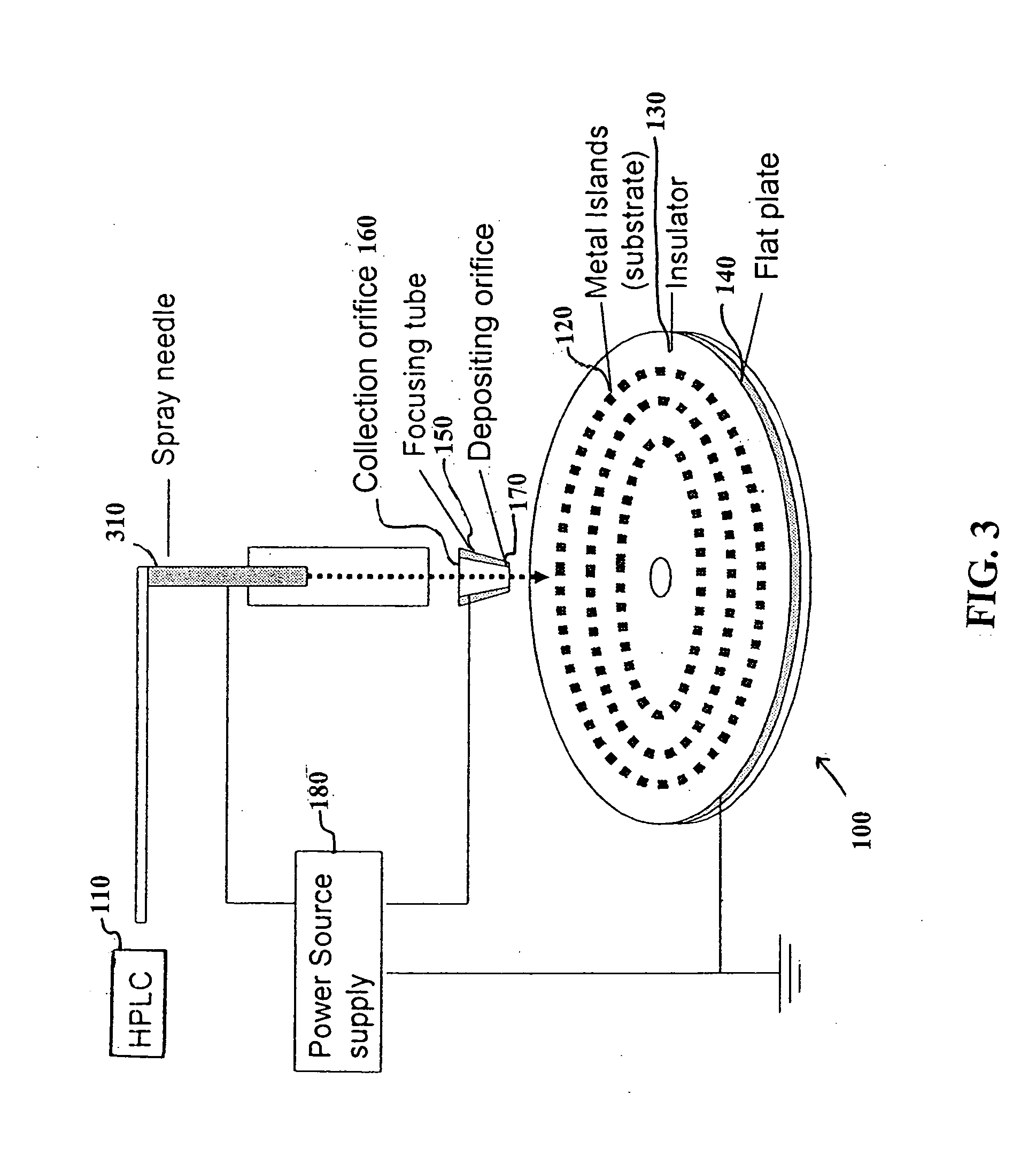
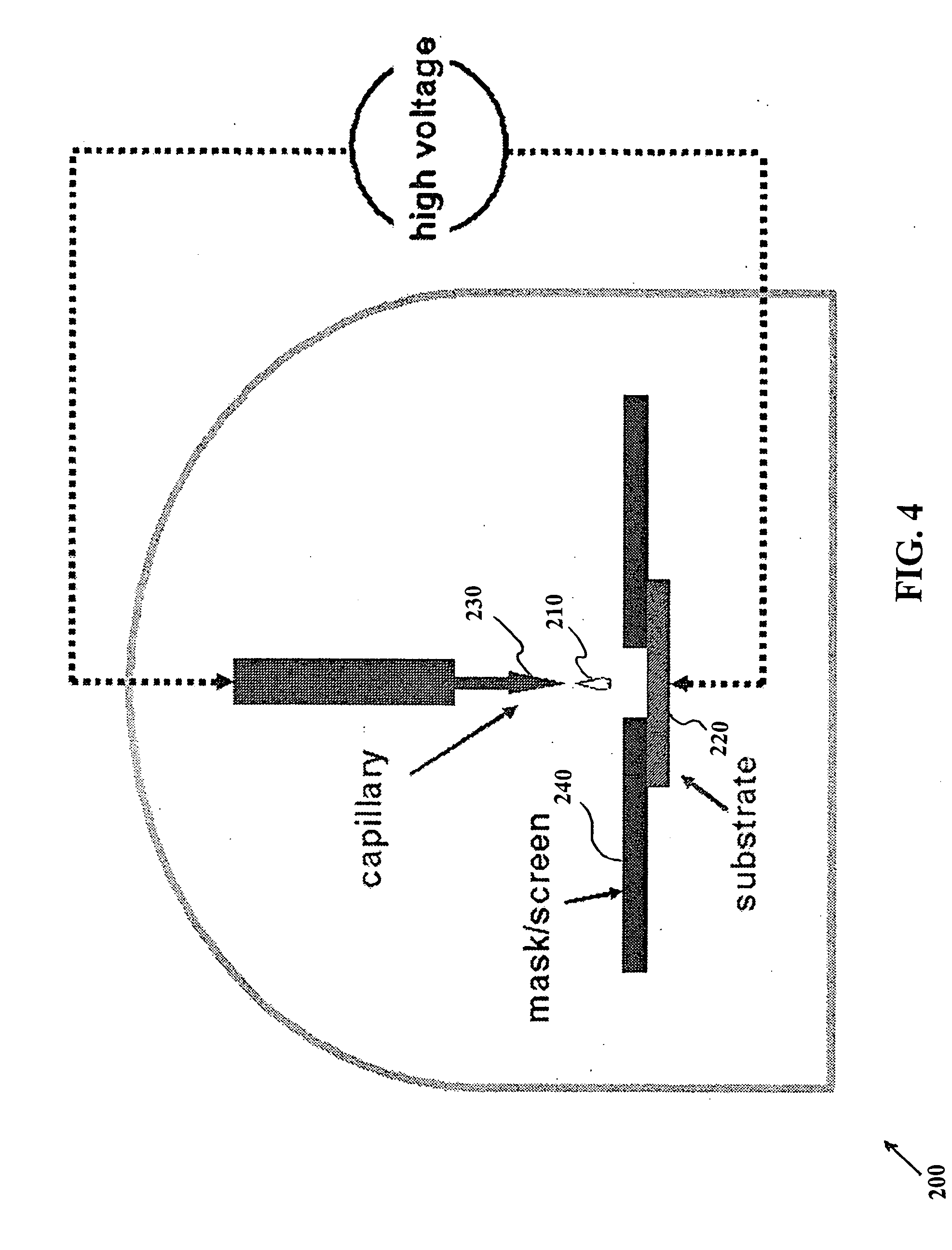
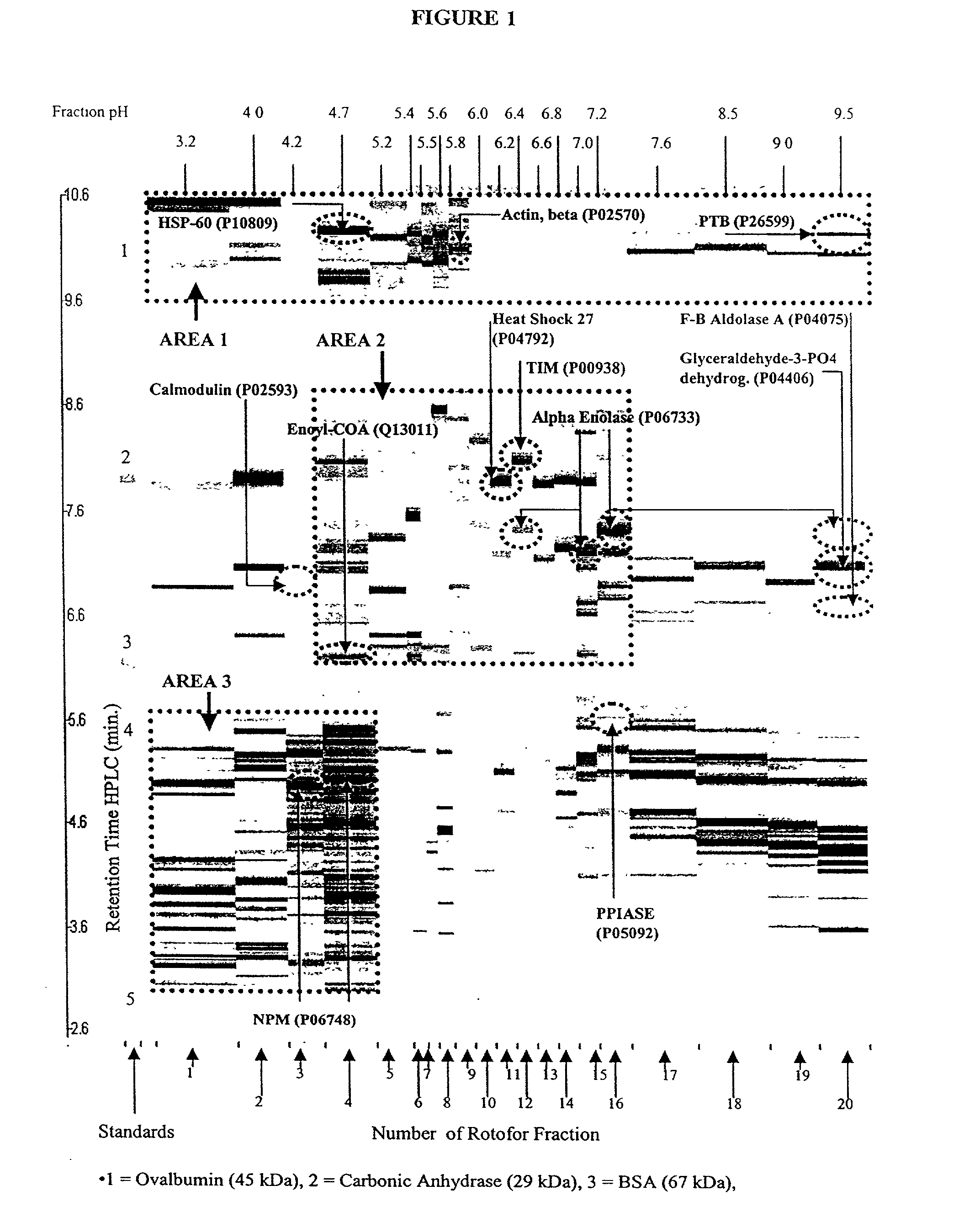
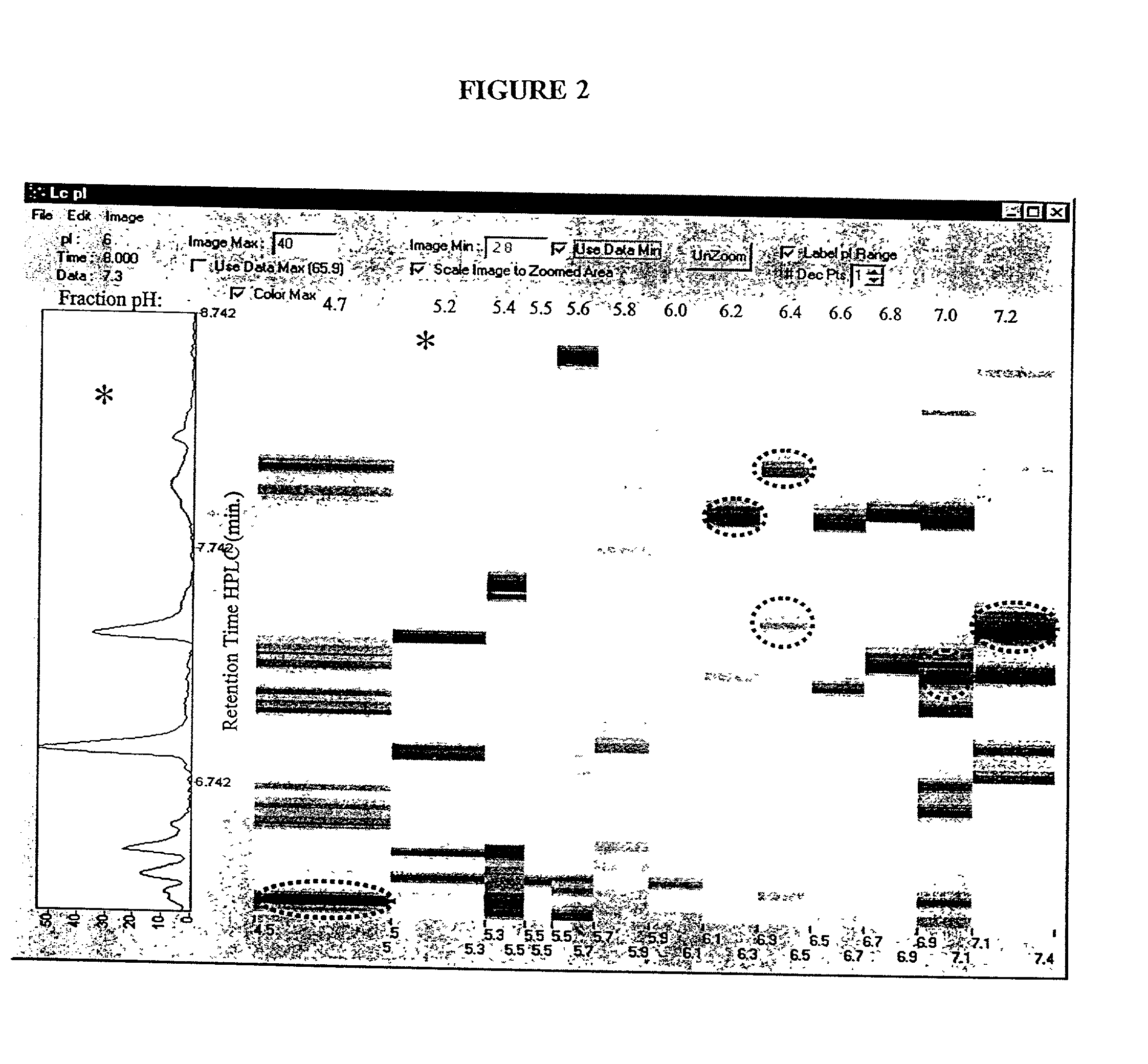
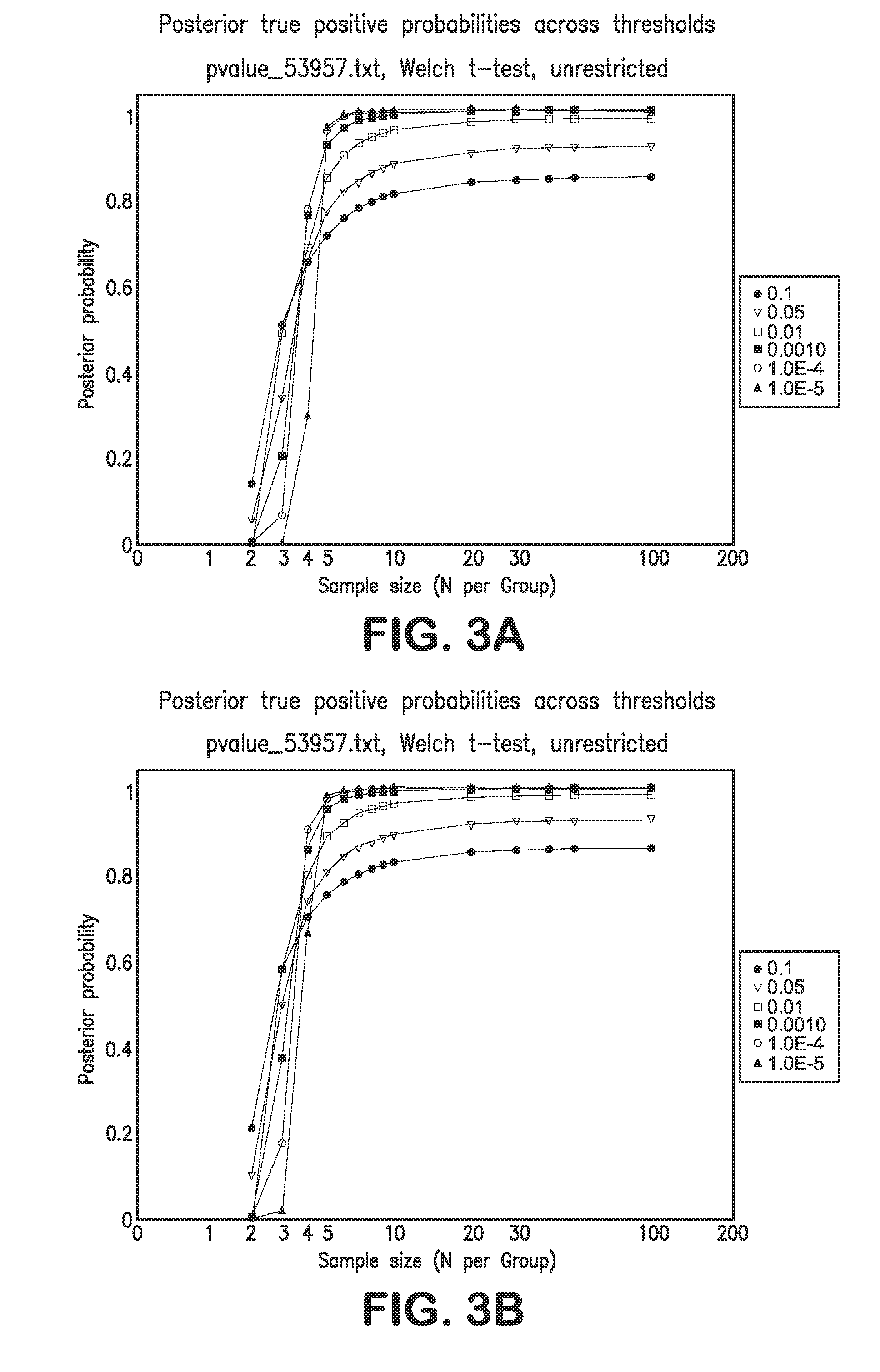
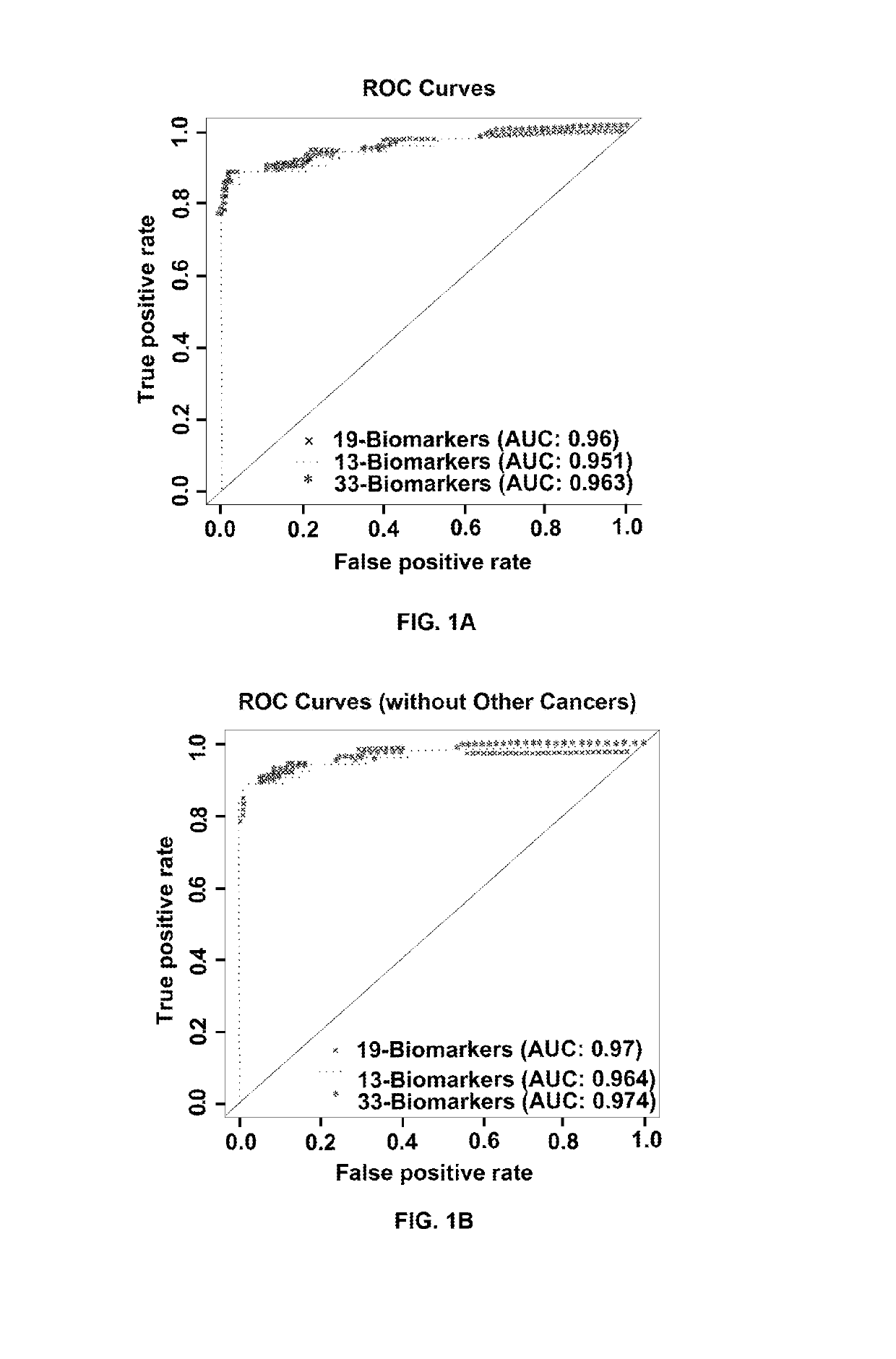
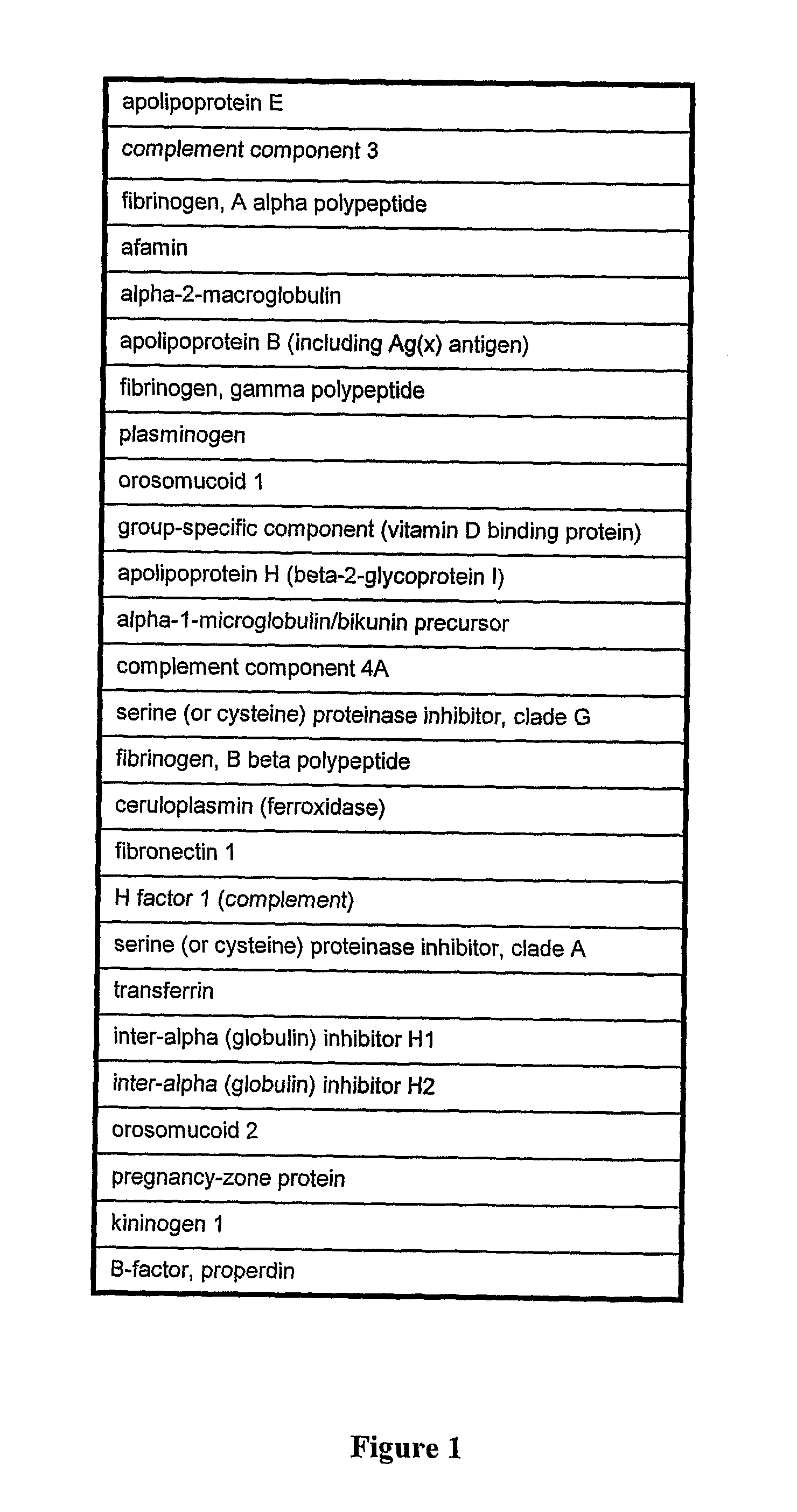
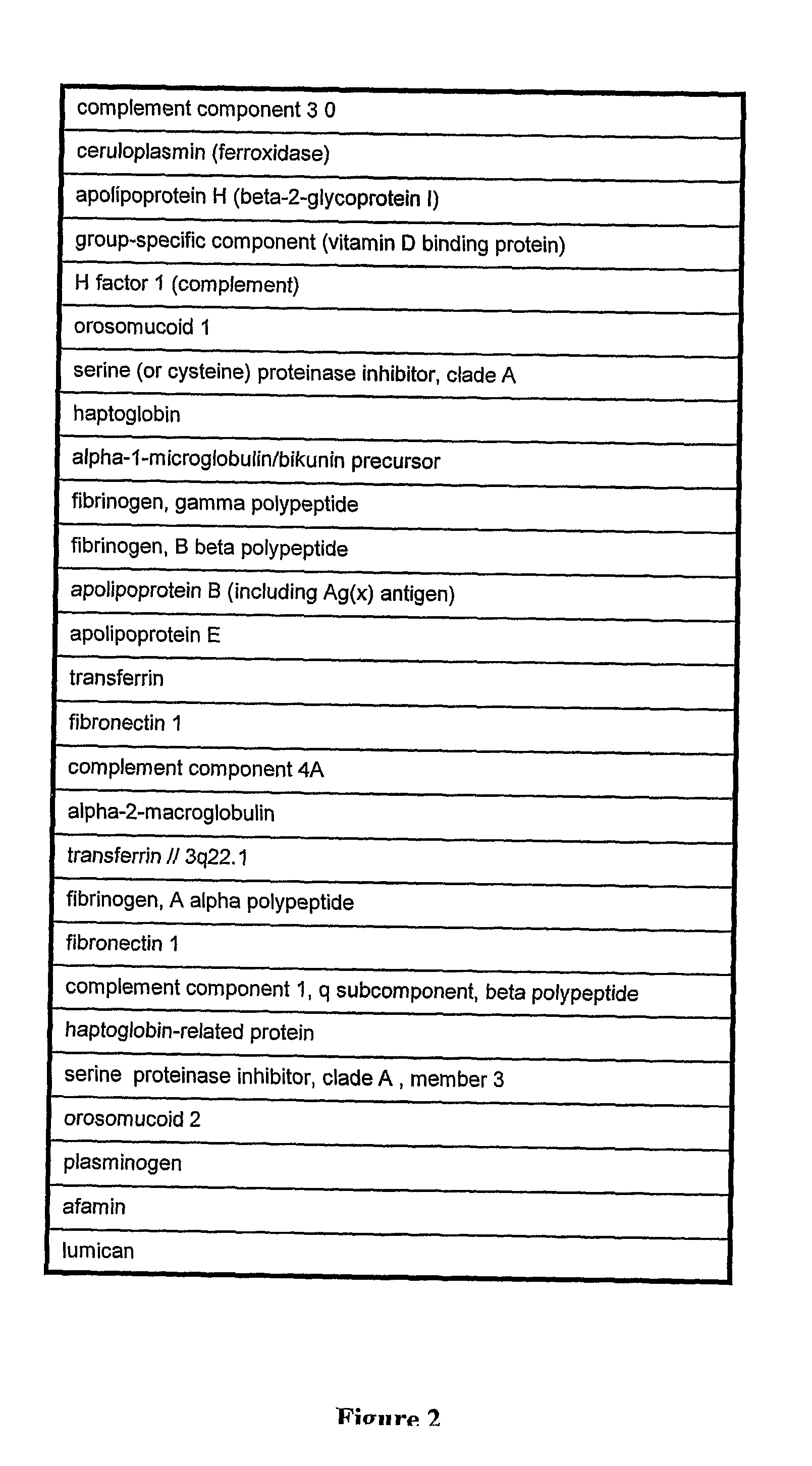
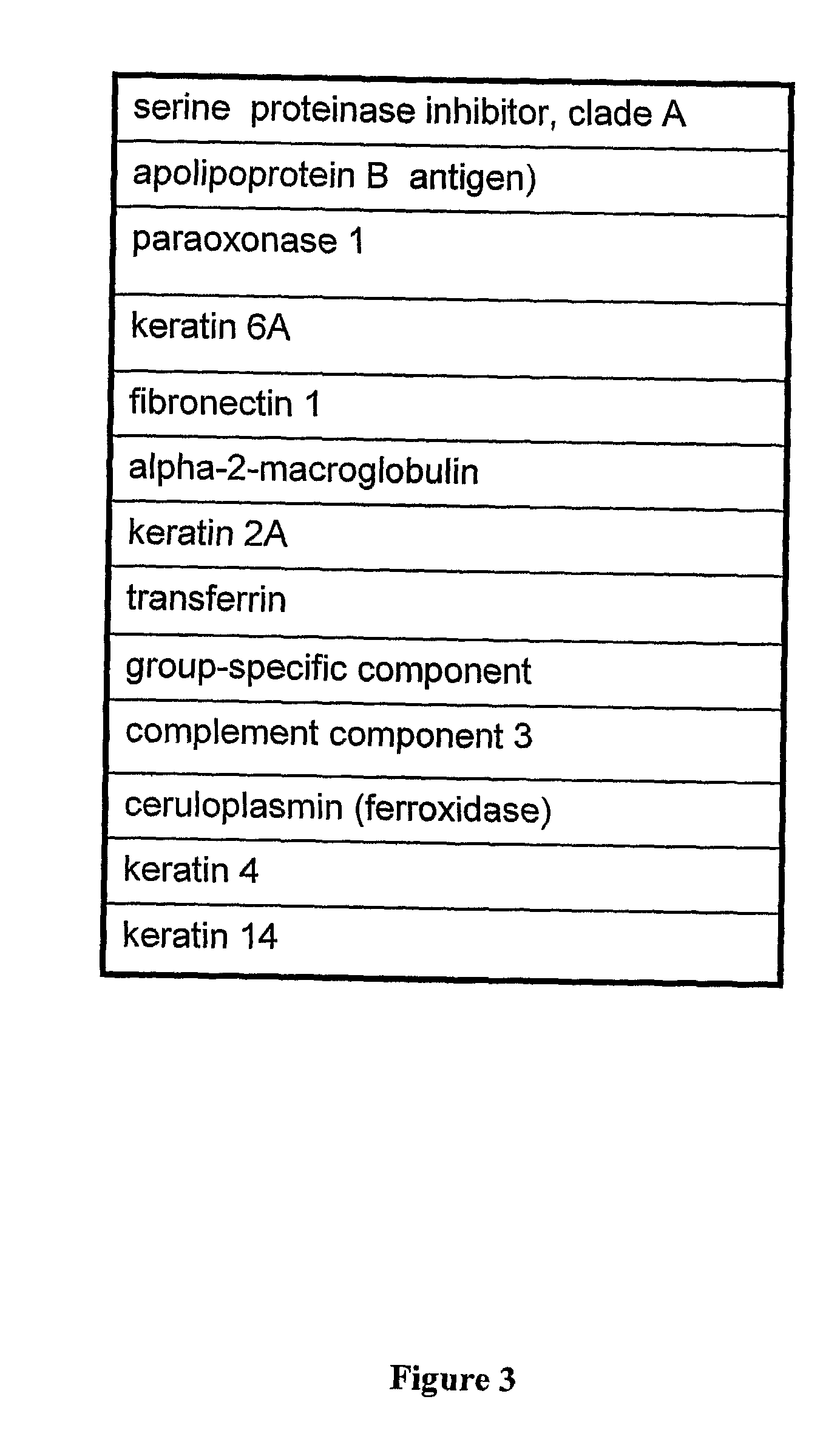
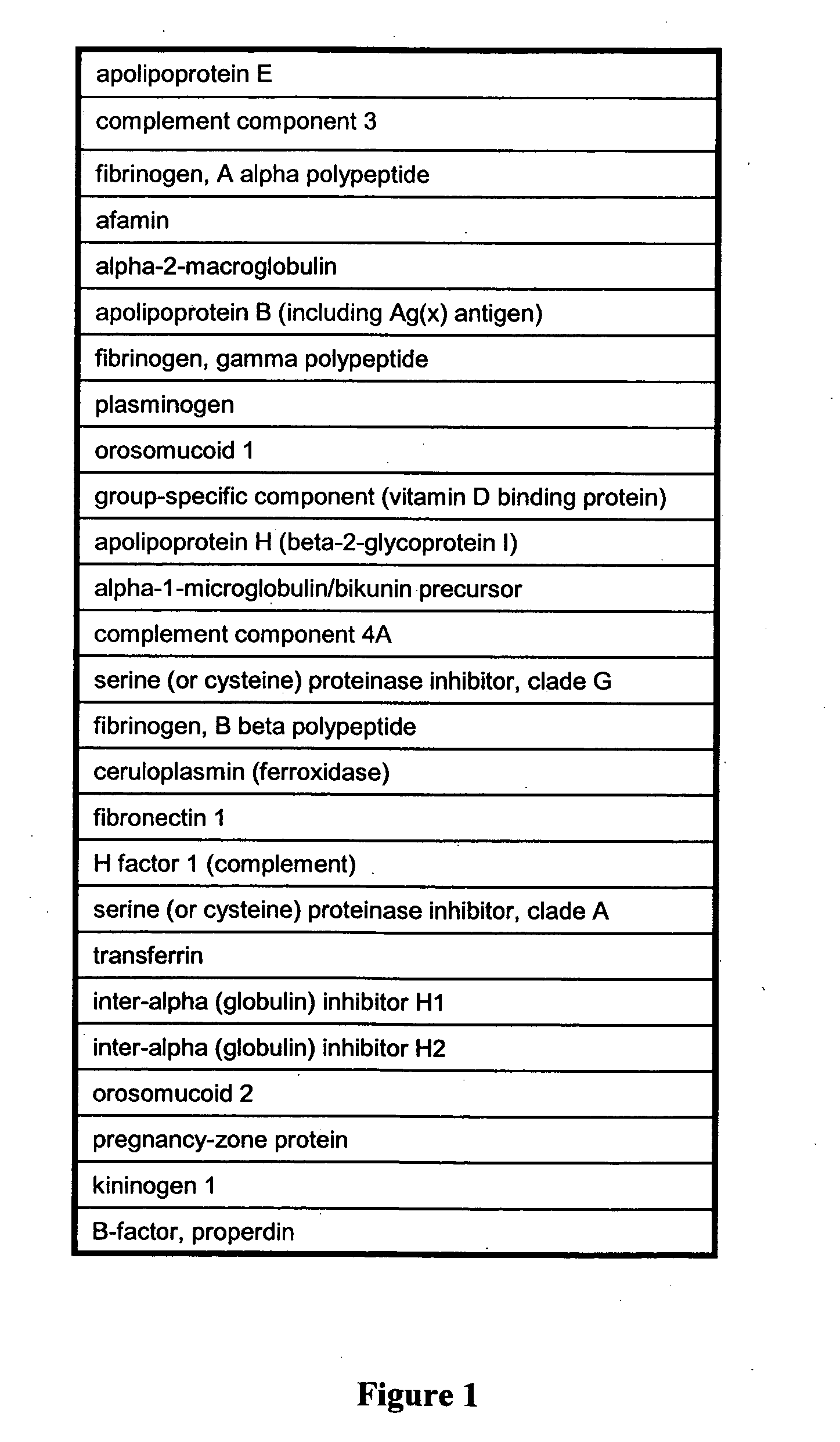
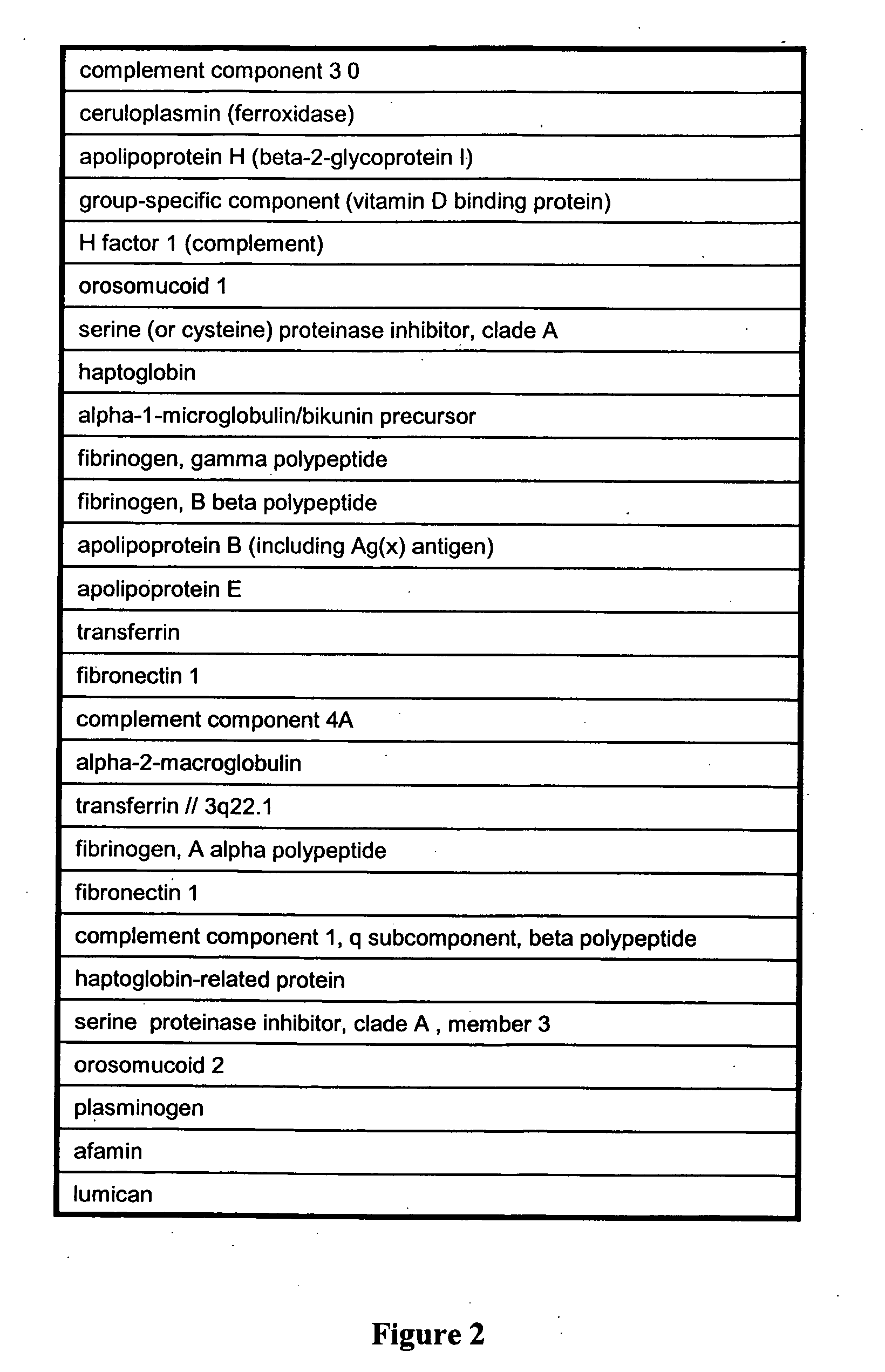
