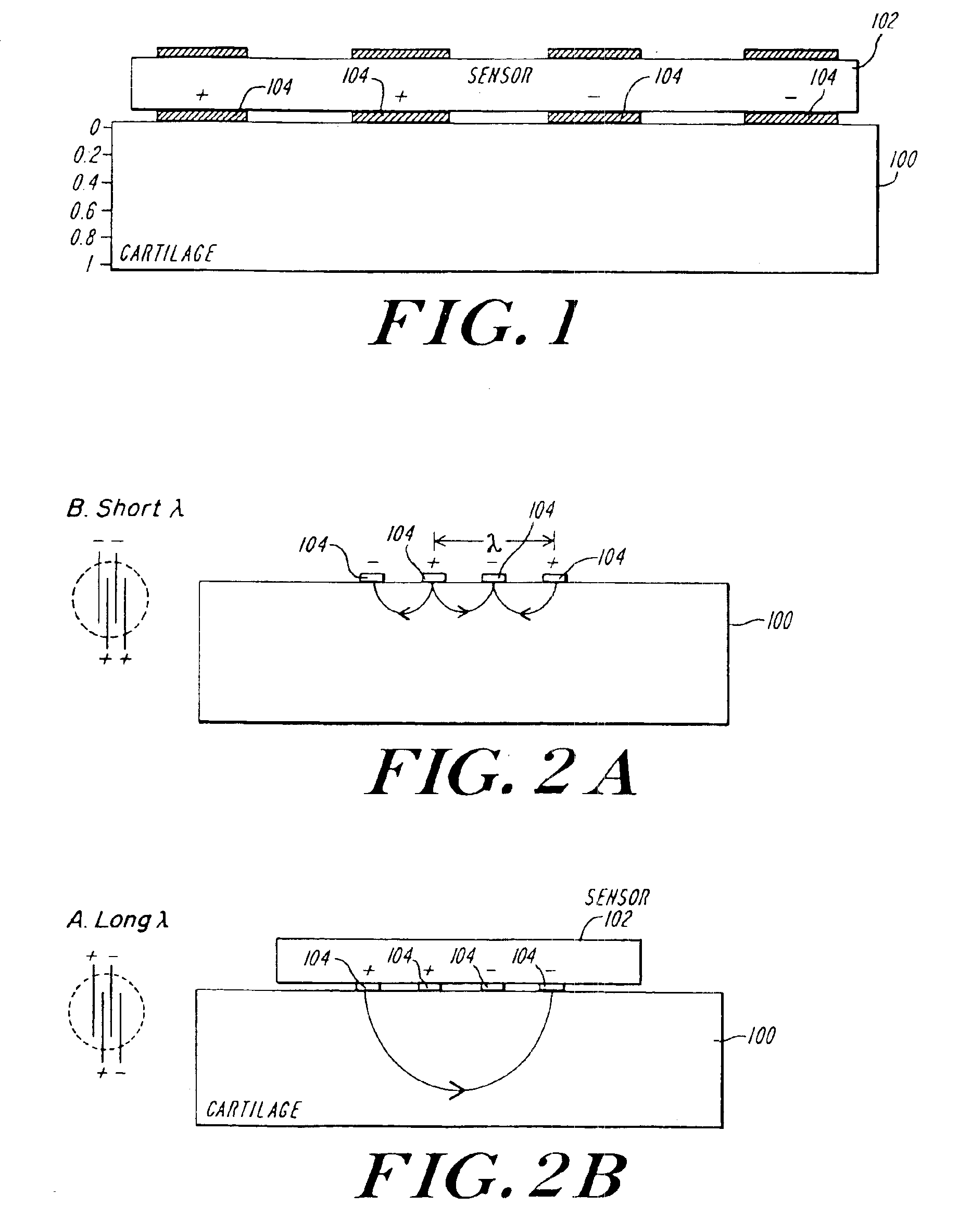
Arthroscopic impedance probe to detect cartilage degeneration
The change in tissue impedance due to the change in the extracellular matrix that results from the degradation of cartilage is utilized to detect degradation of articular cartilage. A probe includes electrodes that apply a current to the articular cartilage which results in a current distribution and electric field within the cartilage, along with an associated voltage drop across the electrodes. The amplitude of this voltage drop is then measured and divided by the current applied to determine the tissue impedance. By measuring the impedance of patient tissue and comparing the detected patient impedance to a normal value for the tissue from clinically normal tissue, a determination of whether the patient tissue is degraded, and a determination of the extent of degradation is possible. Preferably, the impedance is measured using a probe with interdigitated electrodes. By changing which electrodes are utilized, the wavelength of the current distribution changes, allowing the probe to image depth dependent focal lesions.
Owner:MASSACHUSETTS INST OF TECH
Liquid or paste compositions intended to provide elements essential for the synthesis and formation of proteoglycans, in particular, for the treatment of cartilage degradation
The invention relates to the field of pharmaceuticals, dietary supplements and food and, more specifically, to the field of dietary supplements intended for the treatment of degradation of cartilage of any origin. The invention relates to liquid or paste compositions based on glucosamin and chondroitin sulphate, intended to provide elements essential for the synthesis and formation of proteoglycans, in which the chondroitin sulphate / glucosamin combination is stabilised with the addition of carboxylic acids such that the pH of the medium is between 2 and 5 and the chemical degradation rate of the active substances is less than 10% when stored at 25° C. and 60% relative humidity for 10 months for doses of between 300 and 2400 mg chondroitin sulphate and between 500 and 3000 mg glucosamin. The invention is suitable for the treatment of cartilage degradation and, more specifically, arthrosis.
