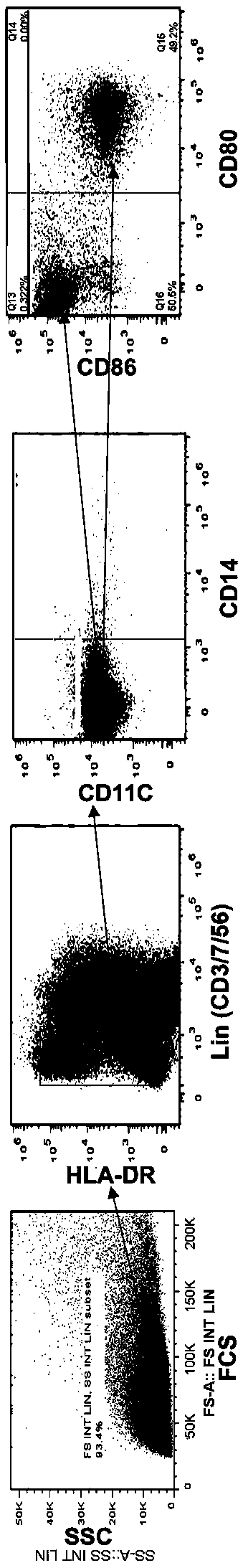
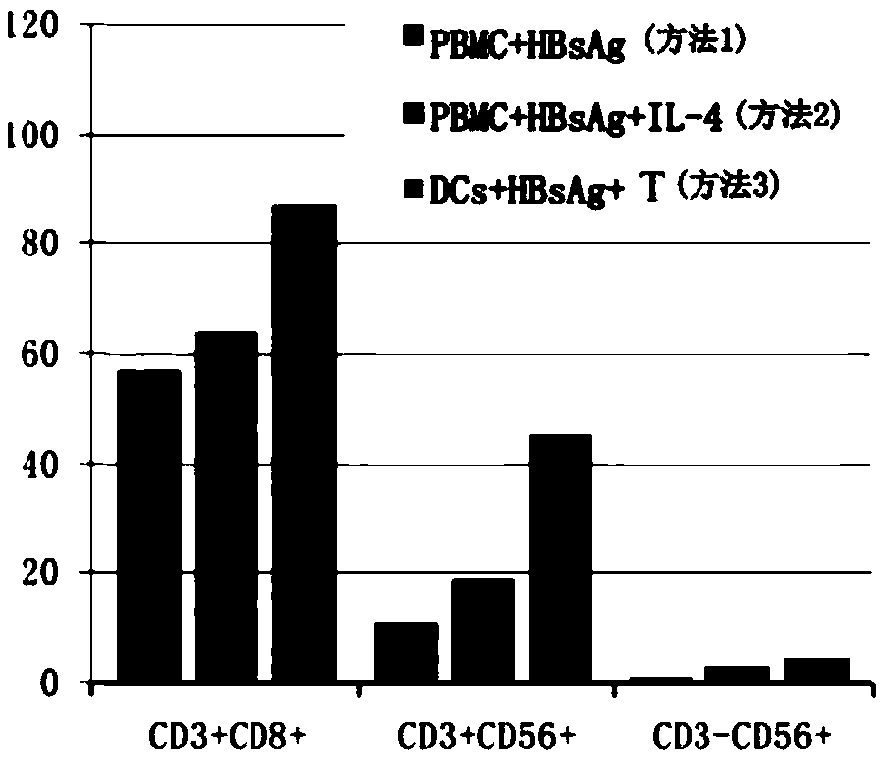
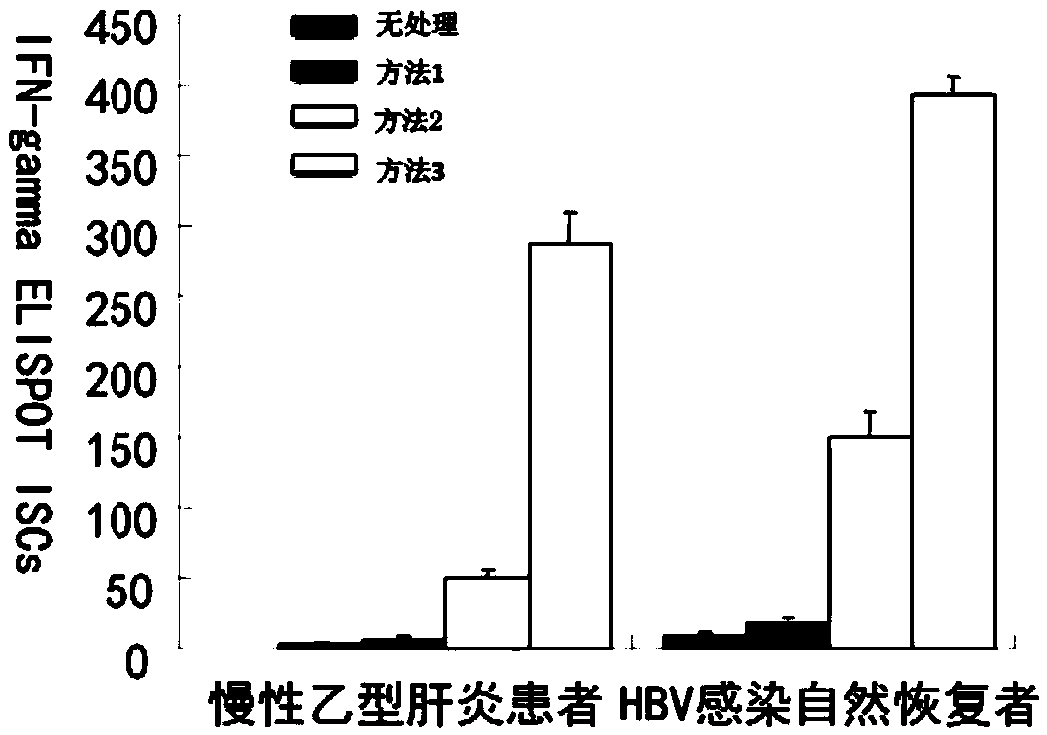
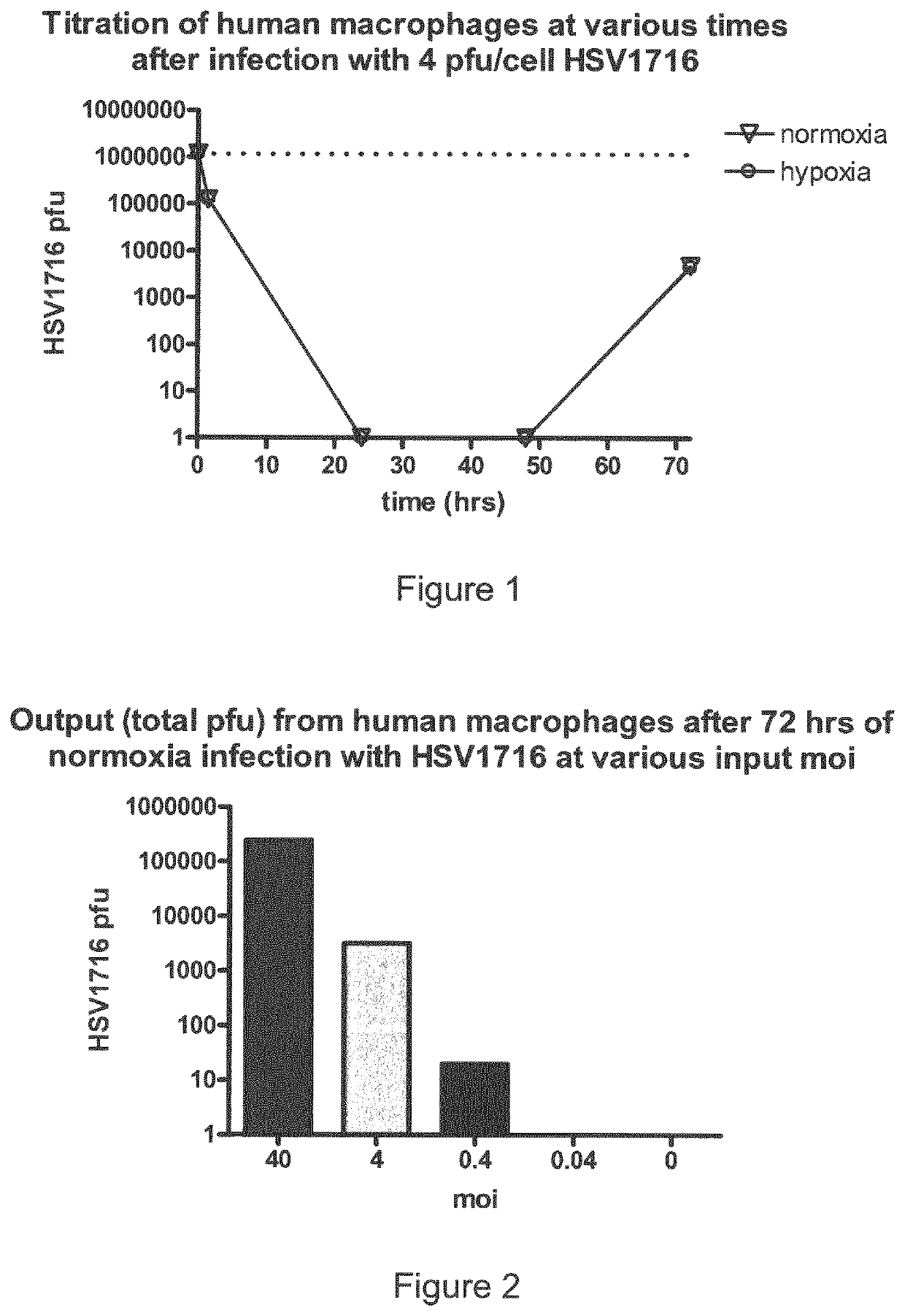
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Monocyte derived" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monocytes are derived from promonocytes in the bone marrow and circulate in the blood for about 24 hours before migrating to the tissues, such as the lung and liver, where they develop into macrophages. adj., adj monocyt´ic. Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Edition.
Porcine DC-SIGN, ICAM-3 and LSECtin and uses thereof
The present invention relates to the cloning, identification and characterization of the unique and entire genomic sequences encoding new porcine DC-SIGN and LSECtin proteins, including the novel nucleotide sequences of the full-length cDNA and genes of both pDC-SIGN gene and pLSECtin. Also provided are the nucleic acid molecules encoding newly discovered porcine ICAM-3 isoforms from porcine monocyte-derived dendritic cells and the use thereof. Specifically, the invention is drawn to an isolated nucleic acid molecule comprising a nucleotide sequence encoding one or more of porcine DC-SIGN, porcine ICAM-3, porcine LSECtin, a complement of the nucleotide sequence or a functional, defined portion of the nucleotide sequence or a protein fusion product linked to a protein that may be of porcine or human origin. Methods for isolating and cloning the new porcine genes and for using the new nucleotide sequences in improved methods for propagating viruses, particularly enveloped viruses, are additionally described herein. The invention further includes new transfected cells or cell lines that can stably express the porcine proteins, new antibodies and the like.
Owner:VIRGINIA TECH INTPROP INC
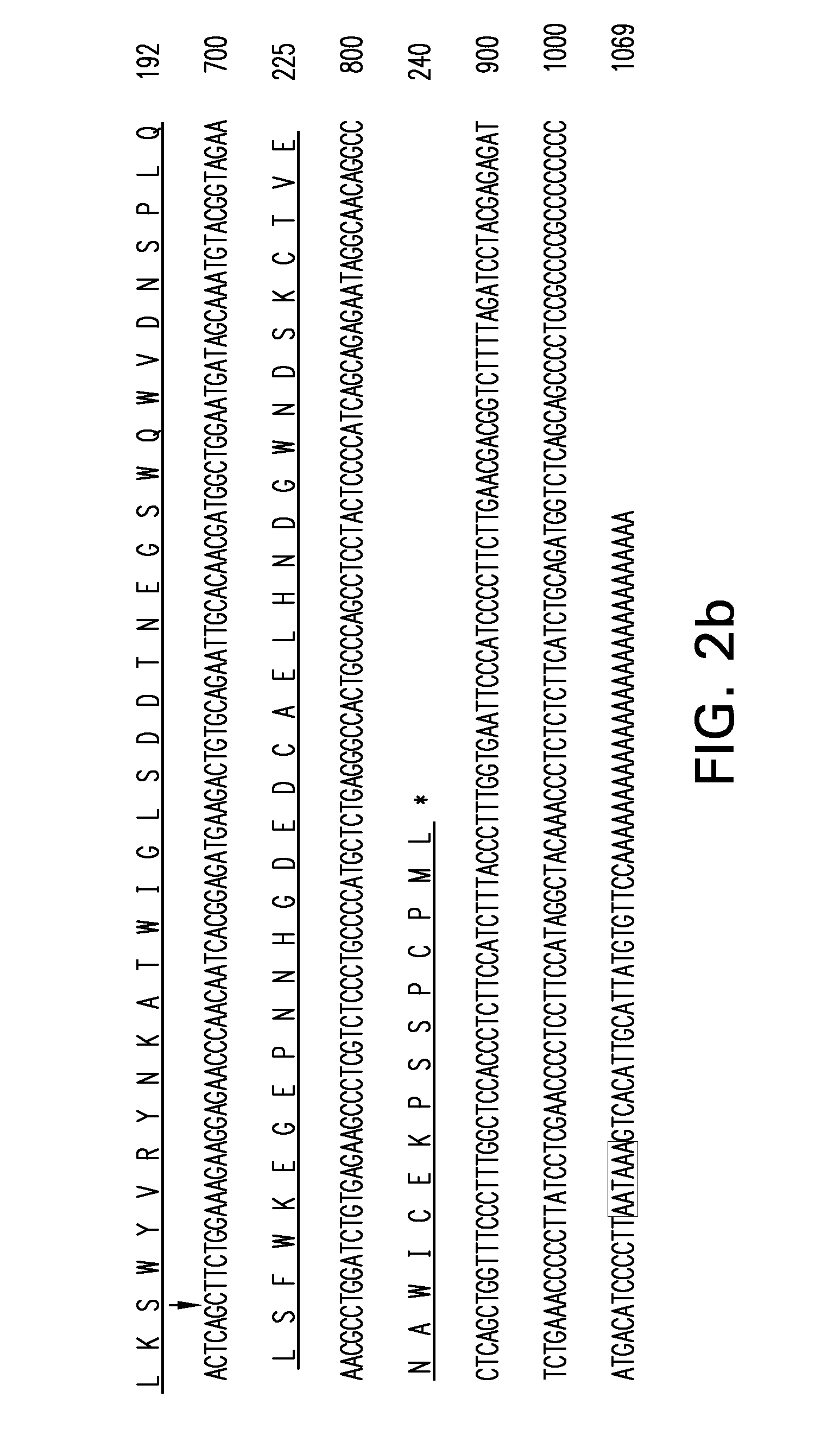
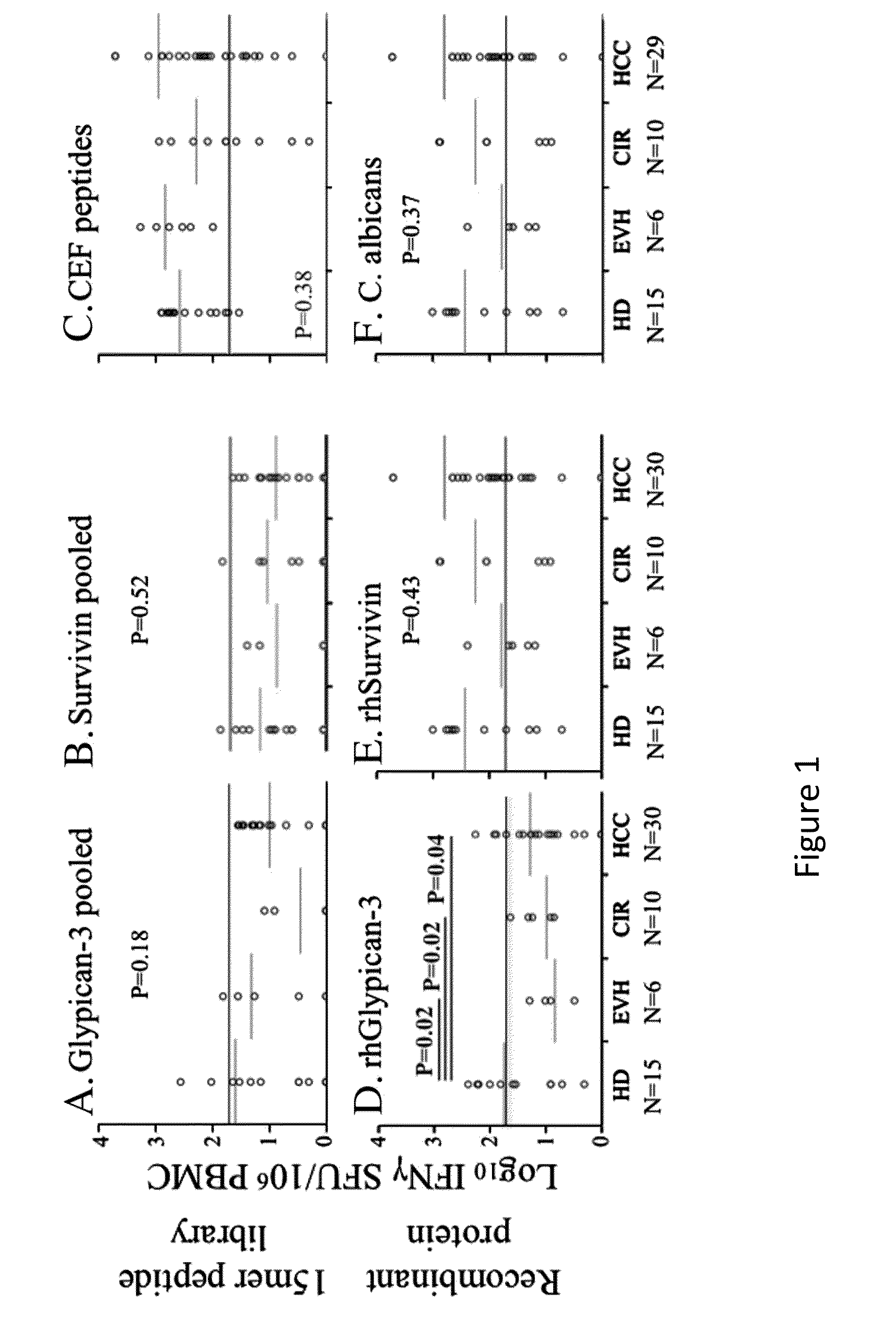
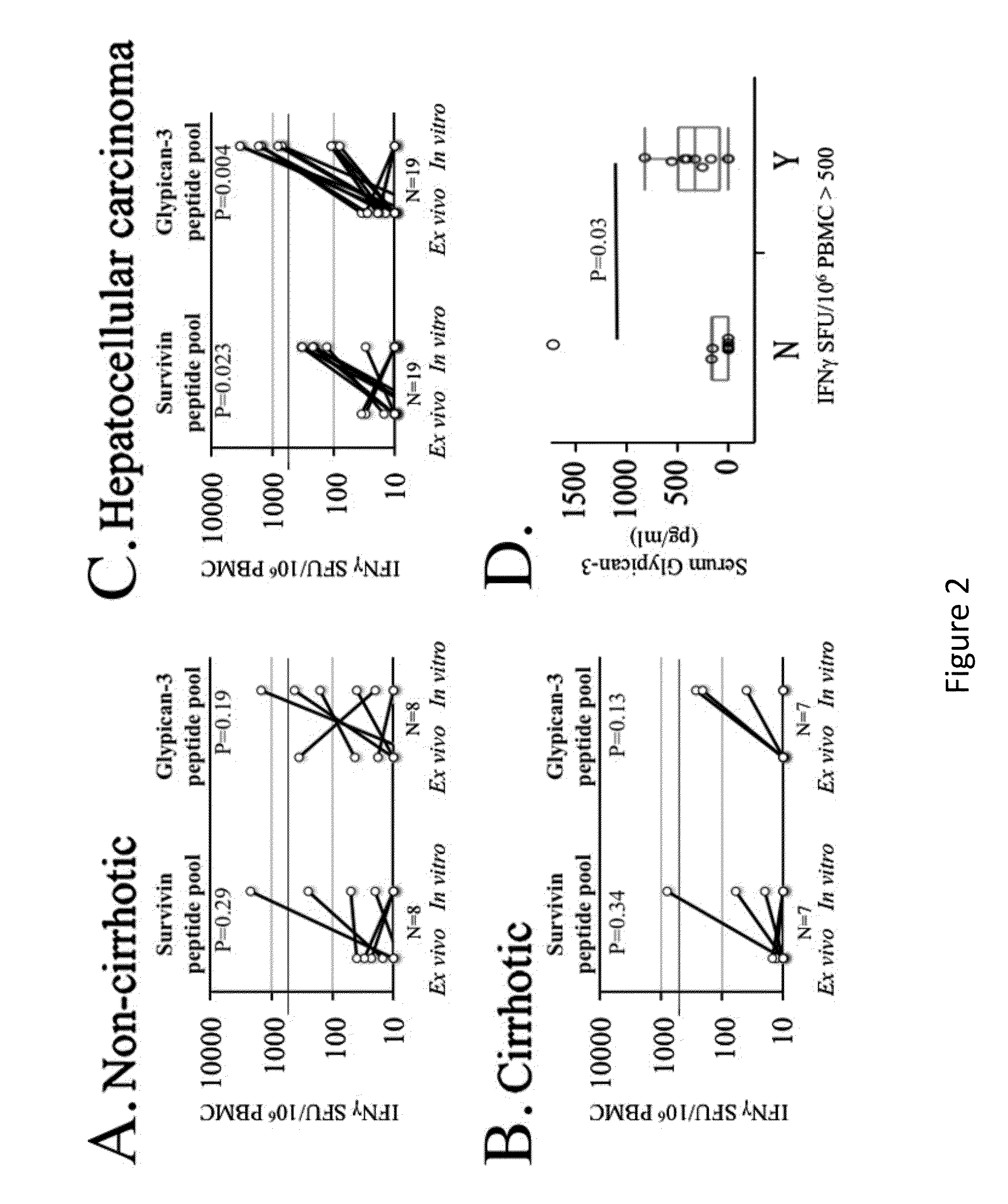
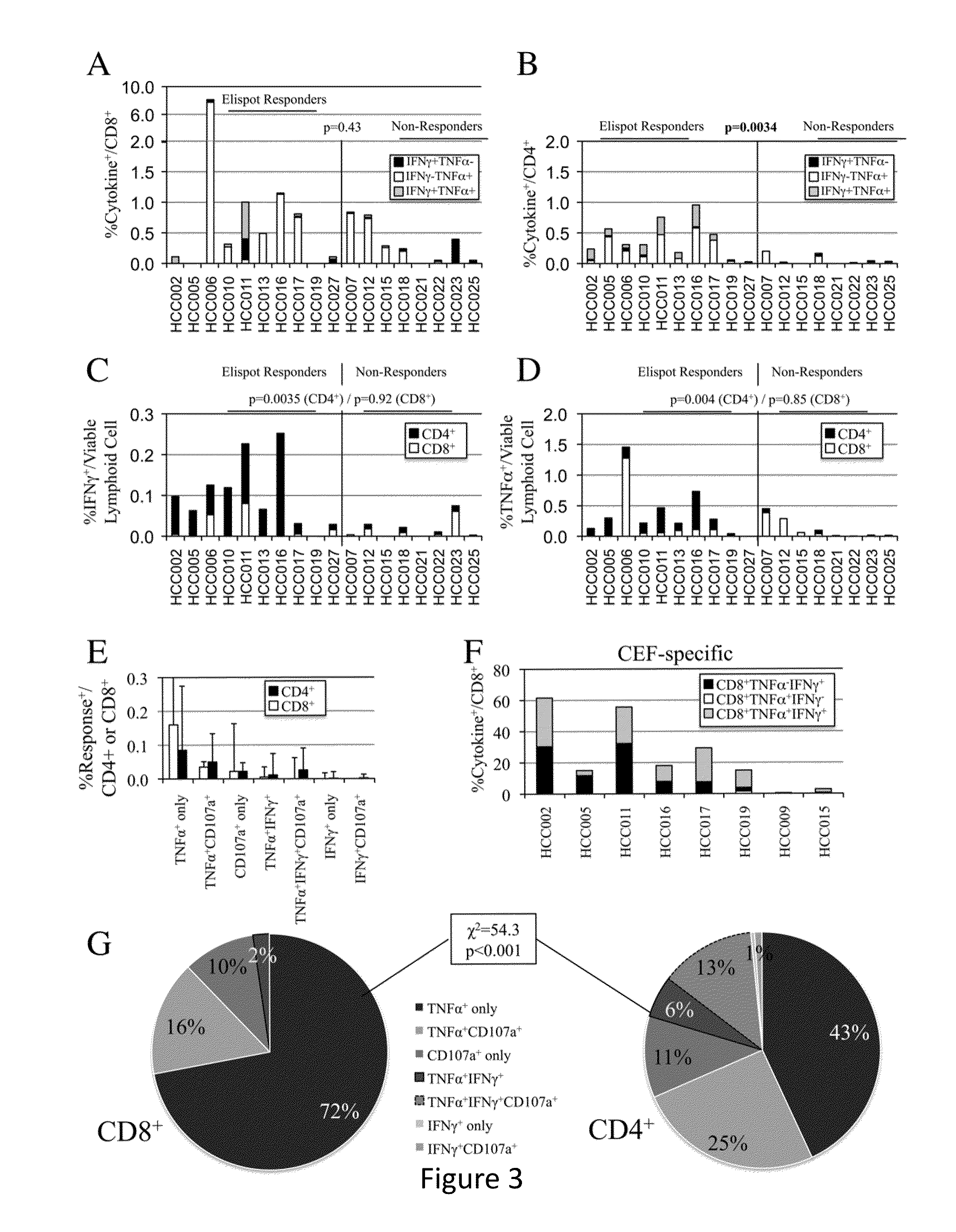
Expansion of Interferon-Gamma-Producing T-Cells Using Glypican-3 Peptide Library
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Human stem cell materials and methods
InactiveUS20050003534A1Reduce riskIncrease the number ofGenetic material ingredientsArtificial cell constructsMammalAdult stem cell
Monocyte derived adult stem cells (MDSCs) isolated from peripheral blood of mammals are provided, along with pharmaceutical compositions containing an MDSC, kits containing a pharmaceutical composition, and methods of preparing, propagating and using MDSCs or differentiated derivatives thereof. The uses of these biological materials include methods of treating disorders or diseases, as well as methods of ameliorating a symptom associated with any such disorder or disease including disorders or diseases associated with platelets.
Owner:CHICAGO UNIV OF
Human stem cell materials and methods
InactiveUS20050260158A1Reduce riskIncrease the number ofBiocideGenetic material ingredientsMammalBiological materials
