Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Pre malignant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pre-malignant: A pre-malignant tumor is not yet cancerous but appears to be developing the properties of cancer. Malignant: Malignant tumors are cancerous. They can grow, spread, and get worse. There is sometimes no clear dividing line between cancerous, precancerous and non-cancerous tumors.
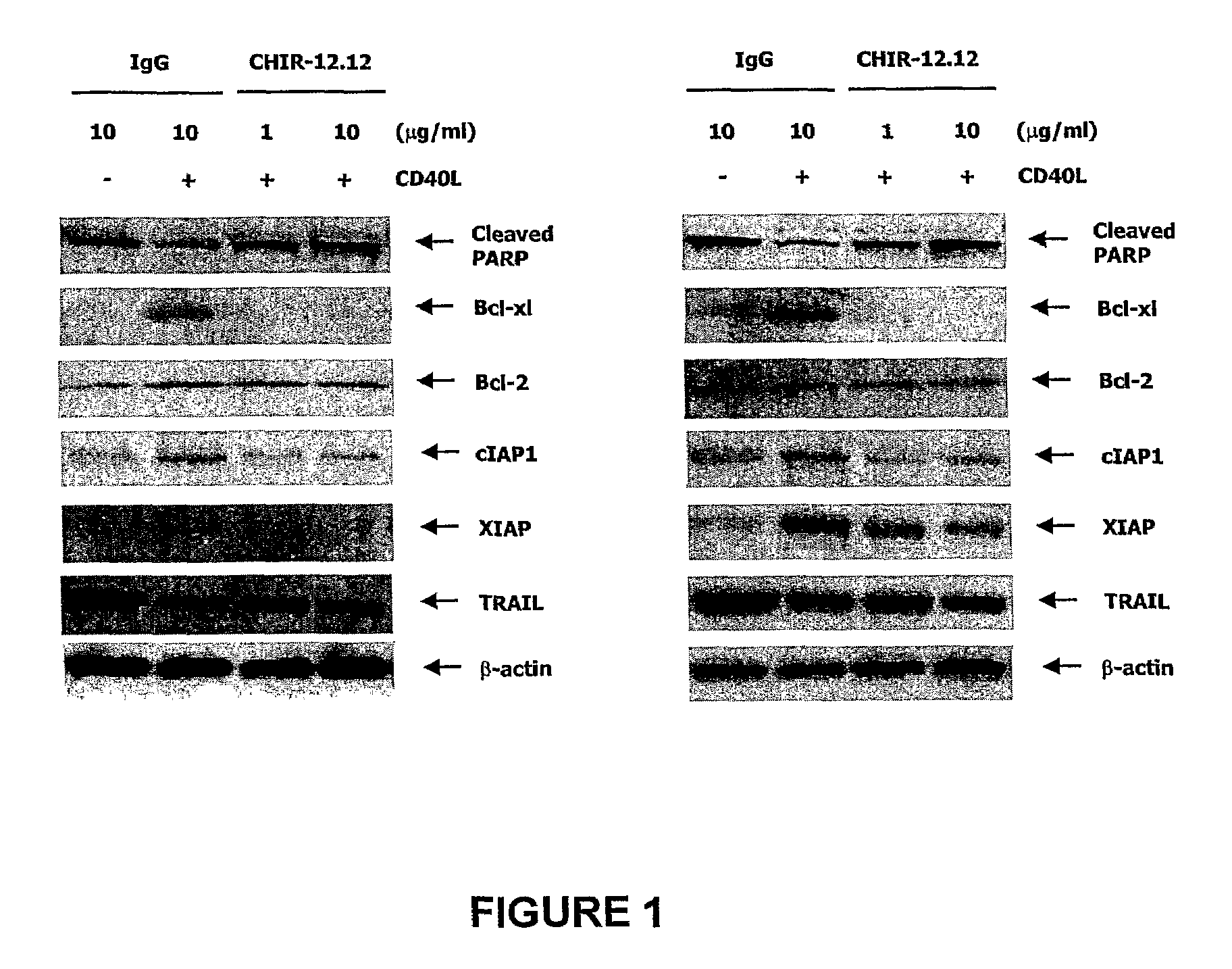
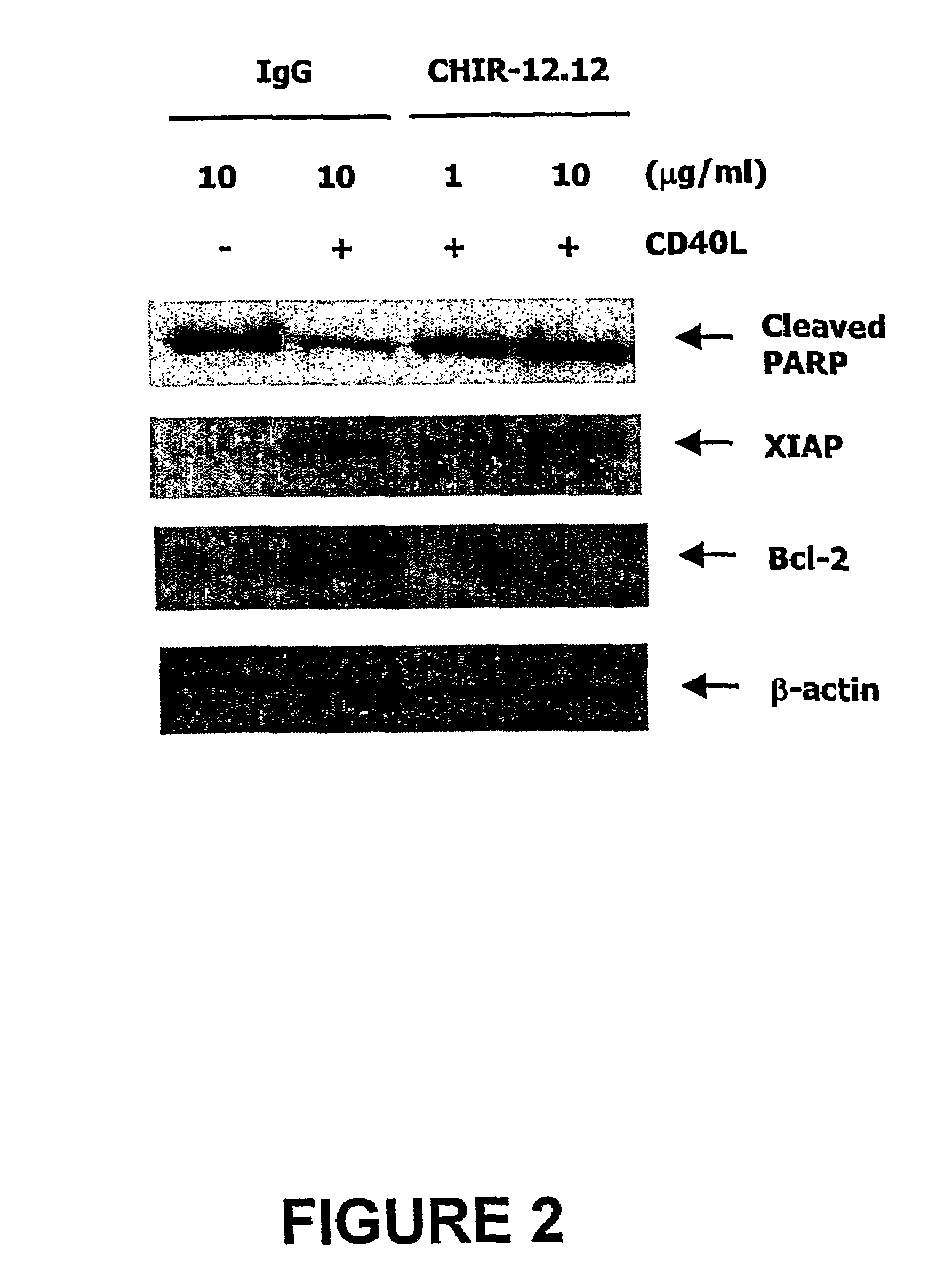
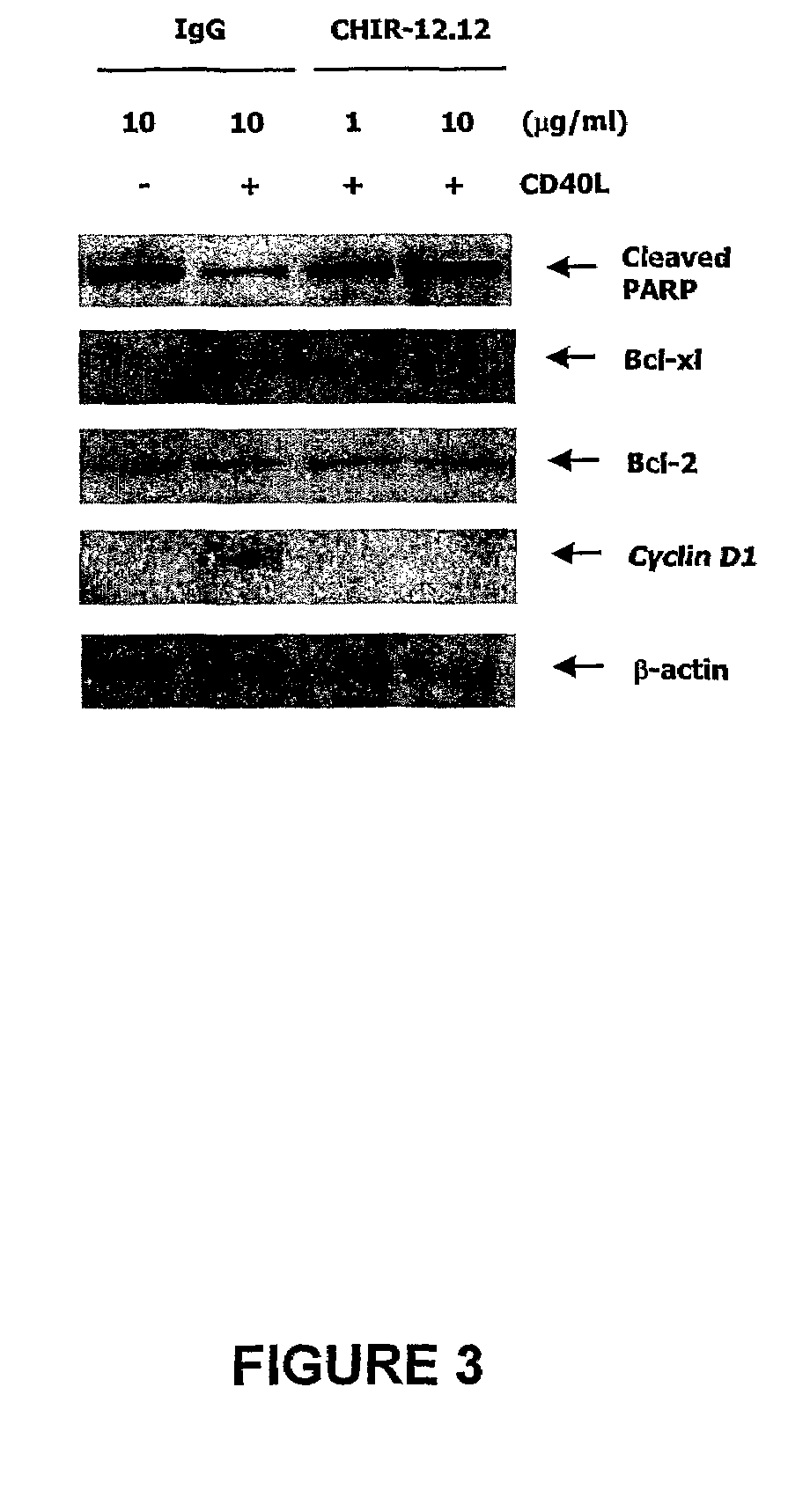
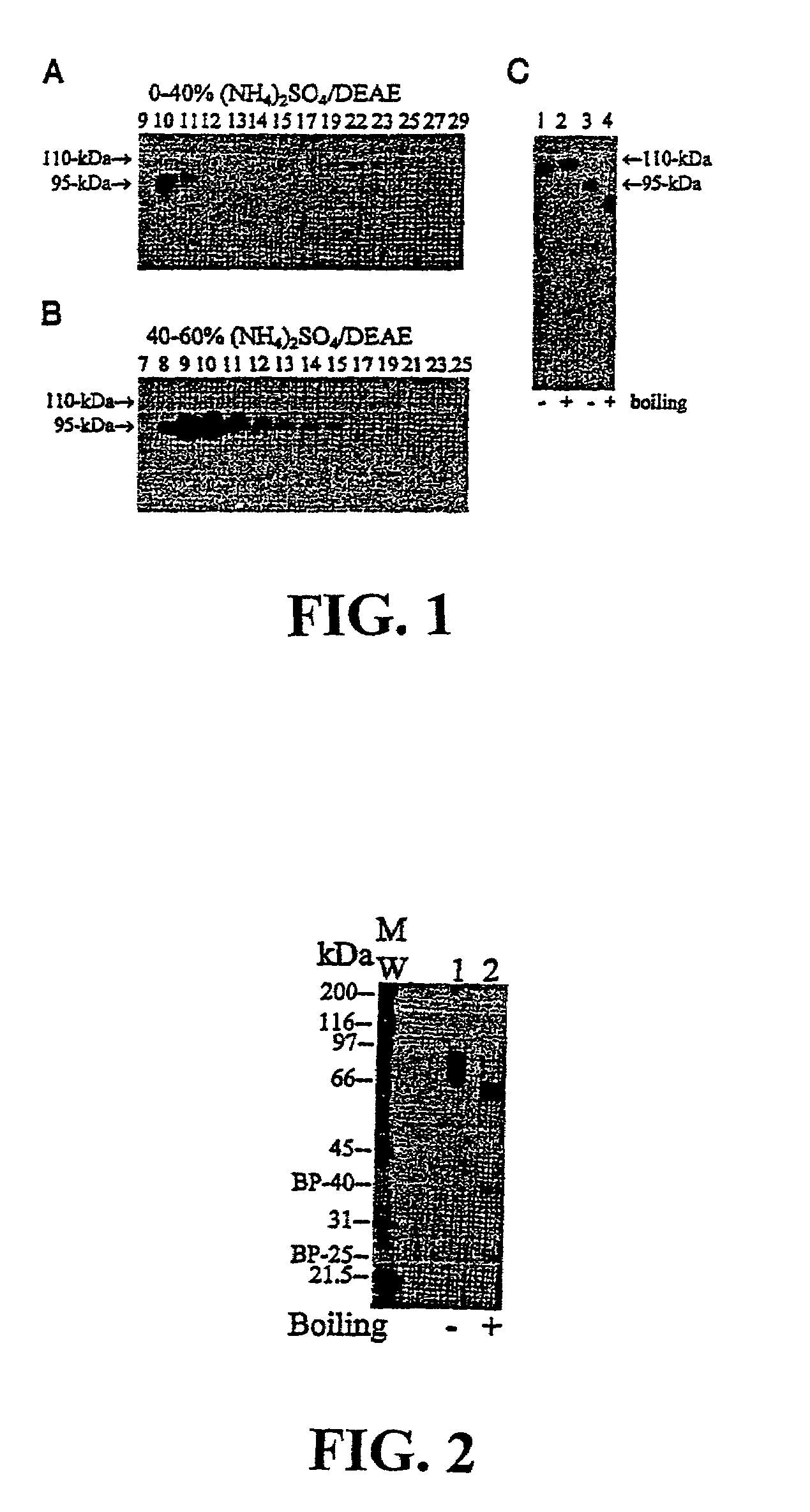
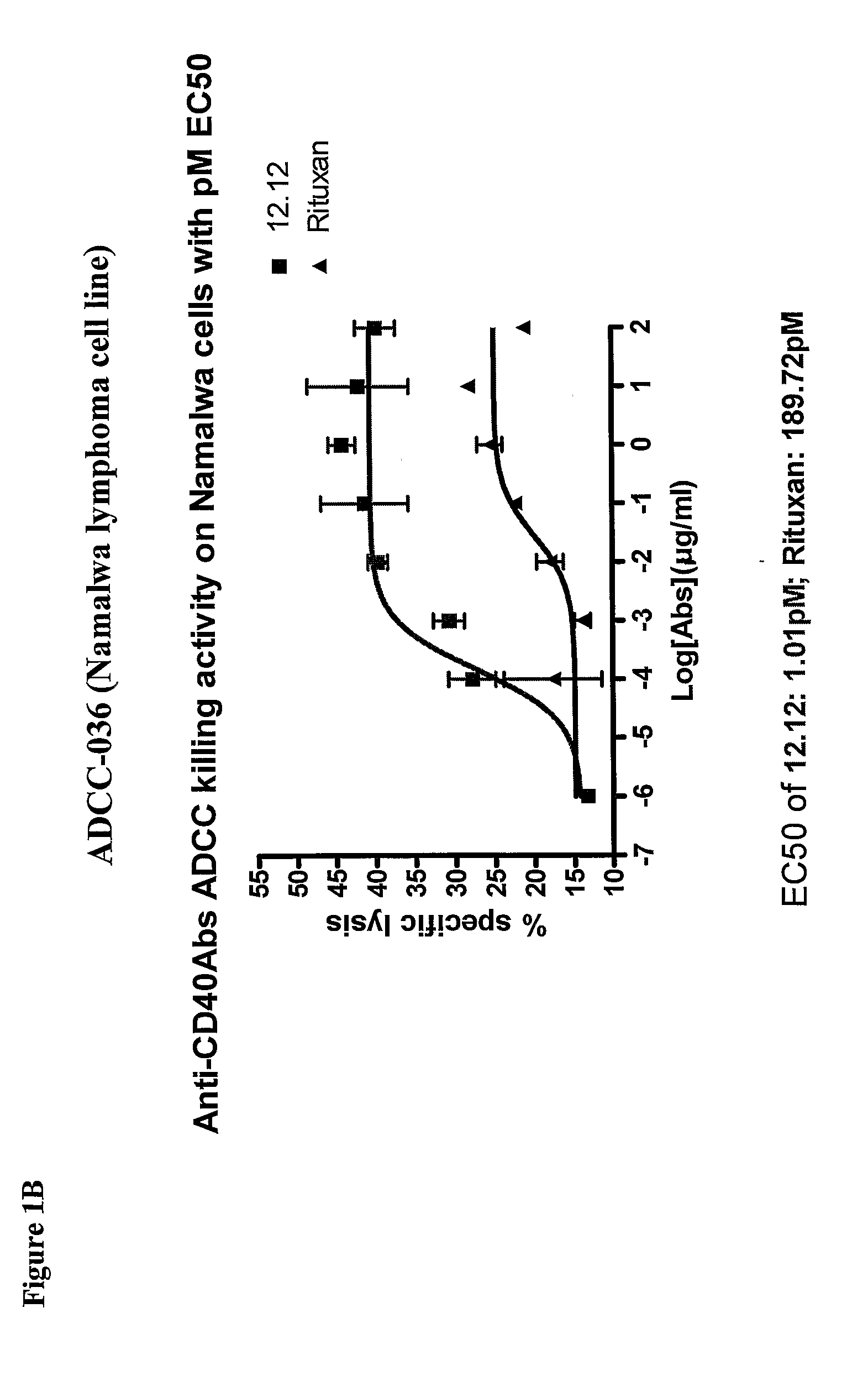
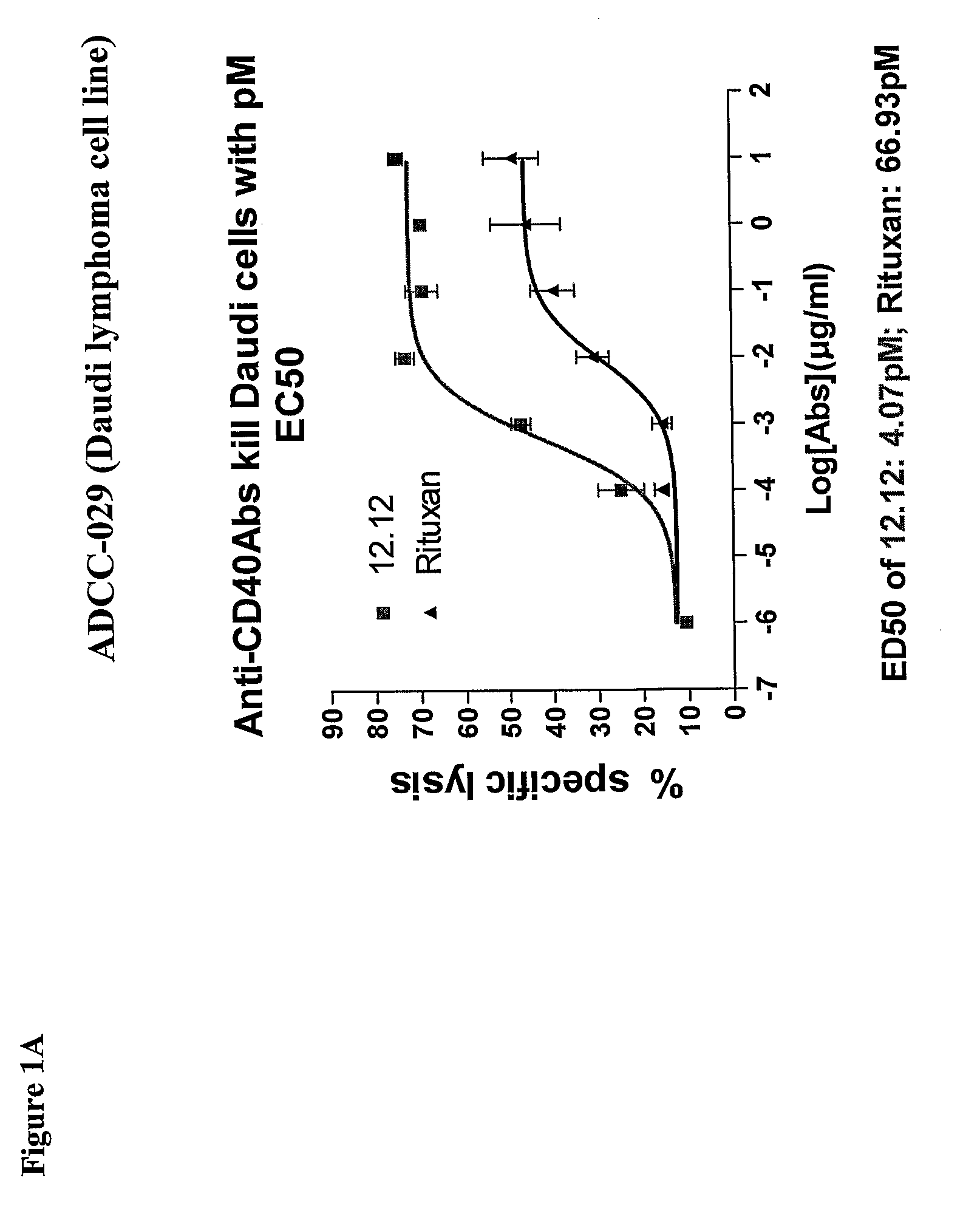
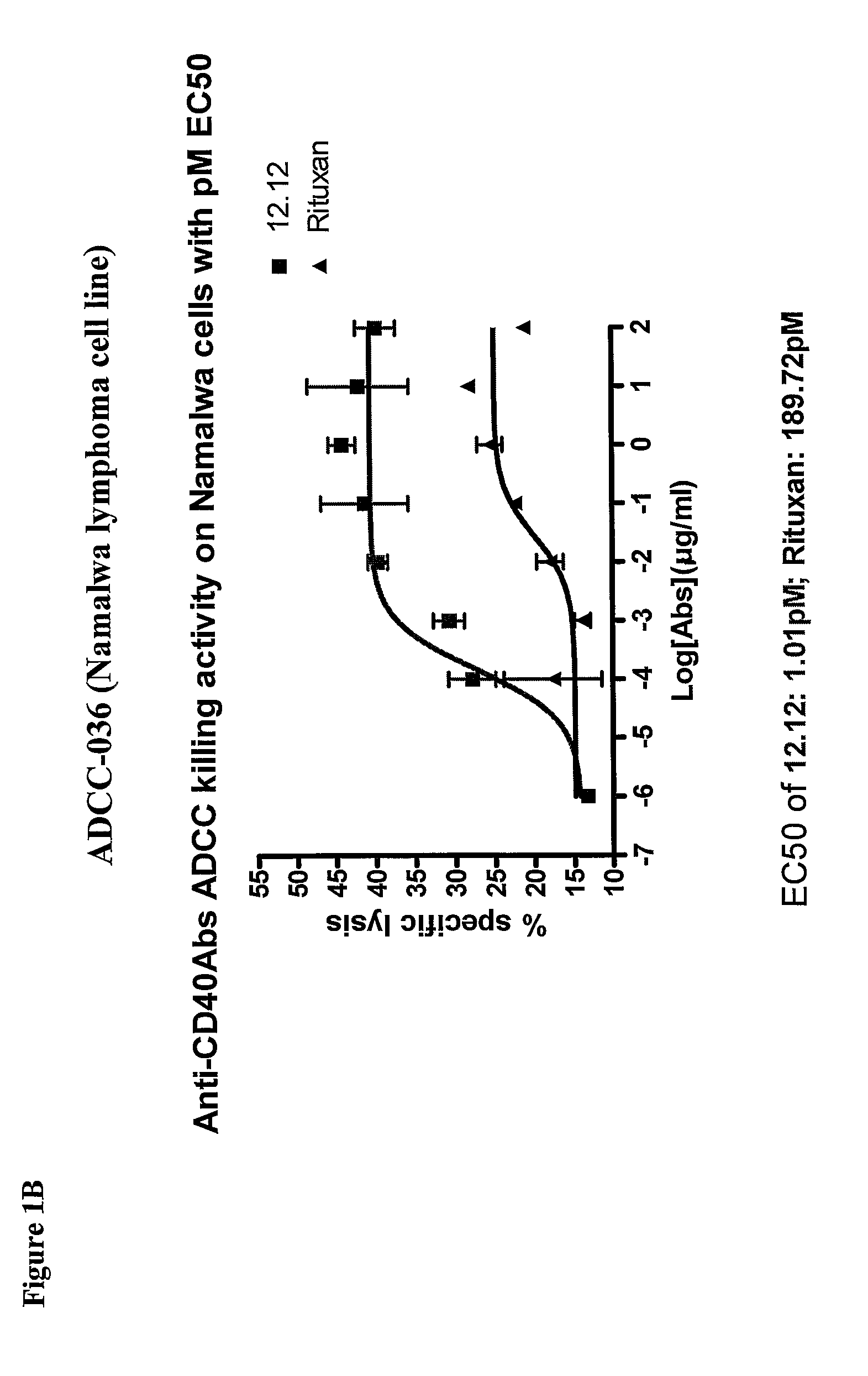
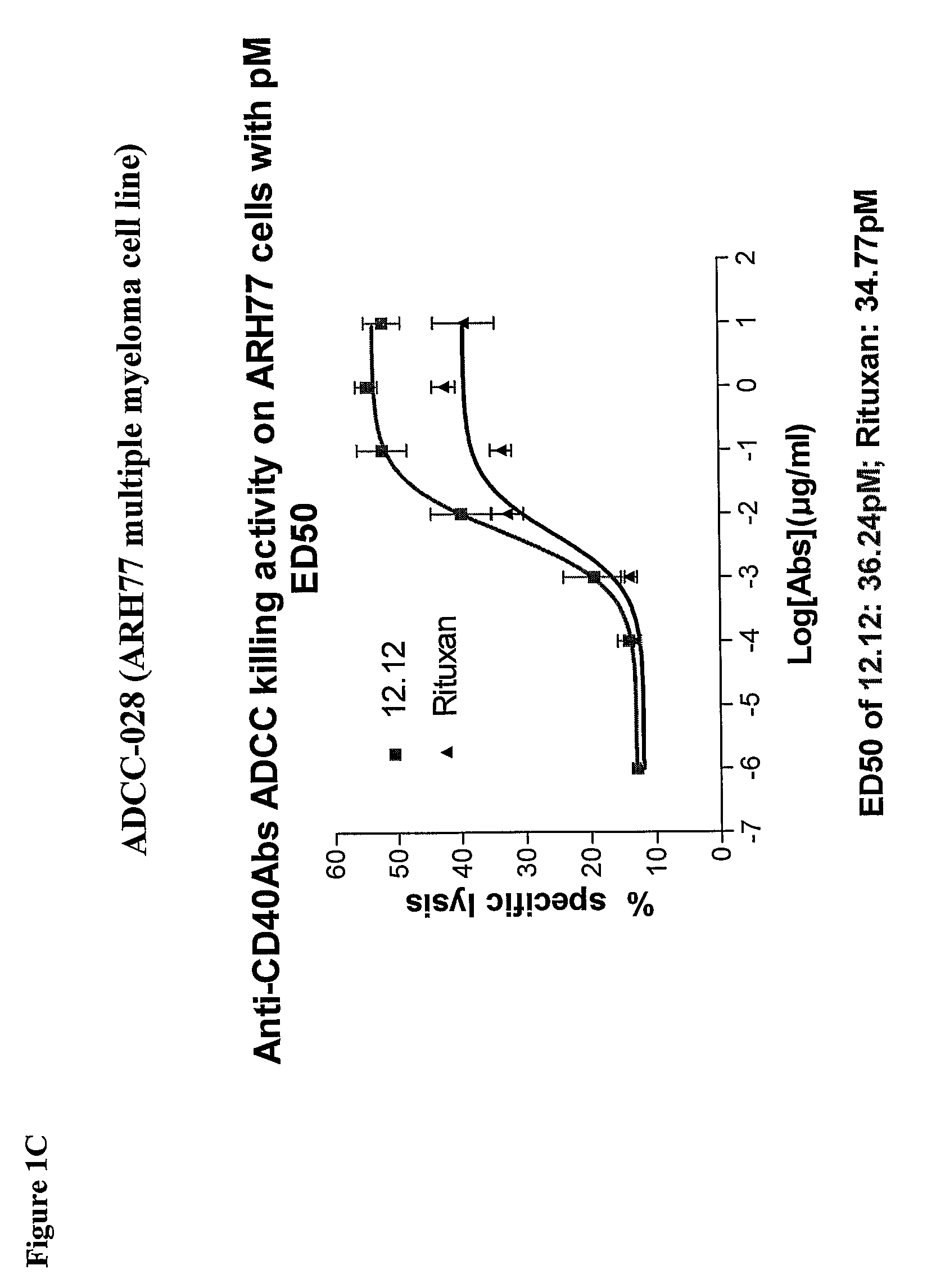
Methods of monitoring the efficacy of anti-CD40 antibodies in treating a subject for a CD40-expressing cancer
InactiveUS8337851B2Microbiological testing/measurementBiological material analysisSignal onApoptosis
Methods for identifying subjects having a cancer or pre-malignant condition that will benefit from anti-CD40 therapeutic agents that modulate CD40L-mediated CD40 signaling are provided. The methods comprise the use of biomarkers of cellular apoptosis, cell proliferation and survival, and CD40 signaling pathways to monitor ex vivo response to one or more anti-CD40 therapeutic agents of interest that modulate CD40 signaling on CD40-expressing neoplastic cells. The ex vivo prognostic assays can be used alone or in conjunction with other prognostic assays to identify candidate subjects who will benefit from treatment with anti-CD40 therapeutic agents. Methods of the invention also comprise the use of these biomarkers to monitor in vivo efficacy of treatment with an anti-CD40 therapeutic agent.
Owner:XOMA TECH LTD
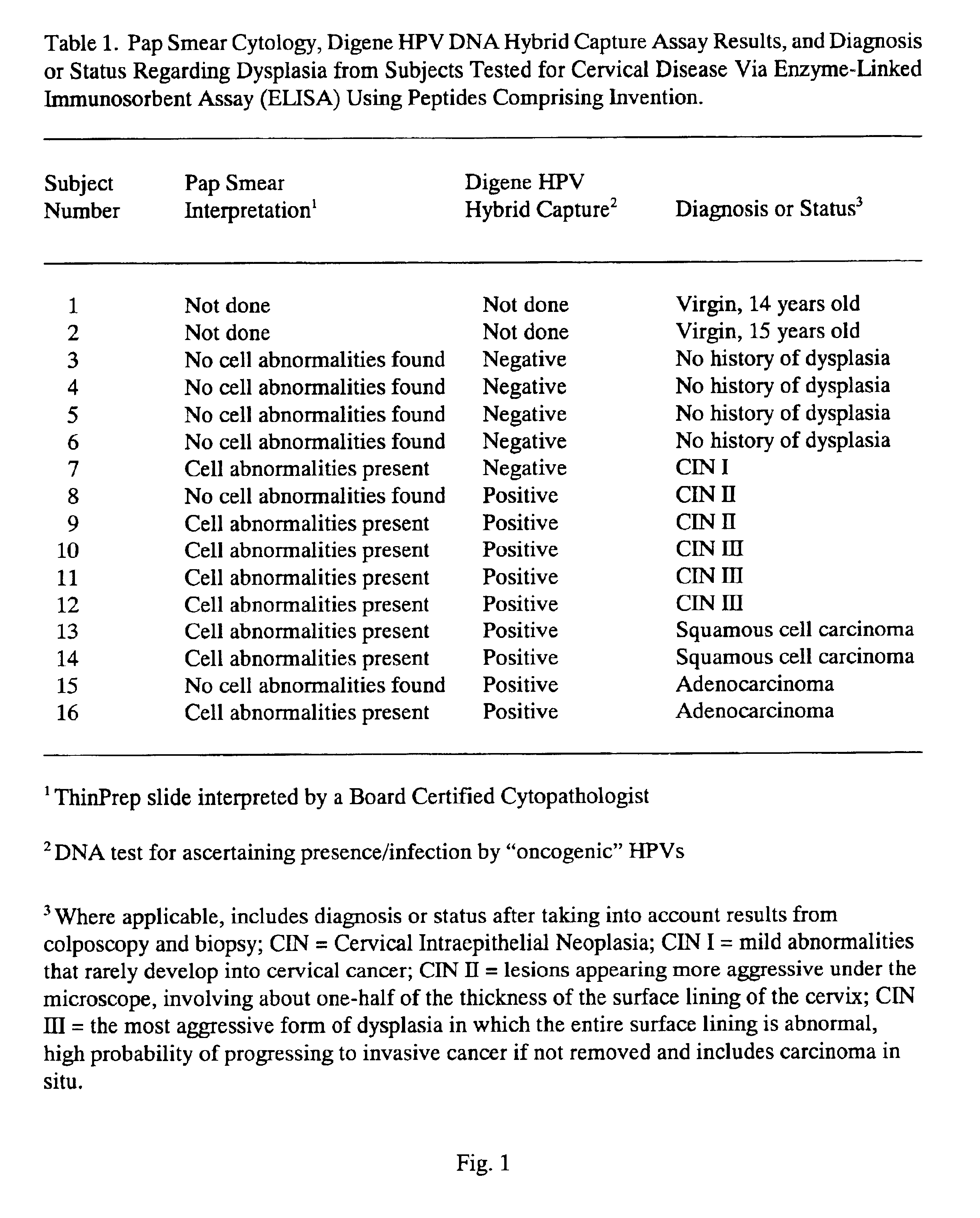
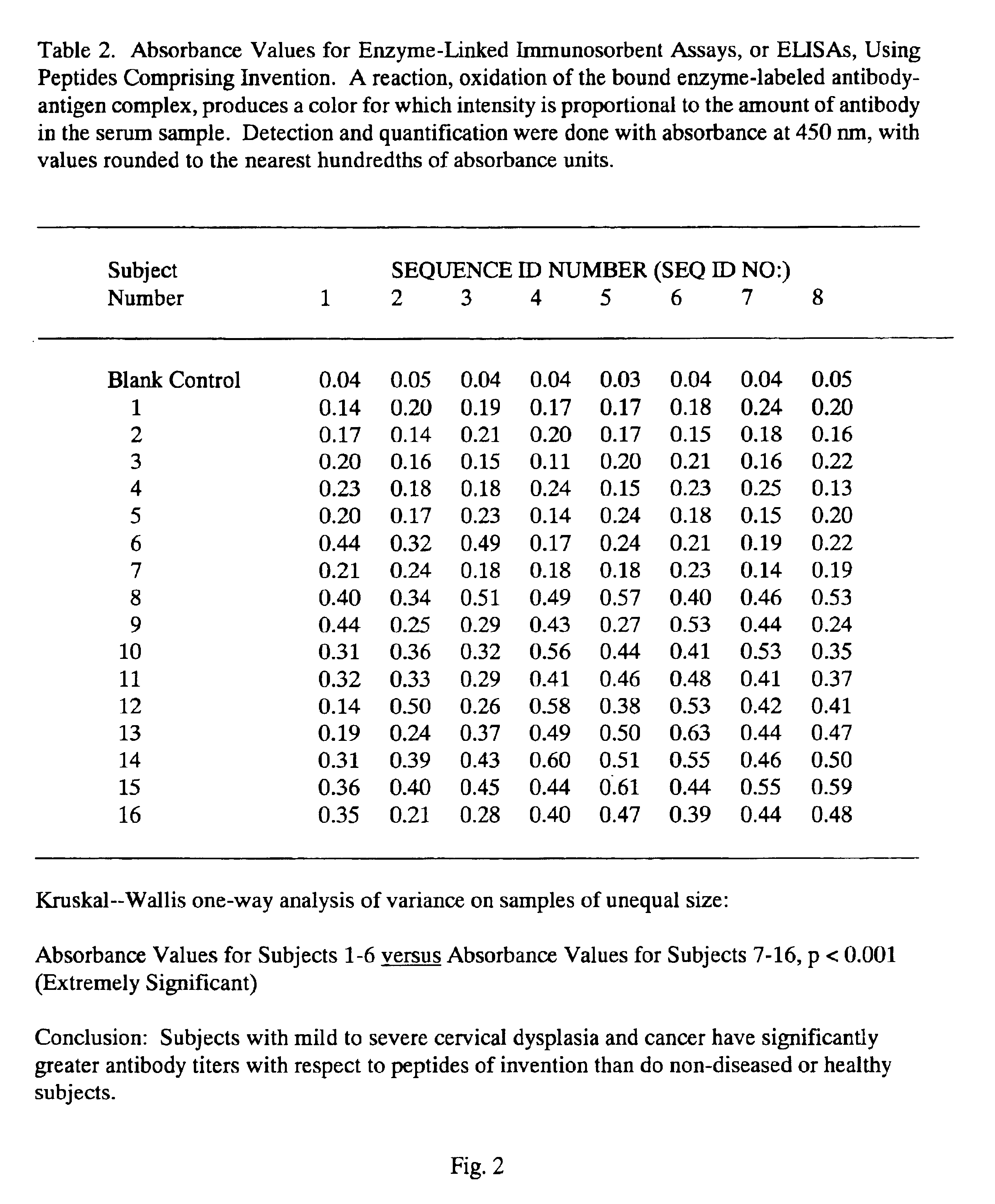
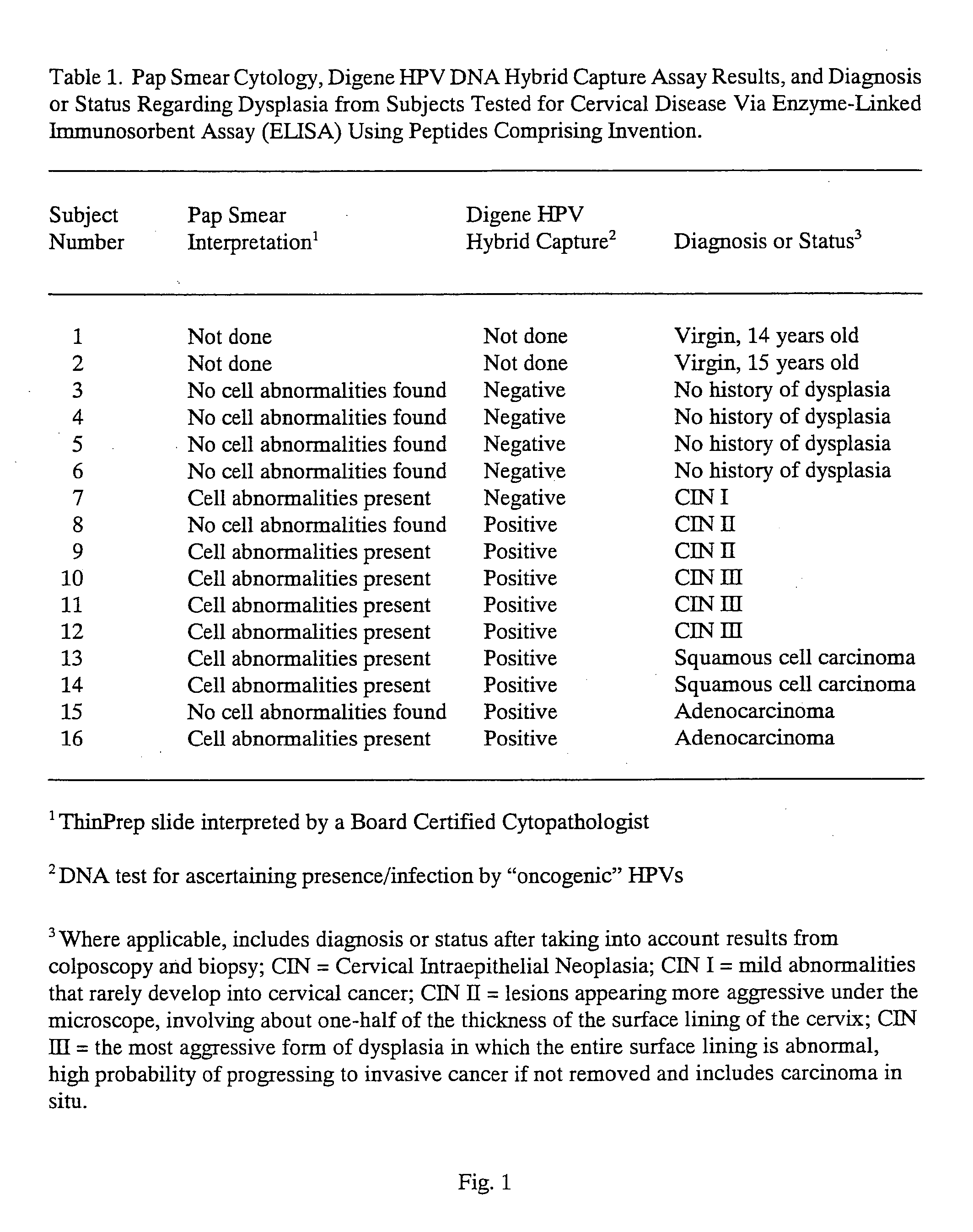
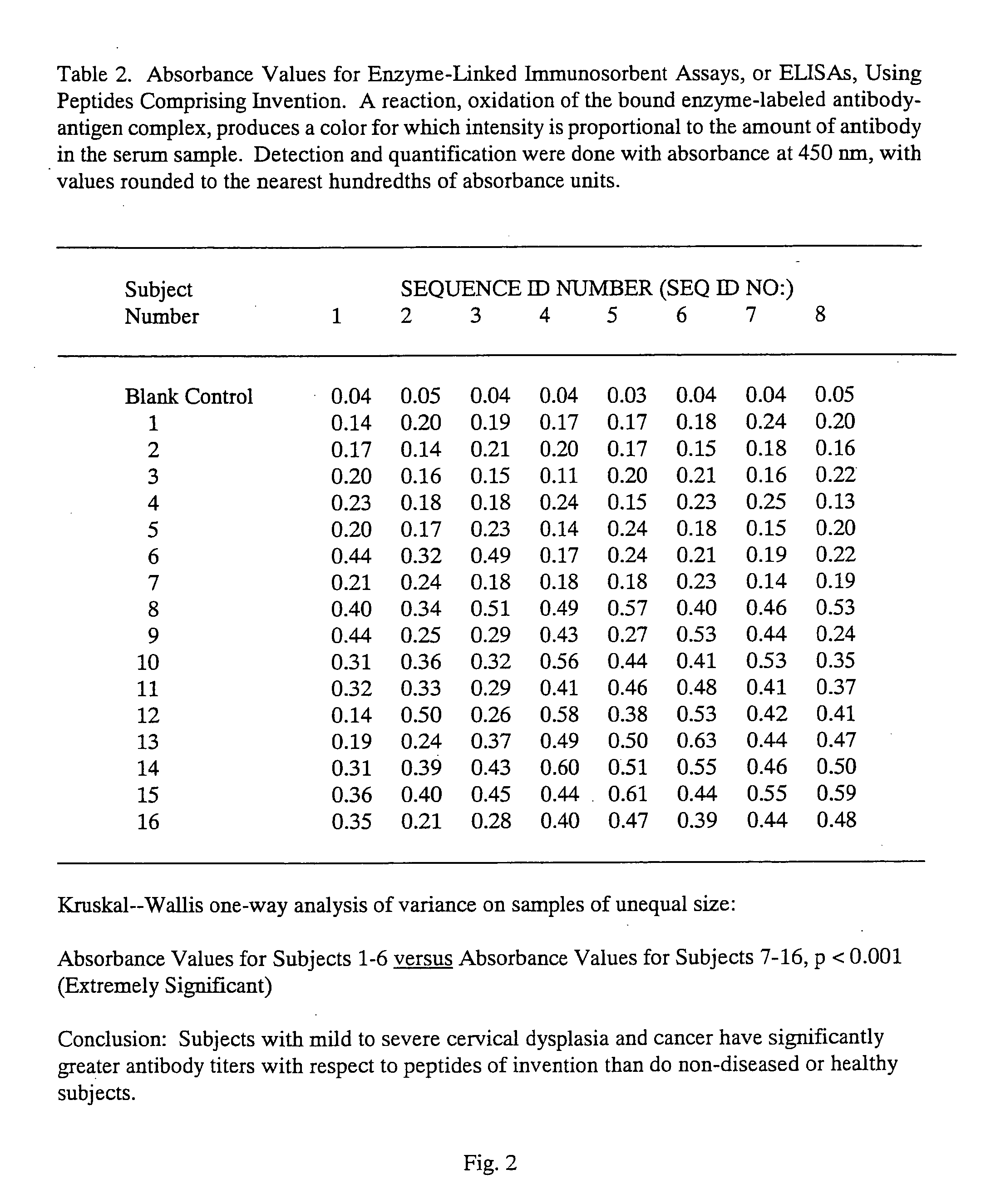
