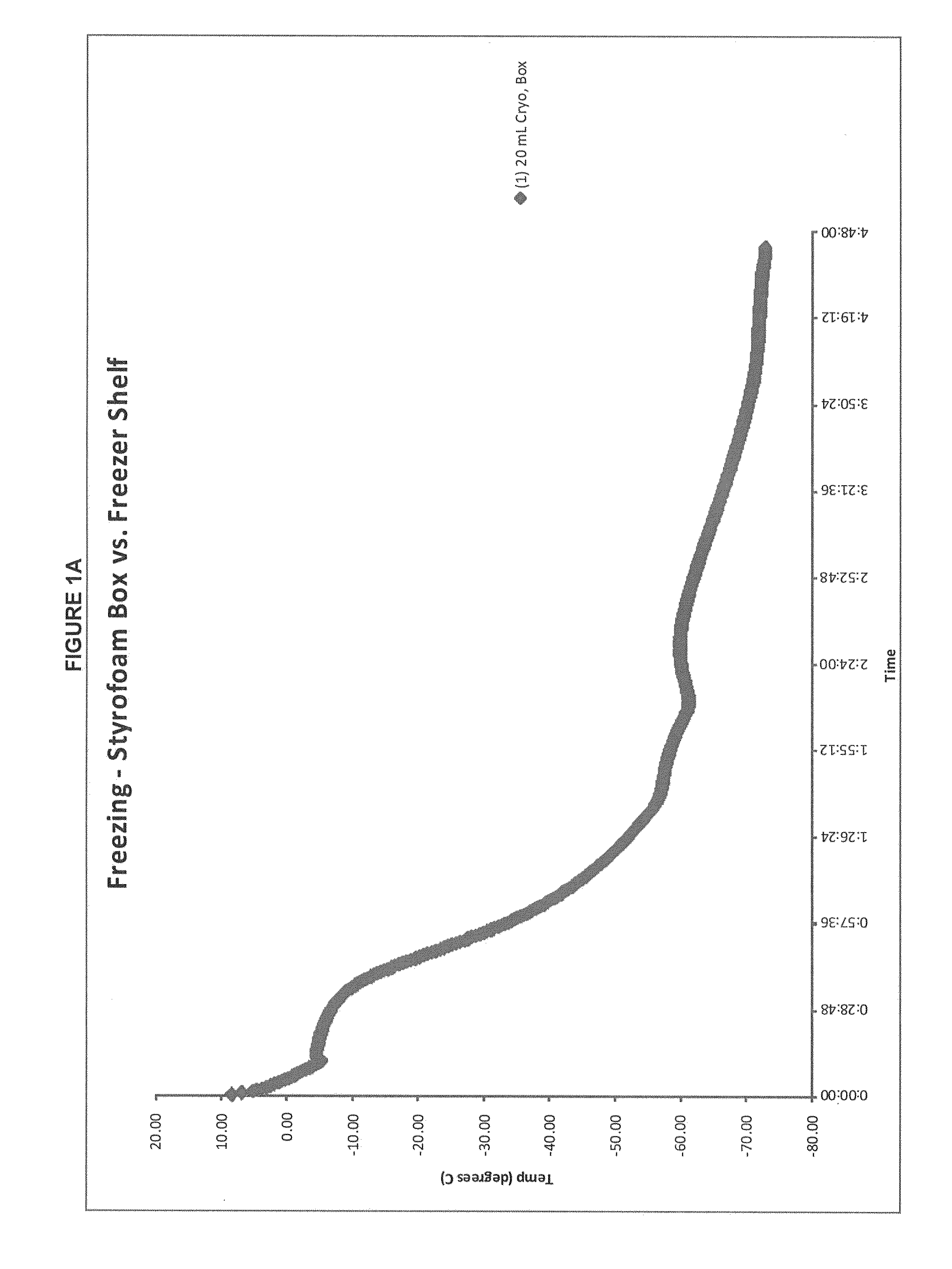
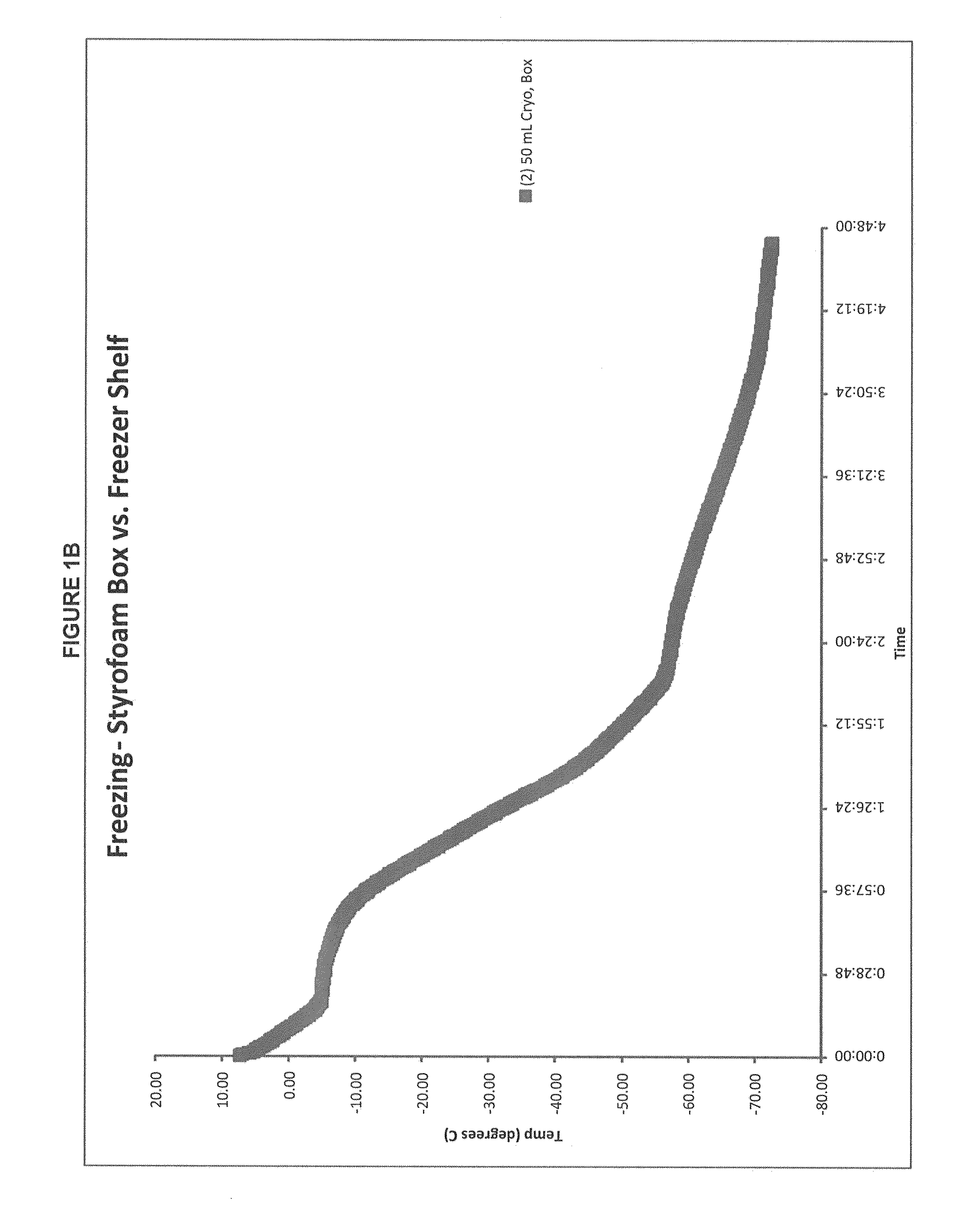
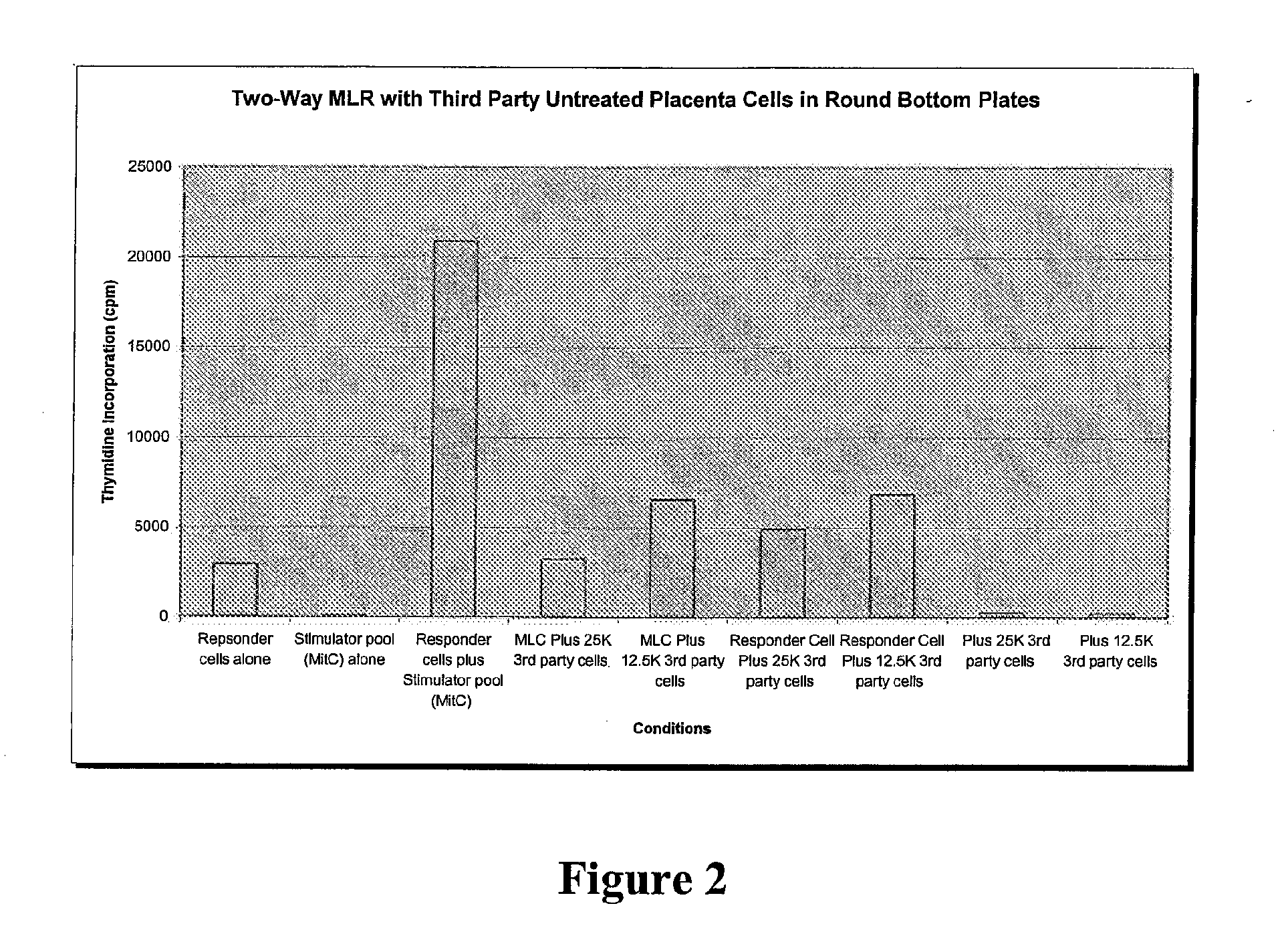
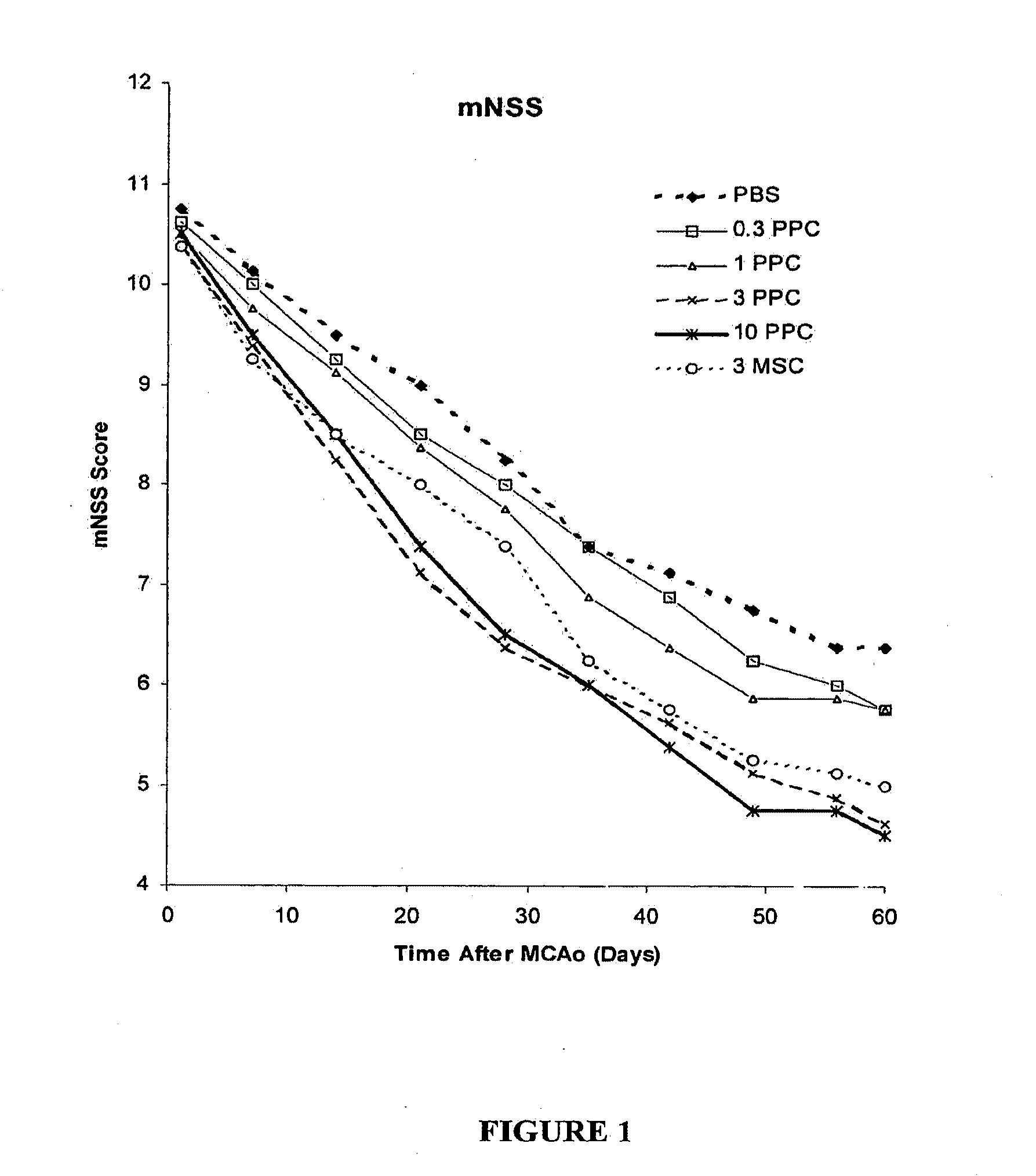
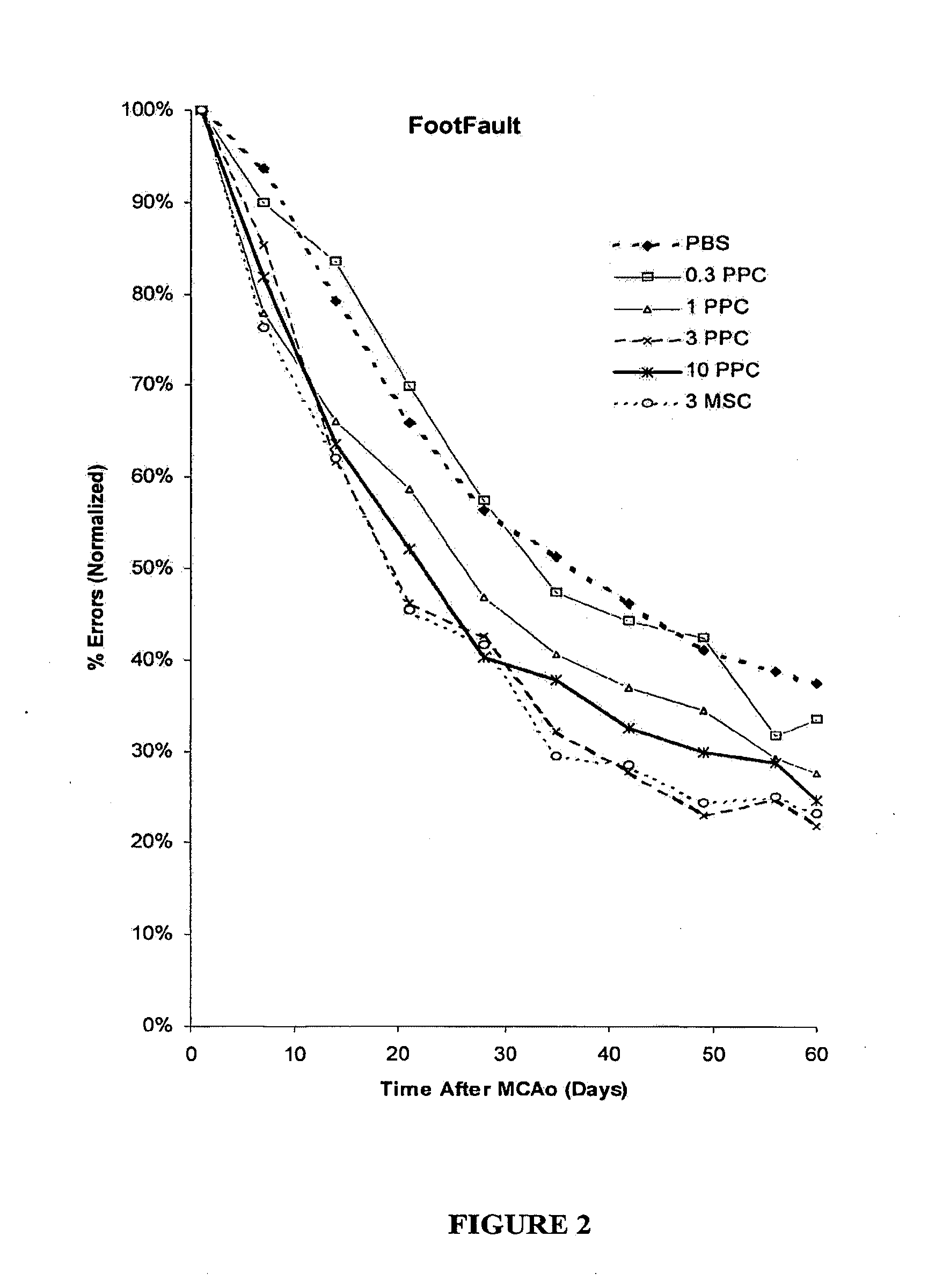
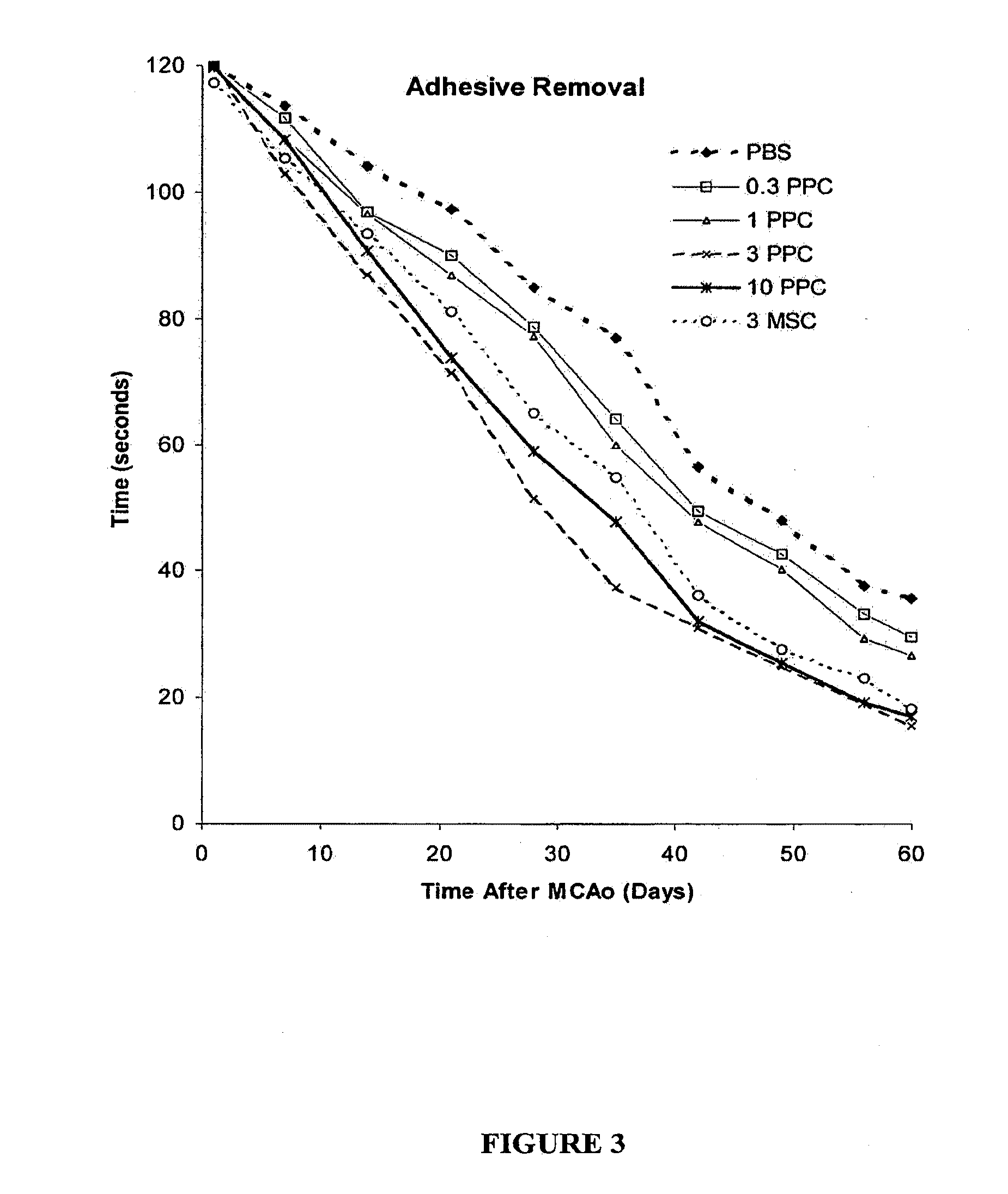
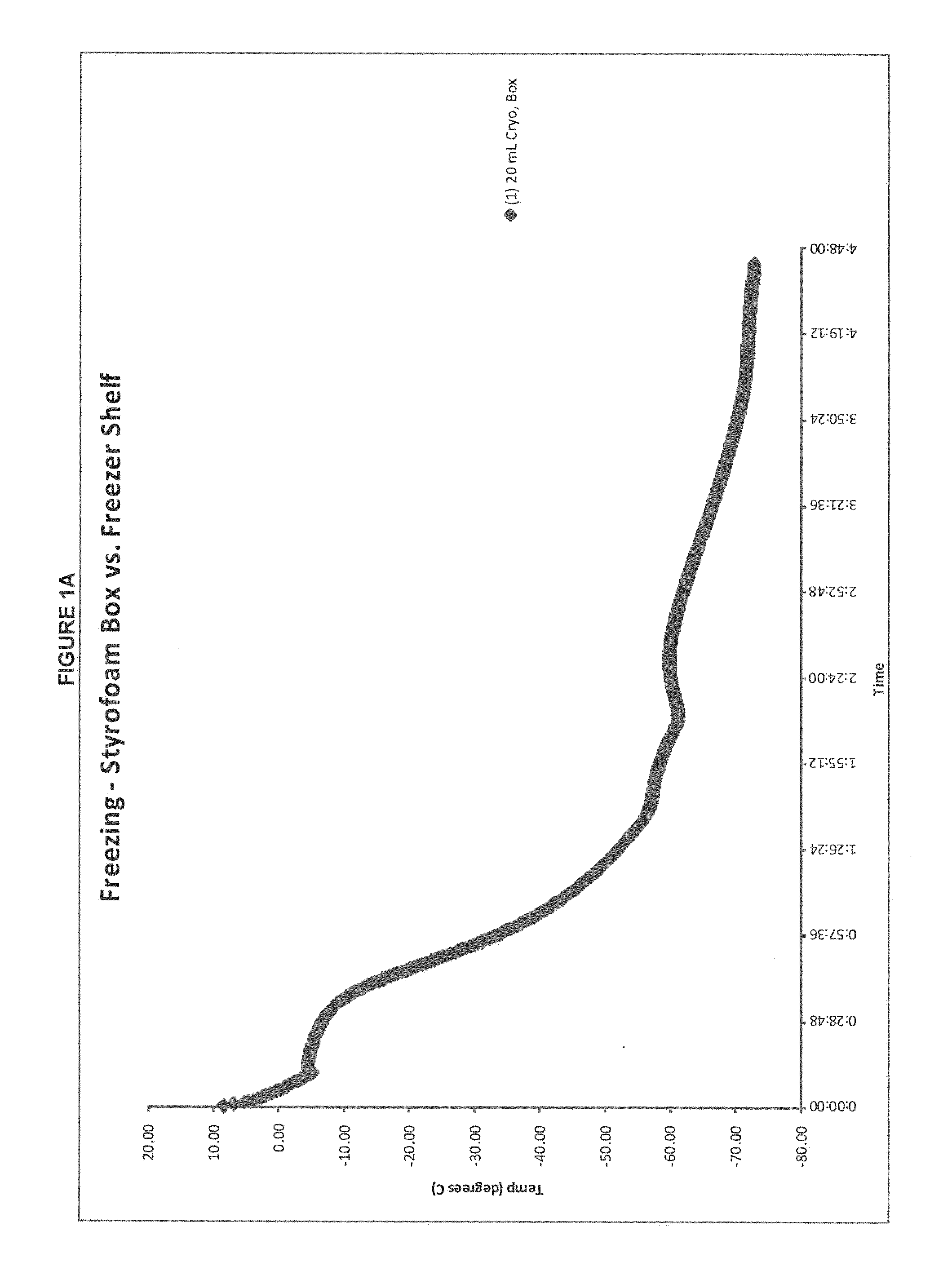
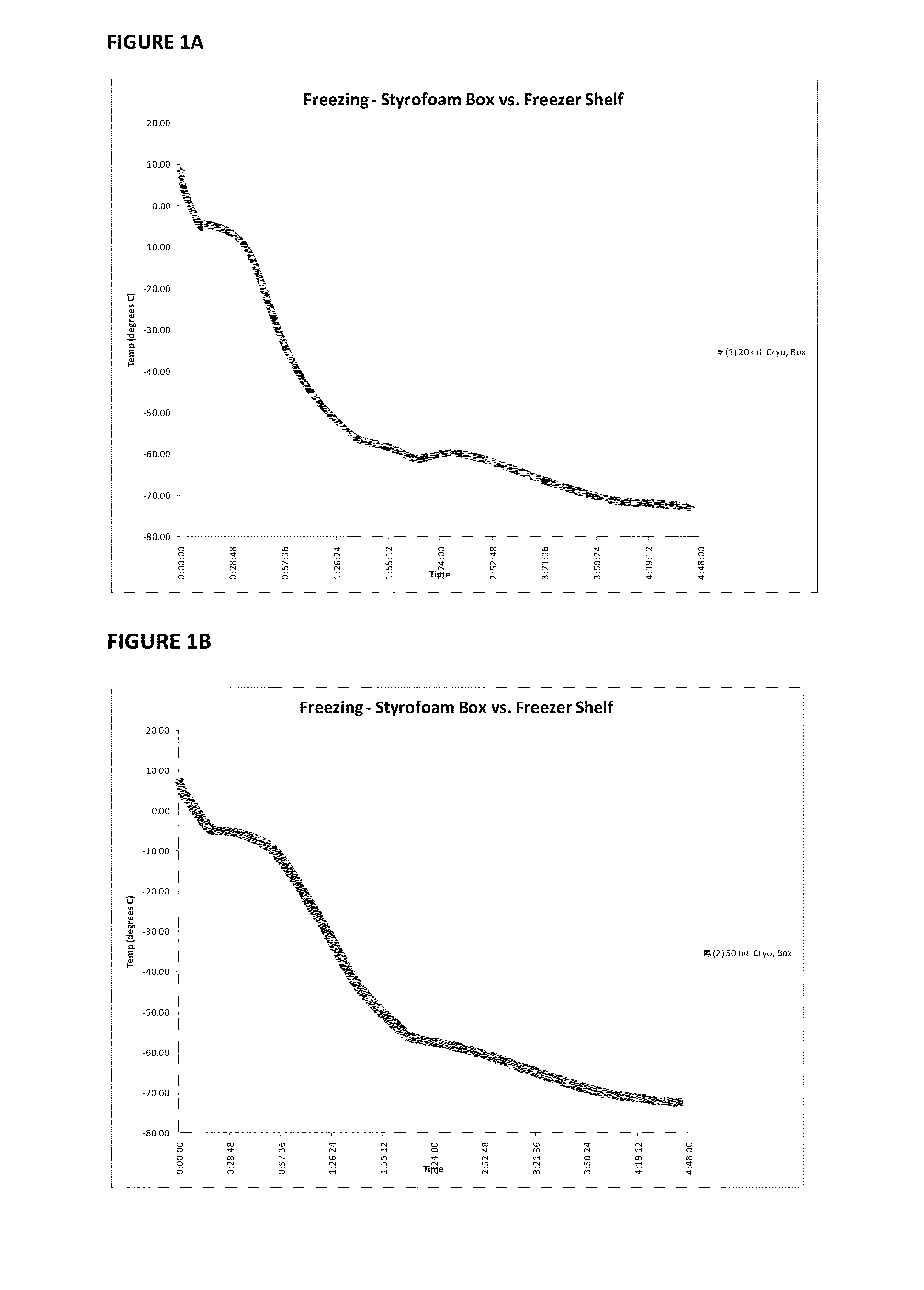
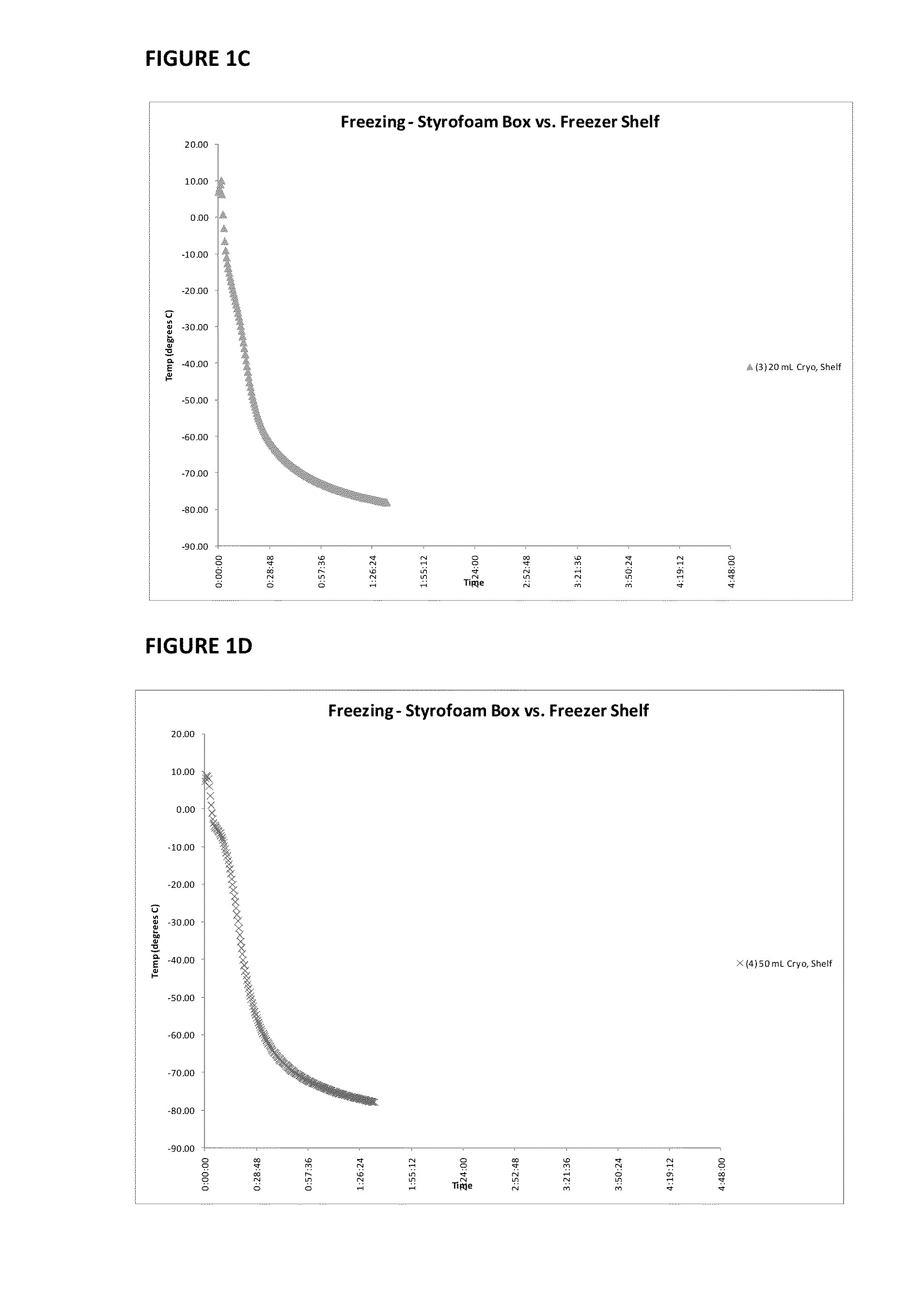
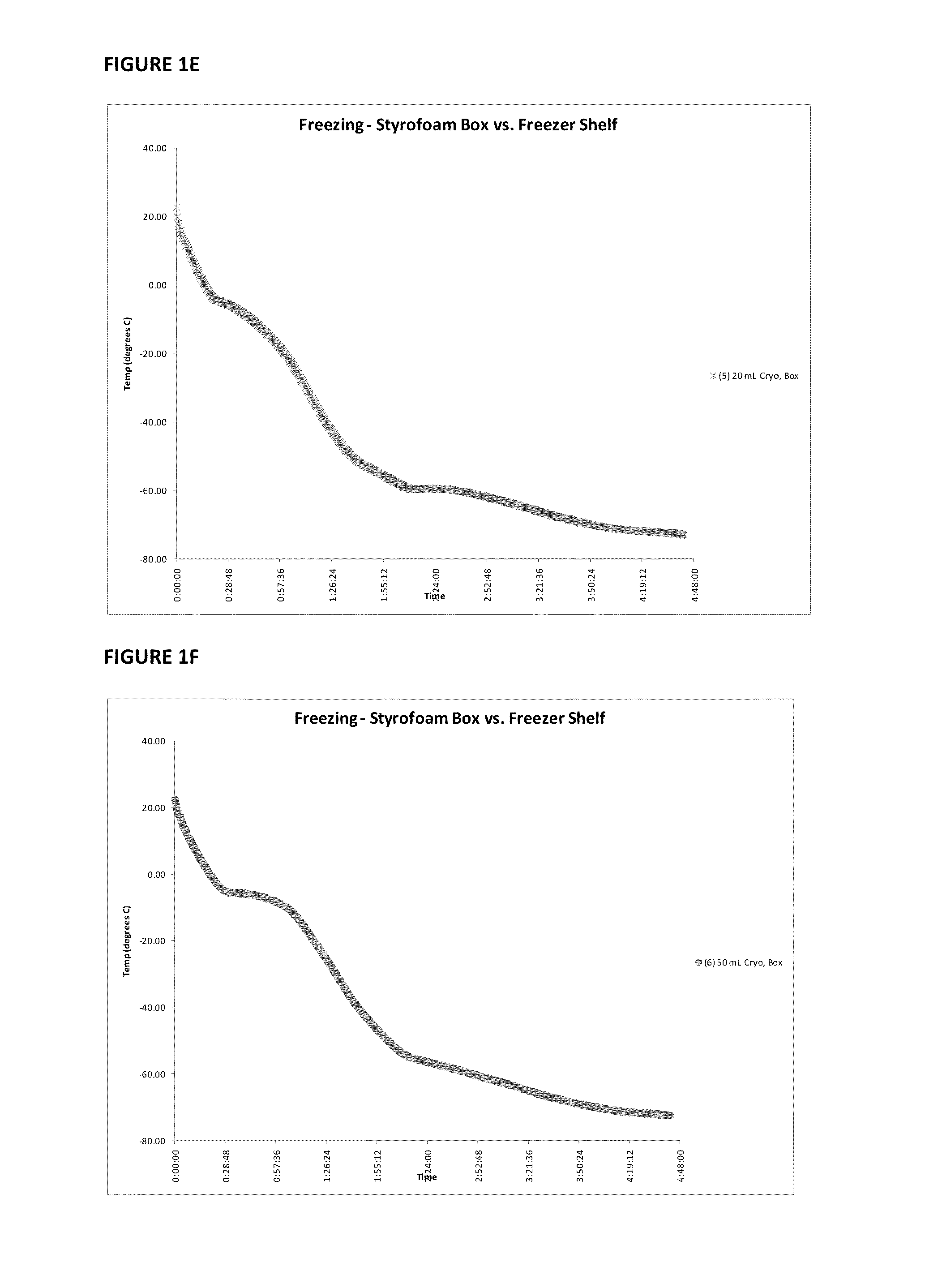
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Placental disc" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
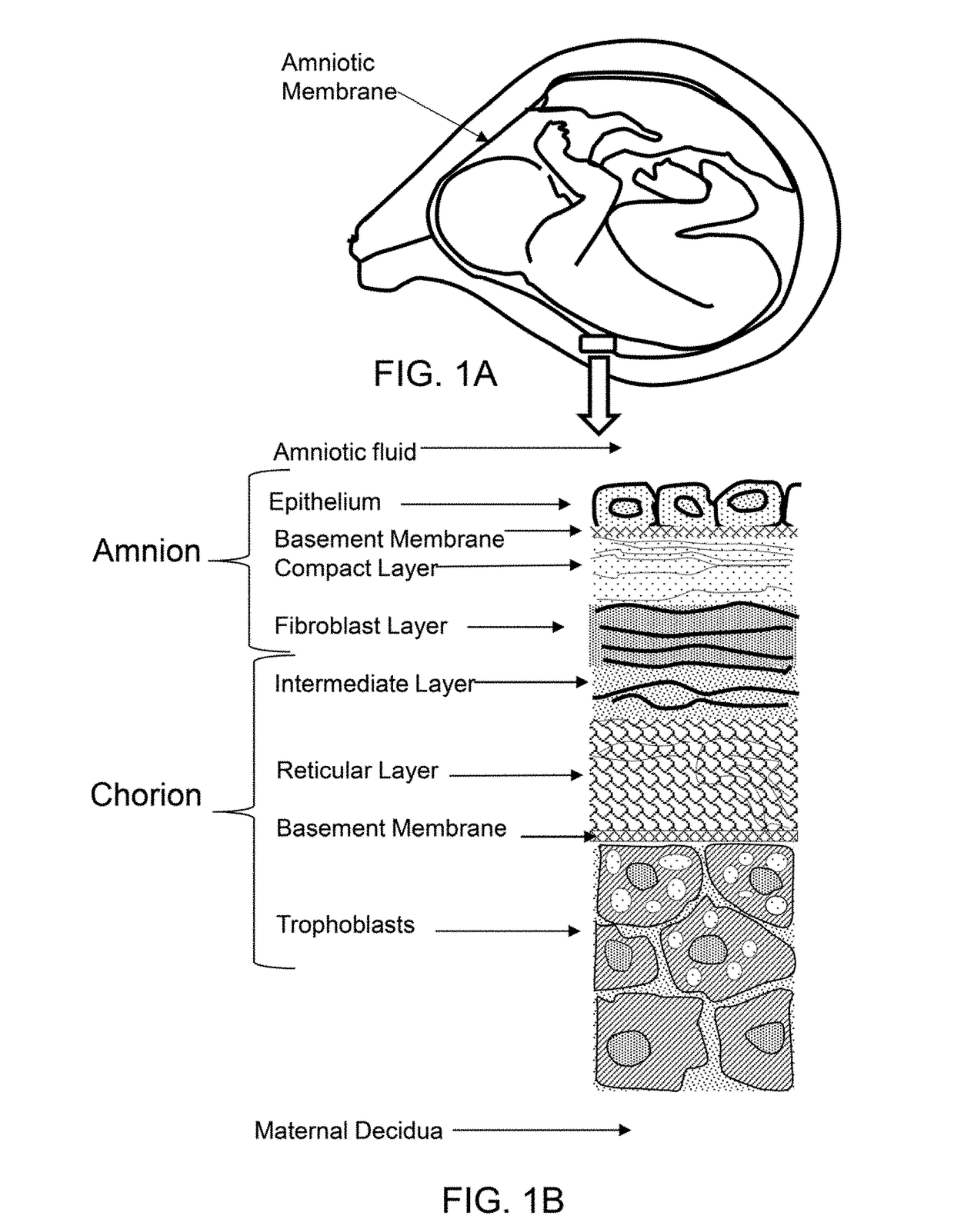
Placenta or placental disc, in mammals, an organ that connects a developing fetus to its mother's uterus Optic disc , in the retina Death-inducing signaling complex
Ocular plug formed from placenta derived collagen biofabric
The present invention relates to ocular plugs formed from a biodegradable material. The plugs comprises a shaft and, optionally, a cap. The ocular plugs are intended to occlude, and to repair, discontinuities in the sclera, whether formed deliberately during injection or surgical foray into the eye, or accidentally. The method further provides methods of making the ocular plug. the invention also provides methods of using the ocular plugs to occlude and repair discontinuities in the sclera, or to deliver biologically active compounds to the sclera or the eye. Finally, the invention provides kits comprising one or more ocular plugs in a container.
