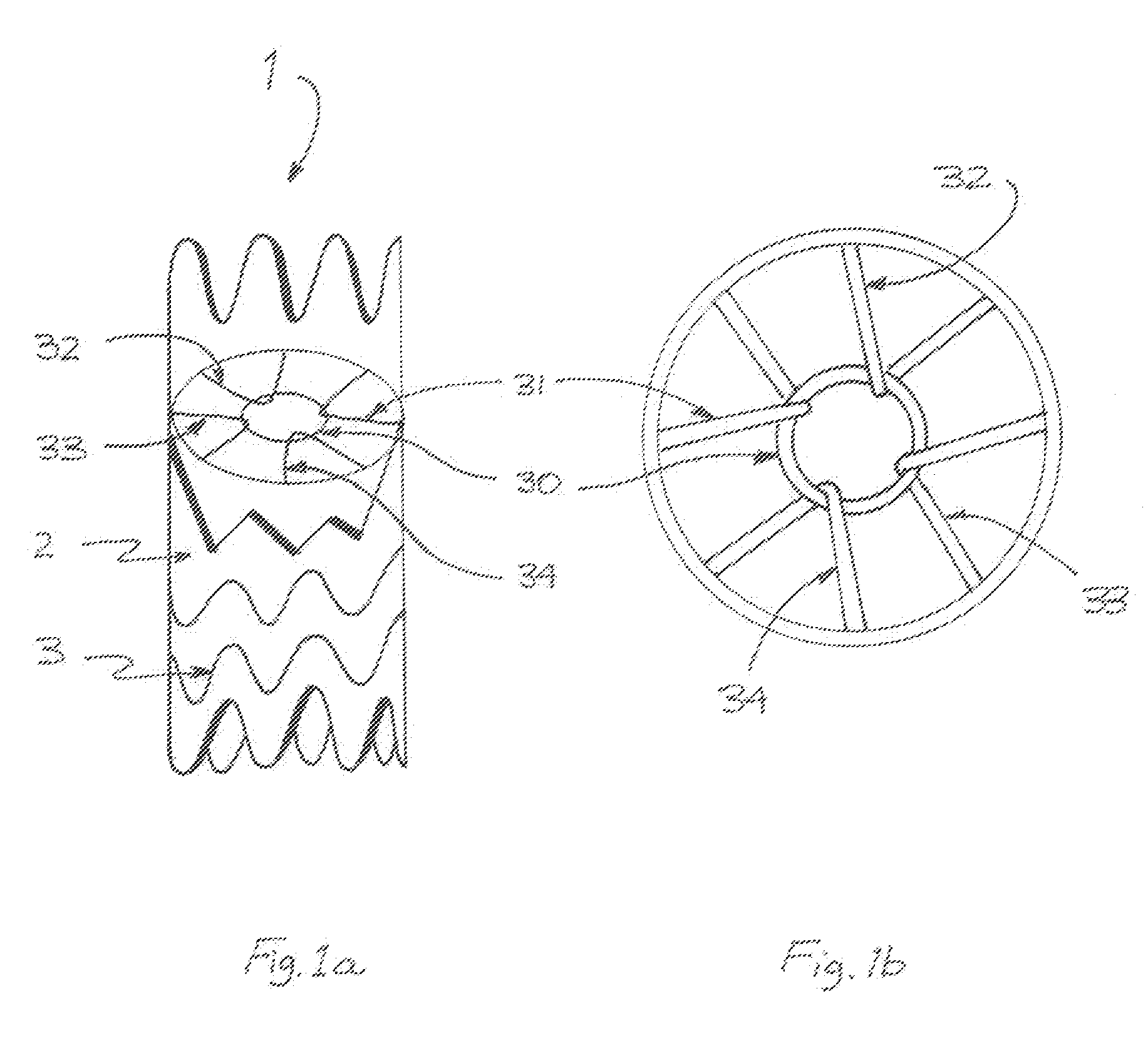
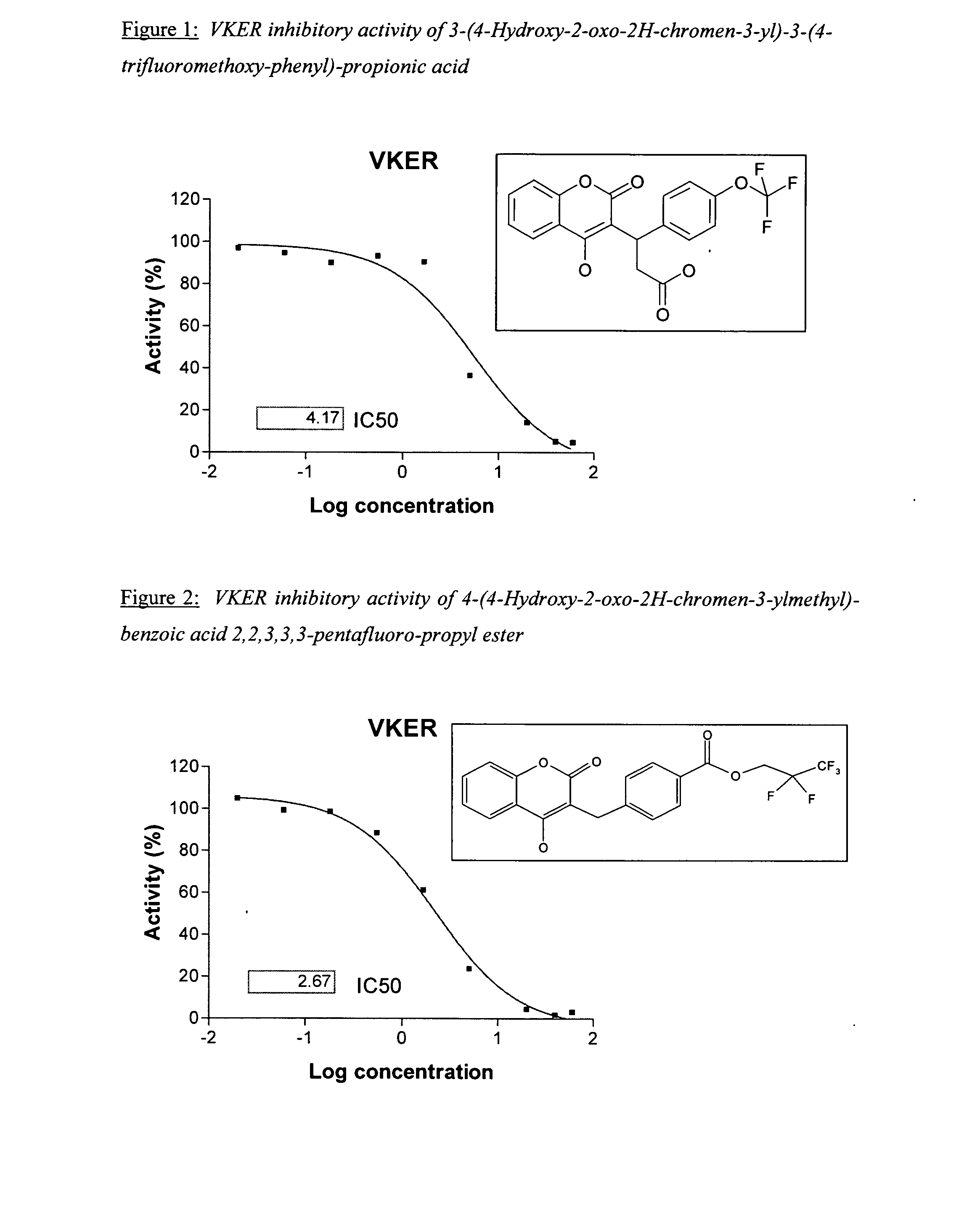
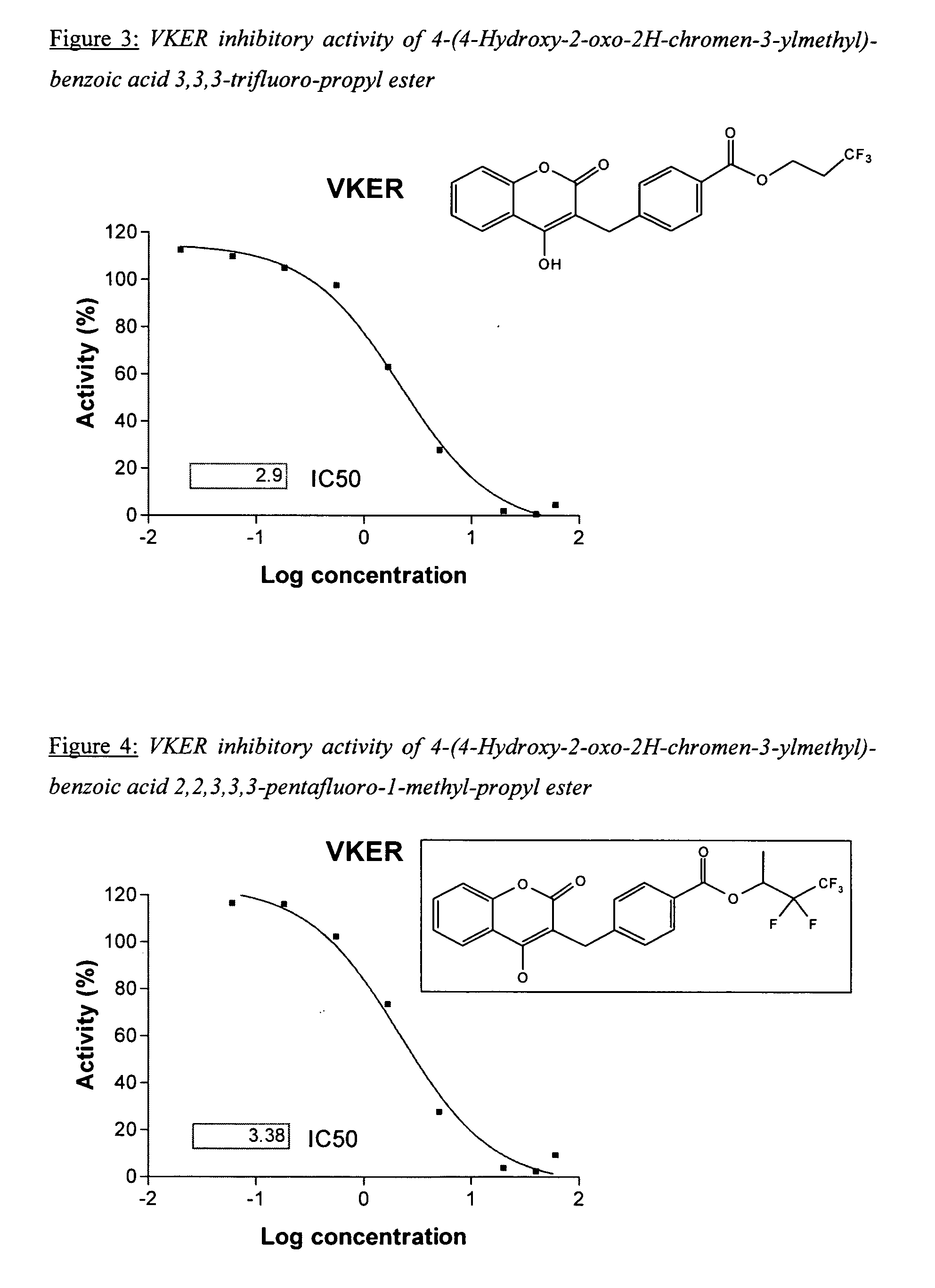
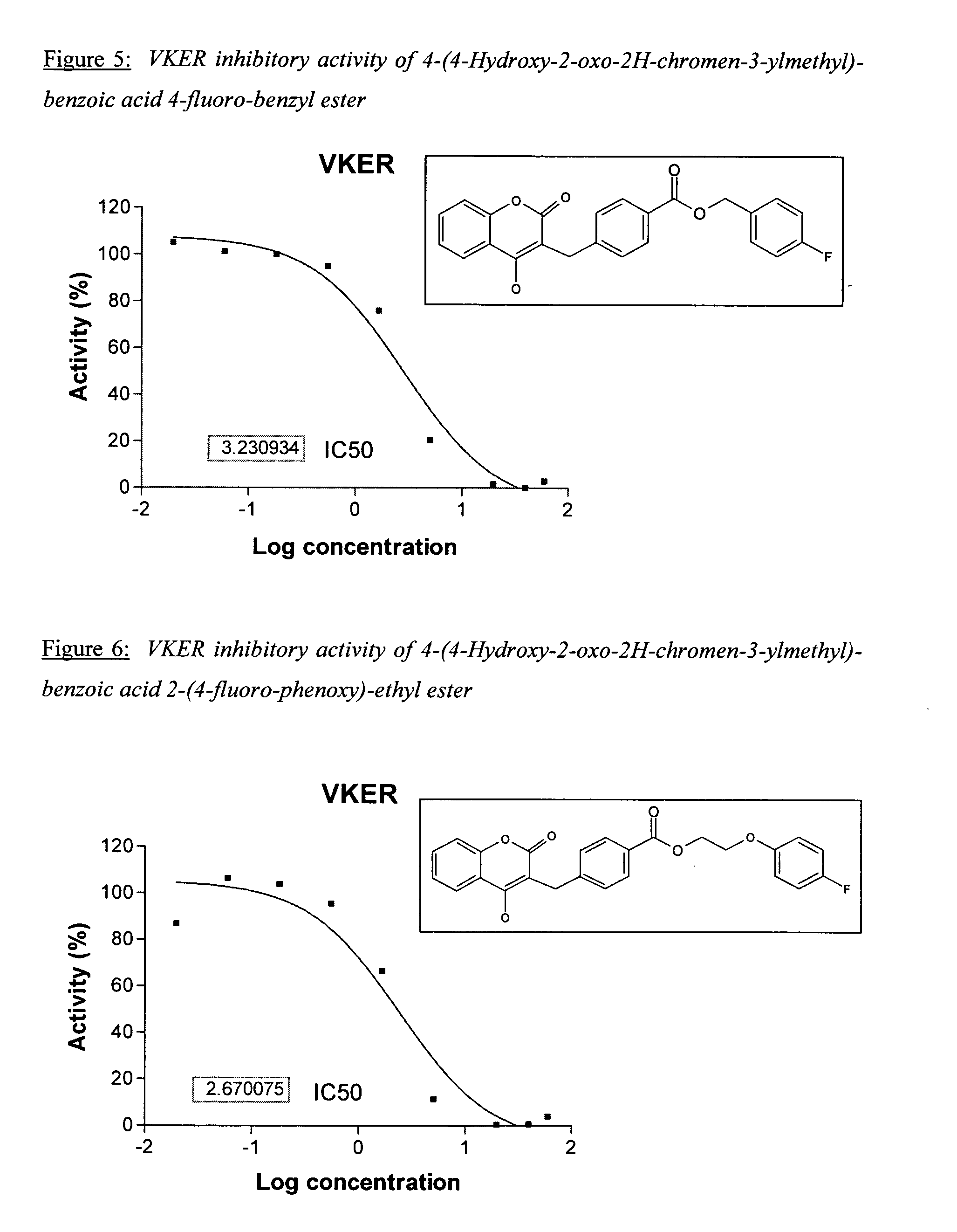
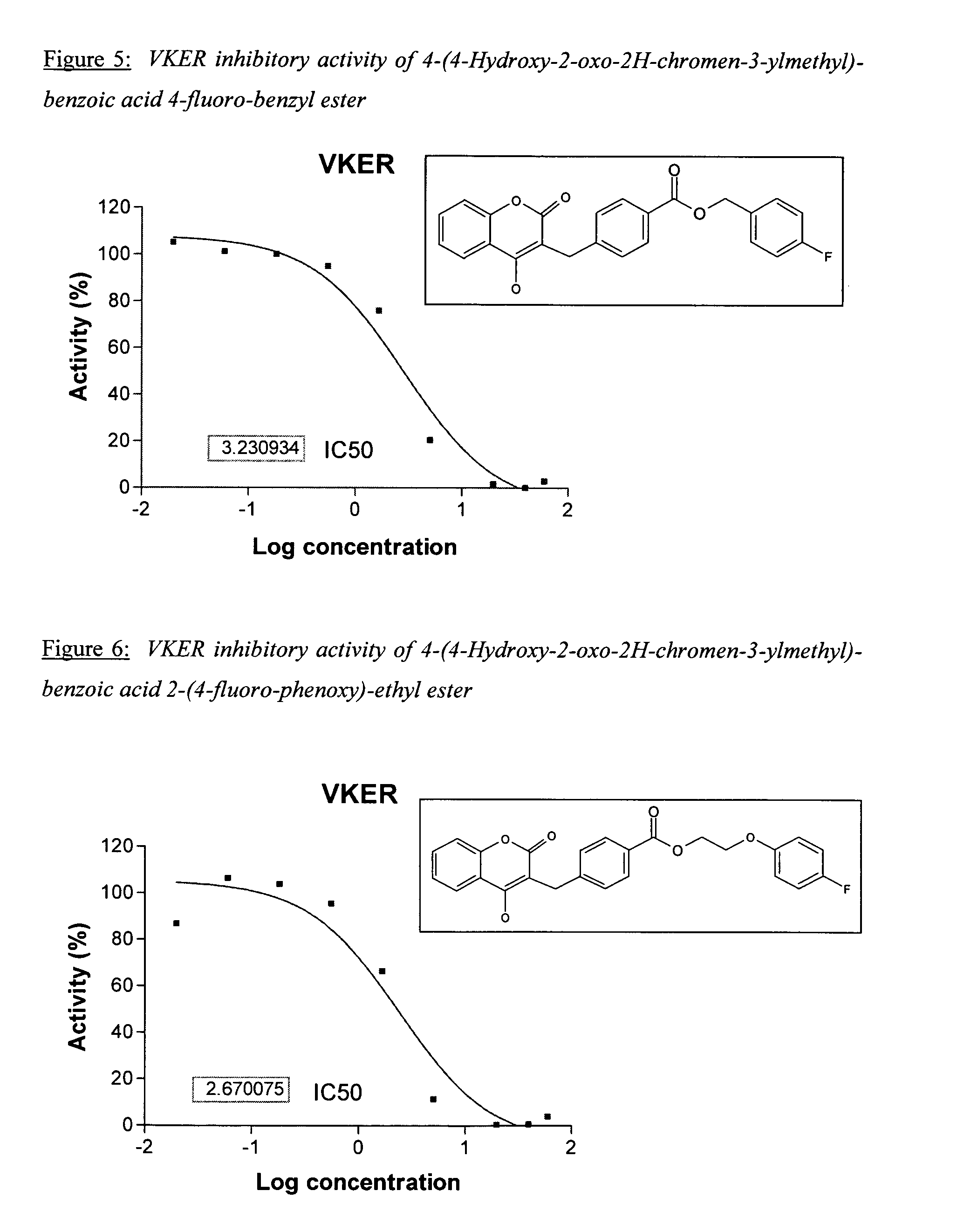
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Long term complications" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Long term complications. The long-term diabetes mellitus complications occur within 10-15 years from the onset of diabetes. The increase of blood sugar level produces changes throughout the body.
