Gene modified mesenchymal stem cell and application thereof
A technology of mesenchymal stem cells and genetic modification, applied in the field of genetically modified cells and tumor treatment, can solve the problems of limiting the role of MSCs, lack of sufficient evidence to show MSCs, and accelerate the development of tumors, etc., to achieve the effect of prolonging the survival period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of genetically modified mesenchymal stem cells
[0058] 1. Construction of lentiviral plasmid vector
[0059] 1) Synthesize and amplify the nucleotide sequences SEQ ID NO:3 and SEQ ID NO:4 encoding the CCL5 and CXCL9 proteins of the present invention, completed by Shanghai Langjing Biotechnology Co., Ltd.;
[0060] 2) Cloning SEQ ID NO: 3 and SEQ ID NO: 4 into the lentiviral expression vector pGreen puro respectively by conventional genetic engineering means to obtain the lentiviral expression vector A containing the nucleotide sequence encoding the CCL5 protein and the lentiviral expression vector A containing the encoding CXCL9 The lentiviral expression vector B of the nucleotide sequence of the protein.
[0061] The specific process of constructing lentiviral expression vector A and lentiviral expression vector B is as follows:
[0062] Insert SEQ ID NO: 3 and SEQ ID NO: 4 into the lentiviral expression vector pGreen puro separately through th...
Embodiment 2
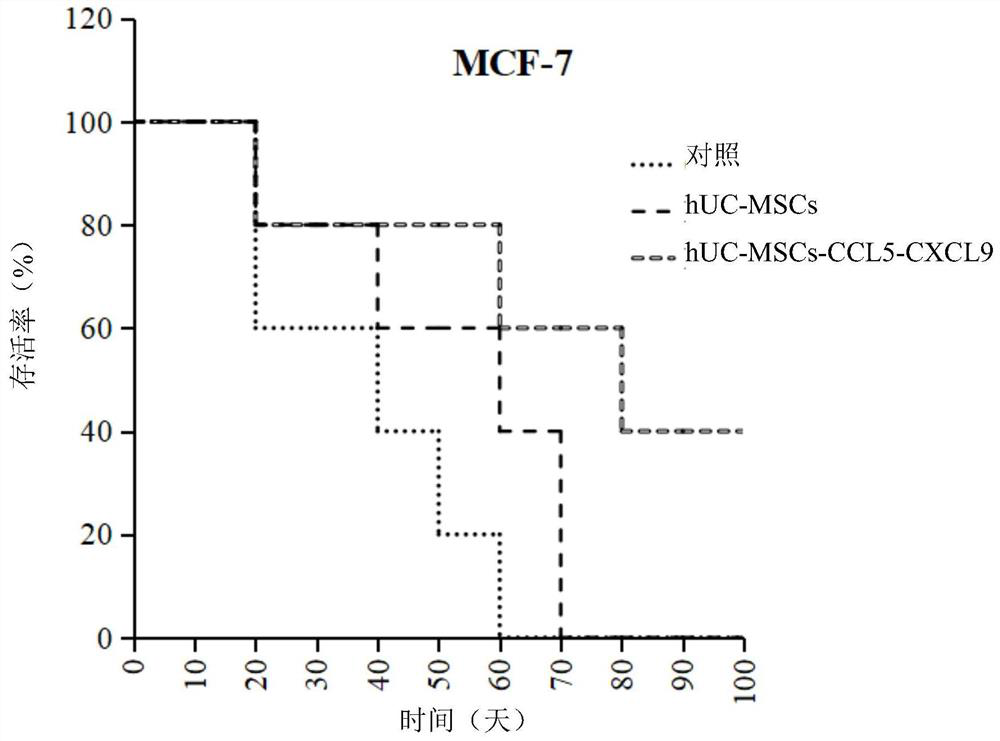
[0089] Example 2 Verification of the effect of the gene-modified mesenchymal stem cells provided by the present invention in treating tumors (breast cancer, pancreatic cancer, liver cancer, lung cancer, gastric cancer, colon cancer) in vivo
[0090] (1) Take 15 female nude mice (6 weeks old, weighing 18-20g, purchased from Guangdong Medical Experimental Animal Center), and inject 5x 10 6 MCF-7 (breast cancer) cells, when the tumor grows to 60mm 3 Size, tumor models were randomly divided into 3 groups:
[0091] a. Control group: intravenous injection of normal saline 200ul / time, once a week, 4 times in total;
[0092] b. hUC-MSCs cell therapy group: 3×10 hUC-MSCs cells were injected into the tail vein 6 One piece / time, 200ul in total, once a week, 4 times in total;
[0093] c.hUC-MSCs-CCL5-CXCL9 cell therapy group: tail vein injection
[0094] hUC-MSCs-CCL5-CXCL9 cells 3×10 6 Each time, a total of 200ul, once a week, a total of 4 times; the survival status of the mice with...
Embodiment 3
[0128] Example 3 Verification of the in vivo tumor prevention effect of the genetically modified mesenchymal stem cells provided by the present invention (1) Take 15 female nude mice (6 weeks old, weighing 18-20 g, purchased from Guangdong Medical Experimental Animal Center), and randomly divide them into 3 groups:
[0129] a. Control group: Inject normal saline 200ul / time into tail vein, once a week, 4 times in total;
[0130] b. hUC-MSCs cell prevention group: 3×10 hUC-MSCs cells were injected into the tail vein 6 One piece / time, 200ul in total, once a week, 4 times in total;
[0131] c.hUC-MSCs-CCL5-CXCL9 cell prevention group: tail vein injection
[0132] hUC-MSCs-CCL5-CXCL9 cells 3×10 6 Each time, a total of 200ul, once a week, a total of 4 times.
[0133] Afterwards, the mice in each group were subcutaneously injected with 5x 10 6 For MCF-7 (breast cancer) cells, the survival status of the mice within 120 days was counted, and the survival rate curve was made. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com