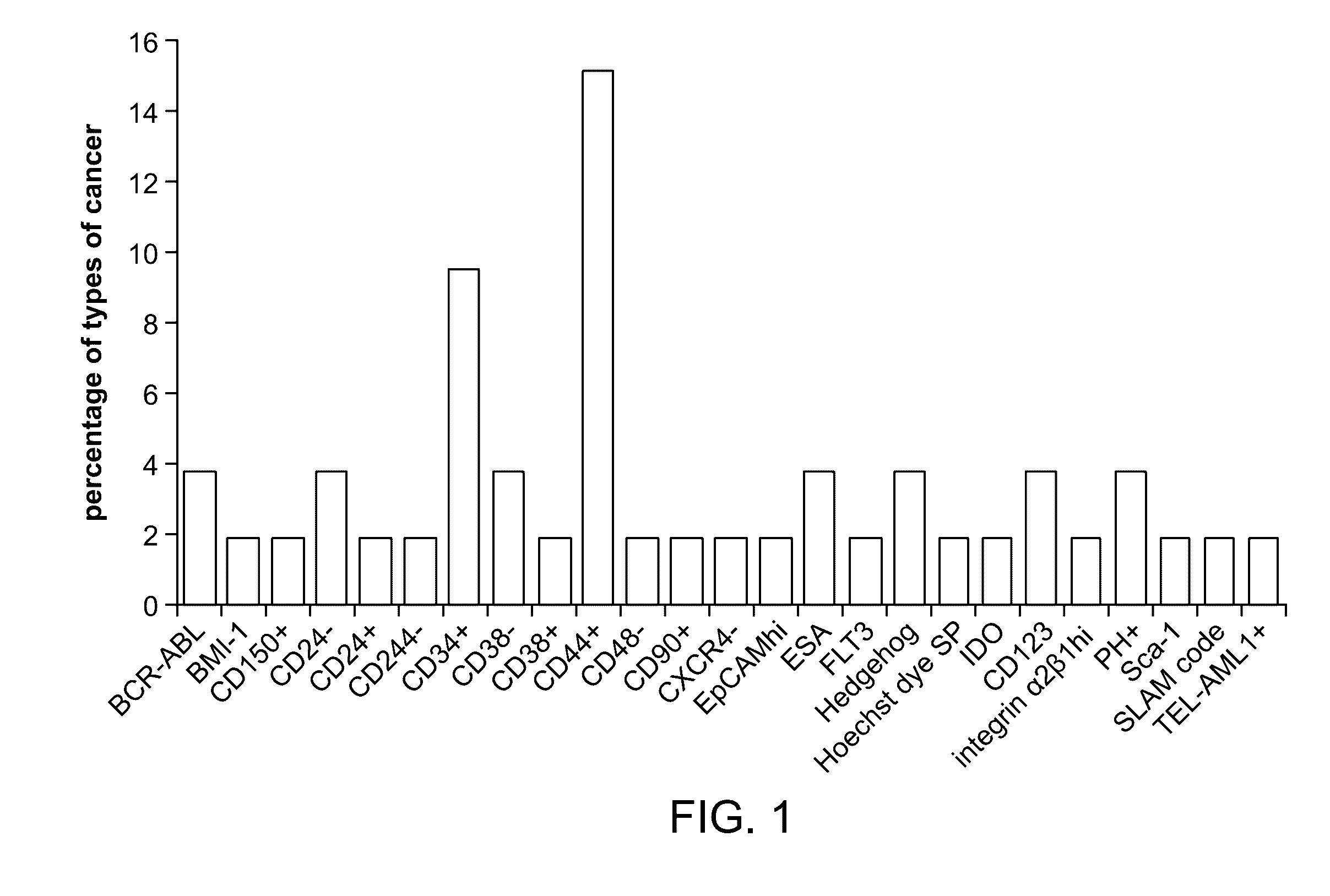
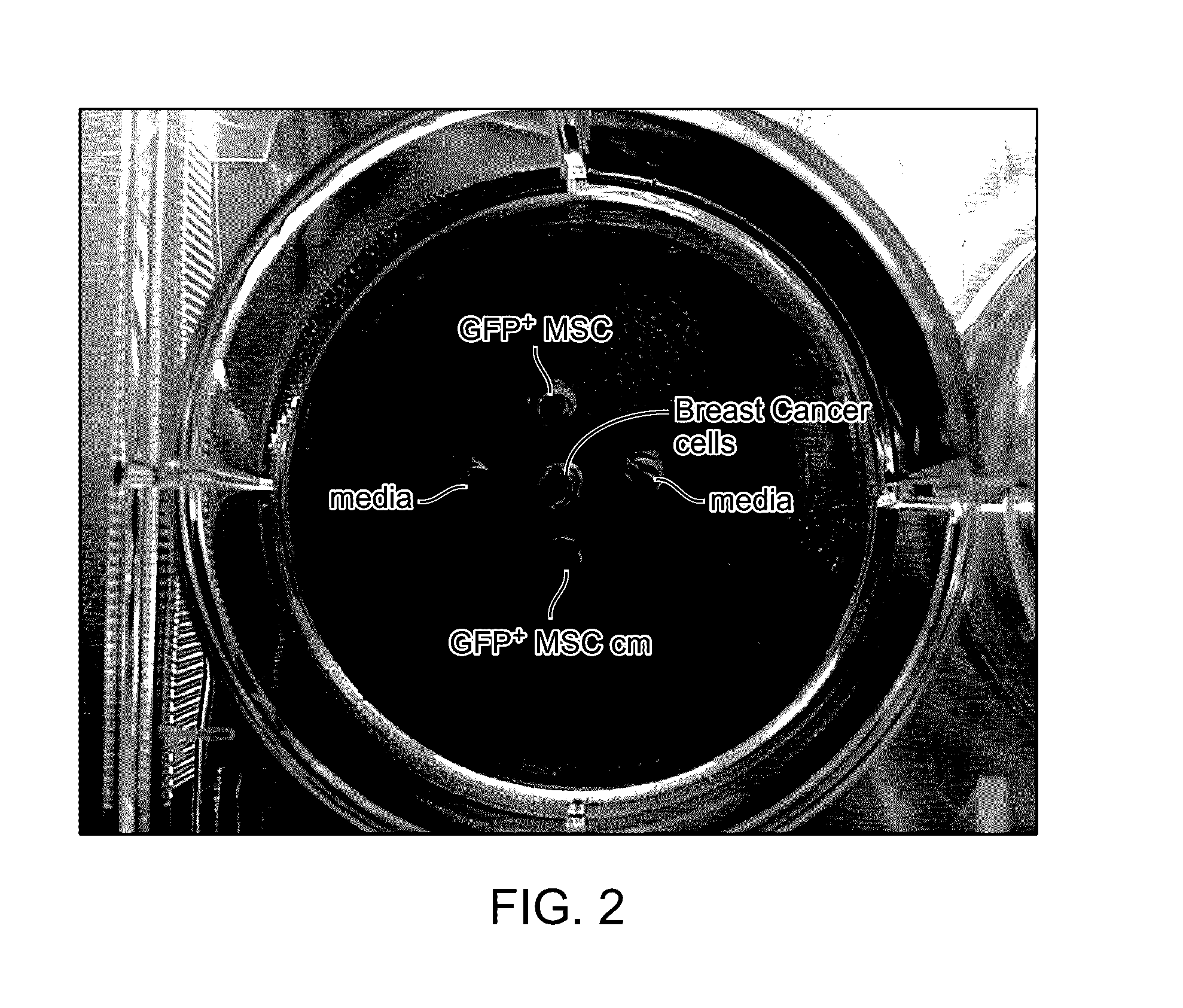
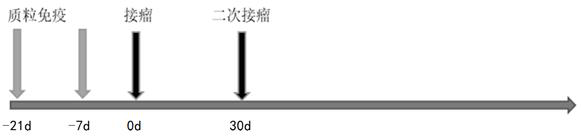
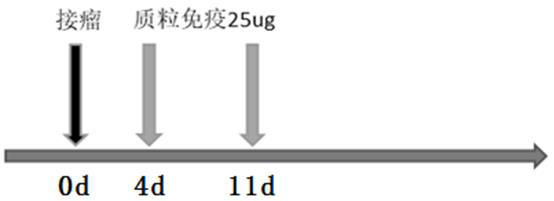
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "CCL5" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemokine (C-C motif) ligand 5 (also CCL5) is a protein which in humans is encoded by the CCL5 gene. It is also known as RANTES (regulated on activation, normal T cell expressed and secreted).
Methods of treating hematological disorders with quinazolinone compounds in selected subjects
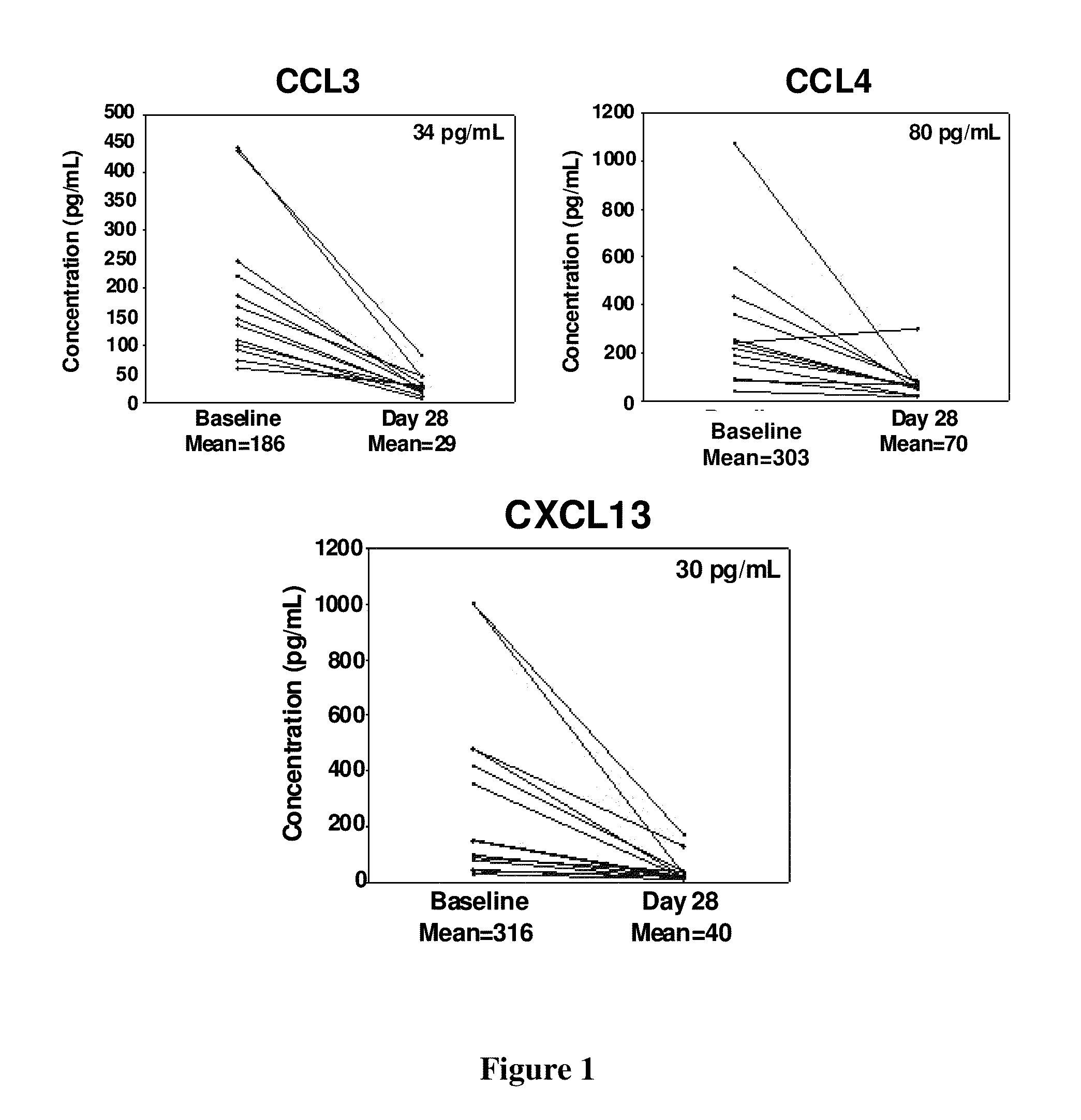
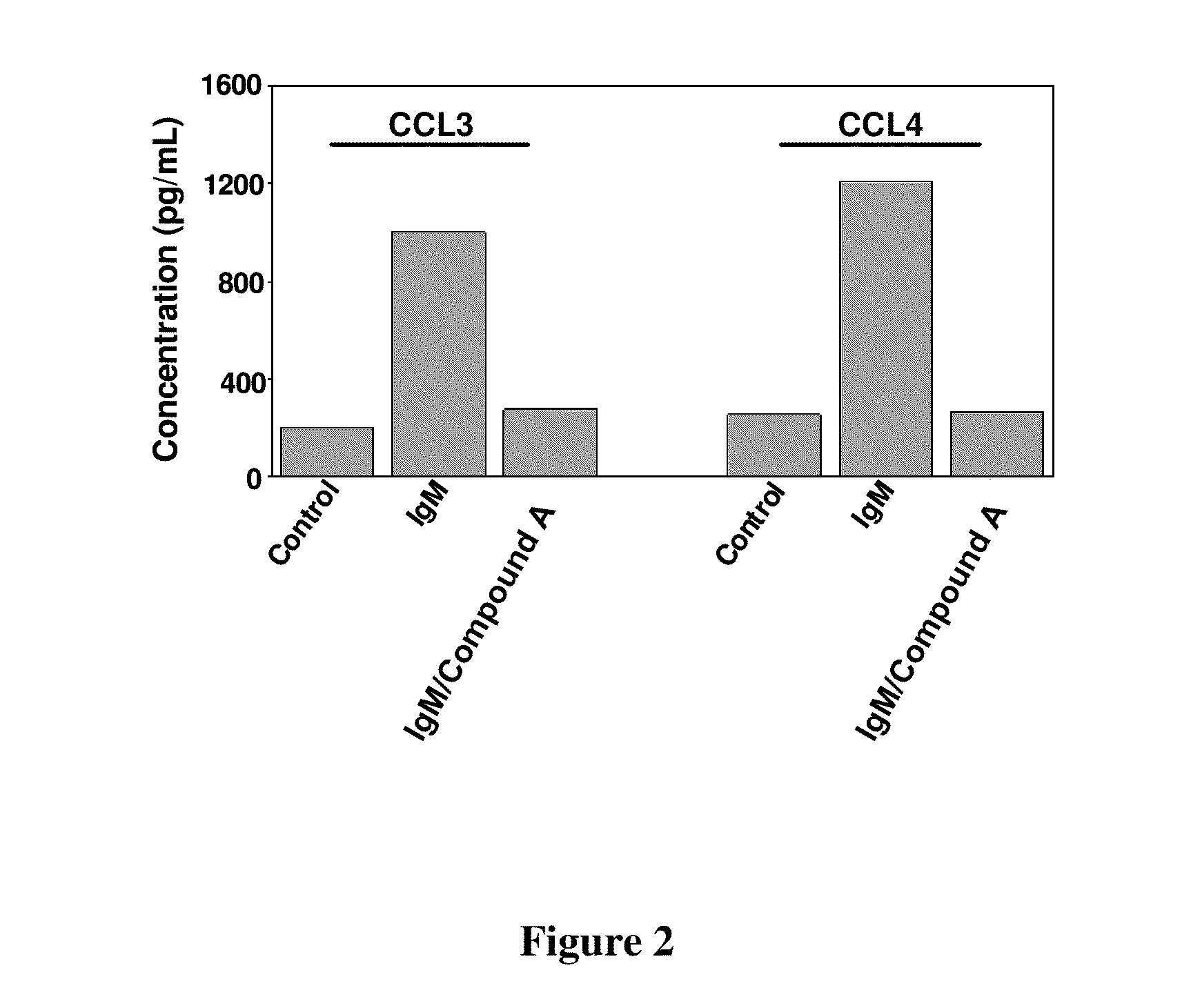
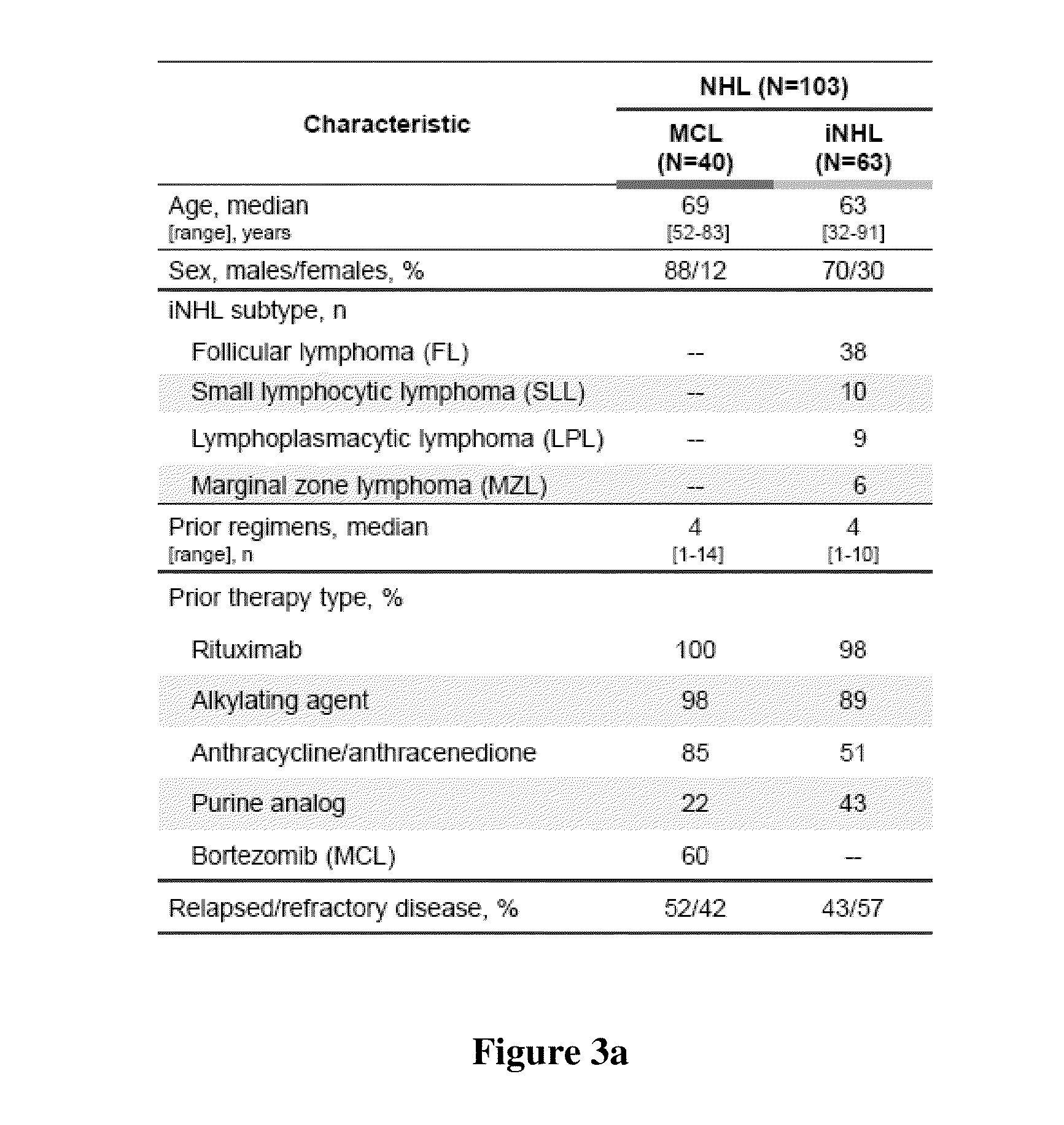
This disclosure relates to methods of selecting a subset of subjects having a hematological disorder and treating the selected group with a PI3K-delta inhibitor. In particular, the methods disclose evaluating levels of characteristic chemokine biomarkers, such as CCL2, CCL3, CCL4, CCL5, CXCL13, CCL17, CCL22, or