Owner:BORGE MATHIEU +2
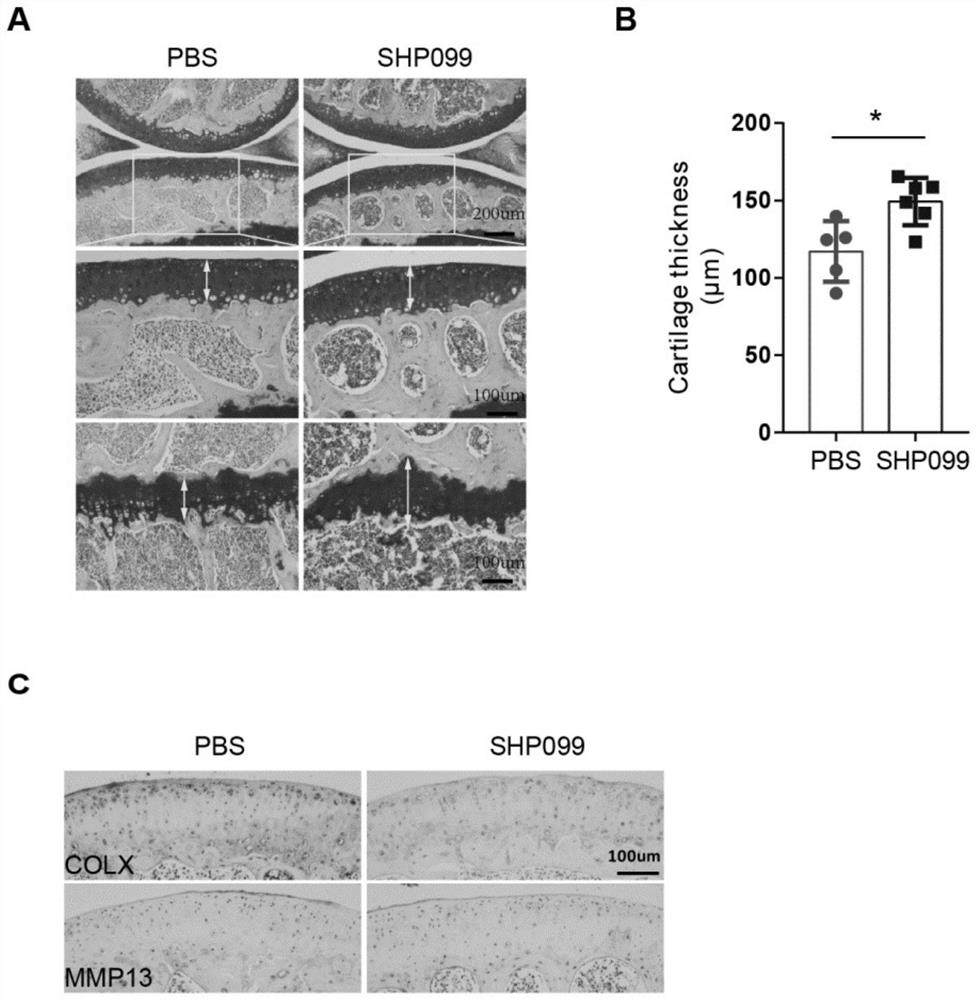
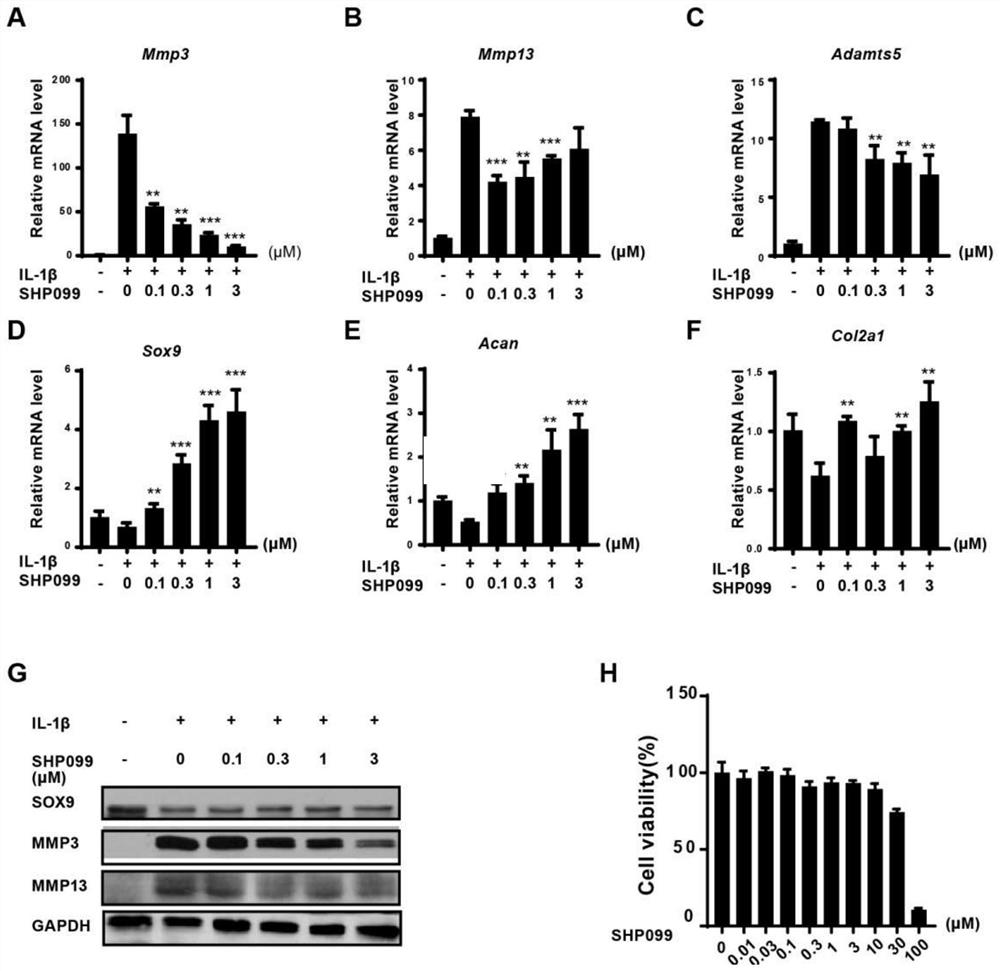
Application of protein tyrosine phosphatase SHP2 inhibitor in preparation of medicine for treating osteoarthritis (OA)
The invention discloses an SHP2 allosteric inhibitor SHP099 for treating osteoarthritis (OA). Aiming at a DMM model and a natural aging induced mouse OA disease model, the SHP099 can effectively inhibit mouse knee joint cartilage defects and promote cartilage repair effects. At the cellular level, the SHP099 remarkably reduces the level of cartilage degrading enzyme under the stimulation of IL-1beta and promotes the expression of cartilage synthesis genes, so that dual regulation on cartilage synthesis and catabolism are realized. In addition, the SHP099 also has the effect of promoting cartilage cell differentiation at a concentration gradient.
Owner:NANJING UNIV
Tetrahydrofuro(3,2-B) pyrrol-3-one derivatives as inhibitors of cysteine proteinases
InactiveUS7799791B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseChagas disease
A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof,wherein: one of R1 and R2 is H, and the other is selected from OR6, SR6, NR6R7, N3, Me, Et, CF3, SOR8 and SO2R8; orR1 and R2 are both H;one of R3 and R4 is H, and the other is selected from tert-butylmethyl, iso-propylmethyl, sec-butyl, tert-butyl, cyclopentyl and cyclohexyl; orR3 and R4 are joined together with the adjacent backbone carbon atom to form a spiro-C5-C6 cycloalkyl group;R6 and R7 are each independently selected from H, C1-8-alkyl and C3-8-cycloalkyl; orR6 and R7 are linked to form a cyclic group together with the nitrogen to which they are attached;R8 is C1-8-alkyl or C3-8-cycloalkyl;R9 is a para-substituted 6-membered monocyclic aryl or heteroaryl ring which includes up to five heteroatoms.The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors
InactiveUS7846935B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseGingival disease
Owner:AMURA THERAPEUTICS
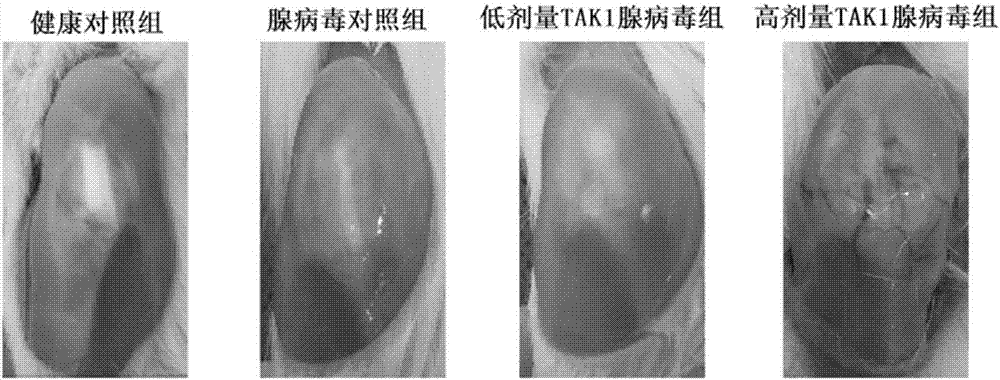
Application of resorcylic acid lactone 5Z-7 in preparation of medicament for treating osteoarthritis
ActiveCN107303295AEffective treatmentFacilitate the processOrganic active ingredientsAntipyreticSide effectArticular cavity
The invention discloses application of resorcylic acid lactone 5Z-7 in preparation of a medicament for treating osteoarthritis and belongs to the field of biological medicines. The resorcylic acid lactone 5Z-7 is a small-molecule inhibitor for TAK1, and activation of immuno-associated pathway, such as NF-kB, p38 and JNK mediated by inflammatory cytolines and a matrix degradation product in synovium and cartilage tissue can be inhibited through targeted inhibition of the enzymatic activity of the TAK1 in an articular cavity, thereby achieving the targets of reducing synovial inflammatory response, inhibiting cartilage degradation and improving the microenvironment of the articular cavity through controlling a series of gene expression related to the osteoarthritis on the downstream of the pathways; the resorcylic acid lactone 5Z-7 is of great significance in delaying the osteoarthritis process and effectively treating the osteoarthritis. In addition, the resorcylic acid lactone 5Z-7 is taken as a small molecule compound, the action target is single and high in specificity, and the resorcylic acid lactone 5Z-7 is free of a toxic or side effect on cartilage tissue through detection, and is safer and more reliable.
