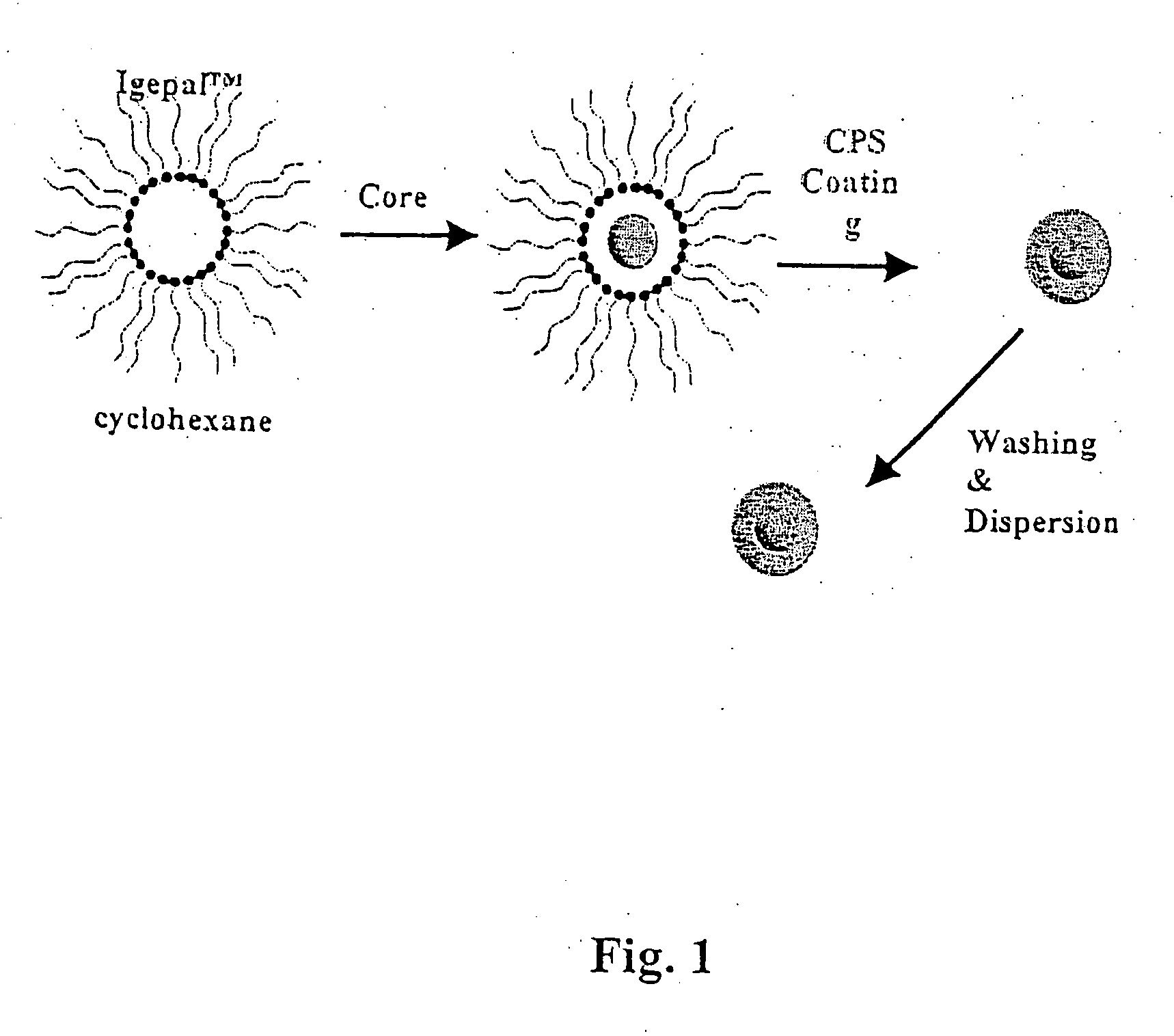
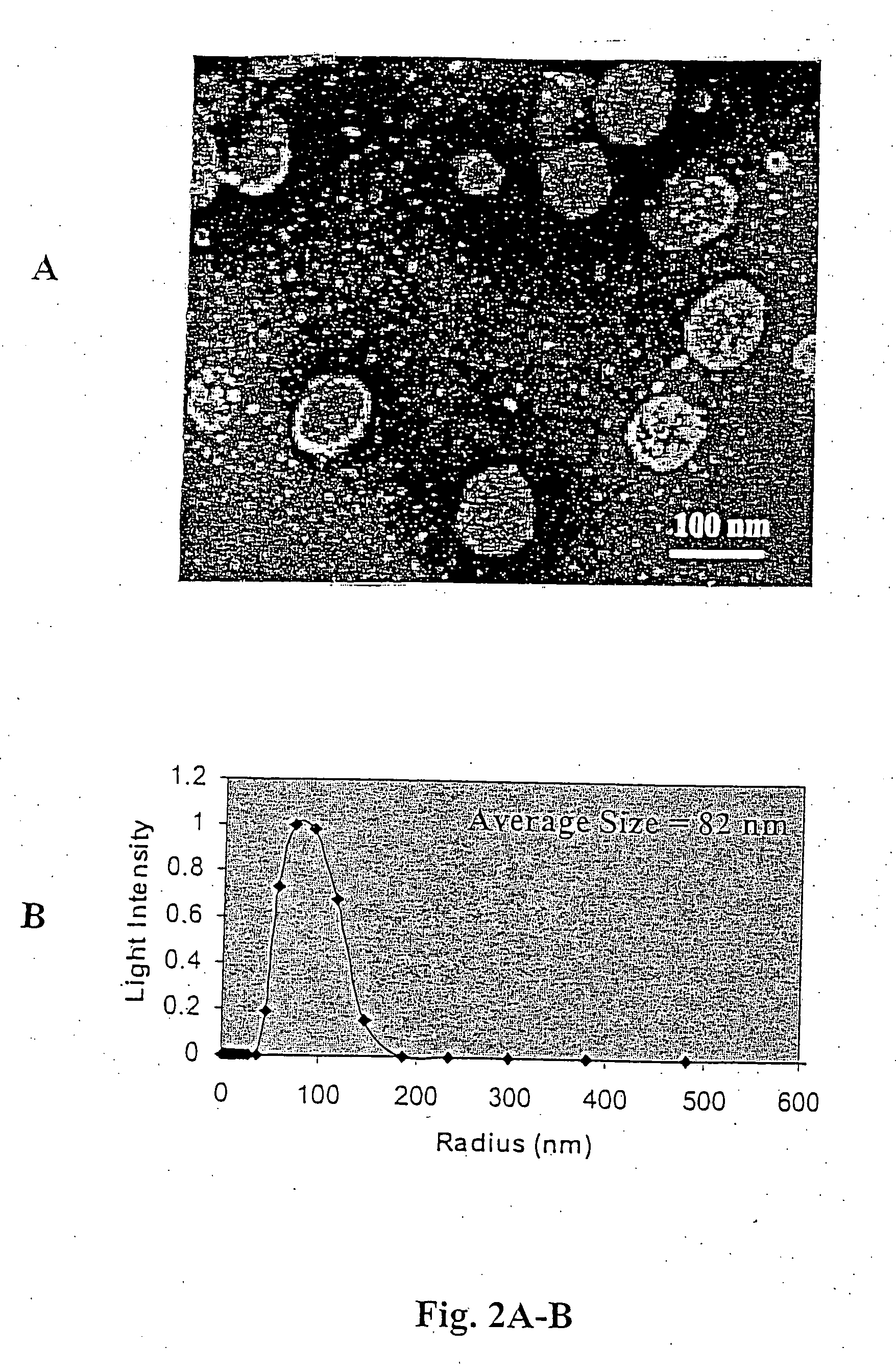
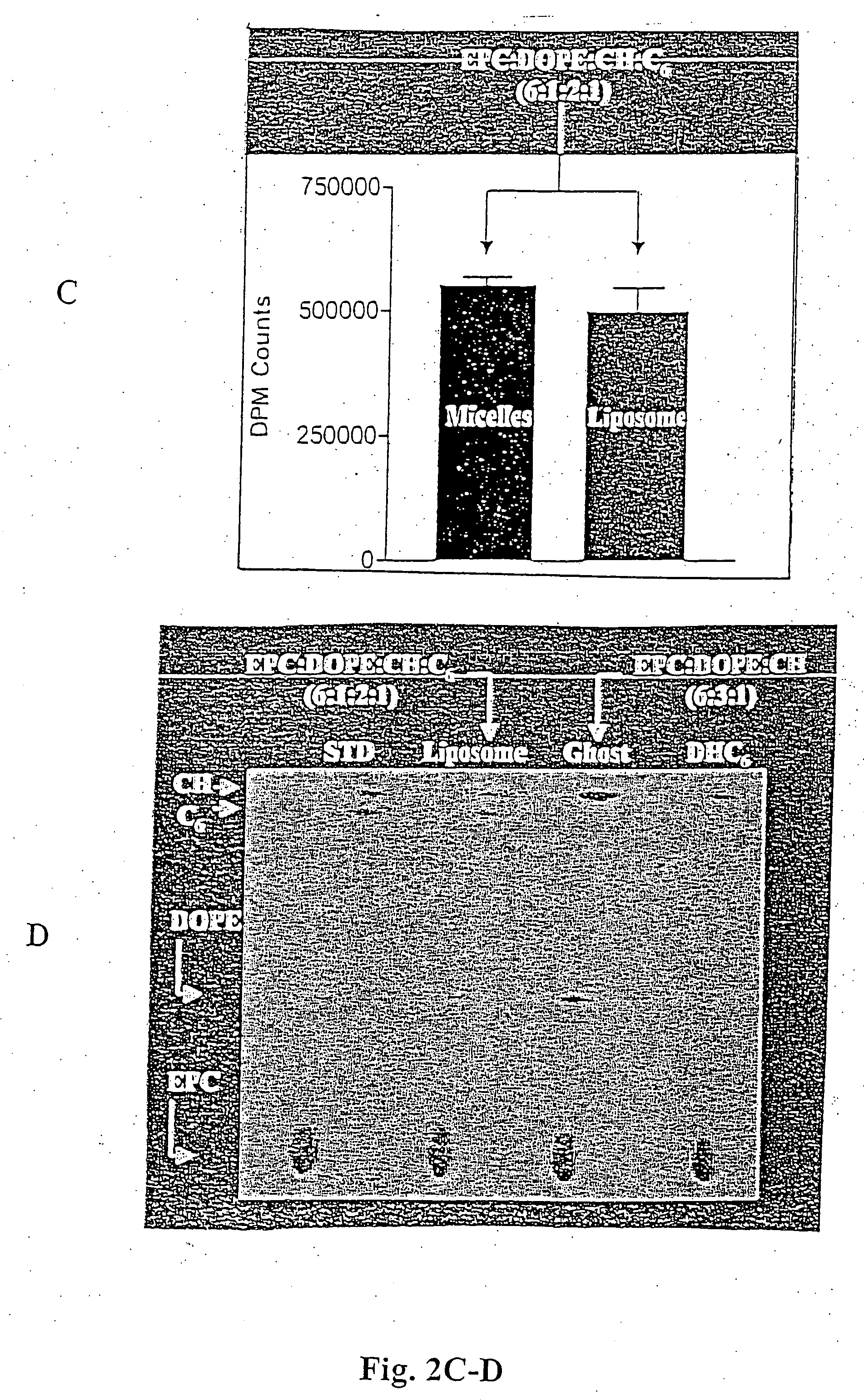
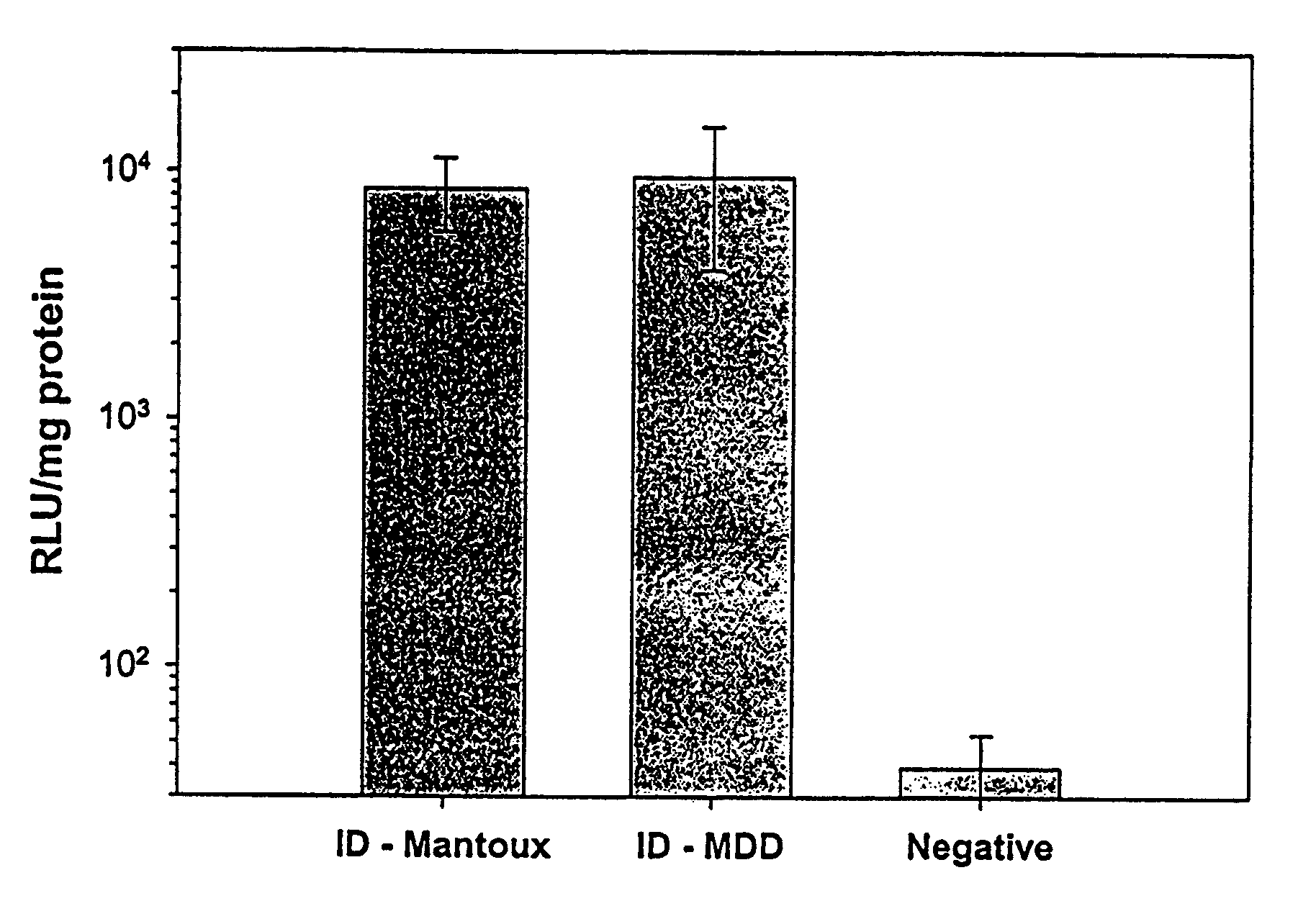
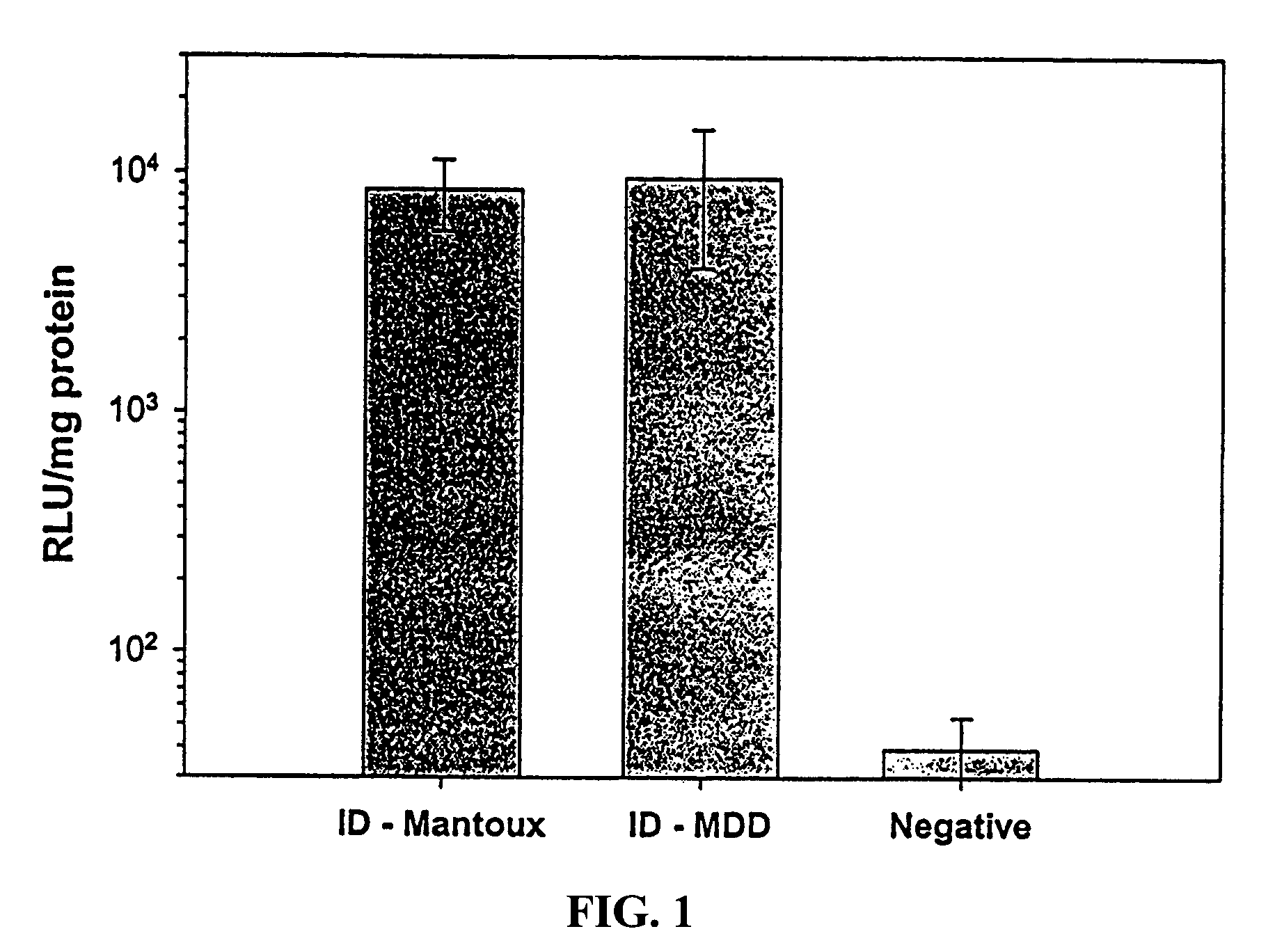
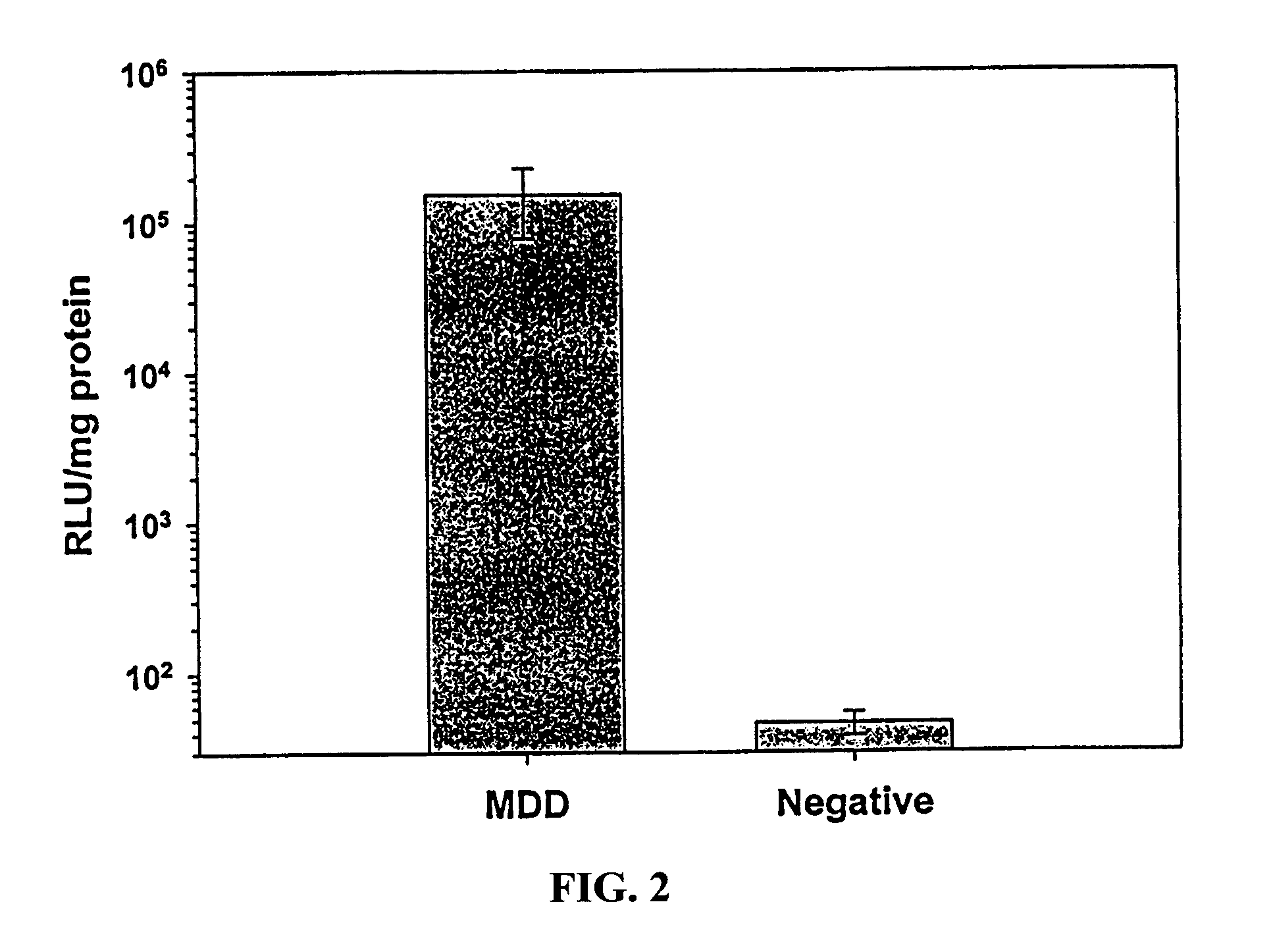
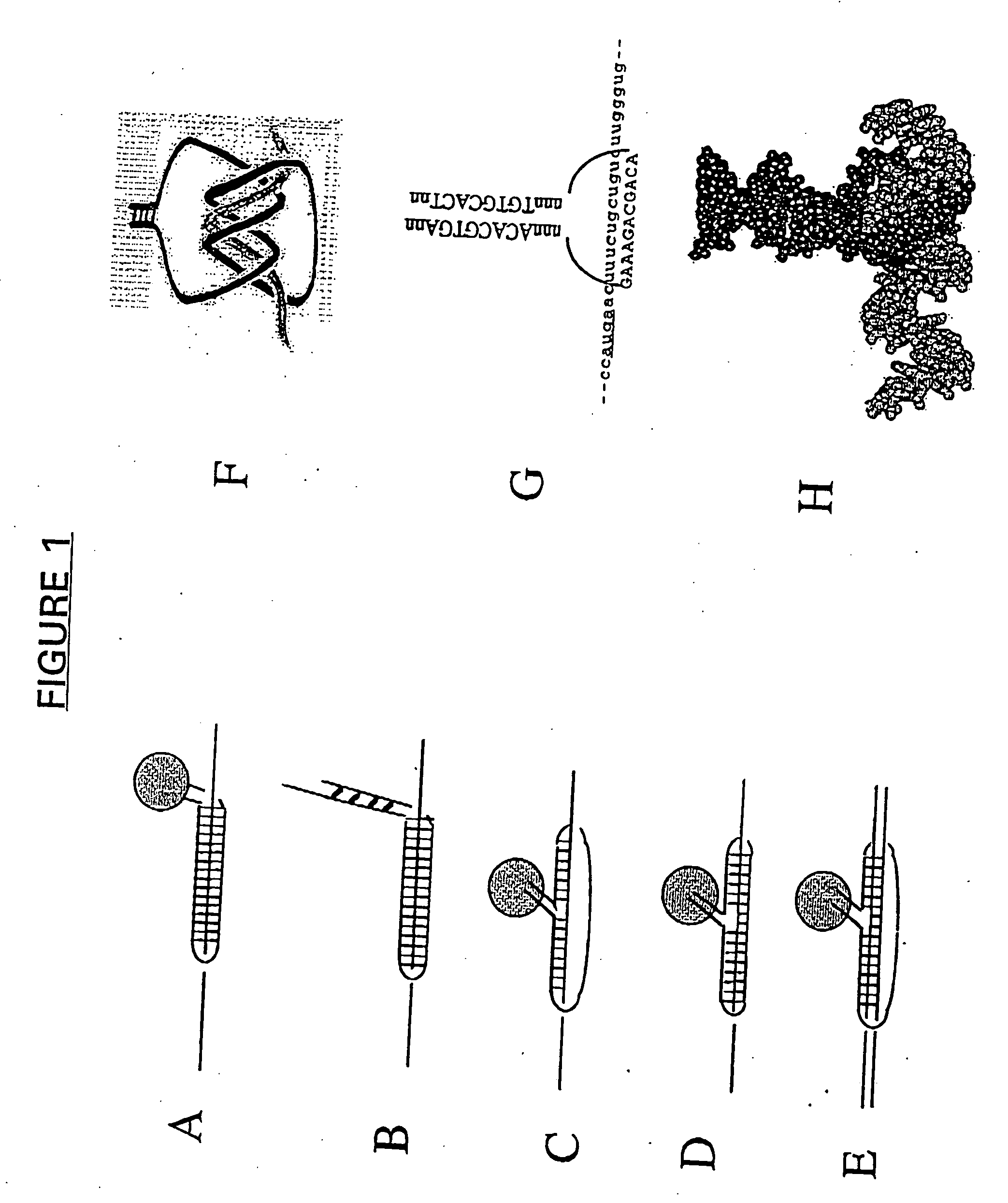
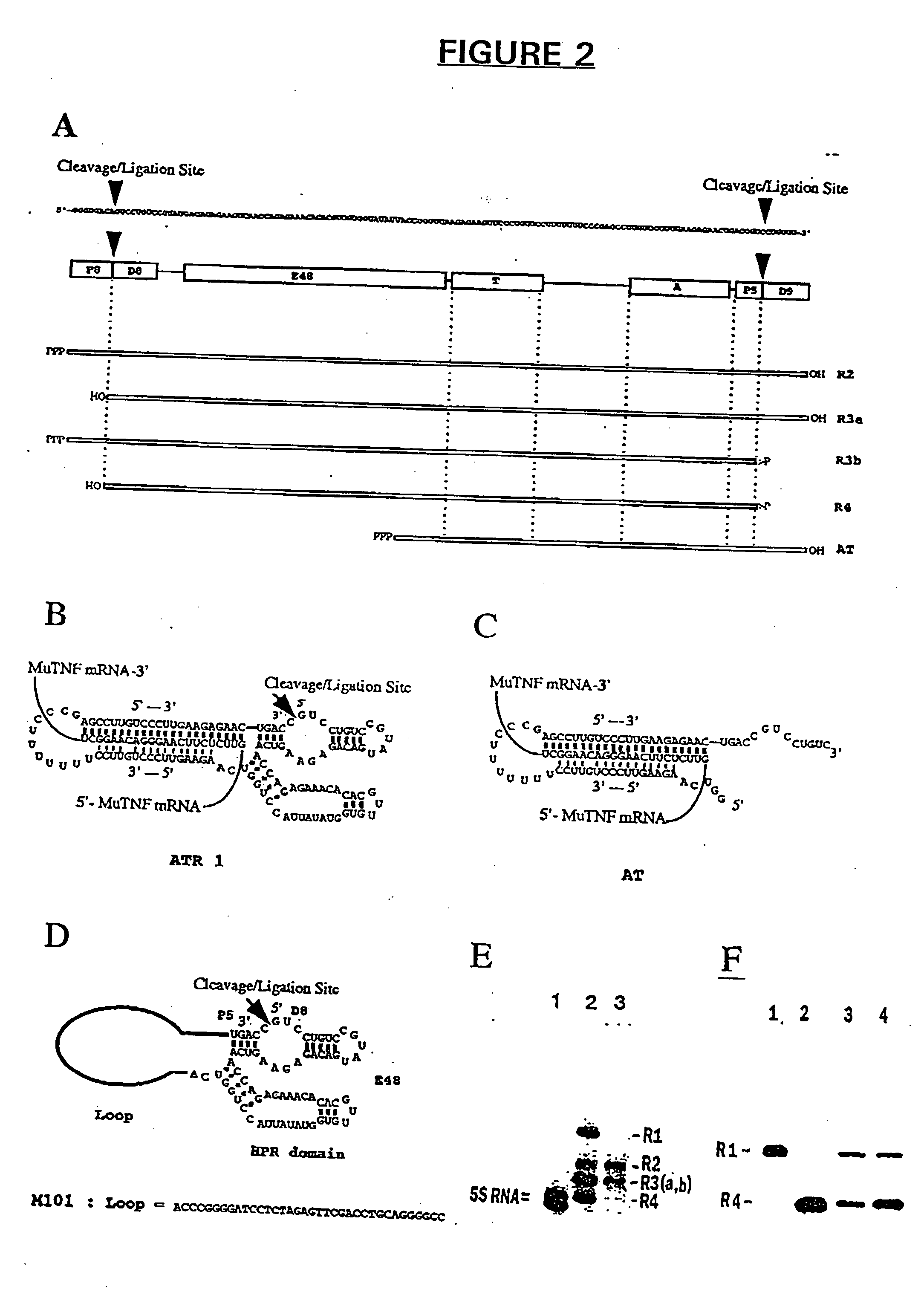
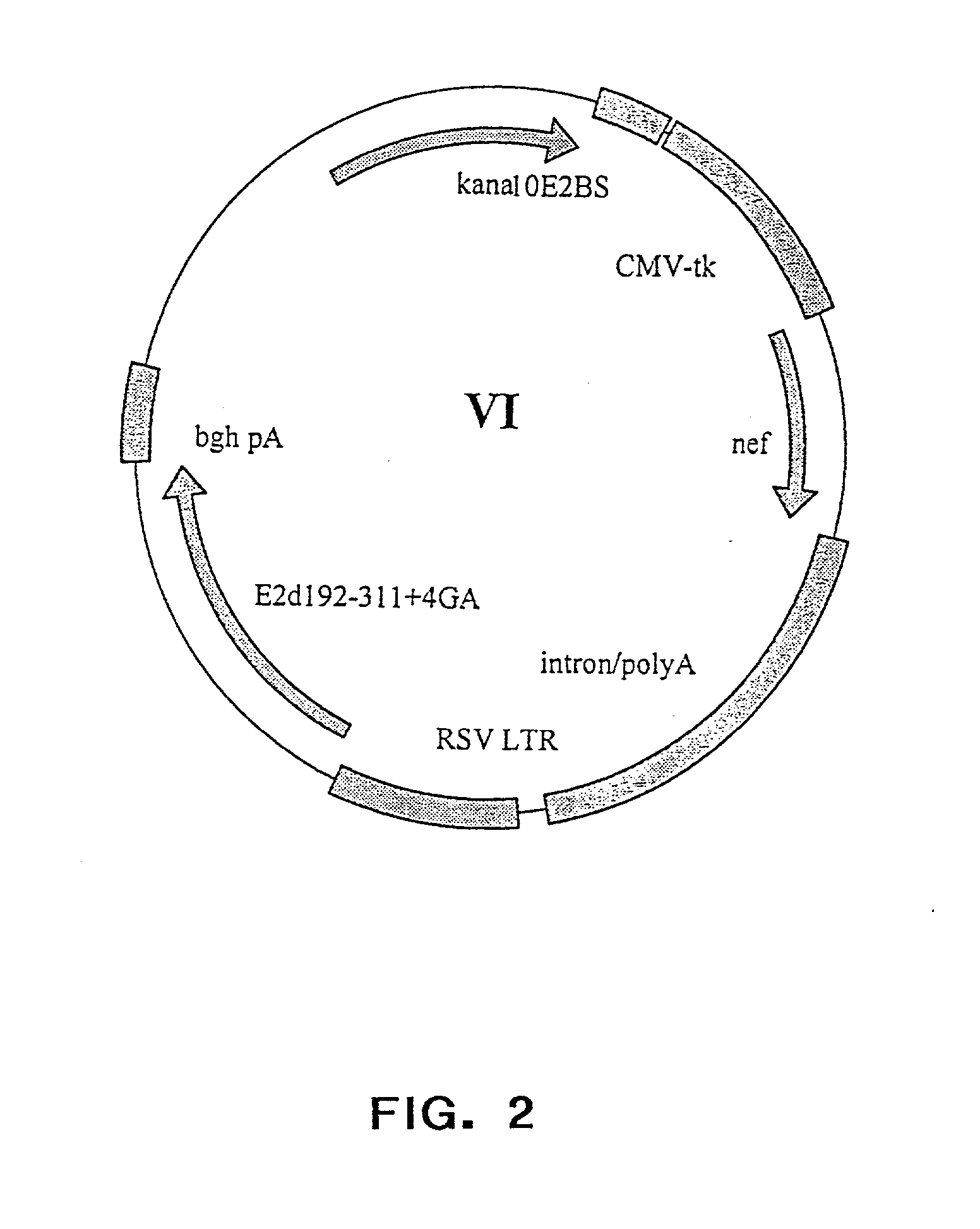
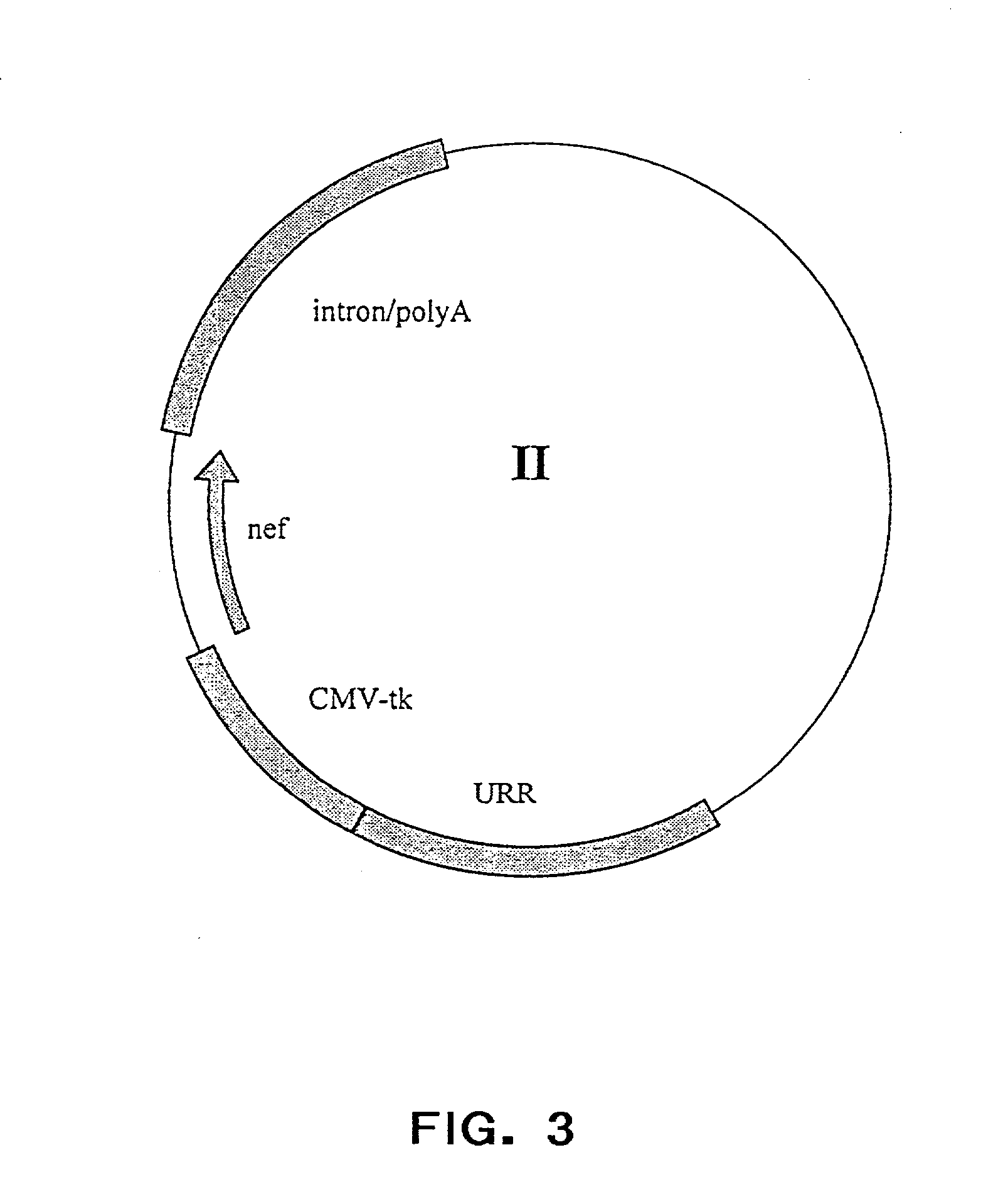
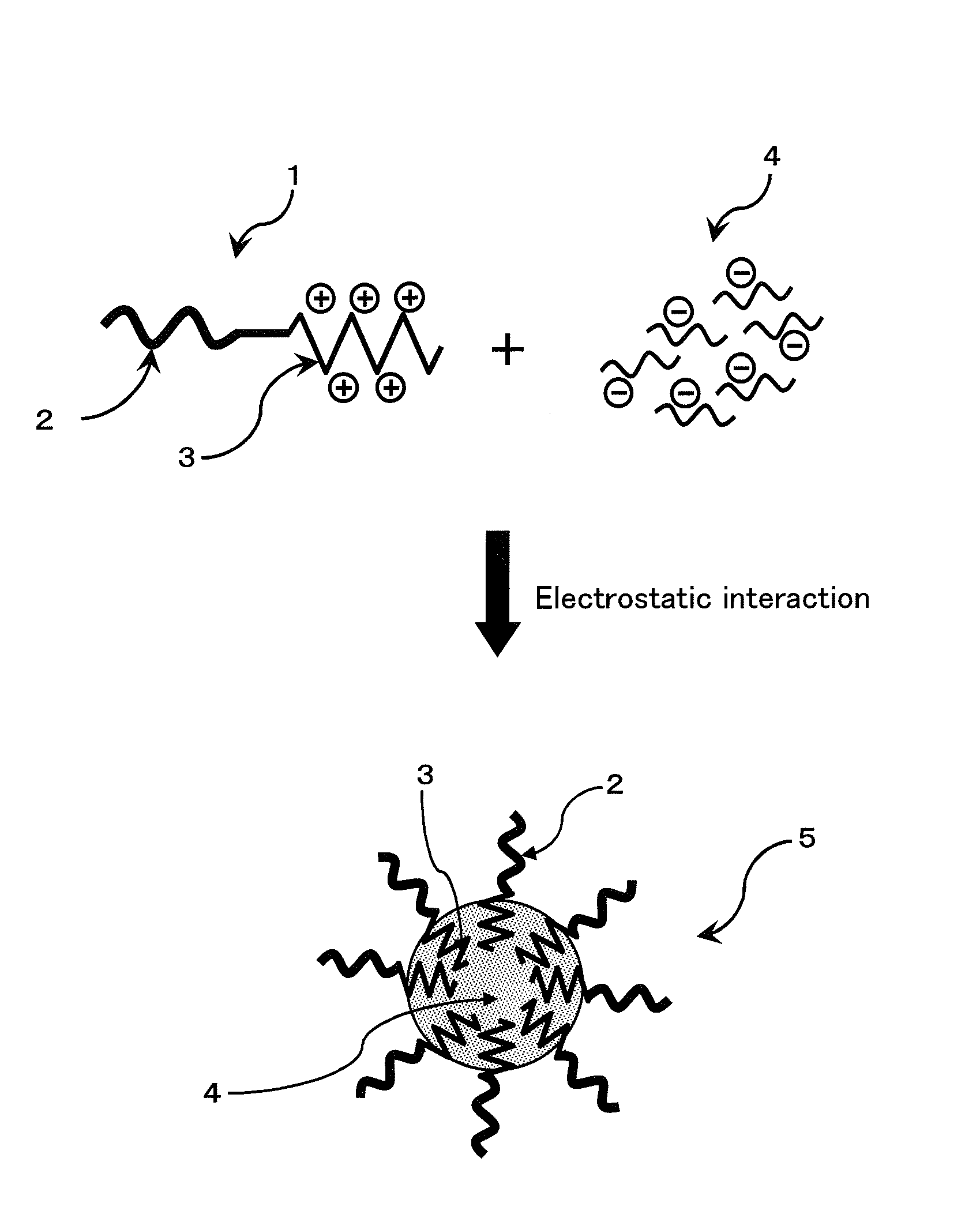
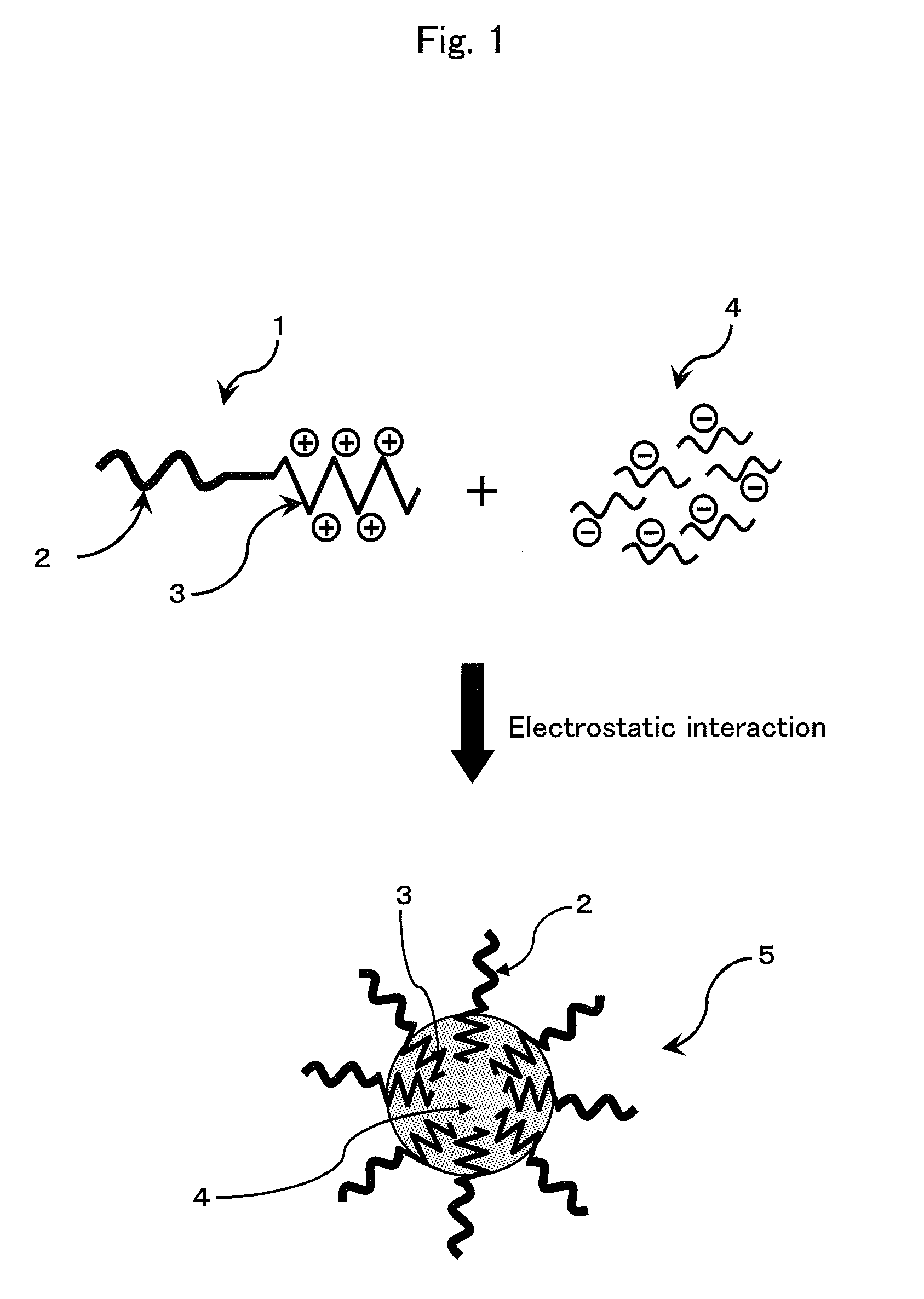
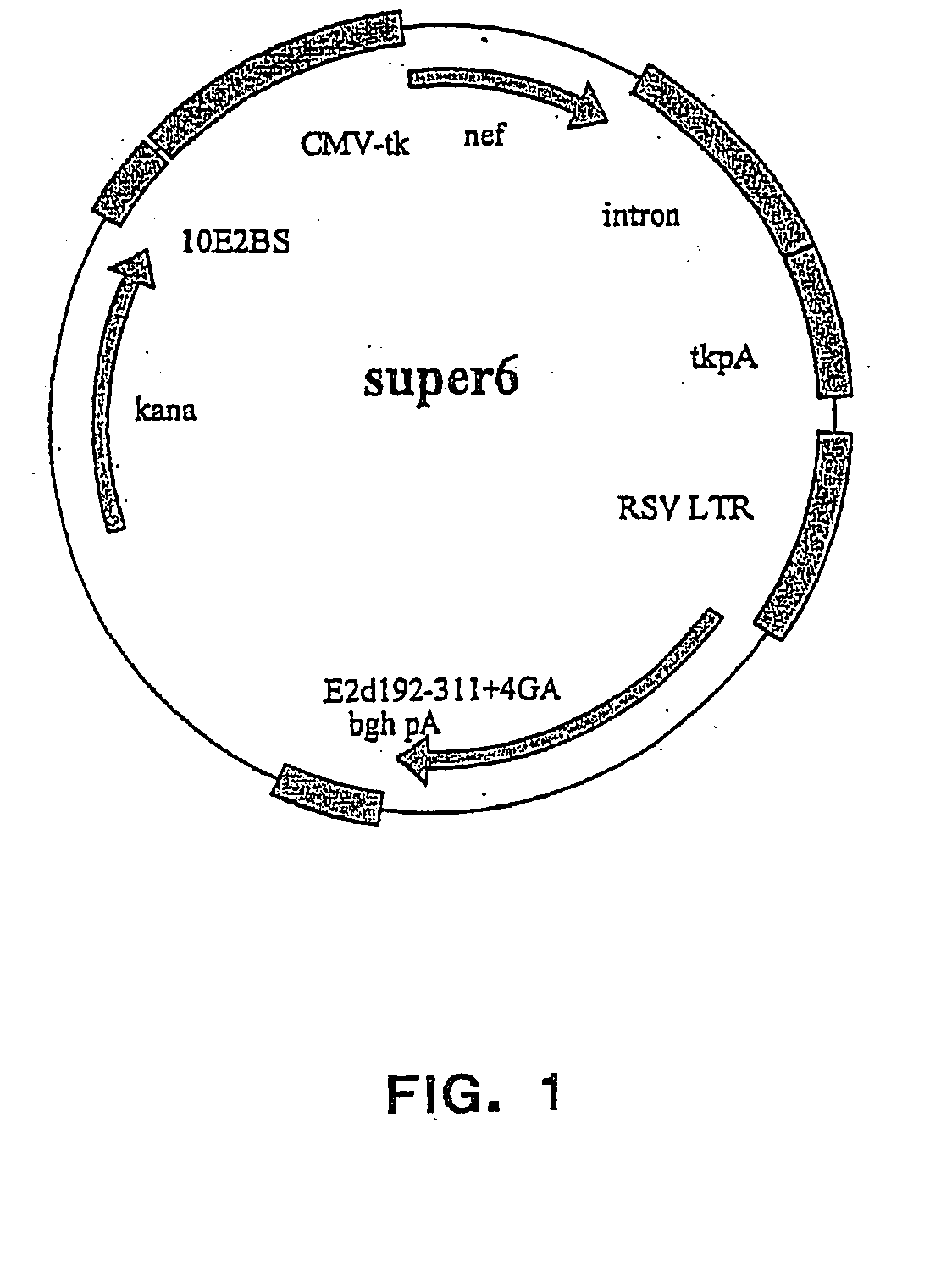
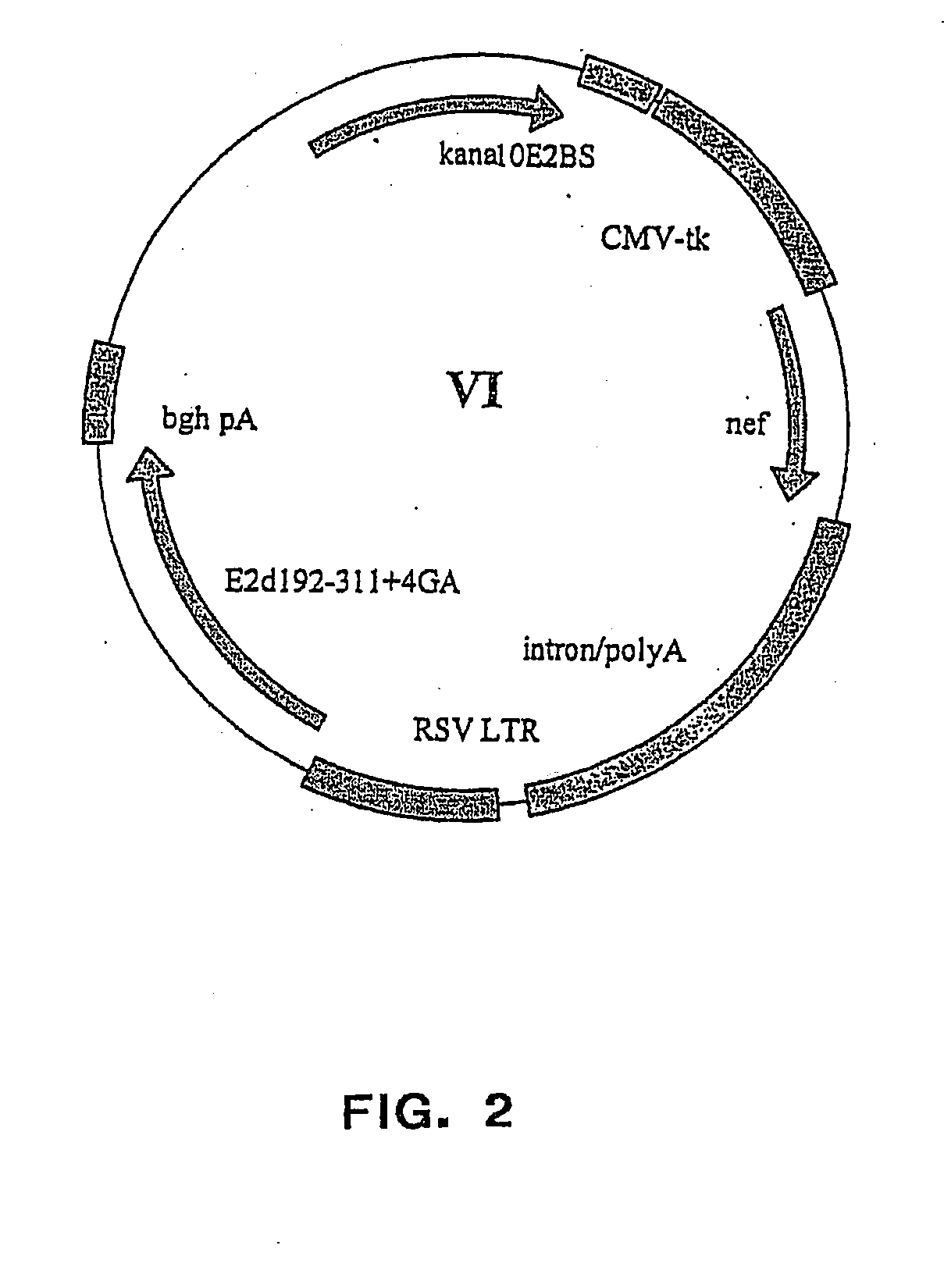
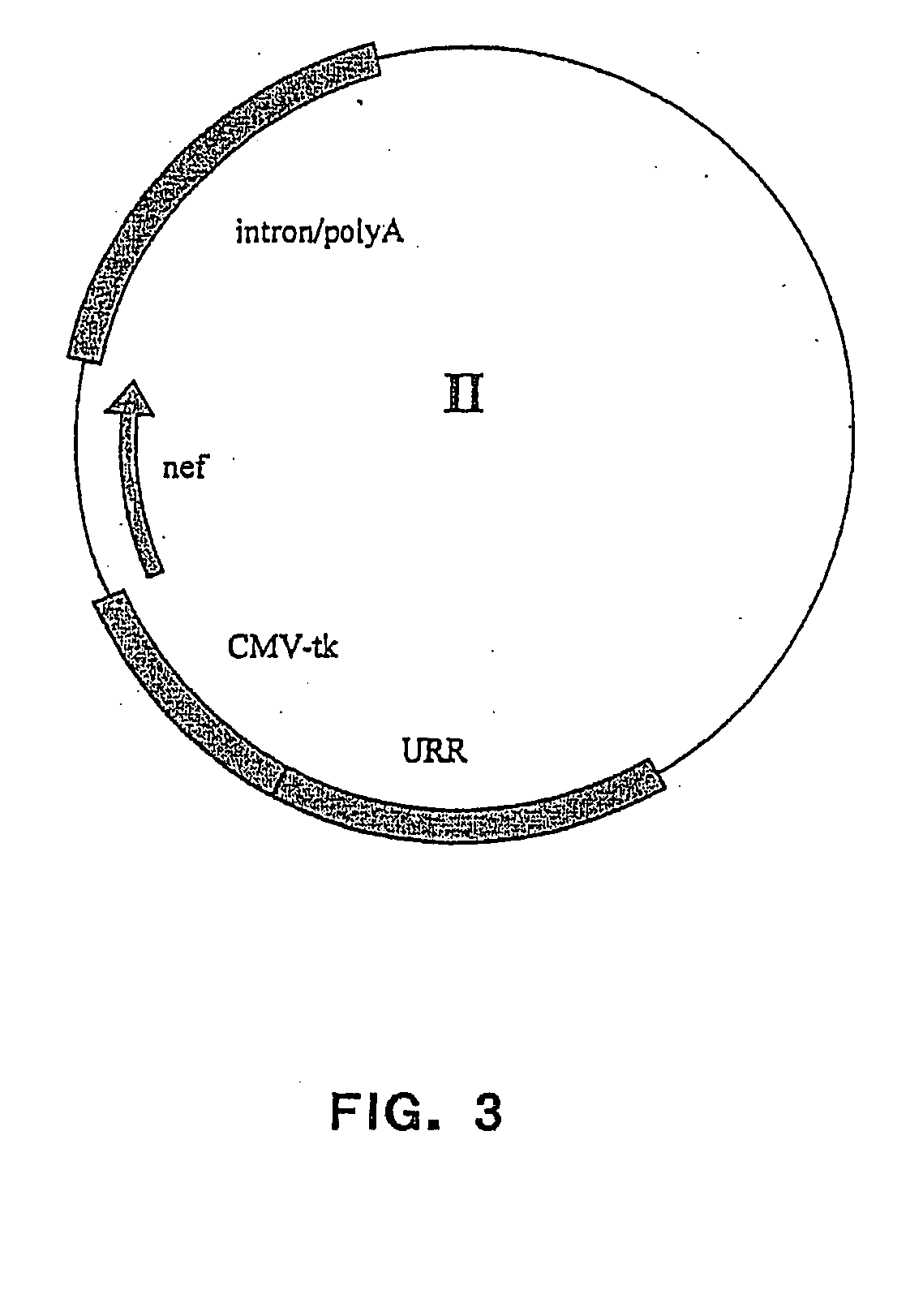
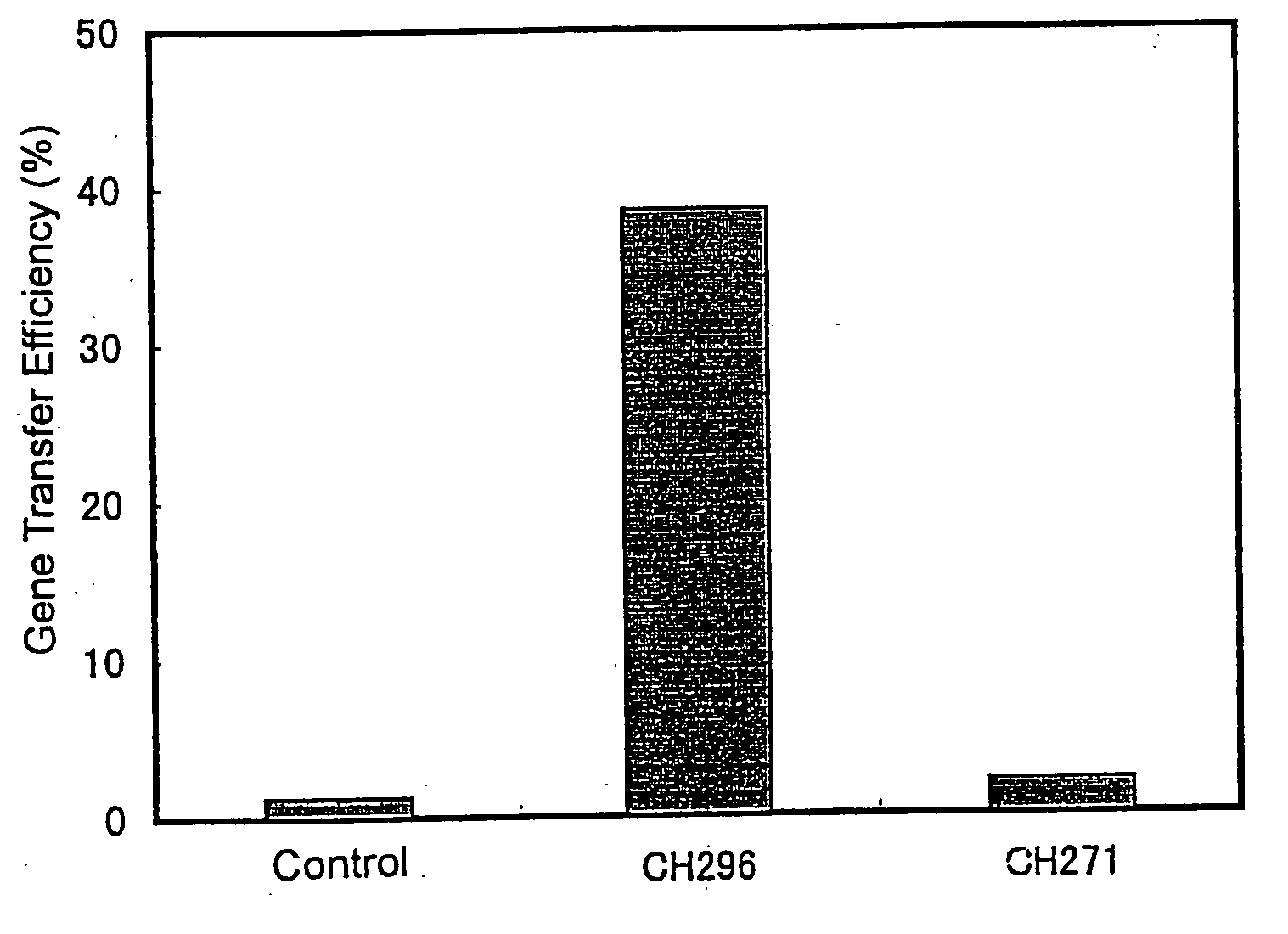
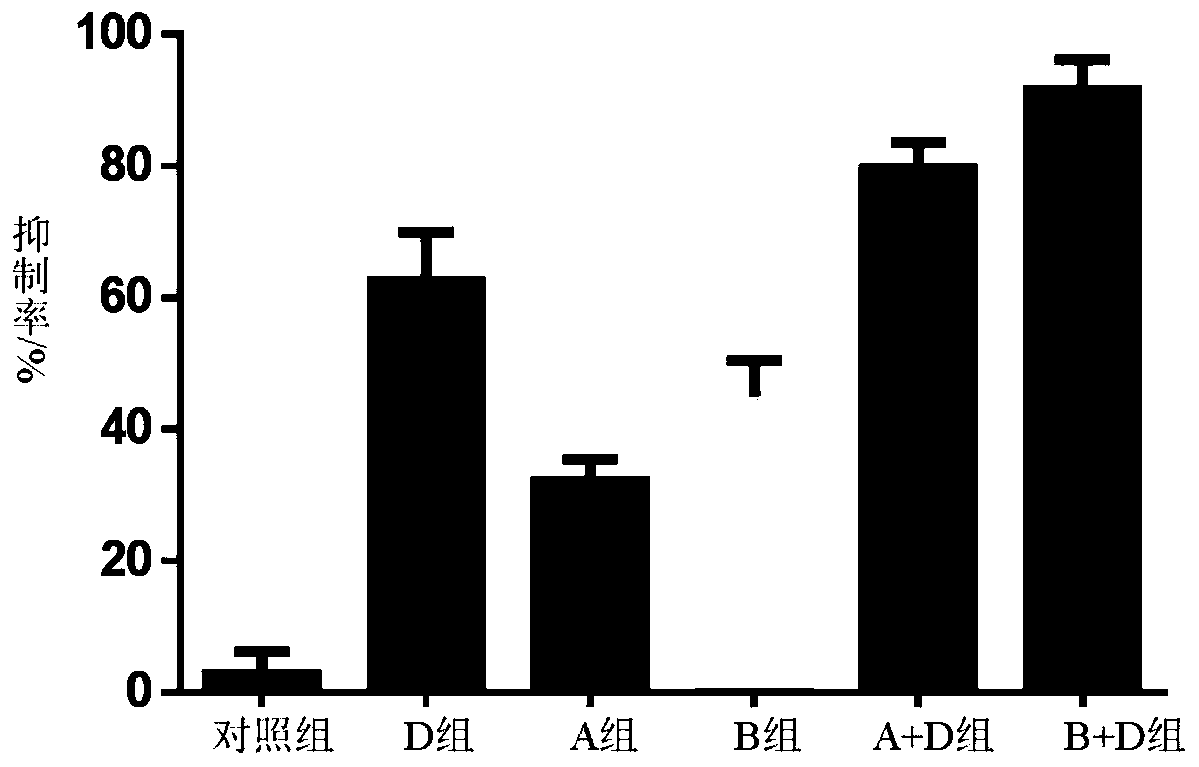
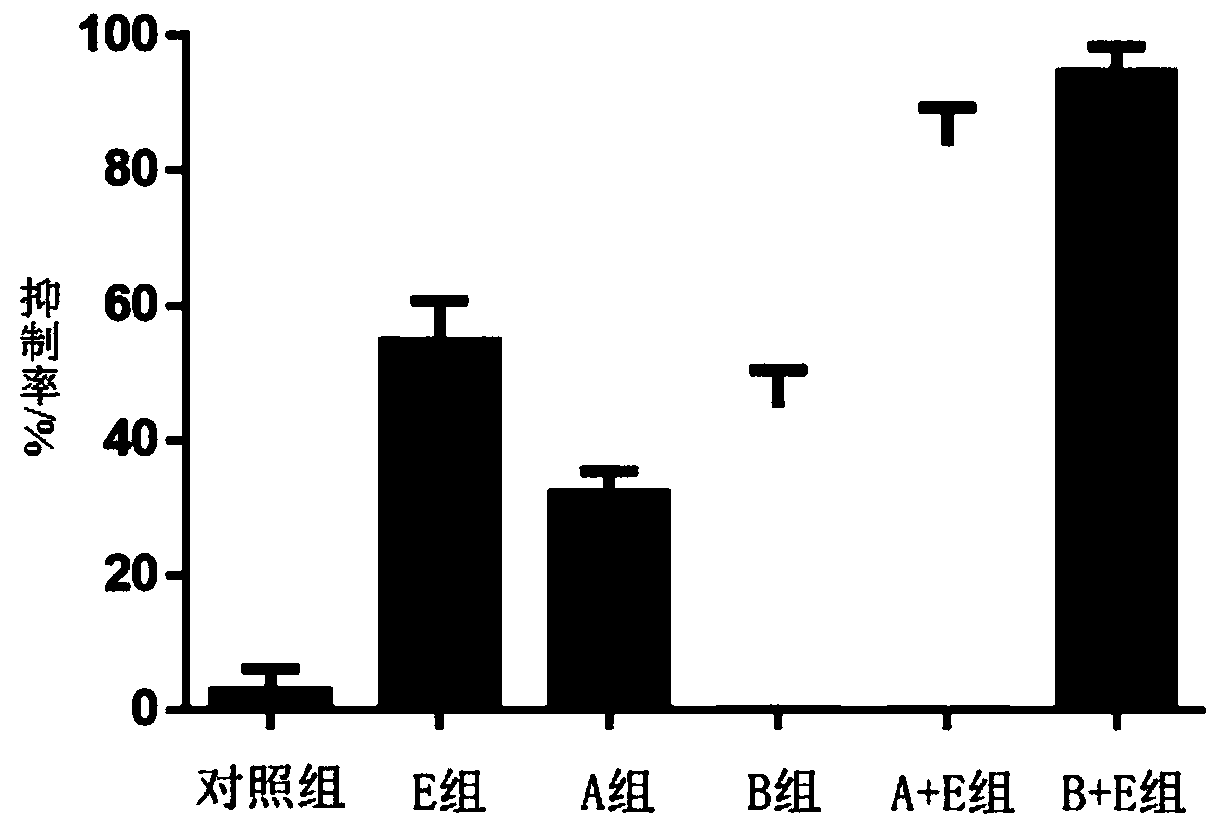
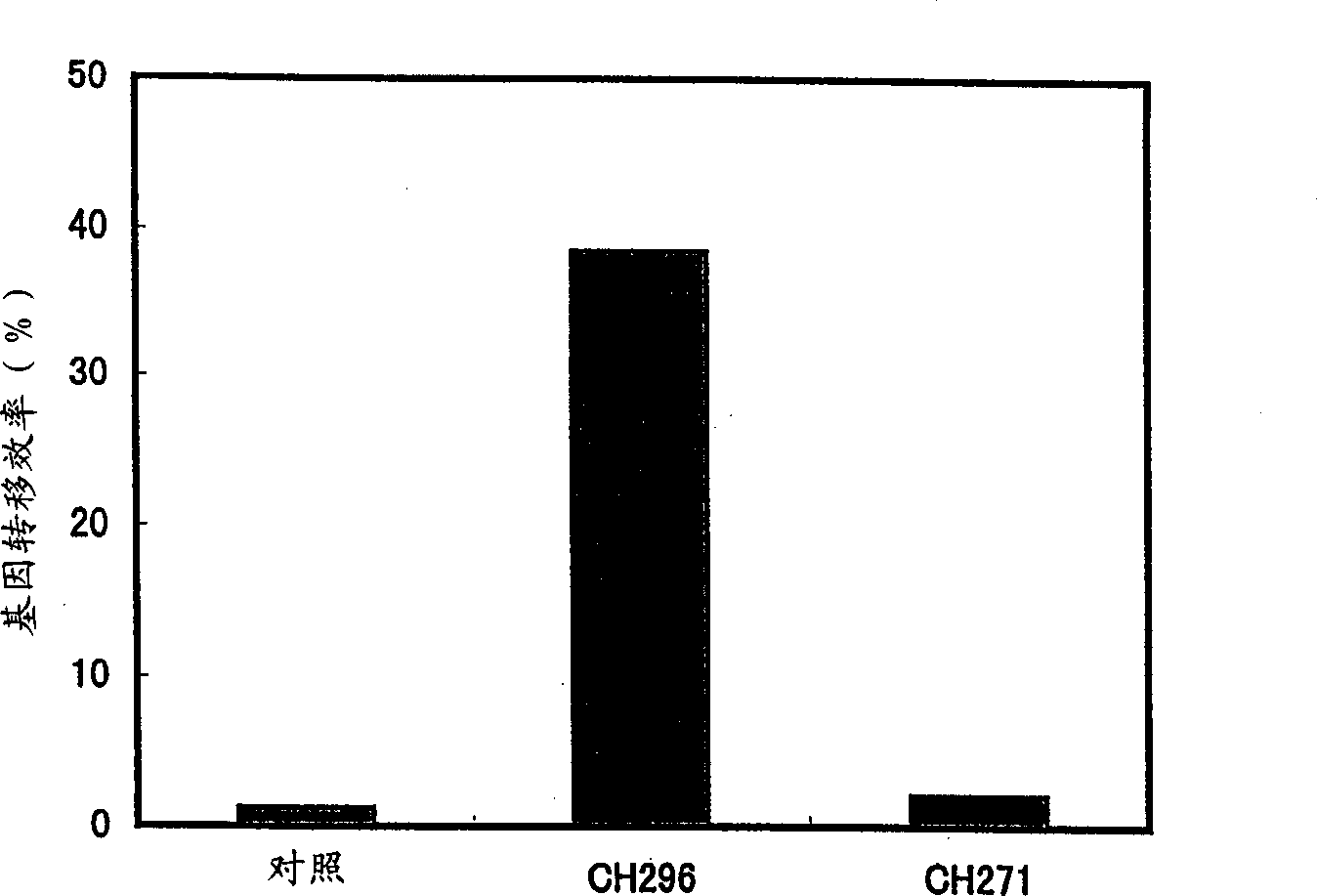
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Gene Therapy Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DNA constructs or co-administered agents used in gene therapy. Gene therapy constructs are used in delivery of genetic material into cells in order to permanently correct an inherited disease or an acquired disease such as cancer.