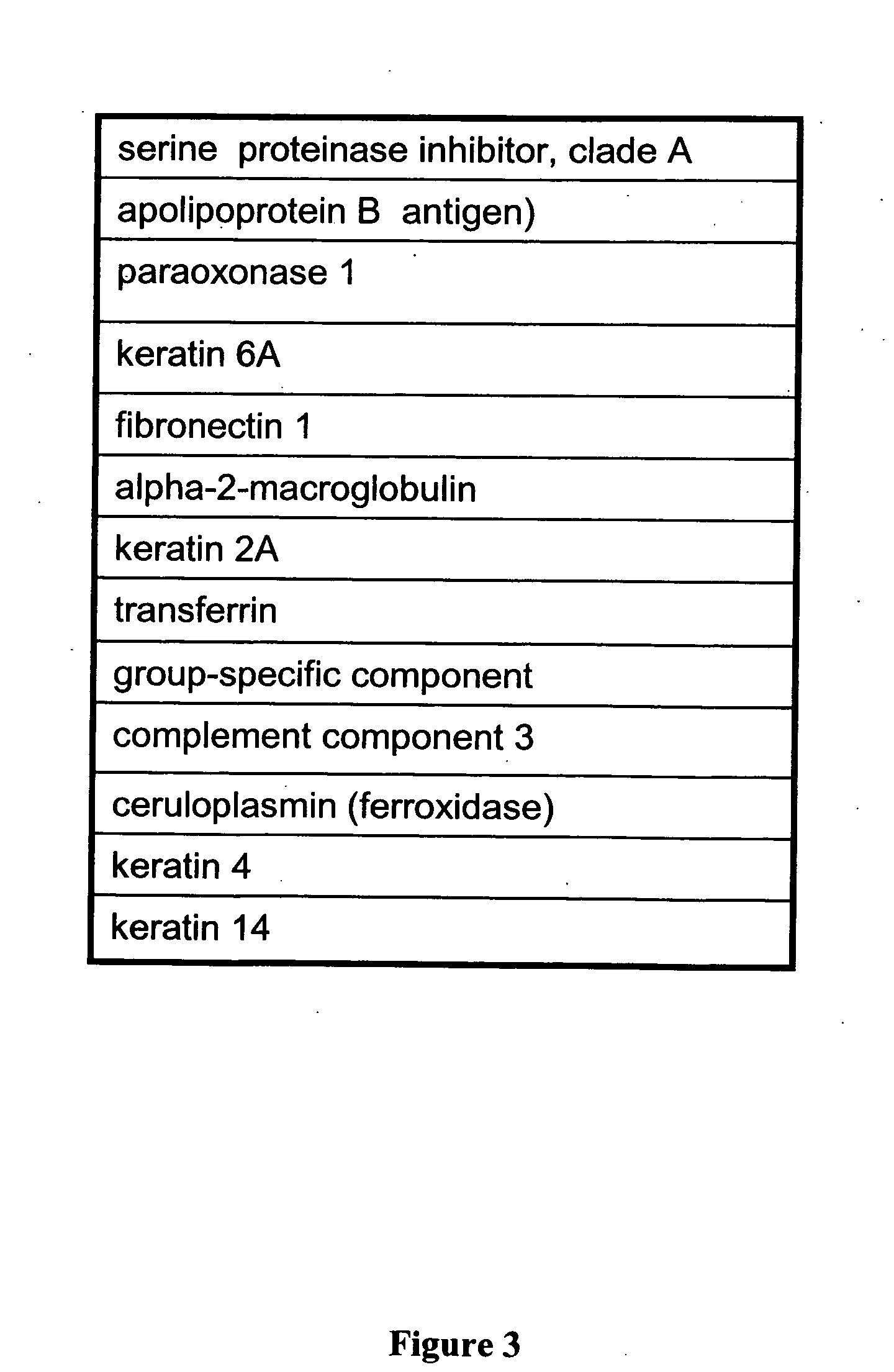
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Protein profile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A protein profile should thus comprise a minimum of proteins to be measured, chosen according to protein physiopathology and the type of abnormality under investigation. Interpretation is based on inter-protein correlations that may appear or disappear depending on the underlying physiopathology.