TNF-alpha to select subjects that would have a greater chance of benefiting from treatment with a PI3K-delta inhibitor. The PI3K-delta inhibitors disclosed in this application are a type of quinazolinone-purinyl family of compounds.
Owner:GILEAD CALISTOGA
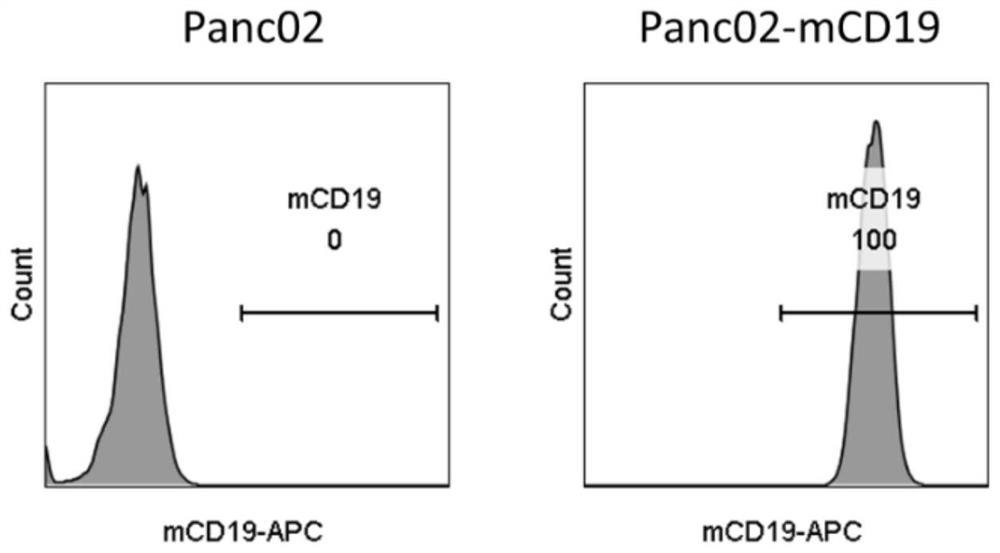
Engineered immune cells and application thereof
InactiveCN112063588AIncreased proliferationProlong survival timeVirusesAntiinfectivesAutoimmune conditionCCL3
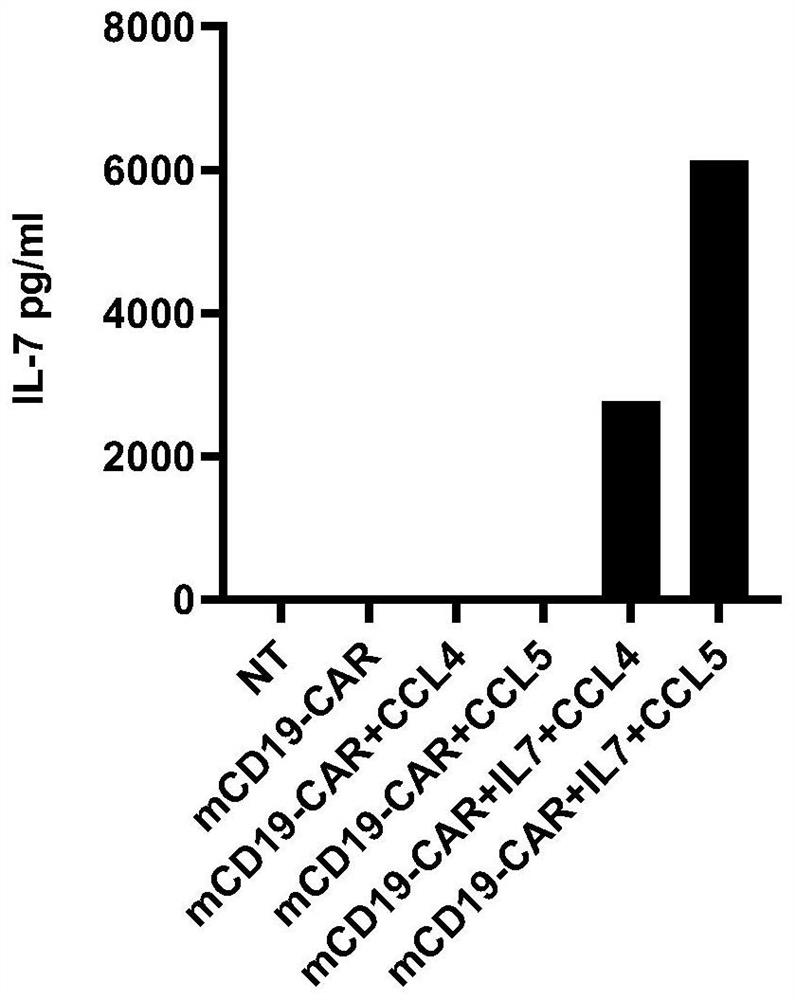
The invention relates to an engineered immune cell, and the engineered immune cell expresses (i) a chimeric receptor, and (ii) exogenous CCL3, CCL4 and / or CCL5. The invention further provides application of the engineered immune cell to treatment of cancers, infections or autoimmune diseases. Compared with a traditional engineered immune cell, the engineered immune cell provided by the invention has the remarkably improved tumor killing activity.