Owner:LIU QING +1
Treatment of stroke and other acute neuraldegenerative disorders using postpartum derived cells
InactiveUS20060233765A1BiocideMammal material medical ingredientsPhysiologyNeuro-degenerative disease
Cells derived from postpartum tissue such as the umbilical cord and placenta, and methods for their use to regenerate, repair, and improve neural tissue, and to improve behavior and neurological function in stroke patients are disclosed.
Owner:DEPUY SYNTHES PROD INC
Placental tissue grafts and improved methods of preparing and using the same
Described herein are tissue grafts derived from the placenta. The grafts are composed of at least one layer of amnion tissue where the epithelium layer has been substantially removed in order to expose the basement layer to host cells. By removing the epithelium layer, cells from the host can more readily interact with the cell-adhesion bio-active factors located onto top and within of the basement membrane. Also described herein are methods for making and using the tissue grafts. The laminin structure of amnion tissue is nearly identical to that of native human tissue such as, for example, oral mucosa tissue. This includes high level of laminin-5, a cell adhesion bio-active factor show to bind gingival epithelia-cells, found throughout upper portions of the basement membrane.
Owner:MIMEDX GROUP
Methods of manufacture of immunocompatible amniotic membrane products
InactiveUS20110206776A1Superior wound healing propertyMaintain good propertiesPeptide/protein ingredientsCulture processObstetricsLigament
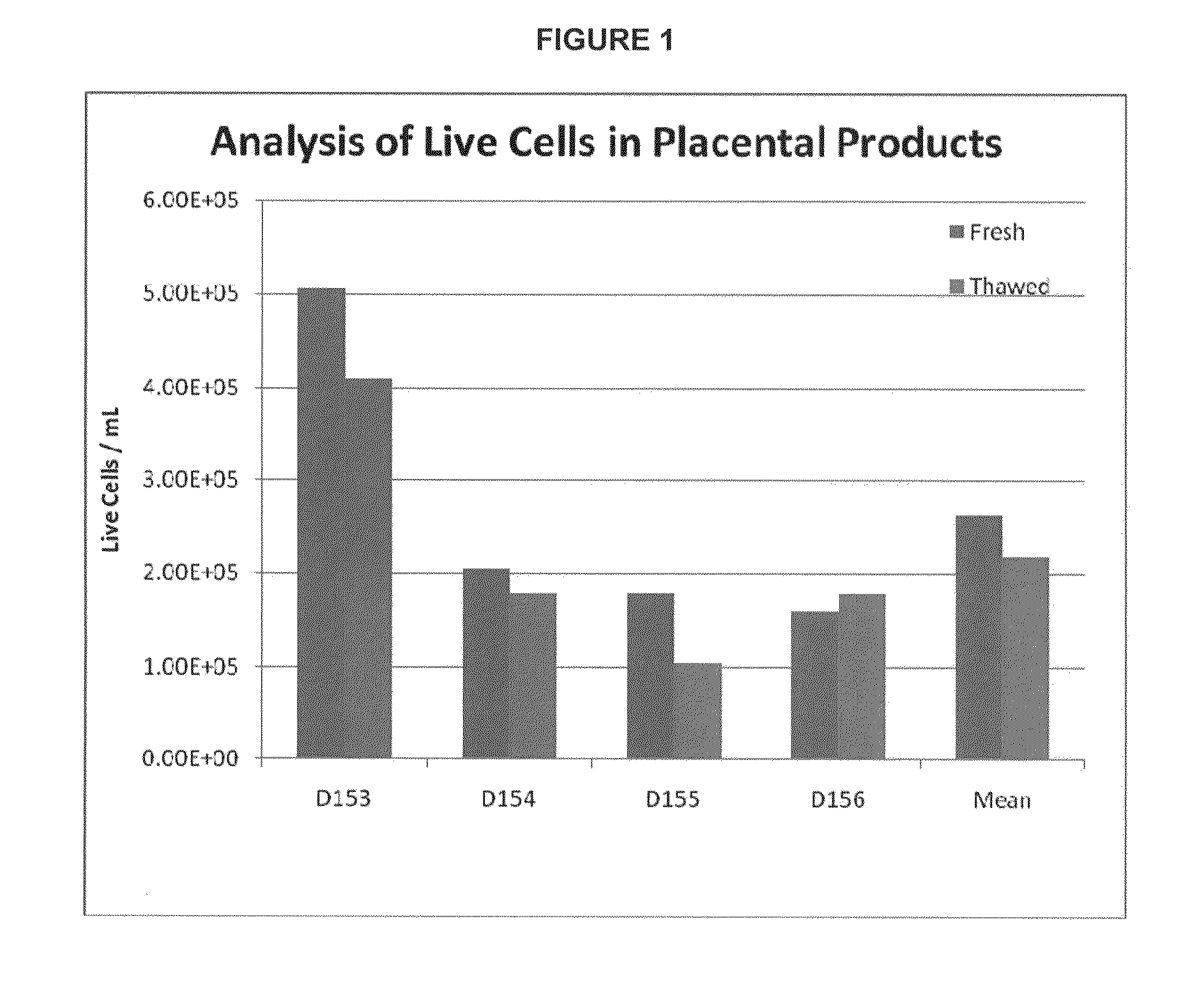
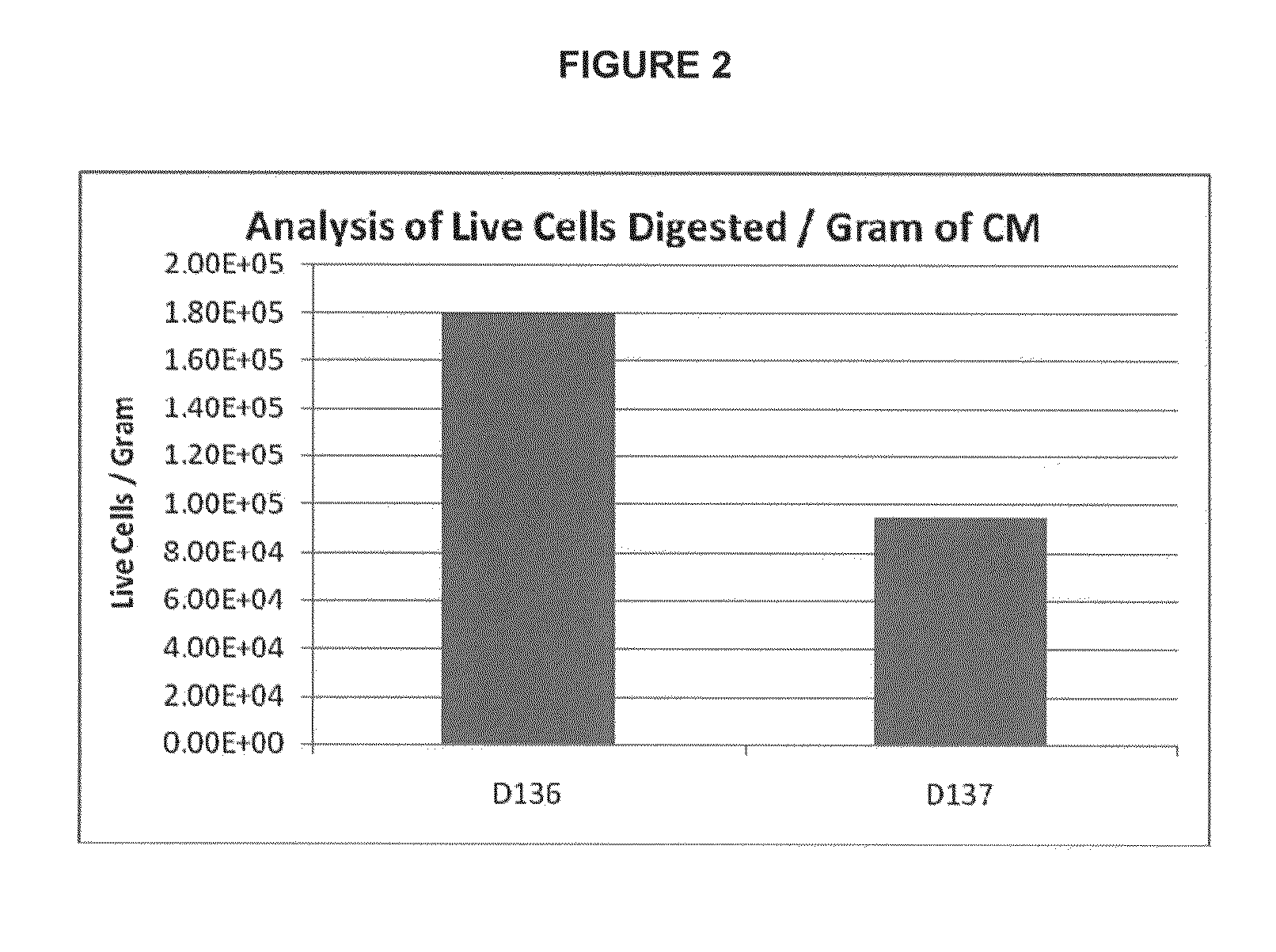
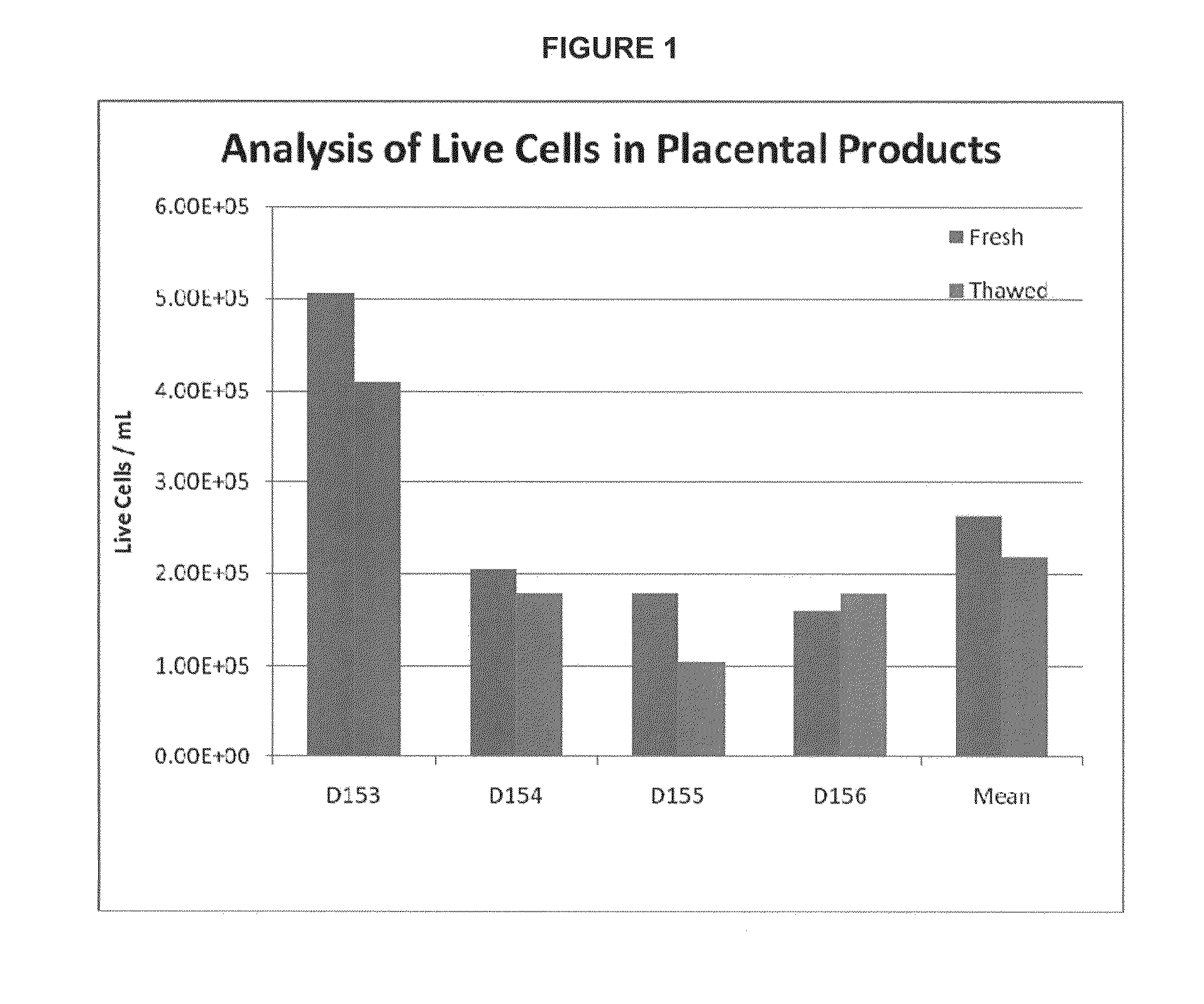
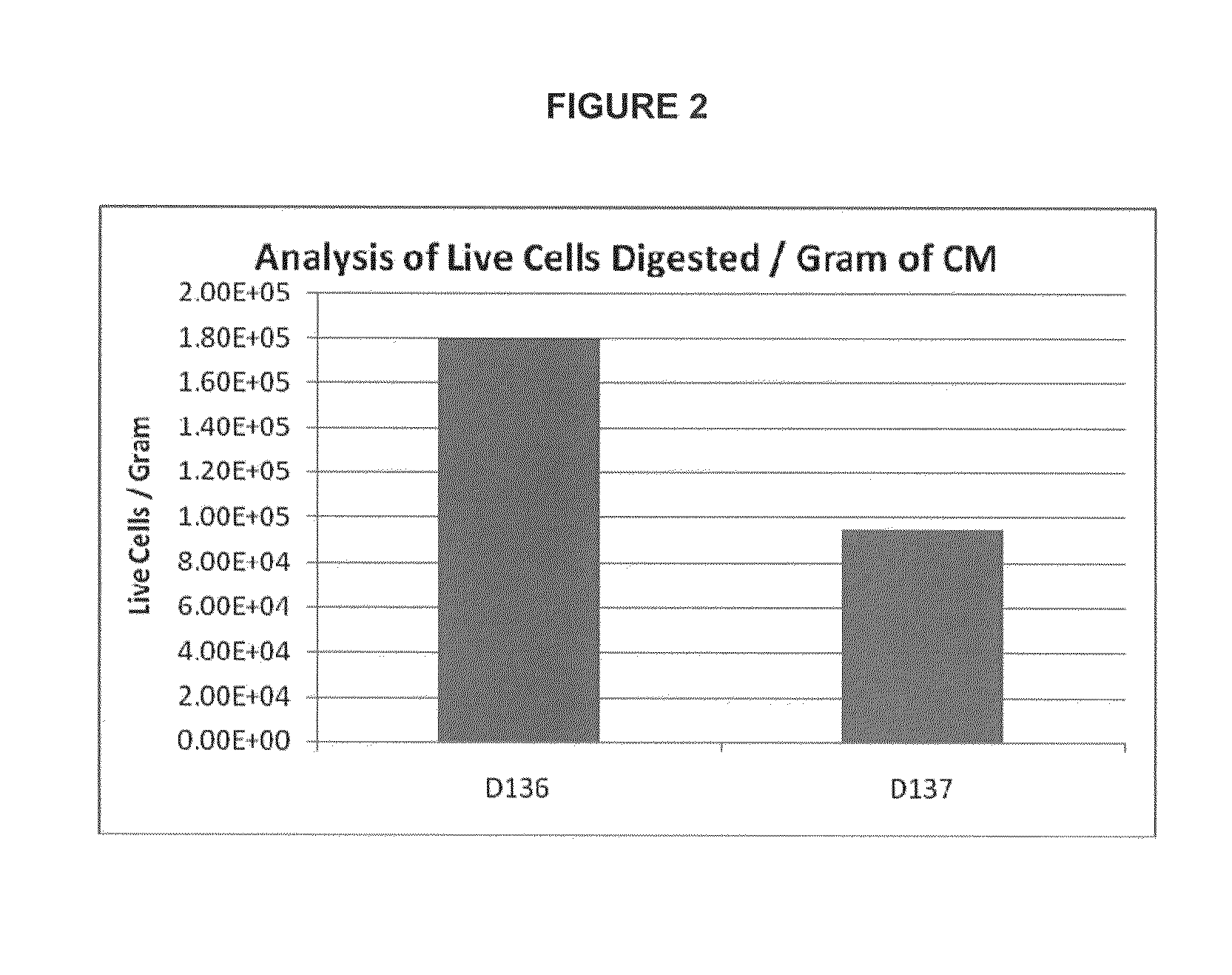
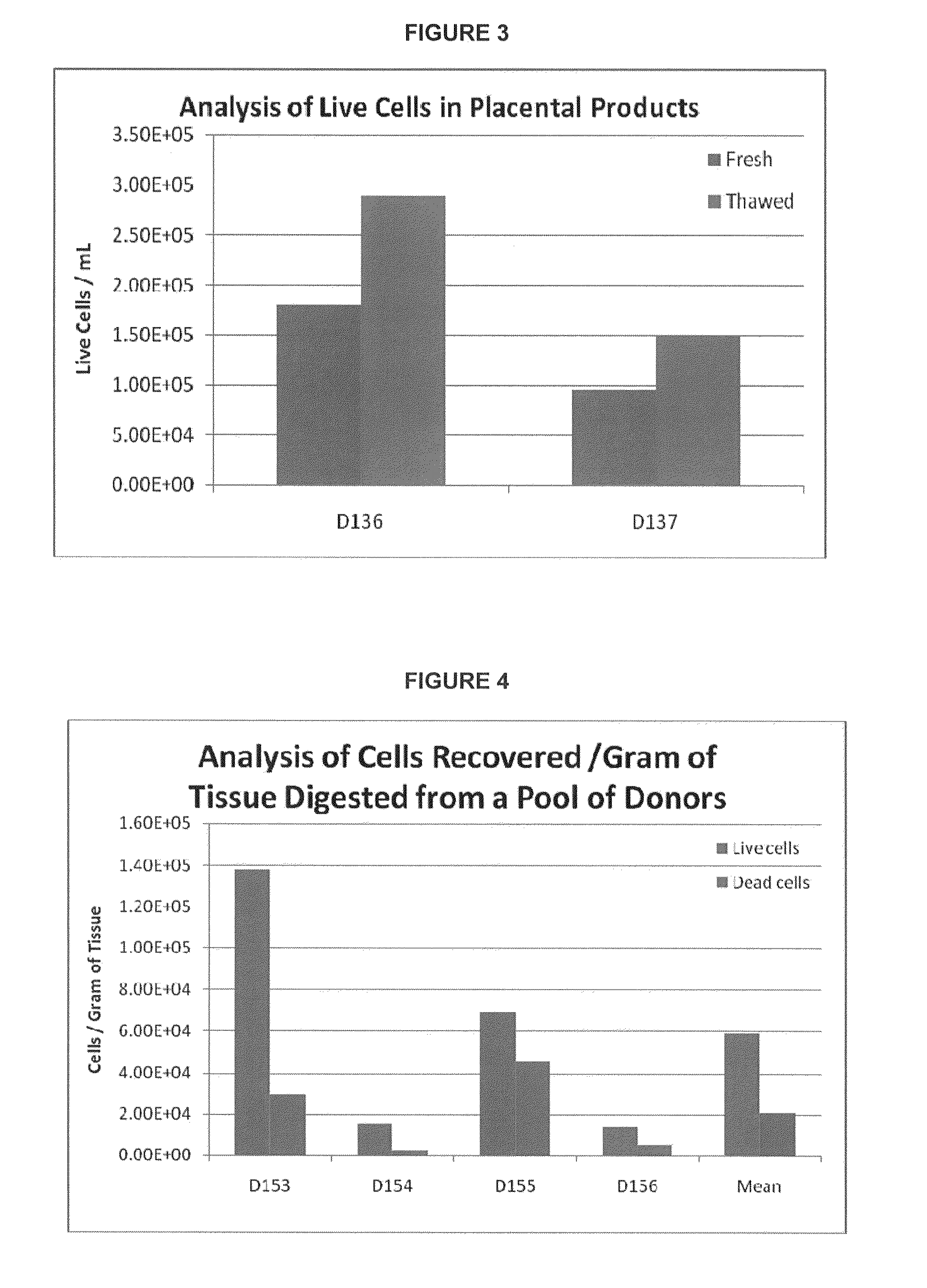
Provided herein is a placental product comprising an immunocompatible amniotic membrane. Such placental products can be cryopreserved and contain viable therapeutic cells after thawing. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