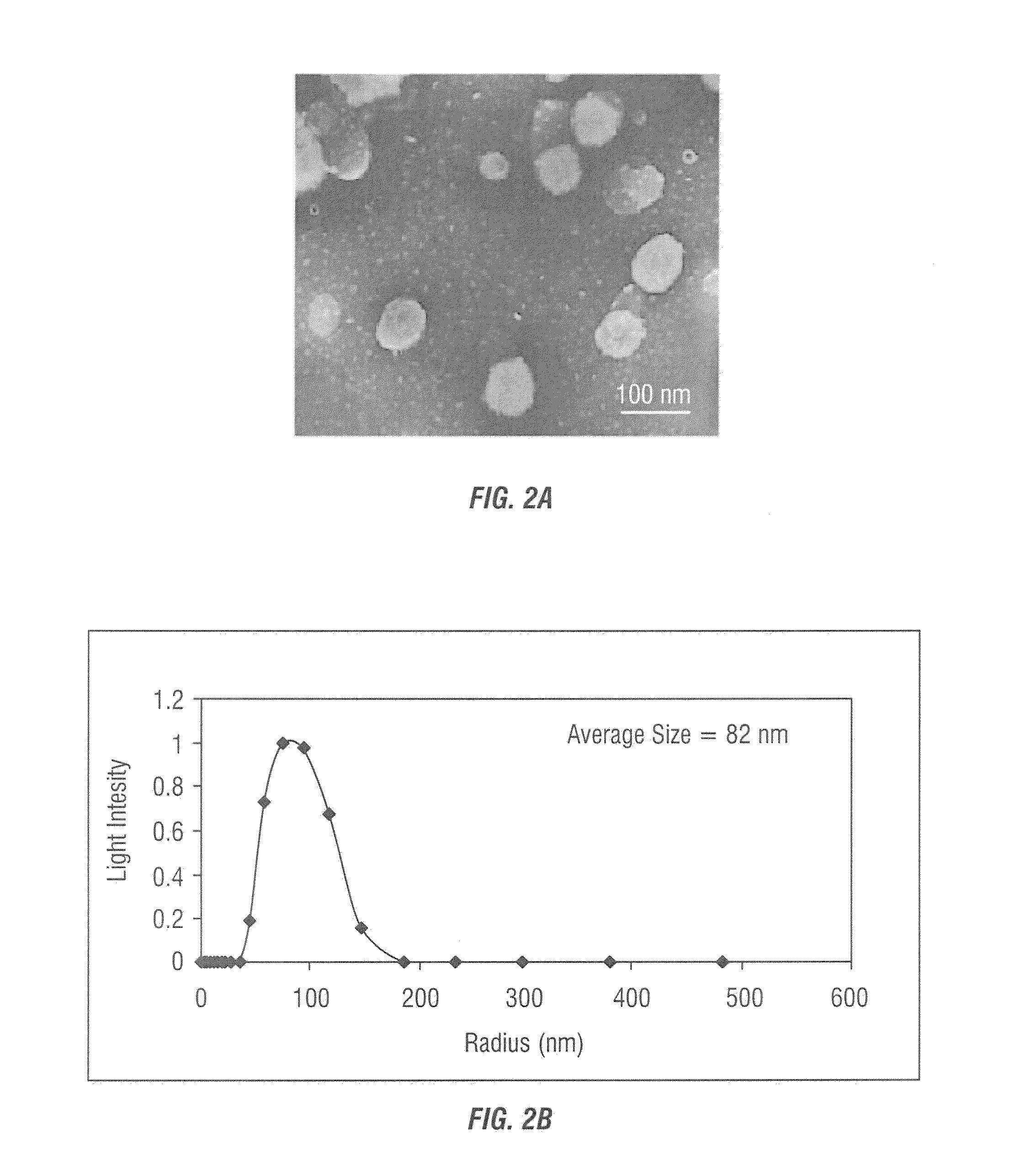
Method and system for systemic delivery of growth arresting, lipid-derived bioactive compounds
ActiveUS20050025820A1Maximizing systemic deliveryCorrection for dispersionOrganic active ingredientsMicroencapsulation basedDendrimerGene Therapy Agent
A system and method for optimizing the systemic delivery of growth-arresting lipid-derived bioactive drugs or gene therapy agents to an animal or human in need of such agents utilizing nanoscale assembly systems, such as liposomes, resorbable and non-aggregating nanoparticle dispersions, metal or semiconductor nanoparticles, or polymeric materials such as dendrimers or hydrogels, each of which exhibit improved lipid solubility, cell permeability, an increased circulation half life and pharmacokinetic profile with improved tumor or vascular targeting.
Owner:PENN STATE RES FOUND
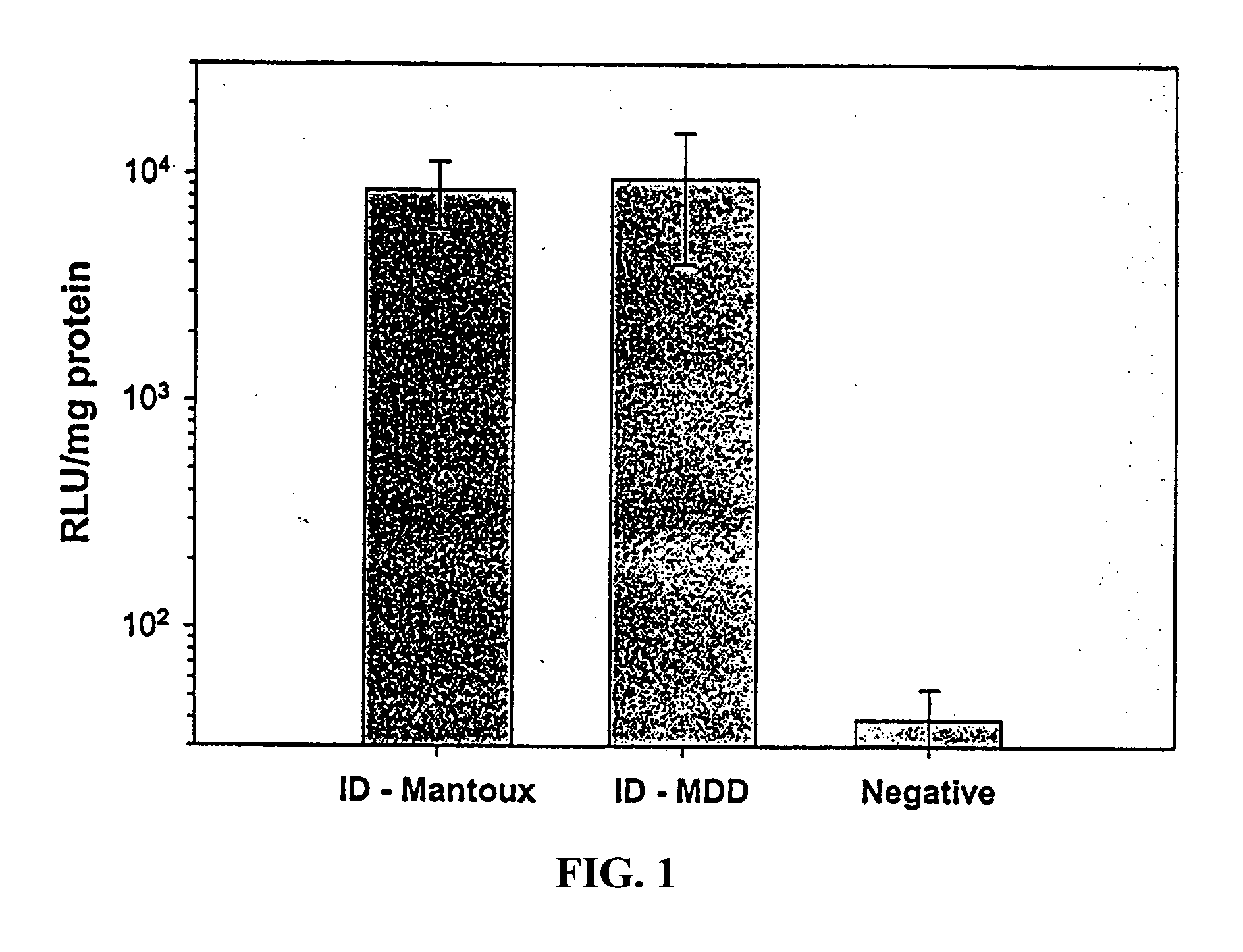
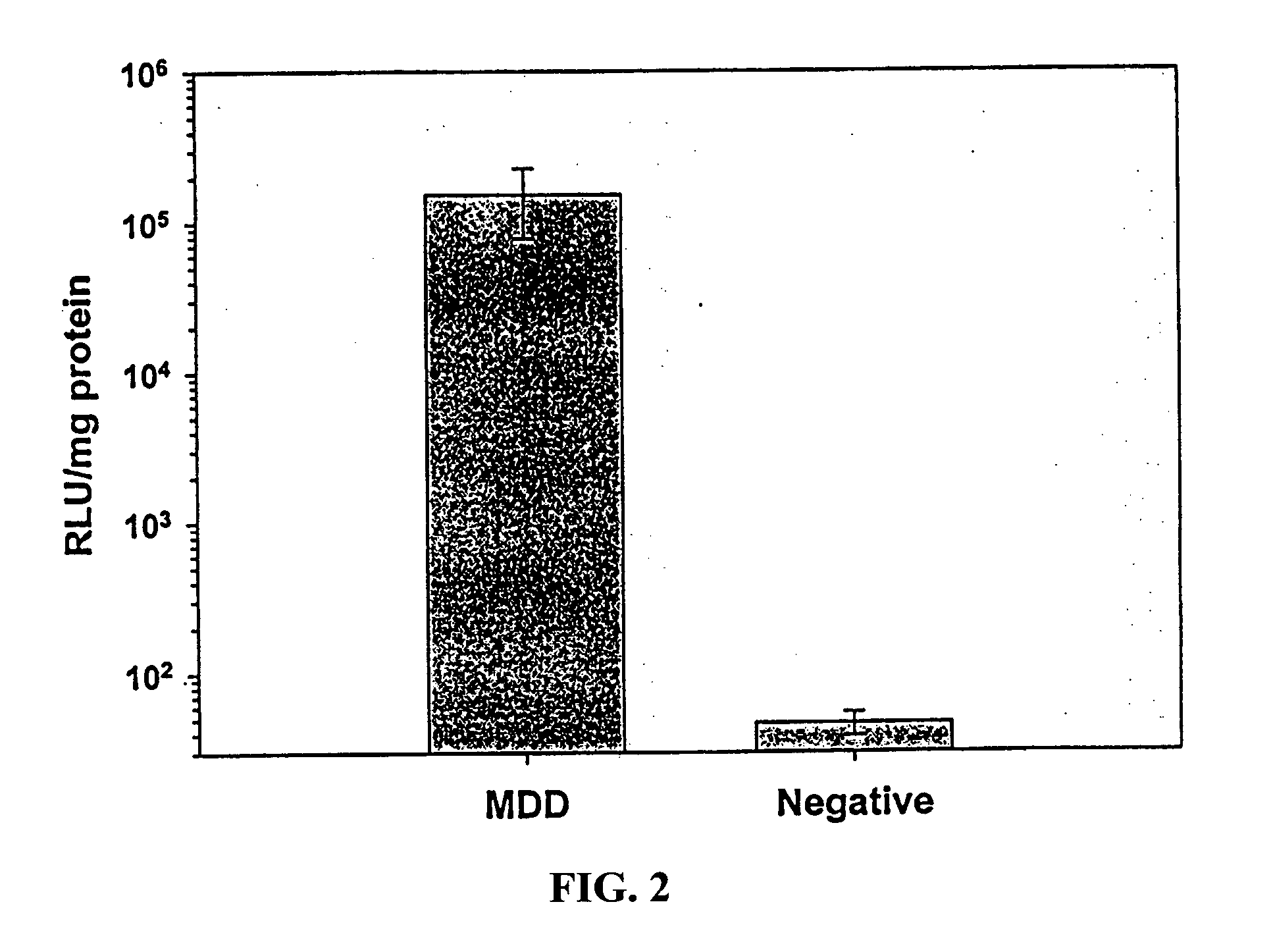