Owner:PEKING UNIV THIRD HOSPITAL
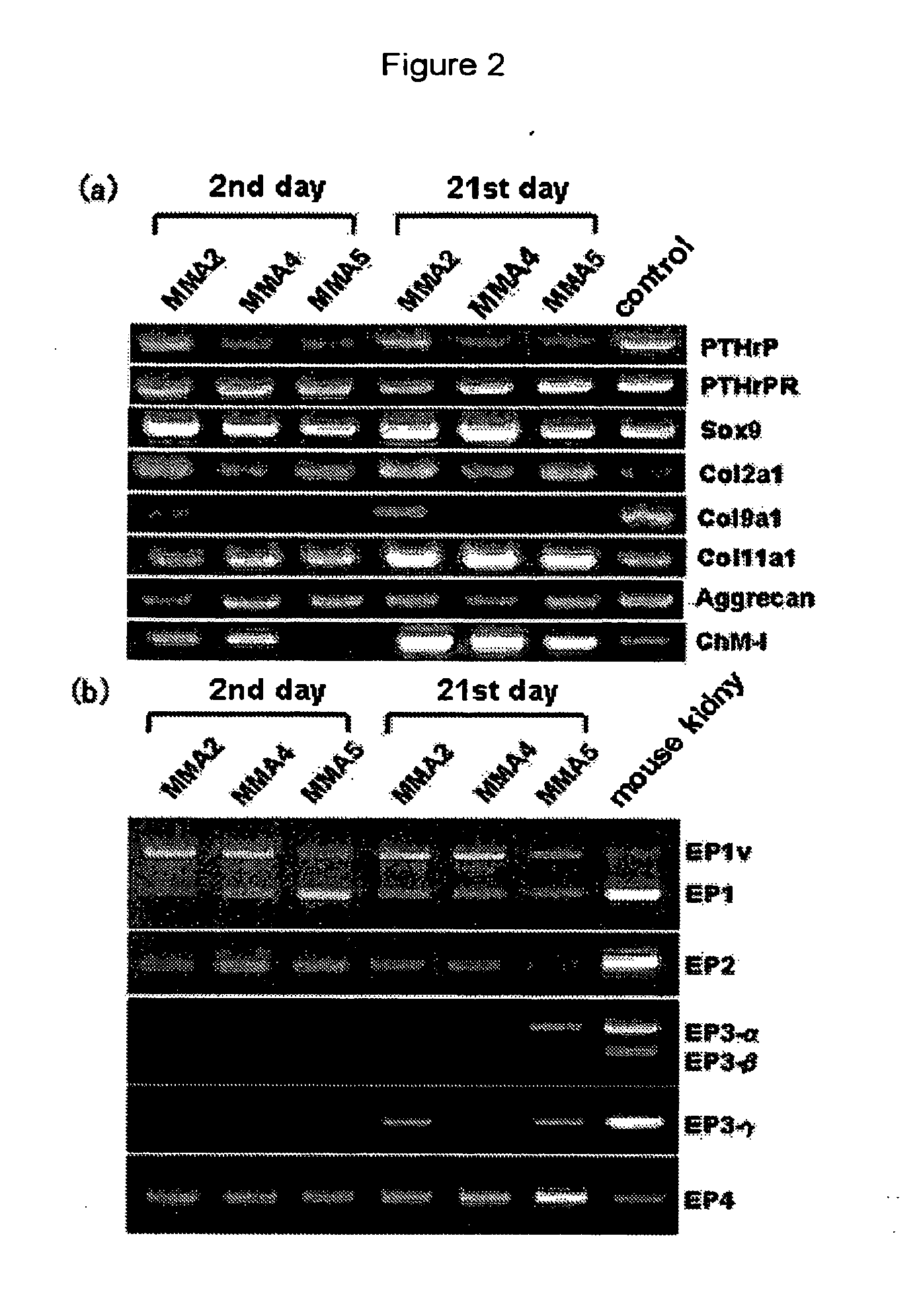
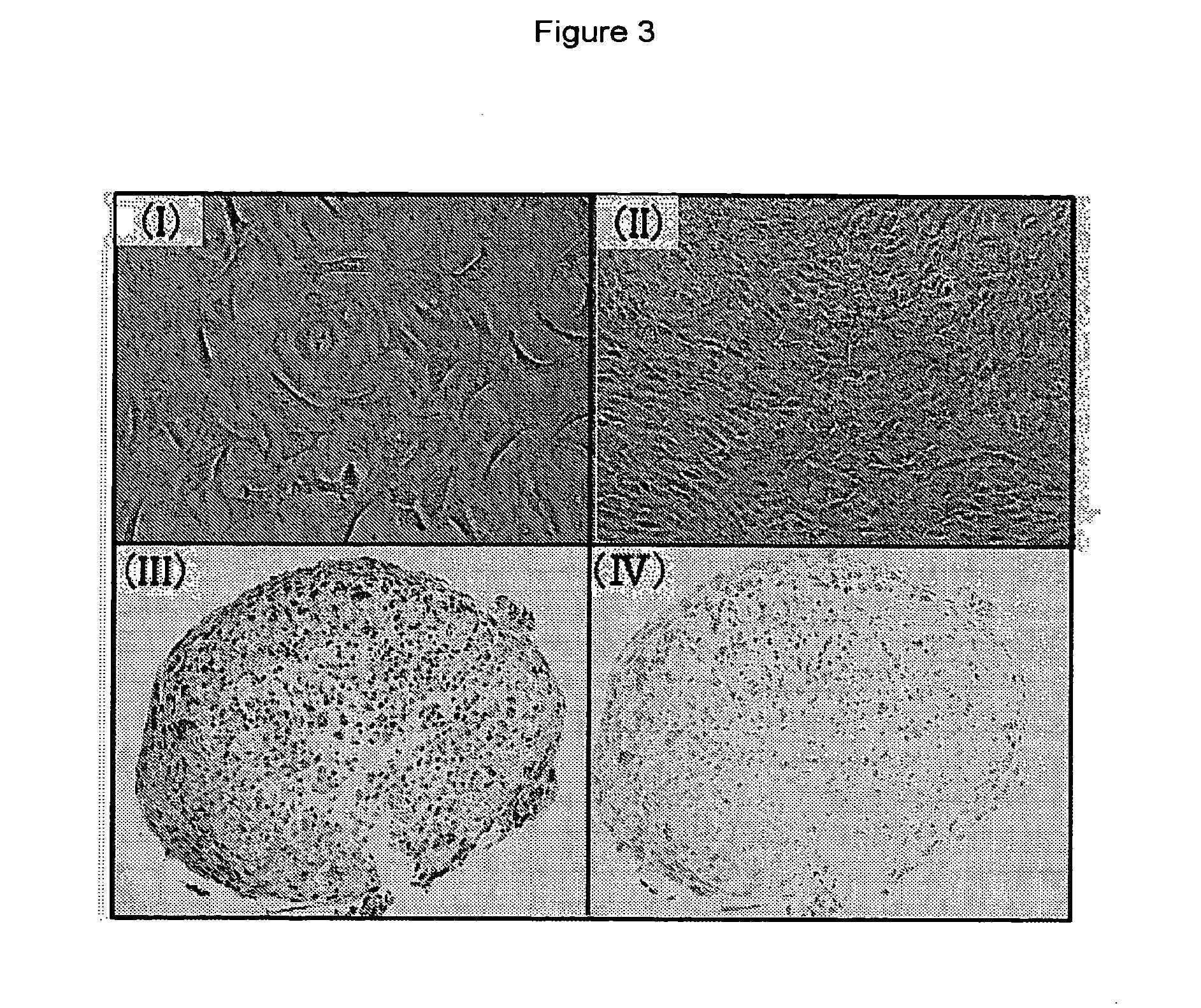
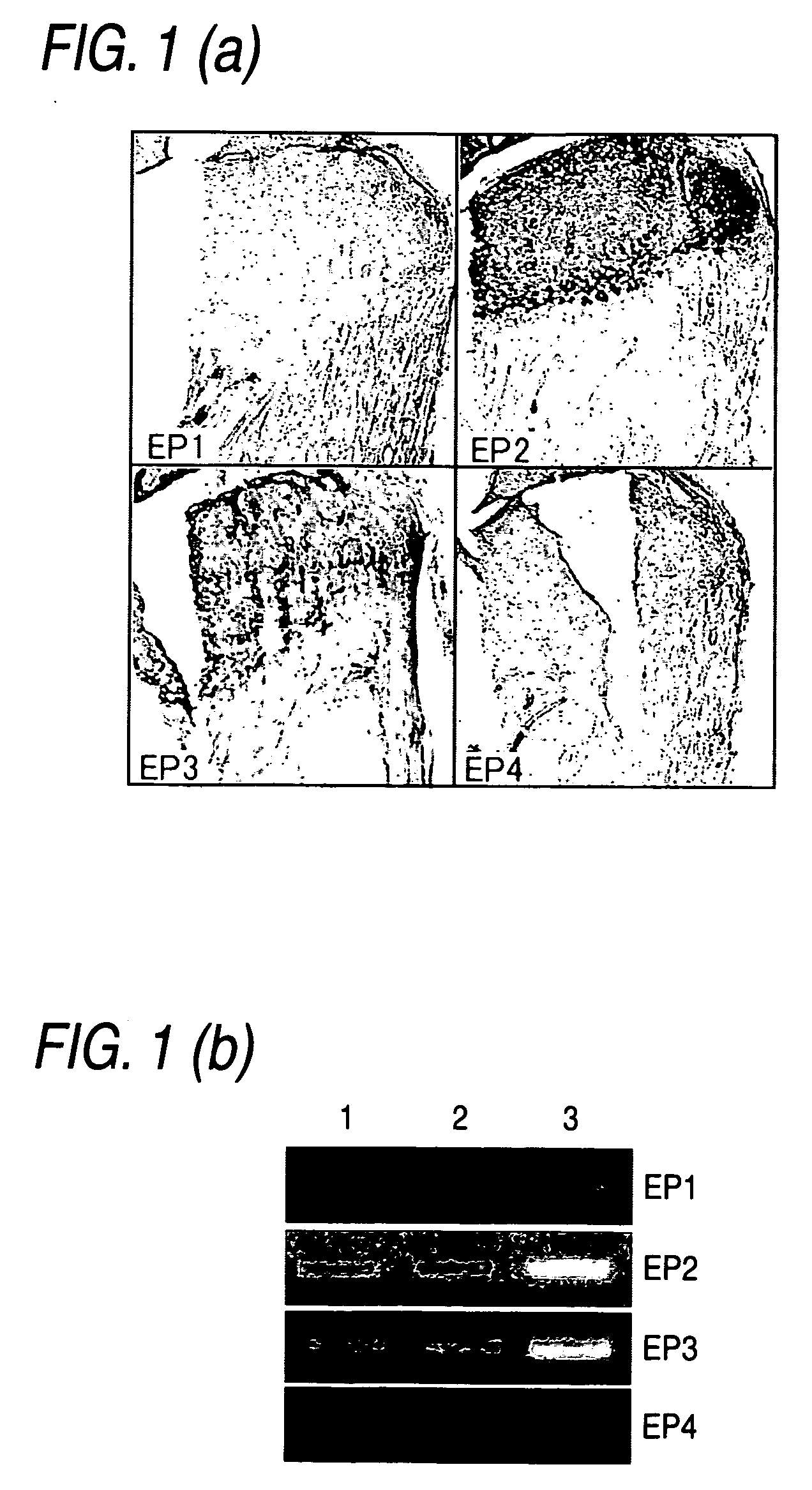
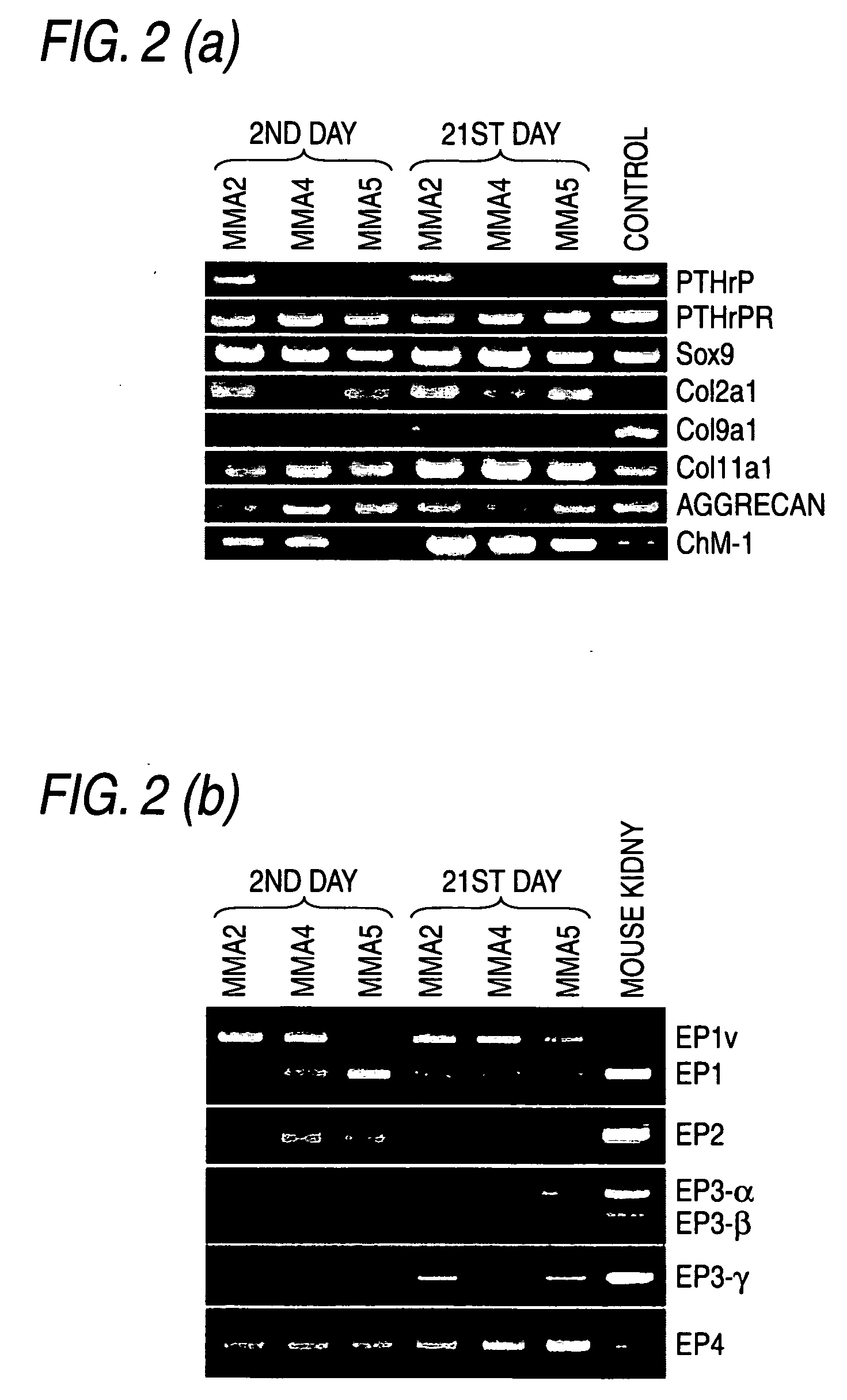
Remedy for cartilage-related diseases
The present invention relates an agent for treating cartilage-related disease comprising as an active ingredient a substance having an EP2 and / or EP3 agonist activity. A substance having an agonist activity to EP2 and / or EP3 has effects of stimulating chondrogenesis, stimulating chondrocyte growth, stimulating chondrocyte differentiation, inhibiting cartilage calcification and inhibiting cartilage degradation, or effects of stimulating integrin mRNA expression, stimulating fibronectin mRNA expression, stimulating D1 mRNA expression and inhibiting osteopontin mRNA expression, and, therefore, is useful as an agent for treating cartilage-related disease.