Monocyte derived adult stem cells (MDSCs) isolated from peripheral blood of mammals are provided, along with pharmaceutical compositions containing an MDSC, kits containing a pharmaceutical composition, and methods of preparing, propagating and using MDSCs or differentiated derivatives thereof. The uses of these biological materials include methods of treating disorders or diseases, as well as methods of ameliorating a symptom associated with any such disorder or disease, including disorders or diseases associated with aberrant function or presence (i.e., level) of pancreatic islet β-cell-like macrophages.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Monocyte-derived dendritic cell subsets
InactiveUS20050123522A1Improve developmentIncreased amenability to transfectionBiocidePeptide/protein ingredientsDiseaseDendritic cell
A novel subset of monocyte-derived dendritic cells are provided. Methods for producing these monocyte-derived dendritic cells and compositions comprising the dendritic cells of the invention are also provided. Methods for inducing an immune response to an antigen of interest using the dendritic cells of the invention are provided. Also provided are methods for therapeutically or prophylactically treating a disease in a subject suffering from the disease using the dendritic cells.
Owner:MAXYGEN
Products For Receptor Mediated Activation And Maturation Of Monocyte-Derived Dendritic Cells By A Phosphorylated Glucomannane Polysaccharide
InactiveUS20080171002A1Easy to identifyOvercome problemsOrganic active ingredientsBiocideDendritic cellPhosphorylation
Phosphorylated glucomannan polysaccharide compositions are shown to effectively enhance healthy immune function. Dosage forms including pills, sprays, functional foods and cosmetics may achieve this benefit while being essentially free of storage protein from nongerminated seeds of Ricinus communis.
Owner:CANTABRIA GROUP LCC
Human Stem Cell Materials and Methods
InactiveUS20080038238A1Reduce riskIncrease the number ofBiocideGenetic material ingredientsMammalAdult stem cell
Monocyte derived adult stem cells (MDSCs) isolated from peripheral blood of mammals is provided, along with pharmaceutical compositions containing an MDSC, kits containing a pharmaceutical composition, and methods of preparing, propagating and using MDSCs or differentiated derivatives thereof. The uses of these biological materials include methods of treating disorders or diseases, as well as methods of ameliorating a symptom associated with any such disorder or disease.
Owner:HUBERMAN ELIEZER +1
Antigen presenting cell assay
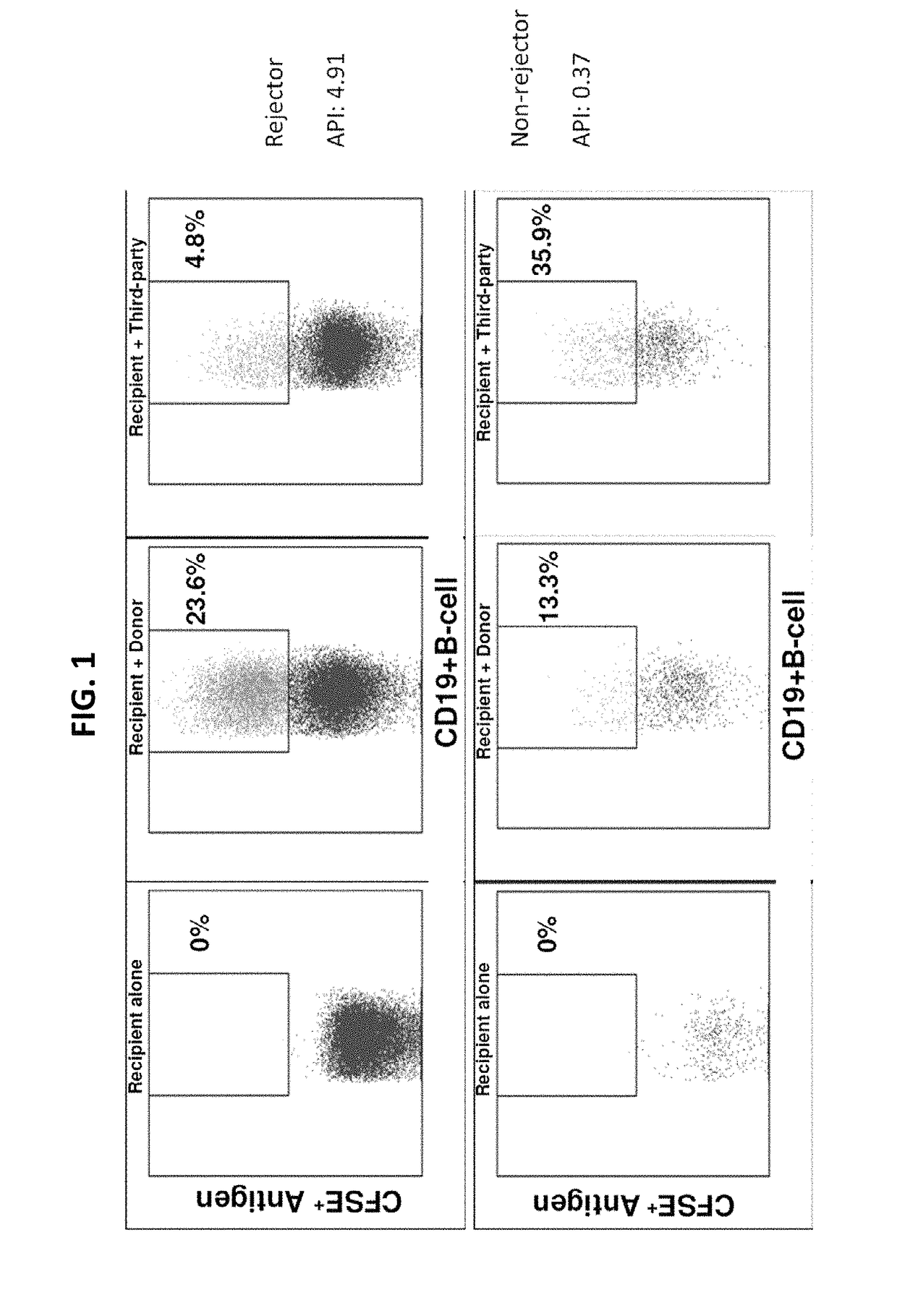
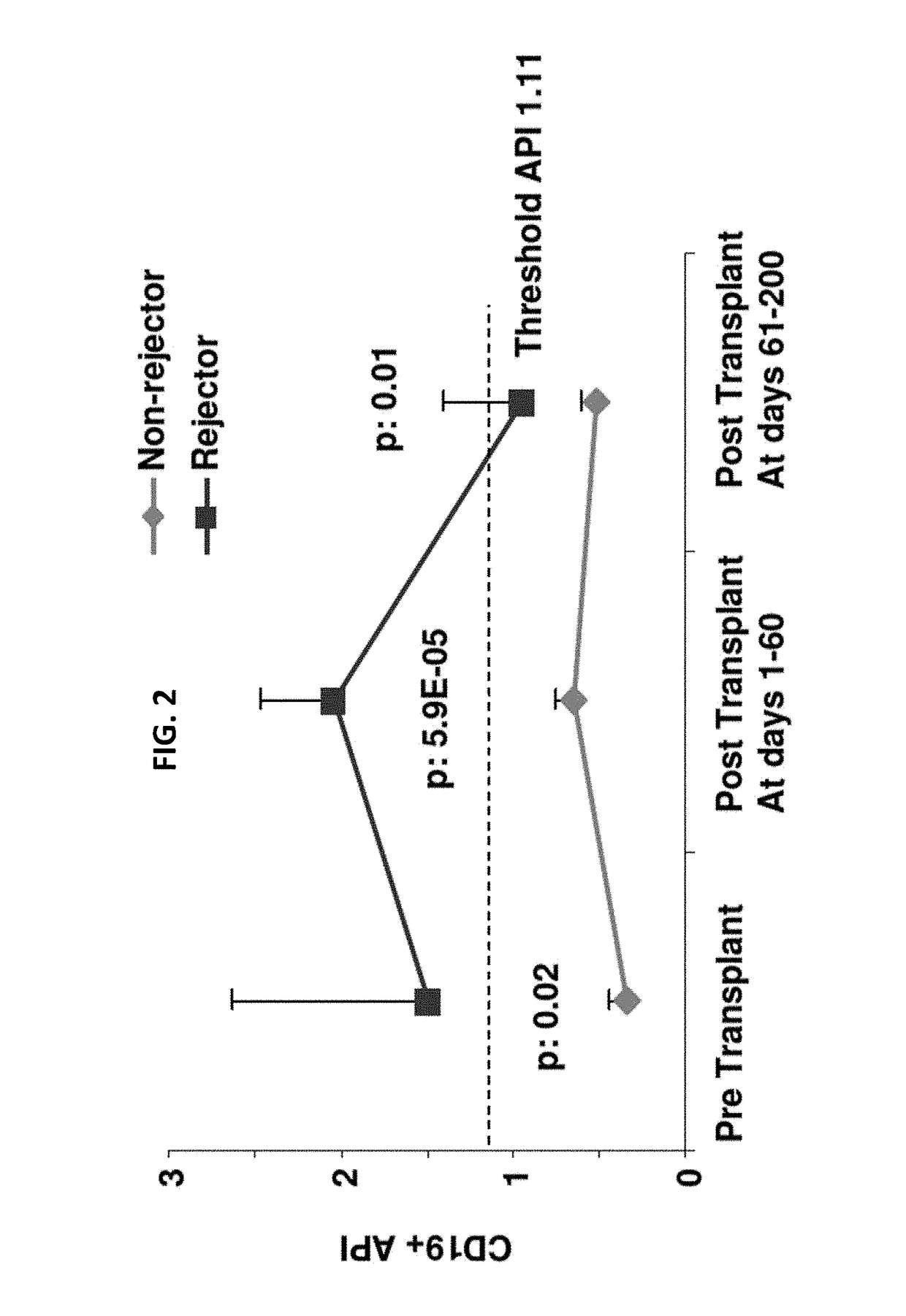
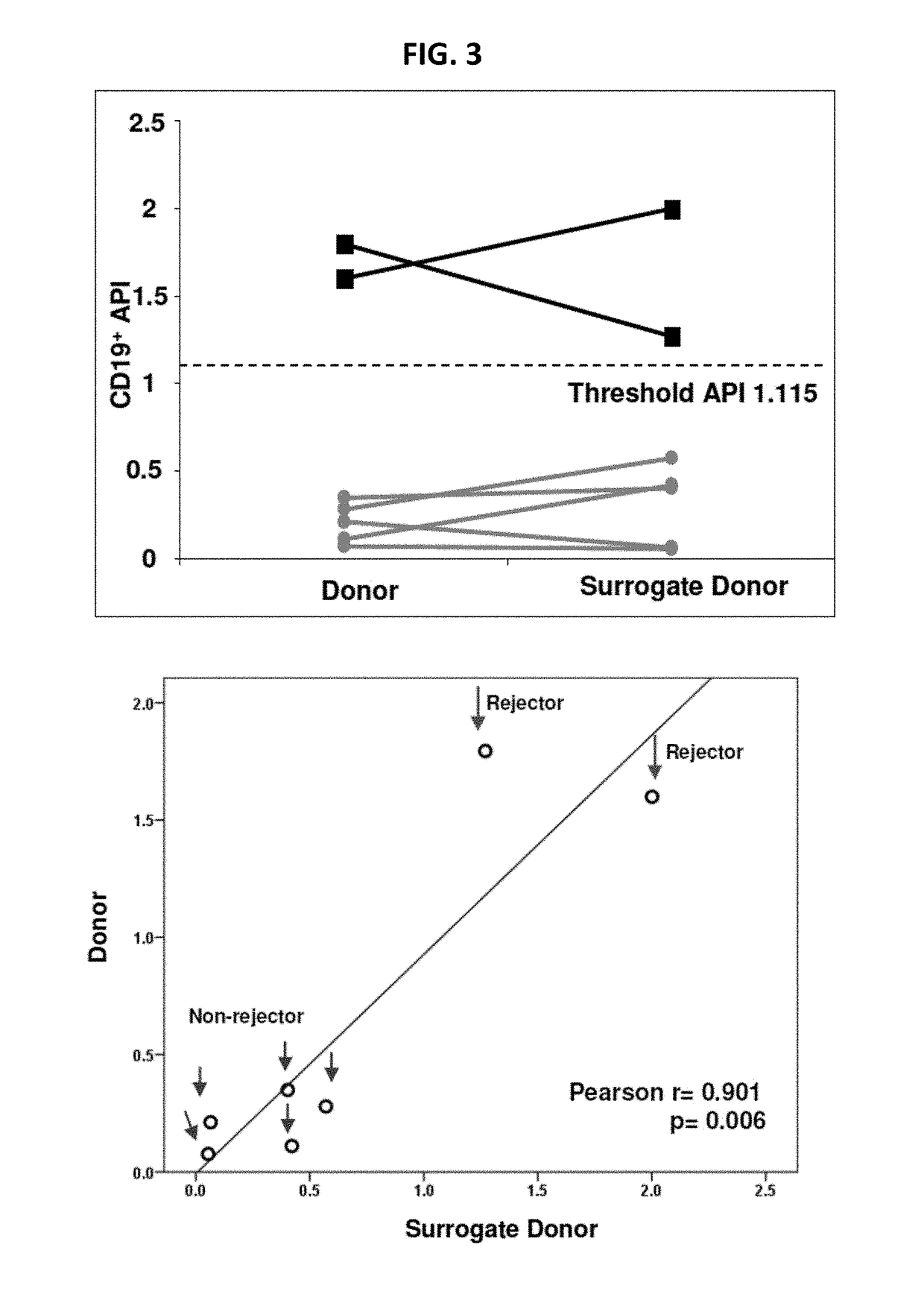
Disclosed herein are methods for diagnosing or predicting acute cellular and / or humoral rejection in a subject. In one example, a method of assessing organ rejection includes contacting a first sample comprising antigen presenting cells (APCs) obtained from a subject in need of or having received an organ transplant with a donor antigen from a donor under conditions sufficient to induce uptake of the donor antigen; contacting a second sample comprising APCs obtained from the subject in need of or having received an organ transplant with a third-party antigen under conditions sufficient to induce uptake of the third-party antigen; and determining an antigen presenting index by determining a ratio of uptake of the donor antigen in the first sample to uptake of the third-party antigen in the second sample, wherein the ratio of greater than one indicates organ rejection and the APCs are monocytes or monocyte-derived cells.