Owner:NANJING BIOHENG BIOTECH CO LTD
Chemotherapy regimen selection
The present invention provides, inter alia, kits for selecting a chemotherapy regimen for a subject. The kits comprise one or more components for detecting the expression of at least one gene from the group of SLC12A7, GZMB, TAF6L, NFIB, METRN, ROPN1B, TTK, CCND1, PTTG1, H2AFZ, WDR45L, DEK, MCM2, USP1, CDT1, TMEM97, RER1, MCM6, LZTFL1, C11orf17, CCL5, XCL1, XCL2, MELK, CTSL2, TPX2, AURKA, CDKN2C, BRP44, PNP, SMC4, NR4A2, C3orf37, MTPAP, CDC25B, ABCF1, MTAP, SNAPC3, RANBP9, COIL, FAM86B1, ITGA6, S100P, RANBP1, PRSS16, SMARCA2, STK24, TSPYL5, SRI, LRP12, CENPF, TUBD1, KIAA1324, DBF4, CCNA2, DLGAP5, FHL1, SIRT3, GTSE1, PCNA, CCNE2, CHD3, CAP1, GPM6B, GUSBP3, GNAI3, LMO4, PSRC1, USP1, STK38, BAT2L1, PMP22, NME5, CENPA, BANK1, and derivatives thereof. Methods for selecting a chemotherapy regimen for a subject are also provided.
Owner:INNOMEDICINE LLC
Molecular methods for assessing post kidney transplant complications
InactiveUS20170184575A1Microbiological testing/measurementDisease diagnosisBacteriuriaRna expression
Methods of screening for expression of an RNA associated with a post-kidney transplant complication include collecting vesicles from urine, isolating vesicle-associated RNA, and analyzing expression patterns. In particular, AIF1, BTN3A3, CCL5, CD48, HAVCR1, or SLC6A6 mRNA expression patterns are analyzed.
Owner:CITY OF SAPPORO +1
Antikine antibodies that bind to multiple CC chemokines
An antikine antibody binds to two, three, four, five or more CC chemokines, such as RANTES / CCL5, MIP-1α / CCL3, MIP-1β / CCL4, or MCP-1 / CCL2. Methods for affinity maturation and humanization of antikine antibodies as well as the production of hybridoma cell lines producing antikine antibodies by sequential immunization are also disclosed.
Owner:VLST +1
Cc-chemokine mutants against liver diseases
InactiveUS20060228327A1Counteracts liver fibrotic injuryImprove suppression propertiesNervous disorderPeptide/protein ingredientsAutoimmune conditionMedicine
CC-Chemokine mutants having reduced Glycosaminoglycans (GAG)-binding properties are effective against liver fibrotic inflammatory and / or autoimmune diseases. Particularly preferred are the mutants of CCL5 / RANTES having reduced GAG-binding properties.
Owner:MERCK SERONO SA
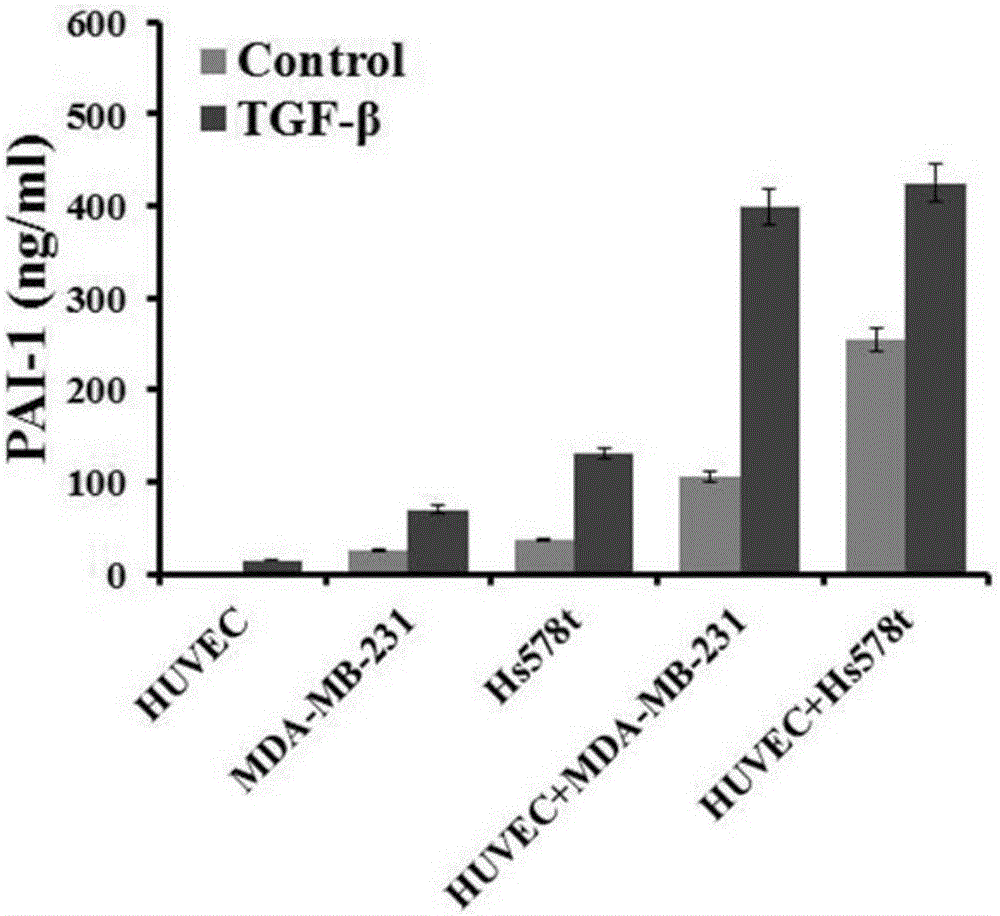
Method for detecting influence of triple negative breast cancer cell on endothelial cell secretion function in epithelial-mesenchymal transition process
ActiveCN106701886AEasy transferPromote secretionCompound screeningApoptosis detectionMda mb 231Factor ii
The invention relates to a method for detecting the influence of a triple negative breast cancer cell on an endothelial cell secretion function in the epithelial-mesenchymal transition process. A triple negative breast cancer cell line MDA-MB-231 and a human umbilical vein endothelial cell HUVEC are selected, and experimental groups including an MDA-MB-231 and HUVEC co-culture group and a control group, namely a MDA-MB-231 individual culture group are set; after the experimental groups and the control group are applied to 10ng / ml TGF-beta stimulation for 36 hours, a TMT quantitative proteome expression profile is applied to analyze differential expressions of secretory proteins in cellular supernatants in the two groups, and secretion results of SERPINE (PAI-1) factors in the co-culture group and the individual culture group are compared. The triple negative breast cancer cell line of the triple negative breast cancer cell in the epithelial-mesenchymal transition process can promote secretion of chemotactic factors CCL5 of endothelial cells and accordingly promotes transfer of the triple negative breast cancer cell.
Owner:管晓翔 +2
Reagent kit for detecting lawful age females synthetic disease genetic susceptibility
InactiveCN101275911AMicrobiological testing/measurementChemiluminescene/bioluminescenceDiseaseFluorescence
The present invention discloses a reagent kit which detects the genetic susceptibility of the synthetic disease of adult woman. The reagent kit comprises a specific primer pair and a specific fluorescent probe pairs which simultaneously detects the SNP polymorphic genotype on the genes of CYBA, CAT, LPL, LEP, ADIPOQ, SOD3, IL3, NOS3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13, CBS, a routine component which is used for the fluorescent quantitative PCR testing and the like. The reagent kit of the invention evaluates the genetic susceptibility of the synthetic disease of adult woman through simultaneously detecting the mononucleotide polymorphism site genotype which is closely linked to the genetic susceptibility of the synthetic disease of adult woman.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Prognostic and treatment response predictive method
PendingUS20210139999A1Easy to sign forImprove predictive performanceMicrobiological testing/measurementBiostatisticsCCL2IL12A
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of at least 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL A in a sample obtained from the tumour of the patient; b) measuring the gene expression of at least 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained from the tumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including with immune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Diagnostic methods and compositions for cancer immunotherapy
PendingCN113260633AOrganic active ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesAntigen Binding Fragment
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The compositions and methods described herein can be used, for example, to determine the propensity of a patient to benefit from treatment with a PD-L1 axis binding antagonist and to treat such patients accordingly. Using the compositions and methods of the disclosure, a patient, such as a human cancer patient, may be determined to be likely to benefit from treatment with a PD-L1 axis binding antagonist if the patient exhibits an elevated pre-treatment expression level of one or more of CST7, NKG7, GZMH, MT-ND4, HLA-H, CCL5, CD8A, CMC1, CD8B, HCST, MT-CYB, MT-ND4L, KLRG1, MT-CO2, MT-ATP6, PLEK, CTSW, HLA-C, LYAR, LITAF, GZMB, KLRD1, FGFBP2, KLRC4-KLRK1, KLRK1, B2M, GZMA, ID2, CX3CR1, PRSS23, GNLY, PRF1, and PATL2. Exemplary PD-L1 axis binding antagonists that may be used in conjunction with the compositions and methods of the disclosure are PD-L1 binding antagonists, such as anti-PD-L1 antibodies and antigen-binding fragments thereof, including atezolizumab, as well as PD-1 binding antagonists, such as anti-PD-1 antibodies and antigen-binding fragments thereof.