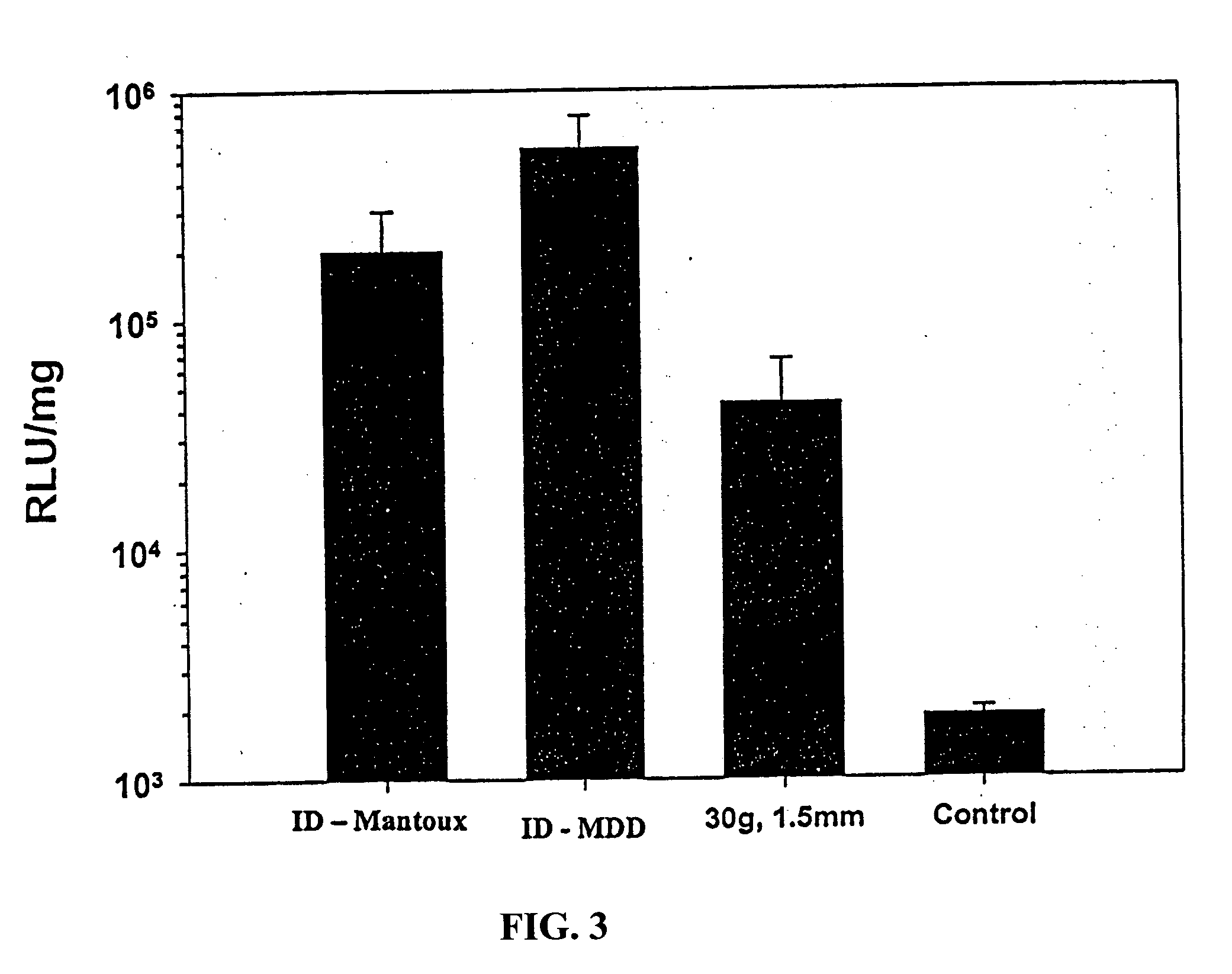
Intradermal delivery of vaccines and gene therapeutic agents via microcannula
InactiveUS7473247B2Efficacious improved responsivenessPrevent leakageSsRNA viruses negative-senseAntibacterial agentsGene Therapy AgentGene
Owner:BECTON DICKINSON & CO
Antisense and antigene therapeutics with improved binding properties and methods for their use
InactiveUS20060074041A1Improved antisenseImproved antigene oligonucleotide compositionOrganic active ingredientsSugar derivativesGene Therapy AgentBase pair
The present invention is directed to novel nucleic acid molecules and methods for their use. More specifically, the novel nucleic acid molecules of the present invention are capable of tightly and specifically interacting with a target molecule of interest not only through standard Watson-Crick base pairing, but also through additional features which allow the antisense molecules to become topologically “locked” onto the target nucleic acid, thereby imparting improved transcription and translation inhibitory properties.