Compositions for treatment of diabetic complications
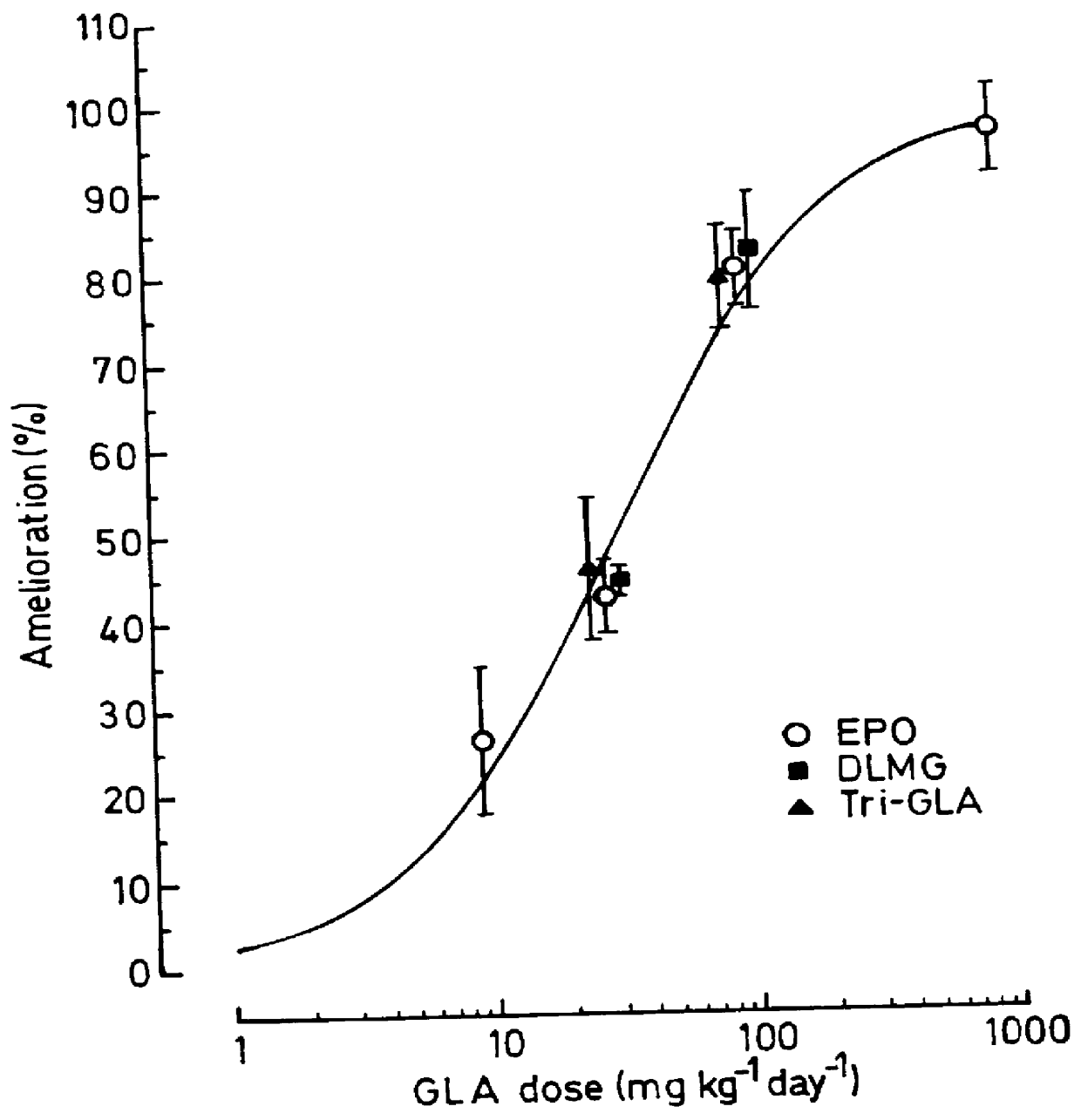
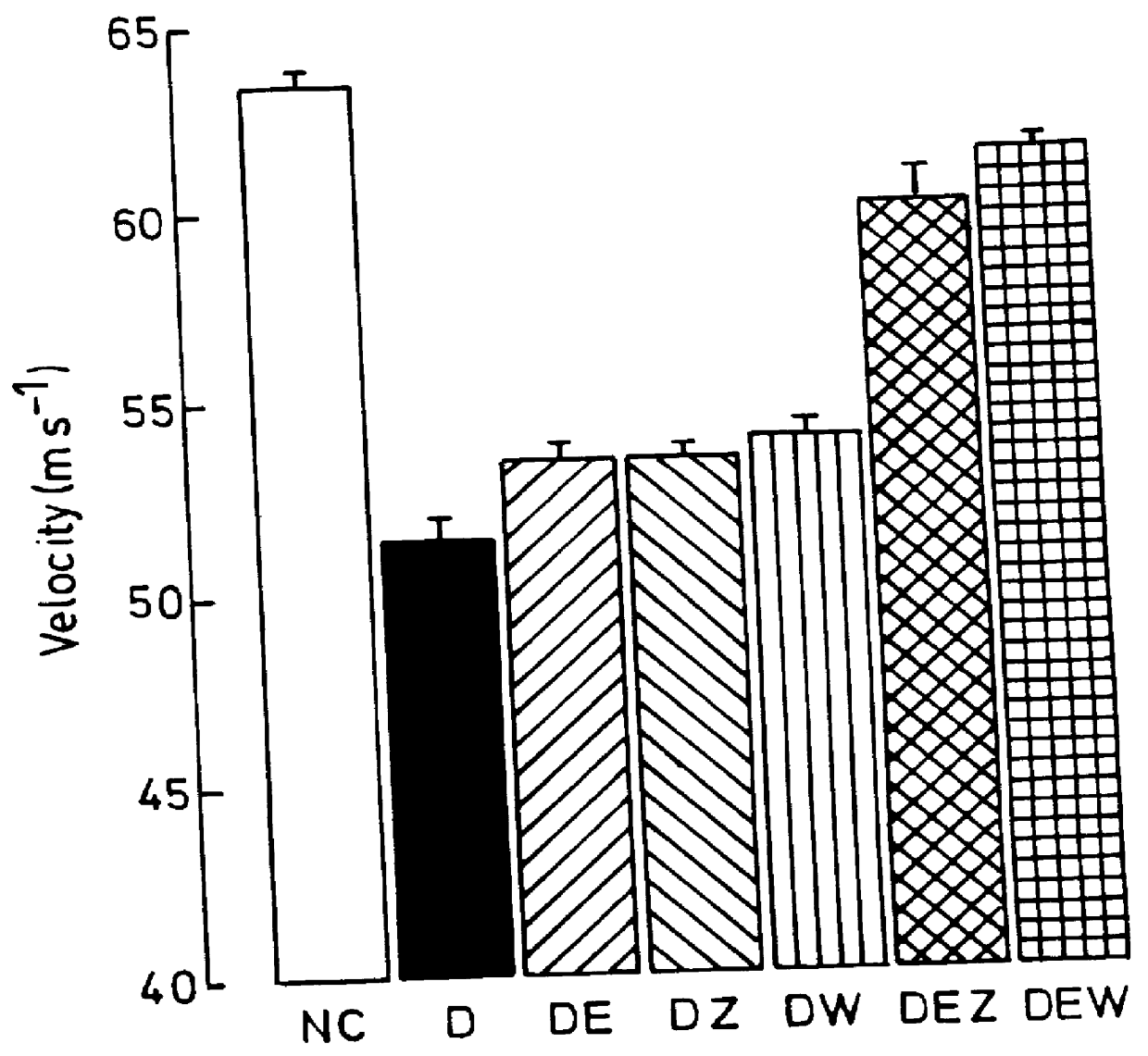
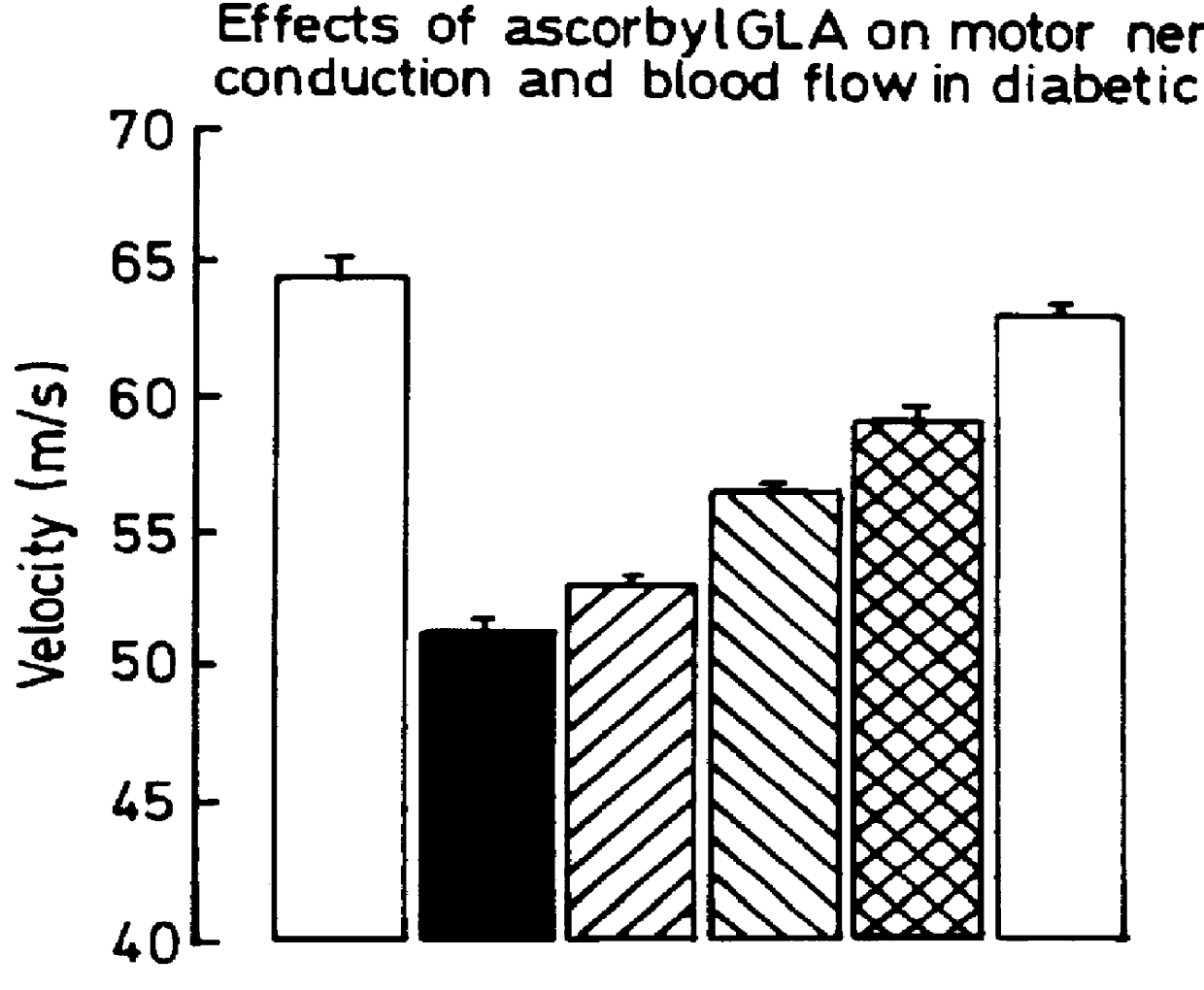
Use of 6-desaturated n-6 fatty acids, especially gammalinolenic acid (GLA), dihomogammalinolenic acid (DGLA) or arachidonic acid (AA), together with a pharmaceutically acceptable material reducing intracellular levels of sorbitol in the body, particularly an aldose reductase inhibitor, in the treatment of (including prophylactic treatment), and in the preparation of medicaments for the treatment of (including prophylactic treatment), the long-term complications of diabetes mellitus. Pharmaceutical compositions of said materials. The ascorbate esters of 6-desaturated n-6 fatty acids (other than GLA or DGLA) per se.
Owner:SCOTIA HLDG
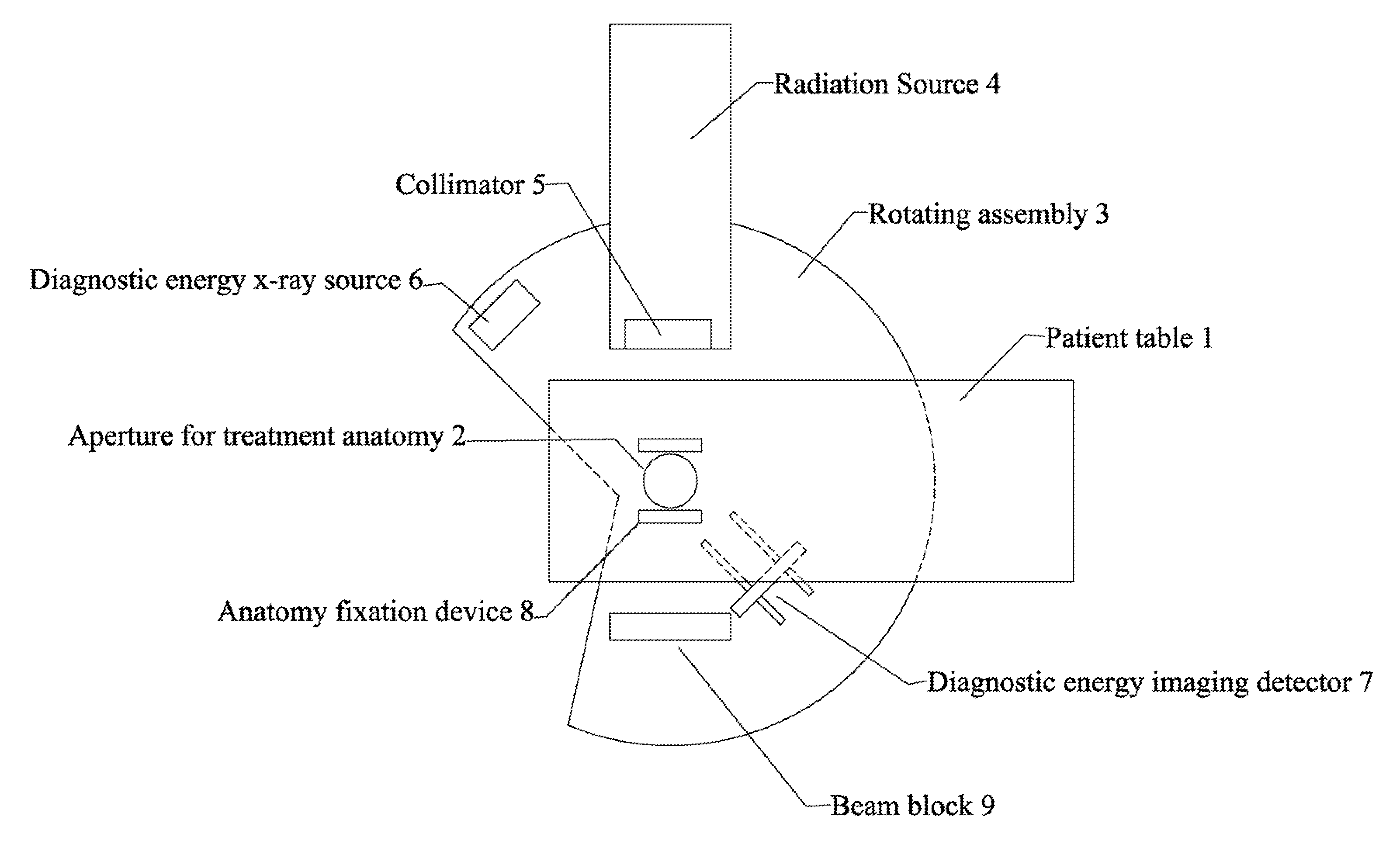
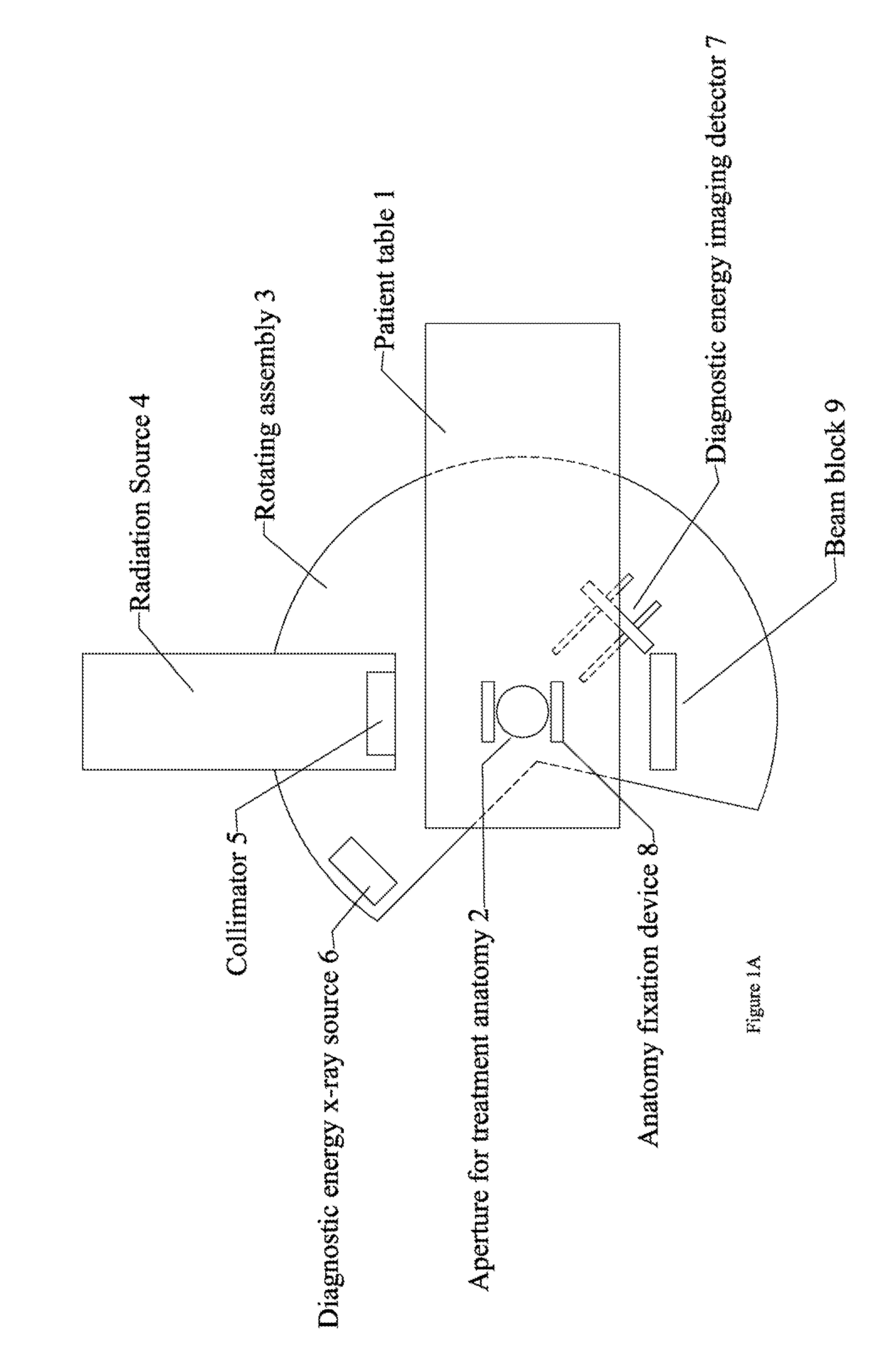
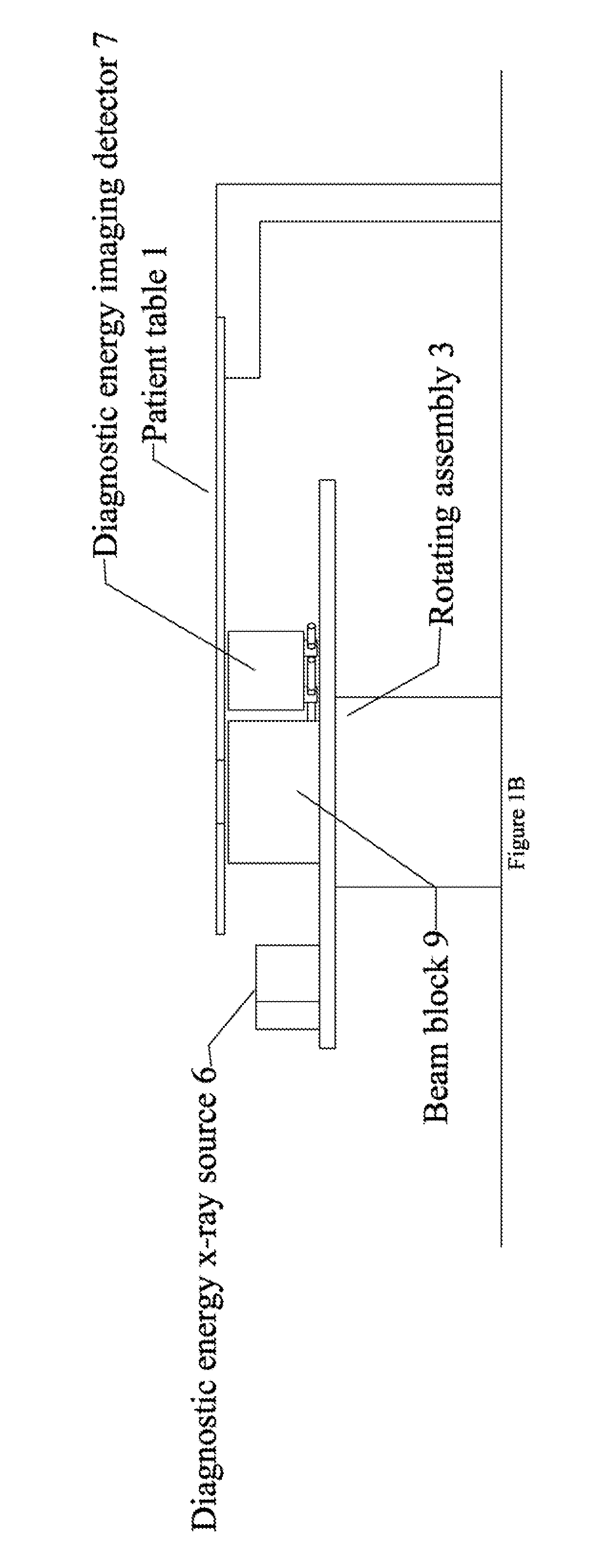
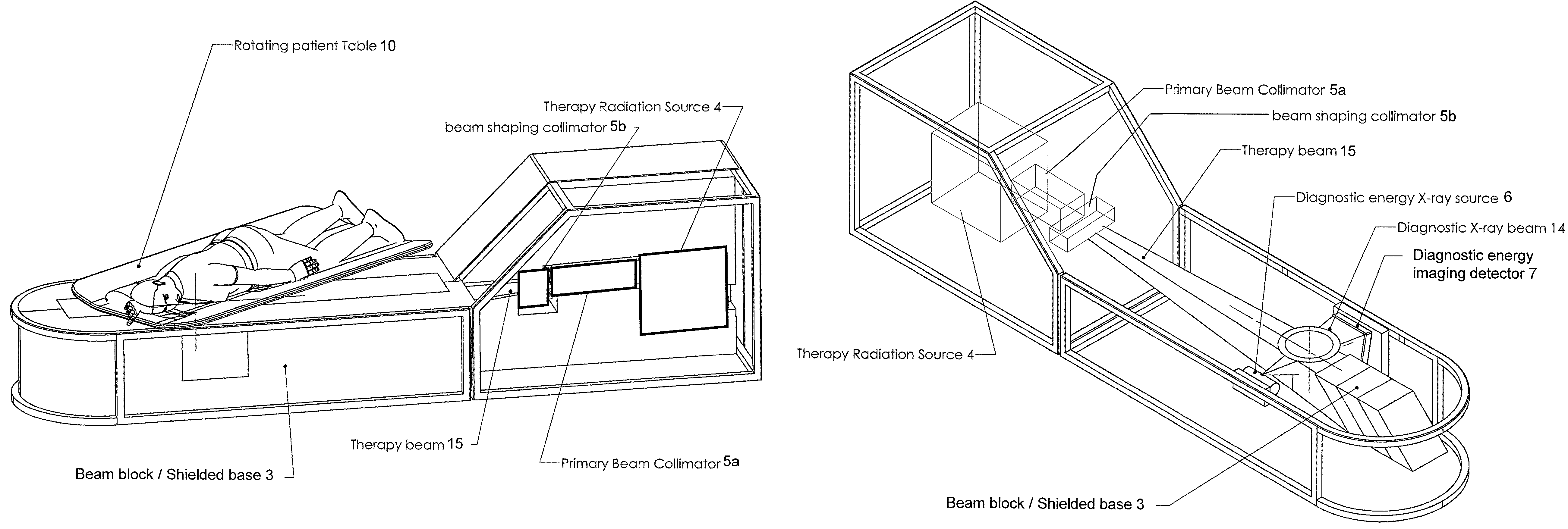
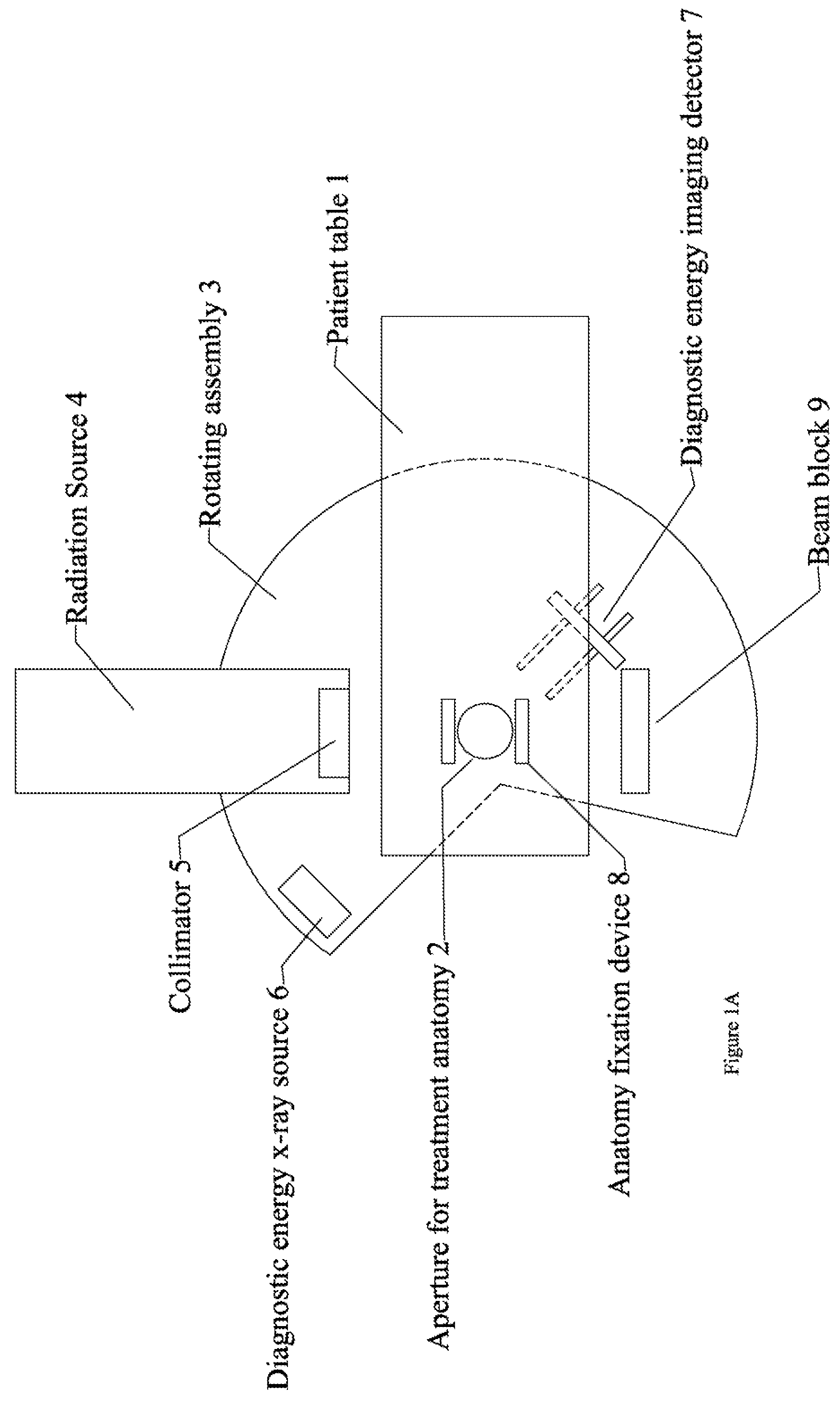
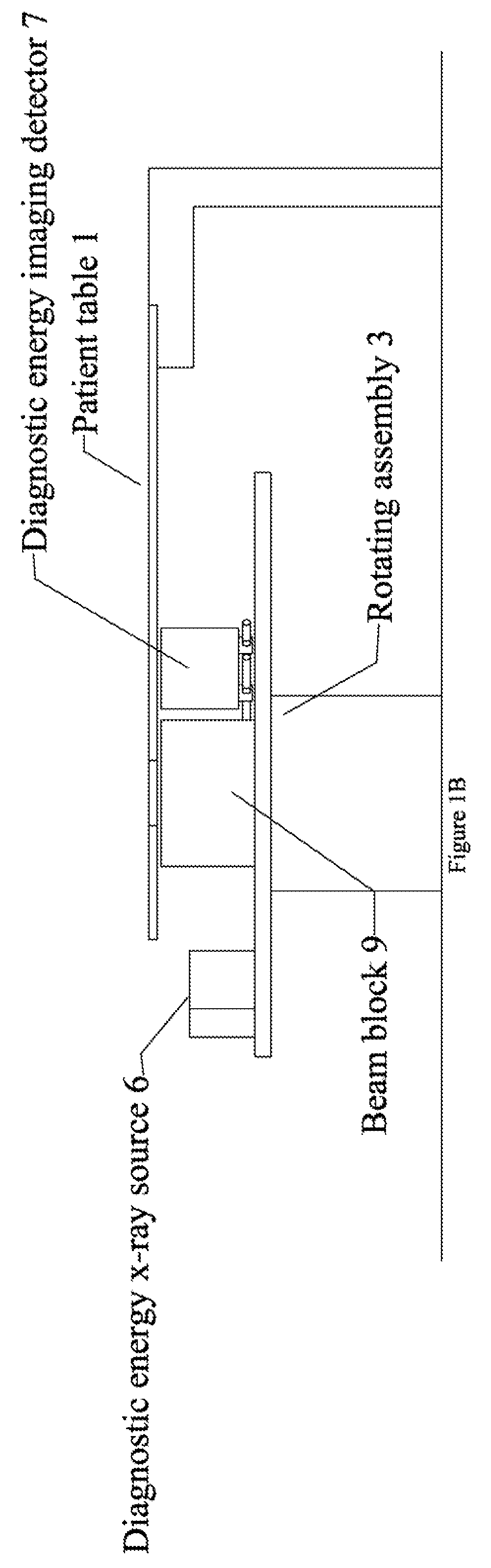
Radiation therapy system for treating breasts and extremities
InactiveUS20070211854A1Maximize separationReduce shielding requirementsPatient positioning for diagnosticsX-ray tube vessels/containerCritical structurePrimary disease
A radiation therapy system optimized for treating extremities such as the breast has unique geometrical features that enable the system to deliver an accurately located prescribed dose to a target volume while eliminating or reducing the collateral dose delivered to the rest of the patient. An optional integral imaging system provides accurate target volume localization for each treatment session. Utilizing the effects of gravity on a prone patient maximizes the separation of a target volume within the breast to adjacent critical structures such as the chest wall, heart and lungs, thereby reducing long term complications not associated with the primary disease. A shielded interface surface between the radiation source and the patient reduces patient dose due to scattered or stray radiation. A shielded enclosure for the radiation sources combined with the shielded interface surface eliminates the need for primary shielding in the room and allows the therapy system to be used in a transportable, mobile facility.
Owner:ORBITAL THERAPY
Method of Treating Stress Hyperglycemia with Human Antibodies to the Glucagon Receptor
ActiveUS20130251728A1Easy to solveLower blood sugar levelsPeptide/protein ingredientsAntibody ingredientsDiseaseDiabetic ketoacidosis
The present invention provides antibodies that bind to the human glucagon receptor, designated GCGR and methods of using same. According to certain embodiments of the invention, the antibodies are fully human antibodies that bind to human GCGR. The antibodies of the invention are useful for lowering blood glucose levels and blood ketone levels and are also useful for the treatment of diseases and disorders associated with one or more GCGR biological activities, including the treatment of diabetes, diabetic ketoacidosis, long-term complications associated with diabetes, or other metabolic disorders characterized in part by elevated blood glucose levels, including stress hyperglycemia.