Owner:ONO PHARMA CO LTD
Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors
InactiveUS7803803B2Improve efficacyDesirable pharmacokinetic propertyAntibacterial agentsBiocideDiseaseCathepsin K
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof,wherein:X is CH or N;one of R1 and R2 is H, and the other is selected from OR6, SR6, NR6R7, N3, Me, Et, CF3, SOR8 and SO2R8;R3 is selected from tert-butylmethyl, iso-propylmethyl, sec-butyl, tert-butyl, cyclopentyl and cyclohexyl;R4 is optionally substituted C1-8 alkyl or optionally substituted C3-8 cycloalkyl;R6 and R7 are each independently selected from H, C1-8-alkyl and C3-8-cycloalkyl, or R6 and R7 are linked to form a cyclic group together with the nitrogen to which they are attached; andR8 is C1-8-alkyl or C3-8-cycloalkyl.The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
Glucosamine chondroitin sulfate calcium-adding tablet and preparation method
InactiveCN111467480ADisperses blood stasis and stops bleedingHas the ability to dissipate blood stasis and stop bleedingPeptide/protein ingredientsHydroxy compound active ingredientsMagnesium stearateStearic acid
The invention discloses a glucosamine chondroitin sulfate calcium-adding tablet and a preparation method. The glucosamine chondroitin sulfate calcium-adding tablet contains 2-amino-2-deoxy-D-glucose,crabshell, shark cartilage powder, pig rib powder, collagen, dimethyl sulfone, calcium phosphate, calcium gluconate, goat milk calcium, magnesium stearate, xylitol, vitamins and functional peptides. The shark cartilage is added, the viscous polysaccharide rich in chondroitin in the shark cartilage can rebuild articular cartilage and is very helpful for cartilage degradation, and the crabshell hasthe functions of dispelling blood stasis, stopping bleeding and removing toxicity for detumescence. The natural calcium extracted from goat milk has high nutritional value, is easy to digest and absorb and high in absorption rate, does not stimulate the stomach, and does not cause any toxic side effects.
Owner:SHANDONG ZHUSHI PHARMA GRP CO LTD
Remedy for Cartilage-Related Diseases
InactiveUS20070270489A1Prevent calcificationPrevent degradationBiocidePeptide/protein ingredientsCalcificationBULK ACTIVE INGREDIENT
The present invention relates an agent for treating cartilage-related disease comprising as an active ingredient a substance having an EP2 and / or EP3 agonist activity. A substance having an agonist activity to EP2 and / or EP3 has effects of stimulating chondrogenesis, stimulating chondrocyte growth, stimulating chondrocyte differentiation, inhibiting cartilage calcification and inhibiting cartilage degradation, or effects of stimulating integrin mRNA expression, stimulating fibronectin mRNA expression, stimulating D1 mRNA expression and inhibiting osteopontin mRNA expression, and, therefore, is useful as an agent for treating cartilage-related disease.
Owner:ONO PHARMA CO LTD
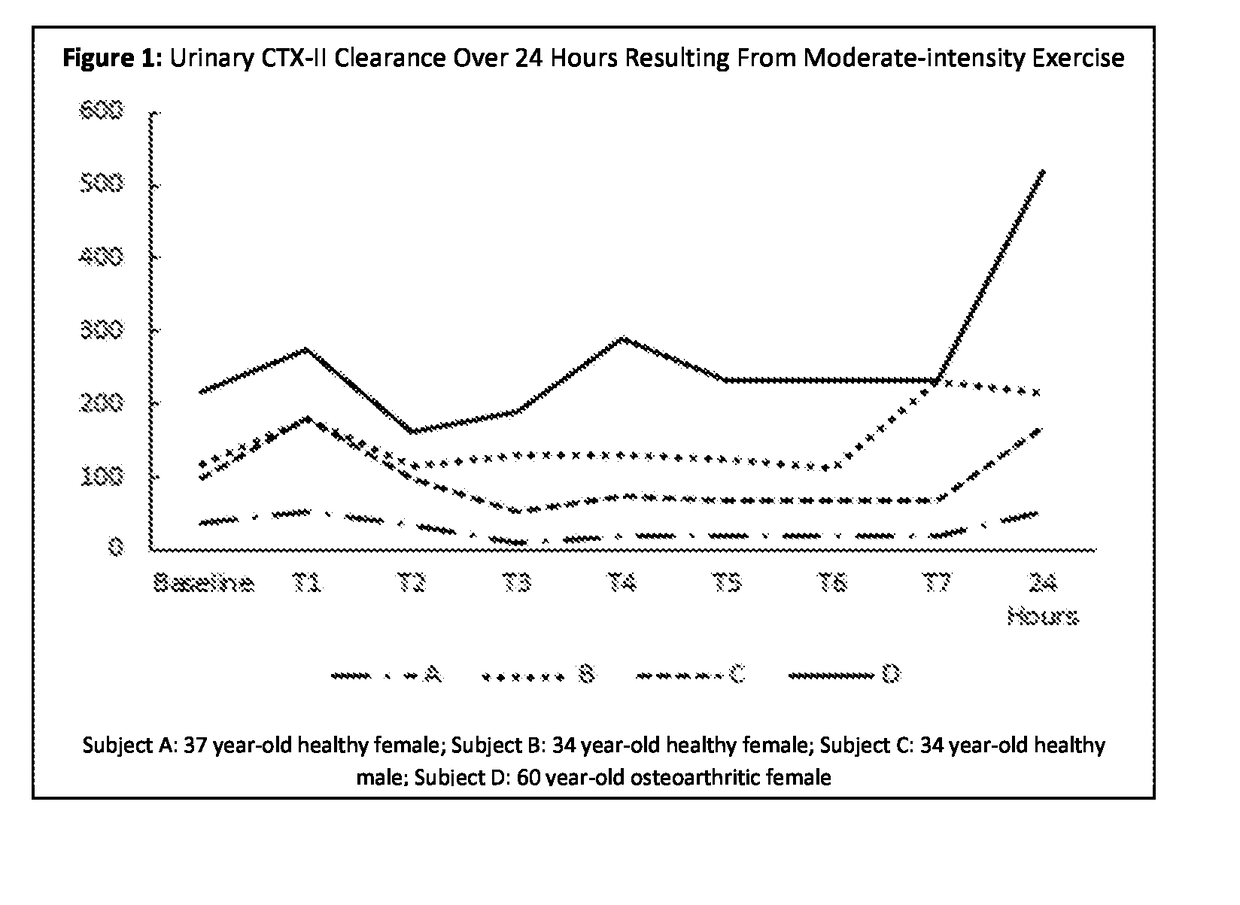
Method for evaluating articular joint therapeutics
ActiveUS20170299611A1Effective conditioningReduce changesDisease diagnosisBird material medical ingredientsMammalOrganism
The invention provides a method for determining the efficacy of compositions used to treat articular joint conditions in mammals. The method includes measuring the change in levels of one or more cartilage degradation biomarkers in a mammal from before exercise and after exercise, then administering a composition used to treat articular joint conditions to the mammal, and measuring the change in levels of one or more cartilage degradation biomarkers in the mammal from before exercise and after exercise.