Compositions and methods for inhibiting adverse immune response in histocompatibility-mismatched transplantation
ActiveUS20070264269A1Effective for adverse immune responseAntipyreticAnalgesicsGraft versus host disease inductionCell based
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
Treatment of stroke and other acute neural degenerative disorders using postpartum-derived cells
Owner:DEPUY SYNTHES PROD INC
Immunocompatible chorionic membrane products
InactiveUS20110212158A1Maintain good propertiesBiocidePeptide/protein ingredientsObstetricsLigament structure
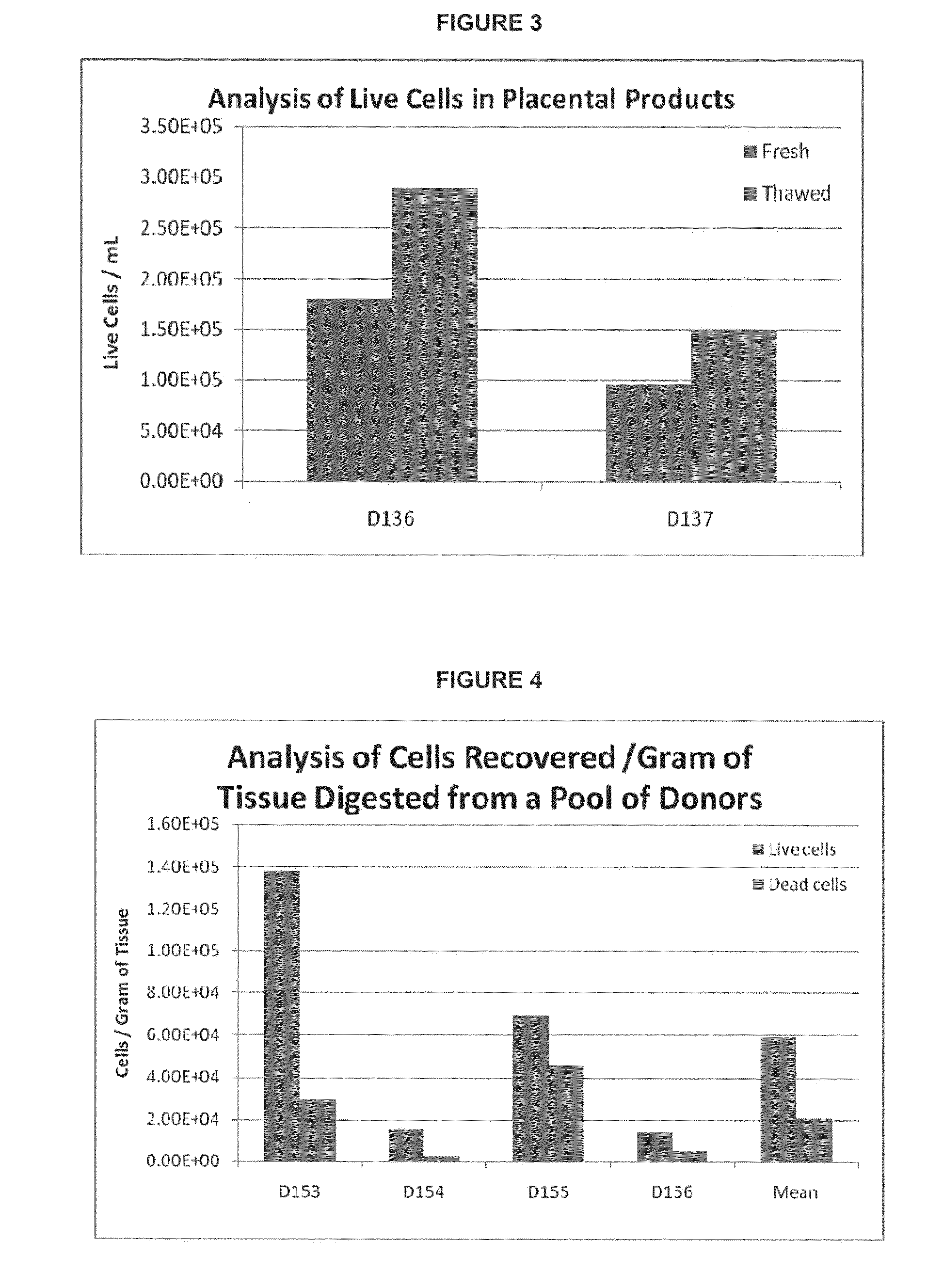
Provided herein is a placental product comprising an immunocompatible chorionic membrane. Such placental products can be cryopreserved and contain viable therapeutic cells after thawing. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
Non-Embryonic Totipotent Blastomere-Like Stem Cells And Methods Therefor
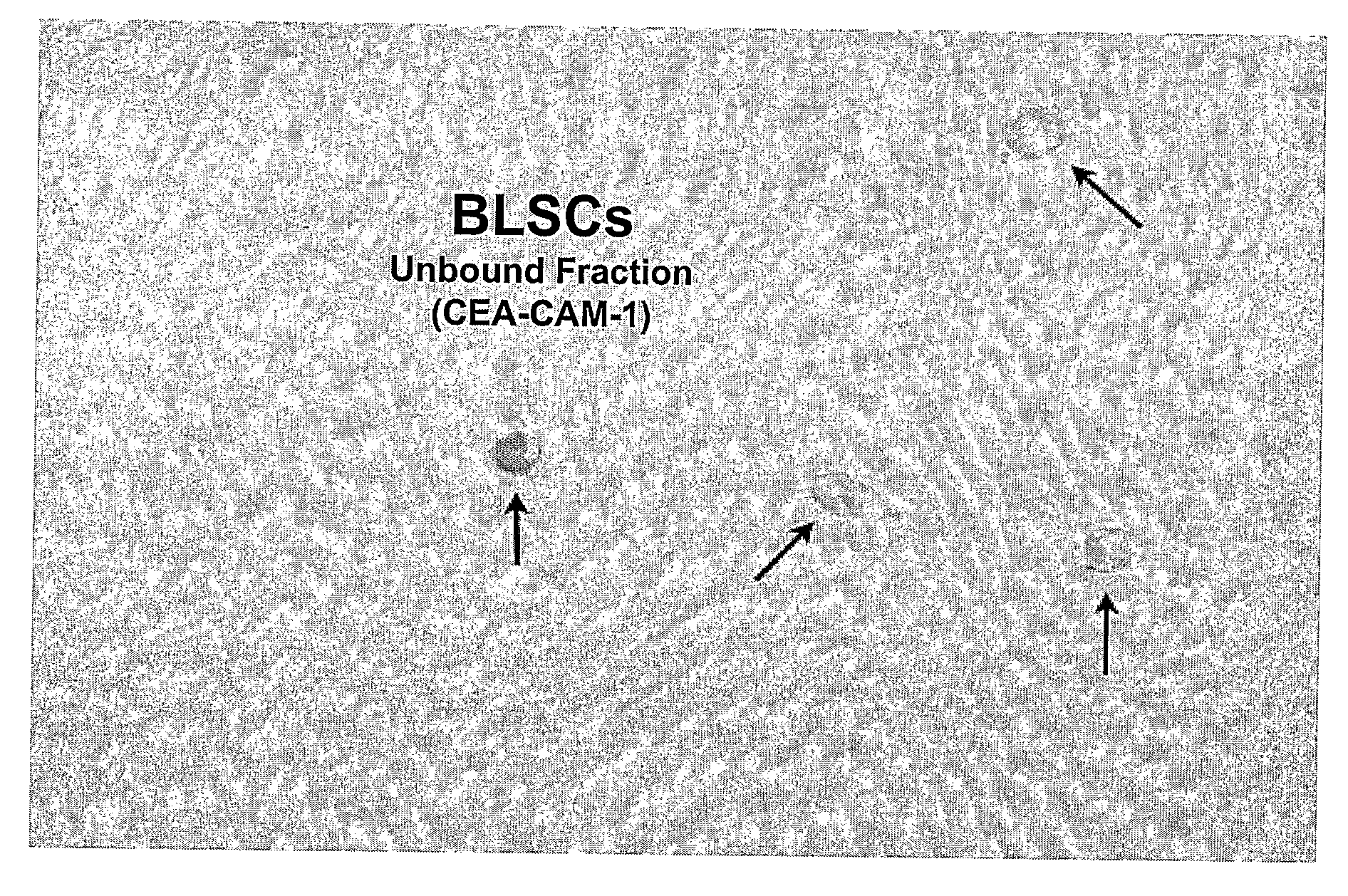
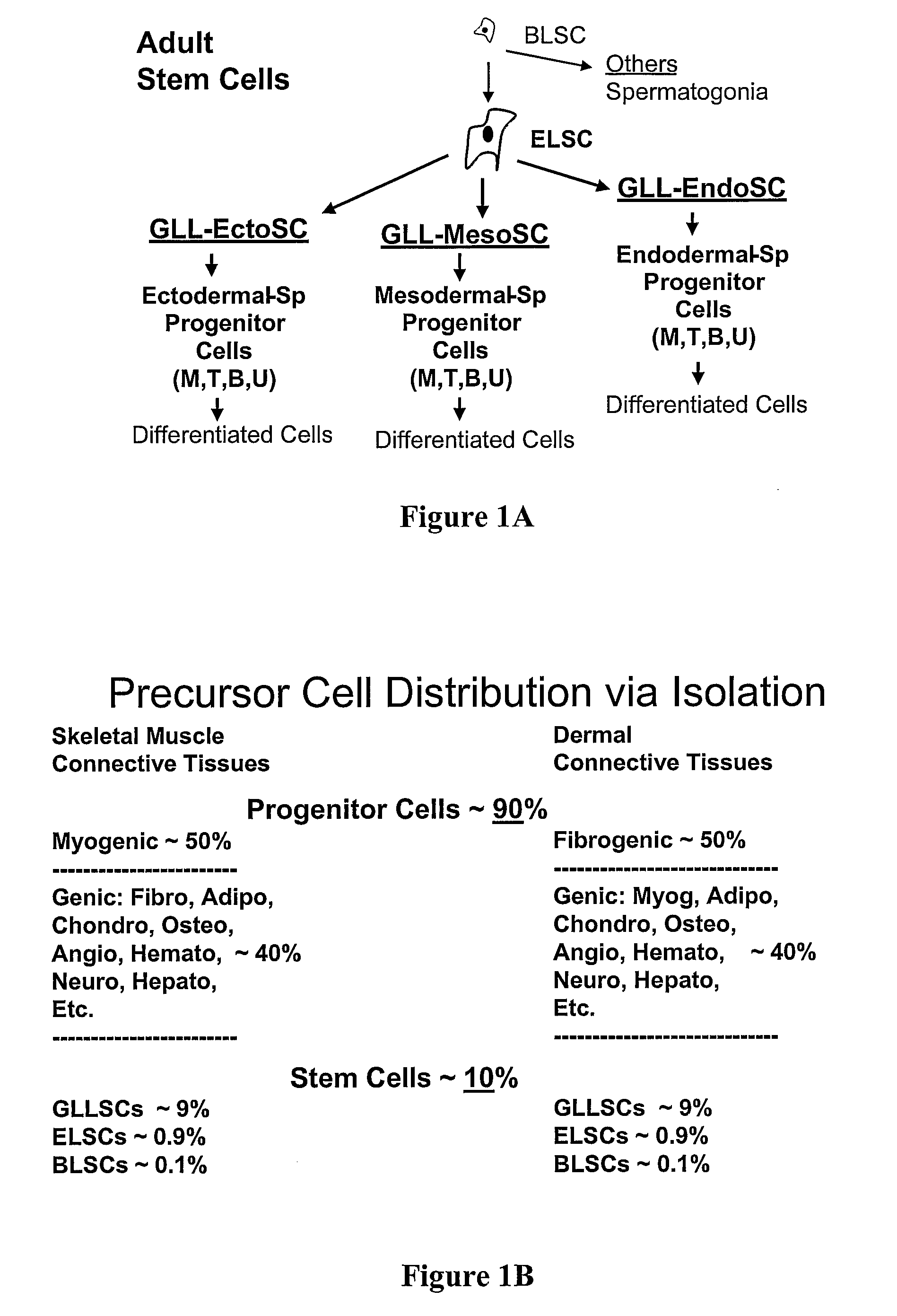
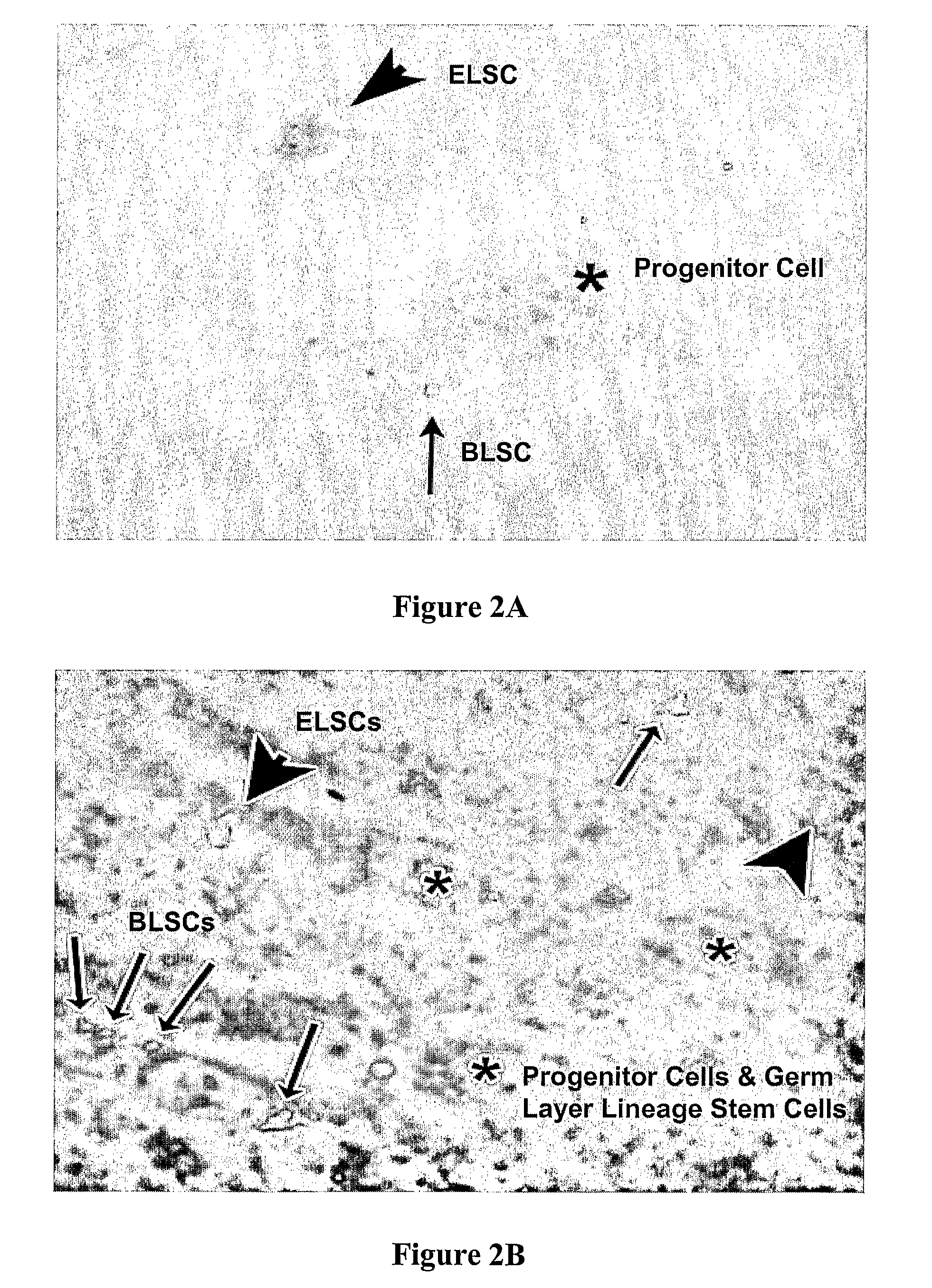
Non-embryonic blastomere-like totipotent stem cells are disclosed. Most preferably, such cells are obtained from various tissues of postnatal mammals (e.g., using tissue biopsied from the mammal), are smaller than 1 μm, have normal karyotype, and do not spontaneously differentiate in serum-free medium without differentiation inhibitors. These non-embryonic blastomere-like totipotent stem cells typically express CD66e, CEA-CAM-1 and telomerase, but do not typically express CD10, SSEA-1, SSEA-3, and SSEA-4. Such blastomere-like totipotent cells can be differentiated into ectodermal, mesodermal, or endodermal tissues, including placental tissues and germ cells. Moreover, when implanted into a mammal, such cells will not be teratogenic.
