Engineered immune cells and application thereof
An immune cell and engineering technology, applied to animal cells, genetically modified cells, cells modified by introducing foreign genetic material, etc., can solve the problem that CAR cells cannot infiltrate tumor tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
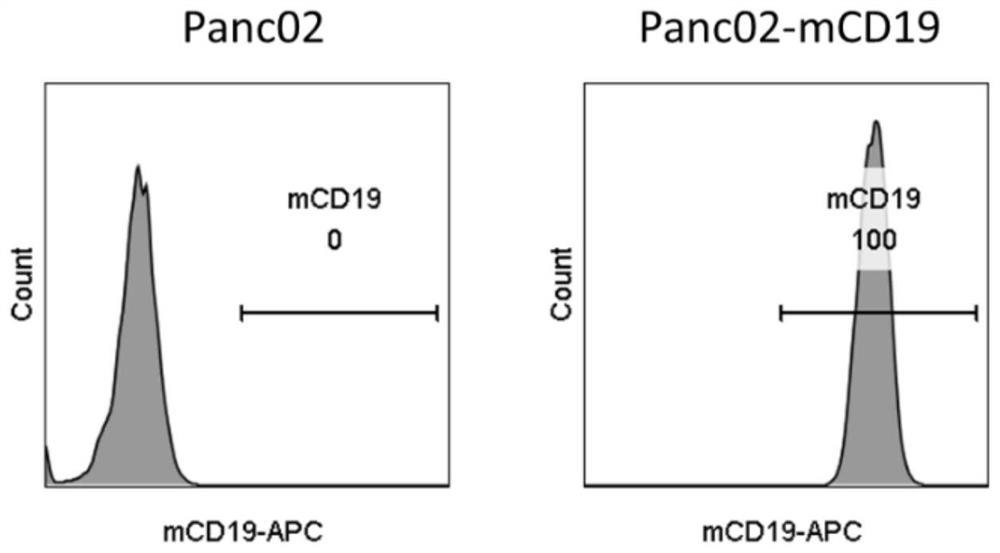
[0126] Example 1 Construction of mouse pancreatic cancer cell line Panc02-mCD19
[0127] 1. Preparation of pLV-mCD19 plasmid
[0128] Using the total mouse spleen mRNA as a template, the total cDNA sequence of the mouse spleen was obtained by reverse transcription PCR, and then the mouse mCD19 sequence containing XbaI and SalI restriction sites was obtained by PCR. Then, the mCD19 gene was recombined into the pLVX vector (PPL, Cat No.: PPL00157-4a) to obtain the pLV-mCD19 plasmid.
[0129] 2. lentiviral packaging
[0130] In a T175 culture flask, 30×10 6 293T cells were inoculated in 30ml of DMEM medium containing 10% fetal bovine serum at a density of 1 cell / flask at 37°C, 5% CO 2 Incubate overnight in the incubator.
[0131] Add 3ml Opti-MEM (Gibco, Cat. No. 31985-070), 34 μg pLV-mCD19 plasmid, 8.5 μg pMD2.G vector (Addgene, Cat. No. 12259) and 17 μg psPAX2 vector (Addgene, Cat. No. 12260) into a sterile tube. Then add 120 μl X-treme GENE HP DNA transfection reagent (R...
Embodiment 2
[0135] Example 2. Preparation of CAR-T cells
[0136] 1. Construction of Retroviral Plasmids
[0137]Artificially synthesized CD19-scFv (SEQ ID NO: 26), CD8a hinge region and transmembrane region (SEQ ID NO: 35 and 29), 41bb intracellular domain (SEQ ID NO: 31) and CD3ζ intracellular domain ( SEQ ID NO: 33) coding sequence fragment, and add XhoI / EcoRI restriction sites at both ends. The fragment was cloned into the MSCV vector to obtain the MSCV-mCD19-CAR plasmid.
[0138] The coding sequence fragments of T2A (SEQ ID NO: 37) and IL-7 (SEQ ID NO: 43) were artificially synthesized sequentially, and EcoRI / SalI restriction sites were added at both ends. The fragment was cloned into the MSCV-mCD19-CAR vector to obtain the MSCV-mCD19-CAR-IL-7 plasmid.
[0139] The coding sequence fragments of T2A (SEQ ID NO: 37) and CCL4 (SEQ ID NO: 83) were artificially synthesized sequentially, and EcoRI / SalI restriction sites were added at both ends. The fragment was cloned into the MSCV-mCD1...
Embodiment 3
[0148] Example 3. Detecting the expression of CAR-T cells
[0149] 1. The expression level of CAR on the cell surface
[0150] Take out the 2 * 10 that embodiment 2 prepares 5 CAR-T cells, using Goat Anti-Rat IgG (H&L) Biotin (BioVision, Cat. No. 6910-250) as the primary antibody, APC Streptavidin (BD Pharmingen, Cat. No. 554067) as the secondary antibody, and detecting CAR T cells by flow cytometry On the expression level of CAR, the results are as follows figure 2 shown.
[0151] It can be seen that compared with the control, CAR positive in mCD19-CAR cells, mCD19-CAR+CCL4 cells, mCD19-CAR+CCL5 cells, mCD19-CAR+IL7+CCL4 cells, and mCD19-CAR+IL7+CCL5 cells The efficiencies are all about 60%, indicating that these cells can effectively express CAR.
[0152] 2. IL-7 expression level
[0153] The supernatant of the CAR-T cells prepared in Example 2 was collected, and according to the manufacturer's suggestion, the IL-7 secretion level in the cells was detected with the Mou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com