Owner:ESM TECH
Composition and method for use in cartilage affecting conditions
ActiveUS8377904B2Decreasing cartilage abnormalityGood for healthBiocidePeptide/protein ingredientsMedicineManganese
Owner:HILLS PET NUTRITION INC
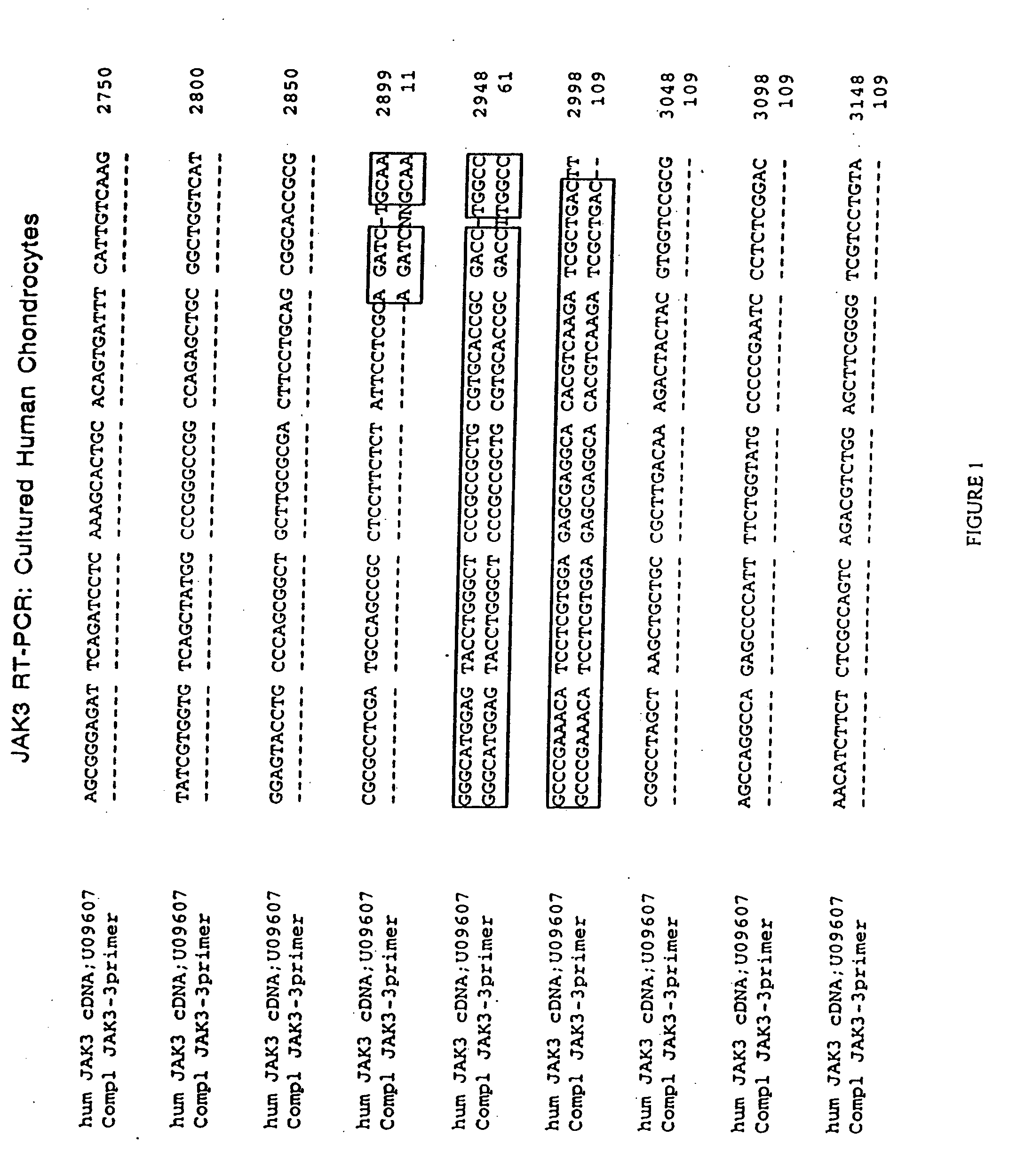
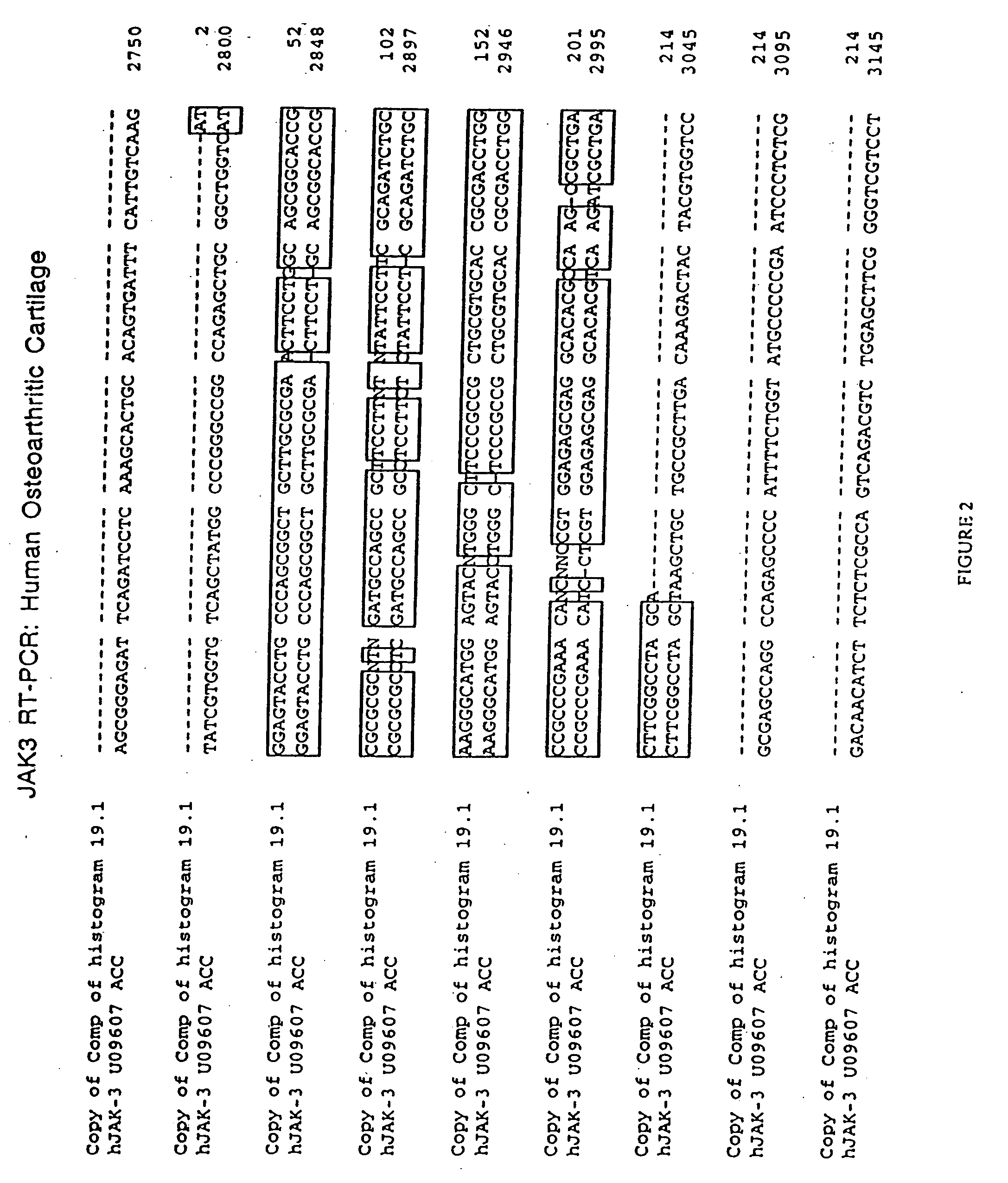
JAK/STAT pathway inhibitors and uses thereof
The role of the JAK / STAT signal transduction pathway cellular mechanisms that lead to the onset and progression of degenerative joint diseases or disorders such as osteoarthritis (OA) is disclosed. Certain known effective OA therapeutics such as hymenialdisine, debromohymenialdisine, and its variants and derivatives are shown to function as JAK3-specific inhibitors, which downregulate steady state mRNA levels of key cellular components involved in cartilage degradation. Another JAK3-specific inhibitor, not previously known as an OA therapeutic, is shown to downregulate steady state mRNA levels of various cellular components involved in cartilage degradation in a manner identical to that of the known OA therapeutics.