Owner:REGENERON PHARM INC
Radiation therapy system for treating breasts and extremities
InactiveUS7526066B2Maximize separationWeaken energyPatient positioning for diagnosticsX-ray tube vessels/containerAnatomical structuresCritical structure
Owner:ORBITAL THERAPY
Absorbable Vascular Filter
An absorbable vascular filter is disclosed for deployment within a vessel for temporary filtering of body fluids. A preferred embodiment is the placement of such absorbable vascular filter within the inferior vena cava (IVC) to filter emboli for the prevention of pulmonary embolism for a limited duration in time. Once protection from PE is complete, the filter is biodegraded according to a planned schedule determined by the absorption properties of the filter components. Hence the temporary absorbable vascular filter obviates the long term complications of permanent IVC filters such as increased deep vein thrombosis, neighboring organ puncture from filter fracture and embolization while also circumventing the removal requirement of metal retrievable IVC filters.
Owner:ADIENT MEDICAL
Osteoinduction of cortical bone allografts by coating with biopolymers seeded with recipient periosteal bone cells
InactiveUS6899107B2Improve clinical outcomesLower immune responseBone implantDiagnosticsBiopolymerBone Cortex
A method by which immune responses to cortical bone grafts and other substrates (e.g., cement, IPN, etc.) can be minimized and at the same time graft osteoinductive potential can be improved, and improved graft substrate materials are disclosed. The method of the invention provides new types of bone grafts that incorporate into host bone more thoroughly and more rapidly, eliminating long-term complications, such as fracture, non-union, infection, and rejection. In the method of the invention, bone grafts or other substrates are modified to have an osteoinductive surface modification that the recipient's body will accept as its own tissue type and therefore will not reject or otherwise cause to fail. The osteoinductive surface modification comprises a biopolymer matrix coating that is seeded with periosteal cells that have been previously harvested either from the graft recipient or from an allogenic or xenogenic donor source.
Owner:DEPUY MITEK INC
Method of reducing the severity of stress hyperglycemia with human antibodies to the glucagon receptor
ActiveUS8771696B2Easy to solveImprove the level ofPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseDiabetic ketoacidosis
The present invention provides antibodies that bind to the human glucagon receptor, designated GCGR and methods of using same. According to certain embodiments of the invention, the antibodies are fully human antibodies that bind to human GCGR. The antibodies of the invention are useful for lowering blood glucose levels and blood ketone levels and are also useful for the treatment of diseases and disorders associated with one or more GCGR biological activities, including the treatment of diabetes, diabetic ketoacidosis, long-term complications associated with diabetes, or other metabolic disorders characterized in part by elevated blood glucose levels, including stress hyperglycemia.