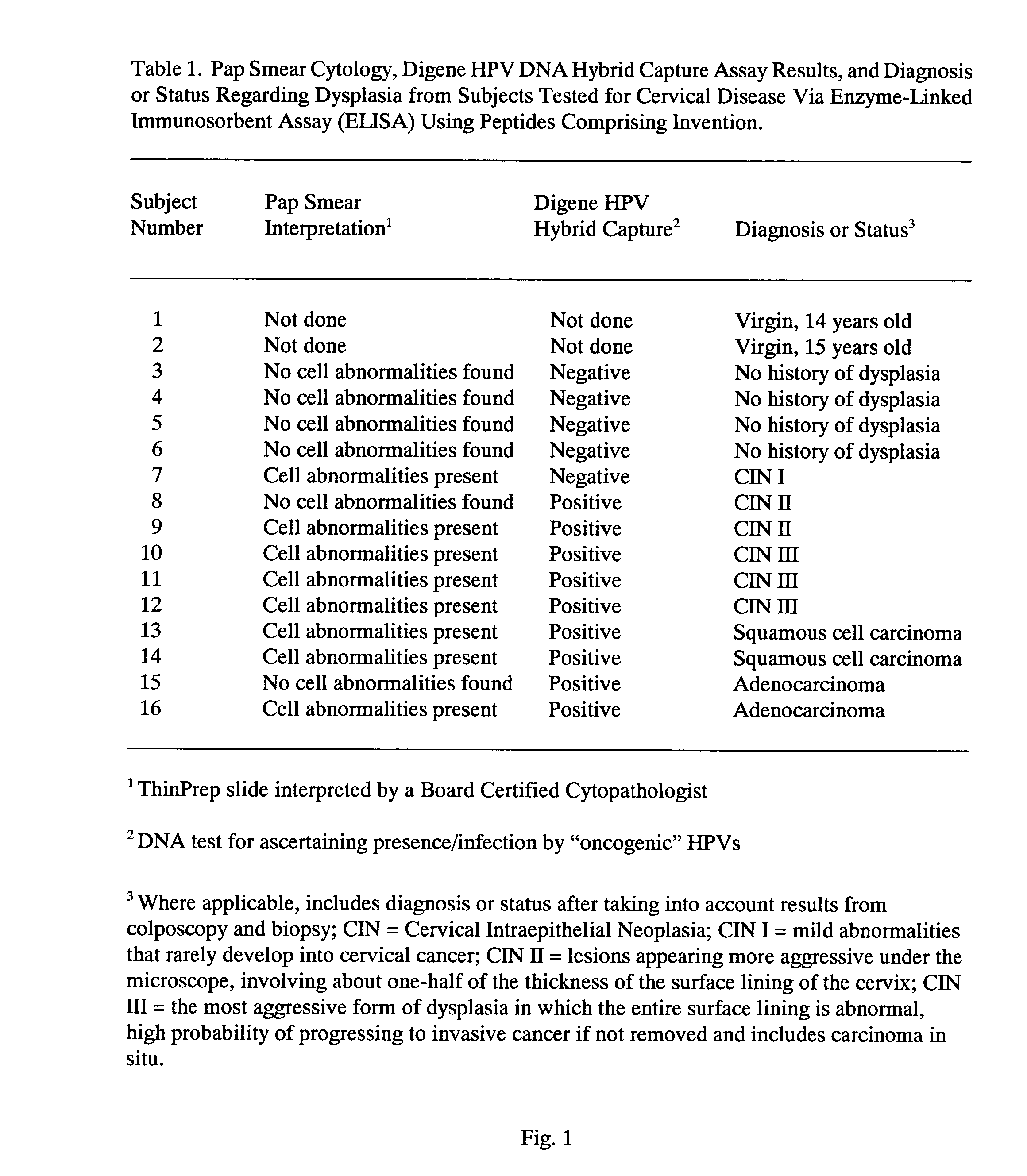
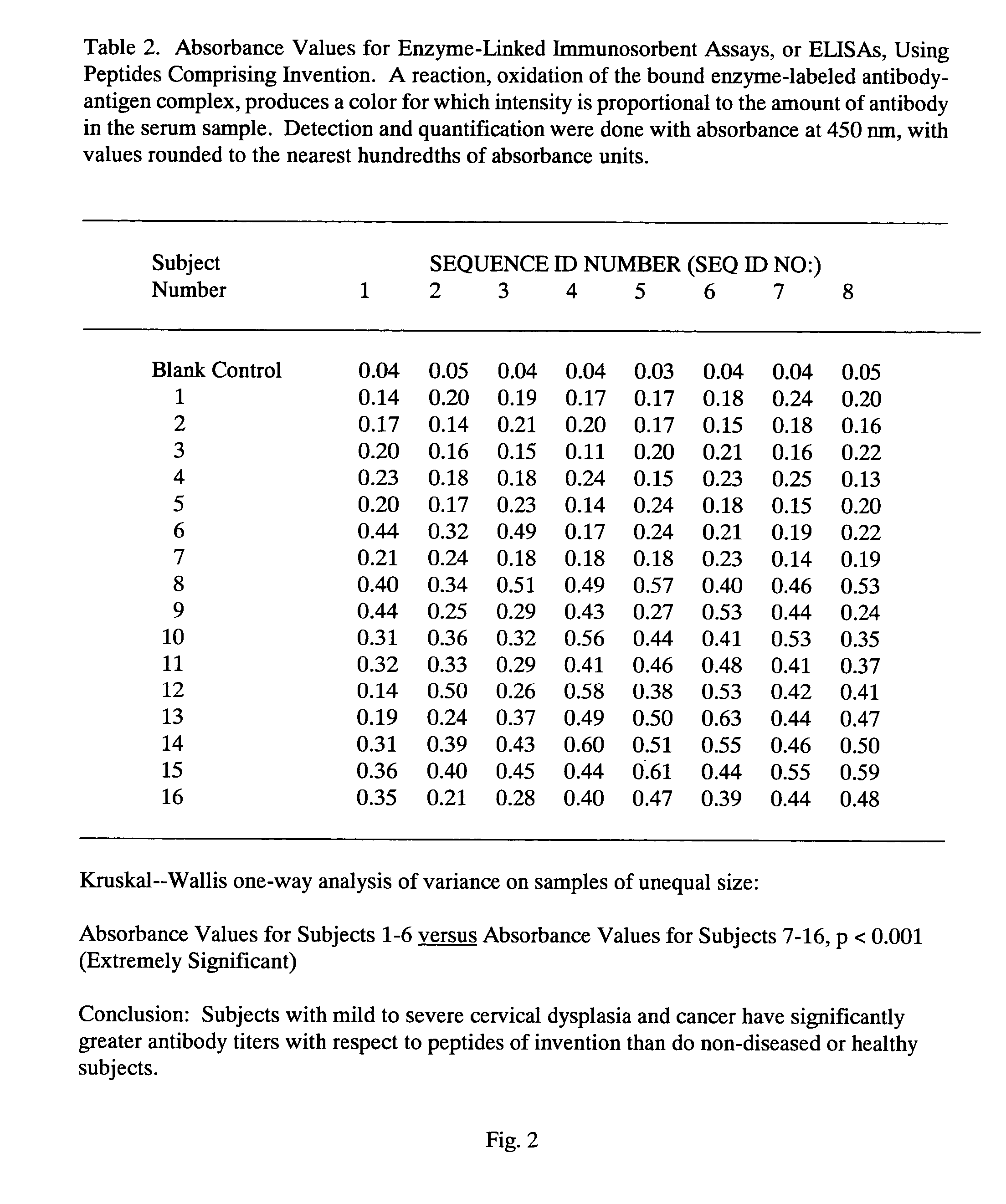
Peptides from the E2, E6, and E7 proteins of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papillomavirus associated cancers
InactiveUS6933123B2Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
Owner:HU YAO XIONG
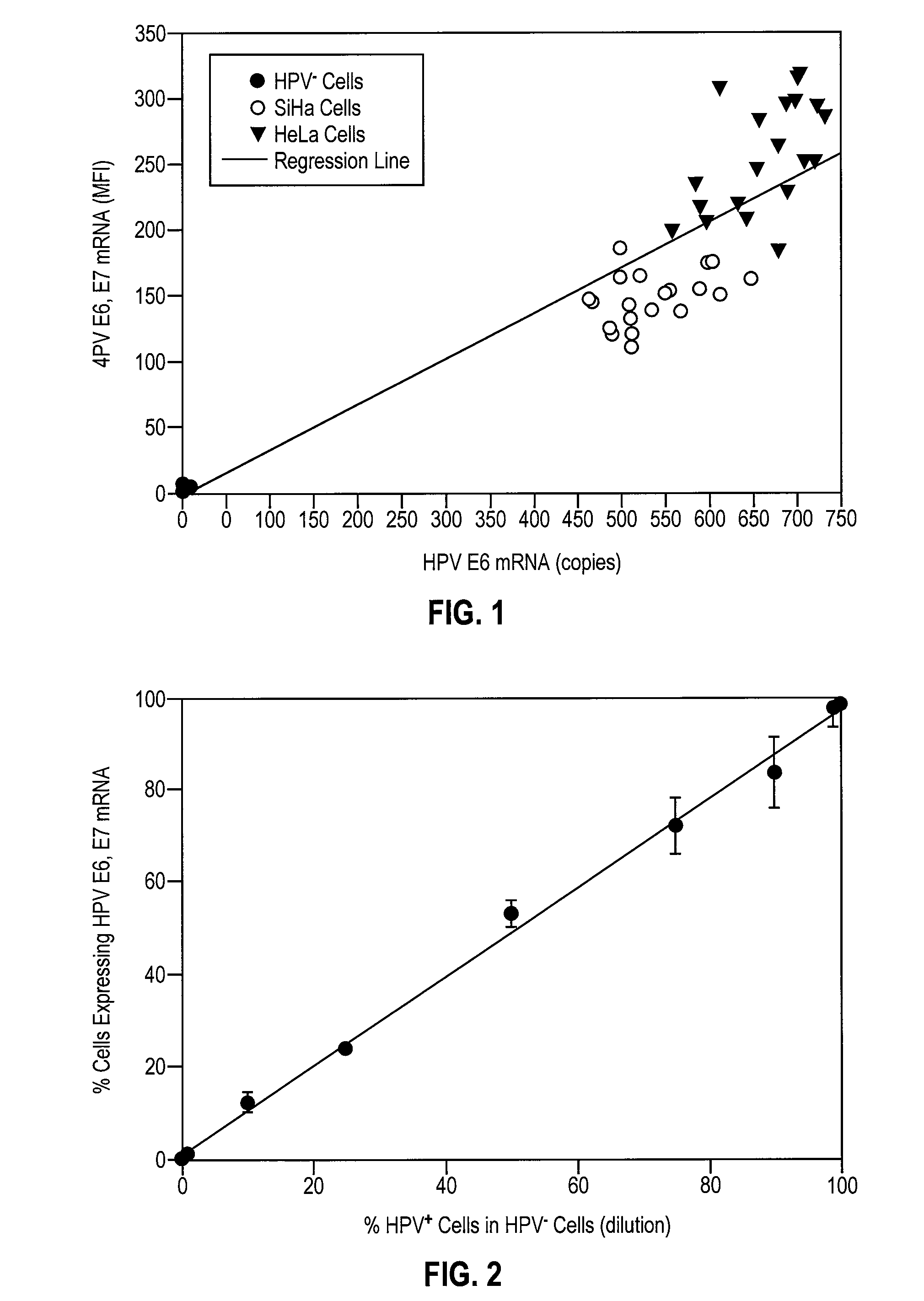
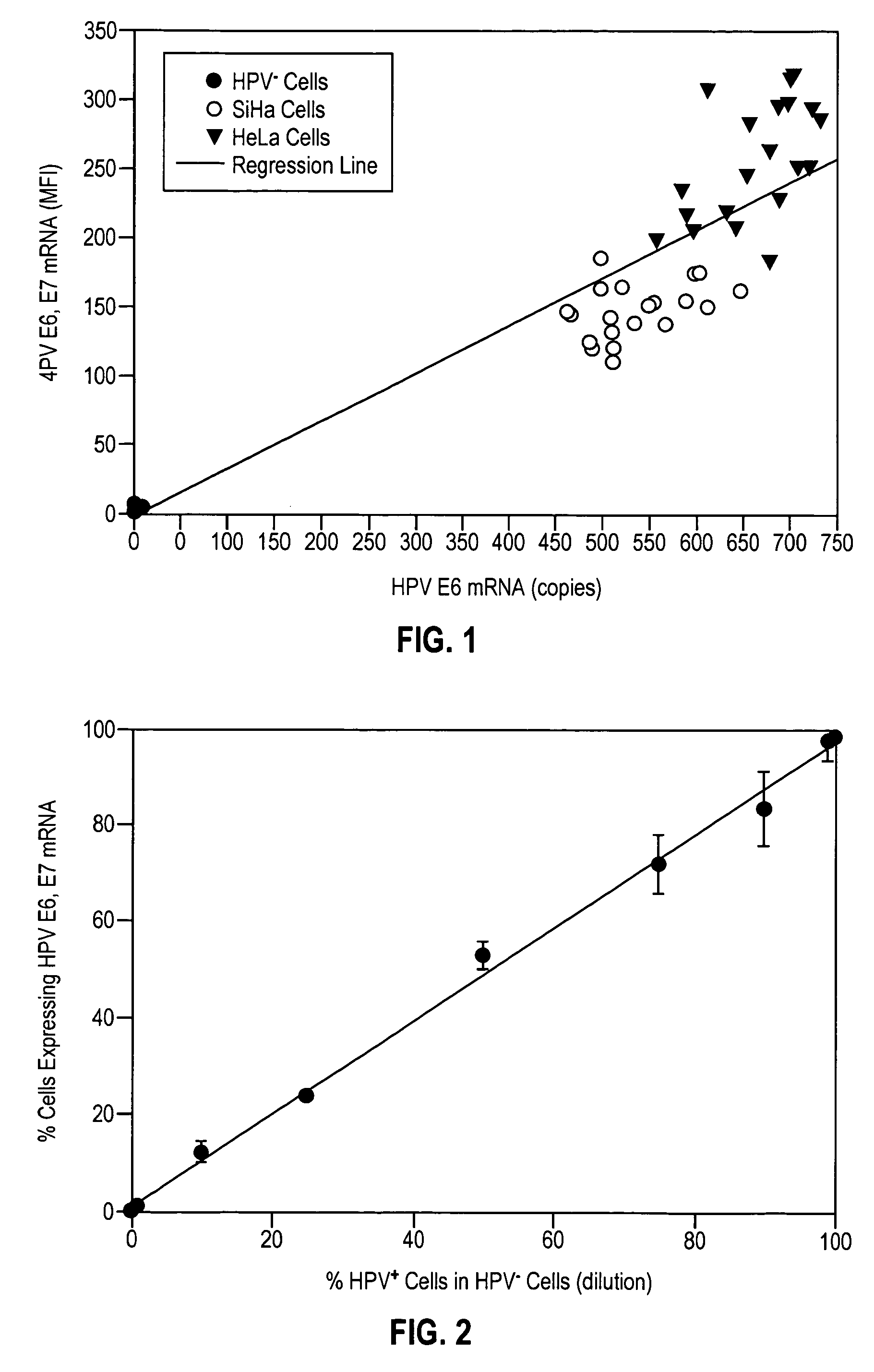
HPV E6, E7 mRNA assay and methods of use thereof
Provided is an HPV E6, E7 mRNA assay, referenced herein as the “In Cell HPV Assay,” that is capable of sensitive and specific detection of normal cervical cells undergoing malignant transformation as well as abnormal cervical cells with pre-malignant or malignant lesions. The In Cell HPV Assay identifies HPV E6, E7 mRNA via in situ hybridization with oligonucleotides specific for HPV E6, E7 mRNA and quantitates the HPV E6, E7 mRNA via flow cytometry. The In Cell HPV Assay can be carried out in less than three hours directly from liquid-based cervical (“LBC”) cytology specimens. The In Cell HPV Assay provides an efficient and highly sensitive alternative to the Pap smear for determining abnormal cervical cytology.
Owner:INCELLDX
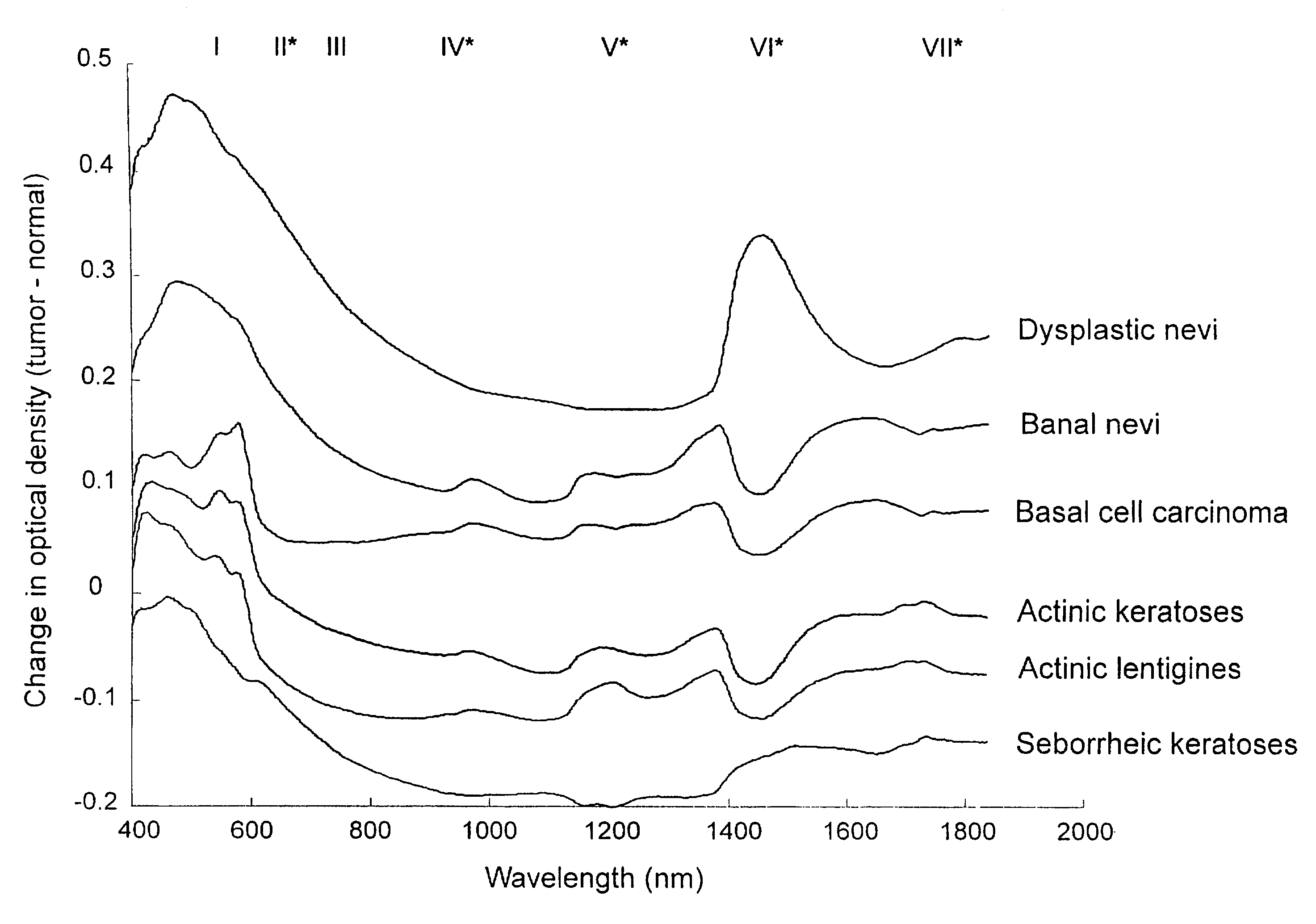
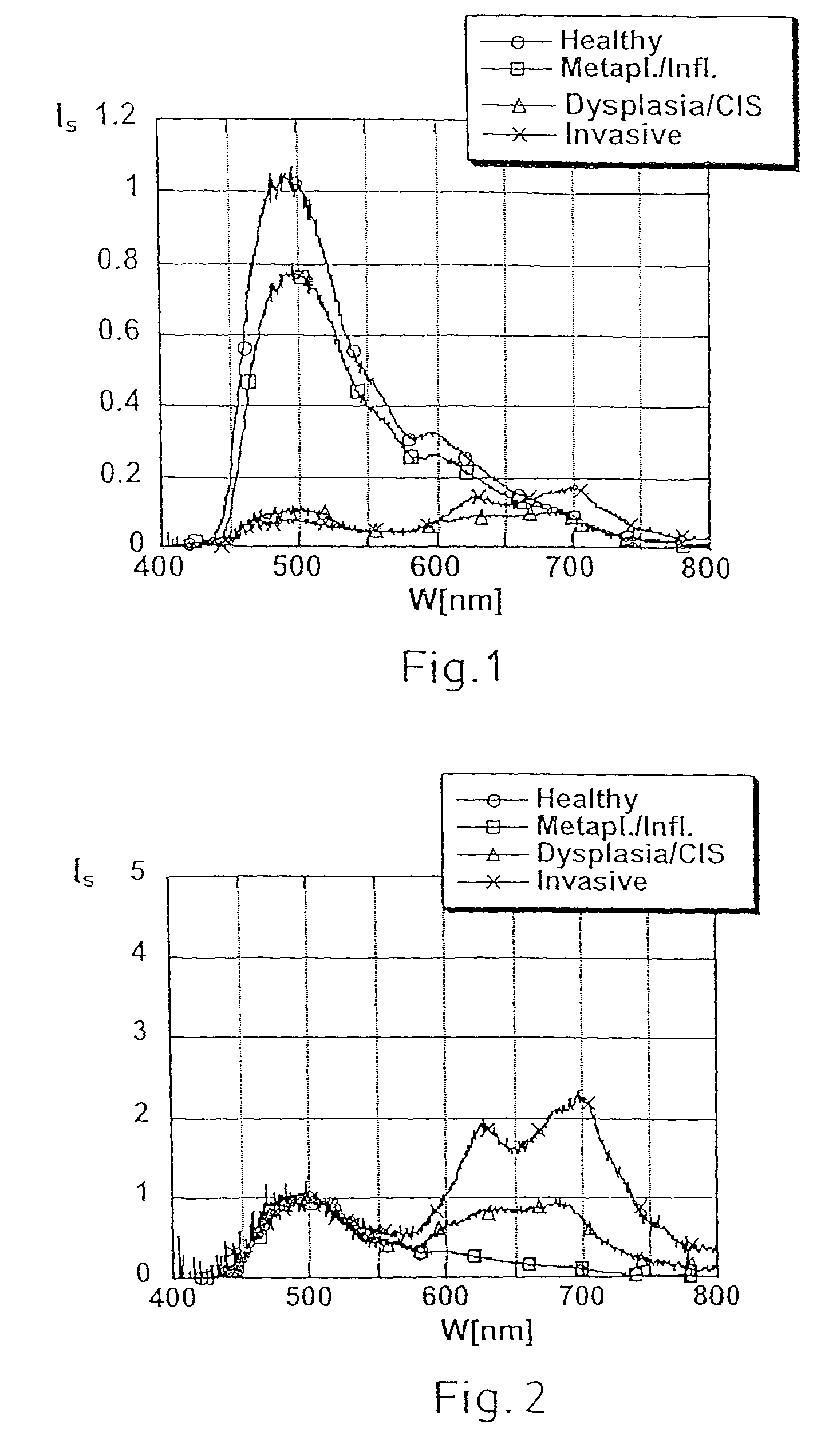
Non-invasive screening of skin diseases by visible/near-infrared spectroscopy
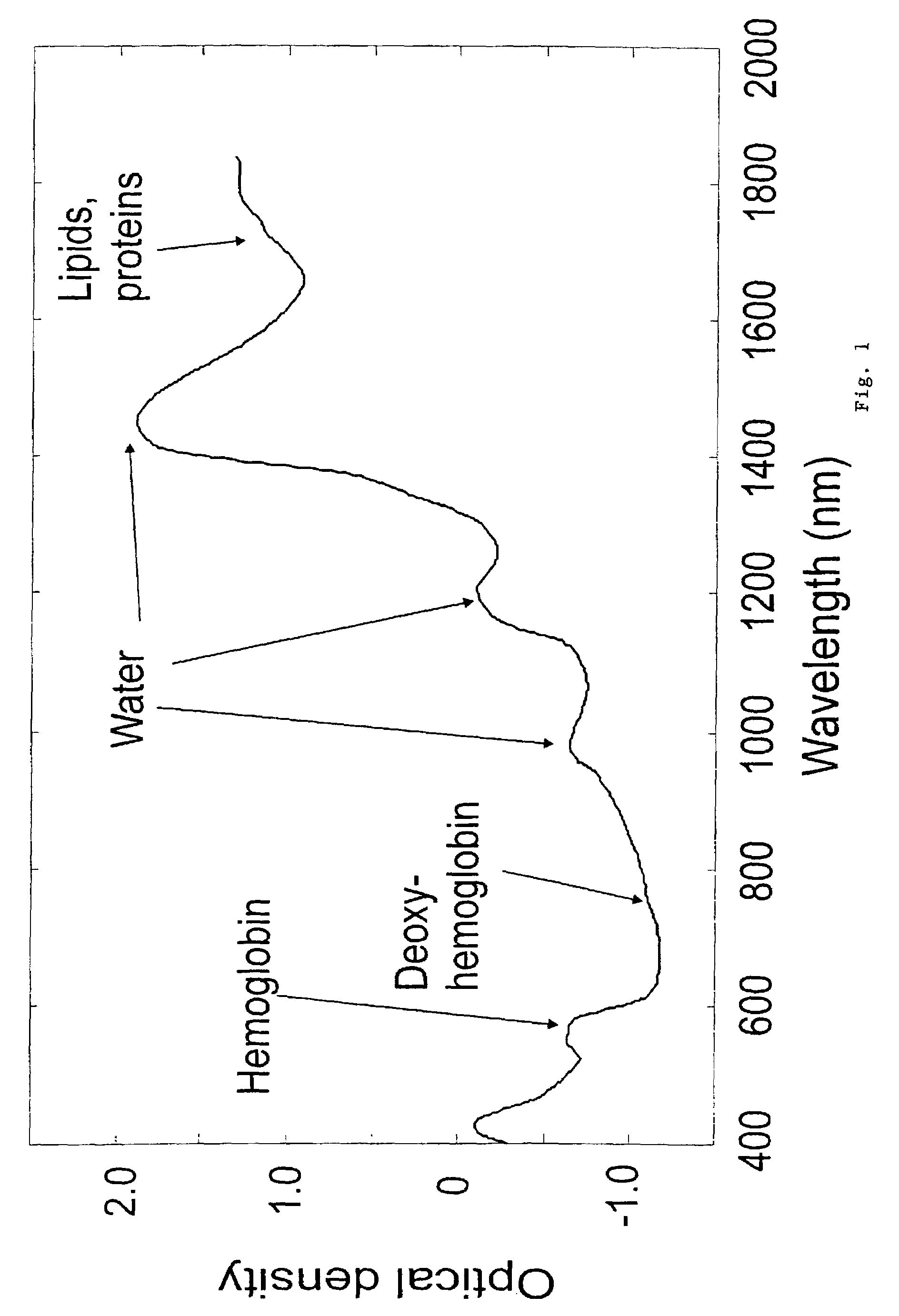
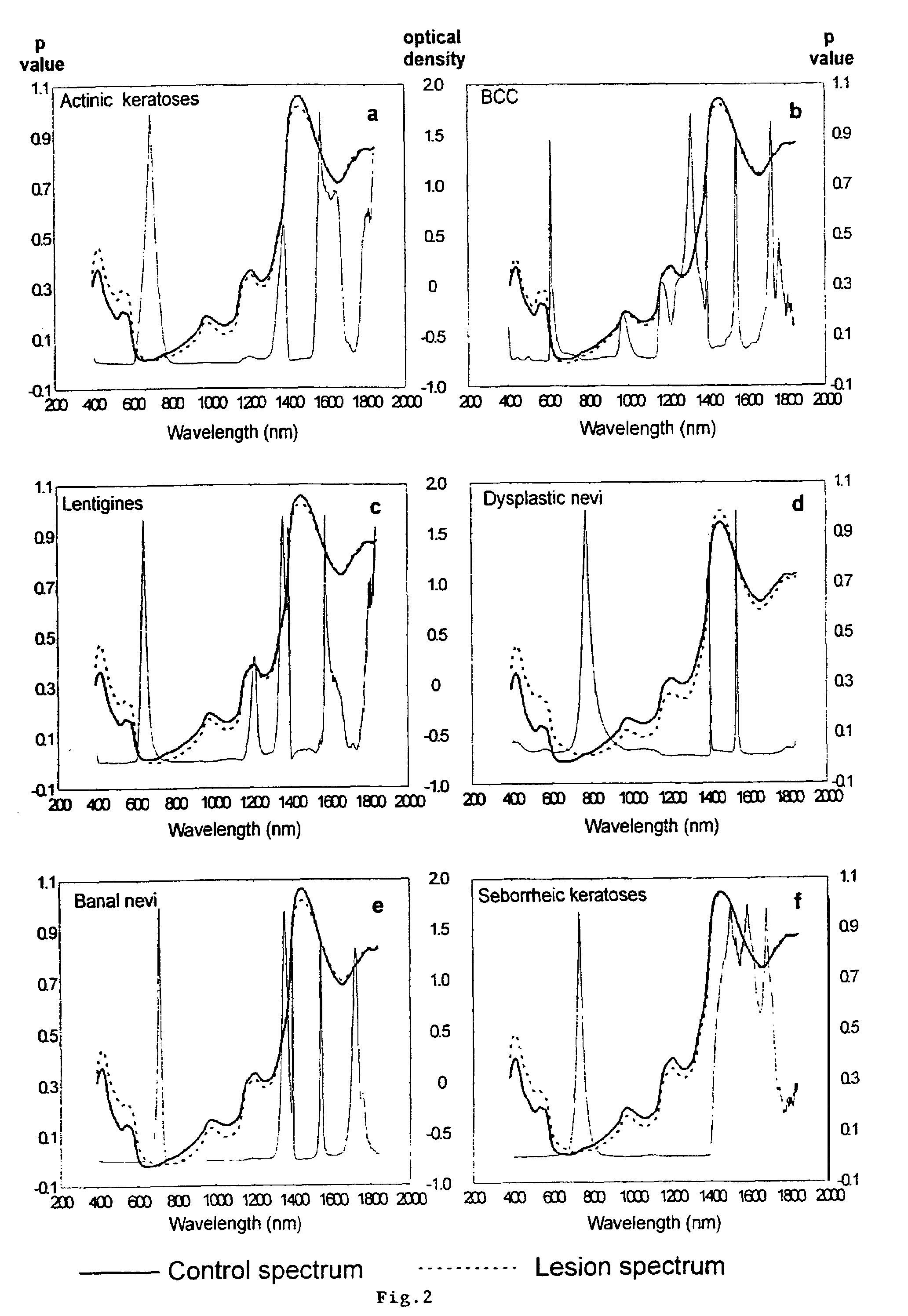
A non-invasive tool for skin disease diagnosis would be a useful clinical adjunct. The purpose of this study was to determine whether visible / near-infrared spectroscopy can be used to non-invasively characterize skin diseases. In-vivo visible- and near-infrared spectra (400-2500 nm) of skin neoplasms (actinic keratoses, basal cell carcinomata, banal common acquired melanocytic nevi, dysplastic melanocytic nevi, actinic lentigines and seborrheic keratoses) were collected by placing a fiber optic probe on the skin. Paired t-tests, repeated