Owner:F HOFFMANN LA ROCHE & CO AG
Reagent kit for detecting man disease genetic susceptibility
The present invention provides a kit for detecting genetic predisposition of adult male syndrome. The kit includes specific primer pairs and specific fluorescent probe pairs for detecting 48 SNP polymorphism genotypes on the genes LPL, TNF-alpha, LEP, ADIPOQ, SOD3, IL3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13 at the same time, and normal components for FQ-PCR detection. The kit of the invention evaluates the genetic predisposition of adult male syndrome by detecting the genotypes of the 48 SNP polymorphism sites at the same time which is tightly relative to the genetic predisposition of adult male syndrome.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Mesenchymal stem cells for targeted cancer therapy
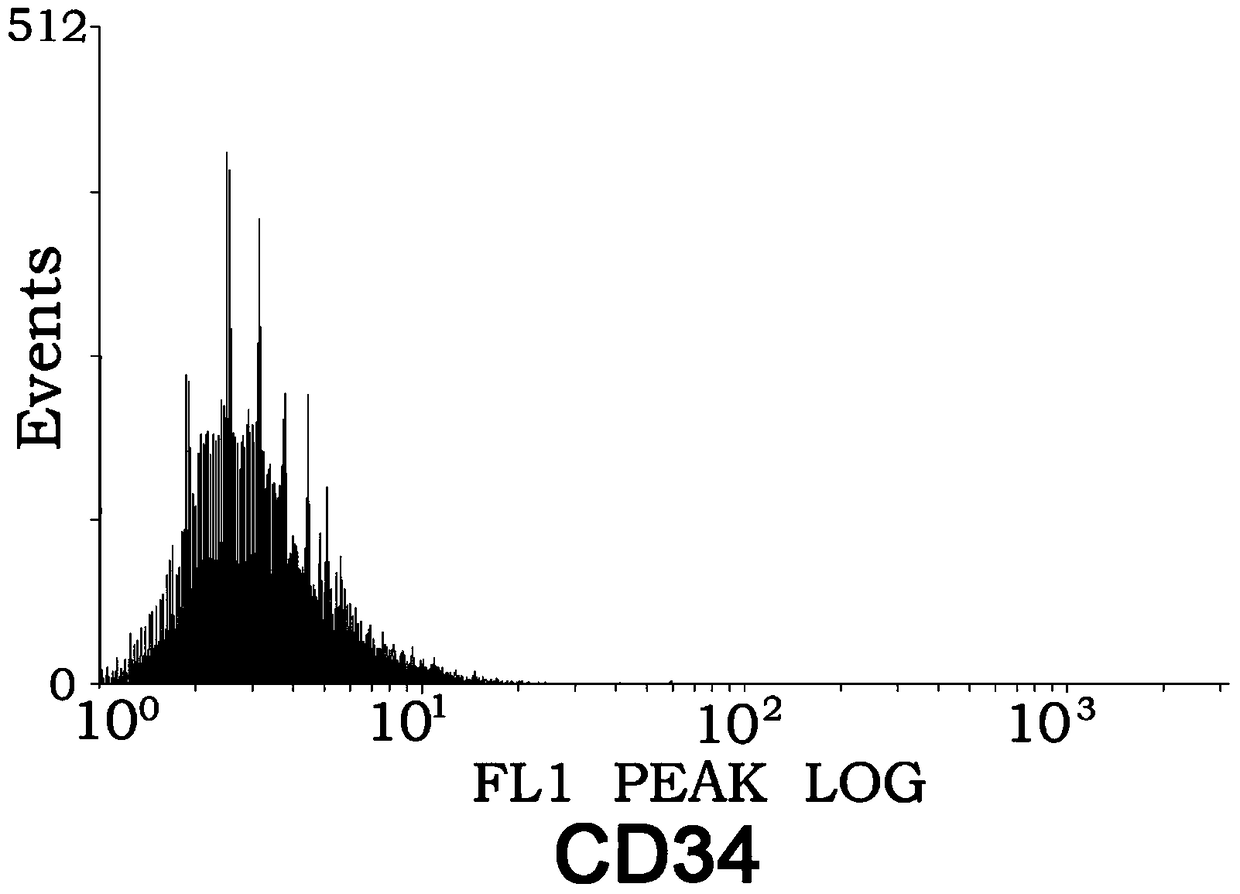
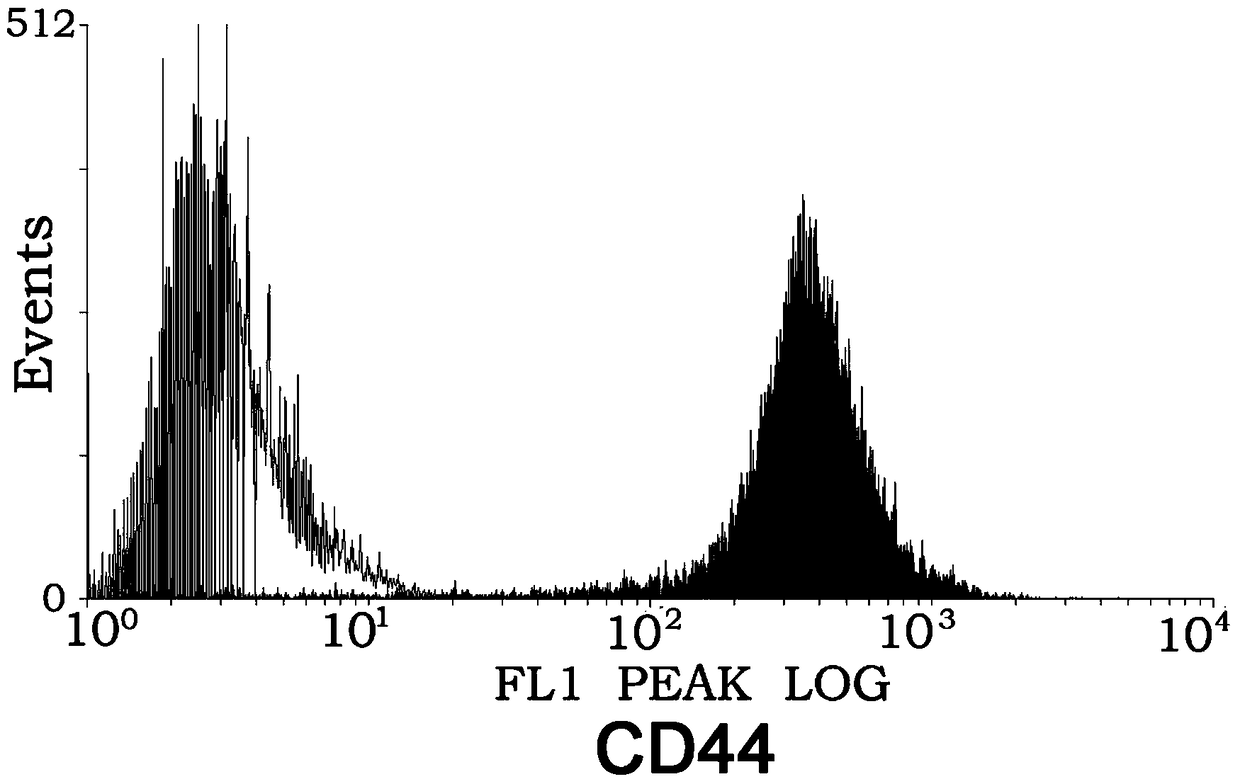
InactiveUS20170000886A1Improve mobilityEnhance metastatic potentialPolypeptide with localisation/targeting motifPeptide/protein ingredientsNucleotideCD44
An isolated polynucleotide comprising the element of: a promoter element that drives expression of the C—C motif ligand 5 (“a CCL5 promoter”) operatively linked to a polynucleotide encoding a fusion polypeptide comprising the Fc and hinge regions of a human IgG CD44 variant 6 (CD44v6) polypeptide is described.
Owner:RGT UNIV OF CALIFORNIA
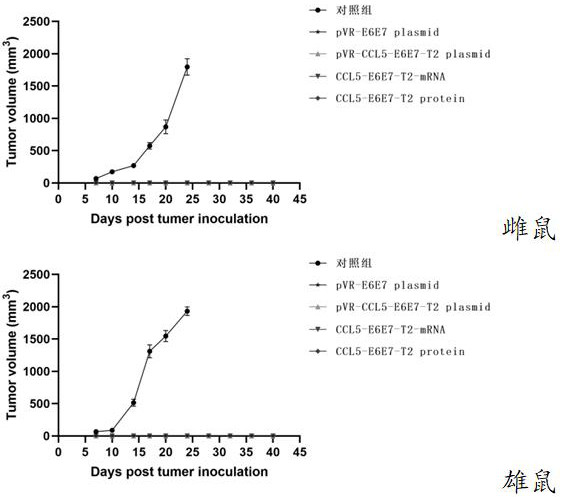
Application of CCL5
ActiveCN114106207AStrong immune boosting effectTrigger immune responseAntibody mimetics/scaffoldsVirus peptidesDiseaseImmune effects
The invention relates to the technical field of biology, and discloses application of CCL5. Different antigen proteins are transferred to the surfaces of the DC cells by utilizing the chemotactic binding capacity of the CCL5 and surface receptors of immune cells such as the DC cells, so that the efficiency of phagocytosis, processing and presentation of various antigen proteins by the DC cells is improved, and the effect of preventing and treating related diseases by the DC cells is improved. A T2 sequence is also added into the antigen, so that the immune effect is enhanced.
Owner:NEWISH TECH (BEIJING) CO LTD
Therapeutic agents for modulating thymic function and/or growth and/or treating various disorders
InactiveUS20160175308A1Increase thymus weightWeight increaseBiocideImmunoglobulinsAutoimmune responsesAgonist
The present disclosure relates to a therapeutic agent for use in a method for modulating the function and / or growth of a thymus in a subject, wherein the therapeutic agent comprises an HER2 or HER1 pathway antagonist or agonist, and / or a CCR / CCL5 antagonist the method involving administering the therapeutic agent to the subject. Also disclosed herein is a therapeutic agent for use in a method for treating a disorder in a subject, the disorder selected from systemic autoimmunity, peripheral autoimmunity and Systemic Lupus Erythematosus,
Owner:UCL BUSINESS PLC
Reagent kit for detecting children disease genetic susceptibility
The present invention provides a kit of detecting genetic predisposition of children syndrome. The kit includes specific primer pairs and specific fluorescent probe pairs for detecting 28 SNP polymorphism genotypes on the genes NOS3, CTLA4, ENOS, ERCC1, ERCC2, MTHFR, MTRR, MTR, MS4A2, XRCC1, ALDH2, TNFA, CCL5, PARP1, OPG, VDR, PON1, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1 at the same time, and normal components for FQ-PCR detection. The kit of the invention evaluates the genetic predisposition of children syndrome by detecting the genotypes of the 28 SNP polymorphism sites at the same time which is tightly relative to the genetic predisposition of children syndrome.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Gene modified mesenchymal stem cell and application thereof
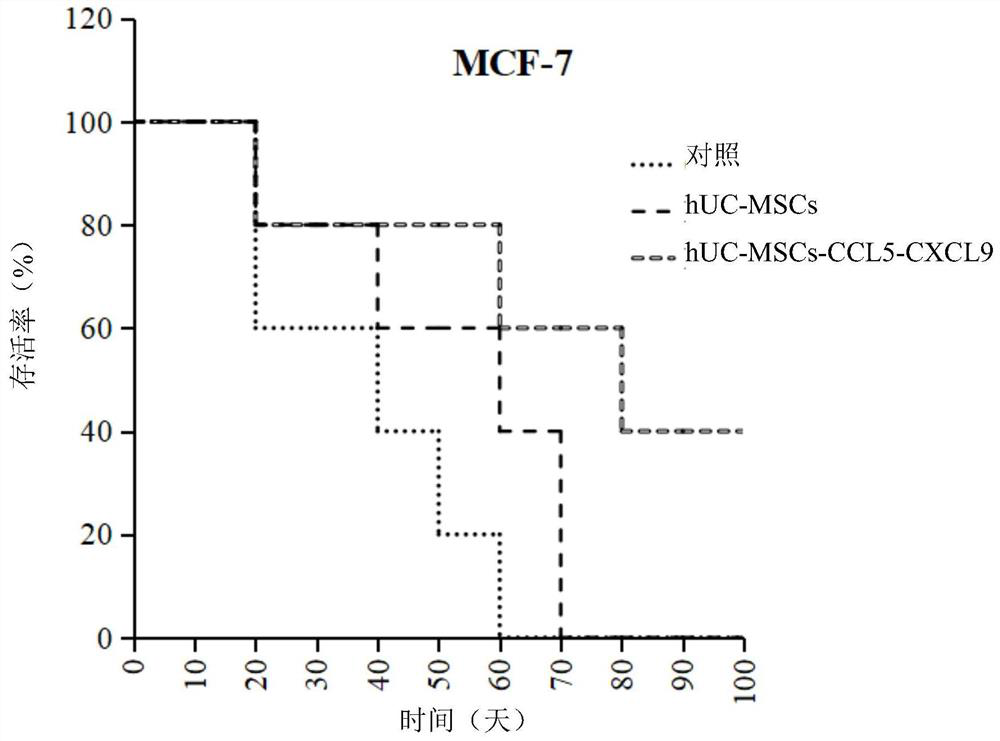
PendingCN112029730AEffective therapeuticEffective preventionChemokinesUnknown materialsMesenchymal stem cellPharmaceutical drug
The invention provides a gene modified mesenchymal stem cell and application thereof. The gene modified mesenchymal stem cell expresses CCL5 and CXCL9 proteins. The invention also provides applicationof the gene modified mesenchymal stem cell in preparation of drugs for treating or preventing tumors, and application of the gene modified mesenchymal stem cell in preparation of vaccines for treating or preventing tumors. The scheme for treating or preventing tumors by using the gene modified mesenchymal stem cell provided by the invention can significantly prolong the lifetime of tumor-bearingmice.