Owner:MORAGA BIOTECH CORP
Immunocompatible chorionic membrane products
InactiveUS20150010609A1Prevent and reduce scarPrevent and reduce and contracture formationBiocidePeptide/protein ingredientsObstetricsDamages tissue
Provided herein is a placental membrane product comprising an immunocompatible chorionic membrane. Such placental membrane products can be cryopreserved and contain therapeutic factors and viable cells after thawing. The placental membrane products are useful in wound healing or tissue repair / regeneration as they are capable of promoting angiogenesis, reducing inflammation, reducing scar formation, and other methods that promote healing. The present technology relates to products to protect injured or damaged tissue, or as a covering to exclude bacteria, to inhibit bacterial activity, or to promote healing or growth of tissue. The field also relates to methods of manufacturing and methods of use of such membrane-derived products.
Owner:OSIRIS THERAPEUTICS
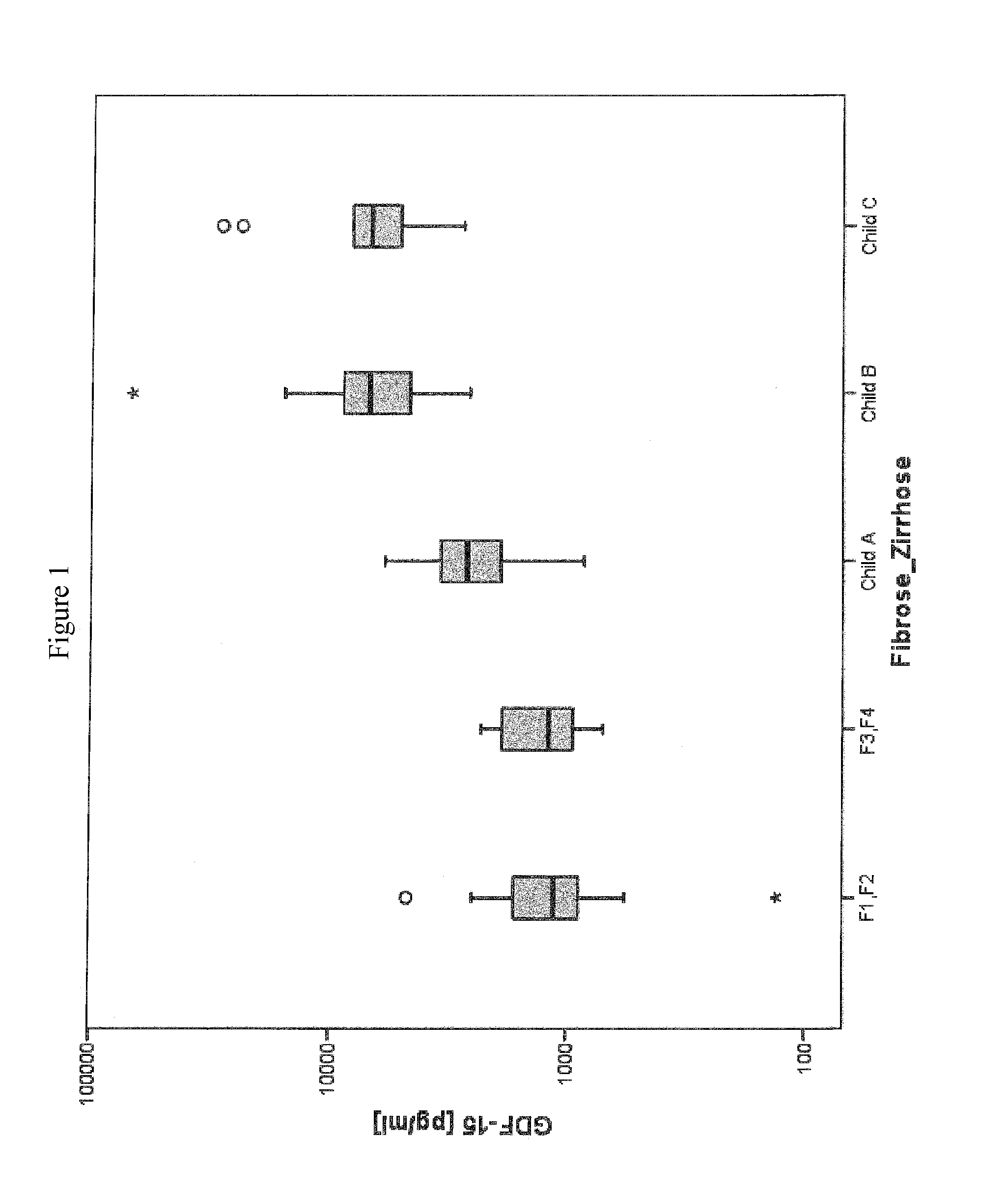
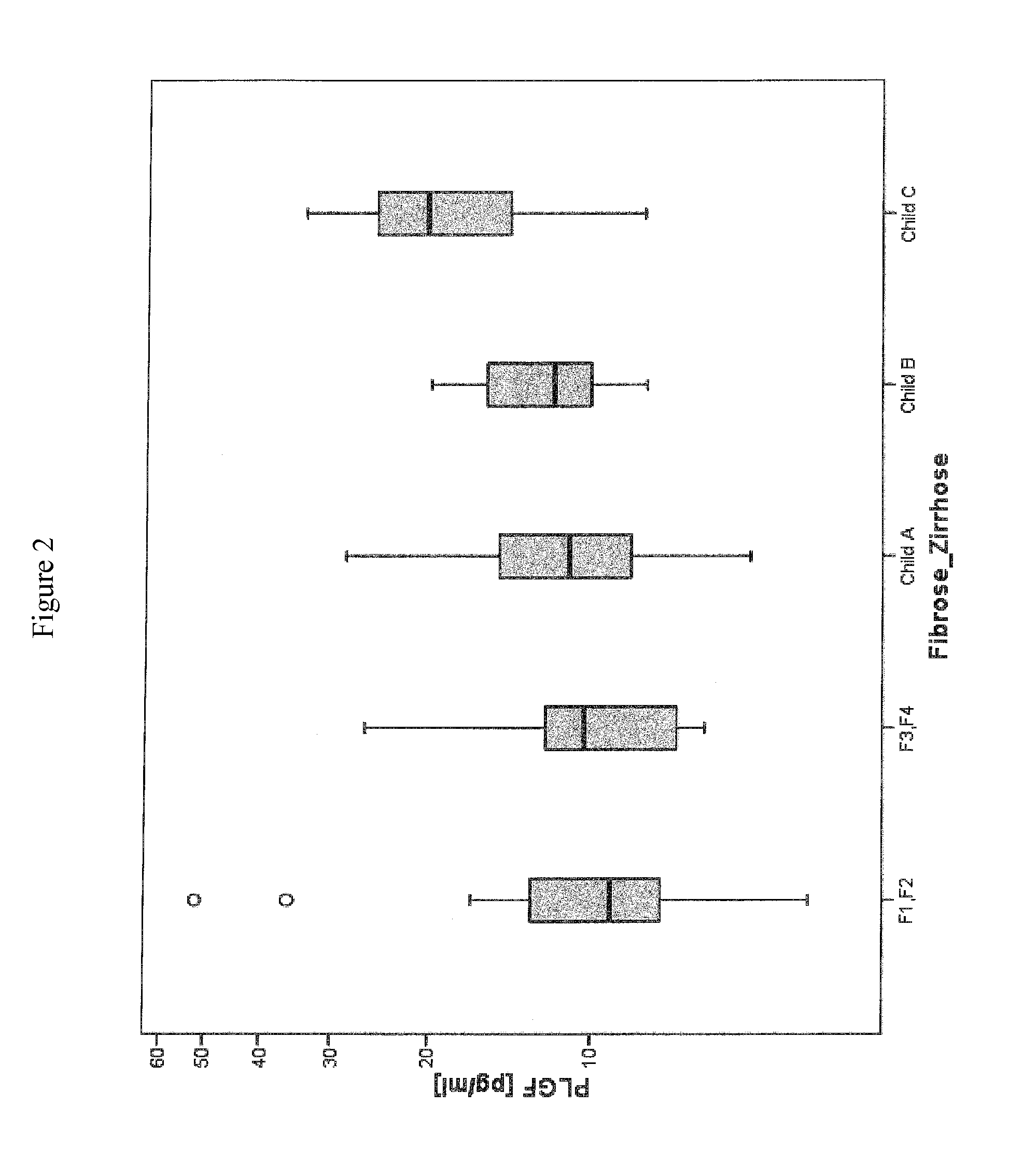
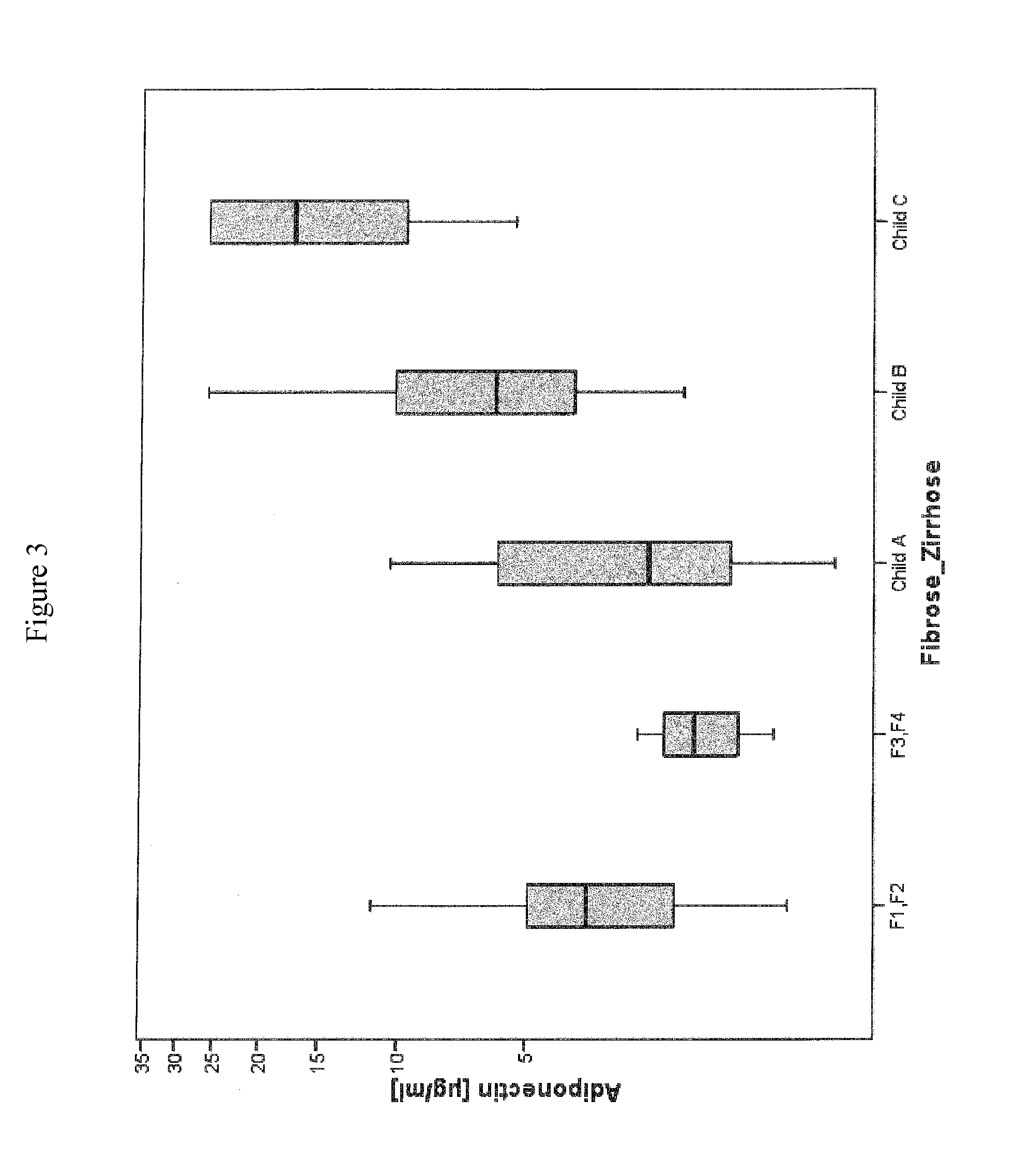
Method for assessment of severity of liver cirrhosis
InactiveUS20110257022A1Peptide librariesLibrary screeningHepatocyte growth factorLiver transplantation
Disclosed is a method for diagnosing whether a subject suffers from a mild or severe form of liver cirrhosis based on determining the amount of GDF-15 (growth differentiation factor 15), PlGF (placental growth factor), and / or hepatocyte growth factor (HGF) in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts). The method may further include determining the amount of adiponectin in a sample from the subject, and comparing the amount to a reference amount for adiponectin. Also described is a method to identifying a subject being susceptible to liver transplantation including determining the amount of GDF-15, PlGF, and / or HGF in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts).