measures analysis of variance and linear discriminant analysis were used to determine whether significant spectral differences existed and whether spectra could be classified according to lesion type. Paired t-tests showed significant differences (p<0.05) between normal skin and skin lesions in several areas of the visible / near-infrared spectrum. In addition, significant differences were found between the lesion groups by analysis of variance. Linear discriminant analysis classified spectra from benign lesions compared to pre-malignant or malignant lesions with high accuracy. Visible / near-infrared spectroscopy is a promising non-invasive technique for the screening of skin diseases.
Owner:NAT RES COUNCIL OF CANADA
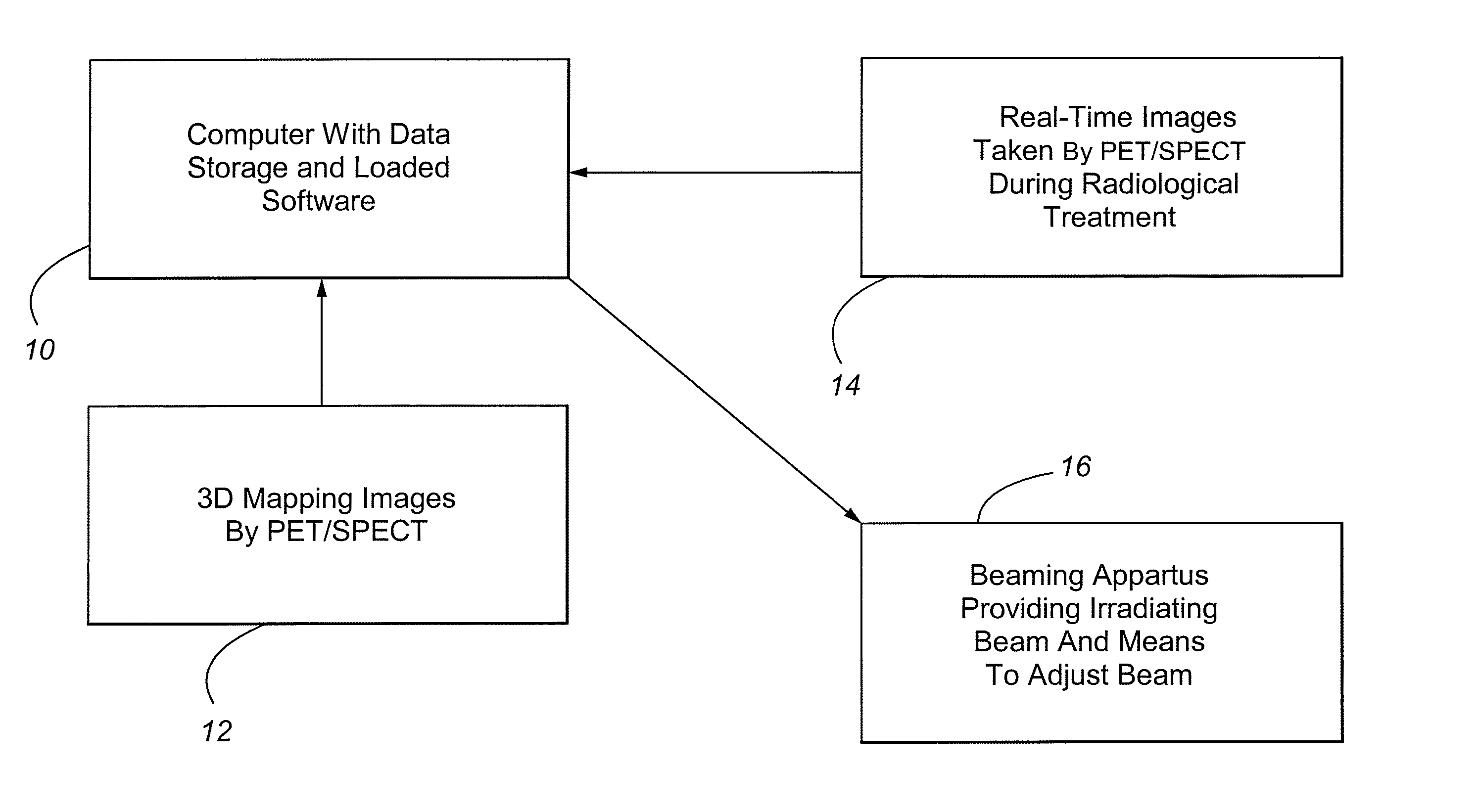
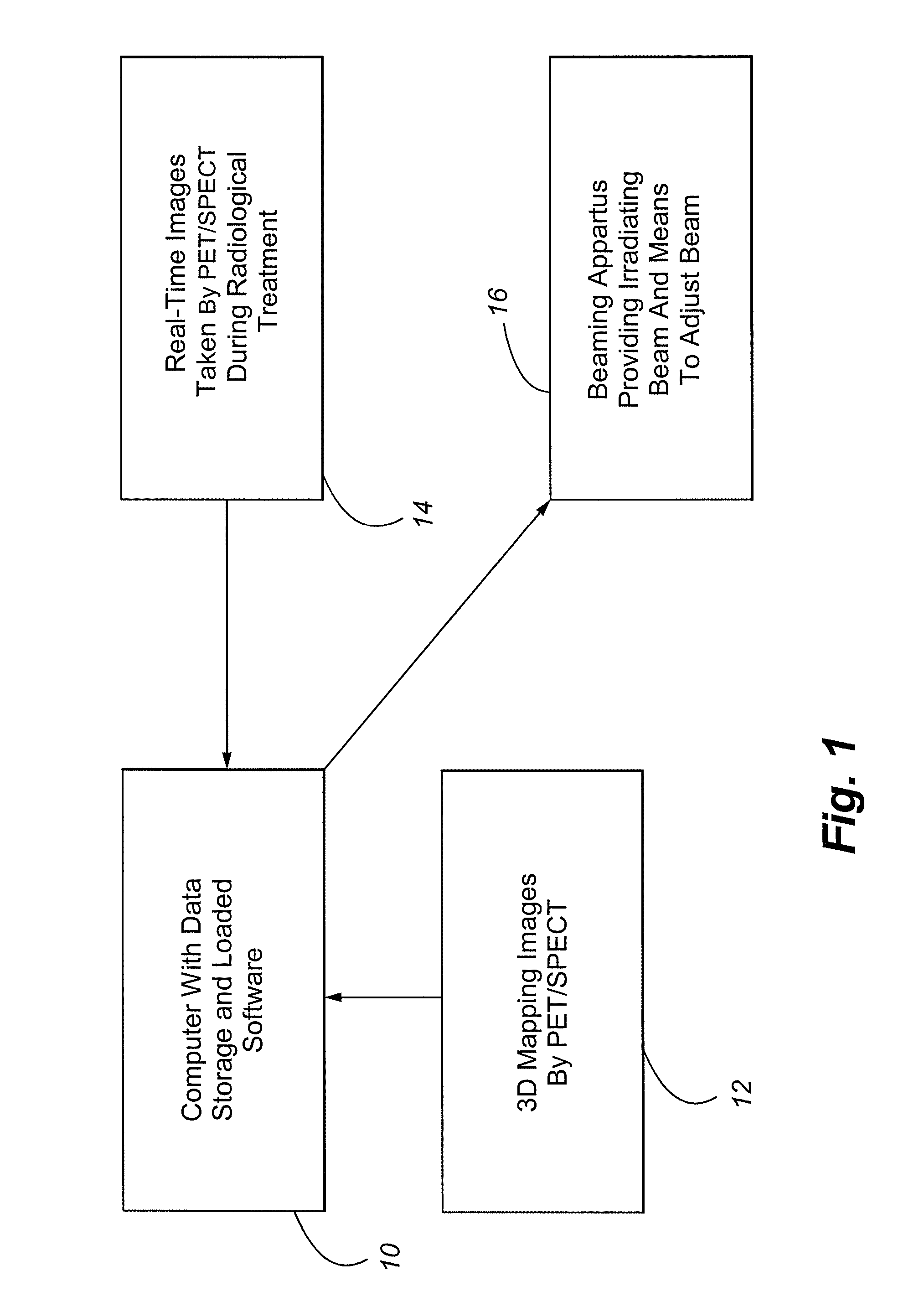
Method and Apparatus Including Use of Metalloporphyrins for Subsequent Optimization of Radiosurgery and Radiotherapy
InactiveUS20070043289A1Selective uptakeEfficient deliveryTelevision system detailsTelevision system scanning detailsRadiosurgeryDual delivery
Owner:ADAIR
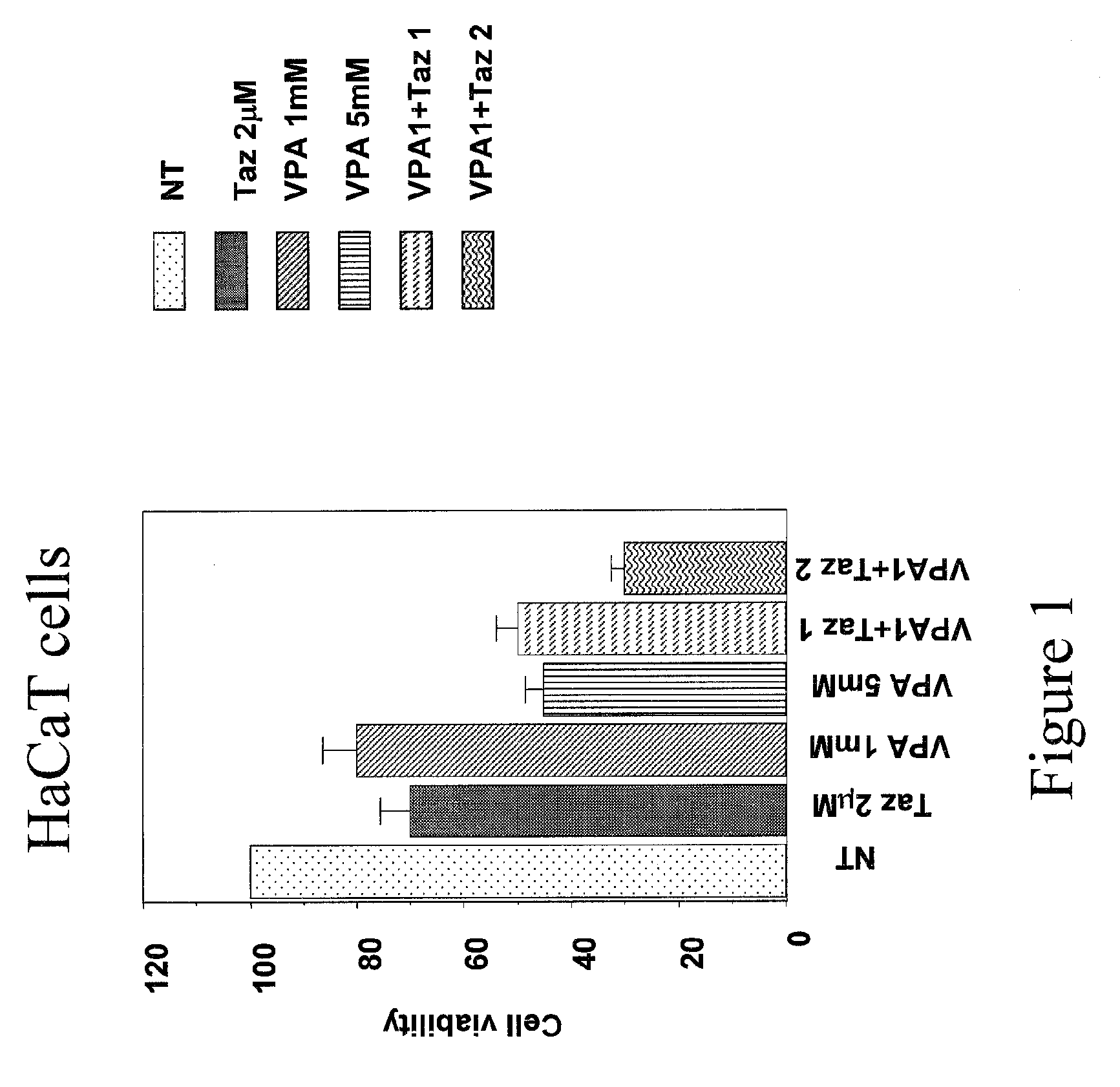
Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Owner:TOPOTARGET GERMANY AG
Novel genes, compositions, kits, and methods for identification, assessment, prevention, and therapy of prostate cancer
InactiveUS20050191673A1Reduced expression levelImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureOncologyNovel gene
The invention relates to newly discovered nucleic acid molecules and proteins associated with prostate cancer including pre-malignant conditions. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human prostate cancers are provided.
Owner:MILLENNIUM PHARMA INC
Genes, compositions, kits, and methods for identification, assessment, prevention, and therapy of prostate cancer
InactiveUS20060068425A1Reduced expression levelHigh expressionCompound screeningTumor rejection antigen precursorsOncologyHuman prostate