Owner:GENZYME CORP
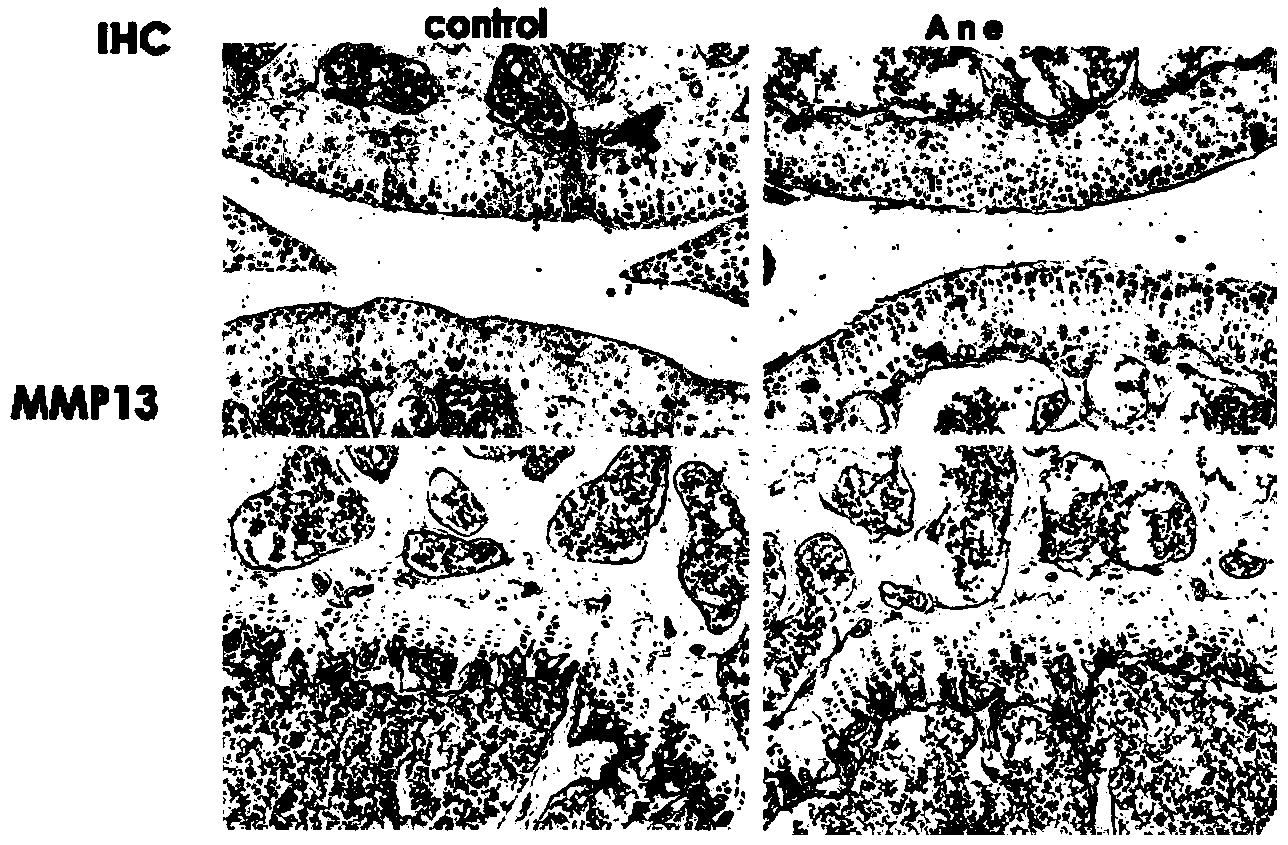
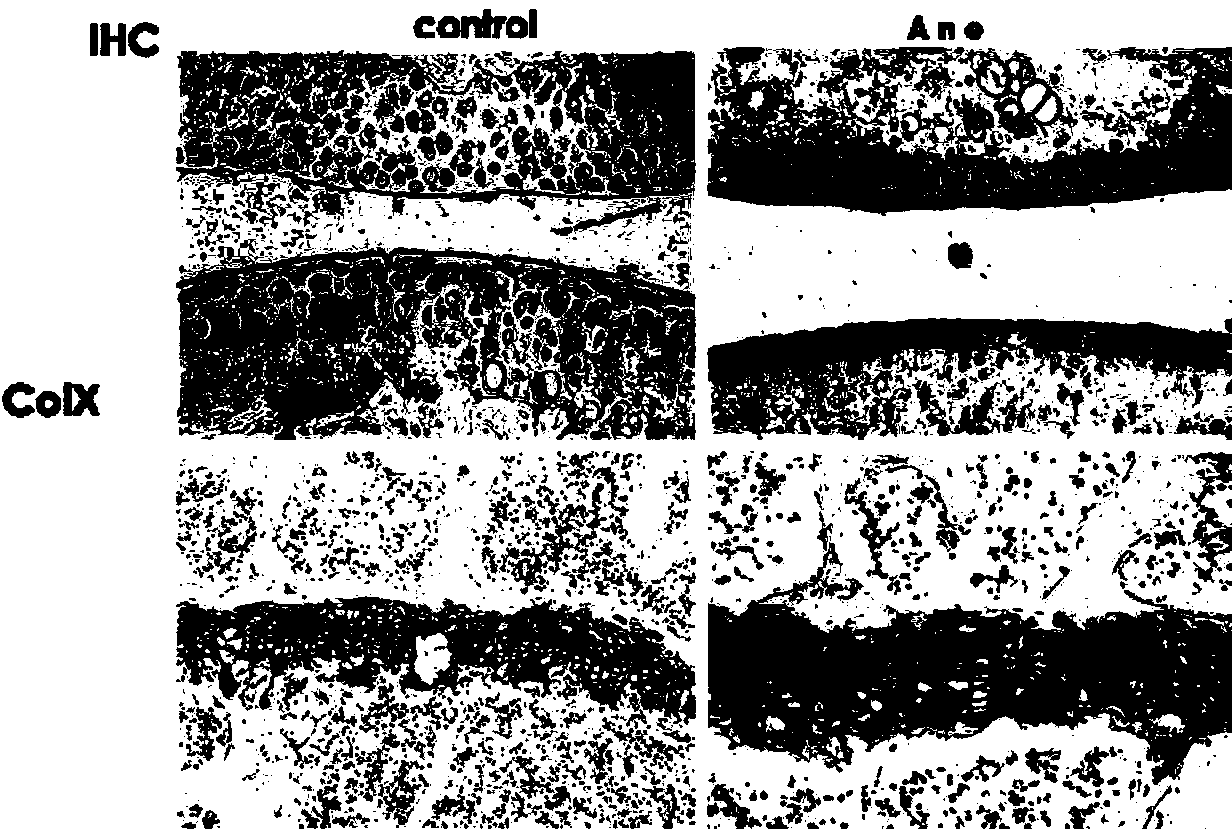
Application of anemonin in preparation of medicines for treating osteoarthritis
The invention discloses application of anemonin in the preparation of medicines for treating osteoarthritis. After a mouse with the osteoarthritis is treated by virtue of anemonin, the degradation of joint cartilages and the hypertrophy of cartilages can be restrained, and the expression quantities of MMP13 and Collagen X are reduced. According to the application, the pharmaceutical use of anemonin is broadened, a new drug for treating the osteoarthritis by preventing the degradation cartilage matrixes and the abnormal hypertrophy of cartilage cells, and diseases can be treated based on the pathogenesis of the osteoarthritis in a targeted manner; the application has a very important significance on the clinic treatment of the osteoarthritis.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Application of rhizoma corydalis pain-relieving dropping pills to treatment of knee osteoarthritis
InactiveCN111514193AImprove securityAvoid degradationAntipyreticAnalgesicsInflammatory factorsPainful joints
The invention belongs to the technical field of medicines, and particularly relates to new application of rhizoma corydalis pain-relieving dropping pills. The inventor accidentally finds that: the rhizoma corydalis pain-relieving dropping pills can inhibit inflammatory factors by being singly used, have the effects of inhibiting cartilage degradation, promoting cartilage cell proliferation, promoting proteoglycan synthesis and the like, and has the effects superior to those of single use of diclofenac sodium sustained-release capsule and the combined use of the diclofenac sodium sustained-release capsule and the rhizoma corydalis pain-relieving dropping pills; and the inventor finds that the rhizoma corydalis pain-relieving dropping pills can obviously relieve arthralgia and ankylosis andpromote the recovery of joint activity when being independently used at the clinical level, and have good clinical treatment effect on KOA, and have obvious effect superior to that of diclofenac sodium. Compared with the diclofenac sodium, the rhizoma corydalis pain-relieving dropping pills can obviously reduce adverse reactions such as gastrointestinal tract irritation and the like when being singly used, and are high in safety after being taken for a long time.
Owner:GANSU LONGSHENRONGFA PHARMACEUTICAL INDUSTRY CO LTD
Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors
InactiveUS8877927B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryDiseaseGingival disease
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof, A compound of formula (I), or a pharmaceutically acceptable salt, hydrate, complex or pro-drug thereof (I), wherein: one of R1 and R2 is H, and the other is selected from F and Cl, or R1 and R2 are both F; R3 is selected from cyclopentyl and cyclohexyl; R4 is an optionally substituted 5- or 6-membered monocyclic or an 8- to 10-membered bicyclic aryl or heteroaryl ring which includes up to four heteroatoms. The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:GRUNENTHAL GMBH
Furo[3. 2-b] pyrrol derivatives
InactiveUS20100216811A1Improve efficacyDesirable pharmacokinetic propertyAntibacterial agentsBiocideGingival diseaseChagas disease
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof, wherein: R3 is tert-butylmethyl, sec-butyl or tert-butyl; X is CH or N; and R4 is optionally substituted C1-8 alkyl or optionally substituted C3-8 cycloalkyl. The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors
InactiveUS7846934B2Improve efficacyDesirable pharmacokinetic propertyBiocideOrganic chemistryGingival diseasePharmaceutical medicine