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Methods of manufacture of therapeutic products comprising vitalized placental dispersions
Owner:OSIRIS THERAPEUTICS
Therapeutic products comprising vitalized placental dispersions
This invention provides a fluid therapeutic placental product comprising placental cells and a placental dispersion comprising placental factors. The placental cells and the placental dispersion are derived from placental tissue. A placental tissue can optionally be an amnion, chorion, or a trophoblast-depleted chorion. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
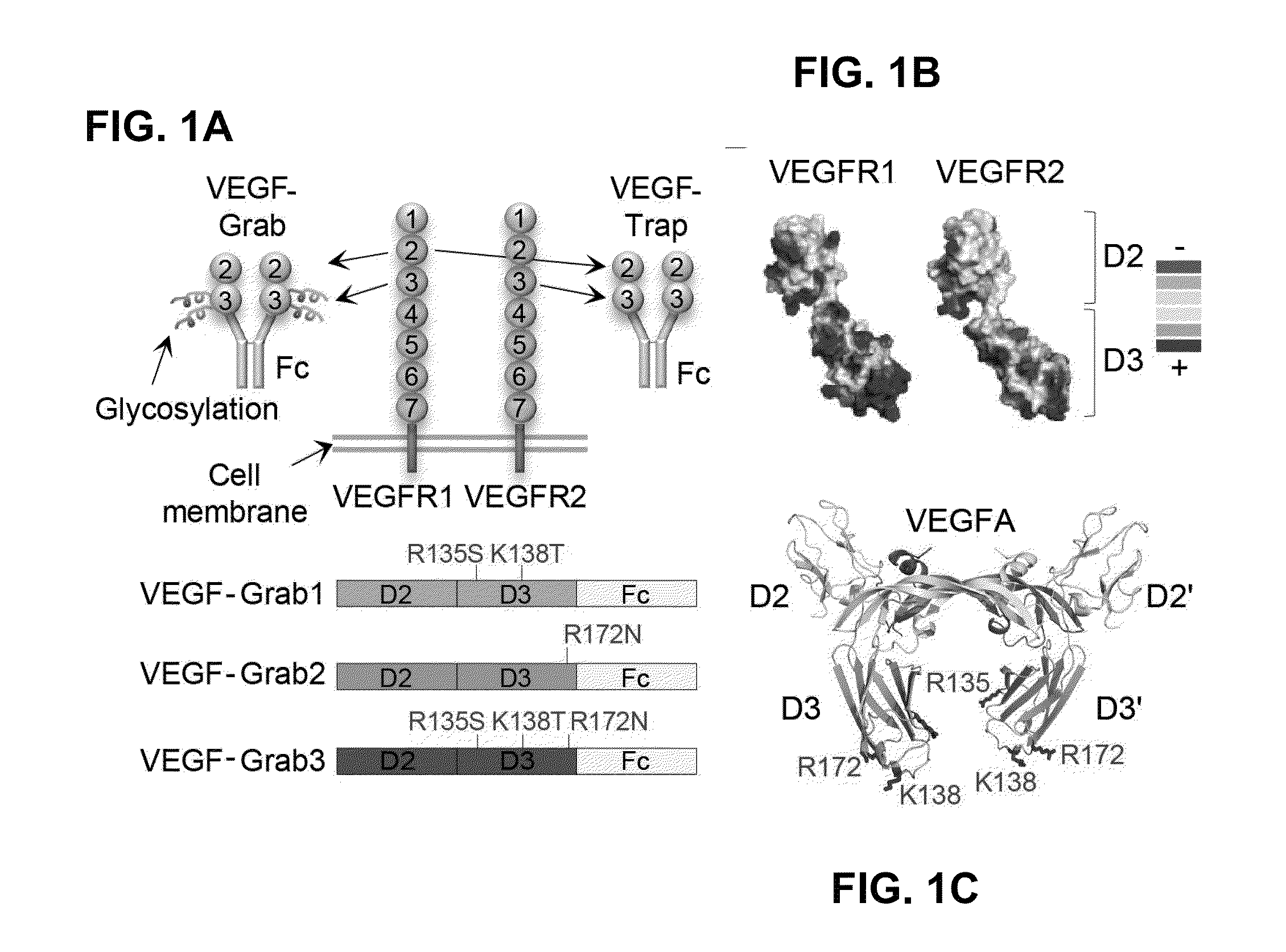
Method of treating eye disease using glycosylated VEGF decoy receptor fusion protein
ActiveUS20160024483A1Improved pharmacokinetic profileStrong and durable anti-angiogenicSenses disorderPeptide/protein ingredientsDecoy receptorsNucleotide
The present application describes an isolated nucleic acid molecule encoding a polypeptide capable of synchronously binding VEGF polypeptide and placenta growth factor (PIGF) polypeptide comprising a nucleotide sequence encoding a VEGFR1 component.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Placental villus plate mesenchymal stem cells and extraction method thereof
InactiveCN106085952APrevent intrusionReduce usageCell dissociation methodsSkeletal/connective tissue cellsFiltrationAntibiotic Y
The invention discloses placental villus plate mesenchymal stem cells. An extraction method of the placental villus plate mesenchymal stem cells includes the following steps that 1, a placental villus plate is obtained; 2, blood vessels and villus tissue are removed; 3, the villus plate is cleaned till the blood color disappears; 4, the villus plate is sheared into 0.5-5 mm<2> tissue microblocks, and the tissue microblocks are cleaned; 5, tissue digestive juice containing pancreatin, collagenase II, collagenase IV and hyaluronidase is added for digestion; 6, filtration and centrifugation are carried out; 7, culture is carried out; 8, cell digestion is carried out with 0.25% pancreatin when the cell confluence reaches 70-90%; 9, subculturing is carried out; 10, detection is carried out; 11, cryopreservation is carried out; 12, database building is carried out. According to the method, the steps are simple, and a large number of mesenchymal stem cells can be rapidly obtained from the placental tissue; the separation process does not need too much cleaning operation; the use of antibiotics is omitted, so that the purity of the placental villus plate mesenchymal stem cells obtained after separation is high.
Owner:四川华皓生物科技有限公司
Dressing including dehydrated placental tissue for wound healing
A dressing for wound healing is provided herein including dehydrated placental tissue, collagen, and oxidized regenerated cellulose. The dehydrated placental tissue may be present in a first layer and the collagen and the oxidized regenerated cellulose may be combined into a second layer. The dehydrated placental tissue may comprise amniotic membrane tissue, chorion tissue, or a combination thereof. The second layer including the collagen and the oxidized regenerated cellulose may comprise about 50% to about 60% collagen by weight and about 40% to about 50% ORC by weight.
Owner:SYSTAGENIX WOUND MANAGEMENT (US) INC
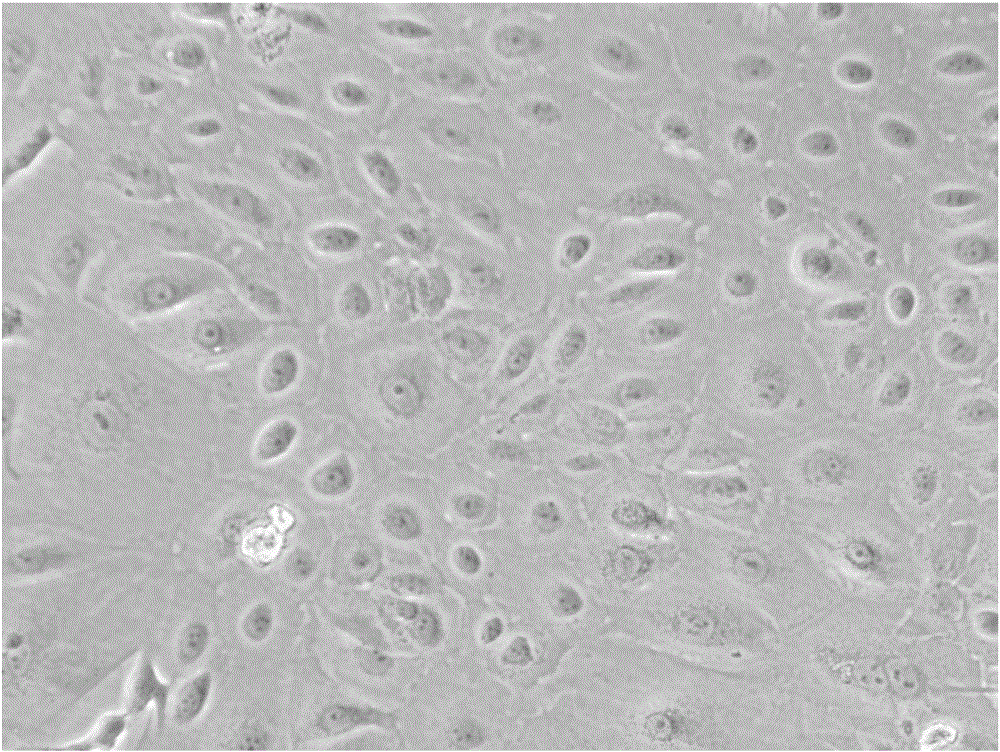
Method for preparing human amniotic membrane epithelial cells from human placenta amnion and application thereof
ActiveCN106520676APromote proliferationHigh purityCulture processMammal material medical ingredientsForcepsDigestion
The invention relates to a method for preparing human amniotic membrane epithelial cells from human placenta amnion and application thereof, in particular to the method for preparing human amniotic membrane epithelial cells from the placenta amnion. The method comprises the following steps that the surface of a placenta is flushed with a basic balanced salt solution repeatedly and the placenta is sterilized; a foetal membrane outer layer amnion is torn and taken into a glass dish by surgical forceps, the grimy blood on the surface of the foetal membrane outer layer amnion is flushed with the basic balanced salt solution, and then the foetal membrane outer layer amnion is sheared into small blocks; the small amnion blocks are evenly attached to a culture dish, the epithelial layer of the amnion faces downwards, and after drying, a complete medium is added for culture; fluid infusion is conducted two days later, and then culture is continued; when epithelial cells are seen to crawl out, a tissue is removed, and medium change is conducted to continue culture; after multiple typical epithelial cell masses occur, the local fusion rate reaches 90%, and continuous cell culture is conducted; and harvesting is conducted, specifically, digestion is conducted by pancreatic enzymes, counting and freezing storage are conducted, and the human amniotic membrane epithelial cells are obtained. The invention further relates to the prepared human amniotic membrane epithelial cells and the application thereof. The method has the advantages showing in the specifications.
Owner:BOYALIFE
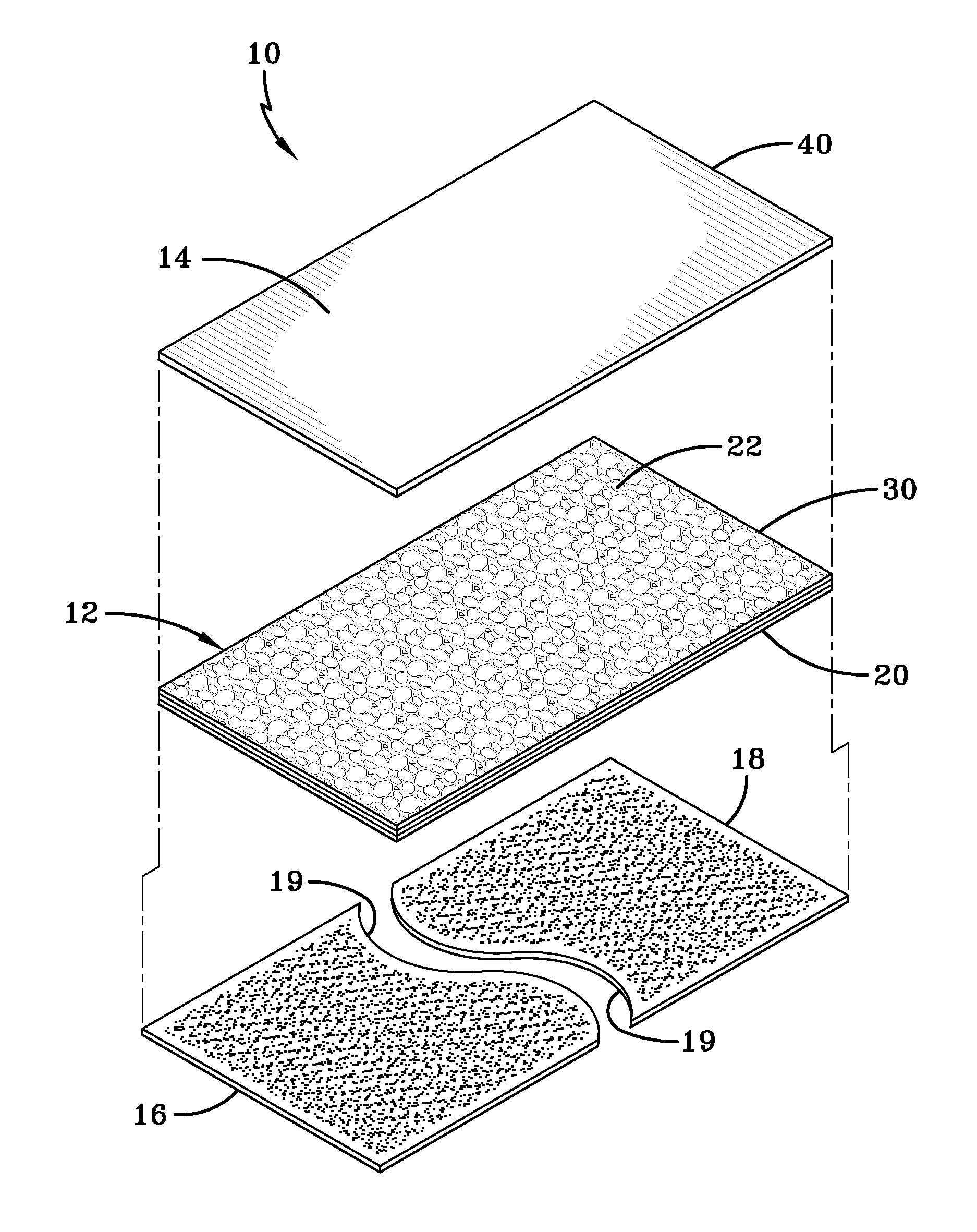
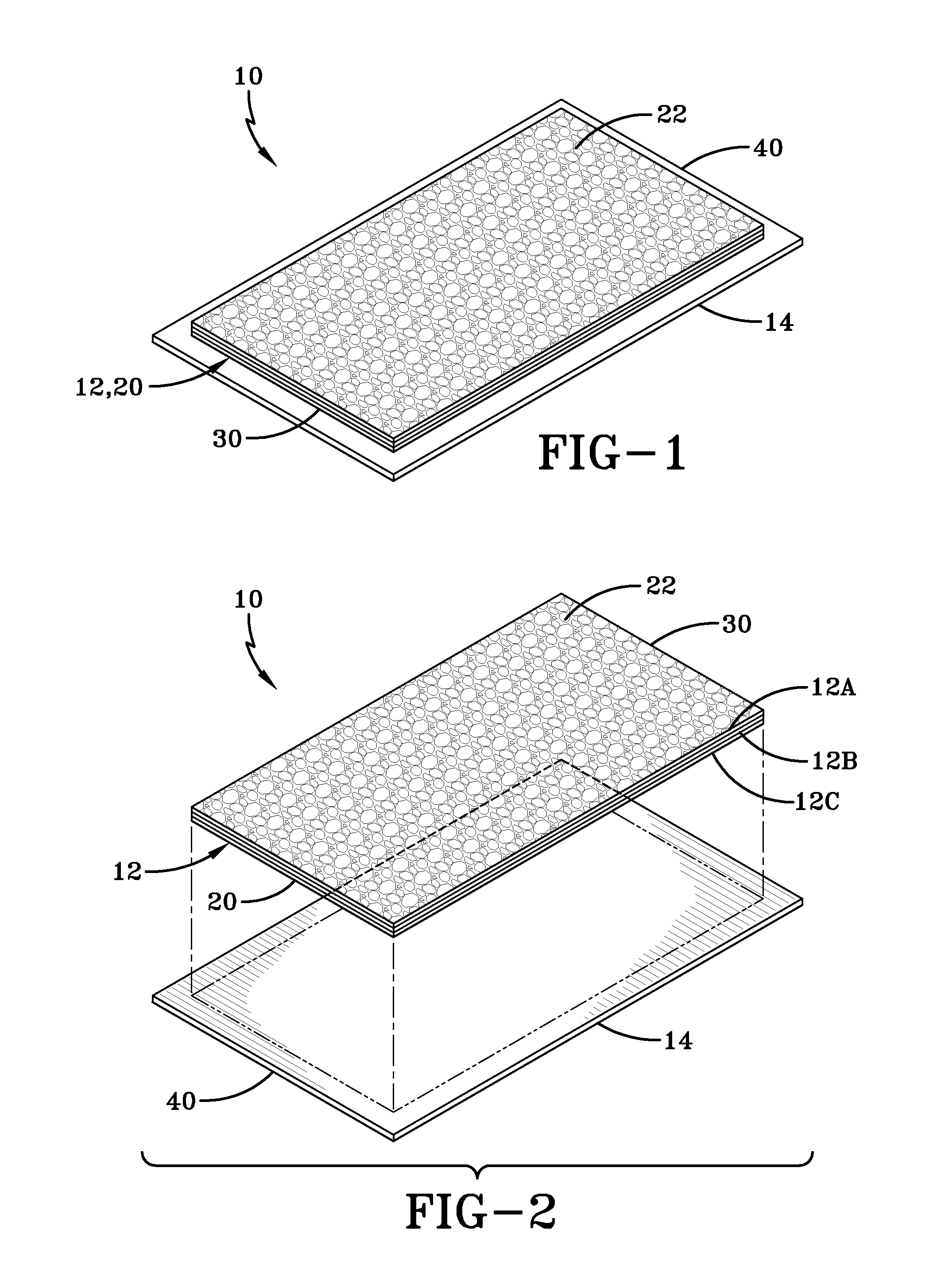
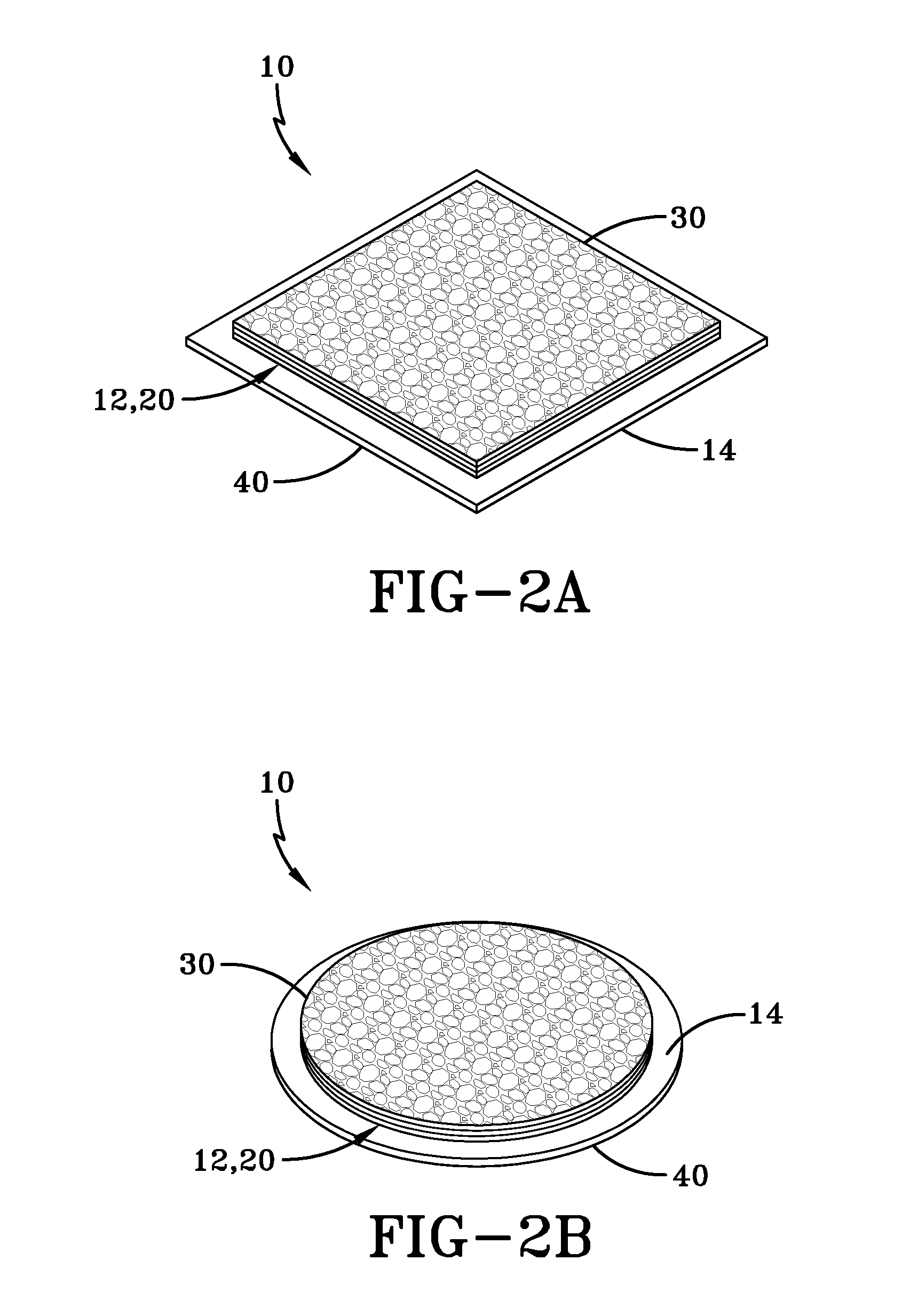
Placental tissue assembly
A placental tissue assembly for treating wounds or surfaces of a patient has one or more layers of placental tissue and a backing material. The one or more layers of placental tissue have a first side and a second side. The backing material is adhered to and covers one of either the first or second sides of the one or more layers of placental tissue to create the assembly. The assembly when applied onto a surface to be treated on the side of the tissue opposite the backing material adheres to the surface with an attachment force greater than an attachment force of the backing material adhered to the first or second side. This allows the backing material to be released leaving the placental tissue affixed to the surface to be treated.