Owner:UNIVERSITY OF PITTSBURGH
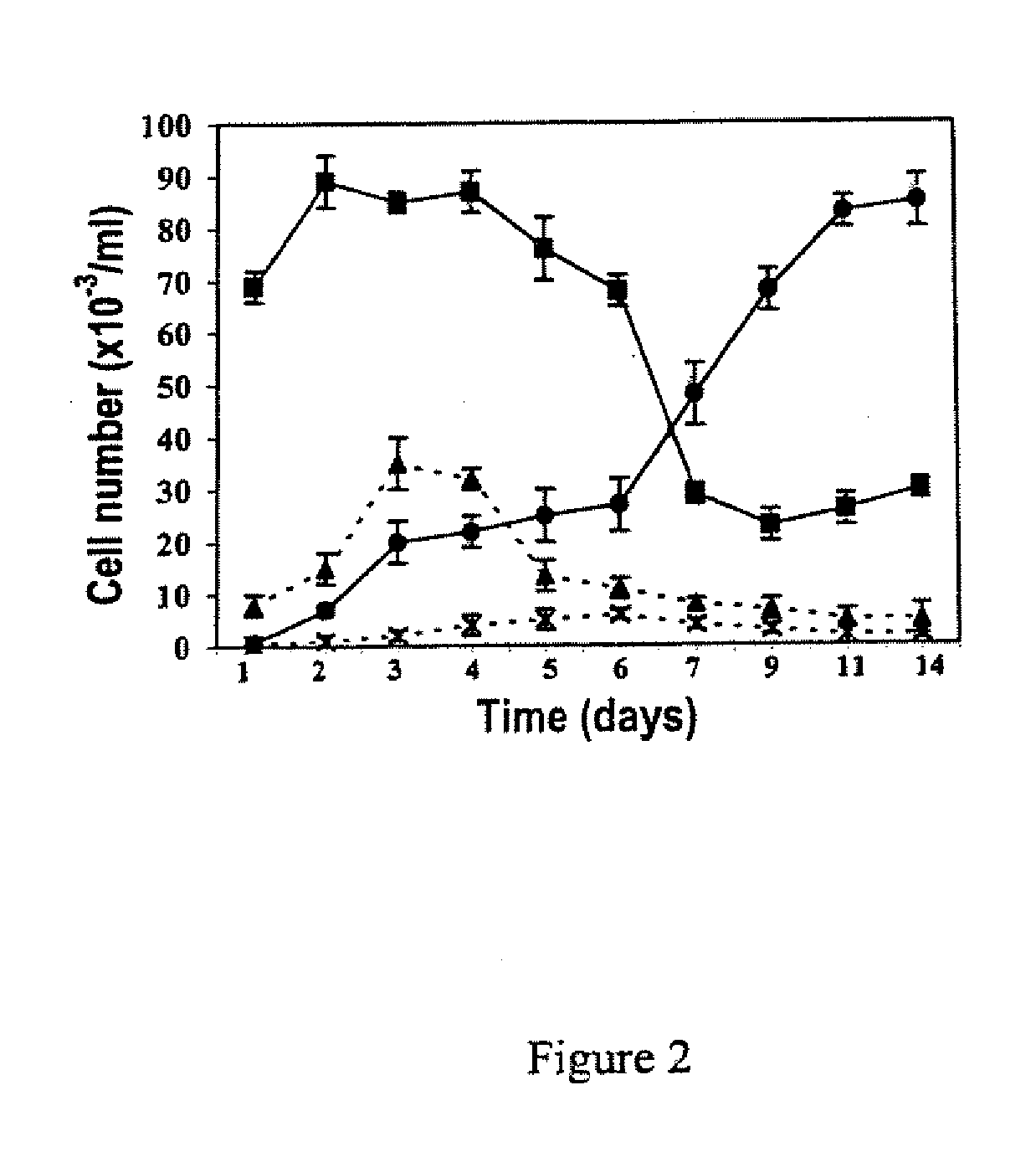
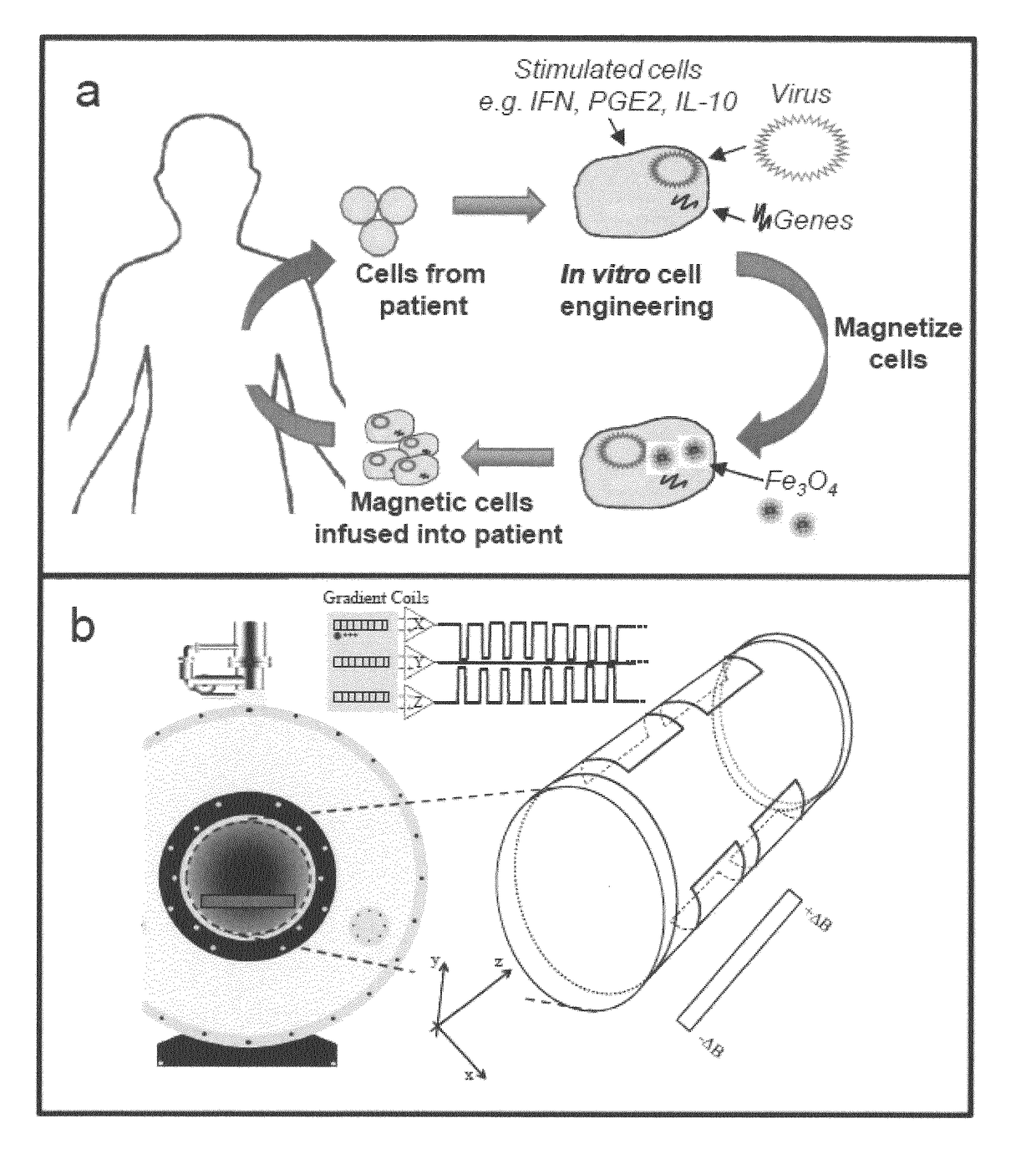
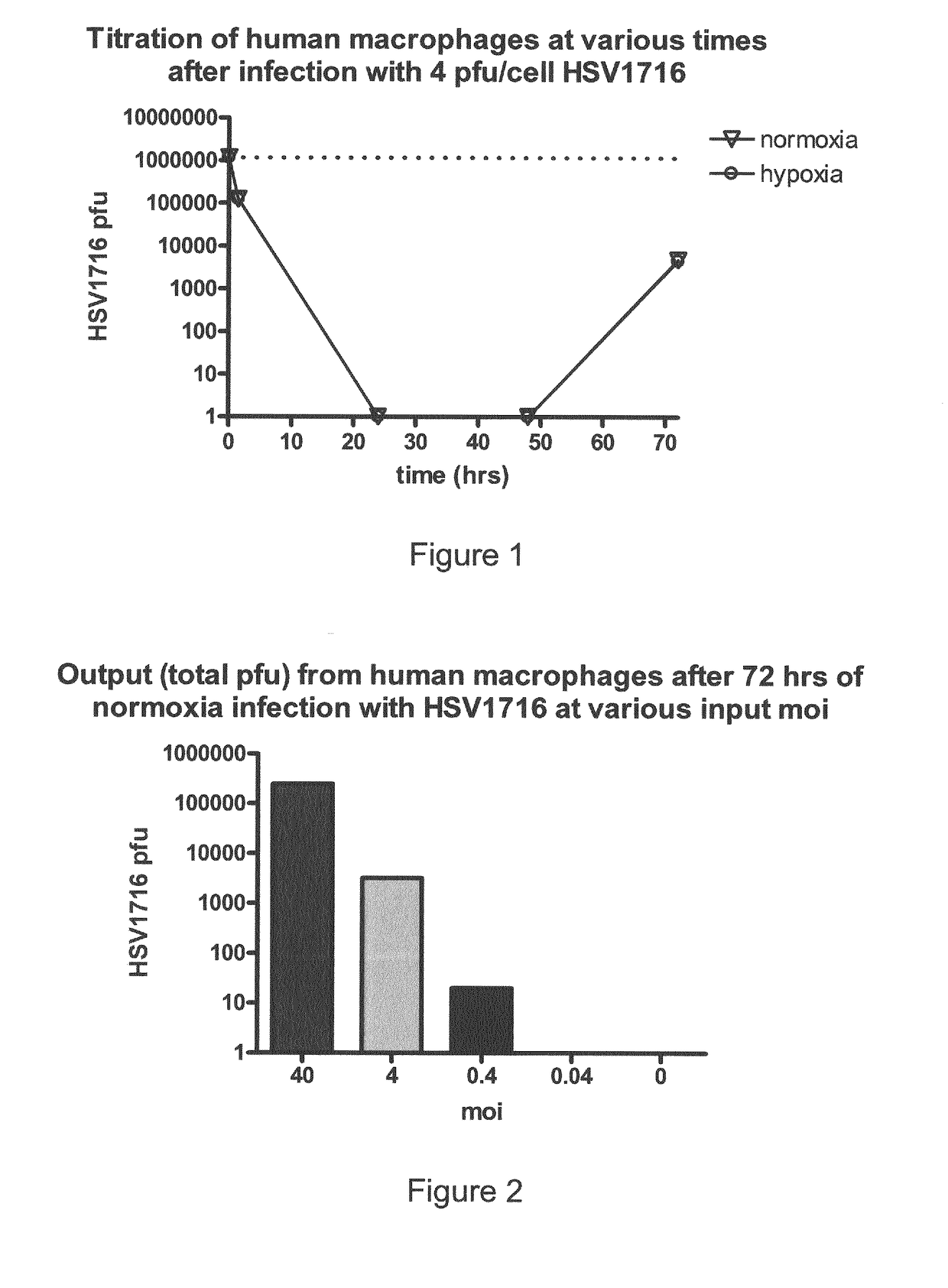
Oncolytic Herpes Simplex Virus Infected Cells
ActiveUS20180071348A1Stimulate immune responseEnhance immune responseHeavy metal active ingredientsEnergy modified materialsInfected cellDisease
A monocyte, monocyte derived cell or macrophage infected with an oncolytic herpes simplex virus is disclosed together with uses of such infected cells in the treatment of diseases such as cancer.
Owner:VIRTTU BIOLOGICS +1
Monocyte-derived stem cells
InactiveUS20100047908A1Artificial cell constructsBlood/immune system cellsLeukocyte inhibitory factorMonocyte derived
Methods for generating multipotent stem cells from adult peripheral blood monocytes are provided. Monocytes may be de-differentiated into monocyte-derived stem cells (MDSCs) by contacting the monocyte with the de-differentation factors, leukocyte inhibitory factor, macrophage colony-stimulating factor, or a combination thereof. The MDSCs may be differentiated into many different types of cells upon contact with the appropriate differentiation factors. Also provided are compositions comprising the MDSCs or differentiated cells derived from the MDSCs.
Owner:OPEXA THERAPEUTICS
Rapid one-step generation of antigen loaded dendritic cell vaccine from precursors
InactiveUS20060239962A1Peptide/protein ingredientsSnake antigen ingredientsLangerhan cellSurface marker
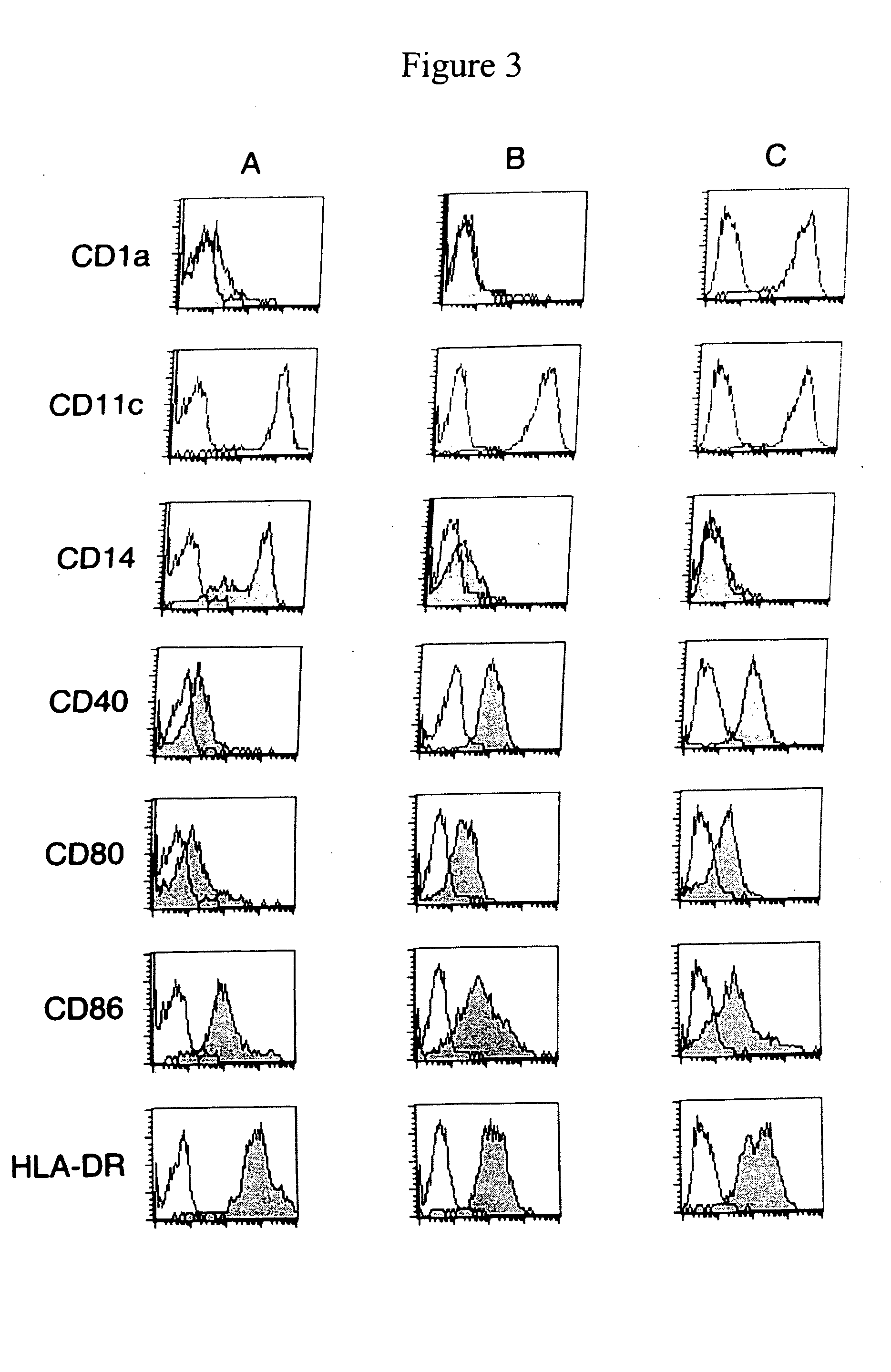
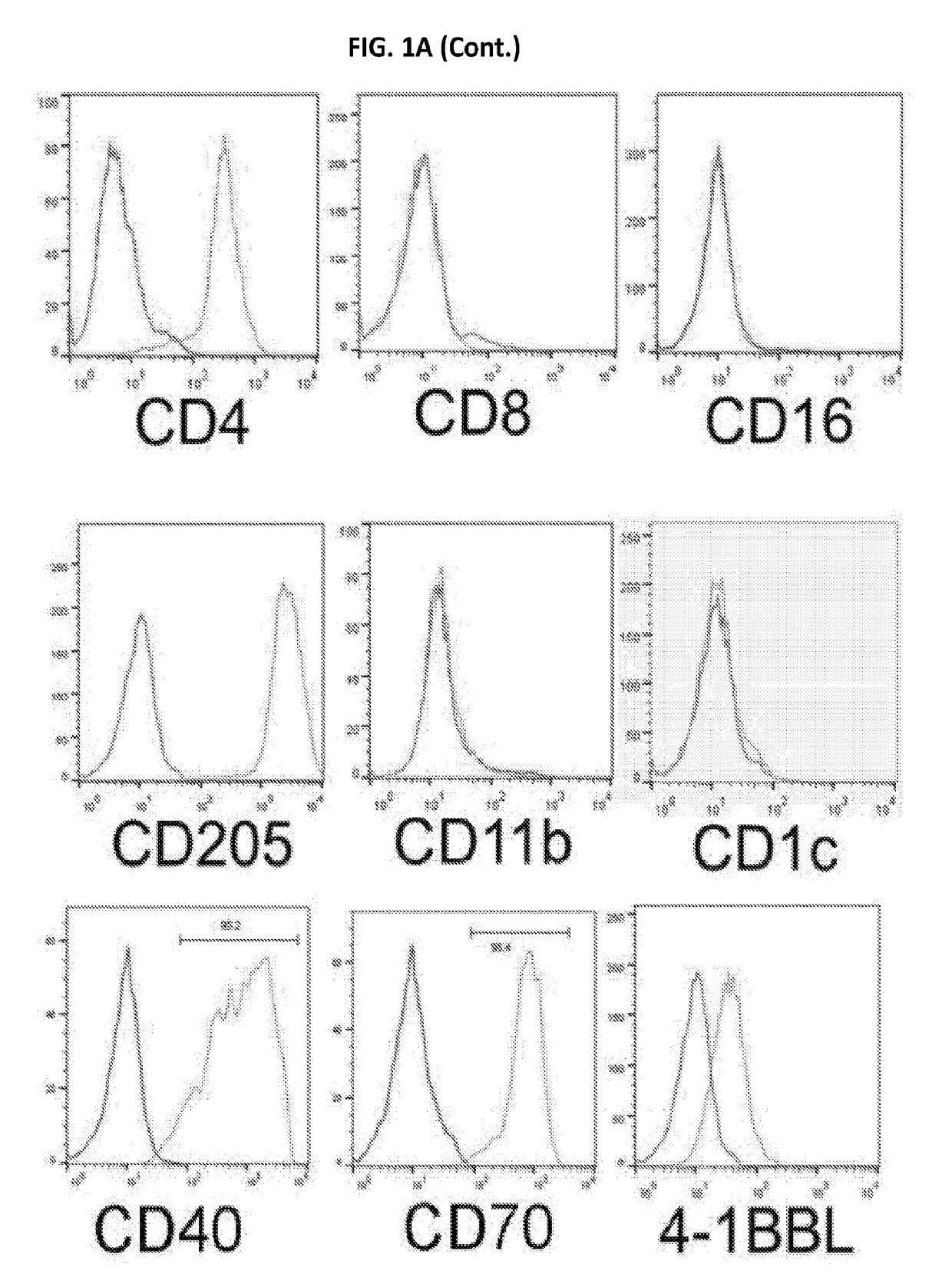
A one-step method for producing antigen loaded antigen-presenting cells from monocytes ex vivo has been found which comprises contacting the monocytes with a composition comprising an activator such as TNF alpha preferably in combination with at least one growth factor such as GM-CSF and at least one soluble or particulate antigen. According to the methods of the present invention, antigen-loaded dendritic cell vaccines can be generated within as little as three (3) days. In another method of the present invention, antigen loaded antigen-presenting cells are produced from monocytes ex vivo by contacting the monocytes with TNF alpha and granulocyte-macrophage colony stimulating factor at one time point to form antigen-presenting cells and then contacting antigen-presenting cells with soluble or particulate antigenic material antigen-presenting cells, wherein the antigen loaded antigen-presenting cells are produced in less than four days. The present invention also includes a vaccine which comprises monocyte-derived antigen loaded antigen-presenting cells, wherein the antigenpresenting cells are composed of two or more subsets selected from the group consisting of Langerhans cells with surface markers (CD 1 a+CD207+); interstitial dendritic cells with surface markers (CD 1a+CD207−); double negative dendritic cells with surface markers 20 (CD 1 a−CD 14−); and dendritic cells with surface markers (CD 14+CD 1 a−CD209+).