The invention relates to newly discovered nucleic acid molecules and proteins associated with prostate cancer including pre-malignant conditions. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human prostate cancers are provided.
Owner:MILLENNIUM PHARMA INC
HPV E6, E7 mRNA assay and methods of use thereof
Provided is an HPV E6, E7 mRNA assay, referenced herein as the “In Cell HPV Assay,” that is capable of sensitive and specific detection of normal cervical cells undergoing malignant transformation as well as abnormal cervical cells with pre-malignant or malignant lesions. The In Cell HPV Assay identifies HPV E6, E7 mRNA via in situ hybridization with oligonucleotides specific for HPV E6, E7 mRNA and quantitates the HPV E6, E7 mRNA via flow cytometry. The In Cell HPV Assay can be carried out in less than three hours directly from liquid-based cervical (“LBC”) cytology specimens. The In Cell HPV Assay provides an efficient and highly sensitive alternative to the Pap smear for determining abnormal cervical cytology.
Owner:INCELLDX
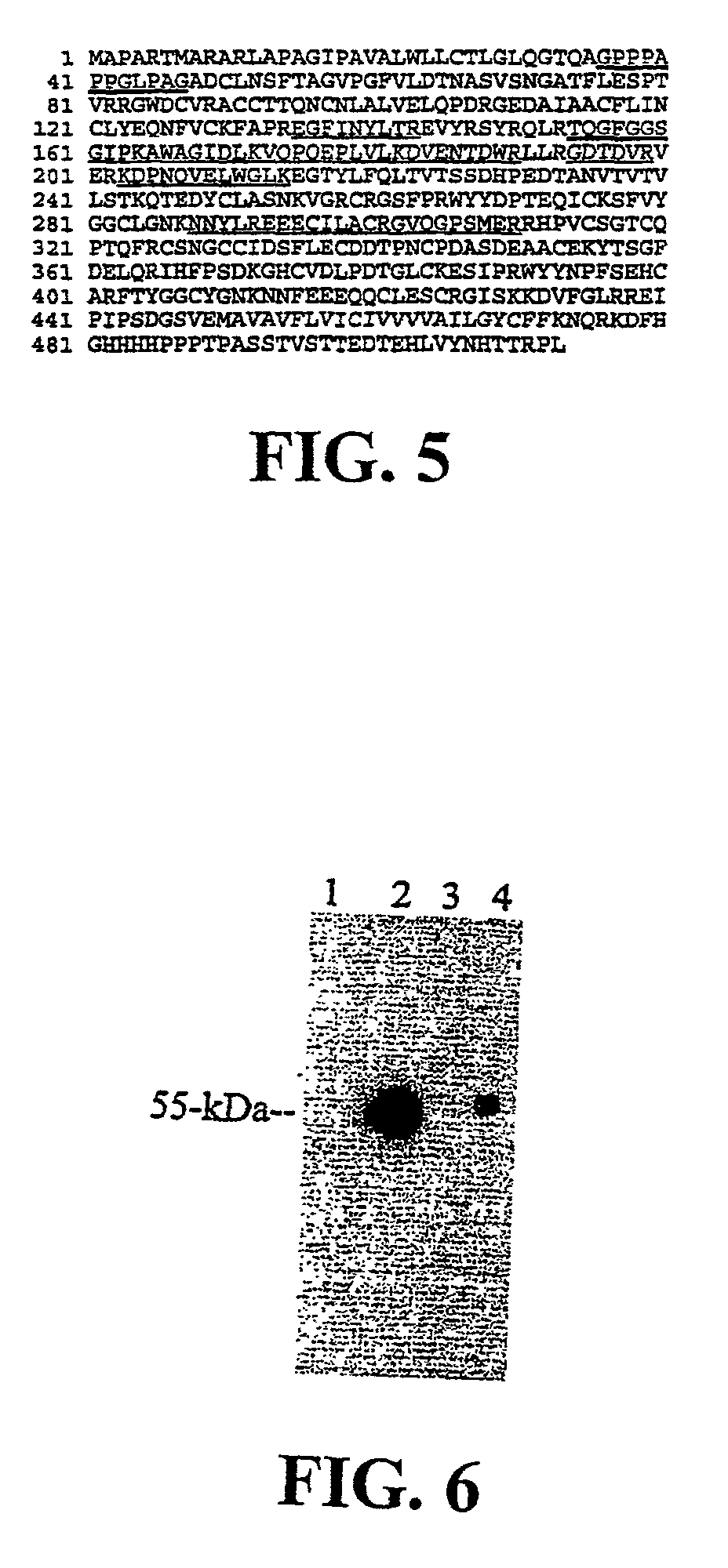
Matriptase, a serine protease and its applications
The invention is directed to a method of detecting a malignancy or a pre-malignant lesion in breast or other tissue, or a pathologic condition, by detecting the presence of single-chain or two-chain forms of matriptase in the tissue. The invention is further directed to a method of treating malignancies, which have the phenotype of matriptase production by administering a tumor formation inhibiting effective amount of concentrate of Bowman-Birk inhibitor (BBIC), or other matriptase inhibitor. The invention also is directed to nucleic acids encoding a matriptase protein or fragments thereof, and their use for structure elucidation and modeling to identify other inhibitors of matriptase, as well as to methods of identifying matriptase modulating agents, including activators and inhibitors.