Owner:SOMAGENICS INC
Use of DF3/MUC1 regulated expression in gene therapy
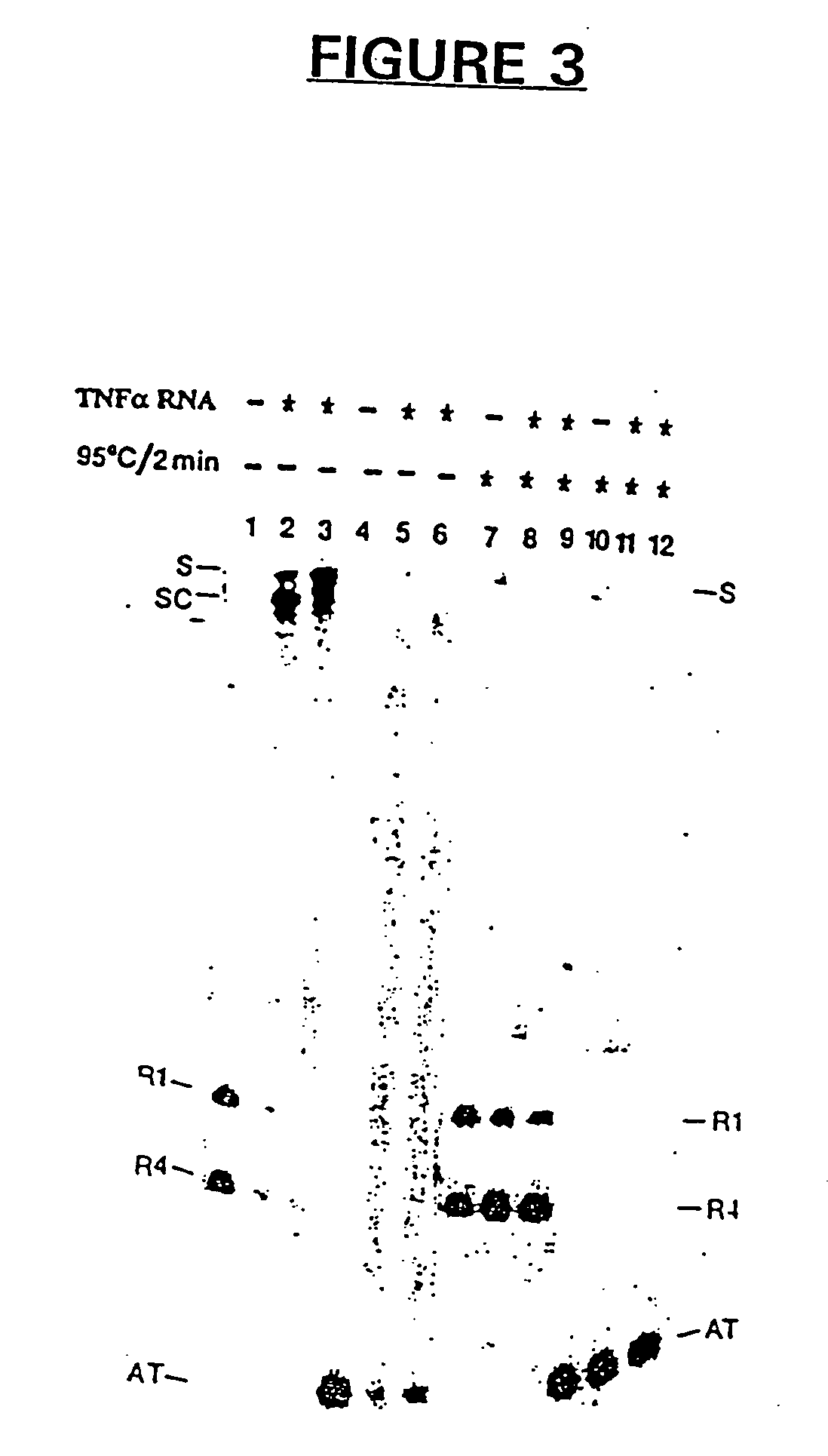
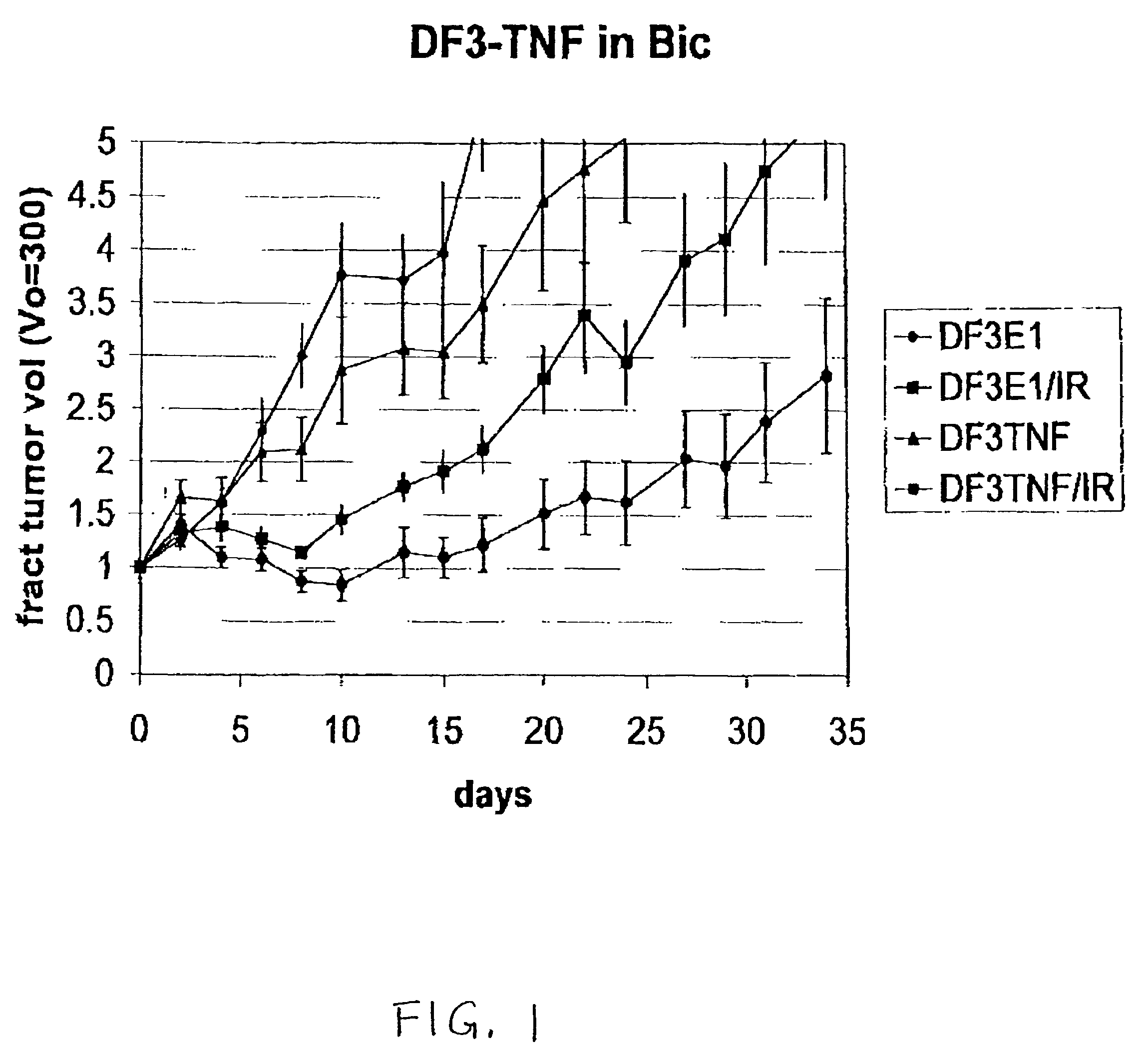
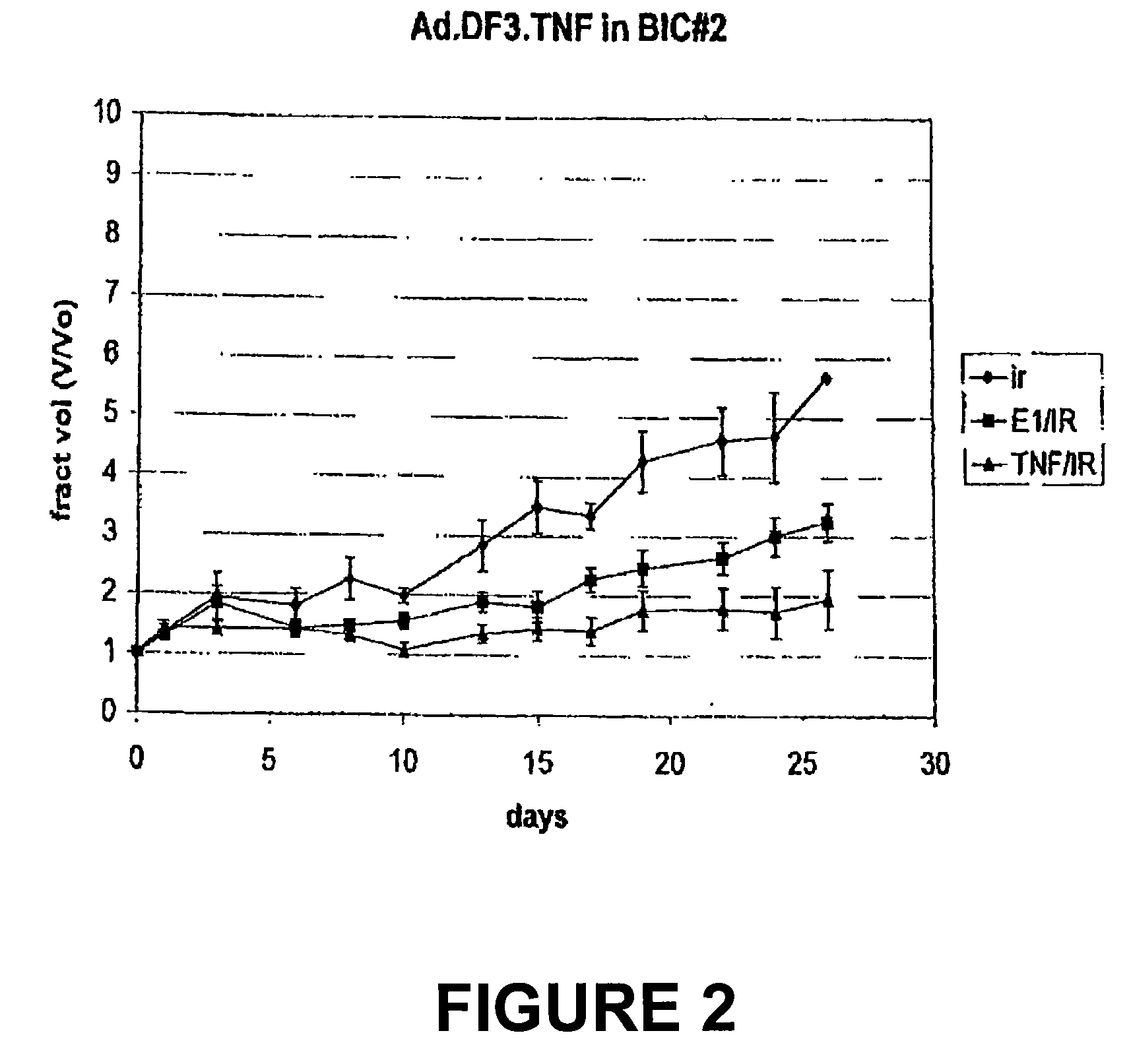
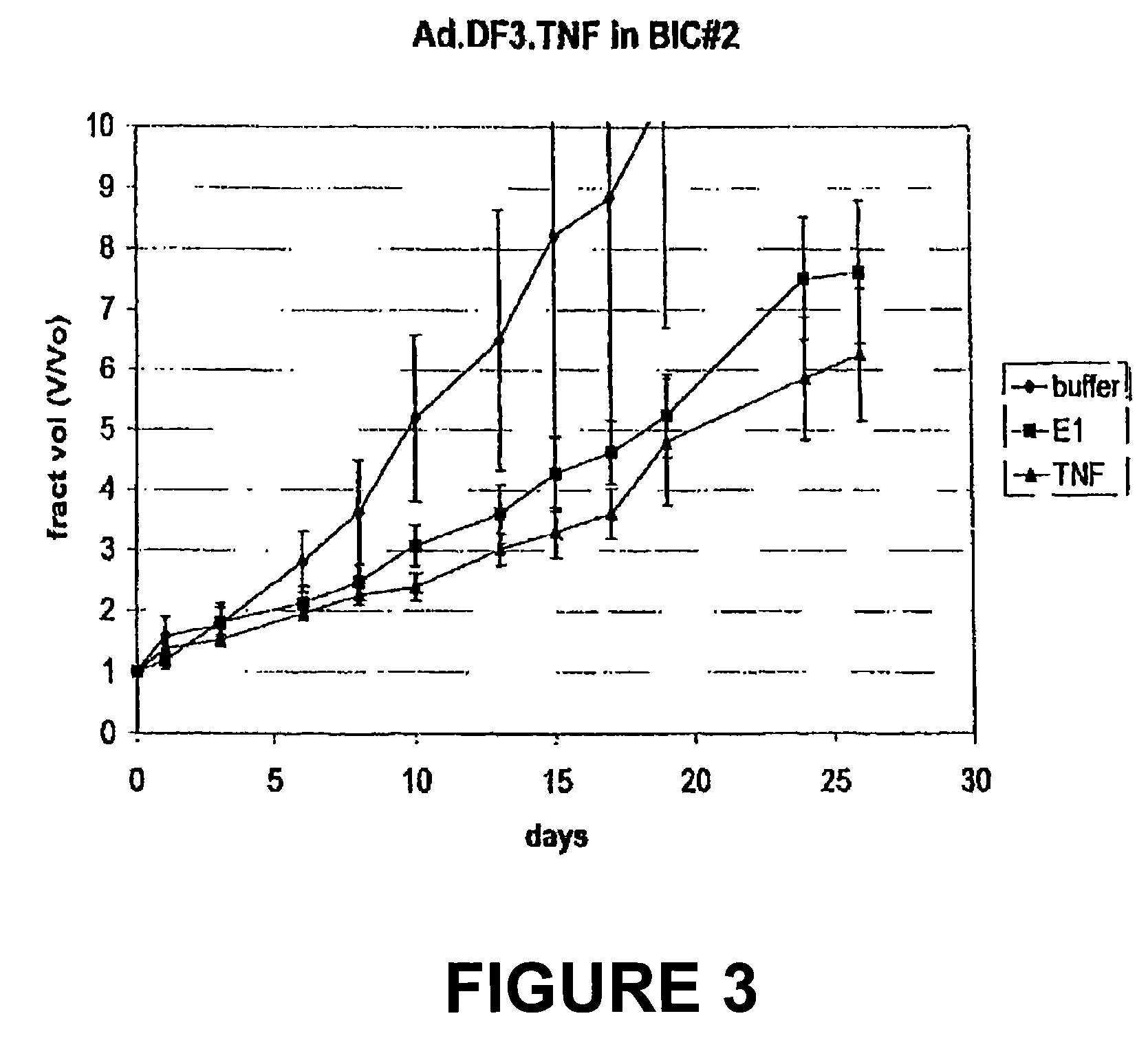
The present invention provides for improved vectors for use in gene therapy. Utilizing the cancer specific DF3 / MUC1 promoter to drive a replication essential gene, vectors are made conditionally replication-competent, permitting wider infection and expression of tumor cells. In addition, therapeutic genes and adjunct therapies further increase anti-tumor efficacy.
Owner:DANA FARBER CANCER INST INC +1
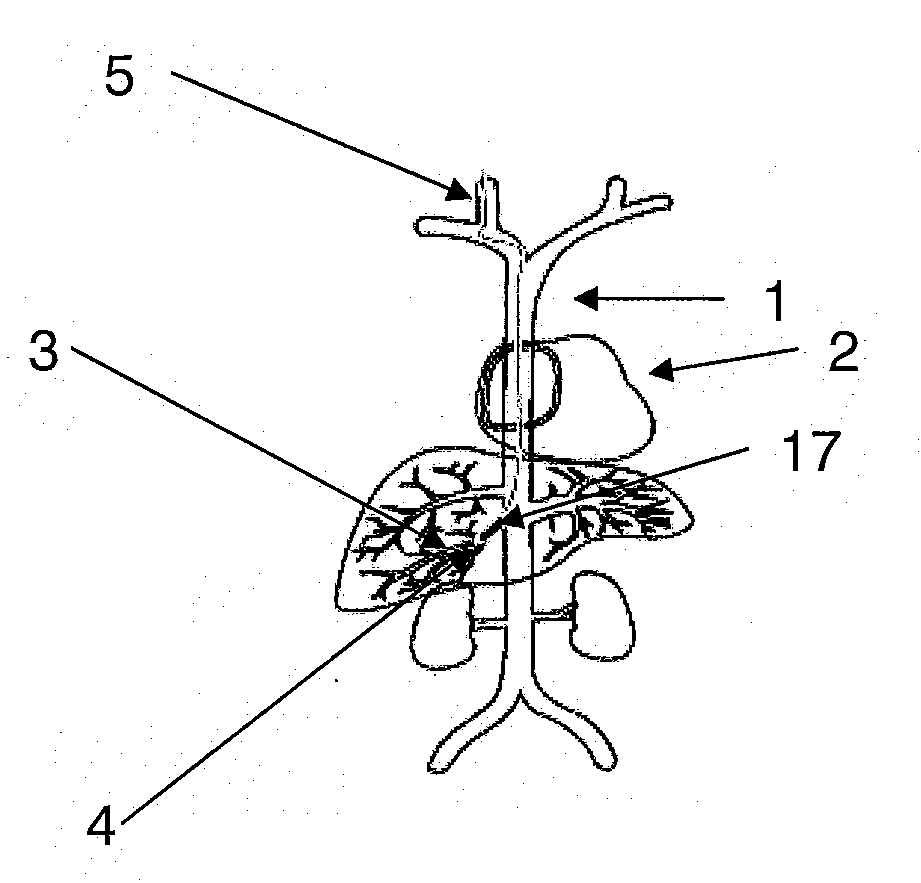
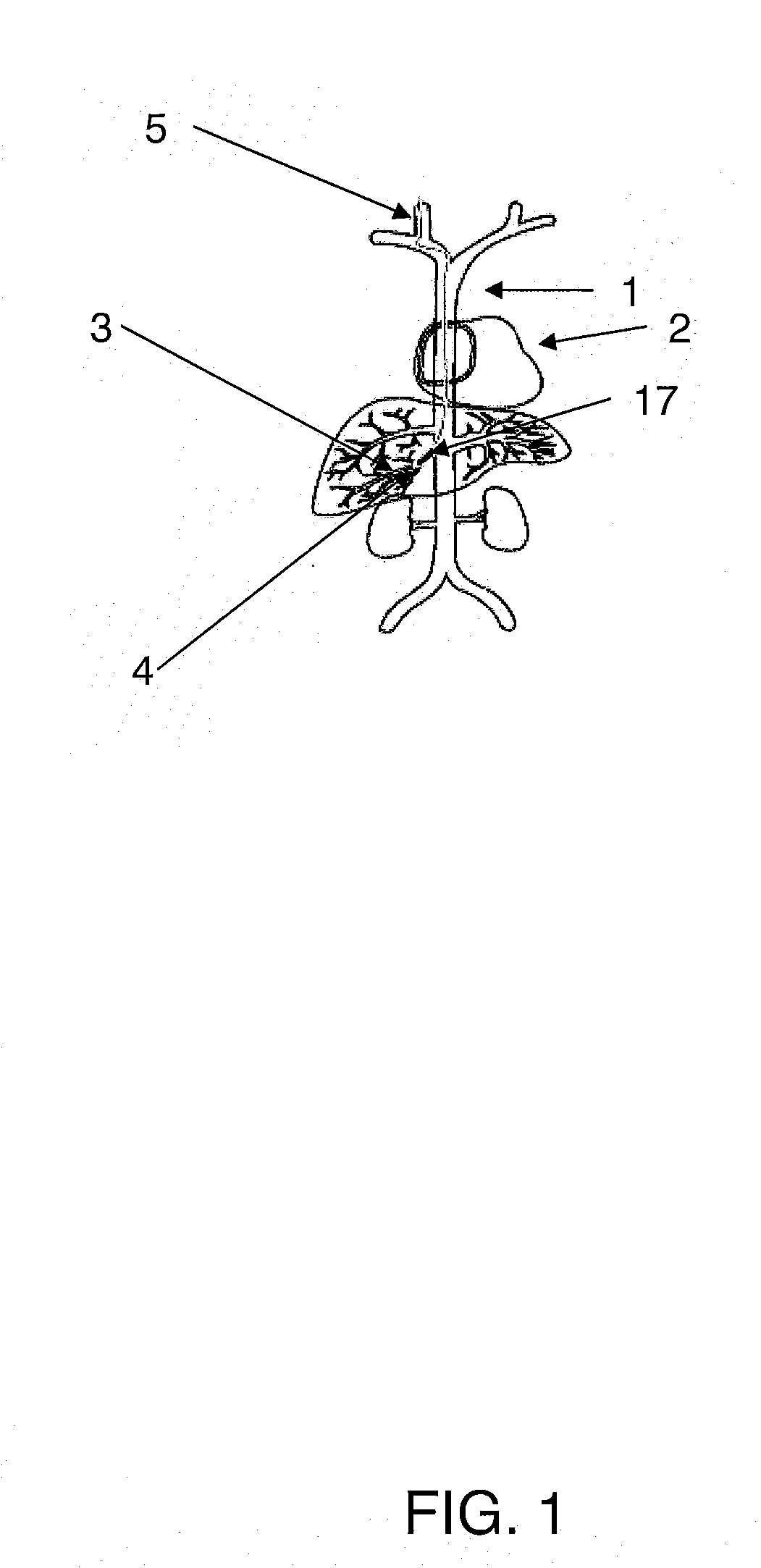
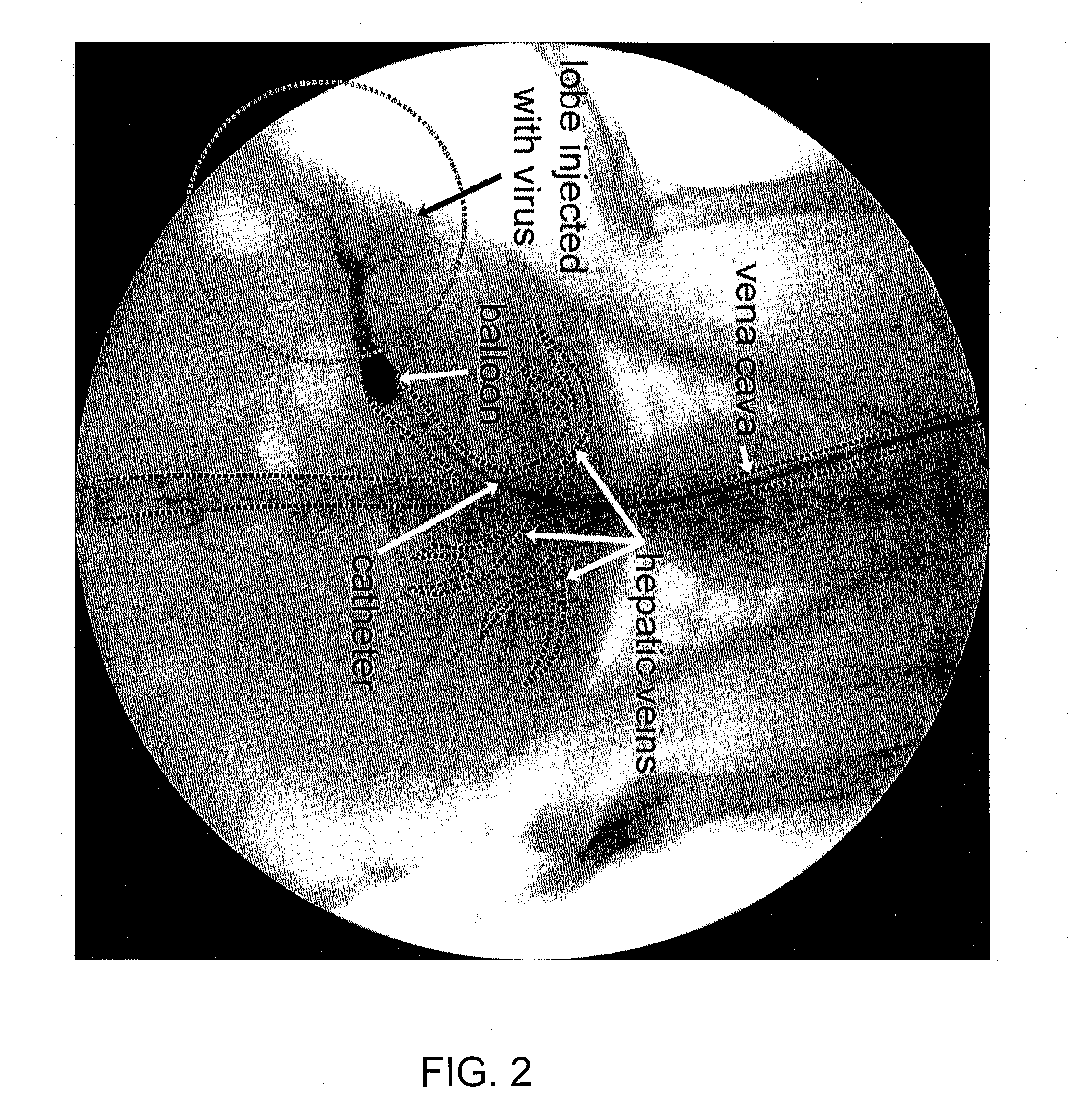
Methods for targeted deliver of genetic material to the liver
InactiveUS20080025952A1Increase pressureProlong residenceBiocideOrganic active ingredientsGenetic MaterialsGene Therapy Agent
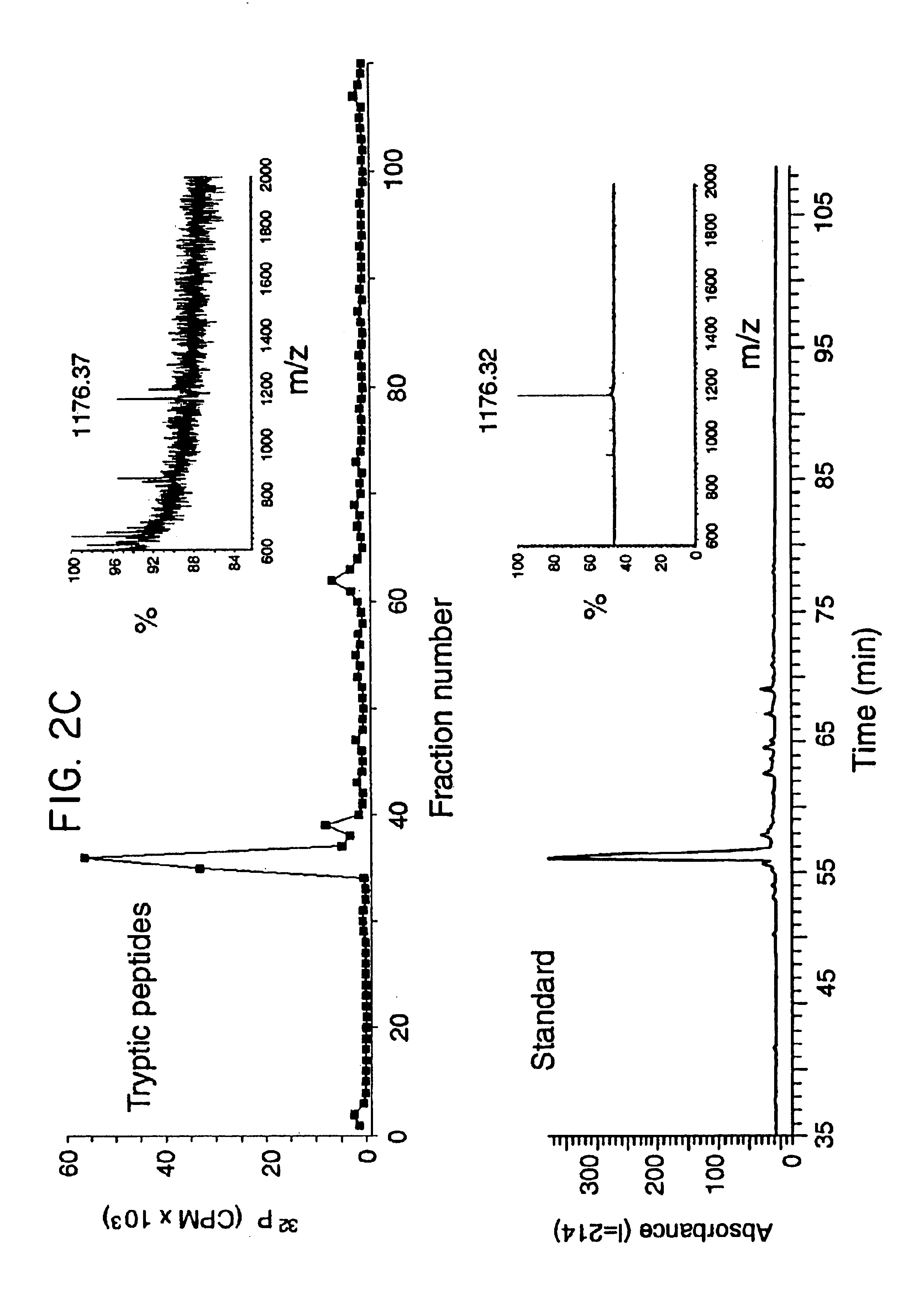
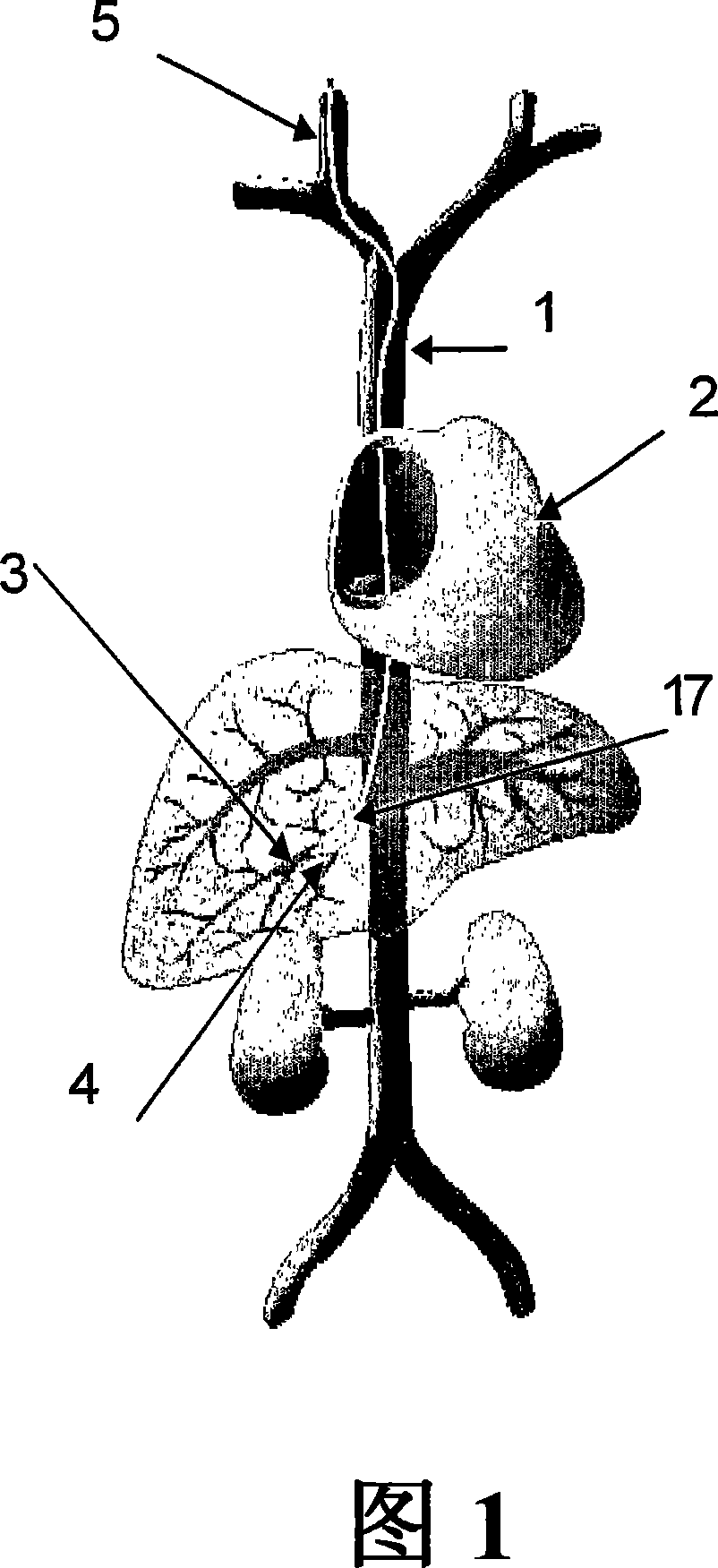
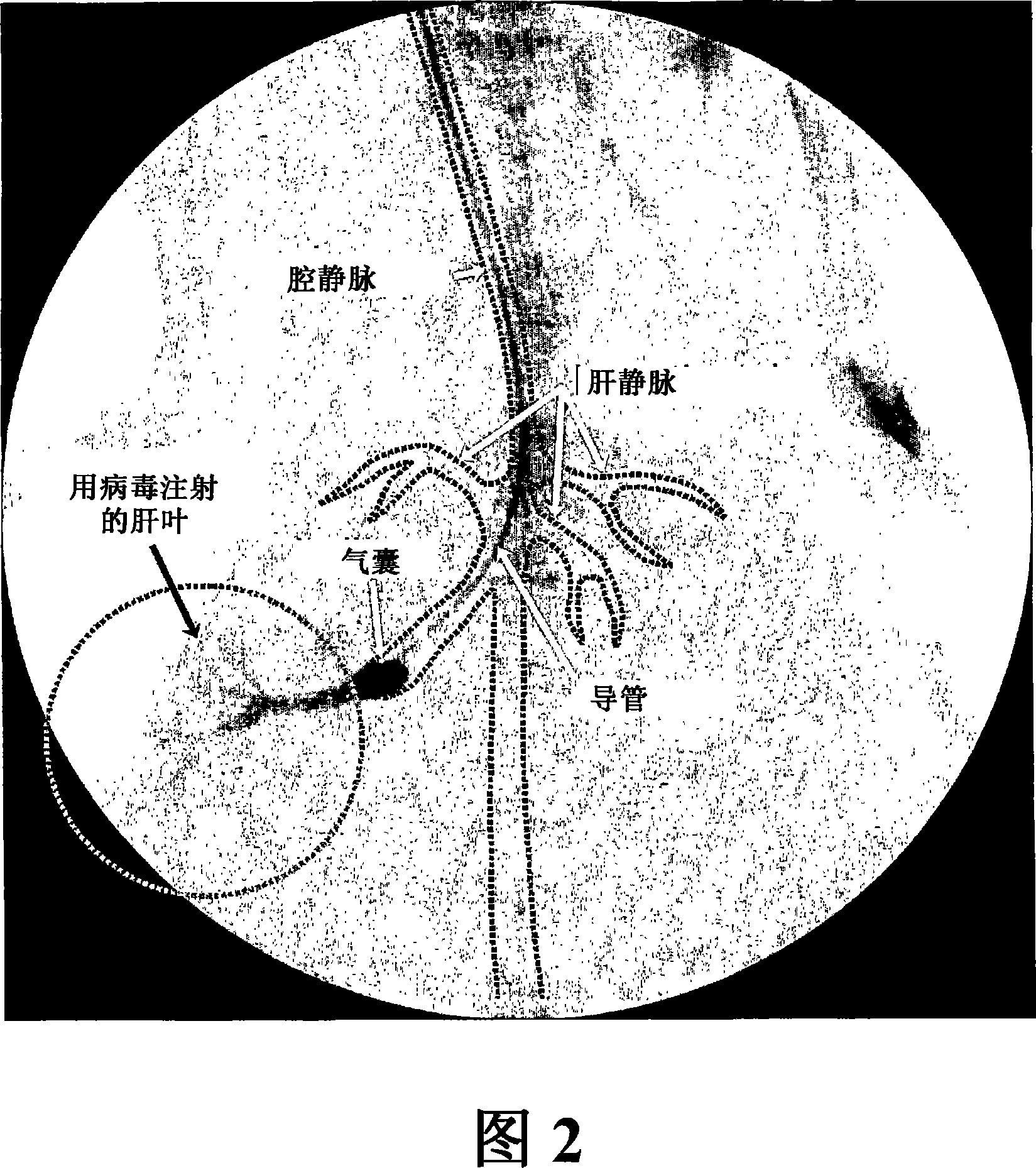
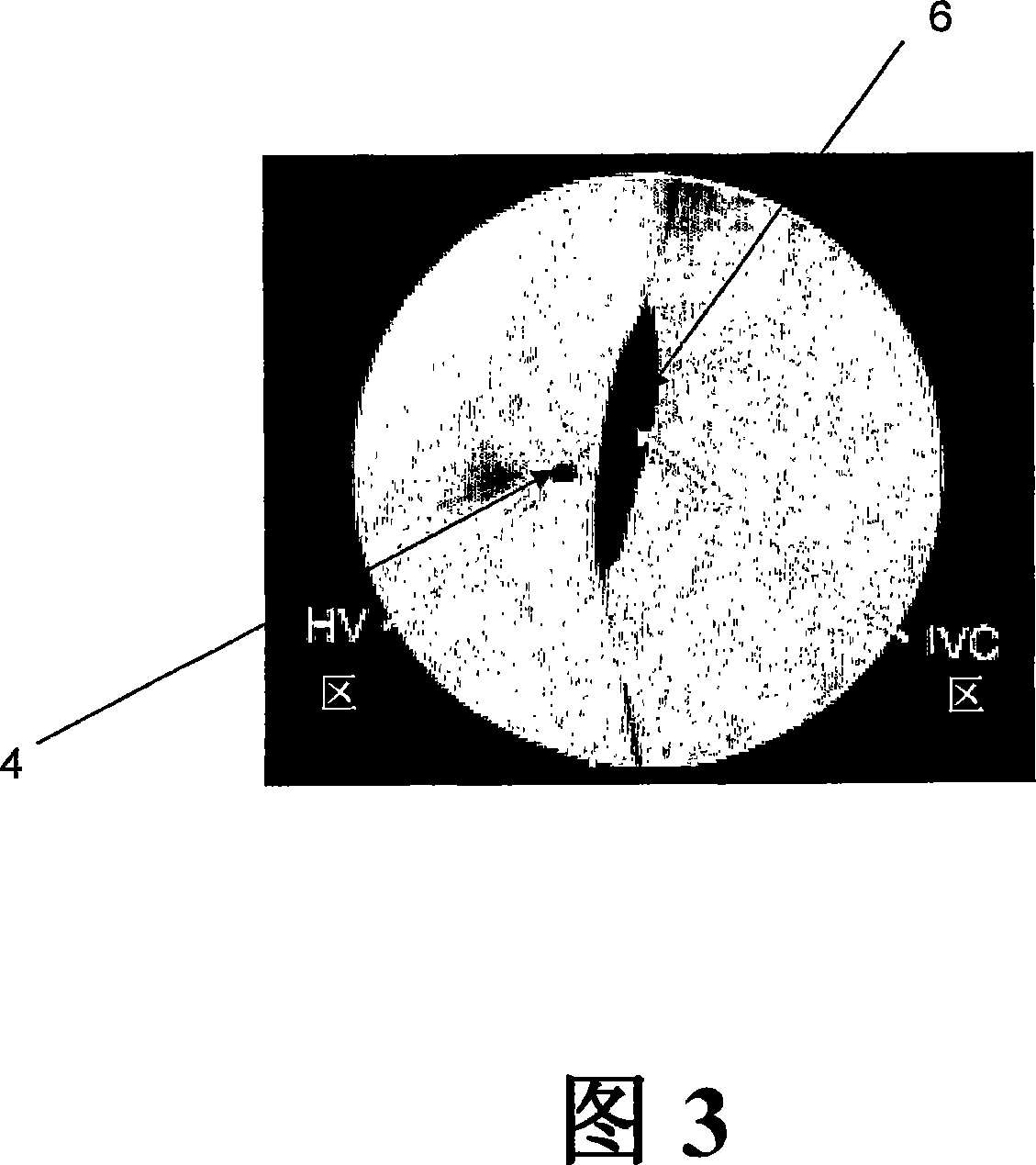
The present invention provides methods for enhanced delivery of various therapeutic agents, such as gene therapy agents, to the vasculature of a target organ in a mammalian subject. The methods for targeted gene therapy in the mammalian liver as a whole, or in a single hepatic lobe, are disclosed. The disclosed methods rely on minimally invasive catheter-based procedures wherein a target organ is isolated and treated locally with a gene therapy agent. The methods offer more efficient and localized transfection of tissue and are well-suited for gene therapy in human subjects.
Owner:SCHEULE RONALD K +1
Novel expression vectors and uses thereof
InactiveUS20030129169A1Increase the number ofAvoiding a severe drawbackBiocideBacteriaBinding siteGene Therapy Agent
The present invention relates to novel vectors, to DNA vaccines and gene therapeutics containing said vectors, to methods for the preparation of the vectors and DNA vaccines and gene therapeutics, and to therapeutic uses of said vectors. More specifically, the present invention relates to novel vectors comprising an expression cassette of a gene of a nuclear-anchoring protein, which contains a DNA binding domain capable of binding to a specific DNA sequence and a functional domain capable of binding to a nuclear component and a multimerized DNA sequence forming a binding site for the nuclear-anchoring protein, and optionally an expression cassette of a gene, genes or a DNA sequence or DNA sequences of interest. The present invention further relates to DNA vaccines and gene therapeutics containing the novel vectors, to methods for the preparation of the novel vectors and the DNA vaccines and gene therapeutics containing the novel vectors, and to the use the vectors in therapy.
Owner:FIT BIOTECH OY PLC
Compositions that specifically bind to colorectal cancer cells and methods of using the same
InactiveUS7135333B1Cell receptors/surface-antigens/surface-determinantsSugar derivativesColorectal tumorAdenocarcinoma
A unique transcription product, CRCA-1, and alternative translation products generated therefrom, are disclosed. The transcript and its translation products are markers for colorectal cells. Screening and diagnostic reagents, kits and methods for metastasized colorectal cancer are disclosed as are reagents, kits and methods for identifying adenocarcinomas as colorectal in origin. Compounds, compositions and methods of treating patients with metastasized colorectal cancer and for imaging metastasized colorectal tumors in vivo are disclosed. Compositions and methods for delivering active compounds such as gene therapeutics and antisense compounds to colorectal cells are disclosed. Vaccines compositions and methods of for treating and preventing metastasized colorectal cancer are disclosed.