Owner:REGENERON PHARM INC
Methods and kits for preventing hypoglycemia
InactiveUS7855177B1Reduce riskReduce incidencePeptide/protein ingredientsMetabolism disorderExcessive weight gainGlycemic
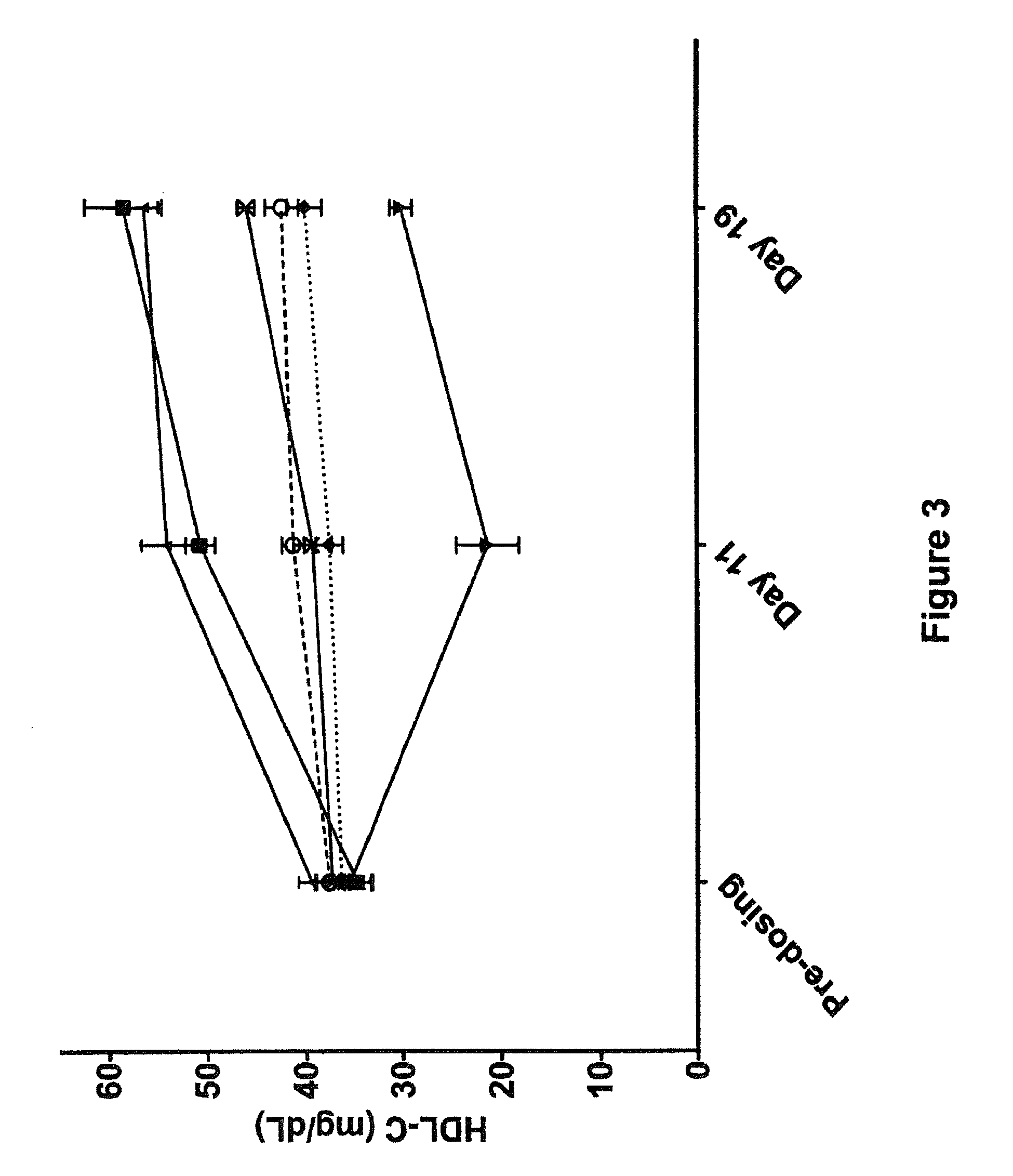
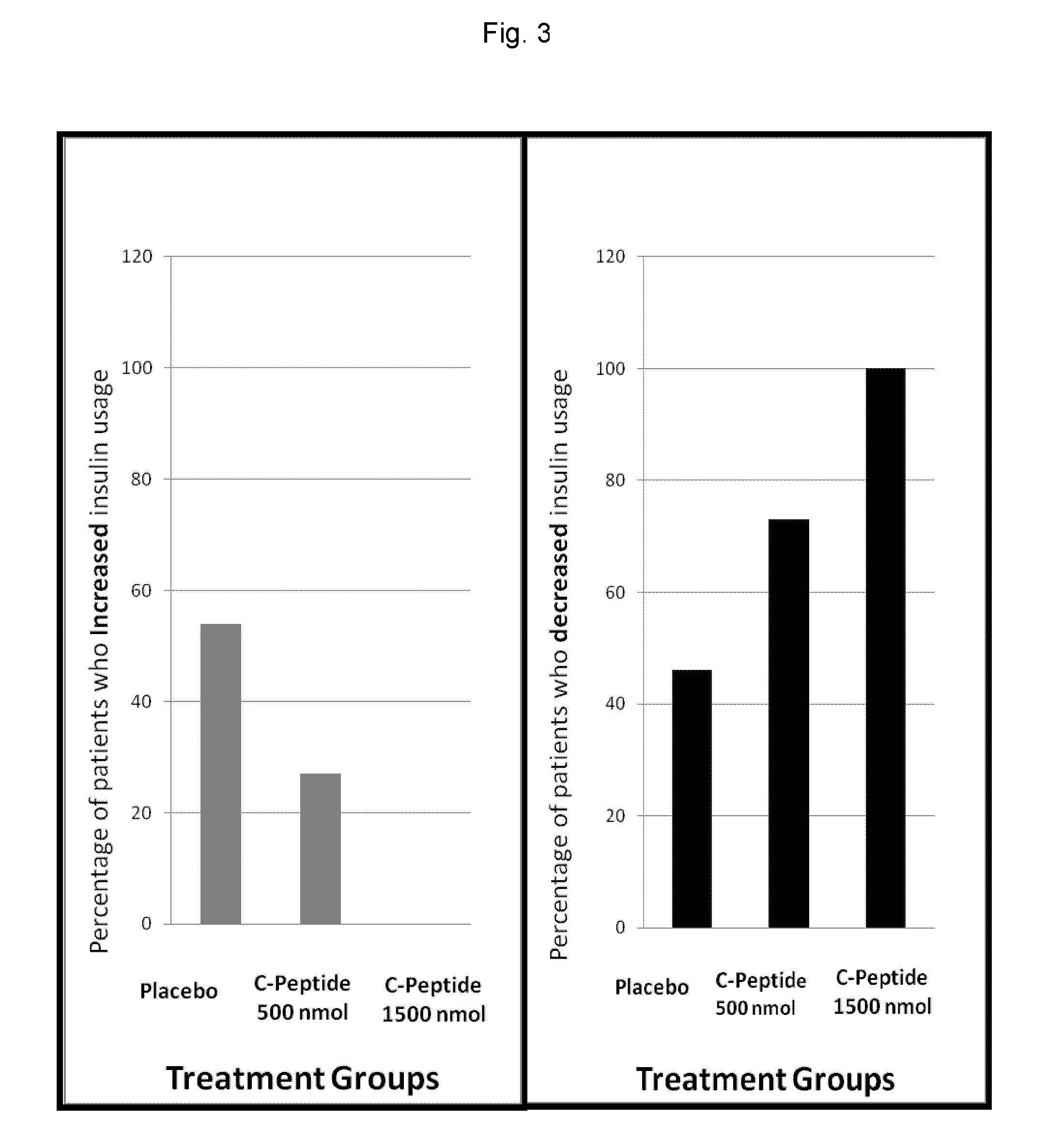
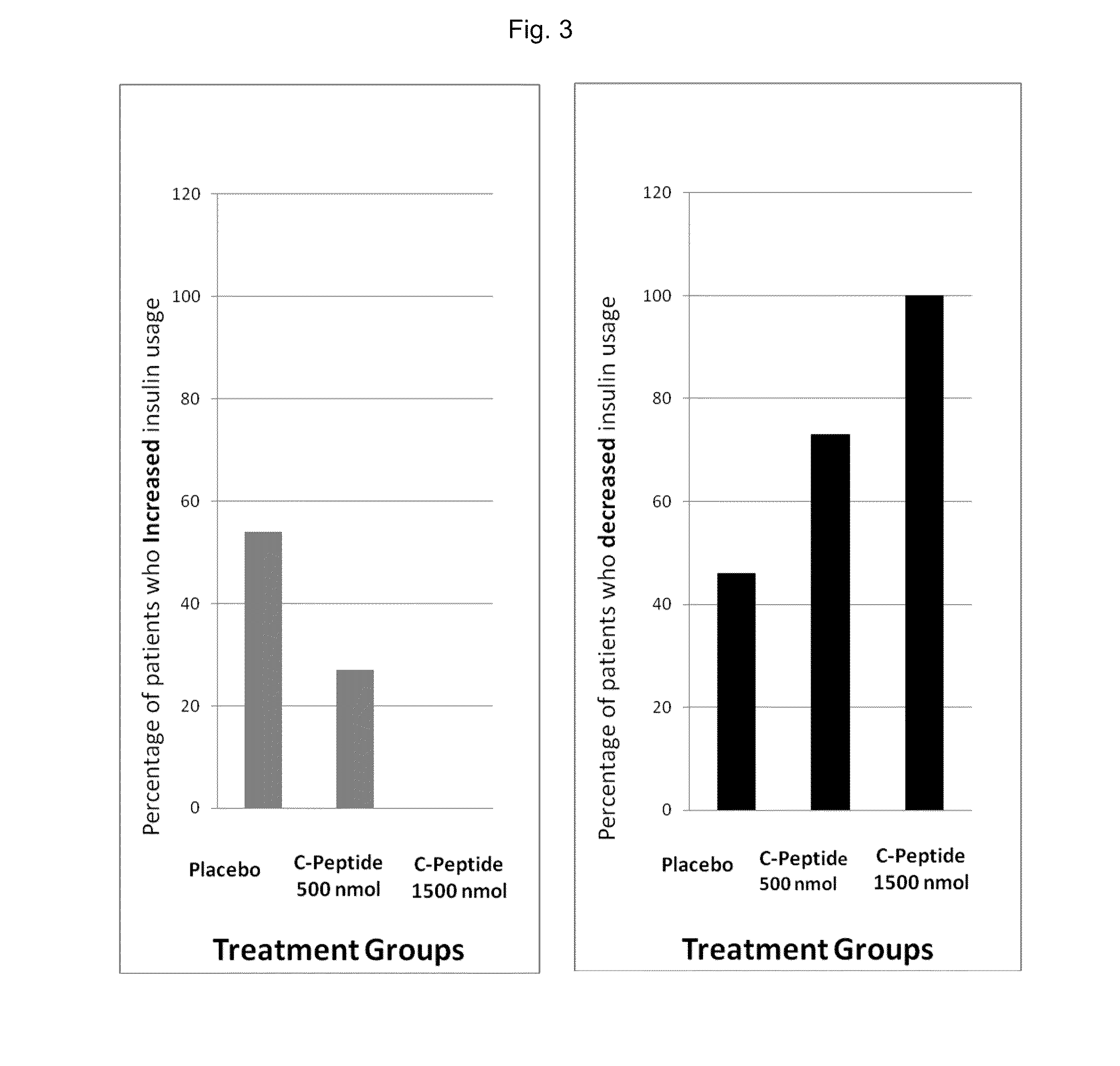
Improved methods and kits for treating the long-term complication of diabetes that reduce the risk of the patient developing hypoglycemia during C-peptide therapy. The use of such methods and kits, can also maintain good glycemic control, and avoid excessive weight gain that may otherwise be associated with excessive insulin administration or caloric intake during C-peptide therapy.
Owner:CEBIX
Human antibodies to the glucagon receptor
The present invention provides antibodies that bind to the human glucagon receptor, designated GCGR and methods of using same. According to certain embodiments of the invention, the antibodies are fully human antibodies that bind to human GCGR. The antibodies of the invention are useful for lowering blood glucose levels and blood ketone levels and are also useful for the treatment of diseases and disorders associated with one or more GCGR biological activities, including the treatment of diabetes, diabetic ketoacidosis and long-term complications associated with diabetes, or other metabolic disorders characterized in part by elevated blood glucose levels.
Owner:REGENERON PHARM INC
Absorbable Vascular Filter
An absorbable vascular filter is disclosed for deployment within a vessel for temporary filtering of body fluids. A preferred embodiment is the placement of such absorbable vascular filter within the inferior vena cava (IVC) to filter emboli for the prevention of pulmonary embolism for a limited duration in time. Once protection from PE is complete, the filter is sequentially biodegraded according to a planned schedule determined by the absorption properties of the filter components. Hence the temporary absorbable vascular filter obviates the long term complications of permanent IVC filters such as increased deep vein thrombosis, while also circumventing the removal requirement of metal retrievable IVC filters.
Owner:ADIENT MEDICAL
Novel mixed bone cement
InactiveCN102688522AReduce in quantitySolve looseImpression capsDentistry preparationsBone cementCancellous bone
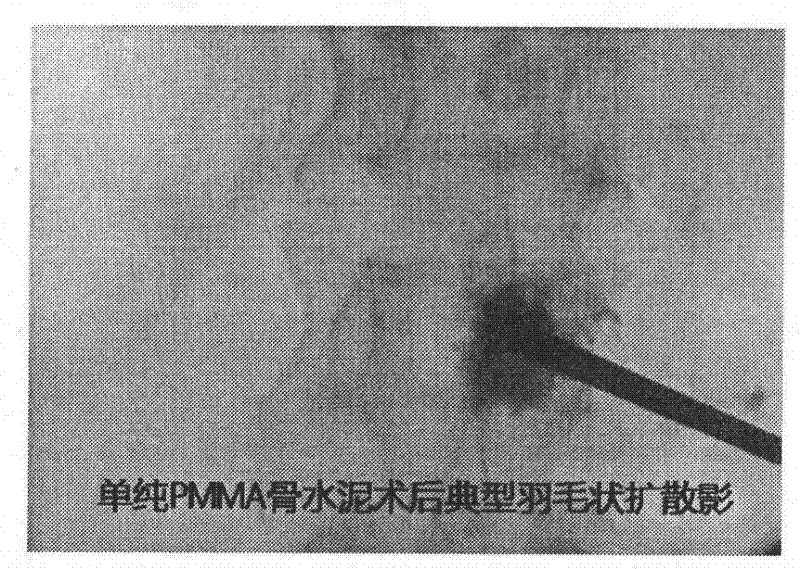
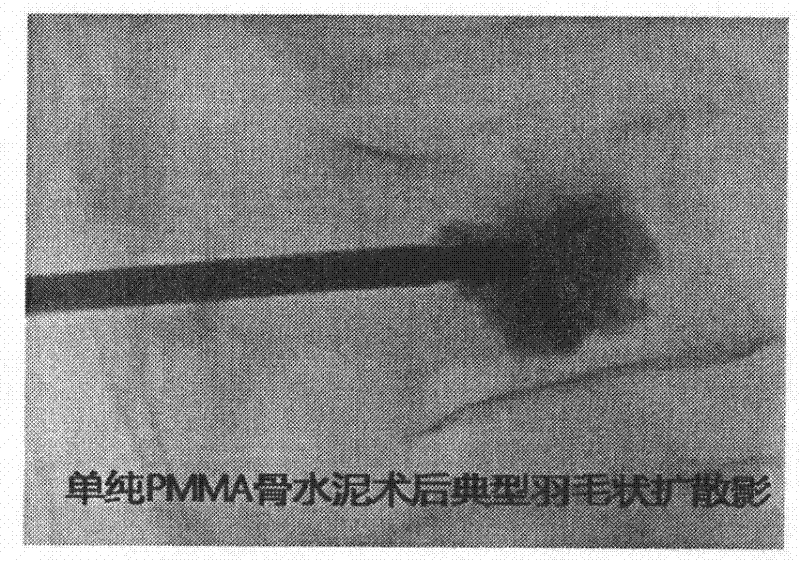
The invention provides novel mixed bone cement. The novel mixed bone cement provided by the invention provides a strong support for the bone at the initial stage, and gradually the rigidity of PMMA bone cement is decreased with the ingrowth of cancellous bone at the later stage, so that the implant has elastic modulus similar tothe cancellous bone, and has both mechanical and biological fixation, thus long-term complications of PMMA bone cement can be reduced. The characteristics of high viscosity and low liquidity of the mixed bone cement are very important in percutaneousvertebroplasty and percutaneous kyphoplasty (PVP and PKP), and the occurrence of bone cement leakage can be reduced.
Owner:SHANGHAI RUIPING BIOTECH TECH
Chronic Traumatic Encephalopathy in Blast-Exposed Individuals
InactiveUS20150119273A1Delay progressIncreased riskLibrary screeningDisease diagnosisCvd riskBrains tissue
The invention is based on the surprising discovery that as few as one episode of blast exposure increases the risk of CTE. Blast exposure is associated with chronic traumatic encephalopathy, impaired neuronal function, and persistent cognitive deficits in blast-exposed military veterans and experimental animals. Early diagnosis and assessment of risk permits physicians to prescribe treatment to reduce or slow progression of impairment before the onset of overt symptoms that become apparent decades after an initial insult or trauma to brain tissue. The invention provides methods and compositions for diagnosis and prognosis of individuals at risk of long term complications related to blast injury or concussive injury.
Owner:TRUSTEES OF BOSTON UNIV +1
Vascular Filter Stent
InactiveUS20120277787A1Prevents pulmonary embolismInhibition of endothelializationStentsSurgeryVeinVascular filter
A vascular filter stent is disclosed for deployment within a vessel for filtering of body fluids. A preferred embodiment is the placement of such vascular filter stent within the inferior vena cava (IVC) to filter emboli for the prevention of pulmonary embolism. By incorporating a stent into the filter design, vessel patency and filter positioning is maintained, while minimizing endothelialization thereby obviating the long term complications of conventional metal VC filters such as filter migration and increased deep vein thrombosis.
Owner:ADIENT MEDICAL
Materials and methods for treating coagulation disorders
The subject invention provides anticoagulant compounds of formula I: and pharmaceutically acceptable salts thereof, wherein R1, R3, n and Ar are as defined herein. The compounds of the subject invention can be used to treat at-risk populations thereby bringing relief of symptoms, improving the quality of life, preventing acute and long-term complications, reducing mortality and treating accompanying disorders. The invention further comprises pharmaceutical compositions comprising the compounds and salts of the invention, as well as methods of using the compounds, salts, and compositions of the invention.