Owner:GEORGETOWN UNIV
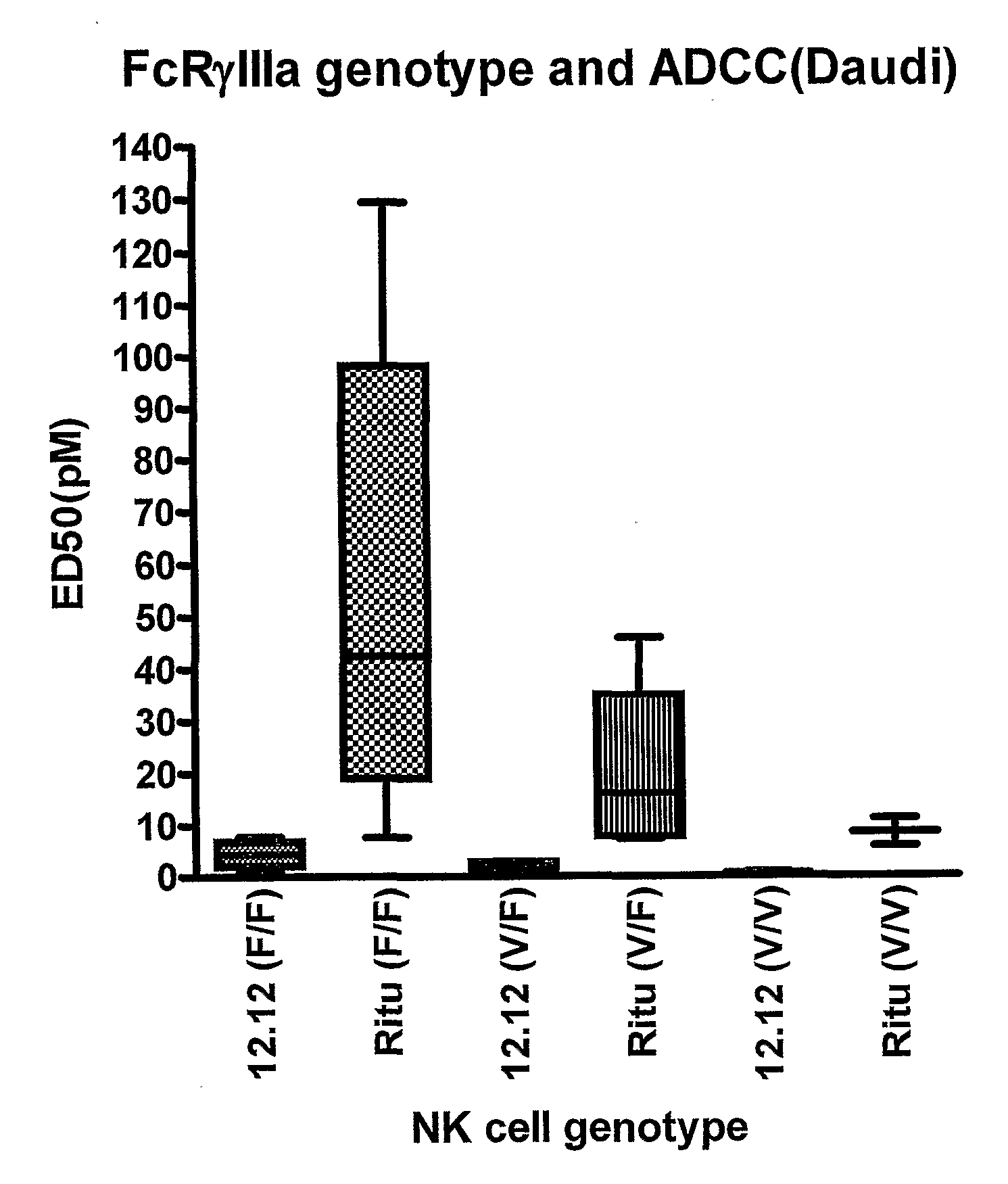
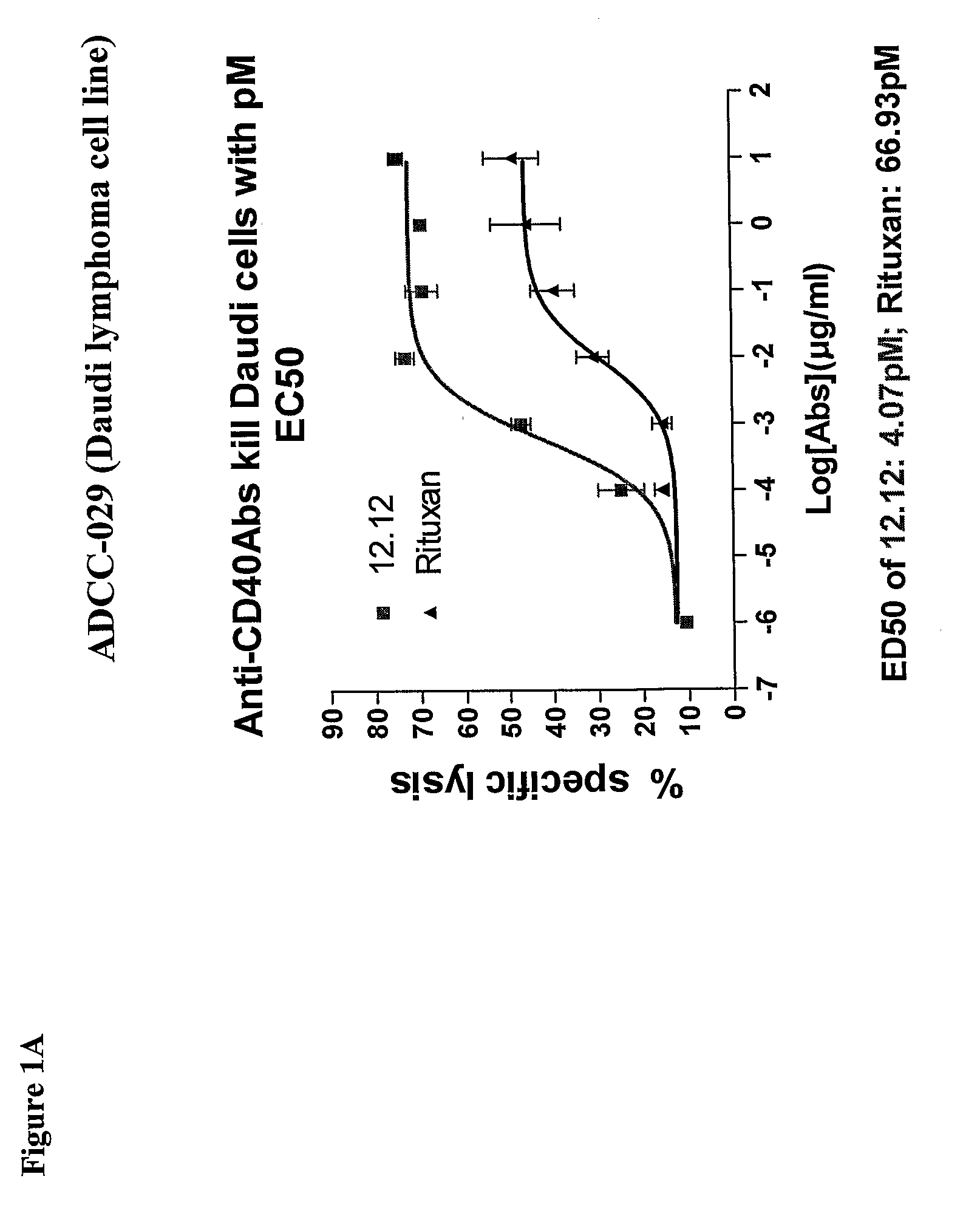
Uses of Anti-cd40 antibodies
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
Genes, compositions, kits and methods for identification, assessment, prevention, and therapy of cervical cancer
InactiveUS7125663B2Reduced expression levelImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureOncologyGene
The invention relates to newly discovered nucleic acid molecules and proteins associated with cervical cancer including pre-malignant conditions such as dysplasia. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human cervical cancers are provided.
Owner:MILLENNIUM PHARMA INC
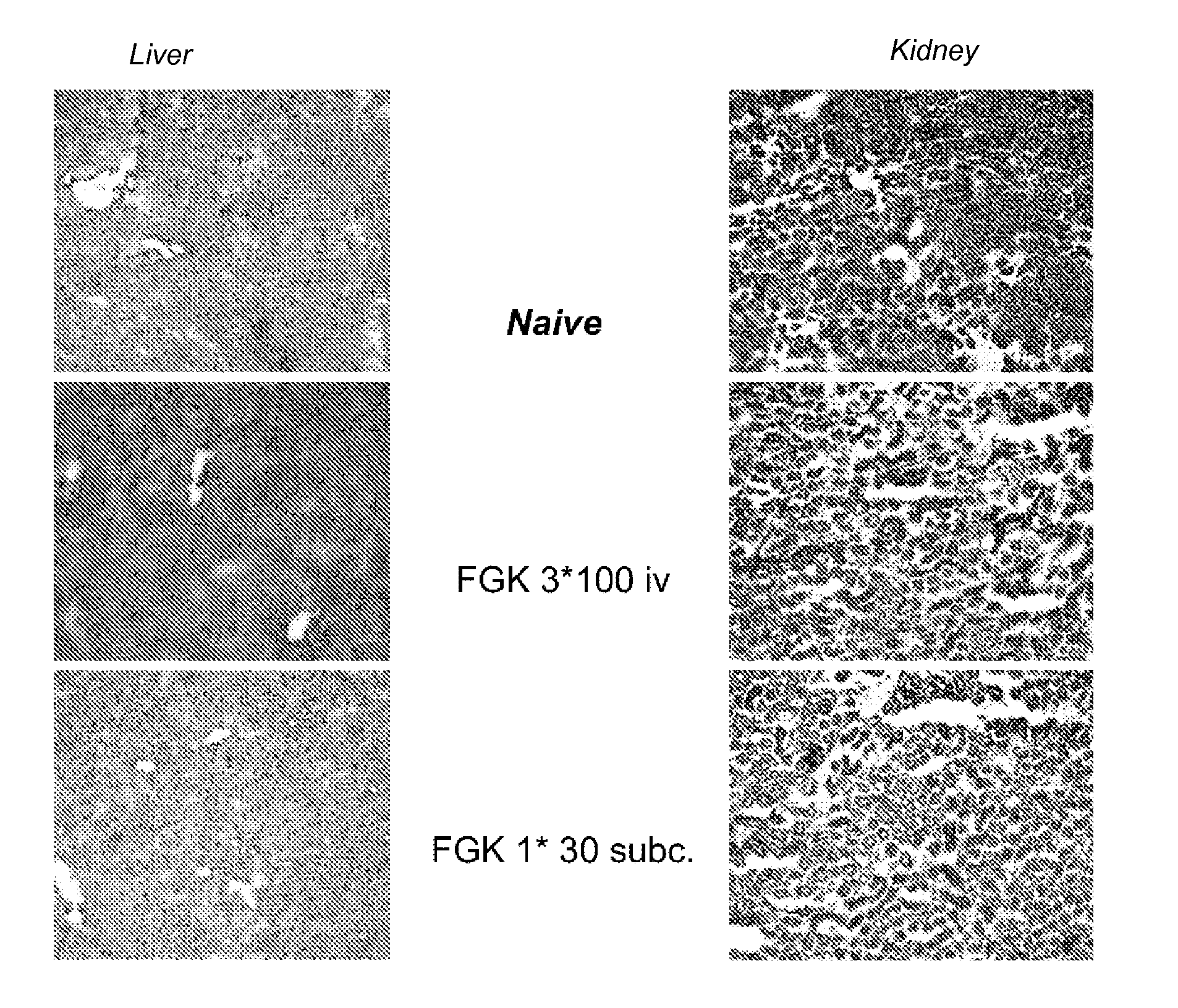
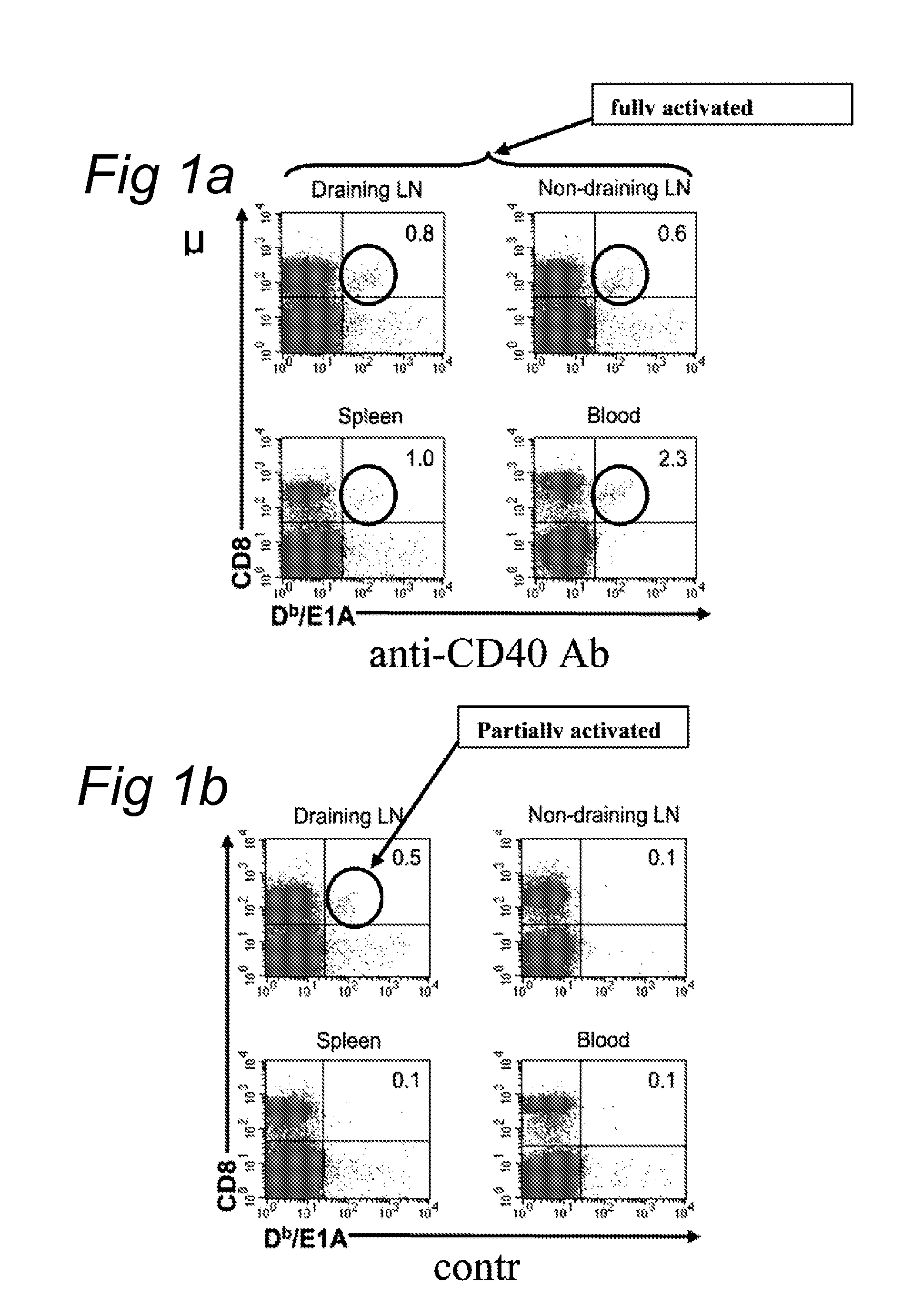
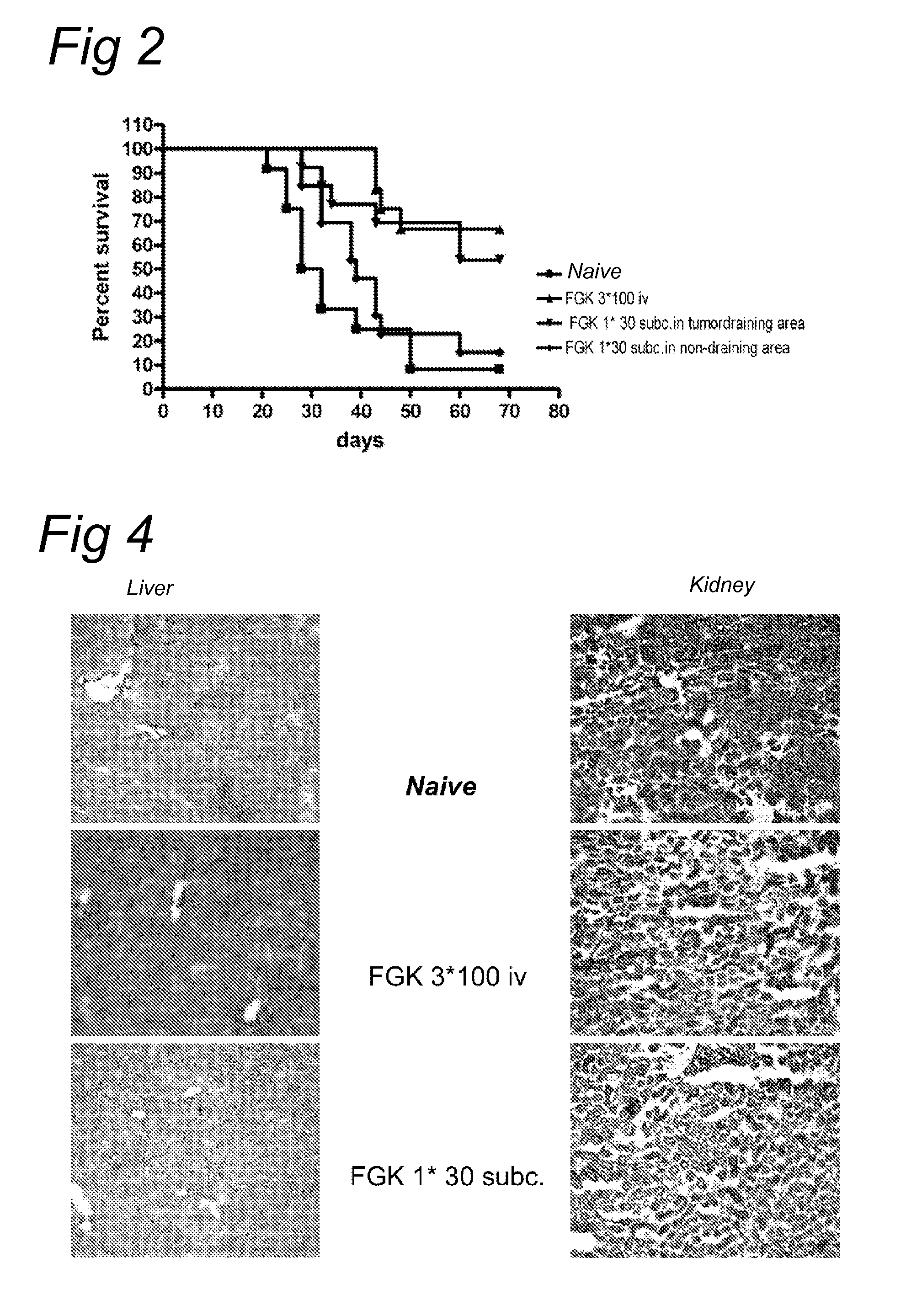
Delivery of a cd40 agonist to a tumor draining lymph node of a subject
InactiveUS20110311525A1Reduce doseLow immunogenicityOrganic active ingredientsPeptide/protein ingredientsAgonistSlow Release Formulation
The invention relates to the use of a CD40 agonist for treating cancer, a pre-malignant disorder or an infectious disease, wherein a CD40 agonist is locally administered and targeted to a tumor draining lymph node of a subject. Optionally, a CD40 agonist is formulated in a slow-release formulation. Optionally, a CTL-activating peptide is further administered.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Novel method for the detection of cancer biomarkers in cervical specimens
InactiveUS20050136405A1Microbiological testing/measurementMaterial analysisTumor BiomarkersProper treatment
Owner:CYTYC CORP
Compositions comprising 5-alpha reductase inhibitors and SERMs and methods of use thereof
InactiveUS20060019989A1Reduce development riskSuppress and inhibit and reduce riskBiocideMetabolism disorder5 Alpha-Reductase InhibitorGynecomastia
This invention provides for combinations of 5 alpha reductase inhibitors and SERMs. These combinations are useful in: 1) preventing prostate carcinogenesis in a subject; 2) preventing the recurrence of, suppressing, inhibiting or reducing the incidence of prostate carcinogenesis in a subject; 3) treating a subject with prostate cancer; 4) suppressing, inhibiting or reducing the incidence of prostate cancer in a subject; 5) treating a subject with pre-malignant lesions of prostate cancer; 6) suppressing, inhibiting or reducing the incidence of pre-malignant lesions of prostate cancer in a subject; 7) reducing the incidence, inhibiting, suppressing, preventing and / or treating androgen-deprivation induced conditions in men suffering from prostate cancer, such as androgen-deprivation induced osteoporosis, bone fractures, loss of bone mineral density (BMD), hot flashes and / or gynecomastia; and 8) treating polycystic ovarian syndrome and reducing the incidence, inhibiting, suppressing, preventing and / or treating diabetes, cardiovascular disease, breast cancer and endometrial cancer in women suffering from polycystic ovarian syndrome.