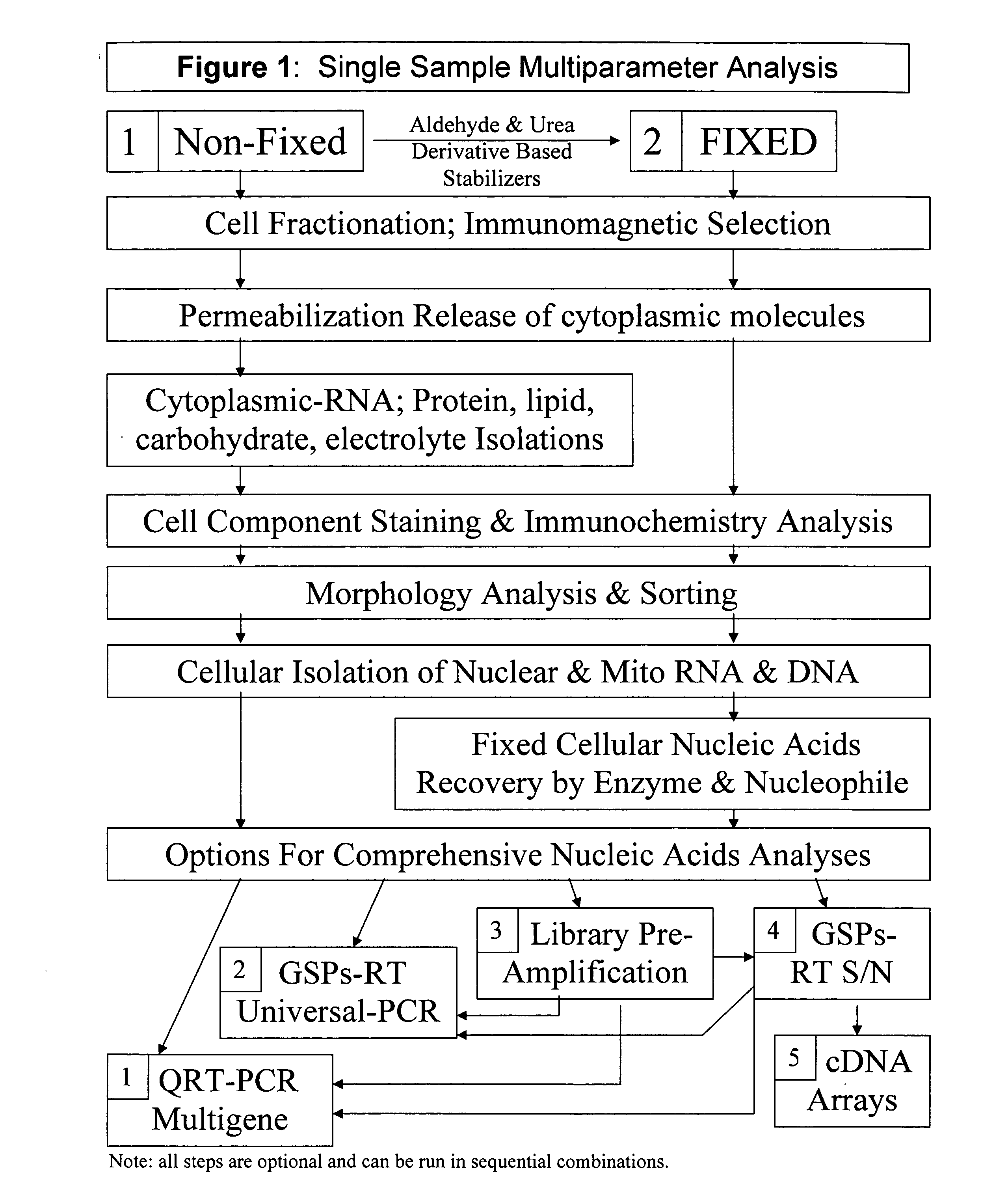
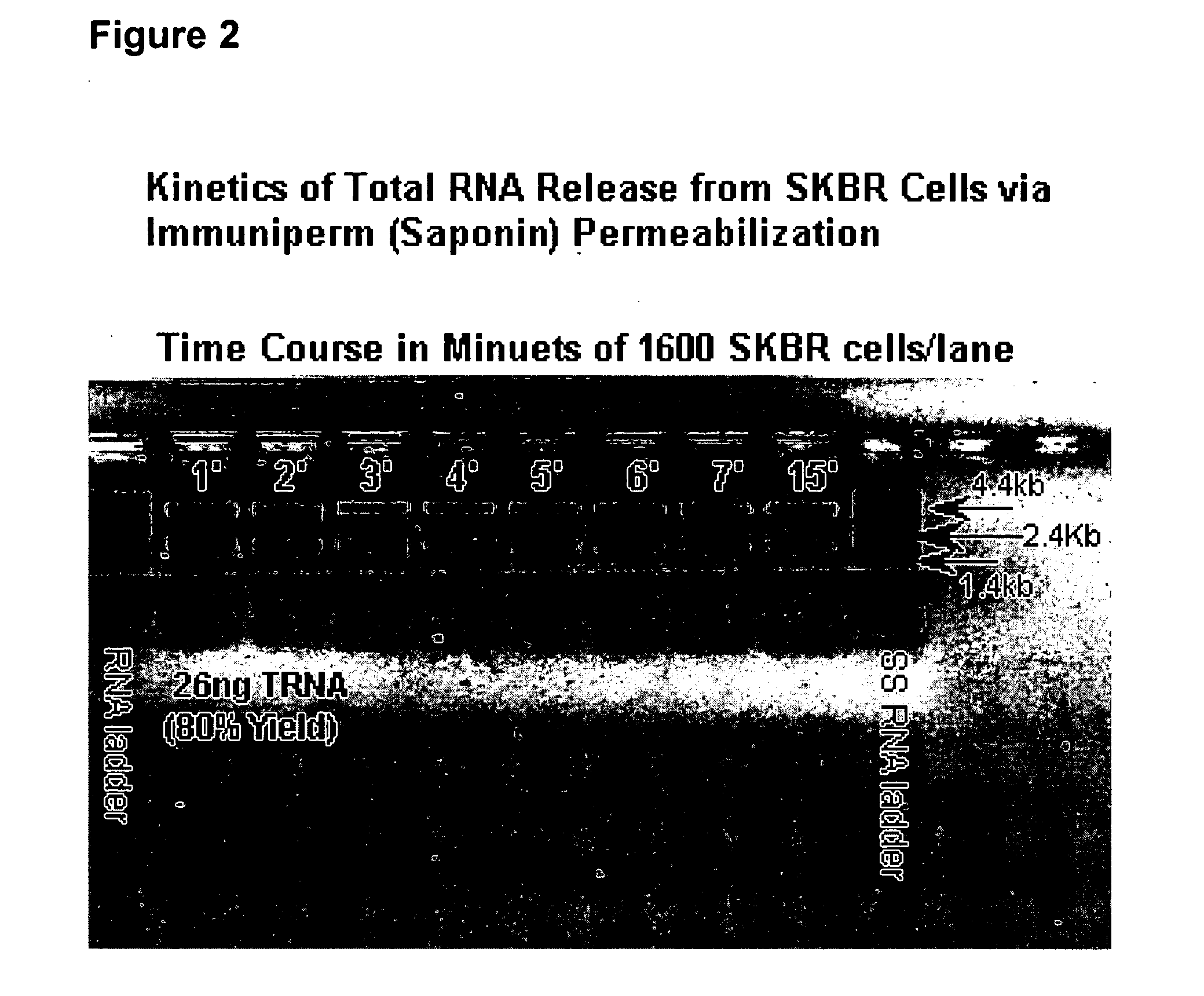
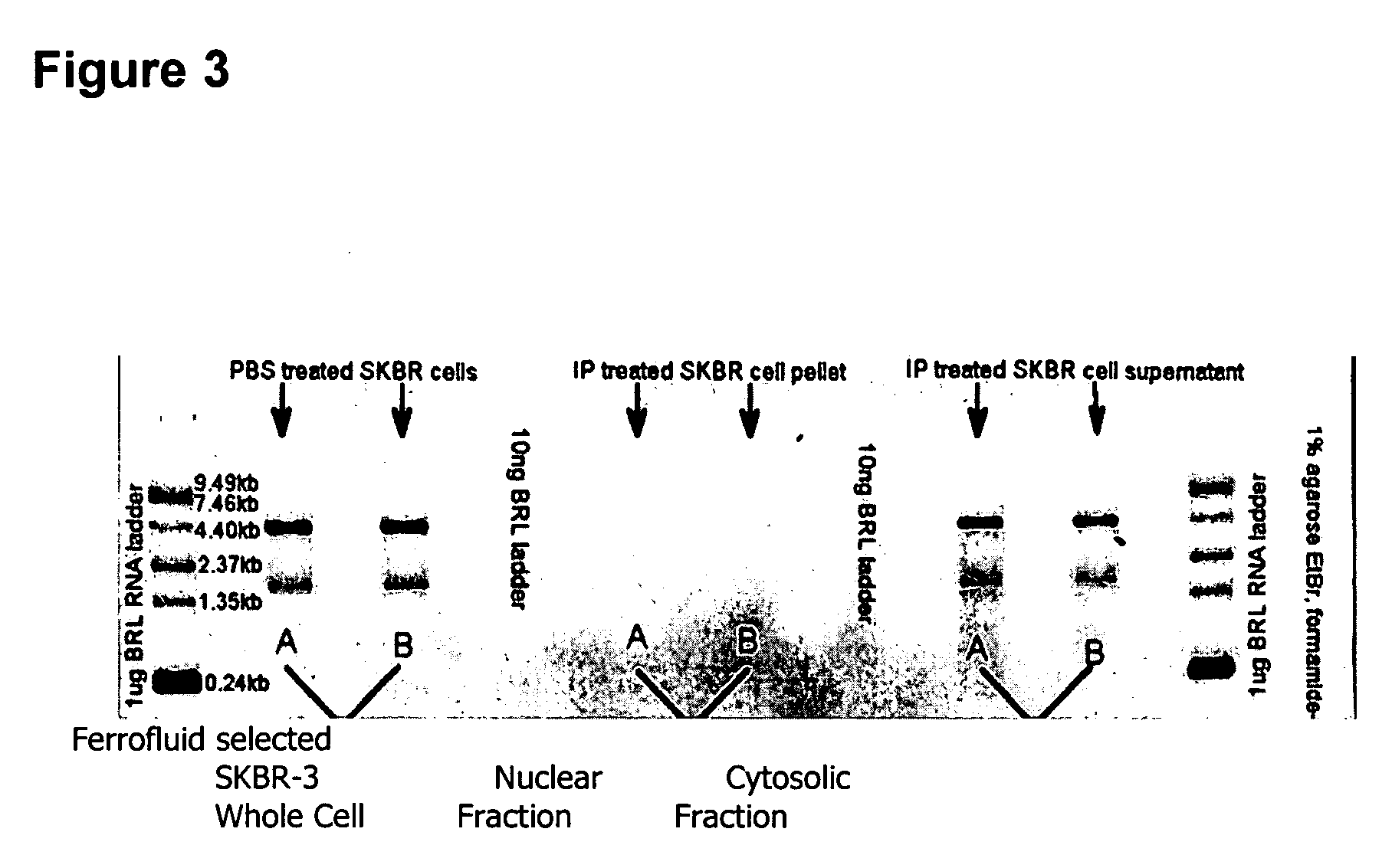
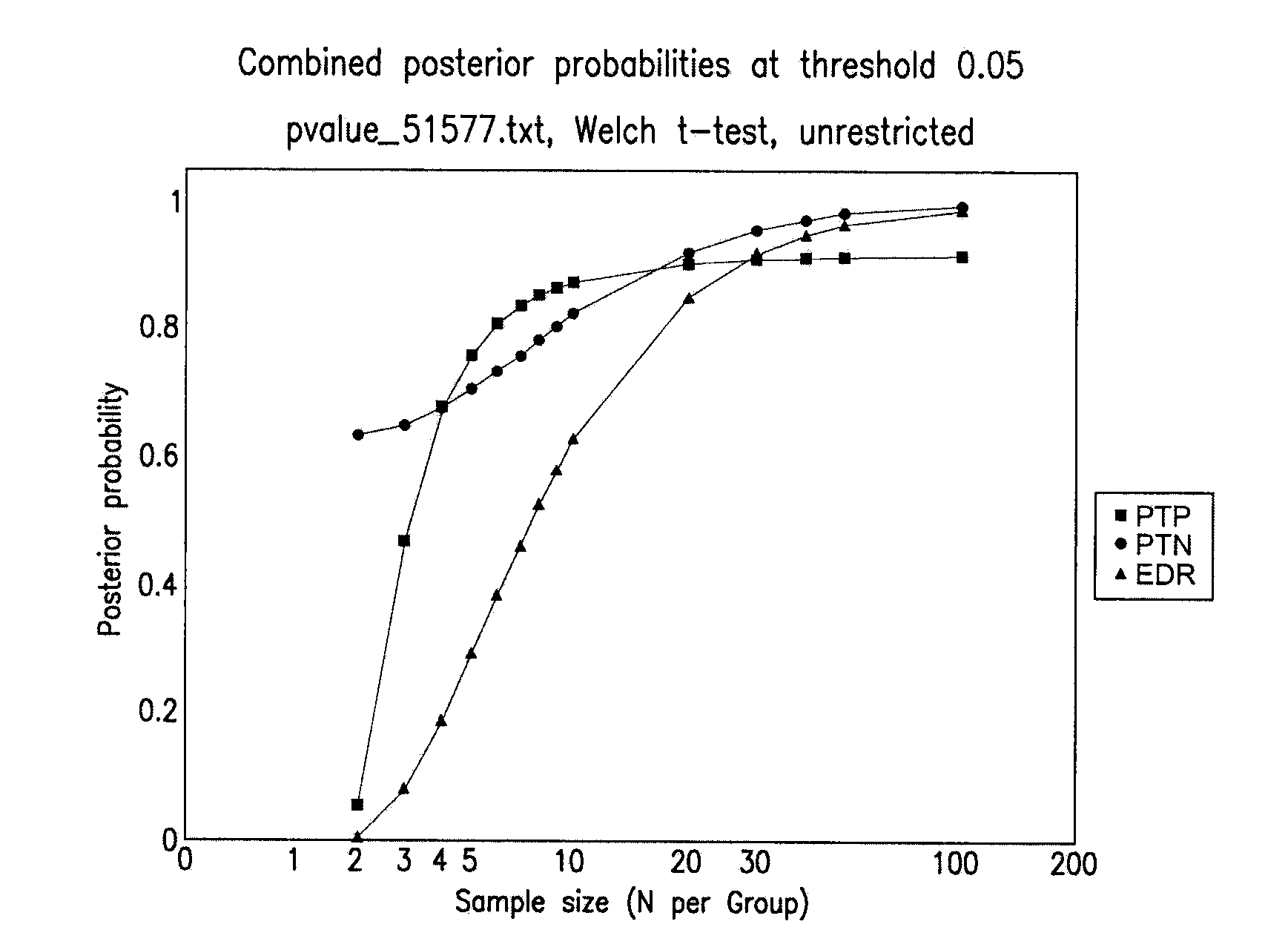
Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample
InactiveUS20060008807A1Microbiological testing/measurementMicroorganism lysisProtein profilingMass spectrometry
A highly sensitive assay is disclosed which utilizes a method for gene specific primed amplification of mRNA libraries from rare cells and rare transcripts found in blood. The assay allows detection of rare mRNA (10 copies / cell) found in 1 to 10 cells isolated through immunomagnetic enrichment. The assay is an improvement over multiplex PCR and allows efficient detection of rare coding sequences for circulating carcinoma cells in the blood. The methods are useful in profiling of cells isolated from tissues or body fluids and serves as an adjunct to clinical diagnosis of diverse carcinomas including early stage detection and classification of circulating tumor cells. Monitoring of nucleic acid and protein profiles of cells either in conventional or microarray formats, facilitates management of therapeutic intervention including staging, monitoring response to therapy, confirmation of remission and detection of regression.