Owner:CADRENAL THERAPEUTICS
Materials and Methods for Treating Coagulation Disorders
The subject invention provides anticoagulant compounds of formula I: and pharmaceutically acceptable salts thereof, wherein R1, R3, n and Ar are as defined herein. The compounds of the subject invention can be used to treat at-risk populations thereby bringing relief of symptoms, improving the quality of life, preventing acute and long-term complications, reducing mortality and treating accompanying disorders. The invention further comprises pharmaceutical compositions comprising the compounds and salts of the invention, as well as methods of using the compounds, salts, and compositions of the invention.
Owner:CADRENAL THERAPEUTICS
Developing biological heart occluder with controllable degradation rate
ActiveCN104001221AJudging the form of releaseDetermine the exact locationSurgeryEngineeringHeat setting
The invention discloses a developing biological heart occluder with controllable degradation rate, which is composed of a monofilament woven support structure and at least two layers of flow-resistant films sewed in the support structure; monofilament is made of a biodegradable high polymer material added with a developing material; the proportion range of the biodegradable high polymer material to the developing material is 70-98 wt% to 30-2 wt%; the particle size range of the developing material is 10 nm to 30 microns; the flow-resistant films are made of non-woven fabrics prepared from the biodegradable high polymer material. According to the invention, long-term complications and potential safety hazards caused by metal preserved in bodies are avoided; because of being manufactured by adopting the monofilament woven structure and the thermal forming method, the developing biological heart occlude is better in compliance and recovery, also good in locating and occluding effect, simple in preparation method and environment-friendly; in addition, experiments prove that the developing biological heart occluder with controllable degradation rate is applied to industrial production and has clinical application prospect.
Owner:MALLOW MEDICAL SHANGHAICO LTD
Degradable soft tissue patch
InactiveCN101066474ADegradability is suitableAvoid long-term complicationsLigamentsMusclesCyclohexanoneTissue repair
The present invention belongs to the field of biomedicine technology, relates to artificial organ, and is especially biodegradable soft tissue patch. The biodegradable soft tissue patch is prepared through weaving porous patch shell in the shape matching the soft tissue to be repaired with poly-p-dioxy cyclohexanone; permeating collagen to cover and seal the pores in the patch shell; covering the side towards the organ with chitosan, sterilizing and drying to complete the patch. The patch of the present invention may be flat one or woven one. The present invention has the active effects, including function of supporting growing tissue in the early stage, and the gradual degradation and absorption to avoid long term complication.
Owner:徐志飞 +3
Anatomical Connection
InactiveUS20080021368A1Minimize momentum lossCatheterMultiple way valvesPulmonary vasculatureWhole body
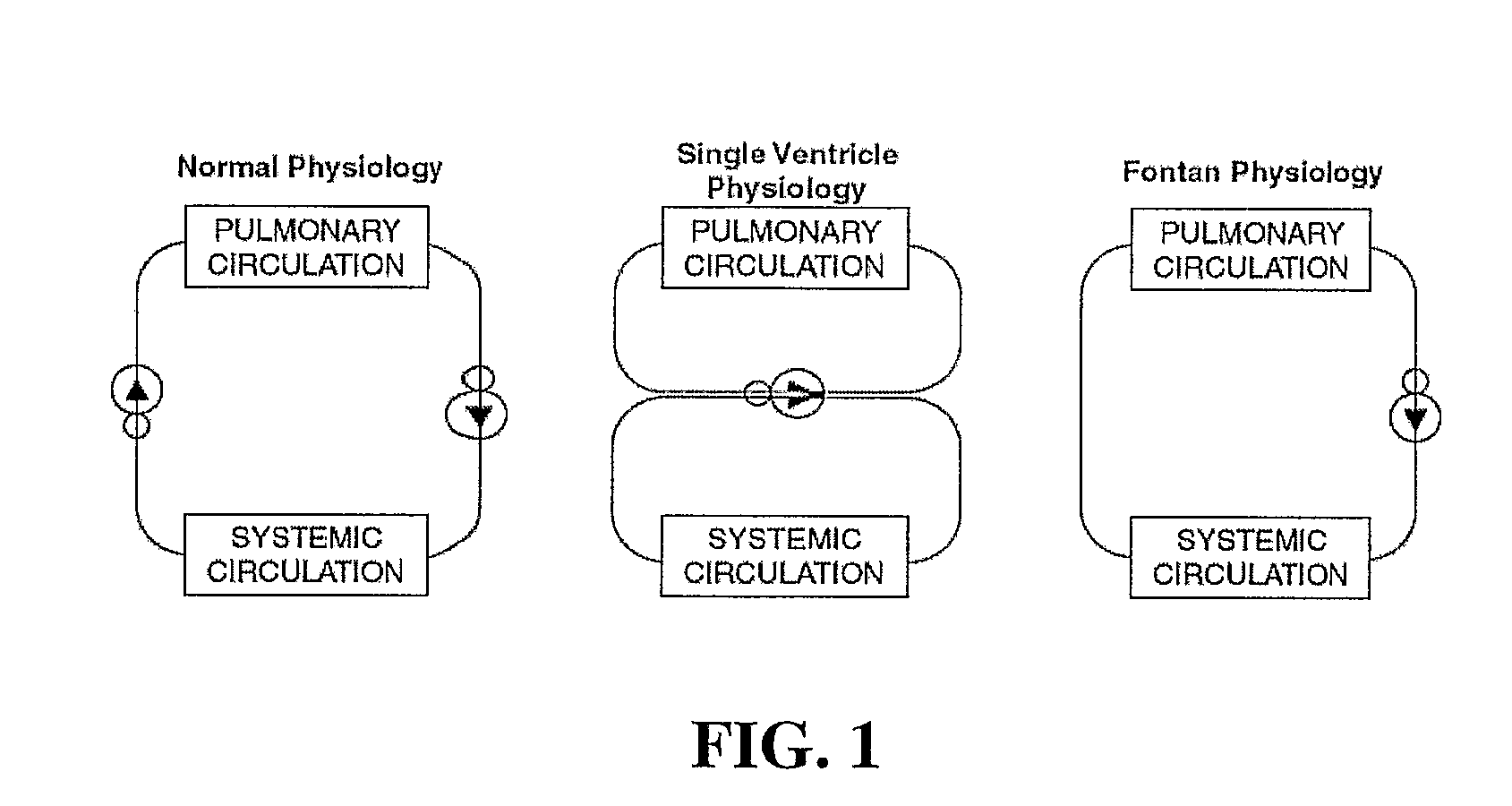
A device for use in the total cavopulmonary connection (TCPC) in order to optimize its hemodynamics. Although the current procedure of choice for single ventricle heart repairs, the TCPC has reduced the post-operative mortality to the level of simpler types of congenital heart disease repairs, Fontan patients are still subjected to serious long-term complications. The TCPC procedure, which restores the vital separation between oxygenated and deoxygenated blood, also leads to an increased workload for the remaining single ventricle, as it is now responsible for pumping the blood through both the systemic and pulmonary circulation. The present device reduces this workload by altering the surgically created design of the TCPC. Improved fluid mechanics and reduced energy dissipation at the connection site translates into less work for the single ventricle and improved transport of deoxygenated blood to the lungs, which may in turn contribute to improved post-operative results and quality of life.
Owner:GEORGIA TECH RES CORP
Materials and methods for treating coagulation disorders
The subject invention provides anticoagulant compounds of formula I:and pharmaceutically acceptable salts thereof, wherein R1, R3, n and Ar are as defined herein. The compounds of the subject invention can be used to treat at-risk populations thereby bringing relief of symptoms, improving the quality of life, preventing acute and long-term complications, reducing mortality and treating accompanying disorders. The invention further comprises pharmaceutical compositions comprising the compounds and salts of the invention, as well as methods of using the compounds, salts, and compositions of the invention.
Owner:CADRENAL THERAPEUTICS
Compositions with enhanced elasticizing activity
Compositions for improving the elasticity of the vagina and of the perineum during the last trimester of pregnancy, include combining a thiolated compound or mixture thereof with an ester of organic acid or mixture thereof. The compositions according to the present invention improve the elastic properties of the vaginal and / or perineal tissues in terms both of increased extensibility and faster elastic recovery. The compositions according to the present invention may decrease risk of trauma of perineal tissues during delivery, as well as risk of rectal or bladder incontinence as a post-partum short / medium / long term complication.
Owner:POLICHEM SA
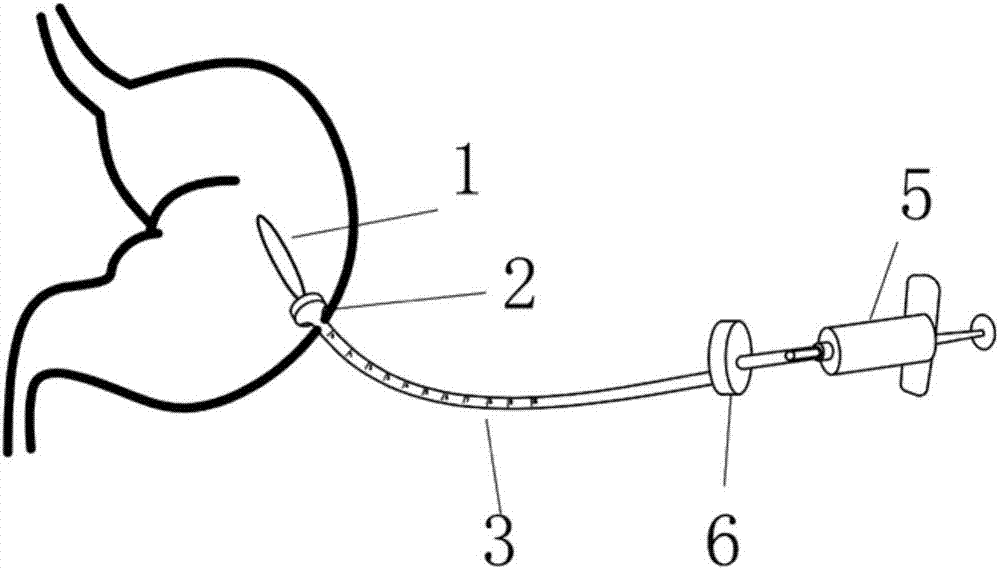
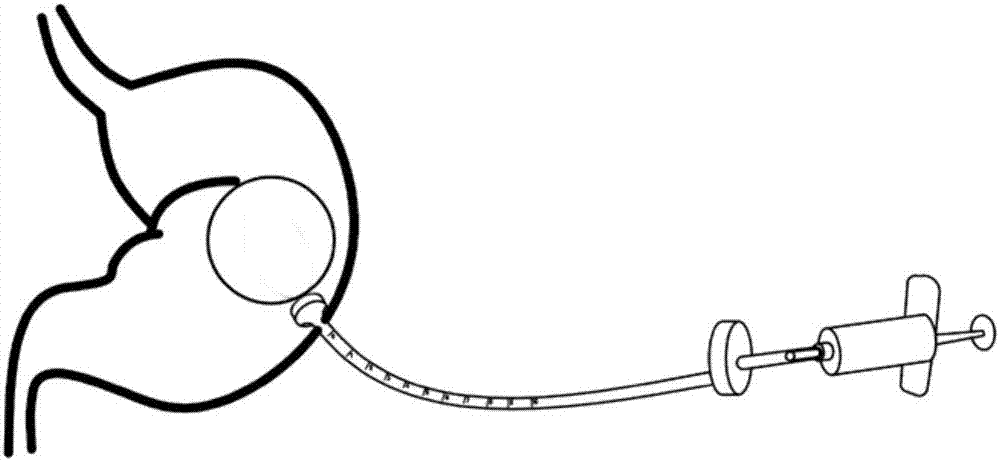
Percutaneous endoscopic gastrostomy gastric airbag implantation device
PendingCN107374793AImprove acid resistanceReduce food intakeNon-surgical orthopedic devicesObesity treatmentGastric fluidAirbag
The invention provides a percutaneous endoscopic gastrostomy gastric airbag implantation device which comprises an airbag. An opening end of the airbag is connected with a catheter, the catheter includes an inner catheter and an outer catheter, the inner catheter is an airbag catheter, the outer catheter is a percutaneous endoscopic gastrostomy catheter, the percutaneous endoscopic gastrostomy catheter is provided with a mushroom head, the airbag is fixed in the stomach by the mushroom head, and an air inlet end of the airbag catheter is detachably connected with a steel ring or a syringe. The gastric airbag catheter is led into the stomach of a patient through the PEG (percutaneous endoscopic gastrostomy) catheter, satiety of the patient can be artificially realized after the stomach airbag is inflated, so that food intake of the patient is decreased, the capacity of the stomach is reduced, the weight of the patient is reduced, 300-1000mL of air can be inflated into the airbag, the airbag has excellent acid resistance and is not easily corroded by stomach juice and food, and the airbag is safe and reliable in the stomach. Compared with traditional operation, the technique has the advantages that the technique is simple and convenient to operate and economical in expense, physical structure functions of digestive tracts cannot be changed, short-term and long-term complications are fewer, postoperative maintenance is simple and convenient and the like. The device has excellent clinical values and social benefits.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Materials and methods for treating coagulation disorders
The subject invention provides anticoagulant compounds of formula I:and pharmaceutically acceptable salts thereof, wherein R1, R3, n and Ar are as defined herein. The compounds of the subject invention can be used to treat at-risk populations thereby bringing relief of symptoms, improving the quality of life, preventing acute and long-term complications, reducing mortality and treating accompanying disorders. The invention further comprises pharmaceutical compositions comprising the compounds and salts of the invention, as well as methods of using the compounds, salts, and compositions of the invention.
Owner:CADRENAL THERAPEUTICS
Absorbable vascular filter
ActiveCN103458826APrevent pulmonary embolismShort retention timeStentsMachines/enginesVeinVascular filter
An absorbable vascular filter is disclosed for deployment within a vessel for temporary filtering of body fluids. A preferred embodiment is the placement of such absorbable vascular filter within the inferior vena cava (IVC) to filter emboli for the prevention of pulmonary embolism for a limited duration in time. Once protection from PE is complete, the filter is biodegraded according to a planned schedule determined by the absorption properties of the filter components. Hence the temporary absorbable vascular filter obviates the long term complications of permanent IVC filters such as increased deep vein thrombosis, neighboring organ puncture from filter fracture and embolization while also circumventing the removal requirement of metal retrievable IVC filters.
Owner:ADIENT MEDICAL
Methods and kits for preventing hypoglycemia
ActiveUS20110098220A1Reduces risk and incidence and severityPeptide/protein ingredientsMetabolism disorderExcessive weight gainGlycemic
The present invention provides improved methods and kits for treating the long-term complication of diabetes that reduce the risk of the patient developing hypoglycemia during C-peptide therapy. The use of such methods and kits, can also maintain good glycemic control, and avoid excessive weight gain that may otherwise be associated with excessive insulin administration or caloric intake during C-peptide therapy.