Owner:BAYLOR RES INST
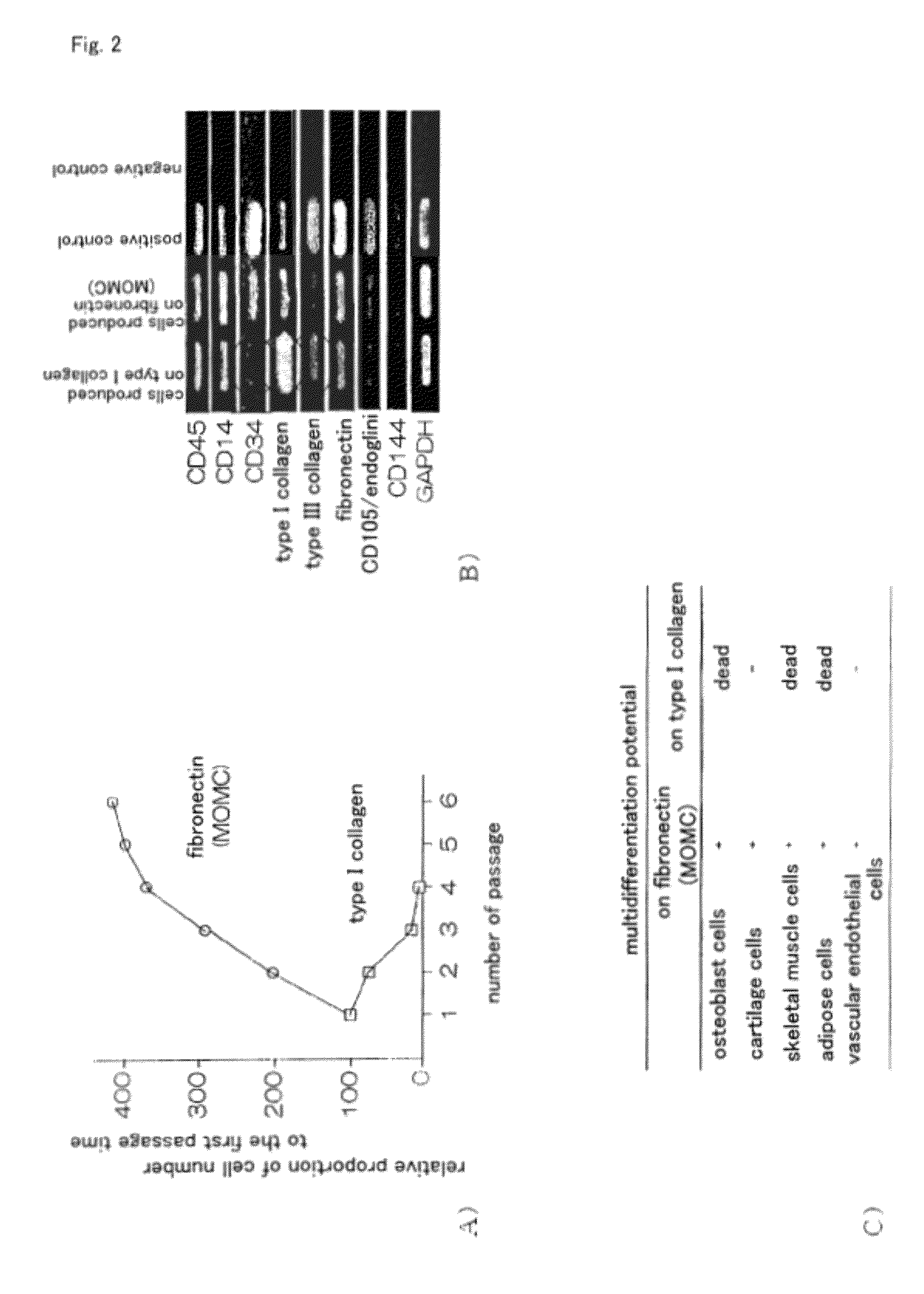
Method for efficient production of monocyte-derived multipotent cell (MOMC)
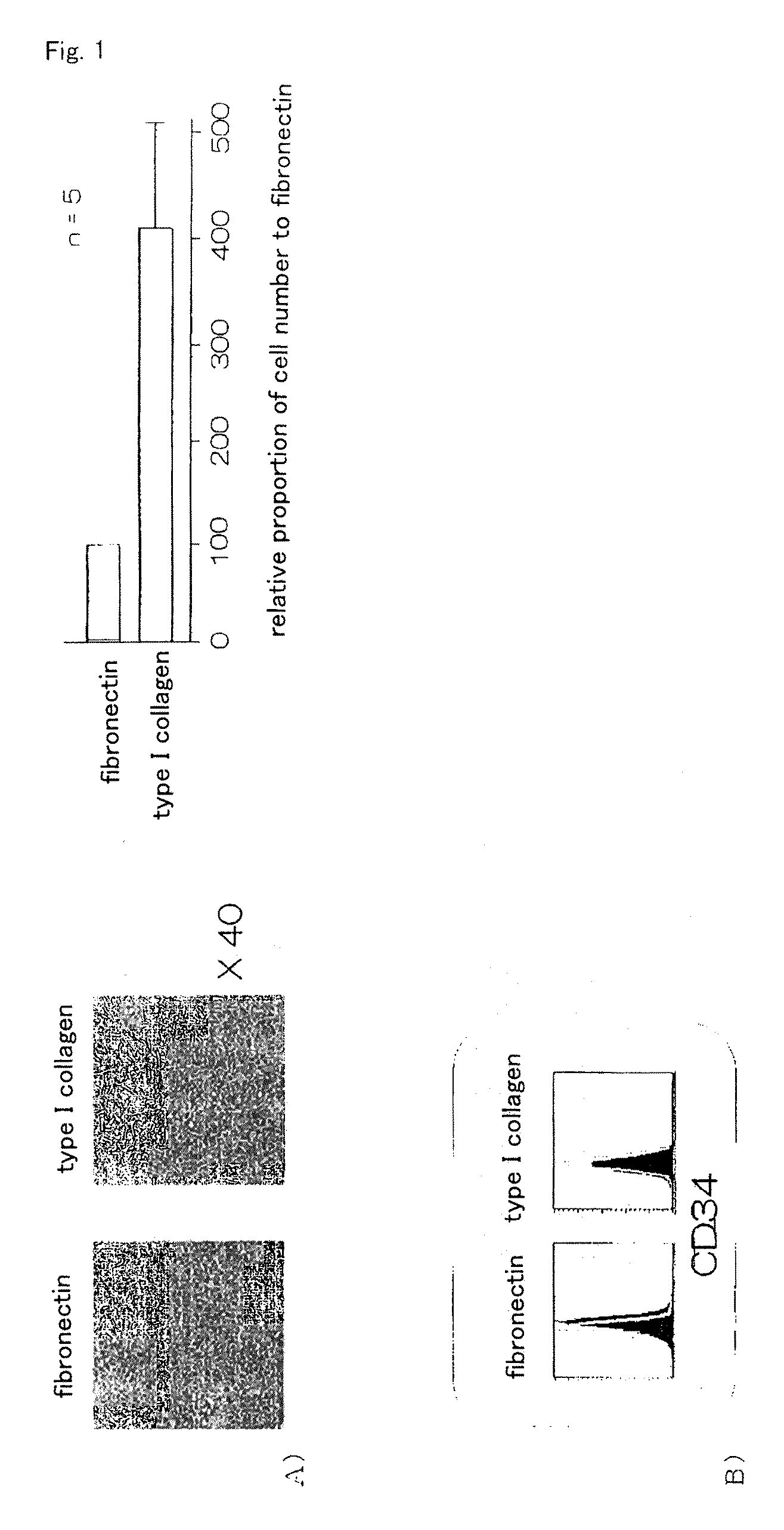
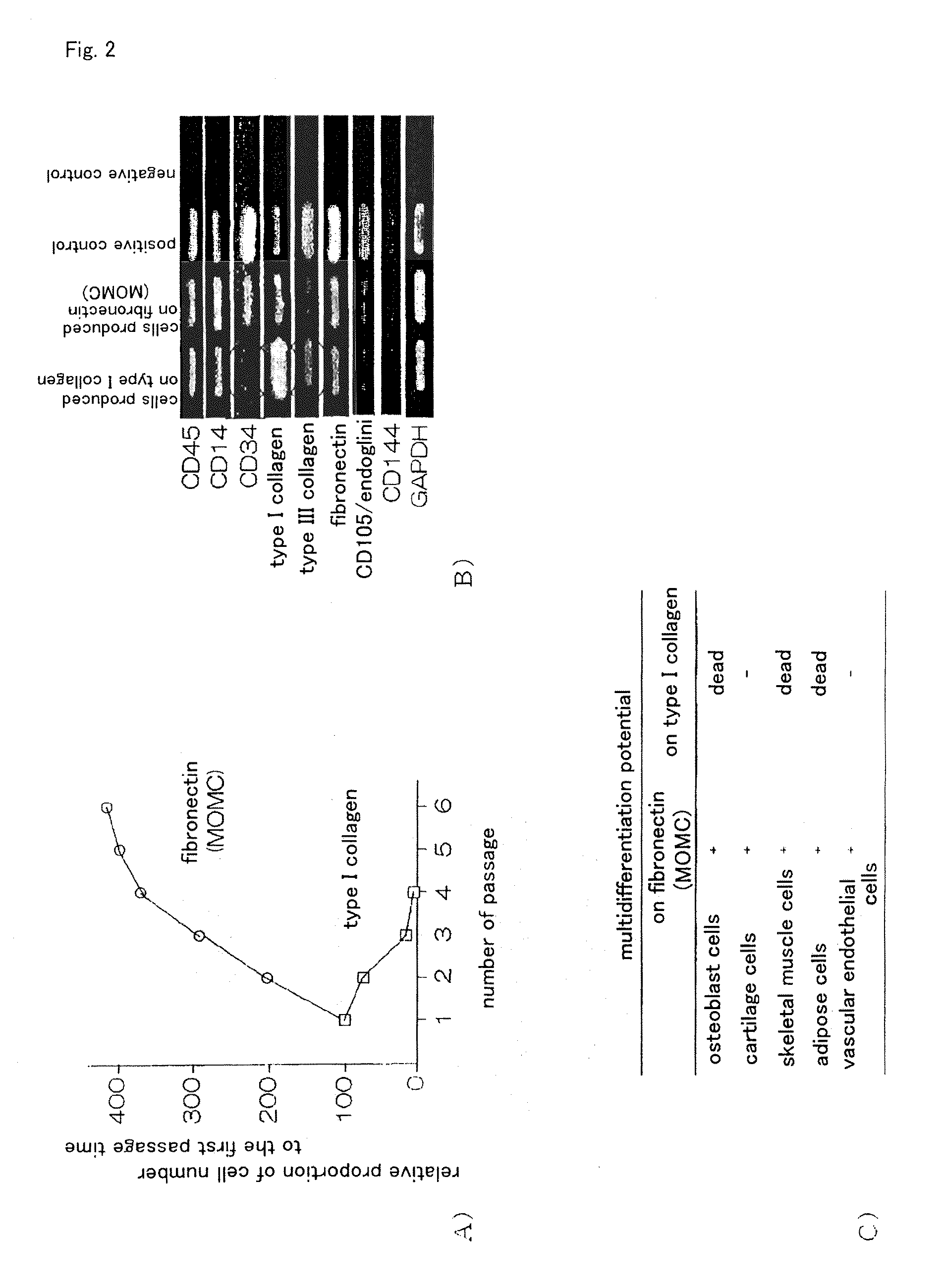
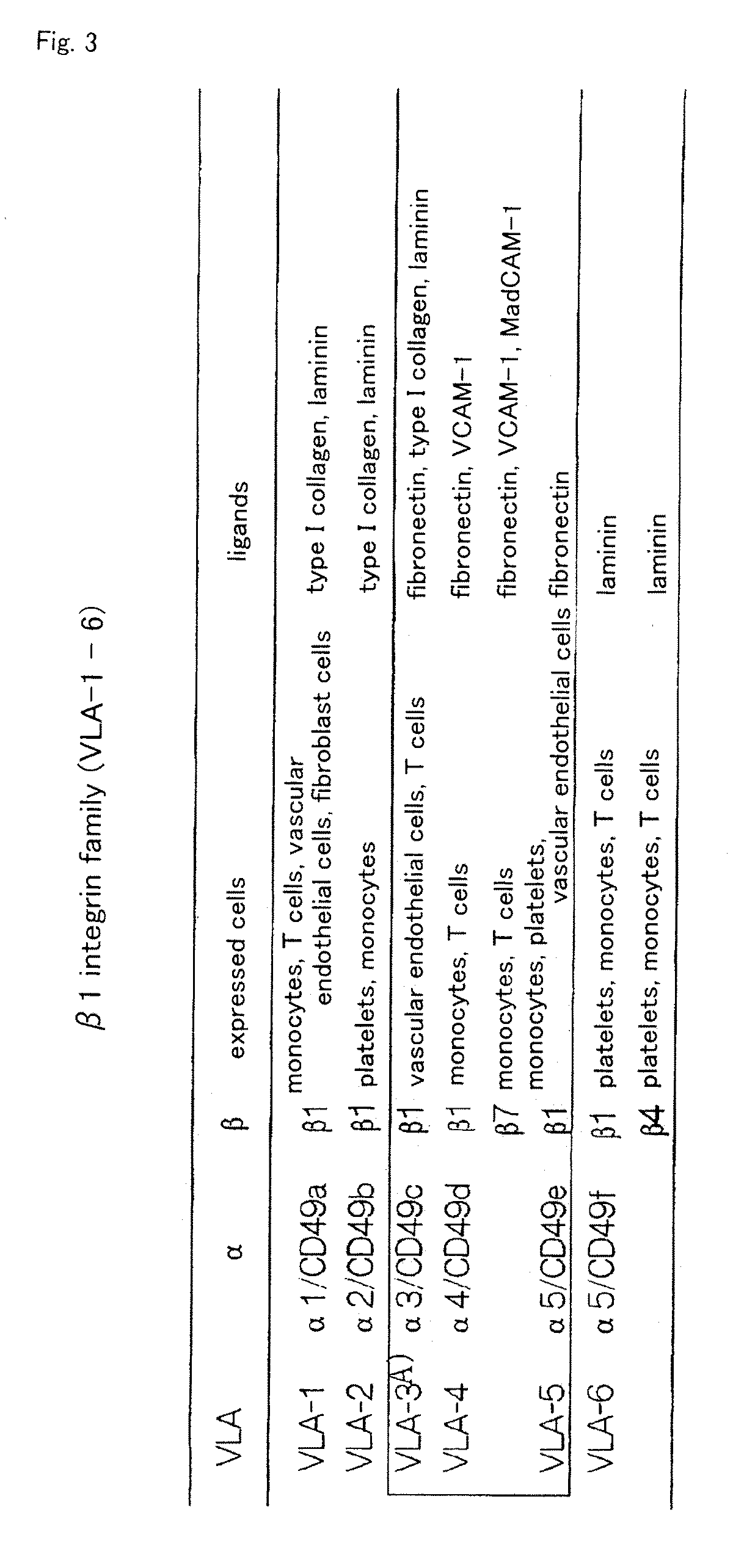
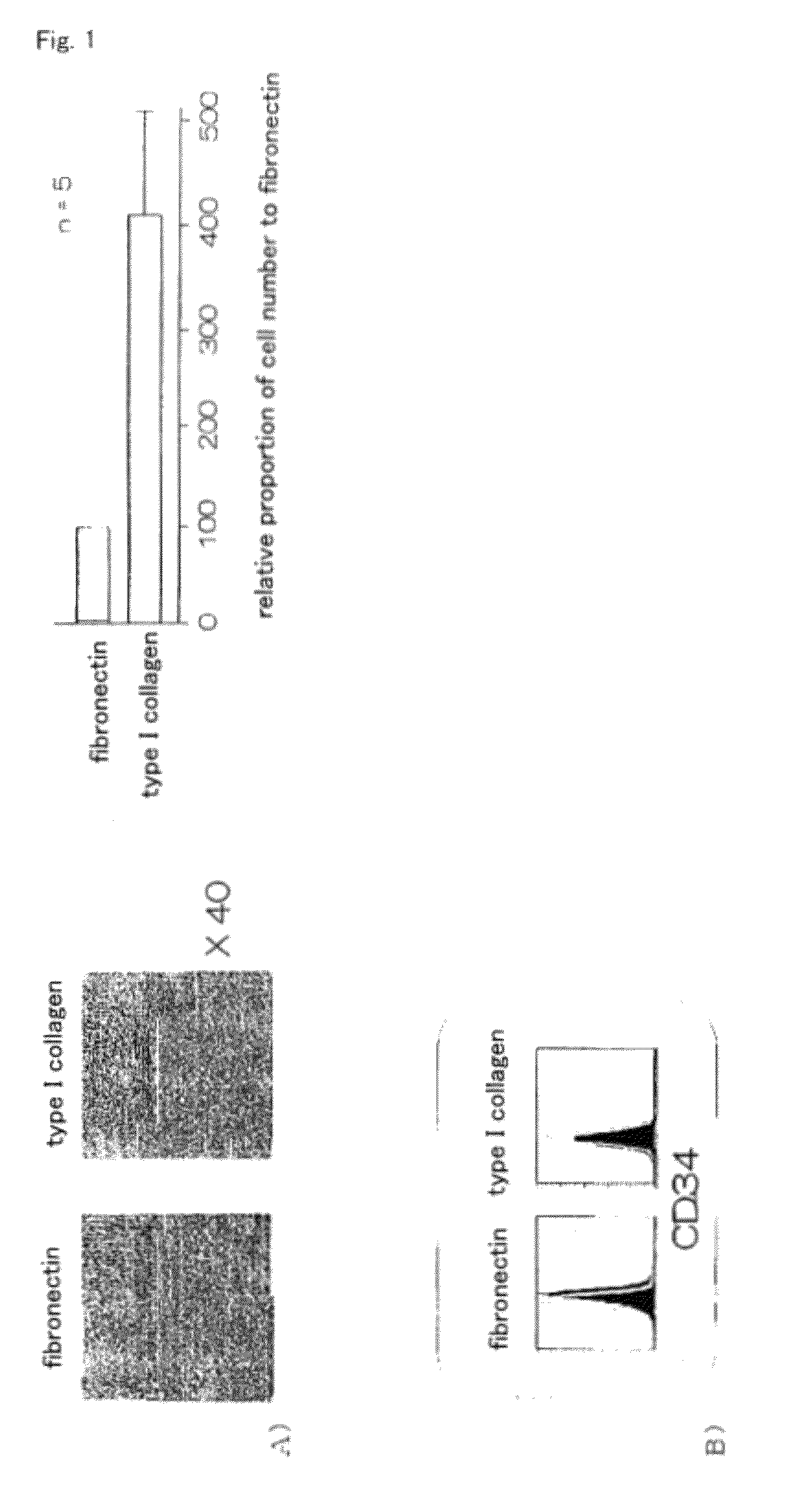
InactiveUS20100261202A1Improve efficiencyA large amountNervous disorderPeptide/protein ingredientsOrgan regenerationFibronectin
It is to provide a practical method for producing efficiently a large amount of MOMC, which is a multipotent cell which is very suitable for cell transplantation for organ regeneration. It was found that by culturing peripheral blood monocytes in vitro on fibronectin in the presence of SDF-1, MOMC can be produced more efficiently, and the present invention has been completed. Specifically, it is a method for producing MOMC by culturing in vitro peripheral blood monocytes expressing CD14 on fibronectin, wherein the in vitro culture is performed in the presence of SDF-1.
