Method for comprehensively utilizing iron vitriol dreg of yellow sodium
A technology of jarosite slag and leaching slag, applied in the direction of flocculation/sedimentation water/sewage treatment, etc., can solve the problems of complex process, no effective recovery of valuable metals, acid consumption, roasting flue gas pollution, etc. Save pollution, shorten processing time, good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
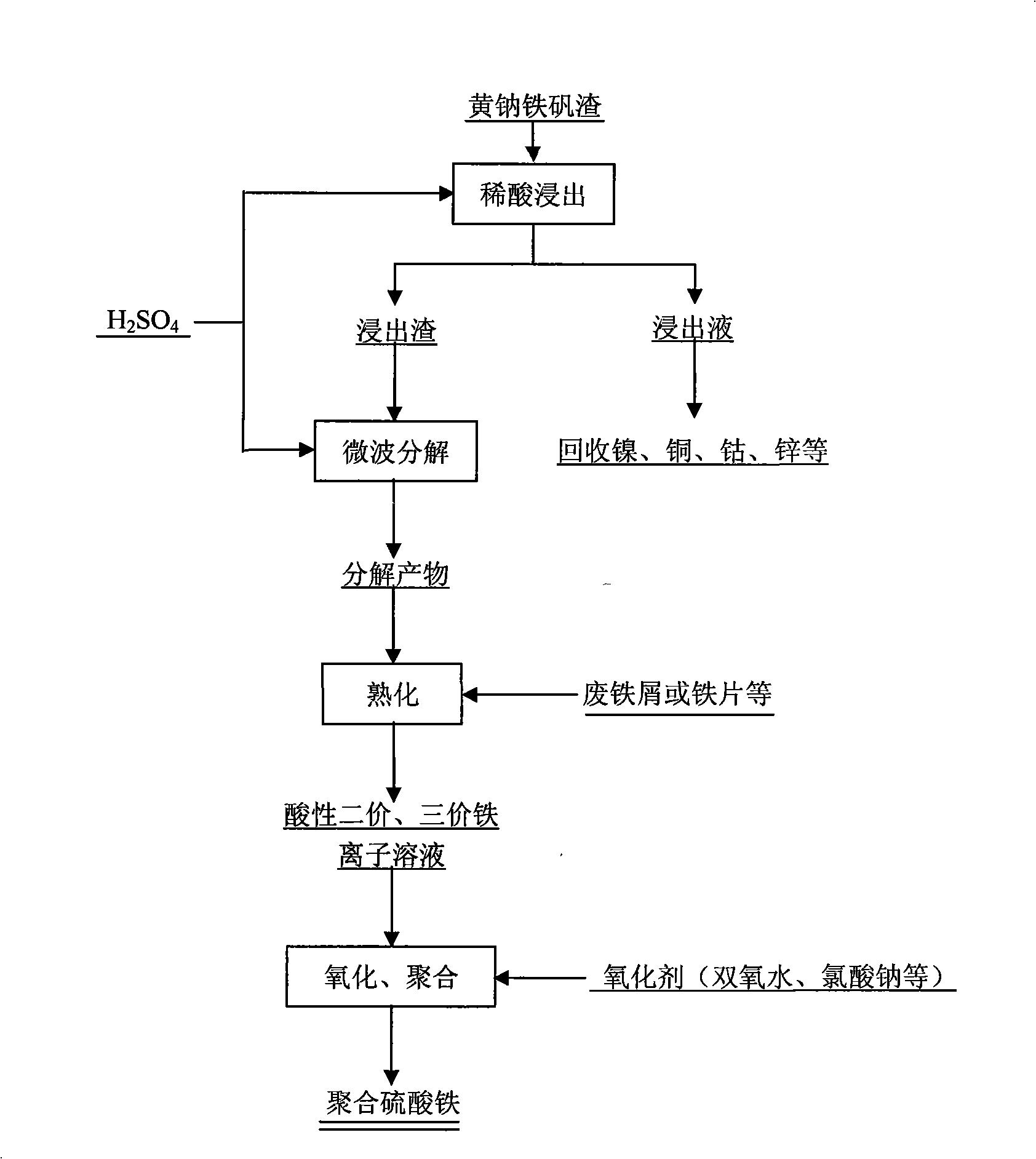
[0030] The valuable metals in the jarosite slag are selectively leached with a sulfuric acid solution with a mass concentration of 1% at a liquid-solid ratio of 3:1, and the average leaching rate of the valuable metals is 80%.
[0031] Fully mix the leached residue with sulfuric acid, the amount of sulfuric acid is 30% of the quality of the jarosite residue, and heat for 15 minutes under microwave radiation to decompose the jarosite, wherein the microwave frequency is 2450±50MHz.
[0032] Add water and scrap iron sheets to the microwave-irradiated material, age for 8 hours, adjust the total iron concentration in the solution to 160g / L, and the ferrous ion content accounts for 20% of the total iron content; then at a temperature of 40°C, use The oxidant hydrogen peroxide oxidizes the divalent iron ions in the above solution to ferric ions, and the reaction time is 1h; the amount of hydrogen peroxide is 1.5 times the amount required to oxidize all the divalent iron ions to ferric...
Embodiment 2
[0034] The valuable metals in the jarosite slag are selectively leached with dilute sulfuric acid with a mass concentration of 3% at a liquid-to-solid ratio of 5:1, and the average leaching rate of valuable metals is 90%.
[0035] Fully mix the leached residue with sulfuric acid, the amount of sulfuric acid is 40% of the quality of the jarosite residue, and heat for 10 minutes under microwave radiation to decompose the jarosite, wherein the microwave frequency is 2450±50MHz.
[0036]Add the microwave irradiated materials to water and scrap iron sheets, age for 5 hours, adjust the total iron concentration in the solution to 170g / L, and the ferrous ion content accounts for 30% of the total iron content; then, at a temperature of 60°C, use The oxidant hydrogen peroxide oxidizes the ferrous ions in the above solution to ferric ions, and the reaction time is 2 hours; the amount of hydrogen peroxide is 1.0 times the amount required to oxidize all the ferric ions to ferric ions. Wate...
Embodiment 3
[0038] The valuable metals in the jarosite slag were selectively leached with dilute sulfuric acid with a mass concentration of 5% at a liquid-to-solid ratio of 10:1, and the average leaching rate of valuable metals was 93%.
[0039] Fully mix the leached residue with sulfuric acid, the amount of sulfuric acid is 60% of the quality of the jarosite residue, and heat for 6 minutes under microwave radiation to decompose the jarosite; the microwave frequency is 916±18MHz.
[0040] Add water and iron filings to the microwave-irradiated material, mature for 2 hours, adjust the total iron concentration in the solution to 180g / L, and the ferrous ion content accounts for 40% of the total iron content; then, at a temperature of 80°C, use an oxidizing agent Sodium chlorate oxidizes the divalent iron ions in the above solution to ferric ions, and the reaction time is 3 hours; the amount of sodium chlorate is 1.0 times the amount required to oxidize all the divalent iron ions to ferric ions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com