The present invention relates to compounds of formula (I), and pharmaceutically acceptable salts thereof (I), wherein: R3 is cyclopentyl or cyclohexyl; X is CH or N; and R4 is optionally substituted C1-8 alkyl or optionally substituted C3-8 cycloalkyl. The invention further relates to pharmaceutical compositions comprising compounds of formula (I), and the use of such compounds in the treatment of a disease selected from osteoporosis, Paget's disease, Chagas's disease, malaria, gingival diseases, hypercalaemia, metabolic bone disease, diseases involving matrix or cartilage degradation, and bone cancer disorders such as bone metastases and associated pain.
Owner:AMURA THERAPEUTICS
Nutraceutical composition and methods of use
ActiveUS20100297254A1High activityPromote growthOrganic active ingredientsBiocideBiological bodyChemical composition
A method of treatment for cartilage degradation in an organism, which includes administering to an organism a composition including a therapeutic amount of an extract from the plant Biota orientalis. Several key components of the extract of Biota orientalis have been identified that have also been shown to have an effect in dramatically reducing and reversing cartilage degradation.
Owner:DACY TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00001.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00002.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl porteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/028210cc-fd6a-4a6c-a658-85011196fcf8/US20100010009A1-20100114-C00003.png)



![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00001.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00002.png)
![Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B]pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14db6556-9167-4d01-9494-1a0c5bb5c3c3/US07846935-20101207-C00003.png)



![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00001.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00002.png)
![Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors Tetrahydrofuro[3,2-B] pyrrol-3-ones as cathepsin K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e552e797-de11-4b11-a941-8beb1053b4a9/US07803803-20100928-C00003.png)





![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a265fe9-8c5f-4847-aca6-8ad8508ffa3f/US08877927-20141104-C00001.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a265fe9-8c5f-4847-aca6-8ad8508ffa3f/US08877927-20141104-C00002.png)
![Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol -3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a265fe9-8c5f-4847-aca6-8ad8508ffa3f/US08877927-20141104-C00003.png)
![Furo[3. 2-b] pyrrol derivatives Furo[3. 2-b] pyrrol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34c2d71e-fc80-4e13-a461-a4e9c5a0287a/US20100216811A1-20100826-C00001.png)
![Furo[3. 2-b] pyrrol derivatives Furo[3. 2-b] pyrrol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34c2d71e-fc80-4e13-a461-a4e9c5a0287a/US20100216811A1-20100826-C00002.png)
![Furo[3. 2-b] pyrrol derivatives Furo[3. 2-b] pyrrol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34c2d71e-fc80-4e13-a461-a4e9c5a0287a/US20100216811A1-20100826-C00003.png)
![Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/481149d7-e564-4592-aafa-e9b5876a063d/US07846934-20101207-C00001.png)
![Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/481149d7-e564-4592-aafa-e9b5876a063d/US07846934-20101207-C00002.png)
![Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors Furo[3,2-B] pyrrol-3-one derivatives and their use as cysteinyl proteinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/481149d7-e564-4592-aafa-e9b5876a063d/US07846934-20101207-C00003.png)