Owner:KEIO UNIV
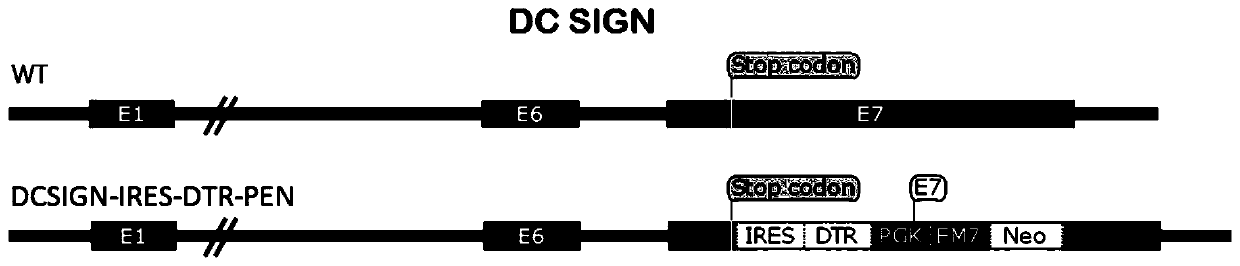
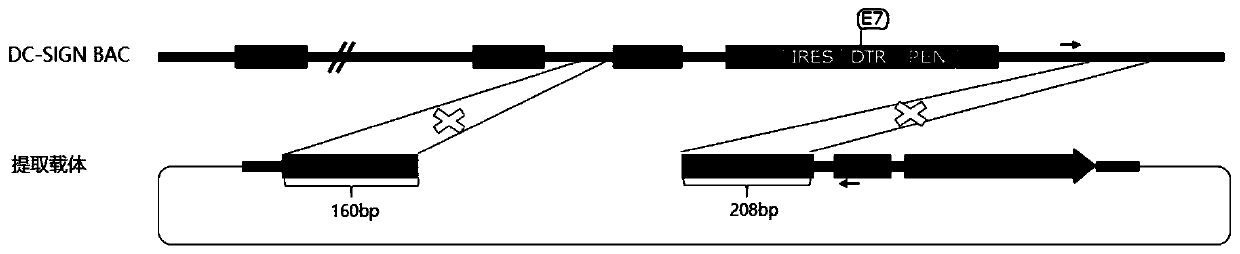
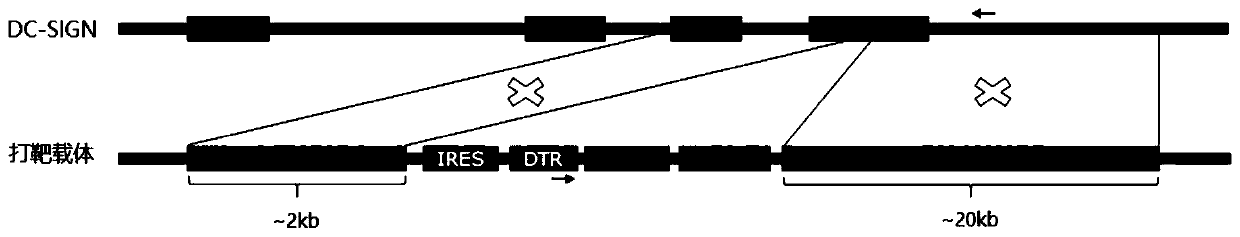
Targeting vector, construction method for transgenic mouse capable of regulating and removing monocyte-derived DC via diphtheria toxin, and application of targeting vector
PendingCN111088280ANormal expressionEasy to removeMicroinjection basedNucleic acid vectorBiotechnologyDendritic cell
The invention relates to the field of animal model construction. The invention discloses a targeting vector, a construction method for a transgenic mouse capable of regulating and removing monocyte-derived DC via the diphtheria toxin, and application of targeting vector. To overcome the defects of existing dendritic cell removal animal models, the invention provides preparation, application and detection methods for a novel inducible DCSIGN-DTR transgenic mouse animal model capable of regulating and removing monocyte-derived DC via the diphtheria toxin, wherein the novel inducible DCSIGN-DTR transgenic mouse animal model is capable of realizing stable passage. The constructed DCSIGN-DTR transgenic mouse animal model for regulating and removing the monocyte-derived DC via the diphtheria toxin has the advantages that monocyte-derived DC removal efficiency reaches 80 to 90%, and the monocyte-derived DC can be better removed; and the function of the monocyte-derived DC is not affected whendiphtheria toxin induction is not used, and no side reaction is caused when the diphtheria toxin induction is used for removing the monocyte-derived DC. Thus, a brand-new monocyte-derived DC removalprinciple and method are provided, and the brand-new monocyte-derived DC inducible removal animal model is provided.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
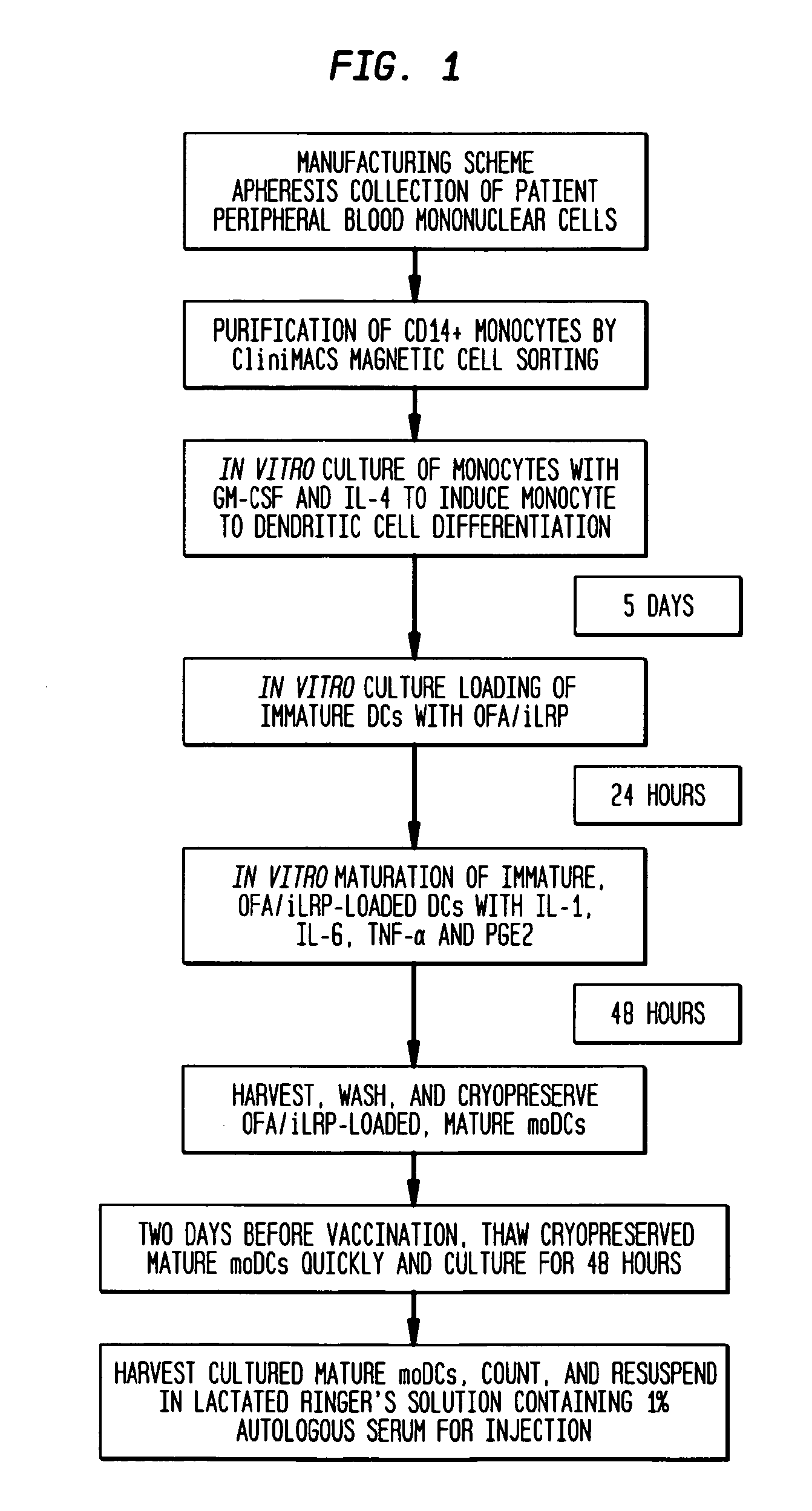
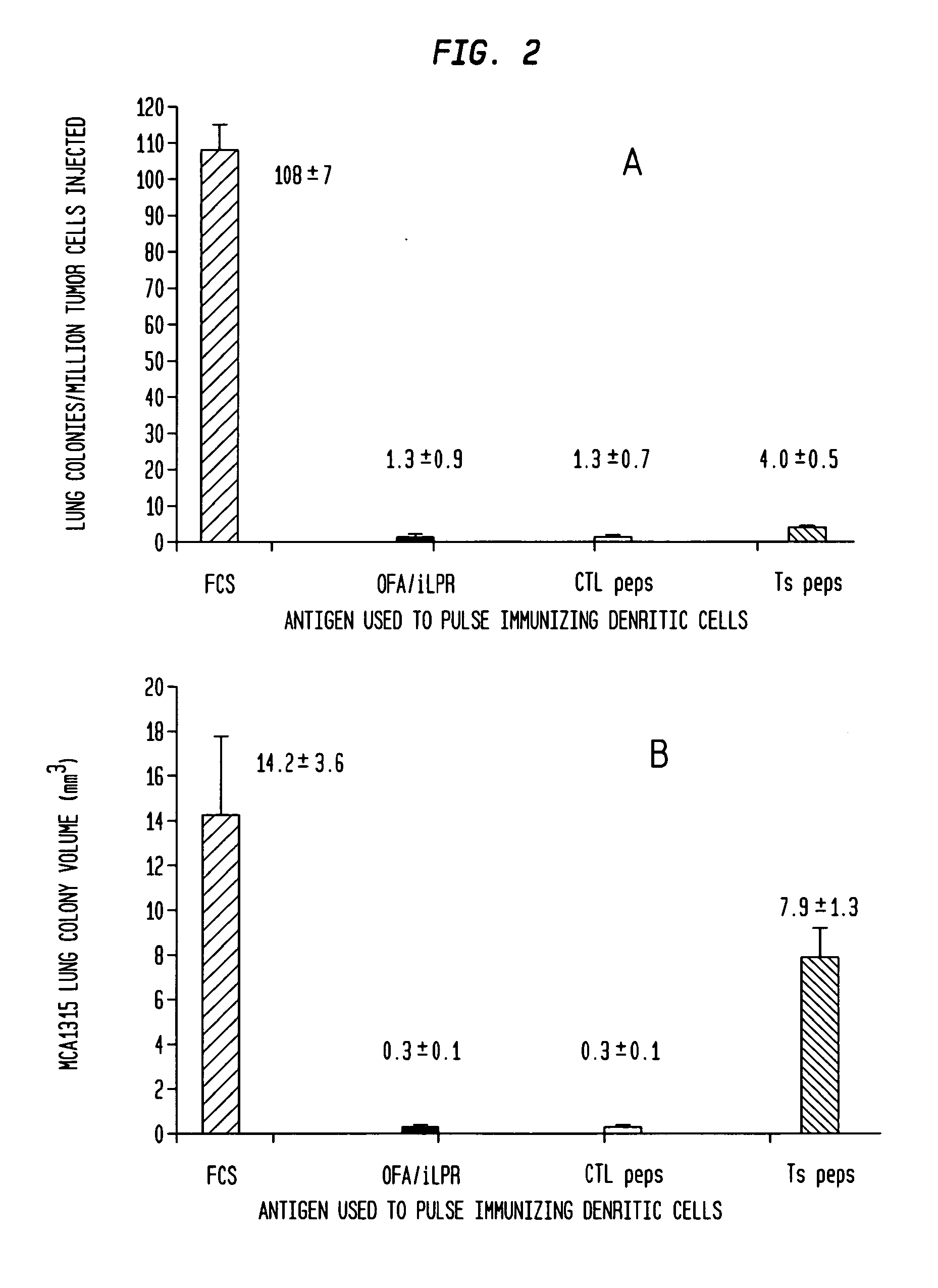
Vaccines with oncofetal antigen/ilrp-loaded autologous dendritic cells and uses thereof
Disclosed are compositions containing isolated monocyte-derived mature dendritic cells loaded with OFA / iLRP, or a fragment thereof that selectively stimulates T cytotoxic lymphocytes, and a carrier, vaccine compositions containing effective dosage amounts of the dendritic cells, methods of making the vaccines, and methods of cancer treatment or therapy that entail administration of the vaccines to cancer patients.
Owner:SOUTH ALABAMA MEDICAL SCI FOUND
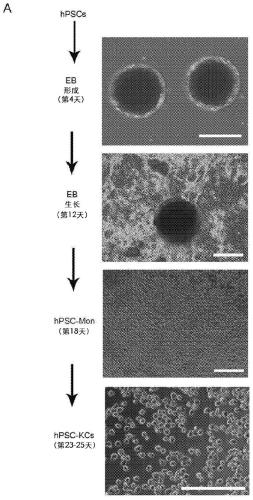
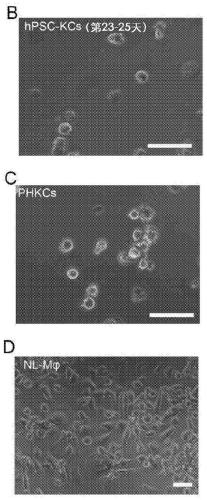
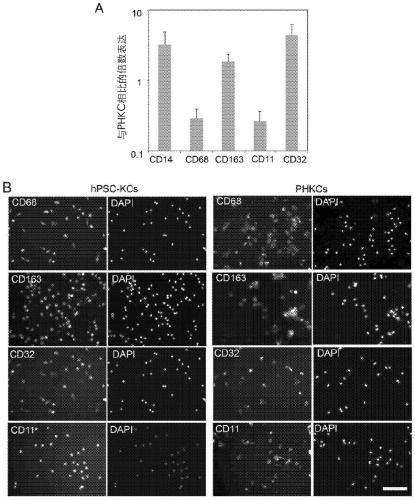
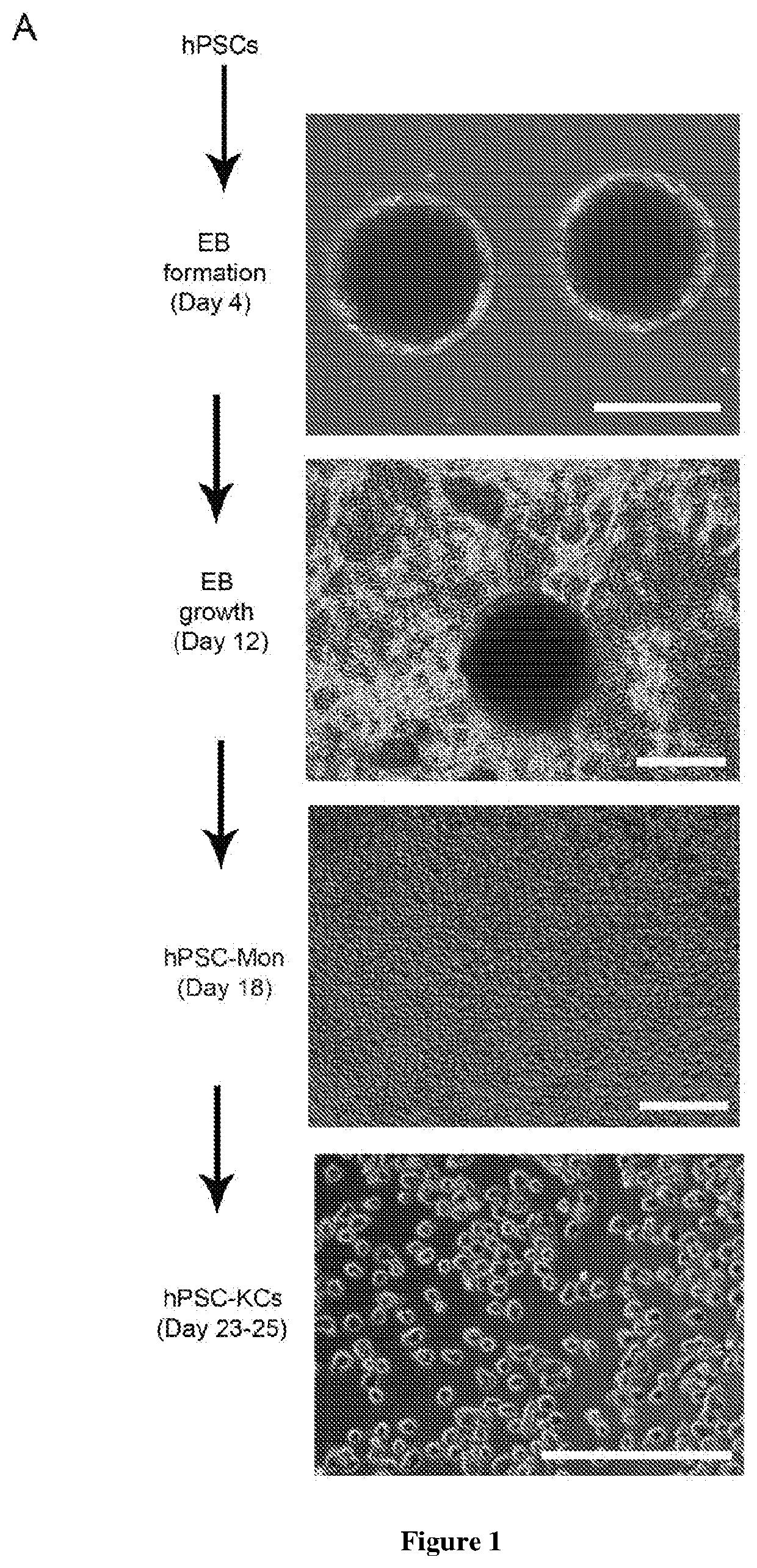
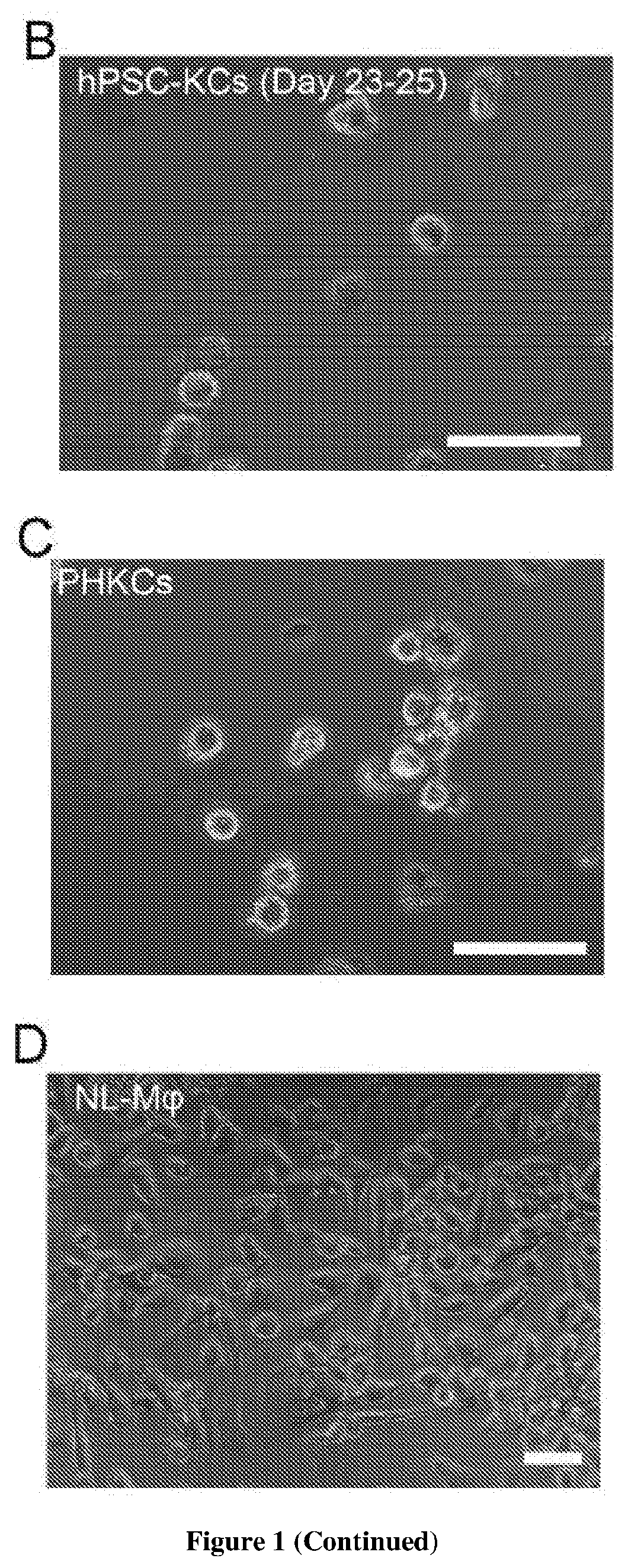
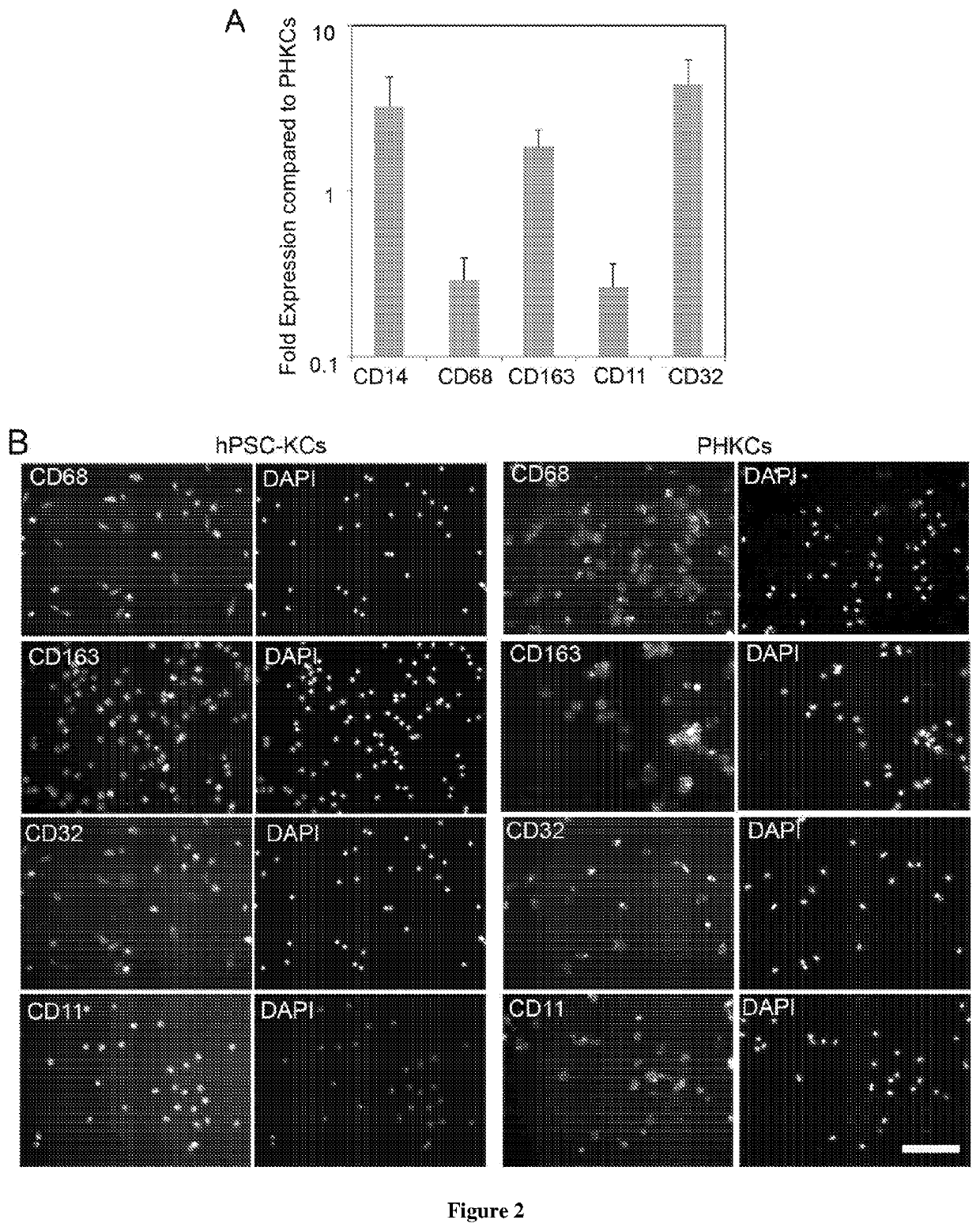
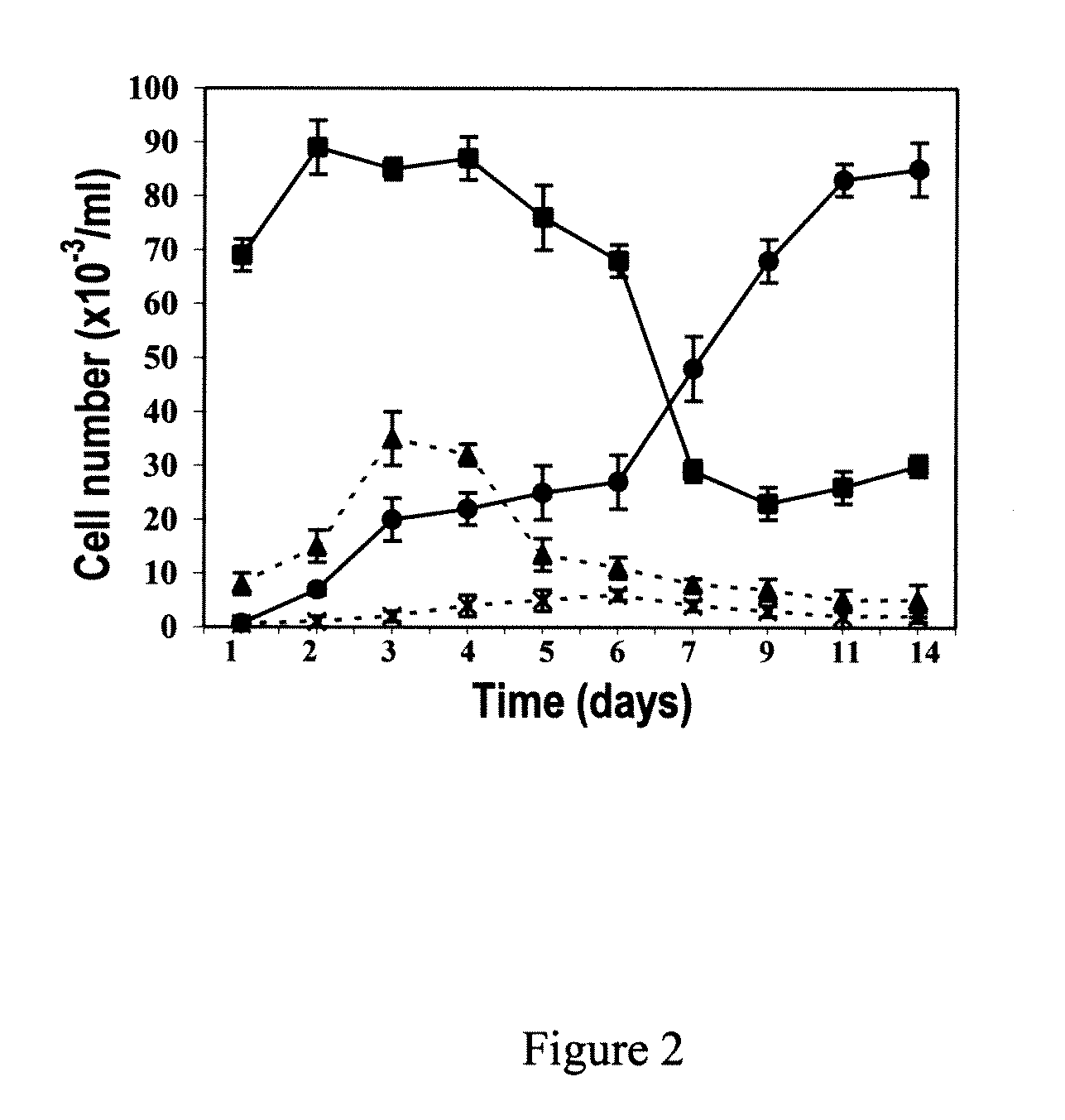
Methods of generating hepatic macrophages and uses thereof