Owner:THOMAS JEFFERSON UNIV
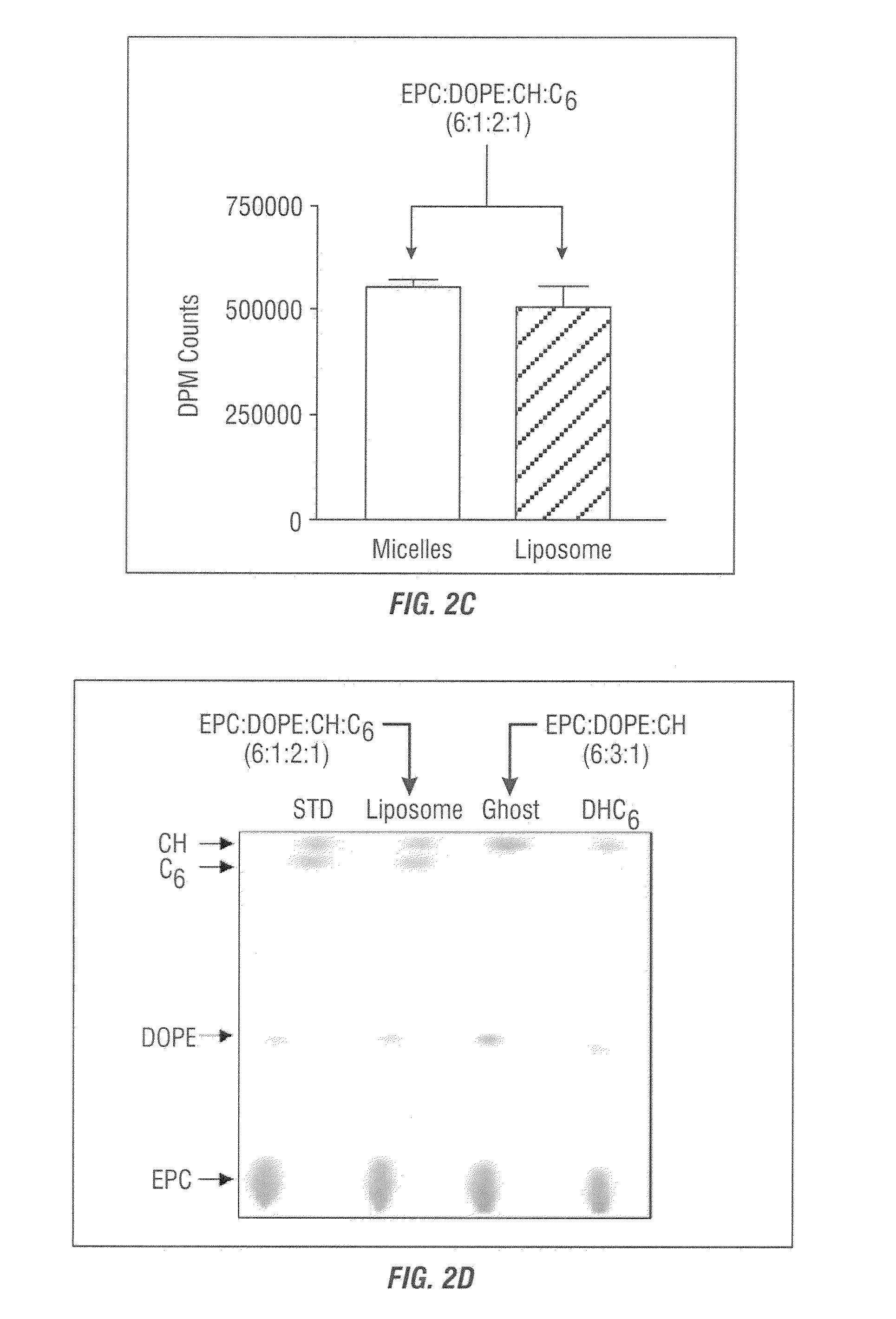
ENVIRONMENT-RESPONDING siRNA CARRIER USING DISULFIDE-CROSS-LINKED POLYMERIC MICELLE
ActiveUS20100121043A1High monodispersibilityImprove stabilityPowder deliverySpecial deliveryCross-linkSide chain
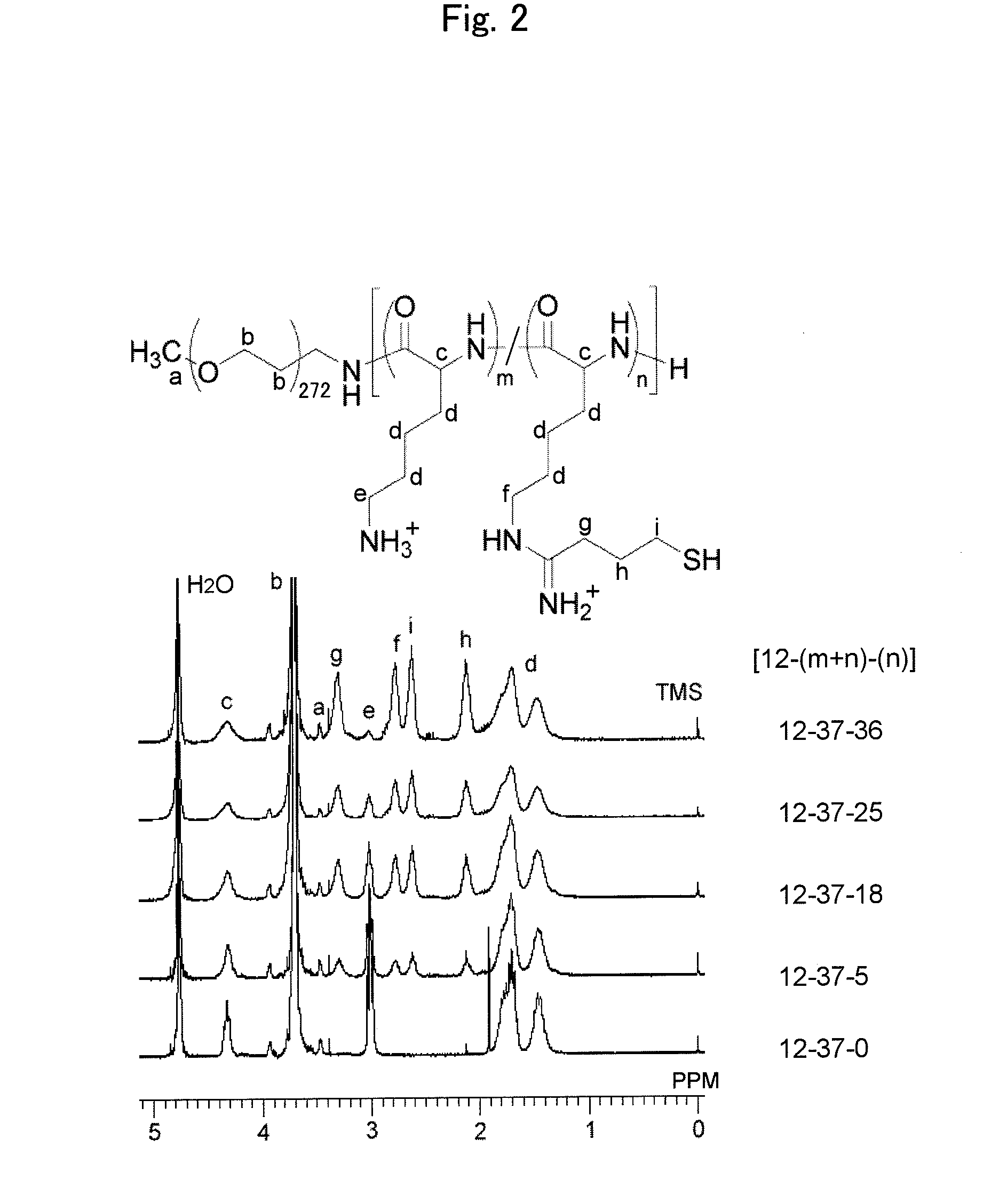
Disclosed is a siRNA-encapsulating polymeric micelle complex (a polyion complex) having high monodispersibility and structural stability and excellent in the ability of transporting siRNA into a cell. Further disclosed are a nucleic acid delivery device, a nucleic acid delivery kit, a pharmaceutical composition, and a gene therapy agent, each of which uses the complex. The polyion complex is characterized by comprising: a block copolymer composed of a polyethylene glycol moiety and a polycation moiety having a thiol group as a side chain at the terminus; and siRNA.
Owner:THE UNIV OF TOKYO
Intradermal delivery of vaccines and gene therapeutic agents via microcannula
InactiveUS20040131641A1Precise positioningImprove responseSsRNA viruses negative-senseAntibacterial agentsGene Therapy AgentGene
Owner:BECTON DICKINSON & CO
Novel expression vectors and uses thereof
InactiveUS20050026137A1Increase the number ofAvoiding a severe drawbackGenetic material ingredientsVirus peptidesEpstein-Barr Virus Nuclear AntigensOrigin of replication
The present invention relates to novel vectors, to DNA vaccines and gene therapeutics containing said vectors, to methods for the preparation of the vectors and DNA vaccines and gene therapeutics containing the vectors, and to therapeutic uses of said vectors. More specifically, the present invention relates to novel vectors comprising (a) an expression cassette of a gene of a nuclear-anchoring protein, which contains (i) a DNA binding domain capable of binding to a specific DNA sequence and (ii) a functional domain capable of binding to a nuclear component and (b) a multimerized DNA sequence forming a binding site for the anchoring protein, and optionally (c) one or more expression cassettes of a DNA sequence of interest. In particular the invention relates to vectors that lack a papilloma virus origin of replication. The nuclear-anchoring protein might be the E2 protein of Bovine Papilloma Virus type 1 or Epstein-Barr Virus Nuclear Antigen 1. The invention also relates to vectors that lack an origin of replication functional in a mammalian cell. The invention further relates to methods for expressing a DNA sequence of interest in a subject.
Owner:FIT BIOTECH OY PLC
Gene therapeutics
Gene therapeutics to be used in treating diseases showing sensitivity to gene therapy, characterized by containing as the active ingredient an efficacious amount of a functional substance which has a function of having an affinity for a virus containing a gene usable in the gene therapy and another function of having an affinity specific for a target cell with a need for the gene transfer, or an efficacious amount of a functional substance which has an affinity for the above virus and an efficacious amount of another functional substance which has an affinity specific for the above cell.