Owner:VIVEX BIOLOGICS GRP INC
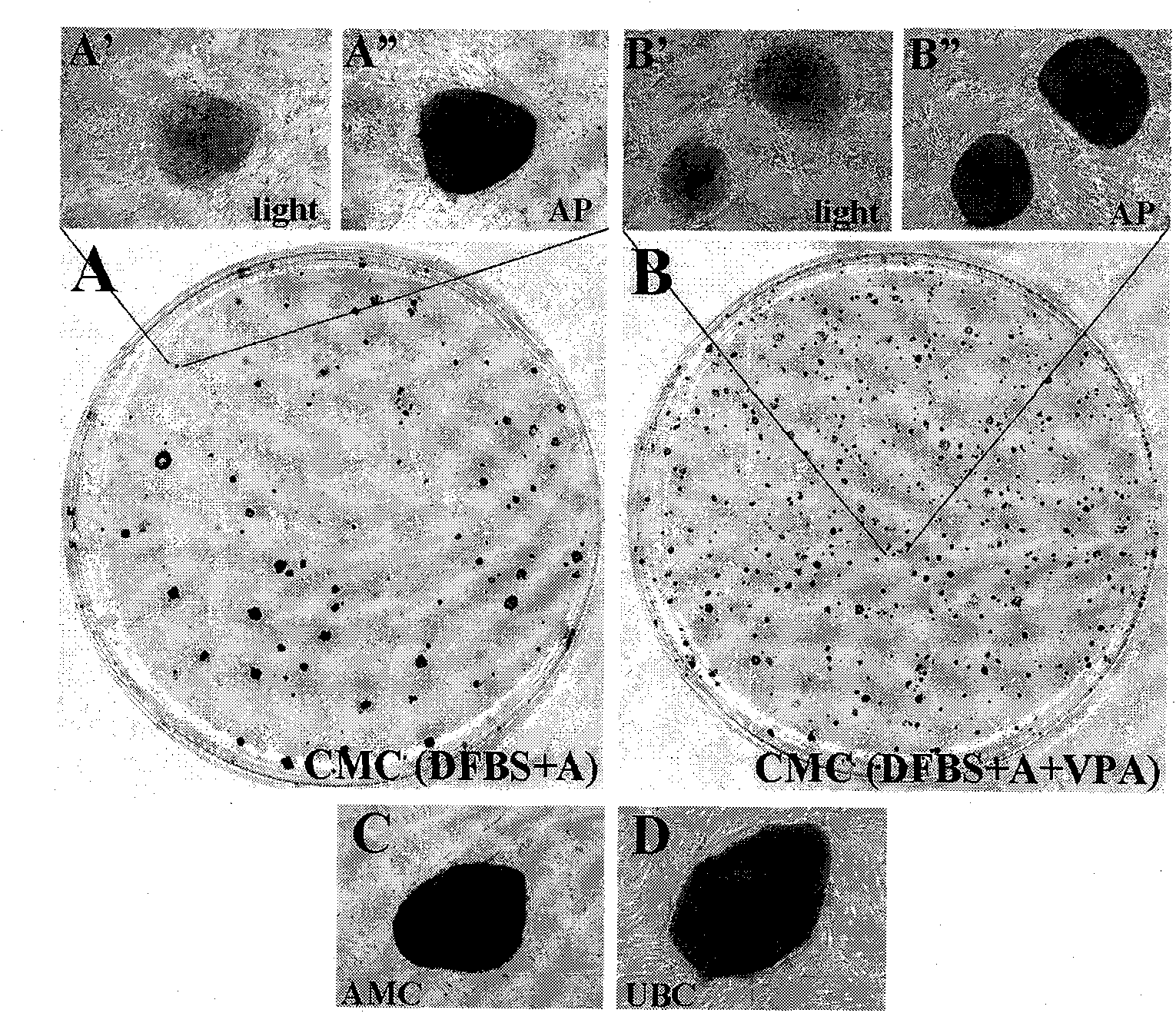
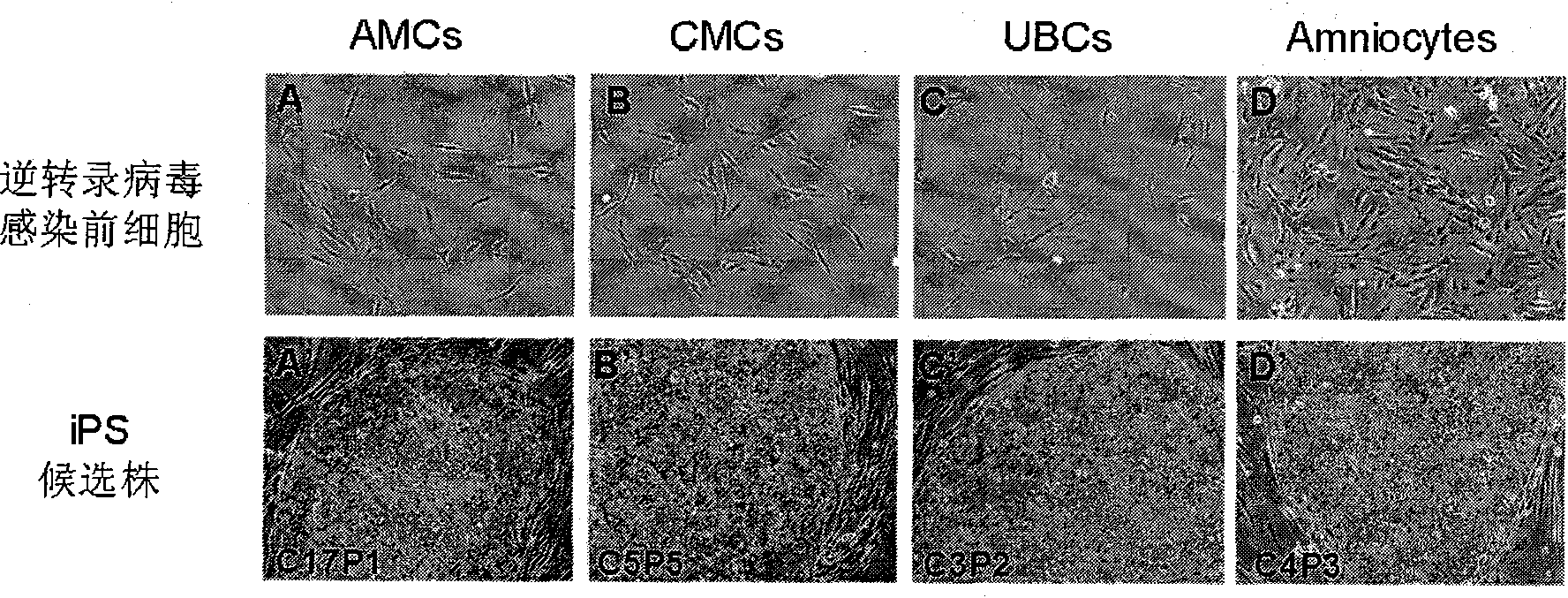
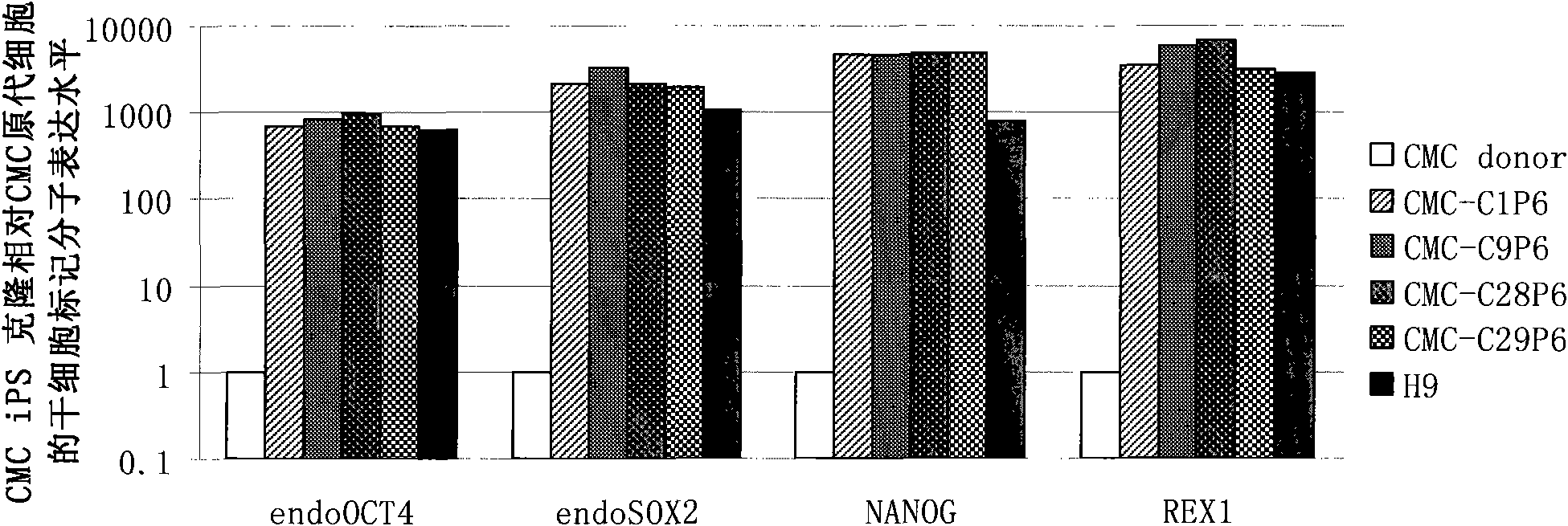
Cell type used for producing induced pluripotent stem (iPS) cells and preparation method and application thereof
InactiveCN101984050AGreat therapeutic application prospectsGood pluripotencyFermentationVector-based foreign material introductionControl orientedDisease
The invention discloses four cell types which have wide heteroplastic transplantation application prospect and can be effectively induced into induced pluripotent stem (iPS) cells. The origins of three cell types are placental tissue, namely amnion mesenchymal cell, chorion mesenchymal cell and umbilical cord mesenchymal cell; and the origin of the other one is amniotic fluid cell. Besides, the invention also discloses an induction reprogramming method for producing cells capable of producing induced pluripotent stem (iPS) cells by efficient induction, including the following steps: cDNA containing pluripotent stem cell factor is respectively introduced into the four primary culture cells; the four primary cells in which cDNA is introduced are respectively cultured on appropriate culture mediums, primary iPS is obtained and then quality of iPS is optimized on appropriate culture mediums, and cloning of pluripotent stem cell can be primarily evaluated. In the invention, three mesenchymal cells of placenta origin and amniotic fluid cell all can be induced into iPS, wherein the chorion mesenchymal cell is compared with fibroblast, efficiency of induction reprogramming is improved by over 100 times, efficiency is about 2.3%, and the efficiency is slightly higher than that of horny cell; and a means which is more effective and more pertinent is provided for building disease model, screening drug and controlling oriented differentiation.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Method of treatment utilizing an acellular amnion derived therapeutic composition
ActiveUS9814746B2Reduce in quantityHigh cure rateCosmetic preparationsAerosol deliveryMedicineAmniotic fluid
Acellular amnion derived therapeutic compositions are described having a number of various compositional embodiments. An acellular amnion derived therapeutic composition has essentially no live or active amniotic cells. The amniotic cells may be destroyed, and the cells and cell debris may be removed from the acellular amnion derived therapeutic composition. An acellular amnion derived therapeutic composition may comprise micronized placental tissue particles, and / or amniotic fluid. An acellular amnion derived therapeutic composition may be a dispersion of micronized amniotic membrane combined with a fluid, such as plasma, saline, amniotic fluid, combinations thereof and the like. An acellular amnion derived therapeutic composition may be combined with a matrix component to form a composite. An acellular amnion derived therapeutic composition may be used in conjunction with a composition comprising viable cells, such as stem cells.
Owner:AMNIO TECH
Multipotent Prenatal Stem Cells
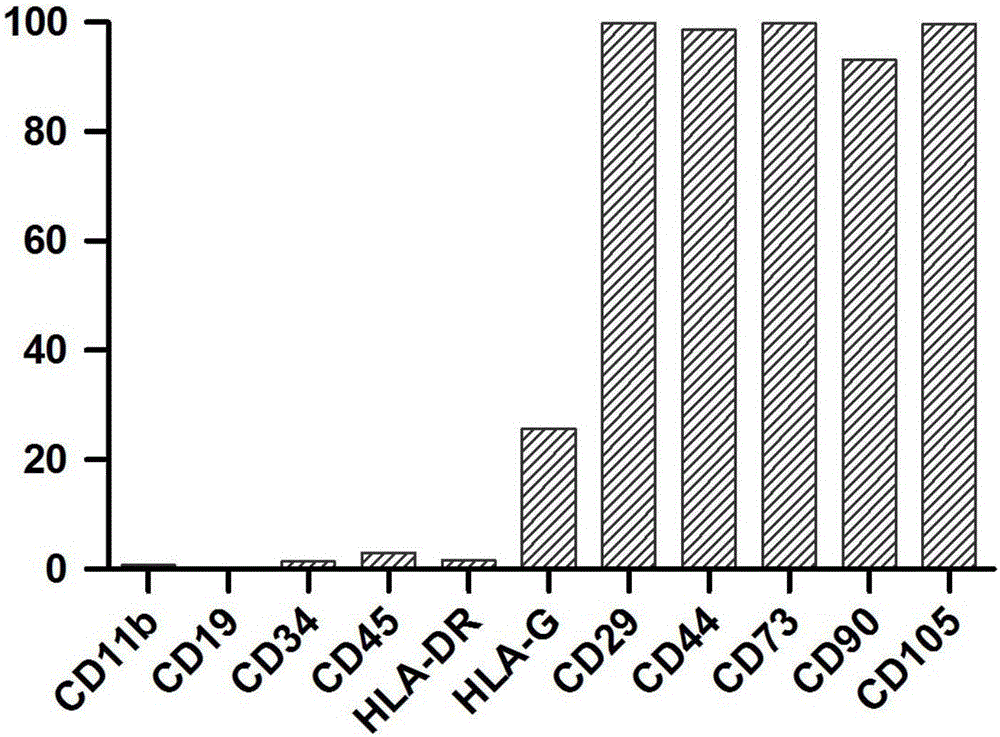
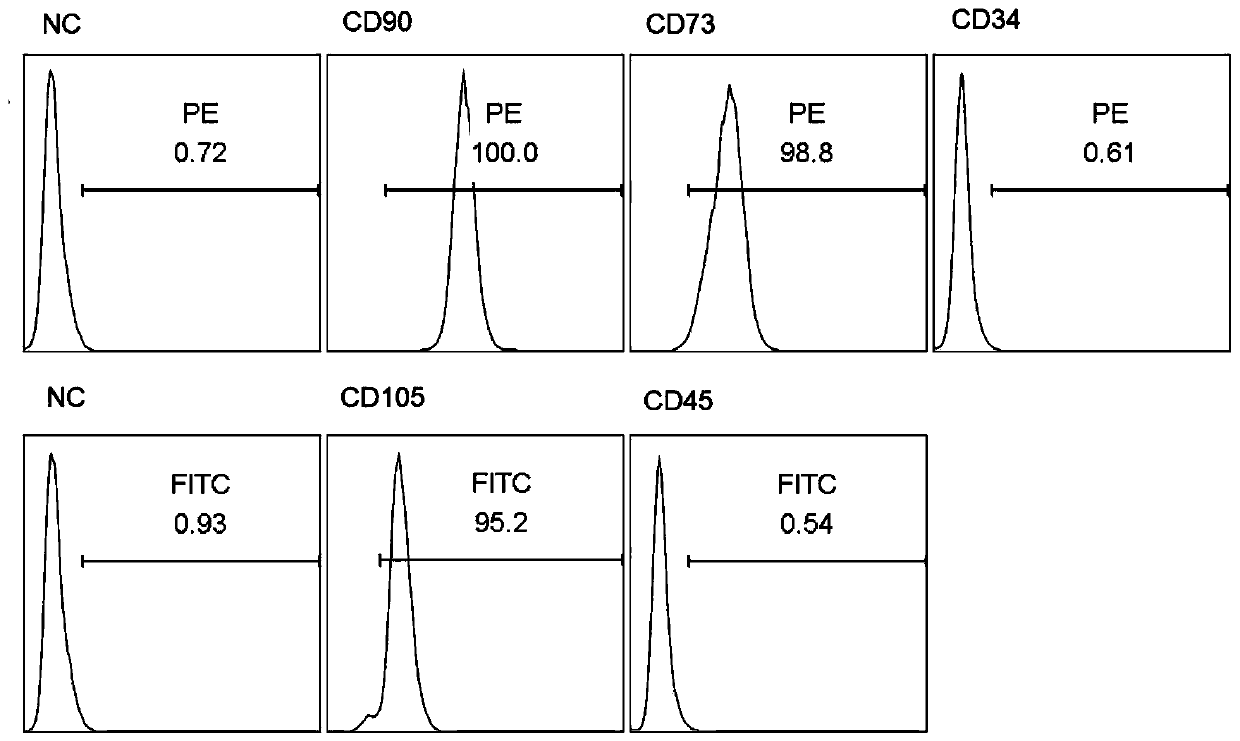
The present invention relates to the isolation, propagation and use of prenatal stem cells. The cells are CD105 negative. In certain embodiments the cells are also SSEA-4 positive or negative, and c-kit negative. The cells may be isolated from a prenatal sample, for instance third trimester amniotic fluid or placental membrane. The cells may be combined with a biologically compatible solution or a biologically compatible matrix and utilized to treat a human subject. The invention also contemplated enriched populations of prenatal stem cells. The enriched cell population may be propagated in the appropriate culture medium and the resulting progeny cells utilized therapeutic applications.