The present disclosure provides a method of deriving hepatic macrophages from stem cell-derived monocytes, through the use of hepatic macrophage culture medium comprising a hepatocyte conditioned medium and a basal medium, wherein the conditioned medium is obtained through culturing hepatocytes in a serum-free culture medium in the presence of an extracellular matrix. Also disclosed is a kit usedfor such a method and hepatic macrophages derived using the method and uses thereof.
Owner:AGENCY FOR SCI TECH & RES
Method for efficient production of monocyte-derived multipotent cell (MOMC)
InactiveUS8216838B2Efficient productionNervous disorderPeptide/protein ingredientsOrgan regenerationCells transplantation
It is to provide a practical method for producing efficiently a large amount of MOMC, which is a multipotent cell which is very suitable for cell transplantation for organ regeneration. It was found that by culturing peripheral blood monocytes in vitro on fibronectin in the presence of SDF-1, MOMC can be produced more efficiently, and the present invention has been completed. Specifically, it is a method for producing MOMC by culturing in vitro peripheral blood monocytes expressing CD14 on fibronectin, wherein the in vitro culture is performed in the presence of SDF-1.
Owner:KEIO UNIV
Combined preparation for the treatment of neoplasic diseases or of infectious diseases
InactiveUS6616925B1Strong cytotoxicityEnhance immune responseBiocideOrganic active ingredientsCytotoxicityWilms' tumor
The present invention relates to combined preparation containing as active substance the following individual components, in the form of a kit-of-parts:monocyte derived cells, particularly cytotoxic macrophages,chemotherapy or immunotherapy drugs,for the simultaneous, separate or sequential use, for the treatment of cancer or infectious diseases.
Owner:I D M IMMUNO DESIGNED MOLECULES
CD40-binding APC-activating molecules
Disclosed are agonist anti-CD40 molecules, including monoclonal antibodies, which can bind to and stimulate professional and non-professional human antigen-presenting cells (“APCs”), enhance the stimulatory effect of CD40L on CD40 positive cells and / or induce phenotypical maturation of monocyte derived dendritic cells. Several such monoclonal antibodies are provided, and cell lines producing them have been deposited at the American Type Culture Collection.
Owner:MYCENAX BIOTECH
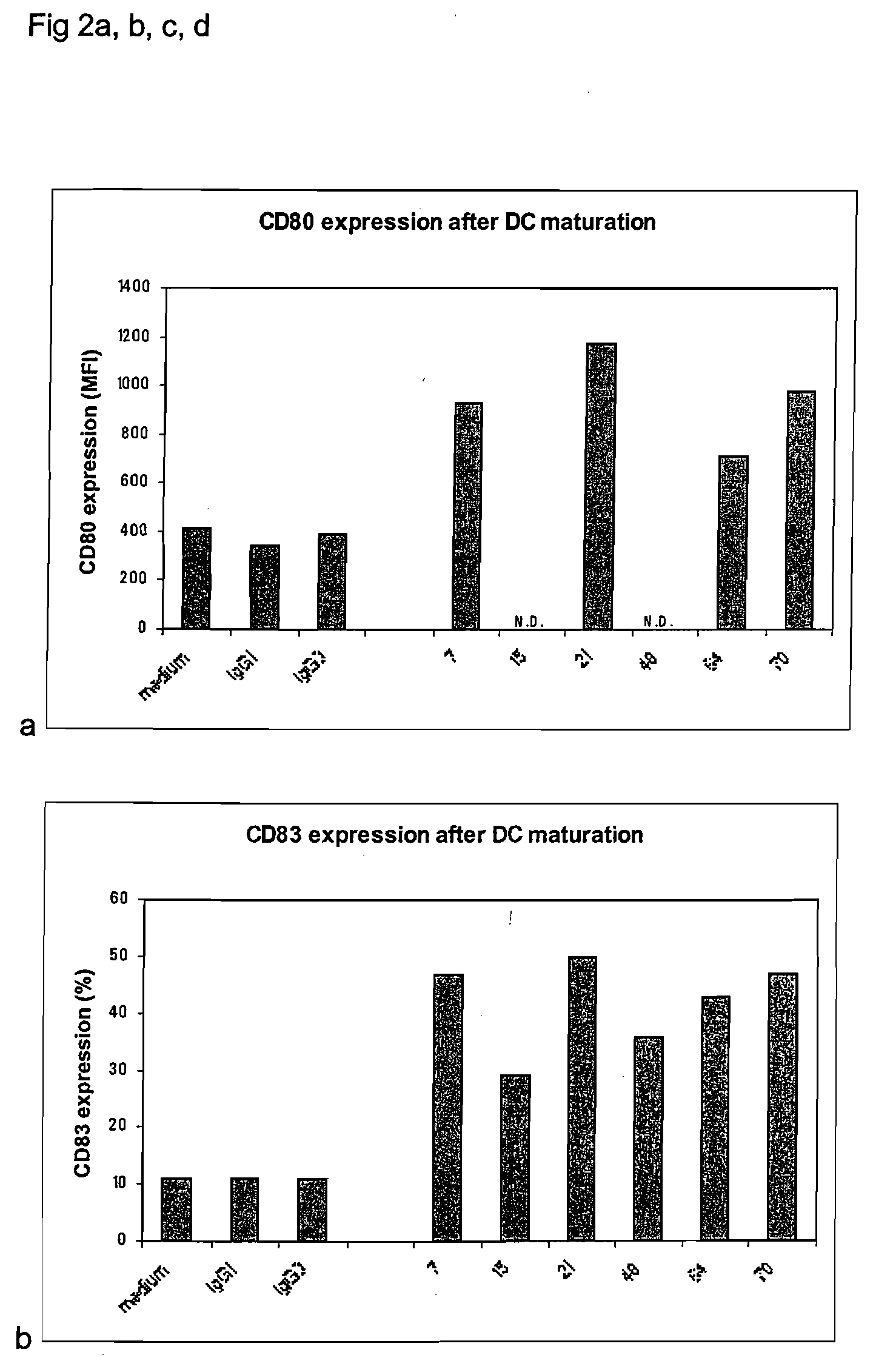
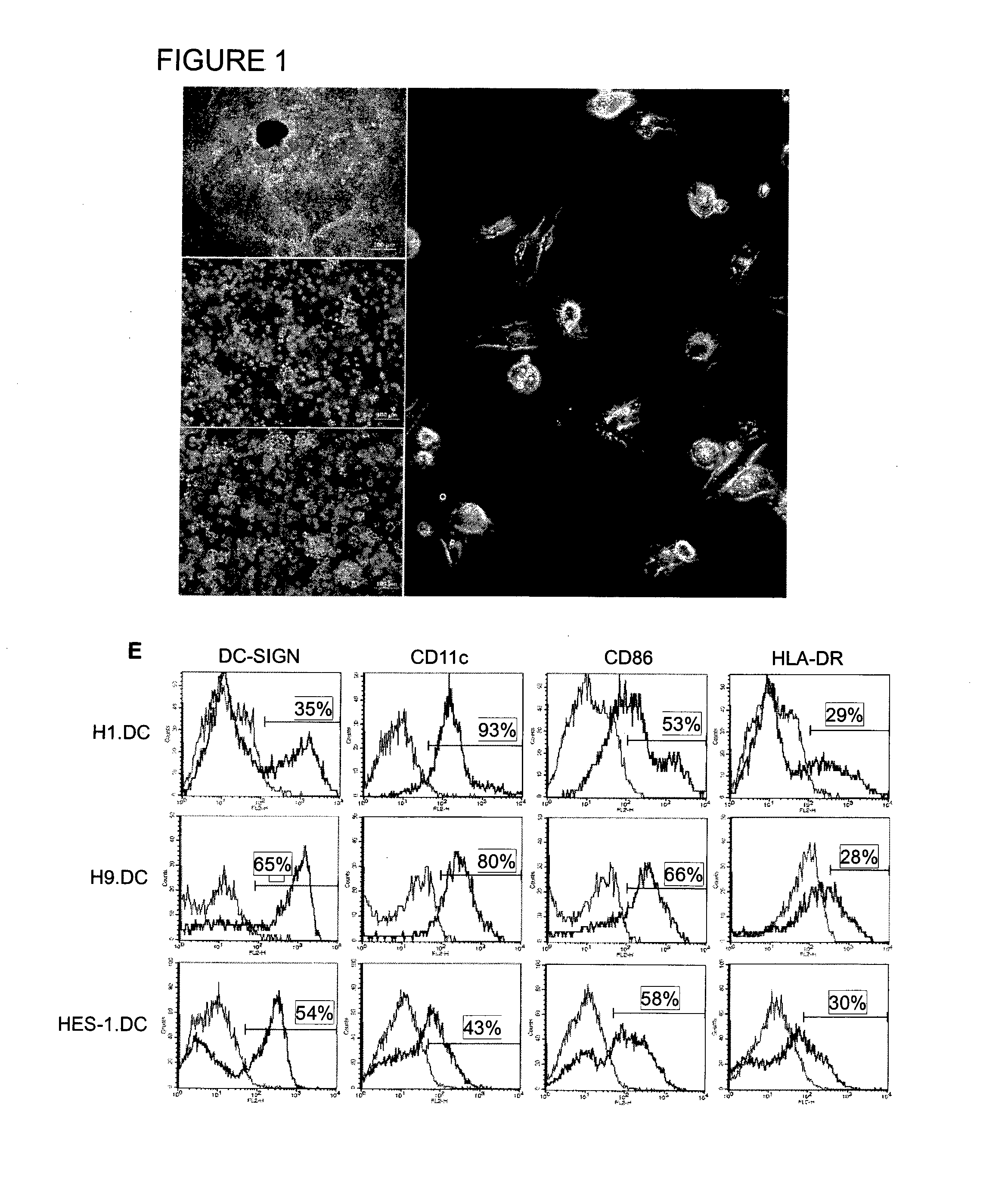
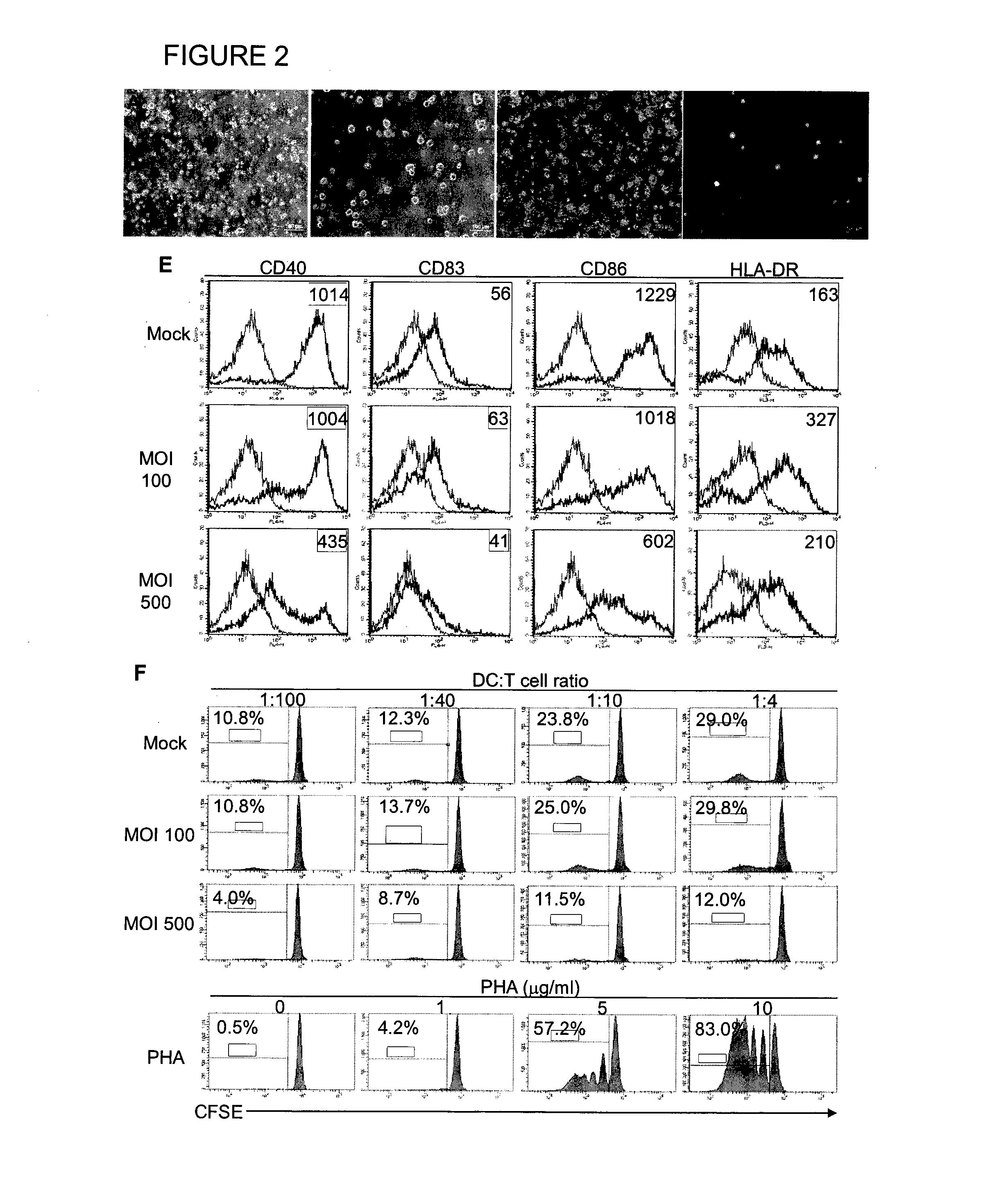
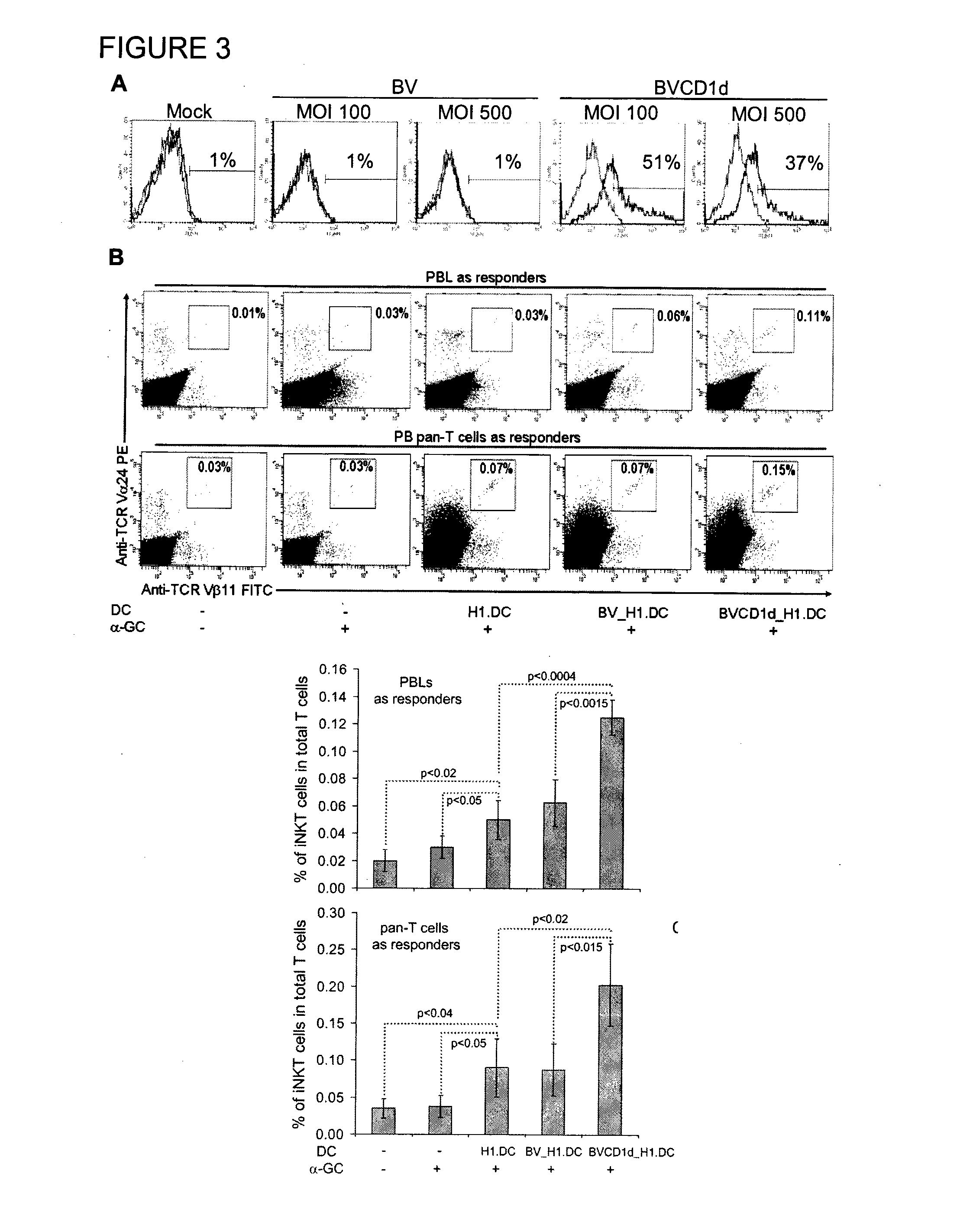
Method of using cd1d over-expression in human dendritic cells to enhance cd8+ t cell-based and invariant natural killer t cell-based antitumor immunity
InactiveUS20130344095A1Genetically modified cellsSnake antigen ingredientsAntitumor immunityDendritic cell