Owner:VERIDEX LCC
Diagnosis of melanoma by nucleic acid analysis
InactiveUS20080274908A1Less traumaticMicrobiological testing/measurementLibrary screeningProtein profilingCuticle
The present invention provides methods for diagnosing melanoma in a subject by analyzing nucleic acid molecules obtained from the subject. The present invention also provides methods for distinguishing melanoma from dysplastic nevi and / or normal pigmented skin. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample.
Owner:DERMTECH INT
Biological profiles and methods of use
InactiveUS20070087448A1Avoid overestimation and underestimationUnderestimation of the illness levelBiological testingMaterial analysis by using resonanceProtein profilingBiological profile
The invention provides methods to diagnose and follow the progression of disease through use of protein profile analysis.
Owner:NELSESTUEN GARY L
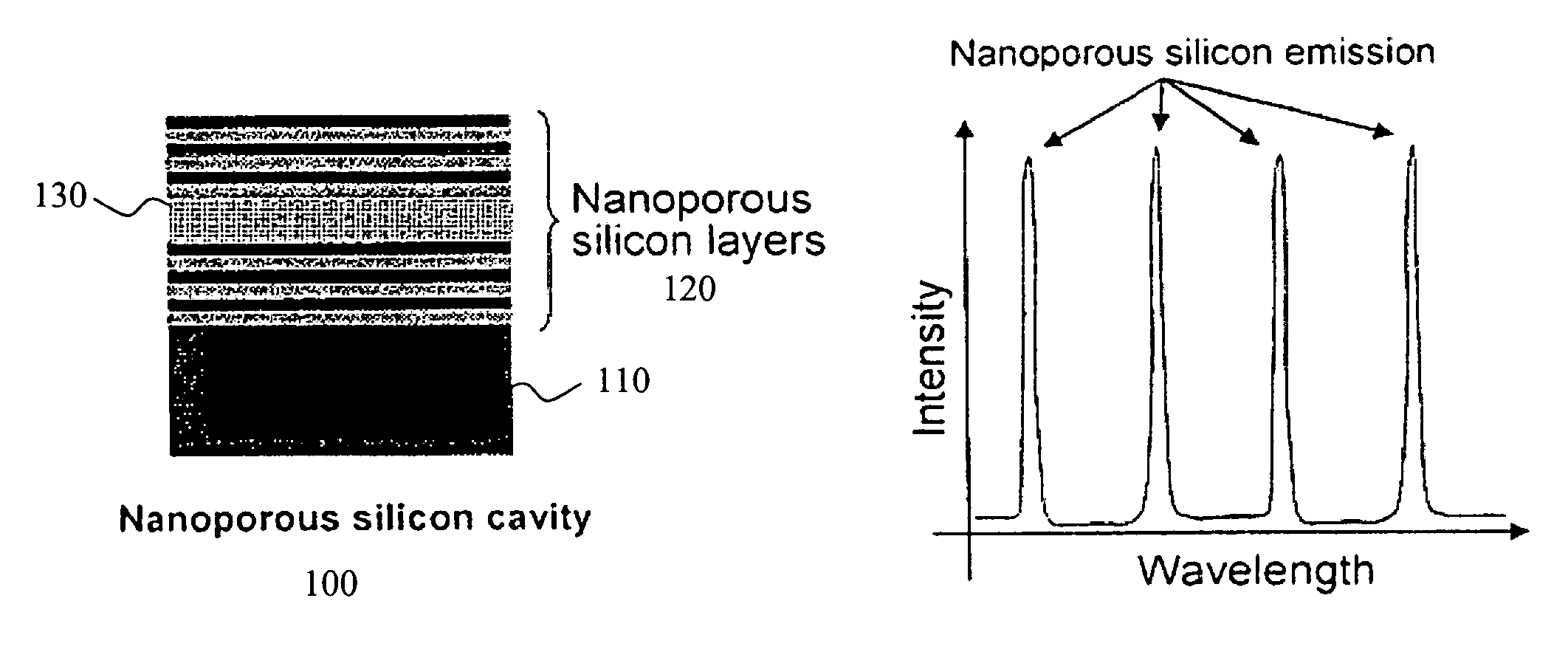
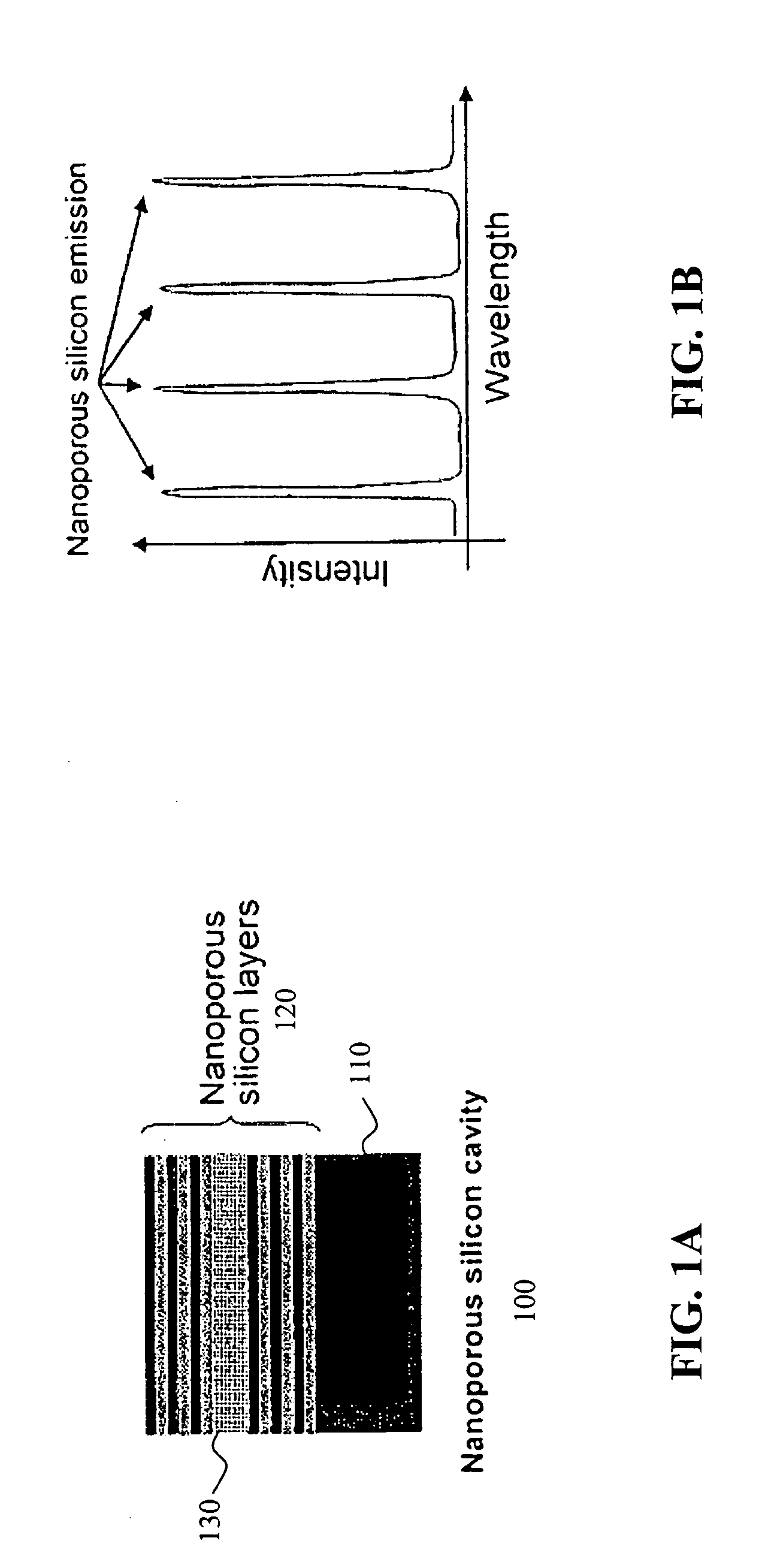
Detection of biomolecules using porous biosensors and Raman spectroscopy
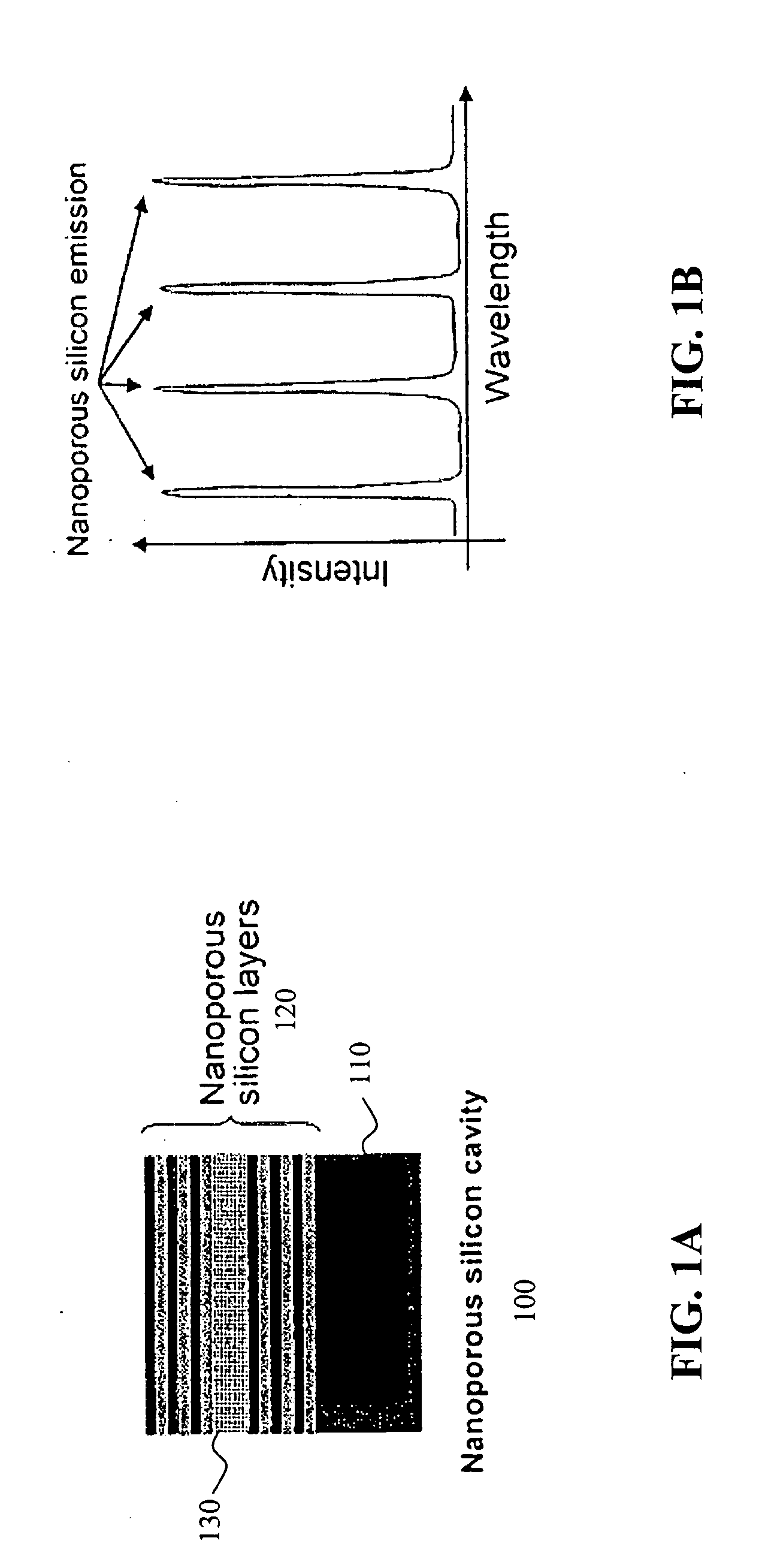
ActiveUS20050196876A1Bioreactor/fermenter combinationsRadiation pyrometryProtein profilingFluorescence
The invention provides methods used to analyze the contents of a biological sample, such as blood serum, with cascade Raman sensing. A fluorescence producing nanoporous biosensor having probes that bind specifically to known analytes is contacted with a biological sample and one or more bound complexes coupled to the porous semiconductor structure are formed. The bound complexes are contacted with a Raman-active probe that binds specifically to the bound complexes and the biosensor is illuminated to generate fluorescent emissions from the biosensor. These fluorescent emissions generate Raman signals from the bound complexes. The Raman signals produced by the bound complexes are detected and the Raman signal associated with a bound protein-containing analyte is indicative of the presence of the protein-containing compound in the sample. The invention methods are useful to provide a protein profile of a patient sample. The invention also provides detection systems useful to practice the invention methods.
Owner:INTEL CORP
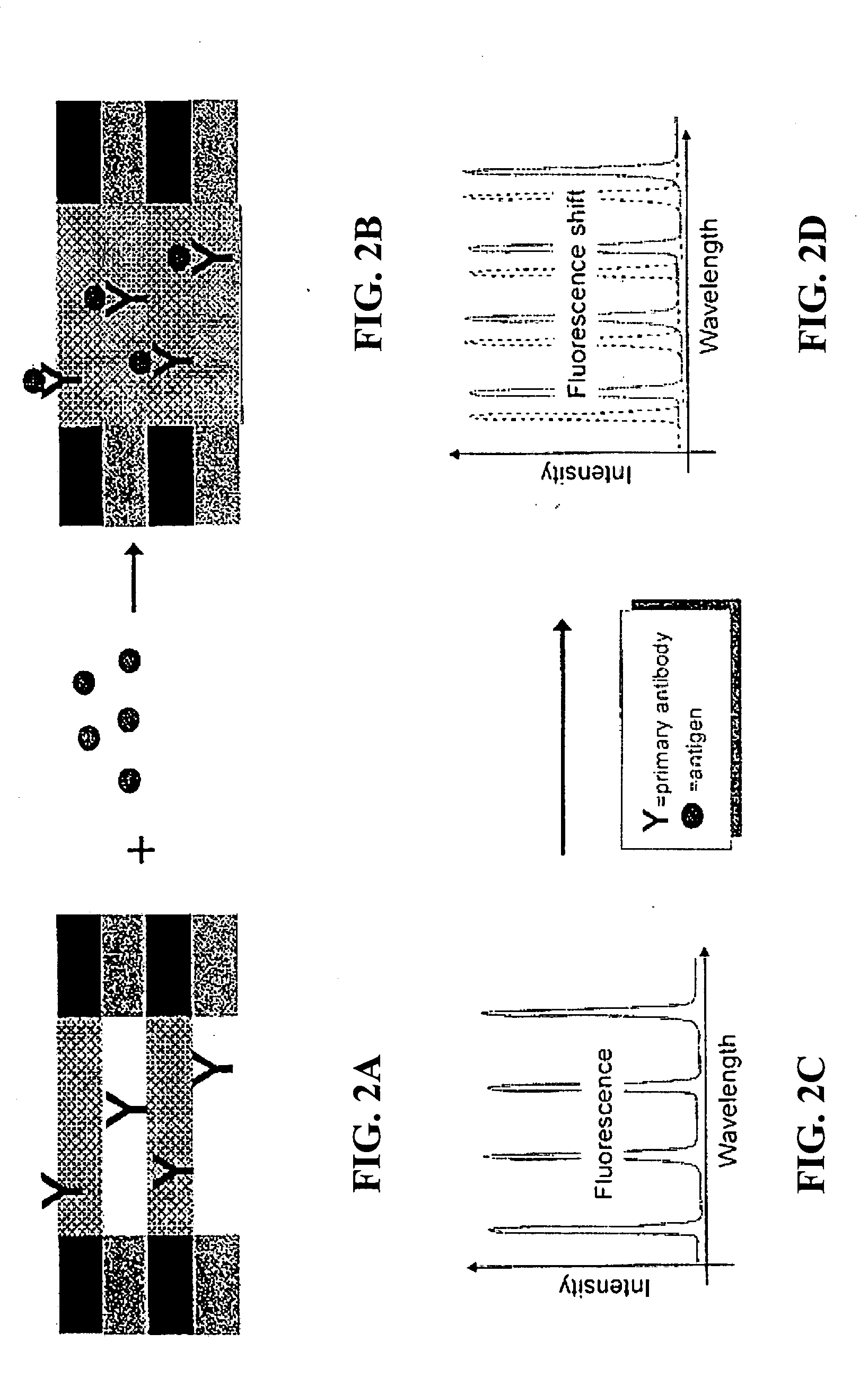
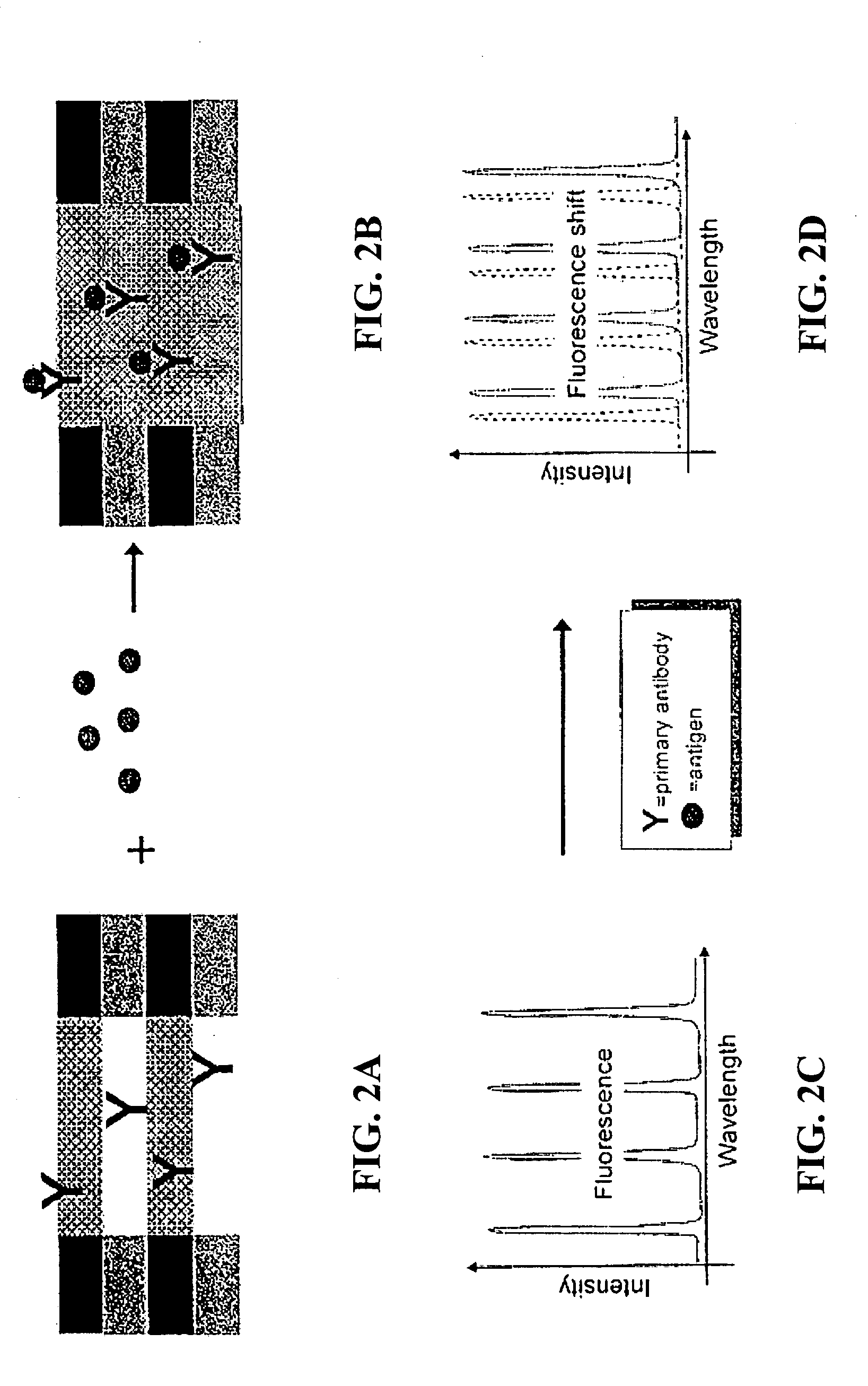
Methods and devices for using Raman-active probe constructs to assay biological samples
InactiveUS20050148100A1Radiation pyrometryMicrobiological testing/measurementDiseaseProtein profiling
Various methods of using Raman-active or SERS-active probe constructs to detect analytes in biological samples, such as the protein-containing analytes in a body fluid are provided. The probe moieties in the Raman-active constructs are selected to bind to and identify specific known analytes in the biological sample or the probe moieties are designed to chemically interact with functional groups commonly found in certain amino acids so that the invention methods provide information about the amino acid composition of protein-containing analytes or fragments in the samples. In some cases, the Raman-active or SERS-active probe constructs, when used in the invention methods, can identify particular protein-containing analytes or types of such analytes so that a protein profile of a patient sample can be made. When compared to a data base of Raman or SERS spectra of normal samples, a disease state of a patient can be identified using the methods disclosed.