Owner:CEBIX
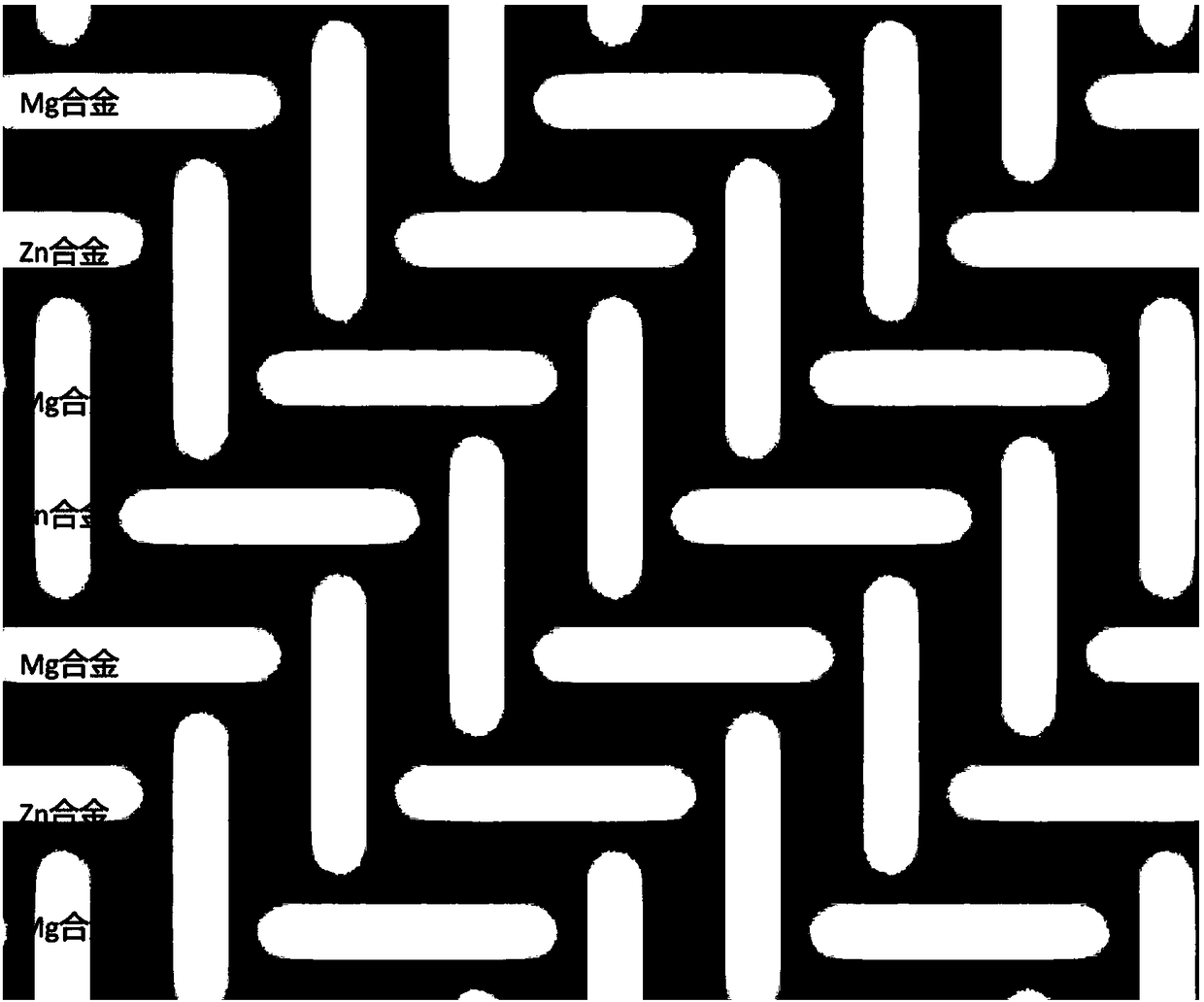
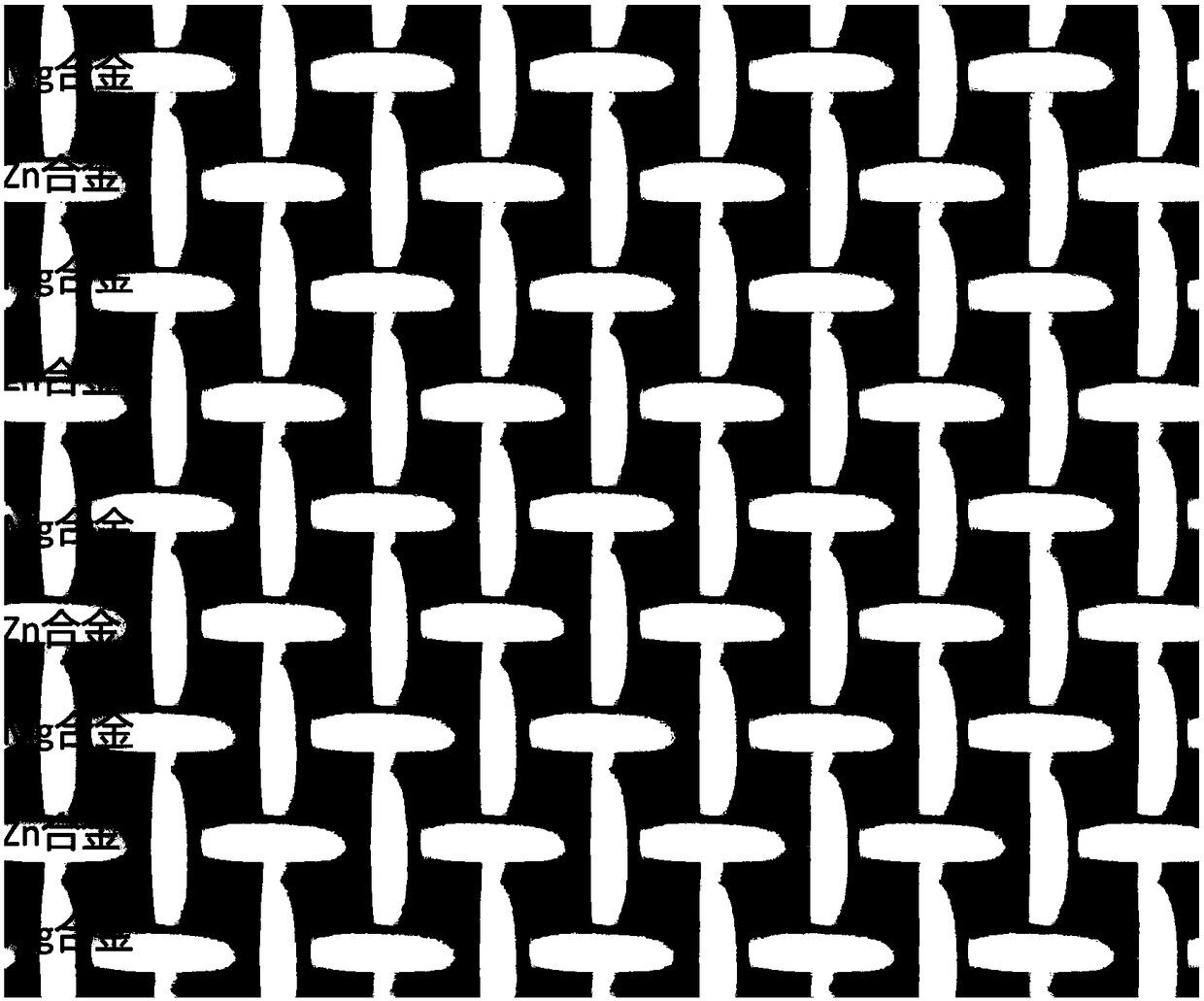
Magnesium alloy and zinc alloy wire hybrid composite sticking patch and application thereof
The invention provides a magnesium alloy and zinc alloy wire hybrid composite sticking patch and application thereof. The magnesium alloy and zinc alloy wire hybrid composite sticking patch is formedthrough mixed weaving of a magnesium alloy wire material and a zinc alloy wire material, and provided with a net structure. The composite sticking patch has the advantage that 1, the sticking patch iscompletely degradable, and absorbed and metabolized by human body in the recovering process from disease, the sticking patch has the good supporting effect in the early implanting stage, and is completely degraded, absorbed and replaced by autologous tissue after tissue recovering is completed in the late stage, and long-term complications caused by a traditional material can be avoided; and 2, by adjusting the size, the appearance and the weaving method of the whole wire material, the composite sticking patches in different types can be obtained, and the sticking patches have different strength and different degradation and absorption time, and can be adaptive to different illness states.
Owner:SHANGHAI JIAO TONG UNIV
Degradable shape memory endoluminal stent and preparation method thereof
InactiveCN107344994AExcellent shape memory performanceEasy to processSurgeryPharmaceutical delivery mechanismIntimal proliferationWhole body
The invention belongs to the technical field of polymer materials, and in particular relates to a degradable shape-memory intraluminal stent and a preparation method thereof. The present invention uses lactide, glycolide or ε-caprolactone, biodegradable polyester and diisocyanic acid as raw materials, and prepares the thermally induced deformation temperature transition point (Ttrans) of about 42°C through bulk melt polymerization. Degradable polymer materials, the obtained materials are processed and shaped above the melting temperature (Tm) to make helical tubular stents for different lumens; under the condition of T>Ttrans, stress is applied, and processed into stents for easy