The manipulation of human dendritic cells (DCs) to induce potent anti-tumor immunity remains an essential subject of study. Here we report that the overexpression of CD1d in human DCs can enhance the priming of naïve CD8+ T cells against tumor antigen. We showed that CD1d can be overexpressed in the human DCs using baculoviral vector carrying the CD1d gene. This CD1d-overexpression is functional as demonstrated by the increased expansion of invariant natural killer T (iNKT) cells while using these modified DCs to present α-galactosylceramide (α-GC). Pulsed with tumor antigenic peptide, these CD1d-overexpressing human DCs showed enhanced capability to prime naïve CD8+ T cells. CD1d-overexpressing human DCs also induced a pro-inflammatory cytokine profile that may favor the priming. Moreover, this CD1d-overexpression strategy can be extrapolated to monocyte-derived human DCs. Therefore, our study suggest that overexpression of CD1d in human DCs may provide a novel strategy to enhance DC immunogenicity and the possible translation into human cancer immunotherapy.
Owner:AGENCY FOR SCI TECH & RES
Compounds, kits and methods for conferring cytoprotection
InactiveUS20090155228A1Inhibit transcriptionEasy to testBiocideArtificial cell constructsDiseaseLipid formation
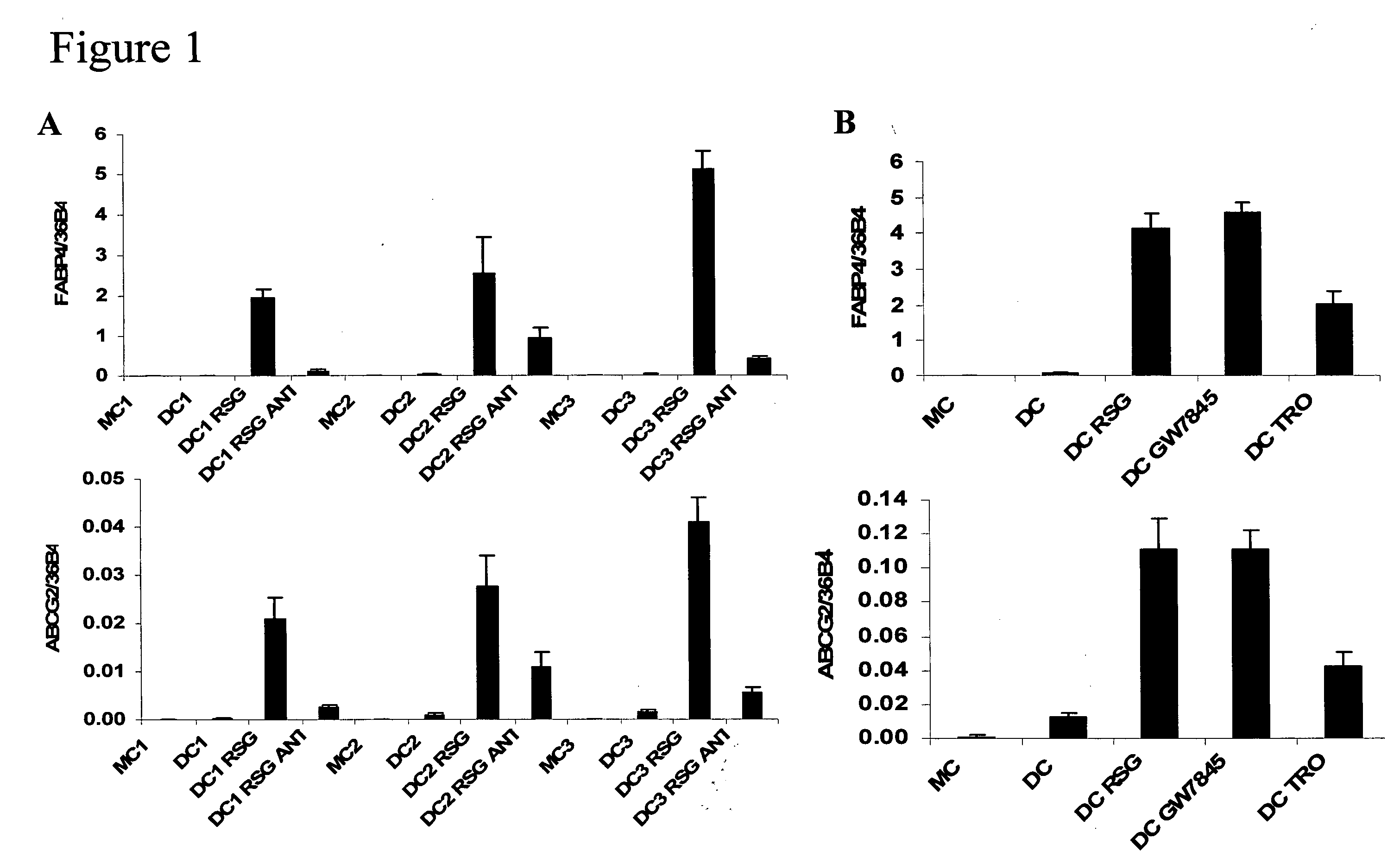
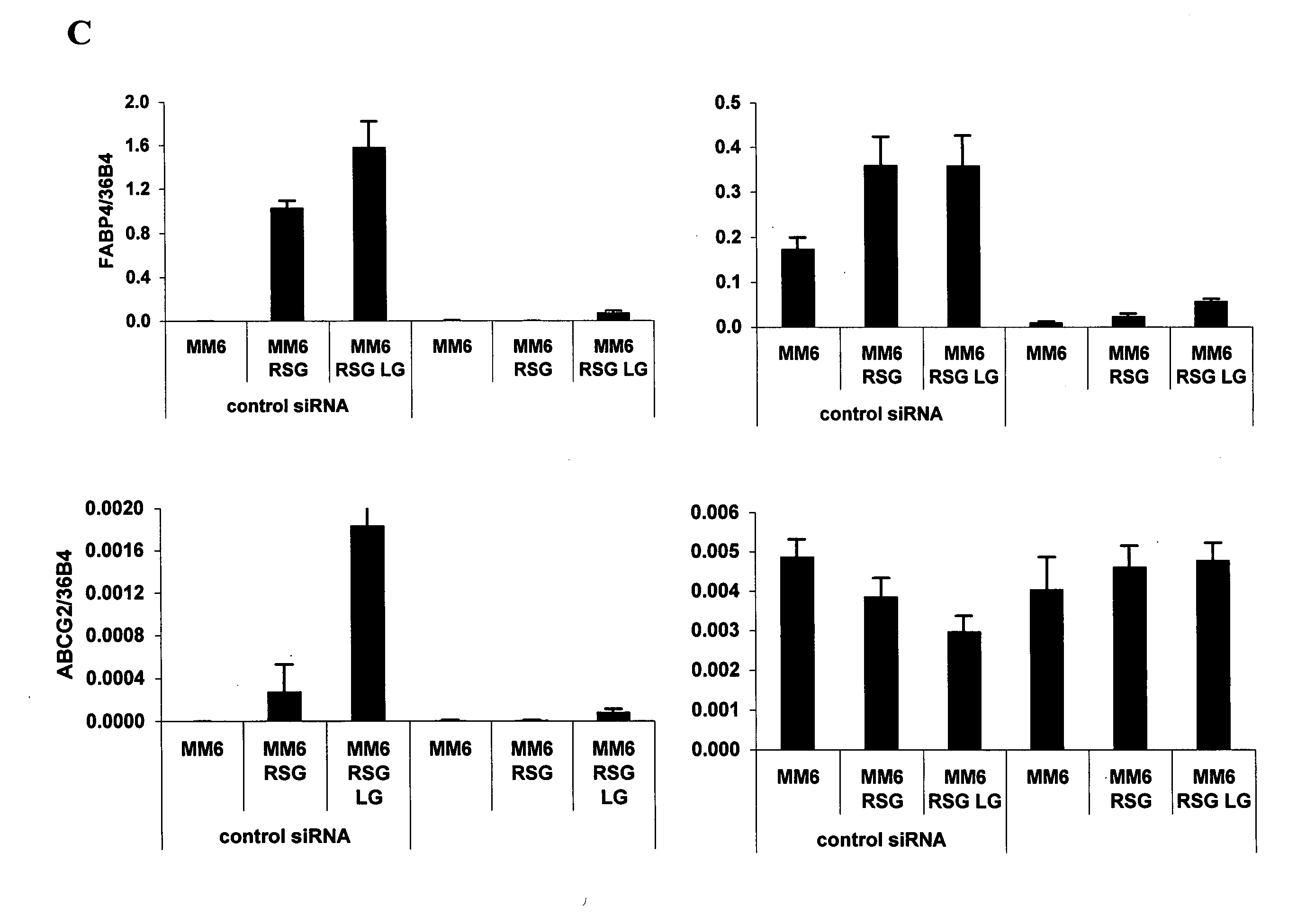
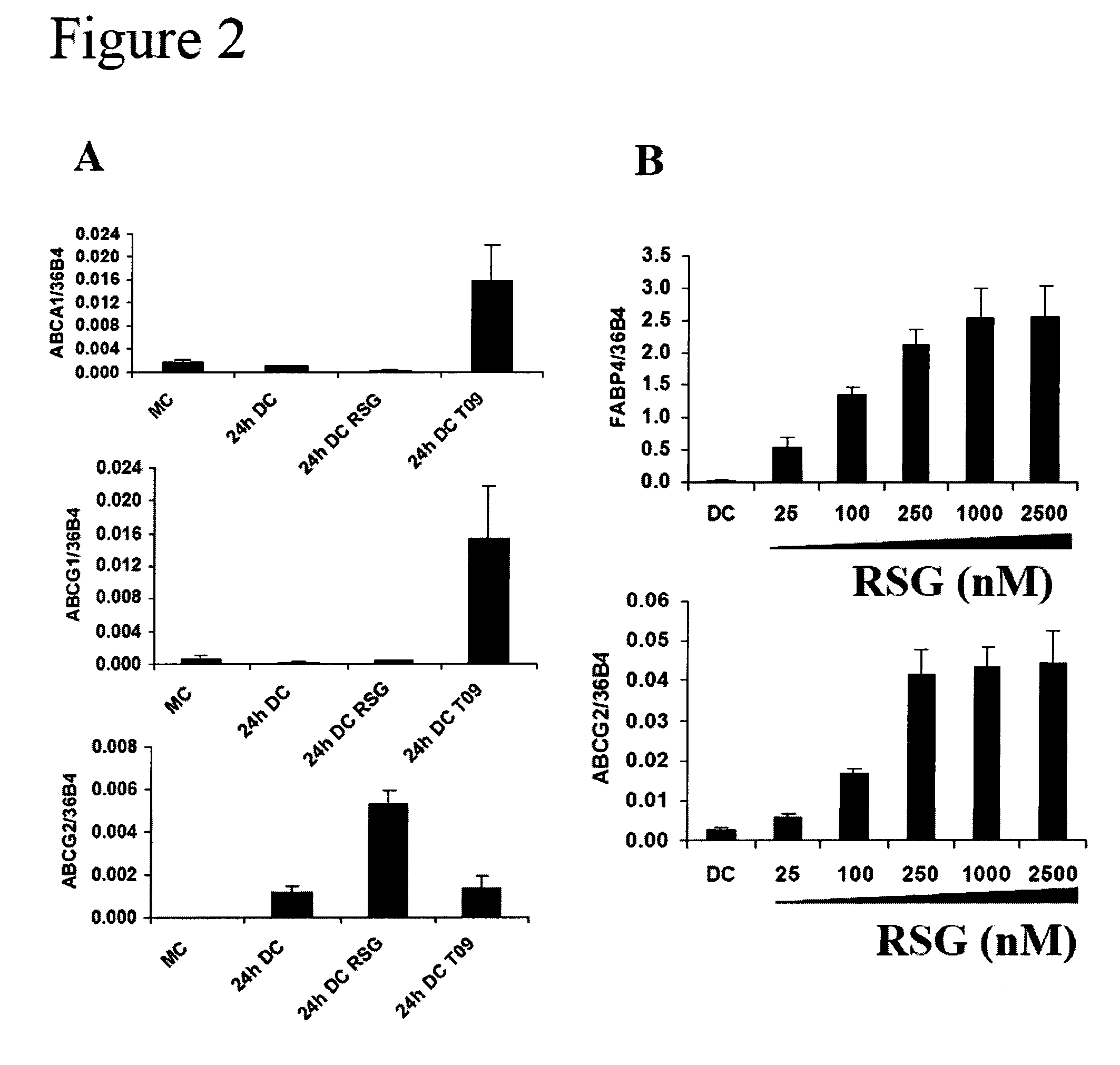
hABCG2, a member of the ATP-Binding Cassette transporters has been identified as a protective pump against endogenous and exogenous toxic agents. ABCG2 was shown to be expressed at high levels in stem cells, and variably regulated during cell differentiation. It is demonstrated herein that functional ABCG2 is expressed in human monocyte-derived dendritic cells by the activation of a nuclear hormone receptor, PPARg. The present results uncovered a mechanism by which up-regulation of functional ABCG2 expression can be achieved via exogenous or endogenous activation of the lipid activated transcription factor, PPARg. Thus the invention relates to combined treatments by PPARg agonists and cytotoxic drugs transportable by ABCG2, various treatments in the field of neoplastic diseases as well as cell therapy, including autologous cell therapy, as well as kits and composition therefor. Method for protecting cells against cytotoxic drugs are also provided.
Owner:UNIVERSITY OF DEBRECEN
Antigen presenting cell assay
Disclosed herein are methods for diagnosing or predicting acute cellular and / or humoral rejection in a subject. In one example, a method of assessing organ rejection includes contacting a first sample comprising antigen presenting cells (APCs) obtained from a subject in need of or having received an organ transplant with a donor antigen from a donor under conditions sufficient to induce uptake of the donor antigen; contacting a second sample comprising APCs obtained from the subject in need of or having received an organ transplant with a third-party antigen under conditions sufficient to induce uptake of the third-party antigen; and determining an antigen presenting index by determining a ratio of uptake of the donor antigen in the first sample to uptake of the third-party antigen in the second sample, wherein the ratio of greater than one indicates organ rejection and the APCs are monocytes or monocyte-derived cells.
