Non-catalytic permeable membrane reactor for producing hydrogen from ammonia-containing tail gas in MOCVD process and application
A membrane reactor, non-catalytic technology, applied in hydrogen production, chemical instruments and methods, specific gas purification/separation, etc., can solve the problems of increased energy consumption, poor selectivity of hydrogen production, high partial pressure, etc., to reduce load and Cost, reduction of exhaust emissions, and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
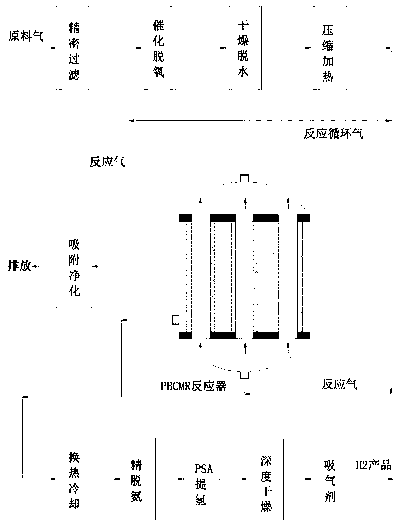
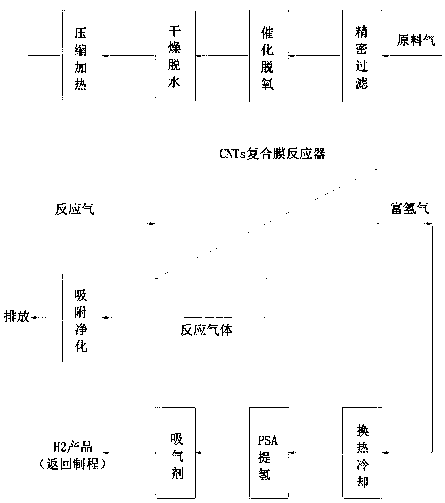
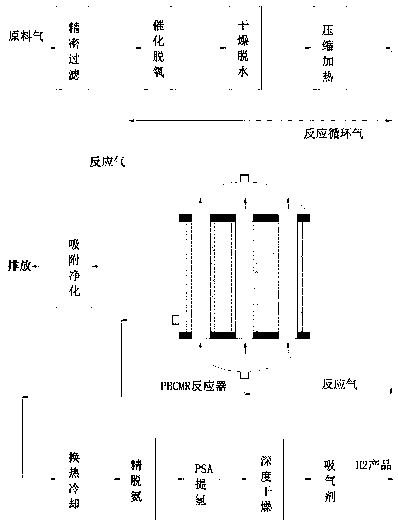
[0031] Such as figure 1 As shown, a non-catalytic permeable membrane reactor for hydrogen production from tail gas containing ammonia in MOCVD process and its application, which contains active components cobalt 15~20% (w / w, the same below), molybdenum 14~16%, containing Catalyst particles composed of 4-6% lanthanum as a promoter component, 3-5% potassium as a promoter, and 53-64% pretreated carbon nanotubes (CNTs), loaded on alumina as a support In the inorganic ceramic membrane tube coated with a metal palladium membrane layer, the thickness of the membrane layer is 1 micron, forming a non-catalytic permeable membrane reactor (PBCMR, Packed Bed Catalytic Membrane Reactor) with a fixed bed of catalyst filled in the membrane tube, according to The following steps carry out the catalytic thermal cracking hydrogen production reaction of ammonia,
[0032] (1) The raw material gas is from the production of LED-GaN epitaxial wafers, and its MOCVD epitaxial tail gas is mainly compo...
Embodiment 2
[0035] The pretreatment of the multilayer carbon nanotube CNTs described in Example 1 is to add about 1 to 2 g of commercially sold CNTs carriers with a specification of 10 nm into a total volume of about 230 to 260 mL of nitric acid with a mass concentration of 30% and about 70% In the mixed solution of nitric acid with mass concentration, heat to 110~120°C, stir evenly, and azeotropically reflux at 110~120°C for 6~8 hours, cool to ambient temperature, vacuum filter, wash with deionized water for two After three times until neutral, the resulting filter cake is dried at 120°C for 1-2 hours, ground into 10-20nm powder, mixed with 1-1.5g of MgO powder, roasted and cooled under nitrogen flow at 630-660°C A mixed carrier of CNTs and MgO is formed for loading active components Co, Mo, cocatalyst La, and promoter K.
Embodiment 3
[0037] On the basis of Examples 1 and 2, the catalyst for hydrogen production by thermal cracking of tail gas containing ammonia in the MOCVD process was prepared by alcohol thermal method, and 2~4g of pretreated multilayer carbon nanotubes (CNTs) and magnesium oxide (MgO) were mixed with the carrier , added to about 50~60mL absolute ethanol solution, heated to 30~50°C and stirred to form a slurry, then added a cobalt-molybdenum bimetallic catalyst with a total volume of about 50~100mL to prepare the precursor as cobalt nitrate (Co( NO3)2) and molybdenum nitrate (Mo(NO3)3) mixed solution, the co-catalyst precursor is a mixed solution of lanthanum nitrate (La(NO3)3) and potassium nitrate (KNO3), and ethanol solution, then stir and mix evenly, and then Add about 10~30mL of ammonia water, adjust the pH of the mixed solution until it is greater than 10, then heat and stir to form a slurry again, and perform ultrasonication on the slurry for 0.5~1h and dry at 100~130°C for 2~4 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com