Owner:TAKARA HOLDINGS
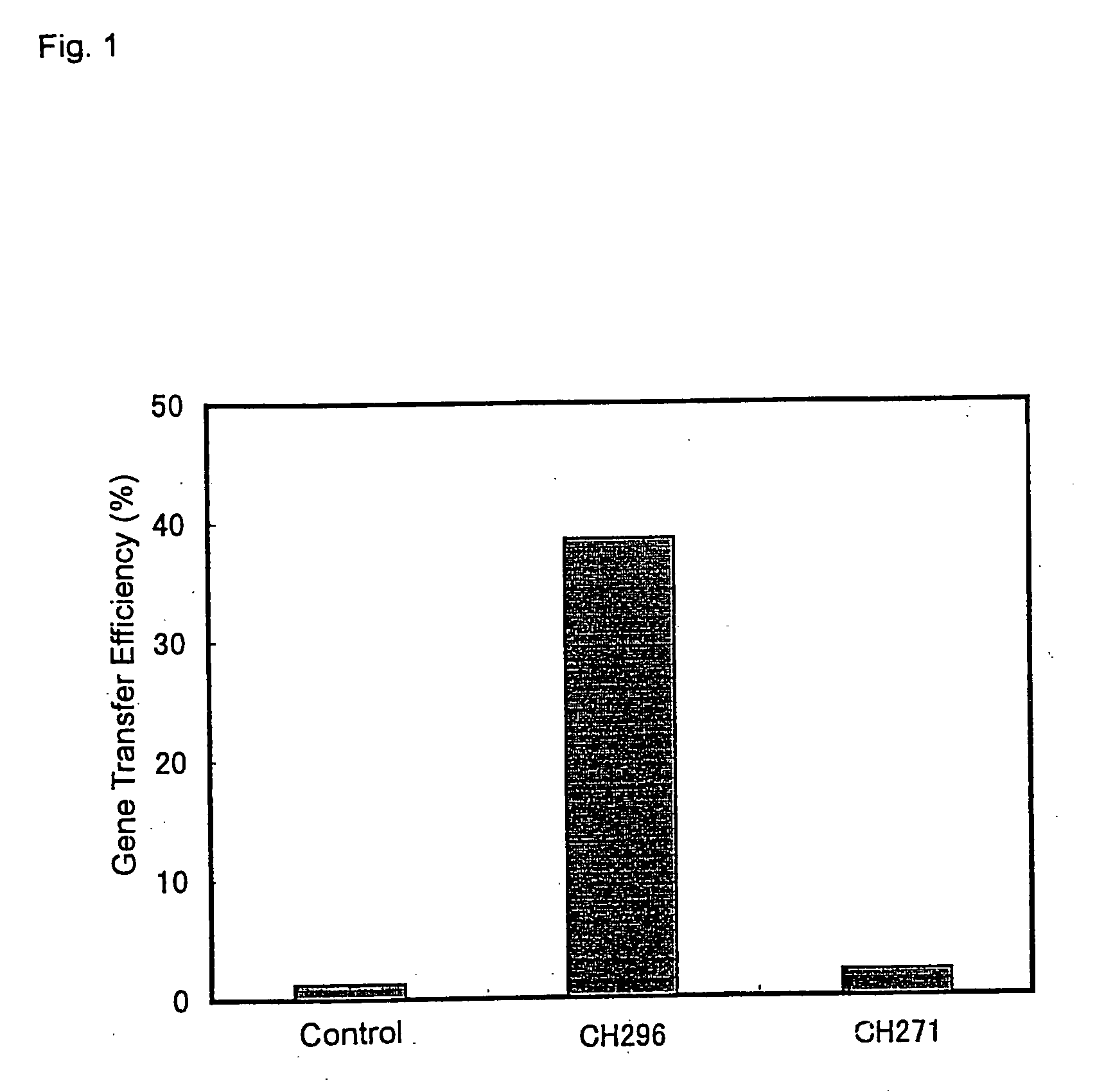
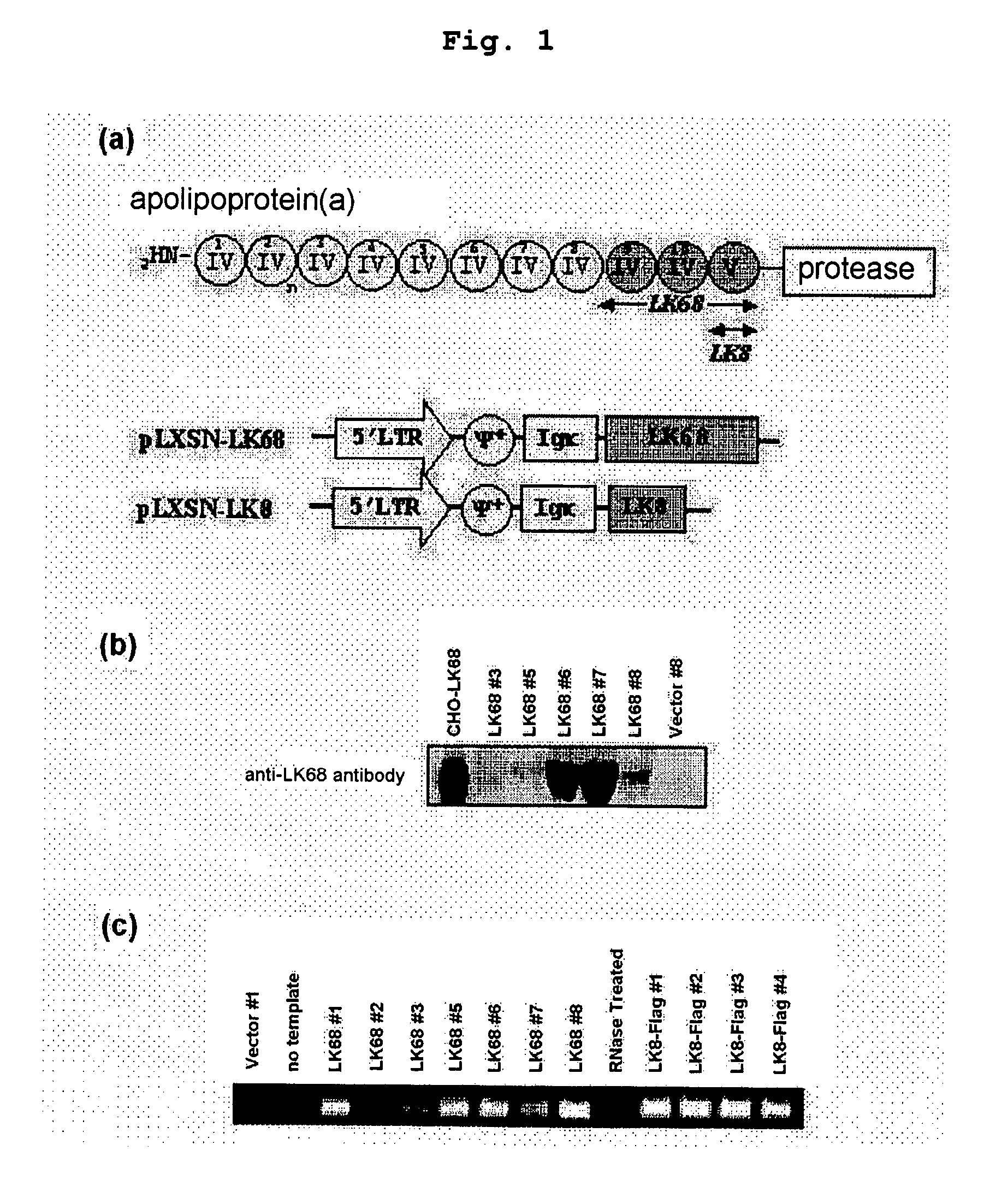
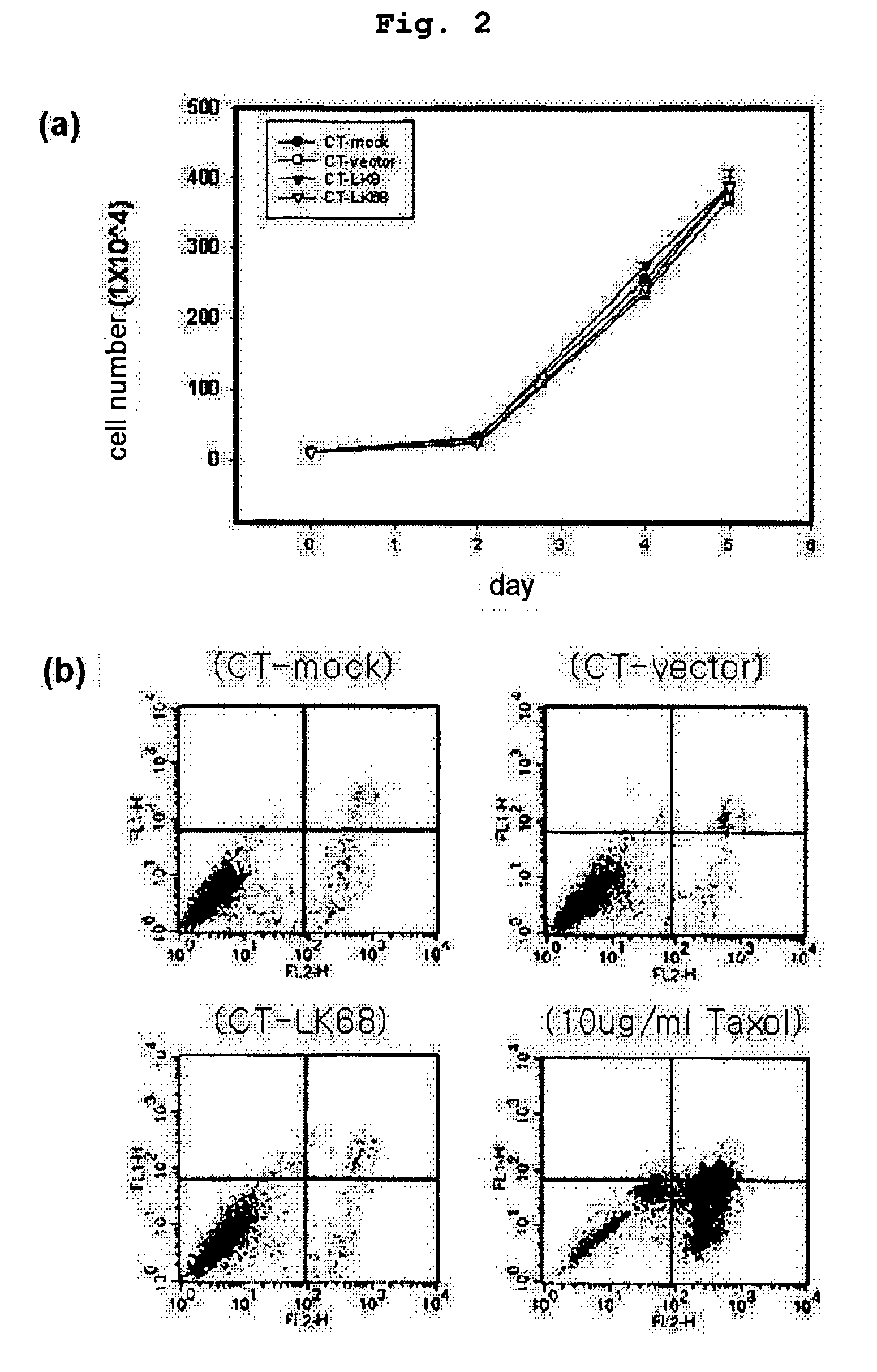
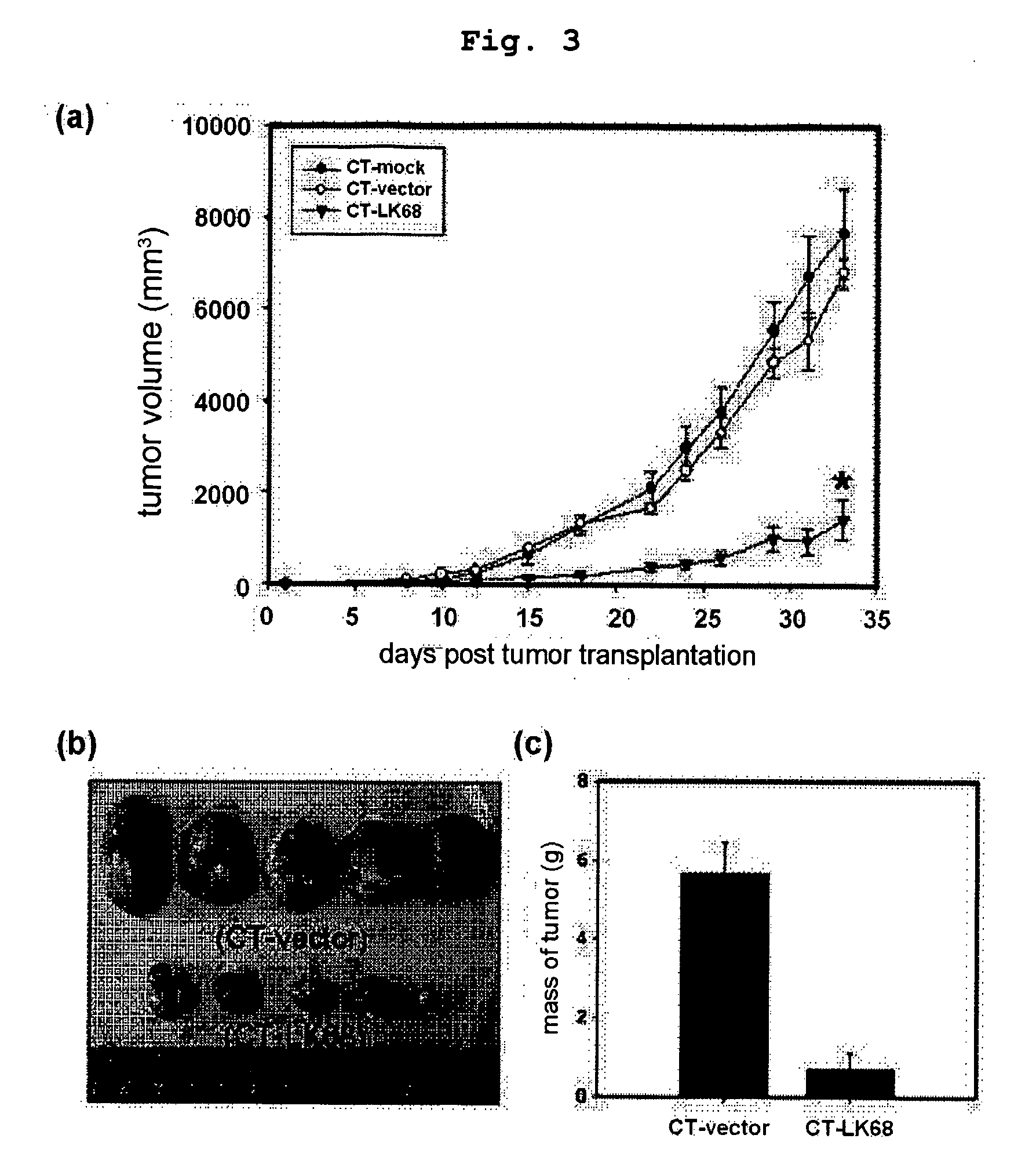
Therapeutic agent for treatment of cancer comprising human apolipoprotein (a) kringles lk68 or lk8 genes as effective ingredient, and method for treating cancer using the same
The present invention relates to an anticancer or an anti-metastatic agent for gene therapy, more precisely, an anticancer or an anti-metastatic agent for gene therapy containing a gene carrier or cells harboring human apolipoprotein (a) kringle KIV9-KIV10-KV (LK68) or KV (LK8) gene as an effective ingredient, and a treatment method for cancer using the same. The agent for gene therapy of the present invention has an inhibiting effect on the growth and the metastasis of a tumor, so it can be effectively used for the prevention and the treatment of various solid tumors as a metastasis inhibitor or a therapeutic agent for primary tumors.
Owner:MOGAM BIOTECH RES INST
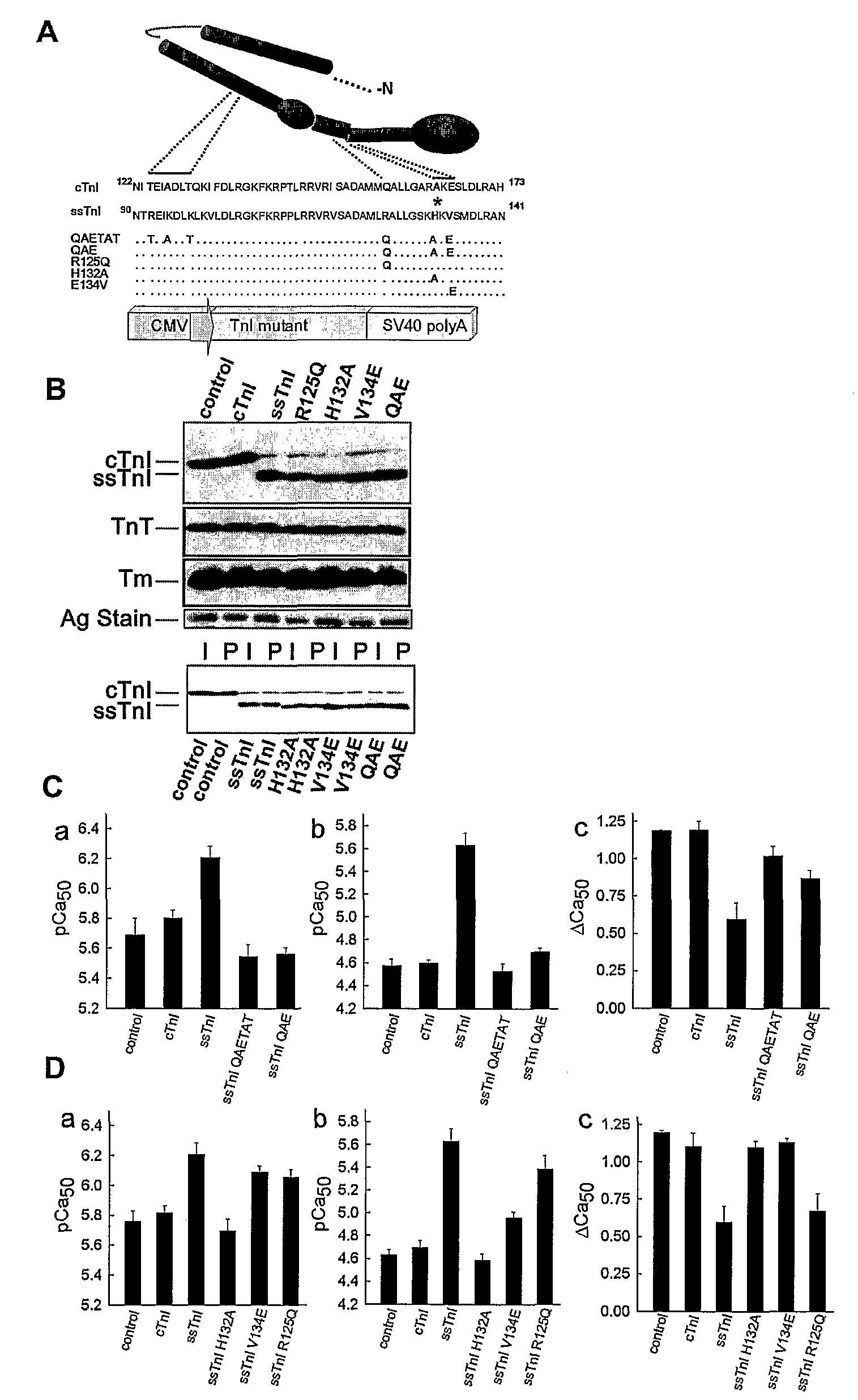
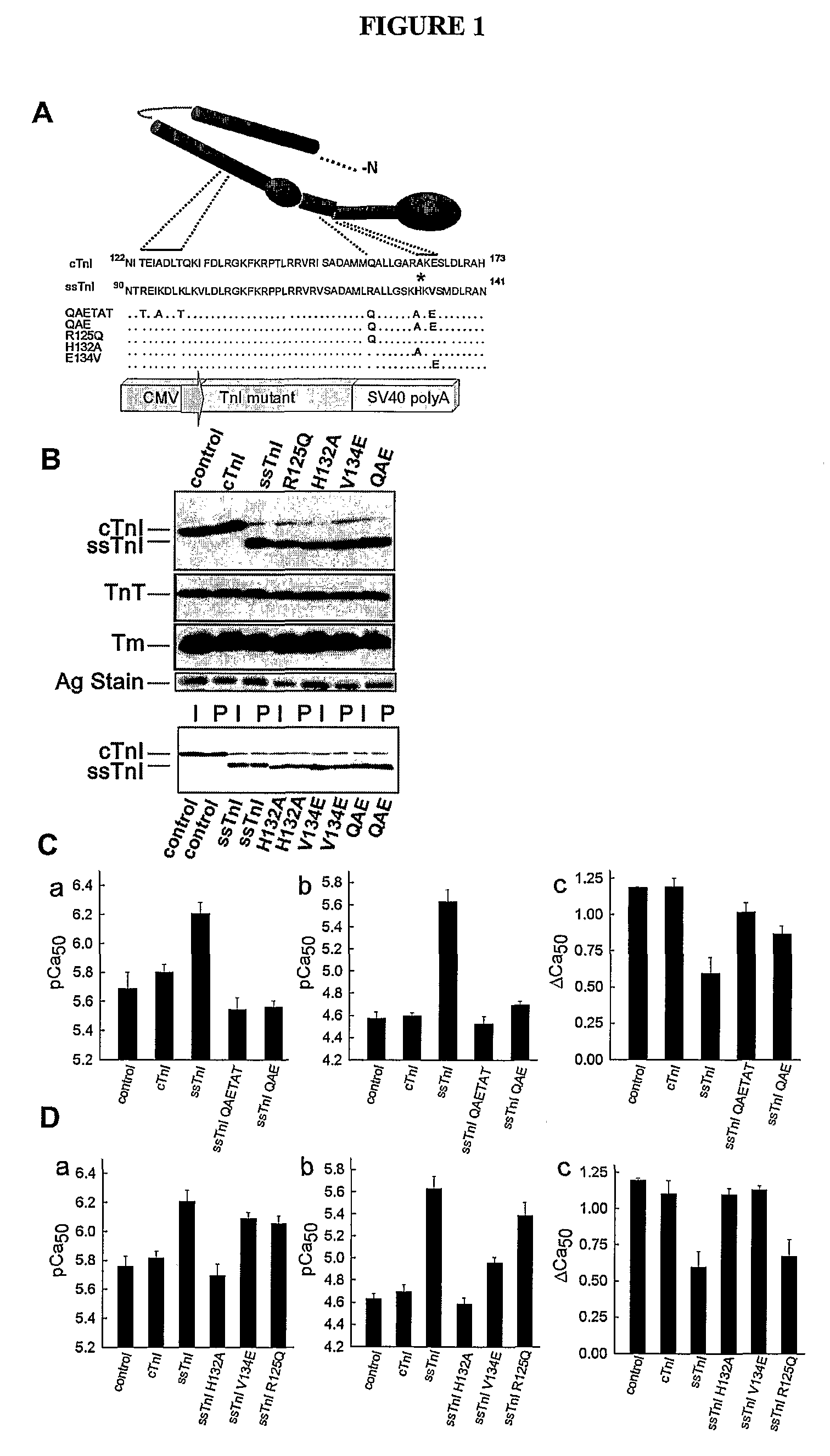
Compositions And Methods For Regulating Cardiac Performance
InactiveUS20080263691A1Physiological function is goodVirusesPeptide/protein ingredientsDiseaseTreatment use
The present invention relates to cardiac performance, in particular to regulating cardiac performance via recombinant troponin I (TnI) protein and nucleic acids encoding recombinant TnI. The present invention provides nucleic acids encoding gain of function TnI proteins (e.g., cTnlA164H), vectors containing such nucleic acids, host cells containing such vectors, transgenic animals carrying a gain of function TnI protein (e.g., a cTnlA164H transgene), and therapeutic agents (e.g., comprising recombinant TnI, TnI analogues, synthetic TnI, or the like) or agents for gene therapy of heart failure or disease for research and therapeutic uses.
Owner:RGT UNIV OF MICHIGAN
Liver cancer superantigen immunogene therapy agent
InactiveCN1401392AEnhanced ability to induce immune rejectionGood curative effectGenetic material ingredientsAntineoplastic agentsCell specificCancer cell
A super-antigen immune gene treater for liver cancer is characterized by that the liver cancer cell specific regulation sequence mediated super-antigen moleculae and co-stimulation moleculae are target expressed in liver cancer cells to specifically enhance the immunogenicity of cancer cell and the recognition action to immunoeffect cells. after the treater is injected to cancer region, it can effectively kill cancer cells.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Compositions that specifically bind to colorectal cancer cells and methods of using the same
InactiveUS7316902B2Cell receptors/surface-antigens/surface-determinantsSugar derivativesColorectal tumorAdenocarcinoma
A unique transcription product, CRCA-1, and alternative translation products generated therefrom, are disclosed. The transcript and its translation products are markers for colorectal cells. Screening and diagnostic reagents, kits and methods for metastasized colorectal cancer are disclosed ars are reagents, kits and methods for identifying adenocarcinomas as colorectal in origin. Compounds, compositions and methods of treating patients with metastasized colorectal cancer and for imaging metastasized colorectal tumors in vivo are disclosed. Compositions and methods for delivering active compounds such as gene therapeutics and antisense compounds to colorectal cells are disclosed. Vaccines compositions and methods of for treating and preventing metastasized colorectal cancer are disclosed.