Owner:深圳市芥至和生物科技有限公司
Prognostic and treatment response predictive method
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of atleast 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL1A in a sample obtained from the tumour of the patient; b)measuring the gene expression of atleast 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained fromthetumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including withimmune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Marker for cancer cell drug resistance, preparation composition for reversing cancer cell drug resistance and application of preparation composition
ActiveCN113512587AIncreased drug resistanceInhibition of drug resistanceMicrobiological testing/measurementBiological material analysisCancer cellOncology
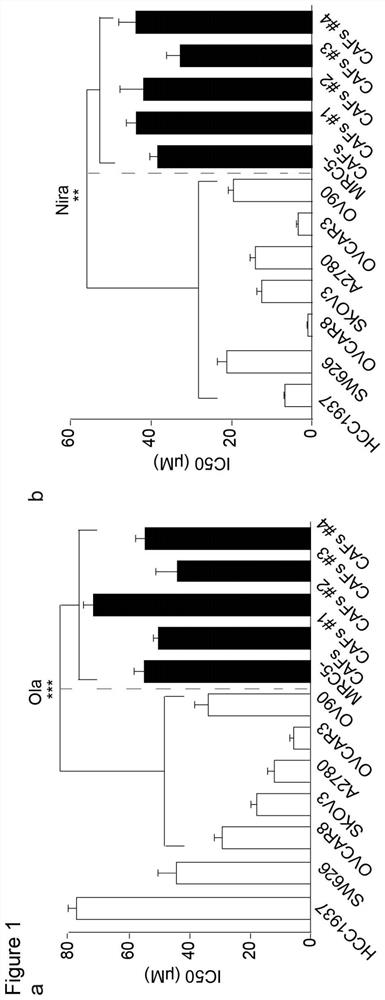
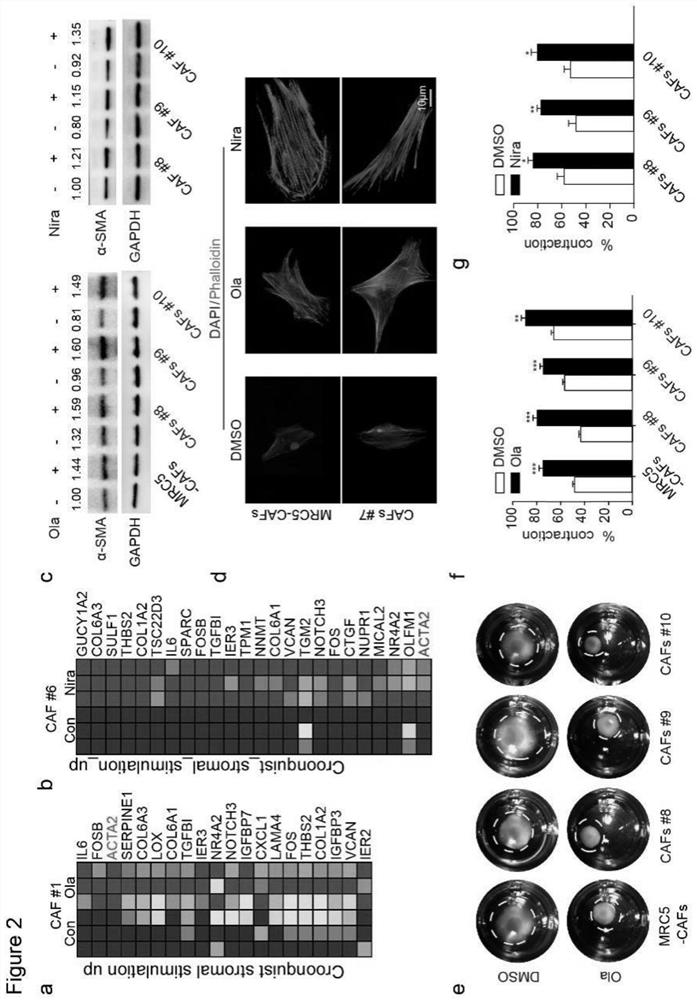
The invention relates to a marker for cancer cell drug resistance, a preparation composition for reversing cancer cell drug resistance and application of the preparation composition. The marker comprises a CCL5 chemotactic factor of cancer-related fibroblasts and a gene coded by the CCL5 chemotactic factor, and the expression level of the CCL5 chemotactic factor is positively correlated with the drug resistance of cancer cells. The preparation combination comprises a PARP inhibitor for inhibiting cancer cell replication and a reversal agent for regulating the cancer cell drug resistance caused by PARP. The reversal agent includes at least one of a regulator that down-regulates the expression of cancer-associated fibroblasts CCL5, a neutralizing antibody of CCL5, and an NF-[kappa] B signal inhibitor. The CCL5 expression related regulator, the CCL5 neutralizing antibody and / or the targeted NF-kappa B inhibitor are / is adopted to reverse the CAFs activation effect induced by the PARP inhibitor, the cancer cell drug resistance caused by the PARP inhibitor is inhibited, and the cancer cell killing effect of the PARP inhibitor is improved.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Chemotherapy regimen selection
The present invention provides, inter alia, kits for selecting a chemotherapy regimen for a subject. The kits comprise one or more components for detecting the expression of at least one gene from the group of SLC12A7, GZMB, TAF6L, NFIB, METRN, ROPN1B, TTK, CCND1, PTTG1, H2AFZ, WDR45L, DEK, MCM2, USP1, CDT1, TMEM97, RER1, MCM6, LZTFL1, C11orf17, CCL5, XCL1, XCL2, MELK, CTSL2, TPX2, AURKA, CDKN2C, BRP44, PNP, SMC4, NR4A2, C3orf37, MTPAP, CDC25B, ABCF1, MTAP, SNAPC3, RANBP9, COIL, FAM86B1, ITGA6, S100P, RANBP1, PRSS16, SMARCA2, STK24, TSPYL5, SRI, LRP12, CENPF, TUBD1, KIAA1324, DBF4, CCNA2, DLGAP5, FHL1, SIRT3, GTSE1, PCNA, CCNE2, CHD3, CAP1, GPM6B, GUSBP3, GNAI3, LMO4, PSRC1, USP1, STK38, BAT2L1, PMP22, NME5, CENPA, BANK1, and derivatives thereof. Methods for selecting a chemotherapy regimen for a subject are also provided.
Owner:INNOMEDICINE LLC
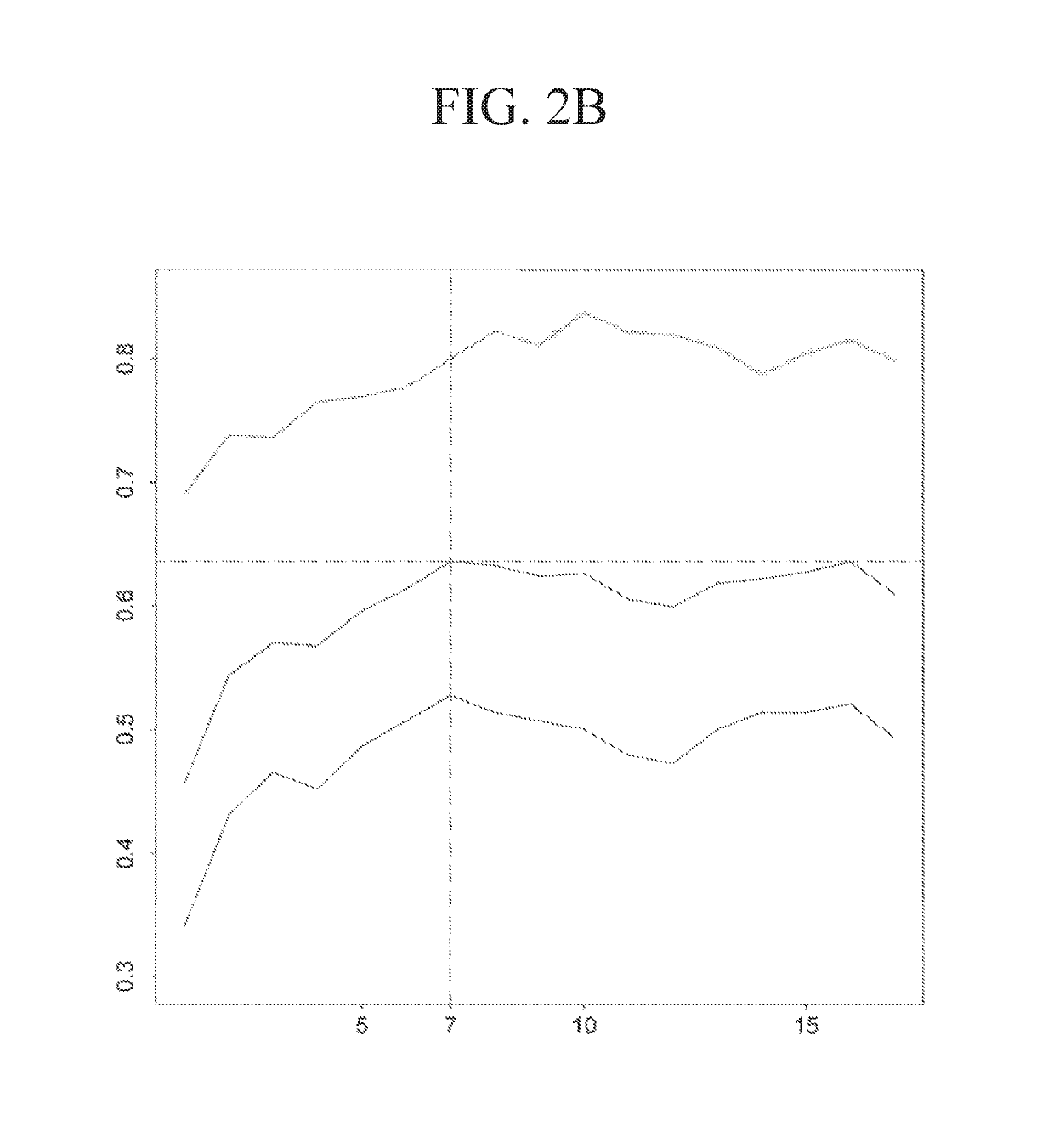
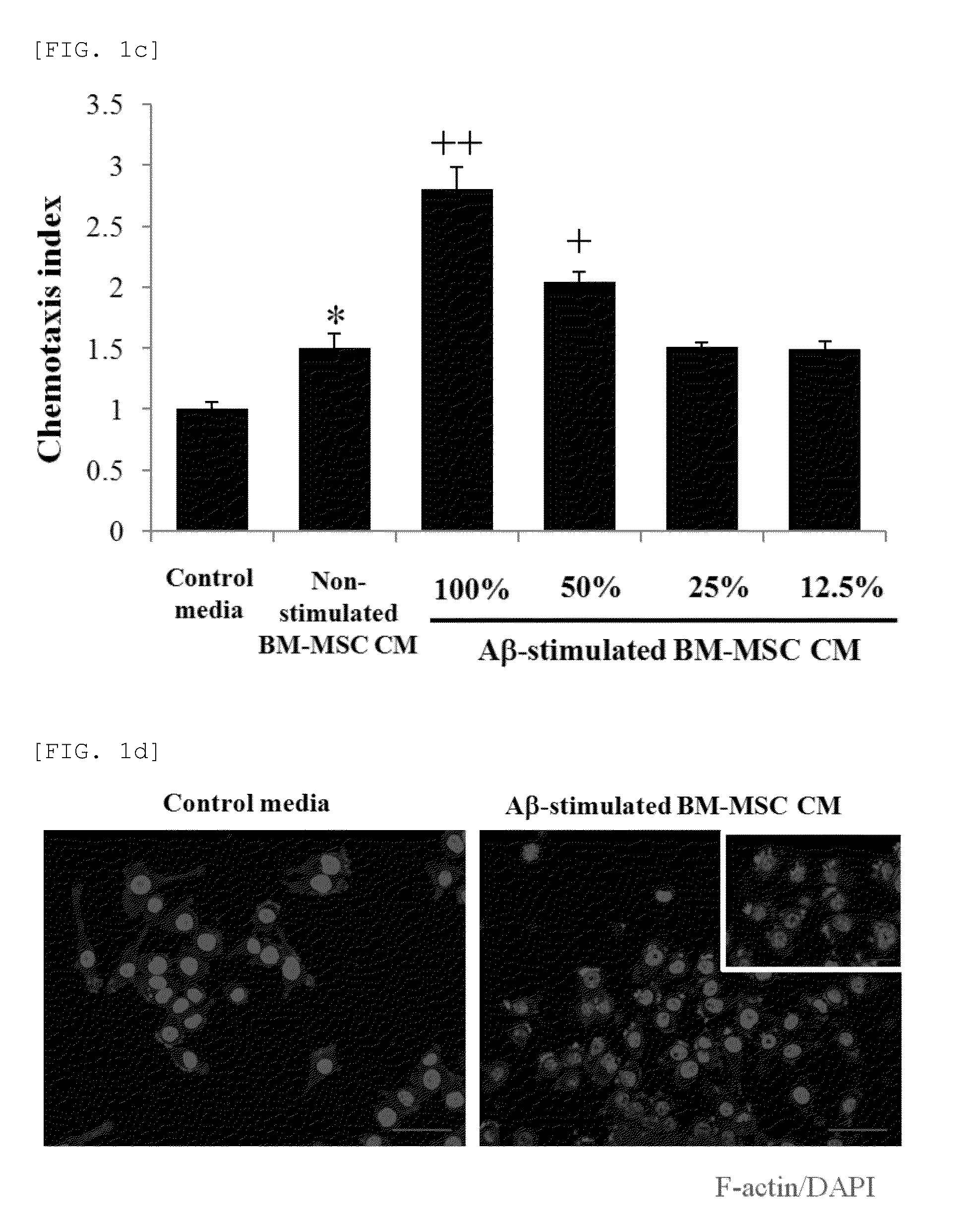
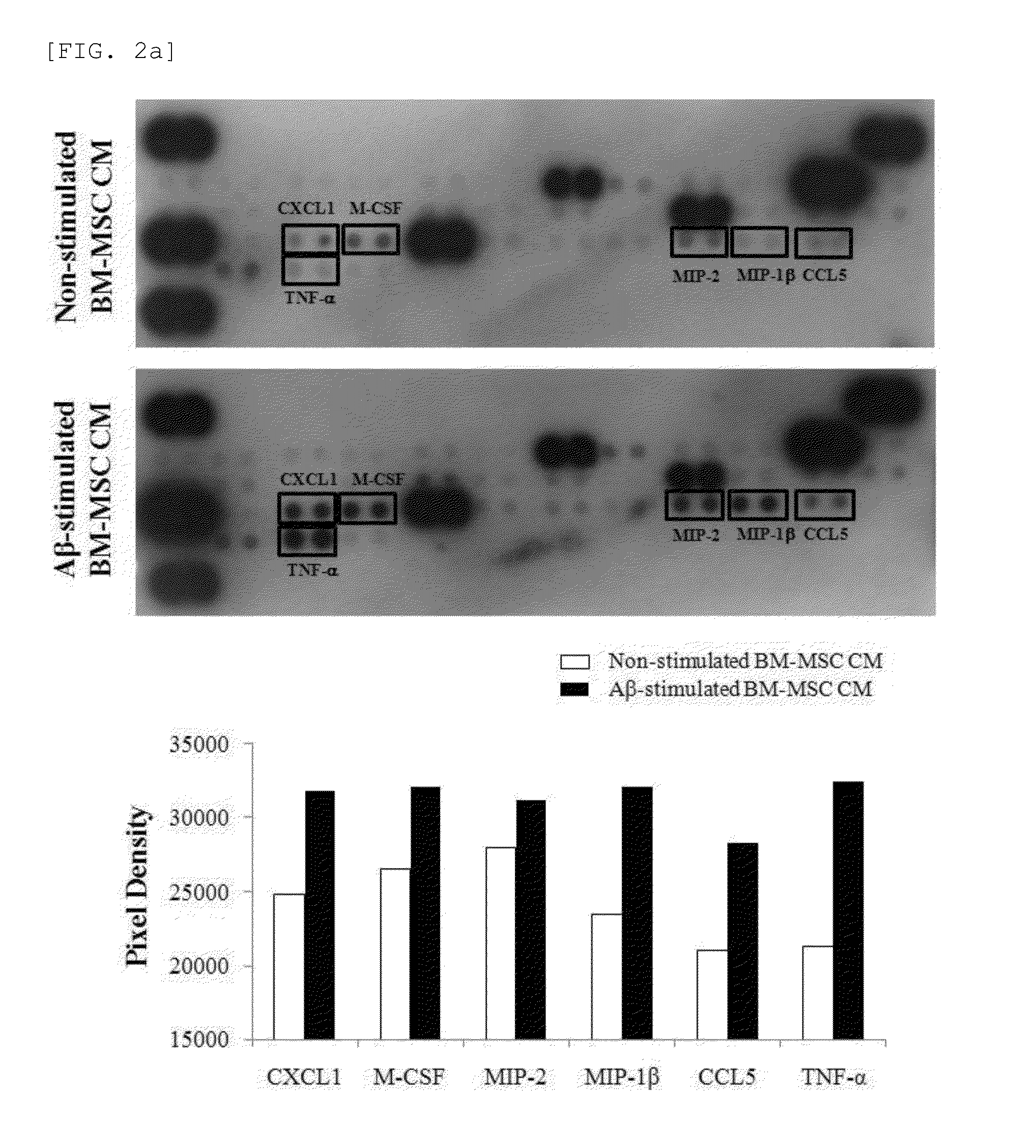
Composition for preventing or treating neurodegenerative diseases containing ccl5
There is provided a composition for preventing or treating neurodegenerative diseases, including one or two or more selected from the group consisting of a chemoattractant CCL5, a CCL5 expression regulator, and a CCL5 activator as an active ingredient, in which by confirming through a morris water maze task that the CCL5 recovers memory loss and improves spatial cognition ability, it has been found that the composition including any one or two or more selected from the group consisting of the CCL5, the CCL5 expression regulator, and the CCL5 activator can be usefully employed as a pharmaceutical composition or a food composition for preventing or treating neurodegenerative diseases.