operation Shape; freeze-set stress relief, off-the-shelf product. The shape-memory intraluminal stent prepared by the method of the present invention has good biocompatibility, provides temporary support for the narrow lumen, has no long-term complications and does not need to be taken out after operation, and finally completely degrades in vivo After metabolism, it can be used as a carrier to carry anti-thrombotic and anti-intimal hyperplasia drugs, without the need for long-term systemic anticoagulation and other advantages.
Owner:HEILONGJIANG XINDA ENTERPRISE GRP
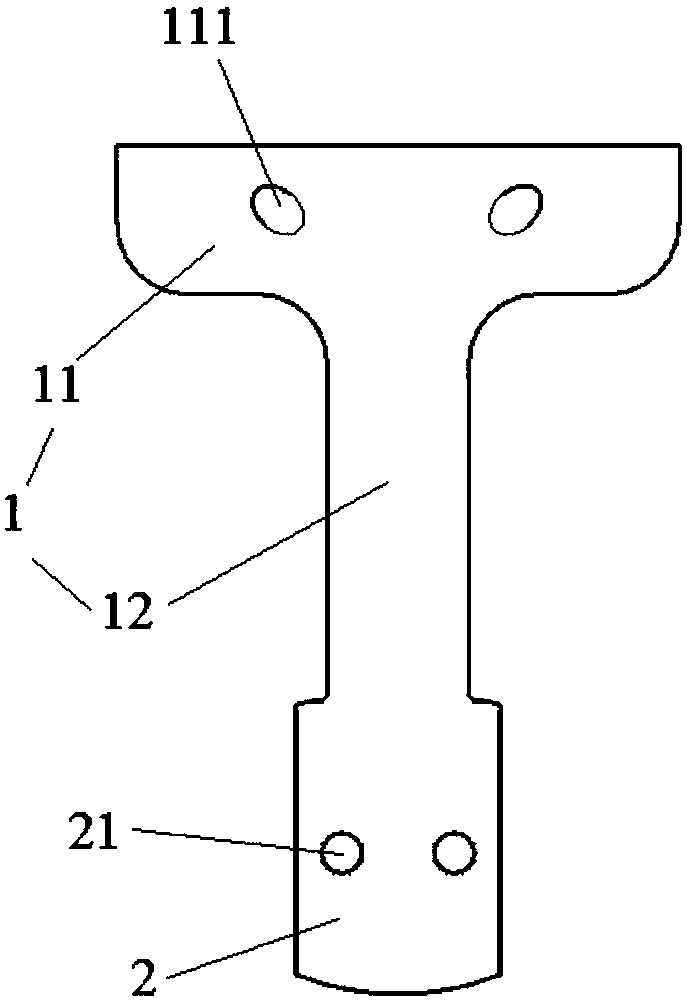
Upper cervical vertebra bearing type reconstruction prosthesis
The invention relates to an upper cervical vertebra bearing type reconstruction prosthesis. The prosthesis comprises a prosthesis body and a fitting part. The prosthesis body comprises a horizontal part and a vertical part, the vertical part is disposed in the middle of the lower end of the horizontal part and vertically connected with the horizontal part, the horizontal part is provided with twofixing holes, and the fixing holes are symmetrically formed in the left and right sides of the front end of the horizontal part. The fixing holes are obliquely upward through holes and run through thefront end and upper end of the horizontal part, and the fitting part is disposed at the lower end of the vertical part and provided with at least one fitting hole. The prosthesis has the advantages that by using a bearing type fixing mode, the prosthesis can perform reconstruction of the occipital neck after atlantoaxial resection, and the problem that only the reconstruction of parts below the dentata can be performed in a traditional method is solved. The contact surface between the upper end of the prosthesis and the anterior arch of atlas or the skull base is large, an effective support is formed, and long-term complications such as sinking displacement are avoided. The surface of the prosthesis is provided with a sponge-like micropore structure, so that the fusion of the prosthesis and adjacent bones is facilitated.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
A developmental biological heart occluder with controllable degradation rate