Owner:PRIME MERGER SUB LLC
Tissue engineering bone based on placenta source filling dry cell and production thereof
InactiveCN1746296ANo immune rejectionHigh yieldBone implantSkeletal/connective tissue cellsMesenchymal stem cellBiomedical engineering
A tissue engineering cartilage based on placenta original filled stem cell and its production are disclosed. The process is carried out by constructing bony tissue from composite biological brace material of placenta original filled stem cell. It can be used to repair bony injure and bony tissue deficit.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Polyvalent Anti-tumor fibroblast vaccine
InactiveUS20170196951A1Skeletal/connective tissue cellsUnknown materialsTumor-Associated FibroblastsTreatment modality
Compositions of matter, of production, and treatment modalities are disclosed for the prevention and / or therapeutic reduction of tumors through induction of immunity against tumor associated fibroblasts and components of tumor microenvironment. In one embodiment of the invention, placentally derived fibroblast cells are cultured under conditions replicating tumor microenvironment. Expression of CD248 on said cultured fibroblasts is used as a marker of effective manipulation. Cells expressing CD248 are utilized a immunogens for stimulation of immunity towards cancer associated fibroblastic cells.
Owner:BATU BIOLOGICS
Preparation method of placental-chorionic-plate-tissue-derived mesenchymal stem cells
PendingCN110684722AFast growthObvious growth advantageCulture processDead animal preservationPlacental discBiophysics
The invention relates to a preparation method of placental-chorionic-plate-tissue-derived mesenchymal stem cells, and belongs to the technical field of cell biology. The preparation method of the placental-chorionic-plate-tissue-derived mesenchymal stem cells comprises the following steps: carrying out tissue separation, namely to obtain a placenta tissue sample, separate out chorion tissue, and carry out washing with a tissue cleaning solution until the chorion tissue is semi-translucent; carrying out tissue cryopreservation, namely to put the washed chorion tissue into a cryopreservation solution, and carry out cooling and cryopreservation according to predetermined procedures; carrying out tissue treatment, namely to take out the chorion tissue after the cryopreservation, soak the chorion tissue with ethanol, remove impurities, and perform shearing so as to obtain tissue masses; and then, carrying out cell culture, namely to put the tissue masses into a culture flask, add a mesenchymal stem cell selection medium so as to obtain a mixture, put the mixture into a CO(2) incubator so as to carry out incubation until cell fusion degree reaches 80+ / -10%, carry out cell passage, and continue amplification culture and / or cryopreservation of the passage cells. The preparation method of the placental-chorionic-plate-tissue-derived mesenchymal stem cells enables long-term storage of placental chorionic plate tissue and mesenchymal stem cells separated from the placental-chorionic plate tissue in liquid nitrogen; and moreover, the placental chorionic plate tissue and the mesenchymalstem cells still have maintained cell activity after reconstruction.
Owner:GUANGDONG VITALIFE BIOTECHNOLOGY CO LTD
Stem cell mediated neuroregeneration and neuroprotection
InactiveUS20170258843A1Reduce redundancyReduced ability to evokeMammal material medical ingredientsProgenitorNervous system
Disclosed are means of inducing neuroregeneration and / or neuroprotection in patients with damage to the nervous system. In one embodiment, placenta derived CD34 positive cells are administered to a patient suffering from a neurological injury, said cells administered alone, or in combination with endothelial progenitor cells that are derived from placental sources. In one embodiment cells are manipulated to decrease immunogenicity by means of gene-editing or RNA interference inducing means. In another embodiment, neuroprotection and / or neuroregeneration is achieved by administration of exosomes derived from placental stem cells.
Owner:ANGIOSTEM INC
Isolation and culture method of placenta mesenchyma precursor stem cells
InactiveCN104630140AStrong differentiation abilityGood source of seed cellsSkeletal/connective tissue cellsEnzyme digestionAntiendomysial antibodies
The invention relates to an isolation and culture method for placenta mesenchyma precursor stem cells. The method includes the processes of placental tissue isolation, combined enzyme digestion, discontinuous gradient density isolation, immunomagnetic bead isolation and mesenchymal precursor stem cell suspension culture. The method concretely comprises the following steps: carrying out placental tissue separation: aseptically collecting placenta through a conventional technology; carrying out combined enzyme digestion; diluting by using 2-5L of PBS or alpha-MEM, and filtering tissue residues by using a 200 mesh filter screen; carrying out discontinuous gradient density isolation; adding a neurotrophic factor receptor mouse anti-human CD271 monoclonal antibody, sorting through a magnetic separation column, and collecting eluted marked cells; carrying out placenta precursor stem cell culture; adding a fresh medium every 2-3d; and allowing placenta precursor stem cells to grow in an embryoid body-like sphere manner within 10-14d. The mesenchyma precursor stem cells obtained in the invention express mesenchymal stem cell signs, also express neural stem cell marker nestin and CD271, and have the characteristics of strong self updating and differentiation capability, and wide application prospect.
Owner:JILIN JIHUI BIOTECH CO LTD
Processing method of B-scan ultrasonic image and device thereof
The invention relates to a video image reorganization processing method. The method comprises the steps that data collection of placenta position, placenta thickness, retroplacental low echo zone, glandulae vesicales, placenta lacuna, placenta basilar part blood flow, one or more cervix uteri blood sinus, cervix uteri shape and cesarean section history is conducted. In addition, the invention further relates to a video data processing module and a device using the processing module.
Owner:PEKING UNIV THIRD HOSPITAL
Human amniotic epithelial cell separation method
ActiveCN104974980AHigh purityHigh differentiation potentialEmbryonic cellsEpithelium surfaceCell activity
Owner:CHONGQING CELL BIOENG TECH CO LTD
Compositions and methods of treatment with amniotic fluid
ActiveUS20200129562A1Promote wound healingReducing and preventing scar formationCosmetic preparationsPowder deliveryMedicinePharmaceutical medicine
The present disclosure provides compositions composed of amniotic fluid and / or modified amniotic fluid, a pharmaceutically acceptable carrier, and optionally a placental tissue graft, micronized placental tissue components, or extracts derived therefrom. Also described are systems and apparatuses for administering or storing said compositions, as well as methods of treatment using said compositions.
Owner:MIMEDX GROUP
Placental villus plate mesenchymal stem cells and clinical preparation method
InactiveCN106119191AHigh yieldHigh purityCell dissociation methodsSkeletal/connective tissue cellsFiltrationRemove blood
The invention discloses placental villus plate mesenchymal stem cells. A clinical preparation method of the placental villus plate mesenchymal stem cells comprises the following steps: (1) acquiring a placental villus plate; (2) removing blood vessels and villus tissues; (3) cleaning the placental villus plate by virtue of sterile physiological saline until the placental villus plate has no blood color; (4) cutting villus plate tissues into 1-5mm[2], and cleaning the cut villus plate tissues by virtue of physiological saline; (5) inoculating tissue blocks into a complete medium, and cultivating the tissue blocks in a moist incubator which is at 37 DEG C and contains 5% of CO2; (6) changing the medium once every 6-11 days, and digesting the cells by virtue of TrypLE<TM> Select when a cell fusion degree is 70-80%; (7) conducting subculture; (8) conducting detection; (9) conducting cryopreservation; and (10) building a data base. According to the clinical preparation method disclosed by the invention, the placental villus plate is taken as a material, such operations as sorting, soaking, filtration and gradient centrifugation are not needed in an extracting process, so that many cells can be obtained by a relatively few of generation times; therefore, cost consumption and separation time can be greatly reduced, and the yield and purity of the mesenchymal stem cells can be improved.
Owner:四川华皓生物科技有限公司
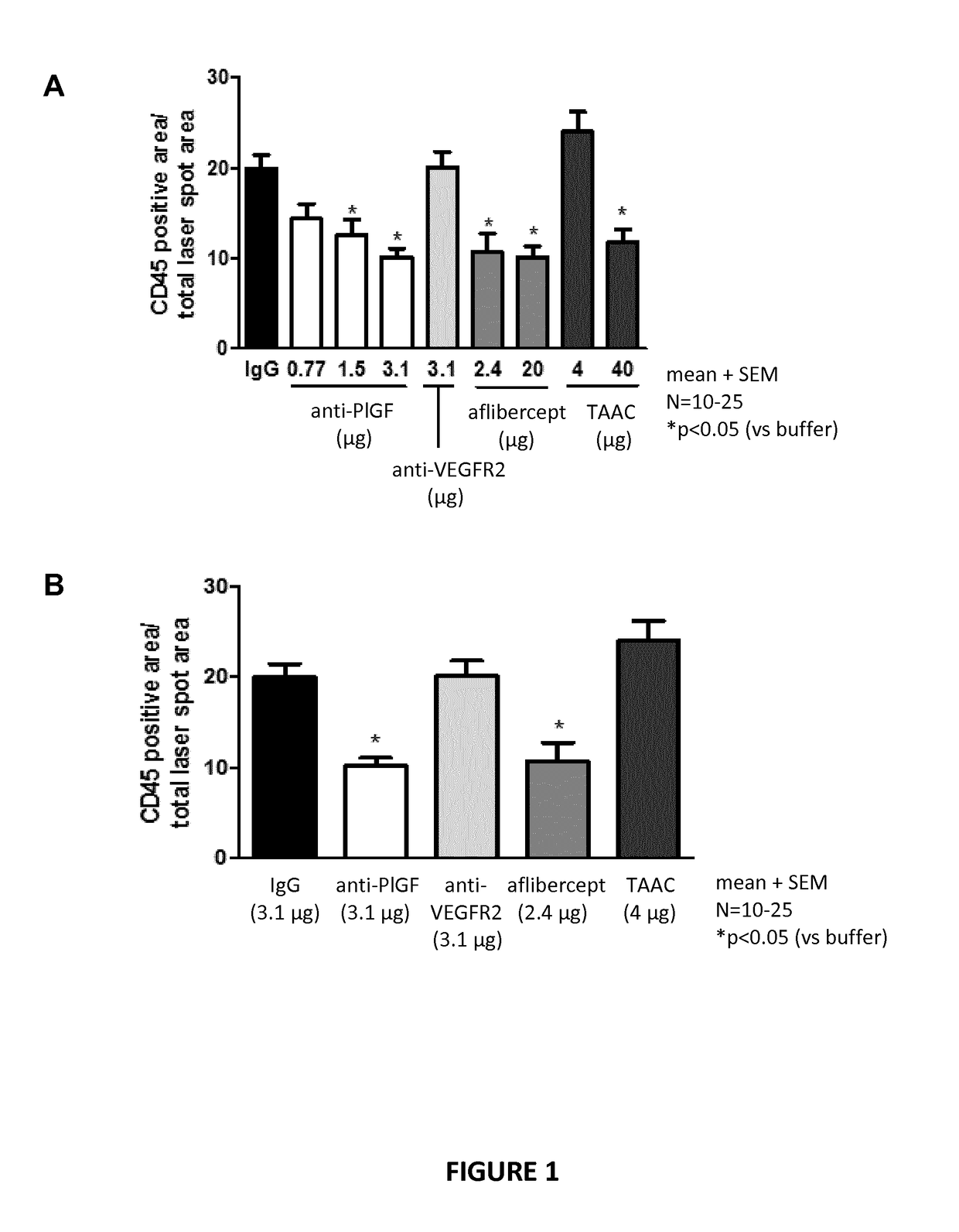
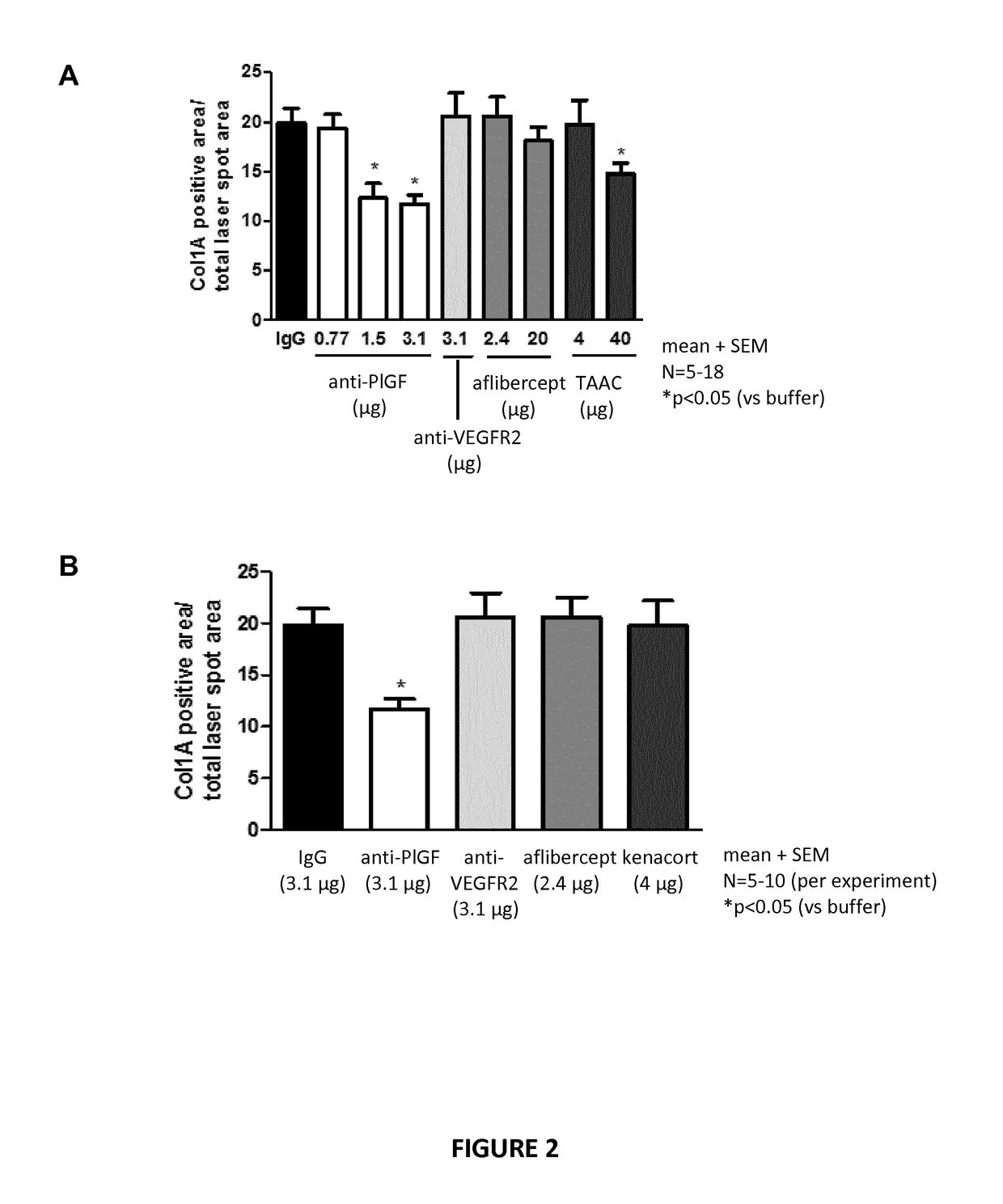
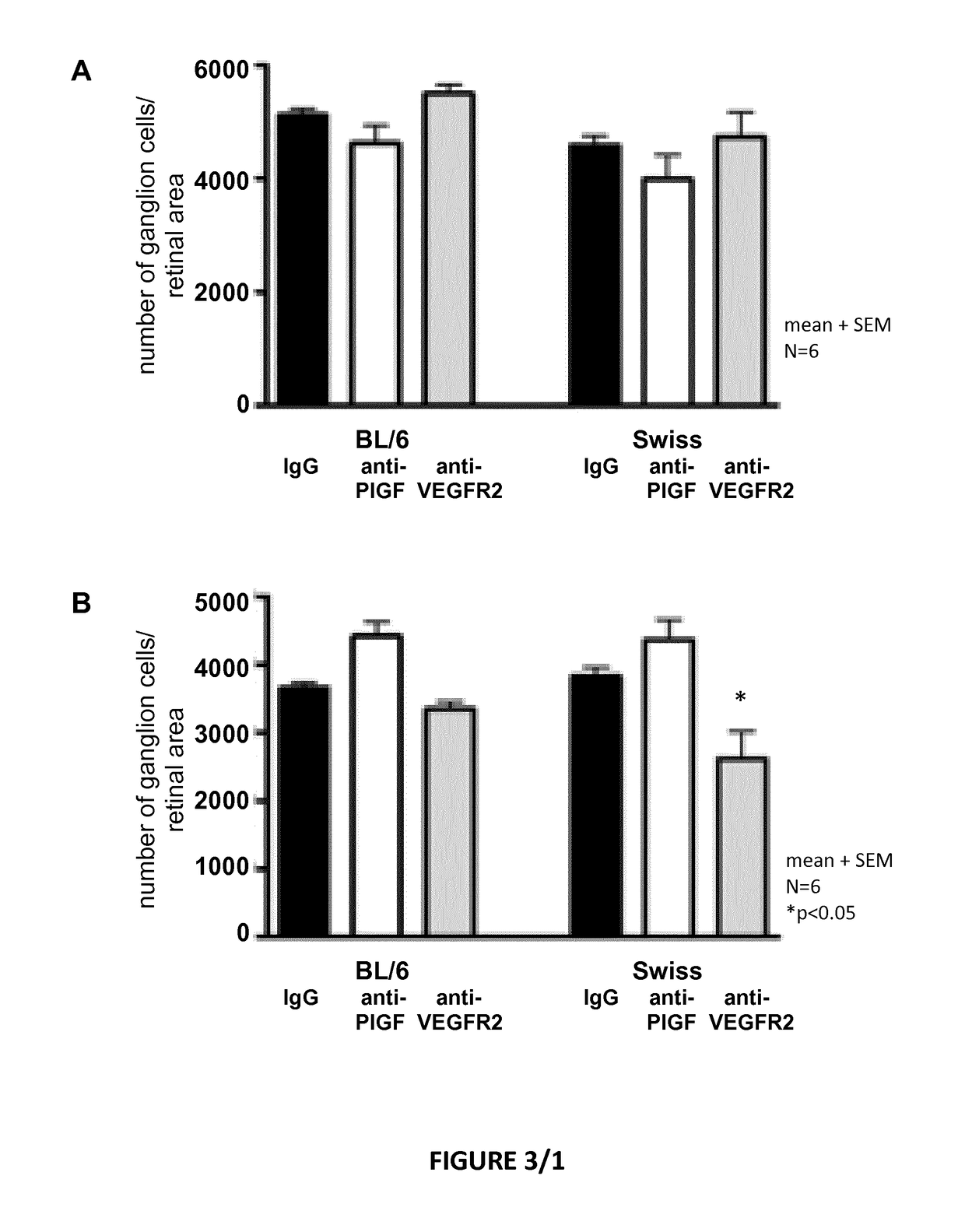
Posterior ocular fibrosis inhibition by antagonizing placental growth factor
InactiveUS20190031762A1Increase vessel maturationIncrease areaSenses disorderImmunoglobulins against growth factorsPlacentaTreatment field
The disclosure is situated in the field of ocular therapies. In particular, in some embodiments the disclosure provides antagonists of placental growth factor and related methods of use for treating, preventing, or delaying posterior ocular fibrosis. In other embodiments, the disclosure provides monospecific placental growth factor (PlGF) antagonist compositions and related methods for maintaining or improving visual acuity of a subject with an eye of which the retina is damaged.
Owner:OXURION NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com