Owner:THOMAS JEFFERSON UNIV
Environment-responding siRNA carrier using disulfide-cross-linked polymeric micelle
ActiveUS8153110B2Improve abilitiesHigh monodispersibilityPowder deliverySpecial deliveryCross-linkSide chain
Owner:THE UNIV OF TOKYO
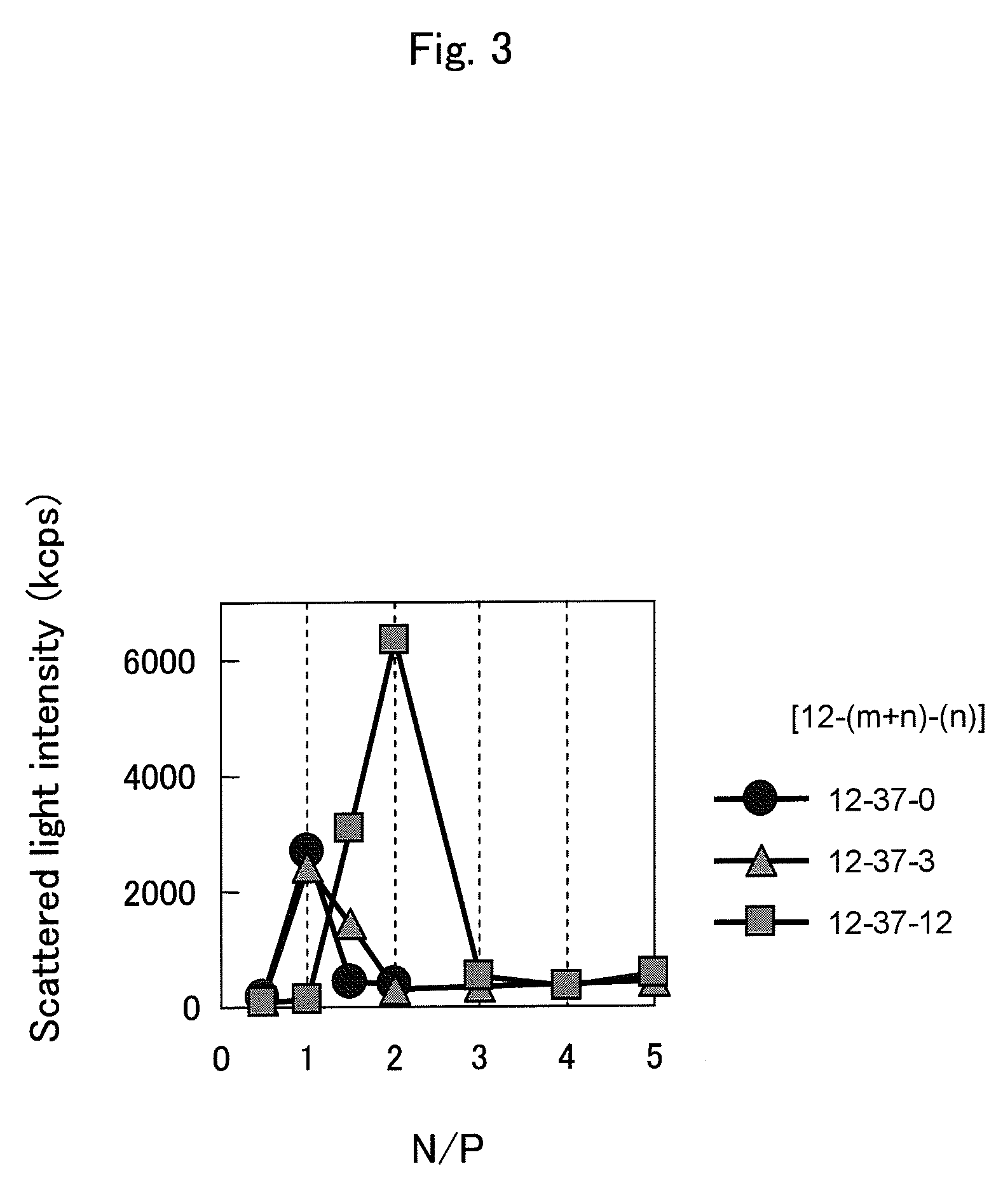
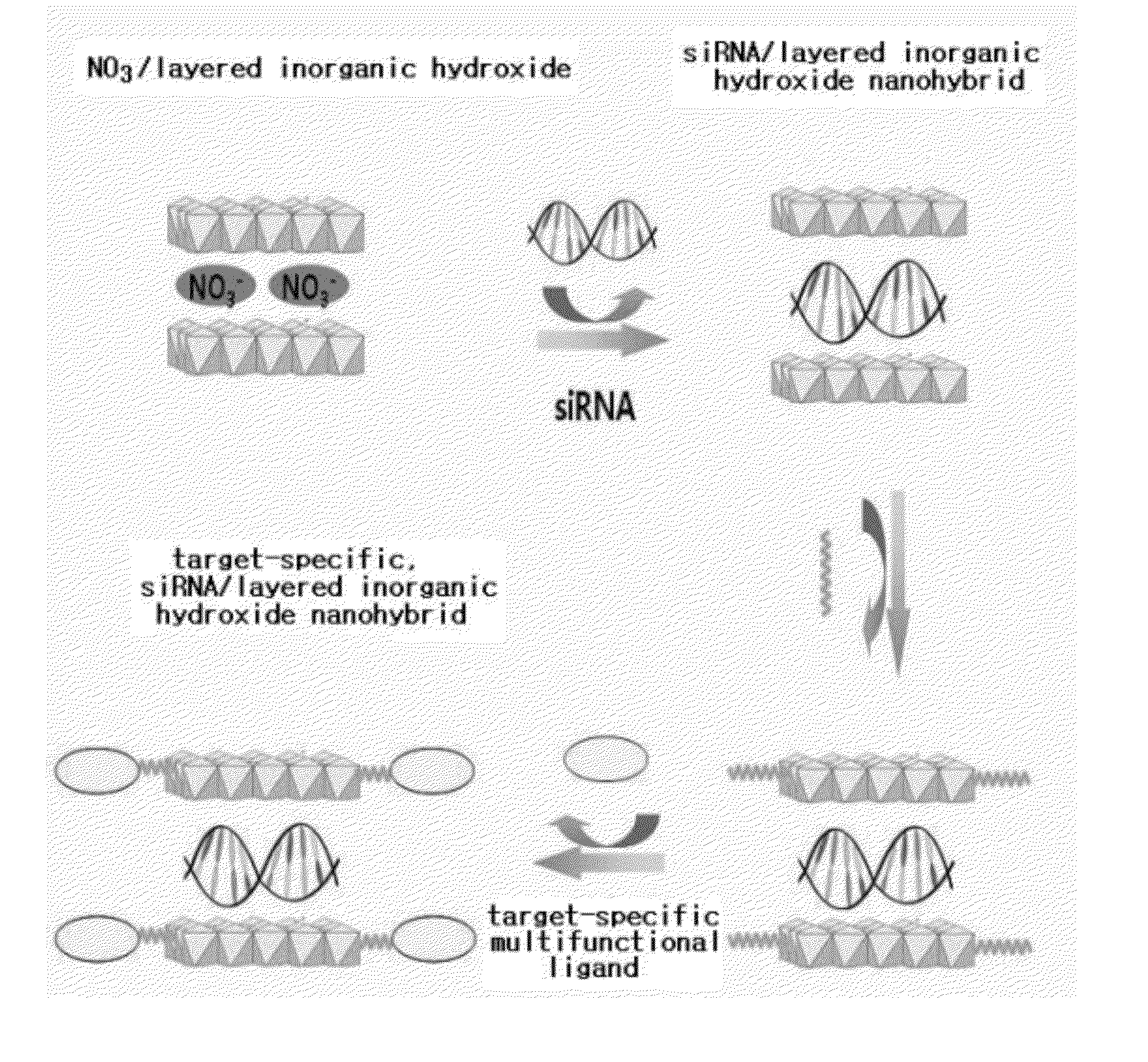
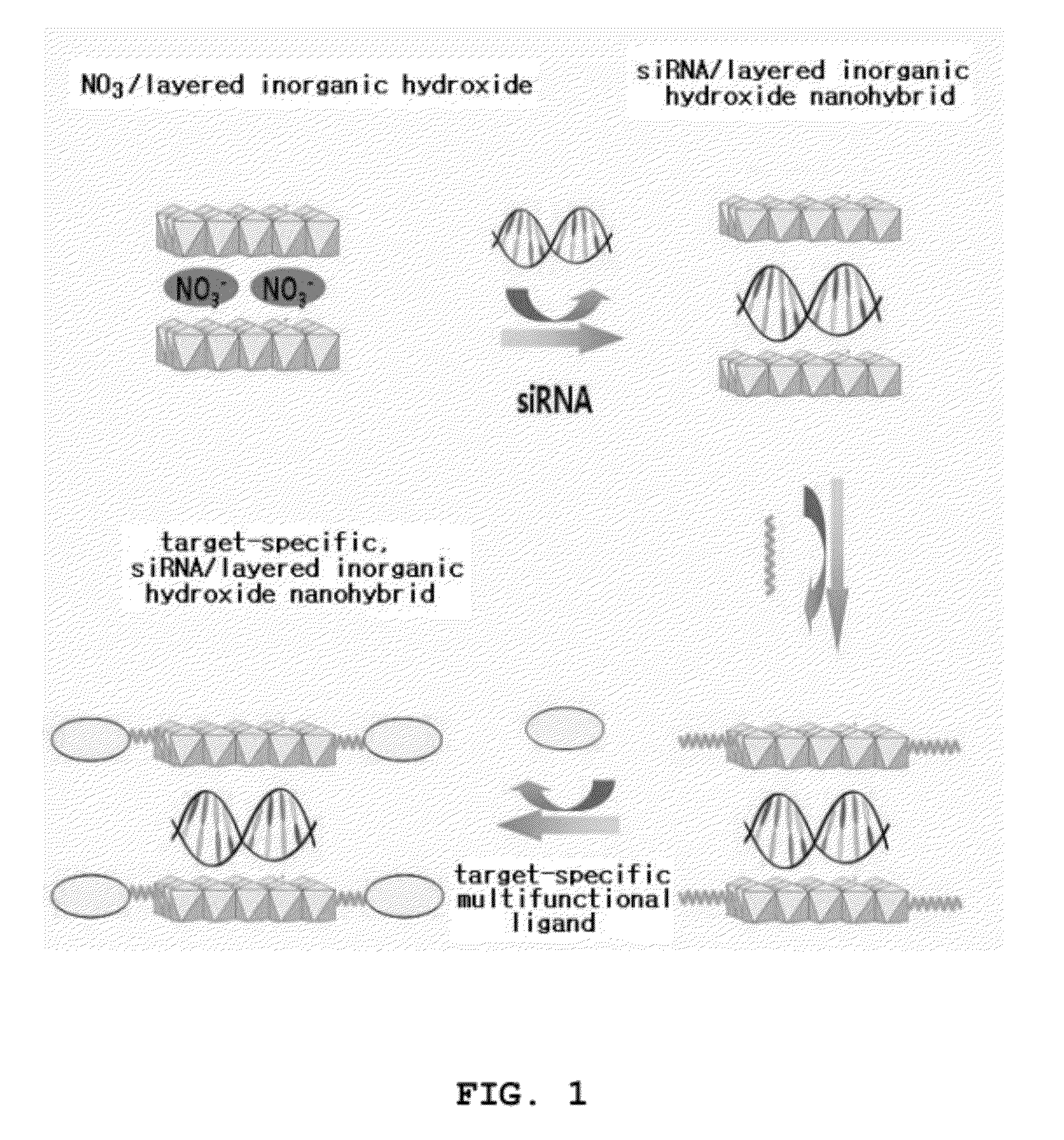
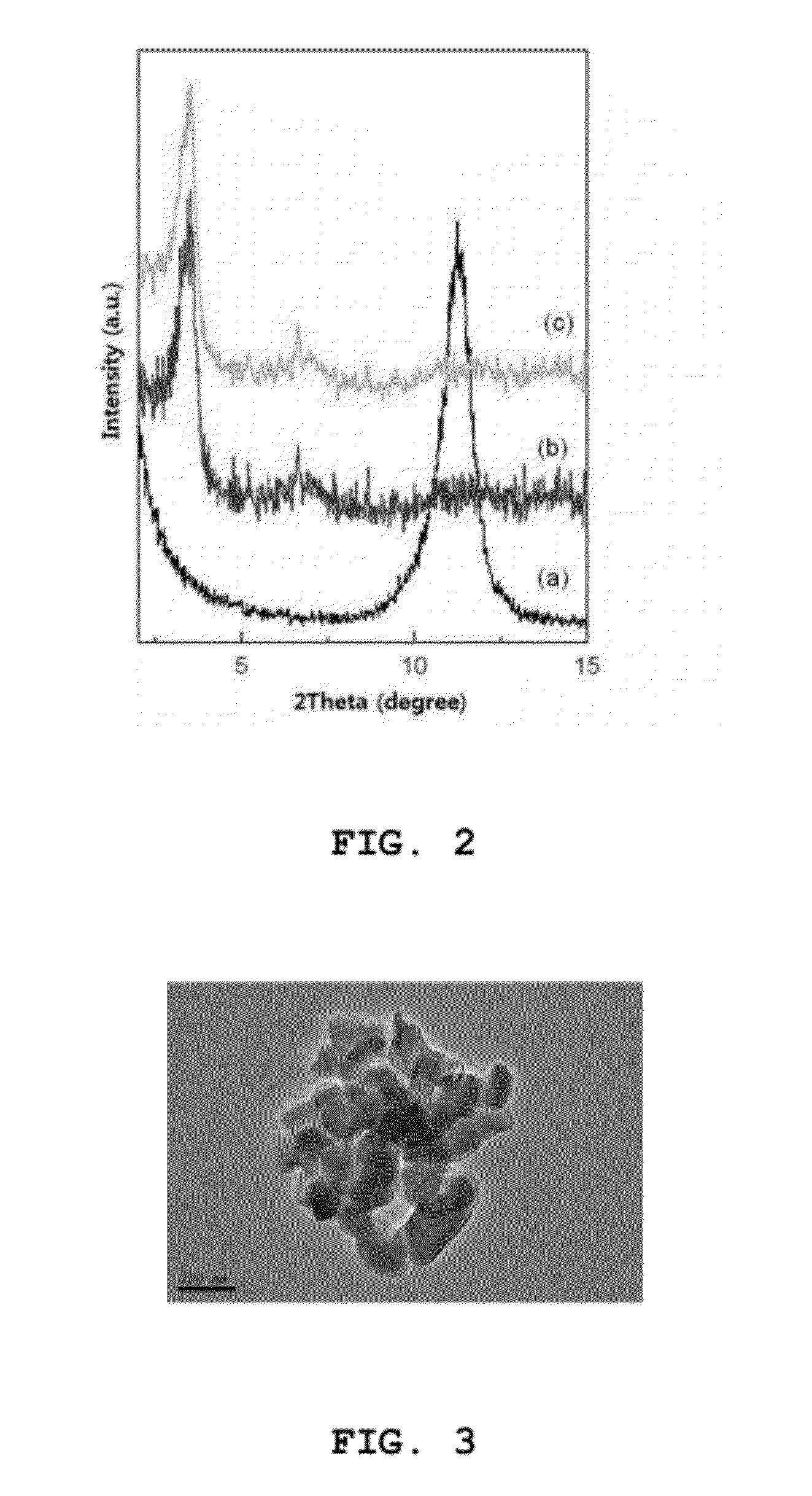
Nano-hybrid of targetable sirna-layered inorganic hydroxide, manufacturing method thereof, and pharmaceutical composition for treating tumor comprising the nano-hybrid
InactiveUS20120220647A1Improve efficiencyOrganic active ingredientsDigestive systemNano hybridGene Therapy Agent
A nanohybrid of the potent gene therapeutic agent siRNA (small interfering RNA) and a target-specific layered inorganic hydroxide, a preparation method thereof, and a pharmaceutical composition for tumor treatment containing the target-specific, siRNA / layered inorganic hydroxide nanohybrid. The nanohybrid increases the in vivo stability of the siRNA, and a target-specific multifunctional ligand, which is bonded to the layered inorganic hydroxide and can bind specifically to a tumor, increases the efficiency of tumor-specific transfer of the siRNA such that the siRNA shows tumor therapeutic activity even at a relatively low dose. Thus, the nanohybrid will be widely useful for target-specific antitumor therapies.
Owner:NANOHYBRID CO LTD
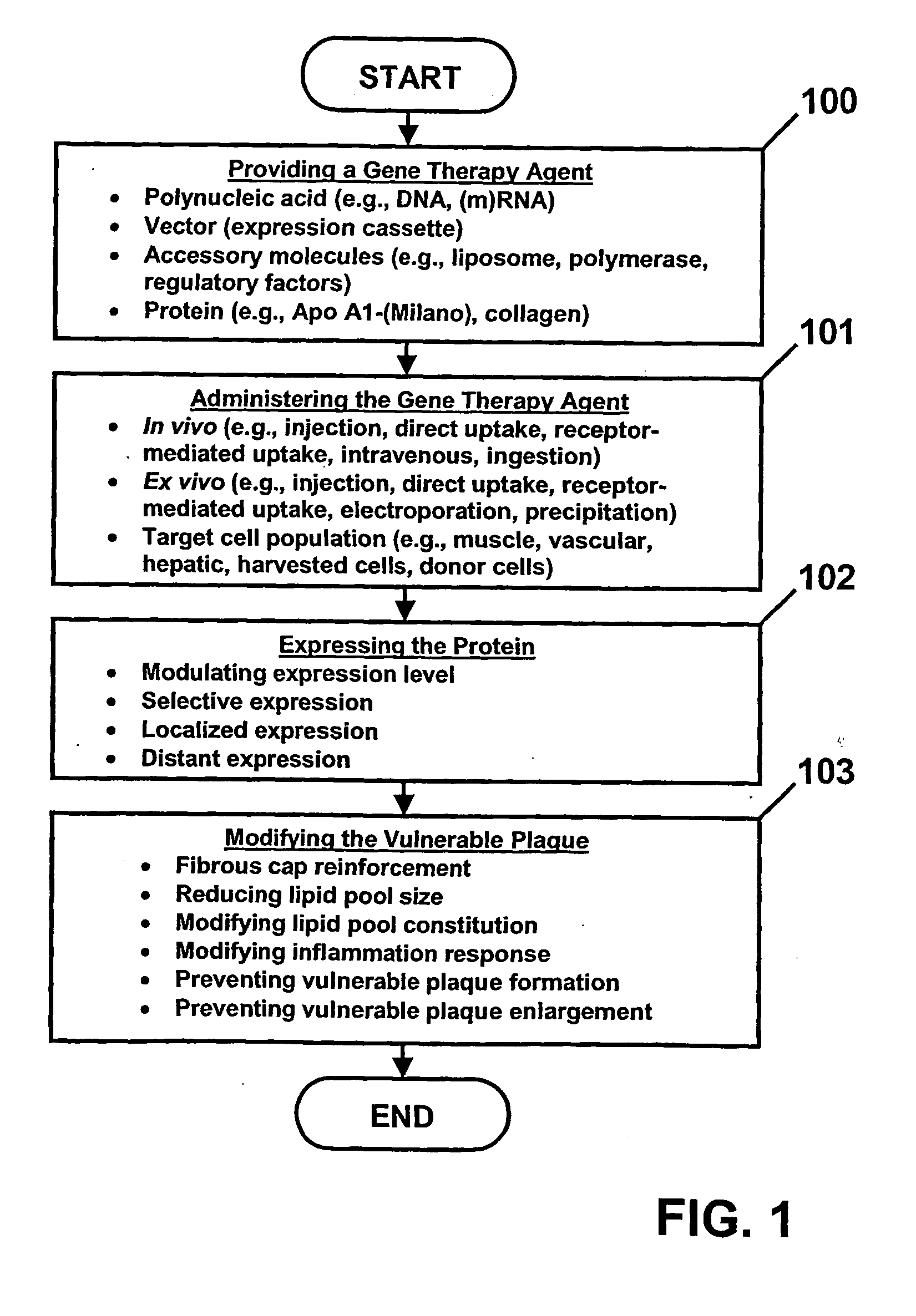
Method and agent for treating vulnerable plaque
InactiveUS20050260157A1Small sizeVulnerable plaque formationBiocideAnimal repellantsVulnerable plaqueGene Therapy Agent
A method and gene therapy agent for treating a vulnerable plaque associated with a blood vessel of a patient is disclosed. The method includes providing at least one gene therapy agent encoding at least one protein. The gene therapy agent is administered to a target cell population. The protein is expressed within the patient from a portion of the target cell population. The vulnerable plaque is modified as a result of the protein expression. The gene therapy agent includes at least one polynucleic acid encoding at least one protein. Administration of the gene therapy agent to a target cell population results in expression of the protein capable of modifying the vulnerable plaque.
Owner:MEDTRONIC VASCULAR INC
Method and system for systemic delivery of growth arresting, lipid-derived bioactive compounds
ActiveUS20130295159A1Maximizing systemic deliveryCorrection for dispersionBiocideMicroencapsulation basedDendrimerLipid formation
A system and method for optimizing the systemic delivery of growth-arresting lipid-derived bioactive drugs or gene therapy agents to an animal or human in need of such agents utilizing nanoscale assembly systems, such as liposomes, resorbable and non-aggregating nanoparticle dispersions, metal or semiconductor nanoparticles, or polymeric materials such as dendrimers or hydrogels, each of which exhibit improved lipid solubility, cell permeability, an increased circulation half life and pharmacokinetic profile with improved tumor or vascular targeting.
Owner:PENN STATE RES FOUND
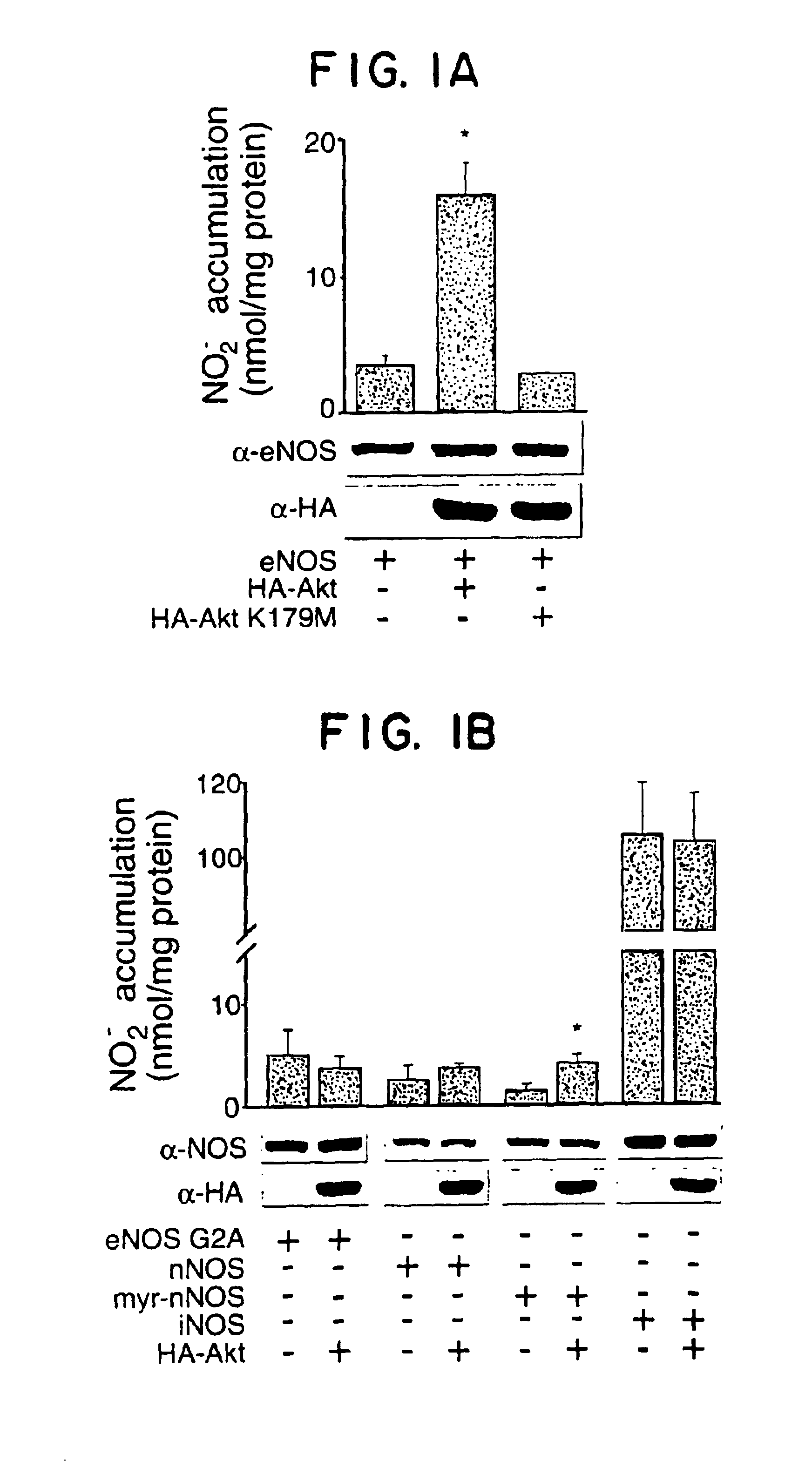
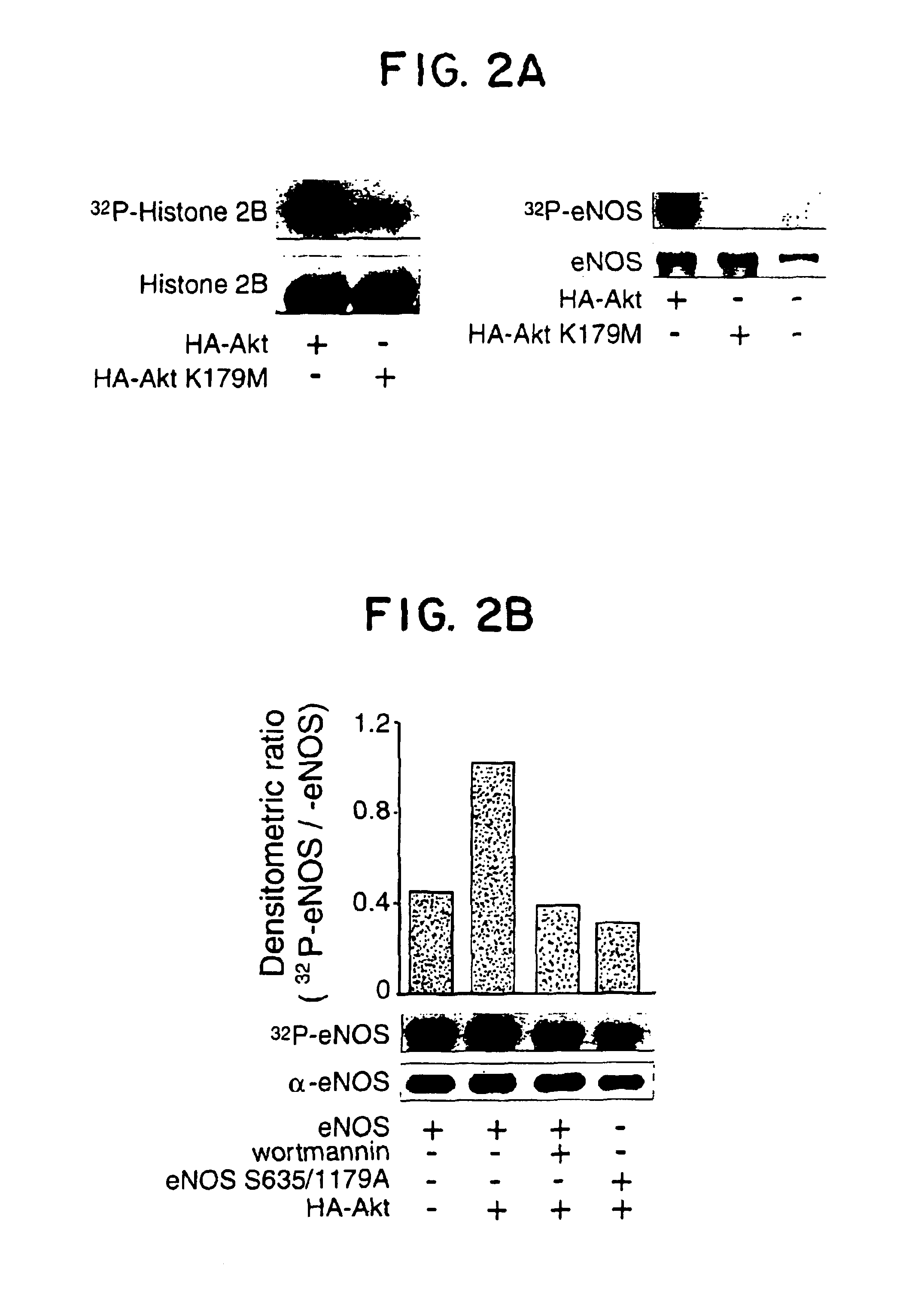
eNOS mutations useful for gene therapy and therapeutic screening
InactiveUS6900038B2Increase basal NO releaseIncrease in reductase activityPeptide/protein ingredientsGenetic material ingredientsDiseasePhosphorylation
The present invention relates to new NOS variants or mutants which contain structural alterations in the site of Akt dependent phosphorylation. The altered NOS proteins or peptides, especially the human eNOS proteins or peptides, Akt proteins or polypeptides and their encoding nucleic acid molecules are useful as gene therapy agents for the treatment of diseases including post angioplasty restenosis, hypertension, atherosclerosis, heart failure, diabetes and diseases with defective angiogenesis. NOS proteins and peptides are also useful in methods of screening for agents which modulate NOS activity.
Owner:YALE UNIV
Methods for targeted delivery of genetic material to the liver
The present invention provides methods for enhanced delivery of various therapeutic agents, such as gene therapy agents, to the vasculature of a target organ in a mammalian subject. The methods for targeted gene therapy in the mammalian liver as a whole, or in a single hepatic lobe, are disclosed. The disclosed methods rely on minimally invasive catheter-based procedures wherein a target organ is isolated and treated locally with a gene therapy agent. The methods offer more efficient and localized transfection of tissue and are wellsuited for gene therapy in human subjects.
Owner:GENZYME CORP
Gene therapy agent for Haemophilia B and its preparation method
The invention relates to site-specific integrating expression vectors for therapy Hemophilia B and to methods of preparing them. A vector of the invention contains a human Factor IX gene in a vector constructed using as a chromosome targeting sequence a polynucleotide without any important physiological function-related gene homologous to DNA on the short arms of human group D and human group G chromosomes. The vector of the invention provides high stability of Factor IX expression, high expression efficiency, no immunogenicity and safety in use.
Owner:XIA JIAHUI
Gene therapeutics for treating bone disorders
In some aspects, the disclosure relates to compositions and methods for modulating ( e.g., increasing and / or decreasing) bone mass in a subject. In some aspects, the disclosure provides isolated nucleic acids, and vectors such as rAAV vectors, configured to express transgenes that promote (e.g., increase) or inhibit (e.g., decrease) activity, differentiation, or function of certain types of bone cells, for example osteoblasts, osteoclasts, osteocytes, etc. In some embodiments, the isolated nucleic acids and vectors described by the disclosure are useful for treating disorders and conditions associated with increased bone mass (e.g., osteopetrosis) or decreased bone mass (e.g., osteoporosis).