Owner:KYUNGPOOK NAT UNIV IND ACADEMIC COOP FOUND
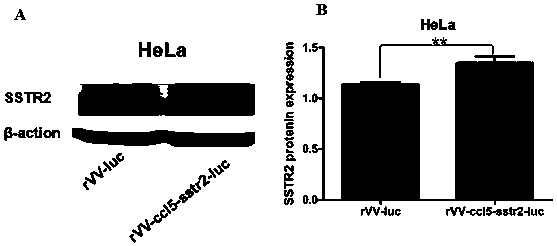
Recombinant poxvirus containing ccl5 and sstr2 genes and preparation method thereof
ActiveCN107164337BMolecular-level technology is simple and easy to implementLarge capacity for inserting foreign genesChemokinesMicroorganism based processesTumor cellsTarget gene
The invention discloses a recombinant vaccinia virus rVV-CCL5-SSTR2-Luc+, containing genes CCL5 and SSTR2, constructed by virtue of a homologous recombination method. By utilizing the characteristic that shuttle plasmids containing human target genes CCL5 and SSTR2 and Luciferase reporter genes have same TK side wings with a vaccinia virus WR strain, homologous recombination is carried out in a vaccinia virus susceptible cell BS-C-1 to integrate the target genes to a vaccinia virus genome, so as to construct the recombinant vaccinia virus rVV-CCL5-SSTR2-Luc+. The recombinant vaccinia virus rVV-CCL5-SSTR2-Luc+ is capable of efficiently expressing exogenous genes, meanwhile, CCL5 is matched with highly expressed CCR5 on the surface of a cell CIK, and SSTR2 has the unique advantage on directional induction of octreotide targeted tumor cells, so that the recombinant vaccinia virus is beneficial to clinical expansion and application and is used for treating various tumors.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE
Signature for predicting clinical outcome in human HER2+ breast cancer
ActiveUS9803245B2Reduced survivalConducive to survivalBiocideOrganic active ingredientsOncologyCD79B
A method of predicting outcome in a subject with for example Her2+ (ERα−) breast cancer comprising: (a) determining a HTICs expression signature comprising determining an expression level of 2 or more HTICS biomarkers selected from Aurkb, Ccna2, Scrn1, Npy, Atp7b, Chaf1b, Ccnb1, Cldn8, Nrp1, Ccr2, C1qb, Cd74, Vcam1, Cd180, Itgb2, Cd72, St8sia4, Kif11, Plk1, Chek1, Mphosph6, Cora1a, Ccl5, Cd3e, Hcls1, Vav1, Plek, Arhgdib, Il2rg, Sash3, Lck, Il2rb, Cybb, Cd79b, Sell, Ccnd2, Tnfrsf1b, Rftn1, Rac2 and Ly86; and (b) calculating a signature score, the signature score comprising a sum of HTICs biomarker expression parameters; wherein a signature score greater than a selected cut-off or control signature score is indicative of a poor outcome (HTICS+) and a signature score less than a selected cut-off is indicative of a good outcome (HTICS−). The methods can be used to prognose outcome and / or select suitable treatment. Arrays and kits for use with the methods are also provided.
Owner:UNIV HEALTH NETWORK
The purpose of ccl5
ActiveCN114106207BStrong immune boosting effectTrigger immune responseAntibody mimetics/scaffoldsVirus peptidesDiseaseImmune effects
The invention relates to the field of biotechnology, and the invention discloses a use of CCL5. The present invention utilizes the chemotactic binding ability of CCL5 and immune cell surface receptors such as DC cells to transport different antigenic proteins to the surface of DC cells, improves the efficiency of phagocytosis, processing and presentation of various antigenic proteins by DC cells, and improves the efficiency of DC cells. The effect of preventing and treating related diseases. The present invention also adds T2 sequence to the antigen so as to enhance the immune effect.