Owner:INTEL CORP
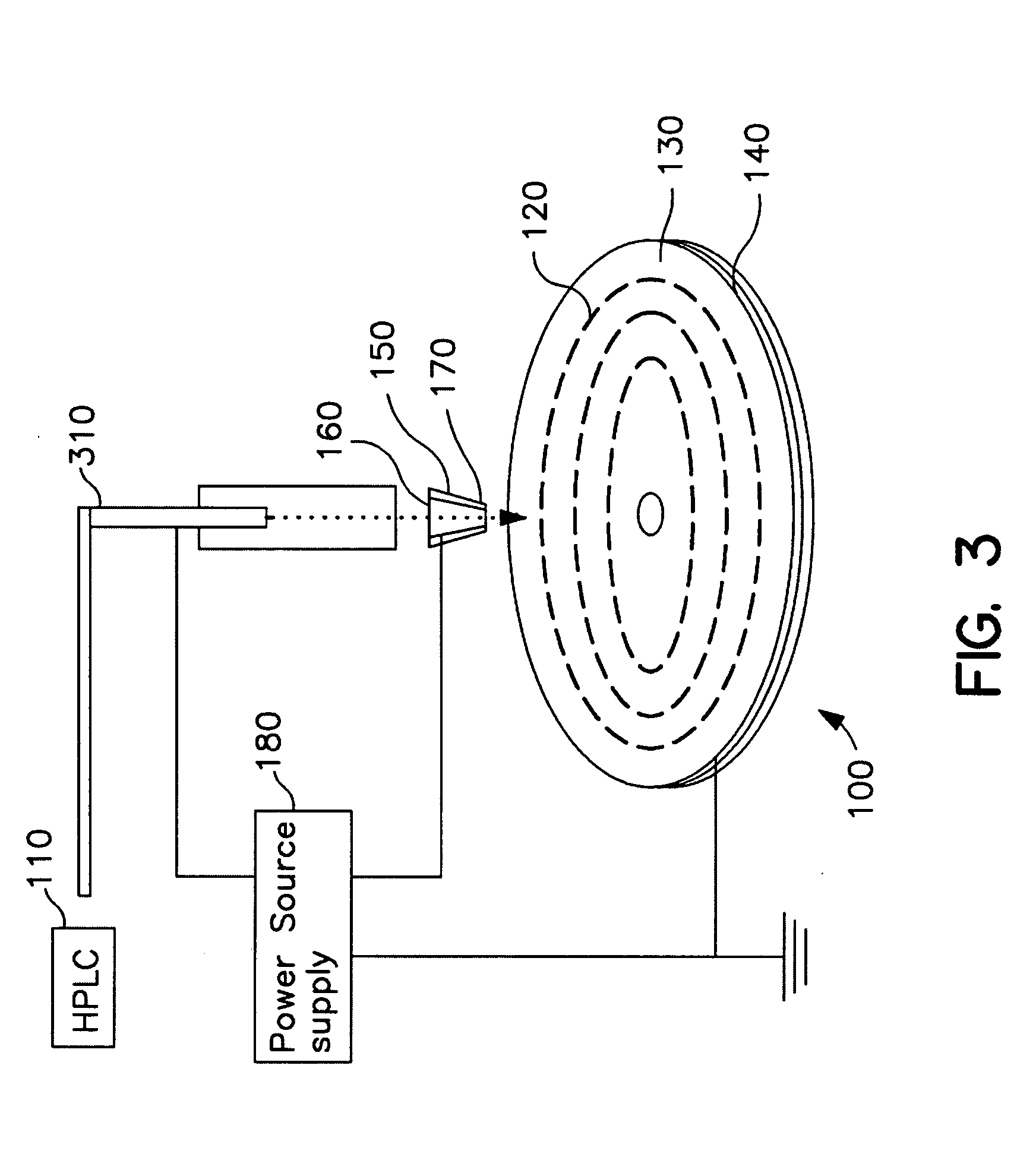
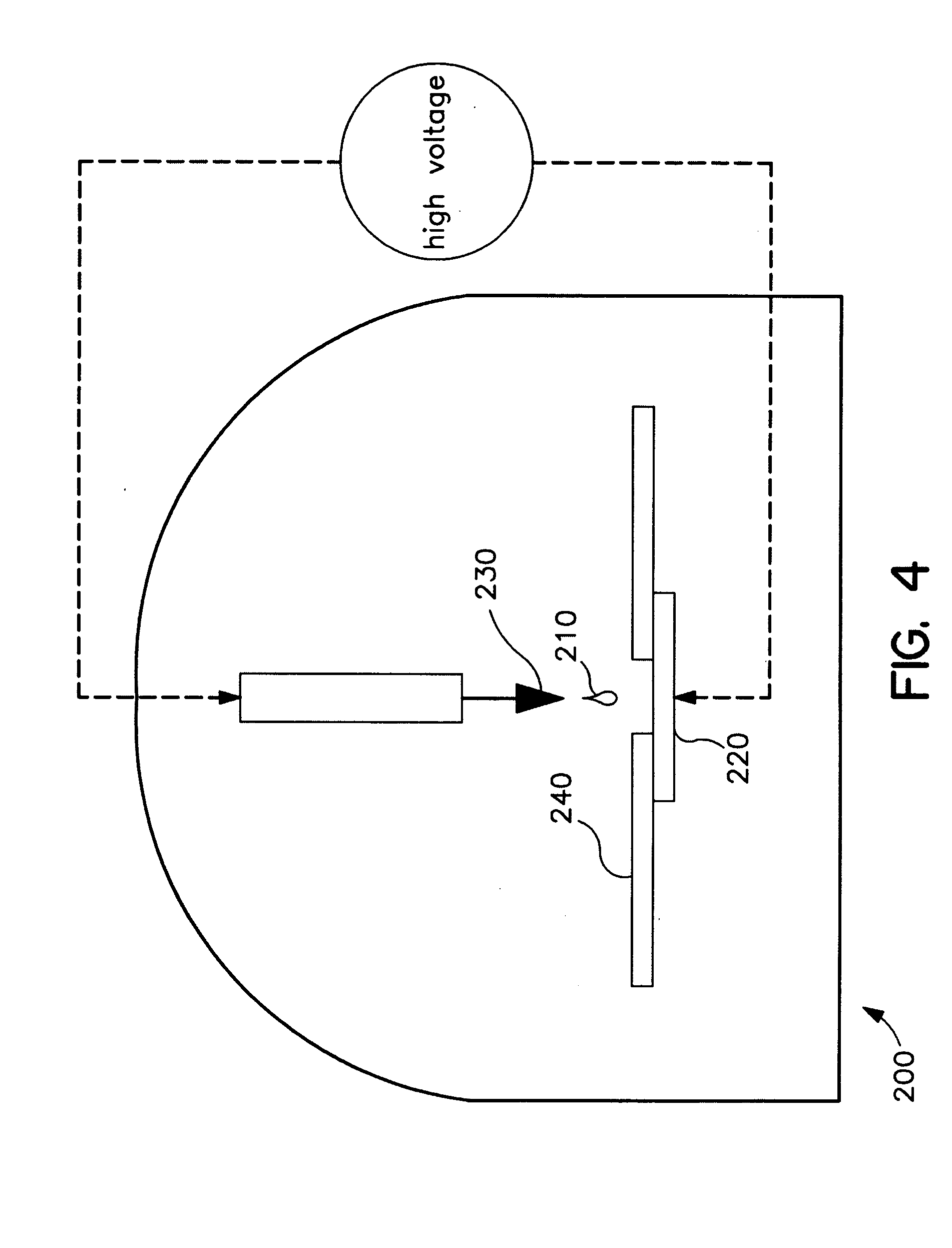
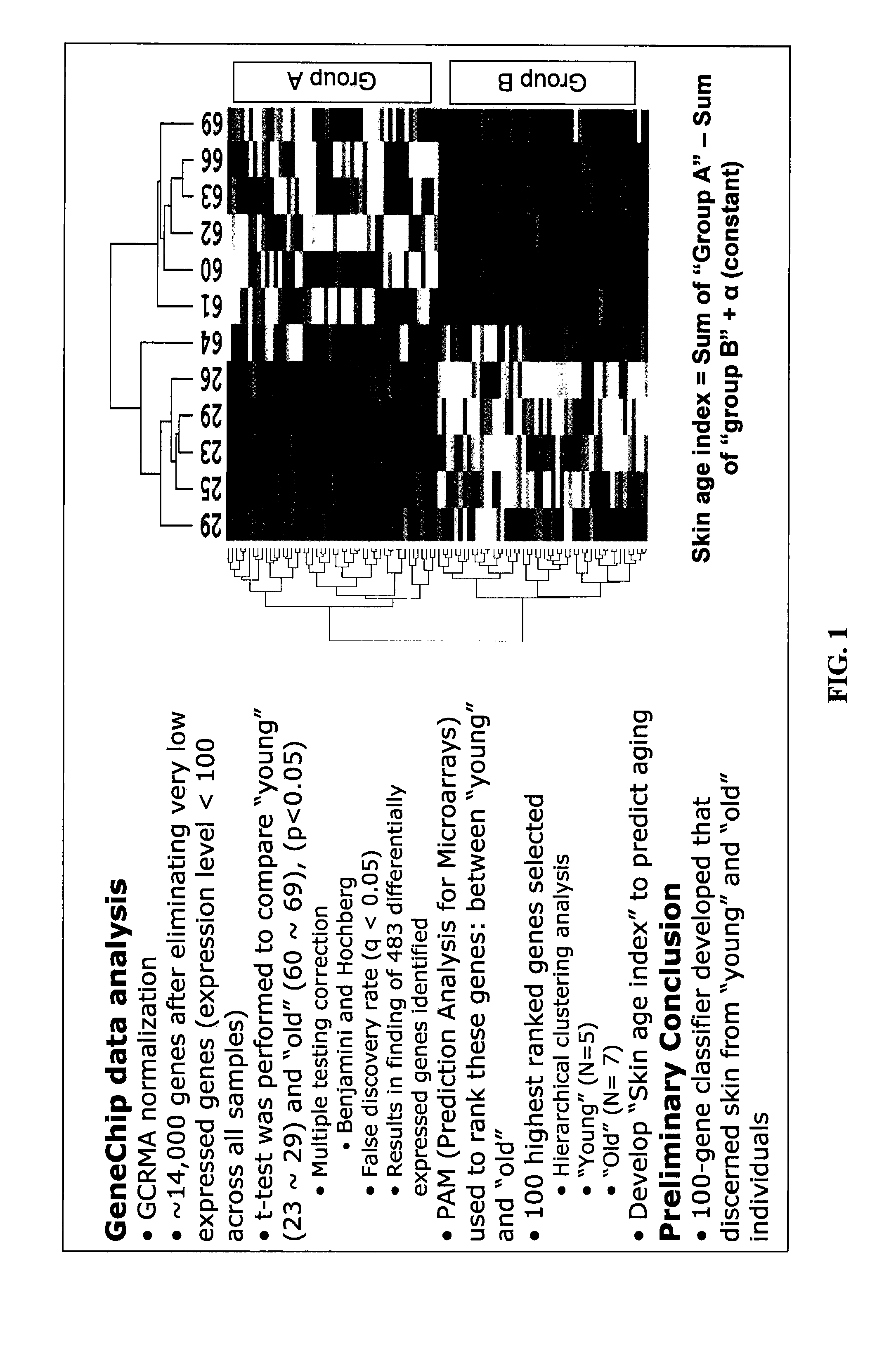
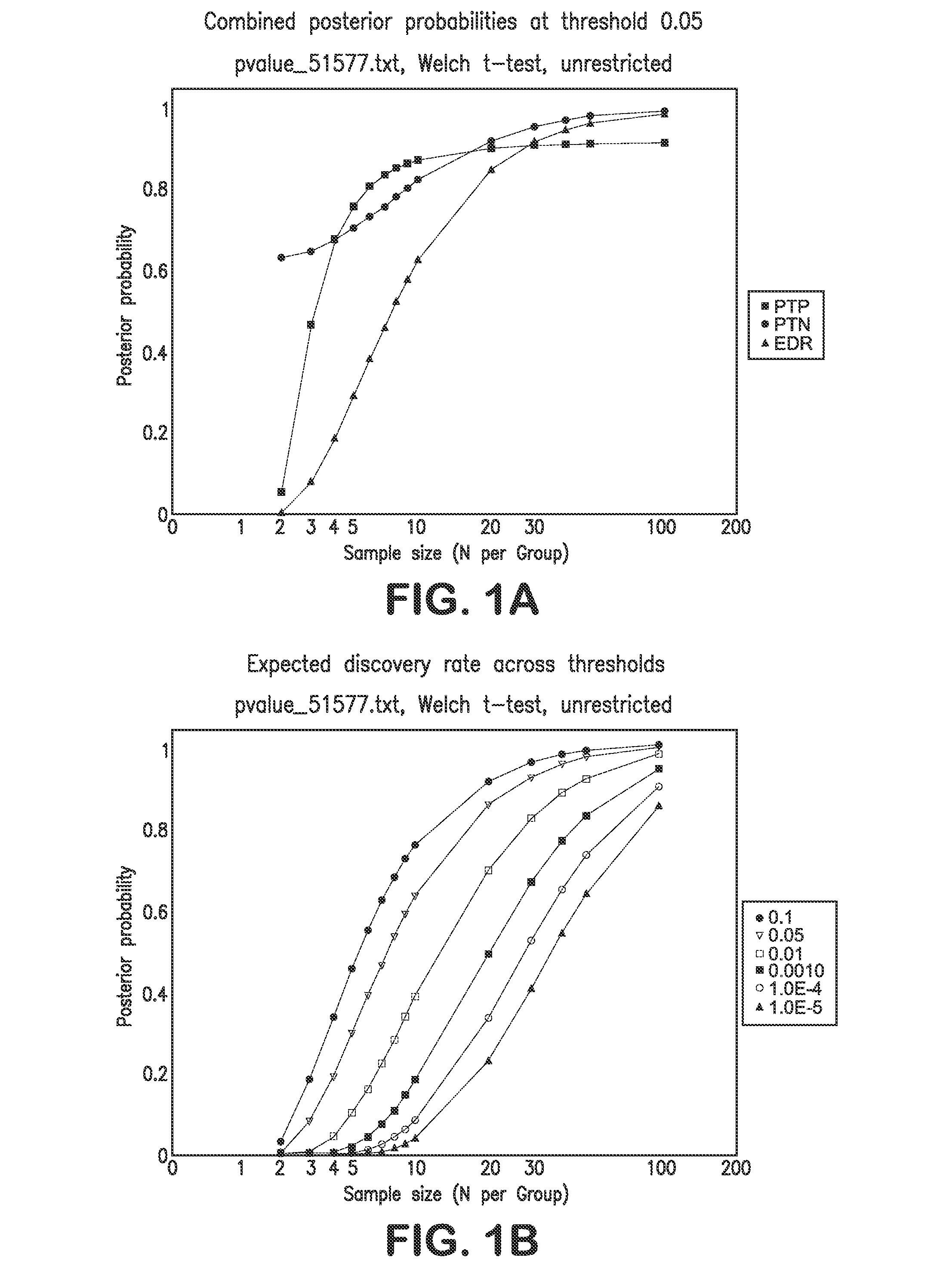
Determining Age Ranges of Skin Samples
InactiveUS20100086501A1Ensure adequate isolationReducing and increasing expression of geneCosmetic preparationsCompound screeningProtein profilingProtein molecules
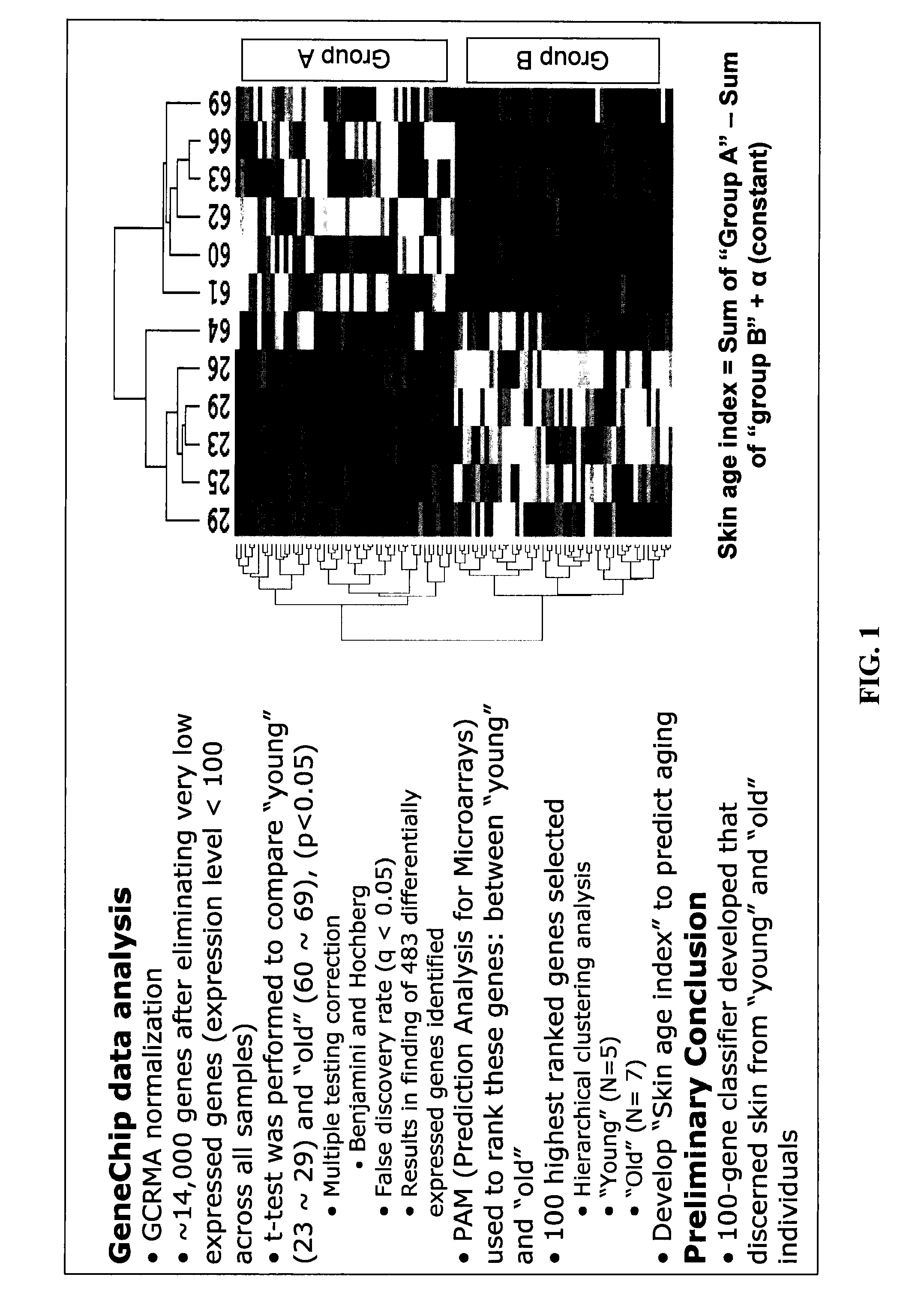
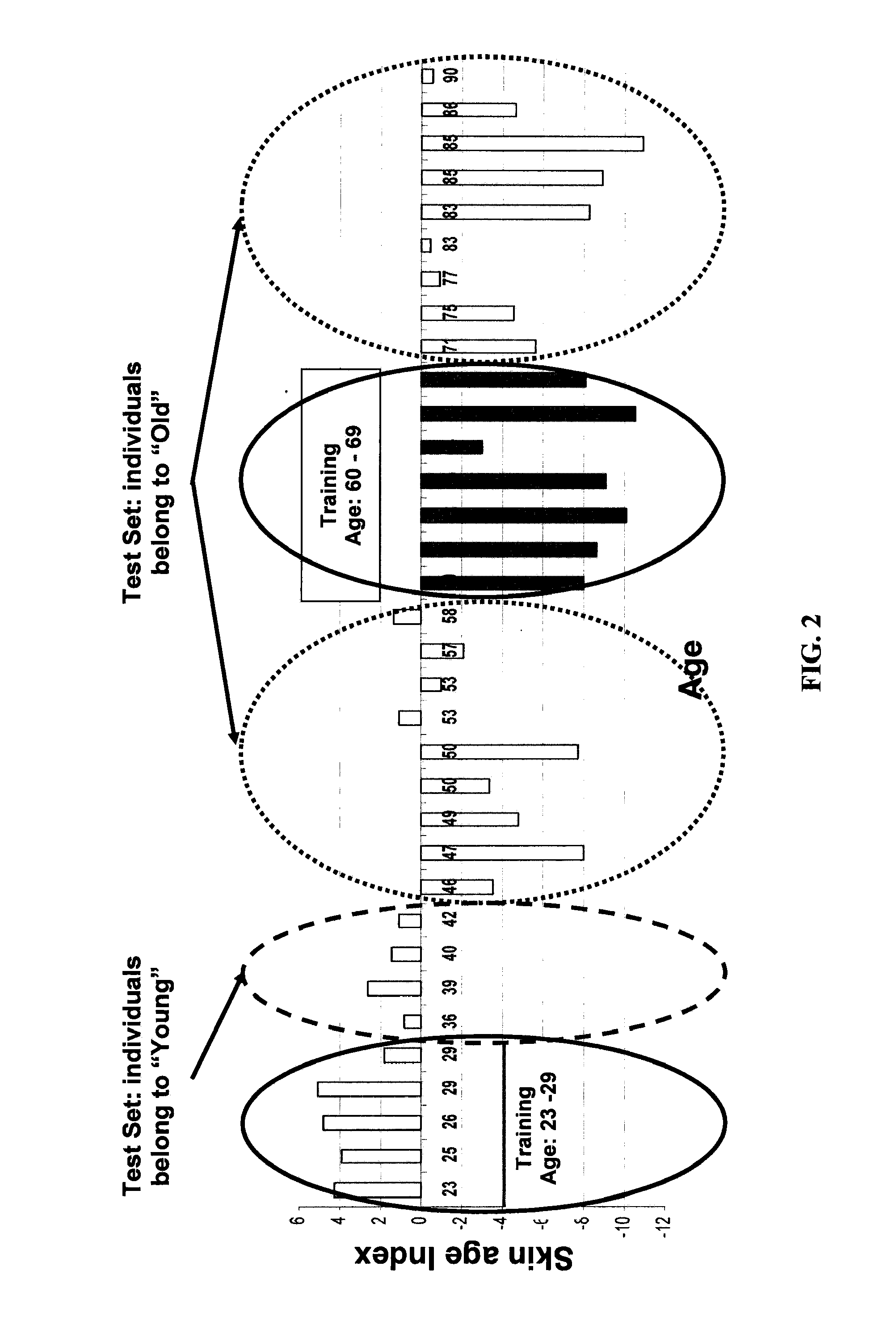
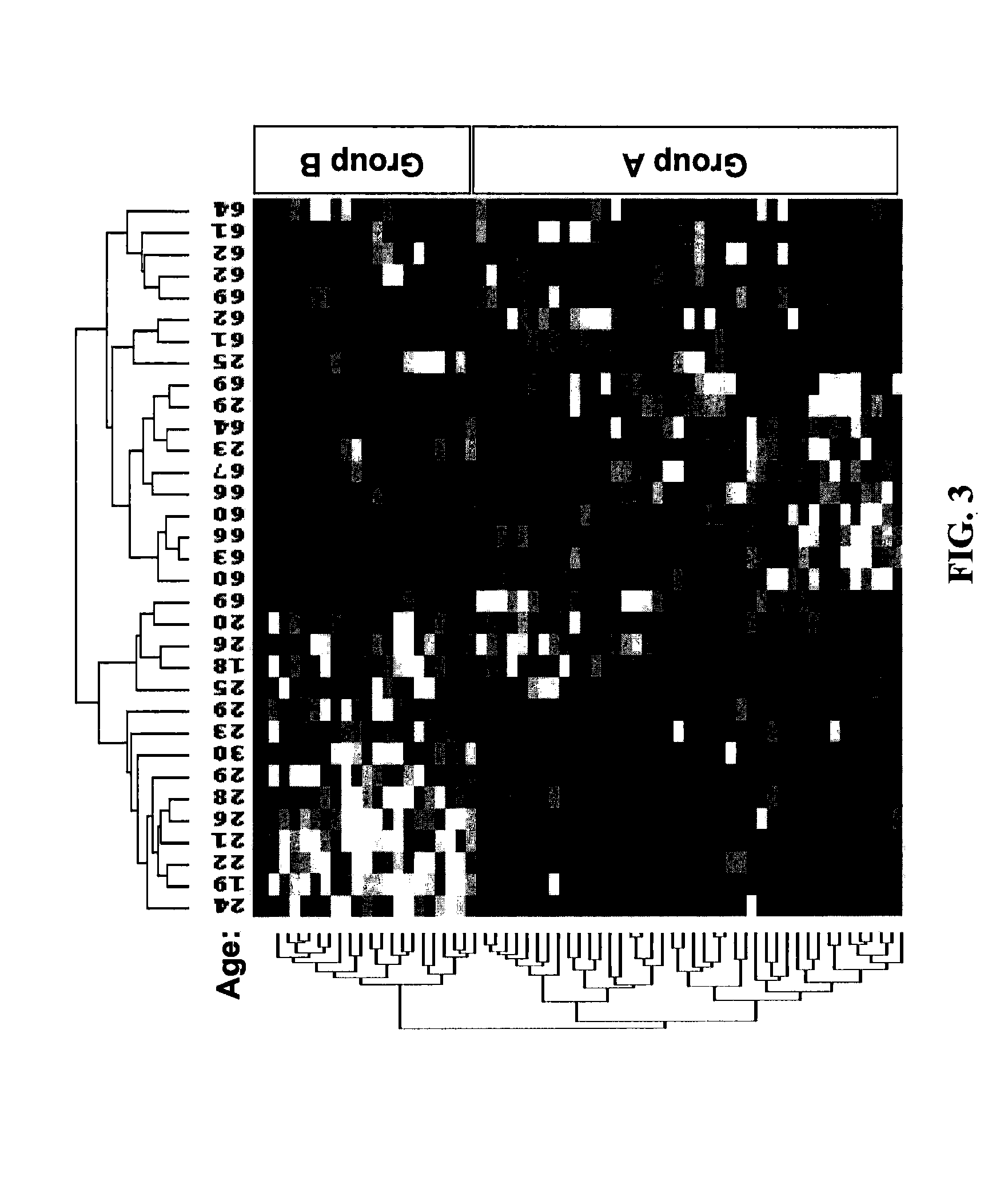
The present invention provides methods for characterizing a skin sample of a subject as belonging to an age range by analyzing nucleic acid or protein molecules obtained from the subject. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample and compare them with a known skin age index. Therapeutic and cosmetic formulations are also provided herein.
Owner:DERMTECH INT
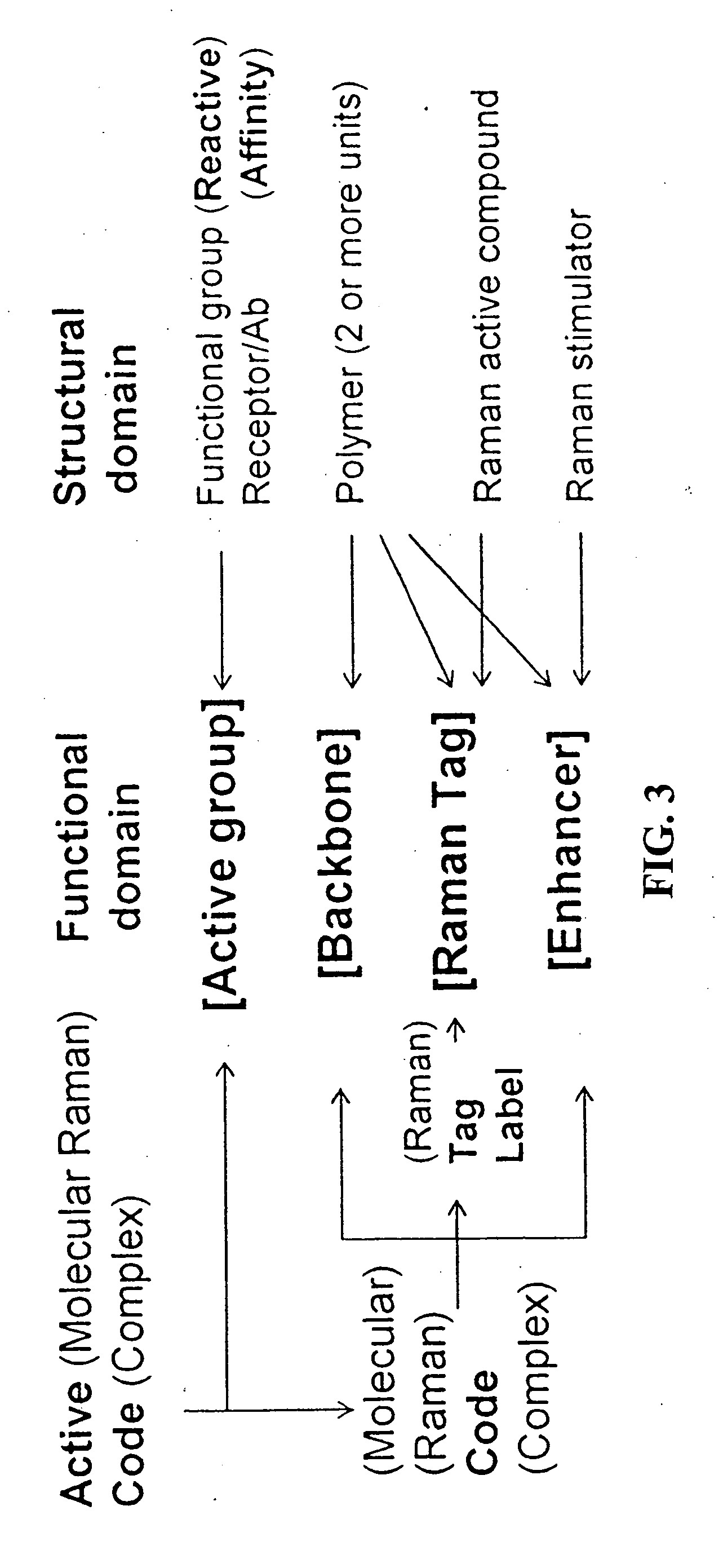
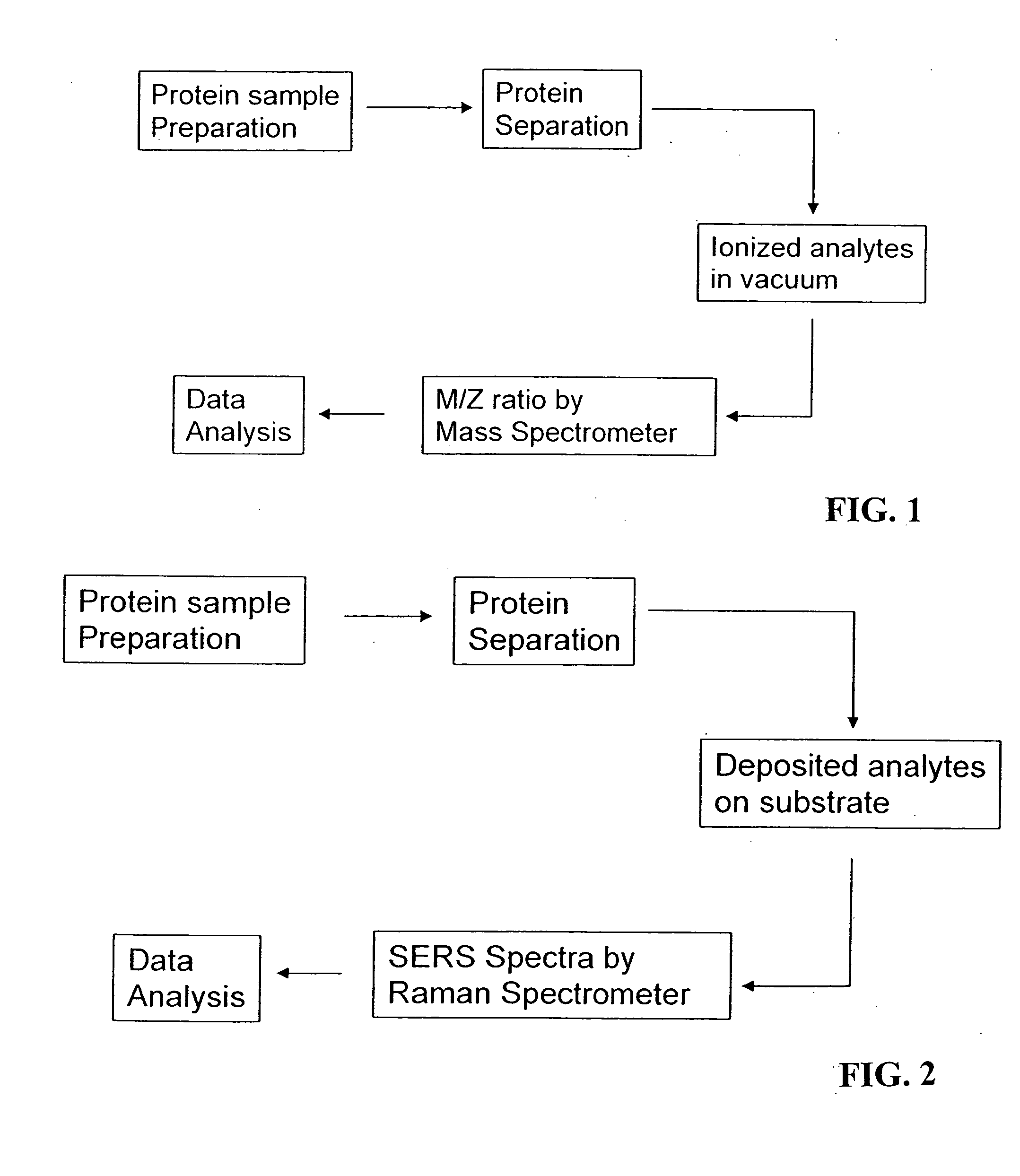
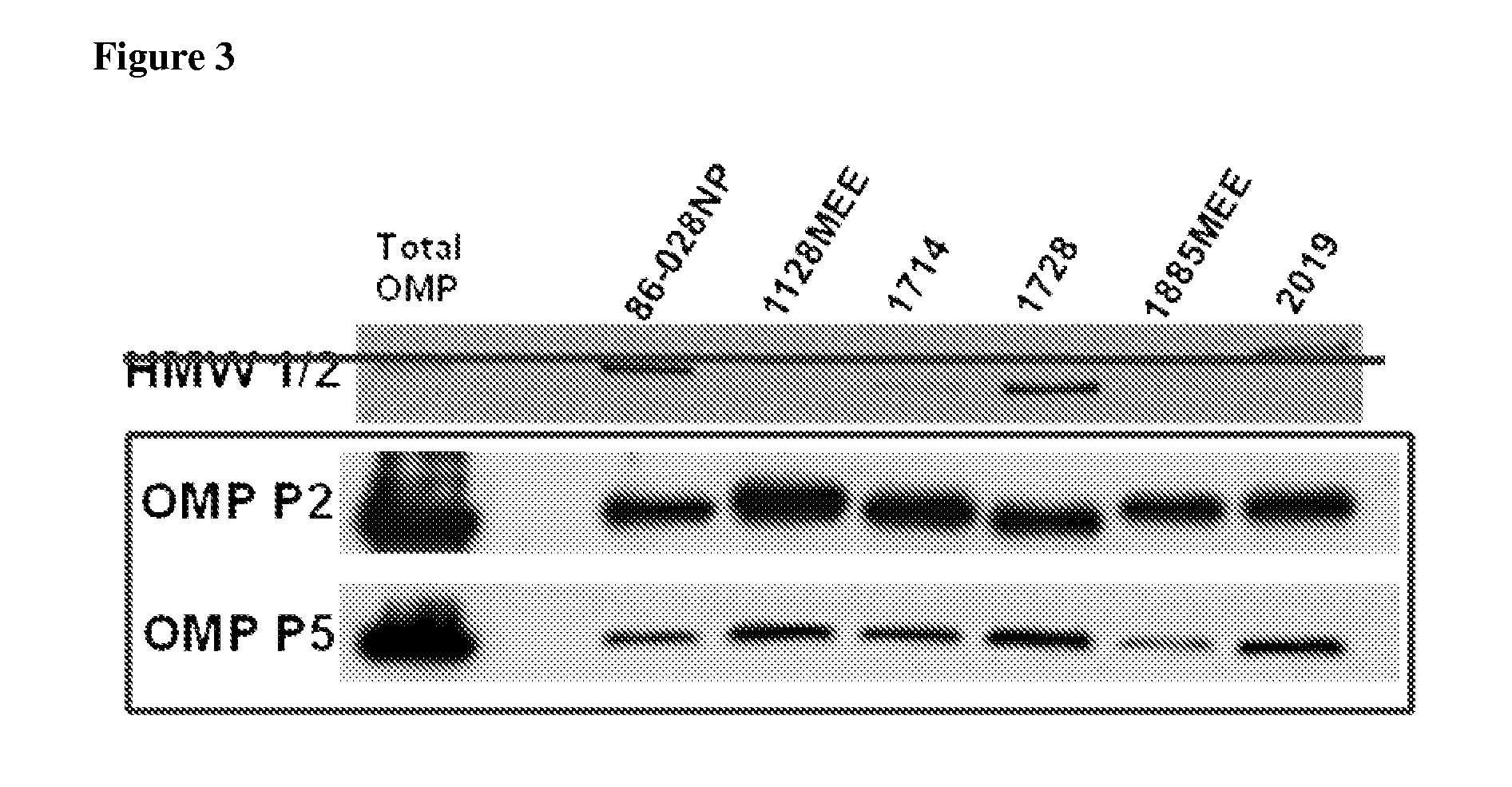
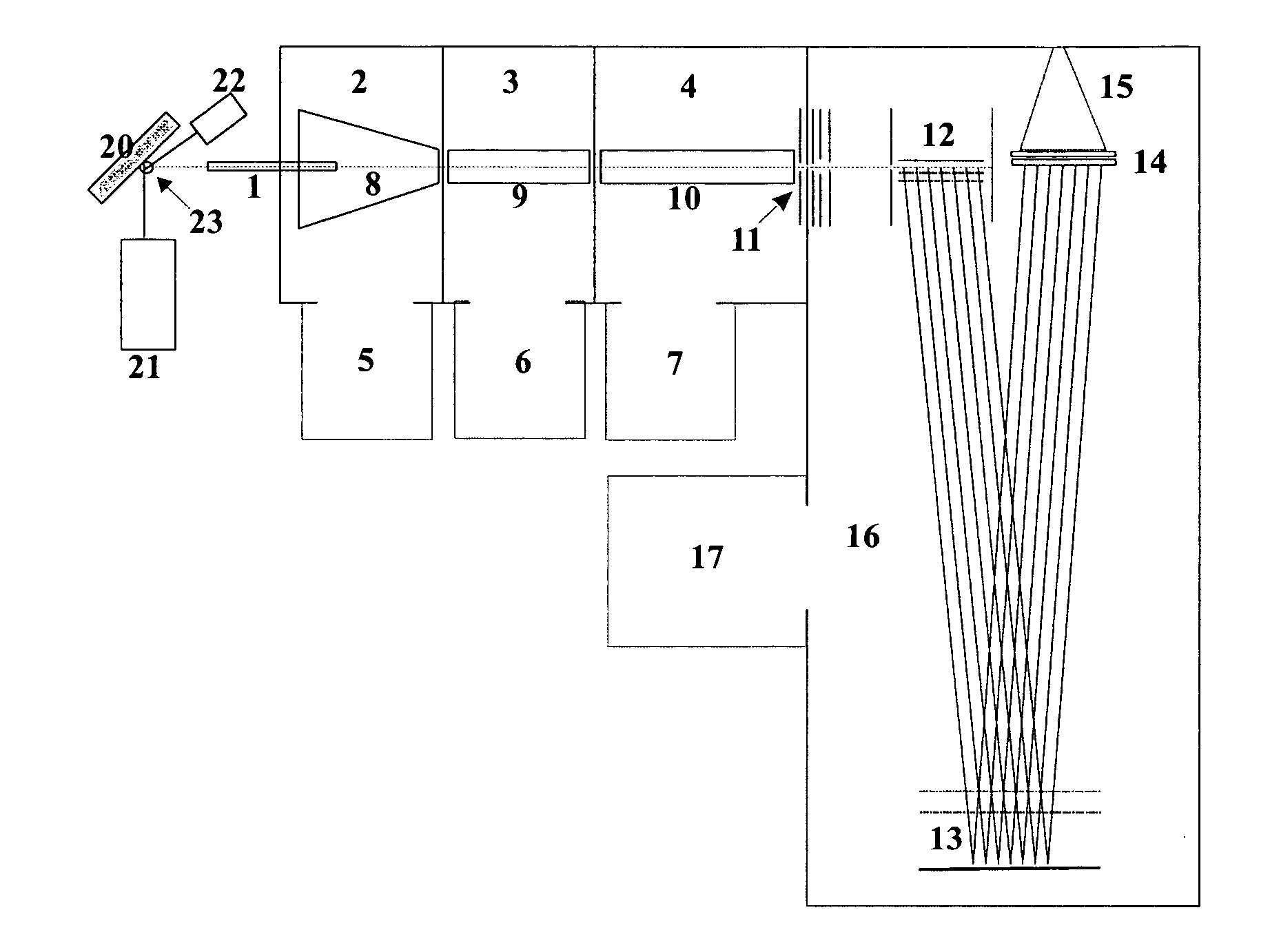
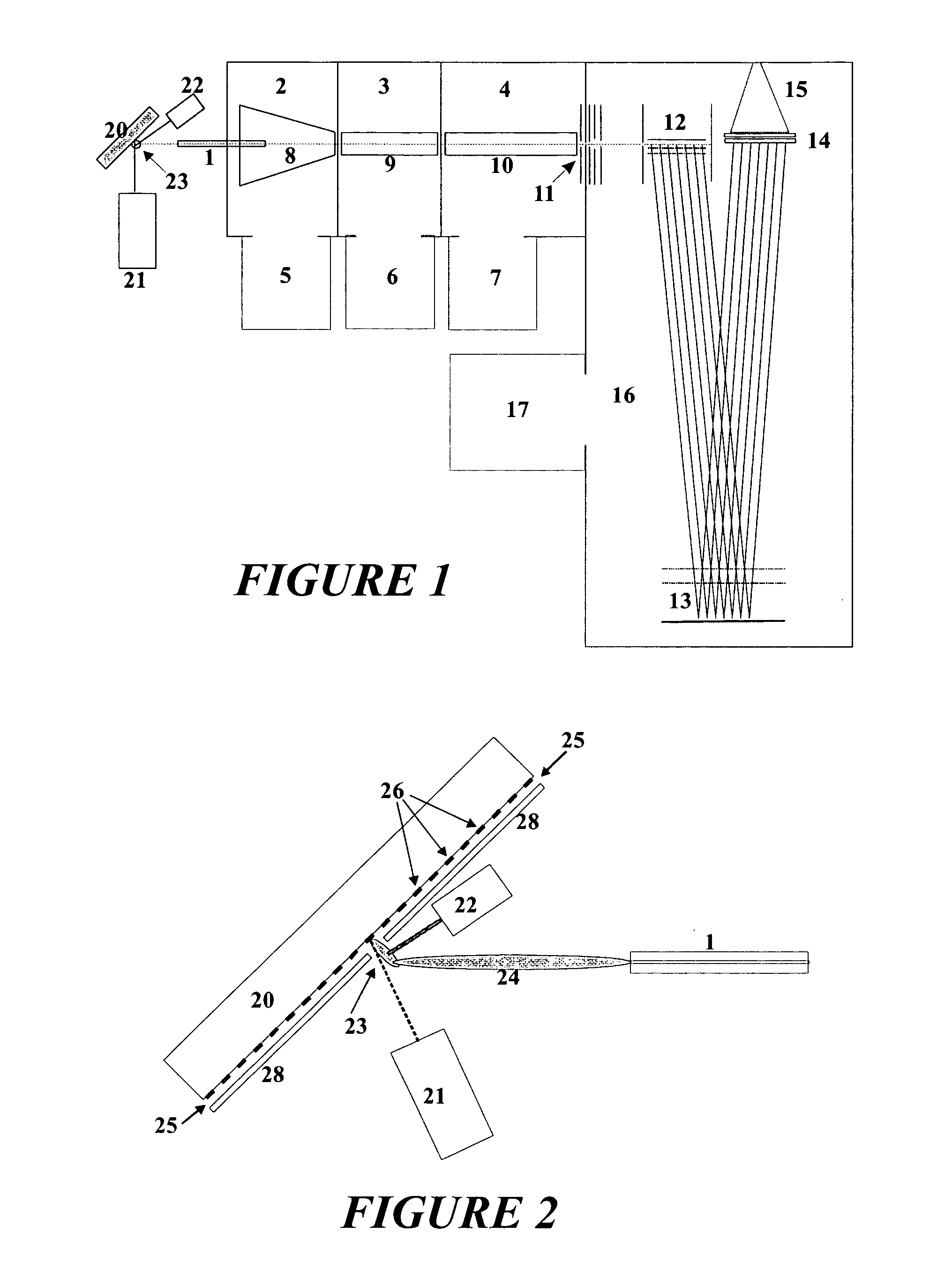
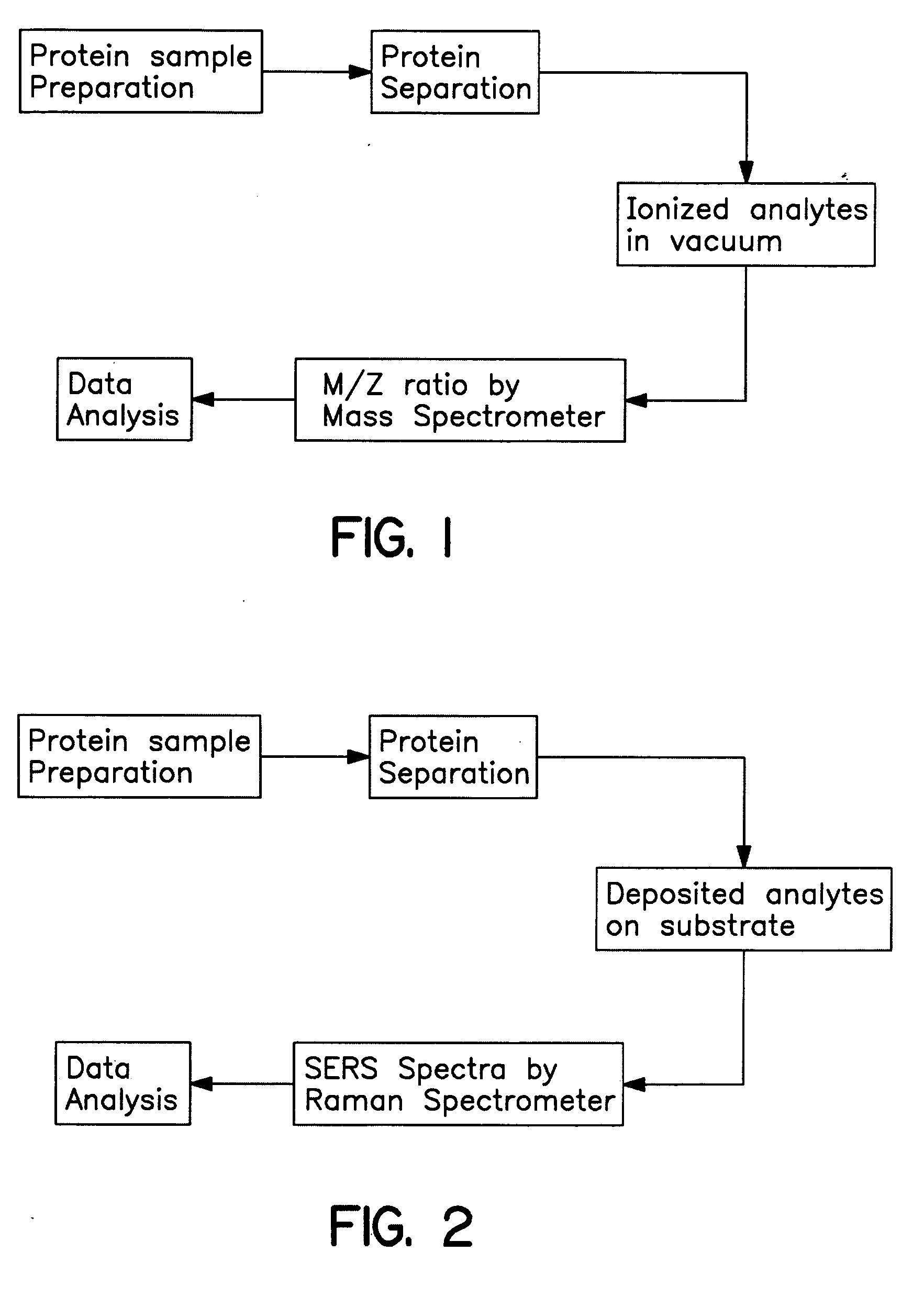
Methods for using raman spectroscopy to obtain a protein profile of a biological sample
The invention provides methods for analyzing the protein content of a biological sample, for example to obtain a protein profile of a sample provided by a particular individual. The proteins and protein fragments in the sample are separated on the basis of chemical and / or physical properties and maintained in a separated state at discrete locations on a solid substrate or within a stream of flowing liquid. Raman spectra are then detected as produced by the separated proteins or fragments at the discrete locations such that a spectrum from a discrete location