Owner:GTX INCORPORATED
System and method of guided treatment within malignant prostate tissue
ActiveUS8548562B2Avoid unnecessary damageImprove accuracyUltrasonic/sonic/infrasonic diagnosticsHeart defibrillatorsNon malignantControl system
Owner:TRACHTENBERG JOHN +2
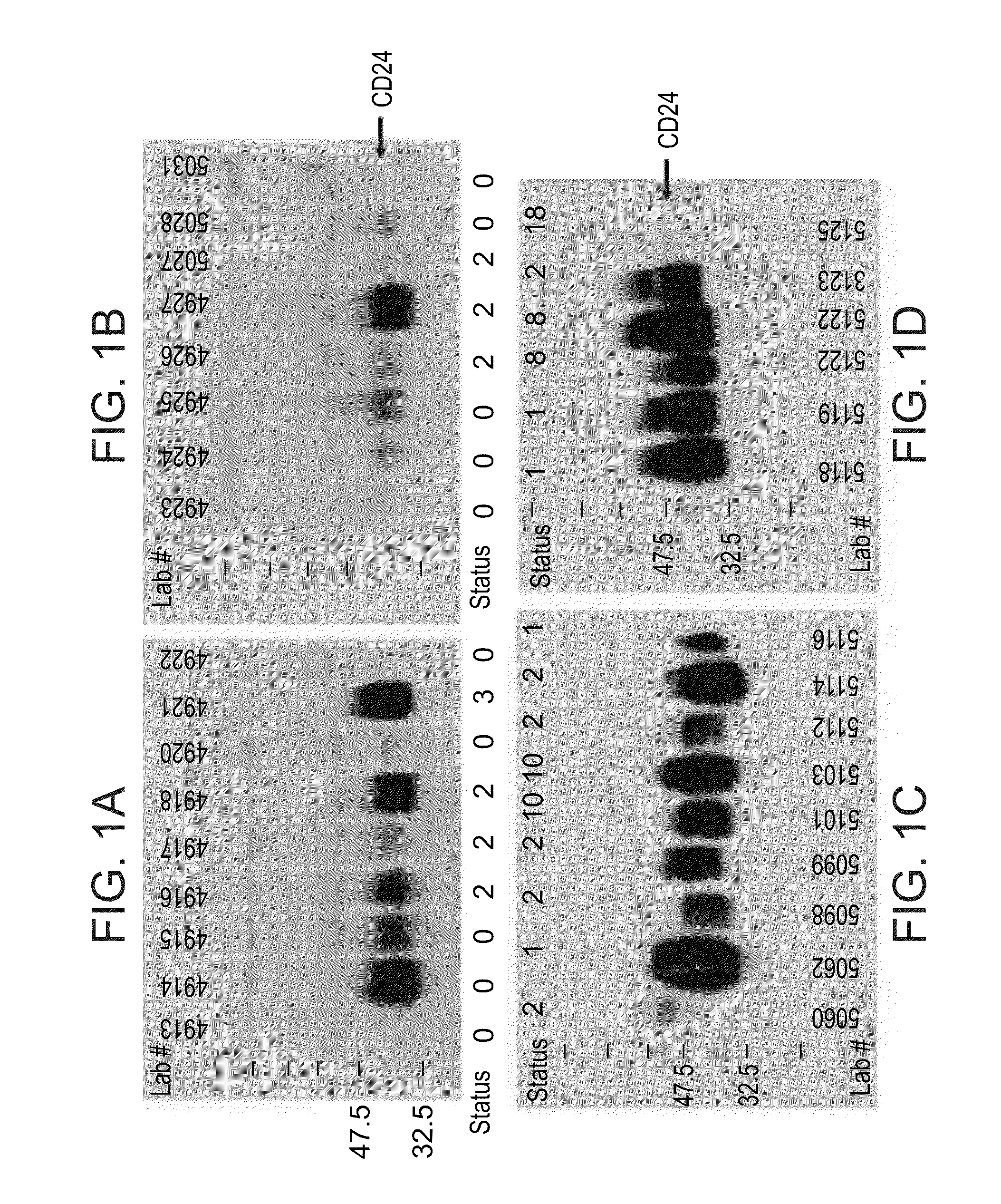
Methods and Kits for Early Detection of Cancer or Predisposition Thereto
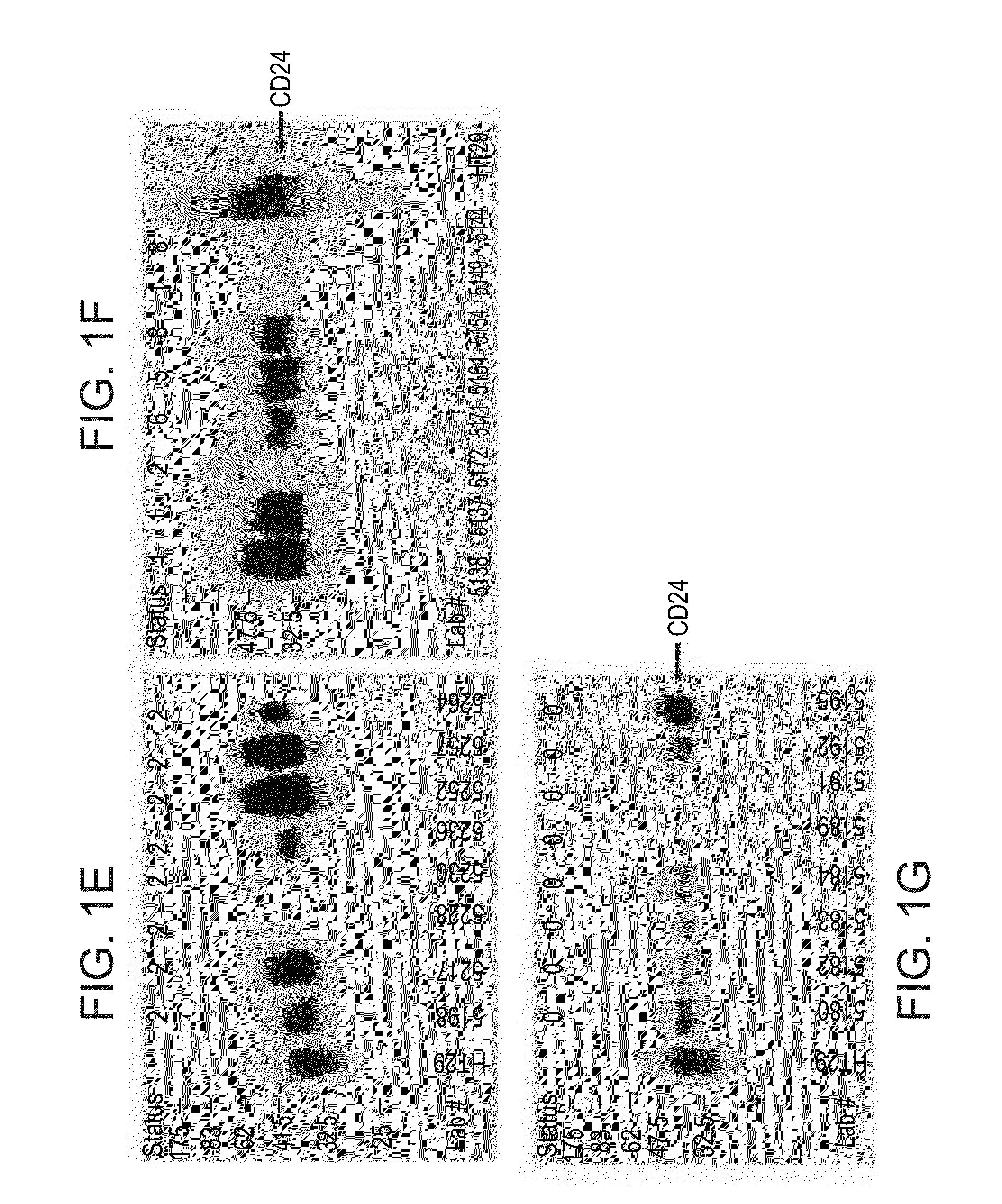
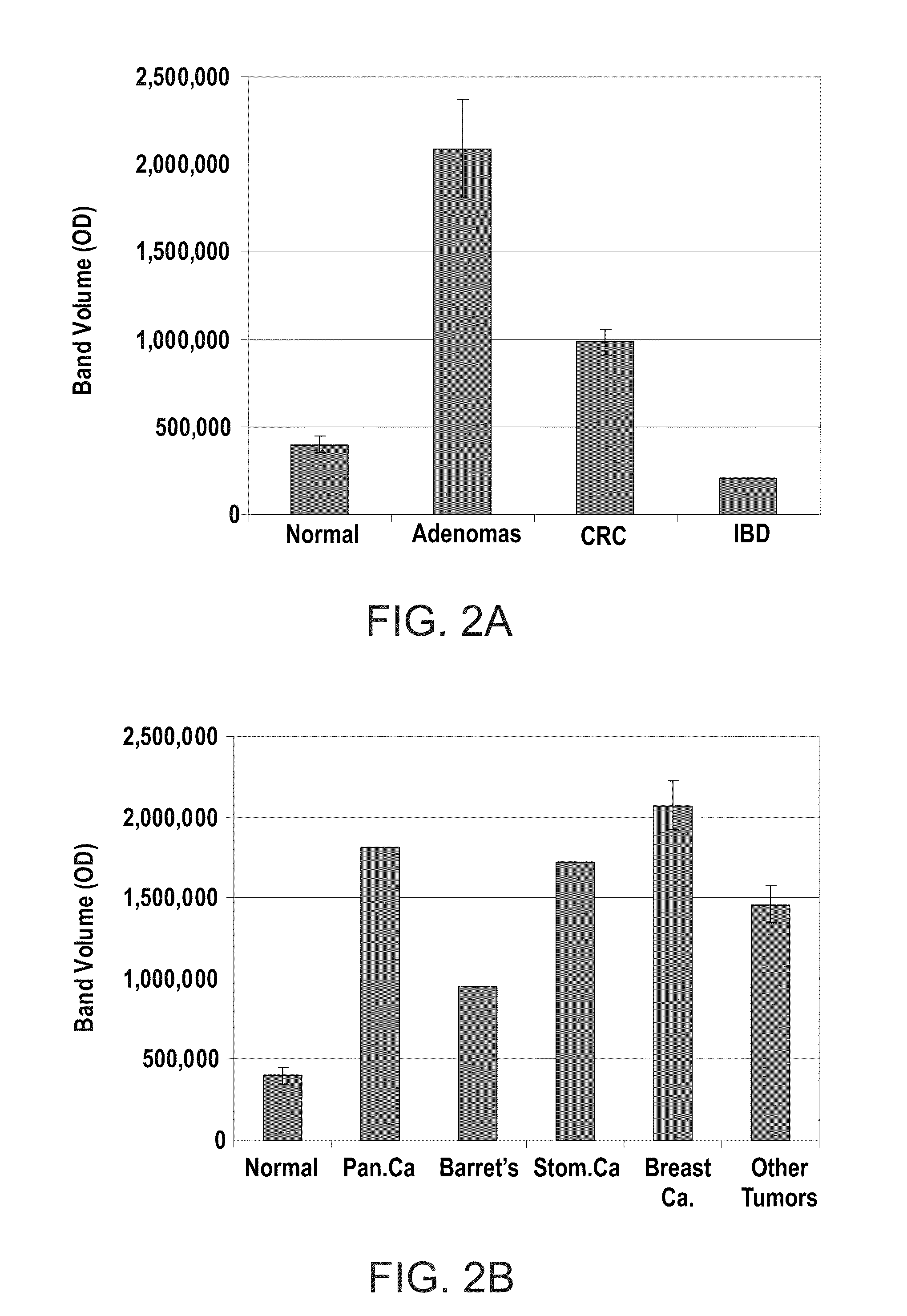
InactiveUS20100062450A1Characteristic be alterMicrobiological testing/measurementDisease diagnosisGastrointestinal cancerCD24
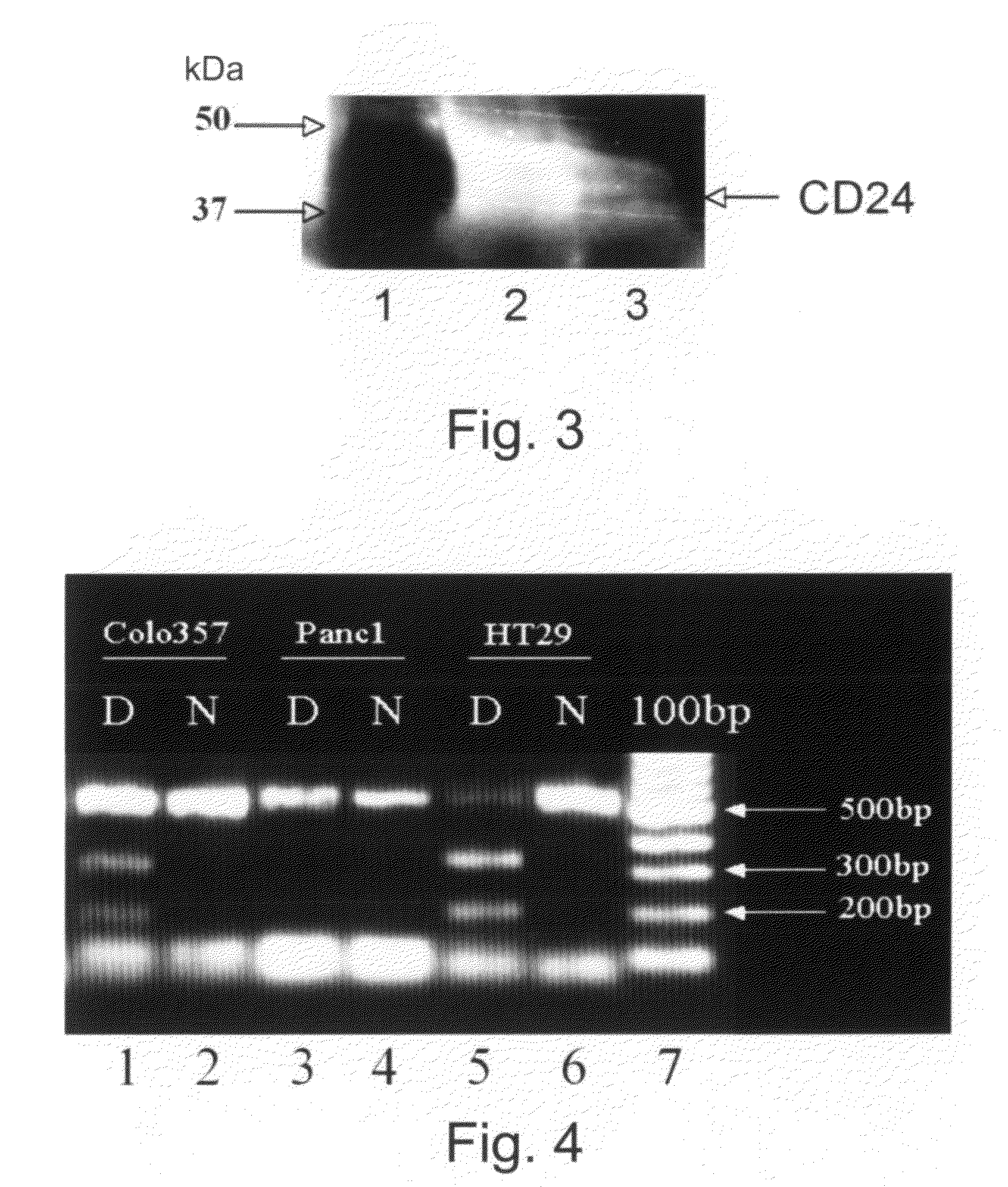
Methods and kits for diagnosing cancer or a pre-malignant lesion by determining the presence and / or level of circulating CD24 of a subject are provided. Also provided are methods and kits for determining if a subject is predisposed to gastrointestinal cancer by the determining the presence or absence, in a homozygous or heterozygous form of cancer associated genotype(s) in the CD24 and / or APC nucleic acid sequences. Also provided are methods and kits for monitoring efficacy of cancer therapy by determining the presence and / or level of circulating CD24 of a subject.
Owner:MEDICAL RES FUND OF TEL AVIV SOURASKY MEDICAL CENT +1
Detection method for human pappilomavirus (HPV) and its application in cervical cancer
InactiveUS20080044809A1High riskMicrobiological testing/measurementVirus peptidesEpithelial cell abnormalityHistone antibody
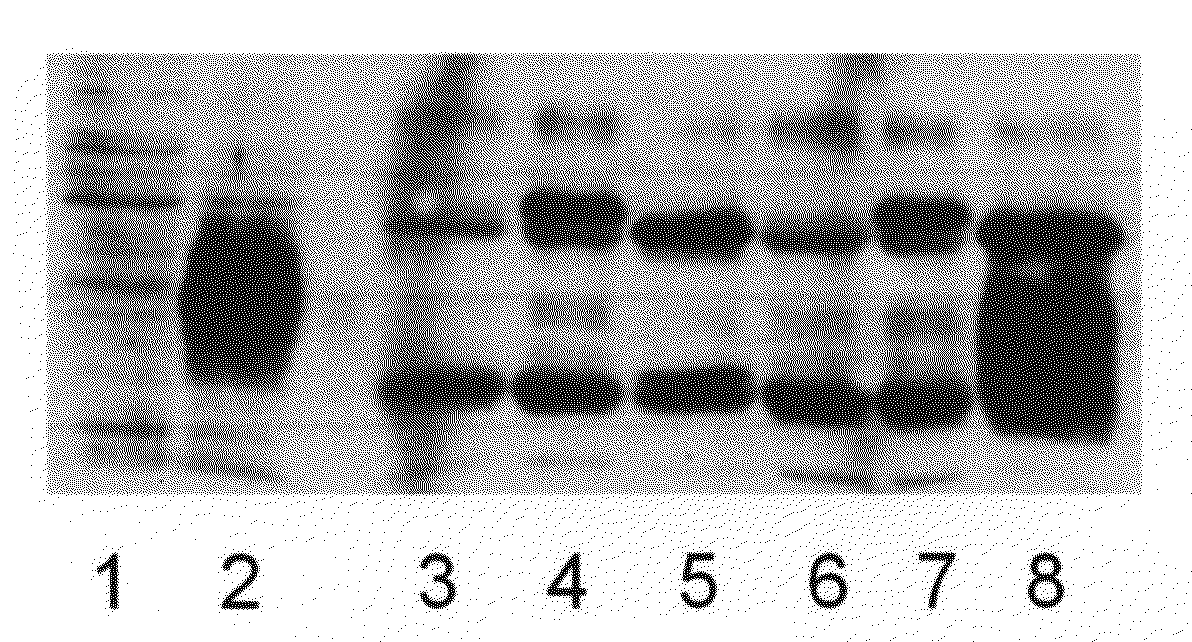
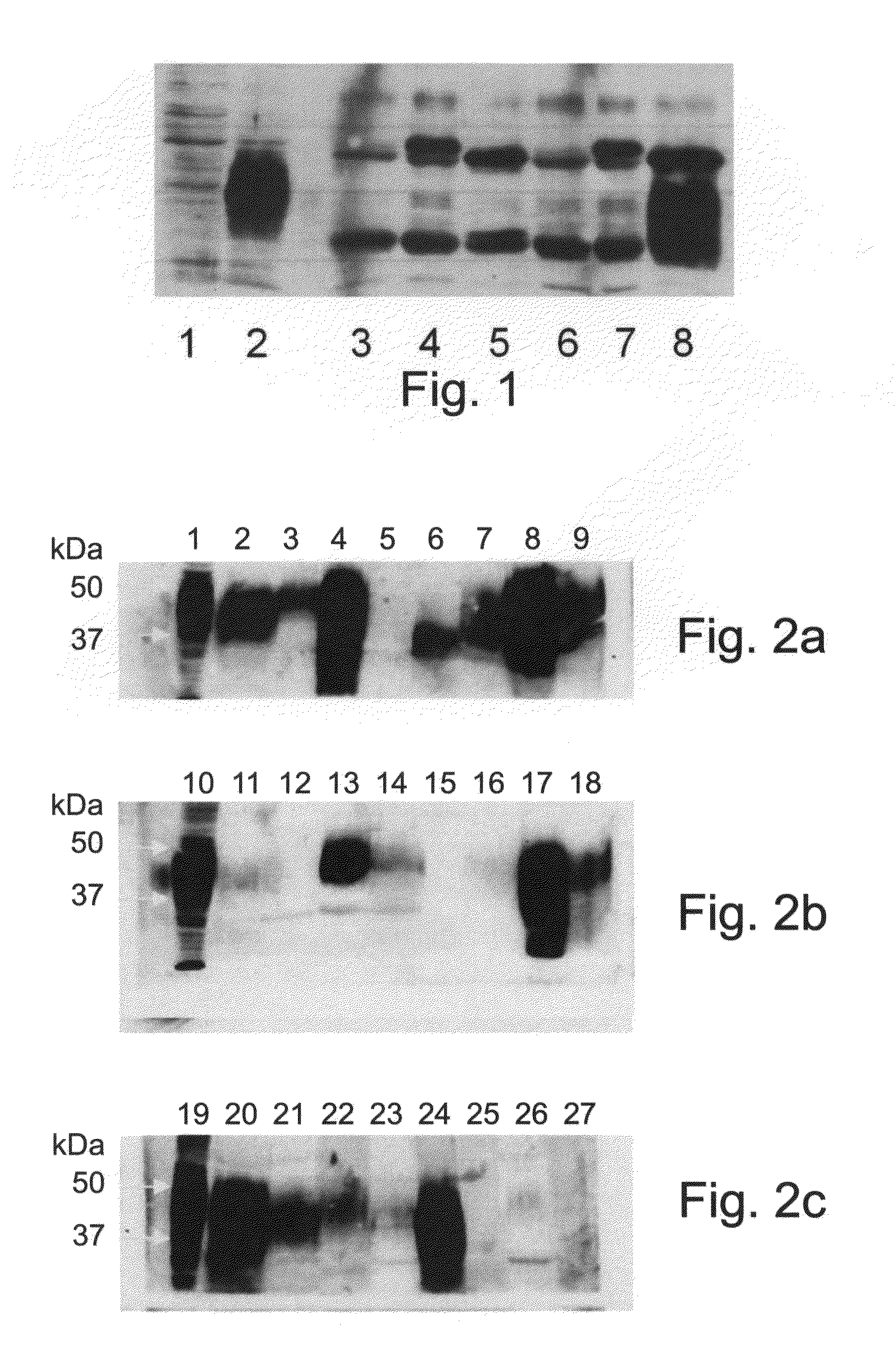
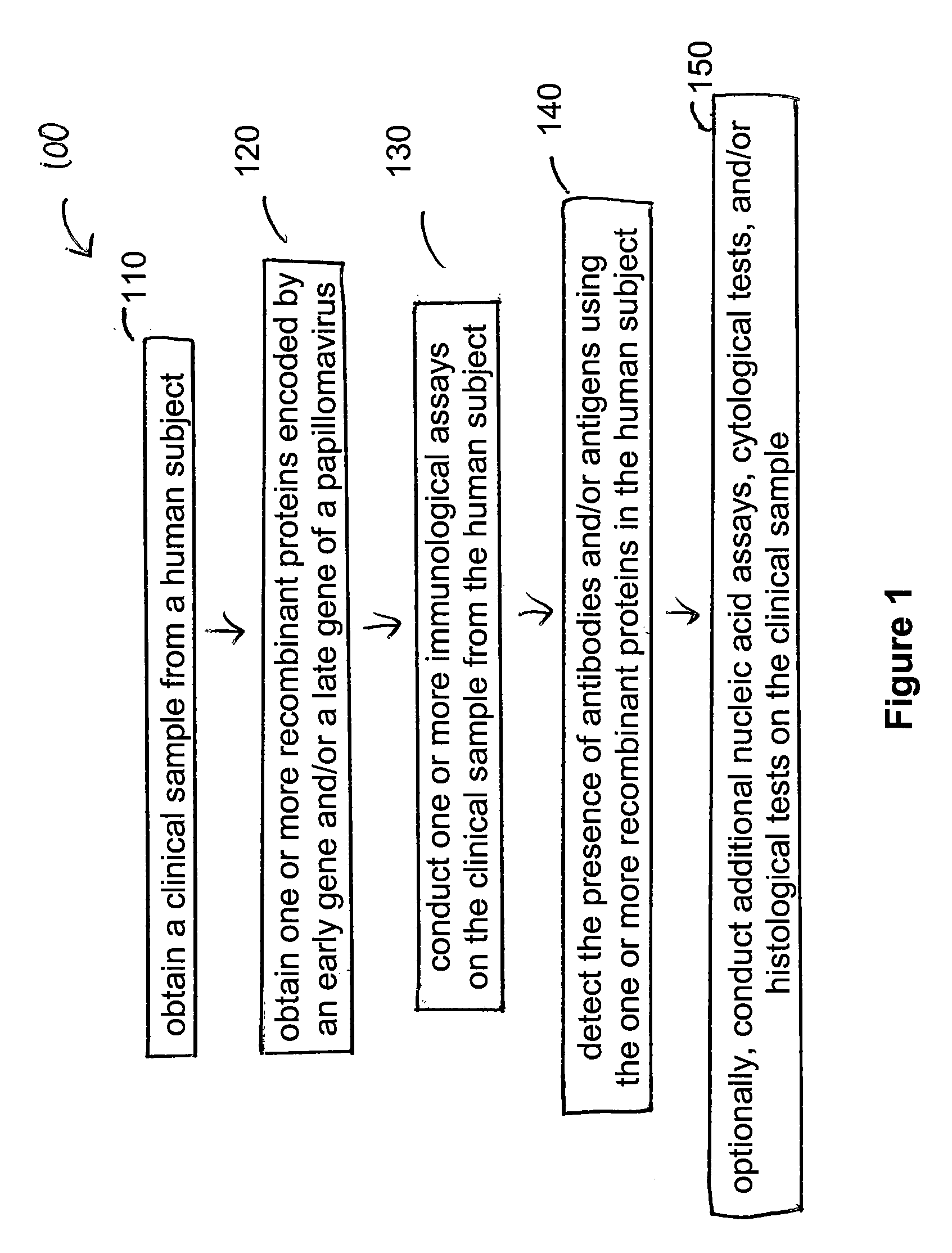
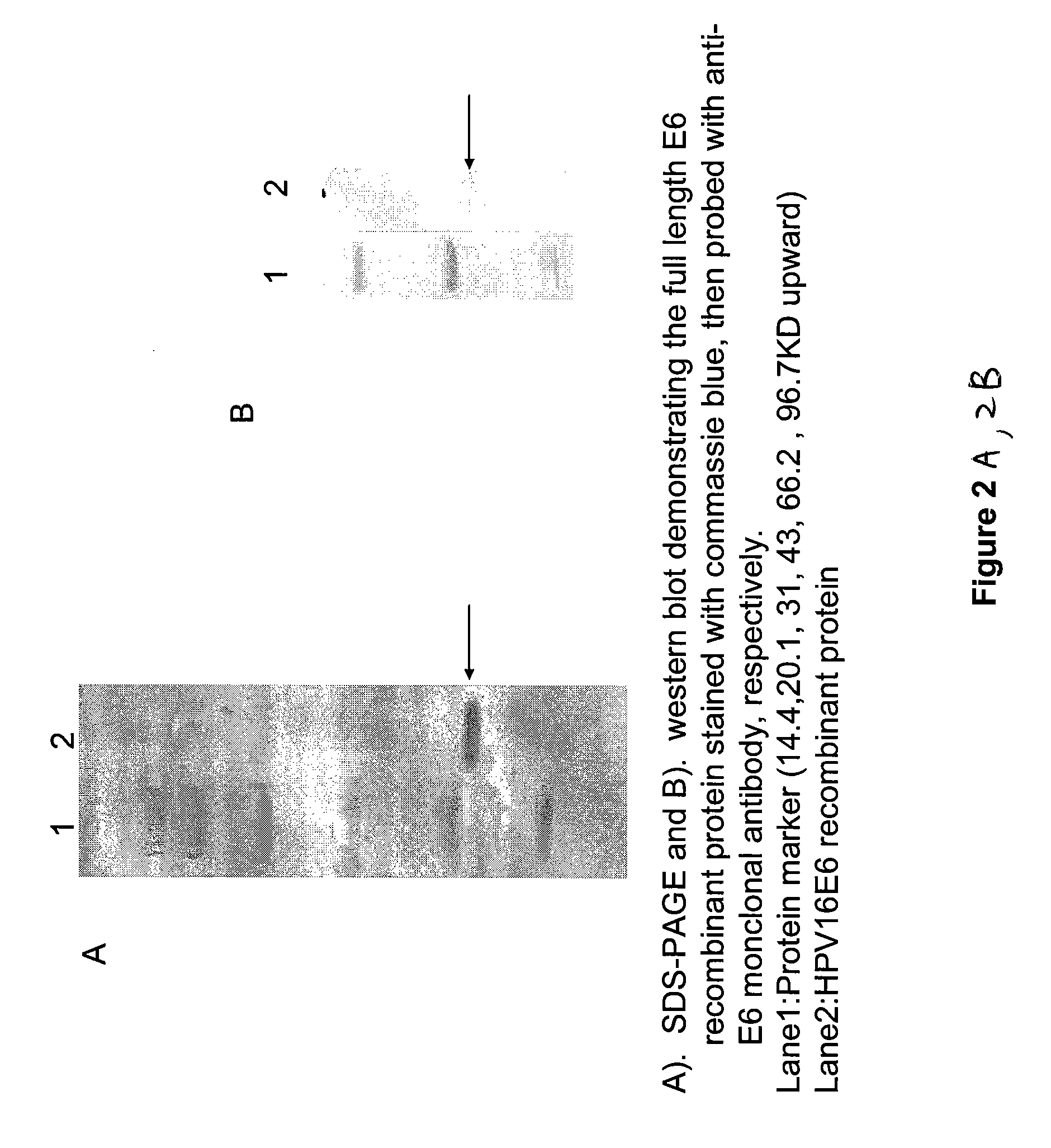
Embodiments of the invention provide methods, assays, and kits for detecting HPV infection and HPV associated epithelial cell abnormalities, most notably those associated with pre-malignant and malignant epithelial cell lesions. Detection of HPV DNAs, genomes, and / or oncoproteins by nucleic acid hybridization assays and immunological assays can be used in early clinical screening for HPV infection and diagnosis for cervical cancer. The polypeptides, recombinant proteins, antibodies, nucleic acids, and various detection methods thereof are particularly useful for diagnosing carcinomas of the uterine cervix and those at risk of developing cervical cancer.