Owner:NEWISH TECH (BEIJING) CO LTD
Antibody
PendingUS20220106402A1Improve anti-tumor activityModulationImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisCell phenotypeWhite blood cell
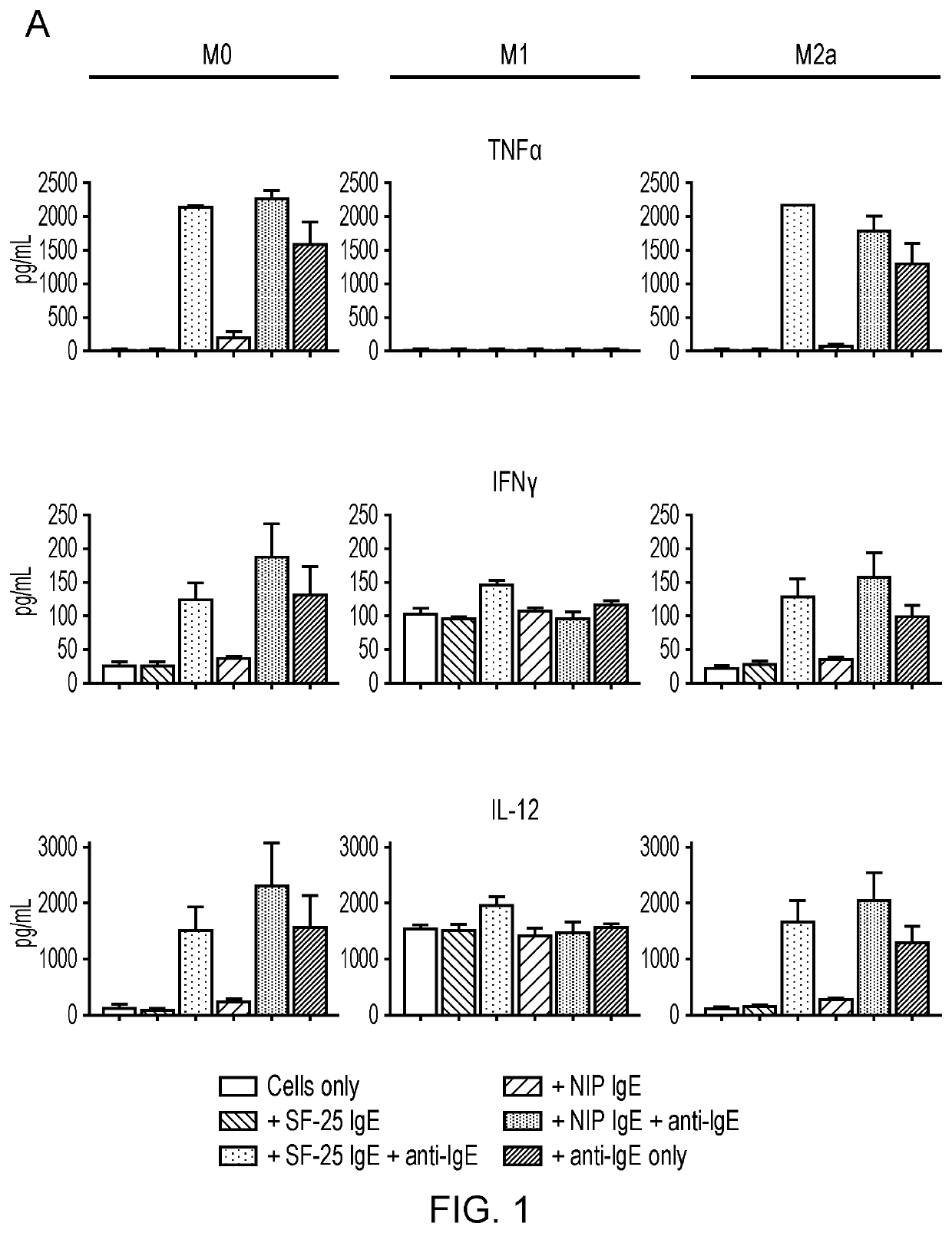
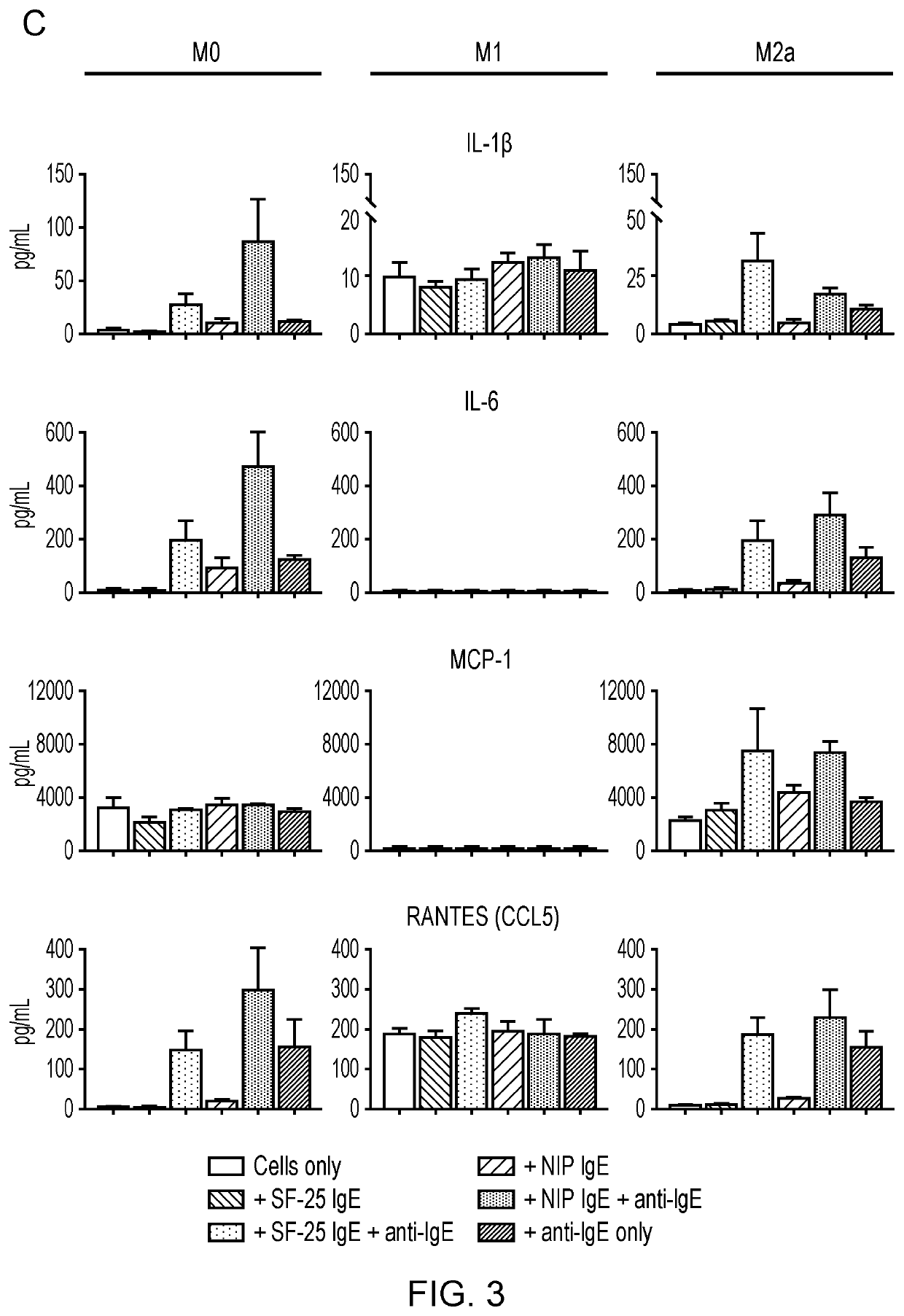
In one aspect, the present invention relates to an immunoglobulin E (IgE) for use in repolarizing macrophages from a first phenotype to an anti-tumor phenotype in the treatment of cancer in a subject; wherein the first phenotype comprises a quiescent (M0) macrophage phenotype or an anti-inflammatory (M2a) macrophage phenotype; and the anti-tumor phenotype comprises a newly polarized macrophage phenotype characterized by expression of the following cytokines and chemokines: tumor necrosis factor alpha (TNFα); interferon-gamma (IFNγ); interleukin-1beta (IL-1β); interleukin-6 (IL-6); Regulated on Activation, Normal T cell Expressed and Secreted (RANTES or CCL5); and / or interleukin-10 (IL-10).
Owner:KING'S COLLEGE LONDON
Method for enhancing chemotactic ability of mesenchymal stem cells and expression of chemokine ccl5
ActiveCN105331580BNo need for transfectionImprove migration abilityMicrobiological testing/measurementSkeletal/connective tissue cellsHuc mscsImmuno modulation
Owner:XIAMEN UNIV
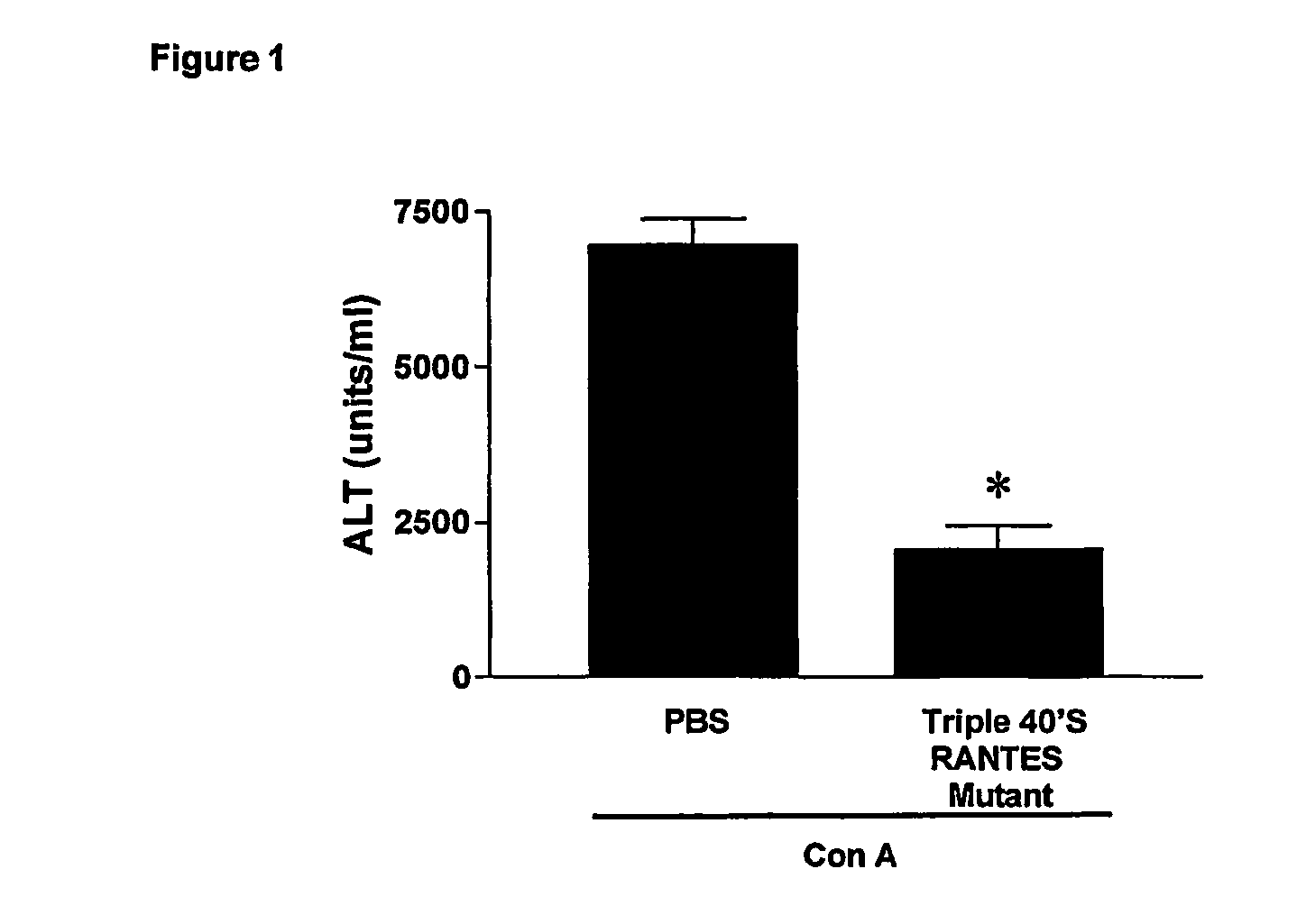
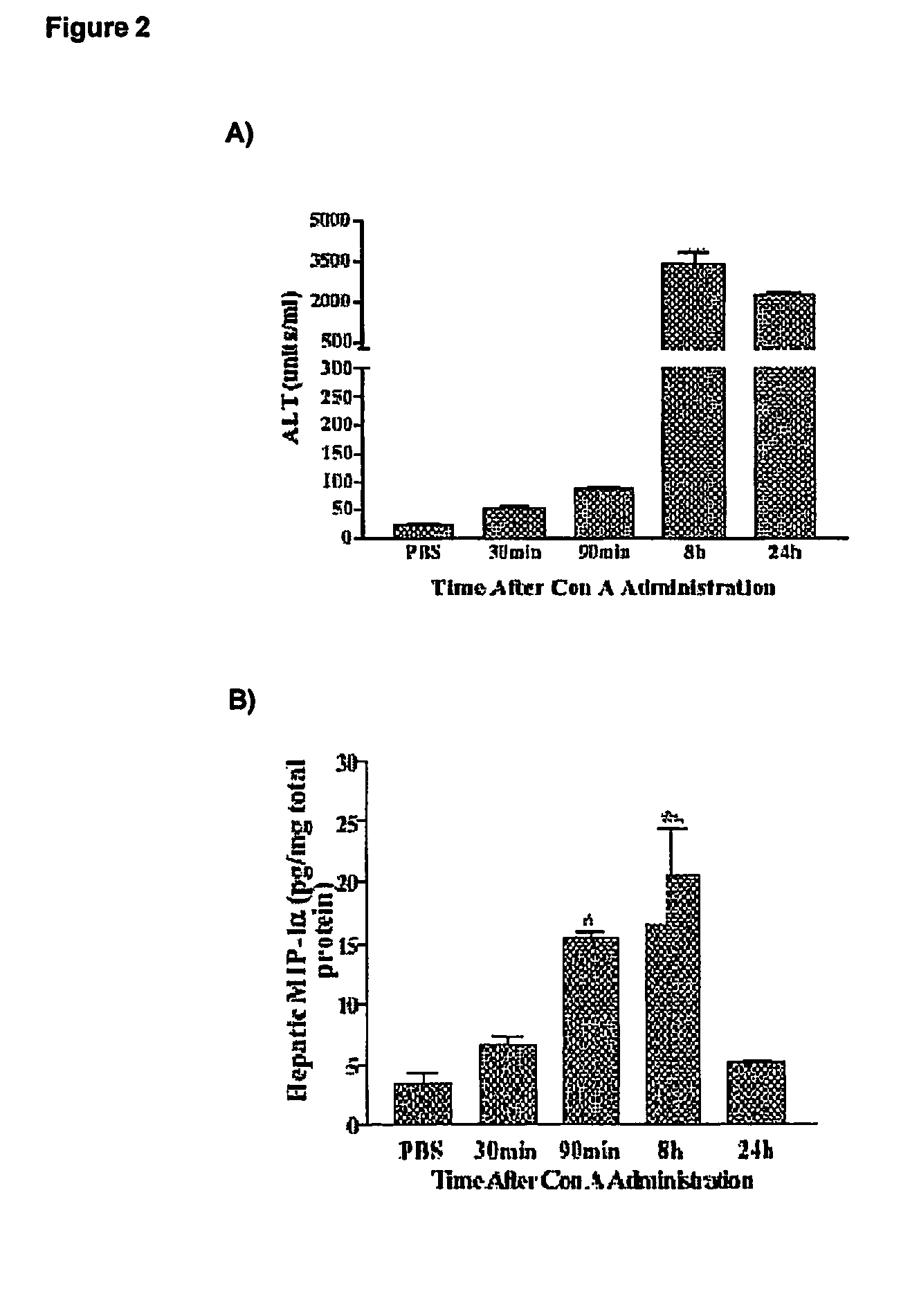
Methods of reducing serum alanine transferase levels in a subject with hepatitis
CC-Chemokine mutants having reduced Glycosaminoglycans (GAG)-binding properties are effective against liver fibrotic inflammatory and / or autoimmune diseases. Particularly preferred are the mutants of CCL5 / RANTES having reduced GAG-binding properties.