provides information about the structure or identity of one or more particular proteins or fragments at the discrete location. The proteins or fragments at discrete locations can be coated with a metal, such as gold or silver, and / or the separated proteins can be contacted with a chemical enhancer to provide SERS spectra. Method and kits for practicing the invention are also provided.
Owner:INTEL CORP
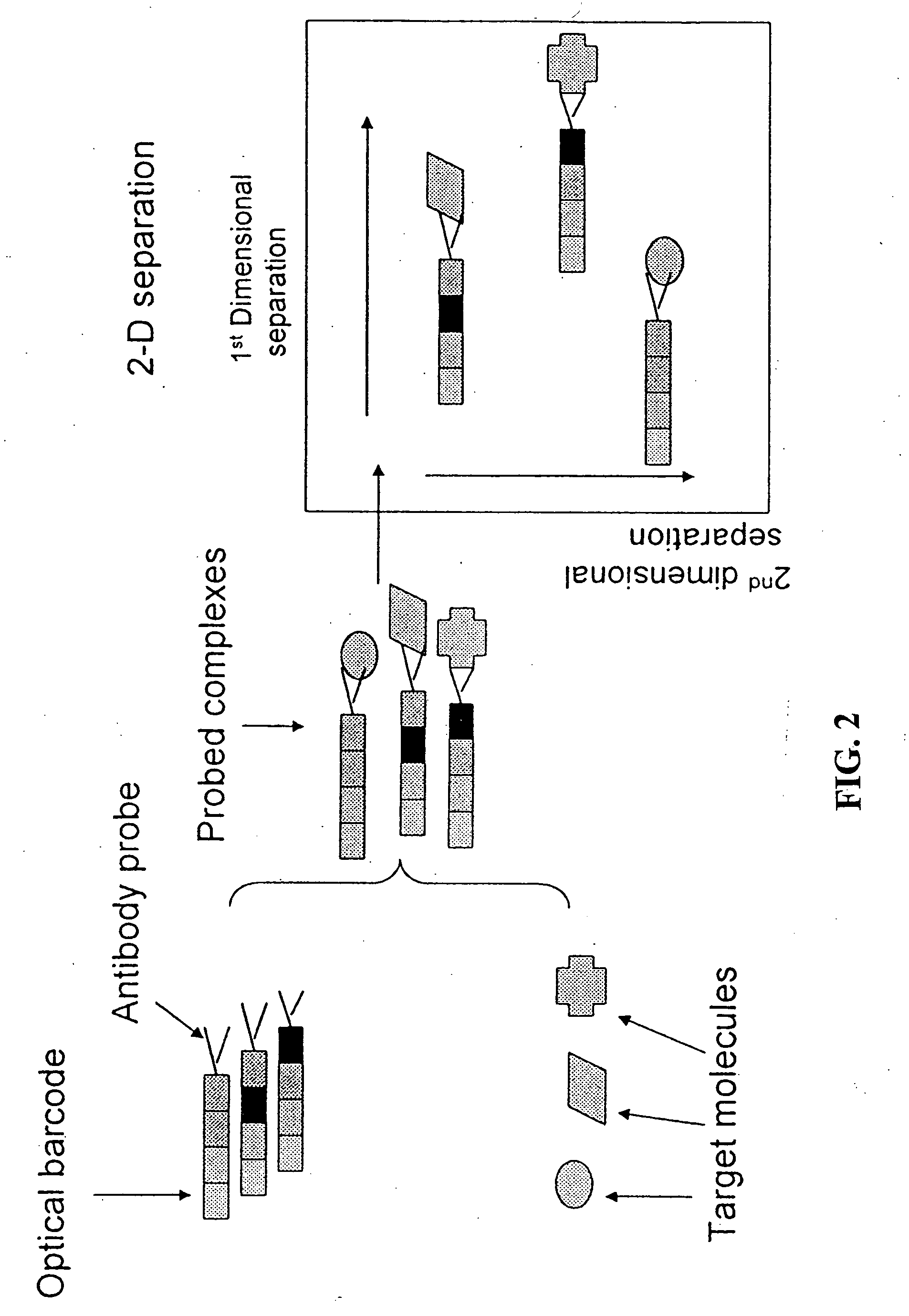
Protein mapping
InactiveUS20040010126A1Easy to separateShorten analysis timeComponent separationMaterial analysis by electric/magnetic meansProtein profilingProtein insertion
The present invention relates to multiphase protein separation methods capable of resolving large numbers of cellular proteins. The methods of the present invention provide protein profile maps for imaging and comparing protein expression patterns. The present invention provides alternatives to traditional 2-D gel separation methods for the screening of protein profiles.
Owner:RGT UNIV OF MICHIGAN
Protein chip for detecting esophageal squamous carcinoma marker and kit box of protein chip
The invention discloses a high-throughput multi-index protein chip for screening esophageal squamous carcinoma specific protein markers, and discloses a kit supporting the use. 55 types of esophageal squamous carcinoma differential proteins in the serum or blood plasma are selected and used as the esophageal squamous carcinoma protein markers and protein contrast prepare the protein chip; and the protein chip comprises a substrate, protein detection indicators and a contrast detection indicator coating, wherein the protein detection indicators are distributed as arrays on the substrate. Detection liquid of a supplementary experiment reagent is filled in