Owner:HEER MEDICAL TECH DEV CO LTD
Composition and method for treatment and chemoprevention of prostate cancer
InactiveUS20050171073A1Effective preventionPreventing prostate cancer relapseBiocideHalogenated hydrocarbon active ingredientsLesionProstate carcinogenesis
This invention relates to compositions and methods of use thereof in the prevention of prostate carcinogenesis in a subject; prevention of the recurrence of, suppression, inhibition or reduction of the incidence of prostate carcinogenesis in a subject; treatment of a subject with prostate cancer; suppression, inhibition or reduction of the incidence of prostate cancer in a subject; treatment of a subject with pre-malignant lesions of prostate cancer; and / or suppression, inhibition or reduction of the incidence of pre-malignant lesions of prostate cancer in a subject.
Owner:UNIV OF TENNESSEE RES FOUND
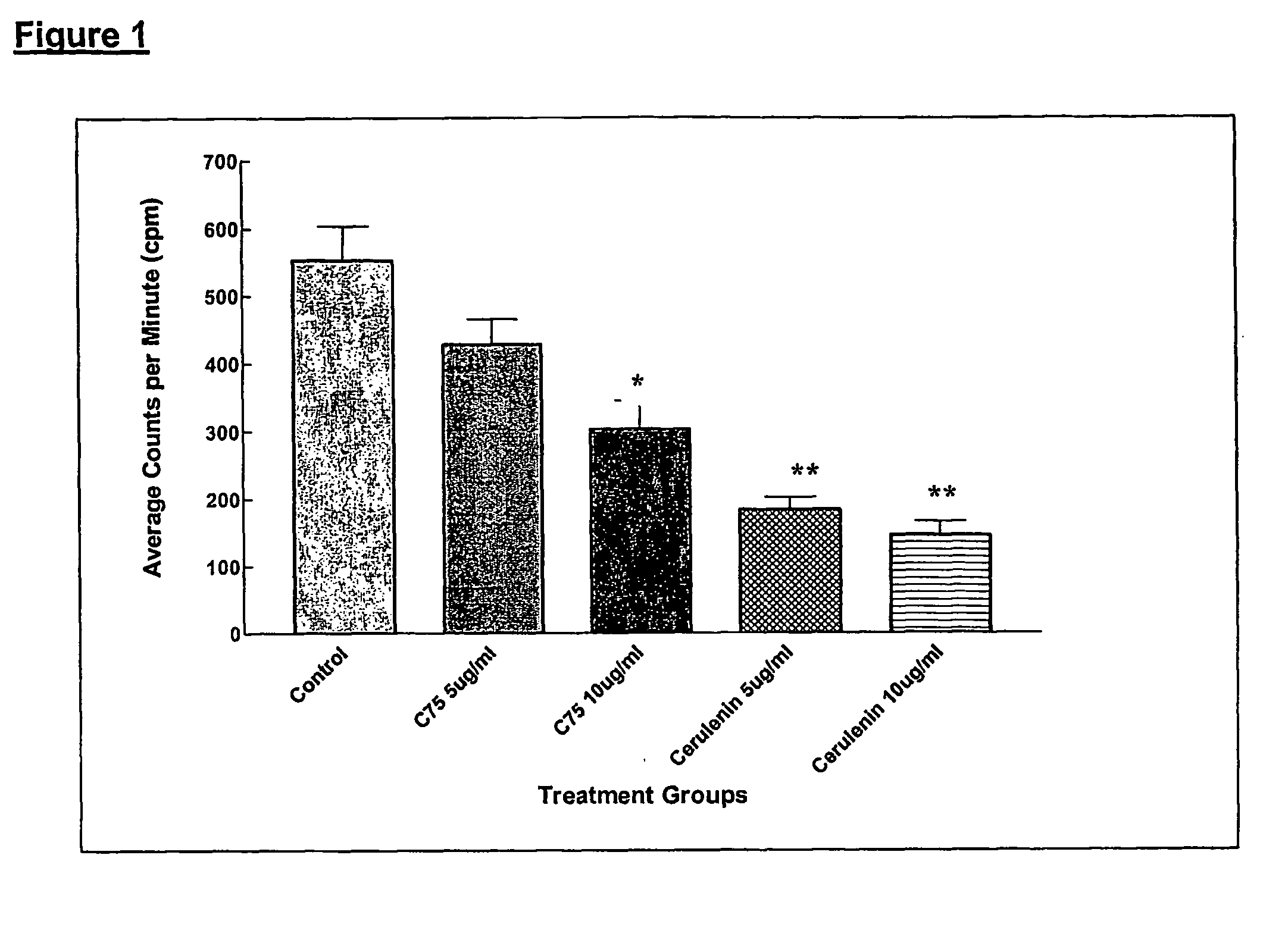
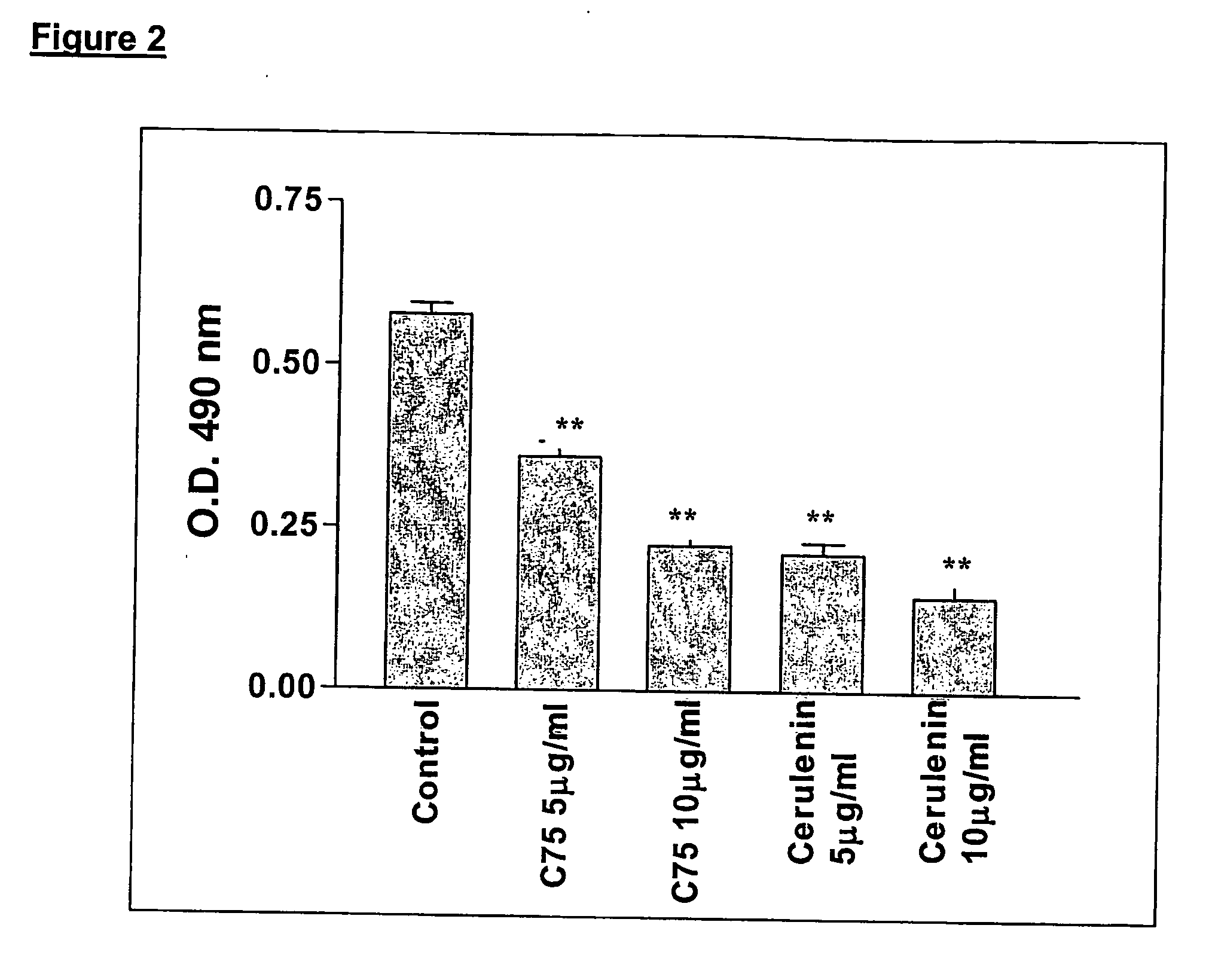
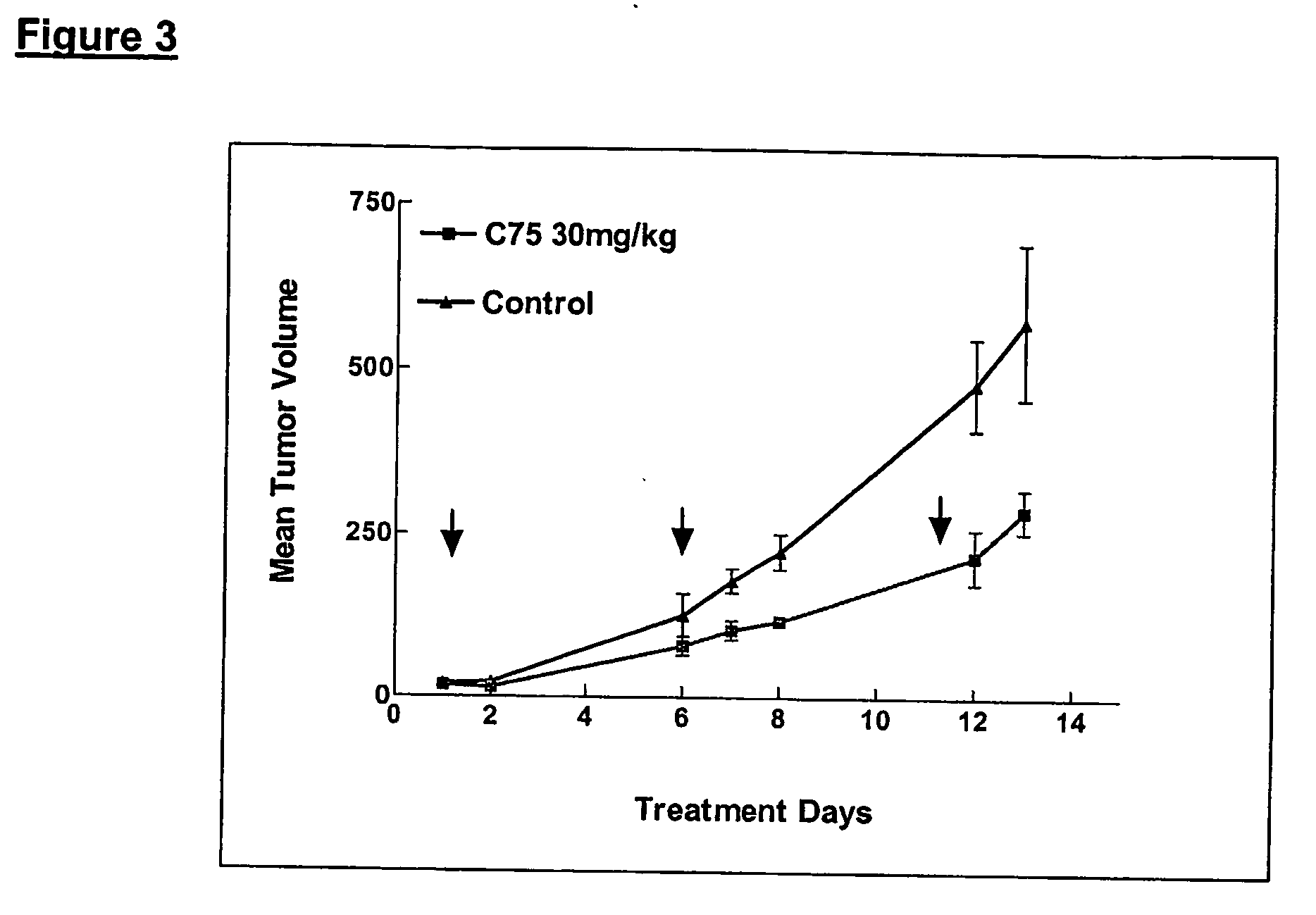
Method for inhibiting cancer development by fatty acid synthase inhibitors
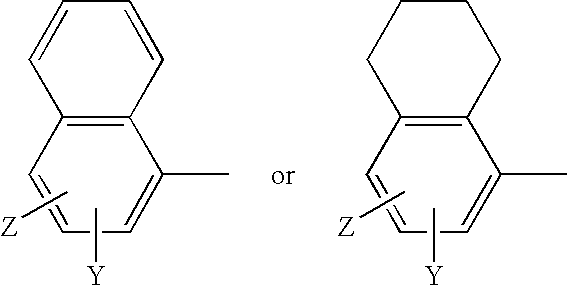
A method for inhibiting or preventing cancer development by the administration of fatty acid synthase (FAS) inhibitors. In particular, the present invention prohibits or delays the development of invasive cancer from pre-malignant (non-invasive) lesions that express FAS. Compositions containing FAS inhibitors also are provided, as well as methods for administering the FAS inhibitors and compositions to patients in need thereof.
Owner:FASGEN +1
Detection of extracellular tumor-associated nucleic acid in blood plasma or serum
InactiveUS8048629B2Enabling detectionFacilitates selection and monitoringMicrobiological testing/measurementGenetic material ingredientsReceptor tyrosine kinase inhibitorBlood plasma
This invention relates to detection of specific extracellular DNA in plasma or serum fractions of human or animal blood associated with neoplastic, pre-malignant or proliferative disease. Specifically, the invention relates to detection tumor-associated DNA, and to those methods of detecting and monitoring tumor-associated DNA found in the plasma or serum fraction of blood by using DNA extraction and amplification with or without enrichment for DNA. The invention allows the selection and monitoring of patients for various cancer therapies including receptor tyrosine kinase inhibitor therapies.