Owner:MERCK SERONO SA
Chemotactic factor as molecular marker for diagnosing rosacea
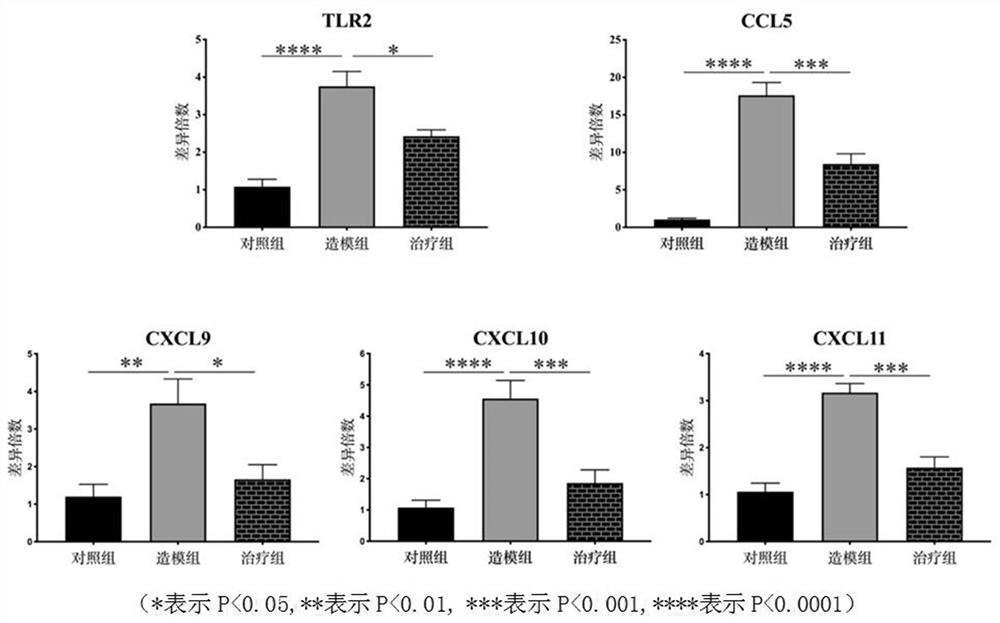
The invention belongs to the field of biological medicine, and particularly relates to the application of a chemotactic factor serving as a rosacea related molecular marker in diagnosis and treatment of the rosacea related molecular marker. The expression of TLR2 and chemotactic factors CCL5, CXCL9, CXCL10 and CXCL11 in rosacea animals induced by LL-37 is increased, and the AhR agonist benvimod can relieve erythema and inflammatory cell infiltration and reduce the expression of the TLR2 and the chemotactic factors. The expression of TLR2 and chemotactic factors CCL5, CXCL9, CXCL10 and CXCL11 in a rosacea cell model induced by LL-37 is increased, and the expression of TLR2 and the chemotactic factors can be reduced by an AhR agonist, namely, benvimod; the up-regulation of the TLR2 can cause the increase of the expression of the chemotactic factor in the cell model, and the AhR agonist benvimod can reduce the expression of the chemotactic factor by down-regulating the expression of the TLR2. The chemotactic factors CCL5, CXCL9, CXCL10 and CXCL11 are expected to become new diagnosis indexes or treatment targets of rosacea diseases.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Engineered immune cells and uses thereof
PendingCN114075550AIncreased proliferationProlong survival timeVirusesAntiinfectivesAutoimmune conditionReceptor
The present invention relates to an engineered immune cell that expresses (i) a chimeric receptor, and (ii) an exogenous CCL3, CCL4 and / or CCL5. The invention also provides application of the engineered immune cell in treatment of cancer, infection or autoimmune diseases. Compared with traditional engineered immune cells, the engineered immune cells provided by the invention have significantly improved tumor killing activity.
Owner:NANJING BIOHENG BIOTECH CO LTD
Diagnostic methods and compositions for cancer immunotherapy
PendingUS20210301023A1Improve responsivenessResponsiveness to treatmentOrganic active ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesAntigen Binding Fragment
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The compositions and methods described herein can be used, for example, to determine the propensity of a patient to benefit from treatment with a PD-L1 axis binding antagonist and to treat such patients accordingly. Using the compositions and methods of the disclosure, a patient, such as a human cancer patient, may be determined to be likely to benefit from treatment with a PD-L1 axis binding antagonist if the patient exhibits an elevated pre-treatment expression level of one or more of CST7, NKG7, GZMH, MT-ND4, HLA-H, CCL5, CD8A, CMC1, CD8B, HCST, MT-CYB, MT-ND4L, KLRG1, MT-CO2, MT-ATP6, PLEK, CTSW, HLA-C, LYAR, LITAF, GZMB, KLRD1, FGFBP2, KLRC4-KLRK1, KLRK1, B2M, GZMA, ID2, CX3CR1, PRSS23, GNLY, PRF1, and PATL2. Exemplary PD-L1 axis binding antagonists that may be used in conjunction with the compositions and methods of the disclosure are PD-L1 binding antagonists, such as anti-PD-L1 antibodies and antigen-binding fragments thereof, including atezolizumab, as well as PD-1 binding antagonists, such as anti-PD-1 antibodies and antigen-binding fragments thereof.
Owner:GENENTECH INC
Immunosuppressant Comprising TSAHC or a Pharmaceutically Acceptable Salts Thereof as an Active Ingredient
PendingUS20220160663A1Suppressed expressionSuppressed secretionDigestive systemAmide active ingredientsIMMUNE SUPPRESSANTSCCL2
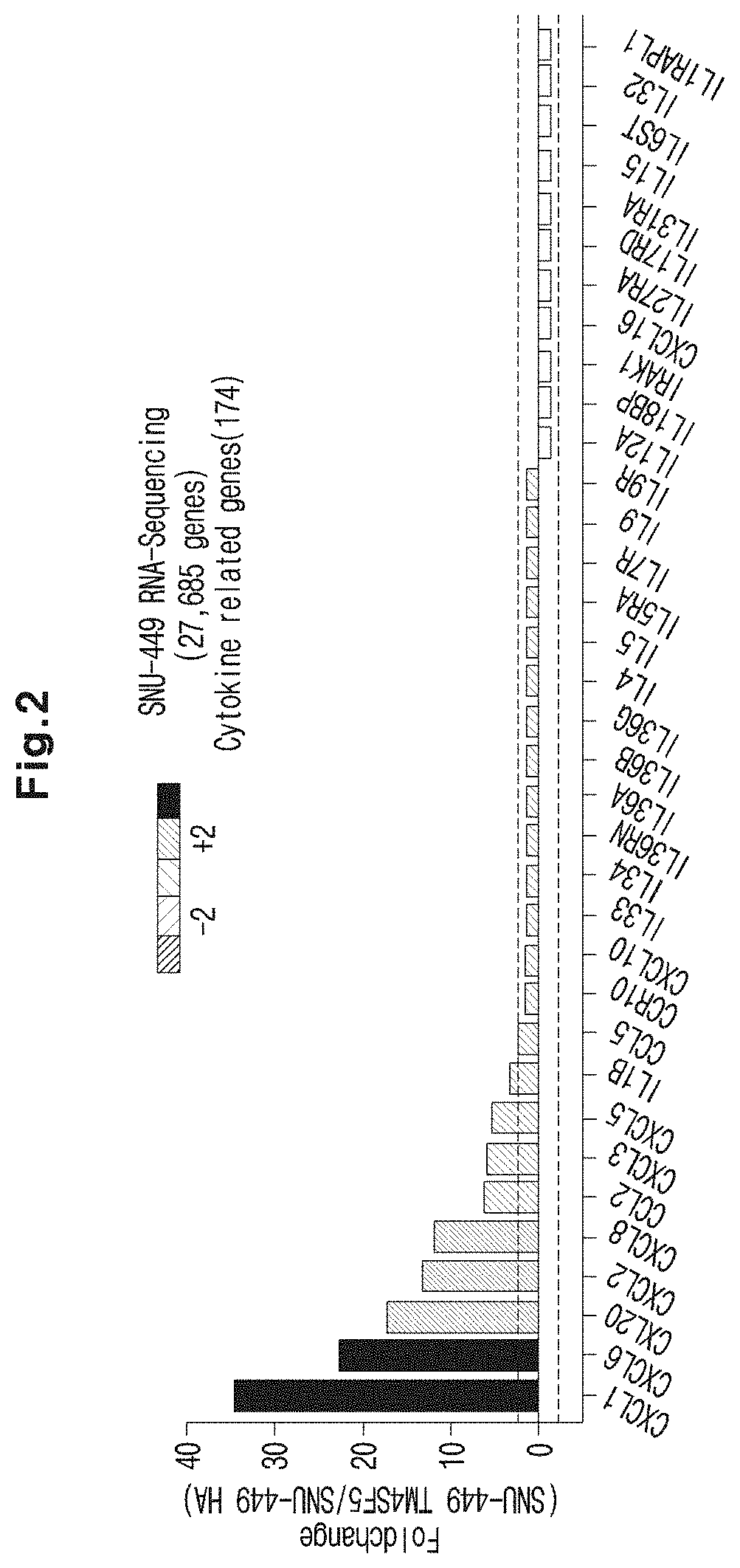
The present invention relates to an immunosuppressant comprising TSAHC or a pharmaceutically acceptable salt thereof as an active ingredient. TSAHC treatment suppressed the expression and secretion of cytokines / chemokines such as IL6, IL1β, TNFα, CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, CCL2, CCL5, CCL20, CXCL10 and CCR10 according to the expression of TM4SF5 gene and protein, and the cytokines or chemokines mediated by the expression of TM4SF5 induced abnormalities in the inflammatory response and metabolic function of liver tissues, hepatic epithelial cells and macrophages, and correlated with liver tissue damage and immune cell recruit in TM4SF5 transgenic animals. Therefore, a composition comprising TSAHC or a pharmaceutically acceptable salt thereof as an active ingredient can be effectively used as an immunosuppressant.
Owner:SEOUL NAT UNIV R&DB FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com