the kit. By adopting the protein chip, the protein profiles of three types of people, namely, normal people, people subjected to esophageal squamous cell carcinoma precancerous lesions, and people subjected to the esophageal squamous carcinoma, can be determined; and means are provided for screening the esophageal squamous carcinoma in the early stage, diagnosing in mid stage and late stage, as well as monitoring the state of illness.
Owner:JIANGSU YUANHUA BIO TECH
Protein profiling platform
Methods and compositions are described for analyzing complex protein mixtures, such as proteomes, using activity-based probes. In particular, probes that specifically react with and bind to the active form of one or more target proteins are employed. Labeled peptides obtained from the labeled active target proteins can be used in screening and identification procedures, and can be related to the identity, presence, amount, or activity of active members of the desired target protein class. The methods and compositions described herein can be used, for example, to provide diagnostic information concerning pathogenic states, in identifying proteins that may act as therapeutic targets, and in drug discovery.
Owner:FROG +5
Diagnosis of Melanoma and Solar Lentigo by Nucleic Acid Analysis
InactiveUS20110160080A1Less traumaticMicrobiological testing/measurementLibrary screeningProtein profilingPigments skin
The present invention provides methods for diagnosing melanoma and / or solar lentigo in a subject by analyzing nucleic acid molecules obtained from the subject. The present invention also provides methods for distinguishing melanoma from solar lentigo and / or dysplastic nevi and / or normal pigmented skin. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample.