ActiveCN104001221BAccurate locationNon-toxicSurgeryX-ray constrast preparationsEngineeringHeat setting
The invention discloses a developing biological heart occluder with controllable degradation rate, which is composed of a monofilament woven support structure and at least two layers of flow-resistant films sewed in the support structure; monofilament is made of a biodegradable high polymer material added with a developing material; the proportion range of the biodegradable high polymer material to the developing material is 70-98 wt% to 30-2 wt%; the particle size range of the developing material is 10 nm to 30 microns; the flow-resistant films are made of non-woven fabrics prepared from the biodegradable high polymer material. According to the invention, long-term complications and potential safety hazards caused by metal preserved in bodies are avoided; because of being manufactured by adopting the monofilament woven structure and the thermal forming method, the developing biological heart occlude is better in compliance and recovery, also good in locating and occluding effect, simple in preparation method and environment-friendly; in addition, experiments prove that the developing biological heart occluder with controllable degradation rate is applied to industrial production and has clinical application prospect.
Owner:MALLOW MEDICAL SHANGHAICO LTD
Filter device
PendingCN109394379ARelieve painReduce cloggingStentsTubular organ implantsEngineeringLong term complications
Owner:SHANGHAI BLUEVASCULAR MEDTECH CO LTD
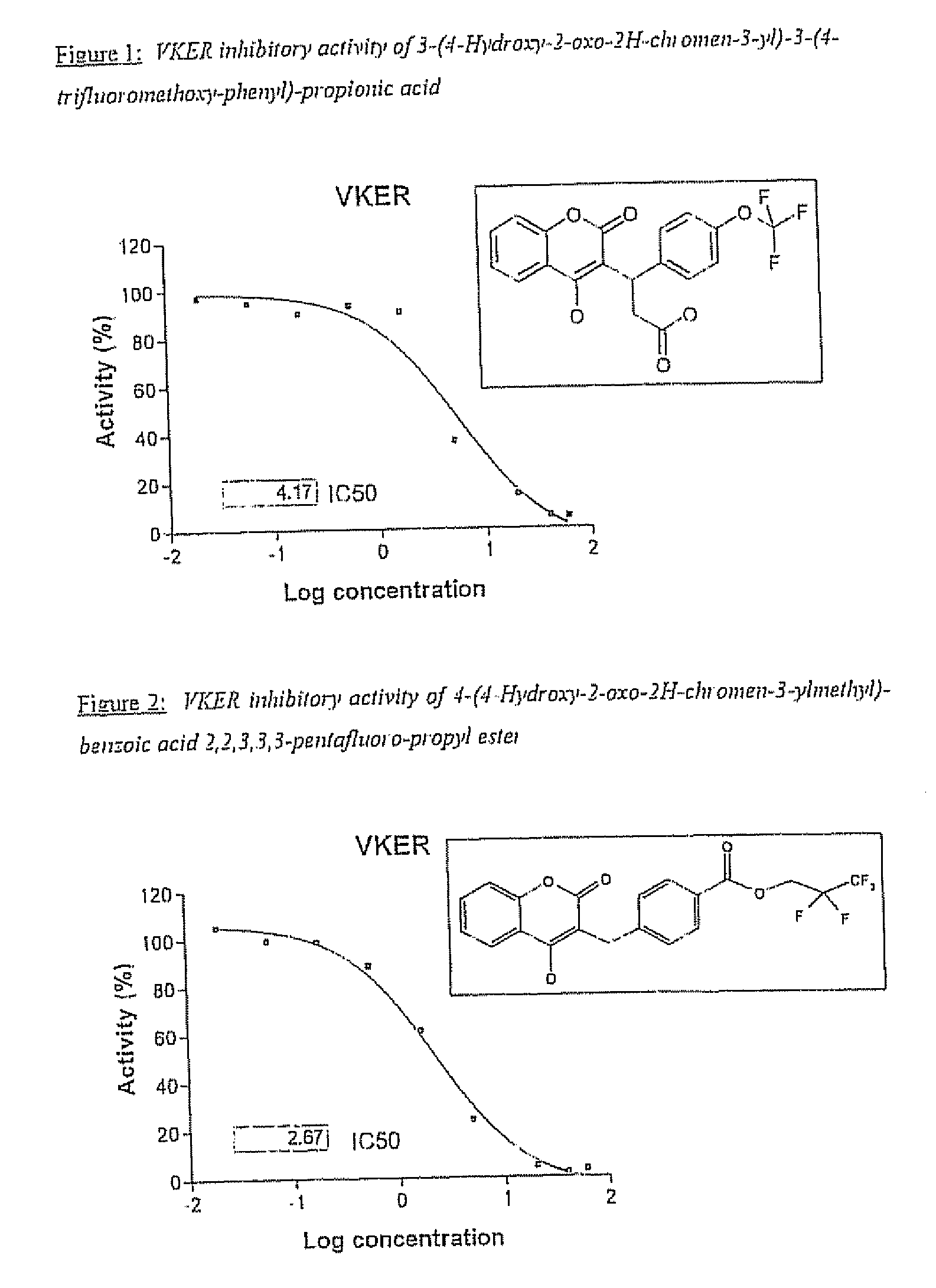
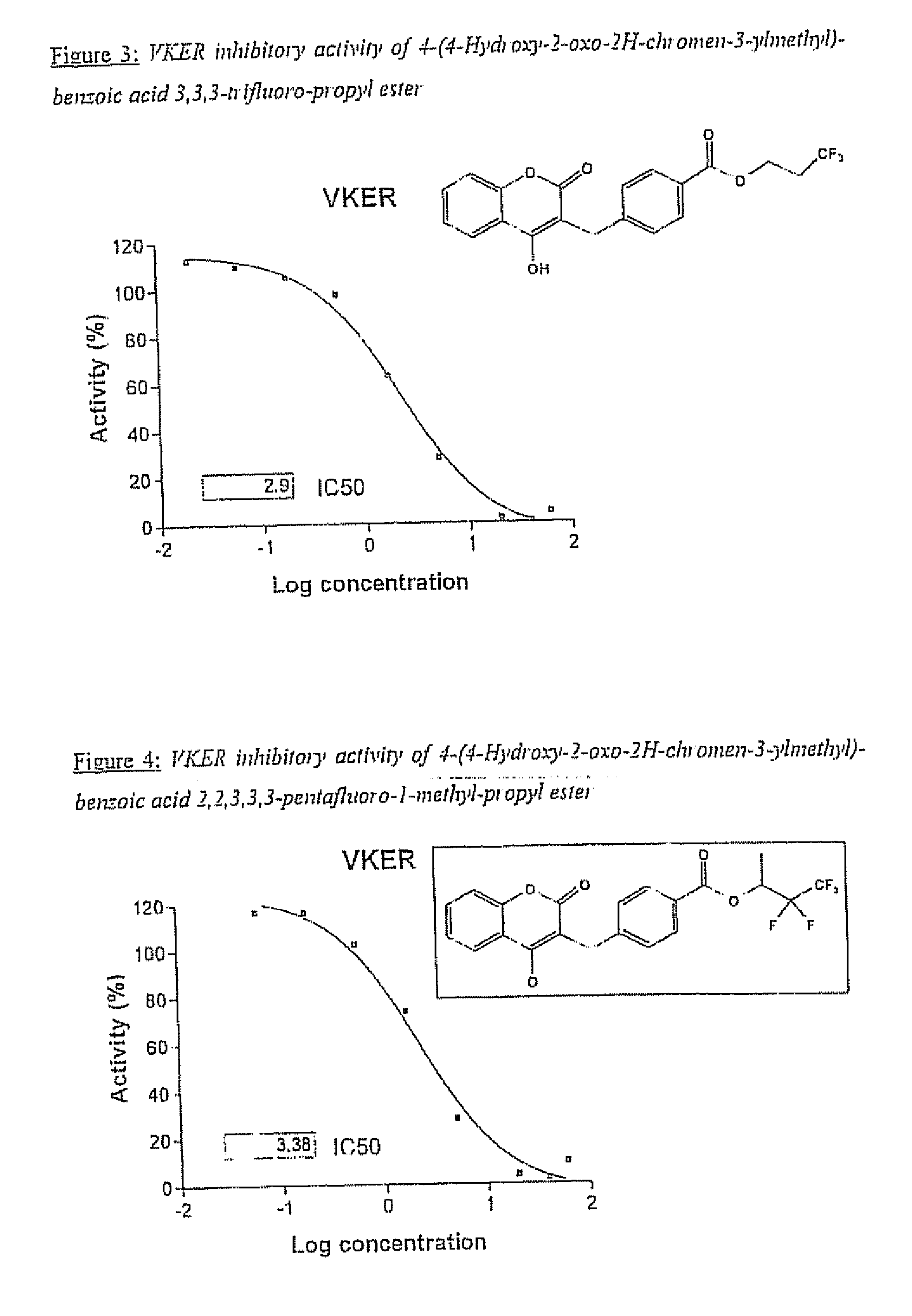
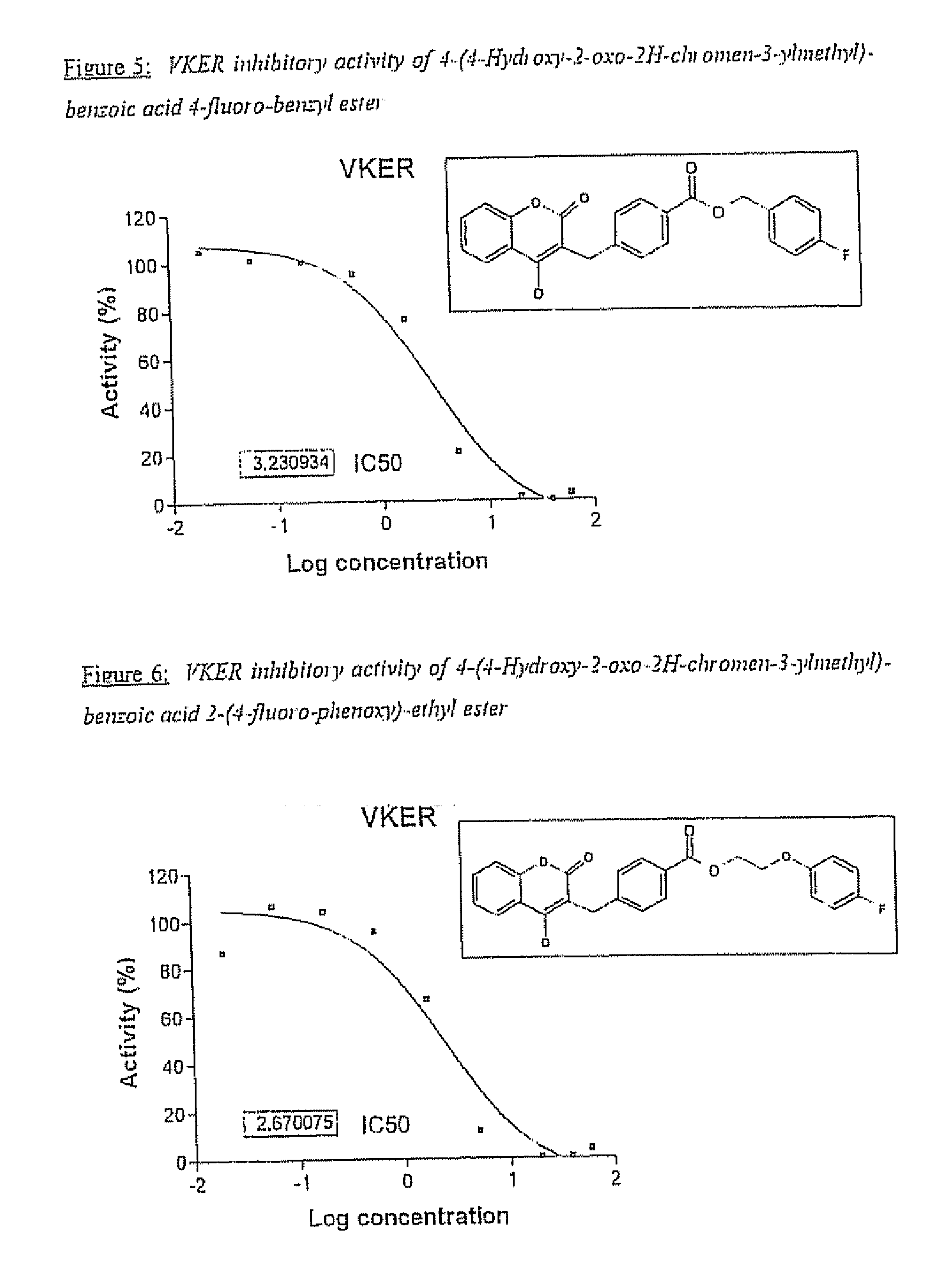
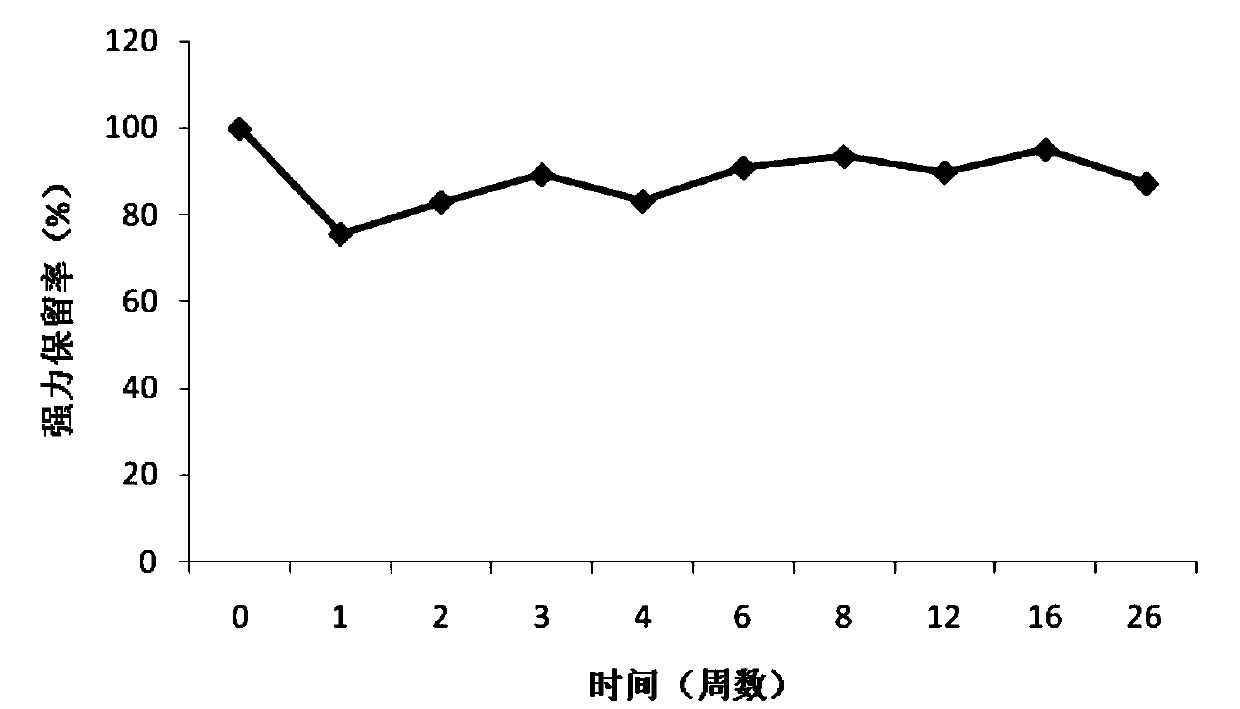
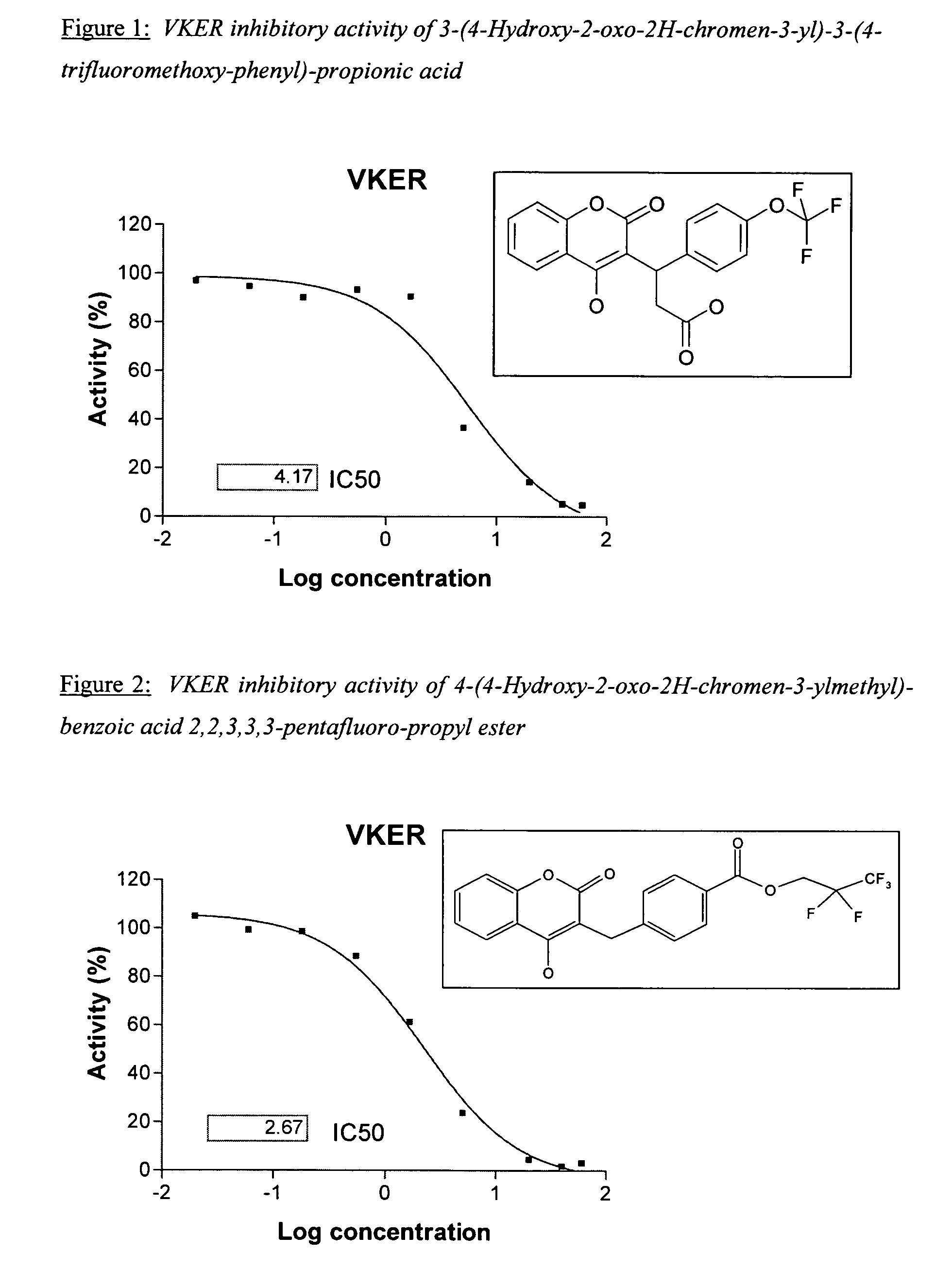
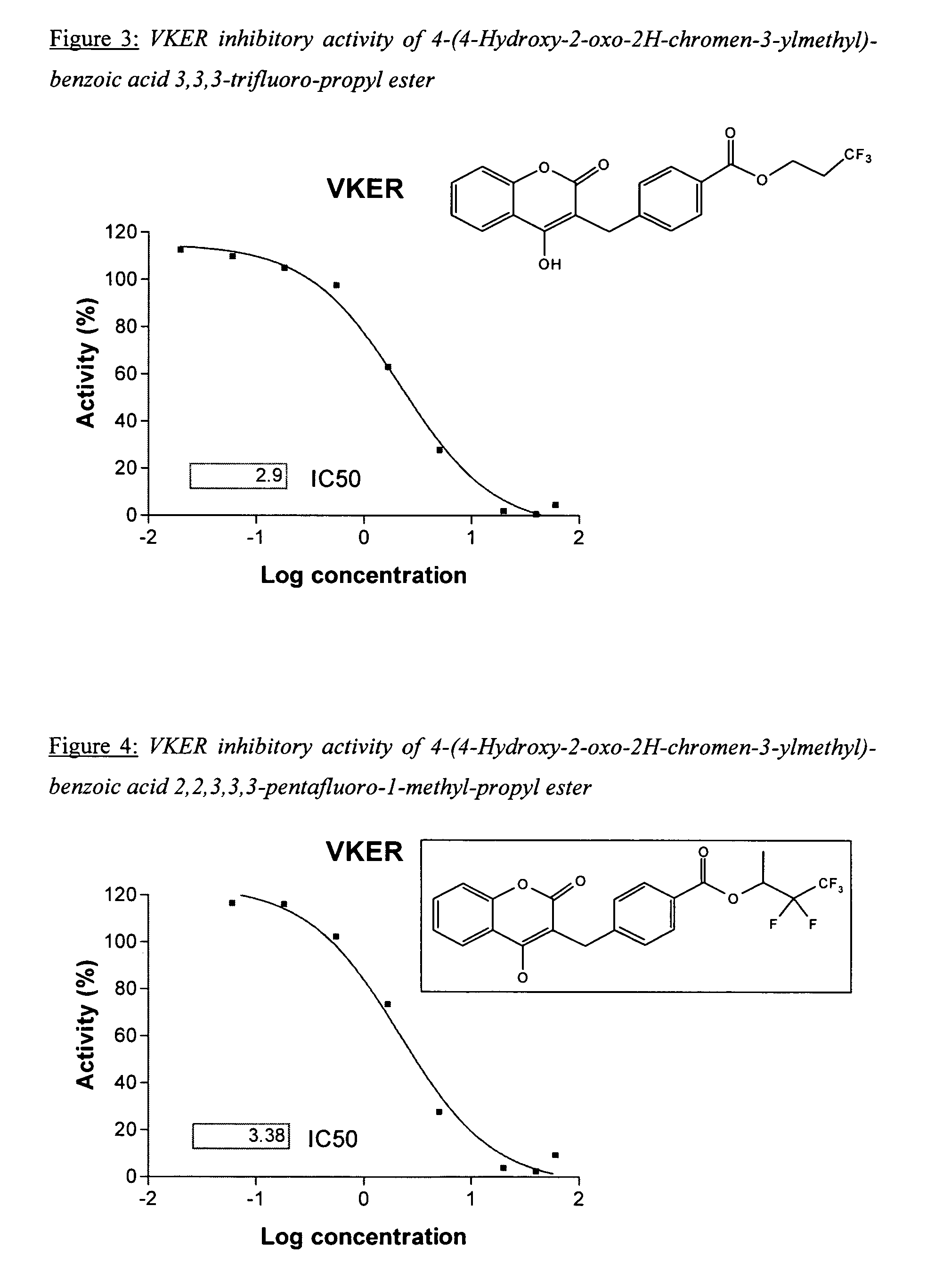
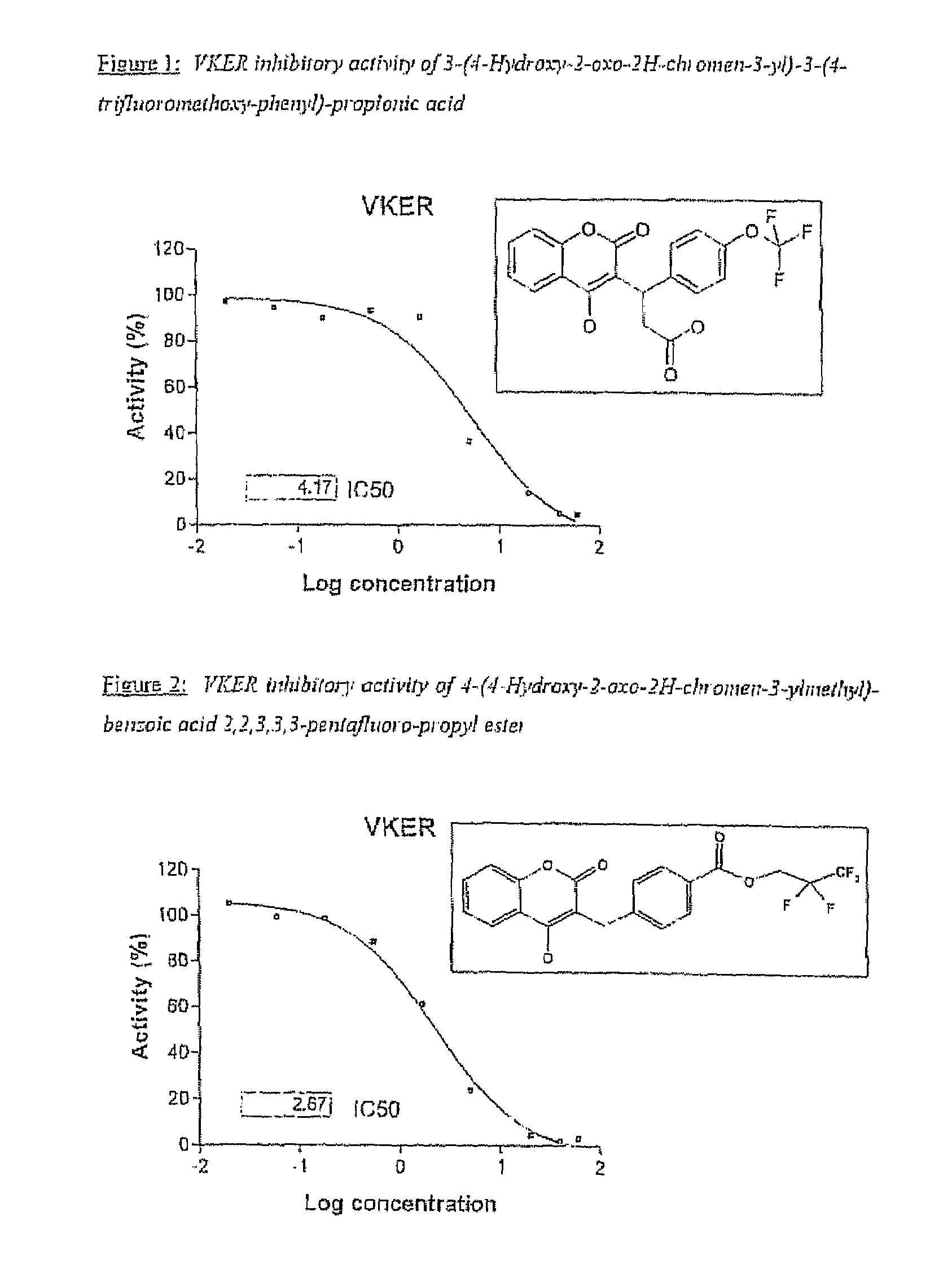
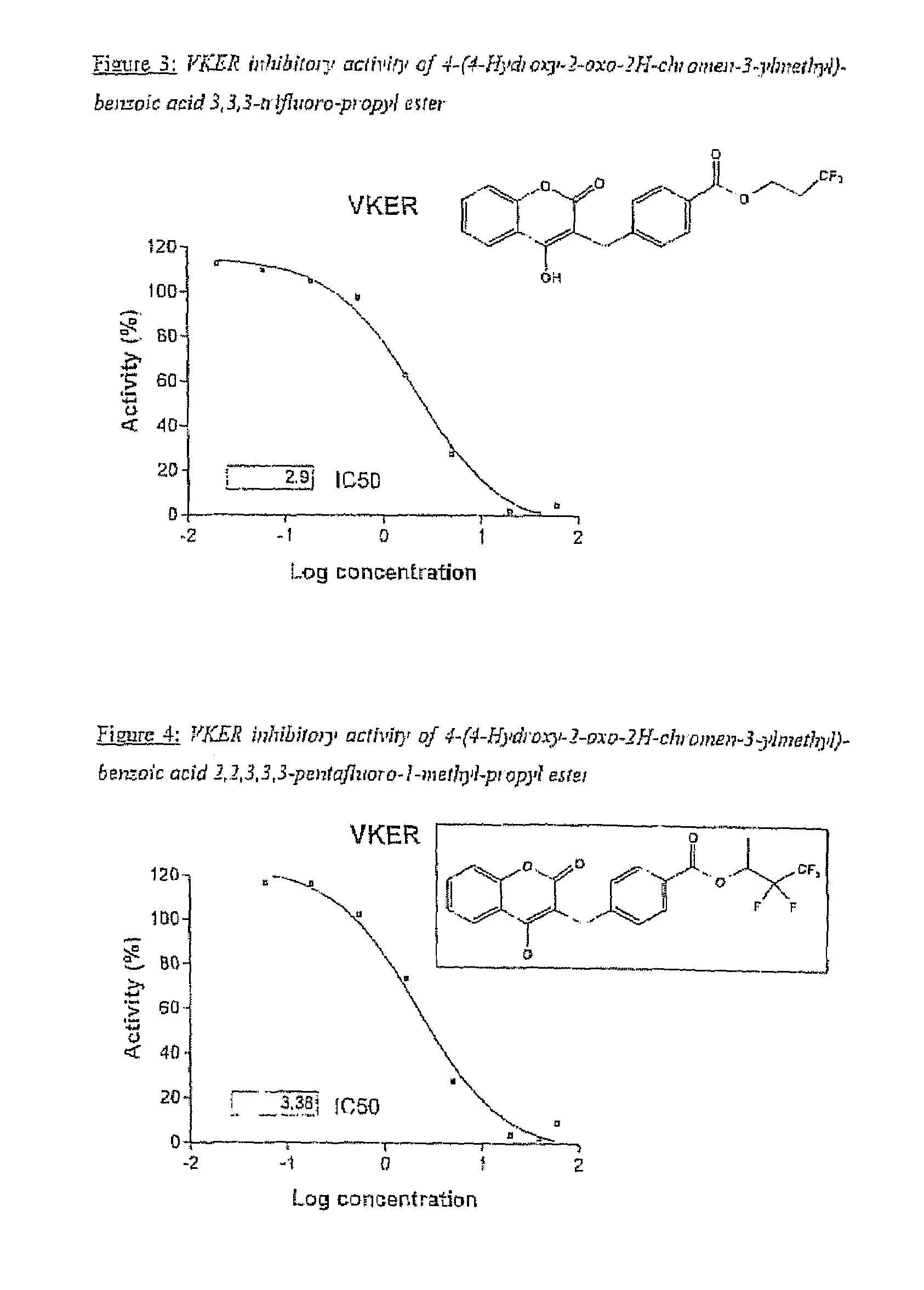
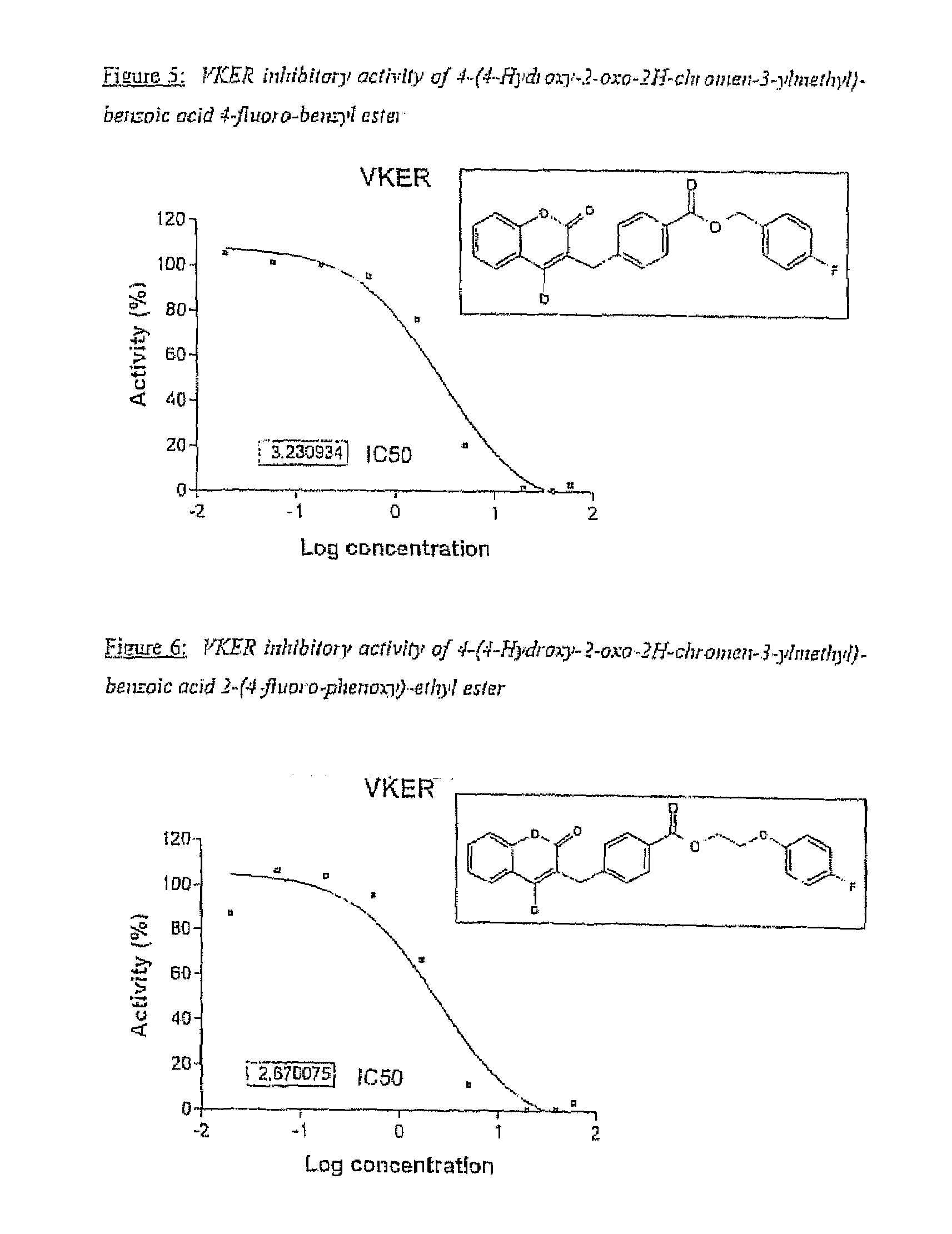
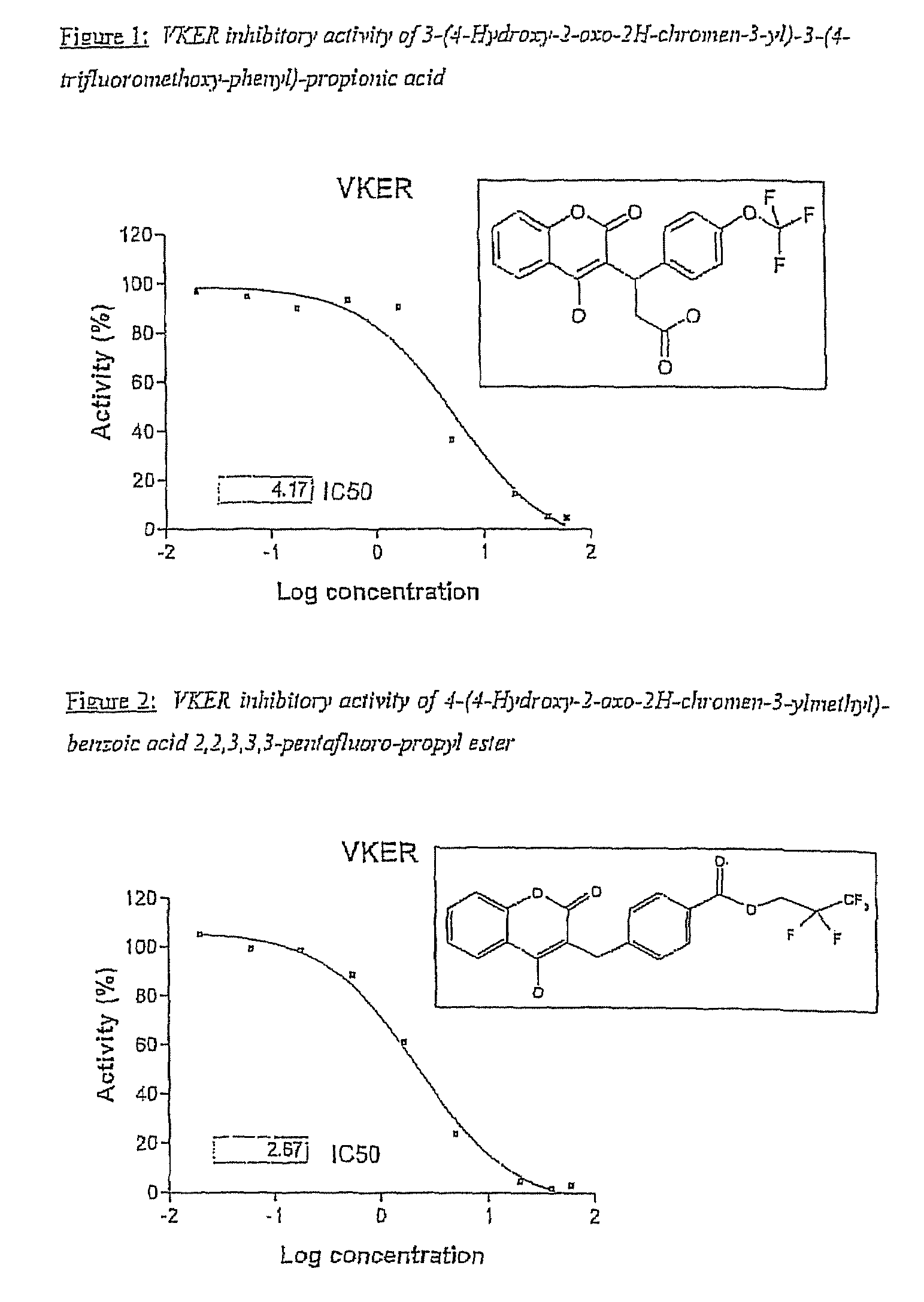
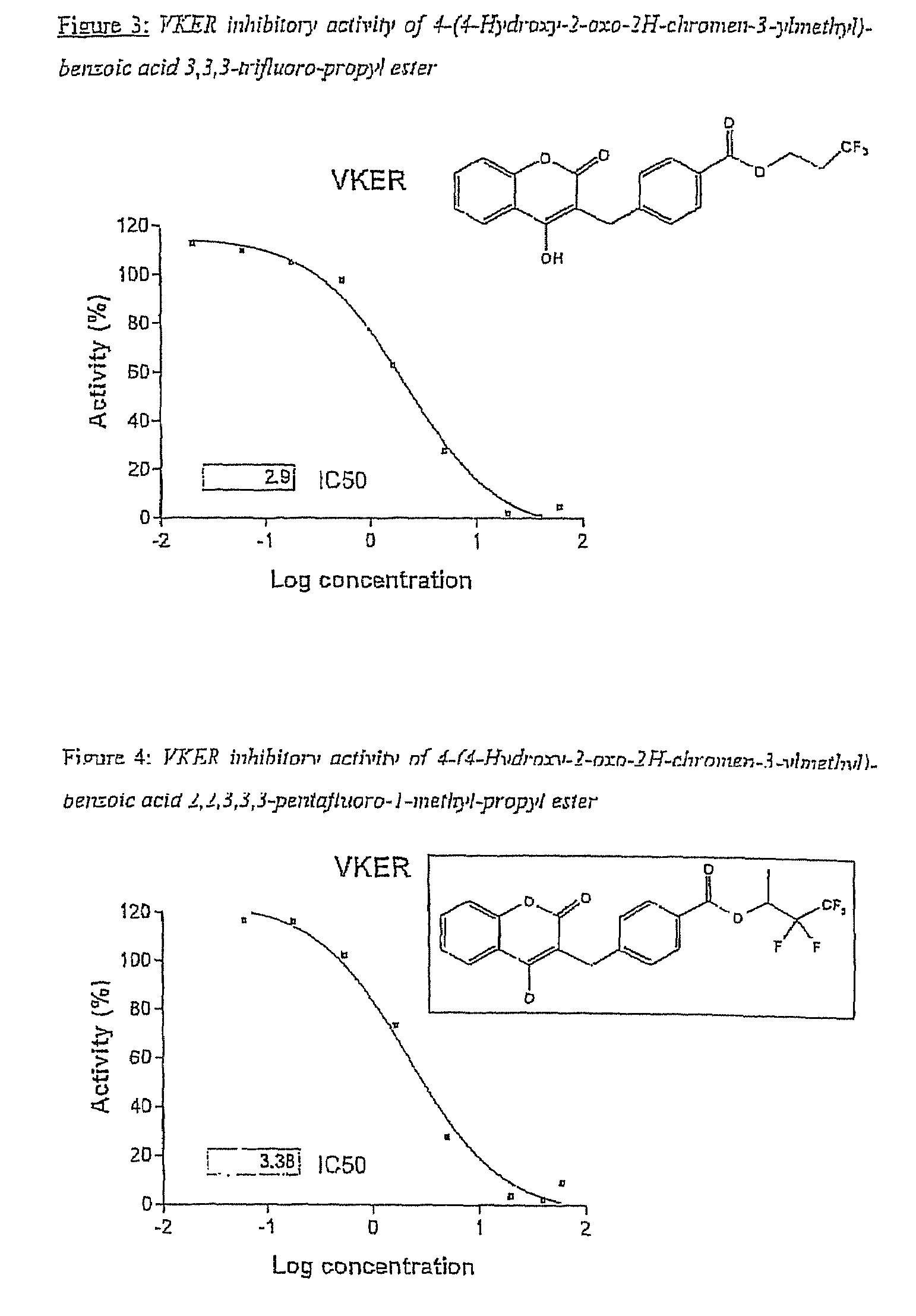
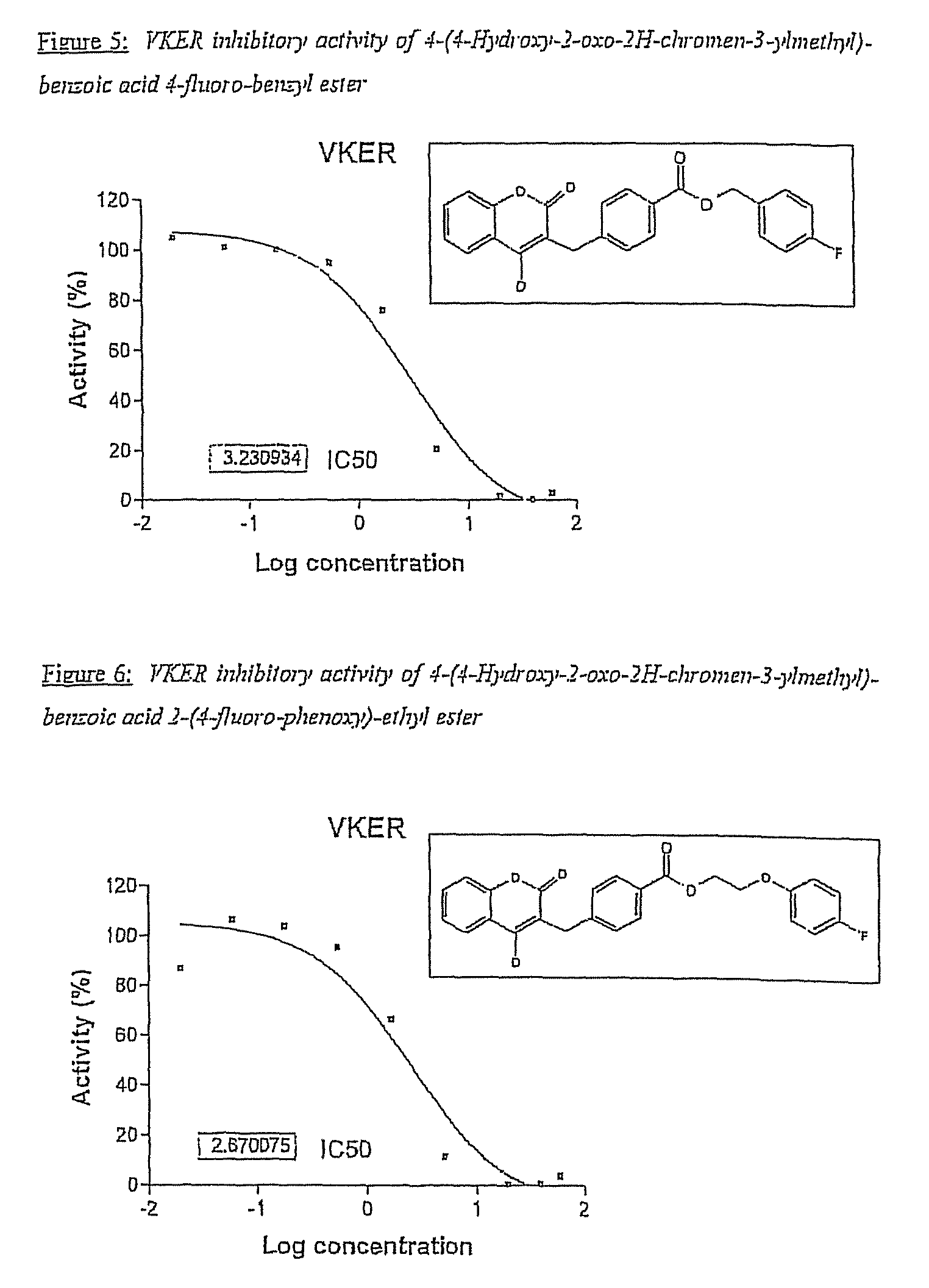
Halogenated ester derivatives of coumarins for the treatment of coagulation disorders
Owner:CADRENAL THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com