Owner:PENN STATE RES FOUND
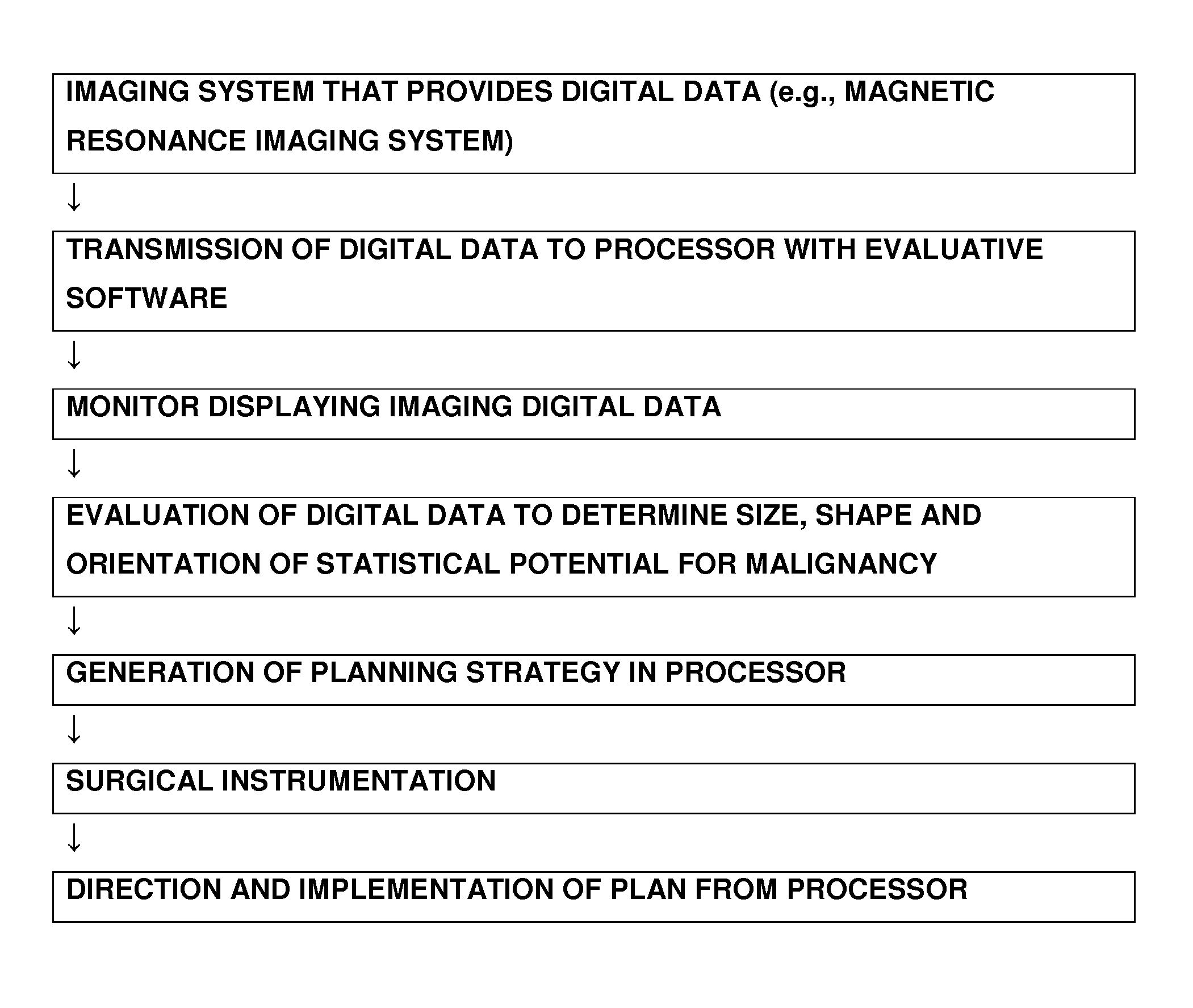
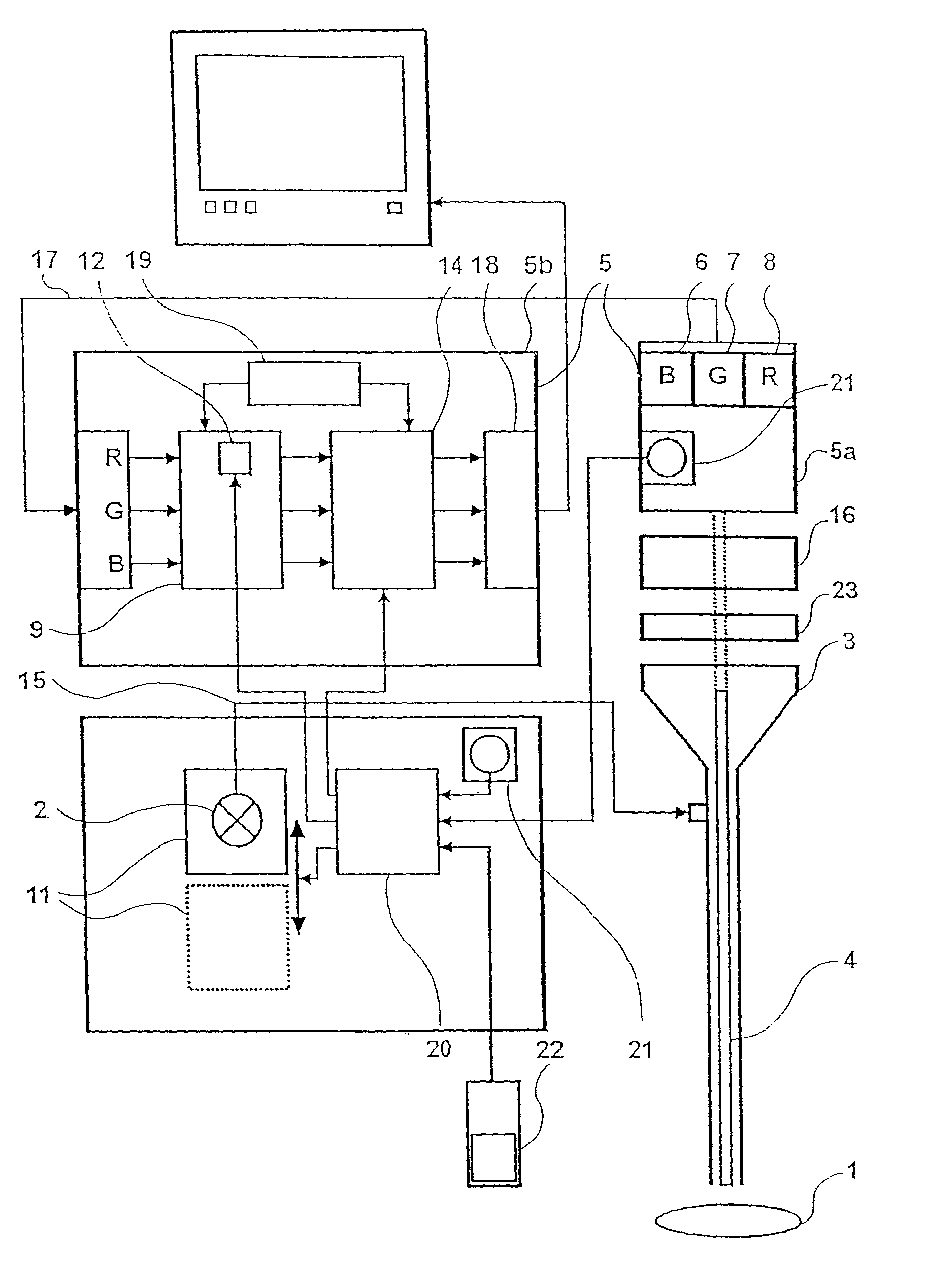
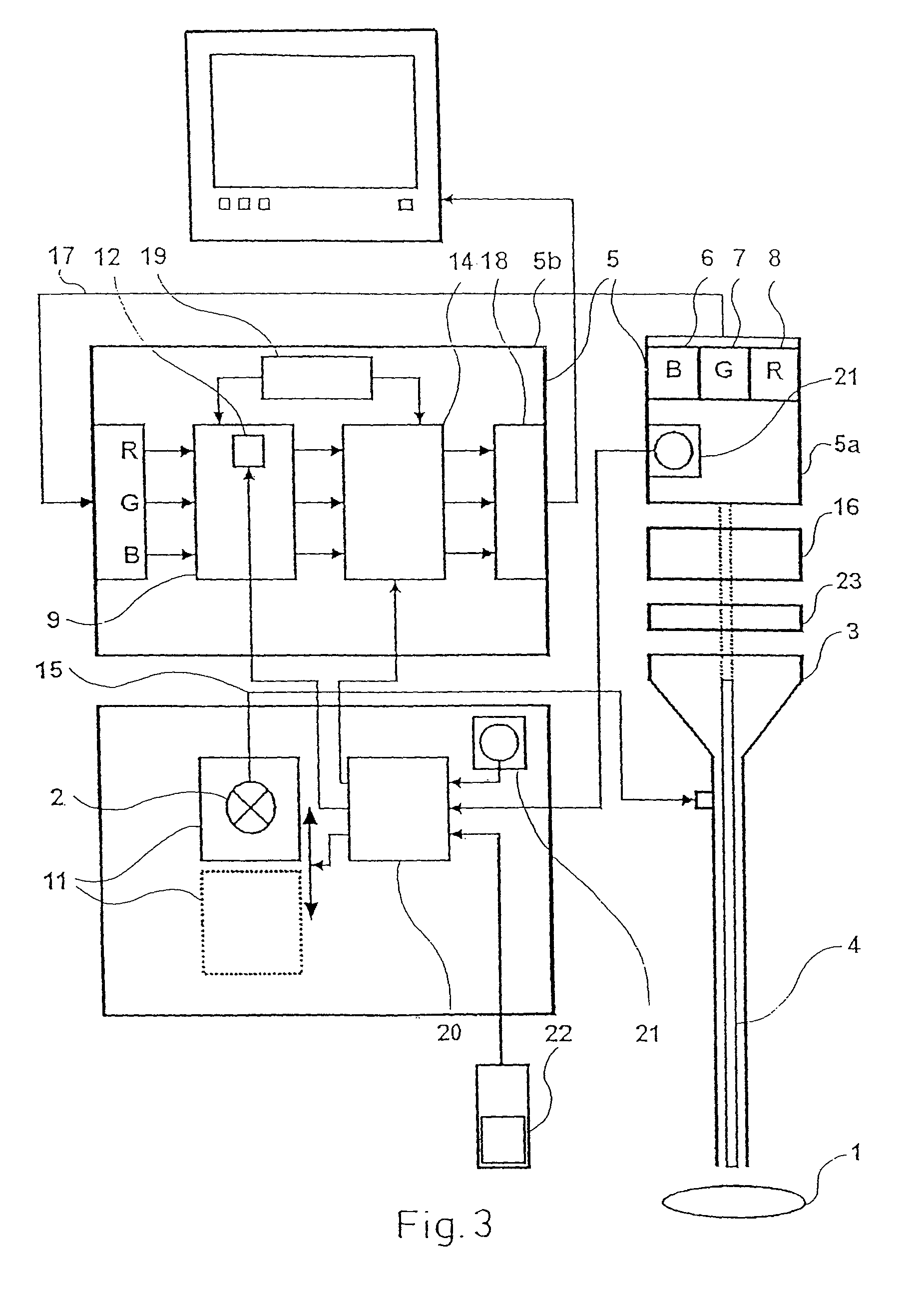
Device for the picture-providing diagnosis of tissue using one of at least two diagnosis modes
InactiveUS8019405B2High color contrastPromote differentiationBronchoscopesLaryngoscopesRed fluorescenceWhite light
A device for picture-providing diagnosis of tissue is selectively operated in a picture-providing white light diagnosis mode and a picture providing auto-fluorescence mode. A color camera having red, green, and blue sensors provides a monitor which picture signals. A light source emits fluorescence excitation light and additionally emits so much red light that the red light remitted by the tissue dominates the red fluorescence light in the picture providing auto-fluorescence mode. The signal from the red sensor of the camera is damped so that normal time appears green and pre-malignant and early malignant tissue appears red.
Owner:RICHARD WOLF GMBH
Methods of diagnosing cancer
A method of diagnosing cancer or a pre-malignant lesion is disclosed. The method comprises determining a level of CD24 expressed on peripheral blood cells of a subject in need thereof, wherein the level of CD24 above a predetermined threshold is indicative of the cancer or the pre-malignant lesion.
Owner:THE MEDICAL RES INFRASTRUCTURE & HEALTH SERVICES FUND OF THE TEL AVIV MEDICAL CENT
Treatment of cancer or pre-malignant conditions using anti-CD40 antibodies
InactiveUS8926979B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsGenotypeHuman patient
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
Diagnostic scanning microscope for information-enriched qualitative histopathology
InactiveUS7885448B2Acquiring/recognising microscopic objectsDiagnostic recording/measuringDiagnostic scanningVisual perception
A microscope array with staggered rows of magnifying imaging systems is used to scan a biological tissue sample in a single linear pass to produce an image and corresponding optical-density data. A conventional computerized algorithm is used to identify, isolate and produce segmented images of nuclei contained in the image. The OD values corresponding to nuclear chromatin are used to identify numerical patterns known to have statistical significance in relation to the health condition of the biological tissue. These patterns are analyzed to detect pre-neoplastic changes in histologically normal-appearing tissue that suggest a risk for the development of a pre-malignant and a potentially malignant lesion. This information is then converted to a visually perceptible form incorporated into the image of the tissue sample and is displayed for qualitative analysis by a pathologist.
Owner:DMETRIX INC
Peptides from the E7 protein of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papilloma virus associated cancers
InactiveUS20050221295A1Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
An isolated protein sequence or peptide from the E2, E6 or E7 early coding region of human papillomavirus (HPV) that is soluble in an aqueous medium, and characterized by a relative paucity of tryptophan, methionine and cysteine residues, and a relative abundance of glycine and asparagine residues. Also disclosed are isolated protein sequences or peptides from the E2, E6 or E7 early coding regions of HPV 16 and 18 and methodologies for detecting or diagnosing cancer or cellular abnormalities. Detection or diagnosis of Cancer or cellular abnormalities may include detecting or diagnosing pre-cancerous or pre-malignant conditions, cervical dysplasia, cervical carcinoma, koilocytosis, hyperkeratosis, intraepithelial lesions, and other cancers. A methodology for detecting or diagnosing cancer or cellular abnormalities comprises the steps of (1) reacting a sample of body fluid or tissue with isolated protein sequences or peptides; (2) forming an antibody-peptide complex; and (3) detecting the antibody-peptide complex.
Owner:HU YAO XIONG
Novel genes, compositions, kits, and methods for identification, assessment, prevention and therapy of cervical cancer
InactiveUS20070003990A1Reduced expression levelImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureOncologyNovel gene
The invention relates to newly discovered nucleic acid molecules and proteins associated with cervical cancer including pre-malignant conditions such as dysplasia. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human cervical cancers are provided.
Owner:MILLENNIUM PHARMA INC
Genes, compositions, kits, and methods for identification, assessment, prevention, and therapy of prostate cancer
The invention relates to newly discovered nucleic acid molecules and proteins associated with prostate cancer including pre-malignant conditions. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human prostate cancers are provided.
Owner:MILLENNIUM PHARMA INC
Peptides from the E2, E6, and E7 proteins of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papillomavirus associated cancers
InactiveUS20040110925A1Reduce the possibilityStrong specificityMicrobiological testing/measurementVirus peptidesTryptophanHyperkeratosis
An isolated protein sequence or peptide from the E2, E6 or E7 early coding region of human papillomavirus (HPV) that is soluble in an aqueous medium, and characterized by a relative paucity of tryptophan, methionine and cysteine residues, and a relative abundance of glycine and asparagine residues. Also disclosed are isolated protein sequences or peptides from the E2, E6 or E7 early coding regions of HPV 16 and 18 and methodologies for detecting or diagnosing cancer or cellular abnormalities. Detection or diagnosis of Cancer or cellular abnormalities may include detecting or diagnosing pre-cancerous or pre-malignant conditions, cervical dysplasia, cervical carcinoma, koilocytosis, hyperkeratosis, intraepithelial lesions, and other cancers. A methodology for detecting or diagnosing cancer or cellular abnormalities comprises the steps of (1) reacting a sample of body fluid or tissue with isolated protein sequences or peptides; (2) forming an antibody-peptide complex; and (3) detecting the antibody-peptide complex.
Owner:HU YAO XIONG
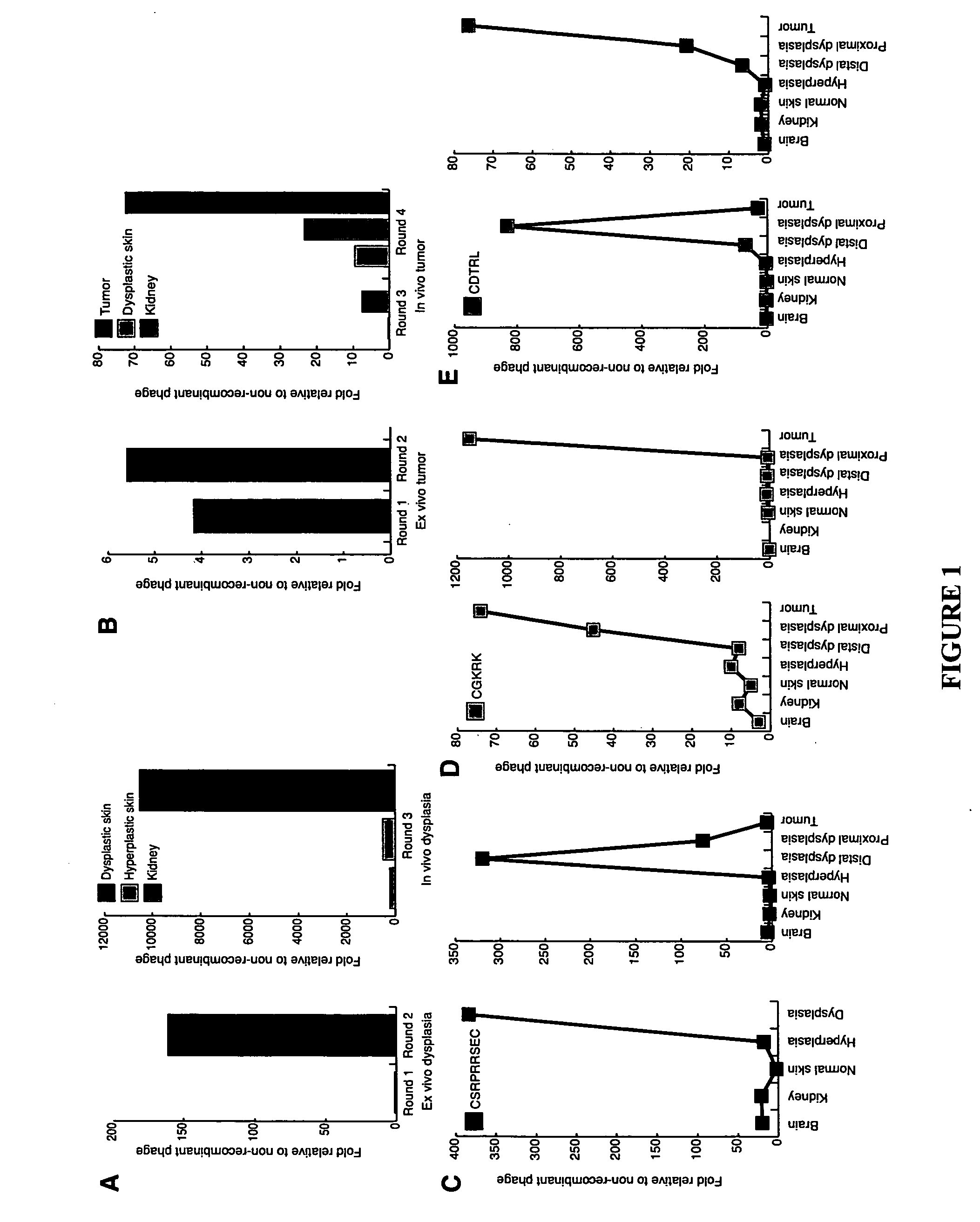
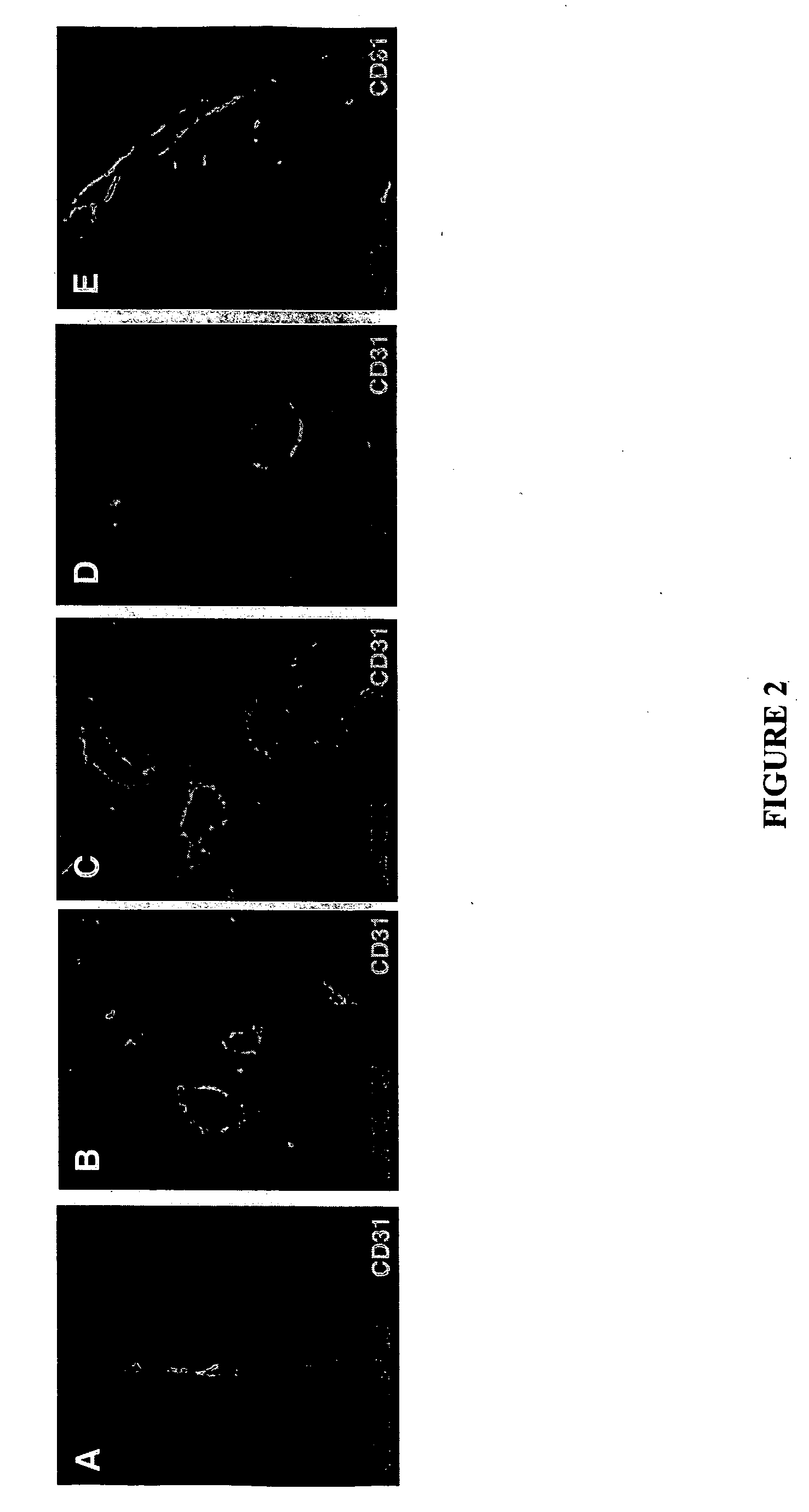
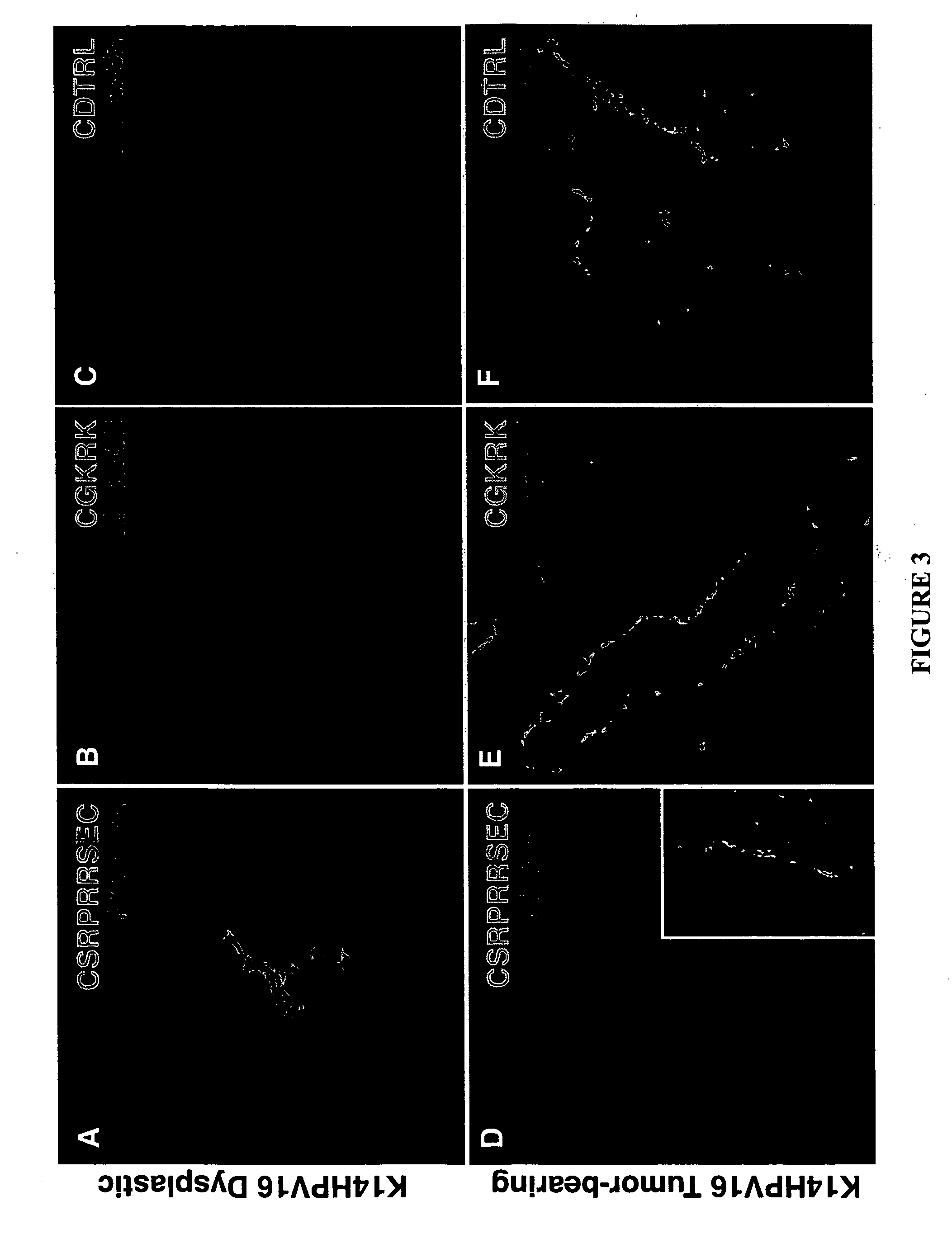
Molecules that selectively home to vasculature of pre-malignant dysplastic lesions or malignancies
InactiveUS20060002854A1Reduce riskDecrease in vasculatureOrganic active ingredientsHybrid immunoglobulinsMalignancyLesion
The present invention provides a conjugate that contains a therapeutic moiety linked to a homing peptide or peptidomimetic which selectively homes to vasculature of pre-malignant dysplastic skin and which includes the amino acid sequence SRPRR (SEQ ID NO: 1) or a conservative variant or peptidomimetic thereof. The present invention further provides a conjugate containing a therapeutic moiety linked to a homing peptide or peptidomimetic which selectively homes to vasculature of malignant skin and which includes the amino acid sequence CGKRK (SEQ ID NO: 6) or the amino acid sequence CDTRL (SEQ ID NO: 7), or a conservative variant or peptidomimetic of one of these sequences.
Owner:BURNHAM INST FOR MEDICAL RES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com