Owner:UNIV OF MASSACHUSETTS
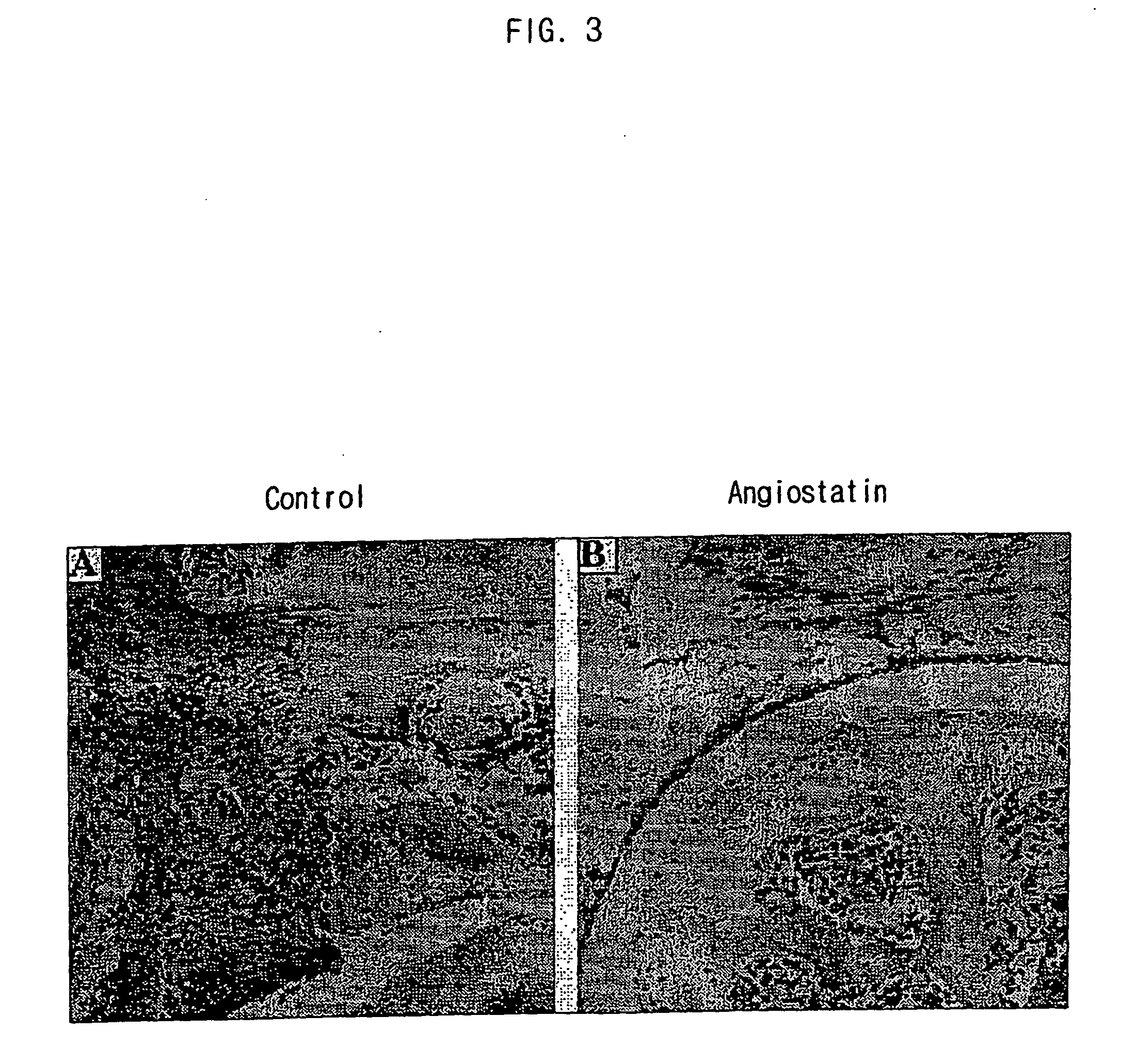
Compositions for gene therapy of rheumatoid arthritis including a gene encoding an anti-angiogenic protein or parts thereof
InactiveUS20050277603A1Improve efficiencyImprove stabilityPeptide/protein ingredientsAntipyreticAngiostatinGene Therapy Agent
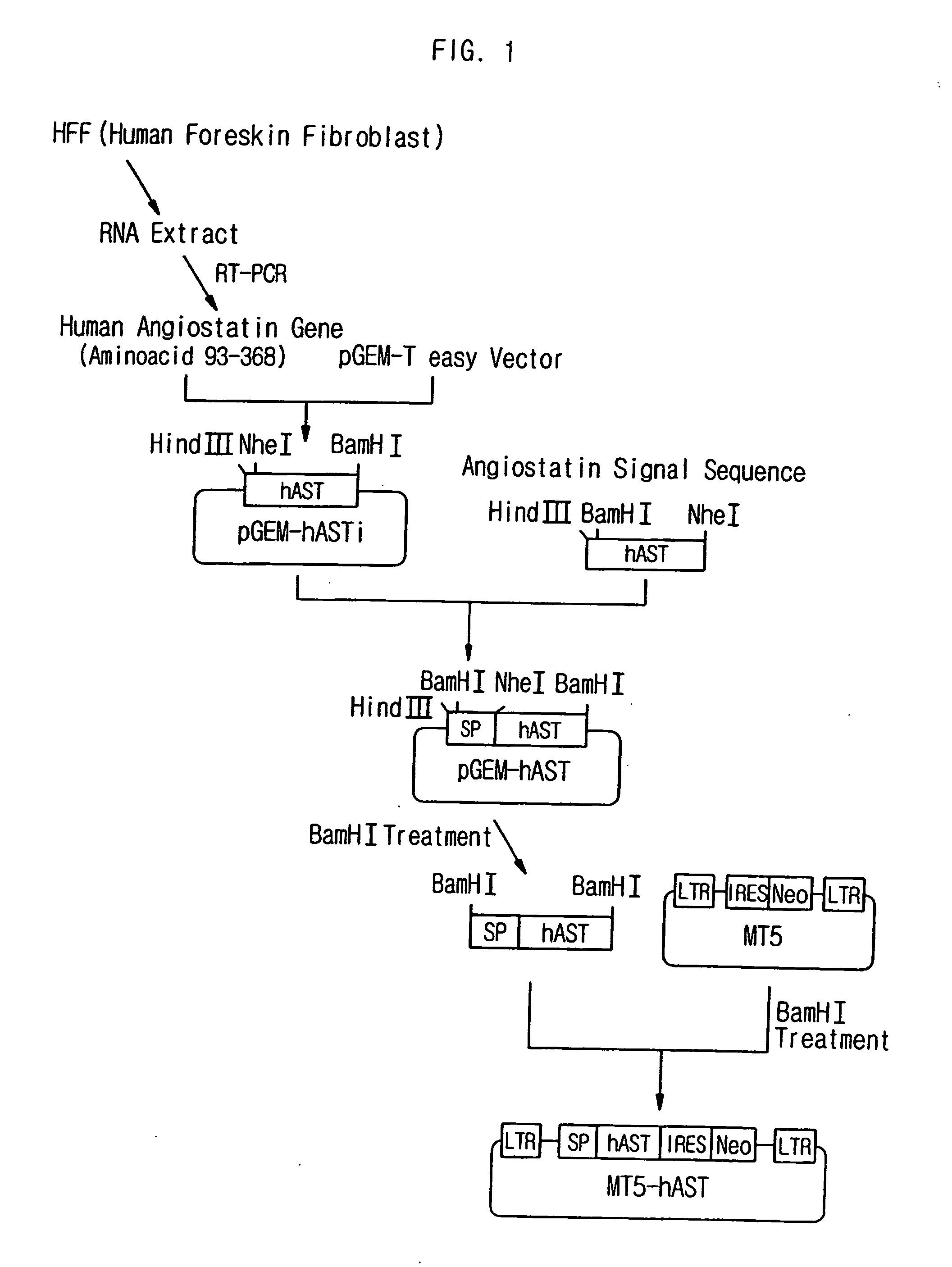
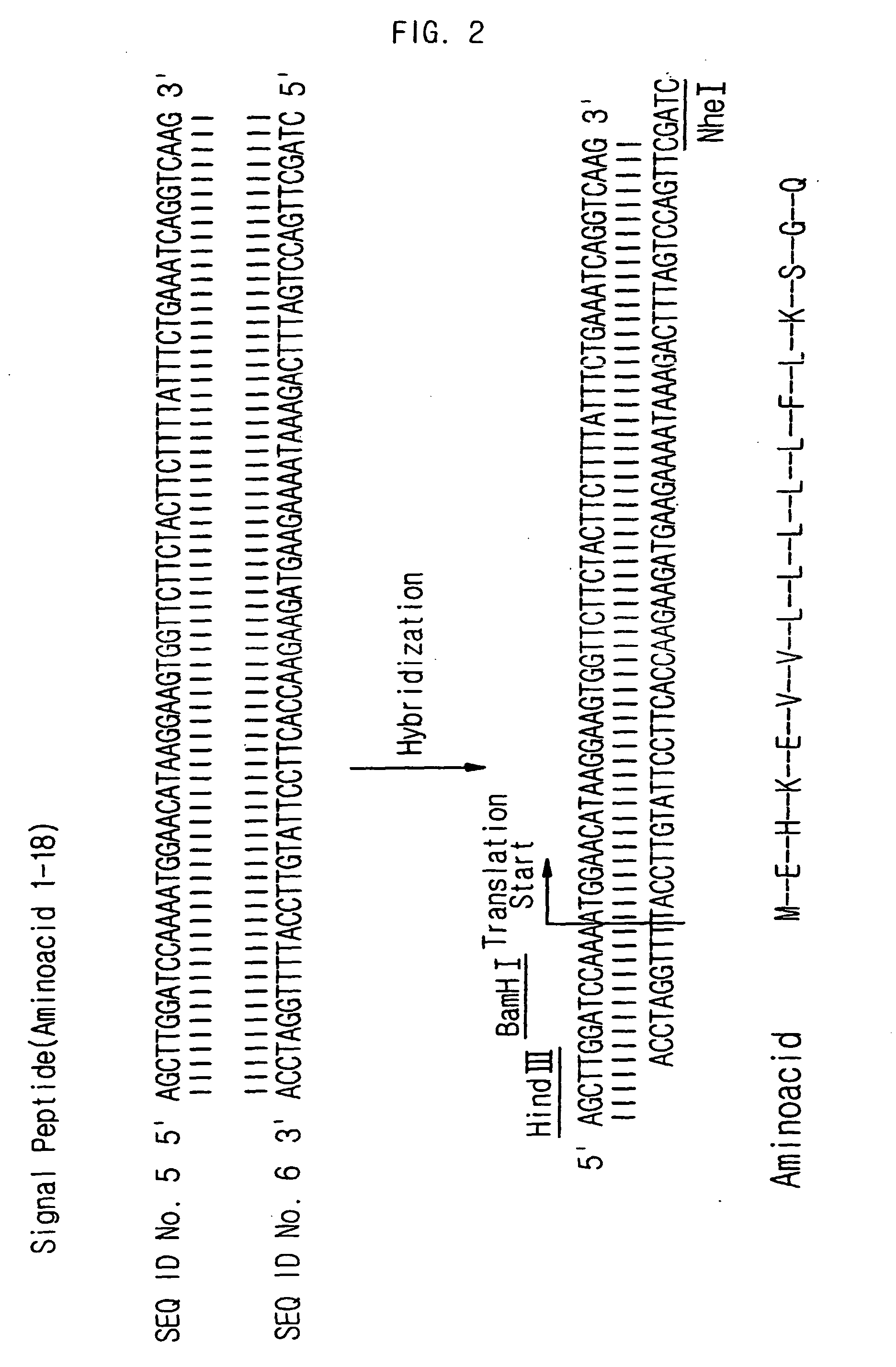
The present invention relates to the compositions for a gene therapy of rheumatoid arthritis including a gene encoding an anti-angiogenic protein or parts thereof. More specifically, the present invention provides a gene therapy of rheumatoid arthritis by preparing a recombinant vector that expresses a gene encoding an anti-angiogenic protein such as angiostatin or parts thereof, and transplanting the recombinant vector or a cell that is transfected or transduced with the recombinant vector into the affected area of a patient, and also provides the compositions for the gene therapy. The compositions for the gene therapy according to the present invention can be used effectively for the treatment of rheumatoid arthritis, for which effective treating methods have not been developed until now, by providing the anti-angiogenic proteins into the knee of a patient continuously to prevent the synovial tissue hyperplasia and the resulting inflammation.
Owner:VIROMED CO LTD
Novel gene therapy agent for haemophilia b and its preparation method
The invention deals with the gene drugs for therapy Hemophilia B and its method of preparation. It contains human source gene vector-FIX recombinant constructed using DNA sequence as leading sequence of therapy gene without important physiological function-related gene on the short arms of human group D,G chromosomes or to which DNA sequence is homologous. This invention also provides the method of preparing gene drug. The therapy gene of gene drug for hemophilia B has a high stability of expression, safety, high expression efficiency and no immunogenecity.
Owner:XIA JIAHUI
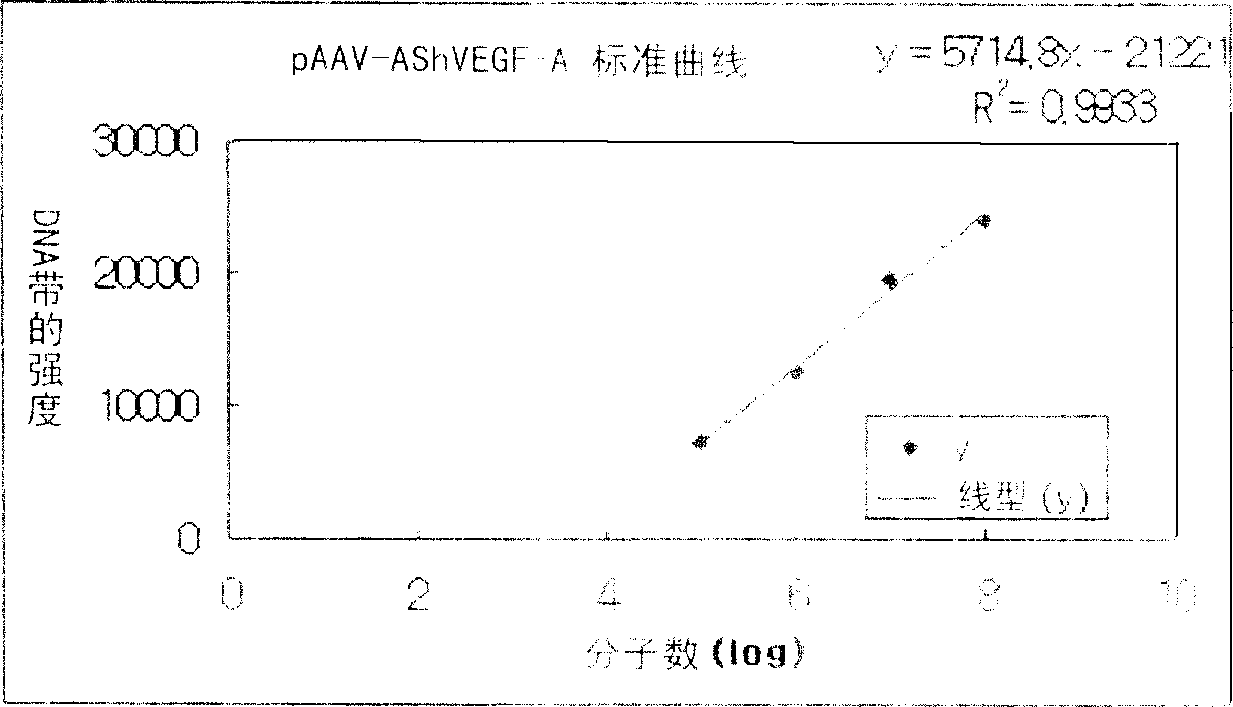
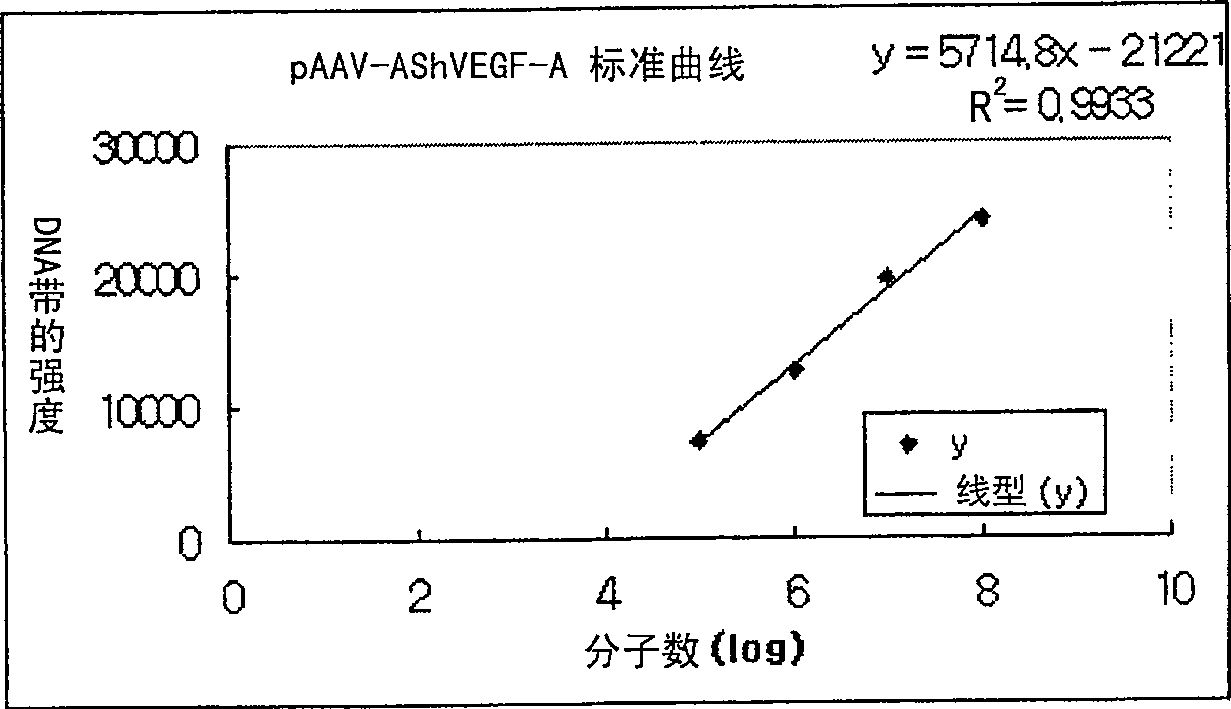
Recombinant adeno-associated virus comprising VEGFR Truncated Soluble cDNA and gene therapeutic agent comprising the same
The present invention relates to a gene therapeutic agent using truncated soluble cDNA of VEGF receptor protein (VEGFR) and adeno-associated virus(AVV) system, and more particularly, relates to a gene therapeutic agent specific to large intestine cancer, bladder cancer and / or lung cancer, comprising a rAAV vector containing truncated soluble cDNA of VEGFR, and a rAAV vector containing the said vector and VEGF-A anti-sense cDNA. The gene therapeutic agent according to the present invention reduces the growth of tumors by inhibiting the expression and function of VEGF involved in angiogenesis necessary for the proliferation and metastasis of tumors. Thus, the inventive gene therapeutic agent can be effectively used to treat cancer at a gene level.
Owner:SCT
Gene therapy and pharmaceutical composition for prevention and treatment of lung cancer
InactiveUS20090169485A1Good curative effectEffective treatmentPowder deliveryOrganic active ingredientsChemical synthesisGene delivery
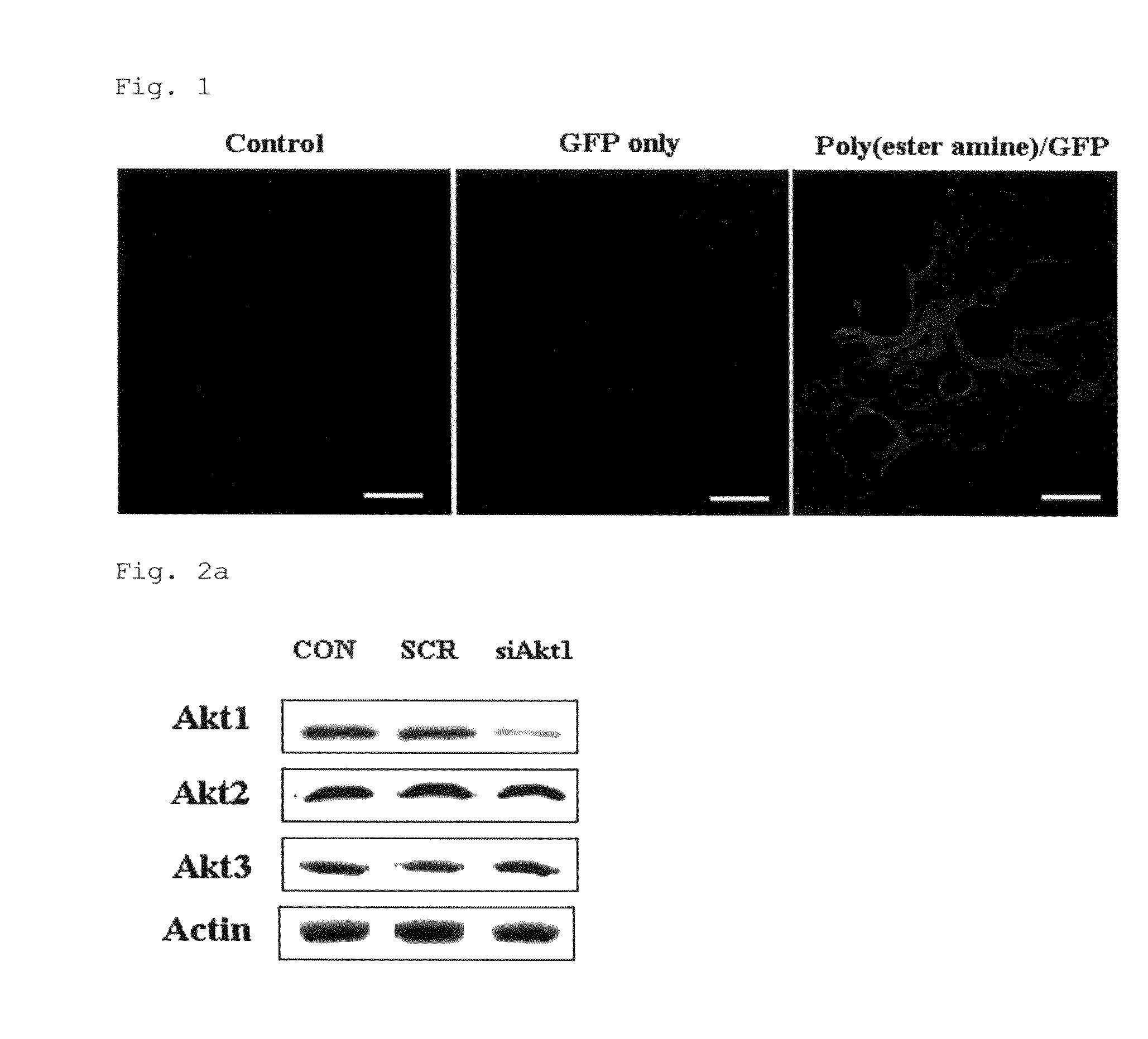
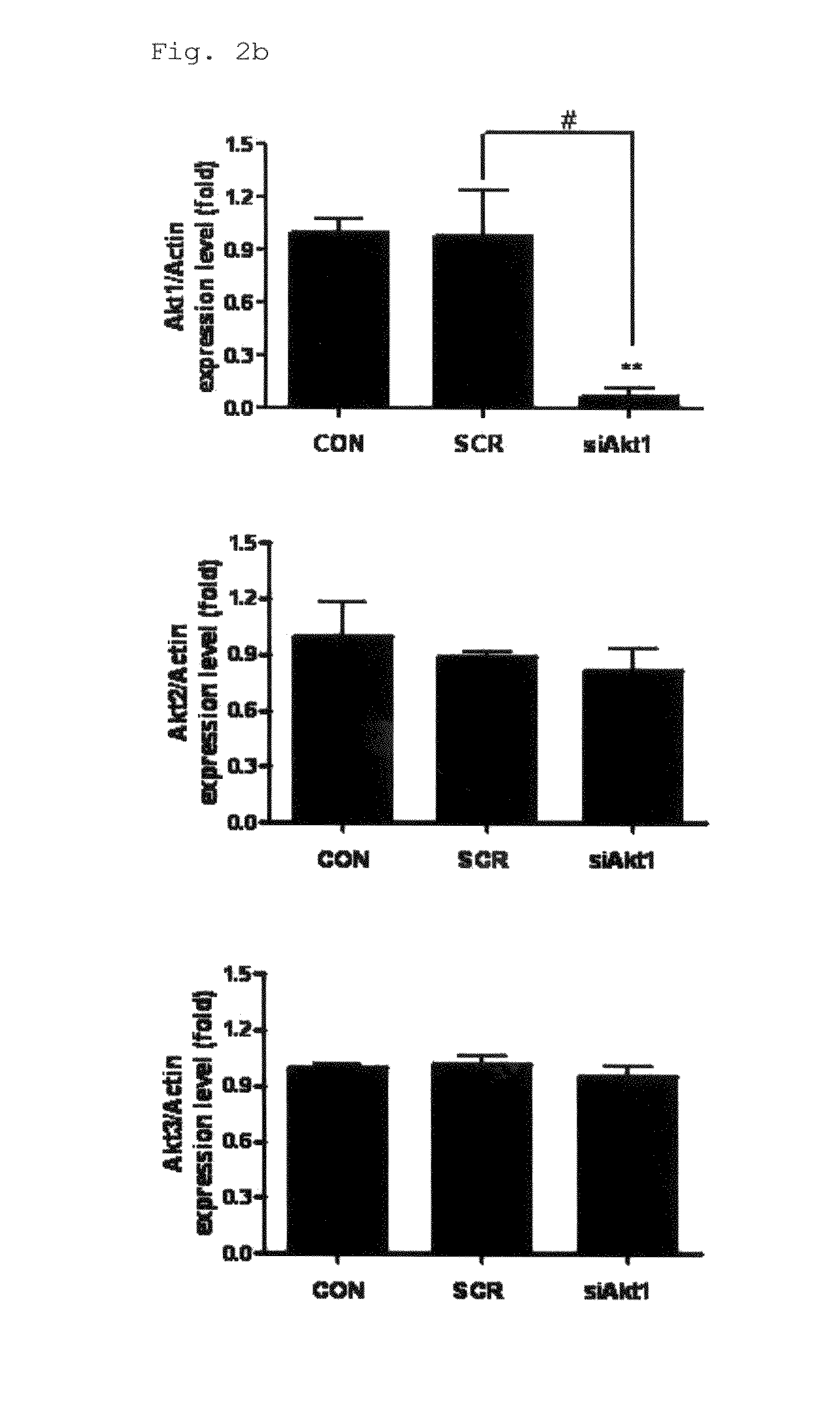
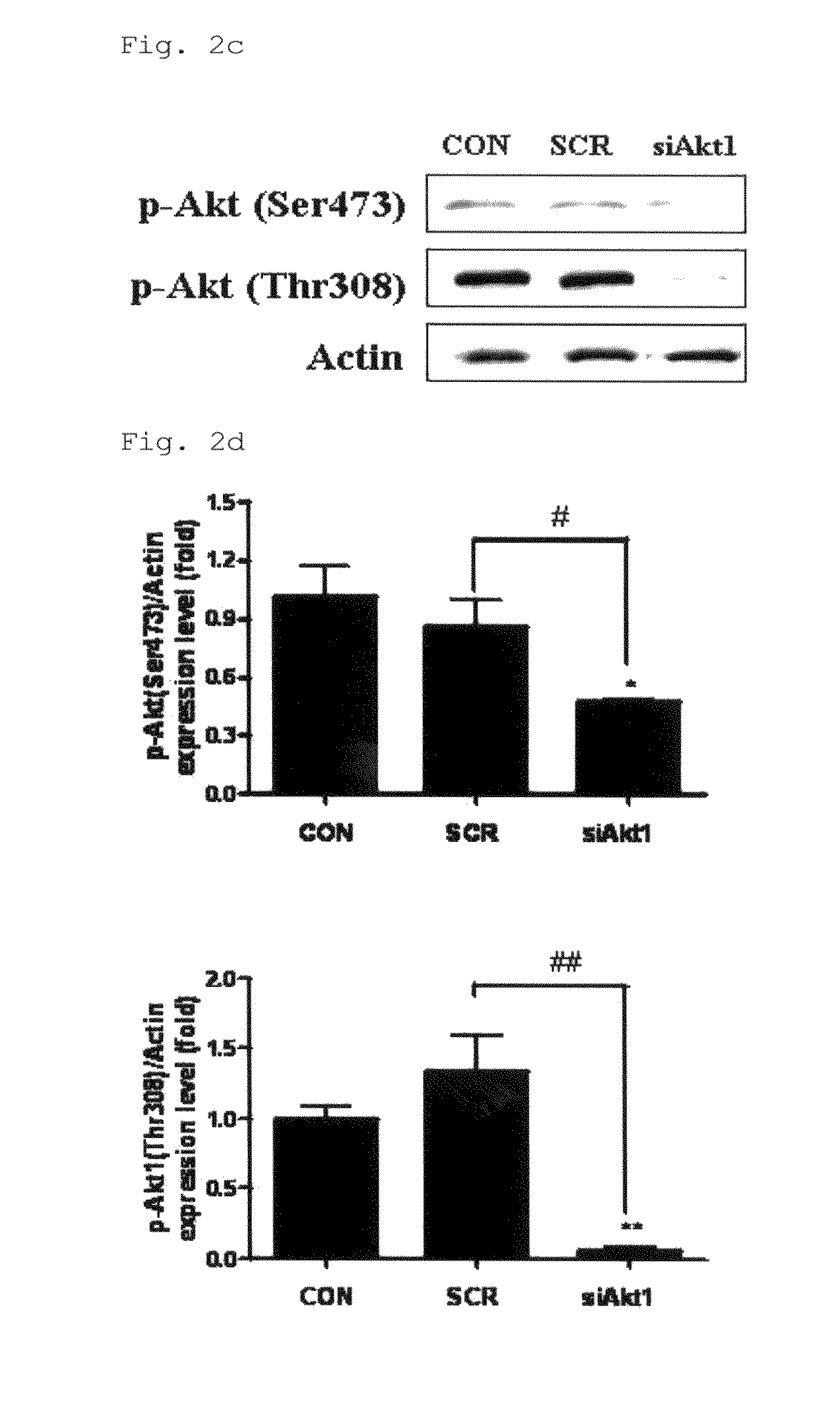
Disclosed herein is a gene therapeutic agent and pharmaceutical composition for the prevention and treatment of lung cancer. For aerosol delivery, chemically synthesized polyester amine is used as a carrier in the gene therapeutic agent. The polyester amine / Akt1 siRNA complex is found to be effectively delivered to the lungs of K-ras null mice through a nose-only inhalation system and to significantly suppress lung cancer progression as denoted by gene delivery efficiency and inhibition of Akt-related signals and cell cycle. Thus, the aerosol delivery of polyester amine-mediated Akt1 siRNA is provided as an effective model for noninvasive gene therapy.
Owner:SEOUL NAT UNIV R&DB FOUND
Recombinant adeno-associated virus comprising VEGFR Truncated Soluble cDNA and gene therapeutic agent comprising the same
The present invention relates to a gene therapeutic agent using truncated soluble cDNA of VEGF receptor protein (VEGFR) and adeno-associated virus(AVV) system, and more particularly, relates to a gene therapeutic agent specific to large intestine cancer, bladder cancer and / or lung cancer, comprising a rAAV vector containing truncated soluble cDNA of VEGFR, and a rAAV vector containing the said vector and VEGF-A anti-sense cDNA. The gene therapeutic agent according to the present invention reduces the growth of tumors by inhibiting the expression and function of VEGF involved in angiogenesis necessary for the proliferation and metastasis of tumors. Thus, the inventive gene therapeutic agent can be effectively used to treat cancer at a gene level.
Owner:SCT
Application of sodium ion channel blocker in preparation of drugs to treat melanoma
ActiveCN109908350ALow costShorten development timeOrganic active ingredientsAntineoplastic agentsExtracellular signalImmunotherapeutic agent
The invention discloses application of a sodium ion channel blocker in the preparation of drugs to treat melanoma. The sodium ion channel blocker is made with any one or two of tetrodotoxin and dehydrated tetrodotoxin. The invention also provides a drug to treat melanoma; the drug comprises the sodium ion channel blocker, a second therapeutic agent and a pharmaceutically acceptable carrier; the second therapeutic agent includes one or more of a chemotherapeutic agent, an immunotherapeutic agent, a gene therapeutic agent and a radiotherapeutic agent. The sodium ion channel blocker can inhibit the proliferation and tumorigenesis of melanoma cells, and can cooperate with the second therapeutic agent, especially mitogen-activated extracellular signal regulation kinase inhibitor, to arrive at better treatment of melanoma.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Gene therapeutics
Gene therapeutics to be used in treating diseases showing sensitivity to gene therapy, characterized by containing as the active ingredient an efficacious amount of a functional substance which has a function of having an affinity for a virus containing a gene usable in the gene therapy and another function of having an affinity specific for a target cell with a need for the gene transfer, or an efficacious amount of a functional substance which has an affinity for the above virus and an efficacious amount of another functional substance which has an affinity specific for the above cell.
Owner:TAKARA HOLDINGS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com