Owner:DERMTECH INT
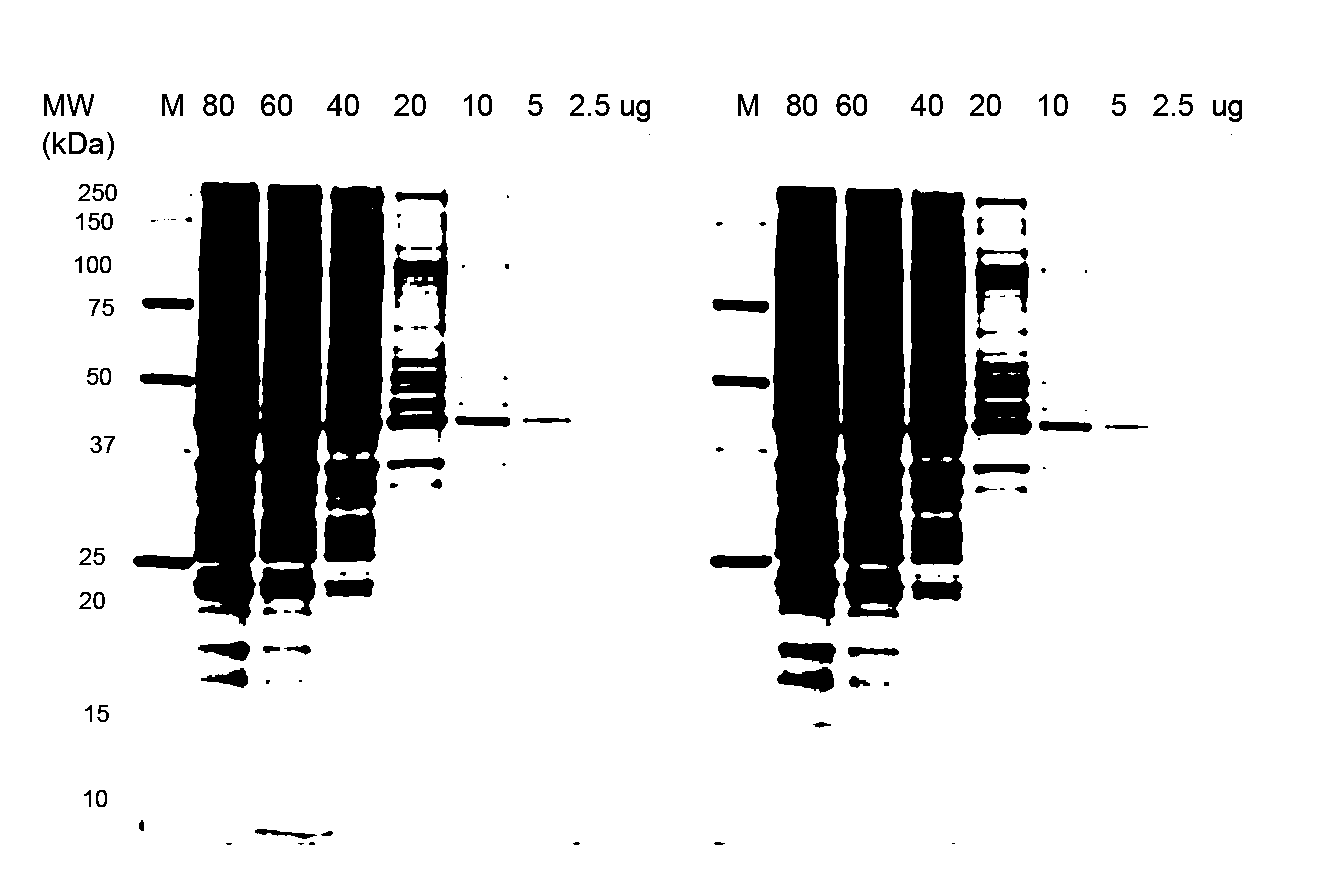
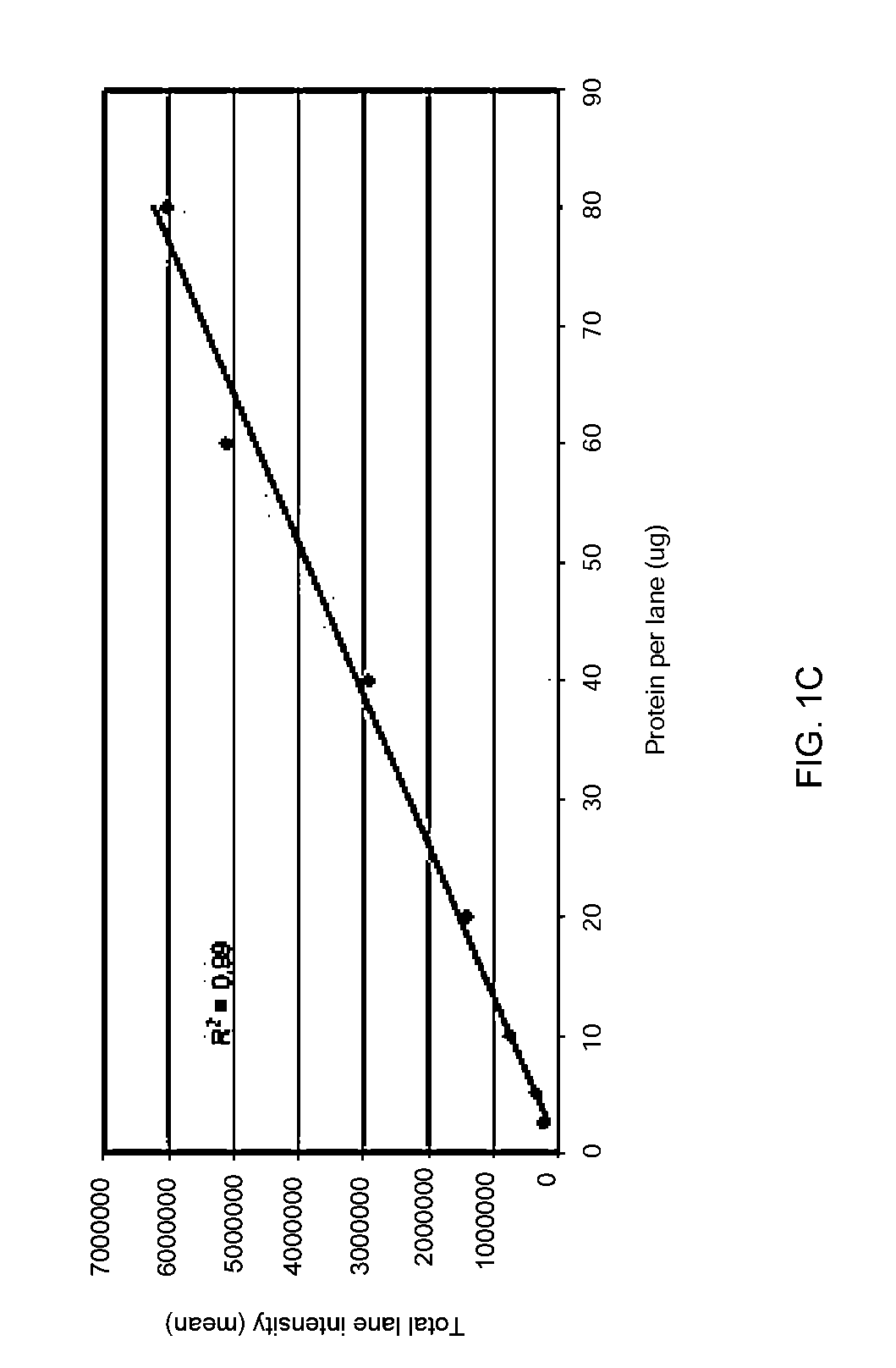
Stain-free protein quantification and normalization
Disclosed herein are methods of protein quantification and normalization using haloalkylated tryptophan fluorescence. Complex protein samples, i.e., samples that each contain 1,000 or more distinct proteins, from diverse sources that do not have common protein profiles are treated with a halo-substituted organic compound (i.e. haloalkane) that reacts with tryptophan residues to form fluorescent products. Irradiation of the samples with ultraviolet light and the detection and quantification of the resultant fluorescent emissions from all proteins in each sample are then used to obtain comparative values for total protein content among the various samples. The values thus obtained are found to be valid indications of comparative total protein content, despite the fact that the tryptophan levels vary widely among the various proteins in any single sample and the samples, due to the diversity of their origins, tend to differ among themselves in the identities and relative amounts of the proteins that they contain. Protein samples are also normalized to correct for differences in sample dilution, sample loading, and protein transfer inconsistencies, by using stain-free detection of total protein in each of the samples, or detection of subsamples within each sample.
Owner:BIO RAD LAB INC
Protein profiles with atmospheric pressure ionization
ActiveUS20060097143A1Accurate massBetter mass massTime-of-flight spectrometersTesting/calibration apparatusProtein profilingDesorption
The invention relates to the acquisition of mass spectra of complex protein mixtures, often called protein profiles, for example to search for biomarkers which indicate stress situations, or to identify microbes. Up to now protein profiles have been acquired using ionization by matrix-assisted laser desorption with high detection sensitivity in linear time-of-flight mass spectrometers, but these display very poor mass resolution and a very poor reproducibility of the mass values. The invention provides methods which produce surprisingly similar mass spectra, but with far higher mass resolution and mass accuracy. Ionization takes place outside the vacuum at ambient pressure, preferably by means of laser desorption and CI post-ionization. Analysis of the ions takes place in a high-resolution mass spectrometer, for example a reflector time-of-flight mass spectrometer with orthogonal ion injection.
Owner:BRUKER DALTONIK GMBH & CO KG
Methods and formulations for diagnosing, monitoring, staging and treating heart failure
Protein profiles useful in diagnosing, monitoring, staging, evaluating treatments and treating and selecting treatments for heart failure are provided.
Owner:QUEENS UNIV OF KINGSTON
Identification of pathogens in body fluids
ActiveUS20100255527A1Microbiological testing/measurementMaterial analysisMicroorganismProtein profiling
Identification of infectious pathogens, particularly viruses, bacteria and other microorganisms is effected with a method whereby pathogens of acute infections can be identified, without first culturing them in external nutrient media, by mass spectrometric measurement of their protein profiles obtained from pathogens directly precipitated from body fluid into pellets by centrifuging. With this method, pathogens which cause acute infections can be identified in less than one hour.
Owner:BRUKER DALTONIK GMBH & CO KG
Using plasma proteomic pattern for diagnosis, classification, prediction of response to therapy and clinical behavior, stratification of therapy, and monitoring disease in hematologic malignancies
The present invention demonstrates that the diagnosis and prediction of clinical behavior in patients with hematologic malignancies, such as leukemia, can be accomplished by analysis of proteins present in a plasma sample. Thus, in particular embodiments the present invention uses plasma to create a diagnostic or prognostic protein profile of a hematologic malignancy comprising collecting plasma samples from a population of patients with hematologic malignancies; generating protein spectra from the plasma samples with or without fractionation; comparing the protein spectra with clinical data; and identifying protein markers in the plasma samples that correlate with the clinical data. Protein markers identified by this approach can then be used to create a protein profile that can be used to diagnose the hematologic malignancy or determine the prognosis of the hematologic malignancy. Potentially these specific proteins can be identified and targeted in the therapy of these malignancies.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Identification of pathogens in body fluids
ActiveUS8450081B2Microbiological testing/measurementOn/in organic carrierProtein profilingMicroorganism
Identification of infectious pathogens, particularly viruses, bacteria and other microorganisms is effected with a method whereby pathogens of acute infections can be identified, without first culturing them in external nutrient media, by mass spectrometric measurement of their protein profiles obtained from pathogens directly precipitated from body fluid into pellets by centrifuging. With this method, pathogens which cause acute infections can be identified in less than one hour.
Owner:BRUKER DALTONIK GMBH & CO KG
Proteomics based diagnostic detection method for chronic sinusitis
ActiveUS20140314876A1Effective choiceEarlyAntibacterial agentsBioreactor/fermenter combinationsProteomics methodsBacteroides
The invention provides for a proteomic approach for identification of specific bacterial protein profiles that may be used in the development of methods for the diagnosis of bacterial chronic sinusitis. The invention provides for methods for determining the presence of pathogenic bacteria in the upper respiratory tract of a subject using protein profiles of the pathogenic bacteria. The invention also provides for methods of diagnosing a bacterial infection of the upper respiratory tract of a subject using protein profiles of a pathogenic bacteria. In addition, the invention provides for devices, immunoassays and kits for identifying pathogenic bacteria in the upper respiratory tract.
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Growth state-specific immunofluorescent probes for determining physiological state and method of use
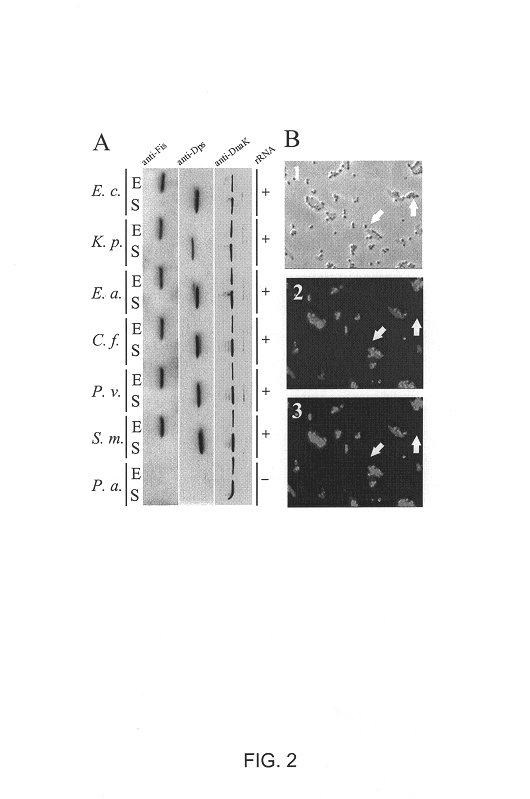
A novel method for examining bacterial growth state is carried out by measuring the levels of conserved cytosolic proteins specific for alternative growth states, using bacterial specific antibody fluorochrome-coupled probe. Utilizing the method of the invention, the cellular growth state of individual bacteria can be determined by measuring the abundance of growth state-specific protein homologs. For example, through use of the protein profiling method of the invention, bacterial VNC state can be distinguished by differentiating growing (exponential phase) from nongrowing or dormant (stationary phase) cells.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Diagnosis of melanoma by nucleic acid analysis
InactiveUS20150005184A1Improve accuracyImprove abilitiesMicrobiological testing/measurementLibrary screeningProtein profilingDysplastic nevus
The present invention provides methods for diagnosing melanoma and / or solar lentigo in a subject by analyzing nucleic acid molecules obtained from the subject. The present invention also provides methods for distinguishing melanoma from solar lentigo and / or dysplastic nevi and / or normal pigmented skin. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample.
Owner:DERMTECH INT
Proteomics based diagnostic detection method for chronic sinusitis
ActiveUS9568472B2Effective choiceEarlyAntibacterial agentsMicrobiological testing/measurementBacteroidesProtein profiling
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Protein profiles with atmospheric pressure ionization
ActiveUS7442921B2Better mass massHigh resolutionTime-of-flight spectrometersTesting/calibration apparatusProtein profilingDesorption
The invention relates to the acquisition of mass spectra of complex protein mixtures, often called protein profiles, for example to search for biomarkers which indicate stress situations, or to identify microbes. Up to now protein profiles have been acquired using ionization by matrix-assisted laser desorption with high detection sensitivity in linear time-of-flight mass spectrometers, but these display very poor mass resolution and a very poor reproducibility of the mass values. The invention provides methods which produce surprisingly similar mass spectra, but with far higher mass resolution and mass accuracy. Ionization takes place outside the vacuum at ambient pressure, preferably by means of laser desorption and CI post-ionization. Analysis of the ions takes place in a high-resolution mass spectrometer, for example a reflector time-of-flight mass spectrometer with orthogonal ion injection.
Owner:BRUKER DALTONIK GMBH & CO KG
Methods for using Raman spectroscopy to obtain a protein profile of a biological sample
The invention provides methods for analyzing the protein content of a biological sample, for example to obtain a protein profile of a sample provided by a particular individual. The proteins and protein fragments in the sample are separated on the basis of chemical and / or physical properties and maintained in a separated state at discrete locations on a solid substrate or within a stream of flowing liquid. Raman spectra are then detected as produced by the separated proteins or fragments at the discrete locations such that a spectrum from a discrete location provides information about the structure or identity of one or more particular proteins or fragments at the discrete location. The proteins or fragments at discrete locations can be coated with a metal, such as gold or silver, and / or the separated proteins can be contacted with a chemical enhancer to provide SERS spectra. Method and kits for practicing the invention are also provided.
Owner:INTEL CORP
Determining age ranges of skin samples
InactiveUS20150259739A1Reducing and increasing expression of geneReduce and increase expressionOrganic active ingredientsBiocideProtein profilingProtein molecules
The present invention provides methods for characterizing a skin sample of a subject as belonging to an age range by analyzing nucleic acid or protein molecules obtained from the subject. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample and compare them with a known skin age index. Therapeutic and cosmetic formulations are also provided herein.
Owner:DERMTECH INT
Diagnosis of melanoma and solar lentigo by nucleic acid analysis
InactiveUS20140154684A1Less traumaticMicrobiological testing/measurementBioinformaticsProtein profilingPigments skin
The present invention provides methods for diagnosing melanoma and / or solar lentigo in a subject by analyzing nucleic acid molecules obtained from the subject. The present invention also provides methods for distinguishing melanoma from solar lentigo and / or dysplastic nevi and / or normal pigmented skin. The methods include analyzing expression or mutations in epidermal samples, of one or more skin markers. The methods can include the use of a microarray to analyze gene or protein profiles from a sample
Owner:DERMTECH INT
Plasma based protein profiling for early stage lung cancer prognosis
The invention provides biomarkers and combinations of biomarkers useful in diagnosing non-small cell lung cancer. Measurements of these biomarkers are inputted into a classification system such as Random. Forest to assist in determining the likelihood that an individual has non-small cell lung cancer. Kits comprising agents for detecting the biomarkers and combination of biomarkers, as well as systems that assist in diagnosing non-small cell lung cancer are also provided.
Owner:LUNG CANCER PROTEOMICS LLC
Protein profile for osteoarthritis
InactiveUS20100292154A1High expressionReduce expressionPeptide librariesSugar derivativesProtein profilingProtein expression profile
The present invention relates to the identification and use of protein expression profiles with clinical relevance to osteoarthritis (OA). In particular, the invention provides the identity of marker proteins whose expression is correlated with OA and OA progression. Methods and kits are described for using these protein expression profiles in the study and / or diagnosis of OA, in the determination of the degree of advancement of OA, and in the selection and / or monitoring of treatment regimens. The invention also relates to the screening of drugs that modulate expression of these proteins or nucleic acid molecules encoding these proteins, in particular for the development of disease-modifying OA agents.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Protein profile for osteoarthritis
ActiveUS20070207480A1Solid foundationCompound screeningApoptosis detectionProtein profilingProtein expression profile
The present invention relates to the identification and use of protein expression profiles with clinical relevance to osteoarthritis (OA). In particular, the invention provides the identity of marker proteins whose expressions are correlated with OA, OA subtype, and / or OA progression. Methods and kits are described for using these protein expression profiles in the study and / or diagnosis of OA, in the determination of the degree of advancement of OA, and in the selection and / or monitoring of treatment regimens. The invention also relates to the screening of drugs that modulate expression of these proteins or nucleic acid molecules encoding these proteins, in particular for the development of disease-modifying OA agents.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Blood Preparation and Profiling
ActiveUS20180306817A1Easy to detectImprove detection levelOmicsDisease diagnosisProtein detectionBlood proteins
The present disclosure relates to methods for generating blood protein profiles in red blood cell-enriched blood samples. The disclosed methods represent a new and improved laboratory technique for producing a protein profile from blood, increasing protein detection.
Owner:SANGUI BIO PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com