Owner:UNIVERSITY OF PITTSBURGH
Methods and pharmaceutical compositions for modulating monocytopoiesis
PendingUS20200216530A1Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsConventional Dendritic CellApoptosis
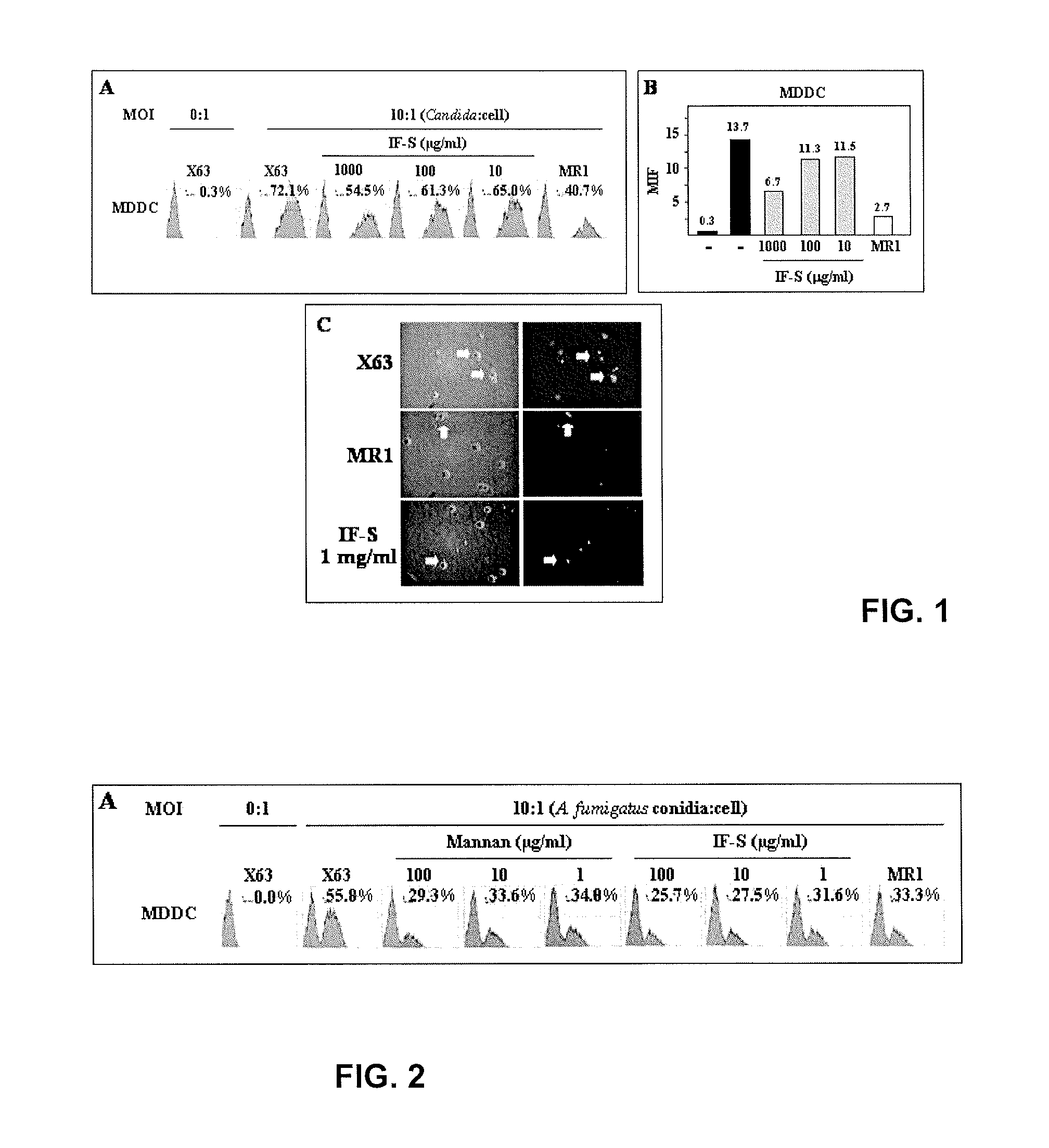
Monocytopoiesis is a hematological process that supplies the periphery with monocytes and subsequently with macrophages and monocyte-derived dendritic cells. Typically, monocytes circulate in the bloodstream for a very short time before undergoing apoptosis, however, stimulatory signals can trigger monocyte survival by inhibiting the apoptotic pathway, and thus contribute to the maintenance of the inflammatory response. Accordingly, there is a need for methods and pharmaceutical compositions for modulating monocytopoiesis. Now, the inventors show that type I interferons signaling promote the differentiation of monocyte-derived phagocytes at the level of their progenitors. Importantly, IFN-alpha and -beta were found to efficiently generate the development of monocyte-derived antigen-presenting cells while having no impact on the precursor activity of conventional dendritic cells. Accordingly, modulators of type I interferon (e.g. neutralizing antibodies or type I IFN polypeptides) would be suitable for modulating monocytopoiesis in subjects in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Methods for generating engineered human primary blood dendritic cell lines
PendingUS20180305666A1Highly potent anti-cancer cytotoxic lymphocytesAntibody mimetics/scaffoldsGenetically modified cellsDendritic cellTax Protein
Owner:UNIV OF MARYLAND BALTIMORE
PORCINE DC-SIGN, ICAM-3 AND LSECtin AND USES THEREOF
InactiveUS20100317054A1Enhancing immunogenic activityBacteriaViruses/bacteriophagesEpidermal Dendritic CellsNucleotide
The present invention relates to the cloning, identification and characterization of the unique and entire genomic sequences encoding new porcine DC-SIGN and LSECtin proteins, including the novel nucleotide sequences of the full-length cDNA and genes of both pDC-SIGN gene and pLSECtin. Also provided are the nucleic acid molecules encoding newly discovered porcine ICAM-3 isoforms from porcine monocyte-derived dendritic cells and the use thereof. Specifically, the invention is drawn to an isolated nucleic acid molecule comprising a nucleotide sequence encoding one or more of porcine DC-SIGN, porcine ICAM-3, porcine LSECtin, a complement of the nucleotide sequence or a functional, defined portion of the nucleotide sequence or a protein fusion product linked to a protein that may be of porcine or human origin. Methods for isolating and cloning the new porcine genes and for using the new nucleotide sequences in improved methods for propagating viruses, particularly enveloped viruses, are additionally described herein. The invention further includes new transfected cells or cell lines that can stably express the porcine proteins, new antibodies and the like.
Owner:VIRGINIA TECH INTPROP INC
Methods of generating hepatic macrophages and uses thereof
The present disclosure provides a method of deriving hepatic macrophages from stem cell-derived monocytes, through the use of hepatic macrophage culture medium comprising a hepatocyte conditioned medium and a basal medium, wherein the conditioned medium is obtained through culturing hepatocytes in a serum-free culture medium in the presence of an extracellular matrix. Also disclosed is a kit used for such a method and hepatic macrophages derived using the method and uses thereof.
Owner:AGENCY FOR SCI TECH & RES
Monocyte-Derived Stem Cells (MDSC) and Methods of Use Thereof
InactiveUS20110070644A1Reduce riskIncrease the number ofBiocideGenetic material ingredientsDiseaseMammal
Monocyte derived adult stem cells (MDSCs) isolated from peripheral blood of mammals is provided, along with pharmaceutical compositions containing an MDSC, kits containing a pharmaceutical composition, and methods of preparing, propagating and using MDSCs or differentiated derivatives thereof The uses of these biological materials include methods of treating disorders or diseases, as well as methods of ameliorating a symptom associated with any such disorder or disease.
Owner:HUBERMAN ELIEZER +1
Construction method of hepatitis vaccine sensitization dendritic cell inducing specific T cell
InactiveCN105368777AImprove immunityDegrade virus geneAntiviralsMammal material medical ingredientsDendritic cellHepatitis vaccine
The invention provides a construction method of a hepatitis vaccine sensitization dendritic cell inducing specific T cell. The method comprises the following steps that (1) an antigen load mononuclear cell derivative dendritic cell is obtained; (2) the T cell is amplified; (3) the specific hepatitis virus T cell is obtained. The method can efficiently present viral antigen.
Owner:黄月华
Oncolytic herpes simplex virus infected cells
ActiveUS10596210B2Stimulate immune responseEnhance immune responseBiocideHeavy metal active ingredientsInfected cellDisease
A monocyte, monocyte derived cell or macrophage infected with an oncolytic herpes simplex virus is disclosed together with uses of such infected cells in the treatment of diseases such as cancer.
Owner:VIRTTU BIOLOGICS +1
Pancreatic islet-like cells
Owner:OPEXA THERAPEUTICS
Method for measuring immunogenicity of protein agent
InactiveUS20190194618A1Improve accuracyImprove efficiencyCompound screeningApoptosis detectionDendritic cellGenotype
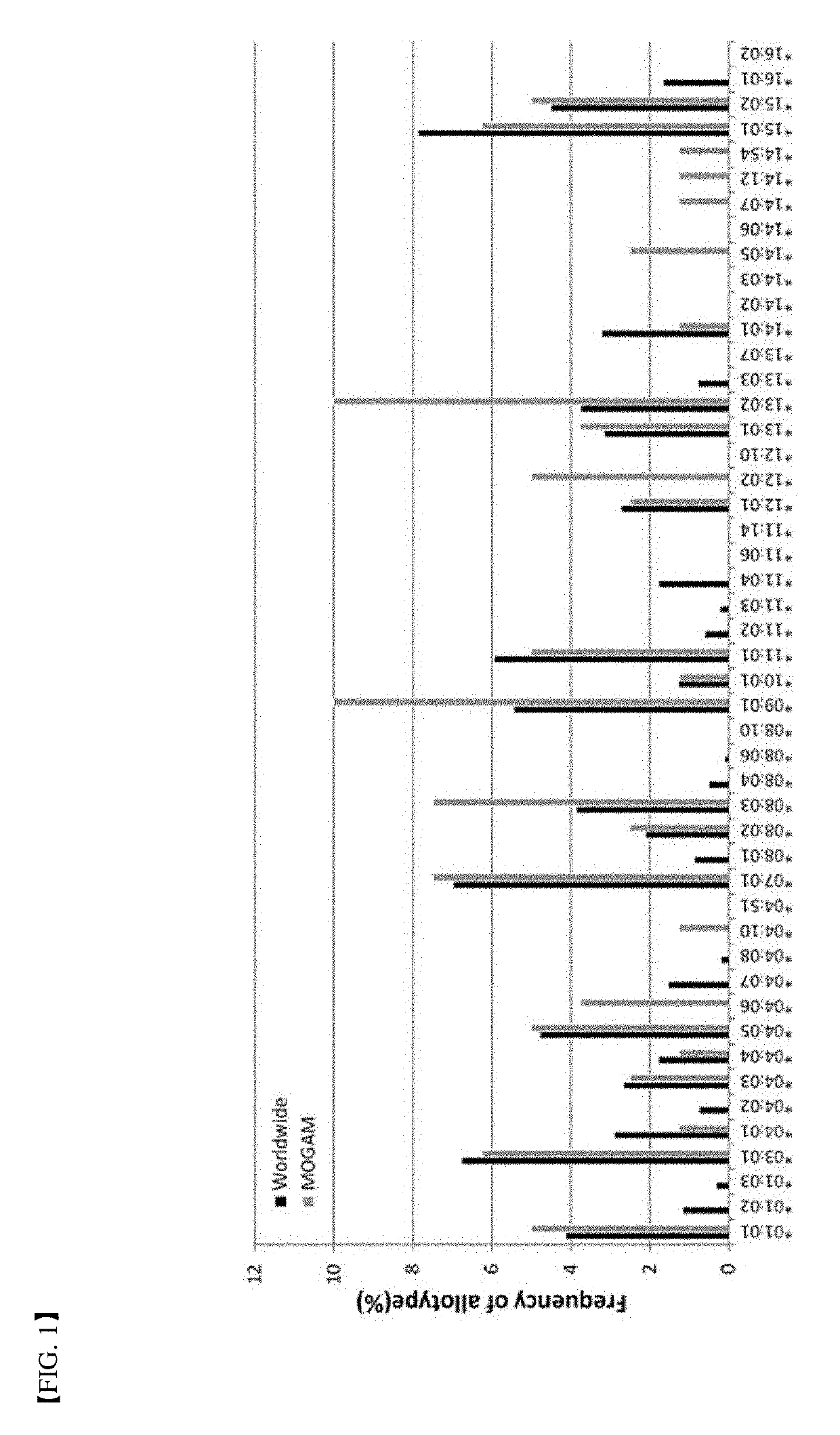
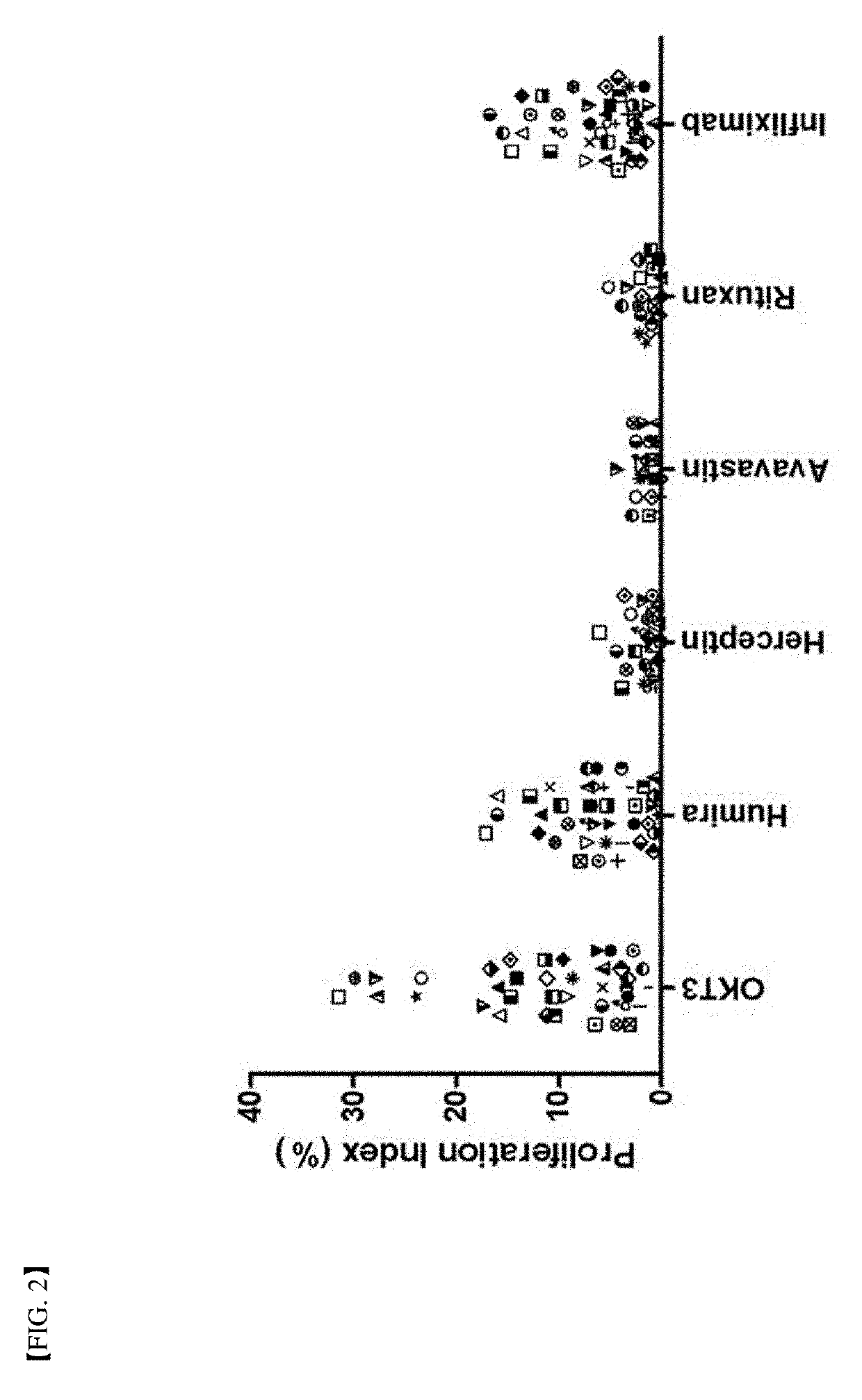
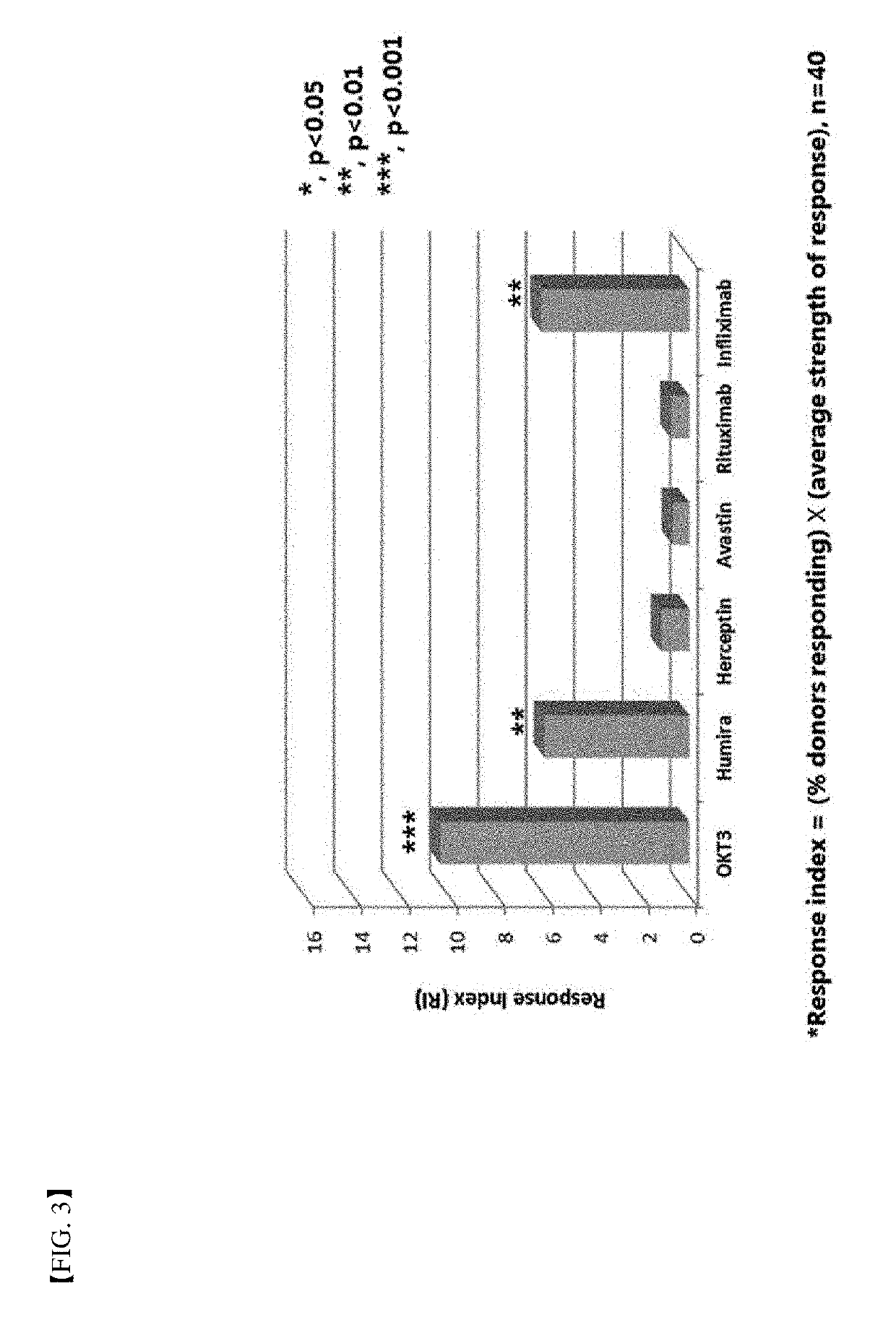
A method for determining immunogenicity of a protein agent. The method includes constructing a library of peripheral blood mononuclear cells having various HLA-DRB1 genotypes; culturing peripheral blood mononuclear cell CD14+ monocyte-derived immature dendritic cells for each genotype in a medium containing a protein to be measured, GM-CSF, IL-4, TNF-α, IL-1β, IL-6 and PGF2 to prepare mature dendritic cells; removing CD8+ T cells from the peripheral blood mononuclear cells for each genotype to prepare CD8+ T cell-free peripheral blood mononuclear cells; co-culturing the mature dendritic cells and the CD8+ T cell-free peripheral blood mononuclear cells at a cell count ratio of approximately 1:5 to 1:20; and quantifying the CD4+ T cells proliferated by co-cultivation per genotype.
Owner:MOGAM INST FOR BIOMEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com